Abstract
Objectives
Despite recent advances in endoscopic equipment and diagnostic techniques, early detection of ulcerative colitis‐associated neoplasia (UCAN) remains difficult because of the complex background of the inflamed mucosa of ulcerative colitis and the morphologic diversity of the lesions. We aimed to describe the main diagnostic patterns for UCAN in our cohort, including lateral extension surrounding flat lesions.
Methods
Sixty‐three lesions in 61 patients with flat‐type dysplasia that were imaged with dye chromoendoscopy (DCE) were included in this analysis. These DCE images were analyzed to clarify the dye‐chromoendoscopic imaging characteristics of flat dysplasia, and the lesions were broadly classified into dysplastic and nondysplastic mucosal patterns.
Results
Dysplastic mucosal patterns were classified into two types: small round patterns with round to roundish structures, and mesh patterns with intricate mesh‐like structures. Lesions with a nondysplastic mucosal pattern were divided into two major types: a ripple‐like type and a gyrus‐like type. Of note, 35 lesions (55.6%) had a small round pattern, and 51 lesions (80.9%) had some type of mesh pattern. About 70% of lesions with small round patterns and 49% of lesions with mesh patterns were diagnosed as high‐grade dysplasia or carcinoma, while about 30% of lesions with small round patterns and 51% of lesions with mesh patterns were diagnosed as low‐grade dysplasia.
Conclusion
When a characteristic mucosal pattern, such as a small round or mesh pattern, is found by DCE, the possibility of UCAN should be considered.
Keywords: dye‐chromoendoscopy, flat‐type dysplasia, ulcerative colitis‐associated neoplasia
INTRODUCTION
Ulcerative colitis (UC) is a chronic persistent inflammatory bowel disease (IBD) of unknown cause with recurrent flare‐ups and remissions, typically starting with mild inflammation of the rectum and progressing to extensive inflammation of the colon. 1 Persistent inflammation can lead to the development of UC‐associated neoplasia (UCAN), in which case there is a need for total colectomy. In fact, the cumulative incidence of UCAN reaches about 7.5–18.4% at 30 years after onset of the disease. 2 , 3 , 4 Hata et al. 5 reported that patients with UC who underwent routine surveillance colonoscopy had a higher survival rate than those who did not. Thus, early detection of UCAN is very important in the management of patients with UC, as is evaluation of inflammatory activity by endoscopic observation. However, despite recent advances in endoscopic equipment and diagnostic techniques, the early detection of UCAN remains difficult because of the complex background of the inflamed mucosa in UC and the morphologic diversity of the lesions.
A consensus statement by the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus (SCENIC) classified UCAN into five types: pedunculated, sessile, superficial elevated, flat, and depressed lesions. 6 In a subsequent report classifying lesions according to these criteria, 64% of high‐grade dysplasia (HGD) lesions were associated with elevated areas (sessile or superficial elevated), while 30% were flat lesions. 7 Although it is considered possible to detect elevated lesions (pedunculated, sessile, and superficial elevated) with white‐light imaging (WLI), flat lesions include so‐called invisible dysplasia that are difficult to detect with WLI. A major challenge for the early diagnosis of UCAN is therefore the detection of these flat lesions.
In recent years, there have been a series of reports regarding endoscopic resection of neoplastic lesions arising within the affected colonic mucosa of UC. Consequently, there is an increasing need to establish diagnostic criteria for determining the extent of lesions. 8 , 9 , 10 , 11 It is not uncommon for tumors that occur within the colitic mucosa of UC to have unusual morphology, even if they are sporadic neoplasia (SN), because they occur against a background of inflamed mucosa. For this reason, it is sometimes difficult to distinguish SN from UCAN on WLI. In such cases, one way to differentiate SN from UCAN is to determine whether or not flat dysplasia extends around the elevated main lesion, although no clear method has currently been established for determining this. Endoscopic resection without recognizing the presence of flat dysplasia surrounding an elevated lesion will result in a positive margin and failure to provide appropriate treatment. The SCENIC consensus statement recommended dye chromoendoscopy (DCE) of the entire colon because it is effective for detecting UCAN, including flat dysplasia, 6 but there is still no report showing the definitive endoscopic characteristics of flat dysplasia on DCE.
In this study we aimed to describe the main diagnostic patterns for UCAN in our cohort, including lateral extension surrounding flat lesions. We selected lesions with flat dysplasia from UCAN cases diagnosed at our hospital and analyzed DCE images to clarify the characteristics of DCE imaging of flat dysplasia.
METHODS
Study design and patients
This single‐center retrospective observational cohort study involved a review of the medical charts and endoscopic records of 158 consecutive UC patients with 211 neoplastic lesions within UC‐affected mucosa who presented at the Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University Hospital (Tokyo, Japan), between January 2001 and August 2022.
Study procedures
Demographic data and disease characteristics of the patients including gender, age at disease onset, age at detection of dysplasia, duration of disease, extent of disease, and Mayo endoscopic subscore (MES) at detection of dysplasia were collected from medical charts. All procedures were performed using a video colonoscope (PCF‐H290ZI, PCF‐H290I, CF‐H290I, CF‐H260AI, or CF‐H260AZI, PCF‐Q240ZI, CF240I; Olympus Medical Systems, Tokyo, Japan). Regular white‐light colonoscopy was performed, followed by 0.1% indigo carmine dye spraying after suspected lesions were recognized. In addition, a concentration of 0.2% was used when the 0.1% solution was deemed insufficient for observation. Detailed endoscopic images of UCAN were collected using an endoscopy reporting system (Solemio ENDO, Solemio QUEV; Olympus, Tokyo, Japan). All colonoscopic examinations were performed by one of five expert endoscopists who were board‐certified fellows of the Japan Gastroenterological Endoscopy Society and who had endoscopy experience for at least 2000 cases with UC.
Definition and evaluation of the endoscopic and pathologic findings
The present study involved the analysis of chromoendoscopic images, with indigo carmine dye‐spraying of flat‐type dysplasia that were entirely flat, and flat lesions spreading around polypoid lesions diagnosed as UCAN after biopsy or endoscopic mucosal resection, endoscopic submucosal dissection (ESD), or surgical resection. Detailed endoscopic observations and analyses of dye chromoendoscopic images were performed by four IBD expert endoscopists, and several types of mucosal patterns were identified and observed intermixed with flat‐type dysplasia. Approximately 20% of lesions were diagnosed by biopsy when UC was endoscopically determined as moderately to severely active (MES 2 or 3), whereas evaluation of the chromoendoscopic mucosal pattern was performed in remission without inflammation in the background mucosa of the lesion (MES 0 or 1) in all cases. Importantly, flat dysplasia refers to a completely flat lesion that falls into class 0‐IIb on the Paris Classification and includes invisible dysplasia. It does not refer to a flat elevated lesion.
Histologic specimens were reviewed and confirmed by at least two experienced pathologists at our hospital, as previously reported. 7 The histologic diagnosis of UCAN was confirmed by routine hematoxylin and eosin staining and p53 immunohistochemistry, and with consideration of overexpression or complete absence of p53 and basal positivity of p53. The expression pattern of Ki‐67 in sporadic lesions was also considered; in these lesions, Ki‐67 expression was typically seen in the luminal aspect of elevated lesions where the expression of p53 was low, whereas its expression was strong and diffuse in UCAN. Regarding dysplasia showing a completely negative pattern of p53, we evaluated p53 staining by comparing dysplastic lesions to nondysplastic lesions. Nondysplastic lesions usually show a wild‐type staining pattern, a typically heterogenous and weak staining pattern, whereas dysplastic lesions show completely negative for p53, which provides a clue to diagnose dysplasia with a completely negative pattern of p53. Based on this evaluation system, there were no discrepancies as for the diagnosis of p53‐completely negative dysplasia cases. However, since there were some discrepancies regarding the degree of dysplasia (low‐grade vs. high‐grade) between two pathologists, we observed all the p53‐completely negative dysplasia specimens carefully together, discussed and determined the degree of the dysplasia considering the nuclear and architectural atypia, and cellular differentiation. Although neoplasms with nuclear and architectural abnormalities are diagnosed as intramucosal carcinoma in Japan, regardless of their invasion status, these lesions were classified as HGD in this study. Cases of sporadic colorectal cancer/adenoma and indefinite colitis‐associated cancer/dysplasia, in which sporadic lesions could not be ruled out, were excluded. In addition, for biopsy specimens among all specimens evaluated in this study, only those cases in which the pathologist judged that the biopsy specimen was a valid and evaluable specimen were included in the evaluation.
Statistical analysis
The results are summarized as median and interquartile range for continuous variables, and as numbers for categorical variables. All statistical analyses were performed using JMP software version 11.1.1 (SAS Institute, Cary, NC, USA).
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki. This retrospective study was approved by the Ethics Committee of the Keio University School of Medicine (Approval number 20150100).
RESULTS
Patient characteristics
A flowchart of the patients included in this study is shown in Figure 1. Finally, 63 lesions in 61 patients with flat‐type dysplasia that had been observed with detailed DCE using high‐definition colonoscopy (PCF‐H290ZI, PCF‐H290I, CF‐H290I, or CF‐H260AZI) were included in this analysis.
Figure 1.

Flowchart of the included patients. SN, sporadic neoplasia; UC, ulcerative colitis; UCAN, ulcerative colitis‐associated neoplasia.
The patients' characteristics are summarized in Table 1. The extent of disease was total colitis in 75.4% of patients and left‐sided colitis in 24.6%. The MES of the background mucosa at the time of UCAN detection was MES 0 in 27 cases (44.3%), MES 1 in 24 cases (39.3%), MES 2 in eight cases (13.1%), and MES 3 in two cases (3.3%).
Table 1.
Patients' characteristics
| Number of patients | 61 |
| Male/female | 43/18 |
| Age at UC onset, median (IQR), years | 30 (24–46) |
| Age at UCAN detection, median (IQR), years | 50 (42–63) |
| Duration of disease, median (IQR), years | 18 (11–24) |
| Clinical type, n (%) | |
| Relapse and remission | 40 (65.6) |
| Chronic persistent | 21 (34.4) |
| First attack | 0 (0.0) |
| Extent of disease, n (%) | |
| Total colitis | 46 (75.4) |
| Left‐sided colitis | 15 (24.6) |
| Mayo endoscopic score at UCAN detection, n (%) | |
| 0 | 27 (44.3) |
| 1 | 24 (39.3) |
| 2 | 8 (13.1) |
| 3 | 2 (3.3) |
IQR, interquartile range; UC, ulcerative colitis; UCAN, ulcerative colitis‐associated neoplasia.
Typical mucosal patterns of flat dysplasia on chromoendoscopy
Representative images of flat‐type dysplasia are shown in Figure 2. For detailed observation of UCAN, endoscopic evaluations were performed on all lesions when the inflammation of UC was at MES 0 or 1 (Fig. 2a). We reviewed and evaluated chromoendoscopic images with indigo carmine dye spraying (Fig. 2b) and near focus images (Fig. 2c) (‘near focus images’ means images taken at a very low magnification or those taken at sufficiently close proximity). The chromoendoscopic mucosal pattern classifications of flat dysplasia and nondysplastic mucosa created on the near focus endoscopic images of all 63 lesions are shown in Figure 3. First, we broadly classified lesions into dysplastic and nondysplastic mucosal patterns. Dysplastic mucosal patterns were then classified into two types: small round patterns (mucosal structures with dotted or fine circular patterns) and mesh patterns (mucosal structure with reticulate pattern). Lesions with a small round pattern were further subdivided into two types according to the concentration of its findings: a dense type and a sparse type. Lesions with a mesh pattern were subdivided into those with a fine mesh and those with an enlarged rough mesh structure. Lesions with a nondysplastic mucosal patten were divided into two major types: a ripple‐like type (relatively uniform wavy pattern structure), and a gyrus‐like type (mucosal structures like the gyri of the cerebral cortex, with relative uniformity).
Figure 2.

Representative chromoendoscopic images of flat dysplasia. (a) White light images, (b) chromoendoscopic images, (c) near focus chromoendoscopic images (images taken at a very low magnification or those taken at sufficiently close proximity).
Figure 3.

Typical chromoendoscopic mucosal patterns of flat dysplasia. Dysplastic mucosal patterns were classified into two types: small round and mesh patterns. The small round pattern was further subdivided into two types: dense and sparse types. The mesh patterns were subdivided into fine mesh and enlarged mesh types. Nondysplastic mucosal pattens were divided into two major types: ripple‐like and gyrus‐like types.
Characteristics of endoscopic findings
The characteristics of endoscopic findings of the 63 lesions are shown in Table 2. Consistent with a previous report, 7 more than 80% of the lesions (84.1%) were located in the distal colon from the sigmoid colon to the rectum. About 50% of the lesions had reddish mucosa and most of them had a small round pattern, as shown in Figure 3. Of note, 55.6% of the lesions had a small round pattern, and 80.9% had some type of mesh pattern. Of the total 63 lesions, part of the area of seven lesions (approximately 11%) had a mixed nondysplastic mucosal pattern, as shown in Figure 4a. However, in 50 patients who underwent endoscopic resection of SN lesions within UC‐affected mucosa during the study period, only nondysplastic patterns were found in the surrounding mucosa (Fig. 4b,c). There were no SN lesions with dysplastic mucosal patterns such as small round or mesh patterns because the peri‐mucosa of SN never contains dysplastic mucosa.
Table 2.
Characteristics of endoscopic findings
| Number of lesions | 63 |
| Location of UCAN, n (%) | |
| Cecum – ascending colon | 1 (1.7) |
| Transverse colon | 4 (6.3) |
| Descending colon | 5 (7.9) |
| Sigmoid colon | 20 (31.7) |
| Rectum | 33 (52.4) |
| Presence of reddish mucosa, n (%) | 32 (50.7) |
| Endoscopic morphologic pattern, n (%) | |
| Small round pattern | 35 (55.6) |
| Dense type | 25 |
| Sparse type | 14 |
| Mesh pattern | 51 (80.9) |
| Fine mesh type | 33 |
| Enlarged mesh type | 24 |
| Nondysplastic pattern | 7 (11.1) |
| Ripple‐like type | 4 |
| Gyrus‐like type | 3 |
UCAN, ulcerative colitis‐associated neoplasia.
Figure 4.
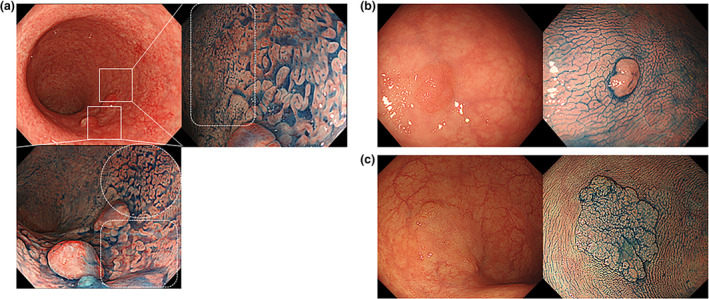
Differences in mucosal pattern surrounding elevated lesions in ulcerative colitis‐associated neoplasia (UCAN) and sporadic neoplasia (SN). (a) Endoscopic images of superficial elevated UCAN with flat‐type dysplasia in the surrounding region. Dye chromoendoscopy shows a mucosal surface with a mesh pattern on the anal side of the superficial elevated lesion (bottom panel, area surrounded by white dotted square), and a mucosal surface with a nondysplastic pattern on the oral side (bottom panel, area surrounded by white dotted circle). On the oral side of the lesion, a mucosal surface with a small round pattern (right panel, surrounded by a white square) is present. (b, c) Endoscopic images of superficial elevated SN. In contrast, the boundaries of the SN are distinct, and only nondysplastic patterns are found in the surrounding mucosa of SN.
Pathologic diagnosis with respect to chromoendoscopic classification
As shown in Table 3, about 70% of lesions with small round patterns were diagnosed as HGD or carcinoma, while about 50% of lesions with mesh patterns were diagnosed as low‐grade dysplasia. Histopathologic evaluation showed that mucosa with a small round pattern showed straight glandular ducts with relatively few branches, and that the glandular duct density tended to be higher in the deeper areas. In lesions with a mesh pattern, many gland ducts with intricate branching and strong deformation were observed, reflecting the pathologic appearance of the mucosal pattern (Fig. 5).
Table 3.
Pathologic diagnosis with respect to chromoendoscopic classification
| Small round pattern (n = 35) | Mesh pattern (n = 51) | |
|---|---|---|
| LGD | 10 (28.6%) | 25 (49.0%) |
| HGD‐carcinoma | 25 (71.4%) | 26 (51.0%) |
HGD, high‐grade dysplasia; LGD, low‐grade dysplasia.
Figure 5.

Representative pathologic images of flat dysplasia. Hematoxylin and eosin (H&E) staining (left panels) and p53 immunohistochemistry (right panels) of ulcerative colitis‐associated neoplasia with a small round pattern (top) and mesh pattern (bottom). Histopathologic evaluation revealed that the mucosa of the small round pattern showed straight glandular ducts with relatively few branches, and that the glandular duct density tended to be higher in the deeper areas. In the mesh pattern, many gland ducts with intricate branching and strong deformation were observed. Scale bar, 100 μm.
DISCUSSION
In this study we examined detailed chromoendoscopic images of flat‐type dysplasia, and identified the mucosal patterns of flat‐type dysplasia on DCE.
Traditionally, random biopsy was considered to be effective for detecting UCAN, but the SCENIC consensus statement published in 2015 recommended that DCE should be used to detect UCAN, 6 and this led to an increased focus on target biopsies. Later, Watanabe et al. 12 reported that there was no difference in tumor detection rate between conventional random biopsy and target biopsy with DCE, suggesting that target biopsy with DCE is useful for detecting UCAN. Nevertheless, there have been no reports clearly describing the characteristic mucosal patterns of UCAN on DCE, and endoscopists are left without guidance on which lesions to target for biopsy. Because diagnostic methods for UCAN on DCE are not well established, it may well be that the importance of DCE has been underestimated. Despite its description in guidelines, in clinical practice DCE is not widely used as a standard observation method for surveillance colonoscopy in patients with UC. 13 , 14 In this report, we clarified the chromoendoscopic imaging characteristics of flat‐type dysplasia, which is difficult to detect on WLI and is sometimes considered invisible. Although pan‐chromoendoscopy has been recommended for detecting UCAN, it is difficult to perform this time‐consuming and burdensome procedure in all cases in clinical practice. We found more than 80% of the lesions in the distal colon, suggesting that a pragmatic approach of using DCE observation in at least the distal colon may improve the detection rate of UCAN in high‐risk patients (e.g. patients with longstanding disease or colorectal stricture). Furthermore, all the mucosal patterns in this study were evaluated during the remission period, because the presence of active inflammation would have affected the mucosal pattern. In fact, 90% of biopsies diagnosed as UCAN were in remission, suggesting that surveillance detection, as well as close examination with DCE, may be more effective when performed in remission.
Several studies have reported on the characteristic endoscopic findings of UCAN. By comparing magnifying colonoscopy with stereomicroscopic images, Shinagawa et al. 15 reported the possibility that pine‐cone and villi patterns may be endoscopic findings characterizing UCAN. Matsumoto et al. 16 and East et al. 17 reported that irregular surface structures and darkening due to obvious capillary vessels are characteristic findings of UCAN, while Nishiyama et al. 18 reported that irregular surface patterns are effective for differentiating UCAN from nontumor lesions. However, all of these reports examined sessile or superficial elevated UCAN and showed how the microvasculature and surface structures of tumors modified by inflammation can be differentiated from conventional endoscopic findings of SN. While it remains important to recognize the endoscopic features of elevated UCAN, we consider that it is critical to detect invisible lesions in order to increase the detection rate of UCAN, and we therefore focused on the mucosal patterns of flat dysplasia in this study. In all cases, the mucosal pattern classification of flat‐type dysplasia that we proposed in this study is based on several mucosal structure patterns confirmed by the very simple method of indigo carmine dye spraying, and we hope that our classification will help in the detection of flat‐type dysplasia in surveillance colonoscopy.
Another reason for our focus on the mucosal pattern of flat‐type dysplasia is the high rate of positive margins in endoscopic resection of UCAN, a rate that has been increasing in recent years. 19 , 20 , 21 , 22 , 23 , 24 The SCENIC consensus statement stated that endoscopic resection of neoplastic lesions in patients with UC may be considered if the boundaries are distinct; 6 and since this statement was made, there have been a number of reports on endoscopic resection of neoplastic lesions in patients with UC. In many of these reports, endoscopic resection was performed without distinguishing between SN and UCAN, but when we focused on the results of ESD for UCAN, we find that the rate of positive margins was higher than that found on ESD of SN. 19 , 20 , 23 , 24 The reason for this high rate of positive margins may be that flat‐type dysplasia surrounding the elevated lesion cannot be accurately diagnosed, even if a negative biopsy was performed around the lesion. Unlike in cases of SN, ESD for UCAN requires a diagnosis of the extent of flat‐type dysplasia, even if it is an elevated lesion, keeping in mind that a field of flat dysplasia may exist in the surrounding area. We expect our results could help to detect flat dysplasia around the main lesion when determining the indications for endoscopic resection.
There are several limitations to this study. First, this was a single‐center retrospective study, and we did not perform pan‐chromoendoscopy; hence, it is undeniable that some UCAN may have been missed, and it is possible that mucosal patterns other than those we proposed may exist. Although multicenter validation is warranted, this is the first study to propose a characteristic mucosal structural pattern for flat‐type dysplasia. Second, the number of cases was small, but the endoscopic diagnosis of flat UCAN is difficult, and the present report covers the largest patient group to date. Third, the sensitivity and specificity of the mucosal pattern classifications were not examined. Because it is still unclear whether the proposed classification can be generalized, it is necessary to verify the sensitivity and specificity of the proposed mucosal pattern through prospective studies in the future.
In conclusion, we proposed a mucosal pattern that may be characteristic of flat‐type dysplasia by DCE. When a characteristic mucosal pattern, such as a small round or mesh pattern, is found by DCE, the possibility of UCAN should be considered.
CONFLICT OF INTEREST
Author K.T. received a grant from the Japanese Foundation for Research and Promotion of Endoscopy. The other authors declare no conflict of interest for this article.
FUNDING INFORMATION
None.
ACKNOWLEDGMENT
We thank Dr Nikki March from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
REFERENCES
- 1. Kobayashi T, Siegmund B, Le Berre C et al. Ulcerative colitis. Nat Rev Dis Primers 2020; 6: 74. [DOI] [PubMed] [Google Scholar]
- 2. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta‐analysis. Gut 2001; 48: 526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett 2014; 345: 235–41. [DOI] [PubMed] [Google Scholar]
- 4. Fumery M, Dulai PS, Gupta S et al. Incidence, risk factors, and outcomes of colorectal cancer in patients with ulcerative colitis with low‐grade dysplasia: A systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2017; 15: 665–74.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hata K, Anzai H, Ikeuchi H et al. Surveillance colonoscopy for ulcerative colitis‐associated colorectal cancer offers better overall survival in real‐world surgically resected cases. Am J Gastroenterol 2019; 114: 483–9. [DOI] [PubMed] [Google Scholar]
- 6. Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc 2015; 81: 489–501.e26. [DOI] [PubMed] [Google Scholar]
- 7. Sugimoto S, Naganuma M, Iwao Y et al. Endoscopic morphologic features of ulcerative colitis‐associated dysplasia classified according to the SCENIC consensus statement. Gastrointest Endosc 2017; 85: 639–46.e2. [DOI] [PubMed] [Google Scholar]
- 8. Kinoshita S, Uraoka T, Nishizawa T et al. The role of colorectal endoscopic submucosal dissection in patients with ulcerative colitis. Gastrointest Endosc 2018; 87: 1079–84. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki N, Toyonaga T, East JE. Endoscopic submucosal dissection of colitis‐related dysplasia. Endoscopy 2017; 49: 1237–42. [DOI] [PubMed] [Google Scholar]
- 10. Yang DH, Rey I. Endoscopic submucosal dissection for colitis‐associated dysplasia. Clin Endosc 2019; 52: 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kinoshita S, Nishizawa T, Yahagi N, Uraoka T. Endoscopic submucosal dissection in patients with ulcerative colitis. Digestion 2019; 99: 27–32. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe T, Ajioka Y, Mitsuyama K et al. Comparison of targeted vs random biopsies for surveillance of ulcerative colitis‐associated colorectal cancer. Gastroenterology 2016; 151: 1122–30. [DOI] [PubMed] [Google Scholar]
- 13. Ballester MP, Mesonero F, Flórez‐Diez P et al. Adherence to endoscopic surveillance for advanced lesions and colorectal cancer in inflammatory bowel disease: An AEG and GETECCU collaborative cohort study. Aliment Pharmacol Ther 2022; 55: 1402–13. [DOI] [PubMed] [Google Scholar]
- 14. Santi G, Michetti P, Froehlich F, Rossel JB, Pittet V, Maillard MH. Adherence to recommendations and quality of endoscopic colorectal cancer surveillance in long‐standing ulcerative colitis. Inflamm Intest Dis 2021; 6: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shinagawa T, Hata K, Morikawa T et al. Pine‐cone and villi patterns are endoscopic signs suggestive of ulcerative colitis‐associated colorectal cancer and dysplasia. Gastrointest Endosc 2019; 89: 565–75.e3. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto T, Kudo T, Jo Y, Esaki M, Yao T, Iida M. Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis: A pilot study. Gastrointest Endosc 2007; 66: 957–65. [DOI] [PubMed] [Google Scholar]
- 17. East JE, Suzuki N, von Herbay A, Saunders BP. Narrow band imaging with magnification for dysplasia detection and pit pattern assessment in ulcerative colitis surveillance: A case with multiple dysplasia associated lesions or masses. Gut 2006; 55: 1432–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishiyama S, Oka S, Tanaka S et al. Clinical usefulness of narrow band imaging magnifying colonoscopy for assessing ulcerative colitis‐associated cancer/dysplasia. Endosc Int Open 2016; 4: E1183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsumoto K, Oka S, Tanaka S et al. Long‐term outcomes after endoscopic submucosal dissection for ulcerative colitis‐associated dysplasia. Digestion 2021; 102: 205–15. [DOI] [PubMed] [Google Scholar]
- 20. Matsui A, Hoteya S, Hayasaka J et al. Real‐world experience of endoscopic submucosal dissection for ulcerative colitis‐associated neoplasia. Inflamm Intest Dis 2021; 6: 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang DH, Kim J, Song EM et al. Outcomes of ulcerative colitis‐associated dysplasia patients referred for potential endoscopic submucosal dissection. J Gastroenterol Hepatol 2019; 34: 1581–9. [DOI] [PubMed] [Google Scholar]
- 22. Kasuga K, Yamada M, Shida D et al. Treatment outcomes of endoscopic submucosal dissection and surgery for colorectal neoplasms in patients with ulcerative colitis. United European Gastroenterol J 2021; 9: 964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishio M, Hirasawa K, Ozeki Y et al. An endoscopic treatment strategy for superficial tumors in patients with ulcerative colitis. J Gastroenterol Hepatol 2021; 36: 498–506. [DOI] [PubMed] [Google Scholar]
- 24. Zeng QS, Zou M, Nie J, Yang JH, Luo ZY, Gan HT. Efficacy and safety of endoscopic submucosal dissection for rectal tumors extending versus not to the dentate line: A systematic review and meta‐analysis. J Clin Gastroenterol 2022; 56: 518–28. [DOI] [PubMed] [Google Scholar]


