Abstract
A commercially available capture enzyme-linked immunosorbent assay (ELISA) for the detection of specific immunoglobulin M (IgM) and IgG antibodies produced during dengue infection (PanBio Dengue Duo) was evaluated with paired serum specimens from 176 patients. Diagnosis was based on a hemagglutination inhibition (HAI) assay, with patients having either primary dengue (n = 90), secondary dengue (n = 58), or no dengue (n = 28) infection. The combined use of IgM and IgG (sensitivity, 99%; specificity, 96%) was superior to the use of IgM alone (sensitivity, 88%; specificity, 96%) or IgG alone (sensitivity, 85%; specificity, 96%). Furthermore, with the first serum sample of the pair of serum samples, the ELISA was able to diagnose significantly more cases of dengue than the HAI assay (55% versus 14%). The results of the IgG capture ELISA gave a significant correlation with those of the HAI assay (r = 0.91; P < 0.0001), and the IgG capture ELISA could be used to distinguish between primary and secondary infection. The best distinction was observed when an IgG cutoff ratio of 3.0 was used, with 88% of primary infections and 98% of secondary infections being correctly classified. This ELISA should prove to be useful in the clinical diagnosis of dengue infection.
Dengue is the most important mosquito-borne disease in the world in terms of morbidity, mortality, and economic costs, with an estimated 100 million cases per year (9). Serology is useful in the diagnosis of dengue infections and in differentiating between primary and secondary infections (3, 4, 7).
Patients with a primary infection produce an immunoglobulin M (IgM) response to dengue virus 3 to 5 days after the onset of fever, and the IgM titer continues to rise for 1 to 3 weeks and is detectable for up to 6 months. Anti-dengue virus IgG antibodies are produced approximately 2 weeks after infection and are maintained for life, although at a hemagglutination inhibition (HAI) assay titer of ≤1:640 (3, 5). In contrast, during secondary infection IgM may take a long time to be detected or may be undetectable, while the IgG titer rises rapidly from 1 to 2 days after the onset of symptoms (3, 4). The HAI assay titer rises to ≥1:2,560, and these levels persist for 30 to 40 days before returning to levels of ≤1:640 (3).
Traditionally, HAI assays have been used for the diagnosis of dengue. The HAI assay requires paired serum specimens collected at least 7 days apart and is considered positive if a fourfold or greater increase in antibody titer is demonstrated (2). Furthermore, a single serum sample demonstrating a titer of ≥1:2,560 is diagnostic of a secondary dengue infection (11).
Doubts concerning the general applicability of the HAI assay have been raised due to variations in the potencies of the hemagglutinins made in different laboratories. Commercially available enzyme-linked immunosorbent assays (ELISAs) offer improvements over the HAI assay for the serological diagnosis of dengue infections. ELISAs reduce interlaboratory variation in dengue serology through the use of a standard calibrator serum sample. Unlike for HAI assays, pretreatment of sera (i.e., acetone extraction) is not required, and a diagnosis can be made from the results for a single serum sample. Differentiation between primary and secondary infections may also be made with a single dilution of serum rather than with a series of dilutions. A commercially available capture ELISA for the detection of IgM and IgG antibodies during dengue infection has recently become available (PanBio Dengue Duo). In this study, the Dengue Duo ELISA has been compared to the HAI assay by using paired serum specimens from patients with or without dengue infection.
MATERIALS AND METHODS
Serum samples.
All serum samples used in this study were submitted for routine pathological investigation at Singapore General Hospital. Paired serum samples from 176 patients suspected of having dengue infection were assayed. Diagnosis was based on the results of an HAI assay, with patients having primary dengue (n = 90), secondary dengue (n = 58), or no dengue (n = 28) infection.
HAI assay.
Kaolin-absorbed sera were tested for antibodies by HAI assay as described previously (2), except the assay was modified to a microtiter plate format. Dengue virus types 1 and 2 were used. Antigens were produced by sucrose-acetone extraction of the brains of suckling mice infected with the following virus strains: dengue virus DEN-1 Hawaii and DEN-2 TR1751.
PanBio Dengue Duo ELISA.
In the PanBio Dengue Duo IgM and IgG capture ELISA, two microtiter plates are supplied; one contains stabilized dengue virus type 1 to 4 antigens and the other contains either anti-human IgM or anti-human IgG bound to separate microwells. Peroxidase-labelled anti-dengue virus-monoclonal antibody (125 μl/well) is added to the antigen plate to solubilize the antigens and form antibody-antigen complexes. Concurrently, 100 μl of patient serum, diluted 1:100 in the diluent provided, is added to each well of an assay plate containing either bound anti-human IgM or anti-human IgG that captures the IgM or the IgG in the patient’s serum, respectively. Both plates are incubated for 1 h at room temperature (antigen plate) or 37°C (assay plate), after which time the assay plate is washed, and 100 μl of the antibody-antigen complexes per well is transferred from the antigen plate to the assay plate. These complexes are then captured by dengue virus-specific IgM or IgG during an incubation for 1 h at 37°C. The plate is then washed and the bound complexes are visualized through the addition of 100 μl of a tetramethylbenzidine substrate per well. After 10 min, the reaction is stopped by the addition of 100 μl of 1 M phosphoric acid per well, and the strips are read at 450 nm with a microtiter plate reader. Positivity is determined by comparison to reference sera containing IgM and IgG provided with the assay kit (cutoff calibrators). The cutoff for sera containing IgM represents the level at which the transient IgM level rises above the background level for populations with active primary and secondary dengue infection, while the cutoff for containing IgG sera represents a level of IgG above that found in patients with primary dengue or past dengue infection. That is, the high IgG response (HAI assay titer, ≥1:2,560) occurs during secondary infection but not primary infection and generally lasts for 30 to 40 days before declining to levels below an HAI assay titer of 1:640 (3). Consequently, a positive sample was defined as having an IgM or IgG sample:calibrator absorbance ratio of ≥1.0 and a negative sample was defined as having both IgM and IgG sample:calibrator absorbance ratios of <1.0.
Data analysis.
Clinical data were correlated with serum antibody levels. The proportions of patients with levels above the designated cutoff for the ELISA were determined. Fisher’s exact test was performed to compare sensitivities and specificities. Spearman’s correlation analysis was performed to compare ELISA ratios and HAI assay titers for individual sera. Analysis of variance (ANOVA) and the Tukey-Kramer multiple comparison test were used to compare the mean IgG ELISA ratios for different HAI assay titers. Statistical analyses were performed by using Instat (Graphpad Software Inc., San Diego, Calif.).
RESULTS
IgM capture ELISA.
By using paired serum specimens, primary dengue infection was detected in 84 of 90 (93%) patients and secondary dengue infection was detected in 46 of 58 (79%) patients by use of IgM alone (ratio, ≥1.0), whereas only 1 of 28 (4%) patients with no infection was found to have IgM antibodies. Consequently, when IgM was used, 130 of 148 patients were diagnosed as having dengue infection (sensitivity, 88%), and the corresponding specificity was 96% (Table 1).
TABLE 1.
Diagnosis of dengue with paired sera
| Method | Specificitya | No. of patients with the following diagnosis by HAI assay/total no. of patients (%):
|
||
|---|---|---|---|---|
| Primary dengue | Secondary dengue | Total | ||
| IgM ratio of ≥1.0 | 27/28 (96) | 84/90 (93) | 46/58 (79) | 130/148 (88) |
| IgG ratio of ≥1.0 | 27/28 (96) | 68/90 (76) | 58/58 (100) | 126/148 (85) |
| IgM ratio of ≥1.0 or IgG ratio of ≥1.0 | 27/28 (96) | 89/90 (99) | 58/58 (100) | 147/148 (99) |
Data represent number of positive serum samples/total number of serum samples (percent).
IgG capture ELISA.
By using paired serum specimens, primary dengue infection was detected in 68 of 90 (76%) patients and secondary dengue infection was detected in 58 of 58 (100%) patients by use of IgG alone (ratio, ≥1.0), whereas only 1 of 28 (4%) patients with no infection was found to have IgG antibodies. Consequently, when IgG was used, 126 of 148 patients were diagnosed as having dengue infection (sensitivity, 85%), and the corresponding specificity was 96% (Table 1).
Combined use of IgM and IgG ELISA.
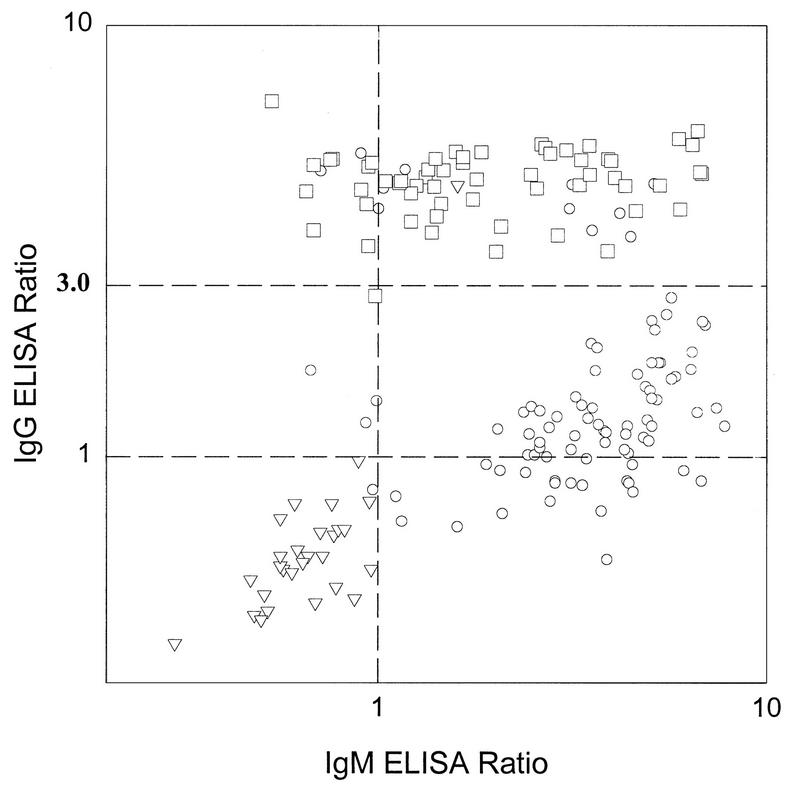
When either an IgM ratio of ≥1.0 or an IgG ratio of ≥1.0 was used to define dengue infection, sensitivity was improved significantly (by Fisher’s exact test, P < 0.0001), while specificity was unchanged. Specificity with paired serum specimens was 96% (27 of 28), while sensitivity was 99% for primary dengue infections (89 of 90), 100% for secondary dengue infections (58 of 58), and 99% for all dengue infections (147 of 148) (Table 1). Of the 6 specimens IgM negative for primary dengue infection, 5 were IgG positive, while all 12 specimens IgM negative for secondary dengue infection were IgG positive (Fig. 1).
FIG. 1.
Comparison of IgM and IgG ELISA ratios for S2 sera from 176 patients. By using the HAI assay as the reference test, 90 patients were diagnosed as having primary dengue (circles), 58 were diagnosed as having secondary dengue (squares), and 28 had no evidence of dengue infection (triangles). ELISA IgM ratios of 1.0 and an IgG ratio of 3.0 are delineated with broken lines.
Only two samples had discrepant results between ELISA and the HAI assay. For one serum sample the IgM ELISA ratio was just below the cutoff (ratio, 0.97), while a fourfold increase in the HAI assay titer was demonstrated (titer range, <10 to 40). For the other serum sample, a significant increase in the IgM titer and a positive IgG titer were demonstrated by ELISA, while the HAI assay showed only a twofold increase in titer, even though it was high (titer range, 320 to 640).
Early diagnosis of dengue infection (use of S1 sera).
Because the HAI assay requires the use of paired serum samples for the diagnosis of primary dengue infection but not secondary dengue, for which the titer of the first (S1) serum specimen is ≥1:2,560, the performance of the ELISA with the S1 serum specimen was evaluated (Table 2). The IgM ELISA detected 53 of 148 (36%) cases of dengue with the S1 serum specimen, while the IgG ELISA detected 47 of 148 (32%) cases of dengue. This was significantly more than the 21 of 148 (14%) cases detected by the HAI assay (by Fisher’s exact test, P = 0.0005). When the combined use of IgM and IgG was used to detect dengue infection, the sensitivity was increased to 82 of 148 (55%), which was significantly higher than that from the use of IgM or IgG alone (by Fisher’s exact test, P < 0.0001) (Table 2). Indeed, for 82 of 84 ELISA-positive serum specimens (98%), dengue infection was subsequently detected through the use of the HAI assay with the second (S2) serum specimen, indicating that the ELISA has a high positive predictive value for dengue infection.
TABLE 2.
Diagnosis of dengue with sera obtained upon admission
| Method | Specificitya | No. of patients with the following diagnosis by HAI assay/total no. of patients (%):
|
||
|---|---|---|---|---|
| Primary dengue | Secondary dengue | Total | ||
| IgM ratio of ≥1.0 | 28/28 (100) | 33/90 (37) | 20/58 (34) | 53/148 (36) |
| IgG ratio of ≥1.0 | 27/28 (96) | 7/90 (8) | 40/58 (69) | 47/148 (32) |
| IgM ratio of ≥1.0 or IgG ratio of ≥1.0 | 27/28 (96) | 39/90 (43) | 43/58 (74) | 82/148 (55) |
| HAI assay titer of ≥2,560 (S1) | 28/28 (100) | 0/90 (0) | 21/58 (36) | 21/148 (14) |
Data represent number of positive serum samples/total number of serum samples (percent).
Distinction between primary and secondary infection.
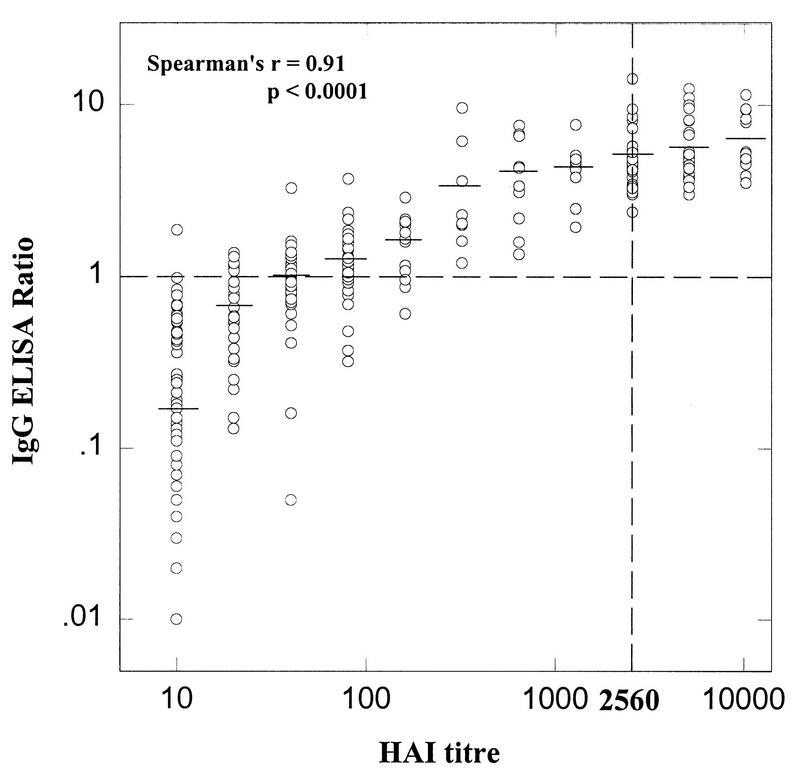
The IgG capture ELISA ratio showed an excellent correlation with the HAI assay titer (Spearman’s r = 0.91; P < 0.0001) (Fig. 2). Furthermore, the mean ELISA ratio was significantly correlated with increasing HAI assay titer (by ANOVA, P < 0.0001) and there was a significant increase in the proportion of patients showing an elevated IgG ratio (ratio, ≥1.0) with increasing HAI assay titer (by Fisher’s exact test, P < 0.0001) (Table 3). The best distinction between primary and secondary dengue was observed when an IgG cutoff of 3.0 was used, with 79 of 90 (88%) of primary infections and 57 of 58 (98%) of secondary infections being correctly classified. By using this cutoff value, there was a highly significant difference between the number of patients with primary and secondary dengue showing elevated IgG titers (by Fisher’s exact test, P < 0.0001).
FIG. 2.
Comparison of IgG ELISA ratio and HAI assay titer for 352 serum specimens (176 paired serum specimens). The broken lines represent an HAI assay titer of 2,560 and an ELISA ratio of 1.0. The mean ELISA ratio for each HAI assay titer is represented by a horizontal bar.
TABLE 3.
Comparison of the Singapore General Hospital HAI assay and PanBio Dengue Duo IgG capture ELISA
| HAI assay titer | No. of serum specimens | Mean ELISA ratioa | % Serum specimens with the following resultb
|
||
|---|---|---|---|---|---|
| ELISA ratio of >1.0 | ELISA ratio of >2.0 | ELISA ratio of >3.0 | |||
| ≥10 | 127 | 0.17 | 4 | 0 | 0 |
| 20 | 26 | 0.68 | 27 | 0 | 0 |
| 40 | 37 | 1.02 | 49 | 3 | 3 |
| 80 | 37 | 1.27 | 76 | 8 | 3 |
| 160 | 14 | 1.64 | 79 | 36 | 0 |
| 320 | 9 | 3.38 | 100 | 67 | 33 |
| 640 | 10 | 4.11 | 100 | 80 | 70 |
| 1280 | 13 | 4.35 | 100 | 92 | 85 |
| 2560 | 29 | 5.18 | 100 | 100 | 97 |
| 5120 | 34 | 5.68 | 100 | 100 | 100 |
| ≥10240 | 16 | 6.40 | 100 | 100 | 100 |
By one-way ANOVA, P < 0.0001 (extremely significant variation between column means). By Tukey-Kramer multiple comparisons test, P < 0.0001 (significant difference between all means).
By chi-square test for trend, P < 0.0001 (significant linear trend between HAI assay titer and the proportion of serum specimens with elevated ELISA ratios); by chi-square test for independence, P < 0.0001 (HAI assay titer and proportion of sera with elevated ELISA ratios are significantly associated).
Use of the IgG:IgM ratio to distinguish between primary and secondary dengue infection was also investigated, because this ratio has been reported to be useful (4). However, the IgG:IgM ratio was not as useful as IgG alone (above), with 80 of 90 (88%) primary infections and 45 of 58 (83%) secondary infections being correctly classified. Indeed, the correct classification of secondary dengue by use of the IgG:IgM ratio was significantly lower than that by use of an IgG ratio of ≥3.0 (by Fisher’s exact test, P = 0.0083).
DISCUSSION
A rapid and accurate method for the detection of dengue fever is important for both the clinician and the patient. The commercially available ELISA described in this report (PanBio Dengue Duo) is suitable for the detection of anti-dengue virus IgM and IgG antibodies in a routine clinical laboratory. The utility of a capture IgM and capture IgG ELISA for the diagnosis of dengue has been reported previously (4, 10). However, the PanBio Dengue Duo ELISA is different from the previously described capture IgM and IgG ELISAs and many other capture ELISAs because serum is incubated in the anti-human antibody plate at the same time that peroxidase-conjugated monoclonal antibody is incubated with antigen. This format decreases the number of assay steps and speeds up the diagnosis of dengue (1). For the PanBio Dengue Duo ELISA, the total assay time is less than 2.5 h.
By using the HAI assay as the reference test, the combined use of IgM and IgG determinations (either IgM or IgG positivity was considered to indicate dengue infection) led to increased sensitivity (99%) in the diagnosis of dengue infection without a decrease in specificity (96%) when paired sera were used. The use of IgM or IgG alone gave significantly lower sensitivities (88 and 85%, respectively). Similar results showing the improvement with the combination of IgM and IgG have been reported previously (4, 10).
High levels of antibody cross-reactivity have been reported for patients with dengue and Japanese encephalitis (JE) virus infections (4, 8). Because the incidence of JE is relatively low in Singapore, sera from patients with JE have not been tested, although the specificity of the PanBio Dengue Duo ELISA for the detection of dengue in these patients should be considered in other countries where JE is more prevalent. The primary cross-reactivity between dengue and JE virus has been reported to occur at the IgG level (8). Consequently, JE virus cross-reactivity in the dengue ELISA should be a concern only for the minority of patients who are IgG positive and IgM negative. In this study, only 12% of patients had this antibody profile.
The ELISA was able to detect 55% of the dengue infections through the use of the first serum sample alone, whereas by the HAI assay dengue could be detected in only 14% of the patients. Consequently, a second serum sample would be needed to be assayed by the ELISA for only 45% of the patients. Previous studies have also suggested that diagnosis based on the IgM titer may take 6 to 7 days after the onset of infection (3, 4, 6, 10). It is important that only 1 of 148 patients with dengue infection was not identified when a second serum sample was tested by the ELISA, and this patient (primary dengue) had an IgM value just below the cutoff value (0.97) as well as a very low HAI assay titer (1:40).
Because secondary dengue may be a more serious form of the disease, the use of the ELISA to distinguish it from primary dengue was also investigated. Traditionally, the HAI assay has been used to distinguish between primary and secondary dengue infections, with a titer greater than or equal to 1:2,560 considered indicative of secondary dengue (11). The results of the IgG capture ELISA used in this study showed an excellent correlation with those of the HAI assay (r = 0.91). Consequently, this ELISA could be used to distinguish between the different states of the disease. When an IgG ELISA ratio of 3.0 was used as the cutoff, 88% of the primary infections and 98% of the secondary infections were correctly classified, and this method was superior to the use of IgG:IgM ratio reported previously (4).
The commercially available assay evaluated in this study should be a useful aid in the diagnosis of dengue infection because it shows excellent sensitivity and specificity and overcomes many of the problems associated with the HAI assay.
REFERENCES
- 1.Chong C F, Ngoh B L, Tan H C, Yap E H, Singh M, Chan L, Chan Y C. A shortened dengue IgM capture ELISA using simultaneous incubation of antigen and peroxidase-labelled monoclonal antibody. Clin Diagn Virol. 1994;1:335–341. doi: 10.1016/0928-0197(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 2.Clarke D H, Casals J. Techniques for hemagglutination and hemagglutination inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 3.Gubler D J. Serological diagnosis of dengue/dengue haemorrhagic fever. Dengue Bull. 1996;20:20–23. [Google Scholar]
- 4.Innis B L, Nisalak A, Nammanitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke C H. An enzyme-linked immunosorbant assay to characterise dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 5.Lam S K. Detection of specific IgM in dengue infection. Southeast Asian J Trop Med Public Health. 1987;18:532–538. [PubMed] [Google Scholar]
- 6.Lam S K. Rapid dengue diagnosis and interpretation. Malay J Pathol. 1993;15:9–12. [PubMed] [Google Scholar]
- 7.Lam S K. Application of rapid laboratory diagnosis in dengue control. Asian Pac J Mol Biol Biotechnol. 1995;3:351–355. [Google Scholar]
- 8.Makino Y, Tadano M, Saito M, Maneekarn N, Sittisombut N, Sirisanthana V, Poneprasert B, Fukunaga T. Studies on serological cross-reaction in sequential flavivirus infections. Microbiol Immunol. 1994;38:951–955. doi: 10.1111/j.1348-0421.1994.tb02152.x. [DOI] [PubMed] [Google Scholar]
- 9.Monath T P. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruechusatawat K, Morita K, Tanaka M, Vongcheree S, Rojanasuphot S, Warachit P, Kanai K, Thongtradol P, Nimnakorn P, Kanungkid S, Igarashi A. Daily observation of antibody levels among dengue patients detected by enzyme-linked immunosorbent assay (ELISA) Jpn J Trop Med Hyg. 1994;22:9–12. [Google Scholar]
- 11.World Health Organization. Dengue haemorrhagic fever diagnosis, treatment and control. Geneva, Switzerland: World Health Organization; 1986. [Google Scholar]