Abstract
Feline immunodeficiency virus (FIV) is a useful model for testing of criteria for AIDS vaccine development. In the protocol we adopted, we used a primary isolate of FIV as a source of antigen and, for challenge, plasma from cats infected with the homologous virus never passaged in vitro. Cat erythrocytes (RBC) were coated with the surface components of freshly harvested and purified FIV by means of biotin-avidin-biotin bridges and used to immunize specific-pathogen-free cats (four doses at monthly intervals; total amount of FIV antigen administered per cat, approximately 14 μg). Immunized cats developed moderate levels of antibodies directed mainly to surface components of the virion and clearly evident lymphoproliferative responses. Four months after the last dose of immunogen, FIV-immunized cats and control cats immunized with bovine serum albumin-coated RBC were challenged. Judged from the results of the subsequent 12-month follow-up, FIV-immunized cats exhibited at least some degree of protection. However, following rechallenge, most of the FIV-immunized animals became virus positive in spite of a booster immunogen dose given 2 months before the second challenge.
Feline immunodeficiency virus (FIV) is extensively used as a model system to identify criteria for the development of effective anti-human immunodeficiency virus vaccines. Previous studies have demonstrated that vaccines based on whole inactivated virus or fixed infected cells can afford protection against experimental challenge with FIV. In contrast, vaccines based on recombinant envelope proteins have given poor results (reviewed in references 4, 14, 20, 31, and 40). This is surprising, as there is solid evidence that epitopes present on envelope proteins are major targets for neutralizing antibodies and other effectors of antiviral immune responses, such as cytotoxic T lymphocytes (3, 7, 13, 18, 21, 34–37). Thus, the possibility exists that failure of subunit vaccines to induce protective immunity is due to inappropriate presentation of the relevant epitopes (29, 33).
In this study, we chose to immunize cats with homologous erythrocytes (RBC) coated with the surface components of FIV particles by means of biotin-avidin-biotin bridges (FIV-RBC) on the following premises: (i) RBC of several mammals can be biotinylated with attachment of just a few thousand biotin molecules without affecting their integrity and in vivo survival (11, 24), (ii) inoculation of RBC coated with antigens via a biotin-avidin-biotin bridge was shown to induce in vivo immune responses similar to or greater than those obtained with complete Freund’s adjuvant (24, 25), and (iii) in a recent study, immunization with minute amounts of a recombinant antigen (150 ng/mouse) bound to RBC protected mice against lethal and latent herpes simplex virus type 1 infection (10). Moreover, we reasoned that the biotin-avidin-biotin bridge used to couple antigens to RBC might not only select in favor of the surface antigens of FIV but also preserve, to a large extent, their native configuration. Recent studies with primate lentiviruses have shown that the native configuration of the surface glycoproteins (SUgp) is extremely important for their immunological functions (reviewed in reference 29).
The results of our study have shown that FIV-RBC elicited significant humoral and cell-mediated immune responses to FIV in spite of the small amount of viral protein injected and that at least the humoral response was preferentially directed to the SUgp. In addition, FIV-RBC-immunized cats exhibited an enhanced, though short-lived, resistance to challenge with ex vivo FIV.
MATERIALS AND METHODS
Production, purification, and biotinylation of FIV for immunogen preparation.
The Pisa-M2 isolate of FIV (FIV-M2) was grown in MBM lymphoid T-cell cultures as previously described (28). To preserve its structural integrity, the virus was concentrated from clarified supernatants by use of a Minitan Filter System (Millipore, Bedford, Mass.), sucrose gradient purified, and biotinylated immediately after harvest from the cultures. For biotinylation, the virus was suspended at 2 mg/ml in phosphate-buffered saline (PBS) containing 1 M sucrose and treated with N-hydroxysuccinimido (NHS)-biotin, a biotin derivative that reacts specifically with exposed NH2 groups, as previously described (11, 25). Briefly, 6 μl of a stock solution of 1-mg/ml NHS-biotin (Pierce, Rockford, Ill.) was added to 1 ml of a virus suspension and the mixture was incubated at 4°C for 1 h. The reaction was blocked by adding Tris/HCl (pH 7.0) to a final concentration of 0.1 M, and after 10 min at 4°C, the virus was disrupted by adding Triton X-100 to a final concentration of 0.02% (vol/vol) and incubating the mixture at 4°C for 15 min. Excess biotin was removed by diluting the viral suspension to 25 ml with PBS and washing it twice with Centricon 10 (Amicon, Beverly, Mass.). Aliquots of biotinylated, disrupted FIV (0.395 mg of protein/ml) were stored at −80°C until use.
Mock antigen.
Mock antigen consisted of bovine serum albumin (BSA) biotinylated at 2 mg/ml exactly the same way as FIV and stored at 0.415 mg of protein/ml.
Biotinylation of cat RBC and quantitation of bound biotin.
Freshly drawn RBC from two specific-pathogen-free cats were washed three times with cold PBS and biotinylated as previously described (11, 24, 25). The number of biotin molecules bound to RBC was determined by measuring the binding of [125I]avidin (1.82 × 105 cpm/μg) as previously described (11). The percentage of RBC that became biotinylated was evaluated by using fluorescein isothiocyanate (FITC)-conjugated streptavidin as a probe. Briefly, 5 μl of an RBC suspension (3 to 5%) in PBS was incubated with 20 μl of FITC-streptavidin (1 mg/ml) diluted 1:100 in PBS. After 30 min at 4°C, the RBC were washed twice in PBS and resuspended to 2 ml; 104 events were analyzed in a flow cytometer (FACScan; Becton Dickinson, Bedford, Mass.).
Coating of biotinylated RBC with FIV and mock antigen.
FIV-RBC and BSA-coated RBC (BSA-RBC) were prepared just before inoculation into cats. Avidin (Boehringer GmbH, Mannheim, Germany) at 0.5 mg/ml was added to a 10% suspension of biotinylated RBC, and following agitation at 4°C for 1 h, excess avidin was removed with three washes in PBS. RBC coupled with avidin-biotin were then used to bind the biotinylated protein antigens. Two hundred micrograms of biotinylated FIV or BSA was added to 10 ml of a 10% suspension of prepared RBC. After 1 h at 4°C, the mixtures were washed three times in abundant PBS to remove unbound components and resuspended at 10%.
Quantitation of FIV and mock antigen bound to RBC.
One hundred micrograms of biotinylated FIV or biotinylated BSA was iodinated with 3 μl of 125I (1 mCi/10 μl) by the chloramine T method, and quantitation was performed by measuring the radioactivity in a Beckman counter. The 125I-labeled, biotinylated FIV (5.57 × 105 cpm/μg) and 125I-labeled, biotinylated BSA (1.25 × 105 cpm/μg) preparations thus obtained were used to determine the amount of each protein that was bound by 1 ml of biotinylated cat RBC with avidin as a bridge.
Immunizations and challenge.
Groups of four 7-month-old, specific-pathogen-free cats (Iffa Credo, L’Arbresle, France) were immunized with either FIV-RBC or BSA-RBC. The animals were housed individually in our climatized animal facility under European Community law conditions, examined once per week, and bled periodically under slight anesthesia. The immunization schedule consisted of 4 doses of 2 ml of 10% coated RBC suspensions given intraperitoneally at monthly intervals. The amounts of FIV and mock antigens received by each cat at the different times are shown in Table 1. Four months after the last immunizing dose, the cats were challenged intravenously with 10 50% cat-infectious doses of FIV-M2 plasma (28). Cats that did not become productively infected after the first challenge (i.e., remained reisolation and serologically negative; see below) were boosted 16 months later with their respective immunogens and after 2 additional months were given a second challenge using the same virus preparation and dose.
TABLE 1.
Amounts of FIV and BSA (mock antigen) proteins administered per cat at each immunization time
| Protein used for immunization | Mean amt of antigen (μg) used for immunizationa no.:
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| FIV | 2.08 ± 0.25 | 2.41 ± 0.29 | 2.50 ± 0.30 | 3.74 ± 0.45 | 3.34 ± 0.29 |
| BSA | 2.62 ± 0.24 | 2.70 ± 0.25 | 3.41 ± 0.31 | 5.50 ± 0.50 | 4.02 ± 0.35 |
The indicated amounts of antigens were present in the 2 ml of coupled RBC suspension used per immunization.
Analysis of anti-FIV immune response in vaccinated cats.
The antibody response to FIV was studied by enzyme-linked immunosorbent assay (ELISA) and immunoblotting. For ELISA, microwells were coated overnight with 100 μl of gradient-purified, disrupted whole FIV-M2 at 2 μg/ml (28). After a postcoating step done with skim milk, serially diluted cat sera were added to the plates. Bound immunoglobulins G (IgG) were revealed with biotinylated mouse anti-cat IgG serum, followed by an antibiotin peroxidase conjugate. Absorbance was read at 450 nm. For immunoblotting analysis, purified, sodium dodecyl sulfate (SDS)-disrupted FIV was separated by SDS–10% polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose sheets. After extensive blocking with skim milk, the blots were incubated with appropriately diluted cat sera. Bound antibodies were visualized by enzyme immunoassay using peroxidase-conjugated mouse anti-cat IgG; the reaction was developed on autoradiographic films (Hyperfilm-ECL; Amersham) by using the enhanced-chemiluminescense (ECL) detection system (Amersham). A serum raised by hyperimmunization of mice with the peptide 517FNKTKAVEMVNIAGNWSCTS536, derived from a conserved region of FIV Env and reactive with the SUgp in immunoblotting (26), was also used in these studies. FIV neutralizing antibodies were tested by using a lymphoid-cell-based assay as previously described (2). Cell-mediated immunity to FIV was monitored by a lymphocyte proliferation assay (28). Briefly, Ficoll-separated peripheral blood mononuclear cells (PBMC; 1.5 × 105) were incubated with 1 μg of purified and sonicated FIV in 200 μl of RPMI 1640 containing 10% heat-inactivated, AB-positive human serum and 2 mM l-glutamine for 4 days and then pulsed with [3H]thymidine for 18 h. The stimulation index (SI) was calculated as the ratio of radioactivity incorporated by PBMC in the presence or absence of FIV antigen. Only SIs of ≥2 were considered indicative of FIV-specific lymphoproliferation.
Follow-up of challenged cats.
Challenged cats were monitored for FIV infection by serology, virus reisolation from PBMC, and detection of proviral DNA by PCR. Diagnostic nested gag PCR was performed on PBMC as previously described (28). Assay sensitivity was 10 copies of the p34TF10 plasmid containing the whole FIV-Pet genome (kindly provided by J. E. Elder, La Jolla, Calif.). DNAs from uninfected cat PBMC and reagent controls were run in parallel, and the positive control (DNA from infected cells) was included in the second step only. At the end of the experiment, cat PBMC were also examined for infectious virus and provirus loads. Proportions of PBMC harboring infectious FIV were assessed by quantitative coculture with MBM cells as previously described (15). Proviral loads were quantitated by competitive PCR using an internal standard derived from the gag gene (32) and expressed as the number of proviruses in 1 μg of PBMC DNA.
RESULTS
Characterization of FIV and mock immunogens.
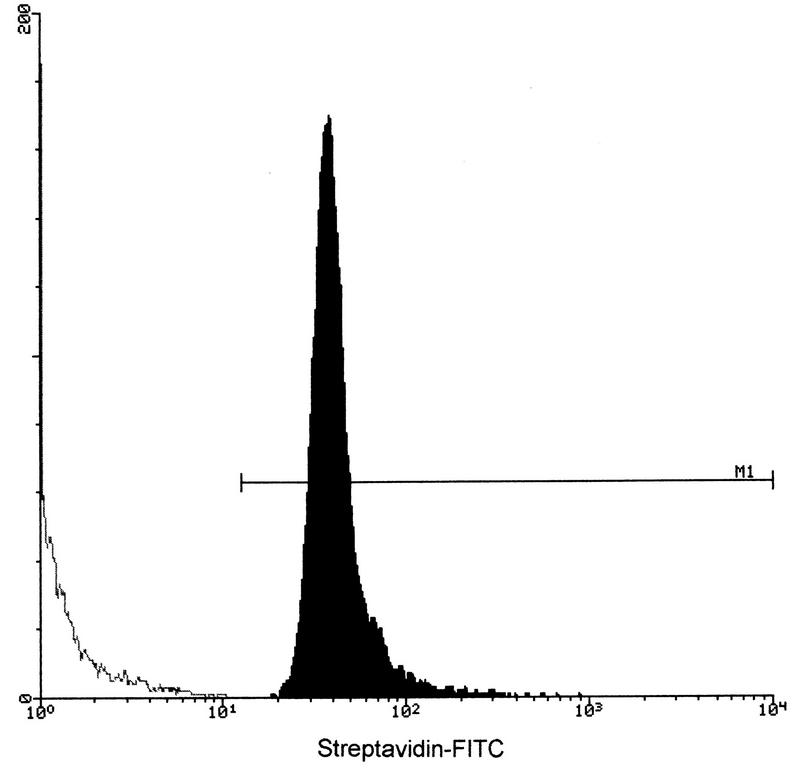
We first measured the proportion of cat RBC that became biotinylated after treatment with NHS-biotin. This was necessary because the procedure used had not been validated for cat RBC previously. Flow cytometric analysis using FITC-streptavidin as a probe showed that the cat RBC were almost all biotinylated and to similar extents (Fig. 1). Using 125I-avidin as a probe, we also estimated that the average number of biotin molecules coupled per single RBC was 30,703 ± 1,018 (mean ± standard deviation [SD] of three independent preparations). We then determined the amount of biotinylated FIV protein and BSA that became bound to biotinylated cat RBC through the avidin bridge by using 125I-labeled, biotinylated antigens. Bound FIV protein was 0.8 ± 0.1 μg/ml of RBC, while bound BSA was 1.1 ± 0.1 μg/ml of RBC (means ± SD of five determinations). Based on these results, it was calculated that the doses of FIV-RBC and BSA-RBC used to immunize cats at the different time points contained between 2.08 and 3.74 μg of FIV proteins and between 2.62 and 5.50 μg of BSA, respectively (Table 1). Thus, each FIV-RBC-immunized cat received approximately 14 μg of FIV protein.
FIG. 1.
Validation of the procedure used for biotinylating cat RBC. Biotin-positive cells were probed with FITC-streptavidin and examined by flow cytometry. M1., biotin-positive cells.
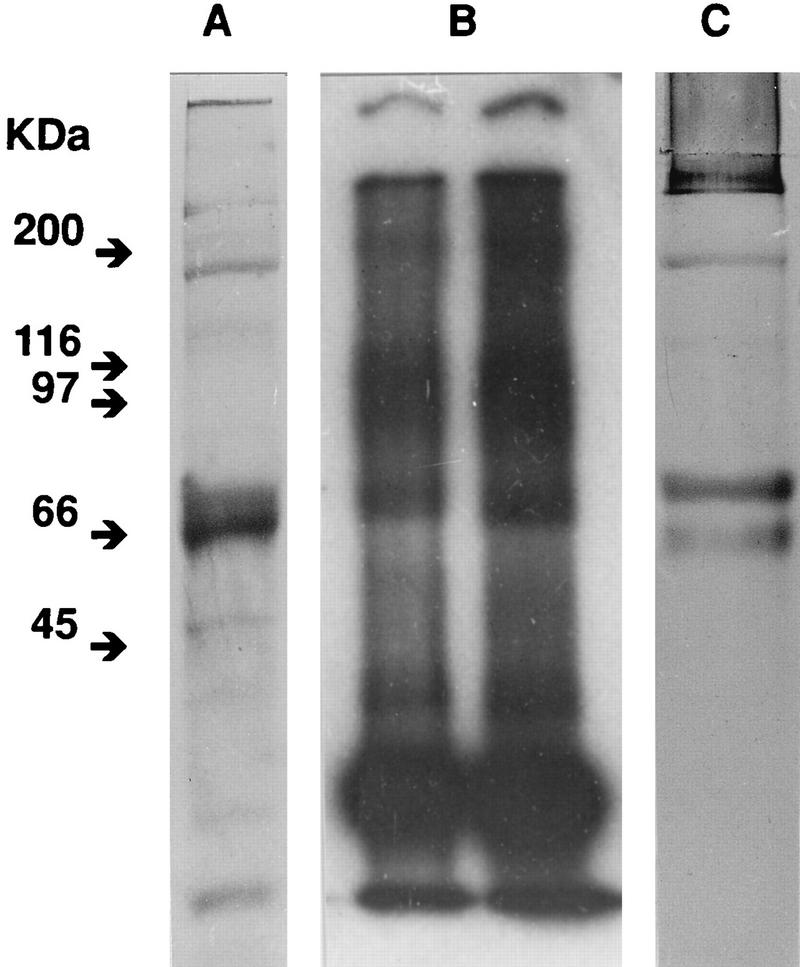
We also attempted to characterize the components of the virus that became biotinylated when whole FIV was treated with NHS-biotin. Freshly prepared, gradient-purified FIV was biotinylated, disrupted with a nonionic detergent exactly as described in Materials and Methods for immunogen preparation, and electrophoresed. Western blotting with streptavidin-peroxidase and ECL revealed three bands that exhibited relatively high levels of biotinylation, two with apparent molecular masses of 65 to 68 kDa, compatible with the SUgp molecule, and one with a mass of 170 kDa. Importantly, no band that might indicate significant biotinylation of the internal 25-kDa p25 capsid protein of FIV was visible in these blots (Fig. 2C). On the other hand, staining of the gels with Coomassie blue and counterstaining with silver demonstrated components with apparent molecular masses similar to those seen by using streptavidin and also other bands with lower masses (Fig. 2A). Note that whereas the 170-kDa band was preferentially stained by Coomassie blue and not by silver, the 65- to 68-kDa bands were preferentially stained by silver, which suggests that they were glycoproteins (12). For comparison, we also performed an autoradiographic analysis of gradient-purified FIV that had been biotinylated, disrupted, and then labeled with 125I to a specific activity of 5.57 × 105 cpm/μg. As expected, the most abundant component in the FIV lysate, by far, was a 25-kDa protein corresponding to capsid protein p25 (Fig. 2B). Based on these results, we tentatively concluded that, in accordance with the rationale of the experiment, biotin had bound considerable amounts of the SUgp and little of the internal viral proteins. This conclusion was corroborated by examining by immunoblotting the specificities of the antibodies produced by cats immunized with FIV-RBC (see below).
FIG. 2.
Characterization of the proteins biotinylated by reacting whole FIV with NHS-biotin. The indicated viral preparations were disrupted in SDS and subjected to SDS–10% PAGE. (A) Staining with Coomassie blue and silver of a gel obtained with biotinylated virus. (B) Autoradiography of a gel obtained with biotinylated FIV that was 125I labeled after disruption (Kodak X-Omat AR film at −70°C in the presence of Du Pont Lightning-Plus intensifying screens). (C) Staining with streptavidin of biotinylated FIV. The gel was blotted onto nitrocellulose, incubated with horseradish peroxidase-conjugated streptavidin, and examined by ECL.
Immune responses elicited by the FIV immunogen.
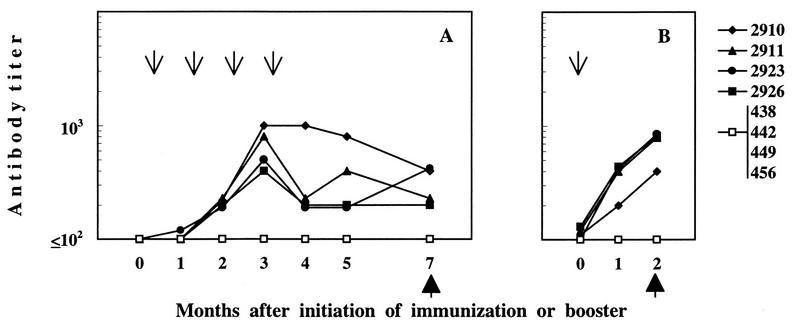
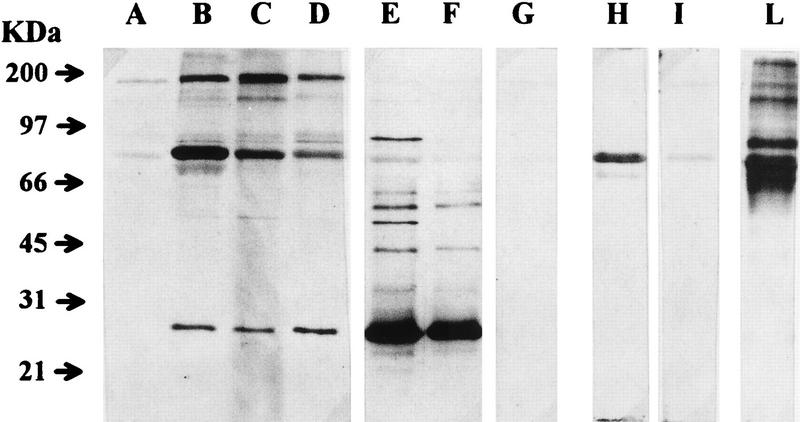
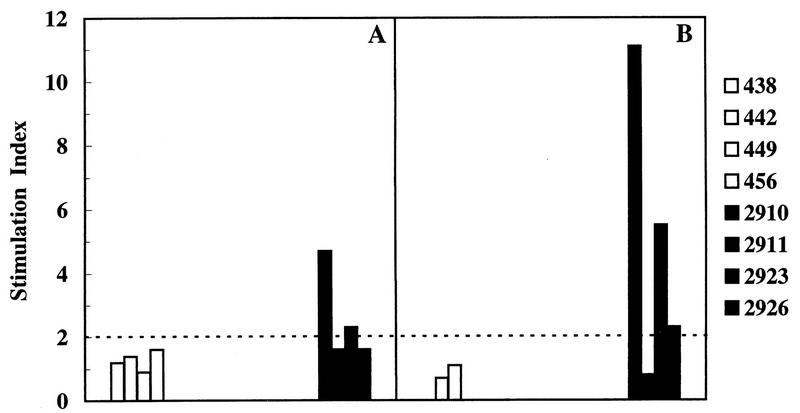
Throughout the experiment, the antibody response of immunized cats was monitored by ELISA with disrupted FIV as the antigen. As shown in Fig. 3A, immunization with FIV-RBC elicited the production of anti-FIV antibodies in all of the cats. Antibodies were first detected 2 months after initiation of immunization; peaked at 3 months, when titers ranged between 1:400 and 1:1,000; and then declined, despite the administration of an additional dose of immunogen. Thus, 4 months after completion of immunization, when the animals were challenged, antibody titers ranged between 1:200 and 1:400. Sera collected at challenge were also examined by immunoblotting for reactivity to denatured FIV proteins. As shown in Fig. 4, the four cats immunized with FIV-RBC (lanes A to D) exhibited various degrees of reactivity, but in all cases, the predominant bands corresponded to proteins of about 70 kDa, compatible with FIV SUgp, and 170 kDa, while the band corresponding to the p25 capsid antigen was relatively weak and remained so even when the blots were developed for prolonged periods (data not shown). This pattern was in stark contrast to that produced by sera of FIV-infected cats (exemplified in lanes E and F of Fig. 4), in which the p25 band was the most dominant by far, whereas high-molecular-weight bands were much fainter than those observed with FIV-RBC immunized cat sera. RBC-BSA-immunized cats were consistently FIV seronegative by both ELISA (Fig. 3A) and immunoblotting (Fig. 4, lane G). These antibody patterns were also compared with the reactivity of a serum obtained by hyperimmunizing mice with a synthetic peptide of the SUgp of FIV and known to stain this protein in immunoblotting. As shown by lane H in Fig. 4, the protein recognized by this serum had an apparent molecular mass similar to that of the protein recognized by the sera of RBC-FIV-immunized cats. Moreover, when probed by a glycoprotein-specific detection system, this protein proved to be glycosylated (Fig. 4, lane L). Consistent with previous results (28), the sera taken at the time of challenge were negative for virus neutralizing antibody, as determined in a lymphoid-cell-based assay (results not shown). Immunized cats were also monitored for FIV-specific cell-mediated immunity by examining the lymphoproliferative response of their PBMC to whole FIV lysate. As shown by the representative results depicted in Fig. 5A, two of four FIV-RBC-immunized cats were positive. In contrast, the four BSA-RBC-immunized cats were constantly negative.
FIG. 3.
ELISA antibody response to disrupted FIV antigen in FIV-RBC-immunized and control cats. Solid symbols indicate individual FIV-RBC-immunized animals. A single open symbol is used for BSA-RBC-immunized cats, as they were all unreactive. The arrows indicate the times when immunizing doses were administered, and the solid arrowheads indicate the times of challenge. (A) Response to primary immunization. (B) Response to a booster FIV-RBC dose given 20 months after completion of the primary immunization.
FIG. 4.
Immunoblot analysis of sera of FIV-RBC-immunized and control cats at the time of challenge. Purified FIV was subjected to SDS-PAGE under reducing conditions, blotted onto nitrocellulose, and reacted with sera from cats immunized with FIV-RBC (lanes A to D), with sera from FIV-infected cats (lanes E and F), with one serum from a BSA-RBC-immunized cat (lane G), and with an SUgp-specific (lane H) or control (lane I) mouse serum. Lane L was stained for glycoproteins by the ECL glycoprotein detection system in accordance with manufacturer’s instructions.
FIG. 5.
Proliferative responses to FIV antigen of individual cats immunized with FIV-RBC (solid bars) or BSA-RBC (open bars). (A) Response at the time of first challenge (4 months after completion of primary immunization). (B) Response at the time of second challenge (2 months after a booster dose of immunogen). The SI is the ratio of radioactivity incorporated by PBMC in the presence of the antigen to that incorporated in the absence of the antigen. Only values of ≥2 were considered indicative of FIV-specific lymphoproliferation. In the absence of antigen, values ranged between 177 and 4,488 cpm in individual cats.
Outcome of challenges.
The above findings demonstrated that immunization with FIV-RBC elicited a significant antibody response against FIV proteins, though it was considerably less pronounced than that observed in a previous successful vaccination experiment in which cats had been immunized with fixed, FIV-infected cells (28). FIV-RBC-immunized cats also developed a cell-mediated response to FIV that was of the same magnitude as that observed in a previous study (28). To assess whether the immune response elicited by FIV-RBC translates into significant protection, we challenged the animals with the same viral strain used for vaccine preparation. The stock of virus used was plasma of cats infected with FIV passaged only in vivo, and the inoculum contained 10 50% cat-infective doses.
Table 2 shows that at the time of challenge, all of the immunized cats were virus negative, as determined by sensitive FIV culture and PCR analysis, thus showing that the procedure used to prepare the FIV-RBC immunogen had effectively inactivated FIV infectivity. Table 2 also shows the results of the virological and serological assays performed on challenged cats at selected times. In the mock-vaccinated group, two cats were repeatedly reisolation and/or serologically positive, as well as PCR positive, and the remaining two cats were repeatedly PCR positive, though they showed no signs of productive FIV replication. Thus, at the end of the 12-month postchallenge follow-up, two of four mock-immunized cats could be scored as productively infected. In contrast, the four animals in the FIV-RBC-vaccinated group showed no evidence of FIV infection throughout the observation period, except for cat 2923, which was PCR positive at 1 month, and, cat 2911 which was PCR positive at 12 months postchallenge. Thus, at the end of the follow-up period, none of the FIV-RBC-immunized cats could be scored as productively infected.
TABLE 2.
Virological and serological markers of FIV infection in FIV-RBC-immunized and mock-immunized cats at the time of challenge and at selected times thereafter
| Cat group and no. | Presence of marker at postchallenge time (mo):
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
1
|
3
|
5
|
7
|
9
|
12
|
||||||||||||||
| PCRa | VIb | PCR | VI | Sc | PCR | VI | S | PCR | VI | S | PCR | VI | S | PCR | VI | S | PCR | VI | S | |
| BSA-RBC immunized | ||||||||||||||||||||
| 438 | − | − | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + | + | − | + |
| 442 | − | − | + | − | − | + | − | − | + | − | − | − | − | − | + | − | − | + | − | − |
| 449 | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − | + | − | − |
| 456 | − | − | − | + | + | + | + | + | − | + | + | + | + | + | + | + | + | − | + | + |
| FIV-RBC immunized | ||||||||||||||||||||
| 2910 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − |
| 2911 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2923 | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2926 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
PBMC were examined for proviral FIV gag sequences by nested PCR.
VI, virus isolation by coculture with MBM cells.
S, serology. Sera were tested in an ELISA for IgG antibody to purified and disrupted FIV. +, antibody titer at least fourfold higher than that at the time of challenge; −, antibody titer not significantly different from that at the time of challenge.
Sixteen months after the above-described challenge, the animals that had escaped productive infection were given one booster dose of the respective immunogens. The antibody and lymphoproliferative levels of the FIV-RBC-immunized animals had, in fact, considerably declined by this time (data not shown). The animals responded promptly to the FIV-RBC booster, so that 2 months later, their anti-FIV ELISA antibody reached higher levels than those observed at the same time after the first challenge (Fig. 3B). This was confirmed by immunoblot analysis, which also showed that the specificity of antibodies was similar to that observed after primary immunization (data not shown). FIV-specific lymphoproliferation was also positive in three of four FIV-RBC-immunized cats (Fig. 5B). As expected, the control cats that had received a booster of mock immunogen remained FIV unreactive (Fig. 3B and 5B). Two months after the booster, FIV-RBC- and BSA-RBC-immunized cats were given a second FIV challenge. As shown in Table 3, this challenge was fully successful since the two mock-immunized animals became productively infected within 1 month. Of the FIV-RBC-immunized animals, only one remained virus free until the end of the 6-month follow-up period, whereas the others became productively infected as readily as the controls. At the end of the experiment, we also measured the infectious virus and the proviral loads found to be associated with the PBMC of infected cats and found no appreciable differences between FIV-RBC- and mock antigen-immunized animals (Table 3).
TABLE 3.
Virological and serological markers of FIV infection in FIV-RBC-immunized and mock-immunized cats at the time of rechallenge and at selected times thereafter
| Cat category and no. | Presence of marker or no. of infected cells at rechallenge time (mo):
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
1
|
3
|
6
|
|||||||||
| PCRa | VIb | PCR | VI | Sc | PCR | VI | S | PCR | VI | Viral loadd | S | |
| BSA-RBC immunized | ||||||||||||
| 442 | + | − | + | + (10) | − | + | + (15) | + | + (1,110) | + (28) | 10 | + |
| 449 | − | − | − | + (14) | − | − | + (15) | + | − | + (28) | 1 | + |
| FIV-RBC immunized | ||||||||||||
| 2910 | − | − | − | − | − | − | − | − | − | − | − | |
| 2911 | − | − | + | + (15) | − | + | + (15) | − | + (4,440) | + (14) | 10 | − |
| 2923 | − | − | − | + (7) | − | + | + (7) | + | + (830) | + (14) | 102 | + |
| 2926 | − | − | − | + (7) | − | + | + (7) | + | + (660) | + (14) | 102 | + |
PBMC were examined for proviral FIV gag sequences by nested PCR. Values in parentheses at 6 months indicate the proviral load present in 1 μg of PBMC DNA as assessed by competitive PCR.
VI, virus isolation by coculture with MBM cells. Values in parentheses indicate days of incubation at the time cultures first became positive.
S, serology. Sera were tested in an ELISA for IgG antibody to purified and disrupted FIV. +, antibody titer at least fourfold higher than that at the time of challenge; −, antibody titer not significantly different from that at the time of challenge.
At this time, PBMC were also examined for infectious virus load by quantitative isolation. Results are expressed as numbers of infected cells per 106 PBMC.
DISCUSSION
Previous studies have shown that vaccine-induced protection against FIV is achievable, though the viral epitopes and the immune mechanisms involved have remained essentially unresolved (reviewed in references 4, 14, 20, 31, and 40). Thus, vaccines composed of fixed cells or whole inactivated virus derived from the FL4 cell line, which is chronically infected with the Petaluma strain of FIV, have repeatedly protected against challenge with tissue culture-adapted virus (5, 17, 19, 41). In addition, we have recently shown that a fixed-cell vaccine prepared with the same primary isolate used in this study effectively, albeit transiently, protected cats against cell-free and cell-associated virus challenges obtained ex vivo, thus showing that vaccine protection can also be efficacious against fully virulent viruses similar to those which circulate in nature (27, 28).
By analogy with other viruses, it is generally believed that the protective antigens of lentiviruses are mainly viral components exposed at the virion surface (6, 16, 29, 30, 33). Characterization of FIV antigens has corroborated this assumption, since all of the FIV structures involved in antibody-mediated neutralization and at least some of the epitopes recognized by cytotoxic T lymphocytes identified to date are located in the SUgp (3, 7, 13, 18, 21, 34–37). It is therefore paradoxical that attempts to vaccinate cats by using recombinant SUgp or synthetic peptides have given poor results and have sometimes enhanced FIV infection following challenge (22, 23, 35, 37, 39). Among the explanations put forward to explain the failure of FIV subunit vaccines to protect and that of similar tested vaccines to protect against other lentiviruses is that the SUgp epitopes important for protection are conformational in nature and that, to induce protective immunity, the relevant viral molecules have to be presented to the immune system in a configuration as similar as possible to that on the native virion (29, 33). That this explanation is plausible is suggested by recent findings showing that vaccination with immunoaffinity-purified SUgp obtained from the FL4 cell line, though less effective than vaccination with whole inactivated FIV in protecting cats against viremia, did, nevertheless, reduce the resulting viral loads (17).
Previous data have shown that coated homologous RBC represent an interesting antigen delivery system (10, 25). In the present study, we have used this approach to immunize cats with enriched, native surface antigens of FIV in an attempt to favor the development of protective immune responses. For immunogen preparation, the proteins exposed on the intact virion were biotinylated by treating freshly harvested, purified, whole FIV with an impermeant biotin derivative specifically reactive with exposed NH2 groups. The virions were then gently disrupted by using a detergent, and the biotinylated proteins obtained were selectively bound to avidin-biotin-coated RBC, which were then used to inoculate cats immediately after coating. The total amount of FIV antigen administered per cat was minimal, approximately 14 μg, and was therefore many-fold smaller than that used in previous studies employing immunoaffinity-purified or recombinant SUgp mixed with potent adjuvants (17, 23). Nonetheless, immunized cats developed clearly evident anti-FIV humoral and cell-mediated immune responses. Anti-FIV antibodies were first detected 1 month after the second dose of immunogen and peaked after the third dose, though they never reached the titers observed with more complex immunogens (27, 28). Antibodies, however, started to decline soon in spite of the administration of a fourth dose of immunogen, so that by 4 months after completion of the immunization cycle, their titers were low. Interestingly, FIV-RBC-immunized animals had immunoblotting profiles that differed considerably from those of FIV-infected cats and even those of cats vaccinated with infected cells (data not shown) in that they produced stronger bands specific for the SUgp and much fainter bands against internal capsid antigen p25, thus showing that FIV-RBC had preferentially stimulated the responses to the surface antigens of FIV. However, the sera taken at the time of challenge lacked detectable FIV neutralizing antibodies, similar to what was observed following vaccination with fixed, infected cells (28). The development of cell-mediated immunity in the vaccines was monitored by examining the lymphoproliferative response of PBMC on repeated occasions. As judged from the proportion of reactive cats and from the SIs observed, this response was as well developed as in previous studies in which we used more complex vaccines and the same assay conditions (27, 28), thus suggesting that the delivery system used may be especially effective in eliciting cell-mediated immune responses, though further studies are needed to prove this point.
To assess whether the immune responses evoked by FIV-RBC had protective value, 4 months after completion of the immunization schedule, FIV-RBC-immunized and control cats were challenged with ex vivo FIV. All four controls became consistently FIV positive by PCR throughout the 12-month follow-up, though only two became productively infected. On the other hand, of the four FIV-RBC-vaccinated cats, none became productively infected and two proved PCR positive only in one occasion during the 12-month follow-up. Thus, although the results of the experiment could not be considered conclusive due to the partial success of the challenge, the outcomes in the groups of animals were sufficiently different as to indicate that FIV-RBC-immunized cats not only had responded immunologically to FIV but were also at least partly protected from FIV challenge.
Sixteen months after the first challenge, the animals that had escaped productive infection (four vaccinated and two mock vaccinated) were given an additional dose of immunogen which elicited a rapid anamnestic immune response. Anti-FIV antibodies, that had become barely detectable with time, increased rapidly so that by 2 months, they reached levels higher than those found at the same time after primary immunization, and lymphoproliferative indices also increased. Two months after the booster, the animals were rechallenged and the mock-vaccinated cats rapidly became virus isolation, as well as PCR, positive. After this challenge, three FIV-RBC-immunized cats became infected as promptly as the controls and with similar viral loads, thus showing that there was little, if any, residual protective immunity. Also, the protection against FIV induced by a fixed-cell vaccine has been shown to be short-lived and difficult to recall once it has waned (27).
The present experiment was conducted by using a series of precautions designed to avoid, or at least minimize, possible artifacts that might be associated with the use of tissue culture-adapted virus for vaccine preparation and challenge, such as reduced virulence of the challenge virus (2, 40) and the presence of similar host cell derived-antigens on the vaccine and the challenge virus (9, 38). Precautions included the use for the vaccine of a preparation of a primary isolate of FIV propagated a limited number of times in vitro and solely in nontransformed lymphoid cells and ex vivo challenge with a virus never passaged in vitro. In previous experiments using the same precautions (27, 28), cellular antigens were ruled out as possible mediators of protective anti-FIV immunity. Thus, it seems unlikely that host cell-derived antigens were significantly involved in the observed protection. However, we also made direct attempts to characterize the FIV antigens that were biotinylated and therefore presumably present in the FIV-RBC inocula. By probing with streptavidin, two proteins with apparent molecular masses of 65 to 68 kDa and one protein with a molecular mass of about 170 kDa, determined by SDS-PAGE, were found to be highly biotinylated. The former proteins had mobility and staining properties compatible with those of the SUgp of FIV; thus, also in the light of the immunoblotting profiles exhibited by the FIV-RBC-immunized cats, we can conclude that the FIV SUgp was well represented in the inocula. The 170-kDa protein was much less abundant, as judged from the size of the streptavidin-stained band, but was markedly immunogenic in cats, as shown by the immunoblots of FIV-RBC-immunized cats. This protein was probably nonviral in origin, since it had an electrophoretic mobility incompatible with those of mature FIV-encoded proteins and was not seen when sera of FIV-infected cats were used. Primate lentiviruses are known to incorporate several cell-derived proteins in their envelope (1, 8), and it is feasible that also FIV does, though no direct information about this issue exists. Thus, it is likely that the 170-kDa protein was cell derived. Further studies are needed to characterize the host cell proteins incorporated by the FIV envelope. The data also showed that internal capsid protein p25, by far the most abundant component of the FIV virion, was poorly represented among the biotinylated proteins, thus confirming that the method used had preferentially biotinylated FIV surface components.
In conclusion, this study has shown that immunization of cats with homologous RBC coated with minute amounts of FIV antigens via biotin-avidin-biotin bridges induced clearly evident FIV-specific immune responses that, judged from immunoblotting profiles, were directed mainly to the SUgp. The results have also shown that, following primary immunization with this method, cats were at least partially protected from ex vivo FIV challenge, thus confirming that the surface antigens of FIV are of paramount importance for the induction of protective immunity. Finally, the results have corroborated observations that vaccine-induced protection against FIV tends to be short-lived and may be difficult to restimulate. Clearly, novel approaches of immunization should be pursued that may provide a response to these important limitations of current immunization methods.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministero della Sanità—Istituto Superiore di Sanità (Progetto Allestimento Modelli Animali per l’AIDS) and the Ministero della Università Ricerca Tecnologica, Rome, Italy.
REFERENCES
- 1.Arthur L O, Bess J W, Jr, Sowder II R C, Benveniste R E, Mann D L, Chermann J-C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 2.Baldinotti F, Matteucci D, Mazzetti P, Giannelli C, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty A J, Willett B J, Gault E A, Jarrett O. A longitudinal study of feline immunodeficiency virus-specific cytotoxic T lymphocytes in experimentally infected cats, using antigen-specific induction. J Virol. 1996;70:6199–6206. doi: 10.1128/jvi.70.9.6199-6206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, Ceccherini-Nelli L, Malvaldi G, Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin Microbiol Rev. 1995;8:87–120. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop S A, Stokes C R, Gruffydd-Jones T J, Whiting C V, Humphries J E, Osborne R, Papanastasopoulou M, Harbour D A. Vaccination with fixed feline immunodeficiency virus (FIV) infected cells: protection, breakthrough and specificity of response. Vaccine. 1996;14:1243–1250. doi: 10.1016/s0264-410x(96)00023-0. [DOI] [PubMed] [Google Scholar]
- 6.Bolognesi D P. The dilemma of developing and testing AIDS vaccines. In: Cooper G M, Temin R G, Sugden B, editors. The DNA provirus: Howard Temin’s scientific legacy. Washington, D.C: American Society for Microbiology; 1995. pp. 301–312. [Google Scholar]
- 7.Cammarota G, Matteucci D, Pistello M, Nicoletti E, Giannecchini S, Bendinelli M. Reduced sensitivity to strain-specific neutralization of laboratory-adapted feline immunodeficiency virus after one passage in vivo: association with amino acid substitutions in the V4 region of the surface glycoprotein. AIDS Res Hum Retroviruses. 1996;12:173–175. doi: 10.1089/aid.1996.12.173. [DOI] [PubMed] [Google Scholar]
- 8.Capobianchi M R, Fais S, Castilletti C, Gentile M, Ameglio F, Cordiali Fei P, Santini S M, Dianzani F. A simple and reliable method to detect cell membrane proteins on infectious human immunodeficiency virus type 1 particles. J Infect Dis. 1994;169:886–889. doi: 10.1093/infdis/169.4.886. [DOI] [PubMed] [Google Scholar]
- 9.Chan W L, Rodgers A, Grief C, Almond N, Ellis S, Flanagan B, Silvera P, Bootman J, Stott J, Kent K, Bomford R. Immunization with class I human histocompatibility leukocyte antigen can protect macaques against challenge infection with SIVmac-32H. AIDS. 1995;9:223–228. [PubMed] [Google Scholar]
- 10.Chiarantini L, Argnani R, Zucchini S, Stevanata L, Zabordi P, Grossi M P, Magnani M, Manservigi R. Red blood cells as delivery system for recombinant HSV-1 glycoprotein B: immunogenicity and protection in mice. Vaccine. 1997;17:276–280. doi: 10.1016/s0264-410x(96)00181-8. [DOI] [PubMed] [Google Scholar]
- 11.Chiarantini L, Magnani M. Immobilization of enzymes and proteins on red blood cells. In: Bekerstaff G F, editor. Methods in molecular biotechnology. Vol. 1. Totowa, N.J: Humana Press; 1996. pp. 143–152. [Google Scholar]
- 12.Dzaudu J K, Deh M E, Barratt D L, Wise G E. Detection of erythrocyte membrane proteins, sialoglycoproteins, and lipids in the same polyacrylamide gel using a double-staining technique. Proc Natl Acad Sci USA. 1984;81:1733–1737. doi: 10.1073/pnas.81.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egberink H F, Keldermans L, Schuurman N, Stam J, Hesselink W, van Vliet A, Verschoor E, Horzinek M, de Ronde A. Monoclonal antibodies to immunodominant and neutralizing domains of the envelope surface protein of feline immunodeficiency virus. J Gen Virol. 1994;75:889–893. doi: 10.1099/0022-1317-75-4-889. [DOI] [PubMed] [Google Scholar]
- 14.Elder J H, Phillips T R. Feline immunodeficiency virus as a model for development of molecular approaches to intervention strategies against lentivirus infections. Adv Virus Res. 1995;45:225–247. doi: 10.1016/s0065-3527(08)60062-7. [DOI] [PubMed] [Google Scholar]
- 15.Giannecchini S, Matteucci D, Mazzetti P, Bendinelli M. Incubation time for feline immunodeficiency virus cultures. J Clin Microbiol. 1996;34:2036–2038. doi: 10.1128/jcm.34.8.2036-2038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilleman M R. Whether and when an AIDS vaccine? Nature Med. 1995;1:1126–1129. doi: 10.1038/nm1195-1126. [DOI] [PubMed] [Google Scholar]
- 17.Hosie M J, Dunsford T H, de Ronde A, Willett B J, Cannon C A, Neil J C, Jarrett O. Suppression of virus burden by immunization with feline immunodeficiency virus Env protein. Vaccine. 1996;14:405–411. doi: 10.1016/0264-410x(95)00193-5. [DOI] [PubMed] [Google Scholar]
- 18.Hosie M J, Flynn J N. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561–7568. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosie M J, Osborne R, Yamamoto J K, Neil J C, Jarrett O. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J Virol. 1995;69:1253–1255. doi: 10.1128/jvi.69.2.1253-1255.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosie M J, Yamamoto J K. Vaccination against FIV. In: Willett B, Jarrett O, editors. Feline immunology and immunodeficiency. Oxford, England: Oxford University Press; 1995. pp. 263–278. [Google Scholar]
- 21.Lombardi S, Garzelli C, La Rosa C, Zaccaro L, Specter S, Malvaldi G, Tozzini F, Esposito F, Bendinelli M. Identification of a linear neutralization site within the third variable region of the feline immunodeficiency virus envelope. J Virol. 1993;67:4742–4749. doi: 10.1128/jvi.67.8.4742-4749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombardi S, Garzelli C, Pistello M, Massi C, Matteucci D, Baldinotti F, Cammarota G, Da Prato L, Bandecchi P, Tozzini F, Bendinelli M. A neutralizing antibody-inducing peptide of the V3 domain of feline immunodeficiency virus envelope glycoprotein does not induce protective immunity. J Virol. 1994;68:8374–8379. doi: 10.1128/jvi.68.12.8374-8379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz H, Hofmann-Lehman R, Bauer-Pham K, Holznagel E, Tozzini F, Bendinelli M, Reubel G, Aubert A, Davis D, Cox B, Young E. FIV vaccine studies. I. Immune response to recombinant FIV env gene products and outcome after challenge infection. Vet Immunol Immunopathol. 1995;46:103–113. doi: 10.1016/0165-2427(94)07010-5. [DOI] [PubMed] [Google Scholar]
- 24.Magnani M, Chiarantini L, Mancini U. Preparation and characterization of biotinylated erythrocytes. Biotechnol Appl Biochem. 1994;20:335–345. doi: 10.1111/j.1470-8744.1994.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 25.Magnani M, Chiarantini L, Vittoria E, Mancini U, Rossi L, Fazi A. Red blood cells as an antigen-delivery system. Biotechnol Appl Biochem. 1992;16:188–194. [PubMed] [Google Scholar]
- 26.Massi C, Lombardi S, Indino E, Matteucci D, La Rosa C, Esposito F, Garzelli C, Bendinelli M. Most potential linear B-cell epitopes of Env glycoproteins of feline immunodeficiency virus are immunologically silent in mice. AIDS Res Hum Retroviruses. 1997;13:1121–1129. doi: 10.1089/aid.1997.13.1121. [DOI] [PubMed] [Google Scholar]
- 27.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Lonetti I, Zaccaro L, Pollera C, Specter S, Bendinelli M. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed-cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J Virol. 1997;71:8368–8376. doi: 10.1128/jvi.71.11.8368-8376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Zaccaro L, Bandecchi P, Tozzini F, Bendinelli M. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing antibodies. J Virol. 1996;70:617–622. doi: 10.1128/jvi.70.1.617-622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore J, Trkola A. HIV type 1 coreceptors, neutralization serotypes, and vaccine development. AIDS Res Hum Retroviruses. 1997;13:733–736. doi: 10.1089/aid.1997.13.733. [DOI] [PubMed] [Google Scholar]
- 30.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9:S117–S136. [PubMed] [Google Scholar]
- 31.Pedersen N C. Feline immunodeficiency virus infection. In: Levy J A, editor. The retroviruses. Vol. 2. New York, N.Y: Plenum Press; 1993. pp. 191–228. [Google Scholar]
- 32.Pistello M, Menzo S, Giorgi M, Da Prato L, Cammarota G, Clementi M, Bendinelli M. Competitive polymerase chain reaction for quantitating feline immunodeficiency virus load in infected cat tissues. Mol Cell Probes. 1994;8:229–234. doi: 10.1006/mcpr.1994.1032. [DOI] [PubMed] [Google Scholar]
- 33.Poignard P, Klasse P J, Sattentau Q J. Antibody neutralization of HIV-1. Immunol Today. 1996;17:239–246. doi: 10.1016/0167-5699(96)10007-4. [DOI] [PubMed] [Google Scholar]
- 34.Richardson J, Fossati I, Moraillon A, Castelot S, Sonigo P, Pancino G. Neutralization sensitivity and accessibility of continuous B cell epitopes of the feline immunodeficiency virus envelope. J Gen Virol. 1996;77:759–777. doi: 10.1099/0022-1317-77-4-759. [DOI] [PubMed] [Google Scholar]
- 35.Rigby M A, Mackay N, Reid G, Osborne R, Neil J C, Jarrett O. Immunogenicity of a peptide from a major neutralising determinant of the feline immunodeficiency virus surface glycoprotein. Vaccine. 1996;14:1095–1102. doi: 10.1016/0264-410x(96)00060-6. [DOI] [PubMed] [Google Scholar]
- 36.Siebelink K H J, Huisman W, Karlas J A, Rimmelzwaan G F, Bosch M L, Osterhaus A D M E. Neutralization of feline immunodeficiency virus by polyclonal feline antibody: simultaneous involvement of hypervariable regions 4 and 5 of the surface glycoprotein. J Virol. 1995;69:5124–5127. doi: 10.1128/jvi.69.8.5124-5127.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siebelink K H J, Tijhaar E, Huisman R C, Huisman W, de Ronde A, Darby I H, Francis M J, Rimmelzwaan G F, Osterhaus A D M E. Enhancement of feline immunodeficiency virus infection after immunization with envelope glycoprotein subunit vaccines. J Virol. 1995;69:3704–3711. doi: 10.1128/jvi.69.6.3704-3711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stott E J. Anti-cell antibody in macaques. Nature. 1991;353:393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- 39.Verschoor E J, Willemse M J, Stam J G, van Vliet A L W, Pouwels H, Chalmers S K, Horzinek M C, Sondermeijer P J A, Hesselink W, de Ronde A. Evaluation of subunit vaccines against feline immunodeficiency virus infection. Vaccine. 1996;14:285–289. doi: 10.1016/0264-410x(95)00205-f. [DOI] [PubMed] [Google Scholar]
- 40.Willett B J, Flynn J N, Hosie M J. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto J K, Hohdatsu T, Holmsted R A, Pu R, Louie H, Zochlinski H A, Acevedo V, Johnson H M, Soulds G A, Gardner M B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J Virol. 1993;67:601–605. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]