Abstract
Adebrelimab is a fully humanized monoclonal antibody against programmed cell death-ligand 1 (PD-L1) that has been approved in combination with chemotherapy as the first-line treatment for extensive-stage small cell lung cancer (ES-SCLC). This approval was driven by the landmark CAPSTONE-1 trial, where adebrelimab demonstrated superior survival outcomes: median overall survival (mOS) improved from 12.8 to 15.3 months (HR=0.72, p=0.0017) and 3-year survival rates doubled (21.1% vs 10.5%) compared to chemotherapy alone. Based on this systemic treatment, addition of sequential thoracic radiotherapy achieved unprecedented mOS of 22.9 months in a Phase II trial. In limited-stage small cell lung cancer (LS-SCLC), the initial results of adebrelimab combined with concurrent chemoradiotherapy are promising. Whether in first-line or later-line treatment, there are numerous ongoing clinical trials to explore the potential of the novel adebrelimab-based regimen, and the results are highly anticipated. Despite ongoing efforts to identify biomarkers that may guide treatment decisions, no validated prognostic or predictive biomarkers are currently available for SCLC. This review summarizes the present role of adebrelimab in SCLC and outlines novel strategies aimed to further improve survival outcome.
Keywords: limited-stage small cell lung cancer, extensive-stage small cell lung cancer, immunotherapy, immunochemotherapy, radiotherapy
Introduction
Lung cancer is one of the most prevalent malignancies globally, with small cell lung cancer (SCLC) accounting for approximately 15% of all cases.1,2 As a type of neuroendocrine cancer, aggressive SCLC is characterized by hostile behavior, including rapid growth, early metastasis, high recurrence rates, and unfavorable prognosis, leading to a median survival of less than 2 years for patients with limited-stage disease and approximately 1 year for those with extensive-stage.1,3,4 For decades, etoposide combined with either carboplatin or cisplatin has become the cornerstone of treatment for chemotherapy-sensitive SCLC, despite its limited efficacy. The overactivation of the programmed cell death-1/programmed death-ligand 1 (PD-1/PD-L1) pathway in tumors is linked to poor prognosis, and as immunotherapy advances, immune checkpoint blockade has improved the clinical outcomes for the population with cancer, including SCLC.5 The success of Phase III IMpower133 and CASPIAN trials led to the approval of atezolizumab and durvalumab, in combination with platinum-based chemotherapy, as standard first-line treatment options for extensive-stage small cell lung cancer (ES-SCLC) by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).6–9 There is no evidence supporting the application of immunotherapy for limited stage small cell lung cancer (LS-SCLC) until the ADRIATIC trial, which is a vital breakthrough with dual positive primary endpoints of positive overall survival (OS) and progression-free survival (PFS) representing immunotherapy has the potential to reshape the treatment landscape of LS-SCLC.10 Despite the improved therapeutic outcomes offered by chemotherapy-immunotherapy combinations, unmet survival needs persist in the management of SCLC. Adebrelimab faces these clinical challenges through its unique antibody design, optimized molecular architecture and innovative integration of other therapeutic strategies.
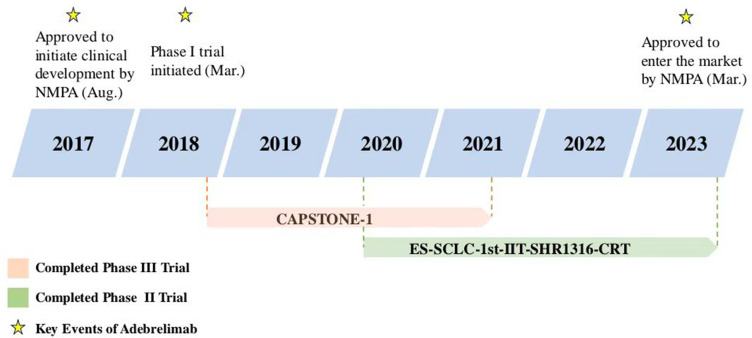
Adebrelimab (SHR-1316) is a novel fully humanized immunoglobulin G4 (IgG4) monoclonal anti-PD-L1 antibody, which has been approved by National Medical Products Administration (NMPA) in China as the first-line treatment in combination with carboplatin and etoposide for adult patients with ES-SCLC (Figure 1).11 In addition, the 2023 Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of SCLC endorse its role for extensive-stage patients with Level 1 recommendation. The results of the safety run-in stage of SHR-1316-III-302 demonstrated the potential of adebrelimab combined with chemoradiotherapy for LS-SCLC. Beyond SCLC, adebrelimab is also being explored as part of combination therapy for other malignancies, including esophageal squamous cell carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer, and colorectal cancer.12–15
Figure 1.
Key milestones in the development of adebrelimab for the treatment of extensive-stage small cell lung cancer. NMPA National Medical Products Administration.
This article summarized the pharmacological properties of adebrelimab (Table 1) and focused on the clinical data and predictive biomarker of adebrelimab in combination with other therapeutics for SCLC treatment.
Table 1.
Key Pharmacological Properties of Adebrelimab
| Pharmacodynamic Properties | Reference | |
|---|---|---|
| Mechanism of action | A fully humanized IgG4 monoclonal antibody designed to target PD-L1, a key component of the PD-1/PD-L1 immune checkpoint signaling pathway | [11] |
| Selectively blocks the interaction of PD-L1 with PD-1 and B7-1 to activate T-cell and improve anti-tumor immune responses | [16,17] | |
| Reactivates anti-tumor immune response without inducing ADCP, ADCR, ADCC, CDC | [18–27] | |
| General profile | Administered as an intravenous infusion | [11] |
| Drug exposure increases dose-proportionally over the dose range 3–20 mg/kg | ||
| Steady state achieved at about 12 weeks after multiple doses | ||
| Systemic accumulation ratio: 1.3 | ||
| Volume of distribution at steady state: 4.35 L | ||
| Clearance: 0.23 L/day | ||
| Terminal elimination half-life: 12 days | ||
| Special populations | Dose modifications not needed for elderly patients | * |
| Clearance clinically unaffected by mild to moderate kidney dysfunction and mild hepatic impairment; dose adjustments not needed in these patients | ||
| Effects of severe kidney dysfunction and moderate to severe hepatic impairment on pharmacokinetics unknown | ||
| Potential drug interactions | Unknown (metabolic drug-drug interactions are not expected) | * |
Note: *Information about special population and potential drug interactions are available from: https://www.shhrp.com.
Abbreviations: IgG4, immunoglobulin G4; PD-L1, programmed cell death-ligand 1; PD-1, programmed death-ligand 1; ADCP, antibody-dependent cellular phagocytosis; ADCR, antibody-dependent cell-mediated cytokine release; ADCC, antibody-dependent cellular cytotoxicity responses; CDC, complement dependent cytotoxicity.
Pharmacological Properties of Adebrelimab
Pharmacodynamic Properties
Adebrelimab (SHR-1316) is a fully-humanized IgG4 monoclonal antibody, different from atezolizumab and durvalumab in the immunoglobulin G subtypes; exhibits high affinity with Kd (dissociation constant) of 27–28 pmol/L.11 As monoclonal antibody against PD-L1, adebrelimab selectively blocks the interaction of PD-L1 with PD-1 and B7.1, activating T-cell and enhancing anti-tumor immune responses.16,17 In addition to signaling blocking, monoclonal antibody drugs can also bind to Fcγ receptors (FcγR) via the Fc region, then mediating immune responses such as complement dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity responses (ADCC), antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cell-mediated cytokine release (ADCR), ultimately leading to the death of the target cells.18–22 As PD-L1 is expressed not only on tumor cells but also on immune cells such as T-cells and B-cells, mitigating Fc-mediated functions is crucial to prevent immune cell exhaustion.23–25 Attributed to the identity as IgG4, adebrelimab is irrelevant to induction of CDC.22,26,27 Moreover, Fc modification is applied to reduce affinity with the FcγRIIIa, FcγRIIa and FcγRI, minimizing the risk of ADCC, ADCP or ADCR, thus enhancing immune efficacy while reducing immune-related adverse reaction. To address the Fab arm exchange (FAE) of IgG4 antibodies, the S228P mutation was implemented in adebrelimab to avoid a bi-specific configuration and subsequent adverse reaction.26,28,29
All the therapeutic proteins are susceptible to immunogenicity. In a phase III study (SHR-1316-III-301) that enrolled patients with ES-SCLC who had not received prior systemic treatment, 44 of 221 (19.9%) evaluable patients developed treatment-induced anti-adebrelimab antibodies at least once.11 Notably, no clinically significant immunogenicity effect on the pharmacokinetics, efficacy, or safety of adebrelimab was observed in this study.
Pharmacokinetic Properties
The pharmacokinetics of adebrelimab as monotherapy was investigated in a Phase I trial (SHR-1316-I-101), which included 41 patients with advanced solid tumors. Adebrelimab was administered intravenously at doses ranging from 3 to 20 mg/kg once every 2 or 3 weeks.30 Following a single dose of adebrelimab, the maximum concentration (Cmax) and the area under the concentration-time curve (AUC) increased in proportion to the administered dose essentially and steady state was reached after approximately 12 weeks of multiple dosing.11 According to the population pharmacokinetic analysis, the steady state volume of distribution was 4.35 L in patients receiving adebrelimab 20 mg/kg every 3 weeks.11 Similar to other antibodies, adebrelimab is expected to be cleared primarily by catabolism. The clearance rate of adebrelimab was 0.23 L/day and the typical elimination half-life was 12 days.11 Population pharmacokinetics based on data from 263 patients identified baseline body weight and albumin levels as significant covariates affecting adebrelimab pharmacokinetics but these covariate effects were not clinically significant or necessary for dose adjustment.31
Therapeutic Efficacy of Adebrelimab
Adebrelimab for Extensive-Stage Small Cell Lung Cancer
First-Line Treatment
The CAPSTONE-1 trial was a multicenter, randomized, double-blind, placebo-controlled, phase III study that evaluated the efficacy of adebrelimab in combination with chemotherapy as the first-line treatment for ES-SCLC.11 From the perspective of trial design, blinded design like IMpower133 minimized assessment bias compared to the open-label CASPIAN trial. However, it is important to note that the CAPSTONE-1 exclusively enrolled Chinese patients with no data from other countries compared to multinational cohorts in IMpower133 and CASPIAN.6,7
Previously untreated patients (n = 462) aged ≥18 years with histologically or cytologically confirmed ES-SCLC according to the Veterans Administration Lung Study Group (VALG) staging system were enrolled. Patients were randomized in a 1:1 ratio to either the adebrelimab group, receiving four–six cycles of adebrelimab (20 mg/kg on day 1 of each 21-day cycle) plus carboplatin and etoposide, or the placebo group, which received the same chemotherapy regimen plus placebo. Subsequently, the patients underwent up to 2 years of maintenance therapy with adebrelimab (20 mg/kg) or a placebo. During the maintenance phase, prophylactic cranial irradiation rather than thoracic radiotherapy was administered. Treatment was continued until disease progression, unacceptable toxicity, patient withdrawal, or investigator decision.11 The Kaplan-Meier method was used to calculate the survival endpoints of which intergroup disparities were compared using a stratified Log rank test and 95% confidence intervals (CIs) calculated via the Brookmeyer-Crowley approach. Hazard ratio and the associated 95% CIs were calculated using a Cox proportional hazards model.11
At baseline, demographics and disease characteristics were comparable between the adebrelimab (n = 230) and placebo (n = 232) treatment groups.11 Across both groups, most patients were male (81%), aged < 65 years (65%, with a median age of 62 years), had stage IV disease (97%), and had a WHO performance status of 1 (86%). 32% of the patients had liver metastases, and 86% of the patients had a PD-L1 tumor proportion score of less than 1%.11
Incorporating adebrelimab to chemotherapy in the first-line treatment of ES-SCLC significantly improved overall survival (OS) and progression-free survival (PFS) compared with placebo plus chemotherapy after a median follow-up of 13.5 months at the time of the final analysis.11 Median overall survival (mOS) was 15.3 months in the adebrelimab group and 12.8 months in the placebo group (HR=0.72 (95% CI 0.58–0.90); p=0.0017). Adebrelimab combined with chemotherapy demonstrated a 28% reduction in the risk of death, closely aligning with the efficacy observed in other PD-L1 inhibitors: atezolizumab showed a 24% risk reduction in IMpower133 and durvalumab achieved 29% in CASPIAN.9,32 The 12-month OS rate was 62.9% in the adebrelimab group compared with 52.0% in the placebo group, while the 24-month OS rate was 31.3% in the adebrelimab group compared with 17.2% in the placebo group. The median progression-free survival (mPFS) was 5.8 months in the adebrelimab group (stratified HR 0.67 (95% CI 0.54–0.83); p <0.0001), and the risk for progression or death was 33% lower compared to placebo group.11 In comparison, atezolizumab plus chemotherapy reduced the risk of disease progression by 23%, whereas durvalumab combined with chemotherapy failed to significantly prolong PFS compared to chemotherapy alone.6,7 The 6-month PFS rate was 49.4% in the adebrelimab group versus 37.3% in the placebo group and the 12-month PFS rate was 19.7% versus 5.9% at 12 months.11 Subgroup analyses further confirmed OS and PFS benefits across all prespecified patient groups. A higher proportion of patients in the adebrelimab group had a confirmed objective response than those in the placebo group (70.4% vs 65.9%, respectively). The percentage of patients who remained in response at 12 months was 20% and 4% respectively.11
The 3-year updated analysis highlights the positive contribution of adebrelimab to long-term survival at the European Society for Medical Oncology Immuno-Oncology Congress (ESMO-IO) by 2023. The OS rate at 36 months demonstrated an improvement of over 10 percentages, with 21.1% in the adebrelimab group compared to 10.5% in the placebo group, reducing the risk of death by 27%.33 The updated PFS rates at 2 years and 3 years were 11.0% and 9.4% respectively, which mean approximately 1 in 10 patients holds the potential for long-term survival in the future. The long-term survival benefit of PD-L1 inhibitors in combination with chemotherapy has also been confirmed in ES-SCLC: the published 3-year estimated OS rate were 16% for atezolizumab plus chemotherapy and 17.6% for durvalumab plus chemotherapy respectively.32,34
Both brain and liver metastases are the common type of complication in ES-SCLC patients associated with poor clinical outcomes.35 Brain metastasis (BM) was underrepresented in the CAPSTONE-1 trial, with only 2% of participants affected, so no data were available for the brain metastasis subgroup.11 Additionally, trials such as IMpower133, CASPIAN, and KEYNOTE-604 have reported brain metastasis rates ranging from 9% to 12%.6,7,36 In contrast, the incidence of BM in SCLC ranges from 40% to 50% during diagnosis and treatment.37,38 These disparities underscore the need for further investigation into the management of brain metastases in ES-SCLC, and the uncertain role of prophylactic cranial irradiation in patients receiving immunotherapy also deserves investigation. In the subgroup without liver metastasis, the CAPSTONE-1 trial outcomes showed that patients receiving adebrelimab plus chemotherapy had significant OS and PFS improvements compared to those who received chemotherapy and placebo (OS: HR= 0.61, 95% CI: 0.46–0.81; PFS: HR= 0.64, 95% CI: 0.50–0.83). However, there was no significant survival benefit in patients with hepatic metastasis, consistent with Impower133 and CASPIAN, suggesting the need for further research to optimize the treatment options for patients with liver metastasis.
Two multicenter observational studies reported the use of 2024 ESMO-IO to assess the efficacy and safety of adebrelimab as a first-line treatment for ES-SCLC in a real-world clinical setting, which is consistent with the results of a prospective study.39,40 The study led by Song et al demonstrated that the mOS and mPFS were 12.4 months and 6.7 months respectively in 114 ES-SCLC patients. The objective response rate (ORR) was 34.21%, which was lower than that of CAPSTONE-1, probably because more patients were aged ≥65 years (62% versus 33%) and were diagnosed with brain metastasis (20% vs 2%) in this study. Meng et al enrolled 188 patients with ES-SCLC who received adebrelimab-based first-line treatments. The mPFS was 6.9m and OS data was not mature, while ORR was 65.4% and disease control rate (DCR) was 85.1%.
First-Line Treatment Combined with Thoracic Radiotherapy
The combination of radiotherapy and immunotherapy has shown the potential to exert a synergistic antitumor effect.41–43 Thoracic radiotherapy (TRT) following chemoimmunotherapy has been investigated in many retrospective studies, proving that TRT could prolong PFS and OS in patients.44–46 The role of TRT as first-line therapy for ES-SCLC remains unclear, and TRT was not permitted in the IMpower133 and CASPIAN trials.6–9 The efficacy of adebrelimab in combination with chemotherapy and TRT was first evaluated in the ES-SCLC-1st-IIT-SHR1316-CRT, a single-arm phase II trial.47
67 eligible patients were previously untreated or had ES-SCLC, with a history of locally treated asymptomatic brain metastases. Patients were administered four to six cycles of adebrelimab intravenously (20 mg/kg on day 1 of each 21-day cycle), etoposide, cisplatin, or carboplatin. Patients with a response sequentially received TRT (≥30 Gy/10 f or≥50 Gy/ 25 f, involved-field irradiation, with 3D conformal radiotherapy (3D-CRT) or intensity-modulated radiation therapy (IMRT)). Subsequently, patients were administered maintenance therapy (≤2 years) with adebrelimab, and prophylactic cranial irradiation (PCI) was allowed during this phase.47
In this trial, 45 patients (67%) underwent sequential TRT. The median OS was 13.4 months in the non-TRT group after a median follow-up of 17.7 months.47 Notably, when sequential thoracic radiotherapy was included, the median OS achieved 22.9 months, representing the best survival data currently available of first-line chemoimmunotherapy for ES-SCLC, and the median PFS was 11.3 months and 4.1 months respectively.47 No difference was observed in either PFS or OS between patients who received hypofractionated or conventional fractionated radiotherapy at different doses. In addition, no obvious difference was found between the subgroups with a BED ≥60 Gy and BED <60 Gy. The ORRs and DCRs in the TRT group were significantly higher than those in the non-TRT group [ORR: 93.3 vs 27.3%; DCR:100 vs 68.2%].47 On this basis, the results of the subsequent phase III trial (NCT06672133) are worthy of anticipation. Apart from sequential thoracic radiotherapy, a phase II trial of adebrelimab-combined chemotherapy with concurrent radiotherapy (NCT06236997) is ongoing.
Later-Line Treatment
Although patients with ES-SCLC are responsive to initial treatment, the disease tends to relapse quickly, and the effectiveness of later-line therapies is limited. A real-world study showed that the median overall survival for patients with SCLC who received three or more lines of treatment was 4.4 months, with a 1-year OS rate of only 11%.48 Current options for later-line treatment mainly focus on chemotherapy drugs with limited efficacy. Novel treatment regimens, especially immunotherapy and targeted therapy, with or without chemotherapy, may offer new hope for SCLC patients. For pretreated ES-SCLC, a Phase 2 trial (ChiCTR2100052533) led by Liu et al assessed the efficacy and safety of adebrelimab plus famitinib, a new multi-target receptor tyrosine kinase inhibitor (TKI). This study employed a Simon two-stage design and selected the ORR as the primary endpoint. In the first stage, adebrelimab (20 mg/kg, administered intravenously every three weeks) and famitinib (20 mg, administered orally once daily) were administered to 13 patients who were unresponsive or intolerant to second-line systemic chemotherapy. Eventually, the ORR was 23.1% (3/13) and three patients achieved confirmed PR. The DCR was 69.2% (9/13) and the median PFS was 4.0 months. Treatment-related adverse events (TRAEs) occurred in 84.6% of the patients, and the most common TRAEs were decreased white blood cell and neutrophil counts. The combination of adebrelimab and famitinib showed promising antitumor activity and safety. In the second stage, the cohort was expanded to include 34 patients for further clinical studies. Another ongoing randomized phase Ib/II study (NCT06332950) aimed to evaluate the safety and efficacy of adebrelimab plus irinotecan liposomes with or without famitinib in patients with ES-SCLC pretreated with immune checkpoint inhibitors. The application of famitinib as a first-line treatment for ES-SCLC (NCT06306560) is also being actively investigated.
Adebrelimab for Limited-Stage Small-Cell Lung Cancer
Over the last few decades, chemotherapy with concurrent radiotherapy (cCRT) has remained the standard treatment for LS-SCLC. Following the transformative impact of immunotherapy on the first-line treatment of ES-SCLC, researchers are shifting their focus to explore the potential of immunotherapy to revolutionize the therapeutic landscape of LS-SCLC. ADRIATIC, a randomized, double-blind, global multicenter phase III clinical trial, suggested that durvalumab consolidation therapy after cCRT substantially improved both OS and PFS in patients with LS-SCLC who had not progressed after standard cCRT.10 Compared to placebo group, durvalumab consolidation group significantly prolonged the mPFS by 7.4 months (16.6 vs 9.2 months; HR=0.76, p=0.02) and the mOS by 22.5 months (55.9 vs 33.4 months; HR=0.73, p=0.01). This groundbreaking finding marks a significant breakthrough in the treatment of LS-SCLC. Therefore, durvalumab has been included in the newly released National Comprehensive Cancer Network (NCCN) Guidelines Version 2.2025 for SCLC as a consolidation therapy option for limited-stage SCLC.
It has been confirmed that immunotherapy, from advanced to limited stages, can provide clinical benefits to patients. Currently, two strategies are under investigation for LS-SCLC: immunotherapy consolidation following concurrent chemoradiotherapy presented by the ADRIATIC trial and concurrent radiotherapy combined with immunochemotherapy followed by immunotherapy maintenance. The ongoing LS-SCLC-MT-IIT-SHR1316 trial (NCT04647357) is exploring the efficacy and safety of adebrelimab as consolidation therapy in LS-SCLC patients without progression after first-line platinum-based cCRT.49 Another active, two-stage, phase III trial (NCT04691063) has evaluated the therapeutic performance of cCRT with adebrelimab in LS-SCLC.50 28 Pathologically confirmed LS-SCLC patients with an ECOG PS score of 0 or 1 and no prior treatment were eligible for participation in SHR-1316-III-302-1b. Etoposide and carboplatin along with adebrelimab (20 mg/kg, day 1) were administered every 3 weeks for four cycles. After two cycles of chemo-immunotherapy, the patients received two additional cycles concurrently with TRT (60 Gy/30 f, 6 weeks), followed by up to 35 cycles of maintenance therapy with adebrelimab. In the safety run-in stage, 10 patients (35.7%) completed 35 cycles of adebrelimab. The majority of patients were male (61%), had stage III disease (93%), and had an ECOG PS of 1 (96%).51 After a median follow-up of 29.4 months, the median OS was not reached, with a 2-year OS rate of 64.3%. The median PFS was 17.9 months for all patients. The confirmed ORR was 92.9% (26/28; 95% CI 76.5–99.1) and DCR was 100% (28/28; 95% CI 87.7–100). The median duration of confirmed response was 20.1 months.51 The outstanding outcomes of first stage proved that immunotherapy starting from induction therapy was feasible and effective for patients with LS-SCLC. A placebo-controlled phase is underway to further evaluate this treatment regimen, and a Chinese multi-cohort trial (ChiCTR2400086633) will compare the efficacy and safety of adebelimab-combined chemotherapy plus concurrent radiotherapy with plus sequential RT.
Tolerability of Adebrelimab
Adebrelimab Plus Chemotherapy
In the CAPSTONE-1 trial, adebrelimab in combination with etoposide and carboplatin suggested manageable tolerability profile in ES-SCLC patients.11 TRAEs of any grade occurred in 100% and 99% of patients in the adebrelimab arm and the placebo arm respectively. Grade ≥3 AEs of any cause were reported in 86% and 85% of the patients in the adebrelimab and placebo groups, respectively. The most frequent grade ≥ 3 AEs were neutropenia (76% in the adebrelimab group vs 75% in the placebo group), leukopenia (46% vs 38%), thrombocytopenia (38% vs 34%), and anemia (28% vs.28%).11 TRAEs led to treatment discontinuation in 5% of patients receiving adebrelimab and 4% of those receiving placebo, with thrombocytopenia being the most common reason (1% in both groups).11 Fatal AEs related to treatment occurred in 2 recipients (0.9%) of each group, attributed to respiratory failure and interstitial lung disease or pneumonia in the adebrelimab plus chemotherapy group.11 Death due to AEs due to any cause occurred in 13 (5.3%) durvalumab treatment in the CASPIAN trial and 3 (1.5%) atezolizumab-treated patients in the IMpower 133 trial, reflecting the tolerable safety of PD-L1 inhibitor combined with chemotherapy.
Immune-mediated AEs were reported in 28% of the patients treated with adebrelimab and 17% of the patients in the placebo arm, with grade 3 or worse events observed in 5% and 3% of the patients in the respective treatment groups. The most common any-grade immune-mediated AEs (≥ 5% incidence) were hypothyroid events (9% in the adebrelimab group vs 6% in the placebo group) and hepatic laboratory abnormalities (7% in the adebrelimab group vs 5% in the placebo group).11
In real-world clinical settings, adebrelimab plus chemotherapy showed a manageable tolerability profile in treatment-naive patients with ES-SCLC. The incidence of grade ≥ 3 AEs was 22.8% and 31.9%, respectively, which was significantly lower than that in the CAPSTONE-1 trial.11,37,38 Hematological toxicities were the most reported grade 3 or 4 AEs.
Adebrelimab Plus Chemotherapy and Radiotherapy
ES-SCLC-1st-IIT-SHR1316-CRT
Recruitment of the TREASURE trial (NCT04462276) was paused due to grade 5 severe adverse events in the atezolizumab with sequential TRT arm; however, adebrelimab in combination with chemotherapy and sequential thoracic radiotherapy was generally well tolerated in patients with ES-SCLC, which may be due to the difference between the design of the gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV).47,52 TRAEs in the study of adebrelimab in combination with sequential thoracic radiotherapy were reported in 90% of patients, with an incidence of grade ≥ 3 TRAEs of 62% and 50% in the TRT and non-TRT arms, respectively. Neutrophil count decreased (48/67, 72%), anemia (46/67, 69%), white blood cell count decreased (43/67, 64%), lymphocyte count decreased (29/67, 43%) and nausea (23/67, 34%) were most commonly reported TRAEs.47 The incidence of serious TRAEs were 13%, the most of which was pneumonitis (2/67, 3%). The discontinuation rate due to AEs was 15%, mostly because of hematological toxicities. No unexpected AEs or treatment-related deaths were observed.47
SHR-1316-III-302-1b
In the safety run-in stage, the tolerability of adebrelimab with cCRT was acceptable: 27 patients developed grade ≥3 TRAEs, all events with ≥10% incidence were hematological toxicities, immune-mediated lung disease of grade 2 occurred in one patient, AEs leading to discontinuation of treatment were reported in one patient (infusion-related reactions), and no TRAE-related deaths occurred.51
Other Ongoing Clinical Trials
The median OS, especially in patients with ES-SCLC receiving first-line chemotherapy combined with immunotherapy, ranges from 12 to 16 months, which remains suboptimal. To further enhance the efficacy while maintaining a reassuring safety profile, numerous studies have explored novel combination therapies based on adebrelimab plus chemotherapy, including targeted therapy and radiotherapy. The most recently initiated clinical trial (NCT06768307) compared the efficacy of concurrent radiotherapy with that of sequential radiotherapy in the context of adebrelimab-combined chemotherapy. In addition to the timing of chest radiotherapy intervention, the appropriate dose needs to be thoroughly investigated. Supported by the promising results from the MATCH and LEAD trials, the ongoing phase III trial (NCT06610734) was designed to evaluate the efficacy and safety of adebrelimab combined with chemotherapy and synchronous low-dose radiotherapy (LDRT).53,54 The phase-III ETER701 trial with an mOS of 19.3 months has powerfully demonstrated that antiangiogenesis agents combined with immunochemotherapy may become a novel and effective first-line treatment strategy for ES-SCLC patients.55 Apatinib, a vascular endothelial growth factor (VEGF) receptor TKI, can inhibit tumor angiogenesis. Two ongoing trials have investigated the application of apatinib combined with adebrelimab in the maintenance stage after immunochemotherapy (NCT06480864 and NCT06614621). Three other trials assessing the efficacy of apatinib plus adebrelimab-combined chemotherapy in the induction stage of ES-SCLC are currently recruiting patients, including ChiCTR2400088296, ChiCTR2500095403, and ChiCTR2400094718. Fluzoparib is a novel Poly ADP-ribose polymerase (PARP) inhibitor that targets PARP enzymes that participate in DNA repair pathways and contribute to the death of tumor cells with homologous recombination deficiency.56 Several ongoing clinical trials are investigating the use of adebrelimab and Fluzoparib in ES-SCLC, including a phase II study (ChiCTR2300070028) that evaluated adebrelimab combined with fluzoparib as maintenance therapy after the induction of immunochemotherapy. In another study (ChiCTR2400083241), fluzoparib was not only incorporated into maintenance therapy, but also brought forward to the induction phase.
Many prospective studies have explored the efficacy of adebrelimab combined with chemotherapy as second-line treatment for ES-SCLC. Two phase II clinical trials (ChiCTR2400082466 and ChiCTR2400089170) aimed to evaluate adebrelimab combined with irinotecan liposomes in patients with advanced ES-SCLC after the first-line treatment. Another clinical trial (ChiCTR2400085196) was designed to compare the therapeutic efficacy of adebrelimab plus docetaxel with that of docetaxel alone in patients with refractory extensive-stage SCLC. In addition, many prospective studies have assessed the application of apatinib in previously treated ES-SCLC patients, showing certain anti-tumor activity with an ORR of 13.6%-17.5% and tolerable toxicities.57–59 The PASSION trial is the first clinical trial to evaluate immunotherapy plus antiangiogenesis agents (camrelizumab plus apatinib) as second-line therapy in extensive-stage SCLC, demonstrating the synergistic effect of this pattern with an ORR of 34%.60 Currently, recruitment is underway for a randomized, multicenter clinical trial (ChiCTR2300078968) aimed to evaluate the efficacy and safety of three treatment models (adebrelimab plus apatinib and nab-paclitaxel, adebrelimab plus apatinib, and apatinib plus nab-paclitaxel) in ES-SCLC progressing after first-line immunochemotherapy. Another single-arm clinical trial (ChiCTR2400087301) recruited patients with SCLC with disease progression after at least two lines of standard treatment regimens (at least one regimen consisting of platinum) to assess the clinical activity of adebrelimab plus apatinib.
In addition to the timing of immunotherapy intervention as previously mentioned, factors relevant to radiotherapy, such as dose and fractionation, may also affect the outcomes of combined treatment in LS-SCLC, which requires further exploration. After a two-cycle induction treatment with adebrelimab and platinum-based chemotherapy in a prospective study (ChiCTR2300077007), patients were assigned to Group A (large segmentation group: 4 Gy×10-15 f) or Group B (hyperfractionated radiotherapy group: 45 Gy/30 f, bid) based on their baseline characteristics and treatment response to explore individualized radiation therapy. Neoadjuvant immunotherapy shows potential, although its efficacy in limited-stage SCLC remains unclear. Pathological complete response (pCR) and major pathological response (MPR) were 47.1% and 70.6% respectively in the NeoSCI cohort study after three cycles of atezolizumab-combined induction chemotherapy and radical surgery.61 There are four active trials investigating the efficacy and safety of adebrelimab plus chemotherapy for neoadjuvant therapy, including NCT06485544, ChiCTR2300074591, ChiCTR2300077557 and ChiCTR2300078911. Furthermore, another ongoing trial (NCT06483282) is investigating apatinib and adebrelimab-combined chemotherapy as induction therapy for resectable SCLC patients.
Tables 2 and 3 summarize other unmentioned ongoing trials of adebrelimab in patients with LS-SCLC and ES-SCLC, respectively.
Table 2.
Ongoing Trials in ES-SCLC Patients
| Identifier | Patients | Phase | Setting | Intervention Arm | Control Arm | Primary Endpoint |
|---|---|---|---|---|---|---|
| NCT06479473 | ES-SCLC N=150 |
I/II | First-line | Adebrelimab + chemotherapy → radiotherapy to all residual lesions + immunotherapy maintenance | Adebrelimab + chemotherapy → immunotherapy maintenance |
PFS |
| ChiCTR2300079125 | ES-SCLC N=50 |
II | First-line | Adebrelimab + chemotherapy → thoracic palliative radiotherapy + immunotherapy maintenance |
- | ORR |
| ChiCTR2400089941 | ES-SCLC responsive to induction N=160 | IV | First-line | Extracranial radiotherapy+ immunotherapy maintenance | Immunotherapy maintenance | PFS OS |
| NCT06597513 | ES-SCLC N=10 |
I/II | First-line | 2 cycles of standard-dose EC → 2 cycles of auto-HSCT-supported Dose-dense Chemotherapy → 4 cycles of standard-dose EC + Adebrelimab → Adebrelimab Maintenance |
- | AE |
| NCT06475209 | ES-SCLC responsive to induction N=60 | II | First-line | Maintenance therapy: Adebrelimab + Apatinib |
- | PFS |
| ChiCTR2300075166 | ES-SCLC N=30 |
II | First-line | Adebrelimab + chemotherapy →Adebrelimab + chest radiation therapy →Adebrelimab + Apatini | - | PFS 1-y and 2-y OS rate |
| ChiCTR2400088006 | ES-SCLC N=128 | - | First-line | Adebrelimab + bevacizumab + chemotherapy → Adebrelimab + bevacizumab maintenance therapy | Adebrelimab + chemotherapy → Adebrelimab + bevacizumab maintenance therapy | 2-y OS rate |
| ChiCTR2400093930 | ES-SCLC pretreated with adebrelimab N=32 |
II | Second-line | Adebrelimub+chemotherapy / targeted therapy | - | ORR |
| NCT04400188 | ES-SCLC pretreated with platinum-based chemotherapy N=25 |
I/II | Later-line | A group: Fluzoparib + temozolomide B group: Fluzoparib + temozolomide + Adebrelimab |
PFS |
Abbreviations: ES-SCLC, extensive-stage small cell lung cancer; PFS, progression-free survival; ORR, objective response rate; OS, overall survival; auto-HSCT-supported Dose-dense Chemotherapy, autologous hematopoietic stem cell transplantation-supported dose-dense chemotherapy; AE, adverse event; 1-y OS rate, 1-year overall survival rate; 2-y OS rate, 2-year overall survival rate.
Table 3.
Ongoing Trials in LS-SCLC Patients
| Identifier | Patients | Phase | Intervention Arm | Control Arm | Primary Endpoint |
|---|---|---|---|---|---|
| NCT05496166 | LS-SCLC N=348 |
III | Adebrelimab + chemotherapy → surgery→ Adebrelimab | Adebrelimab + chemotherapy→radiotherapy → Adebrelimab | PFS |
| NCT06483282 | LS-SCLC N=38 |
II | Adebrelimab + Apatinib + Chemotherapy → surgery → Adebrelimab + Apatinib ± radiotherapy |
- | EFS |
| NCT06527898 | LS-SCLC N=45 |
I/II | Chemotherapy + concurrent hypofractionated radiotherapy → Adebrelimab + chemotherapy → Adebrelimab | - | PFS |
| ChiCTR2400082364 | LS-SCLC N=36 |
II | Hyperfractionated radiotherapy + chemotherapy → adebrelimab maintenance | - | Safety PFS |
| ChiCTR2500095305 | LS-SCLC N=58 |
IV | Adebrelimab + chemotherapy → Adebrelimab + chemotherapy + radiotherapy | - | PFS |
| ChiCTR2400086355 | LS-SCLC N=38 |
- | Adebrelimab + chemotherapy → chemotherapy + radiotherapy → Adebrelimab | - | PFS |
Abbreviations: LS-SCLC, limited-stage small cell lung cancer; PFS, progression-free survival; EFS, event-free survival.
Biomarkers
Given the evident long-term clinical benefits observed in patients treated with adebrelimab, further research is required to identify potential biomarkers. Although PD-L1 expression detected by immunohistochemistry (IHC) is considered an essential predictive marker for the response to immunotherapy in NSCLC patients, it did not appear to predict treatment outcomes in CAPSTONE-1, consistent with previous trials of PD-L1 inhibitors combined with chemotherapy as the first-line treatment for ES-SCLC.11,62,63
In the ES-SCLC-1st-IIT-SHR1316-CRT, biospecimens were collected from 55 patients, including formalin-fixed and paraffin-embedded (FFPE) biopsy tissues. Comprehensive genomic profiling was performed.64 Plasma and peripheral blood mononuclear cells (PBMC) were obtained as liquid biopsies, which can dynamically reflect treatment efficacy. TP53/RB1 co-mutations, commonly found in patients with SCLC, were analyzed in tissues and peripheral blood, indicating poorer clinical outcomes (PFS, tissue, HR=2.85, p=0.071; ctDNA, HR=2.76, p=0.06; OS, tissue, HR=3.35, p=0.032; ctDNA, HR=3.87, p=0.031).64,65 Higher TMB levels (10 muts/Mb) in tissues were associated with longer PFS (HR=0.27, p=0.053) and OS (HR=0.37, p=0.086). Previous studies have suggested that monitoring longitudinal ctDNA changes could aid in the early detection of sustained molecular responses or emerging resistance.66,67 Patients with ctDNA positivity in the peripheral blood collected on day 1 of the seventh cycle in this trial exhibited worse PFS (HR=12.28, p=0.0069), suggesting that ctDNA clearance may play a predictive role in treatment efficacy. Furthermore, a baseline study of PBMCs revealed that patients with a higher baseline of ZNF683+CD8+ T cells had longer PFS and OS than those with a higher baseline of regulatory T cells. These biomarkers were associated with clinical benefits of adebrelimab combined with chemotherapy and TRT; however, further validation is required.
Dosage and Administration of Adebrelimab
The recommended regimen for ES-SCLC involves adebrelimab at a dose of 20 mg/kg in combination with chemotherapy every 3 weeks (21 days) for 4–6 cycles, followed by 20 mg/kg every 3 weeks as monotherapy until disease progression, unacceptable toxicity, or for up to 2 years of maintenance treatment. Adebrelimab was administered as an intravenous infusion 30 minutes prior to chemotherapy on the same day.
Reducing or escalating the dose of adebrelimab is not recommended; however, treatment interruption or discontinuation may be necessary to manage adverse reactions. Data on the administration of adebrelimab during pregnancy were unavailable. Based on animal studies and its classification as an IgG4 antibody capable of crossing the placental barrier, adebrelimab may pose a risk of fetal harm if administered during pregnancy. Women of childbearing potential should use effective contraception during treatment and for at least two months following the final dose.
For comprehensive guidance including warnings, precautions, recommendations of dose adjustments in response to adverse reactions, and information on use in specific populations, local prescription information should be consulted.
Conclusion
In conclusion, the combination of adebrelimab with etoposide and carboplatin significantly improved overall survival compared to chemotherapy alone, and broadened clinical first-line treatment options for patients with ES-SCLC. The unmet clinical demands drive the development of innovative regimens combined with radiotherapy or targeted therapy, which are highly anticipated to bring a breakthrough in survival benefits for LS-SCLC and ES-SCLC in the future. In addition, further researches are needed to continue identifying biomarkers through multi-omics analysis and investigating more effective therapeutic targets.
Abbreviations
SCLC, small cell lung cancer; PD-1, programmed cell death-1; PD-L1, programmed death-ligand 1; ES-SCLC, extensive-stage small cell lung cancer; FDA, Food and Drug Administration; EMA, European Medicines Agency; LS-SCLC, limited-stage small cell lung cancer; IgG4, immunoglobulin G4; SHR-1316, Adebrelimab; NMPA, National Medical Products Administration; CSCO, Chinese Society of Clinical Oncology; NSCLC, non-small cell lung cancer; FcγR, Fcγ receptors; CDC, complement dependent cytotoxicity; ADCC, antibody-dependent cellular cytotoxicity responses; ADCP, antibody-dependent cellular phagocytosis; ADCR, antibody-dependent cell-mediated cytokine release; FAE, Fab arm exchange; Cmax, maximum concentration; AUC, area under the concentration-time curve; VALG, Veterans Administration Lung Study Group; OS, overall survival; PFS, progression-free survival; mOS, median overall survival; HR, hazard ratio; mPFS, median progression-free survival; ESMO-IO, European Society for Medical Oncology Immuno-Oncology Congress; BM, brain metastasis; ORR, objective response rate; DCR, disease control rate; TRT, thoracic radiotherapy; 3D-CRT, 3D conformal radiotherapy; IMRT, intensity-modulated radiation therapy; PCI, prophylactic cranial irradiation; TKI, tyrosine kinase inhibitor; TRAEs, Treatment-related adverse events; cCRT, concurrent radiotherapy; NCCN, National Comprehensive Cancer Network; GTV, gross tumour volume; CTV, the clinical target volume; PTV, planning target volume; LDRT, low-dose radiotherapy; VEGF, vascular endothelial growth factor; PARP, Poly ADP-ribose polymerase; pCR, pathological complete response; MPR, major pathological response; IHC, immunohistochemistry; FFPE, formalin-fixed and paraffin-embedded; PBMC, plasma and peripheral blood mononuclear cell.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Han B, Zheng R, Zeng H, et al. Cancer incidence and mortality in China, 2022. J National Cancer Center. 2024;4(1):47–53. doi: 10.1016/j.jncc.2024.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20(9):624–639. doi: 10.1038/s41571-023-00798-3 [DOI] [PubMed] [Google Scholar]
- 3.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17(12):725–737. doi: 10.1038/nrc.2017.87 [DOI] [PubMed] [Google Scholar]
- 4.Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3. doi: 10.1038/s41572-020-00235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares L, Dvorkin M, Chen YB, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, Phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/s0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 8.Goldman JW, Dvorkin M, Chen YB, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi: 10.1016/s1470-2045(20)30539-8 [DOI] [PubMed] [Google Scholar]
- 9.Liu SV, Reck M, Mansfield AS, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39(6):619–+. doi: 10.1200/jco.20.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Spigel DR, Cho BC, et al. Durvalumab after chemoradiotherapy in limited-stage small-cell lung cancer. N Engl J Med. 2024;391(14):1313–1327. doi: 10.1056/NEJMoa2404873 [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Zhou CC, Yao WX, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–747. doi: 10.1016/s1470-2045(22)00224-8 [DOI] [PubMed] [Google Scholar]
- 12.Chen GL, Gu X, Xue JQ, et al. Effects of neoadjuvant stereotactic body radiotherapy plus adebrelimab and chemotherapy for triple-negative breast cancer: a pilot study. Elife. 2023;12:e91737. doi: 10.7554/eLife.91737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu L, Song Y, Zhao KL, et al. SHR-1316, an anti-PD-L1 antibody, plus chemotherapy as the first-line treatment for advanced esophageal squamous cell carcinoma: a multicentre, phase 2 study. Thoracic Cancer. 2021;12(9):1373–1381. doi: 10.1111/1759-7714.13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan WP, Zhong WZ, Liu YH, et al. Adebrelimab (SHR-1316) in combination with chemotherapy as perioperative treatment in patients with resectable stage II to III NSCLCs: an open-label, multicenter, Phase 1b trial. J Thorac Oncol. 2023;18(2):194–203. doi: 10.1016/j.jtho.2022.09.222 [DOI] [PubMed] [Google Scholar]
- 15.Zhang P, Li XF, Wang X, Yang Y, Wang JF, Cao D. SHR-8068 combined with adebrelimab and bevacizumab in the treatment of refractory advanced colorectal cancer: study protocol for a single-arm, phase Ib/II study. Front Immunol. 2024:151450533. doi: 10.3389/fimmu.2024.1450533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–6587. doi: 10.1158/1078-0432.Ccr-12-1362 [DOI] [PubMed] [Google Scholar]
- 18.Almagro JC, Daniels-Wells TR, Perez-Tapia SM, Penichet ML. Progress and challenges in the design and clinical development of antibodies for cancer therapy. Front Immunol. 2017;8:1751. doi: 10.3389/fimmu.2017.01751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caaveiro JMM, Kiyoshi M, Tsumoto K. Structural analysis of Fc/FcR complexes: a blueprint for antibody design. Immunol Rev. 2015;268(1):201–221. doi: 10.1111/imr.12365 [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 21.Sibéril S, Dutertre CA, Fridman WH, Teillaud JL. FcγR:: the key to optimize therapeutic antibodies? Crit Rev Oncol Hematol. 2007;62(1):26–33. doi: 10.1016/j.critrevonc.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 22.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014:5520. doi: 10.3389/fimmu.2014.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–452. doi: 10.1016/j.immuni.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS. Fc-engineering for modulated effector functions-improving antibodies for cancer treatment. Antibodies. 2020;9(4):64. doi: 10.3390/antib9040064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang XH, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein and Cell. 2018;9(1):63–73. doi: 10.1007/s13238-017-0473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchini R, Karagiannis SN, Jordakieva G, Jensen-Jarolim E. The role of IgG4 in the fine tuning of tolerance in IgE-mediated allergy and cancer. Int J Mol Sci. 2020;21(14):5017. doi: 10.3390/ijms21145017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crescioli S, Correa I, Karagiannis P, et al. IgG4 characteristics and functions in cancer immunity. Curr Allergy Asthma Rep. 2016;16(1):7. doi: 10.1007/s11882-015-0580-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labrijn AF, Buijsse AO, van den Bremer ET, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009;27(8):767–771. doi: 10.1038/nbt.1553 [DOI] [PubMed] [Google Scholar]
- 29.Rispens T, Davies AM, Ooijevaar-de Heer P, et al. Dynamics of inter-heavy chain interactions in human immunoglobulin G (IgG) subclasses studied by kinetic Fab arm exchange. J Biol Chem. 2014;289(9):6098–6109. doi: 10.1074/jbc.M113.541813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.A phase 1 study to evaluate the safety and tolerability of SHR-1316 in subjects with advanced tumors. National Library of Medicine (US). Available from: https://clinicaltrials.gov/study/NCT03474289. Accessed May 20, 2025. [Google Scholar]
- 31.Chen P, Zhang YY, Wang YK, et al. Population pharmacokinetics of adebrelimab - Support of alternative flat dose regimen in extensive-stage small-cell lung cancer. Cpt-Pharmacometrics Systems Pharmacol. 2024;13(7):1238–1251. doi: 10.1002/psp4.13155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN☆. ESMO Open. 2022;7(2):100408. doi: 10.1016/j.esmoop.2022.100408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Y, Wang J, Zhou C, et al. 84P Adebrelimab plus chemotherapy (chemo) as first-line treatment for extensive-stage small-cell lung cancer (ES-SCLC): 3-year update of the phase III CAPSTONE-1 study. Immuno-Oncol Technol. 2023;20:100556. doi: 10.1016/j.iotech.2023.100556 [DOI] [Google Scholar]
- 34.Reck M, Dziadziuszko R, Sugawara S, et al. Five-year survival in patients with extensive-stage small cell lung cancer treated with atezolizumab in the Phase III IMpower133 study and the Phase III IMbrella A extension study. Lung Cancer. 2024;196:107924. doi: 10.1016/j.lungcan.2024.107924 [DOI] [PubMed] [Google Scholar]
- 35.Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 36.Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–+. doi: 10.1200/jco.20.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nugent JL, Bunn PA, Matthews MJ, et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44(5):1885–1893. doi: [DOI] [PubMed] [Google Scholar]
- 38.Hirsch FR, Paulson OB, Hansen HH, Larsen SO. Intracranial metastases in small cell carcinoma of the lung. Prognostic aspects. Cancer. 1983;51(3):529–533. doi: [DOI] [PubMed] [Google Scholar]
- 39.Song Y, Chen B, Chen W, et al. 75P Real-world data of adebrelimab in the first-line treatment of patients with small cell lung cancer. Immuno-Oncol Technol. 2024;24:100818. doi: 10.1016/j.iotech.2024.100818 [DOI] [Google Scholar]
- 40.Wen J, Jiang L, Xu S, et al. 110P Safety and effectiveness of adebrelimab as first-line treatment in extensive-stage small-cell lung cancer: a prospective, real-world study. Immuno-Oncol Technol. 2024;24:100853. doi: 10.1016/j.iotech.2024.100853 [DOI] [Google Scholar]
- 41.Gao Z, Zhao Q, Xu Y, Wang L. Improving the efficacy of combined radiotherapy and immunotherapy: focusing on the effects of radiosensitivity. Radiat Oncol. 2023;18(1):89. doi: 10.1186/s13014-023-02278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucia F, Geier M, Schick U, Bourbonne V. Narrative review of synergistics effects of combining immunotherapy and stereotactic radiation therapy. Biomedicines. 2022;10(6):1414. doi: 10.3390/biomedicines10061414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Liu ZG, Yuan H, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res. 2019;25(6):1709–1717. doi: 10.1158/1078-0432.Ccr-18-2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai Z, Gu X, Xie J, et al. Safety and efficacy of thoracic radiotherapy combined with chemo-immunotherapy in patients with extensive-stage small cell lung cancer: a multicenter retrospective analysis. Transl Lung Cancer Res. 2023;12(10):1987–2000. doi: 10.21037/tlcr-23-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng J, Zhang L, Wang L, et al. Real-world outcomes of PD-L1 inhibitors combined with thoracic radiotherapy in the first-line treatment of extensive stage small cell lung cancer. Radiat Oncol. 2023;18(1):111. doi: 10.1186/s13014-023-02308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Z, Liu J, Wu M, et al. Real-world efficacy and safety of thoracic radiotherapy after first-line chemo-immunotherapy in extensive-stage small-cell lung cancer. J Clin Med. 2023;12(11):3828. doi: 10.3390/jcm12113828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen DW, Zou B, Li BT, et al. Adebrelimab plus chemotherapy and sequential thoracic radiotherapy as fi rst-line therapy for extensive-stage small-cell - cell lung cancer (ES-SCLC): a phase II trial. Eclinicalmedicine. 2024:75102795. doi: 10.1016/j.eclinm.2024.102795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coutinho AD, Shah M, Lunacsek OE, Eaddy M, Willey JP. Real-world treatment patterns and outcomes of patients with small cell lung cancer progressing after 2 lines of therapy. Lung Cancer. 2019;127:53–58. doi: 10.1016/j.lungcan.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 49.A trial of SHR-1316 maintenance therapy for limited stage small cell lung cancer without progression after first line concurrent chemoradiotherapy Treatment. National Library of Medicine (US). Available from: https://clinicaltrials.gov/study/NCT04647357. Accessed May 20, 2025. [Google Scholar]
- 50.A multi-center, randomized, double-blinded, phase III trial of SHR-1316 or placebo in combination with chemo-radiotherapy in patients with limited-stage small-cell lung cancer. National Library of Medicine (US). Available from: https://clinicaltrials.gov/study/NCT04691063. Accessed May 20, 2025. [Google Scholar]
- 51.Cheng Y, Wang H, Min X, et al. 198P Adebrelimab with concurrent chemoradiation (cCRT) for limited-stage small cell lung cancer (LS-SCLC): safety run-in results of a phase III trial. ESMO Open. 2024;9:102771. doi: 10.1016/j.esmoop.2024.102771 [DOI] [Google Scholar]
- 52.Bozorgmehr F, Weykamp F, Overbeck TR, et al. 1988MO Recruitment discontinuation in TREASURE trial (thoracic radiotherapy with atezolizumab in small cell lung cancer extensive disease) due to unexpected safety data. Ann Oncol. 2023;34:S1060. doi: 10.1016/j.annonc.2023.09.1219 [DOI] [Google Scholar]
- 53.Wang H, Yao Z, Kang K, et al. Preclinical study and phase II trial of adapting low-dose radiotherapy to immunotherapy in small cell lung cancer. Med. 2024;5(10):1237–1254.e9. doi: 10.1016/j.medj.2024.06.002 [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Xie Y, Gong Y, et al. 194MO Phase II study of low-dose radiation (LDRT) plus durvalumab (D) and etoposide/platinum (EP) as first-line treatment in ES-SCLC (LEAD): efficacy and safety results. ESMO Open. 2024;9:102767. doi: 10.1016/j.esmoop.2024.102767 [DOI] [Google Scholar]
- 55.Cheng Y, Chen J, Zhang W, et al. Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial. Nat Med. 2024;30(10):2967–2976. doi: 10.1038/s41591-024-03132-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng Y, Arisa O, Peer CJ, Fojo A, Figg WD. PARP inhibitors: a review of the pharmacology, pharmacokinetics, and pharmacogenetics. Semin Oncol. 2024;51(1–2):19–24. doi: 10.1053/j.seminoncol.2023.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Q, Xu JY, Xu YH, et al. Efficacy and safety of apatinib as second or later-line therapy in extensive-stage small cell lung cancer: a prospective, exploratory, single-arm, multi-center clinical trial. Transl Lung Cancer Res. 2022;11(5):832–844. doi: 10.21037/tlcr-22-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Hu X, Jiang J, et al. A prospective study of apatinib in patients with extensive-stage small cell lung cancer after failure of two or more lines of chemotherapy. Oncologist. 2020;25(5):e833–e842. doi: 10.1634/theoncologist.2019-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Y, Huang Z, Lu H, et al. Apatinib in patients with extensive-stage small-cell lung cancer after second-line or third-line chemotherapy: a Phase II, single-arm, multicentre, prospective study. Br J Cancer. 2019;121(8):640–646. doi: 10.1038/s41416-019-0583-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan Y, Zhao J, Wang Q, et al. Camrelizumab plus apatinib in extensive-stage SCLC (PASSION): a multicenter, two-stage, phase 2 trial. J Thorac Oncol. 2021;16(2):299–309. doi: 10.1016/j.jtho.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 61.Duan H, Shi L, Shao C, et al. A multicenter, single-arm, open study of neoadjuvant or conversion atezolizumab in combination with chemotherapy in resectable small cell lung cancer (Cohort Study). Int J Surg. 2023;109(9):2641–2649. doi: 10.1097/js9.0000000000000501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keppens C, Dequeker EM, Pauwels P, Ryska A, Hart N, von der Thüsen JH. PD-L1 immunohistochemistry in non-small-cell lung cancer: unraveling differences in staining concordance and interpretation. Virchows Arch. 2021;478(5):827–839. doi: 10.1007/s00428-020-02976-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Zou B, Li B, et al. P1.06B.10 post-hoc biomarker analysis of adebrelimab plus chemotherapy and sequential radiotherapy as first-line therapy for ES-SCLC. J Thorac Oncol. 2024;19(10):S170. doi: 10.1016/j.jtho.2024.09.308 [DOI] [Google Scholar]
- 65.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. doi: 10.1038/nature14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pellini B, Chaudhuri AA. ctDNA monitoring for small cell lung cancer: ready for prime time? Clin Cancer Res. 2023;29(12):2176–2178. doi: 10.1158/1078-0432.Ccr-23-0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sivapalan L, Iams WT, Belcaid Z, et al. Dynamics of sequence and structural cell-free DNA landscapes in small-cell lung cancer. Clin Cancer Res. 2023;29(12):2310–2323. doi: 10.1158/1078-0432.Ccr-22-2242 [DOI] [PMC free article] [PubMed] [Google Scholar]