Abstract
Well-proven mouse and rat models were used to show that polyclonal antisera to Pneumocystis carinii protect against P. carinii pneumonia. Antibodies were obtained from animals that were allowed to recover from severe P. carinii pneumonia after immunosuppression had been stopped and which then were given a booster injection of P. carinii from the same animal species. Mice immunosuppressed with corticosteroids or antibodies to L3T4+ lymphocytes (which are comparable to CD4 cells of humans) and transtracheally inoculated with mouse P. carinii did not develop P. carinii pneumonia if they were passively immunized with antiserum, while mice immunosuppressed and inoculated by identical procedures but not given antibodies developed severe infections. Rats immunosuppressed with corticosteroids and inoculated with rat P. carinii had less severe infections if they were given rat anti-P. carinii antisera. The polyclonal antisera developed in mice provided greater protection for the mice than the polyclonal rat antisera did for the rats; however, the potencies and compositions of the antisera were not quantitated and probably differed. Since both rats and mice can be protected from P. carinii infections with polyclonal antisera, it may be possible to develop vaccines that will elicit protective antibodies in humans.
Pneumocystis carinii is a leading cause of pneumonia in patients with immune deficiencies, including individuals infected with human immunodeficiency virus and those receiving chemotherapy for malignancies or for transplantation. Although antimicrobics are available for treatment and prophylaxis of P. carinii pneumonia (20), many patients have adverse reactions or fail to respond to the most effective antimicrobial agents (28). In addition, even with prophylaxis, P. carinii continues to be a major cause of illness (23, 24). If a vaccine could be developed that would prevent infections from developing, or reduce infections so that they would be mild or occur later in the course of AIDS, it would be of great clinical utility.
The role of host defenses in preventing the development of P. carinii pneumonia is not well defined. Since individuals with human immunodeficiency virus develop P. carinii pneumonia when their CD4 cell counts fall below 200, cell-mediated immunity has been considered the most important host defense. However, reports of P. carinii pneumonia in children often describe hypogammaglobulinemia or agammaglobulinemia as predisposing factors (7, 19, 23, 24, 30). The role of antibodies in preventing or controlling disease has not been well explained, although a study by Gigliotti and Hughes (13) reported a decrease in severity of P. carinii infection in ferrets given antibody to ferret P. carinii. Also, a study by Harmsen et al. (15) showed that immunized mice cleared P. carinii from their lungs and suggested the possibility that antibodies were responsible for protection. Studies by several researchers have demonstrated the role of T lymphocytes in controlling infections (10, 11, 14), but conclusive studies demonstrating the effects of antibodies have been lacking.
Since a great deal of work with P. carinii was carried out in latently infected rats immunosuppressed for long periods of time with corticosteroids, assessing the contributions of host defenses in the course of disease was not possible because corticosteroids suppress all lymphocytes, including T and B lymphocytes, and influence phagocytosis and inflammation. Inoculated animal models, used in this study, develop severe infections more rapidly and reproducibly than latently infected animals (1, 4). By using them, it has been possible to more clearly determine the effects of drugs on infections (2, 3), study immune responses during the development of infections (5), and test the usefulness of antiserum in providing protection. This report describes the use of polyclonal antisera from convalescent animals that had received a booster injection to prevent or diminish infections in these well-established models.
MATERIALS AND METHODS
Animal models.
To develop P. carinii infections, immunosuppressed animals were transtracheally inoculated as described previously (1, 4, 5). Briefly, BALB/c mice were immunosuppressed and transtracheally inoculated with mouse P. carinii, and virus-free Sprague-Dawley rats from Harlan Colony 202, Indianapolis, Ind., were immunosuppressed and transtracheally inoculated with rat P. carinii. Prior to inoculation, mice were immunosuppressed for 14 days with monoclonal antibody from clone GK1.5, which is directed to L3T4+ cells (comparable to human CD4 cells) (8), in one study and with dexamethasone for 10 days at 1.2 mg/kg of body weight/day in the second study. Prior to inoculation, the rats were immunosuppressed with dexamethansone at 0.36 mg/kg/day for 7 days. Mice were transtracheally inoculated with 106 mouse P. carinii organisms in 0.05 ml of saline, and rats were transtracheally inoculated with 106 rat P. carinii organisms in 0.2 ml of saline; the wounds were closed with clips.
Development of mouse antisera.
The animals developed infections for 10 weeks, at which time severe infections had developed; immunosuppression was stopped, and the animals were allowed to recover. During the second week after the cessation of immunosuppression, the animals were injected intraperitoneally with 0.1 ml of solubilized P. carinii prepared as follows. Heavily infected lung tissue was ground in phosphate-buffered saline (10 mg/ml) and centrifuged slowly (300 × g) to remove large lung debris. The supernatant, containing approximately 105 organisms, was centrifuged at 5,000 × g for 10 min, and the pellet was suspended in 0.1 volume of 1 M urea with 10 mg of dithiothreitol/ml in water, solubilized at 4°C overnight, and diluted 1:10 in phosphate-buffered saline. After an additional 17 days, the animals were anesthetized and exsanguinated by cardiac puncture and sera were evaluated for antibody by enzyme-linked immunosorbent assay (ELISA) by a method that was developed to detect P. carinii in cultures and in animals (9). Briefly, ELISAs were performed in 96-well Corning Easy Wash plates coated with purified mouse P. carinii antigens. The plates were incubated at 35°C, washed three times, blocked, washed, incubated with mouse sera, washed three times, incubated with anti-mouse immunoglobulin G (IgG) tagged with alkaline phosphatase, and washed three times. p-Nitrophenyl phosphate was then added, and the plates were held for color development for approximately 30 min. The plates were read at 405 nm on a Molecular Devices ELISA reader. In the serum pool from the first 16 mice, individual sera had optical densities of from 0.278 ± 0.001 to 0.453 ± 0.011 (normal mouse IgG was 0.20 ± 0.003). In the second serum pool from 19 mice, the optical density range was from 0.283 ± 0.014 to 0.511 ± 0.005 and normal mouse IgG was 0.101 ± 0.003. Western blots were performed to define populations of antibodies. Classes of antibodies were determined and shown to be primarily IgG. The sera were pooled and used to treat mice.
Development of rat antisera.
Rat antisera were developed in approximately the same manner as that used for mouse antiserum. Transtracheally-inoculated Sprague-Dawley rats developed P. carinii infections for 6 weeks; then the dexamethasone immunosuppression was stopped, and the rats were allowed to recover. At the end of the first week after dexamethasone was stopped, the rats were given solubilized rat P. carinii prepared in the same way as solubilized mouse P. carinii except that the pellet contained approximately 106 organisms and the final volume given was 0.3 ml per rat intraperitoneally. The rats were exsanguinated by cardiac puncture 2 weeks after the P. carinii injection, and the individual rat sera were evaluated by ELISA and Western blotting. The rat anti-P. carinii antibody was primarily IgG. Rat sera shown to have antibodies to rat P. carinii antigens were pooled.
Development and evaluation of infections.
After inoculation, the animals were continued on immunosuppression for 6 weeks, allowing infections to become severe. The severity of infections was determined by scoring numbers of organisms in histochemically stained impression smears of lung samples. Animals were anesthetized and exsanguinated by cardiac puncture, and their lungs were removed. A portion of the left lower lobe of each lung was used for the preparation of smears for Giemsa and methenamine-silver nitrate staining. The smears were examined as unknowns by two experienced microscopists and scored according to the following roughly logarithmic scheme (organisms per representative 1,000× microscopic field): 5+, >100; 4+, 11 to 100; 3+, 1 to 10; 2+, 2 to 9 (in 10 fields); 1+, 1 (in 10 to 50 fields); and 0, 0 (in >50 fields).
Evaluation of antisera for major surface glycoprotein specificity.
The mouse serum pool that provided protection was used to blot a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (18) gel of rat P. carinii recombinant major surface glycoprotein (MSG) (21). For controls, normal mouse serum, rat convalescent-phase serum, and normal rat serum were included in the blot. The mouse serum pool also was blotted against a mouse P. carinii antigen prepared from mostly trophozoite forms obtained by differential centrifugation according to a method developed by Merali and Clarkson (22). Individual rat serum samples were tested by ELISA and Western blotting, using as the antigen a rat P. carinii preparation of mostly trophozoite forms prepared by the same differential centrifugation method.
Treatment of animals with antisera.
Immunosuppressed mice were given 60 μl of antiserum pool, and rats were given 400 μl intraperitoneally. For the first mouse study, which used L3T4+ antibody immunosuppression, antiserum was given to one group of mice 1 day prior to P. carinii transtracheal inoculation and at 2 and 4 weeks postinoculation while antiserum was given to a second group of mice only at 3 and 5 weeks after P. carinii inoculations. For the second mouse study, which used dexamethasone immunosuppression, antiserum was given 1 day prior to transtracheal inoculation and at 2 and 4 weeks after inoculation. Immunosuppressed rats were given antiserum 1 day prior to transtracheal inoculation with P. carinii and at 2 and 4 weeks after inoculation. For each of the above studies, there were 10 mice or 8 rats in each antiserum-treated group, 10 mice or 8 rats inoculated at the same time as the antiserum-treated groups but not given antiserum, and 10 mice or 8 rats inoculated at the same time and treated with trimethoprim plus sulfamethoxazole at 50 and 250 mg/kg/day, respectively, in drinking water.
Statistical analysis.
Data were analyzed with the SigmaStat program and the Mann-Whitney rank sum test for nonparametric data.
RESULTS
Untreated dexamethasone-immunosuppressed mice were severely infected and had approximately 50 P. carinii organisms per 1,000× microscopic field detected by Giemsa staining. Untreated L3T4+-immunosuppressed mice also had severe infections, with comparable numbers of organisms detected by Giemsa staining.
Mice immunosuppressed with L3T4+ antibody and mice immunosuppressed with dexamethasone were protected with antisera given prior to transtracheal inoculation. In both immunosuppression groups, zero or few organisms were found in up to 50 1,000× microscopic fields. Differences in infection were statistically significant, with comparisons of the scores of mice treated by antiserum prophylaxis to those of untreated control mice having P values of 0.003 and 0.000, respectively. The group of mice given antiserum only at weeks 3 and 5 had slightly higher infection scores; however, antiserum-treated mice had a statistically significant reduction in numbers of organisms compared to untreated controls (P = 0.023). The drug-treated (trimethoprim plus sulfamethoxazole) control mice were cured (Table 1).
TABLE 1.
P. carinii infection scores with various treatments
| Animal | Immuno- suppression | Scorea when treated with:
|
|||
|---|---|---|---|---|---|
| Antiserum
|
No treat- ment | TMP + SMXb (wk 3–6) | |||
| At inocu- lation and at wk 2 and 4 | At wk 3 and 5 | ||||
| Mouse | L3T4 | 0.5 ± 0.2* | 1.2 ± 0.3** | 3.4 ± 0.4 | 0.0 ± 0.0 |
| Mouse | Dexamethazone | 0.3 ± 0.2*** | ND | 3.6 ± 0.1 | 0.0 ± 0.0 |
| Rat | Dexamethazone | 3.5 ± 0.3**** | ND | 4.7 ± 0.1 | 0.0 ± 0.0 |
Giemsa stain score ± standard error. P values for differences between treated and untreated animals: *, P = 0.003; **, P = 0.023; ***, P = 0.000; ****, P = 0.006. ND, not done.
TMP + SMX, trimethoprim plus sulfamethoxazole.
Rats immunosuppressed with dexamethasone and treated with antiserum prior to inoculation had less severe P. carinii infections than untreated rats. Trimethoprim plus sulfamethoxazole cured rats (infection scores of antiserum-treated rats compared to those of untreated control rats had P values of 0.006). Rat scores are summarized in Table 1.
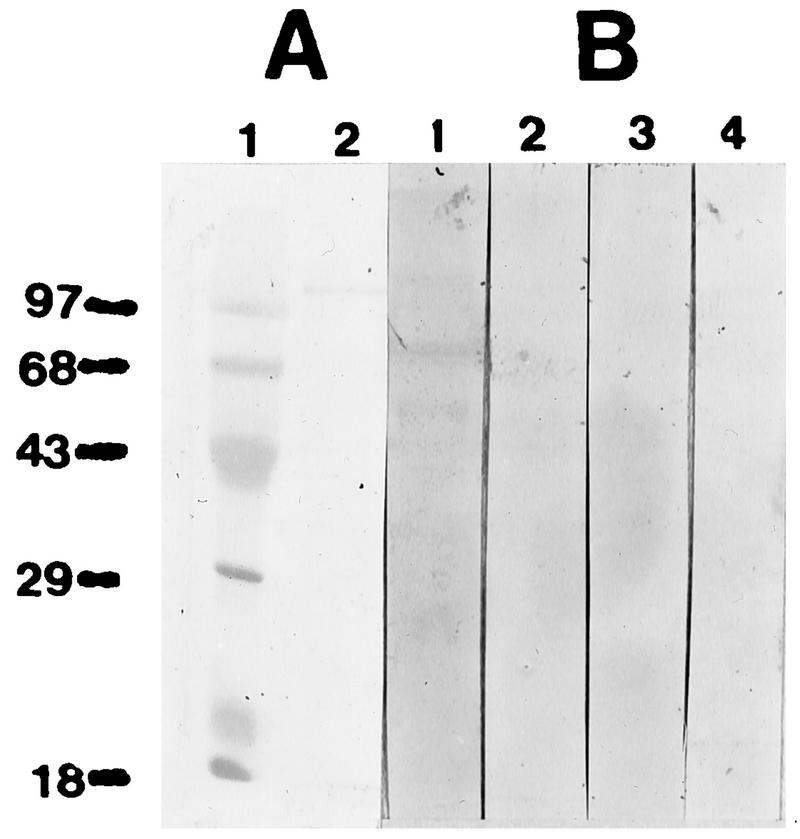
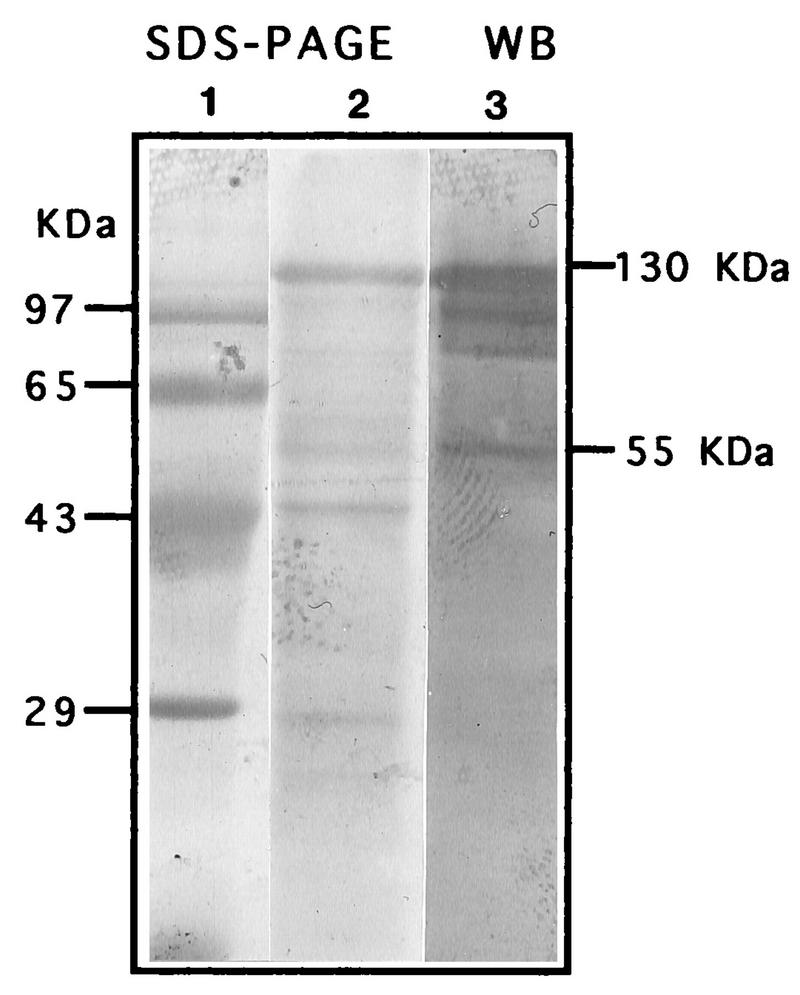
The mouse antiserum did not react with rat P. carinii recombinant MSG. The Western blot showed two bands with the control rat convalescent-phase antiserum, but there was no reaction with normal rat serum or with the polyclonal mouse serum or normal mouse serum (Fig. 1). The mouse serum did react to a mouse P. carinii protein of about 55 kDa as well as the MSG of about 130 kDa and some other constituents of the pool (Fig. 2). The rat serum pool had reactions to the MSG of about 120 kDa and to both a 50- and a 40-kDa band (data not shown).
FIG. 1.
(A) Coomassie blue-stained SDS-PAGE gel with molecular mass markers (in kilodaltons) (lane 1) and recombinant MSG from rat P. carinii (lane 2). (B) Western blot with rat convalescent-phase serum (lane 1), normal rat serum (lane 2), polyclonal mouse serum (lane 3), and normal mouse serum (lane 4).
FIG. 2.
Coomassie blue-stained SDS-PAGE gel with molecular mass markers (lane 1) and mouse P. carinii (lane 2) and Western blot (WB) with mouse polyclonal serum (lane 3).
DISCUSSION
Polyclonal anti-P. carinii antiserum can protect mice and rats from developing severe P. carinii infections. The mice immunosuppressed by antibody to L3T4+ lymphocytes (comparable to CD4 lymphocytes of humans) and given mouse polyclonal antiserum prior to inoculation were most effectively protected from P. carinii pneumonia. This antiserum pool was also effective when given after infections had begun to develop, at 3 weeks postinoculation. In dexamethasone-immunosuppressed mice, the antiserum was effective when given prior to inoculation. Rats were less well protected with the available rat antiserum pool than were mice with their antiserum pool. Still, rats that were treated with polyclonal antiserum at the time of inoculation had less severe infections than the rats that were not treated. The rat antiserum pool may not have been administered in sufficient quantity to provide protection, or the amounts of protective antibodies may have been less in the rat serum pool than in the mouse serum pool.
The mouse antiserum did not react with recombinant rat P. carinii MSG. Some investigators have suggested that MSG is a dominant antigen, and since a previous study had shown cross-reaction of antibodies to surface glycoproteins with mouse and rat P. carinii (6), pure recombinant MSG was used to see if the antisera that provided protection contained antibodies directed to this antigen. The protectve mouse antiserum had antibodies that reacted to mouse P. carinii antigens of 97, 85, and 55 kDa (Fig. 2) in addition to the 130-kDa (MSG) antigens.
Host responses to P. carinii have been studied in many systems in an effort to understand the organism’s pathogenic mechanisms and identify methods to prevent infections or reduce the complications of infection. Although P. carinii was identified as the cause of many serious outbreaks of disease in children in institutions following World War II (12), in children treated for leukemia (21, 25, 29), and in those with risk factors such as malnutrition (17) and immunosuppressive chemotherapies (23, 27), the successful use of trimethoprim plus sulfamethoxazole for prophylaxis in those shown to be at risk (16) discouraged studies of the organism and P. carinii pneumonia. Not until the advent of AIDS did strong interest in P. carinii pneumonia develop. Because patients with AIDS were immunosuppressed by the loss of CD4 lymphocyte function, the study of the role of humoral immunity in preventing P. carinii pneumonia has been largely ignored. The possibility of using passively transferred antisera or a vaccine which could induce protective antibodies offers a new approach to the management of immunosuppressed patients who are at increased risk for P. carinii infections. (Experiments are in progress to test this hypothesis.) Identification of antibodies which afford protection and the antigens which stimulate their formation is ongoing. The goal is to identify a potentially effective immunogen so that soon after detection of HIV positivity, or in anticipation of transplantation, a vaccine could be given to allow antibodies to develop and thus decrease the likelihood of P. carinii pneumonia. Further studies are needed.
REFERENCES
- 1.Bartlett M S, Fishman J A, Queener S F, Durkin M M, Jay M A, Smith J W. A new rat model of Pneumocystis carinii infection. J Clin Microbiol. 1988;26:1100–1102. doi: 10.1128/jcm.26.6.1100-1102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett M S, Fishman J A, Durkin M M, Queener S F, Smith J W. Pneumocystis carinii: improved models to study efficacy of drugs for treatment or prophylaxis of Pneumocystis pneumonia in the rat (Rattus spp.) Exp Parasitol. 1990;70:100–106. doi: 10.1016/0014-4894(90)90089-u. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett M S, Queener S F, Tidwell R R, Milhous W K, Berman J D, Ellis W Y, Smith J W. 8-Aminoquinolines from Walter Reed Army Institute for Research for treatment and prophylaxis of Pneumocystis pneumonia in rat models. Antimicrob Agents Chemother. 1991;35:277–282. doi: 10.1128/aac.35.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett M S, Queener S F, Durkin M M, Shaw M M, Smith J W. Inoculated mouse model of Pneumocystis carinii infection. Diagn Microbiol Infect Dis. 1992;15:129–134. doi: 10.1016/0732-8893(92)90036-s. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett M S, Current W L, Orazi A, Bauer N L, Neiman R S, Queener S F, Smith J W. Comparison of corticosteroid and L3T4+ antibody immunosuppressed mouse models of Pneumocystis carinii pneumonia for evaluation of drugs and leukocytes. Clin Diagn Lab Immunol. 1994;1:511–516. doi: 10.1128/cdli.1.5.511-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer N L, Paulsrud J R, Bartlett M S, Smith J W, Wilde C E., III Pneumocystis carinii organisms obtained from rats, ferrets, and mice are antigenically different. Infect Immun. 1993;61:1315–1319. doi: 10.1128/iai.61.4.1315-1319.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonagura V R, Cunningham-Rundles S, Edwards B L, Ilowite N T, Wedgwood J F, Valacer D J. Common variable hypogammaglobulinemia, recurrent Pneumocystis carinii pneumonia on intravenous γ-globulin therapy and natural killer deficiency. Clin Immunol Immunopathol. 1989;51:216–231. doi: 10.1016/0090-1229(89)90021-4. [DOI] [PubMed] [Google Scholar]
- 8.Dialynas D P, Wilde D B, Marrack P, Pierres A, Wall K A, Havran W, Otten G, Loken M R, Pierres M, Kappler F, Fitch F W. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK 1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen reactivity. Immunol Rev. 1983;74:29–55. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 9.Durkin M M, Bartlett M S, Queener S F, Shaw M M, Lee C H, Smith J W. An enzyme-linked immunosorbent assay for enumeration of Pneumocystis carinii in vitro and in vivo. J Clin Microbiol. 1992;30:3258–3262. doi: 10.1128/jcm.30.12.3258-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuta T, Ueda K, Kyuwa S, Fujiwara K. Effect of T-cell transfer on Pneumocystis carinii infection in nude mice. Jpn J Exp Med. 1984;54:57–64. [PubMed] [Google Scholar]
- 11.Furuta T, Ueda K, Fujihara K, Yamanouchi K. Cellular and humoral immune responses of mice subclinically infected with Pneumocystis carinii. Infect Immun. 1985;47:544–548. doi: 10.1128/iai.47.2.544-548.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gajdusek D C. Pneumocystis carinii—etiologic agent of interstitial plasma cell pneumonia of young and premature infants. Pediatrics. 1957;19:543–565. [PubMed] [Google Scholar]
- 13.Gigliotti F, Hughes W T. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest. 1988;81:1666–1668. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmsen A G, Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990;172:937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmsen A G, Chen W, Gigliotti F. Active immunity to Pneumocystis carinii reinfection in T-cell-depleted mice. Infect Immun. 1995;63:2391–2395. doi: 10.1128/iai.63.7.2391-2395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes W T, McNabb P C, Makres T D, Feldman S. Efficacy of trimethoprim and sulfamethoxazole in the prevention and treatment of Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1974;5:289–293. doi: 10.1128/aac.5.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes W T, Price R A, Sinko F, Havron W S, Kaftos A G, Schonland M, Smythe P M. Protein-calorie malnutrition—a host determinant for Pneumocystis carinii infection. Am J Dis Child. 1974;128:44–52. doi: 10.1001/archpedi.1974.02110260046008. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie S J, Williams P E, Rajagopalan N, Grant I S, Petrie G R, Burns S M, Yap P I. Pneumocystis carinii pneumonia in thymoma with hypogammaglobulinemia: successful outcome of therapy including IV IgG replacement. Scott Med J. 1991;36:50. doi: 10.1177/003693309103600208. [DOI] [PubMed] [Google Scholar]
- 20.Masur H. Prevention and treatment of Pneumocystis pneumonia. N Engl J Med. 1992;327:1855–1860. doi: 10.1056/NEJM199212243272606. [DOI] [PubMed] [Google Scholar]
- 21.Mei Q, Kovacs J A, Hildebrand B, Angus C W. Expression of the major surface glycoprotein of rat-derived Pneumocystis carinii by recombinant baculovirus. J Eukaryot Microbiol. 1996;43:31S. doi: 10.1111/j.1550-7408.1996.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 22.Merali S, Clarkson A B., Jr Polyamine content of Pneumocystis carinii and response to the ornithine decarboxylase inhibitor dl-α-difluoromethylornithine. Antimicrob Agents Chemother. 1996;40:973–978. doi: 10.1128/aac.40.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattison N, Wright T, Herrod H G. Pneumocystis carinii pneumonitis, eosinophilia and hypogammaglobulinemia. Pediatr Infect Dis J. 1987;6:293–294. doi: 10.1097/00006454-198703000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Rao C P, Gelfand E W. Pneumocystis carinii pneumonitis in patients with hypogammaglobulinemia and intact T cell immunity. J Pediatr. 1983;103:410–412. doi: 10.1016/s0022-3476(83)80415-6. [DOI] [PubMed] [Google Scholar]
- 25.Ruebush T K, Weinstein R A, Baehner R L, Wolff D, Bartlett M, Gonzales-Crussi F, Sulzer A J, Schultz M G. An outbreak of Pneumocystis pneumonia in children with acute lymphocytic leukemia. Am J Dis Child. 1978;132:143–148. doi: 10.1001/archpedi.1978.02120270041009. [DOI] [PubMed] [Google Scholar]
- 26.Saah A J, Hoover D R, Peng Y, Phair J P, Visscher B, Kingsley L A, Schrager L K. Predictors for failure of Pneumocystis carinii prophylaxis. JAMA. 1995;273:1197–1202. [PubMed] [Google Scholar]
- 27.Sepkowitz K A, Brown A E, Telzak W W, Cottlieb S, Armstrong D. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA. 1992;267:832–837. [PubMed] [Google Scholar]
- 28.Simonds, R. J., W. T. Hughes, J. Feinberg, and T. R. Navin. 1995. Preventing Pneumocystis carinii pneumonia in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 21(Suppl. 1):S44–S48. [DOI] [PubMed]
- 29.Simone J V. Management of childhood leukemia. Postgrad Med. 1974;55:225–231. doi: 10.1080/00325481.1974.11713767. [DOI] [PubMed] [Google Scholar]
- 30.Staugas R E M, Beard L J, Simmer K, Ferrante A. Hypogammaglobulinemia and depressed natural killer cell cytotoxicity in a patient with Pneumocystis carinii infection. Pediatr Infect Dis J. 1988;7:724–728. doi: 10.1097/00006454-198810000-00012. [DOI] [PubMed] [Google Scholar]