Abstract
Serum cytokine levels were measured in 275 healthy children of different ages (3 to 17 years). Interleukin-1 receptor antagonist (IL-1RA), soluble IL-2R (sIL-2R) (sCD25), IL-6, IL-8, tumor necrosis factor alpha (TNF-α), soluble TNF receptor type II (sTNF-RII) (sCD120b), gamma interferon (IFN-γ), soluble intercellular adhesion molecule 1 (sICAM-1) (sCD54), soluble E selectin (sE-selectin) (ELAM-1; sCD62E), sCD14, and neopterin were measured with commercial test kits. The mean levels of IL-1RA, sIL-2R, TNF-α, sICAM-1, sE-selectin, and sCD14 were higher than in healthy adults. In contrast, IFN-γ and IL-8 were hardly detectable in children and thereby significantly lower than in adults. In the case of TNF-α, sICAM-1, sE selectin, and sCD14, there was a high interindividual variability, apparently unrelated to disease. The profiles of some cytokines, i.e., IL-1RA, IL-6, and TNF-α, showed age-related increases that overlapped with known patterns of physical growth. Of note, sIL-2R and sE-selectin instead declined with time. Because of the remarkable age-dependent variability in healthy pediatric subjects, disease-related changes, as well as therapy-dependent alterations, should be considered with caution.
The detection of cytokines and soluble immunological receptors in sera bears diagnostic relevance in many diseases. In specialized diagnostics, the evaluation of cytokine profiles can improve medical care and facilitate therapeutic decisions not only in diseases such as septicemia, tumors, and systemic inflammation (9) but also in postoperative monitoring of organ transplantation (28). The diagnostic application of these parameters, on the other hand, depends critically on the knowledge of normal serum values. In the case of children, systematic information on normal concentrations is hardly available. The subject of the present study, therefore, was the measurement of diagnostically relevant cytokines, soluble receptors, and mediators in serum in healthy children (3 to 17 years of age). Interleukin-1 receptor antagonist (IL-1RA), soluble IL-2R (sIL-2R) (sCD25), IL-6, IL-8, tumor necrosis factor alpha (TNF-α), soluble TNF receptor type II (sTNF-RII) (sCD120b), gamma interferon (IFN-γ), soluble intercellular adhesion molecule 1 (sICAM-1) (sCD54), soluble E selectin (sE-selectin) (endothelial leukocyte adhesion molecule 1 [ELAM-1]; sCD62E), sCD14, and neopterin were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits. Because the assessment of serum cytokine levels appears to be highly sensitive to the duration and the modality of sample handling, efforts were made to reduce the time and standardize the modalities of the various procedures.
MATERIALS AND METHODS
Sera were obtained from 275 healthy children undergoing corrective surgery or medical checkup (see Table 1 for age distribution). All children had been free of infectious diseases for at least 4 weeks before examination and were not under the influence of any treatment. C-reactive protein was measured to exclude ongoing inflammation. The study was approved by the Research Ethics Committee of the University of Leipzig. Written informed consent was obtained from the parents before entry into the study. All patients with unexpectedly high levels for any of the cytokines were reexamined to verify that no disease had developed immediately after the test.
TABLE 1.
Age distribution of children in the study
| Age (yr) | n |
|---|---|
| 3 | 10 |
| 4 | 23 |
| 5 | 12 |
| 6 | 21 |
| 7 | 16 |
| 8 | 22 |
| 9 | 17 |
| 10 | 20 |
| 11 | 19 |
| 12 | 21 |
| 13 | 18 |
| 14 | 25 |
| 15 | 18 |
| 16 | 23 |
| 17 | 10 |
Blood samples were collected between 8:00 and 9:00 a.m. by venipuncture and centrifuged within 15 min. Serum aliquots were frozen and stored at −70°C until analysis. The specimens were thawed immediately before analysis and used at volumes indicated by the manufacturers.
The analyses were performed with 96-well microtiter plate ELISA kits, except for IL-6 and IL-8, which were analyzed with the Immulite system (DPC Diagnostic Products Corporation, Los Angeles, Calif.), an automatized random access immunoassay system developed for the measurement of immune parameters in single samples (3). The ELISAs were obtained from R&D Systems, Minneapolis, Minn. (IL-1RA, sTNF-RII [p75; sCD120b], and sE-selectin [sCD62E]), Immunotech, Marseille, France (sIL-2R [sCD25] and TNF-α), PerSeptive Diagnostics, Cambridge, Mass. (IFN-γ), T Cell Diagnostics, Cambridge, Mass. (sICAM-1 [sCD54]), and IBL Gesellschaft für Immunchemie und Immunbiologie, Hamburg, Germany (neopterin and sCD14). Test sensitivity was 6.5 pg/ml for IL-1RA, 5 pM for sIL-2R, 2 pg/ml for IL-6, 6.2 pg/ml for IL-8, 5 pg/ml for TNF-α, 0.5 pg/ml for sTNF-RII, 1 pg/ml for IFN-γ, 0.3 ng/ml for sICAM-1, 1 ng/ml for sE-selectin, 1 ng/ml for sCD14, and 0.7 nmol/liter for neopterin.
All ELISA kits provided information on the expected levels in serum in healthy adults, along with technical recommendations for the analyses.
Data were analyzed and plotted with SigmaStat and SigmaPlot scientific software (Jandel Scientific, Erkrath, Germany), using the Mann-Whitney U rank sum test. Two analyses were performed, the first considering all children as a pooled group (n = 275) and the second comparing each age group (Table 1) with adults.
RESULTS
The results of the present analysis in relation to age are summarized in Fig. 1 to 3. Notably, there were no statistically significant differences between females and males (data not shown); accordingly, the data are presented as pooled values.
FIG. 1.
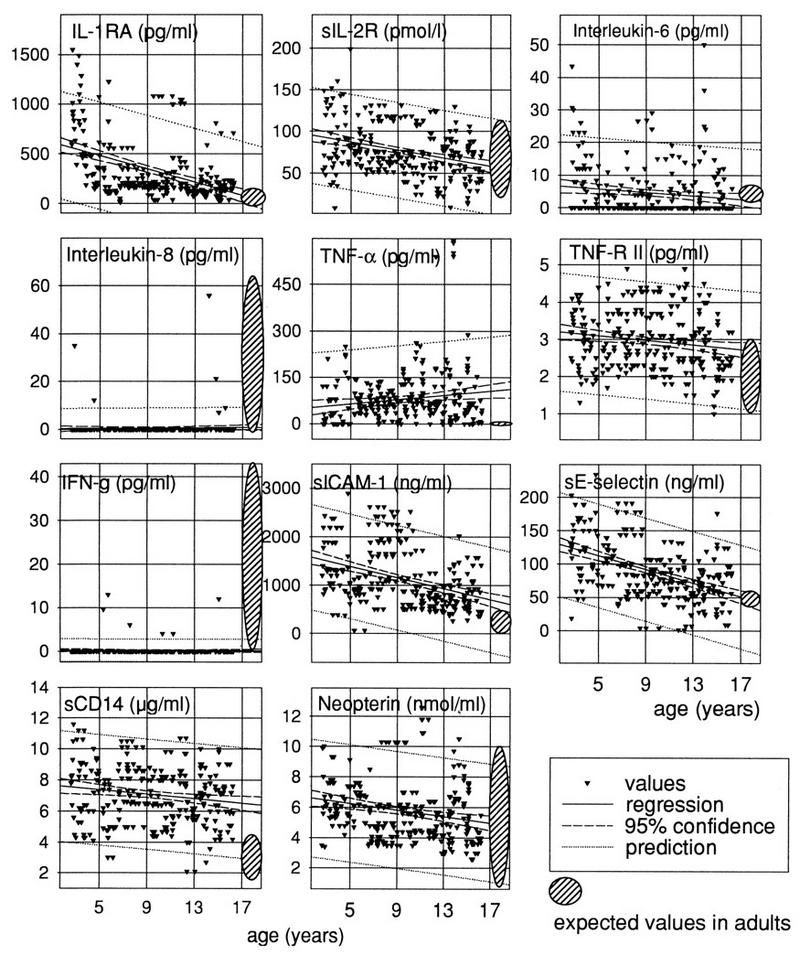
Age-related expression of immunological mediators in childhood (275 children 3 to 17 years of age) (Table 1). Normal values in adult donors are those provided by the suppliers of the respective kits. Note the remarkable degree of interindividual variability for most of the molecules.
FIG. 3.
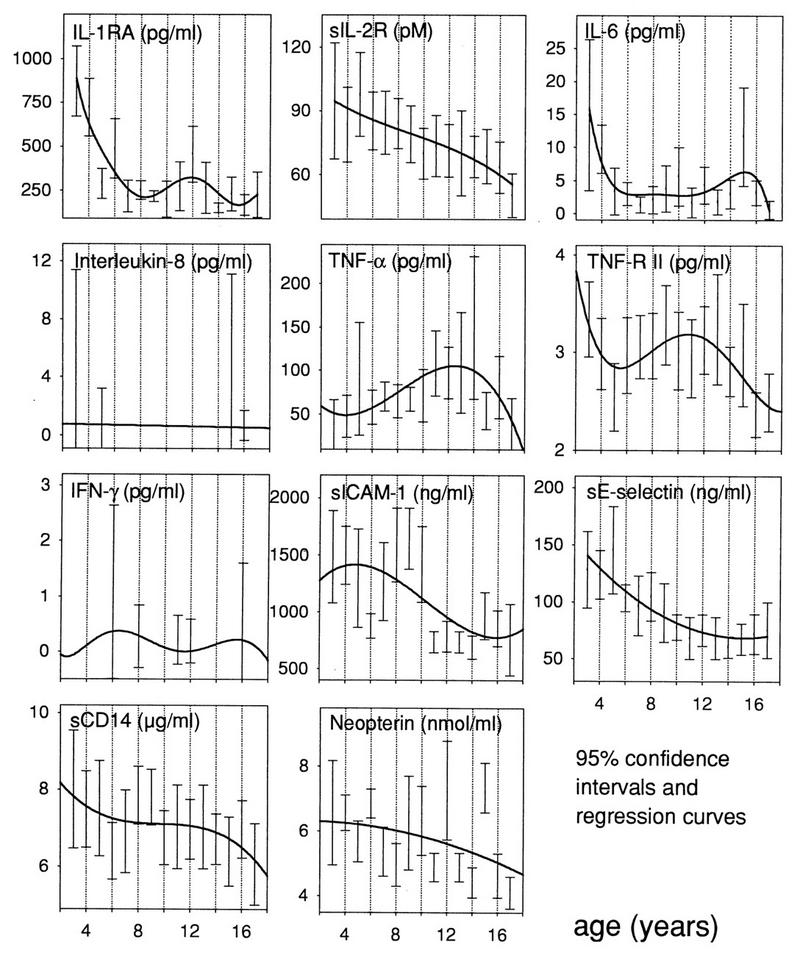
Regression analysis and 95% confidence intervals of measured parameters at different ages. IL-1RA, IL-6, and TNF-α show not only higher values in children than in adults but also a characteristic pattern which resembles the rates of physical growth in childhood. Of note, sIL-2R, sE-selectin, and sCD14 undergo a progressive decline instead.
IL-1RA.
The pattern for IL-1RA consisted of an early peak around 3 years of age (Fig. 3) (P < 0.001 in comparison with healthy adults) and a second peak around age 12 (P < 0.05). Between 7 and 10 years, and after 14 years of age, the values were comparable to those of adults.
sIL-2R.
sIL-2 showed a progressive decline from age 3 onward (Fig. 3); however, due to the large variability at very young ages, there was no significant difference relative to adult levels.
IL-6.
IL-6 peaked around 3 to 4 years of age (P < 0.05 in comparison with adults), as well as at 15 years (P < 0.05) (Fig. 3).
IL-8.
IL-8 was detectable only rarely and always within the range for normal adults (Fig. 1 and 3).
TNF-α.
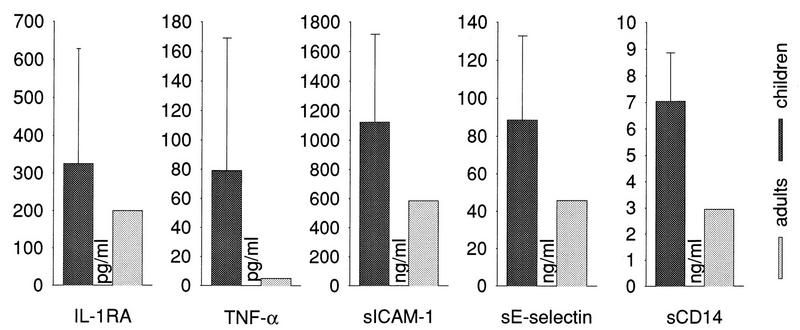
TNF-α showed a progressive increase with age (Fig. 1), with overall levels significantly higher than those in adults (Fig. 2) (P < 0.01). A clear peak was reached at age 13 to 14 (Fig. 3), with a rather sharp fall to adult levels thereafter.
FIG. 2.
Levels (means with standard deviations) of IL-1RA, TNF-α, sICAM-1, sE-selectin, and sCD14 in serum in healthy children and normal adults (see the legend to Fig. 1). For these select molecules, the pooled childhood values (n = 275) were significantly different from the adulthood levels.
sTNF-RII.
The levels of TNF-RII did not significantly differ from those of adults, in spite of apparent peaks at 3 to 4 years and at 10 years of age (Fig. 1 and 3).
IFN-γ.
IFN-γ was hardly detectable in most of the samples; when detected, it remained within the normal adult range (Fig. 1 and 3).
sICAM-1.
The concentration of sICAM-1 was significantly higher in childhood than in adulthood (Fig. 2) (P < 0.05), in particular before age 11 (Fig. 1 and 3) (P < 0.001 before age 11).
sE-selectin.
sE-selectin, on the whole significantly higher than in adulthood (Fig. 2), showed a progressive fall from age 3 to 5 onward (Fig. 1 and 3) (P < 0.05 in comparison with adult levels).
sCD14.
sCD14 was significantly higher in childhood than in adulthood (Fig. 2) (P < 0.05), but with no age-related peaks.
Neopterin.
Neopterin remained within the normal adult range throughout childhood (Fig. 1 and 3).
DISCUSSION
The present study provides valuable information on normal ranges for several cytokines, cytokine receptors, or mediators of potential diagnostic use in pediatrics. The results can be summarized as follows. (i) In general, some molecules (i.e., IL-RA, TNF-α, sICAM-1, sE-selectin, and sCD14) are clearly higher in childhood than in adulthood (Fig. 2), whether or not their profiles present age-related peaks (Fig. 3). (ii) Some cytokines (i.e., IL-RA, IL-6, and TNF-RII) show age-related profiles (Fig. 3), including biphasic patterns that may be related to physical growth (Fig. 3). (iii) Some molecules (i.e., sIL-2R, sICAM-1, and sE-selectin) show a progressive decline after age 3, with no sign of age-related grouping.
The molecules tested in the present study were chosen on the basis of their diagnostic potential. IL-1RA, for example, antagonizes IL-1 by competitive binding to its receptor, acting thereby as an anti-inflammatory molecule (10, 16). Serum IL-1RA levels are regulated on the basis of serum IL-1 levels, and, albeit in a delayed fashion in comparison to IL-1β, significant levels of IL-1RA can be detected. In adults, the diagnostic use of IL-1RA is mainly in IL-1β-dependent diseases, such as inflammatory and autoimmune diseases, septicemia, and septic shock. In contrast to adults, elevated serum IL-RA levels can be detected in healthy children (Fig. 2), especially around 3 and 10 to 12 years of age (Fig. 1), suggesting that IL-1β and/or IL-1RA may play a physiological role in development. Interestingly, this age dependency (Fig. 3) seems to closely match the physical growth rates reported by Prader et al. (29). The hypothesis of a link between IL-1 and bone formation is supported by findings showing IL-1 mRNA in developing bone and cartilage (11).
sIL-2R (sCD25), released from activated lymphocytes and resting monocytes by cleavage of the membrane form of IL-2R (38), is detected in the sera of patients with autoimmune or inflammatory diseases, as well as in different forms of leukemia and lymphoma (30, 38). Increased sIL-2R levels are also closely related to rejection episodes after solid-organ engraftment (1). In contrast to adults, physiologically high concentrations of sIL-R can be found in the sera of healthy children throughout childhood (Fig. 3) (8, 23, 39). At age 15, the levels of sIL-2R decline to those of normal adults (Fig. 1 and 3), suggesting that physiologically high sIL-2R levels are maintained by the enhanced proliferative activity of the immune system during early childhood, especially in connection to the thymic maturation of T cells. With aging and parallel involution of the thymus, sIL-2R then declines to adult levels.
IL-6 is one of the most promising candidates for use in routine diagnostics. It is produced in inflammatory conditions by monocytes, macrophages, fibroblasts, endothelial cells, keratinocytes, lymphocytes, and hepatocytes, as well as in tumors (20). Its secretion is induced by IL-1, TNF, and bacterial endotoxins. Serum IL-6 levels are measured in patients with burns, infections, and autoimmune diseases, or else following solid-organ transplantation. In healthy children, serum IL-6 levels are physiologically (and biphasically) elevated around 3 to 4 and 14 years of age, thus confirming previous observations (6). These findings suggest, as in the case of IL-1RA, that IL-6 may play an important physiological role in childhood, in particular in relation to certain stages of physical growth (Fig. 3) (29). In fact, mRNA for IL-6 has been shown to be expressed in developing cartilage and bone (11).
IL-8 is produced by monocytes, macrophages, fibroblasts, endothelial cells, hepatocytes, and chondrocytes. Following stimulation by other cytokines, IL-8 acts as a chemoattractant especially for polymorphonuclear neutrophils (15). IL-8 is found in high concentrations in the sera of patients with septicemia and also following organ transplantation or during inflammatory and autoimmune diseases. In contrast to the above cytokines, there was hardly any measurable IL-8 in the sera of healthy children (Fig. 1 and 3), indicating that this cytokine is more genuinely involved in inflammation.
TNF-α is produced predominantly by macrophages and lymphocytes (24, 32). This cytokine acts on fibroblasts and endothelial cells, not only inducing inflammatory reactions, fibrosis, and cachexia (5) but also eliciting the production of other inflammatory cytokines and adhesion molecules (5, 22, 37). Because TNF is remarkably unstable, the sera were analyzed very shortly after blood withdrawal, according to a strict standardization protocol. The results of this analysis show that TNF levels are elevated during the entire childhood (Fig. 2), but in a profoundly variable fashion among individuals (Fig. 1). These observations, consistent with similar studies (7, 21), indicate that TNF levels in children must be regarded with greatest caution, requiring careful adjustments to take into account the physiological fluctuations.
sTNF-RII (p75) (sCD120b), a member of the TNF receptor family, is expressed on a large variety of cells (33) and is elevated in inflammation, autoimmune diseases, cancer, and sepsis (17, 27). sTNF-RII was slightly but not significantly increased in the sera of healthy children (Fig. 1 and 3), in agreement with other studies (39). Thus, although sTNF-RII is regulated by TNF-α (36), the present study seems to exclude a direct correlation between serum TNF-α and sTNF-RII in childhood.
IFN-γ is produced by lymphocytes and macrophages. This cytokine acts on lymphocytes, monocytes/macrophages, and connective-tissue cells as an immunoregulatory, as well as proinflammatory, mediator, with B-cell-differentiating and antiviral properties (4). Its levels in serum are mostly low in healthy adults. Indeed, the present study indicates that low levels, or even the absence of immunoreactive IFN-γ, also characterize healthy childhood, with little variability among children. In contrast to most of the above cytokines, therefore, there is no elevation corresponding to particular phases of rapid growth (Fig. 1 and 3); IFN-γ thus appears to be a genuine mediator, and therefore a potential marker, of inflammation and immunity.
sICAM-I (sCD54) is the most important ligand for leukocyte function-associated molecule 1 (LFA-1) and is expressed on a variety of cells including lymphocytes, fibroblasts, keratinocytes, monocytes, and endothelial and dendritic cells (31, 34). The expression of this marker is induced by proinflammatory cytokines, such as IL-1β and TNF-α, as well as lipopolysaccharides. In immunodiagnostics, sICAM-1 measurements are indicated in several autoimmune and inflammatory diseases, including multiple sclerosis, as well as in neoplasia and graft rejection following solid-organ transplantation (13). Our study shows enhanced serum sICAM-I concentrations in healthy children (Fig. 2), especially before age 11 (Fig. 3). This observation differs from reports showing low levels in sera of normal children (18).
sE-selectin (sCD62E, or [ELAM-1]) mediates the adhesion of inflammatory cells to the endothelium at sites of inflammation (19). This is followed by lymphocyte extravasation and diapedesis across the vessel wall. High concentrations of sE-selectin are found in a broad range of inflammatory processes (13). Interestingly, there were high levels during normal childhood (Fig. 2), in particular in early childhood (Fig. 3), whereas other studies found low levels (18). From 12 years of age onward, sE-selectin declines steadily to normal adult levels (Fig. 1). Possibly, this profile parallels or reflects the increased metabolic turnover of connective tissue and accelerated wound healing in childhood, because sE-selectin appears to play a central role in this process (35).
sCD14, which is released by monocytes and macrophages and interacts with bacterial endotoxins (40), is found in high concentrations in the sera of virally infected patients, as well as in septic, polytraumatic, and severely burned patients (25, 26). In the present study, sCD14 was significantly elevated during childhood (Fig. 2) (P < 0.01 except for 6 and 13 years of age), without connection to any disease or trauma.
Neopterin is considered a parameter of increased cellular immunity or inflammatory activity (2). In clinical diagnostics, neopterin is valuable for the monitoring of viral and other infections, malignant diseases, graft survival, and inflammatory diseases (12). In the sera of healthy children, neopterin is always within or slightly below the normal range of adults (Fig. 1 and 3), in agreement with the literature (14), thus representing a quite reliable marker for monitoring the above conditions.
Together, the present findings indicate that cytokine concentrations in the sera of healthy children most often differ from those of adults. In principle, the detection of immunological mediators by ELISA techniques can be influenced by the presence of soluble receptors, inhibitors, antagonists, or binding proteins, as well as by degraded or inactive forms of the cytokines. By investigating a large number of healthy children, carefully excluding children with ongoing diseases, and following strict protocols for the quick and accurate handling of the specimens, the present study clearly documented elevations of IL-1RA, TNF-α, sICAM-1, sE-selectin, and sCD14. Also, these elevations showed mostly age-dependent longitudinal profiles (Fig. 3). Furthermore, the levels of some molecules appear to markedly differ between the first 3 years of life and late childhood. However, considering the limited number of small children examined so far, the examination of larger numbers of small children will be helpful in determining reliable normal cytokine ranges for given age intervals within childhood. These controls, in turn, will be helpful in routine laboratory techniques for the diagnosis and treatment of specific aspects of particular diseases.
ACKNOWLEDGMENTS
We thank Ramona Blaschke, Kati Hofmann, Friedemann Scymanowski, and Antje Voppmann for technical assistance. We thank Jörg Ermann and Eberhard Keller for assistance in preparing the manuscript. We owe special thanks to Ernesta Palombo-Kinne for critical modification of the reviewed manuscript.
Parts of this work were supported by grants from the Deutsche Forschungsgemeinschaft; the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie; the Interdisziplinäres Zentrum für Klinische Forschung; and the Sächsisches Ministerium für Wissenschaft und Kunst.
REFERENCES
- 1.Adams D H, Wang L, Hubscher S G, Elias E, Neuberger J M. Soluble interleukin-2 receptors in serum and bile of liver transplant recipients. Lancet. 1978;i:469–471. doi: 10.1016/s0140-6736(89)91368-8. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Neopterins in clinical medicine. Lancet. 1988;i:509–511. [PubMed] [Google Scholar]
- 3.Babson A L. The Immulite automated immunoassay system. J Clin Immunoassay. 1991;14:83–88. [Google Scholar]
- 4.Baron D, Tyring S K, Fleischmann W R, Coppenhaver D H, Niesel D W, Klimpel G R, Stanton G J, Hughes T K. The interferons: mechanisms of action and clinical application. JAMA. 1991;266:1375–1383. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 6.Brailly H, Montero-Julian F A, Zuber C E, Flavetta S, Grassi J, Houssiau F, van Snick J. Total interleukin-6 in plasma measured by immunoassay. Clin Chem. 1994;40:116–123. [PubMed] [Google Scholar]
- 7.Cetingul N, Yener E, Oztop S, Nisli G. Serum soluble interleukin-2 receptors and tumor necrosis factor-alpha in hematological malignancies of childhood. Acta Paediatr Jpn. 1994;36:49–52. doi: 10.1111/j.1442-200x.1994.tb03128.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan K N, Phillips A D, Walker-Smith J A, MacDonald T T. Serum interleukin-2 receptors in infants and young children. Acta Paediatr. 1995;84:151–155. doi: 10.1111/j.1651-2227.1995.tb13598.x. [DOI] [PubMed] [Google Scholar]
- 9.Daniel V. Diagnostic value of cytokine determination in serum and plasma. Dtsch Med Wochenschr. 1995;120:1171–1174. doi: 10.1055/s-2008-1055462. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello C D. Role of interleukin-1 in infectious diseases. Immunol Rev. 1992;127:119–146. doi: 10.1111/j.1600-065x.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 11.Dodds R A, Merry K, Littlewood A, Gowen M. Expression of mRNA for IL1 beta, IL6 and TGF beta 1 in developing human bone and cartilage. J Histochem Cytochem. 1994;42:733–744. doi: 10.1177/42.6.8189035. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs D, Weiss G, Reibnegger G, Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci. 1992;29:307–341. doi: 10.3109/10408369209114604. [DOI] [PubMed] [Google Scholar]
- 13.Gearing A J, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 14.Griffin D E, Ward B J, Jauregui E, Johnson R T, Vaisberg A. Immune activation during measles: interferon-gamma and neopterin in plasma and cerebrospinal fluid in complicated and uncomplicated disease. J Infect Dis. 1990;161:449–453. doi: 10.1093/infdis/161.3.449. [DOI] [PubMed] [Google Scholar]
- 15.Grob P M, David E, Warren T C, DeLeon R P, Farina P R, Homon C A. Characterization of a receptor for human monocyte-derived neutrophil chemotactic factor/interleukin-8. J Biol Chem. 1990;265:8311–8316. [PubMed] [Google Scholar]
- 16.Hannum C H, Wilcox C J, Arend W P, Joslin F G, Dripps D J, Heimdal P L, Armes L G, Sommer A, Eisenberg S P, Thompson R C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- 17.Haran N, Bar-Khayim Y, Frensdorff A, Barnard G. Tumor necrosis factor (TNF-alpha) binding protein: interference in immunoassays of TNF-alpha. Kidney Int. 1991;40:1166–1170. doi: 10.1038/ki.1991.330. [DOI] [PubMed] [Google Scholar]
- 18.Hatzistilianou M, Athanassiadou F, Agguridaki C, Catriu D. Circulating soluble adhesion molecules in children with acute lymphoblastic leukaemia. Eur J Pediatr. 1997;156:537–540. doi: 10.1007/s004310050657. [DOI] [PubMed] [Google Scholar]
- 19.Hession C, Osborn L, Goff D, Chi-Rosso G, Vassallo C, Pasek M, Pittack C, Tizard R, Goelz S, McCarthy K. Endothelial leukocyte adhesion molecule 1: direct expression cloning and functional interactions. Proc Natl Acad Sci USA. 1990;87:1673–1677. doi: 10.1073/pnas.87.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 21.Ishii E, Ohga S, Yamada S, Sako M, Tasaka H, Kuwano A, Sasaki M, Tsunematsu Y, Ueda K. Prognosis of children with virus-associated hemophagocytic syndrome and malignant histiocytosis: correlation with levels of serum interleukin-1 and tumor necrosis factor. Acta Haematol Jpn. 1991;85:93–99. doi: 10.1159/000204864. [DOI] [PubMed] [Google Scholar]
- 22.Jacob C O. Tumor necrosis factor alpha in autoimmunity: pretty girl or old witch? Immunol Today. 1992;13:122–125. doi: 10.1016/0167-5699(92)90107-i. [DOI] [PubMed] [Google Scholar]
- 23.Jones A C, Besley C R, Warner J A, Warner J O. Variations in serum soluble IL-2 receptor concentration. Pediatr Allergy Immunol. 1994;5:230–234. doi: 10.1111/j.1399-3038.1994.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones E Y, Stuart D I, Walker N P C. Structure of the tumour necrosis factor. Nature. 1989;338:225–228. doi: 10.1038/338225a0. [DOI] [PubMed] [Google Scholar]
- 25.Krüger C, Schütt C, Dietz H, Lüdtke J, Ruf B, Langford A, Pohle H D, Kunze R O F. Elevated levels of soluble CD14 molecules in serum of HIV-1 infected patients. Immunobiology. 1990;181:244. [Google Scholar]
- 26.Krüger C, Schütt C, Obertacke U, Joka T, Müller F E, Knöller J, Knöller W, Schönfled W. Serum CD14 levels in polytraumatized and severely burned patients. Clin Exp Immunol. 1991;85:297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lantz M, Thysell H, Nilsson E, Olsson I. On the binding of tumor necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein I by heparin. J Clin Invest. 1991;88:2026–2031. doi: 10.1172/JCI115530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Pirenne J, Pirenne-Noizat F, de Groote D, Vrindts Y, Lopez M, Gathy R, Jacquet N, Meurisse M, Honore P, Franchimont P. Cytokines and organ transplantation: a review. Nucl Med Biol. 1994;21:545–555. doi: 10.1016/0969-8051(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 29.Prader, A., R. H. Largo, L. Molinari, and C. Issler. 1988. Physical growth of Swiss children from birth to 20 years of age. Helv. Paediatr. Acta 43(Suppl. 52):124–125. [PubMed]
- 30.Rubin L A, Nelson D L. The soluble interleukin-2 receptor: biology, function, and clinical application. Ann Intern Med. 1990;113:619–627. doi: 10.7326/0003-4819-113-8-619. [DOI] [PubMed] [Google Scholar]
- 31.Smith C W, Rothlein R, Hughes B J, Mariscalco M M, Rudloff H E, Schmalstieg F C, Anderson D C. Recognition of an endothelial determinant for CD18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988;82:1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith R A, Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem. 1987;262:6951–6954. [PubMed] [Google Scholar]
- 33.Sprang S R. The divergent receptors for TNF. Trends Biochem Sci. 1991;15:366–368. doi: 10.1016/0968-0004(90)90228-4. [DOI] [PubMed] [Google Scholar]
- 34.Springer T A. Adhesion receptor of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 35.Subramaniam M, Saffaripour S, van de Water L, Frenette P S, Mayadas T N, Hynes R O, Wagner D D. Role of endothelial selectins in wound repair. Am J Pathol. 1997;150:1701–1709. [PMC free article] [PubMed] [Google Scholar]
- 36.Tartaglia L A, Goeddel D V. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 37.Tracey K J, Vlassara H, Cerami A. Cachectin/tumour necrosis factor. Lancet. 1989;i:1122–1126. doi: 10.1016/s0140-6736(89)92394-5. [DOI] [PubMed] [Google Scholar]
- 38.Waldmann T A. The interleukin-2 receptor. J Biol Chem. 1991;266:2681–2684. [PubMed] [Google Scholar]
- 39.Weiss M, Martignoni M, Petropoulou T, Solder B, Belohradsky B H. Increased serum levels of soluble tumor necrosis factor receptor (sTNF-Rs) in children and adolescents with vertically and horizontally transmitted HIV infection. Infection. 1996;24:301–308. doi: 10.1007/BF01743365. [DOI] [PubMed] [Google Scholar]
- 40.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]