In the title compound, the terminal benzimidazole moieties are inclined to one another by about 68°. In the crystal, tetramolecular strands are generated by C—H⋯N hydrogen bonds and C—H⋯π(ring) interactions and are linked by C—H⋯π(ring) and π-stacking interactions.
Keywords: crystal structure, benzimidazole, thioether, hydrogen bond, π-stacking, C—H⋯π(ring) interactions
Abstract
The asymmetric unit of the title compound, C20H22N4OS2, consists of two independent molecules, one of which is disordered. In each molecule, the mean planes of the terminal benzimidazole moieties are inclined to one another by about 68°. In the crystal, tetramolecular strands are generated by C—H⋯N hydrogen bonds and C—H⋯π(ring) interactions and are linked by C—H⋯π(ring) and π-stacking interactions.
1. Chemical context
The benzimidazole ring system consists of a five-membered imidazole ring (with two nitrogens included in the heterocyclic structure) fused to another aromatic ring. This structure gives benzimidazoles significant chemical stability and a range of biological properties (Obaid et al., 2022 ▸), making them a subject of interest in the pharmacological field.
The benzimidazole ring is a well-known motif recognized for its chemical flexibility, allowing effective interaction with various biological targets. As derivatives of this ring, bisbenzimidazoles share interesting physicochemical properties, such as their ability to interact with biological macromolecules like proteins, enzymes, and DNA. These interactions make them promising candidates for the development of drugs aimed at treating various diseases. Several bisbenzimidazole derivatives have been studied for their activity against various pathogens, including parasites and bacteria. Compounds from this class are used in the treatment of parasitic diseases such as giardiasis, amebiasis, and onchocerciasis. Additionally, some studies have revealed that bisbenzimidazoles possess anticancer properties. They work by inhibiting key enzymes in cancer cells, blocking cell division, or inducing mechanisms of programmed cell death (apoptosis). For instance, derivatives like levamisole, used in cancer treatments (Yadav et al., 2018 ▸), have shown potential effects in stimulating the immune system and inhibiting tumor growth. Moreover, emerging research suggests that certain bisbenzimidazoles could be beneficial in the treatment of neurodegenerative diseases like Alzheimer’s disease (Algul et al., 2025 ▸).
Continuing our research in this field (e.g. Missioui et al., 2022 ▸), we synthesized the title compound 1,1′-[oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) via an alkylation reaction. We determined its molecular and crystalline structures, and conducted a Hirshfeld surface analysis to analyze the intermolecular interactions.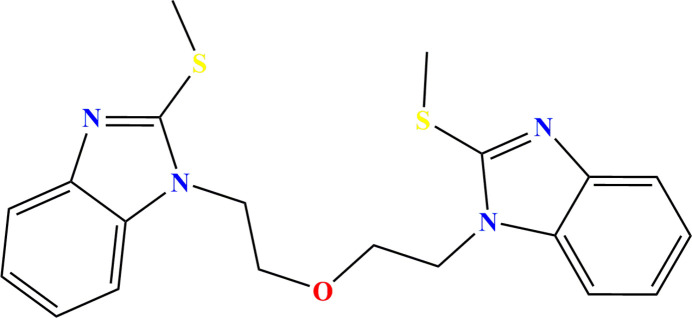
2. Structural commentary
The asymmetric unit consists of two independent molecules, one of which is disordered (Figs. 1 ▸ and 2 ▸). This involves the rotation of one 2-(methylsulfanyl)benzimidazole unit by approximately 170° about the N7—C32 bond in a 0.7200 (13)/0.2800 (13) ratio, while for that at the other end of the molecule a shift of 0.5 Å parallel to the plane of the unit is observed in a 0.775 (6)/0.225 (6) ratio (Fig. 2 ▸). In the ordered molecule, the two benzimidazole units are nearly planar as the dihedral angles between their constituent planes are less than 2°, while the dihedral angle between the mean planes of the benzimidazole units in this molecule is 68.38 (9)° (Fig. 1 ▸) and the C7—N2—C9 —C10 and the C13—N3—C12—C11 torsion angles are 99.1 (3) and 103.6 (3)°, respectively. In the central chain, the N2—C9—C10—O1 and the C11—O1—C10—C9 torsion angles are, respectively, −179.5 (2) and −180.0 (2)°, while the C10—O1—C11—C12 and the O1—C11—C12—N3 torsion angles are, respectively, 179.9 (2) and −60.0 (3)°. One methylsulfanyl group lies nearly in the plane of the five-membered ring to which it is attached [C8—S1—C7—N1 = −1.8 (3)°], but the other is rotated moderately out of the corresponding plane [C14—S2—C13—N4 = −23.2 (3)°]. The benzimidazole units in the disordered molecule were refined as planar rigid groups and the dihedral angle between their mean planes in the major component is 68.12 (11)° but because of the disorder, comparison of its torsion angles with those of the ordered molecule is not useful. In the ordered molecule, bond distances and interbond angles are as expected for the formulation given.
Figure 1.
The ordered molecule in the asymmetric unit of the title compound with labeling scheme and 50% probability ellipsoids.
Figure 2.
The disordered molecule in the asymmetric unit of the title compound showing the overlay of the two components with the minor component depicted with dashed lines.
3. Supramolecular features
In the crystal, the major component of the disordered molecule containing O2 is linked to the molecule containing O1 at −x + 1, −y + 2, −z + 1 by a C35—H34B⋯N4 hydrogen bond (Table 1 ▸) and this two-molecule unit is linked to its counterpart at −x + 1, −y, −z by a C29—H29B⋯Cg1 interaction (Table 1 ▸ and Fig. 3 ▸). These tetramolecular strands are connected by C2—H2⋯Cg5 and C11—H11B⋯Cg11 interactions (Table 1 ▸) as well as by π-stacking interactions between inversion-related N1/C6/C1/N2/C7 rings [centroid–centroid distance = 3.6645 (18) Å, dihedral angle = 0.03 (18)°, slippage = 1.06 Å] to generate the full 3-D structure (Fig. 4 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
Cg1, Cg5 and Cg11 are the centroids of the N5/C26/C21/N6/C27, the C21-C26 and the C1–C6 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯Cg5i | 0.95 | 2.71 | 3.583 (3) | 153 |
| C11—H11B⋯Cg11ii | 0.99 | 2.74 | 3.423 (3) | 126 |
| C29—H29B⋯Cg1iii | 0.99 | 2.70 | 3.489 (3) | 137 |
| C34—H34B⋯N4iv | 0.98 | 2.50 | 3.398 (3) | 153 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Figure 3.
One tetramolecular strand viewed along the c-axis direction with C—H⋯N hydrogen bonds and C—H⋯π(ring) interactions depicted, respectively, by blue and green dashed lines. Hydrogen atoms not involved in these interactions are omitted for clarity.
Figure 4.
Packing viewed along the b-axis direction with C—H⋯N hydrogen bonds and C—H⋯π(ring) and π-stacking interactions depicted, respectively, by blue, green and orange dashed lines. Hydrogen atoms not involved in these interactions are omitted for clarity.
4. Database survey
A search of the Cambridge Structural Database (CSD updated to November 2024 (Groom et al., 2016 ▸)) with the fragment shown in Fig. 5 ▸ (R = C) and restricted to only organic compounds generated seven hits. Four of these contained only one 2-(methylsulfanyl)-1H-benzamidazole moiety and had R = CH2CH2OH (DUNZUI: Akonan et al., 2010 ▸), 5,6-dihydro-2H-pyran-2-one (IHAREP: Hammal et al., 2008 ▸), morpholin-4-methyl (SIMCUN: Abou et al., 2007 ▸) and the ionic compound 1-methyl-2-(methylsulfanyl)-1H-benzimidazol-3-ium iodide (WANXUH: Hasty et al., 2017 ▸). For these, the carbon atom of the methysulfanyl group lies in or very close to the plane of the benzimidazole moiety, while for the first three, the R group projects well out of that plane, which is similar to what is seen in the title molecule. In the asymmetric unit of DUNZUI there are two independent molecules and in its crystal packing, there are π-stacking interactions between five-membered rings of one of these. More extensive π-stacking occurs in WANXUH because of its relatively flat steric profile, while in SIMCUN both rings of the benzimidazole moiety participate in π-stacking interactions.
Figure 5.

The fragment used for the database search.
Two of the other examples are more analogous to the title molecule with two 2-(methylsulfanyl)-1H-benzamidazole moieties bridged by a —(CH2)3— chain (GEVJOH: Yüksektepe et al., 2007 ▸) or by a 1,4-CH2C6H4CH2 unit (UGACEM: Rajakannu et al., 2013 ▸) while the third has two 3-methyl-2-(methylsulfanyl)-1H-benzimidazol-3-ium cations bridged by a 1,3-phenylene group and triflate anions (KEYQUE: Steinke et al., 2023 ▸). In GEVJOH, the dihedral angle between the mean planes of the two benzimidazole units is 74.87 (6)° while the torsion angles corresponding to the C7—N2—C9 —C10 and the C13—N3—C12—C11 torsion in the title molecule are, 87.9 (2) and 93.6 (2)°, respectively, similar to the title compound. For the other two, the bridging units are much less flexible with the mean planes of the benzimidazole units in KEYQUE inclined to that of the central phenylene ring by 60.5 (2) and 86.7 (2)°, respectively. UGACEM has crystallographically-imposed centrosymmetry and the unique benzimidazole is essentially perpendicular to the central phenylene ring.
5. Hirshfeld surface analysis
A Hirshfeld surface analysis of the title compound was performed with CrystalExplorer (Spackman et al., 2021 ▸) to determine the contributions of the several intermolecular interactions in the crystal. Full descriptions of the plots obtained and their interpretations have been published (Tan et al., 2019 ▸). The dnorm surface for the molecule containing O1 (ordered molecule) calculated over the range −0.1811 to 1.3161 in arbitrary units together with several nearest neighbor molecules including the major component of the disordered molecule is shown in Fig. 6 ▸a. The C—H⋯N hydrogen bonds are depicted by red dashed lines and are clearly associated with the dark red spots on the dnorm surface. Fig. 6 ▸b shows the surface for the major component of the disordered molecule calculated over the shape function and showing the characteristic pattern of triangles indicating the presence of the π-stacking interactions (dashed lines) noted in Section 3. The 2-D fingerprint plots for all intermolecular interactions and those delineated into specific contacts are presented in Fig. 7 ▸. The largest contribution is from H⋯H contacts (Fig. 7 ▸b, 52.5% of the total) consistent with the significant hydrogen content of the molecule and the fact that the hydrogen atoms constitute a large portion of its periphery. The next most important contact is C⋯H/H⋯C at 21.9% (Fig. 7 ▸c), which primarily comes from the C—H⋯π(ring) interactions. The N⋯H/H⋯N contacts (Fig. 7 ▸d), contributing 9.0%, appear as a pair of relatively sharp spikes at de + di = 2.88 Å and correspond primarily to the C—H⋯N hydrogen bonds while S⋯H/H⋯S contacts (Fig. 7 ▸e) contribute 8.5%. All other atom⋯atom contacts contribute a total of 8.1% and are considered quite minor.
Figure 6.
Hirshfeld surfaces: (a) the dnorm surface for the ordered molecule with several nearest neighbors with C—H⋯N hydrogen bonds shown as dashed lines; (b) the surface calculated over the shape function for the major component of the disordered molecule showing the π-stacking interaction.
Figure 7.
Fingerprint plots showing: (a) all intermolecular interactions and those delineated into (b) H⋯H, (c) C⋯H/H⋯C, (d) N⋯H/H⋯N and (e) S⋯H/H⋯S contacts.
6. Synthesis and crystallization
To a 50 mL round-bottom flask, 20 mL of dimethylformamide (DMF) were added followed by the successive addition of 0.0122 moles of 2-methylmercaptobenzimidazole, 0.0150 moles of potassium carbonate (K2CO3), 0.0070 moles of 1-chloro-2-(2-chloroethoxy)ethane, and 0.0007 moles of tetrabutylammonium bromide (BTBA). The mixture was stirred at room temperature for 2 h.
The salts were removed by filtration and the solvent was then removed under reduced pressure on a rotary evaporator. The residue obtained was subsequently purified by silica gel column chromatography, using hexane/ethyl acetate (80/20, v/v) as the mobile phase and recrystallized from ethanol, yielding the title compound with a 72% yield as colorless crystals.
1H NMR (300 MHz, CDCl3) (δ, ppm): 2.54 (s, 6H), 3.73 (t, 3J = 7.5 Hz, 4H), 4.33 (t, 3J = 7.9 Hz, 4H), 7.48–7.82 (m, 8Har).; 13C NMR (300 MHz, CDCl3) (δ, ppm): 15.5, 49.4, 71.3, 112.7, 124.4, 127.4, 138.8, 148.1, 171.2. HRMS (ESI–MS) (m/z) 398.54.
7. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. H atoms attached to carbon were placed in calculated positions (C—H = 0.95–0.99 Å) and included as riding contributions with isotropic displacement parameters 1.2–1.5 times those of the attached atoms. In the molecule containing O2, one end is disordered by a modest shift in a 0.775 (6)/0.225 (6) ratio while the other end is disordered by an approximate 170° rotation about the C31—C32 bond in a 0.7200 (13)/0.2800 (13) ratio. In both instances, the disordered portions were refined as rigid groups.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C20H22N4OS2 |
| M r | 398.53 |
| Crystal system, space group | Triclinic, P
|
| Temperature (K) | 100 |
| a, b, c (Å) | 10.6654 (12), 13.5974 (15), 15.7917 (17) |
| α, β, γ (°) | 109.206 (2), 101.687 (2), 107.789 (2) |
| V (Å3) | 1938.8 (4) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.29 |
| Crystal size (mm) | 0.36 × 0.22 × 0.18 |
| Data collection | |
| Diffractometer | Bruker SMART APEX |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| Tmin, Tmax | 0.90, 0.95 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 36334, 10449, 5628 |
| R int | 0.074 |
| (sin θ/λ)max (Å−1) | 0.693 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.068, 0.199, 1.00 |
| No. of reflections | 10449 |
| No. of parameters | 460 |
| No. of restraints | 3 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.32, −1.07 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989025003809/vm2313sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989025003809/vm2313Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989025003809/vm2313Isup3.cml
CCDC reference: 2447131
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
JTM thanks Tulane University for support of the Tulane Crystallography Laboratory. The contributions of the authors are as follows: conceptualization, EME and AM; methodology, AA; investigation, AM and LEG; writing (original draft), JTM and AM; writing (review and editing of the manuscript), YR; formal analysis, AA; supervision, EME; crystal structure determination and validation, JTM; resources, CKM
supplementary crystallographic information
1,1'-[Oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) . Crystal data
| C20H22N4OS2 | Z = 4 |
| Mr = 398.53 | F(000) = 840 |
| Triclinic, P1 | Dx = 1.365 Mg m−3 |
| a = 10.6654 (12) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 13.5974 (15) Å | Cell parameters from 9943 reflections |
| c = 15.7917 (17) Å | θ = 2.6–29.0° |
| α = 109.206 (2)° | µ = 0.29 mm−1 |
| β = 101.687 (2)° | T = 100 K |
| γ = 107.789 (2)° | Column, colourless |
| V = 1938.8 (4) Å3 | 0.36 × 0.22 × 0.18 mm |
1,1'-[Oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) . Data collection
| Bruker SMART APEX diffractometer | 10449 independent reflections |
| Radiation source: fine-focus sealed tube | 5628 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.074 |
| Detector resolution: 8.3333 pixels mm-1 | θmax = 29.5°, θmin = 1.7° |
| φ and ω scans | h = −14→14 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −18→18 |
| Tmin = 0.90, Tmax = 0.95 | l = −21→21 |
| 36334 measured reflections |
1,1'-[Oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) . Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.068 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.199 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.1077P)2] where P = (Fo2 + 2Fc2)/3 |
| 10449 reflections | (Δ/σ)max = 0.003 |
| 460 parameters | Δρmax = 1.32 e Å−3 |
| 3 restraints | Δρmin = −1.07 e Å−3 |
1,1'-[Oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) . Special details
| Experimental. The diffraction data were obtained from 3 sets of 400 frames, each of width 0.5 deg. in omega, collected at phi = 0.00, 90.00 and 180.00 deg. and 2 sets of 800 frames, each of width 0.45 deg in phi, collected at omega = -30.00 and 210.00 deg. The scan time was 30 sec/frame. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. H-atoms attached to carbon were placed in calculated positions (C—H = 0.95 - 0.99 Å). All were included as riding contributions with isotropic displacement parameters 1.2 - 1.5 times those of the attached atoms. In the molecule containing O2, one end is disordered by a modest shift in a 0.775 (6)/0.225 (6) ratio while the other end is disordered by an approximate 180° rotation about the C31—C32 bond in a 0.7200 (13)/0.2800 (13) ratio. In both instances, the disordered portions were refined as rigid groups. |
1,1'-[Oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| S1 | 0.28306 (8) | 0.39883 (6) | 0.31081 (5) | 0.03271 (19) | |
| S2 | 0.28353 (10) | 0.94354 (7) | 0.40847 (8) | 0.0589 (3) | |
| O1 | 0.14040 (17) | 0.69787 (14) | 0.45180 (12) | 0.0265 (4) | |
| N1 | 0.3274 (2) | 0.34561 (18) | 0.46399 (16) | 0.0261 (5) | |
| N2 | 0.3144 (2) | 0.51604 (17) | 0.49496 (15) | 0.0245 (5) | |
| N3 | 0.0989 (2) | 0.90589 (18) | 0.50202 (16) | 0.0287 (5) | |
| N4 | 0.2898 (2) | 1.0698 (2) | 0.58629 (18) | 0.0370 (6) | |
| C1 | 0.3323 (3) | 0.5043 (2) | 0.57981 (18) | 0.0254 (6) | |
| C2 | 0.3387 (3) | 0.5740 (2) | 0.66891 (19) | 0.0301 (6) | |
| H2 | 0.332501 | 0.645270 | 0.681074 | 0.036* | |
| C3 | 0.3544 (3) | 0.5346 (2) | 0.73914 (19) | 0.0347 (7) | |
| H3 | 0.358552 | 0.579523 | 0.801018 | 0.042* | |
| C4 | 0.3641 (3) | 0.4298 (2) | 0.7205 (2) | 0.0331 (7) | |
| H4 | 0.374925 | 0.405259 | 0.770283 | 0.040* | |
| C5 | 0.3586 (3) | 0.3602 (2) | 0.6312 (2) | 0.0306 (6) | |
| H5 | 0.365884 | 0.289325 | 0.619425 | 0.037* | |
| C6 | 0.3419 (3) | 0.3990 (2) | 0.56012 (19) | 0.0256 (6) | |
| C7 | 0.3107 (3) | 0.4180 (2) | 0.42919 (19) | 0.0253 (6) | |
| C8 | 0.2915 (4) | 0.2620 (2) | 0.2606 (2) | 0.0437 (8) | |
| H8A | 0.210903 | 0.203462 | 0.261097 | 0.066* | |
| H8B | 0.378035 | 0.264102 | 0.298536 | 0.066* | |
| H8C | 0.290012 | 0.244072 | 0.194921 | 0.066* | |
| C9 | 0.2979 (3) | 0.6119 (2) | 0.47908 (19) | 0.0266 (6) | |
| H9A | 0.319226 | 0.612552 | 0.421047 | 0.032* | |
| H9B | 0.364700 | 0.684011 | 0.533683 | 0.032* | |
| C10 | 0.1505 (3) | 0.6037 (2) | 0.46773 (19) | 0.0269 (6) | |
| H10A | 0.082889 | 0.531822 | 0.413204 | 0.032* | |
| H10B | 0.128798 | 0.604427 | 0.525953 | 0.032* | |
| C11 | 0.0038 (3) | 0.6962 (2) | 0.44035 (19) | 0.0277 (6) | |
| H11A | −0.019845 | 0.697939 | 0.498150 | 0.033* | |
| H11B | −0.065672 | 0.625561 | 0.385448 | 0.033* | |
| C12 | 0.0002 (3) | 0.7980 (2) | 0.42393 (19) | 0.0299 (6) | |
| H12A | 0.021078 | 0.793568 | 0.364827 | 0.036* | |
| H12B | −0.095464 | 0.795720 | 0.414292 | 0.036* | |
| C13 | 0.2245 (3) | 0.9775 (2) | 0.5057 (2) | 0.0360 (7) | |
| C14 | 0.3995 (4) | 1.0841 (3) | 0.4299 (3) | 0.0683 (12) | |
| H14A | 0.435052 | 1.079617 | 0.376729 | 0.103* | |
| H14B | 0.478065 | 1.115441 | 0.489089 | 0.103* | |
| H14C | 0.348430 | 1.133494 | 0.435353 | 0.103* | |
| C15 | 0.2020 (3) | 1.0574 (2) | 0.6399 (2) | 0.0328 (7) | |
| C16 | 0.2183 (3) | 1.1275 (2) | 0.7317 (2) | 0.0410 (8) | |
| H16 | 0.298217 | 1.197014 | 0.767967 | 0.049* | |
| C17 | 0.1160 (4) | 1.0933 (3) | 0.7681 (2) | 0.0506 (9) | |
| H17 | 0.126520 | 1.139227 | 0.831367 | 0.061* | |
| C18 | −0.0043 (4) | 0.9924 (3) | 0.7150 (2) | 0.0517 (9) | |
| H18 | −0.074229 | 0.972461 | 0.742407 | 0.062* | |
| C19 | −0.0230 (3) | 0.9218 (2) | 0.6238 (2) | 0.0384 (7) | |
| H19 | −0.104208 | 0.853237 | 0.587287 | 0.046* | |
| C20 | 0.0820 (3) | 0.9558 (2) | 0.58821 (19) | 0.0290 (6) | |
| O2 | 0.3432 (2) | 0.27334 (17) | 0.01613 (14) | 0.0388 (5) | |
| S3 | 0.16784 (16) | −0.00367 (13) | 0.02660 (19) | 0.0477 (5) | 0.775 (6) |
| N5 | 0.3973 (3) | 0.04310 (18) | 0.17227 (15) | 0.0359 (8) | 0.775 (6) |
| N6 | 0.44458 (15) | 0.10270 (18) | 0.06022 (10) | 0.0285 (6) | 0.775 (6) |
| C21 | 0.57282 (16) | 0.13722 (15) | 0.12777 (10) | 0.0269 (7) | 0.775 (6) |
| C22 | 0.70801 (15) | 0.1992 (2) | 0.13525 (16) | 0.0274 (8) | 0.775 (6) |
| H22 | 0.726802 | 0.224703 | 0.088098 | 0.033* | 0.775 (6) |
| C23 | 0.81397 (19) | 0.2219 (2) | 0.21508 (18) | 0.0331 (9) | 0.775 (6) |
| H23 | 0.908138 | 0.264333 | 0.223266 | 0.040* | 0.775 (6) |
| C24 | 0.7853 (3) | 0.1837 (2) | 0.28399 (14) | 0.0332 (10) | 0.775 (6) |
| H24 | 0.860720 | 0.200592 | 0.337720 | 0.040* | 0.775 (6) |
| C25 | 0.6500 (3) | 0.1219 (3) | 0.27622 (13) | 0.0349 (10) | 0.775 (6) |
| H25 | 0.631661 | 0.096128 | 0.323322 | 0.042* | 0.775 (6) |
| C26 | 0.5421 (2) | 0.09900 (16) | 0.19659 (11) | 0.0315 (8) | 0.775 (6) |
| C27 | 0.34510 (18) | 0.04853 (11) | 0.09217 (12) | 0.0343 (8) | 0.775 (6) |
| C28 | 0.1014 (6) | −0.1083 (4) | 0.0703 (4) | 0.0574 (16) | 0.775 (6) |
| H28A | −0.000450 | −0.147436 | 0.039998 | 0.086* | 0.775 (6) |
| H28B | 0.143558 | −0.163816 | 0.055384 | 0.086* | 0.775 (6) |
| H28C | 0.124789 | −0.070815 | 0.139505 | 0.086* | 0.775 (6) |
| S3A | 0.1493 (6) | 0.0051 (5) | −0.0063 (5) | 0.0477 (5) | 0.225 (6) |
| N5A | 0.3535 (8) | 0.0386 (7) | 0.1495 (5) | 0.0359 (8) | 0.225 (6) |
| N6A | 0.4310 (6) | 0.1156 (7) | 0.0536 (4) | 0.0285 (6) | 0.225 (6) |
| C21A | 0.5488 (6) | 0.1448 (7) | 0.1284 (4) | 0.0269 (7) | 0.225 (6) |
| C22A | 0.6890 (6) | 0.2104 (10) | 0.1500 (6) | 0.0274 (8) | 0.225 (6) |
| H22A | 0.720515 | 0.243208 | 0.109577 | 0.033* | 0.225 (6) |
| C23A | 0.7804 (7) | 0.2254 (9) | 0.2336 (6) | 0.0331 (9) | 0.225 (6) |
| H23A | 0.877397 | 0.269824 | 0.251334 | 0.040* | 0.225 (6) |
| C24A | 0.7330 (9) | 0.1763 (10) | 0.2925 (5) | 0.0332 (10) | 0.225 (6) |
| H24A | 0.798933 | 0.188189 | 0.349213 | 0.040* | 0.225 (6) |
| C25A | 0.5928 (10) | 0.1110 (11) | 0.2707 (5) | 0.0349 (10) | 0.225 (6) |
| H25A | 0.561761 | 0.077973 | 0.311061 | 0.042* | 0.225 (6) |
| C26A | 0.4993 (8) | 0.0958 (7) | 0.1871 (4) | 0.0315 (8) | 0.225 (6) |
| C27A | 0.3187 (6) | 0.0537 (4) | 0.0717 (4) | 0.0343 (8) | 0.225 (6) |
| C28A | 0.067 (2) | −0.1135 (11) | 0.0191 (15) | 0.0574 (16) | 0.225 (6) |
| H28D | −0.032413 | −0.152168 | −0.019878 | 0.086* | 0.225 (6) |
| H28E | 0.112093 | −0.166856 | 0.004352 | 0.086* | 0.225 (6) |
| H28F | 0.076253 | −0.086085 | 0.086683 | 0.086* | 0.225 (6) |
| C29 | 0.4256 (3) | 0.1246 (2) | −0.02772 (19) | 0.0342 (7) | |
| H29A | 0.329141 | 0.074043 | −0.072048 | 0.041* | |
| H29B | 0.490264 | 0.102472 | −0.059150 | 0.041* | |
| C30 | 0.4495 (3) | 0.2450 (2) | −0.0135 (2) | 0.0330 (6) | |
| H30A | 0.542073 | 0.298032 | 0.035349 | 0.040* | |
| H30B | 0.448728 | 0.252538 | −0.073792 | 0.040* | |
| C31 | 0.3493 (3) | 0.3779 (2) | 0.0136 (2) | 0.0355 (7) | |
| H31A | 0.322927 | 0.368331 | −0.053359 | 0.043* | |
| H31B | 0.446198 | 0.436242 | 0.047735 | 0.043* | |
| C32 | 0.2527 (5) | 0.4171 (3) | 0.0588 (3) | 0.0296 (9) | 0.7195 (12) |
| H32A | 0.164360 | 0.351176 | 0.040756 | 0.036* | 0.7195 (12) |
| H32B | 0.296278 | 0.452162 | 0.128946 | 0.036* | 0.7195 (12) |
| C32A | 0.1981 (13) | 0.3781 (6) | 0.0241 (9) | 0.0296 (9) | 0.2805 (12) |
| H32C | 0.119843 | 0.314492 | −0.031603 | 0.036* | 0.2805 (12) |
| H32D | 0.188617 | 0.368449 | 0.082266 | 0.036* | 0.2805 (12) |
| S4 | 0.39349 (9) | 0.68180 (7) | 0.19358 (6) | 0.0311 (2) | 0.7195 (12) |
| N7 | 0.22350 (13) | 0.49926 (7) | 0.02765 (7) | 0.0211 (5) | 0.7195 (12) |
| N8 | 0.21846 (13) | 0.66717 (8) | 0.03301 (7) | 0.0223 (6) | 0.7195 (12) |
| C33 | 0.27146 (8) | 0.61603 (6) | 0.07802 (5) | 0.0231 (7) | 0.7195 (12) |
| C34 | 0.41965 (18) | 0.82874 (7) | 0.22143 (9) | 0.0458 (12) | 0.7195 (12) |
| H34A | 0.450478 | 0.850518 | 0.173404 | 0.069* | 0.7195 (12) |
| H34B | 0.491032 | 0.878050 | 0.284386 | 0.069* | 0.7195 (12) |
| H34C | 0.331546 | 0.836988 | 0.221551 | 0.069* | 0.7195 (12) |
| C35 | 0.12425 (11) | 0.57701 (9) | −0.05554 (6) | 0.0244 (7) | 0.7195 (12) |
| C36 | 0.03477 (17) | 0.58012 (13) | −0.13155 (8) | 0.0288 (8) | 0.7195 (12) |
| H36 | 0.032523 | 0.649751 | −0.130650 | 0.035* | 0.7195 (12) |
| C37 | −0.04984 (16) | 0.47801 (15) | −0.20768 (7) | 0.0264 (8) | 0.7195 (12) |
| H37 | −0.111558 | 0.477422 | −0.260650 | 0.032* | 0.7195 (12) |
| C38 | −0.04811 (17) | 0.37225 (13) | −0.20983 (8) | 0.0354 (9) | 0.7195 (12) |
| H38 | −0.109548 | 0.303410 | −0.263525 | 0.042* | 0.7195 (12) |
| C39 | 0.04091 (19) | 0.36846 (10) | −0.13557 (9) | 0.0313 (9) | 0.7195 (12) |
| H39 | 0.043377 | 0.298685 | −0.137039 | 0.038* | 0.7195 (12) |
| C40 | 0.12734 (12) | 0.47218 (8) | −0.05802 (6) | 0.0239 (7) | 0.7195 (12) |
| S4A | 0.0421 (3) | 0.39830 (19) | −0.15751 (16) | 0.0311 (2) | 0.2805 (12) |
| N7A | 0.1945 (4) | 0.4857 (2) | 0.02976 (17) | 0.0211 (5) | 0.2805 (12) |
| N8A | 0.1597 (4) | 0.61691 (18) | −0.02063 (18) | 0.0223 (6) | 0.2805 (12) |
| C33A | 0.1366 (2) | 0.50973 (18) | −0.04389 (15) | 0.0231 (7) | 0.2805 (12) |
| C34A | −0.0100 (5) | 0.4769 (3) | −0.22092 (19) | 0.0458 (12) | 0.2805 (12) |
| H34D | 0.073521 | 0.533118 | −0.220876 | 0.069* | 0.2805 (12) |
| H34E | −0.062280 | 0.515944 | −0.189389 | 0.069* | 0.2805 (12) |
| H34F | −0.069224 | 0.424149 | −0.286721 | 0.069* | 0.2805 (12) |
| C35A | 0.2426 (3) | 0.6713 (2) | 0.07740 (18) | 0.0244 (7) | 0.2805 (12) |
| C36A | 0.3022 (5) | 0.7864 (2) | 0.1396 (2) | 0.0288 (8) | 0.2805 (12) |
| H36A | 0.287487 | 0.841980 | 0.119762 | 0.035* | 0.2805 (12) |
| C37A | 0.3831 (5) | 0.8157 (3) | 0.2307 (2) | 0.0264 (8) | 0.2805 (12) |
| H37A | 0.424704 | 0.893413 | 0.274827 | 0.032* | 0.2805 (12) |
| C38A | 0.4068 (5) | 0.7329 (4) | 0.26154 (19) | 0.0354 (9) | 0.2805 (12) |
| H38A | 0.464784 | 0.757051 | 0.324994 | 0.042* | 0.2805 (12) |
| C39A | 0.3472 (6) | 0.6189 (3) | 0.20094 (18) | 0.0313 (9) | 0.2805 (12) |
| H39A | 0.361568 | 0.563534 | 0.221265 | 0.038* | 0.2805 (12) |
| C40A | 0.2647 (4) | 0.5887 (2) | 0.10817 (16) | 0.0239 (7) | 0.2805 (12) |
1,1'-[Oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0413 (4) | 0.0294 (4) | 0.0308 (4) | 0.0179 (3) | 0.0115 (3) | 0.0135 (3) |
| S2 | 0.0604 (6) | 0.0366 (5) | 0.0818 (7) | 0.0194 (4) | 0.0425 (5) | 0.0165 (5) |
| O1 | 0.0267 (9) | 0.0217 (9) | 0.0318 (10) | 0.0118 (8) | 0.0049 (8) | 0.0130 (8) |
| N1 | 0.0244 (11) | 0.0220 (11) | 0.0314 (12) | 0.0103 (9) | 0.0060 (9) | 0.0117 (10) |
| N2 | 0.0258 (11) | 0.0195 (11) | 0.0274 (12) | 0.0105 (9) | 0.0036 (9) | 0.0108 (9) |
| N3 | 0.0279 (12) | 0.0205 (11) | 0.0346 (13) | 0.0108 (9) | 0.0022 (10) | 0.0118 (10) |
| N4 | 0.0293 (13) | 0.0271 (13) | 0.0509 (16) | 0.0102 (10) | 0.0052 (12) | 0.0180 (12) |
| C1 | 0.0237 (13) | 0.0214 (13) | 0.0272 (14) | 0.0074 (11) | 0.0013 (11) | 0.0117 (11) |
| C2 | 0.0361 (15) | 0.0189 (13) | 0.0272 (14) | 0.0122 (11) | −0.0003 (12) | 0.0056 (11) |
| C3 | 0.0446 (17) | 0.0294 (15) | 0.0226 (14) | 0.0157 (13) | 0.0009 (12) | 0.0074 (12) |
| C4 | 0.0398 (16) | 0.0295 (15) | 0.0294 (15) | 0.0150 (13) | 0.0016 (12) | 0.0167 (13) |
| C5 | 0.0317 (14) | 0.0225 (13) | 0.0345 (16) | 0.0112 (11) | 0.0023 (12) | 0.0133 (12) |
| C6 | 0.0221 (13) | 0.0219 (13) | 0.0296 (14) | 0.0083 (11) | 0.0029 (11) | 0.0109 (11) |
| C7 | 0.0225 (13) | 0.0220 (13) | 0.0306 (14) | 0.0098 (11) | 0.0064 (11) | 0.0111 (11) |
| C8 | 0.060 (2) | 0.0313 (16) | 0.0424 (18) | 0.0206 (15) | 0.0217 (16) | 0.0134 (15) |
| C9 | 0.0303 (14) | 0.0197 (13) | 0.0306 (14) | 0.0112 (11) | 0.0059 (11) | 0.0130 (11) |
| C10 | 0.0294 (14) | 0.0190 (12) | 0.0308 (14) | 0.0100 (11) | 0.0050 (11) | 0.0115 (11) |
| C11 | 0.0256 (13) | 0.0232 (13) | 0.0278 (14) | 0.0094 (11) | −0.0004 (11) | 0.0091 (11) |
| C12 | 0.0292 (14) | 0.0244 (14) | 0.0304 (15) | 0.0134 (11) | 0.0000 (11) | 0.0085 (12) |
| C13 | 0.0309 (15) | 0.0252 (15) | 0.0519 (19) | 0.0127 (12) | 0.0090 (14) | 0.0177 (14) |
| C14 | 0.070 (3) | 0.043 (2) | 0.107 (3) | 0.0210 (19) | 0.059 (3) | 0.033 (2) |
| C15 | 0.0362 (15) | 0.0224 (14) | 0.0328 (15) | 0.0101 (12) | −0.0059 (12) | 0.0151 (12) |
| C16 | 0.0485 (19) | 0.0257 (15) | 0.0320 (16) | 0.0055 (13) | −0.0047 (14) | 0.0122 (13) |
| C17 | 0.080 (3) | 0.0287 (16) | 0.0340 (17) | 0.0131 (17) | 0.0156 (17) | 0.0123 (14) |
| C18 | 0.072 (2) | 0.0325 (17) | 0.044 (2) | 0.0095 (17) | 0.0270 (18) | 0.0151 (16) |
| C19 | 0.0450 (18) | 0.0246 (15) | 0.0377 (17) | 0.0072 (13) | 0.0098 (14) | 0.0128 (13) |
| C20 | 0.0314 (14) | 0.0215 (13) | 0.0326 (15) | 0.0105 (11) | −0.0001 (12) | 0.0161 (12) |
| O2 | 0.0555 (13) | 0.0383 (12) | 0.0400 (12) | 0.0330 (11) | 0.0195 (10) | 0.0219 (10) |
| S3 | 0.0413 (6) | 0.0394 (6) | 0.0431 (11) | 0.0092 (5) | 0.0076 (7) | 0.0051 (7) |
| N5 | 0.044 (2) | 0.0291 (14) | 0.0327 (19) | 0.0147 (16) | 0.0109 (17) | 0.0124 (14) |
| N6 | 0.0403 (14) | 0.0231 (13) | 0.0288 (13) | 0.0213 (11) | 0.0092 (11) | 0.0122 (11) |
| C21 | 0.0387 (18) | 0.0241 (14) | 0.0267 (14) | 0.0242 (14) | 0.0103 (13) | 0.0107 (12) |
| C22 | 0.0408 (18) | 0.0338 (17) | 0.0217 (16) | 0.0308 (15) | 0.0161 (13) | 0.0103 (14) |
| C23 | 0.040 (2) | 0.0467 (19) | 0.0254 (19) | 0.0306 (17) | 0.0174 (15) | 0.0143 (16) |
| C24 | 0.042 (3) | 0.0400 (19) | 0.0301 (17) | 0.031 (2) | 0.0099 (17) | 0.0170 (15) |
| C25 | 0.060 (3) | 0.0230 (17) | 0.0246 (16) | 0.023 (2) | 0.0099 (19) | 0.0104 (14) |
| C26 | 0.050 (3) | 0.0226 (15) | 0.0291 (17) | 0.0231 (17) | 0.0136 (17) | 0.0116 (13) |
| C27 | 0.047 (2) | 0.0216 (15) | 0.037 (2) | 0.0199 (15) | 0.0151 (17) | 0.0095 (15) |
| C28 | 0.060 (3) | 0.036 (2) | 0.071 (4) | 0.014 (2) | 0.041 (3) | 0.011 (3) |
| S3A | 0.0413 (6) | 0.0394 (6) | 0.0431 (11) | 0.0092 (5) | 0.0076 (7) | 0.0051 (7) |
| N5A | 0.044 (2) | 0.0291 (14) | 0.0327 (19) | 0.0147 (16) | 0.0109 (17) | 0.0124 (14) |
| N6A | 0.0403 (14) | 0.0231 (13) | 0.0288 (13) | 0.0213 (11) | 0.0092 (11) | 0.0122 (11) |
| C21A | 0.0387 (18) | 0.0241 (14) | 0.0267 (14) | 0.0242 (14) | 0.0103 (13) | 0.0107 (12) |
| C22A | 0.0408 (18) | 0.0338 (17) | 0.0217 (16) | 0.0308 (15) | 0.0161 (13) | 0.0103 (14) |
| C23A | 0.040 (2) | 0.0467 (19) | 0.0254 (19) | 0.0306 (17) | 0.0174 (15) | 0.0143 (16) |
| C24A | 0.042 (3) | 0.0400 (19) | 0.0301 (17) | 0.031 (2) | 0.0099 (17) | 0.0170 (15) |
| C25A | 0.060 (3) | 0.0230 (17) | 0.0246 (16) | 0.023 (2) | 0.0099 (19) | 0.0104 (14) |
| C26A | 0.050 (3) | 0.0226 (15) | 0.0291 (17) | 0.0231 (17) | 0.0136 (17) | 0.0116 (13) |
| C27A | 0.047 (2) | 0.0216 (15) | 0.037 (2) | 0.0199 (15) | 0.0151 (17) | 0.0095 (15) |
| C28A | 0.060 (3) | 0.036 (2) | 0.071 (4) | 0.014 (2) | 0.041 (3) | 0.011 (3) |
| C29 | 0.0439 (17) | 0.0308 (15) | 0.0270 (15) | 0.0225 (13) | 0.0063 (13) | 0.0076 (12) |
| C30 | 0.0437 (17) | 0.0322 (15) | 0.0267 (15) | 0.0243 (13) | 0.0080 (13) | 0.0105 (12) |
| C31 | 0.0514 (18) | 0.0339 (16) | 0.0346 (16) | 0.0293 (14) | 0.0152 (14) | 0.0186 (14) |
| C32 | 0.041 (3) | 0.023 (2) | 0.027 (3) | 0.016 (2) | 0.0091 (19) | 0.0118 (19) |
| C32A | 0.041 (3) | 0.023 (2) | 0.027 (3) | 0.016 (2) | 0.0091 (19) | 0.0118 (19) |
| S4 | 0.0366 (5) | 0.0262 (5) | 0.0259 (5) | 0.0137 (4) | 0.0005 (4) | 0.0104 (4) |
| N7 | 0.0230 (14) | 0.0191 (12) | 0.0247 (12) | 0.0087 (10) | 0.0093 (10) | 0.0120 (10) |
| N8 | 0.0272 (17) | 0.0227 (16) | 0.0187 (16) | 0.0118 (13) | 0.0055 (12) | 0.0102 (13) |
| C33 | 0.0222 (17) | 0.0232 (17) | 0.0217 (17) | 0.0077 (14) | 0.0074 (13) | 0.0083 (14) |
| C34 | 0.054 (3) | 0.033 (2) | 0.036 (2) | 0.024 (2) | 0.003 (2) | 0.0001 (19) |
| C35 | 0.0278 (18) | 0.0218 (17) | 0.0227 (19) | 0.0111 (15) | 0.0054 (15) | 0.0092 (15) |
| C36 | 0.0316 (19) | 0.0306 (19) | 0.0287 (19) | 0.0161 (16) | 0.0084 (15) | 0.0154 (16) |
| C37 | 0.0229 (17) | 0.035 (2) | 0.0168 (17) | 0.0100 (15) | 0.0023 (14) | 0.0100 (16) |
| C38 | 0.036 (2) | 0.031 (2) | 0.029 (2) | 0.0118 (17) | 0.0061 (17) | 0.0058 (17) |
| C39 | 0.043 (2) | 0.0206 (18) | 0.033 (2) | 0.0149 (17) | 0.0077 (17) | 0.0149 (16) |
| C40 | 0.0272 (18) | 0.0255 (18) | 0.0216 (17) | 0.0146 (15) | 0.0075 (14) | 0.0099 (14) |
| S4A | 0.0366 (5) | 0.0262 (5) | 0.0259 (5) | 0.0137 (4) | 0.0005 (4) | 0.0104 (4) |
| N7A | 0.0230 (14) | 0.0191 (12) | 0.0247 (12) | 0.0087 (10) | 0.0093 (10) | 0.0120 (10) |
| N8A | 0.0272 (17) | 0.0227 (16) | 0.0187 (16) | 0.0118 (13) | 0.0055 (12) | 0.0102 (13) |
| C33A | 0.0222 (17) | 0.0232 (17) | 0.0217 (17) | 0.0077 (14) | 0.0074 (13) | 0.0083 (14) |
| C34A | 0.054 (3) | 0.033 (2) | 0.036 (2) | 0.024 (2) | 0.003 (2) | 0.0001 (19) |
| C35A | 0.0278 (18) | 0.0218 (17) | 0.0227 (19) | 0.0111 (15) | 0.0054 (15) | 0.0092 (15) |
| C36A | 0.0316 (19) | 0.0306 (19) | 0.0287 (19) | 0.0161 (16) | 0.0084 (15) | 0.0154 (16) |
| C37A | 0.0229 (17) | 0.035 (2) | 0.0168 (17) | 0.0100 (15) | 0.0023 (14) | 0.0100 (16) |
| C38A | 0.036 (2) | 0.031 (2) | 0.029 (2) | 0.0118 (17) | 0.0061 (17) | 0.0058 (17) |
| C39A | 0.043 (2) | 0.0206 (18) | 0.033 (2) | 0.0149 (17) | 0.0077 (17) | 0.0149 (16) |
| C40A | 0.0272 (18) | 0.0255 (18) | 0.0216 (17) | 0.0146 (15) | 0.0075 (14) | 0.0099 (14) |
1,1'-[Oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) . Geometric parameters (Å, º)
| S1—C7 | 1.749 (3) | S3A—C27A | 1.7474 |
| S1—C8 | 1.805 (3) | S3A—C28A | 1.799 (5) |
| S2—C13 | 1.753 (3) | N5A—C27A | 1.3103 |
| S2—C14 | 1.805 (3) | N5A—C26A | 1.3998 |
| O1—C10 | 1.414 (3) | N6A—C29 | 1.321 (6) |
| O1—C11 | 1.423 (3) | N6A—C27A | 1.3808 |
| N1—C7 | 1.314 (3) | N6A—C21A | 1.3843 |
| N1—C6 | 1.402 (3) | C21A—C22A | 1.3879 |
| N2—C7 | 1.377 (3) | C21A—C26A | 1.4038 |
| N2—C1 | 1.383 (3) | C22A—C23A | 1.3840 |
| N2—C9 | 1.460 (3) | C22A—H22A | 0.9500 |
| N3—C13 | 1.371 (4) | C23A—C24A | 1.3994 |
| N3—C20 | 1.383 (4) | C23A—H23A | 0.9500 |
| N3—C12 | 1.457 (3) | C24A—C25A | 1.3863 |
| N4—C13 | 1.317 (4) | C24A—H24A | 0.9500 |
| N4—C15 | 1.393 (4) | C25A—C26A | 1.3929 |
| C1—C2 | 1.388 (4) | C25A—H25A | 0.9500 |
| C1—C6 | 1.402 (3) | C28A—H28D | 0.9800 |
| C2—C3 | 1.383 (4) | C28A—H28E | 0.9800 |
| C2—H2 | 0.9500 | C28A—H28F | 0.9800 |
| C3—C4 | 1.399 (4) | C29—C30 | 1.508 (4) |
| C3—H3 | 0.9500 | C29—H29A | 0.9900 |
| C4—C5 | 1.392 (4) | C29—H29B | 0.9900 |
| C4—H4 | 0.9500 | C30—H30A | 0.9900 |
| C5—C6 | 1.392 (4) | C30—H30B | 0.9900 |
| C5—H5 | 0.9500 | C31—C32 | 1.495 (5) |
| C8—H8A | 0.9800 | C31—C32A | 1.654 (13) |
| C8—H8B | 0.9800 | C31—H31A | 0.9900 |
| C8—H8C | 0.9800 | C31—H31B | 0.9900 |
| C9—C10 | 1.511 (4) | C32—N7 | 1.449 (3) |
| C9—H9A | 0.9900 | C32—H32A | 0.9900 |
| C9—H9B | 0.9900 | C32—H32B | 0.9900 |
| C10—H10A | 0.9900 | C32A—N7A | 1.449 (4) |
| C10—H10B | 0.9900 | C32A—H32C | 0.9900 |
| C11—C12 | 1.500 (3) | C32A—H32D | 0.9900 |
| C11—H11A | 0.9900 | S4—C33 | 1.7540 |
| C11—H11B | 0.9900 | S4—C34 | 1.8152 |
| C12—H12A | 0.9900 | N7—C40 | 1.3773 |
| C12—H12B | 0.9900 | N7—C33 | 1.3868 |
| C14—H14A | 0.9800 | N8—C33 | 1.3081 |
| C14—H14B | 0.9800 | N8—C35 | 1.4206 |
| C14—H14C | 0.9800 | C34—H34A | 0.9800 |
| C15—C16 | 1.388 (4) | C34—H34B | 0.9800 |
| C15—C20 | 1.402 (4) | C34—H34C | 0.9800 |
| C16—C17 | 1.363 (5) | C35—C36 | 1.3954 |
| C16—H16 | 0.9500 | C35—C40 | 1.4237 |
| C17—C18 | 1.401 (4) | C36—C37 | 1.3726 |
| C17—H17 | 0.9500 | C36—H36 | 0.9500 |
| C18—C19 | 1.375 (4) | C37—C38 | 1.4330 |
| C18—H18 | 0.9500 | C37—H37 | 0.9500 |
| C19—C20 | 1.376 (4) | C38—C39 | 1.3777 |
| C19—H19 | 0.9500 | C38—H38 | 0.9500 |
| O2—C31 | 1.418 (3) | C39—C40 | 1.3966 |
| O2—C30 | 1.420 (3) | C39—H39 | 0.9500 |
| S3—C27 | 1.7474 | S4A—C33A | 1.7540 |
| S3—C28 | 1.798 (5) | S4A—C34A | 1.8152 |
| N5—C27 | 1.3103 | N7A—C40A | 1.3773 |
| N5—C26 | 1.3998 | N7A—C33A | 1.3867 |
| N6—C27 | 1.3808 | N8A—C33A | 1.3081 |
| N6—C21 | 1.3843 | N8A—C35A | 1.4206 |
| N6—C29 | 1.501 (3) | C34A—H34D | 0.9800 |
| C21—C22 | 1.3879 | C34A—H34E | 0.9800 |
| C21—C26 | 1.4038 | C34A—H34F | 0.9800 |
| C22—C23 | 1.3839 | C35A—C36A | 1.3954 |
| C22—H22 | 0.9500 | C35A—C40A | 1.4237 |
| C23—C24 | 1.3994 | C36A—C37A | 1.3726 |
| C23—H23 | 0.9500 | C36A—H36A | 0.9500 |
| C24—C25 | 1.3863 | C37A—C38A | 1.4330 |
| C24—H24 | 0.9500 | C37A—H37A | 0.9500 |
| C25—C26 | 1.3930 | C38A—C39A | 1.3776 |
| C25—H25 | 0.9500 | C38A—H38A | 0.9500 |
| C28—H28A | 0.9800 | C39A—C40A | 1.3966 |
| C28—H28B | 0.9800 | C39A—H39A | 0.9500 |
| C28—H28C | 0.9800 | ||
| C7—S1—C8 | 100.14 (14) | C29—N6A—C21A | 127.8 (3) |
| C13—S2—C14 | 99.88 (16) | C27A—N6A—C21A | 106.1 |
| C10—O1—C11 | 111.49 (18) | N6A—C21A—C22A | 131.6 |
| C7—N1—C6 | 103.9 (2) | N6A—C21A—C26A | 105.4 |
| C7—N2—C1 | 106.1 (2) | C22A—C21A—C26A | 123.0 |
| C7—N2—C9 | 127.7 (2) | C23A—C22A—C21A | 116.4 |
| C1—N2—C9 | 126.2 (2) | C23A—C22A—H22A | 121.8 |
| C13—N3—C20 | 106.6 (2) | C21A—C22A—H22A | 121.8 |
| C13—N3—C12 | 127.9 (3) | C22A—C23A—C24A | 121.4 |
| C20—N3—C12 | 125.5 (2) | C22A—C23A—H23A | 119.3 |
| C13—N4—C15 | 104.2 (2) | C24A—C23A—H23A | 119.3 |
| N2—C1—C2 | 131.7 (2) | C25A—C24A—C23A | 122.0 |
| N2—C1—C6 | 105.5 (2) | C25A—C24A—H24A | 119.0 |
| C2—C1—C6 | 122.7 (2) | C23A—C24A—H24A | 119.0 |
| C3—C2—C1 | 116.8 (2) | C24A—C25A—C26A | 117.4 |
| C3—C2—H2 | 121.6 | C24A—C25A—H25A | 121.3 |
| C1—C2—H2 | 121.6 | C26A—C25A—H25A | 121.3 |
| C2—C3—C4 | 121.0 (3) | C25A—C26A—N5A | 129.7 |
| C2—C3—H3 | 119.5 | C25A—C26A—C21A | 119.9 |
| C4—C3—H3 | 119.5 | N5A—C26A—C21A | 110.3 |
| C5—C4—C3 | 122.1 (3) | N5A—C27A—N6A | 114.0 |
| C5—C4—H4 | 118.9 | N5A—C27A—S3A | 126.5 |
| C3—C4—H4 | 118.9 | N6A—C27A—S3A | 119.5 |
| C6—C5—C4 | 117.1 (2) | S3A—C28A—H28D | 109.5 |
| C6—C5—H5 | 121.5 | S3A—C28A—H28E | 109.5 |
| C4—C5—H5 | 121.5 | H28D—C28A—H28E | 109.5 |
| C5—C6—C1 | 120.2 (2) | S3A—C28A—H28F | 109.5 |
| C5—C6—N1 | 129.4 (2) | H28D—C28A—H28F | 109.5 |
| C1—C6—N1 | 110.3 (2) | H28E—C28A—H28F | 109.5 |
| N1—C7—N2 | 114.2 (2) | N6A—C29—C30 | 109.5 (4) |
| N1—C7—S1 | 126.1 (2) | N6—C29—C30 | 116.4 (2) |
| N2—C7—S1 | 119.69 (19) | N6—C29—H29A | 108.2 |
| S1—C8—H8A | 109.5 | C30—C29—H29A | 108.2 |
| S1—C8—H8B | 109.5 | N6—C29—H29B | 108.2 |
| H8A—C8—H8B | 109.5 | C30—C29—H29B | 108.2 |
| S1—C8—H8C | 109.5 | H29A—C29—H29B | 107.3 |
| H8A—C8—H8C | 109.5 | O2—C30—C29 | 110.0 (2) |
| H8B—C8—H8C | 109.5 | O2—C30—H30A | 109.7 |
| N2—C9—C10 | 110.6 (2) | C29—C30—H30A | 109.7 |
| N2—C9—H9A | 109.5 | O2—C30—H30B | 109.7 |
| C10—C9—H9A | 109.5 | C29—C30—H30B | 109.7 |
| N2—C9—H9B | 109.5 | H30A—C30—H30B | 108.2 |
| C10—C9—H9B | 109.5 | O2—C31—C32 | 110.8 (2) |
| H9A—C9—H9B | 108.1 | O2—C31—C32A | 102.3 (3) |
| O1—C10—C9 | 108.1 (2) | O2—C31—H31A | 109.5 |
| O1—C10—H10A | 110.1 | C32—C31—H31A | 109.5 |
| C9—C10—H10A | 110.1 | O2—C31—H31B | 109.5 |
| O1—C10—H10B | 110.1 | C32—C31—H31B | 109.5 |
| C9—C10—H10B | 110.1 | H31A—C31—H31B | 108.1 |
| H10A—C10—H10B | 108.4 | N7—C32—C31 | 109.8 (3) |
| O1—C11—C12 | 108.8 (2) | N7—C32—H32A | 109.7 |
| O1—C11—H11A | 109.9 | C31—C32—H32A | 109.7 |
| C12—C11—H11A | 109.9 | N7—C32—H32B | 109.7 |
| O1—C11—H11B | 109.9 | C31—C32—H32B | 109.7 |
| C12—C11—H11B | 109.9 | H32A—C32—H32B | 108.2 |
| H11A—C11—H11B | 108.3 | N7A—C32A—C31 | 108.3 (7) |
| N3—C12—C11 | 113.5 (2) | N7A—C32A—H32C | 110.0 |
| N3—C12—H12A | 108.9 | C31—C32A—H32C | 110.0 |
| C11—C12—H12A | 108.9 | N7A—C32A—H32D | 110.0 |
| N3—C12—H12B | 108.9 | C31—C32A—H32D | 110.0 |
| C11—C12—H12B | 108.9 | H32C—C32A—H32D | 108.4 |
| H12A—C12—H12B | 107.7 | C33—S4—C34 | 99.7 |
| N4—C13—N3 | 113.6 (3) | C40—N7—C33 | 105.9 |
| N4—C13—S2 | 126.0 (2) | C40—N7—C32 | 124.77 (17) |
| N3—C13—S2 | 120.4 (2) | C33—N7—C32 | 129.05 (17) |
| S2—C14—H14A | 109.5 | C33—N8—C35 | 103.7 |
| S2—C14—H14B | 109.5 | N8—C33—N7 | 115.1 |
| H14A—C14—H14B | 109.5 | N8—C33—S4 | 125.9 |
| S2—C14—H14C | 109.5 | N7—C33—S4 | 119.0 |
| H14A—C14—H14C | 109.5 | S4—C34—H34A | 109.5 |
| H14B—C14—H14C | 109.5 | S4—C34—H34B | 109.5 |
| C16—C15—N4 | 130.1 (3) | H34A—C34—H34B | 109.5 |
| C16—C15—C20 | 119.5 (3) | S4—C34—H34C | 109.5 |
| N4—C15—C20 | 110.5 (3) | H34A—C34—H34C | 109.5 |
| C17—C16—C15 | 117.9 (3) | H34B—C34—H34C | 109.5 |
| C17—C16—H16 | 121.1 | C36—C35—N8 | 129.5 |
| C15—C16—H16 | 121.1 | C36—C35—C40 | 121.0 |
| C16—C17—C18 | 122.0 (3) | N8—C35—C40 | 109.5 |
| C16—C17—H17 | 119.0 | C37—C36—C35 | 117.0 |
| C18—C17—H17 | 119.0 | C37—C36—H36 | 121.5 |
| C19—C18—C17 | 121.2 (3) | C35—C36—H36 | 121.5 |
| C19—C18—H18 | 119.4 | C36—C37—C38 | 122.1 |
| C17—C18—H18 | 119.4 | C36—C37—H37 | 118.9 |
| C18—C19—C20 | 116.5 (3) | C38—C37—H37 | 118.9 |
| C18—C19—H19 | 121.8 | C39—C38—C37 | 121.3 |
| C20—C19—H19 | 121.8 | C39—C38—H38 | 119.4 |
| C19—C20—N3 | 131.9 (2) | C37—C38—H38 | 119.4 |
| C19—C20—C15 | 123.0 (3) | C38—C39—C40 | 116.8 |
| N3—C20—C15 | 105.1 (2) | C38—C39—H39 | 121.6 |
| C31—O2—C30 | 110.7 (2) | C40—C39—H39 | 121.6 |
| C27—S3—C28 | 98.26 (19) | N7—C40—C39 | 132.2 |
| C27—N5—C26 | 104.2 | N7—C40—C35 | 105.9 |
| C27—N6—C21 | 106.1 | C39—C40—C35 | 121.8 |
| C27—N6—C29 | 129.48 (13) | C33A—S4A—C34A | 99.7 |
| C21—N6—C29 | 124.46 (13) | C40A—N7A—C33A | 105.9 |
| N6—C21—C22 | 131.6 | C40A—N7A—C32A | 125.9 (5) |
| N6—C21—C26 | 105.4 | C33A—N7A—C32A | 127.7 (5) |
| C22—C21—C26 | 123.0 | C33A—N8A—C35A | 103.7 |
| C23—C22—C21 | 116.4 | N8A—C33A—N7A | 115.1 |
| C23—C22—H22 | 121.8 | N8A—C33A—S4A | 125.9 |
| C21—C22—H22 | 121.8 | N7A—C33A—S4A | 119.0 |
| C22—C23—C24 | 121.4 | S4A—C34A—H34D | 109.5 |
| C22—C23—H23 | 119.3 | S4A—C34A—H34E | 109.5 |
| C24—C23—H23 | 119.3 | H34D—C34A—H34E | 109.5 |
| C25—C24—C23 | 122.0 | S4A—C34A—H34F | 109.5 |
| C25—C24—H24 | 119.0 | H34D—C34A—H34F | 109.5 |
| C23—C24—H24 | 119.0 | H34E—C34A—H34F | 109.5 |
| C24—C25—C26 | 117.4 | C36A—C35A—N8A | 129.5 |
| C24—C25—H25 | 121.3 | C36A—C35A—C40A | 121.0 |
| C26—C25—H25 | 121.3 | N8A—C35A—C40A | 109.5 |
| C25—C26—N5 | 129.7 | C37A—C36A—C35A | 117.0 |
| C25—C26—C21 | 119.9 | C37A—C36A—H36A | 121.5 |
| N5—C26—C21 | 110.3 | C35A—C36A—H36A | 121.5 |
| N5—C27—N6 | 114.0 | C36A—C37A—C38A | 122.1 |
| N5—C27—S3 | 126.5 | C36A—C37A—H37A | 118.9 |
| N6—C27—S3 | 119.5 | C38A—C37A—H37A | 118.9 |
| S3—C28—H28A | 109.5 | C39A—C38A—C37A | 121.3 |
| S3—C28—H28B | 109.5 | C39A—C38A—H38A | 119.4 |
| H28A—C28—H28B | 109.5 | C37A—C38A—H38A | 119.4 |
| S3—C28—H28C | 109.5 | C38A—C39A—C40A | 116.8 |
| H28A—C28—H28C | 109.5 | C38A—C39A—H39A | 121.6 |
| H28B—C28—H28C | 109.5 | C40A—C39A—H39A | 121.6 |
| C27A—S3A—C28A | 98.3 (8) | N7A—C40A—C39A | 132.2 |
| C27A—N5A—C26A | 104.2 | N7A—C40A—C35A | 105.9 |
| C29—N6A—C27A | 125.0 (3) | C39A—C40A—C35A | 121.8 |
| C7—N2—C1—C2 | −177.5 (3) | N6A—C21A—C22A—C23A | 178.1 |
| C9—N2—C1—C2 | 0.5 (4) | C26A—C21A—C22A—C23A | 0.3 |
| C7—N2—C1—C6 | 1.4 (3) | C21A—C22A—C23A—C24A | 0.2 |
| C9—N2—C1—C6 | 179.4 (2) | C22A—C23A—C24A—C25A | −0.3 |
| N2—C1—C2—C3 | 178.4 (3) | C23A—C24A—C25A—C26A | −0.2 |
| C6—C1—C2—C3 | −0.3 (4) | C24A—C25A—C26A—N5A | −177.3 |
| C1—C2—C3—C4 | 0.4 (4) | C24A—C25A—C26A—C21A | 0.7 |
| C2—C3—C4—C5 | 0.0 (4) | C27A—N5A—C26A—C25A | 178.0 |
| C3—C4—C5—C6 | −0.4 (4) | C27A—N5A—C26A—C21A | −0.1 |
| C4—C5—C6—C1 | 0.4 (4) | N6A—C21A—C26A—C25A | −179.0 |
| C4—C5—C6—N1 | −177.2 (3) | C22A—C21A—C26A—C25A | −0.8 |
| N2—C1—C6—C5 | −179.1 (2) | N6A—C21A—C26A—N5A | −0.7 |
| C2—C1—C6—C5 | −0.1 (4) | C22A—C21A—C26A—N5A | 177.6 |
| N2—C1—C6—N1 | −1.1 (3) | C26A—N5A—C27A—N6A | 0.9 |
| C2—C1—C6—N1 | 177.9 (2) | C26A—N5A—C27A—S3A | −178.4 |
| C7—N1—C6—C5 | 178.1 (3) | C29—N6A—C27A—N5A | 167.2 (9) |
| C7—N1—C6—C1 | 0.3 (3) | C21A—N6A—C27A—N5A | −1.3 |
| C6—N1—C7—N2 | 0.7 (3) | C29—N6A—C27A—S3A | −13.5 (9) |
| C6—N1—C7—S1 | −178.45 (19) | C21A—N6A—C27A—S3A | 178.0 |
| C1—N2—C7—N1 | −1.4 (3) | C28A—S3A—C27A—N5A | −25.4 (8) |
| C9—N2—C7—N1 | −179.3 (2) | C28A—S3A—C27A—N6A | 155.3 (8) |
| C1—N2—C7—S1 | 177.80 (17) | C27A—N6A—C29—C30 | 112.2 (6) |
| C9—N2—C7—S1 | −0.1 (3) | C21A—N6A—C29—C30 | −81.8 (5) |
| C8—S1—C7—N1 | −1.8 (3) | C27—N6—C29—C30 | 105.3 (3) |
| C8—S1—C7—N2 | 179.1 (2) | C21—N6—C29—C30 | −73.6 (3) |
| C7—N2—C9—C10 | 99.1 (3) | C31—O2—C30—C29 | −169.6 (2) |
| C1—N2—C9—C10 | −78.4 (3) | N6A—C29—C30—O2 | −61.3 (4) |
| C11—O1—C10—C9 | −180.0 (2) | N6—C29—C30—O2 | −68.0 (3) |
| N2—C9—C10—O1 | −179.5 (2) | C30—O2—C31—C32 | −171.2 (3) |
| C10—O1—C11—C12 | 179.9 (2) | C30—O2—C31—C32A | 166.8 (5) |
| C13—N3—C12—C11 | 103.6 (3) | O2—C31—C32—N7 | −160.5 (2) |
| C20—N3—C12—C11 | −76.4 (3) | O2—C31—C32A—N7A | 175.8 (6) |
| O1—C11—C12—N3 | −60.0 (3) | C31—C32—N7—C40 | 80.5 (3) |
| C15—N4—C13—N3 | 0.8 (3) | C31—C32—N7—C33 | −106.9 (3) |
| C15—N4—C13—S2 | −179.9 (2) | C35—N8—C33—N7 | 0.9 |
| C20—N3—C13—N4 | −0.6 (3) | C35—N8—C33—S4 | −178.6 |
| C12—N3—C13—N4 | 179.4 (2) | C40—N7—C33—N8 | −1.4 |
| C20—N3—C13—S2 | −179.87 (19) | C32—N7—C33—N8 | −175.1 (3) |
| C12—N3—C13—S2 | 0.1 (4) | C40—N7—C33—S4 | 178.2 |
| C14—S2—C13—N4 | −23.2 (3) | C32—N7—C33—S4 | 4.5 (3) |
| C14—S2—C13—N3 | 156.0 (3) | C34—S4—C33—N8 | −1.8 |
| C13—N4—C15—C16 | 178.5 (3) | C34—S4—C33—N7 | 178.7 |
| C13—N4—C15—C20 | −0.8 (3) | C33—N8—C35—C36 | 177.9 |
| N4—C15—C16—C17 | −178.7 (3) | C33—N8—C35—C40 | −0.1 |
| C20—C15—C16—C17 | 0.5 (4) | N8—C35—C36—C37 | −177.3 |
| C15—C16—C17—C18 | −1.6 (5) | C40—C35—C36—C37 | 0.4 |
| C16—C17—C18—C19 | 1.4 (6) | C35—C36—C37—C38 | 0.3 |
| C17—C18—C19—C20 | −0.1 (5) | C36—C37—C38—C39 | −1.0 |
| C18—C19—C20—N3 | 178.7 (3) | C37—C38—C39—C40 | 0.9 |
| C18—C19—C20—C15 | −1.0 (4) | C33—N7—C40—C39 | −176.9 |
| C13—N3—C20—C19 | −179.6 (3) | C32—N7—C40—C39 | −2.8 (3) |
| C12—N3—C20—C19 | 0.4 (4) | C33—N7—C40—C35 | 1.1 |
| C13—N3—C20—C15 | 0.0 (3) | C32—N7—C40—C35 | 175.2 (3) |
| C12—N3—C20—C15 | −180.0 (2) | C38—C39—C40—N7 | 177.7 |
| C16—C15—C20—C19 | 0.8 (4) | C38—C39—C40—C35 | −0.1 |
| N4—C15—C20—C19 | −179.8 (2) | C36—C35—C40—N7 | −178.8 |
| C16—C15—C20—N3 | −178.9 (2) | N8—C35—C40—N7 | −0.7 |
| N4—C15—C20—N3 | 0.4 (3) | C36—C35—C40—C39 | −0.6 |
| C27—N6—C21—C22 | −176.9 | N8—C35—C40—C39 | 177.6 |
| C29—N6—C21—C22 | 2.2 (2) | C31—C32A—N7A—C40A | −75.9 (9) |
| C27—N6—C21—C26 | 1.1 | C31—C32A—N7A—C33A | 94.9 (7) |
| C29—N6—C21—C26 | −179.8 (2) | C35A—N8A—C33A—N7A | 0.9 |
| N6—C21—C22—C23 | 178.1 | C35A—N8A—C33A—S4A | −178.6 |
| C26—C21—C22—C23 | 0.3 | C40A—N7A—C33A—N8A | −1.4 |
| C21—C22—C23—C24 | 0.2 | C32A—N7A—C33A—N8A | −173.6 (8) |
| C22—C23—C24—C25 | −0.3 | C40A—N7A—C33A—S4A | 178.2 |
| C23—C24—C25—C26 | −0.2 | C32A—N7A—C33A—S4A | 6.0 (8) |
| C24—C25—C26—N5 | −177.3 | C34A—S4A—C33A—N8A | −1.8 |
| C24—C25—C26—C21 | 0.7 | C34A—S4A—C33A—N7A | 178.7 |
| C27—N5—C26—C25 | 178.0 | C33A—N8A—C35A—C36A | 177.9 |
| C27—N5—C26—C21 | −0.1 | C33A—N8A—C35A—C40A | −0.1 |
| N6—C21—C26—C25 | −179.0 | N8A—C35A—C36A—C37A | −177.3 |
| C22—C21—C26—C25 | −0.8 | C40A—C35A—C36A—C37A | 0.4 |
| N6—C21—C26—N5 | −0.7 | C35A—C36A—C37A—C38A | 0.3 |
| C22—C21—C26—N5 | 177.6 | C36A—C37A—C38A—C39A | −1.0 |
| C26—N5—C27—N6 | 0.9 | C37A—C38A—C39A—C40A | 0.9 |
| C26—N5—C27—S3 | −178.4 | C33A—N7A—C40A—C39A | −176.9 |
| C21—N6—C27—N5 | −1.3 | C32A—N7A—C40A—C39A | −4.4 (8) |
| C29—N6—C27—N5 | 179.6 (2) | C33A—N7A—C40A—C35A | 1.1 |
| C21—N6—C27—S3 | 178.0 | C32A—N7A—C40A—C35A | 173.6 (8) |
| C29—N6—C27—S3 | −1.0 (2) | C38A—C39A—C40A—N7A | 177.7 |
| C28—S3—C27—N5 | −23.16 (19) | C38A—C39A—C40A—C35A | −0.1 |
| C28—S3—C27—N6 | 157.56 (19) | C36A—C35A—C40A—N7A | −178.8 |
| C29—N6A—C21A—C22A | 15.0 (9) | N8A—C35A—C40A—N7A | −0.7 |
| C27A—N6A—C21A—C22A | −176.9 | C36A—C35A—C40A—C39A | −0.6 |
| C29—N6A—C21A—C26A | −166.9 (9) | N8A—C35A—C40A—C39A | 177.6 |
| C27A—N6A—C21A—C26A | 1.1 |
1,1'-[Oxybis(ethane-2,1-diyl)]bis(2-methylsulfanyl-1H-benzo[d]imidazole) . Hydrogen-bond geometry (Å, º)
Cg1, Cg5 and Cg11 are the centroids of the N5/C26/C21/N6/C27, the C21-C26 and the C1–C6 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···Cg5i | 0.95 | 2.71 | 3.583 (3) | 153 |
| C11—H11B···Cg11ii | 0.99 | 2.74 | 3.423 (3) | 126 |
| C29—H29B···Cg1iii | 0.99 | 2.70 | 3.489 (3) | 137 |
| C34—H34B···N4iv | 0.98 | 2.50 | 3.398 (3) | 153 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x, −y+1, −z+1; (iii) −x+1, −y, −z; (iv) −x+1, −y+2, −z+1.
References
- Abou, A., Bany, G. E., Kakou-Yao, R., Seikou, T. & Ebby, N. D. (2007). Acta Cryst. E63, o4218.
- Akonan, L., Molou, K. Y. G., Adohi-Krou, A., Abou, A. & Tenon, A. J. (2010). Acta Cryst. E66, o442. [DOI] [PMC free article] [PubMed]
- Algul, O., Mete, B., Turkmenoglu, B., Saglamtas, R., Alagoz, M. A., Dogen, A., Gulcin, I. & Burmaoglu, S. (2025). J. Mol. Struct.1323, 140800.
- Brandenburg, K. & Putz, H. (2012). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2015). APEX3 and SAINT. Bruker AXS LLC, Madison, Wisconsin, USA.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hammal, L., Bentarzi, Y., Kaoua, R., Bakhta, S., Nedjar-Kolli, B., Andre, C. & Hoffmann, P. (2008). J. Soc. Alger. Chim.18, 45–54.
- Hasty, S. J., Bandara, M. D., Rath, N. P. & Demchenko, A. V. (2017). J. Org. Chem.82, 1904–1911. [DOI] [PMC free article] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst.48, 3–10. [DOI] [PMC free article] [PubMed]
- Missioui, M., Mortada, S., Guerrab, W., Demirtaş, G., Mague, J. T., Ansar, M., El Abbes Faouzi, M., Essassi, E. M., Mehdar, Y. T. H., Aljohani, F. S., Said, M. A. & Ramli, Y. (2022). Ara. J. Chem.15, 103851.
- Obaid, R. J., Mughal, E. U., Naeem, N., Al-Rooqi, M. M., Sadiq, A., Jassas, R. S., Moussa, Z. & Ahmed, S. A. (2022). Process Biochem.120, 250–259.
- Rajakannu, P., Elumalai, P., Mobin, S. M., Lu, K.-L. & Sathiyendiran, M. (2013). J. Organomet. Chem.743, 17–23.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D. & Spackman, M. A. (2021). J. Appl. Cryst.54, 1006–1011. [DOI] [PMC free article] [PubMed]
- Steinke, T., Engelage, E. & Huber, S. M. (2023). Acta Cryst. C79, 26–35. [DOI] [PMC free article] [PubMed]
- Tan, S. L., Jotani, M. M. & Tiekink, E. R. T. (2019). Acta Cryst. E75, 308–318. [DOI] [PMC free article] [PubMed]
- Yadav, S., Narasimhan, B., Lim, S. M., Ramasamy, K., Vasudevan, M., Shah, S. A. A. & Mathur, A. (2018). Egypt. J. Basic Appl. Sci.5, 100-109.
- Yüksektepe, Ç., Çalışkan, N., Genç, M., Servi, S. & Büyükgüngör, O. (2007). Acta Cryst. E63, o100–o102.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2056989025003809/vm2313sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989025003809/vm2313Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989025003809/vm2313Isup3.cml
CCDC reference: 2447131
Additional supporting information: crystallographic information; 3D view; checkCIF report








