Abstract
Human respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infections in infants and young children, as well as an important cause of respiratory tract infections in immunocompromised patients and the elderly, which poses a significant economic and social burden worldwide. In recent years, substantial progress has been made in understanding the structure and function of RSV proteins and the interactions between RSV with host factors which is helpful to the discovery of new therapeutic targets and the development of novel interventions. Although two vaccines and two monoclonal antibodies for RSV prevention have been approved, the antiviral treatment remains an unmet clinical need. In this review, we summarize the structure, protein functional properties, and pathological mechanisms of RSV and the current status of RSV drug development. In addition, remaining challenges and innovative ideas for RSV prevention and treatment have also been highlighted.
Key words: Respiratory syncytial virus (RSV), Infection, Therapeutic targets, Antivirals, Drug development, Viral entry, Viral replication, New strategies
Graphical abstract
This review summarizes the structure, protein functional properties, and pathological mechanisms of respiratory syncytial virus (RSV) and the current status of RSV drug development.
1. Introduction
In 1955, the Walter Reed Army Institute of Research isolated respiratory syncytial virus (RSV) for the first time from chimpanzees with respiratory diseases1. In the following years, RSV was also isolated from infants with severe lower respiratory disease2. Since then, RSV has been recognized as a major cause of lower respiratory tract infection in infants and young children, as well as an important cause of respiratory tract infection in immunodeficient patients and elderly people. In 2019, there were 3.33 million cases of RSV-associated acute lower respiratory tract infection and 26,300 deaths among children aged 0–60 months globally3. RSV disease generates an alarming proportion of overall deaths globally, with 1 in 50 deaths among children aged 0–60 months and about 14,000 deaths each year among people aged 65 and older attributable to RSV. Compared with other respiratory viruses, natural infection with RSV does not induce lasting immunity, and repeated infection with the same subtype of RSV is prone to occur.
RSV is the only member of the genus conformal pneumonitis of the pneumovirus family that can infect humans. The viral particles of RSV range from 100 to 1000 nm in diameter4, have a filamentous envelope, and contain a single strand of negative-sense RNA genome (about 15.2 kb in length) encoding a total of 11 viral proteins5. Similar to influenza viruses, RSV is spread by aerosolized droplets and close contact, with an incubation period of 3–8 days6. After infection, RSV replicates within the nasopharynx and the epithelium of upper respiratory tract and even spreads to the small bronchioles or alveoli of the lower respiratory tract. RSV can cause neutrophil-intensive inflammation of the airway during both upper and lower respiratory tract infections7 and elevate the level of eosinophils in the airways8, which results in airway obstruction and bronchial smooth muscle spasm. In addition, Infants and young children are prone to hyperreactivity after RSV infection, which is closely related to the occurrence of repeated wheezing and asthma.
Ribavirin spray and Synagis (pavizumab) were approved by the FDA in 1986 and 1998, respectively. RSV vaccine, Arexvy, ABRYSVO and mRESVIA have been approved by the US FDA in May 2023, June 2023 and May 2024, respectively, which are limited to the prevention of lower respiratory diseases caused by RSV infection in adults over 60 years. In addition, the second monoclonal antibody for the prevention of RSV disease in infants, Beyfortus (nirsevimab) has been approved by the FDA in June 2023.
In this review, we describe the structure, protein functional properties, and pathological mechanisms of RSV to inform new approaches for the development of prevention and treatment of RSV. Further, we focus on the latest progress in the fight against RSV infection, including the development of small-molecule inhibitors, monoclonal antibodies, and nucleic acid drugs, while also discussing the unresolved problems. In addition, this review highlights innovative ideas for RSV prevention and treatment, including engineered cells, novel antiviral nanomaterials, and fusion biomacromolecules. Finally, the prospect of future RSV research and clinical intervention is prospected.
2. Overview of RSV
2.1. The virion, genome and typing
RSV particles are composed of spiral nucleocapsid and amorphous envelope. From the inside to the outside, there are nucleocapsid, matrix proteins, and lipid envelope respectively. There are transmembrane proteins glycoprotein (G), fusion protein (F), and small hydrophobic protein (SH) on the lipid envelope. The nonglycosylated matrix M protein is present on the inner face of the envelope while the second non-glycosylated matrix protein (M2-1) is inside9. Nucleocapsid proteins include nucleoprotein (N), phosphoprotein (P), and catalytic large subunit (L) (Fig. 1A10).
Figure 1.
Overview RSV. (A) Virion. (B) Genome. (C) Course of infection. (Adapted with permission from Fig. 1 and Box 1 in Ref. 10).
The RSV genome is about 15.2 kb in length and contains 10 genes encoding 11 proteins (Fig. 1B). From 3′- end to 5′- end, there are nonstructural proteins (NS1 and NS2), N, P, M, N, SH, G, F, M2 and L11. With two separate open reading frames that overlap slightly, the M2 encodes the transcription processing factor (M2-1) and transcription regulatory factor (M2-2) proteins. In addition, NS1 at the 3′- end is preceded by a 44-nucleotide specific leader region, while the L gene at the 5′- end is followed by a 155-nucleotide epigenetic region. The replication of RSV is error-prone and has no proofreading mechanism, which allows RSV to rapidly generate single nucleotide polymorphisms and mutations that alter viral virulence, resulting in resistance escape. RSV is divided into two subgroups, A and B12. RSV A and B subpopulations are evolutionary lineages that diverged about 350 years ago and have considerable genotypic variation within them. The main difference was found on the attached glycoprotein G, which conserved only 53% of the amino acid sequence between strains. As for the most common RSV genotypes, 14 genotypes have been identified in A strains (GA 1–713,14, SAA 115, NA 1–416, and ON 1–217,18), and 20 genotypes have been identified in B strains (GB 1–413, SAB 1–415, URU 1–219, and BA 1–1020,21). The A and B strains are common in RSV epidemic season, but the dominant subgroup or genotype varies slightly from year to year and is constantly alternating22.
2.2. Life cycle of RSV
The infection cycle of RSV (Fig. 1C) begins with the attachment of viral particles to the apical surface of ciliated airway epithelial cells. RSV relies on the G protein to attach to the cell surface while the F protein can also interact with immobilized heparin or cellular heparin sulfate to promote attachment23. Then the F protein promotes the fusion of the viral envelope and the cell membrane. After the fusion of viral and cell membranes, the RNP (ribonucleoprotein) complex is released into the host cell cytoplasm, where the viral genome replicates and transcribes, forming inclusion bodies24. These IBs serve as scaffolds formed through RNA and N protein interactions, corresponding to liquid–liquid phase separation compartments that concentrate and enhance the enzyme activity by bringing all components of the polymerase complex together. This condensate can also sequester immune-stimulatory proteins, shielding the viral replication machinery from detection by innate immune sensors25.
Viral mRNA, produced through a discontinuous RNA synthesis mechanism, briefly condenses within IBAGs (inclusion body-associated granules) before being translated into proteins in the cytoplasm. Subsequently, RSV viral particles assemble near the plasma membrane, and the M protein, transported through the Golgi complex secretory pathway along with the F and G proteins, is believed to recruit budding initiation.
Finally, viral particles are released as filamentous particles approximately 130 nm in diameter and ranging from 0.5 to 12 μm in length26. In addition to producing viruses, cells infected with RSV express a significant amount of the F protein, which has been shown to fuse with adjacent cell membranes, leading to the formation of large multinucleated cells. This tendency to form syncytia is one of the most prominent features of RSV infection and contributes to the pathogenic effects on cells.
3. Therapeutics strategy for RSV attachment and entry
RSV G glycoprotein facilitates RSV's attachment through interaction with either CX3C chemokine receptor 1 (CX3CR1) or heparan sulfate proteoglycans (HSPGs)27,28. Subsequent viral entry is mediated by the F glycoprotein. The F glycoprotein undergoes dramatic conformational changes and interacts with nucleoprotein, epidermal growth factor receptor (EGFR), insulin-like growth factor-1 receptor (IGF1R), and intercellular adhesion molecule-1 (ICAM-1)29, driving the fusion of the viral envelope with the host cell membrane10. The F and G play an important role in RSV attachment and entry into the host cells and consequently serve as attractive targets for developing RSV entry inhibitors.
3.1. Targeting G protein
G protein has attracted increasing attention in the treatment of RSV after infection and has become an attractive target30. The replication cycle of RSV begins with G protein-mediated attachment to the apical surface of polarized ciliated airway epithelial cells31, which is facilitated by binding of the G protein CX3C motif to CX3CR1 and other potentially cell surface molecules27,32, 33, 34. The G protein is highly variable among RSV isolates, and therefore its sequence has been used in epidemiological and evolutionary studies. This variability, primarily localized to the mucin-like domains, determines the RSV subgroups (RSV A and RSV B)35.
The expression and glycosylation of G protein were changed when RSV infected different cell lines36,37, and most of the changes were related to the cell specific glycosylation of G protein38. When RSV propagated in primary culture, the molecular weight of G protein was ∼170 kDa, while in HEp-2 cells, the MV of G protein was ∼90 kDa39. In particular, when propagates in Vero cells, the molecular weight of G protein is ∼55 kDa36. Modifications of these G proteins affect RSV infection, permissibility, and host response to infection. Therefore, the type of cell used for RSV propagation is important because host cells vary in their sensitivity to different RSV strains or in their response to infection.
G proteins exist in two forms: the full-length membrane-bound form (mG)40, which is responsible for viral attachment, and the secretory isomer (sG)41, which mediates immune escape. Produced by the expression of the full-length G gene, mG is a type II glycosylated complete membrane protein composed of about 300 amino acids42 (Fig. 2A10). The N-terminal cytoplasmic domain contains a cysteine residue adjacent to the transmembrane (TM) domain, which is palmitoylated after translation (Fig. 2B). The outer domain is located outside the cell and consists of an unglycosylated central conserved domain (CCD) and a heparin-binding domain (HBD), flanked by two highly variable and highly glycosylated mucin-like domains. HBD contains positively charged residues that have been hypothesized to bind to negatively charged heparin sulfate (HS) and other glycosaminoglycans on the surface of host cells. This dependence on glycosaminoglycan-initiated infection does not generalize infection with wild-type RSV because HS is not present on the surface of human ciliated airway epithelial cells and infection still occurs primarily through specific receptor binding43. CCD contains a CX3C chemokine motif (amino acids 182–186) that promotes RSV attachment to susceptible cells carrying the CX3C chemokine receptor CX3CR127.
Figure 2.
Structure of RSV G glycoprotein. (A) Full-length and Soluble RSV G glycoprotein. (B) Domain and post-translational modification of full-length RSV G glycoprotein. (C) Mature secreted RSV glycoprotein G. (Adapted with permission from Fig. 2 in Ref. 10).
The simulation of CX3C by G protein promotes RSV infection44,45 and may alter the chemotaxis of CX3CL1 (fractalkine) in leukocytes46. G protein expression during RSV infection in mice has also been shown to substantially stop NK1.1+ natural killer, CD11b+, and RB6-8C5+ polymorphonuclear cells from trafficking to the lung47. In addition, G proteins appear to be involved in impairment of the Th1 response, reducing the production of Th1 cytokines, particularly IFN-γ48, inhibiting the mRNA expression of several chemokines, including macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, monocyte chemoattractant protein, and IFN-inducer protein 1049. These studies suggest that G protein regulates the immune response of infected hosts50.
sG begins translation at Met48 of the G gene, cleaved at the amino acid position 66, and the cytoplasmic domain and part of the TM domain are removed, allowing ectodomain to be secreted from infected cells (Fig. 2C). sG, which is produced 6 h after virus attachment and accounts for 80% of the total G protein, has most of the neutralizing epitopes on mG, so that sG in the oligomerized state is still able to bind to glycosaminoglycans on the cell surface51. sG maintains the same characteristics as mG in terms of glycosylation and antibody reactivity, acts as an antigenic decoy, binds neutralizing antibodies in the body to resist the immune response52, and reduces the Fc-mediated antiviral activity of white blood cells53.
G-specific antibodies have been shown to induce antibody-dependent cytotoxicity and phagocytosis of RSV-infected cells, have a wide range of functions, and play an important role in anti-RSV34. Rare high-affinity antibodies against the cysteine lasso region of the G protein, such as CB017.5 and CB002.5, have been screened from human B cells. These two antibodies potently neutralize both RSV A and B genotypes, increase IFN-γ levels in bronchoalveolar lavage, and reduce airway hyperresponsiveness and eosinophilic lung infiltration in mouse models of primary and secondary infection54,55. 131-2G, also binds to the central conserved domain of the G protein, is a prominent anti-G protein mab that inhibits the binding and attachment of RSV G protein to host cells and prevents G-protein-mediated chemotaxis, down-regulates virus-induced host inflammatory responses, and promotes viral clearance through antibody-dependent cell-mediated cytotoxicity (ADCC)56. Treatment of RSV-infected BALB/c mice with 131-2G reduced viral titers and leukocyte infiltration57. Compared with anti-F protein mab (143-6C), mice infected with RSV and treated with 131-2G showed more rapid reductions in all evaluated disease measures, including weight loss, number and type of inflammatory cells in the lungs, airway resistance, and mucus production58.
MBX-300 is a negatively charged sulfate lipid with four sulfate groups that targets the RSV G protein and inhibits virus adsorption by inhibiting electrostatic binding59. MBX-300 has been shown to exhibit lower cytotoxicity than Ribavirin in both cultured cells and cotton rat models, and to be more potent against both laboratory strains and clinical isolates. Additionally, MBX-300 also exhibits inhibitory activity against several other viruses, including human metapneumovirus, human rotavirus, human immunodeficiency virus 1, and adenovirus60, 61, 62, 63.
Fujikane et al.64 reported that Ephedrae Herba (EH) and Cinnamomi Cortex (CC), components of Mato, exhibit anti-RSV activity. EH and CC specifically interacted with the central conserved domain (CCD) of the G protein, thereby blocking virus adsorption to the cellular receptor CX3CR1. Genetic mutation of the CX3C motif on the CCD of the G protein reduced the binding ability to EH and CC, suggesting that the CX3C motif is a target of EH and CC. Oral administration of Mato to mice infected with RSV for 5 days resulted in a significant reduction in pulmonary virus titers.
3.2. Targeting F protein
F protein is critical to the entry process of RSV, promoting pH-independent fusion of the viral membrane with the plasma membrane of the host cell, leading to the infection of the host cell65. RSV F protein, a class I fusion glycoprotein, is synthesized as an inactive precursor polypeptide (F0) of 574 amino acids. It contains signal peptide (residues 2–20), signal peptide cleavage site (residues 21–25), F2 region (residues 26–109), peptide cleavage region (residues 110–136), and F1 region (residues 137–574). F1 consists of two heptapeptide repeats: HRA (residues 149–206) and HRB (residues 474–523)66 (Fig. 3A).
Figure 3.
Schematic illustration of the proposed conformational changes during RSV infection for F protein. (A) Domains of full-length RSV F glycoprotein. (B) F-glycoprotein maturation. Initial state: three loose monomers (including pep27) constitute F protein; Furin enzymatic hydrolysis: The action of furan-like protease to remove pep27; Prefusion conformation: three monomers compact together to form a trimer. The 3D structure on the left shows the prefusion and post-fusion trimers. (C) Fusion process between RSV and host cell membrane mediated by RSV F glycoprotein. ① Initial status. ② The pre-fusion state. FP is buried in the F protein. ③ RSV virus particle attachment and semi-fusion: F protein changes conformation and forms long HRA helix. FP is exposed and inserted into the host cell membrane, forming a helical trimer with the three HRA domains, resulting in a semi-fusion between the host cell and the viral membrane. ④ Complete fusion: The HRB helix rearranges and combines with the HRA trimer to form a six-helix bundle, which pulls the cell membrane and the virus membrane to complete fusion. (Adapted with permission from Fig. 1 in Ref. 66).
F0 is cleaved by Furin-like proteases on the trans-Golgi network at sites 109 and 136 to produce three subunits of F2, pep27, and F1. The F2 and F1 subunits are covalently linked by two disulfide bonds to form a heterodimer, and the mature active F protein folds into a compact prefused conformation as a trimer of the F1–F2 heterodimer67 (Fig. 3B). The prefused conformation is a metastable structure that occurs spontaneously on virions and cell surfaces and can be thermally stimulated4,68. During the refolding process, the F1 subunit n-terminal hydrophobic fusion peptide is pulled out of the central cavity of the pre-fused trimer and inserted into the host cell membrane69. After pre-fusion is triggered, a huge conformational change in the F protein is initiated, and the membrane fusion process begins (Fig. 3C). When heptapeptide repeats near the fusion peptide (HRA) bind to those near the viral transmembrane region (HRB), a pre-hairpin intermediate is produced and then further refolded. This folding is irreversible, resulting in the formation of an extremely stable six-helical bundle, which is characteristic of the conformation after RSV F fusion, a process that brings the viral envelope and the host cell membrane closer together to bind70,71. The expression of RSV F on the cell surface of infected cells can also lead to fusion with neighboring cells to form a multinucleated syncytium. The triple symmetric pocket in the central cavity of RSV F is the binding target of most F protein inhibitors, and inhibitor locking to the metastable pre-fusion conformation of RSV F will make membrane fusion difficult to initiate. Unlike G proteins, F proteins are relatively conserved, with a 5% difference between RSV A and RSV B. Therefore, the development of F protein inhibitors makes it easier to defend against immune mutations.
Small molecule inhibitors of F protein interfere with the transition of RSV F protein from the prefusion conformation to the postfusion conformation, block the formation of the fusion-active structure, and stop RSV from entering cells, thereby exerting antiviral activity.
GS-5806 (presatovir) selectively inhibits 75 clinical isolates of RSV A and B subtypes (EC50 = 0.43 nmol/L). The compound maintained potency in primary human airway epithelial cells and exhibited low cytotoxicity in human cell lines and primary cell cultures (selectivity >23,000-fold)72. In healthy adults infected with RSV, treatment with GS-5806 significantly reduced viral load, total mucus produced, and total symptom score73. The bis-substituted benzimidazole derivative BMS-233675 is a potent RSV fusion inhibitor targeting F protein with EC50 and CC50 values of 0.34 and 84 μmol/L, respectively74. After a series of modifications, BMS-433771, with better oral availability and higher anti-RSV activity (average EC50 = 20 nmol/L) was obtained75. Animal experiments showed that the compound was effective in reducing virus titers in the lungs of cotton rats and BALB/c mice when administered 1 h before RSV inoculation76. Through some subtle modifications of BMS-433771, JNJ-53718678 was developed by Jansen Pharmaceuticals. Oral treatment of neonatal lambs with JNJ-53718678 effectively inhibits established acute lower respiratory tract infection in the animals77. In healthy adults, JNJ-53718678 treatment significantly reduced viral load and disease severity78. The clinical trial of JNJ-53718678 in adult and adolescent participants, infected by RSV, who had undergone hematopoietic stem-cell transplantation was terminated for strategic reasons, not because of safety concerns (NCT04332523). Zheng et al. reported an RSV F protein inhibitor, Ziresovir (RO-0529, AK0529). In cell experiments, the EC50 of Ziresovir reached the low nM level for both laboratory and clinically isolated RSV strains. In BALB/c mouse model of RSV infection treated with Ziresovir, viral load was reduced more than one log. Ziresovir has moved to clinical development due to its favorable and balanced preclinical profile of antiviral, DMPK, and toxicological properties79. A study assessing the safety, tolerability, and pharmacokinetics of Ziresovir in healthy subjects was completed in 2022, but the findings are not yet available (NCT04788017). In a phase 3, multicenter, double-blind, randomized, placebo-controlled trial conducted in China, Ziresovir treatment reduced signs and symptoms of bronchiolitis in infants and young children hospitalized with RSV infection and no safety concerns were identified (NCT04231968)80. In a screening of 130,000 compounds, Johnson & Johnson found JNJ-2408068 (R170591)81,82. Although JNJ-2408068 was highly effective in both tissue culture and lung testing in cotton rats, it was found to have long tissue retention and was therefore not suitable for further development as an antiviral compound83,84. After structural optimization, TMC353121 was obtained, which retained the potency of JNJ-2408068, but eliminated its disadvantage of long-term tissue retention. TMC353121 inhibits both virus–cell and cell–cell fusion and could be added as late as 15 h postinfection and still inhibit syncytia formation by 50%85. In HeLa/M cells, RSV replication was effectively inhibited when administered 3 h after infection, and 50% syncytium formation was inhibited even when administered 15 h after infection86. However, pharmacokinetic and pharmacodynamic studies of TMC353121 in RSV-infected cotton rats found that the effective dose of TMC353121 was more than 2000 times higher than the dose required for cultured cells (0.07 ng/mL), partly due to loss of activity caused by binding to serum proteins87. One active biphenyl analogue, CL387626, was discovered by researchers at Wyeth-Ayerst Research. It was effective in inhibiting RSV infection with an IC50 of 0.05 μmol/L88. A single 30 mg/kg dose of CL387626 administered intranasally 4 or 5 days prior to virus challenge, significantly inhibited pulmonary replication of RSV89. Modification of the chemical structure of CL-387626 led to the discovery of RFI-641, which was more effective than CL-387626 in protecting against RSV in different animal models, including African green monkeys, BALB/c mice, and cotton rats90, 91, 92. However, neither CL-387626 nor RFI-641 was further developed. Using a drug repurposing strategy, lonafarnib, a licensed farnesyltransferase inhibitor, and phase III candidate for hepatitis delta virus (HDV) therapy, was identified as an RSV fusion protein inhibitor. Surface plasmon resonance reveals the binding of lonafarnib to RSV fusion protein, and co-crystallography identifies the lonafarnib binding site within RSV F. Oral administration of lonafarnib dose-dependently reduces RSV virus load in a murine infection model using female mice. However, lonafarnib's efficacy is lower compared with the above-mentioned clinical stage inhibitors. Furthermore, lonafarnib also inhibits farnesyltransferases and may therefore have unwanted side effects, particularly when administered orally and at high doses93. 3,4-O-Dicaffeoylquinic acid methyl ester (3,4-DCQAME), which can be isolated from several plants of traditional Chinese medicine, has anti-RSV activity. In RSV-infected mice treated with 3,4-DCQAME, reduced RSV-induced pathological changes and substantial inhibition of viral infection in the lung tissue were observed94.
In addition, significant binding of MDT-637 to the F protein occurred only at 37 °C, while at 22 °C, the binding was significantly reduced. The temperature dependence of binding suggests that VP-14637 may interact with the transient conformation of the F protein95. Kim et al.96 evaluated the antiviral effect of MDT-637 against a variety of genetically diverse RSV strains in vitro and compared it with ribavirin. The results showed that MDT-637 had antiviral activity against a variety of clinical RSV strains of different genotypes and branches in vitro, and may have a better clinical effect than ribavirin in the treatment of RSV infection.
Palivizumab is a humanized monoclonal antibody targeting the F protein. In vitro studies demonstrated that palivizumab effectively neutralizes RSV and exhibits a dose-dependent response. Pretreatment of cotton rats with palivizumab resulted in a 99% reduction of lung RSV titers at a dose of 2.5 mg/kg97. A randomized, double-blind, placebo-controlled study showed that prophylactic administration of palivizumab significantly reduced the rate of high-risk hospitalization among children infected with RSV, leading to the FDA approval of palivizumab in June 1998. Given its high cost, it is critical to know whether palivizumab remains effective in preventing severe RSV disease in children, and its clinical use is still being explored98. In addition, modifications of palivizumab resulted in motavizumab with more potent neutralizing activity. Compared with palivizumab, motavizumab binds to RSV F protein 70-fold better than palivizumab and exhibits approximately 20-fold improvement in neutralizing RSV in vitro. In cotton rats, motavizumab reduced pulmonary RSV titers up to a 100-fold lower level than palivizumab did at the same concentration99,100. MEDI8897 (Nirsevimab), a recombinant antibody optimized from D25, was 50-fold more active than palivizumab in neutralizing multiple RSV A and B strains. At similar serum concentrations, prophylactic administration of MEDI8897 was nine-fold more potent than palivizumab at reducing pulmonary viral loads by > 3 logs in cotton rats infected with either RSV A or B subtypes101. MEDI8897 had a 3-amino acid change (M257Y/S259T/T261E YTE) in the highly conserved fragment crystallizable region, which prolonged the antibody's serum half-life102. MEDI8897 has a good safety profile in healthy preterm infants. Its long half-life and good RSV neutralizing activity resulted in sustained resistance to RSV infection for up to 5 months after a single 50 mg intramuscular injection, supporting its use as a cost-effective option to protect infants against RSV with once-per-RSV-season dosing in the clinic103,104. Nirsevimab was approved in the EU on 3 November 2022 and in the UK on 7 November 2022 for the prevention of RSV lower respiratory tract disease in neonates and infants during their first RSV season105. Clesrovimab (MK-1654), a monoclonal antibody targeting F protein with a longer half-life, has the same YTE mutation as nirsevimab. This monoclonal antibody is in ongoing infant phase 2b/3 trials (NCT04767373) and phase 3 trials (NCT04938830). The mab showed high potency against RSV clinical isolates in vitro and was equally potent against RSV subgroup A and B strains106. Clinical trials have shown that Clesrovimab can reduce viral load and incidence of clinical symptoms after viral infection107. A data analysis assessed the association between serum neutralizing antibodies and clinical endpoints; The study estimated that a single dose of 75 mg could confer protection lasting up to 5 months in more than 75% of term infants.
Nanobodies are derived from camelids (llamas, camels, and dromedaries) and are heavy-chain-only antibodies that are structurally distinct from human antibodies. It is of interest that when the VHH fragment, the variable domain of these camel antibodies, is expressed alone, it retains the specific antigen binding capacity. These therapeutic VHH domains are referred to as nanobodies. Based on a variable domain that targets antigenic site II on the RSV F protein, a nanobody named ALX-0171 has been developed for the treatment of RSV. In preclinical studies, ALX-0171 was 126-fold more potent than palvizumab108. It has successfully completed a phase I clinical trial in healthy adults and has good safety and pharmacokinetics.
Anti-RSV drugs inhibiting entry are listed in Table 1.
Table 1.
anti-RSV drugs inhibiting entry.
| Name | Category | Target | Structure | Development |
|---|---|---|---|---|
| MBX-300 | Small-molecule | RSV G | 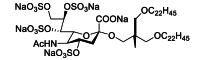 |
Preclinical |
| 131-2G | mAb | RSV G | – | Preclinical |
| GS-5806(presatovir) | Small-molecule | RSV F | 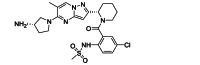 |
Phase 2 |
| MDT-637 | Small-molecule | RSV F |  |
Phase 1 |
| JNJ-53718678 | Small-molecule | RSV F |  |
Phase 2 |
| Ziresovir (AK0529) | Small-molecule | RSV F | 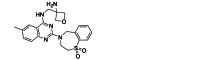 |
Phase 3 |
| TMC353121 | Small-molecule | RSV F | 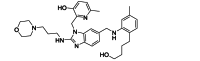 |
Preclinical |
| CL387626 | Small-molecule | RSV F | Preclinical | |
| RFI-641 | Small-molecule | RSV F | 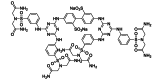 |
Preclinical |
| BMS-233675 | Small-molecule | RSV F | Preclinical | |
| BMS-433771 | Small-molecule | RSV F | 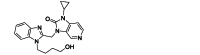 |
Preclinical |
| Lonafarnib | Small-molecule | RSV F | 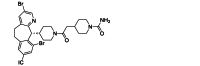 |
Preclinical |
| Palvizumab | mAb | RSV F | – | Accepted in 1998 |
| MEDI8897(Nirsevimab) | mAb | RSV F | – | Accepted in 2022 |
| Clesrovimab (MK-1654) | mAb | RSV F | – | Phase 3 |
| ALX-0171 | mAb | RSV F | – | Phase 2 |
| Motavizumab | mAb | RSV F | – | Phase 3 |
4. Therapeutics strategy for RSV transcription and replication
RSV RNA synthesis comprises viral transcription and replication that are catalyzed by the large protein (L) in coordination with the phosphoprotein polymerase cofactor (P), the nucleoprotein (N), and the M2-1 transcription factor. These activities are essential for the RSV replicative cycle and are thus considered attractive targets for the development of therapeutic agents.
4.1. Targeting L protein
To ensure transcription and replication, the 250 kDa RSV L protein contains three conserved enzymatic domains: the RNA-dependent RNA polymerase (RdRp) domain, the polyribonucleotidyl transferase (PRNTase/capping domain), and the methyltransferase (MTase) domain, followed by the more variable C-terminal domain. In addition, there is another domain in the middle of these domains: the connector domain. Consequently, the RSV L protein possesses all the enzymatic activities required for catalyzing RNA synthesis, which includes activities involved in 5′-RNA capping, N7 and 2′-O-methylations during cap assembly, and 3′-end polyadenylation of each RNA transcript (Fig. 4). The N-terminal RdRp domain of the RSV L protein adopts a right-handed structure, featuring three conserved regions (CR_I, CR_II, and CR_III) and six conserved sequence motifs (motifs a–f). These motifs participate in RNA template regulation, facilitate nucleotide entry, and ensure flexibility of the polymerase109. Motif B harbors a conserved proline-rich sequence, containing most escape mutations for inhibitors, contributing to RSV RdRp's ability to discriminate between natural NTPs and NTP analogs as inhibitors110. The synthesis of RSV RNA requires binding with the tetrameric P protein, which connects the L protein to the encapsulated viral genome. Notably, efficient RNA transcription also requires M2-1111,112.
Figure 4.
Schematic illustration of the proposed mechanisms for replication and transcription initiation for the respiratory Syncytial virus L protein.
Recent low-temperature electron microscopy structures have elucidated the interaction between RSV L and the tetrameric P protein, revealing a tentacle-like arrangement of P, with each monomer adopting a specific conformation113. In addition to the interaction between the P and L proteins, the low-temperature electron microscopy structures provide insights into the structural interaction between the RdRp and capping domains of RSV L protein, indicating a close intertwining of these two domains. This feature demonstrates how the initiation of RNA synthesis by the polymerase is regulated by the capping domain. The structural organization of the L protein provides a framework for understanding the molecular basis of RSV replication and transcription and should facilitate the design of RSV inhibitors.
The compounds targeting the L protein could be mainly categorized as nucleoside inhibitors and non-nucleoside inhibitors. Nucleoside analogues directly inhibit the RdRp enzyme activity of L protein. ALS-8176 is a nucleoside RSV L protein inhibitor with good oral bioavailability and demonstrated excellent anti-RSV efficacy and safety in a phase 2 clinical RSV infection study114. ALS-8176 is a prodrug of ALS-8112. ALS-8112 inhibits all RSV strains in vitro, and its antiviral effect is mediated by the intracellular formation of a 5′-triphosphate metabolite (ALS-8112-TP) that inhibits viral RNA polymerase110. In an RSV infection study, faster RSV clearance and a greater reduction in viral load were observed in the ALS-8176-treated group than placebo group, accompanied by improvements in clinical disease severity115. Remdesivir is a nucleoside analogue prodrug developed by Gilead Sciences, which has been approved for the treatment of COVID-19. Remdesivir has been reported to specifically inhibit RSV RdRp but not human RNA Pol-II in vitro116. In the African green monkey RSV model, 10 mg/kg was administered intravenously once daily, showing a reduction of two orders of magnitude of peak pulmonary viral load. Similarly, approved for the treatment of COVID-19, Molnupiravir is a prodrug of N4-hydroxycytidine (NHC), which was identified by Yoon et al.117 as a potent inhibitor of RSV. NHC is a ribonucleoside analogue with high bioavailability and is well tolerated. Mechanistic studies have shown that NHC is efficiently converted to the active NHC-TP form in disease-associated respiratory tissues, which competes with cytosine ribonucleotides for binding to guanine ribonucleotides and interferes with RNA replication, leading to high viral mutation rates and lethality. In a mouse model, oral administration of this compound was effective against RSV infection, reducing pulmonary viral load and mitigating disease biomarkers117. In addition, there are other nucleoside analogues involved in the inhibition of RSV replication. Ribavirin is a nucleoside agent with activity against specific members of the Paramyxoviridae family and exerts antiviral effects by inhibiting inosine monophosphate dehydrogenase and mutagenizing RNA118. Treatment of RSV infection with Ribavirin resulted in faster clearance of RSV and shorter hospital stay119,120. Inhaled (aerosolized) Ribavirin is approved by the Food and Drug Administration (FDA) for the treatment of infants and children with RSV pneumonia and for the treatment of severe RSV infection in adults121. Oral administration of Ribavirin has not been approved by the FDA for the treatment of RSV infection. Marcelin et al.122 studied oral administration of Ribavirin for RSV infection and found that was a well-tolerated option for RSV infection in moderately to severely immunocompromised patients. But as with other RSV-targeting antiviral agents, resistance to Ribavirin is a concern, which limits its use. Sourimant et al.123 reported that 4′-fluorouridine, a ribonucleoside analogue, exerts an anti-RSV effect by delaying polymerase arrest. The compound showed potent dose-dependent activity against all RSV strains tested, with EC50 values ranging from 0.61 to 1.2 μmol/L. When mice and ferrets were treated within 24 h of RSV infection, once daily oral administration resulted in a significant reduction in viral load in the lungs123.
In addition to nucleoside analogues, RSV drug discovery has also focused on the identification of non-nucleoside inhibitors of L protein. Binding to the connector domain of RSV L, PC786 is a non-nucleoside RSV L protein inhibitor with potent and selective antiviral activity against laboratory or clinical isolates of RSV A and RSV B. Late therapeutic intervention with PC786 rapidly reduced viral load in human respiratory epithelial cells124. In addition, PC786 reduced the level of the inflammatory marker RANTES (mRNA) in RSV-infected fully differentiated human airway epithelial cells, suggesting that PC786 treatment may inhibit virus-induced inflammation125. AZ-27 showed antiviral activity against both A and B RSV types, and no cytotoxicity was detected126. It inhibits the early stages of mRNA transcription, as well as genome replication, by inhibiting the initiation of RNA synthesis at the promoter127. Fordyce et al.128 produced a series of new highly potent RSV inhibitors by replacing cyclopropylamide, a key pharmacophore of AZ-27, with a heteroaromatic group. One of these compounds named 4a has low nanomolar activity against both RSV A and B. In addition, the compound was effective in inhibiting viral replication when administered nasally in rodent models of RSV infection128. Currently, neither AZ-27 nor compound 4a has entered clinical trials. Besides the connector domain, non-nucleoside inhibitors can also target the capping domain. Yu et al.129 reported a non-nucleoside inhibitor JNJ-8003 with sub-nanomolar inhibition potency in both antiviral and polymerase assays. Cryo-EM structure revealed that JNJ-8003 binds to an induced-fit pocket on the capping domain, with multiple interactions consistent with its tight binding and resistance mutation profile129. In addition, DZ7487 is a novel oral nonnucleoside inhibitor specifically designed to target the RSV L protein. Due to its size and structural complexity, it is not yet possible to directly measure DZ7487 binding to L protein RdRp domain. However, the fact that N363T mutation located on the RdRp domain is by far the dominant resistance mutation suggests that DZ7487 may target the RdRp domain. DZ7487 effectively inhibited virus replication in RSV A and B clinical isolates. DZ7487 showed superior efficacy over the nucleoside analogue ALS-8112 in lower airway cells. In BALB/c mice and cotton rats infected with RSV, it effectively inhibited virus replication, reduced viral load in the lungs after infection, and showed significant pathological improvement. Pre-endpoint assessments are currently being conducted before human clinical studies130.
4.2. Targeting N protein
The N protein in the pneumovirus is highly conserved. RSV N protein closely binds to its genome to form ribonucleoprotein complex (RNP), which on the one hand serves to stabilize RSV genome and antisense genome without being degraded by host RNase. On the other hand, it reduces pattern recognition receptors in the cytoplasm of the host such as retinoic acid-inducible gene-I and melanoma differentiation-associated gene5 to evade the host's natural immune response131. In addition, its sequence is highly conserved in all known RSV clades. Therefore, it is a viable option to use it as a target for anti-RSV drug development.
EDP-938 is an RSV N protein inhibitor that works at a post-entry, replication step of the viral life cycle. Human trials have shown that this antiviral drug is rapidly absorbed after oral administration, with a half-life between 11 and 18 h132. Oral doses of up to 600 mg/day or 300 mg/day twice daily for 7 days resulted in a significant decrease in viral load in the lower respiratory tract. EDP-938 was superior to placebo in reducing viral load, total symptoms score, and mucus weight, and it was associated with almost no apparent adverse effects133.
Arrow Therapeutics identified several possible anti-RSV viral compounds, including 1,4 benzodiazepines, among the 20,000 compounds tested in viral neutralization and ELISA assays134, while RSV604 was obtained by structural optimization of these compounds135. It is potent against all clinical isolates of both types A and B, with a low resistance rate in vitro. Sequencing revealed that the resistant virus had mutations in the nucleocapsid protein. In 3D human airway epithelial cell models, RSV604 was able to efficiently penetrate from the basolateral side of the epithelium after mucosal administration and inhibit viral replication136. Mechanistic studies revealed that RSV604 inhibits RSV replication by inhibiting the N protein, and RSV604 did not inhibit viral RNA synthesis in RSV subgenomic replicon cells or RNP-free assays, suggesting that it may act prior to viral replication complex formation137.
4.3. Inhibiting RSV protein translation
The siRNAs are typically 21–23 nucleotide-long double-stranded RNA fragments that interfere with the expression of specific target genes with complementary nucleotide sequences by degrading their mRNA. ALN-RSV01, a siRNA targeting N protein mRNA developed by Alnylam Pharmaceuticals, has significant antiviral activity in mouse models of RSV infection and is potent against both RSV A and B subtypes in vitro138. The phase I clinical study of ALN-RSV01 proved successful with minimal side effects. A phase II trial was also successful, in which ALN-RSV01 was administered daily in the form of nasal spray to healthy adults for two days before and three days after RSV infection, showing the antiviral efficacy of ALN-RSV01 by showing a significantly lower number of RSV infections in ALN-rSV01-treated subjects compared with placebo139.
Deoxyribozymes (Dzs), also known as DNAzymes or DNA enzymes, are synthetic catalytic single-stranded deoxyribonucleic acid molecules with precise substrate recognition ability to cleave sequence-specific mRNA molecules and greater biological stability140. DZ604 is the first reported anti-RSV DNAzyme targeting the initiation signal of the NS2 gene. However, this DNAzyme only reduced virus yield by 85.56% and did not protect RSV-infected cells from cytopathic effect at a concentration of 5 μmol/L. One explanation for the lack of protection of infected cells treated by NS2 targeting DNAzyme could be that the NS2 protein, as a nonstructural protein, is not important for RSV replication141. DZn1133 is also one of them, directly cleaving the initial signal site of the N gene in RSV genomic RNA, thereby reducing the expression of all viral proteins. Among them, the decreased expression of F protein, which is the core of pathological cell fusion activity, and the decreased expression of N protein, which protects viral RNA, maybe the main reasons for its anti-RSV effect. In vitro cell experiments, more than 90% of RSV-infected HEp-2 cells treated by DZn1133 at a concentration of 8 μmol/L were protected from cytopathic effects. In addition, 10 wild strains of RSV, including subgroups A and B, isolated from the patient were significantly inhibited by DZn1133, which had higher anti-RSV activity than ribavirin142.
Antisense oligonucleotides (ASOs) are small synthetic pieces of single-stranded DNA, typically 15 to 30 nucleotides in length. ASOs bind specifically to complementary DNA/RNA sequences by Watson–Crick hybridization. Once bound to the target RNA, ASOs can inhibit the translational process by inducing cleavage machinery or inhibiting mRNA function. Jairath et al. investigated the use of oligodeoxyribonucleotides to inhibit RSV replication in cell culture. Jairath et al.143 investigated the inhibition of RSV replication in cell culture by oligodeoxyribonucleotides. Human epithelial type 2 (HEp-2) cells were infected with RSV A2 strain and treated with the designed oligonucleotide. ELISA and PT-PCR analyses showed that all oligonucleotides inhibited viral antigen production and were more effective than ribavirin. Reverse transcriptase polymerase chain reaction revealed specific deletion of genomic RNA target sequences and detected specific cleavage of genomic target RNA after treating cells with antisense oligonucleotides. These results suggest that antisense oligonucleotides targeting RSV genomic RNA are effective in inhibiting RSV replication and may have therapeutic value143.
Anti-RSV drugs inhibiting propagation are listed in Table 2.
Table 2.
Anti-RSV drugs inhibiting propagation.
| Name | Category | Target | Structure | Development |
|---|---|---|---|---|
| EDP938 | Small-molecule | RSV N |  |
Phase 2 |
| RSV604 | Small-molecule | RSV N | Preclinical | |
| ALS-8176 | Small-molecule | RSV L | 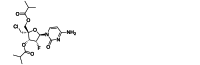 |
Phase 2 |
| Remdesivir | Small-molecule | RSV L | Phase 2 | |
| PC786 | Small-molecule | RSV L | 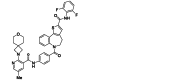 |
Phase 2 |
| AZ-27 | Small-molecule | RSV L |  |
Preclinical |
| JNJ-8003 | Small-molecule | RSV L | 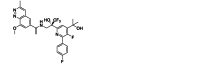 |
Preclinical |
| DZ7487 | Small-molecule | RSV L | Unpublished | Preclinical |
| Ribavirin | Small-molecule | RSV L | 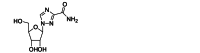 |
Accepted in 1986 |
| Molnupiravir | Small-molecule | RSV L |  |
Phase 2 |
| 4′-Fluorouridine | Small-molecule | RSV L |  |
Preclinical |
| ALN-RSV01 | siRNA | RSV N mRNA | RNA, (CUU GAC UUU GCU AAG AGC CdTdT), complex with RNA (GGC UCU UAG CAA AGU CAA GdTdT) | Phase 2 |
| DZn1133 | Dzs | RSV N mRNA | 5′-TGG GGC AAA GGC TAG CTA CAA CGA ACA AAG ATG-3′ | Preclinical |
| DZ604 | Dzs | RSV NS2 RNA | 5′-ATG GGG CAA AGG CTA GCT ACA ACG AAA ATC AAT TCA-3′ | Preclinical |
| Verdinexor | Small-molecule | RSV M | 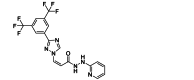 |
Phase 1 |
5. Therapeutics strategy for RSV assembly and budding
5.1. Targeting M protein
RSV matrix protein (M) is the first protein encoded by RSV found in the nucleus, with a total length of 256 amino acids. M protein is a non-glycosylated phosphorylated protein whose monomer consists of an unstructured linking region and two large P-fold structures144. In the virus particle, the M protein is located outside the nucleocapsid and promotes coordination between the structural components of the virus by connecting the lipid bilayer envelope and the nucleocapsid145,146. In host cells, the M protein determines the assembly and budding of RSV (Fig. 5147). F proteins initially accumulate and bud on lipid rafts in the plasma membrane, and then M proteins are recruited to the budding region148. G protein binds to M protein through its cytoplasmic domain, thus promoting its incorporation into the germination zone149. The localization of M proteins depends in part on the viral microfilament network and may interact with actin to promote the movement of other host factors150, including actin depolymerization factor (cofilin-1)151, as well as other host factors such as specialized membrane raft caveolin-1152 and Rablla153, a key component of the apical recirculating endosome. Incorporation of cofilin-1 and caveolin-1 into RSV virions has been observed in cell membrane lipids, suggesting the involvement of cytoskeleton and other host factors in virus germination mediated by M proteins154,155.
Figure 5.
Schematic illustration of the role of M protein in RSV assembly and budding. Viral bud initiation: Glycoprotein F accumulates on the plasma membrane enriched in lipid rafts. Structural protein recruitment: M proteins are recruited through interactions with F and the cell membrane. G is incorporated into the bud by M–G interaction. M assembled into an elongated tubular structure is speculated to provide the force that drives the bud. New capsid transport to budding site: The new ribonucleic capsid (RNPs) is partially transported to the budding site by M–host cytoskeleton (actin/myosin Vb and microtubules) interactions (yellow dashed line) and M2-1. Viral particle maturation: This filament integrates the host membrane into its structure, subsequently producing a mature spherical/filamentous viral particle. (Adapted with permission from Fig. 5 in Ref. 147).
In addition, M proteins also play an important role in RNP transport156. Through direct interactions mediated by M proteins, newborn RNPs9,157, host cytoskeleton proteins, and other cellular proteins158 are transported to the budding site. After the incorporation of RNPs into the newborn virus filaments, the virus particles were released under the control of Rablla159. The virions attract a varying number of host membranes, some of which remain at the end of the microfilaments as appendages or form pockets, eventually producing mature spherical/filamentous virions. The M protein forms a protein sheath around the nuclear shell, and when the expression of other viral proteins is insufficient, the M protein promotes the formation, encapsulation, and release of membrane vesicles to increase the formation of virus-like particles.
At present, although the function of M in the nucleus is not fully determined160, 161, 162, the accumulation of M protein in the nucleus will reduce the virus titer163. Nuclear export of RSV M protein is mediated by nuclear export protein 1 (XPO1) and is essential for RSV assembly and budding while Verdinexor (KPT-335) is a small molecule that inhibits XPO1-mediated nuclear export, thus reducing RSV replication164. In addition, Verdinexor can inhibit the activation of nuclear factor kappa B, thereby reducing the production of cytokines and eliminating virus-associated immunopathology165. KPT-335 is effective against RSV strains A and B and reduces viral replication after prophylactic or therapeutic administration.
So far there are no drug candidates that directly target RSV assembly. However, due to its critical role in assembly and germination, M protein is a promising therapeutic target.
5.2. Targeting NS1, NS2
The non-structural (NS) proteins NS1 and NS2 of RSV are essential proteins for RSV virulence. NS1 and NS2 are considered interferon (IFN) antagonists that strongly inhibit the IFN induction and IFN response pathways by inhibiting or degrading many signaling proteins involved in these processes. Compared with wild-type-infected cells, recombinant NS1 or NS2-deficient viruses showed reduced virulence, and infected cells showed elevated IFN-β mRNA levels, which is consistent with the role of the host antiviral response166, 167, 168, 169, 170. Of the two non-structural proteins, NS1 tends to be a more effective inhibitor of IFN signaling, while NS2 enhances the function of NS1171, 172, 173.
Previous studies have shown that NS regulates the expression level and function of many targets through mitochondrial and nuclear pathways. NS1 inhibits retinoic acid inducible gene I activity by interfering with its activation or interacting with the adaptor protein of the mitochondrial antiviral signaling protein (MAVS)174,175. Goswami et al.176 found that NS proteins assemble a large degradation complex, named “NS-degradasome” (NSD) (Fig. 6A), on the mitochondria in which all NS targets can be found, including RIG-1, TRAF3, IRF3/7, STAT2, etc. The suppression of innate immunity by NS1/2 is enhanced when mitofusin is lost, suggesting that the mitochondrial association is sufficient to activate NSD activity.
Figure 6.
Functional pathways of RSV NS1 and NS2. (A) Schematic illustration of a working NSD assembled by mitochondria. The subunits of the core NSD (green) may be loosely attached. NSD stabilizes by recruiting MAVS to the motional mitochondria, possibly allowing recruitment of other host factors. If juxtaposed in the right space, both NS proteins have the ability to interact with MAVS. It is hypothesized that mitochondrial motion allows rapid clearance of cytoplasmic collection and assembly of complex subunits, and mitochondrial fission provides a larger mitochondrial surface area, allowing more efficient utilization of more MAVS and other membrane structures. (Adapted with permission from Fig. 10. in Ref. 176). (B) NS1 functions as an immune antagonist by binding chromatin at the promoters and enhancers of immune response genes and modulating host gene transcription. (Adapted with permission from Ref. 181).
NS1 inhibits gene expression of the transcription factor IRF3/7 and may enhance STAT2 degradation through a proteasome-mediated process177, 178, 179. Chatterjee et al.180 reported that NS1 had a structural folding similar to the N-terminal domain of the M protein, and observed that RSV infection stimulated increased expression of many genes. RSV-induced gene expression differences included chemokines and cytoskeleton-related genes at early time points of infection, and IFN and inflammatory response genes at later stages of infection. Obviously, NS1 has a significant effect on transcription in host cells, and the mechanism of non-structural proteins affecting transcription in the nucleus during RSV infection has become a current research focus. Pei et al.181 observed that NS1 entered the nucleus of RSV-infected cells. Intranuclear NS1 co-immunoprecipitates with Mediator complex and is associated with chromatin. Chromatin immunoprecipitation revealed that NS1 was enriched and overlapped with promoter, enhancer mesons, and transcription factors of differentially expressed genes during RSV infection. Conversely, mutations in the C-terminal helix of NS1 reduce the effect of NS1 on host gene expression, suggesting that intranuclear NS1 alters the host response to RSV infection by binding to regulatory elements of immune response genes and regulating transcription of host genes (Fig. 6B181). In addition, NS1 is an inhibitor of dendritic cell (DC) maturation172,180,182, 183, 184 and can also inhibit the proliferation of CD103+ CD8+ T cells and Th17 cells185. NS1 and NS2 are thought to inhibit DC maturation and T cell response through defects in immune synapse formation in infected cells. In particular, NS1 promotes the proliferation and activation of Th2 cells, thus promoting the replication and pathogenesis of RSV186.
Studies of the structure of RSV NS1 show that it has secondary and tertiary structural elements similar to the N-terminal domain of the RSV M protein, suggesting that the NS1 protein may have evolved as a structural analogue180. Given that the unique regions of RSV NS1 are important regulators of host gene expression, these unique regions also provide new opportunities for the development of RSV treatment options, such as helio-α3 mutants with the potential to weaken RSV. At present, there are no reported inhibitors against RSV NS1 and NS2. However, NS1 of influenza, which performs a similar function, has related inhibitors. For instance, Woo et al.187 developed a specific DNA aptamer for the influenza A virus NS1 protein. The experiments showed that IFN-β was successfully induced by blocking NS1 protein with a specific aptamer, thus promoting the innate immune response of the host and enhancing the lethality of the host cells against the virus187. This report suggests RSV NS1 and NS2 proteins can be used as targets against RSV.
6. Therapeutics targeting the host
Host-directed therapeutic strategy is an emerging approach in the field of anti-infection. It mainly includes inhibiting the host-dependent factors involved in the life cycle of virus; activating the host's viral defense mechanisms to enhance the protective immune response; regulating signaling pathways that are disrupted by the virus to relieve inflammation; and modulating the local response to infection, limiting the virus niche and promoting tissue repair.
For instance, targeting the receptors that need to interact with RSV to inhibit virus entry. ICAM-1 promotes RSV entry into and infection of human epithelial cells by binding to RSV F protein188. The anti-RSV effect of tiotropium bromide was first reported by Iesato et al.189 Pretreatment with 100 nmol/L tiotropium bromide for 3 days inhibited cell surface ICAM-1 expression and reduced RSV attachment to HEp-2 cells, thereby inhibiting RSV replication. In addition, tiotropium can inhibit RhoA activity and reduce multiple cytokines produced by RSV-infected cells induced by the RhoA signaling pathway, such as IL-1, IL-6, and IL-8, thereby regulating the occurrence of inflammation189. RSV interacts with cell surface HSPGs to initiate infection23,190,191, which provides an attractive target for the development of new inhibitors of RSV infection. Donalisio et al.192 screened and identified SB105-A10 that binds to HSPGs, thereby blocking RSV attachment and infection. The IC50 values of SB105-A10 were detected as 0.35 and 0.25 μmol/L in HEp-2 and A549 cells, respectively, and SB105-A10 was found to bind to both cell types via HSPGs, indicating that its antiviral activity is indeed available by competing with RSV for binding to cell surface HSPGs. Further evaluation of the antiviral effect of SB105-A10 using a highly differentiated human airway epithelial tissue model showed that SB105-A10 significantly reduced RSV infectivity in this model and showed no signs of cytotoxic or proinflammatory effects. These features make SB105-A10 an attractive candidate for further development of RSV inhibitors192. Lingemann et al.193 found that RSV infection triggers the activation of ATP1A1. After signaling by c-src kinase, Tyr845 phosphorylation activates EGFR to mediate RSV entry into cells. In A549 cells, PST2238 (rostafuroxin) can specifically bind to the extracellular domain of ATP1A1 and block RSV-triggered EGFR activation to inhibit RSV entry, thereby playing an anti-RSV effect193. It's worth noting that PST2238 is a drug that has already been shown to be well tolerated in humans, which means it can be further developed as an antiviral agent for RSV194.
In addition, using antioxidants to delay the pathological process caused by RSV is one approach. Regulating the intracellular environment to inhibit the replication of the virus is another. RSV infection can induce intracellular reactive oxygen species (ROS) production, leading to elevated levels of proinflammatory mediators and oxidative stress, which contribute to advancing the pathological process195,196. Treatment with natural antioxidant vitamin A resulted in reduced clinical complications and duration of hospitalization for children with severe RSV infection197,198. The antioxidant resveratrol, a natural polyphenol found in peanuts, grapes, and some berries, has been shown to reduce viral load, airway hypersensitivity, and inflammation in mouse models of RSV infection199. Treatment of RSV infection with ROS scavenger, butylated hydroxyanisole or melatonin, not only reduced pulmonary inflammation but also ameliorated clinical disease in mice200,201. The interaction between RSV and host mitochondria is not fully understood, but treatment of Vero cells with mitoquinone mesylate (MitoQ), a mitochondrial ROS scavenger, has been shown to reduce RSV replication. MitoQ treatment reduced infectious virus titers and genomes in primary human bronchial epithelial cells. In RSV-infected BALB/c mouse models, MitoQ treatment significantly reduced the dense inflammatory cell infiltration in the bronchial periairway and pulmonary perivascular areas202.
Cui et al.203 identified the intracellular calcium ATPase inhibitor cyclopyrazolic acid (CPA) as an RSV inhibitor (EC50 = 4.13 μmol/L). CPA inhibited the replication of RSV subgroup A and B and human parainfluenza virus type 3 (PIV-3). The virus titers of RSV A, RSV B and PIV-3 were reduced by 2.7, 2.2 and 1.8 orders of magnitude at 10 μmol/L, respectively. Investigations about its mechanism of action suggest that CPA may function during viral genome replication and/or transcription. In addition, drugs that increase intracellular calcium concentrations can also inhibit RSV replication203. These findings reveal an important role for intracellular calcium in the RSV life cycle and bring new possibilities for the control of RSV infection.
Since every step of the viral life cycle requires the involvement of host cell factors, every host factor involved is a potential drug target. The development of host-directed treatment strategies for RSV is still in its infancy, but it is still critical for the development of drugs to overcome resistance and achieve broad-spectrum antiviral activity.
Anti-RSV drugs targeting the host are listed in Table 3.
Table 3.
Anti-RSV drugs targeting the host.
| Name | Category | Target | Structure | Development |
|---|---|---|---|---|
| Vitamin A | Antioxidant | Host oxidative responses | 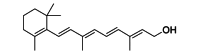 |
Preclinical |
| Resveratrol | Antioxidant | Host oxidative responses | 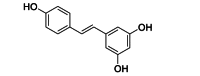 |
Preclinical |
| Melatonin | Antioxidant | Host oxidative responses | 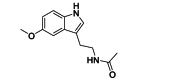 |
Preclinical |
| MitoQ | Antioxidant | Host oxidative responses |  |
Preclinical |
| Tiotropium | Small-molecule | ICAM-1 | 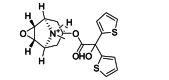 |
Preclinical |
| PST2238 | Small-molecule | ATP1A1 | 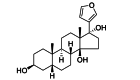 |
Preclinical |
| SB105-A10 | Peptide | HSPGs | ASLRVRIKK | Preclinical |
| CPA | Small-molecule | ATPase | 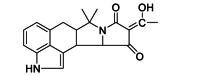 |
Preclinical |
7. New options for RSV prevention and treatment
7.1. Antiviral ENMs
While there have been significant advances in RSV treatment, recent therapeutic methods and drugs remain limited and cannot fully control the diseases caused by RSV. Therefore, there is an urgent need to develop more effective treatment strategies to combat RSV. In comparison to traditional medications, Engineered Nanomaterials (ENMs) offer numerous therapeutic advantages, such as enhanced drug delivery efficiency to the affected areas, reduced side effects on the human body, ease of modulation of physicochemical properties, lower cost, and higher synthesis throughput. These superior characteristics of ENMs can be considered as an emerging solution to the challenges faced in the process of new drug development.
In most studies, the antiviral effects are achieved through the preparation of nanoparticles modified with various functional biomolecules, where the modified coatings can directly inactivate the virus or recognize receptors on the host cell surface, thus blocking virus entry into cells. Functional nanoparticles such as gold nanoparticles204,205, zinc oxide nanoparticles206, carbon nanomaterials207, and nanoclays208 have demonstrated some degree of antiviral properties. The use of metal nanoparticles appears to provide a potential opportunity for novel antiviral treatments. Due to their broad-spectrum antiviral capabilities and ability to overcome drug resistance, metal nanoparticles offer a promising avenue for the development of potential and effective antiviral drugs.
Currently, the development of nanotechnology provides a new platform for the development of potential and effective antiviral drugs. Metal nanoparticles find extensive applications in various fields, including diagnostics, biomarkers, drug delivery systems, and antimicrobial agents, among others. Researchers are continuously exploring efficient nanotechnological solutions for the treatment of RSV virus infections.
Sun et al.209 prepared three types of conjugated silver nanoparticles with coatings using polyvinylpyrrolidone (PVP), bovine serum albumin, and recombinant RSV F protein (RF 412). All three showed antiviral activity against RSV infection in HEp-2 cells. Among them, PVP-coated silver nanoparticles demonstrated effective interaction with the G protein on the envelope of RSV viral particles. Yang et al.210 developed curcumin-modified silver nanoparticles that exhibited highly efficient inhibition against RSV infection, reducing viral titers by approximately two orders of magnitude. These curcumin-modified silver nanoparticles were non-toxic to host cells and could prevent RSV infection of host cells by directly inactivating the virus. Cagno et al.211 and colleagues designed antiviral nanoparticles with long and flexible linkers that mimic HSPGs. These nanoparticles mimic the strong and multivalent binding of viral attachment ligands repeating units, generating a force (190 pN) that leads to irreversible virus deformation, resulting in virus killing. These particles are non-cytotoxic and exhibit nanomolar-level irreversible inhibitory activity against RSV both in vitro and in infected mice.
Researchers have been modifying the monomers of Au/Ag nanoparticles to alter their antiviral action modes, achieving both inhibition and inactivation of viruses, including RSV. However, concerns exist about the unknown clearance mechanisms and potential long-term toxicity of Au/Ag nanoparticles when used as drugs. Despite these concerns, a series of antiviral drugs developed using nanoparticles with multiple functional mechanisms still hold significant application potential. It's important for ongoing research to address the safety and long-term effects of these nanoparticles, as well as to better understand their clearance from the body. The development of effective and safe nanomaterial-based antiviral treatments remains an area of active exploration and innovation in the field of medical research.
Additionally, research has shown that sulfonated cyclodextrins (CDs) exhibit antiviral properties against the HIV virus212. CDs are naturally occurring glucose derivatives with rigid cyclic structures composed of glucose pyranose units linked by α(1–4) glycosidic bonds. CDs have been applied in various commercial applications, including drug delivery, cosmetics, and food products. However, the antiviral effects of CDs have been found to be reversible and virus-specific. Samuel T. Jones and colleagues213 attached highly sulfonated chemical moieties to FDA-approved CD scaffolds, achieving efficient antiviral broad-spectrum molecules. These molecules demonstrated effectiveness against members of the Pneumoviridae family, including RSV A, RSV B, and human metapneumovirus (HMPV), in both in vitro and in vivo studies.
Although ENMs exhibit excellent multifunctionality in antiviral applications, the development of practical therapeutic approaches has been limited in terms of devising new strategies and enhancing both efficacy and safety. While most reported antiviral nanomedicines have shown outstanding performance in mammalian or rodent models, they may elicit adverse or more complex reactions in humans. Therefore, before hastily advancing into clinical trials, further preclinical testing must be conducted using promising animal models such as ferrets, macaques, and other primates that better mimic human disease responses. This will help assess health risks and the antiviral activity of nanomedicines more accurately. Moreover, comprehensive preclinical trials are essential for developing nanomedicines with superior efficacy and broad-spectrum antiviral activity compared to currently approved antiviral drugs. The main targets for designing antiviral nanomedicines are the viral envelope, capsid, and nucleic acids. However, the interactions between ENMs and these targets are not yet fully understood, impeding a deeper comprehension of the mechanisms underlying virus inhibition. Hence, it is essential to elucidate potential interaction mechanisms, including damage caused by the direct binding of ENMs to the virus, interference with virus-host cell interactions, or indirect effects on innate and adaptive immune activation.
7.2. Engineered cells
Currently, the administration of Palivizumab is limited to high-risk individuals and still requires monthly reinfusion to maintain protection. Although new methods have been developed to extend the antibody half-life post-injection, even the most promising of these strategies require lifelong reinfusion to sustain protection. To overcome the need for repeated infusions, alternative strategies for generating long-term immunity have been explored. One approach involves using adenovirus vectors encoding protective antibodies to transduce muscle cells214,215. Another method utilizes lentivirus-encoded antibodies to transduce hematopoietic stem cells, which differentiate into plasma cells secreting antibodies either in vitro before infusion or in vivo after infusion216. A common limitation of both the adenovirus/muscle and lentivirus/stem cell approaches is that the levels of antibodies produced are fixed and do not respond to infections. In contrast, protective antibodies can induce long-lived memory B cells as well as antibody-secreting plasma cells. Memory B cells express a membrane-bound form of antibodies, allowing these cells to rapidly respond to infections and differentiate into additional antibody-secreting cells after infection.
While the use of primary B cells for adoptive cell therapy in generating antibodies has lagged behind other cell types, there has recently been a wave of innovation in this field. Some recent genetic engineering efforts have focused on harnessing the potent protein secretion capabilities of B cells to produce non-antibody therapeutic proteins217,218. Strategies have also been developed to reprogram B cells to produce therapeutic antibodies219. One notable approach in this regard is the work by Howell F. Moffett and colleagues220, who developed an engineered cell strategy using CRISPR/Cas9 to replace endogenous antibodies with antibodies targeting respiratory syncytial virus (RSV) in primary human B cells. Under the control of endogenous regulatory elements, engineered antibodies were effectively expressed in primary B cells, maintaining normal expression and secretion. This work demonstrated the specificity and high efficiency of engineering primary mouse and human cells to produce various effective antiviral antibodies. In these engineered B cells, modified heavy chain loci retained the ability for selective splicing, resulting in cell surface BCR (B cell receptor) and antibody secretion that reached protective levels after adoptive transfer. Using engineered B cells, it was shown that a single transfer of B cells expressing anti-RSV antibodies could provide effective and long-lasting protection against RSV infection in RAG1-deficient mice. This approach offers an opportunity to achieve sterilizing immunity against pathogens that traditional vaccination has failed to induce or maintain protective antibody responses.
8. Conclusions
Since the discovery of respiratory syncytial virus (RSV) in 1955, researchers have accumulated a wealth of knowledge regarding its virus structure, infection mechanisms, life cycle, host responses, community transmission, and clinical management. Our understanding of RSV has facilitated the development of new therapies, but basic scientific research on RSV has lagged behind its clinical advancements. Compared to other viruses, vaccines, drugs, and clinical treatment strategies for RSV still fall short of clinical needs. An encouraging sign is the widespread research into various treatment strategies for RSV, with an increasing number of ongoing clinical trials.
The development and use of antiviral drugs for RSV still face several challenges: 1) Imbalance in virus target research. Currently, a substantial amount of research is focused on the RSV F protein, with insufficient research targeting the G protein and other proteins as potential anti-RSV targets. These proteins play crucial roles in virus infection, replication, and disease initiation, making it particularly important for the innovation paradigm shift in RSV drug research and treatment strategies. 2) Emergence of escape mutants. Like other RNA viruses such as influenza, the high genetic variability of the RSV genome is a major factor contributing to the appearance of escape mutants. Research, clinical trials, and long-term efficacy of single-target anti-RSV drugs may be adversely affected. Therefore, the development of broad-spectrum drugs targeting multiple targets and cocktails should be considered. 3) Drug resistance. With the use of anti-RSV drugs, there is a high probability of evolving drug-resistant mutant strains. The rapid development of drugs targeting drug-resistant mutants remains a challenge. 4) Co-infections. Since the outbreak of COVID-19, cases of RSV co-infections with influenza, HBV, HIV, and other viruses have gained attention. Effective antiviral treatment for virus co-infections requires further research. 5) Insufficient research models. Cell and animal models for RSV play a crucial role in the preclinical testing of candidate RSV drugs. Despite many drugs showing efficacy in preclinical studies, few have progressed to clinical trials. This is partly due to a lack of accurate models for evaluating the pathogenesis of RSV infection. 6) Medicinal chemistry strategies. The medicinal chemistry strategies for the development of antiviral drugs are crucial. There are a lot of medicinal chemistry strategies such as Structure-based drug design, mechanism-based drug design, multivalent interaction-based drug design and drug repurposing221,222. These strategies have accelerated the efficiency of antiviral drug development. But these strategies have their limits. Structure-based drug design relies on techniques that identify the binding between molecules and targets, and skilled operators222. The limitation of drug repurposing is that the number of drugs on the market is limited. The molecular weight of polyvalent molecules is so large that it is hard to be used as oral drugs in clinic223. We look forward to the emergence of approaches to address these limitations and the development of new drug design strategies.
In theory, all viral components can serve as targets for antiviral drugs. Currently, most anti-RSV drugs primarily target viral fusion proteins and replicative enzymes, and genes encoded by the virus are expected to become hotspots for future antiviral drug development. From a viral target perspective, RSV replicates in the cytoplasm and establishes cellular organelles derived from host cell membranes for efficient replication and immune evasion. RdRp is a critical component in the replication machinery of RSV. It differs significantly from the host cell's polymerases, making it a major target for anti-RSV drugs. The three-dimensional structure of the core catalytic domain of RdRp is highly conserved and represents an important target for developing broad-spectrum drugs against RSV and its mutant strains. Furthermore, the 5′ cap modification at the beginning of the RSV genome plays a crucial role in genome replication, translation of viral proteins, particle assembly, and evasion of the host innate immune system. Therefore, targeting the methyltransferase responsible for this cap modification could potentially achieve resistance to RSV and its mutant strains. From a host target perspective, some host proteins are crucial for RSV replication. Targeting these proteins can lead to broad-spectrum anti-RSV effects and may offer higher barriers to resistance development. Additionally, enhancing host restriction factors is a feasible approach to control viral infections. Drugs targeting host factors represent a promising direction for future drug development against RSV.
Author contributions
Ge Yang: Writing – review & editing, Writing – original draft. Guangyu Jiang: Writing – review & editing, Writing – original draft. Jiandong Jiang: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Conceptualization. Yuhuan Li: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Conceptualization.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by foundation CAMS Innovation Fund for Medical Sciences (2021-I2M-1-030 and 2021-I2M-1-048, China), National Natural Science Foundation of China (82394465, 82394464, 82394460, and 82204263) and National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2018ZX09711003, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Jiandong Jiang, Email: jiang.jdong@163.com.
Yuhuan Li, Email: yuhuanlibj@126.com.
References
- 1.Blount R.J., Morris J.A., Savage R.E. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92:544–549. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- 2.Chanock R., Roizman B., Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg. 1957;66:281–290. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- 3.Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liljeroos L., Krzyzaniak M.A., Helenius A., Butcher S.J. Architecture of respiratory syncytial virus revealed by electron cryotomography. Proc Natl Acad Sci U S A. 2013;110:11133–11138. doi: 10.1073/pnas.1309070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Empey K.M., Peebles R.J., Kolls J.K. Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin Infect Dis. 2010;50:1258–1267. doi: 10.1086/651603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins P.L., Graham B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mcnamara P.S., Ritson P., Selby A., Hart C.A., Smyth R.L. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child. 2003;88:922–926. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K., et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 9.Kiss G., Holl J.M., Williams G.M., Alonas E., Vanover D., Lifland A.W., et al. Structural analysis of respiratory syncytial virus reveals the position of M2-1 between the matrix protein and the ribonucleoprotein complex. J Virol. 2014;88:7602–7617. doi: 10.1128/JVI.00256-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battles M.B., Mclellan J.S. Respiratory syncytial virus entry and how to block it. Nat Rev Microbiol. 2019;17:233–245. doi: 10.1038/s41579-019-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Te V.A., Grimes J.M., Fodor E. Structural insights into RNA polymerases of negative-sense RNA viruses. Nat Rev Microbiol. 2021;19:303–318. doi: 10.1038/s41579-020-00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mufson M.A., Orvell C., Rafnar B., Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- 13.Peret T.C., Hall C.B., Schnabel K.C., Golub J.A., Anderson L.J. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79(Pt 9):2221–2229. doi: 10.1099/0022-1317-79-9-2221. [DOI] [PubMed] [Google Scholar]
- 14.Peret T.C., Hall C.B., Hammond G.W., Piedra P.A., Storch G.A., Sullender W.M., et al. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in north America. J Infect Dis. 2000;181:1891–1896. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 15.Venter M., Madhi S.A., Tiemessen C.T., Schoub B.D. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J Gen Virol. 2001;82:2117–2124. doi: 10.1099/0022-1317-82-9-2117. [DOI] [PubMed] [Google Scholar]
- 16.Shobugawa Y., Saito R., Sano Y., Zaraket H., Suzuki Y., Kumaki A., et al. Emerging genotypes of human respiratory syncytial virus subgroup a among patients in Japan. J Clin Microbiol. 2009;47:2475–2482. doi: 10.1128/JCM.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshaghi A., Duvvuri V.R., Lai R., Nadarajah J.T., Li A., Patel S.N., et al. Genetic variability of human respiratory syncytial virus a strains circulating in ontario: a novel genotype with a 72 nucleotide G gene duplication. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano E., Kobayashi M., Tsukagoshi H., Yoshida L.M., Kuroda M., Noda M., et al. Molecular evolution of human respiratory syncytial virus attachment glycoprotein (G) gene of new genotype ON1 and ancestor NA1. Infect Genet Evol. 2014;28:183–191. doi: 10.1016/j.meegid.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Blanc A., Delfraro A., Frabasile S., Arbiza J. Genotypes of respiratory syncytial virus group B identified in Uruguay. Arch Virol. 2005;150:603–609. doi: 10.1007/s00705-004-0412-x. [DOI] [PubMed] [Google Scholar]
- 20.Dapat I.C., Shobugawa Y., Sano Y., Saito R., Sasaki A., Suzuki Y., et al. New genotypes within respiratory syncytial virus group B genotype BA in Niigata, Japan. J Clin Microbiol. 2010;48:3423–3427. doi: 10.1128/JCM.00646-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trento A., Viegas M., Galiano M., Videla C., Carballal G., Mistchenko A.S., et al. Natural history of human respiratory syncytial virus inferred from phylogenetic analysis of the attachment (G) glycoprotein with a 60-nucleotide duplication. J Virol. 2006;80:975–984. doi: 10.1128/JVI.80.2.975-984.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabor D.E., Fernandes F., Langedijk A.C., Wilkins D., Lebbink R.J., Tovchigrechko A., et al. Global molecular epidemiology of respiratory syncytial virus from the 2017–2018 inform-RSV study. J Clin Microbiol. 2020;59 doi: 10.1128/JCM.01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman S.A., Audet S., Beeler J.A. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol. 2000;74:6442–6447. doi: 10.1128/jvi.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rincheval V., Lelek M., Gault E., Bouillier C., Sitterlin D., Blouquit-Laye S., et al. Functional organization of cytoplasmic inclusion bodies in cells infected by respiratory syncytial virus. Nat Commun. 2017;8:563. doi: 10.1038/s41467-017-00655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobe F., Simpson J., Hawes P., Guzman E., Bailey D. Respiratory syncytial virus sequesters NF-kappaB subunit p65 to cytoplasmic inclusion bodies to inhibit innate immune signaling. J Virol. 2020;94 doi: 10.1128/JVI.01380-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forster A., Maertens G.N., Farrell P.J., Bajorek M. Dimerization of matrix protein is required for budding of respiratory syncytial virus. J Virol. 2015;89:4624–4635. doi: 10.1128/JVI.03500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripp R.A., Jones L.P., Haynes L.M., Zheng H., Murphy P.M., Anderson L.J. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2:732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 28.Roberts S.R., Compans R.W., Wertz G.W. Respiratory syncytial virus matures at the apical surfaces of polarized epithelial cells. J Virol. 1995;69:2667–2673. doi: 10.1128/jvi.69.4.2667-2673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tayyari F., Marchant D., Moraes T.J., Duan W., Mastrangelo P., Hegele R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med. 2011;17:1132–1135. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- 30.Power U.F., Nguyen T.N., Rietveld E., de Swart R.L., Groen J., Osterhaus A.D., et al. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J Infect Dis. 2001;184:1456–1460. doi: 10.1086/324426. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Peeples M.E., Boucher R.C., Collins P.L., Pickles R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong K.I., Piepenhagen P.A., Kishko M., Dinapoli J.M., Groppo R.P., Zhang L., et al. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a g protein-dependent manner. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson S.M., Mcnally B.A., Ioannidis I., Flano E., Teng M.N., Oomens A.G., et al. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortjens B., Yasuda E., Yu X., Wagner K., Claassen Y.B., Bakker A.Q., et al. Broadly reactive anti-respiratory syncytial virus G antibodies from exposed individuals effectively inhibit infection of primary airway epithelial cells. J Virol. 2017;91 doi: 10.1128/JVI.02357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia O., Martin M., Dopazo J., Arbiza J., Frabasile S., Russi J., et al. Evolutionary pattern of human respiratory syncytial virus (subgroup A): cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J Virol. 1994;68:5448–5459. doi: 10.1128/jvi.68.9.5448-5459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwilas S., Liesman R.M., Zhang L., Walsh E., Pickles R.J., Peeples M.E. Respiratory syncytial virus grown in vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J Virol. 2009;83:10710–10718. doi: 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajan A., Piedra F.A., Aideyan L., Mcbride T., Robertson M., Johnson H.L., et al. Multiple respiratory syncytial virus (RSV) strains infecting Hep-2 and A549 cells reveal cell line-dependent differences in resistance to RSV infection. J Virol. 2022;96 doi: 10.1128/jvi.01904-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Beato R., Martinez I., Franci C., Real F.X., Garcia-Barreno B., Melero J.A. Host cell effect upon glycosylation and antigenicity of human respiratory syncytial virus G glycoprotein. Virology. 1996;221:301–309. doi: 10.1006/viro.1996.0379. [DOI] [PubMed] [Google Scholar]
- 39.King T., Mejias A., Ramilo O., Peeples M.E. The larger attachment glycoprotein of respiratory syncytial virus produced in primary human bronchial epithelial cultures reduces infectivity for cell lines. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine S., Klaiber-Franco R., Paradiso P.R. Demonstration that glycoprotein g is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68(Pt 9):2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- 41.Hendricks D.A., Baradaran K., Mcintosh K., Patterson J.L. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J Gen Virol. 1987;68(Pt 6):1705–1714. doi: 10.1099/0022-1317-68-6-1705. [DOI] [PubMed] [Google Scholar]
- 42.Wertz G.W., Collins P.L., Huang Y., Gruber C., Levine S., Ball L.A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985;82:4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L., Bukreyev A., Thompson C.I., Watson B., Peeples M.E., Collins P.L., et al. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol. 2005;79:1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kauvar L.M., Harcourt J.L., Haynes L.M., Tripp R.A. Therapeutic targeting of respiratory syncytial virus G-protein. Immunotherapy. 2010;2:655–661. doi: 10.2217/imt.10.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jorquera P.A., Anderson L., Tripp R.A. Understanding respiratory syncytial virus (RSV) vaccine development and aspects of disease pathogenesis. Expert Rev Vaccin. 2016;15:173–187. doi: 10.1586/14760584.2016.1115353. [DOI] [PubMed] [Google Scholar]
- 46.Harcourt J., Alvarez R., Jones L.P., Henderson C., Anderson L.J., Tripp R.A. Respiratory syncytial virus G protein and g protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol. 2006;176:1600–1608. doi: 10.4049/jimmunol.176.3.1600. [DOI] [PubMed] [Google Scholar]
- 47.Johnson C.H., Miao C., Blanchard E.G., Caidi H., Radu G.U., Harcourt J.L., et al. Effect of chemokine receptor CX3CR1 deficiency in a murine model of respiratory syncytial virus infection. Comp Med. 2012;62:14–20. [PMC free article] [PubMed] [Google Scholar]
- 48.Tripp R.A., Moore D., Jones L., Sullender W., Winter J., Anderson L.J. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol. 1999;73:7099–7107. doi: 10.1128/jvi.73.9.7099-7107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tripp R.A., Jones L., Anderson L.J. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J Virol. 2000;74:6227–6229. doi: 10.1128/jvi.74.13.6227-6229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chirkova T., Lin S., Oomens A., Gaston K.A., Boyoglu-Barnum S., Meng J., et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J Gen Virol. 2015;96:2543–2556. doi: 10.1099/vir.0.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Escribano-Romero E., Rawling J., Garcia-Barreno B., Melero J.A. The soluble form of human respiratory syncytial virus attachment protein differs from the membrane-bound form in its oligomeric state but is still capable of binding to cell surface proteoglycans. J Virol. 2004;78:3524–3532. doi: 10.1128/JVI.78.7.3524-3532.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukreyev A., Yang L., Fricke J., Cheng L., Ward J.M., Murphy B.R., et al. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol. 2008;82:12191–12204. doi: 10.1128/JVI.01604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bukreyev A., Yang L., Collins P.L. The secreted g protein of human respiratory syncytial virus antagonizes antibody-mediated restriction of replication involving macrophages and complement. J Virol. 2012;86:10880–10884. doi: 10.1128/JVI.01162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han J., Takeda K., Wang M., Zeng W., Jia Y., Shiraishi Y., et al. Effects of anti-G and anti-F antibodies on airway function after respiratory syncytial virus infection. Am J Respir Cell Mol Biol. 2014;51:143–154. doi: 10.1165/rcmb.2013-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones H.G., Ritschel T., Pascual G., Brakenhoff J., Keogh E., Furmanova-Hollenstein P., et al. Structural basis for recognition of the central conserved region of RSV G by neutralizing human antibodies. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haynes L.M., Caidi H., Radu G.U., Miao C., Harcourt J.L., Tripp R.A., et al. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect Dis. 2009;200:439–447. doi: 10.1086/600108. [DOI] [PubMed] [Google Scholar]
- 57.Miao C., Radu G.U., Caidi H., Tripp R.A., Anderson L.J., Haynes L.M. Treatment with respiratory syncytial virus g glycoprotein monoclonal antibody or F(ab′)2 components mediates reduced pulmonary inflammation in mice. J Gen Virol. 2009;90:1119–1123. doi: 10.1099/vir.0.009308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyoglu-Barnum S., Todd S.O., Chirkova T., Barnum T.R., Gaston K.A., Haynes L.M., et al. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology. 2015;483:117–125. doi: 10.1016/j.virol.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimura K., Mori S., Tomita K., Ohno K., Takahashi K., Shigeta S., et al. Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antivir Res. 2000;47:41–51. doi: 10.1016/s0166-3542(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura M., Terada M., Kamada M., Yokono A., Nakamori S., Ohno T. Mechanistic effect of NMSO3 on replication of human immunodeficiency virus. Antivir Chem Chemother. 2003;14:171–176. doi: 10.1177/095632020301400401. [DOI] [PubMed] [Google Scholar]
- 61.Kaneko H., Kato K., Mori S., Shigeta S. Antiviral activity of NMSO3 against adenovirus in vitro. Antivir Res. 2001;52:281–288. doi: 10.1016/s0166-3542(01)00167-x. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi K., Ohashi K., Abe Y., Mori S., Taniguchi K., Ebina T., et al. Protective efficacy of a sulfated sialyl lipid (NMSO3) against human rotavirus-induced diarrhea in a mouse model. Antimicrob Agents Chemother. 2002;46:420–424. doi: 10.1128/AAC.46.2.420-424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wyde P.R., Moylett E.H., Chetty S.N., Jewell A., Bowlin T.L., Piedra P.A. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by NMSO3 in tissue culture assays. Antivir Res. 2004;63:51–59. doi: 10.1016/j.antiviral.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Fujikane A., Sakamoto A., Fujikane R., Nishi A., Ishino Y., Hiromatsu K., et al. Ephedrae herba and Cinnamomi cortex interactions with G glycoprotein inhibit respiratory syncytial virus infectivity. Commun Biol. 2022;5:94. doi: 10.1038/s42003-022-03046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walsh E.E., Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983;47:171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Z., Pan Y., Jiang S., Lu L. Respiratory syncytial virus entry inhibitors targeting the F protein. Viruses. 2013;5:211–225. doi: 10.3390/v5010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mclellan J.S., Chen M., Leung S., Graepel K.W., Du X., Yang Y., et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yunus A.S., Jackson T.P., Crisafi K., Burimski I., Kilgore N.R., Zoumplis D., et al. Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation. Virology. 2010;396:226–237. doi: 10.1016/j.virol.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mclellan J.S., Ray W.C., Peeples M.E. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013;372:83–104. doi: 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mclellan J.S., Yang Y., Graham B.S., Kwong P.D. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. 2011;85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X., Singh M., Malashkevich V.N., Kim P.S. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc Natl Acad Sci U S A. 2000;97:14172–14177. doi: 10.1073/pnas.260499197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perron M., Stray K., Kinkade A., Theodore D., Lee G., Eisenberg E., et al. Gs-5806 inhibits a broad range of respiratory syncytial virus clinical isolates by blocking the virus-cell fusion process. Antimicrob Agents Chemother. 2015;60:1264–1273. doi: 10.1128/AAC.01497-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Devincenzo J.P., Whitley R.J., Mackman R.L., Scaglioni-Weinlich C., Harrison L., Farrell E., et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 74.Cianci C., Yu K.L., Combrink K., Sin N., Pearce B., Wang A., et al. Orally active fusion inhibitor of respiratory syncytial virus. Antimicrob Agents Chemother. 2004;48:413–422. doi: 10.1128/AAC.48.2.413-422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cianci C., Meanwell N., Krystal M. Antiviral activity and molecular mechanism of an orally active respiratory syncytial virus fusion inhibitor. J Antimicrob Chemother. 2005;55:289–292. doi: 10.1093/jac/dkh558. [DOI] [PubMed] [Google Scholar]
- 76.Cianci C., Genovesi E.V., Lamb L., Medina I., Yang Z., Zadjura L., et al. Oral efficacy of a respiratory syncytial virus inhibitor in rodent models of infection. Antimicrob Agents Chemother. 2004;48:2448–2454. doi: 10.1128/AAC.48.7.2448-2454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roymans D., Alnajjar S.S., Battles M.B., Sitthicharoenchai P., Furmanova-Hollenstein P., Rigaux P., et al. Therapeutic efficacy of a respiratory syncytial virus fusion inhibitor. Nat Commun. 2017;8:167. doi: 10.1038/s41467-017-00170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stevens M., Rusch S., Devincenzo J., Kim Y.I., Harrison L., Meals E.A., et al. Antiviral activity of oral JNJ-53718678 in healthy adult volunteers challenged with respiratory syncytial virus: a placebo-controlled study. J Infect Dis. 2018;218:748–756. doi: 10.1093/infdis/jiy227. [DOI] [PubMed] [Google Scholar]
- 79.Zheng X., Gao L., Wang L., Liang C., Wang B., Liu Y., et al. Discovery of ziresovir as a potent, selective, and orally bioavailable respiratory syncytial virus fusion protein inhibitor. J Med Chem. 2019;62:6003–6014. doi: 10.1021/acs.jmedchem.9b00654. [DOI] [PubMed] [Google Scholar]
- 80.Zhao S., Shang Y., Yin Y., Zou Y., Xu Y., Zhong L., et al. Ziresovir in hospitalized infants with respiratory syncytial virus infection. N Engl J Med. 2024;391:1096–1107. doi: 10.1056/NEJMoa2313551. [DOI] [PubMed] [Google Scholar]
- 81.Andries K., Moeremans M., Gevers T., Willebrords R., Sommen C., Lacrampe J., et al. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antivir Res. 2003;60:209–219. doi: 10.1016/j.antiviral.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 82.Bonfanti J.F., Doublet F., Fortin J., Lacrampe J., Guillemont J., Muller P., et al. Selection of a respiratory syncytial virus fusion inhibitor clinical candidate, part 1: improving the pharmacokinetic profile using the structure-property relationship. J Med Chem. 2007;50:4572–4584. doi: 10.1021/jm070143x. [DOI] [PubMed] [Google Scholar]
- 83.Bonfanti J.F., Meyer C., Doublet F., Fortin J., Muller P., Queguiner L., et al. Selection of a respiratory syncytial virus fusion inhibitor clinical candidate. 2. Discovery of a morpholinopropylaminobenzimidazole derivative (TMC353121) J Med Chem. 2008;51:875–896. doi: 10.1021/jm701284j. [DOI] [PubMed] [Google Scholar]
- 84.Wyde P.R., Chetty S.N., Timmerman P., Gilbert B.E., Andries K. Short duration aerosols of JNJ 2408068 (r170591) administered prophylactically or therapeutically protect cotton rats from experimental respiratory syncytial virus infection. Antivir Res. 2003;60:221–231. doi: 10.1016/j.antiviral.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Roymans D., De Bondt H.L., Arnoult E., Geluykens P., Gevers T., Van Ginderen M., et al. Binding of a potent small-molecule inhibitor of six-helix bundle formation requires interactions with both heptad-repeats of the RSV fusion protein. Proc Natl Acad Sci U S A. 2010;107:308–313. doi: 10.1073/pnas.0910108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olszewska W., Ispas G., Schnoeller C., Sawant D., Van de Casteele T., Nauwelaers D., et al. Antiviral and lung protective activity of a novel respiratory syncytial virus fusion inhibitor in a mouse model. Eur Respir J. 2011;38:401–408. doi: 10.1183/09031936.00005610. [DOI] [PubMed] [Google Scholar]
- 87.Rouan M.C., Gevers T., Roymans D., de Zwart L., Nauwelaers D., De Meulder M., et al. Pharmacokinetics-pharmacodynamics of a respiratory syncytial virus fusion inhibitor in the cotton rat model. Antimicrob Agents Chemother. 2010;54:4534–4539. doi: 10.1128/AAC.00643-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding W.D., Mitsner B., Krishnamurthy G., Aulabaugh A., Hess C.D., Zaccardi J., et al. Novel and specific respiratory syncytial virus inhibitors that target virus fusion. J Med Chem. 1998;41:2671–2675. doi: 10.1021/jm980239e. [DOI] [PubMed] [Google Scholar]
- 89.Wyde P.R., Moore-Poveda D.K., O'Hara B., Ding W.D., Mitsner B., Gilbert B.E. Cl387626 exhibits marked and unusual antiviral activity against respiratory syncytial virus in tissue culture and in cotton rats. Antivir Res. 1998;38:31–42. doi: 10.1016/s0166-3542(98)00002-3. [DOI] [PubMed] [Google Scholar]
- 90.Nikitenko A.A., Raifeld Y.E., Wang T.Z. The discovery of RFI-641 as a potent and selective inhibitor of the respiratory syncytial virus. Bioorg Med Chem Lett. 2001;11:1041–1044. doi: 10.1016/s0960-894x(01)00150-0. [DOI] [PubMed] [Google Scholar]
- 91.Huntley C.C., Weiss W.J., Gazumyan A., Buklan A., Feld B., Hu W., et al. RFI-641, a potent respiratory syncytial virus inhibitor. Antimicrob Agents Chemother. 2002;46:841–847. doi: 10.1128/AAC.46.3.841-847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weiss W.J., Murphy T., Lynch M.E., Frye J., Buklan A., Gray B., et al. Inhalation efficacy of RFI-641 in an african green monkey model of RSV infection. J Med Primatol. 2003;32:82–88. doi: 10.1034/j.1600-0684.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- 93.Sake S.M., Zhang X., Rajak M.K., Urbanek-Quaing M., Carpentier A., Gunesch A.P., et al. Drug repurposing screen identifies lonafarnib as respiratory syncytial virus fusion protein inhibitor. Nat Commun. 2024;15:1173. doi: 10.1038/s41467-024-45241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang W., Li M., Liu Y., Liang N., Yang Z., Zhao Y., et al. Small molecule inhibits respiratory syncytial virus entry and infection by blocking the interaction of the viral fusion protein with the cell membrane. FASEB J. 2019;33:4287–4299. doi: 10.1096/fj.201800579R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Douglas J.L., Panis M.L., Ho E., Lin K.Y., Krawczyk S.H., Grant D.M., et al. Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J Virol. 2003;77:5054–5064. doi: 10.1128/JVI.77.9.5054-5064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y.I., Pareek R., Murphy R., Harrison L., Farrell E., Cook R., et al. The antiviral effects of RSV fusion inhibitor, MDT-637, on clinical isolates, vs its achievable concentrations in the human respiratory tract and comparison to ribavirin. Influenza Other Respir Viruses. 2017;11:525–530. doi: 10.1111/irv.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson S., Oliver C., Prince G.A., Hemming V.G., Pfarr D.S., Wang S.C., et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 98.Garegnani L., Styrmisdottir L., Roson R.P., Escobar L.C., Esteban I., Franco J.V. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst Rev. 2021;11 doi: 10.1002/14651858.CD013757.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu H., Pfarr D.S., Johnson S., Brewah Y.A., Woods R.M., Patel N.K., et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–665. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 100.Mejias A., Chavez-Bueno S., Rios A.M., Aten M.F., Raynor B., Peromingo E., et al. Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the rsv murine model: time versus potency. Antimicrob Agents Chemother. 2005;49:4700–4707. doi: 10.1128/AAC.49.11.4700-4707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu Q., Mclellan J.S., Kallewaard N.L., Ulbrandt N.D., Palaszynski S., Zhang J., et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaj1928. [DOI] [PubMed] [Google Scholar]
- 102.Griffin M.P., Khan A.A., Esser M.T., Jensen K., Takas T., Kankam M.K., et al. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Domachowske J.B., Khan A.A., Esser M.T., Jensen K., Takas T., Villafana T., et al. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J. 2018;37:886–892. doi: 10.1097/INF.0000000000001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hammitt L.L., Dagan R., Yuan Y., Baca C.M., Bosheva M., Madhi S.A., et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837–846. doi: 10.1056/NEJMoa2110275. [DOI] [PubMed] [Google Scholar]
- 105.Keam S.J. Nirsevimab: first approval. Drugs. 2023;83:181–187. doi: 10.1007/s40265-022-01829-6. [DOI] [PubMed] [Google Scholar]
- 106.Tang A., Chen Z., Cox K.S., Su H.P., Callahan C., Fridman A., et al. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat Commun. 2019;10:4153. doi: 10.1038/s41467-019-12137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maas B.M., Lommerse J., Plock N., Railkar R.A., Cheung S., Caro L., et al. Forward and reverse translational approaches to predict efficacy of neutralizing respiratory syncytial virus (RSV) antibody prophylaxis. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Detalle L., Stohr T., Palomo C., Piedra P.A., Gilbert B.E., Mas V., et al. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob Agents Chemother. 2016;60:6–13. doi: 10.1128/AAC.01802-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garriga D., Ferrer-Orta C., Querol-Audi J., Oliva B., Verdaguer N. Role of motif B loop in allosteric regulation of RNA-dependent RNA polymerization activity. J Mol Biol. 2013;425:2279–2287. doi: 10.1016/j.jmb.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 110.Deval J., Hong J., Wang G., Taylor J., Smith L.K., Fung A., et al. Molecular basis for the selective inhibition of respiratory syncytial virus RNA polymerase by 2′-fluoro-4′-chloromethyl-cytidine triphosphate. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Collins P.L., Hill M.G., Cristina J., Grosfeld H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc Natl Acad Sci U S A. 1996;93:81–85. doi: 10.1073/pnas.93.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gilman M., Liu C., Fung A., Behera I., Jordan P., Rigaux P., et al. Structure of the respiratory syncytial virus polymerase complex. Cell. 2019;179:193–204. doi: 10.1016/j.cell.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cao D., Gao Y., Roesler C., Rice S., D'Cunha P., Zhuang L., et al. Cryo-EM structure of the respiratory syncytial virus RNA polymerase. Nat Commun. 2020;11:368. doi: 10.1038/s41467-019-14246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang G., Deval J., Hong J., Dyatkina N., Prhavc M., Taylor J., et al. Discovery of 4′-chloromethyl-2′-deoxy-3′,5′-di-O-isobutyryl-2′-fluorocytidine (ALS-8176), a first-in-class RSV polymerase inhibitor for treatment of human respiratory syncytial virus infection. J Med Chem. 2015;58:1862–1878. doi: 10.1021/jm5017279. [DOI] [PubMed] [Google Scholar]
- 115.Devincenzo J.P., Mcclure M.W., Symons J.A., Fathi H., Westland C., Chanda S., et al. Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med. 2015;373:2048–2058. doi: 10.1056/NEJMoa1413275. [DOI] [PubMed] [Google Scholar]
- 116.Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yoon J.J., Toots M., Lee S., Lee M.E., Ludeke B., Luczo J.M., et al. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00766-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mccurdy L.H., Milstone A., Dummer S. Clinical features and outcomes of paramyxoviral infection in lung transplant recipients treated with ribavirin. J Heart Lung Transpl. 2003;22:745–753. doi: 10.1016/s1053-2498(02)00569-7. [DOI] [PubMed] [Google Scholar]
- 119.Conrad D.A., Christenson J.C., Waner J.L., Marks M.I. Aerosolized ribavirin treatment of respiratory syncytial virus infection in infants hospitalized during an epidemic. Pediatr Infect Dis J. 1987;6:152–158. doi: 10.1097/00006454-198702000-00003. [DOI] [PubMed] [Google Scholar]
- 120.Hall C.B., Walsh E.E., Hruska J.F., Betts R.F., Hall W.J. Ribavirin treatment of experimental respiratory syncytial viral infection. A controlled double-blind study in young adults. JAMA. 1983;249:2666–2670. [PubMed] [Google Scholar]
- 121.Ison M.G., Michaels M.G. RNA respiratory viral infections in solid organ transplant recipients. Am J Transpl. 2009;9(Suppl 4):S166–S172. doi: 10.1111/j.1600-6143.2009.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marcelin J.R., Wilson J.W., Razonable R.R. Oral ribavirin therapy for respiratory syncytial virus infections in moderately to severely immunocompromised patients. Transpl Infect Dis. 2014;16:242–250. doi: 10.1111/tid.12194. [DOI] [PubMed] [Google Scholar]
- 123.Sourimant J., Lieber C.M., Aggarwal M., Cox R.M., Wolf J.D., Yoon J.J., et al. 4′-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Science. 2022;375:161–167. doi: 10.1126/science.abj5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brookes D.W., Coates M., Allen H., Daly L., Constant S., Huang S., et al. Late therapeutic intervention with a respiratory syncytial virus l-protein polymerase inhibitor, PC786, on respiratory syncytial virus infection in human airway epithelium. Br J Pharmacol. 2018;175:2520–2534. doi: 10.1111/bph.14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mirabelli C., Jaspers M., Boon M., Jorissen M., Koukni M., Bardiot D., et al. Differential antiviral activities of respiratory syncytial virus (RSV) inhibitors in human airway epithelium. J Antimicrob Chemother. 2018;73:1823–1829. doi: 10.1093/jac/dky089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tiong-Yip C.L., Aschenbrenner L., Johnson K.D., Mclaughlin R.E., Fan J., Challa S., et al. Characterization of a respiratory syncytial virus l protein inhibitor. Antimicrob Agents Chemother. 2014;58:3867–3873. doi: 10.1128/AAC.02540-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Noton S.L., Nagendra K., Dunn E.F., Mawhorter M.E., Yu Q., Fearns R. Respiratory syncytial virus inhibitor AZ-27 differentially inhibits different polymerase activities at the promoter. J Virol. 2015;89:7786–7798. doi: 10.1128/JVI.00530-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fordyce E., Brookes D.W., Lise-Ciana C., Coates M.S., Hunt S.F., Ito K., et al. Discovery of novel benzothienoazepine derivatives as potent inhibitors of respiratory syncytial virus. Bioorg Med Chem Lett. 2017;27:2201–2206. doi: 10.1016/j.bmcl.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 129.Yu X., Abeywickrema P., Bonneux B., Behera I., Anson B., Jacoby E., et al. Structural and mechanistic insights into the inhibition of respiratory syncytial virus polymerase by a non-nucleoside inhibitor. Commun Biol. 2023;6:1074. doi: 10.1038/s42003-023-05451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guo Q., Qian J., Zeng Q., Zhang L., Zhu X., Zheng J., et al. Characterization of an orally available respiratory syncytial virus l protein polymerase inhibitor DZ7487. Am J Transl Res. 2023;15:1680–1692. [PMC free article] [PubMed] [Google Scholar]
- 131.Liu P., Jamaluddin M., Li K., Garofalo R.P., Casola A., Brasier A.R. Retinoic acid-inducible gene I mediates early antiviral response and toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reina J., Iglesias C. EDP-938, a new antiviral with inhibitory activity against the nucleoprotein of the respiratory syncytial virus. Rev Esp Quimioter. 2023;36:26–29. doi: 10.37201/req/096.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ahmad A., Eze K., Noulin N., Horvathova V., Murray B., Baillet M., et al. EDP-938, a respiratory syncytial virus inhibitor, in a human virus challenge. N Engl J Med. 2022;386:655–666. doi: 10.1056/NEJMoa2108903. [DOI] [PubMed] [Google Scholar]
- 134.Carter M.C., Alber D.G., Baxter R.C., Bithell S.K., Budworth J., Chubb A., et al. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. J Med Chem. 2006;49:2311–2319. doi: 10.1021/jm051185t. [DOI] [PubMed] [Google Scholar]
- 135.Henderson E.A., Alber D.G., Baxter R.C., Bithell S.K., Budworth J., Carter M.C., et al. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. The identification of a clinical candidate. J Med Chem. 2007;50:1685–1692. doi: 10.1021/jm060747l. [DOI] [PubMed] [Google Scholar]
- 136.Chapman J., Abbott E., Alber D.G., Baxter R.C., Bithell S.K., Henderson E.A., et al. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrob Agents Chemother. 2007;51:3346–3353. doi: 10.1128/AAC.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Challa S., Scott A.D., Yuzhakov O., Zhou Y., Tiong-Yip C.L., Gao N., et al. Mechanism of action for respiratory syncytial virus inhibitor RSV604. Antimicrob Agents Chemother. 2015;59:1080–1087. doi: 10.1128/AAC.04119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bitko V., Musiyenko A., Shulyayeva O., Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 139.Devincenzo J., Lambkin-Williams R., Wilkinson T., Cehelsky J., Nochur S., Walsh E., et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2010;107:8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akhtar S., Hughes M.D., Khan A., Bibby M., Hussain M., Nawaz Q., et al. The delivery of antisense therapeutics. Adv Drug Deliv Rev. 2000;44:3–21. doi: 10.1016/s0169-409x(00)00080-6. [DOI] [PubMed] [Google Scholar]
- 141.Teng M.N., Collins P.L. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J Virol. 1999;73:466–473. doi: 10.1128/jvi.73.1.466-473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie Y.Y., Zhao X.D., Jiang L.P., Liu H.L., Wang L.J., Fang P., et al. Inhibition of respiratory syncytial virus in cultured cells by nucleocapsid gene targeted deoxyribozyme (DNAzyme) Antivir Res. 2006;71:31–41. doi: 10.1016/j.antiviral.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 143.Jairath S., Vargas P.B., Hamlin H.A., Field A.K., Kilkuskie R.E. Inhibition of respiratory syncytial virus replication by antisense oligodeoxyribonucleotides. Antivir Res. 1997;33:201–213. doi: 10.1016/s0166-3542(96)01015-7. [DOI] [PubMed] [Google Scholar]
- 144.Money V.A., Mcphee H.K., Mosely J.A., Sanderson J.M., Yeo R.P. Surface features of a mononegavirales matrix protein indicate sites of membrane interaction. Proc Natl Acad Sci U S A. 2009;106:4441–4446. doi: 10.1073/pnas.0805740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Henderson G., Murray J., Yeo R.P. Sorting of the respiratory syncytial virus matrix protein into detergent-resistant structures is dependent on cell-surface expression of the glycoproteins. Virology. 2002;300:244–254. doi: 10.1006/viro.2002.1540. [DOI] [PubMed] [Google Scholar]
- 146.Shahriari S., Gordon J., Ghildyal R. Host cytoskeleton in respiratory syncytial virus assembly and budding. Virol J. 2016;13:161. doi: 10.1186/s12985-016-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hu M., Bogoyevitch M.A., Jans D.A. Impact of respiratory syncytial virus infection on host functions: implications for antiviral strategies. Physiol Rev. 2020;100:1527–1594. doi: 10.1152/physrev.00030.2019. [DOI] [PubMed] [Google Scholar]
- 148.Fleming E.H., Kolokoltsov A.A., Davey R.A., Nichols J.E., Roberts N.J. Respiratory syncytial virus F envelope protein associates with lipid rafts without a requirement for other virus proteins. J Virol. 2006;80:12160–12170. doi: 10.1128/JVI.00643-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ghildyal R., Li D., Peroulis I., Shields B., Bardin P.G., Teng M.N., et al. Interaction between the respiratory syncytial virus G glycoprotein cytoplasmic domain and the matrix protein. J Gen Virol. 2005;86:1879–1884. doi: 10.1099/vir.0.80829-0. [DOI] [PubMed] [Google Scholar]
- 150.Mitra R., Baviskar P., Duncan-Decocq R.R., Patel D., Oomens A.G. The human respiratory syncytial virus matrix protein is required for maturation of viral filaments. J Virol. 2012;86:4432–4443. doi: 10.1128/JVI.06744-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hotulainen P., Paunola E., Vartiainen M.K., Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell. 2005;16:649–664. doi: 10.1091/mbc.E04-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Verkade P., Harder T., Lafont F., Simons K. Induction of caveolae in the apical plasma membrane of Madin–Darby canine kidney cells. J Cell Biol. 2000;148:727–739. doi: 10.1083/jcb.148.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Casanova J.E., Wang X., Kumar R., Bhartur S.G., Navarre J., Woodrum J.E., et al. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin–Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mccurdy L.H., Graham B.S. Role of plasma membrane lipid microdomains in respiratory syncytial virus filament formation. J Virol. 2003;77:1747–1756. doi: 10.1128/JVI.77.3.1747-1756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Radhakrishnan A., Yeo D., Brown G., Myaing M.Z., Iyer L.R., Fleck R., et al. Protein analysis of purified respiratory syncytial virus particles reveals an important role for heat shock protein 90 in virus particle assembly. Mol Cell Proteomics. 2010;9:1829–1848. doi: 10.1074/mcp.M110.001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schmitt A.P., Leser G.P., Waning D.L., Lamb R.A. Requirements for budding of paramyxovirus simian virus 5 virus-like particles. J Virol. 2002;76:3952–3964. doi: 10.1128/JVI.76.8.3952-3964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li D., Jans D.A., Bardin P.G., Meanger J., Mills J., Ghildyal R. Association of respiratory syncytial virus M protein with viral nucleocapsids is mediated by the M2-1 protein. J Virol. 2008;82:8863–8870. doi: 10.1128/JVI.00343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kipper S., Hamad S., Caly L., Avrahami D., Bacharach E., Jans D.A., et al. New host factors important for respiratory syncytial virus (RSV) replication revealed by a novel microfluidics screen for interactors of matrix (M) protein. Mol Cell Proteomics. 2015;14:532–543. doi: 10.1074/mcp.M114.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Utley T.J., Ducharme N.A., Varthakavi V., Shepherd B.E., Santangelo P.J., Lindquist M.E., et al. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. Proc Natl Acad Sci U S A. 2008;105:10209–10214. doi: 10.1073/pnas.0712144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ghildyal R., Baulch-Brown C., Mills J., Meanger J. The matrix protein of human respiratory syncytial virus localises to the nucleus of infected cells and inhibits transcription. Arch Virol. 2003;148:1419–1429. doi: 10.1007/s00705-003-0112-y. [DOI] [PubMed] [Google Scholar]
- 161.Ghildyal R., Ho A., Wagstaff K.M., Dias M.M., Barton C.L., Jans P., et al. Nuclear import of the respiratory syncytial virus matrix protein is mediated by importin beta1 independent of importin alpha. Biochemistry. 2005;44:12887–12895. doi: 10.1021/bi050701e. [DOI] [PubMed] [Google Scholar]
- 162.Ghildyal R., Ho A., Dias M., Soegiyono L., Bardin P.G., Tran K.C., et al. The respiratory syncytial virus matrix protein possesses a Crm1-mediated nuclear export mechanism. J Virol. 2009;83:5353–5362. doi: 10.1128/JVI.02374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shahriari S., Wei K.J., Ghildyal R. Respiratory syncytial virus matrix (M) protein interacts with actin in vitro and in cell culture. Viruses. 2018;10:535. doi: 10.3390/v10100535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jorquera P.A., Mathew C., Pickens J., Williams C., Luczo J.M., Tamir S., et al. Verdinexor (KPT-335), a selective inhibitor of nuclear export, reduces respiratory syncytial virus replication in vitro. J Virol. 2019;93 doi: 10.1128/JVI.01684-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pickens J.A., Tripp R.A. Verdinexor targeting of crm1 is a promising therapeutic approach against RSV and influenza viruses. Viruses. 2018;10:48. doi: 10.3390/v10010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Whitehead S.S., Bukreyev A., Teng M.N., Firestone C.Y., St C.M., Elkins W.R., et al. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73:3438–3442. doi: 10.1128/jvi.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Teng M.N., Whitehead S.S., Bermingham A., St C.M., Elkins W.R., Murphy B.R., et al. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J Virol. 2000;74:9317–9321. doi: 10.1128/jvi.74.19.9317-9321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jin H., Cheng X., Traina-Dorge V.L., Park H.J., Zhou H., Soike K., et al. Evaluation of recombinant respiratory syncytial virus gene deletion mutants in african green monkeys for their potential as live attenuated vaccine candidates. Vaccine. 2003;21:3647–3652. doi: 10.1016/s0264-410x(03)00426-2. [DOI] [PubMed] [Google Scholar]
- 169.Le Nouen C., Brock L.G., Luongo C., Mccarty T., Yang L., Mehedi M., et al. Attenuation of human respiratory syncytial virus by genome-scale codon-pair deoptimization. Proc Natl Acad Sci U S A. 2014;111:13169–13174. doi: 10.1073/pnas.1411290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meng J., Lee S., Hotard A.L., Moore M.L. Refining the balance of attenuation and immunogenicity of respiratory syncytial virus by targeted codon deoptimization of virulence genes. mBio. 2014;5:e1704–e1714. doi: 10.1128/mBio.01704-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schlender J., Bossert B., Buchholz U., Conzelmann K.K. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J Virol. 2000;74:8234–8242. doi: 10.1128/jvi.74.18.8234-8242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Spann K.M., Tran K.C., Chi B., Rabin R.L., Collins P.L. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected] J Virol. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ling Z., Tran K.C., Teng M.N. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J Virol. 2009;83:3734–3742. doi: 10.1128/JVI.02434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boyapalle S., Wong T., Garay J., Teng M., San J.H., Mohapatra S., et al. Respiratory syncytial virus NS1 protein colocalizes with mitochondrial antiviral signaling protein mavs following infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ban J., Lee N.R., Lee N.J., Lee J.K., Quan F.S., Inn K.S. Human respiratory syncytial virus NS 1 targets TRIM25 to suppress RIG-I ubiquitination and subsequent RIG-I-mediated antiviral signaling. Viruses. 2018;10:716. doi: 10.3390/v10120716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goswami R., Majumdar T., Dhar J., Chattopadhyay S., Bandyopadhyay S.K., Verbovetskaya V., et al. Viral degradasome hijacks mitochondria to suppress innate immunity. Cell Res. 2013;23:1025–1042. doi: 10.1038/cr.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lo M.S., Brazas R.M., Holtzman M.J. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of STAT2 expression and alpha/beta interferon responsiveness. J Virol. 2005;79:9315–9319. doi: 10.1128/JVI.79.14.9315-9319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Spann K.M., Tran K.C., Collins P.L. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J Virol. 2005;79:5353–5362. doi: 10.1128/JVI.79.9.5353-5362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ren J., Liu T., Pang L., Li K., Garofalo R.P., Casola A., et al. A novel mechanism for the inhibition of interferon regulatory factor-3-dependent gene expression by human respiratory syncytial virus NS1 protein. J Gen Virol. 2011;92:2153–2159. doi: 10.1099/vir.0.032987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chatterjee S., Luthra P., Esaulova E., Agapov E., Yen B.C., Borek D.M., et al. Structural basis for human respiratory syncytial virus NS1-mediated modulation of host responses. Nat Microbiol. 2017;2 doi: 10.1038/nmicrobiol.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pei J., Beri N.R., Zou A.J., Hubel P., Dorando H.K., Bergant V., et al. Nuclear-localized human respiratory syncytial virus NS1 protein modulates host gene transcription. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang W., Yang H., Kong X., Mohapatra S., San J.H., Hellermann G., et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56–62. doi: 10.1038/nm1174. [DOI] [PubMed] [Google Scholar]
- 183.Gonzalez P.A., Prado C.E., Leiva E.D., Carreno L.J., Bueno S.M., Riedel C.A., et al. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A. 2008;105:14999–15004. doi: 10.1073/pnas.0802555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Munir S., Le Nouen C., Luongo C., Buchholz U.J., Collins P.L., Bukreyev A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J Virol. 2008;82:8780–8796. doi: 10.1128/JVI.00630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Becker Y. Respiratory syncytial virus (RSV)-induced allergy may be controlled by IL-4 and CX3C fractalkine antagonists and CpG ODN as adjuvant: hypothesis and implications for treatment. Virus Genes. 2006;33:253–264. doi: 10.1007/s11262-006-0063-y. [DOI] [PubMed] [Google Scholar]
- 186.Munir S., Hillyer P., Le Nouen C., Buchholz U.J., Rabin R.L., Collins P.L., et al. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Woo H.M., Kim K.S., Lee J.M., Shim H.S., Cho S.J., Lee W.K., et al. Single-stranded DNA aptamer that specifically binds to the influenza virus NS1 protein suppresses interferon antagonism. Antivir Res. 2013;100:337–345. doi: 10.1016/j.antiviral.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 188.Behera A.K., Matsuse H., Kumar M., Kong X., Lockey R.F., Mohapatra S.S. Blocking intercellular adhesion molecule-1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem Biophys Res Commun. 2001;280:188–195. doi: 10.1006/bbrc.2000.4093. [DOI] [PubMed] [Google Scholar]
- 189.Iesato K., Tatsumi K., Saito K., Ogasawara T., Sakao S., Tada Y., et al. Tiotropium bromide attenuates respiratory syncytial virus replication in epithelial cells. Respiration. 2008;76:434–441. doi: 10.1159/000151729. [DOI] [PubMed] [Google Scholar]
- 190.Harris J., Werling D. Binding and entry of respiratory syncytial virus into host cells and initiation of the innate immune response. Cell Microbiol. 2003;5:671–680. doi: 10.1046/j.1462-5822.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 191.Martinez I., Melero J.A. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J Gen Virol. 2000;81:2715–2722. doi: 10.1099/0022-1317-81-11-2715. [DOI] [PubMed] [Google Scholar]
- 192.Donalisio M., Rusnati M., Cagno V., Civra A., Bugatti A., Giuliani A., et al. Inhibition of human respiratory syncytial virus infectivity by a dendrimeric heparan sulfate-binding peptide. Antimicrob Agents Chemother. 2012;56:5278–5288. doi: 10.1128/AAC.00771-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lingemann M., Mccarty T., Liu X., Buchholz U.J., Surman S., Martin S.E., et al. The alpha-1 subunit of the Na+,K+-ATPase (ATP1A1) is required for macropinocytic entry of respiratory syncytial virus (RSV) in human respiratory epithelial cells. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ferrari P., Ferrandi M., Valentini G., Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+–K+–ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R529–R535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 195.El S.C., Bush A.J., Harrison L.M., Aitken J.A., Devincenzo J.P. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204:996–1002. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Garofalo R.P., Kolli D., Casola A. Respiratory syncytial virus infection: mechanisms of redox control and novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:186–217. doi: 10.1089/ars.2011.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dowell S.F., Papic Z., Bresee J.S., Larranaga C., Mendez M., Sowell A.L., et al. Treatment of respiratory syncytial virus infection with vitamin a: a randomized, placebo-controlled trial in Santiago, Chile. Pediatr Infect Dis J. 1996;15:782–786. doi: 10.1097/00006454-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 198.Kawasaki Y., Hosoya M., Katayose M., Suzuki H. The efficacy of oral vitamin a supplementation for measles and respiratory syncytial virus (RSV) infection. Kansenshogaku Zasshi. 1999;73:104–109. doi: 10.11150/kansenshogakuzasshi1970.73.104. [DOI] [PubMed] [Google Scholar]
- 199.Zang N., Xie X., Deng Y., Wu S., Wang L., Peng C., et al. Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J Virol. 2011;85:13061–13068. doi: 10.1128/JVI.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Castro S.M., Guerrero-Plata A., Suarez-Real G., Adegboyega P.A., Colasurdo G.N., Khan A.M., et al. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med. 2006;174:1361–1369. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Komaravelli N., Tian B., Ivanciuc T., Mautemps N., Brasier A.R., Garofalo R.P., et al. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of NRF2. Free Radic Biol Med. 2015;88:391–403. doi: 10.1016/j.freeradbiomed.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hu M., Schulze K.E., Ghildyal R., Henstridge D.C., Kolanowski J.L., New E.J., et al. Respiratory syncytial virus co-opts host mitochondrial function to favour infectious virus production. Elife. 2019;8 doi: 10.7554/eLife.42448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cui R., Wang Y., Wang L., Li G., Lan K., Altmeyer R., et al. Cyclopiazonic acid, an inhibitor of calcium-dependent atpases with antiviral activity against human respiratory syncytial virus. Antivir Res. 2016;132:38–45. doi: 10.1016/j.antiviral.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 204.Baram-Pinto D., Shukla S., Gedanken A., Sarid R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small. 2010;6:1044–1050. doi: 10.1002/smll.200902384. [DOI] [PubMed] [Google Scholar]
- 205.Vonnemann J., Sieben C., Wolff C., Ludwig K., Bottcher C., Herrmann A., et al. Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale. 2014;6:2353–2360. doi: 10.1039/c3nr04449a. [DOI] [PubMed] [Google Scholar]
- 206.Antoine T.E., Mishra Y.K., Trigilio J., Tiwari V., Adelung R., Shukla D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antivir Res. 2012;96:363–375. doi: 10.1016/j.antiviral.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sametband M., Kalt I., Gedanken A., Sarid R. Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl Mater Inter. 2014;6:1228–1235. doi: 10.1021/am405040z. [DOI] [PubMed] [Google Scholar]
- 208.Liang J.J., Wei J.C., Lee Y.L., Hsu S.H., Lin J.J., Lin Y.L. Surfactant-modified nanoclay exhibits an antiviral activity with high potency and broad spectrum. J Virol. 2014;88:4218–4228. doi: 10.1128/JVI.03256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sun L., Singh A.K., Vig K., Pillai S.R., Singh S.R. Silver nanoparticles inhibit replication of respiratory syncytial virus. J Biomed Nanotechnol. 2008;4:149–158. [Google Scholar]
- 210.Yang X.X., Li C.M., Huang C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8:3040–3048. doi: 10.1039/c5nr07918g. [DOI] [PubMed] [Google Scholar]
- 211.Cagno V., Andreozzi P., D'Alicarnasso M., Jacob S.P., Mueller M., Galloux M., et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat Mater. 2018;17:195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 212.Leydet A., Moullet C., Roque J.P., Witvrouw M., Pannecouque C., Andrei G., et al. Polyanion inhibitors of HIV and other viruses. 7. Polyanionic compounds and polyzwitterionic compounds derived from cyclodextrins as inhibitors of HIV transmission. J Med Chem. 1998;41:4927–4932. doi: 10.1021/jm970661f. [DOI] [PubMed] [Google Scholar]
- 213.Jones S.T., Cagno V., Janecek M., Ortiz D., Gasilova N., Piret J., et al. Modified cyclodextrins as broad-spectrum antivirals. Sci Adv. 2020;6 doi: 10.1126/sciadv.aax9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Skaricic D., Traube C., De B., Joh J., Boyer J., Crystal R.G., et al. Genetic delivery of an anti-RSV antibody to protect against pulmonary infection with RSV. Virology. 2008;378:79–85. doi: 10.1016/j.virol.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 215.Schnepp B.C., Johnson P.R. Adeno-associated virus delivery of broadly neutralizing antibodies. Curr Opin HIV AIDS. 2014;9:250–256. doi: 10.1097/COH.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Luo X.M., Maarschalk E., O'Connell R.M., Wang P., Yang L., Baltimore D. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood. 2009;113:1422–1431. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]
- 217.Hung K.L., Meitlis I., Hale M., Chen C.Y., Singh S., Jackson S.W., et al. Engineering protein-secreting plasma cells by homology-directed repair in primary human B cells. Mol Ther. 2018;26:456–467. doi: 10.1016/j.ymthe.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Johnson M.J., Laoharawee K., Lahr W.S., Webber B.R., Moriarity B.S. Engineering of primary human B cells with CRISPR/Cas9 targeted nuclease. Sci Rep. 2018;8 doi: 10.1038/s41598-018-30358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Voss J.E., Gonzalez-Martin A., Andrabi R., Fuller R.P., Murrell B., Mccoy L.E., et al. Reprogramming the antigen specificity of B cells using genome-editing technologies. Elife. 2019;8 doi: 10.7554/eLife.42995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Moffett H.F., Harms C.K., Fitzpatrick K.S., Tooley M.R., Boonyaratanakornkit J., Taylor J.J. B cells engineered to express pathogen-specific antibodies protect against infection. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aax0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gao S., Huang T., Song L., Xu S., Cheng Y., Cherukupalli S., et al. Medicinal chemistry strategies towards the development of effective SARS-CoV-2 inhibitors. Acta Pharm Sin B. 2022;12:581–599. doi: 10.1016/j.apsb.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Erlanson D.A., Fesik S.W., Hubbard R.E., Jahnke W., Jhoti H. Twenty years on: the impact of fragments on drug discovery. Nat Rev Drug Discov. 2016;15:605–619. doi: 10.1038/nrd.2016.109. [DOI] [PubMed] [Google Scholar]
- 223.Liu C., Hu L., Dong G., Zhang Y., Ferreira D.S.E., Liu X., et al. Emerging drug design strategies in anti-influenza drug discovery. Acta Pharm Sin B. 2023;13:4715–4732. doi: 10.1016/j.apsb.2023.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]









