Graphical abstract
Keywords: Sonochemical synthesis, Conducting polymers, Potassium vanadate, Nanofibers, Zinc ion batteries
Highlight
-
•
A 1D nanofiber-structured cathode was designed for aqueous zinc-ion batteries.
-
•
PEDOT-intercalated potassium vanadate nanofiber was synthesized via a sonochemical.
-
•
Oxygen vacancies and PEDOT intercalation enhanced Zn2+ diffusion and kinetics.
-
•
E-PVNF exhibited 182.50mAh g−1 at 15 A g−1 with excellent reversibility.
-
•
E-PVNF maintained 91.87 % capacity retention after 5000 cycles at 10 A g−1.
Abstract
Aqueous zinc-ion batteries (AZIBs) have gained attention as next-generation energy storage systems due to their safety, cost-effectiveness, and eco-friendliness. However, their commercialization is hindered by the structural instability and low electrochemical performance of cathode materials. Herein, we present poly(3,4-ethylenedioxythiophene) (PEDOT)-intercalated potassium vanadate nanofibers (E-PVNF) with oxygen vacancies, synthesized via a sonochemical method. Oxygen vacancies play a crucial role in facilitating Zn2+ diffusion and charge transport by providing additional ion migration channels and enhancing electronic conductivity. The E-PVNF exhibited a high specific capacity of 182.50mAh g−1 even at a high current density of 15 A g−1, significantly outperforming conventional potassium vanadate-based cathodes. To investigate the electrochemical behavior, overpotential and Zn2+ diffusion coefficient (DZn2+) were systematically evaluated as a function of synthesis time. The results revealed a substantial reduction in overpotential and a notable increase in DZn2+, reaching 3.86 × 10−10 cm2 s−1, nearly double that of pristine potassium vanadate. This improvement is attributed to the synergistic effects of PEDOT intercalation and oxygen vacancy engineering, which optimize Zn2+ diffusion pathways and enhance charge transfer. Additionally, while oxygen vacancies facilitate ion and electron transport, they do not directly increase theoretical capacity. This study provides a scalable and effective electrode design strategy for high-performance AZIBs, offering insights into the role of conducting polymer intercalation and oxygen vacancy engineering in improving electrochemical stability and rate capability.
1. Introduction
Aqueous zinc-ion batteries (AZIBs) have attracted growing attention as promising alternatives to lithium-ion batteries for grid-scale and wearable energy storage systems. Their appeal lies in several intrinsic advantages, including enhanced safety due to nonflammable aqueous electrolytes, low cost enabled by abundant zinc resources, and environmental friendliness[[1], [2], [3], [4], [5]]. Despite these benefits, the practical realization of AZIBs remains hindered by fundamental challenges—most notably, the limitations of cathode materials [2,5,6]. In particular, the multivalent Zn2+ ions are accompanied by sluggish diffusion kinetics and significant electrostatic interactions with the host structure, which result in phase instability, severe polarization, and poor long-term cycling performance.
Various cathode materials, including manganese-based oxides, vanadium-based oxides, Prussian blue analogs, molybdenum-based sulfides, and organic compounds, have been extensively explored for AZIB [3,4,7,8]. Among them, vanadium oxides have garnered significant attention due to their high theoretical capacity and the ability to undergo multi-electron redox reactions (V2+, V3+, V4+, and V5+). Furthermore, their layered structure enables facile Zn2+ insertion/extraction, making them a promising candidate for AZIB cathodes. However, despite these advantages, the practical application of vanadium oxides is hindered by several intrinsic limitations. The repeated Zn2+ ion insertion/extraction process induces structural degradation, leading to capacity fading over prolonged cycling. Additionally, their inherently low electrical conductivity and sluggish Zn2+ diffusion contribute to severe polarization and performance deterioration[9,10].
To overcome these challenges, the pre-intercalation of alkali metal ions (Li+, Na+, K+, etc.) into the V–O layers has been explored as an effective strategy, where these ions act as structural “structural pillars” to stabilize the layered framework [11,12]. These alkali metal ions act as electrostatic buffers, alleviating Coulombic interaction during Zn2+ insertion/extraction. By stabilizing the V–O framework and maintaining interlayer spacing, these ions effectively prevent interlayer collapse and mitigate lattice strain, thereby enhancing both structural stability and Zn2+ diffusion kinetics during cycling. Additionally, the presence of pre-inserted metal ions weakens electrostatic interactions between V–O layers, reducing the energy barrier for Zn2+ ion migration and improving electrochemical reversibility. Furthermore, the incorporation of water molecules during the synthesis process induces a polyoxometalate reaction, leading to structural rearrangement [13]. This structural rearrangement promotes the formation of nanostructured morphologies, such as nanorods and nanosheets, which provide a larger electroactive surface area and shorter Zn2+ diffusion paths, thereby enhancing reaction kinetics [[14], [15], [16]]. However, despite these modifications, the insertion of only metal ions and/or H2O molecules into the V–O layers of V2O5 alone remains insufficient to fundamentally resolve its inherently low electrical conductivity, which significantly limits its rate capability [17].
To further address the issue of poor electronic conductivity, oxygen vacancy engineering has emerged as a promising strategy. Oxygen vacancy engineering has been explored as a promising approach to improve electronic conductivity. Oxygen vacancies modify the local electronic environment, modulating electron density and creating defect states that facilitate Zn2+ insertion/extraction, thereby accelerating Zn2+ diffusion [[17], [18], [19]]. Consequently, a combined approach incorporating interlayer 'structural pillars' and oxygen vacancies provides a viable strategy to construct efficient Zn2+ diffusion pathways and modulate charge distribution. This approach also helps maintain structural integrity while enhancing both ionic and electronic conductivity. In addition to direct oxygen vacancy engineering, conductive polymers offer a complementary approach to stabilize and regulate oxygen defect formation in vanadium oxides [20]. Xiong et al. synthesized an oxygen-deficient hydrate vanadium dioxide coated with polypyrrole (PPy) via a one-step hydrothermal method, while Xia et al. increased the interlayer spacing of ammonium vanadate and induced oxygen defects by intercalating poly(3,4-ethylenedioxythiophene) (PEDOT) [19,21]. However, most current techniques require high-temperature treatment under reducing gas atmospheres or involve complex fabrication processes. High-temperature synthesis can potentially disrupt the highly ordered chain structure of conducting polymers, reducing their conductivity and structural stability [20,22].
In this work, we propose a synergistic cathode design strategy that combines PEDOT intercalation with oxygen vacancy engineering in a vanadate framework. Specifically, we developed a sonochemical synthesis method to fabricate PEDOT-intercalated potassium vanadate nanofibers (E-PVNF). Unlike conventional high-temperature processes, the sonochemical approach enables efficient incorporation of PEDOT into the V–O layers while simultaneously inducing structural defects in a controllable and rapid manner. This dual-modification strategy is expected to improve Zn2+ diffusion kinetics, suppress structural collapse, and promote stable charge transport. As a result, E-PVNF demonstrated an outstanding capacity oof 182.50 mAh g−1 even at a high current density of 15 A g−1, significantly outperforming conventional potassium vanadate-based cathodes. Through this approach, we aim to provide new insights into the rational design of high-performance AZIB cathodes by coupling polymer intercalation with defect engineering.
2. Experimental section
2.1. Synthesis procedure
Vanadium pentoxide (V2O5, 99 %), 3, 4-ethylenedioxythiophene (EDOT) (97 %), and potassium persulfate (PPS, K2S2O8, 99 %)) were provided from Sigma-Aldrich. To synthesize potassium vanadate nanofiber (PVNF), 0.4 g of V2O5 (2.2 mmol) was dissolved in 100 mL DI water. Then, it was added to a solution containing 0.22 g PPS (0.88 mmol). The resulting yellow solution was sonicated for 55 min using a Vibracell (20 kHz and 750 W) with a titanium alloy tip (1.3 cm in diameter) immersed 1.5 cm into the solution. After sonication, the solution turned dark red, indicating the formation of potassium vanadate nanofiber (PVNF). To investigate the morphological and structural change of the material during synthesis, samples were collected at various sonication time intervals (0, 3, 4, 10, 15, 30, and 55 min). Each sample was labeled as PV-X, where X represents the sonication time (in min).
The PEDOT-intercalated potassium vanadate nanofiber (E-PVNF) was synthesized by adding 94 μL (0.88 mmol) of EDOT to the PVNF solution, which was subsequently subjected to sonication for 1 h. As the sonication progressed, the solution color changed from dark red to dark green, indicating the formation of E-PVNF. To evaluate the effects of PEDOT intercalation and oxygen vacancy formation on performance, samples were collected at 70, 80, 90, 100, and 120 min during the synthesis process. The collected samples were labeled as EP X, where X represents the sonication time (in minutes). Schematic diagram of the fabrication process for PVNF and E-PVNF is illustrated in Fig.S1.
2.2. Material Characterization
The morphology was studied using a scanning electron microscope (SEM, NANO SEM) equipped with an energy dispersive X-ray spectrometer (EDS) and transmission electron microscopy (TEM, JEM 2100F) equipped with EDS. The crystallinity was explored using X-ray diffraction (XRD, Bruker, D8-Discover) with CuK α 1 radiation (λ = 0.15406 nm). Fourier transform infrared spectroscopy (FT-IR, Nicolet/ iS10 spectrometer) was used to study the chemical bonds of the synthesized samples. X–ray photoelectron spectroscopy (XPS, Kratos, AXIS Supra + ) was employed to determine the chemical composition, oxidation states, and chemical bonding of the synthesized samples.
2.3. Cell fabrication
The cathode was fabricated using an active material, carbon black (Super P, TIMCAL), and poly(vinylidene difluoride) (PVDF, HSV 900, Kynar) with a mass ratio of 7:2:1. To prepare a homogeneous slurry, the powders were mixed using a Thinky Mixer (AR-100, Thinky). The resulting homogeneous slurry was cast onto carbon paper with a loading of 2–3 mg cm−2. The electrolyte was prepared by dissolving 2.58 M zinc trifluoromethanesulfonate (Zn(OTF)2, 98 %) into DI water. A glass fiber separator (GF/C, Whatman) was employed as the separator, and 250 μL of the electrolyte (2.58 M of Zn(OTF)2) was added to the separator.
2.4. Electrochemical measurement
All electrochemical measurements were conducted at battery temperature chamber. The electrochemical impedance spectroscopy (EIS) was conducted on bio-logic (SP-200) with frequency range from 0.1 Hz to 1 MHz. Galvanostatic intermittent titration technique (GITT) was carried out to calculate the Zn2+ diffusion coefficient (DZn2+) and overpotential by repeating galvanostatic charging/discharging at 50 mA g−1 for 10 min, followed by a 10 min open circuit step to allow relaxation back to equilibrium. The DZn2+ was calculated by following equation.
where L is the ion diffusion length, which corresponds to the ion diffusion length. τ is the relaxation time (s), and △Es is the steady-state potential change (V) caused by the current pulse [23,24]. △Et is the potential change (V) during a constant current pulse after removing the iR drop. The Tafel test was conducted from − 0.25 to 0.25 V relative to the open circuit voltage (OCV) at a scan rate of 1 mV s−1.
3. Results and discussions
Fig. S2 shows SEM images demonstrating that when EDOT, V2O5, and PPS are simultaneously added and subjected to ultrasonication, nanofiber structure formation is hindered. Instead of forming well-defined nanofibers, the materials tend to aggregate, suggesting that uncontrolled interactions between the components disrupt the structural transformation required for the formation of vanadate nanofiber. This aggregation phenomenon was also confirmed by morphology analysis according to the change in PPS content during the synthesis process. In a system where all precursors were added at once, it is possible that the heterogeneity of PPS during the morphology formation process caused this structural transformation [13]. To overcome this issue, E-PVNF was fabricated using a two-step process. First, V2O5 was transitioned into PVNF via ultrasonication. During this synthesis process, some oxygen vacancies were formed due to the partial reduction of vanadium species and the structure rearrangement. Then, EDOT was polymerized within the crystal lattice of PVNF through ultrasonication, leading to PEDOT insertion into the V–O interlayer and the formation of secondary oxygen vacancies.
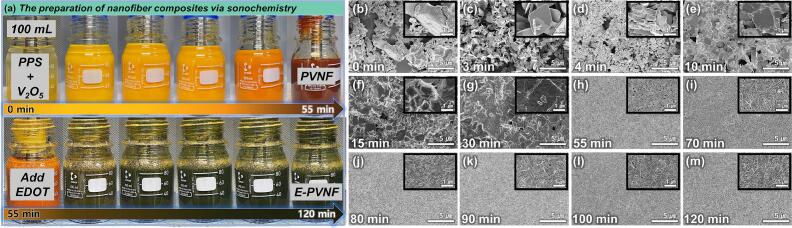
Fig. 1(a) presents photographs illustrating the color change of the active material as a function of synthesis time. PV0 (V2O5) initially consists of [VO5] square pyramidal structures, where four oxygen atoms are positioned in a plane, while the fifth oxygen atom forms a double bond at the apex [[25], [26], [27]]. As pre-intercalation synthesis progresses, the structure undergoes a transformation into a layered V3O8 phase, composed of [VO5] tetragonal pyramids and [VO6] disordered octahedra, leading to a change in the oxidation state of vanadium. Furthermore, ultrasonic-assisted synthesis plays a crucial role in modifying the electronic structure by generating oxygen vacancies through strong cavitation effects [28,29]. The oxidation state transition of vanadium significantly influences the optical properties of the material, inducing a progressive color shift from dark yellow in PV0 (V2O5) to deep red in PV55 (PVNF) [30,31]. Upon the addition of EDOT to the PV55 (PVNF) solution, the polymerization of PEDOT within the hydrated K+ ion framework induces another color shift from dark red to dark green of EP120 (E-PVNF), confirming the successful coexistence of K+ ions and PEDOT.
Fig. 1.
Synthesis time-dependent changes in vanadate nanofibers: (a) Color change of vanadate nanofibers depending on synthesis time. (b) Morphological change with varying synthesis time.
Fig. 1(b)–(m) exhibits the morphological transformation as a function of reaction time. The nanosheet structure of PV0 (V2O5) gradually transitions into a one-dimensional (1D) nanofiber morphology, ultimately achieving a well-defined 1D nanofiber configuration at PV55 (PVNF). Under ultrasonic cavitation, the rapid collapse of microbubbles generates localized high-temperature and high-pressure environments, leading to the fragmentation of layered nanosheets into smaller units [32,33]. These fragments reassemble along specific crystallographic orientations due to surface energy minimization, ultimately forming interconnected 1D nanostructures [34,35]. During EP120 (E-PVNF) synthesis progression, the morphology remains consistent with that of PV55 (PVNF), preserving the 1D nanofiber structure. Notably, no significant bulk deposition of PEDOT was observed on the EP120 (E-PVNF) in comparison to PV55 (PVNF), suggesting that PEDOT was effectively intercalated into the V—O interlayer rather than forming a surface coating (Fig. S3).
Owing to its unique reaction dynamics, the sonochemical method enables the rapid and controlled synthesis of high-quality nanostructures, providing a significant advantage over conventional techniques such as surfactant-assisted templating, reflux, hydrothermal, sol–gel, and electrochemical deposition [36,37]. In particular, hydrothermal and sol–gel processes often struggle to intercalate PEDOT due to their limited solubility and poor stability under high-temperature conditions, typically resulting in surface adsorption or phase separation [20]. Moreover, hydrothermal synthesis is inherently unsuitable for the direct intercalation of organic molecules into inorganic crystal lattices. By contrast, the sonochemical method utilizes cavitation effects to achieve the intercalation of conductive polymers into vanadate within a short time while preserving the nanofiber structure. With its fast synthesis rate and precise structural control, sonochemical synthesis ensures high reproducibility and is considered a promising approach for scalable production [38].
Fig. S4 demonstrates the scale-up of PVNF and E-PVNF preparation via sonochemical synthesis. The cavitation effect generates uniform reaction conditions, promoting the formation of nanofibers with a consistent structural integrity and well-defined morphology. These advantages underscore the potential of sonochemical synthesis as a highly efficient and scalable approach for large-scale production.
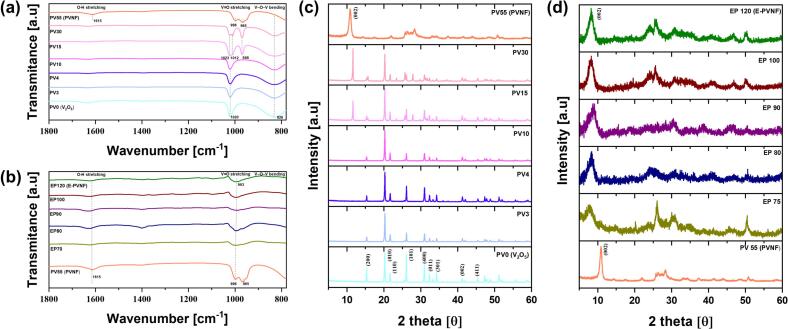
Fig. 2 presents FT-IR spectra and XRD patterns, which were employed to examine the structural evolution and vibrational modes of chemical bonds during the synthesis progression. Fig. 2(a) depicts the FT-IR spectra corresponding to synthesis progression of PV55 (PVNF). The V O stretching vibration is highly sensitive to the intercalation of K+ ions and PEDOT within the V—O layers [39,40]. The V O stretching vibration band of PV0 (V2O5) initially appears at 1020 cm−1. As the reaction progresses, band splitting appears at PV15, resulting in two distinct peaks at 1012 cm−1 and 1023 cm−1. These peaks correspond to the formation of two inequivalent V O stretching modes, associated with distorted octahedral and square pyramidal V O environments, respectively [39,41]. A new peak at 968 cm−1 emerges in PV15, corresponding to V4+=O stretching vibrations attributed to the short-range order of distorted octahedra [42,43]. This feature further reflects the structural and electronic reorganization induced by K+ ion intercalation, which is initiated at PV15. Upon the completion of PVNF synthesis (PV55), V O stretching vibration undergoes redshifts to 998 cm−1 and 965 cm−1, respectively. This red shift is attributed to a partial reduction of V5+ to V4+, reflecting the continuous electronic structure modification occurring during the synthesis progression. Additionally, the V—O—V asymmetric vibration band at 824 cm−1 gradually diminishes at PV55 (PVNF), suggesting a significant structural rearrangement. Furthermore, the band at 1626 cm−1, corresponding to the bending vibrations of O–H groups in water molecules, is clearly observed in PV55 (PVNF), suggesting the presence of either interlayer or surface-bound water molecules at this stage.
Fig. 2.
Physicochemical Characterization as a Function of Synthesis Time: (a) FTIR spectra of PVNF during synthesis progression (PV0–PV55). (b) FTIR spectra of E-PVNF during synthesis progression (PV55–EP120). (c) XRD patterns of PVNF during synthesis progression (PV0–PV55). (d) XRD patterns of E-PVNF during synthesis progression (PV55–EP120).
As EDOT is added, the V=O stretching peak gradually redshifts to 993 cm−1 at EP120 (E-PVNF), indicating a significant reduction of V5+ to V4+ (Fig. 2(b)). This reduction is closely associated with PEDOT polymerization [21]. During the polymerization process, the positively charged S+ in PEDOT chemically interacts with the negatively charged O2− in the PV55 (PVNF), facilitating the generation of oxygen vacancies and the accompanying formation of V4+. Meanwhile, the intercalation of PEDOT and K+ ions within the V—O layer induces structural distortion, leading to a decline in crystallinity. Consequently, as the synthesis progresses, the overall peak intensity decreases.
The evolution of crystalline phases during the PV55 (PVNF) synthesis process is shown in Fig. 2(c). Initially, PV0 (V2O5) exhibits diffraction peaks characteristic of orthorhombic α-V2O5 (JCPDS No. 86–2248), with lattice parameters of a = 11.503 Å, b = 4.369 Å, and c = 3.557 Å [44,45]. With synthesis progression, a gradual phase transition occurs, resulting in the formation of K2V6O16·1.5H2O with a monoclinic structure (JCPDS No. 51–0379), characterized by lattice parameters of a = 12.30 Å, b = 3.60 Å, and c = 16.02 Å [46]. In this structure hydrated K+ acts as pillars between theV6O16, stabilizing the structure, which consists of edge-sharing VO5 square pyramids and VO6 octahedra. The (002) plane, which represents the major peak of PV55 (PVNF), is first observed at PV15 (2θ = 11.61°), which coincides with the emergence of new peaks in the FT-IR spectrum. This correlation suggests that the structural transformation initiates at PV15, marking the onset of phase evolution in the material. As shown in Fig. S5, the (002) diffraction peak shifts to a lower angle (2θ = 10.83°) at PV55 (PVNF), indicating an increase in interlayer spacing due to the K+ insertion. The interlayer distance expands from 7.62 Å to 8.16 Å, suggesting that K+ intercalate into the V − O layer.
During the EP120 (E-PVNF) synthesis process, the intensity of all diffraction peaks significantly decreases as shown in Fig. 2(d). This attenuation is attributed to the reduction in crystallinity due to the incorporation of PEDOT. Furthermore, the diffraction pattern of the EP120 (E-PVNF) shows a peak at 2θ = 8.24°, corresponding to the (002) plane, which is shifted to a lower angle by 2.59° compared to that of PV55 (PVNF). This shift corresponds to an increase in interplanar spacing of EP120 (E-PVNF) from 8.16 to 10.72 Å due to the intercalation of PEDOT within the V − O layers (Fig. S6).
Fig. S7 displays HR-TEM images and EDS mapping for both PV55 (PVNF) and EP120 (E-PVNF). The observed interlayer spacing increases from 8.1 Å to 9.4 Å, aligning well with the d-spacing values determined by XRD analysis. The slight discrepancy between the interlayer spacing values obtained from HR-TEM and XRD may be attributed to differences in measurement techniques, as XRD provides an average structural analysis while HR-TEM captures localized variations at the nanoscale. To gain deeper insight into the distribution of PEDOT within the V − O layers, EDS mapping was performed for PV55 (PVNF) and EP120 (E-PVNF), focusing on the elemental composition of V, O, K, C, and S. In contrast to PV55 (PVNF), EP120 (E-PVNF) exhibits a uniform PEDOT distribution, with C and S signals distinctly identified in the EDS mapping results.
The structures and components of PV55 (PVNF) and EP-120 (E-PVNF) were confirmed by the TGA and derivative thermogravimetry (DTG). As shown in Fig. S8, EP 120 (E-PVNF) shows a second-stage mass loss beginning at ∼ 250 °C, attributed to the thermal degradation of PEDOT. Based on this, the PEDOT content was estimated to be approximately 13 wt%. These results collectively demonstrate the effective incorporation of PEDOT into the vanadate framework.
Fig. 3(a) displays the V 2p spectra during the synthesis progression of PV55 (PVNF). The V 2p1/2 peaks are observed at 516.94 eV (V5+) and 515.63 eV (V4+), while the V 2p3/2 peaks appear at 542.5 eV (V5+) and 523.1 eV (V4+) [47,48]. As shown in Fig. S9, the average oxidation state of vanadium decreases from 4.98 to 4.84 during the synthesis process of PV55 (PVNF), which is attributed to the formation of VO6 octahedral structures, intercalation of water molecules, and charge compensation due to K+ ion insertion. This oxidation state transition directly affects the oxygen bonding environment, as observed in the O 1 s spectra, where peaks at 529.8 eV (lattice oxygen) and 531.1 eV (oxygen vacancies) correspond to the initial bonding state (Fig. 3(b)). The oxygen vacancy concentration increases from 3.88 % to 11.30 % during PV55 (PVNF) synthesis. These vacancies provide additional Zn2+ diffusion pathways, which facilitate Zn2+ transport and improve electrochemical performance. The peak observed at approximately 532.5 eV from PV15 onward originates from water molecules, suggesting progressive water intercalation into the interlayer spacing during synthesis [49,50]. This finding aligns with the FT-IR and XRD results, further confirming the structural evolution of the material.
Fig. 3.
XPS spectra during the synthesis progression of PV55 (PVNF): (a) V 2p (b) O 1 s (c) K 2p. XPS spectra during the synthesis progression of EP120 (E-PVNF): (d) V 2p (e) O 1 s (f) K 2p.
Notably, with the intercalation of PEDOT, the V 2p peaks shift toward lower binding energies, indicating a progressive reduction of V5+ to V4+ due to PEDOT polymerization. As a result, the average oxidation state of vanadium further decreases from 4.84 to 4.75 in the transition from PV55 (PVNF) to EP120 (E-PVNF) (Fig. S10). This reduction occurs because vanadium oxide serves as an oxidizing agent during PEDOT polymerization, accepting electrons and facilitating the formation of V4+ species [42,51]. Consequently, this process also induces the formation of oxygen vacancies within the vanadium oxide layers to compensate for charge imbalance, further modifying the local electronic structure. Accordingly, the oxygen vacancy ratio in EP120 (E-PVNF) increases significantly to 24.82 %. The increased oxygen vacancies weaken V–O bonding by altering the coordination environment of lattice oxygen [52]. This disruption in the local bonding structure reduces electron density around lattice oxygen, leading to a shift in the O 1 s lattice oxygen peak from 529.8 eV to 529.2 eV, as observed in Fig. 3(e), confirming the structural reorganization induced by PEDOT intercalation. In addition, a new peak at 532.6 eV appears, corresponding to the C—O—C/S—O bonding of PEDOT (Fig. 3(e)) [53,54]. This confirms strong electronic interactions between PEDOT and the host matrix, which enhance electrical conductivity and charge transport.
The potassium content increases as the PV55 (PVNF) synthesis progresses, but it gradually decreases as PEDOT polymerization occurs (Fig. 3(e) and (f)). This reduction suggests that the intercalated K+ ions are partially displaced due to the incorporation of PEDOT within the vanadate layers [31].
The S 2p spectrum of E-PVNF was deconvoluted into two characteristic peaks at ∼ 162.8 and ∼ 164.3 eV, corresponding to the S 2p3/2 and S 2p1/2 components of the thiophene ring in PEDOT (Fig. S11). Additionally, a peak at 167.5 eV, assigned to S–O, indicates the possible chemical interaction between PEDOT and vanadate, as shown in Fig. S10. The atomic percentage of S 2p was found to be 2.34 %, confirming successful PEDOT incorporation on the surface of E-PVNF.
To further confirm the presence of V4+ species in PV55 (PVNF) and EP-120 (E-PVNF), electron paramagnetic resonance (EPR) measurements were conducted at room temperature (Fig. S12). The EPR signal of EP-120 (E-PVNF) was stronger than that of PV55 (PVNF), which is consistent with the XPS results. The enhanced EPR signal suggests an increased density of unpaired electrons associated with V4+ species, confirming the partial reduction of V5+ to V4+ during EP120 (E-PVNF) synthesis progression. This V4+ generation is closely linked to the formation of oxygen vacancies, which plays a crucial role in facilitating Zn2+ diffusion by providing additional diffusion pathways and reducing the Zn2+ insertion/extraction energy barrier. This effect ultimately enhances rate capability and cycling stability, highlighting the importance of controlled oxygen vacancy engineering in optimizing electrochemical performance.
The impedance behavior as a function of synthesis time is presented in Fig. S13. EIS behavior varies depending on electrode morphology and electrode–electrolyte interfacial characteristics, thus a clear difference in impedance behavior is observed as the synthesis time increases [55,56]. During PV55 (PVNF) synthesis, the tail in the low-frequency region gradually shortens, indicating that the transformation into a 1D nanofiber structure facilitates Zn2+ diffusion [57]. Furthermore, the charge transfer resistance (Rct) also reduces with longer synthesis time, suggesting improved interphase kinetics. This improvement is attributed to the formation of interconnected ion diffusion pathways, which facilitate Zn2+ transport, and the increased electrochemically active surface area provided by the well-aligned 1D nanofiber morphology [58,59]. During the EP120 (E-PVNF) synthesis, the overall impedance behavior remains similar to that of PV55 (PVNF), but Rct continues to decrease. Specifically, the Rct decreases significantly from 285.4 Ω to 184.6 Ω, clearly indicating improved ionic transport and interfacial charge-transfer kinetics as shown in Table S1. These results demonstrate that increasing oxygen vacancy concentration directly contributes to reduced charge-transfer resistance and enhanced electrochemical performance. This reduction can be attributed to enhanced electrical conductivity due to PEDOT polymerization and formation of oxygen vacancies, all of which synergistically contribute to improved charge transport and electrochemical performance.
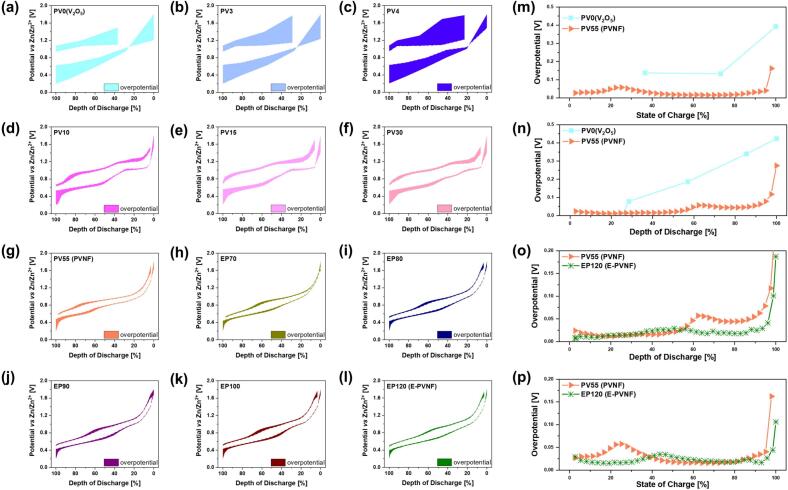
To further investigate ion diffusion and reaction kinetics, GITT measurements were performed. The GITT method measures transient voltage and open-circuit voltage (OCV) changes during charge and discharge using a constant current supply with specified cut-off intervals. This technique effectively retrieves both thermodynamic and kinetic parameters, making it a powerful tool for examining the diffusion coefficient of host materials in battery electrodes[60]. Additionally, GITT enables the calculation of overpotential (η) and internal resistance, which provide insights into reaction kinetics. Electrochemical reactions at the electrode involve multiple sequential steps, including adsorption, mass transfer, and charge transfer processes, all of which contribute to the total overpotential [61,62]. Fig. S14 presents the GITT profile of the third cycle, and the corresponding overpotential at different synthesis stages is summarized in Fig. 4. The total overpotential (η) is calculated by Equation.
Here, Emeas (measured voltage) refers to the voltage recorded at the end of the current pulse, while Eeq (equilibrium voltage) corresponds to the voltage measured at the end of the relaxation period in the galvanostatic titration step, as illustrated in Fig. S15. Fig. 4(a)–(g) presents the overpotential behavior during the third cycle of the PV55 (PVNF) synthesis process, while Fig. 4(m) and (n) provide a comparative analysis of the overpotential under different states of charge (SoC) and depths of discharge (DoD). PV0 (V2O5), PV3, and PV4 exhibit significantly high overpotential during charge/discharge, which is attributed to the extremely sluggish Zn2+ diffusion within the electrode as shown in Fig. 4(a)–(c). However, as the synthesis progresses, the overpotential progressively decreases, reaching its lowest value at PV55 (PVNF). This reduction in overpotential is attributed to the structural and morphological transformation from V2O5 to K2V6O16·1.5H2O. These changes result in an expanded interlayer spacing and the formation of a 1D nanofiber structure, which optimizes Zn2+ diffusion pathways. The Zn2+ diffusion coefficient (DZn2+) is exhibited in Fig. S16. PV0 (V2O5) shows an extremely low DZn2+ of 4.54 × 10−10 cm2 s−1 (discharge) and 1.33 × 10−10 cm2 s−1 (charge). However, as the synthesis progresses, DZn2+ gradually increases due to structural and morphological transformations that enhance Zn2+ transport. At PV55 (PVNF), the DZn2+ significantly increases to 1.96 × 10−9 cm2 s−1(discharge) and 1.63 × 10−9 cm2 s−1 (charge), respectively—more than a magnitude improvement.
Fig. 4.
Overpotential profile during charge/discharge of the 3rd cycle: (a) PV0, (b) PV3, (c) PV4, (d) PV10, (e) PV15, (f) PV30, (g) PV55 (PVNF), (h) EP70, (i) EP80, (j) EP90, (k) EP100, (l) EP120 (E-PVNF). Corresponding overpotential variations as a function of state of charge (SoC) and depth of discharge (DoD): (m–n) for PVNF series, and (o–p) for E-PVNF series.
Similarly, Fig. 4(g)–(l) present the overpotential behavior during the third cycle of the EP120 (E-PVNF) synthesis process, while Fig. 4(o) and (p) integrate individual datasets to provide a comparative analysis of the overpotential across PV55 (PNVF) and EP120 (E-PVNF) as a function of SoC and DoD. A gradual reduction in overpotential was also observed during the EP120 (E-PVNF) synthesis, attributed to (1) enhanced electrical conductivity and expansion of V—O interlayer due to PEDOT polymerization and (2) improved charge transport and Zn2+ diffusion facilitated by oxygen vacancy formation. Consequently, DZn2+ of EP120 (E-PVNF) further increases to 3.86 × 10−9 cm2 s−1 (discharge), 3.75 × 10−9 cm2 s−1 (charge), nearly doubling that of PV55 (PVNF) (Fig. S17). Ultimately, the increase in DZn2+ and the reduction in overpotential effectively lower ion and electron transport resistance, accelerate interphase kinetics, lead to improved rate capability and enhanced long-term cycling stability.
To systematically investigate the effects of PEDOT intercalation and oxygen vacancies, PV55 (PVNF) and EP120 (E-PVNF) were selected as the primary materials for electrochemical performance evaluation. PV55 (PVNF) represents the optimized vanadate nanofiber structure, whereas EP120 (E-PVNF) features a fully intercalated PEDOT framework, introducing both structural and electrochemical modifications. A comparative analysis of these materials was conducted to evaluate their impact on kinetics and charge–discharge performance.
Cyclic voltammograms of PV55 (PVNF) and EP120 (E-PVNF) at various scan rate are shown in Fig. S18. As the scan rate increases, the redox peaks exhibit a slight shift and progressive broadening, primarily due to polarization effects in the electrochemical reaction. Fig. S19 represents the cyclic voltammogram at the third cycle with a scan rate of 0.1 mV s−1. Two distinct redox couples were observed, corresponding to the V3+/V4+ and V4+/V5+ charge transfer reaction. Notably, the voltage difference between the cathodic and anodic peaks of the V3+/V4+ and V4+/V5+ redox couples is slightly reduced, as illustrated in Fig. S20, indicating improved reaction kinetics. This decrease suggests enhanced charge transfer efficiency, which can be attributed to the presence of PEDOT and oxygen vacancies that promote faster electron and ion transport.
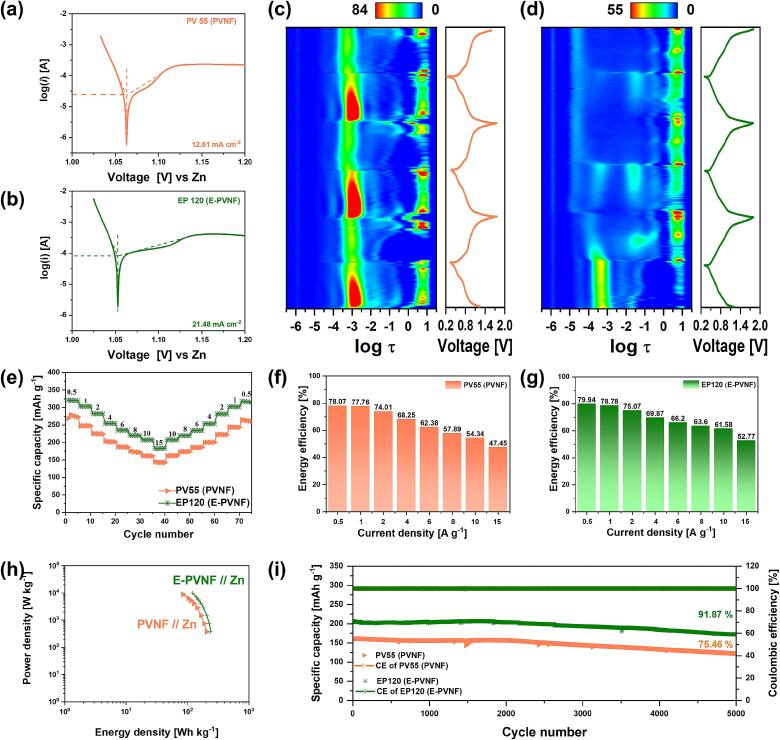
To evaluate the reaction kinetics, half-cell Tafel analysis was conducted to determine the exchange current density (i0). The i0 represents the current density at equilibrium, where oxidation and reduction reactions occur at equal rates, making it a key parameter to judge the kinetics. A higher i0 suggests more efficient charge transfer and rapid electrochemical reactions [63,64]. PV55 (PVNF) exhibited an i0 of 12.61 mA cm−2, whereas EP120 (E-PVNF) exhibited a significantly higher value of 21.48 mA cm−2, indicating a substantial enhancement in charge transfer kinetics due to PEDOT intercalation (Fig. 5(a) and (b)). Thus, PEDOT intercalation not only improves ion diffusion within the electrode but also enhances interfacial reaction kinetics by increasing electrical conductivity and facilitating charge transfer at the electrode–electrolyte interphase, ultimately contributing to overall electrochemical performance.
Fig. 5.
Electrochemical performance of PV55 (PVNF) and EP120 (E-PVNF). (a) Tafel plot of PV55 (PVNF). (b) Tafel plot of EP120 (E-PVNF). (c) In-situ distributed relaxation time (DRT) analysis of PV55 (PVNF) during cycling. (d) In-situ distributed relaxation time (DRT) analysis of EP120 (E-PVNF) during cycling. (e) Rata capability (f) Energy efficacy of PV55 (PVNF). (g) Energy Efficiency of EP120 (E-PVNF). (h) Ragon plot. (i) Cycle performance at 10 A g−1.
To elucidate the synergistic effect of oxygen vacancies and PEDOT intercalation on charge-transfer behavior, we conducted in-situ electrochemical impedance spectroscopy (EIS). In situ EIS enables real-time monitoring of impedance evolution, visualized as a three-dimensional Nyquist diagram composed of real, imaginary, and time axes. For a deeper interpretation, the in-situ EIS data were transformed into the distributed relaxation time (DRT) domain, which enables the visualization of resistance evolution during cycling. The region at τ = –4 to –2 s corresponds to charge transfer processes at the cathode/electrolyte interphase. In this region, EP120 (E-PVNF) exhibited consistently lower resistance than PV55 (PVNF) throughout cycling, indicating enhanced interfacial kinetics (Fig. 5(c) and (d)). Moreover, the DRT contour of EP120 (E-PVNF) showed minimal variation in charge-transfer resistance during Zn2+ insertion and extraction—except for the initial discharge process—suggesting a stable interfacial environment. This distinct behavior is attributed to the co-engineering of PEDOT and oxygen vacancies, which forms interconnected ion/electron transport pathways and expands the V—O interlayer spacing. These structural and electronic modifications facilitate Zn2+ diffusion and improve electron conductivity across the cathode/electrolyte interphase, thereby stabilizing interfacial kinetics over prolonged cycling.
The rate performance further reflects these improvements (Fig. 5 (e) and Fig. S21). At 0.5 A g−1, EP120 (E-PVNF) exhibited a capacity of 320.16 mAh g−1, significantly higher than that of PV55 (PVNF) of 270.7 mAh g−1. Furthermore, even at a high current density of 15 A g−1, EP120 (E-PVNF) maintained an outstanding capacity of 182.50 mAh g−1. This performance enhancement is attributed to the synergistic effects of improved electrical conductivity and interlayer expansion due to PEDOT intercalation, controlled oxygen vacancy formation, and optimized Zn2+ diffusion pathways.
Based on the rate capability analysis, energy efficiency was also evaluated. Energy efficiency serves as an indicator of the reversibility of charge–discharge processes (Fig. 5(f) and (g)) [65]. At a low current density of 0.5 A g−1, both PV55 (PVNF) and EP120 (E-PVNF) exhibited high energy efficiencies approaching 80 %. However, as the current density increased, a distinct difference emerged between the two samples. Even at a high current density of 15 A g−1, EP120 (E-PVNF) maintained an energy efficiency of over 50 %, demonstrating excellent reversibility. This improved reversibility is attributed to enhanced Zn2+ diffusion and kinetics induced by PEDOT intercalation and oxygen vacancy formation, thereby improving the overall reversibility of the electrode.
Nonetheless, it is not necessarily accurate to conclude that oxygen vacancies directly enhance capacity. In fact, the capacity difference between PV55 (PVNF) (392.30 mAh g−1) and EP120 (E-PVNF) (402.85 mAh g−1) remains minimal at very low current densities of 10 mA g−1. This suggests that oxygen vacancies primarily play a role in improving reaction kinetics and electrochemical stability rather than directly increasing capacity (Fig. S22). At high current densities, oxygen vacancies facilitate Zn2+ diffusion, aiding in capacity retention. However, at low current densities, where Zn2+ diffusion is naturally sufficient, their influence becomes less significant.
Besides, EP120 (E-PVNF) exhibits a crucial energy density of 239.33 Wh kg−1 at a power density of 374.12W kg−1, revealing superior advantages in AZIBs as compared to PV55 (PVNF) as shown in Ragon plot (Fig. 5(h)). The detailed values from the Ragon plot are summarized in Table S2. The cells are further tested at a current density of 10 A g−1 to prove the long-term cycle stability of EP120 (E-PVNF). After 5000 cycles, EP120 (E-PVNF) maintains the specific capacity of 177.12mAh g−1 (capacity retention of ∼ 91.87 %), whereas PV55 (PVNF) exhibited a lower retention of 75.46 %. In addition, Fig. S23 presents Coulombic efficiency, where EP120 (E-PVNF) maintains nearly 100 % Coulombic efficiency, confirming its superior electrochemical stability. In addition, compared with other research, EP120 (E-PVNF) have superior cycle performance (Table S3). The superior cycling stability of EP120 (E-PVNF) can be attributed to the synergistic effects of PEDOT intercalation and oxygen vacancies, which enhance structural integrity and charge transfer efficiency. These findings highlight the potential of PEDOT-intercalated vanadate nanofibers, synthesized via a sonochemical method, as a promising cathode material for high-performance AZIBs, offering enhanced electrochemical stability and long-term cycling durability.
In this study, PEDOT-intercalated potassium vanadate nanofibers (E-PVNF) were successfully synthesized using a two-step synthesis process. First, potassium vanadate nanofibers (PVNF) were formed via an ultrasonic synthesis method, followed by the intercalation of PEDOT, which further induced oxygen vacancies. As a result, E-PVNF (EP120) achieved a high specific capacity of 182.50 mAh g−1 at 15 A g−1 and maintained excellent cycling stability, with 177.12 mAh g−1 retained after 5000 cycles at 10 A g−1 (∼91.87 % retention), outperforming the pristine PVNF (PV55).These enhancements were attributed to the structural stabilization of the V–O framework, expansion of interlayer spacing induced by PEDOT intercalation, and the formation of continuous ion diffusion channels and electrically conductive networks enabled by the synergistic effects of oxygen vacancies and PEDOT. Our findings offer a rational electrode design strategy by coupling defect engineering and polymer intercalation, paving the way for advanced cathode materials in next-generation AZIBs.
CRediT authorship contribution statement
Juyeon Han: Writing – original draft, Formal analysis. Yongyeol Park: Writing – original draft, Formal analysis. Ok Sung Jeon: Writing – original draft, Formal analysis. Dongpyo Hong: Data curation. Yuanzhe Piao: Data curation. Young Joon Yoo: Data curation. Sang Yoon Park: Writing – review & editing, Funding acquisition. Se Hun Lee: Writing – review & editing, Funding acquisition. Jeeyoung Yoo: Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by Technology Innovation Program (No. 20020216-K_G012002021601-10054408) funded by the Ministry of Trade, industry & Energy (MI, Korea) and National Research Foundation of Korea (NRF) grant funded by the Korea Government, Ministry of Science and ICT (MSIT) (Nos. 2022R1C1C2011696, 2020R1A2C2103137, 2021R1A5A8033165, RS-2025-00562734), Republic of Korea. This research was supported by the Materials, Components & Equipment Research Program funded by the Gyeonggi Province, and the Ministry of Trade, Industry, and Energy (MOTIE), Republic of Korea, under the “Cooperation program for regional leading industrial complexes”. This research also was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2024-00462805).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2025.107378.
Contributor Information
Sang Yoon Park, Email: yoonpark@kyonggi.ac.kr.
Se Hun Lee, Email: jonathanshsh19@gmail.com.
Jeeyoung Yoo, Email: jyoo@knu.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhou S., Meng X., Chen Y., Li J., Lin S., Han C., Ji X., Chang Z., Pan A. Zinc-Ion anchor induced highly reversible zn anodes for high performance zn-ion batteries. Angewandte Chemie - International Edition. 2024;63 doi: 10.1002/anie.202403050. [DOI] [PubMed] [Google Scholar]
- 2.Jia S., Li L., Shi Y., Wang C., Cao M., Ji Y., Zhang D. Recent development of manganese dioxide-based materials as zinc-ion battery cathode. Nanoscale. 2023;16:1539–1576. doi: 10.1039/d3nr04996e. [DOI] [PubMed] [Google Scholar]
- 3.Kim M., Kim H., Lee S.H., Yu S., Kim W., Bae J.S., Ahn C.Y., Shim H., Lee J.E., Yu S.H. Te hexagonal nanotubes with fast 1-dimensional Zn ion diffusion for high-performance zinc-ion battery cathodes. Chem. Eng. J. 2024;481 doi: 10.1016/j.cej.2023.148256. [DOI] [Google Scholar]
- 4.Zhou T., Xie L., Han Q., Qiu X., Xiao Y., Yang X., Liu X., Yang S., Zhu L., Cao X. Progress and prospect of vanadates as aqueous zn-ion batteries cathodes. Coord. Chem. Rev. 2024;498 doi: 10.1016/j.ccr.2023.215461. [DOI] [Google Scholar]
- 5.Zhu Y., Liang G., Cui X., Liu X., Zhong H., Zhi C., Yang Y. Engineering hosts for Zn anodes in aqueous Zn-ion batteries. Energy Environ Sci. 2023;17:369–385. doi: 10.1039/d3ee03584k. [DOI] [Google Scholar]
- 6.Blanc L.E., Kundu D., Nazar L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule. 2020;4:771–799. doi: 10.1016/j.joule.2020.03.002. [DOI] [Google Scholar]
- 7.Zhang W., Liu J., Cai W., Zhou M., Zhong W., Xiao G., Luo P., Zhao Y., An Q. Engineering d-p orbital hybridization through regulation of interband energy separation for durable aqueous Zn//VO2(B) batteries. Chem. Eng. J. 2023;464 doi: 10.1016/j.cej.2023.142711. [DOI] [Google Scholar]
- 8.Zhang H., Liu X., Chen X., Yang B., Lu Y., Jiang Q., Liu Q. A π-conjugated organic compound with multiple active sites as a cathode material for high-rate aqueous zinc-ion batteries. J. Energ. Storage. 2024;78 doi: 10.1016/j.est.2023.110326. [DOI] [Google Scholar]
- 9.Chen X., Zhang H., Liu J.H., Gao Y., Cao X., Zhan C., Wang Y., Wang S., Chou S.L., Dou S.X., Cao D. Vanadium-based cathodes for aqueous zinc-ion batteries: Mechanism, design strategies and challenges. Energy Storage Mater. 2022;50:21–46. doi: 10.1016/j.ensm.2022.04.040. [DOI] [Google Scholar]
- 10.Liu S., Kang L., Kim J.M., Chun Y.T., Zhang J., Jun S.C. Recent Advances in vanadium-based aqueous rechargeable zinc-ion batteries. Adv. Energ. Mater. 2020;10 doi: 10.1002/aenm.202000477. [DOI] [Google Scholar]
- 11.Lv T., Peng Y., Zhang G., Jiang S., Yang Z., Yang S., Pang H. How About Vanadium-Based Compounds as Cathode Materials for Aqueous Zinc Ion Batteries? Adv. Sci. 2023;10 doi: 10.1002/advs.202206907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan S., Sang Z., Yi Z., Guo J., Zhang X., Li P., Si W.P., Liang J., Hou F. Conductive coating, cation-intercalation, and oxygen vacancies co-modified vanadium oxides as high-rate and stable cathodes for aqueous zinc-ion batteries. EcoMat. 2023;5 doi: 10.1002/eom2.12326. [DOI] [Google Scholar]
- 13.Lee S.H., Koo J.M., Oh S.G., Im S.S. Facile synthesis of ammonium vanadate nanofibers by using reflux in aqueous V2O5 solution with ammonium persulfate. Mater. Chem. Phys. 2017;194:313–321. doi: 10.1016/j.matchemphys.2017.03.053. [DOI] [Google Scholar]
- 14.Su G., Chen S., Dong H., Cheng Y., Liu Q., Wei H., Ang E.H., Geng H., Li C.C. Tuning the electronic structure of layered vanadium pentoxide by pre-intercalation of potassium ions for superior room/low-temperature aqueous zinc-ion batteries. Nanoscale. 2021;13:2399–2407. doi: 10.1039/d0nr07358j. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y., Jiang H., Liu B., Wang X., Zhang Y., Sun J., Wang J. Better engineering layered vanadium oxides for aqueous zinc-ion batteries: Going beyond widening the interlayer spacing. SmartMat. 2024;5 doi: 10.1002/smm2.1231. [DOI] [Google Scholar]
- 16.Fang K., Zhang H., Chen P., Zhang H.Y., Wei Z., Ding L., Ye X.A., Liu J., Liu Y.L., Wang G.G., Yang H.Y. Synergistic structure engineering and electrochemical activation modulating vanadium oxide cathode toward superior zinc-ion storage. Chem. Eng. J. 2024;496 doi: 10.1016/j.cej.2024.153736. [DOI] [Google Scholar]
- 17.Zhang L., Fang D., Wang F., Yi J., Wang M., Hu T., Zhao Y. Interlayer and O-vacancy engineering co-boosting fast kinetics and stable structure of hydrated sodium ammonium vanadate for aqueous zinc-ion battery. Chem. Eng. J. 2025;506 doi: 10.1016/j.cej.2025.159920. [DOI] [Google Scholar]
- 18.Li S., Xu X., Chen W., Zhao J., Wang K., Shen J., Chen X., Lu X., Jiao X., Liu Y., Bai Y. Synergetic impact of oxygen and vanadium defects endows NH4V4O10 cathode with superior performances for aqueous zinc-ion battery. Energy Storage Mater. 2024;65 doi: 10.1016/j.ensm.2023.103108. [DOI] [Google Scholar]
- 19.Zhang Z., Xi B., Wang X., Ma X., Chen W., Feng J., Xiong S. Oxygen Defects engineering of VO2·xH2O nanosheets via in situ polypyrrole polymerization for efficient aqueous zinc ion storage. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202103070. [DOI] [Google Scholar]
- 20.Guo D., Fan Y., Yang Q., Song M., Zhang F., Liu J., Zhu Z., Zhang H. Oxygen-deficient organic/inorganic VOx-PPy cathode composites: Enabling prolonged lifespan of aqueous zinc-ion batteries. Chem. Eng. J. 2025;507 doi: 10.1016/j.cej.2025.160745. [DOI] [Google Scholar]
- 21.Bin D., Huo W., Yuan Y., Huang J., Liu Y., Zhang Y., Dong F., Wang Y., Xia Y. Organic-inorganic-induced polymer intercalation into layered composites for aqueous zinc-ion battery. Chem. 2020;6:968–984. doi: 10.1016/j.chempr.2020.02.001. [DOI] [Google Scholar]
- 22.Luo P., Yu G., Zhang W., Tang H., Zhu D., Chao F., Zhong W., Dong S., An Q. “Triple-synergistic effect” of K+ and PANI co-intercalation enabling the high-rate capability and stability of V2O5 for aqueous zinc-ion batteries. J Colloid Interface Sci. 2024;659:267–275. doi: 10.1016/j.jcis.2023.12.167. [DOI] [PubMed] [Google Scholar]
- 23.Zhai Z., Guo Y., Kang J., Ge Y., Wang L., Yang X., Zhang J., Lu H. Artificially interconnected ion-diffusion nanochannels in ion-indiffusible phase-conversion cathodes for rechargeable aqueous zinc batteries. Energy Storage Mater. 2024;73 doi: 10.1016/j.ensm.2024.103800. [DOI] [Google Scholar]
- 24.Song Z., Zhao Y., Zhou A., Wang H., Jin X., Huang Y., Li L., Wu F., Chen R. Organic intercalation induced kinetic enhancement of vanadium oxide cathodes for ultrahigh-loading aqueous zinc-ion batteries. Small. 2024;20 doi: 10.1002/smll.202305030. [DOI] [PubMed] [Google Scholar]
- 25.Niu X., Li N., Chen Y., Zhang J., Yang Y., Tan L., Wu J., Guo L., Zhu Y. Structure Evolution of V2O5 as Electrode Materials for Metal-Ion Batteries. Batter Supercaps. 2023;6 doi: 10.1002/batt.202300238. [DOI] [Google Scholar]
- 26.Petkov V., Trikalitis P.N., Bozin E.S., Billinge S.J.L., Vogt T., Kanatzidis M.G. Structure of V2O5·nH2O xerogel solved by the atomic pair distribution function technique. J. Am. Chem. Soc. 2002;124:10157–10162. doi: 10.1021/ja026143y. [DOI] [PubMed] [Google Scholar]
- 27.Hu P., Hu P., Vu T.D., Li M., Wang S., Ke Y., Zeng X., Mai L., Long Y. Vanadium Oxide: Phase Diagrams, Structures, Synthesis, and Applications. Chem. Rev. 2023;123:4353–4415. doi: 10.1021/acs.chemrev.2c00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An C., Wang T., Wang S., Chen X., Han X., Wu S., Deng Q., Zhao L., Hu N. Ultrasonic-assisted preparation of two-dimensional materials for electrocatalysts. Ultrason Sonochem. 2023;98 doi: 10.1016/j.ultsonch.2023.106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed M.A., Mohamed A.A. Advances in ultrasound-assisted synthesis of photocatalysts and sonophotocatalytic processes: A review. Iscience. 2024;27 doi: 10.1016/j.isci.2023.108583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi X., Chen D., Wang T., Jiao X. Fast-response, long-cycle life and multi-color electrochromic devices of transition metal-doped potassium vanadate films. Electrochim Acta. 2025;521 doi: 10.1016/j.electacta.2025.145877. [DOI] [Google Scholar]
- 31.Kim J., Lee S.H., Park C., Kim H.S., Park J.H., Chung K.Y., Ahn H. Controlling Vanadate Nanofiber Interlayer via Intercalation with Conducting Polymers: Cathode Material Design for Rechargeable Aqueous Zinc Ion Batteries. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202100005. [DOI] [Google Scholar]
- 32.Priyadarshi A., Khavari M., Bin Shahrani S., Subroto T., Yusuf L.A., Conte M., Prentice P., Pericleous K., Eskin D., Tzanakis I. In-situ observations and acoustic measurements upon fragmentation of free-floating intermetallics under ultrasonic cavitation in water. Ultrason Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X., Das R.S., Bhavya M.L., Garcia-Vaquero M., Tiwari B.K. Acoustic cavitation for agri-food applications: Mechanism of action, design of new systems, challenges and strategies for scale-up. Ultrason Sonochem. 2024;105 doi: 10.1016/j.ultsonch.2024.106850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D.C. Sayle, D.H. Gay, A.L. Rohl, C Richard, A. Catlow, J.H. Harding, M.A. Perrid, P. Nortierc, Computer modelling of V2 0,: surface structures, crystal morphology and ethene sorption, 1996.
- 35.Avansi W., Oliveira C.L.P., Ribeiro C., Leite E.R., Mastelaro V.R. Study of the morphological evolution of vanadium pentoxide nanostructures under hydrothermal conditions. CrstEngComm. 2016;18:7636–7641. doi: 10.1039/c6ce01196a. [DOI] [Google Scholar]
- 36.Li Z., Dong J., Zhang H., Zhang Y., Wang H., Cui X., Wang Z. Sonochemical catalysis as a unique strategy for the fabrication of nano-/micro-structured inorganics. Nanoscale Adv. 2021;3:41–72. doi: 10.1039/d0na00753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbas M., Takahashi M., Kim C. Facile sonochemical synthesis of high-moment magnetite (Fe 3O4) nanocube. J. Nanopart. Res. 2013;15 doi: 10.1007/s11051-012-1354-y. [DOI] [Google Scholar]
- 38.Politi M., Baum F., Vaddi K., Antonio E., Vasquez J., Bishop B.P., Peek N., Holmberg V.C., Pozzo L.D. A high-throughput workflow for the synthesis of CdSe nanocrystals using a sonochemical materials acceleration platform. Digital Discovery. 2023;2:1042–1057. doi: 10.1039/d3dd00033h. [DOI] [Google Scholar]
- 39.Chandrappa G.T., Chithaiah P., Ashoka S., Livage J. Morphological evolution of (NH4)0.5V 2O5• m H2O fibers into belts, triangles, and rings. Inorg. Chem. 2011;50:7421–7428. doi: 10.1021/ic2005858. [DOI] [PubMed] [Google Scholar]
- 40.Y.-J. Liu, J.A. Cowen, T.A. Kaplan, D.C. Degroot, J. Schindler, C.R. Kannewurf, M.G. Kanatzidis, Investigation of the Alkali-Metal Vanadium Oxide Xerogel Bronzes: A^Os-rol^O (A = K and Cs) The synthesis of bronze-like AA2O5V1H2O xerogels (A = K and Cs, 1995. https://pubs.acs.org/sharingguidelines.
- 41.Liu X., Huang C., Qiu J., Wang Y. The effect of thermal annealing and laser irradiation on the microstructure of vanadium oxide nanotubes. Appl. Surf. Sci. 2006;253:2747–2751. doi: 10.1016/j.apsusc.2006.05.041. [DOI] [Google Scholar]
- 42.Lee S.H., Han J., Jeon O.S., Park Y., Hong D., Mirzaei A., Kim J., Shin M.K., Yoo Y.J., Choi M.S., Yoo J., Park S.Y. Synthesis of conducting polymer intercalated sodium vanadate nanofiber composites as active materials for aqueous zinc-ion batteries and NH3 gas sensors at room temperature. Compos. B Eng. 2024;275 doi: 10.1016/j.compositesb.2024.111305. [DOI] [Google Scholar]
- 43.Chithaiah P., Chandrappa G.T., Livage J. Formation of crystalline Na 2V 6O 16· 3H 2O ribbons into belts and rings. Inorg. Chem. 2012;51:2241–2246. doi: 10.1021/ic202260w. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Xu H., He Y., Bian H., Jiang R., Zhao Q., Li D., Wang A., Sun D. Zn-doped V2O5 film electrodes as cathode materials for high-performance thin-film zinc-ion batteries. Solid State Ion. 2024;416 doi: 10.1016/j.ssi.2024.116658. [DOI] [Google Scholar]
- 45.Yang G., Li Q., Ma K., Hong C., Wang C. The degradation mechanism of vanadium oxide-based aqueous zinc-ion batteries. J. Mater. Chem. A Mater. 2020;8:8084–8095. doi: 10.1039/d0ta00615g. [DOI] [Google Scholar]
- 46.Li Y., Yang W., Yang W., Huang Y., Wang G., Xu C., Kang F., Dong L. High-performance zinc-ion batteries enabled by electrochemically induced transformation of vanadium oxide cathodes. J. Energ. Chem. 2021;60:233–240. doi: 10.1016/j.jechem.2021.01.025. [DOI] [Google Scholar]
- 47.Silversmit G., Depla D., Poelman H., Marin G.B., De Gryse R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+) J. Electron. Spectros. Relat. Phenomena. 2004;135:167–175. doi: 10.1016/j.elspec.2004.03.004. [DOI] [Google Scholar]
- 48.Le T.K., Kang M., Han S.W., Kim S.W. Highly intense room-temperature photoluminescence in V 2 O 5 nanospheres. RSC Adv. 2018;8:41317–41322. doi: 10.1039/c8ra06861e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He H., Pan F.C., Liang X.W., Hu Q., Liu S., Hu J., Jun S.C., Lin D., Yamauchi Y., Huo Y. Unveiling the effect of structural water on Zn-ion storage of polyoxovanadate for high-rate and long-life aqueous zinc ion battery. Chem. Eng. J. 2023;462 doi: 10.1016/j.cej.2023.142221. [DOI] [Google Scholar]
- 50.Idriss H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021;712 doi: 10.1016/j.susc.2021.121894. [DOI] [Google Scholar]
- 51.Bi W., Wu Y., Liu C., Wang J., Du Y., Gao G., Wu G., Cao G. Gradient oxygen vacancies in V2O5/PEDOT nanocables for high-performance supercapacitors. ACS Appl. Energy Mater. 2019;2:668–677. doi: 10.1021/acsaem.8b01676. [DOI] [Google Scholar]
- 52.Du M., Miao Z., Li H., Zhang F., Sang Y., Wei L., Liu H., Wang S. Oxygen-vacancy and phosphate coordination triggered strain engineering of vanadium oxide for high-performance aqueous zinc ion storage. Nano Energy. 2021;89 doi: 10.1016/j.nanoen.2021.106477. [DOI] [Google Scholar]
- 53.Alhummiany H., Rafique S., Sulaiman K. XPS analysis of the improved operational stability of organic solar cells using a V2O5 and PEDOT:PSS composite layer: Effect of varied atmospheric conditions. J. Phys. Chem. C. 2017;121:7649–7658. doi: 10.1021/acs.jpcc.6b13016. [DOI] [Google Scholar]
- 54.G. Greczynski, T. Kugler, M. Keil, W. Osikowicz, M. Fahlman, W.R. Salaneck, Photoelectron spectroscopy of thin films of PEDOT-PSS conjugated polymer blend: a mini-review and some new results, 2001. www.elsevier.com/locate/elspec.
- 55.Lazanas A.C., Prodromidis M.I. Electrochemical impedance spectroscopy─A tutorial. ACS Measurement Science Au. 2023;3:162–193. doi: 10.1021/acsmeasuresciau.2c00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lasia A. The Origin of the Constant Phase Element. J. Phys. Chem. Lett. 2022;13:580–589. doi: 10.1021/acs.jpclett.1c03782. [DOI] [PubMed] [Google Scholar]
- 57.Guo J., Che Y., Pedersen K., Stroe D.I. Battery impedance spectrum prediction from partial charging voltage curve by machine learning. Journal of Energy Chemistry. 2023;79:211–221. doi: 10.1016/j.jechem.2023.01.004. [DOI] [Google Scholar]
- 58.Wang L., Yang G., Peng S., Wang J., Yan W., Ramakrishna S. One-dimensional nanomaterials toward electrochemical sodium-ion storage applications via electrospinning. Energy Storage Mater. 2020;25:443–476. doi: 10.1016/j.ensm.2019.09.036. [DOI] [Google Scholar]
- 59.Peng L., Peng H., Wang S., Li X., Mo J., Wang X., Tang Y., Che R., Wang Z., Li W., Zhao D. One-dimensionally oriented self-assembly of ordered mesoporous nanofibers featuring tailorable mesophases via kinetic control. Nat Commun. 2023;14 doi: 10.1038/s41467-023-43963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J., Park S., Hwang S., Yoon W.S. Principles and applications of galvanostatic intermittent titration technique for lithium-ion batteries. J. Electrochem. Sci. Technol. 2022;13:19–31. doi: 10.33961/jecst.2021.00836. [DOI] [Google Scholar]
- 61.Horner J.S., Whang G., Ashby D.S., Kolesnichenko I.V., Lambert T.N., Dunn B.S., Talin A.A., Roberts S.A. Electrochemical modeling of GITT measurements for improved solid-state diffusion coefficient evaluation. ACS Appl Energy Mater. 2021;4:11460–11469. doi: 10.1021/acsaem.1c02218. [DOI] [Google Scholar]
- 62.Pasmay F.N., Litster S. Investigating proton exchange membrane water electrolyzers (PEMWE) performance losses through the galvanostatic intermittent titration technique (GITT) for electrolyzers. J Power Sources. 2025;633 doi: 10.1016/j.jpowsour.2025.236452. [DOI] [Google Scholar]
- 63.van der Heijden O., Park S., Vos R.E., Eggebeen J.J.J., Koper M.T.M. Tafel slope plot as a tool to analyze electrocatalytic reactions. ACS Energy Lett. 2024;9:1871–1879. doi: 10.1021/acsenergylett.4c00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y., Xu X., Sadd M., Kapitanova O.O., Krivchenko V.A., Ban J., Wang J., Jiao X., Song Z., Song J., Xiong S., Matic A. Insight into the critical role of exchange current density on electrodeposition behavior of lithium metal. Adv. Sci. 2021;8 doi: 10.1002/advs.202003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Omenya F., Paiss M., Li X., Reed D. Energy and power evolution over the lifetime of a battery. ACS Energy Lett. 2023;8:2707–2710. doi: 10.1021/acsenergylett.3c00660. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.