Abstract
Female mice treated with dehydroepiandrosterone sulfate early during infection were partially protected (P < 0.05–0.005) from Schistosoma mansoni infection. Hormone treatment did not modify parasite-specific cellular or humoral responses. Serum dehydroepiandrosterone sulfate levels and testosterone infection were negatively correlated, r = −0.621 and r = −0.653, respectively, with schistosome worm burden. The partial resistance to schistosome infection in dehydroepiandrosterone sulfate-treated female mice may be due to the known antischistosomular activity of testosterone.
Schistosomiasis is a parasitic helminth disease that infects >250 million people worldwide. Epidemiologic studies in different geographic areas of the world have consistently demonstrated that the age-related patterns of schistosome infection found in human populations are remarkably similar. In infected human populations, there is a peak of schistosome infection in children, followed by a dramatic decline at a time that is coincident with puberty. Many physiological changes that occur during puberty are hormonally controlled. Thus, hormones that are elevated during puberty could indirectly modulate responses that influence schistosome infection, such as skin thickness, or directly regulate schistosome infection by, for example, elevating protective immune responses. The adrenal steroid hormone dehydroepiandrosterone (DHEA) is implicated in age-related changes in the immune system and susceptibility to certain diseases (3, 12). In the circulation, the majority of DHEA is in the sulfate form, DHEA sulfate (DHEAS), and is converted to DHEA by DHEA sulfatase (9). DHEA is the most abundant hormone in the circulation of males and females, and levels of DHEA(S) in plasma are age related, with the highest levels being present by 20 to 30 years of age, followed by a progressive decline with age (8–10). In animal models, DHEA treatment has been shown to protect against viral (2, 5) and parasitic protozoan (11) infections. The concept that DHEA(S) may influence a parasitic helminth infection has not been addressed. In this study, we have evaluated whether DHEAS has a regulatory role in murine Schistosoma mansoni infection.
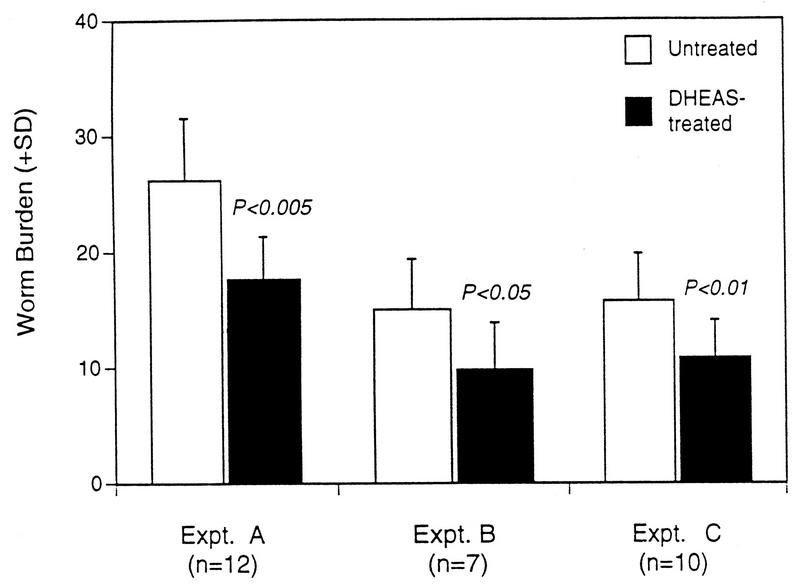
Female mice were placed on DHEAS-supplemented water 14 days prior to challenge with S. mansoni and maintained on DHEAS throughout infection until perfusion (day 47 to 49 after infection). DHEAS treatment was based on original protocols described by Araneo et al. (1). In three separate experiments (A, B, and C), DHEAS-treated mice had significantly lower worm recoveries (P < 0.01 to 0.005) than did control mice (Fig. 1). DHEAS treatment did not modify worm morphology, male/female sex ratio, or the fecundity of the parasites. To determine when DHEAS treatment mediates partial protection against schistosome infection, mice were placed on DHEAS for different periods of time prior to and during infection. DHEAS treatment prior to infection and for the first 2 weeks of infection caused a significantly (P < 0.05) reduced worm burden; in contrast, when DHEAS was administered from the second week of infection until termination no effect on worm burden was observed (data not shown). This data indicates that the effects of DHEAS treatment that partially protect female mice from schistosome infection occur in response to the early migratory larval stage of schistosome infection and that DHEAS is not active against adult worms.
FIG. 1.
S. mansoni worm recovery in three separate experiments in DHEAS-treated and untreated mice. Student’s t test was used to determine statistical differences between hormone-treated and corresponding untreated groups. A total of 7 to 12 mice were used in each experimental group. SD, standard deviation.
Despite DHEAS treatment of female mice repeatedly causing a 30 to 35% lower level of schistosome infection, no immunological parameter examined was distinctly different in DHEAS-treated groups compared to untreated infected mice. There were marginally lower immune responses to parasite antigens, both cellular (interleukin-2 [IL-2], gamma interferon, IL-4, and IL-5) and humoral (immunoglobulin G1 and immunoglobulin G2a), in DHEAS-treated mice (data not shown). The generalized diminished immune responsiveness to parasite antigens in hormone-treated mice compared to untreated infected mice was probably a reflection of the reduced worm burden in these mice and, therefore, the consequential lower in vivo stimulation with worm or egg antigens.
The levels of DHEAS in the serum of mice were assayed by DHEA-S test kits (Genzyme Diagnostics, Cambridge, Mass.). Infected female mice that had been maintained on DHEAS for 9 weeks had increased concentrations of DHEAS (0.295 ± 0.187 μg/ml) in their serum compared to infected mice not treated with hormone (0.104 ± 0.066 μg/ml). Schistosome infection did not alter DHEAS levels (data not shown). The levels of DHEAS in the serum of hormone-treated mice on the day prior to infection had a significant inverse relationship with worm burden (Fig. 2a). Serum DHEAS levels in untreated mice prior to infection were also inversely correlated with worm burden (r = −0.226), but this correlation was not significant. In humans, DHEAS treatment of females is thought to lead to increases in circulating androgenic hormones, in particular testosterone (3). As testosterone is a potential metabolic by-product of DHEAS, we measured serum testosterone in DHEAS-treated mice. Testosterone was assayed by radioimmunoassay with Coat-a-Count Total Testosterone kits (DPC, Los Angeles, Calif.). Female CBA/Ca mice had levels of serum testosterone that were at the lower limit of detection of the testosterone assay used (0.435 ± 0.171 ng/ml). However, female mice that had been treated with DHEAS for 9 weeks had an approximately 13-fold increase in the levels of testosterone in serum (5.683 ± 2.018 ng/ml). DHEAS treatment of female mice clearly elevates testosterone levels, with a positive correlation (r = 0.482) between serum DHEAS concentrations and testosterone levels. Similar to the association between DHEAS and worm burden, the levels of testosterone in serum on the day prior to infection were significantly negatively correlated with worm burden (Fig. 2b). The elevated levels of serum testosterone that we observed in DHEAS-treated female mice are probably the result of the conversion of excess DHEAS to testosterone.
FIG. 2.
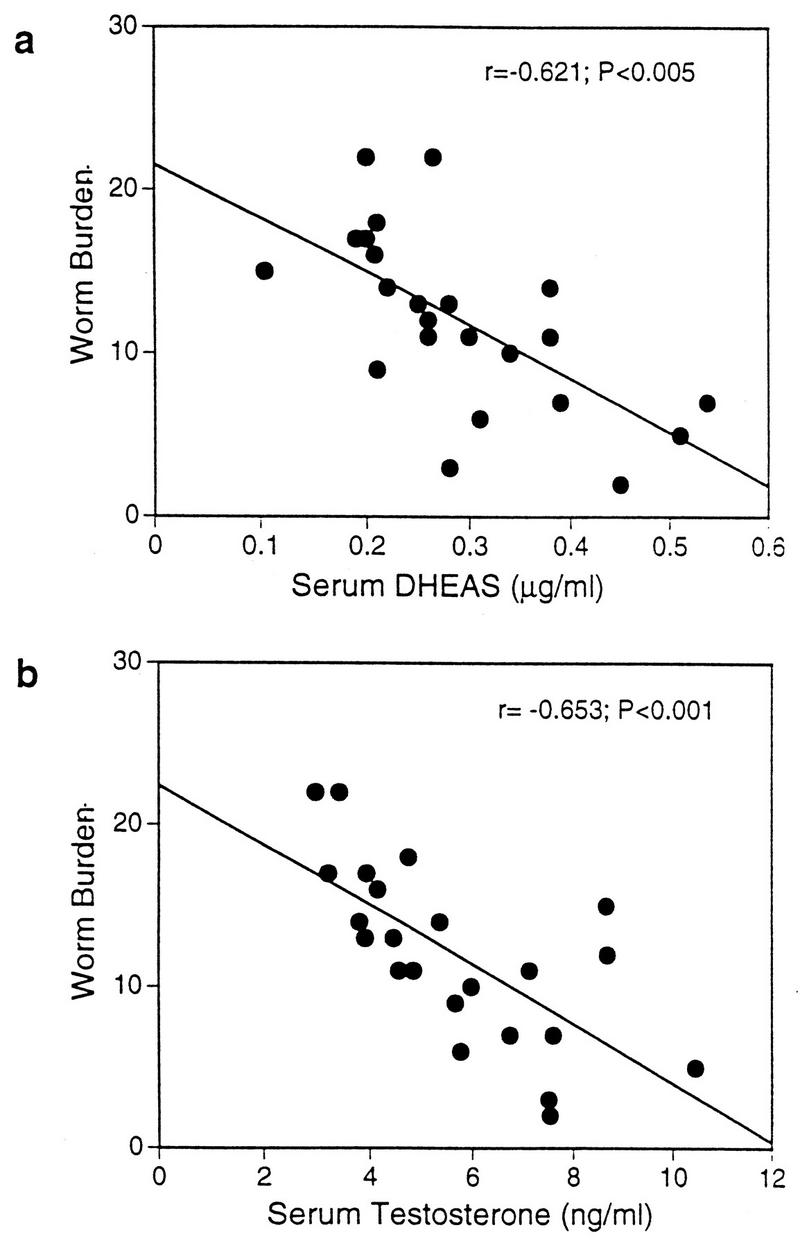
Correlation between the levels in serum of DHEAS (a) or testosterone (b) and worm burden in S. mansoni-infected mice that were treated with DHEAS. Serum was collected on the day prior to infection. Results are from 23 individual mice. DHEAS correlations are expressed as Pearson’s correlation coefficients, whereas nonparametric Spearman’s rank correlations were used for testosterone.
Among mice, females are more susceptible to S. mansoni infection than are males (4). The naturally lower susceptibility of male mice to schistosome infection has been attributed to the presence of more testosterone in male than in female mice (7). Experiments to demonstrate the role of testosterone in susceptibility to schistosome infection have shown that administration of testosterone to female mice early during infection renders females less susceptible to infection and that, conversely, castrated male mice are more susceptible to infection (7). Similarly, DHEAS treatment was effective only against larval stages and had no effect against older worms. The significant negative correlation between serum testosterone and worm burden described in this study has been shown previously (7). The partial resistance of DHEAS-treated female mice to schistosome infection observed in this study may have been mediated by testosterone. In fact, a role for testosterone in human schistosomiasis has been previously reported: Skelly and others (13) described a possible negative correlation between parasite load (fecal egg counts) and testosterone levels in Brazilian male schistosomiasis patients. The mechanism whereby testosterone may influence schistosome infection is not known. The isolation of an adrenal hormone receptor from a schistosome cDNA library may indicate that testosterone may directly impair schistosomular development (7). It is also relevant that DHEA modulates T-cell function by a specific DHEA receptor on T cells (6), and a similar receptor may be present also in schistosomes.
In summary, DHEAS treatment of female mice during early schistosome infection causes partial (30 to 35%) resistance to S. mansoni. The reduced worm burden was not attributable to hormone treatment directly modifying cellular or humoral responses to the parasite. Both the concentration of DHEAS and that of testosterone in the serum of hormone-treated mice negatively correlated with worm burden. Testosterone may be mediating resistance of mice to schistosome infection by a mechanism that is not currently elucidated. We have commenced human studies on Kenyan populations in which schistosome infection is endemic to elucidate the relationship between DHEAS and other hormones, schistosome infection, and antiparasite immunity. Such studies may more accurately address the roles of hormones in schistosome infection.
Acknowledgments
This work was supported by the Medical Research Council (United Kingdom) and The Wellcome Trust (United Kingdom).
REFERENCES
- 1.Araneo B A, Woods M L, Daynes R A. Reversal of immunosenescent phenotype by dehydroepiandrosterone: hormone treatment provides an adjuvant effect on the immunization of aged mice with recombinant hepatitis B surface antigen. J Infect Dis. 1993;167:830–840. doi: 10.1093/infdis/167.4.830. [DOI] [PubMed] [Google Scholar]
- 2.Daneberg H D, Ben-Yehuda A, Zakay-Rones Z, Friedman G. Dehydroepiandrosterone (DHEA) treatment reverses the impaired immune response of old mice to influenza vaccination and protects from influenza infection. Vaccine. 1995;13:1445–1448. doi: 10.1016/0264-410x(95)00063-7. [DOI] [PubMed] [Google Scholar]
- 3.Ebeling P, Koivisto V A. Physiological importance of dehydroepiandrosterone. Lancet. 1994;343:1479–1481. doi: 10.1016/s0140-6736(94)92587-9. [DOI] [PubMed] [Google Scholar]
- 4.Eloi-Sanots S, Olson N J, Correa-Oliveira R, Colley D G. Schistosoma mansoni: mortality, pathophysiology and susceptibility differences in male and female mice. Exp Parasitol. 1992;75:168–175. doi: 10.1016/0014-4894(92)90176-b. [DOI] [PubMed] [Google Scholar]
- 5.Loria R M, Inge T H, Cook S S, Szakal A K, Regelson W. Protection against acute lethal viral infections with the native steroid dehydroepiandrosterone (DHEA) J Med Virol. 1988;26:301–314. doi: 10.1002/jmv.1890260310. [DOI] [PubMed] [Google Scholar]
- 6.Miekle A, Dorchuck R, Araneo B, Strinham J, Evans T, Spruance S, Daynes R A. Presence of a DHEA-specific receptor binding complex in murine T cells. J Steroid Biochem Mol Biol. 1992;42:293–304. doi: 10.1016/0960-0760(92)90132-3. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa M, Fantappie M R, Freeman G L, Eloi-Sanots S, Olson N J, Kovacs W J, Secor W E, Colley D G. Schistosoma mansoni: susceptibility differences between male and female mice can be mediated by testosterone during early infection. Exp Parasitol. 1997;85:233–240. doi: 10.1006/expr.1997.4148. [DOI] [PubMed] [Google Scholar]
- 8.Orentreich N, Brind J L, Rizer R L, Vogelman J H. Age changes and sex differences in serum dehydroepiandrosterone and sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1983;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 9.Parker L N. Control of DHEA secretion. Semin Reprod Endocrinol. 1995;13:275–281. [Google Scholar]
- 10.Phillips G B. Relationship between serum dehydroepiandrosterone sulfate, androsterone, and sex hormones in men and women. Eur J Endocrinol. 1996;134:201–206. doi: 10.1530/eje.0.1340201. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen K R, Healey M C, Cheng L, Yang S. Effects of dehydroepiandrosterone in immunosuppressed adult mice infected with Cryptosporidium parvum. J Parasitol. 1995;81:429–433. [PubMed] [Google Scholar]
- 12.Shealy C N. A review of dehydroepiandrosterone (DHEA) Integr Physiol Behav Sci. 1995;30:308–313. doi: 10.1007/BF02691603. [DOI] [PubMed] [Google Scholar]
- 13.Skelly P J, Secor W E, Reis M G, Ramos E A, Carmo T M, Peixoto E E, Harn D A. Failure of schistosomiasis to significantly decrease testosterone levels in Brazilian men. Am J Trop Med Hyg. 1994;51:40–44. doi: 10.4269/ajtmh.1994.51.40. [DOI] [PubMed] [Google Scholar]