Abstract
Synthetic organic chemists continually draw inspiration from biocatalytic processes to innovate synthetic methodologies beyond existing catalytic platforms. Within this context, although 1,2-amino migration represents a viable biochemical process, it remains underutilized within the synthetic organic chemistry community. Here we present a biomimetic 1,2-amino migration accomplished through the synergistic combination of biocatalytic mechanism and photoredox catalysis. This platform enables the modular synthesis of γ-substituted β-amino acids by utilizing abundant α-amino-acid derivatives and readily available organic molecules as coupling partners. This mild method features excellent substrate and functionality compatibility, affording a diverse range of γ-substituted β-amino acids (more than 80 examples) without the need for laborious multistep synthesis. Mechanistic studies, supported by both experimental observations and theoretical analysis, indicate that the 1,2-amino migration mechanism involves radical addition to α-vinyl-aldimine ester, 3-exo-trig cyclization and a subsequent rearrangement process. We anticipate that this transformation will serve as a versatile platform for the highly efficient construction of unnatural γ-substituted β-amino acids.

Subject terms: Synthetic chemistry methodology, Photocatalysis, Synthetic chemistry methodology
Enzyme-catalysed 1,2-amino migration represents a viable biochemical process that is currently underutilized within the synthetic organic chemistry community. Building upon this biocatalytic mechanism, a biomimetic photoredox-catalysed 1,2-amino migration method has been developed. By integrating photoredox-catalysed conditions, this approach enables the modular synthesis of a diverse library of γ-substituted β-amino acids.
Main
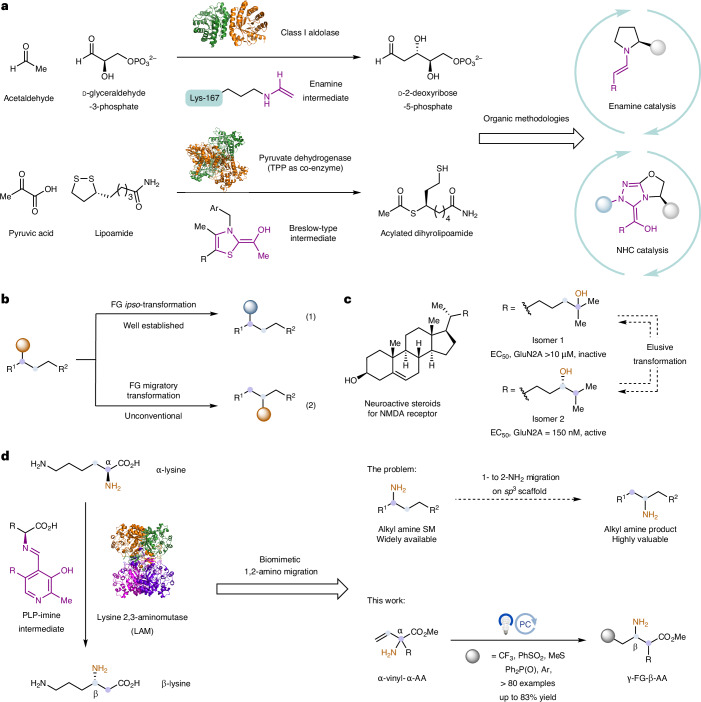
Nature has always been a great source of inspiration for new scientific breakthroughs. In organic chemistry, the inception of innovative synthetic methodologies often stems from scientists investigating and emulating enzymatic processes. For example, class I aldolases such as Escherichia coli d-2-deoxyribose-5-phosphate aldolase (DERA, EC 4.1.2.4) are known to catalyse asymmetric aldol reactions between two aldehydes. This process has been demonstrated to go through an enamine-based intermediate1. The identification of such a mechanism later inspired organic chemists to devise the groundbreaking enamine catalysis (Fig. 1a), which has enabled numerous new enantioselective methods and won the Nobel Prize in 20212,3. In another case, Breslow studied the mechanism behind several enzymatic carbon–carbon (C–C) bond-forming processes involving thiamine pyrophosphate (TPP), and proposed an intermediate derived from the condensation of the thiazole ring in TPP with the reactant carbonyl compound, which was later coined the ‘Breslow-type intermediate’ (Fig. 1a)4. Organic chemists took inspiration from the distinctive reactivity of these intermediates and have further expanded the utilization of these catalysts, giving rise to the field of N-heterocyclic carbene (NHC) catalysis5,6.
Fig. 1. Biomimetic 1,2-amino migration via photoredox catalysis.
a, The development of new organic methods is often inspired by enzymatic transformation. b, FG migratory transformation is a valuable but underdeveloped strategy for molecular modification, especially for late-stage functionalization. c, Regioisomers often pose quite different properties in drug discovery. d, One particular type of FG migratory transformation that intrigued us is 1,2-amino migration, when an amino group is translocated to its neighbouring location on an sp3 scaffold. Given the abundance of alkyl amines as feedstock reagents and their importance as synthetic building blocks, we believe that the chemical community could greatly benefit from such a method. However, to our knowledge, 1,2-amino migration has not yet been established as a practical technique in organic chemistry. Here we have developed a biomimetic photoredox-catalysed 1,2-amino migration method. This method was inspired by a LAM-catalysed α-lysine to β-lysine transformation. AA, amino acid; TPP, thiamine pyrophosphate; NHC, N-heterocyclic carbene; NMDA, N-methyl-d-aspartate; EC50, half maximal effective concentration.
We were motivated by the numerous enzymatic transformations that have not found their purely organic counterparts, and were interested in developing novel biomimetic organic methodologies. One particular class of reactions that we have recently become interested in is functional group (FG) migratory transformation (Fig. 1b). Typically, FG transformation does not involve a positional movement within the molecular skeleton (such as in a ring or carbon chain), and is hence termed an ipso-transformation (Fig. 1b, equation (1))7. A broad range of FG ipso-transformations have been developed, with wide-ranging applications, including cross-coupling chemistry and olefin metathesis8,9. By contrast, an organic transformation that involves the positional movement of a FG along the molecular skeleton is quite unconventional and has not yet received adequate attention (Fig. 1b, equation (2)). We argue that FG migratory transformation can serve as a flexible strategy for molecular modification, especially for the late-stage functionalization of highly complex molecules10–14. One particular application of such a strategy is the interconversion of late-stage regioisomers. As exemplified by the case shown in Fig. 1c, regioisomers often exhibit distinct properties, including bioactivities15. Nevertheless, the synthesis of structurally related regioisomers in medicinal chemistry still requires de novo route planning, which is often time- and resource-consuming. If a late-stage FG migratory transformation could be applied to such a system, it would enable the generation of multiple related regioisomers from one existing isomer in a single operation. In recent years, considerable effort has been dedicated to advancing this type of transformation. There has been notable progress in the community in developing FG migratory transformations of important FGs11,16–19, such as aryl, alkynyl, acyloxy, hydroxyl and cyano groups.
We were particularly interested in one class of FG migratory transformation, namely 1,2-amino migration, in which an amino group is translocated to its adjacent position on an sp3 scaffold (Fig. 1d). Considering the vast availability of alkyl amines as feedstock reagents and their importance as synthetic building blocks20, we anticipate that such a strategy could offer substantial value to the chemical community. To the best of our knowledge, 1,2-amino migration has not yet been developed as a useful method in the field of organic chemistry, and enzymatic processes can indeed provide a viable protocol for the implementation of this concept. Enzyme-catalysed 1,2-amino migration plays a vital role in various biochemical processes, including the biosynthesis of l-β-lysine21. Specifically, the interconversion of l-α-lysine to l-β-lysine is facilitated by lysine 2,3-aminomutase (LAM) in conjunction with pyridoxal 5′-phosphate (PLP) as a co-enzyme (Fig. 1d)22. However, given the specificity of enzyme catalysis, this reaction is only applicable to β-lysine synthesis, with essentially no substrate scope. In this Article we report a biomimetic 1,2-amino migration strategy, leveraging this biocatalytic mechanism and incorporating photoredox-catalysed conditions, for the development of a genetic protocol for a broad range of β-amino-acid syntheses.
Reaction design and optimization studies
Although 1,2-amino migration is a well-studied biochemical process, it remains unexplored by the synthetic organic chemistry community, probably because of the high bond dissociation energy of the C(sp3)–N bond and the non-negligible basicity of the amino group tending to complicate transition-metal-based reactions23,24. Recently, photoredox catalysis has revolutionized the landscape of molecule construction in organic synthesis, particularly in the realms of pharmaceuticals and natural products25–29. Consequently, we wondered whether it might be possible to emulate this naturally occurring process to achieve the modular synthesis of β-amino acids by utilizing visible-light catalysis to unlock the potential of the arylaldehyde to facilitate the 1,2-amino migration of both natural and unnatural α-amino-acid derivatives (Fig. 2a)30. In this catalysis process, the photocatalytically generated free-radical intermediates from feedstock chemicals B (CF3–SO2Na, PhSO2–Na, MeS–SMe, Ph2P(O)–H and Ph–Br) are intercepted by α-vinyl-aldimine esters D derived from the condensation of α-vinyl-α-amino acids A and arylaldehydes, resulting in the formation of the γ-FG-β-radical of α-amino-acid derivatives E. In the key migration step (Fig. 2a, E to F to G), as proposed and further chemically validated by Frey et al.22,31,32, azacyclopropyl carbinyl radical F arises through an analogous 3-exo-trig cyclization of the corresponding alkyl radical E onto an imine group, followed by rearrangement to generate the product-related γ-FG-α-radical of β-amino-acid derivatives G. Finally, a facile reduction followed by acidic work-up regenerates the photocatalyst and releases the product, γ-FG-β-amino acids C.
Fig. 2. Design plan, optimization and control experiments.
a, Design plan for 1,2-amino migration. b, Optimization and control experiments. aReaction conditions: 2 (0.1 mmol), CF3SO2Na (0.15 mmol), PC (1 mol%), K3PO4 (2.0 equiv.), MeCN (3 ml), λmax = 440 nm Kessil (40 W), N2, room temperature, 15 h, then work-up with 1 M HCl in THF, 6 h; 1H NMR yield with 1,3,5-trimethylbenzene as internal standard. b1H NMR yield before work-up with 1 M HCl. cReaction performed on a 0.3 mmol scale with PC (3 mol%), isolated yield. PC, photocatalyst; SET, single-electron transfer; ND, not detected; r.t., room temperature.
Inspired by the biological process, our investigation commenced with a focus on the 1,2-amino migration of α-vinyl-aldimine esters (Fig. 2b). Recognizing the importance of selecting an appropriate arylaldehyde for this transformation, we prepared a range of α-vinyl-aldimine esters (2a–2g) and evaluated the efficiency of 1,2-amino migration with sodium trifluoromethanesulfinate (CF3SO2Na) as model reactant in the presence of K3PO4 and 4CzIPN (1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene) at room temperature with MeCN as solvent. As shown in Fig. 2b, the presence of fluorine-containing arylaldehydes proved pivotal for the success of this reaction, probably due to their strong electron-withdrawing properties, which enhance the electrophilicity of the resulting imines to facilitate the effective trapping of the free-radical intermediates. The reaction proceeded smoothly, yielding γ-CF3-β-Leu-OMe 3 in 45% yield when 3,5-bis(trifluoromethyl)benzaldehyde 1a was used (Fig. 2b, entry 1). Although arylaldehydes 1b and 1c also yielded satisfactory results, albeit with slightly lower yields (entries 2 and 3), the use of other arylaldehydes appeared to impede the reaction progress (entry 4, trace). These results encouraged us to further explore different photocatalysts to improve the efficiency of the 1,2-amino migration process. To our delight, 79% yield was obtained by using Ir(dtbbpy)(ppy)2PF6 as photocatalyst (entry 5). Subsequent control experiments underscored the indispensable roles of both photocatalyst and light to the success of the reaction (entries 6 and 7, respectively). Notably, the yield of 3 was further improved when 3 mol% Ir(dtbbpy)(ppy)2PF6 was used (entry 8, 88% yield, 6.1:1 d.r.). It is worth mentioning that no intramolecular or intermolecular Mannich addition side reactions occurred between the proposed enolate anion intermediate and the electrophilic imine group.
Scope of γ-substituted β-amino acids
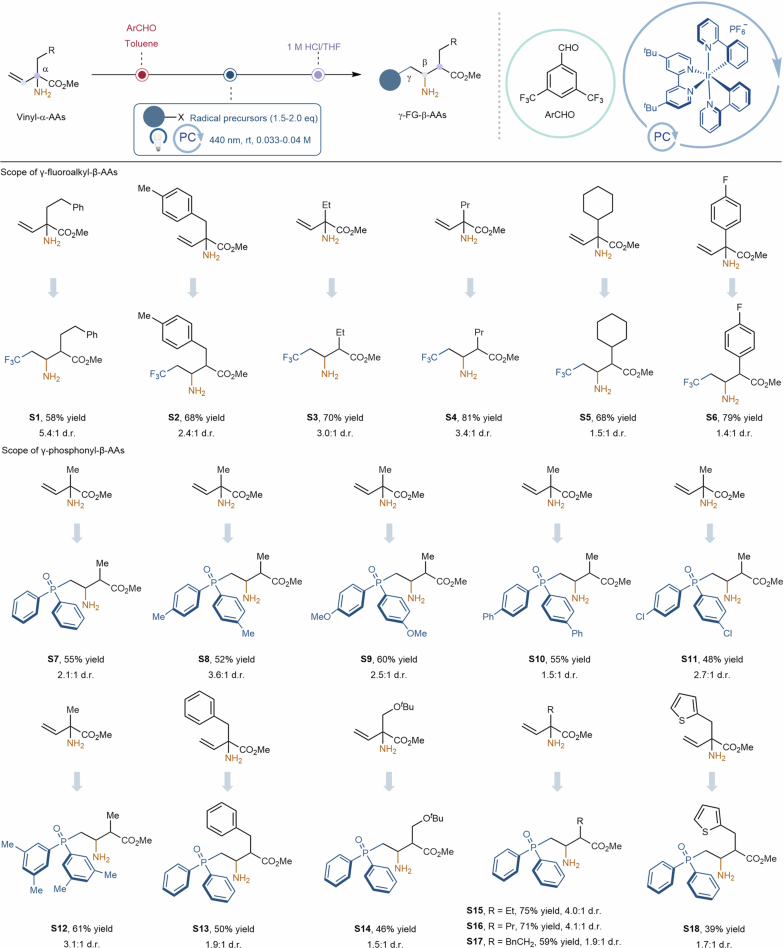
With the optimized reaction conditions in hand, we first performed a series of experiments to examine the scope of the α-amino-acid derivatives by employing CF3SO2Na as the radical precursor (Table 1). In general, γ-CF3-β-amino acids were obtained in exclusive chemo- and regioselectivity under the optimized conditions. A diverse range of natural α-amino-acid derivatives, such as α-Leu-OMe, α-Val-OMe, α-Ala-OMe and α-Phg-OMe, reacted efficiently with CF3SO2Na to afford the corresponding γ-CF3-β-amino acids (3–6, 69–83% yield). We were excited to find that α-Tyr(Me)-OMe, α-Glu-OMe, α-Asp-OMe and α-Ser(tBu)-OMe derivatives were also well tolerated, providing the desired products in satisfactory yields (7–10, 44–73% yield). Subsequently, we further examined the compatibility of the method with unnatural α-amino-acid derivatives. Pleasingly, these intricate substrates proved amenable to the reaction, showcasing the versatility of this protocol in the modification of unnatural α-amino acids (11 and 12, 41–74% yield, respectively). An additional six examples using other unnatural α-amino-acid derivatives for this 1,2-amino migration protocol are detailed in Extended Data Fig. 1. Encouraged by these promising outcomes, the scope of this reaction was further extended to the perfluoroalkylation of α-Leu-OMe derivatives. The hydrodifluoromethylation and perfluorobutylation of α-Leu-OMe derivatives proceeded smoothly, giving the desired products (13 and 14, 34% and 42% yield, respectively) with commercially available CF2HSO2Na and C4F9SO2Na as radical precursors. The observed yield losses are probably attributable to the high liposolubility of the products during extraction from the organic solvent using water.
Table 1.
Synthesis of γ-substituted β-amino acids via 1,2-amino migration
See Supplementary Sections 4.1 to 4.4 for full experimental details, and Extended Data Figs. 1 and 2 for additional examples. All yields are isolated yields in their hydrochloride salt forms except for products 8, 9 and 10, which were isolated in their free amine forms. aReaction condition for the 1,2-amino migration step using CF3SO2Na as the radical precursor: 2 (0.3 mmol), CF3SO2Na (0.45 mmol), Ir(dtbbpy)(ppy)2PF6 (3 mol%), K3PO4 (2.0 equiv.), MeCN (18 ml), λmax = 440 nm Kessil (40 W), N2, r.t., 15 h, then work-up with 1 M HCl in THF, 6 h; isolated yield over three steps from vinyl-α-amino acid. bYield of the one-pot procedure (see Supplementary Section 5 for details).
Extended Data Fig. 1. Additional examples of γ-fluoroalkyl-β-AAs and γ-phosphonyl-β-AAs.
All yields are isolated yield over three steps from vinyl-α-AA in their hydrochloride salt forms except for product S14, which is isolated in its free amine form. See Supplementary Information Section 4.1 to 4.2 for full experimental details.
Organophosphorus and organosulfone compounds are of great importance in medicinal chemistry33, biochemistry34 and catalysis35, as well as for their extensive synthetic utility as synthons36–39. Accordingly, it would be highly desirable to develop a new general protocol for a broad range of densely functionalized P- and S-containing molecules, such as P- and S-containing amino acids40. Importantly, this protocol permits rapid access to the structurally intriguing γ-phosphonyl-β-amino acids (15–19, 42–80% yield) with diarylphosphine oxides as radical precursors via photoredox-enabled 1,2-amino migration (Table 1). An additional 12 examples using other diarylphosphine oxides and other α-vinyl-α-amino-acid derivatives are detailed in Extended Data Fig. 1. Encouraged by our results, this 1,2-amino migration catalytic system has been shown to enable the formation of γ-sulfonyl-β-amino acids (20–23, 45–65% yield) under mild conditions with diverse aromatic and cyclopropyl sodium sulfinates as radical precursors (Table 1). Notably, this strategy could be amenable for the synthesis of γ-thioalkyl-β-amino acids in synthetically useful yield (24, 64% yield) with disulfides as radical precursors. An additional 20 examples for the synthesis of γ-sulfonyl/thioalkyl-β-amino acids are detailed in Extended Data Fig. 2. To our delight, this 1,2-amino migration could also be implemented in a one-pot strategy, albeit affording the desired β-amino acids in slightly lower yields (Table 1 and Extended Data Fig. 2; compound 6, 55% yield; compound 20, 33% yield; compound 21, 32% yield; compound 23, 63% yield; compound S20, 50% yield; compound S29, 50% yield; compound S35, 27% yield).
Extended Data Fig. 2. Additional examples of γ-sulfonyl-β-AAs and γ-thioalkyl-β-AAs.
All yields are isolated yield over three steps from vinyl-α-AA in their hydrochloride salt forms except for product S28, which is isolated in its free amine form. See Supplementary Information Section 4.3 to 4.4 for full experimental details. b The yield of the one-pot procedure, see Supplementary Information Section 5 for details.
Scope of γ-aryl-β-amino acids
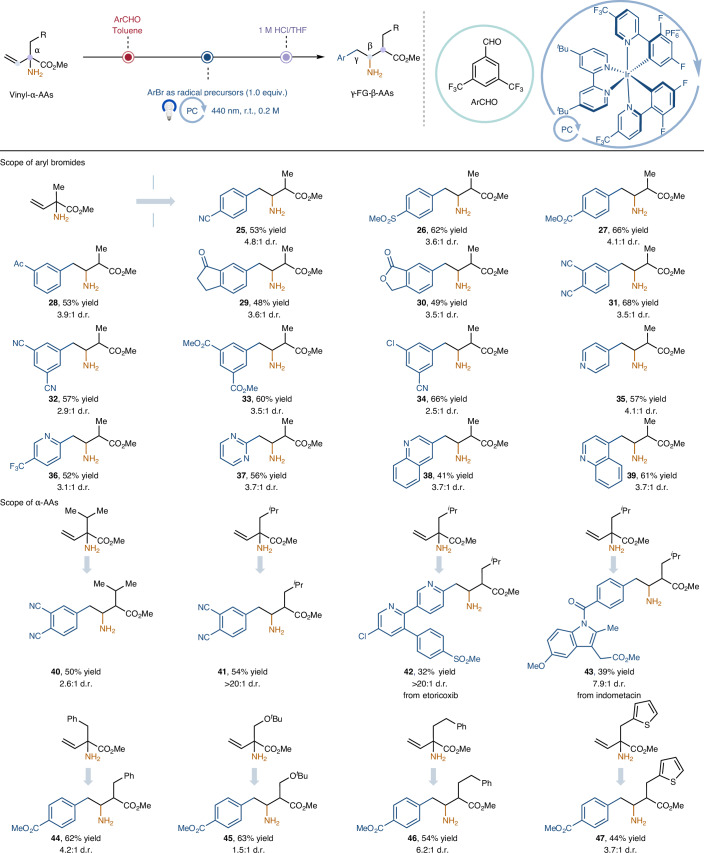
The application of our photoredox-enabled 1,2-amino migration strategy was next extended to the construction of γ-aryl-β-amino acids by utilizing bromoarenes as radical precursors under facile organosilane-mediated bromide abstraction41. As shown in Table 2, we carried out this study by using the α-vinyl-α-Ala-OMe derivative as model substrate. A variety of aryl bromides that incorporate a diverse array of substituents (nitrile, sulfone, ester, ketone, chloride groups) were all found to be competent substrates, giving synthetically useful yields of the desired products (25–34, 48–68% yield). Moreover, regarding the scope of heteroaryl bromides, pyridine- and pyrimidine-derived substrates were functionalized in moderated yields (35–37, 52–57% yield). 3- and 4-bromoquinoline were also readily used to afford their corresponding products in useful yields (38, 41% yield; 39, 61% yield). We next turned our attention to the scope of the α-amino-acid derivatives (Table 2). Pleasingly, natural α-amino-acid derivatives, including α-Val-OMe, α-Leu-OMe, α-Phe-OMe and α-Ser(tBu)-OMe, were viable for the successful delivery of the desired γ-aryl-β-amino acids in moderate to good yields (40–45, 32–63% yield). It is worth noting that pharmaceutical analogues 42 and 43 were effective substrates for late-stage functionalization. Finally, unnatural α-amino-acid derivatives were well tolerated for this transformation, giving products in satisfactory yields (46, 54% yield; 47, 44% yield).
Table 2.
Synthesis of γ-aryl-β-amino acids via 1,2-amino migration
See Supplementary Section 4.5 for full experimental details. All yields are isolated yields in their hydrochloride salt forms except for products 43 and 45, which were isolated in their free amine forms. Reaction condition for the 1,2-amino migration step: 2 (1.5 mmol), aryl bromides (0.3 mmol), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (1 mol%), K2CO3 (2.0 equiv.), (TMS)3SiNH-Ad (1.0 equiv.), MeCN (1.5 ml), λmax = 440 nm Kessil (40 W), N2, r.t., 2 h, then work-up with 1 M HCl in THF, 6 h; isolated yield over two steps from vinyl-α-aldimine esters. TMS, trimethylsilyl; Ad, adamantly.
Synthetic applications
To further demonstrate the potential application of this protocol, we carried out a scaled-up reaction in which 22 mmol α-Ala-OMe derivative was used. The reaction proceeded smoothly to afford γ-CF3-β-Ala-OMe·HCl 5 in 54% yield (Fig. 3a). Derivatization of the obtained β-amino acids 5 led to a shortcut for accessing other appealing molecular frameworks. A telescoped tosylation of 5 followed by reduction and a subsequent Mitsunobu reaction delivered the medicinally relevant azetidine 48 (58% overall yield over three steps). Similarly, tosylation of 5 followed by hydrolysis and intramolecular condensation afforded product 49 (38% overall yield over three steps) featuring a β-lactam structure, a highly privileged motif in biologically active compounds and pharmaceuticals42–44. Additionally, the free carboxylic acid could be prepared by adjusting the protecting groups of compound 5 through benzoyl protection and subsequent hydrolysis. Notably, the resulting carboxylic-acid moiety could serve as a synthetic handle in subsequent functionalization for downstream diversifications, such as the photoredox-catalysed Giese reaction (50, 44% yield)45 and fluorination (51, 80% yield)46. The conversion of this carboxylic acid with diphenylphosphoryl azide is noteworthy, providing the valuable building block vicinal diamine 52 (89% yield) through Curtius rearrangement47. We then proceeded with a preliminary investigation into the asymmetric version of this 1,2-amino migration reaction. We were pleased to achieve moderate enantioselectivities of γ-phosphonyl-β-amino acids 53 (82:18 e.r., 39:61 e.r.) and 54 (80:20 e.r., 27:73 e.r.) using a catalytic chiral (salen)MnIII catalyst. We speculate that the imine group in the γ-FG-β-alkyl radical can coordinate with the chiral (salen)MnIII catalyst, leading to a subsequent (salen)MnIII-induced 3-exo-trig cyclization that generates chirality (details are provided in Supplementary Section 8)48. This approach holds the potential to revolutionize the construction of chiral β-amino acids by simply remoulding the existing α-amino-acid skeletons, a realm that has not been explored (Fig. 3b). Furthermore, the amide condensation reactions of telaprevir block with γ-FG-β-amino-acid derivatives to generate tripeptides 55–58 were also demonstrated, adding intriguing structures to the polypeptide library (Fig. 3c). Leveraging our newly developed protocol with four distinct radical precursors, we successfully achieved the divergent synthesis of four different types of free γ-FG-β-amino acids, 59–62, from a common vinyl-α-amino acid, with good efficiency (48–74% yield; Fig. 3d). The introduction of methyl groups has the potential to substantially enhance the potency of bioactive compounds49. Consequently, we further demonstrated the utility of this protocol in the efficient preparation of a methylated sitagliptin analogue. Beginning with commercially available 1-bromo-2,4,5-trifluorobenzene, we accessed the target β-amino acid 63 from the corresponding α-amino acid in just three steps, achieving a 61% overall yield through this 1,2-amino migration sequence (Fig. 3e). Subsequently, β-amino acid 63 was efficiently elaborated to the target methylated analogue 64 in three steps and 65% overall yield.
Fig. 3. Synthetic applications.
a, Gram-scale preparation of γ-CF3-β-Ala-OMe·HCl 5 and further transformations for the synthesis of compounds 48–52. b, Primary attempts to construct chiral γ-phosphonyl-β-amino acids 53 and 54 using (salen)Mn(III) complex. c, Late-stage modification of a drug block and synthesis of tripeptides 55–58. d, Divergent synthesis of a number of γ-FG-β-amino acids 59–62 from one common vinyl amino acid. e, Synthesis of sitagliptin analogue 64. Supplementary Section 6 presents details of the experimental conditions. All yields are isolated yields in their hydrochloride salt forms except for product 64, which is isolated in its free amine form.
Mechanistic investigations
On the basis of our proposed design plan (Fig. 2a), we performed experimental studies to gain some mechanistic insights into the 1,2-amino migration reaction. Initially, the reaction of α-vinyl-aldimine esters 2n with diphenylphosphine oxide was completely inhibited in the presence of 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) as a radical scavenger, which is consistent with the radical nature of the 1,2-amino migration process (Fig. 4a). Subsequently, utilizing 1,1-diphenylethylene as the radical-trapping reagent under standard conditions (Fig. 4b) resulted in a 24% yield of the inseparable adducts 66 and 67, indicating the involvement of phosphonyl radical intermediates. Conducting the same experiment under standard conditions led to the isolation of both the non-migration product 65 and 1,2-amino migration adduct S14a (Fig. 4c), supporting the mechanistic interpretation that the γ-FG-β-radical of the α-amino-acid intermediate and the γ-FG-α-radical of the β-amino-acid intermediate were generated. These findings further indicate that the reaction probably proceeds through a cyclization–ring-opening sequence involving the intermediate azacyclopropyl carbinyl radical22,31,32. Density functional theory (DFT) calculations were also conducted to support the proposed mechanism based on the experimental observations (Fig. 4d; Supplementary Section 13 presents computational details). The formation of 2IM1 occurs via a radical addition of diphenylphosphine oxide to the vinyl terminal, potentially undergoing 3-exo-trig cyclization to access the intermediate azacyclopropyl carbinyl radical 2IM2. This cyclization is plausible due to the electrophilicity of imine-N and the spatial conformation of 2IM1, with an exergonic conversion by 1.6 kcal mol−1 and a favourable barrier of 12.2 kcal mol−1 (2TS1). Subsequently, 2IM2 proceeds with a ring-opening reaction to yield 2IM3 with an energy release of 8.2 kcal mol−1. The kinetics are favourable, with a significantly low barrier of 5.1 kcal mol−1 (2TS2). To delve deeper into the radical intermediates, a spin density analysis was conducted (Fig. 4e). The distribution of single electrons undergoes substantial changes during the reaction process, delocalizing to the π-systems in 2IM2 and 2IM3. These π-systems may stabilize the radicals, thereby reducing their thermodynamics, particularly the adjacent carbonyl group in 2IM3. Overall, this radical rearrangement has no great barrier and is exergonic, indicating the favourable occurrence of 1,2-amino migration.
Fig. 4. Mechanism investigations.
a, TEMPO-radical inhibition experiment. The reaction was completely inhibited in the presence of TEMPO as a radical scavenger, which is consistent with the radical nature of the 1,2-amino migration process. b, Radical-trapping experiment. The experiments indicate the involvement of phosphonyl radical intermediates. c, Capture of the γ-FG-β-radical of the α-amino-acid derivative and the γ-FG-α-radical of the β-amino-acid derivative. The experiments indicate that the reaction probably proceeds through a cyclization–ring-opening sequence involving the intermediate azacyclopropyl carbinyl radical. d, DFT free-energy profile of 1,2-amino migration. ΔGsol, Gibbs free energy of solvation. e, Spin density isosurface of the radical intermediates. Details are provided in Supplementary Section 13.
Conclusions
In summary, we have developed a biomimetic 1,2-amino migration by harnessing a biocatalytic mechanism and photoredox catalysis for application in the development of a general strategy for the modular synthesis of densely functionalized β-amino acids. This bio-inspired platform exhibits remarkable tolerance towards various FGs, enabling access to an extensive library of γ-substituted β-amino acids, which complements established methods. In addition, the reaction is amenable to late-stage modification of complex molecules and gram-scale synthesis. We anticipate that this broadly applicable approach will provide a robust strategy for the construction of β-amino acids and help alleviate the need to redesign synthetic routes when targeting α-amino-acid modification. Further applications of this 1,2-amino migration strategy are currently under investigation in our laboratory.
Methods
General procedure for the synthesis of γ-fluoroalkyl-β-amino acids
An oven-dried vial (40 ml) containing a stirring bar was charged with 2 (0.3 mmol, 1.0 equiv.), HxCyFzSO2Na (0.45 mmol, 1.5 equiv.), [Ir(dtbpy)(ppy)2]PF6 (8.2 mg, 0.009 mmol, 3 mol%), K3PO4 (0.6 mmol, 127.2 mg, 2.0 equiv.) and CH3CN (18 ml). The vial was sealed and the solution degassed by sparging with nitrogen for 5 min before sealing with parafilm. The reaction was stirred and irradiated using 40-W 440-nm blue light-emitting diode (LED) lamps for 15 h. After completion of the reaction, the mixture was evaporated under vacuum and purified by flash chromatography to provide the imine product. A 20-ml vial was then charged with the imine product, tetrahydrofuran (THF 3 ml) and 1 N HCl (3 ml). The resulting solution was stirred at room temperature for 6 h. After consumption of the starting material was confirmed by thin layer chromatography (TLC) analysis, the solution was poured into water (6.0 ml) and extracted with petroleum ether (10 ml × 2). The water layer was combined and water was removed in vacuo, afforded the corresponding γ-fluoroalkyl-β-amino acids.
General procedure for the synthesis of γ-aryl-β-amino acids
A flame-dried 8-ml reaction vial equipped with a magnetic stir bar was charged with 2 (1.5 mmol, 5.0 equiv.), aryl bromide (0.3 mmol, 1.0 equiv.), [Ir(dFCF3ppy)2(dtbpy)]PF6 (3.4 mg, 0.003 mmol, 1 mol%), (TMS)3SiNH-Ad (0.3 mmol, 119.2 mg, 1.0 equiv.), K2CO3 (0.6 mmol, 82.9 mg, 2.0 equiv.) and CH3CN (1.5 ml). The vial was sealed and the solution degassed by sparging with nitrogen for 5 min before sealing with parafilm. The reaction was stirred and irradiated using 40-W 440-nm blue LED lamps for 2 h. After completion of the reaction, the mixture was evaporated under vacuum and purified by flash chromatography to provide the imine product. A 20-ml vial was then charged with the imine product, THF (3 ml) and 1 N HCl (3 ml). The resulting solution was stirred at room temperature for 6 h. After consumption of the starting material was confirmed by TLC analysis, the solution was poured into water (6.0 ml) and extracted with petroleum ether (10 ml × 2). The water layer was combined and water was removed in vacuo, afforded the corresponding γ-aryl-β-amino-acid products.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-025-01775-2.
Supplementary information
Supplementary protocols, discussions, figures, NMR spectra and HPLC traces.
The xyz coordinates of DFT calculations.
Crystallographic data for compound 68. CCDC reference 2324720.
Crystallographic data for compound 69. CCDC reference 2324723.
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (22171049, 22301054), the Natural Science Foundation of Zhejiang Province (LDQ23B020001), the Hangzhou leading innovation and entrepreneurship team project (TD2022002) and research funds of Hangzhou Institute for Advanced Study, UCAS.
Extended data
Author contributions
X.Z. and Y.L. conceived the idea. X.Z. directed and designed the study. W.F., Y. Cui, B.Z., Y. Chen and L.B. performed the experiments and mechanistic studies. B.Z., Y.L. and X.Z. wrote the manuscript. All authors contributed to the analysis and interpretation of the data.
Peer review
Peer review information
Nature Chemistry thanks Estíbaliz Merino, Peng-Fei Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. The supplementary crystallographic data for this Article can be obtained free of charge from the Cambridge Crystallographic Data Centre (CCDC) under accession nos. CCDC 2324720 (compound 68) and CCDC 2324723 (compound 69). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Competing interests
A Chinese patent application has been filed (applicant: Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences; inventors: X.Z., W.F., Y. Cui, Y. Chen; application no., 202311807786.3; status of application, pending; specific aspect of manuscript covered in patent application, ‘the synthetic methods and development regarding the 1,2-amino migration for the construction of γ-substituted-β-AAs’. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Weitai Fan, Yuang Cui, Beibei Zhan.
Extended data
is available for this paper at 10.1038/s41557-025-01775-2.
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-025-01775-2.
References
- 1.Heine, A. et al. Observation of covalent intermediates in an enzyme mechanism at atomic resolution. Science294, 369–374 (2001). [DOI] [PubMed] [Google Scholar]
- 2.List, B., Lerner, R. A. & Barbas, C. F. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc.122, 2395–2396 (2000). [Google Scholar]
- 3.MacMillan, D. The advent and development of organocatalysis. Nature455, 304–308 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Breslow, R. On the mechanism of thiamine action. IV. Evidence from studies on model systems. J. Am. Chem. Soc.80, 3719–3726 (1958). [Google Scholar]
- 5.Flanigan, D. M., Romanov-Michailidis, F., White, N. A. & Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev.115, 9307–9387 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkinson, M., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature510, 485–496 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Perrin, C. L. & Skinner, G. A. Directive effects in electrophilicaromatic substitution (‘ipso factors’). Nitration of haloanisoles. J. Am. Chem. Soc.93, 3389–3394 (1971). [Google Scholar]
- 8.Wu, X.-F., Anbarasan, P., Neumann, H. & Beller, M. From noble metal to Nobel Prize: palladium-catalyzed coupling reactions as key methods in organic synthesis. Angew. Chem. Int. Ed.49, 9047–9050 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Ogba, O. M., Warner, N. C., O’Leary, D. J. & Grubbs, R. H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev.47, 4510–4544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science363, eaat0805 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Wu, X., Ma, Z., Feng, T. & Zhu, C. Radical-mediated rearrangements: past, present and future. Chem. Soc. Rev.50, 11577–11613 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Wu, Z., Xu, X., Wang, J. & Dong, G. Carbonyl 1,2-transposition through triflate-mediated α-amination. Science374, 734–740 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao, K. & Knowles, R. R. Contra-thermodynamic positional isomerization of olefins. J. Am. Chem. Soc.144, 137–144 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Friese, F. W., Mück-Lichtenfeld, C. & Studer, A. Remote C−H functionalization using radical translocating arylating groups. Nat. Commun.9, 2808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La, D. S. et al. Neuroactive steriod N-methyl-d-aspartate receptor positive allosteric modulators: synthesis, SAR and pharmacological activity. J. Med. Chem.62, 7526–7542 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Edelmann, S. & Lumb, J.-P. A para- to meta-isomerization of phenols. Nat. Chem.16, 1193–1199 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Chen, K. et al. Functional-group translocation of cyano groups by reversible C–H sampling. Nature620, 1007–1012 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Li, W. et al. Distal radical migration strategy: an emerging synthetic means. Chem. Soc. Rev.47, 654–667 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Zhao, G., Yao, W., Mauro, J. N. & Ngai, M.-Y. Excited-state palladium-catalyzed 1,2-spin-center shift enables selective C-2 reduction, deuteration and iodination of carbohydrates. J. Am. Chem. Soc.143, 1728–1734 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger, K. J. & Levin, M. D. Reframing primary alkyl amines as aliphatic building blocks. Org. Biomol. Chem.19, 11–36 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Frey, P. A. Radical mechanisms of enzymatic catalysis. Annu. Rev. Biochem.70, 121–148 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Ballinger, M. D., Reed, G. H. & Frey, P. A. An organic radical in the lysine 2,3-aminomutase reaction. Biochemistry31, 949–953 (1992). [DOI] [PubMed] [Google Scholar]
- 23.Trowbridge, A., Walton, S. M. & Gaunt, M. J. New strategies for the transition-metal-catalyzed synthesis of aliphatic amines. Chem. Rev.120, 2613–2692 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Laurence, C., Brameld, K. A., Graton, J., Le Questel, J.-Y. & Renault, E. The pK(BHX) database: toward a better understanding of hydrogen-bond basicity for medicinal chemists. J. Med. Chem.52, 4073–4086 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev.113, 5322–5363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayanam, J. M. R. & Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev.40, 102–113 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Xuan, J. & Xiao, W.-J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed.51, 6828–6838 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev.116, 10035–10074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature554, 41–49 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Cabrele, C., Martinek, T. A., Reiser, O. & Berlicki, L. Peptides containing β-amino acid patterns: challenges and successes in medicinal chemistry. J. Med. Chem.57, 9718–9739 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Ballinger, M. D., Frey, P. A. & Reed, G. H. Structure of a substrate radical intermediate in the reaction of lysine 2,3-aminomutase. Biochemistry31, 10782–10789 (1992). [DOI] [PubMed] [Google Scholar]
- 32.Han, O. & Frey, P. A. Chemical model for the pyridoxal 5′-phosphate dependent lysine aminomutases. J. Am. Chem. Soc.112, 8982–8983 (1990). [Google Scholar]
- 33.Heidel, K. M. & Dowd, C. S. Phosphonate prodrugs: an overview and recent advances. Future Med. Chem.11, 1625–1643 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horsman, G. P. & Zechel, D. L. Phosphonate biochemistry. Chem. Rev.117, 5704–5783 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Tang, W. & Zhang, X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev.103, 3029–3070 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Ulman, A. et al. New sulfonyl-containing materials for nonlinear optics: semiempirical calculations, synthesis and properties. J. Am. Chem. Soc.112, 7083–7090 (1990). [Google Scholar]
- 37.Trost, B. M. Chemical chameleons. Organosulfones as synthetic building blocks. Bull. Chem. Soc. Jpn61, 107–124 (1988). [Google Scholar]
- 38.Carreno, M. C. Applications of sulfoxides to asymmetric synthesis of biologically active compounds. Chem. Rev.95, 1717–1760 (1995). [Google Scholar]
- 39.Du, X., Cheng-Sánchez, I. & Nevado, C. Dual nickel/photoredox-catalyzed asymmetric carbosulfonylation of alkenes. J. Am. Chem. Soc.145, 12532–12540 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buquoi, J. Q., Lear, J. M., Gu, X. & Nagib, D. A. Heteroarene phosphinylalkylation via a catalytic, polarity-reversing radical cascade. ACS Catal.9, 5330–5533 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le, C., Chen, T. Q., Liang, T., Zhang, P. & MacMillan, D. W. C. A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science360, 1010–1014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng, J., Qi, X., Li, M., Chen, P. & Liu, G. Palladium-catalyzed intermolecular aminocarbonylation of alkenes: efficient access of β-amino acid derivatives. J. Am. Chem. Soc.137, 2480–2483 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Tan, G. et al. Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes. Nat. Chem.14, 1174–1184 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Yue, J. P. et al. Metallaphotoredox-enabled aminocarboxylation of alkenes with CO2. Nat. Catal.6, 959–968 (2023). [Google Scholar]
- 45.Chu, L., Ohta, C., Zuo, Z. & MacMillan, D. W. C. Carboxylic acids as a traceless activation group for conjugate additions: a three-step synthesis of (±)-Pregabalin. J. Am. Chem. Soc.136, 10886–10889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ventre, S., Petronijevic, F. R. & MacMillan, D. W. C. Decarboxylative fluorination of aliphatic carboxylic acids via photoredox catalysis. J. Am. Chem. Soc.137, 5654–5657 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei, J., Zhang, J., Cheng, J. K., Xiang, S.-H. & Tan, B. Modular enantioselective access to β-amino amides by Brønsted acid-catalysed multicomponent reactions. Nat. Chem.15, 647–657 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Larrow, J. F. & Jacobsen, E. N. Asymmetric processes catalyzed by chiral (Salen)metal complexes. Top. Organomet. Chem.6, 123–152 (2004). [Google Scholar]
- 49.Barreiro, E. J., Kümmerle, A. E. & Fraga, C. A. M. The methylation effect in medicinal chemistry. Chem. Rev.111, 5215–5246 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary protocols, discussions, figures, NMR spectra and HPLC traces.
The xyz coordinates of DFT calculations.
Crystallographic data for compound 68. CCDC reference 2324720.
Crystallographic data for compound 69. CCDC reference 2324723.
Data Availability Statement
All data supporting the findings of this study are available within the Article and its Supplementary Information. The supplementary crystallographic data for this Article can be obtained free of charge from the Cambridge Crystallographic Data Centre (CCDC) under accession nos. CCDC 2324720 (compound 68) and CCDC 2324723 (compound 69). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.