Abstract
Inflammation in asthma and other allergic diseases is characterized by excessive production of immunoglobulin E (IgE) and the influx of leukocytes, especially eosinophils. Interleukin 4 (IL-4) and IL-5 are essential for IgE production and eosinophilia, respectively, and are produced by mast cells in allergic conditions, for which glucocorticoids are widely used therapeutically. We assessed the effect of glucocorticoids on IL-4 and IL-5 mRNA production by the RBL-2H3 cell line, an analog of mucosal mast cells. IL-4 and IL-5 mRNAs were induced by an antigen that is used to cross-link receptor bound IgE, by calcium ionophore, or by ionophore with phorbol ester and were markedly inhibited by dexamethasone. In cells activated with ionophore and phorbol ester, 10−6 M dexamethasone reduced the IL-4 and IL-5 mRNA levels to only 12.8 and 5.7%, respectively, of those in cells without dexamethasone, and 10−9 M dexamethasone caused reductions to 27 and 56%, respectively. Hydrocortisone at 10−6 and 10−7 M almost completely inhibited IL-4 and IL-5 mRNA production. Dexamethasone was markedly inhibitory even if it was added after the cells were activated, provided that it was present in the cultures for at least 1.5 h. These studies indicate that the expression of IL-4 and IL-5 mRNAs by mast cells is highly sensitive to glucocorticoids. The data suggest that these inhibitory effects may contribute to the clinical efficacy of glucocorticoids in the therapy of allergic diseases.
Cellular inflammation in asthma and other allergic diseases is characterized by the influx of leukocytes, especially eosinophils. Such inflammation is critical in the airway obstruction in asthma, and secreted products of eosinophils have been shown to be toxic to respiratory mucosa (2). Cytokines are considered to have a major role in the coordination of these cellular inflammatory conditions. In particular, interleukin 4 (IL-4) and IL-5 have prominent roles in allergic reactions. In IL-4 knockout mice immunoglobulin E (IgE) production is totally abolished (17). IL-4 is also important in attracting eosinophils to sites of inflammation (35). This may be achieved by upregulating local endothelial expression of the cell surface adhesion molecule VCAM-1 (33) or inducing the production of the eosinophil chemotactic factor eotaxin (30). IL-5 is a very important cytokine for eosinophils; it accelerates their production and has several actions on mature eosinophils, including priming for activation and prolongation of their life span (31). Antigen-induced eosinophil infiltration into the lungs was absent in IL-5 knockout mice (12). Cells containing mRNA for IL-5, detected by in situ hybridization, have been found at increased frequency in the airways of patients with asthma (15). Thus, production of IL-5 is particularly important in the development of the cellular infiltrate in asthma and other conditions with prominent eosinophilia.
Mast cells have the capacity to produce cytokines in response to cross-linking of receptor-bound IgE by specific allergen (13). Evidence for the mast cell as a source of cytokines in vivo has been obtained by immunohistochemical analysis of bronchial mucosal biopsy specimens from patients with asthma. IL-4, IL-5, IL-6, and tumor necrosis factor alpha were predominantly found in mast cells, although some IL-5 was found in eosinophils (6). In studies in which mRNA was detected in bronchoalveolar lavage fluid and bronchial biopsy specimens from patients with asthma by in situ hybridization, the number of cells expressing mRNA for IL-4 and IL-5 was greatly increased, and expression was found in T cells, mast cells, and eosinophils (43).
Glucocorticoids are widely regarded as the most effective available treatment for asthma, and they are particularly able to suppress cellular inflammation. Glucocorticoids can inhibit the production of most cytokines, and this may be an important general mechanism of their clinical efficacy (2). Glucocorticoids inhibit the expression of IL-4 (42) and IL-5 (28) by T cells. In the present study we have assessed the effects of glucocorticoids on the expression of IL-4 and IL-5 in the RBL-2H3 cell line, an analog of rat mucosal mast cells (34). IL-4 and IL-5 mRNAs were readily induced in these cells after activation by a variety of stimuli. Glucocorticoids markedly inhibited the expression of IL-4 and IL-5 mRNAs, even when the glucocorticoids were added well after the cells were activated. These effects could account, at least in part, for the therapeutic efficacy of glucocorticoids in the management of clinical allergic conditions.
MATERIALS AND METHODS
Cells.
RBL-2H3 cells were obtained from M. A. Beaven, National Institutes of Health, Bethesda, Md., and were cultured and activated as described previously (20, 21). For each aliquot taken from liquid nitrogen, the time course and dose-response of release of granule contents in response to antigen used to cross-link receptor-bound IgE were tested. Aliquots were used for no more than 20 passages. Cells were maintained as monolayers in minimal essential medium (MEM) without CaCl2 (Gibco BRL, Life Technologies, Gaithersburg, Md.) and with 2 mM glutamine (Gibco BRL) and 10% (vol/vol) fetal calf serum (P.A. Biologicals, Sydney, New South Wales, Australia) at 37°C in 5% CO2. There is sufficient calcium in the serum for normal cell growth and secretion (data not shown). Cells were harvested by trypsin treatment, and 0.8 × 106 cells were seeded into each well of 12-well culture dishes and were allowed to form monolayers overnight. Glucocorticoids were then added to the monolayers, and the mixture was incubated overnight prior to activation unless otherwise stated. Overnight glucocorticoid treatment did not affect cell number, cell viability, or total β-hexosaminidase content. Dexamethasone and hydrocortisone were obtained from Sigma (St. Louis, Mo.); the water-soluble form of dexamethasone was used. For experiments involving antigen activation, a 2,4-dinitrophenol (DNP)-specific monoclonal IgE antibody (75 ng/ml; Sigma) was added at the time that the cells were seeded.
Cells were washed twice with MEM (Gibco BRL) containing 200 mg of CaCl2 per liter but without fetal calf serum. All of the following activators were diluted in MEM at the indicated concentrations unless stated otherwise: 100 ng of DNP-bovine serum albumin (BSA) per ml (24 molecules of DNP conjugated with 1 molecule of BSA, kindly donated by H. Metzger, National Institutes of Health, Bethesda, Md.), 1,000 nM A23187 (Sigma), and 50 nM phorbol myristate acetate PMA (Sigma). In preliminary experiments, IL-4 mRNA was elicited to a similar extent by DNP-BSA concentrations of 10 to 1,000 ng/ml (data not shown). The cells were incubated in the solutions for 30 min, and the supernatants were collected for analysis of β-hexosaminidase secretion. The activated cells were then incubated for the remaining time in MEM without CaCl2 and were then lysed for RNA extraction. For samples treated with glucocorticoids overnight, prior to activation, cells were maintained in glucocorticoids throughout the time between activation and RNA extraction.
Analysis of IL-4 and IL-5 mRNAs.
The cells were harvested 4 h after activation unless otherwise stated. Total cellular RNA was extracted as described previously (8), the RNA concentration was estimated by measuring the optical density at 260 nm, and cDNA was prepared as described previously (23) from 1 μg of RNA in 50-μl volumes with oligo(dT) (4 ng/μl) and 4 U of avian myelobastosis virus reverse transcriptase (Promega, Madison, Wis.). PCR was performed with cDNA derived from 0.1 μg of total RNA with 1 U of Taq polymerase (Boehringer Mannheim, Mannheim, Germany) and 250 ng of each amplification primer as described previously (23). PCR was performed in a Gene Machine (Innovonics, Melbourne, Victoria, Australia) for 26 cycles for IL-4, 35 cycles for IL-5, and 22 cycles for β-actin. Denaturation, annealing, and extension conditions were 95°C for 60 s, 58°C for 30 s, and 75°C for 30 s, respectively. The primers, based on published sequences (22, 25, 36), were 5′-ACCTTGCTGTCACCCTGTTC-3′ and 5′-TTGTGAGCGTGGACTCATTC-3′ for IL-4, 5′-CTCTGTTGACGAGCAATGAG-3′ and 5′-CTCTTGCAGGTAATCCAGGA-3′ for IL-5, and 5′-TAACCAACTGGGACGATATG-3′ and 5′-ATACAGGGACAGCACAGCCT-3′ for β-actin. The expected product sizes were 351 bp for IL-4, 239 bp for IL-5, and 202 bp for β-actin. The primers were designed to anneal to different exons, so that any contaminating genomic DNA in the cDNA samples would contain one or more introns and would yield a product larger than that derived from cDNA.
PCR products were electrophoresed in 2% agarose gels and were stained with ethidium bromide. The sizes of the PCR products were determined with reference to molecular size markers (φX174 cleaved with HaeIII; Boehringer Mannheim). In some experiments, the gels were photographed with reversed-image film (Polaroid), and band intensities were measured by laser densitometry (Molecular Dynamics, Sunnyvale, Calif.). In other experiments, the specificities of the products were confirmed by Southern blotting. The gels were transferred to a nylon membrane (Hybond N+; Amersham, Amersham, Buckinghamshire, England) under vacuum, and the membranes were hybridized to oligonucleotide probes designed to anneal to the PCR product but not to the amplification primers. The hybridization probes were 5′-TACCTCCGTGCTTGAAGAAC-3′ for IL-4, 5′-TCAGTATGTCTAGCCCCTGA-3′ for IL-5, and 5′-CAGCCATGTACGTAGCCATC-3′ for β-actin. These were end-labelled with [32P]ATP and T4 polynucleotide kinase (Pharmacia, Uppsala, Sweden). The blots were washed and exposed to X-ray film (DuPont, Wilmington, Del.). After development of the films, band intensities were determined by laser densitometry as described above.
Reverse transcription-PCR (RT-PCR) for β-actin was performed with all samples. Fewer than 5% of the samples had markedly reduced or absent β-actin signals; these samples were excluded from further analysis. Semiquantitative PCR data from time course studies was obtained by performing RT-PCR with all samples and identifying the sample with the strongest signal. Threefold dilutions of this sample were prepared, and PCR was repeated with all samples and these dilutions. The dilutions were used to prepare a standard curve against which the other samples were read. The undiluted specimen of the sample with the strongest signal was defined as having a signal of 100%, and the other signal data were expressed as a percentage of the signal for this sample. Similar procedures were used to obtain semiquantitative data in the experiments with glucocorticoids, except that the signals for the samples which were stimulated and not treated with glucocorticoids were defined as being 100% and were used to prepare the dilutions.
Assessment of release of granule contents.
Secretion of granule contents by RBL-2H3 cells was determined by measuring the release of the granule marker β-hexosaminidase into the medium (26). Culture supernatants were collected 30 min after the cells had been activated. Supernatant (20 μl) was incubated with 20 μl of 5 mM p-nitrophenyl-N-acetyl-d-glucosamide (Sigma) in 0.05 M sodium citrate buffer (pH 4.5) in triplicate in a 96-well plate at 37°C for 2 h. At the end of the incubation, 200 μl of 0.1 M sodium carbonate-sodium bicarbonate buffer (pH 11.0) was added, and the absorbance at 405 nm was read in an enzyme-linked immunosorbent assay plate reader (Diagnostics Pasteur). The release of β-hexosaminidase was expressed as a percentage of the total β-hexosaminidase present in unactivated cells. Any effect of phenol red in the medium was accounted for by the inclusion of medium as a control. The cell lysate was obtained by incubating cells with 1 ml of 0.1% Triton X-100 for 10 min. Spontaneous release in the absence of stimuli was in the range of 2 to 6% of the total β-hexosaminidase and was subtracted from the values given above. Release from cells stimulated with antigen or with the combination of PMA and A23187 (PMA-A23187) was typically in the range of 40 to 50% of total β-hexosaminidase. The means for triplicate samples were determined, and these were used to calculate the means and standard errors of the means (SEMs) for replicate experiments. Data were then expressed as a percentage of the release from stimulated cells not treated with glucocorticoids.
RESULTS
Cytokine expression by RBL-2H3 cells.
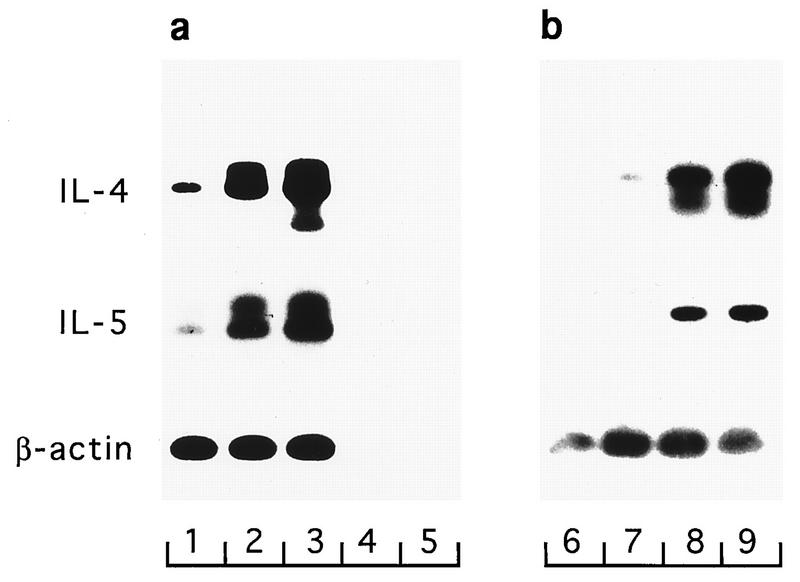
The capacity of RBL-2H3 cells to express cytokines was assessed in response to antigen cross-linking of receptor-bound IgE, to PMA, and to the calcium ionophore A23187. At 4 h after activation, cells were lysed and RT-PCR was performed for IL-4, IL-5, and β-actin mRNA. The sizes of the principal products determined by gel electrophoresis were within 10 bp of their predicted sizes, and their identities were confirmed by Southern transfer and hybridization to radiolabelled oligonucleotide probes (Fig. 1). In the Southern blots, in addition to the principal bands, some samples contained other bands which hybridized specifically. The bands with slower electrophoretic mobilities represent single-stranded PCR products (37); the bands with faster electrophoretic mobilities probably arise from mispriming of specific cDNA.
FIG. 1.
Inducible expression of IL-4 and IL-5 by RBL-2H3 cells. (a) Cells were exposed to no stimulus (lane 1), antigen (lane 2), or PMA-A23187 (lane 3) for 4 h, and RNA was then extracted. RT-PCR was performed, and products were detected by electrophoresis and Southern hybridization. The control RNA, β-actin RNA, was readily detected in all samples, attesting to the integrity of the RNA. Negative controls were omission of reverse transcriptase enzyme from the RT (lane 4, which was otherwise identical to lane 3) and omission of cDNA from the PCR (lane 5). (b) Cells were exposed to no stimulus (lane 6), PMA (lane 7), A23187 (lane 8), or PMA-A23187 (lane 9), and mRNA was detected as described above. Percent β-hexosaminidase releases in this experiment were as follows: no stimulus, 0.5%; PMA stimulus, 2.4%; A23187 stimulus, 43.8%; PMA-A23187 stimulus, 36.7%.
mRNAs for IL-4 and IL-5 were expressed at low levels in unstimulated cells. After stimulation with antigen, mRNA levels for IL-4 and IL-5 were markedly increased. Stimulation with the combination of PMA and A23187 had similar effects (Fig. 1a). IL-4 mRNA was very readily detected, with bands in ethidium bromide-stained gels being visible after only 26 cycles of PCR amplification of cDNA derived from 0.1 μg of total RNA. Stimulation of cells with A23187 alone was almost as effective as PMA-A23187 in inducing IL-4 and IL-5 mRNAs. However, PMA alone had little or no effect (Fig. 1b). The expression of IL-4 and IL-5 mRNAs in response to the different activators correlated with granular secretion, measured by the release of β-hexosaminidase (see the legend to Fig. 1).
To define the peak time of mRNA production, cells were harvested from 15 min to 8 h after activation. There was differential induction of IL-4 and IL-5 (Fig. 2). IL-4 mRNA was induced earlier, with markedly increased production detectable within 1 h. IL-4 mRNA levels peaked at 2 h and returned to baseline levels at 8 h. IL-5 had a slower induction rate, with little change within the first hour, peak expression 4 h after stimulation, and a slower decay of mRNA levels than those for IL-4 mRNA. In these experiments, IL-5 mRNA was detectable in unstimulated cells and IL-5 mRNA levels were not significantly increased by PMA alone. On the basis of these findings, subsequent mRNA harvest was performed 4 h after activation.
FIG. 2.
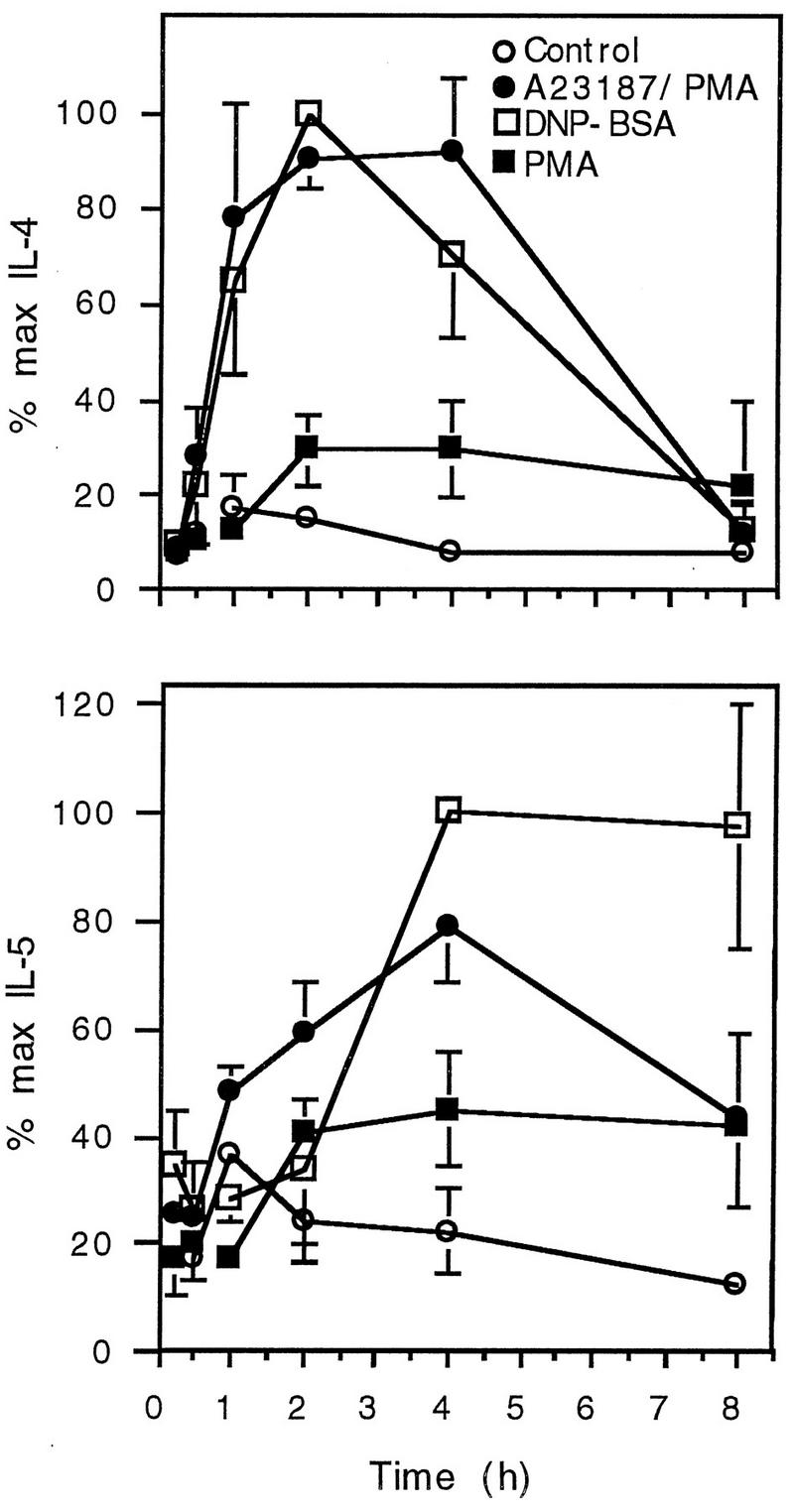
Time course of IL-4 and IL-5 mRNA production. Cells were activated with PMA-A23187 and were incubated for various times before lysis for RNA extraction. IL-4 and IL-5 mRNAs were detected by RT-PCR, and band intensity was determined by laser densitometry. Each value represents the mean and SEM for four (IL-4) or three (IL-5) separate experiments.
Effects of glucocorticoids.
The effect of the synthetic glucocorticoid dexamethasone on the production of IL-4 and IL-5 mRNAs in RBL-2H3 cells was determined. Concentrations of dexamethasone ranging from 10−6 to 10−11 M were tested in the presence of PMA-A23187 (Fig. 3). Dexamethasone at 10−10 M reduced the level of IL-4 mRNA production significantly and at 10−6 to 10−8 M almost abolished it (Fig. 3a), with a 50% inhibitory concentration (IC50) of 10−10.3 M. Dexamethasone at 10−9 M reduced IL-5 mRNA levels significantly and at 10−7 and 10−6 M reduced the mRNA to very low levels (Fig. 3b), with an IC50 of 10−8.8 M. At the higher doses tested, dexamethasone was moderately inhibitory to granule secretion, as measured by β-hexosaminidase release. However, even at doses as high as 10−6 M, only 40 to 50% inhibition of granule release was observed (Fig. 3c).
FIG. 3.
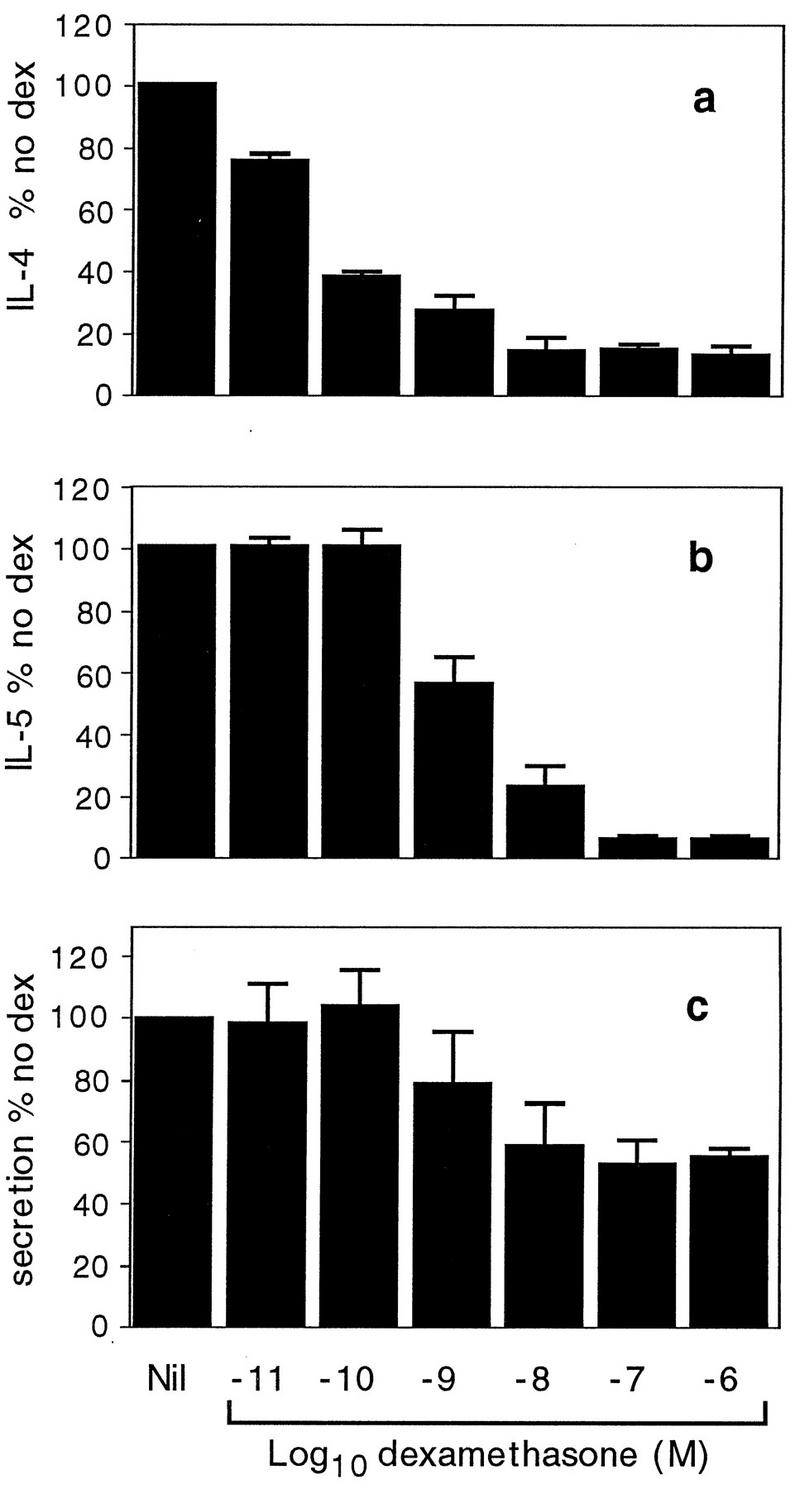
Effects of dexamethasone on cells stimulated with PMA-A23187. Cells were incubated overnight in the presence of various concentrations of dexamethasone and were stimulated with PMA-A23187. Cells were harvested 4 h later for assessment of IL-4 (a) and IL-5 (b) mRNA levels. Supernatants were collected 30 min after activation for determination of β-hexosaminidase secretion (c). Data are expressed as a percentage of the values for the samples from cells given no dexamethasone (dex). Each value represents the mean and SEM for three (a) or two (b and c) separate experiments.
The naturally occurring glucocorticoid hormone hydrocortisone had effects similar to those of dexamethasone. There was a dramatic concentration-dependent decrease in the levels of expression of both IL-4 and IL-5, with the mRNAs of both cytokines essentially being abolished at hydrocortisone concentrations of 10−6 M (Fig. 4a and b). As with dexamethasone, IL-4 mRNA production was more sensitive than IL-5 mRNA production to hydrocortisone. The IC50s were 10−8.9 M for IL-4 and 10−8.0 M for IL-5. Granule secretion was only moderately inhibited by the highest concentrations of hydrocortisone (Fig. 4c).
FIG. 4.
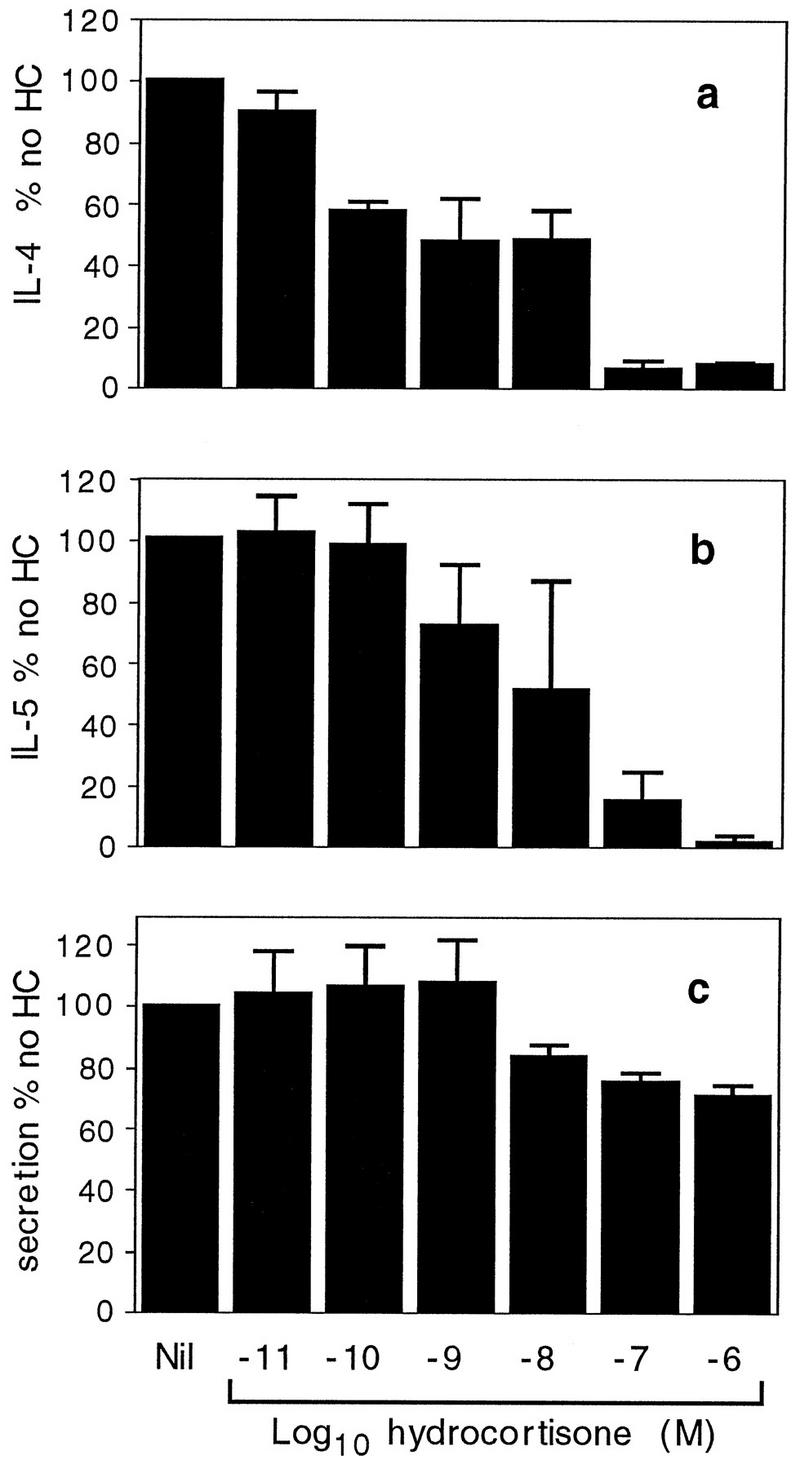
Effects of hydrocortisone. IL-4 mRNA (a), IL-5 mRNA (b), and secretion (c) were determined as described in the legend to Fig. 3, but with hydrocortisone instead of dexamethasone. Data are expressed as a percentage of the values for the samples from cells given no hydrocortisone (HC). Each value represents the mean and SEM for two (a and b) or three (c) separate experiments.
Studies were then performed to determine the effect of dexamethasone on cells activated by antigen-induced cross-linking of IgE receptors. These experiments were performed because this is a more physiological method of mast cell activation than PMA-A23187 stimulation. Cells were incubated with IgE antibody overnight and were exposed to specific antigen. Production of IL-4 and IL-5 mRNAs was markedly inhibited by dexamethasone concentrations from 10−8 to 10−6 M (Fig. 5). The IC50s were 10−8.6 M for IL-4 and 10−8.8 M for IL-5. These effects were similar to those described in Fig. 3 for cells stimulated with PMA-A23187. However, the inhibitory effect of dexamethasone on the secretion of β-hexosaminidase was much greater for cells stimulated with antigen (Fig. 5c) than for cells stimulated with PMA-A23187 (Fig. 3c).
FIG. 5.
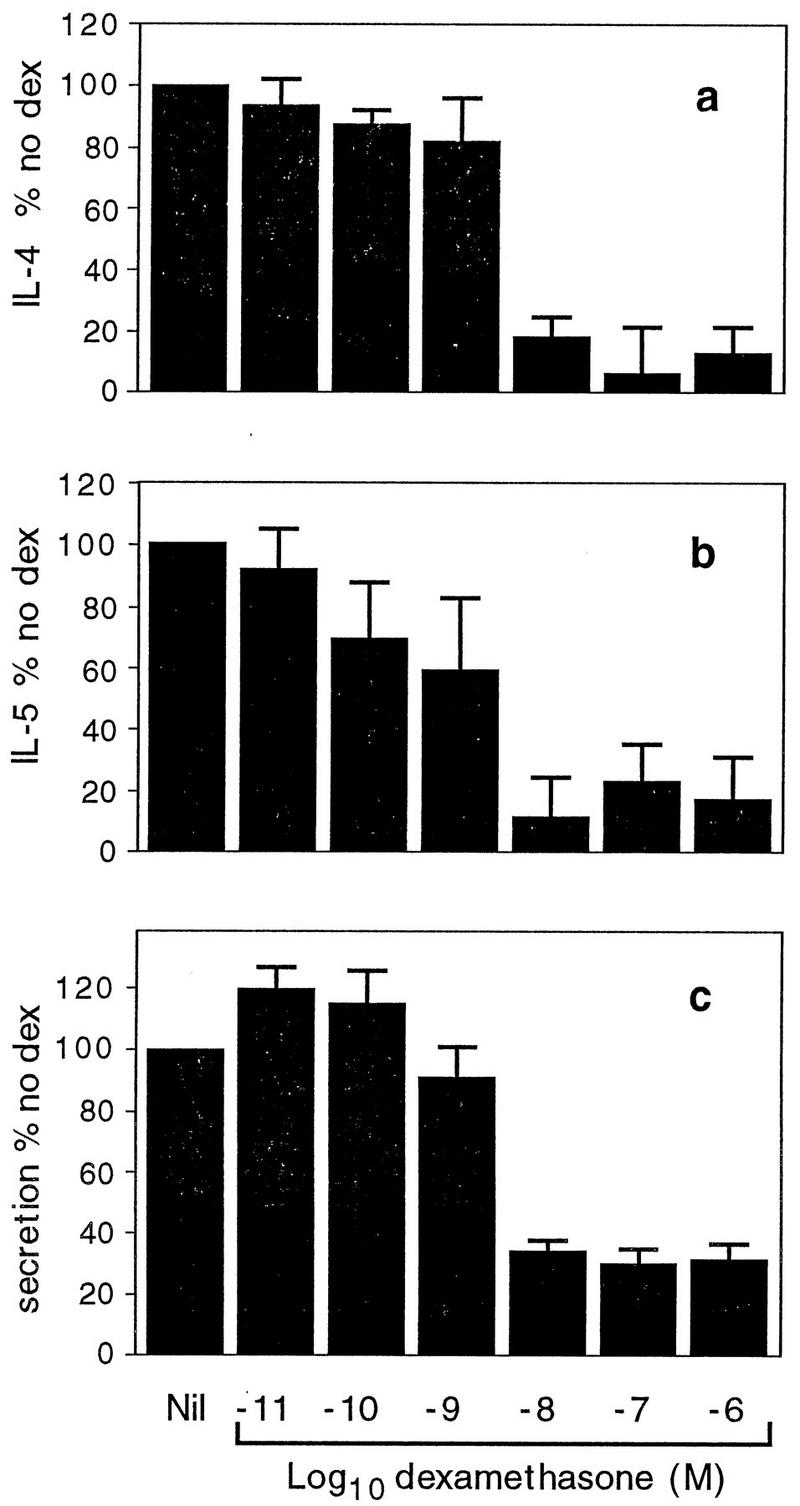
Effect of dexamethasone on cells stimulated with antigen. Cells were activated with DNA-BSA to cross-link receptor-bound IgE. IL-4 mRNA (a), IL-5 mRNA (b), and secretion (c) were determined as described in the legend to Fig. 3. Data are expressed as a percentage of the values for the samples from cells given no dexamethasone (dex). Each value represents the mean and SEM for three (a and b) or five (c) separate experiments.
In the experiments described above, dexamethasone was added overnight, prior to activation of the cells. The effects of adding it at a range of times prior to and after activation were assessed (Fig. 6). Cells were activated at time zero and 4 h later were harvested for RNA extraction. Dexamethasone was added at different times, from 16 h before activation to 4 h after activation, i.e., immediately prior to cell lysis for RNA extraction. Dexamethasone markedly inhibited IL-4 and IL-5 mRNA production when it was added 16 and 2 h prior to activation. It was also markedly inhibitory when it was added at the time of activation and at later times, as late as 2.5 h after activation, which was only 1.5 h before the cells were harvested for RNA extraction (Fig. 6). The effects were variable when dexamethasone was added only 1 h prior to harvest (3 h after activation), and there was no effect when it was added 30 min prior to harvest (3.5 h after activation).
FIG. 6.
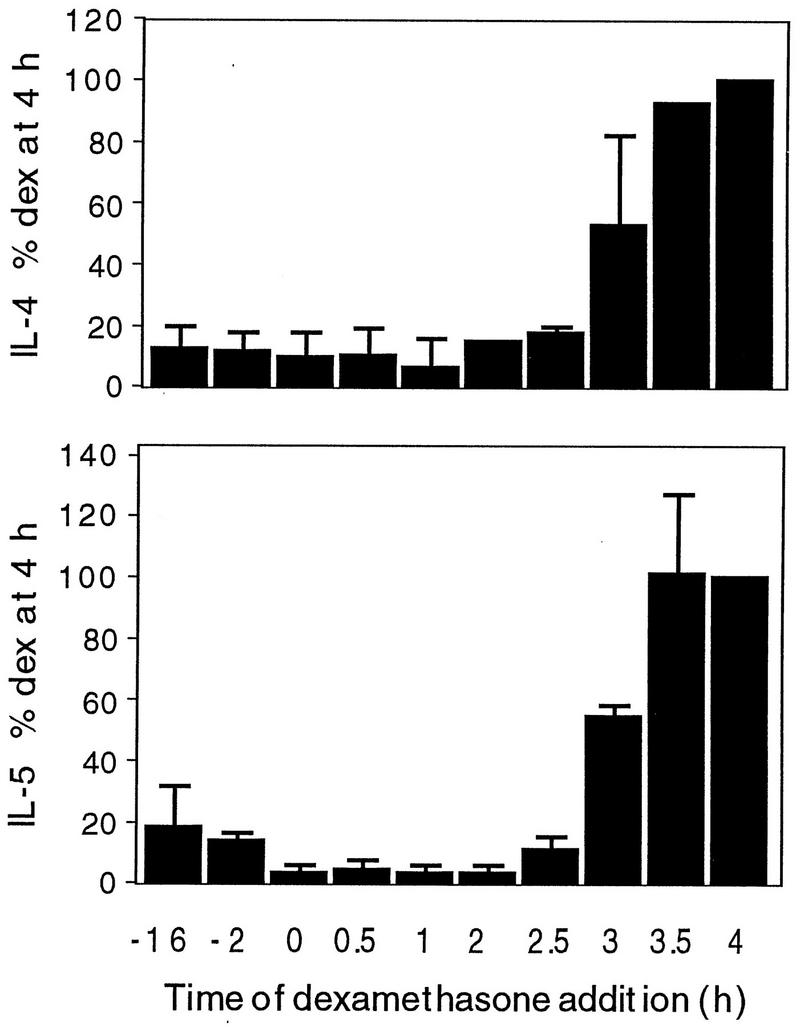
Effect of time of addition of dexamethasone. Cells were activated at time zero and 4 h later were harvested for RNA extraction. Dexamethasone was added at times ranging from 16 h prior to activation (−16) to immediately before cell harvest, i.e., 4 h after activation. RT-PCR was performed for IL-4 and IL-5. Quantitative data were determined as described in the legend to Fig. 3 and were expressed as a percentage of the values for the samples to which dexamethasone (dex) was added 4 h after activation. The data are the means and SEMs of two separate experiments.
DISCUSSION
In this paper we demonstrate that expression of IL-4 and IL-5 can be induced in the RBL-2H3 cell line, an analog of mucosal mast cells, by cross-linking of receptor-bound IgE or by a calcium ionophore. Induction of IL-4 and IL-5 mRNAs was strongly inhibited by the glucocorticoids dexamethasone and hydrocortisone. These findings are consistent with reports that glucocorticoids inhibit the expression or production of a number of cytokines by mast cells, including tumor necrosis factor alpha, IL-1β, IL-3, IL-6, and IL-8 (18, 19, 38, 39, 41). Inhibition of IL-4 mRNA by dexamethasone was recently reported in the human mast cell line HMC-1 (38). In T cells, by contrast, there are conflicting reports on the effect of glucocorticoids on IL-4 production. High concentrations of glucocorticoids were reported to inhibit IL-4 production by human blood T lymphocytes or mononuclear cells (7, 42) and by CD4+ T cells from rat lymph nodes (27). In a study with mice, however, low concentrations of dexamethasone from 10−11 to 10−8 M were reported to enhance IL-4 production by lymph node or spleen cells (10). In the present studies, dexamethasone and hydrocortisone doses from 10−11 to 10−6 M were tested, and there was no evidence of the enhancement of IL-4 or IL-5 mRNA production at any concentration, but there was marked inhibition at higher concentrations (Fig. 3 and 4).
The present findings confirm and extend other recent observations on the effects of glucocorticoids on the production of IL-5 mRNA by mast cells. Dexamethasone inhibits the expression of IL-5 in rat peritoneal mast cells (41) and in human lung explants stimulated with IgE (14). In these studies with cells prepared from tissue, the possibility that glucocorticoids might be acting on another cell population to induce a factor that could inhibit IL-5 mRNA production by mast cells cannot be completely excluded. The use of the RBL-2H3 mast cell line in the present study demonstrates unequivocally that these inhibitory effects of glucocorticoids on IL-5 expression are directly on the mast cells. In cells activated with PMA-A23187, lower concentrations of either dexamethasone or hydrocortisone were required to inhibit IL-4 mRNA compared with the concentrations required to inhibit IL-5 mRNA (Fig. 3 and 4). This effect suggests that the cells may use different signaling pathways for IL-4 and IL-5 mRNA expression. However, differences in glucocorticoid concentrations were not observed in cells activated by antigen (Fig. 5).
These findings raise the possibility that the beneficial therapeutic effects of glucocorticoids in allergic diseases may be mediated, at least in part, by inhibition of production of IL-4 and IL-5 by mast cells. An intriguing and novel observation was that dexamethasone may be added after activation but still markedly reduce the abundance of cytokine mRNA. When dexamethasone was added 2.5 h after activation and the cells were harvested 4 h after activation, the inhibition of IL-4 and IL-5 expression was as marked as when dexamethasone was added prior to activation (Fig. 6). These findings also suggest that in a therapeutic setting, IL-4 and IL-5 production can be inhibited by glucocorticoids after mast cells have been activated by an allergen. This effect may contribute to the clinical efficacy of therapy with glucocorticoids when they are introduced after the onset of an asthma attack.
It is interesting to speculate whether the glucocorticoid concentrations used in the present experiments are similar to those achieved therapeutically. In a pharmacokinetic study with humans, a single oral dose of 20 mg of hydrocortisone, a modest glucocorticoid dose, was followed by a peak concentration in plasma of 0.8 × 10−6 M (11). In the present experiments, IL-4 and IL-5 expression was almost completely abolished by 10−7 and 10−6 M hydrocortisone (Fig. 4). It is difficult to make exact comparisons between concentrations in vivo and in vitro because of differences in protein binding, the more rapid elimination of glucocorticoid in vivo, and different kinetics of receptor occupation during changing extracellular concentrations. Nevertheless, it is reasonable to propose that the concentrations of glucocorticoids achieved in the therapy of allergic conditions are sufficient to inhibit IL-4 and IL-5 mRNA expression. Dexamethasone was approximately 1 order of magnitude more potent than hydrocortisone (Fig. 3 and 4), a difference consistent with previous observations (5).
In T cells, IL-4 and IL-5 mRNA abundance is principally regulated by control of the rate of gene transcription rather than by alterations in mRNA stability (24, 29). From the data presented in Fig. 6, the levels of IL-4 mRNA 4 h after activation were very low in cells given dexamethasone 2.5 h after activation, by which time IL-4 mRNA levels have already peaked (Fig. 2). In activated T cells, the half-life of IL-4 mRNA was 60 min (3). If IL-4 has a similar half-life in mast cells, the findings in Fig. 6 could not be fully accounted for by effects on the rate of gene transcription, and acceleration of mRNA decay might also be involved. In the case of IL-5, peak mRNA expression was not reached until 4 h after activation. In mitogen-stimulated T cells, dexamethasone reduced total mRNA levels by inhibition of IL-5 gene transcription, without affecting mRNA stability (29a). The data therefore suggest that the effects of glucocorticoids on IL-5 in mast cells are based on inhibition of the rate of gene transcription.
The stimuli required for IL-4 and IL-5 gene expression are similar to those required for granule release (Fig. 1b). Thus, cross-linking of IgE receptors or calcium ionophore alone, but not PMA alone, is sufficient for both responses. IgE receptor cross-linking initiates a sequence of intracellular events leading to an increased intracellular calcium ion concentration. These elements of the signaling pathways may be required for both granule release and cytokine mRNA production. In cells stimulated with PMA-A23187, secretion was only partially inhibited by glucocorticoids (Fig. 3 and 4), whereas in cells stimulated with antigen, secretion of granule contents was markedly inhibited (Fig. 5), as has been reported previously (9, 40). The data in Fig. 3 and 4 suggest that the effects of glucocorticoids on cytokine mRNA production may involve components of the signaling pathway not required for granule release. A number of possible mechanisms whereby glucocorticoids might inhibit expression of cytokine genes have been described. Glucocorticoids inhibit the effects of transcription factors AP-1 and NF-κB, which are involved in the expression of cytokine genes. The glucocorticoid receptor-hormone complex interacts directly with AP-1 to prevent it from binding to its DNA motifs in promoter regions (16). Glucocorticoids stimulate production of I-κB, which inhibits the translocation of NF-κB from the cytoplasm to the nucleus (1, 32). Glucocorticoids can also accelerate mRNA degradation, as has been described in the reduction of IL-2 mRNA levels induced with dexamethasone in T cells (4). The effects of glucocorticoids on IL-4 and IL-5 mRNA production in mast cells may be mediated by these and/or other mechanisms.
The relative importance of mast cells as producers of cytokines in allergic conditions is controversial. Mast cells are the most numerous cells that contain IL-4 and IL-5 proteins in immunohistochemical studies with biopsy specimens from asthmatic patients (6). By contrast, in in situ hybridization studies, IL-4 and IL-5 mRNAs were found more frequently in T cells than in mast cells (43). Mast cells may be more effective early in allergic reactions because they are able to be activated rapidly after the arrival of antigen, which cross-links surface-bound IgE. By contrast, before T cells can be activated, antigen must be processed and presented by other cells. The rapid induction of cytokine mRNA described in Fig. 2 is consistent with a role for mast cells in the initiation of clinical allergic reactions.
ACKNOWLEDGMENTS
This project was supported by the Asthma Foundation of New South Wales. R.I.L. was supported by the E. Sternberg Research Fellowship.
REFERENCES
- 1.Auphan N, DiDonato J A, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P J. Anti-inflammatory therapy for asthma. Annu Rev Med. 1993;44:229–242. doi: 10.1146/annurev.me.44.020193.001305. [DOI] [PubMed] [Google Scholar]
- 3.Borger P, Kauffman H F, Postma D S, Vellenga E. IL-7 differentially modulates the expression of IFN-gamma and IL-4 in activated human T lymphocytes by transcriptional and posttranscriptional mechanisms. J Immunol. 1996;156:1333–1338. [PubMed] [Google Scholar]
- 4.Boumpas D T, Anastassiou E D, Older S A, Tsokos G C, Nelson D L, Balow J E. Dexamethasone inhibits human interleukin 2 but not interleukin 2 receptor gene expression in vitro at the level of nuclear transcription. J Clin Invest. 1991;87:1739–1747. doi: 10.1172/JCI115192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braat M C, Oosterhuis B, Koopmans R P, Meewis J M, Van Boxtel C J. Kinetic-dynamic modeling of lymphocytopenia induced by the combined action of dexamethasone and hydrocortisone in humans, after inhalation and intravenous administration of dexamethasone. J Pharmacol Exp Ther. 1992;262:509–515. [PubMed] [Google Scholar]
- 6.Bradding P, Roberts J A, Britten K M, Montefort S, Djukanovic R, Mueller R, Heusser C H, Howarth P H, Holgate S T. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 7.Byron K A, Varigos G, Wootton A. Hydrocortisone inhibition of human interleukin-4. Immunology. 1992;77:624–626. [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Collado-Escobar D, Cunha-Melo J R, Beaven M A. Treatment with dexamethasone down-regulates IgE-receptor-mediated signals and up-regulates adenosine-receptor-mediated signals in a rat mast cell (RBL-2H3) line. J Immunol. 1990;144:244–250. [PubMed] [Google Scholar]
- 10.Daynes R A, Araneo B A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989;19:2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- 11.Derendorf H, Mollmann H, Barth J, Mollmann C, Tunn S, Krieg M. Pharmacokinetics and oral bioavailability of hydrocortisone. J Clin Pharmacol. 1991;31:473–476. doi: 10.1002/j.1552-4604.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 12.Foster P S, Hogan S P, Ramsay A J, Matthaei K I, Young I G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli S J. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 14.Glaum M C, Jaffe J S, Gillespie D H, Raible D G, Post T J, Wang Y, Dimitry E, Schulman E S. IgE-dependent expression of interleukin-5 mRNA and protein in human lung: modulation by dexamethasone. Clin Immunol Immunopathol. 1995;75:171–178. doi: 10.1006/clin.1995.1068. [DOI] [PubMed] [Google Scholar]
- 15.Hamid Q, Azzawi M, Ying S, Moqbel R, Wardlaw A J, Corrigan C J, Bradley B, Durham S R, Collins J V, Jeffrey P K, Quint D J, Kay A B. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonat C, Rahmsdorf H J, Park K K, Cato A C B, Gebel S, Ponta H, Herrlich P. Antitumour promotion and antiinflammation: down modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 18.Leal-Berumen I, Conlon P, Marshall J S. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- 19.Lippert U, Welker P, Kruger-Krasagakes S, Moller A, Henz B M. Modulation of in vitro cytokine release from human leukemic mast cells (HMC-1) by glucocorticoids. Skin Pharmacol. 1996;9:93–98. doi: 10.1159/000211403. [DOI] [PubMed] [Google Scholar]
- 20.Ludowyke R I, Scurr L L, McNally C M. Calcium ionophore-induced secretion from mast cells correlates with myosin light chain phosphorylation by protein kinase C. J Immunol. 1996;157:5130–5138. [PubMed] [Google Scholar]
- 21.Maeyama K, Hohman R J, Metzger H, Beaven M A. Quantitative relationships between aggregation of IgE receptors, generation of intracellular signals, and histamine secretion in rat basophilic leukemia (2H3) cells. Enhanced responses with heavy water. J Biol Chem. 1986;261:2583–2592. [PubMed] [Google Scholar]
- 22.McKnight A J, Barclay A N, Mason D W. Molecular cloning of rat interleukin 4 cDNA and analysis of the cytokine repertoire of subsets of CD4+ T cells. Eur J Immunol. 1991;21:1187–1194. doi: 10.1002/eji.1830210514. [DOI] [PubMed] [Google Scholar]
- 23.Mu H-H, Sewell W A. Enhancement of interleukin-4 production by pertussis toxin. Infect Immun. 1993;61:2834–2840. doi: 10.1128/iai.61.7.2834-2840.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naora H, Young I G. Comparison of the mechanisms regulating IL-5, IL-4, and three other lymphokine genes in the Th2 clone D10.G4.1. Exp Hematol. 1995;23:597–602. [PubMed] [Google Scholar]
- 25.Nudel U, Zakut R, Shani M, Neuman S, Levy Z, Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983;11:1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozawa K, Szallasi Z, Kazanietz M G, Blumberg P M, Mischak H, Mushinski J F, Beaven M A. Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 27.Ramirez F, Fowell D J, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a Th2 cytokine response by CD4(+) T cells in vitro. J Immunol. 1996;156:2406–2412. [PubMed] [Google Scholar]
- 28.Rolfe F G, Hughes J M, Armour C L, Sewell W A. Inhibition of interleukin-5 gene expression by dexamethasone. Immunology. 1992;77:494–499. [PMC free article] [PubMed] [Google Scholar]
- 29.Rolfe F G, Sewell W A. Analysis of human interleukin-5 gene transcription by a novel nuclear run on method based on the polymerase chain reaction. J Immunol Methods. 1997;202:143–151. doi: 10.1016/s0022-1759(96)00245-1. [DOI] [PubMed] [Google Scholar]
- 29a.Rolfe, F. G., and W. A. Sewell. Unpublished data.
- 30.Rothenberg M E, Luster A D, Leder P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in interleukin 4-induced tumor suppression. Proc Natl Acad Sci USA. 1995;92:8960–8964. doi: 10.1073/pnas.92.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanderson C J. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 32.Scheinman R I, Cogswell P C, Lofquist A K, Baldwin A S., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 33.Schleimer R P, Sterbinsky S A, Kaiser J, Bickel C A, Klunk D A, Tomioka K, Newman W, Luscinskas F W, Gimbrone M J, McIntyre B W, et al. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992;148:1086–1092. [PubMed] [Google Scholar]
- 34.Seldin D C, Adelman S, Austen K F, Stevens R L, Hein A, Caulfield J P, Woodbury R G. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci USA. 1985;82:3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tepper R I, Levinson D A, Stanger B Z, Campos-Torres J, Abbas A K, Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- 36.Uberla K, Li W Q, Qin Z H, Richter G, Raabe T, Diamantstein T, Blankenstein T. The rat interleukin-5 gene: characterization and expression by retroviral gene transfer and polymerase chain reaction. Cytokine. 1991;3:72–81. doi: 10.1016/1043-4666(91)90012-3. [DOI] [PubMed] [Google Scholar]
- 37.Valentine J E, Boyle M J, Gough N M, Sewell W A. Presence of single-stranded DNA in PCR products of slow electrophoretic mobility. BioTechniques. 1992;13:222–224. [PubMed] [Google Scholar]
- 38.Warbrick E V, Thomas A L, Williams C M M. The effects of cyclosporin A, dexamethasone and other immunomodulatory drugs on induced expression of IL-3, IL-4 and IL-8 mRNA in a human mast cell line. Toxicology. 1997;116:211–218. doi: 10.1016/s0300-483x(96)03519-6. [DOI] [PubMed] [Google Scholar]
- 39.Wershil B K, Furuta G T, Lavigne J A, Choudhury A R, Wang Z S, Galli S J. Dexamethasone or cyclosporin A suppress mast cell-leukocyte cytokine cascades. Multiple mechanisms of inhibition of IgE- and mast cell-dependent cutaneous inflammation in the mouse. J Immunol. 1995;154:1391–1398. [PubMed] [Google Scholar]
- 40.White M V, Igarashi Y, Lundgren J D, Shelhamer J, Kaliner M. Hydrocortisone inhibits rat basophilic leukemia cell mediator release induced by neutrophil-derived histamine releasing activity as well as by anti-IgE. J Immunol. 1991;147:667–673. [PubMed] [Google Scholar]
- 41.Williams C M M, Coleman J W. Induced expression of mRNA for IL-5, IL-6, TNF-α, MIP-2 and IFN-γ in immunologically activated rat peritoneal mast cells: inhibition by dexamethasone and cyclosporin A. Immunology. 1995;86:244–249. [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C Y, Fargeas C, Nakajima T, Delespesse G. Glucocorticoids suppress the production of interleukin 4 by human lymphocytes. Eur J Immunol. 1991;21:2645–2647. doi: 10.1002/eji.1830211053. [DOI] [PubMed] [Google Scholar]
- 43.Ying S, Durham S R, Corrigan C J, Hamid Q, Kay A B. Phenotype of cells expressing mRNA for Th2-Type (interleukin 4 and interleukin 5) and Th1-type (interleukin 2 and interferon gamma) cytokines in bronchoalveolar lavage and bronchial biopsies from atopic asthmatic and normal control subjects. Am J Respir Cell Mol Biol. 1995;12:477–487. doi: 10.1165/ajrcmb.12.5.7742012. [DOI] [PubMed] [Google Scholar]