Abstract
Apoptosis continues to be controversial in human immunodeficiency virus (HIV)-induced pathogenesis. To investigate whether apoptosis occurs with HIV exposure with or without subsequent infection, levels of apoptosis were measured in cord blood lymphocytes (CBL) from seven newborns delivered to HIV-infected mothers and seven normal, unexposed newborns. Live cells were costained with antibodies to cell surface markers and the DNA dye 7-amino actinomycin D to immunophenotype apoptotic CBL subsets. Apoptosis was measured in fresh and cultured CBL in the presence and absence of CD3 T-cell receptor stimulation. Compared to the CD4+ CBL from HIV-unexposed newborns, CD4+ CBL from six HIV-exposed, noninfected newborns demonstrated significantly greater apoptosis after overnight culture even in the absence of CD3 stimulation. Compared to HIV-unexposed controls, CD8+ CBL from the six HIV-exposed newborns also demonstrated increased levels of apoptosis after overnight culture without stimulation. The one HIV-infected newborn in this study showed the highest levels of CD4+ and CD8+ apoptotic CBL. The data suggest that levels of apoptosis are increased in infants in association with HIV infection. Furthermore, CD4+ and CD8+ cord blood lymphocytes from HIV-exposed infants behaved differently than T lymphocytes from either normal, unexposed infants or an HIV-infected infant. These results suggest that exposure to HIV or HIV-induced factors increases the levels of apoptosis in CBL.
Apoptosis is a process by which cells undergo DNA degradation and ultimately death. It is a critical component of many physiologic processes, including maintaining homeostasis in the immune system. The clinical significance of apoptosis as a mechanism of CD4+ T-cell loss and human immunodeficiency virus (HIV)-induced disease pathogenesis continues to be controversial as reported in studies of adult and pediatric HIV-infected patients (9–11, 13). Since vertical transmission occurs simultaneously with the development of the immune system, apoptosis may be a significant pathogenic mechanism for pediatric HIV disease and may account for the depletion of mature and immature T lymphocytes (6, 9). Only two previous studies have examined HIV-related apoptosis in children (9, 11). A study of peripheral blood mononuclear cells (PBMC) demonstrated that HIV-infected children 1 to 10 years old have greater levels of apoptosis than HIV-exposed but uninfected controls (11). Studies of lymph nodes from HIV-infected children have shown that apoptosis occurs predominantly in uninfected cells, indicating that apoptosis may be related to cellular activation and/or cytokine production and not to direct infection (9). To our knowledge, there are no studies on vertical transmission of HIV that examine apoptosis in young infants exposed to but not infected with HIV. Our data indicate that levels of apoptosis in cord blood lymphocytes (CBL) from HIV-exposed but uninfected newborns are increased compared to those from non-HIV-exposed newborns.
MATERIALS AND METHODS
Subjects.
Seven cord blood specimens from infants of healthy pregnant women randomly chosen served as normal, HIV-unexposed controls. Seven cord blood specimens from infants born to mothers infected with HIV served as the exposed group. All mothers received care in Los Angeles County, Calif., and HIV status and disease stage were determined according to Centers for Disease Control and Prevention guidelines (3, 4). The study was approved by the Human Subject Protection Committee at the University of California Los Angeles.
The study was blinded. Virologic and immunophenotypic information was obtained for the HIV mother-infant pairs only after completion of data analyses. The infants’ clinical statuses were prospectively monitored from birth until definitive classification (4). Clinical and laboratory data of mother and infant pairs are shown in Table 1. All HIV-infected mothers had positive PCRs for HIV type 1 DNA sequences and positive viral cultures of PBMC (2). Two infants (patients 3 and 5) had HIV-positive cultures of CBL but negative PCRs and PBMC cultures at birth. Patient 7 had a positive HIV culture with a low titer (positive at 106 PBMC) and negative PCR at 10 weeks of age and three negative sets of tests subsequently. Thus, the six seroreverter infants, defined as infants who had at least three negative sets of tests for HIV infection (p24 antigen, HIV PCR, HIV culture) between 6 and 18 months of age (4), are included in the data analyses as the HIV-exposed group. Patient 5 was HIV infected (stage A1) as determined by an HIV-positive culture and PCR at 6 weeks of age and thus was excluded from statistical analysis.
TABLE 1.
Clinical and laboratory data on mother-infant pairs
| Characteristic or parameter | Result for patient:
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Maternal HIV status at deliverya | A | A | B | A | B | A | A |
| Maternal CD4+ T cells/mm3 at delivery | 231 | 732 | 247 | 428 | 632 | 461 | 248 |
| ZDV given during pregnancy (trimester[s]) | Yes (1, 2, 3) | Yes (1, 2, 3) | Yes (2, 3) | Yes (3) | No | Yes (3) | Yes (1, 2, 3) |
| ZDV given to mother at delivery | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Method of deliveryb | NSVD | NSVD | NSVD | CS | NSVD | NSVD | Forceps |
| Cord HIV PCR (coculture) | ND (ND) | − (−) | + (+)c | − (ND) | − (+) | − (−) | − (−) |
| Cord CD4+ T cells/mm3 (nlg 1181-4132) | 2,056 | 2,962 | 3,149 | 549d | 2,468 | 1,108 | 2,415 |
| Cord CD8+ T cells/mm3 (nl 573-2223) | 2,228 | 755 | 1,843 | 1,556 | 1,234 | 402 | 1,418 |
| ZDV given to baby postnatally | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| First positive PCR (coculture) | − (−) | − (−) | − (−) | − (−) | + (+) | − (−) | − (+)e |
| HIV status of baby at time of studyf | SR | SR | SR | SR | A | SR | SR |
A, asymptomatic HIV disease; B, symptomatic HIV disease.
NSVD, normal spontaneous vaginal delivery; CS, Cesarean section.
Although the cord PCR and culture were positive (+), patient 3 had negative (−) HIV PCR and cocultures with subsequent PBMC.
Although cord CD4+ T cells were low, patient 4 had normal T-cell numbers in subsequent PBMC.
Although patient 7 had a positive (+) PCR at 10 weeks of age, he had at least three subsequent negative (−) HIV PCR and cocultures.
SR, seroreverter. Patient 5’s first positive studies were at 6 weeks.
nl, normal range.
All six mothers of the HIV-exposed, seroreverter infants received zidovudine (ZDV) during pregnancy. However, the mother of the HIV-infected infant did not. All seven infants received ZDV for the first 6 weeks of life. All mothers’ infections were classified as HIV clinical stage A or B, and the mothers had CD4+ counts of >200 cells/mm3. Absolute numbers of CD4+ CBL were within the normal ranges for our institution (1,181 to 4,132 cells/mm3 [13a]). There were two exceptions; patients 4 (549 cells/mm3) and 6 (1,108 cells/mm3) had decreased numbers of CD4+ CBL but normal CD4+ numbers in subsequent PBMC measurements.
Reagents and MAb.
The following monoclonal antibodies (MAb) conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used: CD5, CD4, and CD8 (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, Calif.). Unconjugated CD3 MAb was used for stimulation of CBL (Coulter, Hialeah, Fla.). Seven-amino actinomycin D (7-AAD) was obtained from Sigma (St. Louis, Mo.), and actinomycin D (AD) was obtained from Boehringer Mannheim (Indianapolis, Ind.).
Cord blood mononuclear cell preparation.
Heparinized blood (0.5 to 4 ml) was obtained from umbilical cord specimens. Initial erythrocyte removal was carried out with 2.5% dextran mixed in equal parts with phosphate-buffered saline (PBS). Mononuclear cells were isolated by density gradient centrifugation over Lymphoprep (Robbins Scientific, Mountain View, Calif.), washed once with PBS, and resuspended in serum-free medium (Iscove’s Modified Dulbecco’s Medium; Irvine Scientific, Santa Ana, Calif.), bovine serum albumin (Sigma) at 1.1 mg/ml, transferrin (Sigma) at 85 μg/ml, glutamine at 2 mM (0.3 mg/ml), and penicillin and streptomycin at 25 U and 25 μg/ml, respectively (14). CBL were cultured overnight at 106 cells/ml in 15-ml culture tubes (1 ml/tube) in medium in the presence or absence of CD3 antibody at 1 μg/ml. Cell viability in the cultures was measured by trypan blue exclusion. Flow cytometric staining and analyses were carried out with freshly isolated cells on day 0 and with cultured cells on day 1.
Immunofluorescent staining and flow cytometry.
To measure apoptosis in combination with cell surface immunophenotyping we used the DNA dye 7-AAD (14). Purified CBL were stained with CD5-FITC/CD8-PE and CD5-FITC/CD4-PE MAb, respectively. Mouse immunoglobulin G1-FITC and immunoglobulin G1-PE (BDIS) were used as isotype control MAb. For determination of apoptotic and dead (late apoptotic or necrotic) cells, CBL were then incubated with 7-AAD (20 μg/ml) in PBS for 20 min at 4°C, washed in PBS, and fixed in 1% paraformaldehyde solution containing AD (20 μg/ml). The samples were submitted for flow cytometric analysis in the paraformaldehyde-AD solution. A FACScan flow cytometer equipped with a standard filter setup (BDIS) was used in these experiments. A minimum of 10,000 events was acquired on each sample. Multiparameter data acquisition and analysis were performed with LYSYS II software (BDIS). For the analysis of apoptotic cells, gates were set on cells staining dimly with 7-AAD; for the analysis of dead cells, gates were set on cells staining brightly with 7-AAD (late apoptotic and/or necrotic cells) (14).
Statistical data analysis.
Control and HIV-exposed CBL were compared for differences in levels of apoptosis in the CD4+ and CD8+ subsets. In addition, CBL exposed to HIV were compared for differential effects of overnight culture and CD3 stimulation. Statistical comparisons were performed by using the Mann-Whitney U test for independent samples (12) and the bootstrap technique (7). Significance was considered at P values of <0.05.
RESULTS
Analysis of apoptotic cells by flow cytometry.
Flow cytometric analysis of apoptosis in CD4+ and CD8+ T-cell subsets of CBL was performed in this study by a technique that was previously described by us (14). First, T-cell subsets were identified by staining of cell surface antigens. Then, staining with the DNA dye 7-AAD permits the discrimination of early apoptotic cells from live cells and late apoptotic or necrotic cells.
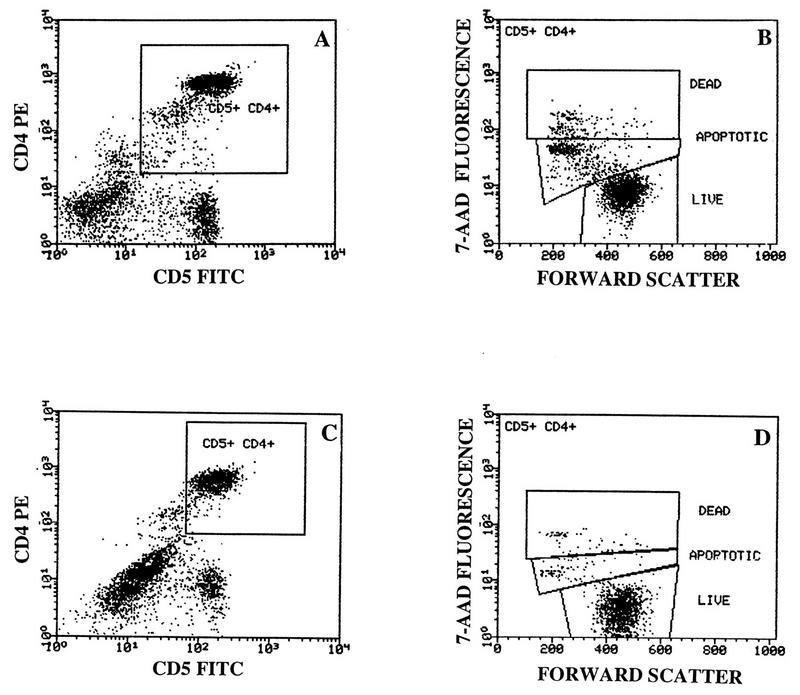
In order to determine the levels of apoptosis in CD4+ and CD8+ T-cell subsets of CBL, cells were immunophenotyped with CD5-FITC/CD4-PE and CD5-FITC/CD8-PE MAb, followed by incubation with 7-AAD. Live cells were defined as 7-AAD− cells. Apoptotic cells were defined as cells staining dimly with 7-AAD, and dead cells were defined as cells staining brightly with 7-AAD. Figure 1 shows a representative example of surface and 7-AAD staining of overnight-cultured CD4+ T cells from an exposed (Fig. 1A and B) and a nonexposed (Fig. 1C and D) newborn. A gate was set on CD5+ CD4+ cells (Fig. 1A and C), and then forward scatter and 7-AAD fluorescence were displayed on this population. In the example shown, the percentages of apoptotic cells in CD4+ CBL were as follows: HIV-exposed, 17% (Fig. 1B); nonexposed, 7% (Fig. 1D).
FIG. 1.
Measurement of apoptosis in CBL subsets by 7-AAD staining. CBL from an HIV-exposed newborn (A and B) and a control newborn (C and D) were cultured overnight in serum-free medium and immunophenotyped with CD5-FITC/CD4-PE followed by staining with 7-AAD. Gates were set on CD5+ CD4+ cells (A and C), and forward scatter and 7-AAD fluorescence were displayed on this population (B and D). Cells undergoing apoptosis were defined as 7-AADdim+ CD5+ CD4+, live cells were defined as 7-AAD−, and dead cells were defined as 7-AADbright+ cells. Percentages of early apoptotic, CD5+ CD4+ cells were 17 and 7% for the HIV-exposed and nonexposed newborn, respectively.
Comparison of CD4+ T-cell apoptosis in control and HIV-exposed CBL.
Levels of apoptosis in CD4+ CBL from cord blood specimens of six HIV-exposed and seven control newborns are summarized in Fig. 2A. Median percent apoptosis is shown. Apoptosis in freshly isolated cells (day 0) was the same in CD4+ CBL from control and HIV-exposed newborns (median = 2%). Apoptosis in CD4+ cells cultured in medium was significantly increased in the CBL from HIV-exposed newborns compared to that in control CBL (13 versus 4%; P < 0.05). However, levels of apoptosis in CD4+ CBL stimulated with CD3 were not significantly different for HIV-exposed newborns and controls (7 versus 4%).
FIG. 2.
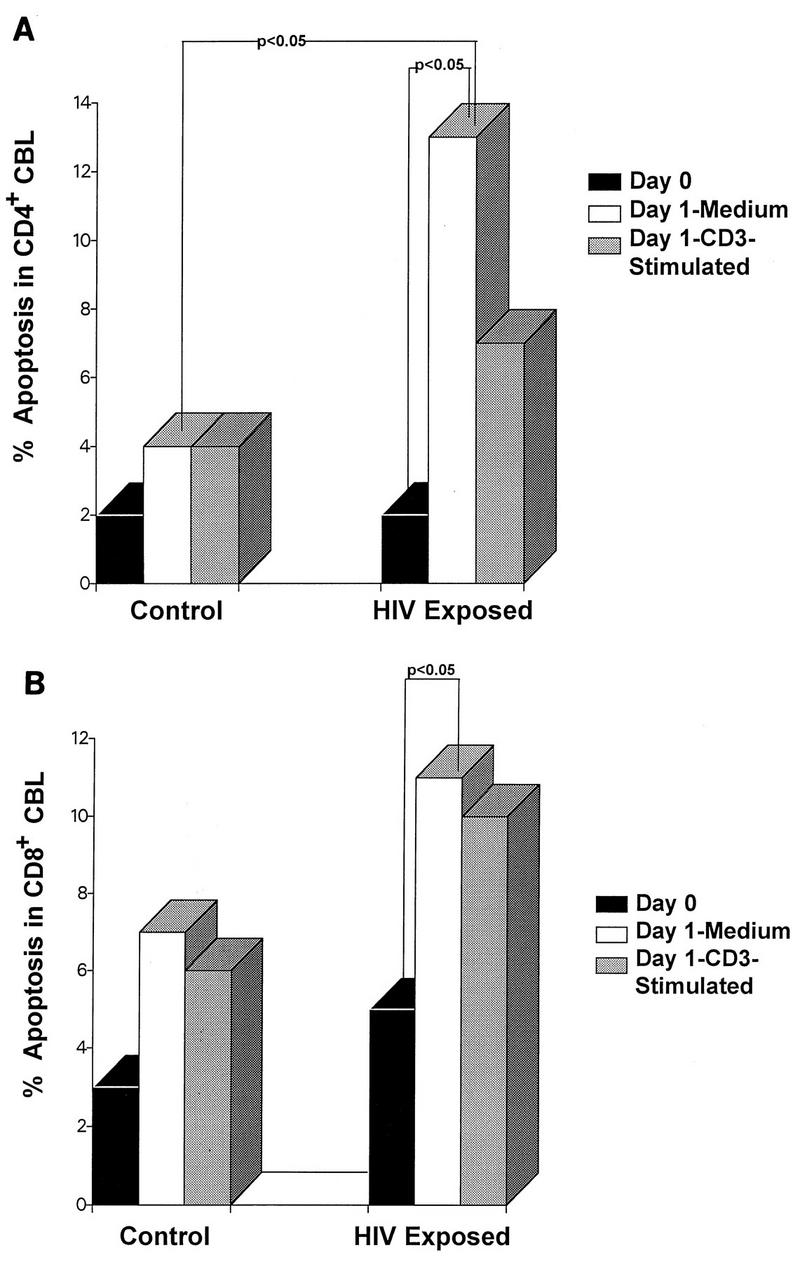
Apoptosis in CD4+ and CD8+ CBL from control and HIV-exposed newborns. Median percent apoptosis is shown for CBL immunophenotyped before in vitro culture (Day 0), after overnight incubation in medium (Day 1-Medium), and after overnight stimulation with CD3 (Day 1-CD3-stimulated). CBL were costained for surface markers and 7-AAD for apoptosis as described in Materials and Methods. Cells undergoing apoptosis were defined as 7- AADdim+ CD5+ CD4+ (A) and as 7-AADdim+ CD5+ CD8+ (B) CBL, respectively, from seven control and six HIV-exposed newborns. The ranges of apoptotic cells were as follows. Ranges for day 0 CD4+ control and HIV-exposed infants were 0 to 5.4 and 1.3 to 3.7%, respectively, and those for CD8+ control and HIV-exposed infants were 1 to 14.6 and 2.9 to 8.0%, respectively. Ranges for day 1 CD4+ control and HIV-exposed infants were 2 to 7.9 and 6 to 24%, respectively, and those for CD8+ control and HIV-exposed infants were 2 to 19.6 and 7 to 13.4%, respectively. Ranges for day 1 with CD3 stimulation CD4+ control and HIV-exposed infants were 2 to 8.1 and 1.2 to 12%, respectively, and those for CD8+ control and CD8+ HIV-exposed infants were 2 to 23 and 2.6 to 15.3%, respectively.
Comparison of CD8+ T-cell apoptosis in control and HIV-exposed CBL.
Levels of apoptosis in CD8+ CBL from six HIV-exposed and seven control newborns are summarized in Fig. 2B. Levels of apoptosis in freshly isolated CD8+ CBL (day 0) from control (3%) and HIV-exposed (5%) newborns were similar. Apoptosis in CD8+ CBL cultured in medium was increased in the CBL from HIV-exposed newborns (11%) compared to that in controls (7%), but these differences did not achieve statistical significance. Levels of apoptosis in CD8+ cells stimulated with CD3 were also not significantly increased in CBL from HIV-exposed newborns (11%) compared to those in controls (6%).
Apoptosis in freshly isolated and cultured CBL.
Levels of apoptosis in freshly isolated CBL and in cultured CBL for both control and HIV-exposed newborns are also presented in Fig. 2. Culturing increased apoptosis for both CD4+ and CD8+ subsets in CBL from HIV-exposed but not from control newborns. Thus, in the cultured CD4+ CBL from the HIV-exposed newborns, apoptosis was significantly increased compared to that in day 0, uncultured cells (13 versus 2%; P < 0.05). Apoptosis was also significantly increased in cultured CD8+ CBL compared to that in fresh CD8+ CBL from the HIV-exposed group (11 versus 5%; P < 0.05).
CD4 and CD8 T-cell apoptosis in CBL from an HIV-infected newborn.
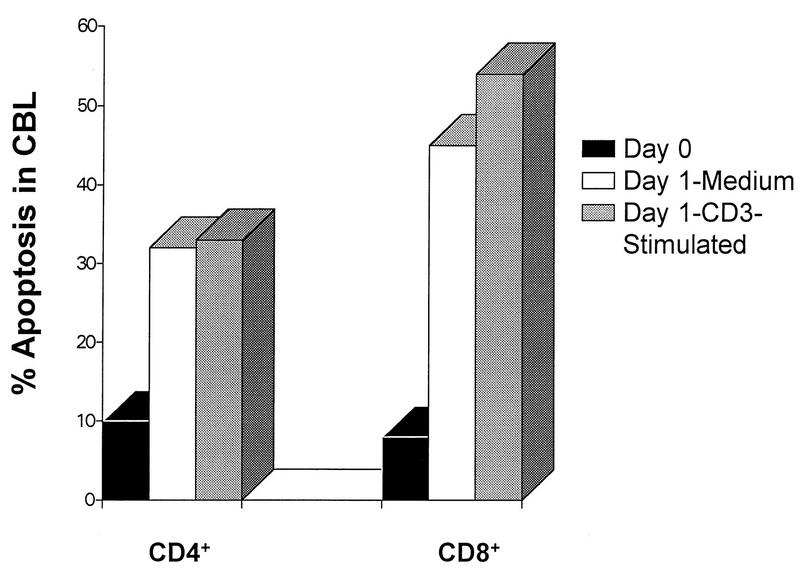
Figure 3 shows the levels of apoptosis in CBL for the one newborn in this study who was HIV infected. Apoptosis in freshly isolated CD4+ CBL (day 0) from the HIV-infected newborn was higher (10%) than those in control (2%) and HIV-exposed (2%) groups. Apoptosis in CD4+ CBL cultured in medium was almost threefold higher in the HIV-infected newborn (32%) than in the CD4+ CBL from HIV-exposed newborns (13%). The apoptosis in CD4+ CBL stimulated with CD3 was fivefold higher in the HIV-infected newborn (33%) than in the CBL from HIV-exposed newborns (7%).
FIG. 3.
Apoptosis in CD4+ and CD8+ cord blood T cells of an HIV-infected newborn. Percent apoptosis for both CD5+ CD4+ and CD5+ CD8+ cells is shown for CBL phenotyped on day 0, after overnight incubation in medium (Day 1-Medium), and after overnight stimulation with CD3 (Day 1-CD3-stimulated). CBL were costained for surface markers and 7-AAD for apoptosis as described in Materials and Methods. Cells undergoing apoptosis were defined as 7- AADdim+ CD5+ CD4+ or as 7-AADdim+ CD5+ CD8+ CBL, respectively. Note the change in the scale of percent apoptotic cells between Fig. 2 and 3.
The levels of apoptosis in freshly isolated CD8+ CBL (day 0) were also higher in CD8+ cells from the HIV-infected newborn (8%) than those in the control (3%) and HIV-exposed groups (5%). Apoptosis in CD8+ CBL cultured in medium was fourfold higher in the HIV-infected newborn (45%) than in the CBL from HIV-exposed newborns (11%). Apoptosis in CD8+ CBL stimulated with CD3 was fivefold higher in the HIV-infected newborn (54%) than in the CBL from HIV-exposed newborns (11%).
Four of the HIV-exposed newborns (patients 1, 3, 5, and 7) in this study were also studied at 6 weeks of age along with 19 other HIV-exposed 6-week-old infants and compared to three age-matched HIV-infected infants. It is noteworthy that the levels of apoptosis in CD8+ PBMC from three HIV-infected infants were significantly higher than levels of apoptosis in CD8+ PBMC from 23 HIV-exposed infants (10 versus 3%; P < 0.05). However, there was no statistically significant difference in the levels of apoptosis in CD4+ PBMC from HIV-infected infants and the HIV-exposed infants at 6 weeks of age (5 versus 3.5%; P > 0.05).
DISCUSSION
The present study is the first to compare apoptosis in HIV-unexposed, HIV-exposed, and HIV-infected newborns. Most importantly, CD4+ CBL from HIV-exposed, uninfected newborns showed significantly greater apoptosis after 1 day of in vitro culture than those obtained from unexposed newborns. CD8+ CBL of HIV-exposed newborns also had increased apoptosis after 1-day cultures in medium compared to those in controls, although these differences were not statistically significant, perhaps due to the small sample size. The HIV-infected infant had the highest levels of CD4+ and CD8+ apoptotic CBL in our study. Levels of apoptosis in fresh and cultured CBL from unexposed newborns were similar to those reported by Aggarwal et al. (1). Lauener et al. reported an increase in apoptotic cells in PBMC obtained from HIV-infected children over 1 year of age compared to levels in HIV-exposed children but did not examine PBMC from nonexposed children (11).
Our data suggest that apoptotic cell death may be related to cellular activation during exposure to HIV, as has been shown for cells obtained from lymph nodes (9, 13). Lymph node biopsies from HIV-infected individuals show a strong correlation between apoptosis and cell activation rather than between apoptosis and clinical stage of HIV disease or viral burden (13). Finkel et al. reported that apoptosis occurs predominantly in uninfected cells in lymph nodes of HIV-infected children (9). It would seem, therefore, that apoptosis is related to cellular activation that occurs with exposure to HIV but is not necessarily directly linked to HIV infection. Activation-induced cell death has been reported to occur during other viral infections, such as Epstein-Barr virus infection, as well as noninfectious diseases, such as lupus erythematosus and B-cell lymphoma (8). The high percentages of apoptotic CD4+ and CD8+ CBL found in one HIV-infected newborn suggest that levels of apoptosis are associated with HIV infection. Although this observation is in agreement with our observations with CD8+ PBMC from infants at 6 weeks of age and with studies reporting a high correlation between level of apoptosis and disease severity in children (9) and adults (10), further studies are needed to assess if there is a direct link between apoptosis and HIV infection.
Overnight culture in the absence of CD3 stimulation yielded higher percentages of apoptotic cells in the CD4+ CBL of HIV-exposed infants than did overnight culture in the presence of CD3. These experiments were meant to determine whether in vivo activation of CBL (presumably by HIV) was sufficient to induce apoptosis or whether CBL need additional in vitro stimulation with CD3 for the process to be enhanced. The findings that levels of apoptosis were highest in cultures of HIV-exposed cells without CD3 stimulation agree with those of Lauener et al. (11) but are in contrast to those of Gougeon et al. (10), who reported that apoptosis in PBMC is enhanced after in vitro activation by a variety of stimuli, including CD3 MAb. Thus, these data suggest that CBL from HIV-exposed infants do not require further stimulation by CD3 to undergo apoptosis whereas PBMC may require such stimulation.
The present study did not address the concern that therapeutic interventions, such as ZDV, may affect the observed levels of apoptosis. Six out of seven mothers in the present study received ZDV during delivery, and six out of seven mothers also received it during prenatal care. Since the effects of ZDV on apoptosis remain unknown, questions regarding its role in apoptosis during HIV infection could not be determined in this study but may be of clinical relevance. It is becoming increasingly difficult to control for such confounding factors, in light of the recommendations to give ZDV to all HIV-infected pregnant women to prevent transmission (5).
Finally, this study has shown that CD4+ and CD8+ T lymphocytes from HIV-exposed infants behave differently from those from either HIV-infected or unexposed, normal infants. Therefore, it seems inappropriate to consider children that were HIV-exposed but did not become infected as immunologically normal controls in studies of vertically transmitted HIV. Studying exposed, uninfected patients, however, may help delineate immunological changes related to exposure to HIV and, as such, may augment the successful care of children infected with HIV.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Health (HD 29341, HD 30629, AI 32440, and AI 28697), by a grant from the Pediatric AIDS Foundation (50548-17-PG), and by a postdoctoral fellowship grant from the Universitywide AIDS Research Program (F92-LA-003).
We are grateful to the physicians and staff participating in the Los Angeles Consortium for the Factors Influencing the Timing of Maternal-Fetal HIV Transmission Project; to Beth Jamieson, Joseph Church, and Ronald Ferdman for their helpful comments; and to Silvia Neagos and Saul Bermudez for technical assistance.
REFERENCES
- 1.Aggarwal S, Gupta A, Nagata S, Gupta S. Programmed cell death (apoptosis) in cord blood lymphocytes. J Clin Immunol. 1997;17:63–73. doi: 10.1023/a:1027340529644. [DOI] [PubMed] [Google Scholar]
- 2.Bryson Y J, Pang S, Wei L S, Dickover R, Diagne A, Chen I S Y. Clearance of HIV infection in a perinatally infected infant. N Engl J Med. 1995;332:833–838. doi: 10.1056/NEJM199503303321301. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1993;41(RR-17):1–35. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morbid Mortal Weekly Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 5.Connor E M, Sperling R S, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 6.Debatin K, Fahrig-Faissner A, Enenkel-Stoodt S, Kreuz W, Benner A, Krammer P H. High expression of APO-1 (CD95) on T lymphocytes from human immunodeficiency virus-1-infected children. Blood. 1994;83:3101–3103. [PubMed] [Google Scholar]
- 7.Efron B, Tibishirani R J. An introduction to the bootstrap. New York, N.Y: Chapman and Hall; 1993. [Google Scholar]
- 8.Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152:3685–3692. [PubMed] [Google Scholar]
- 9.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 10.Gougeon M, Lecoeur H, Dulioust A, Enouf M, Crouvoisier M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 11.Lauener R P, Huttner S, Buisson M, Hossle J P, Albisetti M, Seigneurin J, Seger R A, Nadal D. T-cell death by apoptosis in vertically human immunodeficiency virus-infected children coincides with expansion of CD8+/interleukin-2 receptor−/HLA-DR+ T cells: sign of a possible role for herpes viruses as cofactors? Blood. 1995;86:1400–1407. [PubMed] [Google Scholar]
- 12.Lehmann E L. Nonparametrics. Oakland, Calif: Holden-Day Inc.; 1975. [Google Scholar]
- 13.Muro-Cacho C A, Pantaleo G, Fauci A S. Analysis of apoptosis in lymph nodes of HIV-infected persons. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 13a.Plaeger, S. Unpublished data.
- 14.Schmid I, Uittenbogaart C H, Keld B, Giorgi J V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]