Abstract
Under conditions of environmental stress, prokaryotes and lower eukaryotes such as the yeast Saccharomyces cerevisiae selectively utilize particular subunits of RNA polymerase II (pol II) to alter transcription to patterns favoring survival. In S. cerevisiae, a complex of two such subunits, RPB4 and RPB7, preferentially associates with pol II during stationary phase; of these two subunits, RPB4 is specifically required for survival under nonoptimal growth conditions. Previously, we have shown that RPB7 possesses an evolutionarily conserved human homolog, hsRPB7, which was capable of partially interacting with RPB4 and the yeast transcriptional apparatus. Using this as a probe in a two-hybrid screen, we have now established that hsRPB4 is also conserved in higher eukaryotes. In contrast to hsRPB7, hsRPB4 has diverged so that it no longer interacts with yeast RPB7, although it partially complements rpb4− phenotypes in yeast. However, hsRPB4 associates strongly and specifically with hsRPB7 when expressed in yeast or in mammalian cells and copurifies with intact pol II. hsRPB4 expression in humans parallels that of hsRPB7, supporting the idea that the two proteins may possess associated functions. Structure-function studies of hsRPB4-hsRPB7 are used to establish the interaction interface between the two proteins. This identification completes the set of human homologs for RNA pol II subunits defined in yeast and should provide the basis for subsequent structural and functional characterization of the pol II holoenzyme.
Selective control of mRNA transcription in response to intracellular and extracellular signals occurs at multiple levels, with targets for regulation including gene-specific transcription factors, general transcription factors, and the RNA polymerase II holoenzyme (15, 18, 38, 55, 63). This last mechanism of regulation, involving modification of core RNA polymerase II (pol II) structural composition by altering incorporation of subunits or regulated phosphorylation, has been well documented in prokaryotes and in yeast (17, 31, 59, 62, 70). In higher eukaryotes, the majority of transcriptional control studies have focused on characterizing the expression and modification of gene-specific and general transcription factors. However, a growing body of work on mammalian transcriptional control has demonstrated that mammalian pol II is also subject to modification by phosphorylation of the largest subunit, presumably as a means of regulation (10, 24, 41, 42, 49). In contrast, the issue of subunit variation has not been actively investigated.
Studies of eukaryotic pol II function have depended heavily on paradigms developed through detailed characterization of the yeast Saccharomyces cerevisiae pol II (reviewed in reference 70). Yeast pol II contains 12 subunits (RPB1–12), all of which have been cloned and sequenced and many of which have been subjected to genetic and biochemical functional analysis. Five of these subunits, the common subunits (RPB5, RPB6, RPB8, RPB10, and RPB12), are also incorporated into RNA polymerases I and III (64, 69). Mutational analysis indicates that 10 of the 12 subunits are essential for growth, whereas two, RPB4 and RPB9, are dispensable under moderate growth conditions but required under various suboptimal growth conditions (67, 68). Ten of the 12 subunits are obligate components of all intact RNA pol II molecules, whereas two (RPB4 and RPB7) form a subcomplex that is preferentially incorporated under suboptimal growth conditions such as stationary phase (16, 17, 20). The RPB4-RPB7 subcomplex has been hypothesized to play a stress-protective role (16, 17), potentially by redirecting transcriptional specificity in a manner analogous to sigma factors in prokaryotes (31).
Although human RNA polymerase II is much less well characterized than its yeast counterpart, biochemical and genetic assays of human pol II as well as exploration of the expanding sequence databases have identified homologs for 11 of the 12 defined yeast RNA pol II subunits (1–3, 23, 37, 46, 47, 50, 51, 60, 66). The sole exception, a putative hsRPB4 (human RPB4), evaded detection by the standard approaches used to identify most of the other subunits. In an earlier study, we cloned hsRPB7 based on its ability to induce hyperpolarized budding when overexpressed in S. cerevisiae as part of a screen we performed to identify novel human genes which could regulate cell growth controls (37, 40). In a series of functional characterizations, we demonstrated that hsRPB7 could complement lethality of an rpb7− deletion and weakly conserved the ability to interact with yeast RPB4 (37). Based on this conservation, it seemed likely that RPB4 would similarly have been evolutionarily conserved. We therefore used hsRPB7 as bait in a two-hybrid screen in an attempt to establish the existence of hsRPB4. Here we describe the cloning of hsRPB4 and characterize the hsRPB4 interaction with yeast pol II and hsRPB7, thus completing the set of human homologs of yeast RNA polymerase II subunits and providing the basis for subsequent functional studies.
MATERIALS AND METHODS
Bacterial and yeast strains and mammalian cell lines.
The Escherichia coli DH5αF′ [F′/endA1 hsdR17(rK− mK+)supE44 thi-1 recA1 gyrA (Nalr) relA1Δ(lacZYA-argF) U169 (f80lacD(lacZ)M15)] was used as a host for all cloning constructions. E. coli KC8 [hsdR leuB600 trpC9830 pyrF::Tn5 hisB463 lacDX74 strA galUK] (constructed by K. Struhl [described in references 28 and 29]) was used for purification of library plasmids following two-hybrid library screening. S. cerevisiae EGY191 (MATa ura3 his3 trp131exAop-leu2) was used for library screen and two-hybrid assays (21). The yeast strain WY-4 (MATa his3Δ200 leu2-3 leu2-112 ura3-52 RPB4Δ1::His3), a derivative of N114 (68), was used for hsRPB4 complementation studies. Yeast cells were grown either on YPD rich medium or on complete minimal medium lacking combinations of amino acids or containing zeocin to select for the presence of plasmids (7).
The African green monkey kidney cell line Cos-1 was used for transfection, immunoprecipitation, and immunofluorescence studies of hsRBP4. The HeLa human cervical carcinoma cell line was used for fractionation experiments. All cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum.
Cloning of hsRPB4.
Two-hybrid screening was performed as described in standard protocols (28). The HeLa cell yeast expression library in the vector pJG4-5 which was used for two-hybrid screening has been described previously (29). Briefly, the two-hybrid library was transformed by lithium acetate (56) into the yeast strain EGY191, along with the pEG202-hsRPB7 plasmid (37) as bait and JK103 as LacZ reporter (28). A total of 3.5 × 105 primary transformants were screened, resulting in the identification of five positive clones. Purified library plasmids were sequenced, and two independent clones with homology to yeast RPB4 were identified (4).
To extend a full-length hsRPB4 cDNA, the 1,870-bp cDNA fragment of hsRPB4 obtained by a two-hybrid screen was used as a template for 5′ rapid amplification of cDNA ends (5′-RACE). For this purpose, EG177 (AGCTGCGCTTTGTCTGGATATCATC) and EG185 (GAAGTGTCTCAGCTGTTTCAAACTC) primers were used for amplification from human kidney and human heart 5′-RACE-ready cDNA (Clontech), human fetal brain (a gift of D. Krainc), HL-60 cells, and human heart tissue (gifts of G. Kruh). In additional screening, the 32P-labeled hsRPB4 cDNA was used to directly probe cDNA libraries from human placenta (a gift of J. Chernoff) and HeLa cells (a gift of J. Gyuris). Finally, the hsRPB4 cDNA sequence was used to screen expressed sequence tag (EST) databases maintained by the Institute of Genome Research (33a) and GenBank with the BLAST algorithm (5).
Genomic analysis of hsRPB4.
A cDNA fragment containing the complete open reading frame (ORF) of hsRPB4 was labeled with [α-32P]dCTP (DuPont) with a Random prime II kit (Clontech) and used to probe a human placenta-derived genomic library in the Lambda FIX II vector (Stratagene). Of 11 independent phage, φVII contained the largest insert (∼11 kbp) and encompassed the carboxy-terminal ∼75% of the hsRPB4 cDNA. To obtain more 5′ genomic sequence, a 500-bp DNA fragment from the most upstream intronic sequence present in genomic φVII was labeled as above to reprobe the library, resulting in the isolation of overlapping clone φ3.2A, allowing further upstream sequencing but not reaching the 5′ end of the hsRPB4 cDNA. Finally, oligonucleotides based on the most upstream intronic sequence of φ3.2A were used to isolate an hsRPB4 genomic clone from a human PAC1 library (Genome Systems, Inc.). From this, an 8-kb BamHI-BamHI fragment was subcloned into pBluescript and sequenced by an automated sequencer (Applied Biosystems) in both orientations. The 5′ endpoint of the first exon of hsRPB4 was contained in this PAC1 clone; sequencing was continued for >1 kb upstream of the initiating methionine.
For chromosomal assignment of hsRPB4, metaphase spreads from phytohemagglutinin-stimulated lymphocytes of a healthy male donor were prepared as described by Fan et al. (22). Fluorescence in situ hybridization and detection of immunofluorescence were carried out essentially as previously described (11), with a 1.9-kb cDNA insert encompassing the RBP4 gene. Hybridization sites were detected with fluorescein-labeled avidin (Oncor) and amplified by addition of anti-avidin antibody (Oncor) and a second layer of fluorescein-labeled avidin. The metaphase preparations were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and observed with a Zeiss Axiophot epifluorescence microscope equipped with a cooled charge-coupled-device camera (Photometrics, Tucson, Ariz.) operated by a Macintosh computer work station. Digitized images of DAPI staining and fluorescein signals were captured, pseudocolored, and merged with Oncor Image version 1.6 software.
Plasmid constructions.
To assemble a full-length hsRPB4 cDNA, the 116-bp cDNA fragment of hsRPB4 obtained by 5′-RACE, containing the 5′ coding sequence of hsRPB4 and the anchor primer from the 5′-RACE kit, was subcloned into pUC119 at SmaI (pVK12). This PCR DNA fragment was reamplified from pVK12 to insert usable restriction sites and recloned into pUC119 at SmaI (pVK14). Finally, pVK14 was digested with HindIII and PvuII to obtain this 5′ end fragment, the original clone pJG4-5-hsRPB4 (described in Results) obtained by two-hybrid screen was digested with PvuII and EcoRI to obtain 3′ coding sequence, the two fragments were subcloned into pUC119 digested with HindIII and EcoRI (pUC-hsRPB4), and the whole was completely sequenced. pUC-hsRPB4, matching the genomic amino acid sequence, has been used as a general plasmid to construct all other plasmids containing hsRPB4 described in this study.
For two-hybrid analysis, the LexA-hsRPB7, LexA-RPB7 and pJG4-5/RPB4 (37); LexA-B42 (27); LexA-GAL4 (pSH17-4) and LexA-bicoid (RFHMI) (29) plasmids have been described previously. The pEG202-hsRPB4 clone contains a fusion protein in which LexA protein sequence was followed by amino acids ELGS, followed by the sequence of hsRPB4. Truncations of hsRPB4 (1–47, 1–92, 1–118, and 55–142) and hsRPB7 (1–54, 1–94, and 54–172) described in the text were generated by PCR and inserted into the pEG202 and/or pJG4-5 plasmids and sequenced.
For yeast complementation experiments, the plasmid pYES2-RPB4 was kindly provided by N. Woychik and has been described previously (37, 68). To construct pYES2-hsRPB4, pUC-hsRPB4 was digested with BamHI and EcoRI and subcloned into the similarly digested vector pYES2 (Invitrogen). pZeo-hsRPB7 was constructed by digesting the zeocin-selectable plasmid pHybLex/Zeo (Hybrid Hunter; Invitrogen) to remove LexA and insert hsRPB7 coding sequences. As a negative control for complementation, digested pHybLex/Zeo lacking LexA was additionally self-ligated to create the plasmid pZeoV.
For mammalian expression, to make the pCMV6-HA-hsRPB4 and the pCMV6-HA-hsRPB7 plasmids, a BamHI-EcoRI hsRPB4 DNA fragment from pUC-hsRPB4 and a BamHI-EcoRI hsRPB7 DNA fragment from pUC-hsRPB7 (37) were subcloned into the similarly digested vector pCMV6-HA (a gift of J. Chernoff). To make pCMV6-Myc-hsRPB4, a BamHI-EcoRI hsRPB4 DNA fragment from pUC-hsRPB4 was subcloned into a similarly digested vector, pCMV6-Myc (a gift of J. Chernoff). To make pCMV6-hsRPB7, the DNA fragment encoding the hemagglutinin (HA) tag was removed from plasmid pCMV6-HA-hsRPB7 by digestion with SalI and XbaI, and the ends were filled in with Klenow and religated.
Interaction analysis.
In general, the two-hybrid assay of protein interaction was performed by standard protocols (28). Plasmids expressing appropriate sets of LexA-fused protein, activation-domain fused protein, and LexA operator-LacZ reporter were cotransformed into EGY191 yeast. For all fusion proteins, synthesis of comparable levels of fusion proteins of correct size in yeast was confirmed by Western blot analysis of yeast extracts with polyclonal antiserum specific for LexA (14) or HA (Babco, Richmond, Calif.) as appropriate. Four to six independent colonies for each pair of proteins were tested in analysis of interactions, as indicated. β-Galactosidase assays were performed as described previously (48). Growth on medium without leucine was scored over a period of 5 days.
Northern analysis.
Two oligonucleotides representing antisense to hsRPB4 coding sequence, as well as the 1.9-kb hsRPB4 cDNA, were used sequentially as probes to determine expression of hsRPB4 mRNA in different human tissues by hybridization under standard conditions with a commercially available multiple tissue Northern blot (MTN1; Clontech). Similar loading of lanes was confirmed by reprobing with a 32P-labeled 2.0-kb cDNA fragment of actin (40). Finally, to confirm signals did not represent nonspecific cross-hybridization with contaminating rRNA, a blot containing mRNAs from cell lines used in this study was stained with ethidium bromide to visualize rRNA localization; by subsequent radioactive probe, this was confirmed to be different from the location of the hsRPB4-hybridizing transcripts.
Complementation analysis.
The S. cerevisiae WY-4 strain was transformed with pYES2 vector DNA (Invitrogen), pYES2-RPB4, or pYES2-hsRPB4 DNA by electroporation with a standard protocol and BTX Electro cell manipulator model ECM 600. After transformation, cells were plated on glucose-containing media without uracil and incubated at 23°C for 72 h to select colonies. In two independent assays, three to six colonies from each transformation were resuspended in distilled water, and an equivalent number of cells was spotted on each of four galactose-raffinose plates without uracil, which were then incubated at 23, 30, 34, and 37°C for 1 week to determine growth. Expression of the hsRPB4 protein was confirmed by Western analysis.
Expression and association of hsRPB7 and hsRPB4 in mammalian cells.
Cos cells were transfected with combinations of plasmids as described in Results with DEAE-dextran under standard transfection conditions (7). Coimmunoprecipitations were performed at approximately 48 h following transfection of cells. All steps were carried out at 4°C. To prepare cell lysates, cells were washed twice with 1× phosphate-buffered saline and then lysed in 900 μl of buffer A {5.0 mM MgCl2, 1.0 mM EGTA (pH 7.5), 50 mM Tris (pH 7.8), 10 mM 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate (CHAPS), 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 0.01 mg of aprotinin per ml, 0.01 mg of leupeptin per ml, 0.01 mg of epibestatin per ml} with rotation at 4°C for 30 min. Cells were scraped into the buffer A, and lysates were centrifuged in an Eppendorf microcentrifuge at 10,000 rpm for 10 min to remove insoluble debris. Supernatant from the centrifugation was used in the immunoprecipitation experiments. Protein concentration of the supernatant was measured by bovine serum albumin assay (with a kit from Pierce), and 0.5 mg of total protein in cell lysates was incubated with precipitating antibody (10 μl of anti-HA antiserum) plus 50 μl of 50% protein A-Sepharose beads (Sigma) overnight at 4°C. Beads and associated protein complexes were washed four times in 500 μl of buffer A and analyzed by immunoblotting. A sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (12% polyacrylamide) was used to analyze the products of the immunoprecipitation, which were visualized with antibody to hsRPB7.
To confirm expression of transfected or endogenous hsRPB4 and hsRPB7 proteins in cell lysates, Cos-1 cells were transfected and grown to ∼80% confluence. Five micrograms of cell lysates prepared as for immunoprecipitation was boiled in 2× Laemmli buffer and resolved on an SDS–12% PAGE gel as for immunoprecipitates. Whether for coimmunoprecipitation or direct assay of expression from cell lysates, protein gels were blotted to Immobilon (nylon membrane; Millipore). hsRPB7 and HA-hsRPB4 proteins were detected with either a 1:2,000 dilution of rabbit polyclonal anti-hsRPB7 antiserum (37) or a 1:1,000 dilution of rabbit polyclonal anti-HA antiserum as primary antibody and goat anti-rabbit antibody conjugated to horseradish peroxidase (Amersham) for visualization by enhanced chemiluminescence (DuPont).
Subcellular fractionation.
Cell fractionation was performed essentially as described previously for HeLa cells (25). Protein was assayed with a bovine serum albumin assay (Pierce). Purity of respective fractions was assayed by standard means. To characterize nuclear fraction purity, DNA content was assayed with a Hoefer Model TKO 100 microfluorometer with Hoechst 33258 as described previously (39). 5′ nucleotidase activity, an indicator of the Golgi fraction, was assayed as described previously (7) except that the buffer consisted of 50 mM Tris (pH 7.5), 2 mM MgCl2, 20 mM β-glycerophosphate, 1 mM adenosine, and 0.2 mM AMP. NADPH cytochrome c reductase, an indicator of the endoplasmic reticulum (ER)-containing fraction (61), and lactate dehydrogenase activities, as a measure of cytoplasmic fraction purity (19), were measured as described previously. Following fractionation, equivalent quantities of protein from each fraction (2.5 μg) were run out on an SDS–12% PAGE gel, and hsRPB7 levels were detected by Western analysis as described above.
Immunofluorescence staining.
Cos-1 cells were plated on coverslips 24 h before fixation for immunofluorescence or 24 h before transfection and subsequent immunofluorescence by standard procedures. Primary antibody incubation was performed with anti-hsRPB7 rabbit polyclonal antiserum or with anti-Myc mouse monoclonal antiserum at a dilution of 1:200 for 1 h at room temperature. Secondary antibody for anti-hsRPB7 was biotinylated anti-rabbit antibody (Vector Laboratories), with protein visualized with Texas Red streptavidin (Vector Laboratories). Secondary antibody for coverslips treated with primary anti-Myc antiserum was rhodamine-conjugated anti-mouse antibody. Immunofluorescence pictures were taken with a Zeiss fluorescence microscope.
Preparation of antibody to hsRPB4.
To prepare antibody to hsRPB4, a carboxy terminally derived peptide was sythesized with a leading cysteine to allow conjugation (C-EGRFEDEELQQILD) and was conjugated to KLH as the carrier protein with a kit (Imject; Pierce), as described in the kit protocol. Conjugated peptide was injected into rabbits, and immunoreactive antibody was characterized by standard procedures (30).
Purification of human RNA polymerase II complex.
Human RNA polymerase II complex was purified from a fraction derived from HeLa cell nuclear pellets with an immunoaffinity protocol with anti-carboxy-terminal domain (CTD) monoclonal antibodies as described previously (44). Duplicate samples were resolved by SDS-PAGE; one set was visualized by silver stain by standard methods, while a second set was used for Western analysis with antibody to hsRPB4, and the blot was stripped and reprobed with antibody to hsRPB7.
As a second approach, RNA polymerase II was also purified by conventional chromatography, with the protocol outlined in reference 44 with minor modifications. Approximately 5.5 g of solubilized nuclear pellets was used as starting material. Following purification by a high-pressure liquid chromatography DEAE-5PW column, 50-μl samples of representative fractions were separated by 5 to 20% gradient gel followed by silver stain to identify predominant RNA pol II-containing fractions. Simultaneously, 15-μl samples of matching fractions were used for SDS-PAGE and Western blot analysis with combined antibodies to hsRPB7 and hsRPB4 with goat anti-rabbit alkaline phosphatase-conjugated secondary antibody for visualization. Finally, representative fractions were also assayed for RNA pol II enzymatic activity with the assay for nonspecific transcriptional activity described in reference 54. Assays were carried out in the presence or absence of α-amanitin (2 μl/ml) to confirm activity dependence on RNA pol II.
Nucleotide sequence accession numbers.
The hsRPB4 cDNA sequence and partial flanking genomic sequence have been submitted to GenBank under accession no. U85510. The hsRPB4 genomic sequence has been submitted to GenBank under accession no. U89387.
RESULTS AND DISCUSSION
Cloning of hsRPB4 as a partner protein for the human RNA polymerase II subunit hsRPB7.
We used a LexA-fused hsRPB7 gene as a probe to perform a two-hybrid screen of a HeLa cDNA library. Two of the nine positive clones following a screen of 3.5 × 105 primary transformants demonstrated significant homology to the carboxy-terminal region of yeast RPB4 compared to sequences in the GenBank database (Fig. 1). These library clones represented two independent isolates of a cDNA of 1,855 bp in length, encoding an ORF of 381 bp (127 amino acids). This cDNA was designated hsRPB4. Because this ORF did not contain an initiating methionine and encoded a protein shorter than yeast RPB4, we performed a series of experiments to determine the complete coding sequence of hsRPB4 and to obtain a full-length clone.
FIG. 1.
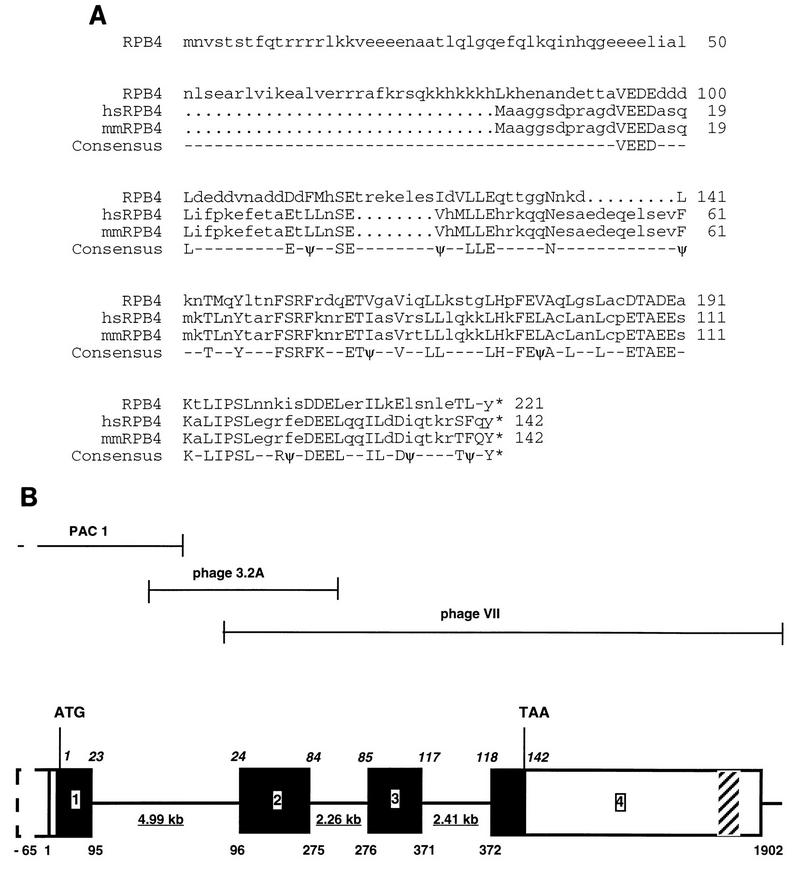
Structure of the hsRPB4 protein and gene. (A) Comparison of hsRPB4 predicted protein sequence with yeast RPB4 and putative mouse RPB4 subunits. Identical amino acid residues are shown in consensus. Conserved hydrophobic amino acids are indicated with ψ. (B) Genomic structure of hsRPB4. The full-length assembled cDNA for hsRPB4 is 1,902 bp. The genomic structure consists of four exons, which span ∼12 kb. Exons are represented as boxes, with translated sequence filled in black; introns are represented as lines. Underlined numbers represent the size of introns. Italicized numbers above the sequence represent amino acid positions relative to exon endpoints. Numbers below the sequence represent nucleotides of the hsRPB4 cDNA relative to exon-intron boundaries: in this case, 1 is taken to represent the first nucleotide of the longest cDNA obtained. The ORF encoding the hsRPB4 protein commences at 23 bp on this scale and extends 426 bp to position 449, followed by 1,453 bp of 3′ untranslated sequences. An Alu J family repeat is present at positions 919 to 1199 bp. The DNA sequence between 1498 and 1822 bp (shown hatched) is strongly homologous to the 40S ribosomal protein S26 and appears to be a novel S26 human pseudogene. An in-frame stop codon is present 65 bp upstream of position 1 of the cDNA, with no intervening splice sequences. The approximate endpoints of the three genomic clones used to generate sequence are shown above the diagram.
With a combination of library screening and PCR-based approaches (Materials and Methods), the hsRPB4 sequence was extended an additional 34 bp of continuous ORF. These were supplemented by overlapping EST databases identified in GenBank and the Institute of Genomic Research, yielding an additional 26 bp of 5′ sequence which incorporated 22 bp of putative 5′ untranslated sequence and an in-frame methionine starting translation of 15 additional amino acids upstream of the start point of the hsRPB4 cDNA isolated in the two-hybrid screen. Supporting the idea that this represented the true 5′ end of the hsRPB4 gene, we additionally identified multiple sequences derived from a related murine cDNA (here designated mmRPB4, Fig. 1A) by scanning the EST databases; of these, the most-5′-extended sequence contained a cDNA endpoint almost identical to that of hsRPB4. Based on these screening results and on Northern analysis in which an hsRPB4 probe hybridized to an mRNA species of ∼2 kb (Fig. 2), it appeared that the human assembled cDNA corresponded to a complete or near-complete hsRPB4 cDNA, with a transcript of 1,902 bp [exclusive of the poly(A) tail] and a 142-amino-acid ORF, encoding a protein with a calculated molecular size of 16.3 kDa. However, because the 5′ untranslated sequence did not contain a stop codon in-frame to the initiating methionine, and because the predicted hsRPB4 protein was substantially shorter than the yeast RPB4 protein (221 amino acids), this did not constitute complete proof of a full-length amino acid sequence.
FIG. 2.
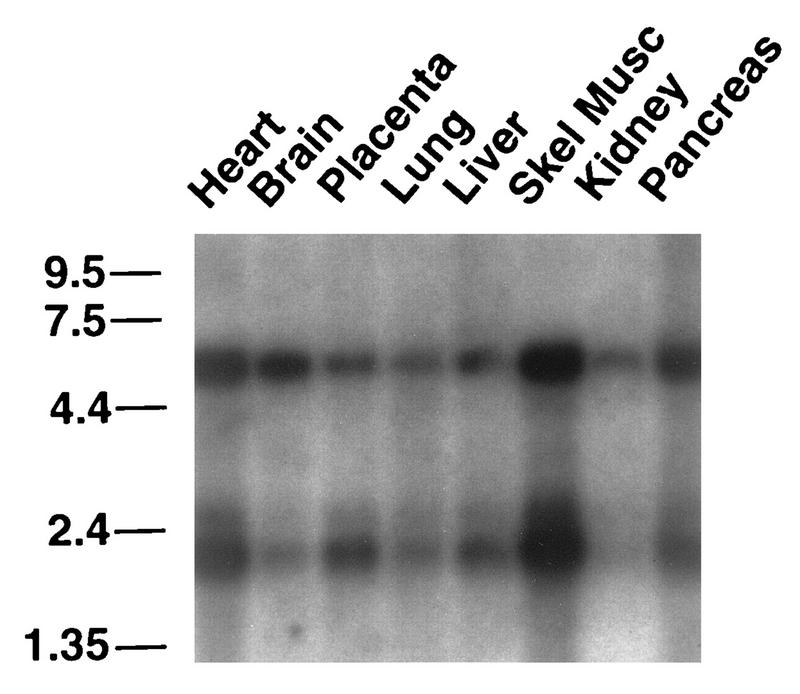
hsRPB4 RNA expression. A multitissue RNA blot was probed with either random primed hsRPB4 coding sequence (shown) or oligonucleotides specific to hsRPB4, with similar results. Reprobe of the same blot with an actin probe (40) confirmed equal load of all lanes.
Therefore, to complement the analysis of the hsRPB4 cDNA and gain additional evidence that we possessed a full-length clone, we undertook an analysis of the genomic structure of the hsRPB4 gene. Using probes based on the hsRPB4 cDNA (see Materials and Methods), we obtained a series of overlapping genomic clones. Sequence analysis of these clones allowed us to establish intron-exon boundaries for the hsRPB4 gene (Fig. 1B), revealing that the hsRPB4 cDNA encompasses four exons and three introns dispersed over 13 kb. Significantly, through this sequencing, we have established that the exon containing the putative hsRPB4 methionine contains an in-frame stop codon 88 bp upstream, confirming that the described hsRPB4 cDNA is full length. Through S1 nuclease assays (37a), we tentatively assign the start site of transcription to a region 118 to 111 bp upstream of the hsRPB4 ATG, although we have not characterized the promoter. Finally, fluorescence in situ hybridization mapping was used to assign the hsRPB4 gene to chromosome 2q21 (results not shown).
The expression pattern of the hsRPB4 mRNA by multitissue Northern blotting reiterates a pattern seen with a number of previously defined subunits of RNA polymerase II (Fig. 2). The hsRPB4 probe hybridized to an mRNA species of ∼2 kb, corresponding to the assembled cDNA, and to a second mRNA species of ∼5.5 kb, which may represent a 5′ or 3′ extended or alternatively spliced form of our current cDNA. While these species are present in all tissues, they are most abundant in skeletal muscle and heart. This pattern is similar to the one we had previously identified for the hsRPB7 transcript (37) as well as those reported for hsRPB6 (47), hsRPB5 (50), and hsRPB11 (23) when similar blots were used. However, this pattern does not correspond to a defective loading of the commercially available blot, as other probes on the same blot yield discrete patterns (40, 58); instead, it suggests that there is a moderate tissue-specific bias to a coordinate regulation of a number of RNA pol II subunits.
Conservation of hsRPB4, mmRPB4, and RPB4 subunits.
Comparison of the aligned RPB4, hsRPB4, and mmRPB4 sequences reveals several features which might be particularly relevant to their functional analysis (Fig. 1). First, the hsRPB4 gene is very highly conserved with its murine homolog, with 98% amino acid identity compared to an average of 85% identity between human and mouse sequences (43). In contrast, because of their shared amino-terminal truncation, both the human and mouse sequences diverge significantly from the yeast RPB4 gene, maintaining only 31% identity (56% similarity) over the entire sequence. This conserved sequence is concentrated in the carboxy-terminal 80 amino acids of the respective proteins. If this is the only region considered, homology rises to 40% identity and 63% similarity. This suggests that evolutionarily conserved essential functions such as docking to the pol II holoenzyme might be predicted to localize to the carboxy-terminal end of the proteins, while the amino-terminal end would contact more divergent proteins.
Second, in spite of their overall sequence divergence, hsRPB4, mmRPB4, and RPB4 conserve a similar charge profile, with total charges in the second percentile of all surveyed proteins (35) and extremely acidic character, with a pI of 4.6 to 4.7. Such a charged, acidic profile has been shown to be present in the transcriptional activation domains of many transcriptional regulatory proteins (52) and is strikingly possessed by a number of other previously defined subunits of RNA polymerase II (hsRPB3, hsRPB6, and hsRPB8), possibly implying interaction with cognate positively charged protein partners, including additional pol II subunits (hsRPB12) or chromatin-associated histones.
hsRPB4 interacts specifically with hsRPB7 and partially complements rpb4− defects in yeast.
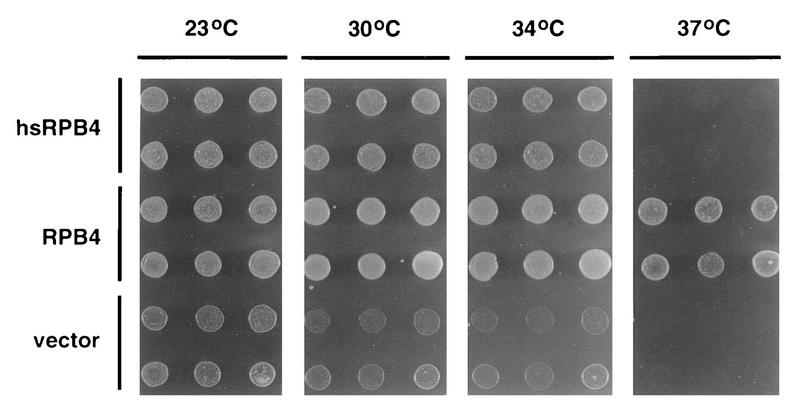
A number of the human homologs of yeast RNA pol II subunits are sufficiently conserved evolutionarily as to complement mutations in their yeast counterparts (37, 46, 60). We sought to determine whether hsRPB4 is able to functionally complement an rpb4 null mutation (Fig. 3). Deletion of RPB4 in S. cerevisiae is not lethal but does result in characteristic heat and cold sensitivity (68) as well as loss of viability at stationary phase (16, 17). In parallel, we transformed the rpb4− strain WY-4 (RPB4Δ1::His3) (a gift of N. Woychik) with vector pYES2, pYES2-RPB4, or pYES2-hsRPB4 and compared the ability of RPB4 and hsRPB4 to support growth at 23°C (permissive for rpb4−), 30°C (semipermissive for rpb4−), 34°C, and 37°C. After 48 to 72 h, all yeast containing RPB4 grew at all temperatures. All yeasts containing only vector were viable at 23°C (although they grew somewhat more slowly than yeast with RPB4) and showed marginal growth at 30 and 34°C and no growth at 37°C. Yeast expressing hsRPB4 displayed an intermediate phenotype, being identical to yeast expressing RPB4 at 23°C and only slightly reduced in growth rate at 30 and 34°C but generally inviable at 37°C. Some degree of colony heterogeneity was observed, with 20 to 30% of hsRPB4-expressing yeast colonies eventually growing at 37°C after several additional days (data not shown). We took this data to be an indication of a partial complementation.
FIG. 3.
hsRPB4 partial complementation of rpb4 null yeast. WY-4 containing pYES2 vector, pYES2-RPB4, or pYES2-hsRPB4 were diluted in suspension, and identical inocula dotted to plates were maintained for 2 days at 23, 30, 34, or 37°C as indicated.
S. cerevisiae RPB4 and RPB7 have previously been shown to associate with pol II as a subcomplex (20), leading us to consider the hypothesis that the partial complementation by hsRPB4 of the rpb4− mutation might derive from poor association with yeast RPB7. Supporting this possibility, in previous work we showed that while yeast RPB4 interacts strongly with RPB7, it interacts only weakly with hsRPB7; perhaps for this reason, hsRPB7 only partially complemented deletions of the rpb7− gene, rescuing lethality but acquiring a heat and cold sensitivity reminiscent of an rpb4− mutation (37).
To test the possibility that the partial ability of hsRPB4 to rescue rpb4− mutation might involve reduced ability or inability of hsRPB4 to interact with RPB7, we used two approaches. First, we repeated the complementation experiment with the addition of human hsRPB7, transforming WY-4 yeast with pYES2 plus pZeoV, pYES2-RPB4 plus pZeoV, pYES2-hsRPB4 plus pZeoV, or pYES2-hsRPB4 plus pZeoV-hsRPB7. In general, it appeared that a slightly higher percentage of pYES-hsRPB4 colonies were viable at 37°C with coexpressed hsRPB7 (data not shown); however, because of the colony heterogeneity of the hsRPB4 phenotype, results of this experiment were equivocal. Second, we used a two-hybrid approach to compare the interactions between RPB4-RPB7, hsRPB4-hsRPB7, RPB4-hsRPB7, and hsRPB4-RPB7 (Table 1). The results indicate that while the RPB4-RPB7 and hsRPB4-hsRPB7 pairs interact strongly and RPB4-hsRPB7 pairs interact weakly, hsRPB4 and RPB7 do not detectably interact, even though all proteins are expressed at equivalent levels in yeast (37a). This finding suggested the human pair of subunits have codiverged from their yeast homologs, maintaining the ability to form a high-affinity subcomplex.
TABLE 1.
Interaction between human and yeast homologs of RPB4 and RPB7
| LexA fusion | Ratio of β-galactosidase values for:
|
||
|---|---|---|---|
| JG4-5 | JG4-5-RPB4 | JG4-5-hsRPB4 | |
| LexA-Bic2-160 | 1.5 | 3.8 | 4.9 |
| LexA-RPB7 | 1 | 98 | 1.4 |
| LexA-hsRPB7 | 2.2 | 15 | 262 |
EGY191 yeast cells were transformed with the pSH18-34 LexA-operator-LacZ-reporter (28) and indicated combinations of LexA-fused and activation domain-fused (pJG4-5) proteins. β-Galactosidase activities were calculated as the average values for six separate colonies. The numbers shown reflect the ratio of β-galactosidase values obtained for different sets of constructs, with the lowest such value standardized to 1. The difference in values between independent colonies assayed did not exceed 15%.
From these results, we conclude that hsRPB4 maintains some ability to associate with the pol II complex in the absence of, or with minimal contribution from, association with an RPB7 or hsRPB7 partner, but that the hsRPB7 partner is likely to enhance this association. hsRPB4 is able to associate with hsRPB7 in the absence of any additional mammalian pol II subunits, suggesting that these proteins form a subcomplex similar to RPB4-RPB7. Further, these results demonstrate that hsRPB4 is a functionally conserved homolog of RPB4 and hence is likely to function as a previously undescribed subunit of human RNA polymerase II.
Functional analysis of hsRPB4 protein interaction domains.
The relatively poor homology observed between amino acids 1 and 140 of RPB4 and amino acids 1 to 60 of hsRPB4 and mmRPB4 suggested that this region might be a good candidate for encompassing the binding site for the interaction with diverged partner proteins RPB7 and hsRPB7. Conversely, the well-conserved carboxy-terminal domain of the proteins might reasonably correspond to a docking site for hsRPB4 with the pol II large complex. We used a two-hybrid approach to examine these issues.
To assign the site of interaction between hsRPB4 and hsRPB7, we analyzed a series of trunctions of the two proteins (hsRPB71–172 [full length], hsRPB71–54, hsRPB71–94, hsRPB754–172; hsRPB41–142 [full length], hsRPB41–47, hsRPB41–92, hsRPB41–118, and hsRPB455–142) for the ability to associate (Table 2). This study indicated that amino acids 1 to 92 on hsRPB4 were sufficient for efficient interaction with hsRPB7, while amino acids 1 to 47 and 55 to 142 were not, suggesting that hsRPB7 association with hsRPB4 required sequences spanning the amino-terminal half of the hsRPB4 protein, as predicted based on the RPB4 and hsRPB4 sequence divergence in this region. In contrast, no truncation of hsRPB7 was observed to associate with hsRPB4, suggesting either that the structure of the hsRPB7 protein was grossly deformed by truncation or that hsRPB4 interacted with multiple regions of the protein.
TABLE 2.
Mapping of the interaction domains of hsRPB4 and hsRPB7
| LexA fusion | β-Galactosidase values fora:
|
|||||
|---|---|---|---|---|---|---|
| hsRPB41–142 | hsRPB41–47 | hsRPB41–92 | hsRPB41–118 | hsRPB455–142 | Vector | |
| hsRPB71–172 | 1,634 | 34 | 2,738 | 3,522 | 71 | 45 |
| hsRPB71–54 | 3 | 3 | 3 | 4 | 3 | 5 |
| hsRPB71–94 | 2 | 3 | 5 | 3 | 3 | 2 |
| hsRPB754–172 | 1 | 6 | 8 | 5 | 7 | 6 |
| Bicoid | 4 | 2 | 3 | 3 | 5 | 3 |
EGY191 yeast cells were transformed with the pSH18-34 LexA operator-LacZ reporter (28) and indicated combinations of LexA-fused and activation domain-fused (pJG4-5) proteins. β-Galactosidase activities were calculated as the average value for four separate colonies. The numbers shown are the β-galactosidase values obtained for different constructs.
Endogenous hsRPB4 and hsRPB7 associate with pol II in the mammalian cell nucleus.
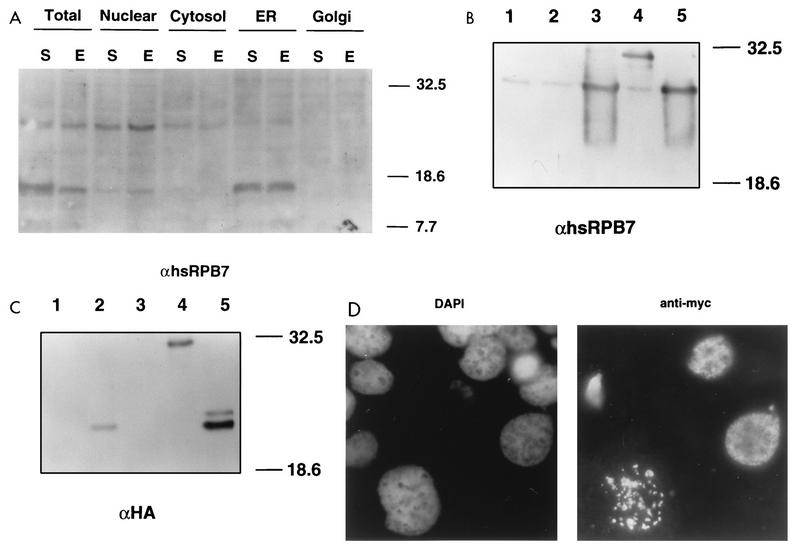
We next characterized the association of hsRPB4 and hsRPB7 in mammalian cells. First we used antibodies to the two proteins to attempt to determine the subcellular localization of the endogenous proteins by immunofluorescence. By this means, anti-hsRPB7 antiserum indicated that while the majority of signal was detected in the cell nuclei, as would be expected for a subunit of RNA pol II, diffuse staining also was noticeable in the cytoplasm, while anti-hsRPB4 signal appeared to be primarily nuclear; it was only marginally detectable (data not shown). We had previously used anti-hsRPB7 antiserum to probe cell lysates and showed that the antibody detected three protein species: a doublet migrating at approximately 17 to 18 kDa (in the expected range for the calculated molecular size of hsRPB7) and a higher-migrating band of approximately 24 to 26 kDa (37). To clarify the immunofluorescence results, we used subcellular fractionation (Fig. 4A) to determine that the larger species, migrating at ∼26–27 kDa was predominantly present in the nuclear fraction with minor localization to cytoplasmic and endoplasmic reticulum (ER) fractions, while the 17-kDa species localized predominantly to the ER. Transfection of mammalian cells with vectors expressing hsRPB7 as native protein or tagged with the HA epitope tag (Fig. 4B and C), followed by visualization with either hsRPB7 or HA-specific antiserum (data not shown), confirmed that the 26 to 27 kDa nuclear species corresponded to the hsRPB7 protein, while the 17 to 18 kDa species was likely to reflect a cross-reactive ER protein unrelated to hsRPB7. To clarify the hsRPB4 localization, we used a second approach, transfecting cells with Myc-tagged (Fig. 4C) or HA-tagged (not shown) hsRPB4 into Cos cells, and confirmed specific expression of the tagged protein as an ∼21 to 23 kDa species. Immunofluorescence visualization of cells transfected with tagged hsRPB4 indicated that at 24 h after transfection, hsRPB4 was entirely nuclear (Fig. 4D).
FIG. 4.
Nuclear colocalization of hsRPB7 and hsRPB4. (A) Human HeLA cells which were starved (incubation in DMEM media with 0.5% fetal bovine serum for 72 h) (S) or exponentially growing (DMEM media with 10% of fetal bovine serum) (E), were fractionated as described in Materials and Methods. Total protein (2.5 μg) in the fractions indicated was resolved on an SDS–12% PAGE gel, and proteins were visualized by with rabbit polyclonal antibody against hsRPB7 followed by goat anti-rabbit antibody conjugated to horseradish peroxidase with enhanced chemiluminescence. The relative mobilities of the molecular size standards are indicated in kilodaltons. (B and C) Whole-cell lysates from Cos-1 cells transiently transfected with a pCMV vector (lane 1) or with pCMV vector expressing HA-tagged hsRPB4 (lane 2), native hsRPB7 (lane 3), HA-tagged hsRPB7 (lane 4), or both pCMV-hsRPB7 and pCMV-HA-hsRPB4 (lane 5) were resolved on duplicate SDS–12% PAGE gels. Blots were probed with rabbit anti-hsRPB7 polyclonal antibody (B) or rabbit polyclonal antibody against the HA tag (C). Proteins were visualized with goat anti-rabbit antibody conjugated to horseradish peroxidase as described in Materials and Methods. The estimated size for hsRPB7 is ∼27 kDa, that for HA-hsRPB7 is ∼32 kDa, and that for HA-hsRPB4 is ∼22 kDa. The mobilities of the molecular size standards are indicated in kilodaltons. (D) Cos-1 cells, transiently transfected with pCMV vector expressing a Myc-hsRPB4 fusion, were incubated 24 h in standard media and stained with DAPI (left) to visualize nuclei and with mouse monoclonal anti-Myc antibody followed by goat anti-mouse immunoglobulin G-rhodamine conjugate. Note that in approximately 10 to 20% of transfected cells HA-hsRPB4 localized to large granular structures in the nucleus. At this time, the most likely explanation for these structures is that they represent aggregates of overexpressed protein, although the possibility that they indicate the association of hsRPB4 with particular nuclear compartments, as reported for other pol II subunits (12, 65), has not been rigorously excluded.
To confirm hsRPB7-hsRPB4 interaction in mammalian cells in vivo, whole-cell lysates were prepared from cells expressing HA-hsRPB4. We then used antibody to HA to perform coimmunoprecipitation, visualizing with antibody to hsRPB7 (Fig. 5). HA-hsRPB4 and endogenous hsRPB7 coimmunoprecipitated efficiently and specifically, supporting the idea that these two proteins function coordinately in vivo. Similar results were subsequently obtained with antibody to hsRPB4, in untransfected cells (data not shown).
FIG. 5.
Association of hsRPB4 and hsRPB7 in mammalian cells. Cos-1 cells transiently transfected with pCMV vector alone or with pCMV vector containing HA-hsRPB4 were immunoprecipitated with rabbit polyclonal antibody specific for HA. Whole-cell lysates (lanes 1 and 2) or immunoprecipitates (lanes 3 and 4) from Cos-1 cells transfected with pCMV vector (lanes 1 and 3) or with pCMV vector expressing HA-hsRPB4 fusion protein (lanes 2 and 4) were resolved on an SDS–12% PAGE gel. Endogenous and coimmunoprecipitated hsRPB7 proteins were visualized with rabbit polyclonal antibody to hsRPB7 as described above. The species migrating at ∼32 kDa in the two lanes of immunoprecipitation is nonspecific.
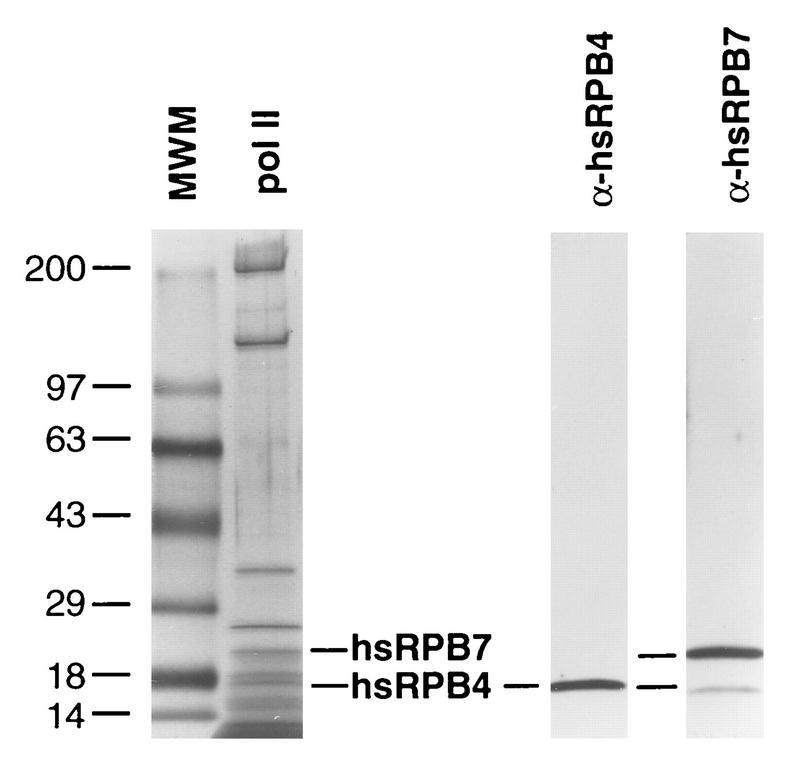
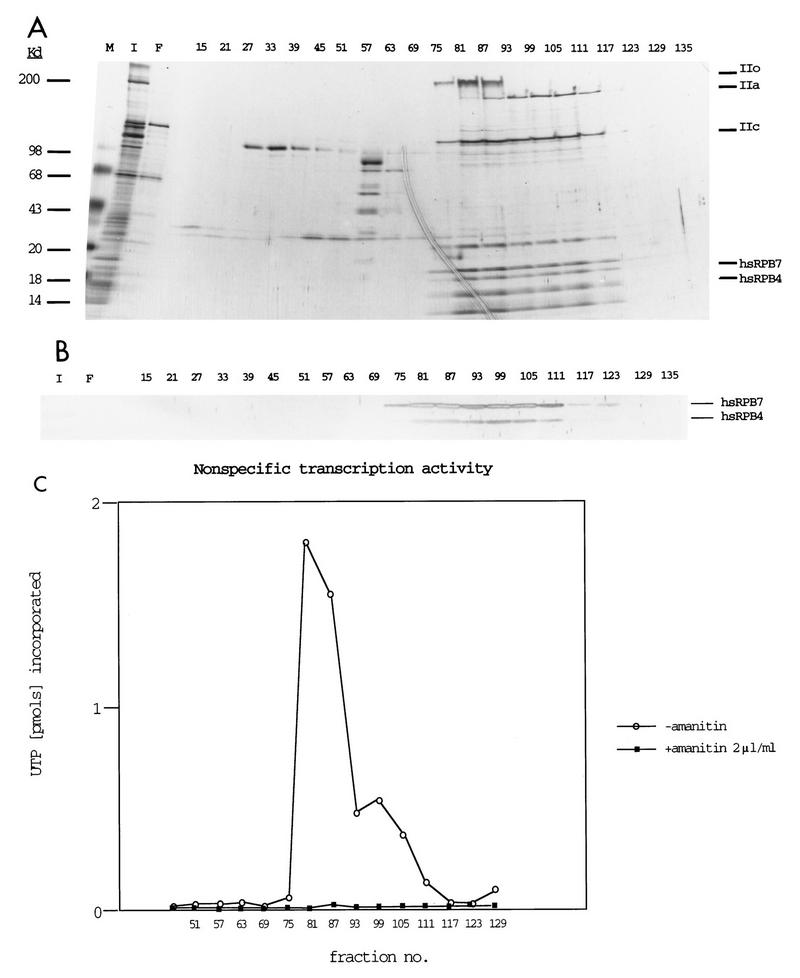
Finally, RPB4 and RPB7 in yeast have been reported to associate substoichiometrically with the remainder of the pol II subunits, with only 10 to 20% of purified pol II complexes containing RPB4-RPB7 in actively growing cells but with 100% of such complexes containing RPB4-RPB7 in stressed or stationary-phase cells (20). Although endogenous hsRPB4 and hsRPB7 were detectable in mammalian cells, it was also of interest to determine if appreciable quantities of these proteins could be detected in association with pol II in cultured mammalian cells. Accordingly, antibody to the CTD of the largest subunit of RNA pol II was used to immunoaffinity purify pol II from HeLa cell nuclei, allowing scrutiny with antibodies to hsRPB4 and hsRPB7 (Fig. 6). By this means, strong signal was observed for both of the proteins. To confirm this result, we additionally utilized conventional chromatographic purification to isolate RNA polymerase II (Fig. 7). Fractions of column-purified pol II were resolved by gradient gel and silver stained to identify the peak of pol II subunit purification (Fig. 7A), while matching fractions were probed in Western analysis with antibodies to hsRPB4 and hsRPB7 (Fig. 7B). Finally, fractions were assayed for nonspecific transcriptional activity in the presence and absence of α-amanitin to confirm the purification of biologically active RNA pol II (Fig. 7C). These assays indicated that peak pol II activity and the hsRPB7 and hsRPB4 subunits cosegregated. Cumulatively, these assays demonstrate that hsRPB4 and hsRPB7 are associated with pol II in actively growing human cells.
FIG. 6.
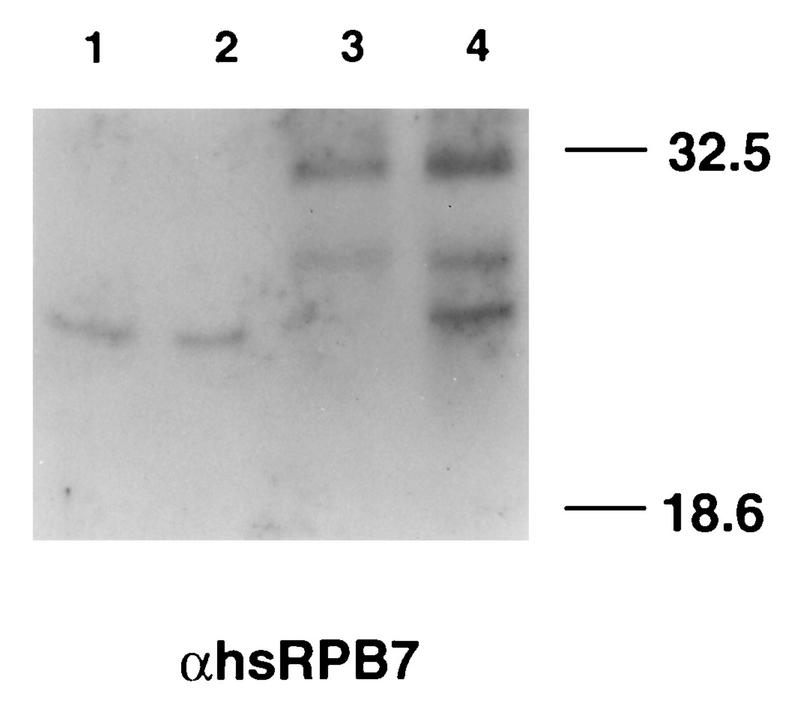
hsRPB4 and hsRPB7 are constituents of anti-CTD immunoaffinity-purified human pol II holoenzyme. Parallel samples of purified human polymerase II complex isolated by immunoprecipitation with anti-CTD (as described in reference 44) were resolved by SDS-PAGE. Left, silver stain of molecular size markers (MWM) and pol II to confirm purification; right, sample was blotted to membrane, probed with antibody to hsRPB4, and then reprobed without intervening strip with antibody to hsRPB7, confirming the presence of these proteins in the complex.
FIG. 7.
hsRPB4 and hsRPB7 are constituents of chromatographically purified transcriptionally active human pol II. (A) Fractions of high-pressure liquid chromatography DEAE-5PW column purified RNA polymerase II (44) were resolved by gradient gel and silver stained. M, molecular size marker; I, input; F, flow- through. The numbers across the top indicate fraction numbers. To the right are shown the positions of marker pol II subunits and approximate migration of hsRPB4 and hsRPB7 (compare with banding pattern in Fig. 6). (B) Parallel fractions resolved by SDS-PAGE and Western blotted, probed with combined antibodies to hsRPB4 and hsRPB7, and visualized with alkaline-phosphatase-conjugated secondary antibody. (C) Assay of nonspecific transcription activity of fractions based on incorporation of UTP into acid-insoluble material in 20 min with φX174 DNA as template, as described in reference 54.
Conclusion.
In this work we report the cloning of hsRPB4 based on interaction with its partner protein, hsRPB7. Through two-hybrid and complementation assays, we establish that hsRPB4 has diverged amino terminally from its yeast homolog RPB4 to the extent that it does not detectably associate with yeast RPB7 and only partially rescues an rpb4− mutation. However, hsRPB4 interacts strongly with hsRPB7 following coexpression of the two proteins in yeast or with endogenous hsRPB7 in the nuclei of human cells. hsRPB4 is also a significant component of RNA polymerase II purified from mammalian cells; we propose that the interaction with the pol II complex is mediated by conserved residues on the carboxy-terminal end of hsRPB4. This characterization completes the identification of human homologs of yeast pol II subunits, a comprehensive summary of whose properties is presented in Table 3.
TABLE 3.
Summary of yeast and human RNA polymerase II subunit compositiona
| S. cere- visiae subunit | Alternate names | Human subunit | Alternate names | No. of amino acids
|
% Amino acid conservation (identity, similarity) | pI (yeast/human) | Comple- menta- tion | Human chromosomal mapping | Reference(s) | |
|---|---|---|---|---|---|---|---|---|---|---|
| S. cere- visiae | Human | |||||||||
| RPB1 | Sc1, B220, RPO21 | hsRPB1 | Hs1, hRPB220, POLR2 A | 1,733 | 1,970 | 56, 73 | 5.4/7.3 | ND | 17p13 | 3, 66 |
| RPB2 | Sc2, B150 | hsRPB2 | Hs2, hRPB140, POLR2 B | 1,224 | 1,174 | 61, 77 | 6.7/6.9 | ND | 4q12 | 1, 3 |
| RPB3 | Sc3, B44 | hsRPB3 | Hs3, hRPB33, POLR2 C | 318 | 275 | 48, 67 | 4.4/4.6 | ND | 16q13-q21 | 3, 51 |
| RPB4 | Sc4, B32 | hsRPB4 | Hs4, “POLR2 D” | 221 | 142 | 31, 56 | 4.7/4.6 | Partial | 2q21 | This work |
| RPB5 | Sc5, ABC27 | hsRPB5 | Hs5, hRPB25, POLR2 E | 215 | 210 | 47, 68 | 9.8/5.5 | No | 19p13.3 | 3, 50 |
| RPB6 | Sc6, ABC23, RPO26 | hsRPB6 | Hs6, hRPB14.4, POLR2 F | 155 | 127 | 56, 77 | 4.4/3.9 | Yes | 22q13.1 | 3, 47, 52a |
| RPB7 | Sc7, B16 | hsRPB7 | Hs7, hRPB19b, POLR2 G | 171 | 172 | 44, 64 | 7.8/5.3 | Partial | ND | 37 |
| RPB8 | Sc8, ABC14.5 | hsRPB8 | Hs8, hRPB17, POL2R H | 146 | 150 | 37, 65 | 4.4/4.3 | Yes | ND | 46, 60 |
| RPB9 | Sc9, B12.6 | hsRPB9 | Hs9, hRPB14.5, POL2R I | 122 | 125 | 45, 67 | 7.9/4.9 | Partial | 19q12 | 2, 3, 46 |
| RPB10 | Sc10β, ABC10β | hsRPB10 | Hs10β, hRPB7.6, POL2R L | 70 | 67 | 75, 82 | 7.8/7.8 | Yes | 11p15 | 4, 46, 60 |
| RPB11 | Sc11, B12.5 | hsRPB11 | Hs11, hRPB14, POL2R J | 120 | 117 | 47, 70 | 5.3/5.7 | ND | ND | 23 |
| RPB12 | Sc10α, RPC10, ABC10α | hsRPB12 | Hs10α, hRPB7.0, POL2R K | 70 | 58 | 39, 60 | 10.1/9.5 | Yes | ND | 60 |
Data from previous related compilations (46, 60) as well as from chromosomal mapping studies and this study are given. ND, not determined.
hRPB19 is a tentative name, and gel-based mobility prediction for hsRPB7 (60) reflects calculated mass; in fact, the observed mobility on the gel is ∼27 kDa, as noted in the text.
The biochemistry of pol II is better established in S. cerevisiae than in human cells. While a number of groups have purified human pol II and associated proteins (26, 32, 36, 45, 51; also reviewed in references 57 and 70), it has been clear from a comparison of results that the number, molecular weight, and quantity of copurified proteins are somewhat variable between different groups and starting sources of material, and it was previously uncertain whether a human RPB4 homolog existed. The ∼27 kDa hsRPB7 species and ∼18- to 19-kDa native hsRPB4 species described in this work are now shown to be present in affinity-purified pol II, conclusively demonstrating their functionality in mammalian cells. The question of what their function might be remains to be answered.
RPB4 and RPB9 are the only subunits of yeast RNA polymerase II that have been shown to possess a null phenotype of viability with cold and temperature sensitivity (67, 68). In subsequent functional studies, RPB9 has been shown to function as a regulator of start site selection in transcriptional initiation (33) and elongation (9), and incorporation of RPB4-RPB7 into yeast pol II has been shown to regulate transcriptional initiation (20). In a further intriguing development, hsRPB7 has recently been identified by two groups as an interacting factor for two proteins involved in transcription, the EWS-Fli oncogenic fusion and the retinoic acid receptor (38a, 59a). In each case, regulation of the levels of hsRPB7 was demonstrated to modulate the activity of the associated transcription factor (38a, 59a). Together, these results support the idea that hsRPB4-hsRPB7 may function as a site of interaction between enhancer-binding transcription factors and the central pol II complex. Because of the variable incorporation of RPB4-RPB7 into pol II in stationary phase, we and other groups have hypothesized that these proteins might act in a manner analogous to bacterial sigma factors (59), to modulate transcription in a manner conducive to promoting stress survival (13, 16, 17, 37). Finally, recent structural studies of yeast RNA pol II in the presence and absence of the RPB4-RPB7 subcomplex have indicated that addition of RPB4-RPB7 to pol II acts to convert pol II from an open complex which allows entry of the enzyme to DNA to a closed complex which is more stably retained during transcriptional elongation (6, 34), leading to the proposal (34) that these proteins may enhance resistance to cellular stresses by facilitating the accumulation of paused transcripts proximal to heat shock promoters (53). If a similar function and mechanism of activity are retained in mammalian cells, as seems likely, then further study of the hsRPB4 and hsRPB7 proteins will be of particular interest in future analyses of the modulation of transcription in response to stress.
ACKNOWLEDGMENTS
This study was supported in part by a grant from the Elsa U. Pardee Foundation and NIH grant CA-70841 (to E.A.G.), by the core NIH grant CA-06927 (to E.A.G. and J.R.T.), and by an appropriation from the Commonwealth of Pennsylvania.
We thank Susan Law, Feng Liu, and Dennis Gately for help with subcellular fractionation, Ilya Serebriiskii for help with the complementation study, and Vadim Bichko for advice and reagents. We are very grateful to Nancy Woychik for reagents and advice and to Danny Reinberg for invaluable help with the pol II purification. We thank Glenn Rall, Claude Kedinger, Marc Vigneron, and Susan Law for helpful comments on the manuscript.
REFERENCES
- 1.Acker J, Wintzerith M, Vigneron M, Kedinger C. Primary structure of the second largest subunit of human RNA polymerase II (or B) J Mol Biol. 1992;226:1295–1299. doi: 10.1016/0022-2836(92)91071-v. [DOI] [PubMed] [Google Scholar]
- 2.Acker J, Wintzerith M, Vigneron M, Kedinger C. Structure of the gene encoding the 14.5 kDa subunit of human RNA polymerase II. Nucleic Acids Res. 1993;21:5345–5350. doi: 10.1093/nar/21.23.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acker J, Wintzerith M, Vigneron M, Kedinger C. A 14.4 KDa acidic subunit of human RNA polymerase II with a putative leucine-zipper. DNA Sequence. 1994;4:329–331. doi: 10.3109/10425179409020860. [DOI] [PubMed] [Google Scholar]
- 4.Acker J, Murroni O, Mattei M G, Kedinger C, Vigneron M. The gene (POLR2L) encoding the hRPB7.6 subunit of human RNA polymerase. Genomics. 1996;32:86–90. doi: 10.1006/geno.1996.0079. [DOI] [PubMed] [Google Scholar]
- 5.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 6.Asturias F J, Meredith G D, Poglitsch C L, Kornberg R D. Two conformations of RNA polymerase II revealed by electron crystallography. J Mol Biol. 1997;272:536–540. doi: 10.1006/jmbi.1997.1273. [DOI] [PubMed] [Google Scholar]
- 7.Ausubel F M, Brent R, Kingston R, Moore D, Seidman J, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 8.Avruch J, Wallach D. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971;233:334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- 9.Awrey D E, Weilbaecher R G, Hemming S A, Orlicky S M, Kane C M, Edwards A M. Transcriptional elongation through DNA arrest sites: a multistep process involving both RNA polymerase II subunit RPB9 and TFIIS*. J Biol Chem. 1997;272:14747–14754. doi: 10.1074/jbc.272.23.14747. [DOI] [PubMed] [Google Scholar]
- 10.Baskaran R, Dahmus M E, Wang J Y J. Tyrosine phosphorylation of mammalian RNA polymerase II carboxy-terminal domain. Proc Natl Acad Sci USA. 1993;90:11167–11171. doi: 10.1073/pnas.90.23.11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell D W, Taguchi T, Jenkins N A, Gilbert D J, Copeland N G, Gilks C B, Zweidler-McKay P, Grimes H L, Tsichlis P N, Testa J R. Chromosomal localization, in man and rodents, of a gene (GFl1) encoding a novel zinc finger protein reveals a new syntenic region between mouse and man. Cytogenet Cell Genet. 1995;70:263–267. doi: 10.1159/000134048. [DOI] [PubMed] [Google Scholar]
- 12.Bregman D B, Du L, van der Zee S, Warren S L. Transcription-dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J Cell Biol. 1995;129:287–298. doi: 10.1083/jcb.129.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brendel V, Karlin S. Applications of statistical criteria in protein sequence analysis: case study of yeast RNA polymerase II subunits. Comput Chem. 1994;18:251–253. doi: 10.1016/0097-8485(94)85020-8. [DOI] [PubMed] [Google Scholar]
- 14.Brent R, Ptashne M. The lexA gene product represses its own promoter. Proc Natl Acad Sci USA. 1980;77:1932–1936. doi: 10.1073/pnas.77.4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buratowski S. The basics of basal transcription by RNA polymerase II. Cell. 1994;77:1–3. doi: 10.1016/0092-8674(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 16.Choder M. A growth rate-limiting process in the last growth phase of the yeast life cycle involves RPB4, a subunit of RNA polymerase II. J Bacteriol. 1993;175:6358–6363. doi: 10.1128/jb.175.19.6358-6363.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choder M, Young R A. A portion of RNA polymerase II molecules has a component essential for stress responses and stress survival. Mol Cell Biol. 1993;13:6984–6991. doi: 10.1128/mcb.13.11.6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conaway R C, Conaway J W. General initiation factors for RNA polymerase II. Annu Rev Biochem. 1993;62:161–190. doi: 10.1146/annurev.bi.62.070193.001113. [DOI] [PubMed] [Google Scholar]
- 19.Deutscher M P. Guide to protein purification. Methods Enzymol. 1990;182:62–63. [PubMed] [Google Scholar]
- 20.Edwards A M, Kane C M, Young R A, Kornberg R D. Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J Biol Chem. 1991;266:71–75. [PubMed] [Google Scholar]
- 21.Estojak J, Brent R, Golemis E A. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y-S, Davis L M, Shows T B. Mapping small DNA sequences by fluorescence in situ hybridization directly on banded metaphase chromosomes. Proc Natl Acad Sci USA. 1990;87:6223–6227. doi: 10.1073/pnas.87.16.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanciulli M, Bruni T, Cerboni C, Bonetto F, Iacobini C, Frati L, Piccoli M, Floridi A, Santoni A, Punturieri A. Cloning of a novel human RNA polymerase II subunit downregulated by doxorubicin: new potential mechanisms of drug related toxicity. FEBS Lett. 1996;384:48–52. doi: 10.1016/0014-5793(96)00277-3. [DOI] [PubMed] [Google Scholar]
- 24.Feaver W J, Svejstrup J W, Henry N L, Kornberg R D. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell. 1994;79:1103–1109. doi: 10.1016/0092-8674(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 25.Frangioni J V, Beahm P H, Shifrin V, Jost C A, Neel B G. The non-transmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560. doi: 10.1016/0092-8674(92)90190-n. [DOI] [PubMed] [Google Scholar]
- 26.Freund E, McGuire P M. Characterization of RNA polymerase type II from human term placenta. J Cell Physiol. 1986;127:432–438. doi: 10.1002/jcp.1041270312. [DOI] [PubMed] [Google Scholar]
- 27.Golemis E A, Brent R. Fused protein domains inhibit DNA binding by LexA. Mol Cell Biol. 1992;12:3006–3014. doi: 10.1128/mcb.12.7.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golemis E A, Gyuris J, Brent R. Interaction trap/two-hybrid system to identify interacting proteins. In: Ausubel F M, Brent R, Kingston R, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1996. pp. 20.1.1–20.1.28. [Google Scholar]
- 29.Gyuris J, Golemis E A, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 30.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 31.Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 32.Hodo H G, III, Blatti S P. Purification using polyethylenimine precipitation and low molecular weight subunit analyses of calf thymus and wheat germ DNA-dependent RNA polymerase II. Biochemistry. 1977;16:2334–2343. doi: 10.1021/bi00630a005. [DOI] [PubMed] [Google Scholar]
- 33.Hull M W, McKune K, Woychik N A. RNA polymerase II subunit RPB9 is required for accurate start site selection. Genes Dev. 1995;99:481–490. doi: 10.1101/gad.9.4.481. [DOI] [PubMed] [Google Scholar]
- 33a.Institute for Genomic Research Consortium. Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature. 1995;377:3–174. [PubMed] [Google Scholar]
- 34.Jensen, G. J., G. Meredith, D. A. Bushnell, and R. D. Kornberg. Structure of wild type yeast RNA polymerase II and location of Rpb4 and Rpb7. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 35.Karlin S, Brendel V. Chance and statistical significance in protein and DNA sequence analysis. Science. 1992;257:39–49. doi: 10.1126/science.1621093. [DOI] [PubMed] [Google Scholar]
- 36.Kedinger C, Gissinger F, Chambon P. Animal DNA-dependent RNA polymerases. Eur J Biochem. 1974;44:421–436. doi: 10.1111/j.1432-1033.1974.tb03500.x. [DOI] [PubMed] [Google Scholar]
- 37.Khazak V, Woychik N, Sadhale P, Brent R, Golemis E A. Human RNA polymerase II subunit hsRPB7 functions in yeast and influences yeast cell morphology and stress survival. Mol Biol Cell. 1995;6:759–775. doi: 10.1091/mbc.6.7.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Khazak, V., and E. A. Golemis. Unpublished data.
- 38.Koleske A J, Young R A. The RNA polymerase II holoenzyme and its implications for gene regulation. Trends Biochem Sci. 1995;20:113–116. doi: 10.1016/s0968-0004(00)88977-x. [DOI] [PubMed] [Google Scholar]
- 38a.Kovar, H. Personal communication.
- 39.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 40.Law S F, Estojak J, Wang B, Mysliwiec T, Kruh G D, Golemis E A. Human enhancer of filamentation 1, a novel p130cas-like docking protein, associates with focal adhesion kinase and induces pseudohyphal growth in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3327–3337. doi: 10.1128/mcb.16.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao S-M, Zhang J, Jeffery D A, Koleske A J, Thompson C M, Chao D M, Viljoen M, van Vuuren H J J, Young R A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 42.Lu H, Glores O, Weinman R, Reinberg D. The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc Natl Acad Sci USA. 1991;88:10004–10008. doi: 10.1073/pnas.88.22.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makalowski W, Zhang J, Boguski M S. Comparative analysis of 1196 orthologous mouse and human full-length mRNA and protein sequences. Genome Res. 1996;6:846–857. doi: 10.1101/gr.6.9.846. [DOI] [PubMed] [Google Scholar]
- 44.Maldonado E, Drapkin R, Reinberg D. Purification of human RNA polymerase II and general transcription factors. Methods Enzymol. 1996;274:72–100. doi: 10.1016/s0076-6879(96)74009-0. [DOI] [PubMed] [Google Scholar]
- 45.Maldonado E, Shiekhattar R, Sheldon M, Cho H, Drapkin R, Rickert P, Lees E, Anderson C W, Linn S, Reinberg D. A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature. 1996;381:86–89. doi: 10.1038/381086a0. [DOI] [PubMed] [Google Scholar]
- 46.McKune K, Moore P A, Hull M W, Woychik N A. Six human RNA polymerase subunits functionally substitute for their yeast counterparts. Mol Cell Biol. 1995;15:6895–6900. doi: 10.1128/mcb.15.12.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKune K, Woychik N A. Functional substitution of an essential yeast RNA polymerase subunit by a highly conserved mammalian counterpart. Mol Cell Biol. 1994;14:4155–4159. doi: 10.1128/mcb.14.6.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 49.Mortillaro M J, Blencowe B J, Wei X, Nakayasu H, Du L, Warren S L, Sharp P A, Berezney R. A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc Natl Acad Sci USA. 1996;93:8253–8257. doi: 10.1073/pnas.93.16.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pati U K, Weissman S M. Isolation and molecular characterization of a cDNA encoding the 23 kDa subunit of human RNA polymerase II. J Biol Chem. 1989;264:13114–13121. [PubMed] [Google Scholar]
- 51.Pati U K, Weissman S M. The amino acid sequence of the human RNA polymerase II 33 kDa subunit hRPB 33 is highly conserved among eukaryotes. J Biol Chem. 1990;265:8400–8403. [PubMed] [Google Scholar]
- 52.Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 52a.Pusch C, Wang Z, Roe B, Blin N. Genomic structure of the RNA polymerase II small subunit (hRPB14.4) locus (POLRF) and mapping to 22q13.1 by sequence identity. Genomics. 1996;34:440–442. doi: 10.1006/geno.1996.0312. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen E B, Lis J T. Short transcripts of the ternary complex provide insight into RNA polymerase II elongational pausing. J Mol Biol. 1995;252:522–535. doi: 10.1006/jmbi.1995.0517. [DOI] [PubMed] [Google Scholar]
- 54.Reinberg D, Roeder R G. Factors involved in specific transcription by mammalian RNA polymerase II: purification and functional analysis of initiation factors IIB and IIE*. J Biol Chem. 1987;262:3310–3321. [PubMed] [Google Scholar]
- 55.Sawadogo M, Sentenac A. RNA polymerase B (II) and general transcription factors. Annu Rev Biochem. 1990;59:711–754. doi: 10.1146/annurev.bi.59.070190.003431. [DOI] [PubMed] [Google Scholar]
- 56.Schiestl R H, Gietz R D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 57.Sentenac A. Eukaryotic RNA polymerases. Crit Rev Biochem. 1985;18:31–91. doi: 10.3109/10409238509082539. [DOI] [PubMed] [Google Scholar]
- 58.Serebriiskii I, Estojak J, Sonoda G, Testa J R, Golemis E A. Association of Krev-1/rap1a with Krit1, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21–22. Oncogene. 1997;15:1043–1049. doi: 10.1038/sj.onc.1201268. [DOI] [PubMed] [Google Scholar]
- 59.Shapiro L. Protein localization and asymmetry in the bacterial cell. Cell. 1993;73:841–855. doi: 10.1016/0092-8674(93)90266-s. [DOI] [PubMed] [Google Scholar]
- 59a.Shemshedini, L. Submitted for publication.
- 60.Shpakovski G V, Acker J, Wintzerith M, Lacroix J-F, Thuriaux P, Vigneron M. Four subunits that are shared by the three classes of RNA polymerase are functionally interchangeable between Homo sapiens and Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:4702–4710. doi: 10.1128/mcb.15.9.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sottocasa G L, Kuylenstierna B, Ernster L, Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. J Cell Biol. 1967;32:415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal ς factor in Escherichia coli: the rpoS gene product ς38 is a second principal ς factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tjian R, Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994;77:5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 64.Treich I, Carles C, Riva M, Sentenac A. RPC10 encodes a new mini subunit shared by yeast nuclear RNA polymerases. Gene Expr. 1992;2:31–37. [PMC free article] [PubMed] [Google Scholar]
- 65.Warren S L, Landolfi A S, Curtis C, Morrow J S. Cytostellin: a novel, highly conserved protein that undergoes continuous redistribution during the cell cycle. J Cell Sci. 1992;103:381–388. doi: 10.1242/jcs.103.2.381. [DOI] [PubMed] [Google Scholar]
- 66.Wintzerith M, Acker J, Vicaire S, Vigneron M, Kedinger C. Complete sequence of the human RNA polymerase II largest subunit. Nucleic Acids Res. 1992;20:910. doi: 10.1093/nar/20.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woychik N A, Lane W S, Young R A. Yeast RNA polymerase II subunit RPB9 is essential for growth at temperature extremes. J Biol Chem. 1991;266:19053–19055. [PubMed] [Google Scholar]
- 68.Woychik N A, Young R A. RNA polymerase II subunit RPB4 is essential for high- and low-temperature yeast cell growth. Mol Cell Biol. 1989;9:2854–2859. doi: 10.1128/mcb.9.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woychik N A, Young R A. RNA polymerase II: subunit structure and function. Trends Biochem Sci. 1990;15:347–351. doi: 10.1016/0968-0004(90)90074-l. [DOI] [PubMed] [Google Scholar]
- 70.Young R A. RNA polymerase II. Annu Rev Biochem. 1991;60:689–715. doi: 10.1146/annurev.bi.60.070191.003353. [DOI] [PubMed] [Google Scholar]