Abstract
Growing evidence suggests that dim light at night (dLAN) may disrupt circadian rhythms and provoke symptoms of anxiety and depression. Due to the inconvenience of pregnancy and caring for infants, there is a high prevalence of dLAN exposure among pregnant and postpartum women. However, the role and circadian mechanism of dLAN on depression, and anxiety during the postpartum period remain unclear. Pregnant mice were housed in either a light-dark cycle (LD; 12 h of 200 lux:12 h of 0 lux) or a light-dLAN cycle (dLAN; 12 h of 200 lux:12 h of 5 lux) during the gestational and postpartum periods. Depression- and anxiety-related symptoms were assessed by the open field test, sucrose preference test, and forced swim test. Hippocampal transcript profiles were examined using multi-timepoint transcriptome analysis to assess the effects of dLAN exposure. Our findings showed that dLAN significantly increased depression-like behaviors, such as decreased sugar preference and increased immobility time, and decreased levels of brain serotonin (5-HT) and brain-derived neurotrophic factor (BDNF) during the postpartum period. In addition, dLAN reduced the amplitude of circadian rest-activity behaviors and nighttime activity levels, and these disruptions were significantly related to depression-like behaviors and low levels of 5-HT. Moreover, dLAN disrupted the expressions of hippocampal circadian genes particularly Per1 in postpartum mice. These findings reveal that dLAN induces depression-like behaviors in postpartum mice, with disruptions in circadian rest-activity rhythms and rhythmic gene expression likely mediating the adverse effects of dLAN on these behaviors.
Subject terms: Depression, Neuroscience
Introduction
Postpartum depression is a common and severe mood disorder that is defined as a major depressive episode that begins within 4 weeks after delivery [1]. 13–22% of women experience postpartum depression within the first year after childbirth [2, 3]. Untreated postpartum depression induces many physical and mental (e.g., suicidality and risky behaviors) and infant negative consequences (e.g., emotional, cognitive, and behavioral development), as well as mother-child interaction problems [4–6]. Over 50% of postpartum depression patients do not receive effective treatment, mainly due to the high underdiagnosis rate of this disease and mothers’ concerns about the potential harm of antidepressants to infants [7, 8]. Even under first-line treatment, only about 54% of patients respond [9]. These challenges emphasize the necessity of identifying the modifiable risk factors of postpartum depression and their underlying mechanisms. In recent years, there has been widespread attention to the impact of environmental factors, such as air pollution and road traffic noise, on the occurrence of postpartum depression [10, 11].
As the most potent zeitgeber, light entrains circadian rhythms, which are believed to modulate diverse brain functions including mood [12, 13]. With the development of modern electricity and lighting technologies, more than 80% of the world’s population lives under light-polluted skies, and exposure to artificial light at night (light pollution) has increased by nearly 50% over the past 25 years, which has become a global environmental issue [14–17]. Exposure to light at night induces circadian rhythms disruption, which is linked to higher risks of psychiatric disorders [18, 19]. Moreover, a randomized controlled trial found that the use of modified spectacles and light bulbs to block blue light at night can alleviate symptoms of postpartum depression [20]. The evidence suggests a close link between light at night and postpartum depression. However, to date, the impact of light at night on the occurrence of postpartum depression remains unclear.
Dim light at night (dLAN) is mainly sourced from night dim light lamps, electronic devices (e.g., smartphones and tablets), and streetlights filtering light [14, 21, 22]. In real-life scenarios, perinatal women often need frequent exposure to dLAN due to discomfort during pregnancy and newborn care [20]. Therefore, the primary purpose of this study was to investigate the effects of dLAN on the occurrence of postpartum depression symptoms in mice. We assessed the effects of dLAN on the circadian rest-activity behavior by obtaining 21-day locomotor activity monitoring data. Furthermore, we explored the association between circadian rest-activity behavior and postpartum depression symptoms. We also examined the extent to which dLAN disrupts circadian oscillations in clock gene expression in the hippocampus.
Materials and methods
Animals
Adult mice (8 weeks old, male and female ICR mice) were obtained from the Guangdong Medical Laboratory Animal Center (Guangzhou, China). Animals showing no indications of illness or physical anomalies were selected for our study. All mice were housed under a standard 12:12 h light/dark cycle (200 lux from white LED lamps), with lights on at 8:00 AM (zeitgeber time 0, ZT0) and lights off at 8:00 PM (ZT12). The ambient temperature was maintained at 22 ± 2 °C, and the mice had ad libitum access to food and water. After a 2-week acclimation period female and male mice were paired in cages at a ratio of 1:1 for mating. The day when the vaginal plug was observed was considered as gestational day 0 (GD0).
Experimental design
Pregnant female mice were housed in cages containing running wheels from GD0, and randomly assigned to the control group (N = 50) and the experimental group (N = 50) using computerized randomization algorithms. The control group was remained under a 12:12 h light/dark cycle, while the experimental group was subjected to a 12:12 h light/dim cycle (dLAN; 200 lux during the light phase and 5 lux during the dim phase). Unless stated otherwise, these lighting conditions were sustained until the experiments ended. One mouse in the control group died unexpectedly. This incident was noted, but no adverse effects were observed in the remaining animals.
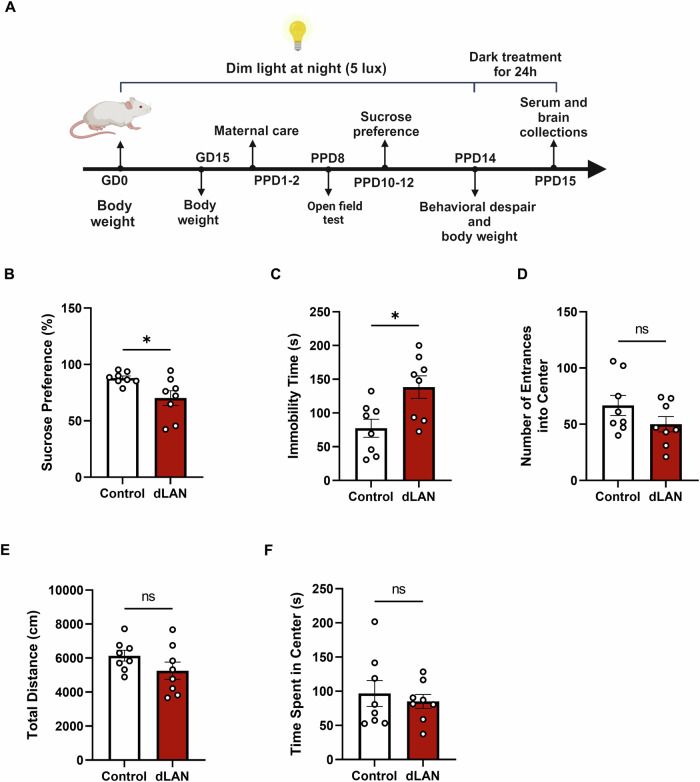
Maternal behavior was monitored on the early postpartum days 1–2 (PPD1-2). Subsequently, open field tests, sucrose preference tests, and forced swimming tests were performed on PPD8, PPD10-12, and PPD14, respectively, to assess postpartum depressive-like behaviors in the mice. Serum and brain tissue samples were collected on PPD15. Body weights were recorded on GD0, GD15, and PPD14. The experimental schedule is shown in Fig. 1A.
Fig. 1. dLAN group mice exhibited postpartum depression-like behaviors.
A Schematic timeline of the experimental design. B Sucrose preference percentage between control and dLAN groups mice on PPD10-12 (t = 2.614, df = 14, p = 0.0204). C Immobile time (s) during FST between control and dLAN groups mice on PPD14 (t = 2.852, df = 14, p = 0.0128). D–F OFT. Number of entrances into the center (t = 1.487, df = 14, p = 0.1591) (D), total distance (cm) (t = 1.475, df = 14, p = 0.1623) (E), time spent in the center (s) (t = 0.5428, df = 14, p = 0.5958) (F) of the OFT between control and dLAN groups mice on PPD8. N = 8 mice per group. Two-tailed unpaired Student’s t-test was used in all of the above experiments. Data are shown as mean ± SEM (*p < 0.05).
Assessment of circadian rhythmic activity
Wheel running activity data over a 21-day period from GD1 to PPD2 in females mice (N = 8 per group) were collected and analysed with ClockLab (Version 6, Actimetrics, Wilmette, IL, USA). A rest period of at least 10 min in wheel running was defined as resting time. We calculated various circadian locomotor activity variables including amplitude and total amount of daily, daytime, and nighttime activity using the activity profile. The 24-h profiles were created by averaging the total counts from a 21-day period over the 24-h day for each mouse, and subsequently calculating group averages. Additionally, we used the ClockLab to calculate cosinor analysis parameters including amplitude (reflecting rhythmic variation magnitude), mesor (the average around which the rhythm oscillates), and Pseudo-F (the indicator of the robustness of rest - activity rhythm). We also used ClockLab to generate analyzed non-parametric parameters including interdaily stability, which measures synchronization to the 24 h light-dark cycle, and intradaily variability, which assesses the fragmentation of rhythmic patterns [23].
Maternal care
On PPD 1 and 2, maternal behaviour was evaluated as previously described [24]. The time postpartum mice spent in nest-building, active nursing (an arched back position) and licking/grooming pups was recorded three times with 2.5 h intervals during the light phase. The duration of each observation was 60 min and each observation commenced 30 min after lights on and concluded 60 min before lights off. In the final stage of the analysis, 4 animals were excluded from the maternal behavior assessment due to inadequate video quality that precluded reliable behavioral scoring.
Behavioral tests
All behavioral measurements were conducted by trained experimenters in a blinded manner. All behavioral tests were performed between 1:00 and 6:00 PM. The selected sample size (N = 8 per group) was sufficient to detect a nearly 25% difference between the two groups with 80% statistical power and a 5% Type I error rate, based on the mean and standard deviation from the pilot study.
Open field test (OFT)
The OFT was carried out in a quiet environment with an open viewing box (50 × 50 × 40 cm) in postpartum mice on PPD8. The apparatus consisted of an outer zone (12.5 × 12.5 cm; 12 squares), a center zone (12.5 × 12.5 cm; 4 squares), a camera fixed at the top, and a software analysis system. The mice were habituated to the experimental environment for 30 min before the commencement of testing. In the beginning, each mouse was gently placed in the center of the apparatus and allowed to explore for 5 min. The activity of the mouse in the experiment box was recorded for 10 min. EthoVision XT 17 software (Noldus Information Technology, Leesburg, VA, USA) was used to analyze the number of entries into the center, the time spent in center and the total distance traveled to assess the anxiety-like behavior of the postpartum mice. Feces were cleaned and the box was wiped with 75% alcohol after the test.
Sucrose preference test (SPT)
To assess anhedonia-like behaviour, a SPT was conducted on PPD10-12 in postpartum mice. Mice were habituated with two bottles of 1% sugar (Sigma-Aldrich, St. Louis, MO, USA) water for 24 h, after which one bottle was replaced with regular water for another 24 h. The SPT was performed subsequently, with two pre-weighed bottles (one with 1% sucrose water and the other with regular water) placed in each cage for 24 h. The position of the bottles was changed every 12 h, and the remaining weights were recorded after the test. The sucrose preference rate was calculated using the consumption of sucrose and regular water: Sucrose Preference Rate (%) = Sucrose consumption / Total fluid consumption × 100%.
Forced swim test (FST)
To assess despair behaviors, forced swim tests were conducted on postpartum mice on PPD14. The forced swim apparatus consisted of a transparent cylinder (20 cm diameter × 40 cm height) filled with water (25 ± 1 °C) up to 15 cm, a camera, and a software analysis system (Noldus Information Technology). At the beginning of the test, each mouse was gently placed into the cylinder. It was defined as immobile when a mouse ceased struggling, floated in the water, or kept its head on the water with slight limbs movement. The total recording time was 6 min, and the immobility time was accumulated during the final 4 min.
Collection of serum and brain tissue
After maternal care and behavioral tests, serum and brain tissue samples were collected after 24 h of constant darkness in the postpartum mice on PPD15 (N = 8 per group). The whole blood samples were collected via cardiac puncture and were allowed to clot on ice for 20 min and followed by centrifugation at 3 000 rpm for 10 min at 4 °C to obtain serum. For brain tissue collection, mice were euthanized using cervical dislocation, and the whole brain of each mouse was removed and quickly washed in ice-cold phosphate buffer saline (PBS) and frozen in liquid nitrogen. All serum and brain samples were stored at −80 °C until analysis.
Collection of hippocampal tissue at multiple time points
Hippocampus samples from postpartum mice on PPD8 were collected after 24 h of constant darkness beginning at ZT0 Samples for RNA-sequencing and quantitative real-time PCR experiments were collected every 4 h over 24 h. The hippocampus was freshly dissected from the brain and flash frozen in liquid nitrogen before being stored at −80 °C.
ELISA
After the behavioral tests, we collected brain tissue and serum for the ELISA experiment (N = 8 per group). Whole-brain tissue homogenates were prepared by diluting the tissue 1:20 (w:v) in PBS, followed by homogenization using a LANYI-GTM homogenizer (Shanghai, China). The homogenates were then centrifuged at 4 000 rpm for 10 min at 4 °C, and the supernatant was collected for analysis. Total protein levels in the brain supernatant were determined using a total protein assay kit. Serotonin (5-HT) and brain-derived neurotrophic factor (BDNF) levels in the brain, as well as oxytocin, estradiol, and progesterone levels in the serum of postpartum mice, were measured using specific quantitative sandwich ELISA kits. All assay kits and reagents were obtained from RGB&CHN (Beijing, China). Each sample and standard were assayed in duplicate.
Hippocampus transcriptomics (mRNA-sequencing)
Total RNA extraction and purification from hippocampal tissue were performed using TRNzol Universal Reagent purchased from Tiangene (Tianjin, China) according to the manufacturer’s instructions, with biological duplicates comprising 17 control and 18 dLAN samples collected every 4 h over 24 h. RNA quality was assessed using the Agilent 5400 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and checked using RNase-free agarose gel electrophoresis. All RNA samples used had a RNA Integrity Number (RIN) > 8; 260/280 ratio > 1.8; and 260/230 ratio > 1.8 (Supplementary Table 1). The libraries were prepared with NEBNext Ultra RNA Library Prep Kit for Illumina (NEB #7530, New England Biolabs, Ipswich, MA, USA). Library size was confirmed by Agilent 5400 Bioanalyzer. 150-bp paired-end sequencing were performed on NovaSeq X Plus platform (Illumina, San Diego, CA, USA) by Novogene Bioinformatic Technology Co., Ltd (Beijing, China). Raw reads were trimmed for adapters and low-quality flanking ends using Fastp (v0.23.1). The clean reads were mapped to the GRCm39 genome (NCBI, Mus musculus, GCF_000001635.27) using HISAT2 (v2.0.5) [25]. Gene coverages were computed using featureCounts with the Gencode M33 annotation (https://www.gencodegenes.org/mouse/release_M33.html), excluding all multi-mapping reads to ensure data reliability [26]. Gene expression levels were normalized using the fragments per kilobase per million (FPKM) method.
To assess the rhythmic of genes, the BIO_CYCLE algorithm was employed [27]. This algorithm yields an output that encompasses the amplitude, phase, and P - value for each transcript. A gene was regarded as circadian when the P - value generated by BIO_CYCLE was less than 0.05 [27, 28]. Heatmaps of rhythmic gene transcripts were constructed using the R version 4.4.1. In these heatmaps, the rows were sorted according to the phase output by BIO_CYCLE and were normalized using row z - scores.
The list of rhythmic genes in the control and dLAN group was analyzed using Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.ad.jp/kegg/) for functional enrichment pathway analysis. Finally, RNA-seq data were partially validated validated by quantitative real-time PCR.
Quantitative real-time PCR
Total RNA was extracted and purified from hippocampal tissue (4 samples/group collected every 4 h over 24 h) via an RNeasy™ Animal RNA Isolation Kit with a Spin Column (R0026, Beyotime, China), and no more than 1 μg RNA was reverse transcribed into cDNA with a BeyoRT™ II First Strand cDNA Synthesis Kit (RNase H minus) (D7168M, Beyotime, China). RT-qPCR was conducted via an ABI 7 300 Real-Time PCR System (Thermo Fisher, Singapore). Relative gene expression was quantified using the threshold cycle value and normalized with ribosomal protein L19 (Rpl19). All results were calculated using the 2^−ΔΔCt method, with the expression level of the control group set as 1 for standardization. The primer information was as follows: Nr1d1-Forward: TCA CCT ATG CCC ATG ACA AGT, Nr1d1-Reverse:TGT GGA GTT GTA GCT GAA GGG; Nr1d2-Forward:TGA ACG CAG GAG GTG TGA TTG, Nr1d2-Reverse:GAG GAC TGG AAG CTA TTC TCA GA; Per1-Forward: CCG CTT ACA GCA GTC TAA TGA, Per1-Reverse:GCA GTT TCC TAT TGG TTG GTC; Clock-Forward:AGA ACT TGG CAT TGA AGA GTC TC, Clock-Reverse:GTC AGA CCC AGA ATC TTG GCT; Rpl19-Forward:CTG AAG GTC AAA GGG AAT GTG TTC, Rpl19-Reverse:TGG TCA GCC AGG AGC TTC TTG.
Statistical analysis
Data were presented as the mean ± standard error of the mean (SEM). Two-tailed unpaired Student’s t-test was used for the analysis of the two groups. Rest-activity rhythms and body weight were analyzed by a repeated measures ANOVA with Bonferroni post-hoc tests. The mRNA expression of clock genes was analyzed by two-way ANOVA followed with Bonferroni post-hoc tests. Statistics were obtained using R version 4.4.1 (R Core Team, Vienna, Austria) and SPSS 28.0 (SPSS Inc., Chicago, IL, USA). Data visualization was performed by R version 4.4.1 or GraphPad Prism 8.0.0 (GraphPad Software, San Diego, CA, USA). For all statistical tests, a p value < 0.05 was considered significant. All experiments were repeated at least three times using different mice, and detailed information was presented in the figure legends.
Results
Effects of dim light at night (dLAN) on depression- and anxiety-like behaviors of postpartum mice
To evaluate the depression-like behavior of postpartum mice, the SPT and FST were performed. Compared with the control group, the test of sucrose intake revealed that dLAN group consumed significantly less sucrose solution, supporting the notion of an anhedonic-like state in postpartum mice exposed to dLAN (Fig. 1B). In the FST, dLAN group showed despair behavior, characterized by a significant increase in immobility time (Fig. 1C). The OFT was used to assess anxiety-like behavior, but no significant differences were observed between the two groups in terms of the number of entries into the center area, the total travel distance, and the time spent in the center region (Fig. 1D–F). We also assessed nest building, active nursing, and licking/grooming behaviors in dams and found that dLAN did not significantly affect maternal care (Fig. S1A-C). Moreover, there were no significant differences in body weight between control and dLAN groups (Fig. S2). These results suggest that postpartum mice exposed to dLAN may exhibit depression-like behavior.
Rest-activity rhythms of female mice is disrupted by dLAN during pregnant and postpartum period
Locomotor activity was assessed using a customized wheel-running system. In the actograms of wheel running activity, dLAN group showed decreased activity unlike the control group (Fig. 2A). The repeated-measures ANOVA (lighting condition: dLAN vs. control; zeitgeber time, ZT) was conducted to analyze the rest - activity rhythms. The results revealed a significant ZT × lighting condition interaction (Fig. 2B), and significant main effects of ZT. The main effect of lighting condition was not significant. During the dLAN phase, mice were less active than those in control group, especially at ZT12, ZT13, ZT22 and ZT23. Nevertheless, at ZT8, mice in dLAN group were more active than in control group. Compared to the control group, the dLAN group exhibited no significant difference in average resting time over a 21-day period during either the light or dark phases (Fig. S3A, B).
Fig. 2. Rest-activity rhythms of female mice were disrupted by dLAN during pregnant and postpartum periods.
A Representative actogram of control (Left) and dLAN group mice (Right). bin = 5 min. White indicates the light phase. Grey indicates the dark phase. B Average resting time/h over 21 days. White indicates the light phase. Grey indicates the dark phase. Repeated measures ANOVA (lighting condition: dLAN vs.control; zeitgeber time, ZT): significant ZT × lighting condition interaction (F23,322 = 3.733, p < 0.001); significant ZT main effect (F23,322 = 36.226, p < 0.001); non-significant lighting condition main effect (F1,14 = 1.596, p = 0.2271). Bonferroni post-hoc: dLAN vs. control showed significant differences at ZT12 (p = 0.026), ZT13 (p = 0.048), ZT22 (p = 0.028), ZT23 (p = 0.022), and ZT8 (p = 0.034). C Daily running wheel counts (t = 3.597, df = 14, p = 0.0029). D Nighttime running wheel counts (t = 3.682, df = 14, p = 0.0025). E Daytime running wheel counts (t = 0.4433, df = 14, p = 0.6643). F Amplitude of control and dLAN mice (t = 2.790, df = 14, p = 0.0145). G Mesor of control and dLAN mice (t = 3.60, df = 14, p = 0.01). H Pseudo - F of control and dLAN mice (t = 3.595, df = 14, p = 0.0029). I–L Correlation plots comparing sucrose preference I, K and immobility time J, L for dLAN group mouse with its locomotor activity amplitude, daily activity (counts), nighttime activity (counts), mesor and Pseudo-F. The red correlation lines (the slope is statistically different from 1, p < 0.05) indicate that the preference for sugar water in dLAN group was positively correlated with amplitude (p = 0.01) and nighttime activity (p = 0.03). The blue correlation lines (the slope is statistically different from 1, p < 0.05) indicate that the immobility time during forced swimming was negatively correlated with amplitude (p = 0.04). N = 8 mice per group. Data are shown as mean ± SEM (*p < 0.05, **p < 0.01).
The daily wheel-running activity and nighttime (active phase) activity revealed a notable decline in the dLAN group compared to the control mice (Fig. 2C, D). The dLAN group exhibited an increased tendency for wheel-running during the daytime period (resting phase), although no statistically significant difference was observed compared to the control group (Fig. 2E). Further cosinor analysis revealed that the amplitude of rhythmic variation in locomotor activity exhibited a significant decline in the dLAN group (Fig. 2F). In addition, decreases were observed in both the mesor and Pseudo-F values (Fig. 2G, H). These findings suggest that the rhythm in the dLAN group was attenuated and rendered less robust. To extend these findings, we applied non-parametric circadian rhythm analyses. The findings indicated that the dLAN group demonstrated an increasing trend in intradaily variability and a concomitant decrease trend in intradaily stability, although these changes did not reach statistical significance when compared to the control group (Fig. S3C, D). Increased variability and reduced stability appear to underpin circadian rhythm fragmentation and instability in mice under dLAN conditions. These results suggest that exposure to dLAN in mice disrupts rest-activity rhythms.
To determine the association between depression-like behavior and disrupted rest-activity rhythms under the dLAN condition, we performed a correlation analysis (Fig. 2I–L). The results showed that the preference for sugar water in dLAN group was positively correlated with activity amplitude and nighttime (active period) running wheel activity, whereas the immobility time during forced swimming was negatively correlated with activity amplitude (Fig. 2I, J). These results suggest that disrupted rest-activity rhythms are associated with the depression-like behavior under dLAN conditions.
Effect of dLAN on hormone, 5-HT, and BDNF levels in postpartum mice
To investigate the effects of dLAN on hormone levels in postpartum mice, we measured serotonin and BDNF levels in the brain, as well as oxytocin, estrogen, and progesterone in the serum. The results showed that the concentrations of 5-HT and BDNF in the dLAN group were significantly lower than those in the control group (Fig. 3A, B). The serum concentrations of oxytocin, estrogen, and progesterone did not exhibit a statistically significant difference between the two groups (Fig. 3C–E). The correlation analysis showed that the levels of 5-HT in dLAN group were positively correlated with the rest - activity rhythm amplitude, and nighttime (active period) running wheel activity (Fig. 3F–I). These results suggest that the reduced concentration of 5-HT is associated with the disrupted rest-activity rhythms under dLAN conditions.
Fig. 3. Effect of dLAN on hormone levels in postpartum mice on PPD15.
A 5-HT concentration in the brain (t = 3.848, df = 14, p = 0.0018). B BDNF concentration in the brain (t = 2.632, df = 14, p = 0.0197). C Oxytocin concentration in serum (t = 0.8314, df = 14, p = 0.4197). D Estrogen concentration in serum (t = 1.060, df = 14, p = 0.3071). E Progesterone concentration in serum (t = 0.0889, df = 14, p = 0.9304). F–I Correlation plots comparing 5-HT F, H and BDNF G, I for dLAN group mouse with its locomotor activity amplitude, daily activity (counts), nighttime activity (counts), mesor and pseudo-F. The red correlation lines (the slope is statistically different from 1, p < 0.05) indicate that the levels of 5-HT in dLAN group were positively correlated with amplitude (p = 0.048) and nighttime activity (p = 0.001). N = 8 mice per group. Two-tailed unpaired Student’ s t-test was used in the above experiments of the two groups. Data are shown as mean ± SEM (*p < 0.05, **p < 0.01).
Effects of dLAN on rhythmic genes expression in the hippocampus
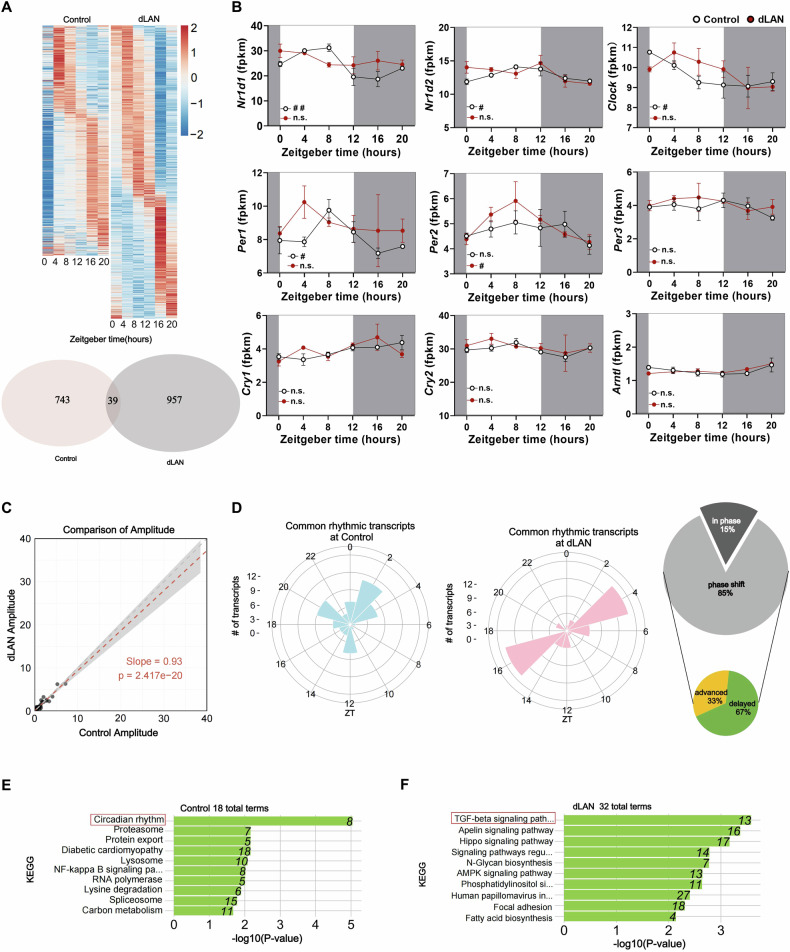
The hippocampus, a brain region known to show robust circadian oscillations, has been implicated in regulating postpartum depression [29–31]. To determine the effects of dLAN on the rhythmic gene expression, we performed RNA-seq on hippocampus samples collected every 4 h over 24 h. We found that 782 and 996 genes with rhythmic patterns in the control and dLAN group, respectively (Fig. 4A and S4A). The overlap of these two genes sets was 39 genes (Fig. 4A). Figure 4B illustrated the nine well-recognized clock genes in the control and dLAN groups. Nr1d1, Nr1d2, Clock, and Per1 exhibited rhythmic oscillations in the control group, but such rhythmic oscillations were lost in the dLAN group (Fig. 4B and S4B). In addition, a comprehensive comparison of the differences between the two experimental groups was performed. The two-way ANOVA results revealed no significant interaction between zeitgeber time and group for nine well-recognized clock genes (Fig. 4B).
Fig. 4. Effects of dLAN on rhythmic gene expression in the hippocampus.
A Gene expression patterns from mRNA-seq were analyzed for circadian rhythms using BIO_CYCLE portal. Heatmaps (top) sorted by phase of gene expression. Each row is one gene with expression level in z-score at 6 time points (columns). Venn Diagram (bottom) shows the number of rhythmic genes in control (red, N = 17 mice) and dLAN (gray, N = 18 mice) postpartum mice hippocampus using the criterion that rhythmic significance is determined by a p value <0.05. B Circadian profiles of circadian clock genes in both control and dLAN hippocampus. White indicates the light phase. Grey indicates the dark phase. Two-way ANOVA (lighting condition: dLAN vs. control; zeitgeber time, ZT): no significant ZT × lighting condition interaction for Nr1d1 (F5, 23 = 2.491, p = 0.061), Nr1d2 (F5, 23 = 1.463, p = 0.240), Clock (F5, 23 = 1.016, p = 0.0431), Per1 (F5, 23 = 0.752, p = 0.593), Per2 (F5, 23 = 0.600, p = 0.701), Per3 (F5, 23 = 0.353, p = 0.875), Cry1 (F5, 23 = 1.366, p = 0.273), Cry2 (F5, 23 = 0.232, p = 0.944), and Arntl (F5, 23 = 1.228, p = 0.328). C The red correlation line (Spearman, slope is statistically different from 1, p = 2.417e–20) indicates that the dLAN group showed an overall reduced amplitude of common rhythmic genes. D Radar plots representing the phase lag of common rhythmic genes in control (left) and dLAN (right) mice. The proportion of common rhythmic genes that exhibit phase shifts during dLAN and the direction of the phase shift induced by dLAN are presented in the form of a small pie chart. Those genes with a phase advancing or delaying by more than 1 h are regarded as phase-advanced genes and phase-delayed genes separately. E, F KEGG analysis of genes that are rhythmic in either control E or dLAN F mice. The data were placed from ZT0 to ZT20 in the heatmaps and radar plots. Data are shown as mean ± SEM. Nonparametric test Bio_Cycle was applied for the detection of the significance of daily oscillation using p value cutoff of 0.05. # and ## indicate p <0.05 and p < 0.01, respectively.
We also we compared the amplitude and phase for the 39 common rhythmic genes between control and dLAN groups (Fig. 4C, D). The amplitude of these common rhythmic genes was lower as seen by the deviation of the regression line from unity (slope = 0.93, p < 0.0001) (Fig. 4C). The amplitude (an indicator for the strength of a rhythm) of the rhythmic genes unique to the dLAN group seemed lower than that of the rhythmic genes unique to the control group (Fig. S4C). Common rhythmic transcripts in control group mainly cluster around ZT2, while these transcripts concentrate at ZT4 and ZT16 in dLAN group (Fig. 4D). 15% of the common rhythmic transcripts were in phase, while a significant 85% exhibited a phase shift. Specifically, 33% of the phase-shift instances are advanced, whereas 67% are delayed. The two polar plots display unique rhythmic transcripts in control and dLAN groups. Unique rhythmic transcripts in control group mainly clustered around ZT14, while the unique transcripts in dLAN group concentrated at ZT4 (Figs. S4D). KEGG analysis showed rhythmic genes of the control group were specifically enriched in the circadian rhythms pathway, while those of dLAN group were primarily enriched in the TGF-β signalings pathway (Fig. 4E, F). KEGG enrichment of rhythmic transcripts unique to the control and dLAN groups was similar to all rhythmic transcripts (Fig. S4E, F).
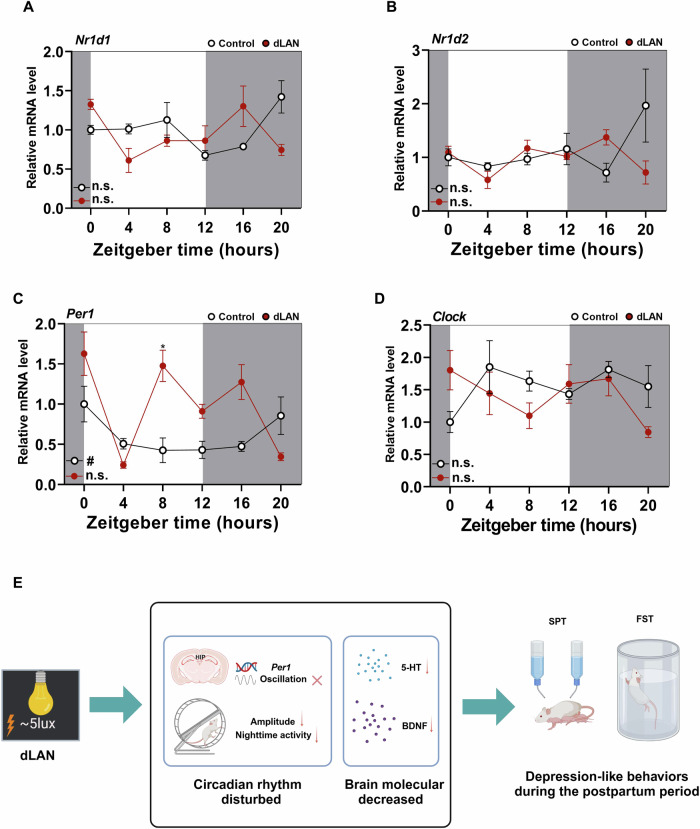
The transcription of four circadian clock genes (Nr1d1, Nr1d2, Per1, Clock) that lost their rhythmicity (Fig. 4B) was further validated by RT-qPCR (Fig. 5A–D). Statistical analysis revealed that the mRNA level of Per1 exhibited significant oscillation only in the control group, while Nr1d1, Nr1d2, and Clock were virtually absent in either group (Fig. 5A–D). Furthermore, the results of the two-way ANOVA revealed significant interactions between ZT and lighting condition for Nr1d1 (Fig. 5A) and Nr1d2 (Fig. 5B), and Per1 (Fig. 5C), but no significant difference between dLAN and control was observed after Bonferroni adjustment for Nr1d1 (Fig. 5A), Nr1d2 (Fig. 5B). The mRNA level of Per1 in the dLAN group was significantly higher at ZT8 compared to the control group (Fig. 5C) even after Bonferroni adjustment.
Fig. 5. dLAN caused the loss of Per1 rhythm in postpartum mice hippocampus.
A–D Transcripts of the circadian clock genes Nr1d1 (A), Nr1d2 (B), Per1 (C), and Clock (D) were analyzed by quantitative PCR. White indicates the light phase. Grey indicates the dark phase. N = 4 mice per time point. Two-way ANOVA (lighting condition: dLAN vs. control; zeitgeber time [ZT]): significant ZT × lighting condition interaction for Nr1d1 (F5,36 = 5.264, p = 0.001), Nr1d2 (F5,36 = 3.233, p = 0.016), Per1 (F5,36 = 7.200, p < 0.001); no significant ZT × lighting condition interaction for Clock (F5,36 = 2.428, p = 0.054); the main effects of both ZT and lighting condition were significant for Per1. Bonferroni post-hoc: dLAN vs. control showed significant differences for Per1 at ZT8 (p = 0.0336). Data are shown as mean ± SEM (*p < 0.05). Nonparametric test Bio_Cycle was applied for the detection of the significance of daily oscillation using p value cutoff of 0.05. # indicate p < 0.05. E The putative mechanisms of dLAN on postpartum depression. Created with BioRender.com.
In summary, this study demonstrates that dLAN leads to the loss of Per1 rhythmicity in the hippocampus, disrupts circadian rest-activity behaviors, and reduces levels of 5-HT and BDNF. This may represent a potential mechanism through which dLAN induces depression-like behaviors during the postpartum period (Fig. 5E).
Discussion
The findings of this study revealed that dLAN induces depression-like behaviors in postpartum mice and reduces the levels of postpartum depression-related hormones including 5-HT and BDNF in the brain. dLAN also leads to disrupted circadian rest-activity behaviors characterized by low amplitude. In addition, these disruptions in circadian rest-activity behaviors were significantly correlated with depression-like behaviors and low levels of 5-HT. Furthermore, dLAN induces a loss of circadian oscillations in the expressions of Per1 mRNA in hippocampal tissue.
No prior study has investigated the effects of dLAN on the onset of postpartum depression. Previous studies have shown that exposure to high levels of nighttime light is associated with elevated risks of various diseases, such as cardiometabolic diseases, cancers, sleep disorders, and major depressive disorder in the general population [32–35]. Previous animal studies reported that dLAN exposure decreased sucrose preference and increased floating duration in mice [36–38]. Another study found that dLAN exposure for 5 weeks induced anhedonia assessed by the sucrose preference test in Wistar rats [39]. However, postpartum depression is distinct from those severe depressive episodes occurring at other times, because of its unique risk factors and pathophysiology [40]. For instance, challenges such as psychosocial stressors related to childbirth and infant care as well as fatigue from circadian and sleep disruptions are commonly associated with postpartum depression [41, 42]. To date, there is a lack of evidence on the relationship between dLAN and postpartum depression. This study provides solid evidence that exposure to dLAN could induce depression-like behaviors, such as decreased sugar preference and increased immobility time, and reduced levels of 5-HT and BDNF in brain during the postpartum period. Our findings were supported by a previous observational human study, which observed significant associations between light at night and higher risks of late pregnancy stress and depression [43]. In addition, significant reductions in levels of 5-HT and BDNF have been found in postpartum depression patients and animal models [44, 45]. BDNF promotes the development and function of serotonergic neurons [46, 47]. Likewise, the rise in 5-HT caused by 5-HT reuptake inhibitors leads to an increase in BDNF expression [48]. Both 5-HT and BDNF possess the ability to modulate the generation and plasticity of neural circuits involved in depression [49].
There is a lack of evidence on the mechanisms underlying the detrimental effects of dLAN on postpartum depression. This study revealed that circadian rhythm disruptions may play a crucial role in the impact of dLAN on the occurrence of postpartum depression symptoms. Circadian rhythms refer to the physiological, psychological, and behavioral fluctuations that occur in an organism over a 24-h period. Circadian rhythm are regulated by a master central clock, predominantly entrained by environmental light cues [50]. Circadian rhythm disruptions lead to broad adverse health outcomes, such as cardiometabolic diseases, neurodegenerative diseases, and mood disorders [51–53]. Our data demonstrate that gestational mice exposed to dLAN exhibited flattened circadian rest-activity behaviors manifested as reduced amplitude/robustness of rhythm and lower nighttime activity levels. In addition, we found that decreased amplitude was related to postpartum depression symptoms. Similarly, previous studies demonstrated that exposure to dLAN decreases nocturnal activity in mice and rats [36, 39, 54]. Notably, a human study of the general population reported that lower amplitude of circadian rest-activity behaviors was significantly associated with increased susceptibility to mood disorders including depression [53].
In addition to disruptions in circadian rest-activity behaviors, alterations in circadian-related gene expressions may mediate the detrimental effects of dLAN on postpartum depression. Circadian rhythms are driven by clock genes, such as Per1, Per2, Per3, Clock, Bmal1, Cryptochromes, and Nr1d1 [55–57], and some of these circadian genes (e.g., Clock and Per3) are closely linked to major depressive disorder [58, 59]. Previously, most animal studies used the single time point examination to investigate the effects of LAN on transcriptome profiles, a method that presents inherent limitations in capturing the dynamic nature of circadian rhythms [60, 61]. Through multi-timepoint transcriptome and RT-qPCR analyses, the present study determined that dLAN induces the disruption of the Per1 rhythmic expression within the hippocampal tissue of postpartum mice. KEGG analyses revealed that the rhythmic genes in the dLAN group were predominantly enriched in the TGF-β signaling pathway. TGF-β signaling regulates both hippocampal neurogenesis and neuroinflammation, which are associated with depression [62, 63]. The target genes for Per1 transcriptional regulators are highly enriched for the TGF-β signaling pathway [64].
In addition, we observed that dLAN not only decreases the amplitude of common rhythmic genes but also induces a significant phase redistribution of these genes. To date, there is limited evidence on the effects of dLAN on the expression profiles of common rhythmic genes. Both amplitude and phase alignment are the most common circadian properties that have been associated with human health [65]. For example, a prospective cohort of 92 614 participants revealed that low amplitude of circadian rest-activity behaviors is linked to higher risks of a broad range of adverse health outcomes [66]. In addition, phase shifts of rhythmic genes have been related to depression [58, 67]. Further research is warranted to investigate the role of amplitude and phase in these rhythmic genes in mediating the detrimental effects of dLAN on postpartum depression.
To date, concerns regarding the teratogenic effects of medications in the suckling and the low efficacy of antidepressants have led to significant limitations in the treatment of postpartum depression [8, 9]. The great challenges in managing postpartum depression emphasize the urgent need to identify modifiable risk factors. Notably, this study has identified dLAN as a novel modifiable factor contributing to postpartum depression. Reducing exposure to dLAN may be an effective strategy for postpartum depression, with potential interventions including the use of light-blocking glasses, lowering indoor light sources below bed level, or employing blackout curtains. Furthermore, this study highlights the importance of circadian rest-activity behaviors in postpartum depression onset. Advances in mobile technology, such as the use of accelerometers, now offer promising approaches to monitor rest-activity patterns and study circadian rhythms in maternal health [68]. Collectively, this study underscores the critical need for further research aimed at improving maternal health and well-being during the postpartum period, particularly from the perspectives of light exposure and circadian rhythm regulation.
Several limitations of this study should be noted. Firstly, we used nocturnal animals to investigate the effects of dLAN on postpartum depression and circadian rhythms, which may not be directly generalized to diurnal humans. Nonetheless, using the nocturnal mammals allows us to control for sleep as a confounding factor [69, 70]. In addition, the circadian systems of both nocturnal mice and humans rely on a network of photosensitive retinal ganglion cells (pRGCs) expressing melanopsin (OPN4), which mediate light responses in an identical manner [71]. Secondly, although we focused on circadian rhythm gene expression in the hippocampus due to its established link to postpartum depression, other brain regions, such as the amygdala and prefrontal cortex, may also play critical roles in the mechanisms by which dLAN induces postpartum depression [72, 73]. Thirdly, while this study provides substantial evidence that disruptions in both behavioral and gene expression circadian rhythms mediate the effects of dLAN on postpartum depression, the specific underlying mechanisms warrant further investigation.
In conclusion, the findings of this study demonstrate that dLAN induces depression-like behaviors in postpartum mice. In addition, disruptions in circadian rest-activity behaviors and circadian gene expressions may mediate the detrimental effects of dLAN on these depression-like behaviors.
Supplementary information
Acknowledgements
HF was supported by the National Science and Technology Innovation 2030 of China-Major Projects (2022ZD0214100), Guangzhou Municipal School (College)-Enterprise Joint Funding Project (2024A03J0214), Guangzhou Science and Technology Plan Project (2025A03J3929), Guangzhou Key R&D Program Agricultural and Social Development Science and Technology Special Project (202206010077). JHZ was supported by the National Key R&D Program of China (2021YFC2501500) and National Natural Science Foundation of China (82171476 & 82341240). YY was supported by the National Science and Technology Innovation 2030 of China-Major Projects (2022ZD0214100), Science and Technology Projects in Guangzhou (2023A03J0578). WW was supported by the Guangzhou Municipal School (College)-Enterprise Joint Funding Project (2025A03J3354). This work was supported by Guangzhou Municipal Key Discipline in Medicine (2025–2027). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
JHZ, HF, and YY conceived and directed the study. BQL, NNZ, and HF designed the study. BQL, NNZ, BL, TL and YMF performed the experiments. BQL, NNZ, HF, WW, NYC and STR analyzed and interpreted the data. BQL, NNZ and HF drafted the manuscript with critical revisions from JHZ and YY. All authors meticulously reviewed the manuscript and provided valuable feedback. BQL, NNZ, and HF contributed equally as co-first authors.
Data availability
Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding authors upon reasonable request.
Code availability
The code employed to produce the results of this study can be obtained upon reasonable request to the corresponding author.
Ethics approval and consent to participate
This study was solely focused on animal research. All animal procedures within this investigation were meticulously carried out in strict accordance with the relevant guidelines and regulations and approved by the Animal Care and Use Committee of Guangzhou Medical University (Approval No.GY2024-411).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bingqin Lin, Nana Zheng, Hongliang Feng.
Contributor Information
Ying Yang, Email: yang.y573@163.com.
Hongliang Feng, Email: hlfeng@link.cuhk.edu.hk.
Jihui Zhang, Email: jihui.zhang@cuhk.edu.hk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-025-03405-4.
References
- 1.Nakano M, Sourander A, Luntamo T, Chudal R, Skokauskas N, Kaneko H. Early risk factors for postpartum depression: a longitudinal Japanese population-based study. J Affect Disord. 2020;269:148–53. [DOI] [PubMed] [Google Scholar]
- 2.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–83. [DOI] [PubMed] [Google Scholar]
- 3.O’hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- 4.Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giallo R, Woolhouse H, Gartland D, Hiscock H, Brown S. The emotional-behavioural functioning of children exposed to maternal depressive symptoms across pregnancy and early childhood: a prospective Australian pregnancy cohort study. Eur Child Adolesc Psychiatry. 2015;24:1233–44. [DOI] [PubMed] [Google Scholar]
- 6.Lutkiewicz K, Bieleninik Ł, Cieślak M, Bidzan M. Maternal-infant bonding and its relationships with maternal depressive symptoms, stress and anxiety in the early postpartum period in a polish sample. Int J Environ Res Public Health. 2020;17:5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 2019;52:165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissman AM, Levy BT, Hartz AJ, Bentler S, Donohue M, Ellingrod VL, et al. Pooled analysis of antidepressant levels in lactating mothers, breast milk, and nursing infants. Am J Psychiatry. 2004;161:1066–78. [DOI] [PubMed] [Google Scholar]
- 9.Molyneaux E, Trevillion K, Howard LM. Antidepressant treatment for postnatal depression. JAMA. 2015;313:1965–6. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Headon KS, Jiao A, Slezak JM, Avila CC, Chiu VY, et al. Association of antepartum and postpartum air pollution exposure with postpartum depression in Southern California. JAMA Netw Open. 2023;6:e2338315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadman T, Strandberg-Larsen K, Calas L, Christiansen M, Culpin I, Dadvand P, et al. Urban environment in pregnancy and postpartum depression: an individual participant data meta-analysis of 12 European birth cohorts. Environ Int. 2024;185:108453. [DOI] [PubMed] [Google Scholar]
- 12.Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie (Berl). 2019;23:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dollish HK, Tsyglakova M, McClung CA. Circadian rhythms and mood disorders: time to see the light. Neuron. 2024;112:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, et al. Health consequences of electric lighting practices in the modern world: a report on the national toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ. 2017;607-608:1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez de Miguel A, Bennie J, Rosenfeld E, Dzurjak S, Gaston KJ. First estimation of global trends in nocturnal power emissions reveals acceleration of light pollution. Remote Sens. 2021;13:3311. [Google Scholar]
- 16.Cinzano P, Falchi F, Elvidge CD. The first world Atlas of the artificial night sky brightness. Mon Not R Astron Soc. 2001;328:689–707. [Google Scholar]
- 17.Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, et al. The new world atlas of artificial night sky brightness. Sci Adv. 2016;2:e1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedrosian TA, Nelson RJ. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 2017;7:e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam SKE, Brown LA, Wilson TS, Tir S, Fisk AS, Pothecary CA, et al. Dim light in the evening causes coordinated realignment of circadian rhythms, sleep, and short-term memory. Proc Natl Acad Sci USA. 2021;118:e2101591118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett S, Alpert M, Kubulins V, Hansler RL. Use of modified spectacles and light bulbs to block blue light at night may prevent postpartum depression. Med Hypotheses. 2009;73:251–3. [DOI] [PubMed] [Google Scholar]
- 21.Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. Limiting the impact of light pollution on human health, environment and stellar visibility. J Environ Manage. 2011;92:2714–22. [DOI] [PubMed] [Google Scholar]
- 22.Chepesiuk R. Missing the dark: health effects of light pollution. Environ Health Perspect. 2009;117:A20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–72. [DOI] [PubMed] [Google Scholar]
- 24.Ebrahimian F, Najdi N, Masrour FF, Salari AA. Swimming exercise strain-dependently affects maternal care and depression-related behaviors through gestational corticosterone and brain serotonin in postpartum dams. Brain Res Bull. 2022;188:122–30. [DOI] [PubMed] [Google Scholar]
- 25.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–8. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30. [DOI] [PubMed] [Google Scholar]
- 27.Agostinelli F, Ceglia N, Shahbaba B, Sassone-Corsi P, Baldi P. What time is it? Deep learning approaches for circadian rhythms. Bioinformatics. 2016;32:i8–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato S, Basse AL, Schönke M, Chen S, Samad M, Altıntaş A, et al. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab. 2019;30:92–110. e4. [DOI] [PubMed] [Google Scholar]
- 29.Pramong R, Wongchitrat P, Govitrapong P, Phansuwan-Pujito P. Development of clock genes expression in rat hippocampus. J Med Assoc Thai. 2015;98:S123–9. [PubMed] [Google Scholar]
- 30.Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms. 2013;28:262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Q, Sun L, Chen Q, Jiao C, Wang Y, Li H, et al. Gut microbiota dysbiosis contributes to depression-like behaviors via hippocampal NLRP3-mediated neuroinflammation in a postpartum depression mouse model. Brain Behav Immun. 2024;119:220–35. [DOI] [PubMed] [Google Scholar]
- 32.Mason IC, Grimaldi D, Reid KJ, Warlick CD, Malkani RG, Abbott SM, et al. Light exposure during sleep impairs cardiometabolic function. Proc Natl Acad Sci USA. 2022;119:e2113290119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney MR, Nichols HB, Jones RR, Olshan AF, Keil AP, Engel LS, et al. Light at night and the risk of breast cancer: findings from the sister study. Environ Int. 2022;169:107495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mead MP, Reid KJ, Knutson KL. Night-to-night associations between light exposure and sleep health. J Sleep Res. 2023;32:e13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min JY, Min KB. Outdoor light at night and the prevalence of depressive symptoms and suicidal behaviors: a cross-sectional study in a nationally representative sample of Korean adults. J Affect Disord. 2018;227:199–205. [DOI] [PubMed] [Google Scholar]
- 36.Bedrosian TA, Weil ZM, Nelson RJ. Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF. Mol Psychiatry. 2013;18:930–6. [DOI] [PubMed] [Google Scholar]
- 37.Fonken LK, Nelson RJ. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav Brain Res. 2013;243:74–8. [DOI] [PubMed] [Google Scholar]
- 38.Walker WH 2nd, Borniger JC, Gaudier-Diaz MM, Hecmarie Meléndez-Fernández O, Pascoe JL, et al. Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol Psychiatry. 2020;25:1080–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutiérrez-Pérez M, González-González S, Estrada-Rodriguez KP, Espítia-Bautista E, Guzmán-Ruiz MA, Escalona R, et al. Dim light at night promotes circadian disruption in female rats, at the metabolic, reproductive, and behavioral level. Adv Biol. 2023;7:e2200289. [DOI] [PubMed] [Google Scholar]
- 40.Batt MM, Duffy KA, Novick AM, Metcalf CA, Epperson CN. Is postpartum depression different from depression occurring outside of the perinatal period? A review of the evidence. Focus (Am Psychiatr Publ). 2020;18:106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mariman A, Hanoulle I, Pevernagie D, Maertens SJ, Dehaene I, Tobback E, et al. Longitudinal assessment of sleep and fatigue according to baby feeding method in postpartum women: a prospective observational study. BMC Pregnancy Childbirth. 2024;24:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McBean AL, Montgomery-Downs HE. What are postpartum women doing while the rest of the world is asleep? J Sleep Res. 2015;24:270–8. [DOI] [PubMed] [Google Scholar]
- 43.Ng CM, Kaur S, Kok EY, Chew WL, Takahashi M, Shibata S. Sleep, light exposure at night, and psychological wellbeing during pregnancy. BMC Public Health. 2023;23:1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Wang X, Zhao Y, Liu A, Zhao T, Zhang Y, et al. Elevated thyroid peroxidase antibody increases risk of post-partum depression by decreasing prefrontal cortex BDNF and 5-HT levels in mice. Front Cell Neurosci. 2016;10:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao X, Wang J, Yao H, Cai Y, Cheng R. Serum BDNF concentration after delivery is associated with development of postpartum depression: a 3-month follow up study. J Affect Disord. 2016;200:25–30. [DOI] [PubMed] [Google Scholar]
- 46.Rumajogee P, Madeira A, Vergé D, Hamon M, Miquel MC. Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms. J Neurochem. 2002;83:1525–8. [DOI] [PubMed] [Google Scholar]
- 47.Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. [DOI] [PubMed] [Google Scholar]
- 49.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. [DOI] [PubMed] [Google Scholar]
- 50.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–102. [DOI] [PubMed] [Google Scholar]
- 51.Molcan L, Sutovska H, Okuliarova M, Senko T, Krskova L, Zeman M. Dim light at night attenuates circadian rhythms in the cardiovascular system and suppresses melatonin in rats. Life Sci. 2019;231:116568. [DOI] [PubMed] [Google Scholar]
- 52.Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18:307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyall LM, Wyse CA, Graham N, Ferguson A, Lyall DM, Cullen B, et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK biobank. Lancet Psychiatry. 2018;5:507–14. [DOI] [PubMed] [Google Scholar]
- 54.Bedrosian TA, Vaughn CA, Galan A, Daye G, Weil ZM, Nelson RJ. Nocturnal light exposure impairs affective responses in a wavelength-dependent manner. J Neurosci. 2013;33:13081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020;21:67–84. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun S, Ma S, Cai Y, Wang S, Ren J, Yang Y, et al. A single-cell transcriptomic atlas of exercise-induced anti-inflammatory and geroprotective effects across the body. Innovation. 2023;4:100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci USA. 2013;110:9950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi SQ, White MJ, Borsetti HM, Pendergast JS, Hida A, Ciarleglio CM, et al. Molecular analyses of circadian gene variants reveal sex-dependent links between depression and clocks. Transl Psychiatry. 2016;6:e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bumgarner JR, Walker WH 2nd, Quintana DD, White RC, Richmond AA, Meléndez-Fernández OH, et al. Acute exposure to artificial light at night alters hippocampal vascular structure in mice. iScience. 2023;26:106996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isaksson C, Ziegler AK, Powell D, Gudmundsson A, Andersson MN, Rissler J. Transcriptome analysis of avian livers reveals different molecular changes to three urban pollutants: soot, artificial light at night and noise. Environ Pollut. 2024;358:124461. [DOI] [PubMed] [Google Scholar]
- 62.Caraci F, Spampinato SF, Morgese MG, Tascedda F, Salluzzo MG, Giambirtone MC, et al. Neurobiological links between depression and AD: the role of TGF-β1 signaling as a new pharmacological target. Pharmacol Res. 2018;130:374–84. [DOI] [PubMed] [Google Scholar]
- 63.Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, et al. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology. 2013;38:872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fishbein AB, Knutson KL, Zee PC. Circadian disruption and human health. J Clin Invest. 2021;131:e148286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng H, Yang L, Ai S, Liu Y, Zhang W, Lei B, et al. Association between accelerometer-measured amplitude of rest-activity rhythm and future health risk: a prospective cohort study of the UK biobank. Lancet Healthy Longev. 2023;4:e200–e10. [DOI] [PubMed] [Google Scholar]
- 67.Sarrazin DH, Gardner W, Marchese C, Balzinger M, Ramanathan C, Schott M, et al. Prefrontal cortex molecular clock modulates development of depression-like phenotype and rapid antidepressant response in mice. Nat Commun. 2024;15:7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishihara K, Horiuchi S, Eto H, Uchida S. The development of infants’ circadian rest-activity rhythm and mothers’ rhythm. Physiol Behav. 2002;77:91–8. [DOI] [PubMed] [Google Scholar]
- 69.Walker WH 2nd, Walton JC, DeVries AC, Nelson RJ. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borniger JC, Weil ZM, Zhang N, Nelson RJ. Dim light at night does not disrupt timing or quality of sleep in mice. Chronobiol Int. 2013;30:1016–23. [DOI] [PubMed] [Google Scholar]
- 71.Foster RG, Hughes S, Peirson SN. Circadian photoentrainment in mice and humans. Biology. 2020;9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghuman A, McEwen A, Tran KH, Mitchell N, Hanstock C, Seres P, et al. Prospective investigation of glutamate levels and percentage gray matter in the medial prefrontal cortex in females at risk for postpartum depression. Curr Neuropharmacol. 2022;20:1988–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, et al. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res. 2013;47:816–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding authors upon reasonable request.
The code employed to produce the results of this study can be obtained upon reasonable request to the corresponding author.