Abstract
The ability to adhere to surfaces and develop as a multicellular community is an adaptation used by most microorganisms to survive in changing environments. Biofilm formation proceeds through distinct developmental phases and impacts not only medicine but also industry and evolution. In organisms such as the opportunistic pathogen Candida albicans, the ability to grow as biofilms is also an important mechanism for persistence, facilitating its growth on different tissues and a broad range of abiotic surfaces used in medical devices. The early stage of C. albicans biofilm is characterized by the adhesion of single cells to the substratum, followed by the formation of an intricate network of hyphae and the beginning of a dense structure. Changes in the transcriptome begin within 30 min of contact with the substrate and include expression of genes related to sulfur metabolism, in particular MET3, and the equivalent gene homologues of the Ribi regulon in Saccharomyces cerevisiae. Some of these changes are initiated early and maintained throughout the process; others are restricted to the earliest stages of biofilm formation. We identify here a potential alternative pathway for cysteine metabolism and the biofilm-associated expression of genes involved in glutathione production in C. albicans.
Medical advances associated with invasive procedures, aggressive immunosuppressive treatments for organ transplantation, and the widespread use of broad-spectrum antibiotics are the most important causes of nosocomial fungal infection. The opportunistic pathogen Candida albicans, the predominant species recovered from hospitalized patients (39), accounts for 10% of hospital-acquired infections; candidiasis is the fourth most common blood-borne infection with an attributable mortality of ca. 38% (14). In immunocompetent individuals, C. albicans occurs as a dimorphic commensal colonizer of mucosal membranes in the oral cavity, gastrointestinal tract, urogenital mucosa, and vagina; in immunocompromised patients, however, this organism can become pathogenic, resulting in proliferative growth on mucosal surfaces and systemically. One of the features that enhances C. albicans' pathogenicity and/or infectivity is its ability to grow tenaciously on the surface of medical devices and abiotic surfaces, as well as on mucosa. Mature biofilms are characterized by the production of a thick extracellular matrix and an altered resistance phenotype to common antifungal agents (11, 12). The basis of the characteristic drug resistance of cells growing in biofilms is a source of much speculation, although recent studies suggest multiple factors involved in this process (5, 53).
Biofilm formation proceeds through characteristic stages that define the process. It begins with the initial adherence of single cells to the substratum, followed by the formation of microcolonies which grow into a confluent but trabeculated monolayer. Once confluence is achieved, the network of yeast cells, pseudohyphae, and hyphae become encased within an extracellular matrix (50). The tenacity of biofilm adherence varies with cell type, growth conditions, and the properties of the abiotic surface. Mutants blocked in either hyphal or yeast morphology can form biofilms, but these differ in their adhesive properties from those produced by wild-type cells (6). The extracellular matrix secreted by cells growing as adherent biofilms differs from the material secreted by planktonically grown cells and from matrices associated with bacterial biofilms (5).
Genome-wide transcription profiling has become an important and powerful tool in cell biology supporting the generation of testable hypotheses for novel processes not yet characterized at the molecular level. To characterize the initiating events in biofilm formation, we performed a detailed transcriptional analysis of the early stages of biofilm development in C. albicans using an Affymetrix oligonucleotide GeneChip representative of the entire genome of C. albicans (36, 37). Contemporaneously, others were performing similar studies by using a PCR-based spotter array composed of 2,002 open reading frames (ORFs), chosen at random from the 6,419 ORFs encoding proteins of >100 amino acids in the C. albicans haploid ORF set (30). In that study (18), gene expression in mature, established biofilms (48 and 72 h) formed in yeast nitrogen base (YNB) under various conditions of flow, oxygenation, and glucose concentration were characterized; 317 ORFs were expressed independently of hyphal formation, whereas 86 were dependent on hyphal formation (18).
The analysis reported here begins with individual cell attachment and ends when the cells have attained confluence during the first 6 h of C. albicans biofilm formation, an interval in the process which differs from later mature stages (11). Our study also differs from that previously reported (18) with respect to conditions used for biofilm formation and the number of ORFs assayed, as well as in the methods of data analysis. We find striking differences in gene expression patterns between nonadherent and adherent cells within 30 min of surface contact; similar and unique patterns of gene expression are also evident in both biofilm and planktonic cells within these early stages of biofilm formation. Our data complement work on gene expression patterns in mature biofilms (18) and indicate that biofilm formation in C. albicans is accompanied by progressive changes in gene expression. Some of these changes are initiated early and maintained throughout the process; others are restricted to the earliest stages of biofilm formation.
MATERIALS AND METHODS
C. albicans strain and media.
The genome sequencing strain SC5314 was used in these studies (20). Cells are routinely maintained at −80°C and plated on agar containing YPD (10 g of yeast extract, 20 g of Bacto peptone, 20 and g of dextrose/liter) before each experiment.
Establishment of biofilms.
A single colony of C. albicans SC5314 was used to inoculate Ham’s F-12 medium (Life Technologies, Gaithersburg, MD) and grown overnight at 30°C in a water shaker. Cells were centrifuged at 2,100 × g for 10 min at room temperature and washed twice in fresh F-12 medium at 30°C. Cells at this point are designated as experimental condition time zero (T = 0). For each time point, biofilms were formed in polystyrene petri dishes (100 mm by 15 mm [Fisher Scientific, Pittsburgh, PA]) using 107 cell/ml of inoculum in fresh F-12 medium (10-ml final volume per plate). Planktonic cultures were prepared by using the same inoculum size in 50 ml of F-12 medium in 250-ml polypropylene flasks, equalizing to the extent possible the surface/volume ratio in biofilm and planktonic cultures. Both cultures were incubated at 37°C in 5% CO2 with rotation at ca. 50 rpm. After 30 min of incubation, medium from the biofilm plates was aspirated, pooled, and centrifuged, and the number of nonadherent cells was determined in a hemocytometer and used for RNA extraction; routinely, >80% of the cell inoculum was firmly attached to the abiotic surface within 30 min. Biofilm cultures were then thoroughly washed three times in prewarmed medium, and fresh F-12 medium added; plates were incubated for 6 h with constant rotation at 37°C and 5% CO2. A total of five time points were assayed for biofilms and equivalent planktonic cultures: T = 30 min, T = 90 min, T = 150 min, T = 270 min, and T = 390 min. A set of biofilm plates corresponding to the five time points described above were stained with phloxine B dye (Sigma, St. Louis, MO), and propidium iodide and visualized by light microscopy.
AFM.
Either cover glass or polystyrene disks (12 mm; Fisher) were submerged in 35-mm wells with 2.5 ml of F-12 medium. Biofilms were formed as described above. Each disk was washed twice with F-12 medium and dried at room temperature for 1 h before imaging. The disks were glued to a metal disk (12 mm) for magnetic attachment to the piezo-scanner of an atomic force microscope (Digital Instruments, St. Barbara, Calif.). The scan rates were ca. 1 Hz, with scan sizes between 15 and 60 μm. All images were flattened manually in x and y directions by using the glass surface as the flattening reference (Fig. 1). Atomic force microscopy (AFM) facilitates highly accurate measurements of the topographic height (z axis of image), whereas the width of features is always subject to a tip-broadening artifact that derives from the shape of the AFM tip (26). We used height and width measurements of the long axis to more accurately describe the dimensional development of the blastoconidia during biofilm formation. The width measurements were corrected for the tip-broadening artifact by applying a tip angle of 35° for DNPS tips as described by Allen et al. (2).
FIG. 1.
AFM. All samples were imaged in the contact mode using a DNPS silicon nitride cantilever with a tip radius of ca. 10 nm (Nanosensors); the use of a porous membrane was not required (1). The scan rates were about 1 Hz with scan sizes between 15 and 60 μm. (A) Cell adhesion (T = 30); (B to E) biofilm development (T = 60 to T = 390). Bar: 10 μm (A and B) and 30 μm (C to E).
RNA extraction.
Before processing the complete set of samples, culture supernatants were collected for glucose determination (Glucose [HK] Assay Kit; Sigma, St. Louis, MO) and pH measurement. After each plate was washed twice with F-12 medium (37°C), cells were scraped from the plate and centrifuged at 2,100 × g at room temperature, and RNA was immediately extracted. Planktonic cells were directly harvested by centrifugation as described above and processed concurrently. After collection by either scraping and/or centrifugation, cells were suspended in 3 ml of extraction buffer (0.1 M Tris-HCl [pH 7.5], 0.1 M LiCl, and 0.02 M dithiothreitol) and transferred to an ice-cold 50-ml tube containing 6 g of acid-washed glass beads, 0.5 ml of 10% sodium dodecyl sulfate, and 5 ml of phenol-chloroform-isoamyl alcohol (25:24:1). The cells were disrupted by five cycles of vortexing (3 min each); between cycles the tubes were incubated alternatively for 3 min at 42°C and then at 0°C. The lysed cells were decanted and centrifuged at 10,000 × g for 10 min at 4°C and subjected to two additional phenol-chloroform-isoamyl alcohol extractions before nucleic acid precipitation in 2.5 volumes of ethanol. Samples were stored at −20°C overnight, and RNA precipitates were collected at 10,000 × g for 10 min. Total RNA was treated with RQ1 DNase (Promega, Madison, WI) for 1 h and cleaned by using an RNeasy Column (QIAGEN, Valencia, CA) prior to cDNA synthesis (Invitrogen, Carlsbad, CA); RNA samples were processed according to the manufacturer's instruction for Affymetrix GeneChips according to the protocol for 15 μg of starting material. A total of twelve samples were obtained per experiment (one from time zero, five from biofilm, and five from planktonic and one from the nonadherent cells at 30 min). The experiment was performed in duplicate, and DNA microarray analysis was carried out from two independent sets of samples.
DNA microarray design and analysis.
A custom high-density oligonucleotide GeneChip manufactured by Affymetrix (Santa Clara, CA) was used; the characteristics of these GeneChips and experimental protocols with C. albicans samples are thoroughly discussed in Lan et al. (36, 37). Gene annotations for the 7,116 open reading frames (ORFs) in the microarray and how they were assigned is described elsewhere (http://agabian.ucsf.edu). RNA labeling, target hybridization, washing, staining, and scanning were performed by using a GeneChip Hybridization Oven 640, a GeneChip Fluidics Station 400, and a GeneArray scanner (Affymetrix) as described previously (36). The technical reproducibility of the chips is very high due to their density, the random distribution of the probes within the matrix, and the synthesis of each probe directly on the substrate.
DNA microarray data analysis.
GeneChips were scanned, and the resulting image files were used to calculate, normalize, and compare the hybridization intensity data by using the Microarray Suite 5.0 software (Affymetrix). The fluorescence signals of each array were normalized by global scaling with a target intensity of 500. The statistical algorithm within this software was used for the absolute analysis of each individual microarray. The single array analysis measures a relative level of expression of a transcript (signal) and determines the detection call indicating whether a transcript is detected (present [P]) or not detected (absent [A]). Absolute analysis of each microarray was followed by comparison analysis between two separate arrays; T = 0 was selected as the baseline for comparison. This estimates the magnitude of fold change (signal log ratio) and the direction of the change (increase, decrease, and no change) in the expression of a transcript across the experimental time course for either biofilm or planktonic cultures. The data derived from each experiment was exported to Microsoft Excel, where the “signal log ratios” are converted to fold changes. For comparative purposes four variables were considered: (i) values with a no-change call, (ii) an absent signal with an increase- or decrease-change call, (iii) fold changes of <2, and (iv) intensity values of <100. If any of these criteria were positive in both planktonic and biofilm data sets, the probe set was excluded from analysis. The final results thus obtained consist of three different data sets: one set is described as the common gene set (CGS); genes in this set are upregulated or downregulated in both planktonic and biofilm cultures with respect to T = 0. The second and third data sets contain the ORFs that are up- or downregulated differentially in either biofilm or planktonic cultures. The complete data set is available online (http://agabian.ucsf.edu).
Reverse transcriptase PCR (RT-PCR).
Reactions were carried out by using SuperScript One Step RT-PCR with Platinum Taq (Invitrogen) and a PTC-200 Peltier Thermal Cycler (MJ Research, Waltham, MA). A total of 1 ng of DNase-treated total RNA was used as a template in each reaction. Primers for each gene are included 5′-GAACACAAATGTCATGAAAC-3′ and 5′-CAAGACTGAGGCACCAGC-3′ for YWP1, 5′-GGTCACTTATTTACCTGATG and 5′-TGTGATTCTACGTTCACCC-3′ for MET3, and 5′-ACACCTGAGTTGACTCCA-3′ and 5′-ACACCTGAGTTGACTCCA-3′ for ORF19.6824. The reactions were performed with 1 cycle of 50°C for 30 min and 94°C for 2 min; 35 cycles of 94°C for 15 s, 50°C for 30 s, and 72°C for 2 min; and 1 cycle at 72°C for 10 min. PCR products were visualized in 1% agarose gels and in some instances qualitatively correlated with signals obtained from DNA microarray intensity data.
RESULTS AND DISCUSSION
In vitro biofilm model for C. albicans.
The surfaces to which C. albicans adheres range from host mucosal membranes and skin to abiotic substrates used in medical devices. Coupled with C. albicans' ability to alter morphology and adapt to a range of environmental conditions, biofilm formation facilitates the transfer to and rapid proliferation of the fungus in the many anatomic sites that, in the immunocompromised host, support its growth. To approximate in vivo conditions, we used F-12 medium, 5% CO2, and 37°C; F-12 medium approximates human physiological conditions regarding fluid constituents, osmolarity, glucose concentration, and pH stability. Each of these factors, as well as the shearing force applied to the petri dish, critically influenced the rate of formation and the adhesive strength of these biofilms (15). These conditions are distinct from those used in a number of other reported biofilm studies (11, 18). The kinetics of glucose consumption were essentially identical in both planktonic and biofilm cultures, with more than half of the glucose being consumed within 4 h (initial concentration of 10 mM) and entirely consumed after 6 h (data not shown). The pH of the medium was stable over the 6 h of the experiment, ranging between 7.6 and 7.8. Approximately 80% of the initial cell inoculum adhered to the petri dish within the 30-min attachment period.
AFM was used to visualize biofilm development. The AFM images shown in Fig. 1 were obtained from glass substrates but are representative of biofilm formation on polystyrene as well (data not shown). Image quality was enhanced when glass disks were used due to the reduced surface roughness of the glass versus polystyrene. AFM images of biofilms provided the opportunity to measure cellular parameters during the early phases of biofilm formation. Hyphal structure showed an approximately cylindrical shape, and the height measurement directly translates into a measurement of the diameter of the hyphae. In contrast, the blastoconidia is an ellipsoid structure with its long axis usually perpendicular to the hyphal axis. At T = 30, C. albicans blastoconidia were on average 1.4 μm high and 2.2 μm wide, and germ tube development (length < 3 μm) was evident. At the density of “seeding” of the biofilms used in these experiments, germ tubes attained lengths up to 10 μm by T = 90. At T = 90, the beginnings of segmentation were evident; hyphae were generally straight and without evidence of branching or budding and the mother cells have increased to ca. 2.2 and 4.7 μm in height and width, respectively. By T = 150 a more intricate network was formed where most hyphae range in length from 20 to 30 μm and form contacts either with neighboring hyphae or mother cells (57) and branching is initiated. The biofilm increased in density with time, and the AFM images at T = 270 show a highly connected network of overlapping hyphae. At this time the pores or potential water channels in the network are reduced to <10 μm in diameter. At T = 390, the pore size is further reduced to an average of ∼1 μm; this pore size is maintained during continued biofilm development and retained in mature biofilms (data not shown).
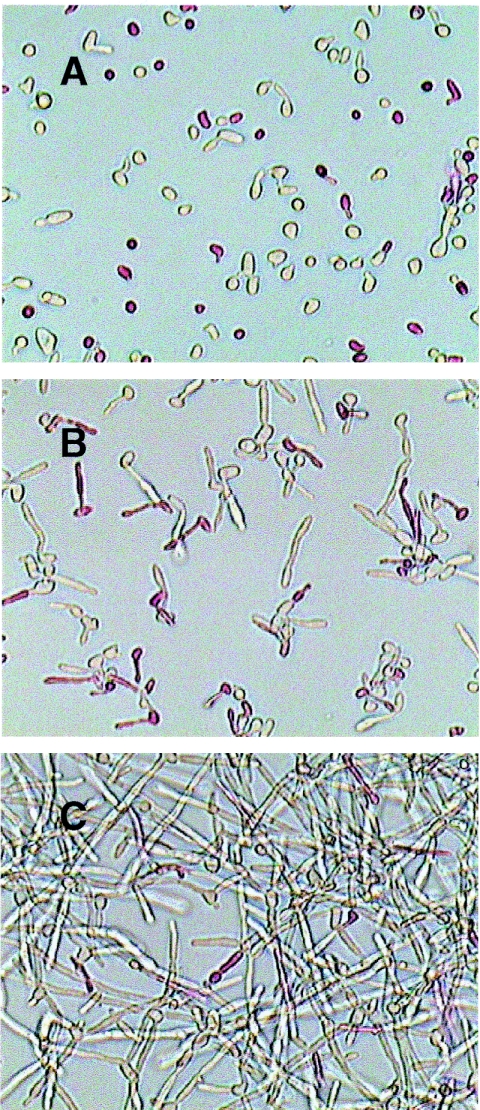
After the initial attachment period at T = 30 in biofilm development we observed what seemed to be a population of significantly larger and more elliptically shaped yeast form cells, reminiscent of the opaque phenotype of the WO-1 strain (56). Phloxine B, a stain that reflects alterations in cell membrane permeability, is used to differentiate white (W) and opaque (O) cells, as well as living and dead colonies in various yeasts (58). Staining of biofilms with Phloxine B produced a different pattern over the course of biofilm development: ca. 30% of the cells stained pink at T = 30 and T = 90 (Fig. 2), suggesting heterogeneity in the population of yeast cells attached to the substratum. However, there was no preferential staining of large versus small cells. At later times (T = 270 and T = 390), the proportion of stained cells decreased until only the tips of the hyphae took up stain (Fig. 2). Propidium iodide, which only stains cells with disrupted cell membranes, did not stain any of the cells throughout the experiment (data not shown), indicating that all cells within the biofilm remained viable for the 6 h of the study. Moreover, during the course of the experiment, biofilms grew as homogeneous hyphal cultures while the planktonic cells became progressively more heterogeneous mixtures of hypha-pseudohypha-yeast forms (see below).
FIG. 2.
Phloxine B staining of C. albicans biofilm formation. A set of five petri dishes corresponding to each datum point in biofilm formation was used for staining with 5 μM Phloxine B (34) and photographed (Arcturus). Differences in staining are evident during the 6 h of biofilm development. (A) T = 30; (B) T = 90; (C) T = 270. Magnification, ×40.
RT-PCR.
Three differentially expressed ORFs, two biofilm (MET3, and ORF19.6824) and one planktonic (YWP1; cyclical expression pattern) were assayed by RT-PCR in addition to DNA microarray analysis. Comparisons of the RT-PCR products with DNA microarray signal values are shown in Fig. 3. MET3 transcripts are detected in both biofilm and planktonic cultures; however, at equal concentrations of total RNA and similar cycling programs, the amounts of product were substantially different in each case and consistent with the pattern obtained from DNA microarray analysis. YWP1 gene expression in planktonic cells is cyclical, both by DNA microarray analysis and RT-PCR, reflecting the initial transition from yeast to hyphal cells and the rapid reappearance of yeast forms in the culture. Conversely, YWP1 gene expression decreases in biofilm cultures and is essentially undetectable through T = 390, both by RT-PCR and DNA microarray analysis, reflecting the hyphal nature of biofilms at this stage of development under our conditions. ORF19.6824, another gene upregulated in biofilm similarly confirms that the expression patterns are equivalent in both types of analysis.
FIG. 3.
Correlation of DNA microarray analysis and RT-PCR. Three genes, which represent a yeast-specific gene (YWP1) and two biofilm upregulated (MET3 and ORF19.6824) genes are detected by RT-PCR and compared to the signal values obtained from the GeneChip data. Amplified products are detected on a 1% agarose gel. Lanes: 0, T = 0; 1 to 5, T = 30 to T = 390 (planktonic); 6 to 10, T = 30 to T = 390 (biofilm).
Gene expression data.
We assayed genome-wide changes in gene expression during the earliest stages of biofilm formation, beginning with the attachment of individual cells to the substratum through the formation of a contiguous cellular layer on the abiotic surface. The transcription data derived from these experiments was compared in several ways. “Vertical” comparisons were made during the time course (i.e., 1 h biofilm versus 1 h planktonic), similar to comparisons made in the study of gene expression in mature biofilms (18). We then went on and compared each vertical comparison longitudinally across the entire time course of the experiment. In these comparisons, genes that are equivalently induced as a function of time and/or morphogenesis in both biofilm and planktonic cultures are not scored since their values cancel each other; ORFs which display variable (i.e., cyclic) patterns of expression during the experimental time course were more difficult to compare across cultures, especially because the adherent early stage biofilms are essentially completely hyphal, whereas the planktonic cultures are heterogeneous with respect to morphologic types. In the “horizontal” comparison we analyzed changes in gene expression as a function of time within either biofilm or planktonic cultures, respectively, using the T = 0 samples as a reference. These two data sets were then compared vertically with one another to identify genes that are regulated in both cultures and genes that are uniquely regulated in either. From these comparisons, a total of 2,492 ORFs whose expression varied during the course of the analysis were identified. Within this group, three different data subsets emerged: differentially upregulated genes in biofilm (41 ORFs; Table 1), differentially upregulated genes in planktonic cultures (25 ORFs; Table 2), and the remainder (2,426 ORFs) comprising a CGS (see Table S1 in the supplemental material) whose expression is similarly regulated in both types of cultures. The DNA microarray data set is derived from two independent experiments and showed a reproducibility of >95%. The genes expressed differentially in either biofilm or planktonic cultures were noted in both replicates. Only one representative set of data is presented here. Finally, we also compared the gene expression profiles of those cells that adhere to the substrate during the 30-min seeding period to the remaining 20% of the cells that did not adhere to the plates (nonadherent cells) (see Table S2 in the supplemental material).
TABLE 1.
Genes and/or ORFs expressed differentially in biofilm cells
| ORFa | Expression (fold change)b at:
|
Gene; ORF (assembly 19)c | ||||
|---|---|---|---|---|---|---|
| T = 30 | T = 90 | T = 150 | T = 270 | T = 390 | ||
| Homology in S. cerevisiae | ||||||
| 6.4694 | 1.0 | 2.8 | 3.3 | 4.5 | 1.0 | SUL1; ORF19.10252 |
| 6.7984 | 22.9 | 22.0 | 16.0 | 26.9 | 12.6 | MET3; ORF19.5025 |
| 6.3204 | 3.2 | 3.8 | 2.6 | 4.0 | 2.4 | MET10; ORF19.4076 |
| 6.6837 | 2.4 | 2.2 | 2.3 | 2.9 | 1.9 | ECM17; ORF19.4099 |
| 6.8195 | 4.1 | 5.4 | 4.0 | 5.0 | 2.2 | MET14; ORF19.946 |
| 6.4514 | 9.9 | 10.3 | 10.6 | 14.5 | 5.4 | MET15; ORF19.5645 |
| 6.2502 | 3.3 | 5.2 | 5.0 | 4.3 | 1.7 | HAL21; ORF19.99 |
| 6.2508 | 3.7 | 5.6 | 5.4 | 4.9 | 1.7 | HAL22; ORF19.105 |
| 6.3134 | 1.4 | 2.8 | 2.5 | 2.5 | 1.8 | ORF19.1159; CYS A homolog |
| 6.6913 | 3.5 | 5.7 | 4.6 | 6.3 | 1.0 | OPT3; ORF19.5673 |
| 6.1607 | −1.9 | 1.0 | 1.0 | 2.4 | 2.8 | GCS1; ORF19.5060 |
| 6.4839 | 3.2 | 6.6 | 5.7 | 8.1 | 9.3 | NCE103; ORF19.1721 |
| 6.7898 | 22.6 | 17.0 | 18.8 | 7.5 | 10.3 | PHO89; ORF19.4599 |
| 6.2299 | 6.4 | 8.6 | 7.9 | 5.7 | 8.9 | ORF19.3936; similar to PHO81 |
| 6.5895 | 6.9 | 5.6 | 4.3 | 7.5 | 4.8 | AAH1; ORF19.2251 |
| 6.7999 | 1.0 | 1.7 | 1.0 | 2.2 | 2.3 | DIM1; ORF19.5010 |
| 6.254 | 1.0 | 1.0 | 1.0 | 2.2 | 2.7 | KRR1; ORF19.8277 |
| 6.2709 | 1.0 | 1.4 | 1.0 | 2.3 | 3.1 | RRP8; ORF19.3630 |
| 6.8114 | 3.8 | 5.7 | 9.4 | 12.6 | 24.3 | ORF19.7214; similar to 1,3-glucosidase |
| 6.3733 | 1.2 | 1.7 | 1.3 | 3.8 | 3.9 | ORF19.3088; similar to S. cerevisiae YDR184 |
| 6.3068 | 1.6 | 2.8 | 2.4 | 3.1 | −1.6 | AGP3; ORF19.3795 |
| 6.2084 | 1.0 | 3.9 | 4.0 | 4.2 | 2.4 | YMC2; ORF19.4733 |
| 6.6487 | 1.7 | 1.7 | 1.5 | 2.1 | 2.6 | ABP140; ORF19.3676 |
| 6.1078 | 2.3 | 2.5 | 2.0 | 2.7 | 1.6 | PUS7; ORF19.9322 |
| 6.972 | 2.1 | 1.6 | 1.7 | 2.5 | 2.2 | POL5; ORF19.13042 |
| 6.9132 | 2.7 | 2.1 | 2.0 | 2.0 | 2.7 | ORF19.5905; similar to S. cerevisiae YBL028C |
| 6.4849 | 6.0 | 5.9 | 4.2 | 2.1 | 3.4 | ORF19.5777; similar to S. cerevisiae YBR096W |
| 6.3387 | 1.0 | 1.0 | 1.0 | 2.7 | 3.2 | ORF19.5049; similar to S. cerevisiae YLR003 |
| 6.337 | 1.0 | 1.5 | 1.4 | 2.6 | 2.3 | ORF19.2527; similar to S. cerevisiae YNL035c |
| 6.2076 | 1.3 | 1.3 | 1.2 | 2.1 | 2.0 | ORF19.1388; similar to S. cerevisiae YER002 |
| No homology in S. cerevisiae | ||||||
| 6.1742 | 5.0 | 26.2 | 32.4 | 24.4 | 79.9 | AMO2; ORF19.3152 |
| 6.7581 | 1.7 | 2.8 | 3.4 | 6.5 | 3.5 | ASM3; ORF19.6037 |
| 6.241 | 2.2 | 1.0 | 3.5 | 98.4 | 86.2 | POL93; ORF19.6078 |
| 6.258 | 1.0 | 2.4 | 2.4 | 1.6 | 2.1 | SSP96; ORF19.5145 |
| 6.2306 | 75.6 | 104.7 | 93.1 | 85.6 | 49.2 | ORF19.1539 |
| 6.8807 | 9.1 | 7.9 | 8.4 | 12.1 | 55.3 | ORF19.6824 |
| 6.4376 | 2.3 | 4.4 | 3.9 | 4.1 | 14.5ORF | ORF19.1797 |
| 6.283 | 6.3 | 7.8 | 7.4 | 5.5 | 1.0 | ORF19.4424 |
| 6.102 | 1.0 | 2.7 | 8.8 | 2.3 | 3.8 | LDG3; ORF19.6486. |
| 6.6784 | 1.0 | 2.5 | 2.1 | 2.9 | 2.5 | ORF19.6675 |
| 6.7184 | 1.3 | 2.5 | 2.6 | 3.2 | 2.9 | ORF19.6920 |
Assembly 6.
Positive or negative values represent the fold changes of up- or downregulated genes, respectively.
Gene names may also be found at http://agabian.ucsf.edu/.
TABLE 2.
Genes or ORFs expressed differentially in planktonic cells
| ORFa | Expression (fold change)b at:
|
Gene; ORF (assembly 19)c | ||||
|---|---|---|---|---|---|---|
| T = 30 | T = 90 | T = 150 | T = 270 | T = 390 | ||
| Homology in S. cerevisiae | ||||||
| 6.4985 | 3.1 | 2.4 | 2.0 | 2.1 | 2.5 | IPL1; ORF19.3474 |
| 6.5762 | 1.8 | 1.0 | 4.0 | 11.4 | 32.9 | BIO32; ORF19.3567 |
| 6.7295 | 2.4 | 1.0 | 1.0 | 4.5 | 5.4 | HSP78; ORF19.882 |
| 6.1952 | 2.1 | 2.5 | 1.9 | 2.8 | 4.9 | SUA72; ORF19.3519 |
| 6.8933 | 140.1 | 24.3 | 16.1 | 1.0 | 16.2 | INO1; ORF19.7585 |
| 6.2039 | 8.9 | 2.9 | 2.6 | 6.9 | 26.2 | ITR2; ORF19.3526 |
| 6.7648 | 5.7 | 10.3 | 8.8 | 12.5 | 16.2 | EXO70; ORF19.6512 |
| 6.677 | 4.2 | 3.7 | 6.4 | 21.7 | 14.1 | CGR1; ORF19.10237 |
| 6.7846 | 3.4 | 2.0 | 2.1 | 3.2 | 6.4 | ORF19.6408; similar to S. cerevisiae YNL064C |
| 6.7979 | 2.0 | 1.9 | 1.8 | 3.8 | 3.7 | ORF19.5030; similar to S. cerevisiae YDR068W |
| 6.6497 | 1.9 | 1.0 | 2.3 | 3.4 | 2.9 | ORF19.428; similar to S. cerevisiae YJL057C |
| 6.7758 | 2.3 | 1.0 | 1.9 | 5.0 | 5.2 | ORF19.3021; similar to S. cerevisiae YMR114C |
| 6.3406 | 2.3 | −2.8 | 1.0 | 7.9 | 10.6 | ORF19.5125; similar to S. cerevisiae YLR149c |
| 6.9062 | 1.0 | −6.5 | −11.3 | 3.8 | 6.5 | ORF19.5975 |
| No homology in S. cerevisiae | ||||||
| 6.1227 | 2.0 | 1.0 | 1.3 | 2.1 | 6.5 | FMO2; ORF19.8477 |
| 6.4504 | 1.3 | −2.3 | −2.2 | 4.4 | 7.8 | CRW3; ORF19.5635 |
| 6.4753 | −2.1 | −2.0 | −2.0 | 2.3 | 4.3 | SOD33; ORF19.2108 |
| 6.3288 | −2.3 | −13.7 | −35.3 | −6.1 | −2.9 | YWP1; ORF19.3618 |
| 6.2131 | 2.4 | 3.8 | 3.3 | 2.8 | 4.4 | ORF19.1049 |
| 6.2106 | 1.2 | 1.0 | 1.0 | 2.1 | 2.2 | ORF19.1482 |
| 6.8 | 5.9 | 7.1 | 7.9 | 11.6 | 8.3 | ORF19.43 |
| 6.81 | 2.6 | 1.0 | 2.2 | 3.7 | 8.8 | ORF19.3742 |
| 6.4587 | 2.2 | 1.0 | 2.6 | 5.8 | 6.5 | ORF19.2806 |
| 6.1755 | 1.0 | 8.2 | 8.5 | 8.5 | 6.6 | ORF19.12172 |
| 6.444 | 1.0 | −1.7 | −1.6 | 2.3 | 3.7 | ORF19.633 |
Assembly 6.
Positive or negative values represent the fold changes of up- or downregulated genes, respectively.
Gene names may also be found at http://agabian.ucsf.edu/.
Differential gene expression in biofilms.
A total of 41 ORFs were expressed more highly during the initial 6 h of biofilm development versus planktonic cultures; their relative levels of gene expression are found in Table 1. Of these 41 ORFs, nine (SUL1, MET10, HAL21, HAL22, MET14, MET15, MET3, ECM17, and ORF19.1159) encode proteins directly involved in sulfur metabolism. The changes in expression of this particular set of ORFs is striking and clearly indicate that not only particular ORFs but also the entire sulfur assimilation pathway is upregulated in early biofilm cultures. Moreover, the kinetics of this induction is also striking, beginning within 30 min of surface contact (Table 1 and Table S2 in the supplemental material) and increasing through the 6 h of the experiment. Unique to the present study and not detected previously (18) are C. albicans HAL21 and HAL22 ORFs, which are also highly upregulated in biofilm cells. HAL21 and HAL22 represent a gene duplication similar to that of HAL2/MET22 in Saccharomyces cerevisiae (45). C. albicans HAL21 and HAL22 encode a phosphatase that catalyzes the hydrolysis of either 3′-phosphoadenosine-5′-phosphate (PAP) and/or 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to AMP and are thus likely to function in sulfur recycling. MET16, which encodes a 3′-phosphoadenylsulfate reductase that reduces 3′-phosphoadenylylsulfate to adenosine-3′,5′-bisphosphate and free sulfite, reported to be upregulated in mature (72 h) biofilms (18), is not upregulated during early biofilm formation. In the absence of concomitant upregulation of MET16, upregulation of HAL21/HAL22 suggests the activation of a futile sulfur cycle in biofilm cells. In plants, accumulation of toxic intermediates such as APS and PAPS is controlled by HAL2, which transfers the sulfur atom to an organic compound (47), typically glutathione (GSH) in most organisms (42, 48). Functionally, GSH not only confers protection against reactive oxygen species but also serves as a storage and transport form of cysteine.
One of the most highly expressed genes in biofilm cells is MET3. The protein encoded by MET3 is the primary activator of sulfur assimilation in the cell. Studies in the fungal pathogen Cryptococcus neoformans indicate that MET3 also plays an important, but not understood role in its virulence and growth. MET3 expression levels in C. neoformans are not regulated by exogenous methionine, although they are in S. cerevisiae (64). In C. albicans, it is reported that MET3 transcription is fully repressed by 1 mM methionine or cysteine (10) and that repression of MET3 expression is detected at 0.1 mM cysteine or methionine. The F-12 media we use contain 0.2 and 0.03 mM cysteine and methionine, respectively, concentrations that are inhibitory for MET3 expression (10). As shown in Fig. 3, the levels of MET3 transcripts in planktonic cells are low, a finding consistent with repression of MET3 as described above. In contrast, even in the presence of what should be inhibitory concentrations of methionine and cysteine, MET3 is induced ∼23-fold by T = 30 in biofilm cells; thus, contact with the abiotic surface appears to override methionine/cysteine repression of MET3. The activation and maintenance of these pathways as a unique attribute in the transcriptome of cells growing as biofilms make these pathways an excellent drug target for the prevention of biofilm formation; one such drug already described elsewhere (3) might be azoxybacillin, a sulfite reductase inhibitor and natural antifungal agent produced by Bacillus cereus. The potential use of this drug will be the target of further investigation.
The identity of the ORFs induced in biofilm cells in the present study further suggests that sulfur assimilation in C. albicans differs substantively from that in S. cerevisiae and more closely resembles sulfur assimilation pathways described in Neurospora crassa and Aspergillus nidulans (40). In these filamentous fungi, sulfur assimilation, especially through the formation of cysteine, follows two biosynthetic pathways: the methionine-cystathionine (CT) pathway and the O-acetyl-serine (OAS) pathway (59). In C. albicans ORF19.1159 encodes a presumptive homologue of the A. nidulans serine O-trans-acetylase [CYSA], the enzyme which converts serine to O-acetylserine as part of the OAS pathway (25); ORF19.7152 encodes a protein with 85% identity to the O-acetylserine sulfhydrylase (CYSK) of Aspergillus, which converts O-acetylserine into cysteine incorporating a sulfur group. These ORFs encode enzymes that are key to the formation of cysteine via the OAS pathway, a pathway not found in S. cerevisiae (46). Our assertion that this pathway is present in C. albicans is strengthened by the observation that, unlike S. cerevisiae, C. albicans met15-null mutants can grow on methionine-depleted media (61). We note that the C. albicans CysA and CysK homologues, ORF19.1159 and ORF19.7152, were not included/assayed in the previous study (18). Although the expression of both of these ORFs is upregulated only in biofilm cultures, there was no differential upregulation of MET6 (homocysteine methyltransferase) and SAM2 (S-adenosylmethionine synthase) suggesting again that the flow of sulfur under these conditions favors the synthesis of cysteine (Fig. 4).
FIG. 4.
Potential second cysteine synthesis pathway in C. albicans. The pathways for the synthesis of homocysteine, methionine, cysteine, and S-adenosylmethionine are shown. In S. cerevisiae (genes in green) the sulfate assimilation pathway flows toward the production of methionine and S-adenosylmethionine. The existence of an alternative route such as the OAS pathway described for A. nidulans is found in C. albicans biofilm cells (ORFs in red).
Complementing the induction of sulfur assimilation pathways is the upregulation of AGP3, an ORF that encodes a putative serine transporter protein and thus would provide a carbon acceptor for sulfur assimilation (7) through the OAS pathway. Similarly, OPT3, a probable oligopeptide transporter, based on its similarity to ISP4 of Arabidopsis, displays kinetics of expression similar to that of SUL1 during early biofilm formation (Table 1), suggesting that OPT3 may play a role in the transport of sulfur-containing compounds (e.g., GSH or GSH-linked xenobiotics), as OPT1 does in S. cerevisiae (8).
Also upregulated during early stages of biofilm development are NCE103, a carbonic anhydrase-like gene (22), and GCS1, a gene encoding γ-glutamylcysteine synthase (4). Both of these enzymes are indirectly related to the sulfur pathway and oxidative metabolism. Mutant strains of NCE103 in S. cerevisiae are unable to grow under normal aerobic conditions, but complementation with the coding region of a carbonic anhydrase gene from Medicago sativa rescue this deficiency (13). In rats, carbonic anhydrase III also functions as a phosphatase upon glutathiolation of one of its cysteine residues (9). Gcs1, the rate-limiting enzyme in GSH biosynthesis, is associated with oxidative metabolism via the de novo production of GSH. In Schizosaccharomyces pombe, Gcs1 is under complex regulation by GSH feedback inhibition, the availability of l-cysteine, and the carbon source (32) and is associated with oxidative metabolism via the de novo production of GSH. Transcripts from both NCE103 and GCS1 are highly upregulated in biofilms; however, the kinetics of GCS1 induction differs from that of the sulfur assimilation pathways and NCE103. Although NCE103 is induced immediately upon attachment of cells to the abiotic surface, the signal values of the NCE103 transcript increase over time, and induction of GCS1 transcription is not observed until 4 h into biofilm growth. At this stage the cells have started to form a second layer and more intricate cell-cell interactions (Fig. 1); more than 50% of the available glucose has been consumed in both biofilm and planktonic cultures and the phloxine B staining pattern of the cells changes (Fig. 2). Taken together, these observations suggest that alterations in membrane structure and permeability and a requirement for GSH-related metabolic activities occur during the transitional period of the early to intermediate stage of biofilm formation. The potential role of GSH in the transient acquisition of drug resistance in biofilms merits some comment. In both mammalian tumor cells (51) and in the eukaryotic pathogen, Plasmodium falciparum (44), changes in GSH levels result in altered sensitivity of these cell types to a variety of drugs. Although this phenomenon remains to be explored in C. albicans, the genomic expression data support the hypothesis that, during biofilm development, alterations in GSH levels may play a role in the drug resistance phenotypes of cells growing as biofilms.
Of the remaining 28 ORFs upregulated in biofilm, 17 have homologues in S. cerevisiae, 5 of which are homologous to ORFs of unknown function; of these 17, 10 encode nuclear proteins (29). Not directly involved in sulfur assimilation but representing a significant second subset of genes expressed in biofilms are ORFs associated with phosphate metabolism: PHO89, PHO81, and ORF19.4424, a PHO2-like gene. In S. cerevisiae PHO89 encodes a membrane Pi transporter that mediates cation-coupled Pi transport acting in concert with a pH-dependent (alkaline) plasma membrane Na+-ATPase (ENA1/Pmr2p) (17). A C. albicans homologue of ENA1, encoded by ORF19.5170, is preferentially upregulated in biofilm cells, along with the alkaline pH-regulated gene PHR1. Other homologues include ORFs associated with pseudohyphal growth (ORF19.5777); mitochondrial transport (YMC2); rRNA processing/Ribi regulon (KRR1, RRP8, and DIM1); tRNA modification (PUS7); an actin filament-binding protein (ABP140); an adenine deaminase (AAH1); and ORF19.7214 (similar to S. cerevisiae YBR056), a glucan 1,3-β-glucosidase. Of the 28 ORFs differentially expressed in early biofilm formation (Table 1), 11 do not have a counterpart in S. cerevisiae; several of these, however, are similar to genes found in other organisms: ORF19.1797, which encodes a protein that contains a GutQ domain characteristic of many phosphosugar isomerases and phosphosugar-binding proteins required for bacterial lipopolysaccharide assembly (60); ORF19.4424, which encodes a protein with a domain similar to one found in the SurE survival protein of bacteria and is most closely related to an acid phosphatase of Yarrowia lipolytica; AMO2, which encodes a protein with similarity to a putative peroxisomal copper amino oxidase from A. niger (16); and SSP96, a protein containing a flavin-binding monooxygenase-like domain. ORF19.6675 displays a low degree of similarity to lipoic acid synthase, and ORF19.6824, a 467-amino-acid protein with a potential helix-loop-helix DNA-binding domain, is upregulated only in biofilm cells. ORF19.6824 appears to be specific for C. albicans, since Southern blot analyses with a collection of DNA from 11 different Candida species and S. cerevisiae failed to show positive hybridization, even at low stringency (data not shown). LDG3 encodes a leucine-aspartic acid-glycine rich protein of unknown function, which belongs to one of the largest gene families in C. albicans (A. Kuo, unpublished data); LGD3 is the only member of this gene family upregulated in biofilms. POL93, which is very similar to TCA8, encodes an RT that is upregulated nearly 100-fold at T = 270 and is classified as a gypsy-like element with an internal primer site similar to a region of the Candida tRNAiMet (21).
Differential gene expression in planktonic cultures.
Although the cells growing as biofilms under the condition used are almost uniformly hyphal, as also observed in biofilm derived from RPMI1640 medium (10), equivalent planktonic cultures consist of a mixed population of hyphal, pseudohyphal, and yeast forms in F-12 media. This qualitative observation was quantitated by comparing the relative gene expression levels of HWP1 and YWP1, hyphal- and yeast-form specific genes, respectively (55). This dichotomy in population structure confounds interpretation of elevated levels of gene expression for the 25 genes differentially expressed in planktonic culture; nevertheless, these bear mention. Of the 25 ORFs, 19 display an increase in fold change during the entire 6 h of analysis; 10 of the 19 have a homologue in S. cerevisiae, including genes involved in metabolism (BIO32 and ITR2), cell cycle (EXO70), yeast-mycelial transition (CGR1), heat shock (HSP78), protein trafficking (ORF19.6408), unknown function (ORF19.3021 and ORF19.5125), and two transcription factors (SUA72 and ORF19.5975). The SUA7 gene in S. cerevisiae has two homologues in C. albicans: SUA71 and SUA72. Although SUA72 is upregulated in planktonic cultures, SUA71 is downregulated (see Table S1 in the supplemental material), suggesting distinct roles for these two almost identical (73% identity at the amino acid level) putative transcription factors. ORF19.5975 is a potential zinc finger protein and is similar to S. cerevisiae Adr1, a putative transcription factor, with respect to the position of zinc ligand and conserved DNA contact residues (52).
The remaining nine ORFs displaying increased mRNA levels during the first 6 h of planktonic growth and which have no homologues in S. cerevisiae include: FMO2, a putative bacterial FAD-containing monooxygenase; CRW3, a potential cell surface antigen with a CFEM (for common in several fungal extracellular membrane proteins) domain present in proteins involved in fungal pathogenesis (35); SOD33, a protein likely similar to a secreted Cu/Zn superoxide dismutase from N. crassa; and six additional ORFs with unknown function (Table 2). It is noteworthy that the expression of five ORFs in the planktonic category cluster with the yeast-form specific gene YWP1: ORF19.1482, ORF19.5975, CRW3, SOD33, and ORF19.633. Of the remaining five planktonic ORFs (of the twenty-five), four show constant fold changes through the entire 6 h, and one (INO1 inositol-1-phosphate synthase) is downregulated (Table 2).
CGS.
The major change in both planktonic and biofilm cultures upon initiation of this protocol is the induction of hyphal morphogenesis. During the 6-h growth period of this experiment, a total of 2,426 ORFs were differentially regulated in both planktonic and biofilm cultures: 1,660 ORFs were upregulated, and 766 were downregulated. A total of 13% of the CGS ORF set (2,426) are ORFs of unclassified function, and almost 20% of the total CGS ORFs have no significant homology to any S. cerevisiae genome sequences (Table 3). Three different patterns of expression in the CGS were noted: group A, ORFs with initial low fold change values that increased over time (T = 390/T = 30, x ≥ 2) (n = 568); group B, ORFs with constant fold change values during the entire 6 h of the experiment (T = 390/T = 30, x = 1) (n = 1,120); group C, ORFs with initial increased fold change values that later decreased (T = 390/T = 30, x ≤ 0.5) (n = 738) (Table S1 in the supplemental material). Functional assignments of ORFs within the three groups were made using MIPS categories based on homology with S. cerevisiae genes (http://mips.gsf.de/proj/yeast/catalogues/funcat/index.html) (Table 3). Overall, the largest proportion of the CGS belongs to group B. Within the classes of gene expression profiles, for groups B and C the largest functional category is that of metabolism followed by protein fate, cellular transport, cell cycle, and DNA processing. Group C ORFs in the categories of cell cycle, DNA processing, and protein fate decreased at later times, as might be expected as the overall cell density increased in the cultures. There is a relative increase in the number of ORFs in group A in the metabolism category due to the expression of ORFs related to amino acid, nitrogen, and sulfur metabolism; this group also includes genes involved in cell rescue, defense, and virulence, including SAP5, SAP6, RBT5, ECM33, ACE2, ALS3, HWP1, TPK2; PLB3, PLB4, PLB5, and ORF19.1586, similar to a bacterial phosphatidylinositol-specific phospholipase C. A more detailed comparison between groups C and A reveals that ORFs related to glycolysis and gluconeogenesis predominate in group C, whereas those associated with the oxidation of fatty acids and ionic homeostasis are upregulated in group A.
TABLE 3.
Functional categories of CGSa
| Description | No. of genes in functional category (%)b
|
||
|---|---|---|---|
| Group A | Group B | Group C | |
| Metabolism | 117 (15) | 194 (11.5) | 146 (13.5) |
| Energy | 28 (3.5) | 28 (1.7) | 29 (2.7) |
| Cell cycle and DNA processing | 31 (4) | 166 (9.8) | 116 (10.8) |
| Transcription | 65 (8) | 92 (5.4) | 54 (5) |
| Protein synthesis | 5 (0.5) | 32 (1.9) | 15 (1.4) |
| Protein fate | 34 (4) | 133 (7.8) | 136 (12.6) |
| Protein with binding function or cofactor requirement | 5 (0.5) | 10 (0.6) | 2 (0.1) |
| Protein activity regulation | 0.0 | 5 (0.3) | 5 (0.5) |
| Cell transport, transport facilitation, and transportation routes | 117 (15) | 163 (9.6) | 125 (11.6) |
| Cellular communication/signal transduction mechanism | 11 (1) | 20 (1.2) | 9 (0.8) |
| Cell rescue, defense, and virulence | 42 (5) | 45 (2.7) | 20 (1.9) |
| Interaction with the cellular environment | 15 (2) | 73 (4.3) | 32 (3) |
| Transposable elements, viral and plasmid proteins | 1 (0.1) | 2 (0.1) | 2 (0.2) |
| Cell fate | 19 (2) | 62 (3.7) | 24 (2.2) |
| Biogenesis of cellular components | 27 (3) | 101 (6) | 48 (4.5) |
| Cell type differentiation | 15 (2) | 79 (4.6) | 33 (3) |
| Subcellular localization | 2 (0.2) | 4 (0.2) | 0 |
| Unclassified proteins | 102 (13) | 212 (12.5) | 120 (11) |
| No good homologies in S. cerevisiae | 161 (20) | 272 (16) | 165 (15) |
C. albicans ORFs or genes were assigned according to the MIPS classification of the S. cerevisiae homologues.
Group A, ORFs with initial low fold change values that increased over time; group B, ORFs with constant fold change values; group C, ORFs with initial increased fold change values that later decreased.
ORFs with no homology to S. cerevisiae but which encode interesting amino acid motifs are also present in group A: for example, ORF19.5760 encodes a 392-amino-acid protein with a Gly-Ser domain at the carboxy terminal similar to that found in lustrin A, human and mouse cornified envelope loricrin, and the extracellular matrix protein keratin (54); ORF19.7104 encodes a mucin-like protein similar to microfilarial sheath protein Shp3 from the nematode Litomosoides (27).
Genes differentially expressed within 30 min of adherence.
Adherence with a hard surface induces the expression of genes involved in the priming of plant fungal pathogens to signals that promote host invasion (33). Perhaps related to this phenomenon, C. albicans displays a thigmotropic response (63) hypothesized to assist the organism in foraging for suitable sources of nutrients (49) and, when pathogenic, in defining the route of tissue penetration (23). It has also been reported that when C. albicans encounters an abiotic surface the transcription of genes encoding efflux pumps MDR1 and CDR1 are rapidly induced, concomitant with the display of enhanced resistance to fluconazole (41). Given this context, we sought to determine whether contact sensing, a common feature in the biology of saprophytic fungi (49), results in a discrete change in gene expression in C. albicans. To answer this question, we analyzed alterations in the C. albicans transcriptome as a consequence of adherence to the abiotic surface by directly comparing gene expression values in T = 30 adherent cells versus T = 30 nonadherent cells (Table S2 in the supplemental material). Surprisingly, within the 30-min period allowed for attachment and adherence, the transcription of 554 ORFs, or nearly 9% of the genome, was altered by a factor of ≥2: 300 ORFs were upregulated in adherent cells. None of these contact-associated changes in gene expression correspond to those noted in the plant pathogen Colletotrichum gloeosporioises. Changes in the expression levels of the transporters CDR1, CDR2, and MDR1 were not observed; however, the experimental protocols used by Mateus et al. (41) and by us differ significantly. The most highly induced gene of known function in Candida biofilm cells is MET3 (∼23-fold at T = 30). Roughly one-third of the 300 genes upregulated upon contact to the abiotic surface have no known function (see Table S2 in the supplemental material); of the remainder, ∼57% are homologous to a subset of genes of the Ribi (for ribosome biogenesis) regulon of S. cerevisiae. This regulon consists of 236 genes primarily encoding proteins involved in ribosome biogenesis, subunits of RNA polymerases I and III, enzymes involved in ribonucleotide metabolism, tRNA synthases, translation factors, and proteins of unknown function predominantly localized in the nucleolus (31) and is coordinately and transiently downregulated in response to environmental or genetic perturbation (19, 28, 43). In C. albicans adhesion to polystyrene results in the upregulation at T = 30 of a majority of the gene homologues in the S. cerevisiae regulon with the exception of genes encoding tRNA synthases and modifying enzymes, genes involved in nucleotide metabolism and translation initiation factors (see Table S2 in the supplemental material). Because the expression of members of this regulon is inherently unstable (24), the levels of the mRNAs measured in our study may alternatively reflect a decrease in their rate of degradation. In general, the regulon is repressed by a wide variety of suboptimal growth conditions, by environmental factors that threaten cell viability, and by defects in the secretory pathway (19, 38, 43). Conversely, transcription of the regulon increases when starved yeast cells are provided with glucose (62) and is generally regulated by pathways such as those which coordinate cell size, progression through the cell cycle, and membrane integrity, with nutrient availability (31, 38). Overall, expression of this regulon in S. cerevisiae seems to be associated with favorable growth conditions; its immediate induction in C. albicans upon adherence to the polystyrene surface may either provide C. albicans with the ability to rapidly respond to a change in environment and/or indicate that growth in the form of a biofilm is preferred.
Other genes of known function upregulated during the first 30 min of incubation include sets that encode proteins involved in sulfur metabolism (CYS3, MET3, MET10, MET14, MET15, ECM17, MXR1, SPE2, and GCS1), phosphate metabolism (PHO8, PHO81, PHO84, PHO86, and PHO89), and iron assimilation (ARN1, CFL9, FTH1, and FRP2).
Final remarks.
Although the induction of sulfur assimilation pathways and the homologues of the Ribi regulon represent the most dramatic differences between planktonic and biofilm cells, involving the coordinate upregulation of functionally related subsets of ORFs, there are additional changes that are also likely to be important in understanding the process of biofilm development. Activation of enzymes involved in oxidative mechanisms such as NCE1O3 and GCS1, together with the expression of newly identified ORFs with no homology in the S. cerevisiae genome, creates new avenues to the search of new pathways during biofilm formation. Surprisingly, none of the genes implicated in azole resistance are upregulated during the first 6 h, but a potential role for GSH in drug resistance is a consideration. The challenge now lies in understanding the functional relevance of these major shifts in the readout of the genomic repertoire when cells grow as communities.
Supplementary Material
Acknowledgments
This study was supported by NIH/NIDCR grants R21 DE15290 and PO1 DE07946 from the National Institutes of Health.
We thank John Lehnen for proofreading the manuscript.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Ahimou, F., A. Touhami, and Y. F. Dufrene. 2003. Real-time imaging of the surface topography of living yeast cells by atomic force microscopy. Yeast 20:25-30. [DOI] [PubMed] [Google Scholar]
- 2.Allen, M. J., N. V. Hud, M. Balooch, R. J. Tench, W. J. Siekhaus, and R. Balhorn. 1992. Tip-radius-induced artifacts in AFM images of protamine-complexed DNA fibers. Ultramicroscopy 42:1095-1100. [DOI] [PubMed] [Google Scholar]
- 3.Aoki, Y., M. Yamamoto, S. M. Hosseini-Mazinani, N. Koshikawa, K. Sugimoto, and M. Arisawa. 1996. Antifungal azoxybacilin exhibits activity by inhibiting gene expression of sulfite reductase. Antimicrob. Agents Chemother. 40:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek, Y. U., Y. R. Kim, H. S. Yim, and S. O. Kang. 2004. Disruption of gamma-glutamylcysteine synthetase results in absolute glutathione auxotrophy and apoptosis in Candida albicans. FEBS Lett. 556:47-52. [DOI] [PubMed] [Google Scholar]
- 5.Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397-403. [DOI] [PubMed] [Google Scholar]
- 6.Baillie, G. S., and L. J. Douglas. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48:671-679. [DOI] [PubMed] [Google Scholar]
- 7.Boer, V. M., J. H. de Winde, J. T. Pronk, and M. D. Piper. 2003. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J. Biol. Chem. 278:3265-3274. [DOI] [PubMed] [Google Scholar]
- 8.Bourbouloux, A., P. Shahi, A. Chakladar, S. Delrot, and A. K. Bachhawat. 2000. Hgt1p, a high-affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 275:13259-13265. [DOI] [PubMed] [Google Scholar]
- 9.Cabiscol, E., and R. L. Levine. 1996. The phosphatase activity of carbonic anhydrase III is reversibly regulated by glutathiolation. Proc. Natl. Acad. Sci. USA 93:4170-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Care, R. S., J. Trevethick, K. M. Binley, and P. E. Sudbery. 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 34:792-798. [DOI] [PubMed] [Google Scholar]
- 11.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandra, J., P. K. Mukherjee, S. D. Leidich, F. F. Faddoul, L. L. Hoyer, L. J. Douglas, and M. A. Ghannoum. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903-908. [DOI] [PubMed] [Google Scholar]
- 13.Clark, D., R. S. Rowlett, J. R. Coleman, and D. F. Klessig. 2004. Complementation of the yeast deletion mutant ΔNCE103 by members of the beta class of carbonic anhydrases is dependent on carbonic anhydrase activity rather than on antioxidant activity. Biochem. J. 379:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 15.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frebort, I., S. Tanaka, K. Matsushita, and O. Adachi. 2000. Cellular localization and metabolic function of n-butylamine-induced amine oxidases in the fungus Aspergillus niger AKU 3302. Arch. Microbiol. 173:358-365. [DOI] [PubMed] [Google Scholar]
- 17.Garciadeblas, B., F. Rubio, F. J. Quintero, M. A. Banuelos, R. Haro, and A. Rodriguez-Navarro. 1993. Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol. Gen. Genet. 236:363-368. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Sanchez, S., S. Aubert, I. Iraqui, G. Janbon, J. M. Ghigo, and C. d'Enfert. 2004. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot. Cell 3:536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of Saccharomyces cerevisiae ura3 and Escherichia coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin, T. J., and R. T. Poulter. 2000. Multiple LTR-retrotransposon families in the asexual yeast Candida albicans. Genome Res. 10:174-191. [DOI] [PubMed] [Google Scholar]
- 22.Gotz, R., A. Gnann, and F. K. Zimmermann. 1999. Deletion of the carbonic anhydrase-like gene NCE103 of the yeast Saccharomyces cerevisiae causes an oxygen-sensitive growth defect. Yeast 15:855-864. [DOI] [PubMed] [Google Scholar]
- 23.Gow, N. A. 1994. Growth and guidance of the fungal hypha. Microbiology 140:3193-3205. [DOI] [PubMed] [Google Scholar]
- 24.Grigull, J., S. Mnaimneh, J. Pootoolal, M. D. Robinson, and T. R. Hughes. 2004. Genome-wide analysis of mRNA stability using transcription inhibitors and microarrays reveals posttranscriptional control of ribosome biogenesis factors. Mol. Cell. Biol. 24:5534-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grynberg, M., J. Topczewski, A. Godzik, and A. Paszewski. 2000. The. Aspergillus nidulans CYSA gene encodes a novel type of serine O-acetyltransferase which is homologous to homoserine O-acetyltransferases. 146:2695-2703. [DOI] [PubMed]
- 26.Habelitz S., M. Balooch, S. J. Marshall, G. Balooch, and G. W. Marshall, Jr. 2002. In situ atomic force microscopy of partially demineralized human dentin collagen fibrils. J. Struct. Biol. 138:227-236. [DOI] [PubMed] [Google Scholar]
- 27.Hirzmann, J., M. Hintz, M. Kasper, T. R. Shresta, A. Taubert, F. J. Conraths, R. Geyer, S. Stirm, H. Zahner, and G. Hobom. 2002. Cloning and expression analysis of two mucin-like genes encoding microfilarial sheath surface proteins of the parasitic nematodes Brugia and Litomosoides. J. Biol. Chem. 277:47603-47612. [DOI] [PubMed] [Google Scholar]
- 28.Hughes, J. D., P. W. Estep, S. Tavazoie, and G. M. Church. 2000. Computational identification of cis-regulatory elements associated with groups of functionally related genes in Saccharomyces cerevisiae. J. Mol. Biol. 296:1205-1214. [DOI] [PubMed] [Google Scholar]
- 29.Huh, W. K., J. V. Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson, J. S. Weissman, and E. K. O'Shea. 2003. Global analysis of protein localization in budding yeast. Nature 425:686-691. [DOI] [PubMed] [Google Scholar]
- 30.Jones, T., N. A. Federspiel, H. Chibana, J. Dungan, S. Kalman, B. B. Magee, G. Newport, Y. R. Thorstenson, N. Agabian, P. T. Magee, R. W. Davis, and S. Scherer. 2004. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 101:7329-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgensen, P., I. Rupes, J. R. Sharom, L. Schneper, J. R. Broach, and M. Tyers. 2004. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 18:2491-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, S. J., H. G. Kim, B. C. Kim, K. Kim, E. H. Park, and C. J. Lim. 2004. Transcriptional regulation of the gene encoding gamma-glutamylcysteine synthetase from the fission yeast Schizosaccharomyces pombe. J. Microbiol. 42:233-238. [PubMed] [Google Scholar]
- 33.Kim, Y. K., Y. Wang, Z. M. Liu, and P. E. Kolattukudy. 2002. Identification of a hard surface contact-induced gene in Colletotrichum gloeosporioides conidia as a sterol glycosyl transferase, a novel fungal virulence factor. Plant J. 30:177-187. [DOI] [PubMed] [Google Scholar]
- 34.Kucsera, J., K. Yarita, and K. Takeo. 2000. Simple detection method for distinguishing dead and living yeast colonies. J. Microbiol. Methods 41:19-21. [DOI] [PubMed] [Google Scholar]
- 35.Kulkarni, R. D., H. S. Kelkar, and R. A. Dean. 2003. An eight-cysteine-containing CFEM domain unique to a group of fungal membrane proteins. Trends Biochem. Sci. 28:118-121. [DOI] [PubMed] [Google Scholar]
- 36.Lan, C. Y., G. Newport, L. A. Murillo, T. Jones, S. Sherer, R. W. Davies, and N. Agabian. 2002. Metabolic specialization associated with phenotypic switching in Candida albicans. Proc. Natl. Acad. Sci. USA 12:14907-14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lan, C. Y., G. Rodarte, L. A. Murillo, T. Jones, R. W. Davis, J. Dungan, G. Newport, and N. Agabian. 2004. Regulatory networks affected by iron availability in Candida albicans. Mol. Microbiol. 53:1451-1469. [DOI] [PubMed] [Google Scholar]
- 38.Li, Y., R. D. Moir, I. K. Sethy-Coraci, J. R. Warner, and I. M. Willis. 2000. Repression of ribosome and tRNA synthesis in secretion-defective cells is signaled by a novel branch of the cell integrity pathway. Mol. Cell. Biol. 20:3843-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchetti, O., J. Bille, U. Fluckiger, P. Eggimann, C. Ruef, J. Garbino, T. Calandra, M. P. Glauser, M. G. Tauber, and D. Pittet. 2004. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000. Clin. Infect. Dis. 38:311-320. [DOI] [PubMed] [Google Scholar]
- 40.Marzluf, G. A. 1997. Molecular genetics of sulfur assimilation in filamentous fungi and yeast. Annu. Rev. Microbiol. 51:73-96. [DOI] [PubMed] [Google Scholar]
- 41.Mateus, C., S. A. Crow, Jr., and D. G. Ahearn. 2004. Adherence of Candida albicans to silicone induces immediate enhanced tolerance to fluconazole. Antimicrob. Agents Chemother. 48:3358-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meister A. 1995. Glutathione metabolism. Methods Enzymol. 251:3-7. [DOI] [PubMed] [Google Scholar]
- 43.Miyoshi, K., C. Shirai, and K. Mizuta. 2003. Transcription of genes encoding trans-acting factors required for rRNA maturation/ribosomal subunit assembly is coordinately regulated with ribosomal protein genes and involves Rap1 in Saccharomyces cerevisiae. Nucleic Acids Res. 31:1969-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller, S. 2004. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol. Microbiol. 53:1291-1305. [DOI] [PubMed] [Google Scholar]
- 45.Murguia, J. R., J. M. Belles, and R. Serrano. 1996. The yeast HAL2 nucleotidase is an in vivo target of salt toxicity. Biol. Chem. 271:29029-29033. [DOI] [PubMed] [Google Scholar]
- 46.Ono, B. I., T. Hazu, S. Yoshida, T. Kawato, S. Shinoda, J. Brzvwczy, and A. Paszewski. 1999. Cysteine biosynthesis in Saccharomyces cerevisiae: a new outlook on pathway and regulation. Yeast 15:1365-1375. [DOI] [PubMed] [Google Scholar]
- 47.Peng, Z., and D. P. Verma. 1995. A rice HAL2-like gene encodes a Ca2+-sensitive 3′(2′),5′-diphosphonucleoside 3′(2′)-phosphohydrolase and complements yeast met22 and Escherichia coli cysQ mutations. J. Biol. Chem. 270:29105-29110. [DOI] [PubMed] [Google Scholar]
- 48.Penninckx, M. J. 2002. An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res. 2:295-305. [DOI] [PubMed] [Google Scholar]
- 49.Perera, T. H., D. W. Gregory, D. Marshall, and N. A. Gow. 1997. Contact-sensing by hyphae of dermatophytic and saprophytic fungi. J. Med. Vet. Mycol. 35:289-293. [DOI] [PubMed] [Google Scholar]
- 50.Ramage, G., K. VandeWalle, J. L. Lopez-Ribot, and B. L. Wickes. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistant in Candida albicans biofilm. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 51.Russo, A., W. DeGraff, N. Friedman, and J. B. Mitchell. 1986. Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer Res. 46:2845-2848. [PubMed] [Google Scholar]
- 52.Schaufler, L. E., and R. E. Klevit. 2003. Mechanism of DNA binding by the ADR1 zinc finger transcription factor as determined by SPR. J. Mol. Biol. 329:931-939. [DOI] [PubMed] [Google Scholar]
- 53.Schinabeck, M. K., L. A. Long, M. A. Hossain, J. Chandra, P. K. Mukherjee, S. Mohamed, and M. A. Ghannoum. 2004. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 48:1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen, X., A. M. Belcher, P. K. Hansma, G. D. Stucky, and D. E. Morse. 1997. Molecular cloning and characterization of lustrin A, a matrix protein from shell and pearl nacre of Haliotis rufescens. J. Biol. Chem. 272:32472-32481. [DOI] [PubMed] [Google Scholar]
- 55.Sohn, K., C. Urban, H. Brunner, and S. Rupp. 2003. EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol. Microbiol. 47:89-102. [DOI] [PubMed] [Google Scholar]
- 56.Soll, D. R. 1997. Gene regulation during high-frequency switching in Candida albicans. Microbiology 143:279-288. [DOI] [PubMed] [Google Scholar]
- 57.Soll, D. R., M. A. Herman, and M. A. Staebell. 1985. The involvement of cell wall expansion in the two modes of mycelium formation of Candida albicans. J. Gen. Microbiol. 131:2367-2375. [DOI] [PubMed] [Google Scholar]
- 58.Suci, P. A., and B. J. Tyler. 2003. A method for discrimination of subpopulations of Candida albicans biofilm cells that exhibit relative levels of phenotypic resistance to chlorhexidine. J. Microbiol. Methods 53:313-325. [DOI] [PubMed] [Google Scholar]
- 59.Takagi, H., K. Yoshioka, N. Awano, S. Nakamori, and B. Ono. 2003. Role of Saccharomyces cerevisiae serine O-acetyltransferase in cysteine biosynthesis. FEMS Microbiol. Lett. 218:291-297. [DOI] [PubMed] [Google Scholar]
- 60.Tzeng, Y. L., A. Datta, C. Strole, V. S. Kolli, M. R. Birck, W. P. Taylor, R. W. Carlson, R. W. Woodard, and D. S. Stephens. 2002. KpsF is the arabinose-5-phosphate isomerase required for 3-deoxy-d-manno-octulosonic acid biosynthesis and for both lipooligosaccharide assembly and capsular polysaccharide expression in Neisseria meningitidis. J. Biol. Chem. 277:24103-24113. [DOI] [PubMed] [Google Scholar]
- 61.Viaene, J., P. Tiels, M. Logghe, S. Dewaele, W. Martinet, and R. Contreras. 2000. MET15 as a visual selection marker for Candida albicans. Yeast 16:1205-1215. [DOI] [PubMed] [Google Scholar]
- 62.Wang, Y., M. Pierce, L. Schneper, C. G. Guldal, X. Zhang, S. Tavazoie, and J. R. Broach. 2004. Ras and Gpa2 mediate one branch of a redundant glucose signaling pathway in yeast. PLoS Biol. 2004 2:610-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watts, H. J., A. A. Very, T. H. Perera, J. M. Davies, and N. A. Gow. 1998. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology 144:689-695. [DOI] [PubMed] [Google Scholar]
- 64.Yang, Z., R. C. Pascon, A. Alspaugh, G. M. Cox, and J. H. McCusker. 2002. Molecular and genetic analysis of the Cryptococcus neoformans MET3 gene and a met3 mutant. Microbiology 148:2617-2625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.