Abstract
Background:
Current guidelines recommend extensively hydrolyzed cow’s milk protein formulas (EHF) as the first-line treatment for infants diagnosed with cow’s milk allergy (CMA). Recently, rice hydrolysate formula (RHF) has emerged as a plant-based alternative with potential advantages in taste, cost-effectiveness, and safety.
Objective:
Our comprehensive systematic review aimed to evaluate the efficacy of RHF in managing CMA and assess the growth standards of children in short-term follow-up.
Methods:
We searched PubMed, Scopus, Cochrane, Embase, and Web of Science databases for relevant studies published until May 2024. Eligible studies were selected based on the inclusion criteria. Data extraction covered study characteristics, Z-scores (weight and length for age, weight for length, body mass index [BMI], and head circumference), tolerance, atopic manifestations, IgE levels, and symptoms-based score (SBS). Quality assessment was performed using appropriate tools for different study designs. Data analysis focused on identifying trends in growth parameters and tolerance outcomes among infants with CMA.
Results:
Seventeen studies, 1695 infants with CMA, and 145 healthy infants were included in the review. Weight-for-age Z-scores varied initially but showed improvement after the first month, except in 1 study. It showed a Z score decreased by an average of 0.69 from the baseline. Length-for-age Z-scores exhibited inconsistency, and RHF tended to have negative effects but performed better than the soy formula. Weight-for-length Z-scores indicated RHF as a reasonable alternative in the first 6 months. RHF gradually enhanced BMI over 6 months. Head circumference Z-scores varied, with RHF showing mixed results compared to cow’s milk protein formula. Tolerance to RHF increased steadily over 2 years. Atopic manifestations at 36 months were moderate for RHF. IgE tests revealed similar sensitization rates across different formulas, and RHF showed effectiveness in symptom reduction over 6 months.
Conclusion:
This comprehensive review suggests that RHF can effectively substitute cow’s milk formula in managing CMA. These formulas are well tolerated in infants, have varying impacts on growth development, and show promise in reducing atopic symptoms.
Keywords: cow’s milk protein allergy, hydrolysate formula, rice, systematic review
Introduction
Food allergies are becoming a more significant health issue. 1 A food allergy is characterized as a harmful health consequence that results from a particular immunological reaction that happens consistently after consuming a certain allergic or specific protein, for example, peanuts, fish, and cow milk. 1 Immunoglobulin (Ig) E-mediated, non-IgE mediated, or mixed responses are known allergic responses.1-3 Among infants and young children under 3 years old, cow’s milk allergy (CMA) is the most common cause of food allergies. It affects 2% to 3% of the population. CMA is characterized by a persistently negative response, with variable onset timings and organ involvement, to 1 or more milk proteins via IgE and/or non-IgE pathways.4-6
Gastrointestinal symptoms are predominant in non-IgE-mediated food allergies, which present challenges in diagnosis, relying primarily on clinical assessments.1,6 Non-IgE-mediated food allergies are categorized by subacute or chronic symptoms, whereas IgE-mediated food allergies are classified by the rapid onset of symptoms ensuing the ingestion (eg, anaphylaxis). 4 For newborns diagnosed with CMA, extensively hydrolyzed casein or whey protein formulas are suggested as a dairy substitute. 4
Soy formula (SF) can trigger allergies in up to 14% of infants with CMA, even though soy tolerance is better in immunoglobulin E (IgE) than non-IgE-mediated CMA.7-9 Furthermore, substantial concentrations of phytoestrogens, like isoflavones, are present in SF.7-9 These phytoestrogens may have undesirable effects like endocrine-disrupting substances. The European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the Nutrition Committee of the French Society of Pediatrics all recommend using SF when parents want to avoid animal products or during CMA after the age of 6 months, when complementary feeding has started, and in the absence of a soy allergy.2,7,8
Since the early 2000s, hydrolyzed rice-protein formulae (RHF) have been used to treat CMA in numerous European nations, including France, Spain, and Italy.7,10 The rice used in RHF is not genetically modified and does not contain phytoestrogens. Apart from the addition of vitamin D2 (cholecalciferol), RHF is entirely plant-based. 7 Foods meant for children under 3 years old are subject to stringent regulations about the levels of pesticides, heavy metals, and arsenic. 7 Interest in growth rates in the first year has been raised by the observation that restricted growth in this period could affect health outcomes in adulthood. 11 Restricted growth in children has both immediate and long-term consequences, including increased morbidity and mortality, poor child development and learning capacity, increased risk of infections and noncommunicable diseases, increased susceptibility to accumulate fat, primarily in the central region of the body, lower fat oxidation, lower energy expenditure, insulin resistance, and a higher risk of developing diabetes, hypertension, dyslipidemia, lowered working capacity, and unfavorable maternal reproductive outcomes in adulthood. Furthermore, children with restricted growth who underwent rapid weight gain after 2 years have a higher chance of being overweight or obese later in life.2,3 Additionally, RHF may be superior to other formulas applied for CMA in terms of taste and cost. This allows more distribution to this formula.1,10
The primary aim of the present study was to investigate the existing literature on RHF, focusing on determining its effectiveness in treating CMA and the growth quality of children who used these formulas for extended periods. Our findings demonstrated the value of RHF in patients with CMA, and we looked at the recent evidence of its application in the treatment, outlining the pros and cons.
Methods
We performed this study in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 12 Our study was registered in PROSPERO, “International Prospective Register of Systematic Reviews” under number: CRD42024540241: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=540241
Search Strategy and Eligibility Criteria
We searched PubMed, Scopus, Cochrane, Embase, and Web of Science databases for relevant studies published until May 2024. There were no limitations regarding the time of publication for the studies included. We used the Medical Subject Headings database to retrieve the synonyms of our search strategy, and the terms were combined using “OR” and “AND” Boolean operators following the Cochrane Handbook for Systematic Reviews. 13 Using a highly sensitive search strategy: (milk OR Dairy) AND (Allergies OR Allergy OR Hypersensitivities OR Hypersensitivity OR intolerance OR immune)) OR CMA) AND ((rice AND formula) OR “Rice protein hydrolysates” OR “Rice protein hydrolysates” OR “Rice protein hydrolyzed” OR “Hydrolyzed rice protein-based formulas”).
The inclusion criteria for our study were as follows: A) The searches were limited to studies that were conducted on human subjects with no language restrictions. B) Studies evaluating the effect of RHF on growth, tolerance, and allergic outcomes in infants and children diagnosed with CMA. The Exclusion criteria: Studies focusing on other hydrolysate formulas or lacking sufficient data on RHF.
Study Selection and Data Extraction
The authors independently assessed the titles and abstracts of all articles using the specific inclusion criteria. They also individually reviewed the full text, including tabulated data and supplements, and resolved differences by referring to other authors to determine if they met the criteria. The final decision on whether to include each paper was made by consensus among all authors. The debate occurred only in 2 papers, and the senior authors (K.S. and H.A.A.I) resolved the issues, with all authors agreeing after discussion. The authors independently extracted data from the studies using Excel data extraction. Specific data were extracted from the included studies, including study characteristics such as study type, number of subjects, age at inclusion, duration of follow-up, number of infants fed RHF and other formulas, and outcome measured. Also, we extracted (weight for age, length for age, weight for length, body mass index [BMI], and head circumference) Z-scores, tolerance, atopic manifestation, IgE tests, and symptom-based score (SBS).
Critical Appraisal Tool and Risk of Bias Assessment
Our included studies were evaluated using different tools according to study design. We used the National Institute of Health Study Quality Assessment Tools (NIH) to assess the quality of the selected observational cohort studies and Studies with no control group. 11 Additionally, we employed the Cochrane risk-of-bias tool for randomized trials (RoB) to evaluate the quality of randomized controlled trials. 12 Two reviewers (A.E. and K.S.) conducted the quality assessment together through a group discussion, and the final decision was made based on their mutual agreement. The NIH tool comprises 14 domains for observational studies and 12 domains for studies with no control group. Each domain was evaluated using the options “yes,” “no,” or “not applicable.” The RoB 2 tool consists of 5 main domains: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result.
Types of Formulae Included in the Study and Definitions of the Main Outcomes
-
A. Types of Formulae included are:
• Rice hydrolysate formula (RHF)
• Extensively hydrolyzed casein formula (EHCF)
• Soy-based formula (SF)
• Amino acid formula (AAF)
• Extensively hydrolyzed whey formula (EHWF)
-
B. Main outcomes:
• Growth parameters, and their measures are weight-for-age, length-for-age, weight-for-length, BMI, and head circumference Z scores
• Allergy parameters and their measures are tolerance, atopic manifestations, IgE levels, and SBS.
Results
Search Results
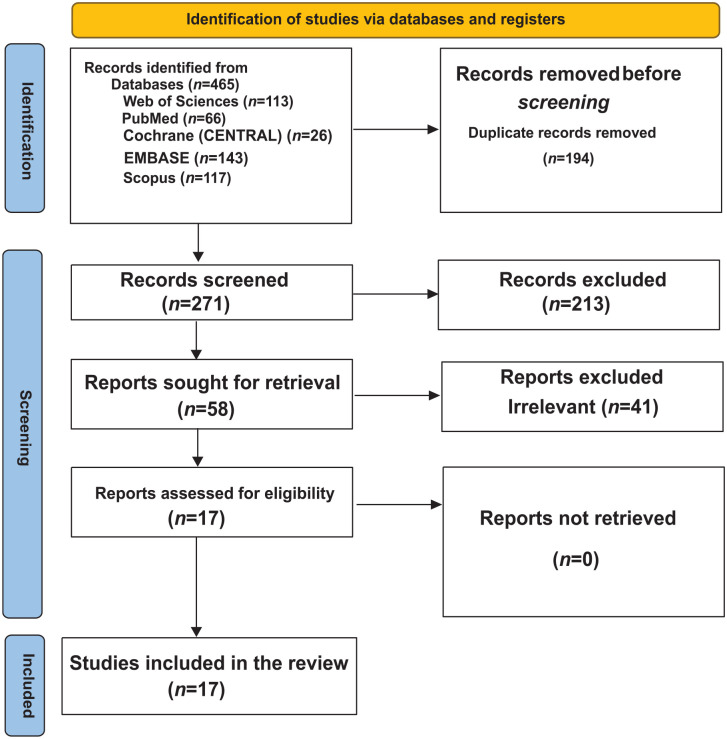
Our search strategy resulted in a total number of 465 studies. After removing duplicates, 271 references remained. Following screening the title and abstract, 58 articles were selected for full-text review. Following the full-text screening, 19 manuscripts were eligible and were included in this review.4,8-10,13-26 The selection process for including studies is shown in (Figure 1).
Figure 1.
PRISMA flow diagram for the search, screening, and article selection process.
Summary of the Included Studies
We reviewed the data of 1722 infants with CMA and 142 healthy infants. Follow-up duration ranged from 1 to 65 months. Absolute weight, height, and head circumference were extracted at different time points (Supplemental Table S1a-S1c). Ten studies4,13-17,21-23,25 were carried out in Italy, 3 studies10,24,26 in Spain, 1 study 18 in France, 1 study 20 done in France and Spain, another study 9 in Belgium, and lastly, 1 study 19 from the United States. The studies measured the growth in CMA infants, tolerance to the formula, allergy, and the duration of CMA (Table 1).
Table 1.
Studies characteristics.
| Study ID | Year | Country | Study type and aim of work | No. of Subjects |
Age at inclusion (Mean ± SD months) | Sex (male %) | Duration of follow-up | Infant health status | No. of infants Fed HRPF | No. of infants Fed another formula for comparison | IgE-Mediated CMA | Control Group | Outcome measure | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D’Auria et al 4 | 2003 | Italy | Randomized Pilot Study. The study evaluated whether RHF supports normal growth and maintains adequate metabolic balance in infants with CMA. |
16 | 6-14 | 56.25% | 6 months | CMA | 8 | 8 SF | No | 8 SF | Growth | Infants in both cohorts exhibited normal growth throughout the study, with no observed adverse reactions. The mean plasma biochemical parameters remained within normal reference ranges and showed no significant differences between the groups. RHF is a nutritionally adequate alternative for infants with CMA. |
| Fiocchi et al 16 | 2003 | Italy | Double-blind, placebo-controlled food challenge. The study aimed to determine if children with cow’s milk and soy allergies could tolerate RHF. |
18 | 5 years (median) | 66.6% | 16 months | CMA | 18 | 18 | Yes | Allergenicity and Tolerance | Thirteen children had positive skin prick tests for casein, 10 for lactalbumin, 8 for rice, and 2 for rice hydrolysate. All tested patients showed positive serology for cow’s milk, with 13 also positive for soy and 7 for rice. The double-blinded, placebo-controlled challenge with RHF was negative in all cases. Children allergic to cow’s milk and soy tolerated RHF without adverse reactions, indicating that rice hydrolysate may be a viable protein source for children with multiple food allergies. | |
| Savino et al 23 | 2005 | Italy | Double-blinded clinical trial, with control
group. The study aimed to compare the growth of infants fed a rice-based hydrolysate formula with those fed a soy formula or an extensively hydrolyzed casein formula during the first 2 years of life. |
88 | 3.27 ± 0.32 | 24 months | CMA | 15 | 17 SF 26 EHCF |
– | 30 infants with AD and without CMA were fed a free diet | Growth | No significant differences were observed in the weight-for-age Z-scores among the RHF, SF, and EHCF groups during the first 2 years of life. | |
| Lasekan et al 19 | 2006 | United States | Randomized, Blinded clinical trial. The study aimed to assess growth, tolerance, and plasma biochemistry in infants fed an experimental rice protein-based formula. |
65 | From birth to 16 weeks of age | 49.2 | 4 months | Healthy | 32 | 35 CMF | No | 35 CMF | Tolerance and Growth | Healthy infants fed an experimental partially hydrolyzed rice protein-based formula exhibited normal growth, tolerance, and plasma biochemistry, comparable to those fed a standard intact milk protein-based formula, despite some variations in amino acid profiles. |
| Fiocchi et al 17 | 2006 | Italy | Prospective, multi-center clinical trial The study evaluated the tolerance of a rice-based hydrolyzed formula in children with cow’s milk allergy. |
100 | 3.17 ± 2.93 years | 58 | CMA | 96 | Yes | Tolerance | The rice-based hydrolyzed formula may be an alternative for children with multiple food allergies, including cow’s milk. | |||
| Agostoni et al 13 | 2007 | Italy | Randomized, prospective, comparative, unblinded
trial. The study investigated whether the type of formula used during the complementary feeding period (6–12 months of age) influences growth differently in infants with CMA. |
125 | 5.3 months (median) | 68.8% | 6 months | CMA | 30 | 32 Soy 31CHy |
yes | 32 BF | Growth | At 6 months, all groups had low weight-for-age (WA) and length-for-age (LA) Z-scores. Infants fed hydrolyzed formulas tended to have greater weight-for-age Z-score improvement between 6 and 12 months. Notably, casein- and rice-based hydrolyzed formulas led to better WA changes than soy formulas. |
| Reche et al 10 | 2010 | Spain | Prospective, Open, Randomized Clinical Trial. The study aimed to compare the clinical tolerance of a new hydrolyzed rice protein formula (HRPF) with an extensively hydrolyzed cow’s milk protein formula (EHF) in infants with IgE-mediated CMA. |
92 | 4.3 | 50 | 24 months | CMA | 41 | 40 EHF | Yes | 40 EHF | Tolerance and Growth | The hydrolyzed rice protein formula (HRPF) was well tolerated by infants with moderate to severe IgE-mediated cow’s milk protein allergy. Children who received this formula demonstrated comparable growth and clinical tolerance development to those who were given an extensively hydrolyzed formula (EHF). |
| Terracciano et al 25 | 2010 | Italy | Prospective, randomized, Cohort, MICMAC. The study aimed to prospectively assess how dietary factors influence the duration of the disease in a randomized cohort. |
72 | 14.1 ± 8.6 | 66.7 | 26 months | CMA | 25 | 29 SF 18 EHF |
Yes | Duration of CMA | The key finding is that infants and children with cow’s milk allergy (CMA) managed with hydrolyzed rice or soy-based formulas develop tolerance significantly earlier than those on an extensively hydrolyzed cow’s milk formula. | |
| Girardet et al 18 | 2010 | France | Prospective, Open, Multicenter clinical
trial Assessing the growth of healthy formula-fed (PPN) infants based on rice protein hydrosylate |
77 | 5 months | Healthy | 77 | No | Growth | Infants fed with PPN based on hydrolyzed rice proteins have satisfactory growth and tolerance. This formula is a new alternative to standard cow and soy milk-based formulas. | ||||
| Nocerino et al 21 | 2012 | Italy | Prospective, Cohort The study sought to evaluate the impact of various dietary therapeutic approaches on the time required for tolerance acquisition in children with CMA. |
225 | 5.75 ± 0.21 | 61.3 | 12 months | CMA | 40 | 36 EHCF 43 EHCF + LGG 50 SF 17 Amino-acid-based formula 39 not receiving any formula |
Yes | Tolerance | The results indicate that a dietary regimen using extensively hydrolyzed cow’s milk formula (EHCF) may be more effective than other dietary approaches in accelerating tolerance acquisition in infants with cow’s milk allergy (CMA). Additionally, the inclusion of Lactobacillus GG appears to enhance this effect. | |
| Berni Canani et al 14 | 2013 | Italy | Prospective, open, Multicenter, nonrandomized
trial. The study aimed to prospectively evaluate the impact of different dietary management strategies on the rate of tolerance acquisition in children with CMA. |
260 | 5.92 | 64.2% | 12 months | CMA | 46 | 55 EHCF 71 EHCF + LGG 55 SF 33 AAF |
(111 IgE)(149 non Ige) | Tolerance | EHCF accelerates tolerance acquisition in children with CMA more effectively than other dietary options, and the addition of LGG further enhances this effect. | |
| Vandenplas et al 9 | 2014 | Belgium | Prospective, Open study without a control group. The effectiveness of a new extensively hydrolyzed rice protein infant formula (RHF) was evaluated in infants with CMA. |
40 | 3.4 ± 1.5 | 52.5 | 6 months | CMA | 40 | Yes | Tolerance and Growth | RHF was tolerated by more than 90 % of children with proven
CMA with a 95 % confidence interval. This RHF is an adequate
and safe alternative to cow milk-based EHF. |
||
| Solar et al 24 | 2016 | Spain | Prospective, blinded, Randomized clinical trial. The study aimed to evaluate the tolerance and efficacy of a new extensively hydrolyzed rice protein formula over 3 months in infants with IgE-mediate CMA. |
50 | 22.7 ± 8.4 weeks | 62 | 3 months | CMA | 50 | Yes | Tolerance and growth | Over 97% of infants tolerated the study formula at the time of introduction. After1 month of follow-up, the infants’ conditions significantly improved. Growth during the 3-month follow-up period followed a normal pattern. | ||
| D’’Auria et al 15 | 2016 | Italy | Randomized trial. The study aimed to prospectively evaluate the impact of different hydrolyzed formulas compared to soy formula on the rate of tolerance acquisition in infants with CMA |
112 | 6 months or more | 56% | 65 months | CMA | 29 | 33 SF 25 eHWF 25 eHCF |
No | Tolerance | Infants with CMA who are fed an extensively hydrolyzed cow’s milk formula achieve tolerance more quickly than those fed vegetable-based formulas, likely due to the tolerogenic effect of residual cow’s milk peptides. | |
| Tormo et al 26 | 2017 | Spain | Prospective, Open study without a control group. The effects of feeding a partially hydrolyzed rice protein formula were examined in children with CMA. |
30 | 1 month | CMA | 30 | No | Tolerance and Growth | Hydrolyzed rice protein formula is a suitable and safe alternative for feeding infants with non-IgE-mediated cow’’s milk protein allergy. | ||||
| Nocerino et al 22 | 2021 | Italy | Prospective, Open, Nonrandomized cohort. The study aimed to determine whether the choice of formula for treating CMA could influence the occurrence of other allergic manifestations and the timing of immune tolerance acquisition |
365 | 65.8 | 36 months | CMA | 73 | 73 EHCF + LGG 73 SF 73 EHWF 73 Amino-acid-based formula |
Yes | Allergenicity and Tolerance | The study’s findings suggest that EHCF combined with L. rhamnosus GG is more effective than other formulas in preventing allergic manifestations (AMs) and promoting immune tolerance in children with CMA. | ||
| Nieto-García et al 20 | 2023 | France and Spain | Prospective, Multicenter, Randomized, Double-blind,
controlled trial. The study assessed the safety, normal growth, and acquisition of tolerance in children with CMA when comparing RHF to EHF. |
105 | 12 months | CMA | Yes | Tolerance and Growth | EHF and RHF demonstrated appropriate growth, successful tolerance acquisition, and good safety profiles in patients with CMA, with no significant differences observed between the 2 formulas. |
Abbreviations: AD, atopic dermatitis; CMF, Cow’’s milk-based formula; EHCF, extensively hydrolyzed casein formula; EHF, extensively hydrolyzed formula; EHWF, extensively hydrolyzed whey formula; LGG, the probiotic L. rhamnosus GG; RHF, rice hydrolyzed formula; SF, soy-based formula.
Weight for Age Z-Score
Six studies reported the weight for age Z-score for Infants fed rice.4,8,9,13,19,23 At the baseline, the mean difference in weight for the age Z-score ranged from −0.71 to 0.28. Two studies showed an increase in Z score in the first month.8,9 However, 1 study revealed that RHF had no effect in the first month but started to enhance after the first month, changing by 0.35° in 1 month. 19 After 6 months of follow-up, 1 study showed that RHF was associated with decreased Z score by an average of 0.69° change from the baseline. 13 Also, to some extent, the results of SF were similar to RHF in the first 6 months. 13 However, for the prolonged effect, RHF increased weight Z score as compared to SF in the 12th month. 13 Another study conducted by Savino et al., 23 covering the second quarter of the first year, showed a slight decrease, contrasting with the results of Lasekan et al., 19 which showed significant improvement at the beginning of the fourth month. For the prolonged effect of RHF, 2 studies showed a rapid decrease in Z score, which refers to the long-term harmful effect of hydrolysate rice in infants. Perhaps this effect is due to the lower protein content, which was later increased.13,23 One study compared EHCF, SF, and RHF to evaluate their impact on growth in infants with IgE-mediated CMA during the complementary feeding period (6–12 months). The findings indicated that both EHCF and RHF showed better weight-for-age Z-score increments compared to SF during this period.
Regarding long-term outcomes, RHF demonstrated a slight preference due to its consistent positive impact on weight-for-age Z-scores. EHCF, while effective in the short term, displayed intermediate results compared to SF and RHF over the follow-up period, suggesting that RHF may provide a better long-term growth benefit for infants with CMA 13 (Supplemental Table S2a-S2c).
Length for Age Z-Score
Five studies, encompassing the first year of life, reported the length for age Z-score in infants fed RHF.3,4,9,13,19 At the baseline, the mean difference in length for the age Z-score ranged from −0.16 to 0.2. Agostoni et al 13 showed a rapid Z score decrease to −0.73 in the sixth month. Two studies8,9 revealed that RHF had no effect on the length. However, another 2 studies showed that RHF had no effect only in the first 4 months, then increased to reach the mean difference (0.13-0.71).13,19 The discrepancy between the studies was high, so we cannot fully understand the effect of RHF on infant length.
Two studies over 12 months reported the length of age Z-score in infants fed SF.4,13 At the baseline, the mean difference ranged from −0.1 to 0.01. One study showed that SF decreased the length for age Z score. However, another study showed a positive effect of SF. Therefore, this difference between results does not lead us to a reliable conclusion. However, RHF tends to have slower growth regarding length but is still better than SF (Supplemental Table S3a and S3b).
Weight for Length Z-Score
Three studies, encompassing the first year, reported the weight for length Z-score.8,9,13 RHF showed variability with a baseline mean difference of weight for length Z-score ranged (-1.1 to 0.12). One study showed a decrease in Z score, but 2 showed an increase in the first 3 months. In the sixth month, the weight for length Z-score reaches (0-0.04), near the normal infants. Interestingly, after the sixth month, the weight for length Z-score increased rapidly to reach 0.24 in 1-year-old, which showed a high weight compared to normal infants. For SF, at birth, the mean difference was −0.2. Over subsequent time points, the mean differences fluctuated, reaching −0.12 at 6 months, −0.18 at 9 months, and −0.3 at 1 year, 13 which refers to its ability to increase weight in the first 6 months and then starting to decrease. For EHCF, the baseline mean difference was −0.37. Similar fluctuations were observed at 6, 9 and 12 months: −0.2, −0.25, and −0.30. 13 All formulas tend to show negative effects after the sixth month. However, RHF can be an alternative formula in the first 6 months (Supplemental Table S4).
BMI for Age Z-Score
Two studies, encompassing the first 6 months, reported the BMI for age Z score.8,9 At the baseline, the mean difference ranged from −0.7 to −1.1. The 2 studies showed a nearly constant increase over time to reach zero in the sixth month. This means that RHF enhanced the BMI gradually over the first 6 months to get a normal infant BMI in the sixth month (Supplemental Table S5).
Head Circumference Z-Score
Two studies over the first 6 months examined the alterations in head circumference in infants.8,20 Vandenplas et al., 9 investigated various time points, reporting mean differences ranging from 0.1 to 0.5 during the first 6 months of the infant’s life. These findings indicate variability in head circumference Z-scores. In the first 3 months, head circumference remained fixed but gradually increased to a mean difference of 0.5 by the sixth month, which is above normal. Lasekan et al., 19 explored the effects of RHF and cow’s milk protein formula on head circumference Z-scores. For the RHF, the mean differences ranged from −0.45 to 0.14 in the first 4 months, while for cow’s milk protein formula, the range was from −0.49 to 0.26 during the same period. These results showed an increased head circumference Z-score in the first 2 weeks of those who received the RHF. Additionally, cow’s milk protein formula showed a rapid increase after 2 weeks to reach 0.26 in the sixth month, with a mean of 0.12 between both formulas (Supplemental Table S6).
Tolerance
Five studies, encompassing the period from the sixth month to the second year of life, reported patients’ acquisition of tolerance.8,10,14,21,25 For RHF, the tolerance increased almost in constant proportion, about (20%-25%) every 6 months, reaching (0.84%-100%) at 24 months.8,10,14,21,25 Also, for EHCF, the tolerance increased in the same manner as RHF, while when Lactobacillus rhamnosus GG (LGG) was added to the EHCF, the proportion increased by about 40% in 6 months.14,21 For SF, the proportion ranged from 12% at the 6 months to (23%-26%) at 12 months, reaching 72% at the end of the study.14,21,25 In the amino acid-based formula (AAF), the proportion ranged from 0.05 in the 6 months to 17% after 6 months.14,21 That means infants who received EHCF + LGG were the most likely to acquire tolerance between other groups. Also, RHF needs 2 years to reach the same tolerance of (EHCF + LGG) in 1 year (Supplemental Table S8).
In the study conducted by Terracciano et al., 25 the duration until the acquisition of tolerance was investigated for various infant formulas. RHF and SF exhibited the same mean difference of 24.3 months. Nocerino et al., 22 reported the incidence of immune tolerance acquisition to cow’s milk proteins in a 36-month prospective cohort study airing at infancy upon diagnosis of the allergy. RHF, SF, and EHWF showed incidences of 0.41, 0.4, and 0.42, respectively, indicating moderate occurrence of adverse immune reactions. The incidence for EHCF was reported at 0.81, providing evidence of the positive effect, with a long duration of around 40 months.22,25 The AAF had a lower incidence of tolerance at 0.19 in a 36-month prospective cohort study starting at infancy, suggesting a relatively worse effect (Supplemental Tables S7 and S9).
Atopic Manifestation at 36 Months
One study reported the incidence of ⩾1 atopic manifestation at 36 months 22 providing comparative data on different formulas. RHF had an incidence of 0.52, indicating moderate adverse reactions. Similarly, SF and EHWF showed comparable rates of 0.58 and 0.51, respectively, suggesting moderate levels of atopic symptoms. In contrast, EHCF exhibited a significantly lower incidence of 0.22, highlighting its superior effectiveness in minimizing allergic manifestations. AAF, however, had the highest incidence at 0.77, indicating a greater likelihood of adverse reactions compared to other formulas. These results emphasize that while EHCF is the most effective in reducing atopic manifestations, RHF demonstrates moderate effectiveness and remains a viable option for CMA management, especially when other formulas are less suitable or poorly tolerated (Supplemental Table S10).
IgE Tests and Symptom-Based Score
One study investigated the incidence of positive skin prick tests (SPT) for IgE-mediated CMA that was assessed across different formula groups at the first and third visits. SPT was performed using fresh cow milk encompassing 3.5% fat applied to the patient’s volar forearm and a 1-mm single peak lancet (ALK, Copenhagen, Denmark), with the histamine dihydrochloride (10 mg/mL) and the isotonic saline (sodium chloride 0.9%) as positive and negative control, respectively. Reactions were based on the largest diameter (in mm) of the wheal and flare at 15 minutes. The SPT result was recorded as “positive” if the wheal was 3 mm or larger, without reaction of the negative control. 14 The RHF and SF groups had a proportion of 82% at both the first and third visits. The EHCF group had a proportion of 91% on the first visit and 70% on the third visit. Additionally, when LGG was added to the EHCF group, it exhibited 88% at the first visit and 55% at the third. The AAF group had a proportion of 85% at both visits 14 (Supplemental Table S11).
Infants who received RHF, SF, or AAF didn’t show any difference in SPT for IgE in both visits, which means that sensitization to a food allergen didn’t alter. However, EHCF showed a moderate decrease. Also, the occurrence of positive atopy patch tests for non-IgE-mediated CMA was investigated across different formula groups at the first and third visits. The RHF group showed 42% of the total at the first visit and 34% at the third. Similarly, the SF group had 59% at the first visit and 43% at the third. The EHCF group exhibited a proportion of 70% of the total at the first visit and 35% at the third visit. Additionally, when LGG was added to the EHCF group, it showed 68% on the first visit and 13% on the third visit. The AAF group exhibited 63% at both the first and third visits 14 . After eliminating the suspected food from the diet, both the RHF and SF groups exhibited a minor improvement. However, the AAF group still showed no discernible effects. In contrast, the EHCF or EHCF + LGG groups demonstrated a significant decrease, indicating their effectiveness surpassing that of the RHF group (Supplemental Table S12).
In the study conducted by Terracciano et al., 25 the impact of different dietary regimens on the duration of CMA was assessed. For the comparison between RHF and SF, the crude hazard ratio (HR) was 1.32, and after adjusting for potential confounders, the adjusted HR showed a slight decrease. In the comparison between RHF and EHCF, the crude HR was 3.01, with a slight increase in the adjusted HR (Supplemental Table S13).
The SBS, which quantifies the number and severity of suspected cow’s milk-related symptoms, was reported by 2 studies.8,9 At inclusion, the mean SBS was 13 to 13.5. Over the course of 1 month, there was a notable reduction in symptoms, reflected in a mean SBS of 3.5 among the same group. At 3 months, it further reduced to 2.4. Finally, at 6 months, the symptoms continued diminishing, resulting in a mean SBS of 1.5 for the same group. These findings collectively suggest a significant improvement in symptom presentation over the first 6 months, emphasizing the effectiveness of the RHF in decreasing the severity of the symptoms (Supplemental Table S14).
Quality Assessment
The Cochrane tool (version 2) was employed for the quality assessment of 11 randomized clinical trials (Figure 2). Out of these, 7 studies were found to have a high risk of bias because of a lack of essential information on allocation, randomization methods, blinding, and missing outcome data. Four cohort studies were evaluated using the NIH quality assessment scale for cohort studies. Out of these, 3 studies were found to have good to high quality, and 1 had low quality due to limitations in methodology (Supplemental Table S15). The remaining 4 studies were assessed using the NIH scale for studies without a control group; 2 were found to be good quality, and 2 were high quality (Supplemental Table S16).
Figure 2.
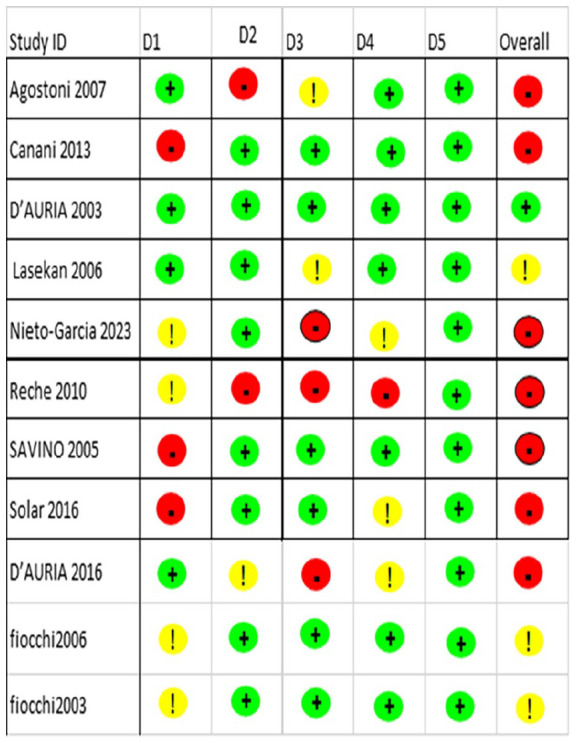
Cochrane risk-of-bias tool for randomized trials (RoB 2).
Green means low risk of bias, Yellow means some concern, and Red indicates high risk of bias.
Discussion
This systematic review explored the efficacy of RHF by assessing various growth parameters, including weight-for-age, length-for-age, weight-for-length, BMI, head circumference, tolerance, atopic manifestations, IgE levels, and SBS in infants with CMA as compared to other formulas for allergy treatment.4,8-10,13-26 The findings reveal fluctuations in growth parameters over different time intervals, indicating a complex impact on growth. The review also addressed the incidence of immune tolerance acquisition and atopic manifestations, providing valuable insights for managing CMA.
The adequacy of infant formula composition should be determined by comparing its effects on physiological parameters, such as growth patterns, and functional outcomes like immune responses in infants fed formulae with those found in healthy populations. 27 The impact of different hydrolysate formulas on growth development was examined in several studies using Z scores for weight, length, and weight for length. Z scores eliminate any potential bias from gender-specific growth differences and small variations in gestational or chronological ages, enabling comparisons of healthy term-born infants to demographic norms based on Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) reference data.19,28,29 By comparing SF and RHF impacts on Weight for Age Z score, similar effects during the initial 6 months were observed, with differences in superiority in the first 2 years.4,13,23 However, the ESPGHAN advises against using SF before 6 months. 19 CMA is one of the main factors influencing growth rates in the first year of life. During the 6-to-12-month period, using hydrolysate formula may reduce local inflammatory responses, which may improve the body’s ability to absorb nutrients from other solid foods and may have an impact on growth.13,30
RHF emerged as a favorable option for the first 6 months, exhibiting positive effects on weight length and BMI Z-scores, in contrast to SF and EHCF, which negatively affected growth. These considerations are within the normal growth rate, not the accelerated growth or obesity exhibited in some formulae. The nutritional drawbacks of SF can be attributed to the reduced absorption of minerals and trace elements due to their phytate content. 31 Furthermore, the RHF enabled a normalization of the growth parameters in the first 6 months. The American Academy of Pediatrics recommended extensively hydrolyzed formulae (EHF) as a preferred therapeutic option, with SF as a second choice. 32 RHF can be recommended as the first-line alternative formula to EHCF or AAF in the dietary management of CMA. 6 However, EHF are substantially more expensive than standard or SF and generally have a bitter taste, which often hampers their acceptability. Therefore, a cost-effective and palatable dietary alternative such as RHF is needed. 9
Infant’s tolerance to substitute formula plays a significant role in formula selection. 13 Despite SF showing better tolerance in IgE compared to non-IgE-mediated cow’s milk protein allergy, both ESPGHAN and an Australian expert recommend not using SF before the age of 6 months.33,34 AAP recommends that the used formula in infants with CMA should be tolerated by at least 90% of children, which was consistent with RHF.7,19,29 In most of our included studies, the achievement of RHF tolerance was around 100% by the age of 2 years.8,10,14,21,25 Clinical tolerance of the RHF was assessed with a SBS, and growth (weight and length) was monitored. In compliance with the present guidelines, this RHF was tolerated by more than 90% of children with a proven CMA with a 95% confidence interval. This RHF can be a safe and satisfactory alternative to cow milk-based EHCF. 9
Healthy infants exhibit good tolerance to rice-based formula, aligning with research findings indicating its tolerability in malnourished infants and those with multiple allergies in both infants and young children.4,6,35 The positive tolerability of RHF in healthy infants is affirmed by its appealing odor, taste, and flavor. 10
Infants with CMP-induced enteropathy or enterocolitis who are sensitive to soy protein should avoid isolated SF. RHF are proven safe and effective for CMA and may be considered as alternatives. RHF is an option for selected infants as those with atopic manifestations or those exhibiting severe allergic manifestations, while 10% to 14% of affected infants with CMA react to soy protein. 34 The initial diagnosis of cow’s milk protein allergy CMA may rely on indicative clinical manifestations, along with SPT and specific IgE determination for milk and protein fractions such as casein, alpha-lactalbumin, and beta-lactoglobulin. 36 Positive SPT to different hydrolyzed formulas was observed in children with CMA. 29 In the current study, EHCF groups exhibited a reduction in IgE levels and SBS compared to RHF, SF, and AAF, which showed no significant difference in sensitization to food allergens. 14 Moreover, the Cow’s Milk-related Symptom Score (CoMiSS) showed a significant decrease in the severity of suspected cow’s milk-related symptoms during the initial 6 months when using RHF.8,9 Infants receiving the rice formula exhibited a tendency for reduced occurrences of spit-up and vomiting in comparison to those fed intact protein milk-based formula, aligning with the trend observed in the reduction of spit-up associated with SF.8,9 While our systematic review offers valuable insights into various hydrolysate formulas, it is essential to acknowledge a notable inherent limitation. The included studies exhibit divergent designs, impeding our ability to conduct a meta-analysis on this subject.
Conclusion
This comprehensive review suggests that RHF can effectively substitute cow’s milk formula in managing CMA. These formulas are well tolerated in infants, have varying impacts on growth development, and show promise in reducing atopic symptoms. Due to the variability of the study, further investigations are necessary to fully understand the efficacy of the RHF.
Supplemental Material
Supplemental material, sj-pdf-1-pdi-10.1177_11795565251332173 for Rice Hydrolysate Formula in Infants and Children Allergic to Cow’s Milk: A Systematic Review of Evidence by Khaled Saad, Amir Aboelgheet, Khalid Hashim, Eman F. Gad, Anas Elgenidy, Ramez M. Odat, Aya Sherif, Ahmed Altaweel, Asmaa B. Zahran, Sara K. Kamal, Abdelrahman Elshimy, Ahmed Samir, Anas Khaled, Ahmed Ibrahim, Amira Elhoufey, Hamad Ghaleb Dailah, Thamer Alruwaili and Hoda Atef Abdelsattar Ibrahim in Clinical Medicine Insights: Pediatrics
Acknowledgments
Not applicable.
ORCID iD: Khaled Saad  https://orcid.org/0000-0002-8473-6116
https://orcid.org/0000-0002-8473-6116
Supplemental Material: Supplemental material for this article is available online.
Statements and Declarations
Ethical Considerations: Not applicable.
Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Author Contributions/CRediT: Conceptualization: KS, EFG, KH, AA, and AA. Methodology: RMO, AA, AS, Alt, ABZ, SKK, AE, AS, AK, AI, AE, and HGD. Writing—original draft: TA, HAAI, and KS. Writing—review and editing: HAAI, RMO, AA, AS, Alt, ABZ, SKK, AE, AS, AK, and AI. Supervision: KS, HAAI, and EFG. All authors have read and approved the final version of the manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Trial Registration: Our study was registered in PROSPERO, “International Prospective Register of Systematic Reviews.” Under number: CRD42024540241: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=540241.
Data Availability: All data generated or analyzed during this study are included in the article and its Supplemental Materials files.
References
- 1. Anania C, Martinelli I, Brindisi G, et al. Hydrolyzed rice formula: an appropriate choice for the treatment of cow’s milk allergy. Clin Med. 2022;11(16):4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saad K, Elgenidy A, Atef M, et al. Cow’s milk-related symptom score for cow’s milk allergy assessment: a meta-analysis for test accuracy. Pediatr Res. 2023;93(4):772-779. [DOI] [PubMed] [Google Scholar]
- 3. Vandenplas Y, Meyer R, Nowak-Wegrzyn A, Salvatore S, Venter C, Vieira MC. The remaining challenge to diagnose and manage cow’s milk allergy: an opinion paper for daily clinical practice. Nutrients. 2023;15(22):4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D’auria E, Salvatore S, Acunzo M, et al. Hydrolysed formulas in the management of cow’s milk allergy: new insights, pitfalls and tips. Nutrients. 2021;13(8):2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flom JD, Sicherer SH. Epidemiology of cow’s milk allergy. Nutrients. 2019;11(5):1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fiocchi A, Barrio-Torres J, Dupont C, et al. Hydrolyzed rice formula for dietary management of infants with cow’s milk allergy. World Allergy Organ J. 2022;15(12):100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bocquet A, Dupont C, Chouraqui JP, et al. Efficacy and safety of hydrolyzed rice-protein formulas for the treatment of cow’s milk protein allergy. Arch Pediatr. 2019;26(4):238-246. [DOI] [PubMed] [Google Scholar]
- 8. Vandenplas Y, De Greef E, Hauser B. Safety and tolerance of a new extensively hydrolyzed rice protein-based formula in the management of infants with cow’s milk protein allergy. Eur J Pediatr. 2014;173(9):1209-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vandenplas Y, De Greef E, Hauser B, et al. An extensively hydrolysed rice protein-based formula in the management of infants with cow’s milk protein allergy: preliminary results after 1 month. Arch Dis Child Educ Pract Ed. 2014;99(10):933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reche M, Pascual C, Fiandor A, et al. The effect of a partially hydrolysed formula based on rice protein in the treatment of infants with cow’s milk protein allergy. Pediatr Allergy Immunol. 2010;21(4):577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH). Study quality assessment tools. 2023. Accessed December 30, 2023. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 12. Cochrane Training. Chapter 8: assessing risk of bias in a randomized trial. 2023. Accessed December 30, 2023. https://training.cochrane.org/handbook/current/chapter-08
- 13. Agostoni C, Fiocchi A, Riva E, et al. Growth of infants with IgE-mediated cow’s milk allergy fed different formulas in the complementary feeding period. Pediatr Allergy Immunol. 2007;18(7):599-606. [DOI] [PubMed] [Google Scholar]
- 14. Berni Canani R, Nocerino R, Terrin G, et al. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013;163(3):771-777.e1. [DOI] [PubMed] [Google Scholar]
- 15. D’Auria E, Pietra B, Mandelli M, Radaelli G, Verduci E, Banderali G. Effect of different hydrolyzed formulas and soy formula on tolerance acquisition in children with cow’s milk allergy. J Pediatr Gastroenterol Nutr. 2016;62(5):728.26465789 [Google Scholar]
- 16. Fiocchi A, Travaini M, D’Auria E, Banderali G, Bernardo L, Riva E. Tolerance to a rice hydrolysate formula in children allergic to cow’s milk and soy. Clin Exp Allergy. 2003;33(11):1576-1580. [DOI] [PubMed] [Google Scholar]
- 17. Fiocchi A, Restani P, Bernardini R, et al. A hydrolysed rice-based formula is tolerated by children with cow’s milk allergy: a multi-centre study. Clin Exp Allergy. 2006;36(3):311-316. [DOI] [PubMed] [Google Scholar]
- 18. Girardet M, Rivero J, Orbegozo T, et al. Tolerance of an infant formula of hydrolyzed rice proteins. Arch Pediatr. 2010;17:1-178. [DOI] [PubMed] [Google Scholar]
- 19. Lasekan JB, Koo WW, Walters J, Neylan M, Luebbers S. Growth, tolerance and biochemical measures in healthy infants fed a partially hydrolyzed rice protein-based formula: a randomized, blinded, prospective trial. J Am Coll Nutr. 2006;25(1):12-19. [DOI] [PubMed] [Google Scholar]
- 20. Nieto-García A, et al. Hydrolyzed rice protein versus extensively hydrolyzed protein formulas in growth and tolerance acquisition of infants with cow’s milk protein allergy: the GRITO study. Presented at: ESPGHAN 55th Annual Meeting; 2023. [Google Scholar]
- 21. Nocerino A, Leone L, Cosenza L, et al. Effects of different dietary regimens on tolerance acquisition in children with cow’s milk allergy: a prospective observational study. Allergy. 2012;67(Suppl 96):166-306.21958323 [Google Scholar]
- 22. Nocerino R, Bedogni G, Carucci L, et al. The impact of formula choice for the management of pediatric cow’s milk allergy on the occurrence of other allergic manifestations: the atopic march cohort study. J Pediatr. 2021;232:183-191.e3. [DOI] [PubMed] [Google Scholar]
- 23. Savino F, Castagno E, Monti G, et al. Z-score of weight for age of infants with atopic dermatitis and cow’s milk allergy fed with a rice-hydrolysate formula during the first two years of life. Acta Paediatr Suppl. 2005;94(449):115-119. [DOI] [PubMed] [Google Scholar]
- 24. Solar A, Ibañez D, Maldonado J, et al. Tolerance and efficacy of extensively hydrolyzed formula for infants with IgE-mediated cow’s milk protein allergy. J Pediatr Gastroenterol Nutr. 2016;62:839-840. [Google Scholar]
- 25. Terracciano L, Bouygue GR, Sarratud T, Veglia F, Martelli A, Fiocchi A. Impact of dietary regimen on the duration of cow’s milk allergy: a random allocation study. Clin Exp Allergy. 2010;40(4):637-642. [DOI] [PubMed] [Google Scholar]
- 26. Tormo R, Cárdenas G, Segurola H. Treatment of non-IgE mediated cow’s milk allergy with a partially hydrolyzed rice infant formula: a prospective study. Nutrition. 2017;64(Suppl 1):1-6. [Google Scholar]
- 27. ESPGHAN Committee on Nutrition. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr. 2005;41(5):584-599. [DOI] [PubMed] [Google Scholar]
- 28. Hamill PV, Drizd TA, Johnson CL, et al. Physical growth: national center for health statistics percentiles. Am J Clin Nutr. 1979;32:607-629. [DOI] [PubMed] [Google Scholar]
- 29. Saad K, Ahmad RA, El-Tellawy MM, et al. Cow milk protein allergy: clinical phenotype and risk factors. Curr Trends Immunol. 2020;21:129-135. [Google Scholar]
- 30. Agostoni C, Axelsson I, Goulet O, et al. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN. J Pediatr Gastroenterol Nutr. 2006;42:352-361. [DOI] [PubMed] [Google Scholar]
- 31. Scientific Committee on Food. Report on the revision of essential requirements of infant formulae and follow-on formulae. SCF/CS/NUT/IF/65; 2003. [Google Scholar]
- 32. Bhatia J, Greer F; American Academy of Pediatrics Committee on Nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121(5):1062-1068. [DOI] [PubMed] [Google Scholar]
- 33. Zeiger RS, Sampson HA, Bock SA, et al. Soy allergy in infants and children with IgE-associated cow’s milk allergy. J Pediatr. 1999;134(5):614-622. [DOI] [PubMed] [Google Scholar]
- 34. Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221-229. [DOI] [PubMed] [Google Scholar]
- 35. Gastanaduy A, Cordano A, Graham GG. Acceptability, tolerance, and nutritional value of a rice-based infant formula. J Pediatr Gastroenterol Nutr. 1990;11:240-246. [DOI] [PubMed] [Google Scholar]
- 36. Antunes J, Borrego LM, Queiroz A, et al. Allergy to extensively hydrolysed formulas. Allergol Immunopathol. 2009;37(5):272-278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pdi-10.1177_11795565251332173 for Rice Hydrolysate Formula in Infants and Children Allergic to Cow’s Milk: A Systematic Review of Evidence by Khaled Saad, Amir Aboelgheet, Khalid Hashim, Eman F. Gad, Anas Elgenidy, Ramez M. Odat, Aya Sherif, Ahmed Altaweel, Asmaa B. Zahran, Sara K. Kamal, Abdelrahman Elshimy, Ahmed Samir, Anas Khaled, Ahmed Ibrahim, Amira Elhoufey, Hamad Ghaleb Dailah, Thamer Alruwaili and Hoda Atef Abdelsattar Ibrahim in Clinical Medicine Insights: Pediatrics