Abstract
Maladaptive implicit emotion regulation has been highlighted as a transdiagnostic characteristic of mood and anxiety disorders. Whilst clinical diagnosis has relied on signs and symptoms, the integration of clinical neurosciences is becoming more important as a means of enhancing assessment, diagnosis, and treatment. Thus, activation likelihood estimation (ALE) meta-analysis was conducted for whole-brain foci comparing implicit emotion regulation in a large sample of patients with mood and anxiety disorders and healthy controls. Twenty-four clinical studies were identified based on established criteria (e.g., DSM-5). ALE meta-analysis reported convergence of hypoactivation in patients (n = 432) in the right medial frontal gyrus (BA9), spreading to the right anterior cingulate gyrus (BA32); and in the left middle temporal gyrus (BA21), spreading to the left superior temporal gyrus (BA22). Convergence of hyperactivation was reported in patients (n = 536) in the left medial frontal gyrus (BA9), spreading to the left superior frontal gyrus and the left middle frontal gyrus. Separate analysis of the mood disorders subgroup further highlighted convergence of hyperactivation in the insula and claustrum. The implications of the current findings are discussed within the context of the Research Domain Criteria (RDoC) framework of developing diagnostic systems that are more predictive of treatment outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-03828-5.
Keywords: Implicit emotion regulation, Emotion regulation, Depression, Anxiety, Meta-analysis, fMRI
Subject terms: Emotion, Neuroscience, Diagnostic markers, Predictive markers, Prognostic markers, Psychiatric disorders
Introduction
An inability to regulate emotions is a central characteristic of many psychiatric disorders. While previous research has largely focused on the role of maladaptive explicit (conscious) emotion regulation, where the goal is to alter the direction, intensity, or duration of an emotional experience, emotion regulation also occurs implicitly (unconsciously), running automatically without monitoring or effort, and can be activated without a conscious strategy, goal, or intent1–10. Importantly, our recent meta-analysis has highlighted the role of maladaptive implicit emotion regulation as a transdiagnostic characteristic of mood and anxiety disorders. It has also been suggested that maladaptive implicit emotion regulation may disrupt the effectiveness of conscious emotion regulation strategies through automatic bottom-up processes like the involuntary shifting of attention towards salient information i.e., rumination, worry, or negative automatic thoughts11.
In healthy individuals, the main neural regions recruited during explicit emotion regulation are the lateral regions of the prefrontal cortex (PFC), such as the ventrolateral prefrontal cortex (vlPFC) and dorsolateral prefrontal cortex (dlPFC)12. Studies investigating the neural underpinnings of maladaptive explicit emotional regulation in mood and anxiety disorders suggest the role of an extended network. In their meta-analysis, Gou et al.13 summarised findings from 28 fMRI studies (656 patients with depression and 680 healthy controls) on explicit emotion dysregulation and reported hyperactivity in the middle temporal gyrus (MTG), superior temporal gyrus (STG), parahippocampal gyrus, and cuneus in patients; regions which interact with the default mode network (DMN), which is known to play a role in self-referential processing, internally focused rumination, and negative cognitions14,15. In contrast, depressed patients also showed hypoactivity in the superior frontal gyrus (SFG), superior temporal gyrus, inferior parietal lobe, and insula; regions which interact with the salience network (SN), which is functional in the filtering of salient information and switching from the DMN to the executive control network, known for its role in higher cognitive processes16. The involvement of a broad spectrum of neural regions was also the main finding of a systematic review of 17 neuroimaging studies (528 patients and 403 healthy controls) on functional connectivity in depressed patients while undertaking an emotional face processing task. Compared to the healthy controls, the processing of negatively valanced faces was associated with reduced effective connectivity from the dlPFC to the amygdala, whereas the processing of happy facial expressions was associated with greater inhibitory connectivity from the ventromedial prefrontal cortex (vmPFC) to the amygdala17. Finally, a meta-analysis of 23 fMRI studies (449 patients and 424 healthy controls) examining disrupted neural facial processing in social anxiety disorder (SAD) reported several altered activations18. Whilst supporting the large body of existing evidence on the role of bilateral amygdala hyperactivity in SAD, increased activation was also seen in areas related to attention (SFG), emotional recognition (superior temporal sulcus), emotion regulation (medial frontal gyrus and subgenual anterior cingulate) and the visual cortex. In contrast, areas that were more active in healthy controls were clusters in the occipital visual cortex (lingual gyrus) and the posterior cingulate.
In healthy individuals, neural regions known to be recruited during implicit emotion regulation are the medial regions of the PFC, including the vmPFC and the anterior cingulate cortex (ACC)12. In patients with mood and anxiety disorders, several neural regions have been identified within the literature, although there are discrepancies regarding the precise regions implicated in implicit emotion regulation. For instance, the vmPFC19,20, dorsomedial prefrontal cortex (dmPFC)21,22, vlPFC, dlPFC19,22–25, the orbitofrontal cortex (OFC)25,26, the ACC23,27, the insula19,23,28, and the amygdala19,20,24,25,29–34 have been identified during implicit emotion regulation. Furthermore, the neural activation in patients during implicit emotion regulation have reported both increased and decreased activation within those regions19,24,29,31,33,35, with many studies being limited by investigation of an isolated region of interest (ROI) determined by a priori hypotheses, which may introduce bias into the literature.
Therefore, the aim of the present activation likelihood estimation (ALE) meta-analysis was to examine neural regions activated during implicit emotion regulation by directly comparing mood and anxiety disorder patients to healthy controls. The findings will offer support to the National Institute of Mental Health (NIHM) Research Domain Criteria (RDoC) framework, where the aim is to develop diagnostic systems that rely on both clinical observations and recent research developments in the clinical neurosciences36. Understanding of the neural underpinnings of implicit emotion regulation in mood and anxiety disorders may not only reveal new insights into their pathophysiology and diagnosis, but also predict better treatment outcomes12,36–39.
Results
Study selection and data extraction
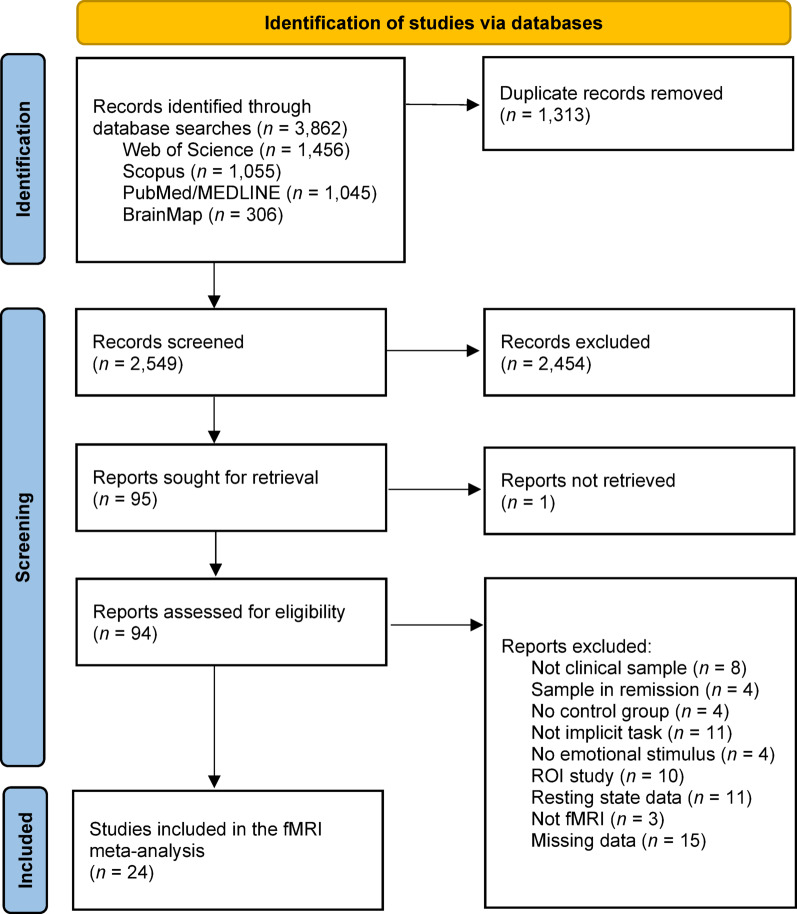
Articles were sought after in the Web of Science (n = 1,456), Scopus (n = 1,055), PubMed/MEDLINE (n = 1,045), and BrainMap (n = 306) databases. The OpenGrey database produced no records. Duplicate records were removed, and the titles and abstracts of the remaining articles were assessed against eligibility criteria, with a total 2,454 records failing to meet inclusion. The final articles (n = 95) were retrieved for full-text screening. Full-text screening resulted in the exclusion of further studies due to several reasons including the sample not meeting a clinical diagnosis or being in remission, no healthy control comparison sample, not investigating implicit emotion regulation, not evaluating responses to emotional stimuli, a ROI study, resting state data, not an fMRI design, and missing data/no access. Authors were contacted to manage missing data, with one response. A final list of neuroimaging studies (n = 24) were eligible for a coordinate-based meta-analysis. The full study selection details are presented in a PRISMA flowchart40 in Fig. 1. A full PRISMA checklist is available in Appendix A of Supplementary Materials.
Fig. 1.
PRISMA flowchart of study selection process. Note. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the study selection process40.
Of the 24 studies (patients: n = 684; healthy controls: n = 579) included in the ALE meta-analysis, eight were major depressive disorder/unipolar depression29,31,37,41–45, two were bipolar disorder37,41, five were generalised anxiety disorder33,42,46–48, six were generalised social phobia/social anxiety disorder27,35,46,49–51, six were panic disorder with/without agoraphobia21,22,32,38,47,52, and three were post-traumatic stress disorder47,53,54. Note that some studies examined more than one psychiatric disorder. Implicit emotion regulation paradigms extracted from the literature searches included implicit emotional response inhibition, implicit attentional cognitive control, implicit cognitive reappraisal, implicit emotional conflict adaptation, implicit emotional regulation, attentional performance with emotional distractors, indirect emotional facial processing, directed attention with disorder specific stimuli, and emotional-associative learning. Characteristics of the studies included in the ALE meta-analysis can be viewed in Table 1.
Table 1.
Characteristics of mood and anxiety disorder studies included in the activation likelihood estimation meta-analysis.
| Author(s) | Sample | Psychiatric disorder(s) | Diagnostic measurement |
Comorbidities | Implicit emotion regulation study characteristics |
|---|---|---|---|---|---|
| Wang et al., 2021 | 26 patients, 25 controls | Panic disorder | DSM-5 | Generalised anxiety disorder, social anxiety disorder, depressive disorder | PD and healthy control participants view negative images preceded by either a negative or non-negative description. Neural activation is observed during the presentation of negative images comparatively between the two (preceding negative and non-negative description) conditions. |
| Thomaes et al., 2012 | 29 patients, 22 controls | Post-traumatic stress disorder | The Structured Clinical Interview for DSM-IV-TR Axis I disorders, the Structured Clinical Interview for Disorders of Extreme Stress Not Otherwise Specified & the Clinician Administered PTSD Scale | Anxiety-disorders, depressive disorders, personality disorders | Implicit emotional conflict regulation in PTSD and healthy control participants. On high emotional conflict (i.e., incongruent [I]) trials, participants must respond to the colour of negative and trauma-related affect labels, irrespective of the affect label which are ‘task-irrelevant’. |
| Yu et al., 2015 | 19 patients, 19 controls | Generalised anxiety disorder | DSM-IV | n/a | Response inhibition in GAD and healthy controls towards negative stimuli. Participants respond to the biological sex of faces (Go/No-Go) irrespective of sad emotional facial expressions, which are ‘task-irrelevant’. |
| Etkin & Schatzberg, 2011 | 57 patients, 32 controls | Generalised anxiety disorder & major depressive disorder | Mini International Neuropsychiatric Interview | Comorbid generalised anxiety disorder & major depressive disorder | Implicit emotional conflict regulation in MDD, GAD, and healthy control participants. Participants must categorise emotional facial expressions, irrespective of overlaid (‘fear’ and ‘happy’) affect labels which are ‘task-irrelevant’. |
| Heitmann et al., 2017 | 24 patients, 24 controls | Generalised social anxiety disorder | Structured Clinical interview for DSM-IV | Major depressive disorder, specific phobia, obsessive-compulsive disorder, general anxiety disorder | SAD and healthy control participants respond to a bar orientation task, irrespective of disorder-related (e.g., giving speech, discussion scene, job interview) scenes. |
| Palm et al., 2011 | 15 patients, 16 controls | Generalised anxiety disorder | Structured Clinical Interview for DSM-IV Disorders | Social phobia, specific phobia, panic attacks | GAD and healthy control participants are presented with anger, disgust, fear, happiness, and sadness emotional facial expressions and must identify the biological sex of the face. |
| Bürger et al., 2017 | 72 patients, 36 controls | Unipolar depression & bipolar disorder | The Structured Clinical Interview for DSM-IV Axis I Disorders |
Panic disorder, agoraphobia, generalised anxiety disorder, social phobia, specific phobia, obsessive-compulsive disorder, post-traumatic stress disorder, somatoform disorder, eating disorder, dysthymia, alcohol abuse, substance abuse |
MDD, BD and healthy control participants must recognise and match facial stimuli, irrespective of angry, fearful, and happy facial expressions, that are ‘task-irrelevant’. |
| Arnone et al., 2012 | 38 patients, 54 controls | Major depressive disorder | Structured Clinical Interview for DSM-IV Axis I Disorders | n/a | MDD and healthy control participants identify the biological sex of sad, fearful, and happy emotional facial expressions, irrespective of the emotional content which is ‘task-irrelevant’. |
| Klumpp et al., 2013 | 29 patients, 27 controls | Generalised social anxiety disorder | Structured Clinical Interview for DSM-IV | n/a | gSAD and healthy control participants match geometric shapes and angry, fearful and happy emotional faces. |
| Gaebler et al., 2013 | 21 patients, 21 controls | Social anxiety disorder | Structured Clinical Interview for DSM-IV Axis I Disorders | Major depression, panic disorder, obsessive-compulsive disorder, dysthymia | SAD and healthy control participants match geometric shapes and faces with the presentation of angry and fearful emotional faces, which are ‘task irrelevant’. |
| Cerullo et al., 2014 | 50 patients, 25 controls | Major depressive disorder, bipolar disorder | Structured Clinical Interview for DSM-IV Axis I Disorders | Panic disorder, post-traumatic stress disorder, generalised anxiety disorder, obsessive-compulsive disorder, social anxiety disorder, substance use | MDD, BP-I, and healthy control participants complete an attentional performance task by responding to circles with the inclusion of distractor unpleasant emotional scenes. |
| Kraus et al., 2018 | 14 patients, 12 controls | Social anxiety disorder | Structured Clinical Interview for DSM-IV Disorders | Obsessive-compulsive disorder, specific phobia | SAD and healthy control participants view fearful emotional facial expressions and respond to the biological sex. |
| Blair et al., 2011 | 25 patients, 23 controls | Social anxiety disorder | Structured Clinical interview for DSM-IV Axis I disorders | n/a | SAD and healthy control participants identify the biological sex of morphing fearful, angry, and happy emotional facial expressions across various intensities. |
| Schwarzmeier et al., 2019 |
10 patients, 10 controls |
Panic disorder | DSM-IV-TR | Unipolar depression, anxiety disorders | PD and healthy control participants complete an agoraphobia symptom provocation task in which biological male facial stimuli are presented with an aversive panic scream. |
| Mazza et al., 2012 |
10 patients, 10 controls |
Post-traumatic stress disorder | Clinician-Administered PTSD Scale for DSM-IV criteria | n/a | PTSD and healthy control participants are presented with happy and sad emotional facial expressions that are followed by ideographs, which must be judged on pleasantness. |
| Korgaonkar et al., 2021 | 22 patients, 33 controls | Panic disorder | Mini International Neuropsychiatric Interview using DSM-IV criteria | Generalised anxiety disorder, major depressive disorder, obsessive-compulsive disorder, social phobia, agoraphobia | Implicit processing of sad, fear, anger, disgust, and happy emotional facial expressions in PD and healthy control participants. |
| Blair et al., 2012 | 50 patients, 18 controls | Generalized anxiety disorder & generalised social phobia | Structural Clinical interview for DSM-IV Axis I disorders | Comorbid generalised anxiety disorder & generalised social phobia | Emotional attention regulation in GAD, SAD, and healthy control participants. Participants are required to complete a number matching task irrespective of the presentation of positive and negative images that are ‘task-irrelevant’. |
| Neumeister et al., 2018 | 60 patients, 60 controls | Panic disorder, generalised anxiety disorder, post-traumatic stress disorder | Structured Clinical Interview for DSM-IV |
Depressive disorder, specific phobia, social anxiety disorder, agoraphobia, eating disorder, somatoform disorder |
Comparing the implicit processing of fearful facial stimuli between PD, GAD, PTSD, and healthy control participants. |
| Mitterschiffthaler et al., 2008 | 17 patients, 17 controls | Major depressive disorder | Structured Clinical interview for DSM-IV Axis I disorders | n/a | MDD and healthy control participants are presented with negative words in red, blue, green, or yellow and must respond to the colour of the negative words. |
| Ruhé et al., 2011 | 22 patients, 22 controls | Major depressive disorder | Structured Clinical interview for DSM-IV Axis I disorders | Anxiety disorders, substance use | MDD and healthy control participants are presented with fearful, angry, and happy emotional facial stimuli. Participants must make judgements based on the biological sex of the faces. |
| Frodl et al., 2009 | 12 patients, 12 controls | Major depressive disorder | DSM-IV | n/a | MDD and healthy control participants are presented with a trio of sad and angry faces. Participants must match the biological sex of faces with a target face. |
| Kaldewaij et al., 2019 | 18 patients, 17 controls | Panic disorder with/without agoraphobia | Structured Clinical Interview for DSM-IV Axis I Disorders | Agoraphobia, specific phobia | PD and healthy control participants identify the biological sex of morphing fearful and happy emotional facial expressions across various intensities irrespective of the emotional content. |
| Feldker et al., 2018 | 26 patients, 26 controls | Panic disorder with/without agoraphobia | Structured Clinical Interview for DSM-IV Axis I Disorders | Agoraphobia, depression, comorbid generalised anxiety disorder, somatic symptom disorder, social phobia, obsessive-compulsive disorder, bulimia nervosa | PD and healthy control participants respond to a bar orientation task, irrespective of panic-related (e.g., chest pain, hyperventilation, crowded areas) scenes. |
| Chechko et al., 2013 | 18 patients, 18 controls | Major depressive disorder | DSM-IV Structured Clinical Interview | n/a | Implicit emotional conflict regulation in MDD and healthy control participants. On high emotional conflict (incongruent [I]) trials, participants must respond to the emotional facial expression irrespective of the overlaid affect labels which are ‘task-irrelevant’. |
Note. Characteristics of mood and anxiety disorder studies (n = 24) investigating implicit emotion regulation included in the meta-analysis. Table reports author(s) and year of publication, sample size, psychiatric disorder(s) [major depressive disorder/unipolar depression, bipolar disorder, generalised anxiety disorder, generalised social phobia/social anxiety disorder, panic disorder with/without agoraphobia, and post-traumatic stress disorder], diagnostic criteria [the Structured Clinical Interview for the Diagnostic and Statistical Manual Of Mental Disorders (DSM), the Structured Clinical Interview for Disorders of Extreme Stress Not Otherwise Specified, the International Classification of Diseases (ICD), and the Mini International Neuropsychiatric Interview], comorbidities, and study characteristics. GAD = generalised anxiety disorder; MDD = major depressive disorder; BP-I = bipolar disorder; SAD = social anxiety disorder; gSAD = generalised social anxiety disorder; PD = panic disorder; PTSD = post-traumatic stress disorder. Under comorbidities n/a represents either no comorbidities or unreported comorbidities.
Quality assessment
To assess the quality of studies, the Newcastle-Ottawa Scale adapted for cross-sectional studies55 was modified for the purpose of this review. The outcome reported that 22 studies were of excellent quality and two were of good quality (see Table 2). The full list of assessment criteria (Appendix B) along with the list of corresponding authors of Table 2 (Appendix C) can be found in the Supplementary Materials.
Table 2.
Quality of mood and anxiety disorder studies included in the activation likelihood estimation meta-analysis.
| Author | [1] | [2] | [3] | [4] | [5] | [6] | [7] | [8] | [9] | [10] | [11] | [12] | [13] | [14] | [15] | [16] | [17] | [18] | [19] | [20] | [21] | [22] | [23] | [24] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Selection: (Maximum 4 stars) |
(1) | Representativeness of the clinical sample | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
| (2) | Selection of the non-clinical sample | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| (3) | Ascertainment of exposure | * | ** | ** | ** | ** | * | ** | ** | ** | ** | ** | ** | ** | * | ** | ** | ** | * | * | ** | ** | ** | ** | ** | |
| (4) | Demonstration that outcome of interest was not present in non-clinical sample | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | ||
|
Comparability (Maximum 2 stars) |
(5) | Comparability of samples in the different outcome groups based on design or analysis. | ** | * | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
|
Outcome: (Maximum 3 stars) |
(6) | Assessment of outcome | ** | ** | * | * | * | ** | * | * | ** | ** | * | * | * | ** | * | * | ** | * | * | * | ** | ** | ** | * |
| (7) | Statistical test | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | |
| Total score | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 10 | 10 | 9 | 9 | 9 | 9 | 9 | 9 | 10 | 7 | 8 | 9 | 10 | 10 | 10 | 9 | ||
Notes. Results of the Newcastle-Ottawa Scale adapted for cross-sectional studies55 reported that 22 studies were of excellent quality and two were of good quality. Full quality assessment criteria (Appendix B) and the list of corresponding authors (Appendix C) can be viewed in the Supplementary Materials.
Activation likelihood estimation meta-analysis
Activation likelihood estimation meta-analyses were conducted for mood and anxiety disorders combined. For exploratory purposes, subgroup analyses were performed for mood and anxiety disorders separately. All significant results for whole-brain foci during implicit emotion regulation are reported in Table 3.
Table 3.
Significant clusters of activation likelihood estimation meta-analysis for whole-brain foci in mood and anxiety disorder patients and healthy controls during implicit emotion regulation at FWE p <.05.
| Anatomical region | Brodmann area | Side | Cluster size | Peak coordinates MNI |
ALE score | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Mood and anxiety disorders | ||||||||
| Hypoactivation in patients (controls > patients) | ||||||||
| Medial frontal gyrus, anterior cingulate gyrus | 9, 32 | R | 928 mm3 | 4 | 38 | 26 | 0.023 | |
| Middle temporal gyrus, superior temporal gyrus | 21, 22 | L | 1,216 mm3 | −64 | −2 | −14 | 0.020 | |
| −56 | −6 | −20 | 0.017 | |||||
| Hyperactivation in patients (patients > controls) | ||||||||
| Medial frontal gyrus, superior frontal gyrus, middle frontal gyrus | 9, 10 | L | 736 mm3 | −22 | 50 | 12 | 0.023 | |
| Mood disorders | ||||||||
| Hypoactivation in patients (controls > patients) | ||||||||
| Medial frontal gyrus, anterior cingulate gyrus | 9, 32 | R, L | 1,128 mm3 | 4 | 38 | 26 | 0.023 | |
| Middle temporal gyrus, superior temporal gyrus | 21, 22 | L | 1440 mm3 | −64 | −2 | −14 | 0.020 | |
| Parahippocampal gyrus, culmen | 36, 35 | L | 648 mm3 | −26 | −34 | −18 | 0.018 | |
| Hyperactivation in patients (patients > controls) | ||||||||
| Medial frontal gyrus, superior frontal gyrus, middle frontal gyrus | 9, 10 | L | 1008 mm3 | −22 | 50 | 12 | 0.023 | |
| Orbital gyrus | 47, 11 | R | 872 mm3 | 20 | 20 | −32 | 0.020 | |
| Inferior frontal gyrus | 44, 45 | L | 512 mm3 | −54 | 22 | 10 | 0.016 | |
| Middle frontal gyrus | 47 | L | 552 mm3 | −46 | 40 | −8 | 0.017 | |
| Medial frontal gyrus | 6 | R, L | 520 mm3 | 4 | −2 | 52 | 0.017 | |
| Anterior cingulate gyrus, cingulate gyrus | 32, 24, 33 | L | 752 mm3 | 0 | 26 | 16 | 0.017 | |
| Insula | 13 | R | 536 mm3 | 42 | 2 | 14 | 0.017 | |
| Lentiform nucleus (putamen) | L | 616 mm3 | −26 | −6 | 6 | 0.017 | ||
| Claustrum | L | 504 mm3 | −38 | −14 | −4 | 0.016 | ||
| Claustrum, insula | R | 536 mm3 | 42 | −14 | −8 | 0.017 | ||
| Anxiety disorders | ||||||||
No significant clusters reported.
Table 3. Significant areas of convergence of hypoactivation and hyperactivation for whole-brain foci in mood and anxiety disorder patients during implicit emotion regulation. A cluster-level family-wise error correction threshold of p <.05 was used, with an uncorrected value of p <.001. A rigorous threshold of 5,000 permutations was applied. Peak coordinates are reported. Locations are reported to be 100% grey matter.
Convergence of hypoactivation in mood and anxiety disorders during implicit emotion regulation
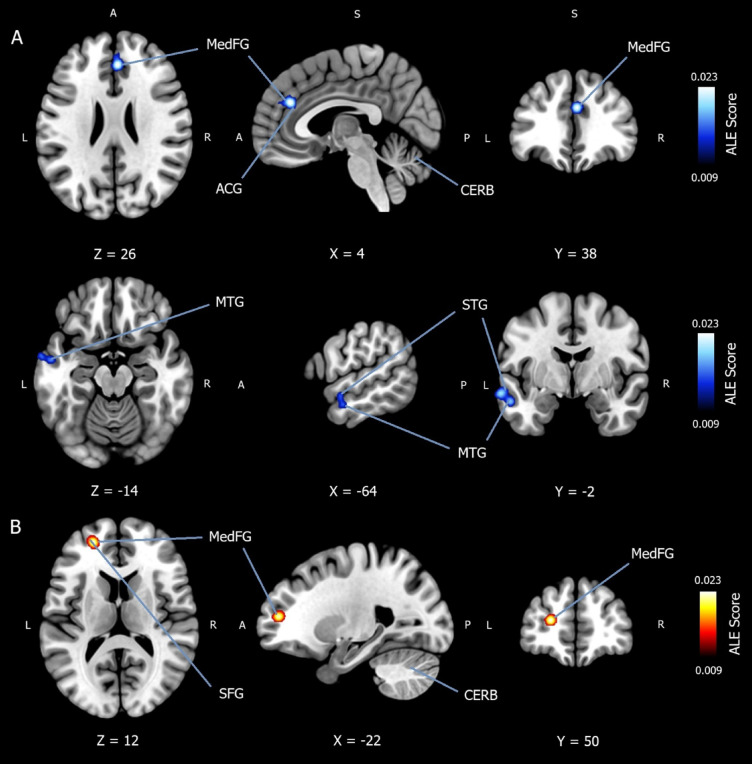
In total, 432 patients were included in the ALE meta-analysis, which converged whole-brain foci for decreased activation in mood and anxiety disorders (controls > patients) during implicit emotion regulation. A total of k = 19 experiments were extracted from the included studies and can be viewed in Appendix E of the Supplementary Materials. Six studies were MDD29,31,37,41,42,45, one was BP41, four were GAD33,42,46,48, three were SAD46,49,51, three were PD22,38,52, and one was PTSD53. Note that some studies examined more than one psychiatric disorder. A cluster-level family-wise error (FWE) correction threshold of p <.05 was used, with an uncorrected value of p <.001. A rigorous threshold of 5,000 permutations was applied. The ALE meta-analysis reported significantly more convergence of hypoactivation (controls > patients) in two cluster regions with three peak coordinates. The first cluster reported a peak coordinate (x = 4, y = 38, z = 26) in the right medial frontal gyrus (BA9), extending over the right anterior cingulate gyrus (BA32). The second cluster yielded two peak coordinates ([x = −64, y = −2, z = −14] and [x = −56, y = −6, z = −20]) in the left MTG (BA21), extending over the left STG (BA22). All locations were reported to be 100% grey matter. The result is available in Table 1. And can be viewed in Fig. 2A.
Fig. 2.
Activation likelihood estimation meta-analysis for convergence of hypoactivation and hyperactivation in mood and anxiety disorder patients during implicit emotion regulation. (A) Activation likelihood estimation (ALE) meta-analysis (n = 432) showing convergence of hypoactivation in patients compared to healthy controls (controls > patients) during implicit emotion regulation. Two cluster regions with three peak coordinates were identified using a cluster-level family-wise error (FWE) correction threshold of p < .05, with an uncorrected value of p <.001. A rigorous threshold of 5,000 permutations was applied. The first cluster reported a peak coordinate (x = 4, y = 38, z = 26) in the right medial frontal gyrus (BA9), extending over the right anterior cingulate gyrus (BA32). The second cluster yielded two peak coordinates ([x = −64, y = −2, z = −14] and [x = −56, y = −6, z = −20]) in the left middle temporal gyrus (BA21), extending over the left superior temporal gyrus (BA22). All locations were reported to be 100% grey matter. MedFG = medial frontal gyrus; SFG = superior frontal gyrus; ACG = anterior cingulate gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; CERB = cerebellum; ALE Score = ALE value. (B) ALE meta-analysis (n = 536) showing convergence of hyperactivation in mood and anxiety disorder patients compared to healthy controls (patients > controls) during implicit emotion regulation. One cluster region with one peak coordinate was identified using a cluster-level FWE correction threshold of p < .05, with an uncorrected value of p < .001. A rigorous threshold of 5,000 permutations was applied. The peak coordinate (x = −22, y = 50, z = 12) was reported in the left medial frontal gyrus (BA9), spreading to both the left superior frontal gyrus (BA10) and the left middle frontal gyrus (BA10). All locations were reported to be 100% grey matter.
Convergence of hyperactivation in mood and anxiety disorders during implicit emotion regulation
In total, 536 patients were included in the ALE meta-analysis, which converged whole-brain foci for increased activation in mood and anxiety disorders (patients > controls) during implicit emotion regulation. A total of k = 24 experiments were extracted from the included studies and can be viewed in Appendix F of the Supplementary Materials. Seven studies were MDD29,31,41–45, one was BP41, four were GAD42,46–48, six were SAD27,35,46,49–51, five were PD21,32,38,47,52, and three were PTSD47,53,54. Note that some studies investigated multiple psychiatric disorders. A cluster-level FWE correction threshold of p <.05 was used, with an uncorrected value of p <.001. A rigorous threshold of 5,000 permutations was applied. The ALE meta-analysis reported significantly more convergence of hyperactivation (patients > controls) in one cluster region with one peak coordinate. The peak coordinate (x = −22, y = 50, z = 12) was reported in the left medial frontal gyrus (BA9), spreading to both the left SFG (BA10) and left middle frontal gyrus (MFG) (BA10). All locations were reported to be 100% grey matter. The result is available in Table 1. And can be viewed in Fig. 2B.
Subgroup analysis for convergence of hypoactivation in mood disorders during implicit emotion regulation
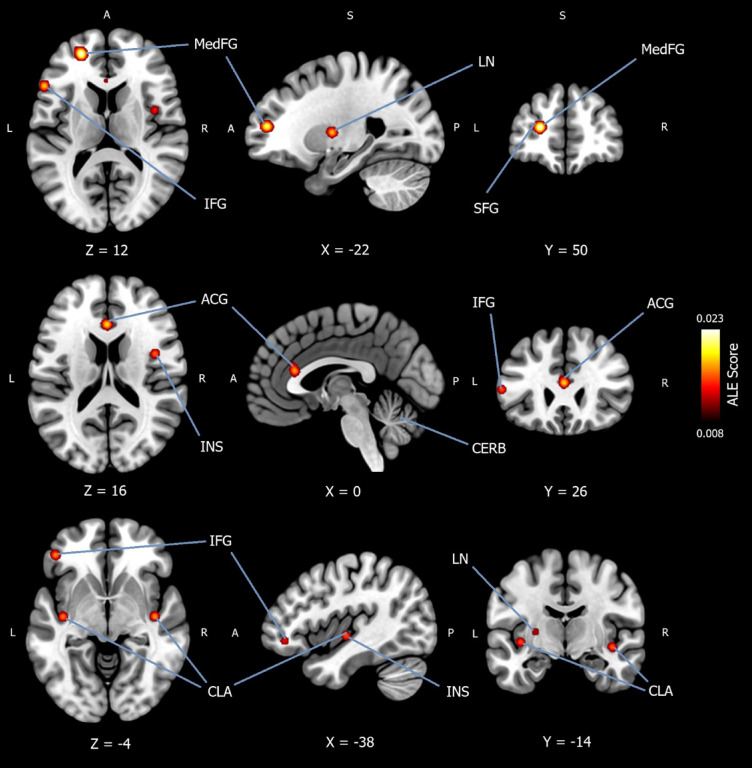
In total, 207 patients were included in the ALE meta-analysis, which converged whole-brain foci for decreased activation in mood disorders (controls > patients) during implicit emotion regulation. A total of k = 8 experiments were extracted from the included studies and can be viewed in Appendix G of the Supplementary Materials. Six studies were MDD29,31,37,41,42,45, and one was BP41. A cluster-level FWE correction threshold of p <.05 was used, with an uncorrected value of p < .001. A rigorous threshold of 5,000 permutations was applied. The ALE meta-analysis reported significantly more convergence of activation (controls > patients) in three cluster regions with five peak coordinates. The first cluster reported a peak coordinate (x = 4, y = 38, z = 26) in the right and left medial frontal gyrus (BA9), extending over the anterior cingulate gyrus (BA32). The second cluster yielded two peak coordinates ([x = −64, y = −2, z = −14] and [x = −56, y = −6, z = −20]) in the left MTG (BA21), extending over the left STG (BA22). Finally, two peak coordinates ([x = −26, y = −34, z = −18] and [x = −16, y = −38, z = −20]) were reported in the parahippocampal gyrus (BA36, BA35), spreading over the culmen. All locations were reported to be 100% grey matter. The result is available in Table 1. And can be viewed in Fig. 3.
Fig. 3.
Subgroup analysis for convergence of hypoactivation in mood disorder patients during implicit emotion regulation. Activation likelihood estimation meta-analysis (n = 225) showing convergence of hypoactivation in mood disorder patients compared to healthy controls (patients > controls) during implicit emotion regulation. Three cluster regions with five peak coordinates were identified using a cluster-level family-wise error correction threshold of p < .05, with an uncorrected value of p < .001. A rigorous threshold of 5,000 permutations was applied. The first cluster reported a peak coordinate (x = 4, y = 38, z = 26) in the right and left medial frontal gyrus (BA9), extending over the anterior cingulate gyrus (BA32). The second cluster yielded two peak coordinates ([x = −64, y = −2, z = −14] and [x = −56, y = −6, z = −20]) in the left middle temporal gyrus (BA21), extending over the left superior temporal gyrus (BA22). Finally, two peak coordinates ([x = −26, y = −34, z = −18] and [x = −16, y = −38, z = −20]) were reported in the parahippocampal gyrus (BA36, BA35), spreading over the culmen. All locations were reported to be 100% grey matter. MedFG = medial frontal gyrus; ACG = anterior cingulate gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; PHG = parahippocampal gyrus; CERB = cerebellum; ALE Score = ALE value.
Subgroup analysis for convergence of hyperactivation in mood disorders during implicit emotion regulation
In total, 225 patients were included in the ALE meta-analysis, which converged whole-brain foci for increased activation in mood disorders (patients > controls) during implicit emotion regulation. A total of k = 10 experiments were extracted from the included studies and can be viewed in Appendix H of the Supplementary Materials. Seven studies were MDD29,31,41–45, and one was BP41. A cluster-level FWE correction threshold of p <.05 was used, with an uncorrected value of p < .001. A rigorous threshold of 5,000 permutations was applied. The ALE meta-analysis reported significantly more convergence of activation (patients > controls) in ten cluster regions with ten peak coordinates. The first peak coordinate (x = −22, y = 50, z = 12) was reported in the left medial frontal gyrus (BA9), spreading to the left SFG (BA10), and left MFG (BA10). Peak coordinates were reported in the right orbital gyrus (x = 20, y = 20, z = −32) (BA47, BA11) and in the left (x = −54, y = 22, z = 10) inferior frontal gyrus (IFG) (BA44, BA45). Peak coordinates (x = −46, y = 40, z = −8) were reported in the left MFG (BA47) and in the right and left (x = 4, y = −2, z = 52) medial frontal gyrus (BA6). Peak coordinates (x = 0, y = 26, z = 16) were reported in the left anterior cingulate gyrus (BA32), spreading to the left cingulate gyrus (BA24, BA33). A peak coordinate (x = 42, y = 2, z = 14) was reported in the right insula (BA13) and in the left lentiform nucleus (putamen) (x = −26, y = −6, z = 6). Finally, peak coordinates were reported in left claustrum (x = −38, y = −14, z = −4) and the right claustrum (x = 42, y = −14, z = −8), spreading to the insula (BA13). All locations were reported to be 100% grey matter. The result is available in Table 1. And can be viewed in Fig. 4.
Fig. 4.
Subgroup analysis for convergence of hyperactivation in mood disorder patients during implicit emotion regulation. Activation likelihood estimation meta-analysis (n = 225) showing convergence of hyperactivation in mood disorder patients compared to healthy controls (patients > controls) during implicit emotion regulation. Ten cluster regions with ten peak coordinates were identified using a cluster-level family-wise error correction threshold of p < .05, with an uncorrected value of p < .001. A rigorous threshold of 5,000 permutations was applied. The first peak coordinate (x = −22, y = 50, z = 12) was reported in the left medial frontal gyrus (BA9), spreading to the left superior frontal gyrus (BA10), and left middle frontal gyrus (BA10). Peak coordinates were reported in the right orbital gyrus (x = 20, y = 20, z = −32) (BA47, BA11) and in the left (x = −54, y = 22, z = 10) inferior frontal gyrus (BA44, BA45). Peak coordinates (x = −46, y = 40, z = −8) were reported in the left middle frontal gyrus (BA47) and in the right and left (x = 4, y = −2, z = 52) medial frontal gyrus (BA6). Peak coordinates (x = 0, y = 26, z = 16) were reported in the left anterior cingulate gyrus (BA32), spreading to the left cingulate gyrus (BA24, BA33). A peak coordinate (x = 42, y = 2, z = 14) was reported in the right insula (BA13) and in the left lentiform nucleus (putamen) (x = −26, y = −6, z = 6). Finally, peak coordinates were reported in left claustrum (x = −38, y = −14, z = −4) and the right claustrum (x = 42, y = −14, z = −8), spreading to the insula (BA13). All locations were reported to be 100% grey matter. MedFG = medial frontal gyrus; SFG = superior frontal gyrus; IFG = inferior frontal gyrus; ACG = anterior cingulate gyrus; INS = insula; LN = lentiform nucleus; CLA = claustrum; CERB = cerebellum; ALE Score = ALE value.
Subgroup analysis for anxiety disorders during implicit emotion regulation
Parameters were set to a cluster-level FWE correction threshold of p <. 05, with an uncorrected value of p < .001. A rigorous threshold of 5,000 permutations was applied. However, no significant clusters were reported. For further exploration, the threshold for analysis was reduced to 1,000 permutations. Yet, no significant clusters were reported. An interpretation of this result is presented in the Discussion. Extracted experiments for both activation contrasts and can be viewed in Appendix I of the Supplementary Materials.
Discussion
Overall, whole-brain ALE meta-analysis reported convergence of hypoactivation during implicit emotion regulation in mood and anxiety disorder patients in two cluster regions. The first region was the right medial frontal gyrus (BA9), which extended over the right anterior cingulate gyrus (BA32). The second cluster region was the left MTG (BA21), extending over the left STG (BA22). In contrast, ALE meta-analysis reported convergence of hyperactivation in mood and anxiety disorder patients in one cluster region. This region was the left medial frontal gyrus (BA9, BA10), spreading to the left MFG and left SFG.
The medial frontal gyrus converges ventral and dorsal attention networks, which involves the mediation of top-down control over emotions, automatic behaviours, impulsivity, and mental states56–61. The anterior cingulate gyrus integrates amygdala and PFC activity regulating emotions, attention, inhibition, uncertainty, reward, and impulsivity62. Collectively, the medial frontal gyrus and the anterior cingulate gyrus form part of the medial prefrontal cortex (mPFC), which is responsible for learning associations between events and their corresponding adaptive emotional response63. To achieve this, the mPFC converges information from subcortical regions and other PFC regions62,64, regulating top-down control of limbic responses towards sensory information; including fear conditioning, attention, emotion regulation, motivation, decision-making, uncertainty, reward, and memory62–67. Previous research has outlined recruitment of the mPFC during implicit emotion regulation in healthy populations12. The results of the present ALE meta-analysis suggest the mPFC is an area of dysfunctional activation in mood and anxiety disorder patients during implicit emotion regulation. Specifically, the present ALE meta-analysis reported convergence of attenuation in patients in the right mPFC (including the ACC) during implicit emotion regulation. Substantial evidence suggests PFC attenuation in patients is a result of an inability to effectively regulate limbic responses due to weak fronto-limbic connectivity30. This has been noted in the ventral and dorsal medial PFC, including the ACC30,68,69, areas outlined in the present review’s findings. Such observations have also been observed in previous studies of mood and anxiety disorder patients during implicit emotion regulation20–23,25,27,33,34,37,38 and is further supported by general amygdala-PFC fear circuitry models and models of emotional dysregulation19,28. The present ALE meta-analysis simultaneously reported convergence of hyperactivation in patients in the left mPFC, which could highlight an adaptive compensatory mechanism. However, further investigation is required to explore this claim. Increased activation in the left mPFC may also be representitive of dysfunctional cognitive-emotional processes such as rumination and worry, as the left hemisphere is typically involved in language processing70,71. This may further be supported by the present findings of the ALE meta-analysis that reported hypoactivation in the left STG, which extended over the MTG; areas involved in language processing, mental states, social perception and memory56,72,73. A previous meta-analysis has also highlighted dysfunction in these regions during explicit emotion regulation in depressed patients13. Given such areas are highlighted in thought-related disorders, this finding may also be linked to the maladaptive nature of rumination and worry72,74. In summary, given the review’s findings and that patients experience difficulties across the functions of the mPFC, this area is highlighted as a ROI for future investigation. A recommendation for future research would be to investigate the nuanced lateralised differences of mPFC activation during implicit emotion regulation in patients.
While the lateral PFC has previously been outlined during implicit emotion regulation in patients19,22,24, this was not highlighted in the present review’s findings. One explanation for this may be that the lateral PFC is mainly involved during emotion regulation that requires higher-order cognitive processes or explicit emotion regulation12. Furthermore, the lateral PFC recruits the mPFC (including the ACC) during the suppression of limbic reactivity62. Therefore, the mPFC may act as a mediator between activation in the lateral PFC and the amygdala, responding quicker than the lateral regions during implicit emotion regulation or possibly overruling lateral PFC processing. In fact, while the lateral PFC is not present in all mammals, the mPFC exists in all mammals and may be an example of the primitive circuitry preserved in humans which supports higher-order cognitive functions that are unique to humans62.
The amygdala was not reported in the findings of the present ALE meta-analysis. This is surprising given the amygdalae consolidate the processing of emotion, motivation, and behaviour, and play a crucial role in emotional reactivity, autonomic responses, mental states, memory, and in the pairing of an emotional response from a stimulus to its emotional value or fear conditioning12,68,75–81. Several previous studies have outlined the amygdala during implicit emotion regulation in patients19,24,29–34. However, the present non-significant finding may collectively reflect the cognitive processes of implicit emotion regulation rather than emotional reactivity or fear conditioning69,79–81. Further research is needed to clarify the exact function of the amygdala during implicit emotion regulation. Research may investigate the several subdivisions of the amygdala which are known to regulate various aspects of behaviour77,81,82.
Although additional experiments are needed to confidently interpret the findings of the separate subgroup analyses for mood and anxiety disorders83, analyses were performed for exploratory purposes. Regarding mood disorders, findings of both hypoactivation and hyperactivation in patients reflected the overall outcomes, with additional areas identified. With regards to convergence of hypoactivation in mood disorder patients, the parahippocampul gyrus (BA36, BA35) and culmen were highlighted. The parahippocampul gyrus is linked to episodic memory, working memory, and temporal memory84. Given that mood disorders are also associated with memory problems, it is certainly plausible that related memory regions are also affected during implicit emotion regulation. The culmen forms part of the cerebellum and while not traditionally linked to emotion processing, recent research suggests a role of the cerebellum in emotional processing, emotional learning, and emotional control85, perhaps due to its connections to the PFC and limbic system. Further research is needed to investigate this with respect to implicit emotion regulation. On the contrary, convergence of hyperactivation in mood disorder patients was observed in several regions including the orbital gyrus (BA47, BA11), IFG (BA44, BA45), insula (BA13), lentiform nucleus, and claustrum. Previous research had highlighted dysfunctional activation during implicit emotion regulation in patients in the OFC25,26 and insula19,23,28. The insula is known to be implicated in sensory processing, valence processing, emotion processing, reward, interoception, and decision-making86. Furthermore, the insula converges sensory, limbic, and PFC regions, including connections to the mPFC and ACC86, areas outlined in this review. Therefore, it is plausible that the insula may be dysfunctional during implicit emotion regulation in patients. Further investigation is needed. Interestingly, the claustrum was highlighted in this review, and while the function of the claustrum remains largely unknown87, it has been linked to consciousness, attention, cognitive control, and salience processing87,88. Given this, the findings of the present ALE meta-analysis, and that the claustrum has extensive connections to the whole cerebral cortex87,88, the claustrum may in fact be recruited during implicit emotion regulation. Hyperactivity of the claustrum in patients may be representative of impaired cognitive control while implicitly regulating emotions or may represent increased neural effort to sustain implicit emotion regulation abilities. Importantly, further investigation is needed to outline the claustrum’s role during implicit emotion regulation.
Regarding anxiety disorders, no significant clusters were reported in this model. This may be due to heterogeneity between anxiety disorders. For instance, neural activity specific to GAD patients has been observed in response to threat in several neural regions compared to SAD and PD patients23. However, it is important to note that there were an insufficient number of studies for adequate power in the anxiety disorder subgroup83, which may account for this non-significant result. Further studies are needed to confidently perform ALE meta-analysis for the anxiety disorders subgroup in order to draw meaning interpretations.
Clinical implications
In addition to traditional diagnostic methods that classify symptoms across populations to establish a diagnosis (i.e., DSM-5), the RDoC framework aims to enhance the assessment, diagnosis, and treatment of psychiatric disorders by investigating the many domains of dysfunction within patients. To achieve this, the RDoC framework integrates findings from neurosciences, genetics, genomics, and behavioural sciences36. The results of the present fMRI meta-analysis reported mPFC dysfunction in patients during implicit emotion regulation, which is suggested to be a transdiagnostic characteristic of mood and anxiety disorders. Therefore, understanding the neural circuitry of the mPFC during implicit emotion regulation may be critical in improving our knowledge of the pathophysiology of mood and anxiety disorders. Such research may provide insights into their future assessment, diagnosis, and treatment. In addition, the right hemisphere appears to be attenuated in patients during implicit emotion regulation, while the left hemisphere shows hyperactivity. This may potentially be compensatory activation which may provide important information on the underlying mechanisms of implicit emotion regulation in patients. It would be pertinent to evaluate how mPFC activity alters over the course of treatment interventions, including both psychopharmacological and cognitive behavioural interventions. In fact, a meta-analysis reported that conscious reappraisal is associated with activation in the mPFC and ACC89. This could also provide information on the transdiagnostic nature of implicit emotion regulation. However, further investigation is required. The current findings offer support to the NIHM framework of developing diagnostic systems that are more predictive of treatment outcomes. Integrating findings from psychiatry and clinical neurosciences will help uncover more about these findings and how they translate to the clinic.
A previous meta-analysis of depressed participants reported dysregulation of the MTG, STG, and parahippocampal gyrus during explicit emotion regulation13. These areas were also highlighted in the present review and warrants further investigation. Along with the main findings of the present ALE meta-analysis, reporting dysregulation of the mPFC in patients during implicit emotion regulation, it appears that dysregulation of the lateral PFC may be related to maladaptive explicit emotion regulation and dysfunction of the medial PFC may be related to maladaptive implicit emotion regulation. This is supported by another meta-analysis that has highlighted neural differences during explicit vs. implicit emotion processing90. Therefore, this is a crucial area of investigation for future research.
Finally, the findings have implications for the effectiveness of explicit emotion regulation strategies. For instance, dysfunctional implicit emotion regulation may affect explicit emotion regulation through automatic spontaneous attention shifts11. Interventions that focus on conscious strategies such as cognitive behavioural therapy or rational emotive behaviour therapy may be constrained by maladaptive implicit emotion processing tendencies. Therefore, it would be important to investigate how dysfunctional implicit emotion regulation affects the success of conscious emotion regulation strategies and interventions. Further investigation is necessary.
Limitations and future directions
The review focused on mood and anxiety disorders due to their shared aetiology and frequent co-morbidity42,91. However, neural differences have been observed between conditions. For instance, MDD patients have exhibited reduced activation in the anterior cingulate gyrus compared with bipolar patients during implicit emotion regulation37. While GAD-specific activity in response to threat has been observed in several neural regions compared to SAD and PD patients23. Future ALE meta-analyses would examine psychiatric conditions separately. Additionally, the severity of a disorder, along with whether the disorder was a first episode or a relapsing disorder was not investigated. Future reviews would consider this. Future reviews would also focus on the inclusion of psychotropic medication and/or active psychotherapy. It may be hypothesised that appropriate intervention stabilises neural dysfunction during implicit emotion regulation. Finally, future meta-analyses may extend focus to other conditions excluded here, such as psychotic disorders or personality disorders, which may provide additional information on the transdiagnostic status of implicit emotion regulation.
The review aimed to investigate implicit emotion regulation across emotion processing in general as opposed to specific categories of emotions. However, neural differences have been reported in response to different emotions13. For instance, during implicit emotion regulation, generalised social phobic patients have exhibited increased amygdala activation in response to angry and contemptuous facial stimuli compared to happy facial stimuli92. Future reviews would examine emotions separately which would highlight important implicit emotion processing differences in patients, or may approach the research question from another perspective such as dimensional emotion systems, alternative to basic emotions.
Finally, research should explore the DMN and SN with respect to implicit emotion regulation in patients. In healthy individuals, the DMN is a large network of regions active when the mind is at rest and typically supressed when engaging in behaviours14. However, PTSD patients have exhibited increased activity during implicit emotion regulation19, while altered DMN activity has been linked to rumination in MDD15. The SN is responsible for the flexible shifting of attention between internal and external tasks. Dysfunctional activation of the SN has also been linked to mood and anxiety disorders16, which may account for difficulties of disengaging from negative information such as rumination and worry. Furthermore, the mPFC and ACC are largely implicated in the DMN and SN, respectively. As these neural regions were outlined in this review, further investigation would improve our understanding of implicit emotion regulation in patients and may lead to improved outcomes of assessment, diagnosis, and treatment.
Conclusion
This review outlines several areas of convergence of both hypoactivation and hyperactivation in mood and anxiety disorder patients during implicit emotion regulation. The overall findings indicate attenuation in the right mPFC and hyperactivation in the left mPFC in patients during implicit emotion regulation. The findings offer important information on the pathophysiology, diagnosis, and treatment of mood and anxiety disorders.
Methods
This meta-analysis was registered with PROSPERO, the International Prospective Register of Systematic Reviews (Registration ID: CRD42022360082). The review followed both neuroimaging meta-analysis protocol83 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)40.
Eligibility criteria
The present fMRI review focused on mood and anxiety disorders due to their common co-morbidity and shared aetiology42,91. Patients were recruited from several demographics including hospitals (mainly outpatients), clinics, and community residents. Patients were only included when a psychiatric diagnosis was based on established diagnostic measurements such as the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5)93 or the International Classification of Disease94. This excluded self-report cutoff measures as a means of a valid psychiatric diagnosis. Included study designs comprised of blinded and unblinded randomised clinical controlled comparison studies and experimental studies. Observational studies and case studies were excluded as they do not typically have a control condition. Healthy control samples acted as the controls in this review and were reported to have no history of psychiatric disorders, which were verified by structured clinical interviews. Functional MRI studies were the focus of this review and studies were included where authors reported whole-brain foci for paradigm-based measures of implicit emotion regulation, contrasted between patients and healthy controls. Thus, ROI studies were excluded. In this review, implicit emotion regulation was defined where participants automatically modulated their emotions without an active strategy. We included tasks that investigated attempts at regulating emotional responses, as opposed to tasks that observe neural responses towards emotional stimuli, which may involve responding to emotions without regulating them. Studies were excluded that investigated explicit forms of emotion regulation (i.e., conscious reappraisal) and studies that did not utilise a behavioural performance task.
Due to inconsistent findings in the fMRI literature, strict exclusion criteria were outlined. While patients with mood and/or anxiety disorder comorbidities were included, patients with a history of psychosis, a neurological condition, personality disorder, suicidal ideation, or psychiatric hospitalisation were excluded. With respect to bipolar disorder where psychosis has a higher prevalence, patients were only included where authors had clearly stated psychosis was not present in their sample. Studies where patients were in remission were excluded due to possible confounding effects. Additionally, studies were excluded if most of the patient sample were affected by psychotropic medications (i.e., SNRIs, SSRIs, or benzodiazepines) on the day of testing, and if they were receiving ongoing psychotherapy. Finally, heart disease and hypertension were excluded due to possible associations with neurodegenerative diseases. Individuals with MRI contraindications were excluded.
Search strategy
Peer-reviewed articles were screened from inception up until the end of April 2024. Searches were performed in databases which included Web of Science, Scopus, PubMed/MEDLINE, and BrainMap. Reference lists were also screened for relevant articles. Due to file-drawer concerns in line with clinical research, grey literature was searched in the OpenGrey database. Titles, abstracts, and keywords were screened using a total of 53 search terms relating to implicit emotion regulation in mood and anxiety disorders. Search terms were truncated and combined with Boolean operators and MeSH searching. An example of search terms included were: “implicit OR automatic OR unconscious OR nonconscious” AND “emotion* regulat*” OR “affect* regulat*” OR “mood regulat*” AND “mood disorder*” OR depress* OR “major depress* disorder” OR unipolar”. Although PTSD and OCD are no longer classified as anxiety disorders in the DSM-5, they were included in the searches due their substantial presentation of anxiety symptoms. Articles sought after were written in English Language due to limited resources for translating articles from other languages. The full search strategy can be found in Appendix D of Supplementary Materials.
Study selection and data extraction
Data extracted included author(s) and year of publication, sample size, psychiatric condition, diagnostic measurement, comorbidities, and study characteristics. To carry out a coordinate-based meta-analysis, whole-brain neural foci (x, y, z) were extracted for paradigm-based measures of implicit emotion regulation and contrasted as either patients (major depressive disorder/unipolar depression, bipolar disorder, generalised anxiety disorder, generalised social phobia/social anxiety disorder, panic disorder, and post-traumatic stress disorder) or healthy controls. Neural foci were extracted as reported by authors. In instances where authors did not provide data for both activation contrasts, available data was extracted. Acquisition space was also extracted. On occasions where a single study included more than one psychiatric condition (i.e., a separate sample of MDD and GAD patients), data were extracted as separate samples. To manage studies with multiple experiments, activation contrasts were only selected if they met the review’s aims. Further following the reviews aims, data was extracted and combined to create an ‘emotion’ variable as opposed to evaluating specific dimensions of emotion processing. Neutral stimulus conditions were omitted from extraction. Article suitability, full-text screening, and data extraction were conducted independently by two blinded reviewers (S.D.P.D and H.C) and disagreements were resolved by a third reviewer (S.C).
Quality assessment
The Newcastle-Ottawa Scale adapted for cross-sectional studies55 was modified for the purpose of this review. The quality of each study was assessed on whether patients were typical of the general population (e.g., severity and comorbidities) and whether healthy controls were comparable (e.g., age, gender or IQ.) Studies were additionally evaluated on whether patients had a verified mental health diagnosis (e.g., DSM-5 by a qualified person) and whether a structured clinical interview was carried out in both patients and healthy controls to confirm or reject diagnosis. Finally, samples were assessed on whether a diagnosis was performed by external persons (i.e., within an outpatient clinic) or by the research team.
Activation likelihood estimation meta-analysis
A meta-analysis of neural correlates was performed using an ALE meta-analysis. ALE meta-analysis determines above-chance convergence of foci between experiments95, or the convergence of differences in neural activation between patients and healthy controls during implicit emotion regulation. To achieve this, the ALE meta-analysis models coordinate locations (x, y, z) as a 3D Gaussian distribution of probability (i.e., empirical model of spatial uncertainty) or the likelihood of activation during implicit emotion regulation between patients and controls. Thus, replacing foci with a probability distribution and computing an ALE score95. Sample sizes were entered using the lowest sample size between patient and control groups, reducing computing bias. A minimum cluster level of 200mm3 was selected for cortical neural regions. For subcortical regions, the minimum cluster size threshold was removed in line with recent studies that have documented subcortical structures smaller than 50mm³ in MDD patients96. A FWE correction threshold of p < .05 was used, with an uncorrected value of p < .001. A strict threshold of 5,000 permutations was selected. If results yielded non-significant results, the threshold of analysis was reduced to 1,000 permutations for exploratory purposes. Only peak coordinates were reported. Overall, two ALE meta-analyses of convergence for whole-brain foci were performed contrasted by convergence of increased activation in patients (patients > controls) and convergence of decreased activation in patients (controls > patients). Four subsequent subgroup ALE meta-analyses were performed for exploratory purposes, which comprised of convergence of increased activation in mood disorder patients (patients > controls), convergence of decreased activation in mood disorder patients (controls > patients), convergence of increased activation in anxiety disorder patients (patients > controls), and convergence of decreased activation in anxiety disorder patients (controls > patients). The ALE meta-analysis was performed with GingerALE version 3.0.2., which generated anatomical labelling of clusters. All coordinates were reported in Montreal Neurological Institute (MNI) space. Studies that reported Talairach coordinates were converted to MNI space using BrainMap (GingerALE). The results were added as an overlay onto a MNI152 template in MRIcroGL. All figures were created in MRIcroGL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
S.D.P.D. conceptualisation, wrote original draft of manuscript, data extraction, quality assessment, activation likelihood estimation meta-analysis, produced tables, produced figures, edited manuscript, registration of review, project administration. H.C. data extraction and review. B.J. conceptualisation and review. S.C. conceptualisation, critical review, critically edited the manuscript, supervision.
Funding statement
This research was done as part of a PhD thesis and did not receive any specific funding from agencies in the public, commercial, or not-for-profit sectors. Stefan Daniel Paul Dalton is supported by the Brunel University London, College of Health, Medicine and Life Sciences Doctoral Research Fund and no other financial support was received during the research and/or the preparation of the manuscript.
Data availability
Raw and generated data, as well as data analysed during this review, are available within this published article and its supplementary materials.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stefan Daniel Paul Dalton, Email: stefandanielpaul.dalton@brunel.ac.uk.
Survjit Cheeta, Email: survjit.cheeta@brunel.ac.uk.
References
- 1.Aldao, A., Nolen-Hoeksema, S. & Schweizer, S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin. Psychol. Rev.30, 217–237 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Bargh, J. A. & Williams, L. E. The nonconscious regulation of emotion. In Handbook of Emotion Regulation 429–445 (The Guilford Press, 2007). [Google Scholar]
- 3.Bartholomew, M. E., Heller, W. & Miller, G. A. Inhibitory control of emotional processing: theoretical and empirical considerations. Int. J. Psychophysiol.163, 5–10 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg, A., Halperin, E., van Zomeren, M. & Gross, J. J. The process model of Group-Based emotion: integrating intergroup emotion and emotion regulation perspectives. Personal Soc. Psychol. Rev.20, 118–141 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Gross, J. J. & Thompson, R. Emotion regulation: conceptual foundations. Handb Emot. Regul.1, 3–27 (2007).
- 6.Gyurak, A., Gross, J. J. & Etkin, A. Explicit and implicit emotion regulation: A Dual-Process framework. Cogn. Emot.25, 400–412 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koole, S. L. & Rothermund, K. I feel better but I don’t know why: the psychology of implicit emotion regulation. Cogn. Emot.25, 389–399 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Mauss, I. B., Cook, C. L. & Gross, J. J. Automatic emotion regulation during anger provocation. J. Exp. Soc. Psychol.43, 698–711 (2007). [Google Scholar]
- 9.Webb, T. L., Miles, E. & Sheeran, P. Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol. Bull.138, 775–808 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Zhang, M. et al. The effects of subliminal goal priming on emotional response Inhibition in cases of major depression. Front Psychol11, (2020). [DOI] [PMC free article] [PubMed]
- 11.Dalton, S. D. P., Cooper, H., Jennings, B. & Cheeta, S. The empirical status of implicit emotion regulation in mood and anxiety disorders: A meta-analytic review. J. Affect. Disord. 380, 256–269 (2025). [DOI] [PubMed] [Google Scholar]
- 12.Etkin, A., Büchel, C. & Gross, J. J. The neural bases of emotion regulation. Nat. Rev. Neurosci.16, 693–700 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Gou, X. et al. The conscious processing of emotion in depression disorder: a meta-analysis of neuroimaging studies. Front Psychiatry14, (2023). [DOI] [PMC free article] [PubMed]
- 14.Menon, V. 20 Years of the default mode network: A review and synthesis. Neuron111, 2469–2487 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tozzi, L. et al. Reduced functional connectivity of default mode network subsystems in depression: Meta-analytic evidence and relationship with trait rumination. NeuroImage Clin.30, 102570 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schimmelpfennig, J., Topczewski, J., Zajkowski, W. & Jankowiak-Siuda, K. The role of the salience network in cognitive and affective deficits. Front Hum. Neurosci17, (2023). [DOI] [PMC free article] [PubMed]
- 17.Jamieson, A. J., Leonards, C. A., Davey, C. G. & Harrison, B. J. Major depressive disorder associated alterations in the effective connectivity of the face processing network: a systematic review. Transl Psychiatry. 14, 1–15 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentili, C. et al. Beyond emotions: A meta-analysis of neural response within face processing system in social anxiety. Exp. Biol. Med.241, 225–237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce, S. E. et al. Altered emotional interference processing in the amygdala and Insula in women with Post-Traumatic stress disorder. NeuroImage Clin.2, 43–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Killgore, W. D. S. et al. Cortico-Limbic responses to masked affective faces across PTSD, panic disorder, and specific phobia. Depress. Anxiety. 31, 150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaldewaij, R., Reinecke, A. & Harmer, C. J. A lack of differentiation in amygdala responses to fearful expression intensity in panic disorder patients. Psychiatry Res. Neuroimaging. 291, 18–25 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Wang, H. Y. et al. Neural basis of implicit cognitive reappraisal in panic disorder: an event-related fMRI study. J. Transl Med.19, 304 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buff, C. et al. Specifically altered brain responses to threat in generalized anxiety disorder relative to social anxiety disorder and panic disorder. NeuroImage Clin.12, 698–706 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonzo, G. A., Huemer, J. & Etkin, A. History of childhood maltreatment augments dorsolateral prefrontal processing of emotional Valence in PTSD. J. Psychiatr Res.74, 45–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenfeld, E. S. et al. Prolonged hemodynamic response during incidental facial emotion processing in inter-episode bipolar I disorder. Brain Imaging Behav.8, 73 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minkova, L. et al. Task-dependent modulation of amygdala connectivity in social anxiety disorder. Psychiatry Res. Neuroimaging. 262, 39–46 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Klumpp, H., Post, D., Angstadt, M., Fitzgerald, D. A. & Phan, K. L. Anterior cingulate cortex and Insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol. Mood Anxiety Disord. 3, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver, S. S., Birn, R. M. & Cisler, J. M. A pilot adaptive neurofeedback investigation of the neural mechanisms of implicit emotion regulation among women with PTSD. Front Syst. Neurosci14, (2020). [DOI] [PMC free article] [PubMed]
- 29.Arnone, D. et al. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and Pharmacological treatment. Am. J. Psychiatry. 169, 841–850 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Burklund, L. J., Craske, M. G., Taylor, S. E. & Lieberman, M. D. Altered emotion regulation capacity in social phobia as a function of comorbidity. Soc. Cogn. Affect. Neurosci.10, 199–208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chechko, N. et al. Brain circuitries involved in emotional interference task in major depression disorder. J. Affect. Disord. 149, 136–145 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Feldker, K. et al. Cardiorespiratory concerns shape brain responses during automatic panic-related scene processing in patients with panic disorder. J. Psychiatry Neurosci.43, 26–36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palm, M. E., Elliott, R., McKie, S., Deakin, J. F. W. & Anderson I. M. Attenuated responses to emotional expressions in women with generalized anxiety disorder. Psychol. Med.41, 1009–1018 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Swartz, J. R. et al. Altered activation of the rostral anterior cingulate cortex in the context of emotional face distractors in children and adolescents with anxiety disorders. Depress. Anxiety. 31, 870–879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaebler, M., Daniels, J. K., Lamke, J. P., Fydrich, T. & Walter, H. Heart rate variability and its neural correlates during emotional face processing in social anxiety disorder. Biol. Psychol.94, 319–330 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Insel, T. et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 167, 748–751 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Bürger, C. et al. Differential abnormal pattern of anterior cingulate gyrus activation in unipolar and bipolar depression: an fMRI and pattern classification approach. Neuropsychopharmacology42, 1399–1408 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korgaonkar, M. S., Tran, J., Felmingham, K. L., Williams, L. M. & Bryant, R. A. Neural correlates of emotional processing in panic disorder. NeuroImage Clin.32, 102902 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waider, J., Araragi, N., Gutknecht, L. & Lesch, K. P. Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive control and emotion regulation: A perspective. Psychoneuroendocrinology36, 393–405 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ372, n71 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerullo, M. A. et al. Bipolar I disorder and major depressive disorder show similar brain activation during depression. Bipolar Disord. 16, 703–712 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Etkin, A. & Schatzberg, A. F. Common abnormalities and Disorder-Specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am. J. Psychiatry. 168, 968–978 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Frodl, T. et al. Neuronal correlates of emotional processing in patients with major depression. World J. Biol. Psychiatry. 10, 202–208 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Mitterschiffthaler, M. T. et al. Neural basis of the emotional Stroop interference effect in major depression. Psychol. Med.38, 247–256 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Ruhé, H., Booij, J., Veltman, D., Michel, M. & Schene, A. Successful Pharmacologic treatment of major depressive disorder attenuates amygdala activation to negative facial expressions: A functional magnetic resonance imaging study. J. Clin. Psychiatry. 73, 451–459 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Blair, K. S. et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and Top-Down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol. Psychiatry. 72, 476–482 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumeister, P. et al. Specific amygdala response to masked fearful faces in post-traumatic stress relative to other anxiety disorders. Psychol. Med.48, 1209–1217 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Yu, F. et al. The neural substrates of response Inhibition to negative information across explicit and implicit tasks in GAD patients: electrophysiological evidence from an ERP study. Front. Psychol.6, 275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blair, K. S. et al. The pathology of social phobia is independent of developmental changes in face processing. Am. J. Psychiatry. 168, 1202–1209 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heitmann, C. Y. et al. Brain activation to task-irrelevant disorder-related threat in social anxiety disorder: the impact of symptom severity. NeuroImage Clin.14, 323–333 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kraus, J. et al. Amygdala reactivity and connectivity during social and non-social aversive stimulation in social anxiety disorder. Psychiatry Res. Neuroimaging. 280, 56–61 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Schwarzmeier, H. et al. Characterizing the nature of emotional-associative learning deficits in panic disorder: an fMRI study on fear conditioning, extinction training and recall. Eur. Neuropsychopharmacol.29, 306–318 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Mazza, M. et al. Neural correlates of automatic perceptual sensitivity to facial affect in posttraumatic stress disorder subjects who survived L’Aquila eartquake of April 6, 2009. Brain Imaging Behav.6, 374–386 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Thomaes, K. et al. Treatment effects on insular and anterior cingulate cortex activation during classic and emotional Stroop interference in child abuse-related complex post-traumatic stress disorder. Psychol. Med.42, 2337–2349 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Wells, G. et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in Meta-Analyses. Control Clin. Trials. 21, 435–443 (2000). [Google Scholar]
- 56.Brunet, E., Sarfati, Y., Hardy-Baylé, M. C. & Decety, J. A PET investigation of the attribution of intentions with a nonverbal task. NeuroImage11, 157–166 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Corbetta, M., Patel, G. & Shulman, G. L. The reorienting system of the human brain: from environment to theory of Mind. Neuron58, 306–324 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kollndorfer, K. et al. Cortical thickness in the right medial frontal gyrus predicts planning performance in healthy children and adolescents. Front Psychol14, (2023). [DOI] [PMC free article] [PubMed]
- 59.Kübler, A., Dixon, V. & Garavan, H. Automaticity and reestablishment of executive Control—An fMRI study. J. Cogn. Neurosci.18, 1331–1342 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Talati, A. & Hirsch, J. Functional specialization within the medial frontal gyrus for perceptual Go/No-Go decisions based on what, when, and where related information: an fMRI study. J. Cogn. Neurosci.17, 981–993 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Wertheim, J. & Ragni, M. The neural correlates of relational reasoning: A Meta-analysis of 47 functional magnetic resonance studies. J. Cogn. Neurosci.30, 1734–1748 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Etkin, A., Egner, T. & Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci.15, 85–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Euston, D. R., Gruber, A. J. & McNaughton, B. L. The role of medial prefrontal cortex in memory and decision making. Neuron76, 1057 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu, P., Chen, A., Li, Y., Xing, X. & Lu, H. Medial prefrontal cortex in neurological diseases. Physiol. Genomics. 51, 432–442 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pati, S., Sood, A., Mukhopadhyay, S. & Vaidya, V. A. Acute Pharmacogenetic activation of medial prefrontal cortex excitatory neurons regulates anxiety-like behaviour. J. Biosci.43, 85–95 (2018). [PubMed] [Google Scholar]
- 66.Smith, I. M., Pang, K. C. H., Servatius, R. J., Jiao, X. & Beck, K. D. Paired-housing selectively facilitates within-session extinction of avoidance behavior, and increases c-Fos expression in the medial prefrontal cortex, in anxiety vulnerable Wistar-Kyoto rats. Physiol. Behav.164, 198–206 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Watanabe, M. [Emotional and motivational functions of the prefrontal cortex]. Brain Nerve Shinkei Kenkyu No Shinpo. 68, 1291–1299 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Barrett, L. F., Bliss-Moreau, E., Duncan, S. L. & Rauch, S. L. Wright, C. I. The amygdala and the experience of affect. Soc. Cogn. Affect. Neurosci.2, 73–83 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wittfoth, D., Pfeiffer, A., Bohne, M., Lanfermann, H. & Wittfoth, M. Emotion regulation through bifocal processing of fear inducing and disgust inducing stimuli. BMC Neurosci.21, 47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirsch, C. R. & Mathews, A. A cognitive model of pathological worry. Behav. Res. Ther.50, 636–646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nolen-Hoeksema, S., Wisco, B. E. & Lyubomirsky, S. Rethinking rumination. Perspect. Psychol. Sci.3, 400–424 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Kaur, A. et al. Structural and Functional Alterations of the Temporal lobe in Schizophrenia: A Literature Review. Cureus 12, (2020). [DOI] [PMC free article] [PubMed]
- 73.Mellem, M. S., Jasmin, K. M., Peng, C. & Martin, A. Sentence processing in anterior superior Temporal cortex shows a social-emotional bias. Neuropsychologia89, 217–224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang, L. et al. Superior Temporal gyrus and cerebellar loops predict nonsuicidal self-injury in major depressive disorder patients by multimodal neuroimaging. Transl Psychiatry. 12, 1–8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adolphs, R. Is the human amygdala specialized for processing social information?? Ann. N Y Acad. Sci.985, 326–340 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Berntson, G. G., Bechara, A., Damasio, H., Tranel, D. & Cacioppo, J. T. Amygdala contribution to selective dimensions of emotion. Soc. Cogn. Affect. Neurosci.2, 123–129 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bzdok, D., Laird, A. R., Zilles, K., Fox, P. T. & Eickhoff, S. B. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum. Brain Mapp.34, 3247–3266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fine, C., Lumsden, J. & Blair, R. J. R. Dissociation between `theory of Mind’ and executive functions in a patient with early left amygdala damage. Brain124, 287–298 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Gupta, R., Koscik, T. R., Bechara, A. & Tranel, D. The amygdala and decision-making. Neuropsychologia49, 760–766 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lanteaume, L. et al. Emotion induction after direct intracerebral stimulations of human amygdala. Cereb. Cortex. 17, 1307–1313 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Sigurdsson, T., Doyère, V., Cain, C. K. & LeDoux, J. E. Long-term potentiation in the amygdala: A cellular mechanism of fear learning and memory. Neuropharmacology52, 215–227 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Pierce, J. E. & Péron, J. The basal ganglia and the cerebellum in human emotion. Soc. Cogn. Affect. Neurosci.15, 599–613 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Müller, V. I. et al. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav Rev.84, 151–161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li, M., Lu, S. & Zhong, N. The Parahippocampal Cortex Mediates Contextual Associative Memory: Evidence from an fMRI Study. BioMed Res. Int. 9860604 (2016). (2016). [DOI] [PMC free article] [PubMed]
- 85.Ciapponi, C. et al. Variations on the theme: focus on cerebellum and emotional processing. Front Syst. Neurosci17, (2023). [DOI] [PMC free article] [PubMed]
- 86.Gogolla, N. The insular cortex. Curr. Biol.27, R580–R586 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Smith, J. B., Lee, A. K. & Jackson, J. The claustrum. Curr. Biol.30, R1401–R1406 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Goll, Y., Atlan, G. & Citri, A. Attention: the claustrum. Trends Neurosci.38, 486–495 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Kalisch, R. The functional neuroanatomy of reappraisal: time matters. Neurosci. Biobehav Rev.33, 1215–1226 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Costafreda, S. G., Brammer, M. J., David, A. S. & Fu, C. H. Y. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res. Rev.58, 57–70 (2008). [DOI] [PubMed] [Google Scholar]
- 91.Beck, R. & Perkins, T. S. Cognitive Content-Specificity for anxiety and depression: A Meta-Analysis. Cogn. Ther. Res.25, 651–663 (2001). [Google Scholar]
- 92.Stein, M. B., Goldin, P. R., Sareen, J., Zorrilla, L. T. E. & Brown, G. G. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch. Gen. Psychiatry. 59, 1027–1034 (2002). [DOI] [PubMed] [Google Scholar]
- 93.Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th Ed. xliv, 947 American Psychiatric Publishing, Inc., Arlington, VA, US, (2013). 10.1176/appi.books.9780890425596
- 94.Organization, W. H. The ICD-10 Classification of Mental and Behavioural Disorders : Diagnostic Criteria for Research (World Health Organization, 1993).
- 95.Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F. & Fox, P. T. Activation likelihood Estimation meta-analysis revisited. Neuroimage59, 2349–2361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tesen, H. et al. Volume of amygdala subregions and clinical manifestations in patients with First-Episode, Drug-Naïve major depression. Front Hum. Neurosci15, (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw and generated data, as well as data analysed during this review, are available within this published article and its supplementary materials.