Abstract
Escherichia coli is the predominant nonpathogenic facultative flora of the human intestine. Some E. coli strains, however, have developed the ability to cause disease of the gastrointestinal, urinary, or central nervous system in even the most robust human hosts. Diarrheagenic strains of E. coli can be divided into at least six different categories with corresponding distinct pathogenic schemes. Taken together, these organisms probably represent the most common cause of pediatric diarrhea worldwide. Several distinct clinical syndromes accompany infection with diarrheagenic E. coli categories, including traveler’s diarrhea (enterotoxigenic E. coli), hemorrhagic colitis and hemolytic-uremic syndrome (enterohemorrhagic E. coli), persistent diarrhea (enteroaggregative E. coli), and watery diarrhea of infants (enteropathogenic E. coli). This review discusses the current level of understanding of the pathogenesis of the diarrheagenic E. coli strains and describes how their pathogenic schemes underlie the clinical manifestations, diagnostic approach, and epidemiologic investigation of these important pathogens.
Escherichia coli is the predominant facultative anaerobe of the human colonic flora. The organism typically colonizes the infant gastrointestinal tract within hours of life, and, thereafter, E. coli and the host derive mutual benefit (169). E. coli usually remains harmlessly confined to the intestinal lumen; however, in the debilitated or immunosuppressed host, or when gastrointestinal barriers are violated, even normal “nonpathogenic” strains of E. coli can cause infection. Moreover, even the most robust members of our species may be susceptible to infection by one of several highly adapted E. coli clones which together have evolved the ability to cause a broad spectrum of human diseases. Infections due to pathogenic E. coli may be limited to the mucosal surfaces or can disseminate throughout the body. Three general clinical syndromes result from infection with inherently pathogenic E. coli strains: (i) urinary tract infection, (ii) sepsis/meningitis, and (iii) enteric/diarrheal disease. This article will review the diarrheagenic E. coli strains, which include several emerging pathogens of worldwide public health importance, and will specifically focus on pathogens afflicting humans. We will particularly concentrate on the E. coli strains whose study has advanced most over the last decade, i.e., enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), and enteroaggregative E. coli (EAEC). Since the categories of diarrheagenic E. coli are differentiated on the basis of pathogenic features, emphasis will be placed on the mechanisms of disease and the development of diagnostic techniques based on virulence factors.
ISOLATION AND IDENTIFICATION
Although assays to identify all categories of diarrheagenic E. coli are available, in many situations it is not necessary to implicate a specific E. coli pathogen in a particular patient. Patients with enterotoxigenic E. coli (ETEC) traveler’s diarrhea, for example, generally resolve their diarrhea long before they come to medical attention for stool culture. Most enteroinvasive E. coli (EIEC) isolates will be missed in the clinical laboratory, yet diarrhea generally resolves and patients respond to empirical antibiotics, such as fluoroquinolones, given for other bacterial diarrheas. Culturing stools for most categories of diarrheagenic E. coli should be performed in cases of persistent diarrhea, especially in travelers, children and the immunocompromised, as well as in outbreak situations. E. coli can be isolated from the stool and sent to a qualified reference laboratory for definitive identification. The indications for culturing for EHEC differ from those for the rest of the diarrheagenic E. coli categories; indications for culturing EHEC are discussed below in greater detail in the EHEC section.
Biochemicals
E. coli is the type species of the genus Escherichia, which contains mostly motile gram-negative bacilli within the family Enterobacteriaceae and the tribe Escherichia (55, 185).
E. coli can be recovered easily from clinical specimens on general or selective media at 37°C under aerobic conditions. E. coli in stool are most often recovered on MacConkey or eosin methylene-blue agar, which selectively grow members of the Enterobacteriaceae and permit differentiation of enteric organisms on the basis of morphology (32).
Enterobacteriaceae are usually identified via biochemical reactions. These tests can be performed in individual culture tubes or by using test “strips” which are commercially available. Either method produces satisfactory results.
For epidemiologic or clinical purposes, E. coli strains are often selected from agar plates after presumptive visual identification. However, this method should be used only with caution, because only about 90% of E. coli strains are lactose positive; some diarrheagenic E. coli strains, including many of the EIEC strains, are typically lactose negative. The indole test, positive in 99% of E. coli strains, is the single best test for differentiation from other members of the Enterobacteriaceae.
Serotyping
Serotyping of E. coli occupies a central place in the history of these pathogens (reviewed in reference 394. Prior to the identification of specific virulence factors in diarrheagenic E. coli strains, serotypic analysis was the predominant means by which pathogenic strains were differentiated. In 1933, Adam showed by serologic typing that strains of “dyspepsiekoli” could be implicated in outbreaks of pediatric diarrhea. In 1944, Kauffman proposed a scheme for the serologic classification of E. coli which is still used in modified form today.
According to the modified Kauffman scheme, E. coli are serotyped on the basis of their O (somatic), H (flagellar), and K (capsular) surface antigen profiles (185, 394). A total of 170 different O antigens, each defining a serogroup, are recognized currently. The presence of K antigens was determined originally by means of bacterial agglutination tests: an E. coli strain that was inagglutinable by O antiserum but became agglutinable when the culture was heated was considered to have a K antigen. The discovery that several different molecular structures, including fimbriae, conferred the K phenotype led experts to suggest restructuring the K antigen designation to include only acidic polysaccharides (394). Proteinaceous fimbrial antigens have therefore been removed from the K series and have been given F designations (494).
A specific combination of O and H antigens defines the “serotype” of an isolate. E. coli of specific serogroups can be associated reproducibly with certain clinical syndromes (Table 1), but it is not in general the serologic antigens themselves that confer virulence. Rather, the serotypes and serogroups serve as readily identifiable chromosomal markers that correlate with specific virulent clones (690).
TABLE 1.
Serotypes characteristic of the diarrheagenic E. coli categories
| Category | Serogroup | Associated H antigen(s) |
|---|---|---|
| ETEC | O6 | H16 |
| O8 | H9 | |
| O11 | H27 | |
| O15 | H11 | |
| O20 | NM | |
| O25 | H42, NM | |
| O27 | H7 | |
| O78 | H11, H12 | |
| O128 | H7 | |
| O148 | H28 | |
| O149 | H10 | |
| O159 | H20 | |
| O173 | NM | |
| EPEC | O55 | H6, NM |
| O86 | H34, NM | |
| O111 | H2, H12, NM | |
| O119 | H6, NM | |
| O125ac | H21 | |
| O126 | H27, NM | |
| O127 | H6, NM | |
| O128 | H2, H12 | |
| O142 | H6 | |
| EHEC | O26 | H11, H32, NM |
| O55 | H7 | |
| O111ab | H8, NM | |
| O113 | H21 | |
| O117 | H14 | |
| O157 | H7 | |
| EAEC | O3 | H2 |
| O15 | H18 | |
| O44 | H18 | |
| O86 | NM | |
| O77 | H18 | |
| O111 | H21 | |
| O127 | H2 | |
| O?a | H10 | |
| EIEC | O28ac | NM |
| O29 | NM | |
| O112ac | NM | |
| O124 | H30, NM | |
| O136 | NM | |
| O143 | NM | |
| O144 | NM | |
| O152 | NM | |
| O159 | H2, NM | |
| O164 | NM | |
| O167 | H4, H5, NM |
O antigen untypeable by conventional methods.
Phenotypic Assays Based on Virulence Characteristics
Identification of diarrheagenic E. coli strains requires that these organisms be differentiated from nonpathogenic members of the normal flora. Serotypic markers correlate, sometimes very closely, with specific categories of diarrheagenic E. coli; however, these markers are rarely sufficient in and of themselves to reliably identify a strain as diarrheagenic. (An exception may be strains of serotype O157:H7, a serotype that serves as a marker for virulent enterohemorrhagic E. coli strains; nevertheless, EHEC of serotypes other than O157:H7 are being identified with increasing frequency in sporadic and epidemic cases.) In addition to its limited sensitivity and specificity, serotyping is tedious and expensive and is performed reliably only by a small number of reference laboratories. Thus, detection of diarrheagenic E. coli has focused increasingly on the identification of characteristics which themselves determine the virulence of these organisms. This may include in vitro phenotypic assays which correlate with the presence of specific virulence traits or detection of the genes encoding these traits.
One of the most useful phenotypic assays for the diagnosis of diarrheagenic E. coli is the HEp-2 adherence assay. The method has recently been reviewed in detail (160). This assay was first described in 1979 by Cravioto et al. (139) and remains the “gold standard” for the diagnosis of EAEC and diffusely adherent E. coli (DAEC). The HEp-2 assay has been modified often since its first description, including such variations as extending the incubation time to 6 h or changing the growth medium during the incubation. However, collaborative studies have shown that the assay performed essentially as first described provides the best ability to differentiate among all three adherent diarrheagenic categories (EPEC, EAEC, and DAEC) (678). The HEp-2 adherence assay entails inoculating the test strain onto a semiconfluent HEp-2 monolayer and incubating it for 3 h at 37°C under 5% CO2. After this incubation time, the monolayer is washed, fixed, stained, and examined by oil immersion light microscopy. The three patterns of HEp-2 adherence (Fig. 1), localized adherence (LA), aggre gative adherence (AA), and diffuse adherence (DA), can be differentiated reliably by an experienced technician. However, the authors have found some strains which yield equivocal results reproducibly in the HEp-2 assay.
FIG. 1.
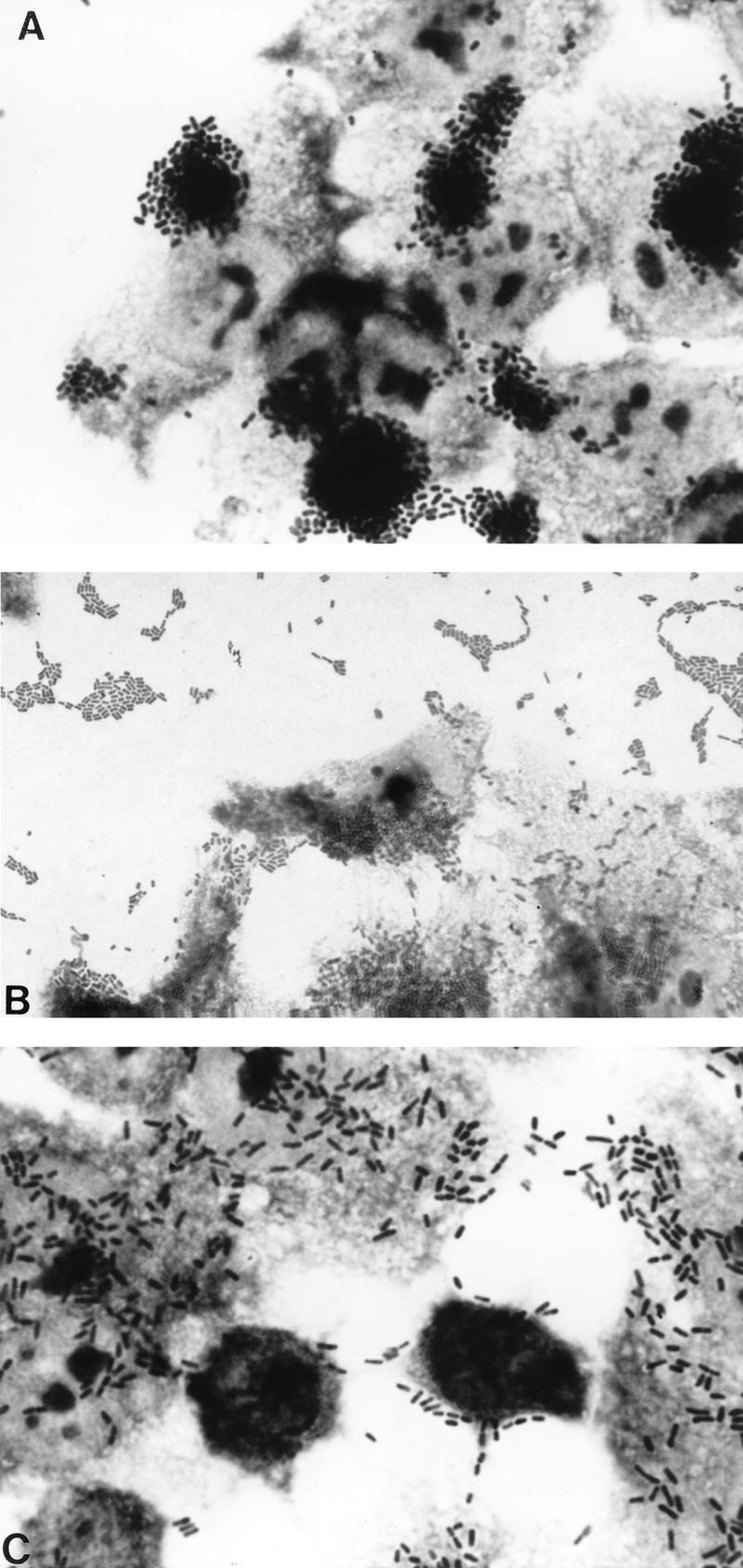
The three HEp-2 adherence patterns manifested by diarrheagenic E. coli. (A) Localized adherence (LA), typical of EPEC. Bacteria form characteristic microcolonies on the surface of the HEp-2 cell. (B) Aggregative adherence (AA), which defines EAEC. Bacteria adhere to each other away from the cells as well as to the cell surface in a characteristic stacked-brick configuration. (C) Diffuse adherence (DA), which defines DAEC. Bacteria are dispersed over the surface of the cell.
Molecular Detection Methods
Diarrheagenic E. coli strains were among the first pathogens for which molecular diagnostic methods were developed. Indeed, molecular methods remain the most popular and most reliable techniques for differentiating diarrheagenic strains from nonpathogenic members of the stool flora and distinguishing one category from another. Substantial progress has been made both in the development of nucleic acid-based probe technologies as well as PCR methods.
Nucleic acid probes.
The use of DNA probes for detection of heat-labile (LT) and heat-stable (ST) enterotoxins in ETEC revolutionized the study of these organisms, replacing cumbersome and costly animal models of toxin detection (455). Since then, gene probes have been introduced for all diarrheagenic categories. Two general methods are commonly used for nucleic acid probe specimen preparation. The first entails the inoculation of purified cultures onto agar plates to produce “colony” blots, in which 30 to 50 such cultures are inoculated per plate. After incubation, the bacterial growth is transferred to nitrocellulose or Whatman filter paper for hybridization (alternatively, the cultures can be grown directly on the nitrocellulose overlying an agar plate). The bacterial growth on the paper can be lysed, denatured, and hybridized with the probe in situ, and then a radiographic image is generated by exposure to X-ray film. Substantial experience by ourselves and others has demonstrated that the colony blot method is reliable and efficient. However, the use of this method requires that the E. coli strain first be isolated from the patient’s stool, which introduces the possibility that any number of E. coli colonies picked from a stool culture may fail to yield the offending pathogenic strain. Over several years of study, we have found that patients symptomatic with E. coli diarrhea generally present with the pathogenic strain as their predominant E. coli strain in the flora. Thus, studies in which three E. coli isolates are tested per diarrheal stool specimen will have acceptable sensitivity. If increased sensitivity is desired or if the study entails a large number of asymptomatic patients, isolating five isolates per specimen may be more appropriate.
An alternative to the use of colony blots is the stool blot method. In this technique, stool samples are spotted directly onto nitrocellulose filters that have been overlaid onto an agar plate (373). After overnight incubation, the filter paper is peeled off the plate, air dried, and treated as above for colony blots. The advantages of this technique include (i) that the E. coli colonies need not be isolated from the stool and (ii) that there may be increased sensitivity if the pathogenic strain represents a minority member of the flora. However, the presence of large numbers of other bacteria decreases the sensitivity of this test, and a threshold number (ca. 105 to 106 per g of stool [461]) of pathogenic organisms must be present to yield definitive results. In addition, the use of stool blots alone does not result in a pure culture of the pathogen, which may be required for verification of phenotypes.
Nucleic acid-based probes themselves can be of two types: oligonucleotide or polynucleotide (fragment probes). DNA fragment (polynucleotide) probes may be derived from genes that encode a particular phenotype or may instead be empirical probes which, through extensive testing, are found to be linked with the presence of a phenotype. Although empirical probes have generated useful results (41, 701), probes which represent the virulence genes themselves are generally superior (241).
Oligonucleotide probes are derived from the DNA sequence of a target gene. Annealing temperatures and other conditions of hybridization and washing need to be determined much more precisely than for polynucleotide probes. Moreover, very slight strain-to-strain differences among the virulence genes may generate false-negative results with oligonucleotide probes. Nevertheless, oligonucleotide probes have the advantage of faster and often cleaner results than those generated by polynucleotide methods, a factor that comes into play especially when screening for very small genes. Recommended oligonucleotide probes are listed in Table 2.
TABLE 2.
Nucleotide sequences of PCR oligonucleotide primers and oligonucleotide probes for diarrheagenic E. coli strains
| Category | Factor | PCR oligonucleotidesa | Reference | Oligonucleotide probe | Reference |
|---|---|---|---|---|---|
| ETEC | STI | TTAATAGCACCCGGTACAAGCAGG | 492 | GCTGTGAATTGTGTTGTAATCC | 457 |
| CTTGACTCTTCAAAAGAGAAAATTAC | GCTGTGAACTTTGTTGTAATCC | ||||
| LT | GGCGACAGATTATACCGTGC | 581 | GCGAGAGGAACACAAACCGG | 581 | |
| CCGAATTCTGTTATATATGTC | |||||
| EPEC | eae | —b | |||
| EAF | CAGGGTAAAAGAAAGATGATAA | 214 | TATGGGGACCATGTATTATCA | 313 | |
| TATGGGGACCATGTATTATCA | |||||
| BFP | AATGGTGCTTGCGCTTGCTGC | 268 | GCTACGGTGTTAATATCTCTGGCG | 462 | |
| GCCGCTTTATCCAACCTGGTA | |||||
| EHEC | eae | CAGGTCGTCGTGTCTGCTAAA | 234 | ACTGAAAGCAAGCGGTGGTG | 691 |
| TCAGCGTGGTTGGATCAACCT (O157:H7-specific) | |||||
| SLTI | TTTACGATAGACTTCTCGAC | 223 | GATGATCTCAGTGGGCGTTC | 270 | |
| CACATATAAATTATTTCGCTC (SLT-I AND II) | |||||
| SLTII | As above | TCTGAAACTGCTCCTGTGTA | 270 | ||
| Plasmid | ACGATGTGGTTTATTCTGGA | 223 | CCGTATCTTATAATAAGACGGATGTTGG | 223 | |
| CTTCACGTCACCATACATAT | |||||
| EIEC | ial | CTGGATGGTATGGTGAGG | 579 | CCATCTATTAGAATACCTGTG | 579 |
| GGAGGCCAACAATTATTTCC | |||||
| EAEC | Plasmid | CTGGCGAAAGACTGTATCAT | 576 | None | |
| CAATGTATAGAAATCCGCTGTT |
Each primer is written 5′-3′. See the text for abbreviations and discussion.
No oligonucleotide primers have yet been described which will detect specifically all human EPEC strains. (See reference 234.)
Whereas the original probe techniques involved radionucleotides to detect probe hybridization, nonisotopic methods are becoming more popular. These include several methods for tagging oligonucleotide probes and a smaller number of effective techniques for detection of polynucleotide probes. These nonisotopic techniques have facilitated the introduction of probe technology into areas where the use of radioisotopes is impractical.
PCR.
PCR is a major advance in molecular diagnostics of pathogenic microorganisms, including E. coli. PCR primers have been developed successfully for several of the categories of diarrheagenic E. coli (listed in Table 2). Advantages of PCR include great sensitivity in in situ detection of target templates. However, substances within stools have been shown to interfere with the PCR, thus decreasing its sensitivity (615); several methods have been used successfully to remove such inhibitors, including Sepharose spin column chromatography and adsorption of nucleic acids onto glass resin (397, 615). Scrupulous attention to proper technique must be maintained to avoid carryover of PCR products from one reaction to the next.
COMMON THEMES IN E. COLI VIRULENCE
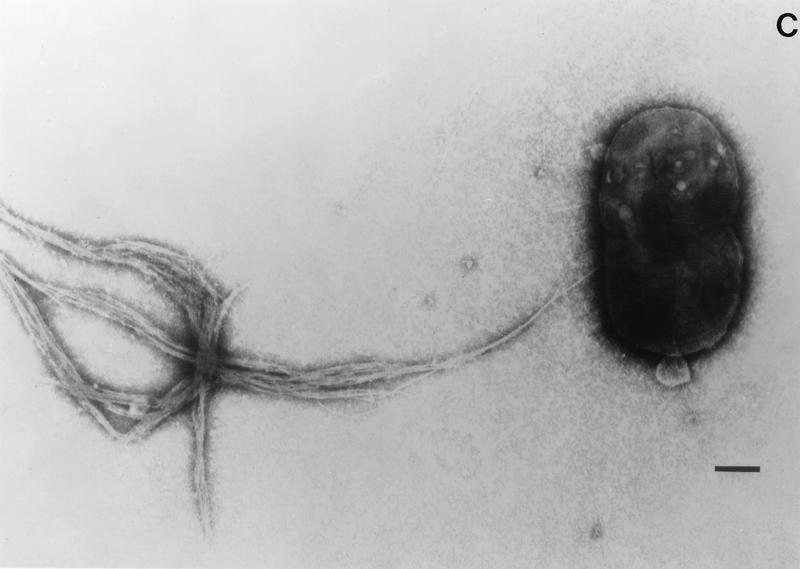
Like most mucosal pathogens, E. coli can be said to follow a requisite strategy of infection: (i) colonization of a mucosal site, (ii) evasion of host defenses, (iii) multiplication, and (iv) host damage. The most highly conserved feature of diarrheagenic E. coli strains is their ability to colonize the intestinal mucosal surface despite peristalsis and competition for nutrients by the indigenous flora of the gut (including other E. coli strains). The presence of surface adherence fimbriae is a property of virtually all E. coli strains, including nonpathogenic varieties. However, diarrheagenic E. coli strains possess specific fimbrial antigens that enhance their intestinal colonizing ability and allow adherence to the small bowel mucosa, a site that is not normally colonized (389, 679). The various morphologies of E. coli fimbriae are illustrated in Fig. 2. The role of fimbrial structures in adherence and colonization is often inferred rather than demonstrated, in part due to the host specificity of most fimbrial adhesins.
FIG. 2.
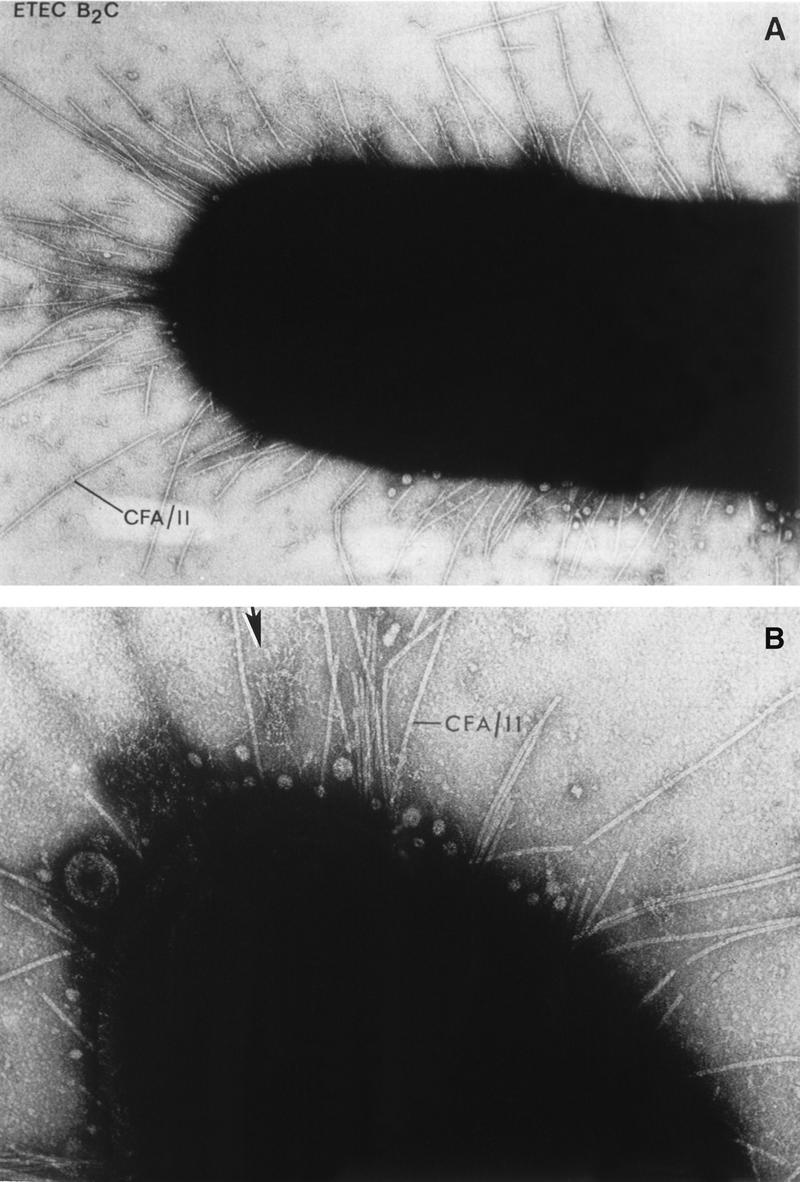
Various morphologies of diarrheagenic E. coli fimbriae as seen by transmission electron microscopy. (A) Rigid fimbrial morphology illustrated by ETEC fimbriae CS1 (labelled CFA/II in the figure). The diameter of individual fimbriae is ca. 7 nm. (B) Flexible fibrillar morphology exemplified by the CS3 component of CFA/II (arrow). Note the typical narrow diameter, ca. 2 to 3 nm, and the coiled appearance. (C) Electron micrograph showing the EPEC bundle-forming pilus expressed by strain E2348/69. Bar, 0.35 μm. Reprinted from reference 245 with permission of the publisher.
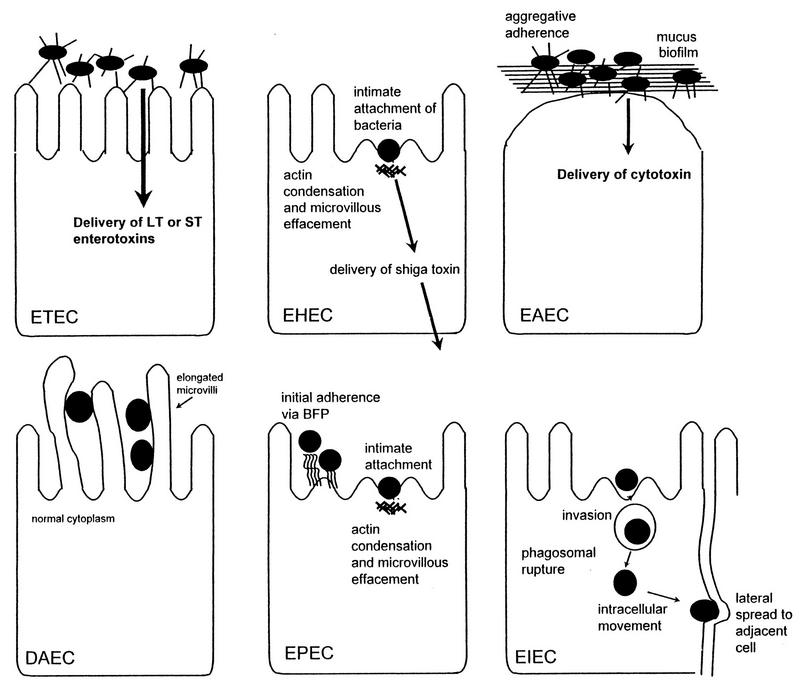
Once colonization is established, the pathogenetic strategies of the diarrheagenic E. coli strains exhibit remarkable variety. Three general paradigms have been described by which E. coli may cause diarrhea; each is described in detail in the appropriate section below: (i) enterotoxin production (ETEC and EAEC), (ii) invasion (EIEC), and/or (iii) intimate adherence with membrane signalling (EPEC and EHEC). However, the interaction of the organisms with the intestinal mucosa is specific for each category. Schematized paradigms are illustrated in Fig. 3.
FIG. 3.
Pathogenic schemes of diarrheagenic E. coli. The six recognized categories of diarrheagenic E. coli each have unique features in their interaction with eukaryotic cells. Here, the interaction of each category with a typical target cell is schematically represented. It should be noted that these descriptions are largely the result of in vitro studies and may not completely reflect the phenomena occurring in infected humans. See the text for details.
The versatility of the E. coli genome is conferred mainly by two genetic configurations: virulence-related plasmids and chromosomal pathogenicity islands. All six categories of diarrheagenic E. coli described in this review have been shown to carry at least one virulence-related property upon a plasmid. EIEC, EHEC, EAEC, and EPEC strains typically harbor highly conserved plasmid families, each encoding multiple virulence factors (275, 467, 701). McDaniel and Kaper have shown recently that the chromosomal virulence genes of EPEC and EHEC are organized as a cluster referred to as a pathogenicity island (431, 432). Such islands have been described for uropathogenic E. coli strains (163) and systemic E. coli strains (75) as well and may represent a common way in which the genomes of pathogenic and nonpathogenic E. coli strains diverge genetically. Plasmids and pathogenicity islands carry clusters of virulence traits, yet individual traits may be transposon encoded (such as ST) (607) or phage encoded (such as Shiga toxin) (485).
In the sections that follow, we will review all aspects of disease due to the different classes of diarrheagenic E. coli. Since diarrheagenic E. coli strains are distinguished and defined on the basis of pathogenetic mechanisms, much of this review will concern the latest advances in our knowledge of the pathogenesis of these organisms.
ENTEROTOXIGENIC E. COLI
ETEC is defined as containing the E. coli strains that elaborate at least one member of two defined groups of enterotoxins: ST and LT (381). ETEC strains were first recognized as causes of diarrheal disease in piglets, where the disease continues to cause lethal infection in newborn animals (reviewed in reference 15). Studies of ETEC in piglets first elucidated the mechanisms of disease, including the existence of two plasmid-encoded enterotoxins. The first descriptions of ETEC in humans reported that certain E. coli isolates from the stools of children with diarrhea elicited fluid secretion in ligated rabbit intestinal loops (642). DuPont et al. subsequently showed that ETEC strains were able to cause diarrhea in adult volunteers (175).
Pathogenesis
ETEC strains are generally considered to represent a pathogenic prototype: the organisms colonize the surface of the small bowel mucosa and elaborate their enterotoxins, giving rise to a net secretory state. Some investigators have reported that ETEC strains may exhibit limited invasiveness in cell cultures, but this has not been demonstrated in vivo (189, 190). ETEC strains cause diarrhea through the action of the enterotoxins LT and ST. These strains may express an LT only, an ST only, or both an LT and an ST. These toxins have recently been reviewed (291, 293, 295, 296, 480, 589), and the reader is referred to these sources for primary references.
Heat-labile toxins.
The LTs of E. coli are oligomeric toxins that are closely related in structure and function to the cholera enterotoxin (CT) expressed by Vibrio cholerae (596). LT and CT share many characteristics including holotoxin structure, protein sequence (ca. 80% identity), primary receptor identity, enzymatic activity, and activity in animal and cell culture assays; some differences are seen in toxin processing and secretion and in helper T-lymphocyte responses (153). There are two major serogroups of LT, LT-I and LT-II, which do not cross-react immunologically. LT-I is expressed by E. coli strains that are pathogenic for both humans and animals. LT-II is found primarily in animal E. coli isolates and rarely in human isolates, but in neither animals nor humans has it been associated with disease. Unless otherwise distinguished by Roman numerals, the term LT below refers to the LT-I form.
(i) LT-I.
LT-I is an oligomeric toxin of ca. 86 kDa composed of one 28-kDa A subunit and five identical 11.5-kDa B subunits (Fig. 4A) (622). The B subunits are arranged in a ring or “doughnut” and bind strongly to the ganglioside GM1 and weakly to GD1b and some intestinal glycoproteins (643). The A subunit is responsible for the enzymatic activity of the toxin and is proteolytically cleaved to yield A1 and A2 peptides joined by a disulfide bond. Two closely related variants of LT-I which exhibit partial antigenic cross-reactivity have been described. These variants are called LTp (LTp-I) and LTh (LTh-I) after their initial discovery in strains isolated from pigs or humans, respectively. The genes encoding LT (elt or etx) reside on plasmids that also may contain genes encoding ST and/or colonization factor antigens (CFAs).
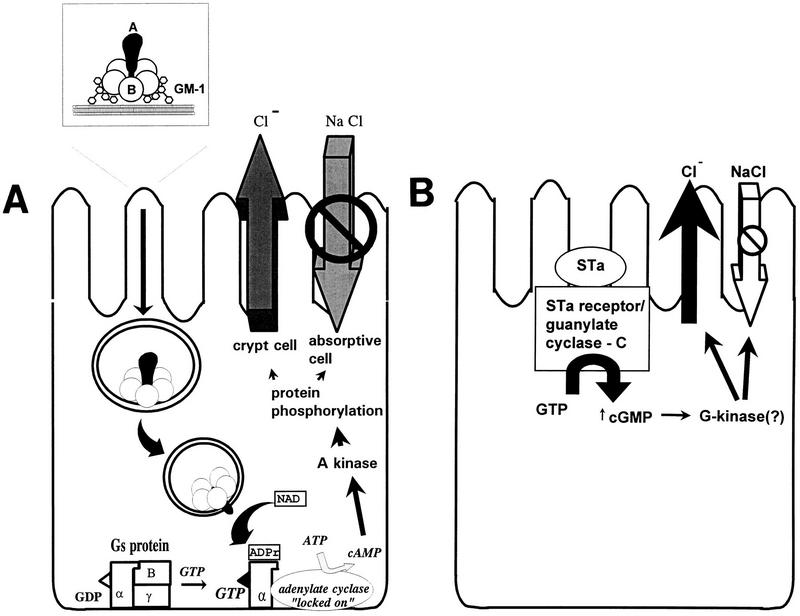
FIG. 4.
Classic mechanisms of action of ETEC toxins (see the text for details and additional proposed mechanisms). (A) LT-I. The LT holotoxin, consisting of one A subunit and five B subunits, is internalized by epithelial cells of the small bowel mucosa via endocytosis. The A1, or catalytic, subunit translocates through the vacuolar membrane and passes through the Golgi apparatus by retrograde transport. In the figure, the A subunit is shown passing through the B subunit ring, but this may not be the case in vivo. A1 catalyzes the ADP-ribosylation of arginine 201 of the α subunit of Gs-protein (which may be apically located); the ADP-ribosylated G-protein activates adenylate cyclase, which elicits supranormal levels of intracellular cAMP. cAMP is an intracellular messenger which regulates several intestinal epithelial cell membrane transporters and other host cell enzymes, as well as having effects on the cytoskeleton. The activation of the cAMP-dependent A kinase results in phosphorylation of apical membrane transporters (especially the cystic fibrosis transmembrane conductance regulator), resulting in secretion of anions (predominantly Cl− by a direct effect, and HCO3− indirectly) by crypt cells and a decrease in absorption of Na+ and Cl− by absorptive cells. cAMP may also have important effects on basolateral transporters and on intracellular calcium levels, both of which may increase the magnitude of the effects on fluid and ion transport. (B) STa. Less is known about the action of ST than of LT. ST is thought to act by binding the ST membrane receptor, GC-C. Activation of GC-C results in increased levels of intracellular cGMP. cGMP exerts its effects in increasing chloride secretion and decreasing NaCl absorption by activating the cGMP-dependent kinase (G-kinase) and/or the cAMP dependent kinase (A-kinase). Other effects of STa in inducing fluid secretion have also been postulated (see the text).
After binding to the host cell membranes, the toxin is endocytosed and translocated through the cell in a process involving trans-Golgi vesicular transport (378). The cellular target of LT is adenylate cyclase located on the basolateral membrane of polarized intestinal epithelial cells. The A1 peptide has an ADP-ribosyltransferase activity and acts by transferring an ADP-ribosyl moiety from NAD to the alpha subunit of the GTP-binding protein, GS, which stimulates adenylate cyclase activity. ADP-ribosylation of the GSα subunit results in adenylate cyclase being permanently activated, leading to increased levels of intracellular cyclic AMP (cAMP). cAMP-dependent protein kinase (A kinase) is thereby activated, leading to supranormal phosphorylation of chloride channels located in the apical epithelial cell membranes. The major chloride channel activated by LT and CT is CFTR (589), the ion channel that is defective in cystic fibrosis. The net result is stimulation of Cl− secretion from secretory crypt cells and inhibition of NaCl absorption by villus tip cells. The increased luminal ion content draws water passively through the paracellular pathway, resulting in osmotic diarrhea.
Although the stimulation of Cl− as a result of increased intracellular levels of cAMP is the classical explanation for the mechanism by which LT and CT cause diarrhea, there is increasing evidence, obtained mostly with CT, that the secretory response to these toxins is considerably more complex (reviewed in reference 589). One alternative mechanism by which these toxins could act involves prostaglandins of the E series (PGE1 and PGE2) and platelet-activating factor. Synthesis and release of arachidonic acid metabolites such as prostaglandins and leukotrienes can stimulate electrolyte transport and intestinal motility. A second alternative mechanism involves the enteric nervous system (ENS), which regulates intestinal motility and ion secretion. Serotonin and vasoactive intestinal polypeptide, both of which can stimulate intestinal epithelial cell secretion via the ENS, are released into the human small bowel after treatment with CT (186). A third potential mechanism could involve a mild intestinal inflammatory response due to CT and LT. CT has been reported to stimulate production of the proinflammatory cytokine interleukin-6 (IL-6), thereby activating the enteric immune system and potentially generating arachidonic acid metabolites that stimulate secretion (433). These alternative secretory mechanisms are supported by a variety of in vitro and in vivo data, and one or more of them could act in concert with the classic mode of action involving cAMP in causing diarrhea due to LT and CT. The similarity of LT and CT is considered sufficiently high to extrapolate mechanistic similarities between the two toxins, and the validity of these assumptions has proven largely correct, with the exception of the failure of LT to release serotonin (660). However, observations made to date for secondary effects of CT have not all been demonstrated for LT, nor has the clinical relevance of these secondary secretory effects been substantiated.
CT and LT have been shown as well to decrease the absorption of fluid and electrolytes from the intestinal lumen (200). Muller et al. have reported that both CT and LT induce cAMP-dependent inhibition of the H+/peptide cotransporter in the human intestinal cell line Caco-2 (456). Interestingly, since the H+/peptide cotransporter does not possess sites for phosphorylation by protein kinase A (PKA), the authors propose that the effect is mediated through PKC. This hypothesis would suggest another novel mechanism of CT and LT and requires substantiation in other systems.
In addition to its enterotoxic properties, LT has the ability to serve as a mucosal adjuvant. Mutants of LT which retain adjuvanticity while eliminating the ADP-ribosyltransferase activity have been constructed (153, 167, 460). Mice immunized orally or intranasally with ovalbumin or fragment C of tetanus toxin together with the mutant LTs have developed higher levels of serum and local antibodies to these antigens than when the antigens are delivered without LT. This property could simplify vaccine development and administration for a variety of pathogens by permitting oral or nasal, rather than parenteral, administration of antigens.
(ii) LT-II.
The LT-II serogroup of the LT family shows 55 to 57% identity to LT-I and CT in the A subunit but essentially no homology to LT-I or CT in the B subunits (229, 271, 518, 589, 612). Two antigenic variants, LT-IIa and LT-IIb, which share 71 and 66% identity in the predicted A and B subunits, respectively, have been described. LT-II increases intracellular cAMP levels by similar mechanisms to those involved with LT-I toxicity, but LT-II uses GD1 as its receptor rather than GM1 (229). As noted above, there is no evidence that LT-II is associated with human or animal disease.
Heat-stable toxins.
In contrast to the large, oligomeric LTs, the STs are small, monomeric toxins that contain multiple cysteine residues, whose disulfide bonds account for the heat stability of these toxins. There are two unrelated classes of STs that differ in structure and mechanism of action. Genes for both classes are found predominantly on plasmids, and some ST-encoding genes have been found on transposons. STa (also called ST-I) toxins are produced by ETEC and several other gram-negative bacteria including Yersinia enterocolitica and V. cholerae non-O1. STa has about 50% protein identity to the EAST1 ST of EAEC, which is described further below. It has recently been reported (564, 706) that some strains of ETEC may also express EAST1 in addition to STa. STb has been found only in ETEC.
(i) STa.
The mature STa is an 18- or 19-amino-acid peptide with a molecular mass of ca. 2 kDa. There are two variants, designated STp (ST porcine or STIa) and STh (ST human or STIb), after their initial discovery in strains isolated from pigs or humans, respectively. Both variants can be found in human ETEC strains. These two variants are nearly identical in the 13 residues that are necessary and sufficient for enterotoxic activity, and of these 13 residues, 6 are cysteines which form three intramolecular disulfide bonds. STa is initially produced as a 72-amino-acid precursor (pre-pro form) that is cleaved by signal peptidase 1 to a 53-amino-acid peptide (533). This form is transported to the periplasm, where the disulfide bonds are formed by the chromosomally encoded DsbA protein (708). An undefined protease processes the pro-STa to the final 18- or 19-residue mature toxin which is released by diffusion across the outer membrane.
The major receptor for STa is a membrane-spanning enzyme called guanylate cyclase C (GC-C), which belongs to a family of receptor cyclases that includes the atrial natriuretic peptide receptors GC-A and GC-B (152, 670). Additional receptors for STa may exist (292, 410), but GC-C is the only receptor identified definitively. GC-C is located in the apical membrane of intestinal epithelial cells, and binding of ligands to the extracellular domain stimulates the intracellular enzymatic activity. A mammalian hormone called guanylin is the endogenous agonist for GC-C (106). Guanylin is a 15-amino-acid peptide which contains four cysteines and is less potent than STa in activating GC-C. Guanylin is presumed to play a role in normal gut homeostasis, and GC-C is apparently used opportunistically by STa to cause diarrhea.
Binding of STa to GC-C stimulates GC activity, leading to increased intracellular cGMP levels (138, 446, 589) (Fig. 4B). This activity leads ultimately to stimulation of chloride secretion and/or inhibition of sodium chloride absorption, resulting in net intestinal fluid secretion. The intermediate steps involved in this process are controversial, and roles for both cGMP-dependent kinases and cAMP-dependent kinases have been reported (589). Ultimately, the CFTR chloride channel is activated, leading to secretion of Cl− ions into the intestinal lumen. In contrast to the 15- to 60-min lag time needed for LT to translocate to and activate the basolateral adenylate cyclase complex, STa acts much faster due to the apical location of its cyclase receptor. Alternative mechanisms of action for STa involving prostaglandins, calcium, and the ENS have been proposed (477, 478), but the evidence for the involvement of these factors is inconsistent. The secretory response to STa may also involve phosphatidylinositol and diacylglycerol release, activation of PKC, elevation of intracellular calcium levels, and microfilament (F-actin) rearrangement (reviewed in reference 589).
(ii) STb.
STb is associated primarily with ETEC strains isolated from pigs, although some human ETEC isolates expressing STb have been reported. STb is initially synthesized as a 71-amino-acid precursor protein, which is processed to a mature 48-amino-acid protein with a molecular weight of 5.1 kDa (23, 171). The STb protein sequence has no homology to that of STa, although it does contain four cysteine residues which form disulfide bonds (23). Unlike STa, STb induces histologic damage in the intestinal epithelium, consisting of loss of villus epithelial cells and partial villus atrophy. The receptor for STb is unknown, although it has been suggested recently that the toxin may bind nonspecifically to the plasma membrane prior to endocytosis (115). Unlike the chloride ion secretion elicited by STa, STb stimulates the secretion of bicarbonate from intestinal cells (589). STb does not stimulate increases in intracellular cAMP or cGMP concentrations, although it does stimulate increases in intracellular calcium levels from extracellular sources (170). STb also stimulates the release of PGE2 and serotonin, suggesting that the ENS may also be involved in the secretory response to this toxin (228, 294).
Colonization factors.
The mechanisms by which ETEC strains adhere to and colonize the intestinal mucosa have been the subject of intensive investigation (for recent reviews, see references 109, 149, 230, and 697). To cause diarrhea, ETEC strains must first adhere to small bowel enterocytes, an event mediated by surface fimbriae (also called pili). Transmission electron microscopy of ETEC strains typically reveals many fimbriae peritrichously arranged around the bacterium; often, multiple fimbrial morphologies can be visualized on the same bacterium (389) (Fig. 2B). A large number of ETEC fimbrial antigens have been characterized (Table 3), although the fimbriae of some ETEC strains have yet to be identified and are only presumed to exist. Clearly, the antigenic heterogeneity conferred by the existence of multiple fimbrial antigens is an obstacle to effective vaccine development.
TABLE 3.
CFAs of human ETEC strains
| Original designation | CS designation | Diameter (nm) | Reference(s) |
|---|---|---|---|
| Rigid rods | |||
| CFA/I | CFA/I | 7 | 321 |
| CS1 | CS1 | 7 | 225, 320, 513 |
| CS2 | CS2 | 7 | 226 |
| CS4 | CS4 | 6 | 698 |
| PCFO159 | CS12 | 7 | 576 |
| PCFO166 | CS14 | 7 | 427 |
| CS17 | CS17 | 7 | 428 |
| PCFO20 | CS18 | 7 | 680 |
| CS19 | CS19 | 7 | 230 |
| CS20 | CS20 | 7 | 671 |
| Bundle-forming | |||
| CFA/III | CS8 | 7 | 634 |
| Longus | CS21 | 7 | 244 |
| Fibrillar | |||
| CS3 | CS3 | 2–3 | 86 |
| CS5 | CS5 | 5 | 127, 411 |
| PCFO148 | CS11 | 3 | 362 |
| PCFO9 | CS13 | 285 | |
| Nonfimbrial | |||
| CS6 | CS6 | 698 | |
| 2230 | CS10 | 147 | |
| 8786 | CS15 | 25 |
ETEC fimbriae confer the species specificity of the pathogen. For example, ETEC strains expressing K99 are pathogenic for calves, lambs and pigs, whereas K88-expressing organisms are able to cause disease only in pigs (109). Human ETEC strains possess their own array of colonization fimbriae, the CFAs (150). The terminology of the CFAs is confusing and inconsistent. However, a uniform scheme has been proposed which would number each putative CFA consecutively according to the year of its initial description (230); the number would be preceded by the initials CS, for coli surface antigen. We support this proposed scheme, and it has been included in Table 3.
The CFAs can be subdivided based on their morphologic characteristics. Three major morphologic varieties exist: rigid rods, bundle-forming flexible rods, and thin flexible wiry structures. CFA/I, the prototype rigid rod-shaped fimbria, is composed of a single protein assembled in a tight helical configuration (308). CFA/III is a bundle-forming pilus with homology to the type 4 fimbrial family (633, 634). CFA/II and CFA/IV are in fact composed of multiple distinct fimbrial structures: CFA/II producers express the flexible CS3 structure either alone or in association with the rod-shaped CS1 or CS2 (389, 597); CFA/IV producers express CS6 in conjunction with CS4 or CS5 (109, 363). A large number of other, less common adhesins have also been found in ETEC strains (150), yet epidemiologic studies suggest that CFA/I, CFA/II, or CFA/IV is expressed by approximately 75% of human ETEC strains worldwide (697). A newly described ETEC fimbria, designated Longus, has been found on a large proportion of human ETEC (244, 246).
The genetics of CFAs have been studied extensively, and these studies have served to illuminate models for fimbrial expression, protein secretion and translocation, and the assembly of bacterial organelles (Fig. 5). CFA genes are usually encoded on plasmids, which typically also encode the enterotoxins ST and/or LT (150). Typical fimbrial gene clusters consist of a series of genes encoding a primary fimbrial subunit protein and accessory proteins which are required for processing, secretion, and assembly of the fimbrial structure itself (150, 308, 319, 370). The pilin structural subunit is usually the predominant immunogen and is thus subject to the greatest antigenic pressure. Pilin subunits accordingly exhibit the greatest sequence variation; however, the N termini of the subunit proteins, as well as the accessory proteins, are generally at least partially conserved. This phenomenon is believed to reflect structure-function requirements (370). Although the actual protein adhesin of some E. coli fimbriae (such as pap and type 1 fimbriae) is a tip protein distinct from the structural protein comprising the stalk, the adhesin of diarrheagenic E. coli fimbriae is generally the stalk protein itself.
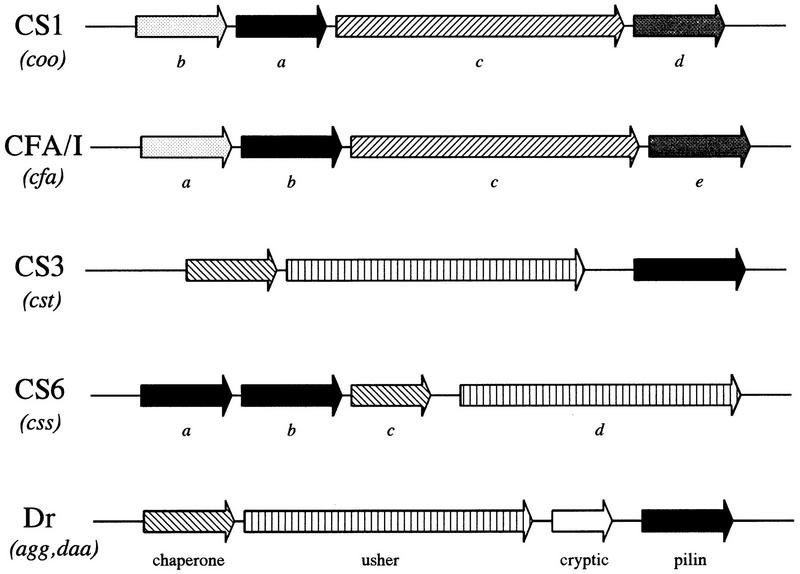
FIG. 5.
Genetics of E. coli fimbriae. Genes required for the expression of functional pili are characteristically linked in gene clusters. The genetic organization of these clusters is illustrated for ETEC fimbriae CS1, CFA/I, CS3, and CS6, and for members of the Dr family, found in DAEC and EAEC. Italicized terms in parentheses represent the gene designations, to be followed by the specific letter under the corresponding arrow to the right. Arrows of similar fill pattern have genetic and functional homology; black arrows represent structural subunits. The known functions of the genes in the Dr cluster are listed below the corresponding genes. These functions can be extrapolated to arrows of similar fill pattern in the CS3 and CS6 gene cluster. The usher and chaperone genes from the Dr, CS6, and CS3 clusters have homology to the genes serving these functions in pap fimbriae: usher proteins are OMPs which serve as pores for the transport and assembly of the fimbrial shaft; fimbrial chaperones bind to the fimbrial subunit proteins in the periplasmic space and prevent premature folding and degradation. CS1 and CFA/I accessory genes, required for assembly and transport of the fimbriae, are homologous to each other but not to CS3, CS6, or the Dr family. CS6 has an unusual organization in that the first two genes of the cluster apparently encode heterologous major subunit proteins (699); the significance of this feature is not yet understood.
Epidemiology
ETEC strains are associated with two major clinical syndromes: weanling diarrhea among children in the developing world, and traveler’s diarrhea. The epidemiologic pattern of ETEC disease is determined in large part by a number of factors: (i) mucosal immunity to ETEC infection develops in exposed individuals; (ii) even immune asymptomatic individuals may shed large numbers of virulent ETEC organisms in the stool; and (iii) the infection requires a relatively high infectious dose (175). These three features create a situation in which ETEC contamination of the environment in areas of endemic infection is extremely prevalent, and most infants in such areas will encounter ETEC upon weaning. The percentage of cases of sporadic endemic infant diarrhea which are due to ETEC usually varies from 10 to 30% (12, 209, 298, 385, 406, 581, 654). School-age children and adults typically have a very low incidence of symptomatic ETEC infection. Characteristically, ST-producing ETEC strains cause the majority of endemic cases (12, 385).
Epidemiologic investigations have implicated contaminated food and water as the most common vehicles for ETEC infection (71, 73, 395, 700). Sampling of both food and water sources from areas of endemic infection have demonstrated strikingly high rates of ETEC contamination (550, 700); this is not unexpected given that 108 CFU of ETEC with buffer must be given to induce high attack rates in volunteers (175, 383). Thus, fecal contamination of water and food sources is the principal reason for the high incidence of ETEC infection throughout the developing world, and the institution of appropriate sanitation is the cornerstone of preventive efforts against this infection.
ETEC infections in areas of endemic infection tend to be clustered in warm, wet months, when multiplication of ETEC in food and water is most efficient (381). Person-to-person transmission was not found to occur during a study of ETEC-infected volunteers housed side by side with volunteers enrolled in an evaluation of influenza vaccine candidates (388).
Although ETEC infection occurs most frequently in infants, immunologically naive adults are susceptible (this stands in contrast to EPEC infection, as described below). Indeed, ETEC is the predominant etiologic agent causing traveler’s diarrhea among adults from the developed world visiting areas where ETEC infection is endemic (21, 70, 174, 422). Studies suggest that 20 to 60% of such travelers experience diarrhea; typically, 20 to 40% of cases are due to ETEC. Predictably, ETEC traveler’s diarrhea occurs most commonly in warm and wet months and among first-time travelers to the developing world (21). Traveler’s diarrhea is usually contracted from contaminated food and water (70, 422, 700).
Clinical Considerations
The clinical characteristics of ETEC disease are consistent with the pathogenetic mechanisms described above. Similar features of the illness have been demonstrated in both volunteers and patients in areas of endemic infection. The illness is typically abrupt in onset with a short incubation period (14 to 50 h) (175, 459). The diarrhea is watery, usually without blood, mucus, or pus; fever and vomiting are present in a minority of patients (175, 381). ETEC diarrhea may be mild, brief, and self-limiting or may result in severe purging similar to that seen in V. cholerae infection (383).
Most life-threatening cases of ETEC diarrhea occur in weanling infants in the developing world. Even though the administration of antibiotics to which ETEC strains are susceptible has been shown to decrease both the duration of diarrhea and the intensity of ETEC excretion (72, 173), effective agents may not be available in areas where the incidence is high; moreover, antibiotic resistance in ETEC strains is an emerging problem, and in many areas (174) effective agents which are safe for children are not readily available. It should be kept in mind, therefore, that the cornerstone of management of ETEC infection is to maintain a normal hydration status. Oral rehydration therapy is often lifesaving in infants and children with ETEC diarrhea.
Travelers to the developing world should also be counseled on the need to maintain hydration when they experience diarrhea. In addition, bismuth subsalicylate or loperamide is effective in decreasing the severity of diarrhea (21); the latter should not be administered to patients with fever or dysentery unless antibiotics are also given. Antibiotics given empirically for traveler’s diarrhea can shorten the duration of the episode (191). Currently, fluoroquinolones (e.g., ciprofloxacin, norfloxacin, and ofloxacin) are the most commonly recommended agents, since increasing antimicrobial resistance to traditional agents has been documented in several areas (173, 174).
Travelers to developing areas are often concerned with the development of traveler’s diarrhea and may seek a means of preventing it. Doxycycline and trimethoprim-sulfamethoxazole have been shown to be effective in this regard, although increasing resistance would suggest that fluoroquinolones administered once daily would be more effective (280). However, the growing problem of antibiotic resistance and the possibility of adverse effects from antimicrobial agents weigh strongly against recommending antimicrobial prophylaxis routinely. Rather, experts have recommended (i) avoiding potentially contaminated food and drink while traveling, (ii) bismuth subsalicylate given four times daily, and (iii) the use of antibiotics empirically if significant diarrhea develops (174).
Oral vaccines against ETEC are being developed by a variety of approaches including the use of killed whole cells, toxoids, purified fimbriae, attenuated ETEC strains, and attenuated Salmonella, Shigella, and V. cholerae strains expressing ETEC antigens (reviewed in references 626 and 630). An oral cholera vaccine containing killed V. cholerae and purified CT B subunit has been reported to provide protection against traveler’s diarrhea due to ETEC (511). This protection is presumably due to the antigenic similarity between LT and CT, although this would not explain the protection against ETEC strains expressing ST. Development of an ETEC vaccine with broad protection is greatly complicated by the numerous intestinal colonization factors expressed by ETEC.
Detection and Diagnosis
Detection of ETEC has long relied on detection of the enterotoxins LT and/or ST. ST was initially detected in a rabbit ligated ileal loop assay (193), but the expense and lack of standardization caused this test to be replaced by the suckling-mouse assay (236), which became the standard test for the presence of STa for many years. The suckling-mouse assay entails the measurement of intestinal fluid in CD4 infant mice after percutaneous injection of culture supernatants.
Several immunoassays have been developed for detection of ST, including a radioimmunoassay (237) and an enzyme-linked immunosorbent assay (ELISA) (144) (available from Denka Seiken, Co. Ltd., Tokyo, Japan). Both of these tests correlate well with results of the suckling-mouse assay and require substantially less expertise (144).
The traditional bioassay for detection of LT involves the use of cell culture, either the Y1 adrenal cell assay or the Chinese hamster ovary (CHO) cell assay. In the Y1 assay, ETEC culture supernatants are added to Y1 cells and the cells are examined for rounding (165). In the CHO cell assay, LT will cause elongation of the CHO cells (265). Immunologic assays are easier to implement in clinical laboratories and include the traditional Biken test (297) as well as newer immunologic methods such as ELISA (709), latex agglutination (304), and two commercially available tests, the reversed passive latex agglutination test (582) and the staphylococcal coagglutination test (116). Both of the commercially available tests are reliable and easy to perform (613).
ETEC strains were among the first pathogenic microorganisms for which molecular diagnostic techniques were developed. As early as 1982 (455), DNA probes were found to be useful in the detection of LT- and ST-encoding genes in stool and environmental samples. Since that time, several advances in ETEC detection have been made, but genetic techniques continue to attract the most attention and use. It should be stressed that there is no perfect test for ETEC: detection of colonization factors is impractical because of their great number and heterogeneity; detection of LT and ST defines an ETEC isolate, yet many such isolates will express colonization factors specific for animals and thus lack human pathogenicity.
The LT polynucleotide probe provides good sensitivity and specificity when labeled with radioisotopes (373, 455) or with enzymatic, nonisotopic detection systems (528). Several different protocols have been published in which nonisotopic labeling methods have proven useful for LT detection (2, 117, 718); we now use a highly reliable alkaline phosphatase-based detection system (Blue Gene; Gibco-BRL) for use in polynucleotide probe colony blot hybridization.
ST polynucleotide probes have had problems of poor sensitivity and specificity, presumably because of the small size of the gene. For this reason, oligonucleotide probes which are generally more sensitive and specific for ST detection have been developed (581) (Table 2 lists the nucleotide sequences of oligonucleotides used for probing and PCR of diarrheagenic E. coli strains). An LT oligonucleotide has also been developed (581), but this reagent has relatively few advantages over an enzymatically detected LT fragment probe. Recently, a trivalent oligonucleotide probe has been proposed which may be of use in detecting the genes encoding LT, ST, and the EHEC Shiga toxin genes (see below); this probe shows promise in an early report (44). ETEC strains are particularly amenable to stool blot hybridization because of the large number of organisms typically shed in the stools of infected individuals (615).
Several PCR assays for ETEC are quite sensitive and specific (177, 374, 492, 581, 615, 654) when used directly on clinical samples or on isolated bacterial colonies. A useful adaptation of PCR is the “multiplex” PCR assay (374, 615), in which several PCR primers are combined with the aim of detecting one of several different diarrheagenic E. coli pathotypes in a single reaction. After multiplex PCR, various reaction products can usually be differentiated by product size, but a second detection step (e.g., nonisotopic probe hybridization) is generally performed to identify the respective PCR products definitively.
ENTEROPATHOGENIC E. COLI
EPEC is an important category of diarrheagenic E. coli which has been linked to infant diarrhea in the developing world. Once defined solely on the basis of O and H serotypes, EPEC is now defined on the basis of pathogenetic characteristics, as described below.
Pathogenesis
Attaching-and-effacing histopathology.
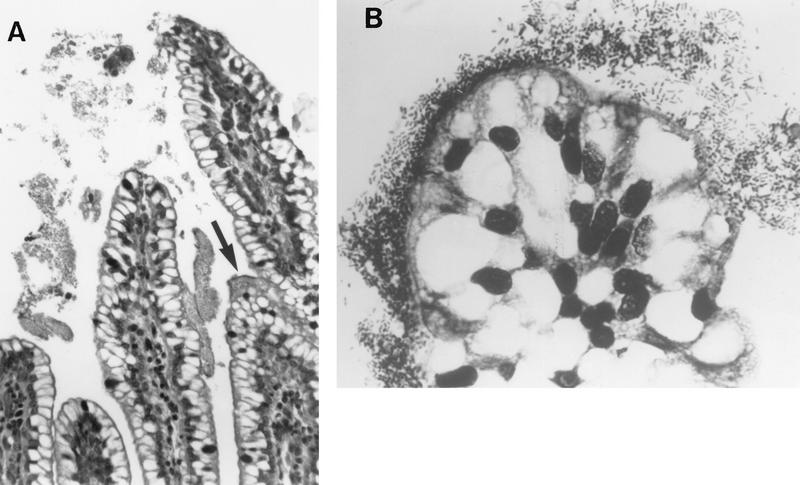
The hallmark of infections due to EPEC is the attaching-and-effacing (A/E) histopathology, which can be observed in intestinal biopsy specimens from patients or infected animals and can be reproduced in cell culture (18, 314, 358, 453, 524, 547, 616, 640, 667, 669) (Fig. 6). This striking phenotype is characterized by effacement of microvilli and intimate adherence between the bacterium and the epithelial cell membrane. Marked cytoskeletal changes, including accumulation of polymerized actin, are seen directly beneath the adherent bacteria; the bacteria sometimes sit upon a pedestal-like structure. These pedestal structures can extend up to 10 μm out from the epithelial cell in pseudopod-like structures (453). This lesion is quite different from the histopathology seen with ETEC strains and V. cholerae, in which the organisms adhere in a nonintimate fashion without causing microvillous effacement or actin polymerization. Although earlier studies had also reported this histopathology, it was not until the report by Moon et al. (453) that the phenotype became widely associated with EPEC and the term “attaching and effacing” was coined.
FIG. 6.
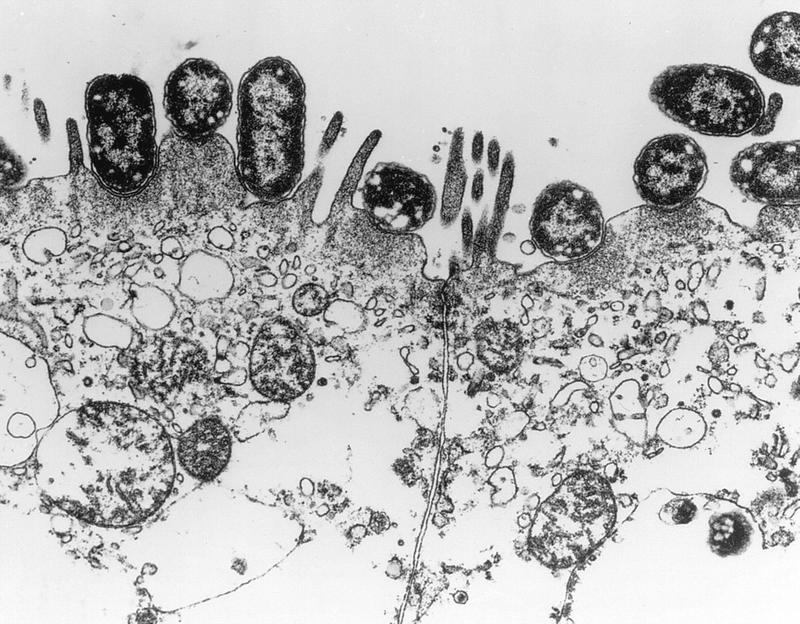
Characteristic EPEC A/E lesion observed in the ileum after oral inoculation of gnotobiotic piglets. Note the intimate attachment of the bacteria to the enterocyte membrane with disruption of the apical cytoskeleton. The appearance of a bacterium sitting on a “pedestal” of cell membrane is quite characteristic. Reprinted from reference 26 with permission of the publisher.
The initial observation by Knutton et al. (359) that the composition of the A/E lesion contained high concentrations of polymerized filamentous actin (F-actin) led to the development of the fluorescent-actin staining (FAS) test. In this test, fluorescein isothiocyanate (FITC)-labeled phalloidin binds specifically to filamentous actin in cultured epithelial cells directly beneath the adherent bacteria. Prior to the development of this test, the A/E histopathology could be detected only by the use of electron microscopy and intact animals or freshly isolated intestinal epithelial cells. Besides providing a diagnostic test for EPEC strains and other organisms capable of causing this histopathology, the FAS test enabled the screening of clones and mutants, leading to the identification of the bacterial genes involved in producing this pathognomonic lesion.
In addition to F-actin, the composition of the A/E lesion includes other cytoskeletal components such as α-actinin, talin, ezrin, and myosin light chain (205). At the tip of the pedestals beneath the plasma membrane are located proteins that are phosphorylated on a tyrosine residue in response to EPEC infection (see below). The formation of the pedestal is a dynamic process, and video microscopy shows that these EPEC pedestals can bend and undulate, alternatively growing longer and shorter while remaining tethered in place on the cell surface (557). Some of the attached EPEC organisms can actually move along the surface of the cultured epithelial cell, reaching speeds up to 0.07 μm/s in a process driven by polymerization of actin at the base of the pedestal. This motility resembles that seen with Listeria spp. (650) inside eukaryotic cells, except that the motile EPEC organisms are located extracellularly. The significance of this motility observed in vitro to the pathogenesis of disease caused by EPEC is unknown. Similar A/E lesions are seen in animal and cell culture models of EHEC (see below) and Hafnia alvei isolated from children with diarrhea (9, 11). However, only a small, highly conserved subset of H. alvei strains produce the A/E lesion (537, 538), and detailed taxonomic studies suggest that the A/E-positive H. alvei strains should not be included in the same species as the A/E-negative H. alvei strains (537). The A/E lesion is also produced by strains of Citrobacter rodentium (formerly Citrobacter freundii biotype 4280) that cause murine colonic hyperplasia (although diarrhea is not seen in infection due to this species) (569). In addition to EPEC and EHEC, a variety of E. coli strains capable of A/E have been isolated from rabbits (102), calves (206), pigs (717), and dogs (172). Thus, EPEC strains are the prototype of an entire family of enteric pathogens that produce A/E lesions on epithelial cells.
Three-stage model of EPEC pathogenesis.
Multiple steps are involved in producing the characteristic A/E histopathology. In 1992, Donnenberg and Kaper (158) proposed a three-stage model of EPEC pathogenesis consisting of (i) localized adherence, (ii) signal transduction, and (iii) intimate adherence (Fig. 7). The temporal sequence of these stages is not certain, and, indeed, the different stages may occur concurrently. Nevertheless, this model has proven to be a robust one that can readily accommodate advances in our understanding of EPEC pathogenesis that have been made since it was first proposed. Additional details on this model can be found in recent reviews (154, 159, 327).
FIG. 7.
Three-stage model of EPEC pathogenesis. (A) The first stage is characterized by initial, relatively distant interaction of bacteria with the enterocyte layer. This initial attachment is thought to be mediated by the bundle-forming pilus. (B) In the second stage, eae and other genes are activated, causing dissolution of the normal microvillar structure. (C) In the third stage, the bacterium binds closely to the epithelial membrane via the protein intimin. Other bacterial gene products mediate further disruption of the cytoskeleton and phosphorylation of cellular proteins. Modified from reference 158 with permission of the publisher.
(i) Localized adherence.
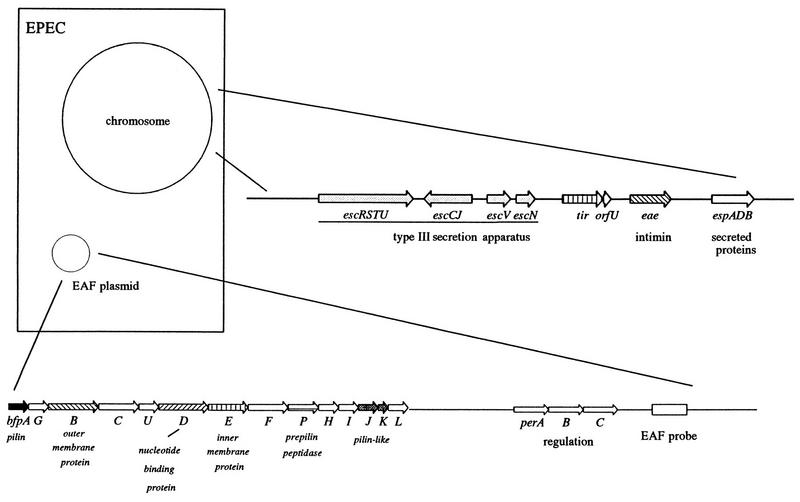
As noted above, adherence to HEp-2 cells was first described by Cravioto et al. for EPEC (139). Baldini et al. (26) showed that the ability of EPEC strain E2348/69 (O127:H6) to adhere in a localized pattern was dependent on the presence of a 60-MDa plasmid. Loss of this plasmid led to loss of the LA phenotype, and transfer of this plasmid to nonadherent E. coli HB101 enabled this strain to adhere to HEp-2 cells. This plasmid was therefore designated the EPEC adherence factor (EAF) plasmid (see below), and a 1-kb fragment from this region was developed as a diagnostic DNA probe (the EAF probe) (27, 461). Although this probe proved to be extremely valuable in diagnosing EPEC (see below) and elucidating the epidemiology of EPEC infections, the exact nature of the adhesin mediating this adherence remained unknown for many years.
The identity of the factor mediating localized adherence was reported in 1991 by Girón et al. (242), who described 7-nm-diameter fimbriae produced by EPEC strains which tended to aggregate and form bundles, thereby suggesting the name “bundle-forming pilus” (BFP). These fimbriae were produced only under certain culture conditions, thereby accounting for the failure of previous investigators to identify them (584). Antiserum prepared against purified BFP significantly, although not completely, reduced the localized adherence of EPEC strain B171 (O111:NM) to HEp-2 cells. BFP are definitely involved in bacterium-to-bacterium adherence in the localized adherence pattern, but there is no definitive proof that BFP mediates actual adherence to epithelial cells. The N-terminal sequence of the purified fimbriae revealed similarity to the TCP pilus of V. cholerae (242) and other members of the type IV fimbrial family. Donnenberg et al. (157) identified the structural gene encoding BFP (bfpA) by using a TnphoA mutant of E2348/69 which no longer conferred localized adherence. Subsequent genetic studies have revealed that a cluster of 13 genes on the EAF plasmid is required for the expression and assembly of BFP (609, 621). Many of these genes encode proteins with similarity to proteins required for type IV pilus biogenesis in other gram-negative pathogens such as V. cholerae and Pseudomonas aeruginosa, but some BFP proteins have no obvious homologs. In addition, expression and assembly of BFP require the global regulator element of EPEC pathogenesis, Per (also called BfpTWV [see below]), and the chromosomal dsbA gene, encoding a periplasmic enzyme that mediates disulfide bond formation (715).
(ii) Signal transduction.
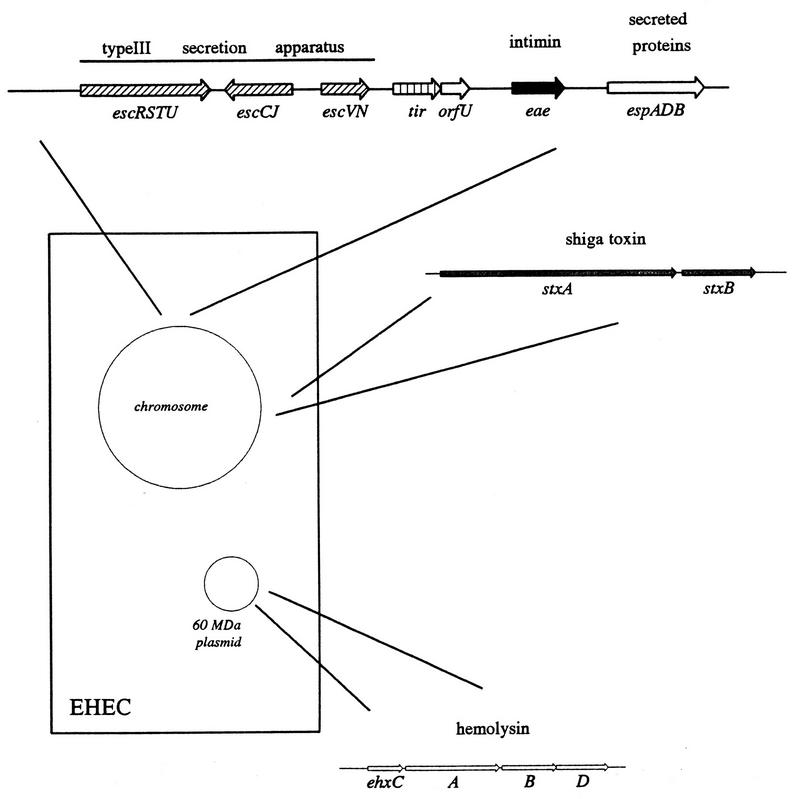
Adherence of EPEC to epithelial cells induces a variety of signal transduction pathways in the eukaryotic cell. The bacterial genes responsible for this signal transduction activity are encoded on a 35-kb pathogenicity island called the locus of enterocyte effacement (LEE), which encodes a type III secretion system, multiple secreted proteins, and a bacterial adhesin called intimin (see below). Mutation of the genes encoding the secreted proteins (espA, espB, and espD) or the genes encoding the type III secretion system (sep and esc) abolishes these multiple signalling events. However, none of these signalling events has been reproduced by the addition of EPEC culture supernatants to epithelial cells, thereby indicating that actual binding of the bacterium is necessary for these changes.
Infection with EPEC induces increases in the intracellular calcium levels [Ca2+i] in cultured epithelial cells to which they are attached (30, 31, 179, 514). The calcium originates from intracellular stores rather than from an influx of extracellular calcium, and buffering of intracellular calcium greatly reduces the polymerization of actin and formation of the A/E lesion (30, 179). The increase in [Ca2+i] has been hypothesized to produce the cytoskeletal changes induced by EPEC via activation of a calcium-dependent, actin-severing protein which could break down actin in the microvillus core (31). Furthermore, since increases in intracellular calcium can inhibit Na+ and Cl− absorption and stimulate chloride secretion in enterocytes (201, 202), these data also suggest that changes in [Ca2+i] may mediate the intestinal secretory response to EPEC. There is evidence that calcium is released from 1,4,5-inositol trisphosphate (IP3)-sensitive stores (31), and several investigators have shown that binding of EPEC to cultured epithelial cells triggers the release of inositol phosphates including IP3 and IP4 in infected cells (179, 212, 360). The increase in the amount of inositol phosphates is consistent with the recently reported activation of phospholipase Cγ1 by EPEC attached to epithelial cells (351).
Adherence of EPEC to epithelial cells results in the phosphorylation of several epithelial cell proteins on serine and threonine residues, the most prominent of which is myosin light chain (407, 409). Activation of at least two kinases, PKC and myosin light chain kinase, has been shown (28, 137, 408, 712). Activation of PKC induces rapid changes in intestinal water and electrolyte secretion in vivo and in vitro (532) and phosphorylation of myosin light chain can lead to increased permeability of tight junctions (408), thereby suggesting additional potential mechanisms of diarrhea due to EPEC.
Binding of EPEC to HeLa cells also induces protein phosphorylation on tyrosine residues (351, 544). The major tyrosine-phosphorylated protein is a 90-kDa protein, called Hp90, inserted into the epithelial cell membrane protein (544). The tyrosine-phosphorylated proteins are part of the A/E lesion, and the distribution of the phosphorylated proteins is restricted to an area immediately beneath the adherent bacteria at the tip of the pedestals (545). Rosenshine et al. (545) have also shown that the tyrosine-phosphorylated Hp90 serves as a receptor for the intimin adhesin (see below). Thus, the signal transduction induced in epithelial cells by EPEC activates receptor binding activity as well as subsequent cytoskeletal rearrangements. The Hp90 protein has recently been shown to be a bacterial protein called Tir (translocated intimin receptor) (352a).
Experiments with polarized epithelial cells such as Caco-2 or T84 show that binding of EPEC results in a decrease in the transepithelial resistance of the monolayers (101, 514, 614). Although an initial report suggested that this drop in resistance involved a transcellular pathway (101), subsequent reports have demonstrated that the paracellular pathway with alterations in tight junctions is involved (514, 614). Buffering of increases in the intracellular calcium concentration completely abrogated the change in resistance (614).
In addition to the effects seen with intestinal epithelial cells, the signal transduction response to EPEC also includes migration of polymorphonuclear leukocytes (PMNs). Using an in vivo system in which polarized T84 intestinal epithelial cells are cocultured with PMNs, Savkovic et al. (565) showed that attachment of EPEC to the epithelial cells caused PMNs to cross the epithelial monolayer. Stimulation of PMN transmigration across intestinal epithelial cells has been shown for invasive organisms such as Salmonella spp. (429) but is unusual for a primarily noninvasive organism such as EPEC. Experimental evidence supports a model in which the binding of EPEC to epithelial cells activates the eukaryotic transcription factor NF-κB, which in turn upregulates the expression of the cytokine IL-8, which is a PMN chemoattractant (565, 566). Neutralizing antibodies to IL-8 ablated ca. 50% of the chemotactic activity, suggesting that other epithelium-derived chemotactic factors are also stimulated by EPEC adherence.
(iii) Intimate adherence.
Intimate adherence of EPEC to epithelial cells is mediated by a 94- to 97-kDa outer membrane protein called intimin. The gene encoding intimin (eae, for E. coli attaching and effacing) was first reported by Jerse et al. (314), who screened TnphoA mutants of EPEC for loss of the A/E phenotype by using the FAS test (the genes involved in EPEC pathogenesis are illustrated in Fig. 8). Although eae mutants cannot adhere intimately to epithelial cells, they can still induce the signal transduction activities described above (212, 544, 565, 618). The eae gene is present in all EPEC, EHEC, C. rodentium, and H. alvei strains capable of producing the A/E histopathology but is absent from E. coli strains in the normal flora, ETEC strains, and other bacteria that do not produce the A/E lesion.
FIG. 8.
Genes involved in EPEC pathogenesis. Genes involved in the pathogenesis of EPEC-induced diarrhea are presented in schematic fashion. Chromosomal virulence genes are clustered within the LEE, which encodes a type III secretory apparatus as well as intimin and a cluster of secreted effector proteins. The EAF plasmid encodes the BFP as well as a cluster of genes required for normal expression of BFP and intimin.
The predicted intimin protein has 31% identity and 50% similarity to the invasin protein of Yersinia species (301). Comparison of the intimin proteins of EPEC strain E2348/69 and EHEC O157:H7 strain EDL933 reveals a striking pattern of sequence conservation among intimin proteins (711). Although the overall protein identity is 83%, the sequence divergence is concentrated in the C-terminal region. The first 75% of the protein (i.e., the first 704 amino acid residues starting from the N terminus) has 94% identity, while the remaining 25% of the residues has only 49% identity (711). The highly divergent C-terminal region is the portion of the molecule that binds to receptors on the epithelial cell (217), and the different intimin sequences can confer different colonization patterns within the intestine (see the section on EHEC, below). There is a growing family of intimin proteins, and sequences have been determined for at least nine intimin proteins from EPEC (8, 314, 398, 687), EHEC (8, 43, 398, 711), C. rodentium (570), H. alvei (217), and E. coli strains pathogenic for rabbits and swine (8). The intimin proteins from these different pathogens are referred to as IntEPEC, IntO26 (from an O26 E. coli strain), IntHA (from Hafnia alvei), etc. The overall pattern for these sequences shows high conservation in the N-terminal region and variability in the C-terminal region.
The role of intimin in human disease was demonstrated by studies in volunteers, who ingested an isogenic eae null mutant of E2348/69 (161). Diarrhea was seen in 11 of 11 volunteers ingesting the wild-type E2348/69 compared to 4 of 11 volunteers ingesting the isogenic mutant (P = 0.002). These results indicate that the eae gene is essential for full virulence of EPEC strain E2348/69 but that additional virulence factors are clearly required for disease. Prior to the discovery of the eae gene, Levine et al. (386) reported that a 94-kDa outer membrane protein (OMP) engendered a strong antibody response in volunteers experimentally infected with EPEC. Subsequent studies showed that this immunogenic 94-kDa OMP is intimin, the product of the eae gene (312). Interestingly, in the volunteer studies conducted by Levine et al. (386) with 10 volunteers, the 9 who became ill upon challenge had no preexisting antibodies to the 94-kDa OMP. In the other volunteer, who did not become ill, antibodies to intimin were present in sera collected prior to challenge. This result hints that intimin may play a role in protective immunity to disease due to EPEC. Secretory immunoglobulin A (IgA) to a 94-kDa OMP of E2348/69 was also found in breast milk from women in a rural Mexican village (143).
Expression of intimin in E. coli K-12 is not sufficient to mediate adherence to epithelial cells (314). However, E. coli K-12 expressing intimin from EPEC strains or E. coli O157:H7 can adhere to epithelial cells when the cells are preinfected with an eae mutant of EPEC (437). The eae mutant itself cannot adhere intimately, but it can provide signals that trigger the epithelial cell to form a functional receptor to which K-12 expressing intimin can adhere. Rosenshine et al. (545) have presented evidence that the EPEC receptor is a tyrosine-phosphorylated 90-kDa membrane protein exposed on the surface of epithelial cells. As discussed above, one of the signal transduction events characteristic of EPEC adhering to epithelial cells is tyrosine phosphorylation of a 90-kDa protein (Tir). When this 90-kDa protein is not tyrosine-phosphorylated, it cannot serve as a receptor. These investigators also showed that purified intimin protein fused to maltose binding protein can bind to membranes extracted from cells preincubated with the eae mutant but not to membranes extracted from cells that have not been infected with this strain. In contrast to these results, Frankel et al. (217, 218) reported that purified intimin-maltose binding protein fusions can adhere to epithelial cells that have not been preincubated with EPEC. These investigators further report that intimin binds to β1 integrins (220), which also serve as receptors for the invasin protein from Yersinia species (379). The reason for these discrepant results is not clear, but it is possible that intimin can bind to more than one receptor, and the question of which receptor is relevant for adherence to intestinal tissue remains to be answered.
Secreted proteins.
A secreted enterotoxin that would explain the mechanism of diarrhea due to EPEC has been unsuccessfully sought for many years (542). It was recently discovered by three independent groups that EPEC strains can secrete proteins into the culture supernatant if grown in cell culture media (273, 309, 350). These proteins, called Esps (for EPEC-secreted proteins), are also produced during the course of disease, since volunteers experimentally infected with EPEC produce antibodies against a number of these proteins (309). However, in contrast to conventional enterotoxins, addition of purified preparations of these secreted proteins has no effect on epithelial cells; only when the proteins are presented to the target epithelial cell by an attached EPEC can they bring about the various signal transduction changes in the epithelial cell outlined above.
At least four proteins are secreted extracellularly by EPEC, and three of these are essential for the A/E histopathology. The proteins that are essential for the A/E phenotype and their apparent molecular masses on sodium dodecyl sulfate-polyacrylamide gel electrophoresis are EspA (25 kDa) (352), EspB (38 kDa; formerly called EaeB) (164, 211, 273, 350), and EspD (40 kDa) (371). Mutation of the espA, espB, or espD gene abolishes the signal transduction in epithelial cells produced by wild-type EPEC and the A/E histopathology. A fourth protein of ca. 110 kDa, called EspC, is homologous to members of the autotransporter protein family, which includes IgA proteases of Neisseria gonorrhoeae and Haemophilus influenzae, Tsh protein produced by avian pathogenic E. coli, SepA of Shigella flexneri, and AIDA-I of DAEC (617). Mutation of the espC gene does not affect signal transduction, A/E histopathology, or any other obvious pathogenic phenotype of EPEC.
The EspA, EspB, and EspD proteins are translated without a conventional N-terminal signal peptide (leader sequence). Jarvis et al. (309) showed that EPEC possesses a type III protein secretion system similar to other specialized protein secretion systems found in a variety of gram-negative human, animal, and plant pathogens. The type III secretion systems are responsible for secretion and translocation of critical virulence determinants such as Shigella Ipa proteins, Yersinia Yops, and proteins involved in invasion by Salmonella spp. (231, 439, 674). In EPEC, the genes encoding this secretion system were initially named sep (for secretion of EPEC proteins), and at least nine sep genes encoding this protein secretion system have been discovered (309, 430). The nomenclature for these genes has recently been revised to correspond with type III secretion systems in Yersinia and other species (188). Those EPEC genes with homologs to Yersinia ysc genes are now called esc, and those type III secretion genes with no homologs will continue to be called sep. Mutation of sepB (escN) abolishes secretion of EspA, EspB, and EspD and abolishes signal transduction and the A/E phenotype (309). Secretion of the 110-kDa EspC protein is not abolished by mutation of sepB (escN) (309), and EspC apparently is secreted extracellularly via a mechanism similar to that used by IgA protease of N. gonorrhoeae (617). Thus, EPEC possesses a specialized protein secretion system that is necessary for translocation of critical proteins from the bacterial cytoplasm to the external environment, where they can interact with epithelial cells. In Yersinia spp., the type III secretion system mediates the injection of the Yop proteins directly into the eukaryotic cell (546), and a similar mechanism could be true for EPEC, whereby the attached EPEC bacterium directly injects one or more of the EspA, EspB, and EspD proteins into the epithelial cell.
Locus of enterocyte effacement.
McDaniel et al. (431) have shown that the eae, espB, and sep (esc) genes are all located within a 35.5-kb chromosomal region of EPEC strain E2348/69 (Fig. 8); subsequent studies showed that espA and espD but not espC are also located in this region (352, 371, 617). This region, called LEE (for locus of enterocyte effacement), is not present in E. coli strains in the normal flora, E. coli K-12, or ETEC. Sequences homologous to the EPEC sequence are also found in other Enterobacteriaceae that cause the A/E phenotype, including EHEC, the rabbit diarrheal pathogen RDEC-1, diarrheagenic H. alvei, and C. rodentium. The LEE region of EPEC E2348/69 is inserted into the E. coli K-12 chromosome at ca. 82 min, where the tRNA for selenocysteine (selC) is located. Interestingly, this location is also the site of insertion for the retronphage φR73 and a large (70 kb) insert (PAI) of uropathogenic E. coli strains containing genes for hemolysin (hly) and P-related fimbriae (prf) (76). The large insert for uropathogenic E. coli has been termed a pathogenicity island (76), and the insertion of the EPEC LEE at the same site suggests that this region of the E. coli chromosome is a hot spot for insertion of virulence factor genes. The G+C content of the LEE is ca. 38%, which is strikingly lower than the 50 to 51% G+C content of the total E. coli genome (431), thus suggesting horizontal transfer of this pathogenicity island into E. coli from another species. The LEE pathogenicity island not only is necessary for the A/E phenotype but also is sufficient. McDaniel and Kaper (432) recently reported that a recombinant plasmid clone containing the entire LEE region with less than 800 bp of flanking DNA is sufficient to confer the A/E phenotype when cloned into K-12 or normal E. coli from the normal flora.
EAF plasmids.
The BFP is encoded on plasmids which range in size from ca. 50 to 70 MDa, called the EAF plasmids. These plasmids share extensive homology among various EPEC strains (467), and the restriction maps of two EAF plasmids have been determined (467, 609). Downstream of the bfp gene cluster is a cluster of three genes encoding a transcriptional activator (Per), which positively regulates several chromosomal and plasmid genes necessary for the pathogenesis of EPEC (see below). Beyond the per genes is a 1-kb restriction fragment that has been extensively used as a diagnostic DNA probe, called the EAF probe (27, 461). Although the DNA sequence of this fragment has been determined (214), the contribution to EPEC pathogenesis of the genes encoded in the EAF probe region is unknown, as is the contribution of genes contained in the major portion of the EAF plasmid. The use of the EAF probe for diagnosis of EPEC is discussed below.
The importance of the EAF plasmid in human disease was shown by Levine et al. (386), who fed strain E2348/69 possessing the EAF plasmid and a derivative of this strain that had lost the plasmid to adult volunteers. Diarrhea occurred in 9 of 10 volunteers who ingested the wild-type strain (mean diarrheal stool volume, 1,178 ml) but in only 2 of 9 volunteers who ingested the cured derivative (mean stool volume, of 433 ml) (P < 0.006). Interestingly, although this plasmid is highly stable in vitro (<1% spontaneous plasmid cure rate), 67% of the challenge strain isolates recovered from volunteer stool specimens had lost this plasmid (386). This high rate of spontaneous cure could affect the diagnosis of EPEC if possession of the EAF plasmid is part of the definition (see below).
Regulation.
As for many other bacterial pathogens, expression of EPEC virulence factors is regulated by a trans-acting protein. A cluster of three open reading frames designated perA, perB, and perC (for plasmid-encoded regulator) encode proteins that form a regulatory complex, which activates the transcription of several genes in the chromosome and on the EAF plasmid. The predicted protein product of the first open reading frame of this region, PerA, has homology to the AraC family of bacterial regulators, including regulators of virulence genes of Shigella spp. (VirF) and ETEC (Rns and CfaD/CfaR) (257). The Per regulator increases expression of the chromosomal eae (257) and espB (eaeB) genes (255), as well as that of genes encoding 50- and 33-kDa OMPs (257). Secretion of EspB is regulated by Per and is induced in response to conditions similar to those in the gastrointestinal tract (349). The bfpA gene is also under per control, as the recently described bfpTVW regulatory gene cluster is in fact allelic with perABC (256, 329, 651). In addition to increasing the expression of these genes, Per decreases the expression of a gene encoding a 20-kDa OMP (257). It has also been reported that Per can repress the expression of intimin during the stationary phase of growth while activating the expression of intimin during exponential growth (357). Thus, there appears to be a global regulatory system for regulation of virulence in EPEC that allows this pathogen to respond to different environmental conditions and different phases of growth, but the details of this regulation have not been elucidated.
Other potential virulence factors.
(i) Other fimbriae.
There have been numerous reports of additional fimbrial structures produced by EPEC, some of which were subsequently shown to be type 1 fimbriae (reviewed in reference 376). Girón et al. (243) reported an extensive characterization of fimbriae produced by EPEC strain B171. In addition to BFP, this strain produced rod-like fimbriae and fibrillae with subunit sizes of 16.5, 15.5, and 14.7 kDa. Their N-terminal amino acid sequence showed homology to F9 and F72 fimbriae of uropathogenic E. coli (both are P fimbriae) and the F1845 fimbria of DAEC, respectively. Antisera prepared against a mixture of all three fimbriae (called FB171) reduced the adherence of strain B171 to HEp-2 cells by ca. 75% (243). Combining anti-FB171 and anti-BFP sera inhibited localized adherence by ca. 100%.
The need for multiple antisera to completely inhibit localized adherence and the results of ultrastructural studies of the “microcolonies” comprising the localized adherence phenotype indicate that this phenomenon is multifactorial (243). Scanning electron micrographs of the microcolonies reveal that multiple bacterium–HEp-2 cell and bacterium-bacterium interactions are involved. Thin fibers resembling fimbriae appear to link bacterial cells and epithelial cells, while rope-like structures resembling BFP may be primarily involved in bacterium-bacterium interactions. Construction of isogenic mutants specifically altered in genes encoding the various fimbrial structures of EPEC will be necessary before the localized adherence phenotype is completely understood.
(ii) EAST1.
As described below, many EAEC strains produce a low-molecular-weight ST called EAST1. Some EPEC strains also produce EAST1, and a survey of E. coli strains by Savarino et al. (564) reported that 14 of 65 EPEC strains tested (22%) hybridized with the astA gene encoding EAST1. Interestingly, strain E2348/69, the prototype EPEC strain used for volunteer studies, contains two copies of the astA gene, one in the chromosome and one in the EAF plasmid. The significance of this toxin in EPEC pathogenesis is unknown, but it is interesting that the EAF-negative EPEC-like organisms responsible for two large outbreaks of disease in adults in Minnesota and Finland (see below) also contained the astA gene. Determination whether EPEC strains containing astA are more frequently isolated from adults than are EPEC strains lacking astA might yield insights into the striking age distribution seen with infections due to EPEC (see below).
(iii) Invasion.
Several investigators have shown that EPEC strains are capable of entering a variety of epithelial cell lines (18, 156, 213, 448). Furthermore, many published photographs of animal and human EPEC infections show apparently intracellular bacteria (453, 524, 667, 669). However, unlike true intracellular pathogens such as Shigella spp., EPEC strains do not multiply intracellularly or escape from a phagocytic vacuole and thus do not appear to be specifically adapted for intracellular survival. EPEC strains do not cause dysentery or a typhoid-like syndrome, and so the clinical significance of cell entry in the pathogenesis of disease due to EPEC is not clear. However, despite the lack of evidence that invasion by EPEC is important in pathogenesis, this phenotype has been very useful in studying the molecular genetics of EPEC because many of the genes involved in invasion are also involved in forming the A/E lesion. Donnenberg et al. (155) have used TnphoA and the gentamicin protection assay to isolate mutants deficient in cell entry. Two categories of noninvasive mutants had insertions located in the bfpA or dsbA loci, both of which are required to produce functional BFP fimbriae (157, 714). A third category of mutants were deficient in intimate adherence, and these mutants had insertions in the eae and espB (eaeB) genes (155, 164). Another category of mutants (category 4 mutants or cfm) were completely deficient in the FAS test and in the ability to induce a tyrosine kinase activity in the host cell; the mutations in these mutants were subsequently shown to be in the sep/esc genes encoding the type III protein secretion system. These results indicate that there is significant overlap between the genes responsible for the invasion process and genes involved in producing attaching and effacing lesions.
Two studies have reported that O111:NM strains contain plasmid sequences that can confer invasiveness upon E. coli K-12 strains containing the cloned fragments (208, 567). Sequences homologous to these cloned genes were present in only a minority of EPEC strains and were apparently not contained in the LEE pathogenicity island. Mutation of the sepZ locus within the LEE abolished the invasion phenotype without affecting the A/E phenotype (527). The relevance of these sequences to the pathogenesis of disease is unknown.
Mechanism of diarrhea.
The impressive advances in our understanding of EPEC pathogenesis at the genetic and cellular levels allow us to present a plausible mechanism for how diarrhea results from infection with EPEC. The dramatic loss of the absorptive microvilli in the A/E lesion could lead to diarrhea via malabsorption. However, the incubation period in adult volunteers can be very short: as little as 2.9 h between ingestion of the organisms and the onset of diarrhea (161). This rapidity suggests that a more active secretory mechanism is involved in diarrhea caused by EPEC and that a variety of intracellular mediators of intestinal ion transport, such as calcium, PKC, inositol phosphates, and tyrosine kinase, are affected by EPEC infection. There have been recent reports of EPEC actively altering ion transport in epithelial cells. Stein et al. (618) found a significant decrease in the transmembrane potential in epithelial cells infected with EPEC. This result suggests that EPEC stimulates either an influx of positive ions or an efflux of negative ions across the membrane. Knutton et al. (361) have found that EPEC can stimulate a rapid but transient increase in short-circuit current (Isc) in intestinal epithelial cells mounted in Ussing chambers; secretion of chloride ions was implicated in this effect (132). In both studies, changes in ion transport did not occur as a result of addition of cell-free culture supernatants of EPEC to epithelial cells; infection with viable bacteria was necessary. These ionic changes were abrogated by mutation of the espB gene but not the eae gene (361, 618).
Mechanisms other than direct stimulation of ion secretion are also possible. As described above, infection of an epithelial cell monolayer by EPEC leads to decreased monolayer resistance, which could result in diarrhea due to increased intestinal permeability. Some clinical reports have noted a local inflammatory response upon biopsy (669), and inflammation has been seen in animal models of EPEC infection (662). As noted above, attachment of EPEC to cultured human intestinal epithelial monolayers induces the transmigration of PMNs (565). Transmigration of PMNs can, in turn, result in increased Isc due to chloride secretion (403), thereby suggesting another mechanism of diarrhea due to EPEC.
These multiple mechanisms could all be involved in diarrhea due to EPEC. The active chloride secretion, perhaps mediated via a pathway involving PKC, could account for the rapid onset of diarrhea. The prolonged diarrhea seen in some patients could result from malabsorption due to loss of the brush border. A local inflammatory response and increased intestinal permeability in response to EPEC infection could also contribute to the diarrhea.
Epidemiology
Age distribution.
The most notable feature of the epidemiology of disease due to EPEC is the striking age distribution seen in persons infected with this pathogen. EPEC infection is primarily a disease of infants younger than 2 years. As reviewed by Levine and Edelman (384), numerous case-control studies in many countries have shown a strong correlation of isolation of EPEC from infants with diarrhea compared to healthy infants. The correlation is strongest with infants younger than 6 months. In children older than 2 years, EPEC can be isolated from healthy and sick individuals, but a statistically significant correlation with disease is usually not found.
EPEC can cause diarrhea in adult volunteers if high inocula (108 to 1010) are given after gastric acid is neutralized with bicarbonate (382). The infectious dose in naturally transmitted infection among infants is not known but is presumed to be much lower. The reason(s) for the relative resistance of adults and older children is not known, but loss of specific receptors with age is one possibility. A similar restriction of disease to young animals is seen with E. coli strains that cause diarrhea by similar A/E mechanisms in weanling rabbits. The fact that EPEC has not been implicated as a cause of traveler’s diarrhea in countries with high incidences of both EPEC and ETEC suggests a physiological basis for this resistance rather than host immunity or exposure. However, several outbreaks of diarrhea due to EPEC have been reported in healthy adults (135, 281, 580, 681), presumably due to ingestion of a large inoculum from a common source. Sporadic disease has also been seen in some adults with compromising factors (diabetics, those with achlorhydria, the elderly) (384, 620).
Transmission and reservoirs.
As with other diarrheagenic E. coli strains, transmission of EPEC is fecal-oral, with contaminated hands, contaminated weaning foods or formula, or contaminated fomites serving as vehicles (384). Unless strict decontamination procedures are followed, admission of an infant to a pediatric ward can result in contamination of crib linen, toys, tabletops, hand towels, scales, carriages, rubber nipples, etc. In one study (543), EPEC was isolated from dust and aerosols, suggesting potential airborne transmission, either directly through inhalation followed by ingestion or indirectly via contamination of other fomites. In the uncommon adult outbreaks, waterborne and foodborne transmission has been reported but no particular type of food has been implicated as more likely to serve as a source of infection. The reservoir of EPEC infection is thought to be symptomatic or asymptomatic children and asymptomatic adult carriers, including mothers and persons who handle infants (384). Numerous studies have documented the spread of infection through hospitals, nurseries, and day care centers from an index case (82, 384, 703). Epidemiologic studies in several countries have shown high backgrounds of asymptomatic carriage; in some studies, as many as 17 to 20% of healthy infants younger than 2 years shed E. coli of EPEC serotypes in their stools (reviewed in reference 384). In symptomatic patients, EPEC can be isolated from stools up to 2 weeks after cessation of symptoms (289). Although animals such as rabbits, pigs (717), and dogs have EPEC-like organisms associated with disease, the serotypes found in these animal pathogens are usually not human serotypes.
EPEC in developed countries.
EPEC once caused frequent outbreaks of infant diarrhea in the United States and the United Kingdom (541). These community-acquired and nosocomial outbreaks were often explosive, with up to 50% mortality (239, 384, 541). EPEC strains are no longer as important a cause of diarrhea in developed countries as they were in the 1940s and 1950s; the reasons for this change in incidence are not clear. The discontinuation of routine serological screening of E. coli by most hospitals contributes in part to the low incidence of EPEC reported in developed countries. However, several outbreaks of diarrhea due to EPEC have been reported in the last two decades in the United States, the United Kingdom, Finland, and other developed countries. These outbreaks frequently occur in day care centers (82, 509) and occasionally occur in pediatric wards (82, 547). An outbreak due to atypical EPEC was recently reported among adults who ate at a gourmet buffet in Minnesota (see below) (281). However, EPEC strains are also associated with sporadic cases of diarrhea in the United States and other developed countries (78, 384, 592). A recent study of E. coli isolated from children with diarrhea in Seattle used diagnostic DNA probes and found a high incidence of EPEC-like organisms in this population (78). This study found that eae-positive, stx-negative strains (usually localized adherence positive but EAF negative) were isolated from 3.6% of specimens, a frequency that exceeded the rates of recovery of Campylobacter spp., E. coli O157:H7, Salmonella spp., Shigella spp., or Yersinia spp. These results suggest that the current importance of EPEC in the United States may be seriously underestimated.
EPEC in developing countries.
In contrast to the limited importance of EPEC in developed countries, EPEC is a major cause of infant diarrhea in developing countries. Numerous case-control studies on six continents have found EPEC to be more frequently isolated from infants with diarrhea than from matched healthy controls (reviewed in references 154 and 384). Particularly in the 0- to 6-month age group, EPEC strains are often the most frequently isolated bacterial diarrheal pathogens. Studies in Brazil (251, 252, 653), Mexico (140, 141), and South Africa (542) have shown that 30 to 40% of infant diarrhea can be attributed to EPEC, and in some studies EPEC infection exceeds rotavirus infection in incidence (140, 251, 252, 540). EPEC strains are an important cause of disease in all settings—nosocomial outbreaks, outpatient clinics, patients admitted to hospitals, community-based longitudinal studies, and urban and rural settings. Several studies have shown that breast feeding is protective against diarrhea due to EPEC (74, 540). Both human colostrum and milk strongly inhibit the adhesion of EPEC to HEp-2 cells in vitro, and the inhibitory activity has been found in both sIgA and oligosaccharide fractions (100, 143).
Clinical Considerations
EPEC causes primarily acute diarrhea, although many cases of protracted EPEC diarrhea have also been reported (154, 384). In the Cincinnati outbreak reported by Rothbaum et al. (547), the duration of hospitalization ranged from 21 to 120 days. The infection can often be quite severe, and many clinical reports emphasize the severity of the disease (82, 547). In outbreaks in the United States and the United Kingdom in the mid-20th century, mortality rates of 25 to 50% were reported (154), and in recent outbreaks from developing countries, 30% mortality was reported (590). In developed countries with the full range of modern treatment available, mortality is much lower although deaths can still result (547). In addition to profuse watery diarrhea, vomiting and low-grade fever are common symptoms of EPEC infection. Fecal leukocytes are seen only occasionally, but more sensitive tests for inflammatory diarrhea such as an anti-lactoferrin latex bead agglutination test are frequently positive with EPEC infection (449). Proximal small intestinal mucosal biopsy specimens often, but not always, show intimately adherent bacteria and the classic A/E histopathology (194, 547, 592). The presence of the A/E lesion is associated with disarrangement of the digestive-absorptive enzyme system, leading to malabsorption of nutrients (194, 289, 547).
As with other diarrheal pathogens, the primary goal of treatment of EPEC diarrhea is to prevent dehydration by correcting fluid and electrolyte imbalances. Oral rehydration may be sufficient for milder cases, but more severe cases require parenteral rehydration. Correction of nutritional imbalance with lactose-free formula or breast milk may be insufficient for some severely ill patients, and total parenteral nutrition may be required (154, 194). A variety of antibiotics have been used to treat EPEC and have proved useful in many cases (154), but multiple antibiotic resistance is common for EPEC (154). Other therapies such as bismuth subsalicylate (204) and specific bovine anti-EPEC milk Igs (447) have also proven useful. There are no vaccines currently available or in clinical trials to prevent disease due to EPEC.
Detection and Diagnosis
Definition of EPEC.
Before discussing the detection and diagnosis of EPEC, it is appropriate to first discuss the characteristics that actually define EPEC. The definition of EPEC has changed drastically in recent years as our knowledge of this pathogen has grown. For many years these organisms were defined only by O serogroups, which were subsequently refined to O:H serotypes. This definition changed as additional serotypes were associated with infantile diarrhea (184). Citing recent pathogenesis data, the Second International Symposium on EPEC in 1995 reached a consensus on the basic characteristics of EPEC (328); the most important of these were the A/E histopathology and the absence of Shiga toxin. Many EHEC strains also produce the A/E lesion; therefore, determining the presence (indicative of EHEC) or absence (indicative of EPEC) of Stx is essential. The possession of specific O and H antigens, which for many years was the sole defining microbiological characteristic of EPEC, is no longer deemed an essential characteristic of EPEC, although the majority of EPEC strains fall into certain well-recognized O:H serotypes.
There is some debate whether EPEC strains that lack the EAF plasmid (which encodes the BFP adhesin and Per regulators) are true pathogens (328). In every case-control study of FAS-positive, eae-positive EPEC strains so far reported, only EAF-positive strains and not EAF-negative strains were significantly associated with diarrhea (183, 252, 254, 387). EAF-negative strains that produce the A/E lesion (i.e., are FAS positive or eae probe positive) can result from loss of the EAF plasmid; indeed, it has been well documented that volunteers who ingest an EAF-positive strain can shed derivatives of the challenge strain that have spontaneously lost the EAF plasmid (386). Furthermore, EAF-negative strains isolated during the course of an epidemiological study could also be derivatives of EHEC that have lost the phages that encode Stx. At present, it is impossible to distinguish strains of these two categories from strains that never possessed the EAF plasmid but can nonetheless cause diarrhea in certain individuals. Moreover, volunteer studies have shown that an EAF plasmid-cured derivative of E2348/69, while significantly attenuated relative to the EAF-positive parent strain, nonetheless caused mild diarrhea in two of nine volunteers (386). EAF-negative, eae-positive strains have been implicated as the causative agents in at least one small pediatric outbreak in the United Kingdom (300, 602) and one large outbreak involving more than 600 individuals in Finland (602, 681). However, the latter outbreak was highly unusual in that a number of adults were affected. In a survey of 925 E. coli strains isolated from patients with diarrhea in the United Kingdom and belonging to EPEC serogroups, fewer than 10% of eae-positive strains hybridized with the EAF probe (585). EAF-negative EPEC strains may be similar to LT-only ETEC strains, which are usually not associated with diarrhea in case-control studies but which have been implicated in outbreaks and sporadic cases. Whether EAF-negative strains possess additional virulence factors that have yet to be discovered or whether there are specific host factors that predispose to disease with these strains is unknown.
Accordingly, a consensus definition was achieved at the Second International Symposium on EPEC: A/E, Stx-negative strains possessing the EAF plasmid would be called “typical EPEC,” while such strains that do not possess the EAF plasmid would be called “atypical EPEC.” Several outbreaks have implicated atypical EPEC as the causative agent. One recent foodborne outbreak involved more than 100 adults who ate at a gourmet buffet in Minnesota (281). The implicated organism was an O39:NM E. coli strain that hybridized with the eae gene plus other genes in the LEE but did not hybridize with the EAF probe. In addition, this strain hybridized with a probe (astA) to EAEC EAST1. In an outbreak of diarrhea in Finnish adults and schoolchildren, an O111 eae-positive, EAF-negative, astA-positive E. coli strain was implicated (602, 681). Unlike the O39:NM strain, the O111 strain possessed a standard EPEC O antigen and also exhibited localized adherence on tissue cells in culture, although it was EAF negative. Note that the Vi antigen reportedly expressed by this strain could not be confirmed in reference laboratories (602).
Diagnostic tests.
Given that EPEC strains, as with other diarrheagenic E. coli strains, are defined on the basis of virulence properties, there are two approaches to the detection of EPEC in the laboratory: phenotypic and genotypic. The phenotypic approach requires the use of cell cultures and fluorescence microscopy, and the genotypic method requires the use of DNA hybridization or PCR.
(i) Phenotypic tests.
The A/E phenotype can be identified by using cultured HEp-2 or HeLa cells and the mushroom toxin phalloidin conjugated to FITC or rhodamine (available from Sigma, Molecular Probes, and other sources) as in the FAS assay originally described by Knutton et al. (359). In brief, the bacterial cells are incubated with the cultured cells for 3 to 6 h, after which cells are fixed and washed as described above for the HEp-2 adherence assay. The cells are permeabilized by treating coverslips with 0.1% Triton X-100 in phosphate-buffered saline (pH 7.2) for 5 min, followed by subjecting them to multiple washes in phosphate-buffered saline and treating them with conjugated phalloidin to stain the filamentous actin. The specimens are then examined under an incident-light fluorescence microscope (at the proper wavelength for FITC or rhodamine conjugate), and the same fields are visualized under phase-contrast microscopy. Punctate spots of intense fluorescence that correspond to bacterial cells under phase-contrast microscopy denote a positive FAS test. To avoid the use of the highly toxic phalloidin, Ismali et al. (303) used a monoclonal antibody directed against α-actinin, which is also concentrated in epithelial cells directly beneath the adherent EPEC. The A/E phenotype can also be identified by electron microscopic examination of intestinal biopsy specimens or of cultured epithelial cells incubated with EPEC.
There are a number of phenotypic tests to determine the other crucial characteristic of EPEC, the lack of Stx expression. These tests are discussed in the section on EHEC below. An excellent phenotypic marker for the presence of the EAF plasmid is localized adherence on HEp-2 or HeLa cells, as described above. An ELISA for the detection of EAF-positive EPEC, based on an antiserum raised against an EAF plasmid-containing strain and absorbed against a plasmid-cured strain, has been described previously (10). However, no subsequent reports have appeared in which this test was described. There are no readily available immunoassays to detect the A/E pattern.
(ii) Genotypic tests.
DNA probes and PCR primers have been developed and evaluated for the three major characteristics of EPEC: A/E, EAF plasmid, and lack of Shiga toxin. DNA tests for genes encoding Shiga toxin are reviewed below. As defined above, typical EPEC strains would possess the eae gene for A/E and the EAF probe or bfp sequences, indicating the presence of the EAF plasmid. Atypical EPEC strains would possess the eae gene only without the EAF plasmid.
(a) eae gene.
Possession of eae sequences correlates with possession of the 35-kb LEE pathogenicity island encoding A/E (431). No exceptions to this generalization have been reported, and so there is no need to test for other sequences in the LEE such as espB and sep unless specific strain differences are sought for molecular epidemiology purposes. Sequence variability has been reported for the espB sequences from different EPEC and EHEC strains (182). As discussed above, sequence variability is also seen in the 3′ end of the eae gene encoding the C-terminal region of intimin. A 1-kb fragment probe originally described by Jerse et al. (314) is derived from sequences encoding the highly conserved N-terminal region. Compared to the A/E phenotype as determined by the FAS test, this probe was 100% sensitive and 98% specific; the two “false-positive” strains possessed the LEE but were FAS negative due to reduced expression of the genes on the LEE (311, 314). This probe is easily used with nonradioactive labeling methods (463). Fragment probes using the variable 3′ end of the eae gene have been reported for O157:H7 EHEC strains (691), but no probes can successfully differentiate all EPEC eae sequences from all EHEC eae sequences.
A variety of eae primers for detecting this gene by PCR have been tested (eae PCR is discussed in the section on EHEC, below). As reported by Gannon et al. (234), primer pairs for the conserved 5′ region amplified fragments from all eae-positive strains whereas primers from the 3′ end were specific for certain serotypes. Primer sequences which will detect eae in both EPEC and EHEC strains are shown in Table 2.
(b) EAF plasmid.
The 1-kb EAF fragment probe originally described by Nataro et al. (461) is from a region of the EAF plasmid with an unknown function. This probe may be used with nonradioactive labeling techniques (238). A sensitive and specific oligonucleotide probe consisting of 21 bases of the EAF fragment was developed by Jerse et al. (313). Franke et al. (214) developed a PCR primer pair to amplify a 397-bp region of the EAF probe. The sequences of these probes and primers are given in Table 2.
The cloning of the bfpA gene allowed the development of DNA probes for specific plasmid-encoded virulence factors. Girón et al. (241) reported an 850-bp bfpA fragment probe that was slightly more sensitive than the EAF probe; among EPEC strains exhibiting localized adherence to HEp-2 cells, the bfpA and EAF probes hybridized with 99 and 96% of the strains, respectively. Trabulsi et al. (658) have reported that bfpA-positive, EAF-negative EPEC strains are found in specific serotypes such as O119:H2. Sohel et al. (608) used a 579-bp bfpA probe cloned from a different EPEC strain which hybridized to a number of Salmonella strains under moderate- but not high-stringency conditions. A 29-base oligonucleotide probe for the bfpA gene was reported by Nagayama et al. (458). This nonradioactive probe, conjugated to alkaline phosphatase, had a sensitivity and specificity of 95.7 and 100%, respectively, with respect to assays for localized adherence. Gunzburg et al. (268) reported a highly sensitive and specific PCR procedure to detect the bfpA gene, and this technique was readily adapted for the diagnosis of EPEC in a field setting in Northern Brazil (654). The sequences for the bfpA oligonucleotide probes and PCR primers are given in Table 2.
In general, there is excellent agreement among the phenotypic characteristics of localized adherence on HEp-2 cells, the presence of bfpA sequences, and EAF probe positivity. However, there are reports of an LA pattern expressed by EPEC strains that are EAF probe negative (78, 586). Whether these strains express another adhesin analogous to BFP is not known, but it has also been shown that increased expression of the chromosomal eae gene encoding intimin in the absence of the EAF plasmid can lead to an LA-like adherence pattern on HEp-2 cells (259). Variability in cell culture assays could also account for some of these discrepancies. The EAF probe has been used in dozens of epidemiologic studies all over the world, and there are several studies showing the significant correlation of possession of EAF probe sequences and disease potential. In view of the many years of data that have accumulated with the EAF probe, we favor the continued use of the EAF probe so that comparisons to previous studies can be readily made. However, the absence of EAF probe sequences in eae-positive strains that lack stx would still allow a diagnosis of atypical EPEC under the definition discussed above.
ENTEROHEMORRHAGIC E. COLI
Origins
The recognition of EHEC as a distinct class of pathogenic E. coli resulted from two key epidemiologic observations. The first was the 1983 report by Riley et al. (539), who investigated two outbreaks of a distinctive gastrointestinal illness characterized by severe crampy abdominal pain, watery diarrhea followed by grossly bloody diarrhea, and little or no fever. This illness, designated hemorrhagic colitis (HC), was associated with the ingestion of undercooked hamburgers at a fast-food restaurant chain. Stool cultures from these patients yielded a previously rarely isolated E. coli serotype, O157:H7. The second key observation was by Karmali et al. (343), also in 1983, who reported the association of sporadic cases of hemolytic uremic syndrome (HUS) with fecal cytotoxin and cytotoxin-producing E. coli in stools. HUS (defined by the triad of acute renal failure, thrombocytopenia, and microangiopathic hemolytic anemia) was already known to be preceded typically by a bloody diarrheal illness indistinguishable from HC. Thus, two key clinical microbiological observations, one based on a rare E. coli serotype and the other based on production of a specific cytotoxin, led to the recognition of a novel and increasingly important class of enteric pathogens causing intestinal and renal disease.
The cytotoxin assay used by Karmali et al. (343) was originally reported by Konowalchuk and colleagues in 1977 (366). These investigators reported that culture filtrates from some strains of E. coli produced a striking, irreversible cytopathic effect on cultured Vero cells that was quite distinct from the noncytopathic effect of ETEC LT on CHO or Y-1 cells. At this same time, O’Brien et al. reported (481, 486) that extracts of certain E. coli strains were cytotoxic for HeLa cells and that this cytotoxic activity could be neutralized by antitoxin prepared against crude Shigella dysenteriae 1 (Shiga) toxin (Stx). They subsequently reported that many E. coli strains isolated from diarrheal illness produced a Shiga-like toxin (SLT), including one of the strains reported by Konowalchuk et al. (366) to produce the Vero cytotoxin (482). O’Brien et al. subsequently showed (483) that Shiga-like toxin and the Vero cytotoxin were the same toxin and that the O157:H7 strains described by Riley et al. produced this toxin. Independently, Johnson et al. (317) reported that E. coli O157:H7 strains isolated from patients with HC in Canada produced a cytotoxin active on Vero cells. Karmali et al. (341) concluded an eventful publication year for this pathogen by proposing that Vero cytotoxin/Shiga-like toxin was the common virulence factor between HC and HUS and was responsible for damage to both intestinal and renal tissue.
As with any organism that is termed an “emerging pathogen,” the question arises whether the organism has always been present and was simply undiagnosed, as with Legionella spp., or is truly a novel pathogen that has recently arisen. After E. coli O157:H7 was recognized as a cause of HC, the Centers for Disease Control and Prevention (CDC) reviewed over 3,000 E. coli strains serotyped between 1973 and 1983 and found only 1 O157:H7 isolate (539). The Public Health Laboratory in the United Kingdom also found only 1 O157:H7 strain among 15,000 E. coli strains serotyped between 1978 and 1982, and the Laboratory Centre for Disease Control in Canada found 6 O157:H7 strains among 2,000 isolates from patients with diarrhea between 1978 and 1982 (148, 263, 317). Therefore, it appears that the presence of O157:H7 strains has genuinely increased in recent years and was not simply missed prior to 1982. However, HUS was a well-known clinical entity prior to 1982. Since its initial description in 1955, numerous outbreaks of HUS gave credence to the hypothesis that HUS was due to a bacterial or viral agent (338). Although Stx-producing S. dysenteriae 1 strains were clearly associated with HUS, stool cultures obtained during many HUS outbreaks yielded E. coli but no recognized pathogens. In a remarkably prescient 1968 article describing HUS in South Africa, Kibel and Barnard (355) suggested that a “mutant strain of E. coli” mutated by a bacteriophage may be responsible for this syndrome. In the 1980s, it was recognized that the Stx is encoded on a bacteriophage in E. coli (484, 587, 605) and that over 100 different E. coli serotypes can express Stx (338). O157:H7 strains are closely related to Stx-negative O55:H7 EPEC strains (690), a serotype that has long been associated with worldwide outbreaks of infant diarrhea, and EHEC and EPEC share many intestinal adherence and other virulence factors (see below). Thus, it appears that non-O157:H7 E. coli strains producing Stx have been around for several decades but it was only with the emergence in the early 1980s of the O157:H7 clone that this pathogenic class of E. coli was recognized.
The discovery of this pathogen along parallel paths of investigation resulted in a parallel nomenclature system, a situation that still exists. The term “verotoxigenic E. coli” or “Vero cytotoxin-producing E. coli” (VTEC) was derived from the 1977 observation by Konowalchuk et al. (366) that these strains produced a toxin that was cytotoxic for Vero cells. An alternative nomenclature is “Shiga toxin-producing E. coli” (STEC [formerly SLTEC]), which reflects the fact that one of the cytotoxins produced by these organisms is essentially identical at the genetic and protein levels to the Stx produced by S. dysenteriae I; the discovery of Stx nearly 100 years ago far pre-dates the description by Konowalchuk. The arguments for and against each term have been published by their proponents (99, 339) and will not be repeated here; usage within the scientific community will ultimately determine whether one or both names will be used. STEC and VTEC are equivalent terms, and both refer to E. coli strains that produce one or more toxins of the Stx family (see below). However, it is not clear that mere possession of genes encoding Stx confer pathogenicity in the absence of other virulence factors (described below). The term “enterohemorrhagic E. coli” (EHEC) was originally coined to denote strains that cause HC and HUS, express Stx, cause A/E lesions on epithelial cells, and possess a ca. 60-MDa plasmid (381, 384). Thus, EHEC denotes a subset of STEC and includes a clinical connotation that is not implied with STEC. Whereas not all STEC strains are believed to be pathogens, all EHEC strains by the above definition are considered to be pathogens. For this review, we will use the term “typical EHEC” to denote STEC strains such as O157:H7 that produce Stx and A/E lesions and possess the 60-MDa plasmid and the term “atypical EHEC” to denote STEC strains that do not produce A/E lesions and/or do not possess the ca. 60-MDa EHEC plasmid.
Pathogenesis
Most of the work on pathogenic factors of E. coli O157:H7 has focused on the Stx, which are encoded on a bacteriophage inserted into the chromosome. Additional potential virulence factors are encoded in the chromosome and on a ca. 60-MDa plasmid found in all EHEC strains of serotype O157:H7.
Histopathology.
The classic intestinal histopathology characteristic of E. coli O157:H7 infection includes hemorrhage and edema in the lamina propria (262). The edema and submucosal hemorrhage in the ascending and transverse colon can be manifested as a “thumbprinting” pattern on barium enema radiography (84, 539). Colonic biopsy specimens from many patients also show focal necrosis and infiltration of neutrophils. The overall pattern resembles a combination of ischemic and infectious injuries similar to those described in toxin-mediated Clostridium difficile-associated colitis, and pseudomembranes are seen in many patients (262).
The classic A/E histopathology has been seen in gnotobiotic piglets (162, 434, 662, 663), infant rabbits (498), and cultured epithelial cells (302, 359) infected with E. coli O157:H7. However, A/E lesions have not yet been reported from clinical specimens. The failure to detect A/E lesions in clinical specimens is thought to be because colonic biopsy specimens are usually collected late in the disease and A/E lesions would be present only early in the course, before the potent cytotoxic effect of Stx occurs (636). In addition, the fear of gastrointestinal hemorrhage during HUS and HC raises the clinical threshold for performing intestinal biopsy.
The cellular responses leading to the A/E histopathology due to EHEC have not been studied as thoroughly as they have been with EPEC. High concentrations of polymerized actin are seen in EHEC mucosal lesions (359), and increased levels of IP3 and intracellular calcium are observed (302). However, in contrast to EPEC strains, EHEC strains fail to induce tyrosine phosphorylation of epithelial cell proteins (302). Interestingly, an eae-negative EHEC strain (that presumably lacked the entire LEE) was still capable of increasing intracellular calcium levels even though A/E lesions were not seen. These results suggest that there are some differences between the cellular response to EPEC and the response to EHEC (302).
As described above for EPEC, the 35-kb LEE pathogenicity island which confers the A/E phenotype for EPEC is also present in E. coli O157:H7. Genes involved in EHEC pathogenesis are illustrated in Fig. 9. The EHEC LEE contains genes encoding intimin, the secreted proteins EspA and EspB, and a type III secretion pathway (310, 431). Interestingly, the sequences of espB genes from two different serotypes of EHEC have only 80% homology at the predicted protein level (182). Whether this sequence divergence in a key effector molecule has any effect on the pathogenesis of disease is not known.
FIG. 9.
Genes involved in EHEC pathogenesis. Genes involved in EHEC pathogenesis are similar to those implicated for EPEC, except for the presence of the Stx-encoding phage on the EHEC chromosome and the presence of the characteristic EHEC 60-MDa plasmid instead of the EAF plasmid of EPEC. The EHEC plasmid is known to encode the enterohemolysin (ehx) as well as a fimbrial antigen potentially involved in colonization.
Similar to EPEC, E. coli O157:H7 induces a host inflammatory response that is apparently linked to the A/E histopathology. In a rabbit model, PMN infiltration in response to E. coli O157:H7 infection was blocked by the addition of anti-CD18 antibody (187). Inhibition of this inflammatory response led to reduction but not elimination of diarrhea in this model. Increased levels of IL-8 have also been reported in cultured epithelial cells infected with EHEC (323). Isogenic E. coli O157:H7 mutants deficient in secretion of EspA or EspB have not been tested in these systems, but based on results with EPEC (565), it is likely that mutation of the genes encoding these factors would eliminate this inflammation. It should be noted, however, that PMN are present in the stools of fewer than 50% of patients with HC (261).
Shiga toxins.
The major virulence factor, and a defining characteristic of EHEC, is Stx. This potent cytotoxin is the factor that leads to death and many other symptoms in patients infected with EHEC. The Stx family has been extensively reviewed (479, 480, 485, 589, 645), and additional primary references will be found in these reviews.
(i) Structure and genetics.
The Stx family contains two major, immunologically non-cross-reactive groups called Stx1 and Stx2. A single EHEC strain may express Stx1 only, Stx2 only, or both toxins or even multiple forms of Stx2. Stx1 from EHEC is identical to Shiga toxin from S. dysenteriae I. (Stx1 from some strains may differ from Stx in one residue, while Stx1 from other strains shows no sequence variation [485, 632].) The prototypical Stx1 and Stx2 toxins have 55 and 57% sequence identity in the A and B subunits, respectively (306). While Stx1 is highly conserved, sequence variation exists within Stx2. The different variants are designated Stx2c, Stx2v, Stx2vhb, Stx2e, etc., and the various subtypes are wholly interchangeable between the Stx and VT nomenclatures (i.e., Stx1 = VT1, Stx2e = VT2e, etc.) (99).
The basic A-B subunit structure is conserved across all members of the Shiga toxin family. For the prototype toxin of this family, Shiga toxin, the single 32-kDa A subunit is proteolytically nicked to yield a ca. 28-kDa peptide (A1) and a 4-kDa peptide (A2); these peptides remain linked by a disulfide bond. The A1 peptide contains the enzymatic activity, and the A2 peptide serves to bind the A subunit to a pentamer of five identical 7.7-kDa B subunits. The B pentamer binds the toxin to a specific glycolipid receptor, globotriaosylceramide or Gb3, which is present on the surface of eukaryotic cells. While Gb3 is the main receptor for Stx, the Stx2e variant uses Gb4 as the receptor. Stx2e is classically associated with pig edema disease rather than human disease, but occasional strains that express only this variant are isolated from patients with HUS or diarrhea (519, 647). After binding, the holotoxin is endocytosed through coated pits and is transported to the Golgi apparatus and then to the endoplasmic reticulum (reviewed in reference 556). The A subunit is translocated to the cytoplasm, where it acts on the 60S ribosomal subunit. Specifically, the A1 peptide is an N-glycosidase that removes a single adenine residue from the 28S rRNA of eukaryotic ribosomes, thereby inhibiting protein synthesis. The resulting disruption of protein synthesis leads to the death of renal endothelial cells, intestinal epithelial cells, Vero or HeLa cells, or any cells which possess the Gb3 (or Gb4 for Stx2e) receptor.
The structural genes for Stx1 and Stx2 are found on lysogenic lambdoid bacteriophages; the genes for Stx2 are chromosomally encoded. (The stxAB genes encoding the A and B subunits are also called sltAB and vtxAB in the literature.) Production of Stx1 from E. coli and S. dysenteriae is repressed by iron and reduced temperature, but expression of Stx2 is unaffected by these factors. During the early 1980s, there were reports of production of low levels of Stx (SLT) by other species such as V. cholerae, V. parahaemolyticus, Campylobacter jejuni, E. coli K-12, and E. coli strains from the normal flora (479). The stx genes are not present in these other species, and other investigators have been unable to confirm these initial observations (94). However, some strains of Citrobacter freundii and Enterobacter spp. produce an Stx2 toxin and contain an stx2 gene with high homology to those found in E. coli (577).
(ii) Stx in intestinal disease.
There is a variety of data showing the involvement of Stx in diarrhea and enterocolitis, beginning with early demonstrations that purified Stx can cause fluid accumulation and histological damage when injected into ligated intestinal loops (reviewed in reference 479). One possible mechanism for fluid secretion in response to Stx involves the selective killing of absorptive villus tip intestinal epithelial cells by Stx (325, 346). In rabbit ileum, the Gb3 receptor is present in much higher concentrations in villus cells than in the secretory crypt cells, and so the death of absorptive cells and preservation of secretory crypt cells could shift the usual balance of intestinal absorption and secretion toward net secretion (325). The available evidence therefore suggests that unlike LT or CT, Stx does not increase active secretion of Cl− ions. Intravenous administration of purified Stx1 or Stx2 to rabbits can produce nonbloody diarrhea (39, 536), suggesting other potential mechanisms of diarrhea besides binding of toxin to villus tip cells.
Support for the role of Stx in intestinal disease also comes from studies with genetically mutated strains of other pathogens. Sjogren et al. (598) used a natural pathogen of rabbits, E. coli RDEC-1, which normally causes nonbloody diarrhea and A/E lesions. This strain, which contains the LEE pathogenicity island, does not normally produce Stx and appears to be a lapine version of a human EPEC strain. These investigators added a bacteriophage expressing Stx1 to RDEC-1 and orally inoculated young rabbits with the hybrid strain. The infection with the Stx-positive strain (RDEC-H19A) was much more severe than that with the toxin-negative strain, with more serious histological lesions including vascular changes, edema, and more severe inflammation. Similarly, Fontaine et al. (210) fed a strain of S. dysenteriae I specifically mutated in stx to monkeys and found that the disease was less severe in monkeys fed the Stx-negative mutant than in those fed the Stx-positive parent strain. Infections with the two strains resulted in equivalent diarrheal stool volumes, but in animals receiving the Stx-positive strain, the stools were consistently more bloody and there was greater destruction of capillary vessels within the connective tissue of the colonic mucosa.
The significance of Stx in intestinal disease can differ according to the animal model used. In piglets, the presence or absence of Stx made no difference to the diarrhea (664) whereas the extent and distribution of the A/E lesion was more important in predicting intestinal symptoms (663). In an infant rabbit model, infection with an O157:H7 strain lacking Stx showed the same changes in ion absorption and secretion as did infection with an O157:H7 expressing Stx (392). In this model, development of the A/E lesion and infiltration of the intestinal tissues with PMNs was crucial to the development of diarrhea (187, 392). The overall conclusion to be drawn from these different studies and different models is that the ability of EHEC to produce the A/E lesions is probably sufficient to cause nonbloody diarrhea but that Stx is essential for the development of bloody diarrhea and hemorrhagic colitis.
(iii) Stx in HUS.
Stx produced in the intestine is assumed to translocate to the bloodstream, although toxin has never been detected in the blood of HUS patients. In polarized intestinal epithelial cells in vitro, Stx moves across the epithelial cell monolayer without obvious cellular disruption, probably through a transcellular, rather than paracellular, pathway (5). Damage of the intestinal epithelium by Stx, bacterial lipopolysaccharide (LPS), or other inflammatory mediators could also aid translocation of the toxin to the bloodstream. This possibility is supported by the fact that patients with bloody diarrhea due to E. coli O157:H7 are more likely to develop HUS than are those with nonbloody diarrhea (261). Although there is no animal model that reproduces the renal histopathology characteristic of HUS following intestinal administration of toxin, intravenous administration of Stx1 or Stx2 in a rabbit model produces vascular lesions in the intestine and central nervous system, organs where there are high concentrations of the Gb3 receptor (39, 536). (Rabbit kidneys lack Gb3 [85], and little or no renal damage is seen in this model [536].) Gb3 is present in high concentrations in human renal tissue (85), and Stx is cytotoxic to human renal endothelial cells in vitro (401). The typical human renal histopathology includes swollen glomerular endothelial cells and deposition of platelets and fibrin within the glomeruli (452). Stx is believed to damage the glomerular endothelial cells, leading to narrowing of capillary lumina and occlusion of the glomerular microvasculature with platelets and fibrin (401). The decreased glomerular filtration rate is presumably responsible for the acute renal failure that is typical of HUS. Traversal of the occluded microvasculature could also injure erythrocytes to produce the fragmented cells that are characteristic of HUS.
Epidemiological data suggest that Stx2 is more important than Stx1 in the development of HUS (reviewed in reference 261). It has been reported that O157:H7 strains that express Stx2 alone are more likely to be associated with progression to HUS than are strains producing Stx1 alone or, curiously, both Stx1 and Stx2 (515). However, other studies have not found a statistically significant association of Stx2-only strains and progression to HUS (126). There are also experimental data for both cultured renal endothelial cells (401) and mice (682) suggesting that Stx2 is more potent in inducing cytotoxicity than is Stx1. As noted above, Stx2 is not a homogeneous class of toxins, and even among variants with identical B subunits (i.e., Stx2 variants other than Stx2e), sequence differences within the A subunit can lead to differences in lethality for mice (440, 507). Some Stx2 variants can be activated by intestinal mucus, and this activation may be associated with greater lethality (440).
Although the simplest mechanism for HUS involves direct cytotoxic action of Stx on renal endothelial cells, there are also several studies that support a role for cytokines in this process. Purified Stx has been reported to induce the expression of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and IL-6 from murine peritoneal macrophages (646) as well as specific synthesis of TNF in the kidney (278). TNF-α and IL-1β can enhance the cytotoxic effect of Stx on human vascular endothelial cells in vitro (345, 400), and these two cytokines, as well as TNF-β and bacterial LPS, have been shown to induce the expression of Gb3 and increase the binding of Stx to human endothelial cells (672). In clinical studies, elevated levels of IL-6 are found in the serum and urine of HUS patients, and the levels of IL-6 correlate with the severity and outcome of disease (344, 673). These studies strongly indicate that cytokines are involved in the disease process, but the relative contributions of direct cytotoxic action by Stx and indirect action via cytokines to the pathology seen in the kidney and other organs remains to be established.
EAST1.
EAST1, first described in EAEC (see below), is also found in many EHEC strains. In one study, all 75 O157:H7 EHEC strains possessed the astA gene encoding EAST1, usually with two gene copies in the chromosome (564); other Stx-producing strains, including 8 (89%) of 9 O26:H11 strains and 12 (52%) of 23 non-O157/O26 strains also carried the astA gene (564). The significance of EAST1 in the pathogenesis of disease due to EHEC is unknown but it could possibly account for some of the nonbloody diarrhea frequently seen in persons infected with these strains.
Enterohemolysin.
The 60-MDa plasmid commonly found in O157:H7 strains contains genes encoding a hemolysin (termed enterohemolysin) (572). Enterohemolysin is found in nearly all O157:H7 strains and is widely distributed among non-O157 Stx-producing E. coli strains. In Germany, approximately 90% of all Stx-positive strains isolated from patients possessed genes encoding this hemolysin (56). In one study of O111:H- Stx-producing E. coli strains isolated from patients, the enterohemolytic phenotype was observed in 16 (88%) of 18 O111:H- strains isolated from patients with HUS but in only 4 (22.2%) of 18 patients with diarrhea without HUS (573). Patients with HUS develop antibodies to enterohemolysin (572), but there are no data indicating that it is involved in pathogenesis of disease. Enterohemolysin belongs to the RTX toxin family, members of which are expressed by uropathogenic E. coli, Pasteurella haemolytica, and other human and animal pathogens (42). The gene encoding the hemolysin (ehxA) has ca. 60% identity to the hlyA gene encoding hemolysin expressed by uropathogenic E. coli (42, 573). The role of enterohemolysin is still subject to speculation. Lysis of erythrocytes in vivo would release heme and hemoglobin, which enhance the growth of E. coli O157:H7 and could serve as a source of iron (see below). In addition to lysing erythrocytes, the toxin lyses bovine but not human leukocytes (42). Two other genetically distinct phage-encoded hemolysins, called Ehly1 and Ehly2, have been reported to be produced by many Stx-producing E. coli strains (58, 571, 624) but there are no data to suggest in vivo expression or any role in pathogenesis for these hemolysins.
Intestinal adherence factors.
The only potential E. coli O157:H7 adherence factor that has been demonstrated to play a role in intestinal colonization in vivo in an animal model is the 94- to 97-kDa OMP intimin, encoded by the eae gene. In conventional and gnotobiotic piglets, O157:H7 strains produce extensive A/E lesions in the large intestine, featuring intimate adherence of the bacteria to the epithelial cells. In contrast, O157:H7 strains specifically mutated in the eae gene no longer produced A/E lesions and, indeed, did not appear to colonize any intestinal site (162, 434, 663). Additional support for a role in human disease is seen with the anti-intimin immune response seen in HUS patients (436) and, by extrapolation, the reduced virulence in volunteers of an EPEC strain mutated in eae (see above). As noted above for the intimin of EPEC, the sequence of the putative receptor binding portion (C-terminal end) of intimin can vary among serotypes, and it was hypothesized that this sequence difference could account for the fact that EPEC is a small bowel pathogen while EHEC is a large bowel pathogen (711). In piglets, wild-type EPEC strains cause A/E lesions in both the small and large intestine while wild-type EHEC strains cause A/E lesions only in the large intestine. When the cloned EPEC eae was introduced into the EHEC eae mutant, the hybrid EHEC strain expressing the EPEC intimin caused A/E lesions in both the small and large intestine. In addition, the volume of diarrhea was greater in piglets infected with the EHEC strain expressing the EPEC intimin than in those infected with wild-type EHEC, indicating that the degree of diarrhea increased with the amount of small bowel colonization. With the ability to change the site of intestinal colonization by substituting EPEC and EHEC genes, these studies demonstrate that, at least in the piglet model, the intimin protein is essential for specific colonization in the large intestine. E. coli O157:H7 produces fimbriae which might aid intestinal adherence (24, 222, 331, 657), but no cloned fimbrial genes have been reported.
The existence of intestinal adherence factors distinct from intimin is suggested by the isolation of Stx-producing E. coli strains of serotypes other than O157:H7 that lack the eae gene but are still associated with bloody diarrhea or HUS in humans. In vitro adherence to cultured epithelial cells has been shown for eae-negative strains of serotypes such as O113:H21 (181, 583, 692), but no specific candidate adhesins have been identified. Other candidate adhesins have been reported for E. coli O157:H7, but none have been well characterized or specifically demonstrated to play a role in adherence in vivo. Sherman et al. (591) reported that a 94-kDa OMP distinct from intimin (399) mediated adherence to HEp-2 epithelial cells, but no further characterization of this factor has been reported. The potential role of LPS in adhesion has been examined, and two groups of investigators have reported that the O157 polysaccharide side chain was not involved in adherence to cultured epithelial cells (68, 130). In fact, loss of the O side chain actually increased rather than decreased the adherence to cultured epithelial cells (68, 130). Tarr (636) reported that a protein with homology to the iron-regulated gene A (irgA) of V. cholerae (248) was involved in adherence of E. coli O157:H7 to HeLa cells, but no further details for this adhesin, designated Iha for (IrgA-homologue adhesin), have yet been reported. Type 1 fimbriae were suggested to be involved in the adherence of some EHEC strains on the basis of inhibition by growth in mannose (176, 551, 695), but a subsequent study reported that growth of EHEC in mannose resulted in catabolite repression (476). Catabolite repression is a global regulatory system that controls the expression of numerous bacterial functions including carbon utilization, motility, sporulation, antibiotic biosynthesis, and, possibly, adherence of EHEC, although mechanisms for the latter are unknown (476).
pO157 plasmid.
All strains of O157:H7 contain a highly conserved plasmid, designated pO157 (574), which varies in size from 93.6 to 104 kb (575). This plasmid is also present in O26:H11 strains and is present in most but not all Stx-producing E. coli strains isolated from humans (56, 390). A 3.4-kb fragment of this plasmid, subsequently shown to encode enterohemolysin (572), was developed by Levine et al. (390) as a diagnostic probe for EHEC. In addition to the enterohemolysin and potential adherence factors described above, this plasmid encodes a catalase-peroxidase, whose function is unknown (93). A possible role of this plasmid in the suppression of production of an exopolysaccharide has also been suggested (222).
The role of this plasmid in the pathogenesis of disease due to EHEC is unknown. In vivo and in vitro studies have reported conflicting results on the role of the plasmid in adherence to epithelial cells. Karch et al. (331) first reported that pO157 was required for the expression of fimbriae and adhesion to epithelial cells. Other investigators have reported that loss of this plasmid either decreased adhesion (657), enhanced adhesion (324), or had no effect on adhesion (222). A study by Hall et al. (276) reported that for one EHEC strain of serotype O103:H2, loss of this plasmid coincided with reduced adhesion to cultured epithelial cells while for another EHEC strain of serotype O5:H-, loss of this plasmid had no effect on adhesion. Dytoc et al. (180) reported in vivo data supporting the involvement of this plasmid in intestinal adherence after oral inoculation of adult rabbits. In this study, E. coli K-12 strain HB101 containing this plasmid adhered to rabbit intestinal cells whereas HB101 without the plasmid did not adhere. In both rabbit (392) and gnotobiotic piglet (664) models of disease, the presence or absence of this plasmid made no difference to the amount of diarrhea, the intestinal histopathology, or the intestinal ion transport. However, a serious limitation to establishing such a role is that there is no suitable animal model that reproduces all aspects of the disease, from intestinal inoculation to bloody diarrhea, to renal involvement (reviewed by Gyles [272]). In these rabbit and piglet studies, the presence or absence of Stx also made no difference, further highlighting the limitations of animal models. (However, a very promising model of HUS involving greyhound dogs has recently been reported [283].) Epidemiological evidence suggests a stronger correlation of the presence of this plasmid with the development of HUS rather than diarrhea. As described above, the enterohemolytic phenotype encoded on this plasmid was observed in 16 (88%) of 18 O111:H- strains isolated from patients with HUS but in only 4 (22.2%) of 18 O111:H- strains isolated from patients with diarrhea without HUS (573).
Despite the uncertainty about the significance of plasmid pO157 in disease, it is in fact widely distributed among human EHEC isolates. The initial study by Levine et al. (390) of the distribution of this plasmid among human isolates (mostly from North America) found that 99% of 107 O157:H7 strains possessed the plasmid, as did 77% of 44 O26:H11 strains. pO157 was also found in 81% of 26 Stx-positive strains of serotypes other than O157:H7 and O26:H11 (390). A subsequent study with a different strain collection from Europe showed similar results, with the plasmid being present in 60% of Stx-positive strains of serotypes other than O157:H7 and O26:H11 (692). Another study in Germany found pO157 in ca. 90% of all Stx-producing E. coli isolates from patients (56). In contrast to the high frequency of the plasmid in human isolates, only a minority of Stx-positive strains of non-O157:H7 serotypes isolated from cattle possess this plasmid (37).
In addition to the 94- to 104-kb pO157 plasmid, a number of other plasmids ranging in size from 2 to 87 kb have been found in strains of E. coli O157:H7 (692). However, no correlation has been seen with possession of any of these plasmids and clinical disease.
Iron transport.
E. coli O157:H7 contains a specialized iron transport system which allows this organism to use heme or hemoglobin as an iron source (377, 450, 655). A 69-kDa outer membrane protein encoded by the chuA (E. coli heme utilization) gene is synthesized in response to iron limitation, and expression of this protein in a laboratory strain of E. coli was sufficient for utilization of heme or hemoglobin as the iron source (655). A gene homologous to chuA is also present in strains of Shigella dysenteriae I but not, interestingly, in other Shigella spp. or in other Shiga toxin-producing E. coli strains such as those of serotype O26:H11 (450, 655). The growth of E. coli O157:H7 is stimulated by the presence of heme and hemoglobin (377, 450), and the lysis of erythrocytes by one or more of the hemolysins reported for this pathogen could release these sources of iron, thereby aiding infection.
Other potential virulence factors.
O157 LPS (as well as LPS from other bacteria) enhances the cytotoxicity of Stx on human vascular endothelial cells in vitro, but its effects in vivo are not clear. There is one report (487) that E. coli O157:H7 can invade cultured intestinal cell lines, but a later report (435) disputed these findings, showing that O157:H7 strains were no more invasive than E. coli strains from the normal flora. Furthermore, there is no in vivo evidence that invasion occurs in humans or in animals.
Epidemiology
The initial description of the EHEC epidemiology has been provided in detail above and illustrates the complexity of recognizing and controlling new and emerging pathogens. The epidemiology of EHEC continues to unfold, and control of disease due to this organism has remained elusive. It is doubtful that we have seen the peak of this epidemic. The salient features of EHEC epidemiology include a reservoir in the intestinal tract of cattle and other animals; transmission by a wide variety of food items, with beef being a major vehicle of infection; and a very low infectious dose, enabling high rates of attack and of person-to-person transmission.
Incidence.
The large outbreaks involving hundreds of individuals are the infections that have garnered the most attention, but sporadic EHEC infections comprise the major disease burden of this pathogen. The frequency of sporadic cases of EHEC infection appears to be on the increase although increased testing and reporting of EHEC complicates this conclusion. Sporadic infections due to E. coli O157:H7 appear to be more common in Canada than in the United States (261), but such infections have already reached major proportions in many areas of the United States. There appears to be a geographic distribution of EHEC infection, being more common in northern than southern states of the United States and more common in western than eastern Canada. The CDC estimates the annual disease burden of E. coli O157:H7 in the United States to be more than 20,000 infections and as many as 250 deaths (84), but the failure of many clinical laboratories to screen for this organism greatly complicates any estimates. In some areas, E. coli O157:H7 is more frequently isolated from routine stool specimens than are Shigella spp. and it is the second or third most frequently isolated pathogen after Campylobacter and/or Salmonella spp. (261, 501). Notably, on a nationwide basis, E. coli O157:H7 is the pathogen most frequently isolated from stool specimens with visible blood (599). Some studies suggest that O157:H7 may cause only 50 to 80% of all EHEC infections (see below), and because EHEC strains of serotypes other than O157:H7 are not routinely sought, the overall incidence of EHEC infections is very difficult to estimate.
The CDC has recently initiated the Foodborne Disease Active Surveillance Network (FoodNet) to continually assess the burden of foodborne disease at several surveillance sites in the United States (114). The results from 1996, the first year of surveillance, show nationwide incidence rates for Campylobacter spp. (25 per 100,000 population), Salmonella spp. (16 per 100,000), Shigella spp. (9 per 100,000), E. coli O157:H7 (3 per 100,000), Yersinia spp. (1 per 100,000), Listeria spp. (0.5 per 100,000), and Vibrio spp. (0.2 per 100,000) (114). These results correlate with previous estimates that E. coli O157:H7 is the fourth most costly foodborne disease in the United States (414). This surveillance network has recently been expanded to include cases of HUS, which will provide additional information on the burden of disease due to non-O157:H7 EHEC in the United States (405).
In addition to its importance in North America, EHEC is an important pathogen in Europe and Japan. In these developed countries of the northern hemisphere, there is a distinct seasonality to infection, with most sporadic cases being reported in the summer. EHEC is also an important pathogen in some countries of the southern hemisphere such as Argentina, Australia, Chile, and South Africa, and non-O157:H7 EHEC serotypes are often more important than O157:H7 serotypes. There is an interesting phenomenon observed in developing countries wherein EHEC is much less frequently isolated than other diarrheagenic E. coli strains, such as ETEC or EPEC. The much lower incidence of EHEC in developing countries than in developed countries does not appear to be a reporting artifact, since EHEC strains have been actively sought in several studies (12, 141).
Animal reservoir.
Stx-producing E. coli can be found in the fecal flora of a wide variety of animals including cattle, sheep, goats, pigs, cats, dogs, chickens, and gulls (57, 263, 315, 683a). However, the great majority of these strains are of serotypes other than O157:H7 and are of questionable pathogenicity (see below). The most important animal species in terms of human infection is cattle. High rates of colonization of stx-positive E. coli have been found in bovine herds in many countries (96, 128, 263, 277, 686). These rates are as high as 60% but are more typically in the range of 10 to 25%. Stx-producing E. coli strains are usually isolated from healthy animals but may be associated with an initial episode of diarrhea in young animals followed by asymptomatic colonization. The isolation rates of O157:H7 are much lower than those of non-O157:H7 serotypes. Surveys of U.S. dairy and beef cattle have found E. coli O157:H7 in 0 to 2.8% of animals, with the highest isolation rates reported from younger rather than older animals (195, 277, 686). The widespread distribution of STEC in animals corresponds to the occurrence of STEC in retail meats. In one study (168), E. coli O157:H7 was isolated from 3.7% of retail beef, 1.5% of pork, 1.5% of poultry, and 2.0% of lamb samples. In another study on STEC in raw meats from grocery stores in Seattle, no O157:H7 isolates were recovered but a high prevalence of non-O157:H7 Stx-producing E. coli strains was found in beef (23%), pork (4%), lamb (48%), veal (63%), chicken (12%), turkey (7%), fish (10%), and shellfish (5%) (554). The isolation of these organisms from fish and shellfish suggests that cross-contamination of food can occur within grocery stores, and at least two outbreaks in Connecticut involving delicatessen products were attributed to deficiencies in supermarket hygiene practices (33).
The reservoir of EHEC in farm animals is further documented by seroepidemiologic surveys, which show that the incidence of elevated O157 LPS antibody levels in serum is threefold higher in Canadian dairy farm families than in urban families (12.5 and 4.7%, respectively) and that the incidence of elevated levels of anti-Stx1 antibodies is sixfold higher in farm families (42 and 7.7%, respectively) (534). The continual exposure of farm families to these organisms has been linked to subclinical infections that serve to immunize against disease (694). The immunizing benefit of farm life was seen in an outbreak of disease due to Stx-positive E. coli O111:NM on a dairy farm in which only visiting urban relatives of residents were ill while members of the resident farm family remained healthy (342).
Transmission.
EHEC can be transmitted by food and water and from person to person. Most cases are caused by ingestion of contaminated foods, particularly foods of bovine origin. In the United States, ingestion of undercooked hamburgers, prepared in a restaurant or in the home, has been a particularly important cause of outbreaks (reviewed in references 261, 263). The largest outbreak so far reported in North America involved hamburgers from a fast-food restaurant chain in December 1992 and January 1993. Of the 732 affected individuals in Washington, Idaho, Nevada, and California, 195 were hospitalized and 4 died (45, 261). Contamination of the hamburgers implicated in these outbreaks was the result in part of modern food-processing technology. Beef from thousands of cattle raised on hundreds of farms is ground together in a single hamburger plant, which then distributes frozen patties to thousands of restaurants in several states.
Consumption of pink ground beef is also an important risk factor for sporadic infections with E. coli O157:H7 in Canada (380) and the United States (438). In one study, hamburgers prepared at home were shown to be an important source of sporadic O157:H7 infections (438), but the authors of this study suggest that many infections resulted not from direct ingestion of undercooked hamburgers but from cross-contamination of other food items by food preparers who did not wash their hands after handling raw ground beef. Other foods of bovine origin, including roast beef and raw milk, and other types of meats, including meat from porcine, avian, and sheep sources, have also been directly linked to outbreaks (261, 263).
The spectrum of vehicles implicated in disease due to EHEC is expanding far beyond the initial hamburger-associated outbreaks. Recent outbreaks have been linked to consumption of mayonnaise (261), unpasteurized apple juice (53, 426), and fermented hard salami (112). The last two vehicles illustrate a notable ability of E. coli O157:H7 to grow in foods of low pH under conditions where other pathogens would not survive. This organism can adapt to acidic conditions to allow it to survive at pH 3.4 for several days (48, 391, 716). Raw vegetables such as lettuce have been the incriminated vehicle in several outbreaks (454), and in the recent outbreak in Japan involving over 9,000 cases, uncooked radish sprouts were implicated in the majority of cases (628). In most of these cases, the fruits or vegetables were believed to be contaminated with cattle feces and the low infectious dose necessary for disease (see below) combined with the consumption of uncooked foods permitted infection. Two recent outbreaks of O157:H7 infection in Michigan and Virginia were associated with consumption of alfalfa sprouts (113). The seeds used to grow the sprouts were from the same distributor, and the infections were probably caused by contaminated alfalfa seeds rather than contamination during the sprouting process. Water sources, including recreational water (347), well water, and even a municipal water system (627), have also been associated with outbreaks. The outbreak due to the municipal water system, which affected 243 individuals and caused four deaths, resulted from an improperly repaired water system which allowed unchlorinated water to be widely distributed. The contamination of preserved foods, vegetables intended to be eaten raw, and water is of particular concern since protection of the public from these sources will be extremely difficult.
A very low infectious dose for EHEC infection has been estimated from outbreak investigations (261). This number, on the order of 100 to 200 organisms for infection, is similar to the number required for Shigella infection and is consistent with the numerous reports of person-to-person transmission in outbreaks (45, 548) and in the institutional setting (46). A low infectious dose is also consistent with waterborne transmission, which has been documented in several reports. In one outbreak of HC and HUS, E. coli O157:H7 was isolated from 21 individuals who swam in the same freshwater lake in Oregon (347). The source of the fecal contamination was hypothesized, but not proven, to be a toddler who was not yet toilet trained.
The duration of shedding of E. coli O157:H7 in feces of infected patients varies widely. In a study of HUS patients in Seattle (639), 66% of patients initially positive for O157:H7 were negative 7 days after diarrhea began, even in the absence of antibiotic therapy. In a study of children with O157:H7 infections in Minnesota child day care facilities, the median duration of shedding was 17 days with a range of 2 to 62 days (46). One adult patient infected with an Stx-positive E. coli O rough:H21 strain was still culture positive 5 months after the termination of a 4-week course of diarrheal illness (493). A study of long-term E. coli O157 shedding in HUS patients in Germany reported a median duration of 21 days with a range of 5 to 124 days (336). In two patients in this study, an Stx gene was apparently lost over several weeks of fecal shedding, which was accompanied by a change in the pulsed-field gel electrophoresis (PFGE) pattern of the O157 isolates. The change in PFGE patterns due to loss of stx genes has implications for the use of molecular epidemiological techniques for distinguishing this organism.
Non-O157:H7 serotypes.
Most outbreaks of EHEC infection have been caused by O157:H7 strains, suggesting that this serotype is in some way more virulent or more transmissible than other serotypes. Nevertheless, other serotypes of Stx-producing E. coli have been implicated in both sporadic disease and outbreaks, and the incidence of disease due to other serotypes is considered to be on the rise (315). Estimation of the true incidence of disease due to non-O157:H7 EHEC is greatly complicated by the need to detect these infections by the presence of Stx or stx genes, since these serotypes are usually sorbitol positive and there are no convenient media such as sorbitol MacConkey agar (see below) that will reliably screen for them. In addition, distinguishing the true pathogens within this group is further complicated by the fact that mere expression of Stx is apparently not sufficient to confer virulence but that other virulence factors, some known and some unknown, are also necessary. Two recent reviews have focused specifically on the non-O157:H7 serotypes of EHEC (315, 638).
E. coli strains belonging to over 200 serotypes can express Stx, but within most serotypes, both Stx-positive and Stx-negative strains can be found (315). More than 50 of these serotypes have been associated with bloody diarrhea or HUS in humans. The most common non-O157:H7 serotypes associated with human disease include O26:H11, O103:H2, O111:NM, and O113:H21 (263). At least 10 outbreaks due to these organisms (including one due to Stx-producing C. freundii) have been reported in Japan, Germany, Italy, Australia, the Czech Republic, and the United States (reviewed in reference 315). These outbreaks have involved 5 to 234 individuals, and for most of them the source of infection could not be determined. It has been suggested that 20 to 25% of HUS cases in North America are due to non-O157:H7 EHEC (315). In many countries such as Chile (488), Argentina (396), and Australia (250), non-O157:H7 EHEC serotypes account for the majority of HUS cases. Non-O157:H7 EHEC strains are also frequently isolated from patients with nonbloody diarrhea. In a Belgian study (520), 62% of the Stx-producing E. coli strains isolated from stool were non-O157:H7, compared to 32% that were O157:H7. In Seattle, non-O157:H7 Stx-producing E. coli strains were found in 1.1% of routine stool specimens, an isolation rate higher than that of Shigella or Yersinia spp. (0.2% each) but lower than that of Campylobacter (2.5%) and Salmonella (3.4%) spp. or E. coli O157:H7 (2.9%) (77). In Boston (3) and Virginia (500), approximately half of all Stx-producing E. coli isolates from patients were of non-O157:H7 serotypes. However, in two case-control studies, non-O157:H7 Stx-producing E. coli strains were recovered at similar rates from patients and healthy controls (91, 505). The recent introduction of commercially available kits to detect Stx should greatly facilitate additional studies that are needed to assess the true incidence of non-O157:H7 EHEC serotypes in human disease.
The significance of isolating a non-O157:H7 Stx-producing E. coli strain from human stool is not as certain as that of isolating an O157:H7 strain. There are several reports of patients from whom such a strain was isolated who had high levels of antibody to the O157 LPS in serum (reviewed in reference 638). The presence of these antibodies suggests that the patient was coinfected with a non-O157:H7 strain and an O157:H7 strain which was not isolated from the stool culture and which may have caused the disease symptoms. In contrast to the probable significance of isolating a non-O157:H7 strain from human specimens, the isolation of non-O157:H7 Stx-producing E. coli from foods is of doubtful significance. As noted above, some surveys have isolated non-O157:H7 Stx-producing E. coli from up to 63% of retail meat samples, which would cause an enormous disease burden if all such strains were pathogenic.
There are at least two additional virulence factors that can help distinguish pathogenic from nonpathogenic EHEC strains: the A/E phenotype and the pO157 plasmid expressing EHEC hemolysin. These characteristics can be detected with the eae and pCVD419 probes, respectively, as described below. Several studies have examined the distribution of the eae and pCVD419 sequences in human and animal strains (37, 57, 315, 398, 692). Although the results varied according to the strain collection analyzed, most non-O157:H7 EHEC strains isolated from human disease are eae positive and/or pCVD419 positive, compared to only a minority of such strains isolated from animal and meat samples. In one study of 208 non-O157:H7 Stx-producing E. coli strains isolated from seven different species of animals, only 1.4% of such strains were positive for eae (57). However, many non-O157:H7 isolates that are clearly associated with outbreaks or sporadic human disease do not possess either eae or the pO157 plasmid (77, 692) indicating that there must be additional, as yet unknown, virulence factors that distinguish pathogenic from nonpathogenic non-O157:H7 EHEC strains.
Clinical Considerations
Few bacterial pathogens can routinely cause such striking clinical syndromes in different organ systems as can EHEC in causing HC and HUS. The dual importance of this pathogen is reflected by the response of the medical community to the 1983 observation by Karmali (343) that linked the production of Stx/Vero cytotoxin to bloody diarrhea and HUS. This paper has been referred to by a gastroenterologist as “one of the most significant contributions to enteric microbiology of this century” (638) and by nephrologists as “arguably the most important paper ever published on HUS” (330).
The most extensive clinical observations have been made with EHEC of the O157:H7 serotype. Some EHEC strains of non-O157:H7 serotypes cause clinical disease indistinguishable from that caused by O157:H7, but as a group they appear to cause less bloody diarrhea and less HUS. Although nonbloody diarrhea, HC, and HUS are the most common clinical syndomes, complications that can arise include cholecystitis, colonic perforation, intussusception, pancreatitis, posthemolytic biliary lithiasis, postinfection colonic stricture, rectal prolapse, appendicitis, hepatitis, hemorrhagic cystitis, pulmonary edema, myocardial dysfunction, and neurological abnormalities (261, 635). The frequencies of the various syndromes for diagnosed cases of O157:H7 infections include ca. 10% nonbloody diarrhea, ca. 90% HC, ca. 10% (of patients younger than 10 years) HUS, and <5% associated intestinal and extraintestinal complications (635). Clinical aspects of disease due to EHEC have been recently and extensively reviewed by multiple authors (84, 261, 474, 515, 625, 635). We will highlight the most important clinical features of disease due to EHEC and refer readers to these reviews for citations in the primary literature.
Clinical disease.
The incubation period of EHEC diarrhea is usually 3 to 4 days, although incubation times as long as 5 to 8 days or as short as 1 to 2 days have been described in some outbreaks. The initial complaint is usually nonbloody diarrhea, although this is preceded by crampy abdominal pain and a short-lived fever in many patients. Vomiting occurs in about half of the patients during the period of nonbloody diarrhea and/or at other times in the illness. Within 1 or 2 days, the diarrhea becomes bloody and the patient experiences increased abdominal pain. This stage usually lasts between 4 and 10 days. In severe cases, fecal specimens are described as “all blood and no stool” (539). In most patients, the bloody diarrhea will resolve without apparent sequelae, but in about 10% of patients younger than 10 years (and in many elderly patients), the illness will progress to HUS.
HUS is defined by a triad of hemolytic anemia, thrombocytopenia, and renal failure; initial clinical manifestations include oligouria or anuria, edema, pallor, and, sometimes, seizures. Most patients will recover with appropriate supportive therapy, but 3 to 5% of affected children will die and about 12 to 30% will have severe sequelae including renal impairment, hypertension, or central nervous system manifestations (261, 515). There are no definitive markers for severity of disease, although host factors and therapeutic interventions are important. Progression to HUS is more likely to occur in patients infected with E. coli O157:H7 who experience bloody diarrhea, fever, and elevated leukocyte count and who are very young or old or were treated with antimotility agents (126, 416, 510). The only bacterial factor that has been associated with more severe disease is the expression of Stx2, as discussed above. It should be noted that thrombotic thrombocytopenic purpura is also associated with O157 infection and appears to be related pathogenetically to HUS (515).
Recurrent cases of HUS are quite uncommon. In a study of HUS cases over a period of 20 years in Utah, HUS occurred twice in the same patient in only 2.6% of all cases (595). Interestingly, although prodromal diarrhea is normal in typical HUS, diarrhea is very uncommon among the small subset of recurrent HUS cases (595). This finding would suggest that the bacterial factors responsible for diarrhea invoke a protective immune response against subsequent diarrheal disease due to EHEC but that a primary EHEC infection may not evoke sufficient protective antitoxic immunity against a subsequent EHEC infection (535).
Isolation of EHEC from extraintestinal sites is very unusual, but rare isolates of E. coli O157:H7 have been recovered from urine, blood, and the glans penis (261). These unusual isolates were from patients with diarrhea. However, Tarr et al. (637) have recently reported an Stx-producing E. coli O103:H2 strain from a urinary tract infection that was unassociated with diarrhea. This infection developed into HUS and suggests that the human uroepithelium can also permit the absorption of Stx.
Treatment.
Treatment of EHEC disease is limited largely to supportive care. Although EHEC strains are generally susceptible to a variety of antibiotics, there are no prospective studies showing conclusively that the use of antibiotics alters the outcome of disease. In a prospective study, Proulx et al. (526) demonstrated a trend toward a lower incidence of HUS in those receiving antibiotics. Consistent with this study, a retrospective study conducted during the 1996 outbreak in Japan indicated that early treatment with one specific antibiotic, fosfomycin, was associated with a reduced risk of HUS (631). There are, however, retrospective studies which suggest that patients who received antibiotics may be at greater risk of developing HUS (108, 510); since these were not prospective, randomized trials, it could be that the patients who were most severely ill were more likely to receive antibiotics. The use of antibiotics may be harmful for two potential reasons: first, lysis of bacteria by some antibiotics leads to increased release of toxin, at least in vitro; second, antibiotic therapy could kill other intracolonic bacteria, thereby increasing the systemic absorption of toxin. While there is controversy about the use of antibiotics, the use of antimotility agents such as loperamide is definitely not indicated in the management of disease due to EHEC; there is evidence that the use of such agents can increase the risk for development of HUS, possibly by delaying intestinal clearance of the organism and thereby increasing toxin absorption (126).
Treatment of renal disease due to EHEC is primarily supportive, except for some experimental therapies currently being evaluated in clinical trials. Current treatment regimens may include dialysis, hemofiltration, transfusion of packed erythrocytes, platelet infusions, and other interventions as clinically indicated. Severe disease may require renal transplant. A promising therapy now being evaluated in clinical trials is Synsorb-Pk, which consists of a chemically synthesized analog of Gb3, the receptor for Stx, coupled to diatomaceous earth (22). This compound would be ingested by patients with bloody diarrhea in the hope that it could absorb toxin from the intestine and prevent the development of HUS. Initial phase I trials have been promising (22), and phase III trials to assess efficacy are in progress.
Vaccines.
There are no currently available vaccines to prevent disease due to EHEC, but a number of experimental approaches are being investigated in animals. Vaccine development has been severely hampered by the lack of an appropriate animal model wherein animals challenged orally with EHEC will develop HUS. A crucial antigen in any potential vaccine is the Stx. Parenteral Stx toxoid vaccines have shown protective effects in rabbits (64) and pigs (80). Attenuated V. cholerae (4, 98) and Salmonella typhimurium (668) vaccine strains that express StxB have been constructed. The V. cholerae constructs have been administered orally to rabbits and have engendered neutralizing serum antibodies and partial protection from the enterotoxic effects of Stx (4). The intestinal adherence factor intimin has also been expressed in attenuated V. cholerae strains (98a, 295). A parenteral vaccine specific for O157 EHEC has been developed based on O157 polysaccharide conjugated to protein carriers (365). An ideal broad-spectrum EHEC vaccine should probably engender both systemic immunity against Stx and local intestinal immunity against intimin and other intestinal colonization factors.
Diagnosis and Detection
General considerations.
(i) Why and when to culture.
With increased emphasis on cost containment in the clinical microbiology laboratory, there is economic pressure not to routinely test stool samples for EHEC. The current lack of a proven treatment that is specific for EHEC infections might also diminish enthusiasm for adding yet another diagnostic test to the laboratory. However, there are compelling clinical, public health, and economic reasons for routinely screening for EHEC on at least some clinical specimens.
The major public health reason for screening for EHEC is to detect outbreaks. Once an outbreak is detected, appropriate public health interventions can be instituted to limit the number of cases and deaths. In the 1993 hamburger-associated outbreak in the northwestern United States, 501 cases and three deaths were reported in Washington State alone (45). Prompt laboratory diagnosis and rapid epidemiological investigation and interventions prevented an estimated 800 additional primary cases (45). Diagnosis of EHEC infections also allows institution of appropriate isolation procedures for infected patients in the hospital or day care settings.
For the individual patient, the benefit of a prompt and accurate EHEC diagnosis can also be substantial. A variety of incorrect diagnoses have been made for patients infected with E. coli O157 including appendicitis, intussusception, primary inflammatory bowel disease, and ischemic colitis (261). Lack of an accurate diagnosis has led to numerous unnecessary and expensive procedures including exploratory surgery, hemicolectomies, colonoscopies, barium enemas, and appendectomies (83). Tarr (635) has written:
“The cost of materials needed to detect E. coli O157:H7 is less than $1.00 per stool sample, and the labor involved in review of the plates is minimal. In this era of managed care, if even one laparotomy or colonoscopy is averted by the timely diagnosis of E. coli O157:H7 infection or if even one severe secondary case of E. coli O157:H7 infection can be prevented, the cost of screening hundreds of stools for this pathogen can be justified. Attempts at economy are better directed at determination of appropriate criteria for requesting a stool culture rather than at limitation of the microbiological evaluation of specimens that are submitted.”
The CDC recommends that clinical microbiology laboratories routinely culture stool specimens from persons with bloody diarrhea or HUS for E. coli O157:H7 with SMAC agar (111). (Other than the presence of blood, there are no obvious indications from a stool specimen whether to seek EHEC. Detection of fecal leukocytes, for example, is not helpful since fecal leukocytes are seen in fewer than half of all patients [635].) The Association of State and Territorial Public Health Laboratory Directors also recommends that clinical laboratories screen at least all bloody stool specimens for E. coli O157:H7 with SMAC agar (83, 136). This group also recommends that E. coli O157:H7 be a nationally reportable disease. (As of February 1997, 42 states require O157 infections to be reported [405].) A Consensus Conference panel of gastroenterologists, microbiologists, epidemiologists, food scientists, and infectious-disease specialists organized by the American Gastroenterological Association Foundation concurred with this recommendation and also recommended that because isolation rates of E. coli O157:H7 from nonbloody stools approach those of Salmonella, Shigella, and Campylobacter spp., all nonbloody stools submitted for the examination of bacterial enteric pathogens also be cultured for E. coli O157:H7 (90). This latter recommendation does not state that all nonbloody stools that enter a clinical microbiology laboratory should be cultured for E. coli O157:H7 but, rather, that once a decision has been made to culture potential pathogens from nonbloody diarrheal stools, E. coli O157:H7 should be sought together with other enteric pathogens. Many considerations are involved in the decision on how extensively the clinical microbiology laboratory should analyze nonbloody stools to diagnose a diarrheal agent. A discussion of these considerations is beyond the scope of this review, but they have been recently reviewed (290).
Two years after the Association of State and Territorial Public Health Laboratory Directors recommended that clinical laboratories screen at least all bloody stool specimens for E. coli O157:H7 with SMAC agar, Boyce et al. (83) surveyed 129 laboratories in the United States that performed stool cultures and found that only 74 (54%) of these laboratories screened all stools or all bloody stools for culture for E. coli O157:H7. Laboratories located in southern states were least likely to culture for O157:H7. Since reported infections with E. coli O157:H7 are more common in the northern states than in the southern states, these authors suggest that laboratory practices may contribute to apparent regional differences in the incidence of O157:H7. Some laboratories that screened all stool samples found no or only rare isolates of O157:H7 among thousands of stool cultures and therefore discontinued the practice (290, 610). Boyce et al. (83) recommend that all laboratories at least perform a pilot study during the warmer months in which all stools submitted for culture are screened for E. coli O157:H7. Depending on the background incidence of this organism, laboratories can then adjust their policies to screen all stools all the time, only bloody stools, or some compromise formula to account for seasonal differences in incidence. As an example of such analysis, one group in Washington State (where the incidence of infection is high) calculated the testing cost per positive isolate as $183 if all stool samples are cultured, $173 if all stool samples with occult blood are cultured, and $40 if only stools with gross blood are cultured (95). This group concluded that for small laboratories, culturing only stools with gross blood provides reasonable sensitivity and cost. The results of a large multicenter study suggested that when the presence of fecal blood was used as the sole criterion for culturing for O157 strains, only 3% of stools would need to be cultured to detect 63% of all O157 infections (599). If either the presence of visible blood or a history of fecal blood were used, more than 90% of cases would be detected by culturing 22% of specimens.
The decision whether to culture for E. coli O157:H7 with inexpensive SMAC agar is only the first decision to be made concerning the diagnosis of EHEC. A second decision must also be made whether to screen for EHEC of serotypes other than O157:H7. As discussed above, such serotypes are increasingly being recognized as pathogens. If the local incidence of HUS is high and culture of bloody stools does not yield O157:H7, screening for EHEC of serotypes other than O157:H7 should be instituted. The decision to screen for non-O157:H7 EHEC involves the detection of Stx by methods that are considerably more expensive than the use of SMAC agar. These toxin-based methods and other diagnostic tests are reviewed below.
(ii) Biosafety issues.
E. coli O157:H7 and other Stx-positive E. coli strains are perhaps the most dangerous enteric pathogens that clinical microbiologists in developed countries are likely to encounter. There have been at least three cases of laboratory-acquired infection with E. coli O157:H7 with serious consequences including renal failure and hemorrhagic colitis (79, 97, 531). No laboratory accident or obvious breach in technique was evident in any of these cases. This level of biohazard is due to the extremely low dose required for infection (see above) and the potent effects of Stx, which are not limited to the intestine. The low infectious dose and the expression of Stx are reminiscent of those for S. dysenteriae I, which most microbiologists in developed countries are unlikely to encounter. There also exists the potential for careless workers to transport the organisms home, where young children could be infected. In the United Kingdom, EHEC will be reclassified from hazard group 2 into hazard group 3, which requires that the same laboratory containment and conditions used to handle Salmonella typhi and Shigella dysenteriae type 1 be used to handle EHEC (525).
(iii) Diagnostic methods.
There are three major categories of methods used to diagnose EHEC infections. These are (i) isolation of E. coli O157 strains from stool samples, (ii) detection of Stx-producing organisms or fecal Stx, and (iii) detection of elevated antibody levels to O157 LPS or other EHEC antigens in serum. Detailed protocols for many of these methods have been reviewed by Smith and Scotland (603). Additional recent reviews for the diagnosis of EHEC from clinical and food specimens have been published by Strockbine et al. (623) and Meng and Doyle (444), respectively. In this section, we will present an overview of diagnostic methods and will review methods for subtyping strains and determining the presence of other virulence factors such as eae or the pO157 plasmid. Although these latter methods are not typically used in clinical microbiology laboratories, they can provide additional important information for diagnostic or epidemiological investigations.
The isolation of E. coli O157:H7 or other Stx-producing E. coli strains from stool specimens depends upon culturing early in the course of disease. Unfortunately, many patients are not extensively evaluated until they have HUS symptoms, which usually begin several days after the onset of diarrhea. In one prospective study of HUS patients in Seattle, Tarr et al. (639) found that persons with HUS sought medical attention an average of 6.5 ± 2.8 days after the onset of diarrhea. When stool samples obtained within 2 days of the onset of diarrhea were cultured for E. coli O157:H7, the recovery rate was 100%. When stools were cultured 3 to 6 days or more than 6 days after diarrhea began, the recovery rate decreased to 91.7 and 33.3%, respectively. Therefore, by the time HUS is manifest, two-thirds of the patients no longer have E. coli O157:H7 in their stools (635). Conversely, as noted above, some patients can shed the organism in their stool for weeks or months after infection, thereby potentially requiring multiple cultures over long periods for a single patient.
Tests for Stx-producing organisms (other than O157) or fecal Stx are generally more expensive than culture methods for detecting O157 strains. In addition, this category of tests may suffer from an additional complication due to the potential instability of the phage carrying the stx genes. Karch et al. (335) tested the stability of stx genes in 45 strains that were stx positive upon initial isolation. After passage on Trypticase soy agar or other common laboratory media, 15 of the 45 strains had lost either stx1 or stx2 genes. The frequency of loss varied according to strain, with strains of serotypes O2:H5 and O73:H34 being particularly prone to toxin gene loss and strains of O26:H11 being very stable. Loss of stx genes has also been observed in serial cultures from individual patients over the course of long-term shedding (336).
There are no common biochemical characteristics that are associated with the great majority of EHEC serotypes. However, there are some biochemical characteristics of E. coli O157:H7 that have been exploited in the isolation and identification of this serotype. An important characteristic is that O157:H7 strains do not ferment d-sorbitol rapidly, in contrast to about 75 to 94% of other E. coli strains (197, 198, 412). E. coli O157 strains also do not ferment rhamnose on agar plates, whereas 60% of non-sorbitol-fermenting E. coli belonging to other serogroups ferment rhamnose (603). (O157 EHEC strains do ferment rhamnose within 1 day in standard tube sugar fermentation tests, as opposed to agar plate tests [603].) One report indicates that more than 90% of E. coli O157:H7 strains give one of two unique biochemical profile numbers on a MicroScan conventional gram-negative identification panel that were not detected with other d-sorbitol-negative E. coli strains (1). Another characteristic of E. coli O157:H7 that distinguishes it from most other serotypes of E. coli is the inability to produce β-glucuronidase, which hydrolyzes 4-methyl-umbelliferyl-d-glucuronide (MUG) and related substrates (649). E. coli O157:H7 also does not grow well at 44 to 45.5°C, which is a temperature commonly used to grow E. coli from food and water samples (529).
Culture techniques.
The agar medium most commonly used for the isolation of E. coli O157:H7 is SMAC agar (198, 412), which is available from multiple commercial sources. This medium contains 1% sorbitol in place of lactose in the standard MacConkey medium. Sorbitol-nonfermenting colonies, indicative of E. coli O157:H7, are colorless on this medium. Multiple sorbitol-nonfermenting colonies (at least 3 and up to 10 [260, 603]) should be selected for testing as potential E. coli O157. SMAC agar is not generally useful for Stx-producing E. coli strains of serotypes other than O157:H7 because there is no known genetic linkage between Stx production and sorbitol fermentation. However, one study found that all 19 Stx-producing E. coli strains isolated from HUS patients in Chile, including some O26, O111, and O55 strains, were sorbitol negative (488). Failure to ferment sorbitol is also not tightly linked to other H types of the O157 serogroup, and several sorbitol-fermenting, Stx-producing O157:H- strains have been isolated from patients with HUS in Germany (270). The recovery rate of E. coli O157:H7 on SMAC agar can be improved by prior enrichment in selective broth for 4 h to overnight rather than direct plating of stool specimens. GN Broth Hajna and trypticase soy broth supplemented with cefixime (50 ng/ml) and vancomycin (40 μg/ml) have both been used with success (332, 555).
There are various modifications of SMAC agar that improve selectivity and differentiation. The inclusion of cefixime and tellurite (CT-SMAC agar) permits the growth of Stx-producing E. coli O157:H7 and Shigella sonnei strains but partially or completely inhibits the growth of most of the other E. coli strains (713). Inclusion of novobiocin has also been reported to increase the selection for E. coli O157:H7 (489). E. coli O157:H7 also does not ferment rhamnose on agar plates, in contrast to the majority of other sorbitol-nonfermenting strains, and so the inclusion of both sorbitol and rhamnose in SMAC agar plus cefixime (CR-SMAC) increases the proportion of colorless colonies on this medium that are actually of the O157:H7 serotype (119). (However, one recent study found that some Stx-positive O157:NM strains did not grow on CT-SMAC [332].) The inability of E. coli O157:H7 to produce β-glucuronidase can be tested by using MUG (649). Hydrolysis of MUG by most non-O157:H7 E. coli strains produces a fluorescent compound, and this substrate has been incorporated into some agar media (629). Various agar media based on one or more of these properties are commercially available (Rainbow Agar O157 [Biolog Inc., Hayward, Calif.], Fluorocult E. coli O157 agar [Merck, Darmstadt, Germany]). One medium, Rainbow Agar, contains chromogenic substrates that are specific for both β-galactosidase and β-glucuronidase, yielding a spectrum of colored colonies ranging from black to gray to red to blue to violet that purportedly differentiates among E. coli O157:H7, E. coli O26:H11, other Stx-producing E. coli serotypes, and Stx-negative E. coli. There is little published information on side-by-side comparisons of these various media.
A nonselective but differential plating medium is enterohemolysin agar (Unipath), which detects the enterohemolysin expressed by about 90% of Stx-producing E. coli isolates from humans (56) (see above). Stool samples are inoculated into tryptic soy broth, and the overnight growth is streaked onto the plates (60). Isolated enterohemolytic colonies are then tested for Stx production (see below). A disadvantage of this approach is the need to read and record hemolytic colonies 3 h after plating (when only α-hemolysin is evident) and again after overnight incubation (when both α-hemolysin and enterohemolysins are evident).
Whatever plating and enrichment method is used, suspected EHEC colonies should be confirmed as E. coli by conventional tests and screened at least for the O157 LPS antigen as described below. Escherichia hermanii is biochemically and serologically similar to E. coli O157 (603) but can be distinguished by cellobiose fermentation (E. coli is negative, and E. hermanii is positive) and growth in the presence of potassium cyanide (E. coli does not grow, and E. hermanii does grow). Stx-producing strains of E. hermanii have not been reported (603). Presumptive identification of E. coli O157:H7 can be reported for confirmed E. coli strains that are sorbitol negative on SMAC agar and agglutinate in O157 antiserum (261). Strains can be forwarded to a reference laboratory for toxin testing and H typing.
Immunoassays.
Most of the immunological assays currently available for EHEC detect either O and H antigens or Stx. The available immunoassays for the O and H antigens are limited largely to detecting EHEC expressing O157 LPS and the H7 flagellar antigen, whereas toxin immunoassays are suitable for detecting both O157 and non-O157 serogroups of EHEC.
(i) O and H antigens.
Colonies can be screened for the O157 LPS directly from SMAC agar or after subculture, whereas screening for the H antigen may require passage through motility medium before testing (see below). Antisera to the O157 LPS and the H7 antigen have been incorporated into a variety of diagnostic kits involving ELISA, latex reagents, colloidal gold-labeled antibody, and other formats and are available from numerous commercial sources (LMD Laboratories, Inc., Carlsbad, Calif.; Oxoid Diagnostic Reagents, Basingstoke, England; Pro-Lab Diagnostics, Round Rock, Tex.; Remel Microbiology Products, Lenexa, Kans.; Difco Laboratories, Detroit, Mich.; Meridian Diagnostics, Cincinnati, Ohio; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.; Neogen Corp., Lansing, Mich.; Denka Seiken Co., Ltd., Tokyo, Japan). More specialized reagents in which antibodies to O157 LPS are already conjugated to fluorescein, peroxidase, or phosphatase are also available (Kirkegaard & Perry Laboratories, Inc.). The CDC has evaluated three commercial latex reagent kits for detecting the O157 antigen (Oxoid, Richmond Hill, and Remel) and one latex reagent kit for detecting the H7 antigen (Remel) (611). The results of this study showed 100% correlation of the commercial O157 kits with the CDC reference antisera. Some false-positive reactions with both the commercial and CDC sera were seen with a strain of C. freundii and strains of Salmonella O group N. (The O157 antigen was previously shown to be identical to the Salmonella O301 antigen [593].) The H7 reagent had a sensitivity of 96% and a specificity of 100% compared to the standard tube agglutination method with CDC reference antisera. Many strains that gave initial negative reactions with the H7 reagent became positive when passed through motility medium to enhance motility (199, 611). (Use of a PCR technique to detect the H7 gene can eliminate the need to pass strains [203].) The CDC study concluded that the commercial latex reagents are good alternatives to standard serologic methods for identifying the E. coli O157 and H7 antigens when the manufacturer directions are closely followed. Additional controls with non-O157 antiserum and latex control reagents should be included to rule out nonspecific agglutination.
Commercially available ELISA kits to detect E. coli O157 antigen directly in stool samples offer testing times of less than 1 hour. These kits are accurate and easy to use in clinical laboratory settings (178, 502). In a study of 605 stool samples, Park et al. (502) found one such kit (E. coli O157 antigen detection ELISA; LMD Laboratories, Inc.) to be more sensitive than direct plating on SMAC agar. Compared to a method involving colony sweeps and immunofluorescence microscopy, this ELISA had a sensitivity of 91.2% and a specificity of 99.5%. These authors found this technique to be faster and easier than a direct immunofluorescence microscopy technique that they had previously reported for detecting O157 antigen directly in stool specimens (501). At least one other ELISA kit for detecting O157 antigen in stools is commercially available (Premier E. coli O157; Meridian Diagnostics, Inc.). Positive results from this ELISA should be considered presumptive and should be confirmed by culture, Stx tests, or PCR (502).
(ii) Shiga toxins.
Numerous immunological assays using various formats have been developed for the detection of Stx and Stx-producing E. coli. A comprehensive review of these techniques, which have been used largely for research purposes, is beyond the scope of this review, and so we limit our discussion to assays that are commercially available. These tests generally take less than 1 h to complete and have the most potential to be adopted by clinical laboratories for detection of non-O157:H7 EHEC.
The first (and, as of this writing, the only) test to be approved by the Food and Drug Administration is the Premier EHEC test (Meridian Diagnostics, Inc.). This test uses monoclonal antibodies directed against Stx1 and Stx2 to capture the antigens and polyclonal anti-Stx antibodies and horseradish peroxidase for detection (3). It is capable of detecting Stx antigen in bacterial cultures, food samples, and fresh or frozen stool samples. Several studies have reported that it is easy to perform, sensitive, and very suitable for use in routine screening for non-O157 EHEC (3, 17, 500, 677). In one study, the Premier EHEC test was positive in 13 of 95 stool samples compared to 6 of 95 samples that were positive with SMAC agar, although it was not as sensitive as PCR, which detected an additional 8 samples (3). Another study (348) found that the Premier EHEC test had a sensitivity and specificity of 100 and 99.7%, respectively, for E. coli O157:H7 compared to the sensitivity and specificity of SMAC agar, which were 60 and 100%, respectively. False-positive reactions due to the presence of (Stx-negative) Pseudomonas aeruginosa in stools were initially reported with this system (59), but this problem has been corrected. This test, like other toxin antigen detection kits, does not identify Stx2e, which is much more common in strains associated with pig edema disease than with human disease (17). This kit is available as a 96-well microtiter plate with detachable microwells so that 1 to 94 samples (plus 2 controls) can be tested at a current list price of $864 per plate.
The VTEC-RPLA test (Oxoid, Inc.) is a reverse passive latex agglutination test for detection of Stx1 and Stx2. Extracts of single colonies contain enough antigen to yield positive reactions in this test, which is performed in 96-well microtiter plates (60). This test has been combined with the enterohemolysin test, in which stool samples are plated on enterohemolysin agar and single, enterohemolytic colonies are tested for toxin in the VTEC-RPLA test (60). An ELISA system to detect Stx1 and Stx2, which uses antibodies against Stx1 and Stx2 to capture the antigen, has recently been developed by LMD Laboratories. Another test using a Gb3 receptor capture system is the VeroTest (MicroCarb, Gaithersburg, Md.). This system purports to detect as little as 10 pg of Stx, a detection level equivalent to that seen with cell culture assays.
(iii) Other antigens.
A monoclonal antibody (4E8C12) that recognizes a 5- to 6-kDa OMP expressed by O157:H7 and O26:H11 strains has been used in the identification of EHEC strains of these serotypes (496). The function of the protein recognized by the antibody is unknown. The monoclonal antibody has been used in both ELISA (497) and dipstick immunoassays (356) to detect E. coli O157:H7 in foods; the capture antibody is a polyclonal antibody against the O157 LPS antigen, and the detection antibody is the 4E8C12 monoclonal antibody. A kit containing this monoclonal antibody (EHEC-Tek; Organon Teknika, Durham, N.C.) is available for detection of O157:H7 in food and environmental samples; additional modifications to this assay have been reported to improve the specificity of the reaction (316). A polyclonal antibody that recognizes products of the pO157 plasmid of E. coli O157:H7 has also been reported as a potential diagnostic technique (656) but is not commercially available.
(iv) Immunomagnetic separation.
Because of the small numbers of E. coli O157 present in many stool samples, immunomagnetic separation (IMS) with commercially available magnetic beads coated with antibody against E. coli O157 has been used (Dynabeads anti-E. coli O157; Dynal, Inc., Lake Success, N.Y.). IMS has also been used to isolate this organism from food and water samples (20, 702, 710) and bovine feces (120). For processing of human feces, a short preenrichment step in GN Broth Hajna (332) or buffered peptone water supplemented with vancomycin, cefixime, and cefsulodin (118) is followed by incubation with anti-E. coli O157 Dynabeads and magnetic separation. The paramagnetic bead-bacterium complex is then resuspended and plated on SMAC, CT-SMAC, or CR-SMAC agar plates. In the study by Karch et al. (332), which used a 4-h preenrichment in GN Broth Hajna prior to IMS, O157 strains were detected at 102 CFU/g of stool in the presence of 107 coliforms in the background flora. For 20 HUS patients with antibody levels in serum indicative of O157 infection, the IMS technique allowed the isolation of O157 strains from 18 samples compared to 13 samples detected by stx PCR, 12 samples detected by stx colony hybridization, and 7 samples detected by direct culture on SMAC or CT-SMAC agar. These authors concluded that IMS is the most sensitive of all detection techniques, even more sensitive than PCR, which required approximately 105 CFU of O157 organisms per g of stool to yield a positive result (compared to 102 for IMS). Several other studies have also concluded that IMS is a highly sensitive technique for detecting E. coli O157 (54, 118, 145). The commercially available magnetic beads are limited to isolating O157 E. coli, but the IMS technique has been adapted to isolate O111 strains in an outbreak setting (506).
(v) Free fecal cytotoxic activity.
Testing for Stx activity in stool samples is an extremely sensitive method to detect the current or recent presence of Stx-producing organisms (338). Several studies have shown the presence of free fecal cytotoxin in samples in which conventional culture techniques failed to yield any Stx-producing organisms (104, 332, 340). In studies in which the two techniques have been compared, testing for fecal toxin is more sensitive than PCR (104, 332), but this discrepancy could be due to the presence of inhibitors of PCR in the stools. For detection of E. coli O157, IMS appears to be more sensitive than testing for free fecal toxin (332), but the O157 IMS assay will not detect Stx-producing strains of serogroups other than O157.
Numerous studies using this technique have contributed much to our understanding of the epidemiology of disease due to Stx-producing strains other than O157:H7. However, testing stool samples (or bacterial cultures) for cytotoxic activity involves cell culture assays that are unavailable or impractical for most clinical microbiology laboratories. Detailed protocols for this technique have been published (337, 603), but in brief, diluted stool samples are centrifuged and a bacterium-free filtrate of the supernatant is added to cell cultures. The use of Vero cells is recommended over HeLa cells, since HeLa cells are less sensitive for some variants of Stx2. The cells are observed for up to 3 days for cytopathic effects, and any cytotoxic activity is confirmed by neutralization with specific anti-Stx serum (337). The same cell culture methods are used to test bacterial cultures for Stx activity. This test has largely been supplanted by the use of immunoassays to detect toxin in stools, but little published information about the relative sensitivities of the cell culture test and the commercially available immunoassays is available. One recent study (348) reported that the Premier EHEC test was at least as sensitive as fecal cytotoxin assays.
DNA probes and PCR.
Most DNA probes and PCR techniques for EHEC are directed toward the detection of genes encoding Stx. While the presence of Stx-producing strains of any serotype in a clinical specimen is assumed to be significant, the mere presence of such strains of non-O157 serogroups in food or other nonclinical samples is of uncertain significance. Frequent loss of stx genes upon subculture can also occur, as described above. For these reasons, probes and PCR techniques for additional EHEC virulence factors can often provide crucial information.
(i) Detection of stx genes.
DNA fragment probes have been used in research studies for nearly 10 years to detect EHEC (475, 693). The use of fragment probes requires two different probes, one for stx1 and one for stx2. With synthetic oligonucleotide probes, at least two probes are usually used for stx1 and stx2 and additional probes specific for stx2c and stx2e are required to detect strains containing these genes (92, 270, 288, 334, 648). One oligonucleotide probe that hybridizes to stx1, stx2, and stx2c at low temperatures (45°C) but only to stx1 at a higher temperature (53°C) has been reported (92).
The PCR technique has been extensively used to detect stx genes either in stx-only techniques or in multiplex PCR techniques incorporating primers for eae, ehx, uidA, or fliC (110, 223, 232, 284, 572) (see below). Numerous primers and PCR protocols have been designed to amplify these genes (89, 233, 305, 318, 333, 491, 492, 505, 523, 549, 661). Some early studies used a single primer pair to detect both stx1 and stx2 (333, 505), but most methods now include two primer pairs that yield different-sized products for stx1 and stx2. Not all primer pairs designed for stx2 will detect all variants of this gene (530), and specific primer pairs for stx2c (549, 661) and stx2e (216, 318, 647) have been designed. A combination of PCR and restriction fragment length polymorphism (RFLP) analysis has been used to distinguish stx2c from stx2 genes (104, 549, 661). In this technique, PCR is performed with a single primer pair capable of amplifying both genes and the amplified product is digested with a restriction endonuclease that will yield different fragment sizes for stx2 and stx2c.
The various PCR techniques are highly sensitive and specific when used with bacterial colonies or cultures, but the use of PCR for direct analysis of stool samples suffers from the same problems with background and inhibitory factors that are seen with other applications of PCR to stool samples. Several studies have used PCR to detect stx genes in stools (89, 104, 145, 332, 505, 530). Using spiked stool samples, Ramotar et al. (530) could detect stx1-containing organisms at 102 CFU per 0.1 g of stool and stx2-containing organisms at 107 CFU per 0.1 g of stool; the reason for the greatly reduced sensitivity for the stx2 genes was unknown. These investigators found that the PCR technique was more sensitive than SMAC agar for the detection of E. coli O157:H7 but was less sensitive than were cell culture assays for free fecal cytotoxin. This study also found excellent correlation between the PCR results and the hybridization of 50 colonies per sample with stx probes. Brian et al. (89) reported a sensitivity of ca. 105 CFU per 0.1 g of stool with equal sensitivity for stx1 and stx2; the sensitivity was increased another 2 log units when a radiolabeled sxt probe was used to hybridize the PCR products in a Southern blot procedure. Paton et al. (505) used a broth enrichment for their PCR procedure but found that ca. 50% of stool samples from healthy children gave positive PCR results. Caprioli et al. (104) reported that a stx PCR technique gave positive results for ca. 50% of stool samples that were positive for free fecal cytotoxin. Karch et al. (332) found that their PCR procedure had a sensitivity of ca. 104 CFU per 0.1 g of stool, which was not as sensitive as IMS for detecting O157 (see above). These studies indicate that PCR offers great potential for detecting EHEC in stool samples but that additional work remains to determine the optimal stool-processing procedure and primer pairs.
PCR techniques have also been used to detect low levels of stx-containing organisms in ground beef and other foods (233, 696), although as noted above, the mere presence of non-O157:H7 stx-positive strains in food samples is of unknown significance. As few as 0.5 to 1 CFU of stx-containing E. coli per g of ground beef can be detected after enrichment in broth, and a quantitative PCR technique has been developed to detect stx1 genes in such samples (696). Another PCR assay designed for detecting E. coli O157:H7 in food and environmental samples is commercially available (BAX for Screening/E. coli O157:H7; Qualicon, Wilmington, Del.). Whether this test detects genes encoding Stx, intimin (eae), H7 antigen (fliC), O157 LPS, the pO157 plasmid, β-glucuronidase, or some other factor is proprietary information.
(ii) Detection of eae genes.
The same eae probes used to identify EPEC can also be used to identify EHEC if they are derived from the highly conserved 5′ end of the gene. The 1-kb fragment probe described by Jerse et al. (314) has been shown to be highly sensitive and specific in many studies. Other double-stranded eae probes or PCR assays have been developed to detect sequences in the 5′ end of eae (234, 330a, 398, 691). As noted above, the 3′ end of the eae gene can vary greatly among serotypes. Specific oligonucleotide probes (398, 691) and PCRs (234, 330a, 398) have been developed to detect sequences in the 3′ end of eae genes from EPEC O127:H6 (234, 578), EHEC O157:H7 (234, 398, 578, 691), and EHEC O111 strains (398). Interestingly, stx-negative O157:H45, O157:H8, and O157:H39 strains possess 3′ eae sequences nearly identical to those found in EPEC O127:H6 but quite different from the O157:H7 eae sequences (578, 691). Primers for eae have been combined with primers for stx1 and stx2 in multiplex PCRs (223, 284).
(iii) Detection of the pO157 plasmid/hemolysin gene.
The pO157 plasmid is present in nearly all O157:H7 strains and many other EHEC strains (see above). A 3.4-kb fragment probe empirically derived from this plasmid (called CVD419) was reported by Levine et al. (390). This probe has been extensively used to identify and characterize EHEC strains despite the unknown function of the sequences contained on this fragment. This fragment was subsequently shown to contain the ehxA (hlyA) genes encoding the EHEC hemolysin (574). Oligonucleotide probes and PCR techniques to detect genes encoding the hemolysin on the pO157 plasmid have also been developed (223, 572).
(iv) Detection of other genes.
PCR techniques to detect the gene (fliC) encoding the H7 antigen have been developed (203, 232). The fliC primer pair has also been combined with primers for stx and eae in a multiplex PCR to allow the specific identification of E. coli O157:H7, O157:NM, and other EHEC strains (232). As noted above, O157:H7 strains do not produce β-glucuronidase. A multiplex PCR which incorporates primers for the mutant glucuronidase gene (uidA) together with primer pairs for stx1 and stx2 in a mismatch amplification mutation assay to identify O157:H7 and the specific stx genes has been developed (110).
Strain subtyping.
E. coli O157:H7 strains form a highly conserved clone that shows low genetic diversity in “housekeeping” genes as assessed by multilocus enzyme electrophoresis analysis (688). This clone is only distantly related to other serotypes of Stx-producing E. coli (688–690) and in fact is more closely related to O55:H7 EPEC strains, which Whittam et al. (690) have proposed as the progenitor of the O157:H7 pathogenic clone. Because the O157:H7 clone is so highly conserved, a variety of techniques have been used to differentiate strains of this serotype for epidemiological studies. These techniques can also be used to differentiate EHEC strains of serotypes other than O157:H7, but serotyping is a more useful marker for these strains than it is for O157:H7 strains. As with other bacterial pathogens, the use of molecular epidemiological techniques can be crucial in investigations of outbreaks due to EHEC, particularly in establishing whether cases are linked to a common source or whether they represent sporadic and unrelated cases.
Plasmid profiles have been used in several studies to distinguish strains of O157:H7, and although they have provided useful information, the authors usually conclude that other methods provide better discrimination (444, 503). Sequence variation within stx genes has been used to distinguish different strains by using specific stx oligonucleotide probes or PCR techniques (104, 216, 288, 318, 549, 648, 661). Phage typing can separate O157:H7 strains into 66 different phage types (227, 354), but this technique is available only in reference centers that possess the typing phage. A combination of phage typing and specific stx gene probes has been used in the United Kingdom as a rapid and discriminatory typing system for investigating E. coli O157 epidemiology (227). Ribotyping has also been applied to this pathogen, but this technique was unable to differentiate among O157:H7 strains (417). Random amplified polymorphic DNA PCR (RAPD-PCR, also called arbitrarily primed PCR) has been successfully used to discriminate among O157:H7 strains (284, 404). This technique uses low-stringency PCR amplification with arbitrarily chosen oligonucleotide primers and allows any laboratory with a PCR machine to distinguish strains of this serotype. Screening for antibiotic resistance patterns may also provide useful subtyping data, but because the use of antibiotics is not indicated for treatment, such information is useful only for epidemiological purposes.
Since EHEC strains contain one or more large (60- to 70-kb) λ-like bacteriophages that contain the stx genes, variations in phage content and chromosomal insertion sites can lead to strain differences that can be detecting by Southern hybridization or PFGE techniques. Hybridization of genomic digests of E. coli O157:H7 strains (separated by conventional gel electrophoresis) with labeled λ DNA provides useful and discriminatory RFLP patterns (264, 503, 553), but this technique yields very complex patterns that can complicate the analysis of large numbers of strains (552). Hybridization of Southern blots with labeled stx gene probes gives less complex but still very sensitive RFLP patterns that are more readily interpreted (264, 552).
PFGE has been used by several groups to investigate the molecular epidemiology of O157:H7 infections (38, 87, 368, 445), and an automated pattern recognition system to analyze the data has been proposed (107). PFGE is a more sensitive but more labor- and equipment-intensive technique than phage typing (38, 368), and the combination of these techniques can offer advantages over the use of either system alone (38). Bender et al. (47) recently used PFGE to subtype E. coli O157:H7 strains in Minnesota and found that routine surveillance by this technique “can identify outbreaks that are not detected by traditional methods and can ascertain whether sudden increases in reported cases are due to sporadic isolated cases or to one or more outbreaks.” The CDC is establishing a national electronic database of PFGE subtypes to facilitate recognition of outbreaks (36).
Serodiagnosis.
Detection of a serum immune response is not usually used for the diagnosis of infections due to other diarrheagenic E. coli strains, but serodiagnostic techniques can provide valuable diagnostic information for EHEC infections, particularly since many cases of HUS are not recognized until after fecal shedding of the organism has ceased. Unfortunately, there is no single antigen that is ideal for use in serodiagnostic assays. Stx represents an obvious choice for an important antigen produced in vivo. However, numerous investigations of the serological response to Stx have yielded disappointing results. A curious phenomenon is that sera from most individuals without any history of infection with Stx-producing E. coli strains contain a substance that is capable of neutralizing Stx2. This nonspecific neutralizing activity has been attributed to a high-density LPS present in serum rather than to specific anti-Stx1 antibodies (105). When ELISAs rather than neutralization tests are conducted to detect antibodies to either Stx1 or Stx2, only a minority of HUS patients showed a response to these toxins (35, 122, 342, 594). Similar results are seen in patients infected with Stx-producing S. dysenteriae I (358a), and it has been suggested that this lack of response may be because Stx is cytotoxic to human B lymphocytes in vitro (131). However, Reymond et al. (534) report that the frequency of VT1 (Stx1) antibodies was about sixfold higher in dairy farm residents than in urban residents. Since dairy farm residents have sustained exposure to the bovine reservoir of STEC, these results suggest that multiple exposures are required to induce detectable antibody responses to Stx, thus greatly limiting the value of this antigen for serodiagnosis. Reymond et al. (535) have recently developed a Western blot technique to detect serum antibodies to Stx. This technique offers several advantages over ELISA and neutralizing-antibody assays, but the authors conclude that it is still not a suitable assay for diagnosing a recent EHEC-associated illness.
For serodiagnostic approaches, the most widely studied antigen is the LPS (35, 69, 121, 125). Chart et al. (125) showed that in one study of 60 patients with HUS, Stx or a Stx-producing E. coli strain could be detected in only 23% of fecal specimens whereas an IgM response to the O157 LPS was detected in 73% of these patients. In an epidemic situation, an immunoassay based on IgG responses to LPS was over 90% sensitive and specific for patients with recent culture-confirmed infection (35). Antibodies to the other antigen of the O157:H7 serotype, the H7 flagellum, were not detected in any of the HUS patients studied by Chart et al. (124). In the same study, some patients showed responses to OMPs, but this response was found to be due to contaminating LPS comigrating with the OMPs. Although the O157 LPS is useful for serodiagnosis, it is difficult to prepare, and the response may be nonspecific since O157 LPS shares epitopes with E. coli O44 and O55 LPS (402, 594) and the LPS of certain serogroups of Salmonella spp., Yersinia enterocolitica, Brucella abortus, and V. cholerae non-O1 strains (reviewed in reference 704). Chart and Rowe (123) reported that five of nine individuals vaccinated with a parenteral cholera vaccine developed antibodies that reacted with O157 LPS; such an antibody response would be considered indicative of infection with E. coli O157 if the recent vaccination history were not known. Detection of serum antibodies reactive with O157 LPS is also useless for detecting infection due to Stx-producing E. coli strains of non-O157 serogroups. Ludwig et al. (402) tested sera from HUS patients against a battery of purified LPS preparations including O157, O26, O55, O111, and O128 and found that six of eight HUS patients whose stool specimens yielded non-O157 STEC isolates exhibited a serologic response to the homologous LPS. Of 99 HUS patients with negative stool cultures for STEC, 82 had serological evidence of infection.
A novel set of immunogenic proteins was recently reported by Jarvis and Kaper (310). These proteins are the 24-kDa EspA and the 37-kDa EspB proteins encoded in the LEE, as described above. Sera from HUS patients contained antibodies that reacted strongly with these proteins, but control patient sera did not react with these proteins. Antigens prepared from O26 EHEC strains reacted as well as did antigens prepared from O157:H7 strains. These proteins should be suitable for serodiagnostic use in a wide variety of EHEC strains of many different serotypes. Another protein encoded in the LEE, intimin, also engenders an antibody response in HUS patients (436), but the secreted EspA and EspB antigens engender stronger antibody responses and are easier to prepare than intimin. There are two potential limitations to the serodiagnostic use of these antigens. First, they can be used only in infections with strains containing the LEE, which appears to be the great majority of strains involved in human disease, and second, there will be cross-reactivity with serum from patients infected with EPEC.
ENTEROAGGREGATIVE E. COLI
The observation by Cravioto et al. (139) of HEp-2 adherence by EPEC was seminal not only for the field of EPEC research but also in that it served as the foundation for the discovery of at least two other categories of diarrheagenic E. coli. Scaletsky et al. (568) and Nataro et al. (468) examined collections of E. coli from studies of diarrhea in the developing world and found, like Cravioto et al., that most EPEC strains adhered to HEp-2 cells. However, these investigators also showed that many E. coli strains that were not of EPEC serogroups adhered to HEp-2 cells as well and, moreover, that the adherence phenotype was clearly distinguishable from that of EPEC. The adherence pattern of EPEC was described as localized adherence (LA), denoting the presence of clusters or microcolonies on the surface of the HEp-2 cells (568). In contrast, non-EPEC did not adhere in the characteristic microcolony morphology, instead displaying a phenotype initially described as diffuse adherence (DA); these E. coli strains were negative with the EAF probe (see the section on EPEC, above).
With these observations in mind, Nataro et al. (466) examined the HEp-2 adherence properties of E. coli isolated from the stools of 154 children with diarrhea and 66 healthy controls in Santiago, Chile. In the course of this study, these investigators were able to subdivide the “diffuse” adherence phenotype into two further categories: aggregative and (true) diffuse (illustrated in Fig. 1). Aggregative adherence (AA) was distinguished by prominent autoagglutination of the bacterial cells to each other; this often occurred on the surface of the cells, as well as on the glass coverslip free from the HEp-2 cells. The sine qua non of AA, however, was the characteristic layering of the bacteria, best described as a stacked-brick configuration. In diffuse adherence (DA), bacteria were seen dispersed over the surface of the HEp-2 cell, with little aggregation and little adherence to the glass coverslip free from the cells. Of 253 EAF probe-negative E. coli strains from Chilean diarrhea patients, 84 (33%) exhibited the AA pattern of adherence, whereas of 134 probe-negative strains from asymptomatic controls, only 20 (15%) were AA positive (P < 0.0002). Proposing a new category of diarrheagenic E. coli, the authors coined the term enteroadherent-aggregative E. coli (later shortened to enteroaggregative E. coli, and abbreviated EAggEC or simply EAEC) to describe strains expressing AA. Diffusely adherent E. coli (DAEC) strains were not associated with diarrhea in this study. Mathewson et al. (419) observed concurrently that E. coli strains that adhered to HEp-2 cells but were not of EPEC serotypes were associated with diarrheal disease in adult travelers to Mexico. Furthermore, these investigators demonstrated that one such strain was capable of causing diarrhea in adult volunteers (418). In these reports, diarrheagenic E. coli strains that adhered to HEp-2 cells but were not of EPEC serotypes were termed “enteroadherent E. coli.” Vial et al. unified these observations by demonstrating that the prototype enteroadherent E. coli strain of Mathewson et al. exhibited the AA phenotype and was thus enteroaggregative (679). The term “enteroadherent” is still frequently used but should now be replaced by the more precise terms enteroaggregative and diffusely adherent (see below).
EAEC strains are currently defined as E. coli strains that do not secrete enterotoxins LT or ST and that adhere to HEp-2 cells in an AA pattern. It is likely that this definition encompasses both pathogenic and nonpathogenic clones, which share a factor(s) conferring a common phenotype. The heterogenous pathogenicity of EAEC in humans has been confirmed in volunteer studies and outbreak investigations (192, 245, 418, 470, 604).
Pathogenesis
The pathogenesis of EAEC infection is not well understood; however, a characteristic histopathologic lesion and several candidate virulence factors have been described.
Histopathology.
Important clues to EAEC pathogenesis may be found by histopathologic examination of infected patients and animal models. EAEC strains characteristically enhance mucus secretion from the mucosa, with trapping of the bacteria in a bacterium-mucus biofilm. Tzipori et al. (665, 666) fed a series of EAEC strains to gnotobiotic piglets; although some of these animals did not experience diarrhea, all animals tested developed an unusual mucoid gel closely adherent to the small intestinal epithelium (Fig. 10). High-power examination of this gel revealed the presence of large numbers of densely packed, aggregating bacteria. In addition, the intestinal epithelium displayed pitting of goblet cells, suggesting stimulation of mucus hypersecretion. Ligated rabbit ileal loops injected with EAEC also display pitting of goblet cells and embedding of aggregating bacteria within a periodic acid-Schiff (PAS)-staining blanket (462, 679). Hicks et al. reported that EAEC strains adhere to sections of pediatric small bowel mucosa in an in vitro organ culture model (286). In this series of experiments, as above, EAEC strains were observed to be embedded within a mucus-containing biofilm. The ability of EAEC to bind mucus has been demonstrated in vitro (684), and volunteers fed EAEC develop diarrhea which is predominantly mucoid (470). The role of excess mucus production in EAEC pathogenesis is unclear; however, the formation of a heavy biofilm may be related to the diarrheagenicity of the organism and, perhaps, to its ability to cause persistent colonization and diarrhea.
FIG. 10.
Interaction of EAEC with the intestinal epithelium. (A) Photomicrograph of the ileum of a gnotobiotic piglet fed EAEC strain 042. The arrow points to the thick mucus gel adhering to the intestinal mucosa (666). (B) High magnification of the ileal mucosa of a piglet fed EAEC strain 17-2 as in panel A. Note the aggregates of bacteria coating the villous surface. The villi are edematous, and the enterocytes themselves appear swollen. Reprinted from reference 665 with permission of the publisher.
In addition to the formation of the characteristic mucus biofilm, experimental evidence suggests that EAEC infection is accompanied by cytotoxic effects on the intestinal mucosa. Vial et al. were the first to show that infection with EAEC strains in rabbit and rat ileal loop models (679) resulted in a destructive lesion demonstrable on light microscopy. The lesion was characterized by shortening of the villi, hemorrhagic necrosis of the villous tips, and a mild inflammatory response with edema and mononuclear infiltration of the submucosa. Transmission electron microscopy showed normal microvillar architecture without invasion of enterocytes; both light and electron microscopy revealed adherent bacteria without formation of the A/E lesion.
Mucosal destruction has been demonstrated in autopsy specimens of ileum from patients who died of EAEC persistent diarrhea during an outbreak in the malnutrition ward of Mexico City hospital (192). Recently, Hicks et al. have shown that EAEC cytotoxicity can be demonstrated in in vitro organ culture by using pediatric intestinal biopsy specimens (286), and Nataro and Sears have shown that EAEC strain 042 elicits cytotoxic effects on T84 cells (human intestinal carcinoma cells) in vitro (465) (Fig. 11). In the T84 cell model, EAEC-induced cytotoxicity was evidenced by a unique phenotype, characterized by vesiculation of the microvillar membrane followed by cell death and exfoliation of cells from the monolayer. In addition, this effect was accompanied by increased vacuole formation and separation of the nucleus from the surrounding cytoplasm. The effects were seen most prominently in areas where EAEC organisms were adhering to the T84 cells. In both the T84 and in vitro organ culture systems, the toxic effects require the presence of genes encoded on the 65-MDa plasmid in addition to those encoding the adherence fimbriae. It should be noted, however, that not all EAEC strains elicit cytotoxic effects on intestinal mucosa. Such strain heterogeneity may account for the inconsistent association of EAEC with diarrhea in epidemiologic investigations and volunteer studies.
FIG. 11.
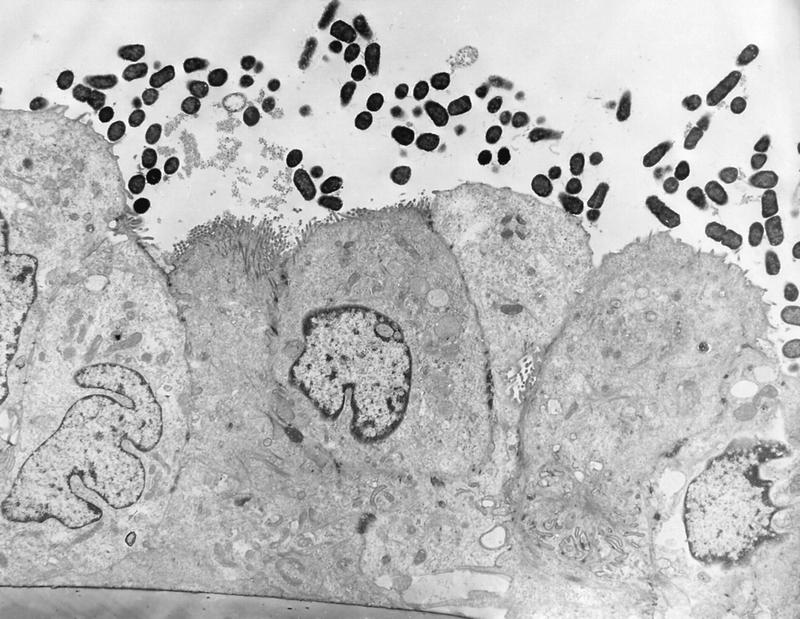
Cytotoxicity of EAEC on T84 cells infected with EAEC strain 042. Note the aggregative adherence of bacteria to the apical membrane, associated with a loss of microvilli and the rounding of the apical membrane. Reprinted from reference 465 with permission of the publisher.
Adherence.
The AA phenotype of EAEC strains has been studied in great detail. Nataro et al. have identified a flexible, bundle-forming fimbrial structure of 2 to 3 nm diameter, designated aggregative adherence fimbriae I (AAF/I) (464). AAF/I mediates HEp-2 adherence and human erythrocyte hemagglutination in strain 17-2. The genes for AAF/I are organized as two separate gene clusters on the 60-MDa plasmid of strain 17-2, separated by 9 kb of intervening DNA (471, 472, 563). Region 1 contains a cluster of genes required for fimbrial synthesis and assembly, including the structural subunit of the fimbria itself. Nucleotide sequence analysis of the region 1 cluster suggests that AAF/I is a member of the Dr family of adhesins, so called because they mediate adherence to the Dr blood group antigen (563). Region 2 encodes a transcriptional activator of AAF/I expression which shows homology to members of the AraC family of DNA binding proteins (472). The AAF/I fimbriae are bundle-forming fimbriae but do not show homology to members of the so-called type 4 class of fimbriae (644).
By using immunogold electron microscopy and a DNA probe derived from the biogenesis cluster of AAF/I, Nataro and coworkers have found that only a minority of EAEC strains express AAF/I (146). A second fimbria (designated AAF/II), which is distinct morphologically and genetically from AAF/I, has now been identified (146). The genes encoding AAF/II are also organized as two unlinked regions; however, in this case, the fimbrial subunit is removed by more than 15 kb from the required biogenesis gene cluster. This latter cluster features the typical organization of the Dr family. Still more AA adhesins are likely to exist.
It has been suggested that AA may be due to factors other than the AAF (149, 683). Debroy et al. have suggested that an afimbrial outer membrane protein may be responsible for AA in some strains (149); Wai et al. have implicated a 38-kDa OMP (683). However, genetic studies have been performed only with the AAFs. The relevance of AAFs to human disease is suggested by the fact that isogenic AAF/II-negative mutants of strain 042 are no longer able to adhere to human intestinal explants in vitro (146). Whether other adherence factors also play a role in adherence to the mucosa or the development of the EAEC biofilm remains to be demonstrated.
EAST1.
While studying the plasmid of strain 17-2, Savarino et al. identified an open reading frame encoding a 4,100-Da homolog of ST (561, 562). The product of this gene, EAST1, is a 38-amino-acid protein which features four cysteine residues, unlike the six residues characteristic of E. coli ST. Of interest is the observation that the eukaryotic membrane protein, guanylin, previously shown to have homology to ST, also contains four cysteine residues. The role of EAST1 in secretion has not yet been determined, although EAST1 clones yield net increases in short-circuit current in the rabbit mucosal Ussing chamber model (561). EAST1 has been found in ca. 40% of EAEC strains, yet toxin genes have not been found with higher frequency in patients with diarrhea than in healthy controls (462). Interestingly, other E. coli categories, notably the EHEC strains, have been shown to elaborate EAST1 with higher frequency than EAEC strains do (see above), and the toxin was reportedly expressed by 38% of nonpathogenic E. coli strains in one study (564).
Invasiveness.
Benjamin et al. have suggested that some EAEC strains may invade intestinal epithelial cells in vitro (49). However, human intestinal explants infected with EAEC strains do not reveal internalization of the bacteria (286), and clinical evidence for a role for invasiveness is as yet lacking.
Cytotoxins.
The toxic effects observed in animal models, human intestinal explants, and T84 cells are not accompanied by internalization of the bacteria or by intimate attachment. Therefore, several groups have attempted to identify secreted cytotoxins in EAEC. Eslava et al. have identified a 108-kDa cytotoxin which elicits destructive lesions in the rat ileal loop (192). This protein was recognized by serum from patients infected with EAEC in the Mexican outbreak described below. Recent data suggest that this protein is a member of the autotransporter family of proteins (322). This protein exhibits enterotoxic activity in the Ussing chamber (473). Moreover, it may be the ca. 120-kDa EAEC supernatant protein shown by Baldwin et al. to elicit rises in intracellular calcium levels in HEp-2 cells (29). A role for calcium as a second messenger of an EAEC toxin is supported by the T84 cell model, in which microvilli are lost via membrane vesiculation (421, 465).
Model of EAEC pathogenesis.
Available data do not permit a full description of EAEC pathogenesis, yet several hypotheses can be formulated. We propose a three-stage model based on in vitro and animal data. Stage I involves initial adherence to the intestinal mucosa and/or the mucus layer. AAF/I and AAF/II are the leading candidates for factors that may facilitate initial colonization. Stage II involves enhanced mucus production, apparently leading to deposition of a thick mucus-containing biofilm encrusted with EAEC. The blanket may promote persistent colonization and perhaps nutrient malabsorption. Stage III, suggested from histopathologic and molecular evidence, includes the elaboration of an EAEC cytotoxin which results in damage to intestinal cells. It is tempting to speculate that malnourished hosts may be particularly impaired in their ability to repair this damage, leading to the persistent-diarrhea syndrome.
The site of EAEC infection in the human intestine has yet to be clearly demonstrated. In vitro organ culture experiments reported by Hicks et al. have shown that EAEC strains are able to adhere to both small and large bowel mucosa (286). Tissue specimens in these studies were derived from pediatric patients and were not formalin fixed prior to incubation with EAEC strains. These features may explain the discrepant results obtained by other investigators (364, 705). The short incubation period observed in some humans challenged with strain 042 (470) (less than 8 h) is also consistent with involvement of the small bowel in diarrheagenicity.
Epidemiology
A growing number of studies have supported the association of EAEC with diarrhea in developing populations, most prominently in association with persistent diarrhea (≥14 days). In several of these studies, EAEC cultured from the stool during the first few days of diarrhea is predictive of a longer duration of illness (157, 282).
The association of EAEC with diarrheal disease appears to be geographic. On the Indian subcontinent, five separate studies have been published which demonstrate the importance of EAEC in pediatric diarrhea (61–63, 326, 508). These studies include hospitalized patients with persistent diarrhea (61), outpatients visiting health clinics (63), and cases of sporadic diarrhea detected during household surveillance (62).
Working in Fortaleza, Brazil, Guerrant and colleagues have demonstrated a consistent association between EAEC and the persistent diarrhea syndrome (196, 393, 521). In this area, EAEC has been implicated in up to 68% of persistent diarrhea cases (196), which represent a disproportionate share of diarrheal mortality. EAEC has also been implicated as a cause of sporadic diarrhea in Mexico, Chile, Bangladesh, and Iran (81, 142, 282, 466).
Gonzalez et al. studied the results of a prospective study of 513 Venezuelan infants with diarrhea and 241 age-matched controls (258). EAEC strains were found in 26.9% of diarrheal patients and 15% of controls (P < 0.0004). The high attributable risk of EAEC infection implicated this category as the major E. coli pathogen in this cohort of infants.
Although most reports have implicated EAEC in sporadic endemic diarrhea, a growing number of reports have described EAEC outbreaks (192, 245, 604). Cobeljic et al. have described an outbreak affecting 19 infants in the nursery of a hospital in Belgrade, Serbia, over a 9-day period in 1995 (129). Of the 19 infants, 12 yielded the same multiply antibiotic resistant EAEC strain of serogroup O4, with an identical plasmid pattern, while 0 of 5 well neonates yielded this organism (P = 0.02). In 16 babies the illness lasted 3 to 9 days (mean, 5.2 days), but in 3 infants, persistent diarrhea developed, lasting 18 to 20 days. Infants with diarrhea typically manifested liquid green stools; in three, mucus was visibly apparent. There was no gross blood. All but three infants required intravenous hydration, but they all survived. The source of infection was unclear.
Eslava et al. have described two outbreaks of EAEC persistent diarrhea occurring in the malnutrition ward of a Mexico City hospital (192); infants who died in these outbreaks were found to have developed necrotic lesions of the ileal mucosa. Smith et al. have reported four outbreaks of EAEC diarrhea in the United Kingdom in 1994 (604). These four outbreaks involved 19, 10, 51, and 53 patients, respectively. Patients in these outbreaks experienced vomiting and diarrhea, usually without fever. Persistent diarrhea occurred in a small number of patients. Each of the outbreaks was associated with consumption of a restaurant meal, but no single source could be implicated. Smith et al. have also reported that at least three outbreaks of diarrhea in the United Kingdom previously attributed to EPEC were actually due to EAEC strains (604). In the United States, EAEC has been linked to diarrhea in human immunodeficiency virus-infected patients (425); however, the precise role for EAEC in AIDS diarrhea is unknown.
Perhaps even more significant than the association of EAEC with persistent diarrhea are the recent data from Brazil (619) and Australia (188) that link EAEC with growth retardation in infants. In each of these studies, the isolation of EAEC from the stools of infants was associated with a low z-score for height and/or weight, irrespective of the presence of diarrheal symptoms. Given the high prevalence of asymptomatic EAEC excretion in some areas (258, 466, 685), such an observation may imply that the contribution of EAEC to the human disease burden is significantly greater than is currently appreciated.
Clinical Features
The clinical features of EAEC diarrhea are becoming increasingly well defined in outbreaks, sporadic cases, and in the volunteer model. As described above, the outbreak in Serbia suggested a watery, mucoid, secretory diarrheal illness with low-grade fever and little to no vomiting. Studies in India also suggest that the illness is most likely be manifested by watery, secretory diarrhea in the absence of fever and without gross blood (62, 508). However, grossly bloody stools have been reported in up to one-third of patients with EAEC diarrhea (142). In volunteers infected with EAEC, the diarrhea was generally mucoid and of low volume without occult blood or fecal leukocytes; all patients remained afebrile. Steiner et al. have found that a large percentage of patients excreting EAEC have detectable fecal lactoferrin (a sensitive indicator of fecal leukocytes) and supranormal levels of IL-8 in the stool (619). This observation suggests that EAEC infection may be accompanied by a subtle form of mucosal inflammation.
Detection and Diagnosis
EAEC infection is diagnosed definitively by the isolation of E. coli from the stools of patients and the demonstration of the AA pattern in the HEp-2 assay. Analysis of small bowel aspirates has not increased the yield (196).
HEp-2 assay.
The HEp-2 assay remains the gold standard for detection of EAEC. Although variations in the AA pattern can be discerned, the presence of bacterial clusters in a stacked-brick configuration should be used to identify EAEC strains.
Different methods for performing the HEp-2 assay have been described (420, 466, 678) (see above). However, comparative studies (678) suggest that the technique as first described by Cravioto et al. (139) (i.e., a single 3-h incubation of bacteria with cells, without a change in medium during the course of the assay) is best able to discriminate the three patterns (AA, DA, and LA). It should also be stressed that AAF adhesins are maximally expressed in static Luria broth cultures at 37°C (464); therefore, the authors incubate all HEp-2 assay inocula in this manner.
DNA probe.
Several lines of evidence suggest that the large plasmids present in most EAEC strains have a high degree of DNA homology (41, 679). From strain 17-2, Baudry et al. (41) selected a 1.0-kb plasmid-derived Sau3a fragment that hybridized with a fragment of similar size from the 65-MDa plasmid of strain 042. In an evaluation of this fragment as a diagnostic probe, 56 (89%) of 63 EAEC strains (by HEp-2 assay) were positive with the EAEC probe by colony blot hybridization; only 2 of 376 strains representing the normal flora and other diarrheagenic categories hybridized with the probe. Subsequent experience with the EAEC probe has revealed that the correlation of probe positivity with AA varies by location. In some studies, the correlation achieves the 89% sensitivity reported by Baudry et al. (41, 326), while in other studies, the sensitivity may be substantially lower (196). The epidemiologic significance of probe-positive versus probe-negative strains is undetermined. The nucleotide sequence of the AA probe represents a cryptic open reading frame which is adjacent to the plasmid replicon (462). A PCR assay with primers derived from the AA probe sequence shows similar sensitivity and specificity (576).
Other tests for EAEC.
Several methods other than the HEp-2 and DNA probe assays have been described. Albert et al. (14) have reported that EAEC probe-positive organisms display an unusual pellicle formation in Mueller-Hinton broth. Similarly, when EAEC strains are grown in polystyrene culture tubes or dishes at 37°C overnight without shaking, a bacterial film is produced on the polystyrene surface and is easily visualized with Giemsa stain (462, 468). Both phenotypes are likely to be due to high surface hydrophobicity, and both of these techniques are convenient substitutes for the DNA probe in assaying EAEC. It should be emphasized, however, that until epidemiologic studies show greater pathogenicity of probe-positive strains over probe-negative strains, the HEp-2 assay should remain the gold standard for EAEC detection.
The critical question in the management of patients from whom EAEC strains are isolated is whether the isolate is responsible for the symptoms. We accept that an EAEC strain is a likely cause of the patient’s diarrhea in three situations: (i) when the patient presents in the course of a documented EAEC outbreak, (ii) when the patient’s isolate can be shown to belong to one of the common EAEC serotypes associated with disease (e.g., O44:H18); and/or (iii) when the patient exhibits persistent diarrhea and stools repeatedly yield an EAEC as the predominant organism in the absence of another enteric pathogen.
A PCR with oligonucleotide primers derived from the probe sequence has also been developed (576). The sensitivity and specificity of the EAEC PCR are similar to those of the AA probe.
ENTEROINVASIVE E. COLI
EIEC strains were first shown to be capable of causing diarrhea in volunteer studies conducted by DuPont et al. in 1971 (175). EIEC strains are biochemically, genetically, and pathogenetically related closely to Shigella spp.; like Shigella spp., EIEC strains are generally lysine decarboxylase negative, nonmotile, and lactose negative (88).
Pathogenesis
The precise pathogenetic scheme of EIEC has yet to be elucidated; however, pathogenesis studies of EIEC suggest that its pathogenetic features are virtually identical to those of Shigella spp. (for reviews of Shigella pathogenesis, the reader is referred to references 249 and 504). Both organisms have been shown to invade the colonic epithelium, a phenotype mediated by both plasmid and chromosomal loci. In addition, both EIEC and Shigella spp. elaborate one or more secretory enterotoxins that may play roles in diarrheal pathogenesis.
Invasiveness.
The current model of Shigella and EIEC pathogenesis comprises (i) epithelial cell penetration, (ii) lysis of the endocytic vacuole, (iii) intracellular multiplication, (iv) directional movement through the cytoplasm, and (v) extension into adjacent epithelial cells (249, 463, 558). When the infection is severe, this sequence of events elicits a strong inflammatory reaction which is manifested grossly as ulceration. The site of Shigella and EIEC infection is the colonic mucosa (558, 559). The interaction of EIEC organisms with eukaryotic cells is shown in Fig. 12.
FIG. 12.
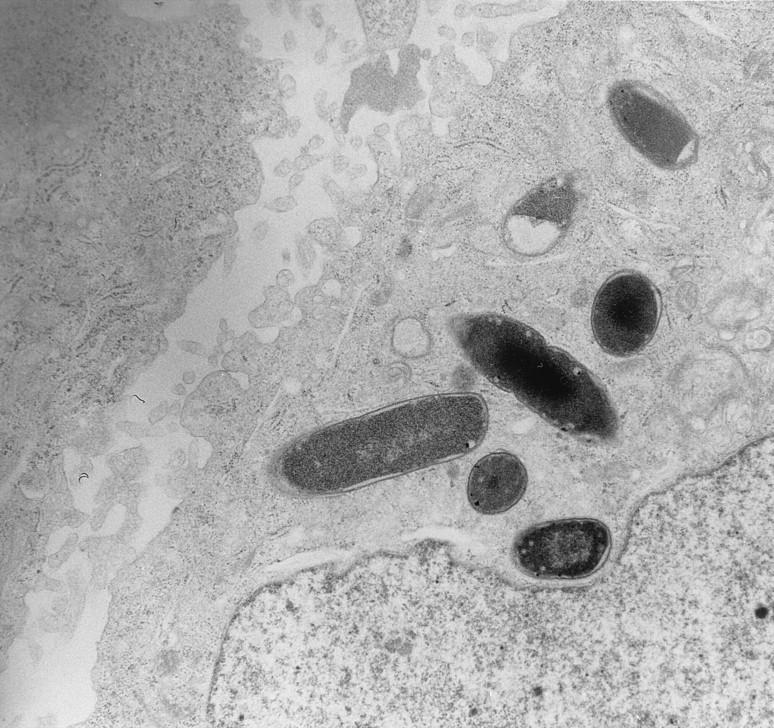
Interaction of EIEC with epithelial cells. Like Shigella, EIEC strains invade intestinal epithelial cells, lyse the phagosomal vacuole, and move through the cytoplasm, ultimately spreading to adjacent epithelial cells. This electron photomicrograph shows an EIEC organism free within the cytoplasm of an infected cell. Photo courtesy of P. Small.
Genes necessary for invasiveness are carried on a 120-MDa plasmid in Shigella sonnei and a 140-MDa plasmid in other Shigella serotypes and in EIEC (40, 560, 601). The invasion-related plasmid has been designated pInv. Figure 13 illustrates the present level of understanding of the plasmid-borne virulence genes of EIEC and Shigella spp. Prominent among these genes are the mxi and spa loci, which encode a so-called type III secretion apparatus (16, 19, 423, 676). This machinery is required for the secretion of multiple proteins which are necessary for full pathogenicity. The Ipa proteins (IpaA to IpaD) are secreted proteins, of which IpaB, IpaC, and IpaD are effectors of the invasion phenotype (40, 274, 442, 443). IpaC has been shown to promote the uptake of Shigella spp. into the eukaryotic cell (415), whereas IpaB is thought to function in the lysis of the phagocytic vacuole (287) and in the induction of apoptosis in macrophages (719).
FIG. 13.
Genes involved in EIEC pathogenesis. Both plasmid and chromosomal genes are involved in conferring pathogenicity on EIEC strains; the genes depicted in the figure have largely been elucidated in Shigella, but most, if not all, also exist in EIEC. The chromosomal locus kcpA activates transcription of the plasmid-borne gene virG, which encodes an OMP required for directional movement through the cytoplasm. The plasmid-borne locus virK increases the surface expression of the VirG protein through an unknown mechanism. A regulatory cascade of virulence genes has been described in which the plasmid locus virF interacts with the chromosomal locus virR to regulate the transcription of the ipa gene cluster of secreted effector proteins. ipa regulation involves the intermediate regulator virB. The mxi and spa loci encode a type III secretory system homologous to that of EPEC and EHEC.
Shigella movement within the cytoplasm appears to be mediated by the nucleation of cellular actin into a “tail” which extends from one pole of the bacterium (7, 558, 675). As additional actin is added to this structure, the bacterium is propelled through the cytoplasm, generally in the lateral direction. VirG (IcsA), is a surface protein which is essential for the nucleation of actin filaments and movement through the cytoplasm and into adjacent cells (247, 558).
Regulation of Shigella virulence is complex and features at least one regulatory cascade. VirR is a chromosomally encoded histone-like protein related to the drdX product (299, 424). VirR apparently acts in concert with VirF, a transcriptional activator encoded on pInv (166). VirF exerts pleiotropic effects, some of which function through the intermediate transcriptional activator VirB (652).
Enterotoxin production.
As described below, both Shigella and EIEC infections are characterized by a period of watery diarrhea that precedes the onset of scanty dysenteric stools containing blood and mucus. Indeed, in most patients with EIEC infection and many with Shigella infection, only watery diarrhea occurs. Nataro et al. (469) have cloned and sequenced a plasmid-borne gene from EIEC (designated sen), which encodes a novel protein with a predicted size of 63 kDa. A mutation in the sen gene causes a significant diminution of the enterotoxic activity of the parent strain. The purified Sen protein elicits rises in Isc levels without having a significant effect on tissue conductance. A role for enterotoxins is unproven, but their presence may explain the characteristic watery diarrhea attributed to EIEC.
Epidemiology
Epidemiologic studies of EIEC mostly describe outbreaks. In sporadic cases, many EIEC strains are probably misidentified as Shigella spp. or nonpathogenic E. coli strains. Documented EIEC outbreaks are usually foodborne or waterborne (375, 413, 606, 659), although person-to-person transmission does occur (279). The infective dose of EIEC in volunteers is higher than that for Shigella spp. (176), and thus the potential for person-to-person transmission is lessened. Endemic sporadic disease occurs in some areas, generally where Shigella spp. are also prevalent, but the epidemiologic features may be different from those of Shigella spp. (353, 641). The incidence of EIEC in developed countries is thought to be low, but occasional foodborne outbreaks, such as one restaurant-associated outbreak involving 370 people in Texas, do occur (259).
Clinical Considerations
The clinical presentation of EIEC disease has been documented from outbreaks, endemic disease, and volunteer studies (176, 375, 413, 606, 641, 659). EIEC infection presents most commonly as watery diarrhea, which can be indistinguishable from the secretory diarrhea seen with ETEC. Only a minority of patients experience the dysentery syndrome, manifested as blood, mucus, and leukocytes in the stool; tenesmus; and fever (413, 606, 641). In two documented EIEC outbreaks, gross blood was observed in 0 and 7% of persons infected (413, 606). Asymptomatic infections due to EIEC are probably unusual.
Detection and Diagnosis
EIEC strains can be difficult to distinguish from Shigella spp and from other E. coli strains, including nonpathogenic strains. In general, identification of EIEC entails demonstrating that the organism possesses the biochemical profile of E. coli, yet with the genotypic or phenotypic characteristics of Shigella spp. The classical phenotypic assay for Shigella and EIEC identification is the Sereny (guinea pig keratoconjunctivitis) test, which correlates with the ability of the strain to invade epithelial cells and spread from cell to cell (367). The ability to form plaques in a HeLa cell monolayer also correlates with these virulence characteristics (441).
Two polynucleotide probes for the detection of EIEC and Shigella spp. have been described. Probe pMR17 is a 17-kb EcoRI fragment derived from pInv of a Shigella flexneri serotype 5 strain (253, 701). ial is a 2.5-kb HindIII fragment isolated from pInv of an EIEC strain (available as a cloned fragment in plasmid pSF55) (600). Both of these probes are virtually 100% sensitive and specific for EIEC strains that have retained their virulence (253). A 21-base oligonucleotide derived from ial is identical in sensitivity and specificity to the polynucleotide probe (221). It should be noted, however, that EIEC strains may lose all or part of the pInv plasmid on in vitro passage or storage, and therefore strains should be hybridized with the probe(s) as soon as possible after they are shed in the feces.
A PCR assay with primers derived from ial was able to detect as few as 10 CFU of S. flexneri in stool without enrichment of the sample (219, 221); this compares with 1,000 CFU detectable by DNA probe. The ial PCR is also effective in a multiplex PCR system to identify EIEC strains simultaneously with other E. coli categories (219).
Pal et al. have developed an ELISA to detect the ipaC gene, which is contained on the Inv plasmid of EIEC and Shigella (499). Using this assay, these investigators identified EIEC and Shigella strains isolated from the stools of children in Kuwait. An advantage of this assay over other methods is that it does not require costly or highly sophisticated equipment and does not use live animals.
DIFFUSELY ADHERENT E. COLI
The term “diffusely adherent E. coli” was initially used to refer to any HEp-2-adherent E. coli strain that did not form EPEC-like microcolonies. With the discovery of EAEC, most authors now recognize DAEC as an independent category of potentially diarrheagenic E. coli.
Pathogenesis
Little is known about the pathogenetic features of DAEC-induced diarrhea. Bilge et al. have described the cloning and characterization of a surface fimbria in this strain, which mediates the DA phenotype (65–67). The genes encoding the fimbria (designated F1845) can be found on either the bacterial chromosome or a plasmid. The fimbrial genes show homology to members of the Dr group of bacterial adhesins.
Benz et al. (51, 52) have described a 100-kDa OMP which is associated with the DA phenotype in one strain of serotype O126:H27. The gene encoding this factor (designated AIDA-I) has been completely sequenced. Use of a DNA probe specific for AIDA-I suggests that this factor is expressed by a minority of DAEC isolates (50).
Yamamoto et al. (707), and Cookson and Nataro (133) have shown that DAEC strains are able to induce finger-like projections extending from the surface of infected Caco-2 or HEp-2 cells (Fig. 14). These projections apparently “embed” the bacteria, providing protection against gentamicin but without complete internalization. A role for this phenotype in pathogenesis has yet to be demonstrated.
FIG. 14.
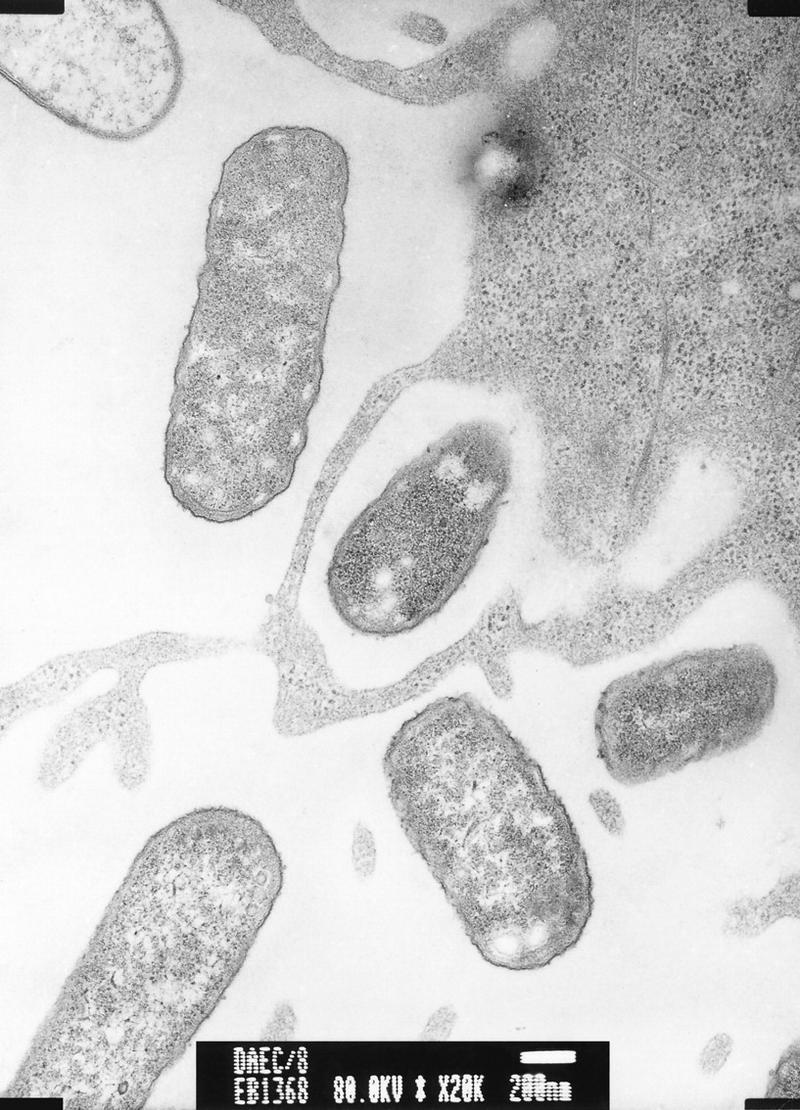
Interaction of DAEC with epithelial cells. DAEC strain C1845 incubated with HEp-2 cells for 8 h. Note the association of the bacteria with the membrane and the formation of long finger-like projections emanating from the cell. These projections wrap around the bacterium in a phenotype termed “embedding.” Invasion is rarely seen. Reprinted from reference 133 with permission of the publisher.
Epidemiology
Several recent studies have implicated DAEC strains as agents of diarrhea, while other studies have not recovered DAEC strains more frequently from diarrheal patients than from asymptomatic controls. An age-dependent susceptibility may explain this observation, because when populations are stratified by age, the association of DAEC with diarrhea is found only in children older than infants (34, 240, 266, 385). Levine et al. showed that the relative risk of DAEC in association with diarrhea increased with age from 1 year to 4–5 years in Santiago, Chile (385). The reason for such an age-related phenomenon is as yet unknown. Other epidemiologic features, such as the mode of acquisition of DAEC infection, are also as yet undetermined.
Jallat et al. have shown that DAEC strains account for a large proportion of diarrheal cases among hospitalized patients in France who have no other identified enteropathogen (307). This report suggests that DAEC strains may be important diarrheal pathogens in the developed world.
Clinical Features
Few epidemiologic or clinical studies permit adequate description of the clinical syndrome associated with DAEC infection. In one study, the majority of patients infected with DAEC had watery diarrhea without blood or fecal leukocytes (522).
Detection and Diagnosis
DAEC strains are defined by the presence of the DA pattern in the HEp-2 adherence assay (574). A 700-bp polynucleotide fragment derived from the daaC gene (66) has been used as a DAEC DNA probe; daaC encodes a molecular usher necessary for expression of the F1845 fimbriae. Approximately 75% of DAEC strains from around the world are positive with this F1845 gene probe (462). However, due to the genetic relatedness of F1845 to other members of the Dr family of adhesins, false-positive reactions with the DA probe may occur, albeit with unknown frequency. No PCR assay has yet been described to identify DAEC.
OTHER CATEGORIES OF E. COLI WHICH ARE POTENTIALLY DIARRHEAGENIC
The six categories of E. coli that are described in the above sections have each been implicated in several diarrhea studies and are now generally accepted as diarrheagenic categories. However, some studies have suggested that there may be still other categories of diarrheagenic E. coli which are quite distinct from those described above. These will be briefly considered.
Gunzberg et al. (266) reported a study of Australian aborigine children with diarrhea in which a significant association was found between diarrheal illness and the presence of a cytotoxic phenotype on HEp-2 cells in the HEp-2 assay. This phenotype, typified by detachment of the cells from the glass within 3 h of incubation with bacteria, was found in organisms regardless of their adherence pattern. This pattern led the investigators to propose that cell-detaching E. coli (CDEC) may be a new category of diarrheagenic E. coli. Elliott et al. (188) have characterized CDEC strains further and have shown that the detaching phenotype appears to be closely associated with the production of the E. coli hemolysin and that these organisms frequently secrete the cytotoxic necrotizing factor (CNF) but do not belong to any of the recognized categories above. Moreover, these investigators have shown that CDEC strains are able to elicit diarrhea and destructive histopathology in the rabbit model. The role of CDEC in human diarrhea has yet to be determined.
Two forms of CNF have been described, CNF1 and CNF2. Both forms are large, monomeric proteins of 110 to 115 kDa that induce multinucleation of eukaryotic cells (reviewed in reference 495). The mechanism of action of CNF has recently been described and appears to be a novel cytotoxic mechanism (207). CNF induces a deamidation of glutamine 63 in the RhoA target protein, producing a glutamate residue at that site. The deamidated RhoA is “locked on” and leads to an increase in the number of stress fibers within the target cell. The affected cells become larger and syncytial and ultimately die. How this mode of action might lead to diarrhea is not known. Most CNF-producing E. coli strains isolated from diarrheal stools have been isolated from animals rather than humans; most human isolates of CNF-producing E. coli have been from extraintestinal infections (103, 151, 495). There is a clear need for case-control studies of CNF-producing E. coli to definitely establish whether these organisms are true human pathogens.
Scott and Kaper (588) and Pickett et al. (516) have cloned and characterized a gene from E. coli which encodes a cytolethal distending toxin (CDT). It has been suggested that the mechanism of action of this toxin involves the GM2/M phase growth arrest of the target cell, which leads to elongation and ultimately to cell death (512). There is a growing CDT family as a result of reports that this toxin, or close homologs, is produced by Campylobacter spp. (517), S. dysenteriae (490), and Haemophilus ducreyi, an agent of genital ulcers (134). The mechanism of CDT action in diarrhea is not known, but partially purified CDT from S. dysenteriae can produce profuse watery diarrhea in a mouse model of diarrhea (490). A controlled study of CDT-producing E. coli infections in Bangladeshi children found that although CDT-positive E. coli strains were isolated from more children with diarrhea than from healthy controls, this difference did not reach statistical significance (13). Moreover, the CDT-positive strains isolated from children with diarrhea usually also possessed virulence properties of other diarrheagenic E. coli strains such as the ability to cause the A/E lesion.
CONCLUSIONS
Our perspective on intestinal E. coli has undergone a remarkable transformation in recent decades and undoubtedly will continue to evolve. Once dismissed as a harmless inhabitant of the intestinal tract, E. coli is now seen as a pathogenic species with remarkable versatility in its ability to cause disease in humans and animals. Outbreaks of disease due to E. coli can affect thousands of individuals and can engender national and international headlines. Pathogen-specific virulence factors have been discovered that adversely affect a wide range of eukaryotic cell processes including protein synthesis, cell division, ion secretion, and transcription. These factors are encoded on a variety of mobile genetic elements such as plasmids, bacteriophages, transposons and pathogenicity islands; this genomic plasticity implies ongoing reassortment of virulence factors that complicates our efforts to categorize the various subgroups into sharply delineated pathotypes. This dynamism promises to present new challenges in the diagnosis, treatment, and prevention of E. coli infections.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-33096 to J.P.N. and AI-21657 and AI-41325 to J.B.K.
REFERENCES
- 1.Abbott S L, Hanson D F, Felland T D, Connell S, Shum A H, Janda J M. Escherichia coli O157:H7 generates a unique biochemical profile on MicroScan conventional gram-negative identification panels. J Clin Microbiol. 1994;32:823–824. doi: 10.1128/jcm.32.3.823-824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdul A A, Faruque S M, Ahmad Q S, Hossain K M, Mahalanabis D, Albert M J. Evaluation of a non-radioactive chemiluminescent method for using oligonucleotide and polynucleotide probes to identify enterotoxigenic Escherichia coli. J Diar Dis Res. 1994;12:113–116. [PubMed] [Google Scholar]
- 3.Acheson D W K, DeBreuker S, Donohue-Rolfe A, Kozak K, Yi A, Keusch G T. Development of a clinically useful diagnostic enzyme immunoassay for enterohemorrhagic Escherichia coli infection. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 109–112. [Google Scholar]
- 4.Acheson D W K, Levine M M, Kaper J B, Keusch G T. Protective immunity to Shiga-like toxin I following oral immunization with Shiga-like toxin I B-subunit-producing Vibrio cholerae CVD 103-HgR. Infect Immun. 1996;64:355–357. doi: 10.1128/iai.64.1.355-357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acheson D W K, Moore R, DeBreucker S, Lincicome L L, Jacewicz M, Skutelsky E, Keusch G T. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun. 1992;64:3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Adam T, Arpin M, Prévost M-C, Gounon P, Sansonetti P J. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J Cell Biol. 1995;129:367–381. doi: 10.1083/jcb.129.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agin T S, Wolf M K. Identification of a family of intimins common to Escherichia coli causing attaching-effacing lesions in rabbits, humans, and swine. Infect Immun. 1997;65:320–326. doi: 10.1128/iai.65.1.320-326.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert M J, Alam K, Islam M, Montanaro J, Rahman A S M H, Haider K, Hossain M A, Kibriya A K M G, Tzipori S. Hafnia alvei, a probable cause of diarrhea in humans. Infect Immun. 1991;59:1507–1513. doi: 10.1128/iai.59.4.1507-1513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert M J, Ansaruzzaman M, Faruque S M, Neogi P K B, Haider K, Tzipori S. An ELISA for the detection of localized adherent classical enteropathogenic Escherichia coli serogroups. J Infect Dis. 1991;164:986–989. doi: 10.1093/infdis/164.5.986. [DOI] [PubMed] [Google Scholar]
- 11.Albert M J, Faruque S M, Ansaruzzaman M, Islam M M, Haider K, Alam K, Kabir I, Robins-Browne R. Sharing of virulence-associated properties at the phenotypic and genetic levels between enteropathogenic Escherichia coli and Hafnia alvei. J Med Microbiol. 1992;37:310–314. doi: 10.1099/00222615-37-5-310. [DOI] [PubMed] [Google Scholar]
- 12.Albert M J, Faruque S M, Faruque A S, Neogi P K, Ansaruzzaman M, Bhuiyan N A, Alam K, Akbar M S. Controlled study of Escherichia coli diarrheal infections in Bangladeshi children. J Clin Microbiol. 1995;33:973–977. doi: 10.1128/jcm.33.4.973-977.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert M J, Faruque S M, Faruque A S G, Bettelheim K A, Neogi P K B, Bhuiyan N A, Kaper J B. Controlled study of cytolethal distending toxin-producing Escherichia coli infections in Bangladeshi children. J Clin Microbiol. 1996;34:717–719. doi: 10.1128/jcm.34.3.717-719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert M J, Qadri F, Haque A, Bhuiyan N A. Bacterial clump formation at the surface of liquid culture as a rapid test for identification of enteroaggregative Escherichia coli. J Clin Microbiol. 1993;31:1397–1399. doi: 10.1128/jcm.31.5.1397-1399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander T J L. Neonatal diarrhoea in pigs. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1994. pp. 151–170. [Google Scholar]
- 16.Allaoui A, Sansonetti P J, Ménard R, Barzu S, Mounier J, Phalipon A, Parsot C. MxiG, a membrane protein required for secretion of Shigella spp. Ipa invasins: involvement in entry into epithelial cells and in intercellular dissemination. Mol Microbiol. 1995;17:461–470. doi: 10.1111/j.1365-2958.1995.mmi_17030461.x. [DOI] [PubMed] [Google Scholar]
- 17.Allerberger F, Rossboth D, Dierich M P, Aleksic S, Schmidt H, Karch H. Prevalence and clinical manifestations of Shiga toxin-producing Escherichia coli infections in Austrian children. Eur J Clin Microbiol Infect Dis. 1996;15:545–550. doi: 10.1007/BF01709361. [DOI] [PubMed] [Google Scholar]
- 18.Andrade J R, Da Veiga V F, De Santa Rosa M R, Suassuna I. An endocytic process in HEp-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989;28:49–57. doi: 10.1099/00222615-28-1-49. [DOI] [PubMed] [Google Scholar]
- 19.Andrews G P, Hromockyj A E, Coker C, Maurelli A T. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infect Immun. 1991;59:1997–2005. doi: 10.1128/iai.59.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AOAC International. FDA bacteriological analytical manual. Arlington, Va: AOAC International; 1995. [Google Scholar]
- 21.Arduino R C, DuPont H L. Traveler’s diarrhoea. Baillieres Clin Gastroenterol. 1993;7:365–385. doi: 10.1016/0950-3528(93)90046-u. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong G D, Rowe P C, Goodyer P, Orrbine E, Klassen T P, Wells G, MacKenzie A, Lior H, Blanchard C, Auclair F, Thompson B, Rafter D J, McLaine P N. A phase I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J Infect Dis. 1995;171:1042–1045. doi: 10.1093/infdis/171.4.1042. [DOI] [PubMed] [Google Scholar]
- 23.Arriaga Y L, Harville B A, Dreyfus L A. Contribution of individual disulfide bonds to biological action of Escherichia coli heat-stable enterotoxin B. Infect Immun. 1995;63:4715–4720. doi: 10.1128/iai.63.12.4715-4720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashkenazi S, Larocco M, Murray B E, Cleary T G. The adherence of verocytotoxin-producing Escherichia coli to rabbit intestinal cells. J Med Microbiol. 1992;37:304–309. doi: 10.1099/00222615-37-5-304. [DOI] [PubMed] [Google Scholar]
- 25.Aubel D A, Darfeuille-Michaud A, Martin C, Joly B. Nucleotide sequence of the nfaA gene encoding the antigen 8786 adhesive factor of enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 1992;77:277–284. doi: 10.1016/0378-1097(92)90169-o. [DOI] [PubMed] [Google Scholar]
- 26.Baldini M M, Kaper J B, Levine M M, Candy D C, Moon H W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2:534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- 27.Baldini M M, Nataro J P, Kaper J B. Localization of a determinant for HEp-2 adherence by enteropathogenic Escherichia coli. Infect Immun. 1986;52:334–336. doi: 10.1128/iai.52.1.334-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldwin T J, Brooks S F, Knutton S, Manjarrez Hernandez H A, Aitken A, Williams P H. Protein phosphorylation by protein kinase C in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1990;58:761–765. doi: 10.1128/iai.58.3.761-765.1990. . (Erratum, 58:2024.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldwin T J, Knutton S, Sellers L, Hernandez H A M, Aitken A, Williams P H. Enteroaggregative Escherichia coli strains secrete a heat-labile toxin antigenically related to E. coli hemolysin. Infect Immun. 1992;60:2092–2095. doi: 10.1128/iai.60.5.2092-2095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin T J, Lee-Delaunay M B, Knutton S, Williams P H. Calcium-calmodulin dependence of actin accretion and lethality in cultured HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1993;61:760–763. doi: 10.1128/iai.61.2.760-763.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldwin T J, Ward W, Aitken A, Knutton S, Williams P H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balows A, Hausler W J, Herrmann K L, Isenberg H D, Shadomy H J. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. [Google Scholar]
- 33.Banatvala N, Magnano A R, Cartter M L, Barrett T J, Bibb W F, Vasile L L, Msher P, Lambert-Fair M A, Green J H, Bean N H, Tauxe R V. Meat grinders and molecular epidemiology: two supermarket outbreaks of Escherichia coli O157:H7 infection. J Infect Dis. 1996;173:480–483. doi: 10.1093/infdis/173.2.480. [DOI] [PubMed] [Google Scholar]
- 34.Baqui A H, Sack R B, Black R E. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children <5 years of age. J Infect Dis. 1992;166:792–796. doi: 10.1093/infdis/166.4.792. [DOI] [PubMed] [Google Scholar]
- 35.Barrett T J, Green J H, Griffin P M, Pavia A T, Ostroff S M, Wachsmuth I K. Enzyme-linked immunosorbent assays for detecting antibodies to Shiga-like toxin I, Shiga-like toxin II, and Escherichia coli O157:H7 lipopolysaccharide in human serum. Curr Microbiol. 1991;23:189–195. [Google Scholar]
- 36.Barrett T J, Hunter S B, Swaminathan B. Abstracts of the 3rd International Symposium on Shiga Toxin (Verocytotoxin)-Producing Escherichia coli Infections. 1997. A national network for molecular subtyping of Escherichia coli O157:H7; p. 111. [Google Scholar]
- 37.Barrett T J, Kaper J B, Jerse A E, Wachsmuth I K. Virulence factors in Shiga-like toxin-producing Escherichia coli isolated from humans and cattle. J Infect Dis. 1992;165:979–980. doi: 10.1093/infdis/165.5.979. [DOI] [PubMed] [Google Scholar]
- 38.Barrett T J, Lior H, Green J H, Khakhria R, Wells J G, Bell B P, Greene K D, Lewis J, Griffin P M. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J Clin Microbiol. 1994;32:3013–3017. doi: 10.1128/jcm.32.12.3013-3017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett T J, Potter M E, Wachsmuth I K. Continuous peritoneal infusion of Shiga-like toxin II (SLT II) as a model for SLT II-induced diseases. J Infect Dis. 1989;159:774–777. doi: 10.1093/infdis/159.4.774. [DOI] [PubMed] [Google Scholar]
- 40.Baudry B, Maurelli A T, Clerc P, Sadoff J C, Sansonetti P J. Localization of plasmid loci necessary for the entry of Shigella flexneri into HeLa cells, and characterization of one locus encoding four immunogenic polypeptides. J Gen Microbiol. 1987;133:3403–3413. doi: 10.1099/00221287-133-12-3403. [DOI] [PubMed] [Google Scholar]
- 41.Baudry B, Savarino S J, Vial P, Kaper J B, Levine M M. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990;161:1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- 42.Bauer M E, Welch R A. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1996;64:167–175. doi: 10.1128/iai.64.1.167-175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beebakhee G, Louie M, De Azavedo J, Brunton J. Cloning and nucleotide sequence of the eae gene homologue from enterohemorrhagic Escherichia coli serotype O157:H7. FEMS Microbiol Lett. 1992;91:63–68. doi: 10.1016/0378-1097(92)90563-4. [DOI] [PubMed] [Google Scholar]
- 44.Begaud E, Germani Y. Detection of enterotoxigenic Escherichia coli in faecal specimens by acetylaminofluorene-labelled DNA probes. Res Microbiol. 1992;143:315–325. doi: 10.1016/0923-2508(92)90023-h. [DOI] [PubMed] [Google Scholar]
- 45.Bell B P, Goldoft M, Griffin P M, Davis M A, Gordon D C, Tarr P I, Bartleson C A, Lewis J H, Barrett T J, Wells J G, Baron R, Kobayashi J. A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers: the Washington experience. JAMA. 1994;272:1349–1353. [PubMed] [Google Scholar]
- 46.Belongia E A, Osterholm M T, Soler J T, Ammend D A, Braun J E, Macdonald K L. Transmission of Escherichia coli O157:H7 infection in Minnesota child day-care facilities. JAMA. 1993;269:883–888. [PubMed] [Google Scholar]
- 47.Bender J B, Hedberg C W, Besser J M, Boxrud D J, Macdonald K L, Osterholm M T. Surveillance for Escherichia coli O157:H7 infections in Minnesota by molecular subtyping. N Engl J Med. 1997;337:388–394. doi: 10.1056/NEJM199708073370604. [DOI] [PubMed] [Google Scholar]
- 48.Benjamin M M, Datta A R. Acid tolerance of enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1995;61:1669–1672. doi: 10.1128/aem.61.4.1669-1672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamin P, Federman M, Wanke C A. Characterization of an invasive phenotype associated with enteroaggregative Escherichia coli. Infect Immun. 1995;63:3417–3421. doi: 10.1128/iai.63.9.3417-3421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benz I, Schmidt M A. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect Immun. 1989;57:1506–1511. doi: 10.1128/iai.57.5.1506-1511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 52.Benz I, Schmidt M A. Isolation and serologic characterization of AIDA-I, the adhesin mediating the diffuse adherence phenotype of the diarrhea-associated Escherichia coli strain 2787 (O126:H27) Infect Immun. 1992;60:13–18. doi: 10.1128/iai.60.1.13-18.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Besser R E, Lett S M, Weber J T, Doyle M P, Barrett T J, Wells J G, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 54.Besser-Wiek J, Boxrud D, Bender J, Sullivan M, Carroll L, Leano F. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Selective media, broth enrichment, immunomagnetic separation, and PCR broth culture assay for detection of Escherichia coli O157:H7 in fecal samples, abstr. C-364; p. 65. [Google Scholar]
- 55.Bettelheim K A. Biochemical characteristics of Escherichia coli. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1994. pp. 3–30. [Google Scholar]
- 56.Beutin L, Aleksic S, Zimmermann S, Gleier K. Virulence factors and phenotypic traits of verotoxigenic strains of Escherichia coli isolated from human patients in Germany. Med Microbiol Immunol (Berlin) 1994;183:13–21. doi: 10.1007/BF00193627. [DOI] [PubMed] [Google Scholar]
- 57.Beutin L, Geier D, Steinrück H, Zimmermann S, Scheutz F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol. 1993;31:2483–2488. doi: 10.1128/jcm.31.9.2483-2488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beutin L, Stroeher U H, Manning P A. Isolation of enterohemolysin (Ehly2)-associated sequences encoded on temperate phages of Escherichia coli. Gene. 1993;132:95–99. doi: 10.1016/0378-1119(93)90519-9. [DOI] [PubMed] [Google Scholar]
- 59.Beutin L, Zimmermann S, Gleier K. Pseudomonas aeruginosa can cause false-positive identification of verotoxin (Shiga-like toxin) production by a commercial enzyme immune assay system for the detection of Shiga-like toxins (SLTs) Infection. 1996;24:267–268. doi: 10.1007/BF01781110. [DOI] [PubMed] [Google Scholar]
- 60.Beutin L, Zimmermann S, Gleire K. Rapid detection and isolation of Shiga-like toxin (verocytotoxin)-producing Escherichia coli by direct testing of individual enterohemolytic colonies from washed sheep blood agar platest in the VTEC-RPLA assay. J Clin Microbiol. 1996;34:2812–2814. doi: 10.1128/jcm.34.11.2812-2814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhan M K, Khoshoo V, Sommerfelt H, Raj P, Sazawal S, Srivastava R. Enteroaggregative Escherichia coli and Salmonella associated with nondysenteric persistent diarrhea. Pediatr Infect Dis J. 1989;8:499–502. doi: 10.1097/00006454-198908000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 63.Bhatnagar S, Bhan M K, Sommerfelt H, Sazawal S, Kumar R, Saini S. Enteroaggregative Escherichia coli may be a new pathogen causing acute and persistent diarrhea. Scand J Infect Dis. 1993;25:579–583. doi: 10.3109/00365549309008546. [DOI] [PubMed] [Google Scholar]
- 64.Bielaszewska M, Karmali M A, Petric M. Localization of verocytotoxin (VT)2 and antigenic cross-reactivity of VT1 and VT2 in the rabbit model. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 249–252. [Google Scholar]
- 65.Bilge S S, Apostol J M, Jr, Aldape M A, Moseley S L. mRNA processing independent of RNase III and RNase E in the expression of the F1845 fimbrial adhesin of Escherichia coli. Proc Natl Acad Sci USA. 1993;90:1455–1459. doi: 10.1073/pnas.90.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bilge S S, Apostol J M, Jr, Fullner K J, Moseley S L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993;7:993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 67.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bilge S S, Vary J C, Jr, Dowell S F, Tarr P I. Role of the Escherichia coli O157:H7 O side chain in adherence and analysis of an rfb locus. Infect Immun. 1996;64:4795–4801. doi: 10.1128/iai.64.11.4795-4801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bitzan M, Karch H. Indirect hemagglutination assay for diagnosis of Escherichia coli O157 infection in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1992;30:1174–1178. doi: 10.1128/jcm.30.5.1174-1178.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Black, R. E. 1990. Epidemiology of traveler’s diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73–S79. [DOI] [PubMed]
- 71.Black R E, Brown K H, Becker S, Abdul Alim A R M, Merson M H. Contamination of weaning foods and transmission of enterotoxigenic Escherichia coli diarrhoea in children in rural Bangladesh. Trans R Soc Trop Med Hyg. 1982;76:259–264. doi: 10.1016/0035-9203(82)90292-9. [DOI] [PubMed] [Google Scholar]
- 72.Black R E, Levine M M, Clements M L, Cisneros L, Daya V. Treatment of experimentally induced enterotoxigenic Escherichia coli diarrhea with trimethoprim, trimethoprim-sulfamethoxazole, or placebo. Rev Infect Dis. 1982;4:540–545. doi: 10.1093/clinids/4.2.540. [DOI] [PubMed] [Google Scholar]
- 73.Black R E, Merson M H, Rowe B, Taylor P R, Abdul Alim A R M, Gross R J, Sack D A. Enterotoxigenic Escherichia coli diarrhoea: acquired immunity and transmission in an endemic area. Bull W H O. 1981;59:263–268. [PMC free article] [PubMed] [Google Scholar]
- 74.Blake P A, Ramos S, Macdonald K L, Rassi V, Gomes T A T, Ivey C, Bean N H, Trabulsi L R. Pathogen-specific risk factors and protective factors for acute diarrheal disease in urban Brazilian infants. J Infect Dis. 1993;167:627–632. doi: 10.1093/infdis/167.3.627. [DOI] [PubMed] [Google Scholar]
- 75.Bloch C A, Rode C K. Pathogenicity island evaluation in Escherichia coli K1 by crossing with laboratory strain K12. Infect Immun. 1996;64:3218–3223. doi: 10.1128/iai.64.8.3218-3223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blum G, Ott M, Lischewski A, Ritter A, Imrich H, Tschäpe H, Hacker J. Excision of large DNA regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect Immun. 1994;62:606–614. doi: 10.1128/iai.62.2.606-614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bokete T N, O’Callahan C M, Clausen C R, Tang N M, Tran N, Moseley S L, Fritsche T R, Tarr P I. Shiga-like toxin-producing Escherichia coli in Seattle children: a prospective study. Gastroenterology. 1993;105:1724–1731. doi: 10.1016/0016-5085(93)91069-t. [DOI] [PubMed] [Google Scholar]
- 78.Bokete T N, Whittam T S, Wilson R A, Clausen C R, O’Callahan C M, Moseley S L, Fritsche T R, Tarr P I. Genetic and phenotypic analysis of Escherichia coli with enteropathogenic characteristics isolated from Seattle children. J Infect Dis. 1997;175:1382–1389. doi: 10.1086/516470. [DOI] [PubMed] [Google Scholar]
- 79.Booth L, Rowe B. Possible occupational acquisition of Escherichia coli O157 infection. Lancet. 1993;342:1298–1299. doi: 10.1016/0140-6736(93)92388-a. [DOI] [PubMed] [Google Scholar]
- 80.Bosworth B T, Samuel J E, Moon H W, O’Brien A D, Gordon V M, Whipp S C. Vaccination with genetically modified Shiga-like toxin IIe prevents edema disease in swine. Infect Immun. 1996;64:55–60. doi: 10.1128/iai.64.1.55-60.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bouzari S, Jafari A, Farhoudi-Moghaddam A A, Shokouhi F, Parsi M. Adherence of non-enteropathogenic Escherichia coli to HeLa cells. J Med Microbiol. 1994;40:95–97. doi: 10.1099/00222615-40-2-95. [DOI] [PubMed] [Google Scholar]
- 82.Bower J R, Congeni B L, Cleary T G, Stone R T, Wanger A, Murray B E, Mathewson J J, Pickering L K. Escherichia coli O114: nonmotile as a pathogen in an outbreak of severe diarrhea associated with a day care center. J Infect Dis. 1989;160:243–247. doi: 10.1093/infdis/160.2.243. [DOI] [PubMed] [Google Scholar]
- 83.Boyce T G, Pemberton A G, Wells J G, Griffin P M. Screening for Escherichia coli O157:H7—a nationwide survey of clinical laboratories. J Clin Microbiol. 1995;33:3275–3277. doi: 10.1128/jcm.33.12.3275-3277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boyce T G, Swerdlow D L, Griffin P M. Current concepts: Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333:364–368. doi: 10.1056/NEJM199508103330608. [DOI] [PubMed] [Google Scholar]
- 85.Boyd B, Lingwood C. Verotoxin receptor glycolipid in human renal tissue. Nephron. 1989;51:207–210. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- 86.Boylan M, Smyth C J, Scott J R. Nucleotide sequence of the gene encoding the major subunit of CS3 fimbriae of enterotoxigenic Escherichia coli. Infect Immun. 1956;29:3297–3300. doi: 10.1128/iai.56.12.3297-3300.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Böhm H, Karch H. DNA fingerprinting of Escherichia coli O157:H7 strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1992;30:2169–2172. doi: 10.1128/jcm.30.8.2169-2172.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brenner D J, Fanning G R, Miklos G V, Steigerwalt A G. Polynucleotide sequence relatedness among Shigella species. Int J Syst Bacteriol. 1973;23:1–7. [Google Scholar]
- 89.Brian M J, Frosolono M, Murray B E, Miranda A, Lopez E L, Gomez H F, Cleary T G. Polymerase chain reaction for diagnosis of enterohemorrhagic Escherichia coli infection and hemolytic-uremic syndrome. J Clin Microbiol. 1992;30:1801–1806. doi: 10.1128/jcm.30.7.1801-1806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brotman M, Giannella R A, Alm P F, Bauman H, Bennett A R, Black R E, Bruhn C M, Cohen M B, Gorbach S L, Kaper J B, Roberts M R, Staneck J L, Taylor S, Troutt H F, Bell B P, Buchanan R L, Durham K, Feng P, Tucker Foreman C, Galler R G, Gravani R B, Hall R H, Hancock D D, Hollingsworth J. Consensus Conference Statement. Escherichia coli O157:H7 infections—an emerging national health crisis, July 11–13, 1994. Gastroenterology. 1995;108:1923–1934. [PubMed] [Google Scholar]
- 91.Brown J E, Echeverria P, Taylor D N, Seriwatana J, Vanapruks V, Lexomboon U, Neill R J, Newland J W. Determination by DNA hybridization of Shiga-like-toxin-producing Escherichia coli in children with diarrhea in Thailand. J Clin Microbiol. 1989;27:291–294. doi: 10.1128/jcm.27.2.291-294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown J E, Sethabutr O, Jackson M P, Lolekha S, Echeverria P. Hybridization of Escherichia coli producing Shiga-like toxin I, Shiga-like toxin II, and a variant of Shiga-like toxin II with synthetic oligonucleotide probes. Infect Immun. 1989;57:2811–2814. doi: 10.1128/iai.57.9.2811-2814.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brunder W, Schmidt H, Karch H. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology. 1996;142:3305–3315. doi: 10.1099/13500872-142-11-3305. [DOI] [PubMed] [Google Scholar]
- 94.Brunton J. Molecular biology and role in disease of the verotoxins (Shiga-like) toxins of Escherichia coli. In: Miller V L, Kaper J B, Portnoy D A, Isberg R R, editors. Molecular genetics of bacterial pathogenesis. Washington, D.C: ASM Press; 1994. pp. 391–404. [Google Scholar]
- 95.Bujak J, Bauer P, Campbell D, Claridge J, Lewis J, Ries A, Stepak P, Turner J, Turner N, Anderson D. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Avoidance of economic entropy in culturing Escherichia coli O157:H7 (ECO157), abstr. C-445. [Google Scholar]
- 96.Burnens A P, Frey A, Lior H, Nicolet J. Prevalence and clinical significance of Vero-toxin-producing Escherichia coli (VTEC) isolated from cattle in herds with and without calf diarrhoea. J Vet Med. 1995;42:311–318. doi: 10.1111/j.1439-0450.1995.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 97.Burnens A P, Zbinden R, Kaempf L, Heinzer I, Nicolet J. A case of laboratory acquired infection with Escherichia coli O157:H7. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;279:512–517. doi: 10.1016/s0934-8840(11)80423-8. [DOI] [PubMed] [Google Scholar]
- 98.Butterton J R, Beattie D T, Gardel C L, Carroll P A, Hyman T, Killeen K P, Mekalanos J J, Calderwood S B. Heterologous antigen expression in Vibrio cholerae vector strains. Infect Immun. 1995;63:2689–2696. doi: 10.1128/iai.63.7.2689-2696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98a.Butterton J R, Ryan E T, Acheson D W, Calderwood S B. Coexpression of the B subunit of Shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio cholerae vaccine strains. Infect Immun. 1997;65:2127–2135. doi: 10.1128/iai.65.6.2127-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calderwood S B, Acheson D W K, Keusch G T, Barrett T J, Griffin P M, Strockbine N A, Swaminathan B, Kaper J B, Levine M M, Kaplan B S, Karch H, O’Brien A D, Obrig T G, Takeda Y, Tarr P I, Wachsmuth I K. Proposed new nomenclature for SLT (VT) family. ASM News. 1996;62:118–119. [Google Scholar]
- 100.Camara L M, Carbonare S B, Silva M L M, Carneiro-Sampaio M M S. Inhibition of enteropathogenic Escherichia coli (EPEC) adhesion to HeLa cells by human colostrum: detection of specific sIgA related to EPEC outer-membrane proteins. Int Arch Allergy Immunol. 1994;103:307–310. doi: 10.1159/000236645. [DOI] [PubMed] [Google Scholar]
- 101.Canil C, Rosenshine I, Ruschkowski S, Donnenberg M S, Kaper J B, Finlay B B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cantey J R, Blake R K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977;135:454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- 103.Caprioli A, Falbo V, Ruggeri F M, Baldassarri L, Bisicchia R, Ippolito G, Romoli E, Donelli G. Cytotoxic necrotizing factor production by hemolytic strains of Escherichia coli causing extraintestinal infections. J Clin Microbiol. 1987;25:146–149. doi: 10.1128/jcm.25.1.146-149.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Caprioli A, Luzzi I, Gianviti A, Russmann H, Karch H. Pheno-genotyping of verotoxin 2 (VT2)-producing Escherichia coli causing haemorrhagic colitis and haemolytic uraemic syndrome by direct analysis of patients’ stools. J Med Microbiol. 1995;43:348–353. doi: 10.1099/00222615-43-5-348. [DOI] [PubMed] [Google Scholar]
- 105.Caprioli A, Luzzi I, Seganti L, Marchetti M, Karmali M, Clarke I, Boyd B. Frequency and nature of verocytotoxin 2 (VT2) neutralizing activity (NA) in human and animal sera. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B. V.; 1994. pp. 353–356. [Google Scholar]
- 106.Carpick B W, Gariépy J. The Escherichia coli heat-stable enterotoxin is a long-lived superagonist of guanylin. Infect Immun. 1993;61:4710–4715. doi: 10.1128/iai.61.11.4710-4715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carson C A, Keller J M, McAdoo K K, Wang D Y, Higgins B, Bailey C W, Thorne J G, Payne B J, Skala M, Hahn A W. Escherichia coli O157:H7 restriction pattern recognition by artificial neural network. J Clin Microbiol. 1995;33:2894–2898. doi: 10.1128/jcm.33.11.2894-2898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carter A O, Borczyk A A, Carlson J A K, Harvey B, Hockin J C, Karmali M A, Krishnan C, Korn D A, Lior H. A severe outbreak of Escherichia coli O157:H7-associated hemorrhagic colitis in a nursing home. N Engl J Med. 1987;317:1496–1500. doi: 10.1056/NEJM198712103172403. [DOI] [PubMed] [Google Scholar]
- 109.Cassels F J, Wolf M K. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J Ind Microbiol. 1995;15:214–226. doi: 10.1007/BF01569828. [DOI] [PubMed] [Google Scholar]
- 110.Cebula T A, Payne W L, Feng P. Simultaneous identification of strains of Escherichia coli serotype O157:H7 and their shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J Clin Microbiol. 1995;33:248–250. doi: 10.1128/jcm.33.1.248-250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Centers for Disease Control. Update. Multistate outbreak of Escherichia coli O157:H7 infections from hamburgers—western United States, 1993. Morbid Mortal Weekly Rep. 1993;42:258–263. [PubMed] [Google Scholar]
- 112.Centers for Disease Control. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami—Washington and California, 1994. Morbid Mortal Weekly Rep. 1995;44:157–160. [PubMed] [Google Scholar]
- 113.Centers for Disease Control. Outbreaks of Escherichia coli O157:H7 infection associated with eating alfafa sprouts—Michigan and Virginia, June–July 1997. Morbid Mortal Weekly Rep. 1997;46:741–744. [PubMed] [Google Scholar]
- 114.Centers for Disease Control and Prevention. Foodborne diseases active surveillance network, 1996. Morbid Mortal Weekly Rep. 1997;46:258–261. [PubMed] [Google Scholar]
- 115.Chao K L, Dreyfus L A. Interaction of Escherichia coli heat-stable enterotoxin B with cultured human intestinal epithelial cells. Infect Immun. 1997;65:3209–3217. doi: 10.1128/iai.65.8.3209-3217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chapman P A, Daly C M. Comparison of Y1 adrenal cell and coagglutination assays for detection of Escherichia coli heat-labile enterotoxin. J Clin Pathol. 1989;42:755–758. doi: 10.1136/jcp.42.7.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chapman P A, Daly C M. Evaluation of non-radioactive trivalent DNA probe (LT, ST1a, ST1b) for detecting enterotoxigenic Escherichia coli. J Clin Pathol. 1993;46:309–312. doi: 10.1136/jcp.46.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chapman P A, Siddons C A. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from cases of bloody diarrhoea, non-bloody diarrhoea and asymptomatic contacts. J Med Microbiol. 1996;44:267–271. doi: 10.1099/00222615-44-4-267. [DOI] [PubMed] [Google Scholar]
- 119.Chapman P A, Siddons C A, Zadik P M, Jewes L. An improved selective medium for the isolation of Escherichia coli O157. J Med Microbiol. 1991;35:107–110. doi: 10.1099/00222615-35-2-107. [DOI] [PubMed] [Google Scholar]
- 120.Chapman P A, Wright D J, Siddons C A. A comparison of immunomagnetic separation and direct culture for the isolation of verocytotoxin-producing Escherichia coli O157 from bovine faeces. J Med Microbiol. 1994;40:424–427. doi: 10.1099/00222615-40-6-424. [DOI] [PubMed] [Google Scholar]
- 121.Chart H. Serodiagnosis of infections caused by Escherichia coli O157:H7 and other VTEC. Serodiagn Immunother Infect Dis. 1993;1:8–12. [Google Scholar]
- 122.Chart H, Law D, Rowe B, Acheson D W K. Patients with haemolytic uraemic syndrome caused by Escherichia coli O157: absence of antibodies to Vero cytotoxin 1 (VT1) or VT2. J Clin Pathol. 1993;46:1053–1054. doi: 10.1136/jcp.46.11.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chart H, Rowe B. Antibody cross-reactions with lipopolysaccharide from E. coli O157 after cholera vaccination. Lancet. 1993;341:1282. doi: 10.1016/0140-6736(93)91187-q. [DOI] [PubMed] [Google Scholar]
- 124.Chart H, Scotland S M, Rowe B. Serum antibodies to Escherichia coli serotype O157:H7 in patients with hemolytic uremic syndrome. J Clin Microbiol. 1989;27:285–290. doi: 10.1128/jcm.27.2.285-290.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chart H, Smith H R, Scotland S M, Rowe B, Milford D V, Taylor C M. Serological identification of Escherichia coli O157:H7 infection in haemolytic uraemic syndrome. Lancet. 1991;337:138–140. doi: 10.1016/0140-6736(91)90801-u. [DOI] [PubMed] [Google Scholar]
- 126.Cimolai N, Basalyga S, Mah D G, Morrison B J, Carter J E. A continuing assessment of risk factors for the development of Escherichia coliO157:H7-associated hemolytic uremic syndrome. Clin Nephrol. 1994;42:85–89. [PubMed] [Google Scholar]
- 127.Clark C A, Heuzenroeder M W, Manning P A. Colonization factor antigen CFA/IV (PCF8775) of human enterotoxigenic Escherichia coli: nucleotide sequence of the CS5 determinant. Infect Immun. 1992;60:1254–1257. doi: 10.1128/iai.60.3.1254-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clarke R C, Wilson J B, Read S C, Renwick S, Rahn K, Johnson R P, Alves D, Karmali M A, Lior H, McEwen S A, Spika J, Gyles C L. Verocytotoxin-producing Escherichia coli (VTEC) in the food chain: preharvest and processing perspectives. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 17–24. [Google Scholar]
- 129.Cobeljic M, Miljkovic-Selimovic B, Paunovic-Todosijevic D, Velickovic Z, Lepsanovic Z, Savic D, Ilic R, Konstantinovic S, Jovanovic B, Kostic V. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol Infect. 1996;117:11–16. doi: 10.1017/s0950268800001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cockerill F, III, Beebakhee G, Soni R, Sherman P. Polysaccharide side chains are not required for attaching and effacing adhesion of Escherichia coli O157:H7. Infect Immun. 1996;64:3196–3200. doi: 10.1128/iai.64.8.3196-3200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cohen A, Madrid Marina V, Estrov Z, Freedman M H, Lingwood C A, Dosch H M. Expression of glycolipid receptors to Shiga-like toxin on human B lymphocytes: a mechanism for the failure of long-lived antibody response to dysenteric disease. Int Immunol. 1990;2:1–8. doi: 10.1093/intimm/2.1.1. [DOI] [PubMed] [Google Scholar]
- 132.Collington, G. K., I. W. Booth, M. S. Donnenberg, J. B. Kaper, and S. Knutton. Attaching and effacing genes encoding secreted signalling proteins of enteropathogenic Escherichia coli (EPEC) are also required for modulation of host cell electrolyte transport. Gut, in press. [DOI] [PMC free article] [PubMed]
- 133.Cookson S T, Nataro J P. Characterization of HEp-2 cell projection formation induced by diffusely adherent Escherichia coli. Microb Pathog. 1996;21:421–434. doi: 10.1006/mpat.1996.0073. [DOI] [PubMed] [Google Scholar]
- 134.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Costin I D, Voiculescu D, Gorcea V. An outbreak of food poisoning in adults associated with Escherichia coli serotype 86:B7:H34. Pathol Microbiol. 1964;27:68–78. doi: 10.1159/000161453. [DOI] [PubMed] [Google Scholar]
- 136.Council of State and Territorial Epidemiologists. CSTE position statement 4. National surveillance of Escherichia coli O157:H7. Atlanta, Ga: Council of State and Territorial Epidemiologists; 1993. [Google Scholar]
- 137.Crane J K, Oh J S. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–3285. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crane J K, Wehner M S, Bolen E J, Sando J J, Linden J, Guerrant R L, Sears C L. Regulation of intestinal guanylate cyclase by the heat-stable enterotoxin of Escherichia coli(STa) and protein kinase C. Infect Immun. 1992;60:5004–5012. doi: 10.1128/iai.60.12.5004-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cravioto A, Gross R J, Scotland S M, Rowe B. An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr Microbiol. 1979;3:95–99. [Google Scholar]
- 140.Cravioto A, Reyes R E, Ortega R. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Rev. 1988;101:123. doi: 10.1017/s0950268800029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cravioto A, Reyes R E, Trujillo F, Uribe F, Navarro A, de la Roca J M, Hernandez J M, Perez G, Vazquez V. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am J Epidemiol. 1990;131:886–904. doi: 10.1093/oxfordjournals.aje.a115579. [DOI] [PubMed] [Google Scholar]
- 142.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 143.Cravioto A, Tello A, Villafán H, Ruiz J, Del Vedovo S, Neeser J-R. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis. 1991;163:1247–1255. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- 144.Cryan B. Comparison of three assay systems for detection of enterotoxigenic Escherichia coli heat-stable enterotoxin. J Clin Microbiol. 1990;28:792–794. doi: 10.1128/jcm.28.4.792-794.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cubbon M D, Coia J E, Hanson M F, Thomson-Carter F M. A comparison of immunomagnetic separation, direct culture and polymerase chain reaction for the detection of verocytotoxin-producing Escherichia coli O157 in human faeces. J Med Microbiol. 1996;44:219–222. doi: 10.1099/00222615-44-3-219. [DOI] [PubMed] [Google Scholar]
- 146.Czeczulin J R, Balepur S, Hicks S, Phillips A, Hall R, Kothary M H, Navarro-Garcia F, Nataro J P. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun. 1997;65:4135–4145. doi: 10.1128/iai.65.10.4135-4145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Darfeuille-Michaud A, Forestier C, Joly B, Cluzei R. Identification of a nonfimbrial adhesive factor of an enterotoxigenic Escherichia coli strain. Infect Immun. 1986;52:468–475. doi: 10.1128/iai.52.2.468-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Day N P, Scotland S M, Cheasty T, Rowe B. Escherichia coli O157:H7 associated with human infections in the United Kingdom. Lancet. 1983;i:825. doi: 10.1016/s0140-6736(83)91887-1. [DOI] [PubMed] [Google Scholar]
- 149.Debroy C, Yealy J, Wilson R A, Bhan M K, Kumar R. Antibodies raised against the outer membrane protein interrupt adherence of enteroaggregative Escherichia coli. Infect Immun. 1995;63:2873–2879. doi: 10.1128/iai.63.8.2873-2879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de Graaf F K, Gaastra W. Fimbriae of enterotoxigenic Escherichia coli. In: Klemm P, editor. Fimbriae: adhesion, genetics, biogenesis, and vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 58–83. [Google Scholar]
- 151.de Rycke J, Gonzalez E A, Blanco J, Oswald E, Blanco M, Boivin R. Evidence for two types of cytotoxic necrotizing factor in human and animal clinical isolates of Escherichia coli. J Clin Microbiol. 1990;28:694–699. doi: 10.1128/jcm.28.4.694-699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de Sauvage F J, Horuk R, Bennett G, Quan C, Burnier J P, Goeddel D V. Characterization of the recombinant human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1992;267:6479–6482. [PubMed] [Google Scholar]
- 153.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Donnenberg M S. Enteropathogenic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 709–726. [Google Scholar]
- 155.Donnenberg M S, Calderwood S B, Donohue-Rolfe A, Keusch G T, Kaper J B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990;58:1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Donnenberg M S, Donohue-Rolfe A, Keusch G T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli(EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989;160:452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- 157.Donnenberg M S, Girón J A, Nataro J P, Kaper J B. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol Microbiol. 1992;6:3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 158.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 160.Donnenberg M S, Nataro J P. Methods for studying adhesion of diarrheagenic Escherichia coli. Methods Enzymol. 1995;253:324–336. doi: 10.1016/s0076-6879(95)53028-2. [DOI] [PubMed] [Google Scholar]
- 161.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Donnenberg M S, Tzipori S, McKee M L, O’Brien A D, Alroy J, Kaper J B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Invest. 1993;92:1418–501424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: American Society for Microbiology; 1996. pp. 135–174. [Google Scholar]
- 164.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Donta S T, Moon H W, Whipp S C. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974;183:334–336. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- 166.Dorman C J. The VirF protein from Shigella flexneri is a member of the AraC transcription factor superfamily and is highly homologous to Rns, a positive regulator of virulence genes in enterotoxigenic Escherichia coli. Mol Microbiol. 1992;6:1575. doi: 10.1111/j.1365-2958.1992.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 167.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Doyle M P, Schoeni J L. Isolation of Escherichia coli O157:H7 from retail fresh meats and poultry. Appl Environ Microbiol. 1987;53:2394–2396. doi: 10.1128/aem.53.10.2394-2396.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Drasar B S, Hill M J. Human intestinal flora. London, United Kingdom: Academic Press, Ltd.; 1974. pp. 36–43. [Google Scholar]
- 170.Dreyfus L A, Harville B, Howard D E, Shaban R, Beatty D M, Morris S J. Calcium influx mediated by the Escherichia coli heat-stable enterotoxin B (STB) Proc Natl Acad Sci USA. 1993;90:3202–3206. doi: 10.1073/pnas.90.8.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dreyfus L A, Urban R G, Whipp S C, Slaughter C, Tachias K, Kupersztoch Y M. Purification of the STb enterotoxin of Escherichia coli and the role of selected amino acids on its secretion, stability and toxicity. Mol Microbiol. 1992;6:2397–2406. doi: 10.1111/j.1365-2958.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 172.Drolet R, Fairbrother J M, Harel J, Hélie P. Attaching and effacing and enterotoxigenic Escherichia coli associated with enteric colibacillosis in the dog. Can J Vet Res. 1994;58:87–92. [PMC free article] [PubMed] [Google Scholar]
- 173.DuPont, H. L. 1995. Pathogenesis of traveler’s diarrhea. Chemotherapy 41(Suppl. 1):33–39. [DOI] [PubMed]
- 174.DuPont H L, Ericsson C D. Prevention and treatment of traveler’s diarrhea. N Engl J Med. 1993;328:1821–1827. doi: 10.1056/NEJM199306243282507. [DOI] [PubMed] [Google Scholar]
- 175.DuPont H L, Formal S B, Hornick R B, Snyder M J, Libonati J P, Sheahan D G, LaBrec E H, Kalas J P. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971;285:1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- 176.Durno C, Soni R, Sherman P. Adherence of vero cytotoxin-producing Escherichia coli serotype O157:H7 to isolated epithelial cells and brush border membranes in vitro: role of type 1 fimbriae (pili) as a bacterial adhesin expressed by strain CL-49. Clin Invest Med. 1989;12:194–200. [PubMed] [Google Scholar]
- 177.du Toit R, Victor T C, van Helden P D. Empirical evaluation of conditions influencing the polymerase chain reaction: enterotoxigenic Escherichia coli as a test case. Eur J Clin Chem. 1993;31:225–231. doi: 10.1515/cclm.1993.31.4.225. [DOI] [PubMed] [Google Scholar]
- 178.Dylla B L, Vetter E A, Hughes J G, Cockerill F R., III Evaluation of an immunoassay for direct detection of Escherichia coli O157 in stool specimens. J Clin Microbiol. 1995;33:222–224. doi: 10.1128/jcm.33.1.222-224.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dytoc M, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 180.Dytoc M, Soni R, Cockerill F, De Azavedo J, Louie M, Brunton J, Sherman P. Multiple determinants of verotoxin-producing Escherichia coli O157 attachment-effacement. Infect Immun. 1993;61:3382–3391. doi: 10.1128/iai.61.8.3382-3391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dytoc M T, Ismaili A, Philpott D J, Soni R, Brunton J L, Sherman P M. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect Immun. 1994;62:3494–3505. doi: 10.1128/iai.62.8.3494-3505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ebel F, Deibel C, Kresse A U, Guzman C A, Chakraborty T. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect Immun. 1996;64:4472–4479. doi: 10.1128/iai.64.11.4472-4479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Echeverria P, Orskov F, Orskov I, Knutton S, Scheutz F, Brown J E, Lexomboon U. Attaching and effacing enteropathogenic Escherichia coli as a cause of infantile diarrhea in Bangkok. J Infect Dis. 1991;164:550–554. doi: 10.1093/infdis/164.3.550. [DOI] [PubMed] [Google Scholar]
- 184.Edelman R, Levine M M. Summary of a workshop on enteropathogenic Escherichia coli. J Infect Dis. 1983;147:1108–1118. doi: 10.1093/infdis/147.6.1108. [DOI] [PubMed] [Google Scholar]
- 185.Edwards P R, Ewing W H. Identification of Enterobacteriaceae. Minneapolis, Minn: Burgess Publishing Co.; 1972. [Google Scholar]
- 186.Eklund S, Brunsson I, Jodal M, Lundgren O. Changes in cyclic 3′5′-adenosine monophosphate tissue concentration and net fluid transport in the cat’s small intestine elicited by cholera toxin, arachidonic acid, vasoactive intestinal polypeptide, and 5-hydroxytryptamine. Acta Physiol Scand. 1987;129:115–125. doi: 10.1111/j.1748-1716.1987.tb08046.x. [DOI] [PubMed] [Google Scholar]
- 187.Elliott E, Li Z, Bell C, Stiel D, Buret A, Wallace J, Brzuszczak I, O’Loughlin E. Modulation of host response to Escherichia coli O157:H7 infection by anti-CD18 antibody in rabbits. Gastroenterology. 1994;106:1554–1561. doi: 10.1016/0016-5085(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 188.Elliott, S. J. 1997. Personal communication.
- 189.Elsinghorst E A, Kopecko D J. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Escherichia coli. Infect Immun. 1992;60:2409–2417. doi: 10.1128/iai.60.6.2409-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Elsinghorst E A, Weitz J A. Epithelial cell invasion and adherence directed by the enterotoxigenic Escherichia coli tib locus is associated with a 104-kilodalton outer membrane protein. Infect Immun. 1994;62:3463–3471. doi: 10.1128/iai.62.8.3463-3471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ericsson C D, DuPont H L, Mathewson J J, West M S, Johnson P C, Bitsura J A M. Treatment of traveler’s diarrhea with sulfamethoxazole and trimethoprim and loperamide. JAMA. 1990;263:257–261. [PubMed] [Google Scholar]
- 192.Eslava C, Villaseca J, Morales R, Navarro A, Cravioto A. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Identification of a protein with toxigenic activity produced by enteroaggregative Escherichia coli, abstr. B-105; p. 44. [Google Scholar]
- 193.Evans D G, Evans D J, Pierce N F. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect Immun. 1973;7:873–880. doi: 10.1128/iai.7.6.873-880.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fagundes-Neto, U. 1996. Enteropathogenic Escherichia coli infection in infants: clinical aspects and small bowel morphological alterations. Rev. Microbiol. Sao Paulo 27(Suppl. 1):117–119.
- 195.Faith N G, Shere J A, Brosch R, Arnold K W, Ansay S E, Lee M S, Luchansky J B, Kaspar C W. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl Environ Microbiol. 1996;62:1519–1525. doi: 10.1128/aem.62.5.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fang G D, Lima A A M, Martins C V, Nataro J P, Guerrant R L. Etiology and epidemiology of persistent diarrhea in northeastern Brazil: a hospital-based, prospective, case-control study. J Pediatr Gastroenterol Nutr. 1995;21:137–144. doi: 10.1097/00005176-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 197.Farmer J J., III . Enterobacteriaceae: introduction and identification. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 438–449. [Google Scholar]
- 198.Farmer J J, III, Davis B R. H7 antiserum-sorbitol fermentation medium: a single-tube screening method for detecting Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1985;22:620–625. doi: 10.1128/jcm.22.4.620-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Feng P, Fields P I, Swaminathan B, Whittam T S. Characterization of nonmotile variants of Escherichia coli O157 and other serotypes by using an antiflagellin monoclonal antibody. J Clin Microbiol. 1996;34:2856–2859. doi: 10.1128/jcm.34.11.2856-2859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Field M, Fromm D, Al-Awqati Q, Greenough W B. Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972;51:796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Field M, Rao M C, Chang E B. Intestinal electrolyte transport and diarrheal disease, part 2. N Engl J Med. 1989;321:879–883. doi: 10.1056/NEJM198909283211307. [DOI] [PubMed] [Google Scholar]
- 202.Field M, Rao M C, Chang E B. Intestinal electrolyte transport and diarrheal disease, part 1. N Engl J Med. 1989;321:800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- 203.Fields P I, Blom K, Hughes H J, Helsel L O, Feng P, Swaminathan B. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J Clin Microbiol. 1997;35:1066–1070. doi: 10.1128/jcm.35.5.1066-1070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Figueroa-Quintanilla D, Salazar-Lindo E, Sack R B, Leon-Barua R, Sarabia-Arce S, Campos-Sanchez M, Eyzaguirre-Maccan E. A controlled trial of bismuth subsalicylate in infants with watery diarrheal disease. N Engl J Med. 1993;328:1653–1658. doi: 10.1056/NEJM199306103282301. [DOI] [PubMed] [Google Scholar]
- 205.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fischer J, Maddox C, Moxley R, Kinden D, Miller M. Pathogenicity of a bovine attaching effacing Escherichia coli isolate lacking Shiga-like toxins. Am J Vet Res. 1994;55:991–999. [PubMed] [Google Scholar]
- 207.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Florentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamination of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 208.Fletcher J N, Embaye H E, Getty B, Batt R M, Hart C A, Saunders J R. Novel invasion determinant of enteropathogenic Escherichia coli plasmid pLV501 encodes the ability to invade intestinal epithelial cells and HEp-2 cells. Infect Immun. 1992;60:2229–2236. doi: 10.1128/iai.60.6.2229-2236.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Flores-Abuxapqui J J, Suarez-Itoil G J, Heredia-Navarrete M R, Puc-Franco M A, Franco-Monsreal J. Frequency of enterotoxigenic Escherichia coli in infants during the first three months of life. Arch Med Res. 1994;25:303–307. [PubMed] [Google Scholar]
- 210.Fontaine A, Arondel J, Sansonetti P J. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox mutant of Shigella dysenteriae 1. Infect Immun. 1988;56:3099–3109. doi: 10.1128/iai.56.12.3099-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Foubister V, Rosenshine I, Donnenberg M S, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli (EPEC) is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC) triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Francis C L, Jerse A E, Kaper J B, Falkow S. Characterization of interactions of enteropathogenic Escherichia coli O127:H6 with mammalian cells in vitro. J Infect Dis. 1991;164:693–703. doi: 10.1093/infdis/164.4.693. [DOI] [PubMed] [Google Scholar]
- 214.Franke J, Franke S, Schmidt H, Schwarzkopf A, Wieler L H, Baljer G, Beutin L, Karch H. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. J Clin Microbiol. 1994;32:2460–2463. doi: 10.1128/jcm.32.10.2460-2463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Reference deleted.
- 216.Franke S, Harmsen D, Caprioli A, Pierard D, Wieler L H, Karch H. Clonal relatedness of Shiga-like toxin-producing Escherichia coli O101 strains of human and porcine origin. J Clin Microbiol. 1995;33:3174–3178. doi: 10.1128/jcm.33.12.3174-3178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Frankel G, Candy D C A, Everest P, Dougan G. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect Immun. 1994;62:1835–1842. doi: 10.1128/iai.62.5.1835-1842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Frankel G, Candy D C A, Fabiani E, Adu-Bobie J, Gil S, Novakova M, Phillips A D, Dougan G. Molecular characterization of a carboxy-terminal eukaryotic-cell binding domain of intimin from enteropathogenic Escherichia coli. Infect Immun. 1995;63:4323–4328. doi: 10.1128/iai.63.11.4323-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Frankel G, Giron J A, Vlamossoi J, Schoolnik G. Multi-gene amplification: simultaneous detection of three virulence genes in diarrheal stool. Mol Microbiol. 1989;3:1729–1734. doi: 10.1111/j.1365-2958.1989.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 220.Frankel G, Lider O, Hershkoviz R, Mould A P, Kachalsky S G, Candy D C A, Cahalon L, Humphries M J, Dougan G. The cell-binding domain of intimins from enteropathogenic Escherichia coli binds to integrins. J Biol Chem. 1996;271:20359–20364. doi: 10.1074/jbc.271.34.20359. [DOI] [PubMed] [Google Scholar]
- 221.Frankel G, Riley L, Giron J A, Valmassoi J, Friedman A, Strockbine N, Falkow S, Schoolnik G A. Detection of Shigella in feces using DNA amplification. J Infect Dis. 1990;161:1252–1256. doi: 10.1093/infdis/161.6.1252. [DOI] [PubMed] [Google Scholar]
- 222.Fratamico P M, Bhaduri S, Buchanan R L. Studies on Escherichia coli serotype O157:H7 strains containing a 60-MDa plasmid and on 60-MDa plasmid-cured derivatives. J Med Microbiol. 1993;39:371–381. doi: 10.1099/00222615-39-5-371. [DOI] [PubMed] [Google Scholar]
- 223.Fratamico P M, Sackitey S K, Wiedmann M, Deng M Y. Detection of Escherichia coli O157:H7 by multiplex PCR. J Clin Microbiol. 1995;33:2188–2191. doi: 10.1128/jcm.33.8.2188-2191.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Reference deleted.
- 225.Froehlich B J, Karakashain A, Melsen L R, Wakefield J C, Scott J R. CooC and CooD are required for assembly of CS1 pili. Mol Microbiol. 1994;12:387–401. doi: 10.1111/j.1365-2958.1994.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 226.Froehlich B J, Karakashian A, Sakellaris H, Scott J R. Genes for CS2 pili of enterotoxigenic Escherichia coli and their interchangeability with those for CS1 pili. Infect Immun. 1995;63:4849–4856. doi: 10.1128/iai.63.12.4849-4856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Frost J A, Cheasty T, Thomas A, Rowe B. Phage typing of Vero cytotoxin-producing Escherichia coli O157 isolated in the United Kingdom: 1989–91. Epidemiol Infect. 1993;110:469–475. doi: 10.1017/s0950268800050895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fujii Y, Kondo Y, Okamoto K. Involvement of prostaglandin E2 synthesis in the intestinal secretory action of Escherichia coli heat-stable enterotoxin II. FEMS Microbiol Lett. 1995;130:259–266. doi: 10.1111/j.1574-6968.1995.tb07729.x. [DOI] [PubMed] [Google Scholar]
- 229.Fukuta S, Magnani J L, Twiddy E M, Holmes R K, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gaastra W, Svennerholm A-M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 231.Galan J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gannon V P J, D’Souza S, Graham T, King R K, Rahn K, Read S. Use of the flagellar H7 gene as a target in multiple PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J Clin Microbiol. 1997;35:656–662. doi: 10.1128/jcm.35.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gannon V P J, King R K, Kim J Y, Golsteyn Thomas E J. Rapid and sensitive method for detection of Shiga-like toxin-producing Escherichia coli in ground beef by using the polymerase chain reaction. Appl Environ Microbiol. 1992;58:3809–3815. doi: 10.1128/aem.58.12.3809-3815.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gannon V P J, Rashed M, King R K, Golsteyn Thomas E J. Detection and characterization of the eae gene of Shiga-like toxin-producing Escherichia coli by using polymerase chain reaction. J Clin Microbiol. 1993;31:1268–1274. doi: 10.1128/jcm.31.5.1268-1274.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Reference deleted.
- 236.Gianella R A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976;14:95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Giannella R A, Drake K W, Luttrell M. Development of a radioimmunoassay for Escherichia coli heat-stable enterotoxin. Infect Immun. 1981;33:186–192. doi: 10.1128/iai.33.1.186-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gicquelais K G, Baldini M M, Martinez J, Maggi L, Martin W C, Prado V, Kaper J B, Levine M M. Practical and economical method for using biotinylated DNA probes with bacterial colony blots to identify diarrhea-causing Escherichia coli. J Clin Microbiol. 1990;28:2485–2490. doi: 10.1128/jcm.28.11.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Giles C, Sangster G, Smith J. Epidemic gastroenteritis of infants in Aberdeen during 1947. Arch Dis Child. 1949;24:45–53. doi: 10.1136/adc.24.117.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Girón J A, Jones T, Millan Velasco F, Castro Munoz E, Zarate L, Fry J, Frankel G, Moseley S L, Baudry B, Kaper J B, et al. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991;163:507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- 241.Girón J A, Donnenberg M S, Martin W C, Jarvis K G, Kaper J B. Distribution of the bundle-forming pilus structural gene (bfpA) among enteropathogenic Escherichia coli. J Infect Dis. 1993;168:1037–1041. doi: 10.1093/infdis/168.4.1037. [DOI] [PubMed] [Google Scholar]
- 242.Girón J A, Ho A S Y, Schoolnik G K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 243.Girón J A, Ho A S Y, Schoolnik G K. Characterization of fimbriae produced by enteropathogenic Escherichia coli. J Bacteriol. 1993;175:7391–7403. doi: 10.1128/jb.175.22.7391-7403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Girón J A, Levine M M, Kaper J B. Longus: a long pilus ultrastructure produced by human enterotoxigenic Escherichia coli. Mol Microbiol. 1994;12:71–82. doi: 10.1111/j.1365-2958.1994.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 245.Girón J A, Qadri F, Jarvis K J, Kaper J B, Albert M J. Monoclonal antibodies specific for the bundle-forming pilus of enteropathogenic Escherichia coli. Infect Immun. 1995;63:4949–4952. doi: 10.1128/iai.63.12.4949-4952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Girón J A, Viboud G I, Sperandio V, Gómez-Duarte O G, Maneval D R, Albert M J, Levine M M, Kaper J B. Prevalence and association of the longus pilus structural gene (lngA) with colonization factor antigens, enterotoxin types, and serotypes of enterotoxigenic Escherichia coli. Infect Immun. 1995;63:4195–4198. doi: 10.1128/iai.63.10.4195-4198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Goldberg M B, Bârzu O, Parsot C, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993;175:2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Goldberg M B, Boyko S A, Butterton J R, Stoebner J A, Payne S M, Calderwood S B. Characterization of a Vibrio cholerae virulence factor homologous to the family of TonB-dependent proteins. Mol Microbiol. 1992;6:2407–2418. doi: 10.1111/j.1365-2958.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 249.Goldberg M B, Sansonetti P J. Shigella subversion of the cellular cytoskeleton: a strategy for epithelial colonization. Infect Immun. 1993;61:4941–4946. doi: 10.1128/iai.61.12.4941-4946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Goldwater P N, Bettelheim K A. The role of enterohaemorrhagic E. coli serotypes other than O157:H7 as causes of disease. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 57–60. [Google Scholar]
- 251.Gomes T A T, Blake P A, Trabulsi L R. Prevalence of Escherichia coli strains with localized, diffuse, and aggregative adherence to HeLa cells in infants with diarrhea and matched controls. J Clin Microbiol. 1989;27:266–269. doi: 10.1128/jcm.27.2.266-269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gomes T A T, Rassi V, Macdonald K L, Ramos S R T S, Trabulsi L R, Vieira M A M, Guth B E C, Candeias J A N, Ivey C, Toledo M R F, Blake P A. Enteropathogens associated with acute diarrheal disease in urban infants in Sao Paulo, Brazil. J Infect Dis. 1991;164:331–337. doi: 10.1093/infdis/164.2.331. [DOI] [PubMed] [Google Scholar]
- 253.Gomes T A T, Toledo R F, Trabulsi L R, Wood P K, Morris J G. DNA probes for identification of enteroinvasive Escherichia coli. J Clin Microbiol. 1987;25:2025–2027. doi: 10.1128/jcm.25.10.2025-2027.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gomes T A T, Vieira M A M, Wachsmuth I K, Blake P A, Trabulsi L R. Serotype-specific prevalence of Escherichia coli strains with EPEC adherence factor genes in infants with and without diarrhea in Sao Paulo, Brazil. J Infect Dis. 1989;160:131–135. doi: 10.1093/infdis/160.1.131. [DOI] [PubMed] [Google Scholar]
- 255.Gomez O, Donnenberg M, Kaper J B. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Plasmid encoded regulator A (PerA) is a transcriptional activator of eaeA and eaeB of enteropathogenic Escherichia coli, abstr. B-310. [Google Scholar]
- 256.Gomez, O., M. S. Donnenberg, and J. B. Kaper. 1994. Unpublished data.
- 257.Gómez-Duarte O G, Kaper J B. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect Immun. 1995;63:1767–1776. doi: 10.1128/iai.63.5.1767-1776.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gonzalez R, Diaz C, Marino M, Cloralt R, Pequeneze M, Perez-Schael I. Age-specific prevalence of Escherichia coli with localized and aggregative adherence in Venezuelan infants with acute diarrhea. J Clin Microbiol. 1997;35:1103–1107. doi: 10.1128/jcm.35.5.1103-1107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gordillo M E, Reeve G R, Pappas J, Mathewson J J, DuPont H L, Murray B E. Molecular characterization of strains of enteroinvasive Escherichia coli O143, including isolates from a large outbreak in Houston, Texas. J Clin Microbiol. 1992;30:889–893. doi: 10.1128/jcm.30.4.889-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gray L D. Escherichia, Salmonella, Shigella, and Yersinia. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 450–456. [Google Scholar]
- 261.Griffin P M. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 739–761. [Google Scholar]
- 262.Griffin P M, Olmstead L C, Petras R E. Escherichia coli O157:H7-associated colitis: a clinical and histological study of 11 cases. Gastroenterology. 1990;99:142–149. doi: 10.1016/0016-5085(90)91241-w. [DOI] [PubMed] [Google Scholar]
- 263.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 264.Grimm L M, Goldoft M, Kobayashi J, Lewis J H, Alfi D, Perdichizzi A M, Tarr P I, Ongerth J E, Moseley S L, Samadpour M. Molecular epidemiology of a fast-food restaurant-associated outbreak of Escherichia coli O157:H7 in Washington State. J Clin Microbiol. 1995;33:2155–2158. doi: 10.1128/jcm.33.8.2155-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Guerrant R L, Brunton L L, Schnaitman T C, Rebhun L I, Gilman A G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974;10:320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gunzberg S T, Chang B J, Elliott S J, Burke V, Gracey M. Diffuse and enteroaggregative patterns of adherence of enteric Escherichia coli isolated from aboriginal children from the Kimberley region of western Australia. J Infect Dis. 1993;167:755–758. doi: 10.1093/infdis/167.3.755. [DOI] [PubMed] [Google Scholar]
- 267.Reference deleted.
- 268.Gunzburg S T, Tornieporth N G, Riley L W. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J Clin Microbiol. 1995;33:1375–1377. doi: 10.1128/jcm.33.5.1375-1377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Reference deleted.
- 270.Gunzer F, Böhm H, Rüssmann H, Bitzan M, Aleksic S, Karch H. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1992;30:1807–1810. doi: 10.1128/jcm.30.7.1807-1810.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Guth B E, Twiddy E M, Trabulsi L R, Holmes R K. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect Immun. 1997;54:529–536. doi: 10.1128/iai.54.2.529-536.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gyles C L. VT toxemia in animal models. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 233–240. [Google Scholar]
- 273.Haigh R, Baldwin T, Knutton S, Williams P H. Carbon dioxide regulated secretion of the EaeB protein of enteropathogenic Escherichia coli. FEMS Microbiol Lett. 1995;129:63–68. doi: 10.1016/0378-1097(95)00136-S. [DOI] [PubMed] [Google Scholar]
- 274.Hale T L, Oaks E V, Formal S B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985;50:620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hales B A, Hart C A, Batt R M, Saunders J R. The large plasmids found in enterohemorrhagic and enteropathogenic Escherichia coli constitute a related series of transfer-defective Inc F-IIA replicons. Plasmid. 1992;28:183–193. doi: 10.1016/0147-619x(92)90050-k. [DOI] [PubMed] [Google Scholar]
- 276.Hall G A, Dorn C R, Chanter N, Scotland S M, Smith H R, Rowe B. Attaching and effacing lesions in vivo and adhesion to tissue culture cells of Vero-cytotoxin-producing Escherichia coli belonging to serogroups O5 and O103. J Gen Microbiol. 1990;136:779–786. doi: 10.1099/00221287-136-4-779. [DOI] [PubMed] [Google Scholar]
- 277.Hancock D D, Besser T E, Kinsel M L, Tarr P I, Rice D H, Paros M G. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington State. Epidemiol Infect. 1994;113:199–207. doi: 10.1017/s0950268800051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Harel Y, Silva M, Giroir B, Weinberg A, Cleary T B, Beutler B. A reporter transgene indicates renal-specific induction of tumor necrosis factor (TNF) by Shiga-like toxin. J Clin Invest. 1993;92:2110–2116. doi: 10.1172/JCI116811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Harris J R, Mariano J, Wells J G, Payne B S, Donnel H D, Cohen M L. Person-to-person transmission in an outbreak of enteroinvasive Escherichia coli. Am J Epidemiol. 1985;122:245–252. doi: 10.1093/oxfordjournals.aje.a114095. [DOI] [PubMed] [Google Scholar]
- 280.Heck J E, Staneck J L, Cohen M B, Weckbach L S, Giannella R A, Hawkins J, Tosiello R. Prevention of travelers’ diarrhea: ciprofloxacin versus trimethoprim/sulfamethoxazole in adult volunteers working in Latin America and the Caribbean. J Travel Med. 1994;1:136–142. doi: 10.1111/j.1708-8305.1994.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 281.Hedberg, C. W., S. J. Savarino, J. M. Besser, C. J. Paulus, V. M. Thelen, L. J. Myers, D. N. Cameron, T. J. Barrett, J. B. Kaper, and M. T. Osterholm. An outbreak of foodborne illness caused by Escherichia coli O39: NM: an agent that does not fit into the existing scheme for classifying diarrheagenic E. coli. J. Infect. Dis., in press. [DOI] [PubMed]
- 282.Henry F J, Udoy A S, Wanke C A, Aziz K M A. Epidemiology of persistent diarrhea and etiologic agents in Mirzapur, Bangladesh. Acta Paediatr Suppl. 1996;381:27–31. doi: 10.1111/j.1651-2227.1992.tb12368.x. [DOI] [PubMed] [Google Scholar]
- 283.Hertzke D M, Cowan L A, Schoning P, Fenwick B W. Glomerular ultrastructural lesions of idiopathic cutaneous and renal glomerular vasculopathy of Greyhounds. Vet Pathol. 1995;32:451–459. doi: 10.1177/030098589503200501. [DOI] [PubMed] [Google Scholar]
- 284.Heuvelink A E, Van de Kar N C A J, Meis J F G M, Monnens L A H, Melchers W J G. Characterization of verocytotoxin-producing Escherichia coli O157 isolates from patients with haemolytic uraemic syndrome in Western Europe. Epidemiol Infect. 1995;115:1–14. doi: 10.1017/s0950268800058064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Heuzenroeder M W, Elliot T R, Thomas C J, Halter R, Manning P A. A new fimbrial type (PCFO9) on enterotoxigenic Escherichia coli O9:H-LT+ isolated from a case of infant diarrhoea in central Australia. FEMS Microbiol Lett. 1990;66:55–60. doi: 10.1016/0378-1097(90)90258-r. [DOI] [PubMed] [Google Scholar]
- 286.Hicks S, Candy D C A, Phillips A D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.High N, Mounier J, Prevost M-C, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hii J H, Gyles C, Morooka T, Karmali M A, Clarke R, DeGrandis S, Brunton J L. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J Clin Microbiol. 1991;29:2704–2709. doi: 10.1128/jcm.29.12.2704-2709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hill S M, Phillips A D, Walker-Smith J A. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut. 1991;32:154–158. doi: 10.1136/gut.32.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hines J, Nachamkin I. Effective use of the clinical microbiology laboratory for diagnosing diarrheal diseases. Clin Infect Dis. 1996;23:1292–1301. doi: 10.1093/clinids/23.6.1292. [DOI] [PubMed] [Google Scholar]
- 291.Hirayama T. Heat-stable enterotoxin of Escherichia coli. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 281–296. [Google Scholar]
- 292.Hirayama T, Wada A, Iwata N, Takasaki S, Shimonishi Y, Takeda Y. Glycoprotein receptors for a heat-stable enterotoxin (STh) produced by enterotoxigenic Escherichia coli. Infect Immun. 1992;60:4213–4220. doi: 10.1128/iai.60.10.4213-4220.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hirst T R. Biogenesis of cholera toxin and related oligomeric enterotoxins. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 123–184. [Google Scholar]
- 294.Hitotsubashi S, Fujii Y, Yamanaka H, Okamoto K. Some properties of purified Escherichia coli heat-stable enterotoxin II. Infect Immun. 1992;60:4468–4474. doi: 10.1128/iai.60.11.4468-4474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hol W G J, Sixma T K, Merritt E A. Structure and function of E. coli heat-labile enterotoxin and cholera toxin B pentamer. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 185–223. [Google Scholar]
- 296.Holmes R K, Jobling M G, Connell T D. Cholera toxin and related enterotoxins of gram-negative bacteria. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 225–255. [Google Scholar]
- 297.Honda T, Taga S, Takeda Y, Miwatani T. Modified Elek test for detection of heat-labile enterotoxin of enterotoxigenic Escherichia coli. J Clin Microbiol. 1981;13:1–5. doi: 10.1128/jcm.13.1.1-5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hoque S S, Faruque A S, Mahalanabis D, Hasnat A. Infectious agents causing acute watery diarrhoea in infants and young children in Bangladesh and their public health implications. J Trop Pediatr. 1994;40:351–354. doi: 10.1093/tropej/40.6.351. [DOI] [PubMed] [Google Scholar]
- 299.Hromockyj A E, Tucker S C, Maurelli A T. Temperature regulation of Shigella virulence: identification of the repressor gene virR, an analogue of hns, and partial complementation by tyrosyl transfer RNA (tRNA1 Tyr) Mol Microbiol. 1992;6:2113–2124. doi: 10.1111/j.1365-2958.1992.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 300.Hughes M H, Greaves J L, Bettelheim K A. Infant diarrhoeae due to Escherichia coli O91 K? H7. J Clin Pathol. 1968;21:387–389. doi: 10.1136/jcp.21.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Isberg R R, Voorhis D L, Falkow S. Identification of Invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 302.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ismaili A, Philpott D J, Dytoc M T, Soni R, Ratnam S, Sherman P M. α-Actinin accumulation in epithelial cells infected with attaching and effacing gastrointestinal pathogens. J Infect Dis. 1995;172:1393–1396. doi: 10.1093/infdis/172.5.1393. [DOI] [PubMed] [Google Scholar]
- 304.Ito T, Kuwahara S, Yokota T. Automatic and manual latex agglutination tests for measurement of cholera toxin and heat-labile enterotoxin of Escherichia coli. J Clin Microbiol. 1983;17:7–12. doi: 10.1128/jcm.17.1.7-12.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Jackson M P. Detection of Shiga toxin-producing Shigella dysenteriae type 1 and Escherichia coli by using polymerase chain reaction with incorporation of digoxigenin-11 −dUTP. J Clin Microbiol. 1991;29:1910–1914. doi: 10.1128/jcm.29.9.1910-1914.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Jackson M P, Neill R J, O’Brien A D, Holmes R K, Newland J W. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol Lett. 1987;44:109–114. doi: 10.1016/0882-4010(87)90106-9. [DOI] [PubMed] [Google Scholar]
- 307.Jallat C, Livrelli V, Darfeuille-Michaud A, Rich C, Joly B. Escherichia coli strains involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J Clin Microbiol. 1993;31:2031–2037. doi: 10.1128/jcm.31.8.2031-2037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Jann K, Hoschutsky H. Nature and organization of adhesins. Curr Top Microbiol Immunol. 1991;151:55–85. doi: 10.1007/978-3-642-74703-8_3. [DOI] [PubMed] [Google Scholar]
- 309.Jarvis K G, Girón J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a specialized secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Jarvis K G, Kaper J B. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Jerse A E, Gicquelais K G, Kaper J B. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect Immun. 1991;59:3869–3875. doi: 10.1128/iai.59.11.3869-3875.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Jerse A E, Martin W C, Galen J E, Kaper J B. Oligonucleotide probe for detection of the enteropathogenic Escherichia coli (EPEC) adherence factor of localized adherent EPEC. J Clin Microbiol. 1990;28:2842–2844. doi: 10.1128/jcm.28.12.2842-2844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Johnson R P, Clarke R C, Wilson J B, Read S C, Rahn K, Renwick S A, Sandhu K A, Alves D, Karmali M A, Lior H, McEwen S A, Spika J S, Gyles C L. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J Food Prot. 1996;59:1112–1122. doi: 10.4315/0362-028X-59.10.1112. [DOI] [PubMed] [Google Scholar]
- 316.Johnson R P, Durham R J, Johnson S T, MacDonald L A, Jeffrey S R, Butman B T. Detection of Escherichia coli O157:H7 in meat by an enzyme-linked immunosorbent assay, EHEC-Tek. Appl Environ Microbiol. 1995;61:386–388. doi: 10.1128/aem.61.1.386-388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Johnson W M, Lior H, Bezanson G S. Cytotoxic Escherichia coli O157:H7 associated with haemorrhagic colitis in Canada. Lancet. 1983;i:76. doi: 10.1016/s0140-6736(83)91616-1. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 318.Johnson W M, Pollard D R, Lior H, Tyler S D, Rozee K R. Differentiation of genes coding for Escherichia coli verotoxin-2 and the verotoxin associated with porcine edema disease (VTE) by the polymerase chain reaction. J Clin Microbiol. 1990;28:2351–2353. doi: 10.1128/jcm.28.10.2351-2353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Jones C H, Dodson K, Hultgren S J. Structure, function, and assembly of adhesive P pili. In: Mobley H L T, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: American Society for Microbiology; 1996. pp. 175–219. [Google Scholar]
- 320.Jordi B J, van Vliet A H M, Willshaw G A, van der Zeijst B A M, Gaastra W. Analysis of the first two genes of the CS 1 fimbrial operon in human enterotoxigenic Escherichia coli of serotype O139:H28. FEMS Microbiol Lett. 1991;80:265–270. doi: 10.1016/0378-1097(91)90607-c. [DOI] [PubMed] [Google Scholar]
- 321.Jordi B J, Willshaw B A, van der Zeijst B A, Gaastra W. The complete nucleotide sequence of region 1 of the CFA/I fimbrial operon of human enterotoxigenic Escherichia coli. DNA Seq. 1992;2:257–263. doi: 10.3109/10425179209020811. [DOI] [PubMed] [Google Scholar]
- 322.Jose J, Jahnig F, Meyer T F. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol Microbiol. 1995;18:377–382. doi: 10.1111/j.1365-2958.1995.mmi_18020378.x. [DOI] [PubMed] [Google Scholar]
- 323.Jung H C, Eckmann L, Yang S, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Junkins A D, Doyle M P. Comparison of adherence properties of Escherichia coli O157:H7 and a 60-megadalton plasmid-cured derivative. Curr Microbiol. 1989;19:21–27. [Google Scholar]
- 325.Kandel G, Donohue-Rolfe A, Donowitz M, Keusch G T. Pathogenesis of Shigella diarrhea XVI. Selective targetting of shiga toxin to villus cells of rabbit jejunum explains the effect of the toxin on intestinal electrolyte transport. J Clin Invest. 1989;84:1509–1517. doi: 10.1172/JCI114327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kang G, Mathan M M, Mathan V I. Evaluation of a simplified HEp-2 cell adherence assay for Escherichia coli isolated from South Indian children with acute diarrhea and controls. J Clin Microbiol. 1995;33:2204–2205. doi: 10.1128/jcm.33.8.2204-2205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Kaper J B. Molecular pathogenesis of enteropathogenic Escherichia coli. In: Miller V L, Kaper J B, Portnoy D A, Isberg R R, editors. Molecular genetics of bacterial pathogenesis. Washington, D.C: ASM Press; 1994. pp. 173–195. [Google Scholar]
- 328.Kaper J B. Defining EPEC. Rev Microbiol Sao Paulo. 1996;27:130–133. [Google Scholar]
- 329.Kaper J B, Gómez-Duarte O G. The per regulator of enteropathogenic Escherichia coli. Mol Microbiol. 1997;23:179–181. doi: 10.1046/j.1365-2958.1997.1841559.x. [DOI] [PubMed] [Google Scholar]
- 330.Kaplan B S, Trompeter R S, Moake J L. Introduction, p. xvii–xxvii. In: Kaplan B S, Trompeter R S, Moake J L, editors. Hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. New York, N.Y: Marcel Dekker, Inc.; 1992. [Google Scholar]
- 330a.Karch H, Böhm H, Schmidt H, Gunzer F, Aleksic S, Heesemann J. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H−. J Clin Microbiol. 1993;31:1200–1205. doi: 10.1128/jcm.31.5.1200-1205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Karch H, Heesemann J, Laufs R, O’Brien A D, Tacket C O, Levine M M. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infect Immun. 1987;55:455–461. doi: 10.1128/iai.55.2.455-461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Karch H, Janetzki-Mittmann C, Aleksic S, Datz M. Isolation of enterohemorrhagic Escherichia coli O157 strains from patients with hemolytic-uremic syndrome by using immunomagnetic separation, DNA-based methods, and direct culture. J Clin Microbiol. 1996;34:516–519. doi: 10.1128/jcm.34.3.516-519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Karch H, Meyer T. Single primer pair for amplifying segments of distinct Shiga-like toxin genes by polymerase chain reaction. J Clin Microbiol. 1989;27:2751–2757. doi: 10.1128/jcm.27.12.2751-2757.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Karch H, Meyer T. Evaluation of oligonucleotide probes for identification of Shiga-like toxin producing Escherichia coli. J Clin Microbiol. 1989;27:1180–1186. doi: 10.1128/jcm.27.6.1180-1186.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Karch H, Meyer T, Rüssmann H, Heesemann J. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect Immun. 1992;60:3464–3467. doi: 10.1128/iai.60.8.3464-3467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Karch H, Rüssmann H, Schmidt H, Schwarzkopf A, Heesemann J. Long-term shedding and clonal turnover of enterohemorrhagic Escherichia coli O157 in diarrheal disease. J Clin Microbiol. 1995;33:1602–1605. doi: 10.1128/jcm.33.6.1602-1605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Karmali M A. Laboratory diagnosis of verotoxin-producing Escherichia coli infections. Clin Microbiol Newsl. 1987;9:65–70. [Google Scholar]
- 338.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Karmali M A, Lingwood C A, Petric M, Brunton J, Gyles C. Maintaining the existing phenotype nomenclatures for E. coli cytotoxins. ASM News. 1996;62:167–169. [Google Scholar]
- 340.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infections by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 341.Karmali M A, Petric M, Lim C, Fleming P C, Steele B T. Escherichia coli cytotoxin, haemolytic-uraemic syndrome, and haemorrhagic colitis. Lancet. 1983;ii:1299–1300. doi: 10.1016/s0140-6736(83)91167-4. [DOI] [PubMed] [Google Scholar]
- 342.Karmali M A, Petric M, Winkler M, Bielaszewska M, Brunton J, van de Kar N, Morooka T, Nair G B, Richardson S E, Arbus G S. Enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Escherichia coli Vero cytotoxin. J Clin Microbiol. 1994;32:1457–1463. doi: 10.1128/jcm.32.6.1457-1463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Karmali M A, Steele B T, Petric M, Lim C. Sporadic cases of haemolytic uremic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet. 1983;i:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 344.Karpman D, Andreasson A, Thysell H, Kaplan B S, Svanborg C. Cytokines in childhood hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Pediatr Nephrol. 1995;9:694–699. doi: 10.1007/BF00868714. [DOI] [PubMed] [Google Scholar]
- 345.Kaye S A, Louise C B, Boyd B, Lingwood C A, Obrig T G. Shiga toxin-associated hemolytic uremic syndrome: interleukin-1 enhancement of Shiga toxin cytotoxicity toward human vascular endothelial cells in vitro. Infect Immun. 1993;61:3886–3891. doi: 10.1128/iai.61.9.3886-3891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Keenan K P, Sharpnack D D, Collins H, Formal S B, O’Brien A D. Morphological evaluation of the effects of Shiga toxin and E. coli Shiga-like toxin on the rabbit intestine. Am J Pathol. 1986;125:69–80. [PMC free article] [PubMed] [Google Scholar]
- 347.Keene W E, McAnulty J M, Hoesly F C, Williams L P, Hedberg K, Oxman G L, Barrett T J, Pfaller M A, Fleming D W. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7 and Shigella sonnei. N Engl J Med. 1994;331:579–584. doi: 10.1056/NEJM199409013310904. [DOI] [PubMed] [Google Scholar]
- 348.Kehl K S, Havens P, Behnke C E, Acheson D W K. Evaluation of the Premier EHEC assay for detection of Shiga toxin-producing Escherichia coli. J Clin Microbiol. 1997;35:2051–2054. doi: 10.1128/jcm.35.8.2051-2054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Kenny B, Abe A, Stein M, Finlay B B. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect Immun. 1997;65:2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kenny B, Lai L, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–324. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 352a.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 353.Ketyi I. Epidemiology of the enteroinvasive Escherichia coli. Observations in Hungary. J Hyg Epidemiol Microbiol Immunol. 1989;33:261–267. [PubMed] [Google Scholar]
- 354.Khakhria R, Duck D, Lior H. Extended phage-typing scheme for Escherichia coli O157:H7. Epidemiol Infect. 1990;105:511–520. doi: 10.1017/s0950268800048135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kibel M A, Barnard P J. The haemolytic-uraemic syndrome: a survey in Southern Africa. S Afr Med J. 1968;42:692–698. [PubMed] [Google Scholar]
- 356.Kim M S, Doyle M P. Dipstick immunoassay to detect enterohemorrhagic Escherichia coli O157:H7 in retail ground beef. Appl Environ Microbiol. 1992;58:1764–1767. doi: 10.1128/aem.58.5.1764-1767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Knutton S, Adu-Bobie J, Bain C, Phillips A D, Dougan G, Frankel G. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect Immun. 1997;65:1644–1652. doi: 10.1128/iai.65.5.1644-1652.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Knutton S, Baldini M M, Kaper J B, McNeish A S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987;55:78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Knutton S, Baldwin T J, Haigh R D, Williams P H, Manjarrez Hernandez A, Aitken A. Intracellular changes in ‘attaching and effacing’ adhesion. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 215–222. [Google Scholar]
- 361.Knutton, S., G. K. Collington, T. J. Baldwin, R. D. Haigh, and P. H. Williams. 1996. Cellular responses to EPEC infection. Rev. Microbiol. Sao Paulo 27(Suppl. 1):89–94.
- 362.Knutton S, Lloyd D R, McNeish A S. Identification of a new fimbrial structure in enterotoxigenic Escherichia coli (ETEC) serotype O148:H28 which adheres to human intestinal mucosa: a potentially new human ETEC colonization factor. Infect Immun. 1987;55:8–92. doi: 10.1128/iai.55.1.86-92.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Knutton S, McConnell M M, Rowe B, McNeish A S. Adhesion and ultrastructural properties of human enterotoxigenic Escherichia coli producing colonization factor antigens III and IV. Infect Immun. 1989;57:3364–3371. doi: 10.1128/iai.57.11.3364-3371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Knutton S, Shaw R K, Bhan M K, Smith H R, McConnell M M, Cheasty T, Williams P H, Baldwin T J. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992;60:2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Konadu E, Robbins J B, Shiloach J, Bryla D A, Szu S C. Preparation, characterization, and immunological properties in mice of Escherichia coli O157 O-specific polysaccharide-protein conjugate vaccines. Infect Immun. 1994;62:5048–5054. doi: 10.1128/iai.62.11.5048-5054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Konowalchuk J, Speirs J I, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977;18:775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kopecko D J. Experimental keratoconjunctivitis (Sereny) assay. In: Clark V L, Bavoil P M, editors. Bacterial pathogenesis, part A. San Diego, Calif: Academic Press, Inc.; 1994. pp. 39–46. [DOI] [PubMed] [Google Scholar]
- 368.Krause U, Thomson-Carter F M, Pennington T H. Molecular epidemiology of Escherichia coli O157:H7 by pulsed-field gel electrophoresis and comparison with that by bacteriophage typing. J Clin Microbiol. 1996;34:959–961. doi: 10.1128/jcm.34.4.959-961.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Reference deleted.
- 370.Kusters J G, Gaastra W. Fimbrial operons and evolution. In: Klemm P, editor. Fimbriae: adhesion, genetics, biogenesis and vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 179–196. [Google Scholar]
- 371.Lai L-C, Wainwright L A, Stone K D, Donnenberg M S. A protein secreted by enteropathogenic Escherichia coli modulates EspB secretion and is required for transduction of signals and for attaching and effacing activities in host cells. 1997. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Lai L-C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Lanata C F, Kaper J B, Baldini M M, Black R B, Levine M M. Sensitivity and specificity of DNA probes with the stool blot technique detection of Escherichia coli enterotoxins. J Infect Dis. 1985;152:1087–1090. doi: 10.1093/infdis/152.5.1087. [DOI] [PubMed] [Google Scholar]
- 374.Lang A L, Tsai Y L, Mayer C L, Patton K C, Palmer C J. Multiplex PCR for detection of the heat-labile toxin gene and Shiga-like toxin I and II genes in Escherichia coli isolated from natural waters. Appl Environ Microbiol. 1994;60:3145–3149. doi: 10.1128/aem.60.9.3145-3149.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Lanyi B, Szita J, Ringelhann A, Kovach K. A waterborne outbreak of enteritis associated with Escherichia coli serotype 124:72:32. Acta Microbiol Hung. 1959;6:77–78. [Google Scholar]
- 376.Law D. Adhesion and its role in the virulence of enteropathogenic Escherichia coli. Clin Microbiol Rev. 1994;7:152–173. doi: 10.1128/cmr.7.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Law D, Kelly J. Use of heme and hemoglobin by Escherichia coli O157 and other Shiga-like-toxin-producing E. coli serogroups. Infect Immun. 1995;63:700–702. doi: 10.1128/iai.63.2.700-702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Lencer W I, Constable C, Moe S, Jobling M G, Webb H M, Ruston S, Madara J L, Hirst T R, Holmes R K. Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol. 1995;131:951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Leong J M, Fournier R S, Isberg R R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Le Saux N, Spika J S, Friesen B, Johnson I, Melnychuk D, Anderson C, Dion R, Rahman M, Tostowarky W. Ground beef consumption in non-commercial settings is a risk factor for sporadic Escherichia coli O157:H7 infection in Canada. J Infect Dis. 1993;167:500–502. doi: 10.1093/infdis/167.2.500. [DOI] [PubMed] [Google Scholar]
- 381.Levine M M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 382.Levine M M, Bergquist E J, Nalin D R, Waterman D H, Hornick R B, Young C R, Sotman S, Rowe B. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 383.Levine M M, Caplan E S, Watermann D, Cash R A, Hornick R B, Snyder M J. Diarrhea caused by Escherichia coli that produce only heat-stable enterotoxin. Infect Immun. 1977;17:78–82. doi: 10.1128/iai.17.1.78-82.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Levine M M, Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- 385.Levine M M, Ferreccio C, Prado V, Cayazzo M, Abrego P, Martinez J, Maggi L, Baldini M M, Martin W, Maneval D, Kay B, Guers L, Lior H, Wasserman S S, Nataro J P. Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Am J Epidemiol. 1993;138:849–869. doi: 10.1093/oxfordjournals.aje.a116788. [DOI] [PubMed] [Google Scholar]
- 385a.Levine M M, McEwen J, Losonsky G, Reymann M, Harari I, Brown J E, Taylor D N, Donohue-Rolfe A, Cohen D, Bennish M, Lim Y L, Arnon R. Antibodies to Shiga holotoxin and to two synthetic peptides of the B. subunit in sera of patients with Shigella dysenteriae 1 dysentery. J Clin Microbiol. 1992;30:1636–1641. doi: 10.1128/jcm.30.7.1636-1641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O’Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 387.Levine M M, Prado V, Robins-Browne R, Lior H, Kaper J B, Moseley S L, Gicquelais K, Nataro J P, Vial P, Tall B. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J Infect Dis. 1988;158:224–228. doi: 10.1093/infdis/158.1.224. [DOI] [PubMed] [Google Scholar]
- 388.Levine M M, Rennels M B, Cisneros L, Highes T P, Nalin D R, Young C R. Lack of person-to-person transmission of enterotoxigenic Escherichia coli despite close contact. Am J Epidemiol. 1980;111:347–355. doi: 10.1093/oxfordjournals.aje.a112906. [DOI] [PubMed] [Google Scholar]
- 389.Levine M M, Ristaino P, Marley G, Smyth C, Knutton S, Boedeker E, Black R, Young C, Clements M L, Cheney C, Patnaik R. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984;44:409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Levine M M, Xu J, Kaper J B, Lior H, Prado V, Tall B, Nataro J, Karch H, Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987;156:175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- 391.Leyer G J, Wang L L, Johnson E A. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl Environ Microbiol. 1995;61:3752–3755. doi: 10.1128/aem.61.10.3752-3755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Li Z, Bell C, Buret A, Robins-Browne R, Stiel D, O’Loughlin E. The effect of enterohemorrhagic Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology. 1993;104:467–474. doi: 10.1016/0016-5085(93)90415-9. [DOI] [PubMed] [Google Scholar]
- 393.Lima, A. A. M., G. Fang, J. B. Schorling, L. De Albuquerque, J. F. McAuliffe, S. Mota, R. Leite, and R. L. Guerrant. 1992. Persistent diarrhea in Northeast Brazil: etiologies and interactions with malnutrition. Acta Paediatr. Scand. 81(Suppl. 381):39–44. [DOI] [PubMed]
- 394.Lior H. Classification of Escherichia coli. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1996. pp. 31–72. [Google Scholar]
- 395.Long K Z, Wood J W, Vasquez Gariby E, Weiss K M, Mathewson J J, de la Cabada F J, DuPont H L, Wilson R A. Proportional hazards analysis of diarrhea due to enterotoxigenic Escherichia coli and breast feeding in a cohort of urban Mexican children. Am J Epidemiol. 1994;139:193–205. doi: 10.1093/oxfordjournals.aje.a116981. [DOI] [PubMed] [Google Scholar]
- 396.Lopez E L, Diaz M, Grinstein S, Devoto S, Mendilaharzu F, Murray B E, Ashkenazi S, Rubeglio E, Woloj M, Vasquez M, Turco M, Pickering L K, Cleary T G. Hemolytic uremic syndrome and diarrhea in Argentine children: the role of Shiga-like toxins. J Infect Dis. 1989;160:469–475. doi: 10.1093/infdis/160.3.469. [DOI] [PubMed] [Google Scholar]
- 397.Lou Q, Chong S K F, Fitzgerald J F, Siders J A, Allen S D, Lee C-H. Rapid and effective method for preparation of fecal specimens for PCR assays. J Clin Microbiol. 1997;35:281–283. doi: 10.1128/jcm.35.1.281-283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Louie M, De Azavedo J, Clarke R, Borczyk A, Lior H, Richter M, Brunton J. Sequence heterogeneity of the eae gene and detection of verotoxin-producing Escherichia coli using serotype-specific primers. Epidemiol Infect. 1994;112:449–461. doi: 10.1017/s0950268800051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Louie M, De Azavedo J C S, Handelsman M Y C, Clark C G, Ally B, Dytoc M, Sherman P, Brunton J. Expression and characterization of the eaeA gene product of Escherichia coli serotype O157:H7. Infect Immun. 1993;61:4085–4092. doi: 10.1128/iai.61.10.4085-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Louise C B, Obrig T G. Shiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect Immun. 1991;59:4173–4179. doi: 10.1128/iai.59.11.4173-4179.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Louise C B, Obrig T G. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J Infect Dis. 1995;172:1397–1401. doi: 10.1093/infdis/172.5.1397. [DOI] [PubMed] [Google Scholar]
- 402.Ludwig K, Bitzan M, Zimmermann S, Kloth M, Ruder H, Müller-Wiefel D E. Immune response to non-O157 vero toxin-producing Escherichia coli in patients with hemolytic uremic syndrome. J Infect Dis. 1996;174:1028–1039. doi: 10.1093/infdis/174.5.1028. [DOI] [PubMed] [Google Scholar]
- 403.Madara J L, Patapoff T W, Gillece-Gastro B, Colgan S P, Parkos C A, Delp C, Mrsny R J. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Madico G, Akopyants N S, Berg D E. Arbitrarily primed PCR DNA fingerprinting of Escherichia coli O157:H7 strains by using templates from boiled cultures. J Clin Microbiol. 1995;33:1534–1536. doi: 10.1128/jcm.33.6.1534-1536.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Mahon B E, Griffin P M, Mead P S, Tauxe R V. Hemolytic uremic syndrome surveillance to monitor trends in infection in Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. Emerg Infect Dis. 1997;3:409–412. doi: 10.3201/eid0303.970329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Mangia A H, Duarte A N, Duarte R, Silva L A, Bravo V L, Leal M C. Aetiology of acute diarrhoea in hospitalized children in Rio de Janeiro City, Brazil. J Trop Pediatr. 1993;39:365–367. doi: 10.1093/tropej/39.6.365. [DOI] [PubMed] [Google Scholar]
- 407.Manjarrez-Hernandez H A, Amess B, Sellers L, Baldwin T J, Knutton S, Williams P H, Aitken A. Purification of a 20-kDa phosphoprotein from epithelial cells and identification as a myosin light chain-phosphorylation induced by enteropathogenic Escherichia coli and phorbol ester. FEBS Lett. 1991;292:121–127. doi: 10.1016/0014-5793(91)80848-w. [DOI] [PubMed] [Google Scholar]
- 408.Manjarrez-Hernandez H A, Baldin T J, Williams P H, Haigh R, Knutton S, Aitken A. Phosphorylation of myosin light chain at distinct sites and its association with the cytoskeleton during enteropathogenic Escherichia coli infection. Infect Immun. 1996;64:2368–2370. doi: 10.1128/iai.64.6.2368-2370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Manjarrez-Hernandez H A, Baldwin T J, Aitken A, Knutton S, Williams P H. Intestinal epithelial cell protein phosphorylation in enteropathogenic Escherichia coli diarrhoea. Lancet. 1992;339:521–523. doi: 10.1016/0140-6736(92)90340-9. [DOI] [PubMed] [Google Scholar]
- 410.Mann E A, Cohen M B, Giannella R A. Comparison of receptors for Escherichia coli heat-stable enterotoxin: novel receptor present in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol. 1993;264:G172–G178. doi: 10.1152/ajpgi.1993.264.1.G172. [DOI] [PubMed] [Google Scholar]
- 411.Manning P A, Higgins G D, Lumb R, Lanser J A. Colonization factor antigens and a new fimbrial type, CFA/V, on O115:H40 and H− strains of enterotoxigenic Escherichia coli in central Australia. J Infect Dis. 1987;156:841–844. doi: 10.1093/infdis/156.5.841. [DOI] [PubMed] [Google Scholar]
- 412.March S B, Ratnam S. Sorbitol-MacConkey medium for detection of Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1986;23:869–872. doi: 10.1128/jcm.23.5.869-872.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Marier R, Wells J G, Swanson R C, Dallhan W, Mehlman I J. An outbreak of enteropathogenic Escherichia coli foodborne disease traced to imported french cheese. Lancet. 1973;ii:1376–1378. [Google Scholar]
- 414.Marks S, Roberts T. E. coli O157:H7 ranks as the fourth most costly foodborne disease. Food Saf. 1993;1993:51–55. [Google Scholar]
- 415.Marquart M E, Picking W L, Picking W D. Soluble invasion plasmid antigen C (IpaC) from Shigella flexneri elicits epithelial cell responses related to pathogen invasion. Infect Immun. 1996;64:4182–4187. doi: 10.1128/iai.64.10.4182-4187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Martin D L, Macdonald K L, White K E, Soler J T, Osterholm M T. The epidemiology and clinical aspects of the hemolytic uremic syndrome in Minnesota. N Engl J Med. 1990;323:1161–1167. doi: 10.1056/NEJM199010253231703. [DOI] [PubMed] [Google Scholar]
- 417.Martin I E, Tyler S D, Tyler K D, Khakhria R, Johnson W M. Evaluation of ribotyping as epidemiologic tool for typing Escherichia coli serogroup O157 isolates. J Clin Microbiol. 1996;34:720–723. doi: 10.1128/jcm.34.3.720-723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Mathewson J J, Johnson P C, DuPont H L. Pathogenicity of enteroadherent Escherichia coli in adult volunteers. J Infect Dis. 1986;154:524–527. doi: 10.1093/infdis/154.3.524. [DOI] [PubMed] [Google Scholar]
- 419.Mathewson J J, Johnson P C, DuPont H L, Morgan D R, Thornton S A, Wood L V, Ericsson C D. A newly recognized cause of traveler’s diarrhea: enteroadherent Escherichia coli. J Infect Dis. 1985;151:471–475. doi: 10.1093/infdis/151.3.471. [DOI] [PubMed] [Google Scholar]
- 420.Mathewson J J, Oberhelman R A, DuPont H L, Javier de la Cabada F, Garibay E V. Enteroadherent Escherichia coli as a cause of diarrhea among children in Mexico. J Clin Microbiol. 1987;25:1917–1919. doi: 10.1128/jcm.25.10.1917-1919.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Matsudaira P, Jammey P. Pieces in the actin-severing puzzle. Cell. 1988;54:139–140. doi: 10.1016/0092-8674(88)90542-9. [DOI] [PubMed] [Google Scholar]
- 422.Mattila L. Clinical features and duration of traveler’s diarrhea in relation to its etiology. Clin Infect Dis. 1994;19:728–734. doi: 10.1093/clinids/19.4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Maurelli A T. Virulence protein export systems in Salmonella and Shigella: a new family or lost relatives. Trends Cell Biol. 1994;4:240–242. doi: 10.1016/0962-8924(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 424.Maurelli A T, Hromockyj A E, Bernardini M L. Environmental regulation of Shigella virulence. Curr Top Microbiol Immunol. 1992;180:95–116. doi: 10.1007/978-3-642-77238-2_5. [DOI] [PubMed] [Google Scholar]
- 425.Mayer H B, Wanke C A. Enteroaggregative Escherichia coli as a possible cause of diarrhea in an HIV-infected patient. N Engl J Med. 1995;332:273–274. doi: 10.1056/NEJM199501263320417. [DOI] [PubMed] [Google Scholar]
- 426.McCarthy M. E. coli O157:H7 outbreak in USA traced to apple juice. Lancet. 1996;348:1299. [Google Scholar]
- 427.McConnell M M, Chart H, Field A M, Hibberd M, Rowe B. Characterization of a putative colonization factor (PCFO166) of enterotoxigenic Escherichia coli of serogroup O166. J Med Microbiol. 1989;135:1135–1144. doi: 10.1099/00221287-135-5-1135. [DOI] [PubMed] [Google Scholar]
- 428.McConnell M M, Hibberd M, Field A M, Chart H, Rowe B. Characterization of a new putative colonization factor (CS 17) from a human enterotoxigenic Escherichia coli of serotype O114:H21 which produces only heat-labile enterotoxin. J Infect Dis. 1990;161:343–347. doi: 10.1093/infdis/161.2.343. [DOI] [PubMed] [Google Scholar]
- 429.McCormick B A, Colgan S P, Delp-Archer C, Miller S I, Madara J L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.McDaniel T K. Ph.D. thesis. Baltimore: University of Maryland; 1996. [Google Scholar]
- 431.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 433.McGee D W, Elson C O, McGhee J R. Enhancing effect of cholera toxin on interleukin-6 intestinal epithelial cells: mode of action and augmenting effect of inflammatory cytokines. Infect Immun. 1993;61:4637–4644. doi: 10.1128/iai.61.11.4637-4644.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.McKee M L, Melton-Celsa A R, Moxley R A, Francis D H, O’Brien A D. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.McKee M L, O’Brien A D. Investigation of enterohemorrhagic Escherichia coli O157:H7 adherence characteristics and invasion potential reveals a new attachment pattern shared by intestinal E. coli. Infect Immun. 1995;63:2070–2074. doi: 10.1128/iai.63.5.2070-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.McKee M L, O’Brien A D. Truncated enterohemorrhagic Escherichia coli (EHEC) O157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to HEp-2 cells. Infect Immun. 1996;64:2225–2233. doi: 10.1128/iai.64.6.2225-2233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.McWhirter E, Handelsman M, De Azavedo J, Brunton J. The enterohemorrhagic Escherichia coli eae gene product intimin is functional when signalling functions are provided by another bacterium. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 269–272. [Google Scholar]
- 438.Mead P S, Finelli L, Lambert-Fair M A, Champ D, Townes J, Hutwager L, Barrett T, Spitalny K, Mintz E. Risk factors for sporadic infection with Escherichia coli O157:H7. Arch Intern Med. 1997;157:204–208. [PubMed] [Google Scholar]
- 439.Mecsas J, Strauss E J. Molecular mechanisms of bacterial virulence: type III secretion and pathogenicity islands. Emerg Infect Dis. 1996;2:271–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Melton-Celsa A R, Darnell S C, O’Brien A D. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect Immun. 1996;64:1569–1576. doi: 10.1128/iai.64.5.1569-1576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Menard R, Sansonetti P J. Shigella flexneri: isolation of non-invasive mutants of gram-negative pathogens. Methods Enzymol. 1994;236:493–509. doi: 10.1016/0076-6879(94)36038-3. [DOI] [PubMed] [Google Scholar]
- 442.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Ménard R, Prévost M C, Gounon P, Sansonetti P, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci USA. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Meng, J., and M. P. Doyle. Microbiology of Shiga toxin-producing Escherichia coli in foods. In J. B. Kaper and A. D. O’Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing Escherichia coli, in press. ASM Press, Washington, D.C.
- 445.Meng J, Zhao S, Zhao T, Doyle M P. Molecular characterisation of Escherichia coli O157:H7 isolates by pulsed-field gel electrophoresis and plasmid DNA analysis. J Med Microbiol. 1995;42:258–263. doi: 10.1099/00222615-42-4-258. [DOI] [PubMed] [Google Scholar]
- 446.Mezoff A G, Giannella R A, Eade M N, Cohen M B. Escherichia coli enterotoxin (STa) binds to receptors, stimulates guanyl cyclase, and impairs absorption in rat colon. Gastroenterology. 1992;102:816–822. doi: 10.1016/0016-5085(92)90163-s. [DOI] [PubMed] [Google Scholar]
- 447.Mietens C, Keinhorst H, Hilpert H, Gerber H, Amster H, Pahud J J. Treatment of infantile E. coli gastroenteritis with specific bovine anti-E. coli milk immunoglobulin. Eur J Pediatr. 1979;132:239–252. doi: 10.1007/BF00496847. [DOI] [PubMed] [Google Scholar]
- 448.Miliotis M D, Koornhof H J, Phillips J I. Invasive potential of noncytotoxic enteropathogenic Escherichia coli in an in vitro Henle 407 cell model. Infect Immun. 1989;57:1928–1935. doi: 10.1128/iai.57.7.1928-1935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Miller J R, Barrett L, Kotloff K, Guerrant R L. A rapid diagnostic test for infectious and inflammatory enteritis. Arch Intern Med. 1994;154:2660–2664. doi: 10.1001/archinte.1994.00420230043006. [DOI] [PubMed] [Google Scholar]
- 450.Mills M, Payne S M. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J Bacteriol. 1995;177:3004–3009. doi: 10.1128/jb.177.11.3004-3009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Reference deleted.
- 452.Moake J L. Haemolytic-uraemic syndrome: basic science. Lancet. 1994;343:393–397. doi: 10.1016/s0140-6736(94)91227-0. [DOI] [PubMed] [Google Scholar]
- 453.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Morgan G M, Newman C, Palmer S R, Allen J B, Shepard W, Rampling A M, Warren R E, Gross R J, Scotland S M, Smith H R. First recognized community outbreak of haemorrhagic colitis due to verotoxin-producing Escherichia coli O157:H7 in the UK. Epidemiol Infect. 1988;101:83–91. doi: 10.1017/s0950268800029241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Moseley S L, Echeverria P, Seriwatana J, Tirapat C, Chaicumpa W, Sakuldaipeara T, Falkow S. Identification of enterotoxigenic Escherichia coli by colony hybridization using three enterotoxin gene probes. J Infect Dis. 1982;145:863–869. doi: 10.1093/infdis/145.6.863. [DOI] [PubMed] [Google Scholar]
- 456.Muller U, Brandsch M, Prasad P D, Fei Y J, Ganapathy V, Leibach F H. Inhibition of the H+/peptide cotransporter in the human intestinal cell line Caco-2 by cyclic AMP. Biochem Biophys Res Commun. 1996;218:461–465. doi: 10.1006/bbrc.1996.0082. [DOI] [PubMed] [Google Scholar]
- 457.Murray B E, Mathewson J J, DuPont H L, Hill W E. Utility of oligodeoxyribonucleotide probes for detecting enterotoxigenic Escherichia coli. J Infect Dis. 1987;155:809–811. doi: 10.1093/infdis/155.4.809. [DOI] [PubMed] [Google Scholar]
- 458.Nagayama K, Bi Z, Oguchi T, Takarada Y, Shibata S, Honda T. Use of an alkaline phosphatase-conjugated oligonucleotide probe for the gene encoding the bundle-forming pilus of enteropathogenic Escherichia coli. J Clin Microbiol. 1996;34:2819–2821. doi: 10.1128/jcm.34.11.2819-2821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Nalin D R, McLaughlin J C, Rahaman M, Yunus M, Curlin G. Enteropathogenic Escherichia coli and idiopathic diarrhoea in Bangladesh. Lancet. 1975;ii:1116–1119. doi: 10.1016/s0140-6736(75)91005-3. [DOI] [PubMed] [Google Scholar]
- 460.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Nataro J P, Baldini M M, Kaper J B, Black R E, Bravo N, Levine M M. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985;152:560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- 462.Nataro, J. P., S. Balepur, S. Hicks, and A. D. Phillips. 1996. Unpublished data.
- 463.Nataro, J. P., and Y. Deng. 1993. Unpublished data.
- 464.Nataro J P, Deng Y, Maneval D R, German A L, Martin W C, Levine M M. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect Immun. 1992;60:2297–2304. doi: 10.1128/iai.60.6.2297-2304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Nataro J P, Hicks S, Phillips A D, Vial P A, Sears C L. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–4768. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Nataro J P, Kaper J B, Robins Browne R, Prado V, Vial P, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 467.Nataro J P, Maher K O, Mackie P, Kaper J B. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1987;55:2370–2377. doi: 10.1128/iai.55.10.2370-2377.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Nataro J P, Scaletsky I C, Kaper J B, Levine M M, Trabulsi L R. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985;48:378–383. doi: 10.1128/iai.48.2.378-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Nataro J P, Seriwatana J, Fasano A, Maneval D R, Guers L D, Noriega F, Dubovsky F, Levine M M, Morris J G., Jr Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect Immun. 1995;63:4721–4728. doi: 10.1128/iai.63.12.4721-4728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Nataro J P, Yikang D, Cookson S, Cravioto A, Savarino S J, Guers L D, Levine M M, Tacket C O. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 471.Nataro J P, Yikang D, Giron J A, Savarino S J, Kothary M H, Hall R. Aggregative adherence fimbria I expression in enteroaggregative Escherichia coli requires two unlinked plasmid regions. Infect Immun. 1993;61:1126–1131. doi: 10.1128/iai.61.3.1126-1131.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Nataro J P, Yikang D, Yingkang D, Walker K. AggR, a transcriptional activator of aggregative adherence fimbria I expression in enteroaggregative Escherichia coli. J Bacteriol. 1994;176:4691–4699. doi: 10.1128/jb.176.15.4691-4699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Navarro-Garcia, F., C. Eslava, J. P. Nataro, and A. Cravioto. 1997. Unpublished data.
- 474.Neill M A. Pathogenesis of Escherichia coli O157:H7 infection. Curr Opin Infect Dis. 1994;7:295–303. [Google Scholar]
- 475.Newland J W, Neill R J. DNA probes for Shiga-like toxins I and II and for toxin-converting bacteriophages. J Clin Microbiol. 1988;26:1292–1297. doi: 10.1128/jcm.26.7.1292-1297.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Nishikawa Y, Scotland S M, Smith H R, Willshaw G A, Rowe B. Catabolite repression of the adhesion of Vero cytotoxin-producing Escherichia coli of serogroups O157 and O111. Microb Pathog. 1995;18:223–229. doi: 10.1016/s0882-4010(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 477.Nzegwu H C, Levin R J. Neurally maintained hypersecretion in undernourished rat intestine activated by E. coli STa enterotoxin and cyclic nucleotides in vitro. J Physiol (London) 1994;479:159–169. doi: 10.1113/jphysiol.1994.sp020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Nzegwu H C, Levin R J. Luminal capsaicin inhibits fluid secretion induced by enterotoxin E-coli STa, but not by carbachol, in vivo in rat small and large intestine. Exp Physiol. 1996;81:313–315. doi: 10.1113/expphysiol.1996.sp003935. [DOI] [PubMed] [Google Scholar]
- 479.O’Brien A D, Holmes R K. Shiga and Shiga-like toxins. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.O’Brien A D, Holmes R K. Protein toxins of Escherichia coli and Salmonella. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 2788–2802. [Google Scholar]
- 481.O’Brien A D, LaVeck G D, Griffin D E, Thompson M R. Characterization of Shigella dysenteriae 1 (Shiga) toxin purified by anti-Shiga toxin affinity chromatography. Infect Immun. 1980;30:170–179. doi: 10.1128/iai.30.1.170-179.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.O’Brien A D, LaVeck G D, Thompson M R, Formal S B. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982;146:763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- 483.O’Brien A D, Lively T A, Chen M E, Rothman S W, Formal S B. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (Shiga) like cytotoxin. Lancet. 1983;i:702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- 484.O’Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 485.O’Brien A D, Tesh V L, Donohue-Rolfe A, Jackson M P, Olsnes S, Sandvig K, Lindberg A A, Keusch G T. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr Top Microbiol Immunol. 1992;180:65–94. doi: 10.1007/978-3-642-77238-2_4. [DOI] [PubMed] [Google Scholar]
- 486.O’Brien A D, Thompson M R, Cantey J R, Formal S B. Abstracts of the 77th Annual Meeting of the American Society for Microbiology 1977. Washington, D.C: American Society for Microbiology; 1977. Production of a Shigella dysenteriae-like toxin by pathogenic Escherichia coli, abstr. B-103. [Google Scholar]
- 487.Oelschlaeger T A, Barrett T J, Kopecko D J. Some structures and processes of human epithelial cells involved in uptake of enterohemorrhagic Escherichia coli O157:H7 strains. Infect Immun. 1994;62:5142–5150. doi: 10.1128/iai.62.11.5142-5150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Ojeda A, Prado V, Martinez J, Arellano C, Borczyk A, Johnson W, Lior H, Levine M M. Sorbitol-negative phenotype among enterohemorrhagic Escherichia coli strains of different serotypes and from different sources. J Clin Microbiol. 1995;33:2199–2201. doi: 10.1128/jcm.33.8.2199-2201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Okrend A J G, Rose B E, Bennett B. A screening method for the isolation of Escherichia coli O157:H7 from ground beef. J Food Prot. 1990;53:249–252. doi: 10.4315/0362-028X-53.3.249. [DOI] [PubMed] [Google Scholar]
- 490.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Olsvik O, Rimstad E, Hornes E, Strockbine N, Wasteson Y, Lund A, Wachsmuth K. A nested PCR followed by magnetic separation of amplified fragments for detection of Escherichia coli Shiga-like toxin genes. Mol Cell Probes. 1991;5:429–435. doi: 10.1016/s0890-8508(05)80014-3. [DOI] [PubMed] [Google Scholar]
- 492.Olsvik O, Strockbine N A. PCR detection of heat-stable, heat-labile, and Shiga-like toxin genes in Escherichia coli. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Rochester, N.Y: Mayo Foundation; 1993. pp. 271–276. [Google Scholar]
- 493.Orr P, Milley D, Colby D, Fast M. Prolonged fecal excretion of verotoxin-producing Escherichia coli following diarrheal illness. Clin Infect Dis. 1994;19:796–797. doi: 10.1093/clinids/19.4.796. [DOI] [PubMed] [Google Scholar]
- 494.Orskov I, Orskov F, Birth-Anderson A, Kanamore M, Svanborg-Eden C. O, K, H and fimbrial antigens in E. coli serotypes associated with pyelonephritis and cystitis. Scand J Infect Dis Suppl. 1982;33:18–25. [PubMed] [Google Scholar]
- 495.Oswald E, Pohl P, Jacquemin E, Lintermans P, van Muylem K, O’Brien A D, Mainil J. Specific DNA probes to detect Escherichia coli strains producing cytotoxic necrotising factor type 1 or type 2. J Med Microbiol. 1994;40:428–434. doi: 10.1099/00222615-40-6-428. [DOI] [PubMed] [Google Scholar]
- 496.Padhye N V, Doyle M P. Production and characterization of a monoclonal antibody specific for enterohemorrhagic Escherichia coli of serotypes O157:H7 and O26:H11. J Clin Microbiol. 1991;29:99–103. doi: 10.1128/jcm.29.1.99-103.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Padhye N V, Doyle M P. Rapid procedure for detecting enterohemorrhagic Escherichia coli O157:H7 in food. Appl Environ Microbiol. 1991;57:2693–2698. doi: 10.1128/aem.57.9.2693-2698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Pai C H, Kelly J K, Meyers G L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Pal T, Al-Sweith N A, Herpay M, Chugh T D. Identification of enteroinvasive Escherichia coli and Shigella strains in pediatric patients by an IpaC-specific enzyme-linked immunosorbent assay. J Clin Microbiol. 1997;35:1757–1760. doi: 10.1128/jcm.35.7.1757-1760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Park C H, Gates K M, Vandel N M, Hixon D L. Isolation of Shiga-like toxin producing Escherichia coli (O157 and non-O157) in a community hospital. Diagn Microbiol Infect Dis. 1996;26:69–72. doi: 10.1016/s0732-8893(96)00180-0. [DOI] [PubMed] [Google Scholar]
- 501.Park C H, Hixon D L, Morrison W L, Cook C B. Rapid diagnosis of enterohemorrhagic Escherichia coli O157:H7 directly from fecal specimens using immunofluorescence stain. Am J Clin Pathol. 1994;101:91–94. doi: 10.1093/ajcp/101.1.91. [DOI] [PubMed] [Google Scholar]
- 502.Park C H, Vandel N M, Hixon D L. Rapid immunoassay for detection of Escherichia coli O157 directly from stool specimens. J Clin Microbiol. 1996;34:988–990. doi: 10.1128/jcm.34.4.988-990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Paros M, Tarr P I, Kim H, Besser T E, Hancock D D. A comparison of human and bovine Escherichia coli O157:H7 isolates by toxin genotype, plasmid profile, and bacteriophage lambda-restriction fragment length polymorphism profile. J Infect Dis. 1993;168:1300–1303. doi: 10.1093/infdis/168.5.1300. [DOI] [PubMed] [Google Scholar]
- 504.Parsot C, Sansonetti P J. Invasion and the pathogenesis of Shigella infections. Curr Top Microbiol. 1996;209:25–42. doi: 10.1007/978-3-642-85216-9_2. [DOI] [PubMed] [Google Scholar]
- 505.Paton A W, Paton J C, Goldwater P N, Manning P A. Direct detection of Escherichia coli Shiga-like toxin genes in primary fecal cultures by polymerase chain reaction. J Clin Microbiol. 1993;31:3063–3067. doi: 10.1128/jcm.31.11.3063-3067.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Paton A W, Ratcliff R M, Doyle R M, Seymour-Murray J, Davos D, Lanser J A, Paton J C. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J Clin Microbiol. 1996;34:1622–1627. doi: 10.1128/jcm.34.7.1622-1627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Paton J C, Paton A W. Survival rate of mice after transient colonization with Escherichia coli clones carrying variant Shiga-like toxin type II operons. Microb Pathog. 1996;20:377–383. doi: 10.1006/mpat.1996.0035. [DOI] [PubMed] [Google Scholar]
- 508.Paul M, Tsukamoto T, Ghosh A R, Bhattacharya S K, Manna B, Chakrabarti S, Nair G B, Sack D A, Sen D, Takeda Y. The significance of enteroaggregative Escherichia coli in the etiology of hospitalized diarrhoea in Calcutta, India, and the demonstration of a new honey-combed pattern of aggregative adherence. FEMS Microbiol Lett. 1994;117:319–326. doi: 10.1111/j.1574-6968.1994.tb06786.x. [DOI] [PubMed] [Google Scholar]
- 509.Paulozzi L J, Johnson K E, Kamahele L M, Clausen C R, Riley L W, Helgerson S D. Diarrhea associated with adherent enteropathogenic Escherichia coli in an infant and toddler center, Seattle, Washington. Pediatrics. 1986;77:296–300. [PubMed] [Google Scholar]
- 510.Pavia A T, Nichols C R, Green D P, Tauxe R V, Mottice S, Greene K D, Wells J G, Siegler R L, Brewer E D, Hannon D, Blake P A. Hemolytic-uremic syndrome during an outbreak of Escherichia coli O157:H7 infections in institutions for mentally retarded persons: clinical and epidemiologic observations. J Pediatr. 1990;116:544–551. doi: 10.1016/s0022-3476(05)81600-2. [DOI] [PubMed] [Google Scholar]
- 511.Peltola H, Siitonen A, Kyrönseppä H, Simula I, Mattila L, Oksanen P, Kataja M J, Cadoz M. Prevention of travellers’ diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet. 1991;338:1285–1289. doi: 10.1016/0140-6736(91)92590-x. [DOI] [PubMed] [Google Scholar]
- 512.Peres S Y, Marches O, Daigle F, Nougayrede J, Herault F, Tasca C, de Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 513.Perez-Casal P, Swartley J S, Scott J R. Gene encoding the major subunit of CS1 pili of human enterotoxigenic Escherichia coli. Infect Immun. 1990;S8:3594–3600. doi: 10.1128/iai.58.11.3594-3600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 515.Pickering L K, Obrig T G, Stapleton F B. Hemolytic-uremic syndrome and enterohemorrhagic Escherichia coli. Pediatr Infect Dis J. 1994;13:459–476. doi: 10.1097/00006454-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 516.Pickett C L, Cottle D L, Pesci E C, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1997;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp cdtb genes. Infect Immun. 1997;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Pickett C L, Twiddy E M, Coker C, Holmes R K. Cloning, nucleotide sequence, and hybridization studies of the type IIb heat-labile enterotoxin gene of Escherichia coli. J Bacteriol. 1989;171:4945–4952. doi: 10.1128/jb.171.9.4945-4952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Pierard D, Huyghens L, Lauwers S, Lior H. Diarrhoeae associated with Escherichia coli producing porcine oedema disease verotoxin. Lancet. 1991;338:762. doi: 10.1016/0140-6736(91)91487-f. [DOI] [PubMed] [Google Scholar]
- 520.Pierard D, Van Etterijck R, Breynaert J, Moriau L, Lauwers S. Results of screening for verocytotoxin-producing Escherichia coli in faeces in Belgium. Eur J Clin Microbiol Infect Dis. 1990;9:198–201. doi: 10.1007/BF01963837. [DOI] [PubMed] [Google Scholar]
- 521.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assman P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Cell. 1983;47:323–330. [Google Scholar]
- 522.Poitrineau P, Forestier C, Meyer M, Jallat C, Rich C, Malpuech G, De Champs C. Retrospective case-control study of diffusely adhering Escherichia coli and clinical features in children with diarrhea. J Clin Microbiol. 1995;33:1961–1962. doi: 10.1128/jcm.33.7.1961-1962.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Pollard D R, Johnson W M, Lior H, Tyler S D, Rozee K R. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J Clin Microbiol. 1990;28:540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Polotsky Y E, Dragunskaya E M, Seliverstova V G, Avdeeva T A, Chakhutinskaya M G, Kétyi I, Vertényi A, Ralovich B, Emody L, Málovics I, Safonova N V, Snigirevskaya E S, Karyagina E I. Pathogenic effect of enterotoxigenic Escherichia coli and Escherichia coli causing infantile diarrhea. Acta Microbiol Acad Sci Hung. 1977;24:221–236. [PubMed] [Google Scholar]
- 525.Promed. 18 January 1997. E. coli O157, advice to lab workers—UK.
- 526.Proulx F, Turgeon J P, Delage G, Lafleur L, Chicoine L. Randomized, controlled trial of antibiotic therapy for Escherichia coli O157:H7 enteritis. J Pediatr. 1992;121:299–303. doi: 10.1016/s0022-3476(05)81209-0. [DOI] [PubMed] [Google Scholar]
- 527.Rabinowitz R P, Lai L-C, Jarvis K, McDaniel T K, Kaper J B, Stone K D, Donnenberg M S. Attaching and effacing of host cells by enteropathogenic Escherichia coli in the absence of detectable tyrosine kinase mediated signal transduction. Microb Pathog. 1996;21:157–171. doi: 10.1006/mpat.1996.0051. [DOI] [PubMed] [Google Scholar]
- 528.Rademaker C M A, Martinez-Martinez L, Perea E J, Jansze M, Fluit A C, Glerum J H, Verhoef J. Detection of enterovirulent Escherichia coli associated with diarrhoea in Seville, southern Spain, with non-radioactive DNA probes. J Med Microbiol. 1993;38:87–89. doi: 10.1099/00222615-38-2-87. [DOI] [PubMed] [Google Scholar]
- 529.Raghubeer E V, Matches J R. Temperature range for growth of Escherichia coli serotype O157:H7 and selected coliforms in E. coli medium. J Clin Microbiol. 1990;28:803–805. doi: 10.1128/jcm.28.4.803-805.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Ramotar K, Waldhart B, Church D, Szumski R, Louie T J. Direct detection of verotoxin-producing Escherichia coli in stool samples by PCR. J Clin Microbiol. 1995;33:519–524. doi: 10.1128/jcm.33.3.519-524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Rao G G, Saunders B P, Masterton R G. Laboratory acquired verotoxin producing Escherichia coli (VTEC) infection. J Hosp Infect. 1996;33:228–230. doi: 10.1016/s0195-6701(96)90008-0. [DOI] [PubMed] [Google Scholar]
- 532.Rao M C, de Jonge H R. Ca and phospholipid-dependent protein kinases. In: Lebenthal E, Duffey M, editors. Textbook of secretory diarrhea. New York, N.Y: Raven Press; 1990. pp. 209–232. [Google Scholar]
- 533.Rasheed J K, Guzman-Versezco L-M, Kupersztoch Y M. Two precursors of the heat-stable enterotoxin of Escherichia coli: evidence of extracellular processing. Mol Microbiol. 1990;4:265–273. doi: 10.1111/j.1365-2958.1990.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 534.Reymond D, Johnson R P, Karmali M A, Petric M, Winkler M, Johnson S, Rahn K, Renwick S, Wilson J, Clarke R C, Spika J. Neutralizing antibodies to Escherichia coli vero cytotoxin 1 and antibodies to O157 lipopolysaccharide in healthy farm family members and urban residents. J Clin Microbiol. 1996;34:2053–2057. doi: 10.1128/jcm.34.9.2053-2057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Reymond D, Karmali M A, Clarke I, Winkler M, Petric M. Comparison of the western blot assay with the neutralizing antibody and enzyme-linked immunosorbent assays for measuring antibody to verocytotoxin 1. J Clin Microbiol. 1997;35:609–613. doi: 10.1128/jcm.35.3.609-613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Richardson S E, Rotman T A, Jay V, Smith C R, Becker L E, Petric M, Olivieri N F, Karmali M A. Experimental verocytotoxemia in rabbits. Infect Immun. 1992;60:4154–4167. doi: 10.1128/iai.60.10.4154-4167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Ridell J, Siitonen A, Paulin L, Lindroos O, Korkeala H, Albert M J. Characterization of Hafnia alvei by biochemical tests, random amplified polymorphic DNA PCR and partial sequencing of 16S rRNA gene. J Clin Microbiol. 1995;33:2372–2376. doi: 10.1128/jcm.33.9.2372-2376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Ridell J, Siitonen A, Paulin L, Mattila L, Korkeala H, Albert M J. Hafnia alvei in stool specimens from patients with diarrhea and healthy controls. J Clin Microbiol. 1994;32:2335–2337. doi: 10.1128/jcm.32.9.2335-2337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 540.Robins-Browne R, Still C S, Miliotis M D, Richardson N J, Koornhof H J, Frieman I, Schoub B D, Lecatsas G, Hartman E. Summer diarrhoea in African infants and children. Arch Dis Child. 1980;55:923–928. doi: 10.1136/adc.55.12.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Robins-Browne R M. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev Infect Dis. 1987;9:28–53. doi: 10.1093/clinids/9.1.28. [DOI] [PubMed] [Google Scholar]
- 542.Robins-Browne R M, Levine M M, Rowe B, Gabriel E M. Failure to detect conventional enterotoxins in classical enteropathogenic (serotyped) Escherichia coli strains of proven pathogenicity. Infect Immun. 1982;38:798–801. doi: 10.1128/iai.38.2.798-801.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Rogers K B. The spread of infantile gastroenteritis in a cubicled ward. J Hyg. 1951;49:140–151. doi: 10.1017/s002217240004403x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 546.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Rothbaum R, McAdams A J, Giannella R, Partin J C. A clinicopathological study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982;83:441–454. [PubMed] [Google Scholar]
- 548.Rowe P C, Orrbine E, Ogborn M, Wells G A, Winther W, Lior H, Manuel D, McLaine P N. Epidemic Escherichia coli O157:H7 gastroenteritis and hemolytic-uremic syndrome in a Canadian Inuit community: intestinal illness in family members as a risk factor. J Pediatr. 1994;124:21–26. doi: 10.1016/s0022-3476(94)70249-7. [DOI] [PubMed] [Google Scholar]
- 549.Rüssmann H, Schmidt H, Heesemann J, Caprioli A, Karch H. Variants of Shiga-like toxin II constitute a major toxin component in Escherichia coli O157 strains from patients with haemolytic uraemic syndrome. J Med Microbiol. 1994;40:338–343. doi: 10.1099/00222615-40-5-338. [DOI] [PubMed] [Google Scholar]
- 550.Ryder R W, Sack D A, Kapikian A Z, McLaughlin J C, Chakraborty J, Mizanur Rahman A S, Merson M H, Wells J G. Enterotoxigenic Escherichia coli and reovirus-like agent in rural Bangladesh. Lancet. 1976;i:659–663. doi: 10.1016/s0140-6736(76)92776-8. [DOI] [PubMed] [Google Scholar]
- 551.Sajjan S U, Forstner J F. Characteristics of binding of Escherichia coli serotype O157:H7 strain CL-49 to purified intestinal mucin. Infect Immun. 1990;58:860–867. doi: 10.1128/iai.58.4.860-867.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Samadpour M. Molecular epidemiology of Escherichia coli O157:H7 by restriction fragment length polymorphism using Shiga-like toxin genes. J Clin Microbiol. 1995;33:2150–2154. doi: 10.1128/jcm.33.8.2150-2154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Samadpour M, Grimm L M, Desai B, Alfi D, Ongerth J E, Tarr P I. Molecular epidemiology of Escherichia coli O157:H7 strains by bacteriophage lambda restriction fragment length polymorphism analysis: application to a multistate foodborne outbreak and a day-care center cluster. J Clin Microbiol. 1993;31:3179–3183. doi: 10.1128/jcm.31.12.3179-3183.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Samadpour M, Ongerth J E, Liston J, Tran N, Nguyen D, Whittam T S, Wilson R A, Tarr P I. Occurrence of Shiga-like toxin-producing Escherichia coli in retail fresh seafood, beef, lamb, pork, and poultry from grocery stores in Seattle, Washington. Appl Environ Microbiol. 1994;60:1038–1040. doi: 10.1128/aem.60.3.1038-1040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Sanderson M W, Gay J M, Hancock D D, Gay C C, Fox L K, Besser T E. Sensitivity of bacteriologic culture for detection of Escherichia coli O157:H7 in bovine feces. J Clin Microbiol. 1995;33:2616–2619. doi: 10.1128/jcm.33.10.2616-2619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Sandvig K, Van Deurs B. Endocytosis and intracellular sorting of ricin and Shiga toxin. FEBS Lett. 1994;346:99–102. doi: 10.1016/0014-5793(94)00281-9. [DOI] [PubMed] [Google Scholar]
- 557.Sanger J M, Chang R, Ashton F, Kaper J B, Sanger J W. Novel form of actin-based motility transports bacteria on the surface of infected cells. Cell Motil Cytoskeleton. 1996;34:279–287. doi: 10.1002/(SICI)1097-0169(1996)34:4<279::AID-CM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 558.Sansonetti P J. Molecular and cellular biology of Shigella flexneri invasiveness: from cell assay systems to shigellosis. Curr Top Microbiol Immunol. 1992;180:1–19. doi: 10.1007/978-3-642-77238-2_1. [DOI] [PubMed] [Google Scholar]
- 559.Sansonetti P J. Escherichia coli, Shigella, antibiotic-associated diarrhea, and prevention and treatment of gastroenteritis. Curr Opin Infect Dis. 1992;5:66–73. [Google Scholar]
- 560.Sasakawa C, Buysse J M, Watanabe H. The large virulence plasmid of Shigella. Curr Top Microbiol Immunol. 1992;180:21–44. doi: 10.1007/978-3-642-77238-2_2. [DOI] [PubMed] [Google Scholar]
- 561.Savarino S J, Fasano A, Robertson D C, Levine M M. Enteroaggregative Escherichia coli elaborate a heat-stable enterotoxin demonstrable in an in vitro rabbit intestinal model. J Clin Invest. 1991;87:1450–1455. doi: 10.1172/JCI115151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Savarino S J, Fasano A, Watson J, Martin B M, Levine M M, Guandalini S, Guerry P. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci USA. 1993;90:3093–3097. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Savarino S J, Fox P, Yikang D, Nataro J P. Identification and characterization of a gene cluster mediating enteroaggregative Escherichia coli aggregative adherence fimbria I biogenesis. J Bacteriol. 1994;176:4949–4957. doi: 10.1128/jb.176.16.4949-4957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Savarino S J, McVeigh A, Watson J, Molina J, Cravioto A, Echeverria P, Bhan M K, Levine M M, Fasano A. Enteroaggregative Escherichia coli heat-stable enterotoxin is not restricted to enteroaggregative Escherichia coli. J Infect Dis. 1996;173:1019–1022. doi: 10.1093/infdis/173.4.1019. [DOI] [PubMed] [Google Scholar]
- 565.Savkovic S D, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli (EPEC) Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 567.Scaletsky I C A, Gatti M S V, Da Silveira J F, DeLuca I M S, Freymuller E, Travassos L R. Plasmid coding for drug resistance and invasion of epithelial cells in enteropathogenic Escherichia coli O111:H- Microb Pathog. 1995;18:387–399. doi: 10.1006/mpat.1995.0035. [DOI] [PubMed] [Google Scholar]
- 568.Scaletsky I C A, Silva M L M, Trabulsi L R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Schauer D B, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Schauer D B, Falkow S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect Immun. 1993;61:4654–4661. doi: 10.1128/iai.61.11.4654-4661.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Schmidt H, Beutin L, Karch H. Hemolytic properties of enterohemorrhagic Escherichia coli O157 strain EDL 933. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 279–282. [Google Scholar]
- 572.Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995;63:1055–1061. doi: 10.1128/iai.63.3.1055-1061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Schmidt H, Karch H. Enterohemolytic phenotypes and genotypes of Shiga toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic uremic syndrome. J Clin Microbiol. 1996;34:2364–2367. doi: 10.1128/jcm.34.10.2364-2367.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Schmidt H, Karch H, Beutin L. The large-sized plasmids of enterohemorrhagic Escherichia coli O157 strains encode hemolysins which are presumably members of the E. coli α-hemolysin family. FEMS Microbiol Lett. 1994;117:189–196. doi: 10.1111/j.1574-6968.1994.tb06763.x. [DOI] [PubMed] [Google Scholar]
- 575.Schmidt H, Kernbach C, Karch H. Analysis of the EHEC hly operon and its location in the physical map of the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology. 1996;142:907–914. doi: 10.1099/00221287-142-4-907. [DOI] [PubMed] [Google Scholar]
- 576.Schmidt H, Knop C, Franke S, Aleksic S, Heeseman J, Karch H. Development of PCR for screening of enteroaggregative Escherichia coli. J Clin Microbiol. 1995;33:701–705. doi: 10.1128/jcm.33.3.701-705.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Schmidt H, Montag M, Bockemühl J, Heesemann J, Karch H. Shiga-like toxin II-related cytotoxins in Citrobacter freundii strains from humans and beef samples. Infect Immun. 1993;61:534–543. doi: 10.1128/iai.61.2.534-543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Schmidt H, Rüssmann H, Karch H. Virulence determinants in nontoxinogenic Escherichia coli O157 strains that cause infantile diarrhea. Infect Immun. 1993;61:4894–4898. doi: 10.1128/iai.61.11.4894-4898.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Schoolnik G K. PCR detection of Shigella species and enteroinvasive Escherichia coli. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Rochester, N.Y: Mayo Foundation; 1993. pp. 277–281. [Google Scholar]
- 580.Schroeder S A, Caldwell J R, Vernon T M, White P C, Granger S I, Bennett J V. A waterborne outbreak of gastroenteritis in adults associated with Escherichia coli. Lancet. 1968;i:737–740. doi: 10.1016/s0140-6736(68)92181-8. [DOI] [PubMed] [Google Scholar]
- 581.Schultsz C. Detection of enterotoxigenic Escherichia coli in stool samples by using nonradioactively labeled oligonucleotide DNA probes and PCR. J Clin Microbiol. 1994;1994:2393–2397. doi: 10.1128/jcm.32.10.2393-2397.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Scotland S M, Flowman R H, Rowe B. Evaluation of a reversed passive latex agglutination test for detection of Escherichia coli heat-labile toxin in culture supernatants. J Clin Microbiol. 1989;27:339–340. doi: 10.1128/jcm.27.2.339-340.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Scotland S M, Knutton S, Said B, Rowe B. Adherence to Caco-2 cells of Vero cytotoxin-producing strains of Escherichia coli belonging to serogroups other than O157. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 257–260. [Google Scholar]
- 584.Scotland S M, Richmond J E, Rowe B. Adhesion of enteropathogenic Escherichia coli (EPEC) to HEp-2 cells is not dependent on the presence of fimbriae. FEMS Microbiol Lett. 1983;20:191–195. [Google Scholar]
- 585.Scotland S M, Smith H R, Cheasty T, Said B, Willshaw G A, Stokes N, Rowe B. Use of gene probes and adhesion tests to characterize Escherichia coli belonging to enteropathogenic serogroups isolated in the United Kingdom. J Med Microbiol. 1996;44:438–443. doi: 10.1099/00222615-44-6-438. [DOI] [PubMed] [Google Scholar]
- 586.Scotland S M, Smith H R, Rowe B. Escherichia coli O128 strains from infants with diarrhea commonly show localized adhesion and positivity in the fluorescent-actin staining test but do not hybridize with an enteropathogenic E. coli adherence factor probe. Infect Immun. 1991;59:1569–1571. doi: 10.1128/iai.59.4.1569-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Scotland S M, Smith H R, Willshaw G A, Rowe B. Vero cytotoxin production in strain of Escherichia coli is determined by genes carried on bacteriophage. Lancet. 1983;ii:216. doi: 10.1016/s0140-6736(83)90192-7. [DOI] [PubMed] [Google Scholar]
- 588.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Senerwa D, Olsvik O, Mutanda L N, Lindqvist K J, Gathuma J M, Fossum K, Wachsmuth K. Enteropathogenic Escherichia coli serotype O111:HNT isolated from preterm neonates in Nairobi, Kenya. J Clin Microbiol. 1989;27:1307–1311. doi: 10.1128/jcm.27.6.1307-1311.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Sherman P, Cockerill F, Soni R, Brunton J. Outer membranes are competitive inhibitors of Escherichia coli O157:H7 adherence to epithelial cells. Infect Immun. 1991;59:890–899. doi: 10.1128/iai.59.3.890-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Sherman P, Drumm B, Karmali M, Cutz E. Adherence of bacteria to the intestine in sporadic cases of enteropathogenic Escherichia coli-associated diarrhea in infants and young children: a prospective study. Gastroenterology. 1989;96:86–94. doi: 10.1016/0016-5085(89)90768-3. [DOI] [PubMed] [Google Scholar]
- 593.Shimada T, Kosako Y, Isshiki Y, Hisatsune K. Enterohemorrhagic Escherichia coli O157:H7 possesses somatic (O) antigen identical with that of Salmonella O301. Curr Microbiol. 1992;25:215–217. doi: 10.1007/BF01570721. [DOI] [PubMed] [Google Scholar]
- 594.Siddons C A, Chapman P A. Detection of serum and faecal antibodies in haemorrhagic colitis caused by Escherichia coli O157. J Med Microbiol. 1993;39:408–415. doi: 10.1099/00222615-39-6-408. [DOI] [PubMed] [Google Scholar]
- 595.Siegler R L, Griffin P M, Barrett T J, Strockbine N A. Recurrent hemolytic uremic syndrome secondary to Escherichia coli O157:H7 infection. Pediatrics. 1993;91:666–668. [PubMed] [Google Scholar]
- 596.Sixma T K, Kalk K H, van Zanten B A, Dauter Z, Kingma J, Witholt B, Hol W G. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J Mol Biol. 1993;230:890–918. doi: 10.1006/jmbi.1993.1209. [DOI] [PubMed] [Google Scholar]
- 597.Sjoberg P O, Lindahl M, Porath J, Wadstrom T. Purification and characterization of CS2, a sialic acid-specific haemagglutinin of enterotoxigenic Escherichia coli. Biochem J. 1988;255:105–111. doi: 10.1042/bj2550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Sjogren R, Neill R, Rachmilewitz D, Fritz D, Newland J, Sharpnack D, Colleton C, Fondacaro J, Gemski P, Boedeker E. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology. 1994;106:306–317. doi: 10.1016/0016-5085(94)90587-8. [DOI] [PubMed] [Google Scholar]
- 599.Slutsker L, Ries A A, Greene K D, Wells J G, Hutwagener L, Griffin P M. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med. 1997;126:505–513. doi: 10.7326/0003-4819-126-7-199704010-00002. [DOI] [PubMed] [Google Scholar]
- 600.Small P L, Falkow S. Development of a DNA probe for the virulence plasmid of Shigella spp. and enteroinvasive Escherichia coli. In: Leive L, Bonventre P F, Morello J A, Silver S D, Wu W C, editors. Microbiology—1986. Washington, D.C: American Society for Microbiology; 1986. pp. 121–124. [Google Scholar]
- 601.Small P L C, Falkow S. Identification of regions on a 230-kilobase plasmid from enteroinvasive Escherichia coli that are required for entry into HEp-2 cells. Infect Immun. 1988;56:225–229. doi: 10.1128/iai.56.1.225-229.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Smith, H. R. 1997. Personal communication.
- 603.Smith H R, Scotland S M. Isolation and identification methods for Escherichia coli O157 and other Vero cytotoxin producing strains. J Clin Pathol. 1993;46:10–17. doi: 10.1136/jcp.46.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Smith H R, Scotland S M, Willshaw G A, Rowe B, Cravioto A, Eslava C. Isolates of Escherichia coli O44:H18 of diverse origin are enteroaggregative. J Infect Dis. 1994;170:1610–1613. doi: 10.1093/infdis/170.6.1610. [DOI] [PubMed] [Google Scholar]
- 605.Smith H W, Green P, Parsell Z. Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens, and pigs. J Gen Microbiol. 1983;129:3121–3137. doi: 10.1099/00221287-129-10-3121. [DOI] [PubMed] [Google Scholar]
- 606.Snyder J D, Wells J G, Yashuk J, Puhr N, Blake P A. Outbreak of invasive Escherichia coli gastroenteritis on a cruise ship. Am J Trop Med Hyg. 1984;33:281–284. doi: 10.4269/ajtmh.1984.33.281. [DOI] [PubMed] [Google Scholar]
- 607.So M, McCarthy B J. Nucleotide sequence of transposon Tn 1681 encoding a heat-stable toxin (ST) and its identification in enterotoxigenic Escherichia coli strains. Proc Natl Acad Sci USA. 1980;77:4011–4015. doi: 10.1073/pnas.77.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Sohel I, Puente J L, Murray W J, Vuopio-Varkila J, Schoolnik G K. Cloning and characterization of the bundle-forming pilin gene of enteropathogenic Escherichia coli and its distribution in Salmonella serotypes. Mol Microbiol. 1993;7:563–575. doi: 10.1111/j.1365-2958.1993.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 609.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Sordillo E M, Nusbaum M E. Screening for Escherichia coli O157:H7—a nationwide survey of clinical laboratories. J Clin Microbiol. 1996;34:1868. doi: 10.1128/jcm.34.7.1868-1869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Sowers E G, Wells J G, Strockbine N A. Evaluation of commercial latex reagents for identification of O157 and H7 antigens of Escherichia coli. J Clin Microbiol. 1996;34:1286–1289. doi: 10.1128/jcm.34.5.1286-1289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Speirs J, Stavric S, Buchanan B. Assessment of two commercial agglutination kits for detecting Escherichia coli heat-labile enterotoxin. Can J Microbiol. 1991;37:877–880. doi: 10.1139/m91-151. [DOI] [PubMed] [Google Scholar]
- 614.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 615.Stacy-Phipps S, Mecca J J, Weiss J B. Multiplex PCR assay and simple preparation method for stool specimens detect enterotoxigenic Escherichia coli DNA during the course of infection. J Clin Microbiol. 1995;33:1054–1059. doi: 10.1128/jcm.33.5.1054-1059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Staley T E, Jones E W, Corley L D. Attachment and penetration of Escherichia coli into intestinal epithelium of the ileum in newborn pigs. Am J Pathol. 1969;56:371–392. [PMC free article] [PubMed] [Google Scholar]
- 617.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Stein M A, Mathers D A, Yan H, Baimbridge K G, Finlay B B. Enteropathogenic Escherichia coli markedly decreases the resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect Immun. 1996;64:4820–4825. doi: 10.1128/iai.64.11.4820-4825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Steiner, T. S., A. A. M. Lima, J. P. Nataro, and R. L. Guerrant. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J. Infect. Dis., in press. [DOI] [PubMed]
- 620.Stevenson J S. Bact.coli D 433 in cases of diarrhoea in adults. Br Med J. 1950;2:195–196. doi: 10.1136/bmj.2.4672.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Stone K D, Zhang H-Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the BFP ultrastructure. Mol Microbiol. 1996;20:325–338. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 622.Streatfield S J, Sandkvist M, Sixma T K, Bagdasarian M, Hol W G J, Hirst T R. Intermolecular interactions between the A and B subunits of heat-labile enterotoxin from Escherichia coli promote holotoxin assembly and stability in vivo. Proc Natl Acad Sci USA. 1992;89:12140–12144. doi: 10.1073/pnas.89.24.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Strockbine, N. A., J. G. Wells, and T. J. Barrett. Isolation of STEC from clinical specimens. In J. B. Kaper and A. D. O’Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli, in press. ASM Press, Washington, D.C.
- 624.Stroeher U H, Bode L, Beutin L, Manning P A. Characterization and sequence of a 33-kDa enterohemolysin (Ehly1)-associated protein in Escherichia coli. Gene. 1993;132:89–94. doi: 10.1016/0378-1119(93)90518-8. [DOI] [PubMed] [Google Scholar]
- 625.Su C, Brandt L J. Escherichia coli O157:H7 infection in humans. Ann Intern Med. 1995;123:698–714. doi: 10.7326/0003-4819-123-9-199511010-00009. [DOI] [PubMed] [Google Scholar]
- 626.Svennerholm A-M, Åhrén C, Jertborn M. Vaccines against enterotoxigenic Escherichia coli infections. I. Oral inactivated vaccines against enterotoxigenic Escherichia coli. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 865–873. [Google Scholar]
- 627.Swerdlow D L, Woodruff B A, Brady R C, Griffin P M, Tippen S, Donnell H D, Geldreich E, Payne B J, Meyer A, Wells J G. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann Intern Med. 1992;117:812–819. doi: 10.7326/0003-4819-117-10-812. [DOI] [PubMed] [Google Scholar]
- 628.Swinbanks D. Japan shuns radishes after ‘possible link’ to E. coli. Nature. 1996;382:567. doi: 10.1038/382567b0. [DOI] [PubMed] [Google Scholar]
- 629.Szabo R A, Todd E C, Jean A. Method to isolate E. coli O157:H7 from food. J Food Prot. 1986;10:768–772. doi: 10.4315/0362-028X-49.10.768. [DOI] [PubMed] [Google Scholar]
- 630.Tacket C O, Levine M M. Vaccines against enterotoxigenic Escherichia coli infections. II. Live oral vaccines and subunit (purified fimbriae and toxin subunit) vaccines. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 875–883. [Google Scholar]
- 631.Takeda T, Tanimura M, Yoshino K, Matsuda E, Uchida H, Ikeda N. Early use of antibiotics for STEC O157 infection reduces the risk of hemolytic uremic syndrome. In: Kaper J B, O’Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli, in press. Washington, D.C: ASM Press; 1998. [Google Scholar]
- 632.Takeda Y. Shiga and Shiga-like (Vero) toxins. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 313–326. [Google Scholar]
- 633.Taniguchi T, Arita M, Sato M, Yamamoto K, Miwatani T, Honda T. Evidence that the N-terminal amino acid sequence of pilus colonization factor antigen III produced by human enterotoxigenic Escherichia coli is similar to that of TcpA pilin of Vibrio cholerae. J Infect Dis. 1994;170:1049–1050. doi: 10.1093/infdis/170.4.1049. [DOI] [PubMed] [Google Scholar]
- 634.Taniguchi T, Fujino Y, Yamamoto K, Miwatani T, Honda T. Sequencing of the gene encoding the major pilin of pilus colonization factor antigen III (CFA/III) of human enterotoxigenic Escherichia coli and evidence that CFA/III is related to type IV pili. Infect Immun. 1995;63:724–728. doi: 10.1128/iai.63.2.724-728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Tarr P I. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin Infect Dis. 1995;20:1–10. doi: 10.1093/clinids/20.1.1. [DOI] [PubMed] [Google Scholar]
- 636.Tarr, P. I. 1997. Personal communication.
- 637.Tarr P I, Fouser L S, Stapleton A E, Wilson R A, Kim H H, Vary J C, Jr, Clausen C R. Hemolytic-uremic syndrome in a six-year-old girl after a urinary tract infection with Shiga-toxin-producing Escherichia coli O103:H2. N Engl J Med. 1996;335:635–637. doi: 10.1056/NEJM199608293350905. [DOI] [PubMed] [Google Scholar]
- 638.Tarr P I, Neill M A. Perspective: The problem of non-O157:H7 Shiga toxin (verocytotoxin)-producing Escherichia coli. J Infect Dis. 1996;174:1136–1139. doi: 10.1093/infdis/174.5.1136. [DOI] [PubMed] [Google Scholar]
- 639.Tarr P I, Neill M A, Clausen C R, Watkins S L, Christie D L, Hickman R O. Escherichia coli O157:H7 and the hemolytic uremic syndrome: importance of early cultures in establishing the etiology. J Infect Dis. 1990;162:553–556. doi: 10.1093/infdis/162.2.553. [DOI] [PubMed] [Google Scholar]
- 640.Taylor C J, Hart A, Batt R M, McDougall C, McLean L. Ultrastructural and biochemical changes in human jejunal mucosa associated with enteropathogenic Escherichia coli (O111) infection. J Pediatr Gastroenterol Nutr. 1986;5:70–73. doi: 10.1097/00005176-198601000-00013. [DOI] [PubMed] [Google Scholar]
- 641.Taylor D N, Echeverria P, Sethabutr O, Pitarangi C, Leksomboon U, Blacklow N R, Rowe B, Gross R, Cross J. Clinical and microbiologic features of Shigella and enteroinvasive Escherichia coli infections detected by DNA hybridization. J Clin Microbiol. 1988;26:1362–1366. doi: 10.1128/jcm.26.7.1362-1366.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Taylor J, Wilkins M P, Payne J M. Relation of rabbit gut reaction to enteropathogenic Escherichia coli. Br J Exp Pathol. 1961;42:43–52. [PMC free article] [PubMed] [Google Scholar]
- 643.Teneberg S, Hirst T R, Ångström J, Karlsson K-A. Comparison of the glycolipid-binding specificities of cholera toxin and porcine Escherichia coli heat-labile enterotoxin: identification of a receptor-active non-ganglioside glycolipid for the heat-labile toxin in infant rabbit small intestine. Glycoconjugate J. 1994;11:533–540. doi: 10.1007/BF00731304. [DOI] [PubMed] [Google Scholar]
- 644.Tennant J M, Mattick J S. Type 4 fimbriae. In: Klemm P, editor. Fimbriae: adhesion, genetics, biogenesis and vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 127–146. [Google Scholar]
- 645.Tesh V L, O’Brien A D. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol Microbiol. 1991;5:1817–1822. doi: 10.1111/j.1365-2958.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 646.Tesh V L, Ramegowda B, Samuel J E. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine peritoneal macrophages. Infect Immun. 1994;62:5085–5094. doi: 10.1128/iai.62.11.5085-5094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Thomas A, Cheasty T, Chart H, Rowe B. Isolation of vero cytotoxin-producing Escherichia coli serotypes O9ab:H- and O101:H-carrying VT2 variant gene sequences from a patient with haemolytic uraemic syndrome. Eur J Clin Microbiol Infect Dis. 1994;13:1074–1076. doi: 10.1007/BF02111832. [DOI] [PubMed] [Google Scholar]
- 648.Thomas A, Smith H R, Rowe B. Use of digoxigenin-labelled oligonucleotide DNA probes for VT2 and VT2 human variant genes to differentiate Vero cytotoxin-producing Escherichia coli strains of serogroup O157. J Clin Microbiol. 1993;31:1700–1703. doi: 10.1128/jcm.31.7.1700-1703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Thompson J S, Hodge D S, Borczyk A A. Rapid biochemical test to identify verocytotoxin-positive strains of Escherichia coli serotype O157. J Clin Microbiol. 1990;28:2165–2168. doi: 10.1128/jcm.28.10.2165-2168.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Tobe T, Schoolnik G K, Sohel I, Bustamante V H, Puente J L. Cloning and characterization of bfpTVW, genes required for the transcriptional activation of bfpA in enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:963–975. doi: 10.1046/j.1365-2958.1996.531415.x. [DOI] [PubMed] [Google Scholar]
- 652.Tobe T, Yoshikawa M, Mizuno T, Sasakawa C. Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by VirF and repression by H-NS. J Bacteriol. 1993;175:6142–6149. doi: 10.1128/jb.175.19.6142-6149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Toledo M R, Alvariza M, Murahovschi J, Ramos S R, Trabulsi L R. Enteropathogenic Escherichia coli serotypes and endemic diarrhea in infants. Infect Immun. 1983;39:586–589. doi: 10.1128/iai.39.2.586-589.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Tornieporth N G, John J, Salgado K, de Jesus P, Latham E, Melo M C, Gunzburg S T, Riley L W. Differentiation of pathogenic Escherichia coli strains in Brazilian children by PCR. J Clin Microbiol. 1995;33:1371–1374. doi: 10.1128/jcm.33.5.1371-1374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Torres A G, Payne S M. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1997;23:825–833. doi: 10.1046/j.1365-2958.1997.2641628.x. [DOI] [PubMed] [Google Scholar]
- 656.Toth I, Barrett T J, Cohen M L, Rumschlag H S, Green J H, Wachsmuth I K. Enzyme-linked immunosorbent assay for products of the 60-megadalton plasmid of Escherichia coli serotype O157:H7. J Clin Microbiol. 1991;29:1016–1019. doi: 10.1128/jcm.29.5.1016-1019.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Toth I, Cohen M L, Rumschlag H S, Riley L W, White E H, Carr J H, Bond W W, Wachsmuth I K. Influence of the 60-megadalton plasmid on adherence of Escherichia coli O157:H7 and genetic derivatives. Infect Immun. 1990;58:1223–1231. doi: 10.1128/iai.58.5.1223-1231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Trabulsi, L. R., L. C. Campos, T. S. Whittam, T. A. T. Gomes, J. Roderigues, and A. G. Goncalves. 1996. Traditional and non-traditional enteropathogenic Escherichia coli serogroups. Rev. Microbiol. Sao Paulo 27(Suppl. 1):1–6.
- 659.Tulloch E F, Ryan K J, Formal S B, Franklin F A. Invasive enteropathic Escherichia coli dysentery. Ann Intern Med. 1973;79:13–17. doi: 10.7326/0003-4819-79-1-13. [DOI] [PubMed] [Google Scholar]
- 660.Turvill J L, Mourad F H, Perrett D, Farthing M J G. Tissue and luminal 5-HT levels in cholera toxin and E. coli heat labile toxin induced secretion. Gastroenterology. 1995;108:A333. [Google Scholar]
- 661.Tyler S D, Johnson W M, Lior H, Wang G, Rozee K R. Identification of verotoxin type 2 variant B subunit genes in Escherichia coli by the polymerase chain reaction and restriction fragment length polymorphism analysis. J Clin Microbiol. 1991;29:1339–1343. doi: 10.1128/jcm.29.7.1339-1343.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Tzipori S, Gibson R, Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989;57:1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Tzipori S, Gunzer F, Donnenberg M S, deMontigny L, Kaper J B, Donohue-Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun. 1995;63:3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Tzipori S, Karch H, Wachsmuth I K, Robins-Browne R M, O’Brien A D, Lior H, Cohen M L, Smithers J, Levine M M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987;55:3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Tzipori S, Montanaro J, Robins-Browne R M, Vial P, Gibson R, Levine M M. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect Immun. 1992;60:5302–5306. doi: 10.1128/iai.60.12.5302-5306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Tzipori, S., and J. P. Nataro. 1997. Unpublished data.
- 667.Tzipori S, Robins-Browne R M, Gonis G, Hayes J, Withers M, McCartney E. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut. 1985;26:570–578. doi: 10.1136/gut.26.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Tzschaschel B D, Guzman C A, Timmis K N, De Lorenzo V. An Escherichia coli hemolysin transport system-based vector for the export of polypeptides: export of Shiga-like toxin IIeB subunit by Salmonella typhimurium aroA. Bio/Technology. 1996;14:765–769. doi: 10.1038/nbt0696-765. [DOI] [PubMed] [Google Scholar]
- 669.Ulshen M H, Rollo J L. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N Engl J Med. 1980;302:99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- 670.Vaandrager A B, Van der Wiel E, Hom M L, Luthjens L H, de Jonge H R. Heat-stable enterotoxin receptor/guanylyl cyclase C is an oligomer consisting of functionally distinct subunits, which are non-covalently linked in the intestine. J Biol Chem. 1994;269:16409–16415. [PubMed] [Google Scholar]
- 671.Valvatne H, Sommerfelt H, Gaastra W, Bhan M K, Grewal H M S. Identification and characterization of CS20, a new putative colonization factor of enterotoxigenic Escherichia coli. Infect Immun. 1996;64:2635–2642. doi: 10.1128/iai.64.7.2635-2642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Van de Kar N C A J, Monnens L A H, Karmali M A, Van Hinsbergh V W M. Tumor necrosis factor and interleukin-1 induce expression of the verotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood. 1992;80:2755–2764. [PubMed] [Google Scholar]
- 673.Van de Kar N C A J, Sauerwein R W, Demacker P N M, Grau G E, Van Hinsbergh V W M, Monnens L A H. Plasma cytokine levels in hemolytic uremic syndrome. Nephron. 1995;71:309–313. doi: 10.1159/000188737. [DOI] [PubMed] [Google Scholar]
- 674.Van Gijsegem F, Genin S, Boucher C. Conservation of secretion pathways for pathogenicity determinants of plant and animal bacteria. Trends Microbiol. 1993;1:175–180. doi: 10.1016/0966-842x(93)90087-8. [DOI] [PubMed] [Google Scholar]
- 675.Vasselon T, Mounier J, Hellio R, Sansonetti P J. Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect Immun. 1992;60:1031–1040. doi: 10.1128/iai.60.3.1031-1040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Venkatesan M M, Buysse J M, Oaks E V. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. J Bacteriol. 1992;174:1990–2001. doi: 10.1128/jb.174.6.1990-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Verma P, Daly J A. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Surveillance and detection of enterohemorrhagic Escherichia coli toxin in a pediatric population in the intermountain West, abstr. C-358; p. 64. [Google Scholar]
- 678.Vial P A, Mathewson J J, DuPont H L, Guers L, Levine M M. Comparison of two assay methods for patterns of adherence to HEp-2 cells of Escherichia coli from patients with diarrhea. J Clin Microbiol. 1990;28:882–885. doi: 10.1128/jcm.28.5.882-885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Vial P A, Robins Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 680.Viboud G I, Binsztein N, Svennerholm A M. A new fimbrial putative colonization factor, PCFO20, in human enterotoxigenic Escherichia coli. Infect Immun. 1993;61:5190–5197. doi: 10.1128/iai.61.12.5190-5197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Viljanen M K, Peltola T, Junnila S Y T, Olkkonen L, Järvinen H, Kuistila M, Huovinen P. Outbreak of diarrhoea due to Escherichia coli O111:B4 in schoolchildren and adults: association of Vi antigen-like reactivity. Lancet. 1990;336:831–834. doi: 10.1016/0140-6736(90)92337-h. [DOI] [PubMed] [Google Scholar]
- 682.Wadolkowski E A, Sung L M, Burris J A, Samuel J E, O’Brien A D. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:3959–3965. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Wai S N, Takade A, Amako K. The hydrophobic surface protein layer of enteroaggregative Escherichia coli strains. FEMS Microbiol Lett. 1996;135:17–22. doi: 10.1111/j.1574-6968.1996.tb07960.x. [DOI] [PubMed] [Google Scholar]
- 683a.Wallace J S, Cheasty T, Jones K. Isolation of vero cytotoxin-producing Escherichia coli O157 from wild birds. J Appl Microbiol. 1997;82:399–404. doi: 10.1046/j.1365-2672.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- 684.Wanke C A, Cronan S, Goss C, Chadee K, Guerrant R L. Characterization of binding of Escherichia coli strains which are enteropathogens to small-bowel mucin. Infect Immun. 1990;58:794–800. doi: 10.1128/iai.58.3.794-800.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Wanke C A, Schorling J B, Barrett L J, Desouza M A, Guerrant R L. Potential role of adherence traits of Escherichia coli in persistent diarrhea in an urban Brazilian slum. Pediatr Infect Dis J. 1991;10:746–751. doi: 10.1097/00006454-199110000-00006. [DOI] [PubMed] [Google Scholar]
- 686.Wells J G, Shipman L D, Greene K D, Sowers E G, Green J H, Cameron D N, Downes F P, Martin M L, Griffin P M, Ostroff S M, Potter M E, Tauxe R V, Wachsmuth I K. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J Clin Microbiol. 1991;29:985–989. doi: 10.1128/jcm.29.5.985-989.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Whittam, T. S., and E. A. McGraw. 1996. Clonal analysis of EPEC serogroups. Rev. Microbiol. Sao Paulo 27(Suppl. 1):7–16.
- 688.Whittam T S, Wachsmuth I K, Wilson R A. Genetic evidence of clonal descent of Escherichia coli O157:H7 associated with hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1988;157:1124–1133. doi: 10.1093/infdis/157.6.1124. [DOI] [PubMed] [Google Scholar]
- 689.Whittam T S, Wilson R A. Genetic relationships among pathogenic Escherichia coli of serogroup O157. Infect Immun. 1988;56:2467–2473. doi: 10.1128/iai.56.9.2467-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Whittam T S, Wolfe M L, Wachsmuth I K, Orskov F, Orskov I, Wilson R A. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect Immun. 1993;61:1619–1629. doi: 10.1128/iai.61.5.1619-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Willshaw G A, Scotland S M, Smith H R, Cheasty T, Thomas A, Rowe B. Hybridization of strains of Escherichia coli O157 with probes derived from the eaeA gene of enteropathogenic E. coli and the eaeA homolog from a Vero cytotoxin-producing strain of E. coli O157. J Clin Microbiol. 1994;32:897–902. doi: 10.1128/jcm.32.4.897-902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Willshaw G A, Scotland S M, Smith H R, Rowe B. Properties of vero cytotoxin-producing Escherichia coli of human origin of O serogroups other than O157. J Infect Dis. 1992;166:797–802. doi: 10.1093/infdis/166.4.797. [DOI] [PubMed] [Google Scholar]
- 693.Willshaw G A, Smith H R, Scotland S M, Field A M, Rowe B. Heterogeneity of Escherichia coli phages encoding Vero cytotoxins: comparison of cloned sequences determining VT1 and VT2 and development of specific gene probes. J Gen Microbiol. 1987;133:1309–1317. doi: 10.1099/00221287-133-5-1309. [DOI] [PubMed] [Google Scholar]
- 694.Wilson J B, Clarke R C, Renwick S A, Rahn K, Johnson R P, Karmali M A, Lior H, Alves D, Gyles C L, Sandhu K S, McEwen S A, Spika J S. Vero cytotoxigenic Escherichia coli infection in dairy farm families. J Infect Dis. 1996;174:1021–1027. doi: 10.1093/infdis/174.5.1021. [DOI] [PubMed] [Google Scholar]
- 695.Winsor D K, Jr, Ashkenazi S, Chiovetti R, Cleary T G. Adherence of enterohemorrhagic Escherichia coli strains to a human colonic epithelial cell line (T84) Infect Immun. 1992;60:1613–1617. doi: 10.1128/iai.60.4.1613-1617.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Witham P K, Yamashiro C T, Livak K J, Batt C A. A PCR-based assay for the detection of Escherichia coli Shiga-like toxin genes in ground beef. Appl Environ Microbiol. 1996;62:1347–1353. doi: 10.1128/aem.62.4.1347-1353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Wolf M K. Occurrence, distribution, and association of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev. 1997;10:569–584. doi: 10.1128/cmr.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Wolf M K, Andrews G P, Tall B D, McConnell M M, Levine M M, Boedeker E C. Characterization of CS4 and CS6 antigenic components of PCF8775, a putative colonization factor complex from enterotoxigenic Escherichia coli E8775. Infect Immun. 1989;57:164–173. doi: 10.1128/iai.57.1.164-173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Wolf M K, DeHaan L A M, Cassels F J, Willshaw G A, Warren R, Boedeker E C, Gaastra W. The CS6 colonization factor of human enterotoxigenic Escherichia coli contains two heterologous major subunits. FEMS Microbiol Lett. 1997;148:35–42. doi: 10.1111/j.1574-6968.1997.tb10263.x. [DOI] [PubMed] [Google Scholar]
- 700.Wood L V, Ferguson L E, Hogan P, Thurman D, Morgan D, DuPont H L, Ericsson C D. Incidence of bacterial enteropathogens in foods from Mexico. Appl Environ Microbiol. 1983;46:328–332. doi: 10.1128/aem.46.2.328-332.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Wood P K, Morris J G, Small P L, Sethabutr O, Toledo M R, Trabulsi L, Kaper J B. Comparison of DNA probes and the Sereny test for identification of invasive Shigella and Escherichia coli strains. J Clin Microbiol. 1986;24:498–500. doi: 10.1128/jcm.24.3.498-500.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Wright D J, Chapman P A, Siddons C A. Immunomagnetic separation as a sensitive method for isolating Escherichia coli O157 from food samples. Epidemiol Infect. 1994;113:31–39. doi: 10.1017/s0950268800051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Wu S-X, Peng R-Q. Studies on an outbreak of neonatal diarrhea caused by EPEC O127:H6 with plasmid analysis restriction analysis and outer membrane protein determination. Acta Paediatr Scand. 1992;81:217–221. doi: 10.1111/j.1651-2227.1992.tb12207.x. [DOI] [PubMed] [Google Scholar]
- 704.Yamada S, Kai A, Kudoh Y. Serodiagnosis by passive hemagglutination test and verotyoxin enzyme-linked immunosorbent assay of toxin-producing Escherichia coli infections in patients with hemolytic-uremic syndrome. J Clin Microbiol. 1994;32:955–959. doi: 10.1128/jcm.32.4.955-959.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Yamamoto T, Echeverria P, Yokota T. Drug resistance and adherence to human intestines of enteroaggregative Escherichia coli. J Infect Dis. 1992;165:744–749. doi: 10.1093/infdis/165.4.744. [DOI] [PubMed] [Google Scholar]
- 706.Yamamoto T, Echeverria P. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect Immun. 1996;64:1441–1445. doi: 10.1128/iai.64.4.1441-1445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Yamamoto T, Kaneko M, Changchawalit S, Serichantalergs O, Ijuin S, Echeverria P. Actin accumulation associated with clustered and localized adherence in Escherichia coli isolated from patients with diarrhea. Infect Immun. 1994;62:2917–2929. doi: 10.1128/iai.62.7.2917-2929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Yamanaka H, Kameyama M, Baba T, Fujii Y, Okamoto K. Maturation pathway of Escherichia coli heat-stable enterotoxin I. Requirement of DsbA for disulfide bond formation. J Bacteriol. 1994;176:2906–2913. doi: 10.1128/jb.176.10.2906-2913.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Yolken R H, Greenberg H B, Merson M H, Sack R B, Kapikian A Z. Enzyme-linked immunosorbent assay for detection of Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1977;5:439–444. doi: 10.1128/jcm.6.5.439-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Yu H, Bruno J G. Immunomagnetic-electrochemiluminescent detection of Escherichia coli O157 and Salmonella typhimurium in foods and environmental water samples. Appl Environ Microbiol. 1996;62:587–592. doi: 10.1128/aem.62.2.587-592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Yu J, Kaper J B. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 712.Yuhan R, Koutsouris A, Hecht G. Enteropathogenic E. coli-induced phosphorylation of myosin light chain increases intestinal epithelial paracellular permeability. Gastroenterol. 1995;108:A948. doi: 10.1016/s0016-5085(97)70006-4. . (Abstract.) [DOI] [PubMed] [Google Scholar]
- 713.Zadik P M, Chapman P A, Siddons C A. Use of tellurite for the selection of verocytotoxigenic Escherichia coli O157. J Med Microbiol. 1993;39:155–158. doi: 10.1099/00222615-39-2-155. [DOI] [PubMed] [Google Scholar]
- 714.Zhang H-Z, Donnenberg M S. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Localized adherence by enteropathogenic Escherichia coli (EPEC) requires the chromosomal dsbA locus, abstr. B-312. [Google Scholar]
- 715.Zhang H-Z, Donnenberg M S. DsbA is required for stability of the type IV pilin of enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:787–797. doi: 10.1046/j.1365-2958.1996.431403.x. [DOI] [PubMed] [Google Scholar]
- 716.Zhao T, Doyle M P, Besser R E. Fate of enterohemorrhagic Escherichia coli O157:H7 in apple cider with and without preservatives. Appl Environ Microbiol. 1993;59:2526–2530. doi: 10.1128/aem.59.8.2526-2530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Zhu C, Harel J, Jacques M, Desautels C, Donnenberg M S, Beaudry M, Fairbrother J M. Virulence properties and attaching-effacing activity of Escherichia coli O45 from swine postweaning diarrhea. Infect Immun. 1994;62:4153–4159. doi: 10.1128/iai.62.10.4153-4159.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Zhu Q Y, Li L Q, Lin W M, Zhou Z J, Liu C J. Detection of genes for heat-stable enterotoxin in Escherichia coli by biotinylated ST-DNA probes. Chin Med J. 1994;107:338–341. [PubMed] [Google Scholar]
- 719.Zychlinsky A, Kenny B, Menard R, Prevost M C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]