Abstract
This study aimed to evaluate the antibacterial efficacy of calcium hydroxide (Ca(OH)2), Ca(OH)2 + resveratrol (RV), Ca(OH)2 + silver nanoparticles(AgNPs), and Ca(OH)2 + salicylic acid (SA) against Enterococcus faecalis (E. faecalis) using culture methods and field emission scanning electron microscopy (FE-SEM). Fifty-four extracted single-rooted teeth were prepared with standardized internal resorption cavities and inoculated with E. faecalis (ATCC 29212). After 21 days of incubation, baseline bacterial counts were determined. The teeth were randomly assigned to five groups (n = 10) based on the applied medicament. After 14 days of incubation, bacterial counts were reassessed, and two samples from each group were analyzed using FE-SEM. Data were analyzed using one-way ANOVA and Duncan’s test (p < .05). All medicament groups significantly reduced bacterial counts (p < .001), with Ca(OH)2 + RV, Ca(OH)2 + AgNPs, and Ca(OH)2 + SA demonstrating superior antibacterial activity compared to Ca(OH)2 alone (p < .001). The saline group exhibited the highest bacterial counts (p < .001). Combining Ca(OH)₂ with RV, AgNPs, or SA significantly enhances antibacterial efficacy in internal root resorption cases. These combinations may serve as effective alternatives to Ca(OH)2 alone in endodontic disinfection. Intracanal medicament application plays a vital role in eliminating microorganisms and enhancing the success of endodontic treatment, particularly in cases of internal root resorption. This study highlights the superior antibacterial efficacy of Ca(OH)2 combined with RV, AgNPs, or SA against E. faecalis. These findings suggest that incorporating these adjuncts into intracanal medicaments may improve disinfection outcomes, offering potential clinical benefits in managing internal root resorption cases.
Keywords: Internal root resorption, Endodontic treatment, E. faecalis, Calcium hydroxide, Resveratrol, Salicylic acid, Silver nanoparticles
Subject terms: Biofilms, Nanoparticles
Introduction
Root resorption is a destructive process caused by inflammatory interactions, leading to tissue demineralisation1. Inflammatory internal root resorption (IRR) is caused by necrotic coronal pulp and progresses due to microbial contamination2,3. While nonsurgical root canal therapy is the primary treatment for IRR, the irregular resorptive cavities complicate complete debridement. Ultrasonic irrigant activation enhances the penetration of irrigation solutions; however, bacteria may still persist4. To enhance disinfection, intracanal antibacterial medicaments are recommended5.
In root canal treatments and IRR, calcium hydroxide (Ca(OH)₂) is widely used as an intracanal medicament due to its high alkalinity, tissue-dissolving ability, and clastic inhibition. Its limited diffusion into dentinal tubules, however, reduces its efficacy against E. faecalis6–8. Alternative intracanal medicaments capable of more effectively eliminating microbes in the root canal system must be thus developed. To enhance the antibacterial activity of Ca(OH)₂, various materials have been added to it and whether they improve its microbial elimination has been evaluated5,9,10.
Resveratrol (3,4′,5-trihydroxystilbene, RV) is a phytoalexin commonly found in various foods and beverages, such as red wine, grapes, peanuts, and strawberries. It exhibits antimicrobial properties in addition to its well-known antioxidant effects and helps prevent certain diseases, such as cancer and coronary heart disease11,12. It also stimulates osteoblast differentiation while inhibiting osteoclastic activity12,13.
In recent years, nanoparticles have been integrated into disinfection strategies due to their distinctive physicochemical properties, including a high surface area-to-mass ratio, nanoscale dimensions, functional versatility, and strong antibacterial efficacy14–16. Research on silver nanoparticles (AgNPs) has demonstrated their potential as an adjunctive agent in endodontic treatment. However, they require prolonged interaction times for effective bacterial elimination, rendering them more suitable as an intracanal medicament than as an irrigant16. Adding AgNPs to Ca(OH)2 also enhances the latter’s antimicrobial efficacy against E. faecalis17,18.
Salicylic acid (SA), a proton pump inhibitor with broad antimicrobial activity, has been reported to be effective against E. faecalis, Candida albicans, and Escherichia coli19,20. It has long been used in the cosmetics industry as a preservative and as an anti-acne treatment21. Since SA is acidic in nature, it must be combined with Ca(OH)₂ to prevent dentin erosion and achieve a neutral pH range. This combination aims to optimise the beneficial properties of both components, including SA’s antibacterial activity, while maintaining the pH within a physiological range that supports stem cell viability and alkaline phosphatase (ALP) function22,23.
Only a few studies have examined the antibacterial efficacy of SA and AgNPs with Ca(OH)₂ against E. faecalis, and none have assessed RV. This in vitro study thus aimed to compare the antibacterial effectiveness of Ca(OH)₂, RV, AgNPs, and SA in disinfecting IRR-affected root dentin using microbiological culture methods and FE-SEM imaging. The null hypothesis stated that there would be no significant differences among the medicaments in terms of their antibacterial efficacy.
Materials and methods
Study design and sample size calculation
This study followed the Preferred Reporting Items for Laboratory Studies in Endodontology (PRILE) 2021 guidelines (Fig. 1) and was approved by the Non-Interventional Clinical Research Ethics Committee of Kutahya Health Sciences University (Ethics No: 2023/12–28).
Fig. 1.
PRILE 2021 flowchart.
The study was performed on extracted human teeth, and its sample size was determined using GPower (v3.1.9.7) following a previous study24. For a one-way ANOVA design with 80% power, an effect size of 0.5, and a Type I error (α) of 0.05, the required sample size was calculated as n = 10 per group (total: 50 teeth), yielding an actual power of 81%. Since four additional samples were allocated for FE-SEM (Hitachi Regulus 8230 FE-SEM, Japan) analysis, the final sample size was 54.
Selection and preparation of samples
In total, 54 extracted single-rooted human maxillary central teeth satisfying the selection criteria—a single root and teeth without caries, fractures, calcifications, resorptions, and anatomic aberrations—were selected. These were selected from the teeth extracted for orthodontic or periodontal reasons with informed consent by the Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Kutahya Health Sciences University. The teeth were examined under a microscope, and unsuitable specimens were excluded. To further assess the canal curvature and Schneider’s angle, CBCT imaging was used. Soft tissue and debris were removed mechanically, followed by disinfection in 0.5% Chloramine-T for 48 h. The teeth were stored in phosphate-buffered saline at 4 °C, and the root lengths were standardised to 18 ± 2 mm using a digital calliper. The crowns were removed with a diamond fissure bur, and the apical patency was confirmed using a #10 K-file.
Root canal shaping and the preparation of IRR cavities
Root canal instrumentation was conducted using the WaveOne Gold file system (size 45/0.05) (Dentsply Sirona, Switzerland) in conjunction with the X-Smart IQ endodontic motor (Dentsply Sirona, Ballaigues, Switzerland), and the WaveOne Gold-specific setting was employed. During instrumentation, 2.5% sodium hypochlorite (NaOCl) (Wizard, Rehber Chemistry, Istanbul, Turkey) was used for irrigation between the file changes. The smear layer was removed using 17% EDTA (Imicryl Ltd., Konya, Turkey) and 2.5% NaOCl, followed by final irrigation with distilled water. The canals were then dried with sterile paper points (Diadent Group International Inc., Chongju, Korea).
IRR cavities were prepared by following a modified version of Gençoğlu ve ark24. and Topçuoğlu ve ark25. To stabilise the sectioned roots and simulate periapical tissue resistance, the roots were placed in Eppendorf tubes filled with silicone impression material (Zhermack, Rovigo, Italy). For accurate realignment, reference marks were made on the mesial and distal surfaces. The roots were transversely sectioned 7 mm coronally from the apex using a thin diamond disc (Sunshine, Langenhagen, Germany), and 2-mm diameter, 1-mm deep resorption cavities were created at the root canal lumen with a diamond round bur (G&Z Instrumente).
After preparing the IRR cavities, the smear layer was removed with 17% EDTA and 5% NaOCl, followed by 37% phosphoric acid for 30 s and rinsing with distilled water (29). The cavities were further cleaned with 5% NaOCl and 17% EDTA, followed by final irrigation. The residual NaOCl was neutralised using 10% sodium thiosulfate (Merck, Darmstadt, Germany) for 5 min.
Using cyanoacrylate glue (Derby Kimya, Istanbul, Turkey), the root halves were reassembled, and paper points were placed inside the canals to prevent adhesive leakage. Two layers of nail varnish were used to seal the root surfaces, while composite resin was used to seal the apical regions (Fig. 2). To confirm IRR cavity uniformity and tooth dimensions, periapical radiographs and CBCT images were taken (Fig. 3).
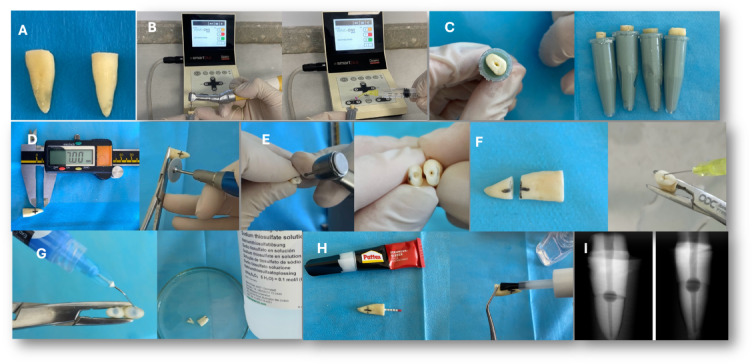
Fig. 2.
(A) Standardization of the samples by decoronating them at 18 ± 2 mm, (B) Preparation and irrigation of the samples, (C) Placement of the samples into Eppendorf tubes filled with silicone impression material, (D) Sectioning of the roots using a fine diamond disc at 7 mm from the apex in the coronal direction, (E) Creation of resorption cavities using a 2 mm diameter round diamond bur, (F) Irrigation of each root half to remove the smear layer, (G) Etching of the resorption cavities with phosphoric acid followed by immersion in 10% sodium thiosulfate for 5 min for neutralization, (H) Reapproximation of the root halves using cyanoacrylate adhesive, with a paper cone placed to prevent leakage into the canal, and coating of the external root surfaces with nail varnish to prevent external contamination, (I) Radiographic image of the teeth with prepared resorption cavities after reassembly.
Fig. 3.
CBCT imaging of teeth with prepared resorption cavities. (A) Measurement of tooth length in the corono-apical direction (B) Measurement of tooth length in the buccolingual direction (C) Measurement of tooth length in the mesiodistal direction (D) Measurement of the resorption cavity diameter.
Sterilisation of root canals
Each root was placed in a 1.5 mL Eppendorf tube containing phosphate-buffered saline (PBS, pH 7.2; Biomatik, Ontario, Canada) and sterilised in an autoclave (121 °C, 15 psi, 15 min) along with silicone-embedding materials. After sterilisation, the samples were incubated at 37 °C for 48 h to confirm the absence of bacterial contamination. FE-SEM imaging was performed on two randomly selected teeth as negative controls to assess dentinal tubule patency, complete microbial elimination, and smear layer removal (Fig. 4).
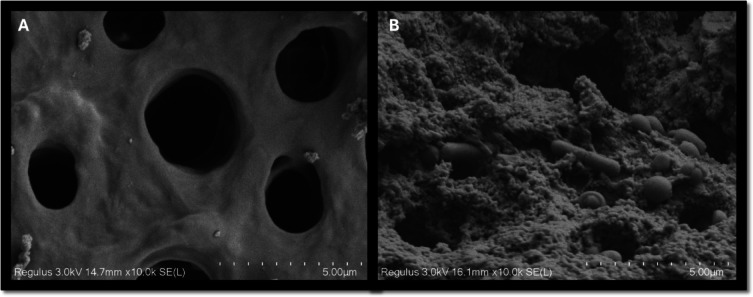
Fig. 4.
(A) FE-SEM images of cross-sections from the resorption cavities of sterilized teeth at 10,000x magnification, (B) FE-SEM images of cross-sections from the resorption cavities of the positive control group at 10,000× magnification.
Contamination of root canals with E. faecalis
E. faecalis ATCC 29,212 was cultured in a brain-heart infusion (BHI) broth (0.25% glucose; Difco Laboratories, Detroit, MI, ABD) at 37 °C for 24 h. A bacterial suspension (3 × 10⁸ cells/mL) was then introduced into the BHI medium containing the roots. To establish contamination, each root canal received 5 mL of sterile BHI mixed with 5 mL of the inoculum. The incubation continued for 21 days at 37 °C and 95% humidity, with fresh cultures added every 48 h. Biofilm formation was confirmed via FE-SEM on two randomly selected samples. Before further procedures, the root surfaces were wiped with 5% NaOCl and repositioned in silicone blocks.
Determination of the minimum inhibitory concentration (MIC) of resveratrol for E. faecalis
The Mueller-Hinton Broth (MHB; Difco Laboratories, Detroit, MI, ABD) was prepared at double strength. A solution of 4 mg RV, 1 g Ca(OH)₂, and 1 g glycerol was prepared. An E. faecalis strain was cultured in a single-strength MHB a day before the experiment. The bacterial suspension was adjusted to the 0.5 McFarland standard (10⁸ CFU/mL) and used for serial dilutions. In 11 Eppendorf tubes, 800 µL of distilled water was added, followed by 800 µL of the RV solution in the first tube. Serial dilutions were performed stepwise to the 12th tube. In a 96-well plate, 100 µL of the diluted solutions and MHB were added and incubated at 37 °C for 24 h. The MIC was determined as 0.5 mg/mL (500 µg) by identifying the first well without bacterial growth.
Experimental groups and application of medicaments
Fifty teeth with established E. faecalis biofilm were randomly assigned to five groups based on the applied medicaments: saline, Ca(OH)₂, AgNP + Ca(OH)₂, SA + Ca(OH)₂, and RV + Ca(OH)₂ (n = 10 per group).
Saline: 20 µL of sterile saline was introduced into the root canal, and the coronal portion was sealed with a cotton pellet and 1 mm Cavit.
Ca(OH)2: 20 µL of Ca(OH)₂ (UltraCal® XS, Ultradent, USA) was applied thrice using a #25 Lentulo spiral for 1 min and sealed with Cavit.
AgNP + Ca(OH)2: A 1:1 mixture of 2 g Ca(OH)₂ and 2 g AgNP solution (Silver Nanopowder/Nanoparticles Dispersion; 99.99% purity, 2200 ppm, 3 nm spherical nanoparticles; Nanografi, Turkey) was applied as above.
SA + Ca(OH)2: A 3.3:1:1 mixture of 6.6 g SA (2-hydroxybenzoic acid (2-(HO)C6H4CO2H), ACS reagent, 99.0% purity; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), 2 g Ca(OH)2, and 2 g distilled water was introduced using the same method.
RV + Ca(OH)2: 10 g Ca(OH)₂ was mixed with 10 g glycerol and 5 mg RV (HY-16561 MedChemExpress, USA) was homogenised and applied using the same method as above.
To maintain 100% humidity, the medicament-treated samples were placed in Eppendorf tubes containing sterile saline-soaked gauze (1 cm × 1 cm), with an additional saline-soaked cotton pellet (300 µL) placed over the dentin blocks. The tubes were sealed with parafilm and incubated at 37 °C in a 100% humid environment for two weeks.
Bacterial count determination and sample collection
To remove the medicaments and collect the final samples, the root canals were irrigated with 2 mL of sterile 0.9% saline using a 30-gauge needle, positioned 1 mm short of the working length. Circumferential filing with a #25 H-file preceded sample collection. A sterile #45 paper point was placed at working length for 1 min to absorb the residual saline. The samples were collected using three paper points and a fine microbrush and transferred aseptically into Eppendorf tubes containing 1.5 mL of sterile saline.
The initial bacterial load (before medicament application) and the final bacterial load (after medicament application) were assessed by collecting the residual saline from the canal lumen using three sterile paper points and a 0.8 mm fine microbrush. Both the initial and final samples were vortexed in an Eppendorf tube for 40 s. Subsequently, 100 µL of the 10⁻² dilution was plated in duplicate on BHI agar. After incubation at 37 °C for 24 h, the colony-forming units (CFUs) were counted.
FE-SEM analysis
Two teeth per group were randomly selected. Using a diamond bur (Komet, USA), shallow longitudinal grooves were created on the root surfaces without penetrating the canal. The roots were split along these grooves using a cement spatula, rinsed with saline, and fixed in 10% formalin for 24 h. After sequential ethanol dehydration (50–100%), the samples were air-dried overnight, mounted on SEM stubs, and gold-palladium coated (300 Å). FE-SEM imaging was performed at 10 kV (Hitachi Regulus 8230, Germany), capturing images from the simulated resorption cavities (Fig. 5).
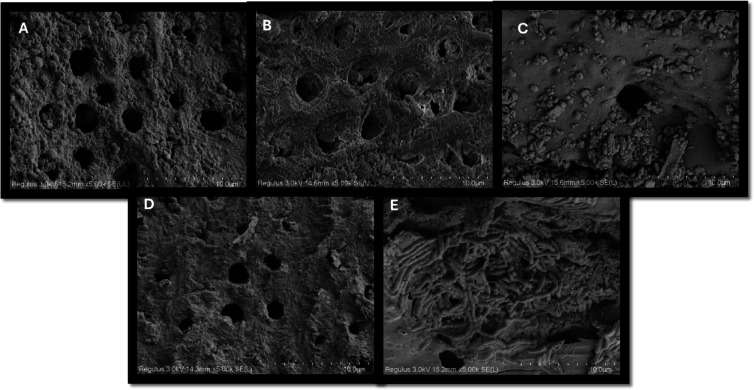
Fig. 5.
FE-SEM Images of groups obtained from resorption cavities after medicament application at 5000× magnification (A) AgNP + Ca(OH)2, (B) RV + Ca(OH)2, (C) SA + Ca(OH)2, (D) Ca(OH)2, (E) Saline.
Statistical analysis
The Shapiro–Wilk test confirmed the normal distribution of continuous variables, allowing for parametric analysis. The descriptive statistics were reported as mean, standard deviation, and range (max–min). The group comparisons were conducted using one-way ANOVA, with Duncan’s test for post-hoc analysis. A p-value < 0.05 was considered statistically significant. Analyses were performed using IBM SPSS (v26, Windows).
Results
MIC of resveratrol
After 24 h of incubation, RV’s MIC was determined to be 0.5 mg/mL, as no visible bacterial growth was observed.
CFU analysis
Following 21 days of incubation, the initial biofilm microorganism count on root canal surfaces averaged 5.12 log₁₀ CFU, with no significant differences among the groups (p > .05) (Table 1; p = 0.183). No bacterial growth was observed in the negative control group. The mean CFU values, standard deviations, and the bacterial count changes (log₁₀) before and after medicament application are detailed in Table 2(p = 0.001). A significant bacterial reduction was observed among the medicament groups (p < .001), with the highest reduction in the RV + Ca(OH)₂ group and the lowest in the saline group (p < .001). The RV + Ca(OH)₂, AgNp + Ca(OH)₂, and SA + Ca(OH)₂ groups demonstrated greater bacterial reduction than Ca(OH)₂ alone (p < .001), though no significant differences were found among these three groups.
Table 1.
Mean CFU, standard deviation, median, and range values of the initial microorganism count.
| Groups | Mean ± Std. Dev. | Range | *p. | |
|---|---|---|---|---|
| Initial microorganism counts | Ca(OH)2 + RV | 5.38 ± 0.81 | 2.62 | 0.183 |
| Ca(OH)2 + AgNPs | 5.29 ± 0.67 | 2.00 | ||
| Ca(OH)2 | 4.88 ± 0.45 | 1.35 | ||
| Ca(OH)2 + SA | 5.19 ± 0.38 | 1.02 | ||
| Saline | 4.86 ± 0.56 | 1.46 |
*Ca(OH)2: calcium hydroxide, AgNPs: silver nanoparticle, SA: salicylic acid, RV: resveratrol.
Significance value italics.
Table 2.
Mean changes (log₁₀) in the bacterial count before and after medicament application across groups.
| Groups | Mean ± Std. Dev. | Range | *p. | |
|---|---|---|---|---|
| Differences before and after medicament application | Ca(OH)2 + RV | 3.13 ± 1.02a | 3.57 | 0.001 |
| Ca(OH)2 + AgNPs | 3.11 ± 0.41a | 1.48 | ||
| Ca(OH)2 | 2.27 ± 0.47b | 1.36 | ||
| Ca(OH)2 + SA | 3.02 ± 0.35 a | 1.20 | ||
| Saline | 0.42 ± 0.58c | 1.44 |
a, b, cIndicate intergroup differences (Tukey post-hoc test).
*Ca(OH)2: calcium hydroxide, AgNPs: silver nanoparticle, SA: salicylic acid, RV: resveratrol.
Significance value bold-italics.
Biofilm assessment via FE-SEM
The FE-SEM analysis confirmed bacterial growth and biofilm formation before medicament application. The post-treatment analysis aligned with the culture method findings, demonstrating that the RV + Ca(OH)₂, AgNp + Ca(OH)₂, and SA + Ca(OH)₂ groups were the most effective against E. faecalis, while Ca(OH)₂ alone and saline exhibited lower efficacy.
To semi-quantitatively assess the biofilm coverage, a four-point scoring system, used by Bhuva ve ark26. and Ordinola-Zapata ve ark27, was applied (Table 3). Two FE-SEM images (750× magnification) from each group (total: 10) were scored (Table 4), corroborating the microbiological culture results.
Table 3.
Four-point scoring system for evaluating biofilms on SEM images.
| Score | Description |
|---|---|
| 1 | Clean dentin or the presence of isolated microbial cells covering less than 5% of the dentin surface. |
| 2 | Isolated residual microbial cells covering 5–33% of the dentin surface, with no residual biofilm layers. |
| 3 | Presence of biofilm structures and microbial cells covering 34–66% of the dentin surface. |
| 4 | Presence of biofilm structures and microbial cells covering 67–100% of the dentin surface |
Table 4.
Score distribution and mean values across the evaluated groups.
| Score 1 | Score 2 | Score 3 | Score 4 | Mean | |
|---|---|---|---|---|---|
|
Positive control (infected teeth) |
0 | 0 | 1 | 3 | 3.75 |
|
Negative control (sterile teeth) |
3 | 1 | 0 | 0 | 1.25 |
| Saline | 0 | 0 | 3 | 1 | 3.25 |
| Ca(OH)2 | 0 | 2 | 2 | 0 | 2.5 |
| Ca(OH)2 + AgNPs | 0 | 3 | 1 | 0 | 2.25 |
| Ca(OH)2 + SA | 0 | 3 | 1 | 0 | 2.25 |
| Ca(OH)2 + RV | 1 | 2 | 1 | 0 | 2.0 |
Ca(OH)2: calcium hydroxide, AgNPs: silver nanoparticle, SA: salicylic acid, RV: resveratrol.
Discussion
The efficacy against E. faecalis of different intracanal medicaments applied to the root dentin for disinfection in cases of IRR—Ca(OH)₂, RV, AgNp, and SA—was evaluated using the microbiological culture method. The greatest bacterial reduction was observed in the RV group, and the lowest in the saline group. The RV + Ca(OH)₂, AgNp + Ca(OH)₂, and SA + Ca(OH)₂ groups demonstrated significantly greater bacterial reduction than Ca(OH)₂ alone, although no statistically significant difference was found among these three groups. The null hypothesis was thus rejected.
Inflammatory IRR is most frequently reported in the maxillary central incisors, particularly in the middle third of the root. Large IRR lesions (≥ 2 mm) detectable on radiographs are often associated with bacterial infection and hard tissue necrosis28. This study thus focused on maxillary central incisors, creating 2 mm resorption cavities in the root’s middle third to simulate clinical conditions. Various methods exist for cavity creation, including drills, burs, and chemicals. While burs ensure standardised cavities, they lack the surface irregularities seen in clinical cases29. To address this, the method of Keskin et al.30, was adopted: Cavities were created using burs, and 37% orthophosphoric acid was then applied to produce a roughened surface.
To ensure reproducibility and minimise variations from bacterial interactions, a single-species biofilm model was used31. The literature indicates that 86% of studies employed mono-species biofilms, with E. faecalis being the most common (92% of mono-species studies; 79% overall)32. E. faecalis was selected for its ability to form biofilms in root canals, penetrate dentinal tubules (up to 1000 μm), survive harsh conditions, and resist antimicrobials33. Due to its slow growth and delayed tubule invasion, short-term cultures yield insufficient bacterial concentrations34. To simulate clinical conditions and ensure mature biofilm formation, a 21-day incubation period was followed35.
The various methods for collecting bacterial samples from root canals include dentin shavings, paper points, syringe aspiration, Gates-Glidden drills, and tooth grinding36–41. While no consensus exists on the most accurate technique, culture-based studies commonly use root canal filing, followed by sterile paper point collection42. Given the presence of resorption cavities in this study, traditional methods alone were deemed insufficient for dislodging bacteria. A fine microbrush was also used in the resorption cavities. To the best of our knowledge, this study is the first to employ a sampling technique using a bonding brush for bacteriological analysis.
According to the results, the addition of RV, SA, and AgNPs to Ca(OH)2 significantly enhanced its antimicrobial efficacy against E. faecalis. Consistent with Koosha et al.43, the SA + Ca(OH)₂ combination exhibited superior antimicrobial activity, biocompatibility with dental pulp stem cells, and biomineralisation potential, as indicated by the increased OCN expression and ALP activity. SA + Ca(OH)₂ may thus improve antimicrobial efficacy and promote biomineralisation, supporting its potential application in resorption treatments.
The results also indicate that RV + Ca(OH)₂ is the most effective intracanal medicament against E. faecalis. RV, a polyphenolic compound found in grapes, is known for its cardioprotective, neuroprotective, anti-inflammatory, antimicrobial, antitumor, antioxidant, osteogenic, and immunomodulatory properties44–47. However, as no studies in dentistry or endodontics have evaluated its antibacterial efficacy, a direct comparison with existing research is not possible. In this study, the RV concentration was determined based on its MIC against E. faecalis (0.5 mg/mL).
Adding AgNPs to Ca(OH)₂ was also found to significantly enhance the latter’s antimicrobial properties. AgNPs exhibit broad-spectrum antimicrobial activity with a low risk of resistance48. Previous studies have shown that the AgNP-Ca(OH)₂ combination improves antibiofilm efficacy and bacterial eradication due to its smaller particle size and larger surface area, which enhance penetration into dentinal tubules9,18,39,49. Moreover, nanoparticles’ high surface-to-volume ratio and ion density facilitate stronger electrostatic interactions with dentin, thereby increasing their antibacterial effects50. While conventional Ca(OH)₂ particles (1–10 μm) struggle to penetrate dentinal tubules (2–2.5 μm)51, nanoparticles achieve significantly deeper penetration into all root canal regions52. In this study, AgNP-Ca(OH)₂ demonstrated superior efficacy against E. faecalis in IRR cavities, thus aligning with the existing literature.
Owing to its high resolution and detailed imaging capabilities, scanning electron microscopy (SEM) is widely used to evaluate infected dentin53–55. A key limitation of imaging techniques in intraradicular biofilm assessment is the subjective selection of observation areas. To address this, we adopted the standardised method described by Bhuva et al.26, ensuring consistent imaging by predetermining the observation regions within the resorption cavities. Additionally, magnification and operational parameters were standardised across all observations. Since microbiological culture methods alone cannot confirm bacterial origin from intraradicular biofilms, our study corroborated the culture data with the FE-SEM imaging of the resorption areas.
This study has several limitations. First, the culture method did not allow for bacterial load comparisons across different root canal regions. Second, using a single-species biofilm does not fully replicate the polymicrobial nature of endodontic infections. Third, the absence of organic tissue in resorption cavities under simulated conditions may not reflect clinical scenarios in which such tissue can influence cleaning and shaping efficacy. Moreover, for standardisation, E. faecalis was introduced without prior cleaning and shaping, which may have affected medicament efficacy. Future studies should assess these medicaments on polymicrobial biofilms in the presence of organic tissue using diverse microbiological methods.
Conclusion
In this study, intracanal medicaments combining RV, AgNPs, and SA with Ca(OH)2 exhibited the highest antibacterial efficacy against E. faecalis in IRR. Given their superior performance over Ca(OH)2, these agents show promise as alternative endodontic medicaments, offering both accessibility and biocompatibility.
Acknowledgements
This research was supported by the Scientific Research Projects Coordination Center (Project number: TDH-2024-158) of Kutahya Health Sciences University. This study was presented orally at the 29th Congress of the Balkan Stomatological Society (BaSS) (24th–26th of April 2025).
Author contributions
M.Ç. and A.K.M. Contributed to conception, design, data acquisition, and interpretation, wrote the main manuscript text, drafted and critically revised the manuscript, prepared all figures. A.G. Contributed to conception, data acquisition, data analysis. All authors reviewed the manuscript.
Funding
This research was supported by the Scientific Research Projects Coordination Center (Project number: TDH-2024-158) of Kutahya Health Sciences University.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Kutahya Health Sciences University (no: : 2023/12–28).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel, S., Ricucci, D., Durak, C. & Tay, F. Internal root resorption: a review. J. Endod. 36(7), 11071121. 10.1016/j.joen.2010.03.014 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Patel, S. et al. ESE position statement on root resorption. Int. Endod J.56(7), 792–801. 10.1111/iej.13916 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Wedenberg, C. & Lindskog, S. Evidence for a resorption inhibitor in dentin. Eur. J. Oral Sci.95(3), 205–211 (1987). [DOI] [PubMed] [Google Scholar]
- 4.Burleson, A., Nusstein, J., Reader, A. & Beck, M. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. J. Endod.33(7), 782–787. 10.1016/j.joen.2007.04.015 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Siqueira, J. F. Jr et al. Efficacy of instrumentation techniques and irrigation regimens in reducing the bacterial population within root canals. J. Endod.28(3), 181–184. 10.1097/00004770-200203000-00009 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Sathorn, C., Parashos, P. & Messer, H. Antibacterial efficacy of calcium hydroxide intracanal dressing: A systematic review and meta-analysis. Int. Endod J.40(1), 2–10. 10.1111/j.1365-2591.2006.01197.x (2007). [DOI] [PubMed] [Google Scholar]
- 7.Dewi, A. et al. Optimal antimicrobial concentration of mixed antibiotic pastes in eliminating Enterococcus faecalis from root dentin. Aust Endod J.47(2), 273–280. 10.1111/aej.12437 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Ordinola-Zapata, R., Noblett, W. C., Perez-Ron, A., Ye, Z. & Vera, J. Present status and future directions of intracanal medicaments. Int Endod J.55(Suppl 3), 613–636. 10.1111/iej.13731 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tülü, G., Kaya, B. Ü., Çetin, E. S. & Köle, M. Antibacterial effect of silver nanoparticles mixed with calcium hydroxide or chlorhexidine on multispecies biofilms. Odontology109(4), 802–811. 10.1007/s10266-021-00601-8 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Teja, K. V. et al. Comparative evaluation of antimicrobial efficacy of different combinations of calcium hydroxide against Enterococcus faecalis. BMC Oral Health23(1), 849. 10.1186/s12903-023-03552-4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baur, J. A. & Sinclair, D. A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov.5(6), 493–506. 10.1038/nrd2060 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Sadruddin, S. & Arora, R. Resveratrol: Biologic and therapeutic implications. J. Cardiometab. Syndr.4(2), 102–106. 10.1111/j.1559-4572.2008.00039.x (2009). [DOI] [PubMed] [Google Scholar]
- 13.Shakibaei, M., Buhrmann, C. & Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem.286(13), 11492–11505. 10.1074/jbc.M110.198713 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sondi, I. & Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria. J. Colloid Interface Sci.275 (1), 177–182. 10.1016/j.jcis.2004.02.012 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Königs, A. M., Flemming, H. C. & Wingender, J. Nanosilver induces a non-culturable but metabolically active state in Pseudomonas aeruginosa. Front. Microbiol.6, 395. 10.3389/fmicb.2015.00395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha, A. & Kishen, A. Antibacterial nanoparticles in endodontics: A review. J. Endod.42(10), 1417–1426. 10.1016/j.joen.2016.05.021 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Afkhami, F., Elahy, S. & Mahmoudi-Nahavandi, A. Spectrophotometric analysis of crown discoloration following the use of silver nanoparticles combined with calcium hydroxide as intracanal medicament. J. Clin. Exp. Dent.9(7), e842–e847. 10.4317/jced.53743 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afkhami, F., Pourhashemi, S. J., Sadegh, M., Salehi, Y. & Fard, M. J. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent.43(12), 1573–1579. 10.1016/j.jdent.2015.08.012 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Farber, B. F. & Wolff, A. G. Salicylic acid prevents the adherence of bacteria and yeast to silastic catheters. J. Biomed. Mater. Res.27(5), 599–602. 10.1002/jbm.820270506 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Farber, B. F. & Wolff, A. G. The use of Salicylic acid to prevent the adherence of Escherichia coli to silastic catheters. J. Urol.149(3), 667–670. 10.1016/s0022-5347(17)36176-1 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Dayal, S., Singh, S. & Sahu, P. Efficacy and safety of 25% trichloroacetic acid peel versus 30% salicylic acid peel in mild-to-moderate acne vulgaris: A comparative study. Dermatol. Pract. Concept.11 (3), e2021063. 10.5826/dpc.1103a63 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi, J. G. et al. Antibacterial activity of hydroxyalkenyl salicylic acids from sarcotesta of Ginkgo biloba against vancomycin-resistant enterococcus. Fitoterapia80 (1), 18–20. 10.1016/j.fitote.2008.09.001 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Monfoulet, L. E. et al. The pH in the microenvironment of human mesenchymal stem cells is a critical factor for optimal osteogenesis in tissue-engineered constructs. Tissue Eng. Part. A20(13–14), 1827–1840 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Gencoglu, N., Yildirim, T., Garip, Y., Karagenc, B. & Yilmaz, H. Effectiveness of different gutta-percha techniques when filling experimental internal resorptive cavities. Int. Endod. J.41(10), 836–842. 10.1111/j.1365-2591.2008.01434.x (2008). [DOI] [PubMed] [Google Scholar]
- 25.Topçuoğlu, H. S. et al. Efficacy of different irrigation techniques in the removal of calcium hydroxide from a simulated internal root resorption cavity. Int. Endod. J.48(4), 309–316. 10.1111/iej.12316 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Bhuva, B. et al. The effectiveness of passive ultrasonic irrigation on intraradicular Enterococcus faecalis biofilms in extracted single-rooted human teeth. Int. Endod. J.43(3), 241–250. 10.1111/j.1365-2591.2009.01672.x (2010). [DOI] [PubMed] [Google Scholar]
- 27.Ordinola-Zapata, R., Bramante, C. M., Aprecio, R. M., Handysides, R. & Jaramillo, D. E. Biofilm removal by 6% sodium hypochlorite activated by different irrigation techniques. Int. Endod. J.47(7), 659–666. 10.1111/iej.12202 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Heboyan, A. et al. Tooth root resorption: A review. Sci. Prog.105(3), 368504221109217. 10.1177/00368504221109217 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Silveira, P. F., Vizzotto, M. B., Montagner, F., da Silveira, H. L. & da Silveira, H. E. Development of a new in vitro methodology to simulate internal root resorption. J. Endod. 40(2), 211–216. 10.1016/j.joen.2013.07.007 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Keskin, C., Keleş, A. & Sarıyılmaz, Ö. Efficacy of glycolic acid for the removal of calcium hydroxide from simulated internal resorption cavities. Clin. Oral Investig.25(7), 4407–4413. 10.1007/s00784-020-03753-z (2021). [DOI] [PubMed] [Google Scholar]
- 31.Du, T. et al. Effect of long-term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals. J. Endod.40(4), 509–514. 10.1016/j.joen.2013.11.026 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Swimberghe, R. C. D., Coenye, T., De Moor, R. J. G. & Meire, M. A. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J.52(5), 604–628. 10.1111/iej.13050 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Stuart, C. H., Schwartz, S. A., Beeson, T. J. & Owatz, C. B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod.32(2), 93–98. 10.1016/j.joen.2005.10.049 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Yang, G. & Chen, W. In vitro effects of Er: YAG laser-activated photodynamic therapy on Enterococcus faecalis in root Canal treatment. Photodiagnosis Photodyn Ther.45, 103992. 10.1016/j.pdpdt.2024.103992 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Stojicic, S., Shen, Y. & Haapasalo, M. Effect of the source of biofilm bacteria, level of biofilm maturation, and type of disinfecting agent on the susceptibility of biofilm bacteria to antibacterial agents. J. Endod.39(4), 473–477. 10.1016/j.joen.2012.11.024 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Rios, A. et al. Evaluation of photodynamic therapy using a light-emitting diode lamp against Enterococcus faecalis in extracted human teeth. J. Endod. 37(6), 856–859. 10.1016/j.joen.2011.03.014 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Schiffner, U., Cachovan, G., Bastian, J., Sculean, A. & Eick, S. In vitro activity of photoactivated disinfection using a diode laser in infected root canals. Acta Odontol. Scand.72(8), 673–680. 10.3109/00016357.2014.898087 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Fonseca, M. B. et al. Photodynamic therapy for root canals infected with Enterococcus faecalis. Photomed. Laser Surg.26(3), 209–213. 10.1089/pho.2007.2124 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Javidi, M., Afkhami, F., Zarei, M., Ghazvini, K. & Rajabi, O. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust. Endod. J.40 (2), 61–65. 10.1111/aej.12028 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Christo, J. E., Zilm, P. S., Sullivan, T. & Cathro, P. R. Efficacy of low concentrations of sodium hypochlorite and low-powered Er,Cr:YSGG laser activated irrigation against an Enterococcus faecalis biofilm. Int. Endod. J.49(3), 279–286. 10.1111/iej.12447 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Bago, I. et al. Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int. Endod. J.46(4), 339–347. 10.1111/j.1365-2591.2012.02120.x (2013). [DOI] [PubMed] [Google Scholar]
- 42.Sathorn, C., Parashos, P. & Messer, H. H. How useful is root canal culturing in predicting treatment outcome? J. Endod.33(3), 220–225. 10.1016/j.joen.2006.11.006 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Koosha, F. et al. Non-cytotoxic root canal dressing with improved antimicrobial efficacy. J. Endod.49(2), 205–211. 10.1016/j.joen.2022.11.007 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Dal-Fabbro, R. et al. Effect of red wine or its polyphenols on induced apical periodontitis in rats. Int. Endod. J.54(12), 2276–2289. 10.1111/iej.13633 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Çelik, N., Işcan Yapar, M., Taghizadehghalehjoughi, A. & Nalcı, K. A. Influence of resveratrol application with pulp-capping materials on the genetic expression levels of stem cells. Int. Endod. J.53(9), 1253–1263. 10.1111/iej.13345 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Paulo, L., Ferreira, S., Gallardo, E. & Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol.26, 1533–1538. 10.1007/s11274-010-0325-7 (2010). [Google Scholar]
- 47.Liu, X. C. et al. Inhibitory effects of resveratrol on orthodontic tooth movement and associated root resorption in rats. Arch. Oral Biol.111, 104642. 10.1016/j.archoralbio.2019.104642 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Kishen, A., Peters, O. A., Zehnder, M., Diogenes, A. R. & Nair, M. K. Advances in endodontics: Potential applications in clinical practice. J. Conserv. Dent.19(3), 199–206. 10.4103/0972-0707.181925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balto, H., Bukhary, S., Al-Omran, O., BaHammam, A. & Al-Mutairi, B. Combined effect of a mixture of silver nanoparticles and calcium hydroxide against Enterococcus faecalis biofilm. J. Endod. 46(11), 1689–1694. 10.1016/j.joen.2020.07.001 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Wu, D., Fan, W., Kishen, A., Gutmann, J. L. & Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod.40(2), 285–290. 10.1016/j.joen.2013.08.022 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Komabayashi, T., D’souza, R. N., Dechow, P. C., Safavi, K. E. & Spångberg, L. S. Particle size and shape of calcium hydroxide. J. Endod.35(2), 284–287. 10.1016/j.joen.2008.11.017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zand, V., Mokhtari, H., Hasani, A. & Jabbari, G. Comparison of the penetration depth of conventional and nano-particle calcium hydroxide into dentinal tubules. Iran. Endod. J.12(3), 366–370. 10.22037/iej.v12i3.16421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George, S., Kishen, A. & Song, K. P. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J. Endod.31(12), 867–872. 10.1097/01.don.0000164855.98346.fc (2005). [DOI] [PubMed] [Google Scholar]
- 54.de Groot, S. D. et al. Laser-activated irrigation within root canals: Cleaning efficacy and flow visualization. Int. Endod. J.42(12), 1077–1083. 10.1111/j.1365-2591.2009.01634.x (2009). [DOI] [PubMed] [Google Scholar]
- 55.Del Carpio-Perochena, A. E. et al. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J. Endod.37(8), 1134–1138 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.