Graphical abstract
Keywords: Cassia seed, Chrysophanol, Aurantio-obtusin, Ultrasonic bidirectional regulation, Nanofiltration
Abstract
Anthraquinone components sublimate during heat treatment refining, causing equipment pipeline contamination and a drop in component output, which is a challenging technical problem for pharmaceutical manufacturers to resolve. Furthermore, the waste liquid generated during pipeline cleaning simultaneously increases production costs and pollutes the environment. Although nanofiltration has the technological advantage of traditional temperature refining, anthraquinone components are absorbed onto the membrane surface during the concentration of cassia seed ethanol extract, leading to membrane pollution and a significant decrease in separation efficiency. Based on the π-π stacking effect and ultrasonic cavitation effect of anthraquinone components, this study proposes the hypothesis of enhancing nanofiltration separation by ultrasonic regulation of the self-assembly of anthraquinone components. The effects of pH, ethanol concentration, ultrasonic power, and the molecular weight cut-off of nanofiltration membranes on the solute rejection and membrane flux were all systematically explored in this work. The separation processes of chrysophanol and aurantio-obtusin were clarified by combining the relationship between ultrasonic power and the existing state. It was discovered that the self-assembly behavior of chrysophanol and aurantio-obtusin was regulated bidirectionally by ultrasonic power. In the range of 100 W − 300 W, the proportion of molecular states of anthraquinone components drops as the particle size distribution of the solution increases. Ultrasound encouraged the π-π stacking effect among anthraquinones, resulting in self-assembly and reduced surface pollution under the cavitation effect, leading to efficient nanofiltration separation. Ultrasonic power showed a logarithmic correlation with the molecular proportion of anthraquinones components in 300 W − 700 W, and ultrasound promoted the breakage of hydrogen bonds between supramolecular structures, resulting in an increase in the molecular proportion and a decrease in solute rejection. The response surface method was used to optimize the separation parameters of ultrasonic-enhanced nanofiltration. Chrysophanol and aurantio-obtusin rejections in cassia seed extract with ethanol concentrations of 35 % − 65 % were both greater than 88 % and 91 %, respectively, as the separation volume increased from 2 L to 20 L. Based on the intermolecular forces of the anthraquinone components in various ethanol solutions, this study used an ultrasonic bidirectional self-assembly ratio to purify cassia seed extract at room temperature through ultrasonic-enhanced nanofiltration, thereby avoiding the problems of component sublimation and environmental contamination brought on by conventional concentration.
1. Introduction
Anthraquinones have sublimation properties, are found in the metabolites of polygonaceae, legumes, rubiaceae, lichens and fungi, and have therapeutic activity such as hemostasis, bactericidal, and diarrhea [[1], [2], [3]]. Anthraquinones are frequently extracted from food or medicinal raw materials using ethanol extraction and concentration under reduced pressure [4]. Heat treatment, however, causes some anthraquinone components to sublimate and adsorb in the concentration equipment pipeline and dissolve in the recovery solvent during the vacuum concentration process. This leads to contamination of the production equipment and recovery solvent, making it difficult to recycle the recovered solvent and raising production costs simultaneously. Additionally, it causes environmental pollution, which makes it challenging to solve the pharmaceutical industry's problems.
Cassia seeds are dried and mature seeds of Senna obtusifolia or Senna tora that contain a variety of active components such as anthraquinones, cassia gum, fatty acids, carbohydrates, proteins, and more. Among them, chrysophanol and aurantio-obtusin, as quality control components, are anthraquinone compounds with the properties of decreasing blood pressure, lowering blood lipids, and protecting the liver [5]. As a result, cassia seed is widely used in the food industry as a homology material. Nanofiltration technology's room-temperature separation advantage has the potential to effectively separate solvents and solutes while simultaneously dealing with component loss and environmental contamination brought on from the sublimation of anthraquinone components as a result of heat treatment [[6], [7], [8]]. Strong van der Waals interactions between molecules are produced by the structure of anthraquinone, which consists of anthracene and quinone groups as well as a highly conjugated electron cloud sharing system [9,10]. Anthraquinone molecules are more likely to aggregate, adsorb, or crystallize as concentration rises [11]. This can easily lead to concentration polarization, adsorption, and membrane fouling during the nanofiltration separation process, which lowers the membrane flux of the nanofiltration membrane and reduces separation efficiency, thus limiting the use of nanofiltration technology [[12], [13], [14]].
Ultrasonic waves are sound waves with frequencies greater than 20 kHz that can cause strong vibration and cavitation effects in the liquid, destroying intermolecular forces and allowing the solute to diffuse from the high concentration region to the low concentration region [[15], [16], [17]]. According to earlier research, the asymmetric collapse of ultrasonic bubbles produces high-speed microjets that support the structure of ordered nanocrystals and lead to the development of uniformly shaped nanomaterials [[18], [19], [20]]. At the same time, ultrasound can cause convection and turbulence in the liquid, speeding the mass transfer process. Under ultrasonic irradiation, ultrasonic power can decrease the degree of self-packing, break the π-π packing between molecules, and speed up the movement of anthraquinone components [[21], [22], [23]]. This might theoretically contribute to improved membrane fouling. But it can also raise the transmittance of the component, which can lead to losses. Consequently, it is yet unclear how ultrasonic waves regulate the assembly of anthraquinone nanocrystals. It restricts how ultrasound-enhanced nanofiltration can be used to separate cassia seed extract. In this study, ultrasonic power, nanofiltration membrane molecular weight cut off (MWCO), solute concentration, and pH were chosen as variables to investigate the separation process of ultrasonic-enhanced nanofiltration, while chrysophanol and aurantio-obtusin rejections, and nanofiltration membrane flux were selected as response values. The response surface method was used to optimize the separation parameters of ultrasonic-enhanced nanofiltration concentrated cassia seed ethanol extract. The relationship between ultrasonic power and mass transfer coefficient of chrysophanol and aurantio-obtusin in nanofiltration was studied in order to clarify the mechanism of ultrasonic-enhanced nanofiltration separation. The sublimation problem brought on by heating and concentration of anthraquinone components, which resulted in low recovery rates of production equipment pipelines and components, has been resolved by the ultrasonic-enhanced nanofiltration separation technology developed in this study. The self-assembly ratio was dynamically modified to guarantee the nanofiltration separation efficiency while raising the membrane flux and decreasing membrane fouling, based on the relationship between ultrasonic power and the π-π stacking effect. A novel study strategy for the regulation of molecular self-assembly is presented through the investigation of this paper.
2. Materials and methods
2.1. Materials
Cassia seeds were purchased from Suzhou Chunhuitang Pharmaceutical Co., LTD., crushed into coarse powder using a JC-FW-100A crusher (Qingdao, China), and extracted twice with 80 % ethanol, each for 1.0 h. The extraction solution was refrigerated at 0–5 ℃ for 12 h, and the contents of chrysophanol and aurantio-obtusin in the filtrate were measured by high performance liquid chromatography (HPLC). The parameters of the ethanol extract of cassia seed were adjusted according to the requirements of the single factor experiment and response surface.
The reference substances, which included aurantio-obtusin (purity 98.3 %) chrysophanol (purity 99.2 %) and 1, 8-dihydroxyanthraquinone (purity 98.0 %) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Methanol and acetonitrile are chromatographically grade, while ethanol, chloroform, sodium hydroxide, ammonium hydroxide, hydrochloric acid, and sulfuric acid are analytically grade. Ultrapure water was produced by the Millipore Direct-Q5 water filtration system.
2.2. Ultrasonic enhanced nanofiltration separation
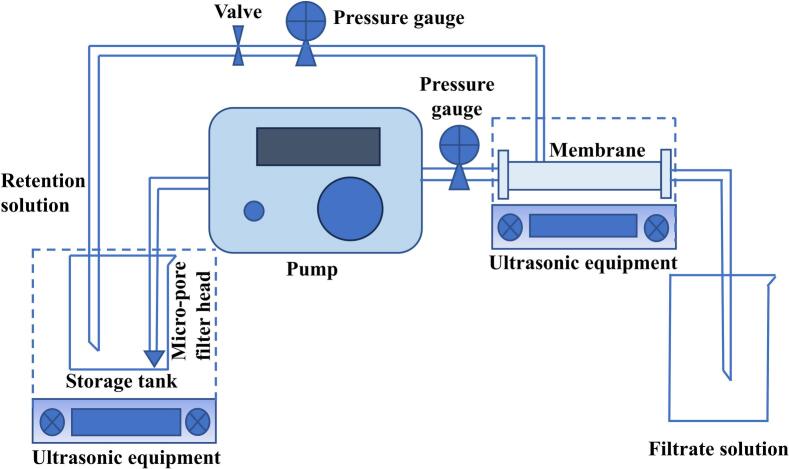
A storage tank is filled with the cassia seed extract. The solution flows through pipes that sequentially feed it through a 0.45 μm micro-pore filter head, a medium-pressure pump, a pressure gauge, a nanofiltration membrane, and a throttle valve. The filtrate tank receives the nanofiltration solution, while the storage tank receives the rejection solution. The ultrasonic-enhanced nanofiltration separation system is shown in Fig. 1. The separation parameters of nanofiltration membrane are shown in Table 1. To reduce the concentration polarization effect during the separation process, when the medium-pressure pump reaches 80 % of its maximum speed, the transmembrane pressure is adjusted through the throttle valve to enhance the effect of tangential filtration on the concentration polarization effect. The transmembrane pressure (TMP) is calculated based on the pressure difference indicated by the pressure gauges before and after the nanofiltration membrane. During the separation process, the storage tank was placed in the SCQ-9200E ultrasonic equipment, which consists of an ultrasonic transducer and an ultrasonic generator, with an ultrasonic frequency of 40 kHz. Among them, the nanofiltration membrane flux was calculated based on the volume of the solution collected at the filtrate end per unit time. The effectiveness of the ultrasound-enhanced nanofiltration separation of the anthraquinone components of cassia seed was assessed by calculating the solute rejection using Eq. (1).
| (1) |
Where C0 and C1 are the component concentrations in cassia seed extract and filtrate.
Fig. 1.
Instrumentation diagram of ultrasonic enhanced nanofiltration separation..
Table 1.
Nanofiltration membranes parameters.
| MWCO | Mode | Brand | Material, specific area |
|---|---|---|---|
| 100 Da | NFX-1812 | Synder filtration (USA) | Polyamide, 0.4 m2 |
| 450 Da | NFW-1812 | Synder filtration (USA) | Polyamide, 0.4 m2 |
| 800 Da | NFG-1812 | Synder filtration (USA) | Polyamide, 0.4 m2 |
For the purpose of comparing the differences between conventional and ultrasound-enhanced nanofiltration separation for the ethanol extract of cassia seed, the conventional separation procedure was performed using the procedure of assembly shown in Fig. 1. During the separation process, the ultrasonic equipment was turned off, and the transmembrane pressure was controlled by a medium-pressure pump and a throttle valve for separation.
2.3. Vacuum concentration of cassia seed extract
Vacuum concentration is a common solvent removal procedure in the production of pharmaceutical and food preparation raw components. Although it offers the technological advantages of high efficiency and energy savings, it is impossible to ignore the impact on heat-sensitive components. Under reduced pressure, 3.0 L of cassia seed extract was concentrated for 30 min using an R210 rotary evaporation equipment. The effect of concentration temperature under reduced pressure of 50 ℃ − 90 ℃ on the transfer rate of anthraquinone components was explored at −0.10 MPa. Simultaneous studies of the effects of vacuum concentration on the transfer rates of chrysophanol and aurantio-obtusin were carried out at 90 °C for 5, 10, 20, 30, and 60 min. Analysis was done on the sublimation law of the anthraquinone components of cassia seed. Eq. (2) was used to calculate the transfer rate after the anthraquinones' concentrations in the extract and concentrated solution were established.
| (2) |
Where C0 and C2 are the component concentrations in cassia seed extract and concentrated solution, and V0 and V2 are the volume of cassia seed extract and concentrated solution.
2.4. Single factor test
Anthraquinones exhibit π-π stacking and a variety of molecular states in extraction solutions due to their π-π conjugated system, planar structure, and strong van der Waals interaction between molecules [[24], [25], [26]]. It is unidentified how ultrasonic power, solute concentration, and pH affect π-π stacking in ethanol solution, despite the fact that nanofiltration concentration can improve π-π stacking.
The pH of the solution was adjusted to 4.00, 5.00, 6.00, 7.00, and 8.00 based on the pKa of chrysophanol (6.63) and aurantio-obtusin (6.32). The ultrasonic power is modified according to the ultrasonic equipment's power range: 0 W, 100 W, 200 W, 300 W, 400 W, 500 W, 600 W, and 700 W. Based on the concentration of aurantio-obtusin and chrysophanol in 80 % ethanol extract, the ethanol solution with different concentrations was diluted five times, producing ethanol concentrations of 70 %, 60 %, 50 %, 40 %, and 30 %, respectively. Based on commercial nanofiltration membrane standards for cassia seed extract concentration at room temperature, nanofiltration membranes with MWCOs of 100 Da, 450 Da, and 800 Da were selected. To separate the 70 % ethanol extract of cassia seed, the separation parameters were fixed at pH 6.00, ultrasonic power 100 W, and 800 Da nanofiltration membrane.
2.5. Experiment design and statistical analysis
Response surface design (RSM) is a method to explore the quadratic relationship between variables and response values and determining the optimal factor configuration, which is frequently used in process optimization and factor interaction. After studying Design-expert 8.06 software and applying the Box-Behnken center combination theory, a three- variable and three-level center combination test method was developed. There were 17 test groups, each of which had three repeated tests. Levels of f variables are marked by (−1, 0, 1). The interaction between variables is computed using Eq. (3), in where Y is the response value, Xi and Xj are the variables, β0 is the constant coefficient, and βi, βii, and βij are the regression coefficients. In model and variable significance analysis, analysis of variance (ANOVA) is used to calculate the statistical significance of equations, while P-value analysis is used for assessing the significance of independent variables and their interactions.
| (3) |
2.6. Molecular state quantitative calculation model
At present, the qualitative analysis of the molecular state of components mainly adopts the spectral method [27,28]. However, the detection process needs to be carried out away from the original solution, and thus the real molecular state of multi-component coexisting solutions cannot be obtained. The mass transfer coefficients of the molecular states of aurantio-obtusin and chrysophanol were fitted using the theory that “the mass transfer coefficient can characterize the ease with which components pass through the nanofiltration membrane pores and is a comprehensive representation of the separation of multiple existing states” [29]. The corresponding component concentrations of chrysophanol and aurantio-obtusin in complex solutions when reaching the same mass transfer coefficient were calculated. The fraction of the molecular state is a percentage of the concentration.
A reference solution of chrysophanol was made based on the ethanol and solute concentrations in the cassia seed extract. According to the pKa 6.63 of chrysophanol, the pH of the solution was adjusted to 4.00 using 5 % hydrochloric acid solution, ensuring that the molecular proportion of chrysophanol was ≥ 99 %. An 800 Da nanofiltration membrane was used to separate the cassia seed extract or chrysophanol solution that was placed in a liquid storage tank. The TMP was set to 0.6, 0.8, 1.0, 1.2, and 1.4 MPa. The chrysophanogen solution and nanofiltration solution were determined with HPLC after the corresponding nanofiltration flux (Jv) and nanofiltration solution were collected. The rejection (R) is calculated using Eq. (1). Based on the relationship among the nanofiltration mass transfer coefficient (k), R and Jv, Eq. (4) fits the linear equation of ln[(1-R)·Jv/R] and k, where 1/k is the slope, ln(DK/δ) is the intercept, DK is the membrane mass transfer performance constant, and δ is the membrane thickness [30].
| (4) |
The power function equation between k and solute concentration (C) is fitted using Eq. (5), where m is the correlation coefficient and n is the power value.
| (5) |
The cassia seed extract was separated by nanofiltration using the power function equations of molecular state concentration and mass transfer coefficient established by the monomer components of chrysophanol, and the power function equations of k and C were fitted. When the k value of the chrysophanol monomer was the same as that of the cassia seed extract, the mass concentration (Ck) of chrysophanol in cassia seed extract compared to the separation environment of the monomer component was calculated using Eq. (5), and the molecular proportion (P) of chrysophanol using Eq. (6). At the same time, the same method was employed for calculating the molecular proportion of aurantio-obtusin in cassia seed extract.
| (6) |
2.7. Effect of ultrasonic power on the molecular state
The ultrasonic wave is a type of mechanical wave that changes the distance between molecules on a regular basis by causing the molecules to vibrate as it travels through the medium [31]. Based on the effect of ultrasonic power on the rejection of aurantio-obtusin and chrysophanol using the response surface method, the internal relationship of ultrasonic regulation separation by changing the molecular state of components was further investigated. The “2.6 Molecular state quantitative calculation model” was used to determine the molecular proportions of aurantio-obtusin and chrysophanol in the extraction solution of cassia seed, while the ultrasonic power ranged from 0 W to 700 W. Anthraquinones' intermolecular π-π stacking was examined in relation to ultrasonic power.
2.8. Sample analysis
2.8.1. Chrysophanol and aurantio-obtusin
The Waters e2695 HPLC with photo-diode array (PDA) detector was used to examine chrysophanol and aurantio-obtusin. Acetonitrile (A)-0.1 % phosphoric acid aqueous solution (B) was used as the mobile phase for the chromatography, which was carried out on a Hedera ODS-2 C18 (4.6 mm × 250 mm, 5 μm) column using gradient elution (0–––15 min, 38 %A; 15–––30 min, 38 %A − 90 %A; 30–––40 min, 90 %A; 40–––45 min, 38 %A). The detection wavelength was 284 nm, with a flow rate of 1.0 mL/min, column temperature of 30 ℃, and injection volume of 10 μL. Aurantio-obtusin and chrysophanol were dissolved in methanol to create a mixed reference solution of 131.65 μg/mL and 246.8 μg/mL, respectively. 0.1, 0.5, 1.0, 1.5, 2.0, and 2.5 mL of the mixed reference solution were precisely measured and put into a 10 mL volumetric bottle with methanol. Using peak area (Y) as the vertical coordinate and concentration (X, μg/mL) as the horizontal coordinate, the standard curves were calculated: aurantio-obtusin (Y = 4706X + 230.1, R2 = 0.999 6) and chrysophanol (Y = 3035X + 120.8, R2 = 0.999 1).
2.8.2. Particle size analysis
Anthraquinone components produce π-π packing in a conjugated system, changing the molecular state into an associated state. Ultrasound speeds up the mobility of anthraquinone molecules, potentially regulating the degree of π-π packing. According to the influence of ultrasonic power on the molecular state of chrysophanol and aurantio-obtusin, an 30 % ethanol solution is used to make chrysophanol and aurantio-obtusin mixed reference solution. The particle size distribution was detected using the Malvern ZEW3600 Zetasizer Nano System (Malvern Instruments, UK), and the effect of ultrasonic power (0 W, 200 W, 300 W and 700 W) on the presence of anthraquinones was analyzed. The ultrasonic power was employed for 10 min. The detection parameters were: wavelength 633 nm, temperature 20 ℃, dispersant ethanol, and refraction 1.360.
2.8.3. Nanofiltration membrane morphology analysis
The micro-characteristics of the nanofiltration membrane separation layer were examined both before and after the ultrasonic-assisted separation of 70 % ethanol extract of cassia seed at ultrasonic powers of 0 W and 700 W in order to confirm the feasibility of ultrasonic-enhanced nanofiltration for the extraction of cassia seed extract. With the following test parameters: Mag 50.00 kx, EHT 10.00 kv, and scale 200 nm, the membrane samples were detected using ZEISS MERLIN Compact ultra-high-resolution field emission scanning electron microscopy (SEM).
3. Results and discussion
3.1. Effect of vacuum concentration on transfer rate
Fig. 2 shows that the transfer rates of aurantio-obtusin and chrysophanol in cassia seed extract varied with temperature. As the temperature increased, chrysophanol's transfer rate decreased more than aurantio-obtusin's. The correlation analysis of transfer rate and heating time revealed a good logarithmic relationship between them, with chrysophanol having a higher absolute value of slope in the logarithmic curve than aurantio-obtusin, indicating that chrysophanol sublimation was more obvious [32]. According to the anthraquinone structures of aurantio-obtusin and chrysophanol, aurantio-obtusin's conjugated system has a stronger electron cloud density and a greater intermolecular π-π packing effect than chrysophanol, which makes it less susceptible to sublimation. The transfer rate is greater than that of chrysophanol at the same temperature.
Fig. 2.
Effect of vacuum concentration on transfer rate, (a) temperature, and (b) time.
3.2. Single factor test of ultrasonic enhanced nanofiltration
3.2.1. pH
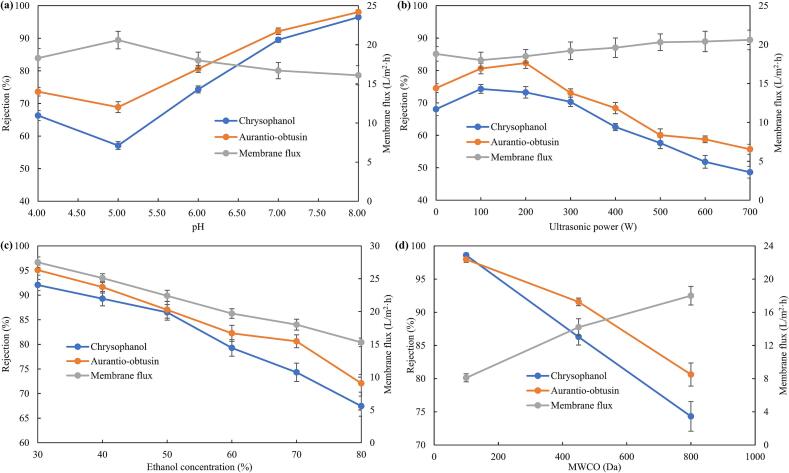
Fig. 3a shows how the rejections of aurantio-obtusin and chrysophanol first decrease and subsequently rise with increasing pH. Both exhibit rejections of over 96 % when pH is close to 8.00. The separation of charged components by nanofiltration is dominated by the Donnan effect, and the pH of the solution may have an impact on the molecular state of anthraquinone components. As a result, the separation behavior and the Donnan effect between the nanofiltration membrane are regulated.
Fig. 3.
Effect of factors on rejections, (a) pH, (b) ultrasonic power, (c) ethanol concentration and (d) MWCO.
The charge repulsion theory of nanofiltration separation is not consistent with the abnormal phenomenon of a decrease in rejection at pH 5.00. According to a thorough analysis of the variation patterns of membrane flux and component rejection data, the membrane flux exhibits a turning-point increase at pH5.00. This suggests that the anthraquinone components increase with the degree of ionization, weakening the π-π packing effect between molecules, which reduces the size of self-assembled molecules and, ultimately, the rejection. Because of hydrogen bonding and van der Waals forces, the ethanol concentration at the interface layer on the nanofiltration membrane's surface is higher than that in the solution while it is in an ethanol solution. Consequently, during the separation process, the ionized anthraquinone components show an “alcohol precipitation” effect, which leads to the phenomena of an ethanol-enhanced nanofiltration charge effect. Thus, as the pH rises above 6.00, the ethanol solution will increase the charge repulsion effect between the component and the nanofiltration membrane, lowering the membrane flux and increasing the rejection [33].
3.2.2. Ultrasonic power
In the nanofiltration separation process, ultrasound can speed up solute molecule movement and increase mass transfer efficiency. Fig. 3b shows that membrane flux and ultrasonic power have a positive association, suggesting that ultrasound can increase separation efficiency by eliminating the contaminated layer on the membrane's surface. The membrane flux of conventional nanofiltration separation dropped from 22.5 L/m2·h to 18.8 L/m2·h when the concentration of 2.0 L of cassia seed extract was reduced to 0.5 L. This suggests that anthraquinone components either adsorbed on the membrane surface or entered the membrane pores, resulting in membrane fouling. Ultrasound speeds up the mass transfer efficiency of membrane separation and the frequency of contact between anthraquinone component molecules as ultrasonic-enhanced nanofiltration separation is employed. Combined with the changing trend of the component rejection, when the ultrasonic power is 0 W − 100 W, the component rejection increases and the membrane flux drops, indicating that some anthraquinone component molecules self-assemble through π-π stacking. The rejection rises as a result of the larger molecules. Macromolecules, on the other hand, have a higher propensity to contaminate the membrane pores. The membrane flux recovered to 20.6 L/m2·h as the ultrasonic power rose from 100 W to 700 W. Ultrasonic increased the effectiveness of component transit through the nanofiltration membrane and promoted the desorption of contaminants. The membrane flux was still less than the initial flux, though, suggesting that certain reversible pollutants that were adsorbed and structured on the membrane pores' surface might be removed by ultrasonic waves. However, some irreversible pollutants that are blocked in the membrane pores cannot be completely cleaned. As the ultrasonic power rose, the cavitation effect caused by the high pressure and temperature disrupted the chemical bond, boosting the free anthraquinone's mass transfer efficiency and lowering rejection [34]. Chrysophanol's molecular weight was smaller than aurantio-obtusin's, and its intermolecular force was also less. The impact of ultrasonic frequency on chrysophanol was thus more obvious.
3.2.3. Ethanol concentration
The effect of 30 %-80 % ethanol concentration on component separation behavior was studied to increase the appropriateness of ultrasonic-enhanced nanofiltration for refining cassia seed extract. Fig. 3c shows that the ethanol concentration dropped from 80 % to 30 %, and that the membrane flux increased as the swelling impact of the nanofiltration membrane reduced. However, the retention rates of casetin and chrysophanol were negatively related with the ethanol concentration, suggesting that the separation of anthraquinone components is not primarily influenced by the decrease in effective separation pore size brought on by membrane swelling. Ethanol molecules wrap anthraquinone components, preventing the π-π stacking effect. As a result, the percentage of self-assembled macromolecules in the solution falls, which lowers the rejection [35].
3.2.4. MWCO
The components were separated by the pore size sieving effect and charge repulsion in nanofiltration. The membrane flux increased linearly as the nanofiltration membrane MWCO increased from 100 Da to 800 Da [36]. The rejections of chrysophanol and aurantio-obtusin decreased from 98.59 % − 74.31 % and 97.96 % − 80.62 %, respectively (Fig. 3d). The rejection of chrysophanol decreases more obvious because its molecular weight is smaller than that of aurantio-obtusin.
To balance the contradiction between component rejection and membrane flux in the separation process, an 800 Da nanofiltration membrane was chosen to concentrate the ethanol extract of cassia seed with the goal of ensuring separation efficiency, and the rejection of anthraquinone components was improved by adjusting the separation parameters. Based on single-factor experimental results, pH, ultrasonic power, and ethanol concentration were chosen as variables, with component rejection and membrane flux as response values. Factor levels ranged from pH 6.00 to 8.00, ultrasonic power from 100 W to 300 W, and ethanol concentration from 30 % to 70 %.
3.3. Statistical analysis and model fitting
The response surface method, which is extensively used in medicine, food, and chemistry, can quantitatively assess the interaction between the variables and response values and then consciously obtain the optimal variables level combination. pH (X1), ultrasonic power (X2), and ethanol concentration (X3) were chosen as variables in accordance with the Box-Behnken design principle, with varying values represented by −1, 0, and 1. Response surface analysis was carried out to explore the influence of variables on the rejection (%) of chrysophanol and aurantio-obtusin, and membrane flux. According to Table 2, the ranges for membrane flux are 16.2 to 28.9 L/m2·h, aurantio-obtusin rejections are 83.50 % to 98.85 %, and chrysophanol rejections are 78.09 % to 94.33 %, respectively. It is evident that different anthraquinones with a similar molecular weight and structural parent nucleus exhibited different levels of sensitivity to the separation parameters under study. According to the Box-Behnken central combination design theory, the regression equation between the response value and the factor variables under investigation is fitted, and the relationship between each variable and the response value is evaluated.
| (7) |
| (8) |
| (9) |
Table 2.
The experimental design and results for response surface analysis.
| Run | X1 | X2 | X3 | Chrysophanol rejection (%) | Aurantio-obtusin rejection (%) | Membrane flux (L/m2·h) |
|---|---|---|---|---|---|---|
| 1 | 0 | −1 | −1 | 94.33 | 98.52 | 25.8 |
| 2 | −1 | 1 | 0 | 79.26 | 84.72 | 24.1 |
| 3 | 0 | 1 | 1 | 86.09 | 90.20 | 19.3 |
| 4 | 0 | 0 | 0 | 89.72 | 93.70 | 18.9 |
| 5 | 0 | 0 | 0 | 87.69 | 91.57 | 18.0 |
| 6 | 0 | 0 | 0 | 88.39 | 93.37 | 17.4 |
| 7 | −1 | 0 | −1 | 90.63 | 95.82 | 28.9 |
| 8 | 1 | −1 | 0 | 92.08 | 97.26 | 17.1 |
| 9 | −1 | 0 | 1 | 78.09 | 83.50 | 17.9 |
| 10 | 1 | 0 | −1 | 93.86 | 94.04 | 21.0 |
| 11 | −1 | −1 | 0 | 81.07 | 86.21 | 22.5 |
| 12 | 0 | 1 | −1 | 89.98 | 93.37 | 26.6 |
| 13 | 0 | −1 | 1 | 88.38 | 93.09 | 16.2 |
| 14 | 0 | 0 | 0 | 87.04 | 93.07 | 18.8 |
| 15 | 0 | 0 | 0 | 88.26 | 92.31 | 19.2 |
| 16 | 1 | 0 | 1 | 94.27 | 98.85 | 16.9 |
| 17 | 1 | 1 | 0 | 85.61 | 91.24 | 20.4 |
According to Eq. (7) and Eq. (8), pH is positively related to rejection, whereas ultrasonic power and ethanol concentration are negatively correlated. Furthermore, pH dominates the separation efficiency of ultrasonic-enhanced nanofiltration, suggesting that charge repulsion and channel sieving still play a major role in nanofiltration separation [37]. The membrane separation layer swells when exposed to ethanol. Thus, ethanol concentration and ultrasonic power had less of an impact on anthraquinones' rejection than pH. The effective separation pore size of the nanofiltration membrane was directly correlated with the membrane flux, as indicated by Eq. (9), which also suggests that the ethanol concentration dominated the nanofiltration membrane flux.
The significance of the model is evaluated by calculating the sum of squares, degrees of freedom, and mean square of each variable. ANOVA also computes the F-value and P-value and compares the mean square of regression with the mean square of residual. According to the variance analysis results in Table 3, the response surface model of the chrysophanol rejection has an F-value of 43.85 and a P-value of less than 0.0001, indicating that the model is valid. The P-values of X1, X2, X3, X1X2, X1X3, X12, X22 and X32 were all less than 0.05, suggesting that there was a significant effect on the chrysophanol rejection. Furthermore, the order of variables influencing the chrysophanol rejection was pH (X1) > ethanol concentration (X3) > ultrasonic power (X2). The precision and reliability of the experimental results are demonstrated by the C.V. value of 1.10 %. Moreover, the P-value of 0.7630 indicated that the lack of fit was not statistically significant. The reasonably excellent agreement between the adjusted R2 of 0.9602 and the projected R2 of 0.9150 indicates that the model may be able to predict the experimental results with considerable precision.
Table 3.
Analysis of variance of regression model.
| Source | df | Chrysophanol |
Aurantio-obtusin |
Membrane flux |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of square | Mean square | F-value | P-value | Sum of Square | Mean Square | F-value | P-value | Sum of square | Mean square | F-value | P-value | ||
| Model | 9 | 372.69 | 41.41 | 43.85 | <0.0001* | 319.67 | 35.52 | 69.51 | <0.0001* | 215.69 | 23.97 | 162.08 | <0.0001* |
| X1 | 1 | 169.00 | 169.00 | 178.95 | <0.0001* | 145.69 | 145.69 | 285.12 | <0.0001* | 42.32 | 42.32 | 286.22 | <0.0001* |
| X2 | 1 | 27.83 | 27.83 | 29.46 | 0.0010* | 30.23 | 30.23 | 59.15 | 0.0001* | 9.68 | 9.68 | 65.47 | <0.0001* |
| X3 | 1 | 60.34 | 60.34 | 63.89 | <0.0001* | 45.65 | 45.65 | 89.34 | <0.0001* | 124.82 | 124.82 | 844.19 | <0.0001* |
| X1X2 | 1 | 5.43 | 5.43 | 5.75 | 0.0476* | 5.13 | 5.13 | 10.04 | 0.0157* | 0.72 | 0.72 | 4.89 | 0.0627 |
| X1X3 | 1 | 41.93 | 41.93 | 44.39 | 0.0003* | 49.91 | 49.91 | 97.68 | <0.0001* | 10.56 | 10.56 | 71.44 | <0.0001* |
| X2X3 | 1 | 1.06 | 1.06 | 1.12 | 0.3244 | 1.28 | 1.28 | 2.50 | 0.1579 | 1.32 | 1.32 | 8.94 | 0.0202* |
| X12 | 1 | 20.36 | 20.36 | 21.55 | 0.0024* | 9.09 | 9.09 | 17.79 | 0.0039* | 2.45 | 2.45 | 16.56 | 0.0048 |
| X22 | 1 | 12.40 | 12.40 | 13.13 | 0.0085* | 9.19 | 9.19 | 17.98 | 0.0038 | 9.01 | 9.01 | 60.91 | 0.0001 |
| X32 | 1 | 37.67 | 37.67 | 39.89 | 0.0004* | 25.65 | 25.65 | 50.19 | 0.0002* | 12.35 | 12.35 | 83.51 | <0.0001* |
| Residual | 7 | 6.61 | 0.94 | 3.58 | 0.51 | 1.04 | 0.15 | ||||||
| Lack of fit | 3 | 1.52 | 0.51 | 0.40 | 0.7630** | 0.62 | 0.21 | 0.28 | 0.8397** | 0.74 | 0.25 | 3.27 | 0.1414** |
| Pure Error | 4 | 5.09 | 1.27 | 2.96 | 0.74 | 0.30 | 0.075 | ||||||
| Cor Total | 16 | 379.30 | 323.25 | 216.72 | |||||||||
* means significant, ** means not significant.
The mathematical model between the variable and the aurantio-obtusin rejection has a F-value of 69.51 and a P-value of less than 0.0001, indicating that the model is valid. Aurantio-obtusin rejection is influenced by the following variables: pH (X1) > ethanol concentration (X3) > ultrasonic power (X2). The P-values of X1, X2, X3, X1X2, X1X3, X12, X22 and X32 were all less than 0.05, suggesting that the aurantio-obtusin rejection was significantly impacted by the investigation variables and the interaction of X1X2 and X1X3. The C.V. value of 0.77 % demonstrates the accuracy and reliability of the experimental results. Moreover, the P-value of 0.8397 indicated that the lack of fit was not statistically significant. The adjusted R2 of 0.9747 and predicted R2 of 0.9552 have a reasonably good agreement, which shows that the model could be able to predict the experimental data with some degree of accuracy.
With an F-value of 64.04 and a P-value of less than 0.0001, the mathematical model between the variable and the membrane flux is shown to be valid. The variables that affect membrane flux are as follows: ethanol concentration (X3) > pH (X1) > ultrasonic power (X2). The P-values of X1, X2, X3, X1X3, X12, X22 and X32 were all less than 0.05, indicating that the interaction of X1X3 and the investigation factors had a significant effect on the membrane flux. The C.V. value of 3.04 % indicates that the experimental results are accurate and reliable. Furthermore, the lack of fit was not statistically significant, according to the P-value of 0.8083. The reasonably excellent agreement between the adjusted R2 of 0.9726 and the predicted R2 of 0.9473 suggests that the model may be able to predict the experimental data with some degree of precision.
The interaction between variables has been analyzed using a three-dimensional response surface diagram to further and clearly evaluate their impacts on membrane flux and rejection of nanofiltration. As shown in Fig. 4a and b, the interaction between pH and ultrasonic power significantly affects the chrysophanol rejection (P < 0.05). As the pH rises, the ionic percentage of chrysophanol in the solution also rises, which causes chrysophanol rejection to increase as a result of nanofiltration charge repulsion. As ultrasonic power grew from 100 W to 300 W, chrysophanol rejection first increased and subsequently dropped. This suggests that ultrasound accelerated the π-π stacking among anthraquinones in cassia seed extract, prompted the formation of intermolecular association compounds in the solution, and increased the rejection of chrysophanol based on the pore size sieving effect of nanofiltration.
Fig. 4.
Response surface diagram of the significant interaction effects, (a) pH and ultrasonic power of chrysophanol, (b) pH and ethanol concentration of chrysophanol, (c) pH and ultrasonic power of aurantio-obtusin, (d) pH and ethanol concentration of aurantio-obtusin, (e) pH and ethanol concentration of membrane flux, and (f) ultrasonic power and ethanol concentration of membrane flux.
When the ultrasonic power above 200 W, the cavitation effect destroys the association state of anthraquinone molecules, resulting in a decline in the rejection. Additionally, the rejection of chrysophanol was significantly impacted by the interaction between pH and ethanol concentration (P < 0.05). The separation layer inflated as the ethanol concentration rose, decreasing the nanofiltration membrane's effective separation pore size. Additionally, the ethanol concentration reduced the degree of intermolecular interaction by interfering with the π-π stacking effect between the anthraquinone components. Consequently, at a pH of 6.00, the rejection decreased as the ethanol concentration increased, indicating that the separation behavior was dominated by the decrease in component association; at a pH of 8.00, the degree of chrysophanol ionization increased, and the separation behavior was dominated by the swelling of the nanofiltration membrane separation layer as the ethanol concentration increased.
Fig. 4c and d shows that the rejection of aurantio-obtusin is significantly affected by the interaction between pH and ethanol concentration, and the interaction between pH and ultrasonic power (P < 0.05). Because aurantio-obtusin and chrysophanol have identical chemical backbone structures, the variables determining their rejection are similar. Both of these variables regulate the separation of anthraquinones' components using nanofiltration in both directions. Controlling the levels of ultrasonic power and ethanol concentration throughout the nanofiltration separation process is essential to raise the anthraquinones' intermolecular motion frequency, improve their association percentage, and ensure the solute's mass transfer efficiency.
Membrane flux is significantly impacted (P < 0.05) by the pH-ethanol concentration interaction, as seen in Fig. 4e and f. Anthraquinones are ionized when pH increases, the charge repulsion effect decreases the solute's mass transfer efficiency, which lowers membrane flux. As the ethanol concentration increased, the separation layer of the nanofiltration membrane swelled, resulting in a smaller effective separation pore size and a lower membrane flux [38].
To improve the applicability of ultrasonic-enhanced nanofiltration separation technology, the system separated 35 %, 45 %, 55 %, and 65 % of a cassia seed ethanol extract solution. The optimal separation parameters using the response surface method were the following: in a 35 % ethanol solution with pH 6.85 and 100 W ultrasonic power, chrysophanol and aurantio-obtusin rejections were 92.13 % and 96.42 %, respectively, with a membrane flux of 24.13 L/m2·h. In a 45 % ethanol solution with pH 7.09 and 100 W ultrasonic power, chrysophanol and aurantio-obtusin rejections were 89.99 % and 94.55 %, respectively, with a membrane flux of 20.03 L/m2·h. In a 55 % ethanol solution with a pH of 7.05 and an ultrasonic power of 294.45 W, chrysophanol and aurantio-obtusin rejections were projected to be 86.86 % and 91.95 %, respectively, with a membrane flux of 19.71 L/m2·h. In a 65 % ethanol solution with a pH of 8.00 and an ultrasonic power of 300 W, chrysophanol and aurantio-obtusin rejections were 88.51 % and 93.72 %, respectively, with a membrane flux of 20.06 L/m2·h.
3.4. Verification experiments
Ultrasonic enhanced nanofiltration was used to separate and verify 35 %, 45 %, 55 %, and 65 % ethanol extracts of cassia seed, respectively. The ethanol extract solution's volume was adjusted to 2.0 L, 5.0 L, 10.0 L, and 20.0 L in order to investigate the effects of solution volume on membrane flux and the rejection of chrysophanol and aurantio-obtusin. Simultaneously, the cassia seed extraction solution underwent vacuum concentration using a rotary evaporation equipment and conventional nanofiltration separation. The impact of the separation process on the efficiency of anthraquinone separation was investigated. Based on the ultrasonic-enhanced nanofiltration separation data shown in Table4, Table5 and Table 6, the rejection and membrane flux results at the series ethanol concentration are practically in accordance with the values that the mathematical model predicted. The cassia seed extract can be refined at room temperature using the separation parameters that were optimized using the response surface method. While the membrane flux showed a downward trend as the solution volume increased, the rejection of chrysophanol and aurantio-obtusin showed a slow increase. This implies that membrane pollution produced by nanofiltration concentration polarization led to a decrease in the effective separation pore size as the separation volume rose. Accordingly, component rejection increased and membrane flux dropped. In practical production applications, the flux reduction problem can be handled by increasing the membrane area.
Table 4.
Effects of different refining processes on rejection (%) or transfer rate (%) of chrysophanol.
| Refining processes | Ethanol concentration | Volume (L) |
|||
|---|---|---|---|---|---|
| 2.00 | 5.00 | 10.00 | 20.00 | ||
| Ultrasonic-enhanced nanofiltration | 35 % | 97.21 ± 0.57 | 96.58 ± 0.82 | 94.58 ± 0.69 | 96.39 ± 1.17 |
| 45 % | 91.21 ± 1.19 | 90.62 ± 1.37 | 89.75 ± 1.41 | 92.15 ± 1.72 | |
| 55 % | 88.02 ± 1.66 | 89.20 ± 1.36 | 88.65 ± 1.70 | 91.40 ± 1.03 | |
| 65 % | 90.10 ± 1.45 | 91.57 ± 0.86 | 91.40 ± 1.27 | 93.71 ± 1.91 | |
| nanofiltration | 35 %* | 84.86 ± 1.07 | 80.74 ± 1.79 | 75.07 ± 2.64 | 65.94 ± 3.82 |
| 45 %* | 78.19 ± 1.82 | 74.43 ± 2.20 | 69.39 ± 2.49 | 63.28 ± 4.46 | |
| 55 %* | 73.53 ± 2.26 | 70.01 ± 2.80 | 67.35 ± 3.35 | 60.02 ± 4.37 | |
| 65 %* | 66.92 ± 2.02 | 62.65 ± 3.52 | 60.61 ± 2.44 | 58.74 ± 3.52 | |
| Vacuum concentration | 35 %* | 68.65 ± 3.87 | 57.86 ± 5.08 | 49.57 ± 5.65 | 42.52 ± 5.78 |
| 45 %* | 74.03 ± 4.55 | 68.46 ± 4.50 | 62.08 ± 4.88 | 56.04 ± 5.39 | |
| 55 %* | 78.46 ± 3.29 | 72.85 ± 4.31 | 68.48 ± 3.80 | 64.64 ± 4.92 | |
| 65 %* | 82.84 ± 2.60 | 78.08 ± 3.65 | 75.30 ± 3.92 | 71.29 ± 4.15 | |
*P < 0.05 versus ultrasonic-enhanced nanofiltration.
Table 5.
Effects of different refining processes on rejection (%) or transfer rate (%) of aurantio-obtusin.
| Refining processes | Ethanol concentration | Volume (L) |
|||
|---|---|---|---|---|---|
| 2.00 | 5.00 | 10.00 | 20.00 | ||
| Ultrasonic-enhanced nanofiltration | 35 % | 96.24 ± 0.65 | 95.75 ± 0.72 | 94.55 ± 1.04 | 94.31 ± 1.25 |
| 45 % | 94.82 ± 0.60 | 93.35 ± 0.84 | 94.13 ± 1.08 | 92.29 ± 1.10 | |
| 55 % | 91.05 ± 0.37 | 92.51 ± 0.81 | 92.02 ± 1.13 | 93.31 ± 1.35 | |
| 65 % | 93.52 ± 0.56 | 94.04 ± 0.32 | 95.54 ± 0.76 | 96.59 ± 0.70 | |
| Nanofiltration | 35 %* | 86.53 ± 1.59 | 80.69 ± 1.76 | 76.33 ± 2.08 | 74.80 ± 2.50 |
| 45 %* | 82.93 ± 1.43 | 77.33 ± 2.51 | 74.20 ± 2.07 | 72.58 ± 2.44 | |
| 55 %* | 79.30 ± 1.11 | 75.97 ± 1.52 | 73.07 ± 1.85 | 71.28 ± 2.70 | |
| 65 %* | 72.24 ± 0.62 | 69.44 ± 1.35 | 68.37 ± 1.72 | 65.42 ± 2.07 | |
| Vacuum concentration | 35 %* | 52.62 ± 2.08 | 48.31 ± 2.27 | 42.08 ± 3.16 | 39.65 ± 3.20 |
| 45 %* | 66.25 ± 1.72 | 61.89 ± 2.09 | 50.40 ± 1.63 | 47.33 ± 2.78 | |
| 55 %* | 72.67 ± 1.24 | 67.04 ± 2.20 | 64.99 ± 2.13 | 62.75 ± 2.67 | |
| 65 %* | 85.20 ± 1.15 | 81.63 ± 1.57 | 78.90 ± 1.62 | 76.14 ± 2.45 | |
*P < 0.05 versus ultrasonic-enhanced nanofiltration.
Table 6.
Effects of ultrasonic-enhanced nanofiltration and nanofiltration on membrane flux (L/m2·h).
| Refining processes | Ethanol concentration | Volume (L) |
|||
|---|---|---|---|---|---|
| 2.00 | 5.00 | 10.00 | 20.00 | ||
| Ultrasonic-enhanced nanofiltration | 35 % | 24.72 ± 1.75 | 23.85 ± 1.36 | 22.16 ± 1.53 | 21.50 ± 1.63 |
| 45 % | 22.70 ± 1.64 | 21.87 ± 1.39 | 21.04 ± 1.85 | 19.60 ± 1.08 | |
| 55 % | 19.55 ± 1.22 | 18.09 ± 1.57 | 17.27 ± 1.95 | 16.26 ± 1.73 | |
| 65 % | 17.20 ± 1.08 | 15.92 ± 1.14 | 15.20 ± 1.35 | 14.52 ± 1.27 | |
| Nanofiltration | 35 %* | 19.07 ± 1.42 | 17.29 ± 2.15 | 14.28 ± 1.47 | 12.55 ± 1.14 |
| 45 %* | 17.26 ± 2.10 | 14.35 ± 1.75 | 12.25 ± 1.08 | 10.86 ± 1.12 | |
| 55 %* | 16.08 ± 1.55 | 13.94 ± 1.43 | 9.76 ± 1.76 | 7.40 ± 1.05 | |
| 65 %* | 14.29 ± 2.07 | 12.51 ± 2.50 | 9.10 ± 2.01 | 6.08 ± 1.30 | |
*P < 0.05 versus ultrasonic-enhanced nanofiltration.
As the separation volume increased, the conventional nanofiltration separation flux declined significantly due to the combined effects of ethanol swelling and pore pollution. This was especially apparent in a 65 % ethanol solution. This suggests that ultrasound can efficiently reduce the surface pollution of the nanofiltration membrane and speed up the solvent mass transfer efficiency in the membrane pores. In contrast to the results of ultrasonic-enhanced nanofiltration, conventional nanofiltration decreased the rejection of aurantio-obtusin and chrysophanol. During nanofiltration, the solute concentration in the retention solution gradually increased. Ultrasound increased the contact frequency between molecules, the proportion of associated states increased, and rejection increased due to the π-π stacking effect. As the concentration of the solute in the retention solution grows, the solution-diffusion effect causes a greater percentage of chrysophanol and aurantio-obtusin to flow through the nanofiltration membrane in the conventional nanofiltration separation [39].
The transfer rates of chrysophanol and aurantio-obtusin decreased as the volume of the 35 % − 65 % ethanol solution increased, and the transfer rates correlated positively with the ethanol concentration. In the experiment, with the same concentration parameters under lower pressure, the concentration time of low concentration ethanol is longer to concentrate the 20.0 L extract to 0.5 L, and pipeline pollution caused by anthraquinone component sublimation is more severe. As a result, the yield and separation efficiency of anthraquinones are much higher with ultrasound-enhanced nanofiltration separation than with heat treatment concentration.
3.5. Effect of ultrasonic power on the molecular state
Ultrasound can bidirectionally regulate the nanofiltration mass transfer behavior of chrysophanol and aurantio-obtusin, although it is difficult to explain the separation behavior in terms of nanofiltration charge effect and pore size sieving. The diversity of component states determines the complexity of nanofiltration separation behavior. The reliability of the quantitative fitting equation of molecular proportion was shown in Table 7, which demonstrates that the correlation coefficients for the mass transfer coefficients of aurantio-obtusin and chrysophanol as well as the power function equation of concentration are both larger than 0.99.
Table 7.
The molecular proportion of three phenolic acid in concentrated solution.
| Ethanol concentration | Chrysophanol |
Aurantio-obtusin |
Power function equations |
|||
|---|---|---|---|---|---|---|
| C (mg/mL) | k (10-6m/s) | C (mg/mL) | k (10-6m/s) | Chrysophanol | Aurantio-obtusin | |
| 30 % | 0.24 | 16.26 | 0.16 | 9.73 |
k = 21.994C0.201 R2 = 0.994 2 |
k = 15.475C0.2383 R2 = 0.990 3 |
| 0.12 | 14.38 | 0.08 | 8.62 | |||
| 0.06 | 12.82 | 0.04 | 7.39 | |||
| 0.03 | 10.91 | 0.02 | 6.11 | |||
| 0.014 | 9.18 | 0.01 | 5.06 | |||
| 50 % | 0.28 | 15.07 | 0.15 | 7.95 |
k = 19.779C0.2193 R2 = 0.995 2 |
k = 12.173C0.2238 R2 = 0.992 3 |
| 0.14 | 13.01 | 0.075 | 6.91 | |||
| 0.07 | 10.72 | 0.0375 | 5.76 | |||
| 0.035 | 9.52 | 0.01875 | 4.96 | |||
| 0.0175 | 8.24 | 0.009375 | 4.32 | |||
| 70 % | 0.36 | 12.57 | 0.22 | 5.51 |
k = 16.298C0.2369 R2 = 0.994 8 |
k = 9.786C0.359 R2 = 0.993 3 |
| 0.18 | 11.12 | 0.11 | 4.52 | |||
| 0.09 | 9.24 | 0.055 | 3.59 | |||
| 0.045 | 7.76 | 0.0275 | 2.66 | |||
| 0.0225 | 6.62 | 0.01375 | 2.07 | |||
Fig. 5 shows how ultrasound affects the molecular proportions of chrysophanol and aurantio-obtusin in cassia seed extract. Increasing the ethanol concentration, which is positively correlated with the molecular proportion of anthraquinones, can decrease the ionization and degree of association of anthraquinones. The effect of ultrasonic power on the proportion of molecular states was negatively associated with the concentration of ethanol, with 30 % ethanol extract showing comparatively satisfactory results.
Fig. 5.
The logarithmic function relationship between ultrasonic power (U) and molecular proportion (P), (a) chrysophanol, and (b) aurantio-obtusin.
The molecular percentage of chrysophanol dropped from 20.46 % to 13.75 % while the ultrasonic power rose from 0 W to 200 W. Instead, the molecular percentage rose to 31.29 % when it exceeded 200 W. The molecular percentage of aurantio-obtusin dropped from 16.45 % to 12.50 % when the ultrasonic power was raised from 0 W to 300 W. The molecular percentage rose to 20.71 % when it surpassed 300 W. This suggests that by controlling the intermolecular forces of anthraquinones, ultrasound may regulate the percentage of self-assembled macromolecules. In line with the previous results [40,41], ultrasound destroys the hydrogen bonds between self-assembly macromolecules as its power increases steadily, increasing the proportion of molecular states.
In order to analyze the mechanism of ultrasonic-regulated π-π stacking effect among anthraquinones and fit the correlation between ultrasonic power and molecular proportion, it is found that there is a logarithmic correlation between the two, and the correlation coefficient is greater than 0.9, indicating that ultrasonic destruction of hydrogen bonds is power-dependent, and the increase of power strengthens the cavitation effect and promotes the break of hydrogen bonds between supramolecular structures. As shown in Fig. 6, the ultrasonic power intervals that produced the supramolecular hydrogen bond disruption of chrysophanol and aurantio-obtusin were 300 W − 700 W and 400 W − 700 W, respectively.
Fig. 6.
Effect of ultrasonic power on particle size distribution of mixed reference solution, (a) 0 W, (b) 200 W, (c)300 W and (d) 700 W.
3.6. Effect of ultrasonic power on the self-assembly of anthraquinone
Chrysophanol and aurantio-obtusin mixed reference solution made with 30 % ethanol was treated with 0 W, 200 W, 300 W, and 700 W ultrasound, respectively, to clarify the regulation rule of ultrasound on the self-assembly of anthraquinone components. The change in the solution's nanoparticle size distribution was shown in Fig. 6. Fig. 6a shows anthraquinones in the mixed reference solution as monomers (1.185 nm) and self-assembly associations (429.9 nm). Anthraquinones' self-assembly behavior is accelerated by increasing the ultrasonic power to 200 W and 300 W, depending on Fig. 5′s showing the impact of ultrasonic power on molecular proportion. The particle sizes of the self-assembly compounds reached 507.8 nm and 1191 nm in Fig. 6b and Fig. 6c, respectively, indicating that the shear and collision effects brought on by ultrasound accelerated the degree of intermolecular interaction, even though the self-assembly between chrysophanol and aurantio-obtusin increased the particle size of the associated state in the solution [42]. As the ultrasonic power reaches 700 W, ultrasonic cavitation breaks the supramolecular compound's hydrogen bond. As seen in Fig. 6d, the monomer component is mostly present in the solution (1.189 nm), while the self-assembly associations' particle size drops to 167.5 nm.
The cavitation effect induced by ultrasound can disrupt hydrogen bonds between molecules and lower the size of micelles. This study demonstrated that the ultrasonic power is related to the bidirectional regulation of the anthraquinone self-assembly process by ultrasound, which is in line with the results of previous research [43,44], when combined with the self-assembly law of anthraquinone components. According to the results of ultrasonic self-assembly adjustment, which were in line with the trend of molecular state proportion change in Fig. 5, anthraquinones' self-assembly process was controlled bidirectionally by ultrasound, and nanofiltration's effectiveness in intercepting anthraquinones was improved [43,45].
3.7. Effect of ultrasonic power on membrane morphology
Ultrasonic waves can disrupt the polymer chains in the membrane separation layer material, resulting in material degradation and changing its mechanical separation capabilities [46]. The morphological changes of the nanofiltration membrane separation layer before and after ultrasonic treatment were studied, and results are shown in Fig. 7. Ultrasonic enhanced nanofiltration was employed to reduce 20.0 L of cassia seed extract to 0.5 L, with a separation time of around 3 h. The roughness of the membrane separation has increased, as seen in Fig. 7b. However, when combined with the rejections of chrysophanol and cassitin in the cassia seed extract, it appears that the nanofiltration membrane's separation performance has remained stable. Although the membrane separation layer is somewhat degraded by ultrasonic radiation, the integrity of the membrane separation layer was not significantly affected. Meanwhile, the extract of cassia seed was treated with an ultrasonic power of 700 W, with the temperature adjusted by flowing water to ≤ 25℃. The chrysophanol and aurantio-obtusin degradation percentages were both less than 2 % following three hours of ultrasonic treatment, suggesting that the ultrasonic-enhanced nanofiltration separation of the cassia seed extract has appropriate application.
Fig. 7.
Effect of ultrasonic power on nanofiltration membrane morphology, (a) 0 W and (b) 700 W.
4. Conclusion
This work resolved pharmaceutical problems such as sublimation and heat-induced environmental contamination by using ultrasound to adjust the self-assembly percentage of anthraquinone components in cassia seed extract. Ultrasonic enhanced the π-π stacking effect between anthraquinones in the range of 100 W to 300 W, which caused the components to self-assemble. It also decreased surface pollution due to the cavitation effect, which led to effective nanofiltration separation. In the 300 W − 700 W range, ultrasonic power exhibited a logarithmic relationship with the molecular proportion of anthraquinones' components. Ultrasonic power also encouraged the breaking of hydrogen bonds between supramolecular structures, which raised the molecular proportion and decreased solute rejection.
The optimal separation parameters utilizing the response surface method, chrysophanol and aurantio-obtusin rejections in cassia seed extract with ethanol concentrations of 35 % − 65 %, were greater than 88 % and 91 %, respectively, as the separation volume increased from 2 L to 20 L. It was evident that ultrasonic enhanced nanofiltration outperformed conventional nanofiltration separation and decompression procedures. Based on the intermolecular forces of the anthraquinone components in various ethanol solutions, this study used an ultrasonic bidirectional self-assembly ratio to purify cassia seed extract at room temperature through ultrasonic-enhanced nanofiltration, thereby avoiding the problems of component sublimation and environmental contamination brought on by conventional concentration.
CRediT authorship contribution statement
Cunyu Li: Writing – review & editing, Project administration, Methodology. Xin Shen: Validation, Writing – review & editing. Ranyun Qiu: Methodology, Writing – review & editing. Dantong Xing: Writing – review & editing. Xinglei Zhi: Writing – review & editing, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 82274106), Innovation Project of State Key Laboratory on Technologies for Chinese Medicine Pharmaceutical Process Control and Intelligent Manufacture (Grant No. NZYSKL240207), Chinese Pharmacy First-Class Scientific Research and Cultivation Project of Nanjing University of Chinese Medicine (Grant No. ZYXPY2024-006) and Longquan City Characteristic Chinese Herbal Medicine Agricultural Science and Technology Park Project (Grant No. 2024KJNY-002).
Footnotes
This article is part of a special issue entitled: ‘Separation Processes’ published in Ultrasonics Sonochemistry.
Contributor Information
Cunyu Li, Email: licunyuok@163.com.
Xinglei Zhi, Email: 12317231@qq.com.
References
- 1.Yagofarov M.I., Balakhontsev I.S., Miroshnichenko E.A., Solomonov B.N. Estimation of sublimation enthalpies of aromatic compounds as a function of temperature. J. Chem. Thermodyn. 2022;174 [Google Scholar]
- 2.Tang T., Yin L., Yang J., Shan G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur. J. Pharmacol. 2007;567:177–185. doi: 10.1016/j.ejphar.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Xie H., He S., Yu Z., Xu H., Wang Z., Li H. Investigation on bowel regulation and constipation relief based on the microbial fermentation solution of cassia seed. J. Ethnopharmacol. 2025;342 doi: 10.1016/j.jep.2025.119412. [DOI] [PubMed] [Google Scholar]
- 4.Miao M., Shi Y., Li Y., Jiang Z., Liu J., Yang S. Non-digestible galactomannan oligosaccharides from Cassia seed gum modulate microbiota composition and metabolites of human fecal inoculum. J. Funct. Foods. 2021;86 [Google Scholar]
- 5.Souza A.A., Ribeiro K.A., Seixas J.R.P.C., Neto J.C.S., Santiago M.G.P.F., Aragão-Neto A.C., Lima-Ribeiro M.H.M., Borba E.F.O., Silva T.G., Kennedy J.F., Albuquerque P.B.S., Carneiro-da-Cunha M.G. Effects including photobiomodulation of galactomannan gel from Cassia grandis seeds in the healing process of second-degree burns. Int. J. Biol. Macromol. 2023;251 doi: 10.1016/j.ijbiomac.2023.126213. [DOI] [PubMed] [Google Scholar]
- 6.Liu N., Zhao X., Wang C., Li Y., Pan S., Huang W., Hakizimana I., Kong W., Wang Y. Application of electrochemical flow-through oxidation technology in the treatment of concentrated water from nanofiltration and reverse osmosis in the coal chemical industry. J. Environ. Chem. Eng. 2024;12 [Google Scholar]
- 7.Li C., Ma Y., Tang S., Xu Y., Zhi X. Recent advances of nanofiltration separation in pharmaceutical field from water to organic solution. J. Mol. Liq. 2024;409 [Google Scholar]
- 8.Wu X., He J., Yin W., Zhang D., Tan J., Zhong Z., Wang X. Research on the removal efficiency and membrane fouling analysis of nanofiltration membranes under the combined pollution of emerging contaminants. J. Water Process Eng. 2024;67 [Google Scholar]
- 9.Abhayawardhana A.D., Sutherland T.C. Heterogeneous proton-coupled electron transfer of a hydroxy-anthraquinone self-assembled monolayer. J. Electroanal. Chem. 2011;653:50–55. [Google Scholar]
- 10.Song J., Zeng J., Chen X., Wang J., Zhang Y., Gao Y., Wang R., Jiang N., Lin Y., Li R. Anti-neuroinflammatory agent rhein lysinate-based self-assembled injectable hydrogel loaded with ZL006 for promoting post-stroke functional recovery. Biomaterials. 2025;318 doi: 10.1016/j.biomaterials.2025.123124. [DOI] [PubMed] [Google Scholar]
- 11.Cho J., Cho J., Lee J., Lee D., Park C., Kim S. Optimization of salting-out crystallization for an efficient in situ separation of synthetic anthraquinone- and azo-type reactive dyes. Sep. Purif. Technol. 2009;68:138–144. [Google Scholar]
- 12.Guo F., Miao J., Xu L., Zhou Q., Deng T. Conductive thin-film nanocomposite nanofiltration membrane comprising N-doped graphene quantum dots with relieved concentration polarization for sulfate separation from high-salinity solution. Desalination. 2023;555 [Google Scholar]
- 13.Li H., Xu S., Wang B., Tian Z., Xu Z., Qian F. A new insight into the effects of DMF solvent activation on the polyamide layers of nanofiltration membranes by molecular simulation. J. Membrane Sci. 2025;718 [Google Scholar]
- 14.Zhang L., Zaoui A., Sekkal W. Adsorption efficiency of highly methylene blue dye concentrations with multilayer chitosan-modified clays for a precise nanofiltration performance of polluted water. J. Water Process Eng. 2024;57 [Google Scholar]
- 15.Lei C., Jacobson B., Hartley J.M., Scott S., Sumarlan I., Feeney A., Prentice P., Ryder K.S., Abbott A.P. Effect of organic solvent additives on the enhancement of ultrasonic cavitation effects in water for lithium-ion battery electrode delamination. Ultrason. Sonochem. 2024;110 doi: 10.1016/j.ultsonch.2024.107049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kianpour G., Salavati-Niasari M., Emadi H. Sonochemical synthesis and characterization of NiMoO4 nanorods. Ultrason. Sonochem. 2013;20:418–424. doi: 10.1016/j.ultsonch.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Chuai S., Zhu X., Ye L., Liu Y., Wang Z., Li F. Study on the mechanism of ultrasonic cavitation effect on the surface properties enhancement of TC17 titanium alloy. Ultrason. Sonochem. 2024;108 doi: 10.1016/j.ultsonch.2024.106957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heydariyan Z., Salavati-Niasari M., Monsef R. Preparation and investigation of TbCrO3/montmorillonite-K10 nanocomposite electrode for electrochemical hydrogen storage application. Fuel. 2024;372 [Google Scholar]
- 19.Mohandes F., Salavati-Niasari M. Sonochemical synthesis of silver vanadium oxide micro/nanorods: Solvent and surfactant effects. Ultrason. Sonochem. 2013;20:354–365. doi: 10.1016/j.ultsonch.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Behpour M., Ghoreishi S.M., Gandomi-Niasar A., Soltani N., Salavati-Niasari M. The inhibition of mild steel corrosion in hydrochloric acid media by two Schiff base compounds. J. Mater. Sci. 2009;44:2444–2453. [Google Scholar]
- 21.Salavati-Niasari M., Davar F., Loghman-Estarki M.R. Controllable synthesis of thioglycolic acid capped ZnS(Pn)0.5 nanotubes via simple aqueous solution route at low temperatures and conversion to wurtzite ZnS nanorods via thermal decompose of precursor. J. Alloy. Compd. 2010;494:199–204. [Google Scholar]
- 22.Amiri M., Pardakhti A., Ahmadi-Zeidabadi M., Akbari A., Salavati-Niasari M. Magnetic nickel ferrite nanoparticles: Green synthesis by Urtica and therapeutic effect of frequency magnetic field on creating cytotoxic response in neural cell lines. Colloid. Surface. b. 2018;172:244–253. doi: 10.1016/j.colsurfb.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M., Du L., Qi L., Li Y., Li Y., Li X. Numerical simulations and electrochemical experiments of the mass transfer of microvias electroforming under ultrasonic agitation. Ultrason. Sonochem. 2018;48:424–431. doi: 10.1016/j.ultsonch.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Mollahosseini A., Abdelrasoul A. Molecular dynamics simulation for membrane separation and porous materials: A current state of art review. J. Mol. Graph. Model. 2021;107 doi: 10.1016/j.jmgm.2021.107947. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H., Wei Y., Lin J. Frustrated π - stacking. Chem Commun. 2024;60:935–942. doi: 10.1039/d3cc04123a. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S., Li W., Qin Y., Chen Y., Liu Z., Li S., Tang L., Zheng H., Tang X. A novel flocculant based on “happy molecules” for the efficient removal of NSAIDs and NOM complexes: Role of parallel π-π stacking force. J. Hazard. Mater. 2025;489 doi: 10.1016/j.jhazmat.2025.137626. [DOI] [PubMed] [Google Scholar]
- 27.Honda A., Nozawa R., Miyamura K. Molecular aggregation by hydrogen bonding in cold-crystallization behavior of mixed nucleobases analyzed by temperature-controlled infrared spectroscopy. RSC Adv. 2024;14:3776–3781. doi: 10.1039/d3ra08293h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J., Jin X., Cheng H., Chen W., Yu W., Wang L. Molecular origin of the plasticizing effect difference of glycerol with other polyols on plasticizing polyvinyl alcohol (PVA) as elucidated by solid-state NMR. Ind. Crop. Prod. 2024;220 [Google Scholar]
- 29.Cui K., Sui N., Huang K. Enhanced separation via confined shear flow in microchannel: A novel insight into the role of confined hydrogen bonds in microfluid and its effect on competitive mass transfer of hydrated ions. Chem. Eng. J. 2024;487 [Google Scholar]
- 30.Li C., Ma Y., Xu Y., Qiu R., Shen X., Huang L., Liu A., Li M., Zheng Y., Zhi X. Ultrasonic-assisted nanofiltration separation recovering salvianolic acid B from ethanol wastewater. Ultrason. Sonochem. 2024;108 doi: 10.1016/j.ultsonch.2024.106967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cha B., Lee S.H., Iqrar S.A., Yi H., Kim J., Park J. Rapid acoustofluidic mixing by ultrasonic surface acoustic wave-induced acoustic streaming flow. Ultrason. Sonochem. 2023;99 doi: 10.1016/j.ultsonch.2023.106575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vahabzadeh A., Fakour M., Ganji D.D., Bakhshi H. Analytical investigation of the one dimensional heat transfer in logarithmic various surfaces. Alex. Eng. J. 2016;55:113–117. [Google Scholar]
- 33.Zhi Y., Zhao X., Shuai A., Jia Y., Cheng X., Lin S., Xiao F., Han L., Chai H., He Q., Liu C. Enhancing rejection of short-chain per- and polyfluoroalkyl substances by tailoring the surface charge of nanofiltration membranes. Water Res. 2025;272 doi: 10.1016/j.watres.2024.122931. [DOI] [PubMed] [Google Scholar]
- 34.Yu X., Zhu K., Hu F., Hu R., Dong W. Effects of ultrasonic treatment on the physicochemical, structural, and functional properties of soluble dietary fiber from coffee peels. Ultrason. Sonochem.1. 2025;114 doi: 10.1016/j.ultsonch.2025.107247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Kuang W., Yu H., Liu D., Liu Y., Kang G., Liang X., Cao Y. Nanofiltration membranes with sandwich-like mixed charge layers for high-efficiency Mg2+/Li+ separation. J. Membrane Sci. 2025;722 [Google Scholar]
- 36.Li H., Xiao S., Zhao X., Yuan J., Yu S. Preparation of high-flux loose nanofiltration membranes for efficient dye/salt separation by controlling interface polymerization through physical and chemical dual constraints. Sep. Purif. Technol. 2025;362 [Google Scholar]
- 37.Hu D., Liu S., Qi L., Liang J., Zhang G. A critical review on ultrasound-assisted adsorption and desorption technology: Mechanisms, influencing factors, applications, and prospects. J. Environ. Chem. Eng. 2024;12 [Google Scholar]
- 38.Deng J., Zhang X., Gu Z., Tong Y., Meng F., Sun L., Liu H., Wang Q. High-efficiency purification of alkali-surfactant-polymer flooding produced water by ultrasonication-ionic liquids combination: Performance and separation mechanism. Sep. Purif. Technol. 2025;363 [Google Scholar]
- 39.Fierro D., Boschetti-de-Fierro A., Abetz V. The solution-diffusion with imperfections model as a method to understand organic solvent nanofiltration of multicomponent systems. J. Membrane Sci. 2012;413–414:91–101. [Google Scholar]
- 40.Ngoc N.L., Takaomi K. Ultrasound stimulus effect on hydrogen bonding in networked alumina and polyacrylic acid slurry. Ultrason. Sonochem. 2010;17:186–192. doi: 10.1016/j.ultsonch.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Sun X., Zhang L., Zhu T., Chen F. Self-assembly of pea peptides prepared by ultrasound-regulated enzymatic hydrolysis. Food Hydrocolloid. 2024;157 [Google Scholar]
- 42.Zhao J., Yang P., Fu J., Wang Y., Wang C., Hou Y., Shi Y., Zhang K., Zhuang W., Ying H. Polymorph control by designed ultrasound application strategy: The role of molecular self-assembly. Ultrason. Sonochem. 2022;89 doi: 10.1016/j.ultsonch.2022.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M., Wang Y., Ding H., Lu M., Gao G., Dong L., Li Q., Chen Y., Li S., Lan Y. Self-assembly of anthraquinone covalent organic frameworks as 1D superstructures for highly efficient CO2 electroreduction to CH4. Sci. Bull. 2021;66:1659–1668. doi: 10.1016/j.scib.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Li C., Zhao C., Ma Y., Chen W., Zheng Y., Zhi X., Peng G. Optimization of ultrasonic-assisted ultrafiltration process for removing bacterial endotoxin from diammonium glycyrrhizinate using response surface methodology. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105215. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Q., Zhang K., Gao G., Jin B., Zi G., Yan R., Li B., Yang B., Yao Y. Crystal and micro-nano structure bidirectional regulation of iron oxalate for high-lithium storage and ultralong-cycling life via ultrasound assistance. Chem. Eng. J. 2024;502 [Google Scholar]
- 46.Safronova E.Y., Pourcelly G., Yaroslavtsev A.B. The transformation and degradation of Nafion® solutions under ultrasonic treatment. The effect on transport and mechanical properties of the resultant membranes. Polym. Degrad. Stabil. 2020;178 [Google Scholar]