Abstract
Phagocytosis and oxidative burst in whole-blood granulocytes were assessed by flow cytometry with Phagotest and Bursttest kits, respectively. Seventy individuals were included in this study: 15 healthy, normal donors, 18 human immunodeficiency virus (HIV) type 1 (HIV-1)-seropositive patients, 19 patients with pulmonary tuberculosis (TB), and 18 patients co-infected with Mycobacterium tuberculosis and HIV-1 (TB-HIV). Granulocyte phagocytosis was assessed by incubating whole blood with fluorescence-labelled Escherichia coli and measuring the proportion of granulocytes with ingested bacteria and the capacity (fluorescence intensity) of each cell to phagocytose E. coli. The percentage of granulocytes converting nonfluorescent dihydrorhodamine to fluorescent rhodamine 123 on production of reactive oxygen intermediates (ROIs) and the mean channel shift were assessed as a measure of oxidative burst. No differences in the proportion of granulocytes that were capable of phagocytosing or producing ROIs in response to E. coli were observed between any of the study groups. Phagocytosis was significantly enhanced in granulocytes from HIV-1-infected individuals. On the other hand, granulocytes from individuals infected with M. tuberculosis alone or in combination with HIV-1 had a significantly reduced capacity to phagocytose E. coli and to produce ROIs in response to E. coli as an agonist. These results provide evidence that granulocytes from individuals with pulmonary TB with or without concomitant infection with HIV-1 have an impaired ability to phagocytose and to undergo oxidative burst, possibly contributing to the enhanced susceptibility to opportunistic infections in these patients.
Of the global burden of coinfection with human immunodeficiency virus (HIV) type 1 (HIV-1) and Mycobacterium tuberculosis, approximately 70% of these individuals reside in sub-Saharan Africa (18). Not only does HIV-1 infection promote the pathogenesis of M. tuberculosis, but clinical data also suggest that M. tuberculosis hastens the course of HIV-1 disease (7, 11, 15, 24, 26). Furthermore, the risk of acquiring new opportunistic infections for patients dually infected with M. tuberculosis and HIV-1 is increased compared with that for individuals infected with HIV-1 alone (28).
Polymorphonuclear neutrophils (PMNs) play an important role in the host defense against bacterial infections and certain fungal infections, and it is thought that depressed neutrophil responses may play a role in the increased susceptibility to secondary infections experienced by HIV-1-infected individuals (9, 22). Defects in PMN chemotaxis (5, 20) and bacterial killing (5, 12, 20) have been shown previously. Other studies of neutrophil function in HIV-1-infected patients have shown discordant data, with some studies suggesting that phagocytosis and the generation of reactive oxygen intermediates (ROIs) are impaired (4, 16, 17), while others have suggested that one or both of these functions are enhanced (2, 17, 27).
Neutrophils from individuals with active pulmonary tuberculosis (TB) have been shown to have an increased capacity for phagocytosis (19) and respiratory burst (10). In contrast Antonaci et al. (1) reported depressed chemotaxis, phagocytosis, and bacterial killing by neutrophils, regardless of whether patients had active or chronic pulmonary TB.
Because very few data showing the impact of M. tuberculosis and HIV-1 coinfection on phagocyte function exist, we considered it important to delineate granulocyte abnormalities that could further shed light on the pathogenesis of TB and HIV-1 infection. Granulocyte function in the presence of TB assumes increasing importance in Africa, where TB is endemic and bacterial superinfection is common (3, 14). The purpose of this study was therefore to investigate the effect of infection with M. tuberculosis or HIV-1, or with both, on granulocyte functions essential to the clearance of secondary microbial infections, namely, phagocytosis and oxidative burst. We demonstrate that whereas granulocyte phagocytosis was enhanced in individuals singly infected with HIV-1, both phagocytosis and oxidative burst were depressed in granulocytes from individuals infected with M. tuberculosis and those dually infected with HIV-1 and M. tuberculosis.
MATERIALS AND METHODS
Patients.
This study included a total of 70 individuals, comprising 15 healthy, normal donors (ND [control] group), 18 HIV-1-seropositive patients from an HIV outpatient clinic in Johannesburg, South Africa (HIV group), 19 patients with pulmonary TB (TB group), and 18 patients coinfected with M. tuberculosis and HIV-1 (TB-HIV group) from a TB hospital in Johannesburg. This study was approved by the University of the Witwatersrand Ethical Committee; the patients were recruited after informed consent was obtained, and the confidentiality of all records was ensured. The healthy volunteers were recruited from among laboratory workers, and they had no known risk factors for HIV-1 infection. The characteristics of the subjects are presented in Table 1. CD4 counts are expressed as percentages of total lymphocytes in addition to absolute counts, because the latter can vary widely due to periodic fluctuations in the leukocyte count and differential (23). All patients in the TB and TB-HIV groups were receiving the standard four-drug anti-TB therapy that included rifampin, isoniazid, pyrazinamide, and ethambutol.
TABLE 1.
Immunological status of individuals within ND, HIV, TB, and TB-HIV groupsa
| Group | No. of subjects | WBCs (no. [103]/μl) | PMNs
|
CD4 cells
|
||
|---|---|---|---|---|---|---|
| No. (103)/μl | % | No./μl | % | |||
| ND | 15 | 6.4 ± 0.5 | 4.3 ± 0.4 | 66.5 ± 2.6 | 752 ± 84.7 | 43.7 ± 2.4 |
| HIV | 18 | 4.7 ± 0.5 | 3.4 ± 0.4 | 71.3 ± 2.6 | 317.3 ± 79.5 | 16.6 ± 2.7 |
| TB | 19 | 9.7 ± 1.2 | 7.7 ± 1.2 | 75.1 ± 1.2 | 671.6 ± 69.9 | 41.9 ± 2.3 |
| TB-HIV | 18 | 6.0 ± 0.6 | 4.0 ± 0.4 | 67.1 ± 3.5 | 302.4 ± 82.8 | 14.7 ± 1.9 |
Values are means ± standard errors of the means. WBC, leukocyte count.
Phagocytosis.
The test for phagocytosis was carried out by using the Phagotest kit (Orpegen, Heidelberg, Germany). One hundred microliters of whole blood was mixed with 2 × 107 fluorescein isothiocyanate (FITC)-labelled Escherichia coli cells at 0°C. Mixtures of heparinized whole blood and bacteria were incubated in a 37°C horizontal shaking water bath for 10 min. As a control, whole blood and FITC-labelled E. coli were incubated at 0°C to reduce the phagocytic potential to a minimum. The fluorescence of the attached bacteria on the cell surface was quenched by using 100 μl of Coomassie brilliant blue. After two washes, erythrocytes were lysed by adding lysing solution for 20 min at room temperature, after which propidium iodide was added to stain the DNAs of the bacteria and the cells.
Oxidative burst.
The production of ROIs was determined with the Bursttest kit (Orpegen). One hundred microliters of whole blood was mixed with 2 × 107 unlabelled E. coli cells at 0°C. Mixtures of heparinized whole blood and bacteria were incubated in a 37°C horizontal shaking water bath for 10 min. As a control, whole blood was incubated with 20 μl of wash solution. Dihydrorhodamine (DHR) was added to the samples at 37°C, and incubation was continued for a further 10 min in order to allow nonfluorescent DHR to convert to fluorescent rhodamine 123 upon the production of ROIs. Lysing solution was added for 20 min at room temperature. After washing, 100 μl of propidium iodide was added to stain the DNAs of the bacteria and the cells.
Flow cytometry.
Ten thousand leukocytes were collected from each sample on a FACSort flow cytometer. The instrument was calibrated and standardized between each analysis by using Calibrite beads (Becton Dickinson, Erembodegen-Aalst, Belgium). All sample analysis was performed with Cellquest software (Becton Dickinson). The granulocyte populations were gated by using their forward- and side-scatter dot plots. During fluorescence-activated cell sorter analysis, free bacteria and aggregates of bacteria are separated from leukocytes on account of their much lower DNA content compared to that of eukaryotic cells. Phagocytosis and oxidative burst were monitored by determining both the proportion of cells fluorescing and the relative fluorescence intensities of the gated granulocytes.
Statistical analysis.
All statistics were determined by using Statgraphics software (STSC, Inc., Rockville, Md.). Differences in cell counts, phagocytosis, and oxidative burst between the ND, HIV, TB, and TB-HIV groups were compared by the nonparametric Mann-Whitney U test. Relationships between various parameters were determined by simple correlation (Spearman’s rank correlation coefficient).
RESULTS
Phagocytosis.
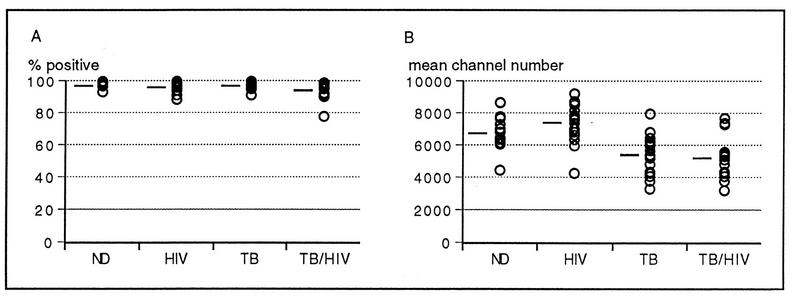
The capacity of granulocytes to phagocytose fluorescent E. coli was measured in anticoagulated whole blood. Figure 1A shows that there were no differences between the four study groups in the percentage of granulocytes able to undergo phagocytosis. When comparing the degree of fluorescence (i.e., efficiency of phagocytosis or uptake of fluorescent E. coli per cell) expressed as a mean channel number (MCN) (Fig. 1B), the fluorescence intensities for the TB and TB-HIV groups were significantly reduced compared to that for the control group (P < 0.01). There was a significant increase (P < 0.05) in the ability of granulocytes from the HIV-1-infected group to phagocytose E. coli compared to that for granulocytes from the ND group. There were also significant differences in granulocyte phagocytic capacity between the HIV and TB groups (P < 0.001) as well as the HIV and TB-HIV groups (P < 0.001). There was no significant difference in phagocytosis between the TB group and the group dually infected with M. tuberculosis and HIV-1 (P > 0.05).
FIG. 1.
Phagocytosis of E. coli by granulocytes from subjects in the ND, HIV, TB, and TB-HIV study groups. (A) Percentage of granulocytes that phagocytose FITC-labelled E. coli after 10 min of incubation. (B) Capacity to phagocytose (relative fluorescence intensity or MCN). Mean values for each group are shown as bars.
Oxidative burst.
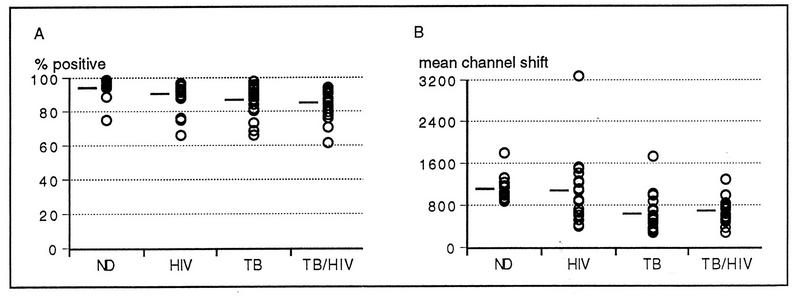
ROI production by whole-blood granulocytes in response to E. coli as a stimulus was measured by conversion of a nonfluorescent substrate to a fluorescent substrate. Figure 2A shows that there were no significant differences between any of the four study groups in the percentage of granulocytes able to undergo oxidative burst (P > 0.05). However, the capacity to produce ROIs was considerably different between the groups (Fig. 2B), with significant differences between the ND and the TB-HIV groups (P < 0.001), the ND and TB groups (P < 0.001), the HIV and TB groups (P < 0.05), and the HIV and TB-HIV groups (P < 0.05). There was no significant difference between the ND group and the HIV-1 group (P > 0.05), even though a wide spread of reactivities was present in the HIV-1 group.
FIG. 2.
ROI production in response to E. coli by granulocytes from subjects in the ND, HIV, TB, and TB-HIV groups. (A) Percentage of granulocytes that convert nonfluorescent DHR to fluorescent rhodamine 123. (B) Fluorescence intensity of ROI production by granulocytes (mean channel shift, sample MCN − MCN of the unstimulated control). Means for each group are indicated as bars.
Relationship between phagocytic and oxidative burst capacity and immunological status.
No correlation was observed within any of the study groups between percent CD4 cells, absolute CD4 count, or leukocyte count and MCN and shift determinations for phagocytosis and oxidative burst, respectively. Furthermore, there was no significant correlation between absolute granulocyte count or percent PMNs and the capacity of granulocytes to phagocytose E. coli or be stimulated to produce ROIs.
Relationship between phagocytic and oxidative burst capacity and time of anti-TB treatment.
The mean duration of anti-TB treatment did not differ significantly between the TB and the TB-HIV groups (P > 0.05). There was no correlation between the ability of granulocytes from individuals with pulmonary TB to phagocytose or produce ROIs and the duration of their anti-TB drug therapy. This was true whether patients had concomitant HIV-1 infection or not. Times of treatment ranged from 1.5 weeks to a maximum of 29.5 weeks. When patients were stratified into two groups on the basis of time of treatment, 18 had received treatment for <2 months and 19 had received treatment for >2 months. There was no significant difference between granulocyte phagocytic capacity or ability to produce ROIs between the groups receiving treatment for <2 months and >2 months (P > 0.05).
DISCUSSION
This study has shown that granulocytes from patients with pulmonary TB or pulmonary TB and HIV-1 disease have impaired function, as measured by phagocytosis and oxidative burst. This appeared to be unrelated to the immune status or the length of anti-TB treatment. Because efficient phagocytosis and the subsequent production of ROIs are important for the intracellular killing of microorganisms by phagocytes, defects in one or both of these functions may lead to deficient killing of intracellular microorganisms, thus predisposing the individual with pulmonary TB to further opportunistic infections. Killing of M. tuberculosis itself by PMNs occurs via nonoxidative means (8), and mycobacterial killing has been shown to be further enhanced by cytokines such as gamma interferon (6) and interleukin-8 (13). This suggests that deficient respiratory burst activity in patients with TB would not necessarily have any impact on the ability of their neutrophils to kill mycobacteria once the mycobacteria were internalized. A direct role for a 25-kDa glycolipoprotein of M. tuberculosis in suppressing bacterial killing, apparently through reduced O2-dependent respiratory burst, has been shown in the absence of any effect on phagocytic potential in vitro (25).
In contrast to the depressed PMN function noted in TB patients, granulocytes from HIV-1-infected individuals showed an enhanced capacity to phagocytose E. coli, in agreement with previous studies (2, 21). This was found for all HIV-1-infected individuals, irrespective of CDC staging, in a study by Schäfer et al. (21) and in our study by using percent CD4 cells or total CD4 cell count as a measure of immune status. Oxidative burst produced by granulocytes from HIV-1-infected individuals, on the other hand, was not significantly altered compared to that produced by granulocytes from healthy donors.
Thus, the PMN defects found in both the TB and the TB-HIV groups were clearly due to factors common to both groups, viz., either pulmonary TB disease or anti-TB drug therapy. Our results would suggest that the persistence of depressed PMN functions, despite anti-TB treatment, may be attributed to the anti-TB therapy rather than the TB infection itself, because the duration of treatment did not appear to result in improved function. Consistent with this possibility is one study that showed decreased neutrophil chemotaxis, phagocytosis, and killing of Candida, regardless of active or chronic disease in TB patients receiving anti-TB treatment (1), and another study that showed a significantly increased phagocytosis capacity for neutrophils from patients with active pulmonary TB prior to treatment (19). It is further interesting that both phagocyte functions in our study were depressed to the same extent in the TB and the TB-HIV groups of patients. This would suggest that PMN functions other than phagocytosis and oxidative killing mechanisms may come into play, consistent with other clinical data showing an increased susceptibility of TB patients to secondary infections when they are coinfected with HIV-1 (3, 14, 28). For example, because phagocytosis and the oxidative burst of PMNs from HIV-1-infected individuals are unimpaired, as confirmed in the present study, it has been suggested by Wenisch et al. (27) that nonoxidative killing mechanisms may be deficient. If this is the case for HIV-1 infection only, then such a difference may underlie the greater susceptibility of TB-HIV groups of patients compared with that of patients with TB only, namely, that HIV-1 coinfection may further unmask polymorphonuclear phagocyte dysfunction in individuals with TB.
This study underscores the importance of a detailed evaluation of anti-TB drug therapy in the context of the direct effects of drugs on the neutrophil functions required for the effective elimination of secondary microbial infections in patients with TB.
ACKNOWLEDGMENTS
This work was supported by the Poliomyelitis Research Foundation and Medical Research Council of South Africa.
We thank D. Spencer at the HIV outpatient clinic and L. Page-Shipp from Rietfontein Hospital, Johannesburg, for cooperation in this study.
REFERENCES
- 1.Antonaci S, Jirillo E, Polignano A, Ventura M T, Sabato R, Bonoma L. Evaluation of phagocyte functions, inflammatory lymphokine activities and in vitro antibody synthesis in patients with active and chronic pulmonary tuberculosis. Cytobios. 1991;67:135–144. [PubMed] [Google Scholar]
- 2.Bandres J C, Trial J, Musher D M, Rossen R D. Increased phagocytosis and generation of reactive oxygen products by neutrophils and monocytes of men with stage 1 human immunodeficiency virus infection. J Infect Dis. 1993;168:75–83. doi: 10.1093/infdis/168.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Brindle R J, Nunn P P, Batchelor B I F, Gathua S N, Kimari J N, Newnham R S, Waiyaki P G. Infection and morbidity in patients with tuberculosis in Nairobi, Kenya. AIDS. 1993;7:1469–1474. doi: 10.1097/00002030-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Chen T P, Roberts R L, Wu K G, Ank B J, Stiehm E R. Decreased superoxide anion and hydrogen peroxide production by neutrophils and monocytes in human-immunodeficiency virus-infected children and adults. Paediatr Res. 1993;34:544–550. doi: 10.1203/00006450-199310000-00032. [DOI] [PubMed] [Google Scholar]
- 5.Ellis M, Gupta S, Galant S, Hakim S, VandeVen C, Toy C, Cairo M S. Impaired neutrophil function in patients with AIDS or AIDS-related complex: a comprehensive evaluation. J Infect Dis. 1988;158:1268–1276. doi: 10.1093/infdis/158.6.1268. [DOI] [PubMed] [Google Scholar]
- 6.Geertsma M F, Nibbering P H, Pos O, Van Furth R. Interferon-γ-activated human granulocytes kill ingested Mycobacterium fortuitum more efficiently than norman granulocytes. Eur J Immunol. 1990;20:869–873. doi: 10.1002/eji.1830200423. [DOI] [PubMed] [Google Scholar]
- 7.Isaksson B, Albert J, Chiodi F, Furucrona A, Krook A, Putkonen P. AIDS two months after primary human immunodeficiency virus infection. J Infect Dis. 1988;158:866–867. doi: 10.1093/infdis/158.4.866. [DOI] [PubMed] [Google Scholar]
- 8.Jones G S, Amirault H J, Andersen B R. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990;162:700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- 9.Magnenat J L, Nicod L P, Auckenthaler R, Junod A F. Mode of presentation and diagnosis of bacterial pneumonia in human immunodeficiency virus-infected patients. Am Rev Respir Dis. 1991;144:917–922. doi: 10.1164/ajrccm/144.4.917. [DOI] [PubMed] [Google Scholar]
- 10.Mandell G L, Fuller L F. Nitroblue tetrazolium dye test: a diagnostic aid in tuberculosis. Am Rev Respir Dis. 1972;106:123–125. doi: 10.1164/arrd.1972.105.1.123. [DOI] [PubMed] [Google Scholar]
- 11.Martin D J, Sim J G M, Sole G J, Rymer L, Shalekoff S, van Niekerk A B N, Becker P, Weilbach C N, Iwanik J, Keddy K, Miller G B, Ozbay B, Ryan A, Viscovic T, Woolf M. CD4+ lymphocyte count in African patients co-infected with HIV and tuberculosis. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:386–390. [PubMed] [Google Scholar]
- 12.Murphy P M, Lane H C, Fauci A S, Gallin J I. Impairment of neutrophil bactericidal capacity in patients with AIDS. J Infect Dis. 1988;158:627–630. doi: 10.1093/infdis/158.3.627. [DOI] [PubMed] [Google Scholar]
- 13.Nibbering P H, Pos O, Stevenhagen A, Van Furth R. Interleukin-8 enhances nonoxidative intracellular killing of Mycobacterium fortuitum by human granulocytes. Infect Immun. 1993;61:3111–3116. doi: 10.1128/iai.61.8.3111-3116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunn P, Brindle R, Carpenter L, Odhiambo J, Wasunna K, Newnham R, Githui W, Gathua S, Omwega M, McAdam K. Cohort study of human immunodeficiency virus infection in patients with tuberculosis in Nairobi, Kenya: analysis of early (6-month) mortality. Am Rev Respir Dis. 1992;146:849–854. doi: 10.1164/ajrccm/146.4.849. [DOI] [PubMed] [Google Scholar]
- 15.Pape J W, Jean S S, Ho J L, Hafner A, Johnson W D., Jr Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 16.Pitrak D L, Bak P M, DeMarais P, Novak R M, Anderson B R. Depressed neutrophil superoxide production in human immunodeficiency virus infection. J Infect Dis. 1993;167:1406–1410. doi: 10.1093/infdis/167.6.1406. [DOI] [PubMed] [Google Scholar]
- 17.Pos O, Stevenhagen A, Meenhorst P L, Kroon F P, Van Furth R. Impaired phagocytosis of Staphylococcus aureus by granulocytes and monocytes of AIDS patients. Clin Exp Immunol. 1992;88:23–28. doi: 10.1111/j.1365-2249.1992.tb03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raviglione M C, Snider D E, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 19.Rieger M, Trnka L, Skvor J, Mison P. Immunoprofile studies in patients with pulmonary tuberculosis. III. Study of haemolytic complement in serum and phagocytic activity of blood neutrophils. Scand J Respir Dis. 1979;60:172–175. [PubMed] [Google Scholar]
- 20.Roilides E, Mertins S, Eddy J, Walsh T J, Pizzo P A, Rubin M. Impairment of neutrophil chemotactic and bactericidal function in children infected with human immunodeficiency virus type 1 and partial reversal after in vitro exposure to granulocyte-macrophage colony-stimulating factor. J Pediatr. 1990;117:531–540. doi: 10.1016/s0022-3476(05)80684-5. [DOI] [PubMed] [Google Scholar]
- 21.Schäfer V, Kreuter J, Rübsamen-Waigmann H, Gerte S, von Briesen H. Influence of HIV-infection on the phagocytic activity of monocytes/macrophages and granulocytes. Clin Diagn Virol. 1994;1:279–287. doi: 10.1016/0928-0197(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 22.Schuchat A, Broome C V, Hightower A, Costa S J, Parkin W. Use of surveillance for invasive pneumococcal disease to estimate the size of the immunosuppressed HIV-infected population. JAMA. 1991;265:3275–3279. [PubMed] [Google Scholar]
- 23.Taylor J M G, Fahey J L, Detels R, Giorgi J V. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. J Acquired Immune Defic Syndr. 1989;2:114–124. [PubMed] [Google Scholar]
- 24.Toossi Z, Sierra-Madero J G, Blinkhorn R A, Mettler M A, Rich E A. Enhanced susceptibility of blood monocytes from patients with pulmonary tuberculosis to productive infection with human immunodeficiency virus type 1. J Exp Med. 1993;177:1511–1516. doi: 10.1084/jem.177.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadee A A, Cohen J D, Rabson A R. Gamma interferon reverses inhibition of leukocyte bactericidal activity by a 25-kilodalton fraction from Mycobacterium tuberculosis. Infect Immun. 1987;55:2777–2782. doi: 10.1128/iai.55.11.2777-2782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallis R S, Vjecha M, Amir-Tahmasseb M, Okwera A, Byekwaso F, Nyole S, Kabengera S, Mugerwa R D, Ellner J J. Influence of tuberculosis on human immunodeficiency virus (HIV-1): enhanced cytokine expression and elevated β2-microglobulin in HIV-1 associated tuberculosis. J Infect Dis. 1993;167:43–48. doi: 10.1093/infdis/167.1.43. [DOI] [PubMed] [Google Scholar]
- 27.Wenisch C, Parschalk B, Zedwitz-Liebenstein K, Graninger W, Rieger A. Dysregulation of the polymorphonuclear leukocyte-Candida spp. interaction in HIV-positive patients. AIDS. 1996;10:983–987. doi: 10.1097/00002030-199610090-00008. [DOI] [PubMed] [Google Scholar]
- 28.Whalen C, Horsburgh C R, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]