Abstract
The human immunoglobulin M (IgM) monoclonal antibody (MAb) 2E9 binds the glucuronoxylomannan (GXM) of Cryptococcus neoformans serotypes A, B, and D. This study was undertaken to determine the opsonic efficacy of 2E9 and its ability to promote the antifungal activity of human polymorphonuclear neutrophils (PMNs) against C. neoformans. We incubated purified PMNs with fluorescein isothiocyanate-labeled C. neoformans cells that were treated with the GXM IgM 2E9, IgM antibodies that do not bind GXM, and rabbit and human factor-B-deficient serum as complement sources. PMN-associated C. neoformans cells fluoresced and were detected with a fluorescence-activated cell sorter. The amount of phagocytosis was defined as the percent fluorescing PMNs, which was 37% for yeast cells opsonized with 2E9 plus rabbit serum and 57% for yeast cells opsonized with 2E9 plus factor-B-deficient serum. Phagocytosis was significantly greater for yeast cells that were treated with 2E9 plus a complement source than for yeast cells treated with the complement sources alone or treated with the control IgMs alone or with the complement sources. Fluorescence quenching and light and electron microscopy of the phagocytosis mixtures revealed that 2E9-opsonized yeast cells were internalized by PMNs. Maximal inhibition of C. neoformans growth occurred when PMNs were cocultured with yeast cells that were opsonized with 2E9 plus a complement source. Our data demonstrate that the human GXM IgM 2E9 can mediate PMN phagocytosis and C. neoformans growth inhibition in vitro. These findings strongly suggest that antibody-mediated deposition of complement components on the cryptococcal capsule can augment PMN complement receptor-mediated antifungal activity. Antibody activation of complement-mediated effector cell antifungal mechanisms may play a role in host defense against cryptococcosis and represents a goal for the use of MAbs to treat or prevent human C. neoformans infections.
Cryptococcus neoformans is an opportunistic fungus that causes meningoencephalitis in 6 to 8% of individuals with AIDS (15). The prognosis of cryptococcosis in these patients is poor because available antifungal agents fail to eradicate their infections. We and others are exploring approaches to therapy that may augment host immunity against C. neoformans. A human monoclonal antibody (MAb), 2E9, that binds the glucuronoxylomannan (GXM) of C. neoformans serotypes A, B, and D was generated from the peripheral-blood lymphocytes of a volunteer who received the investigational glycoconjugate vaccine, GXM-TT (19, 20). This immunoglobulin M (IgM) expresses heavy-chain variable-region idiotypes that are shared by both naturally occurring and GXM-TT-elicited antibodies (28, 45). The GXM epitope specificity of 2E9 is not known. However, a peptide epitope selected by 2E9 from a random phage peptide library inhibits its GXM binding, suggesting that it mimics part of the structure of the 2E9 GXM binding site (53). This peptide mimotope also partially inhibits the GXM binding of GXM-TT-elicited and naturally occurring antibodies from human immunodeficiency virus (HIV)-negative individuals (53), supporting the conclusion that these GXM antibodies and the GXM MAb 2E9 share epitope specificities.
Murine MAbs against GXM can modify the course of experimental cryptococcal infection, and individual MAbs can be either protective, nonprotective, or enhancing, based on their specificity and/or isotype (44), whereas the protective efficacy of polyclonal sera from experimental animals has been inconsistent and unpredictable (12). Murine and lapine antibodies to cryptococcal polysaccharide can enhance the activity of various effector cells against C. neoformans (34, 41). Similarly, polyclonal immune-serum antibodies from some volunteer recipients of GXM-TT (19, 20) can opsonize and promote mononuclear-cell phagocytosis of C. neoformans (54). However, there are marked differences in opsonization between sera from different individuals, and preimmune antibodies are not opsonic (30, 54). These findings may be attributable to the fact that polyclonal sera are heterogeneous with respect to antibody concentration and affinity, and the amounts of antibody used may have been insufficient for biological activity. Therefore, the interpretation of all previous studies addressing the opsonic potential of human sera has been hampered by the use of polyclonal sera (13).
A role for human antibodies in host defense against C. neoformans is supported by some studies (22, 44), although most suggest that complement is the most critical opsonin for immunity to C. neoformans (30, 33). The availability of 2E9 permitted us to investigate the in vitro biological activity of a monospecific GXM antibody that has an epitope specificity and molecular structural features similar to those of naturally occurring serum antibodies and opsonic GXM-TT-elicited antibodies (28, 53). We examined the opsonic efficacy of 2E9 and its ability to promote human polymorphonuclear-neutrophil (PMN) antifungal activity against C. neoformans. PMNs were chosen for study because they mediate phagocytosis and killing of C. neoformans and other pathogenic yeasts (3, 23, 35, 51), their antifungal activity can be superior to that of mononuclear cells (16, 40), and some have proposed that PMNs might play a significant role in host defense against cryptococcosis (35, 40).
MATERIALS AND METHODS
PMN isolation.
Whole blood was obtained from healthy adult volunteers. The protocol for blood donation was approved by the Institutional Review Board of the Albert Einstein College of Medicine, and each donor provided informed consent for venipuncture. PMNs were isolated from whole-blood samples after the mononuclear cells and platelets were removed by density gradient centrifugation over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). The erythrocytes were lysed by treatment with ice-cold isotonic lysing buffer (0.155 M NH4Cl, pH 7.4). The remaining PMNs were washed twice with Hanks’ balanced salt solution (Gibco, Grand Island, N.Y.) and suspended in RPMI 1640 (Gibco) supplemented with 10% fetal calf serum (Harlan Bioproducts for Science, Indianapolis, Ind.).
Yeast cells.
C. neoformans serotype D (strain 24067) was cultured on Sabouraud dextrose agar plates (Difco, Detroit, Mich.) at 30°C and then subcultured to obtain discrete colonies. This strain was chosen because it has been extensively studied in murine-antibody protection studies (41). Serotype-D strains are clinically important and comprise the majority of isolates in some European countries (2, 27). A single yeast colony was transferred to Sabouraud dextrose broth (Difco) and cultured for 72 h at 30°C. The yeast cells were washed three times with phosphate-buffered saline (PBS) and then diluted in the desired medium.
FITC labeling of C. neoformans.
Fluorescein isothiocyanate (FITC) labeling was performed according to published methods (25). Heat-killed yeast cells were washed three times with PBS and incubated for 1 h at room temperature (RT) with 0.1 mg of FITC Isomer I (Sigma Chemical Corporation, St. Louis, Mo.)/ml in 0.1 M NaHCO3 (pH 9.0). The yeast cells were washed three times with PBS, counted in a hemocytometer, and suspended in PBS at a density of 108 yeast cells/ml. Aliquots of the yeast suspension were placed into sterile microcentrifuge tubes and stored at −70°C. FITC-labeled yeast cells were indistinguishable by enzyme-linked immunosorbent assay (ELISA) from unlabeled C. neoformans with respect to the binding of GXM antibodies (data not shown). This was determined as follows. ELISA plates were coated with 1 μg of MAb 2E9/ml, incubated at 37°C for 1 h with dilutions of FITC-labeled and unlabeled heat-killed C. neoformans organisms, and then washed and reincubated with the murine anti-GXM MAb 2H1 (provided by A. Casadevall, Albert Einstein College of Medicine) for 1 h at 37°C. The plates were washed, incubated with alkaline phosphatase-labeled goat anti-mouse IgG (Sigma), washed again, and developed with p-nitrophenylphosphate substrate (Sigma). The optical density (OD) of each of the wells was read at an absorbance of 405 nm in a Ceres 900 ELISA reader (BioTek Instruments, Inc., Winooski, Vt.).
Phagocytosis assay.
FITC-labeled C. neoformans cells (107) were incubated with either 25 μg of 2E9/ml (45), a human myeloma IgM antibody (Calbiochem, San Francisco, Calif.), or a human IgM rheumatoid-factor MAb, RC2 (provided by A. Davidson, Albert Einstein College of Medicine), with or without the complement sources listed below. MAb 2E9 was purified from culture supernatants from a human lymphoblastoid cell line by affinity chromatography with a protein A column based upon the ability of its variable region (VH3) to bind protein A (45). The myeloma antibody and RC2 were tested for staphylococcal protein A (SPA) and GXM binding by ELISA (45). The myeloma antibody bound SPA, indicating that, like 2E9, it is likely to use a VH3 gene element (45, 49), but RC2 did not bind SPA, and neither antibody bound GXM (data not shown). Two complement sources were used in these experiments: baby rabbit serum (Pel-Freez Clinical Systems, Brown Deer, Wis.) and human serum deficient in factor B (Calbiochem). For some experiments, these sera were heated for 30 min at 50°C to inactivate complement components. The alternative complement pathway is inactive in factor-B-deficient serum. Therefore, we chose this complement source to investigate 2E9-mediated classical-pathway activation and human complement-dependent, antibody-mediated opsonization of C. neoformans.
FITC-labeled yeast cells were incubated with each antibody and heated and unheated complement sources for 30 min at 37°C in a shaking water bath, and then the yeast cells were added to 106 freshly isolated PMNs. Each reaction mixture contained 106 PMNs and 107 C. neoformans cells; the effector-to-target ratio (E/T ratio) was 1:10. The phagocytosis mixtures were incubated for 30 min at 37°C, and then 1 ml of ice-cold 0.9% NaCl containing 0.02% EDTA (Sigma) was added. The samples were analyzed by fluorescence-activated cell sorter (FACS) at the FACS Facility of the Cancer Center at the Albert Einstein College of Medicine. Flow cytometry analysis was performed on a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, Calif.), and LYSYSII software was used for data acquisition and analysis. To ensure sufficient numbers of PMNs for analysis, an acquisition gate was created around the PMN cluster based on a bivariate plot, combining forward light scatter and wide-angle scatter. For most experiments, a minimum of 10,000 PMNs were analyzed based on this scatter gate. Logarithmic amplification was used to measure FITC fluorescence. For some experiments, a FACStar PLUS was used for cell sorting of FITC-positive PMNs. For both analysis and sorting, 488-nm excitation was used, and FITC fluorescence was measured with a 530/30-nm band-pass filter. The yeast cells that were attached to the PMNs were discriminated from those that had been internalized by repeating the FACS analysis after the addition of a 2-mg/ml crystal violet solution, which quenches extracellular fluorescence (4). The FACS method used determines the phagocytosis of heat-killed yeast cells. This is an accepted method for studying the phagocytosis of fungi, including C. neoformans and other capsulated pathogens (5, 14, 18, 25). With this method, phagocytosis is reported as the percentage of the total number of PMNs that fluoresce after incubation with FITC-labeled yeast cells. The PMNs that are associated with FITC-labeled yeast cells are identified by their size and their acquisition of a fluorescent appearance. This was determined from the FACS analysis scatter plots as described previously (46).
Inhibition of C. neoformans growth.
C. neoformans cells (104) were treated for 30 min at 37°C with either PBS, 25 μg of the myeloma IgM (Calbiochem)/ml, or 2E9, and 10% heated or unheated baby rabbit serum (Pel-Freez) or 10% factor-B-deficient human serum (Calbiochem). For each experimental condition, C. neoformans cells (104) that were treated with PBS and each of the complement sources and antibodies detailed above were added to freshly isolated human PMNs (104 or 105) and cocultured for 2 h at 37°C. After incubation, the cocultures were pelleted by centrifugation, and the cells were suspended in distilled water for 20 min at 37°C and then lysed by vigorous vortexing. After microscopic confirmation that the cells had been lysed, the lysates were plated on Sabouraud dextrose agar plates (Difco) and incubated for 72 h at RT. Microscopic examination of the surface revealed that each colony contained single cells, indicating that this method successfully disrupted aggregates. The efficacy of this procedure was also confirmed by plating yeast cells that had been incubated with 2E9 alone. Therefore, reductions in CFUs were not a result of insufficient cell lysis or artifactual reduction of CFUs by antibody-mediated agglutination. The C. neoformans CFUs on each plate were counted (each CFU was counted as one colony). Each experimental condition was performed in duplicate, and three replicate plates were used to determine the CFUs from each independent experiment. Experiments with rabbit serum were performed four times, and the experiments with factor-B-deficient serum were performed three times, with cells from two different donors.
2E9-mediated complement activation.
ELISAs were performed to determine if 2E9 could activate complement and deposit C3 on GXM. Polystyrene plates (Corning Glass Works, Corning, N.Y.) were coated with 10 μg of serotype-D GXM (strain 24067)/ml or 1011 φ13 (a GXM peptide mimotope expressed by filamentous phage) particles/ml according to published techniques (45, 53). The following reaction mixtures were incubated for 30 min at RT and then incubated with the ELISA plates for 1 h at 37°C: (i) 7 μg of 2E9/ml and 10% rabbit serum (Pel-Freez Clinical Systems), (ii) 10% rabbit serum, (iii) 7 μg of 2E9/ml, 10% rabbit serum, and 20 μg of GXM/ml, and (iv) 10% rabbit serum and 20 μg of GXM/ml. After incubation, the plates were washed, incubated with goat anti-human C3 (Sigma) for 1 h at 37°C, washed again, and then incubated with alkaline phosphatase-labeled rabbit anti-goat IgG (Sigma) for 1 h at 37°C. After washing, the plates were developed with p-nitrophenylphosphate substrate (Sigma). Rabbit C3 is recognized by goat anti-human C3 (Sigma). The OD of each of the wells was read at an absorbance of 405 nm in a Ceres 900 ELISA reader (BioTek Instruments, Inc.).
Transmission electron microscopy.
Samples were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, postfixed with 1% osmium tetroxide followed by 1% uranyl acetate, dehydrated through a graded series of ethanol, and embedded in LX112 resin (LADD Research Industries, Burlington, Vt.). Ultrathin sections were cut on a Reichert Ultracut E, stained with uranyl acetate followed by lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope at 80 kV. Parallel experiments were performed with FITC-labeled and unlabeled yeast cells. For the studies with live, unlabeled C. neoformans cells, sections were made after 5- and 30-min incubations of 25 μg of 2E9/ml, 10% rabbit serum, and C. neoformans cells as detailed in “phagocytosis assay” above. For the studies with FITC-labeled yeast cells, the PMNs that displayed FITC fluorescence were obtained for sections by cell sorting using the FACS as detailed above.
Statistical analysis.
Statistical analysis of the phagocytosis and growth inhibition data was performed with the nonparametric Mann-Whitney U test, by using Statistica for Windows 4.3 software (StatSoft, Inc.).
RESULTS
Microscopy.
Light and fluorescent microscopy (data not shown) and transmission electron microscopy each revealed that yeast cells opsonized by 2E9 plus rabbit serum were internalized by human PMNs. Transmission electron microscopy was performed with two groups of samples: (i) FITC-labeled PMNs that were obtained by FACS sorting and (ii) PMNs that were cocultured with live yeast cells treated with 2E9 plus rabbit serum and fixed for sectioning at 0, 5, and 30 min after initiation of the cocultures. The ultrastructural studies revealed that some PMNs had two intracellular yeast cells, but most had only one, like that shown in Fig. 1d. The yeast cells being internalized were surrounded by a ruffled membrane (Fig. 1b). The internalized yeast cells were membrane bound, and in some instances their vacuoles were such that there was a large amount of space around the yeast cell (Fig. 1c). Such vacuoles may have been loose, or larger, to accommodate a cell with a larger capsule (compare Fig. 1c and d). In Fig. 1c, a small group of vesicles can be seen at the junction of the yeast cell and the cell membrane.
FIG. 1.
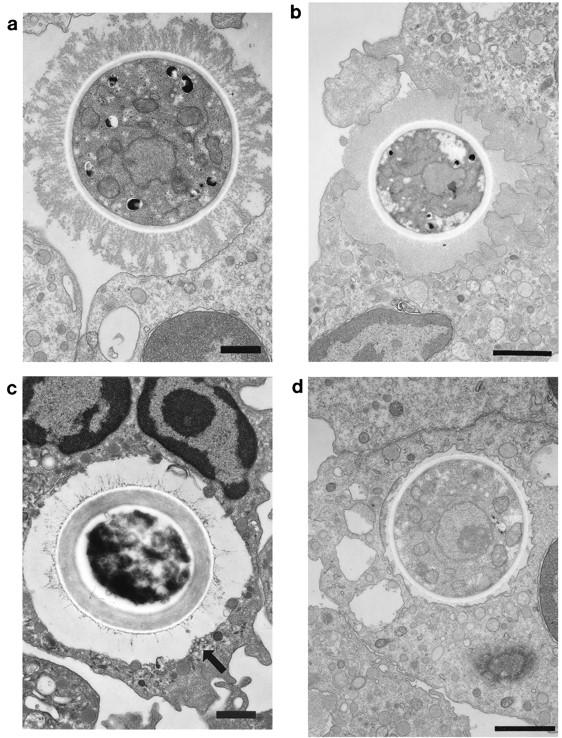
Transmission electron micrographs of sections from human PMNs incubated with 2E9- and rabbit complement-treated C. neoformans cells and processed immediately (a) or cocultured for 5 (b and c) or 30 (d) min. Bars, 1 μm. (a) Human PMN with attached C. neoformans cell. The yeast cell is seen making contact with the PMN membrane. There are no pseudopodia. (b) The yeast cell shown appears to be entering a PMN loosely surrounded by ruffled membrane from the PMN. (c) The yeast cell is seen within the cell surrounded by a large vacuolar membrane. The arrow points to a group of small vesicles beneath the vacuole. (d) A membrane-bound yeast cell is seen in a vacuole. The vacuolar membrane is tighter than that shown in panel c. FACS-separated PMNs that fluoresced had an appearance similar to that of the cells shown.
The electron-microscopic appearance of C. neoformans internalization at the time points selected for study was as follows: at 0 min (immediately after the addition of PMNs to the opsonized yeast cells), the yeast cells were attached to the PMN surface (Fig. 1a); at 5 min, the yeast cells were seen surrounded by the cell membrane as if they had fallen into the cell without being engulfed by pseudopodia (Fig. 1b); and at both 5 and 30 min, the yeast cells were observed enclosed in vacuoles (Fig. 1c and d). We observed no differences in PMN ultrastructure or yeast cell morphology between the phagocytosis mixtures that contained PMNs with live yeast cells and FACS-sorted PMNs with FITC-labeled yeast cells. However, the morphology of the capsules of individual yeast cells was heterogeneous (see Fig. 1a, c, and d).
Phagocytosis of C. neoformans cells by human PMNs.
In preliminary phagocytosis experiments, 2E9 concentrations of 5, 10, and 50 μg/ml plus 10% rabbit serum were evaluated. The level of phagocytosis for 5 μg/ml was 24%; for 10 μg/ml it was 32%, and for 50 μg/ml it was 26%. We subsequently performed experiments with 25 μg of 2E9/ml and found that the average level of phagocytosis produced by this concentration was 30%. Therefore, we chose 25 μg/ml as the concentration for further experiments. No statistical differences in the percentage of PMNs phagocytosing C. neoformans cells were observed between the myeloma antibody plus unheated rabbit serum and the myeloma antibody plus heat-inactivated rabbit serum (Table 1), and the percent PMNs associated with FITC-labeled C. neoformans cells was significantly greater in the presence of 2E9 plus yeast cells treated with unheated rabbit serum (Table 1). Phagocytosis of FITC-labeled C. neoformans cells was not different statistically for unheated and heat-inactivated rabbit serum, 2E9, myeloma antibody, and myeloma antibody plus unheated or heat-inactivated rabbit serum (Table 2). The fluorescence of extracellular yeast cells can be quenched by crystal violet (5), and we used this method to distinguish attached from intracellular yeast cells. These experiments demonstrated that 69% of C. neoformans cells treated with rabbit serum, 82% of cells treated with myeloma antibody plus rabbit serum, and 100% of cells treated with 2E9 plus rabbit serum remained fluorescent after quenching. This indicated that the majority of PMN-associated C. neoformans cells were intracellular.
TABLE 1.
Comparison of the percent PMNs phagocytosing C. neoformans in the presence of 2E9 and human myeloma IgMa
| Serum | % PMNs fluorescing with:
|
Pb | |
|---|---|---|---|
| 2E9 | Myeloma IgM | ||
| Unheated rabbit serum | 34.26 ± 3.43 | 10.52 ± 2.78 | 0.00032 |
| Heated rabbit serum | 6.89 ± 3.43 | 7.04 ± 1.76 | 0.85 |
| Pc | 0.0004 | 0.1151 | |
The E/T ratio for these experiments was 1:10 (PMNs to yeast cells). Each of the experiments for which results are reported was performed five times. P values were determined by the Mann-Whitney U test.
For 2E9 versus the myeloma antibody.
For heated versus unheated rabbit serum.
TABLE 2.
FACS analysis of phagocytosis of C. neoformans by PMNs cocultured with IgM antibodies and rabbit and human complement sourcesa
| Serum and/or antibody | Phagocytosis (% FITC-labeled PMNs) |
|---|---|
| None | 3.41 ± 1.02 |
| 10% heated rabbit serum | 5.98 ± 2.28 |
| 10% unheated rabbit serum | 10.52 ± 2.66 |
| Myeloma IgM | 5.03 ± 1.89 |
| Myeloma IgM + 10% unheated rabbit serum | 10.52 ± 2.78 |
| Myeloma IgM + 10% heated rabbit serum | 7.04 ± 1.76 |
| 2E9 | 4.52 ± 2.48 |
| 2E9 + 10% unheated rabbit serum | 37.04 ± 9.76b |
| 2E9 + 10% heated rabbit serum | 5.71 ± 3.99 |
| 10% factor-B-deficient serum | 12.85 ± 4.34 |
| 2E9 + 10% factor-B-deficient serum | 57.37 ± 21.61c |
| RC2 + 10% factor-B-deficient serum | 11.26 ± 5.23 |
The E/T ratio for these experiments was 1:10 (PMNs to yeast cells). Results for rabbit serum represent five different experiments, and those for factor-B-deficient serum represent three different experiments. P values were determined by the Mann-Whitney U test.
P < 0.01 versus heated rabbit serum.
P < 0.005 versus factor-B-deficient serum.
The alternative complement pathway is inactive in factor-B-deficient serum. Therefore, we chose this complement source to investigate 2E9-mediated classical-pathway activation. The percent phagocytosis was greater when the yeast cells were treated with both 2E9 and factor-B-deficient serum than when they were treated with factor-B-deficient serum alone or with RC2 (the human IgM rheumatoid-factor antibody) plus factor-B-deficient serum (Table 2). Taken together, the results of the phagocytosis experiments indicate that (i) 2E9 binding to C. neoformans cells activated the classical complement pathway in factor-B-deficient serum, (ii) both rabbit and human complement sources supported 2E9-mediated opsonization, and (iii) 2E9 enhanced PMN phagocytosis of C. neoformans.
Growth inhibition studies.
At an E/T ratio of 10:1, coculture of PMNs with yeast cells that had been treated with rabbit serum alone, 2E9 alone, 2E9 plus heated rabbit serum, and 2E9 plus unheated rabbit serum all resulted in reduced C. neoformans CFUs (Table 3). Coculture of PMNs with 2E9 and human factor-B-deficient serum produced a significant reduction in CFUs compared to CFUs obtained with human factor-B-deficient serum alone (Table 3). Additional comparisons between different growth inhibition conditions showed the following: 2E9 plus rabbit serum inhibited growth more than 2E9 alone (P < 0.01); 2E9 plus unheated rabbit serum inhibited growth more than heated rabbit serum alone (P < 0.0001); and 2E9 plus heated rabbit serum inhibited growth more than heated rabbit serum alone (P < 0.005). There was no statistical difference in growth inhibition between unheated rabbit serum alone and heated rabbit serum alone, between 2E9 alone and heated rabbit serum alone, and between 2E9 plus heated rabbit serum and 2E9 alone. Taken together, the results of the growth inhibition experiments indicated that growth inhibition was maximal when yeast cells were opsonized with 2E9 plus an unheated serum complement source.
TABLE 3.
C. neoformans CFUs after coculture of PMNs with 1,000 yeast cells treated with IgM antibodies plus rabbit or human complement sources
| Antibody and/or seruma | CFUb |
|---|---|
| None (PBS) | 727 ± 135 |
| Heated rabbit serum | 633 ± 77 |
| Unheated rabbit serum | 587 ± 47c |
| 2E9 | 541 ± 86c |
| 2E9 + heated rabbit serum | 502 ± 66d |
| 2E9 + unheated rabbit serum | 304 ± 90d |
| None (PBS) | 723 ± 42.9 |
| Factor-B-deficient serum | 723 ± 34.7 |
| Myeloma IgM + factor-B-deficient serum | 756 ± 42.7 |
| 2E9 + factor-B-deficient serum | 350 ± 65e |
Antibodies (2E9 and myeloma antibody) were used at concentrations of 25 μg/ml, and complement sources (unheated rabbit serum, heated rabbit serum, and human factor-B-deficient serum) were used at 10% concentrations. Results for rabbit serum represent three different experiments, and those for factor-B-deficient serum represent two experiments.
The E/T ratio was 10:1 (PMNs to yeast cells).
P < 0.01 versus PBS by the Mann-Whitney U test.
P < 0.005 versus PBS by the Mann-Whitney U test.
P < 0.005 for factor-B-deficient serum versus PBS by the Mann-Whitney U test.
ELISAs to determine 2E9 complement activation.
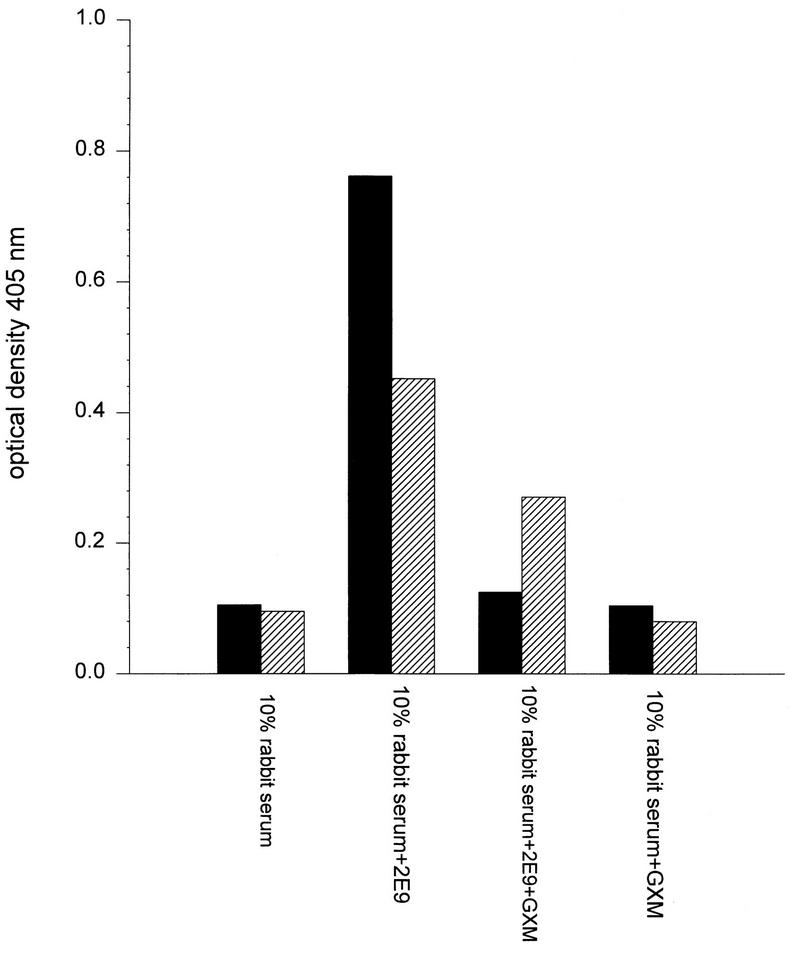
ELISAs demonstrated that 2E9 deposited C3 on solid-phase GXM and a phage expressing a peptide epitope that binds 2E9. Many studies have documented that serotype-D GXM can adhere to polystyrene ELISA plates (9–11, 26, 30). The greatest amount of C3 was detected on both antigens when 2E9 was included in the assay (Fig. 2). Soluble GXM inhibited 2E9 binding to both GXM and φ13 and led to decreased C3 detection, although the reduction in C3 binding to GXM was greater (Fig. 2). These experiments confirmed that 2E9 activation of rabbit complement generated C3 and led to its deposition on both GXM and a GXM phage mimotope.
FIG. 2.
ELISA determination of 2E9 complement activation and C3 deposition on GXM and φ13. C3 deposition is represented by the absorbance at 405 nm of wells that were incubated with goat anti-human C3 as described in the text. The OD at 405 nm is depicted on the y axis for each of the opsonins shown on the x axis. Solid bars, absorbances of GXM-coated wells; diagonally striped bars, absorbances of φ13-coated wells. Each bar represents the average of duplicate wells.
DISCUSSION
The results reported here are the first demonstration that a human IgM can enhance phagocytosis of C. neoformans. A major virulence factor of C. neoformans is its capsular polysaccharide GXM (24). The cryptococcal capsule can activate the alternative complement pathway (33, 44) and deposit complement component C3 at the capsular surface (38). Alternative-pathway complement activation by the cryptococcal capsule is reported to block classical-pathway activation at the cell wall (34). Generation of C3 by activation of the alternative pathway is slower and less efficient than C3 generation that occurs after capsular antibody-mediated activation of the classical complement pathway (36). These observations support the idea that antibody-dependent complement activation may have some advantages for host defense against C. neoformans. There is no known human effector cell IgM Fc receptor, and complement opsonins are required for IgM to mediate phagocytosis. Therefore, our studies strongly suggest that complexes of C. neoformans and the GXM IgM 2E9 enhanced complement receptor-mediated PMN phagocytosis of the opsonized yeast cells by activating complement in both serum sources, which resulted in yeast cell C3 deposition. Both complement sources were opsonic only when 2E9 was also present (Tables 1 and 2). The alternative pathway is inactive in factor-B-deficient serum. It is clear that in this complement source the classical complement pathway was activated by 2E9, although we cannot rule out the possibility that 2E9 activated the alternative pathway of rabbit serum. However, our results strongly suggest that the alternative pathway of rabbit serum is not significantly activated by the cryptococcal capsule (see rabbit serum data in Tables 1 and 2). Our data support the reports of others (33, 36) that an intact alternative pathway is required for phagocytosis of C. neoformans when capsular antibody is not present, because factor-B-deficient serum alone was not opsonic (Table 2).
IgM, a predominant isotype of human antibodies against capsulated pathogens (44), is more efficient in activating the classical complement pathway than other antibody isotypes. It opsonizes (32, 50, 52) and promotes effector cell and/or bactericidal activity against numerous pathogens (39, 48, 52) and has greater protective efficacy than IgG in some experimental infections (7). Pentameric IgM binding to capsular polysaccharides can alter capsular morphology (6) and expose additional capsular C3 binding sites (8, 52), which can facilitate C3 ligand binding to effector cell complement receptors (8, 29). Immune rabbit serum opsonization of C. neoformans has demonstrated that antibody-mediated C3 deposition occurs throughout the capsule and at the cell wall (34). Based on our studies, we propose that activation of the classical complement pathway by 2E9-C. neoformans immune complexes optimized C3 deposition on the yeast cells and promoted C3 ligand binding to PMN complement receptors. This is supported by our ELISAs demonstrating that in the presence of rabbit serum, 2E9 binding to solid-phase GXM and a phage expressing a GXM epitope resulted in C3 deposition on both of these antigens (Fig. 2). Inhibition of C3 deposition by soluble GXM in these experiments confirmed that C3 is deposited on the antigens and suggests that soluble 2E9-GXM complexes might be opsonized and cleared by effector cell complement receptors in vivo. Our ultrastructural studies reinforced the conclusion that PMN phagocytosis of 2E9-opsonized yeast cells was mediated by complement receptors, due to their striking similarity, and near identity, to published reports of mononuclear-cell complement receptor-mediated internalization of opsonized particles (1, 31). The latter, in comparison to Fc receptor-mediated mononuclear-cell internalization, is characterized by an absence of endocytosis at or above the cell membrane (see Fig. 1a), a ruffled phagosome membrane less tightly apposed to the opsonized particle (see Fig. 1b), and small vesicles beneath the vacuole (see Fig. 1c) which are proposed to play a role in phagosome enlargement (1). Therefore, our findings strongly suggest the hypothesis that, at the ultrastructural level, PMN complement-mediated internalization of opsonized yeast cells resembles that of mononuclear phagocytes.
The amount of phagocytosis we observed for 2E9-opsonized C. neoformans was in the same range as the amount of phagocytosis reported for murine-antibody-opsonized Streptococcus pneumoniae by the same FACS method (18). We did not stimulate the PMNs used in our studies as has been done by others (35). Therefore, the phagocytosis we observed probably reflects activation of PMN complement receptors by 2E9-C. neoformans complexes. The degree of phagocytosis for each condition is most likely a function of heterogeneous expression of complement receptors by primary PMNs, the nature of the 2E9-yeast cell complexes formed, and the efficiency of complement opsonin generation by these immune complexes from the serum sources used. Activation of rabbit and human C3 is reportedly similar (43), but rabbit serum does not contain carbohydrate antibodies. The explanation for the difference in phagocytosis between 2E9 plus factor-B-deficient serum and 2E9 plus rabbit serum (Tables 1 and 2) is unknown. The fact that factor-B-deficient serum was associated with more phagocytosis (Table 2) may reflect species differences, additional opsonic properties of GXM binding antibodies in human serum (17, 26, 30), or blockade of classical-pathway complement activation by the alternative pathway of rabbit serum.
The relationship between PMN phagocytosis of 2E9-opsonized yeast cells and C. neoformans growth inhibition was not specifically addressed in our studies. We, like others (41), used different E/T ratios for these experiments (see above). Our data clearly demonstrate that the degree of phagocytosis (Table 2) was correlated with the amount of growth inhibition of C. neoformans (Table 3). However, statistically significant growth inhibition was observed for rabbit serum alone, 2E9 alone, and 2E9 plus heated rabbit serum (Table 3), although statistically significant phagocytosis did not occur for these conditions (Table 2). This might be explained by differences in the E/T ratios used, the activity of mannose binding protein in the rabbit serum (37), or extracellular antifungal mechanisms (21, 42, 47). The purity of our PMN preparations was over 98% based on Wright’s staining. Although mononuclear-cell contamination could have contributed to extracellular C. neoformans growth inhibition (21, 42), our microscopic studies (data not shown) demonstrated that mononuclear cells were rarely, if ever, associated with yeast cells. The conditions used for the growth inhibition experiments were comparable to those reported by others to be optimal for phagocytosis of capsulated C. neoformans (34). The optimal ratio of yeast cells to opsonins was reported to be 125/μl, and in our experiments the ratio was approximately 100 yeast cells/μl of opsonins (complement and antibody). For FACS experiments, the ratio was nearly 500 times greater, which was necessary to achieve the maximum sensitivity of the method (5). However, when larger concentrations of 2E9 were used for the FACS experiments, there was not an increase in phagocytosis (see Materials and Methods). Our findings extend the idea that the number of binding sites for complement opsonins on the cryptococcal capsule is the limiting factor for antibody-dependent complement receptor-mediated biological antifungal activity (34). Taken together with the work of others (34), our data suggest that the ratio of yeast (target) cells to available opsonins is a crucial determinant of antibody-mediated antifungal activity and that it may be equal in importance to the E/T ratio.
In summary, our data demonstrate that the human GXM IgM 2E9 enhances human PMN antifungal activity. Our results support the conclusion that 2E9 activated the classical complement pathway in the serum sources used and enhanced the deposition of opsonic ligands on C. neoformans. Our previous work has shown that 2E9-like antibodies may be markedly decreased in the antibody repertoire of HIV-positive individuals, because their serum antibodies fail to bind a 2E9-selected GXM mimotope (53). Taken together with our present work, our studies of GXM antibody specificity support the idea that 2E9 binds a GXM epitope that may elicit protective capsular antibodies. The biological function of such antibodies, e.g., 2E9, may include activating the classical complement pathway, reversing the cryptococcal capsular blockade of complement activation (36), and enhancing the clearance of circulating yeast cells and soluble GXM by PMNs. Much remains to be learned about the relationship between in vitro and in vivo antibody-mediated biological activity and about the roles that human antibody specificity, idiotype, and isotype play in enhancing effector cell function against C. neoformans. The findings presented here provide the basis for future studies of additional human antibodies and a strategy for identifying the candidate antibodies that may enhance host defense against C. neoformans.
ACKNOWLEDGMENTS
This work was supported by NIH grant RO1AI35370 and by an award from the New York Community Trust for Blood Diseases.
We thank Arturo Casadevall for critical review of the manuscript.
REFERENCES
- 1.Allen L-A, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis by macrophages. J Exp Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett J E, Kwon-Chung K J, Howard D H. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am J Epidemiol. 1977;105:582–586. doi: 10.1093/oxfordjournals.aje.a112423. [DOI] [PubMed] [Google Scholar]
- 3.Bjerknes R. Flow cytometric assay for combined measurement of phagocytosis and intracellular killing of Candida albicans. J Immunol Methods. 1984;72:229–241. doi: 10.1016/0022-1759(84)90451-4. [DOI] [PubMed] [Google Scholar]
- 4.Bjerknes R, Bassoe C F. Human leukocyte phagocytosis of zymosan particles measured by flow cytometry. Acta Pathol Microbiol Immunol Scand Sect C. 1983;91:341–348. [PubMed] [Google Scholar]
- 5.Bjerknes R, Bassoe C F, Sjursen H, Laerum O D, Solberg C O. Flow cytometry for the study of phagocyte functions. Rev Infect Dis. 1989;11:16–24. doi: 10.1093/clinids/11.1.16. [DOI] [PubMed] [Google Scholar]
- 6.Bjornson A B, Detmers P A. The pentameric structure of IgM is necessary to enhance opsonization of Bacteroides thetaiotaomicron and Bacteroides fragilis via the alternative complement pathway. Microb Pathog. 1995;29:117–128. doi: 10.1006/mpat.1995.0051. [DOI] [PubMed] [Google Scholar]
- 7.Brown E J, Hosea S W, Hammer C H, Burch G, Frank M M. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J Clin Invest. 1982;69:85–98. doi: 10.1172/JCI110444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown E J, Joiner K A, Cole R M, Berger M. Localization of complement component 3 on Streptococcus pneumoniae: anticapsular antibody causes complement deposition on the pneumococcal capsule. Infect Immun. 1983;39:403–409. doi: 10.1128/iai.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall A, DeShaw M, Fan M, Dromer F, Kozel T R, Pirofski L. Molecular and idiotypic analysis of antibodies to Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62:3864–3872. doi: 10.1128/iai.62.9.3864-3872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casadevall A, Mukherjee J, Devi S J, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;165:1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 11.Casadevall A, Mukherjee J, Scharff M D. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- 12.Casadevall A, Scharff M D. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38:1695–1702. doi: 10.1128/aac.38.8.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadevall A, Scharff M D. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaka W, Scharringa J, Verheul A F M, Verhoef J, Van Strijp A G, Hoepelman I M. Quantitative analysis of phagocytosis and killing of Cryptococcus neoformans by human peripheral blood mononuclear cells by flow cytometry. Clin Diagn Lab Immunol. 1995;2:753–759. doi: 10.1128/cdli.2.6.753-759.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 16.Davies S F, Clifford D P, Hoidal J R, Repine J E. Opsonic requirements for the uptake of Cryptococcus neoformans by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1982;145:870–874. doi: 10.1093/infdis/145.6.870. [DOI] [PubMed] [Google Scholar]
- 17.DeShaw M, Pirofski L. Antibodies to Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan are ubiquitous in the serum of HIV+ and HIV− individuals. Clin Exp Immunol. 1995;99:425–432. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeVelasco E A, Dekker H A, Antal P, Jalink K P, van Strijp J A, Verheul A F, Verhoef J, Snippe H. Adjuvant Quil A improves protection in mice and enhances opsonic capacity of antisera induced by pneumococcal polysaccharide conjugate vaccines. Vaccine. 1994;12:1419–1422. doi: 10.1016/0264-410x(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 19.Devi S J. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine. 1996;14:841–844. doi: 10.1016/0264-410x(95)00256-z. [DOI] [PubMed] [Google Scholar]
- 20.Devi S J N, Schneerson R, Egan W, Ulrich T J, Bryla D, Robbins J B, Bennett J E. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991;59:3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond R D. Antibody-dependent killing of Cryptococcus neoformans by human peripheral blood mononuclear cells. Nature. 1974;247:148–150. doi: 10.1038/247148a0. [DOI] [PubMed] [Google Scholar]
- 22.Diamond R D, May J E, Kane M A, Frank M M, Bennett J E. The role of the classical and alternative complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974;112:2260–2270. [PubMed] [Google Scholar]
- 23.Diamond R D, Root R K, Bennett J E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972;125:367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- 24.Diamond R E. Cryptococcus neoformans. In: Mandell G, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 2331–2339. [Google Scholar]
- 25.Drevets D A, Campbell P A. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J Immunol Methods. 1991;142:31–38. doi: 10.1016/0022-1759(91)90289-r. [DOI] [PubMed] [Google Scholar]
- 26.Dromer F, Aucouturier P, Clauvel J, Saimot G, Yeni P. Cryptococcus neoformans antibody levels in patients with AIDS. Scand J Infect Dis. 1988;20:283–285. doi: 10.3109/00365548809032452. [DOI] [PubMed] [Google Scholar]
- 27.Dromer F, Mathoulin S, Dupont B, Letenneur L, Ronin O. Individual and environmental factors associated with infection due to Cryptococus neoformans serotype D. French Cryptococcosis Study Group. Clin Infect Dis. 1996;23:91–96. doi: 10.1093/clinids/23.1.91. [DOI] [PubMed] [Google Scholar]
- 28.Fleuridor R, Jefferis R, Mageed R, Pirofski L. Characterization of isotypes and idiotypes in sera from recipients of the GXM-TT vaccine, Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. [Google Scholar]
- 29.Hostetter M K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986;153:682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- 30.Houpt D C, Pfrommer T, Young B J, Larson T A, Kozel T R. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62:2857–2864. doi: 10.1128/iai.62.7.2857-2864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan G. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand J Immunol. 1977;6:797–806. doi: 10.1111/j.1365-3083.1977.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 32.Konishi E, Nakao M. Naturally occurring immunoglobulin M antibodies: enhancement of phagocytic and microbicidal activities of human neutrophils against Toxoplasma gondii. Parasitology. 1992;104:427–432. doi: 10.1017/s003118200006368x. [DOI] [PubMed] [Google Scholar]
- 33.Kozel T R. Opsonization and phagocytosis of Cryptococcus neoformans. Arch Med Res. 1993;24:211–218. [PubMed] [Google Scholar]
- 34.Kozel T R, Highison B A, Stratton C J. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984;43:574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozel T R, Pfrommer G S, Redelman D. Activated neutrophils exhibit enhanced phagocytosis of Cryptococcus neoformans opsonized with normal human serum. Clin Exp Immunol. 1987;70:238–246. [PMC free article] [PubMed] [Google Scholar]
- 36.Kozel T R, Wilson M A, Murphy J W. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun. 1991;59:3101–3110. doi: 10.1128/iai.59.9.3101-3110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhlman M, Joiner K, Ezekowitz A B. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laxalt K A, Kozel T R. Chemotaxigenesis and activation of the alternative complement pathway by encapsulated and nonencapsulated Cryptococcus neoformans. Infect Immun. 1979;26:435–440. doi: 10.1128/iai.26.2.435-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandrell R E, Azmi R H, Granoff D M. Complement-mediated bactericidal activity of human antibodies to poly alpha 2-8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- 40.Miller M F, Mitchell T G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991;59:24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee S, Lee S C, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nabavi N, Murphy J W. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect Immun. 1986;51:556–562. doi: 10.1128/iai.51.2.556-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peake P W, Charlesworth J A, Pussell B A. Activation of rabbit C3: studies of the generation of cleavage products in vitro and of their metabolism in vivo. Complement Inflammation. 1991;8:261–270. doi: 10.1159/000463195. [DOI] [PubMed] [Google Scholar]
- 44.Pirofski L, Casadevall A. Cryptococcus neoformans: paradigm for the role of antibody in immunity. Zentralbl Bakteriol. 1996;284:475–495. doi: 10.1016/s0934-8840(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 45.Pirofski L, Lui R, DeShaw M, Kressel A B, Zhong Z. Analysis of human monoclonal antibodies elicited by vaccination with a Cryptococcus neoformans glucuronoxylomannan capsular polysaccharide vaccine. Infect Immun. 1995;63:3005–3014. doi: 10.1128/iai.63.8.3005-3014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plasman N, Vray B. Quantification of bacterial phagocytosis by flow cytometry and spectrofluorimetry. J Immunol Methods. 1994;174:195–202. doi: 10.1016/0022-1759(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 47.Ramisse F, Binder P, Szatanik M, Alonso J. Passive and active immunotherapy for experimental pneumococcal pneumonia by polyvalent human immunoglobulin or F(ab′)2 fragments administered intranasally. J Infect Dis. 1996;173:1123–1128. doi: 10.1093/infdis/173.5.1123. [DOI] [PubMed] [Google Scholar]
- 48.Robbins J B, Schneerson R, Glode M P, Vann W, Schiffer M, Liu T Y, Parke J C, Huntley C. Cross-reactive antigens and immunity to diseases caused by encapsulated bacteria. J Allergy Clin Immunol. 1975;56:1387–1398. doi: 10.1016/0091-6749(75)90119-0. [DOI] [PubMed] [Google Scholar]
- 49.Sasano M, Burton D R, Silverman G J. Molecular selection of human antibodies with an unconventional bacterial B cell antigen. J Immunol. 1993;151:5822–5839. [PubMed] [Google Scholar]
- 50.Schlesinger L S, Horwitz M A. A role for natural antibody in the pathogenesis of leprosy: antibody in nonimmune serum mediates C3 fixation to the Mycobacterium leprae surface and hence phagocytosis by human mononuclear phagocytes. Infect Immun. 1994;62:280–289. doi: 10.1128/iai.62.1.280-289.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tacker J R, Farhi F, Bulmer G S. Intracellular fate of Cryptococcus neoformans. Infect Immun. 1972;6:162–167. doi: 10.1128/iai.6.2.162-167.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takayanagi T, Kawaguchi H, Yabu Y, Itoh M, Yano K. Inhibition of IgM antibody-mediated aggregation of Toxoplasma gambiense in the presence of complement. Experientia. 1992;48:1002–1006. doi: 10.1007/BF01919153. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Zhong Z, Pirofski L. Peptide epitopes recognized by a human anti-cryptococcal glucuronoxylomannan antibody. Infect Immun. 1997;65:1158–1164. doi: 10.1128/iai.65.4.1158-1164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong Z, Pirofski L. Opsonization of Cryptococcus neoformans by human antiglucuronoxylomannan antibodies. Infect Immun. 1996;64:3446–3450. doi: 10.1128/iai.64.9.3446-3450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]