Abstract
Background
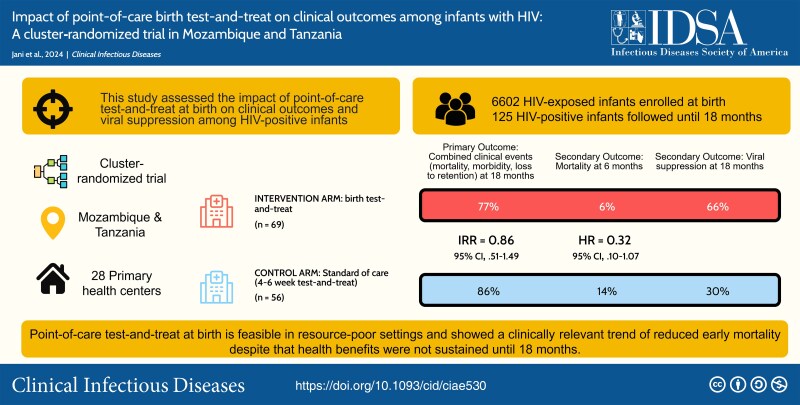
We assessed the impact of point-of-care (PoC) test-and-treat at birth on clinical outcomes and viral suppression among human immunodeficiency virus (HIV)–positive infants in Mozambique and Tanzania.
Methods
This cluster-randomized trial allocated health facilities to intervention, providing PoC testing and antiretroviral treatment (ART) at birth and week 4–8, or control, starting these at week 4–8. The primary outcome was proportions of clinical events (mortality, morbidity, retention, virological failure, toxicity) among HIV-positive infants at month 18. We estimated incidence rate ratios adjusted for timing of HIV detection (aIRR) and reported viral suppression <1000 copies/mL.
Results
Among 6602 neonates enrolled during October 2019–September 2021, 125 were diagnosed with HIV by week 12. In the intervention arm, 38 of 69 (55.1%) were diagnosed at birth. In the control arm, 27 of 56 (48.2%) were retrospectively detected to be HIV-positive at birth, of whom 6 of 56 (10.7%) died or were lost to follow-up before testing. Median age at ART initiation was 6 (intervention) versus 33 days (control). Birth test-and-treat was not associated with a significant reduction in clinical outcomes up to month 18 (53 [76.8%] vs 48 [85.7%]; aIRR, 0.857 [95% confidence interval, .505–1.492]), but showed a 68% relative reduction in 6-month mortality. Viral suppression was poor overall.
Conclusions
PoC test-and-treat at birth is feasible in resource-poor settings and resulted in clinically relevant reduction of early mortality, though improved clinical outcomes were not sustained to month 18. Poor viral suppression may undermine early benefits, calling for better pediatric treatments and adherence interventions.
Clinical Trials Registration. NCT04032522.
Keywords: HIV, point-of-care testing, birth testing, test-and-treat, neonatal treatment
Point-of-care test-and-treat for HIV at birth is feasible in sub-Saharan Africa but does not improve clinical outcomes at month 18, despite reducing mortality at month 6. Poor virologic control undermines survival benefits, requiring improved pediatric treatments and adherence interventions.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/institutional-portal/clinical-infectious-diseases/tidbits/impact-of-point-of-care-birth-test-and-treat-on-clinical-outcomes-among-infants-with-hiv-a-cluster-randomized-trial-in-mozambique-and-tanzania-b24b5824-f7c1-4058-aba0-d1aaeb7c4117/update
An estimated 130 000 children acquire human immunodeficiency virus (HIV) each year globally [1]. Timely access to antiretroviral treatment (ART) is lifesaving for these children, yet global coverage has stagnated below 60% [1]. In the absence of treatment, half of perinatally infected infants die before 24 months of age [2, 3], with a peak of mortality registered by the third month of life [4]. Rapid ART initiation in infants can substantially reduce mortality [5, 6].
Prompt HIV diagnosis is critical to initiate treatment. Most sub-Saharan African countries perform HIV nucleic acid testing in infants aged 4–8 weeks [7]. Point-of-care (PoC) assays for HIV testing are now widely available and are making same day test-and-treat for children scalable to primary healthcare [8].
Point-of-care testing for HIV is feasible and accurate in newborns [9–15]. Birth testing enables earliest HIV diagnosis and linkage to ART with the potential to reduce early mortality and morbidity, prevent development of viral reservoirs, and improve viral control [16, 17]. Neonates who acquire HIV in utero are at elevated risk for early mortality compared to infants acquiring HIV later [2]. National programs in high-burden countries are considering or implementing birth HIV testing in their routine protocols. However, even when available in resource-poor settings, neonatal ART remains mostly in the realm of larger urban hospitals and is not offered in primary healthcare.
Stopping the peak of early mortality in HIV-infected infants will not be achieved by scaling up test-and-treat at 4–8 weeks alone, but will also require diagnosing and treating HIV soon after birth through primary healthcare. Model-based analyses have found that birth PoC testing may improve survival and is cost-effective [18]. However, the effect of birth testing and very early ART on clinical outcomes under health system conditions in sub-Saharan Africa remains uncertain.
We conducted a pragmatic cluster-randomized trial to investigate the clinical impact of PoC HIV testing at birth and 4–8 weeks of age with immediate ART initiation in HIV-positive neonates, compared to the standard-of-care (SoC) of PoC testing and treatment initiation at 4–8 weeks of age.
METHODS
Study Design
In this cluster-randomized trial, 28 primary healthcare facilities in Mozambique (Sofala and Manica provinces) and Tanzania (Mbeya and Songwe regions) were assigned to 1 of 2 study arms. Half (7 per country) implemented PoC early infant diagnosis (EID) at birth and 4–8 weeks with immediate ART initiation; the remaining followed SoC procedures with PoC EID and ART initiation at 4–8 weeks. In Mozambique and Tanzania, 11 of 14 sites and 3 of 14 sites were urban, respectively. We stratified health facilities by country and HIV-positive delivery volume (high/low) for randomization.
Ethical approvals were obtained for Mozambique by the Comité Institucional de Bioética para a Saúde of the Instituto Nacional de Saúde and Comité Nacional de Bioética em Saúde, for Tanzania by the Mbeya Medical Research and Ethics Committee and the Medical Research Coordinating Committee of the National Institute for Medical Research, and for Germany by the Ethics Committee of the Ludwig Maximilians University Hospital.
Participants
Recruitment took place around delivery. Women were at least 18 years of age, documented as diagnosed with HIV, had delivered within the last 72 hours, and consented to home tracing. Women and/or neonates presenting with an emergency requiring immediate medical assistance and stillbirths were excluded from study participation. Mothers provided written informed consent for themselves and their neonates following detailed verbal and written study information. Informed consent and study procedures started at minimum 4 hours after delivery and once mothers had recovered from birth-related distress.
Study Procedures
Participant demographics and history were collected at delivery. Infants allocated to the intervention group underwent PoC testing, either with Abbott mPIMA HIV-1/2 Detect (Abbott, Chicago, Illinois) in Mozambique or Cepheid GeneXpert HIV-1 Qual (Cepheid, Sunnyvale, California) in Tanzania, according to preexisting preferences. PoC analyzers were prioritized for study use and generally placed at the maternity ward and operated by trained nurses/midwives. In Tanzania, analyzers were located at the laboratory at half of the facilities but operated by nurses with laboratory technician support. A single negative test result was considered HIV-negative; positive results were confirmed by a second heel-prick sample. Discrepant or repeatedly invalid results were resolved using Roche TaqMan polymerase chain reaction (Roche Diagnostics, Branchburg, New Jersey). Neonates in the control group had dried blood spot (DBS) sampling at birth for retrospective HIV testing.
Mother–infant pairs were followed up at 4–8 weeks and 12 weeks of age. Testing was performed at each study visit for HIV-negative infants. Infants diagnosed with HIV by week 12 were rolled over into a follow-up schedule at 6, 12, and 18 months. HIV-positive infants received PoC HIV viral load (VL) monitoring at each visit. HIV-exposed, uninfected neonates received infant postnatal prophylaxis according to national guidelines [7].
Neonatal ART initiation was carried out by nurses/midwives and approved by consulting physicians [7]. Starting from birth and at least 2000 g weight, ART consisted of zidovudine, lamivudine, and nevirapine syrups. Neonates weighing <2000 g were deferred from ART initiation. Dosing of ART was adjusted per World Health Organization (WHO) weight bands. In 2022, pediatric dolutegravir (DTG) was introduced with the option to transition infants from lopinavir/ritonavir (LPV/r)–based to DTG-based regimens.
Mothers were asked to report severe medical conditions and death. A clinical diagnosis was applied based on symptoms and limited diagnostic capacities at health centers, or by verbal autopsy in the case of death outside of healthcare settings. We further included severe, unexplained laboratory events as WHO stage 3 events.
Outcome Measures
The primary outcome was the proportion of combined events among infants with HIV at month 18, including death, loss to retention, medical event (hospitalization, severe medical condition), grade III/IV laboratory abnormality, and VL ≥1000 copies/mL. Secondary outcomes included combined events at months 3, 6, and 12. Loss to retention was defined as withdrawal from the study or loss to follow-up after 3 tracing attempts initiated 1 week after a missed visit by community outreach staff. We also compared age at ART initiation, ART safety, VL, and association between VL and other outcomes.
Sample Size
A target sample size of 224 infants with HIV and overall recruitment number of 6000 mother–infant pairs was calculated assuming 4% vertical transmission by week 6, a difference of combined endpoints of 14% (intervention) versus 30% (control), 5% loss to follow-up, intracluster correlation coefficient of 0.01, 80% power, and 2-sided α = .05. These assumptions were based on estimates from previous trials [6, 19].
Statistical Analysis
We compared the frequency of the primary endpoint between study groups using an incidence rate ratio (IRR) with corresponding profile 95% confidence interval (CI) adjusted for health facility, infant age at HIV detection, and whether infants transitioned to a DTG-containing ART regimen. There were no missing data for the primary endpoint.
For single outcomes, we used Kaplan-Meier methods to describe the time-to-event. Cox proportional hazards models with standard errors clustered at the health facility level were used to adjust for infant age at HIV detection and DTG-containing ART regimens. Unadjusted and adjusted hazard ratios (HRs and aHRs) and 95% CIs are presented as summary measures. In the case of multiple medical events per child, only the first occurrence was used.
Infants diagnosed up to 16 weeks (allowing for a 4-week window period for the 12-week visit) were included in vertical transmission percentages. Unknown laboratory and VL values due to nonattendance, inability to collect sufficient blood volume, or laboratory error were reported as unknown. Differences in VL by other outcomes were calculated using mixed-effects models adjusted for health facility clustering, study group, and DTG-containing regimens at 18 months. Grading of laboratory results was performed using the Division of AIDS toxicity tables [20].
Data were double-entered into OpenClinica (version 3.1, Copyright OpenClinica LLC and collaborators) and analyzed using R software (R Foundation for Statistical Computing, Vienna, Austria, version 4.2.1). This study was registered with ClinicalTrials.gov (NCT04032522).
Impact of the COVID-19 Pandemic
The study paused recruitment from 13 May to 27 July 2020 in Mozambique and from 17 April to 25 May 2020 in Tanzania due to coronavirus disease 2019 restrictions. Follow-up study visits still occurred during this time.
Role of the Funding Source
The funders had no role in study design, data collection, analysis or interpretation, manuscript preparation, or decision to submit.
RESULTS
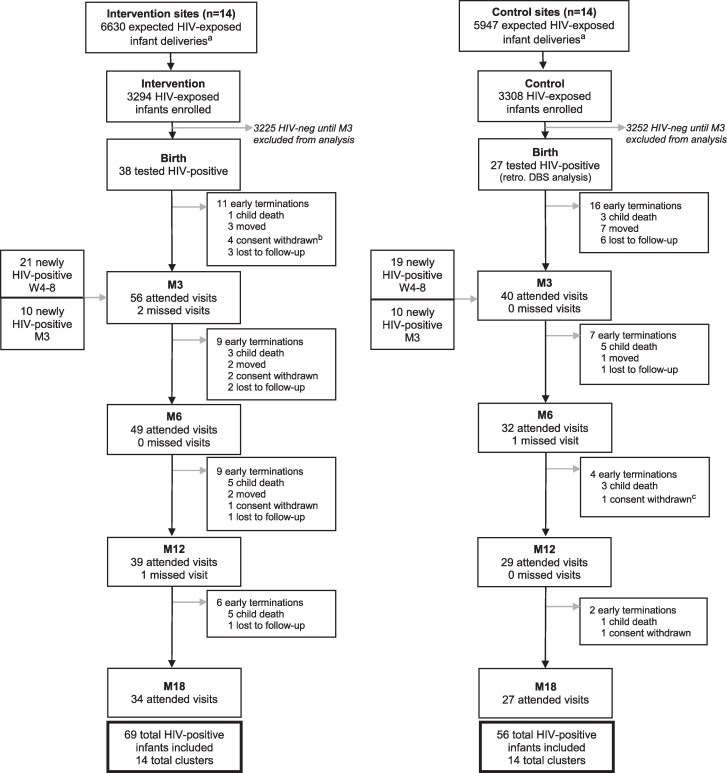
In total, 6602 neonates were enrolled in the study: 3294 (49.9%) in the intervention group and 3308 (50.1%) in the control group (Figure 1). Overall, 4015 (60.8%) were enrolled in Mozambique between October 2019 and July 2021 and 2586 (39.2%) in Tanzania between November 2019 and October 2021. By week 12, 125 infants were diagnosed with HIV (1.89% [95% CI, 1.58%–2.25%]), and 13 (0.2%) had unknown HIV status. Vertical transmission was higher in Mozambique than in Tanzania (2.69% vs 0.66%; P < .001) with 108 (86.4%) infants with HIV from Mozambique and 17 (13.6%) from Tanzania.
Figure 1.
Enrollment and follow-up attendance at birth and months 3–18 with newly HIV-positive infants shown for birth, week 4–8, and month 3. aBased on average HIV-positive deliveries per site. bReasons for withdrawing consent listed as mother/family not accepting the HIV diagnosis of the child (n = 3) or not available (n = 1). cReason for withdrawing consent not available (n = 1). All other instances of consent withdrawn due to mother/family not accepting the child’s HIV diagnosis. Abbreviations: DBS, dried blood spot; HIV, human immunodeficiency virus; M3, month 3; M6, month 6; M12, month 12; M18, month 18; W4-8, week 4–8.
Baseline characteristics were balanced across study groups (Table 1). Among infants with HIV in the intervention group, 38 of 69 (55.1%) were diagnosed at birth, 21 of 69 (30.4%) at 4–8 weeks, and 10 of 69 (14.5%) at 12 weeks of age. In the control group, 40 of 56 (71.4%) were diagnosed at 4–8 weeks and 10 of 56 (17.9%) at 12 weeks of age. Retrospective DBS testing revealed 6 of 56 (10.7%) infants with HIV at birth in the control group died or were lost to follow-up before EID testing. Among infants diagnosed at 4–8 weeks in the control group, 21 of 40 (52.5%) had positive DBS samples at birth. Overall, 27 of 56 (48.2%) infants in the control group tested positive at birth.
Table 1.
Baseline Characteristics of Mothers and Infants With Human Immunodeficiency Virus Included in the Study
| Characteristic | Intervention (n = 69) |
Control (n = 56) |
|---|---|---|
| Infant | ||
| Female sex | 44 (63.8%) | 25 (44.6%) |
| Twin | 5 (7.2%) | 2 (3.6%) |
| Corrected gestational age, wk, median (IQR) | 38 (32–42) | 39 (29–44) |
| Weight, g, mean (SD) | 2850 (492) | 2970 (457) |
| 1-min Apgar score, mean (SD) | 8.31 (0.58) | 8.42 (0.75) |
| 5-min Apgar score, mean (SD) | 9.43 (0.58) | 9.60 (0.69) |
| PoC EID turnaround timea at birth, h, median (IQR) | 19.6 (12.5–27.2) | … |
| Time to ART initiation at birth, h, median (IQR) | 23.0 (16.8–30.4) | … |
| PoC EID turnaround timea | ||
| Within 24 h | 53 (76.8%) | 50 (89.3%) |
| Within 48 h | 66 (95.7%) | 50 (89.3%) |
| Not available (died or LTFU before EID) | 0 (0%) | 6 (10.7%) |
| Mother | ||
| Education | ||
| None | 14 (20.3%) | 7 (12.5%) |
| Primary school | 25 (36.2%) | 29 (51.8%) |
| Secondary school or higher | 30 (43.5%) | 20 (35.7%) |
| Maternal HIV diagnosis | ||
| Before or during first trimesterb of pregnancy | 24 (34.7%) | 25 (44.7%) |
| During second trimesterc of pregnancy | 31 (44.9%) | 21 (37.5%) |
| During third trimesterd of pregnancy | 11 (15.9%) | 7 (12.5%) |
| Unknown | 3 (4.3%) | 3 (5.4%) |
| Time since HIV diagnosis, wk, median (IQR) | 20.3 (15.9–130.5) | 21.9 (15.3–165.8) |
| Maternal ART initiation | ||
| Before second trimesterb of pregnancy | 6 (8.6%) | 12 (21.5%) |
| During second trimesterc of pregnancy | 40 (58.0%) | 23 (41.1%) |
| During third trimesterd of pregnancy | 16 (23.2%) | 15 (26.8%) |
| Unknown/not on ART at delivery | 7 (10.1%) | 6 (10.7%) |
| Time on ART, wk, median (IQR) | 16.5 (12.6–21.5) | 16.7 (7.6–23.9) |
| Maternal ART regimen at delivery | ||
| TDF + 3TC/FTC + DTG | 53 (76.8%) | 41 (73.2%) |
| TDF + 3TC/FTC + EFV | 12 (17.4%) | 11 (19.6%) |
| None | 4 (5.8%) | 4 (7.1%) |
| Maternal VL ≥1000 copies/mL at delivery | 62 (89.9%)e | 50 (89.3%)f |
| Cluster | ||
| Country | ||
| Mozambique | 62 (89.9%) | 46 (82.1%) |
| Tanzania | 7 (10.1%) | 10 (17.9%) |
| Urban | 56 (81.1%) | 40 (71.4%) |
| Rural | 13 (18.8%) | 16 (28.6%) |
| Expected HIV-positive women delivering per year, No. | 4836 | 4377 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; DTG, dolutegravir; EFV, efavirenz; EID, early infant diagnosis; FTC, emtricitabine; HIV, human immunodeficiency virus; IQR, interquartile range; LTFU, lost to follow-up; PoC, point-of-care; SD, standard deviation; TDF, tenofovir disoproxil fumarate; VL, viral load.
aTurnaround time is defined as the time from delivery (at birth) or sample collection (at follow-up visits) to communication of the result to the caregiver and is reported in hours.
bDefined as 1–12 weeks’ gestation.
cDefined as 13–27 weeks’ gestation.
dDefined as ≥28 weeks’ gestation.
ePoC VL assessment.
fLaboratory-based VL assessment.
Early Infant HIV Diagnosis and ART Initiation
Positive HIV test results at all timepoints were available within 24 and 48 hours for 82.4% and 92.8% of infants, respectively. The median turnaround time from delivery to receipt of results by the caregiver for infants diagnosed at birth was 19.6 (interquartile range [IQR], 12.5–27.2) hours.
Overall, 118 (94.4%) infants initiated ART with median age of 6 (IQR, 1.0–33.2) versus 33 (IQR, 31.0–64.5) days in intervention and control groups, respectively. Among infants diagnosed at birth, 35 of 38 (92.1%) initiated ART within 2 days. One neonate died on day 1 before initiating ART, and 2 neonates delayed ART initiation by 2 weeks until reaching minimum weight requirement.
All neonates initiating ART in the first 4 weeks of life received zidovudine, lamivudine, and nevirapine, and 28 of 37 (75.7%) and 31 of 37 (83.8%) switched to abacavir, lamivudine, and LPV/r by week 4–8 and week 12, respectively. Four infants died or were lost to retention before week 4–8. Of those diagnosed and starting ART from week 4–8, 75 of 81 (92.6%) started on abacavir, lamivudine, and LPV/r, 5 of 81 (6.2%) started on zidovudine, lamivudine, and nevirapine and later switched to the former regimen, and 1 infant was lost to retention before switching. When rollout of DTG for infants started, 35 of 118 (29.7%) children were switched within their routine health service delivery to a DTG-containing regimen at a median age of 57 weeks, the majority (23/35 [65.7%]) in the intervention group.
Clinical and Retention Outcomes of Infants With HIV
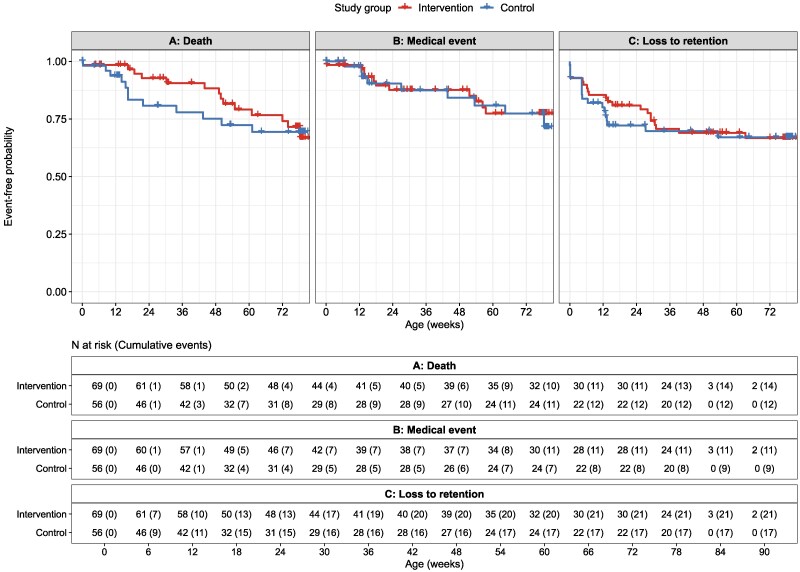
After a median follow-up time of 74.0 (IQR, 14.1–78.6) weeks, 53 (76.8%) and 48 (85.7%) infants died, were lost to retention, experienced a medical event or laboratory abnormality, or were not virally suppressed at 18 months in the intervention and control groups, respectively (IRR, 0.857 [95% CI, .505–1.492]; P = .558; Table 2, Supplementary Table 1). By month 18, 20.3% of infants in the intervention compared to 21.4% in the control groups died (aHR, 0.813 [95% CI, .375–1.762]; P = .599). In total, 15.9% (intervention) versus 14.3% (control) were hospitalized or had a severe medical condition. Loss to retention by month 18 was documented in 30.4% of infants overall and did not differ by group (aHR, 0.976 [95% CI, .388–2.451]; P = .958).
Table 2.
Frequency, Proportion, and Incidence Rate Ratios of Combined Events (Death, Loss to Retention, Any Medical Event or Laboratory Abnormality, and Viral Load ≥1000 Copies/mL at the Last Visit) Among 125 Infants With Human Immunodeficiency Virus at Months 18 (Primary Endpoint), 12, 6, and 3 and Frequency and Proportion of Individual Outcomes Between Study Groups
| Event | No. (%) | aIRR (95% CI)a | P Value | ||
|---|---|---|---|---|---|
| Overall (N = 125) |
Intervention (n = 69) |
Control (n = 56) |
|||
| M3 | |||||
| Death | 4 (3.2) | 1 (1.4) | 3 (5.4) | … | |
| Lost to retentionb | 23 (18.4) | 10 (14.5) | 13 (23.2) | … | |
| Medical eventc | 4 (3.2) | 1 (1.4) | 3 (5.4) | … | |
| Grade III/IV laboratory abnormality | 6 (4.8) | 6 (8.7) | 0 (0) | … | |
| VL ≥1000 copies/mL at M3d | 44 (65.7) | 26 (63.4) | 18 (69.2) | … | |
| Combined evente | 71 (56.8) | 40 (58.0) | 31 (55.4) | 0.908 (.512–1.824) | .940 |
| M6 | |||||
| Death | 12 (9.6) | 4 (5.8) | 8 (14.3) | … | |
| Lost to retentionb | 30 (24.0) | 15 (21.7) | 15 (26.8) | … | |
| Medical eventc | 11 (8.8) | 7 (10.1) | 4 (7.1) | … | |
| Grade III/IV laboratory abnormality | 8 (6.4) | 7 (10.1) | 1 (1.8) | … | |
| VL ≥1000 copies/mL at M6d | 52 (68.4) | 29 (63.0) | 23 (76.7) | … | |
| Combined evente | 98 (78.4) | 52 (75.4) | 46 (82.1) | 0.817 (.511–1.381) | .389 |
| M12 | |||||
| Death | 20 (16.0) | 9 (13.0) | 11 (19.6) | … | |
| Lost to retentionb | 36 (28.8) | 20 (29.0) | 16 (28.6) | … | |
| Medical eventc | 14 (11.2) | 8 (11.6) | 6 (10.7) | … | |
| Grade III/IV laboratory abnormality | 11 (8.8) | 10 (14.5) | 1 (1.8) | … | |
| VL ≥1000 copies/mL at M12d | 34 (51.2) | 20 (50.0) | 14 (53.8) | … | |
| Combined evente | 95 (76.0) | 50 (72.5) | 45 (80.4) | 0.845 (.480–1.515) | .546 |
| M18 | |||||
| Death | 26 (20.8) | 14 (20.3) | 12 (21.4) | … | |
| Lost to retentionb | 38 (30.4) | 21 (30.4) | 17 (30.4) | … | |
| Medical eventc | 20 (16.0) | 11 (15.9) | 9 (16.1) | … | |
| Grade III/IV laboratory abnormality | 20 (16.0) | 12 (17.4) | 8 (14.3) | … | |
| VL ≥1000 copies/mL at M18d | 31 (50.0) | 12 (34.3) | 19 (70.4) | … | |
| Combined evente,f | 101 (80.8) | 53 (76.8) | 48 (85.7) | 0.857 (.505–1.492) | .558 |
Abbreviations: aIRR, adjusted incidence rate ratio; CI, confidence interval; M6, month 6; M12, month 12; M18, month 18; VL, viral load.
aMixed-effects Poisson regression model with random effect for health facility and fixed effects for study group, infant age at first positive test result, and initiated or transitioned to dolutegravir-containing antiretroviral therapy (ART) regimen.
bLoss to retention includes loss to follow-up or voluntary withdrawal from the study due to the mother/family not accepting the HIV diagnosis of the child (7/9 cases) or no further information available (2/9 cases).
cMedical events include any occurrence of hospitalization or severe medical condition during the study period.
dDenominator for VL is infants on ART with a VL test result. M3 excludes infants diagnosed at M3.
eCombined event includes death, loss to retention, any medical or laboratory event (hospitalization, severe medical condition, grade III/IV laboratory abnormality), and VL ≥1000 copies/mL at the last visit.
fPrimary endpoint.
Despite a lack of effect at 18 months of age, we observed clinically relevant, though not statistically significant, differences for early infant mortality up to month 6 (Figure 2 and Supplementary Figure 1), with 4 (5.8%) deaths in the intervention group at a median 17.6 weeks compared to 8 (14.3%) deaths in the control group at a median 14.9 weeks of age (aHR, 0.320 [95% CI, .096–1.068]; P = .064; Table 3).
Figure 2.
Event-free probability of (A) death, (B) medical event (hospitalization or severe medical condition), and (C) loss to retention between study groups in 125 infants with HIV up to month 18. Censored values (+) indicate last known follow-up time for infants still at risk.
Table 3.
Frequency, Proportion, and Hazard Ratios for Death, Medical Event, and Loss to Retention and Between Study Groups Among 125 Infants With Human Immunodeficiency Virus up to 3, 6, 12, and 18 Months of age
| Event | HR (95% CI)a | P Value | aHR (95% CI)b | P Value |
|---|---|---|---|---|
| Death | ||||
| M3 | 0.263 (.027–2.601) | .254 | 0.208 (.022–2.003) | .174 |
| M6 | 0.355 (.107–1.181) | .091 | 0.320 (.096–1.068) | .064 |
| M12 | 0.563 (.233–1.360) | .201 | 0.531 (.219–1.286) | .161 |
| M18 | 0.828 (.383–1.793) | .633 | 0.801 (.369–1.736) | .573 |
| Loss to retentionc | ||||
| M3 | 0.609 (.190–1.953) | .404 | 0.561 (.184–1.707) | .308 |
| M6 | 0.773 (.295–2.023) | .600 | 0.747 (.291–1.920) | .545 |
| M12 | 1.008 (.397–2.562) | .987 | 0.967 (.392–2.385) | .942 |
| M18 | 0.994 (.389–2.538) | .990 | 0.952 (.387–2.343) | .915 |
| Medical eventd | ||||
| M3 | 0.245 (.025–2.362) | .224 | 0.229 (.024–2.216) | .203 |
| M6 | 1.152 (.337–3.939) | .821 | 1.123 (.327–3.859) | .854 |
| M12 | 0.837 (.290–2.416) | .742 | 0.879 (.300–2.572) | .814 |
| M18 | 1.057 (.434–2.573) | .904 | 1.070 (.438–2.612) | .882 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; M3, month 3; M6, month 6; M12, month 12; M18, month 18.
aMixed-effects Cox proportional hazards models with random effect for health facility and fixed effect for intervention group.
bMixed-effects Cox proportional hazards models with random effect for health facility and fixed effects for intervention group and timing of first detected infant HIV positivity.
cLoss to retention includes loss to follow-up or voluntary withdrawal from the study due to the mother/family not accepting the HIV diagnosis of the child (7/9 cases) or no further information available (2/9 cases).
dMedical events include first occurrence of hospitalization or severe medical condition.
Virological Treatment Efficacy and Safety
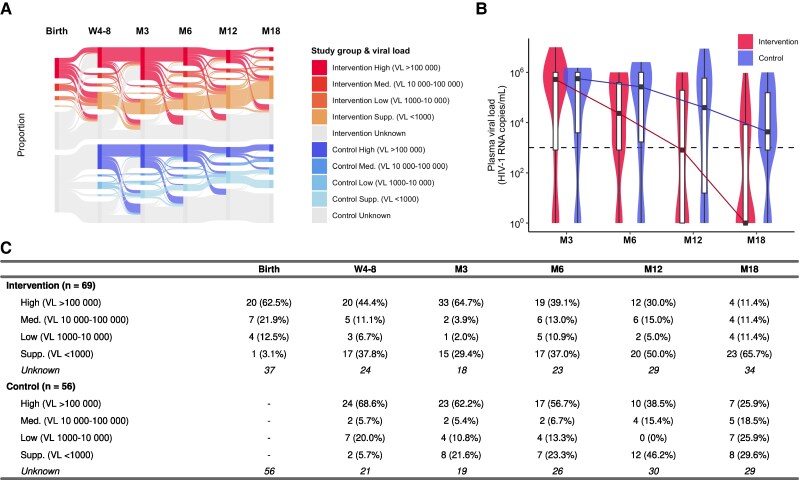
Mean baseline VL available for 32 of 38 (84.2%) neonates at birth was significantly lower than for 75 of 81 (92.6%) infants positive at week 4–8 or 12 (HIV RNA log10 4.95 [standard deviation {SD], 1.11] vs 5.46 [SD, 0.96]; P = .031). A significant difference in viral suppression between groups was observed at month 18 (65.7% vs 29.6%; P = .005; Figure 3). We observed a more rapid reduction of VL between month 3 and 18 in the intervention compared to the control group, with median log10 VL reductions of 1.35 versus 0.33 between month 3 and 6, 1.46 versus 0.83 between month 6 and 12, and 2.90 versus 0.97 between month 12 and 18. Among the 118 infants who started ART, differences in viral suppression were more pronounced at month 18 (Supplementary Table 2).
Figure 3.
A, Sankey diagram showing the proportions of infants in each viral load (VL) category by timepoint (rectangular blocks) and the size and directions of transfers between categories and timepoints (flow lines). The category “Unknown” (gray) includes infants not on antiretroviral therapy, infants who have died or were lost to retention, and infants without VL results. Proportions are calculated without including “Unknown” values in the denominator. B, Violin plots showing the distribution of log10 VL by timepoint between intervention (red) and control groups (blue). Colored lines show the change in medians. The horizontal dashed line at log10 3.0 represents the cutoff value for viral suppression. C, Frequency and proportion of infants in each VL category by timepoint. Abbreviations: HIV-1, human immunodeficiency virus type 1; M3, month 3; M6, month 6; M12, month 12; M18, month 18; Med., medium; Supp., suppressed; VL, viral load; W4-8, week 4–8.
Grade III/IV laboratory abnormalities were reported for infants with low hemoglobin (n = 16), neutropenia (n = 1), and thrombocytopenia (n = 9) after starting ART (Supplementary Table 3). Cases of severe anemia were observed more often at month 3 in the intervention group, possibly related to zidovudine toxicity throughout the first 4 weeks of ART. Anemia was also linked with malnutrition or helminth infection. Episodes of severe pancytopenia were observed in 2 infants around month 3: 1 with malaria who later died and another with no other causes identified other than high VL. No grade III/IV liver enzyme or creatinine elevations were recorded.
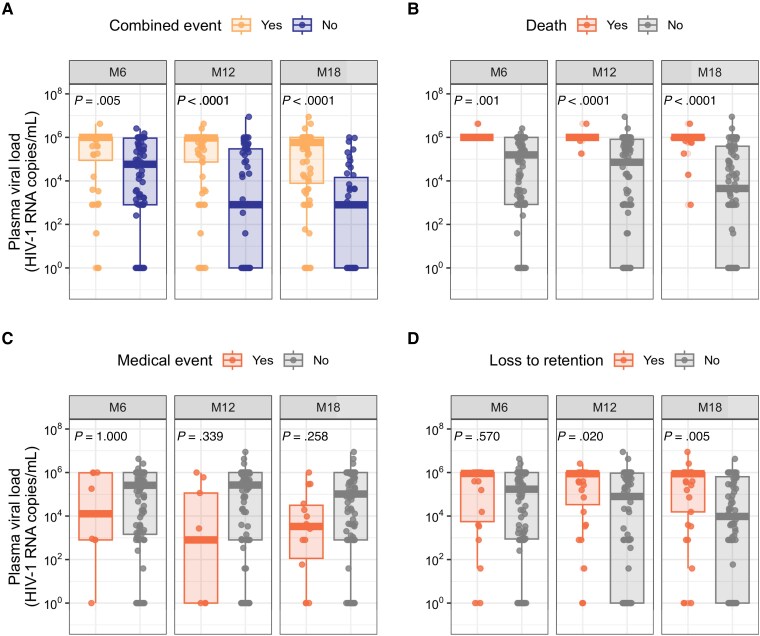
Infants with combined events up to months 6, 12, and 18 were significantly more likely to have higher VLs than infants who did not experience an event. At the most recent blood collection, median log10 VL among infants experiencing a combined event up to month 18 was 5.8 versus 2.9 copies/mL (P < .0001) in those who did not experience an event (Figure 4). Differences in VL were greatest for infants with mortality outcomes up to months 12 and 18; median log10 VL among infants who died by month 18 was 6.0 compared to 3.7 copies/mL among infants alive at month 18 (P < .0001).
Figure 4.
Most recent viral load by occurrence of (A) combined event (death, medical event, grade III/IV laboratory event, HIV viral load >1000 copies/mL at month 18 or the last study visit, or loss to retention) (B, death, C, medical event, and D, loss to retention) cumulatively up to month 6, 12, and 18. Bonferroni-corrected P values calculated using the Mann-Whitney U test. Abbreviations: HIV-1, human immunodeficiency virus type 1; M6, month 6; M12, month 12; M18, month 18.
DISCUSSION
Birth PoC test-and-treat in newborns with HIV was not associated with an overall clinical benefit by month 18 in this study. However, the approach showed a trend toward lower mortality up to 6 months of age. This early health benefit was not sustained likely due to low viral suppression.
High viremia in 89.9% of mothers at delivery was the key driver of HIV transmission, with 78.4% starting ART during pregnancy, 6.4% not on ART, and 4.0% with no documentation of ART available. In sub-Saharan Africa, HIV testing and ART initiation and adherence in pregnant women remain consequential challenges. Testing mothers undergoing ART for HIV VL close to delivery has the potential to identify high-risk cases for vertical transmission [21–23].
Testing newborns for HIV at PoC is feasible in primary and secondary healthcare settings [9–15]. In this study, the vast majority of infants received PoC HIV diagnosis within the first 2 days of life in the intervention group. If testing of newborns had been implemented in control health facilities, 48.2% of infants identified as HIV-positive at 4–8 weeks of age would have been diagnosed at birth. Sadly, 10.7% these infants died or were lost to follow-up before routine testing.
Our study is the first to show successful ART initiation in newborns in peripheral settings in sub-Saharan Africa. Among newborns diagnosed at birth, 92.1% were initiated on ART in the first 2 days of life, showing good ART safety in neonates [24, 25]. A trend toward improved viral suppression in the intervention group persisted until month 6. Although we observed similar viral suppression rates between groups at month 12, viral suppression was significantly greater in the intervention group by month 18. This difference may be biased by a disproportional switch to DTG-containing ART in the intervention group, but it could also suggest long-lasting effects of very early ART [16, 17, 26–28].
We observed clinical benefit with respect to 6-month mortality and combined events until month 3 in infants receiving birth PoC test-and-treat. We argue this trend represents a clinically relevant reduction of early mortality. Further, greater loss to retention in the control group in the first 3 months (23.2% vs 14.5%) could be due to additional early mortality. Combined events and death across all timepoints were significantly associated with high VL, strongly suggesting that poor clinical outcomes are associated with uncontrolled HIV disease. Only 1 infant who died had a recent suppressed VL. Low ART responses were likely related to poor adherence resulting from suboptimal palatability and twice-daily dosing of LPV/r granules, as well as sociobehavioral factors such as inadequate family support, lack of disclosure, or postpartum maternal depression and trauma [19, 29, 30].
The main limitation of this study is the lower than expected number of infants with HIV, especially in Tanzania. Thus, the results presented here reflect the scenario in Mozambique more than in Tanzania. As vertical HIV transmission declines in high-burden countries due to improving prevention of mother-to-child transmission programs and maternal ART coverage during pregnancy [1], reaching large sample sizes is challenging in pediatric trials. Furthermore, PoC EID in our control group was considered optimized SoC, in contrast to DBS EID, which requires sample transport. A DBS-based SoC control may have demonstrated a greater clinical benefit for PoC test-and-treat at birth. Our study, therefore, compares elements related to timing of PoC EID but does not distinguish the benefit of PoC testing against other EID modalities. We cannot exclude bias due to study-supported procurement of pediatric drugs and diagnostics, which exclude interruptions encountered during routine care and treatment services.
Birth PoC test-and-treat can successfully be implemented in primary healthcare in sub-Saharan Africa, but despite promising effects in the first 6 months of life, clinical benefits were not sustained. The main limiting factor affecting favorable outcomes is poor pediatric ART response. The recent implementation of once-daily dispersible DTG for infants >1 month of age and 3000 g weight will likely improve viral control. However, pediatric-specific interventions and novel drug delivery technologies for neonates and low-weight infants are needed. Innovative clinical interventions targeting mothers and newborns around delivery, coupled with sociobehavioral and adherence support, will be essential to reach the Joint United Nations Programme on HIV/AIDS 2030 targets to eliminate pediatric HIV and reduce neonatal mortality in sub-Saharan Africa.
Supplementary Material
Contributor Information
Ilesh V Jani, Instituto Nacional da Saúde, Marracuene, Mozambique.
Issa Sabi, National Institute for Medical Research, Mbeya Medical Research Center, Mbeya, Tanzania.
Kira Elsbernd, Institute of Infectious Diseases and Tropical Medicine, LMU University Hospital, LMU Munich, Germany; Institute for Medical Information Processing, Biometry, and Epidemiology, LMU Munich, Germany.
Bindiya Meggi, Instituto Nacional da Saúde, Marracuene, Mozambique.
Arlete Mahumane, Instituto Nacional da Saúde, Marracuene, Mozambique.
Anange Fred Lwilla, National Institute for Medical Research, Mbeya Medical Research Center, Mbeya, Tanzania.
Kassia Pereira, Instituto Nacional da Saúde, Marracuene, Mozambique.
Siriel Boniface, National Institute for Medical Research, Mbeya Medical Research Center, Mbeya, Tanzania.
Raphael Edom, National Institute for Medical Research, Mbeya Medical Research Center, Mbeya, Tanzania.
Joaquim Lequechane, Instituto Nacional da Saúde, Marracuene, Mozambique.
Falume Chale, Instituto Nacional da Saúde, Marracuene, Mozambique.
Nhamo Chiwerengo, National Institute for Medical Research, Mbeya Medical Research Center, Mbeya, Tanzania.
Nyanda E Ntinginya, National Institute for Medical Research, Mbeya Medical Research Center, Mbeya, Tanzania.
Chishamiso Mudenyanga, Clinton Health Access Initiative, Maputo, Mozambique.
Mariana Mueller, Institute of Infectious Diseases and Tropical Medicine, LMU University Hospital, LMU Munich, Germany.
Martina Rauscher, Immunology, Infection and Pandemic Research, Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Munich, Germany.
Michael Hoelscher, Institute of Infectious Diseases and Tropical Medicine, LMU University Hospital, LMU Munich, Germany; Immunology, Infection and Pandemic Research, Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Munich, Germany; German Center for Infection Research, Partner Site Munich, Germany; Unit of Global Health, Helmholtz Center Munich, German Research Center for Environmental Health, Neuherberg, Germany.
Nuno Taveira, Instituto Universitário Egas Moniz, Lisbon, Portugal.
W Chris Buck, David Geffen School of Medicine, University of California, Los Angeles, California, USA.
Arne Kroidl, Institute of Infectious Diseases and Tropical Medicine, LMU University Hospital, LMU Munich, Germany; German Center for Infection Research, Partner Site Munich, Germany.
the LIFE Study Consortium:
Lise Ellyin, Araújo Patricio, Dadirai Mutsaka, Lara Samuel, Sergey Bocharnikov, Timothy Bollinger, Wilson Simbine, Abhishek Bakuli, Cornelia Lueer, Elmar Saathoff, Fidelina Zekoll, Friedrich Rieß, Otto Geisenberger, Rute Marcelino, Absalao Zumba, Daniel Machavae, Adolfo Vubil, Ana Duajá, Jacinto Adolfo Ndarissone, Joao Manuel, Maria Maviga, Nalia Ismael, Jorge Morais, Nedio Mabunda, Adolfo Vubil, Fatima Mecupa, Amina de Sousa, Abisai Kisinda, Chacha Mangu, Doreen Pamba, Festina Paschal, Hellen Mahiga, Janeth Stephen, Lilian Njovu, Magreth Haule, Oliver Lyoba, Theodora Mbunda, and Willyhelmina Olomi
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. K., I. V. J., I. S., N. T., N. E. N., and M. H. contributed to study design and funding acquisition. I. S., B. M., A. M., K. P., A. F. L., S. B., R. E., J. L., C. M., M. M., and W. C. B. led operational study procedures. F. C., N. C., K. E., and M. M. contributed to data collection and accessed the raw data. K. E. and M. R. performed the analysis. A. K., K. E., and I. V. J. drafted the manuscript; all coauthors had full access to the data, contributed to interpretation, participated in manuscript review, and accept responsibility to submit for publication.
Acknowledgments. The authors gratefully acknowledge the infants and families who participated in the study and dedicated healthcare personnel at the study sites. They also thank Martina Penazzato and Lara Vojnov from the World Health Organization, Landon Myer and Lynn Horn from the University of Cape Town, and Karim Manji from the Muhimbili University of Health and Allied Sciences in Dar es Salaam for external expert advice. The study was conducted at health facilities within the Mozambican and Tanzanian National HIV programs. The Clinton Health Access Initiative (CHAI) provided infrastructural support at the study sites and contingency supplies for pediatric antiretroviral drugs. They thank Lise Ellyin and Helder Mendes from CHAI Mozambique, and Patricia Mbago and Esther Mtumbuka from CHAI Tanzania. The authors also acknowledge the LIFE Study Consortium: Lise Ellyin, Araújo Patricio, Dadirai Mutsaka, Lara Samuel, Sergey Bocharnikov, Timothy Bollinger, Wilson Simbine, Abhishek Bakuli, Cornelia Lueer, Elmar Saathoff, Fidelina Zekoll, Friedrich Rieß, Otto Geisenberger, Rute Marcelino, Absalao Zumba, Daniel Machavae, Adolfo Vubil, Ana Duajá, Jacinto Adolfo Ndarissone, Joao Manuel, Maria Maviga, Nalia Ismael, Jorge Morais, Nedio Mabunda, Adolfo Vubil, Fatima Mecupa, Amina de Sousa, Abisai Kisinda, Chacha Mangu, Doreen Pamba, Festina Paschal, Hellen Mahiga, Janeth Stephen, Lilian Njovu, Magreth Haule, Oliver Lyoba, Theodora Mbunda, Willyhelmina Olomi.
Data availability. Individual participant data that underlie the results presented in this article (text, tables, figures, and Supplementary Material) with labels defining all fields will be made available upon publication to researchers whose proposed use of the data has been approved by the study steering committee. Proposals should be directed to akroidl@lrz.uni-muenchen.de. Researchers wishing to access this data will be asked to sign a data use agreement.
Financial support. This work was supported by the European and Developing Countries Clinical Trials Partnership (RIA2016MC-1615); Unitaid (UCPOC2B); and the German Center for Infection Research (TTU 04.708).
References
- 1. Joint United Nations Programme on HIV/AIDS . UNAIDS data 2023. UNAIDS data 2023. 2023. Available at: https://www.unaids.org/en/resources/documents/2023/2023_unaids_data. Accessed 1 August 2024.
- 2. Becquet R, Marston M, Dabis F, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One 2012; 7:e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–43. [DOI] [PubMed] [Google Scholar]
- 4. Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS 2009; 23:101–6. [DOI] [PubMed] [Google Scholar]
- 5. Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; 382:1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: World Health Organization, 2021. [PubMed] [Google Scholar]
- 8. Luo R, Fong Y, Boeras D, Jani I, Vojnov L. The clinical effect of point-of-care HIV diagnosis in infants: a systematic review and meta-analysis. Lancet 2022; 400:887–95. [DOI] [PubMed] [Google Scholar]
- 9. Jani IV, Meggi B, Mabunda N, et al. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr 2014; 67:e1–4. [DOI] [PubMed] [Google Scholar]
- 10. Jani IV, Meggi B, Vubil A, et al. Evaluation of the whole-blood alere Q NAT point-of-care RNA assay for HIV-1 viral load monitoring in a primary health care setting in Mozambique. J Clin Microbiol 2016; 54:2104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jani I, Meggi B, Loquiha O, et al. Effect of point-of-care testing on antiretroviral therapy initiation rates in infants. In: Conference on Retroviruses and Opportunistic Infections, 13–16 February 2017, Seattle, WA, 2017.
- 12. Jani IV, Meggi B, Loquiha O, et al. Effect of point-of-care early infant diagnosis on antiretroviral therapy initiation and retention of patients. AIDS 2018; 32:1453–63. [DOI] [PubMed] [Google Scholar]
- 13. Meggi B, Vojnov L, Mabunda N, et al. Performance of point-of-care birth HIV testing in primary health care clinics: an observational cohort study. PLoS One 2018; 13:e0198344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meggi B, Bollinger T, Zitha A, et al. Performance of a true point-of-care assay for HIV-1/2 viral load measurement at antenatal and postpartum services. J Acquir Immune Defic Syndr 2021; 87:693–9. [DOI] [PubMed] [Google Scholar]
- 15. Sabi I, Mahiga H, Mgaya J, et al. Accuracy and operational characteristics of Xpert HIV point-of-care testing at birth and different time-points until week 6 in HIV-exposed neonates in Tanzania. Clin Infect Dis 2018; 68:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shiau S, Strehlau R, Technau KG, et al. Early age at start of antiretroviral therapy associated with better virologic control after initial suppression in HIV-infected infants. AIDS 2017; 31:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Millar JR, Bengu N, Vieira VA, et al. Early initiation of antiretroviral therapy following in utero HIV infection is associated with low viral reservoirs but other factors determine viral rebound. J Infect Dis 2021; 224:1925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frank SC, Cohn J, Dunning L, et al. Clinical effect and cost-effectiveness of incorporation of point-of-care assays into early infant HIV diagnosis programmes in Zimbabwe: a modelling study. Lancet HIV 2019; 6:e182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Technau KG, Strehlau R, Patel F, et al. 12-month outcomes of HIV-infected infants identified at birth at one maternity site in Johannesburg, South Africa: an observational cohort study. Lancet HIV 2018; 5:e706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Division of AIDS, National Institute of Allergy and Infectious Diseases. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, Version 2.0. Rockville, MD: National Institute of Allergy and Infectious Diseases, 2014.
- 21. Penazzato M, Townsend CL, Sam-Agudu NA, et al. Advancing the prevention and treatment of HIV in children: priorities for research and development. Lancet HIV 2022; 9:e658–66. [DOI] [PubMed] [Google Scholar]
- 22. Vojnov L, Havlir D, Myer L, Abrams E, Jani I. Same-day test and treat for infants with HIV infection: finally within reach. J Int AIDS Soc 2022; 25:e26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Penazzato M, Kasirye I, Ruel T, et al. Antiretroviral postnatal prophylaxis to prevent HIV vertical transmission: present and future strategies. J Int AIDS Soc 2023; 26:e26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chadwick EG, Pinto J, Yogev R, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. Pediatr Infect Dis J 2009; 28:215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau E, Brophy J, Samson L, et al. Nevirapine pharmacokinetics and safety in neonates receiving combination antiretroviral therapy for prevention of vertical HIV transmission. J Acquir Immune Defic Syndr 2017; 74:493–8. [DOI] [PubMed] [Google Scholar]
- 26. Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis 2014; 59:1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McManus M, Mick E, Hudson R, et al. Early combination antiretroviral therapy limits exposure to HIV-1 replication and cell-associated HIV-1 DNA levels in infants. PLoS One 2016; 11:e0154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luzuriaga K, Mofenson LM. Eliminating pediatric HIV-1 infection. N Engl J Med 2016; 375:193–4. [DOI] [PubMed] [Google Scholar]
- 29. Millar JR, Bengu N, Fillis R, et al. High-frequency failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal needs causing maternal non-adherence. EClinicalMedicine 2020; 22:100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuhn L, Strehlau R, Shiau S, et al. Early antiretroviral treatment of infants to attain HIV remission. EClinicalMedicine 2020; 18:100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.