Abstract
Information about a number of Pneumocystis carinii lipids obtained by the analyses of organisms isolated and purified from infected lungs of corticosteroid-immunosuppressed rats has been reported in recent years. Of the common opportunistic protists associated with AIDS (Cryptosporidium, Toxoplasma, and the microsporidia), more is currently known about the lipids of P. carinii than the others. Lipids that are synthesized by the organism but not by humans are attractive targets for drug development. Thus, the elucidation of Δ7C-24-alykylated sterol and cis-9,10-epoxystearic acid biosyntheses in P. carinii is currently being examined in detail, since these have been identified as P. carinii-specific lipids. The development of low-toxicity drugs that prevent sterol C-24 alkylation and the specific inhibition of the lipoxygenase that forms cis-9,10-epoxystearic acid might prove fruitful. Although humans can synthesize coenzyme Q10, the anti-P. carinii activity and low toxicity of ubiquinone analogs such as atovaquone suggest that the electron transport chain in the pathogen may differ importantly from that in the host. Although resistance to atovaquone has been observed, development of other naphthoquinone drugs would provide a broader armamentarium of drugs to treat patients with P. carinii pneumonia. Studies of bronchoalveolar lavage fluid and of infected lungs have demonstrated that the infection causes a number of chemical abnormalities. Bronchoalveolar lavage fluid obtained after the removal of lung cellular material and the organisms has been shown to contain larger amounts of surfactant proteins and smaller amounts of phospholipids than do comparable samples from P. carinii-free lungs. Increased phospholipase activity, inhibition of surfactant secretion by type II cells, and uptake and catabolism of lipids by the pathogen may explain this phenomenon related to P. carinii pneumonia. Although not yet thoroughly examined, initial studies on the uptake and metabolism of lipids by P. carinii suggest that the organism relies heavily on exogenous lipid nutrients.
PNEUMOCYSTIS CARINII
Pneumocystis carinii is considered the paradigm of opportunistic infectious agents. This unique fungal microbe is ubiquitous worldwide, and most children already test serologically positive for P. carinii, indicating exposure to the organism (109). Immunocompetent humans and mammals are symptom free during the infection. In contrast, immunocompromised or immunodeficient individuals are at high risk for developing life-threatening infections. The organism gained serious attention after being recognized as causing the most prevalent opportunistic infection among AIDS patients. Although dissemination of the organism to extrapulmonary sites is now well documented, the organism proliferates mainly in the lung alveolus, resulting in a pneumonitis referred to as P. carinii pneumonia (PCP).
Because taxonomic information on an infectious agent could provide valuable insight into approaches for the development of effective therapy, it should be noted that since its discovery, workers have disagreed on the classification of P. carinii. Based on nucleotide sequence analyses, P. carinii has been placed with the ascomycetous fungi (20, 101). Although now generally agreed by most investigators that the organism is most closely related to the fungi, it is also acknowledged that it differs from the more commonly studied fungi. Thus, the group of fungus to which P. carinii belongs remains an open question. Recently, the organism has been placed in the Archiascomycetes together with several saprophytic and parasitic plant pathogens such as Taphrina, the causative agent of peach leaf curl disease (1). The taxonomic classification of each of the organisms placed within the Archiascomycetes is typified by a history of controversy and debate. Members of this putative class of fungi may represent the earliest diverging group of ascomycetes (1).
The P. carinii organisms isolated from one mammalian host do not cause infection in another host species (29, 99–101). Hence the organisms found in different mammals probably represent different species. An interim trinomial nomenclature was adopted by P. carinii researchers (99) to distinguish between parasites isolated from different hosts. The organisms from humans are designated P. carinii hominis. Based on karyotype analysis, there appear to be at least two subspecies or strains of organisms isolated from rats. Hence, two groups have been established for organisms infecting rat lungs: P. carinii carinii (“prototype” chromosomal pattern) and P. carinii rattus (“variant” pattern).
The life cycle in the mammalian lung includes two dominant stages, the polymorphic trophozoites and the mature thick-walled cysts (asci), which contain eight intracystic bodies (ascospores) (109). Intermediate stages and thin-walled cysts (containing intracystic bodies resembling trophozoites) are also present. The thick-walled cysts have a trilaminar wall external to the cell surface-limiting membrane, whereas trophozoites lack the wall. All stages in the lungs are surrounded by a characteristic thick glycocalyx (fuzzy coat). While most forms appear to have distinctive tubular extensions of their surfaces, these outpocketings are most elaborate in trophozoites. The organisms adhere to each other and to type I epithelial cells. The absence of adhesion to type II pneumocytes may result from the absence of specific receptors for the organism. The rapid turnover of the apical surface membrane due to active fusion of lung surfactant-containing vesicles and exocytosis of vesicular contents into the alveolar lumen may also inhibit attachment.
ORGANISMS USED FOR BIOCHEMICAL ANALYSES
Although the lack of methods for the continuous axenic subculturing of P. carinii has hampered direct biochemical studies of this pathogen (13, 92), sufficient numbers of organisms can be isolated from infected lungs of animal models to perform the analyses. Also, since organisms in culture and those isolated from fulminant infections can differ in their biochemistry (83), it is important to study infective agents taken directly from their hosts. Lipid analysis of organisms isolated from infected hosts is confounded by the abundance of lung surfactant lipids secreted by the type II pneumocytes. Lung surfactant is particulate and is readily brought down from lung homogenates or bronchoalveolar lavage fluid (BALF) upon standing or by relatively low-speed centrifugation. Insufficient purification thus leads to the coisolation of large amounts of lung surfactant. Lung surfactant is composed mainly of lipids (approximately 80%), with lower concentrations of proteins and carbohydrates. The lipids of lung surfactant are distinct; dipalmitoyl (disaturated) phosphatidylcholine (PC) predominates, although other lipids (cholesterol and phospholipids such as phosphatidylinositol) are also present in substantial amounts. Dipalmitoyl PC is characterized by two fatty acids, 16 carbons in length, with no double bonds (16:0). Furthermore, lung surfactant lipids and proteins bind to the surface of the organisms (71, 72, 78, 81, 82, 117), further complicating the development of purification protocols.
P. carinii Isolated from Infected Lungs of Corticosteroid-Immunosuppressed Rats
Most early biochemical studies performed on P. carinii were done with preparations of undefined purity and organism viability. Most investigators assumed that the lack of visible intact host cells indicated that the organisms used for their studies were free of smaller host particles or molecules adherent to the surface of the organisms. Despite the technical difficulties involved, considerable lipid data have been obtained in the last few years. Most of the studies have been on organisms isolated from the lungs of corticosteroid-immunosuppressed rats.
A useful study that inspired confidence in the interpretation of results obtained by different laboratories was the development of an isolation and purification procedure for rat-derived P. carinii carinii (51). The procedure is as follows. The organisms were grown in viral antibody-negative, P. carinii-free rats that were intratracheally inoculated with known numbers of parasites (6). The resultant purified organism preparations, characterized by several criteria (using microscopic, biochemical, and immunochemical techniques) were found to be 95 to 100% pure (see below). The protocol involved homogenization of infected lungs in the presence of a mucolytic agent. Glutathione, which occurs naturally in relatively high concentrations in fluid lining the alveolar epithelium (7), was chosen for most subsequent studies, although N-acetylcysteine gave comparable results. However, although glutathione is normally found in alveolar fluid, the concentrations used for the organism isolation protocol are much higher. Slight toxicity was observed with ascorbic acid. Independently, dithiothreitol was incorporated into a similar purification scheme as a mucolytic agent (11, 89), but this potent sulfhydryl agent appears to be harsher, as indicated by its deleterious effect on cell viability (indicated by decreases in cellular ATP concentrations [14]). The sulfhydryl agents cause organisms to detach from each other and from the underlying type I pneumocytes (51). Tearing of P. carinii cell surface tubular extensions from the organism and contamination of the organism preparation with host cell membrane fragments and molecules were thus minimized. These observations indicated that disulfide linkages appear to be important in the adhesion of organisms to each other and to epithelial cells. The protocol provides organisms essentially free of adherent molecules such as lung surfactant proteins. Hence, reduction of disulfide bonds may cause detachment of the organisms but may also break other disulfide linkages within the organism surface.
The purity of the organism preparation was quantitatively characterized by the following procedures (hereafter referred to as the defined protocol) (51). (i) No whole host cells or nuclei were detected by light microscopy (phase-contrast optics, differential interference optics, and nuclear staining). By this criterion, the preparation was 100% pure. (ii) Transmission electron microscopy was performed on 28 separate organism preparations. For each, two to four blocks were prepared. Each block was sectioned at two or more different levels, and to ensure random sampling, the first 20 grid squares were photographed at low magnification (maximal area). The micrographs (80 to 100 micrographs per organism preparation) were analyzed by using the most conservative criteria. All isolated membrane fragments in micrographs that lacked a thick glycocalyx were scored as contaminants, even though they could have originated from cytoplasmic organelles of P. carinii. The lowest (not average) estimate by transmission electron microscopy was >95% purity. (iii) The lung surfactant marker molecule surfactant protein A (SP-A) was quantified immunochemically. Monospecific polyclonal antibodies directed against rat SP-A were used in an enzyme-linked immunosorbent assay to quantify SP-A in organism preparations. By using the highest (not average) SP-A value, purity was estimated at >99.5%. (iv) Because lung surfactant contains substantial amounts of lipids and because of the question of host lipid contamination (adsorption versus uptake and incorporation of host lipids by the parasite), exogenous stigmasterol was added at the early homogenization step of the protocol. Stigmasterol was not detected in the preparation prior to the final step. By this criterion, it was estimated that 100% of the lipids extracted from the preparation were from P. carinii. (v) The final organism preparation obtained from infected rat lungs varied according to the parasite load. The total protein content in organism pellets from individual rats typically contained 5 mg of protein. Process controls were also evaluated. These controls are the result of processing the lungs of immunosuppressed rats which were not infected with P. carinii. The mass of final pellets obtained from subjecting the lungs from individual rats to the purification protocol was negligible. Protein analyses of the material gave a maximum (not average) value of <300 μg of protein. Because rats had different parasite loads, it was not possible to calculate the percent purity by this criterion; however, the process control protein content verified that very little host protein (6% maximum) ended up in the final preparation.
These mixed-life-cycle-stage preparations contained about 10 to 30% cysts. The viability of the organisms was estimated at 80 to 95% by a dual-stain live-dead microscopic method (49, 50). Being relatively hydrophobic, calcein acetoxymethyl ester diffuses into cells, where nonspecific esterases cleave the compound, resulting in a fluorescent product which is more hydrophilic and thus is trapped in the cell. Propidium iodide is excluded from healthy cells but can be taken up by cells with damaged cell membranes. In the cell, it fluoresces by intercalating into double-stranded nucleic acids. Moribund organisms exhibiting dual staining were scored as dead. Because the interior of empty cysts is readily accessible to exogenous propidium iodide if these structures contain residual cytoplasmic material including double-stranded nucleic acids, empty cysts were also scored as dead. The protocol, especially the potential breaking of disulfide linkages by glutathione treatment, apparently did not compromise cell membrane integrity to the extent that allows propidium iodide entry. Also, the metabolic functions remain intact, as demonstrated by sustained high ATP levels (10, 51) and the metabolic incorporation of radiolabeled precursors into P. carinii compounds (see below).
By evaluating the protocols used by different investigators (when described in detail), one can now gain insight into the purity of organism preparations. For example, if high-speed centrifugation (e.g., faster than 100 × g) was used throughout the procedure in the absence of a mucolytic agent, it is likely that the organism pellet contained lung surfactant lipid contaminants. If it is reported that a preparation contained only one mammalian host (rat) cell for 1,000 P. carinii cells counted, this represents twice as much host cell contamination (by volume) than the organisms (51). If nuclear DNA is analyzed, it should be kept in mind that a single rat lung cell has >500 times more nuclear DNA than does P. carinii (51). The organism preparations analyzed by other investigators may be of high purity. However, in general, adequate documentation with several criteria is lacking. Although useful qualitative data can be obtained by using less rigorously characterized preparations, certain quantitative data reported for such preparations should be questioned. Reports of lipid analyses in the literature now include studies performed on P. carinii isolated by (i) recovery of organisms present in BALF (78, 79), (ii) differential centrifugation of lung homogenates (94), (iii) homogenization of lungs followed by microfiltration (26, 48), (iv) homogenization of lungs in the presence of a mucolytic sulfhydryl agent followed by a series of low- and high-speed centrifugations and then by microfiltration (22–25, 32–34, 43, 51–56, 68, 91, 103), and (v) coculture of homogenates of P. carinii-infected lungs with one of a number of mammalian cell lines (13, 30, 61) followed by reisolation of cells not adherent to cells of the monolayer. Centrifugation of the culture supernatant is performed to recover suspended organisms (mainly trophozoites) (74–77). The organism preparation reisolated from mammalian cell cultures had a low SP-A content, comparable to that of the defined organism preparations described above (51); however, this preparation has yet to be thoroughly characterized with respect to other potential contaminants.
Different life cycle stages have been separated by counterflow elutriation (18) or gradient centrifugation (11) and by fluorescence-activated cell sorter (FACS) techniques (17). However, lipid analyses of subpopulation of P. carinii have yet to be reported.
In an earlier review of P. carinii biochemistry and metabolism (45), the paucity of available data and the lack of organism preparations that were well characterized by microscopic and biochemical markers precluded a critical evaluation of the literature. Thus, all available publications containing direct or indirect information on the biochemical nature of the pathogen were indiscriminately included. It has subsequently become more feasible to critique the literature.
Human-Derived P. carinii hominis
The procedures used by Sorice et al. for purifying P. carinii hominis were limited to differential centrifugation techniques (94). Apart from that study, only the analysis of P. carinii hominis-infected lungs (without isolation and purification of organisms) has thus far been reported (55). The purification of human-derived organisms is difficult because the organisms appear to be more adhesive to mucoid material and are seen mainly as large clusters in BALF. Only rarely do fresh autopsied human P carinii-infected lungs become available for research purposes; therefore, purification methods for fresh P. carinii hominis preparations are not well developed. For these reasons, elucidation of the biochemical nature of the human pathogen lags behind progress made on organisms from laboratory animal models.
LIPID COMPOSITION OF P. CARINII CARINII
In the lipid composition studies of organisms purified by the defined protocol, the lipids from lungs of normal, untreated rats and from the lungs of corticosteroid-immunosuppressed rats were analyzed and compared with those of the organisms (2, 22–25, 32, 34, 43, 52–56). Lipid analyses comparing the lungs of normal, untreated rats with those of corticosteroid-treated rats represent the only detailed studies available on the effects of steroid immunosuppression of rat lung lipids. Corticosteroid treatment was shown to result in several quantitative changes in the lipid and lung surfactant protein composition in rats not infected with P. carinii. Some reports in the literature do not include data on appropriate controls; others do. This review will focus on data obtained on P. carinii purified by the defined protocol detailed above, from which the most extensive data on the lipids of this organism have thus far been reported.
Fatty Acids of Total Cellular and of Total Extracted Lipids
Total cellular fatty acids include some that are not extracted by organic solvents. For example, fatty acids covalently linked to proteins are not normally extracted by neutral solvent systems. However, these would be released upon hydrolysis of intact cells and then recovered by organic solvents.
Fatty acid composition.
The fatty acid composition of total lipids extracted from freshly isolated organisms closely resembles that of total lipids extracted from the lungs of normal rats and immunosuppressed rat controls (45, 48, 54) (Table 1). A similar profile was also reported for the fatty acids of whole organisms isolated from rat lungs, cocultivated with the WI-38 cell line, reisolated, and then directly hydrolyzed and analyzed for total fatty acids (75). The culture media used for growth of mammalian cell lines included fetal bovine serum, which is known to contain fatty acids (with oleate being more prevalent than palmitate) (102). Following incubation and limited growth in vitro in mammalian cell culture media, the P. carinii total cellular fatty acid composition exhibited relatively higher proportions of oleate (31%) and relatively lower proportions of palmitate (32%) than in freshly isolated organisms (75). No fatty acid composition differences were detected in the WI-38 monolayer cells when the cells were grown with or without P. carinii organisms. It is not clear why differences in the relative proportions of oleate and palmitate in these samples were not detected, since organisms are expected to be attached to the monolayer cells (61, 63, 64, 81, 82). These results might mean that the fatty acid composition of adherent cells may differ from that of cells free in suspension or that only a few P. carinii cells were attached to the monolayer.
TABLE 1.
Fatty acid composition of P. carinii carinii lipidsa
| Fatty acid | Fatty acid content (%) in:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total lipids | Total ester-linked | Total amide-linked | Individual phospholipid classesb
|
||||||||
| LPC | SM | PC | PI | PS | PE | PG | CL | ||||
| 14:0 | 1.1 | 1.5 | 1.7 | 1.3 | 1.2 | 0.9 | 0.7 | 2.3 | 3.3 | 3.6 | 3.6 |
| 15:0 | 0.2 | 0.4 | 0.8 | 1.3 | 0.5 | 0.4 | 0.9 | 5.1 | 3.5 | 4.0 | 5.3 |
| 16:0 | 44.5 | 27.3 | 40.7 | 48.1 | 15.1 | 49.1 | 17.2 | 17.8 | 8.0 | 28.1 | 14.5 |
| 16:1 | 2.1 | 3.2 | 1.5 | 4.4 | 1.0 | 1.4 | 0.8 | 1.9 | 2.8 | 1.3 | 3.4 |
| 17:0 | 0.2 | 0.2 | 0.9 | 1.0 | 0.3 | 0.1 | 0.6 | 8.3 | 0.9 | 5.0 | 1.7 |
| 18:0 | 13.2 | 8.4 | 7.8 | 14.5 | 13.3 | 8.8 | 26.3 | 16.1 | 14.4 | 18.0 | 18.6 |
| 18:1 | 13.4 | 23.9 | 9.0 | 6.8 | 1.4 | 34.2 | 27.2 | 6.5 | 36.7 | 21.7 | 9.8 |
| 18:2 | 1.8 | 7.0 | 2.3 | 2.4 | 0.6 | 4.9 | 3.1 | TRc | 5.3 | 4.1 | 9.1 |
| 20:0 | 0.3 | 0.1 | 1.4 | 1.8 | 6.7 | 0.3 | 1.6 | 5.0 | 1.8 | 1.5 | 1.7 |
| 20:4 | 2.4 | 10.6 | 1.1 | 1.1 | 2.9 | 4.0 | 1.3 | 5.7 | 1.0 | 4.3 | 1.8 |
| 21:0 | 0.2 | NDc | 0.7 | 1.5 | TR | 0.3 | 2.0 | 11.2 | 4.6 | 1.6 | 3.9 |
| 22:0 | 1.7 | 0.1 | 4.6 | 1.8 | 16.7 | 0.4 | 2.3 | 2.0 | 1.1 | 1.1 | 2.7 |
| 22:1 | 0.2 | 1.4 | 0.9 | 4.1 | 0.8 | TR | 2.1 | 7.5 | 3.0 | 2.7 | 6.7 |
| 24:0 | 1.9 | 0.7 | 12.6 | 1.3 | 37.3 | 0.7 | TR | 2.9 | 1.1 | 3.2 | 0.4 |
| 24:1 | ND | ND | 7.0 | 2.3 | 5.4 | 0.4 | 1.0 | 4.6 | 1.5 | 0.8 | 0.9 |
| Othersd | 17.0 | 16.7 | 7.3 | 3.7 | 2.3 | 2.2 | 3.4 | 0.7 | 3.3 | 1.4 | 7.2 |
| S/Ue | 3.23 | 0.82 | 3.17 | 5.54 | 6.60 | 5.08 | 4.61 | 2.75 | 1.44 | 2.55 | 1.95 |
LPC, lyso PC; SM, sphingomyelin; PG, phosphatidylglycerol; CL, cardiolipin.
TR, trace; ND, not detected.
Sum of other minor fatty acids present in the gas chromatograms.
Saturation index (sum of saturated fatty acids/sum of unsaturated fatty acids).
cis-9,10-Epoxyoctadecanoic acid.
To date, only one fatty acid which is present in P. carinii and absent in rat lung controls has been identified (22, 54). This rare fatty acid, cis-9,10-epoxyoctadecanoic acid (Fig. 1), was found in total cellular fatty acids; its distribution among different lipid fractions and individual lipid classes has yet to be examined.
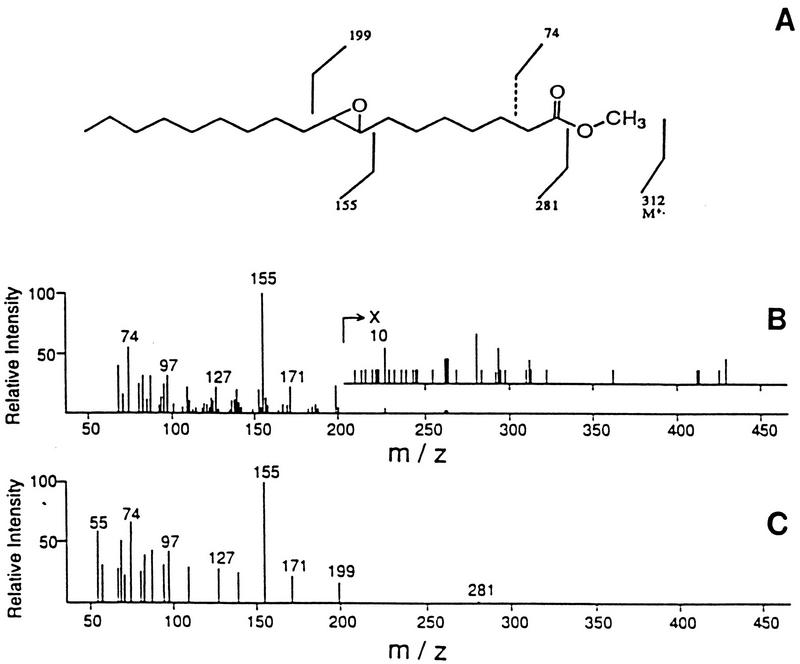
FIG. 1.
cis-9,10-Epoxyoctadecanoic acid was detected among P. carinii carinii fatty acids. (A) Structure of cis-9,10-epoxystearic acid. (B) Methyl ester derivative of the P. carinii epoxy fatty acid verified by GLC-MS. The abundant fragment at m/z 155 represents cleavage at the epoxide ring. (C) Authentic standard showing the same mass spectrum. Reprinted from reference 54 with permission of the publisher.
It is interesting that this fatty acid occurs in high concentrations in the uredospores of rust fungi (105, 106, 110, 111). It has been noted that the lipids of the rust fungi differ from those of other fungi by the presence of cis-9,10-epoxyoctadecanoic acid, the absence of ergosterol, and the presence of high concentrations of Δ7 sterols (see the section on sterols, below). Although the rust fungi are classified as basidiomycetes and are not considered closely related to P. carinii, some researchers have suggested that the rusts and Taphrina spp. have some common features (1, 62). Similar to the rusts and other lower fungi examined, Taphrina deformans does not contain ergosterol (113). However, the major sterol in T. deformans is brassicasterol (ergosta-5,22-diene-3β-ol); no Δ7 sterols were identified in this organism (112).
Neutral Lipids
The neutral-lipid fraction eluted by chloroform (CHCl3) during adsorption column chromatography (silicic acid; Unisil) includes free sterols, steryl esters, triglycerides, free fatty acids, free fatty alcohols, quinones, diglycerides, and monoglycerides. Quantitatively, the neutral-lipid composition of P. carinii carinii was distinct from those of both lung controls. The relatively high proportion of free fatty acids in P. carinii was observed previously in organisms isolated from BALF (78), but they probably had substantial surfactant adhered to their surfaces. Furthermore, the high levels of lyso PC (approximately 10%) reported in that study suggested that lipid degradation probably occurred during the isolation and preparation of organisms and/or during the handling of extracted lipids. Indeed, subsequent analysis of organisms obtained by the defined protocol showed lower lyso PC levels (2 to 6%) (32, 33). However, the relatively high free fatty acid concentrations in P. carinii reported previously (78) were confirmed (33). The relatively low triglyceride level of P. carinii carinii (compared to lung tissue and other cell types), suggested that fatty acids may be a significant carbon source for energy production for the pathogen (see the section on metabolism, below).
Quantitatively, the fatty acid compositions of individually isolated neutral-lipid classes did not differ dramatically from those isolated from rat lung controls (43, 54) (Table 2). The neutral lipids containing fatty acids (e.g., triglycerides and steryl esters) are generally thought to reside in cellular compartments other than membranes. The fatty acids in these P. carinii carinii neutral-lipid classes may be mainly those scavenged from the host as fatty acids or as part of complex lipids.
TABLE 2.
Compositions of the neutral-lipid fraction of P. carinii, whole lungs of normal untreated rats, and whole lungs of corticosteroid-immunosuppressed ratsa
| Lipid class | Lipid content (% by wt) in:
|
||
|---|---|---|---|
| P. carinii carinii | Lungs from normal untreated rats | Lungs from immuno- suppressed rats | |
| Monoglycerides | 0.4 | ND | 0.1 |
| Diglycerides | 2.8 | 0.5 | 0.6 |
| Free sterols | 43.7 | 26.8 | 29.1 |
| Fatty alcohols | 0.5 | 0.5 | 0.4 |
| Free fatty acids | 21.8 | 13.4 | 12.9 |
| Quinones | 0.3 | 0.4 | 0.6 |
| Triglycerides | 13.4 | 51.0 | 37.6 |
| Steryl esters | 17.0 | 7.4 | 18.7 |
Reprinted from reference 23 with permission of the publisher.
ND, not detected.
Sterols.
Polyene antibiotics bind to membrane sterols, and these complexes aggregate and form large pores in the membrane. These complexes are visible by electron microscopy of freeze-fractured preparations as a complex of large intramembranous particles. The surfactant digitonin also binds to free 3-β-hydroxysterols, forming similar particles visible by electron microscopy. Yoshikawa et al. examined filipin-treated (115) and digitonin-treated (116) P. carinii membranes and concluded that trophozoites contained more sterols in their surface membranes than did cysts.
The first direct biochemical evidence of relevance to the understanding of PCP therapy was the report that if P. carinii contained ergosterol, it was present at undetectable levels (45). Because ergosterol is the major sterol found in most fungi, this observation helped explain why polyene antibiotics such as amphotericin B are ineffective as anti-P. carinii drugs. Most commonly used polyene antibiotics have a higher affinity for ergosterol than for cholesterol (present in mammalian host cell membranes). The major sterol in P. carinii was found to be cholesterol (Fig. 2). It is generally believed that cholesterol is scavenged from the host lungs by the pathogen (since cultured cells utilize various organic material in the medium and parasites commonly take up and incorporate nutrients available in the host). In addition to cholesterol, low concentrations of campesterol (ergost-5-en-3-ol), β-sitosterol, and cholest-5-en-3-one were identified in P. carinii and in the lungs of normal rats (normal controls) and immunosuppressed P. carinii-uninfected rats (immunosuppressed controls) (52). It should be noted that the question whether P. carinii synthesizes or scavenges cholesterol and/or these other minor sterols has yet to be experimentally answered.
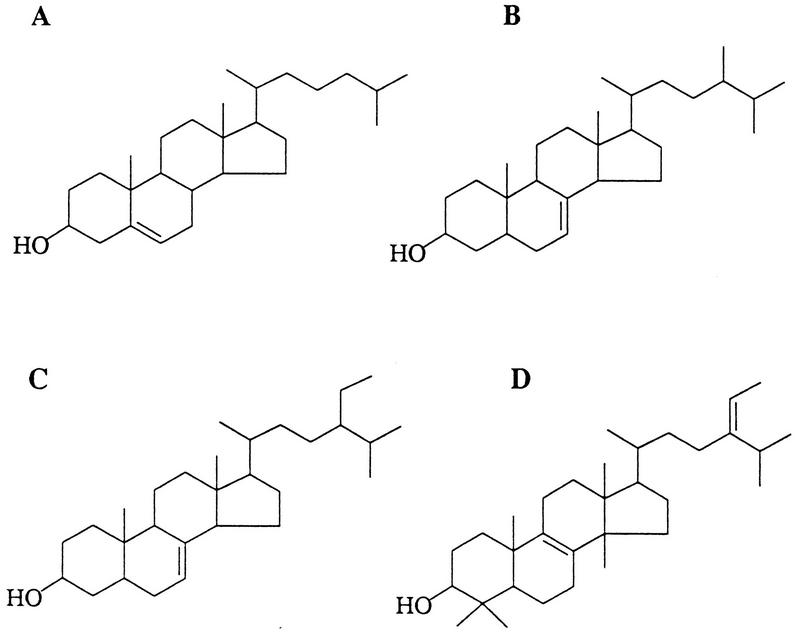
FIG. 2.
Sterols identified in P. carinii carinii organisms or P. carinii hominis-infected lungs. (A) Cholesterol, which is probably scavenged from the host lung, was the most abundant sterol present. (B) Fungisterol (ergost-7-en-3-ol) was among the most abundant of the P. carinii carinii-specific sterols. (C) Stigmast-7-en-3-ol was also present in high concentrations in P. carinii carinii. (D) A C32 sterol, pneumocysterol, was isolated from a human lung infected with P. carinii hominis. Its structure, elucidated by MS and NMR spectroscopy, indicated that a C2 (ethylene) group was present at C-24 of the side chain of lanosterol.
The first evidence that P. carinii contained sterols distinct from those of the host was the detection by high-performance liquid chromatography (HPLC) and gas-liquid chromatography (GLC) of components that were absent in the normal and immunosuppressed rat lung controls (24). Subsequently, at least 24 sterols were resolved by capillary GLC analyses (52). The structural identification of several sterols were elucidated by mass spectrometry (MS) on the basis of elemental compositions, relative molecular masses, and fragmentation patterns (52) (Table 3). The position of the double bond in a sterol molecule can be determined by GLC-MS analysis if the sterol contains only a single double bond. However, if there are two or more double bonds in a sterol molecule, definitive assignment of double-bond positions requires verification by showing that the GLC elution time and mass spectra are the same as those of an authentic standard. Some P. carinii sterols were identified by direct comparison with authentic standards (cholesterol, lanosterol, desmosterol, campesterol, stigmasterol, and β-sitosterol). No P. carinii sterols had mass spectra corresponding to those of authentic ergosterol or brassicasterol (52).
TABLE 3.
Total sterols of P. carinii cariniia
| Sterol | RRTcholb | Wt (%T)c | Wt (%Pc)d |
|---|---|---|---|
| Cholesterol (cholest-5-en-3β-ol) | 1.000 | 78.4 | |
| Unknown | 1.028 | TR | TR |
| Cholest-7-en-3-ol | 1.040 | 0.6 | 4.0 |
| Unknown | 1.060 | 0.1 | 0.6 |
| Desmosterol (cholesta-8,24-dien-3-ol) | 1.074 | 0.2 | 1.29 |
| Unknown | 1.097 | TR | TR |
| Unknown | 1.138 | 0.6 | 4.0 |
| Campesterol (ergost-5-en-3-ol) | 1.169 | 3.7 | |
| Unknown | 1.192 | 0.2 | 1.6 |
| Cholest-5-en-3-one | 1.216 | 1.5 | |
| Unknown | 1.226 | TR | 0.2 |
| Ergosta-dien-3-ol | 1.269 | 1.2 | 8.6 |
| Fungisterol (ergost-7-en-3-ol) | 1.291 | 4.5 | 31.2 |
| Unknown | 1.338 | 0.1 | 0.8 |
| β-Sitosterol (stigmast-5-en-3-ol) | 1.370 | 2.1 | |
| Stigmasta-trien-3-ol | 1.413 | 0.9 | 6.5 |
| Lanosterol (cholesta-7,24-dien-3-ol) | 1.438 | 0.1 | 0.5 |
| Stigmasta-dien-3-ol | 1.471 | 0.3 | 2.3 |
| Stigmast-7-en-3-ol | 1.515 | 1.8 | 12.9 |
| Stigmasta-dien-3-ol | 1.568 | 3.5 | 24.2 |
| Unknown | 1.600 | 0.1 | 0.4 |
| Unknown | 1.642 | TR | 0.2 |
| Unknown | 1.937 | 0.1 | 0.7 |
Adapted from reference 52 with permission of the publisher.
RRTchol, relative retention times with respect to that of cholesterol.
Based on all GLC peaks eluting after cholesterol.
Excluding sterols also found in rat lung controls.
The striking feature of the P. carinii-specific sterols was the presence of several components with a double bond at C-7 (Δ7) of the sterol nucleus, such as fungisterol (ergost-7-en-3-ol). Furlong et al. (26) later confirmed the absence of ergosterol and the identities of some of these sterols; other conclusions differed from those of the earlier report (52). For example, although both groups analyzed authentic brassicasterol (ergosta-5,22-dien-3-ol), Furlong et al. (26) reported its presence in P. carinii whereas Kaneshiro et al. did not (52). The identification of brassicasterol in P. carinii should be regarded as tentative. Also, the former group assigned double-bond positions to some P. carinii-specific polyunsaturated sterols (26) without GLC-MS comparisons with authentic standards, which Kaneshiro et al. (52) did not. The double-bond position assignments of other P. carinii polyunsaturated sterols which were not verified by GLC-MS of authentic standards should also be regarded as tentative.
The detection of C-24-alkylated sterols in P. carinii has identified an attractive drug target because mammals are unable to add an alkyl group at that side chain position. In other systems, the addition of methyl groups at this site on the molecule has been shown to occur via sterol methyl transferases with S-adenosylmethionine (SAM). Therefore, specific inhibition of sterol C-24 alkylation reactions would be expected to affect the pathogen but not the host. Inhibitors of C-24 alkylation have been examined as potential chemotherapeutic agents against kinetoplastid flagellate parasites such as Leishmania spp. and Trypanosoma cruzi (37, 107, 108). It has been suggested that much of the bulk of biomembrane bilayers can be formed by a number of sterol species, including scavenged sterols from the host or environment (37, 86). However, some parasites continue to biosynthesize their own distinct sterols, often in small amounts. It has been proposed that certain membrane functions (e.g., enzymes, pumps, and signal transduction activities) cannot function unless molecules participating in these functions are properly associated with sterols having the precise conformations afforded by the parasite sterols (37). Thus, there are at least two roles for sterols in biomembranes: the formation of the bulk structure of the bilayer, and involvement in activities for which host sterols cannot substitute. The parasite sterols that fulfill the three-dimensional requirements for these special functions have been described as “metabolic” sterols (37).
If P. carinii requires its own sterols for viability and proliferation, inhibition of sterol synthesis by the organism could clear PCP. Further development of sterol C-24 inhibitors would seem warranted because currently available compounds (e.g., 24,25-epiminolanosterol [70, 73]) that inhibit these reactions appear to insert into and perturb host cell membranes. Also, some compounds exhibit dual action, inhibiting both C-24 alkylation and demethylation of lanosterol or other reactions involving the sterol nucleus. Since lanosterol is an intermediate in the cholesterol biosynthetic pathway, host sterol metabolism may be affected by some of these drugs.
The composition of the free sterol fraction of P. carinii resembles that of the total sterols of the organism because most sterols in the organism are not esterified (52). However, the sterols in the steryl ester fraction are different. They have not been subjected to rigorous structural analyses, since this fraction is much smaller than the free sterol fraction. The steryl ester fraction may contain higher concentrations of cholesterol, which would be consistent with the notion that the organism scavenges host cholesterol, and, as in many other cell types examined, steryl esters serve a storage function (not part of membrane bilayers) in these cells. The P. carinii-specific sterols may be concentrated in membrane bilayers rather than lipid storage vesicles in the parasite.
Quinones (coenzyme Q).
Ubiquinone molecules consist of a benzoquinone ring and a polyprenyl chain. The number of five-carbon (isopentyl) groups in the chain forms the basis for homolog designation such as CoQ8, CoQ9, and CoQ10, which has been used as a taxonomic criterion. When P. carinii ubiquinones were isolated by thin-layer chromatography (TLC) and then resolved into individual homologs by HPLC, CoQ10 was the major ubiquinone homolog (23) (Fig. 3). CoQ9 was also detected, but it was present at about half the concentration of CoQ10. In contrast, only CoQ8 and CoQ9 were detected in the lungs of normal and immunosuppressed rats. The structural identities of the rat and P. carinii ubiquinone homologs were verified by comparisons with authentic standards by using fast atom bombardment MS.
FIG. 3.
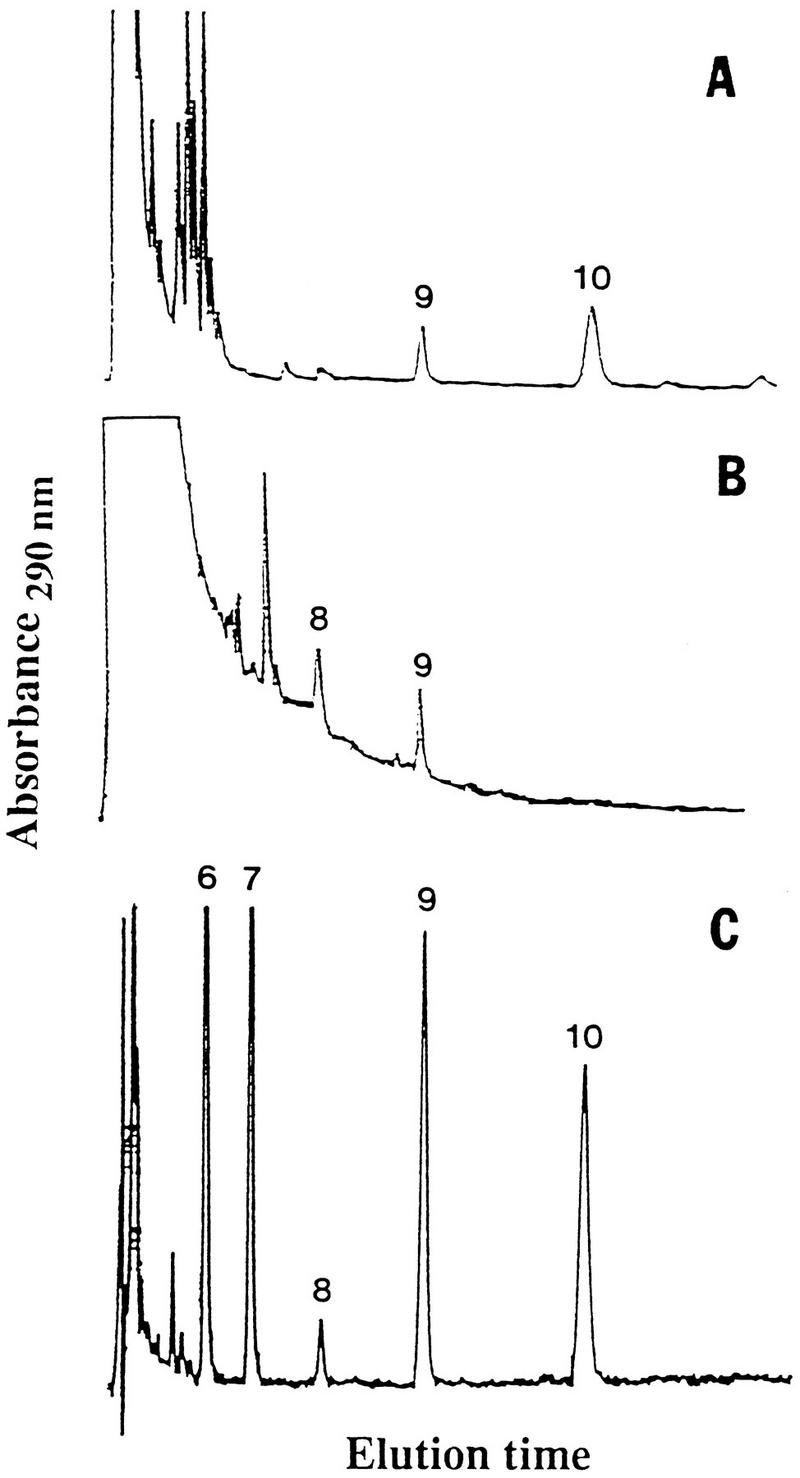
CoQ homologs separated by HPLC. (A) The ubiquinone homologs CoQ10 and CoQ9 detected in P. carinii. (B) Absence of CoQ10 in normal rat lungs. The presence of CoQ8 and CoQ9 was verified by GLC-MS. (C) Ubiquinone standards separated by HPLC. Reprinted from reference 23 with permission of the publisher.
Atovaquone (566C80), an antimalarial naphthoquinone drug, is used clinically to treat PCP (19, 35, 39). In Plasmodium spp., nanomolar concentrations of atovaquone inhibit dihydroorotate dehydrogenase activity, which results in blocking of ATP and pyrimidine synthesis. The drug inhibits P. carinii oxygen consumption, but concentrations in the high micromolar ranges are needed to inhibit the dihydroorotate dehydrogenase of P. carinii (40, 41). Apparently, as an analog of ubiquinone, atovaquone inhibits P. carinii electron transport chain function (35). Several naphthoquinone drugs that are analogs of ubiquinone have broad anti-parasite activity (21, 114).
Polar Lipids
Neutral sphingolipid-glycolipid fraction.
The neutral sphingolipid-glycolipid fraction is commonly obtained by Unisil adsorption column chromatography of the acetone eluate following the removal of the neutral-lipid fraction with chloroform. However, because several components are not completely eluted with acetone, chloroform-methanol (95:5, vol/vol) is used to obtain the “neutral sphingolipid fraction.” The phospholipid cardiolipin also elutes in this fraction but will be discussed together with phospholipids below.
The free ceramides of P. carinii carinii (25) have been identified by TLC. The long-chain bases (LCB) dihydrosphingosine and phytosphingosine plus two additional unidentified components were detected in the ceramides following acid hydrolysis. Sphingosine, the major LCB found in mammalian sphingolipids, was not detected. Further studies, especially on the unidentified LCB, may provide insight into whether sphingolipids are involved in the pathogenesis of PCP. Ceramides and LCB analogs (mycotoxins such as fumonisins) have inflammatory or cytotoxic effects on cells and modulate signal transduction events (36, 69, 88).
Glycolipids that partition into the organic phase during biphasic purification of lipids were analyzed by TLC. Preliminary results indicate that this fraction from P. carinii contained at least five glycolipids whereas both types of rat lung controls contained only four (2). At least one P. carinii glycolipid component exhibited a migration rate that differed from those of rat controls.
Phospholipids.
Since phospholipids are generally found in cells within membrane bilayers, they are expected to be readily distinguishable from their counterparts in the host lung tissue. The phospholipid of the organism contains relatively lower proportions of PC and greater proportions of phosphatidylinositol (PI) and lyso PC than do those of the lungs of immunosuppressed rat controls (32, 33) (Table 4). Earlier analyses of phospholipid profiles obtained with whole P. carinii-infected lung lipids or organisms isolated from BALF were reported (78, 90). Quantitative data were not reported for some P. carinii phospholipids (78); hence, it is difficult to compare data from the earlier study with those obtained from the defined organism preparations.
TABLE 4.
Phospholipid class composition of P. carinii carinii and rat lung controlsa
| Lipid class | Phospholipid content (% phosphate) in:
|
||
|---|---|---|---|
| P. carinii carinii | Rat lung controls
|
||
| Normal | Immunosuppressed | ||
| Phosphatidylcholine | 39.3 | 46.6 | 49.9 |
| Phosphatidylethanolamine | 14.5 | 20.4 | 12.3 |
| Phosphatidylinositol | 11.3 | 2.3 | 4.0 |
| Sphingomyelin | 10.0 | 11.7 | 8.9 |
| Phosphatidylserine | 9.5 | 6.8 | 8.3 |
| Lyso phosphatidylcholine | 6.3 | 2.4 | 4.9 |
| Phosphatidylglycerol | 5.6 | 4.4 | 6.6 |
| Cardiolipin | 3.9 | 5.4 | 5.2 |
Reprinted from reference 33 with permission of the publisher.
The fatty acid composition of the total phospholipid fraction of P. carinii was characterized by a lower saturation index (sum of saturated fatty acids/sum of unsaturated fatty acids) (Table 1) than those for normal and immunosuppressed rat controls (34, 43). The total phospholipid fraction of the organism had lower 16:0 and higher 18:1 fatty acid concentrations than did rat controls.
The fatty acid profiles of individually isolated phospholipid classes of the organism also differed (Table 1) (34). Sphingomyelin contained mainly saturated fatty acids compared to the glycerophospholipids, and its fatty acid composition did not differ from that of the sphingomyelin of rat lung controls. Of the major phospholipid classes analyzed, phosphatidylethanolamine (PE) had the lowest 16:0 content and its overall fatty acid composition was characterized by high levels of unsaturated fatty acids. The saturation index of P. carinii PE was 0.62, which was the lowest for all major phospholipids of the organism or of the lungs from normal and immunosuppressed rat controls.
The phospholipids of P. carinii did not contain detectable levels of phosphonolipids (26, 33). The phospholipids were analyzed by differential hydrolysis procedures in which inorganic phosphate was released from total lipids and compared with the amount of inorganic phosphate released only from lipids with phosphoryl (P—O—C) bonds (33). If phosphonolipids with phosphonyl (P—C) linkages were present, differences between the two values would be detected. In that study, 31P nuclear magnetic resonance (NMR) analyses of P. carinii phospholipids were also performed as independent assays for detecting P—C bonds. P—C linkages were not detected by either method.
The P. carinii glycerophospholipids were also analyzed for the presence of ether linkages. Glyceryl ethers such as platelet-activating factor (acetyl-O-alkyl PC) are known to be potently bioactive (93). These lipids are characterized by a fatty alcohol linked via an ether (C—O—C) bond, which is stronger than an ester bond by which fatty acids are linked to glycerol. Four species of glyceryl ethers were identified: 1-O-octadecyl glycerol, 1-O-octadec-9-enyl glycerol, 1-O-hexadecyl glycerol, and 1-O-hexadec-9-enyl glycerol; platelet-activating factor was not detected (56). The four glyceryl ethers were also present in the lungs of rat controls.
Lipids Associated with Protein and Carbohydrate Macromolecules
Several proteins are now known to be anchored to membrane bilayers by lipid molecules. Lipidated components of biomembranes are known to function in transmembrane signaling events and can activate G proteins and other cytoplasmic proteins during physiological responses to a variety of extracellular stimuli (8). It has been demonstrated that the protein moiety of the P. carinii major surface glycoprotein (MSG; glycoprotein A [gp120]) is encoded by a number of different genes (98). Because the well-characterized variant surface glycoproteins of African trypanosomes and the MSGs of other organisms have inositol lipid anchors, the MSG of P. carinii may have a similar kind of membrane attachment. There is, as yet, no direct biochemical evidence for a PI-type anchor of this family of surface membrane proteins.
Lipidation of membrane proteins also includes prenylation and fatty acylation, which may help anchor proteins into the bilayer and help maintain their functional conformations. Genetic disruption of protein myristoylation inhibits proliferation and viability of fungal cells (42). Hence, lipid modification of proteins has been suggested as an attractive drug target. Several gel electrophoresis-purified glycoprotein bands were analyzed for the presence of lipids. Preliminary results indicated the presence of large amounts of fatty acids, especially 16:0, 18:0, and 18:1, covalently linked to the MSG. In contrast, the purified 56-kDa protein had no detectable fatty acids (57). The 56-kDa protein was thus an appropriate control, which ruled out possible contamination by fatty acids not covalently linked to the purified MSG.
Lipophosphoglycans (LPG) are macromolecules that have the solubility properties of carbohydrates and lack a peptide moiety and phosphonyl linkages (47, 104). These lipidated molecules are efficiently extracted from whole cells with water-saturated butanol (46, 59). Evidence for the presence of LPG in P. carinii was suggested by the use of monoclonal antibodies directed against a Leishmania spp. LPG carbohydrate repeated sequence. The antibody bound to P. carinii surfaces and to a 46-kDa band in a butanol-saturated water extract resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (27). Immunolabeled cells were extracted, and the bulk of the immunolabeled material was eluted from phenyl-Sepharose columns with organic solvents. The major fatty acid associated with this putative P. carinii LPG fraction was 16:0.
LIPIDS OF P. CARINII HOMINIS
Much less information is available on the lipids of organisms infecting human lungs. Lipids extracted from organism preparations prepared by differential centrifugation from frozen, homogenized, and trypsinized infected human lungs were analyzed (94). Monosialoganglioside GM1 and disialoganglioside GD1a were tentatively identified in organism preparation extracts. Identifications were made on the basis of TLC migrations similar to those of authentic standards, followed by sialic acid-positive staining with resorcinol and sugar-positive staining with α-naphthol. These two TLC bands were not detected in analyses of P. carinii-free lungs processed by the same protocol. Rabbit polyclonal antibodies directed against P. carinii hominis bound to gangliosides GM1 and GD1a as shown by dot-blot immunoassays. Binding to these lipids was reduced (GM1, 25% of control; GD1a, 5% of control) but not abolished after the antibody preparation was preabsorbed with GM1 and GD1a. Structural analyses (e.g., by MS) of these gangliosides have yet to be reported.
Sorice et al. (94) also separated P. carinii hominis antigens by nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then incubated Western blots of the gels with polyclonal anti-GM1 antibodies. The antibodies bound to bands equivalent to migrations of 200- and 55-kDa proteins, which were major bands present in P. carinii carinii antigens when analyzed under nonreducing conditions (31). These are probably similar, or related, to Triton-insoluble membrane complexes isolated from other cell systems. The investigators concluded that these bands might include ganglioside micelles, because bands with similar migrations were observed in preparations of GM1 and GD1a in an aqueous buffer solution. The investigators did not perform anti-GM1 antibody binding assays on P. carinii hominis material or the ganglioside micellar material separated under reducing conditions. Furthermore, it was not shown that the commercial anti-GM1 antibodies used fail to bind sulfated gangliosides or other glycolipids. Therefore, their observations could mean that the proteins, especially those within the 200-kDa band, may be covalently linked to glycolipids (e.g., sulfated gangliosides). If these complexes were held together via disulfide bonds, they would be expected to remain intact in the absence of reducing agents during sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Also, the possibility that these are host contaminants cannot yet be ruled out since the organism preparations were of undefined purity. Sialic acid was not consistently detected in analyses of cyst wall material isolated from P. carinii carinii (16). There are currently no data from P. carinii hominis or P. carinii carinii indicating that the organism is capable of synthesizing sialic acid.
Recently, the C31 sterol euphorbol and a rare C32 sterol (which was given the trivial name pneumocysterol) were detected in lipids extracted from lungs infected with P. carinii hominis (55). The pneumocysterol structure was demonstrated by GLC-MS and NMR spectroscopy of the sterol, which was purified by preparative GLC (Fig. 2). Several P. carinii-specific Δ7 sterols previously identified in rat-derived organisms were also present. Whether P. carinii hominis is the source of the C31 and C32 sterols remains to be demonstrated. Humans do not synthesize C-24-alkylated sterols, but it has not been ruled out that these rare sterols were made by other organisms coinfecting the lungs.
LIPID UPTAKE AND METABOLISM
The uptake of fatty acids and complex lipids by P. carinii has been demonstrated by using fluorescent substrates and observing cells by fluorescence optics microscopy or evaluating them by FACS procedures (15, 17, 28, 91). Quantitative data on fatty acid uptake have been obtained using radiolabeled substrates (76, 77). These studies support the idea that the pathogen scavenges host lipids, which are abundant in alveolar lung surfactant of the host. As discussed above, phospholipids and proteins in lung surfactant avidly bind to P. carinii surfaces; fluorescence microscopy with lipid analog probes demonstrated that exogenous lipids not only are adsorbed but also are readily internalized. Furthermore, several radiolabeled lipids and lipid precursors incubated with isolated organisms have now been shown to be transported and metabolized (25, 76, 77, 79).
Fluorescent Lipid Analogs
Fluorescent lipid analogs are used for microscopic visualization of specific lipids within cells because they emit light in a hydrophobic environment, such as membrane bilayers. Fluorescent probes inserted into the head group of lipid molecules make the molecule larger than naturally occurring lipids. Complex lipid analogs with a short-chain fatty acid linked to a fluorescent group also do not have the same precise structures as natural compounds. Although these analogs may not insert into membrane bilayers exactly as those synthesized by the organism, and although some organisms cannot metabolize some analogs, these compounds have proved to be powerful reagents for monitoring the translocation of lipids within cellular compartments.
The C-2 fatty acid-labeled analog 1-palmitoyl-2-(N-4-nitro- benzo-2-oxa-1,3-diazole)-aminocaproyl PC [(palmitoyl C6- NBD)-PC; C6-NBD-PC] and the head group-labeled probe, N-(Texas Red sulfonyl)PE (N-Rh-PE), were used to evaluate the cyst wall structure of P. carinii organisms isolated without a reducing agent (15). After a 20-min incubation at 2°C, the organisms were washed and viewed under fluorescence optics. The lipid analogs labeled trophozoites but not mature cysts. Trophozoite cytoplasm was clearly and intensely fluorescent. Following mild Zymolyase digestion, the cysts were also labeled with these fluorescent lipids. Differential labeling of the two major life cycle stages enabled the development of a technique for isolating cysts by (palmitoyl C6-NBD)-PC labeling and FACS separation (17).
A number of fluorescent lipid analogs of fatty acids and complex lipids were also used to microscopically document the internalization of lipids into the organisms (91). The compounds used in these studies included a number of NBD-tagged (Table 5) and pyrene-tagged lipids (68, 91). These studies were done with organisms isolated by the defined glutathione protocol. The lipid analogs were presented to P. carinii incorporated into either liposomes or micelles. Because fetal bovine serum contains lipids and phospholipase activity (46, 97), the medium selected for the incubations was serum-free RPMI 1640. After the organisms were incubated with the fluorescent probe for 30 min at 2°C to allow insertion of the probe, they were washed and placed at 37°C for 60 min to permit translocation and metabolism of the lipid. Some liposomes containing the lipid probe appeared to remain adsorbed to the surface of the organisms, as suggested by fluorescence in intense punctate patterns, unlike the even fluorescence seen in cytoplasmic components. The punctate surface fluorescence was not removed by the washing procedure, suggesting that insertion of one or more liposome components occurred. In these studies, experiments were not performed to distinguish between adsorption and insertion, which can be examined by adding liposomes without labeled lipids to the washed cells. Removal or reduction of the punctate surface fluorescence would suggest that only adsorption was involved at that stage. After incubation at 37°C, the cells and bathing medium were analyzed for fluorescence intensities. Furthermore, extracted lipids were analyzed by TLC to evaluate the metabolic fate of the fluorescent lipid.
TABLE 5.
Insertion and degradation of fluorescent lipid analogs by P. carinii cariniia
| Fluorescent lipid analog | Insertion of lipid analog | % Degradedb |
|---|---|---|
| C6-NBD-PA | + | 50 |
| C6-NBD-PC | + | 59 |
| C6-NBD-PE | + | 40 |
| C6-NBD-PG | + | 52 |
| C6-NBD-ceramide | + | 66 |
| C6-NBD-fatty acid | − | |
| C12-NBD-fatty acid | + |
Organisms were isolated by the glutathione defined protocol. The probe was inserted by incubation at 2°C for 30 min. After removal of noninserted lipid, translocation and metabolism were allowed to progress at 37°C for 60 min.
After incubation at 37°C, fluorescence (relative fluorescence units) in the bathing medium was compared to that associated with the organisms. Analysis by TLC of the medium demonstrated that the fluorescence was in the free fatty acid fraction.
Organisms became labeled with most of the compounds tested, as determined by observation under fluorescence optics (Table 5). Trophozoites exhibited much greater fluorescence than did cysts, and their cytoplasmic compartments were clearly labeled even before incubation at 37°C. Thus, the lipid probes were not only inserted into a hydrophobic environment at the organism’s surface (and therefore exhibited fluorescence) but were also readily internalized. Free C6-NBD-fatty acid did not insert into the surface membrane of P. carinii. In contrast, C12-NBD-fatty acid inserted into both cysts and trophozoites.
After incubation at 37°C, the surfaces of the intracystic bodies in some cysts were highly fluorescent, but their cytoplasm was not as highly labeled. However, in other cysts, the cytoplasm of intracystic bodies was clearly labeled. The probe could gain direct access to sites within the cyst if the cyst wall were damaged. Indeed, the internal contents of empty cysts (assumed to result from excystation) were readily labeled. The integrity of the cyst wall of organisms labeled with the fluorescent lipid was not evaluated, but independent viability assays of these organism preparations suggest that most mature cysts were alive. Thus, it is highly probable that a variety of lipids translocate into the cytoplasmic compartments of cysts and intracystic bodies, but translocation is slower than into trophozoites.
C12-NBD-fatty acid was not incorporated into P. carinii phospholipid under these experimental conditions. No new phospholipids were detected by TLC analysis of lipids extracted from organisms incubated with any of the NBD-labeled phospholipids and NBD-ceramide examined. This indicated that very little if any metabolism of these analogs occurred during the experimental period. However, fluorescent fatty acids were present in the washing solution of organisms incubated with fluorescent phospholipids. About half of the fluorescence that was associated with the organism at 2°C was subsequently lost during a 60-min incubation at 37°C (Table 5), indicating potent phospholipase activity. The cellular localization of the phospholipase A activity responsible for this degradation was not examined.
In a more recent study, P. carinii was incubated at 37°C for 1 h with different concentrations of the fluorescent fatty acid analog Bodipy-C12 and then washed with a balanced salt solution (28). Fluorescence associated with the cells, measured by flow cytometry or fluorimetry, exhibited saturation kinetics. Fluorescence was observed in lipids other than free fatty acids (phospholipids and other components of the neutral lipid fraction), suggesting that the analog was metabolized and incorporated into complex lipids. However, data demonstrating the reported observations were not shown.
In the same study, the investigators demonstrated that A549 lung epithelial cells in culture also took up Biodipy-C12 (28). After a 1-h incubation with the labeled fatty acid and extensive washing, cells in the monolayer exhibited a punctate fluorescence pattern on their surfaces. The pattern was similar to that observed in experiments on P. carinii with some NBD analogs (described above). After overnight incubation, diffuse fluorescence was observed in the cytoplasm. An experiment was performed to examine whether lipid was transferred from the A549 cells to P. carinii (28). The A549 cells were labeled for 1 h and then washed. Organisms were immediately added, and the monoxenic culture was incubated overnight. Unattached cells suspended in the medium were removed. The cells attached to the A549 cells were detached by treatment with the divalent cation chelator EDTA and were recovered from the supernatant. More fluorescence was found associated with P. carinii organisms that had been attached to the monolayer than with those that were suspended in the medium. It was concluded that lipid transfer occurred. However, since the fluorescence pattern on the A549 cells after a 1-h incubation was punctate, adsorption of liposomes or micelles containing Bodipy-C12 onto the A549 cell surface cannot be ruled out. The uptake by P. carinii of the labeled fatty acid from adsorbed or partially inserted liposomes or micelles on the A549 cell surfaces may have occurred. The conclusion that lipid transfer occurred would have been more convincing if the labeled A549 cells were incubated with label-free liposomes or micelles before organisms were added. Experiments on the transfer of lipids from A549 cells to P. carinii by using unlabeled P. carinii and A549 cells into which label had been clearly incorporated (cells previously incubated overnight and exhibiting intracellular labeling) were apparently not performed.
The ability of EDTA to detach organisms from the A549 monolayer cells suggests that this mammalian cell-pathogen interaction may differ from the situation in the lungs. The adhesion appears to involve mainly ionic interactions that were disrupted by the chelator. The studies on sulfhydryl reagents such as glutathione (described above) suggested that breaking of disulfide linkages is important in the detachment of organisms from the lung epithelium.
Radiolabeled Lipids and Lipid Precursors
Fatty acids.
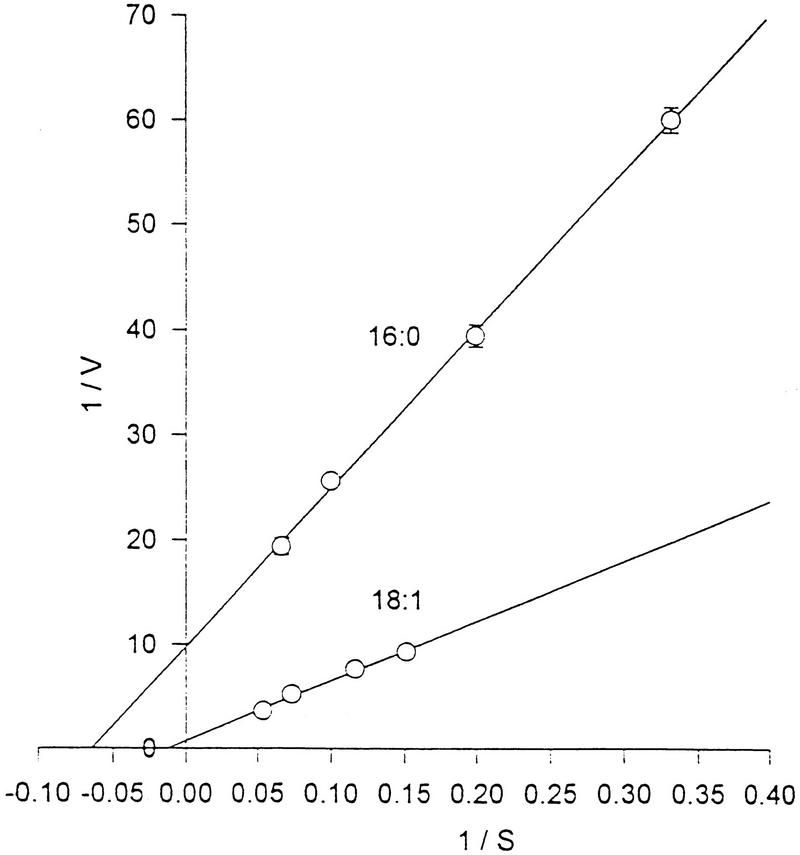
The uptake of 16:0, 18:0, 18:1, and 18:2 fatty acids by rat-derived P. carinii was measured by incubation of the organisms with a radiolabeled fatty acid in a complete cell culture medium (77). Uptake rates at different substrate concentrations resembled first-order kinetics (Fig. 4). Of the four fatty acids, the rate of oleic acid uptake was the highest, consistent with the observation that the oleic acid concentration increased at the highest rate when cells were isolated from rat lungs and placed into a complete cell culture medium with the mammalian cell monolayer. Fatty acid uptake was inhibited by primaquine, a known anti-P. carinii drug. Although no data were shown, it was reported that radiolabeled fatty acids were incorporated in vitro primarily into P. carinii phospholipids (77). Preliminary results on fatty acid metabolism indicated that [14C]16:0 stimulated oxygen uptake and was converted to 14CO2 (74, 76). That the organism transports and metabolizes 18:1 was also demonstrated by the in vitro metabolic radiolabeling of the P. carinii-specific fatty acid cis-9,10-epoxystearic acid (44).
FIG. 4.
Uptake rates of the radiolabeled fatty acids palmitic acid (16:0) and oleic acid (18:1) by P. carinii. The rates resemble first-order kinetics. Reprinted from reference 77 with permission.
Isoprenoids.
The initial evidence for de novo biosynthesis of sterols by P. carinii was the detection of incorporation of the radiolabeled sterol precursors mevalonic acid and squalene into several sterol fractions separated by HPLC (24). Furthermore, the enzyme HMG-CoA reductase, which catalyzes the formation of mevalonic acid from 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA), was detected in P. carinii organism preparations (54). HMG-CoA reductase activity is usually undetectable or present in only small amounts in lung tissue. This was verified by assays on homogenates of lungs from normal and immunosuppressed rats. Hence, the activity observed in purified P. carinii preparations can be attributed to the organism. Lovastatin, a specific inhibitor of HMG-CoA reductase, was effective against the P. carinii enzyme activity, with a 50% inhibitory concentration of 4 nmol (53). Contini et al. (12) reported that the allylamine drug terbinafine, which inhibits biosynthesis of sterols at the squalene epoxidase step, was active against P. carinii. This observation provides further evidence for de novo sterol biosynthesis in the pathogen.
The existence in P. carinii of a branch pathway of isoprenoid biosynthesis leading to polyprenyl group formation was demonstrated by the incorporation in vitro of radiolabeled mevalonic acid into the ubiquione band resolved by TLC (23). It is known that the aromatic ring of ubiquinone is synthesized via the shikimic acid pathway in other systems. The pentafunctional arom gene that codes for much of the shikimic acid pathway was cloned from P. carinii (3). This work suggested that the organism was capable of de novo synthesis of precursors of a number of mammalian essential nutrients (e.g., aromatic amino acids, folic acid, and ubiquinone). Recently, the de novo syntheses of both the polyprenyl chain and the aromatic ring of ubiquinones were studied in detail with radiolabeled precursors (103). Mevalonic acid, p-aminobenzoic acid, shikimic acid, and tyrosine incorporated in vitro into P. carinii ubiquinones. Since CoQ10 was radiolabeled in vitro, these studies demonstrated that this homolog was synthesized by the organism. P. carinii may have a full complement of biosynthetic pathways involved in isoprenoid metabolism.
Phospholipid head groups.
Ethanolamine is the direct precursor of the head group of PE, and choline is the direct precursor of PC and sphingomyelin. Direct incorporation in vitro of radiolabeled ethanolamine and choline into PE and PC, respectively, has been demonstrated (25, 79), but the incorporation of choline into sphingomyelin was not observed (25). From studies of other systems, it is known that PE can be metabolized to PC by methylation of the glycerophospholipid head group. The detection of radiolabeled PC following incubation of P. carinii organisms with radiolabeled ethanolamine demonstrated that the pathogen is capable of this conversion. The radiolabeled head group precursors serine and inositol were also incorporated in vitro into P. carinii phosphatidylserine (PS) and PI, respectively (25). It is also known that the head group of PS can be decarboxylated, resulting in the conversion of PS to PE. Purified P. carinii organisms incubated with radiolabeled serine had radioactive PE, suggesting that P. carinii is also capable of this phospholipid head group conversion.
Sphingolipids.
The biosynthesis of sphingolipids involves the formation of LCB, which is initiated by a condensation reaction that occurs most commonly between serine and palmitoyl-CoA. The formation of ceramides involves the addition of a fatty acid via the serine nitrogen (amide linked). In addition to the incorporation of serine into P. carinii PS, serine was incorporated in vitro into P. carinii sphingolipids (25). The free ceramide fraction, resolved by TLC, was shown to be radioactive by autoradiography. This fraction was recovered and hydrolyzed, and the LCB components were separated by TLC. Dihydrosphingosine and especially phytosphingosine were radioactive. Two other unidentified components were also radioactive. Conversion of free ceramides to sphingomyelin (incorporation of radiolabeled serine into sphingomyelin) was examined, but was not detected in those studies.
HOST-PATHOGEN INTERACTIONS
Phospholipases and Changes in Lung Surfactant
In 1983, Kerbaum et al. examined the composition of BALF of normal rats, cortisone-immunosuppressed control rats, and immunosuppressed rats with PCP (58). The BALF analyzed had been centrifuged at speeds presumably high enough to remove cellular material and particulate lung surfactant. The researchers found decreases in both the phospholipid content and the phospholipid/protein ratio (from 0.50 to 0.18). They interpreted their findings as a possible basis for the respiratory distress accompanying the infection. To find an explanation for the reduced BALF phospholipid, they measured phospholipase activity with PE as a substrate. They found a decrease in enzyme activity in cortisone-treated BALF controls. To compare their values with those of another study (see below), the values have been recalculated here and are expressed in nanomoles of PE hydrolyzed per minute per milligram protein. The activity in immunosuppressed BALF controls was 6.29 nmol/min/mg, and that in normal BALF controls was 9.56 nmol/min/mg. However, BALF from rats with PCP had a higher activity (15.8 nmol/min/mg) than did both controls, indicating that the reduction in the amount of phospholipids resulted from increased phospholipase activity in the lungs of rats with PCP. The addition of a platelet activator preparation previously shown to activate inactive phospholipases increased enzyme activity in BALF from both controls but not in BALF from P. carinii-infected rat lungs. Thus, the researchers concluded that the infection resulted in full activation of phospholipase enzymes in the alveolar fluid.
Subsequently, data confirming the decrease in the amount of BALF phospholipids in lungs of rats with PCP have been accumulated by other investigators (38, 84, 85, 90). Sheehan et al. (90) were among those who confirmed the reduced phospholipid content in BALF was associated with the infection; however, they did not agree with the conclusions of Kernbaum et al. (58) concerning differences in the phospholipase activities of the preparations. Their experimental conditions differed in many parameters, but perhaps the most significant difference may have been the lyophilization and delipidation of BALF, which could have denatured some phospholipase enzymes. From their results (expressed as nanomoles of PC hydrolyzed per minute per milligram of protein), they concluded that dexamethasone immunosuppression itself was the cause of the elevated phospholipase A2 activity. The activities measured were 1 order of magnitude lower than the values obtained in the previous study. The values were as follows: untreated controls, 0.19 nmol/min/mg; immunosuppressed controls, 0.89 nmol/min/mg; and PCP lungs, 0.72 nmol/min/mg (90).
More recently, Hoffman et al. (38) found reduced amounts of total lipids and phospholipids with concomitant increases in phospholipase A activity in BALF of humans with human immunodeficiency virus (HIV) infection and PCP. These effects were correlated with the severity of the infection. Their results were in general agreement with those reported by Kernbaum et al. for corticosteroid-treated rats (58). Elevation of phospholipase activity due to corticosteroid treatment per se was ruled out by the study on BALF from HIV patients with PCP.
P. carinii may also cause decreased lung surfactant synthesis and/or release, which would also lead to a reduction in the amount of phospholipids in BALF. Rice et al. (84) demonstrated that lung surfactant PC synthesis and secretion were lower in alveolar type II cells isolated from lungs of rats with PCP than were those isolated from rat controls. Radiolabeled choline incorporation in vitro into PC of type II cells was depressed, but incorporation into sphingomyelin was enhanced. Also, preliminary results indicated that a similar response in type II cells can be elicited by treatment with purified P. carinii MSG (85).
Unlike lung surfactant phospholipids, surfactant protein levels may be elevated in the BALF from the lungs of patients with PCP (4, 65, 80, 87, 95). This change in the surfactant protein levels appears to be a response specific to P. carinii infection, since it was not seen in the BALF of patients with bacterial pneumonia (5). By using an enzyme-linked immunosorbent assay and polyclonal antisera against purified human SP-A, BALF from HIV-positive PCP patients was found to contain 6.69 μg of SP-A/ml by Phelps and Rose (80), and 10.3 μg/ml by Sternberg et al. (95). However, a major discrepancy in the two studies was in the value for normal controls, which was only 1.5 μg/ml in the former (80) and 14.1 μg/ml in the latter (95) (more like those reported by other workers). Sternberg et al. (96) also found that SP-A concentrations in BALF from HIV-positive individuals without PCP were very low (0.19 μg/ml) and that SP-A concentrations in BALF from HIV-negative patients with PCP were about the same as those in BALF from PCP patients with HIV infections (5.8 μg/ml). They concluded that the SP-A content of BALF was altered both by HIV infection and by P. carinii infection.
An alternative explanation of why BALF from PCP patients may have lower phospholipid concentrations and higher surfactant protein concentrations was recently proposed (33). Corticosteroid treatment itself appeared to stimulate surfactant synthesis. Rice et al. reported that type II pneumocytes isolated from corticosteroid-immunosuppressed P. carinii-free rats secreted more surfactant than did those isolated from untreated controls (85). Consistent with this observation were data reported on whole-lung lipid analyses, in which an increased phospholipid phosphorus-to-protein ratio and increased SP-A levels were observed (33). However, unlike the dramatic increase in the SP-A level observed in P. carinii-infected whole lungs, whole-lung lipids did not have higher levels of lipids. It was reasoned that P. carinii scavenges surfactant lipids but not surfactant proteins and therefore there is a differential reduction in the concentration of lipids and an increase in the concentration of SP-A in the fluid lining the lung alveolus (33). During the preparation of BALF, lipid-laden P. carinii organisms are removed by centrifugation. The lipid content in P. carinii organisms was shown to be more than twice that in rat lungs. The total lipid (milligrams of lipid per milligram of protein) concentrations were as follows: P. carinii, 0.9; lungs from normal rats, 0.24; lungs from immunosuppressed rats not infected with P. carinii, 0.34; and lungs of immunosuppressed rats infected with P. carinii, 0.32 (24). Thus, the bulk of phospholipids initially present in the BALF of untreated or immunosuppressed lungs were probably removed from the alveolar fluid of P. carinii-infected lungs because the lipids become sequestered within the organisms (which were removed by centrifugation). Furthermore, to explain the increased level of SP-A (but not total lipid) in P. carinii-infected whole lung, it was suggested that the organism converts much of the fatty acids it scavenges into CO2, thus eliminating some lipid material from the lung. The low triglyceride and high free fatty acid levels in P. carinii (see above) support the notion that P. carinii utilizes fatty acids for ATP production (23). Conversion in vitro of [14C]glucose to 14CO2 by P. carinii was reported, but this study was done with preparations of questionable purity (79). Preliminary results with organisms from monoxenic cultures suggest that the organism actively converts 14C-labeled fatty acids into 14CO2 (74, 76). Further detailed studies, including radiorespirometry and oxygen consumption measurements with organism preparations of known purity, should elucidate the relative importance of substrates in the various metabolic pathways operative in P. carinii.
Hoffman et al. (38) had expected to find increased levels of lysophosphatides and free fatty acids in BALF of HIV patients with PCP, because phospholipase A activity was elevated in these samples. However, elevation of the levels of the immediate products of phospholipase A action was not detected. Scavenging of lysophosphatides and free fatty acids and catabolism of these products by P. carinii organisms residing in the alveolus would explain this observation.
Although pulmonary dysfunction resulting from the reduced levels of lipids in fluid lining the alveolus may be temporarily relieved by phospholipid replacement, it was suggested that long-term lung surfactant therapy alone would probably not be advisable (33). If lipids are major nutrients utilized by P. carinii for growth and energy production, addition of lipids to their environment would probably lead to enhanced pathogen proliferation.
In contrast to the decreased level of total lipids, phospholipids, and PC of BALF of P. carinii-infected lungs, an increase in sphingomyelin levels has been reported (90, 102). The relative increase in the proportion of sphingomyelin may result from increased synthesis of this sphingolipid (84). Alveolar type II cells isolated from rats with PCP exhibited higher rates of [3H]choline incorporation into sphingomyelin in vitro than did type II cells isolated from normal and immunosuppressed rat controls (84). It is not known if the apparent inability of P. carinii to convert ceramide to sphingomyelin in vitro is involved in this abnormal biochemistry resulting from the host-pathogen interaction. However, if the organism does not synthesize sphingomyelin and hence scavenges this lipid from the rat lung, a reduced sphingomyelin level in BALF would be expected. The observations on sphingomyelin levels and biosynthesis in P. carinii-infected lungs remains a paradox.
Effect of P. carinii on the Function of Lung Cells
Type II cells isolated from lungs of normal untreated rats, immunosuppressed P. carinii-free rats, rats with PCP, and rats with PCP cleared of the infection by trimetroprim-sulfamethoxazole treatment were examined for their responses to secretagogue stimulation (84). The secretagogues used were ATP, a phorbol ester (12-O-tetradecanoylphorbol-13-acetate), and terbutaline. Unlike cells from normal and immunosuppressed rat controls, the cells isolated from rats with active or cleared PCP failed to respond to these compounds. Hence, the infection apparently leads to long-lasting abnormal alveolar epithelial function.
The pathogen-epithelial type II cell interaction may be quite complex. Pesanti (78) reported that in his study of the effects of mixing P. carinii with pneumocytes in vitro, it appeared that type II cells had toxic effects on the organism (78). When P. carinii organisms (isolated from BALF) were incubated with alveolar type II cells, the organisms lysed and disintegrated. This phenomenon was not observed with alveolar macrophage monolayers.
Alveolar macrophages bind, phagocytose, and digest invading microbes, thus clearing the foreign organisms from the lungs. P. carinii adheres to alveolar macrophages (71, 72) and is digested by these cells (60). The fatty acid arachidonic acid, which is a precursor of a number of bioactive compounds, was observed to be released from alveolar macrophages exposed to P. carinii (9). The synthesis and release of cytokines and other factors produced by host cells are expected to become stimulated upon exposure to invading microbes.
Lipid Antigens
The detection of circulating anti-cardiolipin antibodies in PCP patients indirectly indicated that P. carinii hominis synthesized distinct cardiolipin molecules (66, 67). However, Stimmler et al. (96) did not detect significant differences between anti-cardiolipin antibody levels in AIDS patients with PCP and those in patients with other types of opportunistic infections. These workers concluded that the increased antibody levels resulted from the HIV infection and were not a specific response to P. carinii. Thus, it is questionable whether anti-cardiolipin antibody assays would be sufficiently specific for diagnostic purposes.
CONCLUSIONS
Within the last few years, substantial progress has been made in understanding the biochemical nature of P. carinii. Among the more active areas of research has been the elucidation of its lipids. These analyses have identified attractive drug targets. These discoveries were made possible by the development of organism preparations whose purity was thoroughly documented and by systematic biochemical analyses. When gene sequence data indicated a close relationship between P. carinii and the fungi, several groups devoted their efforts to trying to demonstrate the presence of ergosterol in the pathogen. This example demonstrates the danger of approaching the study of organisms (especially the Protista) with preconceived notions and the value of performing basic characterization studies to first find which molecules are in the organism of interest.
The uncommon lipids identified in P. carinii can serve as signatures of the pathogen, which could lead to improved diagnostic methods. Also, several P. carinii lipids which cannot be synthesized by humans have now been identified. Drugs specifically directed against these pathogen lipids, or the enzyme systems required for their biosyntheses, would not be expected to have harmful effects on the host. Experiments on P. carinii carinii lipid metabolism are now feasible and are being performed. These studies should elucidate the biosynthetic pathways operative in the organism. The isolation, purification, and structural characterization of the enzymes (or the genes coding for these enzymes) in P. carinii responsible for the biosyntheses of cis-9,10-epoxystearic acid and C-24-alkylated Δ7 sterols may lead to fruitful drug designs. Other targets may be identified in the future as additional lipid classes in the organism are examined in detail.
The important opportunistic eukaryotic microbes associated with AIDS include Toxoplasma, Cryptosporidium, the microsporidia, Candida, and P. carinii. It is safe to conclude that more is currently known about the lipid biochemistry of P. carinii than about most of the other opportunistic infectious eukaryotic microbes.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants RO1 AI38758 and RO1 AI29316.
REFERENCES
- 1.Alexopoulos C J, Mims C W, Blackwell M. Introductory mycology. 4th ed. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 258–271. [Google Scholar]
- 2.Amit, Z., and E. S. Kaneshiro. Unpublished data.
- 3.Banerji S, Wakefield A E, Allen A G, Maskell D J, Peters S E, Hopkin J M. The cloning and characterization of the arom gene of Pneumocystis carinii. J Gen Microbiol. 1993;139:2901–2914. doi: 10.1099/00221287-139-12-2901. [DOI] [PubMed] [Google Scholar]
- 4.Baughman R P, Hyull W, Whitsett J A. Pneumocystis carinii alters surfactant associated protein-A concentrations found in bronchoalveolar lavage fluid. Clin Res. 1992;40:412A. [PubMed] [Google Scholar]
- 5.Baughman R P, Sternberg R I, Hull W, Buchsbaum J, Whitsett J. Decreased surfactant protein A in patients with bacterial pneumonia. Am Rev Respir Dis. 1993;147:653–657. doi: 10.1164/ajrccm/147.3.653. [DOI] [PubMed] [Google Scholar]
- 6.Boylan C J, Current W L. Improved model of Pneumocystis carinii pneumonia: induced laboratory infection in Pneumocystis-free animals. Infect Immun. 1992;60:1589–1597. doi: 10.1128/iai.60.4.1589-1597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantin A M, North S L, Hubbard R L, Crystal R G. Normal alveolar epithlia fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 8.Casey P J. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 9.Castro M, Morgenthaler T I, Hoffman O A, Standing J E, Rohrbach M S, Limper A H. Pneumocystis carinii induces the release of arachidonic acid and its metabolites from alveolar macrophages. Am J Respir Cell Mol Biol. 1993;9:73–81. doi: 10.1165/ajrcmb/9.1.73. [DOI] [PubMed] [Google Scholar]
- 10.Chen F, Cushion M T. Use of an ATP bioluminescent assay to evaluate viability of Pneumocystis carinii from rats. J Clin Microbiol. 1994;32:2791–2800. doi: 10.1128/jcm.32.11.2791-2800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin K, Merali S, Shaw M M, Bartlett M S, Clarkson A B., Jr Subpopulations of Pneumocystis carinii separated by a Percoll gradient. J Eukaryot Microbiol. 1996;43:53S. doi: 10.1111/j.1550-7408.1996.tb04988.x. [DOI] [PubMed] [Google Scholar]
- 12.Contini C, Manganaro M, Romani R, Tzatzoglou S, Poggesi I, Vullo V, Delia S, De Simone C. Activity of terbinafine against Pneumocystis carinii in vitro and its efficacy in the treatment of experimental pneumonia. J Antimicrob Agents Chemother. 1994;34:727–735. doi: 10.1093/jac/34.5.727. [DOI] [PubMed] [Google Scholar]
- 13.Cushion M T. In vitro studies of Pneumocystis carinii. J Protozool. 1989;36:45–52. doi: 10.1111/j.1550-7408.1989.tb02691.x. [DOI] [PubMed] [Google Scholar]
- 14.Cushion, M. T. Personal communication.
- 15.De Stefano J A, Cushion M T, Sleight R G, Walzer P D. Analysis of Pneumocystis carinii cyst wall. I. Evidence for an outer surface membrane. J Protozool. 1990;37:428–435. doi: 10.1111/j.1550-7408.1990.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 16.De Stefano J A, Cushion M T, Puvanesarajah V, Walzer P D. Analysis of Pneumocystis carinii cyst wall. II. Sugar composition. J Protozool. 1990;37:436–441. doi: 10.1111/j.1550-7408.1990.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 17.De Stefano J A, Sleight R G, Babcock G F, Sramkoski R M, Walzer P D. Isolation of Pneumocystis carinii cysts by flow cytometry. Parasitol Res. 1992;78:179–182. doi: 10.1007/BF00931724. [DOI] [PubMed] [Google Scholar]
- 18.De Stefano J A, Foy J M, Sullivan D W, Dawes S M, Cushion M T, Babcock G F, Sleight R G, van Halbeek H, Walzer P D. Fractionation of Pneumocystis carinii developmental stages by counterflow centrifugal elutriation and sequential filtrations. Parasitol Res. 1994;80:1–9. doi: 10.1007/BF00932616. [DOI] [PubMed] [Google Scholar]
- 19.Dohn M N, Frame P T, Baughman R P, Lafon S W, Smulian A G, Caldwell P, Rogers M D. Open-label efficacy and safety trial of 42 days of 566C80 for Pneumocystis carinii pneumonia in AIDS patients. J Eukaryot Microbiol. 1992;38:220S–221S. [PubMed] [Google Scholar]
- 20.Edman J C, Kovacs J A, Masur H, Santi D, Harwood H J, Sogin M L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature (London) 1988;334:519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- 21.Ellis J E. Coenzyme Q homologs in parasitic protozoa as targets for chemotherapeutic attack. Parasitol Today. 1994;10:296–301. doi: 10.1016/0169-4758(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 22.Ellis J E, Reilly M H, Kaneshiro E S. Identification of an epoxy fatty acid in Pneumocystis carinii lipids. J Eukaryot Microbiol. 1994;41:87S. [PubMed] [Google Scholar]
- 23.Ellis J E, Wyder M A, Zhou L, Gupta A, Rudney H, Kaneshiro E S. Composition of Pneumocystis carinii neutral lipids and identification of coenzyme Q10 as the major ubiquinone homolog. J Eukaryot Microbiol. 1996;43:165–170. doi: 10.1111/j.1550-7408.1996.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 24.Florin-Christensen M, Florin-Christensen J, Wu Y-P, Zhou L, Gupta A, Rudney H, Kaneshiro E S. Occurrence of specific sterols in Pneumocystis carinii. Biochem Biophys Res Commun. 1994;198:236–242. doi: 10.1006/bbrc.1994.1033. [DOI] [PubMed] [Google Scholar]
- 25.Florin-Christensen M, Florin-Christensen J, Kaneshiro E S. Uptake and metabolism of l-serine by Pneumocystis carinii carinii. J Eukaryot Microbiol. 1995;42:669–675. doi: 10.1111/j.1550-7408.1995.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 26.Furlong S T, Samia J A, Rose R M, Fishman J A. Phytosterols are present in Pneumocystis carinii. Antimicrob Agents Chemother. 1994;38:2534–2540. doi: 10.1128/aac.38.11.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furlong S T, Frederique D E, Ghildyal N, Shaw M M, Bartlett M S, McLaughlin G L. Abstracts of the Joint Meeting of the American Society of Parasitology and the Society of Protozoologists, 1996. 1996. Evidence for a lipophosphoglycan from Pneumocystis carinii, abstr. 173; p. 126. [Google Scholar]
- 28.Furlong S T, Koziel H, Bartlett M S, McLaughlin G L, Shaw M M, Jack R M. Lipid transfer from human epithelial cells to Pneumocystis carinii in vitro. J Infect Dis. 1997;175:661–668. doi: 10.1093/infdis/175.3.661. [DOI] [PubMed] [Google Scholar]
- 29.Gigliotti F, Harmsen A G, Haidaris C G, Haidaris P J. Pneumocystis carinii is not universally transmissable between mammalian species. Infect Immun. 1993;61:2886–2890. doi: 10.1128/iai.61.7.2886-2890.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goheen M P, Bartlett M S, Shaw M M, Queener S F, Smith J W. Effects of 8-aminoquinolines on the ultrastructural morphology of Pneumocystis carinii. Int J Exp Pathol. 1993;74:379–387. [PMC free article] [PubMed] [Google Scholar]
- 31.Graves D C, McNabb S J N, Ivey M H, Worley M A. Development and characterization of monoclonal antibodies to Pneumocystis carinii. Infect Immun. 1986;51:125–133. doi: 10.1128/iai.51.1.125-133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Z, Kaneshiro E S. The composition of Pneumocystis carinii phospholipids. J Eukaryot Microbiol. 1994;41:90S. [PubMed] [Google Scholar]
- 33.Guo Z, Kaneshiro E S. Phospholipid composition of Pneumocystis carinii carinii and effects of methylprednisolone immunosuppression on rat lung lipids. Infect Immun. 1995;63:1286–1290. doi: 10.1128/iai.63.4.1286-1290.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Z, Beach D H, Kaneshiro E S. Fatty acid composition of the major phospholipids of Pneumocystis carinii carinii: comparison with those in the lungs of normal and methylprednisolone-immunosuppressed rats. Infect Immun. 1996;64:1407–1412. doi: 10.1128/iai.64.4.1407-1412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutteridge W E. Pneumocystis carinii: potential targets for chemotherapeutic attack. In: Coombs G H, North M J, editors. Biochemical protozoology. London, United Kingdom: Taylor & Francis; 1991. pp. 35–51. [Google Scholar]
- 36.Hannun Y A. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 37.Haughan P A, Goad J L. Lipid biochemistry of trypanosomatids. In: Coombs G H, North M J, editors. Biochemical protozoology. London, United Kingdom: Taylor & Francis; 1991. pp. 312–328. [Google Scholar]
- 38.Hoffman A G D, Lawrence M G, Ognibene F P, Suffredini A F, Lipschik G Y, Kovacs J A, Masur H, Shelhamer J H. Reduction of pulmonary surfactant in patients with human immunodeficiency virus infection and Pneumocystis carinii pneumonia. Chest. 1992;102:1730–1738. doi: 10.1378/chest.102.6.1730. [DOI] [PubMed] [Google Scholar]
- 39.Hughes W T, Gray V L, Gutteridge W E, Latter V S, Pudney M. Efficacy of a hydroxynapthoquinone, 566C80, in experimental parasitic infections of AIDS pneumonitis. Antimicrob Agents Chemother. 1990;34:225–228. doi: 10.1128/aac.34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ittarat I, Asawamahasakda W, Meshnick S R. A preliminary characterization of the Pneumocystis carinii dihydroorotate dehydrogenase. J Eukaryot Microbiol. 1994;41:92S. [PubMed] [Google Scholar]
- 41.Ittarat I, Asawamahasakda W, Bartlett M S, Smith J W, Meshnick S R. Effects of atovaquone and other inhibitors on Pneumocystis carinii dihydroorotate dehydrogenase. Antimicrob Agents Chemother. 1995;39:325–328. doi: 10.1128/aac.39.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson R L, Duromo R J, Langner C A, Rudnick D A, Gordon J I. Genetic and biochemical studies of a mutant of Saccharomyces cerevisiae myristoyl CoA:protein N-myristoyl transferase, nmt72pLeu99→Pro that produces temperature sensitive myristic acid auxotrophy. J Biol Chem. 1993;268:483–494. [PubMed] [Google Scholar]
- 43.Kallam K A, Kaneshiro E S. Detailed analyses of individual neutral lipid classes of Pneumocystis. J Eukaryot Microbiol. 1996;43:51S. doi: 10.1111/j.1550-7408.1996.tb04986.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaneshiro, E. S. Unpublished data.
- 45.Kaneshiro E S, Sleight R G. Biochemistry and metabolism. In: Walzer P D, editor. Pneumocystis carinii pneumonia. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 45–71. [Google Scholar]
- 46.Kaneshiro E S, Wyder M A. Lipophosphoglycan antigen shedding by Leishmania donovani. J Eukaryot Microbiol. 1993;40:336–340. doi: 10.1111/j.1550-7408.1993.tb04925.x. [DOI] [PubMed] [Google Scholar]
- 47.Kaneshiro E S, Jayasimhulu K, Lester R L. Characterizations of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingolipid. J Lipid Res. 1986;27:1294–1303. [PubMed] [Google Scholar]
- 48.Kaneshiro E S, Cushion M T, Walzer P D, Jayasimhulu K. Analysis of Pneumocystis fatty acids. J Protozool. 1989;36:69S–72S. [PubMed] [Google Scholar]
- 49.Kaneshiro E S, Wu Y-P, Cushion M T. Assays for testing Pneumocystis carinii viability. J Protozool. 1991;38:85S–87S. [PubMed] [Google Scholar]
- 50.Kaneshiro E S, Wyder M A, Wu Y-P, Cushion M T. Reliability of calcein methoxy methyl ester and ethidium homodimer or propidium iodide for viability assessment of microbes. J Microbiol Methods. 1993;17:1–16. [Google Scholar]
- 51.Kaneshiro E S, Wyder M A, Zhou L H, Ellis J E, Voelker D R, Langreth S G. Characterization of Pneumocystis carinii preparations developed for lipid analysis. J Eukaryot Microbiol. 1993;40:805–815. doi: 10.1111/j.1550-7408.1993.tb04479.x. [DOI] [PubMed] [Google Scholar]
- 52.Kaneshiro E S, Ellis J E, Jayasimhulu K, Beach D H. Evidence for the presence of “metabolic sterols” in Pneumocystis: identification and initial characterization of Pneumocystis carinii sterols. J Eukaryot Microbiol. 1994;41:78–85. doi: 10.1111/j.1550-7408.1994.tb05938.x. [DOI] [PubMed] [Google Scholar]
- 53.Kaneshiro E S, Ellis J E, Zhou L H, Rudney H, Gupta A, Jayasimhulu K, Setchell K D R, Beach D H. Isoprenoid metabolism in Pneumocystis carinii. J Eukaryot Microbiol. 1995;41:93S. [PubMed] [Google Scholar]
- 54.Kaneshiro E S, Ellis J E, Guo Z, Jayasimhulu K, Maiorano J N, Kallam K A. Characterizations of neutral lipid fatty acids and cis-9,10-epoxy octadecanoic acid in Pneumocystis carinii carinii. Infect Immun. 1996;64:4105–4114. doi: 10.1128/iai.64.10.4105-4114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaneshiro E S, Swonger M, Kreishman G, Brooks E, Jayasimhulu K, Parish E J, Beach D H. Identification of C31 and C32 sterols in Pneumocystis carinii hominis-infected human lungs. J Eukaryot Microbiol. 1996;43:36S. doi: 10.1111/j.1550-7408.1996.tb04972.x. [DOI] [PubMed] [Google Scholar]
- 56.Kaneshiro, E. S., Z. Guo, D. Sul, K. A. Kallam, K. Jayasimhulu, and D. H. Beach. The fatty alcohols and ether lipids of Pneumocystis carinii carinii. Unpublished data. [PubMed]
- 57.Kaneshiro, E. S., K. Jayasimhulu, and J. De Stefano. Lipid modification of Pneumocystis carinii major surface antigen. Unpublished data.
- 58.Kernbaum S, Masliah U, Alcindor L G, Bouton C, Cristol D. Phospholipase activities of bronchoalveolar lavage fluid in rat Pneumocystis carinii pneumonia. Br J Exp Pathol. 1983;64:75–80. [PMC free article] [PubMed] [Google Scholar]
- 59.Korn E D, Dearborn D G, Wright P L. Lipophosphonoglycan of the plasma membrane of Acanthamoeba castellanii. Isolation from whole amoebae and identification of the water-soluble products of acid hydrolysis. J Biol Chem. 1974;249:3335–3341. [PubMed] [Google Scholar]
- 60.Langreth S G, Kaneshiro E S. Abstracts of the 9th International Congress of Protozoology 1993. 1993. Pathogenesis of Pneumocystis carinii pneumonia in viral-negative immunosuppressed rats, an ultrastructural study; p. 70. [Google Scholar]
- 61.Lee C-H, Bauer N L, Shaw M M, Durkin M M, Bartlett M S, Queener S F, Smith J W. Proliferation of rat Pneumocystis carinii on cells sheeted on microcarrier beads in spinner flasks. J Clin Microbiol. 1993;31:1659–1662. doi: 10.1128/jcm.31.6.1659-1662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Edlind T. Phylogeny of Pneumocystis carinii based on beta-tubulin sequence. J Eukaryot Microbiol. 1994;41:97S. [PubMed] [Google Scholar]
- 63.Limper A H, Martin W J., II Pneumocystis carinii: inhibition of lung cell growth mediated by parasite attachment. J Clin Invest. 1990;85:391–396. doi: 10.1172/JCI114451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Limper A H, Standing J E, Hoffman O A, Castro M, Neese L W. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect Immun. 1993;61:4302–4309. doi: 10.1128/iai.61.10.4302-4309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Limper A H, O’Riordan D M, Vuk-Pavlovic Z, Crouch E C. Accumulation of surfactant protein D in the lung during Pneumocystis carinii pneumonia. J Eukaryot Microbiol. 1994;41:98S. [PubMed] [Google Scholar]
- 66.Masala C, Sorice M, DiPrima M A, Lenti L, Misasi R, Contini C, Vullo V. Anticardiolipin antibodies and Pneumocystis carinii pneumonia. Ann Intern Med. 1989;110:749. doi: 10.7326/0003-4819-110-9-749_1. [DOI] [PubMed] [Google Scholar]
- 67.Masala C, Sorice M, Di Prima M A, Lenti L, Misasi R, Mojon M, Contini C, Vullo V. Evidence for shared epitopes between cardiolipin and Pneumocystis carinii. J Infect Dis. 1989;160:736–737. doi: 10.1093/infdis/160.4.736. [DOI] [PubMed] [Google Scholar]
- 68.Mehta, M., and E. S. Kaneshiro. Unpublished data.
- 69.Merrill H A, Jr, van Echten G, Wang E, Sandhoff K. Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J Biol Chem. 1993;268:27299–27306. [PubMed] [Google Scholar]
- 70.Nes W D, Hanners P K, Parish E J. Inhibitors of fungal sterol C-24 alkylation: importance to developmental regulation. Biochem Biophys Res Commun. 1986;139:410–415. doi: 10.1016/s0006-291x(86)80006-7. [DOI] [PubMed] [Google Scholar]
- 71.O’Riordan D M, Standing J E, Limper A H. Pneumocystis carinii glycoprotein A binds macrophage mannose receptors. Infect Immun. 1995;63:779–784. doi: 10.1128/iai.63.3.779-784.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Riordan D M, Standing J E, Kwon K Y, Chang D, Crouch E C, Limper A H. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J Clin Invest. 1995;95:2699–2710. doi: 10.1172/JCI117972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parish E J, Hanners P K, Nes W D. Synthesis and biological evaluation of fungal bioregulators of sterol biosynthesis. In: Stumpf P K, Mudd J B, Nes W D, editors. The metabolism, structure and function of plant lipids. New York, N.Y: Plenum Press; 1987. pp. 103–105. [Google Scholar]
- 74.Paulsrud J R, Glancy T P, Shaw M M, Bartlett M S, Smith J W. Abstracts of the 38th Annual Meeting of the American Society of Tropical Medicine and Hygiene 1989. 1989. Fatty acid metabolism of Pneumocystis carinii in culture, abstr. 330; p. 246. [Google Scholar]
- 75.Paulsrud J R, Queener S F, Bartlett M S, Smith J W. Total cellular fatty acid composition of cultured Pneumocystis carinii. J Clin Microbiol. 1991;31:1899–1902. doi: 10.1128/jcm.31.7.1899-1902.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paulsrud J R, Queener S F. Abstracts of the 47th Annual Meeting of the Society of Protozoologists 1994. 1994. Metabolism of fatty acids and amino acids by cultured Pneumocystis carinii, abstr. P15; p. 83. [Google Scholar]
- 77.Paulsrud J R, Queener S F. Metabolism of fatty acids and amino acids by cultured Pneumocystis carinii. J Eukaryot Microbiol. 1994;41:633–638. doi: 10.1111/j.1550-7408.1994.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 78.Pesanti E L. Phospholipid profile of Pneumocystis carinii and its interaction with alveolar type II epithelial cells. Infect Immun. 1987;55:736–741. doi: 10.1128/iai.55.3.736-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pesanti E L, Cox C. Metabolic and synthetic activities of Pneumocystis carinii in vitro. Infect Immun. 1981;34:908–914. doi: 10.1128/iai.34.3.908-914.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phelps D S, Rose R M. Increased recovery of surfactant protein A in AIDS-related pneumonia. Am Rev Respir Dis. 1991;143:1072–1075. doi: 10.1164/ajrccm/143.5_Pt_1.1072. [DOI] [PubMed] [Google Scholar]
- 81.Pottratz S T, Martin W J., II Role of fibronectin in Pneumocystis carinii attachment to cultured lung cells. J Clin Invest. 1990;85:351–356. doi: 10.1172/JCI114445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pottratz S T, Paulsrud J, Smith J S, Martin W J., II Pneumocystis carinii attachment to cultured lung cells by Pneumocystis gp120, a fibronectin binding protein. J Clin Invest. 1991;88:403–407. doi: 10.1172/JCI115318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Racagni G, de Lema M G, Hernandez G, Machado-Domenech E E. Fetal bovine serum induces changes in fatty acid composition of Trypanosoma cruzi phosphoinositides. Can J Microbiol. 1995;41:951–954. doi: 10.1139/m95-132. [DOI] [PubMed] [Google Scholar]
- 84.Rice W R, Singleton F M, Linke M J, Walzer P D. Regulation of surfactant phosphatidylcholine secretion from alveolar type II cells during Pneumocystis carinii pneumonia in the rat. J Clin Invest. 1993;92:2778–2899. doi: 10.1172/JCI116896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rice W R, Singleton F M, Linke M J, Walzer P D. Control of type II cell function by Pneumocystis carinii. Am Rev Respir Dis. 1993;147:A35. [Google Scholar]
- 86.Rodriguez R J, Low C, Bottema C D K, Parks L W. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1985;837:336–343. doi: 10.1016/0005-2760(85)90057-8. [DOI] [PubMed] [Google Scholar]
- 87.Rose R M, Catalano P J, Koziel H, Furlong S T. Abnormal lipid composition of bronchoalveolar lavage fluid obtained from individuals with AIDS-related lung disease. Am J Respir Crit Care Med. 1994;149:332–338. doi: 10.1164/ajrccm.149.2.8306026. [DOI] [PubMed] [Google Scholar]
- 88.Ross P F, Nelson P E, Richard J L, Oswiler G D, Rice L G, Plattner R D, Wilson T M. Production of fumonisins by Fusarium moniliforme and Fusarium proliferatum isolates associated with equine leukoencephalomalacia and pulmonary edema syndrome in swine. Appl Environ Microbiol. 1990;56:3225–3226. doi: 10.1128/aem.56.10.3225-3226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saric M, Clarkson A B., Jr Ornithine decarboxylase in Pneumocystis carinii and implications for therapy. Antimicrob Agents Chemother. 1994;38:2545–2552. doi: 10.1128/aac.38.11.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheehan P M, Stokes D C, Yeh Y-Y, Hughes W T. Surfactant phospholipids and lavage phospholipase A2 in experimental Pneumocystis carinii pneumonia. Am Rev Respir Dis. 1986;134:526–531. doi: 10.1164/arrd.1986.134.3.526. [DOI] [PubMed] [Google Scholar]
- 91.Sleight R G, Mehta M A, Kaneshiro E S. Uptake and metabolism of fluorescent lipid analogs by Pneumocystis carinii. J Eukaryot Microbiol. 1995;41:111S. [PubMed] [Google Scholar]
- 92.Sloand E, Laughon B, Armstrong M, Bartlett M S, Blumenfeld W, Cushion M, Kalica A, Kovacs J A, Martin W, Pitt E, Pesanti E L, Richards F, Rose R, Walzer P. The challenge of Pneumocystis carinii culture. J Eukaryot Microbiol. 1993;40:188–195. doi: 10.1111/j.1550-7408.1993.tb04902.x. [DOI] [PubMed] [Google Scholar]
- 93.Snyder F. Platelet-activating factor and related lipid mediators. New York, N.Y: Plenum Press; 1987. [Google Scholar]
- 94.Sorice M, Lenti L, Misasi R, Contini C, Cignarella L, Griggi T, Vullo V, Masala C. Evidence for the existence of ganglioside molecules on Pneumocystis carinii from human lungs. Parasitology. 1992;105:1–6. doi: 10.1017/s0031182000073613. [DOI] [PubMed] [Google Scholar]
- 95.Steinberg R I, Whitsett J A, Hull W M, Baughman R P. Pneumocystis carinii alters surfactant protein A concentrations in bronchoalveolar lavage fluid. J Lab Clin Med. 1995;125:462–469. [PubMed] [Google Scholar]
- 96.Stimmler M M, Quismorio F P, Jr, McGehee W G, Boylen T, Sharma O P. Anticardiolipin antibodies in acquired immunodeficiency syndrome. Arch Intern Med. 1989;149:1833–1835. [PubMed] [Google Scholar]
- 97.Stoll L L, Spector A A. Changes in serum influences the fatty acid composition of established cell lines. In Vitro. 1984;20:732–738. doi: 10.1007/BF02618879. [DOI] [PubMed] [Google Scholar]
- 98.Stringer J R. Pneumocystis carinii: what is it, exactly? Clin Microbiol Rev. 1996;9:489–498. doi: 10.1128/cmr.9.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stringer J R the Pneumocystis Workshop. Revised nomenclature for Pneumocystis carinii. J Eukaryot Microbiol. 1994;41:121S–122S. [PubMed] [Google Scholar]
- 100.Stringer J R, Stringer S L, Zhang J, Baughman R, Smulian A G, Cushion M T. Molecular genetic distinction of Pneumocystis carinii from rats and humans. J Eukaryot Microbiol. 1993;40:733–741. doi: 10.1111/j.1550-7408.1993.tb04468.x. [DOI] [PubMed] [Google Scholar]
- 101.Stringer S L, Stringer J R, Blaise M A, Walzer P D, Cushion M T. Pneumocystis carinii: sequence from ribosomal RNA implies a close relationship with fungi. Exp Parasitol. 1989;68:450–461. doi: 10.1016/0014-4894(89)90130-6. [DOI] [PubMed] [Google Scholar]
- 102.Su T H, Natarajan V, Martin W J. Pulmonary surfactant in Pneumocystis carinii pneumonia is associated with a marked increase in sphingomyelin. Am Rev Respir Dis. 1992;145:A246. [Google Scholar]
- 103.Sul D, Kaneshiro E S. Ubiquinone synthesis by Pneumocystis carinii: incorporation of radiolabeled polyprenyl chain and benzoquinone ring precursors. J Eukaryot Microbiol. 1997;44:60S. doi: 10.1111/j.1550-7408.1997.tb05780.x. . polyprenyl chain and the aromatic ring of ubiquinone. Unpublished data. [DOI] [PubMed] [Google Scholar]
- 104.Thomas J R, McConville M J, Thomas-Oates J E, Homan S W, Ferguson M A J, Gorin P A J, Greis K D, Turco S J. Refined structure of the lipophosphoglycan of Leishmania donovani. J Biol Chem. 1992;267:6829–6833. [PubMed] [Google Scholar]
- 105.Tulloch A P. The oxygenated fatty acids of the oil of wheat stem rust uredospores. Can J Chem. 1960;38:204–207. [Google Scholar]
- 106.Tulloch A P, Craig B M, Ledingham G A. The oil of wheat stem rust uredospores. II. The isolation of cis-9,10-epoxyoctadecanoic acid, and the fatty acid composition of the oil. Can J Microbiol. 1959;5:485–491. doi: 10.1139/m59-060. [DOI] [PubMed] [Google Scholar]
- 107.Urbina J A, Vivas J, Lazardi K, Molina J, Payares G, Piras M M, Piras R. Antiproliferative effects of Δ24(25) sterol methyl transferase inhibitors on Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Chemotherapy. 1996;42:294–307. doi: 10.1159/000239458. [DOI] [PubMed] [Google Scholar]
- 108.Urbina J A, Vivas J, Visbal G, Contreras L M. Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes with Δ24(25)-sterol methyl transferase inhibitors and their combinations with ketoconazole. Mol Biochem Parasitol. 1995;79:199–210. doi: 10.1016/0166-6851(95)00117-j. [DOI] [PubMed] [Google Scholar]
- 109.Walzer P D. Pneumocystis carinii pneumonia. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1993. [Google Scholar]
- 110.Weete J D. Lipid biochemistry of fungi and other organisms. New York, N.Y: Plenum Press; 1980. [Google Scholar]
- 111.Weete J D. Structure and function of sterols in fungi. Adv Lipid Res. 1989;23:484–491. [Google Scholar]
- 112.Weete J D, Sancholle M S, Montant C. Effects of triazoles on fungi. II. Lipid composition of Taphrina deformans. Biochim Biophys Acta. 1983;752:19–29. [Google Scholar]
- 113.Weete J D, Fuller M S, Huang M Q, Gandhi S. Fatty acids and sterols of selected hypochytriomycetes and chytridiomycetes. Exp Mycol. 1989;13:183–195. [Google Scholar]
- 114.Yardley V, Snowdon D, Croft S, Hazra B. In vitro activity of diospyrin and derivatives against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei brucei. Phytother Res. 1996;10:559–562. [Google Scholar]
- 115.Yoshikawa H, Morioka H, Yoshida Y. Freeze-fracture localization of filipin-sterol complexes in plasma- and cyto- membranes of Pneumocystis carinii. J Protozool. 1987;34:131–137. doi: 10.1111/j.1550-7408.1987.tb03148.x. [DOI] [PubMed] [Google Scholar]
- 116.Yoshikawa H, Yoshida Y. Distribution of digitonin-sterol complexes in Pneumocystis carinii revealed by freeze-fracture method. Int J Parasitol. 1988;18:39–45. doi: 10.1016/0020-7519(88)90034-3. [DOI] [PubMed] [Google Scholar]
- 117.Zimmerman P E, Voelker D R, McCormack F X, Martin W J. 120kDa surface glycoprotein of Pneumocystis carinii is a ligand for surfactant protein A. J Clin Invest. 1992;89:143–149. doi: 10.1172/JCI115554. [DOI] [PMC free article] [PubMed] [Google Scholar]