Summary
Here, we present a protocol to study neural circuits between the paraventricular thalamus (PVA) and the bed nucleus of the stria terminalis (BNST) in mice by combining calcium imaging with optogenetic stimulation of axon terminals. We describe steps for delivering GCaMP6f and ChrimsonR viruses and implanting the gradient-index (GRIN) lens for use with an Inscopix microscope. We then detail procedures for single-neuron tracking over an extended period through longitudinal recording and data smoothing and processing to enhance analysis.
Subject areas: Behavior, Cell Biology, Neuroscience
Graphical abstract

Highlights
-
•
Cranial window surgery for GCaMP6 and ChrimsonR injection with GRIN lens implantation
-
•
Single-cell in vivo calcium imaging of BNST neurons and PVA terminal stimulation
-
•
Longitudinal calcium imaging and analysis of BNST activity across multiple time points
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Here, we present a protocol to study neural circuits between the paraventricular thalamus (PVA) and the bed nucleus of the stria terminalis (BNST) in mice by combining calcium imaging with optogenetic stimulation of axon terminals. We describe steps for delivering GCaMP6f and ChrimsonR viruses and implanting the gradient-index (GRIN) lens for use with an Inscopix microscope. We then detail procedures for single-neuron tracking over an extended period through longitudinal recording and data smoothing and processing to enhance analysis.
Before you begin
Advances in modern technologies have significantly enhanced classical neuroscience techniques, allowing for precise dissection of neural circuits and identification of key brain regions involved in nociception. Methods such as neural circuit tracing, optogenetics, chemogenetics, and genetic tools now enable researchers to map anatomical connections between specific cell types and link them to behavioral changes.1,2,3 However, these strategies lack direct real-time observation of neural activity. Genetically encoded calcium imaging technique facilitates the visualization of specific neurons, helping to address this issue. Recently, we utilized one-photon calcium imaging to record neuronal activity in the PVA and BNST,4 a circuit implicated in the development of chronic pain. This protocol outlines the procedures for imaging calcium activity in BNST neurons that express GCaMP6f while simultaneously stimulating glutamatergic neuron terminals in the PVA that express ChrimsonR. This methodology can be tailored for different rodent species by adjusting stereotactic parameters and adapted for imaging diverse cell types through the selection of specific mouse models and viral tools. The data analysis techniques are suitable for calcium imaging in brain regions where neurons show high synchronized activity or where significant motion artifacts occur. The aim of this protocol is to increase the number of recorded cells while maintaining a stable field of view across multiple sessions by integrating optogenetic manipulation with calcium signal recording. While only a few studies have attempted longitudinal imaging, most have focused on short-term neural activity recordings. In contrast, our study enables stable calcium signal recording over multiple days.
This protocol can be used to uncover the synaptic structure of neural circuits, identify specific drug targets, and explore the intricate circuit dysfunctions associated with brain disorders, though its applications are not confined to these areas.
Institutional permissions
All the experimental protocol was approved by the Institutional Animal Care and Use Committee of Academia Sinica and followed the National Institutes of Health’s standards and the guidelines from the International Association for the Study of Pain for the use and care of laboratory animals.
Preparation phase
Prepare viruses
Timing: 15 min
-
1.
Purchase the required viral vectors.
-
2.
Aliquot viral vectors: Prepare a pipette, viral stocks (AAV9-CAG-DIO-GCaMP6f-WPRE-SV40 and AAV5-Syn -rc[ChrimsonR-tdTomato]), Eppendorf tubes, and saline solution.
Note: Aliquot 2 μL of the viral vector into PCR tubes; label the tubes clearly and place them in a deep freezer set to −80°C for storage. Prevent repeated cycles of thawing and freezing, store thawed aliquots at 4°C and use within one week. If required, dilute the viral stock with saline to achieve a desired concentration (2 × 1012 vg/mL). Check viral stock concentration for dilution as it may vary across lots.
Set up stereotaxic frame
Timing: 30 min
-
3.
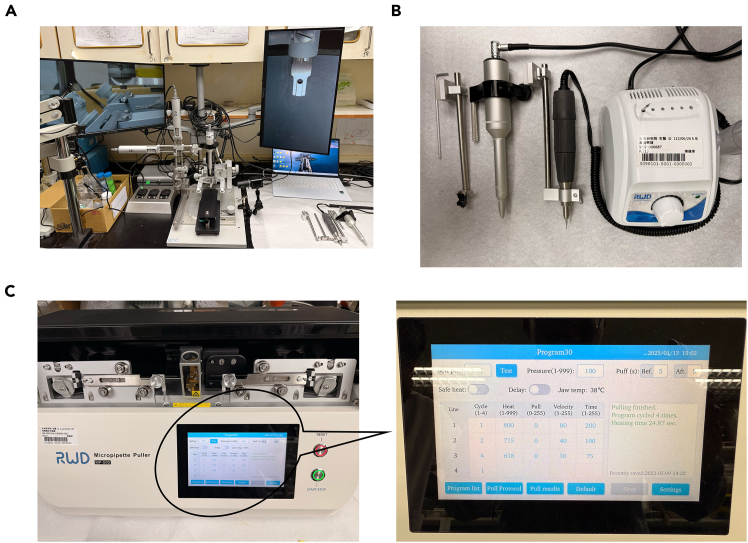
Assemble the stereotaxic apparatus, including: Automated Stereotaxic Instrument, Multi-function holders, Nanoliter microinjection pump, laptop with installed softer for controlling stereotaxic apparatus, camera, monitor and drill bit (tip diameter: 0.5 mm) (Figures 1A and 1B).
-
4.
Inscopix components: GRIN lens, holder, screws, superbond, screwdriver and domes.
Figure 1.
The stereotaxic surgery instruments
(A) Stereotaxic set-up.
(B) Drill, multipurpose holder and micropump.
(C) Micropipette puller and Puller set-up.
Prepare glass pipettes for virus injection
Timing: 10 min
-
5.
Collect glass capillaries with an outer diameter of 1.1 mm along with a micropipette puller and surgical scissors.
-
6.
Place the glass capillary onto the micropipette puller (Figure 1C).
-
7.
Open the program and adjust the parameters to pull and shape the capillaries Trim the tip of each pipette using micro forage.
-
8.
Make sure the diameter at the tip is smaller than 30 μm, and the tapered section extends beyond 1 cm after trimming.
Prepare surgical tools
Timing: 15 min
-
9.
Gather the necessary items: Stainless-steel bowl, Eye lubricant, and Kwik-Sil, a 3 mL syringe, an 30G insulin syringe, sodium pentobarbital, Lidocaine, ketoprofen, chlorhexidine gluconate solution and meloxicam for anesthesia and pain management.
-
10.
Gather materials for surgery: Hair shaver; Adhesive tape; Disinfection supplies: disinfection bags, paper towels, cotton gauze, cotton swabs, saline, absorbable sutures, 75% ethanol; Surgical tools: surgery scissors, scalpel, forceps, scalpel blades, hemostats.
-
11.
Sterilize tools using an autoclave.
Prepare data processing tools
Timing: 30 min
-
12.
Download and install the Inscopix Data Processing Software, Anaconda environment, Visual Studio Code, and the protocol-associated code.
-
13.
This protocol uses custom code for data analysis; refer to the key resources table for download links.
Note: Verify all materials and software are ready before starting surgical or experimental procedures.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| AAV9-CAG-Flex-GCaMP6f-WPRE-SV40 | Chen et al.5 | Addgene viral prep # 100835-AAV9 |
| AAV-EF1a-DIO-GCaMP6f-P2A-nls-dTomato | Jonathan Ting | Addgene viral prep # 51083-AAVrg |
| AAV5-Syn-rc[ChrimsonR-tdTomato] | Klapoetke et al.6 | Addgene viral prep # 62723-AAV5 |
| Chemicals, peptides, and recombinant proteins | ||
| PBS solution | Sigma-Aldrich | P5368 |
| Lidocaine | Sigma-Aldrich | 6108-05-0 |
| Ketoprofen | Sigma-Aldrich | K1751 |
| Chlorhexidine gluconate solution | Sigma-Aldrich | 18472-51-0 |
| Meloxicam | Sigma-Aldrich | 71125-38-7 |
| Sodium pentobarbital | Sigma-Aldrich | 76-74-4 |
| Mineral oil | Sigma-Aldrich | 8042-47-5 |
| Experimental models: Organisms/strains | ||
| VgluT2-Cre (−/−), both sexes, 8–12 weeks old. | Gift from Dr. Chen | N/A |
| Software and algorithms | ||
| Inscopix Data Acquisition Software 1.9.1 | Inscopix | https://inscopix.com/ |
| ImageJ software | ImageJ | https://imagej.net/ij/ |
| PVA-Ca2+-data-analysis | Python | https://doi.org/10.5281/zenodo.13909372 |
| Python 3.9.7 | Anaconda Distribution | N/A |
| Visual Studio Code | Microsoft | N/A |
| Other | ||
| Cameras | Logitech | N/A |
| Isoflurane | Baxter | N/A |
| Automated stereotaxic instrument | RWD | 71000 |
| Multi-function holders | RWD | N/A |
| Holder for Inscopix | RWD | N/A |
| Nanoliter microinjection pump | RWD | N/A |
| Heating pad | MINK | N/A |
| Drill bit | RWD | 78001 Microdrill |
| Injection syringe | Hamilton | 10 μl 1701 Syringe |
| Injection needle | Hamilton | NDL30/50mm |
| nVoke | Inscopix | https://inscopix.com/nvoke-system/ |
| Inscopix microscope Inscopix nVista 3.0 | Inscopix | |
| Integrated lens 0.5 × 6.1 mm | Inscopix | https://inscopix.com/lenses-viruses/ |
| Baseplate cover | Inscopix | 1050-004639 |
| Forceps | Sigma | N/A |
| Surgical scissors | Sigma | N/A |
| Glass capillaries | RWD | 3.5 RWD |
| Micropipette puller | RWD | MP-500 |
| Self-tapping screws | N/A | N/A |
| Eye lubricant (DURATEARS) | Alcon | N/A |
| Kwik-Sil | World Precision Instruments | N/A |
| Super Bond C and B kit | Sun Medical | N/A |
Step-by-step method details
Stereotaxic surgery procedures
Timing: 65 min
Here, we describe the step-by-step procedure for performing stereotaxic surgery on mice. This protocol ensures precise targeting of brain regions.
-
1.Preparation for Surgery.
-
a.Prepare PBS Solution.
-
i.Mix 10× PBS with dabble distilled water to create a working concentration and autoclave prepared 1x PBS.
-
ii.Sterilize a minimum of 50 mL of PBS per mouse using an autoclave for surgical procedures.
-
i.
-
b.Set Up the Surgical Environment (Prepare the Surgical Setup).
-
i.Calibrate the stereotaxic apparatus by ensuring the horizontal and vertical arms are properly aligned with the zero mark, checking for any drift along the X, Y, and Z axes.
-
ii.Arrange all surgical tools, anesthetic supplies, and sterilized materials within easy reach.
-
iii.Confirm the drill bit and other tools are sterilized and in working condition.
-
iv.Open the stereotaxic program controlling the manipulators.
-
i.
-
c.Prepare Mice for Surgery (Anesthetize the Mice).
-
i.Induce anesthesia by administering 2.5% isoflurane to the mice.
-
i.
-
a.
Note: Confirm all instruments and solutions meet sterile requirements before beginning the procedure.
-
2.Procedure for Stereotaxic Surgery.
-
a.Anesthetize and Fix the Mouse.
-
i.Induce anesthesia using 2.5% isoflurane and verify its adequacy by assessing the toe pinch reflex.
-
ii.Position the mouse’s nose under the anesthesia mask delivering 1.5% isoflurane with a flow rate of 1–2 L/min of oxygen to ensure adequate anesthetic delivery.
-
iii.Secure the mouse in the stereotaxic head frame using ear bars.
-
iv.Adjust the Homeothermic system to 35°C to maintain body temperature throughout the procedure.
-
v.Place the mice on a heating pad.Note: Be gentle to avoid injury but ensure the head is firmly fixed. Verify that the head is stable before proceeding.
-
i.
-
b.Expose the Skull.
-
i.Shave the scalp over the surgical area.
-
ii.Clean the surgical area using a chlorhexidine gluconate solution, gently apply 100 mL of lidocaine, and place eye lubricant to the mouse’s eyes to prevent dryness.
-
iii.Make a midline incision along the scalp using a scalpel to expose the skull. Minimize bleeding using sterile gauze or hemostatic agents if necessary.
-
iv.Remove any connective tissue on the skull using a bone scraper to ensure a clear surgical field.Note: Clean the exposed skull thoroughly using sterile saline or ethanol (70%).
-
i.
-
c.Align the Skull.
-
i.Adjust the stereotaxic frame to align the skull horizontally by ensuring the bregma and lambda are at the same vertical height. Use a micromanipulator for fine adjustments.
-
ii.Identify key landmarks and mark on the program controlling the manipulator (bregma, lambda, and the right and left sides of the skull).
-
iii.Mark the bregma with a surgical marker or pencil.
-
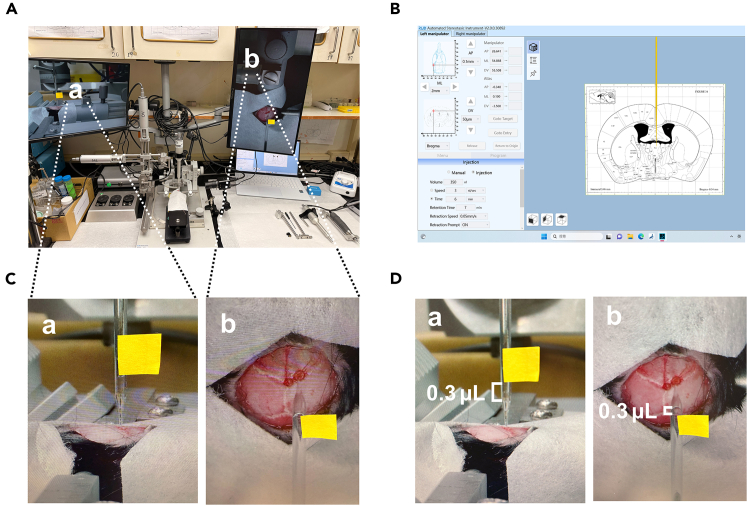
iv.Key the coordinate of each site (PVA and BNST) on the computer interface (Figure 2A).Note: Since we are using an Automated Stereotaxic Instrument, the computer will determine the surgical site.
-
i.
-
d.Drill Craniotomies.
-
i.Key the coordinate of the target; PVA: AP −0.34 mm, ML 0.0 mm, DV −3.5 mm; BNST: AP 0.2 mm, ML 0.95 mm, DV −4.0 mm.
-
ii.Position the manipulator over the target coordinates for each region: (go to the target).
-
iii.Lower the drill bit slowly to the surface of the skull at the marked coordinates. Turn on the drill and carefully drill a hole through the skull. Avoid excessive force to prevent damage to underlying structures.
-
iv.Turn on the drill at a low speed, ensuring minimal pressure to prevent damage to the underlying brain tissue. Gradually increase the speed as necessary, but proceed with caution to avoid perforating the cortical surface (Figures 2A–2C).
-
i.
-
e.Inspect the Surgical Site.
-
i.Confirm that the craniotomy is clean and free of debris.
-
ii.If debris is present, gently flush the area with sterile saline or PBS.Note: Bleeding is managed by applying gentle pressure with a sterile cotton swab or surgical sponge to the affected area. The craniotomy is performed with caution to minimize tissue damage and ensure a clean surgical field.
-
i.
-
a.
Figure 2.
Drill Craniotomies
(A) Program setup to control the drill for precise targeting of the brain region.
(B) Stereotaxic setup after securely fixing the mouse, equipped with a drill for craniotomy and two screens, Screen a and Screen b.
(C) Screen a and Screen b for visualizing from a side view and a top view.
Procedure for viral injections
Timing: 50 min
This step outlines the procedure for performing viral injections in mice, ensuring precise delivery to the targeted brain regions.
-
3.Prepare the Viral Microinjection Setup.
-
a.Remove the drill bit from the manipulator and attach the microinjection pump.
-
b.Fill a 3 mL syringe with mineral oil.
-
c.Connect the syringe to a glass pipette using a needle.
-
d.Slowly fill the glass pipette with mineral oil, ensuring the tip remains fully submerged. Gently tap the pipette to release any trapped air bubbles.
-
e.Attach the glass pipette filled with mineral oil to the microinjection pump.
-
f.Use the computer-assisted pump to remove 50 nL of mineral oil from the glass pipette at a rate of 50 nL/s to check the functionality of the glass pipette.
-
a.
Note: Ensure the glass pipette is straight when attaching it to the microinjection pump. Avoid any air bubble in the glass pipette while filling mineral oil, if there is bubble use new glass pipette
-
4.Load Virus into the Glass Pipette.
-
a.Remove desirable amount of mineral oil (2 μl) and then fill the glass pipette with the virus solution (e.g., AAV-EF1α-DIO-ChrimsonR-tdTomato). Load 400 nL of virus using the pump at a rate of 30 nL/s.
-
b.Test the glass pipette by removing 50 nL of virus to ensure functionality and clean the tip with 70% alcohol.
-
a.
Note: Make sure that there is no air bubble in the glass pipette after loading virus. Remove glass pipette and start from 1. Prepare the Microinjection Setup if an air bubble is present.
-
5.Align the Skull.
-
a.Align the skull by locating the bregma, lambda, and the right and left sides of the skull.
-
a.
-
6.Position the Glass Pipette.
-
a.Use previous coordinate of PVA (AP 0.34 mm, ML 0.0 mm, DV −3.5 mm) and press ‘go to entry’ on your computer.
-
b.Slowly move the manipulator down to the surface of the skull.
-
c.Position the tip of the glass pipette at the center of the drilled hole of stereotaxic surgery procedure.
-
a.
Note: If your reference point is consistent the tip of the pipette will be targeting the center of drilled hole. The setup for viral injections should ensure precise and reliable delivery.
-
7.Target the Injection Site.
-
a.Introduce the glass pipette filled with the virus through the drilled hole.Note: Brain dimpling can occasionally occur if the glass pipette is not sharp enough to penetrate the brain tissue. To handle this, use a new pipette with a sharper tip and insert it slowly at the correct angle to minimize pressure on the cortical surface. If dimpling occurs, reduce the insertion speed and carefully adjust the pipette’s position to avoid causing damage. Applying gentle pressure to the surrounding tissue may help prevent excessive dimpling while preserving the integrity of the brain structure.
-
b.Slowly lower the pipette to the target region by pressing ‘go to entry’ on your computer (adjust the speed of manipulate to 0.1 mm/s).
-
a.
-
8.Mark and Monitor the Virus Level.
- a.
-
b.Use the pressure pump and computer-assisted pump to administer the virus at 20 nL/min. Inject 0.3 μL of virus for each mouse (Figure 3D).
-
9.Remove the Glass Pipette.
-
a.Keep the pipette in place for 10 min to allow diffusion of the virus.
-
b.Gradually remove the pipette and check for blockages.
-
c.Seal the hole by blending and filling it with the Kwik-Sil mixture.
-
a.
Note: Before removing the glass pipette, inspect the virus level on your monitor. Compare the current level to your mark to ensure it reflects the expected decrease. If there is no change, carefully remove the pipette from the brain, clean the tip, and test it. If the pipette is blocked, replace it with a new one and repeat the injection procedure steps (Figure 3D).
-
10.Repeat the Process for BNST Injection.
-
a.Remove the glass pipette and using new glass pipette repeat Viral injections procedure.
-
b.Load the pipette with a new virus solution (e.g., AAV9-CAG-GCaMP6f-WPRE-SV40).
-
c.Remove 50 nL of mineral oil to test the pipette.
-
d.Align the skull using (Refer to stereotaxic surgery procedure).
-
e.Align the glass pipette to the drilled hole over BNST (refer to viral injection procedure).
-
f.Use previous coordinate of BNST (AP 0.20 mm, ML 0.95 mm, DV −4.07 mm) and press go to the sight on your computer.
-
a.
-
11.Target the BNST Region.
-
a.Introduce the pipette to the target site and mark the initial virus level on the screen.
-
a.
-
12.Administer the Virus.
-
a.Inject 0.2 μL of virus at 20 nL/min using the pressure pump.
-
a.
-
13.Remove the Pipette After Injection.
-
a.Keep the pipette in place for 10 min after the injection.
-
b.Gradually remove the pipette and confirm successful delivery by comparing the virus levels before and after the injection.
-
a.
Note: Do not reuse the pipette after the injection to prevent contamination and ensure accurate delivery of the virus.
Figure 3.
Viral vector injection
(A) Stereotaxic setup after securely fixing the mouse, micropump for injection.
(B) Program setup to control the virus injection at the brain region.
(C) Screen a and Screen b display visualizations from the side and top views, respectively, with a marker indicating the virus level in the micropipette.
(D) Screen a and Screen b display visualizations from the side and top views, respectively, showing virus levels. The black indicates the new level after injection.
Procedure for GRIN lens implantation for calcium imaging
Timing: 60 min
This protocol details the step-by-step procedure for implanting a GRIN lens in mice, ensuring accurate placement for optimal calcium imaging in the targeted brain regions.
-
14.Prepare the Setup.
-
a.Remove the microinjection pump and attach the GRIN lens holder with its baseplate attached with the lens to the stereotaxic apparatus.
-
b.Relocate the bregma and confirm the alignment of all points (refer to Step 2 of the stereotaxic surgery procedure).
-
c.Ensure the alignment targets the same drilled hole for BNST, confirming precise bregma relocation.
-
a.
Note: The lens should be centered in the same drilled hole.
-
15.Verify Skull Preparation.
-
a.Confirm that the dura was removed (refer to Step 6 of the stereotaxic surgery procedure).
-
a.
-
16.Implant the GRIN Lens.
-
a.Gently lower the manipulator to the skull holding the GRIN lens (0.5 mm diameter, 6.1 mm length, with baseplate, AC-11191702, Inscopix).
-
b.Use the stereotaxic manipulator to move the lens downward step by step:
-
i.Move down 1 mm at a speed of 0.01 mm/s and wait for 10 min to allow tissue settling.
-
ii.Repeat this process until the lens is positioned 0.1–0.2 mm above the target site (Figures 4A and 4B).
-
i.
-
a.
Note: Use semi-automatic control for precise, gradual movements (adjust the speed of the manipulate).
-
17.Secure the GRIN Lens.
-
a.Affix the lens and its baseplate to the skull using superbond glue and dental cement (first layer of dental cement).
-
b.Use 3M Vetbond along the incision line to secure the skin to the skull.
-
c.Every 5 min later apply a thin coating of superbond for additional stability second layer of dental cement.
-
d.Affix the lens and its baseplate to the skull using superbond glue and dental cement (third layer of dental cement filed after 5 minus of second layer).
-
e.Fill the gap between the screws and the base plate.
-
a.
-
18.Protect the GRIN Lens.
-
a.Attach a baseplate cover (Inscopix) magnetically to the baseplate to safeguard the GRIN lens during non-use periods.
-
a.
-
19.Post-Surgery Recovery.
-
a.Inspect the mice every other day and monitor for proper recovery. After the procedure, return the mice to a clean cage. Administer pain relief via intraperitoneal injection (I.P.) with Ketoprofen at a dose of 2 mg/kg body weight after the surgery. Monitor their behavior, including activity levels, eating, and drinking habits, to assess recovery progress.
-
a.
Note: If the mice show signs of inadequate recovery, such as severe lethargy, labored breathing, inability to eat or drink, or significant weight loss (e.g., >20% body weight reduction), euthanasia should be performed based on humane criteria. Follow institutional guidelines for euthanasia, using CO2 as the method of sacrifice.
-
20.Acclimatization with Dummy Microscope.
-
a.Attach a dummy micro-camera from Inscopix to the baseplate.
-
b.Acclimate the mice to a non-functional (dummy) scope for 7 days, 30 min per day in their home cage to ensure similar behavior during recording sessions.
-
a.
Note: The GRIN lens implantation must target the same coordinates as the viral infusion site for BNST (AP 0.2 mm, ML 0.9 mm, DV −4.0 mm). Ensure the lens is centered in the drilled hole created during stereotaxic surgery. Regular inspection and familiarization of the mice with experimental handling are critical for reliable results.
Figure 4.
GRIN lens implantation
(A) Stereotaxic setup after securely fixing the mouse, equipped with Inscopicx holder for implantation of GRIN lens and two screens, Screen a and Screen b.
(B) Screen a and Screen b for visualizing from a side view and a top view of GRIN lens.
In vivo single-cell calcium imaging
Timing: 60 min
This step outlines the procedure for collecting data during in-vivo calcium imaging in mice. The process ensures accurate recording of neural activity at the single-cell level within the targeted brain regions.
-
21.Miniscope Acclimatization.
-
a.Attach the functional miniscope and acclimate mice in the home cage for 3 additional days:
-
i.Sessions last 30–60 min per day.
-
i.
-
a.
-
22.Recording Chamber Acclimatization.
-
a.Place mice in a 15 cm × 15 cm × 15 cm transparent acrylic recording chamber over a mesh table.
-
b.Acclimate for 3 days with 30-min daily sessions.
-
a.
-
23.Recording Sessions.
-
a.On the recording day, allow 30 min of free exploration in the chamber while recording basal calcium activity.
-
b.Record calcium activity: (Figure 5A).
-
i.Before stimulation: Basal activity.
-
ii.During stimulation: The system can use two separate light sources that emit light at different wavelengths. One light source delivers 620 nm light for optogenetic stimulation PVAVgluT2 terminals in the BNST at a frequency of 10 Hz.
-
iii.Deliver pulsed light paradigms to activate ChrimsonR opsins in PVAVgluT2 neuron terminals at the right BNST.
-
iv.Simultaneously capture calcium activity of right BNST neurons (Methods video S1: Building the slides, related to step 4).
-
v.Post-stimulation: On days 1 (d1), 3 (d3), and 6 (d6) after light termination.
-
vi.Assess the mechanical sensitivity of the mice during the experiment.
-
i.
-
a.
Note: To address poor focus, adjust the objective lens while visualizing fluorescence, starting with superficial landmarks and slowly moving to target neurons; for an obstructed view, clean the imaging window, remove bubbles, and ensure the cranial window is free of debris.
-
24.Sequential Recordings.
-
a.Record calcium activity daily for 7 days to monitor persistent mechanical hypersensitivity.
-
a.
-
25.Mechanical Stimulation.
-
a.Apply monofilament stimuli (0.4 g, 1 g, and 2 g) to the left hind paw:
-
i.Repeat each stimulus 3 times with a 30-s interval between applications. This setup allows for the comparison of mechanical sensitivity before and after light-induced neuronal activation, providing insight into the effects of optogenetic stimulation on pain modulation and sensory changes.
-
ii.Maintain a minimum of 30 s between each set of stimuli.
-
i.
-
a.
-
26.Behavioral Video Recording.
-
a.Use a logitech video camera to capture mouse behavior throughout the experiments.
-
a.
Note: Maintain consistent acquisition parameters and field of view across sessions by using tape to mark the monitoring screen to ensure accurate tracking. This will help selectively label and track flashing cells during optogenetic stimulation and monitor their activity consistently across sessions.
Figure 5.
Field of view, calcium response for mechanical response
(A) Images showing field of view before and after Δf with side and bottom view in a freely behaving mouse (upper panel); calcium responses to a given stimulus after longitudinal registration across different imaging sessions (lower panel).
(B) Schematic showing the location of virus injection and lens placement: ChrimsonR in PVA, ChrimsonR terminals signal and GCaMP6f in BNST.4
Viral injection and GRIN lens placement site verification
Timing: 30 min
This procedure ensures accurate verification of viral infection and GRIN lens placement in mice, confirming precise targeting of the intended brain regions for optimal calcium imaging.
-
27.
After end of experiment anesthetize mice deeply with isoflurane.
-
28.
Perform trans-cardiac perfusion with ice-cold PBS, then 4% paraformaldehyde (PFA) in PBS.
-
29.
Fix brains in PFA 24 h, then transfer to 30% sucrose for cryoprotection.
-
30.
Embed brains in OCT compound and obtain 50 μm coronal slices.
-
31.
Mount slices on slides, cover with a coverslip, and image using fluorescence or confocal microscopy at 10x magnification (Figure 5B).
-
32.
Mask images, exclude animals if viral expression and GRIN lens tip falls outside the target region.
Calcium image data acquisition and preprocessing
Timing: 120 min
This step outlines the procedure for acquiring and preprocessing calcium imaging data for analysis, ensuring clean and accurate data for analysis.
-
33.Image Acquisition.
-
a.Perform imaging at 20 frames per second (20 Hz) using the nVoke system from Inscopix.
-
a.
Note: Adjust the frame rate (e.g., 40 Hz for two layers) if imaging multiple layers.
-
34.Preprocessing Steps.
-
a.Down sampling: Spatial down sampling reduces the resolution of raw images from 1440 × 1080 pixels by a factor of 2.
-
b.Motion Correction: Apply motion correction to remove movement artifacts in Inscopix Data Processing Software (IDPS, v1.9.1).
-
c.Cell Detection: Use PCA/ICA-based methods in Inscopix Data Processing Software (IDPS, v1.9.1) for cell detection.
-
d.Manual Inspection: Inspect spatial footprints of detected cells in the XY image to ensure accurate identification.
-
e.Identify and confirm the Ca2+ transients based on rapid rise and gradual decay patterns (Figure 5).
-
a.
-
35.Noise Removal.
-
a.Smooth calcium traces using a Savitzky-Golay filter (window size: 70 frames, polynomial order: 3) (Figure 6A).
-
a.
-
36.Standardization of Calcium Traces.
-
a.Standardize each neuron’s calcium trace using the formula:
-
b.
-
c.where μbase is the mean and σbase is the standard deviation of fluorescence during the initial 1–3-min baseline (Figure 6B).
-
a.
-
37.Alignment Across Sessions.
-
a.Align videos across sessions to maintain the same field of view for tracing neurons consistently over days or seasons (Figure 6B).
-
a.
Note: Motion artifacts pose significant challenges, especially near ventricular structures. Ensure robust alignment and consistent imaging setups for reliable data. Refer a representative video of longitudinal registration with analyzed heatmap.
-
38.Analyzing the neural responses to ChrimsonR light stimulation.
-
a.Preparation.
-
i.For monitoring spontaneous Ca2+ activity of BNST neurons in response to PVAVgluT2 terminal stimulation.
-
ii.Habituation.
-
iii.Imaging basal activity, light on and post light for 5 min each.
-
i.
-
b.Signal Extraction: Extract a 1-min block of Z-score signal for each imaged neuron during four phases.
-
i.Basal (d1 basal): Before any stimulation.
-
ii.During light (d1 during light): Optogenetic stimulation of PVAVgluT2 terminals (10 Hz, 620 nm).
-
iii.Post-light (d1 post-light): Immediately after light termination.
-
iv.Two days post-light (d3 post-light).
-
i.
-
c.Signal Averaging.
-
i.Average the extracted signals for each neuron across the phases.
-
i.
-
d.Neuron Classification: Compare average block activity of non-basal groups to the basal group.
-
i.Excited neurons: Activity > 2.5 Z-score above basal.
-
ii.Inhibited neurons: Activity < −2.5 Z-score below basal.
-
iii.Non-responsive neurons: Activity within ±2.5 Z-score of basal.
-
i.
-
a.
-
39.Identification and classification of neurons with sensory stimulus-evoked responses.
-
a.Mechanical Stimuli and Recording.
-
i.Administer 0.4 g and 1 g Von Frey monofilament stimuli sequentially in each recording trial before and after light stimulation.
-
ii.Record Ca2+ responses of BNST neurons at the following time points: Baseline (pre-stimulation), 30 min, 1 day, 2 days, 3 days, and 7 days post-light stimulation.
-
i.
-
b.Stimulus Presentation and Timing (Figure 6C).
-
i.Repeat each stimulus three times with a 30-s interval, followed by a 30-s rest.
-
ii.Segment calcium signals into six blocks per stimulus: A 5-s pre-stimulus period and a 30-s post-stimulus period.
-
i.
-
c.Tracking and Normalization.
-
i.Align imaging data to track individual BNST neurons.
-
ii.Normalize all responses and Ca2+ activity to baseline activity.
-
i.
-
d.Data Standardization.
-
i.Standardize responses using a 1–3min global baseline and calculate the Z-score (Fz (t)).
-
ii.Divide the 30-s stimulus period into six 5-s bins and compute the mean Z-score for each bin (Figures 6C and 6D).
-
i.
-
e.Net Response Calculation (Figure 6E).
-
i.For each neuron, calculate the net stimulus response by subtracting the baseline area-under-curve (AUC) from the stimulus response AUC.
-
i.
-
f.Neuron Classification (Figure 6F).
-
i.Stimulus-Excited: If the two highest Z-score bins exceed 2.5 and the net response is positive (> 0).
-
ii.Stimulus-Inhibited: If the two lowest Z-score bins fall below −2.5 and the net response is negative (< 0).
-
iii.No Response: Neurons not meeting the above criteria.
-
i.
-
a.
Figure 6.
Analyzing calcium imaging
(A–F) Screenshot of the program for preprocessing, trace binning, and averaging bins. The full package of custom code can be found in the Data and code availability section.
Expected outcomes
This detailed protocol guides researchers in successfully achieving consistent and robust expression of ChrimsonR and the calcium indicator GCaMP6s in PVAVglut2 neurons and the BNST respectively, while maintaining a stable field of view and minimizing cellular damage (Figure 5A and Methods video S1). Additionally, it offers comprehensive instructions for conducting a precise and secure craniotomy implant using a GRIN lens, ensuring the maintenance of intact neural function with distinct calcium fluctuations in individual cells during each brief light exposure to activate ChrimsonR (Methods video S1). After four weeks of post-surgery, we expect a stable field of view across imaging sessions. This protocol allows for the terminal stimulation of PVAVglut2 neurons at the BNST while simultaneously imaging BNST neurons, enabling the assessment of optical stimulation effects across different sessions and their relationship to behavioral outcomes (Figure 5, Methods video S1). Moreover, this method can be modified to monitor neural activity in diverse deep brain regions, across various mouse strains, and within different brain structures. Lastly, it includes imaging data preprocessing and initial state-space analysis, which can be customized based on specific research objectives. The effectiveness of viral expression and the proper positioning of the GRIN lens can be confirmed after the experiment by examining the lens track and detecting fluorophore signals within the intended brain area (Figure 5).
Limitations
Although we employ automated surgical systems that reduce human error, our overall surgery success rate ranges between 65% and 70% to have clear images for calcium under the GRIN lens. In this protocol, we combine optogenetic stimulation with calcium activity imaging to enable cell- and projection-specific stimulation of PVA projections to the BNST, while simultaneously capturing neuronal activity in the BNST. This approach provides valuable insights into the PVA-BNST connection and its role in sensory pain-related behaviors. However, the strategy does not allow for the interrogation of synaptic strength between the PVA and BNST which may require a detailed analysis of calcium response during and after stimulation or application of slice recording.
Conducting two-site viral infections and lens implantation simultaneously is challenging, as it requires keeping the mice under anesthesia for an extended period while fixed in a stereotaxic apparatus. Prolonged anesthesia poses risks, potentially causing breathing difficulties such as slowed, uneven, or shallow respiration. These issues might signal an overdose of anesthesia or a blocked airway, demanding prompt intervention.
Working near the bregma during the drilling process to target the PVA introduces additional difficulties. The bregma landmark may become obscured, making it challenging to relocate for subsequent procedures. This often necessitates estimation or reliance on previous setups, which increases the likelihood of missing the target.
Troubleshooting
Problem 1
Invisibility of neurons within the field of view (Step2-3).
Potential solution
-
•
Incorrect Region of Interest.
Cause: The imaging area does not include the target region where neurons are located.
Solutions:-
○Verify the accurate stereotaxic coordinates for the targeted brain region.
-
○Ensure proper alignment between the viral injection site and LENS implantation.
-
○
-
•
Poor Focus
Cause: The objective lens is not properly focused on the neuronal layer.
Solutions:-
○Adjust the focus while visualizing the fluorescence signal or structure in real time.
-
○Start by focusing on superficial landmarks, then slowly adjust to locate the target neurons.
-
○
-
•
Insufficient Signal.
Cause: Weak fluorescence from calcium indicators.
Solutions:-
○Increase laser power or excitation light intensity (monitor for photodamage).
-
○Check the indicator concentration and ensure proper delivery into neurons.
-
○Extend time for calcium expression 3weeks to 4 weeks and verify the expression level of genetically encoded calcium indicators (e.g., GCaMP).
-
○
-
•
Obstructed View.
Cause: Tissue damage, debris, or bubbles are obstructing the field of view.
Solutions:-
○Clean the imaging window thoroughly.
-
○Remove bubbles from the preparation.
-
○Ensure the cranial window remains clear and free from debris.
-
○
Problem 2
Focal plane changes (Step 4).
Potential solution
-
•Brain Movement.
-
○Ensure the cranial window is securely fixed to prevent brain movement relative to the microscope.
-
○Use a head-fixation system or stereotaxic frame to minimize motion artifacts.
-
○
-
•Post-Processing Correction.
-
○If minor movement persists, use image stabilization software to correct for drift during post-processing.
-
○
-
•Microscope Stability.
-
○Verify that the microscope stage and objective are stable and free from vibrations.
-
○
-
•Tissue Deformation.
-
○Periodically check and adjust the focus during long recordings to compensate for gradual tissue deformation.
-
○
Problem 3
Dislodgement of dental cement from the surgical region (STEP 1-2).
Potential solution
-
•Surface Preparation.
-
○Thoroughly clean and dry the surgical site to remove any debris, blood, or tissue that could interfere with adhesion.
-
○Remove soft tissue around the skull and gently roughen the bone surface to improve the bond with the dental cement.
-
○
-
•Application Technique.
-
○Apply the dental cement in multiple thin layers rather than a single thick layer to enhance durability and reduce the risk of cracking.
-
○Ensure that the cement fully covers the intended area and forms a secure bond with the skull.
-
○
-
•Curing Process.
-
○Use a high-quality, fast-curing dental cement that is appropriate for in vivo experiments.
-
○Allow the cement to fully cure and harden before applying stress, such as head fixation or imaging procedures.
-
○
-
•Fixation Stability.
-
○Ensure that the head-fixation system is properly adjusted to avoid excessive pressure that could strain or detach the cement.
-
○
-
•Reinforcement and Protection.
-
○For long-term studies, reinforce the cement with an additional adhesive layer or apply a protective coating to prevent wear and detachment over time.
-
○
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Chien-Chang Chen, Ph.D (ccchen@ibms.sinica.edu.tw).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contacts, Selomon Assefa Mindaye (sa80077025@gs.ncku.edu.tw) and Wei-Hsin Chen (vic7538@ibms.sinica.edu.tw).
Materials availability
This study did not generate new unique reagents, plasmids or mouse lines.
Data and code availability
In this study, the code is available at https://doi.org/10.5281/zenodo.13909372 (https://doi.org/10.5281/zenodo.13909372) or on GitHub at the following link: https://github.com/shihche0716/PVA-Ca-data-analysis/tree/v1.0.1.
Acknowledgments
The authors thank the National Laboratory Animal Center, Taiwan. The authors also thank Dr. Jin-Chung Chen, Chang Gung University, for generously sharing VgluT2-Cre mouse line. The authors also thank Hau-Jie Yau for sharing the nVoke3 machine. This work was supported by Academia Sinica grants (AS-IR-(111-113)-05-A) and Taiwan Ministry of Science and Technology grants (MOST 108-2320-B-001 -025 -MY3 and MOST 111-2320-B-001-007-MY3 to C.-C.C. and MOST 111-2811-B-001-009 and 112-2811-B-001-038 to W.-H.C.).
Author contributions
S.A.M., W.-H.C., and C.-C.C. established the protocol. S.A.M. wrote the original draft. S.A.M., W.-H.C., and S.-C.L. contributed to the miniature microscope data analysis. W.-H.C. and C.-C.C. reviewed and edited the manuscript. C.-C.C. supervised the study.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2025.103800.
References
- 1.Campbell E.J., Marchant N.J. The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br. J. Pharmacol. 2018;175:994–1003. doi: 10.1111/bph.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim C.K., Adhikari A., Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 2017;18:222–235. doi: 10.1038/nrn.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yizhar O., Fenno L.E., Davidson T.J., Mogri M., Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Mindaye S.A., Chen W.-H., Lin S.-C., Chen Y.-C., Abdelaziz M.A., Tzeng Y.-S., Shih A.C.-C., Chen S.-Y., Yang S.-B., Chen C.-C. Separate anterior paraventricular thalamus projections differentially regulate sensory and affective aspects of pain. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2024.114946. [DOI] [PubMed] [Google Scholar]
- 5.Chen T.-W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klapoetke N.C., Murata Y., Kim S.S., Pulver S.R., Birdsey-Benson A., Cho Y.K., Morimoto T.K., Chuong A.S., Carpenter E.J., Tian Z., et al. Independent optical excitation of distinct neural populations. Nat. Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In this study, the code is available at https://doi.org/10.5281/zenodo.13909372 (https://doi.org/10.5281/zenodo.13909372) or on GitHub at the following link: https://github.com/shihche0716/PVA-Ca-data-analysis/tree/v1.0.1.