Abstract
A neutralization enzyme immunoassay (N-EIA) was used to determine the neutralizing serum antibody titers to influenza A/Taiwan/1/86 (H1N1) and Beijing/353/89 (H3N2) viruses after vaccination of 51 human immunodeficiency virus (HIV) type 1-infected individuals and 10 healthy noninfected controls against influenza virus infection. Overall, the N-EIA titers correlated well with the hemagglutination-inhibition (HAI) titers that were observed in the same samples in a previous study (F. P. Kroon, J. T. van Dissel, J. C. de Jong, and R. van Furth, AIDS 8:469–476,1994). The N-EIA appeared to be more sensitive than the HAI test. Significantly more fourfold or higher rises in N-EIA titer and higher mean N-EIA titers occurred in HIV-infected individuals with ≥200 CD4+ cells per μl than in those with <200 CD4+ cells per μl.
Symptomatic human immunodeficiency virus (HIV) infection is predominantly characterized by opportunistic infections caused by an impaired T-lymphocyte-mediated immunity. Protection against influenza is primarily mediated by virus-specific antibodies and therefore depends on an intact humoral immune response (1, 7).
Influenza virus infection does not seem to be a major cause of morbidity and mortality in HIV type 1 (HIV-1)-infected individuals. However, many health authorities advise yearly influenza virus vaccinations for these subjects because serious illness and complications from influenza virus infection may occur in these subjects (3, 6, 20, 24).
Except for those with advanced disease, HIV-infected patients can still mount a hemagglutination-inhibiting antibody response after influenza virus vaccination, but the antibody levels achieved are lower than those found in non-HIV-infected individuals (11, 12, 14–16).
It is generally accepted that virus-specific antibodies neutralize the virus by interaction with the viral hemagglutinin (1, 7). The presence of influenza virus-neutralizing antibodies closely parallels immunity to influenza (7). Neutralizing antibodies therefore provide a more functional measure of the immunity to influenza virus infections than hemagglutination-inhibiting antibodies.
The humoral immune response of immunoglobulin G (IgG) immunoglobulins to influenza virus is dependent on the function of CD4+ T-helper cells (25). This T-lymphocyte-dependent humoral response is compromised by HIV-1 infection-induced depletion of CD4+ T-helper cells (for a review, see reference 21). The development of influenza virus-neutralizing (i.e., functionally active) antibodies upon vaccination against influenza virus infection may therefore be of particular relevance for protective immunity to influenza in HIV-infected patients.
The titers of serum neutralizing antibodies to influenza viruses A/Taiwan/1/86 (H1N1) (Taiwan H1N1) and A/Beijing/353/89 (H3N2) (Beijing H3N2) were determined by using a neutralization enzyme immunoassay (N-EIA) (4) after 46 male and 5 female HIV-1-infected subjects (mean age, 39.4 years; age range, 21 to 60 years) from the Infectious Diseases outpatient clinic of the University Hospital Leiden and 10 healthy hospital staff members (mean age, 33.3 years; age range, 24 to 49 years) were vaccinated against influenza virus infection (14).
According to the 1993 Centers of Disease Control and Prevention revised classification for HIV-infected adolescents and adults (5), 5 HIV-infected subjects were classified into group A1 and 1 HIV-infected subject was classified into group C1 (CD4+ T-cell counts, ≥500 cells/μl); 11 subjects were classified into group A2, 4 subjects were classified into group B2, and 2 subjects were classified into group C2 (CD4+ T-cell counts, 200 to 499 cells/μl); and 1 subject was classified into group A3, 9 subjects were classified into group B3, and 18 subjects were classified into group C3 (CD4+ T-cell counts, <200 cells/μl). To show the effect of severe immunosuppression on the neutralizing antibody responses to vaccination against influenza virus infection, the HIV-infected individuals were divided into two groups: those with CD4+ counts of <200 cells/μl (n = 28) and those with CD4+ counts of ≥200 cells/ml (n = 23). None of the patients had active opportunistic infections, and 31 were receiving antiretroviral therapy. The numbers of CD4+ cells, CD8+ cells, and other immunologic parameters have been described previously (14).
All subjects were immunized with a tetravalent influenza split vaccine (Vaxigrip; 1991 and 1992 formula; Institut Mérieux, Lyon, France) between November 1991 and February 1992; a single lot containing 15 μg of virus strains Beijing H3N2, Taiwan H1N1, B/Beijing/1/87, and B/Panama/45/90 was used. A booster was administered 4 weeks after the primary vaccination. The serum samples were collected before the first vaccination against influenza virus infection (day 0), 30 days later, just before the influenza booster, and 60 days after the first vaccination. The samples were coded and stored at −20°C until all specimens had been collected and tested in a blinded fashion in one session.
The N-EIA was performed with the influenza virus strains Taiwan H1N1 and Beijing H3N2. Apart from the extra disinfection of the microtiter plates, the N-EIA was performed with the same reagents and by the same procedures described previously (4). In brief, the serum samples were heat inactivated at 56°C for 1 h and diluted 1/3, 1/10, 1/30, 1/100, 1/300, 1/1,000, and 1/3,000. Three aliquots of 0.025 ml from each dilution were transferred to 96-well microtiter plates, and the plates were incubated for 1 h at 37°C with 0.025 ml of either the Taiwan H1N1 or Beijing H3N2 virus suspensions. Then, LLC-MKD2 monkey kidney cells were added to each well, and the plates were incubated at 37°C for 22 h. Subsequently, the cell monolayers were fixed with 0.050 ml of 0.15% glutaraldehyde per well for 20 min. After removal of the supernatants the plates were disinfected by immersion in 70% ethanol for 10 min. To detect the cell-associated viral antigens, the Taiwan H1N1 and Beijing H3N2 influenza virus A-specific, horseradish peroxidase-labeled (4) monoclonal antibodies 3-15/3-3 and UM 12-67, respectively, were used. The enzyme reaction and measurement of the absorbance values were performed as described previously (4). Virus controls (virus and cells only) and cell controls were each included in six wells in every microtiter plate. Neutralizing antibody titers were defined as those serum dilutions yielding a 50% reduction in the A450 value for the virus control (4). N-EIA titers of serum samples that did not yield a 50% reduction in the absorbance value at dilutions of 1/3 or 1/3,000 were calculated by extrapolation when possible or were entered arbitrarily as 1/1.6 or 1/10,000, respectively.
Statistical data were generated by using the SPSS computer program, version 6.0. For all calculations the hemagglutination-inhibition (HAI) and N-EIA titers were transformed into logarithmic values. One-way analysis of variance (ANOVA) was used for comparison of the group means, followed by the Student Newman-Keuls test for multiple comparisons. The Spearman rank test was used for determination of the coefficients of correlation.
The N-EIA titers correlated well with the HAI titers, which were measured independently in another laboratory in the study of Kroon et al. (14). The overall coefficients of correlation between the N-EIA and HAI titers were 0.93 and 0.80 for the Taiwan H1N1 and Beijing H3N2 strains, respectively (P < 0.001). The coefficients of correlation on days 0, 30, and 60 after vaccination for the Taiwan H1N1 strain were 0.90, 0.91, and 0.88, respectively (all P values were <0.001). For the Beijing H3N2 strain, however, a moderate correlation was observed on day 0 (r = 0.45; P < 0.001). On days 30 and 60 after vaccination the coefficient of correlation was 0.89 (P < 0.001), similar to the results obtained with the Taiwan H1N1 strain. The high levels of correlation (about 0.90) observed between the two assays indicate that the hemagglutination-inhibiting antibodies against influenza A virus strains Taiwan H1N1 and Beijing H3N2 are indeed functionally active. The low level of correlation for the prevaccination titers measured against the Beijing strain (r = 0.45) is related to the substantial number of HAI test-negative serum samples that were found to be positive by N-EIA (Fig. 1). This may be the consequence of the higher sensitivity of the N-EIA compared to that of the HAI test. Alternatively, serum may contain nonimmune factors that can accomplish both HAI and neutralization of influenza viruses (13). Both heat-stable inhibitors (α and γ) and heat-labile inhibitors (β) can prevent hemagglutination, and the β and γ inhibitors also neutralize virus infectivity (2, 9). As a general procedure for prevention of nonspecific HAI, serum samples are heat inactivated and incubated with receptor-destroying enzyme before testing by the HAI test (23). Prior to testing by N-EIA the serum samples were only heat inactivated. Therefore, nonimmune factors, particularly those of the γ class, may have contributed to the neutralization of the influenza A viruses.
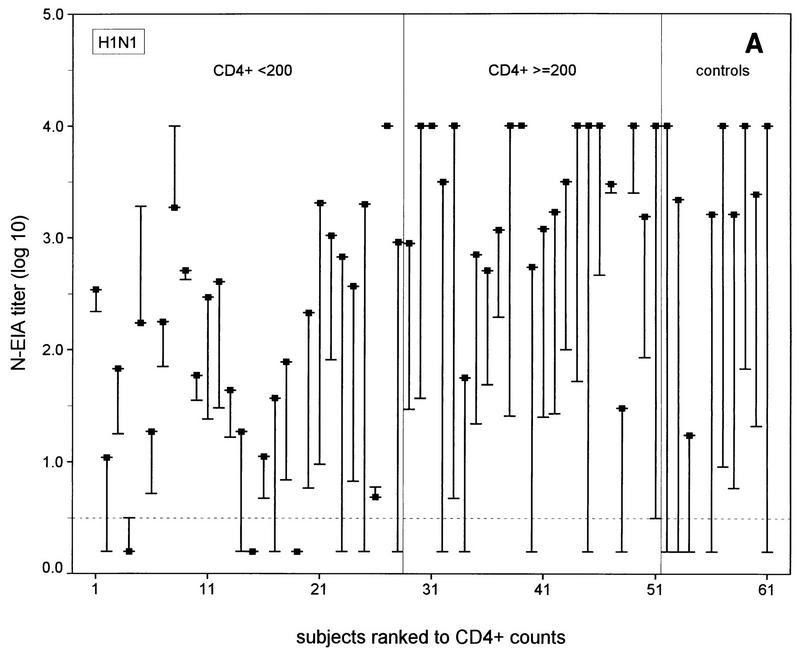
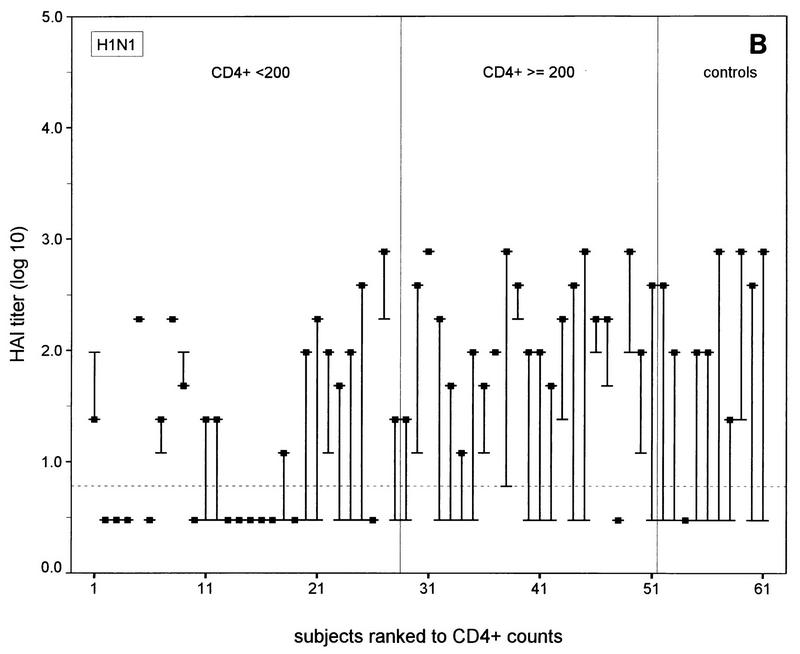
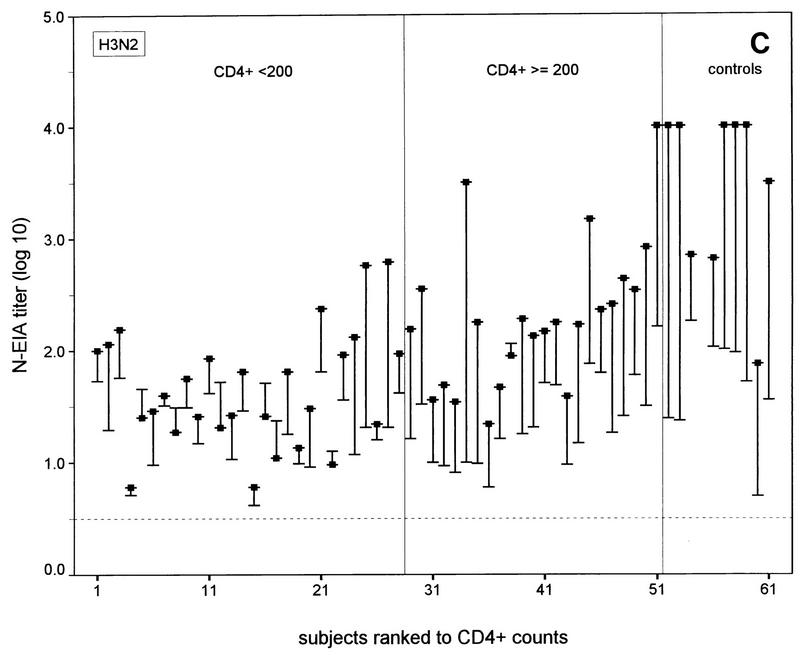
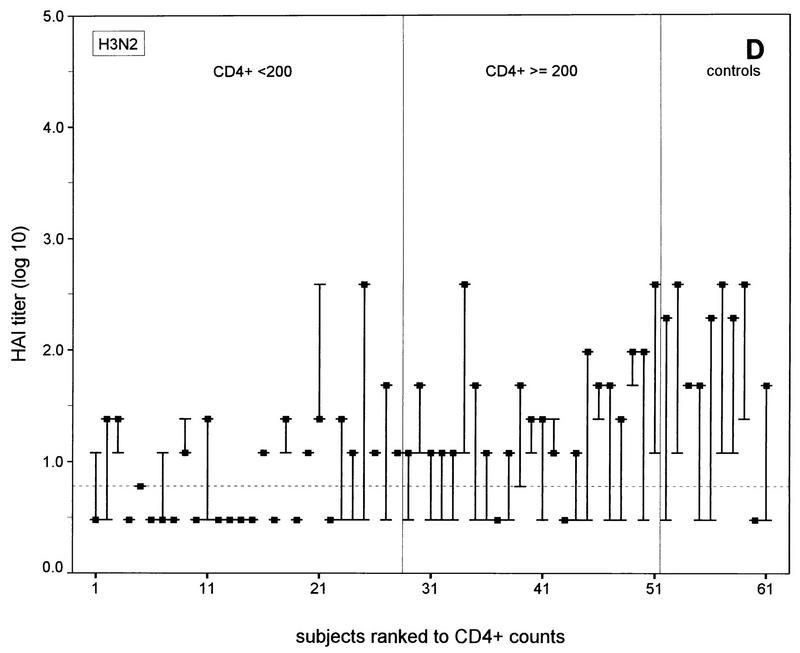
FIG. 1.
N-EIA and HAI test titers before and 30 days after vaccination of individual HIV-1-infected subjects and healthy noninfected controls against influenza A virus infection. (A and C) N-EIA and HAI titers against strain Taiwan H1N1; (B and D) N-EIA and HAI titers against strain Beijing H3N2. The subjects are individually ranked according to increasing CD4+ T-cell counts. Twenty-eight patients had CD4+ counts of <200 cells/μl and 23 subjects had CD4+ counts of ≥200 cells/μl. Of the 10 healthy controls, nine serum samples were available for testing by N-EIA at 30 days after vaccination. The ends of the bars indicate prevaccination titers (dashes) and postvaccination titers (filled squares). The lengths of the bars represent rises in titers for the individual subjects. Horizontal grid lines indicate the minimum levels of detection by the N-EIA and the HAI test. Antibody titers below the levels of detection were assigned arbitrary values of 0.2 and 0.5 for N-EIA and the HAI, respectively.
The N-EIA appeared to be more sensitive than the HAI test: no serum samples that were shown to be positive by the HAI test but negative by neutralization were found. Vice versa, 33 of 120 (28%) and 51 of 120 (43%) serum samples with undetectable hemagglutination-inhibiting antibodies showed neutralizing activity with Taiwan H1N1 and the Beijing H3N2 strains, respectively (Fig. 1). Postvaccination N-EIA titers tended to increase with increasing CD4+ T-cell counts in the HIV-1 infected individuals (Fig. 1A and B).
The prevaccination (arithmetic) mean N-EIA titers to the Taiwan H1N1 strain did not differ significantly between the three groups (P > 0.05; ANOVA), but the prevaccination mean N-EIA titer to the Beijing H3N2 strain was significantly higher (P < 0.05; ANOVA) in the control group compared to the mean N-EIA titers in the two groups of HIV-1-infected individuals (Table 1). At day 30 postvaccination, the mean N-EIA titers for individuals from the group with ≥200 CD4+ T cells/μl were significantly higher than the mean titers for the patients from the group with <200 CD4+ T cells/μl (P < 0.05; ANOVA) for both virus strains. At day 30 the noninfected individuals also showed significantly higher neutralization titers to the Beijing H3N2 strain than the HIV-infected group with ≥200 CD4+ T cells/μl (P <0.05; ANOVA). The booster vaccination at day 30 after primary vaccination did not result in a significant additional enhancement of the mean neutralization titers in any group (data not shown).
TABLE 1.
Neutralizing antibody response to vaccination of healthy and HIV-1-infected individuals against influenza A virus infection
| Vaccinated individuals
|
Mean ± SD log10 N-EIA titer against the following strain on the indicated day:
|
||||||
|---|---|---|---|---|---|---|---|
| Subject category | CD4+ count (cells/μl) | No. of subjects on the following days:
|
|||||
| 0 | 30 | Taiwan H1N1
|
Beijing H3N2
|
||||
| 0 | 30 | 0 | 30 | ||||
| Not HIV-1 infected | 10 | 9 | 0.76 ± 0.60 | 3.38 ± 0.88 | 1.74 ± 0.48a | 3.51 ± 0.79a | |
| HIV-1 infected | ≥200 | 23 | 23 | 1.64 ± 1.22 | 3.37 ± 0.73 | 1.34 ± 0.36 | 2.28 ± 0.59 |
| HIV-1 infected | <200 | 28 | 28 | 1.23 ± 1.12 | 2.03 ± 1.02b | 1.34 ± 0.32 | 1.65 ± 0.53b |
Significantly higher mean N-EIA titer (P < 0.05) compared to those for the other cohorts determined at the same time point.
Significantly lower mean N-EIA titer (P < 0.05) compared to those for other cohorts for measurements at the same time point.
A fourfold or higher rise in the N-EIA or HAI titer was considered an adequate immune response after vaccination against influenza virus infection (18). At 30 days after vaccination adequate neutralizing antibody responses were observed against the Taiwan H1N1 strain in 13 of 28 (46%) of the individuals in the HIV-infected group with <200 CD4+ T cells/μl, 19 of 23 (83%) of the individuals in the HIV-infected group with ≥200 CD4+ T cells/μl (P < 0.01; χ2 test), and 9 of 9 (100%) of the controls. For the Beijing H3N2 strain, these numbers were 4 of 28 (14%) of the individuals in the HIV-infected group with <200 CD4+ T cells/μl group, v 18 of 23 (78%) of the individuals in the HIV-infected group with >200 CD4+ T cells/μl, (P < 0.0005; χ2 test), and 8 of 9 (89%) of the controls. The numbers of subjects in each group with adequate neutralizing antibody responses did not differ significantly from the numbers of subjects with adequate hemagglutination-inhibiting antibody responses measured previously (14) (data not shown). Discrepancies between adequate N-EIA and hemagglutination-inhibiting antibody responses (i.e., no response by N-EIA and an adequate response by the HAI test or vice versa) against the Taiwan H1N1 subtype were observed in 9 of the 51 HIV-infected subjects and against the H3N2 subtype in 8 of the 51 HIV-infected subjects but in none of the controls (Fig. 1).
The present study demonstrates that the recently developed N-EIA is a sensitive, convenient, and objective test for the assessment of influenza A virus neutralizing activities in a large number of serum samples (4). Furthermore, the results obtained by means of a functional antibody assay (N-EIA) support the conclusions drawn from the previous study by Kroon et al. (14).
Determination of the critical levels of virus-neutralizing antibodies that are associated with protection from influenza in HIV-infected individuals, such as has been reported for hemagglutination-inhibiting antibody levels (10), requires large numbers of subjects and meticulous follow-up. Therefore, such a study would hardly be feasible. However, it can be conceived that any neutralizing antibody titer upon vaccination contributes to the protection from serious influenza virus infection.
There is a concern about the transient increase in HIV viremia and the possible effects on the progression of HIV disease after vaccination against influenza virus infection (17, 19, 22). The published data, however, are contradictory (8, 26). At present, the benefits of protection against influenza virus infection seem to outweigh the yet to be established negative effects of vaccination on the progession of HIV infection (3).
REFERENCES
- 1.Ada G L, Jones P D. The immune response to influenza virus infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Anders E M, Hartley C A, Jackson D C. Bovine and mouse β inhibitors of influenza A viruses are mannose binding lectins. Proc Natl Acad Sci USA. 1990;87:4485–4489. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arden N H, Cox N J, Schonberger L B. Prevention and control of influenza. Recommendations of the advisory committee on immunization practices (ACIP) Morbid Mortal Weekly Rep. 1997;46:6–7. [PubMed] [Google Scholar]
- 4.Benne C A, Harmsen M, de Jong J C, Kraaijeveld C A. Neutralization enzyme immunoassay for influenza virus. J Clin Microbiol. 1994;32:987–990. doi: 10.1128/jcm.32.4.987-990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1993;41:1–19. [PubMed] [Google Scholar]
- 6.Cohen J P, Macauly C. Susceptibility to influenza A in HIV-positive patients. JAMA. 1989;261:245. doi: 10.1001/jama.1989.03420020097023. [DOI] [PubMed] [Google Scholar]
- 7.Couch R B, Kasel J A. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 8.Fowke K R, D’Amico R, Chernoff D N, Pottage J C, Benson C A, Sha B E, Kessler H A, Landay A L, Shearer G M. Immunologic and virologic evaluation after influenza vaccination of HIV-1 infected patients. AIDS. 1997;11:1013–1021. doi: 10.1097/00002030-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Gottschalk A, Belyavin G, Biddle F. Glycoproteins as influenza hemagglutinin inhibitors and as cellular virus receptors. In: Gottschalk A, editor. Glycoproteins: their composition, structure and function. New York, N.Y: Elsevier Science Publishing; 1992. pp. 1082–1096. [Google Scholar]
- 10.Hobson D, Curry R L, Beare A S, Ward-Gardner A. The role of serum hemagglutination-inhibiting antibody in protection against challenge with influenza A2 and B viruses. J Hyg Camb. 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang K-L, Ruben F L, Rinaldo C R, Kingsley L, Lyter D W, Ho M. Antibody responses after influenza and pneumococcal immunization in HIV-infected homosexual men. JAMA. 1987;257:2047–2050. [PubMed] [Google Scholar]
- 12.Huengsberg M, Chakraverty M P, Cooper G, Shahmanesh M. Response to influenza immunization in asymptomatic HIV infected men. Genitourin Med. 1995;71:355–357. doi: 10.1136/sti.71.6.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krizanová O, Rathová V. Serum inhibitors of myxoviruses. Curr Top Microbiol Immunol. 1969;47:125–151. doi: 10.1007/978-3-642-46160-6_6. [DOI] [PubMed] [Google Scholar]
- 14.Kroon F P, van Dissel J T, de Jong J C, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccine in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS. 1994;8:469–476. doi: 10.1097/00002030-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Miotti P G, Nelson K E, Dalabetta G A, Farzadegan H, Margolick J, Clements M L. The influence of HIV infection on antibody responses to a two-dose regime of influenza vaccine. JAMA. 1989;262:779–783. [PubMed] [Google Scholar]
- 16.Nelson K E, Clements M L, Miotti P, Cohn S, Polk B F. The influence of human immunodeficiency virus (HIV) infection on antibody responses to influenza vaccines. Ann Intern Med. 1988;109:383–388. doi: 10.7326/0003-4819-109-5-383. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien W A, Grovit-Ferbas K, Namasi A, Ovcak-Derzic S, Wang H-J, Park J, Yeramian C, Mao S-H, Zack J A. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood. 1995;86:1082–1089. [PubMed] [Google Scholar]
- 18.Pereira M S, Chakraverty P, Schild G C, Coleman M T, Dowdle W R. Prevalence of antibody to current influenza viruses and effect of vaccination on antibody response. Br Med J. 1972;4:701–703. doi: 10.1136/bmj.4.5842.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosak B, Voltersvik P, Bjerknes R, Axelsson M, Haaheim L R, Åsjö B. Dynamics of HIV-1 replication following vaccination of HIV+ individuals. Clin Exp Immunol. 1996;104:203–207. doi: 10.1046/j.1365-2249.1996.25732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safrin S, Rush J D, Mills J. Influenza in patients with human immunodeficiency virus infection. Chest. 1990;98:33–37. doi: 10.1378/chest.98.1.33. [DOI] [PubMed] [Google Scholar]
- 21.Schnittman S M, Fauci A S. Human immunodeficiency virus and acquired immunodeficiency syndrome: an update. Adv Intern Med. 1994;39:305–355. [PubMed] [Google Scholar]
- 22.Staprans S I, Hamilton B L, Follansbee S E, Elbeik T, Barbosa P, Grant R M, Femberg M B. Activation of virus replication after vaccination of HIV-1 infected individuals. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subbarao E K, Kawaoka Y, Ryan-Poirier K, Clements M L, Murphy B R. Comparison of different approaches to measuring influenza A virus-specific hemagglutination-inhibition antibodies in the presence of serum inhibitors. J Clin Microbiol. 1992;30:996–999. doi: 10.1128/jcm.30.4.996-999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurn J R, Henry K. Influenza A pneumonitis in a patient infected with the human immunodeficiency virus (HIV) Chest. 1989;95:807–810. doi: 10.1378/chest.95.4.807. [DOI] [PubMed] [Google Scholar]
- 25.Virelizier J L, Postlethwaite R, Schild G C, Allison A C. Antibody responses to antigenic determinants of influenza virus hemagglutinin. I. Thymus dependence of antibody formation and thymus independence of immunological memory. J Exp Med. 1974;140:1559–1570. doi: 10.1084/jem.140.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yerly S, Wunderli W, Wyler C A, Kaiser L, Hirschel B, Suter S, Perrin L H, Siegrist C-A. Influenza immunization of HIV-1 infected patients does not increase HIV-1 viral load. AIDS. 1994;8:1503–1504. doi: 10.1097/00002030-199410000-00022. [DOI] [PubMed] [Google Scholar]