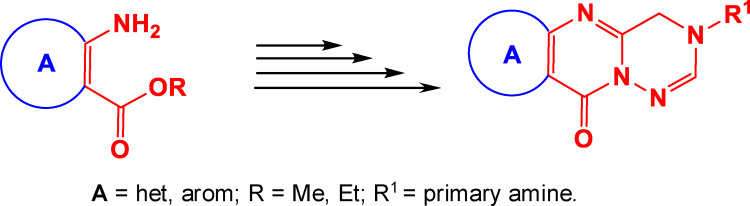
Abstract
The synthesis of pyrimido[2,1‐f][1,2,4]triazines was performed in four steps. Compounds obtained by acylation of the starting amino esters of thieno[2,3‐b]pyridines were reacted with various amines. The resulting amino derivatives underwent cyclization in the presence of hydrazine hydrate leading to new aminomethyl derivatives of thieno[3,2‐d]pyrimidines. Further cyclization of the latter resulted to the synthesis of new unique heterocyclic systems: pyrido[3′′,2′′:4′,5′]thieno[3′,2′:4,5]pyrimido[2,1‐f][1,2,4]triazines. The uniqueness of these systems lies in the fact that even the combination of the last two cycles represents a new heterocyclic system.
Keywords: acylation; aminomethyl derivatives; cyclization; hydrazinolysis; pyrimido[2,1-f][1,2,4]triazine
The synthesis of pyrimido[2,1‐f][1,2,4]triazines opens up new prospects for the synthesis of many new unique heterocyclic systems. The construction of these two cycles on any other cycles and heterocycles leads to completely new heterocyclic systems.
Introduction
Heterocyclic compounds attract an interest of scientists [1] especially medicinal chemists due to their wide range of different pharmacological activities. Among them, many derivatives of condensed thieno[3,2‐d]pyrimidines, exhibiting high biological activity[ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ] can be distinguished. In particular, studies have shown that these compounds possess antimicrobial,[ 13 , 14 , 15 ] antitumor,[ 16 , 17 , 18 , 19 , 20 ] cytotoxic [21] and antiproliferative[ 22 , 23 ] activities. Previously, we have also reported on the synthesis and biological activity of some derivatives of fused thieno[3,2‐d]pyrimidines.[ 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 ] The above observation prompted us to synthesize new 3‐substituted derivatives of fused pyrimidines with suitable functional group, followed by the synthesis of new heterocyclic systems, namely pyrimido[2,1‐f][1,2,4]triazines. It should be noted that obtained new heterocyclic systems are open new directions in the field of fused heterocycles. The derivatives of such fused pentacyclic systems that we synthesized are distinguished by different biological activities. In particular, thiazolo[3,2‐a]pyrimidines, pyrimido[2,1‐b][1,3]thiazines I and triazolopyrimidines II have shown high antimicrobial activity (Figure 1).[ 26 , 27 , 30 ] Considering the significance of heterocyclic compounds, it is highly beneficial to develop universal techniques for synthesizing a wide range of new heterocyclic compounds. This article discusses a technique that is intriguing from both a practical and theoretical perspective.
Figure 1.
The general structures of previously I, II and new III synthesized compounds.
Results and Discussion
Our preliminary goal was the synthesis of 3‐substituted fused pyrimidine derivatives with four cycles 4 based on available suitable functional groups of amino esters 1a,b. [30] By treating amino esters of thieno[2,3‐b]pyridines 1a,b with chloroacetyl chloride followed by substitution of the active chlorine atom with various amines, a novel series of amino derivatives of thieno[2,3‐b]pyridines 3a–c was synthesized. Compounds 3 a–c undergo cyclization in ethanol under the action of hydrazine hydrate [32] leading to aminomethyl derivatives of thieno[3,2‐d]pyrimidines 4 a–c (Scheme 1, Table 1).
Scheme 1.
The synthesis of new 3‐substituted derivatives of fused pyrimidines 4 a–c.
Table 1.
Thieno[2,3‐b]pyridines 3 a–c and thieno[3,2‐d]pyrimidines 4 a–c.
|
N |
Compound |
N |
Compound |
|---|---|---|---|
|
3a |
|
4a |
|
|
3b |
|
4b |
|
|
3c |
|
4c |
|
The presence of NH group in the 3rd position of the pyrimidine ring in compound 4c, enabled a cyclization reaction with triethyl orthoformate to form a new heterocyclic system with 5 rings: 5‐(2‐furyl)‐2,2‐dimethyl‐12‐(pyridin‐3‐ylmethyl)‐1,4,12,13‐tetrahydro‐2H,8H‐pyrano[4′′′,3′′:4′′,5′′]pyrido[3′′,2′′:4′,5′]thieno[3′,2′:4,5]pyrimido[2,1‐f][1,2,4]triazin‐8‐one 5. The resulting heterocyclic system appeared to be new, unique and differs from the pentacyclic heterocyclic systems previously obtained by our group. The uniqueness of compound 5 is attributed to the fact that, in addition to the first three main cycles (pyrano[4,3‐d]thieno[2,3‐b]pyridine), the combination of the last two cycles (pyrimido[2,1‐f][1,2,4]triazine) by itself represents a new heterocyclic system (Scheme 2).
Scheme 2.
Synthesis of new heterocyclic system: 5‐(2‐furyl)‐2,2‐dimethyl‐12‐(pyridin‐3‐ylmethyl)‐1,4,12,13‐tetrahydro‐2H,8H‐pyrano[4′′′,3′′:4′′,5′′]pyrido[3′′,2′′:4′,5′]thieno[3′,2′:4,5]pyrimido[2,1‐f][1,2,4]triazin‐8‐one 5.
It should be noted that there are two conditions for the implementation of this method:
the starting compounds must have vicinal amino and ester groups,
the amine substituted in the 3rd position of the pyrimidine ring (in compounds 4) must be primary amine.
This methodology was subsequently employed for the systems derived from tetrahydroisoquinoline 6a, cyclopenta[c]pyridine 6b, dimethylpyridine 6c, and 2,7‐naphthyridine 6d (Figure 2).[ 31 , 33 ]
Figure 2.
Starting compounds 6a‐d.
The transformations carried out according to Schemes 1 and 2 led to the synthesis of four new heterocyclic systems bearing a pyrimido[2,1‐f][1,2,4]triazine ring, which once again proved that the resulting heterocyclic systems are truly unique (Scheme 3, Table 2).
Scheme 3.
Synthesis of new heterocyclic systems: pyrimido[2,1‐f][1,2,4]trazines 10a–d.
Table 2.
Thieno[2,3‐b]pyridines 8a–d, thieno[3,2‐d]pyrimidines 9a–d and pyrimido[2,1‐f][1,2,4]triazines 10a–d.
|
N |
Compound** |
Yield*(%) |
N |
Compound** |
Yield*(%) |
|---|---|---|---|---|---|
|
8a |
|
79 |
9c |
|
78 |
|
8b |
|
82 |
9d |
|
80 |
|
8c |
|
84 |
10a |
|
61 |
|
8d |
|
71 |
10b |
|
63 |
|
9a |
|
79 |
10c |
|
59 |
|
9b |
|
83 |
10d |
|
57 |
*Yields after recrystallization. **Purity of compounds≤95 %.
The tentative mechanism concerning the reaction between aminoesters and hydrazine hydrate (leading to the synthesis of compounds 4/9) is well‐documented in the existing literature [32] and is illustrated below (Scheme 4). The suggested mechanism for the synthesis of compounds 5/10 can be delineated as follows.
Scheme 4.
Mechanism proposed for the synthesis of 5/10 starting from compounds 3/8.
The structure of the obtained compounds 4, 5, 9 and 10 was confirmed by the NMR, IR, MS spectra, and elemental analyses.
In the 1H NMR spectra of the compounds 4a–c and 9a–d the signals of the ester group are absent and the signals of the NH2 group appear at 5.91–6.16 ppm. In the 1H NMR spectra of the newly synthesized systems 5 and 10a–d, the resonances corresponding to the NH2 and NH functional groups are notably absent; conversely, the resonance associated with the CH group of the triazine ring is observed at a chemical shift of 7.68–7.71 ppm, whereas the signals attributed to the NCH2 and CH2N groups are shifted to a weaker magnetic field, appearing at 4.30–4.38 ppm and 4.60–4.64 ppm, respectively.
In the IR spectrum, absorption of the NH2 and NH groups is absent, whereas absorption of the CO group is observed at 1664–1681 cm−1.
Thus, as a result of the study, five new heterocyclic systems were obtained.
In the future, it is planned to continue studies on fused furans and other heterocycles.
Experimental Section
1H and 13C NMR spectra were recorded in DMSO/CCl4 (1/3) and CDCl3 solutions (300 MHz for 1H and 75 MHz for 13C, respectively) on a Mercury 300VX spectrometer (Varian Inc., Palo Alto, CA, USA). Chemical shifts were reported as δ (parts per million) relative to TMS as internal standard. IR spectra were recorded on Nicolet Avatar 330‐FT‐IR spectrophotometer (Thermo Nicolet, CA, USA) and the reported wave numbers were given in cm‐1. MS spectra were recorded on Waters Q‐Tof. All melting points were determined in an open capillary and were uncorrected. Elemental analyses were performed on an Elemental Analyzer Euro EA 3000. Compounds 1 a,b [30] and 6 a–d[ 31 , 33 ] were already described. Physicochemical data for compound 8d are not given, it did not crystallize and was isolated as an oil (yield 71 %).
General Procedure for the Synthesis of Compounds 2 a,b and 7a–d. To a mixture of ester 1a,b (6a–d for compounds 7a–d) (50 mmol) and triethylamine (60 mmol) in anhydrous benzene (75 mL) chloroacetyl chloride (4.8 mL, 60 mmol), was added dropwise with stirring, and the mixture was stirred for 6 h at 35 °C. After cooling to room temperature and evaporation to dryness, the residue was treated with water (50 mL), and the precipitate was filtered off, washed with water, dried, and recrystallized from ethanol.
General Procedure for the Synthesis of Compounds 3a–c and 8a–d. A mixture of compound 2a,b (7a–d for compounds 8a–d) (5 mmol), the corresponding amine (5.5 mmol) and triethylamine (5.5 mmol) in absolute ethanol (50 mL) was refluxed for 3 h. The mixture was cooled, the solvent was distilled off to dryness, the residue was treated with water (50 mL), and the precipitate was filtered off, washed with water, dried, and recrystallized from ethanol.
General Procedure for the Synthesis of Compounds 4a–c and 9a–d. A mixture of compound 3a–c (8a–d for compounds 9a–d) (5 mmol) and hydrazine monohydrate (50 mmol) in ab‐solute ethanol (50 mL) was refluxed for 5 h. The mixture was cooled, and the precipitate was filtered off, washed with water, dried, and recrystallized from ethanol.
General Procedure for the Synthesis of Compounds 5 and 10a–d. A mixture of compound 4c (9a–d for compounds 10a–d) (1 mmol) and triethyl orthoformate (15 mL) was refluxed for 5 hours. The excess of triethyl orthoformate was distilled off, ethanol (15 mL) was added to the residue, the precipitated crystals filtered off, washed with water, dried and crystallized from ethanol.
Conclusions
As a result of the research, a method was proposed that allows the synthesis of many new heterocyclic compounds. The synthesis of pyrimido[2,1‐f][1,2,4]triazines opens up new prospects for the synthesis of many new unique heterocyclic systems. The construction of these two cycles on any other cycles and heterocycles leads to completely new heterocyclic systems. An important condition for implementation is the presence of vicinal groups of amino esters and primary amines substituted in the 3rd position of aminomethyl derivatives of thieno[3,2‐d]pyrimidines.

Conflict of Interests
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgments
The work was supported by the Science Committee of RA, in the frames of the research project No 21AG‐1D036.
Sirakanyan S. N., Spinelli D., Geronikaki A., Kartsev V. G., Hakobyan E. K., Jughetsyan H. V., Yegoryan H. A., Hovakimyan A. A., ChemistryOpen 2025, e202400379. 10.1002/open.202400379
Contributor Information
Samvel N. Sirakanyan, Email: shnnr@mail.ru.
Elmira K. Hakobyan, Email: hakobyan.elmira@mail.ru.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Charushin V. N., Verbitskiy E. V., Chupakhin O. N., Vorobyeva D. V., Gribanov P. S., Osipov S. N. et al. Russ. Chem. Rev. 2024, 93, RCR5125. [Google Scholar]
- 2. Litvinov V. P., Russ. Chem. Bull. 2004, 53, 487–516. [Google Scholar]
- 3. Zheng G. Z., Bhatia P., Daanen J., Kolasa T., Patel M., Latshaw S., El Kouhen O. F., Chang R., Uchic M. E., Miller L., Nakane M., Lehto S. G., Honore M. P., Moreland R. B., Brioni J. D., Stewart A. O., J. Med. Chem. 2005, 48, 7374–7388. [DOI] [PubMed] [Google Scholar]
- 4. Taltavull J., Serrat J., Gràcia J., Gavaldà A., Andrés M., Córdoba M., Miralpeix M., Vilella D., Beleta J., Ryder H., Pagès L., J. Med. Chem. 2010, 53, 6912–6922. [DOI] [PubMed] [Google Scholar]
- 5. Virupakshi P., Sudhakar B. K., Ravindranath L.K, Venkateswarlu B., AJRC 2017, 10, 280–290. [Google Scholar]
- 6. Kjellerup L., Gordon S., Cohrt K. O., Brown W. D., Fuglsang A. T., Winther A. L., Antimicrob. Agents Chemother. 2017, 61, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romagnoli R., Prencipe F., Oliva P., Baraldi S., Baraldi P. G., Ortega S. S., Chayah M., Salvador M. K., Lopez-Cara L. C., Brancale A., Ferla S., Hamel E., Ronca R., Bortolozzi R., Mariotto E., Mattiuzzo E., Viola G., J. Med. Chem. 2019, 62, 1274–1290. [DOI] [PubMed] [Google Scholar]
- 8. Koteswaraiah M., Praveen Ch., Reddy T. V., Raveendrareddy G., Srinivas U., CDC 2022, 39, 100863. [Google Scholar]
- 9. Yang Y.-Y., Wang W.-Li., Hu X.-T., Chen X., Ni Y., Lei Y.-H., Qiu Q.-Y., Tao L.-Y., Luo T.-W., Wang N.-Y., Bioorg. Chem. 2023, 132, 106356. [DOI] [PubMed] [Google Scholar]
- 10. Zaman G., Ullah S., Uzair M., Batool S., Ahmad H., Ullah F., Pelletier J., Sévigny J., Iqbal J., Hassan A., ChemMedChem 2023, 17, e202300165. [DOI] [PubMed] [Google Scholar]
- 11. Wu Ch., Zhang L., Zhou Zh., Tan L., Wang Zh., Guo C., Wang Y., Eur. J. Med. Chem. 2024, 276, 116649. [DOI] [PubMed] [Google Scholar]
- 12. Zhang H., Lin G., Jia S., Wu J., Zhang Y., Tao Y., Huang W., Song M., Ding K., Ma D., Fan M., Bioorg. Chem. 2024, 148, 107456. [DOI] [PubMed] [Google Scholar]
- 13. Bakhite E. A., Abdel-Rahman A. E., Al-Taifi E. A., Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 513–520. [Google Scholar]
- 14. Tkachenko O. V., Vlasov S. V., Kovalenko S. M., Zhuravel I. O., Chernykh V. P., J. Org. Pharm. Chem. 2013, 11, 15–21. [Google Scholar]
- 15. Saber A. F., El-Dean A. M. K., Redwan Sh.M., Zaki R. M., JCCS 2020, 67, 1239–1246. [Google Scholar]
- 16. Liu Z., Wu Sh., Wang Y., Li R., Wang J., Wang L., Zhao Y., Gong P., Eur. J. Med. Chem. 2014, 87, 782–793. [DOI] [PubMed] [Google Scholar]
- 17. Hafez H. N., Alsalamah S. A., El-Gazzar A.-R. B. A., Acta Pharm. 2017, 67, 275–292. [DOI] [PubMed] [Google Scholar]
- 18. Hu H., Dong Y., Li M., Wang R., Zhang X., Gong P., Zhao Y., Bioorg. Chem. 2019, 90, 103086. [DOI] [PubMed] [Google Scholar]
- 19. Ye T., Han Y., Wang R., Yan P., Chen Sh., Hou Y., Zhao Y., Bioorg. Chem. 2020, 99, 103796. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y., Shen J., Li J. W., Wang Z., Wang Y., Zhu Y., Ding Sh., Chen Y. P., Liu J., New J. Chem. 2023, 47, 21318–21331. [Google Scholar]
- 21. Kandeel M., Abdelhameid M. K., Eman K., Labib M. B., Chem. Pharm. Bull. 2013, 61, 637–647. [DOI] [PubMed] [Google Scholar]
- 22. Temburnikar K. W., Zimmermann S. C., Kim N. T., Ross Ch.R., Gelbmann Ch., Salomon Ch.E., Wilson G. M., Balzarini J., Seley-Radtke K. L., Bioorg. Med. Chem. 2014, 22, 2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farghaly A. M., Aboul Wafa O. M., Baghdadi H. H., Abd El Razik H. A., Sedra S. M. Y., Shamaa M. M., Bioorg. Chem. 2021, 115, 105208. [DOI] [PubMed] [Google Scholar]
- 24. Sirakanyan S. N., Ovakimyan A. A., Noravyan A. S., Minasyan N. S., Dzhagatspanyan I. A., Nazaryan I. M., Hakopyan A. G., Pharm. Chem. J. 2014, 47, 655–659. [Google Scholar]
- 25. Sirakanyan S. N., Akopyan E. K., Paronikyan R. G., Akopyan A. G., Ovakimyan A. A., Pharm. Chem. J. 2016, 50, 296–300. [Google Scholar]
- 26. Sirakanyan S. N., Geronikaki A., Spinelli D., Hakobyan E. K., Kartsev V. G., Petrou A., Hovakimyan A. A., An. Acad. Bras. Cienc. 2018, 90, 1043–1057. [DOI] [PubMed] [Google Scholar]
- 27. Sirakanyan S. N., Spinelli D., Geronikaki A., Kartsev V. G., Hakobyan E. K., Hovakimyan A. A., Curr. Org. Chem. 2018, 22, 2576–2588. [Google Scholar]
- 28. Sirakanyan S. N., Spinelli D., Geronikaki A., Hakobyan E. K., Sahakyan H., Arabyan E., Zakaryan H., Nersesyan L. E., Aharonyan A. S., Danielyan I. S., Muradyan R. E., Hovakimyan A. A., Molecules 2019, 24, 3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sirakanyan S. N., Spinelli D., Geronikaki A., Kartsev V. G., Hakobyan E. K., Hovakimyan A. A., Synth. Commun. 2019, 49, 1262–1276. [Google Scholar]
- 30. Sirakanyan S. N., Spinelli D., Geronikaki A., Hakobyan E. K., Hovakimyan A. A., Synth. Commum. 2019, 49, 2823–2833. [Google Scholar]
- 31. Sirakanyan S. N., Kartsev V. G., Geronikaki A., Spinelli D., Petrou A., Hakobyan E. K., Glamoclija J., Ivanov M., Sokovic M., Hovakimyan A. A., Curr. Top. Med. Chem. 2020, 20, 2192–2209. [DOI] [PubMed] [Google Scholar]
- 32. Reddy A.Ch. Sh., Narsaiah B., Venkataratnam R. V., J. Fluor. Chem. 1995, 74, 1–7. [Google Scholar]
- 33. Sirakanyan S. N., Spinelli D., Geronikaki A., Zuppiroli L., Zuppiroli R., Kartsev V. G., Hakobyan E. K., Yegoryan H. A., Hovakimyan A. A., Int. J. Mol. Sci. 2022, 23, 5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.