Abstract
The design and control of atomic-scale spin structures constitute major challenges for spin-based quantum technology platforms, including quantum dots, color centers, and molecular spins. Here, we showcase a strategy for designing the quantum properties of molecular spin qubits by combining tip-assisted on-surface assembly with electron spin resonance scanning tunneling microscopy (ESR-STM): We fabricate magnetic dimer complexes that consist of an iron phthalocyanine (FePc) molecule and an organometallic half-sandwich complex formed by the FePc ligand and an attached iron atom, Fe(C6H6). The total complex forms a mixed-spin (1/2,1) quantum ferrimagnet with a well-separated correlated ground state doublet, which we utilize for coherent control. As a result of the correlation, the quantum ferrimagnet shows an improved spin lifetime ( > 1.5 μs) as it is partially protected against inelastic electron scattering. Lastly, the ferrimagnet units also enable intermolecular coupling, that can be used to realize both ferromagnetic or antiferromagnetic structures. Thus, quantum ferrimagnets provide a versatile platform to improve coherent control in general and to study complex magnetic interactions.
Subject terms: Scanning probe microscopy, Spintronics, Magnetic properties and materials
Molecular complexes can function as spin qubits, and like other qubit architectures, making molecular spin qubits more resilient against interactions is vital for their use. Here, Huang et al present a molecular complex consisting of iron phthalocyanine, and an adjacent Fe atom, which together form a mixed-spin (1/2,1) quantum ferrimagnet, with improved spin lifetimes.
Introduction
Protecting individual qubits from interaction with the environment is one of the crucial challenges for quantum information processing. For various quantum architectures, a plethora of design strategies were developed that alter the properties of the system in a way that makes it resilient to various interactions. One prominent example constitutes the evolution of superconducting qubits, which transitioned from noisy charge qubits to transmon qubits1, the latter featuring a more robust energy level landscape. Also, for spin-based quantum architectures, a variety of designs were employed, including clock transitions2, singlet-triplet qubits in semiconductor quantum dots3 or chirality-based quantum states4. In particular, for molecular spin qubits, even larger interacting systems have been proposed and realized5–7. Conventionally, these spin systems were enabled by chemical synthesis. An alternative bottom-up route for creating interacting spin structures is on-surface synthesis, monitored and assisted by scanning tunneling microscopy (STM)8. This includes various interacting magnetic spin systems that were shown to form complex spin structures9–16. However, probing the intrinsic spin properties remains challenging and has up to now mostly been indirectly interrogated via the interaction with the substrate conduction electrons, i.e., the Kondo effect. A viable solution to this problem is to decouple the molecular spins from the metallic substrate via thin insulators17–19. This, however, makes it challenging for on-surface chemistry methods8.
A direct way to probe spin properties and to obtain insight into their spin dynamics is to use electron spin resonance in a scanning tunneling microscope (ESR-STM)20. This allows for probing various spin systems, for instance transition metal atoms21, alkali atoms22, rare earth elements23,24, as well as molecular spins17,25,26. Moreover, it permits to control the spin coherently in the time domain27,28. However, short spin lifetimes constitute a major challenge in most spin systems ( < 300 ns17,25,28), which also limits their phase coherence time ≤ . For T1 times, the main limitation remains the scattering with nearby tunneling electrons emanating from the tip and substrate electron baths27,29,30. Thus, one viable strategy is to move to thicker layers of the underlying insulator magnesium oxide (MgO)29, or to employ other routes such as utilizing atomic force microscopy31. An alternative strategy is to make the spin systems intrinsically more robust against sources of noise and relaxation by engineering their magnetic interactions.
In this work, we demonstrate how a tip-assisted assembly of a molecular complex leads to a spin system with improved dynamic spin properties compared to the constituents. The complex consists of an iron phthalocyanine (FePc) molecule and an additional Fe atom. The latter forms together with part of the FePc ligand an organometallic half-sandwich complex. We find that the resulting spin system constitutes a mixed-spin(1/2,1) Heisenberg quantum ferrimagnet32. The ferrimagnet has an improved spin lifetime compared to conventional on-surface spin ½ systems27,28, reaching here up to . This increase is rationalized by employing spin transport calculations, which show that the inelastic scattering channels limiting are suppressed by the correlations in the quantum ferrimagnet. In addition, we demonstrate that multiple complexes can be efficiently coupled either ferromagnetically or antiferromagnetically depending on their relative alignment.
Results
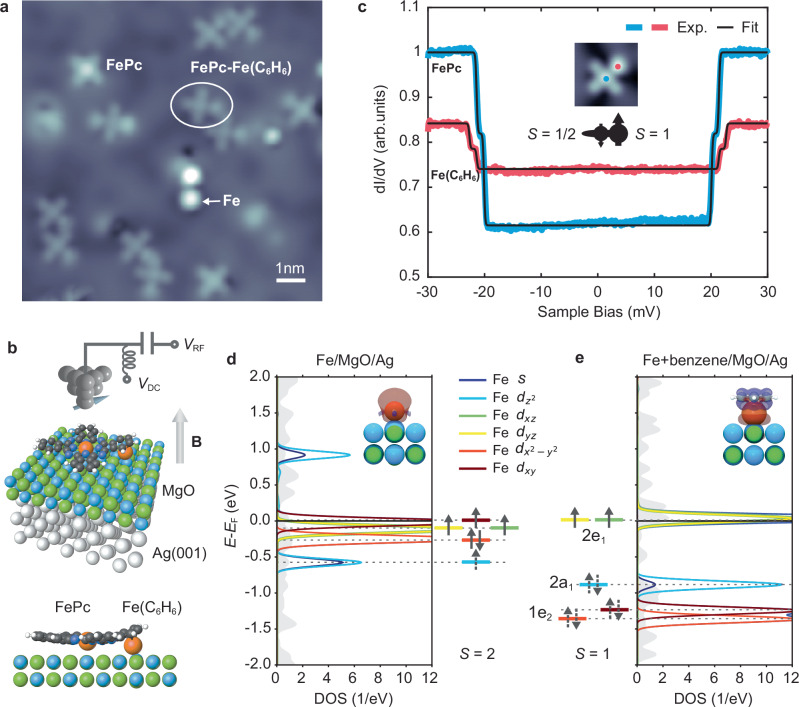
Figure 1a shows an STM topography of the sample system, that consists of individual Fe atoms and FePc molecules on two monolayers (ML) of MgO atop a Ag(001) substrate. FePc molecules were previously shown to be singly charged, resulting in a mostly isotropic spin system25,26, while individual Fe atoms are spin systems with a large out-of-plane magnetic anisotropy 33. From these building blocks, we create molecular complexes in which the two Fe atoms are strongly coupled. We employ a simple tip-assisted assembly scheme, in which an FePc molecule is picked up by the STM tip and subsequently dropped atop the Fe atom (Supplementary Fig. 1). In Fig. 1a we show several of these complexes as well as dimers of complexes (discussed in Fig. 4). Lattice site analysis (Supplementary Fig. 2) as well as density functional theory (DFT) calculations (Fig. 1b and Supplementary Fig. 2b) show that both Fe and FePc adsorb on an oxygen site of MgO with a (2,1) lattice distance. From DFT we also infer that the Fe atom is located underneath the benzene ring (C6H6) of the attached FePc ligand (Fig. 1b), thus mimicking the structure found in organometallic arene complexes, in particular half sandwich or piano stool complexes34. On surfaces, these kinds of systems have however been only rarely synthesized35–37. As a consequence of the shared FePc ligand, the Fe spin under the benzene ring, referred to as Fe(C6H6) in the following, is very close to the FePc central spin. In order to probe the joint magnetic properties of the emerging complexes, we first perform dI/dV measurements on the Fe(C6H6)- and FePc-site (Fig. 1c). These reveal a double-step feature at ~20 meV on both sites. We attribute this to inelastic electron tunneling spectroscopy (IETS) excitations from the magnetic ground state to the excited states. Performing spin transport calculations38, we can reproduce (black lines in Fig. 1c) both the correct position and intensity of the IETS measurements utilizing a Hamiltonian of the form (see Supplementary Section 3).
| 1 |
where meV is the (antiferromagnetic) Heisenberg exchange coupling between the FePc and Fe(C6H6) spins and meV is the out-of-plane magnetic anisotropy of Fe(C6H6). Interestingly, we find that for Fe(C6H6), a spin of is required: Using as in the case of the isolated Fe atom, we are not able to reproduce the experimental data in Fig. 1c (Supplementary Section 3). This reduction of the Fe spin state is additionally supported by remote magnetic sensing experiments (Supplementary Section 4).
Fig. 1. Engineered spin systems in artificial FePc-Fe(C6H6) complexes.
a STM Topographic image showing several molecular FePc-Fe(C6H6) complexes, along with single FePc molecules, Fe atoms and dimers of the complexes (see also Fig. 4) on 2 ML MgO on Ag(001) ( pA, mV, image size: 10.5 × 10.5 nm2, mK). b Sketch of the ESR-STM experiment: A radio frequency (RF) voltage is applied to the tunnel junction in addition to the DC bias voltage . In addition, a magnetic field is applied out-of-plane. The 3D view and side view image show the stable configuration of the FePc-Fe(C6H6) complex calculated by DFT. Orange spheres show the position of the Fe atoms. c dI/dV spectra acquired at the FePc (blue curve) and Fe(C6H6) site (red curve) as indicated in the inset (, , ). The double steps are fitted by inelastic electron spin transport calculations (black lines)38. The central sketch illustrates the overall spin structure. d Projected density of states (PDOS, left) and schematic orbital occupancy (right) for a single Fe atom on 2 ML MgO/Ag(001) obtained from a non-spin-polarized DFT calculation (see inset). It shows the orbitally resolved Fe s and 3d orbitals and their Lowdin charges, i.e., projections on atomic states. A strong hybridization (light and dark blue) is found. The dashed arrows indicate shared electrons of partially filled states. e PDOS (right) and orbital occupancy (left) of Fe with an added benzene ring atop (see inset). Here, the Fe states shift in energy in the presence of the ring and are additionally labeled by the respective molecular orbital states. The plot shows projections onto the dominating 3d states via Lowdin charges, i.e., atomic orbitals (See Supplementary Section 5). The new ligand framework leaves the 2e1 orbitals with dominating contribution singly occupied at the Fermi level resulting in S = 1.
Fig. 4. Spin coupling in a dimer of ferrimagnet complexes.
a Topographic image of two coupled complexes (, , image size: 3 × 3 nm2) with a spacing of 1.04 nm [(3,2) MgO lattice sites]. b Illustration of the magnetic coupling within the spin structure: The small (large) black arrows represent the FePc [Fe(C6H6)] spins. Within each individual complex two spins are antiferromagnetically coupled with strength . Since also the FePc and Fe(C6H6) in different complexes couple antiferromagnetically, the overall coupling between the complexes is ferromagnetic with coupling strength . c Energy level diagram of two coupled spin ½ in the presence of and . The detuning quantifies the distance from the avoided level crossing. The blue (red) arrows represent the dominant ESR transitions at weak (strong) tip fields. d ESR frequency sweep measurement at a fixed showing four peaks to , corresponding to the transitions labeled in (c). All data are measured on the Fe(C6H6) site position marked as a red dot in (a). (, , ). The black line is a fit of four Lorentzians to the data. e ESR frequency sweep measurements at different setpoint currents () showing an avoided level crossing (, , ). The dotted white curves represent fitted ESR transitions using a two-spin model, with and , , . f Simulation of the coupled spin system using a four-spin model (inset) of spins (1, 1/2, 1, 1/2) with antiferromagnetic coupling strengths and . The fitting parameters are: , , , .
To understand the spin state of Fe(C6H6) we employ DFT calculations (Details see Supplementary Section 5): In Fig. 1d we show the non-spin-polarized projected density of states (PDOS) of an individual Fe atoms’ - and -states as discussed elsewhere39, leading to four orbitals ( and ) close to half-filling and consequently . To rationalize the spin state of Fe(C6H6), we employ a simple model by placing a benzene ring atop the Fe atom (Fig. 1e), as it is the case in the complex and in general for organometallic (half) sandwich complexes34. We find that the order of the available states is greatly changed and best described now by those predicted by molecular orbital theory34. The molecular orbital diagram of the complex (Supplementary Fig. 7) nicely illustrates, how, in particular, the e1 states of benzene have a strong overlap with the Fe and orbitals and thus contribute to the stability of the complex.
The resulting two frontier orbitals 2e1 have strong / character and are half-filled, indicating a spin state of . We note that this resembles to a certain degree the frontier orbitals and spin state found in Nickelocene40, which consists of two cyclopentadienyl ligands and a Ni ion. We additionally performed DFT calculations of the full FePc-Fe(C6H6) that show a similar molecular orbital formation (See Supplementary Section 5). These also support that the remarkably strong exchange coupling of meV, the highest observed for spins on MgO, is mediated via the shared FePc ligand.
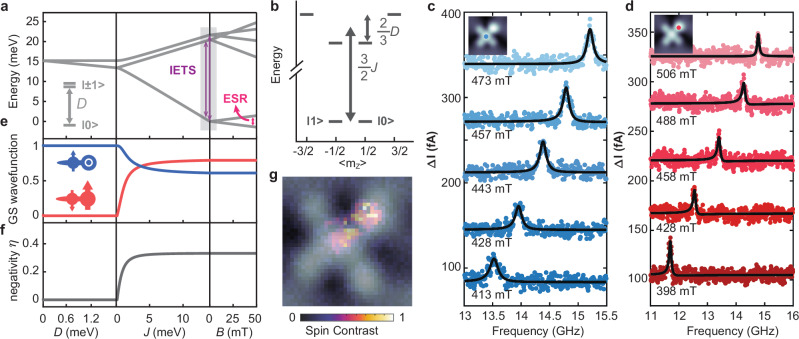
Figure 2a illustrates again the energy level diagram of the FePc-Fe(C6H6) complex under the action of , , and external magnetic field as derived from the spin model in Fig. 1c: For small exchange coupling (), the out-of-plane anisotropy lifts the degeneracies of the Fe(C6H6) states. For increasing exchange coupling (), two state manifolds form with and which are split by an energy . These transitions between the ground state doublet and the excited states quartet are the transitions observed in the IETS measurements in Fig. 1c and are indicated in Fig. 2a. Figure 2b shows the energy diagram as a function of magnetic quantum number Since the excited state quartet is further split by , two distinct steps are observed in Fig. 1c. While IETS measurements are well suited to explore the excited state levels, we additionally perform ESR measurements on the complex (Fig. 2c, d) to reveal low lying excitations. Here, a radio frequency (RF) voltage is applied (Fig. 1b) to drive the transition between the two states and . Measuring the resonance frequency as a function of external magnetic field (out-of-plane) permits to determine the magnetic moment of the complex via the resonance condition , where is Planck’s constant. We can perform these measurements on the FePc site (Fig. 2c) and the Fe(C6H6) site (Fig. 2d) and intriguingly, obtain in both cases , where is the Bohr magneton (Supplementary Section 6 and Supplementary Fig. 12). This suggests that the system acts as one magnetic unit, i.e. a strongly coupled spin system with a ground state that involves both spins: Utilizing again the Hamiltonian in Eq. 1, we plot in Fig. 2e the contribution to the ground state wave function of the coupled system: While in the case of the ground state is given by , for increasing exchange interaction a contribution of is additionally mixed in. In the limiting case of the ground state doublet is given as32:
| 2 |
Fig. 2. Electron spin resonance (ESR) of a single FePc-Fe(C6H6) complex in an STM.
a Energy level diagram resulting from the simulation of a mixed-spin-(1,1/2) Heisenberg ferrimagnet in Eq. 1. The magnetic anisotropy lifts the degeneracy of a spin 1 at zero field. The exchange coupling ( term) with a spin ½ leads to new and multiplets, which are both further separated by the Zeeman energy in a magnetic field . b Energy level diagram at zero field shows the states as a function of the expectation value, revealing a well-separated ground state doublet. c ESR-STM measurements on the FePc site (, ,) and d on the Fe(C6H6) site (, , ) at different magnetic fields. Linear fits to the slope yield a magnetic moment of [FePc site] and [Fe(C6H6) site]. The contributions to the ground state wave function (e) and negativity ( being an Eigenvalue) (f) as a function of , and . The cartoon insets in (e) illustrate the spin of FePc (left) and Fe(C6H6) (right). g A spin density map (pseudocolor) with corresponding topography overlaid (gray) of the complex reveals spin contrast on both the FePc and the Fe(C6H6) site. The spin signal was mapped by acquiring a spectroscopic map close to zero bias [|d2I/dV2(V = 2 mV)|] with a spin-polarized tip and normalized to the highest value (, setpoints: , , ).
This leads to some noteworthy consequences: First, besides their more complicated form, the two states form a two-level system with (See Fig. 2b) which are energetically well separated from the higher energy states. This can be roughly understood from the imbalance of the two spins [e.g., (1/2 + 0) or (−1/2 + 1) for the state], as it is similarly encountered in ferrimagnetism. Second, the two spin states in Eq. (2) are correlated, since they are not separable anymore, as observed similarly for atomic spin systems41,42. To quantify this, we use the negativity , which is employed as a measure for the pairwise entanglement41,43 and which becomes non-zero for non-separable states (See Fig. 2 caption and Supplementary section 3). It approaches for (Fig. 2f)32. Thus, the correlated non-separable ground state with explains well why the same is obtained on both sites in the complex. This is additionally visualized in the spin contrast map in Fig. 2g: Here, the spin density is delocalized over both Fe(C6H6) and FePc sites in the complex.
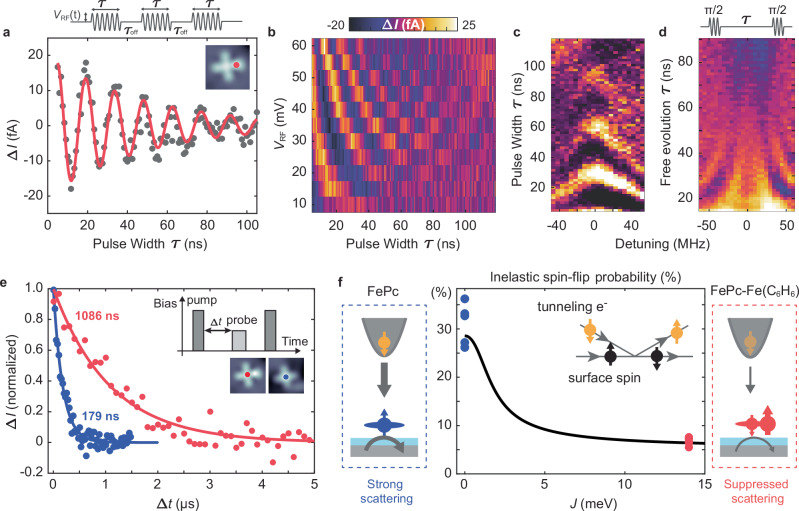
In order to investigate how the unique spin structure of the ferrimagnet complex affects its coherent spin dynamics, we performed pulsed ESR experiments27. Figure 3a illustrates a Rabi oscillation measurement (see Supplementary Section 7) on the Fe(C6H6) site [Phase coherence time ]. Figure 3b shows the power-dependent Rabi oscillations, where we observe a linear increase in the Rabi rate (Supplementary Fig. 13) as expected for an electric field-driven ESR mechanism25,27. In the Chevron pattern of the Rabi oscillation (Fig. 3c), the intensity decreases and the oscillation frequency increases as the system is detuned from the resonance frequency. Ramsey fringe measurements (Fig. 3d) also give a dephasing time () of the same order as and comparable to those found for single on-surface atomic spins27. Thus, they are likely limited by the same sources of decoherence, i.e., -induced tunneling current and fluctuations in the tip magnetic field27. However, we here notably achieve a Rabi frequency of MHz at , which corresponds to a -time of . This exceeds the value observed in other spin-1/2 systems, such as Ti27 and pristine FePc25, by a factor of ~2 at half the voltage. This can originate from multiple sources44–46, including a larger displacement of the surface spin, a different coupling to the magnetic tip, as well as the change in energetic positions of the molecular orbitals (Fig. 1e and Supplementary Section 7).
Fig. 3. Spin dynamics and coherent control of the complex.
a Rabi oscillation measurement performed at the Fe(C6H6) site in the complex with feedback loop on (,, ). Top: RF pulse scheme of width and amplitude . A constant DC voltage was applied during the sequence as an additional spin initialization and readout following the scheme in ref. 27. The solid red curve is a fit to an exponentially decaying sinusoid: for which we obtain . b Rabi oscillation measurements at different . The linear dependence of the Rabi rate on is shown in Supplementary Fig. 13. (at ). c Rabi chevron patterns and d Ramsey fringes with varying detuning . [Setpoint in (c, d): , , , in (c) and in (d). Color scale: [ in (c) and in (d)]. e Pump–probe measurements taken on single FePc (blue) and FePc in the complex (red). Solid lines are exponential fits of the form , resulting in (FePc in complex) and (single FePc) [parameters: , , , , , , (FePc in complex), (single FePc)]. f Inelastic spin-flip probability as a function of (black line) derived from spin transport calculations38 and the states in Eq. (2). The experimental inelastic spin-flip probability extracted from dI/dV measurements (see Supplementary Section 9) on five pristine FePc molecules [blue dots, (31.2 ± 1.9)%] and from five complexes [red dots, (6.7 ± 0.3)%] are shown as well. Inset and sketches to the sides illustrate the spin-flip process in which a tunneling electron exchanges momentum with the on-surface spin.
Additionally, we find a strong enhancement of the relaxation time for which we employ an all electrical pump-probe sequence (Fig. 3e)29,47: Here, a voltage pulse () pumps the spin system into an excited state while after a varying delay time ∆ a second probe pulse () probes, if the spin still is in the excited state. For direct comparison between FePc-Fe(C6H6) and the pristine FePc, we measure on the FePc site for both cases with the same experimental parameters. From exponential-decay fits we obtain (ferrimagnet complex), and (pristine FePc). By performing setpoint-dependent measurements, we conclude that the lifetime in both cases is limited by inelastic scattering with tunneling electrons from both the metallic substrate and tip29 [Supplementary Section 8]. Minimizing the latter, we obtain (ferrimagnet complex) and (pristine FePc) in the limit of large tip-surface distance (Supplementary Fig. 14). To explain the longer lifetime obtained for the complex, we return to its spin model introduced in Fig. 2: The probability for inelastically scattered tunneling electrons results most crucially from the transfer matrix element , where 38. This matrix element connects the initial states in the electron baths and spin system to their final states and . () is the spin vector operator of the local spin (tunneling electron). Thus, contains the inelastic scattering between the two spins, while describes spin-conserving Coulomb potential scattering. Consequently, the probability for inelastic spin-flip scattering depends on the states of the spin system. Figure 3f shows the evolution of this probability as a function of the exchange coupling when using the states in Eq. 2 for (Supplementary Section 9): In the limit of small , the system can be assumed to be in the state of pristine FePc for which we obtain ~29%. This drops to ~6% for the complex as the spin system is transitioning into the correlated ground state. This suggests a decrease by ~4–5, which matches well with the results for the pump-probe data. The reduced inelastic scattering can be intuitively understood as tunneling electrons interacting only with the FePc spin, rather than the complete coupled spin system. In addition to the simulation, we can evaluate the suppressed inelastic scattering from spin-polarized IETS dI/dV measurements (Supplementary Section 9). The probabilities of inelastic scattering obtained from these measurements are additionally shown in Fig. 3f and agree well with the spin transport calculations.
The results of Fig. 3 demonstrate that the FePc-Fe(C6H6) complex allows for coherent manipulation with an enhanced spin lifetime as well as a fast Rabi rate. The latter also benefits from the suppressed inelastic scattering, since this allows us to perform measurements at closer tip-sample distances and thus stronger tip fields without significantly reducing relaxation and coherence. In general, we think that, in particular, the protection makes quantum ferrimagnets a promising system for quantum sensing, simulation, or information processing: The exchange-like interaction between the complex and the tunneling electrons is similar to sources of relaxation in other quantum architectures, such as flip-flop processes with nuclear spins.
However, a crucial ingredient for creating more complex spin structures is the creation of interacting spin systems from individual building blocks. Figure 4a shows a dimer consisting of two closeby FePc-Fe(C6H6) complexes (several more are shown in Fig. 1). A direct advantage of the complex for spin-spin-coupling is the position of the Fe(C6H6) spin at the FePc ligand. This arrangement facilitates coupling with the adjacent complex due to a shorter distance between spins and leads to much larger J coupling than for dimers of pristine FePc26. The coupling scheme is illustrated in Fig. 4b, with the intramolecular coupling as used before and the weaker intermolecular coupling (between ferrimagnets). Treating, for now, the two ferrimagnet complexes as effective spin ½ systems leads to a Hamiltonian of the form26.
| 3 |
Where marks the single-site Zeeman energy of the left and the term of the right complex. Here, is the effective magnetic dipolar coupling between two spins.
To investigate the interacting spin system, we perform ESR measurements on one of the spins for varying tip magnetic field . Since contributes in a Zeeman-like way, but only to one of the complexes26, it allows to tune the coupled spin system through an avoided level crossing as shown in Fig. 4c. Here, we depict the energy level diagram of two coupled spin-½ as a function of and . We additionally highlight the dominating ESR transitions below ( and ) and above ( and ) the point of no detuning .
At the latter, the single-site Zeeman energies for the complex on the left () and right () are equal. These kinds of measurements have been realized before on regular coupled spin ½ systems, i.e., Ti atoms48,49 and pristine FePc26. We show in the following that also the more complex ferrimagnetic spins can be coupled as well and in fact constitute more flexible magnetic building blocks. An ESR spectrum measured on the Fe(C6H6) site within the left complex reveals four ESR peaks (Fig. 4d), corresponding to those labeled in Fig. 4c. Figure 4e shows an ESR map measurement as a function of frequency and tip setpoint current . The latter is in good approximation 46 and can tune the frequency and intensity of the four ESR transitions. The evolution shows good agreement with the predictions of an effective two-spin model (Fig. 4e, see Supplementary Section 10) which suggests that the two complexes can be described as two coupled effective spin ½ with intermolecular coupling strength . This makes them suitable building blocks for larger spin systems. However, a notable difference compared to previous works on atomic and molecular spins is that is negative, constituting a ferromagnetic (FM) exchange interaction between the two effective spins. This can be rationalized when considering the internal structure of each complex (Fig. 4b). Here, the coupling between all adjacent spins remains antiferromagnetic, as it is most frequently found for superexchange interaction. However, the two ferrimagnet complexes can also be coupled antiferromagnetically (AFM, ) when positioning the two Fe(C6H6) centers in each complex closest together (Supplementary Figs. 18, 19 and Supplementary Section 10).
To capture minute details in the position and intensity of the peaks (see Supplementary Section 10), we employ a simulation of the full four-spin system (Fig. 4f), where all magnetic dipolar couplings are calculated based on the distances between all spin centers (Supplementary Fig. 20). The full four-spin model does not show significant deviations from the two-spin model for both the FM case in Fig.4f and the AFM case discussed in Supplementary Fig. 19. Only the exchange coupling differs for the effective two-spin model compared to a four-spin model, since it now incorporates the different spin structure of the complexes. Thus, we conclude that the complexes can be effectively treated as spin ½ systems and that they can serve as fundamental building blocks for larger spin structures.
Discussion
Our measurements highlight how both atomic and molecular building blocks can be combined to tailor new molecular spin structures. A crucial ingredient is the bond formation between the Fe and the benzene ring, which, instead of using tip-assisted on-surface assembly, could also be created by a thermally driven on-surface self-assembly. This was already successfully demonstrated on metal substrates50,51 by employing thermal sample annealing. We also expect that these systems could be directly synthesized in fully chemically derived molecular ferrimagnets.
In particular, the intricate connection between the emerging correlated spin state of the complex and the improvement in its dynamic properties reveals a key strategy to protect spins on surfaces from the main source of relaxation, i.e., scattering by nearby electron spins. Combining this protection with an increased MgO layer thickness29 and a remote spin readout28 could allow to further enhance and . For a remote spin readout, the demonstration of efficient spin-spin coupling (Fig. 4) is an important prerequisite, which also offers the opportunity to design larger spin structures or quantum simulators with both AFM and FM coupling. Lastly, we note that the concept of strongly coupled quantum ferrimagnets employed here is not unique to assembled molecular spins on surfaces: The ingredients for the spin system are quite universal and could also be realized by spins in other quantum architectures, including quantum dots, color centers or fully chemically derived molecular ferrimagnets.
Methods
Sample preparation
All sample preparation was carried out in situ at a base pressure of <5 × 10−10 mbar. The Ag(001) surface was prepared through several cycles of argon-ion sputtering and annealing through e-beam heating. For MgO growth, the sample was heated up to 510 °C and exposed to an Mg flux for 10 min in an oxygen environment at 10−6 mbar, leading to an MgO coverage of ~50% and layer thicknesses ranging from two to five monolayers. FePc was evaporated onto the sample held at room temperature using a home-built Knudsen cell at a pressure of 9 × 10−10 mbar for 90 s. Electron-beam evaporation of Fe was carried out for 21 s onto the cold sample. We determined the thickness of MgO layers through point-contact measurements on single Fe atoms29. All experiments were carried out using a Unisoku USM1600 STM inside a home-built dilution refrigerator with a base temperature of 50 mK. An effective spin temperature of ~300 mK was estimated from ESR measurements of Fe dimers. Here, the intensities of ESR peaks of the coupled electron spin states depend on temperature52, which we take as an estimate of the Boltzmann distribution in the experiment.
Pulsed electron spin resonance measurements
Spin-polarized tips were prepared by picking up individual Fe atoms from the surface. The spin polarization was tested through the asymmetry in dI/dV spectra of FePc with respect to voltage polarity. Magnetic tips showing a high spin contrast were subsequently tested in continuous-wave (CW) ESR-STM measurements. The RF voltage was applied to the tip side of the junction using an RF generator (Rohde & Schwarz SMB100B). The RF voltage was combined with the DC tunnel bias using a Diplexer (Marki Microwave MDPX-0305). Note that while the bias voltage was applied to the STM tip, all bias signs were inverted in the manuscript to follow the conventional definition of bias voltage with respect to the sample bias.
For pulsed ESR measurements, we followed the manipulation scheme introduced in ref. 27. An arbitrary waveform generator (Zurich Instruments HDAWG) gated the output of the RF generator to generate the desired pulsed ESR scheme. We used a digital lock-in amplifier (Stanford Research Systems SR860) to read out the ESR signal using an on/off modulation scheme at 323 Hz. During the Rabi or Ramsey measurements, the feedback loop was set to low-gain values. In total, these measurements took ~3 s per data point, so that a typical Rabi or Ramsey measurement, including three iterations of 100 points, took around 15 min. All measurements were conducted by using schemes described in ref. 27, in which a DC background current is applied in lock-in A and B cycles, while RF pulses were only applied in lock-in A cycles. Linear backgrounds that originate from an additional rectification for increasing pulse lengths27 have been subtracted.
For pump-probe measurements in Fig. 3 and Supplementary Fig. 14, we altered the tunnel current by changing the bias voltage to zero once the feedback loop had been disengaged. Consequently, all measurements have been performed at constant tunnel conductance and constant tip-atom distance, which in particular helped to keep the influence of the tip field constant. For each measurement, the lockin A cycles include both pump and probe pulse voltages while the lock-in B cycles are left empty, with no DC voltages applied.
DFT calculations
All periodic-cell density functional theory (DFT) calculations were performed using plane-wave basis and pseudopotentials in Quantum Espresso version 7.153,54. All pseudopotentials use the Perdew–Burke–Ernzerhof (PBE) parametrization for the exchange and correlation potential55. We mimic the experiment by placing the Fe on top of MgO (100), which is supported by a silver (100) substrate. The bulk lattice constants for silver and MgO with PBE are aAg = 4.16 Å and aMgO = 4.25 Å, which results in a lattice mismatch of about 2% (experimental value: 2.9%55). To construct the surface slab, we used four layers of Ag with the lateral lattice constant fixed to that of the PBE bulk silver and added up to two layers of MgO. We created lateral supercells of about 20 × 20 Å and 15 Å of vacuum were used to pad the cell in z-direction. All calculations used a k-grid equivalent to 15 × 15 × 1 k-points of the 1 × 1 × 1 unit cell. All 3d plots in the main text were created using OVITO ref. 56. To account for dispersive forces we used the revised VV10 functional in all calculations57. We used non-spin-polarized (similar to open-shell restricted Hartree-Fock) calculations for the calculation of the crystal-field splittings and molecular orbital order, as well as spin-polarized (similar to unrestricted Hartree-Fock) calculations to confirm the spin-states of the adsorbates. The calculated STM topographic images (Supplementary Fig. 22) use the Tersoff-Hamann approach as implemented in the STMpw code58. They show generally good agreement with respect to the individual features of Fe and FePc in the dimer at the respective biases.
Supplementary information
Source data
Acknowledgements
P.W. acknowledges funding from the Emmy Noether Program of the DFG (WI5486/1-1), financing from the Baden-Württemberg Foundation Program on Quantum Technologies (Project AModiQuS) and support from the Centre for Integrated Quantum Science and Technology (IQST). P.G. and P.W. acknowledge financial support from the Hector Fellow Academy (Grant No. 700001123). C.W. and J.R. acknowledge support from the Institute for Basic Science (IBS-R027-D1). The authors acknowledge the use of Blender for rendering the images in Fig. 1b and Supplementary Fig. 2b.
Author contributions
P.W. and W.H. conceived the research. W.H., M.S., P.G., K.H.A.-Y., D.S., C.S., W.W. and P.W. set up the experiment and conducted the measurements. W.H., M.S., P.G., K.H.A.-Y. and P.W. analyzed the experimental data. C.W. performed the DFT calculations. J.R. participated in the modeling of the spin systems. W.H., M.S., P.G., K.H.A.-Y., C.S., W.W. and P.W. discussed the results. W.H. and P.W. wrote the manuscript with input from all authors. W.W. and P.W. supervised the project.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data supporting the findings of this study are available in the article. Source data are provided with this paper.
Code availability
The code for the DFT calculated STM images is available from Zenodo58 and the code for DFT calculations is available from Quantum Espresso page (https://www.quantum-espresso.org/).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-60409-w.
References
- 1.Krantz, P. et al. A quantum engineer’s guide to superconducting qubits. Appl. Phys. Rev.6, 021318 (2019).
- 2.Shiddiq, M. et al. Enhancing coherence in molecular spin qubits via atomic clock transitions. Nature531, 348–351 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Petta, J. R. et al. Coherent manipulation of coupled electron spins in semiconductor quantum dots. Science309, 2180–2184 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Trif, M., Troiani, F., Stepanenko, D. & Loss, D. Spin-electric coupling in molecular magnets. Phys. Rev. Lett.101, 217201 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Bertaina, S. et al. Quantum oscillations in a molecular magnet. Nature453, 203–206 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Ardavan, A. et al. Will spin-relaxation times in molecular magnets permit quantum information processing? Phys. Rev. Lett.98, 057201 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Troiani, F. & Affronte, M. Molecular spins for quantum information technologies. Chem. Soc. Rev.40, 3119–3129 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Clair, S. & de Oteyza, D. G. Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis. Chem. Rev.119, 4717–4776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra, S. et al. Observation of fractional edge excitations in nanographene spin chains. Nature598, 287–292 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Song, S. et al. Highly entangled polyradical nanographene with coexisting strong correlation and topological frustration. Nat. Chem.16, 938–944 (2024). [DOI] [PubMed] [Google Scholar]
- 11.Li, C. et al. Strong signature of electron-vibration coupling in molecules on Ag(111) triggered by tip-gated discharging. Nat. Commun.14, 5956 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan, Q. et al. Bottom-up synthesis and characterization of porous 12-atom-wide armchair graphene nanoribbons. Nano Lett.24, 10718–10723 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Vegliante, A. et al. Tuning the spin interaction in nonplanar organic diradicals through mechanical manipulation. ACS Nano18, 26514–26521 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drost, R., Kezilebieke, S., Lado, J. L. & Liljeroth, P. Real-space imaging of triplon excitations in engineered quantum magnets. Phys. Rev. Lett.131, 086701 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Wang, Y. et al. Thermal spin-crossover and temperature-dependent zero-field splitting in magnetic nanographene chains. Preprint at arXiv:2407.20996 (2024).
- 16.Villalobos, F. et al. Globally aromatic odd-electron π-magnetic macrocycle. Chem11, 102316 (2025).
- 17.Kovarik, S. et al. Spin torque–driven electron paramagnetic resonance of a single spin in a pentacene molecule. Science384, 1368–1373 (2024). [DOI] [PubMed] [Google Scholar]
- 18.Gao, Y. et al. On-surface synthesis of a doubly anti-aromatic carbon allotrope. Nature623, 977–981 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong, Q. et al. Constructing covalent organic nanoarchitectures molecule by molecule via scanning probe manipulation. Nat. Chem.13, 1133–1139 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann, S. et al. Electron paramagnetic resonance of individual atoms on a surface. Science350, 417–420 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Chen, Y., Bae, Y. & Heinrich, A. J. Harnessing the quantum behavior of spins on surfaces. Adv. Mater.35, 2107534 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Kovarik, S. et al. Electron paramagnetic resonance of alkali metal atoms and dimers on ultrathin MgO. Nano Lett.22, 4176–4181 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Reale, S. et al. Electrically driven spin resonance of 4f electrons in a single atom on a surface. Nat. Commun.15, 5289 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czap, G. et al. Direct electrical access to the spin manifolds of individual lanthanide atoms. ACS Nano19, 3705–3713 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willke, P. et al. Coherent spin control of single molecules on a surface. ACS Nano15, 17959–17965 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Zhang, X. et al. Electron spin resonance of single iron phthalocyanine molecules and role of their non-localized spins in magnetic interactions. Nat. Chem.14, 59–65 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Yang, K. et al. Coherent spin manipulation of individual atoms on a surface. Science366, 509–512 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Wang, Y. et al. An atomic-scale multi-qubit platform. Science382, 87–92 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Paul, W. et al. Control of the millisecond spin lifetime of an electrically probed atom. Nat. Phys.13, 403–407 (2017). [Google Scholar]
- 30.Willke, P. et al. Probing quantum coherence in single-atom electron spin resonance. Sci. Adv.4, eaaq1543 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sellies, L. et al. Single-molecule electron spin resonance by means of atomic force microscopy. Nature624, 64–68 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Čenčariková, H. & Strečka, J. Unconventional strengthening of the bipartite entanglement of a mixed spin-(1/2,1) Heisenberg dimer achieved through Zeeman splitting. Phys. Rev. B102, 184419 (2020). [Google Scholar]
- 33.Baumann, S. et al. Origin of perpendicular magnetic anisotropy and large orbital moment in Fe atoms on MgO. Phys. Rev. Lett.115, 237202 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Crabtree, R. H. The Organometallic Chemistry of the Transition Metals (John Wiley & Sons, 2009).
- 35.Huttmann, F., Schleheck, N., Atodiresei, N. & Michely, T. On-surface synthesis of sandwich molecular nanowires on graphene. J. Am. Chem. Soc.139, 9895–9900 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Mathialagan, S. K. et al. On-surface synthesis of organolanthanide sandwich complexes. Adv. Sci.11, 2308125 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briganti, M. et al. Mixed-sandwich titanium(III) qubits on Au(111): electron delocalization ruled by molecular packing. Nano Lett.22, 8626–8632 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ternes, M. Spin excitations and correlations in scanning tunneling spectroscopy. N. J. Phys.17, 063016 (2015). [Google Scholar]
- 39.Wolf, C., Delgado, F., Reina, J. & Lorente, N. Efficient ab initio multiplet calculations for magnetic adatoms on MgO. J. Phys. Chem. A124, 2318–2327 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Ormaza, M. et al. Efficient spin-flip excitation of a nickelocene molecule. Nano Lett.17, 1877–1882 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Ternes, M. Probing magnetic excitations and correlations in single and coupled spin systems with scanning tunneling spectroscopy. Prog. Surf. Sci.92, 83–115 (2017). [Google Scholar]
- 42.Muenks, M., Jacobson, P., Ternes, M. & Kern, K. Correlation-driven transport asymmetries through coupled spins in a tunnel junction. Nat. Commun.8, 14119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peres, A. Separability criterion for density matrices. Phys. Rev. Lett.77, 1413–1415 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Lado, J. L., Ferrón, A. & Fernández-Rossier, J. Exchange mechanism for electron paramagnetic resonance of individual adatoms. Phys. Rev. B96, 205420 (2017). [Google Scholar]
- 45.Phark, S.-h et al. Electric-field-driven spin resonance by on-surface exchange coupling to a single-atom magnet. Adv. Sci.10, 2302033 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, K. et al. Tuning the exchange bias on a single atom from 1 mT to 10 T. Phys. Rev. Lett.122, 227203 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Loth, S., Etzkorn, M., Lutz, C. P., Eigler, D. M. & Heinrich, A. J. Measurement of fast electron spin relaxation times with atomic resolution. Science329, 1628–1630 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Veldman, L. M. et al. Free coherent evolution of a coupled atomic spin system initialized by electron scattering. Science372, 964–968 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Kot, P. et al. Electric control of spin transitions at the atomic scale. Nat. Commun.14, 6612 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang, W. et al. Probing magnetism in self-assembled organometallic complexes using Kondo spectroscopy. ACS Nano19, 1190–1197 (2025). [DOI] [PubMed] [Google Scholar]
- 51.Pacchioni, G. E. et al. Two-orbital Kondo screening in a self-assembled metal–organic complex. ACS Nano11, 2675–2681 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Choi, T. et al. Atomic-scale sensing of the magnetic dipolar field from single atoms. Nat. Nanotechnol.12, 420–424 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter21, 395502 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Giannozzi, P. et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter29, 465901 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Dal Corso, A. Pseudopotentials periodic table: from H to Pu. Comput. Mater. Sci.95, 337–350 (2014). [Google Scholar]
- 56.Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the open visualization tool. Model. Simul. Mater. Sci. Eng.18, 015012 (2010). [Google Scholar]
- 57.Sabatini, R., Gorni, T. & de Gironcoli, S. Nonlocal van der Waals density functional made simple and efficient. Phys. Rev. B87, 041108 (2013). [Google Scholar]
- 58.Lorente, N. & Robles, R. STMpw. Zenodo10.5281/ZENODO.3581159 (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in the article. Source data are provided with this paper.
The code for the DFT calculated STM images is available from Zenodo58 and the code for DFT calculations is available from Quantum Espresso page (https://www.quantum-espresso.org/).