Abstract
Gram-positive anaerobic cocci (GPAC) are a heterogeneous group of organisms defined by their morphological appearance and their inability to grow in the presence of oxygen; most clinical isolates are identified to species in the genus Peptostreptococcus. GPAC are part of the normal flora of all mucocutaneous surfaces and are often isolated from infections such as deep organ abscesses, obstetric and gynecological sepsis, and intraoral infections. They have been little studied for several reasons, which include an inadequate classification, difficulties with laboratory identification, and the mixed nature of the infections from which they are usually isolated. Nucleic acid studies indicate that the classification is in need of radical revision at the genus level. Several species of Peptostreptococcus have recently been described, but others still await formal recognition. Identification has been based on carbohydrate fermentation tests, but most GPAC are asaccharolytic and use the products of protein degradation for their metabolism; the introduction of commercially available preformed enzyme kits affords a physiologically more appropriate method of identification, which is simple and relatively rapid and can be used in routine diagnostic laboratories. Recent reports have documented the isolation in pure culture of several species, notably Peptostreptococcus magnus, from serious infections. Studies of P. magnus have elucidated several virulence factors which correlate with the site of infection, and reveal some similarities to Staphylococcus aureus. P. micros is a strongly proteolytic species; it is increasingly recognized as an important pathogen in intraoral infections, particularly periodontitis, and mixed anaerobic deep-organ abscesses. Comparison of antibiotic susceptibility patterns reveals major differences between species. Penicillins are the antibiotics of choice, although some strains of P. anaerobius show broad-spectrum β-lactam resistance.
Gram-positive anaerobic cocci (GPAC) are better known to most bacteriologists as peptococci or peptostreptococci; most clinical isolates are identified to species in the genus Peptostreptococcus. GPAC are a major part of the normal human flora and are frequently recovered from human clinical material (35, 84, 136, 251); they constituted 24 to 31% of all isolates in four surveys of anaerobic pathogens (28, 138, 196, 295). However, they have been little studied (186, 196, 262, 273) and their importance as human pathogens is insufficiently recognized. Many factors have contributed to this lack of interest. The classification is unsound and has been confused by many loosely defined taxa (136, 166, 167, 190); of the five species most frequently reported from clinical infections, two are known to be genetically heterogeneous (136, 167, 190, 202). Routine diagnostic laboratories can rarely give adequate resources to the isolation of slowly growing anaerobes; until recently, identification schemes relied on the availability of gas-liquid chromatography (GLC) (136, 251, 254) and therefore were impractical for most laboratories. From the clinician’s standpoint, most GPAC are cultured from polymicrobial infections (35, 129, 196, 273), often with well-recognized pathogens such as microaerophilic streptococci or fusobacteria, and their isolation is relatively unimportant—the patient has usually been given therapy that is effective against anaerobes long before the GPAC are cultured. Therefore, there has been little laboratory or clinical interest in the field.
Several recent advances have made the subject somewhat less impenetrable. Taxonomic studies involving nucleic acid techniques (67, 142, 166, 167, 188) should soon lead to a revision of the classification, better identification schemes and, eventually, a closer association between taxon and clinical context. With the introduction of preformed enzyme kits (59, 192–194), a simple, relatively rapid method of identification is now available for use in routine laboratories. Surveys of the clinical importance of GPAC have defined more clearly the pathogenic potential of different species (14, 33, 35, 196). The importance of Peptostreptococcus micros in intraoral infections is now recognized (110, 185, 221, 267). Studies of the pathogenicity of P. magnus have led to the description of virulence factors, some of which may have industrial applications (149, 158, 205, 286). This review discusses what is known of the biology of GPAC, describes recent advances, and defines areas in particular need of further study.
Terminology and Definition of GPAC
The study of GPAC has suffered from a proliferation of synonyms; at various stages, the terms “anaerobic streptococcus,” “anaerobic coccus,” “Peptococcus and Peptostreptococcus,” and “anaerobic gram-positive coccus” have been used to describe them. As the classification has evolved, the precise meaning of some of these terms has changed; for instance, until 1974 both Peptococcus and Peptostreptococcus included species of microaerophilic streptococci (134). At present, most species of clinical importance are classified in the genus Peptostreptococcus (35, 84, 136). However, this review will use the acronym GPAC; not only is it shorter, but the genus Peptostreptococcus is a phylogenetically heterogeneous taxon and will undergo radical revision (67, 167, 188). Many studies have preferred to use the term GPAC (85, 190, 192) or AGPC (anaerobic gram-positive coccus) (82, 84, 117, 142, 261). Furthermore, the term GPAC is useful in the routine diagnostic laboratory, because it gives a broad morphological description of organisms isolated under specified atmospheric conditions; it is a term of convenience, nothing more. Watt and Jack (272) defined anaerobic cocci as “cocci that grow well under satisfactory conditions of anaerobiosis and do not grow on suitable solid media in 10% CO2 in air even after incubation for 7 days at 37°C.” This is a valuable working definition, which will be used in this review.
CLASSIFICATION
These organisms have been fair game for amateur taxonomists. Hare (114)
Development until 1980
An awareness of the many changes in nomenclature (Table 1) makes it easier to assess previous identification schemes and old clinical reports. The classification has always been very unsatisfactory; at least 40 species have been described (114), but many were poorly defined. Some species, for instance Peptococcus activus, were not included in the 1980 Approved Lists of Bacterial Names because no strains were available (136, 237). Others are now recognized as synonyms, e.g., Peptococcus aerogenes for Peptostreptococcus asaccharolyticus (136, 237), or have been placed on the list of nomina rejicienda, for instance, Peptococcus anaerobius, now known as P. magnus (136).
TABLE 1.
Changes in classification of genus Peptococcus and genus Peptostreptococcus from 1974 to 1997
| Genus | Classification of:
|
||||
|---|---|---|---|---|---|
| Rogosa, 1974 (225) | Skerman et al., 1980 (237) | Holdeman Moore et al., 1986 (135) | Ezaki et al., 1992 (84) | 1997 | |
| Peptococcus | P. niger (T)a | P. niger | P. niger | P. niger | P. niger |
| P. activus | |||||
| P. aerogenes | |||||
| P. anaerobius | |||||
| P. asaccharolyticus | P. asaccharolyticus | ||||
| P. constellatus | |||||
| P. glycinophilus | |||||
| P. indolicus | |||||
| P. magnus | |||||
| P. prevotii | |||||
| P. saccharolyticus | |||||
| Peptostreptococcus | P. anaerobius (T) | P. anaerobius | P. anaerobius | P. anaerobius | P. anaerobius |
| P. asaccharolyticus | P. asaccharolyticus | P. asaccharolyticus | |||
| P. barnesae | P. barnesae | ||||
| ‘P. harei’b | |||||
| P. heliotrinreducens | P. heliotrinreducens | P. heliotrinreducens | |||
| P. hydrogenalis | P. hydrogenalis | ||||
| P. indolicus | P. indolicus | P. indolicus | |||
| ‘P. ivorii’b | |||||
| P. lacrimalis | |||||
| P. lactolyticus | |||||
| P. lanceolatus | |||||
| P. magnus | P. magnus | P. magnus | |||
| P. micros | P. micros | P. micros | P. micros | P. micros | |
| ‘P. octavius’b | |||||
| P. parvulus | P. parvulus | ||||
| P. prevotii | P. prevotii | P. prevotii | |||
| P. productus | P. productus | P. productus | P. productus | P. productus | |
| P. tetradius | P. tetradius | P. tetradius | |||
| P. vaginalis | |||||
(T) denotes the type species of the genus.
Recently proposed species (188).
The genera Peptococcus and Peptostreptococcus were described by Kluyver and van Niel in 1936 (154). They were separated on the basis of morphological characteristics: peptococci, the anaerobic equivalent of staphylococci, were arranged in clumps and were assigned to the tribe Micrococcaceae, whereas peptostreptococci, as anaerobic streptococci, were arranged in chains and were placed in the tribe Streptococcaceae. This distinction is influenced by so many variables, for instance the composition of the medium, that it is unreliable (84, 136); however, the scheme lasted until the application of DNA hybridization techniques in 1983 (87). A later scheme by Prevot in 1948 (218), based on microscopic appearance, divided GPAC into eight genera; the identification to the species level was based on criteria such as production of indole, liquefaction of gelatin, and growth in litmus milk. Most of these tests are now disregarded. A different approach was taken by Hare and coworkers (114, 116, 263). Their classification of anaerobic cocci used fermentation and gas production from five carbohydrates and five organic acids, combined with serological tests, from which they distinguished 10 groups of anaerobic cocci. Since they found that the results of carbohydrate fermentation reactions varied with the quantity of fatty acids in the medium, the tests were standardized by careful definition of their basal media. These authors were able to place 90% of nearly 400 human strains in well-defined groups (114). Commendably, they did not add more Latin names to a classification already burdened by loosely defined species. Hare’s scheme unfortunately included both microaerophilic and overtly aerobic groups, but its merits have been demonstrated by the identification of Hare group III with P. hydrogenalis (190) and the recent proposal of Hare group VIII as “P. octavius” (188).
In 1971, Rogosa (224) united three genera, Peptococcus, Peptostreptococcus, and Ruminococcus, in a new family of strictly anaerobic gram-positive cocci or coccobacilli, the Peptococcaceae; he added Sarcina in 1974 (225). He noted that peptococci and most peptostreptococci could use the products of protein decomposition as their sole energy source whereas ruminococci and sarcinae required the presence of carbohydrates for fermentation. He characterized ruminococci by the digestion of cellulose and the fermentation of cellobiose and sarcinae by the conspicuous arrangement of cells in packets. He noted the heterofermentative ability of most peptostreptococci and proposed that this property was of sufficient taxonomic importance that it should be used to distinguish them from the streptococci, which were characterized by the homofermentation of carbohydrates to form lactic acid. Holdeman and Moore (134) therefore transferred Peptococcus morbillorum, Peptococcus constellatus, and Peptostreptococcus intermedius to the genus Streptococcus, thus removing all microaerophilic species from the Peptococcaceae; this important revision was later supported by analysis of cell wall structure (276) and nucleic acid studies (142). Holdeman and Moore also described a new genus from human feces, Coprococcus, which they assigned to the family Peptococcaceae; coprococci, like ruminococci, used fermentable carbohydrates for growth, but they formed different metabolic end products, notably butyric acid. When the Approved Lists of Bacterial Names were published in 1980 (237), the present classification was beginning to take shape; seven species were recognized in the genus Peptococcus, and four were recognized in the genus Peptostreptococcus (Table 1).
Application of Nucleic Acid Techniques
The introduction of nucleic acid techniques in the 1980s soon led to a major revision of the classification (87) but regrettably little consensus, because the two investigations employing DNA-DNA hybridization techniques (87, 142) came to conflicting conclusions. Ezaki et al. (87) studied 65 strains, mainly from human clinical specimens, which they characterized by standard biochemical methods (carbohydrate fermentation reactions, volatile fatty acid [VFA] patterns detected by GLC, and tests for enzyme activity with the API ZYM commercial kit) and taxonomic techniques (cellular fatty acid analysis, determination of guanine-plus-cytosine content of DNA, and DNA-DNA homology experiments). They noted that the G+C content of Peptococcus niger, the type strain of the genus Peptococcus, was 51 mol%, but for other species in the genus Peptococcus, they recorded a G+C content of 29 to 34 mol%, a range they considered to be unacceptably large for one genus. Comparison of DNA-DNA homology values and cellular fatty acid profiles led them to reclassify four species of Peptococcus in the genus Peptostreptococcus, retaining only P. niger in the genus Peptococcus.
The data from the study of Ezaki et al. (87) finally disposed of the spurious distinction between peptococci and peptostreptococci based on cellular arrangement, and the new scheme was adopted in Bergey’s Manual of Systematic Bacteriology in 1986 (136). However, this revision was almost immediately challenged by a study of Huss et al. (142), which used similar techniques. The results of G+C contents and DNA-DNA hybridization values often differed between the two studies; for instance, Ezaki et al. recorded a binding value of 46% between ATCC 14963T and ATCC 9321T (the type strains of P. asaccharolyticus and P. prevotii, respectively), which indicated a distinct relationship, whereas Huss et al. detected no DNA homology between these strains. These studies also recorded significantly different values for the G+C contents of certain key strains, for instance, ATCC 14963T and ATCC 29427T, the type strains of P. asaccharolyticus and P. indolicus, respectively. Huss et al. assessed the degree of relatedness at the genus level by use of DNA-rRNA cistron similarity studies; they concluded that P. anaerobius DSM 20357 (not the type strain) was more closely related to the type strains of Eubacterium tenue and Clostridium lituseburense than to other species of GPAC. The conclusions of Huss et al. were not generally accepted at the time but they have been confirmed by later studies (67, 82, 211).
The application of nucleic acid techniques has led to the recognition that several species are only very remotely related to most species of GPAC. Analysis of nucleic acid relatedness data and cell wall peptidoglycan structure revealed that Peptococcus saccharolyticus should be transferred to the genus Staphylococcus (150). Most strains of Staphylococcus saccharolyticus will grow only under anaerobic conditions on primary isolation but on subculture will grow in an aerobic atmosphere including 10% CO2 (81); S. saccharolyticus is therefore not an anaerobic coccus by the definition of Watt and Jack (272), but it is a potential source of confusion. Peptostreptococcus parvulus was reclassified in the genus Streptococcus by Cato (57), but recent comparisons of 16S rRNA sequence data have led to its reassignment to a new genus, Atopobium, with two species that were previously placed in the genus Lactobacillus (68). Peptostreptococcus heliotrinreducens is also only very remotely related to other GPAC (167, 190) and should probably be classified with oral, asaccharolytic species at present in the genus Eubacterium (102, 216, 268).
Within the Peptococcaceae, P. magnus, P. micros, and P. anaerobius are generally accepted as being phylogenetically valid species. Peptococcus glycinophilus was shown to be a synonym of P. micros by Cato et al. (58), who compared the soluble cell protein patterns of the type strains by polyacrylamide gel electrophoresis (PAGE). The classification of Peptostreptococcus productus is under active review. P. productus is a strongly saccharolytic species with a distinctive oval cell morphology and a G+C content of 44 to 45 mol%, much higher than that of other peptostreptococci. Recently it was reclassified in the genus Ruminococcus (82). However, this study did not examine the type species of the genus Ruminococcus; other workers (219, 289) have demonstrated at least two unrelated groups in the genus. Until there is a definitive revision of the classification of ruminococci, Willems and Collins (289) have recommended that P. productus be retained in its present taxonomic position.
Several species of butyrate-producing GPAC have recently been described (83, 166, 188); they have all been assigned to the genus Peptostreptococcus as a placement of convenience. P. asaccharolyticus and P. prevotii, the two butyrate-producing species commonly reported from human pathological material, have long been recognized as genetically heterogeneous (87, 117, 166, 202). Until recently, standard identification schemes (128, 133, 254) assigned all indole-positive strains of GPAC to the species P. asaccharolyticus or P. indolicus; however, the type strains of both species are asaccharolytic. P. hydrogenalis was described by Ezaki et al. (83) from saccharolytic, indole-positive strains previously identified as P. asaccharolyticus; Hare group III (114, 116, 192, 263) is a synonym of P. hydrogenalis (190, 193). Another group of indole-positive, saccharolytic strains, the ‘trisimilis’ group, differs from P. hydrogenalis in whole-cell composition (190) and biochemical activity (186, 192) and probably merits species status. A group of strains biochemically similar to P. asaccharolyticus but differing in 16S rRNA sequence and whole-cell composition has recently been proposed as “Peptostreptococcus harei” (188, 190).
The species P. prevotii is still often used as a loose description for all strains of indole-negative, butyrate-producing GPAC (203), although Rogosa recognized that the group was heterogeneous and recommended that P. prevotii be placed on the list of nomina rejicienda in 1974 (225). Other species in this group have now been described: P. tetradius in 1983 (87) and P. vaginalis, P. lactolyticus, and P. lacrimalis in 1992 (166). However, there is strong evidence (166, 167, 190, 192) that related species of clinical importance still await formal description.
The Case for a Radical Revision
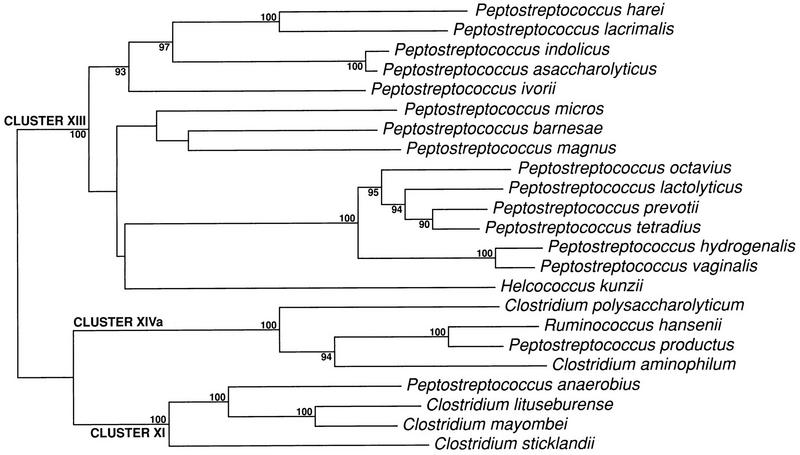
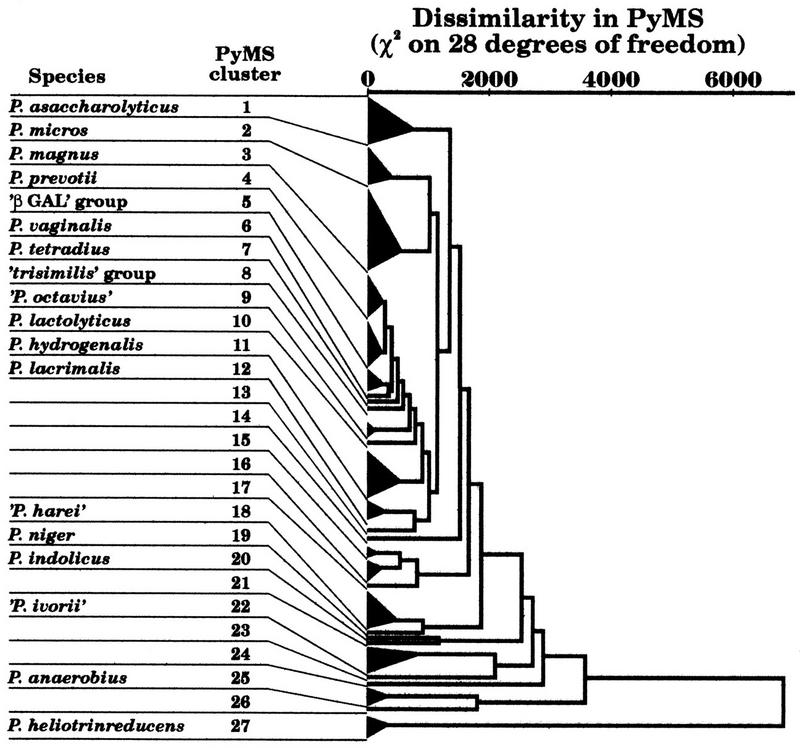
The present situation is that the genus Peptostreptococcus contains 13 recognized species, with a G+C range from 28 to 37 mol%, except for P. productus (44 to 45 mol%); there is now but one species in the genus Peptococcus, Peptococcus niger, which has a G+C content of 50 to 51 mol% (84). A recent study (190) compared conventional techniques with whole-cell composition by pyrolysis mass spectrometry (PyMS) (Fig. 1); it has led to the proposal of three new species: “P. harei,” “P. ivorii,” and “P. octavius” (Fig. 2) (188). Several further undescribed groups of strains formed distinct clusters by both techniques; these included βGAL (β-galactosidase) strains (192) and the ‘trisimilis’ group, both of which probably merit species status.
FIG. 1.
Clustering of 26 reference and 101 clinical strains of GPAC on the basis of whole-cell composition as assessed by PyMS. Numbered but unnamed clusters did not contain type strains of recognized or proposed species. Adapted from reference 190 with permission of the publisher.
FIG. 2.
Phylogenetic tree constructed by the neighbor-joining method, showing the position of Peptostreptococcus species within Clostridium rRNA clusters XI, XIII, and XIVa (67). Significant bootstrap values (90% and higher), expressed as a percentage of 500 replications, are indicated at the branching points. Adapted from reference 188 with permission of the publisher.
Recent studies based on comparisons of 16S rRNA sequence data have confirmed that Peptococcus niger (the type species of the family Peptococcaceae), is not related to other GPAC (67, 167), and they agree with those of Huss et al. (142) that P. anaerobius, the type species of the genus Peptostreptococcus, is more closely related to some members of the genus Clostridium than to other GPAC (67, 82, 163, 211). Li et al. (167) have recommended that the genus Peptostreptococcus be divided into at least seven genera. The most comprehensive survey, by Collins et al. (67), compared over 100 reference strains of low-G+C content gram-positive anaerobes, mainly type strains of the genus Clostridium. Collins et al. proposed splitting clostridia and related groups into 19 clusters based on 16S rRNA gene analysis. Of the 11 species in the genus Peptostreptococcus in this survey, 9 were assigned to cluster XIII, which also contained Helcococcus kunzii but no recognized species of Clostridium. Cluster XI contained one species of GPAC, P. anaerobius, with E. tenue and 18 species of Clostridium. P. productus was assigned to cluster XIVa with Streptococcus hansenii and species of Clostridium, Coprococcus, and Ruminococcus. Sarcina ventriculi and Sarcina maxima fell into a redefined genus Clostridium (cluster I) and were found to be closely related to Clostridium perfringens (288). Atopobium parvulum was placed in the Actinomycetales. A possible revised structure for GPAC based on these data is presented in Table 2.
TABLE 2.
Possible revised classification of GPAC based on 16S rRNA sequence analysis (67, 188) with type strains and G+C contentsa
| Genus | Species
|
Type strain
|
|||||
|---|---|---|---|---|---|---|---|
| Name | Terminal VFAb | G+C content (mol%)c | Major energy
sourceb
|
G+C content (mol%)c | Synonym(s)d | ||
| Carbohydrates | Proteins | ||||||
| Cluster XI | |||||||
| Peptostreptococcus | anaerobius (T) | IC (IV) | 33–34 | w | w | 34 | ATCC 27337/GIFU 7882/NCTC 11460/VPI 4330 |
| Cluster XIII | |||||||
| Genus 1 | prevotii | B | 29–33 | + | + | 33 | ATCC 9321/DSM 20548/GIFU 7658/NCTC 11806 |
| tetradius | B | 30–32 | ++ | + | 32 | ATCC 35098/DSM 2951/GIFU 7672 | |
| hydrogenalis | B | 30–31 | ++ | w | 30 | DSM 7454/GIFU 7662 | |
| lactolyticus | B | 34 | ++ | + | 34 | DSM 7456/GIFU 8586 | |
| vaginalis | B | 28–30 | + | + | 30 | DSM 7457/GIFU 12669 | |
| ‘octavius’ | C | 26–31 | + | w | 28 | NCTC 9810 | |
| Genus 2 | asaccharolyticus | B | 30–34 | − | + | 32 | ATCC 14963/DSM 20463/GIFU 7656/NCIB 10074/NCTC 11461 |
| indolicus | B | 32–34 | − | + | 34 | ATCC 29427/DSM 20464/GIFU 7848/NCTC 11088/VPI 11004 | |
| Genus 3 | magnus | A | 32–34 | −/w | ++ | 32 | ATCC 15794/DSM 20470/GIFU 7629/NCTC 11804 |
| Genus 4 | micros | A | 28–29 | − | ++ | 28 | ATCC 33270/DSM 20468/GIFU 7824/NCTC 11808/VPI 5464 |
| Genus 5 | barnesae | A(B) | 34–35 | − | w | 34 | DSM 3244 |
| Genus 6 | lacrimalis | B | 30–31 | − | ++ | 30 | DSM 7455/GIFU 7667 |
| Genus 7 | ‘harei’ | B | 25 | − | + | 25 | DSM 10020 |
| Genus 8 | ‘ivorii’ | IV | 28 | − | w | 28 | DSM 10022 |
| Cluster I | |||||||
| Clostridium | (S.) ventriculi | A | 28–31 | ++ | − | 31 | DSM 286 |
| (S.) maxima | B | 28–30 | ++ | − | 29 | DSM 316 | |
| Cluster IV | |||||||
| Ruminococcus | flavefaciens (T) | A | 39–44 | ++ | − | NK | ATCC 19208 |
| Cluster XIVa | |||||||
| ? | productus | A | 44–45 | ++ | − | 45 | ATCC 27340/GIFU 7707/NCTC 11829/VPI 4299 |
| ? | (R.) torques | A | 40–42 | ++ | − | 41 | ATCC 27756 |
| Coprococcus | eutactus (T) | B | 39–42 | ++ | − | 41 | ATCC 27759 |
| Not in clostridial subphylum | |||||||
| Peptococcus | niger (T) | C | 50–51 | − | − | 50 | ATCC 27731/DSM 20475/GIFU 7850/NCTC 11805/VPI 7953 |
| Atopobium | parvulum | A | 46 | ++ | w | 46 | ATCC 33793/DSM 20469/GIFU 7866/VPI 0546 |
| ?Eubacterium | heliotrinreducens | A | 34–37 | − | ++ | 36 | ATCC 29202/DSM 20476/NCTC 11029 |
R., Ruminococcus, S., Sarcina. A, acetate; B, butyrate; IV, isovalerate; IC, isocaproate; C, n-caproate. (T), type species of genus. −, negative; w, weakly positive; +, positive; ++, strongly positive; NK, not known.
Data on production of VFAs and major energy sources from Holdeman Moore et al. (135, 136) and Murdoch and Mitchelmore (192).
Data on G+C contents from Ezaki et al. (84, 86, 87), Li et al. (166), and Murdoch et al. (186, 188).
ATCC, American Type Culture Collection, Rockville, Md.; DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany; NCTC, National Collection of Type Cultures, London, United Kingdom; NCIB, National Collection of Industrial Bacteria, Aberdeen, United Kingdom; GIFU, Gifu University School of Medicine, Gifu, Japan; VPI, Virginia Polytechnic Institute, Blacksburg.
It is evident that the genus Peptostreptococcus requires radical revision (67, 163, 167). The continual changes in nomenclature are extremely confusing, and the constant addition of new species, although essential, makes the situation even more complicated for those not familiar with the field. Furthermore, several clinically important species still await formal description (166, 190, 192). It is difficult to escape the conclusion that the lack of a sound and stable classification has substantially contributed to the neglect of GPAC.
LABORATORY ISOLATION AND MAINTENANCE
Proper collection and transport of specimens is crucial for recovery of anaerobes in the laboratory. Finegold (90)
Most species in the genus Peptostreptococcus have been isolated from human clinical material and are not extremely oxygen sensitive; they are strict anaerobes in that they need an anaerobic atmosphere for multiplication, but the very limited data available suggest that many clinical strains are moderately aerotolerant. Fourteen clinical strains studied by Tally et al. (258) all survived exposure to atmospheric oxygen for 8 h; nine (63%) survived more than 72 h. Investigations (187) of four fresh clinical isolates of P. magnus and P. micros revealed that 1% of cells were still viable after exposure to air for 48 h.
Aspirates or tissue are generally considered to be the best specimens for culture of obligate anaerobes; swabs are less satisfactory (172). Specimens should be delivered to the laboratory as quickly as possible and must not be allowed to dry out, because moisture is important in maintaining viability (20). Anaerobic transport systems are necessary to optimize recovery rates (6, 20, 84, 243) and are essential for swabs; Mangels (172) presents an excellent overview of the subject. According to Watt and Smith (273), growth is best in the temperature range from 35 to 37°C and is enhanced by the presence of 10% CO2 in the atmosphere; a palladium catalyst should be present to remove traces of oxygen.
The nutritional requirements of Peptostreptococcus spp. have been very little studied and would repay investigation; given the heterogeneity of the genus, it is difficult to make generalizations. Commercial nonselective solid media vary in their ability to support the growth of fresh clinical isolates; in one study (119), Fastidious Anaerobe Agar (Lab M, Bury, United Kingdom) consistently gave the best growth. Sodium oleate (Tween 80; final concentration, 0.02%) enhances the growth of some species (114) but is not essential; neither are vitamin K nor hemin supplements (136). Satellitism of some fresh clinical isolates of the βGAL group has been noted around colonies of P. magnus on unenriched blood-containing media (187). Solid media that do not contain blood, such as Gifu anaerobic medium (Nissui, Tokyo, Japan) can support growth well (84). Ezaki et al. (84) stated that prereduced media are necessary for growth, but this is not my experience (187). Viability in chopped-meat broth depends on the commercial source; some preparations appear not to support growth at all, but most strains will survive in high-quality products for several months, if not years, at room temperature on the open bench, providing a very convenient method of short-term storage (187). For long-term storage, Holdeman Moore et al. (136) recommend lyophilization of cultures in the early stationary phase of growth in a medium containing less than 0.2% fermentable carbohydrate. GPAC retain viability well at −80°C or in liquid nitrogen (136).
Ruminococci, coprococci, and sarcinae are more sensitive to oxygen and will grow only under well-maintained anaerobic conditions (84). Carbohydrates are essential for the growth of ruminococci and sarcinae and stimulate the growth of coprococci (84). Most strains will not grow under the conditions encountered in routine laboratories; Bryant (53) and Ezaki et al. (84) give excellent guidelines for their isolation and culture.
Unfortunately, there have been few attempts to develop selective media for GPAC. This is a subject that deserves investigation; because most clinical isolates of GPAC grow relatively slowly on standard media and are usually present in mixed culture, they are frequently overgrown by other organisms, and many clinical studies will therefore give a falsely low estimate of their frequency. However, because GPAC are heterogeneous, a single medium is unlikely to support the growth of all representatives yet be reasonably selective. GPAC are very susceptible to most antibiotics, including, quite often, neomycin and polymyxin (187); they are usually resistant to bicozamycin (241, 270), but this broad-spectrum agent may no longer be available. Wren (292) showed that nalidixic acid-Tween blood agar (10 mg of nalidixic acid per liter, 0.1% Tween 80) gave better isolation than neomycin blood agar (75 mg of neomycin per liter) but recommended that a combination of different media be used to maximize recovery rates. Petts et al. (213) reported that oxolinic acid was superior to nalidixic acid for suppression of staphylococci but permitted the growth of nonsporing anaerobes, including GPAC. Ezaki et al. (84) recommended phenylethylalcohol agar (Difco, Detroit, Mich.), but Turng et al. (265) reported that 79% of strains of P. micros did not grow on PEA. Recently, Turng et al. (265) have described P. micros medium (PMM), a selective and differential medium for P. micros, which contains colistin-nalidixic acid agar (Difco), a selective base for gram-positive cocci, supplemented with glutathione and lead acetate. Strains of P. micros can use the reduced form of glutathione to form hydrogen sulfide, which reacts with lead acetate to form a black precipitate under the colony.
LABORATORY IDENTIFICATION
The absence of clear guidelines for characterisation [of GPAC] is a constant source of discouragement to the clinical microbiologist and this has held back studies on the role of these organisms in health and disease. Watt and Jack (272)
These comments were written in 1977; the situation has improved since but is still far from satisfactory; since there is no simple, accessible, and generally accepted identification scheme, few routine laboratories identify clinical isolates to the species level.
Identification to the Group Level
Grouping a clinical isolate can be surprisingly difficult; the microbiologist must decide whether an organism is a strict anaerobe and whether it is a coccus or a rod. In addition, many strains retain Gram’s stain poorly and therefore can appear gram negative. GPAC must be distinguished from microaerophilic organisms, most of which, from human clinical specimens, will be streptococci. Other species which can be misidentified as GPAC include Staphylococcus saccharolyticus and Gemella morbillorum. Microaerophilic strains of these species may appear only on anaerobic plates on primary incubation but will grow in 10% CO2 on repeated subculture. However, routine laboratories cannot wait 7 days to detect growth in an aerobic environment; a simple, reliable test is needed to distinguish strict anaerobes from microaerophiles. A cheap but effective technique is to apply a 5-μg metronidazole disc to the edge of the inoculum; two studies (192, 272) have reported that all strains of GPAC examined after incubation for 48 h showed zones of inhibition of 15 mm or greater whereas microaerophilic strains showed no zones. Metronidazole-resistant strains of GPAC have been reported (78, 230, 236, 280), but they appear to be rare.
Streptococci and gemellae are strongly saccharolytic, unlike most GPAC, and produce large quantities of lactic acid, which can be detected by GLC. S. saccharolyticus is a normal member of the skin flora; strains ferment several carbohydrates, produce urease and catalase, and reduce nitrate (81, 84). Some GPAC, particularly strains of P. asaccharolyticus, decolorize rapidly with Gram’s stain and can be confused with gram-negative anaerobes such as veillonellae; testing for vancomycin susceptibility with a 5-μg disc appears to be a simple and reliable method of separation (129, 192). The cell morphology of older cultures of GPAC can be very irregular, with many coccobacillary and rod-like forms, and can lead to possible confusion with lactobacilli or clostridia; P. anaerobius is particularly prone to pleomorphism, but its close relationship to some species of clostridia (67) shows that morphological distinctions are often arbitrary. The problem is similar for the facultative catalase-negative, gram-positive cocci and is well covered by Facklam and Elliott (88); the distinction relies considerably on the microbiologist’s judgement.
Identification to the Species Level
Early identification schemes depended on microscopic appearance, colonial morphology, ability to form gas from carbohydrates and organic acids, and carbohydrate fermentation reactions (114, 116, 218, 225). However, these tests proved to be of limited value for a group of bacteria that are often pleomorphic and usually asaccharolytic.
Gas-liquid chromatography.
In the 1970s, GLC was introduced for the detection of fatty acid by-products of metabolism (133, 254). Unfortunately, GPAC produce a very limited range of VFAs, but GLC can be used to classify them into three groups (Table 3): an acetate group containing several species, notably P. magnus and P. micros, which produce acetic acid or no VFAs at all; a large butyrate group, containing eight recognized species and a large number of unrecognized groups, which product butyric acid as their major terminal VFA; and a caproate group, whose members produce large quantities of longer-chain VFAs. The most important species in this last group is P. anaerobius, the only species of GPAC to produce a major terminal peak of isocaproic acid; GLC is a reliable method for its identification (133, 192). GLC is also useful for identifying the rarely isolated Peptococcus niger and the recently proposed “P. octavius” (188), which produce n-caproic acid, and “P. ivorii,” also recently proposed (188), which produces a terminal peak of isovaleric acid. Few workers except Ezaki et al. (87) have studied production of nonvolatile fatty acids (NVFAs), but it appears to be of little value except to exclude strains of streptococci, which, by definition, form large quantities of lactic acid (113). The results of GLC correlate very closely with biochemical tests (192) and whole-cell composition (190). Both liquid and solid media can be used for the detection of VFAs (285). Unfortunately, GLC is time-consuming, and the capital equipment is costly; although it is beyond the capabilities of most diagnostic laboratories, most identification manuals (133, 136, 251, 254) have recommended schemes in which GLC is an essential step.
TABLE 3.
Differential characteristics of Peptostreptococcus and Peptococcus speciesa
| Species (no. of strains examined) | Terminal VFA | Production
ofb
|
Carbohydrate fermentation
reactionsc
|
Production of
saccharolytic and proteolytic enzymesb:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indole | Urease | ALP | ADH | Glucose | Lactose | Raffinose | Ribose | Mannose | αGAL | βGAL | αGLU | βGUR | ArgA | ProA | PheA | LeuA | PyrA | TyrA | HisA | ||
| P. magnus (n = 116) | A | − | − | d | d | −/w | − | − | − | − | − | − | − | − | + | − | − | + | + | −/w | −/w |
| P. micros (n = 31) | A | − | − | + | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + |
| P. heliotrinreducens (n = 6) | A | − | − | − | + | − | − | − | − | − | − | − | − | − | d | + | + | + | − | w | w |
| P. productus (n = 1) | A | − | − | − | − | + | + | + | d | d | + | − | + | − | − | − | − | − | − | − | − |
| P. barnesae (n = 1) | A(B) | w | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| P. asaccharolyticus (n = 52) | B | d | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | d | − | d | w |
| P. indolicus (n = 6) | B | + | − | + | − | − | − | − | − | − | − | − | − | − | + | − | + | + | − | w | + |
| “P. harei” (n = 13) | B | d | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | −/w | − | w | + |
| P. lacrimalis (n = 1) | B | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | + | − | d | + |
| “trisimilis” group (n = 4) | B | + | − | d | − | + | w | −/w | −/w | + | − | d | − | − | − | − | − | − | + | − | − |
| P. hydrogenalis (n = 14) | B | + | d | −/w | − | + | + | + | − | + | − | − | d | − | − | − | − | − | − | − | − |
| P. prevotii (type strain) | B | − | + | − | − | − | − | + | + | + | + | − | + | + | + | − | − | − | + | w | + |
| P. tetradius (type strain) | B | − | + | − | − | + | − | − | − | + | − | − | + | + | + | − | w | + | w | w | w |
| prevotii/tetradius group (n = 34) | B | − | ?d | − | − | d | − | d | d | d | d | d | + | + | + | − | d | d | d | d | d |
| P. lactolyticus (n = 1) | B | − | + | − | − | + | + | − | − | + | − | + | − | − | + | − | − | − | − | − | − |
| P. vaginalis (n = 29) | B | ?d | − | −/w | + | + | − | − | − | d | − | − | − | − | + | − | − | + | − | − | + |
| βGAL group (n = 24) | B | − | − | w | d | + | + | − | + | + | − | w/+ | −/w | − | + | − | − | + | w | − | − |
| “P. ivorii” (n = 4) | IV | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| P. anaerobius (n = 63) | IC(IV) | − | − | − | − | + | − | − | − | w | − | − | + | − | − | + | − | − | − | − | − |
| “P. octavius” (n = 6) | C | − | − | − | − | + | − | − | + | + | − | − | − | − | − | + | − | − | w | − | − |
| P. niger (n = 1) | C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
A, acetate; B, butyrate; IV, isovalerate; IC, isocaproate; C, n-caproate; ALP, alkaline phosphatase; ADH, arginine dihydrolase; αGAL, α-galactosidase; βGAL, β-galactosidase; αGLU, α-glucosidase; βGUR, β-glucuronidase; ArgA, arginine arylamidase (AMD); ProA, proline AMD; PheA, phenylalanine AMD; LeuA, leucine AMD; PyrA, pyroglutamyl AMD; TyrA, tyrosine AMD; HisA, histidine AMD. −, >90% negative; w, weakly positive; +, >90% positive; d, different reactions.
Standard identification schemes.
Further identification has relied mainly on the fermentation of carbohydrates and other standard bacteriological tests such as nitrate reduction and urease production (129, 254), which may be valuable for other groups of bacteria but are of little use for GPAC. Most schemes (84, 129, 133, 251, 254) include testing for indole production, but its reliability is doubtful (190, 192). Since relatively few species of GPAC produce acid from carbohydrates (85, 136), many strains have been characterized on the basis of negative reactions (129, 254). Some recent schemes (129, 251) still base differentiation of P. magnus and P. micros on cell size, a highly unsatisfactory method of discrimination; its use requires the prior application of GLC and may lead to confusion with P. heliotrinreducens and asaccharolytic eubacteria (187). Colony morphology is a useful guide, particularly for many strains of P. micros, which exhibit a characteristic halo (195, 254), but this feature is not completely reliable (192). To quote a review from 1987 (262), “simple and reliable methods for the differentiation and taxonomy of these organisms have not yet been described.”
Preformed enzyme kits.
Identification methods should be appropriate to the metabolism of the organisms concerned. It has long been accepted that most species of GPAC are strongly proteolytic and use the products of protein decomposition as a major energy source (154, 225). In 1985, Ezaki and Yabuuchi (85) examined 77 strains of GPAC, comparing their amidase and oligopeptidase activities with VFA production and carbohydrate fermentation reactions. These tests were performed in an aerobic atmosphere at 37°C with a 4-h incubation period, conditions which are much more convenient for the routine diagnostic laboratory than those for carbohydrate fermentation tests. They found that the carbohydrate fermentation reactions were almost always negative but that most groups studied produced numerous positive peptidase reactions; in particular, P. magnus and P. micros were reliably differentiated by several tests. Peptidase activity correlated well with VFA production. These authors also examined 21 strains of microaerophilic streptococci and S. saccharolyticus, which they could easily distinguish by their strong saccharolytic activity and generally weak proteolytic activity. Furthermore, they were able to differentiate “classic” strains of P. asaccharolyticus from a group of saccharolytic indole-positive organisms, which they later described as P. hydrogenalis (83). This study showed that proteolytic enzyme profiles (PEPs) could distinguish clearly and reproducibly among recognized species of GPAC; it also revealed the value of PEPs for distinguishing undescribed groups of GPAC from recognized taxa.
This seminal study contributed to the development in the 1980s of several commercial preformed enzyme kits, for instance RapID ANA (Innovative Diagnostic Systems, Atlanta, Ga.), Vitek ANI card (Vitek Systems, Hazelwood, Mo.), ATB 32A (API-bioMérieux, Basingstoke, United Kingdom) (now renamed Rapid ID 32 A), and AN-IDENT (Analytab Products, Plainview, N.Y.). These kits incorporate a range of saccharolytic and proteolytic enzymes, carbohydrate fermentation reactions, “classic” tests such as tests for indole production and nitrate reduction, and others such as for alkaline phosphatase (ALP) and arginine dihydrolase (ADH) activity. However, they are designed to identify as wide a range of anaerobes as possible, and they include many tests of little relevance for GPAC. Furthermore, they are often marketed as “rapid” kits and neglect to mention that a heavy inoculum of the organism is required; subculture onto one or several plates for 48 h is usually necessary before an adequate inoculum can be harvested. Data comparing different agar bases and sources of blood are not available. The databases accompanying the kits are often incomplete or inaccurate, partly because long-recognized species such as P. prevotii are heterogeneous. Most importantly, the saccharolytic and proteolytic reactions can be very qualitative, which makes the tests difficult for inexperienced microbiologists to read and prone to variations in inoculum; this in turn reduces the confidence of laboratory workers in their value. It is unfortunate that kits do not always include color charts to make end points easier to read (203).
Many studies in the 1980s (4, 106, 148, 193, 194, 198, 249) compared commercial PEP kits with “gold standard” identification by conventional schemes (133, 254). Most of them examined a wide variety of anaerobic bacteria but relatively few strains of each group—rarely more than 30 strains of GPAC. Second-generation kits have now been marketed and evaluated (Table 4). Using the RapID-ANA II panel, Celig and Schreckenberger (59) were able to identify 71 of 73 strains (97%) of anaerobic cocci and microaerophilic streptococci correctly; the only 2 strains that were inadequately identified were the 2 strains of P. prevotii tested. Marler et al. (174) identified only 90 of 126 strains (72%) of GPAC to the species level; 37% of the strains of P. anaerobius were misidentified, but the incorrect tests were not detailed. Three of the four more recent evaluations (153, 169, 192, 203) of the ATB 32A kit (now renamed Rapid ID 32 A) have reported favorably. In the largest study (192), which included a comprehensive collection of type and reference strains, Murdoch and Mitchelmore were able to place 246 of 256 clinical strains (96%) in 1 of 10 PEP groups. However, they noted much greater heterogeneity in some groups, particularly in the butyrate-producing cocci, than was accepted at the time. For eight clinical strains which they characterized as Hare group III by their PEP, they later identified as P. hydrogenalis when the PEPs were compared with that of the type strain; similarly, a large group of indole-negative, butyrate-producing clinical strains, the ADH group, was subsequently identified as P. vaginalis. Both groups were informally recognized by their PEPs (197, 263) before either species was formally described; later comparison of the type strain and clinical strains by PyMS confirmed identification (190). These observations confirm the observations of Ezaki et al. (85, 87) that PEPs are much more powerful than conventional methods for characterizing GPAC. However, Ng et al. (203) tested the same system and reported that 8 of the 11 genital tract isolates of P. micros examined showed weak peptidase activity and did not form ALP; as a result, only 11 (58%) of 19 strains of P. micros were correctly identified. As would be expected, the 12 isolates that Ng et al. identified as P. prevotii by conventional techniques were heterogeneous; their PEPs were fully documented in the report (203), and several can be placed in recognized species such as P. vaginalis and P. tetradius (186, 192). The Rapid ID 32 A kit is not approved by the Food and Drug Administration for diagnostic use in the United States, but it can be purchased for research purposes. Users should be aware that the more recently recognized species of GPAC are not at present in the database and therefore that species such as P. hydrogenalis and P. vaginalis cannot be identified by means of a profile number.
TABLE 4.
Recent evaluations of preformed enzyme kits for the identification of the major species of GPAC
| Reference | System tested | No. of strains tested | Successful
identification to species level ofa:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| P. anaero- bius | P. asaccharo- lyticus | P. magnus | P. micros | P. prevotii | P. tetradius | P. hydro- genalis | |||
| Kitch and Appelbaum, 1989 (153) | ATB 32A | 20 | 6/6 (100) | 3/3 (100) | 7/7 (100) | 4/4 (100) | |||
| Looney et al., 1990 (169) | ATB 32A | 24 | 6/7 (83) | 6/7 (83) | 6/6 (100) | 4/4 (100) | |||
| Celig and Schreckenberger, 1991 (59) | RapID ANA II | 55 | 7/7 (100) | 24/24 (100) | 13/13 (100) | 6/6 (100) | 0/2 (0) | 3/3 (100) | |
| Marler et al., 1991 (174) | RapID ANA II | 126 | 15/24 (62) | 25/29 (86) | 26/27 (96) | 20/20 (100) | 4/25 (16) | 0/1 (0) | |
| Murdoch and Mitchelmore, 1991 (192) | ATB 32A | 295b | 47/47 (100) | 50/50 (100) | 87/87 (100) | 31/31 (100) | 1/1 (100) | 7/7 (100) | 9/9 (100) |
| van Dalen et al., 1993 (266) | ATB 32A | 39 | 4/4 (100) | 1/1 (100) | 34/34 (100) | ||||
| Ng et al., 1994 (203) | Rapid ID 32 A | 100 | 20/20 (100) | 25/25 (100) | 23/24 (96) | 11/19 (58) | 1/12 (8) | ||
Number of positive identifications/number of strains tested (percent positive).
Total strains tested; 63 (21%) strains were identified to other species or were not placed in recognized species.
Thus, these reports differ markedly in their appraisal of preformed enzyme kits. Most reports conclude that PEPs represent a considerable advance in identification methods in terms of speed, simplicity, and discrimination; furthermore, the kits are readily available in routine diagnostic laboratories. An identification scheme incorporating their use has been described (192). However, Ng et al. concluded that conventional methods rather than PEPs should be used to identify P. magnus, P. micros, and P. prevotii. It is clear that the peptidase activity of vaginal strains of P. micros requires further investigation. The studies by Marler et al. (174) and Ng et al. (203) recorded very poor success rates for the identification of “P. prevotii” because PEPs are much more discriminatory than conventional schemes (129, 251) and therefore can characterize recently described species such as P. vaginalis. Further reports would be valuable if they characterize indole-negative, butyrate-producing strains as far as possible rather than labelling them as P. prevotii.
Preliminary examination of the Rapid ID 32 STREP kit (API-bioMérieux) (187) indicates that this system may be of value for characterizing saccharolytic, butyrate-producing organisms in the P. prevotii/tetradius group (genus 1, Table 2); it allows clear differentiation of P. hydrogenalis and “trisimilis” strains.
The challenge now is to develop a more comprehensive classification, so that most clinical strains can be allocated to clearly defined, phylogenetically valid species; and to develop more reliable and discriminatory tests for difficult groups, particularly butyrate-producing strains.
Other techniques.
Few other identification tests are of value. Graves et al. (104) described a simple but useful method for the provisional identification of P. anaerobius; they showed that strains of P. anaerobius are susceptible to sodium polyanethol sulfonate (SPS; Liquoid) but that other species of GPAC are resistant. Later evaluations (192, 284) have confirmed that this test is both sensitive and specific. SPS paper discs are commercially available. Other antibiotic susceptibility tests, for susceptibility to novobiocin (296) or as used for gram-negative anaerobic rods (255), do not appear to be helpful.
Other approaches to the identification of GPAC have had limited success. Harpold and Wasilauskas (117) used high-pressure liquid chromatography (HPLC) to detect amino acid utilization by GPAC and reported that different species gave reproducible patterns. Krausse and Ullmann (155) used HPLC to detect the production of VFAs and NVFAs. Both reports drew attention to the potential of HPLC for automated microbial identification, but neither appears to have been followed up, perhaps because the capital costs of the equipment might be too high for routine diagnostic laboratories. Wells and Field (277) examined the long-chain fatty acid profiles of GPAC and concluded that they might be of taxonomic value but were not reliable for species designation. Serological studies have described an indirect fluorescent-antibody test (69), the analysis of EDTA-soluble extracts of cell surface components (240), and the use of antisera against whole cells raised in rabbits (291), but they have not been taken further.
The sophisticated requirements of oral microbiological research have led to the development of highly sensitive and specific DNA probes that can give rapid identification of individual colonies. Radiolabelled probes for P. anaerobius and P. micros have been described (297, 298), and more recently, a digoxigenin-labelled probe for P. micros has been reported (108). Probes are still prohibitively expensive for most routine diagnostic laboratories, in terms of cost of equipment and consumables, but they are invaluable in appropriate research institutions, particularly in studies of the complex ecology of the gingival crevice (245); they will be used more widely in the future.
COMPOSITION OF THE NORMAL FLORA
Virtually the only source of anaerobes participating in infection is the indigenous flora of mucosal surfaces and, to a much lesser extent, the skin. Finegold (90)
GPAC are part of the normal flora of the mouth, upper respiratory and gastrointestinal tracts, female genitourinary system, and skin (7, 84, 126, 201, 251). Since many commensal groups are rarely isolated from clinical specimens (84, 91, 183), there has been little stimulus to study them. Consequently, many commensal organisms cannot be assigned to recognized species and few reports of the normal flora have attempted identification to the species level. Analysis is complicated by conflicting data; for example, reports disagree about the presence of P. anaerobius in the oral and vaginal flora (123, 126, 136, 201, 241).
GPAC constitute 1 to 15% of the normal oral flora (201, 251, 253); they are found mainly in plaque and the gingival sulcus. P. micros is usually considered to be the predominant species of GPAC (136, 221), although, in a recent study (168) it was not isolated from gingival samples of 25 individuals with healthy sulci. Most workers would agree that P. anaerobius is part of the gingival flora (201, 235), and P. magnus has been reported to be a member (241), but Holdeman Moore et al. (136) stated that neither are members of the normal flora. It is possible that these species are regularly present but in undetectable numbers; the threshold for detection of GPAC is relatively high because of the lack of a selective medium.
The gastrointestinal tract hosts a wide variety of GPAC, including most recognized species of Peptostreptococcus and poorly studied groups such as Coprococcus, Ruminococcus, and Sarcina (7, 71, 91, 136, 183). Using optimal anaerobic techniques, Finegold et al. (91) and Moore and Holdeman (183) showed that P. productus is one of the commonest organisms in the gastrointestinal flora. Fecal composition is related to age (201, 247) and diet (71, 91). Finegold et al. associated high counts of P. micros with American rather than Japanese diets, while Crowther (71) reported that Sarcina ventriculi was common in the gastrointestinal tracts of vegetarians, in counts of up to 108/g of feces, but very infrequent in persons whose diets contained animal products.
Large numbers of GPAC can be found in the female genitourinary tract; counts vary with physiological processes such as the stage of the menstrual cycle and pregnancy (177), making assessment of the normal flora extremely complicated. P. tetradius, P. lactolyticus, and P. vaginalis were first described from vaginal discharges (87, 166); P. anaerobius, P. asaccharolyticus, P. hydrogenalis, P. magnus, P. micros, P. prevotii, and Peptococcus niger have also been isolated (11, 136, 201). Bartlett et al. (11) reported carriage rates of 40% and mean counts of 108.7/g in normal vaginal secretions; the most common species were P. magnus, P. asaccharolyticus, and P. prevotii. In a valuable recent study, Hillier et al. (126) reported carriage rates in pregnant women of 92% and mean counts of 104.2 CFU/ml; the most common species, in terms of both carriage rates and counts, were P. asaccharolyticus followed by P. magnus. Hill et al. (123) noted higher counts of GPAC in the vaginal flora of prepubertal girls than in the flora of normal adult women; the major species were P. tetradius and P. anaerobius. Unfortunately, neither Hill nor Hillier reported finding strains of P. vaginalis because satisfactory methods for its identification were not available at the time.
The skin flora contains GPAC (7, 90, 251), but its composition at the species level appears to have been little studied. Murdoch et al. (196) suggested that P. magnus, P. asaccharolyticus, and P. vaginalis are probably components because they can often be isolated from superficial wound infections.
It is clear that few investigations have been carried out on this difficult subject. Further studies would be very welcome; they should aim to characterize as many isolates as possible to the species level and to carry out quantitative counts when appropriate. The ability to identify recently described species, rather than to place strains in poorly defined species such as P. prevotii is essential.
CLINICAL IMPORTANCE
GPAC are commonly present in human clinical specimens; data from four surveys of anaerobic infections (28, 138, 196, 295) are consistent that they account for about 25 to 30% of all anaerobic isolates (Table 5). They can be cultured from a wide variety of sites, particularly abscesses and infections of the mouth, skin and soft tissues, bone and joints, and upper respiratory and female genital tracts (Fig. 3) (35, 90, 92).
TABLE 5.
Most common species of Peptostreptococcus isolated in comparative anaerobic surveys
| Reference | No. of isolates (% of total
Peptostreptococcus isolated)a
of:
|
Total no. (%) of Peptostrepto- coccus isolatesa | Total no. of anaerobes isolated | ||||
|---|---|---|---|---|---|---|---|
| P. anaerobius | P. asaccharolyticusb | P. magnus | P. micros | P. prevotiib | |||
| Wren et al., 1977 (295) | 44 (13) | 33 (10) | 84 (26) | 21 (6) | 31 (10) | 326 (26) | 1,271 |
| Holland et al., 1977 (138) | 17 (14) | 23 (18) | 48 (38) | NAc | 21 (17) | 125 (31) | 408 |
| Rosenblatt, 1985d (227) | 35 | 72 | 176 | 31 | 30 | NA | NA |
| Brook, 1988 (28) | 285 (18) | 293 (18) | 318 (20) | 74 (5) | 233 (15) | 1,600 (24) | 6,557 |
| Brook, 1994e (33) | 46 | 103 | 74 | 51 | 56 | 659 | NA |
| Murdoch et al., 1994f (196) | 27 (16) | 24 (14) | 55 (33) | 23 (14) | 0 | 209 (27) | 782 |
Percentages for individual species are based on total Peptostreptococcus isolated; percentages for total Peptostreptococcus isolated are based on total anaerobes isolated.
Species affected by recent taxonomic changes.
NA, not available.
Data available only for the 15 most frequently isolated anaerobic species.
Pediatric survey. For this report, figures for percentages of total Peptostreptococcus isolated are not quoted since only 330 (50%) of 659 strains were identified to the species level.
Percentages based on the 168 strains available for examination. Strains of P. asaccharolyticus do not include “P. harei.”
FIG. 3.
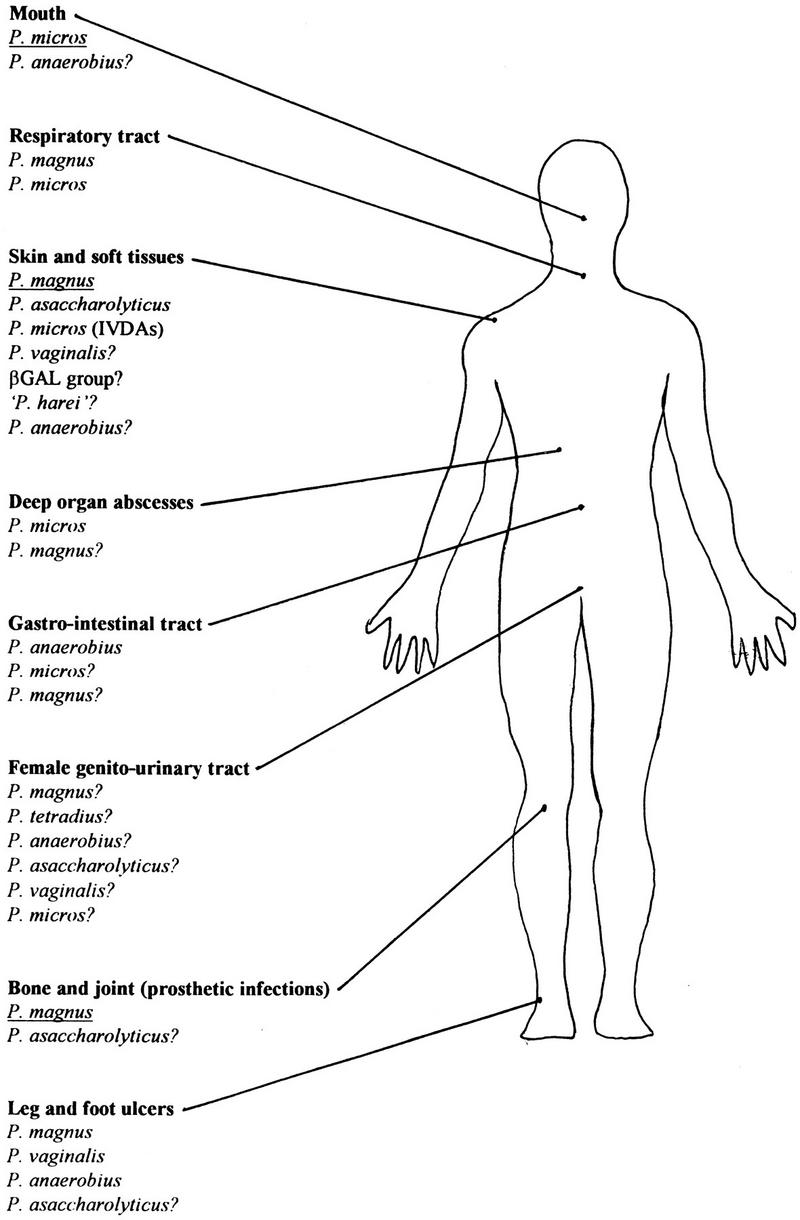
Sites of pathology for species of GPAC. Species of major importance are underlined, ? denotes uncertain pathology.
Most infections involving GPAC are polymicrobial (35, 90), particularly abscesses and those developing from mucocutaneous surfaces. However, there are many instances of their isolation in pure culture; most relate to P. magnus (14, 32, 38, 51, 64, 72, 77, 94, 141, 173, 196, 207, 214, 217, 231), but there are also reports of P. anaerobius (181), P. asaccharolyticus (196), P. indolicus (13), P. micros (210, 278), P. vaginalis (189, 196), and “P. harei” (188).
When a report employs the term “anaerobic streptococcus,” it will be used in the following discussion, because this term is likely to include microaerophilic streptococci such as Streptococcus intermedius and Streptococcus constellatus, which were removed from the genus Peptostreptococcus in 1974 (134). Otherwise the term GPAC is used, with the caveat that some organisms formerly included in the group would not be described as such nowadays. Data from mucocutaneous sites will be presented first, followed by data from sites that are usually sterile.
Oral and Respiratory Tract Infections
The flora of the oral cavity is extremely complex and has been intensively studied. GPAC have been relatively neglected, but the importance of P. micros is now recognized.
Most dental infections are polymicrobial but are dominated by anaerobic organisms (260). The crevicular fluid is probably the major source of nutrients in the subgingival ecosystem; it contains relatively high concentrations of glycoproteins and peptides but low concentrations of carbohydrates; consequently, the flora is largely asaccharolytic (235). P. micros would appear well adapted to this serum-rich, oxygen-depleted environment, and many studies have associated it with periodontitis, but it is not always detected at active sites. Haffajee et al. (109) isolated it from only 5 of 33 adults with active periodontal disease, but in these patients it constituted 5 to 10% of the mean bacterial count. Moore et al. (184) reported P. micros counts of >1% in the supragingival flora of 21 young adults with severe periodontitis; it was present in 48% of subgingival samples, more often than at supragingival sites, and raised subgingival counts correlated with gingival inflammation. Likewise, Socransky et al. (245) isolated P. micros from 24% of subgingival plaque samples and linked it to gingival redness, deeper pockets, and loss of periodontal attachment. However, in a related study, Haffajee et al. (110) associated P. micros with deep progressing pockets but not raised subgingival temperature. It has been suggested that periodontal disease is episodic rather than a continual progression; a longitudinal study by Rams et al. (221) found a higher prevalence in patients with active disease (47%) than in patients with inactive disease (14%). In a prospective study of adults with chronic periodontitis by Moore et al. (185), P. micros, Campylobacter rectus, and Fusobacterium nucleatum were the only species isolated from all patients, but no quantitative difference was detected between the flora of active and inactive sites. A recent comparison of the subgingival microflora of mobile and nonmobile teeth (103) reported higher counts of C. rectus and P. micros around mobile teeth; the authors suggested that tooth mobility might create a subgingival environment conducive to overgrowth by periodontal pathogens.
One approach has been to identify organisms, termed risk markers, which occur more frequently than others in active disease or whose presence is linked to a less favorable outcome. Dzink et al. (76) compared the cultivable subgingival flora from sites with active periodontal disease and inactive sites; they reported that a site was more likely to be active if Bacteroides forsythus, Porphyromonas gingivalis, P. micros, C. rectus, or Prevotella intermedia was isolated. Another approach has been to associate groups of potential pathogens, termed species clusters, with dental disease. Socransky et al. (244) associated P. micros with the presence of several species, including Prevotella melaninogenica and P. gingivalis, but found a very strong negative correlation with another putative periodontal pathogen, Bacteroides forsythus. This observation may help to explain why P. micros is not always present in patients with active periodontitis.
Periodontitis can dramatically worsen in patients with human immunodeficiency virus (HIV). Rams et al. (220) reported that although the subgingival flora of 14 patients with HIV was usually qualitatively unchanged, counts of P. micros from normal sites were raised and those from infected sites were very high; other putative pathogens such as C. rectus were also more common. The authors suggested that P. micros can act as an opportunist pathogen in HIV-positive patients.
Dental implants may be susceptible to plaque-induced gingivitis, which can progress to peri-implantitis. Alcoforado et al. (1) cultured a wide range of organisms from failing implants; P. micros was the most common isolate, accounting for 24% of the cultivable flora. Rosenberg et al. (226) reported major differences between the flora of implant failures attributed to trauma and those due to infection. Implant sites with traumatic failure exhibited a microflora consistent with periodontal health and did not yield P. micros. In contrast, implant sites with infectious failure yielded a wide variety of periodontal pathogens; P. micros was isolated from 75% of sites and made up more than 10% of the cultivable flora.
Thus, there is good evidence that P. micros is associated with periodontal infections, but it is extremely difficult to prove causation in a microbial flora as complex as that of the subgingival plaque. The development of techniques such as DNA probes (108, 297, 298) may help to resolve this problem. The evidence implicating other GPAC is much weaker. In a cross-sectional study by Moore et al. (184), P. anaerobius was a major component (>1%) of the supragingival flora but was less common in the subgingival flora. However, in a later report, the same workers (182) associated P. anaerobius with both gingivitis and periodontitis. Wade et al. (269) isolated P. micros, P. anaerobius, P. asaccharolyticus, and rarely P. productus from subgingival plaque in patients with chronic periodontitis.
P. micros is often implicated in other oral infections (260). In a quantitative study of 10 endodontic abscesses by Williams et al. (290), P. micros was the most common organism (accounting for 18% of all isolates) and was the predominant organism in five abscesses. A quantitative study of 50 acute dentoalveolar abscesses by Lewis et al. (164) yielded a mainly anaerobic flora; along with “Streptococcus milleri,” Prevotella oralis, and Prevotella melaninogenica, GPAC (30%) were the most common organisms; unfortunately, they were not identified to the species level. Pericoronitis is an acute condition in which the soft tissues investing the crown of a partially erupted tooth become inflamed; Wade et al. (267) reported that in a group of 20 patients with pericoronitis, P. micros was the second most common organism. Recent investigations have shown that anaerobes, including GPAC, are able to invade cementum via periapical periodontal tissue (152) and nonexposed dental pulps (140), possibly via dentinal tubules. Dental infections can extend to cause brain abscesses (92), either directly or by hematogenous spread.
Two recent studies (147, 179) of peritonsillar abscesses have distinguished a group of patients with mainly aerobic infections, in which Streptococcus pyogenes predominated, from a larger group with mixed, primarily anaerobic infections. Both studies reported that the most frequent isolates in the second group were P. micros, “Streptococcus milleri,” and Prevotella and Fusobacterium species. Surface swabs do not reliably reflect the organisms present in the tonsillar core (180); P. micros is significantly more common in the core.
The complications of peritonsillar abscess can be serious and occasionally fatal. A recent case report (63) described a peritonsillar abscess that yielded at least eight organisms, including P. anaerobius and P. asaccharolyticus. The case was complicated by a retropharyngeal abscess, which yielded a similar range of organisms, and anaerobic myonecrosis, in which seven organisms including P. anaerobius were cultured from neck muscle. This case illustrates the extraordinary range of organisms that can be isolated from intraoral abscesses.
There have been relatively few reports of GPAC from the upper respiratory tract in health or disease. Thomas and Hare (263) isolated strains of several Hare groups from the nasal flora; the predominant group, Hare group VIII, has recently been recognized as “P. octavius.” Brook (24) compared the flora of 25 children with purulent nasopharyngitis with that of 25 controls and showed a statistically significant increase in counts of GPAC as well as Streptococcus pneumoniae, Haemophilus influenzae, and Fusobacterium and Prevotella spp. A more recent report (50) noted that anaerobes, including GPAC, predominated in chronic but not acute sinusitis. Data from surveys by Brook (34) and Murdoch et al. (196) (Table 6) indicate that the major species are P. magnus and P. micros. Other reports have shown the importance of GPAC in retropharyngeal abscesses (23), wound infections after head and neck surgery for cancer (46), chronic suppurative otitis media (49), and tracheostomy site wounds (21). Brook (34) isolated a wide range of GPAC from children with otitis media; 16 of 33 GPAC (48%) isolated from patients with acute otitis were recovered in pure culture, compared with 4 of 71 strains (6%) isolated from patients with chronic otitis.
TABLE 6.
Analysis of GPAC reported from clinical studies
| Site of isolation and reference(s) | No. of culturepositive specimens | No. of specimens with anaerobes | No. of specimens with GPAC | Total no. of strains isolated | Total no. of anaer- obes isolated | Total no. of GPAC isolated | No. of isolates of:
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. anaer- obius | P. asaccha- rolyticusa | P. mag- nus | P. mi- cros | P. pre- votiia | Other species | Unidentified species | |||||||
| All abscesses | |||||||||||||
| Brook, 1994 (pediatric) (33) | 538 | NAc | 205 | NA | NA | 230 | 19 | 57 | 22 | 15 | 22 | 0 | 95 |
| Murdoch et al., 1994b (196) | 67 | 11 | 10 | 15 | 13 | 0 | 9d | 9 | |||||
| Endodontic abscesses | |||||||||||||
| Williams et al., 1983 (290) | 10 | 9 | 6 | 45 | 37 | 7 | 1 | 0 | 0 | 5 | 0 | 0 | 1 |
| Peritonsillar abscesses | |||||||||||||
| Jokipii et al., 1988 (146) | 42 | 28 | 13 | 133 | 51 | 15 | 3 | 1 | 0 | 0 | 0 | 0 | 11 |
| Jousimies-Somer et al., 1993 (147) | 122 | 102 | NA | 550 | 407 | 44 | 6 | 0 | 2 | 34 | 0 | 0 | 2 |
| Mitchelmore et al., 1995 (179) | 45 | 38 | NA | 138 | 112 | 36 | 5 | 0 | 0 | 26 | 1 | 0 | 4 |
| Sinusitis | |||||||||||||
| Brook, 1994 (pediatric) (33) | 45 | NA | 22 | NA | NA | 22 | 1 | 5 | 4 | 5 | 3 | 0 | 4 |
| Murdoch et al., 1994b (196) | 23 | 0 | 2 | 10 | 6 | 0 | 2e | 3 | |||||
| Pediatric otitis media | |||||||||||||
| Brook, 1994 (33) | 383 | NA | 94 | NA | NA | 102 | 6 | 11 | 11 | 8 | 10 | 0 | 56 |
| Pleuropulmonary infections | |||||||||||||
| Marina et al., 1993b (173) | 116 | NA | 605 | 404 | 39 | 5 | 3 | 7 | 19 | 3 | 0 | 2 | |
| Brook and Frazier, 1993 (39) | 197 | 70 | NA | 343 | 127 | 27 | 3 | 1 | 12 | 1 | 2 | 0 | 8 |
| Abdominal infections | |||||||||||||
| Wren et al., 1977b (295) | 306 | NA | NA | 576 | 110 | 10 | 9 | 31 | 6 | 10 | 9f | 35 | |
| Brook, 1994 (pediatric) (33) | 115 | NA | 82 | NA | NA | 91 | 3 | 8 | 12 | 15 | 2 | 0 | 51 |
| Infected hemorrhoids | |||||||||||||
| Brook and Frazier, 1996 (43) | 19 | 18 | NA | 68 | 39 | 13 | 6 | 2 | 0 | 0 | 1 | 0 | 4 |
| Suppurative genitourinary infections | |||||||||||||
| Brook, 1989 (30) | 103 | 96 | NA | 275 | 189 | 52 | 16 | 11 | 9 | 1 | 8 | 0 | 7 |
| Obstetric and gynecological infections | |||||||||||||
| Brook, 1988 (28) | 871 | NA | NA | NA | 1,328 | 455 | 125 | 119 | 52 | 9 | 51 | 0 | 99 |
| High vaginal swabs | |||||||||||||
| Wren et al., 1977b (295) | 241 | NA | NA | 441 | 113 | 27 | 9 | 17 | 10 | 7 | 7g | 36 | |
| Post-caesarean endometritis | |||||||||||||
| Hillier et al., 1990 (131) | 26 | NA | 16 | 149 | 65 | 34 | 5 | 6 | 11 | 0 | 4 | 8h | 0 |
| Superficial infections | |||||||||||||
| Wren et al., 1977b (295) | 180 | NA | NA | 323 | 110 | 7 | 15 | 36 | 5 | 14 | 2i | 31 | |
| Brook and Frazier, 1990 (37) | 1,260 | 860 | NA | 3,172 | 2,012 | 528 | 68 | 84 | 133 | 21 | 78 | 0 | 144 |
| Murdoch et al., 1994b (196) | 71 | 4 | 9 | 28 | 0 | 0 | 15j | 15 | |||||
| Summanen et al., 1995 (non-IVDU) (252) | 74 | 25 | NA | 222 | 106 | 26 | 4 | 4 | 8 | 5 | 5 | 0 | 0 |
| Summanen et al., 1995 (IVDU) (252) | 86 | 45 | NA | 304 | 131 | 20 | 0 | 0 | 3 | 15 | 0 | 0 | 2 |
| Diabetic foot infections | |||||||||||||
| Wheat et al., 1986k (283) | 54 | 22 | 19 | 179 | 50 | 30 | 3 | 3 | 12 | 1 | 9 | 0 | 2 |
| Johnson et al., 1995 (145) | 46 | 34 | NA | 285 | 103 | 51 | 7 | 10 | 23 | 0 | 5 | 0 | 6 |
| Nonpuerperal breast infections | |||||||||||||
| Brook, 1988 (26) | 41 | 34 | NA | 90 | 62 | 21 | 3 | 4 | 5 | 0 | 1 | 2l | 6 |
| Edmiston et al., 1990 (77) | 52 | 41 | NA | 221 | 142 | 68 | 3 | 12 | 23 | 9 | 14 | 0 | 7 |
| Giamarellou et al., 1994 (98) | 38 | 14 | NA | 60 | 30 | 18 | 0 | 0 | 11 | 0 | 7 | 0 | 0 |
| Cellulitis | |||||||||||||
| Brook and Frazier, 1995 (42) | 274 | 128 | NA | 582 | 335 | 110 | 13 | 10 | 40 | 5 | 21 | 0 | 21 |
| Necrotizing fasciitis | |||||||||||||
| Brook and Frazier, 1995 (41) | 81 | 73 | NA | 375 | 270 | 82 | 12 | 12 | 24 | 7 | 12 | 0 | 15 |
| Mediastinitis | |||||||||||||
| Brook and Frazier, 1996 (44) | 17 | 14 | 7 | 42 | 29 | 7 | 0 | 0 | 3 | 2 | 2 | 0 | 0 |
| Post-thoracotomy sternal wound infections | |||||||||||||
| Brook, 1989 (32) | 65 | 15 | NA | 87 | 19 | 10 | 1 | 0 | 4 | 0 | 4 | 0 | 1 |
| External otitis | |||||||||||||
| Brook et al., 1992 (45) | 46 | 12 | 10 | 67 | 22 | 11 | 2 | 0 | 4 | 1 | 2 | 0 | 2 |
| Pediatric bite wounds | |||||||||||||
| Brook, 1987 (22) | 39 | 32 | NA | 156 | 75 | 27 | 0 | 4 | 5 | 0 | 0 | 0 | 18 |
| Pediatric burns | |||||||||||||
| Brook, 1994 (33) | 180 | NA | 22 | NA | NA | 25 | 0 | 6 | 6 | 0 | 3 | 0 | 10 |
| Anaerobic bacteremia | |||||||||||||
| Topiel and Simon, 1986b (264) | 12 | 12 | 0 | 5 | 1 | 5 | 0 | 0 | 1 | ||||
| Brook, 1989b (31) | 296 | NA | 352 | 44 | 1 | 10 | 7 | 2 | 13 | 0 | 11 | ||
| Pediatric intracranial abscesses | |||||||||||||
| Brook, 1981 (17) | 19 | 17 | NA | 51 | 34 | 7 | 0 | 2 | 1 | 0 | 1 | 0 | 3 |
| Anaerobic joint infections | |||||||||||||
| Fitzgerald et al., 1982b (94) | 46 | NA | 72 | 37 | 5 | 1 | 19 | 4 | 7 | 0 | 1 | ||
| Brook and Frazier, 1993b (38) | 65 | 15 | 67 | 15 | 1 | 1 | 6 | 0 | 2 | 0 | 5 | ||
| Anaerobic osteomyelitis | |||||||||||||
| Brook and Frazier, 1993b (38) | 73 | NA | 122 | 41 | 2 | 2 | 12 | 2 | 9 | 0 | 14 | ||
P. asaccharolyticus and P. prevotii have been affected by taxonomic changes; clinical data must therefore be interpreted cautiously.
These studies analyzed only specimens that yielded anaerobes or GPAC alone.
NA, not available.
P. heliotrinreducens, five strains; P. indolicus, one strain; βGAL group, two strains; P. vaginalis, one strain.
“P. octavius”, two strains.
P. productus, five strains; A. parvulum, four strains.
P. productus, four strains; A. parvulum, three strains.
P. tetradius, seven strains; P. niger, one strain.
P. productus, two strains.
P. heliotrinreducens, 1 strain; P. vaginalis, 6 strains; βGAL group, 6 strains; P. hydrogenalis, 2 strains.
Only data on ‘reliable specimens’ (those judged free of surface contamination) are included.
P. tetradius, 2 strains.
Anaerobic infections of the lower respiratory tract are relatively common but underdiagnosed; GPAC form one of the major pathogenic groups (8). The same species as in intraoral disease are found, and it is likely that the gingival crevice is the site of origin of many of these organisms (8). However, since transtracheal aspiration is now rarely used, it is difficult to obtain uncontaminated specimens to determine the pathogens responsible. The most common clinical syndromes are aspiration pneumonia, lung abscess, and empyema (8); the course of infection is usually indolent with abscess formation, but a more rapid presentation resembling pneumococcal pneumonia can occur. Early studies in laboratory animals by Smith (238, 239) demonstrated that combinations of anaerobes were essential to induce lung abscess formation. In a classic study of 83 cases of empyema by Bartlett et al. (9), anaerobes were cultured in 76% of patients and aerobes were cultured in only 65%. A similar study by the same workers (10) of 54 patients with aspiration pneumonia documented anaerobes in 93% of patients and aerobes in 54%. In both series, the predominant organisms were Prevotella spp., Fusobacterium nucleatum, Peptostreptococcus spp., and microaerophilic streptococci. However, the peptostreptococci were not fully identified in these studies, and it is likely that some strains would now be classified in the genus Streptococcus (134). Four cases of empyema reported by Murdoch et al. (195) all yielded a similar flora; the GPAC were all identified as P. micros. Recent investigations (62, 173, 196) support the predominance of P. micros but have also reported isolation of P. anaerobius, P. magnus, P. vaginalis, and P. heliotrinreducens. However, an analysis of 197 cases of empyema in a younger age group (39) revealed that aerobes (particularly S. aureus and S. pneumoniae) were more common than anaerobes; the predominant species of GPAC was P. magnus. Several factors, including the age of the patient and the cause of the empyema, could account for these differences; further reports would be valuable. P. magnus has been isolated in pure culture from a patient with necrotizing pneumonia (207).
Intra-Abdominal Infections
Anaerobes constitute more than 99% of the fecal flora, and counts of GPAC often exceed 1010/g (90, 91). However, to quote Moore et al. (183), “it is most significant that many of the more numerous fecal organisms have not been reported from human infections.” As an example, P. productus is probably the commonest GPAC in the normal gut flora (91, 183), but it is very rarely cultured from properly obtained clinical specimens (136, 196). In a summary of five studies of intra-abdominal sepsis (92), GPAC accounted for 5% of strains, a much lower percentage than that of gram-negative anaerobes. Few reports have attempted to identify intraabdominal GPAC to the species level. In two surveys of anaerobic infections, Brook (28, 34) isolated strains of most species of GPAC, but unidentified strains comprised the largest group in both reports. In a more recent survey (196), intra-abdominal sites yielded 38 strains (18% of all isolates), 23 of which were from abscesses; the most common species was P. anaerobius (11 isolates), but almost all species of GPAC were recovered. P. anaerobius was also the commonest anaerobe in a recent series of infected hemorrhoids (43).
In 1972, Sabbaj et al. (229) reported a series of 47 liver abscesses, including 15 that were purely anaerobic; “anaerobic streptococci” or anaerobic cocci were the most common isolates after microaerophilic streptococci. Brook and Frazier (40) described 14 pediatric liver abscesses that yielded 29 organisms, mostly anaerobes; GPAC (5 isolates) were the most common organisms, but only one strain (P. magnus) was identified.
Infections of the Genitourinary Tract
Infection of the urinary tract by anaerobes appears to be an uncommon event (92). GPAC have been isolated from several sites, most frequently superficial abscesses (29, 30, 196), but rarely from infections of the upper tract (34). Brook (30) documented 103 localized suppurative genitourinary infections, mostly skin related, in which mixed infections of GPAC and “Bacteroides” group spp. were the most common combination of organisms; P. anaerobius was the most common species of GPAC.
GPAC are commonly involved in gynecological and obstetric sepsis (28, 35, 92), but they have received much less study than gram-negative anaerobic rods, probably because the classification and identification methods are less well established. The association between bacterial vaginosis (BV) and a range of gynecological and obstetric infections is at present a very active area of research.
Gynecological sepsis.
The normal adult vaginal flora contains about 105 to 107 bacteria/ml of fluid and is dominated (>95%) by lactobacilli (79, 126); the low pH (<4.5) and the ability of many lactobacilli to form hydrogen peroxide (126) appear to be important factors in its maintenance. BV represents a condition of bacterial overgrowth with replacement of the lactobacilli by a more anaerobic flora; bacterial counts are 100 to 1,000 times higher than normal (79). The classical indicator organism is Gardnerella vaginalis (79), but BV is now clearly associated with increased carriage of Mycoplasma hominis and raised counts of anaerobes, particularly gram-negative rods (126, 139, 256); these include several Prevotella species, notably Prevotella bivia and Prevotella disiens, Porphyromonas and Mobiluncus species, and F. nucleatum. The role of GPAC in BV is less clear.
A quantitative comparison of pregnant women with normal flora and BV (126) showed a significant increase in the proportion of BV-positive women with carriage rates of GPAC >105 CFU/ml; carriage rates and counts of P. tetradius were increased, but no significant change was detected for other species of GPAC. Hill and Livengood (122) isolated GPAC from 90% of 33 nonpregnant women with BV at mean counts of 108/ml of vaginal fluid; P. asaccharolyticus, P. prevotii, and P. tetradius were present at carriage rates of 33 to 58% and bacterial counts of 107.5 to 108. Treatment with intravaginal clindamycin led to cure in 92% of patients with BV and significant decreases in BV-associated organisms, including GPAC.
BV is now implicated in the development of pelvic inflammatory disease (PID) (79, 80, 256) and postsurgical complications such as vaginal cuff cellulitis following hysterectomy (79). Until recently, PID was little studied because it was difficult to obtain uncontaminated specimens from the upper genital tract (UGT), but the widespread availability of laparoscopy has increased the accessibility of the UGT. Neisseria gonorrhoeae and Chlamydia trachomatis are regarded as the most important primary pathogens, but the role of anaerobic bacteria is less clear; there is considerable debate about whether anaerobes can act as primary pathogens or only as secondary invaders when the UGT is already damaged.
Sweet et al. (256) reported a laparoscopic study of the UGT flora of 188 women hospitalized for acute PID; they recovered P. asaccharolyticus from 93 women (50%) and P. anaerobius from 72 (38%). In a related study of a similar group of patients (257), GPAC were the most common group of organisms, accounting for 18% of all isolates; unfortunately they were not identified to the species level, and many UGT specimens were taken via the lower genital tract.
Soper et al. (246) studied 84 women with acute salpingitis by using laparoscopy for tubal cultures and endometrial biopsy. Of their patients, 62% had BV. Culture of the UGT often yielded anaerobes, which were always BV-associated organisms. However, they isolated N. gonorrhoeae or C. trachomatis from 77% of patients and concluded that these organisms were responsible for most of their cases of acute salpingitis; they doubted whether anaerobes alone often initiated PID. GPAC accounted for 14 of the 18 anaerobes from endometrial samples, but only one patient, who had acute endometritis, yielded anaerobes alone (P. tetradius and P. asaccharolyticus). One histologically normal endometrial biopsy specimen yielded P. anaerobius and P. asaccharolyticus.
Tubo-ovarian abscess is a rare complication of PID. In 1940, Altemeier (3) isolated “anaerobic streptococci” from nearly 90% of patients with chronic tubo-ovarian abscesses. More recently, a review by Landers and Sweet (161) concluded that the most common organisms recovered from this condition were Escherichia coli, Bacteroides fragilis, and GPAC.
Obstetric infections.
“Anaerobic streptococci” were associated with puerperal infection by Schottmüller in 1910 (233); later reports by Schwarz and Dieckmann (234), Brown (52), and Colebrook and Hare (65) clearly established their importance. Infection classically spread from the placental site via the uterine veins to the broad ligaments, often causing thrombophlebitis, abscess formation, and infertility (114); in contrast to S. pyogenes infection, septicemia and metastatic infection were rare. Other pathogens such as gram-negative anaerobes and aerobic streptococci were usually present. Fortunately, improved obstetric practices and the ready availability of antibiotics have made puerperal fever a rare disease in the developed world. However, it is still common in the Third World, although it has been very little investigated. A unique study by Hare and Polunin (115) suggests that GPAC may play an important role. They compared the Murut, a tribe in the interior of British North Borneo (now Sarawak), whose high rates of infertility were leading to depopulation, with the Dusun, a neighboring, unaffected tribe. Gynecological abnormalities consistent with postabortion fever or postpartum fever were common in the Murut but not the Dusun. The authors reported that the cervical carriage rate of the predominant organism, Hare group I (now P. hydrogenalis [190]), was 33% in infertile members of the Murut but 3% in members of the Dusun.
Recently, there has been intense interest in the link between BV and many obstetric complications, including chorioamnionitis (127, 275), amniotic fluid infection (124), post-caesarean endometritis (275), preterm labor (139) and delivery (79, 130), low neonatal birth weight (130), and intrauterine growth retardation (97); there is now a large and complex literature on the subject. A recent trial (118) concluded that treatment with metronidazole and erythromycin reduced the rates of premature delivery in women with BV.
Unfortunately, GPAC have received less investigation than some other groups of anaerobes, notably Prevotella species. Some workers (97, 130) have not attempted to isolate them, and others (127, 177) have not identified them to the species level. In a series of studies by Hillier and coworkers, GPAC have been recovered from chorioamnion cultures in patients with chorioamnionitis (125, 127) and from endometrial specimens in patients with post-Caesarean endometritis (131, 275). Infection of the chorioamnion with GPAC was related to preterm delivery but not to histological chorioamnionitis (125); GPAC were the most common organisms except for ureaplasmas. A wide range of GPAC (Table 6) were recovered from the UGT of women with post-caesarean endometritis (131), but since samples were taken per cervix, contamination by the vaginal flora cannot be excluded. Holst et al. (139) isolated a range of organisms, including P. anaerobius and P. asaccharolyticus, more frequently from BV-positive women who had preterm delivery than from BV-positive women who went to term or from term labor controls. However, other BV-associated organisms, notably F. nucleatum and Mobiluncus species, were more clearly associated with premature delivery than were GPAC.
Several obstetric conditions predispose to the seeding of GPAC into the bloodstream; GPAC bacteremia has been reported after septic abortion (242), post-caesarean endomyometritis (75), and postpartum (264) and is discussed more fully below.
In conclusion, GPAC are more common in BV than in the normal vaginal flora, but few studies have presented data on the individual species involved. GPAC have been isolated from several UGT complications linked to BV, but it has not yet been possible to prove that they are pathogenic or even important components of a synergistic group of pathogens. The obvious parallel is with the role of P. micros in the development of periodontitis—it is possible to show association but very difficult to prove cause; in comparison, the evidence for a role for GPAC in BV-related gynecological and obstetric disease is at present weaker. However, early studies, which reported more florid pathology than is usually seen nowadays, do support the contention that GPAC are pathogenic rather than simply being passengers. Although it is time-consuming and expensive, future studies should attempt to identify GPAC to the species level; the place of P. hydrogenalis and P. vaginalis deserves proper investigation. If GPAC are grouped rather than fully identified, the opportunity to reveal interesting pathogenic relationships may be lost (126).
Superficial and Soft Tissue Infections
GPAC are part of the normal skin flora (7, 90, 251); when specimens are properly transported and processed, they are often isolated from superficial and soft tissue infections and postoperative surgical wounds (14, 28, 35, 196). However, superficial infections are usually mixed, and it is often difficult to assess what is a pathogen and what is merely an opportunistic colonist. In some conditions such as cellulitis, adequate specimens can rarely be obtained and treatment is empirical. As a result, it is often difficult to determine the pathogens responsible for soft tissue infections.
An early survey of GPAC by Pien et al. (215) described 85 isolates, of which 42 were from surgical wounds, 19 were from foot or leg ulcers, and 16 were from skin infections; pure cultures were obtained from patients with hydradenitis suppurativa and infected epidermoid cysts. The classic study by Bourgault et al. (14) established the preeminence of P. magnus; of 183 infections that yielded P. magnus, the largest group of 57 strains (31% of total) were from soft tissue infections, 12 in pure culture. In a retrospective report on 14 years’ experience of superficial infections, Brook and Frazier (37) isolated GPAC most often from abscesses of the neck and perirectal region, and wounds of the neck, external genitalia, and inguinal region. They concluded that the location of the infection was paramount in determining the organisms involved; superficial infections of the trunk most often yielded P. magnus, but P. asaccharolyticus and P. anaerobius were most common in perirectal abscesses and infections of the leg and external genitalia. In a survey by Murdoch et al. (196), 33% of GPAC were recovered from superficial sites; almost half were identified as P. magnus, though most species of GPAC were isolated. P. magnus was the major species at superficial sites from the upper body, such as minor abscesses, infected sebaceous cysts, and postoperative wound infections; nine strains were isolated in pure culture. P. asaccharolyticus and P. vaginalis predominated in specimens from the leg and pelvis. A recent investigation by Summanen et al. (252) compared superficial abscesses in intravenous drug users (IVDUs) with a normal population. In the latter group, the expected range of species was isolated, with P. magnus commonest, but in the IVDUs, P. micros accounted for 15 of 20 strains of GPAC. The frequency of fusobacteria, veillonellae, and other oral flora in the IVDUs led the authors to conclude that the mouth was the source of these pathogens.
GPAC are strongly associated with diseases of the feet, particularly in diabetics. Sanderson (231) described three diabetics with infections of the foot from whom P. magnus was isolated in pure culture; GPAC were also isolated in mixed culture from nine other patients, eight of whom were diabetics. In the series of Bourgault et al. (14), 29 patients (14%) had infected foot ulcers; a foot abscess from a diabetic yielded a pure culture of P. magnus. A prospective study by Wheat et al. (283) of 54 diabetics with foot infections, in which great care was taken to avoid contamination from surface flora, reported that most infections were heavily mixed; GPAC, cultured from 36% of specimens, were the major anaerobic group, and P. magnus was the most common anaerobe. A recent study (145) reached similar conclusions. In an ongoing study of decubitus ulcers, Wren (293) has confirmed the predominance of P. magnus and observed a strong association with S. aureus.
GPAC have been implicated in the development of breast abscesses. In a study by Edmiston et al. (77), GPAC were the most common organisms in both acute and chronic nonpuerperal breast abscesses and P. magnus was the commonest single organism (10% of all isolates). Krepel et al. (157, 158) have demonstrated extracellular enzyme production by some of these strains. Bourgault et al. (14) and Edmiston et al. (77) noted breast abscesses which yielded pure cultures of P. magnus. Other reports (26, 98) confirm these observations. Sturm (250) suggested two main sources of pathogens: the first, the skin, would account for the importance of S. aureus; and the second, the vaginal flora, would explain the polymicrobial anaerobic nature of many of these infections.
In a series of reports by Brook and coworkers, GPAC were the most common anaerobic group isolated from patients with cellulitis (42), necrotizing fasciitis (41), mediastinitis (44), pyomyositis (36), pediatric bite wounds (22), anaerobic conjunctivitis associated with contact lenses (27), post-thoracotomy sternal wound infections (32), and external otitis (45). GPAC were isolated from all body sites in patients with cellulitis and necrotizing fasciitis and were associated with gangrene, necrosis, and gas in the tissues; P. magnus was the most common anaerobe.
Thus, GPAC are the most common anaerobes cultured from soft tissue infections. P. magnus is often present in pure culture, notably in infected foot ulcers in diabetics, superficial abscesses, and postoperative wound infections. We recommend that diagnostic laboratories provide effective anaerobic transport systems and incubate appropriate soft tissue specimens for 5 days, so that GPAC and other slow-growing anaerobes will be detected. Organisms from important abscesses and pure cultures should be identified to the species level.
Septicemia and Cardiovascular Infections
GPAC are sometimes isolated from blood cultures, but they appear to cause significant morbidity much less often than do gram-negative anaerobes, particularly Bacteroides spp. In an analysis of anaerobic blood cultures taken over 12 years (31), GPAC were responsible for 9% of all isolates and 12% of isolates from clinically significant cases, compared to 20 and 33% for B. fragilis alone. Of 12 patients with GPAC bacteremia described by Topiel and Simon (264), 8 were postpartum and aged under 35, but the other 4 were aged over 50 and had multiple pathologic findings. Only one patient developed hypotension, and none died. In an analysis of pyrexia following septic abortion (242), 76 patients had positive blood cultures, of which 38 (50%) yielded “anaerobic streptococci”; these patients developed a variety of complications, but none died. In a series of blood cultures from 200 patients with post-caesarean endomyometritis (75), GPAC were the most common isolates but anaerobic gram-negative rods, particularly B. fragilis, caused greater morbidity. Thus, obstetric patients appear at particular risk of GPAC bacteremia.
Cases of endocarditis have very rarely been documented. Felner and Dowell (89) analyzed 33 cases of anaerobic endocarditis, 2 (6%) of which yielded GPAC; since this report was from a reference laboratory, it may not reflect the true picture. Two cases (one fatal) caused by P. magnus (64, 217) and single cases caused by P. micros (278) and P. anaerobius (181) have been reported. P. magnus has been isolated in pure culture from two vascular grafts by Bourgault et al. (14) and from a case of purulent pericarditis complicated by mediastinitis (214).
Central Nervous System Infections
Central nervous system infections involving GPAC usually present as abscesses and yield a characteristic anaerobic flora. Predisposing factors can be identified in about 85% of cases (92); the most common routes are by direct spread from infections of the middle ear, sinuses, and mouth (usually teeth) and by hematogenous spread from pleuropulmonary infections or via cardiovascular abnormalities. Heineman and Braude in 1963 (120) established the importance of anaerobes in brain abscesses; they recovered anaerobes alone from 10 (56%) abscesses of 18. The most common organisms were “anaerobic streptococci,” which were cultured from 12 patients; a preexisting focus of chronic infection was present in the sinuses, ear, or lung of 14 patients (78%). In an analysis of 19 pediatric intracranial abscesses (17), Brook reported that microaerophilic streptococci, GPAC, Prevotella spp., and fusobacteria were the most common organisms. A review of 14 series reported the isolation of “anaerobic streptococci” or Peptostreptococcus spp. from 92% of abscesses and Peptococcus spp. from 27% (92). Unfortunately, these organisms were rarely identified further. Murdoch et al. (195) described three brain abscesses which yielded P. micros, fusobacteria, “Bacteroides” group spp., and microaerophilic streptococci. A more recent report (121) described a case which yielded P. micros and F. nucleatum.
Anaerobes are rarely the cause of meningitis; gram-negative rods appear to be more common than GPAC (92). Brown et al. (51) reported a case secondary to head and neck surgery which yielded P. magnus in pure culture.
Musculoskeletal Infections
Anaerobic osteomyelitis is rarely reported but is probably underdiagnosed because specimens are inadequately processed. It is particularly associated with three conditions: traumatic fractures of long bones, peripheral vascular disease, and osteomyelitis of the cranial and facial bones developing from sinus, ear, or dental infections (38). In a recent survey, Brook and Frazier (38) isolated GPAC from all three groups of patients; most infections were polymicrobial, but the authors noted an association between P. magnus and osteomyelitis of the hands or feet secondary to peripheral vascular disease. Wheat et al. (283) reported that osteomyelitis often complicated diabetic foot infections; the most common anaerobe was P. magnus. Papasian et al. (210) described a case of lumbar osteomyelitis which yielded pure cultures of P. micros. Bourgault et al. (14) recorded six cases of osteomyelitis following femoral fractures which yielded P. magnus in pure culture; internal fixation had been carried out in five of the cases.
Septic arthritis due to anaerobes is also rare. GPAC are associated with low-grade septic arthritis after orthopedic procedures, typically replacements of the hip and knee joints (14, 94). In Bourgault’s series, P. magnus was isolated from 32 of 222 specimens obtained from patients with bone and joint infections (14%); it was recovered in pure culture from 18 patients, in 15 of whom foreign bodies were present. These observations were expanded by Fitzgerald et al. (94), who described 43 patients with clinically, radiologically, and microbiologically documented anaerobic arthritis; the hip or knee was involved in almost 90% of cases. Patients with postoperative or posttraumatic infections were usually infected with GPAC, but in a smaller group with chronic debilitating diseases, “Bacteroides” group spp. were more common. P. magnus was isolated from 40% of patients and was the most common organism by far. Aggressive surgery and prolonged antibiotic therapy were required for eradication; delayed treatment often resulted in permanent loss of joint function. Other cases caused by P. magnus have since been reported (38, 72, 141).
It is important that clinicians be aware that anaerobes can cause arthritis; these infections are chronic and cause much morbidity, and it is likely that many cases are overlooked (38).
PATHOGENESIS AND VIRULENCE FACTORS
The pathogenicity of GPAC has been little studied. Two main lines of research have been pursued: attempts to define virulence factors; and animal models that re-create mixtures of organisms which can then be correlated with quantifiable measures such as abscess size or mortality. The most valuable studies have combined the two approaches.
Most species of GPAC express their pathogenicity via a synergistic interaction with facultative organisms or other anaerobes. However, it is technically difficult to evaluate the relative importance of different components in mixed infections. In the preantibiotic era, Smith carried out classic studies on the pathogenesis of anaerobic lung infections by using experimental animals (238, 239). Injection of pus into the trachea and culture of the resulting lung abscesses led to the isolation of 17 different organisms. A series of experiments showed that monomicrobial challenge with any of these organisms did not result in abscess formation, but a combination of an “anaerobic streptococcus,” an anaerobic spirochete, a probable Fusobacterium, and an anaerobic gram-negative rod was necessary and sufficient to reproduce the disease. Smith thus demonstrated the principle of microbial synergy in the development of anaerobic abscesses. In 1958, Mergenhagen et al. (178) reported synergy between “anaerobic streptococci” and S. aureus in causing progressive gangrene in rabbits; neither component was effective on its own, but cells of S. aureus could be replaced by a dialyzable cell filtrate of S. aureus. This work was extended in the 1980s by Brook and coworkers (18, 19, 25, 47, 48), who used a subcutaneous abscess model in the mouse. They demonstrated synergy of GPAC with facultative and anaerobic bacteria, including S. aureus, P. aeruginosa, several species of streptococci and members of the Enterobacteriaceae, in their ability to induce abscesses, enhance the growth of bacterial components, and increase mortality (19, 25). These studies examined recent clinical isolates of P. anaerobius, P. asaccharolyticus, P. magnus, P. micros, and P. prevotii; they did not demonstrate clear differences between species of GPAC, but small numbers of strains were examined. More recently, Baumgartner et al. (12) used a mouse model to study the potential of oral anaerobes to form abscesses; one of the three strains of P. anaerobius examined was pathogenic in pure culture, but three strains of P. micros were not. Another study by Lewis et al. (165) concluded that anaerobic gram-negative rods from dental infections were more abscessogenic than “Streptococcus milleri” or GPAC; the GPAC were unidentified.
Few studies have tried to explain the mechanisms of microbial synergy. Hypotheses have included mutual protection from phagocytosis (143) and lowering of oxidation-reduction potentials in host tissue (178). Ingham et al. (143) reported that a wide variety of anaerobes were able to inhibit phagocytosis of an indicator strain of Proteus mirabilis; anaerobic gram-negative rods were much more effective than “anaerobic streptococci.” Mergenhagen (178) noted that the infecting dose of “anaerobic streptococci” was significantly reduced when the inoculum was supplemented with chemical reducing agents.
Anaerobic bacteria elaborate a variety of potential virulence factors, including superoxide dismutase, immunoglobulin proteases, coagulation-spreading factors such as coagulase and hyaluronidase, and adherence factors (92); most of the work has been done on clostridia and gram-negative anaerobes. Steffen and Hentges (248) investigated the hydrolytic enzyme activity of fresh abdominal isolates of a range of anaerobes; the eight strains of GPAC examined were much less active than the gram-negative anaerobes, but gelatinase, collagenase, and hyaluronidase were sometimes formed. Marshall and Kaufman (175) reported the formation of several extracellular enzymes (DNase, RNase, coagulase, and hemolysins) by fresh strains of P. asaccharolyticus, P. anaerobius, P. prevotii, and P. indolicus but not P. magnus; enzyme production was variable for all species. Tam and Chan (259) described the production of hyaluronidase by unidentified GPAC isolated from diseased periodontal pockets. Brook showed that the ability to induce capsule formation was an important virulence mechanism (48); capsulated organisms were able to seed more successfully to distant organs (19).
Most work has centered on P. magnus, which elaborates a range of putative virulence factors. Myhre (199) reported that 15 (42%) of 36 strains of P. magnus bound significant amounts of human serum albumin; binding was mediated by a specific protein receptor on the cell wall which showed affinities to proteins elaborated by β-hemolytic streptococci (160, 199). None of a range of other anaerobes, including B. fragilis or C. perfringens, possessed this property. Krepel et al. (157, 158) compared the production of proteolytic enzymes by clinical strains of P. magnus from different sites. They reported that strains from nonpuerperal breast abscesses and diabetic foot infections had significantly greater enzymatic activity, particularly collagenase and gelatinase, than did strains from patients with intra-abdominal sepsis; they proposed that these enzymes could have an important adjunctive effect on the development of soft tissue infections. Björck and coworkers have recently studied protein L, a cell surface protein formed by some strains of P. magnus. Protein L binds to the κ light-chain variable domain of human and many mammalian immunoglobulin molecules (205) and triggers the release of histamine and leukotriene C4 from mast cells and basophilic granules (212). Only 4 (13%) of 30 clinical strains of P. magnus examined by Kastern et al. (149) expressed protein L; all 4 belonged to a group of seven strains isolated from the vaginal flora of women with BV. The correlation with BV indicates that protein L may be a virulence determinant, but this remains to be tested in a satisfactory animal model (149); it may be of value as an immunochemical agent (74, 286). Recently, de Château and Björck (73) have described a mosaic albumin-binding protein, protein PAB, which shows substantial relatedness to protein L and protein G, a virulence factor elaborated by some β-hemolytic streptococci. New techniques for identification of strains synthesizing protein L and albumin-binding proteins have also been developed (204).
The association of P. micros with oral infections has led to some recent interest. Murdoch et al. (195) suggested that the strong proteolytic activity of P. micros could be important in abscess development. Carlsson et al. (56) compared the ability of 37 species of oral bacteria to form hydrogen sulfide from glutathione; P. micros was by far the most active organism tested. Glutathione is a tripeptide involved in the intracellular defense against reactive oxygen metabolites, whereas hydrogen sulfide is cytotoxic. van Dalen et al. (266) described a rough morphotype of P. micros from plaque samples, which differed from the “normal” smooth morphotype in colony morphology, hemolytic ability, and its hydrophilic rather than hydrophobic nature. Most interestingly, electron microscopy revealed that cells of the rough morphotype possessed long fibrillar structures, which appeared to aggregate with each other and which were absent from cells of the smooth morphotype.
It is evident that little is known about the pathogenesis of most infections which yield GPAC or the mechanisms by which GPAC cause disease. The investigations initiated by Krepel et al. (157, 158), comparing fresh clinical isolates from different sites, and more thorough studies of virulence factor formation by different species would be valuable lines of inquiry. The ability of some strains of P. magnus to form both a protein that inhibits immunoglobulin activity and enzymes that permit spread through subcutaneous tissues invites comparison with S. aureus.
SUSCEPTIBILITY TO ANTIBIOTICS
Analysis of antibacterial susceptibility patterns is complex because many different methods are used and comparison is often difficult; there is no generally accepted method for anaerobic susceptibility testing in routine laboratories which is cheap, simple, and accurate (228, 281). The measurement of MICs by the agar dilution method (16, 151, 200) is the most widely used standard as a quantitative technique, but it is not practical in the routine diagnostic laboratory; when shifts due to technical variation are taken into account, this method has an unacceptably high degree of inherent error (281, 282). The broth disk elution method is no longer considered acceptable by the National Committee for Clinical Laboratory Standards (200). The breakpoint method (16) is not suitable for laboratories that test small numbers of organisms; when MICs cluster around the breakpoint, the test is not accurate enough to avoid interbatch variability. Broth microdilution testing is economical, but GPAC are often too fastidious to grow in the medium. Of the systems that utilize a continuous concentration gradient, the disk diffusion technique is generally considered unsatisfactory for fastidious and slow-growing organisms (281). A recent study (282) examined the spiral gradient end-point method; light growth was experienced with many strains of GPAC, and the agreement rate with the agar dilution method was only 86%. The Etest (AB Biodisk, Solna, Sweden) is simple and easy to use but relatively expensive; studies comparing Etest MICs with those obtained by agar incorporation techniques (61, 70) have reported good agreement.
Fortunately, GPAC are usually susceptible to all of the antibiotics used to treat anaerobic infections (Tables 7 and 8) (77, 92, 96); this predictability contributed to the recommendation in 1988 (93) that routine susceptibility testing for anaerobes was not required except in well-defined circumstances. Some authorities (99, 228) have disagreed strongly, citing the recent increase in resistance of fusobacteria to penicillins. Several studies (2, 15, 230, 236, 294) have observed major differences between species of GPAC (Tables 9 to 12) but unfortunately these reports are not at all consistent between themselves; it is not clear how much these differences are due to true geographical variations and how much to methodological differences, or, indeed, to individual error. Regrettably, most reports have presented data for GPAC as a group rather than for individual species, even when interesting resistance patterns have been detected (223, 230). It is highly desirable that future investigations should present data on different species separately; in particular, P. anaerobius is only distantly related to other GPAC and, although the available data are scanty, its susceptibility patterns appear to be distinct.
TABLE 7.
Antibiotic susceptibility patterns of GPACa
| Reference and MIC type | No. of strains tested | MIC (mg/liter) ofa:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ | Pen | Amp | AmpSul | Pip | PipTaz | Cefox | Ctx | Imi | Ery | Clin | Chlor | Cip | Vanc | ||
| Watt et al., 1979 (274) | 94 | ||||||||||||||
| Range | 0.15–10 | <0.04–>20 | <0.04–10 | ||||||||||||
| MIC50 | 0.6 | 2.5 | 0.15 | ||||||||||||
| MIC90 | 1.25 | 10 | 1.25 | ||||||||||||
| Edson et al., 1982 (78) | 101 | ||||||||||||||
| MIC50 | <0.78 | <0.78 | <0.78 | <0.78 | 3.12 | ||||||||||
| MIC90 | >25 | <0.78 | 1.56 | <0.78 | 6.25 | ||||||||||
| Watt and Brown, 1986 (271) | 36b | ||||||||||||||
| Range | 0.125–2 | <0.06–8 | 0.125–8 | <0.015–2 | 0.125–>16 | 0.03–2 | 0.5–8 | ||||||||
| MIC50 | 0.5 | 0.5 | 0.5 | 0.25 | 2 | 0.125 | 1 | ||||||||
| MIC90 | 1 | 4 | 8 | 1 | 16 | 0.25 | 8 | ||||||||
| Greenwood and Palfreyman, 1987 (105) | 50 | ||||||||||||||
| Range | 0.015–4 | 0.06–0.5 | |||||||||||||
| MIC50 | 0.12 | 0.25 | |||||||||||||
| MIC90 | 1.0 | 0.5 | |||||||||||||
| Wexler and Finegold, 1988 (280) | 25 | ||||||||||||||
| Range | 0.12–>256 | 0.06–8 | 0.06–16 | 0.06–1 | 0.06–>256 | 1–64 | |||||||||
| MIC90 | 256 | 8 | 4 | 0.12 | 256 | 8 | |||||||||
| Panichi et al., 1990 (208) | 38 | ||||||||||||||
| Range | 0.125–1 | 0.125–0.5 | 0.125–4 | 0.125–8 | 0.25–128 | 0.125–2 | 0.125–4 | ||||||||
| MIC50 | 0.125 | 0.125 | 0.25 | 2 | 2 | 0.125 | 0.125 | ||||||||
| MIC90 | 0.5 | 8 | 4 | 4 | 8 | 0.25 | 2 | ||||||||
| Sanchez et al., 1992 (230) | 103 | ||||||||||||||
| MIC50 | 0.5 | 0.06 | 3 | 0.125 | 0.19 | ||||||||||
| MIC90 | 32 | 0.2 | >256 | 2 | 0.75 | ||||||||||
| Wexler et al., 1993 (281) | 20 | ||||||||||||||
| Range | 0.125–2 | 0.125–8 | 0.06–0.5 | 0.06–1 | |||||||||||
| MIC50 | 0.5 | 0.125 | 0.06 | 0.125 | |||||||||||
| MIC90 | 2 | 0.5 | 0.06 | 1 | |||||||||||
| Pankuch et al., 1993 (209) | 58 | ||||||||||||||
| MIC50 | 2 | 0.125 | 0.125 | 0.25 | 0.25 | 1 | |||||||||
| MIC90 | 4 | 1 | 1 | 2 | 2 | 4 | |||||||||
| Goldstein et al., 1993 (100) | 29 | ||||||||||||||
| Range | <0.06–2 | <0.06–1 | <0.06–4 | <0.06–4 | <0.015–1 | <0.06–>128 | |||||||||
| MIC50 | 0.25 | 0.125 | <0.06 | <0.06 | <0.015 | 0.125 | |||||||||
| MIC90 | 2 | 0.25 | 1 | 2 | 0.06 | 2 | |||||||||
| Citron et al., 1995 (60) | 32c | ||||||||||||||
| Range | 0.25–128 | <0.06–2 | <0.06–2 | 0.125–8 | <0.06–16 | <0.06–32 | 0.06–>128 | <0.06–>128 | 0.5–16 | ||||||
| MIC50 | 0.5 | 0.25 | 0.25 | 0.125 | 0.5 | 1 | 4 | 0.25 | 4 | ||||||
| MIC90 | 16 | 1 | 1 | 4 | 4 | 16 | >128 | >128 | 8 | ||||||
MZ, metronidazole; Pen, penicillin G; Amp, ampicillin; AmpSul, ampicillin-sulbactam; Pip, piperacillin; PipTaz, piperacillin-tazobactam; Cefox, cefoxitin; Ctx, cefotaxime; Imi, imipenem; Ery, erythromycin; Clin, clindamycin; Chlor, chloramphenicol; Cip, ciprofloxacin; Vanc, vancomycin.
Includes eight strains of “P. saccharolyticus” and one Veillonella sp.
Includes eight strains of Veillonella spp.
TABLE 8.
Antibiotic susceptibility patterns of GPAC by using breakpointsa
| Reference | No. of strains tested | Susceptibility to following antibiotic
(breakpoint) (mg/liter)b
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ (16) | Pen (varies) | Amp (varies) | AmpSul (16) | AmoxCl (8/4) | Pip (varies) | TicClav (64/2) | Cefox (32) | Ctx (64) | CfpSul (16) | Imi (8) | Ery (8) | Clin (varies) | Chlor (16) | Cip (varies) | ||
| Wexler and Finegold, 1988 (280) | 25 | 88 | 100 (16) | 100 | 100 | 100 | 84 (4) | 96 | ||||||||
| Edmiston et al., 1990 (77) | ||||||||||||||||
| P. asaccharolyticus | 12 | 100 | 100 (4) | 100 | 100 | 58 | 100 (4) | 100 (4) | ||||||||
| P. magnus | 23 | 96 | 100 (4) | 100 | 96 | 100 | 87 (4) | 100 (4) | ||||||||
| P. micros | 9 | 100 | 100 (4) | 100 | 100 | 100 | 100 (4) | 100 (4) | ||||||||
| Panichi et al., 1990 (208) | 38 | 100 | 90 (32) | 100 (128) | 100 | 100 | 100 | 100 (8) | ||||||||
| Pankuch et al., 1993 (209) | 58 | 93 | 100 (64) | 100 | 91 (4) | 81 (2) | ||||||||||
| Goldstein et al., 1993 (100) | 29 | 100 | 100 | 100 (64) | 100 | 100 | 90 (4) | |||||||||
| Wexler et al., 1993 (281) | 20 | 100 | 100 | 100 | 100 (4) | |||||||||||
| Civen et al., 1995 (62) | 6 | 100 | 100 (4) | 100 | 100 (64) | 100 | 100 | 100 | 100 (4) | 100 | ||||||
| Citron et al., 1995 (60) | 32 | 94 | 100 (8) | 100 | 97 (64) | 100 | 100 | 100 | 97 | 50 | 78 (4) | 100 | ||||
MZ, metronidazole; Pen, penicillin G; Amp, ampicillin; AmpSul, ampicillin-sulbactam; AmoxCl, amoxicillin-clavulanic acid; Pip, piperacillin; TicClav, ticarcillin-clavulanic acid; Cefox, cefoxitin; Ctx, cefotaxime; CfpSul, cefoperazone-sulbactam; Imi, imipenem; Ery, erythromycin; Clin, clindamycin; Chlor, chloramphenicol; Cip, ciprofloxacin.
Numbers in parentheses after the antibiotics give breakpoints (milligrams per liter); when they vary, they are given in parentheses in the body of the table.
TABLE 9.
Antibiotic susceptibility patterns of P. anaerobius
| Reference and MIC type | No. of strains tested | MIC (mg/liter)
ofa:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ | Pen | Amox | AmoxCl | Pip | PipTaz | Cefox | Imi | Clin | Cip | Trova | ||
| Aldridge et al., 1983 (2) | 20 | |||||||||||
| Range | <0.25–>64 | <0.125–16 | <0.125–1 | |||||||||
| MIC50 | 2 | 0.125 | 0.125 | |||||||||
| Sanchez et al., 1992 (230) | 8 | |||||||||||
| MIC50 | 0.05 | |||||||||||
| Reig and Baquero, 1994 (222) | 4 | |||||||||||
| Range | 1–8 | 2–>16 | 2–>16 | 0.25–32 | 0.5–32 | |||||||
| Mode | 8 | >16 | >16 | |||||||||
| Bowker et al., 1996 (15) | 9 | |||||||||||
| Range | <0.008–4 | <0.008–8 | <0.008–32 | 0.03–64 | 0.06–16 | <0.008–1 | 0.008–0.5 | <0.008–4 | 0.03–1 | |||
| MIC50 | 0.06 | 0.03 | 0.12 | 0.5 | 0.25 | 0.03 | 0.06 | 0.5 | 0.06 | |||
MZ, metronidazole; Pen, penicillin G; Amox, amoxicillin; AmoxCl, amoxicillin-clavulanic acid; Pip, piperacillin; PipTaz, piperacillin-tazobactam; Cefox, cefoxitin; Imi, imipenem; Clin, clindamycin; Cip, ciprofloxacin; Trova, trovafloxacin.
TABLE 12.
Antibiotic susceptibility patterns of P. micros
| Reference and MIC type | No. of strains tested | MIC (mg/liter)
ofa:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ | Pen | Amox | AmoxCl | Pip | PipTaz | Cefox | Imi | Clin | Chlor | Cip | Trova | ||
| Aldridge et al., 1983 (2) | 5 | ||||||||||||
| Range | <0.25 | <0.125 | <0.125 | ||||||||||
| MIC50 | <0.25 | <0.125 | <0.125 | ||||||||||
| Sanchez et al., 1992 (230) | 13 | ||||||||||||
| MIC50 | 0.094 | ||||||||||||
| MIC90 | 0.5 | ||||||||||||
| Rams et al., 1992 (221) | 49 | ||||||||||||
| Range | <0.5–>8 | <0.06–2 | <1–>16 | <0.25–>4 | <0.5–8 | ||||||||
| MIC50 | <0.5 | <0.06 | 2 | <0.25 | 1 | ||||||||
| MIC90 | 4.0 | 0.25 | 4 | 1 | 4 | ||||||||
| Sheikh et al., 1993 (236) | 23 | ||||||||||||
| Range | 0.06–>128 | 0.06–2 | 0.008–0.125 | 0.008–64 | |||||||||
| MIC50 | 1 | 0.25 | 0.008 | 0.03 | |||||||||
| MIC90 | >128 | 2 | 0.125 | 64 | |||||||||
| Reig and Baquero, 1994 (222) | 4 | ||||||||||||
| Range | 0.03 | 0.06–0.5 | 0.06–0.12 | 0.06–0.12 | 0.06 | ||||||||
| Mode | 0.03 | 0.25 | 0.12 | 0.12 | 0.06 | ||||||||
| Bowker et al., 1996 (15) | 12 | ||||||||||||
| Range | <0.008–0.015 | <0.008–0.03 | <0.008–0.12 | <0.008–0.25 | <0.008–0.5 | <0.008–0.03 | <0.008–0.25 | <0.008–0.5 | 0.015–0.06 | ||||
| MIC50 | <0.008 | <0.008 | <0.008 | 0.015 | 0.25 | 0.015 | <0.008 | 0.25 | 0.03 | ||||
| MIC90 | 0.015 | 0.015 | 0.03 | 0.12 | 0.25 | 0.03 | 0.03 | 0.5 | 0.06 | ||||
MZ, metronidazole; Pen, penicillin G; Amox, amoxicillin; AmoxCl, amoxicillin-clavulanic acid; Pip, piperacillin; PipTaz, piperacillin-tazobactam; Cefox, cefoxitin; Imi, imipenem; Clin, clindamycin; Chlor, chloramphenicol; Cip, ciprofloxacin; Trova, trovafloxacin.
Most evidence suggests that P. asaccharolyticus, P. magnus, and P. micros are almost always susceptible to penicillins (Tables 7 to 12) (2, 15, 144, 222). However, some strains of P. anaerobius are much more resistant (2, 15, 187, 222). A recent report of results obtained by the Etest method (294) also observed high rates of resistance in P. magnus and P. micros. Of the cephalosporins, cefoxitin is probably the most effective; some strains are only moderately susceptible (60, 271, 280) but complete resistance appears to be rare (15, 187, 208). The activity of cefotaxime appears often to be borderline (60, 208, 271). Occasional strains of P. anaerobius are resistant to both cefoxitin and cefotaxime (187). Ceftazidime is unreliable (144, 208). The limited data available on ceftriaxone (208, 280), cefoperazone (280), and cefotetan (281) indicate moderate efficacy. GPAC appear to be uniformly and exquisitely susceptible to imipenem and meropenem (2, 15, 101, 236, 281). β-Lactamase production by GPAC has never been satisfactorily documented (96, 144). Sturm (250) reported that 3 of 31 strains from breast abscesses broke down the chromogenic cephalosporin nitrocefin (Oxoid, Basingstoke, United Kingdom), but he did not fully identify these strains. A few other workers (2, 187) appear to have examined penicillin-resistant GPAC for β-lactamase activity, but they have been unsuccessful. When MIC data on β-lactam agents alone are compared with data on β-lactam/β-lactamase inhibitor combinations, the values are similar or identical, e.g., amoxicillin and amoxicillin-clavulanate (222), piperacillin and piperacillin-tazobactam (209, 222), cefoperazone and cefoperazone-sulbactam (280), and ampicillin and ampicillin-sulbactam (60). Thus, resistance is most likely to be mediated via modified penicillin-binding proteins (96, 222).
Metronidazole susceptibility is a controversial area. Watt and Jack (272) reported that the strictly anaerobic cocci they examined were all susceptible to metronidazole but the microaerophilic strains were all resistant; they did not (contra some workers [279]) include this property in their definition of an anaerobic coccus. Several authorities maintain that almost all GPAC are susceptible (92, 96, 200), but resistance has frequently been reported (78, 230, 251, 280). Unfortunately, the possibility that some resistant strains were microaerophilic streptococci cannot be dismissed, because their species identity has rarely been revealed; in one study that reported a resistance rate of 12% (280), the authors acknowledged that microaerophilic strains were probably responsible. However, in a recent multicenter study, Sheikh et al. (236) reported MICs at which 90% of the isolates were inhibited (MIC90s) of 128 mg/liter for P. asaccharolyticus and P. micros (Tables 10 and 12). In the report of Bowker et al. (15), strains of P. asaccharolyticus, P. magnus, and P. micros were all exquisitely susceptible to metronidazole but two of the nine strains of P. anaerobius tested were of intermediate susceptibility (MIC, 2 to 4 mg/liter). Some strains, usually belonging to the prevotii/tetradius group or βGAL group, are partially or even completely resistant by disk testing (187). Further data to clarify this important issue would be extremely valuable.
TABLE 10.
Antibiotic susceptibility patterns of P. asaccharolyticusa
| Reference and MIC type | No. of strains tested | MIC (mg/liter)
ofa:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ | Pen | Amox | AmoxCl | Pip | PipTaz | Cefox | Imi | Clin | Cip | Trova | ||
| Aldridge et al., 1983 (2) | 5 | |||||||||||
| Range | 0.25–8 | <0.125 | <0.125 | |||||||||
| MIC50 | 0.5 | <0.125 | <0.125 | |||||||||
| Sanchez et al., 1992 (230) | 14 | |||||||||||
| MIC50 | 0.064 | |||||||||||
| MIC90 | >256 | |||||||||||
| Sheikh et al., 1993 (236) | 45 | |||||||||||
| Range | 0.03–>128 | 0.008–8 | <0.008–0.5 | 0.008–>128 | ||||||||
| MIC50 | 0.5 | 0.06 | 0.008 | 0.06 | ||||||||
| MIC90 | 128 | 0.25 | 0.03 | 0.5 | ||||||||
| Reig and Baquero, 1994 (222) | 12 | |||||||||||
| Range | 0.03–4 | 0.03–8 | 0.03–4 | 0.06–0.5 | 0.06–0.25 | |||||||
| Mode | 0.03 | 0.12 | 0.12 | 0.06 | 0.06 | |||||||
| Bowker et al., 1996 (15) | 13 | |||||||||||
| Range | <0.008–0.03 | <0.008 | <0.008 | <0.008–0.06 | <0.008–0.12 | <0.008–0.03 | <0.008–128 | <0.008 | 0.03–1 | |||
| MIC50 | 0.015 | <0.008 | <0.008 | 0.015 | 0.06 | <0.008 | 0.06 | <0.008 | 0.25 | |||
| MIC90 | 0.03 | <0.008 | <0.008 | 0.06 | 0.12 | 0.015 | 1 | <0.008 | 1 | |||
MZ, metronidazole; Pen, penicillin G; Amox, amoxicillin; AmoxCl, amoxicillin-clavulanic acid; Pip, piperacillin; PipTaz, piperacillin-tazobactam; Cefox, cefoxitin; Imi, imipenem; Clin, clindamycin; Cip, ciprofloxacin; Trova, trovafloxacin.
Erythromycin and the newer macrolides clarithromycin and azithromycin have very similar efficacy and are probably not active enough to be recommended (60, 230, 274). Clindamycin is widely used as an antianaerobic agent, but several groups (60, 77, 209, 280, 294) have reported that resistance is fairly common; there appears to be considerable geographical variation within individual countries, for instance Spain (96) and the United States (101), which may be dependent on local usage. Ohm-Smith et al. (206) described selection of clindamycin-resistant anaerobes, particularly GPAC, following clindamycin therapy for pelvic soft tissue infections. Recently, Reig et al. (223) and Sanchez et al. (230) have described inducible macrolide-lincosamide resistance in several species of GPAC; they recommended that erythromycin susceptibility be assessed before individual isolates are reported as susceptible to clindamycin. The disk diffusion method or the Etest method can be used (223, 230). The few data available on individual species (15, 230, 236) are often conflicting, e.g., for P. asaccharolyticus and P. micros (Tables 10 and 12). The sum of these observations is that clindamycin is clearly inferior to penicillins for therapy against GPAC.
Few data are available on glycopeptides, but resistance has not been reported; teicoplanin appears to be slightly more effective than vancomycin (105). Most strains are susceptible to chloramphenicol, although acetyltransferase activity in GPAC has been recorded (96). Several studies (100, 209, 271) indicate that early quinolones such as ciprofloxacin have only moderate activity, but more recent agents such as trovafloxacin (15), clinafloxacin (170), and Bay y3118 (209, 281) are extremely active. However, in a recent study (15) which presented data by species, isolates of P. magnus, P. micros, and P. asaccharolyticus were consistently susceptible to ciprofloxacin but isolates of P. anaerobius were often borderline or resistant.
The available data are often so contradictory that it is difficult to piece together a coherent pattern. However, it appears that a penicillin or (probably) metronidazole is effective first-line therapy for GPAC; the superior penetration of metronidazole into pus (159) is an advantage. A β-lactamase inhibitor will add little to penicillin therapy for GPAC, although it may be essential for associated organisms in mixed infections. Susceptibility to clindamycin varies widely; local geographical variations should be taken into account, and erythromycin susceptibility should also be tested. Cephalosporins are usually but not always effective. Chloramphenicol has been neglected. Carbapenems are extremely active, and the newer quinolones show promise.
GENUS PEPTOSTREPTOCOCCUS
Definition of the Genus
The genus Peptostreptococcus is genetically and phenotypically so heterogeneous (67) that it is difficult to construct a definition beyond that supplied by Watt and Jack (272); the genus is now little more than a placement of convenience for anaerobes of a certain cell morphology which cannot multiply in an aerobic environment.
Cells vary in size from 0.3 to 2.0 μm and can be arranged in chains, pairs, tetrads, or clumps; most species are present either as chains or clumps. Most species retain Gram’s stain well, but some present a characteristic decolorized appearance after incubation for 48 h. They are obligate anaerobes, but some strains are aerotolerant; this aspect of their biology has been very little studied. They do not form spores. The ability to utilize carbohydrates varies greatly; some species are asaccharolytic, but a few are strongly saccharolytic. For most species, the products of protein digestion appear to be the principal energy source.
The G+C content varies from 27 to 37 mol%. The type species is P. anaerobius.
P. anaerobius
P. anaerobius is the type species of the genus Peptostreptococcus (136). At present it is classified with other species of GPAC on the basis of G+C content and DNA relatedness (87), but nucleic acid studies have revealed that it is only distantly related to other species in the genus (142, 163, 167). Collins et al. (67) placed it in cluster XI with Eubacterium tenue and 10 species of Clostridium, but they considered that it represented a relatively distinct subline within the cluster which was probably worthy of genus status.
The cell morphology is usually coccobacillary, with a diameter of 0.5 to 0.7 μm, and is often highly pleomorphic; the cells are arranged in chains (136, 192). Growth on enriched blood agar is more rapid than with other species of GPAC; most strains form distinctive colonies, 1 mm in diameter after 24 h, which are grey with slightly raised off-white centers and which usually give off a distinctive, sickly sweet odor (192). Some carbohydrates may be weakly fermented, but proteolytic activity is feeble (Table 3). Of 47 strains studied by Murdoch et al. (192) using the ATB 32A kit (API-bioMérieux; now renamed Rapid ID 32A), all produced α-glucosidase and 43 (90%) produced proline arylamidase; other saccharolytic or proteolytic enzymes were not detected. A variety of VFAs are formed in peptone-yeast-glucose broth (136) or Robertson’s cooked-meat broth (192): acetic, isobutyric, butyric, isovaleric, and isocaproic acids. In Robertson’s cooked-meat broth, 90 to 95% of strains produce a major, terminal peak of isocaproic acid, but with 5 to 10% of strains, isocaproic acid is not detected and the terminal peak is of isovaleric acid (192). With experience, presumptive identification can be made by a combination of cellular and colonial morphology and odor. Graves et al. (104) reported that 27 strains of P. anaerobius were all susceptible to SPS but 43 other strains of GPAC were resistant; they suggested that SPS disk testing provided a simple method of presumptive identification. Others (192, 284) have confirmed the reliability of this test. Babcock (5) developed a tyrosine-containing medium for identification of P. anaerobius, but because cultures required incubation for up to 72 h to detect crystal formation, this medium has been little used.
P. anaerobius is recognized as part of the gastrointestinal flora (136, 201), but the data regarding the oral and vaginal flora are contradictory (123, 126, 136, 201); further reports would be valuable. It accounted for 13 to 18% of all isolates of GPAC in four surveys (Table 5). It has been isolated from a wide variety of human clinical specimens: Holdeman Moore et al. (136) list abscesses of the brain, jaw, pleural cavity, ear, pelvic, urogenital, and abdominal regions, as well as blood, spinal and joint fluid, and cases of osteomyelitis. It has been recovered from specimens from periodontitis and intraoral sepsis (182, 184, 269) and is one of the most common species of GPAC in infections of the abdominal cavity and the female genitourinary tract (28, 30, 196) (Table 6). In a recent survey (196), strains were almost always isolated with members of the fecal flora such as coliforms and enterococci; strains were occasionally isolated from superficial sites prone to fecal contamination. Isolation in pure culture is rare but has been reported from two cases of pleural empyema (39, 295) and a recent case of prosthetic valve endocarditis (181).
There have been few reports on pathogenicity. Using a subcutaneous abscess model in the mouse, Brook et al. demonstrated synergy of P. anaerobius with facultative and anaerobic bacteria in their ability to induce abscesses, enhance the growth of bacterial components, and increase mortality (19, 25); capsule formation was an important virulence determinant (48). Baumgartner et al. (12) reported that a fresh clinical isolate was able to form an abscess in a mouse model. It is therefore difficult at present to assess the pathogenicity of P. anaerobius; it may be a significant component of mixed infections, or it may be present more often as a passenger.
Antibiotic data are unfortunately scanty (Table 9). All 35 strains examined by Holdeman Moore et al. (136) were susceptible to clindamycin; one was resistant to chloramphenicol, two were resistant to erythromycin, four were resistant to penicillin, and four were resistant to tetracycline. Occasional strains are resistant to a range of β-lactam agents including cefotaxime and cefoxitin (2, 15, 187, 222); modified penicillin-binding proteins are the most likely mechanism of resistance, since β-lactamase activity has not been clearly demonstrated (2, 62, 187, 250) and combination with a β-lactamase inhibitor does not restore susceptibility (15, 222). Two of the nine strains studied by Bowker et al. (15) had intermediate susceptibility to metronidazole (MICs, 2 to 4 mg/liter). The strains examined by Wren (294) with Etest strips were all susceptible to metronidazole, penicillin, chloramphenicol, and clindamycin.
In summary, P. anaerobius is only distantly related to other GPAC and is easily identified by a variety of techniques. It is part of the gastrointestinal flora and is commonly isolated from human clinical specimens, particularly from the abdominal cavity and genitourinary tract, but usually in heavily mixed growth; its place in the normal flora and infections of the mouth is less clear. There are few reports on pathogenicity or antibiotic susceptibility; some strains are resistant to a variety of β-lactam agents. The limited data on P. anaerobius are often contradictory, and several aspects of its biology deserve further study.
P. asaccharolyticus
P. asaccharolyticus is frequently reported from human clinical specimens, but strains previously recognized on phenotypic grounds as P. asaccharolyticus are genetically diverse (84, 87, 136, 188). Hence, the biology and pathogenicity of this important species are poorly defined.
Until recently, all butyrate-producing GPAC that formed indole were identified as P. asaccharolyticus or P. indolicus; unfortunately, although the type strain of P. asaccharolyticus is asaccharolytic (87, 136), standard identification texts (128, 136, 254) did not attempt to distinguish between asaccharolytic and strongly saccharolytic strains. Ezaki et al. clarified the taxonomy by describing a group of saccharolytic strains as P. hydrogenalis (83); a similar saccharolytic group, termed “trisimilis,” has been informally described (186, 190). Ezaki et al. (87) also recognized a group of genetically distinct asaccharolytic strains (homology group 2) which may correspond to the recently proposed “P. harei” (188). To complicate matters further, the type strain of P. asaccharolyticus is highly atypical in its whole-cell composition (190) and some biochemical properties (192). Recent 16S rRNA sequence data (67, 188) indicate a close genetic relationship to P. indolicus in Clostridium cluster XIII.
The cellular morphology is characteristic: the cell size is more uniform (cell diameter of 0.5 to 0.9 μm) than is observed with most species of GPAC, and cells occur in clumps, retaining Gram’s stain poorly and often resembling strains of neisseriae (192). The colony morphology is also distinctive; after 5 days of incubation on enriched blood agar, colonies are 2 to 3 mm in diameter, glistening, low convex, usually whitish to lemon-yellow, and often have a characteristic musty odor (192). Typical strains are asaccharolytic but usually form several arylamidases (Table 3); the reproducibility of proteolytic enzyme reactions between laboratories can be poor (187). Coagulase is not produced. Authorities (87, 136) differ on whether nitrate is reduced. According to standard identification manuals (84, 129, 136, 251), strains of P. asaccharolyticus form indole, but several lines of evidence, including whole-cell protein electrophoresis (261), HPLC (117), PEP studies (192), and PyMS (190), indicate that a proportion of typical strains, perhaps 10 to 20%, are indole-negative. All strains form butyric acid as their terminal VFA.
The identification of P. asaccharolyticus is contentious. Indole-positive strains must be distinguished from P. hydrogenalis, P. indolicus, “P. harei,” and unrecognized groups. With experience, the cellular and colony morphology and smell are useful guides to identification. P. hydrogenalis is strongly saccharolytic and very weakly proteolytic; cells of this species usually vary greatly in size but retain Gram’s stain well. P. indolicus is phenotypically similar to P. asaccharolyticus and can be difficult to differentiate, but production of coagulase is reported as a characteristic feature (87, 132); Ezaki et al., but not Holdeman Moore et al., also recommend testing for nitrate reduction (84, 87, 136). The cell and colony morphology readily distinguish strains of P. asaccharolyticus from “P. harei” (188, 192), but biochemical tests to distinguish these species are poorly defined. Indole-negative strains of P. asaccharolyticus present a major problem, since most identification schemes (84, 129, 136, 251) use indole production as a key test for the differentiation of butyrate-producing GPAC. Strains showing the characteristic cell and colony morphology, with the correct PEP except for a negative indole reaction, can probably be identified as P. asaccharolyticus; examination of the whole-cell composition of four such strains by PyMS confirmed their identity (190).
P. asaccharolyticus is part of the normal flora of the genitourinary and gastrointestinal tracts (7, 123, 126, 201, 241) and possibly also of the skin (196), but it appears to be rare in the oral flora. Hillier et al. (126) reported that over 75% of pregnant women were carriers in the vaginal flora; it was one of the most common organisms, with counts of 104 to 105 CFU/ml, which were similar in women with normal flora and BV. However, the identification techniques used in this report depended heavily on carbohydrate fermentation tests, and strains of P. hydrogenalis were not reported. It is one of the most common species of GPAC in human clinical material (Table 5). Brook (28) associated it with obstetric and gynecological infections. Holdeman Moore et al. (136) reported “strains homologous with the type strain” from vaginal discharge, skin abscess, and peritoneal abscess. Murdoch et al. (196) reported that a submandibular sebacous cyst and an ischiorectal abscess yielded pure cultures. There are few reports on pathogenicity or susceptibility to antibiotics (Table 10). Brook et al. demonstrated synergy with facultative and anaerobic bacteria in the ability to induce abscesses, enhance the growth of bacterial components, and increase mortality (19, 25); capsule formation was an important virulence determinant (48). Data on antibiotic susceptibility are conflicting. The strains examined by Bowker et al. (15) were extremely susceptible to antibiotics, but more than 10% of the strains examined by Sheikh et al. (236) were resistant to metronidazole. Reig and Baquero (222) reported strains with borderline susceptibility to penicillins. Sanchez et al. (230), Sheikh et al. (236), and Bowker et al. (15) noted only occasional resistance to clindamycin, but Wren (294) reported 12% clindamycin resistance.
P. asaccharolyticus is clearly one of the most important species of GPAC, but studies of its biology will be held back until a phylogenetically valid species is defined and simple, accessible tests for its identification are available. In 1986, Holdeman Moore et al. (136) warned that “reports of isolation and incidence of P. asaccharolyticus in the present literature should be interpreted cautiously.” These comments still apply.
P. barnesae
Three strains of a new species of purinolytic GPAC were isolated from chicken feces by Schiefer-Ullrich and Andreesen in 1985 (232). 16S rRNA sequence data place P. barnesae in Clostridium cluster XIII of Collins et al. (67, 188). The type strain, DSM 3244T, forms cells with diameters of 0.5 to 0.9 μm which usually arranged in pairs, and it grows poorly on enriched blood agar to form white colonies with diameters of up to 1 mm after incubation for 5 days (186). It is asaccharolytic but feebly indole positive; it utilizes purines and glycine. Acetate is the principal VFA, but small quantities of butyrate are also formed under certain conditions (84). Human isolates have not yet been reported.
P. heliotrinreducens
The little-studied species P. heliotrinreducens was isolated from the ruminal flora of a sheep by Lanigan (162), who placed it in the genus Peptococcus; it was later transferred to the genus Peptostreptococcus on the basis of its G+C content (86, 136). Recent 16S rRNA sequence data and PyMS studies indicate that it should not be classified in the genus Peptostreptococcus (167, 190).
The type strain is a coccobacillus, with a diameter of 0.3 to 0.6 μm, which grows very slowly on enriched blood agar to form translucent, glistening colonies, with diameters of up to 1 mm, after incubation for 5 days (192). It does not utilize carbohydrates but reduces nitrates and pyrrolizidine alkaloids. The only VFAs formed are small amounts of acetate and, with special techniques, butyrate (136, 192). Small quantities of the NVFAs lactate and succinate are also formed (102). P. heliotrinreducens is rarely included in standard identification manuals. Identification by PEP with the ATB 32A kit (API-bioMérieux) appears straightforward (192); the type strain is strongly proteolytic and forms ADH but not ALP. Its slow growth, cell morphology, and proteolytic activity could lead to misidentification as P. micros; however, strains of P. micros form ALP but not ADH and usually have a distinctive colony morphology.
Five strains identified as P. heliotrinreducens with PEPs similar to the type strain have been isolated from human clinical material, all from polymicrobial anaerobic abscesses (of the subcutaneous tissue of a diabetic, tooth, abdominal wall, perianal region, and pleural empyema) (191). These strains were susceptible to metronidazole, vancomycin, penicillin, cefoxitin, clindamycin, erythromycin, and novobiocin by disk testing; two were resistant to chloramphenicol (187). A recent study (102) involving PyMS and artificial neural networks indicated that these strains were dissimilar from the type strain; two representative strains were very similar to organisms from dentoalveolar abscesses first described as Eubacterium C2 (268) but now placed in the newly described Eubacterium exiguum (216). There are no other clinical reports of P. heliotrinreducens, probably because of the difficulties posed by culture and identification.
P. hydrogenalis
In 1983, Ezaki et al. (87) reported that three saccharolytic strains of GPAC, provisionally identified on phenotypic grounds as P. asaccharolyticus, formed a distinct group on the basis of DNA-DNA hybridization data. These strains, originally designated Peptostreptococcus group A-1, were formally described as P. hydrogenalis in 1990 (83). 16S rRNA sequence data indicate a close relationship to other butyrate-producing, saccharolytic species such as P. prevotii and P. tetradius in Clostridium cluster XIII of Collins et al. (67). Comparisons of PEPs (192, 193) and whole-cell composition by PyMS (190) indicate that P. hydrogenalis corresponds to Hare group III of Thomas and Hare (263).
The cells are very variable in size (diameter, 0.5 to 1.8 μm); they are usually arranged in clumps but sometimes in tetrads and short chains (83, 192). Strains grow on enriched blood agar to form unremarkable, grey-white convex colonies, with diameters of 2 to 3 mm, after incubation for 5 days; an unpleasant odor is noted with some strains but can be present with other species of GPAC, particularly P. vaginalis (192). Strains of P. hydrogenalis are strongly saccharolytic and produce indole (83), but their proteolytic activity is very weak (83, 85, 186, 192); this combination of characteristics is distinctive and permits identification by use of the PEP. Examination with the ATB 32A kit reveals that some strains also produce urease and α-glucosidase. Butyrate is the terminal VFA formed; copious production of hydrogen from peptone-yeast extract is characteristic (83). The “trisimilis” group also contains saccharolytic indole- and butyrate-producing strains but can be distinguished by slower colony growth and formation of the proteolytic enzyme pyroglutamyl arylamidase (190, 192); preliminary investigations with the Rapid ID 32 STREP kit indicate that production of acid from trehalose and hydrolysis of hippurate may also be helpful.
Ezaki et al. (83) isolated the type strain from human feces and other strains from human vaginal discharges; it is part of the normal prepubertal vaginal flora (123). Thomas and Hare (263) isolated strains of Hare group III from nose, skin, and particularly vaginal specimens. Murdoch et al. (196) reported strains from a purulent sternotomy wound (cultured with Escherichia coli) and an infected pacemaker site (with an unidentified strain of GPAC); it accounted for only 1% of isolates in this survey, but it has since been isolated from other infected superficial sites, including a cellulitic leg ulcer, ingrowing toenails, and inflamed foot ulcers (187). It has not yet been reported in pure culture, and its pathogenicity has not been studied. Very few antibiotic susceptibility data are available; the nine strains examined by Murdoch and Magee (190) were all susceptible to vancomycin by disk testing, but single strains were moderately resistant to metronidazole, penicillin, chloramphenicol, or clindamycin (187).
P. indolicus
P. indolicus is unique amongst recognized species of GPAC in that it is rarely isolated from human pathological specimens but is an important veterinary pathogen (171). Nucleic acid studies (67, 142, 188) indicate that P. indolicus is closely related to but distinct from P. asaccharolyticus; in a revised classification of the group, it would probably merit placement in the same genus (67).
Few human strains are available for study, but their cell and colony morphology are similar to those of strains of P. asaccharolyticus (192); both species show similar patterns of biochemical activity, with moderate proteolytic activity but inability to ferment sugars (136). Hoi-Sorensen (132) examined 274 strains, mostly of bovine origin, and noted that 267 (97%) produced a cell-associated peptocoagulase that coagulated rabbit and calf plasma. Ezaki et al. (87) compared four strains of P. indolicus with 15 strains of P. asaccharolyticus and proposed that the key distinguishing features were production of peptocoagulase, reduction of nitrate to nitrite, and formation of propionate from lactate by P. indolicus. However, Holdeman Moore et al. (136) reported that some strains of both species were able to reduce nitrate. Butyrate is the terminal VFA produced. P. indolicus has been isolated in pure culture from the skin lesion of a sheepherder (13) and from a superficial abscess (196); it causes a synergistic infection, bovine summer mastitis, with S. aureus (132, 171). Hoi-Sorensen (132) reported that 20 animal strains were all susceptible to penicillin, tetracycline, and chloramphenicol.
P. lacrimalis
P. lacrimalis is a recently described species of unknown pathogenic potential. The type strain of P. lacrimalis, GIFU 7667, was differentiated in 1992 from P. prevotii by Li et al. (166) on the basis of DNA-DNA hybridization studies; recent 16S rRNA sequence data (67) confirm its validity. The peptidoglycan type of the cell wall differs from P. prevotii, P. tetradius, and P. lactolyticus (166). GIFU 7667T forms small cells with diameters of 0.5 to 0.7 μm, which occur in short chains or clumps (166). It grows slowly on enriched blood agar to form pink-white colonies, with diameters of 1 to 2 mm after incubation for 5 days, which exhibit marked variation in size (186). The two strains in the original description by Li et al. (166) were asaccharolytic and inactive in classic biochemical tests but showed strong proteolytic activity. Butyrate is the terminal VFA. The PEP as revealed in the ATB 32A commercial kit is distinctive and should afford a straightforward method of identification (Table 3) (186). Both strains described by Li et al. were cultured from discharges from human eyes. Previous isolates are likely to have been identified as P. prevotii, but other strains have not yet been reported (192).
P. lactolyticus
Using DNA-DNA hybridization studies, Li et al. (166) described P. lactolyticus in the same report as P. vaginalis and P. lacrimalis. Comparisons of 16S rRNA sequence data (67) and cell wall peptidoglycan type (166) indicate that P. lactolyticus is closely related to P. vaginalis, P. prevotii, and P. tetradius.
The type strain grows very slowly on enriched blood agar to form translucent colonies, 1 mm in diameter, after incubation for 5 days; the cells are very variable in size (diameter, 0.5 to 1.5 μm) and occur in clumps and short chains (186). The two strains described show strong saccharolytic and proteolytic activity and produce urease, ALP, and β-galactosidase (166), a distinctive combination of properties that enables easy identification with the ATB 32A commercial kit (186). Butyrate is the terminal VFA produced. Both strains were isolated from human vaginal discharges; the slow growth on standard laboratory media may explain why these are the only strains so far reported. Other data are not available.
P. magnus
P. magnus is by far the most common species of GPAC in human clinical specimens (28, 196, 227, 295) and probably the most pathogenic: two studies (14, 196) and numerous case reports (51, 64, 72, 141, 207, 214, 217) have documented its isolation in pure culture from a wide variety of anatomical sites. However, readily available methods for differentiation from P. micros are still not generally agreed, a problem of great importance since both species are frequently isolated from human clinical specimens.
P. magnus was classified in the genus Peptococcus because of its cellular arrangement (136) until its G+C content and data from DNA-DNA homology studies led Ezaki et al. (87) to transfer it to the genus Peptostreptococcus. Recent 16S rRNA sequence analyses (67) place it in Clostridium cluster XIII but indicate that it forms a separate lineage worthy of generic status. “Peptostreptococcus variabilis,” described by Foubert and Douglas in 1948 (95), is probably a synonym, although some data from analysis of soluble cellular proteins (58) and whole-cell protein electrophoresis (261) indicate that it may be a valid species.
Strains of P. magnus are very variable in both cell and colonial morphology (192). The typical gram-stained appearance is of clumps of cells with diameter of 0.8 to 1.6 μm, with the largest, most strongly stained cells in the center and smaller, variability stained cells on the periphery; the latter are possibly dead cells which are disintegrating. However, some strains exhibit a cellular arrangement consistently similar to that of strains of P. asaccharolyticus, with very decolorized cells of uniform size. Most strains grow slowly on enriched blood agar to form colonies with diameters of 1 to 2 mm after incubation for 5 days; there is often marked variation in the size and color on one plate, with some colonies convex and whitish while others are flatter and translucent, an appearance that often leads to the assumption that the culture is mixed. Some strains consistently produce white colonies, similar to those of many staphylococci, but others produce translucent, grey, or occasionally yellowish colonies. When two fresh clinical isolates were exposed to air, 1% of cells were still viable after 48 h, indicating that resting cells may be relatively aerotolerant (187). Saccharolytic activity is very limited, with weak acid sometimes formed from glucose and fructose (136), but proteolytic activity is strong (192). Acetate is the only VFA formed (136).
The differentiation of P. magnus and P. micros has long been a major source of contention. Colony morphology is useful for presumptive identification but is not completely reliable (195, 251, 254). Many biochemical tests have been put forward, but none is both generally accepted and practicable in a routine diagnostic laboratory. According to recent standard manuals (129, 251), acetate-forming GPAC which form cells with diameters of more than 0.6 μm can be presumptively identified as P. magnus; however, few laboratories have ready access to GLC equipment, so this method is of limited use. Ezaki et al. (87) reported that 22 of 23 strains of P. magnus examined formed catalase or liquified gelatin, but the type strain of P. micros was negative for both tests; they suggested that these tests could be “helpful.” Cato et al. (58) recommended a comparison of soluble-cell protein patterns by PAGE, but this technique is unlikely to be of use outside a research laboratory. Cato et al. (58) also compared 10 reference strains of P. magnus by using the API ZYM kit (Analytab Products) and reported that none formed ALP; Holdeman Moore et al. (136) recommended this test in Bergey’s Manual of Systematic Bacteriology in 1986. However, with the same commercial kit, Ezaki et al. (87) reported that 23 strains all produced ALP, although some did so only weakly; 2 strains, including the type strain, were common to both studies. Of 87 strains, 6 (7%), including the type strain, produced ALP when examined by Murdoch and Mitchelmore (192) with the ATB 32A kit. These reports are clearly incompatible and indicate that differentiation by ALP production alone is not reliable.
The PEP uses a range of tests and is therefore potentially a more powerful and discriminatory method of identification (Table 3). Six of the seven recent reports (Table 4) have concluded that preformed enzyme kits differentiate extremely well between these two species. However, in one study by Ng et al. (203), 8 of 11 strains of P. micros from the female genital tract were misidentified as P. magnus because they gave weak peptidase reactions and did not form ALP; the 8 strains of P. micros from other sites and 23 of 24 strains of P. magnus were correctly identified. Surprisingly, these observations led these workers to conclude that all strains of P. magnus and P. micros should be identified by a combination of GLC and cell size. In summary, most of the available evidence indicates that P. magnus and P. micros can be reliably distinguished by a combination of colony morphology and PEP, supported by Gram-stained cell morphology to assess the cell size; GLC is not required.
According to Holdeman Moore et al. (136), P. magnus is part of the normal flora of the urogenital tract but is only very rarely present in the fecal flora or gingival crevices. However, other workers (84, 91, 201) have reported that it is common in stool specimens. It is part of the prepubertal vaginal flora (123). Of 171 pregnant women, 50% were carriers in their vaginal flora (126); women with normal flora and those with BV had similar counts of 104 to 105 CFU/ml. Murdoch et al. (196) noted its frequent occurrence in superficial skin infections and suggested that it might be part of the normal skin flora.
P. magnus is one of the most common anaerobic pathogens; it accounted for 5 to 12% of all anaerobic isolates and 20 to 38% of all isolates of GPAC in four surveys (Table 5) (28, 138, 196, 295). It has been recovered in pure culture from a range of serious infections, some of which have been fatal; reports have described cases of native-valve endocarditis (64), paravalvular abscess round a bioprosthetic aortic valve (217), purulent pericarditis complicated by mediastinitis (214), meningitis after neurosurgery (51), and anaerobic necrotizing pneumonia complicated by pyopneumothorax (207). Several reports noted that the patient was previously well with no apparent cause of serious infection. Bourgault et al. (14) documented 222 infections over 3 years which yielded P. magnus; it was particularly associated with soft tissue and bone/joint infections and was isolated in pure culture from 32 patients (including bone and joint, 18 patients; soft tissue, 10 patients; vascular, 2 patients). Fitzgerald et al. (94) described some of these orthopedic cases in a series of 43 patients with anaerobic septic arthritis; P. magnus was the most common anaerobe in posttraumatic and postoperative cases, causing a chronic, low-grade but painful infection which usually necessitated aggressive surgery and prolonged courses of antibiotics for successful eradication. Other infections of a prosthetic implant (72) and a previously normal joint (141) and infections secondary to trauma (38) have been reported. Many reports (14, 37, 196, 252, 295) have noted a strong association with soft tissue infections, particularly wound infections and superficial abscesses, in which it is often present in pure culture (14, 196). P. magnus was the predominant anaerobe in investigations of nonpuerperal breast abscesses (77, 98) and diabetic foot infections (145, 231, 283); peripheral infections secondary to vascular disease frequently progressed to osteomyelitis (38, 283). Case series have recorded pure cultures of P. magnus from a breast abscess (77), a specimen of infected pleural fluid (173), a postthoracotomy sternal wound infection (32), and three diabetic foot infections (231). It has been recovered from upper respiratory tract infections such as sinusitis and pediatric otitis media (33, 196), but other species of GPAC appear to be more common in infections of the mouth, central nervous system, abdomen, and female genitourinary tract (28, 33, 196). Bourgault et al. (14) and Murdoch et al. (196) noted that (unlike most species of GPAC) P. magnus was isolated more often with facultative organisms than with other obligate anaerobes.
The importance of P. magnus has stimulated several studies of its pathogenicity. Using a mouse abscess model, Brook et al. demonstrated synergy with facultative and anaerobic bacteria in the ability to induce abscesses, enhance the growth of bacterial components, and increase mortality (19, 25); capsule formation was an important virulence determinant (48). Of 36 strains examined by Myhre (199), 15 (42%) were able to bind human serum albumin via a specific protein receptor on the cell wall; the significance of this observation is unclear. Krepel et al. (157, 158) reported that strains of P. magnus from nonpuerperal breast abscesses and diabetic foot infections had significantly greater enzymatic activity than did strains from intra-abdominal sepsis. Björck and coworkers have recently studied protein L, a cell surface protein formed by some strains of P. magnus, which binds to the κ light-chain variable domain of immunoglobulin molecules (205) and triggers the release of histamine from mast cells and basophilic granules (212). They have correlated the isolation of vaginal strains forming protein L with the presence of BV and have postulated that protein L may be a virulence determinant (149).
There are relatively few reports on antibiotic susceptibility (2, 15, 77, 222, 236) (Table 11). Susceptibility to clindamycin varies widely and may be related to local usage (96, 101); Wren (294) reported 9% resistance, and Sanchez et al. reported >10% (230). Wren (294) also reported 16% resistance to penicillin. Otherwise, significant resistance has rarely been encountered. These data indicate that metronidazole and β-lactam agents should be effective against P. magnus but clindamycin should be used with caution.
TABLE 11.
Antibiotic susceptibility patterns of P. magnus
| Reference and MIC type | No. of strains tested | MIC (mg/liter)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ | Pen | Amox | AmoxCl | TicCl | Pip | PipTaz | Cefox | Imi | Clin | Cip | Trova | ||
| Aldridge et al., 1983 (2) | 10 | ||||||||||||
| Range | <0.25–1 | <0.125–0.25 | 0.125–0.25 | ||||||||||
| MIC50 | <0.25 | 0.125 | 0.125 | ||||||||||
| Sanchez et al., 1992 (230) | 38 | ||||||||||||
| MIC50 | 0.038 | ||||||||||||
| MIC90 | >256 | ||||||||||||
| Reig and Baquero, 1994 (222) | 24 | ||||||||||||
| Range | 0.03–0.25 | 0.03–2 | 0.03–2 | 0.06–0.5 | 0.06–0.25 | ||||||||
| Mode | 0.03 | 0.12 | 0.12 | 0.25 | 0.12 | ||||||||
| Sheikh et al., 1993 (236) | 14 | ||||||||||||
| Range | 0.06–2 | 0.016–0.5 | <0.008–0.06 | 0.016–64 | |||||||||
| MIC50 | 0.25 | 0.06 | 0.008 | 0.03 | |||||||||
| MIC90 | 0.5 | 0.5 | 0.06 | 1 | |||||||||
| Johnson et al., 1995 (145) | 20 | ||||||||||||
| MIC50 | 1 | 0.12 | 1 | 0.5 | |||||||||
| MIC90 | 16 | 1 | 4 | 8 | |||||||||
| Bowker et al., 1996 (15) | 36 | ||||||||||||
| Range | 0.008–0.06 | 0.015–0.06 | 0.015–0.25 | 0.12–0.5 | 0.06–0.5 | 0.008–0.12 | 0.06–8 | 0.015–0.5 | 0.015–0.25 | ||||
| MIC50 | 0.015 | 0.06 | 0.12 | 0.25 | 0.25 | 0.03 | 0.25 | 0.12 | 0.06 | ||||
| MIC90 | 0.06 | 0.06 | 0.25 | 0.5 | 0.5 | 0.06 | 1 | 0.5 | 0.25 | ||||
MZ, metronidazole; Pen, penicillin G; Amox, amoxicillin; AmoxCl, amoxicillin-clavulanic acid; TicCl, ticarcillin-clavulanic acid; Pip, piperacillin; PipTaz, piperacillin-tazobactam; Cefox, cefoxitin; Imi, imipenem; Clin, clindamycin; Cip, ciprofloxacin; Trova, trovafloxacin.
P. magnus can clearly act as a primary pathogen, causing a range of infections in previously well patients, ranging from infected sebaceous cysts to life-threatening infections such as endocarditis. It is probably the commonest anaerobe in soft tissue and bone/joint infections, particularly when prosthetic implants are present. Patients with vascular disease such as diabetics also appear to be at significant risk. The limited evidence suggests that it may be relatively aerotolerant; it is more often isolated with aerobes than with obligate anaerobes. It elaborates a range of interesting virulence factors that merit fuller study. Other species of GPAC are usually isolated in heavily mixed culture from specimens with low oxygen tensions; this is less often the case with P. magnus, which shows interesting parallels in its pathogenicity to S. aureus.
P. micros
P. micros was little studied until recently, but its importance in a range of oral infections is now recognized. Its strong proteolytic activity may be significant in the development of mixed extraoral anaerobic abscesses. However, simple tests for its differentiation from P. magnus are still not generally agreed.
Its classification has presented few problems. Collins et al. (67) assigned P. micros to Clostridium cluster XIII with most other species of Peptostreptococcus but considered that it probably constitutes a separate genus. P. glycinophilus is now regarded as a synonym of P. micros; comparison of the type strains by Cato et al. (58) revealed 84% DNA homology and identical protein patterns by PAGE.
The cells are 0.3 to 0.7 μm in diameter and are usually arranged in pairs and chains (136), but they can occur in clumps when they have been cultured on solid media (192). Strains grow very slowly on enriched blood agar to form highly characteristic colonies with diameters of 1 mm after incubation for 5 days: they are typically white (but sometimes grey), glistening, and domed and are often surrounded by a distinctive yellow-brown halo of discolored agar up to 2 mm wide (192, 195, 266). van Dalen et al. (266) described a rough (Rg) morphotype from the gingival plaque of patients with periodontitis, which forms dry, white, hemolytic colonies with crinkled edges; the PEPs are identical to those of the smooth (Sm) morphotype, and the Rg type readily changes to a Sm-like variant (RgSm) in broth culture. Very recent data from PAGE, PyMS, and 16S rRNA sequences (156) indicate that the two morphotypes constitute distinct clusters. The cells may be relatively aerotolerant; when two fresh clinical isolates were exposed to air for 48 h, 1% of cells were still viable (187). Strains of P. micros are asaccharolytic but form ALP and are very strongly proteolytic; they produce acetic acid or no VFAs at all (136, 192). Differentiation from P. magnus has been contentious and is discussed in the section on that species; the available evidence (Tables 3 and 4) indicates that identification can readily be achieved by a combination of colony morphology and PEP, supported by the use of a Gram-stained film to check the cell size; GLC is not required. However, proteolytic enzyme production may vary between anatomical sites. van Dalen et al. (266) noted variable formation by periodontal strains of proline and leucylglycine AMD but consistently positive reactions for phenylalanine and glutamylglutamyl AMD, whereas Ng et al. (203) reported that most vaginal strains formed leucylglycyl AMD but not proline, phenylalanine, or glutamylglutamyl AMD. Confusion with P. heliotrinreducens is also possible for workers without experience of these species, particularly since they are isolated from similar clinical specimens and their cell size is similar; they can be differentiated by colony morphology and PEP, particularly the production of ADH and ALP (192). When haloes are present, the colony morphology of P. micros is distinctive enough to make a presumptive identification (195). Recently, Turng et al. (265) have described PMM, a selective and differential medium which exploits the ability of P. micros to use the reduced form of glutathione to form hydrogen sulfide.
P. micros is part of the normal flora of the gingival crevice (136, 221) and the gastrointestinal tract (7, 91); there are conflicting reports on its status in the normal vaginal flora (123, 126, 136, 201, 241). It is increasingly recognized as an important oral pathogen; this subject is discussed in the section on oral and respiratory tract infections, above. P. micros comprised 5 to 14% of all GPAC in three surveys of anaerobes (28, 196, 295) (Table 5); it has been isolated from many anatomical sites outside the mouth (Table 6), but few case details are available. Holdeman Moore et al. (136) reported that it is “frequently isolated from human clinical specimens including brain, lung, jaw, head, neck and bite abscesses, spinal fluid, blood, and abscesses of other body sites,” but they did not provide supporting data; they hypothesized that extraoral abscesses might develop by hematogenous spread from a primary oral reservoir, most probably the gingival sulcus. Finegold (90) states that P. micros is part of the predominant flora of intra-abdominal infections and of skin and soft tissue abscesses, particularly in IVDUs. Of 23 strains analyzed by Murdoch et al. (195, 196), 15 were cultured from deep sites above the diaphragm but none were from superficial sites. The authors observed a strong association with soft tissue abscesses, including two brain abscesses, two pleural empyemas, and two anorectal abscesses, but also noted its isolation from intrauterine contraceptive devices and patients with chronic sinusitis. In contrast to P. magnus, it was always recovered with other anaerobes but only half the strains were isolated with facultative organisms. These reports (195, 196) noted that P. micros was often part of a distinctive flora which typically included microaerophilic streptococci, Fusobacterium spp., and “Bacteroides” group spp., forming a species cluster analogous to those found in dental disease (244). Summanen et al. (252) compared the bacteria of superficial abscesses in IVDUs and those with no similar history; P. micros was the most common species of GPAC in the first group, probably because oral pathogens were responsible. There is one case report of its culture in pure growth from a paravertebral abscess (210) and one recent case of native valve endocarditis (278).
Brook used a mouse abscess model to demonstrate synergy with facultative and anaerobic bacteria in the ability to induce abscesses, enhance the growth of bacterial components, and increase mortality (19, 25); capsule formation was an important virulence determinant (48). Recent work by Carlsson et al. (56) has shown the ability of P. micros to form hydrogen sulfide from glutathione; high concentrations of hydrogen sulfide, a cytotoxic agent, can be present in periodontal pockets. Cells of the rough morphotype described by van Dalen et al. (266) were hemolytic and possessed fibrillar structures that appeared to aggregate with each other.
Most studies (2, 15, 77, 136, 179, 222) have reported that P. micros is usually highly susceptible to antibiotics (Table 12). However, Rams et al. (220) examined 49 subgingival strains from patients with periodontitis and found that some were resistant to metronidazole and cefoxitin. More recently, Sheikh et al. (236) reported that at least 10% of the strains in a multicenter study were completely resistant to metronidazole and clindamycin, and Wren (294), using the Etest, observed a resistance rate to penicillin of 8%. Marked geographical variations or methodological differences would seem to be the most likely explanations for these apparently conflicting data.
In summary, P. micros is a strongly proteolytic organism, typically isolated from intraoral sepsis and extraoral abscesses and associated with microaerophilic streptococci and fastidious anaerobes. Its isolation from intrauterine contraceptive devices and intranasal specimens (196) indicates that it may be present in a wider range of disease processes and anatomical sites.
P. prevotii
Until 1992, the only recognized species of indole-negative, butyrate-producing GPAC were P. prevotii and P. tetradius (136); as a result, early surveys of human anaerobic isolates (28, 138, 227, 295) reported that P. prevotii was one of the most common species of GPAC in human clinical material. However, nucleic acid studies (67, 87, 166) and comparison of PEPs (192–194) have established that butyrate-producing GPAC are a very heterogeneous group. It is likely that strictly defined strains of P. prevotii are only occasionally recovered from human pathological specimens (189, 190, 192).
Foubert and Douglas (95) described P. prevotii in 1948 on the basis of six strains, some of which were saccharolytic and others were not. In the 8th edition of Bergey’s Manual of Determinative Bacteriology (225), published in 1974, Rogosa considered that P. prevotii was a “composite description of two organisms” and recommended that the name be rejected, as were many other poorly defined species at the time. However, the taxon persisted as a placement of convenience. With the description of P. tetradius (87), P. vaginalis, P. lacrimalis, P. lactolyticus (166), and “P. harei” (188), the taxonomy of the butyrate-producing cocci is becoming more firmly established, but several further species of clinical importance await description (84, 166, 190, 192). Comparisons of 16S rRNA sequence data (67, 188) and peptidoglycan structure (166) reveal that P. prevotii is closely related to P. tetradius, P. hydrogenalis, P. lactolyticus, and P. vaginalis in Clostridium cluster XIII.
Cells of the type strain, ATCC 9321T, vary markedly in size (diameter, 0.6 to 1.5 μm) and are usually arranged in clumps or tetrads (136, 192). Colonies are also very variable but are typically 2 mm in diameter, matte grey, and low convex after incubation for 5 days on enriched blood agar (192). ATCC 9321T produces urease and shows weak saccharolytic and proteolytic activity. Butyrate is the terminal VFA formed.
ATCC 9321T can be distinguished from the type strain of P. tetradius, GIFU 7672T, by production of α-galactosidase and acid from raffinose (192); however, not enough strains of either species have been reliably identified that these distinctions can be generalized. Indole-negative strains of P. asaccharolyticus can be distinguished by their distinctive colony morphology and inability to ferment sugars (192). Strains of P. lactolyticus, P. vaginalis, and other saccharolytic, butyrate-producing GPAC can be differentiated by their PEPs.
Strains identified as P. prevotii have been isolated from the fecal flora (91, 201) and during many infections (Table 7), but the uncertainties surrounding its classification make its clinical importance difficult to assess. Hillier et al. (126) recently reported that carriage in the vaginal flora of pregnant women increased from 32% in those with normal flora to 49% in women with BV; the mean counts also increased. However, since P. vaginalis was not differentiated in this study, the identification of these organisms must be open to doubt. Gulletta et al. (107) described a case of bacteremia in an immunocompromised patient from whom “Peptococcus prevotii” was cultured. Antibiotic susceptibility data have been reported (2, 77, 107, 222, 294).
Much work remains to be done on the classification and identification of saccharolytic, butyrate-producing GPAC. We recommend that clinical isolates not be identified as P. prevotii unless their PEP is almost identical to that of ATCC 9321T, because they may belong to the βGAL group (see below) or other unrecognized groups. Such strains are best described as “prevotii/tetradius group.” The pathogenicity of P. prevotii sensu stricto cannot yet be assessed, but at present it appears not to be a common human isolate.
P. productus
P. productus is a major part of the bowel flora (136) but is rarely isolated from human pathological specimens. It is a strongly saccharolytic organism with a distinctive oval cell morphology; the type strain has a G+C ratio of 45 mol% (84, 87). These characteristics are highly atypical of the genus Peptostreptococcus, which has a G+C range of 28 to 37 mol% (84). Its classification is at present under active review.
Holdeman Moore et al. (136) noted considerable phenotypic heterogeneity within the species P. productus and questioned whether it was a phylogenetically valid species. They also observed that the genus Ruminococcus comprised at least two groups: the first included highly fermentative species with ovoid cell morphology, to which P. productus showed strong similarities, and the second, which contained Ruminococcus flavefaciens, the type species of the genus, comprised species with spherical cells. Comparisons of 16S rRNA sequence data led Collins et al. (67) to place P. productus in Clostridium cluster XIVa, a group containing representatives of the genera Clostridium, Ruminococcus, and Coprococcus but no other recognized species of Peptostreptococcus. Ezaki et al. (82) recently reassigned P. productus to the genus Ruminococcus on the basis of 16S rRNA sequence data. However, this study did not examine ATCC 19208T, the type strain of R. flavefaciens; further work (219, 289) has demonstrated that the genus Ruminococcus contains at least two unrelated groups and that P. productus and R. flavefaciens are in different groups. Willems and Collins (289) have recommended that P. productus be retained in its present taxonomic position until a definitive revision of the classification of ruminococci is made.
Cells of P. productus are ovoid, measuring 0.6 to 0.9 by 0.8 to 2.0 μm, and occur in pairs or chains (136). Strains grow rapidly on enriched blood agar to form glistening grey colonies, with diameters of 1 mm, after incubation for 24 h. They are very strongly saccharolytic, forming acid from many carbohydrates including cellobiose; the presence of fermentable carbohydrates greatly stimulates growth. They do not show proteolytic activity (85, 136). Acetate is the only VFA produced. The PEP of the type strain, examined with using the ATB 32A kit, is very distinctive: tests for several saccharolytic enzymes (α-galactosidase, β-galactosidase, α-arabinosidase, and α-glucosidase) are strongly positive, but other tests are negative (192).
P. productus is one of the most common organisms in the human fecal flora (91, 183). Holdeman Moore et al. (136) stated that it was rarely, if ever, found in properly collected clinical specimens; Marui (176) identified 1 strain in a study of 278 clinical strains, and several surveys (28, 196, 227) have not reported it at all. However, the type strain was isolated from the blood of a patient with septicemia (87), and Wren et al. (295) identified 11 strains from abdominal and genitourinary sites (Table 6). It has been cultured from the subgingival microflora of patients with chronic periodontitis (269). The type strain is susceptible to metronidazole, vancomycin, and cefoxitin but resistant to penicillin and clindamycin by disk testing (187).
P. tetradius
Described by Ezaki et al. in 1983, from strains previously assigned to “Gaffkya anaerobia” (87), P. tetradius has been little studied because of difficulties with its identification, in particular its differentiation from P. prevotii. Analyses of 16S rRNA sequence data and peptidoglycan structure indicate that these species are closely related (67, 166, 188); however, studies of whole-cell composition by PyMS (190), which have compared type strains with human isolates with similar biochemical activity, indicate that several saccharolytic, butyrate-producing groups closely related to these species still await formal description.
The cell morphology is not distinctive; the cells are very variable in size (diameter, 0.5 to 1.8 μm) and occur in clumps and tetrads (186). The colony morphology is very similar to that of several other species of GPAC, with matte grey, low convex colonies with diameters up to 2 mm after incubation for 5 days but showing marked variation in size and shape. P. tetradius is strongly saccharolytic but weakly proteolytic; urease production is characteristic, but indole is not formed (87). Murdoch and Mitchelmore (192) suggested that production of urease and the saccharolytic enzymes α-glucosidase and β-glucuronidase appeared to be a distinctive combination of properties which might be of use to distinguish this species and P. prevotii from other recognized species of GPAC, but they cautioned that only a small number of strains had been available for examination. Recent investigations comparing a larger number of strains (187) indicate that PEPs can be used to characterize a variety of saccharolytic groups which show similarities to P. prevotii and P. tetradius (Table 3). Ezaki et al. (84) considered that the ability of strains of P. tetradius to ferment a wider range of sugars and to form ALP differentiated it most reliably from P. prevotii.
Of the five strains initially described by Ezaki et al. (87), four, including the type strain, were cultured from vaginal discharges; the fifth was from a patient with otorrhoea. Hill et al. (123) reported that P. tetradius was the most common species of GPAC in the vaginal flora of prepubertal girls. In a study by Hillier et al. (126), P. tetradius was cultured from the vaginal flora of pregnant women with normal flora (21% carriage; mean count, 103.7 CFU/ml), but the carriage rates and mean counts were significantly higher in women with BV (41% carriage; mean count, 105 CFU/ml). Marui (176) recovered 6 strains (2%) from 278 GPAC studied. In Murdoch’s survey (196), three strains were isolated, all from vaginal discharges; P. tetradius accounted for only 1.5% of 168 strains of GPAC examined. Soper et al. (246) reported the isolation of P. tetradius and P. asaccharolyticus from the endometrial biopsy specimen of a woman with acute endometritis. There appear to be no studies yet of pathogenicity.
It is now well established that P. tetradius is a constituent of the vaginal flora, but its role in disease will probably not be clarified until the classification of P. prevotii, P. tetradius, and other saccharolytic species is resolved and satisfactory methods for their identification are described. At present, it does not appear to be a common isolate. Organisms from clinical material that are biochemically similar but not identical to P. prevotii or P. tetradius should not be identified to the species level; they are best described as “prevotii/tetradius group” strains.
P. vaginalis
Li et al. (166) described P. vaginalis in 1992 from strains cultured from human vaginal discharges. Unlike P. lacrimalis and P. lactolyticus, which were proposed in the same study, many other strains have since been isolated (189, 196); it is likely that P. vaginalis is one of the more pathogenic species of GPAC.
The original species proposal (166), based on DNA-DNA hybridization data, has been supported by 16S rRNA sequence comparisons (67), which indicate that P. vaginalis is closely related to P. hydrogenalis, P. prevotii, and P. tetradius; the cell wall peptidoglycan structure is most similar to that of P. hydrogenalis (166).
The cells vary in size (diameter, 0.5 to 1.5 μm) and are usually arranged in clumps or tetrads (166, 192). The colony morphology is unremarkable; colonies are 2 to 3 mm in diameter, grey-white, and low convex after incubation for 5 days on enriched blood agar. Some strains produce an unpleasant odor similar to that formed by some strains of P. hydrogenalis (192). There is moderate saccharolytic activity, but peptones and oligopeptides are the major energy source (166, 186). Butyrate is the terminal VFA produced.
P. vaginalis is easily identified by its distinctive PEP with the ATB 32A kit (186, 192): of 27 strains described by Murdoch and Kelly (189), most or all the strains were strongly positive for ADH and for arginine, leucine, and histidine AMD; none of the strains formed urease; fermentation of mannose and production of catalase and ALP were variable. However, all six strains described by Li et al. (166) formed ALP. Two indole-forming strains which may be members of this species have been reported (192); four others have recently been isolated (187).
Of the strains originally described by Li et al. (166), four were isolated from the vaginal flora, one was from an umbilical smear and the type strain was cultured from an ovarian abscess. Murdoch et al. (196) documented 10 strains, of which 5 were from postsurgical wound infections (of the leg, inguinal region, and trunk) and 1 was from a pleural empyema; 5 strains were isolated with S. aureus. Since P. vaginalis accounted for 6% of the GPAC in this survey, the authors suggested that a large proportion of organisms identified as P. prevotii in previous surveys (28, 277, 295) might have been strains of P. vaginalis. In a recent report (189), 10 strains were recovered from superficial sites, most frequently from leg ulcers with S. aureus. P. vaginalis has been isolated in pure culture from an infected skin graft on the leg (196) and a superficial abscess of the upper arm (189). These observations indicate that P. vaginalis is probably part of the normal flora of the female genitourinary tract and possibly the skin; it merits detailed study.
Very few antibiotic susceptibility data are yet available. The 10 strains described by Murdoch and Kelly (189) were susceptible to metronidazole, vancomycin, penicillin, and clindamycin by the disk method (192), except for one isolate, which was resistant to clindamycin. Two strains examined by Bowker et al. (15) were susceptible to metronidazole, clindamycin, and all β-lactam agents tested.
Recently Proposed Species
It is clear that the taxonomy of GPAC is incomplete and that many strains isolated from human clinical material, perhaps 10% (196), cannot be placed in recognized species. Three species have recently been proposed (188); they have been assigned to the genus Peptostreptococcus as a placement of convenience pending revision of the classification.
“P. harei.”
Murdoch and Mitchelmore (192) were able to distinguish two groups of P. asaccharolyticus on phenotypic grounds; typical strains, including the type strain, were designated group A, and a smaller group of nine strains were designated group B. Analysis of whole-cell composition by PyMS (190) supported this division. Comparison of the 16S rRNA sequence of two group B strains with recognized type strains of Peptostreptococcus has led to the recent proposal of P. harei sp. nov. (188) and its assignment to Clostridium cluster XIII; it appears most closely related to P. lacrimalis. “P. harei” may correspond to P. asaccharolyticus homology group 2, described by Ezaki et al. (87) on the basis of DNA-DNA hybridization data.
The cells show considerable variation in size (diameter, 0.5 to 1.5 μm) and shape (circular, oval, or elliptical). Colonies grow very slowly on enriched blood agar; after incubation for 48 h they are barely visible, but after 5 days they form flat, translucent, nonhemolytic colonies, with diameters of 1 mm. Like P. lacrimalis, they show little or no ability to ferment carbohydrates but moderate proteolytic activity. Indole and catalase production are variable, but none of the strains studied forms urease, ALP, coagulase, or ADH. The terminal VFA formed is butyric acid. Strains can be distinguished from other species of GPAC except for P. asaccharolyticus by their PEP; strains of “P. harei” and P. asaccharolyticus are easily separated by their clearly different cell and colony morphology.
The type strain was isolated from pus from a sacral sore; antral washouts, pus from the peritoneal cavity, and abscesses of the face and breast have yielded further strains (188). Two strains have been recovered in pure culture, from a thigh abscess and a submandibular abscess. These observations indicate the possible clinical importance of “P. harei.”
Antibiotic susceptibility testing performed by the disk method (192) indicated that the nine isolates studied (190) were susceptible to metronidazole, vancomycin, penicillin, cefoxitin, chloramphenicol, and clindamycin, except for one isolate which was resistant to penicillin, clindamycin, and chloramphenicol and a second isolate which was resistant to chloramphenicol alone (187).
“P. ivorii.”
In a study of 256 clinical strains (192), 4 strains showing superficial similarity to P. anaerobius were grouped by their inability to form α-glucosidase and formation of isovaleric acid as the terminal VFA. Analysis of the whole-cell composition (190) and sequencing of 16S rRNA (188) have confirmed that they are distinct from P. anaerobius and that they should be assigned to Clostridium cluster XIII but in a distinct lineage.
Cells are cocci of variable size (diameter, 0.4 to 1.5 μm) arranged in clumps. The strains grow slowly on enriched blood agar to form yellow-white, low convex colonies, 1 to 2 mm in diameter after incubation for 5 days. The four strains studied show little or no activity in classical biochemical tests, except that two form catalase. Carbohydrates are not fermented. Saccharolytic enzymes are not detected with the ATB 32A preformed enzyme kit; the only proteolytic enzyme formed is proline AMD. Several VFAs are formed, including large quantities of butyric and isovaleric acids.
Strains of “P. ivorii” are easily identified. If GLC is available, they are readily distinguished from other GPAC, except for the small minority (5 to 10%) of P. anaerobius strains that form isovaleric acid as their terminal VFA; strains of P. anaerobius characteristically produce α-glucosidase and have very different cell and colony morphologies. Two strains of “P. ivorii,” like P. anaerobius, were susceptible to SPS. The PEP is distinctive, since few species of GPAC form proline AMD. Strains of “P. octavius” have similar PEPs but ferment several carbohydrates.
Strains of “P. ivorii” have been isolated from a leg ulcer, a preputial sac in a patient with balanitis, and an intrauterine contraceptive device, all in mixed culture. This species does not appear to be as clinically significant as “P. harei.” Antibiotic susceptibility testing by the disk method (192) indicated that all four isolates were susceptible to metronidazole, vancomycin, penicillin, cefoxitin, and chloramphenicol and that three strains were moderately resistant to clindamycin and erythromycin (187).
“P. octavius.”
Hare group VIII was described by Thomas and Hare (263) on the basis of fermentation reactions and gas production from five carbohydrates and five organic acids, combined with serological tests. Murdoch and Mitchelmore (192) identified two clinical strains as Hare group VIII by comparison of their PEPs with two reference strains, NCTC 9810 and NCTC 9820; analysis of whole-cell composition by PyMS (190) supported this identification. 16S rRNA sequencing studies (188) confirm that Hare group VIII is a distinct species; designated “P. octavius,” it appears closely related to P. lactolyticus, P. prevotii, and P. tetradius.
Cells of “P. octavius” are 0.7 to 0.9 μm in diameter and are arranged in clumps; the cell morphology is similar to strains of P. asaccharolyticus, except that Gram-stained cells of P. octavius decolorize much less. After incubation for 5 days, colonies on enriched blood agar are 1 to 2 mm in diameter, yellowish-white, glistening, circular, raised, and entire (186). “P. octavius” is moderately saccharolytic but weakly proteolytic (188). The VFA profile is very distinctive: large quantities of butyric acid, smaller quantities of isovaleric acid, and a large terminal peak of n-caproic acid are present. Identification by PEP appears to be reliable, but more strains need to be studied; examination by GLC is useful. With experience, the cell morphology supports identification.
Fifteen strains examined by Thomas and Hare (263) and both strains examined by Murdoch et al. (196) were from the nasal flora. Thomas and Hare cultured other strains from the skin (three strains) and vagina (one strain). These observations indicate that “P. octavius” may be part of the normal flora of the upper respiratory tract. Thomas and Hare did not culture any strains from infective processes, but one has recently been isolated in pure culture from an infected hip prosthesis (112). Four isolates tested by the disk method (192) were susceptible to vancomycin, cefoxitin, erythromycin, and clindamycin; single strains were only moderately susceptible to metronidazole, penicillin, and chloramphenicol.
Other Well-Defined Groups
The “trisimilis” group.
When Murdoch and Mitchelmore (192) identified a group of clinical strains as Hare group III by their PEP with the ATB 32A kit, they noted that three strains formed a subgroup characterized by formation of pyroglutamyl AMD, weak indole production, and very slow growth. Analysis of the whole-cell composition by PyMS (190) confirmed that these strains were distinct from Hare group III (now recognized as P. hydrogenalis). Preliminary analysis with the Rapid ID 32 STREP kit (187) revealed that four “trisimilis” strains all formed β-glucosidase and acid from trehalose but did not hydrolyze hippurate whereas seven strains of P. hydrogenalis all hydrolyzed hippurate but were negative for the other two tests.
The cells are 0.8 to 1.5 μm in diameter and are arranged in clumps and tetrads (186). After incubation for 5 days on enriched blood agar, the colonies are 1 to 2 mm in diameter, grey with whiter centers, entire, and circular. The four strains studied are strongly saccharolytic but weakly proteolytic; they do not form urease but are weakly catalase positive. The terminal VFA is butyric acid.
Strains have been isolated from a blood culture, a swab taken from an infected mastoid wound (with P. vaginalis), a labial cyst (with P. magnus), and pus from an infected sternotomy wound (with E. coli). Disk testing (192) revealed that these isolates were susceptible to vancomycin, cefoxitin, and clindamycin but only moderately susceptible to penicillin; one strain was moderately susceptible to metronidazole and resistant to chloramphenicol (187). Nucleic acid-based studies of this group would be valuable.
The βGAL group.
Murdoch and Mitchelmore (192) described a loose group of 11 strains characterized by their PEP, members of which consistently formed acid from several carbohydrates (glucose, fructose, and mannose and often lactose, trehalose, cellobiose, and ribose), the saccharolytic enzyme β-galactosidase (βGAL), and ALP, but not catalase, indole, or urease. Production of other saccharolytic enzymes was variable, but the proteolytic enzymes arginine and leucine AMD were consistently present. Enzyme production was not consistent if freshly poured plates were not used. All strains formed butyric acid as their terminal VFA. Analysis by PyMS (190) indicated that this group was relatively heterogeneous but related to other saccharolytic strains in the prevotii/tetradius group; however, βGAL strains were easily differentiated from all recognized species by their PEPs. 16S rRNA analysis of two representative strains (66) reveals that each represents an undescribed species in Clostridium cluster XIII (67); they are most closely related to P. lactolyticus in the prevotii/tetradius group.
The cell morphology of these strains was unremarkable, usually consisting of clumps of medium-large to small cocci. After incubation for 5 days on enriched blood agar, colonies were 1 to 2 mm in diameter and similar in appearance to other butyrate-producing cocci; they varied markedly in size and shape. On primary isolation, several strains formed satellites around colonies of staphylococci or, in one instance, P. magnus; they were then easily overlooked. On repeat isolation, they lost this characteristic.
The most interesting feature of this group is their association with significant infections. In the survey by Murdoch et al. (196), βGAL strains comprised 5% of all isolates and were cultured from abscesses of the breast and buttock, an infected pacemaker site (with P. hydrogenalis), pus from an abdominal wound, infected bed sores, and an infected skin graft site. One strain was isolated in pure culture from an inguinal abscess. Other strains have since been isolated. Further taxonomic studies of these strains would be valuable.
GENUS PEPTOCOCCUS
Peptococcus niger is now the sole remaining representative of the genus.
P. niger
P. niger was designated the type species first of the genus Peptococcus (154) and later of the family Peptococcaceae (224). It is only remotely related to other species of GPAC (67) and is rarely cultured from human pathological specimens; its main interest lies in its taxonomic significance.
Hall (111) described Micrococcus niger in 1930 from a single strain of GPAC which formed black colonies on blood agar; it was isolated from the urine of an elderly woman. When Kluyver and van Niel proposed the genus Peptococcus (154), they designated P. niger the type species. The type strain was subsequently lost. In 1975, Wilkins et al. (287) reported a similar strain, which formed black pigment, from the navel of a healthy astronaut involved in the Skylab program; they designated a neotype strain, ATCC 27731. In 1983, with the transfer of three species from the genus Peptococcus to the genus Peptostreptococcus, P. niger became the sole member of the genus (87). Its G+C ratio is 50 to 51 mol%, much higher than that of GPAC in Clostridium cluster XIII of Collins et al. (67); comparisons of 16S rRNA sequence data reveal that P. niger is not part of the clostridial subphylum.
The neotype strain forms cells 0.3 to 1.3 μm in diameter arranged singly, in pairs, and in clumps; it grows very slowly on enriched blood agar to form raised, grey colonies 1 mm in diameter after incubation for 5 days (186). Both Hall (111) and Wilkins et al. (287) observed that the black colonies noted on initial culture of fresh isolates became grey on exposure to air; after several laboratory transfers, cultures from blood agar plates no longer produced pigment. However, in meat infusion-peptone agar deeps, black colonies were formed by both fresh isolates and strains that would no longer form pigment on blood agar plates (287). The type strain forms catalase but is inactive in other classical biochemical tests; carbohydrates are not fermented (287), and proteolytic and saccharolytic enzymes have not been detected (85, 192). Its most distinctive feature is its VFA profile, which includes acetic, isobutyric, butyric, isovaleric, and terminal n-caproic acids (135, 287).
P. niger appears to be a rare constituent of the normal flora of the human umbilicus. Wilkins et al. (287) cultured it from the navels of 5 (3%) of 150 healthy individuals but not from 40 other skin sites. Recently, Hillier et al. (126) reported its presence in the vaginal flora of 20% of pregnant women; the carriage rates and counts were similar in women with normal flora and BV. It is rarely isolated from clinical specimens: Wilkins et al. (287) cultured strains from a rectal abscess, a pilonidal cyst, and a “vaginal area swab,” and Marui (176) reported finding 1 strain of P. niger among 278 GPAC examined, but it has not been isolated in other surveys (196, 227, 295). Its very slow growth and lack of distinctive biochemical features may be partly responsible.
Holdeman Moore et al. (135) stated that the strains were susceptible to penicillin, erythromycin, clindamycin, chloramphenicol, and tetracycline. Hillier and Moncla (129) reported data on four strains; one was resistant to metronidazole, and two were resistant to lincomycin.
GENUS RUMINOCOCCUS
The genus Ruminococcus is genetically heterogeneous (84, 219, 289): analyses of 16S rRNA sequence data (219, 289) assign several species to Clostridium cluster XIVa of Collins et al. (67) and others, including the type species, R. flavefaciens, to cluster IV. P. productus is closely related to the ruminococci placed in cluster XIVa (219, 289). The G+C content varies from 39 to 46 mol%.
The cell morphology can be ovoid or spheroid, with ends that can be flat, rounded, or pointed, depending on the species (53); unlike sarcinae, cells are arranged in pairs or chains and do not form spores; many strains retain Gram’s stain poorly and therefore appear gram negative. Ruminococci are strongly saccharolytic; most species require carbohydrates for growth, do not utilize peptides, and use ammonia as their nitrogen source. The ability to digest cellulose may be an important marker (53). The major VFA formed is acetic acid, which distinguishes them from coprococci. Most strains will not grow on standard laboratory media; Bryant (53) and Ezaki et al. (84) describe conditions for their isolation. Several species are found in the human gastrointestinal tract, often in large numbers (91, 183); others are present in the soil. Ruminococci have very rarely been isolated from human clinical specimens (53, 294), but laboratory procedures may be partly responsible; few diagnostic laboratories use culture methods adequate for their isolation.
GENUS COPROCOCCUS
In 1974, Holdeman and Moore (134) proposed the genus Coprococcus for novel organisms isolated in an investigation of the fecal flora. The new genus comprised species of nonsporing GPAC for which fermentable carbohydrates were required or highly stimulatory and which formed butyric acid as their terminal VFA. The latter property differentiates them from ruminococci, although Ezaki et al. (82) suggested that they might be better classified together. The genus has a G+C content of 39 to 42 mol%; the type species is C. eutactus (137). Coprococci are unlikely to grow on commercial media; Holdeman et al. (133) and Ezaki et al. (84) describe the specialized media required for their isolation. The three species, C. eutactus, C. comes, and C. catus, were originally described from the human gastrointestinal tract but have not yet been reported from clinical specimens.
GENUS SARCINA
The genus Sarcina is unique because the cell morphology reveals gram-positive cocci characteristically arranged in packets of eight which can form spores. The genus was described in 1842 and was incorporated in the family Peptococcaceae by Rogosa in 1974 (225). However, recent 16S rRNA sequence data reveal that the type strains of both species, S. ventriculi and S. maxima, fall into Clostridium cluster I of Collins et al. (67, 288). Since the genus Sarcina is older, it has nomenclatural priority, but it is likely to be included in an emended genus Clostridium (288).
Sarcinae are strict anaerobes that require fermentable carbohydrates; they will grow in a pH range between 1 and 9.8 (55). Canale-Parola (54, 55), Crowther (71), and Ezaki et al. (84) provide information for primary isolation and identification. S. ventriculi, the type species, is widespread in the soil and is common in the feces of vegetarians (71, 84). It has been found in the gastric contents and feces of patients with gastrointestinal disorders (71) and in abdominal specimens (295); if pathological conditions such as pyloric stenosis retard the flow of food to the small intestine, S. ventriculi can thrive in the stomach (55), but its pathogenic status is unclear.
GENUS ATOPOBIUM
Atopobium parvulum is an obligately anaerobic gram-positive coccus arranged in pairs or short chains; it is therefore a species of GPAC by the definition of Watt and Jack (272), but it is more closely related to the lactic acid bacteria than to the genus Peptostreptococcus (68).
P. parvulus was reclassified in the genus Streptococcus by Cato (57) because the type strain produced large quantities of lactic acid when growth was stimulated by Tween 80. The G+C content is 46 mol%, compared to 27 to 37 mol% for most species at present in the genus Peptostreptococcus (87). The genus Atopobium was proposed in 1992 by Collins and Wallbank (68) on the basis of 16S rRNA sequence data to include S. parvulus and two anaerobic species previously in the genus Lactobacillus.
A. parvulum is strongly saccharolytic but weakly proteolytic; its major metabolic product is lactic acid (113). The type strain will grow slowly on enriched blood agar to form translucent colonies smaller than 1 mm in diameter after incubation for 5 days; its PEP is distinctive (192, 193). The principal habitat is most likely to be the gastrointestinal tract; it has been found in normal fecal specimens (91), abdominal abscesses and wounds, and high vaginal swabs (295). The two strains examined by Hardie (113) from the respiratory tract were susceptible to penicillin, clindamycin, erythromycin, tetracycline, and chloramphenicol (113). The type strain is susceptible to metronidazole (192).
CONCLUDING REMARKS
GPAC account for 25 to 30% of anaerobes from human clinical material, but knowledge of their biology has lagged far behind that of other important anaerobic groups. As a comparison, until 10 years ago, three genera, Bacteroides, Clostridium, and Peptostreptococcus, accounted for more than 75% of anaerobes in four clinical surveys (28, 138, 227, 295). The taxonomy of the gram-negative anaerobic rods has been intensively investigated, and several clearly defined genera are now recognized; identification criteria for the group are well established, permitting species-level investigations of their clinical role in, e.g., complications of BV. More than 100 species of clostridia have been described and preparations for revision of the genus are well advanced (67); simple and accessible methods of identification are generally available. The situation for GPAC is quite different; much of the work on their classification has been a part of broader studies on clostridia, while standard manuals often recommend identification methods that were developed for other anaerobic groups and are physiologically inappropriate for GPAC. Many areas still require basic research.
(i) Radical revision of the classification is overdue. P. anaerobius is clearly not closely related to other species of GPAC. P. asaccharolyticus and P. prevotii are inadequately defined, and several species of clinical importance, particularly saccharolytic, butyrate-producing groups closely related to P. prevotii and P. tetradius, still await description (Table 2).
(ii) The development of selective media is an urgent priority. Potential selective agents such as oxolinic acid (213), aztreonam, and novobiocin should be evaluated. The recent development of PMM (265) may be a significant advance.
(iii) Identification schemes for differentiating P. magnus from P. micros usually require GLC and therefore cannot be used in most routine laboratories. Future identification schemes for butyrate-producing strains must place less weight on the formation of indole (190, 192). Carbohydrate fermentation reactions may prove valuable for prevotii/tetradius group strains (genus 1, Table 2) but probably not for other groups in Clostridium cluster XIII. The pioneering study by Ezaki and Yabuuchi (85) examined several peptidase tests which could be useful for the identification of asaccharolytic groups. Identification by PEP relies on predictable enzyme expression; more data on the influence on enzyme production of the agar base or source of blood would be welcome, since either could affect the PEP significantly.
(iv) Little is known about the metabolism of individual species. Comparison of their proteolytic properties (Table 3) reveals that most butyrate-producing GPAC fall into two groups, a group of species which forms arginine AMD and often leucine, tyrosine, and histidine AMD but never proline AMD; and a second group, which forms longer-chain VFAs and proline AMD but never arginine, leucine, tyrosine, or histidine AMD. These groups appear to have divergent metabolic pathways; the implications could have considerable significance in the ecology and energy requirements of individual species. Comparative studies could lead to the development of better methods of identification.
(v) The normal flora, particularly of the skin, is little known, and the available data are often contradictory.
(vi) Further data are required on the clinical importance and pathogenicity of individual species, particularly P. vaginalis, P. hydrogenalis, and “P. harei.” Several putative virulence factors have been described for P. magnus; these studies should be extended to other species.
(vii) Little is known about the mechanisms of antibiotic resistance, even for β-lactam agents. Potential metronidazole resistance is clinically relevant but poorly defined.
All studies should aim to present data for individual species rather than for GPAC as a group.
GPAC are being linked to an increasing range of clinical infections, particularly of musculoskeletal and soft tissue origin, where they are difficult to culture and easily overlooked. We therefore recommend that (i) laboratories provide effective anaerobe transport systems; (ii) appropriate specimens be incubated under anaerobic conditions for prolonged periods, preferably for 5 days, before being discarded; (iii) when GPAC are isolated in pure culture or from important specimens such as deep organ abscesses, they be identified to the species level; (iv) publication of case reports be encouraged, particularly for species other than P. magnus.
Much remains to be discovered about the basic microbiology of GPAC.
Identification Scheme for GPAC
The following scheme should enable reasonably experienced workers to identify at least 80% of clinical strains within 48 h of isolation.
Primary isolation.
Enriched blood agar should be used for culturing GPAC. It is useful to test for metronidazole susceptibility by placing a 5-μg disk on the primary inoculum. The disk must be placed on the edge of the plate and well away from secondary streaking; otherwise, growth of extremely susceptible organisms such as fusobacteria may be suppressed completely.
Primary plates should be examined after incubation for 48 h and 5 days. By using a combination of cell and colony morphology and smell, it is possible to make a presumptive identification from primary plates of many strains of P. anaerobius, P. micros, and P. asaccharolyticus, comprising 25 to 50% of clinical isolates. However, P. magnus does not appear to have features that permit easy characterization.
Subculture.
Further identification requires subculture onto enriched blood agar, which should be as freshly prepared as possible. A quarter plate must also be incubated in an aerobic atmosphere containing 6% CO2 to detect capnophilic organisms. If GLC is available for the detection of VFAs, a broth culture can be inoculated.
After 48 h, PEP kits can be inoculated and read (Table 3); a purity plate for the PEP kit must also be inoculated. The best results with PEP kits are achieved with fresh plates and the correct inoculum (MacFarland no. 4). It is advisable to record colony and cell morphology again, to check that they are consistent with identification by PEP. If the PEP does not give a clear identification, the broth culture can be examined by GLC to detect VFA production. GLC of unidentified strains will usually place them in the butyrate group, which contains many undescribed species.
It is advisable to check both aerobic and anaerobic plates again after 5 days (and to record observations), particularly if the PEP does not give a clear identification; it is all too frequent that the culture is mixed! Colony morphology and smell are most distinctive after 5 days and help to distinguish “P. harei” from P. asaccharolyticus. However, by 5 days, many strains (such as P. magnus, P. tetradius, and βGAL group strains) will show marked heterogeneity in their colony morphology.
A significant proportion of clinical isolates, perhaps 10%, cannot yet be identified to phylogenetically valid species.
Modifications.
For slow-growing strains (colony diameter, <1 mm after incubation for 48 h), harvesting an adequate inoculum for the ATB 32A kit will require inoculation of two or three plates.
When presumptive strains of P. anaerobius are subcultured, an SPS disk should be placed on the primary streaking; the plate can be inspected after 18 h. Organisms susceptible to SPS with appropriate cell and colony morphology do not need further characterization. Some strains of P. ivorii are susceptible to SPS, but they do not form α-glucosidase or the VFA isocaproic acid.
If the microscopic appearance from the primary plate suggests a very decolorized strain of GPAC, for instance P. asaccharolyticus or P. indolicus, a disk containing 5 μg of vancomycin can be used to check the Gram reaction.
ACKNOWLEDGMENTS
Many people, notably Ivor Mitchelmore, John Magee, Jeremy Hardie, Mike Wren, and D. C. E. Speller, have given much appreciated advice and support. I am grateful to William Wade, Ian Phillips, A. P. MacGowan, and R. A. Howe for reading sections of early manuscripts. John Magee and M. D. Collins kindly prepared Fig. 1 and 2, respectively. Ray Feeney gave me essential and uncomplaining support on the computer. I thank D. S. Reeves and S. Tabaqchali and the staff of the Microbiology Departments of Southmead Hospital, Bristol, and St. Bartholomew’s Hospital, London, for their support and patience. Glen Tillotson initiated me into the study of GPAC.
Grants from the Sir Jules Thorn Trust and Showering Fund, Southmead Hospital, Bristol, were essential for my personal support.
REFERENCES
- 1.Alcoforado G A, Rams T E, Feik D, Slots J. Microbial aspects of failing osseointegrated dental implants in humans. J Parodontol. 1991;10:11–18. [PubMed] [Google Scholar]
- 2.Aldridge K E, Sanders C V, Lewis A C, Marier R L. Susceptibility of anaerobic bacteria to β-lactam antibiotics and β-lactamase production. J Med Microbiol. 1983;16:75–82. doi: 10.1099/00222615-16-1-75. [DOI] [PubMed] [Google Scholar]
- 3.Altemeier W A. The anaerobic streptococci in tubo-ovarian abscess. Am J Obstet Gynecol. 1940;39:1038–1042. [Google Scholar]
- 4.Appelbaum P C, Kaufmann C S, Depenbusch J W. Accuracy and reproducibility of a four-hour method for anaerobe identification. J Clin Microbiol. 1985;21:894–898. doi: 10.1128/jcm.21.6.894-898.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babcock J B. Tyrosine degradation in presumptive identification of Peptostreptococcus anaerobius. J Clin Microbiol. 1979;9:358–361. doi: 10.1128/jcm.9.3.358-361.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron, E. J., M. L. Vaisanen, M. McTeague, C. A. Strong, and S. M. Finegold. 1993. Comparison of the Accu-Cul-Shure System and a swab placed in a B-D Port-a-Cul tube for specimen culture and transport. Clin. Infect. Dis. 16(Suppl. 4):S325–S327. [DOI] [PubMed]
- 7.Bartlett J G. Anaerobic cocci. In: Mandell G L, Douglas R G, Bennett J E, editors. The principles and practice of infectious diseases. 3rd ed. New York, N.Y: Churchill Livingstone, Inc.; 1990. pp. 1867–1869. [Google Scholar]
- 8.Bartlett, J. G. 1993. Anaerobic bacterial infections of the lung and pleural space. Clin. Infect. Dis. 16(Suppl. 4):S248–S255. [DOI] [PubMed]
- 9.Bartlett J G, Gorbach S L, Finegold S M. The bacteriology of aspiration pneumonia. Am J Med. 1974;56:202–207. doi: 10.1016/0002-9343(74)90598-1. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett J G, Gorbach S L, Thadepalli H, Finegold S M. Bacteriology of empyema. Lancet. 1974;i:338–340. doi: 10.1016/s0140-6736(74)93079-7. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett J G, Onderdonk A B, Drude E, Goldstein C, Anderka M, Alpert S, McCormack W M. Quantitative bacteriology of the vaginal flora. J Infect Dis. 1977;136:271–277. doi: 10.1093/infdis/136.2.271. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner J C, Falkner W A, Beckerman T. Experimentally induced infection by oral anaerobic microorganisms in a mouse model. Oral Microbiol Immun. 1992;7:253–256. doi: 10.1111/j.1399-302x.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 13.Bourgault A M, Rosenblatt J E. First isolation of Peptococcus indolicusfrom a human clinical specimen. J Clin Microbiol. 1979;9:549–550. doi: 10.1128/jcm.9.4.549-550.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourgault A-M, Rosenblatt J E, Fitzgerald R H. Peptococcus magnus: a significant human pathogen. Ann Intern Med. 1980;93:244–248. doi: 10.7326/0003-4819-93-2-244. [DOI] [PubMed] [Google Scholar]
- 15.Bowker K E, Wootton M, Holt H A, Reeves D S, MacGowan A P. The in-vitro activity of trovafloxacin and nine other antimicrobials against 413 anaerobic bacteria. J Antimicrob Chemother. 1996;38:271–281. doi: 10.1093/jac/38.2.271. [DOI] [PubMed] [Google Scholar]
- 16.British Society for Antimicrobial Chemotherapy. Guide to sensitivity testing. London, United Kingdom: Academic Press; 1991. [Google Scholar]
- 17.Brook I. Bacteriology of intracranial abscess in children. J Neurosurg. 1981;54:484–488. doi: 10.3171/jns.1981.54.4.0484. [DOI] [PubMed] [Google Scholar]
- 18.Brook I. Encapsulated anaerobic bacteria in synergistic infections. Microbiol Rev. 1986;50:452–457. doi: 10.1128/mr.50.4.452-457.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brook I. Bacteraemia and seeding of capsulate Bacteroidesspp. and anaerobic cocci. J Med Microbiol. 1987;23:61–67. doi: 10.1099/00222615-23-1-61. [DOI] [PubMed] [Google Scholar]
- 20.Brook I. Comparison of two transport systems for recovery of aerobic and anaerobic bacteria from abscesses. J Clin Microbiol. 1987;26:592–594. doi: 10.1128/jcm.25.10.2020-2022.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brook I. Microbiological studies of tracheostomy site wounds. Eur J Respir Dis. 1987;71:380–383. [PubMed] [Google Scholar]
- 22.Brook I. Microbiology of human and animal bite wounds in children. Pediatr Infect Dis J. 1987;6:29–32. doi: 10.1097/00006454-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Brook I. Microbiology of retropharyngeal abscesses in children. Am J Dis Child. 1987;141:202–204. doi: 10.1001/archpedi.1987.04460020092034. [DOI] [PubMed] [Google Scholar]
- 24.Brook I. Aerobic and anaerobic bacteriology of purulent nasopharyngitis in children. J Clin Microbiol. 1988;26:592–594. doi: 10.1128/jcm.26.3.592-594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brook I. Enhancement of growth of aerobic, anaerobic, and facultative bacteria in mixed infections with anaerobic and facultative Gram-positive cocci. J Surg Res. 1988;45:222–227. doi: 10.1016/0022-4804(88)90068-6. [DOI] [PubMed] [Google Scholar]
- 26.Brook I. Microbiology of non-puerperal breast abscesses. J Infect Dis. 1988;157:377–379. doi: 10.1093/infdis/157.2.377. [DOI] [PubMed] [Google Scholar]
- 27.Brook I. Presence of anaerobic bacteria in conjunctivitis associated with wearing contact lenses. Ann Ophthalmol. 1988;20:397–399. [PubMed] [Google Scholar]
- 28.Brook I. Recovery of anaerobic bacteria from clinical specimens in 12 years at two military hospitals J. Clin Microbiol. 1988;26:1181–1188. doi: 10.1128/jcm.26.6.1181-1188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brook I. Aerobic and anaerobic microbiology of Bartholin’s abscess. Surg Gynecol Obstet. 1989;169:32–34. [PubMed] [Google Scholar]
- 30.Brook I. Anaerobic bacteria in suppurative genitourinary infections. J Urol. 1989;141:889–893. doi: 10.1016/s0022-5347(17)41040-8. [DOI] [PubMed] [Google Scholar]
- 31.Brook I. Anaerobic bacterial bacteremia: 12-year experience in two military hospitals. J Infect Dis. 1989;160:1071–1075. doi: 10.1093/infdis/160.6.1071. [DOI] [PubMed] [Google Scholar]
- 32.Brook I. Microbiology of post-thoracotomy sternal wound infection. J Clin Microbiol. 1989;27:806–807. doi: 10.1128/jcm.27.5.806-807.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brook I. Peptostreptococcal infection in children. Scand J Infect Dis. 1994;26:503–510. doi: 10.3109/00365549409011807. [DOI] [PubMed] [Google Scholar]
- 34.Brook I. The role of anaerobic bacteria in perinephric and renal abscesses in children. Pediatrics. 1994;93:261–264. [PubMed] [Google Scholar]
- 35.Brook I. Anaerobic cocci. In: Mandell G L, Bennett J E, Dolin R, editors. The principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone, Inc.; 1995. pp. 2204–2206. [Google Scholar]
- 36.Brook I. Pyomyositis in children caused by anaerobic bacteria. J Pediatr Surg. 1996;31:394–396. doi: 10.1016/s0022-3468(96)90745-9. [DOI] [PubMed] [Google Scholar]
- 37.Brook I, Frazier E H. Aerobic and anaerobic bacteriology of wounds and cutaneous abscesses. Arch Surg. 1990;125:1445–1451. doi: 10.1001/archsurg.1990.01410230039007. [DOI] [PubMed] [Google Scholar]
- 38.Brook I, Frazier E H. Anaerobic osteomyelitis and arthritis in a military hospital. Am J Med. 1993;94:221–28. doi: 10.1016/0002-9343(93)90115-6. [DOI] [PubMed] [Google Scholar]
- 39.Brook I, Frazier E H. Aerobic and anaerobic microbiology of empyema. Chest. 1993;103:1502–1507. doi: 10.1378/chest.103.5.1502. [DOI] [PubMed] [Google Scholar]
- 40.Brook I, Frazier E H. Role of anaerobic bacteria in liver abscesses in children. Pediatr Infect Dis J. 1993;12:743–747. doi: 10.1097/00006454-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Brook I, Frazier E H. Clinical and microbiological features of necrotizing fasciitis. J Clin Microbiol. 1995;33:2382–2387. doi: 10.1128/jcm.33.9.2382-2387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brook I, Frazier E H. Clinical features and aerobic and anaerobic microbiological characteristics of cellulitis. Arch Surg. 1995;130:786–792. doi: 10.1001/archsurg.1995.01430070108024. [DOI] [PubMed] [Google Scholar]
- 43.Brook I, Frazier E H. Aerobic and anaerobic microbiology of infected hemorrhoids. Am J Gastroenterol. 1996;91:333–335. [PubMed] [Google Scholar]
- 44.Brook I, Frazier E H. Microbiology of mediastinitis. Arch Intern Med. 1996;156:333–336. [PubMed] [Google Scholar]
- 45.Brook I, Frazier E H, Thompson D H. Aerobic and anaerobic microbiology of external otitis. Clin Infect Dis. 1992;15:955–958. doi: 10.1093/clind/15.6.955. [DOI] [PubMed] [Google Scholar]
- 46.Brook I, Hirokawa R. Microbiology of wound infection after head and neck cancer surgery. Ann Otol Rhinol Laryngol. 1989;98:323–325. doi: 10.1177/000348948909800501. [DOI] [PubMed] [Google Scholar]
- 47.Brook I, Walker R I. Pathogenicity of anaerobic gram-positive cocci. Infect Immun. 1984;45:320–324. doi: 10.1128/iai.45.2.320-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brook I, Walker R I. The role of encapsulation in the pathogenesis of anaerobic gram-positive cocci. Can J Microbiol. 1985;31:176–180. doi: 10.1139/m85-033. [DOI] [PubMed] [Google Scholar]
- 49.Brook I, Yocum P. Quantitative bacterial cultures and β-lactamase activity in chronic suppurative otitis media. Ann Otol Rhinol Laryngol. 1989;98:293–297. doi: 10.1177/000348948909800411. [DOI] [PubMed] [Google Scholar]
- 50.Brook I, Yocum P, Frazier E H. Bacteriology and β-lactamase activity in acute and chronic maxillary sinusitis. Arch Otolaryngol Head Neck Surg. 1996;122:418–422. doi: 10.1001/archotol.1996.01890160058011. [DOI] [PubMed] [Google Scholar]
- 51.Brown M A, Greene J N, Sandin R L, Vincent A L. Case report: anaerobic meningitis caused by Peptostreptococcus magnusafter head and neck surgery. Am J Med Sci. 1994;308:184–185. doi: 10.1097/00000441-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Brown T K. The incidence of puerperal infection due to anaerobic streptococci. Am J Obstet Gynecol. 1930;20:300–309. [Google Scholar]
- 53.Bryant M P. Genus Ruminococcus. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins, Co.; 1986. pp. 1093–1097. [Google Scholar]
- 54.Canale-Parola E. Biology of the sugar-fermenting sarcinae. Bacteriol Rev. 1970;34:82–97. doi: 10.1128/br.34.1.82-97.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canale-Parola E. Genus Sarcina. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1100–1103. [Google Scholar]
- 56.Carlsson J, Larsen J T, Edlund M-B. Peptostreptococcus microshas a uniquely high capacity to form hydrogen sulfide from glutathione. Oral Microbiol Immunol. 1993;8:42–45. doi: 10.1111/j.1399-302x.1993.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 57.Cato E P. Transfer of Peptostreptococcus parvulus (Weinberg, Nativelle, and Prevot 1937) Smith 1957 to the Genus Streptococcus: Streptococcus parvulus(Weinberg, Nativelle, and Prevot 1937) comb. nov., nom. rev., emend. Int J Syst Bacteriol. 1983;33:82–84. [Google Scholar]
- 58.Cato E P, Johnson J L, Hash D E, Holdeman L V. Synonomy of Peptococcus glycinophilus (Cardon and Barker 1946) Douglas 1957 with Peptostreptococcus micros (Prevot 1933) Smith 1957 and electrophoretic differentiation of Peptostreptococcus micros from Peptococcus magnus(Prevot 1933) Holdeman and Moore 1972. Int J Syst Bacteriol. 1983;33:207–210. [Google Scholar]
- 59.Celig D M, Schreckenberger P C. Clinical evaluation of the RapID-ANA II panel for identification of anaerobic bacteria. J Clin Microbiol. 1991;29:457–462. doi: 10.1128/jcm.29.3.457-462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Citron, D. M., E. J. C. Goldstein, M. A. Kenner, L. B. Burnham, and C. B. Inderlied. 1995. Activity of ampicillin/sulbactam, ticarcillin/clavulanate, clarithromycin, and eleven other antimicrobial agents against anaerobic bacteria isolated from infections in children. Clin. Infect. Dis. 20(Suppl. 2):S356–S360. [DOI] [PubMed]
- 61.Citron D M, Ostovari M I, Karlsson A, Goldstein E J. Evaluation of the E test for susceptibility testing of anaerobic bacteria. J Clin Microbiol. 1991;29:2197–2203. doi: 10.1128/jcm.29.10.2197-2203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Civen, R., H. Jousimies-Somer, M. Marina, L. Borenstein, H. Shah, and S. M. Finegold. 1995. A retrospective review of cases of anaerobic empyema and update of bacteriology. Clin. Infect. Dis. 20(Suppl. 2):S224–S229. [DOI] [PubMed]
- 63.Civen, R., M.-L. Vaisanen, and S. M. Finegold. 1993. Peritonsillar abscess, retropharyngeal abscess, mediastinitis, and nonclostridial anaerobic myonecrosis: a case report. Clin. Infect. Dis. 16(Suppl. 4):S299–S303. [DOI] [PubMed]
- 64.Cofsky R D, Seligman S J. Peptococcus magnusendocarditis. South Med J. 1985;78:361–362. doi: 10.1097/00007611-198503000-00035. [DOI] [PubMed] [Google Scholar]
- 65.Colebrook L, Hare R. The anaerobic streptococci associated with puerperal fever. J Obstet Gynaecol. 1933;40:609–630. [Google Scholar]
- 66.Collins, M. D. 1997. Personal communication.
- 67.Collins M D, Lawson P A, Willems A, Cordoba J J, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow J A E. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 68.Collins M D, Wallbanks S. Comparative sequence analyses of the 16S rRNA genes of Lactobacillus minutus, Lactobacillus rimae and Streptococcus parvulus: proposal for the creation of a new genus, Atopobium. FEMS Microbiol Lett. 1992;95:235–240. doi: 10.1016/0378-1097(92)90435-q. [DOI] [PubMed] [Google Scholar]
- 69.Collins M L Z, Falkler W A, Hall E R, Graham M B. Serological studies of peptostreptococci using an indirect fluorescent antibody test. J Dent Res. 1989;68:1508–1512. doi: 10.1177/00220345890680110801. [DOI] [PubMed] [Google Scholar]
- 70.Croco, J. L., M. E. Erwin, J. M. Jennings, L. R. Putnam, and R. N. Jones. 1995. Evaluation of the Etest for determinations of antimicrobial spectrum and potency against anaerobes associated with bacterial vaginosis and peritonitis. Clin. Infect. Dis. 20(Suppl. 2):S339–S341. [DOI] [PubMed]
- 71.Crowther J S. Sarcina ventriculiin human faeces. J Med Microbiol. 1971;4:343–349. doi: 10.1099/00222615-4-3-343. [DOI] [PubMed] [Google Scholar]
- 72.Davies U M, Leak A M, Dave J. Infection of a prosthetic knee joint with Peptostreptococcus magnus. Ann Rheumat Dis. 1988;47:866–868. doi: 10.1136/ard.47.10.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Château M, Björck L. Protein PAB, a mosaic albumin-binding bacterial protein representing the first contemporary example of module shuffling. J Biol Chem. 1994;269:12147–12151. [PubMed] [Google Scholar]
- 74.de Château M, Nilson B H, Erntell M, Myhre E, Magnusson C G, Akerstrom B, Björck L. On the interaction between Protein L and immunoglobulins of various mammalian species. Scand J Immunol. 1993;37:399–405. doi: 10.1111/j.1365-3083.1993.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 75.DiZerega G S, Yonekura M L, Keegan K, Roy S, Nakamura R, Ledger W. Bacteremia in post-cesarean section endomyometritis: differential response to therapy. Obstet Gynecol. 1980;55:587–590. [PubMed] [Google Scholar]
- 76.Dzink J L, Socransky S S, Haffajee A D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988;15:316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 77.Edmiston C E, Walker A P, Krepel C J, Gohr C. The non-puerperal breast infection: aerobic and anaerobic microbial recovery from acute and chronic disease. J Infect Dis. 1990;162:695–699. doi: 10.1093/infdis/162.3.695. [DOI] [PubMed] [Google Scholar]
- 78.Edson R S, Rosenblatt J E, Lee D T, McVey E A. Recent experience with antimicrobial susceptibility of anaerobic bacteria; increasing resistance to penicillin. Mayo Clin Proc. 1982;57:737–741. [PubMed] [Google Scholar]
- 79.Eschenbach, D. A. 1993. Bacterial vaginosis and anaerobes in obstetric-gynecologic infections. Clin. Infect. Dis. 16(Suppl. 4):S282–S287. [DOI] [PubMed]
- 80.Eschenbach D A, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes K K. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158:819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 81.Evans C A, Mattern K L, Hallam S L. Isolation and identification of Peptococcus saccharolyticusfrom human skin. J Clin Microbiol. 1978;7:261–264. doi: 10.1128/jcm.7.3.261-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ezaki T, Li N, Hashimoto Y, Miura H, Yamamoto H. 16S ribosomal DNA sequences of anaerobic cocci and proposal of Ruminococcus hansenii comb. nov. and Ruminococcus productuscomb. nov. Int J Syst Bacteriol. 1994;44:130–136. doi: 10.1099/00207713-44-1-130. [DOI] [PubMed] [Google Scholar]
- 83.Ezaki T, Liu S-L, Hashimoto Y, Yabuuchi E. Peptostreptococcus hydrogenalissp. nov. from human fecal and vaginal flora. Int J Syst Bacteriol. 1990;40:305–306. doi: 10.1099/00207713-40-3-305. [DOI] [PubMed] [Google Scholar]
- 84.Ezaki T, Oyaizu H, Yabuuchi E. The anaerobic gram-positive cocci. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. Berlin, Germany: Springer-Verlag KG; 1992. pp. 1879–1892. [Google Scholar]
- 85.Ezaki T, Yabuuchi E. Oligopeptidase activity of gram-positive anaerobic cocci used for rapid identification. J Gen Appl Microbiol. 1985;31:255–266. [Google Scholar]
- 86.Ezaki T, Yabuuchi E. Transfer of Peptococcus heliotrinreducens to the genus Peptostreptococcus: Peptostreptococcus heliotrinreducensLanigan 1983 comb. nov. Int J Syst Bacteriol. 1986;36:107–108. [Google Scholar]
- 87.Ezaki T, Yamamoto N, Ninomiya K, Suzuki S, Yabuuchi E. Transfer of Peptococcus indolicus, Peptococcus asaccharolyticus, Peptococcus prevotii and Peptococcus magnus to the genus Peptostreptococcus and proposal of Peptostreptococcus tetradiussp. nov. Int J Syst Bacteriol. 1983;33:683–698. [Google Scholar]
- 88.Facklam R, Elliott J A. Identification, classification and clinical relevance of catalase-negative, gram-positive cocci, excluding the streptococci and enterococci. Clin Microbiol Rev. 1995;8:479–495. doi: 10.1128/cmr.8.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Felner J M, Dowell V R. Anaerobic bacterial endocarditis. N Engl J Med. 1970;22:1188–1192. doi: 10.1056/NEJM197011262832203. [DOI] [PubMed] [Google Scholar]
- 90.Finegold S M. Anaerobic infections in humans: an overview. Anaerobe. 1995;1:3–9. doi: 10.1016/s1075-9964(95)80340-8. [DOI] [PubMed] [Google Scholar]
- 91.Finegold S M, Attebery H R, Sutter V L. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am J Clin Nutr. 1974;27:1456–1469. doi: 10.1093/ajcn/27.12.1456. [DOI] [PubMed] [Google Scholar]
- 92.Finegold S M, George W L. Anaerobic infections in humans. New York, N.Y: Academic Press, Inc.; 1989. [Google Scholar]
- 93.Finegold S M the NCCLS working group on anaerobic susceptibility testing. Susceptibility testing of anaerobic bacteria. J Clin Microbiol. 1988;26:1253–1256. doi: 10.1128/jcm.26.7.1253-1256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fitzgerald R H, Rosenblatt J E, Tenney J H, Bourgault A-M. Anaerobic septic arthritis. Clin Orthoped. 1982;164:141–148. [PubMed] [Google Scholar]
- 95.Foubert E L, Douglas H C. Studies on the anaerobic micrococci. I. Taxonomic considerations. J Bacteriol. 1948;56:25–34. doi: 10.1128/JB.56.1.25-34.1948. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Rodriguez J A, Garcia-Sanchez J E, Munoz-Bellido J L. Antimicrobial resistance in anaerobic bacteria: current situation. Anaerobe. 1995;1:69–80. doi: 10.1006/anae.1995.1001. [DOI] [PubMed] [Google Scholar]
- 97.Germain M, Krohn M A, Hillier S L, Eschenbach D A. Genital flora in pregnancy and its association with intrauterine growth retardation. J Clin Microbiol. 1994;32:2162–2168. doi: 10.1128/jcm.32.9.2162-2168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giamarellou H, Soulis M, Antoniadou A, Gogas J. Periareolar nonpuerperal breast infection: treatment of 38 cases. Clin Infect Dis. 1994;18:73–76. doi: 10.1093/clinids/18.1.73. [DOI] [PubMed] [Google Scholar]
- 99.Goldstein E J C. The importance of susceptibility testing of anaerobes: a clinician’s perspective. Antimicrob Newsl. 1992;8:49–52. [Google Scholar]
- 100.Goldstein, E. J. C. 1993. Patterns of susceptibility to fluoroquinolones among anaerobic bacterial isolates in the United States. Clin. Infect. Dis. 16(Suppl. 4):S377–S381. [DOI] [PubMed]
- 101.Goldstein E J C, Citron D M, Cherubin C E, Hillier S L. Comparative susceptibility of the Bacteroides fragilisgroup species and other anaerobic bacteria to meropenem, imipenem, piperacillin, cefoxitin, ampicillin/sulbactam, clindamycin and metronidazole. J Antimicrob Chemother. 1993;31:363–372. doi: 10.1093/jac/31.3.363. [DOI] [PubMed] [Google Scholar]
- 102.Goodacre R, Hiom S J, Cheeseman S L, Murdoch D A, Weightman A J, Wade W G. Identification and discrimination of oral asaccharolytic Eubacteriumspecies by pyrolysis mass spectrometry and artificial neural networks. Curr Microbiol. 1996;32:77–84. doi: 10.1007/s002849900014. [DOI] [PubMed] [Google Scholar]
- 103.Grant D A, Flynn M J, Slots J. Periodontal microbiota of mobile and non-mobile teeth. J Periodontol. 1995;66:386–390. doi: 10.1902/jop.1995.66.5.386. [DOI] [PubMed] [Google Scholar]
- 104.Graves M H, Morello J A, Kocka F E. Sodium polyanethol sulfonate sensitivity of anaerobic cocci. Appl Microbiol. 1974;27:1131–1133. doi: 10.1128/am.27.6.1131-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Greenwood D, Palfreyman J. Comparative activity of LY146032 against anaerobic cocci. Eur J Clin Microbiol Infect Dis. 1987;6:682–684. doi: 10.1007/BF02013071. [DOI] [PubMed] [Google Scholar]
- 106.Gulletta E, Amato G, Nani E, Covelli I. Comparison of two systems for identification of anaerobic bacteria. Eur J Clin Microbiol Infect Dis. 1985;4:282–285. doi: 10.1007/BF02013653. [DOI] [PubMed] [Google Scholar]
- 107.Gulletta E, Amato G, Nani E, Covelli I. Bacteremia caused by Peptococcus prevotiiin an immunocompromised host. Microbiologica. 1986;9:105–108. [PubMed] [Google Scholar]
- 108.Gunaratnam M, Smith G L F, Socransky S S, Smith C M, Haffajee A D. Enumeration of subgingival species on primary isolation plates using colony lifts. Oral Microbiol Immunol. 1992;7:14–18. doi: 10.1111/j.1399-302x.1992.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 109.Haffajee A D, Socransky S S, Dzink J L, Taubman M A, Ebersole J L. Clinical, microbiological and immunological features of subjects with refractory periodontal diseases. J Clin Periodontol. 1988;15:390–398. doi: 10.1111/j.1600-051x.1988.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 110.Haffajee A D, Socransky S S, Smith C, Dibart S. Microbial risk indicators for periodontal attachment loss. J Periodont Res. 1991;26:293–296. doi: 10.1111/j.1600-0765.1991.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 111.Hall I C. Micrococcus niger, a new pigment forming anaerobic coccus recovered from urine in a case of general arteriosclerosis. J Bacteriol. 1930;20:407–415. doi: 10.1128/jb.20.6.407-415.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hall, V. 1996. Personal communication.
- 113.Hardie J M. Anaerobic streptococci. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1066–1068. [Google Scholar]
- 114.Hare R. The anaerobic cocci. In: Waterson A P, editor. Recent advances in medical microbiology. London, United Kingdom: Churchill; 1967. pp. 285–317. [Google Scholar]
- 115.Hare R, Polunin I. Anaerobic cocci in the vagina of native women in British North Borneo. J Obstet Gynaecol Br Emp. 1960;67:985–989. doi: 10.1111/j.1471-0528.1960.tb09256.x. [DOI] [PubMed] [Google Scholar]
- 116.Hare R, Wildy P, Billett F S, Twort D N. The anaerobic cocci: gas formation, fermentation reactions, sensitivity to antibiotics and sulphonamides. Classification. J Hyg. 1952;50:295–319. doi: 10.1017/s0022172400019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harpold D J, Wasilauskas B L. Rapid identification of obligately anaerobic gram-positive cocci using high-pressure liquid chromatography. J Clin Microbiol. 1987;25:996–1001. doi: 10.1128/jcm.25.6.996-1001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hauth J C, Goldenberg R L, Andrews W W, DuBard M B, Copper R L. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med. 1995;333:1732–1736. doi: 10.1056/NEJM199512283332603. [DOI] [PubMed] [Google Scholar]
- 119.Heginbothom M, Fitzgerald T C, Wade W G. Comparison of solid media for cultivation of anaerobes. J Clin Pathol. 1990;43:253–256. doi: 10.1136/jcp.43.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heineman H S, Braude A I. Anaerobic infection of the brain. Observations on eighteen consecutive cases of brain abscess. Am J Med. 1963;35:682–697. doi: 10.1016/0002-9343(63)90139-6. [DOI] [PubMed] [Google Scholar]
- 121.Henson J W, Ferraro M J. Case records of the Massachusetts General Hospital. Case 43-1993. A 71-year-old woman with confusion, hemianopia and an occipital mass. N Engl J Med. 1993;329:1335–1341. doi: 10.1056/NEJM199310283291809. [DOI] [PubMed] [Google Scholar]
- 122.Hill G B, Livengood C H., III Bacterial vaginosis-associated microflora and effects of topical intravaginal clindamycin. Am J Obstet Gynecol. 1994;171:1198–1204. doi: 10.1016/0002-9378(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 123.Hill, G. B., K. K. St. Claire, and L. T. Gutman. 1995. Anaerobes predominate among the vaginal microflora of prepubertal girls. Clin. Infect. Dis. 20(Suppl. 2):S269–S270. [DOI] [PubMed]
- 124.Hillier, S. L., M. A. Krohn, E. Cassen, T. R. Easterling, L. K. Rabe, and D. A. Eschenbach. 1995. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clin. Infect. Dis. 20(Suppl. 2):S276–S282. [DOI] [PubMed]
- 125.Hillier S L, Krohn M A, Kiviat N B, Watts D H, Eschenbach D A. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165:955–961. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 126.Hillier, S. L., M. A. Krohn, L. K. Rabe, S. J. Klebanoff, and D. A. Eschenbach. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin. Infect. Dis. 16(Suppl. 4):S273–S281. [DOI] [PubMed]
- 127.Hillier S L, Martius J, Holmes K K, Eschenbach D A. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 128.Hillier S L, Moncla B J. Anaerobic gram-positive cocci. In: Balows A, Hausler W J, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 533–537. [Google Scholar]
- 129.Hillier S L, Moncla B J. Peptostreptococcus, Proprionibacterium, Eubacterium, and other nonsporeforming anaerobic gram-positive bacteria. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 587–602. [Google Scholar]
- 130.Hillier S L, Nugent R P, Eschenbach D A, Krohn M A, Gibbs R S, Martin D H, Klebanoff M A. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 131.Hillier, S. L., D. H. Watts, M. F. Lee, and D. A. Eschenbach. 1990. Etiology and treatment of post-Cesarean-section endometritis after cephalosporin prophylaxis. J. Reprod. Med. 35(Suppl.):S322–S328. [PubMed]
- 132.Hoi-Sorenson G. Micrococcus indolicus. Some biochemical properties, and the demonstration of six antigenically different types. Acta Vet Scand. 1973;14:301–326. doi: 10.1186/BF03547448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holdeman L V, Cato E P, Moore W E C. Anaerobe laboratory manual. 4th ed. Blacksburg: Anaerobe Laboratory, Virginia Polytechnic Institute and State University; 1977. [Google Scholar]
- 134.Holdeman L V, Moore W E C. New genus, Coprococcus, twelve new species, and emended descriptions of four previously described species of bacteria from human feces. Int J Syst Bacteriol. 1974;24:260–277. [Google Scholar]
- 135.Holdeman Moore L V, Johnson J L, Moore W E C. Genus Peptococcus. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1082–1083. [Google Scholar]
- 136.Holdeman Moore L V, Johnson J L, Moore W E C. Genus Peptostreptococcus. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1083–1092. [Google Scholar]
- 137.Holdeman Moore L V, Moore W E C. Genus Coprococcus. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1097–1099. [Google Scholar]
- 138.Holland J W, Hill E O, Altemeier W A. Numbers and types of anaerobic bacteria isolated from clinical specimens since 1960. J Clin Microbiol. 1977;5:20–25. doi: 10.1128/jcm.5.1.20-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holst E, Goffeng A R, Andersch B. Bacterial vaginosis and vaginal micro-organisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol. 1994;32:176–186. doi: 10.1128/jcm.32.1.176-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoshino E, Ando N, Sato M, Kota K. Bacterial invasion of non-exposed dental pulp. Int Endod J. 1992;25:2–5. doi: 10.1111/j.1365-2591.1992.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 141.Hunter T, Chow A W. Peptostreptococcus magnusseptic arthritis—a report and review of the English literature. J Rheumatol. 1988;15:1583–1584. [PubMed] [Google Scholar]
- 142.Huss V A R, Festl H, Schleifer K H. Nucleic acid hybridisation studies and deoxyribonucleic acid base compositions of anaerobic gram-positive cocci. Int J Syst Bacteriol. 1984;34:95–101. [Google Scholar]
- 143.Ingham H R, Sisson P R, Tharagonnet D, Selkon J B, Codd A A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977;ii:1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- 144.Johnson, C. C. 1993. Susceptibility of anaerobic bacteria to β-lactam antibiotics in the United States. Clin. Infect. Dis. 16(Suppl. 4):S371–S376. [DOI] [PubMed]
- 145.Johnson, S., F. Lebahn, L. R. Peterson, and D. N. Gerding. 1995. Use of an anaerobic collection and transport swab device to recover anaerobic bacteria from infected foot ulcers in diabetics. Clin. Infect. Dis. 20(Suppl. 2):S289–S290. [DOI] [PubMed]
- 146.Jokipii A M M, Jokipii L, Sipila P, Jokinen K. Semiquantitative culture results and pathogenic significance of obligate anaerobes in peritonsillar abscesses. J Clin Microbiol. 1988;26:957–961. doi: 10.1128/jcm.26.5.957-961.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jousimies-Somer, H., S. Savolainen, A. Makitie, and J. Ylikoski. 1993. Bacteriologic findings in peritonsillar abscesses in young adults. Clin. Infect. Dis. 16(Suppl. 4):S292–S298. [DOI] [PubMed]
- 148.Karachewski N O, Busch E L, Wells C L. Comparison of PRAS II, RapID ANA, and API 20A systems for identification of anaerobic bacteria. J Clin Microbiol. 1985;21:122–126. doi: 10.1128/jcm.21.1.122-126.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kastern W, Holst E, Nielsen E, Sjöbring U, Björck L. Protein L, a bacterial immunoglobulin-binding protein and a possible virulence determinant. Infect Immun. 1990;58:1217–1222. [Google Scholar]
- 150.Kilpper-Baelz R, Schleifer K H. Transfer of Peptococcus saccharolyticus Foubert and Douglas to the genus Staphylococcus: Staphylococcus saccharolyticus(Foubert and Douglas) comb. nov. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt I Orig Reihe C. 1981;2:324–331. [Google Scholar]
- 151.King A, Phillips I. European collaborative study of reproducibility of quantitative sensitivity testing of anaerobes. J Antimicrob Chemother. 1988;21:425–438. doi: 10.1093/jac/21.4.425. [DOI] [PubMed] [Google Scholar]
- 152.Kiryu T, Hoshino E, Iwaku M. Bacteria invading periapical cementum. J Endod. 1994;20:169–172. doi: 10.1016/S0099-2399(06)80328-6. [DOI] [PubMed] [Google Scholar]
- 153.Kitch T T, Appelbaum P C. Accuracy and reproducibility of the 4-hour ATB 32A method for anaerobe identification. J Clin Microbiol. 1989;27:2509–2513. doi: 10.1128/jcm.27.11.2509-2513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kluyver A J, van Niel C B. Prospects for a natural system of classification of bacteria. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt II. 1936;94:369–403. [Google Scholar]
- 155.Krausse R, Ullmann U. Identification of anaerobic bacteria using high-performance liquid chromatography. Zentralbl Bakteriol Mikrobiol Hyg Ser A. 1987;266:340–352. doi: 10.1016/s0176-6724(87)80252-3. [DOI] [PubMed] [Google Scholar]
- 156.Kremer B H A, Magee J T, van Dalen P J, van Steenbergen T J M. Characterization of smooth and rough morphotypes of Peptostreptococcus micros. Int J Syst Bacteriol. 1997;47:363–368. doi: 10.1099/00207713-47-2-363. [DOI] [PubMed] [Google Scholar]
- 157.Krepel C J, Gohr C M, Edmiston C E, Farmer S G. Anaerobic pathogenesis: collagenase production by Peptostreptococcus magnusand its relationship to site of infection. J Infect Dis. 1991;163:1148–1150. doi: 10.1093/infdis/163.5.1148. [DOI] [PubMed] [Google Scholar]
- 158.Krepel C J, Gohr C M, Walker A P, Farmer S G, Edmiston C E. Enzymatically active Peptostreptococcus magnus: association with site of infection. J Clin Microbiol. 1992;30:2330–2334. doi: 10.1128/jcm.30.9.2330-2334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kucers A, Bennett N M. The use of antibiotics. 3rd ed. London, United Kingdom: Heinemann; 1979. [Google Scholar]
- 160.Lämmler C, Alaboudi A, Hildebrand A. Characterization of albumin-binding properties of Peptostreptococcus magnus. Can J Microbiol. 1989;35:614–618. doi: 10.1139/m89-098. [DOI] [PubMed] [Google Scholar]
- 161.Landers D V, Sweet R L. Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis. 1983;5:876–884. doi: 10.1093/clinids/5.5.876. [DOI] [PubMed] [Google Scholar]
- 162.Lanigan G W. Peptococcus heliotrinreducenssp. nov., a cytochrome-reducing anaerobe which metabolises pyrrolizidine alkaloids. J Gen Microbiol. 1976;94:1–10. doi: 10.1099/00221287-94-1-1. [DOI] [PubMed] [Google Scholar]
- 163.Lawson P A, Llop-Perez P, Hutson R A, Hippe H, Collins M D. Towards a phylogeny of the clostridia based on 16S rRNA sequences. FEMS Microbiol Lett. 1993;113:87–92. doi: 10.1111/j.1574-6968.1993.tb06493.x. [DOI] [PubMed] [Google Scholar]
- 164.Lewis M A O, MacFarlane T W, McGowan D A. Quantitative bacteriology of acute dento-alveolar abscesses. J Med Microbiol. 1986;21:101–104. doi: 10.1099/00222615-21-2-101. [DOI] [PubMed] [Google Scholar]
- 165.Lewis M A O, MacFarlane T W, McGowan D A, MacDonald D G. Assessment of the pathogenicity of bacterial species isolated from acute dentoalveolar abscesses. J Med Microbiol. 1988;27:109–116. doi: 10.1099/00222615-27-2-109. [DOI] [PubMed] [Google Scholar]
- 166.Li N, Hashimoto Y, Adnan S, Miura H, Yamamoto H, Ezaki T. Three new species of the genus Peptostreptococcus isolated from humans: Peptostreptococcus vaginalis sp. nov., Peptostreptococcus lacrimalis sp. nov. and Peptostreptococcus lactolyticussp. nov. Int J Syst Bacteriol. 1992;42:602–605. doi: 10.1099/00207713-42-4-602. [DOI] [PubMed] [Google Scholar]
- 167.Li N, Hashimoto Y, Ezaki T. Determination of 16S ribosomal RNA sequences of all members of the genus Peptostreptococcusand their phylogenetic position. FEMS Microbiol Lett. 1994;116:1–6. doi: 10.1111/j.1574-6968.1994.tb06666.x. [DOI] [PubMed] [Google Scholar]
- 168.Lie M A, Danser M M, van der Weijden G A, Timmerman M F, de Graaff J, van der Velden U. Oral microbiota in subjects with a weak or strong response in experimental gingivitis. J Clin Periodontol. 1995;22:642–647. doi: 10.1111/j.1600-051x.1995.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 169.Looney W J, Gallusser A J C, Modde H K. Evaluation of the ATB 32A system for identification of anaerobic bacteria isolated from clinical specimens. J Clin Microbiol. 1990;28:1519–1524. doi: 10.1128/jcm.28.7.1519-1524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.MacGowan, A. P. 1997. Personal communication.
- 171.Madsen M, Aalbaek B, Hansen J W. Comparative bacteriological studies on summer mastitis in grazing cattle and pyogenes mastitis in stabled cattle in Denmark. Vet Microbiol. 1992;32:81–88. doi: 10.1016/0378-1135(92)90009-i. [DOI] [PubMed] [Google Scholar]
- 172.Mangels J. Anaerobic transport systems: are they necessary? Clin Microbiol Newsl. 1994;16:13–16. [Google Scholar]
- 173.Marina, M., C. A. Strong, R. Civen, E. Molitoris, and S. M. Finegold. 1993. Bacteriology of anaerobic pleuropulmonary infections: preliminary report. Clin. Infect. Dis. 16(Suppl. 4):S256–S262. [DOI] [PubMed]
- 174.Marler L M, Siders J A, Wolters L C, Pettigrew Y, Skitt B K, Allen S D. Evaluation of the new RapID-ANA II system for the identification of clinical anaerobic isolates. J Clin Microbiol. 1991;29:874–878. doi: 10.1128/jcm.29.5.874-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Marshall R, Kaufman A K. Production of deoxyribonuclease, ribonuclease, coagulase and hemolysins by anaerobic gram-positive cocci. J Clin Microbiol. 1981;13:787–788. doi: 10.1128/jcm.13.4.787-788.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marui T. Characterization of peptococci and peptostreptococci isolated from human clinical specimens and their taxonomical problems. Acta Sch Med Univ Gifu. 1981;29:1071–1082. [Google Scholar]
- 177.McDonald H M, O’Loughlin J A, Jolley P T, Vigneswaran R, McDonald P J. Changes in vaginal flora during pregnancy and association with preterm birth. J Infect Dis. 1994;170:724–728. doi: 10.1093/infdis/170.3.724. [DOI] [PubMed] [Google Scholar]
- 178.Mergenhagen S E, Thonard J C, Scherp H W. Studies on synergistic infection. I. Experimental infection with anaerobic streptococci. J Infect Dis. 1958;103:33–34. doi: 10.1093/infdis/103.1.33. [DOI] [PubMed] [Google Scholar]
- 179.Mitchelmore I J, Prior A J, Montgomery P Q, Tabaqchali S. Microbiological features and pathogenesis of peritonsillar abscesses. Eur J Clin Microbiol Infect Dis. 1995;14:870–877. doi: 10.1007/BF01691493. [DOI] [PubMed] [Google Scholar]
- 180.Mitchelmore I J, Reilly P G, Hay A J, Tabaqchali S. Tonsil surface and core cultures in recurrent tonsillitis: prevalence of anaerobes and β-lactamase producing organisms. Eur J Clin Microbiol Infect Dis. 1994;13:542–548. doi: 10.1007/BF01971304. [DOI] [PubMed] [Google Scholar]
- 181.Montejo M, Ruiz-Irastorza G, Aguirrebengoa K, Amutio E, Hernandez J L, Aguirre C. Prosthetic valve endocarditis caused by Peptostreptococcus anaerobius. Clin Infect Dis. 1995;20:1431. doi: 10.1093/clinids/20.5.1431. [DOI] [PubMed] [Google Scholar]
- 182.Moore L V H, Moore W E C, Cato E P, Smibert R M, Burmeister J A, Best A M, Ranney R R. Bacteriology of human gingivitis. J Dent Res. 1987;66:989–995. doi: 10.1177/00220345870660052401. [DOI] [PubMed] [Google Scholar]
- 183.Moore W E C, Holdeman L V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974;27:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Moore W E C, Holdeman L V H, Smibert R M, Hash D E, Burmeister J A, Ranney R R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982;38:1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moore W E C, Moore L V H, Ranney R R, Smibert R M, Burmeister J A, Schenkein H A. The microflora of periodontal sites showing active destructive progression. J Clin Periodontol. 1991;18:729–739. doi: 10.1111/j.1600-051x.1991.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 186.Murdoch D A. Gram-positive anaerobic cocci. In: Collier L H, editor. Topley and Wilson’s microbiology and microbial infections. 9th ed. Vol. 2. London, United Kingdom: Edward Arnold; 1998. pp. 783–797. [Google Scholar]
- 187.Murdoch, D. A. Unpublished data.
- 188.Murdoch D A, Collins M D, Willems A, Hardie J M, Young K A, Magee J T. Description of three new species of the genus Peptostreptococcus from human clinical specimens: Peptostreptococcus harei sp. nov., Peptostreptococcus ivorii sp. nov. and Peptostreptococcus octaviussp. nov. Int J Syst Bacteriol. 1997;47:781–787. [Google Scholar]
- 189.Murdoch D A, Kelly M. Peptostreptococcus prevotii or Peptostreptococcus vaginalis? Identification and clinical importance of a new species of Peptostreptococcus. Anaerobe. 1997;3:23–26. doi: 10.1006/anae.1996.0060. [DOI] [PubMed] [Google Scholar]
- 190.Murdoch D A, Magee J T. A numerical taxonomic study of the Gram-positive anaerobic cocci. J Med Microbiol. 1995;43:148–155. doi: 10.1099/00222615-43-2-148. [DOI] [PubMed] [Google Scholar]
- 191.Murdoch D A, Mitchelmore I J. Isolation of Peptostreptococcus heliotrinreducensfrom human polymicrobial abscesses. Lett Appl Microbiol. 1989;9:223–225. [Google Scholar]
- 192.Murdoch D A, Mitchelmore I J. The laboratory identification of gram-positive anaerobic cocci. J Med Microbiol. 1991;34:295–308. doi: 10.1099/00222615-34-5-295. [DOI] [PubMed] [Google Scholar]
- 193.Murdoch D A, Mitchelmore I J, Nash R A, Hardie J M, Tabaqchali S. Preformed enzyme profiles of reference strains of gram-positive anaerobic cocci. J Med Microbiol. 1988;27:65–70. doi: 10.1099/00222615-27-1-65. [DOI] [PubMed] [Google Scholar]
- 194.Murdoch D A, Mitchelmore I J, Tabaqchali S. Identification of gram-positive anaerobic cocci by use of systems for detecting preformed enzymes. J Med Microbiol. 1988;25:289–293. doi: 10.1099/00222615-25-4-289. [DOI] [PubMed] [Google Scholar]
- 195.Murdoch D A, Mitchelmore I J, Tabaqchali S. Peptostreptococcus microsin polymicrobial abscesses. Lancet. 1988;i:594. doi: 10.1016/s0140-6736(88)91393-1. [DOI] [PubMed] [Google Scholar]
- 196.Murdoch D A, Mitchelmore I J, Tabaqchali S. The clinical importance of gram-positive anaerobic cocci isolated at St Bartholomew’s Hospital, London, in 1987. J Med Microbiol. 1994;41:36–44. doi: 10.1099/00222615-41-1-36. [DOI] [PubMed] [Google Scholar]
- 197.Murdoch D A, Wilks M, Mitchelmore I J, Tabaqchali S. Identification of gram-positive anaerobic cocci by gas-liquid chromatography and preformed enzyme profile using a commercial kit, ATB 32A. In: Borriello S P, editor. Clinical and molecular aspects of anaerobes. Petersfield, United Kingdom: Wrightson Biomedical; 1990. pp. 285–291. [Google Scholar]
- 198.Murray P R, Weber C J, Niles A C. Comparative evaluation of three identification schemes for anaerobes. J Clin Microbiol. 1985;22:52–55. doi: 10.1128/jcm.22.1.52-55.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Myhre E B. Surface receptors for human serum albumin in Peptococcus magnusstrains. J Med Microbiol. 1984;18:189–195. doi: 10.1099/00222615-18-2-189. [DOI] [PubMed] [Google Scholar]
- 200.National Committee for Clinical Laboratory Standards. National Committee for Clinical Laboratory Standards, Villanova, Pa. 1990. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 2nd ed. Approved standard. Publication M11-A2. [Google Scholar]
- 201.Neut C, Lesieur V, Romond C, Beerens H. Analysis of gram-positive anaerobic cocci in oral, fecal and vaginal flora. Eur J Clin Microbiol Infect Dis. 1985;4:435–437. doi: 10.1007/BF02148708. [DOI] [PubMed] [Google Scholar]
- 202.Ng L-K, Dillon J-A R. Molecular fingerprinting of isolates of the genus Peptostreptococcus using rRNA genes from Escherichia coli and P. anaerobius. J Gen Microbiol. 1991;137:1323–1331. doi: 10.1099/00221287-137-6-1323. [DOI] [PubMed] [Google Scholar]
- 203.Ng J, Ng L-K, Chow A W, Dillon J-A R. Identification of five Peptostreptococcusspecies isolated predominantly from the female genital tract by using the Rapid ID 32 A system. J Clin Microbiol. 1994;32:1302–1307. doi: 10.1128/jcm.32.5.1302-1307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ng J, Ng L, Holst E, Dillon J R. Differentiation of Protein-L containing and albumin-binding Peptostreptococcus magnusisolates by DNA amplification, ribotyping and pulse field gel electrophoresis. Anaerobe. 1996;2:95–102. [Google Scholar]
- 205.Nilson B H, Solomon A, Björck L, Akerstrom B. Protein L from Peptostreptococcus magnusbinds to the κ light chain variable domain. J Biol Chem. 1992;267:2234–2239. [PubMed] [Google Scholar]
- 206.Ohm-Smith M J, Sweet R L, Hadley W K. Occurrence of clindamycin-resistant anaerobic bacteria isolated from cultures taken following clindamycin therapy. Antimicrob Agents Chemother. 1986;30:11–14. doi: 10.1128/aac.30.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Panagou P, Papandreou L, Bouros D. Severe anaerobic necrotizing pneumonia complicated by pyopneumothorax and anaerobic monoarthritis due to Peptostreptococcus magnus. Respiration. 1991;58:223–225. doi: 10.1159/000195932. [DOI] [PubMed] [Google Scholar]
- 208.Panichi, G., R. di Rosa, P. Enrico, and S. Babudieri. 1990. Anaerobic bacteria and bacterial infections: perspectives on treatment and resistance in Italy. Rev. Infect. Dis. 12(Suppl. 2):S152–S156. [DOI] [PubMed]
- 209.Pankuch G A, Jacobs M R, Appelbaum P C. Susceptibilities of 428 gram-positive and -negative anaerobic bacteria to Bay y3118 compared with their susceptibilities to ciprofloxacin, clindamycin, metronidazole, piperacillin, piperacillin-tazobactam, and cefoxitin. Antimicrob Agents Chemother. 1993;37:1649–1654. doi: 10.1128/aac.37.8.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Papasian C J, McGregor D H, Hodges G R, Kennedy J. Peptostreptococcal vertebral osteomyelitis. J Clin Microbiol. 1986;24:633–635. doi: 10.1128/jcm.24.4.633-635.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Paster B J, Russell J B, Yang C M J, Chow J M, Woese C R, Tanner R. Phylogeny of the ammonia-producing ruminal bacteria Peptostreptococcus anaerobius, Clostridium sticklandii, and Clostridium aminophilumsp. nov. Int J Syst Bacteriol. 1993;43:107–110. doi: 10.1099/00207713-43-1-107. [DOI] [PubMed] [Google Scholar]
- 212.Patella V, Casolaro V, Björck L, Marone G. Protein L: a bacterial immunoglobulin-binding protein that activates human basophils and mast cells. J Immunol. 1990;145:3054–3061. [PubMed] [Google Scholar]
- 213.Petts D N, Champion W, Raymond G. Oxolinic acid as a selective agent for the isolation of non-sporing anaerobes from clinical material. Lett Appl Microbiol. 1988;6:65–67. [Google Scholar]
- 214.Phelps R, Jacobs R A. Purulent pericarditis and mediastinitis due to Peptococcus magnus. JAMA. 1985;254:947–948. [PubMed] [Google Scholar]
- 215.Pien F D, Thompson R L, Martin W J. Clinical and bacteriologic studies of anaerobic, gram-positive cocci. Mayo Clin Proc. 1972;47:251–257. [PubMed] [Google Scholar]
- 216.Poco S E, Nakazawa F, Ikeda T, Sato M, Sato T, Hoshino E. Eubacterium exiguumsp. nov., isolated from human oral lesions. Int J Syst Bacteriol. 1996;46:1120–1124. doi: 10.1099/00207713-46-4-1120. [DOI] [PubMed] [Google Scholar]
- 217.Pouëdras P, Donnio P Y, Sire J M, Avril J L. Prosthetic valve endocarditis and paravalvular abscess caused by Peptostreptococcus magnus. Clin Infect Dis. 1992;15:185. doi: 10.1093/clinids/15.1.185. [DOI] [PubMed] [Google Scholar]
- 218.Prevot A R. Manuel de classification et de determination des bacteries anaerobies. 2nd ed. Paris, France: Masson; 1948. [Google Scholar]
- 219.Rainey F A, Janssen P H. Phylogenetic analysis by 16S ribosomal DNA sequence comparison reveals two unrelated groups of species within the genus Ruminococcus. FEMS Microbiol Lett. 1995;129:69–74. doi: 10.1016/0378-1097(95)00138-U. [DOI] [PubMed] [Google Scholar]
- 220.Rams T E, Andriolo M, Feik D, Abel S N, McGivern T M, Slots J. Microbiological study of HIV-related periodontitis. J Periodontol. 1991;62:74–81. doi: 10.1902/jop.1991.62.1.74. [DOI] [PubMed] [Google Scholar]
- 221.Rams T E, Feik D, Listgarten M A, Slots J. Peptostreptococcus microsin human periodontitis. Oral Microbiol Immunol. 1992;7:1–6. doi: 10.1111/j.1399-302x.1992.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 222.Reig M, Baquero F. Antibacterial activity of clavulanate and tazobactam on Peptostreptococcusspecies. J Antimicrob Chemother. 1994;33:358–359. doi: 10.1093/jac/33.2.358. [DOI] [PubMed] [Google Scholar]
- 223.Reig M, Moreno A, Baquero F. Resistance of Peptostreptococcusspp. to macrolides and lincosamides: inducible and constitutive phenotypes. Antimicrob Agents Chemother. 1992;36:662–664. doi: 10.1128/aac.36.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rogosa M. Peptococcaceae, a new family to include the gram-positive, anaerobic cocci of the genera Peptococcus, Peptostreptococcus, and Ruminococcus. Int J Syst Bacteriol. 1971;21:234–237. [Google Scholar]
- 225.Rogosa M. Family III. Peptococcaceae. In: Buchanan R E, Gibbons N E, editors. Bergey’s manual of determinative bacteriology. 8th ed. Baltimore, Md: The Williams & Wilkins Co.; 1974. pp. 517–527. [Google Scholar]
- 226.Rosenberg E S, Torosian J P, Slots J. Microbial differences in 2 clinically distinct types of failures of osseointegrated implants. Clin Oral Impl Res. 1991;2:135–144. doi: 10.1034/j.1600-0501.1991.020306.x. [DOI] [PubMed] [Google Scholar]
- 227.Rosenblatt J E. Anaerobic cocci. In: Lennette E H, Balows A, Hausler W J, Shadomy E P, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 445–449. [Google Scholar]
- 228.Rosenblatt, J. E., and I. Brook. 1993. Clinical relevance of susceptibility testing of anaerobic bacteria. Clin. Infect. Dis. 16(Suppl. 4):S446–S448. [DOI] [PubMed]
- 229.Sabbaj J, Sutter V L, Finegold S M. Anaerobic pyogenic liver abscess. Ann Intern Med. 1972;77:629–638. doi: 10.7326/0003-4819-77-4-629. [DOI] [PubMed] [Google Scholar]
- 230.Sanchez M L, Jones R N, Croco J L. Use of the E-Test to assess macrolide-lincosamide resistance patterns among Peptostreptococcusspecies. Antimicrob Newsl. 1992;8:45–49. [Google Scholar]
- 231.Sanderson P J. Infection of the foot with Peptococcus magnus. J Clin Pathol. 1977;30:266–268. doi: 10.1136/jcp.30.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Schiefer-Ullrich H, Andreesen J R. Peptostreptococcus barnesaesp. nov., a Gram-positive, anaerobic, obligately purine-utilising coccus from chicken feces. Arch Microbiol. 1985;143:26–31. [Google Scholar]
- 233.Schottmüller H. Zur Bedeutung einiger Anaëroben in der Pathologie, insbesondere bei puerperalen Erkrankungen. Mitt Grenzgeb Med Chir. 1910;21:450–490. [Google Scholar]
- 234.Schwarz O, Dieckman W J. Anaerobic streptococci: their role in puerperal infection. South Med J. 1926;19:470–479. [Google Scholar]
- 235.Shah, H. N., and S. E. Gharbia. 1995. The biochemical milieu of the host in the selection of anaerobic species in the oral cavity. Clin. Infect. Dis. 20(Suppl. 2):S291–S300. [DOI] [PubMed]
- 236.Sheikh, W., D. H. Pitkin, and H. Nadler. 1993. Antibacterial activity of meropenem and selected comparative agents against anaerobic bacteria at seven North American centers. Clin. Infect. Dis. 16(Suppl. 4):S361–S366. [DOI] [PubMed]
- 237.Skerman V B D, MacGowan V, Sneath P H A. Approved lists of bacterial names. Int J Syst Bacteriol. 1980;30:225–240. [Google Scholar]
- 238.Smith D T. Experimental aspiratory abscess. Arch Surg. 1927;14:231–239. [Google Scholar]
- 239.Smith D T. Fusospirochetal disease of the lungs produced with cultures from Vincent’s angina. J Infect Dis. 1930;46:303–310. [Google Scholar]
- 240.Smith G L F, Cumming C G, Ross P W. Analysis of EDTA-soluble cell surface components of Gram-positive anaerobic cocci. J Gen Microbiol. 1986;132:1591–1597. doi: 10.1099/00221287-132-6-1591. [DOI] [PubMed] [Google Scholar]
- 241.Smith G L F, Cumming C G, Ross P W. Survival of Gram-positive anaerobic cocci on swabs and their isolation from the mouth and vagina. J Clin Pathol. 1986;39:93–98. doi: 10.1136/jcp.39.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Smith J W, Southern P M, Lehmann J H. Bacteremia in septic abortion: complications and treatment. Obstet Gynecol. 1970;35:704–707. [PubMed] [Google Scholar]
- 243.Smyth A G, McDowell D B, Stassen L F A. An in-vitro study of the comparative viability on different swab types of simulated specimens of bacteria commonly present in oro-facial infections. Br J Oral Maxillofac Surg. 1993;31:161–164. doi: 10.1016/0266-4356(93)90116-e. [DOI] [PubMed] [Google Scholar]
- 244.Socransky S S, Haffajee A D, Dzink J L, Hillman J D. Associations between microbial species in subgingival plaque samples. Oral Microbiol Immunol. 1988;3:1–7. doi: 10.1111/j.1399-302x.1988.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 245.Socransky S S, Haffajee A D, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the sampled site. J Clin Periodontol. 1991;18:766–775. doi: 10.1111/j.1600-051x.1991.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 246.Soper D E, Brockwell N J, Dalton H P, Johnson D. Observations concerning the microbial etiology of acute salpingitis. Am J Obstet Gynecol. 1994;170:1008–1017. doi: 10.1016/s0002-9378(94)70094-x. [DOI] [PubMed] [Google Scholar]
- 247.Stark P L, Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol. 1982;15:189–203. doi: 10.1099/00222615-15-2-189. [DOI] [PubMed] [Google Scholar]
- 248.Steffen E K, Hentges D J. Hydrolytic enzymes of anaerobic bacteria isolated from human infections. J Clin Microbiol. 1981;14:153–156. doi: 10.1128/jcm.14.2.153-156.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stenson M J, Lee D T, Rosenblatt J E, Contezac J M. Evaluation of the AnIdent system for the identification of anaerobic bacteria. Diagn Microbiol Infect Dis. 1986;5:9–15. doi: 10.1016/0732-8893(86)90086-6. [DOI] [PubMed] [Google Scholar]
- 250.Sturm A W. Mobiluncusspecies and other anaerobic bacteria in non-puerperal breast abscesses. Eur J Clin Microbiol Infect Dis. 1989;8:789–792. doi: 10.1007/BF02185846. [DOI] [PubMed] [Google Scholar]
- 251.Summanen P, Baron E J, Citron D M, Strong C, Wexler H M, Finegold S M. Wadsworth anaerobic bacteriology manual. 5th edn. Belmont, Calif: Star Publishing Co.; 1993. [Google Scholar]
- 252.Summanen, P. H., D. A. Talan, C. Strong, M. McTeague, R. Bennion, J. E. Thompson, and S. M. Finegold. 1995. Bacteriology of skin and soft-tissue infections: comparison of infections in intravenous drug users and individuals with no history of intravenous drug use. Clin. Infect. Dis. 20(Suppl. 2):S279–S282. [DOI] [PubMed]
- 253.Sutter, V. L. 1984. Anaerobes as normal oral flora. Rev. Infect. Dis. 6(Suppl. 1):S62–S63. [DOI] [PubMed]
- 254.Sutter V L, Citron D M, Edelstein M A C, Finegold S M. Wadsworth anaerobic bacteriology manual. 4th edn. Belmont, Calif: Star Publishing Co.; 1985. [Google Scholar]
- 255.Sutter V L, Finegold S M. Antibiotic disc susceptibility tests for rapid presumptive identification of Gram-negative anaerobic bacilli. Appl Microbiol. 1971;21:13–20. doi: 10.1128/am.21.1.13-20.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sweet, R. L. 1995. Role of bacterial vaginosis in pelvic inflammatory disease. Clin. Infect. Dis. 20(Suppl. 2):S271–S275. [DOI] [PubMed]
- 257.Sweet R L, Roy S, Faro S, O’Brien W F, Sanfilippo J S, Seidlin M. Piperacillin and tazobactam versus clindamycin and gentamicin in the treatment of hospitalised women with pelvic infection. Obstet Gynecol. 1994;83:280–286. [PubMed] [Google Scholar]
- 258.Tally F P, Stewart P R, Sutter V L, Rosenblatt J E. Oxygen tolerance of fresh clinical anaerobic bacteria. J Clin Microbiol. 1975;1:161–164. doi: 10.1128/jcm.1.2.161-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tam Y-C, Chan E C S. Phase variation of hyaluronidase-producing peptostreptococci associated with periodontal disease. J Dent Res. 1983;62:1009–1012. doi: 10.1177/00220345830620090201. [DOI] [PubMed] [Google Scholar]
- 260.Tanner, A., and N. Stillman. 1993. Oral and dental infections with anaerobic bacteria: clinical features, predominant pathogens, and treatment. Clin. Infect. Dis. 16(Suppl. 4):S304–S309. [DOI] [PubMed]
- 261.Taylor E A, Jackman P J H, Phillips I T. The differentiation of asaccharolytic anaerobic Gram-positive cocci by protein electrophoresis. J Med Microbiol. 1991;34:339–348. doi: 10.1099/00222615-34-6-339. [DOI] [PubMed] [Google Scholar]
- 262.Taylor E A, Phillips I T. The taxonomy of the asaccharolytic anaerobic Gram-positive cocci. In: Borriello S P, Hardie J M, editors. Recent advances in anaerobic bacteriology. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 259–270. [Google Scholar]
- 263.Thomas C G A, Hare R. The classification of anaerobic cocci and their isolation in normal human beings and pathological processes. J Clin Pathol. 1954;7:300–304. doi: 10.1136/jcp.7.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Topiel M S, Simon G L. Peptococcaceae bacteremia. Diagn Microbiol Infect Dis. 1986;4:109–117. doi: 10.1016/0732-8893(86)90144-6. [DOI] [PubMed] [Google Scholar]
- 265.Turng B F, Guthmiller J M, Minah G E, Falkler W A. Development and evaluation of a selective and differential medium for the primary isolation of Peptostreptococcus micros. Oral Microbiol Immunol. 1996;11:356–361. doi: 10.1111/j.1399-302x.1996.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 266.van Dalen P J, van Steenbergen T J M, Cowan M M, Busscher H J, de Graaff J. Description of two morphotypes of Peptostreptococcus micros. Int J Syst Bacteriol. 1993;43:787–793. doi: 10.1099/00207713-43-4-787. [DOI] [PubMed] [Google Scholar]
- 267.Wade W G, Gray A R, Absi E G, Barker G R. Predominant cultivable flora in pericoronitis. Oral Microbiol Immunol. 1991;6:310–312. doi: 10.1111/j.1399-302x.1991.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 268.Wade W G, Lewis M A O, Cheeseman S L, Absi E G, Bishop P A. An unclassified Eubacteriumtaxon in acute dento-alveolar abscess. J Med Microbiol. 1994;40:115–117. doi: 10.1099/00222615-40-2-115. [DOI] [PubMed] [Google Scholar]
- 269.Wade W G, Moran J, Morgan J R, Newcombe R, Addy M. The effects of antimicrobial acrylic strips on the subgingival microflora in chronic periodontitis. J Clin Periodontol. 1992;19:127–134. doi: 10.1111/j.1600-051x.1992.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 270.Watt B, Brown F V. A selective agent for anaerobic cocci. J Clin Pathol. 1983;36:605–606. doi: 10.1136/jcp.36.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Watt B, Brown F V. Is ciprofloxacin active against clinically important anaerobes? J Antimicrob Chemother. 1986;17:605–613. doi: 10.1093/jac/17.5.605. [DOI] [PubMed] [Google Scholar]
- 272.Watt B, Jack E P. What are anaerobic cocci? J Med Microbiol. 1977;10:461–468. doi: 10.1099/00222615-10-4-461. [DOI] [PubMed] [Google Scholar]
- 273.Watt B, Smith G L F. Anaerobic cocci. In: Parker M T, Collier L H, editors. Topley and Wilson’s principles of bacteriology, virology and immunology. 8th ed. London, United Kingdom: Edward Arnold; 1990. pp. 247–253. [Google Scholar]
- 274.Watt B, Young O, McCurdy G. The susceptibility of anaerobic cocci from clinical samples to six antimicrobial agents. J Infect. 1979;1:143–149. [Google Scholar]
- 275.Watts D H, Krohn M A, Hillier S L, Eschenbach D A. Bacterial vaginosis as a risk factor for post-Cesarean endometritis. Obstet Gynecol. 1990;75:52–58. [PubMed] [Google Scholar]
- 276.Weiss N. Cell wall structure of anaerobic cocci. Rev Inst Pasteur Lyon. 1981;1:53–59. [Google Scholar]
- 277.Wells C L, Field C R. Long-chain fatty acids of peptococci and peptostreptococci. J Clin Microbiol. 1976;4:515–521. doi: 10.1128/jcm.4.6.515-521.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wenisch C, Wiesinger E, Werkgartner T, Makristathis A, Graninger W. Treatment of Peptostreptococcus microsendocarditis with teicoplanin. Clin Infect Dis. 1995;21:446–447. doi: 10.1093/clinids/21.2.446. [DOI] [PubMed] [Google Scholar]
- 279.Wexler, H. M. 1993. Susceptibility testing of anaerobic bacteria—the state of the art. Clin. Infect. Dis. 16(Suppl. 4):S328–S333. [DOI] [PubMed]
- 280.Wexler H M, Finegold S M. In vitro activity of cefoperazone plus sulbactam compared with that of other antimicrobial agents against anaerobic bacteria. Antimicrob Agents Chemother. 1988;32:403–406. doi: 10.1128/aac.32.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wexler H M, Molitoris E, Finegold S M. In vitro activity of Bay Y3118 against anaerobic bacteria. Antimicrob Agents Chemother. 1993;37:2509–2513. doi: 10.1128/aac.37.11.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wexler H M, Molitoris E, Murray P R, Washington J, Zabransky R J, Edelstein P H, Finegold S M. Comparison of spiral gradient endpoint and agar dilution methods for susceptibility testing of anaerobic bacteria: a multilaboratory collaborative evaluation. J Clin Microbiol. 1996;34:170–174. doi: 10.1128/jcm.34.1.170-174.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wheat L J, Allen S D, Henry M, Kernek C B, Siders J A, Kuebler T, Fineberg N, Norton J. Diabetic foot infections. Arch Intern Med. 1986;146:1935–1940. [PubMed] [Google Scholar]
- 284.Wideman P A, Vargo V L, Citronbaum D, Finegold S M. Evaluation of the sodium polyanethol sulfonate disk test for the identification of Peptostreptococcus anaerobius. J Clin Microbiol. 1976;4:330–333. doi: 10.1128/jcm.4.4.330-333.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wiggins R J, Wilks M, Tabaqchali S. Analysis by gas liquid chromatography of production of volatile fatty acids by anaerobic bacteria grown on solid medium. J Clin Pathol. 1985;38:933–936. doi: 10.1136/jcp.38.8.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wikstrom M, Sjöbring U, Drakenberg T, Forsen S, Björck L. Mapping of the immunoglobulin light chain-binding site of Protein L. J Mol Biol. 1995;250:128–133. doi: 10.1006/jmbi.1995.0364. [DOI] [PubMed] [Google Scholar]
- 287.Wilkins T D, Moore W E C, West S E H, Holdeman L V. Peptococcus niger(Hall) Kluyver and van Niel 1936: emendation of description and designation of neotype strain. Int J Syst Bacteriol. 1975;25:47–49. [Google Scholar]
- 288.Willems A, Collins M D. Phylogenetic placement of Sarcina ventriculi and Sarcina maxima within Group I Clostridium, a possible problem for future revision of the genus Clostridium. Request for an opinion. Int J Syst Bacteriol. 1994;44:591–593. doi: 10.1099/00207713-44-3-591. [DOI] [PubMed] [Google Scholar]
- 289.Willems A, Collins M D. Phylogenetic analysis of Ruminococcus flavefaciens, the type species of the genus Ruminococcus, does not support the reclassification of Streptococcus hansenii and Peptostreptococcus productusas ruminococci. Int J Syst Bacteriol. 1995;45:572–575. doi: 10.1099/00207713-45-3-572. [DOI] [PubMed] [Google Scholar]
- 290.Williams B L, McCann G F, Schoenknecht F D. Bacteriology of dental abscesses of endodontic origin. J Clin Microbiol. 1983;18:770–774. doi: 10.1128/jcm.18.4.770-774.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wong M, Catena A, Hadley W K. Antigenic relationships and rapid identification of Peptostreptococcusspecies. J Clin Microbiol. 1980;11:515–521. doi: 10.1128/jcm.11.5.515-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wren M W D. Multiple selective media for the isolation of anaerobic bacteria from clinical specimens. J Clin Pathol. 1980;33:61–65. doi: 10.1136/jcp.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wren, M. W. D. 1996. Personal communication.
- 294.Wren M W D. Anaerobic cocci of clinical importance. Br J Biomed Sci. 1996;53:294–301. [PubMed] [Google Scholar]
- 295.Wren M W D, Baldwin A W F, Eldon C P, Sanderson P J. The anaerobic culture of clinical specimens: a 14-month study. J Med Microbiol. 1977;10:49–61. doi: 10.1099/00222615-10-1-49. [DOI] [PubMed] [Google Scholar]
- 296.Wren M W D, Eldon C P, Dakin G H. Novobiocin and the differentiation of peptococci and peptostreptococci. J Clin Pathol. 1977;30:620–622. doi: 10.1136/jcp.30.7.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yasui S. Development and clinical application of DNA probe specific for Peptostreptococcus micros. Bull Tokyo Med Dent Univ. 1989;36:49–62. [PubMed] [Google Scholar]
- 298.Yasui S, Reynolds H S, Zambon J J, Genco R J, Potts T V. Development of specific DNA probe for the identification of Peptostreptococcus anaerobius. J Dent Res. 1989;68:256. [Google Scholar]