Abstract
Astroviruses are a new family of positive-stranded RNA viruses that cause gastroenteritis in a wide range of animals and in humans. Seven types of astrovirus, tentatively considered serotypes, have been distinguished by enzyme-linked immunosorbent assays (ELISA) or immunoelectron microscopy, but it is unclear whether the serotype designation is used properly. To test human sera for the presence of neutralizing antibodies and to type field strains, neutralization tests (NT) using CaCo2 tissue-culture-adapted astrovirus strains 1 to 7 and the corresponding rabbit reference sera were developed. In rabbits, neutralizing antibodies were predominantly serotype specific, with the exception of low-level cross-reactivity in astrovirus serotype 4 reference serum with astrovirus serotype 1 virus. Similarly, in humans, no evidence of cross-reactivity was found for the serotype combinations tested (all except the combination 1 and 7 and the combination 6 and 7). Typing by NT was concordant with typing by ELISA and genotyping, with one exception. The seroprevalence rates of neutralizing antibodies in an age-stratified sample of the population in Utrecht Province (n = 242) were 91% for astrovirus serotype 1, 69% for astrovirus serotype 3, 56% for astrovirus serotype 4, 36% for astrovirus serotype 5, 31% for astrovirus serotype 2, 16% for astrovirus serotype 6, and 10% for astrovirus serotype 7. Acquisition of antibodies was slower among persons seropositive for astrovirus serotype 5 than among those seropositive for astrovirus serotypes 1 to 4, suggesting that the epidemiology of serotype 5 astrovirus is different from that of astrovirus serotypes 1 to 4.
Astroviruses are a recently classified new family of nonenveloped, single-stranded RNA viruses, evolutionarily related to the Caliciviridae and Picornaviridae (5). Astroviruses have been found in fecal samples from humans, cattle, sheep, pigs, cats, and ducks. In most species, these viruses cause gastroenteritis, except for the duck astrovirus, which may cause fulminant hepatitis with a mortality as high as 25% (13). In calves, astrovirus infections are asymptomatic, although they lead to infection and cytopathologic changes in M cells (19).
In humans, astroviruses like other enteric viruses are transmitted primarily through the fecal-oral route (including food- and waterborne transmission) and occasionally by aerosols (13). Clinically, astrovirus infections are similar to other viral causes of gastroenteritis, although astrovirus-associated disease is usually milder, especially in adults (8). In infants, astrovirus disease may require hospitalization, especially in 6- to 12-month-old babies (16); the disease may be complicated for several weeks by a malabsorption syndrome (10). It has been postulated that the incidence of astrovirus-associated gastroenteritis has been underestimated and that astrovirus infections may be one of the common infections of childhood (1, 13). This view is supported by the finding that 75% of children between 5 and 10 years of age have antibodies to astrovirus, as determined by immunoelectron microscopy (IEM) (7). Infections in volunteers with a prechallenge titer of antibodies to astrovirus did not result in diarrhea, suggesting a correlation of astrovirus-specific antibodies (as determined by IEM) with protective immunity (10). It is unknown if humans develop neutralizing antibodies to astrovirus, as has been demonstrated in rabbits immunized with astroviruses 1, 3, and 5 grown in LLCMK cells (4).
Serotyping is complicated because several antigenically distinct types of astrovirus have been identified. To date, seven types of astrovirus have been distinguished based on IEM, enzyme-linked immunosorbent assay (ELISA), and genomic sequencing, but their antigenic relationships have only partially been established by neutralization assays (3, 4, 9, 11, 12, 15). Therefore, we developed neutralization assays for astrovirus types 1 to 7 to study the homotypic and heterotypic immune responses in immunized rabbits and in different age groups of naturally infected humans. In addition, the results of typing of field strains by neutralization assay were compared with those of ELISA and genotyping.
MATERIALS AND METHODS
Reference reagents and sera.
Astrovirus types 1 to 7 and sera from rabbits immunized with these viruses were kindly provided by J. Kurtz (John Radcliffe Hospital, Oxford, United Kingdom). The reference virus stocks had been passaged three to six times in CaCo2 cells when used in the neutralization assay. Human sera were obtained from an ongoing surveillance system of infectious diseases, in which sera had been collected from a random sample of people of all age groups living in Utrecht Province, The Netherlands, for determination of antibodies to a wide range of microorganisms. For our study, sera were divided on the basis of age groups: <1 year, 1 to 4 years, and 5-year age groups from 5 through 79 years of age. Only sera that were available in sufficient quantities for all neutralization assays were used. There were between 14 and 16 sera in each group, with the exception of the youngest age group (<1 year) (12 sera) and the oldest (75 to 79 years) (13 sera). A total of 242 sera were tested.
CaCo2 cell culture and neutralization assays.
Cultivation of astroviruses in a human colon carcinoma cell line, CaCo2 (ATCC HTB 37), was performed by using a modified version of the protocol of Willcocks et al. (18). CaCo2 cells (passage numbers 80 to 100) were plated at 3 × 106/25-cm2 flask and were incubated in Wistar medium (WM) supplemented with 15% fetal bovine serum (FBS), 0.2 M glutamine, 0.084% sodium bicarbonate, and antibiotics. An important modification of the original protocol (18) was the optimal time for infection, which was determined empirically at between 6 and 17 days postseeding, when cells had become confluent. Prior to infection, the monolayers were rinsed three times with WM without FBS. Virus or stool suspensions were prepared in WM plus 10 μg of trypsin IX (Sigma, Zwijndrecht, The Netherlands) per ml and incubated for 1 h at 37°C, after which the inoculum was diluted with WM to a final concentration of 3 μg of trypsin per ml and added to the cell monolayer. After 1 h at 37°C, the inoculum was removed, and WM supplemented with 3 μg of trypsin per ml was added. The optimal concentration of trypsin was chosen to yield sufficient levels of released progeny virus while the cytopathic effect (CPE) was still visible. At higher concentrations of trypsin, the monolayer was disrupted prior to onset of CPE. The cells were incubated at 37°C until full CPE developed (usually at day 3 or 4) and were harvested by two cycles of freeze-thawing. The suspension was clarified by low-speed centrifugation and stored at −70°C. For titrations, virus preparations were serially diluted (10-fold) in WM plus 10 μg of trypsin per ml and inoculated in 10 wells per dilution in 96-well plates. The bicarbonate concentration of WM was increased to 0.25% for use of the 96-well plates in a CO2 incubator. Titers were expressed as the reciprocal of the highest dilution giving CPE in 50% of the wells after 5 days (50% tissue culture infective dose [TCID50]). For neutralization assays, 100 TCID50 of each astrovirus serotype was added to serial twofold dilutions of sera in triplicate wells. After a 1-h incubation at 37°C the mixture was added to the rinsed monolayers. Following a 1-h incubation at 37°C, the inoculum was removed, and WM supplemented with 3 μg of trypsin per ml was added. The plates were monitored microscopically for the presence of CPE daily until 1 week after inoculation and frozen at −20°C for testing by ELISA. Neutralization titers are expressed as the reciprocal of the highest serum dilution giving full protection. Rabbit pre- and postimmunization sera were included in each test as controls. In addition, dilutions of serum without virus were added to some wells to assay for toxicity of sera.
Confirmation by ELISA.
To confirm the microscopic readings of the results of the neutralization assay and virus titration assays, plates were freeze-thawed three times, and the contents of the wells were transferred without any further treatment to a 96-well plate for testing by ELISA. The plates had been coated overnight at 4°C with 5 μg of the astrovirus group-specific monoclonal antibody IG5 per ml, kindly provided by I. Sharp, Colindale, United Kingdom. Before the culture fluids were added, the binding sites had been saturated with phosphate-buffered saline plus 5% FBS for 1 h at 37°C, and the plates were washed three times with phosphate-buffered saline supplemented with 0.05% Tween 20. Negative-control reactions were done in parallel in wells coated with an equivalent amount of a monoclonal antibody to influenza virus. The monoclonals were prepared by ammonium sulfate precipitation of ascites fluid. After a 2-h incubation the cell lysates were removed, and the plates were washed again.
Hyperimmune rabbit antiserum to astrovirus serotype 1 was added as detector, followed by horseradish peroxidase-labelled goat antibody to rabbit immunoglobulin G (Sigma). The substrate used was tetramethylbenzidine, and plates were read at 450 nm. Samples were considered ELISA positive when they had a positive/negative ratio (A450 in wells coated with astrovirus monoclonal antibody/A450 in wells coated with influenza monoclonal antibody) of 3 or higher, with a minimal difference between positive and negative signals of 0.300.
Astrovirus typing by the neutralization test.
For serotyping of astrovirus strains, we used the neutralization assay as described above with rabbit sera as reference reagents. A coded panel of CaCo2-adapted astrovirus isolates was provided by the Centers for Disease Control and Prevention (CDC) (Atlanta, Ga.) for typing. The isolates had previously been typed by immunologic and genetic methods (15). The viruses were passaged once in CaCo2 cells, titrated to calculate the size of the inoculum needed, and typed in the neutralization assays. After the neutralization assays were completed, the results were sent to the CDC and decoded.
RESULTS
Optimization of CaCo2 cell culture and neutralization assay.
The conditions described in Materials and Methods enabled reading of the results of the neutralization assays and virus titration assays by microscopically monitoring the development of CPE. Typically, CPE started to develop at the margins of holes made in the monolayer by the incubation with trypsin and consisted of rounding of cells, detachment of cells from the monolayer, and clumping of detached cells. Initially, titrations and neutralization assays were confirmed by ELISA. Results were very similar, with occasionally slightly higher titers in virus titrations because of detection of low-level virus yield by ELISA in individual wells that had not (yet) resulted in visible CPE (data not shown). Therefore, in subsequent experiments we read the neutralization assays by CPE scoring only. All astrovirus reference strains were grown to sufficiently high titers for use in the neutralization assay, although differences of as much as 3 log units were observed between virus types. The minimum yield was 105.7 TCID50 per ml for astrovirus serotype 7, and the maximum yield was 109.2 TCID50 for astrovirus serotype 6.
Typing of reference strains with rabbit sera.
Reference sera from rabbits that had been immunized parenterally with astrovirus serotypes 1 to 7 were assayed for levels of neutralizing antibodies to homologous (the astrovirus serotype used for immunization) and heterologous (the other serotypes) astroviruses (Table 1). The same sera had been used in an ELISA typing system that correlates well with genotyping (15). High levels of neutralizing antibodies were detected in sera from all rabbits, but only to the homologous virus. A low level of cross-reactivity was observed for astrovirus serotype 1 in astrovirus serotype 4 reference serum, but the homologous reaction was more than 256-fold higher. Astrovirus serotype 1 serum did not show cross-reactivity with any virus at the lowest dilution of serum tested (i.e., 1:40).
TABLE 1.
Titers of (cross-)neutralizing antibodies in sera from rabbits immunized with astrovirus serotypes 1 to 7a
| Rabbit reference serum | Titer
|
||||||
|---|---|---|---|---|---|---|---|
| ASV-1 | ASV-2 | ASV-3 | ASV-4 | ASV-5 | ASV-6 | ASV-7 | |
| ASV-1 | 20,480 | <40 | <40 | <40 | <40 | <40 | <40 |
| ASV-2 | <40 | 10,240 | <40 | <40 | <40 | <40 | <40 |
| ASV-3 | <40 | <40 | 2,560 | <40 | <40 | <40 | <40 |
| ASV-4 | 40 | <40 | <40 | 10,240 | <40 | <40 | <40 |
| ASV-5 | <40 | <40 | <40 | <40 | 640 | <40 | <40 |
| ASV-6 | <40 | <40 | <40 | <40 | <40 | 81,920 | <40 |
| ASV-7 | <40 | <40 | <40 | <40 | <40 | <40 | 2,560 |
ASV, astrovirus.
Typing of field strains with rabbit sera.
A coded panel consisting of 13 astrovirus isolates from different populations and a negative-control specimen was obtained from the CDC. The astrovirus isolates had previously been typed by ELISA and genotyping as described elsewhere (15) and were tested in the neutralization assay at 100 TCID50 per well, with rabbit reference sera. The serotyping by NT was concordant with antigenic typing by ELISA and phylogenetic grouping for all but one sample (93%). One sample had been typed as serotype 7 by ELISA and genotyping but was not neutralized by any of the astrovirus sera. The CPE that was observed for this sample was different from the CPE that was observed for other astroviruses. Further evaluation by A. Ras in the Diagnostic Laboratory for Infectious Diseases and Perinatal Screening (National Institute for Public Health and the Environment [RIVM], Bilthoven, The Netherlands) revealed that the sample also contained an enterovirus (data not shown).
Seroprevalence study.
The seroprevalence of neutralizing antibodies to astrovirus serotypes 1 to 7 was determined by using sera from a randomized cross-sectional sample of the population of Utrecht Province. Overall, the percentage of persons with neutralizing antibodies was highest for astrovirus serotype 1 (91%), followed by serotype 3 (69%), serotype 4 (56%), serotype 5 (36%), serotype 2 (31%), serotype 6 (16%), and serotype 7 (10%). The seroprevalence increased with age, but acquisition of antibodies appeared to be slower for persons seropositive for serotype 5 virus than for those seropositive for serotypes 1 to 4 (Fig. 1). The difference was significant when percentages of seropositive persons younger or older than 20 years were compared for astrovirus serotypes 1 to 4 and serotype 5 (chi-square test, P < 0.0001). The seroprevalence of antibodies to serotypes 6 and 7 was too low for our analysis and was excluded from this comparison.
FIG. 1.
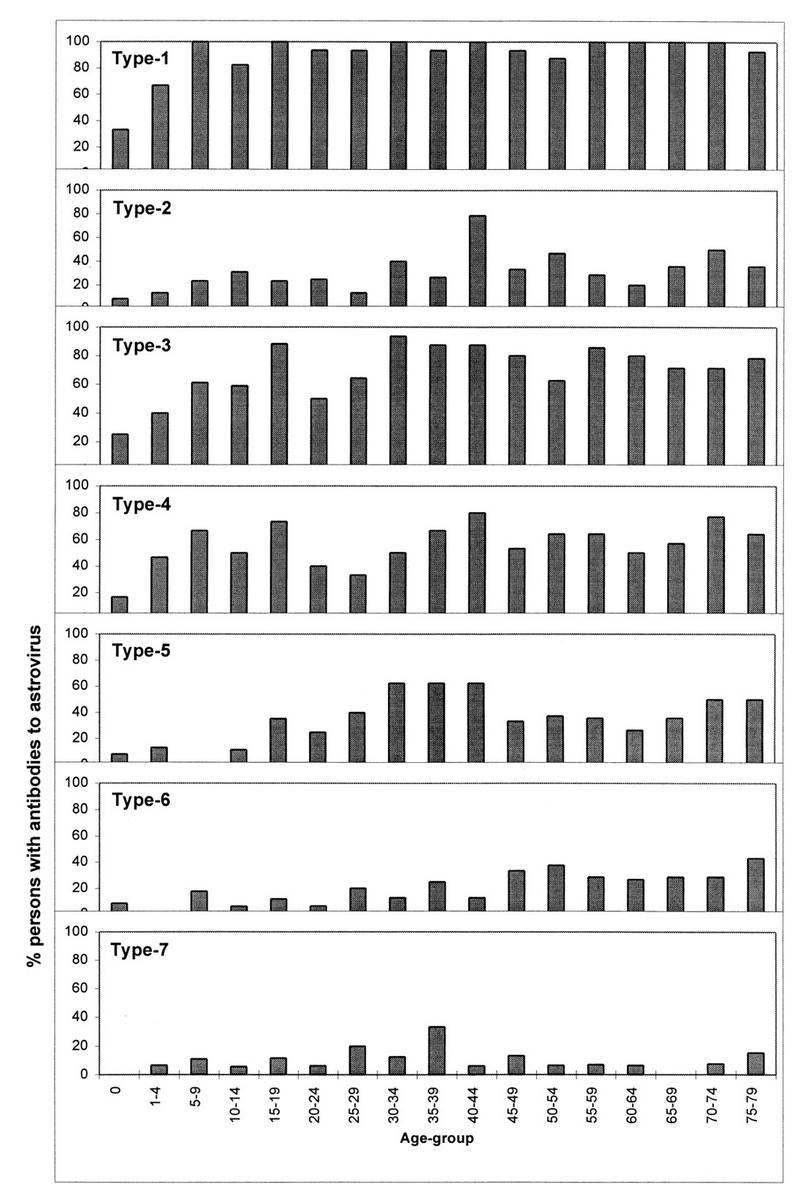
Age-stratified seroprevalence (percentage of sera tested per age group) of neutralizing antibodies to astrovirus serotypes 1 to 7.
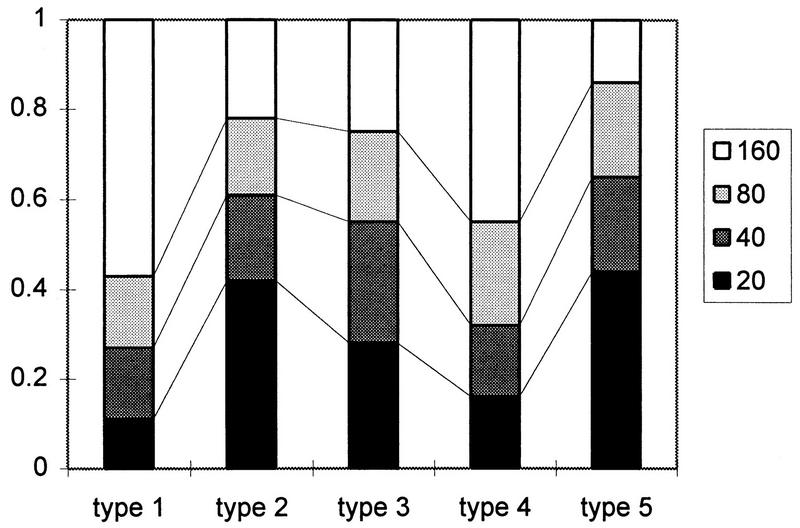
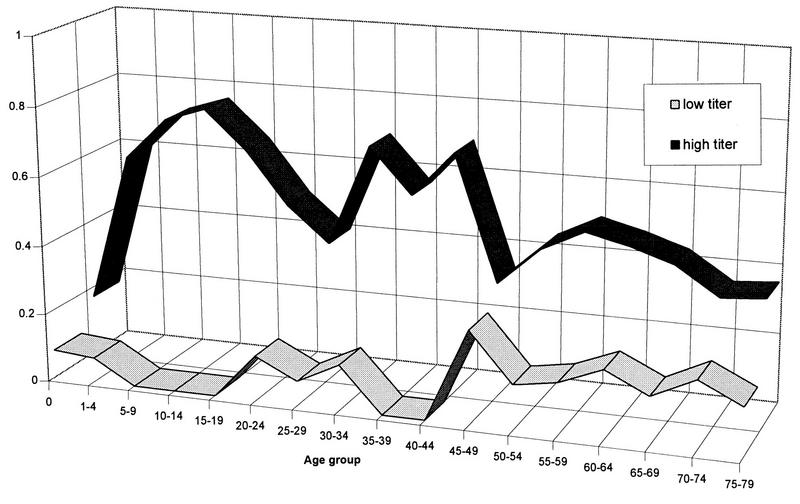
In addition, we looked at levels of antibodies for the seropositive persons for serotypes 1 to 5 (Fig. 2). More than 50% of sera positive for astrovirus serotype 1 antibodies had high titers (160 or more). Similarly, almost half of the astrovirus serotype 4-positive sera had high titers. Sera positive for astrovirus serotype 2 and 3 antibodies had a fairly even distribution of all titer levels. Antibodies to astrovirus serotype 5 were present at low titers. The higher titers were found less frequently in older persons but were seemingly clustered in certain age groups for antibodies to astrovirus serotype 1 (Fig. 3) and astrovirus serotype 4. For the other serotypes of astrovirus, only a few sera had high levels of antibodies, and they were not clustered as clearly as for astrovirus serotypes 1 and 4. High levels of antibodies to astrovirus serotype 5 were never found in the younger age groups.
FIG. 2.
Fraction of positive sera per titer level for astrovirus serotypes 1 to 5.
FIG. 3.
Age-stratified distribution (fraction of astrovirus-positive sera per group) of high (>160) and low (=20) titers of antibody to astrovirus serotype 1.
Cross-reactivity in human sera.
We tried to examine whether sera from humans had cross-reactive neutralizing antibodies by calculating Spearman rank order correlations for all possible combinations of antibody serotypes (Table 2). The underlying assumption was that—if neutralizing antibodies were cross-reactive—there would be a positive correlation between the level (titer) of antibody against one astrovirus serotype and the titer of antibody against the heterologous astrovirus. For these calculations, samples with titers 160 or higher (i.e., the maximal serum dilution that was tested) were excluded since they bias the data set; a cumulation of samples is seen at a single point (160), which does not reflect the true situation (sera with a range of titers). Including these sera in the analysis would artificially create a (false) correlation. An insufficient number of samples were available for calculation of the correlation coefficient between serotypes 1 and 7 and between serotypes 6 and 7 (NA in Table 2). No significant correlations were found for any of the virus combinations tested.
TABLE 2.
Correlations for pairs of antibodies to astrovirus in sera from humans
| Antibody | Correlation coefficient (and P value)
|
|||||
|---|---|---|---|---|---|---|
| ASV-2 | ASV-3 | ASV-4 | ASV-5 | ASV-6 | ASV-7 | |
| ASV-1 | −0.25 (0.28) | 0.14 (0.28) | 0.11 (0.56) | −0.08 (0.62) | −0.33 (0.17) | NAa |
| ASV-2 | −0.29 (0.09) | −0.19 (0.36) | 0.002 (0.99) | 0.14 (0.66) | 0.57 (0.11) | |
| ASV-3 | 0.22 (0.17) | 0.05 (0.71) | −0.34 (0.14) | −0.08 (0.76) | ||
| ASV-4 | 0.12 (0.52) | 0.18 (0.56) | 0.13 (0.75) | |||
| ASV-5 | −0.32 (0.21) | −0.29 (0.34) | ||||
| ASV-6 | NA | |||||
NA, not applicable due to insufficient number of samples.
DISCUSSION
Astroviruses have been classified into seven distinct antigenic groups by IEM, immunofluorescence testing, ELISA, and genotyping, but it remains unclear if the groups are true serotypes (3, 9, 11, 12, 15). By definition, for a true serotype, the homologous/heterologous neutralization titer ratio should be higher than 16 (2). By this criterion, based on our results all seven previously distinguished types of human astrovirus can be considered true serotypes. This confirms and extends earlier findings by Hudson et al. (4), who obtained the same results for astrovirus serotypes 1, 2, and 5 by plaque reduction neutralization assay. Hudson et al. (4) found a high level of cross-reactivity in rabbit reference serum 5 with astrovirus serotype 2, whereas we found low levels of cross-neutralizing antibodies in hyperimmune serum for astrovirus serotype 4 with astrovirus serotype 1. The reason for this discrepancy remains unclear. Low levels of neutralization of a heterologous astrovirus, as we observed, may be caused by steric hindrance, since high levels of nonneutralizing cross-reactive antibodies have been detected in the same rabbit sera by ELISA (3, 4). Hudson et al. (4) used less-stringent cutoff criteria for neutralization (80% reduction in plaque assay against 75 to 100 PFU) than we did (complete neutralization of 100 TCID50), which may explain slight differences in neutralization, especially at low titers.
We tested 242 sera from humans for the presence of neutralizing antibodies to astrovirus serotypes 1 to 7 and looked for associations between test results to determine whether cross-reactivity occurred in humans naturally infected with astrovirus. We found no evidence of cross-reactivity. However, with the present serum collection, we were not able to distinguish primary from secondary infections. It is conceivable that repeated infections may boost heterologous neutralizing antibody titers, since low levels of cross-reactivity were found in the reference rabbit sera (4). Such repeat infections may not be common, as most high antibody titers were found in association with the youngest age groups; typically, with repeat infection with viruses of the same serotype, one would expect booster responses and an increase in the prevalence of high antibody titers with age. It would be interesting to test pre- and postinfection sera from volunteers to determine whether the presence of preexisting neutralizing antibodies is correlated with protection from infection. Experimental infections of adult volunteers with preexisting antibodies, as determined by ELISA, resulted in mild disease or asymptomatic infection (8).
When virus neutralization assays were used, all but one of the samples from a coded panel of field strains were typed in agreement with the results of ELISA and genotyping (15). In the ELISA, the same Oxford rabbit reference sera that we tested in our assays were used. Previous attempts of strain typing by ELISA resulted in high levels of cross-reactivity when rabbit sera were used as detector antibody (3). The astrovirus typing ELISA uses the rabbit sera as capture antibodies, which might explain the different results: the use of serum as a capture antibody may require higher-affinity binding than use as a detector, thus increasing the stringency of the assay. Alternatively, nonspecific binding of the viruses may result in conformation changes of viral epitopes. Whatever the mechanism is, the results of the recently described typing ELISA (15) correlated well with our typing by virus neutralization assays and may be useful for future studies.
The correlation between typing by neutralization assay and genotyping of the capsid region may be fortuitous, although it suggests that this region contains at least one important neutralizing epitope. It has been shown that astroviruses of serotype 1 in this region of the capsid gene exhibit as much as 7% nucleotide sequence divergence over a 15-year period, which might be expected for a genomic region coding for proteins that are under immune pressure for a highly prevalent virus (16). Arguing against this hypothesis is the fact that codon changes were not found in the study (16). Recently, a neutralizing monoclonal antibody against astrovirus serotype 2 has been described (17). Characterization of neutralization escape mutants may help to resolve the viral epitopes that induce neutralizing antibodies.
We found substantial differences in seroprevalence for the different astrovirus serotypes. Our data suggest that astrovirus serotype 1 is most prevalent, followed by serotypes 3 and 4 (intermediate prevalence, 50 to 70%), serotypes 2 and 5 (low prevalence, 30 to 40%), and serotypes 6 and 7 (very low prevalence, 10 to 20%). We have insufficient virologic data to study the correlation with virus typing for The Netherlands, but our data are consistent with findings elsewhere. Kriston et al. (6) found a high seroprevalence (90%) for astrovirus serotype 1 by the immunofluorescence test and a low seroprevalence for astrovirus serotype 6 (10 to 30%). Several investigators have found predominantly astrovirus serotype 1, with a few percent astrovirus serotypes 2, 3, and 4, and rarely serotypes 5, 6, and 7 (9, 11, 12, 14, 15). Noel and Cubitt (14) found a distribution of serotypes in the United Kingdom that would match our seroprevalence data (86% for serotype 1, 1% for serotype 2, 8% for serotype 3, and 6% for serotype 4). In a smaller survey, Willcocks et al. (18) found a predominance of astrovirus serotype 1 strains in one year but predominantly serotype 4 strains in another year. They showed that most infections occurred in young children, a finding similar to our data for serotypes 1 to 4. The different seroprevalence of serotype 5 antibodies has to our knowledge not previously been reported and, if confirmed, suggests a difference in the epidemiology of astrovirus serotype 5.
In conclusion, we found that astrovirus infections are quite common in The Netherlands, especially with astrovirus serotype 1. Astrovirus infections induce serotype-specific neutralizing antibodies in humans of all age groups, and these antibodies may persist for a prolonged period. The seroprevalence of antibodies to astrovirus serotype 5 suggests that the epidemiology of serotype 5 is different from that of astrovirus serotypes 1 to 4.
ACKNOWLEDGMENTS
We thank J. Noel (CDC) for compiling a coded panel of astrovirus strains, J. Kurtz (John Radcliffe Hospital) for providing astrovirus reference strains and rabbit reference sera, M. Conyn (RIVM) for providing the human sera, and J. de Jong (RIVM) for fruitful discussions.
REFERENCES
- 1.Echeverria P, Hoge C W, Bodhidatta L, Tungtaem C, Herrmann J, Imlarp S, Tamura K. Etiology of diarrhea in a rural community in western Thailand: importance of enteric viruses and enterovirulent Escherichia coli. J Infect Dis. 1994;169:916–919. doi: 10.1093/infdis/169.4.916. [DOI] [PubMed] [Google Scholar]
- 2.Grandien M, Forsgren M, Ehrnst A. Enteroviruses and reoviruses. In: Schmidt N J, Emmons R W, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. 6th ed. Washington, D.C: APHA Inc.; 1989. pp. 513–578. [Google Scholar]
- 3.Herrmann J E, Hudson R W, Perron-Henry D M, Kurtz J B, Blacklow N R. Antigenic characterization of cell-cultivated astrovirus serotypes and development of astrovirus-specific monoclonal antibodies. J Infect Dis. 1988;158:182–185. doi: 10.1093/infdis/158.1.182. [DOI] [PubMed] [Google Scholar]
- 4.Hudson R W, Herrmann J E, Blacklow N R. Plaque quantitation and virus neutralization assays for human astroviruses. Arch Virol. 1989;108:33–38. doi: 10.1007/BF01313740. [DOI] [PubMed] [Google Scholar]
- 5.Jiang B, Monroe S S, Koonin E V, Stine S E, Glass R I. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc Natl Acad Sci USA. 1993;90:10539–10543. doi: 10.1073/pnas.90.22.10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriston S, Willcocks M, Carter M, Cubitt W. Seroprevalence of astrovirus types 1 and 6 in London, determined using recombinant virus antigen. Epidemiol Infect. 1996;41:159–164. doi: 10.1017/s0950268800001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz J, Lee T. Astrovirus gastroenteritis age distribution of antibody. Med Microbiol Immunol. 1978;166:227–230. doi: 10.1007/BF02121154. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz J, Lee T, Craig J W, Reed S E. Astrovirus infections in volunteers. J Med Virol. 1979;3:221–230. doi: 10.1002/jmv.1890030308. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz J, Lee T W. Human astrovirus serotypes. Lancet. 1984;ii:1405. doi: 10.1016/s0140-6736(84)92101-9. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz J B, Lee T W. Astroviruses: human and animal. Ciba Found Symp. 1987;128:92–107. doi: 10.1002/9780470513460.ch6. [DOI] [PubMed] [Google Scholar]
- 11.Lee T W, Kurtz J B. Human astrovirus serotypes. J Hyg Camb. 1982;89:539–540. doi: 10.1017/s0022172400071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee T W, Kurtz J B. Prevalence of human astrovirus serotypes in the Oxford region 1976–1992, with evidence for two new serotypes. Epidemiol Infect. 1994;112:187–193. doi: 10.1017/s0950268800057551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui S M. Astroviruses. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press Ltd.; 1995. pp. 1035–1045. [Google Scholar]
- 14.Noel J, Cubitt D. Identification of astrovirus serotypes from children treated at the Hospitals for Sick Children, London 1981–93. Epidemiol Infect. 1994;113:153–159. doi: 10.1017/s0950268800051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel J S, Lee T W, Kurtz J B, Glass R I, Monroe S S. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J Clin Microbiol. 1995;33:797–801. doi: 10.1128/jcm.33.4.797-801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palombo E A, Bishop R F. Annual incidence, serotype distribution, and genetic diversity of human astrovirus isolates from hospitalized children in Melbourne, Australia. J Clin Microbiol. 1996;34:1750–1753. doi: 10.1128/jcm.34.7.1750-1753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Fauquier A, Carrascosa A L, Carrascosa J L, Otero A, Glass R I, Lopez J A, San-Martin C, Melero J A. Characterization of a human astrovirus serotype 2 structural protein (VP26) that contains an epitope involved in virus neutralization. Virology. 1994;201:312–320. doi: 10.1006/viro.1994.1296. [DOI] [PubMed] [Google Scholar]
- 18.Willcocks M M, Carter M J, Laidler F R, Madeley C R. Growth and characterization of human fecal astrovirus in a continuous cell line. Arch Virol. 1990;113:73–81. doi: 10.1007/BF01318354. [DOI] [PubMed] [Google Scholar]
- 19.Woode G N, Pohlenz J F, Gourley N E K, Fagerland J A. Astrovirus and Breda virus infections of dome cell epithelium of bovine ileum. J Clin Microbiol. 1984;19:623–630. doi: 10.1128/jcm.19.5.623-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]