Full text
PDF

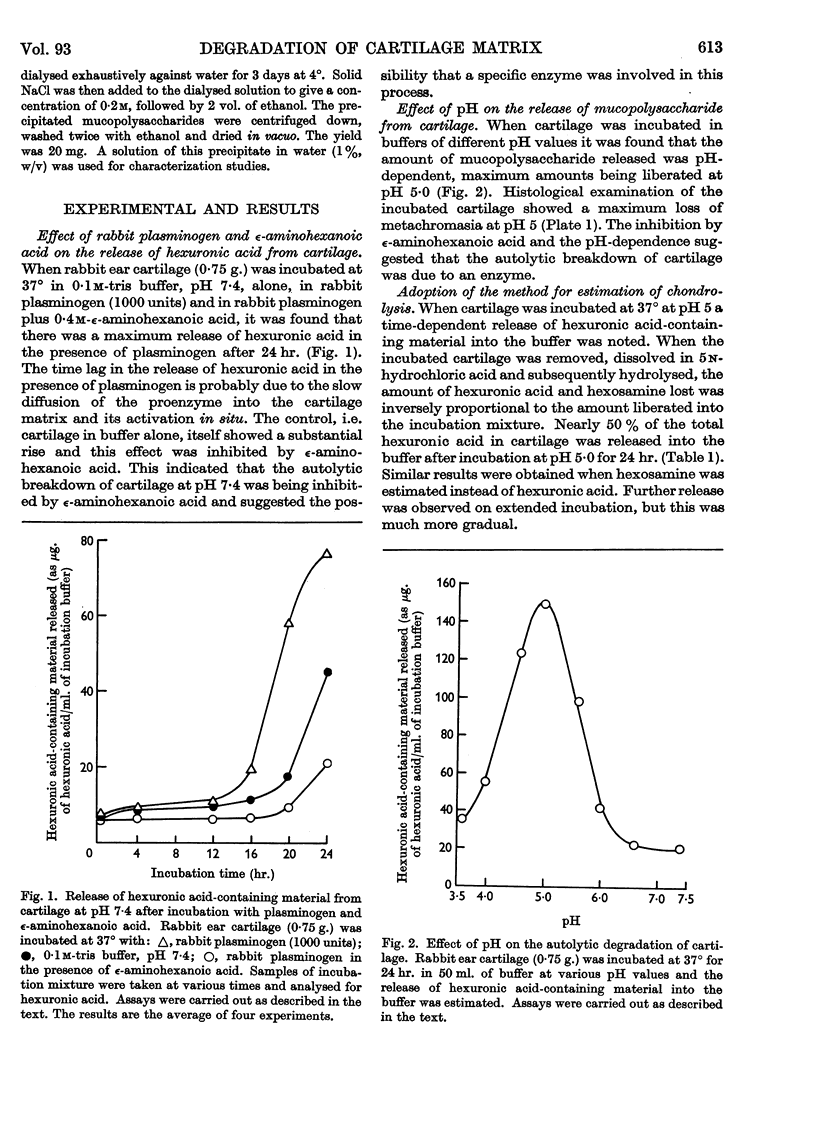






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. xi-Aminocaproic acid: an inhibitor of plasminogen activation. J Biol Chem. 1959 Apr;234(4):832–837. [PubMed] [Google Scholar]
- ANDERSON A. J., LACK C. H., ALI S. Y. Glycoprotein excretion following the injection of papain into the rabbits. Nature. 1963 Feb 16;197:707–707. doi: 10.1038/197707a0. [DOI] [PubMed] [Google Scholar]
- ANDERSON A. J. Some studies on the relationship between sialic acid and the mucopolysaccharide-protein complexes in human cartilage. Biochem J. 1962 Feb;82:372–381. doi: 10.1042/bj0820372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAZIN S., DELAUNAY A. [The "in-vitro" effect of various enzymes on cartilage fragments obtained from rabbitears]. Ann Inst Pasteur (Paris) 1961 Apr;100:481–489. [PubMed] [Google Scholar]
- COOPER W. D., LORING H. S. The purine and pyrimidine composition of the tobacco mosaic virus and the Holmes masked strain. J Biol Chem. 1954 Dec;211(2):505–515. [PubMed] [Google Scholar]
- DAVIDSON F. M. The activation of plasminogen by staphylokinase: comparison with streptokinase. Biochem J. 1960 Jul;76:56–61. doi: 10.1042/bj0760056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., BEAUFAY H. Tissue fractionation studies. 10. Influence of ischaemia on the state of some bound enzymes in rat liver. Biochem J. 1959 Dec;73:610–616. doi: 10.1042/bj0730610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- DI FERRANTE N. Turbidimetric measurement of acid mucopolysaccharides and hyaluronidase activity. J Biol Chem. 1956 May;220(1):303–306. [PubMed] [Google Scholar]
- DINGLE J. T. Aetiological factors in the collagen diseases. Lysosomal enzymes and the degradation of cartilage matrix. Proc R Soc Med. 1962 Feb;55:109–111. [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A., FELL H. B. Studies on the mode of action of excess of vitamin A. 1. Effect of excess of vitamin A on the metabolism and composition of embryonic chick-limb cartilage grown in organ culture. Biochem J. 1961 Jun;79:497–500. doi: 10.1042/bj0790497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A., FELL H. B. Studies on the mode of action of excess of vitamin A. 1. Effect of excess of vitamin A on the metabolism and composition of embryonic chick-limb cartilage grown in organ culture. Biochem J. 1961 Jun;79:497–500. doi: 10.1042/bj0790497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELL H. B., DINGLE J. T. Studies on the mode of action of excess of vitamin A. 6. Lysosomal protease and the degradation of cartilage matrix. Biochem J. 1963 May;87:403–408. doi: 10.1042/bj0870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACK C. H., ANDERSON A. J., ALISY Action of plasmin on cartilage matrix in vivo. Nature. 1961 Sep 30;191:1402–1403. doi: 10.1038/1911402a0. [DOI] [PubMed] [Google Scholar]
- LACK C. H. Chondrolvsis. Ann Phys Med. 1961 Aug;6:93–99. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARKS N., LAJTHA A. PROTEIN BREAKDOWN IN THE BRAIN. SUBCELLULAR DISTRIBUTION AND PROPERTIES OF NEUTRAL AND ACID PROTEINASES. Biochem J. 1963 Dec;89:438–447. doi: 10.1042/bj0890438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. B., ROSEMAN S., DORFMAN A. Determination of the chondroitinase activity of bovine testicular preparations. J Biol Chem. 1951 Jan;188(1):327–334. [PubMed] [Google Scholar]
- MOWRY R. W. Improved procedure for the staining of acidic polysaccharides by Müller's colloidal (hydrous) ferric oxide and its combination with the Feulgen and the periodic acid-Schiff reactions. Lab Invest. 1958 Nov-Dec;7(6):566–576. [PubMed] [Google Scholar]
- MUIR H. The nature of the link between protein and carbohydrate of a chondroitin sulphate complex from hyaline cartilage. Biochem J. 1958 Jun;69(2):195–204. doi: 10.1042/bj0690195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMAN R. E., LOGAN M. A. The determination of hydroxyproline. J Biol Chem. 1950 May;184(1):299–306. [PubMed] [Google Scholar]
- PARTINGTON F. R., WOOD G. C. The role of non-collagen components in the mechanical behaviour of tendon fibres. Biochim Biophys Acta. 1963 Mar 5;69:485–495. doi: 10.1016/0006-3002(63)91298-8. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE S. M., DAVIS H. F., ADAIR G. S. The chemistry of connective tissues. 6. The constitution of the chondroitin sulphate-protein complex in cartilage. Biochem J. 1961 Apr;79:15–26. doi: 10.1042/bj0790015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTER J. L., McCLUSKEY R. T., WEISSMANN G., THOMAS L. The effects of papain on cartilage in vivo: factors influencing the distribution of papain protease following intravenous injection. Ann N Y Acad Sci. 1960 Jun 30;86:929–942. doi: 10.1111/j.1749-6632.1960.tb42850.x. [DOI] [PubMed] [Google Scholar]
- THOMAS L. Reversible collapse of rabbit ears after intravenous papain, and prevention of recovery by cortisone. J Exp Med. 1956 Aug 1;104(2):245–252. doi: 10.1084/jem.104.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]



