Abstract
Human enteroviruses have traditionally been typed according to neutralization serotype. This procedure is limited by the difficulty in culturing some enteroviruses, the availability of antisera for serotyping, and the cost and technical complexity of serotyping procedures. Furthermore, the impact of information derived from enterovirus serotyping is generally perceived to be low. Enteroviruses are now increasingly being detected by PCR rather than by culture. Classical typing methods will therefore no longer be possible in most instances. An alternative means of enterovirus typing, employing PCR in conjunction with molecular genetic techniques such as nucleotide sequencing or nucleic acid hybridization, would complement molecular diagnosis, may overcome some of the problems associated with serotyping, and would provide additional information regarding the epidemiology and biological properties of enteroviruses. We argue the case for developing a molecular typing system, discuss the genetic basis of such a system, review the literature describing attempts to identify or classify enteroviruses by molecular methods, and suggest ways in which the goal of molecular typing may be realized.
The enteroviruses comprise a large genus belonging to the Picornaviridae. Sixty-six immunologically distinct serotypes are known to cause infections in humans. Most infections are mild or asymptomatic, and as a result enteroviruses are considered by many to be unimportant as human pathogens and unworthy of sustained investigation in the light of other viral infections of greater perceived public health importance. However, enteroviruses may also result in serious or even fatal disease. Enteroviruses are the most commonly implicated viral agents of acute myocarditis and aseptic meningitis. Congenital infections occur, although their frequency is unknown. Infection in neonates is frequently life-threatening. The enterovirus group includes the polioviruses (PV), the cause of paralytic poliomyelitis, which still causes significant disability in many parts of the world. There is now growing evidence that enteroviruses also cause or contribute to common chronic diseases, including dilated cardiomyopathy, one of the commonest indications for cardiac transplantation, insulin-dependent diabetes mellitus, and chronic fatigue syndrome.
Typing of viruses is required primarily to provide information on the relationship between viruses, to identify virus types with increased virulence or specific disease attributes, for epidemiological investigations, and to allow correlation with viral immunity. New molecular technologies have spawned the development of molecular typing methods for several virus groups that are not amenable to other means of investigation and have raised the possibility of simpler typing methods for other virus groups that have traditionally been typed on a phenotypic basis.
Based on their ability to replicate in cells of human and primate origin, infectivity and pathogenicity in different animal species, and antigenic differences, human enteroviruses were originally subclassified into PV serotypes 1 to 3, coxsackievirus groups A and B (CAV, serotypes 1 to 22 and 24; CBV, serotypes 1 to 6) and echoviruses (ECV; serotypes 1 to 7, 9, 11 to 27, and 29 to 33) (reviewed in references 269 and 287). The value of these distinctions has to some extent been diminished by the availability of recently described cell lines permissive for a wider range of enteroviruses and by the intrinsic variability of biological phenotypes observed among naturally occurring enterovirus strains. Since 1970, newly identified serotypes have not been assigned to the above groups but, rather, have been numerically classified as enterovirus serotypes (ENV) 68 to 71 (279).
Enterovirus infection in humans may result in a wide range of acute symptoms involving the cardiac and skeletal muscle, central nervous system (CNS), pancreas, skin, and mucous membranes (Table 1) (reviewed in references 269 and 289). Poliomyelitis caused by PV has been successfully eliminated from many parts of the world by a successful World Health Organization (WHO)-sponsored Poliomyelitis Eradication Initiative (PEI), but other enterovirus infections remain frequent and sometimes serious causes of morbidity. Enterovirus infection has also been associated with such chronic diseases as dilated cardiomyopathy and chronic myocarditis (17, 49, 54, 197, 303, 305), chronic fatigue syndrome (16, 77, 136, 482), insulin-dependent diabetes mellitus (reviewed in references 25, 26, and 481), motor neuron disease (464), and postpoliomyelitis syndrome (400). Evidence from studies in murine models indicates that chronic enterovirus infection is characterized by restricted genome replication and gene expression, although some controversy about the role of enterovirus infection in chronic disease in humans remains (reviewed in reference 301). Chronic infections also occur in immunodeficient patients (264).
TABLE 1.
Clinical manifestations of enterovirus infection
| Syndrome | Commonly implicated serotype(s) | Reference(s) |
|---|---|---|
| Asymptomatic infection | All serotypes | 223, 269 |
| Paralytic poliomyelitis | PV 1 to PV 3 | 270, 288, 290, 330 |
| ENV 70 | 225, 226, 455 | |
| ENV 71 | 75 | |
| CAV 7 | 145, 454 | |
| Aseptic meningitis/meningoencephalitis | PV, CBV, CAV, ECV | 38, 87, 263, 378, 379 |
| ENV 71 | 10, 393 | |
| Acute myocarditis | CBV | 142, 236 |
| Bornholm disease (pleurodynia) | CBV | 69, 145 |
| Hand, foot, and mouth disease | CAV 16 | 367, 460 |
| ENV 71 | 56, 151 | |
| Herpangina | CAV, CBV, ECV | 69, 145, 460 |
| Exanthem | CAV, CBV | 460 |
| Acute hemorrhagic conjunctivitis | ENV 70 | 242 |
| CAV 24 (variant) | 283 | |
| Neonatal multisystem disease | CBV, ECV | 4–6, 150, 200 |
| Nonspecific febrile/respiratory illness | CAV, CBV, ECV | 69, 86, 90 |
Due to the diversity of disease manifestations, few of which are unique to enterovirus infections, diagnoses cannot be made on clinical grounds alone. Although specific therapy for enterovirus infections is not yet available, diagnosis is nonetheless important to distinguish between enterovirus-induced disease and other treatable causes of clinically similar disease, to identify PV outbreaks, and to assess the prognosis. A number of potential benefits in patient management accrue from a specific diagnosis of enterovirus infection (reviewed in reference 67). Traditionally, enteroviruses have been detected by isolation in cell culture, and their serotypic identity has been established by neutralization of infectivity with serotype-specific antisera. However, recent developments in molecular detection technology make it probable that enterovirus diagnosis will increasingly be achieved by non-culture-based methods, particularly nucleic acid amplification methods such as PCR. This review assesses the need for a molecular system for typing enteroviruses which is compatible with molecular diagnostic methods and which might eventually replace serotyping methods. Salient aspects of enterovirus genome structure and variability, which form the genetic basis of such a molecular typing system, are discussed. Possible applications of and approaches to molecular typing for enteroviruses are also discussed.
THE NEED FOR TYPING OF ENTEROVIRUSES
In uncomplicated situations, serotypic identification of an enterovirus isolate generally takes 1 to 2 weeks and usually has little impact on patient management. Thus, for clinical diagnosis it is often sufficient to establish a diagnosis of an “enterovirus infection” without further specifying the serotype (296, 381). However, in certain situations, serotypic identification is necessary or at least useful, and with the ability to type enteroviruses more rapidly by using molecular techniques, serotypic identification will possibly have greater impact on patient management.
Correct Identification of Poliovirus
The eradication of poliomyelitis and wild PV by the year 2000 is the aim of the WHO PEI (213, 468). Polio vaccination can then be stopped, but only when the eradication of wild PV has been documented convincingly. Therefore, identifying enterovirus strains as PV or nonpolio enteroviruses (NPEV) is extremely important. Since most PV infections are asymptomatic, demonstrating the absence of cases of paralytic poliomyelitis is an unreliable marker of eradication, particularly as the goal of eradication is approached (110), and it must be accompanied by virological monitoring. However, the extensive use of live PV (Sabin) vaccine results in fecal shedding of vaccine strain virus, which can survive sewage treatment (128) and persist in the environment for several months (389). The use of the Sabin vaccine also results in rare cases of vaccine-associated paralytic poliomyelitis (21, 315), probably caused by reversion to neurovirulence of vaccine strain virus as a result of a point mutation or recombination with wild PV (239). The ability to identify wild and vaccine-derived PV in clinical and environmental samples is therefore necessary to monitor the effectiveness of polio vaccination strategies (47, 286, 341, 423), to identify and monitor PV outbreaks (81, 167, 326, 411, 444), to diagnose cases of vaccine-associated paralytic poliomyelitis, and to exclude other enteroviruses as a cause of poliomyelitis-like disease (20, 75, 104, 114, 126, 143, 144, 225, 268, 454). A network of WHO-sponsored polio laboratories has been established to coordinate this task.
Studying Relationships between Enterovirus (Sub)type and Disease Manifestation
Although the disease spectra of the different serotypes overlap considerably, certain disease syndromes are caused by one or a few serotypes. The most striking example is of course the PV, which are the main cause of paralytic poliomyelitis. A typing method which gave a complete correlation between virus type and pathogenicity or virulence for other enterovirus syndromes would be difficult to achieve and would require a more thorough knowledge of the viral determinants of pathogenesis. However, some associations between enterovirus serotype and disease syndrome have been observed (145, 293, 416), and the use of sensitive molecular detection methods has recently proved of value in further delineating these associations. Thus, most cases of enteroviral meningitis have been found to be caused by certain CBV and ECV serotypes (38) whereas most cases of viral myocarditis are associated with CBV (196, 208, 312). Such knowledge may simplify virological diagnosis and identify enterovirus serotypes and diseases for which future effort should be directed toward understanding pathogenesis and designing therapeutic or preventive measures.
Identification of New Enterovirus Types or Variants
The ability to recognize previously unknown enterovirus types (39, 393) or strains (242) provides a basis for understanding changing patterns in the epidemiology and clinical manifestations of enterovirus infections. This has been of particular importance when the new type may cause poliomyelitis-like illness (10, 75, 225). Molecular detection methods are increasingly accepted as a means of identifying and characterizing previously unknown, noncultivable microorganisms and establishing their etiologic relationship to disease (reviewed in reference 116). This approach may also identify additional enterovirus types. One recent study identified novel enterovirus sequences, possibly representing previously unrecognized enterovirus types, in patients with chronic fatigue syndrome (77, 123).
Typing of Enteroviruses in Neonates and Immunodeficient Patients
Patients with hypo- or agammaglobulinemia are susceptible to chronic enterovirus infections which may cause persistent central nervous system infection, arthritis, dermatomyositis, or chronic enteritis (296), the most severe complication being a chronic meningoencephalitis (264). Some patients have been successfully treated with intravenous or intrathecal immunoglobulins with a high neutralizing-antibody titer against the infecting enterovirus serotype (106, 112, 264, 267). Immunoglobulin therapy has also been used in limited trials to treat neonates with fulminant enterovirus infections (5). This treatment was associated with a significant reduction in levels of viremia and viruria only when the immune globulin administered had a neutralization titer to the patient’s isolate of at least 800. However, studies of immune globulin therapy in neonatal infections thus far have been too small to allow its widespread use to be recommended. Conversely, typing of enterovirus isolates has been used to exclude persistent enterovirus infection of the central nervous system in children with rapidly recurring episodes of aseptic meningitis due to repeated infection with different serotypes (9).
Epidemiological Monitoring
Enteroviruses have a worldwide distribution. Within a given geographic locality, some serotypes may be endemic, with little or only gradual change in the range of serotypes present from year to year. In temperate climates there is increased circulation in summer and early fall. In contrast, other serotypes may be introduced periodically, causing epidemics, with very few isolations reported in intervening years (294; reviewed in reference 295). Epidemiological surveillance plays a crucial role in the control of infectious diseases (356), and obtaining information on the serotypes of enteroviruses detected in clinical and environmental samples has formed an important part of surveillance programs (154, 170, 263, 416). Such knowledge may help in understanding changing patterns of enterovirus infection and disease association and may also facilitate the rapid diagnosis of enterovirus infections. This may reduce unnecessary hospitalization and other medical expenditure (11, 363), allow immune globulin batches with high titers to frequently circulating serotypes to be reserved for intravenous therapy of neonates or immunodeficient patients (5), and guide the formulation of antigens for serological diagnosis (11, 91).
CONVENTIONAL METHODS FOR DIAGNOSIS AND TYPING OF ENTEROVIRUSES AND THEIR LIMITATIONS
Enterovirus diagnosis is achieved by culturing virus from clinical specimens. When this is unsuccessful or not possible, demonstration of seroconversion, a fourfold or greater rise in type-specific virus-neutralizing antibody titers, or virus-specific immunoglobulin M (IgM) antibody in serum and/or cerebrospinal fluid (CSF) may provide evidence of infection. Methods for enterovirus diagnosis are detailed in references 138, 269, 296, and 381.
Virus Isolation
Isolation of an enterovirus from affected organs and associated body fluids (e.g., myocardium, pericardial fluid, or CSF) provides the strongest evidence of an enteroviral etiology. However, enteroviruses are rarely isolated from myocardium except in infants with fulminant infection, although the causal relationship between enteroviruses and acute myocarditis was originally established on this basis (reviewed in reference 142). Virus isolation from blood components is also useful and provides evidence of systemic infection (88, 345, 346). Detection of enterovirus in the alimentary tract (throat swabs, rectal swabs, or stool specimens) provides only circumstantial evidence of etiology, since viral shedding at these sites may occur in the absence of symptoms, particularly in infants and during epidemic seasons (223). However, stool is the most sensitive specimen for enterovirus detection, and stool culture is recommended for diagnosis of PV infections (470). Culturing enteroviruses from sewage and other environmental samples is of particular use for environmental monitoring and epidemiological studies.
Although virus isolation has been regarded as the “gold standard” for enterovirus identification, virus isolation procedures are only poorly standardized and virus isolation data may vary considerably between laboratories. Sensitivity is highly dependent on the type and quality of the specimen, the timing of specimen collection, and storage before arrival in the virus laboratory (138, 296). The choice of cell types used for enterovirus isolation is also important. No single cell line currently in use supports the growth of all known enterovirus serotypes (381). Therefore, to ensure maximal isolation efficiency, combinations of cell lines are generally used (68, 89). A presumptive identification of the enterovirus group can often be based upon growth of the isolate in different cell lines (193). Many enteroviruses grow well in primary monkey kidney cells. However, the supply of these cells is limited. CAV serotypes are difficult to grow in cell culture. Most have been propagated in RD cells (392, 459), but in practice isolation from clinical material is often unsuccessful (248). The use of suckling mice for isolation of CAV has been more successful (248), but because of practical difficulties this technique is rarely used. As a consequence, CAV infections are probably underdiagnosed and underrepresented in epidemiological surveys (416). PV may be selectively isolated in cultures of recombinant murine cells expressing the human PV receptor gene (169, 336). At present, there is no universal consensus on the optimal combination and number of cell lines required for enterovirus isolation, and different combinations are used in different laboratories.
Other factors affecting the efficiency of isolation are the physiological condition of the cells and whether blind passages are performed. Blind passages are necessary in some cases before a cytopathic effect in cell culture or paralysis in suckling mice becomes apparent. This may be due to the low initial titer of virus or the requirement for adaptation to growth in the isolation system (248). Under optimal conditions, a positive enterovirus isolation can be reported within a few days, but a presumptive diagnosis may require more than 14 days when dealing with difficult isolates and even longer for samples containing mixtures of viruses.
Enterovirus Serotyping
Following the isolation of an enterovirus, the serotypic identity can be determined by neutralization of infectivity with serotype-specific antisera. Typing by neutralization with reference antisera for all 66 serotypes individually is clearly impractical. To overcome this problem, equine type-specific hyperimmune sera have been mixed to give intersecting pools containing different combinations of individual antisera (138, 269, 391). Enterovirus isolates are incubated with each antiserum pool and then reinoculated onto susceptible cells. After incubation for several days, the neutralization pattern is recorded. From this, the enterovirus serotype can be inferred, since pools are designed so that neutralization patterns are distinct for each individual serotype. Finally, the suspected enterovirus type can be confirmed by neutralization with the type-specific single antiserum. The Lim Benyesh-Melnick (LBM) pool scheme consists of eight pools (designated A to H) containing antisera to 42 different enterovirus types (241, 271). Antibodies against 19 additional CAV serotypes in seven additional pools (designated J to P) can be used if identification with pools A to H cannot be achieved (272). Alternative intersecting pools have been developed at the National Institute of Public Health and the Environment (RIVM) in the Netherlands, which also allow identification of enterovirus types 68 to 71 (201). The RIVM pools are now used in the WHO polio laboratory network and are candidates to replace LBM pools.
There are several drawbacks to the use of intersecting pools for typing of enteroviruses. First, the method is time-consuming, labor-intensive, and costly. Second, the supply of antisera is limited, and WHO has advocated a conservative approach to the use of pools, which should not be used to type every clinical isolate (273). Third, the problem of “untypeable” enteroviruses is frequently encountered (296). There are several reasons for this. (i) Untypeable isolates may contain mixtures of enteroviruses. In the tropics, up to 50% of stool samples from infants may contain enteroviruses (224), and the frequency of mixed infections is presumably high. To resolve mixtures, isolates are purified by three serial terminal dilutions or plaque purifications. (ii) Not all enterovirus serotypes can be identified with intersecting pools (CAV 3, 11, 15, 17, and 24 and enterovirus types 68 to 71 cannot be typed using LBM pools). (iii) Enteroviruses sometimes form aggregates which can be neutralized only after treatment with sodium deoxycholate or chloroform or by ultrafiltration to dissociate the aggregates (202, 456). (iv) The isolates may be so-called prime strains. These are antigenic variants of recognized serotypes which are neutralized poorly or not at all by antiserum to the homologous prototype strain. However, antiserum raised against the prime strain will neutralize both prime and prototypic strains (269). This requires inoculation of experimental animals with the isolate to make an antiserum, which must then be tested for neutralization of the prototypes. (v) Isolates that are genuinely untypeable after excluding the above possible reasons for failure to type may represent a new or previously unrecognized enterovirus type. However, when first attempts at typing isolates using intersecting pools is unsuccessful, the additional labor and expense required to achieve serotypic identification are usually considered prohibitive and such isolates remain untyped.
The standardization of enterovirus serotyping is problematic, and results derived from the LBM typing scheme may be influenced by the experience of laboratory personnel. This is illustrated by results of a small-scale quality control scheme on enterovirus isolation. A panel of 10 samples was sent out to 17 laboratories. None of the 11 participating laboratories that performed virus isolation and serotyping reported correct results for all the samples (449).
Because of the practical problems and limited clinical relevance of enterovirus serotyping, the number of laboratories offering this service has steadily decreased. At present, typing is performed mainly in research and reference laboratories. Attempts have been made to shorten the time required to identify and type enterovirus isolates by detecting the virus by enzyme immunoassays with type-specific antisera (480), by using immunoelectron microscopy with polyvalent and type-specific antisera (237, 310), or by using early detection of enterovirus antigens in cell culture by indirect immunofluorescence or enzyme immunoassay with monoclonal antibodies to group-reactive (218, 433, 483, 484) or type-specific (27, 79, 218) determinants. Type-specific monoclonal antibodies have been found useful for identification of CBV isolates, including prime strains (229). Although these methods can provide a positive identification more rapidly than neutralization assays, none are in widespread use.
Serotyping is, however, used by the WHO polio laboratory network for the serotypic identification of PV and intratypic differentiation of vaccine or vaccine-derived strains from wild PV strains (reviewed in reference 450). Intratypic differentiation is based on the detection of antigenic differences between vaccine strains and currently circulating wild PV strains, and it may be achieved by neutralization of infectivity with cross-absorbed polyclonal antisera specific for either Sabin vaccine strains or common wild strains (327, 451, 469) or panels of vaccine-derived and/or wild PV strain-specific monoclonal antibodies (85, 101, 113, 189, 327). The neutralization test involving polyclonal antisera has now been replaced by an enzyme immunoassay (131, 327, 443), which allows a more conservative use of antisera.
Serological Diagnosis
Serological diagnosis of enterovirus infections is complicated by the large number of serotypes; the occurrence of anamnestic, heterotypic antibody responses; and the lack of a uniformly cross-reactive enterovirus group antigen. The requirement for appropriately timed paired sera to demonstrate a diagnostically significant rise in antibody titer further limits the utility of enterovirus serologic testing. The demonstration of enterovirus-specific IgM antibody in a single serum, indicative of a current antigenic stimulus, provides evidence of recent infection (11, 32, 33, 45, 59, 96, 111, 119, 256, 262, 388, 430), but results must be interpreted with caution, since background levels of IgM in the general population may be high (277), particularly during epidemic seasons. In addition, the persistence of IgM responses for some time beyond the end of acute infection has been observed in patients with chronic disease (305). Thus, while detection of IgM in serum may be more sensitive than virus isolation in detecting enterovirus infection (32, 96), this provides only circumstantial evidence of an etiologic role in concurrent symptoms. However, detection of virus-specific IgM in CSF is more likely to indicate a causal relationship to CNS symptoms and is more sensitive than isolation of PV from stool for confirming a diagnosis of paralytic poliomyelitis (311, 369). Determination of virus-specific IgM in serum has proved useful in monitoring a recent PV outbreak in the Netherlands (326) and in defining the epidemiology of NPEV outbreaks (11, 134).
ENTEROVIRUS DETECTION BY PCR
Genetic similarities among enterovirus serotypes allow the use of generic cDNA hybridization probes derived from one enterovirus serotype to detect genomes of a wide range of enteroviruses (49, 181, 198, 434; reviewed in reference 377). Hybridization technology has also been successfully used to detect enterovirus RNA in clinical material from patients with suspected enterovirus infection (16, 17, 49, 84, 107, 197). Also, the use of in situ hybridization in particular may provide useful information on the cellular distribution of virus infection and its relationship to inflammatory or necrotic lesions (83, 219, 220). However, nucleic acid hybridization technology is laborious and is not always sufficiently sensitive for diagnosis, for example, in the detection of enterovirus RNA in CSF (reviewed in reference 377). PCR has therefore been explored as a straightforward yet sensitive means of enterovirus genome detection. Comparison of published enterovirus genome sequences has allowed highly conserved sequences to be identified. These have been exploited as primer recognition sequences for reverse transcriptase PCR (RT-PCR) assays capable of detecting most or all enteroviruses, including those which cannot readily be propagated in cell culture (63, 178, 196, 376, 494) and untypeable isolates (394). When carefully optimized, these assays are at least as sensitive as cell culture systems for the detection of enteroviruses in clinical specimens (2, 7, 57, 132, 133, 153, 192, 302, 313, 324, 380, 382, 390, 427, 478, 494) and environmental samples (1, 98, 228, 348, 436, 437). Results can be obtained in as little as 5 h (382) and may therefore have a greater impact on clinical decision-making. It is also possible to detect enterovirus RNA by RT-PCR in specimens from which infectious virus is never or only rarely recovered by culture, such as myocardial tissue from patients with acute myocarditis or dilated cardiomyopathy (196, 208, 258, 395) and in formalin-fixed, paraffin-embedded tissue (161, 240, 304, 312, 358, 403, 465).
RT-PCR will therefore assume a major role in the laboratory diagnosis of enterovirus infections. An enterovirus PCR test kit for the diagnosis of enteroviral meningitis, now commercially available from Roche Molecular Systems, will assist in the transition to PCR-based diagnosis (199, 382, 478). This test is based on single-step reverse transcription and amplification of enterovirus RNA, and it incorporates enzymatic elimination of amplicon contamination, thus harmonizing and simplifying laboratory procedures and reducing the risk of false positivity due to carryover of previously amplified nucleic acid. However, quality assurance procedures will be required to ensure test sensitivity, specificity, and reliability; experience with PCR assays for other viruses has revealed considerable interlaboratory variation in sensitivity, specificity, and reliability, which is partially but not completely alleviated by the use of standardized methods or commercial assays (46, 93, 185, 351, 442, 485, 486).
A limitation of most PCR methods described thus far for the diagnosis of enterovirus infections is the inability to perform serotyping or other subclassification of enteroviruses. When such information is required, it may therefore be necessary to supplement PCR detection with traditional culture methods to provide a virus isolate for typing purposes (109). However, due to the greater sensitivity of PCR, cell culture in many cases will not yield a virus isolate. As an alternative approach, nucleotide sequence analysis of PCR products may allow the typing of viruses detected only by PCR (reviewed in reference 78). A few studies have compared the nucleotide sequence of enterovirus PCR products with published enterovirus sequences to type or classify enteroviruses in the absence of a virus isolate (19, 208, 303, 304, 312). Other methods of assessing sequence differences have also been described, including restriction fragment length polymorphism (RFLP) analysis of PCR products (PCR-RFLP) (24, 196, 313, 396), hybridization with type-specific probes (63, 94, 421), or single-strand conformation polymorphism (SSCP) analysis of PCR products (PCR-SSCP) (121). A molecular typing system based on such methods could minimize the requirement for isolating enteroviruses in culture for most common diagnostic and epidemiological applications and may provide additional biological information which could not have been obtained by serotyping methods.
GENETIC BASIS OF MOLECULAR TYPING FOR ENTEROVIRUSES
Development of a molecular typing system for enteroviruses requires an understanding of the structure and function of the enterovirus genome and a knowledge of the variability of genome sequences among enteroviruses.
Enterovirus Genome Structure
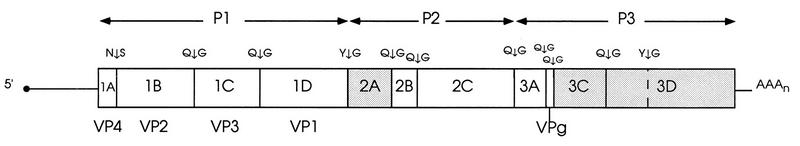
The enterovirus genome is a single-stranded RNA molecule, approximately 7,500 nucleotides long, of positive polarity (Fig. 1) (reviewed in references 160 and 383). An approximately 750-nucleotide 5′ untranslated region (5′ UTR) is followed by a long open reading frame coding for an approximately 2,100-amino-acid polyprotein. This is followed by a short 3′ untranslated region (3′ UTR) and a poly(A) tail.
FIG. 1.
Genetic organization of poliovirus type 1 (Mahoney), the type member of genus Enterovirus of Picornaviridae. The polyprotein encoded by the single open reading frame is shown as an elongated rectangle, the 5′ and 3′ noncoding regions are shown as lines, and the genome-linked protein (VPg) is indicated by a black circle. Cleavage sites between individual viral proteins are shown above the genome at appropriate locations; these proteins are described within the rectangle according to the L434 nomenclature (383a); the capsid proteins 1AB, 1A, 1B, 1C, and 1D are commonly referred to as VP0, VP4, VP2, VP3, and VP1, respectively. The proteinases 2Apro, 3Cpro, and 3CDpro are represented by shaded boxes. The structural protein precursor P1 and the nonstructural protein precursors P2 and P3 are indicated above the polyprotein. Reprinted from reference 160 with permission of the publisher.
5′ UTR.
Several important functions have been mapped to the 5′ UTR. For PV, the first 100 nucleotides (approximately) play a role in viral replication (13). Enteroviruses use internal initiation of translation rather than the ribosome-scanning model proposed for cellular mRNAs (333). This internal initiation has been shown to require a large portion (nucleotides 130 to about 600) of the PV 5′ UTR (40, 152), but the exact ribosome-binding sequence is not known. Point mutations in the 5′ UTR have been shown to affect virulence (55, 204, 278, 299, 438), temperature sensitivity, and plaque morphology (354).
Open reading frame.
The open reading frame following the 5′ UTR is translated into a polyprotein which is co- and posttranslationally cleaved to give four structural proteins (VP4, VP2, VP3, and VP1), which form the viral capsid, and seven nonstructural proteins (217). VP1 to VP3 are partially exposed on the virion surface, while VP4 is completely internalized in infectious virions. Three or four neutralization determinants have been identified for each PV serotype by using monoclonal antibody neutralization escape mutants, and they have been mapped to VP1, VP2, and/or VP3 (reviewed in reference 278). Neutralization determinants have also been identified on VP1 for CBV 3 (149) and CBV 4 (266, 359). Determinants of attenuation of virulence (52, 278, 355), virion thermostability (278), altered host range (82), in vitro cell tropism (245), persistent infection (53, 331), and plaque morphology (354, 488) have also been mapped to the capsid-encoding region.
Some functions of the nonstructural proteins and their precursor forms are known. Protein 2A is one of the viral proteinases that cleaves the polyprotein in trans between proteins VP1 and 2A and frees the capsid protein precursor from the rest of the polyprotein (432). Proteinase 2A has also been shown to participate in host cell shutoff by indirectly inducing cleavage of the cellular p220 protein, which is an important factor in cap-dependent initiation of translation (412). The specific functions of 2B and 2C are not known, although protein 2C and its precursor form 2BC have been found in the replication complex of PV (41), and protein 2C has a helicase activity. Protein 3AB is a precursor of 3B, the small polypeptide covalently linked to the 5′ UTR of picornavirus RNA molecules (115). Protein 3C is the second viral proteinase which does most of the polyprotein processing (155, 156), while 3D is the RNA-dependent RNA polymerase (447).
3′ UTR.
The coding region is followed by a 70- to 100-nucleotide 3′ UTR. This region is important in the initiation of the minus-strand RNA synthesis, but no specific sequences have been identified for polymerase binding. Several secondary and tertiary structures (pseudoknots) have been proposed for this region (182, 342) and supported by biochemical studies (186, 335). A genomic poly(A) tail with an average length of 75 nucleotides follows the 3′ UTR of all enteroviruses (18).
Genetic Relationships among Human Enteroviruses
Complete genome sequences for a number of human enterovirus serotypes have been published (51, 60, 92, 139, 173, 174, 179, 182, 190, 217, 221, 232, 246, 317, 337, 339, 353, 384, 413, 414, 419, 431, 487, 493) (Table 2). Partial sequence data are available for the majority of the remaining serotypes (23, 103, 105, 130, 177, 339, 342, 349, 372, 461) (Table 2). While sequence comparisons partially support the classical subgrouping of enterovirus serotypes into PV, CAV, CBV, and ECV, in many cases genetic relationships do not correlate with this division. Nucleotide sequence comparison of the several genomic regions indicates that while serotypes within the PV and CBV groups are genetically related, CAV and ECV are genetically diverse subgroups. ECV types 22 and 23 differ significantly from the rest of the enteroviruses genetically (179, 415). Although these viruses are still currently classified as enteroviruses (279), the International Committee on Taxonomy of Viruses Picornavirus Study Group has recently proposed that they be reclassified into a separate genus, provisionally designated human orphanovirus (HOV) types 1 and 2, respectively. The remaining ECV serotypes are genetically related to the CBV group (177).
TABLE 2.
| Enterovirus strain description | Abbreviation | GenBank accession no. | Reference |
|---|---|---|---|
| CAV 1/T.T | CAV 1 | X87584 | 349 |
| CAV 2/Fleetwood | CAV 2 | L28146 | 349 |
| CAV 3/J.Ol. | CAV 3 | X87586 | 349 |
| CAV 5/G.S. | CAV 5 | X87588 | 349 |
| CAV 7/AB-IV | CAV 7 | X87589 | 349 |
| CAV 8/C.D. | CAV 8 | X87590 | 349 |
| CAV 9/Griggs | CAV 9 | D00627 | 60 |
| CAV 10/M.K. | CAV 10 | X87591 | 349 |
| CAV 11/Belgium 1 | CAV 11 | X87592 | 349 |
| CAV 13/Flores | CAV 13 | X87594 | 349 |
| CAV 14/G-14 | CAV 14 | X87595 | 349 |
| CAV 16/G-10 | CAV 16 | U05876 | 339 |
| CAV 18/G-13 | CAV 18 | X87598 | 349 |
| CAV 20/IH Pool 35 | CAV 20 | X87600 | 349 |
| CAV 20b/Cecil P2647 | CAV 20b | X87602 | 349 |
| CAV 21/Coe | CAV 21 | D00538 | 174 |
| CAV 24/EH 24/70 | CAV 24 | D90457 | 419 |
| CBV 1/Japan | CBV 1 | M16560 | 182 |
| CBV 3/Nancy | CBV 3 | M33854 | 221 |
| CBV 4/JVB | CBV 4 | X05690 | 190 |
| CBV 5/1954/UK/85 | CBV 5 | X67706 | 487 |
| ECV 1/Farouk | ECV 1 | L76395 | 342 |
| ECV 6/Finland/88 | ECV 6/FIN/88 | L76399 | 342 |
| ECV 7/Wallace | ECV 7 | L76401 | 342 |
| ECV 7/Finland/87 | ECV 7/FIN/87 | L76400 | 342 |
| ECV 11/Gregory | ECV 11 | X80059 | 92 |
| ECV 12/Travis | ECV 12 | X79047 | 232 |
| ECV 27/Bacon | ECV 27 | L76396 | 342 |
| ECV 30/Finland/85 | ECV 30/FIN/85 | L76397 | 342 |
| L76398 | 342 | ||
| ENV 68/Fermon | ENV 68 | X87604 | 349 |
| ENV 69/Toluca-1 | ENV 69 | X87605 | 349 |
| ENV 70/J670/71 | ENV 70 | D00820 | 384 |
| ENV 71/MS7423/87 | ENV 71/87 | U22522 | 51 |
| PV 1/Mahoney | PV 1m | V01148 | 353 |
| PV 1/1400/Mexico/80 | PV 1/MEX/80 | L76404 | 342 |
| PV 1/15/Hong Kong/81 | PV 1/HK/81 | L76402 | 342 |
| PV 1/NE-459/Spain/82 | PV 1/SPA/82 | L76409 | 342 |
| PV 2/Lansing | PV 21 | M12197 | 233 |
| PV 2/Sabin | PV 2s | X00595 | 431 |
| PV 2/299/USA-Ca/52 | PV 2/USA/52 | L76412 | 342 |
| PV 2/364/India/56 | PV 2/IND/56 | L76393 | 342 |
| PV 2/LS2575/Kuwait/80 | PV 2/KUW/80 | L76403 | 342 |
| PV 3/Leon/37 | PV 31 | K01392 | 413 |
| PV 3/23127/Finland/84 | PV 3f | X04468 | 173 |
| PV 3/Saukett E | PV 3sa-E | L23846 | 175 |
| PV 3/1620/Netherlands/58 | PV 3/NET/58 | L76406 | 342 |
| PV 3/17206/Netherlands/70 | PV 3/NET/70 | L76407 | 342 |
| PV 3/21267/Marocco/77 | PV 3/MAR/77 | L76405 | 342 |
| PV 3/2141/Switzerland/80 | PV 3/SWI/80 | L76410 | 342 |
| PV 3/20800/Turkey/81 | PV 3/TUR/81 | L76411 | 342 |
| PV 3/F29/Singapore/86 | PV 3/SIN/86 | L76408 | 342 |
| PV 3/30865/Bolivia/86 | PV 3/BOL/86 | L76394 | 342 |
| PV 3/50/USSR/87 | PV 3/USSR/87 | L76413 | 342 |
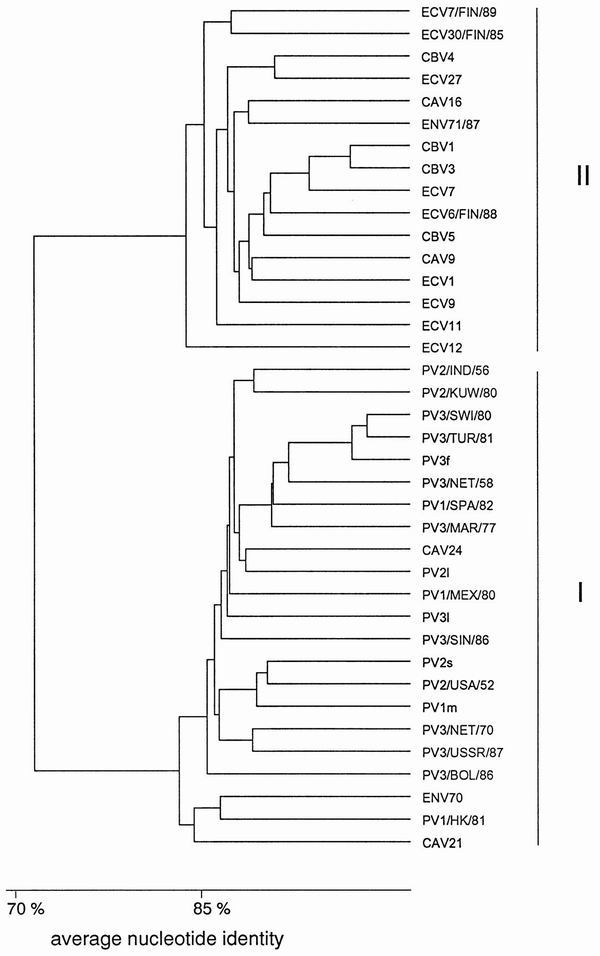
The UTRs are the most highly conserved parts of the enterovirus genome. For example, the three Sabin strains of PV have 85% nucleotide identity in the 5′ UTR and approximately 90% identity in the 3′ UTR (413, 431). Toyoda et al. (431) showed that the 5′ UTR could be divided into conserved (nucleotides 1 to 650) and hypervariable (nucleotides 651 to 750) regions according to the variation seen among the three Sabin strains. Three computer-predicted secondary structures have been proposed for the conserved part of the 5′ UTR, and this model is supported by biochemical studies (334, 366, 410). The nucleotide sequence variation seen in this region of all sequenced enteroviruses shows mainly compensatory base changes that support the proposed stem-loop structures (105, 177, 340, 342, 372). Particularly between nucleotides 440 and 600 of the enterovirus genome, there are a number of short nucleotide stretches showing almost perfect conservation among all enterovirus groups, as well as in some cases the genetically more distant rhinoviruses. These have been exploited as primer and probe recognition sequences for broadly reactive diagnostic PCR assays. Comparison of the 5′ UTR of human enteroviruses reveals two clusters with a minimum nucleotide identity of 77% within each cluster (Fig. 2): PV, CAV 21, CAV 24, and ENV 70 form one cluster (I); and all CBV serotypes, CAV 9, CAV 16, ENV 71, ECV 11, ECV 12, and all other partially sequenced ECV serotypes form a second cluster (II) (177, 180, 342).
FIG. 2.
Dendrogram based on the nucleotide identity of the 5′ UTR. Sequences used for comparison are given in Table 2.
While the need to maintain some identical nucleotide stretches and the secondary structure seems to restrict the variation in the 5′ UTR, the functional polypeptide structure places the main constraint on variation in the coding region, although higher-order structural requirements may also restrict variation. Most of the variation seen in the protein coding region is silent; i.e., the predicted amino acid sequence is not altered. Each of the two clusters based on sequence similarity in the 5′ UTR can be further subdivided into two separate clusters based on nucleotide and amino acid sequence similarity in the coding region. When the partial nucleotide sequence of VP2 protein is compared, CAV 2, CAV 3, CAV 5, CAV 7, CAV 8, CAV 10, CAV 14, CAV 16, and ENV 71 form one cluster (A); all CBV serotypes, CAV 9, ECV 11, ECV 12, and ENV 69 form another cluster (B); all PV serotypes, CAV 1, CAV 11, CAV 13, CAV 17, CAV 18, CAV 20, CAV 21, and CAV 24 form the third cluster (C); and ENV 68 and ENV 70 form the fourth cluster (D) (349) (Fig. 3). The clustering of enteroviruses is the same regardless of which protein is used in the comparison, provided that complete protein-coding sequences are used in the analysis (342). Sequence variation is highest in VP1, which codes for the major antigenic sites and the most type-specific neutralization determinants. The amino acid similarity is at least 71% within a cluster and ranges from 53 to 68% between clusters in the capsid-coding region (180, 342).
FIG. 3.
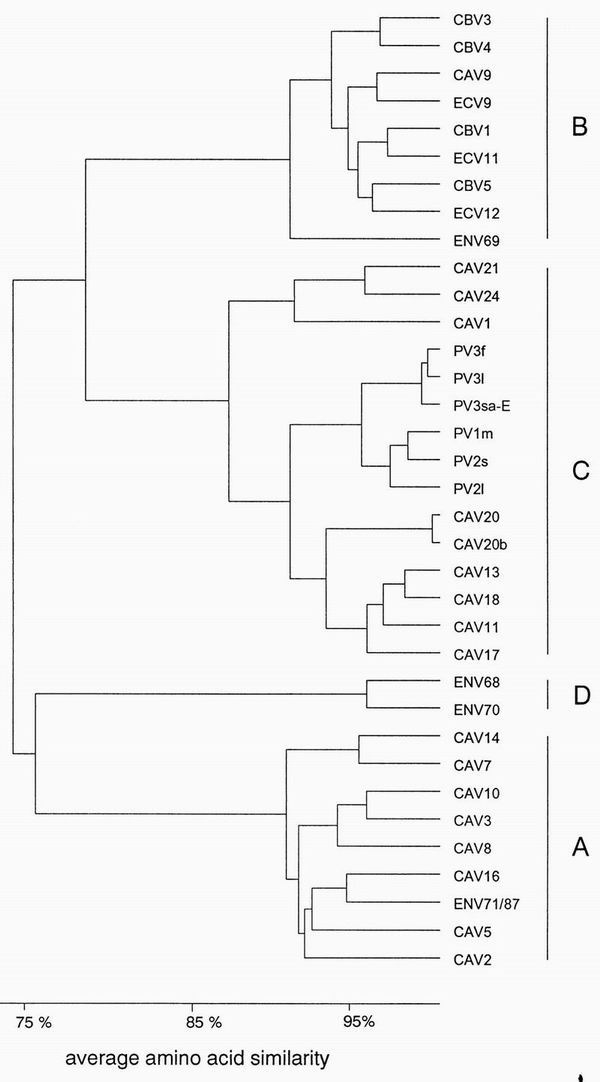
Genetic relationships among human enteroviruses. Dendrogram based on amino acid similarity of a 70-amino-acid sequence in the junction of VP4 and VP2. Sequences used for comparison are given in Table 2.
The nonstructural proteins show considerably less variation than the structural proteins do. While variation in the capsid proteins may be advantageous, enabling the virus to escape from host immune responses, variation in the nonstructural proteins, many of which are enzymes, might be deleterious. In general, amino acid similarity in nonstructural proteins is high, usually up to 90% within a cluster, being highest in the 3D protein, which is the RNA polymerase.
Clustering of the 3′ UTR is the same as that seen in the coding region, with a minimum nucleotide identity of 76% within a cluster (342). The most prominent variation between the clusters is its length: PV-like viruses have the shortest 3′ UTR (69 to 72 nucleotides), while CBV-like viruses have the longest (102 to 106 nucleotides) and CAV 16-like viruses and ENV 70 have an intermediate-length 3′ UTR (82 to 86 nucleotides). A similar secondary structure can be predicted for all the members in each cluster, although there are minor differences between clusters. Two stem-loop structures can be predicted for ENV 70 and the PV-like viruses, and three stem-loop structures can be predicted for the CBV- and CAV 16-like viruses (335, 342). It seems that the need to maintain some identical nucleotides as well as the secondary structure limits the variation in the 3′ UTR.
Intratypic Genetic Variation in Enteroviruses
In common with many other RNA viruses, enteroviruses exhibit a high degree of intratypic sequence heterogeneity, resulting from the relatively high rate of nucleotide misincorporation during viral RNA replication both in vitro and in vivo (163, 164, 215, 318, 457). The sequence variation with a single serotype is, in many genomic regions, often as high as that observed between serotypes (105, 216, 227, 340, 495) (Table 3). The structural proteins, especially the antigenic sites, show greatest intratypic variation. This has been shown both by nucleotide sequence analysis (215) and by demonstration of the differential reactivity of isolates with panels of neutralizing monoclonal antibodies (85, 171, 215, 332, 344, 458). On comparing VP1 coding sequences of epidemiologically unrelated isolates of a single serotype, up to 25% nucleotide divergence may be observed, corresponding to up to 17% divergence in the amino acid sequence (Table 3). Intratypic sequence variation is observed to a lesser extent in the untranslated and nonstructural genes (Table 3).
TABLE 3.
Intratypic nucleotide and amino acid sequence diversity of selected regions of enterovirus genomes
| Serotype | % Maximum nucleotide (amino acid) sequence divergence
|
Reference(s) | ||
|---|---|---|---|---|
| 5′ UTR | Capsid (VP1) | NSa proteins | ||
| PV 1 | 22.6 (8) | 365 | ||
| ECV 30 | 10–30 | 103, 105 | ||
| CBV 1 | 11 | 25 (5) | 105, 495 | |
| CBV 3 | 11 | 125 | ||
| CBV 4 | 20 | 12 | 172 | |
| CBV 5 | 16 | 227 | ||
| CAV 9 | 24.4 (17) | 61 | ||
| CAV 24v | 12 (4.4) | 183 | ||
| ENV 70 | 11 (3.9) | 422, 475 | ||
NS, nonstructural.
POSSIBLE APPLICATIONS OF MOLECULAR TYPING OF ENTEROVIRUSES
A molecular typing system for enteroviruses will provide a valuable alternative to the existing serological methods for identifying enterovirus types and for epidemiological monitoring. Molecular typing may also prove useful for studying other properties of enteroviruses which cannot be addressed by traditional typing methods.
Genotypic Classification of Enteroviruses
Several virus groups that have not been amenable to serological investigation due to difficulties in laboratory propagation have been classified according to molecular genetic criteria. These include the human papillomaviruses (102), the human polyomavirus JC (22, 148, 479), hepatitis C viruses (HCV) (320, 321, 407, 408, 417, 418), and hepatitis D viruses (473), and subtypes of human immunodeficiency virus type 1 (HIV-1) (463). Genotypic classification has proved invaluable for epidemiological investigation and for studying differences in biological phenotype such as virulence and antiviral susceptibility.
HCV provides a useful analogy for molecular typing systems. HCV has a positive-sense RNA genome of approximately 10,000 kb and was discovered by molecular cloning techniques. Based on sequence information, a growing number of distinct HCV genotypes and subtypes have been described. At present, 6 major genotypes and more than 50 subtypes have been differentiated (407, 408, 417, 418, 429). HCV genotyping is useful for epidemiological analysis and for prediction of the likely response of chronic HCV infection to alpha interferon therapy (reviewed in reference 406). Identification of HCV genotypes is thus of clinical importance. While nucleotide sequencing remains the gold standard for genotype determination, other differentiating molecular methods used include PCR-RFLP analysis (95, 265, 309), genotype-specific PCR (64, 320–322), and group-specific PCR followed by genotype-specific hybridization or reverse hybridization (line probe assay) (357, 417, 418, 428, 452). Some methods originally used genomic regions now known to have too little variability to differentiate all types and subtypes. However, difficulty in accommodating the increasing number of newly characterized genotypes and subtypes presents problems for all these typing methods (418).
As described above, human enteroviruses can be classified into two major phylogenetic groups based on 5′ UTR sequence similarity and into four major groups based on similarity in the coding region or the 3′ UTR. Recently, a PCR assay combined with amplicon sequencing which allows enteroviruses to be assigned to one of the four clusters has been described (19). It has been suggested that this should form the basis of an alternative genetic classification of enteroviruses (19, 180, 349). A region covering part of the 5′ UTR, the VP4, and the amino terminus of the VP2 protein was amplified, and sequences obtained from the VP2-coding region were analyzed. This allowed the correct genetic classification of virus isolates in clinical samples, although some PCR products derived directly from clinical samples did not yield unambiguous sequence data. Electrophoretic sizing of 3′ UTR-derived PCR products may provide an alternative means for differentiating the major enterovirus subgroups.
Classification of enteroviruses based on phylogenetic relationships will be useful in studying viral genetics, taxonomy, evolution, and epidemiology. However, phylogenetic classification may not correlate consistently with disease phenotype. For example, NPEV types that cause paralytic disease do not show a close phylogenetic relationship to PV (51). Also, a phylogenetic classification would not provide information on serotypic identity. For these reasons, such a classification may be of limited clinical value. In no other case has conventional virus taxonomy based on serotypic classification been replaced by a genotypic classification, although alternative genotypic classification methods have been described for adenoviruses (reviewed in reference 401). In this situation, however, an initial genotypic classification of adenoviruses may simplify the subsequent task of serotypic identification by limiting the number of neutralization tests required (210). Similarly, an initial genotypic classification of enteroviruses may assist in subsequent identification of enterovirus serotypes or other biological phenotypes discussed below.
Identification of Enterovirus Serotypes
Despite the large number of enterovirus serotypes and the high degree of antigenic variation, the serotypes appear to be stable, probably reflecting the conservation of epitopes that are critical for capsid structure or function (reviewed in reference 260). It should therefore be possible to identify the genetic determinants of epitopes that define each individual serotype. The argument in favor of retaining a serotypic classification of enteroviruses is greater than simply one of familiarity. The concept of serotype is an important biological phenotype which is closely related to immunity and, in some cases, to clinical presentation. Serotype-specific identification is therefore of paramount importance in PV surveillance, and this would also be true of any other serotype for which vaccination subsequently becomes available. The potential need for serotypic identification in the context of the infected neonate or immunodeficient patient has already been discussed.
The serotype of an enterovirus is defined by neutralizing epitopes generally located on surface-exposed loops of the virus capsid proteins, particularly on VP1 but also on VP2 and VP3 (260, 280). Several PV neutralization determinants are conformation dependent and in some cases are formed by amino acid residues from different capsid proteins in close proximity on the virion surface. Therefore, it would be difficult to predict the precise neutralization characteristics of an enterovirus from nucleotide sequence data. Most linear epitopes identified to date are cross-reactive and nonneutralizing (58, 168, 386, 387). However, important neutralization determinants formed from uninterrupted colinear amino acid residues have been identified for PV (278, 280), CBV 3 (149), and CBV 4 (266, 359), and it may be possible to identify “signature” neutralizing epitopes from the nucleotide sequence of the relevant genomic region which permit serotypic identification. It would be necessary to identify epitopes conserved among a wide range of field isolates for the development of molecular typing reagents. Capsid protein residues which do not form part of neutralization epitopes may nonetheless influence antigenicity by stabilizing the local tertiary structure of the antigenic site (328), and there may be sufficient constraint on their genetic variability for such capsid regions to be useful in serotypic identification. Relatively highly conserved sequences have been identified in VP4- and VP2-encoding regions, and these have been used to design broadly reactive PCR assays from which information on the serotype or genetic group could be obtained by analysis of the intervening sequence (130, 177, 211, 238, 303, 312, 324, 349, 386, 422, 477). However, these assays are less broadly reactive than those which target the 5′ UTR. Because of sequence variability in capsid-encoding regions, it is unlikely that a single primer pair will allow the amplification of all serotypes, and the use of degenerate or multiplex primers will probably be necessary. Recent improvements in PCR technology make this approach feasible (42, 404). Selection of primers that target relatively highly conserved capsid regions flanking more variable regions which distinguish individual serotypes may allow the use of several multiplex primer pairs for the amplification of a wide range of enteroviruses. Use of an initial genotyping assay, as discussed above, may reduce the number of multiplex primers required for subsequent serotypic identification, which could be achieved by molecular analysis of PCR products by methods described above. In view of the large number of enterovirus serotypes, a subsequent line probe assay analogous to that developed for HCV genotyping (417, 418) or microchip analysis as used to study resistance to HIV protease inhibitors (231) would be practically advantageous. In these assays, the virus type is identified in a single post-PCR reverse hybridization with type-specific oligonucleotides immobilized in lines on a membrane support or in high-density arrays on a microchip.
Other genomic regions that do not encode serotype-specific determinants are more conserved across serotypes and may evolve or undergo recombination (122, 127, 281) independently of virus serotype. It is therefore unlikely that serotypic identification of field enterovirus isolates could be based on the nucleotide or deduced amino acid sequence of nonstructural genomic regions. Some have attempted to identify enterovirus serotypes on this basis (reviewed in reference 306), for example, by analyzing PCR products generated with enterovirus group-reactive primers by high-stringency hybridization (63), PCR-RFLP analysis (196, 313), PCR-SSCP analysis (121), or nucleotide sequence determination (304, 312). However, the serotypic sensitivity and specificity of these discriminatory mechanisms have not been fully evaluated by analysis of a large number and wide range of serologically characterized field enterovirus strains. PCR-RFLP has been found useful only for PV and CBV, in part due to lack of sequence data for most ECV and CAV serotypes (196). Identification of all 66 human enterovirus serotypes by PCR-RFLP is unlikely to be practical: one suggested restriction enzyme digestion flow chart requires a total of nine different restriction enzymes and allows the identification of only 15 serotypes (196). Another drawback of RFLP analysis is that single point mutations within the recognition sequence may give rise to unclassifiable results. The use of PCR-SSCP for the analysis of enterovirus genotypes by using PCR products from the 5′ UTR has also been described (121). Different electrophoretic profiles were reported for 14 enterovirus serotypes investigated. However, this method is also very susceptible to minor sequence variation. Furthermore, SSCP analysis requires additional equipment and is time-consuming.
Attempts have been made to devise genetic typing methods for numerous other virus groups that have been classified by serological methods. Serological relationships among virus groups classified by genetic methods have also been studied. Genetic typing methods that show good correlation with serological classification methods have been reported for influenza virus types (76, 471) and influenza virus type A hemagglutinin and neuraminidase subtypes (471), some but not all hepatitis B virus subtypes (108, 314, 323), human herpesvirus types (214, 261, 426, 445), human T-cell lymphotropic viruses (439, 441), HIV types (405, 440), human polyomavirus BK groups (191), and serotypes of rotaviruses (135, 140), adenoviruses (ocular serotypes) (385), HCV (409, 428, 446, 490), dengue viruses (235, 298, 397, 398), astroviruses (316), hantaviruses (350), California group bunyaviruses (43), and foot-and-mouth disease viruses (250, 368, 448). Only partial correlation has been observed between HIV-1 subtypes and gp120 serotypes, but no correlation between HIV-1 subtype and neutralization type was observed (65, 230, 292), presumably reflecting a dependence of neutralization determinants on conformational structures which cannot be predicted from primary nucleotide or deduced amino acid sequences. Partial correlation was also observed for antigenic types of Norwalk and related small, round, structured viruses (14). A method for genetic typing of human rhinoviruses has been described (297), but this was not designed to correlate closely with serotypic classification.
The extent to which these genetic typing methods mirror serotyping methods depends on the degree of phylogenetic similarity between serotypes in the genomic region targeted by genetic typing methods (which affects typing specificity) and the extent of genetic variation within serotypes (which affects sensitivity), particularly with field strains. There is a high degree of phylogenetic similarity among enterovirus serotypes in most genomic regions, while the regions showing greatest divergence among serotypes also show greatest variability within serotypes. The human enterovirus group is considerably larger than most of the above virus groups, and the development of a molecular typing system which identifies all, or even the most important, serotypes presents a formidable challenge.
Intratypic Differentiation of Poliovirus
Currently used methods to differentiate vaccine or vaccine-derived strains from wild PV strains rely on genetic or antigenic differences between currently circulating wild PV strains and those from which the oral Sabin vaccine strains were derived, as a result of continuous genetic drift. A number of molecular methods have been developed, including oligonucleotide mapping of RNase T1-digested viral RNA by two-dimensional gel electrophoresis (318), hybridization of viral RNA with vaccine- or wild-strain-specific synthetic oligonucleotide probes (94) or riboprobes (97), PCR amplification of viral RNA with primers specific for Sabin vaccine strains (476) or current wild strains (307, 308, 477), and PCR with enterovirus group-reactive primers for PCR amplification followed by RFLP analysis (24, 122, 396), high-stringency hybridization with vaccine-strain-derived PCR product probes (421), or nucleotide sequence determination (365). Chumakov and coworkers (73, 74, 361, 362, 420) have also described a PCR-RFLP assay to identify and quantify the presence of neurovirulent revertants of Sabin vaccine strain virus in vaccine lots as a means of ensuring vaccine safety, while Guillot et al. (147) have described a site-specific PCR to detect the excretion of such revertants by recipients of Sabin polio vaccine.
Molecular methods for intratypic differentiation of PV currently used by the WHO polio laboratory network include RNA probe hybridization, strain-specific PCR, and PCR-RFLP analysis. In a recent study comparing the reliability of these and currently used serological methods (enzyme immunoassay with cross-absorbed polyclonal antisera and virus neutralization with strain-specific monoclonal antibodies) in five WHO laboratories with a coded panel of wild and vaccine strain viruses, all the methods gave correct results in 91.9 to 97.8% of tests (443). The RNA probe hybridization assay and enzyme immunoassay with polyclonal antisera performed best, but most discrepancies were obtained by laboratories using techniques that had only recently been introduced. However, only the enzyme immunoassay was able to detect and confirm the presence of both Sabin-like and wild non-Sabin-like viruses in samples containing mixtures of vaccine and wild PV. It was recommended that at least two methods of intratypic differentiation based on fundamentally different principles (e.g., antigenic differences or sequence differences) be used, with nucleotide sequence determination being used to resolve discrepant results.
Although these methods have proved successful, their use has thus far been restricted to analysis of PV which have first been isolated in cell culture and characterized as PV by standard methods. The use of molecular biological methods for both detection and (if possible simultaneous) intratypic differentiation of PV in clinical and environmental samples may lead to further improvement in the streamlining, standardization, and sensitivity of test procedures. This would require an assay that detects all PV types and strains, but not other enteroviruses, to identify PV-containing samples for subsequent intratypic differentiation. Given the extent of genetic variation among wild PV strains and their phylogenetic similarity to some NPEV strains, designing such a PV-specific assay presents theoretical difficulties. However, a number of promising methods have been described. Using primers located in the 5′ UTR designed to selectively amplify PV, Abraham et al. (3) reported a sensitivity of 100% on testing 81 PV isolates and a specificity of 96% on testing 50 NPEV isolates, while Egger et al. (109) reported a sensitivity of 100% on testing 81 PV isolates when two primer pairs located in the P2-P3 region were used and a specificity of 98% on testing 45 NPEV isolates. Using primers in the VP1-2A region, either alone or in combination with general enterovirus-reactive primers, Chezzi (66) reported 100% sensitivity and specificity on testing 125 PV and 38 NPEV isolates. Finally, Kilpatrick et al. (211) used primers containing mixed-base or deoxyinosine residues designed to recognize VP1-encoding regions unique to PV and successfully detected all of 158 wild or vaccine-related PV strains but none of 49 NPEV reference strains. Although these tests were applied to virus isolates, it may be possible to improve their sensitivity for direct detection in clinical material by further optimization of reaction conditions or by using the PV-specific primers as nested primers in a two-stage amplification with enterovirus group-reactive primers in the initial amplification.
An alternative approach to poliovirus-specific detection may lie in the use of immunocapture PCR, in which PV strains are selectively recovered from test specimens by attachment to paramagnetic particles or other solid phase by means of PV-specific antibody or possibly PV receptor protein. This method has been used for detection of hepatitis A virus (100, 137, 187, 291, 347) and group-specific detection of group A rotaviruses (141) in fecal and environmental samples. Achieving PV specificity during sample processing in this way would allow the use of enterovirus group-reactive primers for the initial screening assay. Intratypic differentiation would then be performed by one of the above methods.
Molecular Epidemiology
The ability to identify small differences in nucleotide sequence between virus isolates provides a sensitive tool for molecular epidemiological studies, especially of RNA viruses with a high rate of sequence evolution in nature (99, 165, 338, 375). Conventional virological techniques allow the characterization of enterovirus serotypes and intratypic differentiation of PV but do not provide sufficient information to study molecular epidemiological or evolutionary relationships between isolates or strains or to identify the source of a virus. Phylogenetic analysis of virus isolates provides the necessary information for this type of study. Genetic differences between strains may be demonstrated by oligonucleotide mapping of viral RNA (207, 282, 285), by PCR-RFLP (24, 453), or, more commonly nowadays, by nucleotide sequence determination of PCR products (307, 308, 365), which provides the most detailed and informative discrimination between strains. This method typically uses primers that target sequences which are relatively conserved among isolates of a given serotype, thus allowing the amplification of most isolates, but which flank a more variable region in the VP1-2A junction region of the genome, thus providing maximal discrimination potential between unrelated strains. The concept of PV genotype has been used to describe isolates of a single serotype which show at least 85% sequence identity in this genomic region and are therefore considered to be closely related (365).
Molecular epidemiological investigation has been used within the PEI (reviewed in reference 206) to (i) determine the sources of imported viruses (247, 307, 308, 343), (ii) monitor the pathways of virus transmission (15, 308, 365, 491), (iii) monitor the progress of control activities (423, 444), (iv) identify reservoirs sustaining virus transmission (176, 307, 365), (v) develop molecular reagents for the rapid detection of wild PV in clinical and environmental samples (94, 307, 308, 477), and (vi) provide critical evidence that PV eradication has been achieved (212, 329, 472).
Phylogenetic analysis has also been used to study the epidemiology of other enterovirus serotypes. By using this approach, it has been possible to estimate the date of emergence of newly arising enterovirus serotypes or strains (284, 285, 422) and to study their subsequent molecular evolution and global spread (50, 183, 184, 207, 243, 244), to confirm the common source of isolates from a single enterovirus outbreak (103, 105, 374) and study their genetic relationship to strains from previous outbreaks (154, 184, 227), and to identify cocirculating strains of a single serotype (172). Most of these studies have involved nucleotide sequence analysis of PCR products. However, for identification and monitoring of enterovirus outbreaks, PCR-RFLP or PCR-SSCP could allow the rapid identification of all PCR-positive samples with an identical pattern (the “outbreak pattern”), thus yielding valuable epidemiological information and reducing the requirement for complete nucleotide sequence analysis.
Resolution of Enterovirus Mixtures
The need to identify and resolve mixed enterovirus infections is evident when investigating the etiology of paralytic poliomyelitis. Failure to identify a dual PV-NPEV infection or failure to detect a wild PV or NPEV infection in a Sabin vaccine recipient may lead to misattribution of the poliomyelitis etiology, resulting in the failure to notice wild PV circulation and the delay of preventive measures. The ability to identify wild PV in sewage and other environmental samples containing a high background of NPEV or Sabin vaccine viruses is also crucial to epidemiological monitoring. The presence of multiple enterovirus types may be suspected when serotype determination by neutralization with intersecting pools or type-specific antisera is unsuccessful. Samples containing mixed Sabin and wild PV may be identified during intratypic differentiation procedures, as described above. However, recently conducted quality assurance procedures have found that failure to correctly identify enterovirus mixtures is widespread among diagnostic and reference laboratories (449). It is vital for certification of PV elimination that all PV strains are correctly identified, even when they occur in mixtures of enteroviruses.
The use of PCR-based diagnostic techniques may provide an opportunity to improve this detection rate. Methods for selective amplification of PV may be appropriate for epidemiological monitoring, where information on the possible presence of other enteroviruses is not required. However, methods that allow the specific, simultaneous identification of individual components of enterovirus mixtures may be necessary for etiologic investigation of poliomyelitis cases or other clinical syndromes. This could be achieved by performing amplification with broadly reactive or multiplex primers, followed by molecular differentiation of each component. Some molecular differentiation methods may not detect the presence of multiple viruses. Direct nucleotide sequence analysis of PCR products produces a consensus nucleotide sequence and cannot detect minor components that comprise less than 25% of the original virus mixture (276), while PCR-RFLP analysis may give an uninterpretable result. Other methods may be more useful: multiple HCV infections have been detected with type-specific reverse hybridization probes (417, 418) or nested primers (120). Subtractive hybridization or amplification methods such as representational difference analysis (249), in which a predominant species amplified by PCR is used to enrich for minor components present in the original specimen, may also be useful if the complexity of current methods can be reduced. In one sense, all enterovirus samples are mixtures, because, like other RNA viruses, they exist as a quasispecies population. Molecular typing methods must be sufficiently robust to allow unequivocal identification of the major component(s). Because typing methods such as those discussed here based on sequence analysis or probe recognition detect consensus sequences, the presence of quasispecies does not appear to interfere.
Identification of Virulence Determinants
The wide range and severity of disease syndromes associated with enterovirus infection in humans and experimental animals are explained in part by the influence of numerous host-related factors, including age (146, 209, 252), sex (37, 253), nutritional factors (28–31, 251, 466, 467), immunodeficiency (264, 474), genetic constitution (70), preexisting immunity (223) or maternal antibody (86), and exercise (124, 166). However, the association of certain enterovirus serotypes and strains with particular disease syndromes indicates that virus-encoded factors also have a significant influence on disease manifestation. Identifying such virus-encoded factors is a worthwhile but challenging exercise. Most studies to date have been conducted with experimentally infected laboratory animals.
Cell culture and animal model studies.
The search for determinants of virulence has been conducted on another level by studying enterovirus variants that show altered pathogenicity in experimental animals compared with that of the parental virus from which they were derived. Thus, the PV Sabin vaccine strains have been compared with their neurovirulent progenitors and revertants in primates and, more recently, in transgenic mice expressing the human PV receptor. By comparing nucleotide and amino acid sequences and studying the in vitro replication kinetics and in vivo virulence phenotype of chimeric viral genomes constructed from homologous neurovirulent and vaccine strain genomes, mutations that affect neurovirulence (8, 48, 55, 71, 204, 234, 254, 255, 299, 325, 337, 360, 425, 462) and temperature sensitivity (48, 71, 325, 424) have been mapped, in some cases to single nucleotides (255; reviewed in reference 278). By using a similar experimental rationale, loss of cardiovirulence in a murine model upon passage of a cardiovirulent CBV 3 strain was mapped to a single nucleotide transition at nucleotide position 234 in the 5′ UTR (438), although in a separate study of a different noncardiovirulent CBV 3 strain, no attenuating lesion could be identified in the 5′ UTR (489) or VP1-encoding region (488). In a third study with clinical CBV 3 isolates as well as laboratory-passaged strains, sequence analysis of a 300-base region of the 5′ UTR indicated that murine cardiovirulence was associated with an A residue at nucleotide position 565 whereas nonvirulent isolates had a U or C residue at this position (125). Another study involving a nonvirulent neutralization escape mutant of a cardiovirulent CBV 3 strain identified an amino acid change in the VP2-encoding region which resulted in attenuation of cardiovirulence, despite having no effect upon virus replication in the heart (222). The nonvirulent variant also failed to induce the production of tumor necrosis factor alpha upon infection of monocytes in vitro. In a murine model of CBV 4-induced pancreatitis, loss of pancreatic, hepatic, and cardiac virulence was mapped to a nucleotide change resulting in a single amino acid change in VP1 (52), with an additional minor determinant of pancreatic virulence being identified in VP4 (355).
From these studies in cell culture and animal models, it has been postulated that enterovirus virulence is determined by a number of factors, including virion thermostability, encapsidation efficiency, the specificity of cell receptors present on the virion surface which restricts the range of cell types that can be infected, and the efficiency of viral genome transcription or translation within a given cell type. Mutations affecting these functions may result in a decreased virus load, either at the site of initial virus replication or in the target organ, and may offer the immune system an advantage in restricting initial virus replication, limiting viremic spread, and eliminating virus and virus-infected cells from target organs (8, 117, 125, 234, 438).
Determinants of enterovirus virulence in humans.
Investigations in which inbred or transgenic animals of uniform age, sex, and physiological status are infected with plaque-purified or cloned enteroviruses may be of value in identifying regions of the viral genome which influence disease expression. However, attenuating mutations resulting from cell culture passage of virus may not always be found in naturally occurring field strains (435). It is also probable that virulence determinants are more heterogeneous and less easily identifiable among the continually evolving enterovirus strains that cause natural infections in the genetically and physiologically diverse human population. At present, there is little information in this field, and the difficulty in identifying virulence determinants in humans should not be underestimated.
The occurrence of enterovirus epidemics may provide an opportunity to study and compare enterovirus strains which are relatively homogeneous in genetic constitution and disease association. Only a few such studies have been reported. In a comparison of two strains of ENV 71, one associated with outbreaks of hand, foot, and mouth disease and the other associated with outbreaks of aseptic meningitis or paralytic disease, significant variation of the 5′ UTR sequence was observed (492) even though the two strains were serologically indistinguishable. This variation suggested that the observed difference in neurovirulence between these strains was due to genetic differences, possibly in the 5′ UTR.
Another study in which partial 5′ UTR sequences of ECV and PV were compared found that part of an oligopyrimidine tract known to be involved in efficient binding to rRNA in neurovirulent PV strains was completely conserved in neurovirulent ECV serotypes but was variable in less neurovirulent ECV serotypes (372). It was hypothesized that this may provide a basis for explaining the differences in the observed neurovirulent potential of different ECV.
Need for further study.
The above hypotheses must be tested further by studying larger numbers of field enterovirus strains isolated from patients with well-defined clinical syndromes. This could be accomplished with an appropriately designed molecular typing system. On the basis of current knowledge of molecular determinants of virulence and tropism, a typing system targeting such candidate genomic regions as the 5′ UTR and the capsid-encoding region may, after comparison of a sufficiently large number of enteroviruses from different patient groups, permit the identification of consensus sequences associated with a particular disease phenotype. The biological effect of such consensus sequences could then be studied in cell culture and, where possible, in animal models with chimeric viruses generated by intertypic genome recombination or site-directed mutagenesis. Although this is a formidable task, the information provided by such investigations would further increase our understanding of the pathogenesis of enterovirus infection. This information may provide a rationale for the design of recombinant live-attenuated enterovirus vaccines (62) or antiviral prophylactic or therapeutic measures.
Classification of Enteroviruses Based on Receptor Usage
Viral infection of host cells is initiated by binding of the virus to a specific cell surface protein. The requirement of a virus for a specific cell surface protein and the cellular and tissue distribution of this protein clearly may influence viral tropism and disease manifestation (162). It is presumed that enteroviruses have specific regions in their symmetrical capsid structure, called viral attachment proteins (VAP), which recognize specific features of selected cellular membrane proteins as receptors. Such specific receptors are an important determinant of the host range and cell and tissue tropism of a virus. These receptors are considered to possess two functions: first, to attach virions, and second, to eclipse virus infectivity. The identification and characterization of enterovirus receptors has been the object of intensive investigation in recent years, because knowledge of virus-receptor interaction as the initial step of infection offers an opportunity for chemical or immunologic intervention before the viral genome has reached relative stability within the cell. The two most obvious possibilities for antiviral therapy based on knowledge of virus receptors involve the blocking of virus-receptor interactions with either antireceptor antibodies or the soluble receptor and the development of pharmacological agents that specifically block the virus binding at the level of either the cell receptor or the VAP. However, cell receptors have been identified for only a small number of enterovirus types thus far.
For PV, the receptor is known to be a member of the Ig superfamily (274). The integrin VLA-2 has been reported to be the receptor for ECV 1 (36), and decay-accelerating factor (DAF/CD55), a membrane protein of 70 kDa, has been reported to be the receptor for ECV 3, 6, 7, 11, 12, 20, and 21 (34) and ENV 70 (203). Like the major group of human rhinoviruses, CAV 13, CAV 15, CAV 18, and CAV 21 can use intercellular cell adhesion molecule 1 as the receptor (80) whereas CAV 9 interacts with the vitronectin receptor (371). From infected HeLa cells, a CBV-receptor complex containing virus capsid proteins bound to a cellular protein of 46 kDa was purified (35, 257). Shafren et al. (399) reported that DAF/CD55 might also be a cell attachment receptor for serotypes B1, B3, and B5. However, competition experiments revealed that all six serotypes of CBV compete for a common receptor protein. A 100-kDa cellular protein that also has the ability to bind CBV 1 to CBV 6 was recently identified by Raab de Verdugo et al. (352).
Some information is available on the regions of the enterovirus capsid involved in cell attachment. PV strains are hypothesized to attach to target cells in a manner similar to human rhinoviruses via a “canyon” which is defined by multiple viral capsid peptides (373). A similar surface structure thought to serve as the primary and secondary receptor-binding site has also been described for CBV 3 (300).
It appears that the attachment of CAV 9, ECV 22, and ECV 23 is mediated by a portion of VP1 which contains a highly conserved 3-amino-acid sequence, the RGD (Arg-Gly-Asp) motif (60, 415). In CAV 9, this RGD motif is located within a relative 15-amino-acid insert in comparison to other enteroviruses. The RGD motif is known to play a role in cell adhesion in many proteins present in extracellular matrices and in the blood. In all three RGD-containing enteroviruses as well as in foot-and-mouth disease virus (259), blocking experiments with specific peptides showed the significance of the RGD motif for the infection of cells. However, CAV 9 can also infect some cells independently of the RGD motif (370). The identification of capsid regions of other enteroviruses involved in receptor binding would provide the basis for a molecular classification of enteroviruses according to cell receptor usage. In the future, this may lead to a greater understanding of the influence of receptor specificity on viral tropism and pathogenicity and may allow the targeting of antiviral therapy with agents designed to interfere with specific virus-receptor interactions.
Determination of Sensitivity or Resistance to Antiviral Agents
A range of antipicornavirus agents have been described in the literature (reviewed in references 12 and 319). However, to date, none appear likely to play a potential role in clinical practice. Most act by noncovalent interaction with virus capsids, resulting in inhibition of viral attachment to cell receptors or inhibition of disassembly following cell entry. Kraus et al. (232) isolated a rhodanine-resistant variant of ECV 12 by sequential passage in the presence of rhodanine, an inhibitor of virion disassembly. Sequence analysis of the resistant variant and parental virus revealed seven amino acid differences, six of which were located in the capsid-encoding region. The amino acid differences responsible for the rhodanine-resistant phenotype have not yet been identified. Similarly, resistance to enviroxime, an inhibitor of picornavirus RNA replication, has been mapped to the 3A protein, and amino acid substitutions conferring resistance have been mapped for human rhinovirus 14, PV 1 (158), and CBV 3 (159). If clinically useful antiviral agents were identified, it would be necessary to monitor drug resistance. Since resistance might result from numerous different mutations, it may not prove possible to devise a molecular typing system which identifies every possible mutation. Experience with drug resistance in HIV and cytomegalovirus infections has found that most cases of resistance are caused by one or more of a few common mutations, which can be readily identified by nucleotide sequencing of PCR products (188, 194, 364), differential hybridization of amplified viral RNA to oligonucleotide probes (129), or point mutation assays (44, 118, 205). However, these tests may fail to predict resistance resulting from other less common mutations (402). Prediction of drug susceptibility based on nucleotide sequence data will become increasingly difficult as new combination and sequential chemotherapeutic strategies are introduced.
Formation of a Database of Enterovirus Genome Sequences
Molecular sciences are heavily dependent on the use of computers for accumulation, processing, analysis, storage, and dissemination of experimental data, and the role of transnational nucleic acid and protein sequence databases in these processes is crucial (reviewed in reference 275). Due to the increasing use of general sequence databases and the requirement for specialist functions, a number of specialist databases which cross-reference with major sequence databases have been formed (195). Even if an enterovirus molecular typing system were to use methods other than complete nucleotide sequencing of PCR products, it is likely that nucleotide sequencing of representative samples would still be required for ongoing quality assurance, discrepancy analysis, and resolution of untypeable samples. A database of enterovirus sequences generated by molecular detection and typing methods would facilitate the quality assurance of detection and typing reagents and provide a resource for the study of viral epidemiology, evolution, taxonomy, and molecular pathogenesis.
MOLECULAR TYPING OF ENTEROVIRUSES: THE WAY FORWARD
The development of a universally applicable molecular typing system for enteroviruses will require considerable effort to generate additional molecular sequence data, to provide reference reagents, to research laboratory and computer methods required for the development of a typing system, and to raise the funding required to make this work possible.
Identification of Priorities for Molecular Typing
Molecular typing methods for viruses should aim to provide clinically and biologically useful information about field viruses, particularly with regard to virulence, viral epidemiology, and virus serotype identification. The extent to which molecular classification systems for viruses have aimed toward or achieved these goals varies, and in some cases further development and refinement of the methods are required. For enteroviruses, to these general aims must be added the requirement to differentiate wild PV and NPEV, at least until global eradication of wild PV is verified.
A genotyping method based on phylogenetic relationships would not achieve all these objectives but would be of value for epidemiological studies and may be useful as an initial step in further typing procedures. Phylogenetic typing is also a realistic intermediate target for enterovirus molecular typing, and methods such as those described by Arola et al. (19), based on nucleotide sequence analysis of a target region located in the 5′ UTR and the VP4- and VP2-encoding region, should be pursued further.
Even if wild PV eradication is achieved by the year 2000, a period of continued surveillance will be required to verify this. The ability to differentiate between wild and vaccine-derived PV and NPEV is therefore likely to be required until at least 2010. In view of this, molecular detection of wild PV should also be considered a goal of research and development of molecular typing, which should be achievable by methods discussed above.
In this review, we have considered a number of biological characteristics of enteroviruses for which typing methods may be useful. In most cases, our understanding of these characteristics is incomplete. The development of molecular typing methods based on these biological phenotypes will therefore require further preliminary research to identify the relevant molecular determinants and will doubtless prove more refractory to genetic analysis, but it should nonetheless be considered a longer-term aim. Without such objectives, it is doubtful whether a molecular typing system would become widely used.
Establishment of a Collection of Representative Enterovirus Strains
It will be apparent that the development of molecular typing will require access to prototype enterovirus strains; field enterovirus isolates, including well-characterized isolates, prime strains, and untypeable isolates collected during recent years and from different geographical regions; and clinical specimens in which enteroviruses have been detected by culture or by PCR. Relevant demographic and clinical information about the source of clinical isolates and specimens must also be recorded to allow statistical correlation between genotypic and phenotypic features of viruses. Such a collection would provide a resource for the generation of molecular sequence data and for the evaluation of subsequently developed molecular typing assays.
Augmentation of Enterovirus Molecular Sequence Data
Although the volume of molecular sequence data available for human enterovirus genomes has increased in recent years, much additional sequence data is still required for many prototype strains, particularly for ECV and CAV. Another important area where information is needed is the degree of sequence variation within individual serotypes. Limited information on this is available only for a few serotypes (Table 3). A third relevant area is the study of sequences obtained directly by PCR amplification from patient specimens and their comparison with sequences of the corresponding cell culture isolates, since selective susceptibility of cultured cells and the process of adaptation to growth in cell culture may result in selection of a virus population which is substantially different in genetic constitution and biological phenotype from those of the original field strain (72, 74, 157).
The genomic regions that should be targeted for sequence analysis will be determined by the objectives of molecular typing. Phylogenetic classification may be based on the sequence of the VP2-encoding region or the length of the 3′ UTR, as described above. Classification based on biological features such as serotype, receptor usage, tissue tropism, or virulence will require sequence information from the genomic regions that influence these properties. VP1 and VP2 proteins contain the major neutralizing epitopes, and also the VAP involved in cell receptor interaction, and this genomic region should also be investigated as a target for molecular typing methods. Observations that mutations in VP3 and VP4 may also affect virion surface features and biological properties suggest that this genomic region should also be analyzed. The 5′ UTR is also of special interest in view of its role in the regulation of transcription and translation of the viral genome and its influence on virulence, and this region should be considered an important target for comparative sequence analysis. In contrast, the nonstructural protein-encoding region is relatively conserved in sequence and possibly also in function among enteroviruses and is not generally believed to contribute significantly to the diversity of enterovirus biology. It is therefore likely that a detailed knowledge of the structure and function of the 5′ half of the enterovirus genome would provide a sufficient basis for understanding the observed biological variation of enteroviruses and for designing molecular typing systems to classify and identify enteroviruses according to this variation.
The most straightforward way to obtain additional sequence information is probably by direct sequencing of PCR products generated with consensus, degenerate, or multiplex primers whenever possible (177, 349). This method could be applied to prototype strains and clinical isolates as well as to PCR-positive patient specimens. For example, amplification of the complete 5′ UTR may readily be achieved; sequencing of PCR products could then be performed with both external PCR primers and internal forward and reverse primers. The two inner primers could be located in the region around base 450, which is highly conserved throughout the enterovirus genus. Multiplex or degenerate PCR primers would probably be required to allow the detection of the two major 5′ UTR phylogenetic subgroups (Fig. 2), as suggested by Pöyry et al. (342).
These molecular sequence data would provide a basis for the development of molecular typing methods involving current or future nucleic acid or protein technologies.
Design and Development of a Molecular Typing System
Development of a typing system will require a strong research base to determine the molecular basis of distinguishing the biological characteristics of enteroviruses. Evaluation will be required to determine the reliability of identifying specific genomic sequences as specific markers of virus type. This is likely to prove most difficult when biological features are associated with higher-order structures dependent on the interaction of noncontiguous nucleotide or amino acid sequences. Refinement of target sequences and optimization of reagents and methods over time will undoubtedly be required. Ongoing evaluation and quality assurance will also be necessary to ensure that typing methods retain specificity and sensitivity in identifying current enterovirus strains.
It is envisaged that molecular typing of enteroviruses would be conducted primarily in regional or national reference centers, as is often the case with current serotyping methods. Specialist procedures, such as intratypic differentiation of PV, would probably still be performed in WHO reference laboratories. While methods for epidemiological investigation of specific outbreaks may be tailored to local or temporary requirements, a universally applicable molecular typing system is desirable for most typing purposes. A molecular typing system for enteroviruses should therefore be simpler to perform and interpret than serotyping by neutralization and should be more amenable to standardization and quality control. Reagents should be temperature stable and the test should be robust enough to be performed in diverse climates. Results should be rapidly available, to maximize clinical utility. Molecular typing methods should be adaptable, to accommodate emerging and evolving virus strains, and should be cost-effective.
To realize these goals, research and development should make full use of recent advances in PCR technology and of automation of sample preparation, PCR, and post-PCR processing, including nucleotide sequencing. The choice of post-PCR analysis in particular will be influenced by these constraints. Electrophoretic analysis is difficult to standardize and automate. PCR-RFLP, PCR-SSCP, and other electrophoretic sizing procedures should therefore be avoided. Methods that require multiple parallel tests to identify different virus types will be both labor- and reagent-intensive and unlikely to be cost-effective or to give rapid results. A rapid and definitive typing result is most likely to be achieved by reverse hybridization of the PCR product to solid-phase-bound, type-specific probes in a microtiter plate-based, microchip, or line probe assay or by direct nucleotide sequence analysis of PCR products. The latter has been made more feasible by the recent introduction of rapid, cheaper automated methods. Sequence analysis may be required for epidemiological studies and quality assurance, whereas probe hybridization analysis may be sufficient and more practical for other purposes and will probably be necessary for identification of mixed infections. However, the ongoing development of DNA and protein technology may provide other possible methods in the near future.
Establishment of a Network of Laboratories
It will be apparent that the work required to develop a reliable and useful molecular typing system for enteroviruses is extensive and will require considerable long-term funding. A strategy to raise this funding must address the aforementioned perception of enteroviruses as relatively unimportant agents of human disease and the current reality of decreased funding in most areas of basic research. At present, enterovirus research is conducted mainly in laboratories with independent funding studying such aspects as pathogenesis, structure and molecular biology, epidemiology, diagnosis, and biodiversity. All these areas of interest have relevance to the design of molecular typing methods. A collaborative network of such laboratories would build on the expertise of individual research groups and provide the critical mass required to sustain central facilities required for archiving reference reagents and sequence data and coordination of research efforts. It is also to be hoped that such a grouping would achieve greater prominence in areas of public education and so convince the medical and scientific communities, as well as the general public, of the need to continue research into this important genus of viruses and the potential benefits of new ways of typing human enteroviruses.
ACKNOWLEDGMENT
We thank Pam Gregory, Secretary to the European Union Concerted Action on Virus Meningitis and Encephalitis, for secretarial and administrative assistance.
Appendix
The other members of the European Union Concerted Action on Virus Meningitis and Encephalitis are as follows: Maria Ciardi, Instituto di Malattie Infettive, University of Rome, Rome, Italy; Paola Cinque, Division di Malattie Infettive, Ospedale San Raffaele, Milan, Italy; José M. Echevarría, C.N.M., Instituto de Salud Carlos III, Madrid, Spain; Bent Faber Vestergaard, Department of Virology, Statens Seruminstitut, Copenhagen, Denmark; Marianne Forsgren, Clinical Virology F68, Huddinge University Hospital, Huddinge, Sweden; Giuseppe Gerna, Viral Diagnostic Service, IRCCS Policlinico San Matteo, Pavia, Italy; Monica Grandien, Swedish Institute for Infectious Disease Control, Stockholm, Sweden; Tapani Hovi, Enterovirus Laboratory, National Public Health Institute, Helsinki, Finland; Nikolai Kirkby, Department of Virology, Statens Seruminstitut, Copenhagen, Denmark; Paul Klapper, Manchester Central Laboratory Services, Manchester Royal Infirmary, Manchester, United Kingdom; Marjaleena Koskiniemi, Department of Virology, University of Helsinki, Helsinki, Finland; Pierre Lebon, Service de Bacteriologie, Virologie et Hygiene, Hôpital Saint Vincent de Paul, Paris, France; Annika Linde, Swedish Institute for Infectious Disease Control, Stockholm, Sweden; Philippe Monteyne, Laboratoire de Neurochimie, Université Catholique de Louvain, Brussels, Belgium; Elisabeth Puchhammer-Stockl, Institute of Virology, University of Vienna, Vienna, Austria; Christian J. M. Sindic, Laboratoire de Neurochimie, Université Catholique de Louvain, Brussels, Belgium; Clive Taylor, Department of Virology, Public Health Laboratory, Newcastle General Hospital, Newcastle-upon-Tyne, United Kingdom; Volker ter Meulen, Institut für Virologie und Immunobiologie, Universität Würzburg, Würzburg, Germany; and Thomas Weber, Department of Neurology, Marienkrankenhaus Hamburg, Hamburg, Germany.
REFERENCES
- 1.Abbaszadegan M, Huber M S, Gerba C P, Pepper I L. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl Environ Microbiol. 1993;59:1318–1324. doi: 10.1128/aem.59.5.1318-1324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abebe A, Johansson B, Abens J, Strannegård Ö. Detection of enteroviruses in faeces by polymerase chain reaction. Scand J Infect Dis. 1992;24:265–273. doi: 10.3109/00365549209061331. [DOI] [PubMed] [Google Scholar]
- 3.Abraham R, Chonmaitree T, McCombs J, Prabhakar B, Lo Verde P T, Ogra P L. Rapid detection of poliovirus by reverse transcription and polymerase chain reaction amplification: application for differentiation between poliovirus and nonpoliovirus enteroviruses. J Clin Microbiol. 1993;31:395–399. doi: 10.1128/jcm.31.2.395-399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abzug M J. Perinatal enterovirus infections. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 231–238. [Google Scholar]
- 5.Abzug M J, Keyserling H L, Lee M L, Levin M J, Rotbart H A. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clin Infect Dis. 1995;20:1201–1206. doi: 10.1093/clinids/20.5.1201. [DOI] [PubMed] [Google Scholar]
- 6.Abzug M J, Levin M J, Rotbart H A. Profile of enterovirus disease in the first two weeks of life. Pediatr Infect Dis J. 1993;12:820–824. doi: 10.1097/00006454-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Abzug M J, Loeffelholz M, Rotbart H A. Diagnosis of neonatal enterovirus infection by polymerase chain reaction. J Pediatr. 1995;126:447–450. doi: 10.1016/s0022-3476(95)70466-3. [DOI] [PubMed] [Google Scholar]
- 8.Agol V, Drozdov S G, Ivannikova T A, Kolesnikova M S, Korolev M B, Tolskaya E A. Restricted growth of attenuated poliovirus strains in cultured cells of a human neuroblastoma. J Virol. 1989;63:4034–4038. doi: 10.1128/jvi.63.9.4034-4038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aintablian N, Pratt R D, Sawyer M H. Rapidly recurrent enteroviral meningitis in non-immunocompromised infants caused by different viral strains. J Med Virol. 1995;47:126–129. doi: 10.1002/jmv.1890470204. [DOI] [PubMed] [Google Scholar]
- 10.Alexander J P, Baden L, Pallansch M A, Anderson L J. Enterovirus 71 infections and neurologic disease—United States, 1977–1991. J Infect Dis. 1994;169:905–908. doi: 10.1093/infdis/169.4.905. [DOI] [PubMed] [Google Scholar]
- 11.Alexander J P, Jr, Chapman L E, Pallansch M A, Stephenson W T, Török T J, Anderson L J. Coxsackievirus B2 infection and aseptic meningitis: a focal outbreak among members of a high school football team. J Infect Dis. 1993;167:1201–1205. doi: 10.1093/infdis/167.5.1201. [DOI] [PubMed] [Google Scholar]
- 12.Al-Nakib W. Prospects for treatment and prevention of virally induced heart disease. In: Banatvala J E, editor. Viral infections of the heart. London, United Kingdom: Edward Arnold; 1993. pp. 231–239. [Google Scholar]
- 13.Andino R, Rieckhof G E, Achacoso P L, Baltimore D. Poliovirus RNA synthesis utilizes an RNA complex formed around the 5′-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ando T, Monroe S S, Gentsch J R, Jin Q, Lewis D C, Glass R I. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and Southern hybridization. J Clin Microbiol. 1995;33:64–71. doi: 10.1128/jcm.33.1.64-71.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anonymous. Genomic analysis of type 3 wild poliovirus isolates in southern Alberta. Can Commun Dis Rep. 1993;19:96–99. [PubMed] [Google Scholar]
- 16.Archard L C, Bowles N E, Behan P O, Bell E J, Doyle D J. Postviral fatigue syndrome: persistence of enterovirus RNA in muscle and elevated creatine kinase. J R Soc Med. 1988;81:326–329. doi: 10.1177/014107688808100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archard L C, Freeke C, Richardson P, Meany B, Olsen E, Morgan-Capner P, Rose M, Taylor P, Banner N, Yacoub M, Bowles N. Persistence of enterovirus RNA in dilated cardiomyopathy: a progression from myocarditis. In: Schultheiss H-P, editor. New concepts in viral heart disease. Berlin, Germany: Springer-Verlag KG; 1988. pp. 349–362. [Google Scholar]
- 18.Armstrong J A, Edmonds M, Nakazato H, Phillips B S, Vaughan M H. Polyadenylic acid sequences in the virion RNA of poliovirus and eastern equine encephalitis virus. Science. 1972;176:526–528. doi: 10.1126/science.176.4034.526. [DOI] [PubMed] [Google Scholar]
- 19.Arola A, Santti J, Ruuskanen O, Halonen P, Hyypiä T. Identification of enteroviruses in clinical specimens by competitive PCR followed by genetic typing using sequence analysis. J Clin Microbiol. 1996;34:313–318. doi: 10.1128/jcm.34.2.313-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assaad F, Cockburn W C. Four-year study of WHO virus reports on enterovirus other than poliovirus. Bull W H O. 1972;46:329–336. [PMC free article] [PubMed] [Google Scholar]
- 21.Assaad F, Cockburn W C. The relation between acute persisting spinal paralysis and poliomyelitis vaccine—results of a ten year enquiry. Bull W H O. 1982;60:231–242. [PMC free article] [PubMed] [Google Scholar]
- 22.Ault G S, Stoner G L. Two major types of JC virus defined in progressive multifocal leukoencephalopathy brain by early and late coding region DNA sequences. J Gen Virol. 1992;73:2669–2678. doi: 10.1099/0022-1317-73-10-2669. [DOI] [PubMed] [Google Scholar]
- 23.Bailly J L, Borman A M, Peigue-Lafeuille H, Kean K M. Natural isolates of ECHO virus type 25 with extensive variations in IRES sequences and different translational efficiencies. Virology. 1996;215:83–96. doi: 10.1006/viro.1996.0009. [DOI] [PubMed] [Google Scholar]
- 24.Balanant J, Guillot S, Candrea A, Delpeyroux F, Crainic R. The natural genomic variability of polioviruses analysed by a restriction fragment length polymorphism assay. Virology. 1991;184:645–654. doi: 10.1016/0042-6822(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 25.Banatvala J E. Insulin-dependent (juvenile-onset, type 1) diabetes mellitus: Coxsackie B viruses revisited. Prog Med Virol. 1987;34:33–54. [PubMed] [Google Scholar]
- 26.Barrett-Connor E. Is insulin dependent diabetes mellitus caused by Coxsackie B infection? A review of the epidemiologic evidence. Rev Infect Dis. 1985;7:207–215. doi: 10.1093/clinids/7.2.207. [DOI] [PubMed] [Google Scholar]
- 27.Bastis D, Simonet S, Patterson M A, Neill S. Identification of enteroviruses by indirect immunofluorescence using monoclonal antibodies. Clin Diagn Virol. 1995;3:83–93. doi: 10.1016/0928-0197(94)00025-p. [DOI] [PubMed] [Google Scholar]
- 28.Beck M A, Kolbeck P C, Rohr L H, Shi Q, Morris V C, Levander O A. Vitamin E deficiency intensifies the myocardial injury of coxsackievirus B3 infection of mice. J Nutr. 1994;124:345–358. doi: 10.1093/jn/124.3.345. [DOI] [PubMed] [Google Scholar]
- 29.Beck M A, Kolbeck P C, Rohr L H, Shi Q, Morris V C, Levander O A. Benign human enterovirus becomes virulent in selenium-deficient mice. J Med Virol. 1994;43:166–170. doi: 10.1002/jmv.1890430213. [DOI] [PubMed] [Google Scholar]
- 30.Beck M A, Kolbeck P C, Shi Q, Rohr L H, Morris V C, Levander O A. Increased virulence of a human enterovirus (coxsackievirus B3) in selenium-deficient mice. J Infect Dis. 1994;170:351–357. doi: 10.1093/infdis/170.2.351. [DOI] [PubMed] [Google Scholar]
- 31.Beck M A, Shi Q, Morris V C, Levander O A. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- 32.Bell E J, McCartney R A, Basquill D, Chaudhuri A K R. μ-antibody capture ELISA for the rapid diagnosis of enterovirus infections in patients with aseptic meningitis. J Med Virol. 1986;19:213–217. doi: 10.1002/jmv.1890190303. [DOI] [PubMed] [Google Scholar]
- 33.Bendig J W A, Molyneaux P. Sensitivity and specificity of μ-capture ELISA for detection of enterovirus IgM. J Virol Methods. 1996;59:23–32. doi: 10.1016/0166-0934(95)01997-9. [DOI] [PubMed] [Google Scholar]
- 34.Bergelson J M, Chan M, Solomon K, St. John N F, Lin H, Finberg R W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for many echoviruses. Proc Natl Acad Sci USA. 1994;91:6245–6249. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 36.Bergelson J M, St. John N, Kawaguchi S, Chan M, Stubdal H, Modlin J, Finberg R W. Infection by echoviruses 1 and 8 depends on the alpha 2 subunit of human VLA-2. J Virol. 1993;67:6847–6852. doi: 10.1128/jvi.67.11.6847-6852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berkovich S, Ressel M. Effect of sex on susceptibility of adult mice to coxsackie B2 infection. Arch Gesamte Virusforsch. 1967;22:246–251. doi: 10.1007/BF01240519. [DOI] [PubMed] [Google Scholar]
- 38.Berlin L E, Rorabaugh M L, Heldrich F, Roberts K, Doran T, Modlin J F. Aseptic meningitis in infants <2 years of age: diagnosis and etiology. J Infect Dis. 1993;168:888–892. doi: 10.1093/infdis/168.4.888. [DOI] [PubMed] [Google Scholar]
- 39.Bharucha E P, Mondkar V P, Wardia N H, Irani P F, Katrak S M. Neurological complications of a new conjunctivitis. Lancet. 1972;ii:970–971. doi: 10.1016/s0140-6736(72)92492-0. [DOI] [PubMed] [Google Scholar]
- 40.Bienkowska-Szewczyk K, Ehrenfeld E. An internal 5′ noncoding region required for translation of poliovirus RNA in vitro. J Virol. 1988;62:3068–3072. doi: 10.1128/jvi.62.8.3068-3072.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bienz K, Egger D, Pfister T, Troxler M. Structural and functional characterization of the poliovirus replication complex. J Virol. 1992;66:2740–2747. doi: 10.1128/jvi.66.5.2740-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birch D E, Kolmodin L, Wong J, Zangenberg G A, Zoccoli M A, McKinney N, Young K K Y, Laird W J. Simplified hot start PCR. Nature. 1996;381:445–446. doi: 10.1038/381445a0. [DOI] [PubMed] [Google Scholar]
- 43.Black W C, Vanlandingham D N, Sweeney W P, Wasieloski L P, Calisher C H, Beaty B J. Typing of LaCrosse, snowshoe hare, and Tahyna viruses by analyses of single-strand conformation polymorphisms of the small RNA segments. J Clin Microbiol. 1995;33:3179–3182. doi: 10.1128/jcm.33.12.3179-3182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boivin G, Chou S, Quirk M R, Erice A, Jordan M C. Detection of ganciclovir resistance mutations and quantitation of cytomegalovirus (CMV) DNA in leukocytes of patients with fatal disseminated CMV disease. J Infect Dis. 1996;173:523–528. doi: 10.1093/infdis/173.3.523. [DOI] [PubMed] [Google Scholar]
- 45.Boman J, Nilsson B, Juto P. Serum IgA, IgG, and IgM responses to different enteroviruses as measured by a coxsackie B5-based indirect ELISA. J Med Virol. 1992;38:32–35. doi: 10.1002/jmv.1890380108. [DOI] [PubMed] [Google Scholar]
- 46.Bootman J S, Kitchin P A. Reference preparations in the standardisation of HIV-1 PCR - an international collaborative study. J Virol Methods. 1994;49:1–8. doi: 10.1016/0166-0934(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 47.Böttiger M, Herrström E. Isolation of polioviruses from sewage and their characteristics: experience over two decades in Sweden. Scand J Infect Dis. 1992;24:151–155. doi: 10.3109/00365549209052605. [DOI] [PubMed] [Google Scholar]
- 48.Bouchard M J, Lam D-H, Racaniello V R. Determinants of attenuation and temperature sensitivity in the type 1 poliovirus Sabin vaccine. J Virol. 1995;69:4972–4978. doi: 10.1128/jvi.69.8.4972-4978.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowles N E, Richardson P J, Olsen E G J, Archard L C. Detection of coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;i:1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- 50.Brandful J A, Takeda N, Yoshii T, Miyamura K, Mingle J A, Addy E T, Yamazaki S. A study of the evolution of coxsackievirus A24 variant in Ghana by viral RNA fingerprinting analysis. Res Virol. 1991;142:57–65. doi: 10.1016/0923-2516(91)90028-2. [DOI] [PubMed] [Google Scholar]
- 51.Brown B A, Pallansch M A. Complete nucleotide sequence of enterovirus 71 is distinct from poliovirus. Virus Res. 1995;39:195–205. doi: 10.1016/0168-1702(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 52.Caggana M, Chan P, Ramsingh A. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J Virol. 1993;67:4797–4803. doi: 10.1128/jvi.67.8.4797-4803.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calvez V, Pelletier I, Borzakian S, Colbere-Garapin F. Identification of a region of the poliovirus genome involved in persistent infection of HEp-2 cells. J Virol. 1993;67:4432–4435. doi: 10.1128/jvi.67.7.4432-4435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cambridge G, MacArthur C G C, Waterson A P, Goodwin J F, Oakley C M. Antibodies to coxsackie B viruses in primary congestive cardiomyopathy. Br Heart J. 1979;41:692–696. doi: 10.1136/hrt.41.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cann A J, Stanway G, Hughes P J, Minor P D, Evans D M A, Schild G C, Almond J W. Reversion to neurovirulence of the live attenuated Sabin type 3 oral poliovirus vaccine. Nucleic Acids Res. 1984;12:7787–7792. doi: 10.1093/nar/12.20.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao Y Y, Yang X X, Li J, Ren L P, Lin W H, Guo Y N, Lu Z W, Li J F. Study on the etiology of hand, foot and mouth disease (HFMD) in China. Acta Acad Med Sin. 1985;7:333–336. [PubMed] [Google Scholar]
- 57.Casas I, Klapper P E, Cleator G M, Echevarría J E, Tenorio A, Echevarría J M. Two different PCR assays to detect enteroviral RNA in CSF samples from patients with acute aseptic meningitis. J Med Virol. 1995;47:378–385. doi: 10.1002/jmv.1890470414. [DOI] [PubMed] [Google Scholar]
- 58.Cello J, Samuelson A, Stålhandske P, Svennerholm B, Jeansson S, Forsgren M. Identification of group-common linear epitopes in structural and nonstructural proteins of enteroviruses by using synthetic peptides. J Clin Microbiol. 1993;31:911–916. doi: 10.1128/jcm.31.4.911-916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan D, Hammond G W. Comparison of serodiagnosis of group B coxsackie virus infections by an immunoglobulin M capture enzyme immunoassay versus microneutralization. J Clin Microbiol. 1985;21:830–834. doi: 10.1128/jcm.21.5.830-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang K H, Auvinen P, Hyypiä T, Stanway G. The nucleotide sequence of coxsackievirus A9: implications for receptor binding and enterovirus classification. J Gen Virol. 1989;70:3269–3280. doi: 10.1099/0022-1317-70-12-3269. [DOI] [PubMed] [Google Scholar]
- 61.Chang K H, Day C, Walker J, Hyypiä T, Stanway G. The nucleotide sequences of wild-type coxsackievirus A9 strains imply that an RGD motif in VP1 is functionally significant. J Gen Virol. 1992;73:621–626. doi: 10.1099/0022-1317-73-3-621. [DOI] [PubMed] [Google Scholar]
- 62.Chapman, N. M., and S. Tracy. 1995. Can recombinant DNA technology provide useful vaccines against viruses which induce heart disease? Eur. Heart J. 16(Suppl. O):144–146. [DOI] [PubMed]
- 63.Chapman N M, Tracy S, Gauntt C J, Fortmueller U. Molecular detection and identification of enteroviruses by using enzymatic amplification and nucleic acid hybridization. J Clin Microbiol. 1990;28:843–850. doi: 10.1128/jcm.28.5.843-850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chayama K, Tsubota A, Arase Y, Saitoh S, Koida I, Ikeda K, Matsumoto T, Kobayashi M, Iwasaki S, Koyama S, Morinaga T, Kumada H. Genotypic subtyping of hepatitis C virus. J Gastroenterol Hepatol. 1993;8:150–156. doi: 10.1111/j.1440-1746.1993.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 65.Cheingsong-Popov R, Lister S, Callow D, Kaleebu P, Beddows S, Weber J. Serotyping HIV type 1 by antibody binding to the V3 loop: relationship to viral genotype. WHO Network for HIV Isolation and Characterization AIDS Res Hum Retroviruses. 1994;10:1379–1386. doi: 10.1089/aid.1994.10.1379. [DOI] [PubMed] [Google Scholar]
- 66.Chezzi C. Rapid diagnosis of poliovirus infection by PCR amplification. J Clin Microbiol. 1996;34:1722–1725. doi: 10.1128/jcm.34.7.1722-1725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chonmaitree T, Baldwin C D, Lucia H L. Role of the virology laboratory in diagnosis and management of patients with central nervous system disease. Clin Microbiol Rev. 1989;2:1–14. doi: 10.1128/cmr.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chonmaitree T, Ford C, Sanders C, Lucia H L. Comparison of cell cultures for rapid isolation of enteroviruses. J Clin Microbiol. 1988;26:2576–2580. doi: 10.1128/jcm.26.12.2576-2580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chonmaitree T, Mann L. Respiratory infections. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 255–270. [Google Scholar]
- 70.Chow L H, Gauntt C J, McManus B M. Differential effects of myocarditic variants of coxsackievirus B3 in inbred mice. A pathologic characterization of heart tissue damage. Lab Invest. 1991;64:55–64. [PubMed] [Google Scholar]
- 71.Christodoulou C, Colbère-Garapin F, Macadam A, Taffs L F, Marsden S, Minor P, Horaud F. Mapping of mutations associated with neurovirulence in monkeys infected with Sabin 1 poliovirus revertants selected at high temperature. J Virol. 1990;64:4922–4929. doi: 10.1128/jvi.64.10.4922-4929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chumakov K M, Dragunsky E M, Norwood L P, Douthitt M P, Ran Y, Taffs R E, Ridge J, Levenbrook I S. Consistent selection of mutations in the 5′-untranslated region of oral poliovirus vaccine upon passaging in vitro. J Med Virol. 1994;42:79–85. doi: 10.1002/jmv.1890420115. [DOI] [PubMed] [Google Scholar]
- 73.Chumakov K M, Norwood L P, Parker M L, Dragunsky E M, Ran Y, Levenbrook I S. RNA sequence variants in live poliovirus vaccine and their relation to neurovirulence. J Virol. 1992;66:966–970. doi: 10.1128/jvi.66.2.966-970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chumakov K M, Powers L B, Noonan K E, Roninson I B. Correlation between amount of virus with altered nucleotide sequence and the monkey test for acceptability of oral poliovirus vaccine. Proc Natl Acad Sci USA. 1991;88:199–203. doi: 10.1073/pnas.88.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chumakov K M, Voroshilova M, Shindarov L, Lavrova I, Gracheva L, Koroleva G, Vasilenko S, Brodvarova I, Nikolova M, Gyurova S, Gacheva M, Mitov G, Ninov N, Tsylka E, Robinson I, Frolova M, Bashkirtsev V, Martiyanova L, Rodin V. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol. 1979;60:329–340. doi: 10.1007/BF01317504. [DOI] [PubMed] [Google Scholar]
- 76.Claas E C, Sprenger M J, Kleter G E, van Beek R, Quint W G, Masurel N. Type-specific identification of influenza viruses A, B and C by the polymerase chain reaction. J Virol Methods. 1992;39:1–13. doi: 10.1016/0166-0934(92)90120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clements G B, McGarry F, Nairn C, Galbraith D N. The detection of enterovirus specific RNA in serum: the relationship to chronic fatigue. J Med Virol. 1995;45:151–166. doi: 10.1002/jmv.1890450208. [DOI] [PubMed] [Google Scholar]
- 78.Clewley J P. Derivation and interpretation of viral nucleotide sequences from clinical specimens. Rev Med Microbiol. 1995;6:26–38. [Google Scholar]
- 79.Cohen-Abbo A, Culley B S, Sannella E C, Wright P F. Diagnostic tests for poliovirus infection: a comparison of neutralization and immunofluorescence for the identification and typing of stool isolates. J Virol Methods. 1995;52:35–39. doi: 10.1016/0166-0934(94)00132-z. [DOI] [PubMed] [Google Scholar]
- 80.Colonno R J, Callahan P I, Long W J. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986;57:7–12. doi: 10.1128/jvi.57.1.7-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Conyn-van Spaendonck M A E, Oostvogel P M, van Loon A M, van Wijngaarden J K, Kromhout D. Circulation of poliovirus during the poliomyelitis outbreak in the Netherlands in 1992–1993. Am J Epidemiol. 1996;143:929–935. doi: 10.1093/oxfordjournals.aje.a008836. [DOI] [PubMed] [Google Scholar]
- 82.Couderc T, Hogle J, Le Blay H, Horaud F, Blondel B. Molecular characterization of mouse-virulent poliovirus type 1 Mahoney mutants: involvement of polypeptides VP1 and VP2 located on the inner surface of the capsid protein shell. J Virol. 1993;67:3808–3817. doi: 10.1128/jvi.67.7.3808-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Couderc T, Christodoulou C, Kopecka H, Marsden S, Taffs L F, Crainic R, Horaud F. Molecular pathogenesis of neural lesions induced by poliovirus type 1. J Gen Virol. 1989;70:2907–2918. doi: 10.1099/0022-1317-70-11-2907. [DOI] [PubMed] [Google Scholar]
- 84.Cova L, Kopecka H, Aymard M, Girard M. Use of cRNA probes for the detection of enteroviruses by molecular hybridization. J Med Virol. 1988;24:11–18. doi: 10.1002/jmv.1890240103. [DOI] [PubMed] [Google Scholar]
- 85.Crainic R, Couillin P, Blondel B, Cabau N, Boue A, Horodniceanu F. Natural variation of poliovirus neutralization epitopes. Infect Immun. 1983;41:1217–1225. doi: 10.1128/iai.41.3.1217-1225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dagan R. Nonpolio enteroviruses and the febrile young infant: epidemiologic, clinical and diagnostic aspects. Pediatr Infect Dis J. 1996;15:67–71. doi: 10.1097/00006454-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 87.Dagan R, Jenista J A, Menegus M A. Association of clinical presentation, laboratory findings, and virus serotypes with the presence of meningitis in hospitalized infants with enterovirus infection. J Pediatr. 1988;113:975–978. doi: 10.1016/s0022-3476(88)80566-3. [DOI] [PubMed] [Google Scholar]
- 88.Dagan R, Jenista J A, Prather S L, Powell K R, Menegus M A. Viremia in hospitalized children with enterovirus infections. J Pediatr. 1985;106:397–401. doi: 10.1016/s0022-3476(85)80663-6. [DOI] [PubMed] [Google Scholar]
- 89.Dagan R, Menegus M A. A combination of four cell types for rapid detection of enteroviruses in clinical specimens. J Med Virol. 1986;19:219–228. doi: 10.1002/jmv.1890190304. [DOI] [PubMed] [Google Scholar]
- 90.Dagan R, Menegus M A. Nonpolio enteroviruses and the febrile infant. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 239–254. [Google Scholar]
- 91.Dagan R, Prather S L, Powell K R, Menegus M A. Neutralizing antibodies to non-polio enteroviruses in human immune serum globulin. Pediatr Infect Dis J. 1983;2:454–456. doi: 10.1097/00006454-198311000-00009. [DOI] [PubMed] [Google Scholar]
- 92.Dahllund L, Nissinen L, Pulli T, Hyttinen V-P, Stanway G, Hyypiä T. The genome of echovirus 11. Virus Res. 1995;35:215–222. doi: 10.1016/0168-1702(94)00104-k. [DOI] [PubMed] [Google Scholar]
- 93.Damen M, Cuypers H T M, Zaaijer H L, Reesink H W, Schaasberg W P, Gerlich W H, Niesters H G M, Lelie P N. International collaborative study on the second EUROHEP HCV-RNA reference panel. J Virol Methods. 1996;58:175–185. doi: 10.1016/0166-0934(96)02011-3. [DOI] [PubMed] [Google Scholar]
- 94.Da Silva E E, Pallansch M A, Holloway B P, Oliveira M J C, Schatmayr H G, Kew O M. Oligonucleotide probes for the specific detection of the wild poliovirus type 1 and 3 endemic to Brazil. Intervirology. 1991;32:149–159. doi: 10.1159/000150195. [DOI] [PubMed] [Google Scholar]
- 95.Davidson F, Simmonds P, Ferguson J C, Jarvis L M, Dow B C, Follett E A, Seed C R, Krusius T, Lin C, Medgyesi G A, Kiyokawa H, Olim G, Duraisamy G, Cuypers T, Saeed A A, Teo D, Conradie J, Kew M C, Lin M, Nuchaprayoon C, Ndimbie O K, Yap P-L. Survey of major genotypes and subtypes of hepatitis C virus using RFLP of sequences amplified from the 5′ non-coding region. J Gen Virol. 1995;76:1197–1204. doi: 10.1099/0022-1317-76-5-1197. [DOI] [PubMed] [Google Scholar]
- 96.Day C, Cumming H, Walker J. Enterovirus-specific IgM in the diagnosis of meningitis. J Infect. 1989;19:219–228. doi: 10.1016/s0163-4453(89)90689-0. [DOI] [PubMed] [Google Scholar]
- 97.De L, Nottay B, Yang C-F, Holloway B P, Pallansch M, Kew O. Identification of vaccine-related polioviruses by hybridization with specific RNA probes. J Clin Microbiol. 1995;33:562–571. doi: 10.1128/jcm.33.3.562-571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Leon R, Shieh C, Baric S, Sobsey M D. Advances in water analysis and treatment. Proceedings of the Water Quality Technology Conference. Denver, Colo: American Water Works Association; 1990. Detection of enteroviruses and hepatitis A virus in environmental samples by gene probes and polymerase chain reaction; pp. 833–853. [Google Scholar]
- 99.Delwart E L, Busch M P, Kalish M L, Mosley J W, Mullins J I. Rapid molecular epidemiology of human immunodeficiency virus transmission. AIDS Res Hum Retroviruses. 1995;11:1081–1093. doi: 10.1089/aid.1995.11.1081. [DOI] [PubMed] [Google Scholar]
- 100.Deng M Y, Day S P, Cliver D O. Detection of hepatitis A in environmental samples by antigen-capture PCR. Appl Environ Microbiol. 1994;60:1927–1933. doi: 10.1128/aem.60.6.1927-1933.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deshpande J M, Dave K H. Intratypic differentiation of poliovirus type 1 isolates. Indian J Med Res. 1991;93:202–207. [PubMed] [Google Scholar]
- 102.De Villiers E M. Human pathogenic papillomavirus types: an update. In: zur Hausen H, editor. Human pathogenic papillomaviruses. Berlin, Germany: Springer-Verlag KG; 1994. pp. 1–12. [Google Scholar]
- 103.Diedrich S, Driesel G, Schreier E. Sequence comparison of echovirus type 30 isolates to other enteroviruses in the 5′ noncoding region. J Med Virol. 1995;46:148–152. doi: 10.1002/jmv.1890460212. [DOI] [PubMed] [Google Scholar]
- 104.Dietz V, Andrus J, Olive J M, Cochi S, Dequadros C. Epidemiology and clinical characteristics of acute flaccid paralysis associated with non-polio enterovirus isolation—the experience in the Americas. Bull W H O. 1995;73:597–603. [PMC free article] [PubMed] [Google Scholar]
- 105.Drebot M A, Nguan C Y, Campbell J J, Lee S H S, Forward K R. Molecular epidemiology of enterovirus outbreaks in Canada during 1991–1992: identification of echovirus 30 and coxsackievirus B1 strains by amplicon sequencing. J Med Virol. 1994;44:340–347. doi: 10.1002/jmv.1890440406. [DOI] [PubMed] [Google Scholar]
- 106.Dwyer J M, Erlendsson K. Intraventricular gamma-globulin for the management of enterovirus encephalitis. Pediatr Infect Dis J. 1988;7:530–533. [PubMed] [Google Scholar]
- 107.Easton A J, Eglin R P. The detection of coxsackievirus RNA in cardiac tissue by in situ hybridization. J Gen Virol. 1988;69:285–291. doi: 10.1099/0022-1317-69-2-285. [DOI] [PubMed] [Google Scholar]
- 108.Echevarría J E, Tenorio A, Courouce A M, Leon P, Echevarría J M. Polymerase chain reaction can resolve some undefined cases of hepatitis B virus antigenic subtyping. J Med Virol. 1994;42:217–223. doi: 10.1002/jmv.1890420302. [DOI] [PubMed] [Google Scholar]
- 109.Egger D, Pasamontes L, Ostermayer M, Bienz K. Reverse transcription multiplex PCR for differentiation between polio- and enteroviruses from clinical and environmental samples. J Clin Microbiol. 1995;33:1442–1447. doi: 10.1128/jcm.33.6.1442-1447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eichner M, Dietz K. Eradication of poliomyelitis: when can one be sure that polio virus transmission has been terminated? Am J Epidemiol. 1996;143:816–822. doi: 10.1093/oxfordjournals.aje.a008820. [DOI] [PubMed] [Google Scholar]
- 111.El-Hagrassy M M O, Coltart D J, Banatvala J E. Coxsackie-B-virus-specific IgM responses in patients with cardiac and other diseases. Lancet. 1980;ii:1160–1162. doi: 10.1016/s0140-6736(80)92595-7. [DOI] [PubMed] [Google Scholar]
- 112.Erlendsson K, Swartz T, Dwyer J M. Successful reversal of echovirus encephalitis in X-linked hypogammaglobulinemia by intraventricular administration of immunoglobulin. N Engl J Med. 1985;312:351–353. doi: 10.1056/NEJM198502073120605. [DOI] [PubMed] [Google Scholar]
- 113.Ferguson M, Qui Y-H, Minor P D, Magrath D A, Spitz M, Schild G C. Monoclonal antibodies specific to the Sabin vaccine strain of poliovirus type 3. Lancet. 1982;i:122–124. doi: 10.1016/s0140-6736(82)91091-1. [DOI] [PubMed] [Google Scholar]
- 114.Figueroa J P, Ashley D, King D, Hull B. An outbreak of acute flaccid paralysis in Jamaica associated with echovirus type 22. J Med Virol. 1989;29:315–319. doi: 10.1002/jmv.1890290418. [DOI] [PubMed] [Google Scholar]
- 115.Flanegan J B, Petterson R F, Ambros V, Hewlett M J, Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5′ terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci USA. 1977;74:961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fredericks D N, Relman D A. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Freistadt M S, Eberle K E. Correlation between poliovirus type 1 Mahoney replication in blood cells and neurovirulence. J Virol. 1996;70:6486–6492. doi: 10.1128/jvi.70.9.6486-6492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frenkel L M, Wagner L E, Jr, Atwood S M, Cummins T J, Dewhurst S. Specific, sensitive, and rapid assay for human immunodeficiency virus type 1 pol mutations associated with resistance to zidovudine and didanosine. J Clin Microbiol. 1995;33:342–347. doi: 10.1128/jcm.33.2.342-347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frisk G, Torfason E G, Diderholm H. Reverse radioimmunoassays of IgM and IgG antibodies to coxsackie B viruses in patients with acute myopericarditis. J Med Virol. 1984;14:191–200. doi: 10.1002/jmv.1890140302. [DOI] [PubMed] [Google Scholar]
- 120.Fujimura Y, Ishimoto S, Shimoyama T, Narita N, Kuze Y, Yoshioka A, Fukui H, Tanaka T, Tsuda F, Okamoto H, Miyakawa Y, Mayumi M. Genotypes and multiple infections with hepatitis C virus in patients with haemophilia A in Japan. J Viral Hepatitis. 1996;3:79–84. doi: 10.1111/j.1365-2893.1996.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 121.Fujioka S, Koide H, Kitaura Y, Duguchi H, Kawamura K. Analysis of enterovirus genotypes using single-strand conformation polymorphisms of polymerase chain reaction products. J Virol Methods. 1995;51:253–258. doi: 10.1016/0166-0934(94)00112-t. [DOI] [PubMed] [Google Scholar]
- 122.Furione M, Guillot S, Otelea D, Balanant J, Candrea A, Crainic R. Polioviruses with natural recombinant genomes isolated from vaccine-associated paralytic poliomyelitis. Virology. 1993;196:199–208. doi: 10.1006/viro.1993.1468. [DOI] [PubMed] [Google Scholar]
- 123.Galbraith D N, Nairn C, Clements G B. Phylogenetic analysis of short enteroviral sequences from patients with chronic fatigue syndrome. J Gen Virol. 1995;76:1701–1707. doi: 10.1099/0022-1317-76-7-1701. [DOI] [PubMed] [Google Scholar]
- 124.Gatmaitan B G, Chason J L, Lerner A M. Augmentation of the virulence of murine coxsackievirus B3 myocardiopathy by exercise. J Exp Med. 1970;131:1121–1136. doi: 10.1084/jem.131.6.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gauntt C J, Pallansch M A. Coxsackievirus B3 clinical isolates and murine myocarditis. Virus Res. 1996;41:89–99. doi: 10.1016/0168-1702(95)01250-8. [DOI] [PubMed] [Google Scholar]
- 126.Gear J H S. Non polio causes of polio-like paralytic syndromes. Rev Infect Dis. 1984;6:S379–S384. doi: 10.1093/clinids/6.supplement_2.s379. [DOI] [PubMed] [Google Scholar]
- 127.Georgescu M-M, Delpeyroux F, Tardy-Panit M, Balanant J, Combiescu M, Combiescu A A, Guillot S, Crainic R. High diversity of poliovirus strains isolated from the central nervous system from patients with vaccine-associated paralytic poliomyelitis. J Virol. 1994;68:8089–8101. doi: 10.1128/jvi.68.12.8089-8101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gerba C P. Virus survival in wastewater treatment. In: Goddard M, Butler M, editors. Viruses and waste water treatment. Oxford, United Kingdom: Pergamon Press; 1981. pp. 39–48. [Google Scholar]
- 129.Gingeras T R, Prodanovich P, Latimer T, Guatelli J C, Richman D D, Barringer K J. Use of self-sustained sequence replication amplification reaction to analyze and detect mutations in zidovudine-resistant human immunodeficiency virus. J Infect Dis. 1991;164:1066–1074. doi: 10.1093/infdis/164.6.1066. [DOI] [PubMed] [Google Scholar]
- 130.Gjøen K, Bruu A L, Ørstavik I. Intratypic genome variability of echovirus type 30 in part of the VP4/VP2 coding region. Arch Virol. 1996;141:901–908. doi: 10.1007/BF01718164. [DOI] [PubMed] [Google Scholar]
- 131.Glikmann G, Pedersen M, Pedersen I. Intratypic differentiation of poliovirus strains by enzyme-linked immunosorbent assay (ELISA): poliovirus type 2 and poliovirus type 3. J Virol Methods. 1987;18:25–36. doi: 10.1016/0166-0934(87)90107-8. [DOI] [PubMed] [Google Scholar]
- 132.Glimåker M, Abebe A, Johansson B, Ehrnst A, Olcén P, Strannegård Ö. Detection of enteroviral RNA by polymerase chain reaction in faecal samples from patients with aseptic meningitis. J Med Virol. 1992;38:54–61. doi: 10.1002/jmv.1890380112. [DOI] [PubMed] [Google Scholar]
- 133.Glimåker M, Johansson B, Olcén P, Ehrnst A, Forsgren M. Detection of enteroviral RNA by polymerase chain reaction in cerebrospinal fluid from patients with aseptic meningitis. Scand J Infect Dis. 1992;25:547–557. doi: 10.3109/00365549309008542. [DOI] [PubMed] [Google Scholar]
- 134.Goldwater P N. Immunoglobulin M capture immunoassay in investigation of coxsackievirus B5 and B6 outbreaks in South Australia. J Clin Microbiol. 1995;33:1628–1631. doi: 10.1128/jcm.33.6.1628-1631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark H F, Forrester B, Fang Z-Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gow J W, Behan W M H, Clements G B, Woodall C, Riding M, Behan P O. Enteroviral RNA sequences detected by polymerase chain reaction in muscle of patients with postviral fatigue syndrome. Br Med J. 1991;302:692–696. doi: 10.1136/bmj.302.6778.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Graff J, Ticehurst J, Flehmig B. Detection of hepatitis A virus in sewage sludge by antigen capture polymerase chain reaction. Appl Environ Microbiol. 1993;59:3165–3170. doi: 10.1128/aem.59.10.3165-3170.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grandien M, Forsgren M, Ehrnst A. Enteroviruses. In: Lennette E H, Lennette D A, Lennette E T, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. 7th ed. Washington, D.C: American Public Health Association; 1995. pp. 279–297. [Google Scholar]
- 139.Gratsch T E, Righthand V F. Construction of a recombinant cDNA of echovirus 6 that established a persistent in vitro infection. Virology. 1994;201:341–348. doi: 10.1006/viro.1994.1299. [DOI] [PubMed] [Google Scholar]
- 140.Green K Y, Sears J F, Taniguchi K, Midthun K, Hoshino Y, Gorziglia M, Nishikawa K, Urasawa S, Kapikian A Z, Chanock R M, Flores J. Prediction of human rotavirus serotype by nucleotide sequence analysis of the VP7 protein gene. J Virol. 1988;62:1819–1823. doi: 10.1128/jvi.62.5.1819-1823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grinde B, Jonassen T O, Ushijima H. Sensitive detection of group A rotaviruses by immunomagnetic separation and reverse transcription-polymerase chain reaction. J Virol Methods. 1995;55:327–338. doi: 10.1016/0166-0934(95)00070-x. [DOI] [PubMed] [Google Scholar]
- 142.Grist N R. Coxsackie virus infections of the heart. In: Waterson A P, editor. Recent advances in clinical virology 1. Edinburgh, United Kingdom: Churchill Livingstone; 1977. pp. 141–150. [Google Scholar]
- 143.Grist N R, Bell E J. Enteroviral etiology of the paralytic poliomyelitis syndrome. Arch Environ Health. 1970;21:382. doi: 10.1080/00039896.1970.10667255. [DOI] [PubMed] [Google Scholar]
- 144.Grist N R, Bell E J. Paralytic poliomyelitis and nonpolio enteroviruses: studies in Scotland. Rev Infect Dis. 1984;6:S385–S386. doi: 10.1093/clinids/6.supplement_2.s385. [DOI] [PubMed] [Google Scholar]
- 145.Grist N R, Bell E J, Assaad F. Enteroviruses in human disease. Prog Med Virol. 1978;24:114–157. [PubMed] [Google Scholar]
- 146.Grodums E I, Dempster G. The age factor in experimental coxsackie B3 infection. Can J Microbiol. 1959;5:595–603. doi: 10.1139/m59-073. [DOI] [PubMed] [Google Scholar]
- 147.Guillot S, Otelea D, Delpeyroux F, Crainic R. Point mutations involved in the attenuation/neurovirulence alternation in type 1 and 2 oral polio vaccine strains detected by site-specific polymerase chain reaction. Vaccine. 1994;12:503–507. doi: 10.1016/0264-410x(94)90307-7. [DOI] [PubMed] [Google Scholar]
- 148.Guo J, Kitamura T, Ebihara H, Sugimoto C, Kunitake T, Takehisa J, Qun Na Y, Al-Ahadi M N, Hallin A, Kawabe K, Taguchi F, Yogo Y. Geographical distribution of the human polyomavirus JC virus types A and B and isolation of a new type from Ghana. J Gen Virol. 1996;77:919–927. doi: 10.1099/0022-1317-77-5-919. [DOI] [PubMed] [Google Scholar]
- 149.Haarmann C M, Schwimmbeck P L, Mertens T, Schultheiss H P, Strauer B E. Identification of serotype-specific and nonserotype-specific B-cell epitopes of coxsackie B virus using synthetic peptides. Virology. 1994;200:381–389. doi: 10.1006/viro.1994.1202. [DOI] [PubMed] [Google Scholar]
- 150.Haddad J, Gut J P, Wending M J, Astruc D F, Jernite M, Obert G, Messer J. Enterovirus infections in neonates. A retrospective study of 21 cases. Eur J Med. 1993;2:209–214. [PubMed] [Google Scholar]
- 151.Hagiwara A, Tagaya I, Yoneyama T. Epidemic of hand, foot and mouth disease associated with enterovirus type 71 infection. Intervirology. 1978;9:60–63. doi: 10.1159/000148922. [DOI] [PubMed] [Google Scholar]
- 152.Haller A A, Semler B L. Linker-scanning mutagenesis of the internal ribosome entry site of poliovirus RNA. J Virol. 1992;66:5075–5086. doi: 10.1128/jvi.66.8.5075-5086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Halonen P, Rocha E, Hierholzer J, Holloway B, Hyypiä T, Hurskainen P, Pallansch M. Detection of enteroviruses and rhinoviruses in clinical specimens by PCR and liquid-phase hybridization. J Clin Microbiol. 1995;33:648–653. doi: 10.1128/jcm.33.3.648-653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hamby B B, Pallansch M A, Kew O M. Reemergence of an epidemic coxsackievirus B5 genotype. J Infect Dis. 1987;156:288–292. doi: 10.1093/infdis/156.2.288. [DOI] [PubMed] [Google Scholar]
- 155.Hanecak R, Semler B L, Anderson C W, Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci USA. 1982;79:3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hanecak R, Semler B L, Ariga H, Andersson C W, Wimmer E. Expression of a cloned gene segment of poliovirus in E. coli: evidence for autocatalytic production of the viral proteinase. Cell. 1984;37:1063–1073. doi: 10.1016/0092-8674(84)90441-0. [DOI] [PubMed] [Google Scholar]
- 157.Hartig P C, Webb S R. Coxsackievirus B4 heterogeneity: effect of passage on neutralization and mortality. Acta Virol. 1986;30:475–486. [PubMed] [Google Scholar]
- 158.Heinz B A, Vance L M. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J Virol. 1995;69:4189–4197. doi: 10.1128/jvi.69.7.4189-4197.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heinz B A, Vance L M. Sequence determinants of 3A-mediated resistance to enviroxime in rhinoviruses and enteroviruses. J Virol. 1996;70:4854–4857. doi: 10.1128/jvi.70.7.4854-4857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hellen C U T, Wimmer E. Enterovirus genetics. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 25–72. [Google Scholar]
- 161.Hilton D A, Variend S, Pringle J H. Demonstration of coxsackie virus RNA in formalin-fixed sections from childhood myocarditis cases by in situ hybridization and the polymerase chain reaction. J Pathol. 1993;170:45–51. doi: 10.1002/path.1711700108. [DOI] [PubMed] [Google Scholar]
- 162.Holland J J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- 163.Holland J J, De La Torre J C, Steinhauer D A. RNA virus populations as quasispecies. Curr Top Microbiol Immunol. 1992;176:1–20. doi: 10.1007/978-3-642-77011-1_1. [DOI] [PubMed] [Google Scholar]
- 164.Holland J J, Spindler K, Horodyski F, Grabau E, Nichol S, Vandepol S. Rapid evolution of RNA genomes. Science. 1982;215:1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- 165.Holmes E C, Zhang L Q, Robertson P, Cleland A, Harvey E, Simmonds P. The molecular epidemiology of human immunodeficiency virus type 1 in Edinburgh. J Infect Dis. 1995;171:45–53. doi: 10.1093/infdis/171.1.45. [DOI] [PubMed] [Google Scholar]
- 166.Horstmann D M. Acute poliomyelitis. Relation of physical activity at the time of onset to the course of the disease. JAMA. 1950;142:236. doi: 10.1001/jama.1950.02910220016004. [DOI] [PubMed] [Google Scholar]
- 167.Hovi T, Cantell K, Huovilainen A, Kinnunen E, Kuronen T, Lapinleimu K, Pöyry T, Roivainen M, Salama N, Stenvik M, Silander A, Thoden C-J, Salaminen S, Weckström P. Outbreak of paralytic poliomyelitis in Finland: widespread circulation of antigenically altered poliovirus type 3 in a vaccinated population. Lancet. 1986;i:1427–1432. doi: 10.1016/s0140-6736(86)91566-7. [DOI] [PubMed] [Google Scholar]
- 168.Hovi T, Roivainen M. Peptide antisera targeted to a conserved sequence in poliovirus capsid protein VP1 cross-react widely with members of the genus Enterovirus. J Clin Microbiol. 1993;31:1083–1087. doi: 10.1128/jcm.31.5.1083-1087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hovi T, Stenvik M. Selective isolation of poliovirus in recombinant murine cell culture expressing the human poliovirus receptor gene. J Clin Microbiol. 1994;32:1366–1368. doi: 10.1128/jcm.32.5.1366-1368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hovi T, Stenvik M, Rosenlew M. Relative abundance of enterovirus serotypes in sewage differs from that in patients—clinical and epidemiologic implications. Epidemiol Infect. 1996;116:91–97. doi: 10.1017/s0950268800058982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hu D, Dong D, Tang E, Zhang M, Cao Y. Antigenic analysis of coxsackievirus B3 with monoclonal antibodies. Jpn J Med Sci Biol. 1993;46:87–93. doi: 10.7883/yoken1952.46.87. [DOI] [PubMed] [Google Scholar]
- 172.Hughes M S, Hoey E M, Coyle P V. A nucleotide sequence comparison of coxsackievirus B4 isolates from aquatic samples and clinical specimens. Epidemiol Infect. 1993;110:389–398. doi: 10.1017/s0950268800068333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hughes P J, Evans D M A, Minor P D, Schild G C, Almond J W, Stanway G. The nucleotide sequence of a type 3 poliovirus isolated during a recent outbreak of poliomyelitis in Finland. J Gen Virol. 1986;67:2093–2102. doi: 10.1099/0022-1317-67-10-2093. [DOI] [PubMed] [Google Scholar]
- 174.Hughes P J, North C, Minor P D, Stanway G. The complete nucleotide sequence of coxsackievirus A21. J Gen Virol. 1989;70:2943–2952. doi: 10.1099/0022-1317-70-11-2943. [DOI] [PubMed] [Google Scholar]
- 175.Huovilainen A, Kinnunen L, Pöyry T, Laaksonen L, Roivainen M, Hovi T. Poliovirus type 3/Saukett: antigenic and structural correlates of sequence variation in the capsid proteins. Virology. 1994;199:228–232. doi: 10.1006/viro.1994.1116. [DOI] [PubMed] [Google Scholar]
- 176.Huovilainen A, Mulders M N, Agboatwalla M, Pöyry T, Stenvik M, Hovi T. Genetic divergence of poliovirus strains isolated in the Karachi region of Pakistan. J Gen Virol. 1995;76:3079–3088. doi: 10.1099/0022-1317-76-12-3079. [DOI] [PubMed] [Google Scholar]
- 177.Huttunen P, Santti J, Pulli T, Hyypiä T. The major echovirus group is genetically coherent and related to coxsackie B viruses. J Gen Virol. 1996;77:715–725. doi: 10.1099/0022-1317-77-4-715. [DOI] [PubMed] [Google Scholar]
- 178.Hyypiä T, Auvinen P, Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989;70:3261–3268. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- 179.Hyypiä T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, Auvinen P, Kinnunen L, Stanway G. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci USA. 1992;89:8847–8851. doi: 10.1073/pnas.89.18.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hyypiä T, Hovi T, Knowles N J, Stanway G. Classification of enteroviruses based on molecular and biological properties. J Gen Virol. 1997;78:1–11. doi: 10.1099/0022-1317-78-1-1. [DOI] [PubMed] [Google Scholar]
- 181.Hyypiä T, Stålhandske P, Vainionpää R, Pettersson U. Detection of enteroviruses by spot hybridization. J Clin Microbiol. 1984;19:436–438. doi: 10.1128/jcm.19.3.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Iizuka N, Kuge S, Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987;156:64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- 183.Ishiko H, Takeda N, Miyamura K, Kato N, Tanimura M, Lin K H, Yin-Murphy M, Tam J S, Mu G F, Yamazaki S. Phylogenetic analysis of a coxsackievirus A24 variant: the most recent worldwide pandemic was caused by progenies of a virus prevalent around 1981. Virology. 1992;187:748–759. doi: 10.1016/0042-6822(92)90477-7. [DOI] [PubMed] [Google Scholar]
- 184.Ishiko H, Takeda N, Miyamura K, Tanimura M, Yamanaka T, Kasuga K, Oda K, Imai K, Yamamoto Y, Mochida Y, Uchida K, Nakagawa H, Yamazaki S. Phylogenetically different strains of a variant of coxsackievirus A 24 were repeatedly introduced but discontinued circulating in Japan. Arch Virol. 1992;126:179–193. doi: 10.1007/BF01309694. [DOI] [PubMed] [Google Scholar]
- 185.Jackson J B, Drew J, Lin H J, Otto P, Bremer J W, Hollinger F B, Wolinsky S M. Establishment of a quality assurance program for human immunodeficiency virus type 1 DNA polymerase chain reaction assays by the AIDS Clinical Trials Group, ACTG PCR Working Group, and the ACTG PCR Virology Laboratories. J Clin Microbiol. 1993;31:3123–3128. doi: 10.1128/jcm.31.12.3123-3128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jacobson S J, Konings D A M, Sarnow P. Biochemical and genetic evidence for a pseudoknot structure at the 3′ terminus of the poliovirus RNA genome and its role in viral RNA amplification. J Virol. 1993;67:2961–2971. doi: 10.1128/jvi.67.6.2961-2971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jansen R W, Siegl G, Lemon S M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci USA. 1990;87:2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Japour A J, Welles S, D’Aquila R T, Johnson V A, Richman D D, Coombs R W, Reichelderfer P S, Kahn J O, Crumpacker C S, Kuritzkes D R. Prevalence and clinical significance of zidovudine resistance mutations in human immunodeficiency virus isolated from patients after long-term zidovudine treatment. AIDS Clinical Trials Group 116B/117 Study Team and the Virology Committee Resistance Working Group. J Infect Dis. 1995;171:1172–1179. doi: 10.1093/infdis/171.5.1172. [DOI] [PubMed] [Google Scholar]
- 189.Jarzabek Z, Zabicka J, John A, Howlett J, Dunn G, Wood D J. Application of monoclonal antibody panels in the virological and epidemiological review of poliomyelitis in Poland, 1981–1990. Bull W H O. 1992;70:327–333. [PMC free article] [PubMed] [Google Scholar]
- 190.Jenkins O, Booth J B, Minor P D, Almond J W. The complete nucleotide sequence of coxsackie B4 and its comparison to other members of the picornaviridae. J Gen Virol. 1987;68:1835–1848. doi: 10.1099/0022-1317-68-7-1835. [DOI] [PubMed] [Google Scholar]
- 191.Jin L, Gibson P E, Booth J C, Clewley J P. Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J Med Virol. 1993;41:11–17. doi: 10.1002/jmv.1890410104. [DOI] [PubMed] [Google Scholar]
- 192.Johnston S L, Sanderson G, Pattemore P K, Smith S, Bardin P G, Bruce C B, Lambden P R, Tyrrell D A J, Holgate S T. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–117. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Johnston S L, Siegel C S. Presumptive identification of enteroviruses with RD, HEp-2, and RMK cell lines. J Clin Microbiol. 1990;28:1049–1050. doi: 10.1128/jcm.28.5.1049-1050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jung M, Agut H, Candotti D, Ingrand D, Katlama C, Huraux J M. Susceptibility of HIV-1 isolates to zidovudine: correlation between widely applicable culture test and PCR analysis. J Acquired Immune Defic Syndr. 1992;5:359–364. [PubMed] [Google Scholar]
- 195.Kamel N N. A profile for molecular biology databases and information resources. Comput Appl Biosci. 1992;8:311–321. doi: 10.1093/bioinformatics/8.4.311. [DOI] [PubMed] [Google Scholar]
- 196.Kämmerer U, Kunkel B, Korn K. Nested polymerase chain reaction for specific detection and rapid identification of human picornaviruses. J Clin Microbiol. 1994;32:285–291. doi: 10.1128/jcm.32.2.285-291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kandolf R, Canu A, Klingel K, Kirschner P, Schönke H, Mertsching J, Zell R, Hofschneider P H. Molecular studies on enteroviral heart disease. In: Brinton M A, Heinz F X, editors. New aspects of positive-strand RNA viruses. Washington, D.C: American Society for Microbiology; 1990. pp. 340–348. [Google Scholar]
- 198.Kandolf R, Hofschneider P H. Molecular cloning of the genome of a cardiotropic coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci USA. 1987;84:6272–6276. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kao S-Y, Niemiec T M, Loeffelholz M J, Dale B, Rotbart H A. Direct and uninterrupted RNA amplification of enteroviruses with colorimetric microwell detection. Clin Diagn Virol. 1995;3:247–257. doi: 10.1016/s0928-0197(94)00041-7. [DOI] [PubMed] [Google Scholar]
- 200.Kaplan M H, Klein S W, McPhee J, Harper R G. Coxsackie virus infections in infants younger than three months of age: a serious childhood illness. Rev Infect Dis. 1983;5:1019–1032. doi: 10.1093/clinids/5.6.1019. [DOI] [PubMed] [Google Scholar]
- 201.Kapsenberg J G. Picornaviridae: the enteroviruses (polioviruses, coxsackieviruses, echoviruses) In: Lennette E H, Halonen P, Murphy F A, editors. Laboratory diagnosis of infectious diseases. Principles and practice. 2. Viral, rickettsial, and chlamydial diseases. New York, N.Y: Springer-Verlag; 1988. pp. 692–722. [Google Scholar]
- 202.Kapsenberg J G, Ras A, Korte J. Improvement of enterovirus neutralization by treatment with sodium deoxycholate or chloroform. Intervirology. 1980;12:329–334. doi: 10.1159/000149092. [DOI] [PubMed] [Google Scholar]
- 203.Karnauchow T M, Tolson D L, Harrison B A, Altman E, Lublin D M, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55) J Virol. 1996;70:5143–5152. doi: 10.1128/jvi.70.8.5143-5152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kawamura N, Kohara M, Abe S, Komatsu T, Tago K, Arita M, Nomoto A. Determinants in the 5′ noncoding region of poliovirus Sabin 1 RNA that influence the attenuation phenotype. J Virol. 1989;63:1302–1309. doi: 10.1128/jvi.63.3.1302-1309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kaye S, Loveday C, Tedder R S. A microtitre format point mutation assay: application to the detection of drug resistance in human immunodeficiency virus type-1 infected patients treated with zidovudine. J Med Virol. 1992;37:241–246. doi: 10.1002/jmv.1890370402. [DOI] [PubMed] [Google Scholar]
- 206.Kew O M, Mulders M N, Lipskaya G Y, Dasilva E E, Pallansch M A. Molecular epidemiology of polioviruses. Semin Virol. 1995;6:401–414. [Google Scholar]
- 207.Kew O M, Nottay B K, Hatch M H, Hierholzer J C, Obijeski J F. Oligonucleotide fingerprint analysis of enterovirus 70 isolates from the 1980 to 1981 pandemic of acute hemorrhagic conjunctivitis: evidence for a close genetic relationship among Asian and American strains. Infect Immun. 1983;41:631–635. doi: 10.1128/iai.41.2.631-635.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Khan M, Why H, Richardson P, Archard L C. Nucleotide sequencing of polymerase chain reaction products shows the presence of Coxsackie-B3 virus in endomyocardial biopsies from patients with myocarditis or dilated cardiomyopathy. Biochem Soc Trans. 1994;22:176S. doi: 10.1042/bst022176s. [DOI] [PubMed] [Google Scholar]
- 209.Khatib R, Chason J L, Silberberg B K, Lerner A M. Age-dependent pathogenicity of group B coxsackieviruses in Swiss-Webster mice: infectivity for myocardium and pancreas. J Infect Dis. 1980;141:394–403. doi: 10.1093/infdis/141.3.394. [DOI] [PubMed] [Google Scholar]
- 210.Kidd A H, Jönsson M, Garwicz D, Kajon A E, Wermenbol A G, Verweij M J, de Jong J C. Rapid subgenus identification of human adenovirus isolates by a general PCR. J Clin Microbiol. 1996;34:622–627. doi: 10.1128/jcm.34.3.622-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kilpatrick D R, Nottay B, Yang C-F, Yang S-J, Mulders M N, Holloway B P, Pallansch M A, Kew O M. Group-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residues at positions of codon degeneracy. J Clin Microbiol. 1996;34:2990–2996. doi: 10.1128/jcm.34.12.2990-2996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim-Farley R J, Bart K J, Schonberger L B, Orenstein W A, Nkowane B M, Hinman A R, Kew O M, Hatch M H, Kaplan J E. Poliomyelitis in the USA: virtual elimination of disease caused by wild virus. Lancet. 1984;ii:1315–1317. doi: 10.1016/s0140-6736(84)90829-8. [DOI] [PubMed] [Google Scholar]
- 213.Kim-Farley R the Expanded Programme on Immunization Team. Global immunization. Annu Rev Public Health. 1992;13:223–237. doi: 10.1146/annurev.pu.13.050192.001255. [DOI] [PubMed] [Google Scholar]
- 214.Kimura H, Shibata M, Kuzushima K, Nishikawa K, Nishiyama Y, Morishima T. Detection and direct typing of herpes simplex virus by polymerase chain reaction. Med Microbiol Immunol. 1990;179:177–184. doi: 10.1007/BF00195248. [DOI] [PubMed] [Google Scholar]
- 215.Kinnunen L, Huovilainen A, Pöyry T, Hovi T. Rapid molecular evolution of wild type 3 poliovirus during infection in individual hosts. J Gen Virol. 1990;71:317–324. doi: 10.1099/0022-1317-71-2-317. [DOI] [PubMed] [Google Scholar]
- 216.Kinnunen L, Pöyry T, Hovi T. Genetic diversity and rapid evolution of poliovirus in human hosts. Curr Top Microbiol Immunol. 1992;176:49–61. doi: 10.1007/978-3-642-77011-1_4. [DOI] [PubMed] [Google Scholar]
- 217.Kitamura N, Semler B L, Rothberg P G, Larsen G R, Adler C J, Dorner A J, Emini E A, Hanecak R, Lee J J, van der Werf S, Anderson C W, Wimmer E. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981;291:547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- 218.Klespies S L, Cebula D E, Kelley C L, Galehouse D, Maurer C C. Detection of enteroviruses from clinical specimens by spin amplification shell vial culture and monoclonal antibody assay. J Clin Microbiol. 1996;34:1465–1467. doi: 10.1128/jcm.34.6.1465-1467.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Klingel K, Kandolf R. Molecular in situ localization techniques in diagnosis and pathogenicity studies of enteroviral heart disease. Clin Diagn Virol. 1996;5:157–166. doi: 10.1016/0928-0197(96)00217-6. [DOI] [PubMed] [Google Scholar]
- 220.Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci USA. 1992;89:314–318. doi: 10.1073/pnas.89.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Klump W M, Bergmann I, Muller B C, Ameis D, Kandolf R. Complete nucleotide sequence of infectious coxsackievirus B3 cDNA: two initial 5′ uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990;64:1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Knowlton K U, Joen E-S, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70:7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kogon A, Spigland I, Frothingham T G, Elveback L, Williams C, Hall C E, Fox J P. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VII. Observations on viral excretion, seroimmunity, intrafamilial spread and illness association in coxsackie and echovirus infections. Am J Epidemiol. 1969;89:51–61. doi: 10.1093/oxfordjournals.aje.a120915. [DOI] [PubMed] [Google Scholar]
- 224.Kok P W, Leeuwenburg J, Tukei P, van Wezel A L, Kapsenberg J G, van Steenis G, Galazka A, Robertson S E, Robinson D. Serological and virological assessment of oral and inactivated poliovirus vaccines in a rural population in Kenya. Bull W H O. 1992;70:93–103. [PMC free article] [PubMed] [Google Scholar]
- 225.Kono R, Miyamura K, Tajiri E, Sasagawa A, Phuapradit P. Virological and serological studies of neurological complications of acute hemorrhagic conjunctivitis in Thailand. J Infect Dis. 1977;135:706–713. doi: 10.1093/infdis/135.5.706. [DOI] [PubMed] [Google Scholar]
- 226.Kono R, Miyamura K, Tajiri E, Shiga S, Sasagawa A, Irani P F, Katrak S M, Wadia N H. Neurologic complications associated with acute hemorrhagic conjunctivitis infection and its serologic confirmation. J Infect Dis. 1974;129:590–593. doi: 10.1093/infdis/129.5.590. [DOI] [PubMed] [Google Scholar]
- 227.Kopecka H, Brown B, Pallansch M. Genotypic variation in coxsackievirus B5 isolates from three different outbreaks in the United States. Virus Res. 1995;38:125–136. doi: 10.1016/0168-1702(95)00055-u. [DOI] [PubMed] [Google Scholar]
- 228.Kopecka H, Dubrou S, Prevot J, Marechal J, López-Pila J M. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction, and hybridization. Appl Environ Microbiol. 1993;59:1213–1219. doi: 10.1128/aem.59.4.1213-1219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Korn, K. Unpublished observation.
- 230.Kostrikis L G, Cao Y, Ngai H, Moore J P, Ho D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kozal M J, Shah N, Shen N, Yang R, Fucini R, Merigan T C, Richman D D, Morris D, Hubbell E, Chee M, Gingeras T R. Extensive polymorphisms observed in HIV-1 clade B protease gene using high-density oligonucleotide arrays. Nat Med. 1996;2:753–759. doi: 10.1038/nm0796-753. [DOI] [PubMed] [Google Scholar]
- 232.Kraus W, Zimmermann H, Zimmermann A, Eggers H J, Nelsen-Salz B. Infectious cDNA clones of echovirus 12 and a variant resistant against the uncoating inhibitor rhodanine differ in seven amino acids. J Virol. 1995;69:5853–5858. doi: 10.1128/jvi.69.9.5853-5858.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.La Monica N, Meriam C, Racaniello V R. Mapping of sequences required for mouse neurovirulence of poliovirus type 2 Lansing. J Virol. 1986;57:515–525. doi: 10.1128/jvi.57.2.515-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.La Monica N, Racaniello V R. Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. J Virol. 1989;53:2357–2360. doi: 10.1128/jvi.63.5.2357-2360.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lanciotti R S, Calisher C H, Gubler D J, Chang G J, Vorndam A V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lansdown A B G. Viral infections and diseases of the heart. Prog Med Virol. 1978;24:70–113. [PubMed] [Google Scholar]
- 237.Lee T W, Megson B, Kurtz J B. Enterovirus typing by immune electronmicroscopy. J Med Microbiol. 1996;44:151–153. doi: 10.1099/00222615-44-2-151. [DOI] [PubMed] [Google Scholar]
- 238.Leparc I, Aymard M, Fuchs F. Acute, chronic and persistent enterovirus and poliovirus infections: detection of viral genome by seminested PCR amplification in culture-negative samples. Mol Cell Probes. 1994;8:487–495. doi: 10.1006/mcpr.1994.1070. [DOI] [PubMed] [Google Scholar]
- 239.Li J, Zhang L B, Yoneyama T, Yoshida H, Shimizu H, Yoshii K, Hara M, Nomura T, Yoshikura H, Miyamura T, Hagiwara A. Genetic basis of the neurovirulence of type 1 polioviruses isolated from vaccine-associated paralytic patients. Arch Virol. 1996;141:1047–1054. doi: 10.1007/BF01718608. [DOI] [PubMed] [Google Scholar]
- 240.Li Y, Yang Y, Chen H. Detection of enteroviral RNA in paraffin-embedded myocardial tissue from patients with Keshan by nested PCR. Chin Med J. 1995;75:344–345. [PubMed] [Google Scholar]
- 241.Lim K A, Benyesh-Melnick M. Typing of viruses by combination of antiserum pools. Application to typing of enteroviruses (Coxsackie and ECHO) J Immunol. 1960;84:309–317. [PubMed] [Google Scholar]
- 242.Lim K H, Yin-Murphy M. An epidemic of conjunctivitis in Singapore in 1970. Singapore Med J. 1971;12:247–249. [PubMed] [Google Scholar]
- 243.Lin K H, Takeda N, Miyamura K, Yamazaki S, Chen C W. The nucleotide sequence of 3C proteinase region of the coxsackievirus A24 variant: comparison of the isolates in Taiwan in 1985–1988. Virus Genes. 1991;5:121–131. doi: 10.1007/BF00571927. [DOI] [PubMed] [Google Scholar]
- 244.Lin K H, Wang H L, Sheu M M, Huang W L, Chen C W, Yang C S, Takeda N, Kato N, Miyamura K, Yamazaki S. Molecular epidemiology of a variant of coxsackievirus A24 in Taiwan: two epidemics caused by phylogenetically distinct viruses from 1985 to 1989. J Clin Microbiol. 1993;31:1160–1166. doi: 10.1128/jcm.31.5.1160-1166.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lindberg A M, Crowell R L, Zell R, Kandolf R, Pettersson U. Mapping of the RD phenotype of the Nancy strain of coxsackievirus B3. Virus Res. 1992;24:187–196. doi: 10.1016/0168-1702(92)90006-u. [DOI] [PubMed] [Google Scholar]
- 246.Lindberg A M, Stålhandske P O K, Pettersson U. Genome of coxsackievirus B3. Virology. 1987;156:50–63. doi: 10.1016/0042-6822(87)90435-1. [DOI] [PubMed] [Google Scholar]
- 247.Lipiskaya G Y U, Cherkasova E A, Belova G I, Maslova S V, Kutateladze T N, Drozdov S G, Mulders M N, Pallaansch M A, Kew O M, Agol V I. Geographical genotypes (geotypes) of poliovirus case isolates from the former Soviet Union: relatedness to other known poliovirus genotypes. J Gen Virol. 1995;76:1687–1699. doi: 10.1099/0022-1317-76-7-1687. [DOI] [PubMed] [Google Scholar]
- 248.Lipson S M, Walderman R, Costello P, Szabo K. Sensitivity of rhabdomyosarcoma and guinea pig embryo cell cultures to field isolates of difficult-to-cultivate group A coxsackieviruses. J Clin Microbiol. 1988;26:1298–1303. doi: 10.1128/jcm.26.7.1298-1303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lisitsyn N, Lisitsyn N, Wigler M. Cloning the differences between two complex genomes. Science. 1993;259:946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- 250.Locher F, Suryanarayana V V, Tratschin J D. Rapid detection and characterization of foot-and-mouth disease virus by restriction and nucleotide sequence analysis of PCR products. J Clin Microbiol. 1995;33:440–444. doi: 10.1128/jcm.33.2.440-444.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Loria R M, Kibrick S, Madge G. Infection in hypercholesteremic mice with coxsackievirus B. J Infect Dis. 1976;133:655–662. doi: 10.1093/infdis/133.6.655. [DOI] [PubMed] [Google Scholar]
- 252.Lyden D, Olszewski J, Huber S. Variation in susceptibility of Balb/c mice to coxsackievirus group B type 3-induced myocarditis with age. Cell Immunol. 1987;105:332–339. doi: 10.1016/0008-8749(87)90081-5. [DOI] [PubMed] [Google Scholar]
- 253.Lyden D C, Olszewski J, Feran M, Job L P, Huber S A. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–438. [PMC free article] [PubMed] [Google Scholar]
- 254.Macadam A J, Ferguson G, Arnold C, Minor P D. An assembly defect as a result of an attenuating mutation in the capsid proteins of the poliovirus type 3 vaccine strain. J Virol. 1991;65:5225–5231. doi: 10.1128/jvi.65.10.5225-5231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Macadam A J, Stone D M, Almond J W, Minor P D. The 5′ noncoding region and virulence of poliovirus vaccine strains. Trends Microbiol. 1994;2:449–454. doi: 10.1016/0966-842x(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 256.Magnius L O, Saleh L H, Vikerfors T, Norder H. A solid-phase reverse immunosorbent test for the detection of enterovirus IgM. J Virol Methods. 1988;20:73–82. doi: 10.1016/0166-0934(88)90042-0. [DOI] [PubMed] [Google Scholar]
- 257.Mapoles J E, Krah D L, Crowell R L. Purification of a HeLa cell receptor protein for group B coxsackieviruses. J Virol. 1985;55:560–566. doi: 10.1128/jvi.55.3.560-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Martin A B, Webber S, Fricker F J, Jaffe R, Demmler G, Kearney D, Zhang Y H, Bodurtha J, Gelb B, Ni J, Bricker J T, Towbin J A. Acute myocarditis. Rapid diagnosis by PCR in children. Circulation. 1994;90:330–339. doi: 10.1161/01.cir.90.1.330. [DOI] [PubMed] [Google Scholar]
- 259.Mason P W, Rieder E, Baxt B. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc Natl Acad Sci USA. 1994;91:1932–1936. doi: 10.1073/pnas.91.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mateu M G. Antibody recognition of picornaviruses and escape from neutralization: a structural view. Virus Res. 1995;38:1–24. doi: 10.1016/0168-1702(95)00048-u. [DOI] [PubMed] [Google Scholar]
- 261.Matsumoto T, Yamada O, Itagaki A, Ishida S, Kamahora T, Kurimura T. Rapid DNA diagnosis of herpes simplex virus serotypes. J Virol Methods. 1992;40:119–125. doi: 10.1016/0166-0934(92)90013-4. [DOI] [PubMed] [Google Scholar]
- 262.McCartney R A, Banatvala J E, Bell E J. Routine use of μ-antibody-capture ELISA for the serological diagnosis of coxsackie B virus infections. J Med Virol. 1986;19:205–212. doi: 10.1002/jmv.1890190302. [DOI] [PubMed] [Google Scholar]
- 263.McIntyre J P, Keen G A. Laboratory surveillance of viral meningitis by examination of cerebrospinal fluid in Cape Town, 1981–9. Epidemiol Infect. 1993;111:357–371. doi: 10.1017/s095026880005706x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.McKinney R E, Katz S L, Wilfert C M. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9:334–356. doi: 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- 265.McOmish F, Yap P L, Dow B C, Follett E A, Seed C, Keller A J, Cobain T J, Krusius T, Kolho E, Naukkarinen R, Lin C, Lai C, Leong S, Medgyesi G A, Héjjas M, Kiyokawa H, Fukada K, Cuypers T, Saeed A A, Al-Rasheed A M, Lin M, Simmonds P. Geographical distribution of hepatitis C virus genotypes in blood donors: an international collaborative survey. J Clin Microbiol. 1994;32:884–892. doi: 10.1128/jcm.32.4.884-892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.McPhee F, Zell R, Reimann B Y, Hofschneider P H, Kandolf R. Characterization of the N-terminal part of the neutralizing antigenic site I of coxsackievirus B4 by mutation analysis of antigen chimeras. Virus Res. 1994;34:139–151. doi: 10.1016/0168-1702(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 267.Mease P J, Ochs H D, Wedgwood R J. Successful treatment of echovirus meningoencephalitis and myositis-fasciitis with intravenous immune globulin therapy in a patient with X-linked agammaglobulinemia. N Engl J Med. 1981;304:1278–1281. doi: 10.1056/NEJM198105213042107. [DOI] [PubMed] [Google Scholar]
- 268.Melnick J L. Enterovirus type 71 infections: a varied clinical pattern sometimes mimicking paralytic poliomyelitis. Rev Infect Dis. 1984;6:S387–S390. doi: 10.1093/clinids/6.supplement_2.s387. [DOI] [PubMed] [Google Scholar]
- 269.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 655–712. [Google Scholar]
- 270.Melnick J L. Current status of poliovirus infections. Clin Microbiol Rev. 1996;9:293–300. doi: 10.1128/cmr.9.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Melnick J L, Rennick V, Hampil B, Schmidt N J, Ho H H. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 42 enteroviruses. Bull W H O. 1973;48:263–268. [PMC free article] [PubMed] [Google Scholar]
- 272.Melnick J L, Schmidt N J, Hampil B, Ho H H. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 19 group A coxsackievirus serotypes. Intervirology. 1977;8:172–181. doi: 10.1159/000148892. [DOI] [PubMed] [Google Scholar]
- 273.Melnick J L, Wimberly I L. Lyophilized combination pools of enterovirus equine antisera: new LBM pools prepared from reserves of antisera stored frozen for two decades. Bull W H O. 1985;63:543–550. [PMC free article] [PubMed] [Google Scholar]
- 274.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 275.Mewes H W, Doelz R, George D G. Sequence databases: an indispensable source for biotechnological research. J Biotechnol. 1994;35:239–256. doi: 10.1016/0168-1656(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 276.Meyer R F, Brown F. Sequence identification of antigenic variants in plaque isolates of foot-and-mouth disease virus. J Virol Methods. 1995;55:281–283. doi: 10.1016/0166-0934(95)00064-2. [DOI] [PubMed] [Google Scholar]
- 277.Miller N A, Carmichael H A, Calder B D, Behan P O, Bell E J, McCartney R A, Hall F C. Antibody to coxsackie B virus in diagnosing postviral fatigue syndrome. Br Med J. 1991;302:140–143. doi: 10.1136/bmj.302.6769.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Minor P D. The molecular biology of poliovaccines. J Gen Virol. 1992;73:3065–3077. doi: 10.1099/0022-1317-73-12-3065. [DOI] [PubMed] [Google Scholar]
- 279.Minor P, Brown F, Domingo E, Hoey E, King A, Knowles N, Lemon S, Palmenberg A, Ruekert R R, Stanway G, Wimmer E, Yin-Murphy M. Picornaviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy. Classification and nomenclature of viruses. Sixth report of the International Committee on Taxonomy of Viruses Virology Division, International Union of Microbiological Societies. Springer-Verlag, Vienna, Austria. (Arch. Virol. Suppl. 10.) 1995. pp. 329–336. [Google Scholar]
- 280.Minor P D, Ferguson M, Evans D M A, Almond J W, Icenogle J P. Antigenic structure of polioviruses of serotypes 1, 2, and 3. J Gen Virol. 1986;67:1283–1291. doi: 10.1099/0022-1317-67-7-1283. [DOI] [PubMed] [Google Scholar]
- 281.Minor P D, John A, Ferguson M, Icenogle J P. Antigenic and molecular evolution of the vaccine strain of type 3 poliovirus during the period of excretion of primary vaccinee. J Gen Virol. 1986;67:693–706. doi: 10.1099/0022-1317-67-4-693. [DOI] [PubMed] [Google Scholar]
- 282.Minor P D, Schild G C, Ferguson M, Mackay A, Magrath D I, John A, Yates P J, Spitz M. Genetic and antigenic variation in type 3 polioviruses: characterization of strains by monoclonal antibodies and T1 oligonucleotide mapping. J Gen Virol. 1982;61:167–176. doi: 10.1099/0022-1317-61-2-167. [DOI] [PubMed] [Google Scholar]
- 283.Mirkovic N, Schmidt J, Yin-Murphy M, Melnick J L. Enterovirus etiology of the 1970 Singapore epidemic of acute conjunctivitis. Intervirology. 1974;4:119–127. doi: 10.1159/000149850. [DOI] [PubMed] [Google Scholar]
- 284.Miyamura K, Takeda N, Tanimura M, Ogino T, Yamazaki S, Chen C W, Lin K H, Lin S Y, Ghafoor A, Murphy M. Evolutionary study on the coxsackievirus A 24 variant causing acute hemorrhagic conjunctivitis by oligonucleotide mapping analysis of RNA genome. Arch Virol. 1990;114:37–51. doi: 10.1007/BF01311010. [DOI] [PubMed] [Google Scholar]
- 285.Miyamura K, Tanimura M, Takeda N, Kono R, Yamazaki S. Evolution of enterovirus 70 in nature: all isolates were recently derived from a common ancestor. Arch Virol. 1986;89:1–14. doi: 10.1007/BF01309875. [DOI] [PubMed] [Google Scholar]
- 286.Miyamura K, Yamashita K, Yamadera S, Kato N, Akatsuka M, Hara M, Inouye S, Yamazaki S. Poliovirus surveillance: isolation of polioviruses in Japan, 1980–1991. A report of the National Epidemiological Surveillance of Infectious Agents in Japan. Jpn J Med Sci Biol. 1992;45:203–214. doi: 10.7883/yoken1952.45.203. [DOI] [PubMed] [Google Scholar]
- 287.Modlin J F. Introduction. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 1606–1613. [Google Scholar]
- 288.Modlin J F. Polioviruses. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 1613–1620. [Google Scholar]
- 289.Modlin J F. Coxsackieviruses, echoviruses, and newer enteroviruses. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 1620–1636. [Google Scholar]
- 290.Modlin J F. Poliomyelitis and poliovirus immunization. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 195–220. [Google Scholar]
- 291.Monceyron C, Grinde B. Detection of hepatitis A virus in clinical and environmental samples by immunomagnetic separation and PCR. J Virol Methods. 1994;46:157–166. doi: 10.1016/0166-0934(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 292.Moore J P, Cao Y, Leu J, Qin L, Korber B, Ho D D the WHO and NIAID Networks for HIV Isolation and Characterization. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–444. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Moore M. From the Centers for Disease Control. Enteroviral disease in the United States, 1970–1979. J Infect Dis. 1982;146:103–108. doi: 10.1093/infdis/146.1.103. [DOI] [PubMed] [Google Scholar]
- 294.Moore M, Kaplan M H, McPhee J, Bregman D J, Klein S W. Epidemiologic, clinical, and laboratory features of Coxsackie B1-B5 infections in the United States, 1970–79. Public Health Rep. 1984;99:515–522. [PMC free article] [PubMed] [Google Scholar]
- 295.Morens D M, Pallansch M A. Epidemiology. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 3–23. [Google Scholar]
- 296.Morens D M, Pallansch M A, Moore M. Polioviruses and other enteroviruses. In: Belshe R B, editor. Textbook of human virology. St. Louis, Mo: Mosby Year Book; 1991. pp. 427–497. [Google Scholar]
- 297.Mori J, Clewley J P. Polymerase chain reaction and sequencing for typing rhinovirus RNA. J Med Virol. 1994;44:323–329. doi: 10.1002/jmv.1890440403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Morita K, Tanaka M, Igarashi A. Rapid identification of dengue virus serotypes by using polymerase chain reaction. J Clin Microbiol. 1991;29:2107–2110. doi: 10.1128/jcm.29.10.2107-2110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Moss E G, O’Neill R E, Racaniello V R. Mapping of attenuating sequences of an avirulent poliovirus type 2 strain. J Virol. 1989;63:1884–1890. doi: 10.1128/jvi.63.5.1884-1890.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Muckelbauer J K, Kremer M, Minor I, Diana G, Dutko F J, Groarke J, Pevear D C, Rossman M G. The structure of coxsackievirus B3 at 3.5 Å resolution. Structure. 1995;3:653–667. doi: 10.1016/s0969-2126(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 301.Muir P, Archard L C. There is evidence for persistent enterovirus infections in chronic medical conditions in humans. Rev Med Virol. 1994;4:245–250. [Google Scholar]
- 302.Muir P, Nicholson F, Jhetam M, Neogi S, Banatvala J E. Rapid diagnosis of enterovirus infection by magnetic bead extraction and polymerase chain reaction detection of enterovirus RNA in clinical specimens. J Clin Microbiol. 1993;31:31–38. doi: 10.1128/jcm.31.1.31-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Muir P, Nicholson F, Illavia S J, McNeil T S, Ajetunmobi J F, Dunn H, Starkey W G, Reetoo K N, Cary N R B, Parameshwar J, Banatvala J E. Serological and molecular evidence of enterovirus infection in patients with end-stage dilated cardiomyopathy. Heart. 1996;76:243–249. doi: 10.1136/hrt.76.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Muir P, Nicholson F, Spencer G T, Ajetunmobi J F, Starkey W G, Khan M, Archard L C, Cairns N J, Anderson V E R, Leigh P N, Howard R S, Banatvala J E. Enterovirus infection of the central nervous system of humans: lack of association with chronic neurological disease. J Gen Virol. 1996;77:1469–1476. doi: 10.1099/0022-1317-77-7-1469. [DOI] [PubMed] [Google Scholar]
- 305.Muir P, Nicholson F, Tilzey A J, Signy M, English T A H, Banatvala J E. Chronic relapsing pericarditis and dilated cardiomyopathy: serological evidence of persistent enterovirus infection. Lancet. 1989;i:804–807. doi: 10.1016/s0140-6736(89)92270-8. [DOI] [PubMed] [Google Scholar]
- 306.Mulders M N, Koopmans M P G, van der Avoort H G A M, van Loon A M. Detection and characterization of polioviruses—the molecular approach. In: Becker Y, Darai G, editors. PCR: protocols for diagnosis of human and animal virus diseases. Berlin, Germany: Springer-Verlag KG; 1995. pp. 137–156. [Google Scholar]
- 307.Mulders M N, Lipskaya G Y, van der Avoort H G A M, Koopmans M P G, Kew O M, van Loon A M. Molecular epidemiology of wild poliovirus type 1 in Europe, the Middle East, and the Indian subcontinent. J Infect Dis. 1995;171:1399–1405. doi: 10.1093/infdis/171.6.1399. [DOI] [PubMed] [Google Scholar]
- 308.Mulders M N, van Loon A M, van der Avoort H G A M, Reimerink J H J, Ras A, Bestebroer T M, Drebot M A, Kew O M, Koopmans M P G. Molecular characterization of a wild poliovirus type 3 epidemic in the Netherlands (1992 and 1993) J Clin Microbiol. 1995;33:3252–3256. doi: 10.1128/jcm.33.12.3252-3256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Nakao T, Enomoto N, Takada N, Takada A, Date T. Typing of hepatitis C virus genomes by restriction fragment length polymorphism. J Gen Virol. 1991;72:2105–2112. doi: 10.1099/0022-1317-72-9-2105. [DOI] [PubMed] [Google Scholar]
- 310.Narang H K, Codd A A. Enterovirus typing by immune electron microscopy using low-speed centrifugation. J Clin Pathol. 1980;33:191–194. doi: 10.1136/jcp.33.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Nibbeling R, Reimerink J H J, Agboatwala M, Naquib T, Ras A, Poelstra P, van der Avoort H G A M, van Loon A M. A poliovirus type-specific IgM antibody-capture enzyme-linked immunosorbent assay for the rapid diagnosis of poliomyelitis. Clin Diagn Virol. 1994;2:113–126. doi: 10.1016/0928-0197(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 312.Nicholson F, Ajetunmobi J F, Li M, Shackleton E A, Starkey W G, Illavia S J, Muir P, Banatvala J E. Molecular detection and serotypic analysis of enterovirus RNA in archival specimens from patients with acute myocarditis. Br Heart J. 1995;74:522–527. doi: 10.1136/hrt.74.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nicholson F, Meetoo G, Aiyar S, Banatvala J E, Muir P. Detection of enterovirus RNA in clinical samples by nested polymerase chain reaction for rapid diagnosis of enterovirus infection. J Virol Methods. 1994;48:155–166. doi: 10.1016/0166-0934(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 314.Nicholson W J, Black S H, Simmonds P, Chung C W, Aw D, Peutherer J F. Comparison of hepatitis B virus subtyping of d/y determinants by radioimmunoprecipitation assay and the polymerase chain reaction. J Med Virol. 1992;36:21–27. doi: 10.1002/jmv.1890360105. [DOI] [PubMed] [Google Scholar]
- 315.Nkowane B M, Wassilak S G F, Orenstein W A. Vaccine-associated paralytic poliomyelitis. United States 1973 through 1984. JAMA. 1987;257:1335–1340. [PubMed] [Google Scholar]
- 316.Noel J S, Lee T W, Kurtz J B, Glass R I, Monroe S S. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J Clin Microbiol. 1995;33:797–801. doi: 10.1128/jcm.33.4.797-801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nomoto A, Omata T, Toyoda H, Kuge S, Horie H, Kataoka Y, Genba Y, Nakano Y, Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci USA. 1982;79:5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nottay B K, Kew O M, Hatch M H, Heyward J T, Obijeski J F. Molecular variation of type 1 vaccine-related and wild polioviruses during replication in humans. Virology. 1981;108:405–423. doi: 10.1016/0042-6822(81)90448-7. [DOI] [PubMed] [Google Scholar]
- 319.O’Connell J, Albin R, Blum D, Grint P, Schartz J. Development of antiviral agents for picornavirus infections. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 419–434. [Google Scholar]
- 320.Okamoto H, Kobata S, Tokita H, Inoue T, Woodfield G D, Holland P V, Al-Knawy B A, Uzunalimoglu O, Miyakawa Y, Mayumi M. A second-generation method of genotyping hepatitis C virus by the polymerase chain reaction with sense and antisense primers deduced from the core gene. J Virol Methods. 1996;57:31–45. doi: 10.1016/0166-0934(95)01960-x. [DOI] [PubMed] [Google Scholar]
- 321.Okamoto H, Sugiyama Y, Okada S, Kurai K, Akahane Y, Sugai Y, Tanaka T, Sato K, Tsuda F, Miyakawa Y, Mayumi M. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992;73:673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- 322.Okamoto H, Tokita H, Sakamoto M, Horikita M, Kojima M, Iizuka H, Mishiro S. Characterization of the genomic sequence of type V (or 3a) hepatitis C virus isolates and PCR primers for specific detection. J Gen Virol. 1993;74:2385–2390. doi: 10.1099/0022-1317-74-11-2385. [DOI] [PubMed] [Google Scholar]
- 323.Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo R I, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69:2575–2583. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- 324.Olive D M, Al-Mufti S, Al-Mulla W, Khan M A, Pasca A, Stanway G, Al-Nakib W. Detection and differentiation of picornaviruses in clinical samples following genomic amplification. J Gen Virol. 1990;71:2141–2147. doi: 10.1099/0022-1317-71-9-2141. [DOI] [PubMed] [Google Scholar]
- 325.Omata T, Kohara M, Kuge S, Komatsu T, Abe S, Semler B L, Kameda A, Itoh H, Arita M, Wimmer E, Nomoto A. Genetic analysis of the attenuation phenotype of poliovirus type 1. J Virol. 1986;58:348–358. doi: 10.1128/jvi.58.2.348-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Oostvogel P M, van Wijngaarden J K, van der Avoort H G, Mulders M N, Conyn-van Spaendonck M A, Rumke H C, van Steenis G, van Loon A M. Poliomyelitis outbreak in an unvaccinated community in The Netherlands, 1992–93. Lancet. 1994;344:665–670. doi: 10.1016/s0140-6736(94)92091-5. [DOI] [PubMed] [Google Scholar]
- 327.Osterhaus A D M E, van Wezel A, Hazendonk T, Uytdehaag F, van Asten J, van Steenis B. Monoclonal antibodies to polioviruses. Comparison of intratypic strain differentiation of polioviruses using monoclonal antibodies versus cross absorbed antisera. Intervirology. 1983;20:129–136. doi: 10.1159/000149381. [DOI] [PubMed] [Google Scholar]
- 328.Page G S, Mosser A G, Hogle J M, Filman D J, Rueckert R R, Chow M. Three-dimensional structure of poliovirus serotype 1 neutralizing determinants. J Virol. 1988;62:1781–1794. doi: 10.1128/jvi.62.5.1781-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Pan American Health Organization. Strategies for the certification of the eradication of wild poliovirus transmission in the Americas. Bull Pan Am Health Organ. 1993;27:287–296. [PubMed] [Google Scholar]
- 330.Paul J. A history of poliomyelitis virus. New Haven, Conn: Yale University Press; 1971. [Google Scholar]
- 331.Pavio N, Buc-Caron M-H, Colbère-Garapin F. Persistent poliovirus infection of human fetal brain cells. J Virol. 1996;70:6395–6401. doi: 10.1128/jvi.70.9.6395-6401.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Peigue-Lafeuille H, Fuchs F, Gharabaghi F, Chambon M, Aymard M. Impact on routine diagnosis of echovirus infections of intratypic differentiation and antigenic variation in echovirus type 25 studied by using monoclonal antibodies. J Clin Microbiol. 1990;28:2291–2296. doi: 10.1128/jcm.28.10.2291-2296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Pelletier J, Kaplan G, Racaniello V R, Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5′ noncoding region. Mol Cell Biol. 1988;8:1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Pilipenko E V, Blinov V M, Romanova L I, Sinyakov A N, Malsova S V, Agol V I. Conserved structural domains in the 5′ untranslated region of picornaviral genomes: an analysis of the segment controlling translation and neurovirulence. Virology. 1989;168:201–209. doi: 10.1016/0042-6822(89)90259-6. [DOI] [PubMed] [Google Scholar]
- 335.Pilipenko E V, Maslova S V, Sinyakov A N, Agol V I. Towards identification of cis-acting elements involved in the replication of enterovirus and rhinovirus RNAs: a proposal for the existence of tRNA-like terminal structures. Nucleic Acids Res. 1992;20:1739–1745. doi: 10.1093/nar/20.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pipkin P A, Wood D J, Racaniello V R, Minor P D. Characterisation of L cells expressing the human poliovirus receptor for the specific detection of polioviruses in vitro. J Virol Methods. 1993;41:333–340. doi: 10.1016/0166-0934(93)90022-j. [DOI] [PubMed] [Google Scholar]
- 337.Pollard S R, Dunn G, Cammack N, Minor P D, Almond J W. Nucleotide sequence of a neurovirulent variant of the type 2 oral poliovirus vaccine. J Virol. 1989;63:4949–4951. doi: 10.1128/jvi.63.11.4949-4951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Power J P, Lawlor E, Davidson F, Holmes E C, Yap P L, Simmonds P. Molecular epidemiology of an outbreak of infection with hepatitis C virus in recipients of anti-D immunoglobulin. Lancet. 1995;345:1211–1213. doi: 10.1016/s0140-6736(95)91993-7. [DOI] [PubMed] [Google Scholar]
- 339.Pöyry T, Hyypiä T, Horsnell C, Kinnunen L, Hovi T, Stanway G. Molecular analysis of coxsackievirus A16 reveals a new genetic group of enteroviruses. Virology. 1994;202:5313–5319. doi: 10.1006/viro.1994.1423. [DOI] [PubMed] [Google Scholar]
- 340.Pöyry T, Kinnunen L, Hovi T. Genetic variation in vivo and proposed functional domains of the 5′ noncoding region of poliovirus RNA. J Virol. 1992;66:5313–5319. doi: 10.1128/jvi.66.9.5313-5319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pöyry T, Stenvik M, Hovi T. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. Appl Environ Microbiol. 1988;54:371–374. doi: 10.1128/aem.54.2.371-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Pöyry T, Kinnunen L, Hyypiä T, Brown B, Horsnell C, Hovi T, Stanway G. Genetic and phylogenetic clustering of enteroviruses. J Gen Virol. 1996;77:1699–1717. doi: 10.1099/0022-1317-77-8-1699. [DOI] [PubMed] [Google Scholar]
- 343.Pöyry T, Kinnunen L, Kapsenberg J, Kew O, Hovi T. Type 3 poliovirus/Finland/1984 is genetically related to common Mediterranean strains. J Gen Virol. 1990;71:2535–2541. doi: 10.1099/0022-1317-71-11-2535. [DOI] [PubMed] [Google Scholar]
- 344.Prabhakar B S, Haspel M V, McClintock P R, Notkins A L. High frequency of antigenic variants among naturally occurring human coxsackie B4 virus isolates identified by monoclonal antibodies. Nature. 1982;300:374–376. doi: 10.1038/300374a0. [DOI] [PubMed] [Google Scholar]
- 345.Prather S L, Dagan R, Jenista J A, Menegus M A. The isolation of enteroviruses from blood: a comparison of four processing methods. J Med Virol. 1984;14:221–227. doi: 10.1002/jmv.1890140305. [DOI] [PubMed] [Google Scholar]
- 346.Prather S L, Jenista J A, Menegus M A. The isolation of nonpolio enteroviruses from serum. Diagn Microbiol Infect Dis. 1984;2:353–357. doi: 10.1016/0732-8893(84)90069-5. [DOI] [PubMed] [Google Scholar]
- 347.Prévot J, Dubrou S, Maréchal J. Detection of human hepatitis A virus in environmental water by an antigen-capture polymerase chain reaction method. Water Sci Technol. 1993;27:227–233. [Google Scholar]
- 348.Puig M, Jofre J, Lucena F, Allard A, Wadell G, Girones R. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl Environ Microbiol. 1994;60:2963–2970. doi: 10.1128/aem.60.8.2963-2970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Pulli T, Koskimies P, Hyypiä T. Molecular comparison of coxsackie A virus serotypes. Virology. 1995;212:30–38. doi: 10.1006/viro.1995.1450. [DOI] [PubMed] [Google Scholar]
- 350.Puthavathana P, Lee H W, Kang C Y. Typing of hantaviruses from five continents by polymerase chain reaction. Virus Res. 1992;26:1–14. doi: 10.1016/0168-1702(92)90142-v. [DOI] [PubMed] [Google Scholar]
- 351.Quint W G, Heijtink R A, Schirm J, Gerlich W H, Niesters H G. Reliability of methods for hepatitis B virus DNA detection. J Clin Microbiol. 1995;33:225–228. doi: 10.1128/jcm.33.1.225-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Raab de Verdugo U, Selinka H-C, Huber M, Kramer B, Kellermann J, Hofschneider P H, Kandolf R. Characterisation of a 100-kilodalton binding protein for the six serotypes of coxsackie B viruses. J Virol. 1995;69:6751–6757. doi: 10.1128/jvi.69.11.6751-6757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Racaniello V R, Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci USA. 1981;78:4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ramsingh A I, Caggana M, Ronstrom S. Genetic mapping of the determinants of plaque morphology of coxsackievirus B4. Arch Virol. 1995;140:2215–2226. doi: 10.1007/BF01323241. [DOI] [PubMed] [Google Scholar]
- 355.Ramsingh A I, Collins D N. A point mutation in the VP4 coding sequence of coxsackievirus B4 influences virulence. J Virol. 1995;69:7278–7281. doi: 10.1128/jvi.69.11.7278-7281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Raska K. Epidemiologic surveillance in the control of infectious disease. Rev Infect Dis. 1983;5:1112–1117. doi: 10.1093/clinids/5.6.1112. . (Editorial.) [DOI] [PubMed] [Google Scholar]
- 357.Ravaggi A, Zonaro A, Marin M G, Puoti M, Albertini A, Cariani E. Distribution of viral genotypes in Italy determined by hepatitis C virus typing by DNA immunoassay. J Clin Microbiol. 1994;32:2280–2284. doi: 10.1128/jcm.32.9.2280-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Redline R W, Genest D R, Tycko B. Detection of enteroviral infection in paraffin-embedded tissue by the RNA polymerase chain reaction technique. Am J Clin Pathol. 1991;96:568–571. doi: 10.1093/ajcp/96.5.568. [DOI] [PubMed] [Google Scholar]
- 359.Reimann B-Y, Zell R, Kandolf R. Mapping of a neutralizing antigenic site of coxsackievirus B4 by construction of an antigenic chimera. J Virol. 1991;65:3475–3480. doi: 10.1128/jvi.65.7.3475-3480.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ren R, Moss E G, Racaniello V R. Identification of two determinants that attenuate vaccine-related type 2 poliovirus. J Virol. 1991;66:1377–1382. doi: 10.1128/jvi.65.3.1377-1382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Rezapkin G V, Chumakov K M, Lu Z, Ran Y, Dragunsky E M, Levenbook I S. Microevolution of Sabin 1 strain in vitro and genetic stability of oral poliovirus vaccine. Virology. 1994;202:370–378. doi: 10.1006/viro.1994.1353. [DOI] [PubMed] [Google Scholar]
- 362.Rezapkin G V, Norwood L P, Taffs R E, Dragunsky E M, Levenbook I S, Chumakov K M. Microevolution of type 3 Sabin strain of poliovirus in cell cultures and its implications for oral poliovirus vaccine quality control. Virology. 1995;211:377–384. doi: 10.1006/viro.1995.1420. [DOI] [PubMed] [Google Scholar]
- 363.Rice S K, Heinl R E, Thornton L L, Opal S M. Clinical characteristics, management strategies, and cost implications of a statewide outbreak of enterovirus meningitis. Clin Infect Dis. 1995;20:931–937. doi: 10.1093/clinids/20.4.931. [DOI] [PubMed] [Google Scholar]
- 364.Richman D D, Guatelli J C, Grimes J, Tsiatis A, Gingeras T. Detection of mutations associated with zidovudine resistance in human immunodeficiency virus by use of the polymerase chain reaction. J Infect Dis. 1991;164:1075–1081. doi: 10.1093/infdis/164.6.1075. [DOI] [PubMed] [Google Scholar]
- 365.Rico-Hesse R, Pallansch M A, Nottay B K, Kew O M. Geographic distribution of wild poliovirus type 1 genotypes. Virology. 1987;160:311–322. doi: 10.1016/0042-6822(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 366.Rivera V M, Welsh J D, Maizel J V. Comparative sequence analysis of the 5′ noncoding region of the enteroviruses and rhinoviruses. Virology. 1988;165:42–50. doi: 10.1016/0042-6822(88)90656-3. [DOI] [PubMed] [Google Scholar]
- 367.Robinson C R, Doane F W, Rhodes A J. Report of an outbreak of febrile illness with pharyngeal lesions and exanthems, Toronto, summer 1957—isolation of group A Coxsackie virus. Can Med Assoc J. 1958;79:615–621. [PMC free article] [PubMed] [Google Scholar]
- 368.Rodriguez A, Martinez-Salas E, Dopazo J, Davila M, Saiz J C, Sobrino F. Primer design for specific diagnosis by PCR of highly variable RNA viruses: typing foot-and-mouth disease virus. Virology. 1992;189:363–367. doi: 10.1016/0042-6822(92)90717-4. [DOI] [PubMed] [Google Scholar]
- 369.Roivainen M, Agboatwalla M, Stenvik M, Rysä T, Akram D S, Hovi T. Intrathecal immune response and virus-specific immunoglobulin M antibodies in laboratory diagnosis of acute poliomyelitis. J Clin Microbiol. 1993;31:2427–2432. doi: 10.1128/jcm.31.9.2427-2432.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Roivainen M, Piirainen L, Hovi T. Efficient RGD-independent entry process of coxsackievirus A9. Arch Virol. 1996;141:1909–1919. doi: 10.1007/BF01718203. [DOI] [PubMed] [Google Scholar]
- 371.Roivainen M, Piirainen L, Hovi T, Virtanen J, Riikonen T, Heino J, Hyypiä T. Entry of coxsackievirus A9 into host cells: specific interactions with alpha v beta 3 integrin, the vitronectin receptor. Virology. 1994;203:357–365. doi: 10.1006/viro.1994.1494. [DOI] [PubMed] [Google Scholar]
- 372.Romero J R, Rotbart H A. Sequence analysis of the downstream 5′ nontranslated region of seven echoviruses with different neurovirulence phenotypes. J Virol. 1995;69:1370–1375. doi: 10.1128/jvi.69.2.1370-1375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Rossmann M G, Arnold E, Erickson J W, Frankenberger E W, Griffith P J, Hecht H, Johnsson J E, Kramer G, Luo M, Mosser A G, Rueckert R R, Sherry B, Vriend G. Structure of a common cold virus and functional relationship to other picornaviruses. Nature. 1985;317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 374.Rossouw E, Tsilimigras C W, Schoub B D. Molecular epidemiology of a coxsackievirus B3 outbreak. J Med Virol. 1991;34:165–171. doi: 10.1002/jmv.1890340306. [DOI] [PubMed] [Google Scholar]
- 375.Rota J S, Heath J L, Rota P A, King G E, Celma M L, Carabana J, Fernandez-Munoz R, Brown D, Jin L, Bellini W J. Molecular epidemiology of measles virus: identification of pathways of transmission and implications for measles elimination. J Infect Dis. 1996;173:32–37. doi: 10.1093/infdis/173.1.32. [DOI] [PubMed] [Google Scholar]
- 376.Rotbart H A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990;28:438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Rotbart H A. Nucleic acid detection systems for enteroviruses. Clin Microbiol Rev. 1991;4:156–168. doi: 10.1128/cmr.4.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Rotbart H A. Meningitis and encephalitis. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 271–289. [Google Scholar]
- 379.Rotbart H A. Enteroviral infections of the central nervous system. Clin Infect Dis. 1995;20:971–981. doi: 10.1093/clinids/20.4.971. [DOI] [PubMed] [Google Scholar]
- 380.Rotbart H A, Kinsella J P, Wasserman R L. Persistent enterovirus infection in culture-negative meningoencephalitis: demonstration by enzymatic RNA amplification. J Infect Dis. 1990;161:787–791. doi: 10.1093/infdis/161.4.787. [DOI] [PubMed] [Google Scholar]
- 381.Rotbart H A, Romero J R. Laboratory diagnosis of enteroviral infections. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 401–418. [Google Scholar]
- 382.Rotbart H A, Sawyer M H, Fast S, Lewinski C, Murphy N, Keyser E F, Spadoro J, Kao S-Y, Loeffelholz M. Diagnosis of enteroviral meningitis by using PCR with a colorimetric microwell detection assay. J Clin Microbiol. 1994;32:2590–2592. doi: 10.1128/jcm.32.10.2590-2592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Rueckert R R. Picornaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 609–654. [Google Scholar]
- 383a.Rueckert R R, Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984;50:957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Ryan M D, Jenkins O, Hughes P J, Brown A, Knowles N J, Booth D, Minor P D, Almond J W. The complete nucleotide sequence of enterovirus type 70: relationships with other members of the picornaviridae. J Gen Virol. 1990;71:2291–2299. doi: 10.1099/0022-1317-71-10-2291. [DOI] [PubMed] [Google Scholar]
- 385.Saitoh-Inagawa W, Oshima A, Aoki K, Itoh N, Isobe K, Uchio E, Ohno S, Nakajima H, Hata K, Ishiko H. Rapid diagnosis of adenoviral conjunctivitis by PCR and restriction fragment length polymorphism analysis. J Clin Microbiol. 1996;34:2113–2116. doi: 10.1128/jcm.34.9.2113-2116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Samuelson A, Forsgren M, Johansson B, Wahren B, Sallberg M. Molecular basis for serological cross-reactivity between enteroviruses. Clin Diagn Lab Immunol. 1994;1:336–341. doi: 10.1128/cdli.1.3.336-341.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Samuelson A, Forsgren M, Sallberg M. Characterization of the recognition site and diagnostic potential of an enterovirus group-reactive monoclonal antibody. Clin Diagn Lab Immunol. 1995;2:385–386. doi: 10.1128/cdli.2.3.385-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Samuelson A, Skoog E, Forsgren M. Aspects on the serodiagnosis of enterovirus infections by ELISA. Serodiagn Immunother Infect Dis. 1990;4:395–406. [Google Scholar]
- 389.Sattar S A. Viral survival in receiving waters. In: Goddard M, Butler M, editors. Viruses and waste water treatment. Oxford, United Kingdom: Pergamon Press; 1981. pp. 91–108. [Google Scholar]
- 390.Schlesinger Y, Sawyer M H, Storch G A. Enteroviral meningitis in infancy: potential for polymerase chain reaction in patient management. Pediatrics. 1994;94:157–162. [PubMed] [Google Scholar]
- 391.Schmidt N F, Guenther R W, Lennette E H. Typing of ECHO virus isolates by immune serum pools. The “Intersecting Serum Scheme.”. J Immunol. 1961;87:623–626. [PubMed] [Google Scholar]
- 392.Schmidt N J, Ho H H, Lennette E H. Propagation and isolation of group A coxsackieviruses in RD cells. J Clin Microbiol. 1975;2:183–185. doi: 10.1128/jcm.2.3.183-185.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Schmidt N J, Lennette E H, Ho H H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis. 1974;129:304–309. doi: 10.1093/infdis/129.3.304. [DOI] [PubMed] [Google Scholar]
- 394.Schnurr D, Dondero M, Holland D, Connor J. Characterization of echovirus 22 variants. Arch Virol. 1996;141:1749–1758. doi: 10.1007/BF01718297. [DOI] [PubMed] [Google Scholar]
- 395.Schwaiger A, Umlauft F, Weyrer K, Larcher C, Lyons J, Mühlberger V, Dietze O, Grûnewald K. Detection of enterovirus ribonucleic acid in myocardial biopsies from patients with idiopathic dilated cardiomyopathy by polymerase chain reaction. Am Heart J. 1993;126:406–410. doi: 10.1016/0002-8703(93)91058-m. [DOI] [PubMed] [Google Scholar]
- 396.Schweiger B, Schreier E, Böthig B, López-Pila J M. Differentiation of vaccine and wild-type polioviruses using polymerase chain reaction and restriction enzyme analysis. Arch Virol. 1994;134:39–50. doi: 10.1007/BF01379105. [DOI] [PubMed] [Google Scholar]
- 397.Seah C L K, Chow V T K, Chan Y C. Semi-nested PCR using NS3 primers for the detection and typing of dengue viruses in clinical serum specimens. Clin Diagn Virol. 1995;4:113–120. doi: 10.1016/0928-0197(94)00063-z. [DOI] [PubMed] [Google Scholar]
- 398.Seah C L K, Chow V T K, Tan H C, Chan Y C. Rapid, single-step RT-PCR typing of dengue viruses using five NS3 gene primers. J Virol Methods. 1995;51:193–200. doi: 10.1016/0166-0934(94)00104-o. [DOI] [PubMed] [Google Scholar]
- 399.Shafren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Sharief M K, Hentges R, Ciardi M. Intrathecal immune response in patients with the post-polio syndrome. N Engl J Med. 1991;325:749–755. doi: 10.1056/NEJM199109123251101. [DOI] [PubMed] [Google Scholar]
- 401.Sharp I R, Wadell G. Adenoviruses. In: Zuckerman A J, Banatvala J E, Pattison J R, editors. Principles and practice of clinical virology. 3rd ed. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1995. pp. 287–308. [Google Scholar]
- 402.Sheehy N, Desselberger U. Sequence analysis of reverse transcriptase genes of zidovudine (AZT)-resistant and -sensitive human immunodeficiency virus type 1 strains. J Gen Virol. 1993;74:223–228. doi: 10.1099/0022-1317-74-2-223. [DOI] [PubMed] [Google Scholar]
- 403.Shimizu H, Schnurr D P, Burns J C. Comparison of methods to detect enteroviral genome in frozen and fixed myocardium by polymerase chain reaction. Lab Invest. 1994;71:612–616. [PubMed] [Google Scholar]
- 404.Shuber A P, Grondin V J, Klinger K W. A simplified procedure for developing multiplex PCRs. Genome Res. 1995;5:488–493. doi: 10.1101/gr.5.5.488. [DOI] [PubMed] [Google Scholar]
- 405.Sidhu M K, Rashidbaigi A, Liao M J, Testa D. Distinction between HIV-1 and HIV-2 nucleotide sequences by PCR and restriction enzyme analysis. BioTechniques. 1995;18:20–24. [PubMed] [Google Scholar]
- 406.Simmonds P. Variability of hepatitis C virus. Hepatology. 1995;21:570–583. doi: 10.1002/hep.1840210243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Simmonds P, Alberti A, Alter H, Bonino F, Bradley D W, Brechot C, Brouwer J T, Chan S-W, Chayama K, Chen D-S, Choo Q-L, Colombo M, Cuypers H T M, Date T, Dusheiko G M, Esteban J I, Fay O, Hadziyannis S J, Han J, Hatzakis A, Holmes E C, Hotta H, Houghton M, Irvine B, Kohara M, Kolberg J A, Kuo G, Lau J Y N, Lelie P N, Maertens G, McOmish F, Miyamura T, Misokami M, Nomoto A, Prince A M, Reesink H W, Rice C, Roggendorf M, Schalm S W, Shikata T, Shimotohno K, Stuyver L, Trépo C, Weiner A, Yap P L, Urdea M S. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology. 1994;19:1321–1324. [PubMed] [Google Scholar]
- 408.Simmonds P, Holmes E C, Cha T-A, Chan S-W, McOmish F, Irvine B, Beall E, Yap P L, Kolberg J, Urdea M S. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74:2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 409.Simmonds P, Rose K A, Graham S, Chan S W, McOmish F, Dow B C, Follett E A, Yap P L, Marsden H. Mapping of serotype-specific, immunodominant epitopes in the NS-4 region of hepatitis C virus (HCV): use of type-specific peptides to serologically differentiate infections with HCV types 1, 2, and 3. J Clin Microbiol. 1993;31:1493–1503. doi: 10.1128/jcm.31.6.1493-1503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Skinner M A, Racaniello V R, Dunn G, Cooper J, Minor P D, Almond J W. New model for the secondary structure of the 5′ non-coding RNA of poliovirus is supported by biochemical and genetic data that also shows that RNA secondary structure is important in neurovirulence. J Mol Biol. 1989;207:379–392. doi: 10.1016/0022-2836(89)90261-1. [DOI] [PubMed] [Google Scholar]
- 411.Slater P E, Orenstein W A, Morag A, Avni A, Handsher R, Green M S, Costin C, Yarrow A, Rishpon S, Havkin O, Ben-Zvi T, Kew O M, Rey M, Epstein I, Swartz T A, Melnick J L. Poliomyelitis outbreak in Israel in 1988: a report and two commentaries. Lancet. 1990;335:1192–1198. doi: 10.1016/0140-6736(90)92705-m. [DOI] [PubMed] [Google Scholar]
- 412.Sonenberg N. Poliovirus translation. Curr Top Microbiol Immunol. 1990;161:23–47. doi: 10.1007/978-3-642-75602-3_2. [DOI] [PubMed] [Google Scholar]
- 413.Stanway G, Hughes P J, Mountford R C, Reeve P, Minor P D, Schild G C, Almond J W. Comparison of the complete nucleotide sequences of the genomes of the neurovirulent poliovirus P3/Leon/37 and its attenuated Sabin vaccine derivative P3/Leon12a1b. Proc Natl Acad Sci USA. 1984;81:1539–1543. doi: 10.1073/pnas.81.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Stanway G, Cann A J, Hauptmann R, Hughes P, Clarke L D, Mountford R C, Minor P D, Schild G C, Almond J W. The nucleotide sequence of poliovirus type 3 leon 12 a1b: comparison with poliovirus type 1. Nucleic Acids Res. 1983;11:5629–5643. doi: 10.1093/nar/11.16.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Stanway G, Kalkkinen N, Roivainen M, Ghazi F, Khan M, Smyth M, Meurman O, Hyypiä T. Molecular and biological characteristics of echovirus 22, a representative of a new picornavirus group. J Virol. 1994;68:8232–8238. doi: 10.1128/jvi.68.12.8232-8238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Strikas R A, Anderson L J, Parker R A. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970–1983. J Infect Dis. 1986;153:346–351. doi: 10.1093/infdis/153.2.346. [DOI] [PubMed] [Google Scholar]
- 417.Stuyver L, Rossau R, Wyseur A, Duhamel M, Vanderborght B, van Heuverswyn H, Maertens G. Typing of hepatitis C virus isolates and characterization of new subtypes using a line probe assay. J Gen Virol. 1993;74:1093–1102. doi: 10.1099/0022-1317-74-6-1093. [DOI] [PubMed] [Google Scholar]
- 418.Stuyver L, Wyseur A, van Arnheim W, Hernandez F, Maertens G. Second-generation line probe assay for hepatitis C virus genotyping. J Clin Microbiol. 1996;34:2259–2266. doi: 10.1128/jcm.34.9.2259-2266.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Supanaranond K, Takeda N, Yamazaki S. The complete nucleotide sequence of a variant of coxsackie virus A24, an agent causing acute haemorrhagic conjunctivitis. Virus Genes. 1992;6:149–158. doi: 10.1007/BF01703064. [DOI] [PubMed] [Google Scholar]
- 420.Taffs R E, Chumakov K M, Rezapkin G V, Lu Z, Douthitt M, Dragunsky E M, Levenbook I S. Genetic stability and mutant selection in Sabin 2 strain of oral poliovirus vaccine grown under different cell culture conditions. Virology. 1995;209:366–373. doi: 10.1006/viro.1995.1268. [DOI] [PubMed] [Google Scholar]
- 421.Takeda N, Sakae K, Agboatwalla M, Isomura S, Hondo R, Inouye S. Differentiation between wild and vaccine-derived strains of poliovirus by stringent microplate hybridization of PCR products. J Clin Microbiol. 1994;32:202–204. doi: 10.1128/jcm.32.1.202-204.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Takeda N, Tanimura M, Miyamura K. Molecular evolution of the major capsid protein VP1 of enterovirus 70. J Virol. 1994;68:854–862. doi: 10.1128/jvi.68.2.854-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Tambini G, Andrus J K, Marques E, Boshell J, Pallansch M, de Quadros C A, Kew O. Direct detection of wild poliovirus circulation by stool surveys of healthy children and analysis of community waste water. J Infect Dis. 1993;168:1510–1514. doi: 10.1093/infdis/168.6.1510. [DOI] [PubMed] [Google Scholar]
- 424.Tardy-Panit M, Blondel B, Martin A, Tekaia F, Horaud F, Delpeyroux F. A mutation in the RNA polymerase of poliovirus type 1 contributes to attenuation in mice. J Virol. 1993;67:4630–4638. doi: 10.1128/jvi.67.8.4630-4638.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Tatem J M, Weeks-Levy C, Giorgiu A, DiMichelle S J, Gargacz E J, Racaniello V R, Cano F R. A mutation present in the amino terminus of Sabin 3 poliovirus VP1 protein is attenuating. J Virol. 1992;66:3194–3197. doi: 10.1128/jvi.66.5.3194-3197.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Tenorio A, Echevarría J E, Casas I, Echevarría J M, Tabares E. Detection and typing of human herpesviruses by multiplex polymerase chain reaction. J Virol Methods. 1993;44:261–269. doi: 10.1016/0166-0934(93)90061-u. [DOI] [PubMed] [Google Scholar]
- 427.Thorén A, Widell A. PCR for the diagnosis of enteroviral meningitis. Scand J Infect Dis. 1994;26:249–254. doi: 10.3109/00365549409011792. [DOI] [PubMed] [Google Scholar]
- 428.Tisminetzky S, Gerotto M, Pontisso P, Chemello L, Prescott L E, Rose K A, Baralle F, Simmonds P, Alberti A. Comparison of genotyping and serotyping methods for the identification of hepatitis C virus types. J Virol Methods. 1995;55:303–307. doi: 10.1016/0166-0934(95)00067-x. [DOI] [PubMed] [Google Scholar]
- 429.Tokita H, Okamoto H, Iizuka H, Kishimoto J, Tsuda F, Lesmana L A, Miyakawa Y, Mayumi M. Hepatitis C variants from Jakarta, Indonesia classifiable into novel genotypes in the second (2e and 2f), tenth (10a) and eleventh (11a) genetic groups. J Gen Virol. 1996;77:293–301. doi: 10.1099/0022-1317-77-2-293. [DOI] [PubMed] [Google Scholar]
- 430.Torfason E G, Frisk G, Diderholm H. Indirect and reverse radioimmunoassay and their apparent specificities in detection of antibodies to enteroviruses in human sera. J Med Virol. 1984;13:13–18. doi: 10.1002/jmv.1890130103. [DOI] [PubMed] [Google Scholar]
- 431.Toyoda H, Kohara M, Kataoka Y, Suganuma T, Omata T, Imura N, Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes: implication for genetic relationships, gene function and antigenic determinants. J Mol Biol. 1984;174:561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- 432.Toyoda H, Nicklin M J H, Murray M G, Anderson C W, Dunn J J, Studier F W, Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986;45:761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- 433.Trabelsi A, Grattard F, Nejmeddine M, Aouni M, Bourlet T, Pozzetto B. Evaluation of an enterovirus group-specific anti-VP1 monoclonal antibody, 5-D8/1, in comparison with neutralization and PCR for rapid identification of enteroviruses in cell culture. J Clin Microbiol. 1995;33:2454–2457. doi: 10.1128/jcm.33.9.2454-2457.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Tracy S. A comparison of the genomic homologies in the coxsackievirus B group: use of fragments of the cloned coxsackievirus B3 genome as probes. J Gen Virol. 1984;65:2167–2172. doi: 10.1099/0022-1317-65-12-2167. [DOI] [PubMed] [Google Scholar]
- 435.Tracy S, Chapman N M, Romero J, Ramsingh A I. Genetics of coxsackievirus B cardiovirulence and inflammatory heart muscle disease. Trends Microbiol. 1996;4:175–179. doi: 10.1016/0966-842x(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 436.Tsai Y-L, Sobsey M D, Sangermano L R, Palmer C J. Simple method of concentrating enteroviruses and hepatitis A virus from sewage and ocean water for rapid detection by reverse transcriptase-polymerase chain reaction. Appl Environ Microbiol. 1993;59:3488–3491. doi: 10.1128/aem.59.10.3488-3491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Tsai Y-L, Tran B, Sangermano L R, Palmer C J. Detection of poliovirus, hepatitis A virus, and rotavirus from sewage and ocean water by triplex reverse transcriptase PCR. Appl Environ Microbiol. 1994;60:2400–2407. doi: 10.1128/aem.60.7.2400-2407.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Tu Z, Chapman N M, Hufnagel G, Tracy S, Romero J R, Barry W H, Zhao L, Currey K, Shapiro B. The cardiovirulent phenotype of coxsackievirus B3 is determined at a single site in the genomic 5′ nontranslated region. J Virol. 1995;69:4607–4618. doi: 10.1128/jvi.69.8.4607-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Tuke P W, Luton P, Garson J A. Differential diagnosis of HTLV-I and HTLV-II infections by restriction enzyme analysis of ‘nested’ PCR products. J Virol Methods. 1992;40:163–173. doi: 10.1016/0166-0934(92)90065-l. [DOI] [PubMed] [Google Scholar]
- 440.Udaykumar A, Heredia, Soriano V, Bravo R, Epstein J S, Hewlett I K. Enhanced diagnostic efficiency of the polymerase chain reaction by coamplification of multiple regions of HIV-1 and HIV-2. J Virol Methods. 1994;49:37–46. doi: 10.1016/0166-0934(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 441.Vallejo A, García Sáiz A. Typing human T-cell lymphotropic virus (HTLV-I and HTLV-II) by nested polymerase chain reaction: application to clinical specimens. J Virol Methods. 1995;51:9–17. doi: 10.1016/0166-0934(94)00093-v. [DOI] [PubMed] [Google Scholar]
- 442.Vandamme A M, Fransen K, Debaisieux L, Marissens D, Sprecher S, Vaira D, Vandenbroucke A T, Verhofstede C. Standardisation of primers and an algorithm for HIV-1 diagnostic PCR evaluated in patients harbouring strains of diverse geographical origin. The Belgian AIDS Reference Laboratories. J Virol Methods. 1995;51:305–316. doi: 10.1016/0166-0934(94)00126-2. [DOI] [PubMed] [Google Scholar]
- 443.Van der Avoort H G A M, Hull B P, Hovi T, Pallansch M A, Kew O M, Crainic R, Wood D J, Mulders M N, van Loon A M. Comparative study of five methods for intratypic differentiation of polioviruses. J Clin Microbiol. 1995;33:2562–2566. doi: 10.1128/jcm.33.10.2562-2566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Van der Avoort H G A M, Reimerink J H J, Ras A, Mulders M N, van Loon A M. Isolation of epidemic poliovirus from sewage during the 1992–3 type 3 outbreak in the Netherlands. Epidemiol Infect. 1995;114:481–491. doi: 10.1017/s0950268800052195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.VanDevanter D R, Warrender P, Bennett L, Schultz E R, Coulter S, Garber R L, Rose T R. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol. 1996;34:1666–1671. doi: 10.1128/jcm.34.7.1666-1671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Van Doorn L-J, Kleter B, Pike I, Quint W. Analysis of hepatitis C virus isolates by serotyping and genotyping. J Clin Microbiol. 1996;34:1784–1787. doi: 10.1128/jcm.34.7.1784-1787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Van Dyke T A, Flanegan J B. Identification of poliovirus polypeptide p63 as a soluble RNA-dependent RNA polymerase. J Virol. 1980;35:732–740. doi: 10.1128/jvi.35.3.732-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Vangrysperre W, De Clercq K. Rapid and sensitive polymerase chain reaction based detection and typing of foot-and-mouth disease virus in clinical samples and cell culture isolates, combined with a simultaneous differentiation with other genomically and/or symptomatically related viruses. Arch Virol. 1996;141:331–344. doi: 10.1007/BF01718403. [DOI] [PubMed] [Google Scholar]
- 449.Van Loon, A. M., G. M. Cleator, and A. Ras for the European Union Concerted Action on Virus Meningitis and Encephalitis. External quality assessment of enterovirus detection and typing. Bull. W. H. O., in press. [PMC free article] [PubMed]
- 450.Van Loon A M, Ras A, Poelstra P, Mulders M, van der Avoort H. Intratypic differentiation of polioviruses. In: Kurstak E, editor. Measles and poliomyelitis. Vienna, Austria: Springer-Verlag KG; 1993. pp. 359–369. [Google Scholar]
- 451.Van Wezel A L, Hazendonk A G. Intratypic serum differentiation of poliomyelitis virus strains by strain-specific antisera. Intervirology. 1979;11:2–8. doi: 10.1159/000149005. [DOI] [PubMed] [Google Scholar]
- 452.Viazov S, Zibert A, Ramakrishnan K, Widell A, Cavicchini A, Schreier E, Roggendorf M. Typing of hepatitis C virus isolates by DNA enzyme immunoassay. J Virol Methods. 1994;48:81–91. doi: 10.1016/0166-0934(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 453.Vonsover A, Handsher R, Neuman M, Guillot S, Balanant J, Rudich H, Mendelson E, Swartz T, Crainic R. Molecular epidemiology of type 1 polioviruses isolated in Israel and defined by restriction fragment length polymorphism assay. J Infect Dis. 1993;167:199–203. doi: 10.1093/infdis/167.1.199. [DOI] [PubMed] [Google Scholar]
- 454.Voroshilova M K, Chumakov M P. Poliomyelitis-like properties of AB-IV-coxsackie A7 group of viruses. Prog Med Virol. 1959;2:106–170. [Google Scholar]
- 455.Wadia N H, Katrak S M, Misra V P, Wadia P N, Miyamura K, Hashimoto K, Ogino T, Hikiji T, Kono R. Polio-like motor paralysis associated with acute hemorrhagic conjunctivitis in an outbreak in 1981 in Bombay, India: clinical and serologic studies. J Infect Dis. 1983;147:660–668. doi: 10.1093/infdis/147.4.660. [DOI] [PubMed] [Google Scholar]
- 456.Wallis C, Melnick J L. Virus aggregation as the cause of the nonneutralizable persistent fraction. J Virol. 1967;1:478–488. doi: 10.1128/jvi.1.3.478-488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Ward C D, Flanegan J B. Determination of the poliovirus RNA polymerase error frequency at eight sites in the viral genome. J Virol. 1992;66:3784–3793. doi: 10.1128/jvi.66.6.3784-3793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Webb S R, Kearse K P, Foulke C L, Hartig P C, Prabhakar B S. Neutralization epitope diversity of coxsackievirus B4 isolates detected by monoclonal antibodies. J Med Virol. 1986;20:9–15. doi: 10.1002/jmv.1890200103. [DOI] [PubMed] [Google Scholar]
- 459.Wecker I, ter Meulen V. RD cells in the laboratory diagnosis of enteroviruses. Med Microbiol Immunol. 1977;163:233–240. doi: 10.1007/BF02125507. [DOI] [PubMed] [Google Scholar]
- 460.Wenner H A. Virus diseases associated with cutaneous eruptions. Prog Med Virol. 1973;16:269–336. [PubMed] [Google Scholar]
- 461.Werner G, Rosenwirth B, Bauer E, Seifert J-M, Werner F-J, Besemer J. Molecular cloning and sequence determination of the genomic regions encoding protease and genome-linked protein of three picornaviruses. J Virol. 1986;57:1084–1093. doi: 10.1128/jvi.57.3.1084-1093.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Westrop G D, Wareham K A, Evans D M A, Dunn G, Minor P D, Magrath D I, Taffs F, Marsden S, Skinner M A, Schild G C, Almond J W. Genetic basis of attenuation of the Sabin type 3 oral poliovirus vaccine. J Virol. 1989;63:1338–1344. doi: 10.1128/jvi.63.3.1338-1344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.WHO Network for HIV Isolation and Characterization. HIV type 1 variation in World Health Organization-sponsored vaccine evaluation sites: genetic screening, sequence analysis, and preliminary biological characterization of selected viral variants. AIDS Res Hum Retroviruses. 1994;10:1387–1400. doi: 10.1089/aid.1994.10.1327. [DOI] [PubMed] [Google Scholar]
- 464.Woodall C J, Riding M H, Graham D I, Clements G B. Sequences specific for enteroviruses detected in spinal cord from patients with motor neurone disease. Br Med J. 1994;308:1541–1543. doi: 10.1136/bmj.308.6943.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Woodall C J, Watt N J, Clements G B. Simple technique for detecting RNA viruses in single sections of wax embedded tissue. J Clin Pathol. 1993;46:276–277. doi: 10.1136/jcp.46.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Woodruff J F. The influence of post-weaning undernutrition on coxsackievirus B-3 infection of adult mice. I. Viral persistence and increase in severity of lesions. J Infect Dis. 1970;121:137–163. doi: 10.1093/infdis/121.2.137. [DOI] [PubMed] [Google Scholar]
- 467.Woodruff J F. The influence of post-weaning undernutrition on coxsackievirus B-3 infection of adult mice. II. Alteration of host defence mechanisms. J Infect Dis. 1970;121:164–181. doi: 10.1093/infdis/121.2.164. [DOI] [PubMed] [Google Scholar]
- 468.World Health Assembly. Global eradication of poliomyelitis by the year 2000. Resolution WHA41.28. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]
- 469.World Health Organization. Markers of poliovirus strains isolated from cases temporally associated with the use of live poliovirus vaccine. Report on a WHO collaborative study. J Biol Stand. 1981;9:163–185. doi: 10.1016/s0092-1157(81)80021-2. [DOI] [PubMed] [Google Scholar]
- 470.World Health Organization. Manual for the virological investigation of poliomyelitis. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 471.Wright K E, Wilson G A R, Novosad D, Dimock C, Tan D, Weber J M. Typing and subtyping of influenza viruses in clinical samples by PCR. J Clin Microbiol. 1995;33:1180–1184. doi: 10.1128/jcm.33.5.1180-1184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Wright P F, Kim Farley R J, de Quadros C A, Robertson S E, Scott R M, Ward N A, Henderson R H. Strategies for the global eradication of poliomyelitis by the year 2000. N Engl J Med. 1991;325:1774–1779. doi: 10.1056/NEJM199112193252504. [DOI] [PubMed] [Google Scholar]
- 473.Wu J-C, Choo K-B, Chen C-M, Chen T-Z, Huo T-I, Lee S-D. Genotyping of hepatitis D virus by restriction-fragment length polymorphism and relation to outcome of hepatitis D. Lancet. 1995;346:939–941. doi: 10.1016/s0140-6736(95)91558-3. [DOI] [PubMed] [Google Scholar]
- 474.Wyatt H V. Poliomyelitis in hypogammaglobulinemics. J Infect Dis. 1973;128:802. doi: 10.1093/infdis/128.6.802. [DOI] [PubMed] [Google Scholar]
- 475.Yamazaki K, Oishi I, Minekawa Y. Nucleotide sequence analysis of recent epidemic strains of enterovirus 70. Microbiol Immunol. 1995;39:429–432. doi: 10.1111/j.1348-0421.1995.tb02224.x. [DOI] [PubMed] [Google Scholar]
- 476.Yang C F, De L, Holloway B P, Pallansch M A, Kew O M. Detection and identification of vaccine-related polioviruses by the polymerase chain reaction. Virus Res. 1991;20:159–179. doi: 10.1016/0168-1702(91)90107-7. [DOI] [PubMed] [Google Scholar]
- 477.Yang C F, De L, Yang S J, Ruiz-Gómez J, Cruz J R, Holloway B P, Pallansch M A, Kew O M. Genotype-specific in vitro amplifications of sequences of the wild type 3 polioviruses from Mexico and Guatemala. Virus Res. 1992;24:277–296. doi: 10.1016/0168-1702(92)90124-r. [DOI] [PubMed] [Google Scholar]
- 478.Yerly S, Gervaix A, Simonet V, Caflisch M, Perrin L, Wunderli W. Rapid and sensitive detection of enteroviruses in specimens from patients with aseptic meningitis. J Clin Microbiol. 1996;34:199–201. doi: 10.1128/jcm.34.1.199-201.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Yogo Y, Iida T, Taguchi F, Kitamura H T, Aso Y. Typing of human polyomavirus JC virus on the basis of restriction fragment length polymorphisms. J Clin Microbiol. 1991;29:2130–2138. doi: 10.1128/jcm.29.10.2130-2138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Yolken R H, Torsch V M. Enzyme-linked immunosorbent assay for detection and identification of coxsackieviruses A. Infect Immun. 1981;31:742–750. doi: 10.1128/iai.31.2.742-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Yoon J W. The role of viruses and environmental factors in the induction of diabetes. Curr Top Microbiol Immunol. 1990;164:95–123. doi: 10.1007/978-3-642-75741-9_6. [DOI] [PubMed] [Google Scholar]
- 482.Yousef G E, Bell E J, Mann G F, Murugesan V, Smith D G, McCartney R A, Mowbray J F. Chronic enterovirus infection in patients with postviral fatigue syndrome. Lancet. 1988;i:146–150. doi: 10.1016/s0140-6736(88)92722-5. [DOI] [PubMed] [Google Scholar]
- 483.Yousef G E, Brown I N, Mowbray J F. Derivation and biochemical characterization of an enterovirus group-specific monoclonal antibody. Intervirology. 1987;28:163–170. doi: 10.1159/000150012. [DOI] [PubMed] [Google Scholar]
- 484.Yousef G E, Mann G F, Brown I N, Mowbray J F. Clinical and research application of an enterovirus group-reactive monoclonal antibody. Intervirology. 1987;28:199–205. doi: 10.1159/000150017. [DOI] [PubMed] [Google Scholar]
- 485.Zaaijer H L, Cuypers H T M, Reesink H W, Winkel I N, Gerken G, Lelie P N. Reliability of polymerase chain reaction for detection of hepatitis C virus. Lancet. 1993;341:722–724. doi: 10.1016/0140-6736(93)90488-3. [DOI] [PubMed] [Google Scholar]
- 486.Zeuzem S, Ruster B, Roth W K. Clinical evaluation of a new polymerase chain reaction assay (Amplicor HCV) for detection of hepatitis C virus. Z Gastroenterol. 1994;32:342–347. [PubMed] [Google Scholar]
- 487.Zhang G, Wilsden G, Knowles N J, McCauley J W. Complete nucleotide sequence of a coxsackie B5 virus and its relationship to swine vesicular disease virus. J Gen Virol. 1993;74:845–853. doi: 10.1099/0022-1317-74-5-845. [DOI] [PubMed] [Google Scholar]
- 488.Zhang H, Blake N W, Ouyang X, Pandolfino Y A, Morgan-Capner P, Archard L C. A single amino acid substitution in the capsid protein VP1 of Coxsackievirus B3 (CVB3) alters plaque phenotype in Vero cells but not cardiovirulence in a mouse model. Arch Virol. 1995;140:959–966. doi: 10.1007/BF01314972. [DOI] [PubMed] [Google Scholar]
- 489.Zhang H Y, Yousef G E, Cunningham L, Blake N W, Yang X O, Bayston T A, Kandolf R, Archard L C. Attenuation of a reactivated cardiovirulent coxsackievirus B3: the 5′-nontranslated region does not contain major attenuation determinants. J Med Virol. 1993;41:129–137. doi: 10.1002/jmv.1890410208. [DOI] [PubMed] [Google Scholar]
- 490.Zhang Z X, Yun Z B, Chen M, Sönnerborg A, Sällberg M. Evaluation of a multiple peptide assay for typing of antibodies to the hepatitis C virus: relation to genomic typing by the polymerase chain reaction. J Med Virol. 1995;45:50–55. doi: 10.1002/jmv.1890450110. [DOI] [PubMed] [Google Scholar]
- 491.Zheng D-P, Zhang L-B, Fang Z-Y, Yang C-F, Mulders M, Pallansch M A, Kew O M. Distribution of wild type 1 poliovirus genotypes in China. J Infect Dis. 1993;168:1361–1367. doi: 10.1093/infdis/168.6.1361. [DOI] [PubMed] [Google Scholar]
- 492.Zheng Z-M, He P-J, Caueffield D, Neumann M, Specter S, Baker C C, Bankowski M J. Enterovirus 71 isolated from China is serologically similar to the prototype E71 BrCr strain but differs in the 5′-noncoding region. J Med Virol. 1995;47:161–167. doi: 10.1002/jmv.1890470209. [DOI] [PubMed] [Google Scholar]
- 493.Zimmermann H, Eggers H J, Zimmermann A, Kraus W, Nelsensalz B. Complete nucleotide sequence and biological properties of an infectious clone of prototype echovirus 9. Virus Res. 1995;39:311–319. doi: 10.1016/0168-1702(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 494.Zoll G J, Melchers W J G, Kopecka H, Jambroes G, van der Poel H J A, Galama J M D. General primer-mediated polymerase chain reaction for detection of enteroviruses: application for diagnostic routine and persistent infections. J Clin Microbiol. 1992;30:160–165. doi: 10.1128/jcm.30.1.160-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Zoll J, Galama J, Melchers W. Intratypic genome variability of the coxsackievirus B1 2A protease region. J Gen Virol. 1994;75:687–692. doi: 10.1099/0022-1317-75-3-687. [DOI] [PubMed] [Google Scholar]