Abstract
Objective
Patients with dysphagia due to nasopharyngeal carcinoma (NPC) after radiotherapy often have chewing difficulty. Kinematic analysis of mandibular movements may provide clinically useful information for the chewing function. However, current kinematic device costs limited clinical application, and specialized software is required for control and data processing. This study aimed to mandibular kinematics parameter recognition using a self-developed Nswallow 2D motion capture software. To investigate whether differences in kinematic data of mandibular movements during mastication can be used as an indicator of masticatory dysfunction in NPC patients, and to examine relationship with mastication efficiency.
Method
Thirty-three patients with early-stage NPC after radiotherapy and thirty-seven healthy controls were recruited. The self-developed Nswallow 2D motion capture software was used to automatically mark and capture the facial parts of the participants. We tracked jaw kinematic during chewing and analyzed the characteristics of kinematic data of mandibular movements during chewing tasks. Meanwhile, the masticatory efficiency using two-color chewing gum was analyzed by the ViewGum software.
Result
Significant differences were observed in the mastication time [ Total Masticatory Time (NPC 13.500 (11.600, 16.400); HC 10.533 (9.716, 12.250) & Chewing Sequence Duration (NPC 0.614 (0.582, 0.701); HC 0.544 (0.480, 0.586)], speed of mandibular motion [Maximum Chewing Speed (NPC 23.740 (17.775, 25.906); HC 28.467 (24.009, 38.600) & Average Chewing Speed (NPC 11.844 (10.395,13.285); HC 18.169 (16.586, 19.632)], and Mandibular Motion Amplitude [NPC 7.159 (5.887, 7.869); HC 9.184 (7.541;11.141)] between two groups (P < 0.000). Logistic regression analysis and receiver operating characteristic curve analyses were performed based on the above data as explanatory variables. Among them, the Average Chewing Speed exhibited the highest area under the ROC curve, the odds ratio was 3.629, and the cutoff value was 14.28, with a sensitivity of 90.91%, a specificity of 80.00%, and an area under the curve of 0.9255. The masticatory efficiency in the NPC group significantly decreased compared to the healthy control group (P < 0.000). Linear regression analysis showed that Average Chewing Speed negatively affects masticatory efficiency.
Conclusion
The Nswallow 2D motion capture software represents an easy-to-use and affordable system that can be utilized to assess masticatory function in patients with NPC. The Average Chewing Speed of chewing is a highly sensitive kinematic indicator for evaluating mastication efficiency. Furthermore, the Average Chewing Speed could serve as a screening test for patients with mastication disorder of nasopharyngeal cancer and provide dietary guidance for such individuals.
Keywords: Nasopharyngeal carcinoma, Dysphagia, Mastication, Kinematics, Mastication efficiency
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor that occurs in the nasopharynx and nasopharyngeal epithelia [1]. Radiation therapy has been demonstrated to be effective [2]. However, many complications might be associated with radiation therapy, including dysphagia and trismus [3], as well as muscle fibrosis which impaired mastication capabilities [4]. These complications can potentially result in gradually worse of swallowing, speech production, chewing ability, and other orofacial motor functions. Early and accurate assessment of motor function impairment can facilitate the comprehension of patients’ current functional status, enabling early intervention and enhancing the quality of life [5]. Oral movement assessments through clinical observation usually was the main screening test, which may be easy overlook for patients with minor impairment during early stage. The face-to-face assessment also could pose the risk of droplet transmission of respiratory infections.
Computer vision technology has the potential to fully or partially automate the clinical examination of oral and maxillofacial dysfunction, thus providing an accurate and objective assessment. Researchers have introduced various approaches for precisely measuring the kinematic characteristics of the tongue, jaw, and lips (such as position and speed during speech production) [6]. These advancements facilitate effective monitoring in the early-stage of orofacial dyskinesia in patients [7].
A deep learning-based model combined with a 3D camera can accurately localize facial landmarks in videos of patients performing various speech tasks [5–8]. However, the clinical applicability of these techniques is constrained due to their reliance on costly and user-unfriendly systems [9]. The 2D Markerless Systems are a relatively convenient and cost-effective technique. Research has demonstrated that 2D features, extracted from color cameras only, are as informative as 3D features, extracted from color and depth cameras [9], implying that 2D video analysis may serve as a superior evaluation method. Therefore, this study developed an accessible face tracking software system to assess orofacial motor function. The recorded videos of participants were obtained during chewing movement and facial markers was determined before collection through the software. The aim was to investigate the differences of mandibular kinematics data during chewing between NPC patients and healthy participants, and further to clarify the potential indicators for evaluating masticatory dysfunction that influence mastication efficiency.
The role of task
Task-specific policies are imposed based on the intended goal of a given motor behavior. The effects on the masticatory motor task in NPC patients are expected to vary depending on the demands placed on muscle strength, endurance, range of motion, speed, and coordination throughout the progression of the disease. For example, in the early stages of dysphagia in patients with NPC, they still retain the ability to eat orally and do not report any speech difficulties. However, impaired motor performance only becomes apparent when attempting a challenging motor task. The focus of our study was on the movement of the lower jaw, which plays a crucial role in mastication and is distinct from the tongue. This approach allows for easier quantification of its impact on masticatory efficiency within a clinical context.
Methods
Participants
Consecutive patients admitted to a hospital underwent rehabilitation at a hospital outpatient clinic in Guangzhou, China, between March 2022 and January 2023 participated in this cross-sectional study. In this study, there were 33 patients with NPC after radiotherapy (20 males and 13 females, age 54.18 ± 9.700). The duration of radiotherapy for NPC varied from 2 to 24 years, while the number of radiotherapy sessions ranged from 21 to 45.
All participants included in this study met the following criteria: (1) diagnosed with NPC and treated with radiotherapy or chemoradiotherapy; complicated with dysphagia which was identified with videofluoroscopic swallowing study (VFSS); (2) FOIS (Functional Oral Intake Scale) ≥ 4; (3) has no history of orofacial trauma or surgery; (3) having at least 26 teeth; and (4) a maximum DMFT (decayed missing filled teeth) score of 4 and an Angle Class I occlusion. Patients were excluded if they fulfilled the following criteria: (1) presence of tumor recurrence or metastasis; (2) presence of other neurological diseases affecting oral movements; (3) unstable vital signs; (4) no consent from patients or family members; and (5) a history of oral cancer, dental disorders causing painful chewing, or ill-fitting dentures.
Meanwhile, 37 age-matched healthy participants (15 males and 22 females, aged 51.97 ± 6.702) were recruited for this study. The research protocol was approved by the Research Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (Approval No: CUHK Attached SAN Medical Ethics ([2021]02–200-01). This study was registered at Chictr.org in January 2022 (Chinese Clinical Trial Registry Unique Identifier ChiCTR2200056026, registration time 30/01/2022). This study began in March 2022 and concluded in January 2023. All participants involved in this study provided written informed consent for the use of their medical data and any remaining specimens. We accessed the software's data records during February–March 2023. Importantly, we only accessed anonymized medical records and sample information and did not approach or obtain any information that identified participants. All participants'identity information was removed or encrypted before data extraction to ensure privacy protection during the study.
Experimental procedure
Nswallow motion capture system
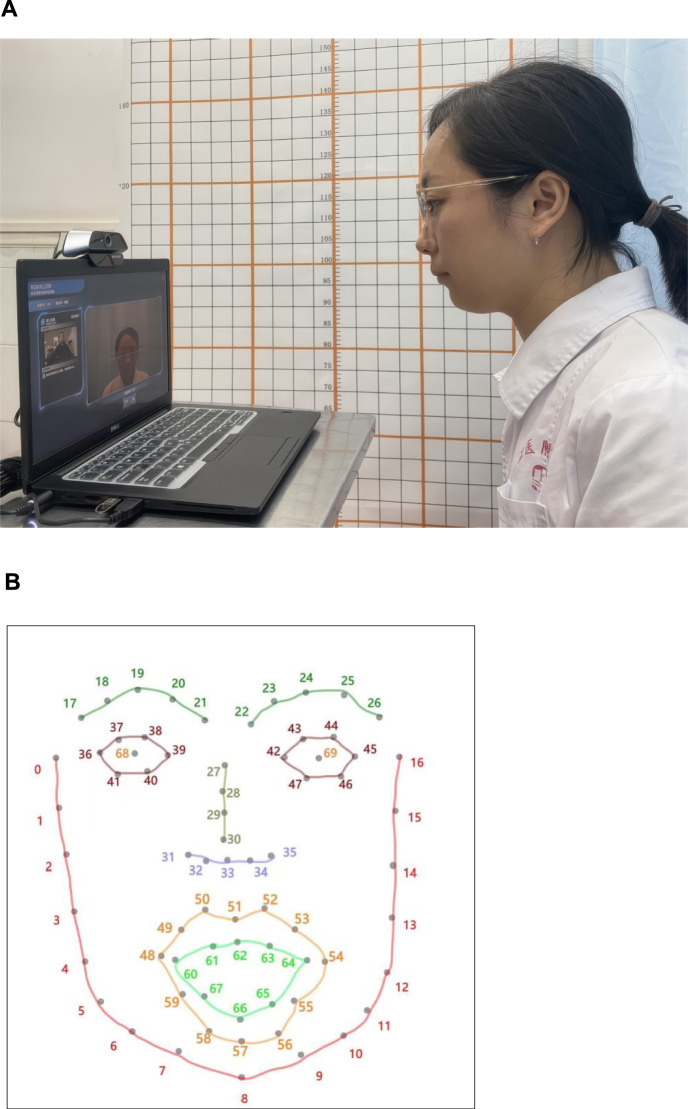
During each session, the movements of jaw were recorded using the two-dimensional motion capture system. This system consisting of analytical software (Nswallow) and a camera [Exilim EX-F1, Casio, Tokyo, Japan, resolution 640 × 480 pixels at 50 frames per second (fps)]. All participants were filmed at a face-camera distance ranging from 30 to 50 cm and at a height close to that of the participants’ eyes [10]. A continuous light source was positioned adjacent to the camera for providing consistent illumination (Fig. 1A). Throughout the task, participants were required to maintain their gaze toward the camera and minimize excessive head movements.
Fig. 1.
A Environment used for recording the video. B Face model used in this work
Facial marker
In this study, an Nswallow software was developed that utilizes a CMU model incorporating a face detector to accurately locate and track facial feature points. We utilized the facial marker points obtained from a previously validated deep learning-based facial alignment model. These markers are acquired from the model and are not distributed on the face [5]. The distribution of these markers is as follows: 10 for eyebrows, 14 for eyes, 9 for noses, 20 for lips (12 for external contours and 8 for internal contours), and 17 for facial contour, as shown in Fig. 1B. The markers were concealed during recording. The target site investigated in this study was referred to as site 8 and site 33. That is, the point position of jaw central (8 th Marker points = JC) and nose tip (33rd Marker points = NT) is extracted as the key point detection.
Chewing task
Food of chewing task was provided by the investigators. Specimens of 30 mm length were prepared from Wrigley's Doublemint-F5® gums (Wrigley's Doublemint-F5®; Azure and Pink; The Wrigley Company; Guangzhou, China) in the flavors “Sour Berry” (azure color) and “Watermelon” (pink color). We cut strips from both colors and manually stuck them together, resulting in a test strip that measures 30 × 18 × 3 mm. Prior to each chewing cycle, participants were instructed to thoroughly remove any food debris from their mouth and clean any soft dirt from the tooth surface. They were then asked to naturally chew two-colored gums for a total of 20 cycles.
Masticatory efficiency
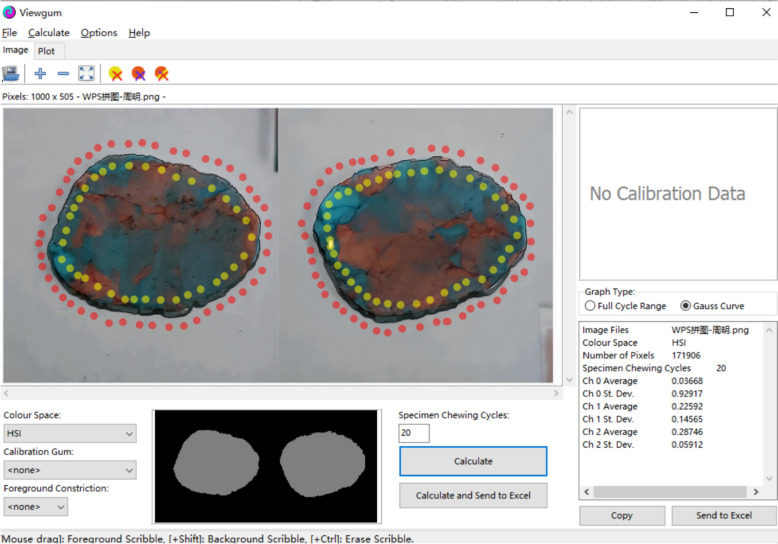
Masticatory efficiency was evaluated with a previously validated color-mixing ability test. The resulting bolus above was retrieved from the oral cavity and flattened into a 1 mm-thick wafer by pressing it with a custom-made polyvinyl chloride plate that had a milled depression of 1 mm × 50 mm × 50 mm [11]. The digitized images were arranged side by side to form a single 1000-pixel image for analysis of masticatory efficiency using specialized software program (ViewGum®; dHAL Software) [12]. The software converts the compound image of both sides of the specimen into the HIS color space and calculates the standard deviation of Hue = sqrt (Variance of Hue, VOH) of the image. VOH can be used as a measure of masticatory efficiency. The greater the hue difference of each pixel in the mixed image, the more insufficient chewing leads to uneven color mixing, resulting in higher VOH, and vice versa, lower VOH is observed when chewing is sufficient, as shown in Fig. 2.
Fig. 2.
Image from masticatory efficiency analysis using software program (ViewGum)
Data processing
The movement data were segmented into chewing sequences based on continuous digital video recordings. After the gum was inserted into the oral cavity, the chewing process commenced at the point of maximum jaw closure, with a prerequisite that each chewing sequence must consist of 20 complete cycles. The principal investigator determined the start and end frames of each event by rigorously examining video data based on rule-based criteria. After initially parsing the data files, the chewing sequence was further refined to exclude non-chewing movements (such as tooth clearance) and swallowing-related motions.
Data filtering
Prior to analysis, motion signals were digitally low-pass filtered (flp = 10 Hz) using zero-phase-shift forward and reverse digital filters (Butterworth, 8-pole). Each signal was high-pass filtered (FHP = 0.20 Hz) to remove high-frequency components from the displacement signal. The application of high-pass filtering was essential due to the upward shift in mandible mean position caused by mass breakdown. Without high-pass filtering, the low-frequency component of the chewing signal would overpower the spectrum, thereby obscuring the high-frequency component associated with frequency of chew.
Measurements
The kinematic measurements were acquired for each task:
Mandibular motion amplitude: The straight-line distance between the minimum and maximum mandibular positions in the two anchor movement trajectories of the tip of the nose and jaw was defined. This measurement was used to indicate the amplitude of mandibular movement during chewing.
Total masticatory time: The initiation of mandibular marker descent was recorded, followed by the completion of 20 chewing cycles and the measurement of the time taken to return to the initial position, which was defined as the overall duration of mastication.
Chewing sequence duration: The initiation time of mandibular marker descent was recorded, and the duration until its return to the initial position was defined as a chewing cycle.
Maximum Chewing Speed: The algorithmic determination of the maximum velocity (mm/s) of jaw movement in the time history is based on identifying the peak value of the first derivative of the two-dimensional path distance.
Average Chewing Speed: Average Chewing Speed was calculated as the mean value of the first derivative of the two-dimensional distance movement history.
Statistical analysis
IBM SPSS 22.0 software was used to analyze the data. The measurements data were presented as frequencies or mean (standard deviations). Age and BMI were tested by t test; χ2 tests were used to compare gender. In the analysis of kinematic data, independent sample t-tests were conducted on Total Masticatory Time and Chewing Sequence Duration, and non-parametric analyses by Wilcoxon Signed Ranks test were performed to examine Maximum Chewing Speed, Average Chewing Speed, and Mandibular Motion Amplitude. Logistic regression analysis was performed to identify sensitive parameters that could effectively distinguish patients with early-stage NPC using kinematic parameters exhibited significant differences between the two groups as explanatory variables. The significance level was set at p < 0.05. Receiver operating characteristic (ROC) curves were employed to calculate the area under the curve (AUC) and assess test accuracy. Additionally, simple linear regression analysis was performed to examine the relationship between kinematic parameters with significant differences and masticatory efficiency.
Sample size calculation
Based on pre-experimental data, we used G*Power (Windows Version 3.1.9.7) to calculate the required sample size, with an effect size of 0.76, α of 0.05, and power (1-β) of 0.8. Additionally, considering the approximately 15% rate of loss to follow-up and the possibility of missing data, a minimum sample size of 68 participants was required.
Results
Characteristics of the participants
In this study, a total of 202 participants were recruited from the rehabilitation outpatient department, out of whom 102 participants met the inclusion criteria. 32 participants were solely assessed and excluded due to a lack of recorded video footage. Additionally, 7 participants could not fully capture their facial chewing movements due to significant body shaking during recording, while three participants spoke during the chewing process. Participants characteristics are shown in Table 1.
Table 1.
Characteristics of study participants
| Healthy control group (n = 37) | NPC group (n = 33) | P value | |
|---|---|---|---|
| Age (years) | 51.97 ± 6.702 | 54.18 ± 9.700 | 0.267 |
| Sex:male/female (n) | 15/22 | 20/13 | 0.159 |
| BMI (kg/m2) | 21.85 ± 4.10 | 19.78 ± 4.33 | 0.044 |
| Radiotherapy sessions (n) | – | 38 (33.40) | – |
| Years from irradiation (years) | – | 10.91 ± 6.26 | – |
Kinematic analysis of mandibular movement
The values obtained from analyzing mastication movement were compared between the two groups, and it was found that the NPC group had significantly longer masticatory time (Total Masticatory Time & Chewing Sequence Duration) than those of the healthy control group (P < 0.000). Furthermore, both Mandibular Motion Amplitude and speed of mandibular motion (Maximum Chewing Speed & Average Chewing Speed) were found to be significantly lower in the NPC group compared to those in the healthy control group (P < 0.000). It is worth mentioning that the Mandibular Motion Amplitude in individual NPC patients is larger than that of normal individuals. The comparison of kinematic analysis results is shown in Table 2.
Table 2.
Comparison of kinematic analysis results. Presented as the M (P25, P75) Percentile number
| Mastication parameters | NPC group (n = 33) | Healthy control group (n = 37) | Z | P value |
|---|---|---|---|---|
| Total masticatory time (s) | 13.500 (11.600, 16.400) | 10.533 (9.716, 12.250) | − 4.312 | < 0.000 |
| Chewing sequence duration (ms) | 0.614 (0.582, 0.701) | 0.544 (0.480, 0.586) | − 4.583 | < 0.000 |
| Mandibular motion amplitude (mm) | 7.863 (7.524, 8.611) | 9.184 (7.541, 11.141) | − 2.124 | 0.034 |
| Maximum speed (mm/s) | 23.775 (17.775, 29.236) | 28.467 (24.009, 38.600) | − 3.006 | 0.003 |
| Average speed (mm/s) | 11.394 (9.098, 12.874) | 18.169 (16.586, 19.632) | − 6.606 | < 0.000 |
| VOH [SDHue] | 0.864 (0.773, 0.896) | 0.609 (0.500, 0.669) | − 6.036 | < 0.000 |
Masticatory efficiency
Compared to the healthy control group, the NPC group exhibited a significant increase in VOH [SDHue] (P < 0.000), indicating a pronounced decrease in masticatory efficiency. The comparison of masticatory efficiency results is shown in Table 2.
Linear regression analysis and logistic regression analysis
This study employed a phased modeling approach. Initially, significant variables were identified through multiple linear regression analysis, followed by the construction of a logistic regression model to examine the relationships among the variables. The detailed findings are presented below. The results of the multiple linear regression analysis indicated that the regression equation was statistically significant. Specifically, the Total Masticatory Time (β = 0.070, p = 0.039), Chewing Sequence Duration (β = 0.273, p = 0.026), and Maximum Chewing Speed (β = 0.006, p = 0.046) were found to have a significant positive influence on masticatory efficiency. Conversely, the Mandibular Motion Amplitude (β = − 0.097, p = 0.023) and Average Chewing Speed (β = − 0.355, p = 0.010) of mandibular movement exhibited a significant negative impact on masticatory efficiency. Meanwhile, the variance inflation factor (VIF) values of all independent variables in this study ranged from 1.176 to 1.781, which is substantially lower than the stringent threshold of 10 (typically, VIF values below 5 are considered acceptable). Collectively, these findings suggest that there is no substantial multicollinearity issue among the explanatory variables.
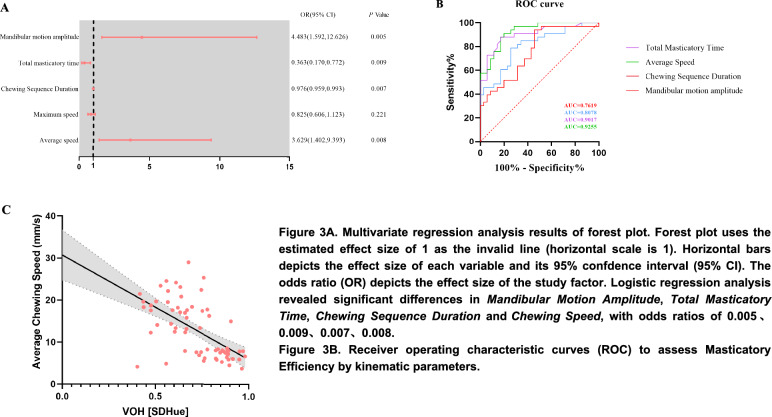
Based on these findings, a logistic regression analysis was conducted using the aforementioned variables that exhibited significant disparities as explanatory factors. This study found significant variations in both the Mandibular Motion Amplitude, the Average Chewing Speed and mastication time (Total Masticatory Time & Chewing Sequence Duration). The odds ratios for these variables were determined to be 4.483, 0.363, 0.976, and 3.629, respectively, as depicted in Fig. 3A. Among them, the Average Chewing Speed exhibited the highest area under the ROC curve, and the cutoff value was 14.28, with a sensitivity of 90.91%, a specificity of 80%, and an area under the curve of 0.9255 (Fig. 3B). Linear regression analysis showed that Average Chewing Speed negatively affects masticatory efficiency (Fig. 3C).
Fig. 3.
A. Multivariate regression analysis results of forest plot. Forest plot uses the estimated effect size of 1 as the invalid line (horizontal scale is 1). Horizontal bars depicts the effect size of each variable and its 95% confdence interval (95% CI). The odds ratio (OR) depicts the effect size of the study factor. Logistic regression analysis revealed significant differences in Mandibular Motion Amplitude, Total Masticatory Time, Chewing Sequence Duration and Average Chewing Speed, with odds ratios of 0.005, 0.009, 0.007, 0.008. B. Receiver operating characteristic curves (ROC) to assess Masticatory Efficiency by kinematic parameters. C. Results of linear regression analysis of Average Chewing Speed-Mastication Efficiency (VOH [SDHue])
Discussion
In this study, we developed a straightforward and non-invasive video-based method to investigate the relevant kinematic characteristics of mastication in patients with NPC and explore the relationship between mastication-related kinematic parameters and masticatory efficiency.
The kinematic differences in mastication movement
This study revealed that individuals with NPC exhibited a prolonged Total Masticatory Time and Chewing Sequence Duration. Additionally, a significant decrease was observed in speed of mandibular motion (Maximum Chewing Speed & Average Chewing Speed), Mandibular Motion Amplitude, and masticatory efficiency. These kinematic data are considered characteristic features of masticatory movements in the analysis of masticatory disorders.
Nasopharyngeal tumors mainly originate from the cervical fascia and then spread laterally to invade the medial/lateral pterygoid muscles and/or other masticatory muscles [13, 14]. After undergoing radiation therapy, patients may develop fibrosis in the masticatory muscles, which can result in reduced muscle elasticity and strength; this affects the masticatory movement, leading to decreased chewing endurance and potential muscle damage or fatigue. Consequently, this can lead to a decrease in chewing speed and require an extended chewing time to compensate for completing the same task. Another study demonstrated a significant association between masticatory muscle activity and both chewing power and occlusal force, with the former showing a positive correlation with masticatory efficiency. When the motor function of chewing is well preserved, it enables the accomplishment of rapid and forceful chewing, thereby enhancing masticatory efficiency [15]. After undergoing radiation treatment, the mastication-related muscles strength significantly reduced, resulting in insufficient force generation for food chewing. This can affect the fluency and coordination of the chewing movement. Therefore, it might require a longer duration and slower speed to compensate for the adaptation of chewing movement to changes in muscle function, ensuring optimal food mastication [16]. Other studies have also demonstrated a strong positive correlation between stable masticatory movement patterns and masticatory efficiency. In other words, unnecessary and irregular mandibular movements may diminish overall mastication efficiency [15]. Furthermore, saliva performs the crucial function of lubricating and moistening food during chewing. Patients diagnosed with NPC often encounter xerostomia and reduced salivary secretion [17]. Consequently, the decrease in saliva production can also render the act of chewing challenging. Patients with NPC may have impaired oral mucosa and restricted tongue mobility, further affecting their ability to chew normally.
However, we occasionally observed instances of greater mandibular motion during chewing in NPC patients (6.06%) even than that in healthy participants. Among them, 33 patients with nasopharyngeal carcinoma were identified, and 2 patients exhibited a greater amplitude of jaw movement compared with the healthy control group (n = 37). In this study, a number of remaining teeth in patients were largely within the normal range. However, radiotherapy may lead to adjustments in the mandibular movement trajectory in some patients due to changes in dentin sensitivity or periodontal ligament proprioception. In addition, this might be attributed to a local compensatory mechanism resulting from tongue atrophy while tooth function remains preserved, as evidenced by the mandibular-tooth linkage compensation. During chewing, tongue movement aids in thoroughly mixing food and saliva. Patients with NPC after radiotherapy may lose tongue flexibility to some extent. They were prone to adjust a greater range of jaw movement for mixing. It may also be compounded by the pathological factors associated with treatment. As the primary treatment modality for nasopharyngeal carcinoma, radiotherapy not only impacts the tongue muscles but may also induce secondary changes in the masticatory muscles and temporomandibular joint. For instance, radiation fibrosis can lead to abnormal tension in the masticatory muscle groups, such as the masseter and temporalis muscles.
Temporospatial data and mastication efficacy
Furthermore, both multivariate analysis and AUC results demonstrated that mastication time (Total Masticatory Time & Chewing Sequence Duration), Mandibular Motion Amplitude, and Average Chewing peed could serve as reliable indicators for identifying patients with masticatory dysfunction. The exceptional predictive performance of Average Chewing Speed is particularly noteworthy, which emerged as the most sensitive parameter for evaluating masticatory efficiency. And the results of multivariate analysis and the area under the ROC curve indicate a strong association between Average Chewing Speed and masticatory efficiency.
Studies have reported that an increase in the Average Chewing Speed can enhance food refinement and promote uniform mixing [18, 19], thereby improving mastication efficiency. The increase in chewing speed decreases the dwell time of food in the oral cavity, leading to a more rapid and uninterrupted chewing motion. This results in more comprehensive exposure of the food to saliva, enhancing the blending of enzymes in the saliva with the food. Maintaining a consistent chewing speed also enhances the activation of the masticatory muscles, improves chewing force, and facilitates better food processing and fragmentation. Therefore, the Average Chewing Speed of NPC patients could be an indicator of their overall chewing ability.
This study further uncovers an abnormal movement pattern in NPC patients: some patients exhibit a negative correlation between increased speed and decreased efficiency. Specifically, tongue atrophy impairs the ability to effectively propel food during mastication. Patients compensate through a technique termed “quick empty chewing,” yet due to insufficient tongue coordination, food tends to accumulate in the anterior region of the mouth. Some patients adopt a “high-frequency, low-power” exercise regimen as a compensatory mechanism. Nevertheless, although this approach increases chewing speed, the actual grinding force remains inadequate. This study identified that the average chewing speed was the most sensitive parameter for predicting impaired masticatory efficiency (with a significantly higher maximum AUC compared to other indicators), thereby offering a novel clinical indicator for assessing the masticatory efficiency of NPC patients.
Evaluation methods of masticatory movement and masticatory efficiency
This study is based on facial recognition and tracking the movement of the target points to obtain accurate and detailed information about jaw movement displacement, speed, and time during chewing [20]. The application of this technique has been instrumental in assessing the temporal characteristics of jaw movement and detecting impairments in mandibular control in neurodegenerative diseases [9, 21, 22]. Studies have observed a decrease in jaw movement speed preceding changes in speech rate and speech articulation among ALS patients [23, 24]. The jaw movement was evaluated through the motion analysis of mandibular landmarks. It is worth to note that the unmarked facial recognition and tracking system software used in this study is superior to previous research methods: firstly, as it does not require placing markers on the face that interfere with lip movement; secondly, using headgear to track the marker makes it difficult to chew normally. Furthermore, motion capture enables the detection of small, subtle movements that are typically not easily discerned, which is more sensitive to alterations compared to observation-based assessments. Through our results, the recorded chewing data were stable and discernible.
Masticatory efficiency was used as a metric to objectively assess the participants'ability to chew and quantify the actual chewing function [12]. The two-color chewing gum, which had been utilized in previous studies [12], was employed for evaluation to compare the degree of color mixing in chewing products. This measure is widely utilized as a simple yet effective indicator of masticatory efficiency when screening patients with masticatory disorders [25]. Compared to traditional chewing, chewing gum is more widely accepted by people and may lead to increased unconscious chewing behavior [26]. Additionally, chewing gum is less likely to be accidentally swallowed, preventing the risk of choking and aspiration. Previous studies used rice crackers as the testing material; however, this can present challenges in gathering crushed samples post-chewing, especially when dealing with tiny particles and less mobile or sensitive oral structures. The advantage of this measure is that it allows for checking whether the bolus has been sufficiently formed.
In addition, patients with nasopharyngeal carcinoma were included in this study, whereas prior research on mandibular movement and masticatory efficiency has predominantly focused on healthy individuals or patients with common masticatory dysfunctions, such as dentition defects or temporomandibular joint disorders. By incorporating this specific population, this study elucidates the clinical significance of disease-specific alterations in mandibular motor parameters and introduces novel quantitative metrics for evaluating tumor recovery.
Limitations
This study presents some limitations. Firstly, the sample size of this study is small, which may have resulted in underpowered analysis, and the recruited patients showed variable chewing function, and suffered different method of radiotherapy. Secondly, the video collected in this study was shot in a controlled and standardized environment; however, we did not assess the predictive performance under different environmental conditions or lighting sources. Thus, potential measurement biases might compromise the integrity of our findings and limit their generalizability. Third, the current study confined its analysis to a single food item, and it remains inconclusive whether other foods might exhibit similar outcomes. Additionally, the experiment was only conducted once, resulting in limited verification. Further validation is required for these findings due to the excessively cautious nature of this approach. In future, a more comprehensive analysis could be achieved by expanding the sample size and incorporating long-term follow-up data.
Conclusions
The quantitative information on mandibular movement in NPC presented in this study may contribute to the identification and measurement of masticatory disorders. NPC patients were compared with healthy participants to elucidate the lower speed and longer duration of mandibular movement. The Average Chewing Speed of chewing is a highly sensitive kinematic indicator for evaluating mastication efficiency. Furthermore, the Average Chewing Speed of chewing could serve as a screening test for patients with mastication disorder of nasopharyngeal cancer and provide dietary guidance for such individuals.
Acknowledgements
The authors would like to thank the medical staff of the Rehabilitation Department of the Third Affiliated Hospital of Sun Yat-sen University for their support and assistance to this research.
Abbreviations
- NPC
Nasopharyngeal carcinoma
- VFSS
Videofluoroscopic swallowing study
- FOIS
Functional Oral Intake Scale
- AUC
Area under the curve
- ROC
Receiver operating characteristic
Author contributions
All authors contributed to the study conception and design. Conceptualization: [WXM], [XCQ]. Methodology: [WXM], [YC], [WZH]. Software design: [ZYSY], [WLF]. Assessment: [XCQ], [ZF]. Data acquisition and analysis: [YC], [ZF]. Writing-original draft preparation: [YC], [WZH]. Writing-review and editing: [YC], [WZH], [WZH]. Funding acquisition: [WXM]. Supervision: [WXM].
Funding
This study was funded by Youth Fund of the National Natural Science Foundation of China (Grant No: 81802236).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Third Affiliated Hospital of Sun Yat-sen University ([2021]02-200-01) and was conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for publication
All subjects and/or their legal guardian(s) have given informed consent for their identifying information/images to be published in online open-access publications.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chen Yang and Zhenhai Wei have contributed equally to this work.
References
- 1.Wang YH, Cheng HZ, Liu K, Cai BL, Luo Y, Kan D, et al. Clinical therapeutic effects of acupuncture in treating patients with dysphagia after radiotherapy in nasopharyngeal carcinoma: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021;100(26): e26410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang ZQ, Feng XD, Ge CL, Yang Y, Liang N, Ye Q, et al. The long-term survival of the doublet regimen of concurrent chemoradiation therapy for locoregionally advanced nasopharyngeal carcinoma: a retrospective study. Radiat Oncol. 2022;17(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Toledo IP, Pantoja LLQ, Luchesi KF, Assad DX, De Luca CG, Guerra ENS. Deglutition disorders as a consequence of head and neck cancer therapies: a systematic review and meta-analysis. Support Care Cancer. 2019;27(10):3681–700. [DOI] [PubMed] [Google Scholar]
- 4.Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):199–212. [DOI] [PubMed] [Google Scholar]
- 5.Bandini A, Rezaei S, Guarin DL, Kulkarni M, Lim D, Boulos MI, et al. A new dataset for facial motion analysis in individuals with neurological disorders. IEEE J Biomed Health Inform. 2021;25(4):1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearney E, Giles R, Haworth B, Faloutsos P, Baljko M, Yunusova Y. Sentence-level movements in Parkinson’s disease: loud, clear, and slow speech. J Speech Lang Hear Res. 2017;60(12):3426–40. [DOI] [PubMed] [Google Scholar]
- 7.Bologna M, Berardelli I, Paparella G, Marsili L, Ricciardi L, Fabbrini G, et al. Altered kinematics of facial emotion expression and emotion recognition deficits are unrelated in Parkinson’s disease. Front Neurol. 2016;7:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandini A, Namasivayam A, Yunusova Y. Video-based tracking of jaw movements during speech: preliminary results and future directions. Interspeech 2017. 2017. p. 689–93.
- 9.Guarin DL, Dempster A, Bandini A, Yunusova Y, Taati B. Estimation of orofacial kinematics in Parkinson’s disease: comparison of 2D and 3D markerless systems for motion tracking. 2020 15th IEEE international conference on automatic face and gesture recognition (FG 2020)2020. p. 540–3.
- 10.Bandini A, Orlandi S, Giovannelli F, Felici A, Cincotta M, Clemente D, et al. Markerless analysis of articulatory movements in patients with Parkinson’s disease. J Voice. 2016. 10.1016/j.jvoice.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Halazonetis DJ, Schimmel M, Antonarakis GS, Christou P. Novel software for quantitative evaluation and graphical representation of masticatory efficiency. J Oral Rehabil. 2013;40(5):329–35. [DOI] [PubMed] [Google Scholar]
- 12.Campos Sugio CY, Mosquim V, Jacomine JC, Zabeu GS, de Espíndola GG, Bonjardim LR, et al. Impact of rehabilitation with removable complete or partial dentures on masticatory efficiency and quality of life: a cross-sectional mapping study. J Prosthet Dent. 2022;128(6):1295–302. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Y, Pan J, Chen Y, Lin S, Zong J, Chen Y, et al. The prognosis of nasopharyngeal carcinoma involving masticatory muscles: a retrospective analysis for revising T subclassifications. Medicine (Baltimore). 2015;94(4): e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang M, Zhou P, Liao X, Xu M, Wang R. Prognostic value of masticatory muscle involvement in nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Oral Oncol. 2017;75:100–5. [DOI] [PubMed] [Google Scholar]
- 15.Unno M, Shiga H, Kobayashi Y. The relationship between masticatory path pattern and masticatory efficiency in gumi-jelly chewing. Nihon Hotetsu Shika Gakkai Zasshi. 2005;49(1):65–73. [DOI] [PubMed] [Google Scholar]
- 16.Kosaka T, Kida M, Kikui M, Hashimoto S, Fujii K, Yamamoto M, et al. Factors influencing the changes in masticatory performance: the Suita study. JDR Clin Trans Res. 2018;3(4):405–12. [DOI] [PubMed] [Google Scholar]
- 17.Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873–9. [DOI] [PubMed] [Google Scholar]
- 18.Woda A, Foster K, Mishellany A, Peyron MA. Adaptation of healthy mastication to factors pertaining to the individual or to the food. Phys Behav. 2006;89(1):28–35. [DOI] [PubMed] [Google Scholar]
- 19.Miquel-Kergoat S, Azais-Braesco V, Burton-Freeman B, Hetherington MM. Effects of chewing on appetite, food intake and gut hormones: a systematic review and meta-analysis. Phys Behav. 2015;151:88–96. [DOI] [PubMed] [Google Scholar]
- 20.Green JR, Wilson EM, Wang YT, Moore CA. Estimating mandibular motion based on chin surface targets during speech. J Speech Lang Hear Res. 2007;50(4):928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson EM, Green JR, Weismer G. A kinematic description of the temporal characteristics of jaw motion for early chewing: preliminary findings. J Speech Lang Hear Res. 2012;55(2):626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simione M, Wilson EM, Yunusova Y, Green JR. Validation of clinical observations of mastication in persons with ALS. Dysphagia. 2016;31(3):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green JR, Yunusova Y, Kuruvilla MS, Wang J, Pattee GL, Synhorst L, et al. Bulbar and speech motor assessment in ALS: challenges and future directions. Amyotroph Later Scler Front Degener. 2013;14(7–8):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yunusova Y, Green JR, Lindstrom MJ, Ball LJ, Pattee GL, Zinman L. Kinematics of disease progression in bulbar ALS. J Commun Disord. 2010;43(1):6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schimmel M, Leemann B, Herrmann FR, Kiliaridis S, Schnider A, Müller F. Masticatory function and bite force in stroke patients. J Dent Res. 2010;90(2):230–4. [DOI] [PubMed] [Google Scholar]
- 26.Schimmel M, Christou P, Miyazaki H, Halazonetis D, Herrmann FR, Muller F. A novel colourimetric technique to assess chewing function using two-coloured specimens: validation and application. J Dent. 2015;43(8):955–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.