Abstract
The cell wall of Candida albicans not only is the structure in which many biological functions essential for the fungal cells reside but also is a significant source of candidal antigens. The major cell wall components that elicit a response from the host immune system are proteins and glycoproteins, the latter being predominantly mannoproteins. Both the carbohydrate and protein moieties are able to trigger immune responses. Although cell-mediated immunity is often considered to be the most important line of defense against candidiasis, cell wall protein and glycoprotein components also elicit a potent humoral response from the host that may include some protective antibodies. Proteins and glycoproteins exposed at the most external layers of the wall structure are involved in several types of interactions of fungal cells with the exocellular environment. Thus, coating of fungal cells with host antibodies has the potential to influence profoundly the host-parasite interaction by affecting antibody-mediated functions such as opsonin-enhanced phagocytosis and blocking the binding activity of fungal adhesins for host ligands. In this review, the various members of the protein and glycoprotein fraction of the C. albicans cell wall that elicit an antibody response in vivo are examined. Although a number of proteins have been shown to stimulate an antibody response, for some of these species the response is not universal. On the other hand, some of the studies demonstrate that certain cell wall antigens and anti-cell wall antibodies may be the basis for developing specific and sensitive serologic tests for the diagnosis of candidasis, particularly the disseminated form. In addition, recent studies have focused on the potential for antibodies to cell wall protein determinants to protect the host against infection. Hence, a better understanding of the humoral response to cell wall antigens of C. albicans may provide the basis for the development of (i) effective procedures for the serodiagnosis of disseminated candidiasis and (ii) novel prophylactic (vaccination) and therapeutic strategies for the management of this type of infection.
The dimorphic fungus Candida albicans is both a commensal and opportunistic pathogen of humans. Predisposing factors for candidiasis include immunosuppressive, antibiotic, and cytotoxic therapies; the presence of intravenous catheters and indwelling devices; very low birth weight; AIDS; diabetes; and drug abuse. Depending on the underlying host defect, this microorganism is able to cause a variety of infections that range from mucosal candidiasis to life-threatening disseminated candidiasis (15, 61, 214).
C. albicans pathogenicity also depends on a complex array of microorganism-related putative virulence factors. These include the yeast-to-mycelium transition, antigenic variability, phenotypic switching, adhesion to host cells and tissues, cell surface hydrophobicity, molecular mimicry, and production of extracellular enzymes (24–26, 50, 64, 113, 233, 246, 277). Most of the biological functions related to pathogenicity and virulence reside in the fungal cell wall, whose main features are presented later in greater detail.
As the outermost cellular structure, the cell wall plays an essential role in the interactions with the host, including the triggering and modulation of the anti-Candida host immune responses, which appear to rely on a complex interplay between natural and adaptive immunity (89). Fungal antigens may stimulate specific cell-mediated and humoral immune responses. The importance of cellular defense mechanisms for protection against fungal infections is supported by the clinical observation that most invasive fungal infections occur in patients with defective cellular immunity (81, 155). The role of antibodies in resistance to candidiasis is poorly understood, and reports exist in the literature providing evidence both in favor of and against the importance of antibody immunity as a main host defense mechanism against fungi. However, several authors have suggested the need to reexamine the contribution of antibodies in fungal infections (28, 60, 185, 189), and there is renewed interest in the study of the host antibody response to Candida and the possibility of immunointervention as a feasible prophylactic or therapeutic approach for the management of candidiasis.
Another controversial aspect is the laboratory diagnosis of Candida infections. The diagnosis of invasive candidiasis is usually difficult to establish by clinical criteria; therefore, culture techniques and serodiagnostic tests for antigen and antibody detection have been used as laboratory aids to diagnosis. However, blood cultures for Candida species generally exhibit low sensitivity (129, 214, 240), and the tests for determination of marker antigens need further fine-tuning to improve their sensitivity and specificity so that they will be valuable in guiding clinical treatment decisions (203, 238, 240). Likewise, standard immunological tests to detect anti-Candida antibodies usually have low specificity and/or sensitivity (i.e., in most cases they failed to discriminate between disseminated and superficial candidiasis), since they mainly recognize antibodies against Candida cell wall mannan, which are ubiquitous in human sera (129, 306, 318). Furthermore, the crude preparations of candidal antigens cannot be standardized enough to allow good test reproducibility among laboratories (129). A simple, reliable, and easy-to-perform serological assay for the diagnosis of systemic Candida infections is still not available but is urgently needed. In this context, the detection of defined fungal antigens and/or antibodies to such antigens may provide a suitable procedure for diagnosis of invasive candidiasis.
In this review, we will focus specifically on the antibody response to defined protein and glycoprotein C. albicans cell wall components in humans and in animal models of experimentally induced candidiasis (Table 1), with special emphasis on the implications for developing novel diagnostic, prophylactic, and therapeutic techniques for candidiasis. Since these proteins and glycoproteins are integral candidal components, we will first consider their main biological features in the context of their natural environment within the fungal cell. Readers who wish to know more about basic aspects of cell wall biology are referred to a number of excellent recent reviews discussing the composition and structure, physiological role, and antigenic composition of the cell wall of C. albicans (26, 36, 41, 62, 88, 210, 233, 259, 260, 265, 266).
TABLE 1.
Antibody response to C. albicans cell wall proteins and mannoproteins
| Componenta | Immunoglobulin isotypeb | Epitope | Antibodies detected in:
|
Comments | Reference(s) | ||
|---|---|---|---|---|---|---|---|
| Humans
|
Animal models | ||||||
| Healthy | Patients | ||||||
| Carbohydrates | |||||||
| Mannan | IgA, IgE, IgG, IgM | Multiple | + | + | + | Antigen-based serodiagnostic test; strain serotyping is defined by specificity of antibodies to fine structural antigenic motifs of mannan | 6, 12, 19, 37, 62, 104, 111, 118, 128, 129, 151, 154, 211, 220, 238, 240, 253, 303 |
| O-linked | + | + | 112 | ||||
| β-1,2 linked | + | + | 119, 231 | ||||
| Various, acid stable | + | 106 | |||||
| HMW-MP | IgG3 | Unknown | + | + | 119, 231 | ||
| 14–18-kDa moiety | IgG3? | β-1,2 linked | + | + | Antibodies cross-react with β-1,2 linked mannooligosaccharides of mannan | 119, 231 | |
| Blood group I-related antigens | IgM | Carbohydrate other than mannose | + | + | Antibodies present as part of response to blood group antigens and are presumed to be independent of the fungus | 202 | |
| Proteins | |||||||
| SAP | IgG, IgA | Unknown | + | + | + | Elevated antibody levels in patients may be due to contaminating mannan | 37, 123, 172, 205, 236, 245 |
| Hsp90 | IgG, IgM | Unknown, LKVIRK | − | + | Antibodies associated with recovery from infection | 186, 190–193 | |
| Hsp70 | IgG | Unknown | + | + | + | Antibodies may recognize epitopes among microbes and self | 46, 47, 152, 161 |
| Heat shock mannoproteins | IgA | Polysaccharide | + | Apparently induce secretory immune response during mucosal candidiasis | 226 | ||
| Enolase | IgG, IgE | Unknown | NDc | + | + | Antigen-based serodiagnostic test; species differences in epitopes recognized | 84, 124–126, 183, 199, 252, 253, 281, 286, 305, 306, 309, 310 |
| PGK | IgG, IgE | Unknown | Not a universal immunogen/allergen | 124 | |||
| GAPDH | IgM, IgG? | Unknown | ND | + | 98 | ||
| mp58 | IgG, IgM? | Unknown | ND | + | + | Positive in patients with systemic but not superficial candidiasis | 169, 262 |
HMW-MP, high-molecular-mass mannoproteins.
Not all Ig isotypes may have been tested.
ND, not detected.
C. ALBICANS CELL WALL
The cell wall may be envisaged as a dynamic and plastic, multilayered structure located external to the plasma membrane. The presence of the wall is essential to several aspects of the biology and, as noted above, the pathogenicity of C. albicans. Thus, the cell wall is the structure responsible for maintaining the shape that characterizes each growth form (yeast and hyphae) of the fungus, and it acts as a permeability barrier that protects the protoplast against physical and osmotic injuries. In addition, the cell wall plays nutritional roles and is the structure that mediates the initial interaction between the microorganism and the environment.
Composition, Structure, and Functions
The major components (80 to 90%) of the cell wall of C. albicans are carbohydrates: (i) mannan or polymers of mannose covalently associated with proteins to form glycoproteins, also referred to as mannoproteins; (ii) β-glucans that are branched polymers of glucose containing β-1,3 and β-1,6 linkages; and (iii) chitin, which is an unbranched homopolymer of N-acetyl-d-glucosamine (GlcNAc) containing β-1,4 bonds. Proteins (6 to 25%) and lipids (1 to 7%) are present as minor wall constituents (26, 36, 265, 266). Yeast cells and germ tubes are similar in their cell wall composition, although the relative amounts of β-glucans, chitin, and mannan may vary with the morphology (26, 259).
β-Glucans and chitin are the structural components of the wall. Quantitatively, β-glucans contribute to 47 to 60% by weight of the cell wall. Chitin is a minor (0.6 to 9%) but structurally important component, particularly associated with cell-cell connections in the ring between parent and daughter cells, in the bud scar, and in the septa of dividing independent cells.
Mannose polymers do not exist as such but are found in covalent association with proteins (mannoproteins) (26, 36, 259, 265, 266). They are the main material of the amorphous cell wall matrix in which the structural polymers (β-glucans and chitin) are embedded, and they represent 40% of the total cell wall carbohydrate. The mannoproteins of C. albicans share several general features with the mannoproteins of Saccharomyces cerevisiae, a model organism that is one of the most thoroughly investigated yeasts in this regard. The structure of the carbohydrate component of C. albicans mannoproteins is described in detail below. Although mannose is the main monosaccharide component of C. albicans cell wall glycoproteins, there is evidence that other sugar residues are also present in some wall glycoprotein species (see below).
Electron microscopy studies have shown the existence of several layers in the cell wall of C. albicans. The structural appearance is variable and seems to be related to the strain examined, growth conditions, morphology, and the experimental conditions used to prepare the specimens (26, 36, 233). Several cytochemical and cytological studies indicate that the cell wall layering is essentially due to the distribution of mannoproteins at various levels within the wall structure (36). The microfibrillar polysaccharides, glucan and chitin, appear to be more concentrated in the inner cell wall layer, adjacent to the plasma membrane. Proteins and glyco(manno)proteins fill the network of structural polymers and appear to be dominant in the outermost cell wall layer, which has a fibrillar or flocculent aspect (36, 259).
The different cell wall components interact with each other to give rise to the overall architecture of the cell wall. Besides hydrogen and hydrophobic bonds, there is also experimental evidence for the presence of covalent linkages between different components (249, 260). In this context, Surarit et al. (288) reported the presence of glycosidic linkages between glucan and chitin in the nascent wall of C. albicans. On the other hand, recent evidence indicates that mannoproteins may establish covalent associations with β-1,3- and β-1,6-glucans through phosphodiester linkages (139, 140, 251), suggesting that such associations may play a key role in configuring the final cell wall structure characteristic for each growth form (yeast and mycelium) of C. albicans (249, 257–260). Finally, interactions between mannoproteins and chitin also appear to exist in the wall of C. albicans cells (176). Hence, it is possible that the so-called layering, as concluded mainly from electron microscopy observations, is the result of quantitative differences in the proportions of the individual wall components (β-glucans, chitin, and mannoproteins) in each layer rather than absolute qualitative differences (212). Nevertheless, since proteins and mannoproteins appear to be involved in a variety of essential processes for C. albicans such as morphogenesis and several pathogenicity-related aspects, and since they may be responsible for the hydrophobic or hydrophilic status of the cell surface (24–26, 36, 65, 66, 114–116, 121, 162, 249, 257–260), an asymmetric distribution of wall protein and glycoprotein constituents as a consequence of the physiologic role played by each moiety should not be ruled out (41).
A complex array of protein-containing components has been solubilized from isolated cell wall preparations and from intact cells of both candidal growth forms by different treatments (29–31, 33, 39, 71, 162, 176, 178, 227–229, 285). Analysis of these components has revealed quantitative and qualitative differences in the protein composition of yeast and mycelial cell walls. Some components have been characterized as high-molecular-mass (>120-kDa) mannoproteins that appear to be covalently linked to the structural polysaccharides. These species, which contain large amounts of carbohydrate and consequently could be major elicitors of anti-candidal host immunity, seem to play also an important and active role in modulating the organization of the different cell wall constituents to obtain the final supramolecular structure of the wall that is specific for each morphologic form of C. albicans (31, 34, 70, 95, 96, 164, 167, 179, 180, 228, 259, 285). Several studies have identified 20 to 40 polypeptide species in the medium-to-low-molecular-mass (from 80 to 15 kDa) range (33, 39, 71). Most molecules that appear to be present in the outermost wall layers and that exhibit receptor-like activities and adhesin properties are medium- and low-molecular-mass species (41). The high-molecular-mass species seem to be homogeneously distributed on the cell surface (31, 34, 95), whereas protein and mannoprotein moieties that may represent receptors for different host ligands exhibit clustering or asymmetric cell surface distribution (32, 163, 181). This further supports the contention, noted above, that differences in the distribution or topological location of wall mannoproteins appear to be related to the distinct functional or structural role played by individual species.
Variability and Dynamics of Cell Wall Antigens
The expression, distribution, chemical characteristics, and behavior observed in vitro and the biological properties of C. albicans cell wall-bound proteins and glycoproteins appear to be dependent on multiple environmental and organism-related factors. These include growth conditions, growth state, morphology of the cells, strain and serotype, phenotypic switching, cell surface hydrophobic or hydrophilic status, and the nature of the biological specimens (intact cells or isolated wall preparations) that are subjected to analysis (3, 5, 14, 21, 29, 35, 59, 96, 114, 115, 120, 122, 164, 167, 179, 229, 233, 278). Particularly, differential expression of antigens at the cell surface level of both C. albicans growth forms has received considerable attention from numerous research groups. A number of different possibilities have been suggested to explain the changes in the cell wall composition observed during the morphologic transition. Possible explanations are as follows: (i) form-specific components reflect de novo synthesis of proteins; (ii) topological rearrangement of preexisting cell wall constituents occurs; (iii) differences are due to major quantitative variations in the composition of surface components on both fungal morphologies; (iv) changes in the glycosylation pattern of the same molecule occur depending on the cell morphology considered; and (v) a combination of all the above could occur (21, 31, 33, 42, 215, 287, 301, 308). Since the ability to grow in the filamentous form appears to be one of the important virulence traits in C. albicans, and since the presence of hyphal elements in deep tissues is related to infection (214), special emphasis has been directed towards the characterization of hypha-specific moieties and their possible use as antigen markers in the serodiagnosis of systemic candidiasis (208, 209, 230, 235, 307). In this context, the ideal marker antigen should be expressed by all C. albicans strains under all different environmental conditions. However, since the cell wall appears to be a highly dynamic “organelle” that exhibits both the capacity of differentially expressing variable constituents useful for switching between the commensal and pathogenic lifestyles and the capacity for modulating and/or evading the immune host defense barriers, this variability must be taken into consideration when trying to identify potential marker antigens of infection. Therefore, this variability accounts, at least partly, for the poor specificity and sensitivity displayed by some of the methods currently available for immunodiagnosis of systemic candidiasis.
Structural peculiarities in the cell wall mannan (see below) are responsible for the antigenic specificity of C. albicans serotype A and B strains (111). Antigenic variability in the epitopes that accounts for this specificity has been detected also. In fact, there is evidence suggesting that both serotypes are determined phenotypically rather than genotypically. Poulain et al. (234) showed that serotype B yeast cells and germ tubes express antigen 6 (the factor characteristic for serotype A strains) in infected tissues and that germ tubes of serotype B strains, but not the mother yeast cells from which the germ tubes emanate, express the antigen in vitro. Kobayashi et al. (144) have shown that serotype A strains behave as serotype B strains at pH 2 since they do not express both factors 5 and 6, the latter being specific for serotype A C. albicans cells. Barturen et al. (14) reported that the expression of antigens responsible for serotype A specificity is modulated by the pH of the culture medium. These observations may be related to the activity of the enzyme 1,2-β-mannosyltransferase, which appears to be suppressed or lowered at low pH (148).
This antigenic variability detected for epitopes conferring serotype specificity is also extended to other polysaccharide epitopes. The surface antigen makeup of C. albicans is in general variable both in vitro and in vivo, as already indicated. Recently, De Bernardis et al. (56) studied the expression of a polysaccharide epitope in yeast and mycelial cells in a model of candidal vaginitis in rats. This epitope is shared by a family of strongly antigenic cell wall mannoproteins including a 65-kDa component, which is recognized as a main target of cell-mediated anti-Candida immunity in humans (99, 294, 296). They showed that this epitope is efficiently incorporated in all layers of the cell wall and abundantly expressed on the yeast cell surface soon (1 h) after the vaginal challenge but is no longer expressed on the surface of most of the cells harvested after 24 h of intravaginal growth. This represents the first clear indication of cell surface antigenic variation of C. albicans during active infection. Since mannans are strongly immunogenic and immunomodulatory constituents of C. albicans, changes associated with the composition of these wall polymers and epitope expression on the cell surface may theoretically represent a mechanism that modulates the host immune response.
CARBOHYDRATE COMPONENT
As stated above, β-glucans, chitin, and mannan are the three major polysaccharide components of the cell wall of C. albicans. Although β-glucans are present in greater abundance than mannan in the wall of this fungal species (44), they are immunologically less active (233).
Most of the mannan is found as large N-linked polymers containing several hundred mannose residues associated with high-molecular-mass species, yet smaller N-linked moieties and/or O-linked mannooligosaccharides are associated with smaller glycoproteins. Antibodies to all these carbohydrate immunodeterminants are readily detectable in serum samples. Although mannose appears to be the major monosaccharide constituent, the potential for other complex carbohydrates that may be also present in candidal cell wall glycoproteins to elicit an antibody response has been reported.
Mannan
Initial studies focusing on the characterization of antigen patterns for the serologic classification or identification of medically important Candida species demonstrated that cell wall mannan is the main candidal antigen responsible for the specificity of the different serologic reactions (91, 111, 282, 302, 303). Consequently, considerable effort has been expended to determine the specific chemical structure of mannan and to define the epitopes present in this wall component that may account for serospecificity (for a review, see reference 210).
As mentioned above, mannan does not exist as such in the wall structure but exists in covalent association with proteins (mannoproteins) (36, 62, 265, 266). However, the term “mannan” has also been used to refer to the main soluble immunodominant component present in the outer cell wall layer of C. albicans. This component, also called phosphomannoprotein or phosphopeptidemannan complex (Fig. 1), contains homopolymers of d-mannose (as the main component), 3 to 5% protein, and 1 to 2% phosphate (239). A detailed structure of this cell wall constituent has emerged from several studies (10, 144–149, 267–274). The mannose polymers are linked to proteins by N-glycosidic bonds (through two GlcNAc [di-N-acetylchitobiose] units) to asparagine residues and by O-glycosidic, alkali-labile linkages to threonine or serine residues. The N-glycosidically linked carbohydrate has a backbone chain of α-1,6-linked mannopyranosyl residues with oligosaccharide branches containing mannopyranosyl residues with α-1,2, α-1,3, β-1,2, β-1,6, and single α-1,6-linked mannose units and phosphodiester bonds (87, 90, 145, 150, 271). Single mannose residues and short, unbranched mannose oligosaccharides constitute the O-glycosidically linked sugar component (222).
FIG. 1.
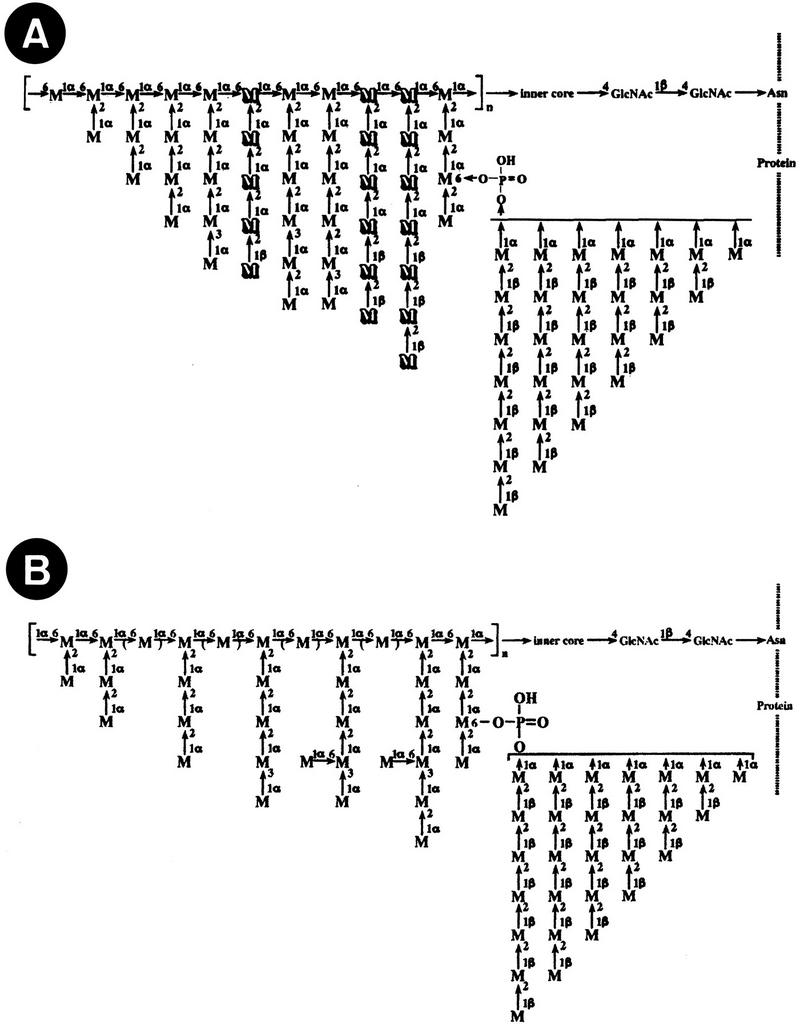
Structures of C. albicans mannan (M, d-mannopyranose units; GlcNAc, N-acetylglucosamine units; Asn, asparagine residue). (A) Representative structure of serotype A strain mannan. Side chains shown by the outlined letter M indicate putative serotype A-specific structures containing β-1,2-linked mannopyranosyl residues at the nonreducing terminal (i.e., antigenic factor 6); other side chains depicted are common in mannans from both serotypes. Reprinted from reference 267 with permission of the publisher. (B) Representative structure of serotype B strain mannan. Single mannose units linked by α-1,6 bonds to the oligosaccharide branches containing α-1,2- and α-1,3-linked mannopyranosyl residues make a serotype B-specific epitope (i.e., antigenic factor 4); α-1,6-linked mannose residues in brackets indicate the partial absence of these units in mannan from C. albicans B cells. Reprinted from reference 271 with permission. Structures corresponding to other antigenic factors present in mannans from both C. albicans serotypes are described in the text.
The antigenic specificity of serotypes A and B of C. albicans, described by Hasenclever and Mitchell (111), appears to be determined by structural peculiarities of the carbohydrate moiety of mannan present in the cell wall of strains belonging to a particular serotype. In this context, Kobayashi et al. (147) showed that oligomannosyl side chains containing both β-1,2 and α-1,2 linkages were serotype A-specific epitopes (factor 6) whereas β-1,2-linked oligomannosyl side chains, which are attached to phosphate, serve as major common epitopes (factor 5) for both C. albicans serotypes (267). Thus, individual oligomannosides obtained by depolymerization of the phosphopeptidemannan complex, represent epitopes that have been described as the basis of rabbit polyclonal antibody specificities (267). Further characterization of epitopes recognized by antisera to other C. albicans antigenic factors revealed that antiserum to factor 1 is directed to the O-linked mannose chains of the cell wall mannoproteins whereas the epitope recognized by antisera to factor 9 is the α-1,6-linked mannose backbone of the outer chain of the N-linked oligosaccharide moiety (10). Single mannose units linked by α-1,6 bonds to the oligosaccharide branches containing α-1,2- and α-1,3-linked mannopyranosyl residues make the epitope corresponding to antigenic factor 4 (271). The structures defining the different antigenic factors mentioned above are depicted in Fig. 1.
The mannan component appears to be involved in the adherence of C. albicans cells to macrophages located in the splenic marginal zone and in the subcapsular and medullary sinuses of peripheral lymph node tissue of mice (40, 51, 109, 135–137, 157). Kanbe et al. (136) showed that the mannan portion of the phosphomannoprotein isolated from yeast-form C. albicans cells by β-mercaptoethanol extraction and concanavalin A-agarose affinity chromatography contained the adhesins responsible for attachment to macrophages in both types of tissues. Li and Cutler (157) identified one adhesin site on the acid-labile portion of the mannoprotein as a β-1,2-linked tetramannosyl residue (Ag10G). However, the results indicated that additional adhesins in the phosphomannoprotein complex were involved in the adherence to macrophages. In fact, Kanbe and Cutler (135) later reported evidence of strong adhesin activity in the acid-stable moiety of the phosphomannoprotein complex. The adhesin site implicated is a carbohydrate but is devoid of epitopes for factors 5 and 6 or for Ag10G, which contains the β-1,2-linked oligomannosyl adhesin site. The diagnostic implications of using these carbohydrate epitopes or monoclonal antibodies against them to detect specific antibody or antigen, respectively, in sera from patients with disseminated candidiasis need to be investigated. In any case, immunization of mice with the mannan adhesin fraction implicated in adhesion to macrophages elicits protective antibody responses against experimental Candida infection (106) (see below).
Anti-mannan antibodies have been shown to be ubiquitous in human sera, presumably because the immune system can be stimulated as a result of colonization by C. albicans in the absence of disease (45, 62, 129). In any defined population, levels of anti-mannan antibodies are usually distributed about a mean; sera with the highest levels give positive precipitin tests when tested against cell wall mannan (128). When anti-mannan antibodies were measured in serially drawn sera from neutropenic patients, a frequency distribution plot showed that the levels of antibodies from patients with invasive candidiasis were elevated and tended to skew the normal distribution curve to the right. However, a clear bimodal distribution of these antibody levels was not observed; therefore, after establishing a cutoff value for anti-cell wall mannan antibodies, the best sensitivity value was about 65% (102).
The applications for the diagnosis of disseminated candidiasis by detection of (i) mannan that circulates during infection or (ii) anti-mannan antibodies have been discussed elsewhere (129, 238, 240). Although most of the recent serological tests for invasive candidiasis are directed to the detection of specific circulating cell wall-bound or cytoplasmic candidal antigens other than mannan, special emphasis has been put on the detection of mannan, as a major cell wall antigen, by different procedures including counterimmunoelectrophoresis, radioimmunoassay, enzyme-linked immunosorbent assay (ELISA), and latex agglutination (11, 12, 19, 23, 57, 74, 82, 85, 92, 94, 131, 143, 154, 156, 198, 211, 220, 256, 311–313). Sensitivities of 23 to 100% and specificities of 92 to 100% have been reported for ELISAs for mannan detection (57). Although early studies showed good sensitivity and specificity of latex-agglutination tests (92), recent studies have been less favorable (19, 23, 154), particularly for sera from patients with malignancies (74). A commercial test for mannan detection, the Cand-Tec system (Ramco Laboratories Inc., Houston, Tex.) has been shown to be relatively insensitive (30 to 50%) compared to other methods (6, 154, 220). Another commercial system to detect Candida mannan in serum, the Pastorex Candida test (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France [118]), appeared promising, but Gutiérrez et al. (104), who conducted a prospective clinical trial, found a 0% sensitivity for this test. The sensitivity of the Pastorex system was improved when serial assays with multiple consecutive serum samples were used (103). In a recent study, Savolainen et al. (253) examined the reactivity of immunoglobulin E (IgE), IgG, and IgM antibodies to mannan by a radioallergosorbent test (RAST) and ELISA and to proteins (some of which are in the cell wall) by immunoblotting and concluded that (i) the IgE titer rose early and was more useful for detection than were IgG and IgM titers, (ii) RAST and immunoblotting analyses were both required for maximum sensitivity and specificity, and (iii) invasive disease could be distinguished from mucosal colonization by this procedure.
Most serologic studies used whole phosphopeptidemannan complexes as antigenic preparations and did not identify individual epitopes recognized by different antibodies. To determine the molecular basis of recognition of oligomannoside antigenic motifs by human and animal (in experimentally induced candidiasis models) immune systems, Faille et al. (76) developed a method of rendering oligomannosides antigenic by coupling them to a carrier molecule that was able to bind to conventional substrates for immunoanalysis. The oligomannosides were coupled to a lipid, 4-hexadecylaniline by a method leading to a mole-to-mole binding whose efficiency can be visualized and assessed by thin-layer chromatography. The hybrid molecules (neoglycolipids [NGL]) exhibited both immunogenicity and antigenicity.
Trinel et al. (298) constructed NGL from three families of oligomannosides released by sequential depolymerization of C. albicans phosphopeptidemannan by acid hydrolysis (NGLH), β-elimination (NGLO), and acetolysis (NGLA) and studied their reactivity by ELISA with six monoclonal antibodies reacting with polysaccharide moieties of C. albicans mannoproteins. The six monoclonal antibodies were also tested by immunoblotting against germ tube antigens obtained by alkali extraction under reducing conditions. By this extraction method, the authors obtained highly standardized profiles of C. albicans germ tube cell wall and cytoplasmic proteins and mannoproteins ranging from 300 to 10 kDa (119). Comparing the epitope mapping of antibodies by ELISA with NGL and by immunoblotting with the extract, they provided evidence that the reactivity of monoclonal antibodies with NGLH (corresponding to homopolymers of β-1,2-linked mannopyranosyl units [77, 268]) was correlated with reactivity with a 14- to 18-kDa C. albicans antigen. Conversely, monoclonal antibodies reacting with NGLA also reacted with polydisperse C. albicans high-molecular-mass mannoprotein species (298). Oligomannosides released after mild acetolysis have been described as being composed of from 1 to more than 11 mannosyl residues (75) linked mainly through α-1,2 and α-1,3 bonds (146, 150, 268, 273) and to a lesser extent through β-1,2 bonds (145, 268).
By using the method of NGL construction, an analysis of the human antibody response to C. albicans-derived oligomannosides was undertaken to define the molecular basis of the recognition of the mannan molecule by human Igs (75, 112, 231, 299). Construction of NGL from C. albicans O-linked oligomannosides provided evidence that human antibodies reacted with these residues (112). O-linked oligomannosides released by mild-alkali degradation contained one to seven mannose residues, among which the quantitatively major components, mannobiose and mannotriose, were shown to contain exclusively α-1,2 linkages (112). The pool of oligomannosides was converted to NGL and tested by ELISA with human sera. The sera were from patients who had seroconverted during the course of a mycological and serological survey for candidiasis as detected by indirect immunofluorescence and co-counterimmunoelectrophoresis assays (232). The results of ELISA-NGLO tests appeared to correlate with the results of ELISA on the original phosphopeptidemannan molecule and with the results of routine serologic tests (112). Subsequently, Poulain et al. (231) selected sera with different IgG3 levels against NGLH and NGLA from patients with candidiasis to check the previously correlated reactivity between NGLH and the 14- to 18-kDa antigen and between NGLA and high-molecular-mass mannoprotein species (298). Patient sera recognized saccharide epitopes distributed over the 14- to 18-kDa antigen. These epitopes, which were identified by IgG3, are thought to consist of β-1,2-linked oligomannosides because of the observed reactivity of the same sera with NGL constructed from these residues (231). Subsequent analysis has led to the identification of the 14- to 18-kDa antigen as a phospholipomannan because of its resistance to proteolysis, its ability to be extracted by chloroform-methanol-water, and its content of phosphate, mannose, and palmitic acid as revealed by metabolic labelling with radioactive probes (299). Poulain et al. (231) also concluded that the oligomannosides distributed over high-molecular-mass mannoprotein moieties, which are susceptible to release from mannan by acetolysis, may act as epitopes for IgG3. Hernando et al. (119) tested sera from patients with candidiasis and from healthy individuals by immunoblotting for reactivity with high-molecular-mass mannoprotein species and the 14- to 18-kDa antigen described above that is present in the extracts of yeast cells and germ tubes. All sera tested recognized high-molecular-mass moieties, and recognition by control sera was invariably associated with reactivity with the 14- to 18-kDa antigen. However, despite a high level of antibodies against high-molecular-mass mannoproteins, some serum samples failed to react with the 14- to 18-kDa antigen or lost this reactivity during the course of the disease. Hernando et al. (119) also found specific recognition of bands in the molecular mass range of 20 to 54 kDa. This is in agreement with the generally accepted contention that antibodies directed to medium- to low-molecular-mass (60- to 29-kDa) antigens may be of value for serodiagnosis (93, 281, 318).
Nonmannan Carbohydrates
Undoubtedly, mannose is the main monosaccharide present in C. albicans cell wall glycoproteins. Nevertheless, some experimental evidence indicates that wall glycoproteins may contain sugar residues other than mannose (e.g., sialic acid, galactose, and fucose) that may define additional functional and/or antigenic motifs (2, 29, 130, 202, 308) and raises the possibility that antibodies to other carbohydrate antigens are present in sera from colonized or infected individuals. Antibodies to the human blood group I antigen reacted with C. albicans cells in vitro and in infected tissue sections and cross-reacted with protein moieties present in β-mercaptoethanol extracts from yeast and hyphal cells, suggesting that complex carbohydrates related to the blood group I antigen are present at the surface of Candida cells (202). Blood group-related antigens such as the I and i antigenic determinants, the precursors of the ABH system, are developmentally regulated (105). The I antigen is the precursor of the H antigen, to which it is converted by fucosylation. Blood group-related antigens are present not only on human erythrocytes but also on epithelial and endothelial cells and in soluble form in plasma and other body fluids (48). Hence, the spectrum of individuals who have antibodies to the I antigen that will react with the analogous antigenic determinants on the surface of fungal cells will be the same as the distribution of anti-I antibodies in the population. The extent to which the presence of cross-reactive antigens on a commensal/opportunistic pathogen such as C. albicans may contribute to the development of host antibodies is unclear. In any case, anti-I antibodies are cold-reactive Igs, and the extent to which they may interact with the fungus in vivo is yet unknown.
PROTEIN COMPONENT
Numerous C. albicans proteins and glycoproteins play their structural and physiological role outside the plasma membrane barrier (secreted species). They can be divided into two broad categories: moieties whose primary destination is considered to be the cell wall, and molecules whose final destination is the exocellular environment (41). Members of both categories may elicit a host humoral response, and antibodies to several secreted candidal proteins have been detected in sera from both human patients and animals with experimentally induced candidiasis (Table 1).
Secreted Proteins
A complex assortment of hydrolytic enzymes such as proteinases (secreted aspartyl proteinase), phospholipases, acid phosphatase, chitinases, esterase, and glucoamylase can be found in culture filtrates of C. albicans cells (41). Although some of the proteins present in culture filtrates may represent products specifically secreted, some others may be components shed from the cell wall or, alternatively, released from the cytoplasm as a consequence of spontaneous cell lysis (see below). Several of the enzymes mentioned above are putative virulence factors of C. albicans (26, 41, 50); consequently, neutralizing antibodies to them could represent an effective host defense barrier. Unfortunately, except for aspartyl proteinase, the humoral response of the host to these candidal moieties is essentially unexamined.
Antigenic components released by C. albicans cells may be of potential utility as specific marker antigens for the serodiagnosis of systemic candidiasis. During the 1960s, yeast phase cultures incubated for up to 4 weeks were widely used to obtain soluble antigens (170, 174, 182, 276). These antigens cross-reacted with antigen preparations from the cell wall and the cytoplasm and may therefore be considered autolytic products rather than secreted components (182, 291). More recently, Torosantucci et al. (295) studied the material released after 24 h by yeast- and mycelial-phase C. albicans cells growing in a chemically defined simple medium. These authors characterized concanavalin A-reactive mannoprotein constituents and demonstrated that cells of both growth forms released comparable amounts of such mannoproteins. No qualitative differences in individual molecular species between the two extracts were found (295).
Later, Aguiar et al. (1) characterized the exocellular antigens obtained from concentrated filtrates of mycelial- and yeast-phase cultures grown on synthetic medium. The only difference between the extracts was the presence in mycelial culture supernatants of a high-molecular-mass, polysaccharide-rich component, migrating in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels at a position that would correspond to proteins with molecular masses of 245 to 265 kDa. The authors suggested that this molecular entity may correspond to the previously described 260-kDa mycelium-specific high-molecular-mass mannoprotein species (31). Sera from immunized rabbits clearly recognized medium- to low-molecular-mass antigens (21 to 44 kDa) in the culture filtrate supernatants (1).
Recently, we have characterized a complex array of protein and mannoprotein components released into a chemically defined simple medium by cells of both C. albicans morphologies (165). A short incubation time (6 to 8 h) was used to prevent potential contamination with cytosolic components released by autolysis. In addition to high-molecular-mass (i.e., >180 kDa) species, which may include the 260-kDa high-molecular-mass mannoprotein moiety (1, 31), several mannoprotein species within a molecular mass range of 58 to 180 kDa were also detected. Qualitative and quantitative differences in the electrophoretic patterns of the materials released by yeast and mycelial cells along the entire range of molecular mass were resolved by the 5 to 15% slab gradient gels used in this study (165). The soluble material cross-reacted with polyclonal antisera to known cell wall components, including the 58-kDa fibrinogen-binding mannoprotein (mp58) that we described (32, 33, 168).
Other candidal cell surface-related moieties that have been purified and characterized from culture supernatants are the receptor for the complement C3d fragment (255), a fucoside-binding adhesin with lectin-like properties (49, 63, 297), an exo-β-glucanase activity (43, 171), and enolase (284). Except for enolase and preliminary observations in the case of the mp58 receptor (see below), the literature contains no reports of studies on the serologic response in humans and/or animal models to other candidal moieties present in supernatant filtrates of C. albicans cultures. The presence of cell wall-bound components in the extracellular medium could be a consequence of an imbalance in degradation and synthesis occurring during cell wall expansion (101, 259, 260) and/or of fibril shedding. This imbalance could occur during changes in hydrophobicity related to environmental and nutritional influences (114, 115), which may result in the liberation of minor but significant amounts of protein and mannoprotein wall components (165). If this situation also occurs in vivo, the release of wall-associated antigenic moieties may trigger a complex humoral response in the host.
Secreted aspartyl proteinase.
An extracellular proteinase activity from C. albicans, reported by Staib (279), Remold et al. (241), and Rüchel (243), has been characterized as a carboxyl proteinase. Among putative virulence factors in candidiasis, this secreted aspartyl proteinase (SAP) ranks with those which are most widely investigated (246), since several observations suggest a major role for SAP in the pathogenesis of candidiasis (16, 17, 52, 54, 55, 68, 69, 132–134, 173, 216, 237, 244, 247, 248).
MacDonald and Odds (172) compared the titers of antibodies to candidal SAP and to cytoplasmic antigens in the sera of healthy individuals, patients with candidiasis, and other hospitalized patients without candidiasis. The levels of anti-SAP antibodies in human sera were higher in patients with candidiasis than in healthy individuals, while the antibody titer against candidal cytoplasmic antigens was almost equal in the two groups. The authors concluded that SAP could be a specific antigen for the serological diagnosis of candidiasis (172). Detection of anti-SAP antibodies for serodiagnosis has been attempted (213). Elevated levels of IgG antibodies to SAP were also observed in patients with invasive or disseminated candidiasis (236, 245).
Ishiguro et al. (123) studied the serological characteristics of patients with C. albicans vaginal infection. The frequency of detection of antibodies and their binding patterns against cellular antigens did not differ greatly between healthy females and patients with vaginitis. In contrast, IgG antibodies against SAP purified from culture medium were found at a higher frequency among patients who carried C. albicans in their vaginal discharge than among healthy individuals. Vaginal infection by C. albicans might contribute to a rise in the level of anti-proteinase antibodies. However, antibody production may be stimulated as a result of gastrointestinal colonization or other types of infection, and this may explain why 18 and 13% of healthy females and patients in whom the absence of Candida was assessed by microbiological culture and supported by clinical signs and symptoms, respectively, had anti-proteinase antibodies.
The purified enzyme used in the studies by Ishiguro et al. (123) was contaminated with mannan. Later, Morrison et al. (204) purified SAP and removed mannan by column chromatography with sequential anion-exchange, gel permeation, and linear gradient anion-exchange steps. The polyclonal antibodies prepared against this purified SAP were used in a competitive binding-enzyme immunoassay in an immunosuppressed-rabbit model of disseminated candidiasis (205). This assay was able to detect SAP antigenuria within 24 h of intravenous challenge and could discriminate between gastrointestinal C. albicans colonization and disseminated candidiasis.
In a study by Cassone et al. (37) demonstrating that antibodies mediate protection in rat vaginal candidiasis (see below), anti-proteinase IgA antibodies were found to be present in the vaginal fluid from most of the infected animals. The presence of anti-proteinase antibodies in the vaginal fluid may be expected, taking into account that this enzyme is actively produced in vivo by C. albicans cells during infection (52). Preadsorption of the vaginal fluid from infected rats with C. albicans cells previously grown in vitro under conditions in which synthesis of SAP was not induced significantly reduced the ability of the vaginal fluid to transfer protection to healthy animals. Hence, it seems likely that a major protective role in this model is played by anti-mannan antibodies (37). However, the possibility of a protective effect exerted by specific anti-SAP antibodies cannot be completely ruled out (53).
Immunosuppressive and B-cell mitogenic protein.
Tavares et al. (293) have purified from the supernatants of C. albicans cultures an immunosuppressive B-cell mitogenic (ISM) protein that plays an important role in the survival of the microorganism in the host. They showed that the immunosuppressive and B-cell mitogenic properties of this protein, designated p43, were quantitatively associated with the host susceptibility to C. albicans infection. Moreover, treatment of C57BL/6 mice with p43 decreases the resistance of the host and subsequently facilitates fungal growth. The loss of the capacity to produce p43 by C. albicans correlates with the loss of the fungal virulence. Immunosuppressive B-cell mitogenic proteins produced by bacteria and viruses have been described previously (8, 9, 80). These ISM proteins are virulence factors because the immunosuppression, a consequence of B-cell overstimulation induced by such proteins, is crucial for the survival of the producing microorganisms in the host (158). These observations suggest that a strategy of vaccine development may involve the neutralization of microbial metabolites or substances that are virulence factors like the ISM protein. Tavares et al. (292) investigated whether neutralization of p43 by previous selective immunization of the host with this protein would protect against C. albicans infection. They found that immunization of BALB/c mice with p43 fully protected the mice against the fungal infection and that passive administration of specific anti-p43 antibodies significantly protected the animals against challenge with living microorganisms. However, antibodies to p43 have been not detected in naturally infected human individuals.
Heat Shock Proteins
All living organisms have the ability to respond to sudden changes of temperature by increasing their production of a set of proteins, the so-called heat shock proteins (hsps). C. albicans is no exception to this rule (317). hsps are also immunodominant antigens and major targets of host immune response during different types of infections (141, 142, 177, 316). An interesting feature of some hsps of C. albicans is that they are not confined to the intracellular compartment but are also present at the cell (wall) surface. Members of the hsp70 family of proteins of this fungus, along with the 47-kDa fragment of hsp90, have been reported as being present in the cell wall (161, 197). As components of fungal cell walls, hsps may play roles similar to those described for hsps in the cytoplasm (160). This surface location implies that the antigenic determinants are naturally exposed and may be responsible for the immunodominant nature of C. albicans hsps. Moreover, the presence of hsps at the candidal cell surface may facilitate potential protective roles of host antibodies.
In any case, the ubiquitous nature of hsps, along with the high degree of homology among them, poses interesting challenges to the immune system of the host. First, the presence of epitopes shared by a number of infectious agents may provide the immune system with a universal signal for infection, and antibodies to these conserved regions could provide some natural resistance to infection, bridging the gap between innate and acquired immunity. Second, epitopes shared by the parasite and the host may trigger deleterious autoimmune responses (141). This could also be true for the immune responses against highly conserved immunodominant glycolytic enzymes (see below).
hsp90.
Immunoblotting experiments with sera from patients with systemic candidiasis showed the presence of a 47-kDa immunodominant antigen in whole-cell extracts of the fungus (186, 190, 191). This 47-kDa antigen was further identified as a heat-stable breakdown product of hsp90 (187). Monovalent antibodies against the 47-kDa fragment were used in immunoelectron microscopy to demonstrate its cell wall location (197). Antibody to the 47-kDa antigen is present in serum samples from a high proportion of patients with chronic mucocutaneous candidiasis and with AIDS (20, 186, 193). Patients who recover from systemic candidiasis produce a major antibody response to the 47-kDa component, whereas patients with fatal cases have little antibody or falling titers (186, 190, 191, 193). In a mouse model of systemic candidiasis, prior administration of sera from two infected patients containing antibodies to hsp90 resulted in decreased mortality rates (194). Epitope mapping of C. albicans hsp90 with patient sera revealed that patients recovering from systemic candidiasis produce antibodies against both fungus-specific and conserved epitopes of hsp90 (192). In particular, a highly conserved oligopeptide epitope (LKVIRK) was recognized by sera from all patients with antibody to the 47-kDa antigen (192). Moreover, when given prophylactically, a murine monoclonal antibody raised against this epitope reduced mortality in a mouse model of invasive candidiasis (194). The LKVIRK epitope is central to the proposed protein-binding site of human hsp90, suggesting a possible mechanism whereby the monoclonal antibody was protective. If extraneous circulating candidal hsp90 binds to serum proteins, causing them to malfunction, an antibody preventing this would be beneficial (185). Thus, it was concluded that autoantibodies against hsp90 can protect against infection. “Human recombinant antibodies” are entirely human in origin, thus avoiding problems inherent to the human anti-mouse antibody response; therefore they are the antibodies of choice for an Ig-based therapy for candidiasis. In this context, the protective potential of a human recombinant antibody to the LKVIRK motif was assessed in two models of murine invasive candidiasis. A statistically significant improvement in survival (acute model) or renal clearance of infection (chronic model) was apparent in both experiments (195, 196).
C. albicans hsp90 circulates in body fluids of patients with disseminated candidiasis, and the 47-kDa antigen was isolated from patient sera by affinity chromatography (191). An enzyme-linked immunodot assay with affinity-purified antibody to the 47-kDa moiety was capable of detecting circulating antigen in the patient sera (186). By using this assay, systemic candidiasis was detected in 77% of neutropenic patients and in 87% of nonneutropenic patients. The sensitivity and specificity of detection were improved over those of other commercially available products.
In another study, Zoller et al. (318) used purified somatic antigens of C. albicans, including a 47-kDa component, in enzyme immunoassays for antibody detection in sera from patients with confirmed disseminated candidiasis. The assay had a sensitivity of 81.5% and a specificity of 97%. However, the identity of this 47-kDa antigen remains to be resolved, since it could actually be enolase (see below).
hsp70.
Two members of the hsp70 family of proteins (Ssa1p and Ssa2p) have been found in C. albicans (72, 152, 161). A cell wall location of at least one member of this family of proteins has been shown for both S. cerevisiae and C. albicans (160, 161). The presence of hsp70s in the cell wall and surface implies that the antigen is present in intact cells, without the necessity for cellular disruption, and is readily exposed to the host immune defenses. La Valle et al. (152) reported that sera from three healthy individuals contained antibodies to C. albicans Ssa1p and suggested that the presence of such antibodies could contribute to protection against infection. It was also pointed out that the variability in this generally conserved protein was predominantly in the C-terminal region, which is also the immunodominant region (142). In the case of the product of C. albicans SSA2 gene, it was reported that serum samples from both healthy people and patients with candidiasis contained antibodies to the C-terminal portion of Ssa2p (161). Constantino et al. (47) have analyzed the humoral response of CBA/H mice to systemic infection with C. albicans. The major antibody response identified was directed against a protein antigen of 96 kDa (which was not induced by heat shock) and against a 75-kDa candidal hsp, which, according to these authors, appears to be a member of the hsp70 family. Due to the high homology among the different members of the hsp70 family, the antibody responses reported by the different groups could result from a combination of antibodies to the different hsp70s present in C. albicans, as well as to hsp70s from other organisms. These reactivity patterns are also likely to be the result of combination of antibodies to conserved epitopes and epitopes unique to individual C. albicans hsp70s.
Other heat shock proteins.
Swoboda et al. (290) probed hsps (with molecular masses of 38 to 42, 66 to 68, 70 to 72, and 74 to 76 kDa) of C. albicans isolated by ATP affinity chromatography in Western immunoblots and ELISA experiments with sera from patients with oral and/or esophageal C. albicans infections. These hsps were recognized by the different serum samples assayed; in addition, the levels of IgA correlated with the severity of candidal infection in each case. Heat shock mannoproteins with approximate molecular masses of 180 to 200, 130 to 150, 90 to 110, and 60 to 70 kDa have been identified as being involved in the secretory immune response during mucosal candidiasis (226). These molecules were overexpressed after a temperature shift from 25 to 37°C. The antigenic determinants recognized by secretory IgA antibodies in saliva and vaginal washings were polysaccharide in nature. The 180- to 200-kDa component also enhanced tumor necrosis factor secretion by a murine macrophage cell line (221).
Glycolytic Enzymes
Glycolytic enzymes are abundant immunodominant antigens during C. albicans infections, and C. albicans enolase (2-phospho-d-glycerate hydrolase; EC 4.2.1.11) phosphoglycerate kinase (PGK; EC 2.7.2.3), alcohol dehydrogenase (ADH; EC 1.1.1.1), pyruvate kinase (PYK; EC 2.7.1.40), aldolase (EC 4.1.2.13), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; EC 1.2.1.12) have been described as major allergens or immunogens during candidiasis (97, 98, 104, 123, 124, 199, 263, 281, 286, 289, 304–306, 310). All of these enzymes appear to be highly conserved.
The presence of glycolytic enzymes in the cell wall of C. albicans has been reported recently. Thus, enolase was found to be associated with glucan in the inner layers of the cell wall as well as in culture supernatants (7). PGK was found in the cell wall and at the outermost cell surface (4). The presence of ADH in the wall of C. albicans has been suggested also (219). Finally, our group has recently found evidence indicating the presence in C. albicans of a wall-associated, enzymatically active form of GAPDH, which appears to be located at the most external cell surface layer (97, 98). The cell wall location of other glycolytic enzymes such as PYK or aldolase has not been examined.
It has recently been suggested that the cytoplasm may be the origin of several protein species (including enolase and hsp70) found in the cell wall of C. albicans and that these moieties may mask other essential cell surface antigens, thus subverting specific host immune responses to such antigens (73). However, although the localization of presumably “intracellular” glycolytic enzymes in the wall of C. albicans cells is intriguing, the presence of glycolytic enzymes on microbial surfaces is not unprecedented. Thus, GAPDH has been identified as a constitutive protein component of the cell wall of Streptococcus pyogenes, where it not only is enzymatically active but also serves as a binding protein for fibronectin, lysozyme, myosin, and actin (218, 314), and in the cell wall of another yeast species, Kluyveromyces marxianus, where its concentration increases substantially upon induction of flocculation (78, 79). In addition, Goudot-Crozet et al. (100) presented evidence for an active form of GAPDH on the surface of Schistosoma mansoni and showed that sera from subjects with low susceptibility to infection by this parasite reacted with this protein while sera of susceptible individuals had little or no reactivity. ADH is also a surface protein of Entamoeba histolytica (315). Finally, PGK and triose-phosphate isomerase have been found on the surface of S. mansoni (153, 275) and have been suggested as potential candidates for vaccine development against this parasite.
Glycolytic enzymes of C. albicans are immunogens during candidiasis. Of these, enolase seems to be the most immunodominant antigen in humans. Enolase stimulates both humoral and cellular immune responses in mice, and these responses are indicative of C. albicans proliferation in the host. Antibodies to other glycolytic enzymes (PYK, ADH, PGK, and GAPDH) have also been found in the sera of patients with different forms of candidiasis. Enolase, PGK, aldolase, and ADH of C. albicans are also allergens for allergic patients.
Enolase.
Despite the considerable heterogeneity of the humoral responses to antigens of C. albicans in humans (175, 190, 281), several immunodominant antigens have been identified. These include enolase, the glycolytic enzyme that catalyzes the reversible dehydration of 2-phospho-d-glycerate to high-energy phosphoenolpyruvate. Strockbine et al. (281) characterized the antigenic components in cytoplasmic extracts of C. albicans that were recognized by sera from patients with disseminated candidiasis. They found that these patients had circulating antibodies directed against a 48-kDa protein antigen purified by anion-exchange chromatography from the candidal extract. Individuals colonized with C. albicans or without evidence of candidiasis did not have detectable levels of antibodies to this antigen, which was subsequently identified as enolase (84, 183). Circulating anti-enolase antibodies may have potential value for the diagnosis of candidiasis (199, 305, 306). Thus, an ELISA with purified C. albicans enolase as the target was devised to detect antibodies in sera from patients with proven candidiasis. Statistical analysis of the results obtained indicated that the assay was able to discriminate between invasive infection and simple colonization (305). However, the test suffered from low sensitivity. Using purified candidal enolase as the antigen in immunoblotting experiments, another group detected anti-enolase IgG antibodies in serial samples drawn from 92.5% of the patients with systemic candidiasis examined, with a specificity of 95% (199).
Antigenemia with the 48-kDa antigen as detected by ELISA was observed in a murine model of disseminated candidiasis in the absence of fungemia and correlated with deep tissue infection (309). To investigate the expression of this candidal cytoplasmic antigen in the serum of patients with cancer, who are at high risk for deep invasive candidiasis, Walsh et al. (310) conducted a prospective clinical trial with patients from four medical oncology centers over a 2-year period. They concluded that C. albicans enolase antigenemia is a marker for deep tissue invasion even in the absence of fungemia. The serum enolase immunoassay complemented rather than replaced blood cultures for the diagnosis of such infections (240, 310). The assay was very specific (96%), but the sensitivity was low (only 54% in patients with proven deep tissue invasion). Testing of multiple samples improved the sensitivity for antigen detection to 85% for patients with proven deep tissue infection and to 64% for patients with proven candidemia (310). A commercial assay (Directigen; Becton Dickinson) for the detection of candidal enolase has been evaluated, in comparison with other serodiagnostic reagents for invasive Candida infection, by Gutiérrez et al. (104). They showed that the most useful markers in patients with first-time C. albicans sepsis are IgM antibodies to blastoconidial antigens. However, for adequate detection of invasive candidiasis, they proposed that levels of both IgG and IgM antibodies and of circulating 48-kDa antigen should be determined.
Some major protein allergens of C. albicans have been identified by immunoblotting with anti-human IgE antibodies. In one initial study, 77% of the serum samples from asthmatic patients reacted with fractions containing a 46-kDa protein (252). Ishiguro et al. (124) also focused on the identification and characterization of IgE binding of Candida antigens. Several moieties with molecular masses of 175, 125, 46, 43, and 37 kDa were detected. The 46-, 43-, and 37-kDa antigens were purified, and their polypeptide sequences were found to have significant levels of homology to the S. cerevisiae glycolytic enzymes enolase, PGK, and aldolase, respectively. Although enolase from S. cerevisiae, in addition to the C. albicans enzyme, is a major allergen (125, 151), S. cerevisiae enolase did not react with IgE of patient sera, suggesting that IgE antibodies whose levels are elevated in allergic patients recognized a limited set of epitopes. In this context, characterization of candidal enolase antigenic motifs eliciting IgE responses revealed that the C-terminal portion of the protein was the more immunogenic (126). The molecular mass and cytoplasmic localization point to the possibility that the 46-kDa allergen detected by Savolainen et al. (252) was also enolase.
Recently, the reactivity toward mannan of antibodies (IgE, IgG, and IgM) present in postoperative patients with invasive candidiasis has been examined by different methods including immunoblotting (253). Several protein antigens were detected by this technique, none by all patient sera, although a 46-kDa band, believed to be enolase was the most conspicuous immunogen detected (253). Enolase thus appears to be an immunodominant protein both in allergic reactions to fungal antigens and in fungal infections. In this context, Breitenbach et al. (22) have reported that serum from a patient allergic to the molds Alternaria alternata and Cladosporium herbarum reacted equally well with the enolases from Alternaria, Saccharomyces, and Candida, concluding that enolases are highly conserved major fungal allergens.
Since a Th2 cell response that predominates over a Th1 response has been suggested to underlie the IgE response in atopic individuals (58), and since disseminated candidiasis in murine models is associated with the Th2 response (242), IgEs may be a better indicator of infection than other Ig classes. In one study (253), IgE titers rose early during infection and the greatest sensitivity (93%) and specificity (73%) were seen with the combined use of two immunoassays (RAST and immunoblotting) for IgE detection, while IgG and IgM detection resulted in lower sensitivities and specificities. Whether IgE responses are similar and potentially useful for diagnosis in immunocompromised patients has not been examined.
The humoral and cellular immune responses to enolase have been studied in mice (286). Both lymphocyte activation and antibody production to enolase were evident in germ-free mice colonized with C. albicans. Immune responses arising as a result of gastrointestinal tract colonization can be studied in this model because the alimentary tracts of germ-free adult mice are easily colonized by oral administration of C. albicans and the microorganism does not persist in the bloodstream or invade internal organs (13). Moreover, lymphocytes from intravenously challenged mice responded to enolase, and in these animals enolase was the immunodominant humoral antigen. This study demonstrated that enolase stimulates cellular and humoral responses and that specific immune responses to enolase are sensitive indicators of the presence of proliferating C. albicans in mice (286).
Sundstrom and Aliaga (283) isolated and sequenced a clone coding for C. albicans enolase from a cDNA library. Comparison of the predicted C. albicans enolase sequence with that of crystallized S. cerevisiae enolase (280) showed extensive homology in regions of secondary structure, thus illustrating the similarity in the architecture of the two enzymes. The protein product of the cloned cDNA has been purified as a recombinant protein fused to glutathione S-transferase and has been shown to have enolase enzymatic activity. Consistent with the presumed cytoplasmic location of this enzyme, no reactivity of whole cells with antiserum to the fusion protein was observed by immunofluorescence and no enolase activity was detected when intact cells were used in the enzymatic assays (284). These results indicated that enolase was not present on the surface of C. albicans, however, enolase was detected by radioimmunoprecipitation in cell wall extracts obtained after digestion of the wall β-glucan network with β-glucanases (Zymolyase). The enzyme was also found in culture supernatants. The presumed cytoplasmic location of enolase suggests that enolase released from fungal cells spontaneously or as a consequence of damage inflicted by host cells and factors may account for the circulating levels of the enzyme in the bloodstream of patients with disseminated candidiasis. Moreover, the release of enolase may be important in defining the selective stimulation of the host antifungal responses during infection. In this context, it has been recently reported that anti-enolase antibodies may have an immunoprotective effect in mice (304).
Although the cytoplasmic location of enolase appeared to be established, Angiolella et al. (7) have recently shown that this enzyme is also present in the cell wall of C. albicans. These authors studied the effect of cilofungin, a lipopeptide antibiotic affecting β-1,3-d-glucan synthesis, and showed that subinhibitory, nonlytic doses of this antibiotic inhibited the incorporation of a 46- to 48-kDa glucan-associated protein (46K protein), which seems to be a cell wall-associated form of enolase, into the growing cell wall. Several lines of evidence supported this contention: (i) the 46K protein exhibited cross-reactivity toward anti-enolase antibodies; (ii) two internal peptide fragments of the purified 46K protein were identical in amino acid sequence to the peptide sequence of the recombinant enolase protein; and (iii) by immunoelectron microscopy with anti-enolase antibodies, the 46K protein was clearly detected in the inner layers of the fungal cell wall (7, 83, 184, 283). The presence of the protein in the inner layers but not in the outermost regions of the cell wall is consistent with previous results of Sundstrom and Aliaga (284). Results reported by Angiolella et al. (7) strongly suggested that enolase is a bona fide cell wall protein, in contrast to previous results obtained by other authors (73, 261). In any case, it remains to be demonstrated whether the cell wall-associated form of enolase is enzymatically active, what its role in the cell wall could be, if the postulated cell-wall location of the enzyme contributes to its antigenic properties, and/or if the wall-associated form may account for the enolase found in the external milieu.
Other glycolytic enzymes.
A number of recent reports indicate that in addition to enolase, several other candidal glycolytic enzymes act as elicitors of the host immune response. IgE antibodies to candidal antigens present in 41% of serum samples from allergic patients reacted with PGK (124). The IgE response seemed to be directed against specific epitopes of the C. albicans PGK, since the antibodies did not cross-react with the homologous enzyme from S. cerevisiae (124). Similar to enolase (7), evidence indicating that PGK is also a bona fide component of the cell wall of C. albicans has recently been reported (4).
A dual location (cytoplasm and cell wall) has been suggested for the candidal ADH (219). Although the presence of this enzyme in the wall of C. albicans cells requires further confirmation, ADH has been shown to be a major allergen and to elicit an immune response during candidiasis. Thus, IgE antibodies from allergic patients reacted with ADH (263), and antibodies to candidal ADH have been reported to be present in the sera of patients with superficial candidiasis (289). An immunoblot analysis of C. albicans components cross-reacting with human IgE antibodies revealed the existence of an immunodominant candidal allergen that was recognized by 77% of serum samples obtained from asthmatic patients (264) and that was identified as ADH (263).
Recently, a cDNA clone coding for a protein exhibiting 76% homology to the GAPDH from S. cerevisiae has been isolated by immunoscreening of a cDNA library with a pooled antiserum preparation obtained by mixing sera from two patients with systemic candidiasis confirmed by blood culture and five neutropenic patients with a high level of anti-C. albicans IgM antibodies (97, 98). The most reactive isolated clone contained a 0.9-kb cDNA encoding a polypeptide immunoreactive only with sera from patients with confirmed systemic candidiasis. The deduced amino acid sequence from the cDNA clone corresponded to the GAPDH of C. albicans, and the enzyme was found to be present and enzymatically active both at the outer surface of the wall and in the cytoplasm of C. albicans cells (97, 98).
Finally, IgE antibodies present in sera from allergic patients have also been found to react with aldolase (124), and serum from individuals affected by superficial candidiasis contains antibodies to candidal PYK (289). However, as noted above, these proteins are not known to be in the wall of C. albicans cells.
Receptors and Binding Proteins for Host Ligands
C. albicans displays a large repertoire of cell wall-bound receptor-like molecules that mediate the interaction between the fungus and the host cells and tissues. Although there is detailed information about the biochemical characteristics and functional features of most of these candidal adhesins and receptors (24–26, 41, 65, 66, 121, 219), information on the cell-mediated and/or humoral host responses to these moieties is scant. Among the molecules displaying receptor-like characteristics, perhaps two of the best characterized are the C3d receptor mannoprotein (CR2) and the 58-kDa fibrinogen binding mannoprotein (mp58). Although several similarities appear to exist between these two candidal cell wall mannoproteins (27, 32, 159, 255), the receptors appear to be biochemically and functionally independent entities (166).
The ability of C. albicans cells to bind serum proteins such as fibrinogen was initially described by different authors (18, 217, 300). Later, our group identified, in β-mercaptoethanol extracts from intact C. albicans yeast and hyphae, mp58 which specifically binds fibrinogen (32) and which appears to be heterogeneously distributed on the surface of cells in vivo (181). This molecule has N- and O-glycosidically linked mannose; O-linked sugar residues seem to be involved in the interaction of the molecule with fibrinogen (32). By using a polyclonal monospecific antiserum raised against the mp58 species (32) as a probe, it was found that the mp58 species is expressed by C. albicans cells both in culture and in infected human tissues (32, 166, 168, 181). In addition, a large number of C. albicans clinical isolates examined contained cell wall-bound functional (i.e., as detected by their ability to bind fibrinogen) mp58-like moieties (169, 262). The presence of antibodies to the mp58 species in sera from patients with different types of candidiasis has also been investigated by immunoblotting. All sera from patients with confirmed systemic candidiasis reacted with this antigen, whereas none of the sera from control individuals and patients with superficial candidiasis contained detectable levels of antibodies to the mp58 (169, 262). These results suggest the potential usefulness of mp58 as a marker antigen for the serodiagnosis of systemic candidiasis.
The ability of C. albicans to rosette antibody-sensitized erythrocytes coated with complement fragments C3d and iC3b was initially reported by Heidenreich and Dierich (117) and later reported by other investigators (for a review of this topic, see references 26 and 121). With respect to candidal cell surface moieties that may represent receptors for C3d, a 60-kDa component that exhibits the ability to bind C3d has been detected in both growth forms of the fungus (27, 138, 159, 255, 308). The candidal receptor for C3d is expressed in vivo by fungal cells in kidney tissue sections and peritoneal lavage fluid from infected mice (138) and also appears to be immunogenic in vivo, eliciting a cell-mediated response (86). However, a serologic host response to this C. albicans cell wall component, which may play a role in virulence and pathogenesis, has not yet been evaluated.
ANTIBODIES TO CELL WALL ANTIGENS: PROTECTIVE AND DIAGNOSTIC VALUE
Clinical observations indicate that antibodies appear to play an important role in host defense against disseminated candidiasis, because individuals with defects in cell-mediated immunity mechanisms are particularly prone to superficial but not disseminated candidiasis (for reviews, see references 185 and 189). The role of the host humoral response to C. albicans in providing protection against disease, however, remains contentious. In this context, the literature is full of conflicting data that either support or refute the importance of anti-Candida antibodies as effective mechanisms for fighting infection. In a recent review, Casadevall (28) pointed out that the antibody response to Candida is complex, with the presence in immune sera of protective, nonprotective, and deleterious (infection-enhancing) antibodies that may be isotype and epitope specific. As a result, immunoprotection experiments with polyclonal antibodies that may differ in their isotype and in their recognition of epitopes are difficult to interpret and usually lead to inconclusive observations. Also, a number of variables must be considered when searching for and assessing protective antibody effects. Adding further complexity to this problem is the fact that antibodies may exert protective effects by an assortment of mechanisms (which may be difficult to evaluate) and that the fungus may use a variety of “tricks” to evade the host immune defenses (mainly antigenic variability and immunomodulation). However, in recent years, there has been increasing evidence that some Candida-specific antibodies can be immunoprotective during infection, thus suggesting the viability of an immunotherapy and/or vaccine approach for the treatment and management of candidiasis (28, 60, 185, 189).
All fungal antigens that appear to be potential elicitors of these immunoprotective antibody responses are secreted proteins or C. albicans cell wall-bound components (Table 2). These include several adhesins, most of which are wall mannoproteins, which play a key role in attachment of the fungus to host tissues and cells or to inert surfaces or materials (reviewed in references 24–26, 41, 50, 65, 66, and 121).
TABLE 2.
Putative protective antibodies to Candida cell wall components: models and strategies
| Component | Infection or vaccination | Antibodies | Model | Comments | Reference(s) |
|---|---|---|---|---|---|
| Mannan | Infection | Polyclonal | Rat vaginitis | Passive transfer with antibodies to mannan and SAP | 37, 53 |
| β-1,2 linked oligomannoside | Vaccination with liposomal-packed component | Polyclonal | Mouse and mouse vaginitis | Correlation between agglutinating antibodies and resistance | 106, 108 |
| Passive transfer | Monoclonal (IgG) | Mouse and mouse vaginitis | Apparent epitope and antibody specificity; agglutination not sufficient for protection | 106, 108 | |
| Vaccination with mannan-protein conjugate | Polyclonal | Mouse vaginitis | Correlation between agglutinating antibodies and resistance | 110 | |
| Yeast killer toxin receptor (β-1,6 glucan) | Infection; vaccination with anti-idiotypic antibodies | Polyclonal (IgA) | Rat vaginitis | Direct candidacidal activity | 38, 224, 225 |
| SAP | Infection | Polyclonal (IgA) | Rat vaginitis | Polyclonal antibodies contained both anti-mannan and anti-SAP immunoglobulins; anti-SAP antibodies may contribute to resistance | 37, 53 |
| hsp90 | Human infection | Polyclonal | Human and mouse | Presence of antibody correlates with protection; passive transfer reduces mortality rate | 188, 193, 194 |
| LKVIRK epitope | Monoclonal (IgG) | Mouse | Prophylaxis reduced mortality | 194 | |
| Human infection | Human recombinant antibody | Mouse | Reduced mortality in acute infection and reduced organ burden in chronic model | 196 | |
| hsp70 | Human infection | Polyclonal | Human | Proposed as a general intermediate mechanism between innate and specific immunity | 141 |
| hsp75 | Infection | Polyclonal (IgG) | Mouse | High levels of antibodies correlated with resolution of infection | 46, 47 |
| Non-hsp 96-kDa moiety | Infection | Polyclonal (IgG) | Mouse | High levels of antibodies correlated with resolution of infection | 47 |
| Enolase | Vaccination | Polyclonal | Mouse | Passive transfer in a lethal but not acute model | 304 |
| p43 immunosup- ressive factor | Vaccination | Polyclonal | Mouse | Neutralization of immunosuppressive properties; no lethal model | 292 |
Several authors have found that the carbohydrate portion of many candidal cell surface adhesins and receptors is important in fungal adherence to host epithelial cells and proteins (32, 40, 51, 65, 66, 109, 135–137, 157, 200, 201, 250). Han and Cutler (106) immunized mice with a mannan fraction (previously encapsulated into liposomes) implicated in the adhesion of C. albicans cells to macrophages (see the section on carbohydrate component, above) (135) to induce protective antibody responses (106). Circulating agglutinins specific for this fraction correlated with an increased resistance to disseminated candidiasis. Specific circulating antibodies that recognized the acid-labile mannan portion of the phosphomannoprotein adhesin complex were at least partly responsible for the protection. Encapsulation of the antigen in lipid was necessary to produce the protective response, thus suggesting that some undetermined coadjuvant factors may participate in triggering the host humoral response.
In the same study, these authors tested two monoclonal antibodies specific for different mannan epitopes in the adhesin fraction. Both antibodies agglutinated Candida cells, but only one of them protected mice against disseminated candidiasis. The protective antibody recognized the acid-labile adhesin, while the nonprotective antibody recognized a different epitope in the fraction. Although further experimentation is necessary to determine the mechanism by which anti-adhesin antibodies protect the host against disseminated candidiasis, the authors suggest that such antibodies may alter the adherence of yeast cells in vivo or may enhance phagocytosis (106). Taking into account that patients with disseminated candidiasis vary not only in whether they express a good humoral response to candidal antigens but also in the antigen specificity and class of antibodies produced and the epitopes they recognize on particular antigens (189), these observations underscore the importance of defining precisely which fungal epitopes can induce protective responses and indicate that only antibodies with certain specificities are protective. In this context, it has been reported recently that a mannan vaccine (liposome-encapsulated cell surface mannans) and the protective monoclonal antibody previously described by Han and Cutler (106, 107) protect mice against Candida vaginitis (108) but that a mannan-protein conjugate vaccine can also induce a protective immune response against the same infection in mice (110). Hence, the diagnostic implications of using these carbohydrate epitopes or monoclonal antibodies against them to detect specific antibody or antigen, respectively, in sera from patients with disseminated candidiasis needs to be investigated.
Polonelli et al. (224) demonstrated that secretory anti-idiotype IgA elicited by intravaginal vaccination with a monoclonal antibody with specificity for a yeast killer toxin from Pichia anomala (which kills Candida cells) protected rats from infectious challenge with C. albicans by molecular mimicking of yeast killer toxin activity as its internal image. In this case, the protective antibodies were not anti-Candida antibodies. Consistent with this observation was a previous report from this group showing that yeast killer toxin-like anti-idiotypic antibodies specifically react with the C. albicans killer toxin receptor (223). The killer toxin receptor is β-1,6 glucan and not a wall-associated protein or glycoprotein (254). Later, Polonelli et al. (225) showed that exposure to C. albicans bearing the killer toxin receptor stimulated the production of antibodies that were functionally equivalent to anti-idiotypic antibodies because they were also able to confer a significant protection against Candida cells in a model of rat vaginitis. Antibodies that mimicked killer toxin were found in unvaccinated mice that were exposed to the fungus. Such antibodies were recovered from the vaginal fluid of women with candidiasis, and, once purified, they were able to afford passive protection in the animal model. The authors speculated that the host immune response may exploit the killer toxin receptor of microbial pathogens to produce microbicidal antibodies (38). In any case, additional clinical studies (with a large number of subjects) are required to demonstrate a correlation between infection or simple colonization by C. albicans and levels of antibodies to anti-killer toxin.
In another study, antibodies to the cell wall carbohydrate component were also implicated in mediating protection in rat vaginal candidiasis. Cassone et al. (37) showed that the spontaneous clearing of the initial infection rendered the animals highly resistant to a second vaginal challenge with the fungus. The vaginal fluid of these Candida-resistant rats contained antibodies to mannan constituents and SAP and was capable of transferring a degree of protection against Candida cells to naive rats. This protection was mediated by the immunoglobulins present in the vaginal fluid. These results suggest that acquired anti-candidal protection in this vaginitis model is mediated at least in part by antibodies, among which those directed against the mannan antigen(s) might play a key role.
Recently, a protective role for anti-enolase antibodies has been suggested (304). Thus, passive protection against a subacute but lethal challenge with C. albicans cells in a murine system was provided by repeated administration of immune serum. The protective effect appeared not to cause the initial clearance of fungi but to afford protection at some later stage in infection.
As noted in a previous section of this review, antibody to the p43 immunosuppressive factor provided protection against a fungal challenge following vaccination with the factor or passive transfer of anti-factor serum (292).
As discussed above, a protective role for antibodies to hsp90 has been suggested on the basis of both correlates of antibody titers in patients recovering from candidiasis and passive-protection experiments in murine models (186, 188, 190–193, 196). A human recombinant monoclonal antibody to an epitope recognized by all positive sera has been found to provide passive protection in mice (196). hsp70s are immunodominant antigens that elicit an antibody response in different types of microbial infection (141, 142, 177). Such responses to conserved epitopes may confer a level of cross-species protection intermediate between innate and acquired immunity (141).
The potential of additional candidal cell wall proteins to provide targets for protective antibodies has been pointed out by Casadevall (28) and Matthews and colleagues (185, 189). We have previously shown that Fab fragments of a monoclonal antibody to a putative structural protein of the wall of C. albicans germ tubes (31) inhibited morphogenesis in vitro (34). Casadevall (28) also emphasized that protective antibodies are epitope and isotype specific. On the one hand, this raises questions about whether complex antigen sources such as intact cells or cell extracts will be successful in producing a general protective antibody response; on the other hand, it suggests that specific antigens and epitopes may be successful in this regard. In addition, antibodies may be able to enhance the efficacy of antifungal drugs. Certain monoclonal antibodies to the glucuronoxylomannan of Cryptococcus neoformans not only provide passive protection against infection but also enhance the efficacy of antifungal drugs (67, 206, 207). Preliminary experiments with C. albicans suggest that a monoclonal antibody to the acid-labile mannan adhesin can prolong survival in infected mice receiving nontherapeutic doses of amphotericin B and fluconazole (107).
Another controversial issue is the diagnosis of candidiasis, mostly in its invasive form. Systemic candidiasis is difficult to diagnose antemortem because of the lack of a typical clinical picture; in addition, although advances in blood culture methods have improved the detection of fungemia, routine blood cultures are relatively insensitive and make take several days to establish the diagnosis of candidemia (129, 240). The need for a rapid noninvasive test for the diagnosis of invasive Candida infections has focused the interest of many research groups for more than a quarter of a century (reviewed in references 57, 129, 209, and 240). In this context, the potential of several candidal antigens and antibodies to them, including several cell wall-associated antigenic species, has been examined. The principal cell wall components that have been used in these tests are mannan and enolase in both antigen and antibody detection schemes. Other antigens have also been detected in immunoblot analyses. The diagnostic applications of these species were described more extensively in previous sections. As discussed elsewhere in this review, some of these tests in the hands of their developers provided both sensitivity and specificity. Thus, the answer to the general question whether serologic tests for candidiasis can be diagnostic is yes. However, a distinct question is whether these tests are clinically useful and feasible to perform. In this area, the answer is more ambiguous. The predictive value of serologic tests generally increases when serial serum samples are obtained, tests for more than one antigen or antibody are used, or serological tests are used in conjunction with blood culture procedures. A successful commercially available test, which simply does not exist to date, will require both high specificity and high sensitivity to discriminate between colonization and infection. New tests must improve on previous methods in these areas and be more timely, allowing a more rapid diagnosis of the invasive forms of the disease. This is clearly an aspect in which our increasing understanding of the antibody response to Candida could have direct implications. Thus, detection of specific immunodominant antigens, rather than undefined antigenic preparations, and/or detection of antibodies to such antigens may prove suitable for diagnosis of invasive candidiasis. The ideal “antigenic marker” for deep infection needs to meet certain criteria in order to be useful in the diagnosis of candidiasis. First, it has to be expressed in all strains of C. albicans and under different growing conditions. Second, it has to be expressed and trigger an immune response during infection, preferably at the early stages. Finally, it should not elicit a major immune response during the interactions of C. albicans with the host as a component of the normal human flora. As mentioned earlier in this review in the discussion of individual cell wall molecules, a number of cell wall antigens with potential utility as antigenic markers have been identified, characterized, cloned, and sequenced. With their amino acid sequences readily available and the application of new epitope-mapping techniques, it is now easier to identify specific epitopes within these antigens and to adapt this knowledge to the development of improved serologic tests for the diagnosis of candidiasis.
SUMMARY AND OUTLOOK
Morbidity and mortality rates associated with systemic infections by C. albicans remain unacceptably high, the main reasons being the difficulties in the diagnosis and treatment of this type of infection. Many research laboratories have examined the utility of detecting antigenemia with candidal moieties or host antibodies produced to fungal antigens for diagnosis of disseminated candidiasis, and studies towards this objective continue in several laboratories (127, 253, 305). However, although urgently needed, an early, accurate, and reliable serology-based procedure for diagnosis is not commercially available and in general use, nor are alternative approaches for the therapy and prophylaxis of candidiasis available. Clearly, the development of these innovative tools can directly benefit from a better understanding of the mechanisms of pathogenicity displayed by C. albicans, especially the role of the antibody response during disseminated candidiasis. As stated above, cell wall proteins and mannoproteins of C. albicans are major elicitors of the host immune response, and our increasing knowledge of the identity and expression of these components may assist in the development of innovative tools leading to a better management of candidiasis.
The demonstration of protective antibody effects has benefited from major advances in different fields such as (i) our increasing knowledge of the pathogenicity mechanisms associated with Candida infections, which can help identify opportunities for immunointervention; (ii) the identification and characterization of individual cell wall moieties displaying antigenic properties, which may represent potential candidates for vaccine development; and (iii) the advent of the monoclonal antibody and antibody engineering technologies, which have renewed interest in serum therapy as a safe, inexpensive alternative to treat infections. As stated by Matthews and Burnie (189), a number of questions need to be answered when assessing the therapeutic potential of antibodies to candidal antigens (e.g., which types of candidal infections and groups of patients would respond to antibodies, which Candida species are potentially treatable with which antibodies, and whether there is synergism between antibodies to different antigens and between antibodies and antifungal agents). However, it is expected that the currently expanding knowledge in these areas could ultimately result in the design of innovative prophylactic and therapeutic strategies for these infections.
ACKNOWLEDGMENTS
Work in the laboratory of J.P.M. was supported by grants from DIGICyT (PM92-0246) and CICyT (SAF95-0595), Ministerio de Educación y Ciencia, and FISSS (93/0801), Ministerio de Sanidad y Consumo, Spain. Work in the laboratory of W.L.C. has been supported by Public Health Service grant AI23416 from the National Institutes of Health. The support of grant CRG931457 (NATO Collaborative Research Grants Programme) to W.L.C. and J.P.M. is also acknowledged. J.L.L.R. acknowledges support from Public Health Service grant 1R29 AI42401-01 and a Pfizer Medical Education grant.
REFERENCES
- 1.Aguiar J M, Baquero F, Jones J M. Candida albicans exocellular antigens released into a synthetic culture medium: characterization and serological response in rabbits. J Gen Microbiol. 1993;139:3005–3010. doi: 10.1099/00221287-139-12-3005. [DOI] [PubMed] [Google Scholar]
- 2.Alaei S, Larcher C, Ebenbichler C, Prodinger W M, Janatova J, Dierich M P. Isolation and biochemical characterization of the iC3b receptor of Candida albicans. Infect Immun. 1993;61:1395–1399. doi: 10.1128/iai.61.4.1395-1399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alloush H M, López-Ribot J L, Chaffin W L. Dynamic expression of cell wall proteins of Candida albicans revealed by probes from cDNA clones. J Med Vet Mycol. 1996;34:91–97. [PubMed] [Google Scholar]
- 4.Alloush H M, López-Ribot J L, Masten B J, Chaffin W L. 3-Phosphoglycerate kinase: a glycolytic enzyme present in the cell wall of Candida albicans. Microbiology. 1997;143:321–330. doi: 10.1099/00221287-143-2-321. [DOI] [PubMed] [Google Scholar]
- 5.Anderson J, Mihalik R, Soll D R. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J Bacteriol. 1990;172:224–235. doi: 10.1128/jb.172.1.224-235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson T J, Bryant H E, Church D L. Evaluation of a Candida antigen detection method (Cand-Tec): experience from a university teaching hospital. Can J Infect Dis. 1992;3:167–172. doi: 10.1155/1992/725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angiolella L, Facchin M, Stringaro A, Maras B, Simonetti N, Cassone A. Identification of a glucan-associated enolase as a main cell wall protein of Candida albicans and an indirect target of lipopeptide antimycotics. J Infect Dis. 1996;173:684–690. doi: 10.1093/infdis/173.3.684. [DOI] [PubMed] [Google Scholar]
- 8.Arala-Chaves M P, Ribeiro A S, Santarem M M G, Coutinho A. Strong mitogenic effect for murine B lymphocytes of an immunosuppressor substance released by Streptococcus intermedius. Infect Immun. 1986;54:543–548. doi: 10.1128/iai.54.2.543-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arala-Chaves M P, Ribeiro A S, Vilanova M, Porto M T, Santarem M M G, Lima M. Correlation between B-cell mitogenicity and immunosuppressor effects of a protein released by porcine monocytes infected with African swine fever virus. Am J Vet Res. 1988;49:1955–1961. [PubMed] [Google Scholar]
- 10.Ataoglu H, Zueco J, Sentandreu R. Characterization of epitopes recognized by Candida factor 1 and 9 antisera by use of Saccharomyces cerevisiae mnn mutants. Infect Immun. 1993;61:3313–3317. doi: 10.1128/iai.61.8.3313-3317.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Axelsen N H, Kirkpatrick C H. Simultaneous characterization of free candida antigens and candida precipitins in a patient’s serum by means of crossed immunoelectrophoresis with intermediate gel. J Immunol Methods. 1973;2:245–249. doi: 10.1016/0022-1759(73)90050-1. [DOI] [PubMed] [Google Scholar]
- 12.Bailey J W, Sada E, Brass C, Bennet J E. Diagnosis of systemic candidiasis by latex agglutination for serum antigen. J Clin Microbiol. 1985;21:749–752. doi: 10.1128/jcm.21.5.749-752.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balish E, Balish M J, Salkowski C A, Lee K W, Bartizal K R. Colonization of congenitally athymic, gnotobiotic mice by Candida albicans. Appl Environ Microbiol. 1984;47:647–652. doi: 10.1128/aem.47.4.647-652.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barturen B, Bikandi J, San Millán R, Moragues M D, Régulez P, Quindós G, Pontón J. Variability in expression of antigens responsible for serotype specificity in Candida albicans. Microbiology. 1995;141:1535–1543. doi: 10.1099/13500872-141-7-1535. [DOI] [PubMed] [Google Scholar]
- 15.Bodey G P. Candidiasis. Pathogenesis, diagnosis and treatment. New York, N.Y: Raven Press; 1993. [Google Scholar]
- 16.Borg M, Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun. 1988;56:626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borg M, Rüchel R. Demonstration of fungal proteinase during phagocytosis of Candida albicans and Candida tropicalis. J Med Vet Mycol. 1990;28:3–14. [PubMed] [Google Scholar]
- 18.Bouali A, Robert R, Tronchin G, Senet J M. Characterization of binding of human fibrinogen to the surface of germ-tubes and mycelium of Candida albicans. J Gen Microbiol. 1987;133:545–551. doi: 10.1099/00221287-133-3-545. [DOI] [PubMed] [Google Scholar]
- 19.Bougnoux M E, Hill C, Moissenet D, Feuilhade de Chauvin M, Bonnay M, Vicens-Sprauel I, Pietri F, McNeil M, Kaufman L, Dupouy-Camet J, Bohuon C, Andremont A. Comparison of antibody, antigen, and metabolite assays for hospitalized patients with disseminated or peripheral candidiasis. J Clin Microbiol. 1990;28:905–909. doi: 10.1128/jcm.28.5.905-909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burford-Mason A P, Matthews R C, Williams J R B. Transient abrogation of immunosuppression in a patient with chronic mucocutaneous candidiasis following vaccination with Candida albicans. J Infect. 1987;14:147–157. doi: 10.1016/s0163-4453(87)91977-3. [DOI] [PubMed] [Google Scholar]
- 21.Brawner D L, Cutler J E. Variability in expression of cell surface antigens of Candida albicans during morphogenesis. Infect Immun. 1986;51:337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breitenbach M, Simon B, Probst G, Oberkofler H, Ferreira F, Briza P, Achatz G, Unger A, Ebner C, Kraft D, Hirschwehr R. Enolases are highly conserved fungal allergens. Int Arch Allergy Immunol. 1997;113:144–117. doi: 10.1159/000237521. [DOI] [PubMed] [Google Scholar]
- 23.Cabezudo I, Pfaller M, Gerarden T, Koontz F, Wentzel R, Gingrinch R, Heckman K, Burns C P. Value of the Cand-Tec candida antigen assay in the diagnosis and therapy of systemic candidiasis in high-risk patients. Eur J Clin Microbiol Infect Dis. 1989;8:770–777. doi: 10.1007/BF02185843. [DOI] [PubMed] [Google Scholar]
- 24.Calderone R A. Molecular interactions at the interface of Candida albicans and host cells. Arch Med Res. 1993;24:275–279. [PubMed] [Google Scholar]
- 25.Calderone R A. Recognition between Candida albicans and host cells. Trends Microbiol. 1993;1:55–58. doi: 10.1016/0966-842x(93)90033-n. [DOI] [PubMed] [Google Scholar]
- 26.Calderone R A, Braun P C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991;55:1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderone R A, Linehan L, Wadsworth E, Sandberg A L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988;56:252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casanova M, Chaffin W L. Cell wall glycoproteins of Candida albicans as released by different methods. J Gen Microbiol. 1991;137:1045–1051. doi: 10.1099/00221287-137-5-1045. [DOI] [PubMed] [Google Scholar]
- 30.Casanova M, Chaffin W L. Phosphate-containing proteins and glycoproteins of the cell wall of Candida albicans. Infect Immun. 1991;59:808–813. doi: 10.1128/iai.59.3.808-813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casanova M, Gil M L, Cardeñoso L, Martínez J P, Sentandreu R. Identification of wall-specific antigens synthesized during germ tube formation by Candida albicans. Infect Immun. 1989;57:262–271. doi: 10.1128/iai.57.1.262-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casanova M, López-Ribot J L, Monteagudo C, Llombart-Bosch A, Sentandreu R, Martínez J P. Identification of a 58-kilodalton cell surface fibrinogen-binding mannoprotein from Candida albicans. Infect Immun. 1992;60:4221–4229. doi: 10.1128/iai.60.10.4221-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casanova M, López-Ribot J L, Martínez J P, Sentandreu R. Characterization of cell wall proteins from yeast and mycelial cells of Candida albicans by labelling with biotin: comparison with other techniques. Infect Immun. 1992;60:4898–4906. doi: 10.1128/iai.60.11.4898-4906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casanova M, Martínez J P, Chaffin W L. Fab fragments from a monoclonal antibody against a germ tube mannoprotein block the yeast-to-mycelium transition in Candida albicans. Infect Immun. 1990;58:3810–3812. doi: 10.1128/iai.58.11.3810-3812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casanova M, Martínez J P, Chaffin W L. Identification of germ tube cell wall antigens of Candida albicans. J Med Vet Mycol. 1991;29:269–272. doi: 10.1080/02681219180000391. [DOI] [PubMed] [Google Scholar]
- 36.Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- 37.Cassone A, Boccanera M, Adriani D, Santoni G, De Bernardis F. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect Immun. 1995;63:2619–2624. doi: 10.1128/iai.63.7.2619-2624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassone A, Conti S, De Bernardis F, Polonelli L. Antibodies, killer toxins and antifungal immunoprotection: a lesson from nature. Immunol Today. 1997;18:164–169. doi: 10.1016/s0167-5699(97)84662-2. [DOI] [PubMed] [Google Scholar]
- 39.Chaffin W L, Stocco D M. Cell wall proteins of Candida albicans. Can J Microbiol. 1983;29:1438–1444. doi: 10.1139/m83-220. [DOI] [PubMed] [Google Scholar]
- 40.Chaffin W L, Collins B, Marx J N, Cole G T, Morrow K J., Jr Characterization of mutant strains of Candida albicans deficient in expression of a surface determinant. Infect Immun. 1993;61:3449–3458. doi: 10.1128/iai.61.8.3449-3458.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaffin, W. L., J. L. López-Ribot, M. Casanova, D. Gozalbo, and J. P. Martinez. Cell wall and associated proteins of Candida albicans: identification, function, and expression. Microbiol. Mol. Biol. Rev., in press. [DOI] [PMC free article] [PubMed]
- 42.Chaffin W L, Skudlarek J, Morrow K J. Variable expression of a surface determinant during proliferation of Candida albicans. Infect Immun. 1988;56:302–309. doi: 10.1128/iai.56.2.302-309.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chambers R S, Broughton M J, Cannon R D, Carne A, Emerson G W, Sullivan P A. An exo-β-(1,3)-glucanase of Candida albicans: purification of the enzyme and molecular cloning of the gene. J Gen Microbiol. 1993;139:325–334. doi: 10.1099/00221287-139-2-325. [DOI] [PubMed] [Google Scholar]
- 44.Chattaway F W, Holmes M R, Barlow A J E. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968;51:367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- 45.Chew W H, Theus T L. Candida precipitins. J Immunol. 1967;98:220–224. [PubMed] [Google Scholar]
- 46.Constantino P J, Gare N F, Warmington J P. Humoral immune responses to systemic Candida albicans infection in inbred mouse strains. Immunol Cell Biol. 1995;73:125–133. doi: 10.1038/icb.1995.20. [DOI] [PubMed] [Google Scholar]
- 47.Constantino P J, Franklyn K M, Gare N F, Warmington J R. Production of antibodies to antigens of Candida albicans in CBA/H mice. Infect Immun. 1994;62:1400–1405. doi: 10.1128/iai.62.4.1400-1405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coon J S, Weinstein R S. Blood group-related antigens as markers of malignant potential and heterogeneity in human carcinomas. Hum Pathol. 1986;17:1089–1106. doi: 10.1016/s0046-8177(86)80413-0. [DOI] [PubMed] [Google Scholar]
- 49.Critchley I A, Douglas L J. Isolation and partial characterization of an adhesin from Candida albicans. J Gen Microbiol. 1987;133:629–636. doi: 10.1099/00221287-133-3-629. [DOI] [PubMed] [Google Scholar]
- 50.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 51.Cutler J E, Brawner D L, Hazen K C, Jutila M A. Characteristics of Candida albicans adherence to mouse tissue. Infect Immun. 1990;58:1902–1908. doi: 10.1128/iai.58.6.1902-1908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Bernardis F, Agatensi L, Ross I K, Emerson G W, Lorenzini R, Sullivan P A, Cassone A. Evidence for a role for secreted aspartate proteinase of Candida albicans in vulvovaginal candidiasis. J Infect Dis. 1990;161:1276–1283. doi: 10.1093/infdis/161.6.1276. [DOI] [PubMed] [Google Scholar]
- 53.De Bernardis F, Boccanera M, Adriani D, Santoni G, Cassone A. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Protective role of antibodies in experimental candidal vaginitis, abstr. F70. [Google Scholar]
- 54.De Bernardis F, Chiani P, Ciccozzi M, Pellegrini G, Ceddia T, D’Offizzi G, Quinti I, Sullivan P A, Cassone A. Elevated aspartic proteinase secretion and experimental pathogenicity of Candida albicans isolates from oral cavities of subjects infected with human immunodeficiency virus. Infect Immun. 1996;64:466–471. doi: 10.1128/iai.64.2.466-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Bernardis F, Lorenzini F, Verticchio R, Agatensi L, Cassone A. Isolation, acid proteinase secretion, and experimental pathogenicity of Candida parapsilosis from outpatients with vaginitis. J Clin Microbiol. 1989;27:2598–2603. doi: 10.1128/jcm.27.11.2598-2603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Bernardis F, Molinari A, Boccanera M, Stringaro A, Robert R, Senet J M, Arancia G, Cassone A. Modulation of cell surface associated mannoprotein antigen expression in experimental candidal vaginitis. Infect Immun. 1994;62:509–519. doi: 10.1128/iai.62.2.509-519.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Repentigny L, Reiss E. Current trends in immunodiagnosis of candidiasis and aspergillosis. Rev Infect Dis. 1984;6:301–312. doi: 10.1093/clinids/6.3.301. [DOI] [PubMed] [Google Scholar]
- 58.Del Prete G. Human Th1 and Th2 lymphocytes: their role in the pathophysiology of atopy. Allergy. 1992;47:450–455. doi: 10.1111/j.1398-9995.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 59.Deslauriers N, Michaud J, Carré B, Léveillée C. Dynamic expression of cell-surface antigens probed with Candida albicans-specific monoclonal antibodies. Microbiology. 1996;142:1239–1248. doi: 10.1099/13500872-142-5-1239. [DOI] [PubMed] [Google Scholar]
- 60.Dixon D M, Cox R, Cutler J, Deepe G. Researchers use molecular immunology and technology to combat fungal pathogens. ASM News. 1996;62:81–84. [Google Scholar]
- 61.Dixon D M, McNeil M M, Cohen M L, Gellin B G, LaMontagne J R. Fungal infections: a growing threat. Public Health Rep. 1996;111:226–235. [PMC free article] [PubMed] [Google Scholar]
- 62.Domer J E. Candida cell wall mannan: a polysaccharide with diverse immunologic properties. Crit Rev Microbiol. 1989;17:33–51. doi: 10.3109/10408418909105721. [DOI] [PubMed] [Google Scholar]
- 63.Douglas L J. Adhesion of Candida species to epithelial surfaces. Crit Rev Microbiol. 1987;15:27–43. doi: 10.3109/10408418709104446. [DOI] [PubMed] [Google Scholar]
- 64.Douglas L J. Candida proteinases and candidosis. Crit Rev Biotechnol. 1988;8:121–129. doi: 10.3109/07388558809150541. [DOI] [PubMed] [Google Scholar]
- 65.Douglas L J. Mannoprotein adhesins of Candida albicans. In: Bennet J E, Hay R J, Peterson P K, editors. New strategies in fungal disease. Edinburgh, United Kingdom: Churchill Livingstone; 1992. pp. 34–50. [Google Scholar]
- 66.Douglas, L. J. 1995. Adhesin-receptor interactions in the attachment of Candida albicans to host epithelial cells. Can. J. Bot. 73(Suppl. 1):S1147–S1153.
- 67.Dromer F, Charreire J. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococosis and mechanism of action. J Infect Dis. 1991;163:114–120. doi: 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- 68.Edison A M, Manning-Zweerink M. Comparison of the extracellular proteinase activity produced by a low-virulence mutant of Candida albicans and its wild-type parent. Infect Immun. 1988;56:1388–1390. doi: 10.1128/iai.56.5.1388-1390.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Maghrabi E A, Dixon D M, Burnett J W. Characterization of Candida albicans epidermolytic proteases and their role in yeast-cell adherence to keratinocytes. Clin Exp Dermatol. 1990;15:183–191. doi: 10.1111/j.1365-2230.1990.tb02069.x. [DOI] [PubMed] [Google Scholar]
- 70.Elorza M V, Mormeneo S, García de la Cruz F, Gimeno C, Sentandreu R. Evidence for the formation of covalent bonds between macromolecules in the domain of the wall of Candida albicans mycelial cells. Biochem Biophys Res Commun. 1989;162:1118–1125. doi: 10.1016/0006-291x(89)90789-4. [DOI] [PubMed] [Google Scholar]
- 71.Elorza M V, Murgui A, Sentandreu R. Dimorphism in Candida albicans: contribution of mannoproteins to the architecture of yeast and mycelial cells. J Gen Microbiol. 1985;131:2209–2216. doi: 10.1099/00221287-131-9-2209. [DOI] [PubMed] [Google Scholar]
- 72.Eroles P, Sentandreu M, Elorza M V, Sentandreu R. Cloning of a cDNA fragment encoding part of a 70 kDa heat shock protein of Candida albicans. FEMS Microbiol Lett. 1995;128:95–100. doi: 10.1111/j.1574-6968.1995.tb07506.x. [DOI] [PubMed] [Google Scholar]
- 73.Eroles P, Sentandreu M, Elorza M V, Sentandreu R. The highly immunogenic enolase and Hsp70p are adventitious Candida albicans cell wall proteins. Microbiology. 1997;143:313–320. doi: 10.1099/00221287-143-2-313. [DOI] [PubMed] [Google Scholar]
- 74.Escuro R S, Jacobs M, Gerson S L, Machicao A R, Lazarus H M. Prospective evaluation of a candida antigen detection test for invasive candidiasis in immunocompromised adult patients with cancer. Am J Med. 1989;87:621–627. doi: 10.1016/s0002-9343(89)80393-6. [DOI] [PubMed] [Google Scholar]
- 75.Faille C, Mackenzie D W R, Michalski J C, Poulain D. The evaluation of an enzyme immunoassay using neoglycolipids constructed from Candida albicans oligomannosides to define the specificity of anti-mannan antibodies. Eur J Clin Microbiol Infect Dis. 1992;11:438–446. doi: 10.1007/BF01961859. [DOI] [PubMed] [Google Scholar]
- 76.Faille C, Michalski J C, Strecker G, Mackenzie D W R, Camus D, Poulain D. Immunoreactivity of neoglycolipids constructed from oligomannosidic residues of Candida albicans cell wall. Infect Immun. 1990;58:3537–3544. doi: 10.1128/iai.58.11.3537-3544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Faille C, Wieruszeski J M, Lepage G, Michalski J C, Poulain D, Strecker G. 1H-NMR spectroscopy of mannooligosaccharides of the β-1,2-linked series released from the phosphopeptidomannan of Candida albicans VW32 (serotype A) Biochem Biophys Res Commun. 1991;181:1251–1258. doi: 10.1016/0006-291x(91)92073-s. [DOI] [PubMed] [Google Scholar]
- 78.Fernandes P A, Keen J N, Findlay J B C, Moradas-Ferreira P. A protein homologous to glyceraldehyde-3-phosphate dehydrogenase is induced in the cell wall of flocculent Kluyveromyces marxianus. Biochim Biophys Acta. 1992;1159:67–73. doi: 10.1016/0167-4838(92)90076-p. [DOI] [PubMed] [Google Scholar]
- 79.Fernandes P A, Sousa M, Moradas-Ferreira P. Flocculation of Kluyveromyces marxianus is induced by a temperature upshift. Yeast. 1993;9:859–866. doi: 10.1002/yea.320090806. [DOI] [PubMed] [Google Scholar]
- 80.Ferreira P, Soares R, Ribeiro A, Arala-Chaves M P. Correlation between specific immunosuppression and polyclonal B cell activation induced by a protein secreted by Streptococcus mutans. Scand J Immunol. 1988;27:549–554. doi: 10.1111/j.1365-3083.1988.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 81.Fidel P A, Jr, Sobel J D. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335–348. doi: 10.1128/cmr.9.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Filice G, Yu B, Armstrong D. Immunodiffusion and agglutination tests for Candida in patients with neoplastic disease. J Infect Dis. 1977;135:349–357. doi: 10.1093/infdis/135.3.349. [DOI] [PubMed] [Google Scholar]
- 83.Franklyn K M, Warmington J R. Cloning and nucleotide sequence analysis of the Candida albicans enolase gene. FEMS Microbiol Lett. 1993;111:101–108. doi: 10.1111/j.1574-6968.1993.tb06368.x. [DOI] [PubMed] [Google Scholar]
- 84.Franklyn K M, Warmington J R, Ott A K, Ashman R B. An immunodominant antigen of Candida albicans shows homology to the enzyme enolase. Immunol Cell Biol. 1990;68:173–178. doi: 10.1038/icb.1990.24. [DOI] [PubMed] [Google Scholar]
- 85.Fujita S, Matsubara F, Matsuda T. Enzyme-linked immunosorbent assay measurement of fluctuations in antibody titer and antigenemia in cancer patients with and without candidiasis. J Clin Microbiol. 1986;23:568–575. doi: 10.1128/jcm.23.3.568-575.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukayama M, Wadsworth E, Calderone R A. Expression of the C3d-binding protein (CR2) from Candida albicans during experimental candidiasis as measured by lymphoblastogenesis. Infect Immun. 1992;60:8–12. doi: 10.1128/iai.60.1.8-12.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukazawa Y. Antigenic structure of Candida albicans. In: Kurstak E, Marquis G, Auger P, de Repentigny L, Montplaisir S, editors. Immunology of fungal diseases. New York, N.Y: Marcel Dekker, Inc.; 1989. pp. 37–62. [Google Scholar]
- 88.Fukazawa Y, Kagaya K. Molecular bases of adhesion of Candida albicans. J Med Vet Mycol. 1997;35:87–99. doi: 10.1080/02681219780000971. [DOI] [PubMed] [Google Scholar]
- 89.Fukazawa Y, Cassone A, Bistoni F, Howard D H, Kagaya K, Murphy J W, Cenci E, Lane I E, Menacci A, Puccetti P, Romani L, Spaccapelo R, Tonneti L, Wu-Hsieh B A. Mechanisms of cell-mediated immunity in fungal infections: a review. J Med Vet Mycol. 1994;32:123. doi: 10.1080/02681219480000781. [DOI] [PubMed] [Google Scholar]
- 90.Fukazawa Y, Nishikawa A, Suzuki M, Shinoda T. Immunochemical basis of the specificity of the yeast: immunochemical determinants of several antigenic factors of yeast. In: Preusser H J, editor. Medical mycology. Stuttgart, Germany: Gustav Fischer Verlag; 1980. pp. 126–136. [Google Scholar]
- 91.Fukazawa Y, Shinoda T, Tsuchiya T. Response and specificity of antibodies for Candida albicans. J Bacteriol. 1968;95:754. doi: 10.1128/jb.95.3.754-763.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fung J C, Donta S T, Tilton R C. Candida detection system (CAND-TEC) to differentiate between Candida albicans colonization and disease. J Clin Microbiol. 1986;24:542–547. doi: 10.1128/jcm.24.4.542-547.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gatermann S, Heesemann J, Laufs R. Identification of Candida albicans antigens recognized by sera of patients with candidiasis. Mykosen. 1986;29:343–354. doi: 10.1111/j.1439-0507.1986.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 94.Gentry L O, Wilkinson I D, Lea A S, Price M F. Latex agglutination test for detection of Candida antigen in patients with disseminated disease. Eur J Clin Microbiol. 1983;2:122–128. doi: 10.1007/BF02001577. [DOI] [PubMed] [Google Scholar]
- 95.Gil M L, Casanova M, Martínez J P. Changes in the cell wall glycoprotein composition of Candida albicans associated to the inhibition of germ tube formation by EDTA. Arch Microbiol. 1994;161:489–494. doi: 10.1007/BF00307769. [DOI] [PubMed] [Google Scholar]
- 96.Gil M L, Casanova M, Martínez J P, Sentandreu R. Antigenic cell wall mannoproteins in Candida albicans isolates and in other Candida species. J Gen Microbiol. 1991;137:1053–1061. doi: 10.1099/00221287-137-5-1053. [DOI] [PubMed] [Google Scholar]
- 97.Gil-Navarro I, Gil M L, Cervera A M, Casanova M, Martínez J P, Gozalbo D. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is a surface antigen, abstr. F51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gil-Navarro I, Gil M L, Casanova M, O’Connor J E, Martínez J P, Gozalbo D. The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is a surface antigen. J Bacteriol. 1997;179:4992–4999. doi: 10.1128/jb.179.16.4992-4999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gómez M J, Torosantucci A, Arancia S, Maras B, Parisi L, Cassone A. Purification and biochemical characterization of a 65-kilodalton mannoprotein (MP65), a main target of anti-Candida cell-mediated immune responses in humans. Infect Immun. 1996;64:2577–2584. doi: 10.1128/iai.64.7.2577-2584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goudot-Crozel V, Caillol D, Djabali M, Dessein A J. The major parasite surface antigen associated with human resistance to schistosomiasis is a 37-kDa glyceraldehyde-3P-dehydrogenase. J Exp Med. 1989;170:2065–2080. doi: 10.1084/jem.170.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gozalbo D, Elorza M V, Sanjuán R, Marcilla A, Valentín E, Sentandreu R. Critical steps in fungal cell wall synthesis: strategies for their inhibition. Pharmacol Ther. 1993;60:337–345. doi: 10.1016/0163-7258(93)90015-6. [DOI] [PubMed] [Google Scholar]
- 102.Greenfield R A, Bussey M J, Stephens J L, Jones J M. Serial enzyme-linked immunosorbent assays for antibody to Candida antigens during induction chemotherapy for acute leukemia. J Infect Dis. 1983;148:275–283. doi: 10.1093/infdis/148.2.275. [DOI] [PubMed] [Google Scholar]
- 103.Gutiérrez J, Liébana J. Immunological methods for the detection of structural components and metabolites of bacteria and fungi in blood. Clinical effectiveness. Ann Biol Clin. 1993;51:83–90. [PubMed] [Google Scholar]
- 104.Gutiérrez J, Maroto C, Piédrola G, Martín E, Pérez J A. Circulating Candida antigens and antibodies: useful markers of candidemia. J Clin Microbiol. 1993;31:2550–2552. doi: 10.1128/jcm.31.9.2550-2552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hakomori S. Philip Levine Award Lecture. Blood group glycolipid antigens and their modifications as human cancer antigens. Am J Clin Pathol. 1984;82:635–648. doi: 10.1093/ajcp/82.6.635. [DOI] [PubMed] [Google Scholar]
- 106.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han Y, Cutler J E. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Monoclonal antibody B6.1 protects neutropenic mice and enhances therapeutic potential of antifungal drugs against disseminated candidiasis, abstr. F87. [Google Scholar]
- 108.Han Y, Cutler J E. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Antibodies induced by Candida albicans surface mannans and monoclonal antibody B6.1 specific for the mannans help mice resist Candida vaginitis, abstr. F72. [Google Scholar]
- 109.Han Y, Rooijen N, Cutler J E. Binding of Candida albicans yeast cells to mouse popliteal lymph node tissue is mediated by macrophages. Infect Immun. 1993;61:3244–3249. doi: 10.1128/iai.61.8.3244-3249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Han Y, Ulrich M A, Cutler J E. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Candida albicans surface mannan-protein conjugates induce a protective immune response against candidiasis, abstr. F71. [Google Scholar]
- 111.Hasenclever H F, Mitchel W O. Antigenic studies of Candida. I. Observation of two antigenic groups in Candida albicans. J Bacteriol. 1961;82:570–573. doi: 10.1128/jb.82.4.570-573.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hayette M P, Strecker G, Faille C, Dive D, Camus D, Mackenzie D W R, Poulain D. Presence of human antibodies reacting with Candida albicans O-linked oligomannosides revealed by using an enzyme-linked immunosorbent assay and neoglycolipids. J Clin Microbiol. 1992;30:411–417. doi: 10.1128/jcm.30.2.411-417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hazen K C. Cell surface hydrophobicity of medically important fungi, specially Candida species. In: Doyle R J, Rosenberg M, editors. Microbial cell surface hydrophobicity. Washington, D.C: American Society for Microbiology; 1990. pp. 249–295. [Google Scholar]
- 114.Hazen K C, Hazen B W. Hydrophobic surface protein masking by the opportunistic fungal pathogen Candida albicans. Infect Immun. 1992;60:1499–1508. doi: 10.1128/iai.60.4.1499-1508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hazen K C, Hazen B W. Surface hydrophobic and hydrophilic protein alterations in Candida albicans. FEMS Microbiol Lett. 1993;107:83–88. doi: 10.1111/j.1574-6968.1993.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 116.Hazen K C, Lay J G, Hazen B W, Fu R C G, Murthy S. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990;58:3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heidenreich F, Dierich M P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985;50:598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herent P, Stynen D, Hernando F, Fruit J, Poulain D. Retrospective evaluation of two latex agglutination tests for detection of circulating antigens during invasive candidosis. J Clin Microbiol. 1992;30:2158–2164. doi: 10.1128/jcm.30.8.2158-2164.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hernando F L, Cailliez J C, Trinel P A, Faille C, Mackenzie D W R, Poulain D. Qualitative and quantitative differences in recognition patterns of Candida albicans protein and polysaccharide antigens by human sera. J Med Vet Mycol. 1993;31:219–226. [PubMed] [Google Scholar]
- 120.Hernando F L, Estévez J J, Cebrian M, Poulain D, Pontón J. Identification of Candida albicans cell wall antigens lost during subculture in synthetic media. J Med Vet Mycol. 1993;31:227–237. [PubMed] [Google Scholar]
- 121.Hostetter M K. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial cells. Clin Microbiol Rev. 1994;7:29–42. doi: 10.1128/cmr.7.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hostetter M K, Lorenz J S, Preus L, Kendrick K E. The iC3b receptor on Candida albicans: subcellular localization and modulation of receptor expression by glucose. J Infect Dis. 1990;161:761–768. doi: 10.1093/infdis/161.4.761. [DOI] [PubMed] [Google Scholar]
- 123.Ishiguro A, Homma M, Sukai T, Higashide K, Torii S, Tanaka K. Immunoblotting analysis of sera from patients with candidal vaginitis and healthy females. J Med Vet Micol. 1992;30:281–292. doi: 10.1080/02681219280000371. [DOI] [PubMed] [Google Scholar]
- 124.Ishiguro A, Homma M, Torii S, Tanaka K. Identification of Candida albicans antigens reactive with immunoglobulin E antibody of human sera. Infect Immun. 1992;60:1550–1557. doi: 10.1128/iai.60.4.1550-1557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ito K, Ishiguro A, Kanbe T, Tanaka K, Torii S. Detection of IgE antibody against Candida albicans enolase and its crossreactivity to Saccharomyces cerevisiae enolase. Clin Exp Allergy. 1995;25:522–528. doi: 10.1111/j.1365-2222.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 126.Ito K, Ishiguro A, Kanbe T, Tanaka K, Torii S. Characterization of IgE-binding epitopes on Candida albicans enolase. Clin Exp Allergy. 1995;25:529–535. doi: 10.1111/j.1365-2222.1995.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 127.Jarvis W R. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis. 1995;20:1526–1530. doi: 10.1093/clinids/20.6.1526. [DOI] [PubMed] [Google Scholar]
- 128.Jones J M. Quantitation of antibody against cell wall mannan and a major cytoplasmic antigen of Candida in rabbits, mice, and humans. Infect Immun. 1980;30:78–89. doi: 10.1128/iai.30.1.78-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones J M. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3:32–45. doi: 10.1128/cmr.3.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones L, Hobden C, O’Shea P. Use of a real time fluorescent probe to study the electrostatic properties of the cell surface of Candida albicans. Mycol Res. 1995;99:969–976. [Google Scholar]
- 131.Kahn F W, Jones J M. Latex agglutination test for detection of Candida antigens in sera of patients with invasive candidiasis. J Infect Dis. 1986;153:579–585. doi: 10.1093/infdis/153.3.579. [DOI] [PubMed] [Google Scholar]
- 132.Kaminishi H, Hamatake H, Cho T, Tamaki T, Suenaga N, Fujii T, Hagihara Y, Maeda H. Activation of blood clotting factors by microbial proteinases. FEMS Microbiol Lett. 1994;121:327–332. doi: 10.1111/j.1574-6968.1994.tb07121.x. [DOI] [PubMed] [Google Scholar]
- 133.Kaminishi H, Miyaguchi H, Tamaki T, Suenaga N, Hisamatsu M, Mihashi I, Matsumoto H, Maeda H, Hagihara Y. Degradation of humoral host defense by Candida albicans proteinase. Infect Immun. 1995;63:984–988. doi: 10.1128/iai.63.3.984-988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaminishi H, Tanaka M, Cho T, Maeda H, Hagihara Y. Activation of the plasma kallikrein-kinin system by Candida albicans proteinase. Infect Immun. 1990;58:2139–2143. doi: 10.1128/iai.58.7.2139-2143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kanbe T, Cutler J E. Evidence for adhesin activity in the acid-stable moiety of the phosphomannoprotein cell wall complex of Candida albicans. Infect Immun. 1994;62:1662–1668. doi: 10.1128/iai.62.5.1662-1668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kanbe T, Han Y, Redgrave B, Riesselman M H, Cutler J E. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph node tissue. Infect Immun. 1993;61:2578–2584. doi: 10.1128/iai.61.6.2578-2584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanbe T, Jutila M A, Cutler J E. Evidence that Candida albicans binds via a unique adhesion system on phagocytic cells in the marginal zone of the mouse spleen. Infect Immun. 1992;60:1972–1978. doi: 10.1128/iai.60.5.1972-1978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kanbe T, Li R K, Wadsworth E, Calderone R A, Cutler J E. Evidence for the expression of the C3d receptor of Candida albicans in vitro and in vivo by immunofluorescence and immunoelectron microscopy. Infect Immun. 1991;59:1832–1838. doi: 10.1128/iai.59.5.1832-1838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kapteyn J C, Montijn R C, Dijkgraaf G J, Klis F M. Identification of β-1,6-glucosylated cell wall proteins in yeast and hyphal forms of Candida albicans. Eur J Cell Biol. 1994;65:402–407. [PubMed] [Google Scholar]
- 140.Kapteyn J C, Montijn R C, Dijkgraaf G J, van den Ende H, Klis F M. Covalent association of β-1,3-glucan with β-1,6-glucosylated mannoproteins in cell walls of Candida albicans. J Bacteriol. 1995;177:3788–3792. doi: 10.1128/jb.177.13.3788-3792.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kauffmann S H E. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 142.Kauffmann S H E, Schoel B. Heat shock proteins as antigen in immunity against infection and self. In: Morimoto R I, Tissieres A, Georgopoulos G, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 495–531. [Google Scholar]
- 143.Kerkering T M, Espinel-Ingroff A, Shadomy S. Detection of Candida antigenemia by counterimmunoelectrophoresis in patients with invasive candidiasis. J Infect Dis. 1979;140:659–664. doi: 10.1093/infdis/140.5.659. [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi H, Giummelly P, Takahashi S, Ishida M, Sato J, Takaku M, Nishidate Y, Shibata N, Okawa Y, Suzuki S. Candida albicans serotype A strains grow in yeast extract added Sabouraud liquid medium at pH 2.0, elaborating mannans without β-1,2 linkage and phosphate group. Biochem Biophys Res Commun. 1991;175:1003–1009. doi: 10.1016/0006-291x(91)91664-x. [DOI] [PubMed] [Google Scholar]
- 145.Kobayashi H, Shibata N, Mitobe H, Ohkubo Y, Suzuki S. Structural study of phosphomannan of yeast-form cells of Candida albicans J-1012 strain with special reference to application of mild acetolysis. Arch Biochem Biophys. 1989;272:364–375. doi: 10.1016/0003-9861(89)90230-0. [DOI] [PubMed] [Google Scholar]
- 146.Kobayashi H, Shibata N, Nakada M, Chaki S, Mizugami K, Ohkubo Y, Suzuki S. Structural analysis of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch Biochem Biophys. 1990;278:195–204. doi: 10.1016/0003-9861(90)90248-w. [DOI] [PubMed] [Google Scholar]
- 147.Kobayashi H, Shibata N, Suzuki S. Evidence for oligomannosyl residues containing both β-1,2 and α-1,2 linkages as a serotype A-specific epitope(s) in mannans of Candida albicans. Infect Immun. 1992;60:2106–2109. doi: 10.1128/iai.60.5.2106-2109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kobayashi H, Takahashi S I, Shibata N, Miyauchi M, Ishida M, Sato J, Maeda K, Suzuki S. Structural modification of cell wall mannans of Candida albicans serotype A strains grown in yeast extract-Sabouraud liquid medium under acidic conditions. Infect Immun. 1994;62:968–973. doi: 10.1128/iai.62.3.968-973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kobayashi H, Takaku M, Nishidate Y, Takahashi S, Takikawa M, Shibata N, Okawa Y, Suzuki S. Structure of the d-mannan of the pathogenic yeasts, Candida stellatoidea ATCC 20408 (type II) strain, in comparison with that of C. stellatoidea ATCC 36232 (type I) strain. Carbohydr Res. 1992;231:105–116. doi: 10.1016/0008-6215(92)84012-h. [DOI] [PubMed] [Google Scholar]
- 150.Kogan G, Pavliak V, Masler L. Structural studies of mannan of the cell wall of pathogenic yeast Candida albicans serotypes A and B and Candida parapsilosis. Carbohydr Res. 1988;172:243–253. doi: 10.1016/s0008-6215(00)90858-9. [DOI] [PubMed] [Google Scholar]
- 151.Kortenkangas-Savolainen O, Kalimo K, Lammintausta K, Savolainen J. IgE-binding components of baker’s yeast (Saccharomyces cerevisiae) recognized by immunoblotting analysis. Simultaneous IgE-binding to mannan and 46-48 kDa allergens of Saccharomyces cerevisiae and Candida albicans. Clin Exp Allergy. 1993;23:179–184. doi: 10.1111/j.1365-2222.1993.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 152.La Valle R, Bromuro C, Ranucci L, Muller H M, Crisanti A, Cassone A. Molecular cloning and expression of a 70-kilodalton heat shock protein of Candida albicans. Infect Immun. 1995;63:4039–4045. doi: 10.1128/iai.63.10.4039-4045.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee K W, Shalaby K A, Thakur A, Medhat A, Karim A M, LoVerde P T. Cloning of the gene for phosphoglycerate kinase from Schistosoma mansoni and characterization of its gene product. Mol Biochem Parasitol. 1995;71:221–231. doi: 10.1016/0166-6851(95)91598-o. [DOI] [PubMed] [Google Scholar]
- 154.Lemieux C, St-Germain G, Vincelette J, Kaufman L, de Repentigny L. Collaborative evaluation of antigen detection by a commercial latex agglutination test and enzyme immunoassay in the diagnosis of invasive candidiasis. J Clin Microbiol. 1990;28:249–253. doi: 10.1128/jcm.28.2.249-253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Levitz S M. Overview of host defenses in fungal infections. Clin Infect Dis. 1992;14:S37–S42. doi: 10.1093/clinids/14.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 156.Lew M A, Siber G R, Donahue D M, Maiorca F. Enhanced detection with an enzyme-linked immunosorbent assay of candida mannan in antibody containing serum after heat extraction. J Infect Dis. 1982;145:45–56. doi: 10.1093/infdis/145.1.45. [DOI] [PubMed] [Google Scholar]
- 157.Li R K, Cutler J E. Chemical definition of an epitope adhesin molecule on Candida albicans. J Biol Chem. 1993;268:18293–18299. [PubMed] [Google Scholar]
- 158.Lima M, Bandeira A, Portnoi D, Ribeiro D, Arala-Chaves M P. Protective effect of a T-cell-dependent, immunosuppressive, B-cell-mitogenic protein (F3′EP-Si, or P90) produced by Streptococcus intermedius. Infect Immun. 1992;60:3571–3578. doi: 10.1128/iai.60.9.3571-3578.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Linehan L, Wadsworth E, Calderone R A. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988;56:1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.López-Ribot J L, Chaffin W L. Members of the hsp70 family of proteins in the cell wall of Saccharomyces cerevisiae. J Bacteriol. 1996;178:4724–4726. doi: 10.1128/jb.178.15.4724-4726.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.López-Ribot J L, Alloush H M, Masten B J, Chaffin W L. Evidence for the presence in the cell wall of Candida albicans of a protein related to the hsp70 family. Infect Immun. 1996;64:3333–3340. doi: 10.1128/iai.64.8.3333-3340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.López-Ribot J L, Casanova M, Martínez J P, Sentandreu R. Characterization of cell wall proteins of yeast and hydrophobic mycelial cells of Candida albicans. Infect Immun. 1991;59:2324–2332. doi: 10.1128/iai.59.7.2324-2332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.López-Ribot J L, Casanova M, Monteagudo C, Sepúlveda P, Martínez J P. Evidence for the presence of a high-affinity laminin receptor-like molecule on the surface of Candida albicans yeast cells. Infect Immun. 1994;62:742–746. doi: 10.1128/iai.62.2.742-746.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.López-Ribot J L, Navarro D, Sepúlveda P, Nogueira J M, Casanova M, Martínez J P. A comparative study on cell wall antigens and cell surface hydrophobicity in clinical isolates of Candida albicans. Mycopathologia. 1994;127:1–13. doi: 10.1007/BF01104005. [DOI] [PubMed] [Google Scholar]
- 165.López-Ribot J L, Gozalbo D, Sepúlveda P, Casanova M, Martínez J P. Preliminary characterization of the material released to the culture medium by Candida albicans yeast and mycelial cells. Antonie Leeuwenhoek. 1995;68:195–201. doi: 10.1007/BF00871815. [DOI] [PubMed] [Google Scholar]
- 166.López-Ribot J L, Martínez J P, Chaffin W L. Comparative study of the C3d receptor and 58-kilodalton fibrinogen-binding mannoproteins of Candida albicans. Infect Immun. 1995;63:2126–2132. doi: 10.1128/iai.63.6.2126-2132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.López-Ribot J L, Casanova M, Gil M L, Martínez J P. Common and form-specific cell wall antigens of Candida albicans as released by chemical and enzymatic treatments. Mycopathologia. 1996;134:13–20. doi: 10.1007/BF00437047. [DOI] [PubMed] [Google Scholar]
- 168.López-Ribot J L, Monteagudo C, Sepúlveda P, Casanova M, Martínez J P, Chaffin W L. Expression of the fibrinogen binding mannoprotein and the laminin receptor of Candida albicans in vitro and in infected tissues. FEMS Microbiol Lett. 1996;142:117–122. doi: 10.1111/j.1574-6968.1996.tb08417.x. [DOI] [PubMed] [Google Scholar]
- 169.López-Ribot J L, Sepúlveda P, Casanova M, Navarro D, Martínez J P. Abstracts of the American Society for Microbiology Conference on Candida and Candidiasis: Biology, Pathogenesis, and Management. Washington, D.C: American Society for Microbiology; 1996. Expression of the fibrinogen-binding mannoprotein in clinical strains of Candida albicans and immunoblotting analysis of sera from patients with candidiasis, abstr. A16. [Google Scholar]
- 170.Louria D B, Brayton R G, Finkel G. Studies on the pathogenesis of experimental Candida albicans infections in mice. Sabouraudia. 1963;2:271–283. [Google Scholar]
- 171.Luna-Arias J P, Andaluz E, Ridruejo J C, Olivero I, Larriba G. The major exoglucanase from Candida albicans: a non-glycosylated secretory monomer related to its counterpart from Saccharomyces cerevisiae. Yeast. 1991;7:833–841. doi: 10.1002/yea.320070808. [DOI] [PubMed] [Google Scholar]
- 172.MacDonald F, Odds F C. Inducible proteinase of Candida albicans in diagnostic serology and in the pathogenesis of systemic candidosis. J Med Microbiol. 1980;13:423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- 173.Maeda H, Maruo K, Kaminishi H, Hagihara Y. Microbial proteases as a universal trigger of kinin generation in microbial infections. In: Bönner G, Fritz H, Schoelkens B, Dietze G, editors. Recent progress on kinins. Basel, Switzerland: Birkhäuser Verlag; 1992. pp. 362–369. [Google Scholar]
- 174.Mankowski Z T. Production of glycoprotein by Candida albicans in a synthetic medium and its influence on the growth of newborn mice. Mycopathol Mycol Appl. 1968;36:113–118. doi: 10.1007/BF02051419. [DOI] [PubMed] [Google Scholar]
- 175.Manning-Zweerink M, Maloney C S, Mitchell T G, Weston H. Immunoblot analyses of Candida albicans associated antigens and antibodies in human sera. J Clin Microbiol. 1986;23:46–52. doi: 10.1128/jcm.23.1.46-52.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marcilla A, Elorza M V, Mormeneo S, Rico H, Sentandreu R. Candida albicans mycelial cell wall structure: supramolecular complexes released by Zymolyase, chitinase and β-mercaptoethanol. Arch Microbiol. 1991;155:312–319. doi: 10.1007/BF00243448. [DOI] [PubMed] [Google Scholar]
- 177.Maresca B, Kobayashi G S. Hsp70 in parasites as an inducible protective protein and as an antigen. Experientia. 1994;50:1067–1074. doi: 10.1007/BF01923463. [DOI] [PubMed] [Google Scholar]
- 178.Marot-Leblond A, Robert R, Aubry J, Ezcurra P, Senet J M. Identification and immunochemical characterization of a germ tube specific antigen of Candida albicans. FEMS Immunol Med Microbiol. 1993;7:175–186. doi: 10.1111/j.1574-695X.1993.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 179.Martínez J P, Gil M L, Casanova M, López-Ribot J L, García de Lomas J, Sentandreu R. Wall mannoproteins in cells from colonial phenotypic variants of Candida albicans. J Gen Microbiol. 1990;136:2421–2432. doi: 10.1099/00221287-136-12-2421. [DOI] [PubMed] [Google Scholar]
- 180.Martínez J P, López-Ribot J L, Gil M L, Sentandreu R, Ruiz-Herrera J R. Inhibition of the dimorphic transition of Candida albicans by the ornithine decarboxylase inhibitor 1,4-diaminobutanone: alterations in the glycoprotein composition of the cell wall. J Gen Microbiol. 1990;136:1937–1943. doi: 10.1099/00221287-136-10-1937. [DOI] [PubMed] [Google Scholar]
- 181.Martínez J P, López-Ribot J L, Chaffin W L. Heterogeneous surface distribution of the fibrinogen-binding protein on Candida albicans. Infect Immun. 1994;62:709–712. doi: 10.1128/iai.62.2.709-712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Masler L, Sikl D, Bauer S, Sandula J. Extracellular polysaccharide-protein complexes produced by selected strains of Candida albicans. Folia Microbiol. 1966;11:373–378. doi: 10.1007/BF02887694. [DOI] [PubMed] [Google Scholar]
- 183.Mason A B, Brandt M E, Buckley H R. Enolase activity associated with a Candida albicans cytoplasmic antigen. Yeast. 1989;5:S231–S240. [PubMed] [Google Scholar]
- 184.Mason A B, Buckley H R, Gorman J A. Molecular cloning and characterization of the Candida albicans enolase gene. J Bacteriol. 1993;175:2632–2639. doi: 10.1128/jb.175.9.2632-2639.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Matthews R C. Pathogenicity determinants of Candida albicans: potential targets for immunotherapy? Microbiology. 1994;140:1505–1511. doi: 10.1099/13500872-140-7-1505. [DOI] [PubMed] [Google Scholar]
- 186.Matthews R C, Burnie J P. Diagnosis of systemic candidiasis by an enzyme-linked dot immunobinding assay for a circulating immunodominant 47-kilodalton antigen. J Clin Microbiol. 1988;26:459–463. doi: 10.1128/jcm.26.3.459-463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Matthews R C, Burnie J P. Cloning of a cDNA sequence encoding a major fragment of the 47 kilodalton stress protein homologue of Candida albicans. FEMS Microbiol Lett. 1989;60:25–30. doi: 10.1016/0378-1097(89)90071-2. [DOI] [PubMed] [Google Scholar]
- 188.Matthews R C, Burnie J P. The role of hsp90 in fungal infection. Immunol Today. 1992;13:345–348. doi: 10.1016/0167-5699(92)90169-8. [DOI] [PubMed] [Google Scholar]
- 189.Matthews R C, Burnie J P. Antibodies against Candida: potential therapeutics? Trends Microbiol. 1996;4:354–358. doi: 10.1016/0966-842x(96)10056-1. [DOI] [PubMed] [Google Scholar]
- 190.Matthews R C, Burnie J P, Tabaqchali S. Immunoblot analysis of the serological response in systemic candidiasis. Lancet. 1984;ii:1415–1418. doi: 10.1016/s0140-6736(84)91618-0. [DOI] [PubMed] [Google Scholar]
- 191.Matthews R C, Burnie J P, Tabaqchali S. Isolation of immunodominant antigens from the sera of patients with systemic candidosis and characterization of the serological response to Candida albicans. J Clin Microbiol. 1987;25:230–237. doi: 10.1128/jcm.25.2.230-237.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Matthews R C, Burnie J P, Lee W. The application of epitope mapping to the development of a new serological test for systemic candidiasis. J Immunol Methods. 1991;143:73–79. doi: 10.1016/0022-1759(91)90274-j. [DOI] [PubMed] [Google Scholar]
- 193.Matthews R C, Burnie J P, Smith D, Clark I, Midgley J, Conolly M, Gazzard B. Candida and AIDS: evidence for protective antibody. Lancet. 1988;ii:263–266. doi: 10.1016/s0140-6736(88)92547-0. [DOI] [PubMed] [Google Scholar]
- 194.Matthews R C, Burnie J P, Howat D, Rowland T, Walton F. Autoantibody to hsp90 can mediate protection against systemic candidosis. Immunology. 1991;74:20–24. [PMC free article] [PubMed] [Google Scholar]
- 195.Matthews R C, Hodgetts S, Burnie J P. Patient-derived phage antibody display library as a source of human recombinant antibodies to candidal hsp90. Serodiagn Immunother Infect Dis. 1994;6:213–217. [Google Scholar]
- 196.Matthews R C, Hodgetts S, Burnie J P. Preliminary assessment of a human recombinant antibody to hsp90 in the treatment of murine invasive candidiasis. J Infect Dis. 1995;171:1668–1671. doi: 10.1093/infdis/171.6.1668. [DOI] [PubMed] [Google Scholar]
- 197.Matthews R C, Wells C, Burnie J P. Characterization and cellular localization of the immunodominant 47-kDa antigen of Candida albicans. J Med Microbiol. 1988;27:227–232. doi: 10.1099/00222615-27-4-227. [DOI] [PubMed] [Google Scholar]
- 198.Meckstroth K L, Reiss E, Keller J W, Kaufman L. Detection of antibodies and antigenemia in leukemic patients with candidiasis by enzyme-linked immunosorbent assay. J Infect Dis. 1981;144:24–32. doi: 10.1093/infdis/144.1.24. [DOI] [PubMed] [Google Scholar]
- 199.Mitsutake K, Kohno S, Miyazaki T, Miyazaki H, Maesaki S, Koga H. Detection of Candida enolase antibody in patients with candidiasis. J Clin Lab Anal. 1994;8:207–210. doi: 10.1002/jcla.1860080405. [DOI] [PubMed] [Google Scholar]
- 200.Miyakawa Y, Kagaya K, Kuribayashi T, Suzuki M, Fukazawa Y. Isolation and chemical and biological characterization of antigenic mutants of Candida albicans serotype A. Symp Yeasts. 1989;89:S225–S229. [PubMed] [Google Scholar]
- 201.Miyakawa Y, Kuribayashi T, Kagaya K, Suzuki M. Role of specific determinants in mannan of Candida albicans serotype A in adherence to human buccal epithelial cells. Infect Immun. 1992;60:2493–2499. doi: 10.1128/iai.60.6.2493-2499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Monteagudo C, Sepúlveda P, López-Ribot J L, Real E, Casanova M, Chaffin W L, Martínez J P. Abstracts of the American Society for Microbiology Conference on Candida and Candidiasis: Biology, Pathogenesis, and Management. Washington, D.C: American Society for Microbiology; 1996. Blood group I antigen detection in Candida albicans cell wall glycoproteins, abstr. A08. [Google Scholar]
- 203.Morhart M, Rennie R, Ziola B, Bow E, Louie T J. Evaluation of enzyme immunoassay for Candida cytoplasmic antigens in neutropenic cancer patients. J Clin Microbiol. 1994;32:766–776. doi: 10.1128/jcm.32.3.766-776.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Morrison C J, Hurst S F, Bragg S L, Kuykendall R J, Diaz H, McLaughlin D W, Reiss E. Purification and characterization of the extracellular aspartyl proteinase of Candida albicans: removal of extraneous proteins and cell wall mannoprotein and evidence for lack of glycosylation. J Gen Microbiol. 1993;139:1177–1186. doi: 10.1099/00221287-139-6-1177. [DOI] [PubMed] [Google Scholar]
- 205.Morrison C J, Hurst S F, Bragg S L, Pruitt W R, Gorelkin L, Reiss E. Program and Abstracts of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1992. Aspartyl proteinase (AP): an antigenic marker in the diagnosis of systemic candidiasis by enzyme immunoassay (EIA), abstr. 1062; p. 287. [Google Scholar]
- 206.Mukherjee J, Yuan R, Casadevall A, Scharff M D. Immunoglobulin G3 blocking antibodies to the fungal pathogen Cryptococcus neoformans. J Exp Med. 1996;183:1905–1909. doi: 10.1084/jem.183.4.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mukherjee J, Zuckier S, Scharff M D, Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucoronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994;38:580–587. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Navarro D, Monzonis E, López-Ribot J L, Sepúlveda P, Casanova M, Nogueira J M, Martínez J P. Diagnosis of systemic candidiasis by enzyme immunoassay detection of specific antibodies to mycelial phase cell wall and cytoplasmic candidal antigens. Eur J Clin Microbiol Infect Dis. 1993;12:839–846. doi: 10.1007/BF02000404. [DOI] [PubMed] [Google Scholar]
- 209.Navarro D, Casanova M, Martínez J P. Systemic candidiasis: current diagnostic methods. Clin Adv Treat Fungal Infect. 1993;4:6–13. [Google Scholar]
- 210.Nelson R D, Shibata N, Podzorski R P, Herron M J. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev. 1991;4:1–19. doi: 10.1128/cmr.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ness M J, Vaughan W P, Woods G L. Candida antigen latex test for detection of invasive candidiasis in immunocompromised patients. J Infect Dis. 1989;159:495–502. doi: 10.1093/infdis/159.3.495. [DOI] [PubMed] [Google Scholar]
- 212.Odds F C. Morphogenesis in Candida albicans. Crit Rev Microbiol. 1985;12:45–93. doi: 10.3109/10408418509104425. [DOI] [PubMed] [Google Scholar]
- 213.Odds F C. Candida albicans proteinase as a virulence factor in the pathogenesis of Candida infections. Zentralbl Bakteriol Mikrobiol Hyg Ser A. 1985;260:539–542. doi: 10.1016/s0176-6724(85)80069-9. [DOI] [PubMed] [Google Scholar]
- 214.Odds F C. Candida and candidosis. A review and bibliography. 2nd ed. London, United Kingdom: Bailliere Tindall; 1988. [Google Scholar]
- 215.Ollert M K, Calderone R A. A monoclonal antibody that defines a surface antigen on Candida albicans hyphae cross-reacts with yeast cell protoplasts. Infect Immun. 1990;58:625–631. doi: 10.1128/iai.58.3.625-631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ollert W, Söhnchen R, Korting H C, Ollert U, Bräutigam S, Bräutigam W. Mechanisms of adherence of Candida albicans to cultured human epidermal keratinocytes. Infect Immun. 1993;61:4560–4568. doi: 10.1128/iai.61.11.4560-4568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Page S, Odds F C. Binding of plasma proteins to Candida species in vitro. J Gen Microbiol. 1988;134:2693–2702. doi: 10.1099/00221287-134-10-2693. [DOI] [PubMed] [Google Scholar]
- 218.Pancholi V, Fischetti V A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pendrak M L, Klotz S A. Adherence of Candida albicans to host cells. FEMS Microbiol Lett. 1995;129:103–114. doi: 10.1111/j.1574-6968.1995.tb07566.x. [DOI] [PubMed] [Google Scholar]
- 220.Phillips P, Dowd A, Jewesson P, Radigan G, Tweeddale M G, Clarke A, Geere I, Kelly M. Nonvalue of antigen detection immunoassays for diagnosis of candidemia. J Clin Microbiol. 1990;28:2320–2326. doi: 10.1128/jcm.28.10.2320-2326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pitzurra L, Polonelli L, Cantelli C, Gerloni M, Pontón J, Bikandi J, Blasi E. Candida albicans stress mannoprotein, SMP200, enhances tumour necrosis factor secretion in the murine macrophage cell line ANA-I. J Med Vet Mycol. 1996;34:219–222. doi: 10.1080/02681219680000371. [DOI] [PubMed] [Google Scholar]
- 222.Podzorski R P, Gray G R, Nelson R D. Different effects of Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J Immunol. 1990;144:707–716. [PubMed] [Google Scholar]
- 223.Polonelli L, Conti S, Campani L, Gerloni M, Castagnola M, Morace G, Chezzi C. Detection by immunofluorescent anti-idiotypic antibodies of yeast killer toxin cell wall receptors. J Immunol Methods. 1990;132:205. doi: 10.1016/0022-1759(90)90031-p. [DOI] [PubMed] [Google Scholar]
- 224.Polonelli L, De Bernardis F, Conti S, Boccanera M, Gerloni M, Morace G, Magliani W, Chezzi C, Cassone A. Idiotypic intravaginal vaccination to protect against candidal vaginitis by secretory, yeast killer toxin-like anti-idiotypic antibodies. J Immunol. 1994;152:3175–3182. [PubMed] [Google Scholar]
- 225.Polonelli L, De Bernardis F, Conti S, Boccanera M, Magliani W, Gerloni M, Cantelli C, Cassone A. Human natural yeast killer toxin-like candidacidal antibodies. J Immunol. 1996;156:1880–1885. [PubMed] [Google Scholar]
- 226.Polonelli L, Gerloni M, Conti S, Fisicaro P, Cantelli C, Portincasa P, Almondo F, Barea P L, Hernando F L, Pontón J. Heat-shock mannoproteins as targets of secretory IgA in Candida albicans. J Infect Dis. 1994;169:1401–1405. doi: 10.1093/infdis/169.6.1401. [DOI] [PubMed] [Google Scholar]
- 227.Pontón J, Jones J M. Analysis of cell wall extracts of Candida albicans by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot techniques. Infect Immun. 1986;53:565–572. doi: 10.1128/iai.53.3.565-572.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pontón J, Jones J M. Identification of two germ-tube-specific cell wall antigens of Candida albicans. Infect Immun. 1986;54:864–868. doi: 10.1128/iai.54.3.864-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pontón J, Marot-Leblond A, Ezkurra P A, Barturen B, Robert R, Senet J M. Characterization of Candida albicans cell wall antigens with monoclonal antibodies. Infect Immun. 1993;61:4842–4847. doi: 10.1128/iai.61.11.4842-4847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pontón J, Quindós G, Arilla M C, Mackenzie D W. Simplified adsorption method for detection of antibodies to Candida albicans germ tubes. J Clin Microbiol. 1994;32:217–219. doi: 10.1128/jcm.32.1.217-219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Poulain D, Faille C, Delaunoy C, Jacquinot P M, Trinel P A, Camus D. Probable presence of β-1,2-linked oligomannosides that act as human immunoglobulin G3 epitopes and are distributed over a Candida albicans 14- to 18-kilodalton antigen. Infect Immun. 1993;61:1164–1166. doi: 10.1128/iai.61.3.1164-1166.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Poulain D, Fruit J, Founier L, Del Cas E, Vernes A. Application of co-counterimmunoelectrophoresis to the diagnosis of systemic candidiasis. Importance and limitation of the method. Eur J Clin Microbiol. 1986;5:427–434. doi: 10.1007/BF02075699. [DOI] [PubMed] [Google Scholar]
- 233.Poulain D, Hopwood V, Vernes A. Antigenic variability of Candida albicans. Crit Rev Microbiol. 1985;12:223–270. doi: 10.3109/10408418509104430. [DOI] [PubMed] [Google Scholar]
- 234.Poulain D, Tronchin G, Vernes A, Popeye R, Biguet J. Antigenic variations of Candida albicans in vivo and in vitro relationships between P antigens and serotypes. Sabouraudia. 1983;21:99–112. [PubMed] [Google Scholar]
- 235.Quindós G, Pontón J, Cisterna R, Mackenzie D W. Value of detection of antibodies to Candida albicans germ tube in the diagnosis of systemic candidiasis. Eur J Clin Microbiol Infect Dis. 1990;9:178–183. doi: 10.1007/BF01963834. [DOI] [PubMed] [Google Scholar]
- 236.Ray T L, Payne C D. Detection of Candida acid proteinase (CAP) antibodies in systemic candidiasis by enzyme immunoassay. Clin Res. 1987;35:711A. [Google Scholar]
- 237.Ray T L, Payne C D. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: a role for Candida acid proteinase. Infect Immun. 1988;56:1942–1949. doi: 10.1128/iai.56.8.1942-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Reboli A C. Diagnosis of invasive candidiasis by a dot immunobinding assay for Candida antigen detection. J Clin Microbiol. 1993;31:518–523. doi: 10.1128/jcm.31.3.518-523.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Reiss E. Immunochemistry of fungal antigens. B. Opportunistic pathogens. In: Murphy J W, Friedman H, Bendinelli M, editors. Fungal infections and immune responses. New York, N.Y: Plenum Press; 1993. pp. 439–468. [Google Scholar]
- 240.Reiss E, Morrison C. Nonculture methods for diagnosis of disseminated candidiasis. Clin Microbiol Rev. 1993;6:311–323. doi: 10.1128/cmr.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Remold H, Fasold H, Staib F. Purification and characterization of a proteolytic enzyme from Candida albicans. Biochim Biophys Acta. 1968;167:399–406. doi: 10.1016/0005-2744(68)90219-2. [DOI] [PubMed] [Google Scholar]
- 242.Romani L, Cenci E, Mencacci A, Bistoni F, Pucetti P. T helper cell dichotomy to Candida albicans: implications for pathology, therapy, and vaccine design. Immunol Res. 1995;14:148–162. doi: 10.1007/BF02918174. [DOI] [PubMed] [Google Scholar]
- 243.Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981;659:99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- 244.Rüchel R. Cleavage of immunoglobulins by pathogenic yeast of the genus Candida. Microbiol Sci. 1986;3:316–319. [PubMed] [Google Scholar]
- 245.Rüchel R, Böning-Stutzer B, Mari A. A synoptical approach to the diagnosis of candidosis, relying on serological antigen and antibody test, on culture, and on evaluation of clinical data. Mycoses. 1988;31:87–106. [PubMed] [Google Scholar]
- 246.Rüchel R, De Bernardis F, Ray T L, Sullivan P A, Cole G T. Candida acid proteinases. J Med Vet Mycol. 1992;30:123–132. [PubMed] [Google Scholar]
- 247.Rüchel R, Ritter B, Schaffrinski M. Modulation of experimental systemic murine candidosis by intravenous pepstatin. Zentrabl Bakteriol. 1990;273:391–403. doi: 10.1016/s0934-8840(11)80443-3. [DOI] [PubMed] [Google Scholar]
- 248.Rüchel R, Zimmermann F, Böning-Stutzer B, Helmchen U. Candidiasis visualized by proteinase directed immunofluorescence. Virchows Arch Pathol Anat. 1991;419:199–202. doi: 10.1007/BF01626348. [DOI] [PubMed] [Google Scholar]
- 249.Ruiz-Herrera J. Fungal cell wall: structure, synthesis and assembly. Boca Raton, Fla: CRC Press, Inc.; 1992. [Google Scholar]
- 250.Sandin R L, Rogers A L, Paterson R J, Beneke E S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982;35:79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sanjuán R, Zueco J, Stock R, Font de Mora J, Sentandreu R. Identification of glucan-mannoprotein complexes in the cell wall of Candida albicans using a monoclonal antibody that reacts with a (1,6)-β-glucan epitope. Microbiology. 1995;141:1545–1551. doi: 10.1099/13500872-141-7-1545. [DOI] [PubMed] [Google Scholar]
- 252.Savolainen J, Viander M, Koivikko A. IgE-, IgA- and IgG-antibody responses to carbohydrate and protein antigens of Candida albicans in asthmatic children. Allergy. 1990;45:54–63. doi: 10.1111/j.1398-9995.1990.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 253.Savolainen J, Rantala A, Nermes M, Lehtonen L, Viander M. Enhanced IgE response to Candida albicans in postoperative invasive candidiasis. Clin Exp Allergy. 1996;26:452–460. [PubMed] [Google Scholar]
- 254.Sawant A D, Ahern D G. Involvement of a cell wall receptor in the mode of action of an anti-Candida toxin of Pichia anomala. Antimicrob Agents Chemother. 1990;34:1331–1335. doi: 10.1128/aac.34.7.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Saxena A, Calderone R A. Purification and characterization of the extracellular C3d-binding protein of Candida albicans. Infect Immun. 1990;58:309–314. doi: 10.1128/iai.58.2.309-314.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Segal E, Berg R A, Pizzo P A, Bennett J E. Detection of Candida antigen in sera of patients with candidiasis by an enzyme-linked immunosorbent assay-inhibition technique. J Clin Microbiol. 1979;10:116–118. doi: 10.1128/jcm.10.1.116-118.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sentandreu R, Elorza M V, Mormeneo S, Sanjuan R, Iranzo M. Possible roles of mannoproteins in the construction of Candida albicans cell wall. In: Vanden Bosche H, Odds F C, Kerridge D, editors. Dimorphic fungi in biology and medicine. New York, N.Y: Plenum Publishing Co.; 1993. pp. 169–175. [Google Scholar]
- 258.Sentandreu R, Herrero E, Martínez J P, Elorza M V. Yeast cell wall glycoproteins. NATO ASI Ser Ser H. 1991;53:229–239. [Google Scholar]
- 259.Sentandreu R, Martínez J P, Elorza M V, Mormeneo S. Relationships between dimorphism, cell wall structure, and surface activies in Candida albicans. In: Prasad R, editor. Candida albicans: cellular and molecular biology. Berlin, Germany: Springer-Verlag KG; 1991. pp. 72–88. [Google Scholar]
- 260.Sentandreu R, Mormeneo S, Ruiz-Herrera J. Biogenesis of the fungal cell wall. In: Wessels J G H, Meinhardt F, editors. The Mycota. I. Growth, differentiation and sexuality. Heidelberg, Germany: Springer-Verlag KG; 1994. pp. 111–124. [Google Scholar]
- 261.Sentandreu M, Elorza M V, Valentin E, Sentandreu R, Gozalbo D. Cloning of cDNAs coding for Candida albicans cell surface proteins. J Med Vet Mycol. 1995;33:105–111. [PubMed] [Google Scholar]
- 262.Sepúlveda P. Caracterización de adhesinas y marcadores antigénicos de la superficie celular de Candida albicans. Ph.D. thesis. Valencia, Spain: University of Valencia; 1995. [Google Scholar]
- 263.Shen H D, Choo K B, Lee H H, Hsieh J C, Lin W L, Lee W R, Han S H. The 40-kilodalton allergen of Candida albicans is an alcohol dehydrogenase: molecular cloning and immunological analysis using monoclonal antibodies. Clin Exp Allergy. 1991;21:675–681. doi: 10.1111/j.1365-2222.1991.tb03195.x. [DOI] [PubMed] [Google Scholar]
- 264.Shen H D, Choo K B, Tang R B, Lee C F, Yeh J Y, Han S H. Allergenic components of Candida albicans identified by immunoblot analysis. Clin Exp Allergy. 1989;19:191–196. doi: 10.1111/j.1365-2222.1989.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 265.Shepherd M G. Cell envelope of Candida albicans. Crit Rev Microbiol. 1987;15:7–25. doi: 10.3109/10408418709104445. [DOI] [PubMed] [Google Scholar]
- 266.Shepherd M G, Poulter R T M, Sullivan P A. Candida albicans: biology, genetics, and pathogenicity. Annu Rev Microbiol. 1985;39:579–614. doi: 10.1146/annurev.mi.39.100185.003051. [DOI] [PubMed] [Google Scholar]
- 267.Shibata N, Arai M, Haga E, Kikuchi T, Najima M, Satoh T, Kobayashi H, Suzuki S. Structural identification of an epitope of antigenic factor 5 in mannans of Candida albicans NIH B-729 (serotype B) and J-1012 (serotype A) as β-1,2-linked oligomannosyl residues. Infect Immun. 1992;60:4100–4110. doi: 10.1128/iai.60.10.4100-4110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shibata N, Fukasawa S, Kobayashi H, Tojo M, Yonezu T, Ambo A, Ohkubo Y, Suzuki S. Structural analysis of phospho-d-mannan-protein complexes isolated from yeast and mold form cells of Candida albicans NIH A-207 serotype A strain. Carbohydr Res. 1989;187:239–253. doi: 10.1016/0008-6215(89)80006-0. [DOI] [PubMed] [Google Scholar]
- 269.Shibata N, Hisamichi K, Kikuchi T, Kobayashi H, Okawa Y, Suzuki S. Sequential nuclear magnetic resonance assignment of β-1,2-linked mannooligosaccharides isolated from the phosphomannan of the pathogenic yeast Candida albicans NIH B-792 strain. Biochemistry. 1992;31:5680–5686. doi: 10.1021/bi00139a036. [DOI] [PubMed] [Google Scholar]
- 270.Shibata N, Ichikawa T, Tojo M, Takahashi M, Ito N, Okubo Y, Suzuki S. Immunochemical study on the mannan of Candida albicans NIH A-207, NIH B-792 and J-1012 strains prepared by fractional precipitation with cetyltrimethyl-ammonium bromide. Arch Biochem Biophys. 1985;243:338–348. doi: 10.1016/0003-9861(85)90511-9. [DOI] [PubMed] [Google Scholar]
- 271.Shibata N, Ikuta K, Imai T, Satoh Y, Satoh R, Suzuki A, Kojima C, Kobayashi H, Hisamichi K, Suzuki S. Existence of branched side chains in the cell wall mannan of pathogenic yeast, Candida albicans. Structure-antigenicity relationships between the cell wall mannans of Candida albicans and Candida parapsilosis. J Biol Chem. 1995;270:1113–1122. doi: 10.1074/jbc.270.3.1113. [DOI] [PubMed] [Google Scholar]
- 272.Shibata N, Kobayashi H, Takahashi S, Okawa Y, Hisamichi K, Suzuki S, Suzuki S. Structural study on a phosphorylated mannotetraose obtained from the phosphomannan of Candida albicans NIH B-792 strain by acetolysis. Arch Biochem Biophys. 1991;290:535–542. doi: 10.1016/0003-9861(91)90578-7. [DOI] [PubMed] [Google Scholar]
- 273.Shibata N, Kobayashi H, Tojo M, Suzuki S. Characterization of phosphomannan-protein complexes isolated from viable cells of yeast and mycelial forms of Candida albicans NIH B-792 strain by the action of Zymolyase 100-T. Arch Biochem Biophys. 1986;251:697–708. doi: 10.1016/0003-9861(86)90379-6. [DOI] [PubMed] [Google Scholar]
- 274.Shibata S, Plessas N, Goldstein I J. Production and characterization of antisera raised to the manninotrionate group (α-d-Galp-(1-6)-α-d-Galp-(1-6)-d-gluconate) Carbohydr Res. 1989;189:301–307. doi: 10.1016/0008-6215(89)84106-0. [DOI] [PubMed] [Google Scholar]
- 275.Shoemaker C, Gross A, Gebremichael A, Harn D. cDNA cloning and functional expression of the Schistosoma mansoni protective antigen triose-phosphate isomerase. Proc Natl Acad Sci USA. 1992;89:1842–1846. doi: 10.1073/pnas.89.5.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sikl D, Masler L, Bauer S. Mannan from the extracellular surface of Candida albicans Berkhourt. Experientia. 1964;20:456. doi: 10.1007/BF02152149. [DOI] [PubMed] [Google Scholar]
- 277.Soll D R. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992;5:183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Staab J F, Ferrer C A, Sundstrom P M. Developmental expression of a tandemly repeated, proline- and glutamine-rich amino acid motif on hyphal surfaces of Candida albicans. J Biol Chem. 1996;271:6298–6305. doi: 10.1074/jbc.271.11.6298. [DOI] [PubMed] [Google Scholar]
- 279.Staib F. Serum proteins as nitrogen source for yeast-like fungi. Sabouraudia. 1965;4:187–193. doi: 10.1080/00362176685190421. [DOI] [PubMed] [Google Scholar]
- 280.Stec B, Lebioda L. Refined structure of yeast apo-enolase at 2.25 A resolution. J Mol Biol. 1990;211:235–248. doi: 10.1016/0022-2836(90)90023-F. [DOI] [PubMed] [Google Scholar]
- 281.Strockbine N A, Largen M T, Zweibel S M, Buckley H R. Identification and molecular weight characterization of antigens from Candida albicans that are recognized by human sera. Infect Immun. 1984;43:715–721. doi: 10.1128/iai.43.2.715-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Summers D F, Grollman A, Hasenclever H F. Polysaccharide antigens of Candida cell wall. J Immunol. 1964;92:491–499. [PubMed] [Google Scholar]
- 283.Sundstrom P M, Aliaga G R. Molecular cloning of cDNA and analysis of protein secondary structure of Candida albicans enolase, an abundant, immunodominant glycolytic enzyme. J Bacteriol. 1992;174:6789–6799. doi: 10.1128/jb.174.21.6789-6799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sundstrom P M, Aliaga G R. A subset of proteins found in culture supernatants of Candida albicans includes the abundant, immunodominant, glycolytic enzyme enolase. J Infect Dis. 1994;169:452–456. doi: 10.1093/infdis/169.2.452. [DOI] [PubMed] [Google Scholar]
- 285.Sundstrom P M, Kenny G E. Enzymatic release of germ tube-specific antigens from cell walls of Candida albicans. Infect Immun. 1985;49:609–614. doi: 10.1128/iai.49.3.609-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sundstrom P M, Jensen J, Balish E. Humoral and cellular responses to enolase after alimentary tract colonization or intravenous immunization with Candida albicans. J Infect Dis. 1994;170:390–395. doi: 10.1093/infdis/170.2.390. [DOI] [PubMed] [Google Scholar]
- 287.Sundstrom P M, Tam M R, Nichols E J, Kenny G E. Antigenic differences in the surface mannoproteins of Candida albicans as revealed by monoclonal antibodies. Infect Immun. 1988;56:601–606. doi: 10.1128/iai.56.3.601-606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Surarit R, Gopal P K, Shepherd M G. Evidence for a glycosidic linkage between chitin and glucan in the cell wall of Candida albicans. J Gen Microbiol. 1988;134:1723–1730. doi: 10.1099/00221287-134-6-1723. [DOI] [PubMed] [Google Scholar]
- 289.Swoboda R K, Bertram G, Hollander H, Greenspan D, Greenspan J S, Gow N A R, Gooday G W, Brown A J P. Glycolytic enzymes of Candida albicans are nonubiquitous immunogens during candidiasis. Infect Immun. 1993;61:4263–4271. doi: 10.1128/iai.61.10.4263-4271.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Swoboda R K, Miyasaki S, Greenspan D, Greenspan J S. Heat-inducible ATP-binding proteins of Candida albicans are recognized by sera of infected patients. J Gen Microbiol. 1993;139:2995–3003. doi: 10.1099/00221287-139-12-2995. [DOI] [PubMed] [Google Scholar]
- 291.Taschdjian C L, Kozinn P J, Okas A, Caroline L, Halle A. Serodiagnosis of systemic candidiasis. J Infect Dis. 1967;117:180–187. doi: 10.1093/infdis/117.2.180. [DOI] [PubMed] [Google Scholar]
- 292.Tavares D, Ferreira P, Vilanova M, Videira A, Arala-Chaves M P. Immunoprotection against systemic candidiasis in mice. Internat Immunol. 1995;7:785–796. doi: 10.1093/intimm/7.5.785. [DOI] [PubMed] [Google Scholar]
- 293.Tavares D, Salvador A, Ferreira P, Arala-Chaves M P. Immunological activities of a Candida albicans protein which plays an important role in the survival of the microorganism in the host. Infect Immun. 1993;61:1881–1888. doi: 10.1128/iai.61.5.1881-1888.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Torosantucci A, Bromuro C, Gómez M J, Ausiello C M, Urbani F, Cassone A. Identification of a 65-kDa mannoprotein as a main target of human cell mediated immune response to Candida albicans. J Infect Dis. 1993;168:427–435. doi: 10.1093/infdis/168.2.427. [DOI] [PubMed] [Google Scholar]
- 295.Torosantucci A, Gómez M J, Casalinuovo I, Cassone A. Biochemical and antigenic characterization of mannoprotein constituents released from yeast and mycelial forms of Candida albicans. J Med Vet Mycol. 1991;29:361–372. doi: 10.1080/02681219180000591. [DOI] [PubMed] [Google Scholar]
- 296.Torosantucci A, Palma C, Boccanera M, Ausiello C M, Spagnoli G C, Cassone A. Lymphoproliferative and cytotoxic responses of human peripheral blood mononuclear cells to mannoprotein constituents of Candida albicans. J Gen Microbiol. 1990;136:2155–2163. doi: 10.1099/00221287-136-11-2155. [DOI] [PubMed] [Google Scholar]
- 297.Tosh F D, Douglas L J. Characterization of a fucoside-binding adhesin of Candida albicans. Infect Immun. 1992;60:4734–4739. doi: 10.1128/iai.60.11.4734-4739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Trinel P A, Faille C, Jacquinot P M, Cailliez J C, Poulain D. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect Immun. 1992;60:3845–3851. doi: 10.1128/iai.60.9.3845-3851.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Trinel P A, Borg-von-Zepelin M, Lepage G, Jouault T, Mackenzie D W R, Poulain D. Isolation and primary characterization of the 14- to 18-kDa Candida albicans antigen as phospholipomannan containing β-1,2-linked oligomannosides. Infect Immun. 1993;61:4398–4405. doi: 10.1128/iai.61.10.4398-4405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tronchin G, Robert R, Bouali A, Senet J M. Immunocytochemical localization of in vitro binding of human fibrinogen to Candida albicans germ tube and mycelium. Ann Inst Pasteur Microbiol. 1987;138:177–187. doi: 10.1016/0769-2609(87)90194-3. [DOI] [PubMed] [Google Scholar]
- 301.Tronchin G, Bouchara J P, Robert R. Dynamic changes of the cell wall surface of Candida albicans associated with germination and adherence. Eur J Cell Biol. 1989;50:285–290. [PubMed] [Google Scholar]
- 302.Tsuchiya T, Fukazawa Y, Kawakita S. Significance of serological studies on yeast. Mycopathol Mycol Appl. 1965;26:1. doi: 10.1007/BF02098585. [DOI] [PubMed] [Google Scholar]
- 303.Tsuchiya T, Taguchi M, Fukazawa Y, Shinoda T. Serological characterization of yeast as an aid in identification and classification. Methods Microbiol. 1984;16:75–126. [Google Scholar]
- 304.van Deventer A J M, Goessens W H F, van Vliet H J A, Verbrugh H A. Abstracts of the American Society for Microbiology Conference on Candida and Candidiasis: Biology, Pathogenesis, and Management. Washington, D.C: American Society for Microbiology; 1996. Anti-enolase antibodies protective against systemic candidiasis in mice, abstr. B50. [DOI] [PubMed] [Google Scholar]
- 305.van Deventer A J M, van Vliet H J A, Hop W C J, Goessens W H F. Diagnostic value of anti-Candida enolase antibodies. J Clin Microbiol. 1994;32:17–23. doi: 10.1128/jcm.32.1.17-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.van Deventer A J M, van Vliet H J A, Voogd L, Hop W C J, Goessens W H F. Increased specificity of antibody detection in surgical patients with invasive candidiasis with cytoplasmic antigens depleted of mannan residues. J Clin Microbiol. 1993;31:994–997. doi: 10.1128/jcm.31.4.994-997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Villalba R, González A I, Linares M J, Casal M, Torres A. Detection of antibodies to Candida albicans germ tube as a possible aid in diagnosing systemic candidiasis in bone marrow transplant patients. Eur J Clin Microbiol Infect Dis. 1993;12:347–349. doi: 10.1007/BF01964431. [DOI] [PubMed] [Google Scholar]
- 308.Wadsworth E, Prasad S C, Calderone R A. Analysis of mannoprotein from blastoconidia and hyphae of Candida albicans with a common epitope recognized by anti-complement receptor type 2 antibodies. Infect Immun. 1993;61:4675–4681. doi: 10.1128/iai.61.11.4675-4681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Walsh T J, Buckley H R, Hom-Eng M, Johnson D E, Maret M, Gary P. 10th Congress of the International Society for Human and Animal Mycology. 1988. Analysis of a 48-kilodalton cytoplasmic antigen of Candida albicans in experimental candidiasis, abstr. O111. [Google Scholar]
- 310.Walsh T J, Hathorn J W, Sobel J D, Merz W G, Sánchez V, Maret S M, Buckley H R, Pfaller M A, Schaufele R, Silva C, Navarro E, Lecciones L, Chandraseka P, Lee J, Pizzo P A. Detection of circulating Candida enolase by immunoassay in patients with cancer and invasive candidiasis. N Engl J Med. 1991;324:1026–1031. doi: 10.1056/NEJM199104113241504. [DOI] [PubMed] [Google Scholar]
- 311.Warren R C, Bartlett A, Bidwell D E, Richardson M D, Voller A, White L O. Diagnosis of invasive candidosis by enzyme immunoassay of serum antigen. Br Med J. 1977;1:1183–1185. doi: 10.1136/bmj.1.6070.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Weiner M H, Coats-Stephen M. Immunodiagnosis of systemic candidiasis: mannan antigenemia detected by radioimmunoassay in experimental and human infections. J Infect Dis. 1979;140:989–993. doi: 10.1093/infdis/140.6.989. [DOI] [PubMed] [Google Scholar]
- 313.Weiner M H, Yount W J. Mannan antigenemia in the diagnosis of invasive Candida infection. J Clin Invest. 1976;58:1045–1053. doi: 10.1172/JCI108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Winram S B, Lottenberg R. The plasmin-binding protein Plr of group A streptococci is identified as glyceraldehyde-3-phosphate dehydrogenase. Microbiology. 1996;142:2311–2320. doi: 10.1099/13500872-142-8-2311. [DOI] [PubMed] [Google Scholar]
- 315.Yang W, Li E, Kairong T, Stanley S L., Jr Entamoeba histolytica has an alcohol dehydrogenase homologous to the multifunctional adhE gene product of Escherichia coli. Mol Biochem Parasitol. 1994;64:253–260. doi: 10.1016/0166-6851(93)00020-a. [DOI] [PubMed] [Google Scholar]
- 316.Young D, Lathigra R, Hendrix R, Sweetser D, Young R A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci USA. 1988;85:4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zeuthen M L, Howard D H. Thermotolerance and the heat-shock response in Candida albicans. J Gen Microbiol. 1989;135:2509–2518. doi: 10.1099/00221287-135-9-2509. [DOI] [PubMed] [Google Scholar]
- 318.Zoller L, Kramer I, Kappe R, Sonntag H G. Enzyme immunoassay for invasive Candida infections: reactivity of somatic antigens of Candida albicans. J Clin Microbiol. 1991;29:1860–1867. doi: 10.1128/jcm.29.9.1860-1867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


