Abstract
trans-Sialidase inhibition assay (TIA) was employed in a population at high risk of Trypanosoma cruzi infection. From 20 serum samples that were negative by conventional serologic and parasitologic assays, 18 (90%) were reactive in TIA, providing further evidence of the higher sensitivity of TIA and suggesting that the actual prevalence of T. cruzi infection might be underestimated.
The American trypanosomiasis, Chagas’ disease, is a chronic illness that affects about 16 million people in the Americas. After the acute phase of the disease, parasites are rarely found in the blood and diagnosis is mainly based on serology. Conventional serology employs crude preparations of parasites, although some relevant antigens have been cloned and tested as diagnostic tools (9). Serology seems to be equally sensitive to or even more sensitive than PCR for detecting infection in patients (2, 13). A new serological test, trans-sialidase inhibition assay (TIA), is under development (6–8). The assay is based on the detection of antibodies able to inhibit the activity of the trans-sialidase, a virulence factor from Trypanosoma cruzi (12). The enzyme is absent in other protozoan parasites, such as Trypanosoma rangeli, Leishmania spp., and Plasmodium spp., that are frequently found in geographical regions where T. cruzi is present. These neutralizing antibodies are detected during both the acute and chronic phases of the human infection (7, 8, 10).
Epidemiological surveys of the population at risk of infection are performed through conventional serology. Very often, when people living in the same house are tested, only some of them are recorded as infected even though all were exposed to similar risk factors. Since TIA seems to be a more sensitive technique (7, 8), it was employed in a population at very high risk of infection but which was serologically negative when tested by conventional T. cruzi assays.
TIA employs a recombinant trans-sialidase (3, 4) that is preincubated with the serum to be tested, and then the remnant ability to transfer the sialyl residue from sialyllactose to [14C]lactose is evaluated. At present we have tested about 60 normal human sera obtained from the regions of endemicity; they show variable degrees of trans-sialidase inhibition, ranging from −10 to 30%, as reported previously (7, 8). To perform TIA with experimental samples, normal human pooled serum was employed as a negative control and the value of inhibition obtained was taken as 0% (6–8).
Blood samples were obtained from the Sanapa and Angaite Amerindian communities in western Paraguay (150 samples). The seroprevalence of T. cruzi infection among this group was 82%. The people of this group live in houses built with palm leaves, where large numbers of infected vector bugs (70 to 300 triatomines per house) are present. However, some people in the community are consistently negative by the normally employed enzyme-linked immunosorbent and immunofluorescence tests, and also by parasitological tests (xenodiagnosis and hemoculture). Sera from 20 such serologically negative persons (20 to 40 years old) that had been living in the same place for the last 14 to 30 years were further analyzed by TIA and by a dot spot assay employing recombinant T. cruzi antigens that are able to detect chronic (antigens 1, 2, and 30 [5, 9]) or acute (SAPA antigen [1, 8, 9, 11]) cases of human Chagas’ disease.
The dot spot assay confirmed the negative results obtained by conventional serology in 16 of the 20 samples. These 16 individuals would be considered not infected with T. cruzi by present criteria. Sera from the 20 individuals were then analyzed by TIA. As shown in Fig. 1, 18 of the 20 sera were TIA positive, 15 of them having values ranging from 68 to 98%. The serum components were fractionated by protein A-agarose (Life Technologies, Gaithersburg, Md.), and the inhibitory activity was found in the immunoglobulin fraction (not shown).
FIG. 1.
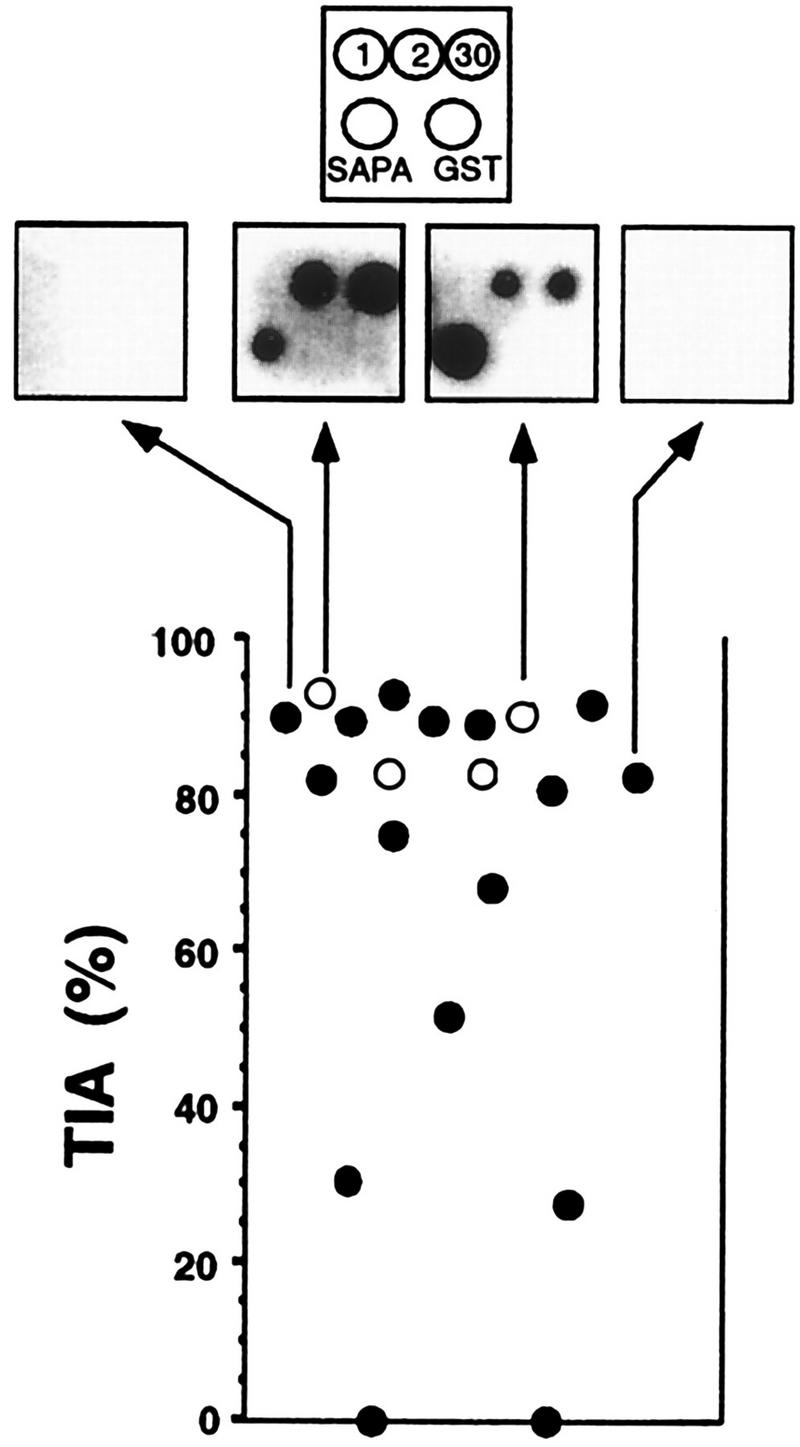
TIA of sera from Paraguayan aborigines. Assayed serum samples were from persons negative for T. cruzi infection by conventional serological and parasitological tests. The value of 0% was assigned to the inhibition obtained with pooled serum samples from areas of nonendemicity. The positions of the dot spots of recombinant antigens 1, 2, 30, and SAPA and of the glutathione S-transferase (GST) from Schistosoma japonicum, expressed by the pGEX vector without insert (Pharmacia, Uppsala, Sweden) and used as a negative control, are indicated in the top box. Open circles denote the samples giving positive dot spot reactions. The results from two positive and two negative sera in the dot spot assay are also shown.
The TIA results found were similar to those observed with sera obtained from chagasic patients (7, 8). We have shown that these antibodies remain for long periods of time (14 years) in acute-stage patients successfully treated with benznidazole even after T. cruzi infection is no longer detected by conventional serologic and parasitological tests (8). TIA results for the Amerindians are compatible with those findings and suggest that the subjects were in contact with the parasite, which might support the possibility of spontaneous cure or the establishment of a cryptic infection. In fact, the subjects tested meet the present criteria for absence of T. cruzi infection by either conventional serology or parasitological tests. However, they should be rejected as blood or organ donors and should be monitored for the appearance of chronic Chagas’ disease pathologies. The present study also suggests that the actual seroprevalence of T. cruzi infection might be underestimated.
Acknowledgments
This study was supported by grants from the World Bank/UNDP/WHO Special Program for Research and Training in Tropical Diseases (TDR), the Swedish Agency for Research Cooperation with Developing Countries (SAREC), the Universidad de Buenos Aires, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and the Argentina and the Japan International Cooperation Agency (project on Chagas’ and other infectious diseases). The research of A. C. C. Frasch was supported in part by an International Research Scholars Grant from the Howard Hughes Medical Institute and the International Atomic Energy Agency, Vienna, Austria.
REFERENCES
- 1.Affranchino J L, Ibañez C F, Luquetti A O, Rassi A, Reyes M B, Macina R A, Åslund L, Pettersson U, Frasch A C C. Identification of a Trypanosoma cruzi antigen that is shed in the acute phase of Chagas disease. Mol Biochem Parasitol. 1989;34:221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- 2.Avila H A, Pereira J B, Thieman O, De Paiva E, De Grave W, Morel C M, Simpson L. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J Clin Microbiol. 1993;31:2421–2426. doi: 10.1128/jcm.31.9.2421-2426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buschiazzo A, Frasch A C C, Campetella O. Medium scale production and purification to homogeneity of a recombinant trans-sialidase from Trypanosoma cruzi. Cell Mol Biol. 1996;42:703–710. [PubMed] [Google Scholar]
- 4.Campetella O, Uttaro A, Parodi A J, Frasch A C C. A recombinant Trypanosoma cruzi trans-sialidase lacking the amino acid repeats retains the enzymatic activity. Mol Biochem Parasitol. 1994;64:337–340. doi: 10.1016/0166-6851(94)00036-0. [DOI] [PubMed] [Google Scholar]
- 5.Ibáñez C F, Affranchino J L, Macina R A, Reyes M B, Leguizamón S, Camargo M E, Åslund L, Pettersson U, Frasch A C C. Multiple Trypanosoma cruzi antigens containing tandemly repeated amino acid sequence motifs. Mol Biochem Parasitol. 1988;30:27–34. doi: 10.1016/0166-6851(88)90129-6. [DOI] [PubMed] [Google Scholar]
- 6.Leguizamón M S, Campetella O, González-Cappa S M, Frasch A C C. Mice infected with Trypanosoma cruzi produce antibodies against the enzymatic domain of trans-sialidase that inhibit its activity. Infect Immun. 1994;62:3441–3446. doi: 10.1128/iai.62.8.3441-3446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leguizamón M S, Campetella O, Russomando G, Almirón M, Guillén I, González-Cappa S M, Frasch A C C. Antibodies inhibiting Trypanosoma cruzi trans-sialidase activity in sera from human infections. J Infect Dis. 1994;170:1570–1574. doi: 10.1093/infdis/170.6.1570. [DOI] [PubMed] [Google Scholar]
- 8.Leguizamón M S, Russomando G, Luquetti A, Rassi A, Almirón M, González-Cappa S M, Frasch A C C, Campetella O. Long-lasting antibodies detected by a trans-sialidase inhibition assay of sera from parasite-free, serologically cured chagasic patients. J Infect Dis. 1997;175:1272–1275. doi: 10.1086/593697. [DOI] [PubMed] [Google Scholar]
- 9.Pastini A C, Iglesias S I, Carricarte V C, Guerin M E, Sánchez D O, Frasch A C C. Immunoassay with recombinant Trypanosoma cruzi antigens potentially useful for screening donated blood and diagnosing Chagas disease. Clin Chem. 1994;40:1893–1897. [PubMed] [Google Scholar]
- 10.Pereira-Chioccola V L, Schenkman S, Kloetzel J K. Sera from chronic Chagasic patients and rodents infected with Trypanosoma cruzi inhibit trans-sialidase by recognizing its amino-terminal and catalytic domain. Infect Immun. 1994;62:2973–2978. doi: 10.1128/iai.62.7.2973-2978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes M B, Lorca M, Muñoz P, Frasch A C C. Fetal IgG specificities against Trypanosoma cruzi antigens in infected newborns. Proc Natl Acad Sci USA. 1990;87:2846–2850. doi: 10.1073/pnas.87.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schenkman S, Eichinger D, Pereira M E A, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 13.Wincker P, Bosseno M F, Britto C, Yaksic N, Cardoso M A, Morel C M, Breniere S F. High correlation between Chagas’ disease serology and PCR-based detection of Trypanosoma cruzi kinetoplast DNA in Bolivian children living in an endemic area. FEMS Microbiol Lett. 1994;124:419–423. doi: 10.1111/j.1574-6968.1994.tb07318.x. [DOI] [PubMed] [Google Scholar]


