Abstract
Viruses constitute a significant group of pathogens that have caused numerous fatalities and substantial economic losses in recent years, particularly with the emergence of coronaviruses. While the impact of SARS-CoV-2 appears to be diminishing in daily life, only a limited number of drugs have received approval or emergency use authorization for its treatment. Given the high mutation rate of viral genomes, host-directed agents (HDAs) have emerged as a preferred choice due to their broad applicability and lasting effectiveness. In contrast to direct-acting antivirals (DAAs), HDAs offer several advantages, including broad-spectrum antiviral activities, potential efficacy against future emerging viruses, and a lower likelihood of inducing drug resistance. In our review article, we have synthesized known host-directed antiviral targets that span diverse cellular pathways and mechanisms, shedding light on the intricate interplay between host cells and viruses. Additionally, we have provided a brief overview of the development of HDAs based on these targets. We aim for this comprehensive analysis to offer valuable perspectives and insights that can guide future antiviral research and drug development efforts.
Keywords: Antiviral, Host-directed target, Virus-host interaction, miRNAs, IRFs, Hsps, Ubiquitin–proteasome system, Drug development
Graphical abstract
This review provides a summary of the host-directed antiviral targets identified to date, spanning a wide array of cellular pathways and processes, elucidating the intricate nature of host-virus interactions. Additionally, we have examined the advancements in inhibitors and drug research centered on these host-directed antiviral targets.
1. Introduction
Viruses, estimated to encompass approximately 1031 diverse species, have evolved in tandem with their hosts, notably humans, engaging in an enduring and subtle conflict1. Although most viruses do not lead to lethal outcomes in humans, several pandemics caused by viruses have occurred in the past 20 years, including severe acute respiratory syndrome coronavirus (SARS-CoV) (2002), “swine” influenza (2009), Middle East respiratory syndrome coronavirus (MERS-CoV) (2012), H1N1 chikungunya (2014), Ebola (2014), Zika (ZIKV) (2015), and most recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2019)2,3. The impact of SARS-CoV-2 has been profound, leading to a significant number of infections and fatalities, prompting a three-year disruption in both society and economics. Despite substantial progress in understanding SARS-CoV-2 and mitigating its virulence and morbidity, the emergence of viruses as high-risk pathogens in future outbreaks remains a foreseeable concern.
The conventional method for controlling virus-induced pandemics is vaccination, a convenient and cost–effective long-term measure4, 5, 6. However, challenges such as the expenses associated with low-temperature preservation and transportation, inadequate or absent immune responses in certain populations, and the limited effectiveness for individuals already infected render vaccination an incomplete solution for specific viruses7. An alternative option involves utilizing chemicals or natural substrate mimics inspired by virus–host interactions. Viruses possess a constrained genome size, encoding structural and non-structural proteins essential for their replication and propagation. Targeting virus-encoded proteins or other viral factors, such as the genome, represents an optimal approach, as the absence of human homologs would likely result in minimal cytotoxicity. Nevertheless, the heightened mutational frequency of viruses can render pathogen-targeted therapeutic interventions ineffective, particularly under selective pressures.
As a result, the scientific community is increasingly shifting towards an alternative strategy known as HDAs, which offer supplementary and compensatory options for combating viruses. In comparison to DAAs, HDAs present several advantages. Viruses are obligate parasitic pathogens that depend on host resources and cellular machinery for their survival. Targeting host-directed factors provides a broad-spectrum antiviral strategy, given that multiple viruses may utilize many of the same host factors during replication8. Additionally, compounds that interfere with host targets create a higher barrier to antimicrobial resistance due to the relatively stable host genome. However, the development of HDAs relies heavily on a comprehensive understanding of viral pathogenesis, which can be particularly challenging in unexpected pandemic scenarios. Understanding virus–host interactions and associated cellular pathways aids in the development of effective HDA strategies and facilitates the management of public health emergencies. This approach allows for the repurposing of existing drugs and offers insights into viral evolution and adaptation mechanisms within hosts.
In this review, we have compiled potential cellular targets involved in virus-host interactions, including microRNAs, transcription and nuclear factors, heat shock proteins, kinases and their associated signaling pathways, metabolism-related targets, the ubiquitin–proteasome system and ubiquitylation, and other host-directed targets. Furthermore, we have analyzed the progress in developing inhibitors and drugs based on these host-directed antiviral targets. While certain targets are known to be associated with specific viral infections, they may offer valuable insights applicable to other viral pathogenesis scenarios. Host-directed antiviral targets related to host immune and inflammation responses, which have been extensively covered elsewhere9, 10, 11, are not discussed in this review.
2. MicroRNAs (miRNAs)
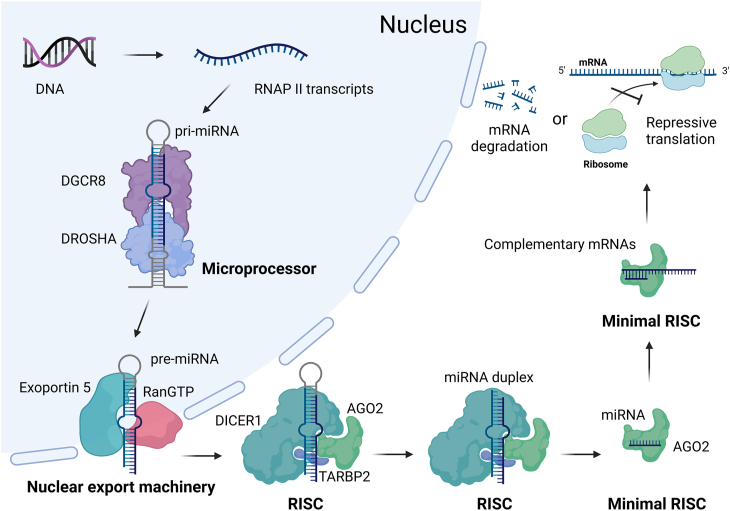
miRNAs are short, non-coding RNA sequences typically consisting of around 20 oligoribonucleotides. They are derived from larger RNA polymerase II (RNAP II) transcripts known as primary miRNAs (pri-miRNAs). A pri-miRNA can undergo processing to generate multiple miRNAs or a single miRNA. miRNAs combine with various components to form the RNA-induced silencing complex (RISC), with argonaute proteins playing a crucial role, particularly argonaute 2 (AGO2)12, 13, 14. miRNAs guide RISC to target mRNAs, primarily by recognizing the 3′UTRs of mRNAs, although they may also bind to the 5′UTR or the coding region15, 16, 17, 18. The degree of complementarity between miRNAs and their target mRNAs dictates the fate of the mRNAs: direct degradation by AGO2 in cases of high complementarity, or translational repression in cases of lower complementarity (Fig. 1)14,15.
Figure 1.
The biogenesis of miRNA. RNAP II transcripts (pri-miRNAs) undergo processing into miRNAs by the microprocessor and RISC, and meanwhile are transported out of the nucleus via nuclear export machinery. miRNAs associate with AGO2 to form the minimal RISC, guiding it to target mRNAs primarily by recognizing the 3′UTRs of mRNAs. The level of complementarity between miRNAs and their target mRNAs determines the fate of the mRNAs: direct degradation (high complementarity) or translational repression (lower complementarity). (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
Compared to other regulatory factors, miRNAs have a relatively recent history. The first discovery of miRNAs dates back to 1993 in Caenorhabditis elegans, with the identification of the miRNA lin-4, followed by let-719,20. Through extensive research efforts, miRNAs were eventually acknowledged as a conserved group of endogenous regulatory factors present in various species, spanning animals and plants, and were officially termed miRNAs21, 22, 23, 24. Over the course of approximately two decades, there has been a substantial expansion in the understanding of miRNAs, ranging from the identification of novel miRNAs to the elucidation of their roles in physiology and disease. The microRNA database miRbase now contains 38,589 entries, including 1917 miRNA entries from Homo sapiens (as of November 23, 2024)25. The discovery of miRNAs has significantly transformed the scientific comprehension of gene regulation, leading to Victor Ambros and Gary Ruvkun jointly receiving the Nobel Prize in Physiology or Medicine “for the discovery of microRNA and its role in post-transcriptional gene regulation”26. miRNAs introduce a novel mechanism of post-transcriptional gene regulation and play crucial roles in various cellular processes such as development, differentiation, proliferation, apoptosis, homeostasis, stress responses, and immune responses, particularly in IFN-mediated immune activation during viral infections14,15. Through interactions with their target mRNAs, miRNAs either degrade or transcriptionally repress these mRNAs via RISC, thereby shaping the cellular expression profile and influencing cellular evolution. Notably, based on reported and predicted miRNA targets, miRNAs are known to regulate over half of the protein-coding genes in humans16.
In the context of viral infections, miRNAs play a dual role. On one hand, they can initiate an antiviral response by directly interacting with the viral genome or altering host mRNA expression patterns. Conversely, viruses may exploit host miRNAs to establish a more conducive cellular environment for their replication.
2.1. Antiviral miRNAs
Building upon prior research, McCaskill et al. expanded on the antiviral properties of miRNA mimics against influenza A virus (IAV) and respiratory syncytial virus (RSV). They pinpointed miR-124, miR-24, and miR-744 as targeting the p38 mitogen-activated protein kinase (MAPK) signaling pathway, specifically honing in on the MAPK-activated protein kinase 2 (MK2) (Fig. 2). Meanwhile, MK2 was identified as a versatile antiviral target with potential for the development of therapies against both IAV and RSV27.
Figure 2.
The antiviral mechanism of miR-124, miR-24, and miR-744. The p38 MAPK pathway receives upstream signal transduction, including signals from GPCR, DNA damage, oxidative stress, UV radiation, inflammatory cytokines, FasL, and TGF-β, and regulates downstream effectors, activating transcription and leading to cytokine production, apoptosis, and other cellular responses. miR-124, miR-24, and miR-744 have been identified as targeting the p38 MAPK signaling pathway, specifically focusing on the MK2 kinase. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
A recent study has unveiled a novel pathway of IFN-mediated antiviral response that governs cholesterol biosynthesis through miR-342-5p. This miRNA employs a multi-hit strategy by repressively modulating key regulators (SREBF2 and miR-33) and crucial enzymes (IDI1 and SC4MOL) within the sterol pathway (Fig. 3). Leveraging the significance of this pathway, miR-342-5p achieves broad-spectrum antiviral activity against human cytomegalovirus (HCMV), herpes simplex virus 1 (HSV1), and IAV (H1N1)28.
Figure 3.
The mechanism of miR-342-5p regulating sterol metabolism. miR-342-5p targets the mevalonate-sterol biosynthesis pathway through a multi-hit mechanism, suppressing the pathway at various functional levels: transcriptionally viaSREBF2, post-transcriptionally via miR-33, and enzymatically viaIDI1 and SC4MOL. This leads to a reduction in intermediate sterol pathway metabolites and total cholesterol levels. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
Several other antiviral miRNAs demonstrate diverse mechanisms in combating viral infections. For instance, miR-223 targets the Forkhead Box Protein O3 (FOXO3) to combat vesicular stomatitis virus (VSV), miR-let-7c targets the HO-1 transcriptional repressor Bach1 to fight against hepatitis C virus (HCV), and hsa-miR-1-3p directly inhibits the supportive host factor ATP6V1A to counteract influenza virus H1N129, 30, 31. These examples showcase the varied ways in which miRNAs can be utilized in antiviral strategies, whether by modulating the host's immune response or by disrupting the virus's ability to exploit host factors for replication.
2.2. Proviral miRNAs
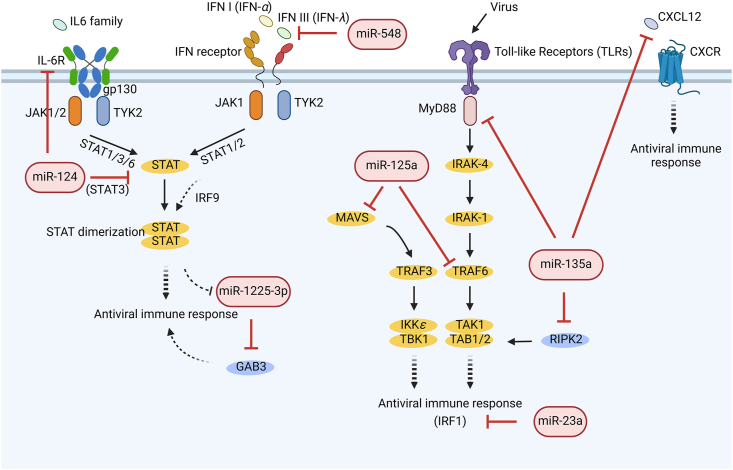
Host miRNAs can modulate proviral activity by interfering with host antiviral immune responses, with their effects varying depending on the specific virus. For instance, miR-124, known for its antiviral activity, has been found to exhibit proviral activity against enterovirus 71 (EV71) by targeting IL-6R and STAT3 mRNAs (Fig. 4)32. This highlights the need for further exploration into the diverse roles miRNAs play across various viral pathogens.
Figure 4.
The proviral mechanisms of miRNAs. The IL-6 family/IL-6R, IFN I/IFN receptors, TLRs, and CXCL12/CXCR pathways mediate diverse antiviral immune responses. In contrast, miRNAs such as miR-124, miR-1225-3p, miR-548, miR-23a, miR-135a, and miR-125a disrupt these antiviral responses at various levels. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
Another miRNA, miR-1225-3p, is down-regulated by type I interferon through the IFN/JAK/STAT signaling pathway. Its inhibition leads to increased expression of growth factor receptor-bound protein 2-associated binding protein 3 (GAB3) (Fig. 4), which enhances antiviral responses against multiple IFN-susceptible viruses, including HCV, Sendai virus (SeV), and Newcastle disease virus33. The study suggests that the downregulation of miR-1225-3p may serve as a mechanism by which host cells defend against viral infection by boosting the antiviral response. This discovery not only elucidates the biological function of miR-1225-3p but also proposes a novel antiviral regulatory pathway involving miRNA and GAB3.
Two other miRNAs, miRNA-548 and miRNA-23a, are also engaged in the IFN response by targeting IFN-λ1 and IRF1 (Fig. 4), respectively, thereby promoting the replication of EV71 and VSV, or human HSV1 separately34,35.
Toll-like Receptors (TLRs) are pivotal pattern recognition receptors for pathogens that mediate innate immune responses against viruses. For instance, miR-135a targets myeloid differentiation primary response 88 (MyD88) in the TLR signaling pathway, along with related serine/threonine kinase 2 (RIPK2) and the antiviral chemokine CXCL12 (Fig. 4), thereby preferentially regulating HCV propagation36. Similarly, miR-125a, upregulated in response to HCV infection, suppresses the expression of mitochondrial antiviral signaling (MAVS) and TNF receptor-associated factor 6 (TRAF6) (Fig. 4), both crucial components of the antiviral IFN response and TLR signaling pathway37.
Furthermore, proviral miRNAs like miR-214 facilitate viral infections by enhancing host supportive factor thrombin while simultaneously targeting the antiviral factor 2′,5′-oligoadenylate synthetase38. This regulatory effect of miR-214 may create a conducive environment for viral replication while evading the host immune response.
The modulation of gene expression by miRNAs can occur through various mechanisms. Some miRNAs directly target mRNAs32, while others influence positive or negative regulatory factors28,31,35,38. This diversity in miRNA-mediated regulation enables precise control of gene expression in response to viral infections.
It is crucial to recognize that the antiviral or proviral factors and signaling pathways regulated by miRNAs are not mutually exclusive. For example, FOXO3, MAVS, TRAF6, and GAB3 are all linked to the IFN-mediated antiviral response30,33,37, emphasizing the multi-hit regulatory nature of miRNAs. Furthermore, miRNAs and the IFN pathway can directly or indirectly regulate each other, forming a regulatory loop or network against viral infections30,35. This intricate interplay between miRNAs and the IFN pathway adds an additional layer of control to the host's antiviral defenses.
This multi-layer regulatory mechanism may assist host cells in effectively combating viral infections while preventing excessive immune responses that could lead to damage. Simultaneously, this complex regulatory network may offer viruses multiple avenues to evade the host's immune defenses, contributing to the ongoing evolutionary battle between host and virus. Exploring the interaction between these miRNAs and their targets, whether from hosts or viruses, is crucial for developing novel antiviral therapeutic strategies. Modulating miRNAs or their targets in immune responses could enhance the host's antiviral capabilities or mitigate pathological damage caused by viral infections.
As a relatively new and efficient post-transcriptional genetic regulatory mechanism, it was soon discovered that miRNAs are encoded not only in living organisms but also in viruses39. Compared to viral protein-mediated gene regulation, viral miRNAs offer several advantages in intervening with cellular activities and promoting viral replication, such as occupying much less space in the limited genome and being less likely to induce immune responses due to their non-immunogenic nature14,40. Overall, viral miRNA-mediated gene regulation creates a conducive cellular environment for virus replication and propagation by aiding immune evasion during early viral entry and infection, counteracting apoptosis, and maintaining viral latency in later infected cells41, 42, 43. Both cellular and viral miRNAs have the potential to serve as targets for antiviral interventions due to their extensive role in post-transcriptional regulation44.
2.3. Strategies for targeting miRNAs
In the realm of drug development, the viable strategy for targeting miRNAs in antiviral therapy encompasses several key elements. Firstly, the utilization of miRNA mimics and anti-miRNA therapy plays a pivotal role. miRNA mimics are employed to restore or enhance the function of miRNAs that are diminished or under-expressed in diseases, while anti-miRNAs function by binding to overexpressed endogenous miRNAs, thereby silencing their activity. Secondly, miRNAs are harnessed for viral inhibition, exemplified by Miravirsen, an antisense RNA strand employing locked nucleic acid (LNA) technology to target the 5′ end of miR-122 for treating HCV infection. miR-122 is a supportive host factor for HCV infection and related hepatocellular carcinoma45. Miravirsen has exhibited efficient liver delivery, reduced cholesterol accumulation, and diminished HCV titers. Even though the clinal trial of Miravirsen was ceased, there are still more successors on the way, such as RG-101, the next-generation GalNAc-conjugated antagomiR against miR-122 under Phase II clinical trial46. Furthermore, disease-specific miRNA drug development is exemplified by Phase II clinical trials for MRG-201 and MRG-106, miR-29 analogs for scleroderma, and antimiR-155 nucleotides for fungoid cutaneous T-cell lymphoma.
Nevertheless, targeting miRNAs for antiviral therapy presents several challenges. Firstly, there is difficulty in target selection and validation due to the intricate nature of miRNAs targeting multiple genes simultaneously, making it exceedingly complex to accurately discern their mechanisms of action. Despite the availability of advanced algorithms, extensive sequence data, and tools like the MiRBase database to aid in predicting miRNA–mRNA binding sites, the functions of most miRNAs remain ambiguous and cannot be validated across all biological contexts. Secondly, concerns regarding off-target effects and safety arise from the multi-target nature of miRNAs, potentially leading to unintended consequences during treatment, such as the inhibition or activation of non-target genes, resulting in adverse reactions. For instance, anti-miRNA therapy may inadvertently impact certain tumor suppressor genes or genes crucial for normal cell homeostasis, disrupting fundamental cellular functions. Lastly, delivery and toxicity issues pose significant challenges as the delivery system for miRNA therapy must ensure effective targeting of the drug to the intended tissue with minimal toxicity. Currently, the development of delivery systems remains a hurdle, necessitating the reduction of side effects while ensuring efficacy. In conclusion, while antiviral therapy targeting miRNAs holds substantial promise, numerous challenges including target selection, off-target effects, and delivery system development must be addressed before clinical application can be realized.
In addition to their antiviral potential, miRNAs can serve as clinical biomarkers for chronic and persistent viral infections, including human immunodeficiency virus, human papillomavirus, and HCV. In some instances, miRNAs can also function as predictive biomarkers for prognosis, particularly for carcinogenic viruses47, 48, 49, 50, 51, 52, 53. As modern medical practices increasingly focus on precise and personalized healthcare, miRNA profiles could offer a robust diagnostic tool for identifying pathogen-resistant or susceptible populations. However, current research in this area predominantly concentrates on plants and livestock54, 55, 56, 57, 58, 59, 60, 61, 62. Furthermore, specific miRNAs combined with oncolytic viruses can be formulated as novel targeted anti-cancer agents63, 64, 65, holding promise for the development of more effective and targeted cancer therapies.
The methodologies employed to identify cellular or viral miRNAs and investigate their functional roles can inspire other genetic research endeavors. These methods encompass next-generation sequencing technology, genome-wide miRNA functional screening, in silico prediction or bioinformatics-based integrative analysis, transcriptomics analysis, gene reporter assays, and various other techniques66, 67, 68, 69, 70. These approaches provide valuable tools for unraveling the intricate roles of miRNAs in viral infections and other biological processes, contributing to the advancement of new antiviral strategies and drug targets.
3. Transcription and nuclear factors
Transcription factors play a pivotal role in governing gene expression, exerting a profound impact on cellular adaptation in reaction to internal and external cues. Acting as key regulatory proteins, they act as mediators that bridge various cellular antiviral responses, rendering them significant focal points for antiviral investigations. Despite being historically deemed challenging to target pharmacologically, recent research and clinical trials are progressively endorsing a reassessment of transcription factors as promising targets within the antiviral domain71,72. Nevertheless, caution is warranted when targeting transcription factors due to their broad-reaching effects on gene expression and diverse cellular functions.
3.1. Interferon regulatory factors (IRFs)
Upon viral infection, host cells typically initiate the innate immune response by engaging pattern recognition receptors and subsequent adaptor proteins like STING and MAVS, along with effector kinases. These signaling cascades culminate in interferon-mediated antiviral signaling, a process tightly regulated by IRFs, notably IRF3 and IRF773. Given their pivotal role in antiviral defenses, IRFs are frequently targeted by viral proteins (Fig. 5). For instance, rotavirus (RV) NSP1, pestivirus Npro, pseudorabies virus (PRV) US3, and H1N1 IAV PA have all been identified as antagonists of IRF374, 75, 76, 77. Similarly, duck hepatitis A virus (DHAV)-1 3C, PRV UL24, and H1N1 IAV NS2 have been reported to interfere with IRF778, 79, 80. The direct interaction between these viral proteins and IRFs disrupts the activation and downstream signaling of IRFs, sometimes leading to their degradation. This manipulation empowers the virus to effectively subvert the host's innate antiviral response, thereby facilitating its replication and spread.
Figure 5.
The IRF-mediated antiviral response and their regulation by viruses. IRF3 and IRF7 are activated by upstream signal transduction triggered by stimuli like DNA, RNA, and LPS from intracellular or extracellular sources, leading to the activation of the interferon response. Given their critical functions in antiviral responses, various viruses have developed specific proteins that target IRF3 and IRF7. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
IRFs and their associated antiviral responses are under the regulation of host restriction factors to uphold physiological homeostasis. However, this regulatory framework also presents opportunities for viruses to manipulate the network and undermine host immune defenses (Fig. 5). For instance, NBR1 acts as a cargo receptor, sequestering IRF3 into autophagosomes for degradation, a process that is exploited by SeV to enhance viral replication81. Similarly, A20, induced by the IAV NS1 protein, suppresses the IRF3 signaling pathway82, enabling viruses to evade the host's antiviral response.
AGO2, another restriction factor, impedes the assembly of the transcriptional complex involving IRF3 and CBP/p300 in the nucleus. H5N1 infection disrupts the nuclear distribution of AGO2, leading to the activation of the IFN signaling pathway83.
Additional restriction factors like human noncoding RNA nc886, Rubicon, and Fas-associated factor 1 (FAF1) have been identified to negatively modulate the IRF3-mediated antiviral pathway84, 85, 86. However, their specific interactions with viral infections remain unclear and necessitate further exploration.
In addition to exploiting cellular factors, viruses can encode their own IRF to subvert the host immune response and promote pathogenesis87. These viral IRF homologs mimic host IRF functions, enabling the virus to manipulate antiviral signaling pathways and create a conducive environment for viral replication. Investigating viral IRF homologs offers valuable insights into host–virus interactions and viral strategies to evade immune responses, potentially guiding the development of innovative antiviral approaches.
A comprehensive understanding of the interplay among host restriction factors, IRFs, and viral proteins is essential for delineating host–virus dynamics. This knowledge may inspire the design of therapies aimed at restoring IRF function and bolstering the host's antiviral response88,89. Exploring the repercussions of IRF inhibition by viral proteins can shed light on the broad impact of viruses on the host immune system and response to viral infections. Furthermore, unraveling how host restriction factors regulate IRF-mediated antiviral pathways can offer insights into maintaining homeostasis and preventing excessive immune responses that could lead to tissue damage.
Moreover, IRFs could potentially serve as biomarkers for antiviral therapy90. Monitoring IRF expression levels or activation status during viral infections may enable clinicians to evaluate the efficacy of antiviral treatments and make informed decisions regarding patient care.
3.2. Zinc finger antiviral protein (ZAP)
Metal ions play crucial roles in biological systems, with Zn(II) ions being particularly essential for approximately 10% of encoded proteins91,92. This section delves into a specific group of Zn(II)-binding proteins that possess one or multiple domains coordinating with Zn(II), known as zinc finger domains. Zinc finger domains represent common DNA-binding domains in transcription factors, playing a pivotal role in gene regulation. These domains are prevalent in cellular proteins and contribute to the resilience of animals and plants against external stress93, 94, 95, 96, 97, 98, 99.
One notable example is ZAP, also referred to as zinc finger CCCH-type antiviral protein 1 or inactive poly (ADP-ribose) polymerase 13 (PARP13). ZAP comprises three integrated domains: the N-terminal domain, the central domain, and a third PARP-like domain, with various signal peptides and cofactor binding sites intersecting these domains. The N-terminal domain contains four C3H1-type zinc finger domains that bind to RNA. In conjunction with diverse cofactors like poly(A)-specific ribonuclease PARN and the decapping complex DCP1–DCP2, this domain facilitates the degradation of viral mRNAs from both ends100. The central domain of ZAP includes a fifth zinc finger domain and two WWE domains that bind to ADP-ribose. Although the PARP-like domain of ZAP reportedly lacks ADP-ribosyltransferase activity, the binding of ADP-ribose enhances its antiviral function. Conversely, a single Q668R mutation disrupts poly(ADP-ribose) (PAR) binding and reduces ZAP's antiviral efficacy101. The absence of ADP-ribosyltransferase activity is attributed to the closure of the NAD+ binding cleft, facilitated by a newly formed short α-helix (Asp803–His807), the hydrogen bond between His810 and Tyr826, and the absence of PARP consensus residues crucial for nicotinamide anchoring and catalysis (Fig. 6)102.
Figure 6.
The domain arrangement of ZAP and schematic of each domain's function. The comparison of PARP consensus residues is sourced from a recent report102. The full-length structure of ZAP is revealed through the AlphaFold-predicted model, while the complex structures of the N-terminal domain with RNA, the central domain with ADP-ribose, and the alignment of the PARP-like domain with PARP15 are elucidated by PDB entries 6UEJ, 7TJQ, 2X5Y (and 3BLJ for PARP15), respectively. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
ZAP typically identifies viral genomes through ZAP-responsive elements (ZREs) that consist of high GC content. Consequently, frequent CpG nucleotides, which invariably result in high GC content, are considered targets of ZAP. Viruses, adapting over time to host antiviral immune pressures, have shown a gradual decrease in CpG dinucleotides, a trend observed in viruses like SARS-CoV-2 and related species103. Analyzing and comparing CpG dinucleotides in viral genomes can aid in vaccine development104. To effectively exert its antiviral function, ZAP must discern between host and viral genomes based on CpG dinucleotide content. Moreover, ZAP's role in IFN-mediated antiviral responses involves recognizing CpG dinucleotides, influencing the gene expression of related interferon-stimulated genes (ISGs) and interferon-repressed genes (IRGs)105. However, CpG dinucleotides alone do not solely determine ZAP recognition, as evidenced by the lack of a positive correlation between lentiviral vector production and CpG abundance106. The precise targeting mechanism of ZAP remains to be fully elucidated.
Furthermore, a multitude of nuclear factors partake in host antiviral defenses and host–virus interactions. Some of these factors are integral to IFN signaling or other antiviral immune responses, including various ISGs and modulators107,108. Others play crucial roles in normal cellular functions, even during viral infections, such as importin proteins, mRNA splicing factors, or complexes84,109, 110, 111, 112, 113. Effector molecules implicated in other diseases, like p53 linked to cancer, also exhibit activity in viral infections114, 115, 116. As scientific understanding advances, more instances of such crossovers are anticipated to emerge.
Despite the significant involvement of transcription and nuclear factors in host–virus interactions, targeting these factors poses challenges due to their structural diversity, limited druggable sites, nuclear localization, and multifaceted cellular functions. Nonetheless, with technological progress, certain factors previously deemed "undruggable” have shown promise for drug development. These advancements offer valuable insights and avenues for crafting innovative antiviral therapeutic strategies and identifying potential drug targets.
4. Heat shock proteins (Hsps)
Hsps have long been acknowledged for their significance in viral infections since the previous century117, 118, 119, 120, 121, 122. The interplay between Hsps and viral proteins, along with the roles of Hsps in viral replication and transcription, has been progressively explored. Hsps, a class of molecular chaperones, play pivotal roles in various cellular processes beyond the well-studied heat shock response. Operating at the core of the protein quality control system, Hsps, in conjunction with their adaptable co-chaperones or complexes, facilitate the proper folding, refolding, and degradation of specific client proteins. These diverse functions of Hsps are intricately tied to their ATPase activity, with the affinity for client proteins being modulated by their ATP-bound state. Additionally, a variety of co-chaperones or complexes regulate the interactions of Hsps with client proteins based on their specificity, collectively overseeing the diverse cellular functions of Hsps123, 124, 125, 126, 127.
Given the central involvement of Hsps in cellular processes and the reliance of viruses on host translation machinery, the association of Hsps with viral infections is expected. Among the array of Hsps, Hsp70 and Hsp90 have been extensively studied. Multiple investigations have demonstrated that Hsp70 plays a role in various stages of the viral infection cycle, encompassing entry, replication, assembly, and release from host cells (Fig. 7). Hsp70 also influences viral protease activity and stabilizes viral proteins through its chaperone function128, 129, 130, 131, 132, 133, 134. Hsp90 collaborates in these processes, particularly during the assembly phase131. The proviral impact of Hsp70 is evident across a spectrum of viruses, including several flaviviruses (such as ZIKV, dengue, yellow fever, West Nile, and Japanese encephalitis viruses), primate lentiviruses (such as HIV-1, HIV-2, and simian immunodeficiency viruses SIVMAC and SIVAGM), avian virus CELO, porcine epidemic diarrhea virus (PEDV), Hepatitis B virus (HBV), and IAV. Different viruses utilize distinct viral proteins to engage with Hsp70, enhancing or relocating its expression to facilitate specific stages in their replication cycles. Furthermore, the induced expression and proviral impact of Hsp70 have been observed in virus-infected plants135.
Figure 7.
The proviral effects of Hsp70 impact multiple stages of the viral infection cycle, such as entry, replication, assembly, and release, and involve various viruses, with their interacting proteins specified in the brackets. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
While Hsps have been associated with proviral effects in numerous viral infections, several studies have indicated that Hsps can also exhibit antiviral effects, potentially through the degradation of viral proteins136, 137, 138, 139. Nonetheless, chemical inhibitors, antibodies, or competitive recombinant Hsp proteins have been demonstrated to reverse the proviral function of Hsps in viral replication, suggesting their therapeutic potential against viruses130,140, 141, 142, 143, 144, 145, 146.
4.1. Representative Hsp70 inhibitors
It is anticipated that Hsp70 inhibitors will exhibit broad-spectrum antiviral activity. The high affinity of Hsp70 for ADP and the conformational state of Hsp70 make the ATP-binding site challenging to access, posing difficulties in designing inhibitors that target these sites. Several representative Hsp70 inhibitors are detailed in Supporting Information Table S1.
MKT-077 functions by inhibiting Hsp70 through disrupting its interaction with nucleotide exchange factors, leading to the release of Hsp70 binding substrates147. MKT-077 and its analogues JG-18 and JG-40 have been shown to inhibit the transmission of DENV, with no observed toxicity to host cells at concentrations that effectively inhibit viral replication129. Another inhibitor, HS-72, selectively targets Hsp70i (the inducible isoform of Hsp70) by focusing on an allosteric site. HS-72 has been demonstrated to inhibit DENV entry primarily by disrupting the binding of Hsp70i to the DENV receptor complex148. On the other hand, IMB-DM122, a derivative of the natural compound oxymatrine, acts as a downregulator of Hsc70 (the constitutive isoform of Hsp70). IMB-DM122 effectively reduces the encapsidation of Hsc70 into HCV virion particles by targeting the Hsc70 mRNA 3′ untranslated region sequence and destabilizing the mRNA. This action limits HCV assembly and restricts its ability to infect cells149.
4.2. Representative Hsp90 inhibitors
Several representative Hsp90 inhibitors are listed in Table S1, with the most renowned being geldanamycin150. Geldanamycin was the first Hsp90 inhibitor identified to bind to the N-terminal ATP-binding pockets. It has been reported to exhibit antiviral activity against a range of viral infections, including HSV2, HSV1, HCMV, EBOV, HIV1, and influenza viruses. However, due to its poor water solubility and severe liver toxicity, geldanamycin has undergone structural modifications. Modified 17-AAG has been shown to inhibit RSV replication at concentrations as low as 1.9 nmol/L151.
Radicicol, an Hsp90 inhibitor derived from Monosporium bonorden152, destabilizes the newly synthesized L protein (the large subunit of VSV polymerase) by inhibiting Hsp90. This action has been demonstrated to inhibit the replication of SV5, HPIV-2, HPIV-3, SV41, and La Crosse Bunyavirus153. Gedunin, a noncompetitive Hsp90 inhibitor versus ATP, has also been reported to inhibit DENV in vitro with an EC50 value of 10 μmol/L154.
Many Hsp90 inhibitors in clinical trials are linked to serious side effects such as cardiotoxicity and gastrointestinal toxicity. Subtype-selective Hsp90 inhibitors may present a novel strategy for developing broad-spectrum antivirals.
In a study, interfering with the upstream regulator of Hsps, heat shock transcription factor 1 (HSF1), using its dominant-negative mutant (mHSF1), reversed Hsp70's anti-apoptotic function and induced cancer cell death155. This approach could be a valuable consideration for intervening in the proviral influence of Hsps, alongside the use of chemical inhibitors or antibodies. Moreover, leveraging the interaction of Hsps with viral proteins could enable the use of viral proteins or even entire virions for delivering Hsps, allowing them to exert their cytoprotective effects156.
5. Kinases and their related signaling pathways
Kinases, a group of post-translational regulators, play a crucial role in connecting various intracellular or intercellular signaling pathways and are involved in numerous physiological events. The study of cellular kinases dates back to the 1950s, and their wide-ranging and critical involvement in life sciences led to researchers Edmond H. Fischer and Edwin G. Krebs being awarded the Nobel Prize in Physiology or Medicine in 1992 for their discoveries related to “reversible protein phosphorylation as a biological regulatory mechanism”157. The term “kinome” was later introduced as a counterpart to the “genome”, underscoring the significance of kinases and advocating for their systematic study158. Cellular kinases and their associated signaling pathways serve as important regulatory factors, functioning not only in physiological conditions but also in diseases such as viral infections and cancer159,160. While most kinase inhibitors are approved for use as anti-cancer agents, recent research has increasingly recognized their potential antiviral effects, particularly through drug repurposing during various virus-caused pandemics161, 162, 163, 164, 165.
It is worth noting that viruses also encode their own kinases, although this discovery occurred much later than the identification of host kinases166, 167, 168. Viral kinases play a vital role in pathogenesis, although they are not the primary focus of this review, and only a brief introduction will be provided regarding their role in viral infections.
5.1. IKKε and TBK1
During viral infections, cellular kinases can play a crucial role in mediating antiviral responses by regulating related signaling pathways. For example, kinases such as IKKε and TBK1 are involved in RIG-I/MAVS and TLR signaling pathways.
In the context of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV), the nucleoprotein (NP) binds to the kinase domain (KD) of IKKε. This interaction blocks the autocatalytic activity of IKKε, thereby preventing the IRF3-mediated antiviral response to SeV infection. This mechanism is conserved among various arenaviruses, including the Old World arenavirus Lassa virus (LASV) and New World arenaviruses from clades A (White Water Arroyo virus (WWAV)), B (Junin virus (JUNV)), and C (Latino virus (LATV))169.
Similarly, in the case of HSV1, the tegument protein UL46 interacts with TBK1. This interaction leads to a reduction in TBK1 activation and its downstream signaling. UL46 specifically inhibits the dimerization of TBK1 and interferes with its interaction with IRF3, ultimately inhibiting IRF3 activation and the subsequent production of type I interferons (IFN-I)170.
5.2. p38 MAPK
The p38 MAPK signaling pathway is involved in a variety of cellular processes in response to environmental stresses and inflammatory cytokines, encompassing proliferation, development, differentiation, transformation, and apoptosis. While p38 MAPK can facilitate antiviral immune responses by stimulating transcription and cytokine production, promoting immune cell proliferation and differentiation, it can also support viral replication by modulating translation and the cell cycle. Early apoptosis during viral infection often impedes productive virus proliferation. Viral proteins like HIV-1 Nef interact with kinases (such as the CAMKIIδ–ASK-1 complex) within the pathway, disrupting p38 MAPK-mediated apoptosis. Competitive peptide inhibitors targeting Nef binding domains to CAMKIIδ have reversed this effect, restoring p38 MAPK phosphorylation and apoptosis171. Effective antiviral treatments have been demonstrated by targeting other kinases in the p38 MAPK pathway, including p38 itself (SB203580 and SB202190), MSK1/2 (H89 and SB747651A), and MNK1 (CGP57380) (Table S1)172,173. Given its influence on cytokine production, inhibiting this pathway could prevent virus-induced cytokine storms and potentially enhance prognosis.
5.3. RIPK3 and MLKL
Necroptosis, akin to apoptosis, serves as an early defense mechanism against viral infections and is recognized as a component of innate immunity. This process relies on two key effectors: receptor-interacting protein kinase 3 (RIPK3) and the pseudokinase mixed-lineage kinase-domain-like (MLKL). Certain poxviruses carry MLKL homologs in their genomes, which imitate the function of MLKL and bind RIPK3, thereby obstructing cellular MLKL activation and preventing necroptotic cell death174. However, in the later stages of viral infections, viruses tend to promote apoptosis and necroptosis to hasten replication and dissemination. Following 6 h of Rhinovirus infection, the phosphorylation of MLKL (indicative of necroptosis) coincides with caspase cleavage (an apoptotic marker), leading to compromised plasma membrane integrity and the release of alarmin molecules into the culture media. The application of inhibitors targeting both necroptosis and apoptosis reversed the cellular damage induced by the release of viral progeny175.
5.4. AMPK
The AMPK signaling pathway, functioning as a cellular energy sensor, plays a pivotal role in various cellular processes such as cell growth, proliferation, autophagy, stress response, and metabolic reprogramming (Fig. 8). These processes are crucial for viral propagation and can be exploited by viruses. Manipulating this pathway offers a means to regulate viral infections. For instance, metformin, a biguanide derivative prescribed for type 2 diabetes mellitus (T2DM), was repurposed for COVID-19 treatment during the pandemic. Subsequent research elucidated its antiviral mechanism, which involves AMPK activation and associated metabolic reprogramming176. The intricate nature of the AMPK pathway in viral infections underscores the necessity for caution when devising AMPK-targeted antiviral strategies. While AMPK activators may impede viral replication in certain scenarios, they could potentially facilitate viral replication or exacerbate inflammatory responses in others176,177. Therefore, comprehending the specific mechanisms of AMPK in distinct viral infections is paramount for the development of efficacious antiviral therapies.
Figure 8.
The vital role of the AMPK signaling pathway in cellular progress. This pathway integrates various signaling factors to govern diverse and essential cellular processes, encompassing cytoskeletal signaling, acute metabolic regulation, transcriptional control of metabolism, as well as protein metabolism and autophagy. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
5.5. Pyruvate kinase (PK)
Another instance pertinent to energy metabolism and viral infections involves PK. Like AMPK, PK plays a pivotal role in cellular processes, exhibiting versatility in its interactions with viruses. Pyruvate kinase muscle type 2 (PKM2) has been identified as an antiviral factor by incorporating into virions and hindering their ability to assimilate cellular tRNAs such as tRNALys3, tRNALys1,2, and tRNAAsn, thereby diminishing HIV-1 virion infectivity178. In the case of tomato bushy stunt virus (TBSV), the virus exploits PK to boost ATP levels, aiding in the function of recruited cellular DEAD-box helicases and consequently enhancing the production of viral (+)RNA progeny179. Furthermore, IAV NP and matrix protein (M1) both interact with pyruvate kinase along with another host glycolytic enzyme, alpha-enolase180,181. Nevertheless, the study did not elaborate on the implications of these interactions in viral infections.
5.6. Others
Focal adhesion kinase (FAK), a non-receptor tyrosine kinase, and casein kinase 2 (CK2), a ubiquitous serine/threonine kinase, are additional kinases that hold pivotal roles in both cellular processes and viral infections. These kinases have been observed to interact with IAV proteins, specifically NP and NS1, respectively, enhancing their polymerase activity182,183.
The identification of more kinases involved in virus-host interactions enhances our understanding of viral infections. Delving into these interactions offers valuable insights for crafting targeted antiviral therapies that disrupt viral pathogenesis while preserving essential cellular functions. Various viruses or subtypes manipulate different kinases and associated signaling pathways, potentially influencing their cellular or tissue tropism and host specificity, thereby elucidating why certain populations exhibit more susceptibility or resistance phenotypes184. This knowledge can aid in tailoring antiviral agents to specific virus types or subtypes and even in devising personalized antiviral treatments when necessary. Conversely, some viruses utilize redundant cellular kinases, enabling them to develop resistance by switching to alternative kinases upon treatment with a single kinase inhibitor185,186. In such scenarios, broad-spectrum kinase inhibitors or combination therapies may be more effective.
5.7. The problems and development challenges of kinase inhibitors in antiviral therapy
The repurposing of kinase inhibitors as antivirals, despite their broad antiviral potential, presents significant concerns and limitations. Kinases, integral for cellular function regulation, raise toxicity worries when targeted by inhibitors. The narrow therapeutic window of current antiviral drugs, with a small margin between efficacy and toxicity, restricts their clinical application. Operating within a well-defined therapeutic window is crucial to minimize potential toxicity. Most kinase inhibitors, designed to target conserved ATP-binding sites shared across kinase families, exhibit cross-inhibitory activity against multiple kinases, posing specificity challenges. Host kinases adaptor protein 2 (AP2)-associated protein kinase 1 (AAK1) inhibitors, for instance, affect various kinases beyond AAK1, complicating the assessment of their antiviral effects. Future research aims to identify highly selective AAK1 inhibitors to elucidate the antiviral mechanism. Given AAK1's structural resemblance to numerous kinases, developing selective and specific AAK1 inhibitors presents a challenging task for pharmaceutical chemists. Balancing the adverse effects of AAK1 inhibitors on normal cellular transport, crucial for cell function, is another obstacle. When formulating antiviral AAK1 inhibitors, maintaining a safe and effective compound window is paramount. Developing allosteric kinase inhibitors encounters hurdles in hits discovery, optimization, and activity evaluation. While drug repurposing appears promising during emergencies, challenges encompass considerations of prior knowledge of pharmacokinetics/pharmacodynamics, safety profiles, delivery routes, and formulation of repurposed candidates.
In essence, drug resistance and the narrow therapeutic window of kinase inhibitors in antiviral therapy underscore the hurdles in developing kinase inhibitors tailored for antiviral use. Overcoming these challenges demands multidisciplinary collaboration and innovative strategies to enhance the effectiveness of antiviral treatments.
6. Metabolism-related targets
In the realm of antiviral strategies, while many miRNAs and kinases primarily function as upstream regulatory factors, downstream effectors also hold promise as antiviral targets due to their pivotal roles in cellular antiviral responses. Targeting these effectors, however, may yield a less significant impact compared to disrupting an upstream regulator. In addition to effectors, proteases, complexes, or pathways involved in the host's fundamental metabolism are sometimes viewed as potential targets in viral infections. These elements play critical roles in providing energy and essential materials for host cells, making them attractive targets for antiviral interventions.
6.1. Angiotensin-converting enzyme 2 (ACE2)
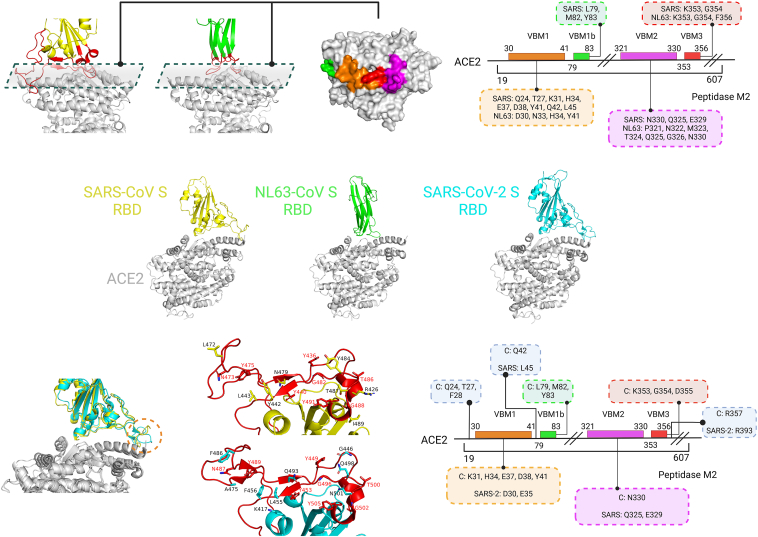
Proteases play a significant role in viral infections, with ACE2 serving as a prominent example in the context of SARS-CoV and SARS-CoV-2 during viral entry and fusion processes. In normal physiological conditions, ACE2 functions by catalyzing the conversion of angiotensin I to angiotensin 1–9 and angiotensin II to angiotensin 1–7, acting as a negative regulator within the renin-angiotensin system. In 2003, ACE2 was identified as a receptor for the SARS coronavirus187, and subsequently, the complex structure of ACE2 with the SARS-CoV spike (S) receptor-binding domain (RBD) was elucidated (Fig. 9)188. Its structure was later resolved with NL63-CoV S RBD and SARS-CoV-2 S RBD as well189,190. ACE2 binds to the “up” conformation of one or multiple RBDs, triggering conformational changes in the S2 subunits. This process leads to the formation of a six-helix bundle (6-HB), creating a conducive environment for viral fusion and entry191,192. Virus-binding motifs (VBMs) (Fig. 9) within ACE2 have been proposed to explain how NL63-CoV S and SARS-CoV S can bind to the same receptor despite lacking structural homology in their RBD cores or receptor-binding motifs (RBMs)189. The binding mode between ACE2 and the RBD from SARS-CoV-2 S closely resembles that of SARS-CoV S, with minor differences at the distal end (Fig. 9)190. By examining the contacting residues from ACE2, many of them can be categorized as VBMs, offering a potential strategy for developing broad-spectrum vaccines or blocking agents that target these critical interaction points. This understanding of the interaction between ACE2 and viral spike proteins provides insights that could aid in the development of therapeutics with broader efficacy against related coronaviruses.
Figure 9.
The binding modes between ACE2 and the RBDs from the S proteins of SARS-CoV, NL63-CoV, and SARS-CoV-2. The upper left panel illustrates the binding mode between ACE2 and the S RBD from SARS-CoV or NL63-CoV, with VBMs distinguished by different colors. The upper right section displays the location of residues in ACE2's VBMs, color-coded to match the left side, and highlights contacting residues within a labeled dotted box (mirrored in the lower right diagram). The lower left section depicts the interaction between ACE2 and the S RBD from SARS-CoV or SARS-CoV-2, featuring RBMs in red, contacting residues represented as sticks, and equivalent residues marked in red. The letter ‘C’ signifies the shared contacting residues between SARS-CoV and SARS-CoV-2 during their ACE2 interaction. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
6.2. Furin, TMPRSS2, and cathepsin W
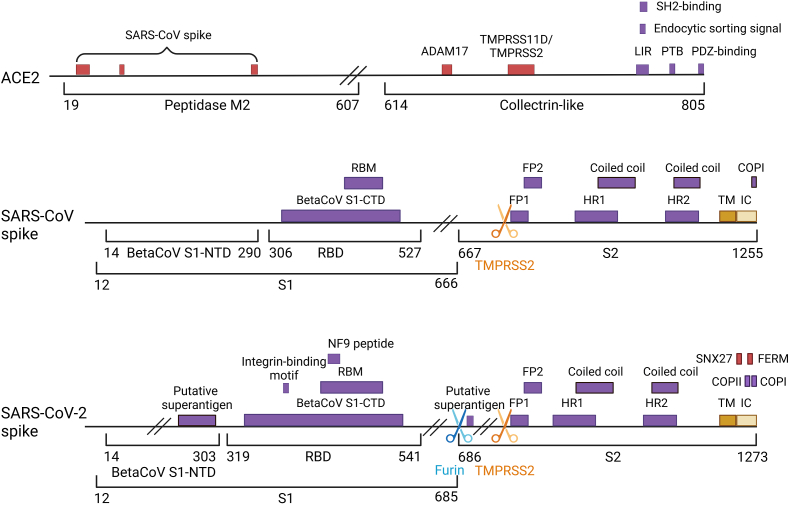
In addition to ACE2, two ubiquitous endoproteases, furin and TMPRSS2, play crucial roles in the activation of SARS-CoV-2 S and viral entry. Furin recognizes the multibasic motif (R-X-R/K-R↓), cleaving the S protein into non-covalently bound subunits (S1 and S2), while TMPRSS2 targets the monobasic cleavage site (R/K↓) within S2, exposing the fusion peptide and facilitating the formation of the 6-HB structure (Fig. 10)193,194. Notably, the furin-mediated cleavage process is present in SARS-CoV-2 S but absent in the related SARS-CoV, potentially imparting greater flexibility to SARS-CoV-2 S and enhancing its infectivity upon ACE2 binding195. Conversely, the recognition site of TMPRSS2 is more conserved across coronaviruses, underscoring its critical role in viral fusion and entry. Furthermore, TMPRSS2 enhances SARS-CoV entry by processing ACE2 at residues 697 to 716 (Fig. 10), independently of S protein activation196.
Figure 10.
The domain arrangement of ACE2 and the S glycoprotein from SARS-CoV and SARS-CoV-2. Domains are depicted as purple boxes, while the transmembrane (TM) and intracellular (IC) regions are shown as dark and light yellow boxes, respectively. Interacting domains are illustrated as red boxes, with corresponding interacting proteins labeled above. The recognition sites of the two proteases, furin and TMPRSS2, are denoted by blue and orange arrow symbols, respectively. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
Beyond SARS-CoV-2, furin and TMPRSS2 are implicated in the pathogenesis of various viruses. Furin facilitates the entry and infectivity of viruses such as HIV-1, ZIKV, measles, and IAV197, while TMPRSS2 is involved in the entry of human parainfluenza viruses (HPIVs) and SeV198. Additionally, cathepsin W, a lysosomal cysteine protease, is involved in the release of IAV from late endosomes, although the specific substrate and mechanism of action remain to be fully elucidated199,200.
6.2.1. Representative TMPRSS2 inhibitors
Camostat mesylate (Table S1) demonstrates promising antiviral activity as a TMPRSS2 inhibitor. In vitro studies have revealed its potent antiviral effects against both influenza virus type A and type B. Notably, treatment with camostat mesylate significantly reduced SARS-CoV entry into Calu-3 cells tenfold201, indicating its potential as an antiviral agent for combating SARS-CoV-2 infection. Several clinical trials have been approved to assess its efficacy and safety in human subjects (ClinicalTrials.gov Identifier: NCT04730206, NCT04657497, NCT04355052, NCT04608266, and NCT04455815, among others).
Nafamostat mesylate (Table S1), structurally akin to camostat mesylate, has shown inhibitory effects on the entry of SARS-CoV, MERS-CoV, and SARS-CoV-2 S proteins into host cells202. Moreover, it has demonstrated continued efficacy against SARS-CoV-2 variants, including lineages B.1.1.7 and B.1.351203. Multiple clinical trials have been registered to evaluate the efficacy of nafamostat mesylate in treating COVID-19 (ClinicalTrials.gov Identifier: NCT04390594, NCT04418128, NCT04352400, NCT04628143, and NCT04623021).
Bromhexine (Table S1), an inhibitor of TMPRSS2, exhibits favorable effects in treating cytopathies induced by SARS-CoV-2 infection204. Furthermore, numerous clinical trials have been sanctioned to assess its efficacy and safety in COVID-19 patients (ClinicalTrials.gov Identifier: NCT04355026, NCT04273763, NCT04405999, NCT04424134, and NCT04340349).
Collectively, these results underscore the promising potential of targeting TMPRSS2 for drug development aimed at combating COVID-19.
6.3. Lipid metabolism
Lipids, a vital group of biomolecules, play diverse essential roles within cells, serving as integral membrane components, energy reservoirs, and mediators of signal transduction. It has been elucidated that lipids and lipid metabolism are intricately involved in nearly every stage of viral infection. During early infection, lipids facilitate the endocytosis and fusion processes of viruses, sometimes acting as cellular receptors for viral entry. After virus entry and the initiation of replication, viruses may induce alterations in membrane structure to compartmentalize processes, aiding in the establishment of viral replication complexes and conferring resistance against cellular proteolysis. At this stage, viruses may also manipulate cellular lipid metabolism, potentially for energy provision, storage of viral components, and intracellular transport. In the later stages of infection, newly synthesized lipids can serve as structural frameworks for viral assembly, directly incorporating into virions, facilitating virus release from host cells, and influencing the pathogenicity of viral progeny. Detailed reviews on this subject can be found in references205, 206, 207.
Numerous factors associated with lipid metabolism have emerged as potential targets for antiviral interventions, including acyl-coenzyme A: cholesterol acyltransferase (ACAT), fatty acid synthase (FASN), oxysterol-binding protein (OSBP), the cholesterol uptake receptor NPC1L1, and diacylglycerol O-acyltransferase 1 (DGAT1)208, 209, 210, 211, 212.
DGAT1 plays a pivotal role in the production of infectious particles of HCV, facilitating the interaction between two viral proteins, NS5A and capsid protein core213,214. Additionally, DGAT1 contributes to the development of steatosis as a complication of HCV infection215. Inhibition or depletion of DGAT1 has been shown to impede HCV infection and safeguard mice from core-induced steatosis. Notably, pradigastat (Table S1), a specific DGAT1 inhibitor with potential anti-HCV properties in vitro, exhibited no significant effect in patients during a randomized clinical trial216. This underscores the need for further investigation into DGAT1 inhibitors as potential anti-HCV agents.
Identifying a critical intersection that is essential for viral replication but dispensable and redundant for host lipid metabolism is a fundamental step in developing host-directed broad-spectrum antiviral targets. This strategy necessitates a comprehensive understanding of the intricate interplay between viral infection and host lipid metabolism, alongside careful consideration of potential side effects and toxicity concerns.
6.4. Purine and pyrimidine biosynthesis pathways
The purine and pyrimidine biosynthesis pathways represent potential antiviral targets due to their role in providing essential materials for the extensive DNA or RNA synthesis necessary for viral replication.
Dihydrofolate reductase (DHFR) is a crucial enzyme in folate metabolism and DNA precursor synthesis217,218. Various reports have highlighted DHFR inhibitors with antiviral properties against a range of viruses, including HIV, ZIKV, influenza virus, and RSV219, 220, 221, 222.
Inosine-5′-monophosphate dehydrogenase (IMPDH) is a key enzyme in the de novo biosynthesis of guanine nucleotides. Inhibiting IMPDH disrupts gene synthesis for DNA and RNA viruses, thereby impeding viral replication. IMPDH inhibitors, such as mycophenolic acid, its prodrug mycophenolate mofetil, and mizoribine (Table S1), exhibit broad-spectrum antiviral activity against both DNA and RNA viruses in vitro223. For instance, mycophenolic acid, a non-competitive IMPDH inhibitor, demonstrates antiviral efficacy closely linked to intracellular GTP levels, suggesting that IMPDH inhibition plays a crucial role in its antiviral mechanism224,225.
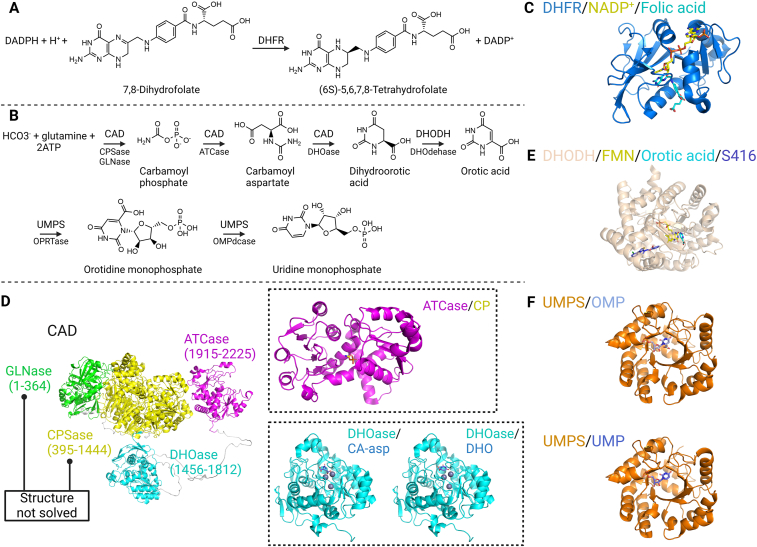
Alternatively, the pyrimidine biosynthesis pathway has emerged as an antiviral target in various high-throughput phenotype screens, focusing on three proteases: the multifunctional protein CAD, dihydroorotate dehydrogenase (DHODH), and the bifunctional uridine monophosphate synthetase (UMPS) (Fig. 11). Corresponding inhibitors have shown effectiveness against diverse viruses, including RSV, hepatitis D virus (HDV), and Ebola virus (EBOV)226, 227, 228. While these proteases and pathways are traditionally considered targets for anti-tumor therapies, they have regained significance in the development of host-directed antivirals229.
Figure 11.
Host-directed targets in the purine and pyrimidine biosynthesis pathways. Panels (A) and (B) display the catalytic activities of four proteases (DHFR, CAD, DHODH, UMPS), with specific activities of multifunctional proteases indicated below the arrows. Panels (C–F) showcase complex structures of these proteases with their substrates, products, or inhibitors. Specifically, the structure of DHFR is detailed by PDB 4M6K with NADP+ and folic acid. The full-length CAD structure is depicted by the AlphaFold-predicted model, colored by domain, with specific domains detailed using PDB models. Notably, the GLNase domain and CPSase domain are yet to be resolved. The ATCase domain is illustrated by PDB 5G1P (CP denotes the substrate carbamoyl phosphate), while the DHOase domain is depicted by PDB 4C6I (CA-asp and DHO represent the substrate carbamoyl aspartate and the product dihydroorotic acid, respectively). The structure of DHODH is elaborated by PDB 6M2B, featuring the product orotic acid, the cofactor flavin mononucleotide (FMN), and the inhibitor S416. Lastly, the structure of UMPS is detailed by PDB 7AM9 with its substrate OMP and by PDB 7ASQ with the product UMP. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
Due to its pivotal role in the pyrimidine biosynthesis pathway, DHODH is currently a favored host target for antiviral drug development230. GSK983 (Table S1) hinders viral replication and impedes the proliferation of rapidly dividing cells by targeting DHODH. Through phenotypic screening, a potent new small molecule (RYL-634, Table S1) has been identified as an effective DHODH inhibitor, displaying remarkable potency against HIV, DENV, EV71, and ZIKV. An analog of RYL-634, RYL-687 (Table S1), has also demonstrated efficacy against Ebola virus infection in vitro231. A recent study by Cordsmeier et al.232 assessed DHODH inhibitors (Table S1) against monkeypox virus (MPXV) (sourced from diagnostic samples), vaccinia virus (VACV), and cowpox virus (CPXV). The inhibitors demonstrated activity against all three viruses, leading the authors to speculate that they might exhibit inhibitory effects against orthopoxviruses in general. DHODH inhibitors offer significant advantages when used in conjunction with DAAs. The combined use of DHODH inhibitors and DAAs results in cumulative inhibition over the disease course compared to using a single drug alone. Combining DHODH inhibitors with nucleoside analogue DAAs enhances the incorporation efficiency of nucleoside analogues.
Beyond the purine and pyrimidine biosynthesis pathways, various nucleotide metabolism-related proteins or complexes have been implicated in viral replication and transport. These include Mx GTPases, RNA helicase A, ATPase/RNA helicase X-linked DEAD-box polypeptide 3 (DDX3), guanylate-binding protein 1 (GBP1), Ras-related protein Rab-5A, and the SKI complex233, 234, 235, 236, 237, 238. These proteins or complexes modulate diverse cellular processes, exhibiting either proviral or antiviral activities by interacting with the viral genome or associating with viral structural or non-structural proteins. They influence the stability of the replication complex, disrupt virus fusion, trafficking, and egress from cells, or regulate innate immunity.
DDX3 is a cytokine crucial for DNA and RNA viral replication, making it a valuable host target for broad-spectrum antiviral drug development. Several DDX3 inhibitors targeting ATP-binding sites have been developed, showing promising anti-HIV activity in vitro. However, inhibitors that target ATP-binding sites may also inhibit other kinases with similar sites. To circumvent this issue, inhibitors have been designed to target the RNA binding sites of DDX3. The first DDX3 inhibitors targeting the RNA binding site, such as DDX3 inhibitors 1 and 2 (Table S1), can impede the helicase and ATPase functions of DDX3. DDX3 stands out as a promising target for broad-spectrum antiviral drug development, with DDX3 inhibitor 3 (Table S1) serving as a promising starting point for further detailed research239, 240, 241, 242.
6.5. Others
Additional metabolism-related targets, such as GAPDH in glycolysis, sirtuins involved in epigenetic modifications regulation, and cyclophilin A (CypA) in cellular responses to oxidative stress, have emerged as potential antiviral targets following phenotypic screening and subsequent inhibition assays243, 244, 245. Their implication in antiviral mechanisms is unsurprising, given their conserved roles in host biological processes.
For example, CypA plays a crucial role in HCV replication. Cyclosporin A (CsA, Table S1) exhibits a strong binding affinity with CypA and is an approved immunosuppressive drug. The antiviral response observed in the combination group of CsA and IFN-α2b proved significantly more effective. CypA and NIM811 (Table S1), an analogue with a methyl-isoleucine at position 4 of CsA, both demonstrated anti-HCV activity in vitro246,247.
Overall, targeting downstream effectors and cellular metabolic pathways presents a robust antiviral strategy with potentially broad applicability. This strategy should be implemented with careful consideration of potential side effects.
7. The ubiquitin–proteasome system and ubiquitylation
Ubiquitination, an indispensable posttranslational modification for cellular proteins, involves attaching the 76-amino acid protein ubiquitin (Ub) to specific proteins. This process is orchestrated by three crucial enzymes: ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3), and antagonized by ubiquitin hydrolases/deubiquitinating enzymes (DUBs). Ubiquitin can be conjugated to targeted proteins at seven internal lysine residues (K6, K11, K27, K29, K33, K48, K63) or the amino-terminal methionine, resulting in diverse ubiquitination modifications. These modifications can lead to the degradation of modified targets by the proteasome or facilitate various cellular processes (Fig. 12)248. The ubiquitin–proteasome system plays a pivotal role in upholding protein homeostasis, serving as a cellular defense mechanism against pathogens and diseases like neurodegenerative disorders, cancer, and viral infections, where the accumulation of misfolded and aggregated proteins is a common hallmark of their pathogenesis249. Similar to other conserved regulatory or metabolic pathways, the ubiquitin–proteasome pathway can function as a cellular antiviral mechanism by breaking down viral components. Nevertheless, viruses have developed strategies to evade and exploit this pathway, diminishing antiviral responses as a tactic in the enduring co-evolutionary struggle between viruses and their hosts250,251.
Figure 12.
The scheme of ubiquitin–proteasome system. Ubiquitination relies on three pivotal enzymes: ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3). Ubiquitin (Ub) can attach to targeted proteins via internal lysines (K6, K11, K27, K29, K33, K48, K63) or the amino-terminal methionine, resulting in a spectrum of ubiquitination modifications. This process can be counteracted by ubiquitin hydrolases/deubiquitinating enzymes (DUBs). These modifications may trigger the degradation of modified targets by the proteasome or facilitate diverse cellular processes. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
7.1. Tripartite motif (TRIM) family
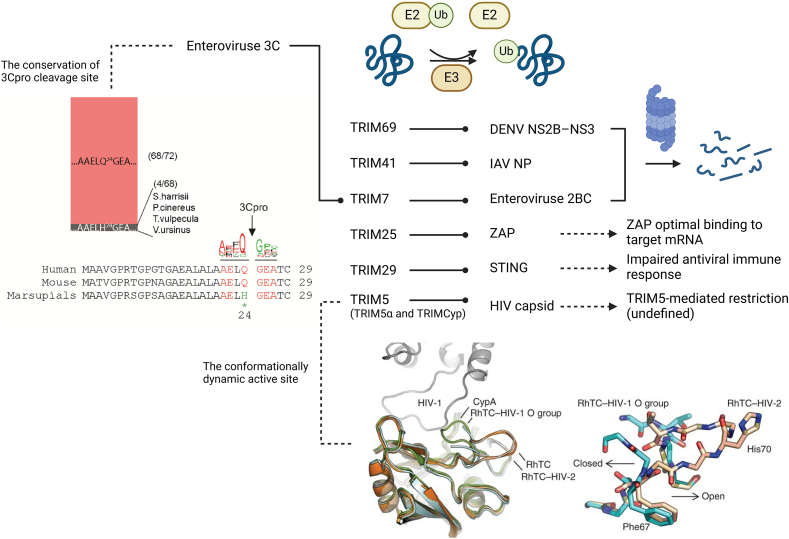
Within the extensive array of over 600 E3 ubiquitin ligases, the TRIM family stands out for its significant involvement in viral infections (Fig. 13)251. TRIM proteins can exert antiviral effects by directly marking viral proteins for degradation. For instance, TRIM69 targets the DENV NS2B-NS3 protease complex, TRIM41 targets the IAV NP, and TRIM7 targets the enterovirus 2BC protein252, 253, 254. Moreover, TRIM proteins can influence the interaction of other restriction factors with viral components. For example, TRIM25 facilitates K63-linked polyubiquitination of ZAP, enhancing its binding to target mRNA255. On the other hand, TRIM proteins may also exhibit proviral effects by dampening immune responses. For instance, TRIM29 mediates K48 ubiquitination of STING, thereby compromising the innate immune response triggered by DNA viruses and cytosolic DNA, particularly affecting the STING–TBK1–IRF3 signaling pathway256. A continuous co-evolutionary interplay is evident between TRIM proteins and viruses. TRIM5 has developed broad-spectrum antiviral activity with a flexible active site, prone to conformational changes when targeting the HIV capsid257. In response, virus-encoded proteases have evolved to counteract the TRIM-mediated degradation of crucial proteins. For example, while TRIM7 targets the enterovirus 2BC protein for degradation, it is concurrently cleaved by the enterovirus 3C protease, with the cleavage site being conserved across mammals (except in marsupials)254. The involvement of TRIM proteins in antiviral responses underscores their potential as targets for antiviral interventions.
Figure 13.
The intensive involvement of TRIMs in viral infection and the co-evolution between TRIMs and viruses. TRIM proteins play a role in targeting viral proteins for proteasomal degradation, coordinating with restriction factors, and modulating the antiviral immune response through ubiquitination. Specifically, Enterovirus 3C acts to counteract the restriction imposed by TRIM7, with its cleavage site being conserved among mammals (except in marsupials)254. Additionally, Rhesus macaque TRIMCyp (RhTC) has developed a conformationally dynamic binding site (loop 66–74) for the HIV capsid, leading to the acquisition of broad-spectrum antiviral activity257. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
7.2. APOBEC3G-Vif-E3 ubiquitin ligase complex
The APOBEC3G-Vif-E3 ubiquitin ligase complex represents a significant antiviral target. APOBEC3G, an innate host antiviral protein initially known as CEM15, exhibits cytidine deaminase activity, leading to detrimental hypermutations in retroviral DNA (Fig. 14A). Notably, APOBEC3G can manifest its antiviral function independently of its enzymatic activity258. Moreover, APOBEC3G disrupts the interaction between the Moloney leukemia virus 10 (MOV10) protein and AGO2, thereby impeding the normal assembly of the miRNA-inducing silencing complex (miRISC) and inhibiting miRNA-mediated translation repression259. However, HIV and related retroviruses encode the Vif protein, which thwarts the incorporation of APOBEC3G into progeny virions. Vif recruits the ElonginB/C-Cullin5 E3 ubiquitin ligase to target APOBEC3G for proteasomal degradation (Fig. 14A and B)260, 261, 262, 263, 264, 265. Beyond ubiquitin-dependent inhibition, Vif may employ various strategies to target APOBEC3G/APOBEC3, including interference with its synthesis and transcriptional regulation266, 267, 268, 269, although the precise mechanisms remain unclear.
Figure 14.
The APOBEC3G (A3G)-Vif-E3 ubiquitin ligase complex. (A) The mechanisms of action of APOBEC3G and Vif. (B) The structure of the APOBEC3G/RNA complex in association with HIV-1 Vif, CBF-β, and the host Cullin–RING ubiquitin ligase (CRL) complex. The model depicting the APOBEC3G/RNA/Vif/CBF-β/CRL complex is derived from a recent publication278. (C) A comparative analysis of human APOBEC3 isoforms. The structures of human APOBEC3 isoforms are represented using PDB 5KEG, AlphaFold model (UniProt Q9UH17), PDB 3VOW, AlphaFold model (UniProt Q96AK3), AlphaFold model (UniProt Q8IUX4), PDB 8CX0, and PDB 8FVI, respectively. The Root Mean Square Deviation (RMSD) and sequence identity are computed with APOBEC3G as the reference. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
Subsequent research has broadened the antiviral spectrum of APOBEC3G to encompass HBV, foamy viruses (FVs), xenotropic murine leukemia virus-related virus (XMRV), and more270, 271, 272. Investigations into the antiviral activities of APOBEC3G isoforms (such as APOBEC3A, APOBEC3B, APOBEC3C, APOBEC3DE, APOBEC3F, APOBEC3H) (Fig. 14C) and their specific contributions have been thorough273, 274, 275, 276, 277. The structure and mode of action of APOBEC3G have been continuously explored, with recent advancements including the elucidation of the cryo-EM structure of the human APOBEC3G/HIV-1 Vif/CBF-β/ELOB/ELOC complex (Fig. 14B)278.
Given the critical reliance of viral Vif functions on the APOBEC3G/Vif/CBF-β/CRL complex formation, compounds disrupting the interaction between Vif and the E3 ubiquitin ligase or between Vif and APOBEC3G have demonstrated efficacy as antiviral agents279, 280, 281, 282, 283. Additionally, the transcription cofactor core-binding factor beta (CBF-β) plays a pivotal role in Vif-mediated APOBEC3G inhibition, making the CBF-β-Vif interface a promising target for antiviral agent development.
Two compounds, IMB-26 and IMB-35 (Table S1), have been discovered to directly bind to APOBEC3G (A3G) and disrupt its interaction with Vif, thereby rescuing A3G from Vif-mediated degradation284. Both compounds exhibited A3G-dependent anti-HIV-1 activity. IMB-26 also demonstrated potent antiviral effects against HCV in vitro by stabilizing intracellular A3G.
Furthermore, two Vif inhibitors, RN-18 and RN-19 (Table S1), have been identified. These compounds inhibited HIV-1 replication specifically in A3G-positive cells, with IC50 values exceeding 100 μmol/L in A3G-negative cells285. Treatment with compound RN-18 not only elevated the levels of A3G but also resulted in the degradation of Vif.
7.3. Others
Various E3 ubiquitin ligases can be co-opted by viral proteins to enhance virus replication. For instance, the PRV protein UL13 recruits the E3 ligase RNF5 to target STING, evading STING-mediated interferon production286. The non-structural protein 5 (NS5) of ZIKV utilizes the host CRL3–ZSWIM8 complex to degrade STAT2, inhibiting the host's antiviral immune response287. Similarly, the E3 ubiquitin ligase TRAF6 exerts a proviral effect on tick-borne flaviviruses (TBFVs) through its interaction with non-structural protein 3 (NS3), although the exact mechanism remains unclear288.
Apart from mediating protein degradation, E3 ligases can display antiviral activity by disrupting virus assembly. For example, the E3 ligase MARCH8 hinders the integration of VSV and HIV glycoproteins into virions, reducing their infectivity289.
Beyond the proteasome pathway, ubiquitination regulates various cellular processes like autophagy and signal transduction. The aforementioned E3 ligase MARCH8 participates in PABPC4-mediated ubiquitin modification of viral N proteins, leading to autolysosome degradation in eight coronaviruses290. Ubiquitination of NF-κB essential modulator (NEMO) by the linear ubiquitin chain assembly complex (LUBAC) activates downstream NF-κB signaling and innate immune responses against viruses. However, viral proteins can disrupt this process to thwart host antiviral activities by targeting LUBAC or NEMO ubiquitination. For instance, the NS3 protein of HCV interacts with LUBAC, competitively impeding LUBAC's binding to NEMO, inhibiting NEMO's linear ubiquitination and NF-κB activation, aiding HCV's immune evasion291.
Sumoylation, a post-translational modification closely linked to ubiquitination, regulates diverse cellular functions, including antiviral responses292, 293, 294. Viral proteins can exploit sumoylation to evade host defenses. For example, the human adenovirus protein E1B-55K enhances the interaction between the SUMO-targeted ubiquitin ligase (STUbL) RNF4 and the antiviral factor Daxx, counteracting Daxx's antiviral effect by promoting its proteasomal degradation295.
The ubiquitin–proteasome system presents a promising target for developing host-directed antiviral agents. Exploiting viruses’ reliance on this pathway and its role in protein homeostasis may lead to novel broad-spectrum antiviral strategies, such as proteolysis-targeting chimera (PROTAC) technology296. This approach not only addresses drug resistance but also targets traditionally undruggable proteins. Additionally, ubiquitin-specific proteases (USPs) modulate type I interferon production, with antiviral USPs (USP2b, USP3, USP18, USP25, UL36USP, and HAUSP) and proviral USPs (USP4, USP13, USP15, and USP17) playing crucial roles in antiviral immunity297. Understanding how viruses manipulate the ubiquitin–proteasome system is essential for designing targeted antiviral interventions.
8. Other host-directed targets
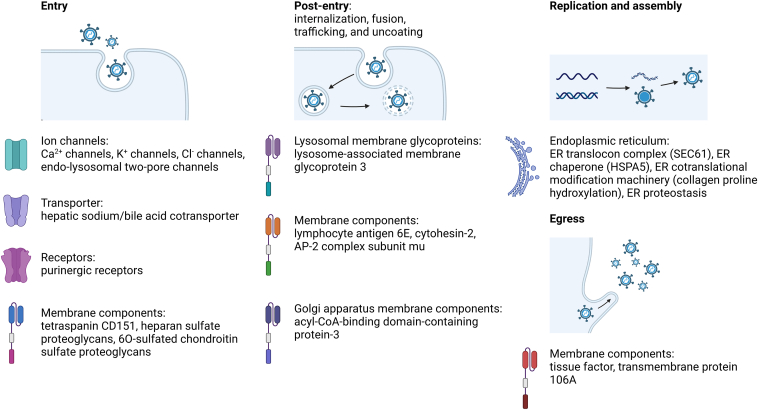
Successful viral infection involves a series of steps, including entry into host cells, release of the viral genome from endosomes, and intracellular trafficking for replication. During these processes, various cellular factors are exploited for viral purposes (Fig. 15). These factors include ion channels, transporters, receptors, and membrane components that facilitate virus entry, such as Ca2+ channels, K+ channels, Cl− channels, endo-lysosomal two-pore channels (TPCs), hepatic sodium/bile acid cotransporter (NTCP), purinergic receptors, and the tetraspanin CD151298, 299, 300, 301, 302, 303. Cell-based assays have identified HBV entry inhibitors that target NTCP, including propranolol, progesterone, vanitaracin A, proscillaridin A, NTI-007, and fasiglifam (Table S1)304, 305, 306, 307, 308.
Figure 15.
Other host-directed antiviral targets from this review and their roles in the process of viral infection. (Created in BioRender. Gu, X. (2025) https://BioRender.com/z08y439).
Certain membrane components, such as heparan sulfate proteoglycans (HSPGs) and 6-O-sulfated chondroitin sulfate proteoglycans (CSPGs), can serve as non-selective attachment sites for virus entry. These proteoglycans play roles in various viral infections by facilitating entry through electrostatic interactions between the negatively charged heparan sulfate moieties of HSPGs and the positively charged amino acids on virus surfaces309, 310, 311. As a result, interventions that target HSPGs or competitively bind to virion particles have demonstrated antiviral effects312,313. Targeting HSPGs could potentially offer a broad-spectrum antiviral approach due to the non-specific nature of the interaction between HSPGs and viruses. However, this approach may be associated with challenges such as low performance and systemic toxicity. Similarly, certain antiviral chemicals like salicylanilide niclosamide act through electrostatic interactions, independent of a direct target. This compound blocks virus entry by acidifying the micro-environment314. While this method represents a novel approach to antiviral defense, it is limited by the issues related to non-specific interactions.
Viruses exploit various proteins that facilitate post-entry processes such as internalization, fusion, trafficking, and uncoating. These proteins include membrane components from lysosomes (lysosome-associated membrane glycoprotein 3 (LAMP3)), cell surfaces (lymphocyte antigen 6E (LY6E), cytohesin-2 (CYTH2), and AP-2 complex subunit mu (AP2M1)), and the Golgi apparatus (acyl-CoA-binding domain-containing protein-3 (ACBD3))315, 316, 317, 318, 319.
The endoplasmic reticulum (ER) plays a critical role in maintaining intracellular homeostasis and is a prime target during viral infections due to the substantial production of viral proteins. Viruses often exploit the ER to aid in their replication and assembly processes. Chemical or genetic interventions target various aspects of ER functions. These interventions can affect the ER translocon complex (SEC61), ER chaperone (HSPA5), ER cotranslational modification machinery (collagen proline hydroxylation), and ER proteostasis320, 321, 322, 323. Targeting ER functions has proven to be an effective strategy for antiviral therapy, highlighting the significance of the ER in viral replication.
During virus egress from host cells, certain membrane constituents can be integrated into virion particles. These components can either enhance viral infectivity, such as tissue factor (TF), or conversely, trap virions on the cell surface, like transmembrane protein 106A (TMEM106A), potentially restricting their spread324,325.
Specific cellular architectures or their associated proteins and components of the cytoskeleton play crucial roles in host-virus interactions. Structures like filopodia, tight junction (TJ) proteins, and the protein tubulin provide physical support for virus attachment, intracellular trafficking, and virion budding326, 327, 328. In some instances, these structures and proteins can even act as cellular receptors for virus entry, as seen with TJ proteins like claudin-1 and occludin in the context of HCV infection327.
While targeting these factors shows promise for developing antiviral agents, their highly conserved biological functions in host cells present challenges. One potential approach is using prodrugs that are selectively activated in virus-infected cells, such as combretastatin peptide hybrids326. This strategy allows for targeted drug delivery, reducing off-target effects. However, when utilizing prodrugs, factors like hydrolytic stability, permeability, and metabolic clearance rate must be carefully considered, as they can impact the efficacy and safety of these compounds. Thoughtful design and optimization of prodrugs are essential to ensure their stability, efficient activation in target cells, and appropriate clearance from the body to minimize potential side effects.
9. Chemistry strategies that can be employed to reduce the toxicity of drugs targeting these host factors
Host proteins, being more conserved than viral proteins, exhibit lower susceptibility to mutation, rendering drugs targeting host proteins less prone to resistance—an essential attribute for developing broad-spectrum antivirals, given the significant challenge of resistance faced by current antiviral treatments.
Despite numerous host factors playing pivotal roles in the viral life cycle, only a limited subset has been explored as antiviral targets, primarily due to concerns raised by HDAs. Targeting host proteins can potentially lead to unintended toxicity issues, as these proteins may be vital for specific cellular functions. Design strategies focusing on regions that interact with viral proteins or nucleic acids without disrupting other host protein interactions can enhance the precision of drug design. For instance, employing structure-based drug design using crystal structure information enables the development of drugs that selectively bind to viral proteins, thereby reducing non-specific interactions with host proteins. Analyzing interactions between viral and host proteins can help identify key proteins shared among closely related coronaviruses, aiding in the screening of drug candidates with potent antiviral effects.
To mitigate the toxicity of drugs targeting host proteins, several chemistry strategies can be employed: 1) Covalent inhibitors enhance drug efficacy by forming covalent bonds with the target, potentially reducing the required dosage and frequency of administration, thereby minimizing host toxicity. The nucleophilic nature of proteins, with abundant hydroxyl, sulfhydryl, amino, and other groups, allows them to interact effectively with electrophilic groups present in covalent binding compounds like Michael receptors, halogens, carbonyls, and isocyanogens, leading to altered protein conformation and subsequent inhibition of protein activity329. 2) The development of PROTAC molecules offers a method to selectively degrade host proteins, thereby reducing drug toxicity. Comprising target protein-specific ligands and E3 ubiquitin ligase recruitment ligands connected by linkers, PROTAC molecules facilitate the ubiquitination and subsequent degradation of the target protein330. 3) Addressing the functional deficiency of RNA binding proteins, which underlies various diseases, poses a challenge with conventional drugs. Inspired by targeted protein degradation techniques, a novel approach for the targeted degradation of RNA-binding proteins, known as RNA-PROTAC, has been developed331. Its successful application in anti-tumor research is anticipated to inspire innovative ideas for antiviral drug design. 4) Ribonuclease targeting chimeras (RIBOTACs) represent a novel strategy for degrading RNA by selectively binding to RNAs, particularly those forming complex secondary and tertiary structures, and subsequently activating the ribonuclease L (RNase L). 5) Utilizing synthetic carbohydrate receptors (SCRs) to disrupt the interaction between viruses and host cell membrane proteins offers a method to reduce viral infectivity, thereby inhibiting the virus life cycle. SCRs, potent inhibitors of highly glycosylated envelope viruses, exhibit a dual mode of action by impeding viral invasion and aiding in viral clearance through interference with sugar molecules in the viral envelope. 6) The combination of HDAs and DAAs results in additive inhibition throughout the disease progression compared to individual agents. In the realm of pharmaceutical chemistry, the design of dual-target inhibitors or degraders can effectively reduce drug side effects. These strategies employ diverse mechanisms to decrease drug toxicity to the host while maintaining robust viral inhibition, offering a range of pathways for the development of broad-spectrum antiviral drugs.
10. The role of artificial intelligence in identifying and validating host proteins as antiviral targets
Artificial intelligence (AI) demonstrates significant promise in identifying and validating host proteins as targets for antiviral therapies, playing a crucial role in drug repurposing and the development of innovative antiviral treatments. AI technology excels in analyzing vast datasets to unveil hidden connections among existing drugs, disease targets, and potential therapies. This capability expedites the identification of drug targets, particularly during sudden infectious disease outbreaks, and empowers research into diseases with intricate mechanisms like cancer, neurodegenerative disorders, and autoimmune conditions. AI also proved invaluable in drug repurposing by uncovering new indications for existing drugs through the analysis of novel associations between drugs and diseases. This approach leverages dosage and safety data of known drugs, expediting clinical trials and substantially reducing development timelines and costs. AI accelerates drug repurposing through various strategies such as virtual screening, target identification, structure-based drug design, and natural language processing. Machine learning (ML) algorithms facilitate swift drug development, including the repurposing of existing drugs, to identify new or approved antiviral medications capable of inhibiting pathogens like SARS-CoV-2. The application of AI in structural biology, drug repurposing, and development, particularly in the context of COVID-19 research, offers a comprehensive view of AI's applications and inspires researchers to harness its potential in combating the pandemic. AI and ML-based methodologies for drug repurposing encompass network algorithms applied to knowledge maps containing relationships among diverse medical entities (e.g., diseases, drugs, proteins) to pinpoint pertinent host protein targets or regions within host interaction networks that can be targeted. AI also contributes to the structural elucidation of SARS-CoV-2 proteins, a critical step in drug discovery. By predicting the structures of infectious proteins, AI identifies potential drugs that could effectively target these proteins, proposing new chemical compounds for further evaluation as potential therapeutics. Moreover, AI employs quantitative structure-activity relationship (QSAR) modeling to establish quantitative mathematical models correlating chemical structure with biological activity, repurposing them for other disease conditions. The utilization of AI in determining drug dosages, assessing administration effects, predicting bioactive substances, and monitoring drug release aids in identifying bioactive compounds tailored to specific disease-associated targets332.
11. Discussion
For viral infections, direct-acting agents remain pivotal in treatment, with HDAs serving as a valuable complementary strategy rather than a sole alternative. As an adjunct approach, HDAs can help control viral replication, mitigate resistance development, and offer symptomatic relief by modulating dysregulated host responses, particularly in cases of hyper-inflammation.
In theory, any host factor playing a specific role in physiological conditions could be a potential target for HDAs, given viruses’ reliance on cellular machinery for proliferation. However, due to technological constraints and the intricacies of the host genome, many of these factors remain undiscovered, representing a key objective in future antiviral research. Recent advancements have unveiled numerous host factors involved in viral infection through genomic, transcriptomic, and proteomic analyses, alongside high-throughput screening and chemical or genetic interventions. It is crucial to discern whether observed changes are causal or consequential and whether they stem from virus propagation, host defense mechanisms, or limitations in screening methods, a consideration that should always be borne in mind and may explain many undisclosed or misidentified targets333. Moreover, the credibility of genomic, transcriptomic, and proteomic analyses would be strengthened by independent research findings, clinical reports, and consistent, thorough investigations. Despite certain cellular factors initially deemed undruggable, such as transcription factors, kinases, and miRNAs, technological and scientific progress has overturned this perception, emphasizing the importance of recognizing both current and future “undruggable” targets.
Innovative solutions beyond traditional targeted therapies have emerged, including the synthetic lethality strategy, PROTACs, lysosome-targeting chimeras (LYTACs), alternative regulation of upstream or downstream effectors, topology-matching design, genome modification technology, and the development of supportive databases and computational methods334, 335, 336, 337, 338, 339, 340, 341, 342, 343, 344. These advancements promise a wider array of options for combating diseases and broadening the spectrum of antiviral targets.
The intricate and dynamic relationship between viruses and their hosts is shaped by the selective pressures and defensive mechanisms of each entity. Viruses have evolved diverse strategies to evade host immunity, such as encoding homologous proteins and RNAs that counteract or mimic host functions and exploiting cellular factors for viral propagation. Notably, under prolonged inhibition, resistant viruses may switch to different isoforms or entirely bypass the initially dependent pathway185,345. This dynamic host-virus interplay appears to be a pervasive phenomenon, potentially reflecting a delicate balance between host and parasites. The adaptability of viruses to exploit alternative pathways underscores the challenges in developing effective antiviral therapies and the significance of comprehending the intricate interplay between viruses and hosts.
Author contributions
Xiaoxia Gu and Weiguang Sun conceived the review topic. Xiaoxia Gu drafted and edited the manuscript. Mengzhu Zheng, Ya Gao, Shuang Lin, Xiaotian Zhang, Chunmei Chen, Hucheng Zhu, Weiguang Sun, and Yonghui Zhang revised the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by the National Program for Support of Top-notch Young Professionals (Grant No. 0106514050 to Yonghui Zhang, China), the National Natural Science Foundation of China (Grant Nos. 82273811 and U22A20380 to Yonghui Zhang), the National Key Research and Development Program of China (Grant No. 2021YFA0910500 to Yonghui Zhang), and TongjiRongcheng Center for Biomedicine, Huazhong University of Science and Technology. The figures were created using BioRender (https://biorender.com/) and are licensed under a BioRender Academic Publication License. We extend our sincere gratitude to the anonymous reviewers for their invaluable comments, which have significantly enhanced this paper.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2025.03.011.
Contributor Information
Hucheng Zhu, Email: zhuhucheng@hust.edu.cn.
Weiguang Sun, Email: weiguang_sun@hust.edu.cn.
Yonghui Zhang, Email: zhangyh@mails.tjmu.edu.cn.
Appendix A. Supporting information
The following is the Supporting Information to this article:
References
- 1.Breedlove B. Hunters searching among starry nights and at the edges of life. Emerg Infect Dis. 2020;26:187–188. [Google Scholar]
- 2.Morens D.M., Fauci A.S. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020;182:1077–1092. doi: 10.1016/j.cell.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C., Hu L., Dong G., Zhang Y., Ferreira da Silva-Júnior E., Liu X., et al. Emerging drug design strategies in anti-influenza drug discovery. Acta Pharm Sin B. 2023;13:4715–4732. doi: 10.1016/j.apsb.2023.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou Y., Chen M., Bian Y., Zheng X., Tong R., Sun X. Advanced subunit vaccine delivery technologies: from vaccine cascade obstacles to design strategies. Acta Pharm Sin B. 2023;13:3321–3338. doi: 10.1016/j.apsb.2023.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu B., Gao Y., An X., Wang H., Huang Y. Localized delivery of nanomedicine and antibodies for combating COVID-19. Acta Pharm Sin B. 2023;13:1828–1846. doi: 10.1016/j.apsb.2022.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng C., Li Y., Ferdows B.E., Patel D.N., Ouyang J., Tang Z., et al. Emerging vaccine nanotechnology: from defense against infection to sniping cancer. Acta Pharm Sin B. 2022;12:2206–2223. doi: 10.1016/j.apsb.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Lancet Microbe The Lancet Microbe at 2: what is past is prologue. Lancet Microbe. 2022;3 doi: 10.1016/S2666-5247(22)00095-7. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Hu Y., Zheng M. Enterovirus A71 antivirals: past, present, and future. Acta Pharm Sin B. 2022;12:1542–1566. doi: 10.1016/j.apsb.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar N., Sharma S., Kumar R., Tripathi B.N., Barua S., Hinh L., et al. Host-directed antiviral therapy. Clin Microbiol Rev. 2020;33 doi: 10.1128/CMR.00168-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann S.H.E., Dorhoi A., Hotchkiss R.S., Bartenschlager R. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov. 2018;17:35–56. doi: 10.1038/nrd.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallis R.S., O'Garra A., Sher A., Wack A. Host-directed immunotherapy of viral and bacterial infections: past, present and future. Nat Rev Immunol. 2023;23:121–133. doi: 10.1038/s41577-022-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai X., Hagedorn C.H., Cullen B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y., Kim M., Han J., Yeom K.H., Lee S., Baek S.H., et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen B.R. Viruses and microRNAs. Nat Genet. 2006;38(Suppl):S25–S30. doi: 10.1038/ng1793. [DOI] [PubMed] [Google Scholar]
- 15.Grassmann R., Jeang K.T. The roles of microRNAs in mammalian virus infection. Biochim Biophys Acta. 2008;1779:706–711. doi: 10.1016/j.bbagrm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skalsky R.L., Cullen B.R. Viruses, microRNAs, and host interactions. Annu Rev Microbiol. 2010;64:123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lytle J.R., Yario T.A., Steitz J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci U S A. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman R.C., Farh K.K., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart B.J., Slack F.J., Basson M., Pasquinelli A.E., Bettinger J.C., Rougvie A.E., et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 21.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 23.Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 24.Lee R.C., Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 25.Kozomara A., Birgaoanu M., Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2018;47:D155–D162. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burki T. 2024 Nobel Prize awarded for work on microRNAs. Lancet. 2024;404:1507–1508. doi: 10.1016/S0140-6736(24)02303-1. [DOI] [PubMed] [Google Scholar]
- 27.McCaskill J.L., Ressel S., Alber A., Redford J., Power U.F., Schwarze J., et al. Broad-spectrum inhibition of respiratory virus infection by microRNA mimics targeting p38 MAPK signaling. Mol Ther Nucleic Acids. 2017;7:256–266. doi: 10.1016/j.omtn.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson K.A., Hsieh W.Y., Forster T., Blanc M., Lu H., Crick P.J., et al. An interferon regulated microRNA provides broad cell-intrinsic antiviral immunity through multihit host-directed targeting of the sterol pathway. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng S., Wang J., Wei S., Li C., Zhou K., Hu J., et al. Endogenous cellular microRNAs mediate antiviral defense against influenza A virus. Mol Ther Nucleic Acids. 2018;10:361–375. doi: 10.1016/j.omtn.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L., Song Y., He L., Wan X., Lai L., Dai F., et al. MicroRNA-223 promotes type I interferon production in antiviral innate immunity by targeting Forkhead box protein O3 (FOXO3) J Biol Chem. 2016;291:14706–14716. doi: 10.1074/jbc.M115.700252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W.C., Wei C.K., Lee J.C. MicroRNA-let-7c suppresses hepatitis C virus replication by targeting Bach1 for induction of haem oxygenase-1 expression. J Viral Hepat. 2019;26:655–665. doi: 10.1111/jvh.13072. [DOI] [PubMed] [Google Scholar]
- 32.Chang Z., Wang Y., Bian L., Liu Q., Long J.E. Enterovirus 71 antagonizes the antiviral activity of host STAT3 and IL-6R with partial dependence on virus-induced miR-124. J Gen Virol. 2017;98:3008–3025. doi: 10.1099/jgv.0.000967. [DOI] [PubMed] [Google Scholar]
- 33.Cheng M., Niu Y., Fan J., Chi X., Liu X., Yang W. Interferon down-regulation of miR-1225-3p as an antiviral mechanism through modulating Grb2-associated binding protein 3 expression. J Biol Chem. 2018;293:5975–5986. doi: 10.1074/jbc.RA117.000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Xie J., Xu X., Wang J., Ao F., Wan Y., et al. MicroRNA-548 down-regulates host antiviral response via direct targeting of IFN-λ1. Protein Cell. 2013;4:130–141. doi: 10.1007/s13238-012-2081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ru J., Sun H., Fan H., Wang C., Li Y., Liu M., et al. miR-23a facilitates the replication of HSV-1 through the suppression of interferon regulatory factor 1. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sodroski C., Lowey B., Hertz L., Jake Liang T., Li Q. MicroRNA-135a modulates hepatitis C virus genome replication through downregulation of host antiviral factors. Virol Sin. 2019;34:197–210. doi: 10.1007/s12250-018-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J., Zhang Y., Su Y., Tian L., Qin P., Xu X., et al. MicroRNA-125a targets MAVS and TRAF6 to modulate interferon signaling and promote HCV infection. Virus Res. 2021;296 doi: 10.1016/j.virusres.2021.198336. [DOI] [PubMed] [Google Scholar]
- 38.Patil R.N., Karpe Y.A. Uncovering the roles of miR-214 in hepatitis E virus replication. J Mol Biol. 2020;432:5322–5342. doi: 10.1016/j.jmb.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Pfeffer S., Zavolan M., Grässer F.A., Chien M., Russo J.J., Ju J., et al. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 40.Sarnow P., Jopling C.L., Norman K.L., Schütz S., Wehner K.A. MicroRNAs: expression, avoidance and subversion by vertebrate viruses. Nat Rev Microbiol. 2006;4:651–659. doi: 10.1038/nrmicro1473. [DOI] [PubMed] [Google Scholar]
- 41.Mishra R., Kumar A., Ingle H., Kumar H. The interplay between viral-derived miRNAs and host immunity during infection. Front Immunol. 2020;10:3079. doi: 10.3389/fimmu.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruscella P., Bottini S., Baudesson C., Pawlotsky J.M., Feray C., Trabucchi M. Viruses and miRNAs: more friends than foes. Front Microbiol. 2017;8:824. doi: 10.3389/fmicb.2017.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuo Y., Gao G., Shi J.A., Zhou X., Wang X. miRNAs: biogenesis, origin and evolution, functions on virus–host interaction. Cell Physiol Biochem. 2013;32:499–510. doi: 10.1159/000354455. [DOI] [PubMed] [Google Scholar]
- 44.Hennig T., Prusty A.B., Kaufer B.B., Whisnant A.W., Lodha M., Enders A., et al. Selective inhibition of miRNA processing by a herpesvirus-encoded miRNA. Nature. 2022;605:539–544. doi: 10.1038/s41586-022-04667-4. [DOI] [PubMed] [Google Scholar]
- 45.van der Ree M.H., van der Meer A.J., de Bruijne J., Maan R., van Vliet A., Welzel T.M., et al. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antivir Res. 2014;111:53–59. doi: 10.1016/j.antiviral.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Anthiya S., Griveau A., Loussouarn C., Baril P., Garnett M., Issartel J.P., et al. MicroRNA-based drugs for brain tumors. Trends Cancer. 2018;4:222–238. doi: 10.1016/j.trecan.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Abdelkhalek Z.S., Abdalla M.S., Fathy M.M., Elbaz T.M., Abdelaziz A.O., Nabeel M.M., et al. Role of circulating microRNA-21 and microRNA-215 in the diagnosis of hepatitis C related hepatocellular carcinoma. J Infect Dev Ctries. 2021;15:997–1003. doi: 10.3855/jidc.12230. [DOI] [PubMed] [Google Scholar]
- 48.Elfert A.Y., Salem A., Abdelhamid A.M., Salama A., Sourour D.A., Shaker O., et al. Implication of miR-122, miR-483, and miR-335 expression levels as potential signatures in HCV-related hepatocellular carcinoma (HCC) in Egyptian patients. Front Mol Biosci. 2022;9 doi: 10.3389/fmolb.2022.864839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honegger A., Schilling D., Sueltmann H., Hoppe-Seyler K., Hoppe-Seyler F. Identification of E6/E7-dependent microRNAs in HPV-positive cancer cells. Methods Mol Biol. 2018;1699:119–134. doi: 10.1007/978-1-4939-7435-1_10. [DOI] [PubMed] [Google Scholar]
- 50.Martelli F., Mencarini J., Rocca A., Della Malva N., Bartolozzi D., Giannecchini S. Polyomavirus microRNA in saliva reveals persistent infectious status in the oral cavity. Virus Res. 2018;249:1–7. doi: 10.1016/j.virusres.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Naaman H., Rall G., Matullo C., Veksler-Lublinsky I., Shemer-Avni Y., Gopas J. miRNA-124 is a link between measles virus persistent infection and cell division of human neuroblastoma cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rashad N.M., El-Shal A.S., Shalaby S.M., Mohamed S.Y. Serum miRNA-27a and miRNA-18b as potential predictive biomarkers of hepatitis C virus-associated hepatocellular carcinoma. Mol Cell Biochem. 2018;447:125–136. doi: 10.1007/s11010-018-3298-8. [DOI] [PubMed] [Google Scholar]
- 53.Riazalhosseini B., Mohamed R., Apalasamy Y.D., Langmia I.M., Mohamed Z. Circulating microRNA as a marker for predicting liver disease progression in patients with chronic hepatitis B. Rev Soc Bras Med Trop. 2017;50:161–166. doi: 10.1590/0037-8682-0416-2016. [DOI] [PubMed] [Google Scholar]
- 54.Ghanbari M., Eini O., Ebrahimi S. Differential expression of MYB33 and AP2 genes and response of Ty resistant plants to beet curly top Iran virus infection in tomato. J Plant Pathol. 2016;98:555–562. [Google Scholar]
- 55.Heidari M., Zhang H., Sunkara L. MDV-induced differential microRNA expression in the primary lymphoid organ of thymus. Microb Pathog. 2022;170 doi: 10.1016/j.micpath.2022.105688. [DOI] [PubMed] [Google Scholar]
- 56.Heidari M., Zhang L., Zhang H. MicroRNA profiling in the bursae of Marek's disease virus-infected resistant and susceptible chicken lines. Genomics. 2020;112:2564–2571. doi: 10.1016/j.ygeno.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Hong Y., Truong A.D., Lee J., Vu T.H., Lee S., Song K.-D., et al. Exosomal miRNA profiling from H5N1 avian influenza virus-infected chickens. Vet Res. 2021;52:36. doi: 10.1186/s13567-021-00892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong Y.H., Hue D., Lillehoj H.S., Song K.D., Oh J.D. Differential regulation of microRNA transcriptome in chicken lines resistant and susceptible to necrotic enteritis disease. Poult Sci. 2014;93:1383–1395. doi: 10.3382/ps.2013-03666. [DOI] [PubMed] [Google Scholar]
- 59.Luo J., Mitra A., Tian F., Chang S., Zhang H., Cui K., et al. Histone methylation analysis and pathway predictions in chickens after MDV infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mondal D., Chakrabarty U., Dutta S., Mallik A., Mandal N. Identification and characterization of novel microRNAs in disease-resistant and disease-susceptible Penaeus monodon. Fish Shellfish Immunol. 2021;119:347–372. doi: 10.1016/j.fsi.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 61.Sattar S., Song Y., Anstead J.A., Sunkar R., Thompson G.A. Cucumis melo microRNA expression profile during aphid herbivory in a resistant and susceptible interaction. Mol Plant-Microbe Interact. 2012;25:839–848. doi: 10.1094/MPMI-09-11-0252. [DOI] [PubMed] [Google Scholar]
- 62.Tian F., Luo J., Zhang H., Chang S., Song J. miRNA expression signatures induced by Marek's disease virus infection in chickens. Genomics. 2012;99:152–159. doi: 10.1016/j.ygeno.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Brachtlova T., van Ginkel J.-W., Luinenburg M.J., de Menezes R.X., Koppers-Lalic D., Pegtel D.M., et al. Expression of oncolytic adenovirus-encoded RNAi molecules is most effective in a pri-miRNA precursor format. Mol Ther Oncolytics. 2020;19:332–343. doi: 10.1016/j.omto.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elsedawy N.B., Nace R.A., Russell S.J., Schulze A.J. Oncolytic activity of targeted picornaviruses formulated as synthetic infectious RNA. Mol Ther Oncolytics. 2020;17:484–495. doi: 10.1016/j.omto.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sano M., Nakasu A., Ohtaka M., Nakanishi M. A Sendai virus-based cytoplasmic RNA vector as a novel platform for long-term expression of microRNAs. Mol Ther Methods Clin Dev. 2019;15:371–382. doi: 10.1016/j.omtm.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clerget G., Abel Y., Rederstorff M. Small non-coding RNAs: a quick look in the rearview mirror. Methods Mol Biol. 2015;1296:3–9. doi: 10.1007/978-1-4939-2547-6_1. [DOI] [PubMed] [Google Scholar]
- 67.Andrés-León E., Gómez-López G., Pisano D.G. Prediction of miRNA–mRNA interactions using miRGate. Methods Mol Biol. 2017;1580:225–237. doi: 10.1007/978-1-4939-6866-4_15. [DOI] [PubMed] [Google Scholar]
- 68.Barta T., Peskova L., Hampl A. miRNAsong: a web-based tool for generation and testing of miRNA sponge constructs in silico. Sci Rep. 2016;6 doi: 10.1038/srep36625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blondal T., Brunetto M.R., Cavallone D., Mikkelsen M., Thorsen M., Mang Y., et al. Genome-wide comparison of next-generation sequencing and qPCR platforms for microRNA profiling in serum. Methods Mol Biol. 2017;1580:21–44. doi: 10.1007/978-1-4939-6866-4_3. [DOI] [PubMed] [Google Scholar]
- 70.Filip R., Desrochers G.F., Lefebvre D.M., Reed A., Singaravelu R., Cravatt B.F., et al. Profiling of microRNA targets using activity-based protein profiling: linking enzyme activity to microRNA-185 function. Cell Chem Biol. 2021;28:202–212. doi: 10.1016/j.chembiol.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bushweller J.H. Targeting transcription factors in cancer—from undruggable to reality. Nat Rev Cancer. 2019;19:611–624. doi: 10.1038/s41568-019-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lambert M., Jambon S., Depauw S., David-Cordonnier M.H. Targeting transcription factors for cancer treatment. Molecules. 2018;23:1479. doi: 10.3390/molecules23061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 74.Yi C.Y., Zhao Z.Z., Wang S.Y., Sun X., Zhang D., Sun X.M., et al. Influenza A virus PA antagonizes interferon-β by interacting with interferon regulatory factor 3. Front Immunol. 2017;8:1051. doi: 10.3389/fimmu.2017.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gottipati K., Holthauzen L.M.F., Ruggli N., Choi K.H. Pestivirus N-pro directly interacts with interferon regulatory factor 3 monomer and dimer. J Virol. 2016;90:7740–7747. doi: 10.1128/JVI.00318-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie J.Y., Zhang X.B., Chen L., Bi Y.J., Idris A., Xu S.J., et al. Pseudorabies virus US3 protein inhibits IFN-β production by interacting with IRF3 to block its activation. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.761282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao B., Shu C., Gao X., Sankaran B., Du F., Shelton C.L., et al. Structural basis for concerted recruitment and activation of IRF-3 by innate immune adaptor proteins. Proc Natl Acad Sci U S A. 2016;113:E3403–E3412. doi: 10.1073/pnas.1603269113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai Y.L., Xia X.Y., Cheng A.C., Wang M.S., Ou X.M., Mao S., et al. DHAV-1 blocks the signaling pathway upstream of type I interferon by inhibiting the interferon regulatory factor 7 protein. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.700434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X.L., Zhang M.L., Ye C., Ruan K.Y., Xu A.Y., Gao F., et al. Inhibition of the DNA-sensing pathway by pseudorabies virus UL24 protein via degradation of interferon regulatory factor 7. Vet Microbiol. 2021;255 doi: 10.1016/j.vetmic.2021.109023. [DOI] [PubMed] [Google Scholar]
- 80.Zhang B., Liu M.X., Huang J.X., Zeng Q.Y., Zhu Q.Y., Xu S., et al. H1N1 influenza A virus protein NS2 inhibits innate immune response by targeting IRF7. Viruses. 2022;14:2411. doi: 10.3390/v14112411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai Y.T., Zhu Y., Zheng J.Q., Zhang Y.C., Chen W. NBR1 mediates autophagic degradation of IRF3 to negatively regulate type I interferon production. Biochem Biophys Res Commun. 2022;623:140–147. doi: 10.1016/j.bbrc.2022.07.043. [DOI] [PubMed] [Google Scholar]
- 82.Feng W.J., Sun X.N., Shi N., Zhang M.L., Guan Z.H., Duan M. Influenza a virus NS1 protein induced A20 contributes to viral replication by suppressing interferon-induced antiviral response. Biochem Biophys Res Commun. 2017;482:1107–1113. doi: 10.1016/j.bbrc.2016.11.166. [DOI] [PubMed] [Google Scholar]
- 83.Wang S.Y., Sun X., Yi C.Y., Zhang D., Lin X., Sun X.M., et al. AGO2 negatively regulates type I interferon signaling pathway by competition binding IRF3 with CBP/p300. Front Cell Infect Microbiol. 2017;7:195. doi: 10.3389/fcimb.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song S., Lee J.J., Kim H.J., Lee J.Y., Chang J., Lee K.J. Fas-associated factor 1 negatively regulates the antiviral immune response by inhibiting translocation of interferon regulatory factor 3 to the nucleus. Mol Cell Biol. 2016;36:1136–1151. doi: 10.1128/MCB.00744-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee Y.S., Bao X.Y., Lee H.H., Jang J.J., Saruuldalai E., Park G., et al. Nc886, a novel suppressor of the type I interferon response upon pathogen intrusion. Int J Mol Sci. 2021;22:2003. doi: 10.3390/ijms22042003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim J.H., Kim T.H., Lee H.C., Nikapitiya C., Uddina M.B., Park M.E., et al. Rubicon modulates antiviral type I interferon (IFN) signaling by targeting IFN regulatory factor 3 dimerization. J Virol. 2017;91 doi: 10.1128/JVI.00248-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Myoung J., Lee S.A., Lee H.R. Beyond viral interferon regulatory factors: immune evasion strategies. J Microbiol Biotechnol. 2019;29:1873–1881. doi: 10.4014/jmb.1910.10004. [DOI] [PubMed] [Google Scholar]
- 88.Bedard K.M., Wang M.L., Proll S.C., Loo Y.M., Katze M.G., Gale M., et al. Isoflavone agonists of IRF-3 dependent signaling have antiviral activity against RNA viruses. J Virol. 2012;86:7334–7344. doi: 10.1128/JVI.06867-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji Z.L., Li F.F., Xia Z.Q., Guo X.C., Gao M.J., Sun F., et al. The scorpion venom peptide Smp76 inhibits viral infection by regulating type-I interferon response. Virol Sin. 2018;33:545–556. doi: 10.1007/s12250-018-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang M., Jiang Y.F., Xiao X.Q., Peng M.L., Peng F., Gong G.Z. Differences in IP-10, TLR4 and IRF5/3 between SVR and non-SVR HCV-1 patients treated with PEG-IFN and ribavirin. Mol Med Rep. 2017;15:2318–2324. doi: 10.3892/mmr.2017.6229. [DOI] [PubMed] [Google Scholar]
- 91.Kluska K., Adamczyk J., Krężel A. Metal binding properties, stability and reactivity of zinc fingers. Coordin Chem Rev. 2018;367:18–64. [Google Scholar]
- 92.Wang G., Zheng C. Zinc finger proteins in the host-virus interplay: multifaceted functions based on their nucleic acid-binding property. FEMS Microbiol Rev. 2020;45 doi: 10.1093/femsre/fuaa059. [DOI] [PubMed] [Google Scholar]
- 93.Bai H.R., Lin P., Li X., Liao X.Q., Wan L.H., Yang X.H., et al. DgC3H1, a CCCH zinc finger protein gene, confers cold tolerance in transgenic chrysanthemum. Sci Hortic. 2021;281 [Google Scholar]
- 94.Han G.L., Yuan F., Guo J.R., Zhang Y., Sui N., Wang B.S. AtSIZ1 improves salt tolerance by maintaining ionic homeostasis and osmotic balance in Arabidopsis. Plant Sci. 2019;285:55–67. doi: 10.1016/j.plantsci.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 95.Ma G.M., Zhang Y., Li X.Y. Dufulin enhances salt resistance of rice. Pestic Biochem Physiol. 2022;188 doi: 10.1016/j.pestbp.2022.105252. [DOI] [PubMed] [Google Scholar]
- 96.Nagel C., Machulla A., Zahn S., Soppa J. Several one-domain zinc finger μ-proteins of Haloferax volcanii are important for stress adaptation, biofilm formation, and swarming. Genes. 2019;10:361. doi: 10.3390/genes10050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rui P.H., Yang X.C., Xu S.Q., Wang Z.Q., Zhou X.P., Jiang L., et al. FvZFP1 confers transgenic Nicotiana benthamiana resistance against plant pathogens and improves tolerance to abiotic stresses. Plant Sci. 2022;316 doi: 10.1016/j.plantsci.2021.111176. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H.J., Zhao T.Y., Zhuang P.T., Song Z.Q., Du H., Tang Z.Z., et al. NbCZF1, a novel C2H2-type zinc finger protein, as a new regulator of SsCut-induced plant immunity in Nicotiana benthamiana. Plant Cell Physiol. 2016;57:2472–2484. doi: 10.1093/pcp/pcw160. [DOI] [PubMed] [Google Scholar]
- 99.Zuo H.L., Yang L.W., Zheng J.F., Su Z.Q., Weng S.P., He J.G., et al. A single C4 zinc finger-containing protein from Litopenaeus vannamei involved in antibacterial responses. Fish Shellfish Immunol. 2018;81:493–501. doi: 10.1016/j.fsi.2018.07.053. [DOI] [PubMed] [Google Scholar]
- 100.Zhu Y., Chen G., Lv F., Wang X., Ji X., Xu Y., et al. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc Natl Acad Sci U S A. 2011;108:15834–15839. doi: 10.1073/pnas.1101676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xue G., Braczyk K., Gonçalves-Carneiro D., Dawidziak D.M., Sanchez K., Ong H., et al. Poly(ADP-ribose) potentiates ZAP antiviral activity. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1009202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karlberg T., Klepsch M., Thorsell A.-G., Andersson C.D., Linusson A., Schüler H. Structural basis for lack of ADP-ribosyltransferase activity in poly(ADP-ribose) polymerase-13/zinc finger antiviral protein. J Biol Chem. 2015;290:7336–7344. doi: 10.1074/jbc.M114.630160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Subramanian S. The long-term evolutionary history of gradual reduction of CpG dinucleotides in the SARS-CoV-2 lineage. Biology. 2021;10:52. doi: 10.3390/biology10010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jaglan A., Satija S., Singh D., Phartyal R., Verma M. Intra-genomic heterogeneity in CpG dinucleotide composition in dengue virus. Acta Trop. 2022;232 doi: 10.1016/j.actatropica.2022.106501. [DOI] [PubMed] [Google Scholar]
- 105.Shaw A.E., Rihn S.J., Mollentze N., Wickenhagen A., Stewart D.G., Orton R.J., et al. The antiviral state has shaped the CpG composition of the vertebrate interferome to avoid self-targeting. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sertkaya H., Hidalgo L., Ficarelli M., Kmiec D., Signell A.W., Ali S., et al. Minimal impact of ZAP on lentiviral vector production and transduction efficiency. Mol Ther Methods Clin Dev. 2021;23:147–157. doi: 10.1016/j.omtm.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parisien J.-P., Lenoir J.J., Alvarado G., Horvath C.M. The human STAT2 coiled-coil domain contains a degron for Zika virus interferon evasion. J Virol. 2022;96 doi: 10.1128/JVI.01301-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu Y., Yang X., Yao Z., Dong X., Zhang D., Hu Y., et al. C19orf66 interrupts Zika virus replication by inducing lysosomal degradation of viral NS3. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xia S., Robertus J.D. X-ray structures of NS1 effector domain mutants. Arch Biochem Biophys. 2010;494:198–204. doi: 10.1016/j.abb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saito A., Ferhadian D., Sowd G.A., Serrao E., Shi J., Halambage U.D., et al. Roles of capsid-interacting host factors in multimodal inhibition of HIV-1 by PF74. J Virol. 2016;90:5808–5823. doi: 10.1128/JVI.03116-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ashraf U., Tengo L., Le Corre L., Fournier G., Busca P., McCarthy A.A., et al. Destabilization of the human RED-SMU1 splicing complex as a basis for host-directed antiinfluenza strategy. Proc Natl Acad Sci U S A. 2019;116:10968–10977. doi: 10.1073/pnas.1901214116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rivera-Serrano E.E., Fritch E.J., Scholl E.H., Sherry B. A cytoplasmic RNA virus alters the function of the cell splicing protein SRSF2. J Virol. 2017;91 doi: 10.1128/JVI.02488-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gagne B., Tremblay N., Park A.Y., Baril M., Lamarre D. Importin β1 targeting by hepatitis C virus NS3/4A protein restricts IRF3 and NF-κB signaling of IFNB1 antiviral response. Traffic. 2017;18:362–377. doi: 10.1111/tra.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rivas C., Aaronson S.A., Munoz-Fontela C. Dual role of p53 in innate antiviral immunity. Viruses. 2010;2:298–313. doi: 10.3390/v2010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X., Liu Y., Li K., Hao Z. Roles of p53-mediated host–virus interaction in coronavirus infection. Int J Mol Sci. 2023;24:6371. doi: 10.3390/ijms24076371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cardozo C.M., Hainaut P. Viral strategies for circumventing p53: the case of severe acute respiratory syndrome coronavirus. Curr Opin Oncol. 2021;33:149–158. doi: 10.1097/CCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Marco A., Carattoli A., Rozera C., Fortini D., Giorgi C., Belardo G., et al. Induction of the heat-shock response by antiviral prostaglandins in human cells infected with human immunodeficiency virus type 1. Eur J Biochem. 1998;256:334–341. doi: 10.1046/j.1432-1327.1998.2560334.x. [DOI] [PubMed] [Google Scholar]
- 118.De Marco A., Santoro M.G. Antiviral effect of short hyperthermic treatment at specific stages of vesicular stomatitis virus replication cycle. J Gen Virol. 1993;74(Pt 8):1685–1690. doi: 10.1099/0022-1317-74-8-1685. [DOI] [PubMed] [Google Scholar]
- 119.Morozov A., Subjeck J., Raychaudhuri P. HPV16 E7 oncoprotein induces expression of a 110 kDa heat shock protein. FEBS Lett. 1995;371:214–218. doi: 10.1016/0014-5793(95)00884-c. [DOI] [PubMed] [Google Scholar]
- 120.Ohgitani E., Kobayashi K., Takeshita K., Imanishi J. Biphasic translocation of a 70 kDa heat shock protein in human cytomegalovirus-infected cells. J Gen Virol. 1999;80:63–68. doi: 10.1099/0022-1317-80-1-63. [DOI] [PubMed] [Google Scholar]
- 121.Tatem J., Stollar V. Effect of Sindbis virus infection on induction of heat shock proteins in Aedes albopictus cells. J Virol. 1989;63:992–996. doi: 10.1128/jvi.63.2.992-996.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Theodorakis N.G., Morimoto R.I. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987;7:4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chadli A., Graham J.D., Abel M.G., Jackson T.A., Gordon D.F., Wood W.M., et al. GCUNC-45 is a novel regulator for the progesterone receptor/hsp90 chaperoning pathway. Mol Cell Biol. 2006;26:1722–1730. doi: 10.1128/MCB.26.5.1722-1730.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mayer M.P. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 2013;38:507–514. doi: 10.1016/j.tibs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 125.Radons J. The human HSP70 family of chaperones: where do we stand?. Cell Stress Chaperones. 2016;21:379–404. doi: 10.1007/s12192-016-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rauch J.N., Gestwicki J.E. Binding of human nucleotide exchange factors to heat shock protein 70 (Hsp70) generates functionally distinct complexes in vitro. J Biol Chem. 2014;289:1402–1414. doi: 10.1074/jbc.M113.521997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Retzlaff M., Stahl M., Eberl H.C., Lagleder S., Beck J., Kessler H., et al. Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Rep. 2009;10:1147–1153. doi: 10.1038/embor.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glotzer J.B., Saltik M., Chiocca S., Michou A.I., Moseley P., Cotten M. Activation of heat-shock response by an adenovirus is essential for virus replication. Nature. 2000;407:207–211. doi: 10.1038/35025102. [DOI] [PubMed] [Google Scholar]
- 129.Taguwa S., Maringer K., Li X., Bernal-Rubio D., Rauch J.N., Gestwicki J.E., et al. Defining Hsp70 subnetworks in dengue virus replication reveals key vulnerability in flavivirus infection. Cell. 2015;163:1108–1123. doi: 10.1016/j.cell.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pujhari S., Brustolin M., Macias V.M., Nissly R.H., Nomura M., Kuchipudi S.V., et al. Heat shock protein 70 (Hsp70) mediates Zika virus entry, replication, and egress from host cells. Emerg Microbes Infect. 2019;8:8–16. doi: 10.1080/22221751.2018.1557988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seo H.W., Seo J.P., Jung G. Heat shock protein 70 and heat shock protein 90 synergistically increase hepatitis B viral capsid assembly. Biochem Biophys Res Commun. 2018;503:2892–2898. doi: 10.1016/j.bbrc.2018.08.065. [DOI] [PubMed] [Google Scholar]
- 132.Park J.Y., Ryu J., Park J.E., Hong E.J., Shin H.J. Heat shock protein 70 could enhance porcine epidemic diarrhoea virus replication by interacting with membrane proteins. Vet Res. 2021;52:138. doi: 10.1186/s13567-021-01006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Manzoor R., Kuroda K., Yoshida R., Tsuda Y., Fujikura D., Miyamoto H., et al. Heat shock protein 70 modulates influenza A virus polymerase activity. J Biol Chem. 2014;289:7599–7614. doi: 10.1074/jbc.M113.507798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gurer C., Cimarelli A., Luban J. Specific incorporation of heat shock protein 70 family members into primate lentiviral virions. J Virol. 2002;76:4666–4670. doi: 10.1128/JVI.76.9.4666-4670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen Z., Zhou T., Wu X., Hong Y., Fan Z., Li H. Influence of cytoplasmic heat shock protein 70 on viral infection of Nicotiana benthamiana. Mol Plant Pathol. 2008;9:809–817. doi: 10.1111/j.1364-3703.2008.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Broquet A.H., Lenoir C., Gardet A., Sapin C., Chwetzoff S., Jouniaux A.M., et al. Hsp70 negatively controls rotavirus protein bioavailability in Caco-2 cells infected by the rotavirus RF strain. J Virol. 2007;81:1297–1304. doi: 10.1128/JVI.01336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hyskova V., Belonoznikova K., Cerovska N., Ryslava H. HSP70 plays an ambiguous role during viral infections in plants. Biol Plant. 2021;65:68–79. [Google Scholar]
- 138.Iordanskiy S., Zhao Y.Q., DiMarzio P., Agostini I., Dubrovsky L., Bukrinsky M. Heat-shock protein 70 exerts opposing effects on Vpr-dependent and Vpr-independent HIV-1 replication in macrophages. Blood. 2004;104:1867–1872. doi: 10.1182/blood-2004-01-0081. [DOI] [PubMed] [Google Scholar]
- 139.Yu L., Ye L., Zhao R., Liu Y.F., Yang S.J. HSP70 induced by Hantavirus infection interacts with viral nucleocapsid protein and its overexpression suppresses virus infection in Vero E6 cells. Am J Transl Res. 2009;1:367–380. [PMC free article] [PubMed] [Google Scholar]
- 140.Fallouh H., Mahana W. Antibody to heat shock protein 70 (HSP70) inhibits human T-cell lymphoptropic virus type I (HTLV-I) production by transformed rabbit T-cell lines. Toxins. 2012;4:768–777. doi: 10.3390/toxins4100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao J., Xiao S., Liu X., Wang L., Ji Q., Mo D., et al. Inhibition of HSP70 reduces porcine reproductive and respiratory syndrome virus replication in vitro. BMC Microbiol. 2014;14:64. doi: 10.1186/1471-2180-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Howe M.K., Haystead T.A.J. New indications for HSP90 and HSP70 inhibitors as antiviral drugs. Heat Shock Protein Based Therapies. 2015;9:175–196. [Google Scholar]
- 143.Khachatoorian R., Riahi R., Ganapathy E., Shao H., Wheatley N.M., Sundberg C., et al. Allosteric heat shock protein 70 inhibitors block hepatitis C virus assembly. Int J Antimicrob Agents. 2016;47:289–296. doi: 10.1016/j.ijantimicag.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu N., Fu J., Wang H., Lu L. Quercetin counteracts the pro-viral effect of heat shock response in grass carp cells with its therapeutic potential against aquareovirus. Aquacult Res. 2021;52:3164–3173. [Google Scholar]
- 145.Nimgaonkar I., Archer N.F., Becher I., Shahrad M., LeDesma R.A., Mateus A., et al. Isocotoin suppresses hepatitis E virus replication through inhibition of heat shock protein 90. Antivir Res. 2021;185 doi: 10.1016/j.antiviral.2020.104997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li C., Chu H., Liu X., Chiu M.C., Zhao X., Wang D., et al. Human coronavirus dependency on host heat shock protein 90 reveals an antiviral target. Emerg Microbes Infect. 2020;9:2663–2672. doi: 10.1080/22221751.2020.1850183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rinaldi S., Assimon V.A., Young Z.T., Morra G., Shao H., Taylor I.R., et al. A local allosteric network in heat shock protein 70 (Hsp70) links inhibitor binding to enzyme activity and distal protein–protein interactions. ACS Chem Biol. 2018;13:3142–3152. doi: 10.1021/acschembio.8b00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Howe M.K., Speer B.L., Hughes P.E., Loiselle D.R., Vasudevan S., Haystead T.A.J. An inducible heat shock protein 70 small molecule inhibitor demonstrates anti-dengue virus activity, validating Hsp70 as a host antiviral target. Antivir Res. 2016;130:81–92. doi: 10.1016/j.antiviral.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peng Z.G., Fan B., Du N.N., Wang Y.P., Gao L.M., Li Y.H., et al. Small molecular compounds that inhibit hepatitis C virus replication through destabilizing heat shock cognate 70 messenger RNA. Hepatology. 2010;52:845–853. doi: 10.1002/hep.23766. [DOI] [PubMed] [Google Scholar]
- 150.Ji X., Li Z. Medicinal chemistry strategies toward host targeting antiviral agents. Med Res Rev. 2020;40:1519–1557. doi: 10.1002/med.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Geller R., Andino R., Frydman J. Hsp90 inhibitors exhibit resistance-free antiviral activity against respiratory syncytial virus. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Roe S.M., Prodromou C., O'Brien R., Ladbury J.E., Piper P.W., Pearl L.H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 153.Connor J.H., McKenzie M.O., Parks G.D., Lyles D.S. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology. 2007;362:109–119. doi: 10.1016/j.virol.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li F., Jin F., Wang Y., Zheng D., Liu J., Zhang Z., et al. Hsp90 inhibitor AT-533 blocks HSV-1 nuclear egress and assembly. J Biochem. 2018;164:397–406. doi: 10.1093/jb/mvy066. [DOI] [PubMed] [Google Scholar]
- 155.Wang J.H., Yao M.Z., Gu J.F., Sun L.Y., Shen Y.F., Liu X.Y. Blocking HSF1 by dominant-negative mutant to sensitize tumor cells to hyperthermia. Biochem Biophys Res Commun. 2002;290:1454–1461. doi: 10.1006/bbrc.2002.6373. [DOI] [PubMed] [Google Scholar]
- 156.Wheeler D.S., Dunsmore K.E., Wong H.R. Intracellular delivery of HSP70 using HIV-1 Tat protein transduction domain. Biochem Biophys Res Commun. 2003;301:54–59. doi: 10.1016/s0006-291x(02)02986-8. [DOI] [PubMed] [Google Scholar]
- 157.The Nobel Prize . Physiology or medicine. 1992. https://www.nobelprize.org/prizes/medicine/1992/summary/ Available from: [Google Scholar]
- 158.Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 159.König R., Stertz S., Zhou Y., Inoue A., Hoffmann H.H., Bhattacharyya S., et al. Human host factors required for influenza virus replication. Nature. 2010;463:813–817. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Muranyi W., Haas J., Wagner M., Krohne G., Koszinowski U.H. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science. 2002;297:854–857. doi: 10.1126/science.1071506. [DOI] [PubMed] [Google Scholar]
- 161.García-Cárceles J., Caballero E., Gil Cmartínez A. Kinase inhibitors as underexplored antiviral agents. J Med Chem. 2022;65:935–954. doi: 10.1021/acs.jmedchem.1c00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gutierrez-Chamorro L., Felip E., Ezeonwumelu I.J., Margelí M., Ballana E. Cyclin-dependent kinases as emerging targets for developing novel antiviral therapeutics. Trends Microbiol. 2021;29:836–848. doi: 10.1016/j.tim.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 163.Malekinejad Z., Baghbanzadeh A., Nakhlband A., Baradaran B., Jafari S., Bagheri Y., et al. Recent clinical findings on the role of kinase inhibitors in COVID-19 management. Life Sci. 2022;306 doi: 10.1016/j.lfs.2022.120809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Naik R.R., Shakya A.K., Aladwan S.M., El-Tanani M. Kinase inhibitors as potential therapeutic agents in the treatment of COVID-19. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.806568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raghuvanshi R., Bharate S.B. Recent developments in the use of kinase inhibitors for management of viral infections. J Med Chem. 2022;65:893–921. doi: 10.1021/acs.jmedchem.0c01467. [DOI] [PubMed] [Google Scholar]
- 166.Levinson A.D., Oppermann H., Levintow L., Varmus H.E., Bishop J.M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978;15:561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- 167.Tan K.B. Comparative study of the protein kinase associated with animal viruses. Virology. 1975;64:566–570. doi: 10.1016/0042-6822(75)90135-x. [DOI] [PubMed] [Google Scholar]
- 168.Leader D.P. Viral protein kinases and protein phosphatases. Pharmacol Ther. 1993;59:343–389. doi: 10.1016/0163-7258(93)90075-o. [DOI] [PubMed] [Google Scholar]
- 169.Pythoud C., Rodrigo W.W.S.I., Pasqual G., Rothenberger S., Martinez-Sobrido L., de la Torre J.C., et al. Arenavirus nucleoprotein targets interferon regulatory factor-activating kinase IKKε. J Virol. 2012;86:7728–7738. doi: 10.1128/JVI.00187-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.You H., Zheng S., Huang Z., Lin Y., Shen Q., Zheng C. Herpes simplex virus 1 tegument protein UL46 inhibits TANK-binding kinase 1-mediated signaling. mBio. 2019;10 doi: 10.1128/mBio.00919-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kumar P., Rawat K., Sharma T., Kumari S., Saxena R., Kumar B., et al. HIV-1 Nef physically associate with CAMKIIδ–ASK-1 complex to inhibit p38MAPK signalling and apoptosis in infected cells. Life Sci. 2019;224:263–273. doi: 10.1016/j.lfs.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 172.Kumar R., Khandelwal N., Thachamvally R., Tripathi B.N., Barua S., Kashyap S.K., et al. Role of MAPK/MNK1 signaling in virus replication. Virus Res. 2018;253:48–61. doi: 10.1016/j.virusres.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chander Y., Kumar R., Khandelwal N., Singh N., Shringi B.N., Barua S., et al. Role of p38 mitogen-activated protein kinase signalling in virus replication and potential for developing broad spectrum antiviral drugs. Rev Med Virol. 2021;31 doi: 10.1002/rmv.2217. [DOI] [PubMed] [Google Scholar]
- 174.Petrie E.J., Sandow J.J., Lehmann W.I.L., Liang L.Y., Coursier D., Young S.N., et al. Viral MLKL homologs subvert necroptotic cell death by sequestering cellular RIPK3. Cell Rep. 2019;28:3309–3319.e5. doi: 10.1016/j.celrep.2019.08.055. [DOI] [PubMed] [Google Scholar]
- 175.Mukhopadhyay U., Patra U., Chandra P., Saha P., Gope A., Dutta M., et al. Rotavirus activates MLKL-mediated host cellular necroptosis concomitantly with apoptosis to facilitate dissemination of viral progeny. Mol Microbiol. 2022;117:818–836. doi: 10.1111/mmi.14874. [DOI] [PubMed] [Google Scholar]
- 176.Bhutta M.S., Gallo E.S., Borenstein R. Multifaceted role of AMPK in viral infections. Cells. 2021;10:1118. doi: 10.3390/cells10051118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Silwal P., Kim J.K., Yuk J.M., Jo E.K. AMP-activated protein kinase and host defense against infection. Int J Mol Sci. 2018;19:3495. doi: 10.3390/ijms19113495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mouree K.R., Kishimoto N., Iga N., Kirihara C., Yamamoto K., Takamune N., et al. Virion-packaged pyruvate kinase muscle type 2 affects reverse transcription efficiency of human immunodeficiency virus type 1 by blocking virion recruitment of tRNALys3. Biol Pharm Bull. 2018;41:612–618. doi: 10.1248/bpb.b17-00991. [DOI] [PubMed] [Google Scholar]
- 179.Chuang C., Prasanth K.R., Nagy P.D. The glycolytic pyruvate kinase is recruited directly into the viral replicase complex to generate ATP for RNA synthesis. Cell Host Microbe. 2017;22:639–652.e7. doi: 10.1016/j.chom.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 180.Mishra S., Goyal P., Kumar D., Chaudhari R., Rajala M.S. Experimental validation of influenza A virus matrix protein (M1) interaction with host cellular alpha enolase and pyruvate kinase. Virology. 2020;549:59–67. doi: 10.1016/j.virol.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 181.Kumar D., Tiwari K., Rajala M.S. Analysis of A549 cell proteome alteration in response to recombinant influenza A virus nucleoprotein and its interaction with cellular proteins, a preliminary study. Acta Virol. 2017;61:56–65. doi: 10.4149/av_2017_01_56. [DOI] [PubMed] [Google Scholar]
- 182.Elbahesh H., Bergmann S., Russell C.J. Focal adhesion kinase (FAK) regulates polymerase activity of multiple influenza A virus subtypes. Virology. 2016;499:369–374. doi: 10.1016/j.virol.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 183.Patil A., Anhlan D., Ferrando V., Mecate-Zambrano A., Mellmann A., Wixler V., et al. Phosphorylation of influenza A virus NS1 at serine 205 mediates its viral polymerase-enhancing function. J Virol. 2021;95 doi: 10.1128/JVI.02369-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marschall M., Strojan H., Kiener R., Wangen C., Sonntag E., Mueller R., et al. Differential upregulation of host cell protein kinases by the replication of α-, β- and γ-herpesviruses provides a signature of virus-specific signalling. J Gen Virol. 2020;101:284–289. doi: 10.1099/jgv.0.001370. [DOI] [PubMed] [Google Scholar]
- 185.Chander Y., Kumar R., Verma A., Khandelwal N., Nagori H., Singh N., et al. Resistance evolution against host-directed antiviral agents: buffalopox virus switches to use p38-γ under long-term selective pressure of an inhibitor targeting p38-α. Mol Biol Evol. 2022;39 doi: 10.1093/molbev/msac177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Johnson J.R., Crosby D.C., Hultquist J.F., Kurland A.P., Adhikary P., Li D., et al. Global post-translational modification profiling of HIV-1-infected cells reveals mechanisms of host cellular pathway remodeling. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 189.Wu K., Li W., Peng G., Li F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc Natl Acad Sci U S A. 2009;106:19970–19974. doi: 10.1073/pnas.0908837106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 191.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang X., Xia S., Zhu Y., Lu L., Jiang S. Pan-coronavirus fusion inhibitors as the hope for today and tomorrow. Protein Cell. 2021;12:84–88. doi: 10.1007/s13238-020-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78:779–784. doi: 10.1016/j.molcel.2020.04.022. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wu C.-R., Yin W.-C., Jiang Y., Xu H.E. Structure genomics of SARS-CoV-2 and its Omicron variant: drug design templates for COVID-19. Acta Pharmacol Sin. 2022;43:3021–3033. doi: 10.1038/s41401-021-00851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Braun E., Hotter D., Koepke L., Zech F., Groß R., Sparrer K.M.J., et al. Guanylate-binding proteins 2 and 5 exert broad antiviral activity by inhibiting furin-mediated processing of viral envelope proteins. Cell Rep. 2019;27:2092–2104. doi: 10.1016/j.celrep.2019.04.063. e10. [DOI] [PubMed] [Google Scholar]
- 198.Abe M., Tahara M., Sakai K., Yamaguchi H., Kanou K., Shirato K., et al. TMPRSS2 is an activating protease for respiratory parainfluenza viruses. J Virol. 2013;87:11930–11935. doi: 10.1128/JVI.01490-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Edinger T.O., Pohl M.O., Yangueez E., Stertz S. Cathepsin W is required for escape of influenza A virus from late endosomes. mBio. 2015;6 doi: 10.1128/mBio.00297-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guenther S.C., Martinez-Romero C., Borau M.S., Pham C.T.N., Garcia-Sastre A., Stertz S. Proteomic identification of potential target proteins of cathepsin W for its development as a drug target for influenza. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.00921-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hoffmann M., Schroeder S., Kleine-Weber H., Mueller M.A., Drosten C., Poehlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee J., Lee J., Kim H.J., Ko M., Jee Y., Kim S. TMPRSS2 and RNA-dependent RNA polymerase are effective targets of therapeutic intervention for treatment of COVID-19 caused by SARS-CoV-2 variants (B.1.1.7 and B.1.351) Microbiol Spectr. 2021;9 doi: 10.1128/spectrum.00472-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.He Y., Zhou J., Gao H., Liu C., Zhan P., Liu X. Broad-spectrum antiviral strategy: host-targeting antivirals against emerging and re-emerging viruses. Eur J Med Chem. 2024;265 doi: 10.1016/j.ejmech.2023.116069. [DOI] [PubMed] [Google Scholar]
- 205.Ketter E., Randall G. Virus impact on lipids and membranes. Annu Rev Virol. 2019;6:319–340. doi: 10.1146/annurev-virology-092818-015748. [DOI] [PubMed] [Google Scholar]
- 206.Heaton N.S., Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011;19:368–375. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mazzon M., Mercer J. Lipid interactions during virus entry and infection. Cell Microbiol. 2014;16:1493–1502. doi: 10.1111/cmi.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hu L., Li J., Cai H., Yao W., Xiao J., Li Y.-P., et al. Avasimibe: a novel hepatitis C virus inhibitor that targets the assembly of infectious viral particles. Antivir Res. 2017;148:5–14. doi: 10.1016/j.antiviral.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 209.Oslob J.D., Johnson R.J., Cai H., Feng S.Q., Hu L., Kosaka Y., et al. Imidazopyridine-based fatty acid synthase inhibitors that show anti-HCV activity and in vivo target modulation. ACS Med Chem Lett. 2013;4:113–117. doi: 10.1021/ml300335r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Barretto N., Sainz B., Hussain S., Uprichard S.L. Determining the involvement and therapeutic implications of host cellular factors in hepatitis C virus cell-to-cell spread. J Virol. 2014;88:5050–5061. doi: 10.1128/JVI.03241-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Meutiawati F., Bezemer B., Strating J.R.P.M., Overheul G.J., Žusinaite E., van Kuppeveld F.J.M., et al. Posaconazole inhibits dengue virus replication by targeting oxysterol-binding protein. Antivir Res. 2018;157:68–79. doi: 10.1016/j.antiviral.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 212.Schuster C., Lefèvre M., Baumert T.F. Triglyceride synthesis and hepatitis C virus production: identification of a novel host factor as antiviral target. Hepatology. 2011;53:1046–1048. doi: 10.1002/hep.24177. [DOI] [PubMed] [Google Scholar]
- 213.Herker E., Harris C., Hernandez C., Carpentier A., Kaehlcke K., Rosenberg A.R., et al. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat Med. 2010;16:1295–1298. doi: 10.1038/nm.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Camus G., Herker E., Modi A.A., Haas J.T., Ramage H.R., Farese R.V., et al. Diacylglycerol acyltransferase-1 localizes hepatitis C virus NS5A protein to lipid droplets and enhances NS5A interaction with the viral capsid core. J Biol Chem. 2013;288:9915–9923. doi: 10.1074/jbc.M112.434910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Harris C., Herker E., Farese R.V., Ott M. Hepatitis C virus core protein decreases lipid droplet turnover: a mechanism for core-induced steatosis. J Biol Chem. 2011;286:42615–42625. doi: 10.1074/jbc.M111.285148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gane E., Stedman C., Dole K., Chen J., Meyers C.D., Wiedmann B., et al. A diacylglycerol transferase 1 inhibitor is a potent hepatitis C antiviral in vitro but not in patients in a randomized clinical trial. ACS Infect Dis. 2017;3:144–151. doi: 10.1021/acsinfecdis.6b00138. [DOI] [PubMed] [Google Scholar]
- 217.Klon A.E., Héroux A., Ross L.J., Pathak V., Johnson C.A., Piper J.R., et al. Atomic structures of human dihydrofolate reductase complexed with NADPH and two lipophilic antifolates at 1.09 Å and 1.05 Å resolution. J Mol Biol. 2002;320:677–693. doi: 10.1016/s0022-2836(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 218.Anderson D.D., Quintero C.M., Stover P.J. Identification of a de novo thymidylate biosynthesis pathway in mammalian mitochondria. Proc Natl Acad Sci U S A. 2011;108:15163–15168. doi: 10.1073/pnas.1103623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Beck S., Zhu Z., Oliveira M.F., Smith D.M., Rich J.N., Bernatchez J.A., et al. Mechanism of action of methotrexate against Zika virus. Viruses. 2019;11:338. doi: 10.3390/v11040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Francesconi V., Giovannini L., Santucci M., Cichero E., Costi M.P., Naesens L., et al. Synthesis, biological evaluation and molecular modeling of novel azaspiro dihydrotriazines as influenza virus inhibitors targeting the host factor dihydrofolate reductase (DHFR) Eur J Med Chem. 2018;155:229–243. doi: 10.1016/j.ejmech.2018.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Karabulut S., Sizochenko N., Orhan A., Leszczynski J. A DFT-based QSAR study on inhibition of human dihydrofolate reductase. J Mol Graph Model. 2016;70:23–29. doi: 10.1016/j.jmgm.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 222.Tonelli M., Naesens L., Gazzarrini S., Santucci M., Cichero E., Tasso B., et al. Host dihydrofolate reductase (DHFR)-directed cycloguanil analogues endowed with activity against influenza virus and respiratory syncytial virus. Eur J Med Chem. 2017;135:467–478. doi: 10.1016/j.ejmech.2017.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Markland W., McQuaid T.J., Jain J., Kwong A.D. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother. 2000;44:859–866. doi: 10.1128/aac.44.4.859-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Leyssen P., Balzarini J., Clercq E.D., Neyts J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J Virol. 2005;79:1943–1947. doi: 10.1128/JVI.79.3.1943-1947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Smee D.F., Bray M., Huggins J.W. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir Chem Chemother. 2001;12:327–335. doi: 10.1177/095632020101200602. [DOI] [PubMed] [Google Scholar]
- 226.Bonavia A., Franti M., Pusateri Keaney E., Kuhen K., Seepersaud M., Radetich B., et al. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV) Proc Natl Acad Sci U S A. 2011;108:6739–6744. doi: 10.1073/pnas.1017142108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Verrier E.R., Weiss A., Bach C., Heydmann L., Turon-Lagot V., Kopp A., et al. Combined small molecule and loss-of-function screen uncovers estrogen receptor alpha and CAD as host factors for HDV infection and antiviral targets. Gut. 2020;69:158–167. doi: 10.1136/gutjnl-2018-317065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Martin S., Chiramel A.I., Schmidt M.L., Chen Y.-C., Whitt N., Watt A., et al. A genome-wide siRNA screen identifies a druggable host pathway essential for the Ebola virus life cycle. Genome Med. 2018;10:58. doi: 10.1186/s13073-018-0570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xiong R., Zhang L., Li S., Sun Y., Ding M., Wang Y., et al. Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein cell. 2020;11:723–739. doi: 10.1007/s13238-020-00768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Boschi D., Pippione A.C., Sainas S., Lolli M.L. Dihydroorotate dehydrogenase inhibitors in anti-infective drug research. Eur J Med Chem. 2019;183 doi: 10.1016/j.ejmech.2019.111681. [DOI] [PubMed] [Google Scholar]
- 231.Gong M., Yang Y., Huang Y., Gan T., Wu Y., Gao H., et al. Novel quinolone derivatives targeting human dihydroorotate dehydrogenase suppress Ebola virus infection in vitro. Antivir Res. 2021;194 doi: 10.1016/j.antiviral.2021.105161. [DOI] [PubMed] [Google Scholar]
- 232.Cordsmeier A., Herrmann A., Gege C., Kohlhof H., Korn K., Ensser A. Molecular analysis of the 2022 mpox outbreak and antiviral activity of dihydroorotate dehydrogenase inhibitors against orthopoxviruses. Antivir Res. 2025;233 doi: 10.1016/j.antiviral.2024.106043. [DOI] [PubMed] [Google Scholar]
- 233.Haller O., Arnheiter H., Pavlovic J., Staeheli P. The discovery of the antiviral resistance gene Mx: a story of great ideas, great failures, and some success. Annu Rev Virol. 2018;5:33–51. doi: 10.1146/annurev-virology-092917-043525. [DOI] [PubMed] [Google Scholar]
- 234.Wang Y., Chen X., Xie J., Zhou S., Huang Y., Li Y.-P., et al. RNA helicase A is an important host factor involved in dengue virus replication. J Virol. 2019;93 doi: 10.1128/JVI.01306-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Brai A., Fazi R., Tintori C., Zamperini C., Bugli F., Sanguinetti M., et al. Human DDX3 protein is a valuable target to develop broad spectrum antiviral agents. Proc Natl Acad Sci U S A. 2016;113:5388–5393. doi: 10.1073/pnas.1522987113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Glitscher M., Himmelsbach K., Woytinek K., Schollmeier A., Johne R., Praefcke G.J.K., et al. Identification of the interferon-inducible GTPase GBP1 as a major restriction factor for hepatitis E virus. J Virol. 2021;95 doi: 10.1128/JVI.01564-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mo S., Tang W., Xie J., Chen S., Ren L., Zang N., et al. Respiratory syncytial virus activates Rab5a to suppress IRF1-dependent lambda interferon production, subverting the antiviral defense of airway epithelial cells. J Virol. 2021;95 doi: 10.1128/JVI.02333-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Weston S., Baracco L., Keller C., Matthews K., McGrath M.E., Logue J., et al. The SKI complex is a broad-spectrum, host-directed antiviral drug target for coronaviruses, influenza, and filoviruses. Proc Natl Acad Sci U S A. 2020;117:30687–30698. doi: 10.1073/pnas.2012939117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Maga G., Falchi F., Radi M., Botta L., Casaluce G., Bernardini M., et al. Toward the discovery of novel anti-HIV drugs. second-generation inhibitors of the cellular ATPase DDX3 with improved anti-HIV activity: synthesis, structure–activity relationship analysis, cytotoxicity studies, and target validation. Chemmedchem. 2011;6:1371–1389. doi: 10.1002/cmdc.201100166. [DOI] [PubMed] [Google Scholar]
- 240.Bol G.M., Vesuna F., Xie M., Zeng J., Aziz K., Gandhi N., et al. Targeting DDX3 with a small molecule inhibitor for lung cancer therapy. EMBO Mol Med. 2015;7:648–669. doi: 10.15252/emmm.201404368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Samal S.K., Routray S., Veeramachaneni G.K., Dash R., Botlagunta M. Ketorolac salt is a newly discovered DDX3 inhibitor to treat oral cancer. Sci Rep. 2015;5:9982. doi: 10.1038/srep09982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Garbelli A., Radi M., Falchi F., Beermann S., Zanoli S., Manetti F., et al. Targeting the human DEAD-box polypeptide 3 (DDX3) RNA helicase as a novel strategy to inhibit viral replication. Curr Med Chem. 2011;18:3015–3027. doi: 10.2174/092986711796391688. [DOI] [PubMed] [Google Scholar]
- 243.Gale T.V., Horton T.M., Hoffmann A.R., Branco L.M., Garry R.F. Host proteins identified in extracellular viral particles as targets for broad-spectrum antiviral inhibitors. J Proteome Res. 2019;18:7–17. doi: 10.1021/acs.jproteome.8b00204. [DOI] [PubMed] [Google Scholar]
- 244.Koyuncu E., Budayeva H.G., Miteva Y.V., Ricci D.P., Silhavy T.J., Shenk T., et al. Sirtuins are evolutionarily conserved viral restriction factors. mBio. 2014;5 doi: 10.1128/mBio.02249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chatterji U., Bobardt M., Selvarajah S., Yang F., Tang H., Sakamoto N., et al. The isomerase active site of cyclophilin A is critical for hepatitis C virus replication. J Biol Chem. 2009;284:16998–17005. doi: 10.1074/jbc.M109.007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Inoue K., Sekiyama K., Yamada M., Watanabe T., Yasuda H., Yoshiba M. Combined interferon α2b and cyclosporin A in the treatment of chronic hepatitis C: controlled trial. J Gastroenterol. 2003;38:567–572. doi: 10.1007/s00535-002-1104-5. [DOI] [PubMed] [Google Scholar]
- 247.Ma S., Boerner J.E., TiongYip C., Weidmann B., Ryder N.S., Cooreman M.P., et al. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with alpha interferon. Antimicrob Agents Chemother. 2006;50:2976–2982. doi: 10.1128/AAC.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cockram P.E., Kist M., Prakash S., Chen S.H., Wertz I.E., Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591–605. doi: 10.1038/s41418-020-00708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tang Q., Wu P., Chen H., Li G. Pleiotropic roles of the ubiquitin–proteasome system during viral propagation. Life Sci. 2018;207:350–354. doi: 10.1016/j.lfs.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Luo H. Interplay between the virus and the ubiquitin–proteasome system: molecular mechanism of viral pathogenesis. Curr Opin Virol. 2016;17:1–10. doi: 10.1016/j.coviro.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hage A., Rajsbaum R. To TRIM or not to TRIM: the balance of host–virus interactions mediated by the ubiquitin system. J Gen Virol. 2019;100:1641–1662. doi: 10.1099/jgv.0.001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bagga T., Tulsian N.K., Mok Y.K., Kini R.M., Sivaraman J. Mapping of molecular interactions between human E3 ligase TRIM69 and dengue virus NS3 protease using hydrogen–deuterium exchange mass spectrometry. Cell Mol Life Sci. 2022;79:233. doi: 10.1007/s00018-022-04245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Patil G., Zhao M., Song K., Hao W., Bouchereau D., Wang L., et al. TRIM41-mediated ubiquitination of nucleoprotein limits influenza A virus infection. J Virol. 2018;92 doi: 10.1128/JVI.00905-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fan W., McDougal M.B., Schoggins J.W. Enterovirus 3C protease cleaves TRIM7 to dampen its antiviral activity. J Virol. 2022;96 doi: 10.1128/jvi.01332-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zheng X., Wang X., Tu F., Wang Q., Fan Z., Gao G. TRIM25 is required for the antiviral activity of zinc finger antiviral protein. J Virol. 2017;91 doi: 10.1128/JVI.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li Q., Lin L., Tong Y., Liu Y., Mou J., Wang X., et al. TRIM29 negatively controls antiviral immune response through targeting STING for degradation. Cell Discov. 2018;4:13. doi: 10.1038/s41421-018-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Caines M.E.C., Bichel K., Price A.J., McEwan W.A., Towers G.J., Willett B.J., et al. Diverse HIV viruses are targeted by a conformationally dynamic antiviral. Nat Struct Mol Biol. 2012;19:411–416. doi: 10.1038/nsmb.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shindo K., Takaori-Kondo A., Kobayashi M., Abudu A., Fukunaga K., Uchiyama T. The enzymatic activity of CEM15/APOBEC-3G is essential for the regulation of the infectivity of HIV-1 virion but not a sole determinant of its antiviral activity. J Biol Chem. 2003;278:44412–44416. doi: 10.1074/jbc.C300376200. [DOI] [PubMed] [Google Scholar]
- 259.Liu C., Zhang X., Huang F., Yang B., Li J., Liu B., et al. APOBEC3G inhibits microRNA-mediated repression of translation by interfering with the interaction between Argonaute-2 and MOV10. J Biol Chem. 2012;287:29373–29383. doi: 10.1074/jbc.M112.354001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sheehy A.M., Gaddis N.C., Choi J.D., Malim M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 261.Zhang H., Yang B., Pomerantz R.J., Zhang C., Arunachalam S.C., Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mangeat B., Turelli P., Caron G., Friedli M., Perrin L., Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 263.Harris R.S., Bishop K.N., Sheehy A.M., Craig H.M., Petersen-Mahrt S.K., Watt I.N., et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 264.Mariani R., Chen D., Schröfelbauer B., Navarro F., König R., Bollman B., et al. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 265.Sheehy A.M., Gaddis N.C., Malim M.H. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- 266.Kao S., Khan M.A., Miyagi E., Plishka R., Buckler-White A., Strebel K. The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity. J Virol. 2003;77:11398–11407. doi: 10.1128/JVI.77.21.11398-11407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Stopak K., de Noronha C., Yonemoto W., Greene W.C. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol Cell. 2003;12:591–601. doi: 10.1016/s1097-2765(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 268.Anderson B.D., Harris R.S. Transcriptional regulation of APOBEC3 antiviral immunity through the CBF-β/RUNX axis. Sci Adv. 2015;1 doi: 10.1126/sciadv.1500296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Binning J.M., Smith A.M., Hultquist J.F., Craik C.S., Caretta Cartozo N., Campbell M.G., et al. Fab-based inhibitors reveal ubiquitin independent functions for HIV Vif neutralization of APOBEC3 restriction factors. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Turelli P., Mangeat B., Jost S., Vianin S., Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science. 2004;303:1829. doi: 10.1126/science.1092066. [DOI] [PubMed] [Google Scholar]
- 271.Delebecque F., Suspène R., Calattini S., Casartelli N., Saïb A., Froment A., et al. Restriction of foamy viruses by APOBEC cytidine deaminases. J Virol. 2006;80:605–614. doi: 10.1128/JVI.80.2.605-614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Paprotka T., Venkatachari N.J., Chaipan C., Burdick R., Delviks-Frankenberry K.A., Hu W.S., et al. Inhibition of xenotropic murine leukemia virus-related virus by APOBEC3 proteins and antiviral drugs. J Virol. 2010;84:5719–5729. doi: 10.1128/JVI.00134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chen H., Lilley C.E., Yu Q., Lee D.V., Chou J., Narvaiza I., et al. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr Biol. 2006;16:480–485. doi: 10.1016/j.cub.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 274.Holmes R.K., Koning F.A., Bishop K.N., Malim M.H. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation: comparisons with APOBEC3G. J Biol Chem. 2007;282:2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- 275.Mbisa J.L., Bu W., Pathak V.K. APOBEC3F and APOBEC3G inhibit HIV-1 DNA integration by different mechanisms. J Virol. 2010;84:5250–5259. doi: 10.1128/JVI.02358-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hultquist J.F., Lengyel J.A., Refsland E.W., LaRue R.S., Lackey L., Brown W.L., et al. Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-deficient HIV-1. J Virol. 2011;85:11220–11234. doi: 10.1128/JVI.05238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Refsland E.W., Hultquist J.F., Harris R.S. Endogenous origins of HIV-1 G-to-A hypermutation and restriction in the nonpermissive T cell line CEM2n. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Li Y.-L., Langley C.A., Azumaya C.M., Echeverria I., Chesarino N.M., Emerman M., et al. The structural basis for HIV-1 Vif antagonism of human APOBEC3G. Nature. 2023;615:728–733. doi: 10.1038/s41586-023-05779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cen S., Peng Z.-G., Li X.-Y., Li Z.-R., Ma J., Wang Y.-M., et al. Small molecular compounds inhibit HIV-1 replication through specifically stabilizing APOBEC3G. J Biol Chem. 2010;285:16546–16552. doi: 10.1074/jbc.M109.085308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Huang W., Zuo T., Jin H., Liu Z., Yang Z., Yu X., et al. Design, synthesis and biological evaluation of indolizine derivatives as HIV-1 VIF-ElonginC interaction inhibitors. Mol Divers. 2013;17:221–243. doi: 10.1007/s11030-013-9424-3. [DOI] [PubMed] [Google Scholar]
- 281.Ma L., Zhang Z., Liu Z., Pan Q., Wang J., Li X., et al. Identification of small molecule compounds targeting the interaction of HIV-1 Vif and human APOBEC3G by virtual screening and biological evaluation. Sci Rep. 2018;8:8067. doi: 10.1038/s41598-018-26318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pery E., Sheehy A., Nebane N.M., Brazier A.J., Misra V., Rajendran K.S., et al. Identification of a novel HIV-1 inhibitor targeting Vif-dependent degradation of human APOBEC3G protein. J Biol Chem. 2015;290:10504–10517. doi: 10.1074/jbc.M114.626903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zuo T., Liu D., Lv W., Wang X., Wang J., Lv M., et al. Small-molecule inhibition of human immunodeficiency virus type 1 replication by targeting the interaction between Vif and ElonginC. J Virol. 2012;86:5497–5507. doi: 10.1128/JVI.06957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Cen S., Peng Z.G., Li X.Y., Li Z.R., Ma J., Wang Y.M., et al. Small molecular compounds inhibit HIV-1 replication through specifically stabilizing APOBEC3G. J Biol Chem. 2010;285:16546–16552. doi: 10.1074/jbc.M109.085308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nathans R., Cao H., Sharova N., Ali A., Sharkey M., Stranska R., et al. Small-molecule inhibition of HIV-1 Vif. Nat Biotechnol. 2008;26:1187–1192. doi: 10.1038/nbt.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kong Z., Yin H., Wang F., Liu Z., Luan X., Sun L., et al. Pseudorabies virus tegument protein UL13 recruits RNF5 to inhibit STING-mediated antiviral immunity. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ren W., Fu C., Zhang Y., Ju X., Jiang X., Song J., et al. Zika virus NS5 protein inhibits type I interferon signaling via CRL3 E3 ubiquitin ligase-mediated degradation of STAT2. Proc Natl Acad Sci U S A. 2024;121 doi: 10.1073/pnas.2403235121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Youseff B.H., Brewer T.G., McNally K.L., Izuogu A.O., Lubick K.J., Presloid J.B., et al. TRAF6 plays a proviral role in tick-borne flavivirus infection through interaction with the NS3 protease. iScience. 2019;15:489–501. doi: 10.1016/j.isci.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tada T., Zhang Y., Koyama T., Tobiume M., Tsunetsugu-Yokota Y., Yamaoka S., et al. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins. Nat Med. 2015;21:1502–1507. doi: 10.1038/nm.3956. [DOI] [PubMed] [Google Scholar]
- 290.Jiao Y., Kong N., Wang H., Sun D., Dong S., Chen X., et al. PABPC4 broadly inhibits coronavirus replication by degrading nucleocapsid protein through selective autophagy. Microbiol Spectr. 2021;9 doi: 10.1128/Spectrum.00908-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chen Y., He L., Peng Y., Shi X., Chen J., Zhong J., et al. The hepatitis C virus protein NS3 suppresses TNF-α-stimulated activation of NF-κB by targeting LUBAC. Sci Signal. 2015;8 doi: 10.1126/scisignal.aab2159. [DOI] [PubMed] [Google Scholar]
- 292.Flotho A., Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 293.Hendriks I.A., D'Souza R.C.J., Yang B., Verlaan-de Vries M., Mann M., Vertegaal A.C.O. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat Struct Mol Biol. 2014;21:927–936. doi: 10.1038/nsmb.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Jackson Stephen P., Durocher D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell. 2013;49:795–807. doi: 10.1016/j.molcel.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 295.Müncheberg S., Hay R.T., Ip W.H., Meyer T., Weiß C., Brenke J., et al. E1B-55K-mediated regulation of RNF4 SUMO-targeted ubiquitin ligase promotes human adenovirus gene expression. J Virol. 2018;92 doi: 10.1128/JVI.00164-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bekes M., Langley D.R., Crews C.M. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21:181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zhu H.H., Zhao X.B., Hu W.W., Chen W.L. Research progress on ubiquitin-specific protease in antiviral immunity. J Zhejiang Univ (Med Sci) 2015;44:578–583. doi: 10.3785/j.issn.1008-9292.2015.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Charlton F.W., Pearson H.M., Hover S., Lippiat J.D., Fontana J., Barr J.N., et al. Ion channels as therapeutic targets for viral infections: further discoveries and future perspectives. Viruses. 2020;12:844. doi: 10.3390/v12080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Filippini A., D’Amore A., Palombi F., Carpaneto A. Could the inhibition of endo-lysosomal two-pore channels (TPCs) by the natural flavonoid naringenin represent an option to fight SARS-CoV-2 infection?. Front Microbiol. 2020;11:970. doi: 10.3389/fmicb.2020.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z., et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1 doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Fälth M., et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 302.Ferrari D., Idzko M., Mueller T., Manservigi R., Marconi P. Purinergic signaling: a new pharmacological target against viruses?. Trends Pharmacol Sci. 2018;39:926–936. doi: 10.1016/j.tips.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 303.Hochdorfer D., Florin L., Sinzger C., Lieber D. Tetraspanin CD151 promotes initial events in human cytomegalovirus infection. J Virol. 2016;90:6430–6442. doi: 10.1128/JVI.00145-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kaneko M., Watashi K., Kamisuki S., Matsunaga H., Iwamoto M., Kawai F., et al. A novel tricyclic polyketide, vanitaracin A, specifically inhibits the entry of hepatitis B and D viruses by targeting sodium taurocholate cotransporting polypeptide. J Virol. 2015;89:11945–11953. doi: 10.1128/JVI.01855-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Matsunaga H., Kamisuki S., Kaneko M., Yamaguchi Y., Takeuchi T., Watashi K., et al. Isolation and structure of vanitaracin A, a novel anti-hepatitis B virus compound from Talaromyces sp. Bioorg Med Chem Lett. 2015;25:4325–4328. doi: 10.1016/j.bmcl.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 306.Okuyama-Dobashi K., Kasai H., Tanaka T., Yamashita A., Yasumoto J., Chen W., et al. Hepatitis B virus efficiently infects non-adherent hepatoma cells via human sodium taurocholate cotransporting polypeptide. Sci Rep. 2015;5 doi: 10.1038/srep17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhang J., Fu L.L., Tian M., Liu H.Q., Li J.J., Li Y., et al. Design and synthesis of a novel candidate compound NTI-007 targeting sodium taurocholate cotransporting polypeptide [NTCP]–APOA1–HBx–Beclin1-mediated autophagic pathway in HBV therapy. Bioorg Med Chem. 2015;23:976–984. doi: 10.1016/j.bmc.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 308.Nio Y., Akahori Y., Okamura H., Watashi K., Wakita T., Hijikata M. Inhibitory effect of fasiglifam on hepatitis B virus infections through suppression of the sodium taurocholate cotransporting polypeptide. Biochem Biophys Res Commun. 2018;501:820–825. doi: 10.1016/j.bbrc.2018.04.199. [DOI] [PubMed] [Google Scholar]
- 309.Seitz S., Iancu C., Volz T., Mier W., Dandri M., Urban S., et al. A slow maturation process renders hepatitis B virus infectious. Cell Host Microbe. 2016;20:25–35. doi: 10.1016/j.chom.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 310.Koganti R., Memon A., Shukla D. Emerging roles of heparan sulfate proteoglycans in viral pathogenesis. Semin Thromb Hemost. 2021;47:283–294. doi: 10.1055/s-0041-1725068. [DOI] [PubMed] [Google Scholar]
- 311.Fons N.R., Kines R.C., Thompson C.D., Day P.M., Lowy D.R., Schiller J.T. Chondroitin sulfate proteoglycans are de facto cellular receptors for human papillomavirus 16 under high serum conditions. J Virol. 2022;96 doi: 10.1128/jvi.01857-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ray B., Ali I., Jana S., Mukherjee S., Pal S., Ray S., et al. Antiviral strategies using natural source-derived sulfated polysaccharides in the light of the COVID-19 pandemic and major human pathogenic viruses. Viruses. 2022;14:35. doi: 10.3390/v14010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hu Y., Jo H., DeGrado W.F., Wang J. Brilacidin, a COVID-19 drug candidate, demonstrates broad-spectrum antiviral activity against human coronaviruses OC43, 229E, and NL63 through targeting both the virus and the host cell. J Med Virol. 2022;94:2188–2200. doi: 10.1002/jmv.27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jurgeit A., McDowell R., Moese S., Meldrum E., Schwendener R., Greber U.F. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhou Z., Xue Q., Wan Y., Yang Y., Wang J., Hung T. Lysosome-associated membrane glycoprotein 3 is involved in influenza A virus replication in human lung epithelial (A549) cells. Virol J. 2011;8:384. doi: 10.1186/1743-422X-8-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mar K.B., Rinkenberger N.R., Boys I.N., Eitson J.L., McDougal M.B., Richardson R.B., et al. LY6E mediates an evolutionarily conserved enhancement of virus infection by targeting a late entry step. Nat Commun. 2018;9:3603. doi: 10.1038/s41467-018-06000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yi C., Cai C., Cheng Z., Zhao Y., Yang X., Wu Y., et al. Genome-wide CRISPR-Cas9 screening identifies the CYTH2 host gene as a potential therapeutic target of influenza viral infection. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110559. [DOI] [PubMed] [Google Scholar]
- 318.Yuan S., Chu H., Huang J., Zhao X., Ye Z.W., Lai P.M., et al. Viruses harness YxxØ motif to interact with host AP2M1 for replication: a vulnerable broad-spectrum antiviral target. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Klima M., Chalupska D., Różycki B., Humpolickova J., Rezabkova L., Silhan J., et al. Kobuviral non-structural 3A proteins act as molecular harnesses to hijack the host ACBD3 protein. Structure. 2017;25:219–230. doi: 10.1016/j.str.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 320.Heaton Nicholas S., Moshkina N., Fenouil R., Gardner Thomas J., Aguirre S., Shah Priya S., et al. Targeting viral proteostasis limits influenza virus, HIV, and dengue virus infection. Immunity. 2016;44:46–58. doi: 10.1016/j.immuni.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Patrick Reid S., Shurtleff A.C., Costantino J.A., Tritsch S.R., Retterer C., Spurgers K.B., et al. HSPA5 is an essential host factor for Ebola virus infection. Antivir Res. 2014;109:171–174. doi: 10.1016/j.antiviral.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 322.Aviner R., Li K.H., Frydman J., Andino R. Cotranslational prolyl hydroxylation is essential for flavivirus biogenesis. Nature. 2021;596:558–564. doi: 10.1038/s41586-021-03851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Almasy K.M., Davies J.P., Lisy S.M., Tirgar R., Tran S.C., Plate L. Small-molecule endoplasmic reticulum proteostasis regulator acts as a broad-spectrum inhibitor of dengue and Zika virus infections. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2012209118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Mao D., Yan F., Zhang X., Gao G. TMEM106A inhibits enveloped virus release from cell surface. iScience. 2022;25 doi: 10.1016/j.isci.2022.103843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Sutherland M.R., Simon A.Y., Shanina I., Horwitz M.S., Ruf W., Pryzdial E.L.G. Virus envelope tissue factor promotes infection in mice. J Thromb Haemost. 2019;17:482–491. doi: 10.1111/jth.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Richter M., Leuthold M.M., Graf D., Bartenschlager R., Klein C.D. Prodrug activation by a viral protease: evaluating combretastatin peptide hybrids to selectively target infected cells. ACS Med Chem Lett. 2019;10:1115–1121. doi: 10.1021/acsmedchemlett.9b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zeisel M.B., Turek M., Baumert T.F. Tight junctions and viral entry. Future Virol. 2010;5:263–271. [Google Scholar]
- 328.Aliyu I.A., Kumurya A.S., Bala J.A., Yahaya H., Saidu H. Proteomes, kinases and signalling pathways in virus-induced filopodia, as potential antiviral therapeutics targets. Rev Med Virol. 2021;31 doi: 10.1002/rmv.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Xu S.J., Ding D., Zhang X.J., Liu X.Y., Zhan P. Novel targets and strategies in antiviral drug discovery. Acta Pharm Sin. 2022;57:903–916. [Google Scholar]
- 330.Xu S., Ding D., Liu X., Zhan P. Universal strategies and methodologies in broad-spectrum antiviral drug discovery. Acta Pharmacol Sin. 2022;57:1289–1300. [Google Scholar]
- 331.Ghidini A., Clery A., Halloy F., Allain F.H.T., Hall J. RNA-PROTACs: degraders of RNA-binding proteins. Angew Chem Int Ed Engl. 2021;60:3163–3169. doi: 10.1002/anie.202012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Singh A. Artificial intelligence for drug repurposing against infectious diseases. Artif Intell Chem. 2024;2 [Google Scholar]
- 333.Bieniasz P. Repurposing a bacterial immune system to discover antiviral targets. N Engl J Med. 2017;376:1290–1291. doi: 10.1056/NEJMcibr1616057. [DOI] [PubMed] [Google Scholar]
- 334.Dang C.V., Reddy E.P., Shokat K.M., Soucek L. Drugging the 'undruggable' cancer targets. Nat Rev Cancer. 2017;17:502–508. doi: 10.1038/nrc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Banik S.M., Pedram K., Wisnovsky S., Ahn G., Riley N.M., Bertozzi C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature. 2020;584:291–297. doi: 10.1038/s41586-020-2545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Neklesa T.K., Winkler J.D., Crews C.M. Targeted protein degradation by PROTACs. Pharmacol Ther. 2017;174:138–144. doi: 10.1016/j.pharmthera.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 337.Huang A., Garraway L.A., Ashworth A., Weber B. Synthetic lethality as an engine for cancer drug target discovery. Nat Rev Drug Discov. 2020;19:23–38. doi: 10.1038/s41573-019-0046-z. [DOI] [PubMed] [Google Scholar]
- 338.Lundstrom K. Are viral vectors any good for RNAi antiviral therapy?. Viruses. 2020;12:1189. doi: 10.3390/v12101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Bloom K., Mussolino C., Arbuthnot P. Transcription activator-like effector (TALE) nucleases and repressor TALEs for antiviral gene therapy. Curr Stem Cell Rep. 2015;1:1–8. [Google Scholar]
- 340.Abbott T.R., Dhamdhere G., Liu Y., Lin X., Goudy L., Zeng L., et al. Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell. 2020;181:865–876. doi: 10.1016/j.cell.2020.04.020. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Xu S., Ding D., Zhang X., Sun L., Kang D., Huang B., et al. Newly emerging strategies in antiviral drug discovery: dedicated to Prof. Dr. Erik De Clercq on occasion of his 80th anniversary. Molecules. 2022;27:850. doi: 10.3390/molecules27030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Hengphasatporn K., Plaimas K., Suratanee A., Wongsriphisant P., Yang J.M., Shigeta Y., et al. Target identification using homopharma and network-based methods for predicting compounds against dengue virus-infected cells. Molecules. 2020;25:1883. doi: 10.3390/molecules25081883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Qureshi A., Thakur N., Tandon H., Kumar M. AVPdb: a database of experimentally validated antiviral peptides targeting medically important viruses. Nucleic Acids Res. 2014;42:D1147–D1153. doi: 10.1093/nar/gkt1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Thakur A., Kumar M. AntiVIRmiR: a repository of host antiviral miRNAs and their expression along with experimentally validated viral miRNAs and their targets. Front Genet. 2022;13 doi: 10.3389/fgene.2022.971852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.van der Schaar H.M., van der Linden L., Lanke K.H.W., Strating J.R.P.M., Puerstinger G., de Vries E., et al. Coxsackievirus mutants that can bypass host factor PI4KIIIβ and the need for high levels of PI4P lipids for replication. Cell Res. 2012;22:1576–1592. doi: 10.1038/cr.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.