In the context of the development of synthetic routes that facilitate the incorporation of β-amino acids into peptide synthesis, the synthesis, crystal structure and Hirshfeld surface analysis are reported of fluorenylmethoxycarbonyl (Fmoc) protected β-amino butyric acid. The importance of pH control in the reaction employing Fmoc-N3 is demonstrated with another β-amino acid analogue from which Fmoc carbamate was identified as the major product.
Keywords: crystal structure, Fmoc-β-amino butyric acid and Fmoc carbamate
Abstract
In the context of the development of synthetic routes that facilitate the incorporation of β-amino acids into peptide synthesis, the synthesis, crystal structure and Hirshfeld surface analysis are reported of fluorenylmethoxycarbonyl (Fmoc) protected β-amino butyric acid, namely, 3-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid, C19H19NO4. The importance of pH control in the reaction employing Fmoc-N3 is demonstrated with another β-amino acid analogue from which Fmoc carbamate was identified as the major product.
1. Chemical context
The increasing application of non-natural amino acids, particularly β-amino acids, in drug development, specifically peptide drugs necessitates the development of an economical and cost-effective method for producing modified β-amino acids. Modern peptide synthesis predominantly employs solid-phase peptide synthesis (SPPS) using the Fmoc strategy to mask the reactivity of the amine group with a temporary protective group (Behrendt et al., 2016 ▸). Peptide chain elongation can then be performed through sequential cycles involving the removal of the protective group, followed by the coupling of N-protected amino acids (Hlebowicz et al., 2005 ▸; Isidro-Llobet et al., 2007 ▸). This strategy allows efficient and controlled peptide assembly. Fmoc chemistry, while seemingly straightforward, presents challenges, particularly with β-amino acids due to the additional α-carbon, which serves as a potential reactive site. During the optimization of the synthesis of Fmoc-β-amino acids using alternatives to Fmoc-Cl, Fmoc-R-β-aminobutyric acid (Fmoc-R-βABA) 1 was synthesized to a high yield and purity after the in situ preparation of Fmoc-N3 (Cruz et al., 2004 ▸). When the same technique was applied to the synthesis of Fmoc-dl-β-phenylalanine, Fmoc-carbamate 2 was obtained. 
2. Structural commentary
Compound 1 crystallizes in the orthorhombic space group P212121, its asymmetric unit comprising of a single molecule (Fig. 1 ▸). The tricyclic fluorenyl group is planar (r.m.s. deviation 0.025 Å). The carbamate group adopts the trans geometry and is planar (r.m.s. deviation 0.005 Å). The absolute configuration of an R stereogenic centre is confirmed to a high degree of certainty. The Hooft parameter of −0.05 (14) demonstrates that a single enantiomer is present. Compound 2 crystallizes in the orthorhombic space group Pca21, its asymmetric unit comprising of two molecules (Fig. 2 ▸). The two tricyclic fluorenyl groups are both planar (r.m.s. deviation of C16–C28 = 0.020 Å and C1–C13 = 0.025 Å). The carbamate group is planar (r.m.s. deviation 0.001 Å). The absolute configuration of an R stereogenic centre is confirmed to a high degree of certainty. The Hooft parameter of −0.05 (14) demonstrates that a single enantiomer is present.
Figure 1.
The molecular structure of Fmoc-β-amino butyric acid 1 showing 50% displacement ellipsoids
Figure 2.
The molecular structure of Fmoc carbamate 2 showing 50% displacement ellipsoids
3. Supramolecular features
In the molecular packing of crystal 1 (Fig. 3 ▸), two hydrogen-bonded (Table 1 ▸) chains are observed (Fig. 4 ▸). One chain forms from the carbamate hydrogen atom (H1) and the carbonyl oxygen atom (O1) of an adjacent molecule (−1 + x, y, z) and continues parallel to the a axis (Fig. 4 ▸). The second chain observed is formed by the carboxylic acid group hydrogen atom (H4) and the carbonyl oxygen atom (O3) of the adjacent molecule ( + x,
+ x,  − y, 1 − z). These chains are not linear but have an angle of 47.9 (10)° between the carboxylic acid planes of each molecule. Hydrogen-bond statistical analysis (Mercury 2024.1.0; Macrae et al., 2020 ▸) was performed, which highlighted the hydrogen bonds to be not unusual. The transamide chain hydrogen bond [2.844 (2) Å] is a little below average in distance (2.97 Å) from 1428 hits. The carboxylic acid chain hydrogen bond [2.656 (2) Å] was found to be of average distance (2.66 Å) from 3072 hits.
− y, 1 − z). These chains are not linear but have an angle of 47.9 (10)° between the carboxylic acid planes of each molecule. Hydrogen-bond statistical analysis (Mercury 2024.1.0; Macrae et al., 2020 ▸) was performed, which highlighted the hydrogen bonds to be not unusual. The transamide chain hydrogen bond [2.844 (2) Å] is a little below average in distance (2.97 Å) from 1428 hits. The carboxylic acid chain hydrogen bond [2.656 (2) Å] was found to be of average distance (2.66 Å) from 3072 hits.
Figure 3.
Packing of 1 as viewed along the a axis
Table 1. Hydrogen-bond geometry (Å, °) for 1.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O4—H4⋯O3i | 0.94 (4) | 1.72 (4) | 2.656 (2) | 177 (3) |
| N1—H1⋯O1ii | 0.88 (3) | 2.04 (3) | 2.844 (3) | 152 (2) |
Symmetry codes: (i) 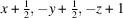 ; (ii)
; (ii)  .
.
Figure 4.
Hydrogen bonding present in 1
Strong face–face π–π interactions (as investigated with the CSD Materials Aromatics Analyser tool) are observed in the packing of 1, running parallel to the a axis. The rings C1–C6 and C8–13 form columns stacking on top and below with symmetry equivalents (x, y, z and −1 + x, y, z), with a centroid–centroid distance of 4.8393 (2) Å and twist plane angle of 0.0 (3)°. These interactions can be visualised later in the Hirshfield surface analysis plot (Fig. 7, 2nd from top) showing the C⋯H/H⋯C interactions primarily around the Fmoc group.In the molecular packing of crystal 2 (Fig. 5 ▸), the two molecules in the asymmetric unit dimerize with hydrogen bonds (Table 2 ▸) formed between the carbamate hydrogen atom (H2A) and oxygen atom (O1), as well as the carbamate hydrogen atom (H1B) and oxygen atom (O3). The other carbamate hydrogen atoms of each of the two molecules then form a further hydrogen bond to an adjacent molecule to form a 1D hydrogen-bonded network (Fig. 6 ▸). The carbamate hydrogen atom (H1A) forms a hydrogen bond with oxygen atom (O1) (x, −1 + y, z) and the carbamate hydrogen atom (H2B) with oxygen atom (O3) (x, 1 + y, z). Hydrogen-bond statistical analysis (Mercury 2024.1.0; Macrae et al., 2020 ▸) was performed, which allowed comparison to 3268 structures. This showed that the dimeric hydrogen bonds (N2—H2A⋯O1) and (N1—H1B⋯O3) were not unusual. The hydrogen bonds (N1—H1A⋯O1) and (N2—H2B⋯O3) were found to be unusual on account of their hydrogen bonds having shorter lengths and more acute angles [2.8549 (3) Å, 149 (3)° and 2.827 (3) Å, 152 (3)°, respectively] below the mean (2.94 Å and 173.35°). A strong face–face π–π interaction is observed in the packing of 2 between the rings C17–C22 and C23–-28 (x, −1 + y, z) as well as between C2–C7 and C8–C13(x, 1 + y, z) columns stacking on top and below with symmetry equivalents), with a centroid–centroid distance of 4.4182 (15) Å and relative orientation of 2.55 (9)°. These interactions can also be visualised later in the Hirshfield surface analysis plot (Fig. 8, 2nd from top) showing the ⋯H/H⋯C interactions around the Fmoc group.
Figure 5.
Packing of 2 as viewed along the b axis
Table 2. Hydrogen-bond geometry (Å, °) for 2.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1B⋯O3 | 0.91 (3) | 2.06 (3) | 2.955 (3) | 169 (3) |
| N1—H1A⋯O1i | 0.86 (3) | 2.08 (3) | 2.849 (3) | 149 (3) |
| N2—H2A⋯O1 | 0.85 (3) | 2.13 (3) | 2.967 (3) | 169 (3) |
| N2—H2B⋯O3ii | 0.87 (4) | 2.03 (4) | 2.827 (3) | 152 (3) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Figure 6.
Hydrogen bonding between the carbamate groups present in 2
4. Database survey
A search of the Cambridge Structural Database (CSD version 5.46, November 2024; Groom et al., 2016 ▸) highlighted that the crystal structures of compounds 1 and 2 have not been reported. Searches for structural motifs similar to 1 began with Fmoc-protected α-amino acids and discovered 51 results of various natural amino acids, synthetic derivatives and co-crystals, the most closely related structure being CUWKIO (Valle et al., 1984 ▸), a Fmoc-protected alanine monohydrate that differs in structure by the one carbon atom as well as co-crystallizing with a molecule of water. Despite crystallizing in the same space group as 1, CUWKIO has a different set of hydrogen bonds formed within the crystal structure. Instead of the amide hydrogen-bonded chains seen in 1, the amide H atom of CUWKIO forms a hydrogen bond to the carboxylic acid carbonyl whereas the co-crystallized water forms a hydrogen bond to the amide carbonyl. The co-crystallized water then satisfies its remaining hydrogen-bond formation with the carboxylic acid carbonyl and the carboxylic acid hydrogen is satisfied by forming a hydrogen bond to the water. In total, four hydrogen bonds are reported in CUWKIO in contrast to 1, which has two. Aromatic interactions, as investigated with the CSD Materials Aromatics Analyser tool, highlight a change in packing of the π–π interactions of the Fmoc groups. Strong face-face interactions in 1 are no longer present but instead strong edge-to-face interactions seen in CUWKIO. Further investigation of structural similarities lead us to look for Fmoc-protected β-amino acids, the structural motif of which was found the related structure BOMRAY (Ahmad Wani et al., 2014 ▸), which differs as there is a spiro-centred carbon with cyclic six-membered ring at the β carbon, in contrast to 1, which has the methyl group as well as being a racemate. BOMRAY crystallises in the P space group and the primary hydrogen-bond interactions are a not unusual dimerization of the carboxylic acid as well as a long amide N—H to carboxylic acid carbonyl bond. The amide carbonyl in this case only forms a long weak intermolecular interaction with a CH2 group. Analysis of the aromatic interactions in BOMRAY reveals strong face-face π-stacking interactions, albeit with an offset, meaning only one of the phenyl rings is involved in the interaction.
space group and the primary hydrogen-bond interactions are a not unusual dimerization of the carboxylic acid as well as a long amide N—H to carboxylic acid carbonyl bond. The amide carbonyl in this case only forms a long weak intermolecular interaction with a CH2 group. Analysis of the aromatic interactions in BOMRAY reveals strong face-face π-stacking interactions, albeit with an offset, meaning only one of the phenyl rings is involved in the interaction.
5. Hirshfeld surface analysis
In order to visualise the intermolecular interactions in 1 and 2, Hirshfeld surface analysis was carried out using CrystalExplorer 21.5 (Spackman et al., 2021 ▸) and visualised via two-dimensional fingerprint plots (McKinnon et al., 2007 ▸). The Hirshfeld surface analysis of 2 was carried out with the asymmetric unit of the two molecules. The left columns of Fig. 7 ▸ (top) and 8 (top) show the Hirshfeld surfaces of 1 and 2, respectively, each mapped with the function dnorm, which is the sum of the distances from a surface point to the nearest interior (di) and exterior(de) atom, normalized by the van der Waals (vdW) radii of the corresponding atom (rvdW). Contacts shorter than the sum of their vdW radii are shown in red, those longer in blue and those approximately equal to their vdW radii in white. In the structure of 1, Fig. 7 ▸, the shortest contacts, with the most intense red spots, are shown to be the hydrogen-bonding sites, as shown in Fig. 4 ▸, the carboxylic acid and amide. The fingerprint plots (Tan et al., 2019 ▸) for 1 and 2 are given in the right columns of Figs. 7 ▸ and 8 ▸ and the intermolecular interactions shown in Tables 3 ▸ and 4 ▸, respectively. The overall fingerprint plots are shown first (top) followed by those delineated into C⋯H/H⋯C, H/H, N⋯H/H⋯N and O⋯H/H⋯O. For 1, the most important overall contribution is H⋯H, Fig. 7 ▸, contributing 51.8% with the tip of de = di at 1.12 Å. The shortest interactions, the hydrogen bonding at the carboxylic acid and amide sites, as highlighted by the dnorm surface plot, are clearly visualized in the bottom O⋯H/H⋯O plot with the shortest distances. The C⋯H⋯π interactions are shown in the the surface of the C⋯H/H⋯C highlighted figure as well as the characteristic wings revealed in the fingerprint plot. For structure 2, while the dimerization of the amine and carbonyl within the asymmetric unit is occluded from the view, the surface map shows the brightest red spots corresponding to the hydrogen bond formed from the amine to carbonyl, clearly highlighted in Fig. 8 ▸ (bottom) surface displaying O⋯H/H⋯O contacts and its corresponding plot demonstrating the shortest distance. In 2, the most important contribution again is H⋯H, contributing 51.9% with the tip of de = di at 1.16 Å.
Figure 7.

Hirshfeld surfaces of 1 mapped with dnorm (left image of each pair) with the corresponding two-dimensional fingerprint plot (right image of each pair) showing firstly all contributions and then the major contributions of C⋯H/H⋯C, H⋯H, N⋯H/H⋯N and O⋯H/H⋯O contacts.
Figure 8.

Hirshfeld surfaces of 2 mapped with dnorm (left image of each pair) with the corresponding two-dimensional fingerprint plot (right image of each pair) showing firstly all contributions and then the major contributions of C⋯H/H⋯C, H⋯H, N⋯H/H⋯N and O⋯H/H⋯O contacts.
Table 3. Summary of the percentages of intermolecular contacts contributed to the HSA surface of Fmoc-protected β-aminobutyric acid 1.
| Inside atom | Outside atom | Total contributions | |||
|---|---|---|---|---|---|
| C | H | N | O | ||
| C | 2.0 | 13.0 | 0.0 | 0.2 | 15.3 |
| H | 9.8 | 51.8 | 0.5 | 9.8 | 71.9 |
| N | 0.0 | 0.5 | 0.0 | 0.0 | 0.5 |
| O | 0.2 | 11.6 | 0.0 | 0.5 | 12.3 |
| Total contributions | 12.0 | 77.0 | 0.5 | 10.5 |
Table 4. Summary of the percentages of intermolecular contacts contributed to the HSA surface of Fmoc carbamate 2.
| Inside atom | Outside atom | Total contributions | |||
|---|---|---|---|---|---|
| C | H | N | O | ||
| C | 2.6 | 17.9 | 0.0 | 0.1 | 20.7 |
| H | 13.6 | 51.9 | 1.2 | 4.9 | 71.6 |
| N | 0.0 | 1.2 | 0.0 | 0.2 | 1.4 |
| O | 0.1 | 5.5 | 0.1 | 0.6 | 6.4 |
| Total contributions | 16.3 | 76.6 | 1.3 | 5.8 |
6. Synthesis and crystallization
Fmoc-Cl was initially derivatized into Fmoc-N3 to prepare it for addition to solutions of the β-amino acid. Specifically, 10 mmol of Fmoc-Cl were dissolved in dioxane (5 ml), while 12 mmol of NaN3 were dissolved in a 2:1 mixture of dioxane/water (10 ml). The Fmoc-Cl solution was then added to the NaN3 solution, and the resulting mixture was stirred at 323 K for 2 h.>
For the synthesis of 1, (Fig. 9 ▸)11 mmol of R-βABA were dissolved in a 2:1 mixture of dioxane/10% NaHCO3, which maintains the pH at 8–9. The Fmoc-N3 solution was cautiously added in three portions to the β-amino acid over a period of 1 h. The reaction mixture was then stirred for 15 h at room temperature. Following the reaction, the mixture was poured into 5 mL of ice-cold water and subjected to three extractions with petroleum ether. The aqueous layers were separated using a separation funnel and chilled on ice for 2 h. Subsequently, the aqueous layer was acidified to pH 1 with 2 M HCl. The resulting precipitate was filtered and washed with ice-cold water until a pH of about 5 was attained. The collected white solid was placed in a petri dish covered with a paper towel and left to dry overnight within the fume hood. X-ray quality single crystals were grown by recrystallization from ethyl acetate/pet ether. NMR: 1H NMR (400 MHz, DMSO) δ = 12.18 (s, 1H), 7.89 (dd, J = 7.3, 5.9, 3H), 7.73–7.62 (m, 3H), 7.47–7.26 (m, 7H), 4.63 (d, J = 6.2, 1H), 4.38–4.17 (m, 3H), 3.88 (hept, J = 6.8, 1H), 2.50–2.42 (m, 1H), 2.30 (dd, J = 15.4, 7.3, 1H), 1.10 (d, J = 6.6, 2H). 13C NMR (101 MHz, DMSO) δ 128.03, 127.32, 125.56, 125.21, 120.29, 69.65, 65.78, 65.43, 47.14, 47.14, 46.44, 44.33, 41.51, 41.51, 41.16, 40.11, 21.12, 20.77. MP:120-125 °C HRMS Analysis: m/z (ES+) 326.1401. C19H20NO4 requires 326.1387.
Figure 9.
The synthesis of the title compounds.
For the synthesis of 2, 11 mmol of dl-β-phenylalanine were dissolved in a 2:1 mixture of dioxane/10% NaHCO3 along with NH4OH (1 mL), which maintains the pH at 12. As above, the Fmoc-N3 solution was cautiously added in three portions to the β-amino acid over a period of 1 h. The reaction mixture was then stirred for 15 h at room temperature. Following the reaction, the mixture was poured into 5 mL of ice-cold water and subjected to three extractions with petroleum ether. The aqueous layers were separated using a separation funnel and chilled on ice for 2 h. Subsequently, the aqueous layer was acidified to pH 1 with 2 M HCl. The resulting precipitate was filtered and washed with ice-cold water until a pH of about 5 was attained. The collected white solid was placed in a Petri dish covered with a paper towel and left to dry overnight within the fume hood. X-ray quality single crystals were grown by recrystallization from ethyl acetate/pet ether. M.p. 471–473 K NMR: 1H NMR (400 MHz, DMSO) δ = 7.89 (d, J = 7.5, 2H), 7.70 (d, J = 7.4, 2H), 7.46–7.38 (m, 2H), 7.34 (td, J = 7.4, 1.2, 2H), 6.75 (s, 1H), 6.55 (s, 1H), 4.28 (d, J = 1.6, 1H), 4.27 (s, 1H), 4.22 (dd, J = 8.0, 5.7, 1H). 13C NMR (101 MHz, DMSO) δ = 157.20, 157.13, 144.45, 143.05, 141.21, 139.90, 137.90, 129.39, 128.06, 127.76, 127.52, 125.62, 121.85, 120.57, 120.49, 110.19, 65.48, 47.22. HRMS Analysis: m/z (ES+) C15H13NO2 requires 239.26.
7. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. All carbon-bound H atoms were positioned geometrically and refined as riding, with aromatic C—H = 0.95 Å, sp3 C—H = 1.00 Å, sp3 C—H2 0.99 Å and with Uiso(H) = 1.2 Ueq(C) and sp3 C—H3 = 0.98 Å with Uiso(H) = 1.5Ueq(methyl C). Hydrogen atoms involved in hydrogen-bonding interactions were refined isotropically.
Table 5. Experimental details.
| 1 | 2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C19H19NO4 | C15H13NO2 |
| M r | 325.35 | 239.26 |
| Crystal system, space group | Orthorhombic, P212121 | Orthorhombic, Pca21 |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 4.8393 (2), 12.4928 (4), 27.3101 (9) | 15.3560 (3), 5.0400 (1), 31.0254 (7) |
| V (Å3) | 1651.07 (10) | 2401.19 (9) |
| Z | 4 | 8 |
| Radiation type | Cu Kα | Cu Kα |
| μ (mm−1) | 0.75 | 0.71 |
| Crystal size (mm) | 0.3 × 0.03 × 0.01 | 0.25 × 0.03 × 0.01 |
| Data collection | ||
| Diffractometer | Bruker D8 Venture Photon III | Bruker D8 Venture Photon III |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| Tmin, Tmax | 0.664, 0.753 | 0.683, 0.753 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 25780, 2922, 2590 | 18874, 4069, 3754 |
| R int | 0.081 | 0.051 |
| (sin θ/λ)max (Å−1) | 0.596 | 0.596 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.032, 0.073, 1.06 | 0.030, 0.068, 1.05 |
| No. of reflections | 2922 | 4069 |
| No. of parameters | 226 | 341 |
| No. of restraints | 0 | 1 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.12, −0.17 | 0.14, −0.17 |
| Absolute structure | Flack x determined using 992 quotients [(I+)−(I−)]/[(I+)+(I−)] (Parsons et al., 2013 ▸) | Flack x determined using 1617 quotients [(I+)−(I−)]/[(I+)+(I−)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | −0.05 (14) | −0.26 (13) |
Supplementary Material
Crystal structure: contains datablock(s) 1, 2. DOI: 10.1107/S2056989025003810/vu2009sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989025003810/vu20091sup2.hkl
Supporting information file. DOI: 10.1107/S2056989025003810/vu20091sup4.cml
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989025003810/vu20092sup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
MAM is grateful to his family for support of his PhD studies.
supplementary crystallographic information
3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid (1) . Crystal data
| C19H19NO4 | Dx = 1.309 Mg m−3 |
| Mr = 325.35 | Cu Kα radiation, λ = 1.54178 Å |
| Orthorhombic, P212121 | Cell parameters from 3582 reflections |
| a = 4.8393 (2) Å | θ = 3.2–66.6° |
| b = 12.4928 (4) Å | µ = 0.75 mm−1 |
| c = 27.3101 (9) Å | T = 100 K |
| V = 1651.07 (10) Å3 | Needle, colourless |
| Z = 4 | 0.3 × 0.03 × 0.01 mm |
| F(000) = 688 |
3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid (1) . Data collection
| Bruker D8 Venture Photon III diffractometer | 2590 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.081 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | θmax = 66.8°, θmin = 3.2° |
| Tmin = 0.664, Tmax = 0.753 | h = −5→5 |
| 25780 measured reflections | k = −14→14 |
| 2922 independent reflections | l = −32→31 |
3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid (1) . Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.032 | w = 1/[σ2(Fo2) + (0.0304P)2 + 0.2044P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.073 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.12 e Å−3 |
| 2922 reflections | Δρmin = −0.17 e Å−3 |
| 226 parameters | Absolute structure: Flack x determined using 992 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 0 restraints | Absolute structure parameter: −0.05 (14) |
| Primary atom site location: dual |
3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid (1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. For 1, a needle crystal with dimensions 0.01 x 0.026 x 0.3 mm was selected and for 2, a needle crystal with dimensions 0.012 x 0.026 x 0.25 was selected. Intensity data for each was collected on a Bruker Venture Photon III diffractometer operating with a CuKα microfocus X-ray source with the crystal mounted in fomblin oil on a MicroMount (MiTeGen, USA) and cooled to 100 K in a stream of cold nitrogen gas using an Oxford Cryosystems 700 Cryostream. Data were corrected for absorption using empirical methods (SADABS; Bruker, 2023) based upon symmetry equivalent reflections combined with measurements at different azimuthal angles (Krause et al., 2015). The crystal structures were solved and refined against F2 values using ShelXT (Sheldrick, 2015a) for solution and ShelXL (Sheldrick, 2015b) for refinement accessed via the Olex2 program (Dolomanov et al., 2009). Non-hydrogen atoms were refined anisotropically. |
3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid (1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.6114 (3) | 0.65660 (13) | 0.60381 (6) | 0.0260 (4) | |
| O2 | 0.2958 (3) | 0.76326 (12) | 0.64093 (6) | 0.0254 (4) | |
| O3 | −0.0146 (4) | 0.30716 (13) | 0.53156 (6) | 0.0309 (4) | |
| O4 | 0.3887 (4) | 0.38097 (14) | 0.51041 (7) | 0.0331 (4) | |
| H4 | 0.422 (8) | 0.314 (3) | 0.4965 (14) | 0.078 (12)* | |
| N1 | 0.1735 (4) | 0.67252 (15) | 0.57425 (7) | 0.0215 (4) | |
| H1 | 0.005 (6) | 0.691 (2) | 0.5827 (9) | 0.032 (7)* | |
| C1 | 0.4148 (5) | 0.89471 (18) | 0.70131 (8) | 0.0230 (5) | |
| H1A | 0.227847 | 0.885997 | 0.716402 | 0.028* | |
| C2 | 0.4183 (5) | 0.99043 (18) | 0.66703 (8) | 0.0231 (5) | |
| C3 | 0.2681 (5) | 1.0088 (2) | 0.62449 (9) | 0.0309 (6) | |
| H3 | 0.133824 | 0.958606 | 0.613666 | 0.037* | |
| C4 | 0.3181 (6) | 1.1019 (2) | 0.59806 (10) | 0.0372 (7) | |
| H4A | 0.216012 | 1.115393 | 0.568994 | 0.045* | |
| C5 | 0.5141 (6) | 1.1755 (2) | 0.61339 (10) | 0.0374 (7) | |
| H5 | 0.546508 | 1.238106 | 0.594522 | 0.045* | |
| C6 | 0.6638 (6) | 1.15843 (19) | 0.65610 (10) | 0.0326 (6) | |
| H6 | 0.797817 | 1.208928 | 0.666704 | 0.039* | |
| C7 | 0.6136 (5) | 1.06620 (19) | 0.68294 (9) | 0.0250 (5) | |
| C8 | 0.7377 (5) | 1.02758 (18) | 0.72875 (9) | 0.0239 (5) | |
| C9 | 0.9348 (5) | 1.0736 (2) | 0.75932 (10) | 0.0304 (6) | |
| H9 | 1.010016 | 1.142041 | 0.751993 | 0.037* | |
| C10 | 1.0195 (6) | 1.0182 (2) | 0.80057 (9) | 0.0346 (6) | |
| H10 | 1.151438 | 1.049315 | 0.822029 | 0.042* | |
| C11 | 0.9134 (6) | 0.9177 (2) | 0.81081 (9) | 0.0322 (6) | |
| H11 | 0.977890 | 0.879868 | 0.838730 | 0.039* | |
| C12 | 0.7136 (5) | 0.8712 (2) | 0.78084 (9) | 0.0273 (6) | |
| H12 | 0.639459 | 0.802678 | 0.788335 | 0.033* | |
| C13 | 0.6254 (5) | 0.92702 (18) | 0.73987 (8) | 0.0229 (5) | |
| C14 | 0.5056 (5) | 0.79107 (19) | 0.67675 (9) | 0.0249 (5) | |
| H14A | 0.686512 | 0.801056 | 0.660463 | 0.030* | |
| H14B | 0.523900 | 0.733348 | 0.701367 | 0.030* | |
| C15 | 0.3773 (5) | 0.69342 (17) | 0.60606 (9) | 0.0213 (5) | |
| C16 | 0.2032 (5) | 0.58643 (17) | 0.53839 (8) | 0.0212 (5) | |
| H16 | 0.404379 | 0.576644 | 0.531365 | 0.025* | |
| C17 | 0.0920 (5) | 0.48249 (18) | 0.56036 (9) | 0.0255 (5) | |
| H17A | 0.172825 | 0.473954 | 0.593435 | 0.031* | |
| H17B | −0.110285 | 0.490126 | 0.564482 | 0.031* | |
| C18 | 0.1454 (5) | 0.38223 (18) | 0.53224 (9) | 0.0230 (5) | |
| C19 | 0.0586 (6) | 0.6161 (2) | 0.49085 (9) | 0.0314 (6) | |
| H19A | −0.139338 | 0.625852 | 0.497011 | 0.047* | |
| H19B | 0.084998 | 0.558750 | 0.466822 | 0.047* | |
| H19C | 0.136876 | 0.682848 | 0.478078 | 0.047* |
3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid (1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0159 (8) | 0.0299 (9) | 0.0323 (9) | 0.0033 (7) | −0.0010 (7) | −0.0065 (8) |
| O2 | 0.0174 (8) | 0.0265 (8) | 0.0322 (9) | 0.0008 (7) | −0.0031 (7) | −0.0098 (7) |
| O3 | 0.0281 (9) | 0.0220 (8) | 0.0427 (11) | −0.0025 (7) | 0.0001 (8) | −0.0051 (8) |
| O4 | 0.0280 (10) | 0.0227 (9) | 0.0486 (12) | 0.0002 (8) | 0.0117 (9) | −0.0072 (8) |
| N1 | 0.0147 (10) | 0.0212 (10) | 0.0287 (11) | 0.0010 (8) | −0.0011 (9) | −0.0044 (9) |
| C1 | 0.0195 (12) | 0.0233 (12) | 0.0263 (13) | −0.0004 (10) | −0.0001 (10) | −0.0031 (10) |
| C2 | 0.0202 (12) | 0.0246 (12) | 0.0246 (12) | 0.0042 (10) | 0.0031 (10) | −0.0036 (10) |
| C3 | 0.0278 (14) | 0.0346 (14) | 0.0304 (13) | 0.0074 (12) | −0.0007 (11) | −0.0066 (12) |
| C4 | 0.0404 (16) | 0.0419 (16) | 0.0292 (14) | 0.0167 (14) | −0.0002 (12) | 0.0046 (12) |
| C5 | 0.0420 (16) | 0.0315 (15) | 0.0385 (16) | 0.0098 (13) | 0.0110 (13) | 0.0094 (12) |
| C6 | 0.0316 (15) | 0.0262 (13) | 0.0402 (15) | 0.0026 (12) | 0.0074 (12) | 0.0010 (12) |
| C7 | 0.0225 (12) | 0.0235 (12) | 0.0290 (13) | 0.0040 (10) | 0.0045 (10) | −0.0028 (10) |
| C8 | 0.0212 (12) | 0.0236 (12) | 0.0267 (13) | 0.0007 (10) | 0.0029 (10) | −0.0059 (10) |
| C9 | 0.0253 (14) | 0.0266 (13) | 0.0394 (15) | −0.0021 (11) | 0.0021 (11) | −0.0098 (12) |
| C10 | 0.0307 (14) | 0.0397 (16) | 0.0336 (15) | 0.0050 (12) | −0.0056 (12) | −0.0159 (13) |
| C11 | 0.0351 (15) | 0.0360 (14) | 0.0254 (13) | 0.0116 (12) | −0.0032 (11) | −0.0072 (11) |
| C12 | 0.0311 (14) | 0.0249 (12) | 0.0258 (13) | 0.0037 (11) | 0.0039 (11) | −0.0036 (10) |
| C13 | 0.0204 (12) | 0.0239 (12) | 0.0243 (12) | 0.0014 (10) | 0.0038 (10) | −0.0044 (10) |
| C14 | 0.0199 (12) | 0.0268 (13) | 0.0279 (13) | −0.0010 (10) | −0.0042 (10) | −0.0063 (11) |
| C15 | 0.0209 (12) | 0.0179 (11) | 0.0251 (12) | −0.0031 (10) | 0.0011 (10) | −0.0005 (10) |
| C16 | 0.0175 (11) | 0.0203 (11) | 0.0258 (12) | −0.0001 (9) | −0.0011 (10) | −0.0032 (10) |
| C17 | 0.0269 (12) | 0.0220 (12) | 0.0276 (13) | −0.0005 (10) | 0.0043 (10) | −0.0026 (10) |
| C18 | 0.0222 (13) | 0.0206 (11) | 0.0261 (13) | 0.0003 (10) | −0.0025 (10) | 0.0025 (10) |
| C19 | 0.0382 (16) | 0.0261 (13) | 0.0300 (14) | 0.0002 (12) | −0.0060 (12) | −0.0010 (11) |
3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid (1) . Geometric parameters (Å, º)
| O1—C15 | 1.224 (3) | C7—C8 | 1.469 (3) |
| O2—C14 | 1.452 (3) | C8—C9 | 1.392 (3) |
| O2—C15 | 1.350 (3) | C8—C13 | 1.402 (3) |
| O3—C18 | 1.216 (3) | C9—H9 | 0.9500 |
| O4—H4 | 0.94 (4) | C9—C10 | 1.385 (4) |
| O4—C18 | 1.320 (3) | C10—H10 | 0.9500 |
| N1—H1 | 0.88 (3) | C10—C11 | 1.384 (4) |
| N1—C15 | 1.340 (3) | C11—H11 | 0.9500 |
| N1—C16 | 1.462 (3) | C11—C12 | 1.394 (4) |
| C1—H1A | 1.0000 | C12—H12 | 0.9500 |
| C1—C2 | 1.519 (3) | C12—C13 | 1.386 (3) |
| C1—C13 | 1.520 (3) | C14—H14A | 0.9900 |
| C1—C14 | 1.523 (3) | C14—H14B | 0.9900 |
| C2—C3 | 1.389 (3) | C16—H16 | 1.0000 |
| C2—C7 | 1.407 (3) | C16—C17 | 1.528 (3) |
| C3—H3 | 0.9500 | C16—C19 | 1.521 (3) |
| C3—C4 | 1.391 (4) | C17—H17A | 0.9900 |
| C4—H4A | 0.9500 | C17—H17B | 0.9900 |
| C4—C5 | 1.385 (4) | C17—C18 | 1.492 (3) |
| C5—H5 | 0.9500 | C19—H19A | 0.9800 |
| C5—C6 | 1.390 (4) | C19—H19B | 0.9800 |
| C6—H6 | 0.9500 | C19—H19C | 0.9800 |
| C6—C7 | 1.387 (3) | ||
| C15—O2—C14 | 115.19 (17) | C10—C11—C12 | 121.1 (2) |
| C18—O4—H4 | 110 (2) | C12—C11—H11 | 119.4 |
| C15—N1—H1 | 117.4 (18) | C11—C12—H12 | 120.7 |
| C15—N1—C16 | 120.36 (19) | C13—C12—C11 | 118.5 (2) |
| C16—N1—H1 | 117.6 (18) | C13—C12—H12 | 120.7 |
| C2—C1—H1A | 110.5 | C8—C13—C1 | 110.4 (2) |
| C2—C1—C13 | 102.15 (19) | C12—C13—C1 | 129.2 (2) |
| C2—C1—C14 | 113.24 (19) | C12—C13—C8 | 120.4 (2) |
| C13—C1—H1A | 110.5 | O2—C14—C1 | 107.37 (19) |
| C13—C1—C14 | 109.72 (19) | O2—C14—H14A | 110.2 |
| C14—C1—H1A | 110.5 | O2—C14—H14B | 110.2 |
| C3—C2—C1 | 129.7 (2) | C1—C14—H14A | 110.2 |
| C3—C2—C7 | 119.9 (2) | C1—C14—H14B | 110.2 |
| C7—C2—C1 | 110.3 (2) | H14A—C14—H14B | 108.5 |
| C2—C3—H3 | 120.6 | O1—C15—O2 | 123.3 (2) |
| C2—C3—C4 | 118.8 (3) | O1—C15—N1 | 125.1 (2) |
| C4—C3—H3 | 120.6 | N1—C15—O2 | 111.6 (2) |
| C3—C4—H4A | 119.4 | N1—C16—H16 | 108.3 |
| C5—C4—C3 | 121.1 (3) | N1—C16—C17 | 109.11 (19) |
| C5—C4—H4A | 119.4 | N1—C16—C19 | 110.34 (18) |
| C4—C5—H5 | 119.7 | C17—C16—H16 | 108.3 |
| C4—C5—C6 | 120.6 (3) | C19—C16—H16 | 108.3 |
| C6—C5—H5 | 119.7 | C19—C16—C17 | 112.4 (2) |
| C5—C6—H6 | 120.7 | C16—C17—H17A | 108.1 |
| C7—C6—C5 | 118.7 (3) | C16—C17—H17B | 108.1 |
| C7—C6—H6 | 120.7 | H17A—C17—H17B | 107.3 |
| C2—C7—C8 | 108.5 (2) | C18—C17—C16 | 116.76 (19) |
| C6—C7—C2 | 120.9 (2) | C18—C17—H17A | 108.1 |
| C6—C7—C8 | 130.6 (2) | C18—C17—H17B | 108.1 |
| C9—C8—C7 | 130.9 (2) | O3—C18—O4 | 123.5 (2) |
| C9—C8—C13 | 120.4 (2) | O3—C18—C17 | 123.0 (2) |
| C13—C8—C7 | 108.7 (2) | O4—C18—C17 | 113.4 (2) |
| C8—C9—H9 | 120.5 | C16—C19—H19A | 109.5 |
| C10—C9—C8 | 118.9 (2) | C16—C19—H19B | 109.5 |
| C10—C9—H9 | 120.5 | C16—C19—H19C | 109.5 |
| C9—C10—H10 | 119.7 | H19A—C19—H19B | 109.5 |
| C11—C10—C9 | 120.5 (2) | H19A—C19—H19C | 109.5 |
| C11—C10—H10 | 119.7 | H19B—C19—H19C | 109.5 |
| C10—C11—H11 | 119.4 | ||
| N1—C16—C17—C18 | −170.6 (2) | C9—C8—C13—C12 | −1.4 (3) |
| C1—C2—C3—C4 | −177.1 (2) | C9—C10—C11—C12 | −1.9 (4) |
| C1—C2—C7—C6 | 176.9 (2) | C10—C11—C12—C13 | 1.0 (4) |
| C1—C2—C7—C8 | −2.4 (3) | C11—C12—C13—C1 | 179.4 (2) |
| C2—C1—C13—C8 | −0.7 (2) | C11—C12—C13—C8 | 0.7 (3) |
| C2—C1—C13—C12 | −179.5 (2) | C13—C1—C2—C3 | −179.9 (2) |
| C2—C1—C14—O2 | −67.4 (3) | C13—C1—C2—C7 | 1.9 (2) |
| C2—C3—C4—C5 | 0.2 (4) | C13—C1—C14—O2 | 179.18 (18) |
| C2—C7—C8—C9 | −178.4 (2) | C13—C8—C9—C10 | 0.5 (4) |
| C2—C7—C8—C13 | 1.9 (3) | C14—O2—C15—O1 | −1.0 (3) |
| C3—C2—C7—C6 | −1.5 (4) | C14—O2—C15—N1 | −179.49 (19) |
| C3—C2—C7—C8 | 179.2 (2) | C14—C1—C2—C3 | 62.2 (3) |
| C3—C4—C5—C6 | −0.8 (4) | C14—C1—C2—C7 | −116.0 (2) |
| C4—C5—C6—C7 | 0.2 (4) | C14—C1—C13—C8 | 119.7 (2) |
| C5—C6—C7—C2 | 0.9 (4) | C14—C1—C13—C12 | −59.1 (3) |
| C5—C6—C7—C8 | −179.9 (2) | C15—O2—C14—C1 | 161.10 (18) |
| C6—C7—C8—C9 | 2.3 (4) | C15—N1—C16—C17 | 90.3 (3) |
| C6—C7—C8—C13 | −177.3 (3) | C15—N1—C16—C19 | −145.8 (2) |
| C7—C2—C3—C4 | 0.9 (4) | C16—N1—C15—O1 | 11.4 (4) |
| C7—C8—C9—C10 | −179.0 (2) | C16—N1—C15—O2 | −170.19 (18) |
| C7—C8—C13—C1 | −0.7 (3) | C16—C17—C18—O3 | −147.0 (2) |
| C7—C8—C13—C12 | 178.2 (2) | C16—C17—C18—O4 | 36.3 (3) |
| C8—C9—C10—C11 | 1.1 (4) | C19—C16—C17—C18 | 66.7 (3) |
| C9—C8—C13—C1 | 179.6 (2) |
3-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}butanoic acid (1) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O4—H4···O3i | 0.94 (4) | 1.72 (4) | 2.656 (2) | 177 (3) |
| N1—H1···O1ii | 0.88 (3) | 2.04 (3) | 2.844 (3) | 152 (2) |
Symmetry codes: (i) x+1/2, −y+1/2, −z+1; (ii) x−1, y, z.
(2). Crystal data
| C15H13NO2 | Dx = 1.324 Mg m−3 |
| Mr = 239.26 | Cu Kα radiation, λ = 1.54178 Å |
| Orthorhombic, Pca21 | Cell parameters from 6701 reflections |
| a = 15.3560 (3) Å | θ = 3.2–65.9° |
| b = 5.0400 (1) Å | µ = 0.71 mm−1 |
| c = 31.0254 (7) Å | T = 100 K |
| V = 2401.19 (9) Å3 | Needle, colourless |
| Z = 8 | 0.25 × 0.03 × 0.01 mm |
| F(000) = 1008 |
(2). Data collection
| Bruker D8 Venture Photon III diffractometer | 3754 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.051 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | θmax = 66.8°, θmin = 2.9° |
| Tmin = 0.683, Tmax = 0.753 | h = −18→18 |
| 18874 measured reflections | k = −5→5 |
| 4069 independent reflections | l = −36→36 |
(2). Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.030 | w = 1/[σ2(Fo2) + (0.0291P)2 + 0.3089P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.068 | (Δ/σ)max < 0.001 |
| S = 1.05 | Δρmax = 0.14 e Å−3 |
| 4069 reflections | Δρmin = −0.16 e Å−3 |
| 341 parameters | Absolute structure: Flack x determined using 1617 quotients[(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 1 restraint | Absolute structure parameter: −0.26 (13) |
| Primary atom site location: dual |
(2). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(2). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.53087 (12) | 0.7151 (3) | 0.47451 (7) | 0.0233 (4) | |
| O2 | 0.42477 (11) | 0.4384 (3) | 0.45115 (6) | 0.0198 (4) | |
| N1 | 0.53609 (17) | 0.2741 (4) | 0.48787 (8) | 0.0230 (5) | |
| H1A | 0.5141 (19) | 0.123 (6) | 0.4816 (10) | 0.019 (7)* | |
| H1B | 0.589 (2) | 0.295 (6) | 0.5007 (11) | 0.031 (8)* | |
| C1 | 0.32636 (17) | 0.5633 (5) | 0.39449 (9) | 0.0176 (6) | |
| H1 | 0.293372 | 0.398198 | 0.401756 | 0.021* | |
| C2 | 0.26475 (15) | 0.7768 (5) | 0.37850 (9) | 0.0176 (5) | |
| C3 | 0.19497 (17) | 0.8955 (5) | 0.39955 (9) | 0.0226 (6) | |
| H3 | 0.179526 | 0.844026 | 0.427999 | 0.027* | |
| C4 | 0.14832 (17) | 1.0913 (6) | 0.37805 (11) | 0.0263 (6) | |
| H4 | 0.100259 | 1.173510 | 0.391963 | 0.032* | |
| C5 | 0.17085 (18) | 1.1689 (5) | 0.33649 (9) | 0.0248 (6) | |
| H5 | 0.138392 | 1.303942 | 0.322415 | 0.030* | |
| C6 | 0.24063 (19) | 1.0499 (5) | 0.31541 (10) | 0.0221 (6) | |
| H6 | 0.256156 | 1.102689 | 0.287037 | 0.027* | |
| C7 | 0.28718 (16) | 0.8528 (5) | 0.33653 (9) | 0.0176 (5) | |
| C8 | 0.36178 (15) | 0.6916 (5) | 0.32254 (8) | 0.0171 (5) | |
| C9 | 0.40625 (17) | 0.6884 (5) | 0.28345 (9) | 0.0212 (6) | |
| H9 | 0.391122 | 0.808222 | 0.261028 | 0.025* | |
| C10 | 0.4733 (2) | 0.5061 (5) | 0.27793 (11) | 0.0251 (7) | |
| H10 | 0.503783 | 0.499597 | 0.251306 | 0.030* | |
| C11 | 0.49616 (17) | 0.3337 (5) | 0.31090 (10) | 0.0237 (6) | |
| H11 | 0.542294 | 0.210925 | 0.306624 | 0.028* | |
| C12 | 0.45228 (17) | 0.3385 (5) | 0.35021 (9) | 0.0205 (6) | |
| H12 | 0.468555 | 0.221720 | 0.372863 | 0.025* | |
| C13 | 0.38445 (19) | 0.5168 (5) | 0.35564 (10) | 0.0170 (6) | |
| C14 | 0.37677 (17) | 0.6619 (5) | 0.43372 (9) | 0.0194 (5) | |
| H14A | 0.417409 | 0.805151 | 0.425237 | 0.023* | |
| H14B | 0.336087 | 0.732822 | 0.455632 | 0.023* | |
| C15 | 0.50003 (18) | 0.4899 (5) | 0.47137 (9) | 0.0165 (6) | |
| O3 | 0.71790 (12) | 0.3289 (3) | 0.51774 (6) | 0.0235 (4) | |
| O4 | 0.81702 (12) | 0.6052 (3) | 0.54788 (6) | 0.0203 (4) | |
| N2 | 0.71138 (16) | 0.7720 (4) | 0.50730 (8) | 0.0201 (5) | |
| H2A | 0.663 (2) | 0.759 (5) | 0.4946 (10) | 0.019 (7)* | |
| H2B | 0.730 (2) | 0.928 (7) | 0.5147 (11) | 0.033 (9)* | |
| C16 | 0.91776 (18) | 0.4654 (5) | 0.60216 (9) | 0.0182 (6) | |
| H16 | 0.948852 | 0.635510 | 0.595949 | 0.022* | |
| C17 | 0.98232 (16) | 0.2503 (5) | 0.61506 (9) | 0.0187 (5) | |
| C18 | 1.05075 (16) | 0.1434 (5) | 0.59156 (9) | 0.0229 (6) | |
| H18 | 1.062974 | 0.204782 | 0.563253 | 0.028* | |
| C19 | 1.10103 (18) | −0.0548 (6) | 0.61018 (11) | 0.0282 (7) | |
| H19 | 1.148311 | −0.128562 | 0.594474 | 0.034* | |
| C20 | 1.08324 (19) | −0.1464 (5) | 0.65126 (11) | 0.0300 (7) | |
| H20 | 1.118190 | −0.283160 | 0.663310 | 0.036* | |
| C21 | 1.0147 (2) | −0.0408 (5) | 0.67526 (10) | 0.0248 (7) | |
| H21 | 1.002632 | −0.103184 | 0.703529 | 0.030* | |
| C22 | 0.96431 (16) | 0.1588 (5) | 0.65662 (9) | 0.0187 (5) | |
| C23 | 0.88995 (16) | 0.3076 (5) | 0.67375 (8) | 0.0183 (5) | |
| C24 | 0.84808 (18) | 0.2916 (6) | 0.71340 (9) | 0.0232 (6) | |
| H24 | 0.865756 | 0.164739 | 0.734293 | 0.028* | |
| C25 | 0.7798 (2) | 0.4652 (6) | 0.72182 (11) | 0.0274 (7) | |
| H25 | 0.751130 | 0.458980 | 0.748942 | 0.033* | |
| C26 | 0.75307 (19) | 0.6472 (5) | 0.69102 (10) | 0.0266 (6) | |
| H26 | 0.706041 | 0.763484 | 0.697254 | 0.032* | |
| C27 | 0.79414 (17) | 0.6619 (5) | 0.65113 (9) | 0.0225 (6) | |
| H27 | 0.775450 | 0.786606 | 0.630113 | 0.027* | |
| C28 | 0.86269 (19) | 0.4917 (5) | 0.64261 (10) | 0.0178 (6) | |
| C29 | 0.86563 (17) | 0.3768 (5) | 0.56314 (9) | 0.0186 (5) | |
| H29A | 0.905150 | 0.311282 | 0.540284 | 0.022* | |
| H29B | 0.825305 | 0.231754 | 0.571180 | 0.022* | |
| C30 | 0.74632 (18) | 0.5541 (5) | 0.52380 (9) | 0.0164 (5) |
(2). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0227 (10) | 0.0147 (9) | 0.0326 (11) | −0.0011 (7) | −0.0087 (8) | −0.0015 (8) |
| O2 | 0.0215 (10) | 0.0167 (9) | 0.0211 (10) | −0.0041 (7) | −0.0075 (8) | 0.0043 (7) |
| N1 | 0.0222 (13) | 0.0150 (12) | 0.0318 (14) | −0.0029 (9) | −0.0085 (11) | 0.0005 (10) |
| C1 | 0.0160 (14) | 0.0192 (13) | 0.0176 (14) | −0.0019 (11) | −0.0010 (11) | 0.0023 (10) |
| C2 | 0.0145 (11) | 0.0163 (12) | 0.0220 (14) | −0.0035 (9) | −0.0055 (10) | −0.0027 (11) |
| C3 | 0.0177 (13) | 0.0271 (14) | 0.0231 (15) | −0.0014 (11) | −0.0005 (11) | −0.0039 (11) |
| C4 | 0.0146 (13) | 0.0256 (14) | 0.0388 (18) | 0.0021 (11) | −0.0022 (12) | −0.0103 (14) |
| C5 | 0.0194 (13) | 0.0216 (13) | 0.0334 (16) | 0.0018 (10) | −0.0108 (11) | −0.0008 (11) |
| C6 | 0.0236 (15) | 0.0216 (13) | 0.0212 (15) | −0.0006 (11) | −0.0047 (12) | 0.0012 (11) |
| C7 | 0.0135 (12) | 0.0169 (13) | 0.0225 (14) | −0.0027 (9) | −0.0037 (10) | −0.0020 (10) |
| C8 | 0.0145 (12) | 0.0159 (13) | 0.0208 (14) | −0.0041 (10) | −0.0024 (10) | 0.0004 (10) |
| C9 | 0.0209 (13) | 0.0214 (13) | 0.0213 (14) | −0.0013 (11) | 0.0002 (11) | 0.0037 (10) |
| C10 | 0.0197 (16) | 0.0308 (16) | 0.0248 (18) | −0.0006 (10) | 0.0063 (13) | −0.0013 (12) |
| C11 | 0.0180 (13) | 0.0226 (14) | 0.0304 (16) | 0.0019 (11) | 0.0001 (11) | −0.0014 (11) |
| C12 | 0.0192 (13) | 0.0186 (13) | 0.0235 (15) | −0.0011 (10) | −0.0038 (11) | 0.0018 (11) |
| C13 | 0.0157 (15) | 0.0160 (13) | 0.0194 (15) | −0.0034 (9) | −0.0047 (12) | −0.0014 (10) |
| C14 | 0.0200 (13) | 0.0183 (13) | 0.0199 (14) | 0.0007 (9) | −0.0044 (11) | 0.0033 (11) |
| C15 | 0.0160 (13) | 0.0188 (13) | 0.0149 (14) | 0.0002 (9) | 0.0005 (11) | −0.0020 (10) |
| O3 | 0.0211 (10) | 0.0142 (9) | 0.0351 (12) | −0.0008 (7) | −0.0083 (8) | −0.0002 (8) |
| O4 | 0.0226 (10) | 0.0144 (9) | 0.0239 (10) | −0.0017 (7) | −0.0079 (8) | 0.0020 (8) |
| N2 | 0.0195 (12) | 0.0145 (11) | 0.0263 (13) | −0.0004 (9) | −0.0079 (10) | 0.0012 (9) |
| C16 | 0.0163 (12) | 0.0185 (13) | 0.0198 (15) | −0.0012 (9) | −0.0030 (11) | 0.0020 (11) |
| C17 | 0.0150 (11) | 0.0190 (12) | 0.0223 (14) | −0.0031 (9) | −0.0043 (10) | −0.0002 (10) |
| C18 | 0.0163 (12) | 0.0278 (14) | 0.0248 (15) | −0.0030 (10) | −0.0019 (11) | −0.0032 (11) |
| C19 | 0.0163 (14) | 0.0311 (15) | 0.0372 (18) | 0.0008 (11) | −0.0036 (12) | −0.0071 (13) |
| C20 | 0.0256 (14) | 0.0213 (14) | 0.0432 (19) | 0.0054 (11) | −0.0156 (14) | −0.0015 (12) |
| C21 | 0.0239 (16) | 0.0251 (14) | 0.0255 (16) | −0.0028 (11) | −0.0117 (13) | 0.0028 (12) |
| C22 | 0.0183 (13) | 0.0172 (12) | 0.0205 (14) | −0.0033 (9) | −0.0056 (11) | −0.0008 (10) |
| C23 | 0.0183 (12) | 0.0190 (13) | 0.0176 (14) | −0.0053 (10) | −0.0044 (10) | 0.0000 (10) |
| C24 | 0.0260 (14) | 0.0268 (15) | 0.0167 (13) | −0.0093 (11) | −0.0030 (10) | −0.0014 (11) |
| C25 | 0.0258 (17) | 0.0327 (16) | 0.0236 (18) | −0.0113 (12) | 0.0054 (14) | −0.0104 (12) |
| C26 | 0.0208 (13) | 0.0251 (15) | 0.0340 (17) | −0.0021 (11) | 0.0041 (12) | −0.0124 (12) |
| C27 | 0.0197 (13) | 0.0202 (13) | 0.0275 (15) | −0.0009 (10) | −0.0020 (12) | −0.0033 (11) |
| C28 | 0.0147 (14) | 0.0186 (14) | 0.0201 (16) | −0.0043 (9) | −0.0015 (12) | −0.0022 (10) |
| C29 | 0.0202 (13) | 0.0166 (13) | 0.0189 (13) | 0.0030 (10) | −0.0025 (10) | 0.0028 (10) |
| C30 | 0.0153 (13) | 0.0197 (13) | 0.0142 (14) | −0.0006 (10) | −0.0003 (11) | −0.0004 (11) |
(2). Geometric parameters (Å, º)
| O1—C15 | 1.234 (3) | O3—C30 | 1.230 (3) |
| O2—C14 | 1.450 (3) | O4—C29 | 1.451 (3) |
| O2—C15 | 1.340 (3) | O4—C30 | 1.343 (3) |
| N1—H1A | 0.86 (3) | N2—H2A | 0.85 (3) |
| N1—H1B | 0.91 (3) | N2—H2B | 0.87 (4) |
| N1—C15 | 1.323 (4) | N2—C30 | 1.325 (3) |
| C1—H1 | 1.0000 | C16—H16 | 1.0000 |
| C1—C2 | 1.516 (4) | C16—C17 | 1.523 (4) |
| C1—C13 | 1.518 (4) | C16—C28 | 1.519 (4) |
| C1—C14 | 1.526 (4) | C16—C29 | 1.518 (4) |
| C2—C3 | 1.390 (4) | C17—C18 | 1.388 (4) |
| C2—C7 | 1.400 (4) | C17—C22 | 1.397 (4) |
| C3—H3 | 0.9500 | C18—H18 | 0.9500 |
| C3—C4 | 1.390 (4) | C18—C19 | 1.389 (4) |
| C4—H4 | 0.9500 | C19—H19 | 0.9500 |
| C4—C5 | 1.391 (4) | C19—C20 | 1.383 (5) |
| C5—H5 | 0.9500 | C20—H20 | 0.9500 |
| C5—C6 | 1.391 (4) | C20—C21 | 1.395 (4) |
| C6—H6 | 0.9500 | C21—H21 | 0.9500 |
| C6—C7 | 1.388 (4) | C21—C22 | 1.395 (4) |
| C7—C8 | 1.470 (4) | C22—C23 | 1.466 (4) |
| C8—C9 | 1.392 (4) | C23—C24 | 1.390 (4) |
| C8—C13 | 1.397 (4) | C23—C28 | 1.403 (4) |
| C9—H9 | 0.9500 | C24—H24 | 0.9500 |
| C9—C10 | 1.391 (4) | C24—C25 | 1.391 (4) |
| C10—H10 | 0.9500 | C25—H25 | 0.9500 |
| C10—C11 | 1.387 (4) | C25—C26 | 1.387 (5) |
| C11—H11 | 0.9500 | C26—H26 | 0.9500 |
| C11—C12 | 1.394 (4) | C26—C27 | 1.391 (4) |
| C12—H12 | 0.9500 | C27—H27 | 0.9500 |
| C12—C13 | 1.386 (4) | C27—C28 | 1.383 (4) |
| C14—H14A | 0.9900 | C29—H29A | 0.9900 |
| C14—H14B | 0.9900 | C29—H29B | 0.9900 |
| C15—O2—C14 | 117.54 (19) | C30—O4—C29 | 116.43 (19) |
| H1A—N1—H1B | 124 (3) | H2A—N2—H2B | 119 (3) |
| C15—N1—H1A | 119 (2) | C30—N2—H2A | 118.4 (19) |
| C15—N1—H1B | 116 (2) | C30—N2—H2B | 121 (2) |
| C2—C1—H1 | 110.4 | C17—C16—H16 | 110.5 |
| C2—C1—C13 | 102.5 (2) | C28—C16—H16 | 110.5 |
| C2—C1—C14 | 110.3 (2) | C28—C16—C17 | 102.0 (2) |
| C13—C1—H1 | 110.4 | C29—C16—H16 | 110.5 |
| C13—C1—C14 | 112.7 (2) | C29—C16—C17 | 110.1 (2) |
| C14—C1—H1 | 110.4 | C29—C16—C28 | 113.0 (2) |
| C3—C2—C1 | 129.3 (3) | C18—C17—C16 | 129.1 (2) |
| C3—C2—C7 | 120.6 (2) | C18—C17—C22 | 120.4 (2) |
| C7—C2—C1 | 110.2 (2) | C22—C17—C16 | 110.4 (2) |
| C2—C3—H3 | 120.7 | C17—C18—H18 | 120.6 |
| C4—C3—C2 | 118.5 (3) | C17—C18—C19 | 118.8 (3) |
| C4—C3—H3 | 120.7 | C19—C18—H18 | 120.6 |
| C3—C4—H4 | 119.5 | C18—C19—H19 | 119.5 |
| C3—C4—C5 | 121.1 (3) | C20—C19—C18 | 120.9 (3) |
| C5—C4—H4 | 119.5 | C20—C19—H19 | 119.5 |
| C4—C5—H5 | 119.8 | C19—C20—H20 | 119.6 |
| C4—C5—C6 | 120.4 (3) | C19—C20—C21 | 120.9 (3) |
| C6—C5—H5 | 119.8 | C21—C20—H20 | 119.6 |
| C5—C6—H6 | 120.6 | C20—C21—H21 | 120.9 |
| C7—C6—C5 | 118.9 (3) | C22—C21—C20 | 118.2 (3) |
| C7—C6—H6 | 120.6 | C22—C21—H21 | 120.9 |
| C2—C7—C8 | 108.4 (2) | C17—C22—C23 | 108.7 (2) |
| C6—C7—C2 | 120.5 (2) | C21—C22—C17 | 120.7 (3) |
| C6—C7—C8 | 131.1 (3) | C21—C22—C23 | 130.6 (3) |
| C9—C8—C7 | 130.2 (2) | C24—C23—C22 | 130.6 (2) |
| C9—C8—C13 | 120.7 (2) | C24—C23—C28 | 120.6 (2) |
| C13—C8—C7 | 109.0 (2) | C28—C23—C22 | 108.7 (2) |
| C8—C9—H9 | 120.7 | C23—C24—H24 | 120.7 |
| C10—C9—C8 | 118.6 (3) | C23—C24—C25 | 118.6 (3) |
| C10—C9—H9 | 120.7 | C25—C24—H24 | 120.7 |
| C9—C10—H10 | 119.7 | C24—C25—H25 | 119.7 |
| C11—C10—C9 | 120.7 (3) | C26—C25—C24 | 120.7 (3) |
| C11—C10—H10 | 119.7 | C26—C25—H25 | 119.7 |
| C10—C11—H11 | 119.6 | C25—C26—H26 | 119.5 |
| C10—C11—C12 | 120.8 (2) | C25—C26—C27 | 121.0 (3) |
| C12—C11—H11 | 119.6 | C27—C26—H26 | 119.5 |
| C11—C12—H12 | 120.6 | C26—C27—H27 | 120.6 |
| C13—C12—C11 | 118.7 (3) | C28—C27—C26 | 118.8 (3) |
| C13—C12—H12 | 120.6 | C28—C27—H27 | 120.6 |
| C8—C13—C1 | 109.9 (2) | C23—C28—C16 | 110.2 (2) |
| C12—C13—C1 | 129.7 (3) | C27—C28—C16 | 129.5 (3) |
| C12—C13—C8 | 120.4 (3) | C27—C28—C23 | 120.4 (3) |
| O2—C14—C1 | 107.6 (2) | O4—C29—C16 | 107.33 (19) |
| O2—C14—H14A | 110.2 | O4—C29—H29A | 110.2 |
| O2—C14—H14B | 110.2 | O4—C29—H29B | 110.2 |
| C1—C14—H14A | 110.2 | C16—C29—H29A | 110.2 |
| C1—C14—H14B | 110.2 | C16—C29—H29B | 110.2 |
| H14A—C14—H14B | 108.5 | H29A—C29—H29B | 108.5 |
| O1—C15—O2 | 123.1 (2) | O3—C30—O4 | 123.3 (2) |
| O1—C15—N1 | 124.4 (3) | O3—C30—N2 | 124.2 (3) |
| N1—C15—O2 | 112.5 (2) | N2—C30—O4 | 112.5 (2) |
| C1—C2—C3—C4 | 179.2 (3) | C16—C17—C18—C19 | −179.5 (3) |
| C1—C2—C7—C6 | −178.9 (2) | C16—C17—C22—C21 | 179.5 (2) |
| C1—C2—C7—C8 | 1.5 (3) | C16—C17—C22—C23 | −1.2 (3) |
| C2—C1—C13—C8 | 2.8 (3) | C17—C16—C28—C23 | −2.3 (3) |
| C2—C1—C13—C12 | −178.2 (3) | C17—C16—C28—C27 | 178.2 (3) |
| C2—C1—C14—O2 | 171.9 (2) | C17—C16—C29—O4 | −170.1 (2) |
| C2—C3—C4—C5 | −0.4 (4) | C17—C18—C19—C20 | 0.4 (4) |
| C2—C7—C8—C9 | 178.4 (2) | C17—C22—C23—C24 | −179.6 (2) |
| C2—C7—C8—C13 | 0.4 (3) | C17—C22—C23—C28 | −0.3 (3) |
| C3—C2—C7—C6 | 0.6 (4) | C18—C17—C22—C21 | 0.1 (4) |
| C3—C2—C7—C8 | −179.0 (2) | C18—C17—C22—C23 | 179.4 (2) |
| C3—C4—C5—C6 | 0.4 (4) | C18—C19—C20—C21 | −0.5 (4) |
| C4—C5—C6—C7 | 0.1 (4) | C19—C20—C21—C22 | 0.3 (4) |
| C5—C6—C7—C2 | −0.6 (4) | C20—C21—C22—C17 | −0.1 (4) |
| C5—C6—C7—C8 | 179.0 (3) | C20—C21—C22—C23 | −179.2 (3) |
| C6—C7—C8—C9 | −1.2 (5) | C21—C22—C23—C24 | −0.3 (5) |
| C6—C7—C8—C13 | −179.2 (3) | C21—C22—C23—C28 | 178.9 (3) |
| C7—C2—C3—C4 | −0.2 (4) | C22—C17—C18—C19 | −0.2 (4) |
| C7—C8—C9—C10 | −177.3 (3) | C22—C23—C24—C25 | 178.0 (3) |
| C7—C8—C13—C1 | −2.1 (3) | C22—C23—C28—C16 | 1.7 (3) |
| C7—C8—C13—C12 | 178.8 (2) | C22—C23—C28—C27 | −178.7 (2) |
| C8—C9—C10—C11 | −0.9 (4) | C23—C24—C25—C26 | 1.1 (4) |
| C9—C8—C13—C1 | 179.7 (2) | C24—C23—C28—C16 | −179.0 (2) |
| C9—C8—C13—C12 | 0.5 (4) | C24—C23—C28—C27 | 0.6 (4) |
| C9—C10—C11—C12 | 0.3 (4) | C24—C25—C26—C27 | −0.4 (4) |
| C10—C11—C12—C13 | 0.8 (4) | C25—C26—C27—C28 | −0.2 (4) |
| C11—C12—C13—C1 | 179.9 (3) | C26—C27—C28—C16 | 179.6 (3) |
| C11—C12—C13—C8 | −1.2 (4) | C26—C27—C28—C23 | 0.1 (4) |
| C13—C1—C2—C3 | 178.0 (2) | C28—C16—C17—C18 | −178.6 (3) |
| C13—C1—C2—C7 | −2.6 (3) | C28—C16—C17—C22 | 2.1 (3) |
| C13—C1—C14—O2 | −74.2 (3) | C28—C16—C29—O4 | 76.7 (3) |
| C13—C8—C9—C10 | 0.5 (4) | C28—C23—C24—C25 | −1.2 (4) |
| C14—O2—C15—O1 | −2.3 (4) | C29—O4—C30—O3 | 6.6 (4) |
| C14—O2—C15—N1 | 176.8 (2) | C29—O4—C30—N2 | −173.0 (2) |
| C14—C1—C2—C3 | −61.8 (3) | C29—C16—C17—C18 | 61.2 (3) |
| C14—C1—C2—C7 | 117.6 (2) | C29—C16—C17—C22 | −118.1 (2) |
| C14—C1—C13—C8 | −115.7 (2) | C29—C16—C28—C23 | 115.9 (2) |
| C14—C1—C13—C12 | 63.3 (4) | C29—C16—C28—C27 | −63.7 (4) |
| C15—O2—C14—C1 | 150.3 (2) | C30—O4—C29—C16 | −158.2 (2) |
(2). Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1B···O3 | 0.91 (3) | 2.06 (3) | 2.955 (3) | 169 (3) |
| N1—H1A···O1i | 0.86 (3) | 2.08 (3) | 2.849 (3) | 149 (3) |
| N2—H2A···O1 | 0.85 (3) | 2.13 (3) | 2.967 (3) | 169 (3) |
| N2—H2B···O3ii | 0.87 (4) | 2.03 (4) | 2.827 (3) | 152 (3) |
Symmetry codes: (i) x, y−1, z; (ii) x, y+1, z.
References
- Ahmad Wani, N., Gupta, V. K., Kant, R., Aravinda, S. & Rai, R. (2014). Acta Cryst. E70, 272–277. [DOI] [PMC free article] [PubMed]
- Behrendt, R., White, P. & Offer, J. (2016). J. Pept. Sci.22, 4–27. [DOI] [PMC free article] [PubMed]
- Bruker (2023). APEX5 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Cruz, L. J., Beteta, N. G., Ewenson, A. & Albericio, F. (2004). Org. Process Res. Dev.8, 920–924.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hlebowicz, E., Andersen, A., Andersson, L. & Moss, B. (2005). J. Pept. Res.65, 90–97. [DOI] [PubMed]
- Isidro-Llobet, A., Just–Baringo, X., Ewenson, A., Alvarez, M. & Albericio, F. (2007). PeptideScience, 88, 733-737. [DOI] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst.48, 3–10. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst.53, 226–235. [DOI] [PMC free article] [PubMed]
- McKinnon, J. J., Jayatilaka, D. & Spackman, M. A. (2007). Chem. Commun. 3814–3816. [DOI] [PubMed]
- Tan, S. L., Jotani, M. M. & Tiekink, E. R. T. (2019). Acta Cryst. E75, 308–318. [DOI] [PMC free article] [PubMed]
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D. & Spackman, M. A. (2021). J. Appl. Cryst.54, 1006–1011. [DOI] [PMC free article] [PubMed]
- Valle, G., Bonora, G. M. & Toniolo, C. (1984). Can. J. Chem.62, 2661–2666.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2. DOI: 10.1107/S2056989025003810/vu2009sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989025003810/vu20091sup2.hkl
Supporting information file. DOI: 10.1107/S2056989025003810/vu20091sup4.cml
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989025003810/vu20092sup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report









