Abstract
Background
Trauma-induced coagulopathy (TIC) occurs in a quarter of trauma patients and is associated with death due to uncontrolled bleeding. Current guidelines recommend viscoelastic testing (VET) to assess coagulopathy and guide transfusions. The Quantra with the QStat Cartridge is a point-of-care (POC) VET device that measures changes in clot stiffness (CS) during coagulation and fibrinolysis using ultrasound detection of resonance. This study aimed to evaluate the performance of the QStat Cartridge in trauma patients compared with rotational thromboelastometry (ROTEM) delta and thromboelastography (TEG) 6s VET devices.
Methods
A multicenter prospective observational study was conducted in adult patients meeting criteria for a full trauma team response at eight US level 1 trauma centers. Citrated blood samples drawn on arrival at the hospital or after blood transfusions were analyzed in parallel on QStat, ROTEM or TEG. Correlation between QStat and equivalent VET measurements was assessed by linear regression. Concordance was assessed by agreement of results relative to device-specific normal reference ranges.
Results
259 severely injured patients were enrolled, yielding 271 samples for analysis. Moderate to strong correlations between QStat and corresponding ROTEM and TEG measurements were observed (r=0.64–0.88). The concordance between CS results was 84.5% for QStat CS and EXTEM A10 and 83.3% for CS and citrated rapid TEG maximum amplitude. For fibrinogen-related results, concordance was 81.5% for QStat fibrinogen contribution to clot stiffness (FCS) and FIBTEM A10 and 93.8% for FCS and citrated functional fibrinogen maximum amplitude. For fibrinolysis measurements, the overall agreement between QStat clot stability to lysis and EXTEM ML or CK-LY30 was 97.5% and 92.9%, respectively.
Conclusion
QStat provides comparable information to the ROTEM delta and TEG 6s in trauma patients and can be useful for diagnosing TIC and guiding treatment. The Quantra’s simplicity of use, ability to deploy at the POC, and rapid availability of results may provide clinicians with a faster, more convenient means to assess and manage TIC.
Level of evidence
Diagnostic test, level II.
Trial registration number
Keywords: blood coagulation tests, fibrinolysis, hemorrhage
WHAT IS ALREADY KNOWN ON THIS TOPIC
Current guidelines for the management of trauma patients recommend the use of whole blood viscoelastic testing (VET) to monitor hemostasis and guide goal-directed resuscitation. In single-center studies, the Quantra System with the QStat Cartridge has shown similar diagnostic performance to rotational thromboelastometry (ROTEM) in severely injured trauma patients.
WHAT THIS STUDY ADDS
This multicenter study evaluated the performance of the QStat Cartridge in trauma patients compared with both the ROTEM delta and thromboelastography 6s VET devices. The study found that the QStat Cartridge provided comparable detection of coagulopathies associated with severe trauma as the other VET devices with a faster time to actionable results.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides further validation of the clinical utility of the Quantra System with the QStat Cartridge in the trauma setting.
Background
Approximately 25% of severely injured patients present to the emergency department (ED) with evidence of trauma-induced coagulopathy (TIC), a condition associated with uncontrolled bleeding and 35–50% mortality.1 Therefore, rapid assessment and appropriate management of coagulopathy is critical for survival. The initial evaluation of TIC is focused on characterizing coagulation deficits to guide transfusions and prevent death from hemorrhage.2 Once the patient’s hemostatic condition is stabilized, the concern shifts toward the risk for thrombotic complications, as the coagulation status of trauma patients often transitions from a hypocoagulable to a hypercoagulable state.1
The pathophysiology of TIC is complex and multifaceted, including compromised thrombin generation, platelet dysfunction, fibrinogen depletion and dysfunction, and dysregulated fibrinolysis.1 Standard coagulation tests are insufficient to reflect this complexity as they only assess thrombin generation, whereas whole blood viscoelastic testing (VET) devices provide a rapid comprehensive analysis of the dynamics of clot formation including time to initiation, clot strength and dissolution.3 Consequently, VET has emerged as a superior diagnostic and monitoring tool in trauma.4,7 Guidelines for the management of bleeding and coagulopathy after trauma recommend the early and repeat monitoring of hemostasis using VET for guiding goal-directed resuscitation.2 8 9
Thromboelastometry (ROTEM; Werfen, Bedford, MA) and thromboelastography (TEG; Haemonetics, Boston, MA) are two whole blood VET methods that have been implemented to monitor global hemostatic function in trauma patients and guide goal-directed transfusion of blood products.46,8 Although marketed for use at the point of care, the initial ROTEM delta and TEG 5000 devices required manual patient blood sample and reagent handling, which hampered wide-scale implementation in trauma and critical care settings.10 Subsequently, these devices have been supplanted by more portable automated cartridge-based bedside systems (ROTEM sigma and TEG 6s).10
The Quantra System is an FDA-cleared cartridge-based point-of-care VET device that measures changes in clot stiffness by quantification of shear modulus using high-frequency ultrasound (Sonic Estimation of Elasticity via Resonance (SEER) sonorheometry).11 The Quantra QPlus Cartridge is indicated for use in cardiac and major orthopedic surgery and includes tests for assessing coagulation factor activity, presence of heparin anticoagulation, fibrinogen contribution and platelet contribution to clot stiffness.12 The QPlus Cartridge has been validated in a number of prospective studies which evidenced a strong correlation of its clot stiffness, platelet and fibrinogen measurements with those determined by TEG or ROTEM-based devices and/or with standard coagulation tests.13,18 The Quantra QStat Cartridge includes a test to assess fibrinolysis in addition to tests for assessing coagulation factor activity, fibrinogen contribution and platelet contribution to clot stiffness.12 Previously, the QStat Cartridge has been evaluated favorably in a multicenter study in patients undergoing liver transplant and in single-center studies in trauma patients in comparison to ROTEM delta as well as standard coagulation tests.19,23 The objective of this study was to evaluate the performance of the QStat Cartridge in trauma patients in a large multicenter setting in comparison to ROTEM delta and TEG 6s. We hypothesized that the QStat Cartridge would show equivalent diagnostic performance to both devices.
Methods
Study design
This was a multicenter, prospective, observational study evaluating the performance of the Quantra Hemostasis Analyzer with the QStat Cartridge in adult trauma patients in comparison to ROTEM delta and TEG 6s, two other FDA-cleared VET platforms. This assessment includes correlation and concordance between QStat measurements with equivalent ROTEM delta and TEG 6s measurements.
The study was conducted at eight American College of Surgeons’ designated level 1 trauma centers in the USA from March 2020 through July 2022. Seven of the eight centers were academic institutions. Three centers were located in large urban areas and five in small to medium-sized urban areas. The study was registered on ClinicalTrials.gov (NCT04312958). The study was reviewed and approved by a central institutional review board (IRB). Each site registered the study with their local IRB, which elected to either retain local oversight of the study or to cede oversight to the central IRB.
Study population
The study population consisted of adult patients (>18 years) experiencing major trauma who met local criteria for a full trauma team response. Details were collected in the study electronic Case Report Form regarding the type and extent of the subject’s injuries, vital signs on presentation in the ED, and other information regarding the traumatic event to confirm that the subject met inclusion criteria. Patients were excluded from the study if incarcerated, pregnant, concurrently enrolled in a study that could have confounded the results, affected by a condition that, in the opinion of the clinical team, would have posed additional risks, or if required, written consent could not be obtained.
Sample collection, testing and data processing
For each enrolled subject, whole blood samples were collected in 2.7 mL evacuated tubes containing 3.2% sodium citrate on arrival in the ED and in some cases, at later time points after administration of blood products. Samples for QStat analysis were collected in parallel with samples drawn for analysis on the ROTEM delta or TEG 6s. ROTEM delta testing (INTEM, EXTEM, and FIBTEM assays) was performed at seven sites, and TEG 6s testing (Global Hemostasis with Lysis Cartridge or Global Hemostasis Cartridge) was done at three sites. Two sites performed testing on both comparator devices. Quantra testing was performed by trained clinical or research staff at the point of care in the trauma operating room (OR) (two sites), ancillary laboratory near the OR (one site), or in a research laboratory (five sites). ROTEM or TEG 6s testing was performed in a clinical laboratory by trained laboratory professionals (four sites), in a research laboratory by trained research staff (three sites), or in the trauma OR at the point of care by clinical staff (one site).
Data for each subject were collected using the Medrio eClinical electronic data capture software (Medrio LLC, San Francisco, CA). Data were source verified by study monitors who reviewed source documents available at each of the study sites. The principal investigator at each site reviewed and approved each completed and monitored subject electronic casebook. After the database lock, a SAS-ready export file was created for statistical analysis.
Information documented in the study database from the patient medical chart included patient demographics (age, gender, race); the reason for admittance to the hospital/injury characteristics; trauma severity score (depending on site, either Injury Severity Score (ISS) or Revised Trauma Score (RTS)); time blood samples were collected; time diagnostic tests were performed; results of diagnostic tests performed including the Quantra with the QStat Cartridge, ROTEM delta, TEG 6s and standard laboratory coagulation testing (when available); and blood products and relevant medications administered within 24 hours of Quantra testing.
Quantra and QStat Cartridge
The Quantra is a fully integrated and automated analyzer that performs whole blood coagulation tests at the point of care.11 12 The Quantra uses SEER sonorheometry, an ultrasound-based technology, to measure the change in stiffness (shear modulus expressed in hectopascals) of a citrated whole blood sample during the process of coagulation and fibrinolysis.11 The QStat Cartridge, developed for use with the Quantra analyzer, is a single-use disposable containing lyophilized reagents that performs four measurements in parallel on citrated whole blood samples.12 The cartridge outputs five measurements: clot time (CT), clot stiffness (CS), fibrinogen contribution to clot stiffness (FCS), platelet contribution to clot stiffness (PCS) and clot stability to lysis (CSL).12 CSL is calculated as the normalized difference between the clot stiffness change during a period of 15 minutes after maximum clot stiffness is achieved, assessed in the presence or absence of tranexamic acid (TXA). The CSL reportable range extends from 100% (no fibrinolysis) to 10% (significant fibrinolysis). A CSL value below the threshold of 90% indicates that the reduction in CS is due to the influence of fibrinolysis.
The Quantra analyzers and the QStat Cartridges used in the study were labelled for investigational use only, and the results generated by the system were blinded to the clinical team and not used to alter or influence existing standards of care.
Statistical methods
All analyses were performed using SAS V.9.4 (SAS Institute) or R V.4.1.2. Descriptive analyses included demographics, trauma characteristics, and summary statistics for clinical endpoints. These were generated using the eligible subject set consisting of all enrolled subjects whose data are included in the study database. Samples were eligible for inclusion in the analysis data set if data from the Quantra and at least one of the ROTEM or TEG assays were available to allow at least one Quantra to ROTEM or TEG comparison.
Linear regression models were used to evaluate the relationship between device measurements. The analyses performed included Quantra CT versus ROTEM INTEM CT or TEG 6s citrated kaolin reaction time (CK-R); Quantra CS versus ROTEM EXTEM A10 or TEG 6s citrated rapid TEG maximum amplitude (CRT-MA); Quantra FCS versus ROTEM FIBTEM A10 or TEG 6s citrated functional fibrinogen maximum amplitude (CFF-MA); and Quantra PCS versus ROTEM PLATEM A10 (EXTEM A10–FIBTEM A10). Because the relationship between clot stiffness (A in millimeters, mm) and clot elasticity (G in hectopascals) is not linear, ROTEM and TEG clot stiffness amplitudes were converted to clot elasticity units using the following formula:hectopascals (hPa)=(5×A)/(100-A).24 Point estimates of Pearson’s correlation coefficient were reported and presented with linear regression fits for each of these models.
To assess the concordance between device clot time and clot stiffness measurements, paired test results were assigned into normal, hypocoagulable or hypercoagulable categories based on whether the result was within or outside a given measurement’s normal reference range as provided by the manufacturer. For clot stiffness measurements, only total clot stiffness (EXTEM A10 and CRT-MA) and fibrinogen contribution to clot stiffness (FIBTEM A10 and CFF-MA) measurements were considered because there are no valid reference ranges for the platelet component of ROTEM delta (EXTEM A10–FIBTEM A10) and TEG 6s (CRT-MA–CFF-MA).
To assess the concordance between device fibrinolysis measurements, paired test results were assigned into lysis positive and lysis negative categories based on the following definitions:
Lysis positive: QStat CSL<90%, ROTEM EXTEM ML>15%, TEG 6s CK-LY30>2.6%.
Lysis negative: QStat CSL>90%, ROTEM EXTEM ML≤15%, TEG 6s CK-LY30≤2.6%.
The CSL threshold of 90% represents the lower 95% CI around the lower limit of the normal reference range. The CK-LY30 threshold of 2.6% represents the upper limit of the normal reference range. The EXTEM ML threshold of 15% has been reported previously in the trauma literature.25 Overall agreements of assignments were calculated with a 95% CI generated using a non-parametric bootstrap approach with resampling and replacement performed 5000 times.
The time to actionable results was determined for Quantra and TEG 6s as the mean time from test initiation (device start time) to the time that clot time (CT, R) and clot stiffness (CS, CRT-MA) results were available. A similar time to result comparison was not performed for the ROTEM because all ROTEM delta tests were run for 60 minutes to enable calculation of EXTEM ML.
Sample size determination
Enrollment for this study was targeted at 254 subjects, so that a minimum of 127 samples (one per subject) could be obtained per device arm to enable a comparison of Quantra to ROTEM delta and Quantra to TEG 6s. The target sample size was informed by a simulation study conducted to estimate the number of samples that would yield sufficient power to achieve greater than 80% overall agreement between the fibrinolysis measurements by the Quantra versus ROTEM or TEG 6s devices. A bootstrap method was applied to simulate 10 000 clinical trials informed by data from a pilot study. The sample size estimated using this approach was 115, with 80% power. Assuming a dropout rate of 10%, the total number of subjects required for enrollment per study device arm was 127.
Results
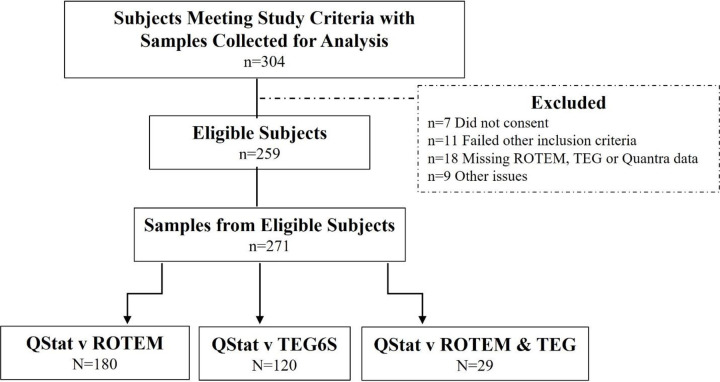
The eligible study population consisted of 259 trauma patients from a total of 304 subjects enrolled across all sites (figure 1). Subjects were excluded after enrollment due to deferred consent not obtained (n=7), inclusion criteria no longer met (n=11), no data available for either Quantra, ROTEM delta or TEG 6s (n=18), and other reasons (n=9). From the eligible subject set, a total of 271 paired samples were obtained containing either matching Quantra and ROTEM delta results (n=180), Quantra and TEG 6s results (n=120) or results across all three platforms (n=29). The majority of samples (55.3%) were collected on admittance to the ED. The remainder was drawn later, either before (6.7%) or after (38.0%) administration of blood products.
Figure 1. Flow diagram of subject selection and sample collection. ROTEM, rotational thromboelastometry; TEG, thromboelastography.
Table 1 provides a description of the study population. Most subjects were male (81.1%), with a median age (IQR) of 43 years (31–58). Blunt and penetrating injuries accounted for 51.0% and 39.4% of cases, respectively. Injury severity was assessed by either the ISS or RTS. Nearly half of subjects (48%) had an ISS>25, or RTS<11, indicative of severe injuries requiring an immediate response.
Table 1. Description of the study cohort (n=259).
| Variable | Value |
|---|---|
| Male sex | 210 (81.1) |
| Age (years) | 43 (31, 58) |
| Race | |
| Caucasian | 164 (63.3) |
| Black/African American | 48 (18.5) |
| Asian | 11 (4.3) |
| Other | 36 (13.9) |
| Admission characteristics | |
| Systolic blood pressure | 124 (104, 146) |
| Heart rate | 98 (94, 100) |
| Respiration rate | 20 (16, 23) |
| Body temperature | 36.5 (36.1, 36.8) |
| Mechanism of injury | |
| Blunt | 132 (51.0) |
| Penetrating | 102 (39.4) |
| Other | 25 (9.6) |
| Traumatic brain injury | 91 (35.1) |
| Injury score | |
| Injury Severity Score (ISS), n=98 | 23 (9, 33) |
| Revised Trauma Score (RTS), n=126 | 11 (8, 12) |
| Missing, n=35 | |
| GCS at admission | 14 (4, 15) |
| Intubated | 81 (31.3) |
| CPR performed | 19 (7.3) |
| Massive transfusion | 72 (31.7) |
| Outcome | |
| Discharged from hospital within 15 days | 171 (66.0) |
| Still admitted after 15 days | 42 (16.2) |
| Death during hospital stay | 46 (17.8) |
Data expressed as number (%) or median (IQR).
CPR, cardiopulmonary resuscitation; GCS, Glasgow Coma Scale.
Summary statistics for the comparison of QStat and ROTEM delta or QStat and TEG 6s clot time and clot stiffness measurements are shown in table 2. All three devices identified coagulopathies typically observed in trauma patients, although to varying degrees. These included low clot stiffness values and enhanced thrombin generation characterized by shorter times to clot initiation relative to normal reference ranges. QStat CS values were low for 39.9% and 30.7% of samples, whereas the percentage of low EXTEM A10 and CRT-MA values was 33.9% and 14.9%, respectively. Additionally, 49.4% and 45.4% of samples analyzed on QStat had a short CT value, while INTEM CT and CK-R values were short for 13.4% and 33.6% of samples, respectively.
Table 2. Quantra QStat, ROTEM delta and TEG 6s parameters: summary statistics and concordance.
| Comparison | n | Overall concordance% (95% CI) | QStat | ROTEM delta | P value |
|---|---|---|---|---|---|
| QStat CT vs. INTEM CT (mean, SD) | 164 | 53.70% (45.7% to 61.4%) | 125 (31.8) sec | 167 (48.5) sec | |
| Below RR, n (%) | 81 (49.4) | 22 (13.4) | <0.001* | ||
| Above RR, n (%) | 11 (6.7) | 22 (13.4) | 0.066 | ||
| Within RR, n (%) | 72 (43.9) | 120 (73.2) | <0.001* | ||
| RR: CT: 121–175 s; INTEM CT: 121–208 s | |||||
| QStat CS vs. EXTEM A10 (mean, SD) | 168 | 84.50% (78.0% to 89.5%) | 16.7 (7.4) hPa | 50 (10.0) mm | |
| Below RR, n (%) (‘hypocoagulable’) | 67(39.9) | 57 (33.9) | 0.309 | ||
| Above RR, n (%) (‘hypercoagulable’) | 3 (1.8) | 5 (3.0) | 0.721 | ||
| Within RR, n (%) (‘normal’) | 98 (58.3) | 106 (63.1) | 0.434 | ||
| RR: CS: 14.0–35.4 hPa; EXTEM A10: 47–67 mm | |||||
| QStat FCS vs. FIBTEM A10 (mean, SD) | 168 | 81.50% (74.7% to 86.9%) | 1.9 (1.3) hPa | 14 (6.7) mm | |
| Below RR, n (%) (‘hypocoagulable’) | 11 (15.5) | 16 (9.5) | 0.138 | ||
| Above RR, n (%) (‘hypercoagulable’) | 5 (3.0) | 13 (7.8) | 0.090 | ||
| Within RR, n (%) (‘normal’) | 137 (81.5) | 139 (82.7) | 0.887 | ||
| RR: FCS: 0.9–4.2 hPa; FIBTEM A10: 7–22 mm | |||||
| Comparison | n | Overall concordance | QStat | TEG 6s | P value |
| QStat CT vs. CK-R (mean, SD) | 119 | 65.50% (56.2% to 73.9%) | 130 (34.9) sec | 5.4 (1.9) min | |
| Below RR, n (%) | 54 (45.4) | 40 (33.6) | 0.087 | ||
| Above RR, n (%) | 11 (9.2) | 5 (4.2) | 0.196 | ||
| Within RR, n (%) | 54 (45.4) | 74 (62.2) | 0.014* | ||
| RR: CT: 121–175 s; CK-R: 4.6–9.1 min | |||||
| QStat CS vs. CRT-MA (mean, SD) | 114 | 83.30% (74.9% to 89.4%) | 16.7 (6.4) hPa | 57.5 (6.8) mm | |
| Below RR, n (%) (‘hypocoagulable’) | 35 (30.7) | 17 (14.9) | 0.007* | ||
| Above RR, n (%) (‘hypercoagulable’) | 1 (0.8) | 0 (0) | 1.00 | ||
| Within RR, n (%) (‘normal’) | 78 (68.4) | 97 (85.1) | 0.005* | ||
| RR: CS: 14.0–35.4 hPa; CRT-MA: 52–70 mm | |||||
| QStat FCS vs. CFF-MA (mean, SD) | 113 | 93.80% (87.2% to 97.3%) | 1.9 (1.2) hPa | 18.5 (5.4) mm | |
| Below RR, n (%) (‘hypocoagulable’) | 16 (14.2) | 21 (18.6) | 0.472 | ||
| Above RR, n (%) (‘hypercoagulable’) | 4 (3.5) | 4 (3.5) | 1.00 | ||
| Within RR, n (%) (‘normal’) | 93 (82.3) | 88 (77.9) | 0.505 | ||
| RR: FCS: 0.9–4.2 hPa; CFF-MA: 15–32 mm |
Values are expressed as mean (SD), then number of samples (%) below or above the reference range (RR) for each respective parameter. The manufacturer’s RR for each parameter is indicated in bold. Overall concordance refers to overall percent agreement (95% CI) between three categories: below RR, above RR, within RR.
Statistical significance defined as p<0.05.
CFF-MA, citrated functional fibrinogen maximum amplitude; CK-R, citrated kaolin reaction time; CRT-MA, citrated rapid TEG maximum amplitude; CS, clot stiffness; CT, clot time; FCS, fibrinogen contribution to clot stiffness; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
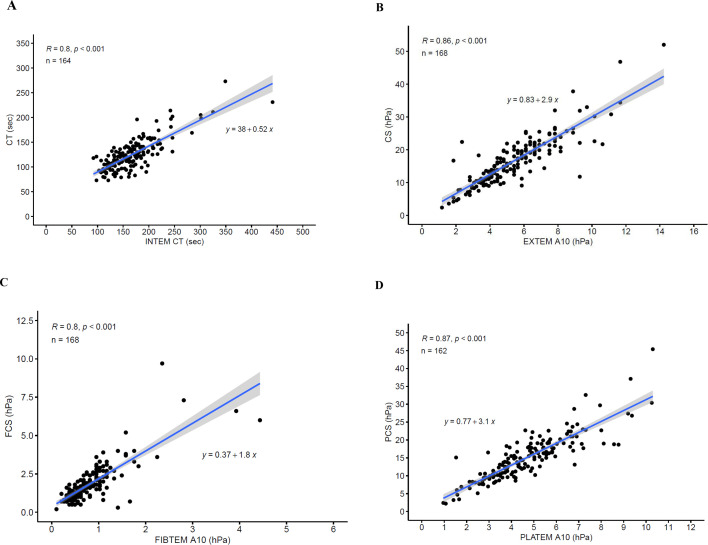
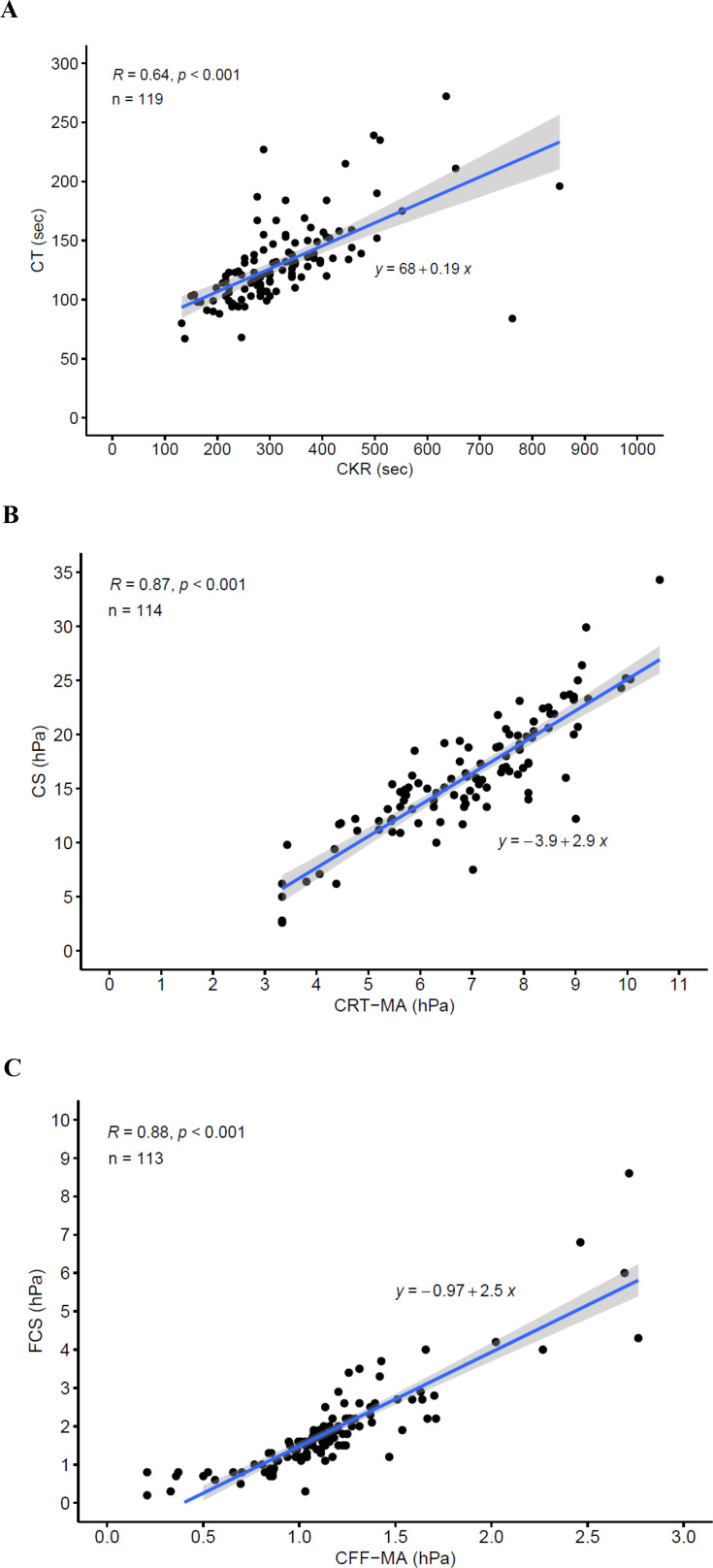
Linear regression analysis demonstrated moderate to strong positive correlations between QStat measurements and the corresponding ROTEM delta and TEG 6s measurements with r values ranging from 0.80 to 0.88 for clot stiffness measurements and 0.64 to 0.80 for clot time measurements (table 3). Scatter plots of the comparisons are shown in figure 2 (QStat vs. ROTEM delta) and figure 3 (QStat vs. TEG 6s).
Table 3. Correlation of Quantra QStat with ROTEM delta and TEG 6s parameters.
| Comparison | n | R value (P value) | Regression equation |
|---|---|---|---|
| Quantra QStat vs. ROTEM delta | |||
| CT (sec) vs. INTEM CT (sec) | 164 | 0.80 (<0.001) | CT=0.52 (INTEM CT)+38 |
| CS (hPa) vs. EXTEM A10 (hPa) | 168 | 0.86 (<0.001) | CS=2.9 (EXTEM A10)+0.83 |
| FCS (hPa) vs. FIBTEM A10 (hPa) | 168 | 0.80 (<0.001) | FCS=1.8 (FIBTEM A10)+0.37 |
| PCS (hPa) vs. PLATEM A10 (hPa) | 162 | 0.87 (<0.001) | PCS=3.1 (PLATEM A10)+0.77 |
| Quantra QStat vs. TEG 6s | |||
| CT (sec) vs. CK-R (min) | 119 | 0.64 (<0.001) | CT=0.19 (CK-R)+68 |
| CS (hPa) vs. CRT-MA (hPa) | 114 | 0.87 (<0.001) | CS=2.9 (CRT-MA)−3.9 |
| FCS (hPa) vs. CFF-MA (hPa) | 113 | 0.88 (<0.001) | FCS=2.5 (CFF-MA)−0.97 |
Clot stiffness values for ROTEM delta and TEG 6s (amplitude in millimeters) have been converted to elasticity in units of hectopascals (hPa) by a validated formula (14).
CFF-MA, citrated functional fibrinogen maximum amplitude; CK-R, citrated kaolin reaction time; CRT-MA, citrated rapid TEG maximum amplitude; CS, clot stiffness; CT, clot time; FCS, fibrinogen contribution to clot stiffness; PCS, platelet contribution to clot stiffness; PLATEM A10, EXTEM A10−FIBTEM A10; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
Figure 2. Scatter plots showing correlation between Quantra QStat and related ROTEM delta measurements. (A) QStat CT vs. INTEM CT. (B) QStat CS vs. EXTEM A10. (C) QStat FCS vs. FIBTEM A10. (D) QStat PCS vs. PLATEM A10. The CS values for ROTEM delta (EXTEM and FIBTEM A10) were converted from clot amplitude in units of millimeters to elasticity in units of hectopascals using a validated conversion formula (24). PLATEM A10 is not a parameter output by the ROTEM delta but was calculated offline by subtracting FIBTEM A10 from EXTEM A10 after conversion to hectopascals. CS, clot stiffness; CT, clot time; FCS, fibrinogen contribution to clot stiffness; PCS, platelet contribution to clot stiffness; ROTEM, rotational thromboelastometry.
Figure 3. Scatter plots showing correlation between Quantra QStat and related TEG 6s measurements. (A) QStat CT vs. CK-R. (B) QStat CS vs. CRT-MA. (C) QStat FCS vs. CFF-MA. The CS values for TEG 6s (CRT-MA and CFF-MA) were converted from clot amplitude in units of millimeters to elasticity in units of hectopascals using a validated conversion formula (24). CFF-MA, citrated functional fibrinogen maximum amplitude; CK-R, citrated kaolin reaction time; CRT-MA, citrated rapid TEG maximum amplitude; CS, clot stiffness; CT, clot time; FCS, fibrinogen contribution to clot stiffness; TEG, thromboelastography.
Concordance between device clot time and clot stiffness measurements based on assignment as below, above or within the manufacturer’s normal reference ranges is shown in table 2. The concordance between QStat and the corresponding ROTEM delta and TEG 6s test results was strongest for the clot stiffness measurements. Results for CS and EXTEM A10 and for CS and CRT-MA were concordant for 84.5% and 83.3% of samples, respectively. A similar strong concordance was observed for the fibrinogen-related measurements, with 81.5% concordance for FCS and FIBTEM A10 and 93.8% for FCS and CFF-MA. The concordance between clot time results across the devices was weaker, with results for CT and INTEM CT concordant for 53.7% of samples and results for CT and CK-R for 65.5% of samples.
Concordance between the device clot lysis measurements is shown in table 4. QStat CSL and ROTEM delta EXTEM ML were concordant for 97.5% of samples. Both devices categorized 6.9% of samples as fibrinolysis positive. QStat CSL and TEG 6s CK-LY30 were concordant for 92.9% of samples. QStat CSL categorized 7.1% as fibrinolysis positive, whereas this was 9.4% for CK-LY30.
Table 4. Concordance of Quantra QStat with ROTEM delta and TEG 6s fibrinolysis parameters.
| ROTEM delta EXTEM | ||
|---|---|---|
| ML>15% (fibrinolysis+) | ML≤15% (fibrinolysis−) | |
| Quantra QStat | ||
| CSL<90% (fibrinolysis+) | 9 | 2 |
| CSL≥90% (fibrinolysis−) | 2 | 147 |
| Overall concordance | 97.5% (93.3% to 99.2%) | |
| TEG 6s CK-LY30 | ||
| LY30>2.6% (fibrinolysis+) | LY30≤2.6% (fibrinolysis−) | |
| Quantra QStat | ||
| CSL<90% (fibrinolysis+) | 4 | 2 |
| CSL≥90% (fibrinolysis−) | 4 | 75 |
| Overall concordance | 92.9% (84.7% to 97.1%) | |
Concordance is expressed as % (95% CI).
CK-LY30, citrated kaolin percent clot lysis at 30 min after maximum amplitude; CSL, clot stability to lysis; ML, maximum lysis (ie, percent clot lysis at 60 min); ROTEM, rotational thromboelastometry; TEG, thromboelastography.
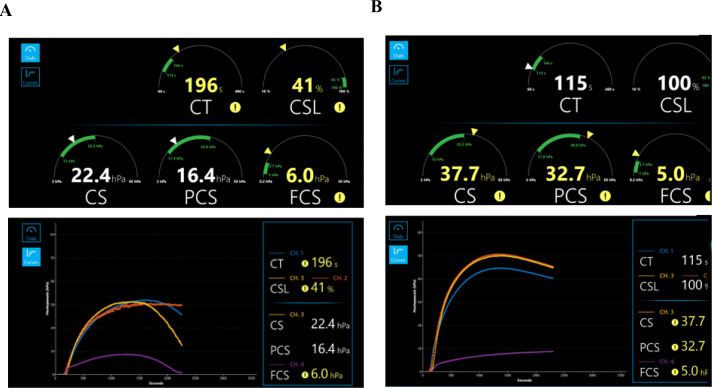
An analysis of the discordant lysis samples revealed six samples that were lysis positive based on ROTEM or TEG test results but lysis negative based on Quantra. Of these, five samples showed evidence of clot retraction on the Quantra. This is observed as a similar reduction in CS both in the presence and absence of TXA that was mistaken for fibrinolysis positive on the other two devices. Figure 4 shows Quantra QStat test results of two trauma patients, one with a fibrinolysis positive signal and the other a fibrinolysis negative signal due to clot retraction. The remaining discordant sample gave a false positive signal for fibrinolysis on the ROTEM due to a mechanical error of the system.
Figure 4. QStat differentiates fibrinolysis from clot retraction. QStat dials and curves generated for samples from two trauma patients: (A) indicating the presence of fibrinolysis and (B) indicating the absence of fibrinolysis with evidence of clot retraction. The CSL parameter is calculated as the normalized difference between the CS change after maximum CS assessed in the presence and absence of TXA. (A) Sample shows a fibrinolysis positive signal (CSL is 41%, below the threshold of 90%), with a rapid decline in CS in the absence of TXA (channel 3—yellow), while there is no decline in the presence of TXA (channel 2—orange). (B) Sample shows a negative fibrinolysis signal (CSL is 100%, above the threshold of 90%). Although there is a decline in CS in the absence of TXA (channel 3—yellow), this decline is similar in the presence of TXA (channel 2—orange). Consequently, the observed decline in CS is not due to fibrinolysis but is caused by clot retraction. CS, clot stiffness; CSL, clot stability to lysis; CT, clot time; FCS, fibrinogen contribution to clot stiffness; PCS, platelet contribution to clot stiffness; TXA, tranexamic acid.
An analysis of the time to actionable results, determined as the mean time from test initiation (start time) to the time that final CT and CS results were available, showed that for the QStat Cartridge, final CT and CS results were available on average at 2.6 and 9.0 minutes after test initiation, respectively. By comparison, results for the corresponding TEG 6s measurements CK-R and CRT-MA were available at 5.0 and 29.3 minutes.
Discussion
Previously, the Quantra QStat System has been compared with the ROTEM delta VET device and conventional coagulation tests in severely injured trauma patients in single-center studies.19,21 This study is the first multicenter study comparing Quantra QStat with ROTEM delta and TEG 6s VET devices in this clinical setting. Based on correlation and concordance analysis, the study results showed that the Quantra QStat System provides comparable information as the other two VET devices.
Linear regression analysis exhibited a strong correlation between Quantra QStat and equivalent ROTEM delta measurements, with r values of 0.80–0.87, in line with previous single-center cohort studies in severely injured trauma patients and patients who had a liver transplant.19,23 Our study also showed a similar moderate to strong correlation between Quantra QStat and equivalent TEG 6s measurements (r values 0.64–0.88).
Correlation does not mean concordance, and shorter clot time and higher clot stiffness values were observed on Quantra in comparison to ROTEM or TEG. The differences in clot times and clot stiffnesses observed in our study are consistent with previous reports in cardiac surgery,13,18 trauma19 20 and liver transplantation.23 In all these comparisons, despite the conversion of ROTEM and TEG amplitudes expressed in millimeters to shear modulus units of hectopascals, clot stiffness values on Quantra are twofold to threefold higher. This is likely caused by differences in methodology, such as frequency of the oscillating force and applied shear stress, which may cause a downward bias for ROTEM and TEG estimates compared with SEER sonorheometry.26
Because of methodological differences, a ‘gold standard’ for VET does not exist, and in each clinical setting device-specific measurement threshold and target values will be needed to guide transfusion.27 We therefore analyzed the concordance between device clot time and clot stiffness measurements based on assignment as within, below or above the normal reference range. This showed a high degree of concordance between total clot stiffness results as well as fibrinogen-related clot stiffness results, whereas concordance between clot time results was only moderate. Concordance between platelet-related clot stiffness values could not be analyzed because ROTEM delta and TEG 6s do not report a comparable platelet clot stiffness measurement with validated normal reference ranges.
Despite this moderate to high level of concordance, some heterogeneity between devices was observed. Based on total clot stiffness, Quantra and ROTEM similarly categorized samples as hypocoagulable, whereas TEG categorized fewer samples as hypocoagulable. Based on clot time, Quantra and TEG identified more samples with short CT than did ROTEM. Short clot times are observed in trauma patients experiencing severe tissue damage, and they reflect rapid activation of the coagulation cascade triggered by the release of large amounts of tissue factor.28 A consequence of such rapid clot formation is the subsequent consumption of clotting factors leading to uncontrolled hemorrhage. This heterogeneity across devices may reflect differences in sensitivity for detecting TIC. Moreover, our concordance analysis was based on the manufacturer-reported normal reference range, which is an arbitrary threshold for depicting hypocoagulable or hypercoagulable states in severely injured trauma patients. In at least one study, the use of normal reference values as transfusion thresholds has led to overtransfusions.29 Simultaneous testing of laboratory markers of coagulopathy such as PT-INR, platelet count and fibrinogen may have shed further light on this heterogeneity, but this was not part of the current study objectives. Previous studies in severely injured trauma patients in which both QStat and standard laboratory tests were conducted showed the capability of the Quantra measurements to detect TIC with good accuracy.20 21
Based on the device-specific thresholds for predicting clot lysis, hyperfibrinolysis was observed in 6.9% (Quantra and ROTEM) to 9.4% (TEG) of samples. A similar low frequency was reported in a previous single-center trauma cohort.19 There was a strong concordance between the devices for categorizing samples as hyperfibrinolytic for Quantra versus ROTEM and for Quantra versus TEG. Previous comparative studies between Quantra and ROTEM in trauma and liver transplantation reported similar concordance.19 23
Analysis of the discordant samples for which ROTEM or TEG predicted hyperfibrinolysis but the QStat CSL measurement did not, highlights the importance of measuring clot lysis with and without fibrinolysis inhibition to rule out a reduction in clot stiffness attributable to platelet-mediated clot retraction rather than to clot lysis.30 Interpretation of clot lysis results based on a single test may falsely identify a decline in clot stiffness that is caused by clot retraction as fibrinolysis, as previously reported for kaolin-activated TEG.31 The Quantra CSL measurement integrates the decline in clot stiffness in the presence and absence of TXA and, therefore, automatically corrects for the presence of clot retraction. This type of interpretation is also possible with ROTEM delta if APTEM (with the fibrinolysis inhibitor aprotinin) is run in parallel with EXTEM, but this differential must be assessed offline by the user.
We compared the time to actionable results across devices and found that final CT and CS results were available on average at two to three times faster than the corresponding TEG 6s measurements CK-R and CRT-MA. In contrast to TEG and ROTEM, the Quantra CSL measurement is not calculated at a fixed time point but is assessed based on the change in clot stiffness during a period of 15 minutes after maximum clot stiffness has been obtained. A previous single-center trauma study reported that on average the Quantra CSL measurement was available within 44.2 minutes from test initiation.19 Apart from a rapid turnaround time, other operational advantages of the Quantra QStat System include its automated cartridge-based concept, which eliminates any manual pipetting of blood sample and reagent, and a unique primary output in the form of dial indicators (figure 4).12 The latter enables easy reading and interpretation of results, which has been reported as a barrier with curve-based TEG and ROTEM outputs that require operator training.32 33
Our study has several limitations. Although ROTEM delta or TEG 6s test results were used for the clinical management of the enrolled subjects, the clinicians were blinded to the Quantra QStat test results, and consequently, its direct clinical impact could not be determined. Not all samples were collected on ED admission, with a considerable proportion (44.7%) at later time points, most notably after administration of blood products. This difference in the timing of blood collection may have resulted in a very heterogeneous population with respect to the degree and phenotype of TIC, which may present as hypocoagulable or hypercoagulable.1 Phenotypic TIC characterization by simultaneous testing with standard laboratory tests was not performed as this was primarily a method comparison study of VET devices. The study focused on the determination of hyperfibrinolysis based on device-specific measurement thresholds. For TEG and ROTEM, fibrinolysis measurement thresholds have also been defined for the detection of hypofibrinolysis (fibrinolysis shutdown), which has been documented as a frequent phenomenon in trauma with worse outcomes.33 34 The study was not designed to compare the identification of hypofibrinolysis because a threshold for the Quantra CSL measurement has not been established.
In conclusion, the present multicenter study shows that the Quantra QStat Cartridge provides comparable information as the ROTEM delta and TEG 6s in trauma patients. This supports the use of QStat as an aid to assess coagulation and fibrinolysis status in trauma patients and guide treatment. Several studies have shown how Quantra measurements could predict commonly used ROTEM or laboratory-based transfusion thresholds in surgical patients and trauma patients.20 23 35 The Quantra’s simplicity of use, ability to deploy at the point of care, and availability of rapid, easy-to-interpret results may provide clinicians with a faster, more reliable means to assess coagulation/fibrinolysis status in trauma patients. However, future studies are needed to define and validate a transfusion algorithm to guide optimal patient care.
Supplementary material
Acknowledgements
The authors thank Robert Wiard and Mirta Sanchez-Illan for support with clinical study management, Maxwell Marion-Spencer, MS, for support with statistical analyses, and Wim Houdijk, PhD, for medical writing support.
Footnotes
Funding: The study was funded by HemoSonics, LLC.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: reply Reply
A central institutional review board (IRB) approved the protocol with a waiver of written informed consent (Advarra, Pro 00041164). Each site registerd the study with their local IRB which elected to either retain local oversight of the study or cede oversight to the central IRB. Two local IRBs approved a deferred consent process.
Data availability statement
Data are available upon reasonable request.
References
- 1.Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, Schöchl H, Hunt BJ, Sauaia A. Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021;7:30. doi: 10.1038/s41572-021-00264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossaint R, Afshari A, Bouillon B, Cerny V, Cimpoesu D, Curry N, Duranteau J, Filipescu D, Grottke O, Grønlykke L, et al. The European guideline on management of major bleeding and coagulopathy following trauma: sixth edition. Crit Care. 2023;27:80. doi: 10.1186/s13054-023-04327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen T, Haas T, Cushing MM. The strengths and weaknesses of viscoelastic testing compared to traditional coagulation testing. Transfusion. 2020;60 Suppl 6:S21–8. doi: 10.1111/trf.16073. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263:1051–9. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zipperle J, Schmitt FCF, Schöchl H. Point-of-care, goal-directed management of bleeding in trauma patients. Curr Opin Crit Care. 2023;29:702–12. doi: 10.1097/MCC.0000000000001107. [DOI] [PubMed] [Google Scholar]
- 6.Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ Jr, Mattox KL, Suliburk J. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74:378–85. doi: 10.1097/TA.0b013e31827e20e0. [DOI] [PubMed] [Google Scholar]
- 7.Baksaas-Aasen K, Van Dieren S, Balvers K, Juffermans NP, Næss PA, Rourke C, Eaglestone S, Ostrowski SR, Stensballe J, Stanworth S, et al. Data-driven Development of ROTEM and TEG Algorithms for the Management of Trauma Hemorrhage: A Prospective Observational Multicenter Study. Ann Surg. 2019;270:1178–85. doi: 10.1097/SLA.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 8.Bugaev N, Como JJ, Golani G, Freeman JJ, Sawhney JS, Vatsaas CJ, Yorkgitis BK, Kreiner LA, Garcia NM, Aziz HA, et al. Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy: Practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2020;89:999–1017. doi: 10.1097/TA.0000000000002944. [DOI] [PubMed] [Google Scholar]
- 9.Christoffel J, Maegele M. Guidelines in trauma-related bleeding and coagulopathy: an update. Curr Opin Anaesthesiol. 2024;37:110–6. doi: 10.1097/ACO.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 10.Volod O, Bunch CM, Zackariya N, Moore EE, Moore HB, Kwaan HC, Neal MD, Al-Fadhl MD, Patel SS, Wiarda G, et al. Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices. J Clin Med. 2022;11:860. doi: 10.3390/jcm11030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrante EA, Blasier KR, Givens TB, Lloyd CA, Fischer TJ, Viola F. A Novel Device for the Evaluation of Hemostatic Function in Critical Care Settings. Anesth Analg. 2016;123:1372–9. doi: 10.1213/ANE.0000000000001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volod O, Viola F. The Quantra System: System Description and Protocols for Measurements. Methods Mol Biol. 2023;2663:743–61. doi: 10.1007/978-1-0716-3175-1_50. [DOI] [PubMed] [Google Scholar]
- 13.Groves DS, Welsby IJ, Naik BI, Tanaka K, Hauck JN, Greenberg CS, Winegar DA, Viola F. Multicenter Evaluation of the Quantra QPlus System in Adult Patients Undergoing Major Surgical Procedures. Anesth Analg. 2020;130:899–909. doi: 10.1213/ANE.0000000000004659. [DOI] [PubMed] [Google Scholar]
- 14.DeAnda A, Levy G, Kinsky M, Sanjoto P, Garcia M, Avandsalehi KR, Diaz G, Yates SG. Comparison of the Quantra QPlus System With Thromboelastography in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2021;35:1030–6. doi: 10.1053/j.jvca.2020.11.058. [DOI] [PubMed] [Google Scholar]
- 15.Demailly Z, Wurtz V, Barbay V, Surlemont E, Scherrer V, Compère V, Billoir P, Clavier T, Besnier E. Point-of-Care Viscoelastic Hemostatic Assays in Cardiac Surgery Patients: Comparison of Thromboelastography 6S, Thromboelastometry Sigma, and Quantra. J Cardiothorac Vasc Anesth. 2023;37:948–55. doi: 10.1053/j.jvca.2023.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Baulig W, Akbas S, Schütt PK, Keul W, Jovic M, Berdat P, von Felten S, Steigmiller K, Ganter MT, Theusinger OM. Comparison of the resonance sonorheometry based Quantra® system with rotational thromboelastometry ROTEM® sigma in cardiac surgery - a prospective observational study. BMC Anesthesiol. 2021;21:260. doi: 10.1186/s12871-021-01469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baryshnikova E, Di Dedda U, Ranucci M. A Comparative Study of SEER Sonorheometry Versus Standard Coagulation Tests, Rotational Thromboelastometry, and Multiple Electrode Aggregometry in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2019;33:1590–8. doi: 10.1053/j.jvca.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Zghaibe W, Scheuermann S, Munting K, Blaudszun G, Besser M, Ortmann E, Klein AA. Clinical utility of the Quantra® point-of-care haemostasis analyser during urgent cardiac surgery. Anaesthesia. 2020;75:366–73. doi: 10.1111/anae.14942. [DOI] [PubMed] [Google Scholar]
- 19.Michelson EA, Cripps MW, Ray B, Winegar DA, Viola F. Initial clinical experience with the Quantra QStat System in adult trauma patients. Trauma Surg Acute Care Open . 2020;5:e000581. doi: 10.1136/tsaco-2020-000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossetto A, Wohlgemut JM, Brohi K, Davenport R. Sonorheometry versus rotational thromboelastometry in trauma: a comparison of diagnostic and prognostic performance. J Thromb Haemost. 2023;21:2114–25. doi: 10.1016/j.jtha.2023.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Duclos G, Fleury M, Grosdidier C, Lakbar I, Antonini F, Lassale B, Arbelot C, Albaladejo P, Zieleskiewicz L, Leone M. Blood coagulation test abnormalities in trauma patients detected by sonorheometry: a retrospective cohort study. Res Pract Thromb Haemost. 2023;7:100163. doi: 10.1016/j.rpth.2023.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soucy-Proulx M, Kato H, Coeckelenbergh S, Naili Kortaia S, Herboulier L, Pittau G, Pham P, Lemoine A, Duranteau J, Roullet S. Sonorheometry Device Thresholds in Liver Transplantation: An Observational Retrospective Study. J Clin Med. 2024;13:696. doi: 10.3390/jcm13030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flores AS, Forkin KT, Brennan MM, Kumar SS, Winegar DA, Viola F. Multicenter evaluation of the Quantra with the QStat Cartridge in adult patients undergoing liver transplantation. Liver Transpl. 2023;29:1216–25. doi: 10.1097/LVT.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon C, Ranucci M, Hochleitner G, Schöchl H, Schlimp CJ. Assessing the Methodology for Calculating Platelet Contribution to Clot Strength (Platelet Component) in Thromboelastometry and Thrombelastography. Anesth Analg. 2015;121:868–78. doi: 10.1213/ANE.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gall LS, Brohi K, Davenport RA. Diagnosis and Treatment of Hyperfibrinolysis in Trauma (A European Perspective) Semin Thromb Hemost. 2017;43:224–34. doi: 10.1055/s-0036-1598001. [DOI] [PubMed] [Google Scholar]
- 26.Corey FS, Walker WF. Sonic Estimation of Elasticity via Resonance: A New Method of Assessing Hemostasis. Ann Biomed Eng. 2016;44:1405–24. doi: 10.1007/s10439-015-1460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolliger D, Kamber F, Mauermann E. Same Same but Different: Viscoelastic Hemostatic Assays in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2021;35:1037–9. doi: 10.1053/j.jvca.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Savage SA, Zarzaur BL, Pohlman TH, Brewer BL, Magnotti LJ, Croce MA, Lim GH, Martin AC. Clot dynamics and mortality: The MA-R ratio. J Trauma Acute Care Surg. 2017;83:628–34. doi: 10.1097/TA.0000000000001637. [DOI] [PubMed] [Google Scholar]
- 29.Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, Banerjee A, Sauaia A. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82:114–9. doi: 10.1097/TA.0000000000001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katori N, Tanaka KA, Szlam F, Levy JH. The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thrombelastography. Anesth Analg. 2005;100:1781–5. doi: 10.1213/01.ANE.0000149902.73689.64. [DOI] [PubMed] [Google Scholar]
- 31.Arnolds DE, Scavone BM. Thromboelastographic Assessment of Fibrinolytic Activity in Postpartum Hemorrhage: A Retrospective Single-Center Observational Study. Anesth Analg. 2020;131:1373–9. doi: 10.1213/ANE.0000000000004796. [DOI] [PubMed] [Google Scholar]
- 32.Morton S, Galea J, Uprichard J, Hudson A. The practicalities and barriers of using TEG6s in code red traumas: an observational study in one London major trauma centre. CJEM. 2019;21:361–4. doi: 10.1017/cem.2018.426. [DOI] [PubMed] [Google Scholar]
- 33.Gasciauskaite G, Malorgio A, Castellucci C, Budowski A, Schweiger G, Kolbe M, Grande B, Noethiger CB, Spahn DR, Roche TR, et al. User Perceptions of ROTEM-Guided Haemostatic Resuscitation: A Mixed Qualitative-Quantitative Study. Bioengineering (Basel) 2023;10:386. doi: 10.3390/bioengineering10030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore HB, Moore EE, Neal MD, Sheppard FR, Kornblith LZ, Draxler DF, Walsh M, Medcalf RL, Cohen MJ, Cotton BA, et al. Fibrinolysis Shutdown in Trauma: Historical Review and Clinical Implications. Anesth Analg. 2019;129:762–73. doi: 10.1213/ANE.0000000000004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naik BI, Tanaka K, Sudhagoni RG, Viola F. Prediction of hypofibrinogenemia and thrombocytopenia at the point of care with the Quantra® QPlus® System. Thromb Res. 2021;197:88–93. doi: 10.1016/j.thromres.2020.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.