Abstract
Introduction
The COVID-19 pandemic, driven by the SARS-CoV-2 virus, has had a significant global impact, with over 775 million cases reported and more than 7 million deaths as of July 2024. In Chile, approximately 5.4 million people have been infected, with a substantial proportion experiencing persistent symptoms known as post-COVID-19 syndrome. This study aims to estimate the prevalence of post-COVID-19 syndrome in Punta Arenas, Chile, and to explore the associated symptoms, mainly focusing on psychological, physical and molecular impacts on the affected population.
Methods and analysis
This cross-sectional study will use stratified random sampling to select a representative sample of 282 adults from Punta Arenas. Participants eligible for the study are those who had tested positive for SARS-CoV-2 by reverse transcription-quantitative PCR between July 2022 and July 2023. Data collection will include comprehensive clinical assessments, psychological evaluations and laboratory analyses of inflammatory biomarkers. Standardised instruments will be used to ensure consistency and reliability in measuring persistent symptoms. Statistical analyses will include descriptive statistics, regression models and subgroup analyses to identify risk factors and the prevalence of post-COVID-19 syndrome.
Ethics and dissemination
The Human Research Ethics Committee of the Clinical Hospital of the University of Chile approved the study protocol (Memorandum No 007/2023). We will present the results in peer-reviewed publications and national and international professional and academic meetings.
Trial registration number
Keywords: Cross-Sectional Studies, Prevalence, COVID-19, Post-Acute COVID-19 Syndrome
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study location is an isolated region with low population mobility, enabling a precise prevalence assessment.
Recruitment from the Chilean Ministry of Health Epidemiological Surveillance System database ensures a representative sample.
A limitation of the study is the challenge of distinguishing whether self-reported symptoms are solely due to persistent COVID-19 or are influenced by other underlying illnesses or conditions.
The study relies on participants’ recall of symptoms and medical history; there is a potential for recall bias.
Introduction
The SARS-CoV-2 virus pandemic has left a profound global footprint, affecting over 775 million individuals and tragically claiming more than 7 million lives as of July 2024.1 2 In Chile, the infection has been documented in approximately 5.4 million people with a prevalence that could reach over 28% of the population.3,5
COVID-19 is characterised by multisystemic impact, affecting the respiratory, gastrointestinal, cardiovascular and neurological systems.6,9 While many individuals develop mild symptoms, with 36% of infections reported as asymptomatic infections in a seroprevalence study conducted in Chile,3 approximately 15% of cases can progress to severe forms requiring hospitalisation and around 5% can suffer life-threatening complications.10 A subset of infected individuals can develop a postinfection syndrome characterised by persistent and fluctuating symptoms. This syndrome has been known by various terms, including ‘persistent COVID-19’, ‘post-COVID-19 syndrome’, ‘chronic COVID-19 syndrome’, or ‘post-acute sequelae of SARS-CoV-2 infection’.11,13 The term post-COVID-19 will be used in the remainder of this study. Post-COVID-19 constitutes a syndrome linked to a spectrum of previous asymptomatic, moderate or severe infections.6 12 14 The WHO officially defined post-COVID-19 syndrome as the persistent manifestation of symptoms up to 3 months following a history of probable or confirmed SARS-CoV-2 infection lasting at least 2 months.11 15 16 These symptoms can arise during or after the initial disease and include physiological, psychological and cognitive impairments, significantly impacting individuals’ daily life.17,21
While associated risk factors are still under investigation, current evidence suggests an elevated likelihood of developing post-COVID-19 syndrome among individuals aged 35–70 years, predominantly women, with a history of underlying conditions such as type 2 diabetes mellitus, obesity, previous Epstein-Barr virus infection, presence of autoantibodies and various social health determinants.2022,26 The post-COVID-19 syndrome is a diagnosis ‘by exclusion’ characterised by the persistence of symptoms for over 3 months following the initial infection and requiring the ruling out of alternative conditions.11 27 28 Worldwide, the prevalence of post-COVID-19 syndrome is estimated to be between 6% and 7% in the adult population and 1% in the paediatric population. However, reported prevalence ranges from 2% to 45%.313 29,31 The reasons for this widespread variation across different populations are as yet unclear.32,36 Several factors have been proposed, including individual as well as the environment, in particular the study design and location.3 27 37 38
Subantarctic Chile was one of the most affected regions in the world at the beginning of the COVID-19 pandemic.39 On the one hand, the low mobility rates of this territory, because of its unique geographical isolation and extreme latitude, enable high traceability of cases, facilitating recruitment and reducing variability induced by migratory flows.20 39 This context highlights the need for comprehensive prevalence estimates across the country. Despite this significant burden, a gap remains in understanding the long-term impact of COVID-19 on this population. Additionally, the estimation of post-COVID-19 syndrome prevalence is made more difficult due to different methodological approaches, hindering cross-study comparisons. Therefore, adherence to standardised strategies is essential.
The aim of this study is to estimate the prevalence and linked symptoms of post-COVID-19 syndrome in the city of Punta Arenas, Chile. The design is based on collecting a representative sample using age-based stratification and includes a comprehensive psychological assessment. Data collection includes clinical, epidemiological, psychological, respiratory, physical and molecular variables. This strategy will help identify the most prevalent persistent symptoms, ascertain their duration and gauge their impact on affected individuals’ quality of life. In addition, the study will assess inflammatory biomarkers in blood, including cytokines and B-cell immune repertoire, to explore their potential association with post-COVID-19 syndrome and disease severity.1440,46 The results could contribute to inform decision-making for post-pandemic management.
Methods and analysis
The methods of this study were designed in concordance with the WHO definition of post-COVID-19 syndrome47 and are reported following the Strengthening the Reporting of Observational Studies in Epidemiology statement for cross-sectional studies.48
Study aims and design
The post-COVID-19 syndrome study is a cross-sectional observational study with a population-based approach and stratified random sampling. The primary aim is to estimate the prevalence of post-COVID-19 syndrome following SARS-CoV-2 infection in adult patients who tested positive for COVID-19 by reverse transcription-quantitative PCR (RT-qPCR). The secondary aims are exploratory and include: (1) describing and establishing the frequency of physical and psychological signs and symptoms, (2) identifying individuals who meet the WHO case definition of post-COVID-19 syndrome in Chile, (3) exploring risk factors associated with post-COVID-19 syndrome and (4) exploring inflammatory and molecular biomarkers associated with post-COVID-19 syndrome. The study is carried out by the Centro Asistencial Docente e Investigación de la Universidad de Magallanes (CADI-UMAG) in Punta Arenas, Chile. Participant recruitment began in May 2023 and is ongoing, with an expected completion date of December 2024. Participants are being contacted at three separate points in time.
Population and eligibility
To be eligible for participation in this transversal study, an individual must satisfy the following criteria: (1) be 18 years or older, (2) have a history of a confirmed or positive diagnosis of SARS-CoV-2 infection by RT-qPCR between July 2022 and July 2023 and reside in Punta Arenas, (3) be registered in the Local Health Authority-Secretaría Regional Ministerial de Salud (SEREMI) epidemiological surveillance system database and (4) be able to understand and provide written informed consent. The study will also include participants who report persistent symptoms associated with positive SARS-CoV-2 infection test results, categorised as self-reported post-COVID-19 syndrome condition cases.
Sample size
A sample size determination was carried out to ascertain the number of participants required to achieve the desired precision in prevalence estimates, ensuring a 95% CI, as per:
In equation (1), p represents the expected prevalence rate, while d denotes the precision level. The post-COVID-19 syndrome prevalence rates reported in the literature were used as inputs to calculate the required sample sizes. Based on official WHO guidelines,47 the expected prevalence of post-COVID-19 syndrome in the general population was estimated at 20%, and this estimate was used as the basis for the sample size calculation at the time the study was designed. It is anticipated that 20% of individuals may experience persistent symptoms (eg, fatigue, pain, concentration problems and others) for at least 3 months. This estimation allows for the calculation of a sample size that considers the entire population of the area, along with a current inability to precisely determine prior exposure to the virus. A margin of error of ±5% in estimating the prevalence with a 95% CI, and a potential 12.5% sample loss was also considered. This potential anticipated sample loss could be due to participants declining some or all specimen collections post consent or it becoming impossible to re-contact patients during follow-up visits at CADI. This sample size estimation was calculated using the Scalex SP calculator.49
Based on the above estimates and calculations, it was determined that the study would have to enrol a total of 282 participants. This sample size represents approximately 0.30% of the regional population aged 18 years and older. The sample was stratified into three age groups: youth (18–29 years, 14.6%), adulthood (30–64 years, 48%) and older adulthood (≥65 years, 11.4%), based on census data from the Chilean Institute of Statistics.50 Recruitment will continue beyond the initial target to ensure robust and accurate estimates of post-COVID-19 syndrome.
Recruitment of participants
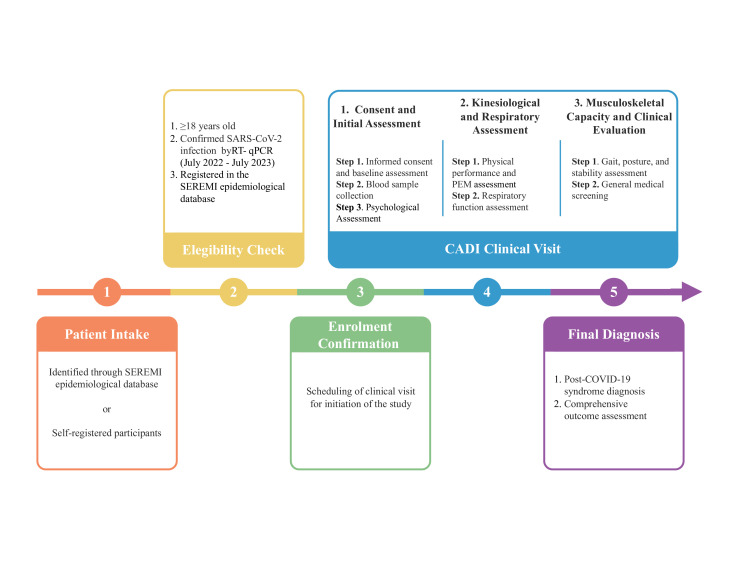
Figure 1 shows the flow diagram of participant recruitment in the post-COVID-19 syndrome study. Participants will be recruited through the following two ways:
Figure 1. Recruitment and assessment process in the post-COVID-19 syndrome study. The diagram illustrates the recruitment, eligibility assessment and clinical visits of the participants. Participants complete three stages of clinical visits: baseline assessments, kinesiological and respiratory evaluations and musculoskeletal analysis. The final step will be a comprehensive diagnosis, outcome analysis and estimation of the prevalence of post-COVID-19 syndrome. CADI, Centro Asistencial Docente e Investigación; RT-qPCR, reverse transcription-quantitative PCR; PEM, post-exertional malaise; SEREMI, Local Health Authority-Secretaría Regional Ministerial de Salud.
Via community health services message
Individuals who tested positive for SARS-CoV-2 and were registered with the Ministry of Health Services in Punta Arenas will be invited to participate in the post-COVID-19 syndrome study. The initial call for participants will be made via e-mail and will include a general adherence survey sent to a sample of 4000 people from the Local Health Authority-SEREMI database in Punta Arenas. From those who respond, 282 SARS-CoV-2 positive individuals, aged 18 years and above, will be selected in the order of response. Registration to participate will be conducted through the post-COVID-19 syndrome study website or via email.
Self-registered participants
Individuals interested in participating in the post-COVID-19 syndrome study will be able to self-enrol. However, only those with a positive SARS-CoV-2 test recorded in the Local Health Authority-SEREMI database will be eligible for inclusion through this method. The study population will be frequency-matched to the proportional stratified sampling distribution according to the age ranges defined in this study, and cases will be randomly selected.
Self-registration will be one strategy to minimise selection bias if enrolment rates are low. If many eligible participants refuse to enrol, the resulting sample may differ substantially from the target population, potentially introducing bias into the results. To minimise this, we will work closely with healthcare workers at each site to facilitate enrolment and will engage local patient associations and the community to raise awareness and encourage participation.
Data collection
The study data will be collected and managed using the Research Electronic Data Capture (REDCap) system, hosted on a secure server at the University of Magallanes. REDCap is a secure, web-based software platform designed to support data acquisition for research studies using devices and REDCap software.51 Continuous automated and manual data validation measures will be implemented throughout the survey fieldwork to optimise data integrity. Before the initiation of fieldwork, the survey team will undergo training on data collection protocols and data security measures. All collected data, including demographic information such as age, gender, history of current or past COVID-19 diagnosis and treatment, history of household COVID-19 contact, symptoms and laboratory results and source documentation, will be entered into an electronic case report file within REDCap. Additional data will be collected from individuals reporting symptoms suggestive of post-COVID-19 syndrome to assess health-seeking behaviour and barriers to care related to their symptoms. These fields are detailed in the online supplemental material. The principal investigators will ensure that the data entered into REDCap are accurate, complete and reliable, with range checks applied to data values.
Data deidentification
Each participant will be assigned a unique subject ID code number (post-COVID-19 XXX) consecutively on inclusion. This study number will be entered into the data collection form. Data collectors will generate a separate list containing the study number and the participant’s name. This list will be used to identify potential participants for the second component of the study (the survey). The list with identifying information will remain at the participating organisation and will not be disclosed to the research team. ID numbers linked to sensitive patient data (including name, ID number and date of enrolment) will be stored in a secured file, separate from other data, according to Chilean law requirements on data protection and research involving human subjects (Act No 20584, Act No 19628 and Act No 20120). Deidentified data will be incorporated into the source documentation, which will be stored in locked cabinets and retained for a maximum of 5 years in the confidential storage room 1010 at CADI-UMAG.
Data sources and measurements
Table 1 outlines the primary study domains and response variables to be assessed through standardised questionnaires, physical assessments and laboratory analyses. Participants will attend three visits at CADI-UMAG, each lasting up to 90 min. A multidisciplinary team will assist during the initial visit to ensure thorough and accurate data collection.
Table 1. Summary of study visits, domains and assessment instruments*.
| Visit | Step | Domain | Instrument | Purpose | Timing |
|---|---|---|---|---|---|
| V1 | S1 | Sociodemographic | Survey form | Baseline characterisation | Baseline |
| Medical history | Medical history form | Identify risk/modifying factors | |||
| Quality of life | QoL instrument | Assess functional impact | |||
| Health behaviours | Behavioural questionnaire | Lifestyle-related recovery factors | |||
| S2 | Blood biomarkers | Clinical lab panel | General physiological status | ||
| Immune profiling | Research lab panel | Immune system evaluation | |||
| S3 | Cognitive function | WAIS-IV | Cognitive sequelae detection | ||
| Mental health | Standardised scales | Psychological impact | |||
| Sleep quality | Sleep Quality Index | Evaluate sleep-related symptoms | |||
| V2 | S1 | Physical performance | FAS, InBody, dynamic handgrip. | Functional impact assessment | Follow-up |
| PEM | DePaul PEM section | Evaluate PEM response | |||
| Respiratory function | Pulmonary tools | Pulmonary sequelae | |||
| V3 | S1 | Gait and posture | 3D photogrammetry | Motor system evaluation | |
| S2 | Integrated assessment | All instruments used | Diagnostic confirmation of post-COVID | Final visit |
See online supplemental table 1 for full variable details and measurement methods.
FAS, Fatigue Assessment Scale; InBody, body composition analyser; PEM, post-exertional malaise; QoL, quality of life; WAIS-IV, Wechsler Adult Intelligence Scale (fourth edition).
Clinic visit 1: initial patient assessment
Informed consent will be obtained from all participants before their assessment during the initial clinic visit. Baseline data collection will include demographic variables. A structured questionnaire, divided into five sections of categorised, multiple-choice clinical questions, will be used. Comprehensive laboratory evaluations will be conducted, including a complete blood count, inflammatory biomarkers in blood and an analysis of B and T cell immune repertoires. Additionally, standardised questionnaires will assess psychological and cognitive functions .52,58
Informed consent
In compliance with Chilean legal requirements, this study is classified as low-risk research involving human subjects, necessitating written informed consent from all participants. Data collectors will explain the study, providing participants with an explanatory statement and a consent form. Given the study’s focus on prevalence, an open call will be made to the general population. To accommodate participants with varying health conditions and potential impairments, different consent options will be provided:
Participants capable of providing consent: the study will be clearly explained, and written consent will be obtained by the investigating physicians after allowing time for reflection.
Participants unable to provide written consent: consent will be sought from a trusted individual or, if unavailable, a family member. The participant’s refusal will be respected, and written consent will be obtained from this representative.
Written consent will be secured whenever possible on the day of study admission to facilitate prompt procedures, such as blood tests. Participants will be informed of their rights to access, modify, oppose or cancel their participation. A revocation consent form will be provided, and on withdrawal, the participant will be excluded from further study procedures, although previously collected data will still be analysed. The research team will promptly communicate significant findings to participants.
Clinic visit 2: kinesiological and respiratory assessment
During the second visit, participants will undergo kinesiological assessments, including musculoskeletal capacity tests such as fatigue evaluation, body composition analysis and handgrip strength measurement using a dynamometer (administered by a healthcare professional). Additionally, participants will complete a standardised questionnaire assessing post-exertional malaise (PEM), characterised by the delayed or prolonged exacerbation of symptoms before and after physical activity to evaluate baseline status and immediate postactivity changes.59,61
This evaluation complements the fatigue and physical function assessments 62 63 . Anthropometric measurements will include height, measured with a stadiometer, and weight and body composition, assessed through bioelectrical impedance analysis (using the InBody 770 device) .64,66 Furthermore, respiratory assessments will be conducted, including pulmonary function tests and spirometry, covering seven categorised respiratory parameters. 67,70
Clinic visit 3: functional movement and clinical assessment
Musculoskeletal function will be assessed using the 6-minute walk test (measuring distance covered), heart rate variability, stability evaluation, postural control, electroencephalography and electromyography, covering six categorised items. Any abnormalities detected during these assessments, including previously undiagnosed mental health issues, will be reported by the medical staff. Participants experiencing significant fatigue or functional decline after the test may be re-evaluated for PEM using the same standardised questionnaire from clinic visit 2 to monitor potential symptom exacerbation 71 72 .
Following these assessments, a medical evaluation will be conducted to diagnose the presence or absence of post-COVID-19 syndrome. Diagnosis will be based on clinical criteria following WHO guidelines.2 47 Participants not diagnosed with post-COVID-19 syndrome will either be discharged or referred to a healthcare centre for further evaluation or specialised care as needed.
Outcome measures
Primary outcome
The primary outcome measure will be to determine the prevalence of persistent symptoms in individuals who tested positive for SARS-CoV-2 in Punta Arenas. The severity of symptoms—including fatigue, pain, dyspnoea and cognitive impairment—will be assessed using standardised questionnaires with population-based norm cut-off scores for clinically significant severity. These assessments will be based on the 10 symptoms identified by the WHO, according to the Delphi consensus, which is associated with long covid (post-COVID-19 syndrome).2 47
Secondary outcomes
Assess the impact of persistent COVID-19 symptoms on daily life and well-being, including psychological, physical and respiratory aspects.
Explore inflammatory and molecular biomarkers in blood samples to investigate their association with the persistence and severity of COVID-19 symptoms.
Biobank storage and accessory research
After laboratory analysis, blood residues (serum and plasma, at least 1 mL each) will be stored in the Molecular Medicine Laboratory—CADI-UMAG Biobank at −80°C and labelled with the subject ID number, thus deidentifying the samples. If future use of these samples is required, approval will be sought from the corresponding ethics committee, and the participant will be recontacted to approve the use of their samples.
Statistical analysis
Data will be processed and analysed using the R statistical programming language. Descriptive analyses will be conducted, where continuous variables will be summarised using either the mean and SD or the median and IQR, depending on the data distribution. For categorical variables, proportions will be calculated. Depending on the data distribution, we will apply one-sample or two-sample t-tests or, alternatively, Wilcoxon and Mann-Whitney tests. In addition, univariable and multivariable regression models, along with χ2 and Fisher’s exact tests, will be employed. The study will also use a general linear mixed model, with subject IDs as the random effect. For questionnaire assessments, rank correlation methods will be applied. Statistical significance will be set at a threshold of p<0.05. If sufficient data are available, we will create subgroups based on post-COVID-19 syndrome status within the substudies to evaluate within-group associations across all parameters.
Patient and public involvement
Primary care clinics will be involved from the planning phase, collaborating on the development of educational materials to ensure message clarity and relevance. Patients and the community are considered key stakeholders in the project.
Community dissemination will begin prior to recruitment through a targeted communication campaign (local radio and press) led by the local health authority and the research team. The campaign will outline the study’s objectives, the research questions and the relevance of the findings to encourage participation. Information will be provided via town hall meetings and leaflets distributed in pharmacies and consultation offices. On study completion, results will be presented to the community and local authorities, emphasising public health implications related to post-COVID-19 syndrome. All communications will be simplified for public understanding.
Protocol amendments and dissemination policy
This protocol is the first version, completed on 10 May 2023. The trial has been registered at ClinicalTrials.gov (NCT05855382). Amendments will be published under this registration number. If required, the online version at ClinicalTrials.gov will be updated. The study will be performed in accordance with the Declaration of Helsinki and the principles of local legal and regulatory requirements. The trial status is recruiting; recruitment began in May 2023. The expected date of completion is December 2024.
Ethics and dissemination
The Human Research Ethics Committee of the Clinical Hospital of the University of Chile approved the study protocol, as stated in Memorandum No 007/2023. The observational design entails minimal risk to participants. No direct benefits to participants are anticipated, and neither incentives for participation will be provided nor reimbursement for expenses. Personal data will be handled according to Chilean laws and regulations (Act No. 19 628), and data will be anonymised as described above. At enrolment, potential participants will be fully informed on all aspects of the study and will be prompted to share their full contact details for the next follow-up clinic visit. It is possible that post-COVID-19 syndrome screening may elicit some anxiety among participants. To mitigate this risk, experienced clinicians within the study teams will be available to educate and support participants. Screening results will be made available to participants rapidly to minimise their wait time, and medical professionals will present these results.
We will present the results of peer-reviewed publications and national and international professional and academic meetings. We will organise seminars with relevant stakeholders (health policymakers and authorities, experts and academics) and hold town hall meetings with the local community. We will coordinate with the local health authority for press releases and radio broadcasts to disseminate the study’s purpose, introduce the research team and share milestones.
Supplementary material
Acknowledgements
The authors would acknowledge the support provided by the Chilean Ministry of Health (Local Health Authority - SEREMI, Regional of Magallanes), Francisca Sanfuentes Praga, the Head of the Public Health Department, Vivian Garay Vukasovic and their teams.
Footnotes
Funding: This study was funded by the Regional Innovation Fund (FIC-R, BIP 40036196-0) of the Regional Government of Magallanes and Chilean Antarctic. Additional support was provided by the Health Research Fund (FONIS SA22I0135) of the National Agency for Research and Development (ANID), Chile, through the Centro Asistencial Docente y de Investigación of the University of Magallanes (CADI-UMAG); and by the Associative Research Program (PIA FB0001) of the National Commission for Scientific and Technological Research (CONICYT), Chile, through the Centre for Biotechnology and Bioengineering (CeBiB). The work of MN, JP and LSV is funded by the ANILLO Program (ATE220016) of the National Agency for Research and Development (ANID), Chile.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-093844).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
References
- 1.Zeng Z, Geng X, Wen X, et al. Novel receptor, mutation, vaccine, and establishment of coping mode for SARS-CoV-2: current status and future. Front Microbiol. 2023;14:1232453. doi: 10.3389/fmicb.2023.1232453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization COVID-19 epidemiological update - 13 August 2024. https://www.who.int/publications/m/item/covid-19-epidemiological-update-edition-170 n.d. Available.
- 3.Vial P, González C, Icaza G, et al. Seroprevalence, spatial distribution, and social determinants of SARS-CoV-2 in three urban centers of Chile. BMC Infect Dis. 2022;22:99. doi: 10.1186/s12879-022-07045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayala A, Vargas C, Elorrieta F, et al. Inequity in mortality rates and potential years of life lost caused by COVID-19 in the Greater Santiago, Chile. Sci Rep. 2023;13:16293. doi: 10.1038/s41598-023-43531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gobierno de Chile Cifras Oficiales COVID-19. 2024. https://www.gob.cl/pasoapaso/cifrasoficiales Available.
- 6.Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. 2022;54:1473–87. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemi F, Guralnik E, Vang J, et al. Guidelines for Triage of COVID-19 Patients Presenting With Multisystemic Symptoms. Qual Manag Health Care. 2023;32:S3–10. doi: 10.1097/QMH.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–54. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao R, Qiu Y, He J-S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–78. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst Rev. 2022;5:CD013665. doi: 10.1002/14651858.CD013665.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boix V, Merino E. Post-COVID syndrome. The never ending challenge. Med Clin (Engl Ed) 2022;158:178–80. doi: 10.1016/j.medcle.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yong SJ, Liu S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev Med Virol. 2022;32:e2315. doi: 10.1002/rmv.2315. [DOI] [PubMed] [Google Scholar]
- 13.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–7. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merad M, Blish CA, Sallusto F, et al. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–7. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 15.WHO . A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–31. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carfì A, Bernabei R, Landi F, et al. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324:603–5. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanne JH. Covid-19: Even mild infections can cause long term heart problems, large study finds. BMJ. 2022;376:o378. doi: 10.1136/bmj.o378. [DOI] [PubMed] [Google Scholar]
- 20.Sarmiento Varón L, González-Puelma J, Medina-Ortiz D, et al. The role of machine learning in health policies during the COVID-19 pandemic and in long COVID management. Front Public Health. 2023;11:1140353. doi: 10.3389/fpubh.2023.1140353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou T, Sawano M, Arun AS, et al. Internal Tremors and Vibrations in Long COVID: A Cross-Sectional Study. Am J Med. 2024 doi: 10.1016/j.amjmed.2024.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal J, Ghosh A, Bhatt SP, et al. High prevalence of post COVID-19 fatigue in patients with type 2 diabetes: A case-control study. Diabetes Metab Syndr. 2021;15:102302. doi: 10.1016/j.dsx.2021.102302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4:e532–41. doi: 10.1016/S2589-7500(22)00048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Shi L, Liu D, et al. Changes in Epstein-Barr virus infections in the children before and after the COVID-19 pandemic in Zhengzhou, China. J Med Virol. 2023;95:e28597. doi: 10.1002/jmv.28597. [DOI] [PubMed] [Google Scholar]
- 25.Trihandini I, Muhtar M, Karunia Sakti DA, et al. The effect of long-haul COVID-19 toward domains of the health-related quality of life among recovered hospitalized patients. Front Public Health. 2023;11:1068127. doi: 10.3389/fpubh.2023.1068127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adar S, Konya PŞ, Akçin Aİ, et al. Evaluation and follow-up of pain, fatigue, and quality of life in COVID-19 patients. Osong Public Health Res Perspect. 2023;14:40–50. doi: 10.24171/j.phrp.2022.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim C, Moon JY, Kim SH, et al. Prevalences and Interrelationships of Post COVID-19 Fatigue, Sleep Disturbances, and Depression in Healthy Young and Middle-Aged Adults. J Clin Med. 2024;13:2801. doi: 10.3390/jcm13102801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence (NICE) COVID-19 rapid guideline: managing the long-term effects of covid-19. 2024. https://www.ncbi.nlm.nih.gov/books/NBK567261/ Available. [PubMed]
- 29.O’Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine. 2023;55:101762. doi: 10.1016/j.eclinm.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallek M, Adorjan K, Behrends U, et al. Post-COVID syndrome. Deutsches Ärzteblatt International. 2023 doi: 10.3238/arztebl.m2022.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sk Abd Razak R, Ismail A, Abdul Aziz AF, et al. Post-COVID syndrome prevalence: a systematic review and meta-analysis. BMC Public Health. 2024;24:1785. doi: 10.1186/s12889-024-19264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C, Haupert SR, Zimmermann L, et al. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J Infect Dis. 2022;226:1593–607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson MM, Qasmieh SA, Kulkarni SG, et al. The Epidemiology of Long Coronavirus Disease in US Adults. Clin Infect Dis. 2023;76:1636–45. doi: 10.1093/cid/ciac961. [DOI] [PubMed] [Google Scholar]
- 34.Petersen MS, Kristiansen MF, Hanusson KD, et al. Prevalence of long COVID in a national cohort: longitudinal measures from disease onset until 8 months’ follow-up. Int J Infect Dis. 2022;122:437–41. doi: 10.1016/j.ijid.2022.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlis RH, Santillana M, Ognyanova K, et al. Prevalence and Correlates of Long COVID Symptoms Among US Adults. JAMA Netw Open . 2022;5:e2238804. doi: 10.1001/jamanetworkopen.2022.38804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen A, David JK, Maden SK, et al. Human Leukocyte Antigen Susceptibility Map for Severe Acute Respiratory Syndrome Coronavirus 2. J Virol. 2020;94:e00510-20. doi: 10.1128/JVI.00510-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachelet VC, Silva-Ayarza I, Lizana FJ, et al. SARS-CoV-2 humoral immune response in patients with cardiovascular risk factors: the COmmunity Cohort Study protocol. BMJ Open. 2022;12:e061345. doi: 10.1136/bmjopen-2022-061345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizrahi B, Sudry T, Flaks-Manov N, et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ. 2023;380:e072529. doi: 10.1136/bmj-2022-072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González-Puelma J, Aldridge J, Montes de Oca M, et al. Mutation in a SARS-CoV-2 Haplotype from Sub-Antarctic Chile Reveals New Insights into the Spike’s Dynamics. Viruses. 2021;13:883. doi: 10.3390/v13050883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laidlaw BJ, Ellebedy AH. The germinal centre B cell response to SARS-CoV-2. Nat Rev Immunol. 2022;22:7–18. doi: 10.1038/s41577-021-00657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghofrani Nezhad M, Jami G, Kooshkaki O, et al. The Role of Inflammatory Cytokines (Interleukin-1 and Interleukin-6) as a Potential Biomarker in the Different Stages of COVID-19 (Mild, Severe, and Critical) J Interferon Cytokine Res. 2023;43:147–63. doi: 10.1089/jir.2022.0185. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez L, Tan Z, Lakshmikanth T, et al. Immune system perturbations in patients with severe long COVID. J Immunol. 2023;210:233. doi: 10.4049/jimmunol.210.Supp.233.07. [DOI] [Google Scholar]
- 43.Zhu Z, Wang P, Jia X, et al. B-Cell Receptor Features and Database Establishment in Recovered COVID-19 Patients by Combining 5’-RACE with PacBio Sequencing. Front Biosci (Landmark Ed) 2023;28:40. doi: 10.31083/j.fbl2802040. [DOI] [PubMed] [Google Scholar]
- 44.Santopaolo M, Gregorova M, Hamilton F, et al. Prolonged T-cell activation and long COVID symptoms independently associate with severe COVID-19 at 3 months. Elife. 2023;12:e85009. doi: 10.7554/eLife.85009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature New Biol. 2020;584:463–9. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein J, Wood J, Jaycox JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature New Biol. 2023;623:139–48. doi: 10.1038/s41586-023-06651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization (WHO) Clinical case definition: COVID-19 post COVID-19 condition. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 Available.
- 48.STROBE. https://www.strobe-statement.org n.d. Available.
- 49.Naing L, Nordin RB, Abdul Rahman H, et al. Sample size calculation for prevalence studies using Scalex and ScalaR calculators. BMC Med Res Methodol. 2022;22:209. doi: 10.1186/s12874-022-01694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Instituto Nacional de Estadísticas (INE) C Censo de Población y Vivienda. Regiones INE Magallanes. https://regiones.ine.cl/magallanes/estadisticas-regionales/sociales/censos-de-poblacion-y-vivienda/censo-de-poblacion-y-vivienda n.d. Available.
- 51.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brenner LA. In: Encyclopedia of clinical neuropsychology. Kreutzer JS, DJ CB, editors. New York, NY: Springer; 2011. Beck anxiety inventory; pp. 359–61. [Google Scholar]
- 53.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 54.Sanz J, Perdigó AL, Vázquez C. Adaptación española del Inventario para la Depresión de Beck-II (BDI-II): 2. Propiedades psicométricas en población general. Clin Salud. 2003;14:249–80. [Google Scholar]
- 55.Beck AT, Steer RA, Brown G. Beck depression inventory–II (BDI-II) [database record] APA PsycTests; 1996. [Google Scholar]
- 56.Carralero García P, Hoyos Miranda FR, Deblas Sandoval Á, et al. Calidad del sueño según el Pittsburgh Sleep Quality Index en una muestra de pacientes recibiendo cuidados paliativos. Medicina Paliativa. 2013;20:44–8. doi: 10.1016/j.medipa.2012.05.005. [DOI] [Google Scholar]
- 57.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 58.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–36. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Global Burden of Disease Long COVID Collaborators. Wulf Hanson S, Abbafati C, et al. Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328:1604–15. doi: 10.1001/jama.2022.18931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jason LA, Dorri JA. ME/CFS and Post-Exertional Malaise among Patients with Long COVID. Neurol Int. 2022;15:1–11. doi: 10.3390/neurolint15010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parums DV. Long COVID or Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) and the Urgent Need to Identify Diagnostic Biomarkers and Risk Factors. Med Sci Monit. 2024;30:e946512. doi: 10.12659/MSM.946512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J Psychosom Res. 2003;54:345–52. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 63.Michielsen HJ, De Vries J, Van Heck GL, et al. Examination of the dimensionality of fatigue. Eur J Psychol Assess. 2004;20:39–48. doi: 10.1027/1015-5759.20.1.39. [DOI] [Google Scholar]
- 64.Jayanama K, Putadechakun S, Srisuwarn P, et al. Evaluation of Body Composition in Hemodialysis Thai Patients: Comparison between Two Models of Bioelectrical Impedance Analyzer and Dual-Energy X-Ray Absorptiometry. J Nutr Metab. 2018;2018:4537623. doi: 10.1155/2018/4537623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mathiowetz V, Rennells C, Donahoe L. Effect of elbow position on grip and key pinch strength. J Hand Surg Am. 1985;10:694–7. doi: 10.1016/s0363-5023(85)80210-0. [DOI] [PubMed] [Google Scholar]
- 66.Keech A, Sandler CX, Vollmer-Conna U, et al. Capturing the post-exertional exacerbation of fatigue following physical and cognitive challenge in patients with chronic fatigue syndrome. J Psychosom Res. 2015;79:537–49. doi: 10.1016/j.jpsychores.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 68.Volianitis S, McConnell AK, Jones DA. Assessment of maximum inspiratory pressure. Prior submaximal respiratory muscle activity ('warm-up’) enhances maximum inspiratory activity and attenuates the learning effect of repeated measurement. Respiration. 2001;68:22–7. doi: 10.1159/000050458. [DOI] [PubMed] [Google Scholar]
- 69.Larson JL, Covey MK, Vitalo CA, et al. Maximal inspiratory pressure. Learning effect and test-retest reliability in patients with chronic obstructive pulmonary disease. Chest. 1993;104:448–53. doi: 10.1378/chest.104.2.448. [DOI] [PubMed] [Google Scholar]
- 70.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutherland DH. The evolution of clinical gait analysis. Part II kinematics. Gait Posture. 2002;16:159–79. doi: 10.1016/s0966-6362(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz MH, Rozumalski A. The Gait Deviation Index: a new comprehensive index of gait pathology. Gait Posture. 2008;28:351–7. doi: 10.1016/j.gaitpost.2008.05.001. [DOI] [PubMed] [Google Scholar]