Abstract
At this stage in the COVID-19 pandemic, most infections are “breakthrough” infections that occur in individuals with prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure. To refine long-term vaccine strategies against emerging variants, we examined both innate and adaptive immunity in breakthrough infections. We performed single-cell transcriptomic, proteomic, and functional profiling of primary and breakthrough infections to compare immune responses from unvaccinated and vaccinated individuals during the SARS-CoV-2 Delta wave. Breakthrough infections were characterized by a less activated transcriptomic profile in monocytes and natural killer cells, with induction of pathways limiting monocyte migratory potential and natural killer cell proliferation. Furthermore, we observed a female-specific increase in transcriptomic and proteomic activation of multiple innate immune cell subsets during breakthrough infections. These insights suggest that prior SARS-CoV-2 vaccination prevents overactivation of innate immune responses during breakthrough infections with discernible sex-specific patterns and underscore the potential of harnessing vaccines in mitigating pathologic immune responses resulting from overactivation.
INTRODUCTION
Vaccination has greatly reduced the number and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections since its introduction approximately 1 year into the COVID-19 pandemic. Given that nearly everyone has been previously infected or fully vaccinated, the number of SARS-CoV-2 infections in this previously exposed population, known as breakthrough infections, has also increased. Because breakthrough infections will continue to contribute to the spread of SARS-CoV-2 in high-risk populations and could also contribute to Long Covid (1), there is a need to improve our understanding of immune responses during breakthrough infections to inform more robust vaccination and treatment strategies and to enhance our understanding of how prior exposure affects the coordination of immune responses.
Numerous groups have found that prior vaccination boosts patients’ adaptive immune responses during COVID-19 breakthrough infection (2–7). Despite this, delayed spike protein–specific humoral and cellular adaptive immunity and reduced proportions of B cells and naïve CD4+ T cells have been reported in breakthrough infections (8–10). Although the success of vaccinations has conventionally been attributed to adaptive immune memory, trained immunity, in which the responsiveness of innate immune cells is affected by prior vaccination or infection, has been more recently described in a growing body of literature (11–16). For example, enhanced antiviral platelet signatures have been found in Omicron breakthrough patients (17). Another group reported a higher percentage of antigen-presenting monocytes, mature monocytes, and mature neutrophils and a lower percentage of activated neutrophils in SARS-CoV-2 breakthrough infections (18). BNT162b2 mRNA SARS-CoV-2 vaccination has been shown to activate both monocytes and natural killer (NK) cells (19, 20). As with other severe viral infections (e.g., HIV and hantavirus), adaptive NK cell expansion has been observed after SARS-CoV-2 infection (21, 22). However, the role of NK cells in the context of SARS-CoV-2 breakthrough infection is not clear despite ample evidence that dysfunctional innate immune responses are associated with COVID-19 severity (23–27).
Prior work has also demonstrated that dendritic cells (DCs) and NK cells shape adaptive immune responses, chiefly through their priming, activation, and regulatory functions (28–30). In addition, the role of DCs in priming adaptive immune cells has been leveraged in numerous vaccine designs, including vaccines targeting cancer, influenza, and HIV-1 (31–34). However, the complex immunomodulatory roles of these innate immune cells after vaccination and infection and their impact on adaptive immune responses remain to be further elucidated. To better understand both innate and adaptive immune functions during breakthrough infection, we compared immune responses between vaccinated and unvaccinated participants infected during the SARS-CoV-2 Delta wave in April through December of 2021. We performed single-cell RNA sequencing (scRNA-seq), mass cytometry, and Olink plasma proteomics on the peripheral immune cells and plasma samples collected from unvaccinated and vaccinated SARS-CoV-2–infected participants during the acute phase of infection.
RESULTS
Both innate and adaptive immune responses are transcriptionally distinct between breakthrough and primary infection
To investigate how prior vaccination alters the peripheral immune response in SARS-CoV-2 breakthrough infections, we profiled peripheral immune cells in a cohort of healthy controls (HCs); unvaccinated, SARS-CoV-2–infected (UVI, primary infection) participants; and vaccinated, SARS-CoV-2–infected (VI, breakthrough infection) participants (Fig. 1A and table S1). HCs were uninfected at the time of sample collection 30 weeks after their second dose of either the Pfizer BNT162b2 or the Moderna (Spikevax) COVID-19 mRNA vaccine. All vaccinated (VI) participants had also received a full course of two doses of the Pfizer or Moderna COVID-19 mRNA vaccine except for one participant who received a full course of one dose of the Janssen COVID-19 vaccine. In these vaccinated participants, samples were drawn at a mean of 17.5 weeks postvaccination with a range of 2 to 33.5 weeks postvaccination (fig. S1A). Infected participants were recruited from April to December 2021, during the SARS-CoV-2 Delta variant wave, and had mild or moderate SARS-CoV-2 infection with a mean World Health Organization (WHO) score of 3.3 (SD = 0.57); no individuals required mechanical ventilation. There were no significant differences in the days since symptom onset at sample collection between the UVI and VI groups (7.5 and 6.3, respectively, Student’s t test, P value = 0.44; fig. S1B). UVI and VI participants with current immunosuppressant treatments were excluded from the study, and the two infected groups did not show significant differences in comorbidities or smoking history (P value > 0.2; table S2). Peripheral blood mono-nuclear cells (PBMCs) from these donors were processed for both mass cytometry and scRNA-seq (Fig. 1A). Transcriptomic analyses were assessed ex vivo; mass cytometry analyses were performed in the presence and absence of an optimized stimulation cocktail of Toll-like receptor (TLR) agonists and phorbol 12-myristate 13-acetate and ionomycin to stimulate multiple immune cell subsets (tables S3 and S4). In addition, plasma samples were used for Olink proteomic assessments (Fig. 1A).
Fig. 1. Transcriptomic differences in innate and adaptive immunity are observed between primary and breakthrough infections.
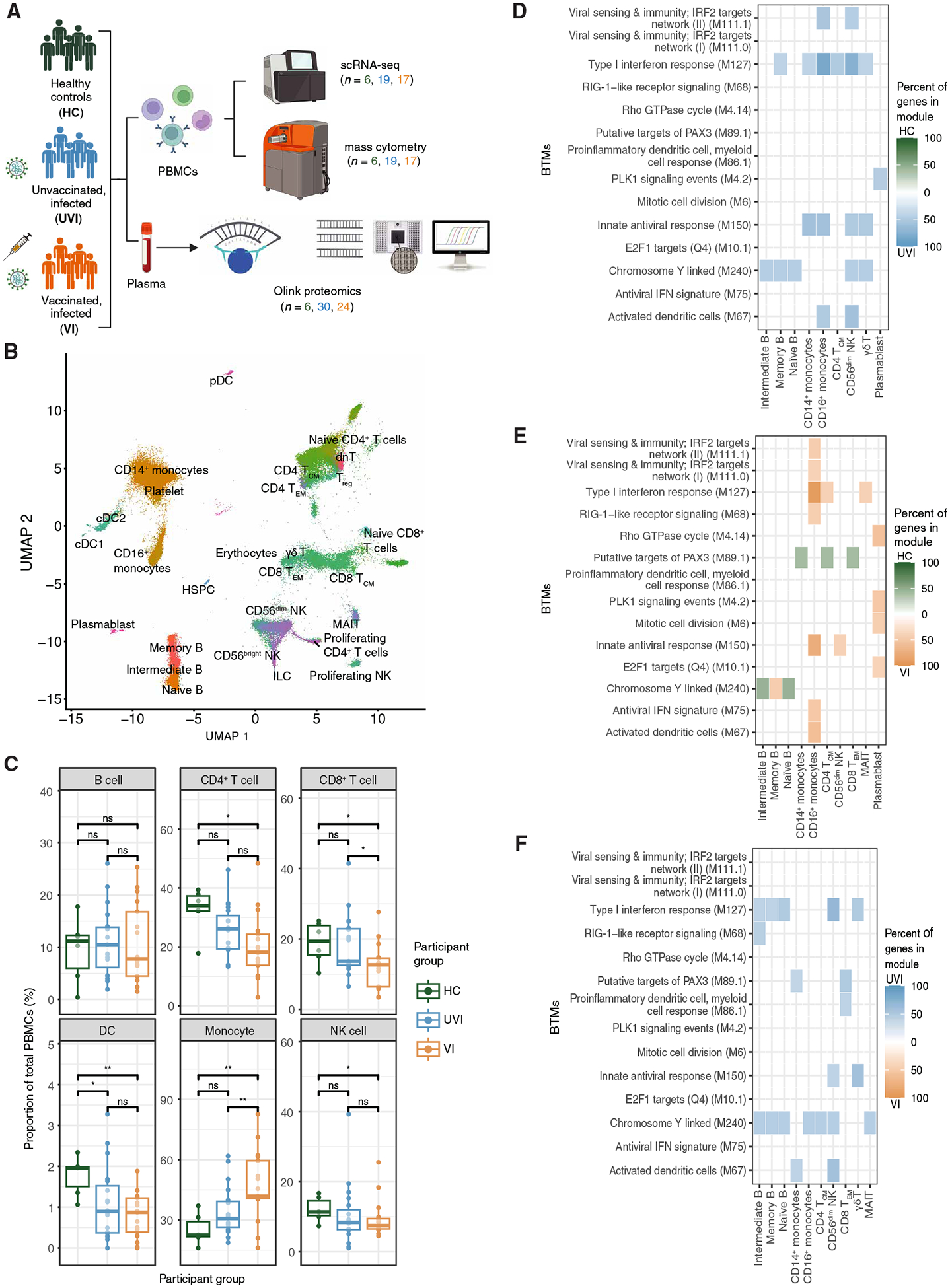
(A) Pipeline of PBMC and plasma sample processing and number of participants analyzed per group. All subsequent figures use the same abbreviations and color scheme for participant groups. (B) Uniform manifold approximation and projection (UMAP) of scRNA-seq dataset annotated by mapping to PBMC populations in (35) and colored by cell type. (C) Proportion of cell types out of total PBMCs in each sample in scRNA-seq dataset (n = 6 HCs, n = 19 UVI participants, n = 17 VI participants). (D to F) Heatmap displaying the top 20 enriched BTMs in PBMC subsets identified by overrepresentation analysis. Percent of genes in module was calculated by determining the number of genes enriched in each participant group out of the total number of measured module genes for each pairwise comparison: HC versus UVI (D), HC versus VI (E), and UVI versus VI (F). BTMs with an adjusted P value < 0.05, with fewer than two genes, and that scored <10% for percent of genes in the module were removed from the plot. ns, not significant; *P ≤ 0.05 and **P ≤ 0.01 by t test with Benjamini-Hochberg’s correction for multiple hypothesis testing. pDC, plasmacytoid dendritic cell; dnT, double-negative T cell; ILC, innate lymphoid cell; MAIT, mucosal-associated invariant T cell; Treg, regulatory T cell; γδT, gamma delta T cell; IRF, interferon regulatory factor; RIG, retinoic acid–inducible gene; GTPase, guanosine triphosphatase; IFN, interferon; PLK1, Polo-like kinase 1.
To identify the transcriptional state of the innate and adaptive immune cell subsets in each participant group, we mapped the scRNA-seq dataset to an annotated PBMC reference and did not observe participant-level batch effects (Fig. 1B and fig. S1C) (35). Consistent with previous findings, both infected groups had reduced DC proportions compared with HCs (Fig. 1C) (27, 36, 37). In VI participants, there was also a significant decrease in the proportion of CD4+ T cells and NK cells compared with that in HCs (adjusted P value ≤ 0.05; Fig. 1C). Compared with both HCs and UVI participants, VI participants exhibited a significant decrease in the proportion of CD8+ T cells and a corresponding increase in the proportion of monocytes (adjusted P value ≤ 0.05; Fig. 1C).
To better understand how vaccination alters the response to SARS-CoV-2 infection, we used single-cell differential gene expression testing to identify transcriptomic differences between primary and breakthrough infections by comparing the HC, UVI, and VI groups. These differentially expressed genes were further analyzed using blood transcriptional modules (BTMs) sourced from a reference gene set database dedicated to studying blood transcriptional immune responses (38). Compared with HCs, samples from both infected groups up-regulated genes enriched in viral sensing and type I interferon response BTMs in multiple cell types, including B cells, monocytes, T cells, and NK cells (Fig. 1, D and E). In addition, samples from VI participants up-regulated gene expression of cell cycle and translation-related BTMs in plasmablasts relative to HC samples (Fig. 1E). Despite shared activation of antiviral pathways, multiple B cell subsets, CD14+ monocytes, CD56dim NK cells, and gamma delta T cells exhibited further up-regulation of genes enriched in type I interferon response and activation-related BTMs in samples from UVI participants compared with VI participants (Fig. 1F). Thus, prior vaccination affects both innate and adaptive immunity in their transcriptional response to SARS-CoV-2 infection.
Monocyte transcriptomic profiles are more activated in SARS-CoV-2–infected participants without prior vaccination
Because breakthrough infections were associated with a greater proportion of monocytes compared with primary infections, we first investigated the monocyte compartment (Fig. 2A). The frequencies of classical CD14+ and nonclassical CD16+ monocytes did not differ among samples from the HC, UVI, and VI groups (fig. S2A). Classical CD14+ monocytes from both UVI and VI participants up-regulated genes that are enriched in type I interferon response, innate antiviral response, and activation-related BTMs relative to those from HCs (fig. S2, B and C). Compared with CD14+ monocytes in HCs, CD14+ monocytes in VI participants down-regulated multiple major histocompatibility complex genes enriched in antigen presentation BTMs (fig. S2C). This is consistent with prior work demonstrating down-regulation of HLA-DR (human leukocyte antigen–DR isotype) in patients with COVID-19 (39, 40). When directly comparing CD14+ monocytes from UVI and VI participants, CD14+ monocytes from UVI participants further up-regulated genes that are enriched in type I interferon response, innate antiviral response, and activation-related BTM modules, suggesting a greater proinflammatory CD14+ monocyte response in primary infection (Fig. 2B). Consistent with this, CD14+ monocytes up-regulated an interferon-stimulated gene (ISG) hub in UVI participants consisting of interferon-induced protein with tetratricopeptide repeats 2 (IFIT2), IFIT3, sterile alpha motif domain containing 9 like (SAMD9L), SAMD9, XIAP-associated factor 1 (XAF1), interferon-induced protein 44–like (IFI44L), 2′−5′-oligoadenylate synthetase 2 (OAS2), interferon alpha–inducible protein 6 (IFI6), and MX dynamin–like GTPase 1 (MX1) relative to VI participants (Fig. 2C).
Fig. 2. Monocytes are transcriptionally more activated in breakthrough infections.

(A) UMAP projection of monocytes in the scRNA-seq dataset colored by monocyte subsets. (B) Heatmap of the top 40 enriched BTMs in monocyte subsets calculated as in Fig. 1F (n = 19 UVI participants and n = 17 VI participants). (C and D) Gene network display of differentially expressed genes in CD14+ monocytes (C) and CD16+ monocytes (D) was mapped to known protein-protein networks derived from the human STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) database. Each gene is assigned a score derived from a β-uniform mixture model fitted to the P values generated from differential gene expression analysis. The highest scoring subgraph was generated with log fold change (logFC) relative to each participant group indicated by color scale (orange: up-regulated in samples from VI participants; blue: up-regulated in samples from UVI participants). MAPK, mitogen-activated protein kinase; MHC, major histocompatibility complex; TF, transcription factor; AP-1, activator protein 1.
Although metabolism-related BTMs were not identified in the top 40 differential BTMs, we performed a targeted analysis of metabolism-related BTMs given the known role of immunometabolism in vaccination and trained immunity (41–43). CD14+ monocytes from VI participants down-regulated genes in lipid metabolism, purine nucleotide biosynthesis, and respiratory electron transport chain BTMs compared with CD14+ monocytes from UVI participants, suggesting that prior vaccination altered metabolic profiles during breakthrough infection (fig. S2D).
Nonclassical CD16+ monocytes from both infected groups up-regulated genes enriched in proinflammatory BTMs compared with those from HCs as expected (fig. S2, B and C). CD16+ monocytes from UVI participants up-regulated genes enriched in antigen presentation and inositol phosphate metabolism BTMs compared with CD16+ monocytes from VI participants (Fig. 2B). The inositol phosphate metabolism BTM includes pantothenate kinase 3 (PANK3), which catalyzes the first step in coenzyme A (CoA) synthesis. CoA has been shown to enhance the magnitude of TLR-driven proinflammatory responses in macrophages through metabolic and epigenetic programming (44). Network analysis of CD16+ monocytes revealed an ISG hub that includes gene members up-regulated in samples from both UVI and VI participants, suggesting that SARS-CoV-2 infection elicited a robust ISG response in CD16+ monocytes regardless of vaccination status (Fig. 2D). Collectively, our findings demonstrated that vaccination attenuates inflammatory profiles in both classical CD14+ and nonclassical CD16+ monocytes in VI participants.
We next evaluated monocyte function ex vivo. Although monocytes from both UVI and VI participants responded to stimulation, unstimulated monocytes in UVI participants exhibited elevated interferon-gamma inducible protein 10 [IP-10 (CXCL10) protein] compared with unstimulated monocytes in VI participants (Fig. 3A and fig. S3). In addition, monocytes from VI participants had reduced protein expression of CD123 [interleukin-3RA (IL-3RA)] in both unstimulated and stimulated conditions relative to UVI participants (Fig. 3A and fig. S3). IL-3 has previously been shown to promote inflammation in murine and human sepsis (45). Thus, the reduced protein expression of IP-10 and CD123 in monocytes from VI participants is consistent with a reduced inflammatory monocyte response in breakthrough infections compared with primary infection.
Fig. 3. Protein-level differences are observed in monocytes and plasma between primary and breakthrough infections.
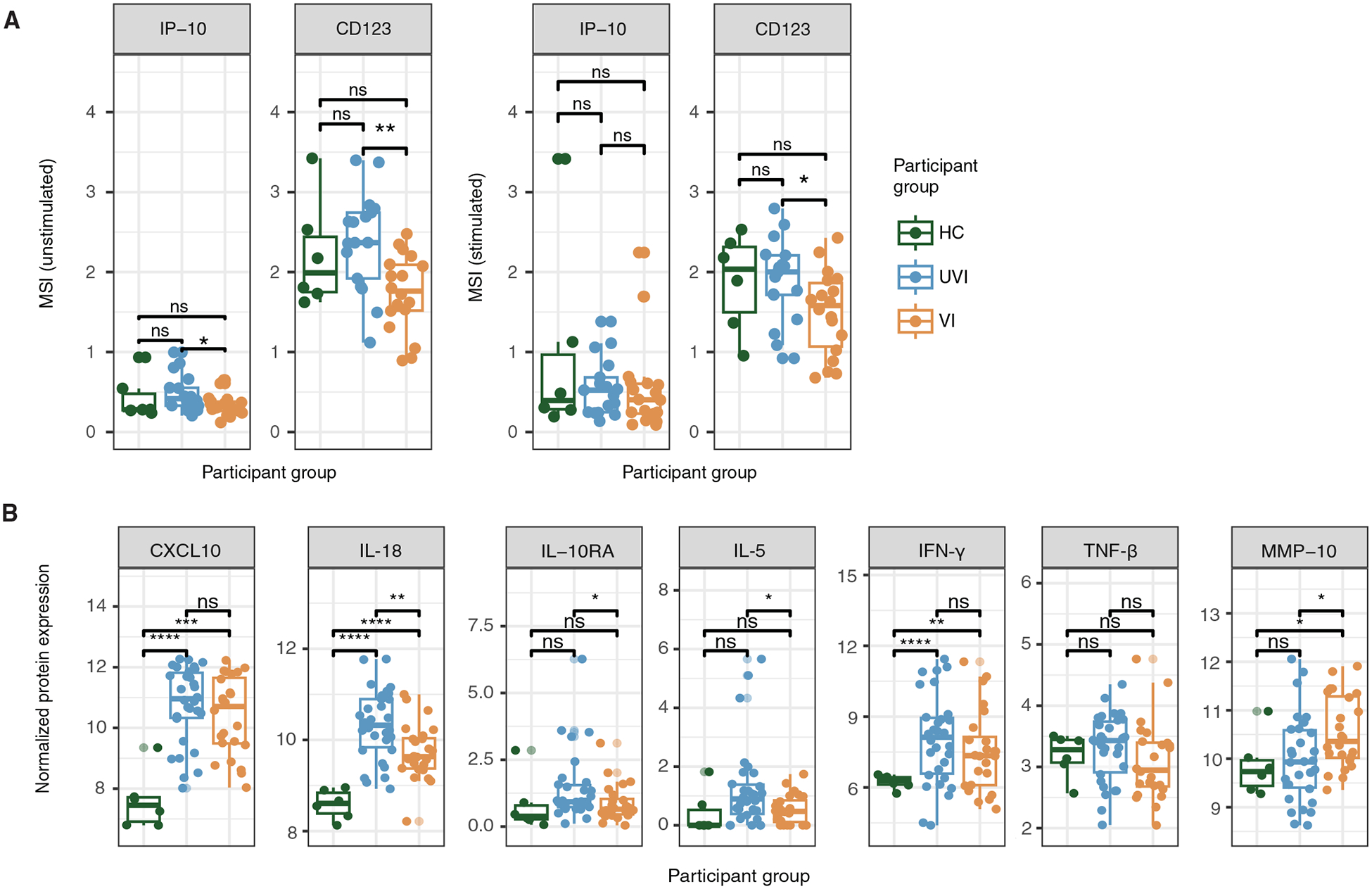
(A) Box plots depicting the arcsinh-transformed mean signal intensity (MSI) of IP-10 and CD123 protein expression from the mass cytometry dataset (n = 6 HCs, n = 19 UVI participants, n = 17 VI participants). (B) Box plots depicting normalized expression of select plasma proteins from the Olink assay (n = 6 HCs, n = 30 UVI participants, n = 24 VI participants). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 by the Wilcoxon rank sum test with Benjamini-Hochberg’s correction for multiple hypothesis testing.
We further investigated inflammatory differences in UVI and VI participants by performing a plasma proteomic assay using Olink’s inflammation panel. As expected during acute SARS-CoV-2 infection, plasma from both UVI and VI participants had elevated concentrations of several inflammatory proteins, including CXCL10, IL-18, and interferon-γ, compared with plasma from HCs (Fig. 3B and fig. S4). In UVI participants, plasma protein concentrations of IL-18, IL-10RA, and IL-5 were significantly elevated compared with those in VI participants (adjusted P value ≤ 0.05; Fig. 3B). In contrast, matrix metalloproteinase-10 (MMP-10) protein expression was significantly elevated in plasma from VI participants compared with that in both HCs and UVI participants (adjusted P value ≤ 0.05; Fig. 3B). MMP-10 has been shown to play a protective role in acute Pseudomonas aeruginosa lung infection by promoting the conversion of M1-like proinflammatory macrophages to M2-like immunosuppressive macrophages (46).
To explore potential cellular communication pathways driving differential activation of monocytes during acute primary versus breakthrough SARS-CoV-2 infection, we used MultiNicheNet to infer receptor-ligand interactions and to assess downstream signaling (47). This analysis inferred that monocytes primarily received signals from CD4+ T cells, CD8+ T cells, and other monocytes (Fig. 4, A and B). Transforming growth factor beta 1 (TGFB1) emerged as a ligand signaling to multiple receptors on monocytes from UVI participants (Fig. 4, A and C). All of these interactions correlated with increased gene expression of matrix metalloproteinase 9 (MMP9) and vascular endothelial growth factor A (VEGFA), which can stimulate monocyte migration (48–50), in samples from UVI participants (Fig. 4C and fig. S5A). In contrast, in samples from VI participants, signaling by granulin precursor (GRN) was correlated with down-regulated MMP9 and VEGFA gene expression (Fig. 4, D and E and fig. S5B). In addition, target gene thioredoxin-interacting protein (TXNIP) was down-regulated in monocytes from UVI participants and up-regulated in monocytes from VI participants (Fig. 4, C to E). TXNIP inhibits thioredoxin, a redox chemoattractant released in infection and inflammation (51). On the protein level, monocytes in UVI participants significantly up-regulated expression of the chemokine receptor CCR4 upon stimulation compared with those in VI participants (adjusted P value ≤ 0.05), suggesting greater migratory potential (Fig. 4F). Collectively, these findings demonstrated that breakthrough infection in VI participants resulted in a less activated transcriptomic profile in monocytes than primary infection in UVI participants.
Fig. 4. Differential cell-cell interactions suggest increased monocyte migration in primary infections and down-regulated migration in breakthrough infections.

(A and B) Circos plots of the top 15 predicted differential cell-cell communication received by monocytes from all annotated PBMC coarse cell types in UVI (n = 19) (A) and VI (n = 17) (B) participants. (C and D) Heatmaps depicting the top target genes correlated to the up-regulated cell-cell interactions received by monocytes in UVI (C) and VI (D) participants. Pearson correlation coefficients were calculated between the log-transformed normalized pseudobulk expression values of the genes encoding for each ligand-receptor pair of interest and the corresponding downstream target gene in the receiver cell type. (E) Box plots depicting scaled expression of cell-cell interactions’ target genes from scRNA-seq dataset. (F) Box plots depicting the arcsinh-transformed MSI of CCR4 protein expression in monocytes under both unstimulated and stimulated conditions from the mass cytometry dataset (n = 19 UVI participants and n = 17 VI participants). *P ≤ 0.05 and ****P ≤ 0.0001 by the Wilcoxon rank sum test with Benjamini-Hochberg’s correction for multiple hypothesis testing.
Transcriptomic activation in NK cells differs between primary and breakthrough infections
We next investigated transcriptomic differences in the NK cell compartment among the HC, UVI, and VI groups. We identified three distinct NK cell clusters annotated as CD56dim, CD56bright, or proliferating NK cells (Fig. 5A). As expected, infection resulted in an increase in the proportion of proliferating NK cells in UVI participants compared with that in HCs (Fig. 5B). In addition, protein expression of CD38 and CD57, markers of NK activation and differentiation, respectively, was significantly increased in samples from both infection groups compared with HCs (adjusted P value ≤ 0.05; Fig. 5C and fig. S6, A and B), supporting the active role of NK cells in response to SARS-CoV-2 infection.
Fig. 5. NK cells are transcriptionally more activated in breakthrough infections.
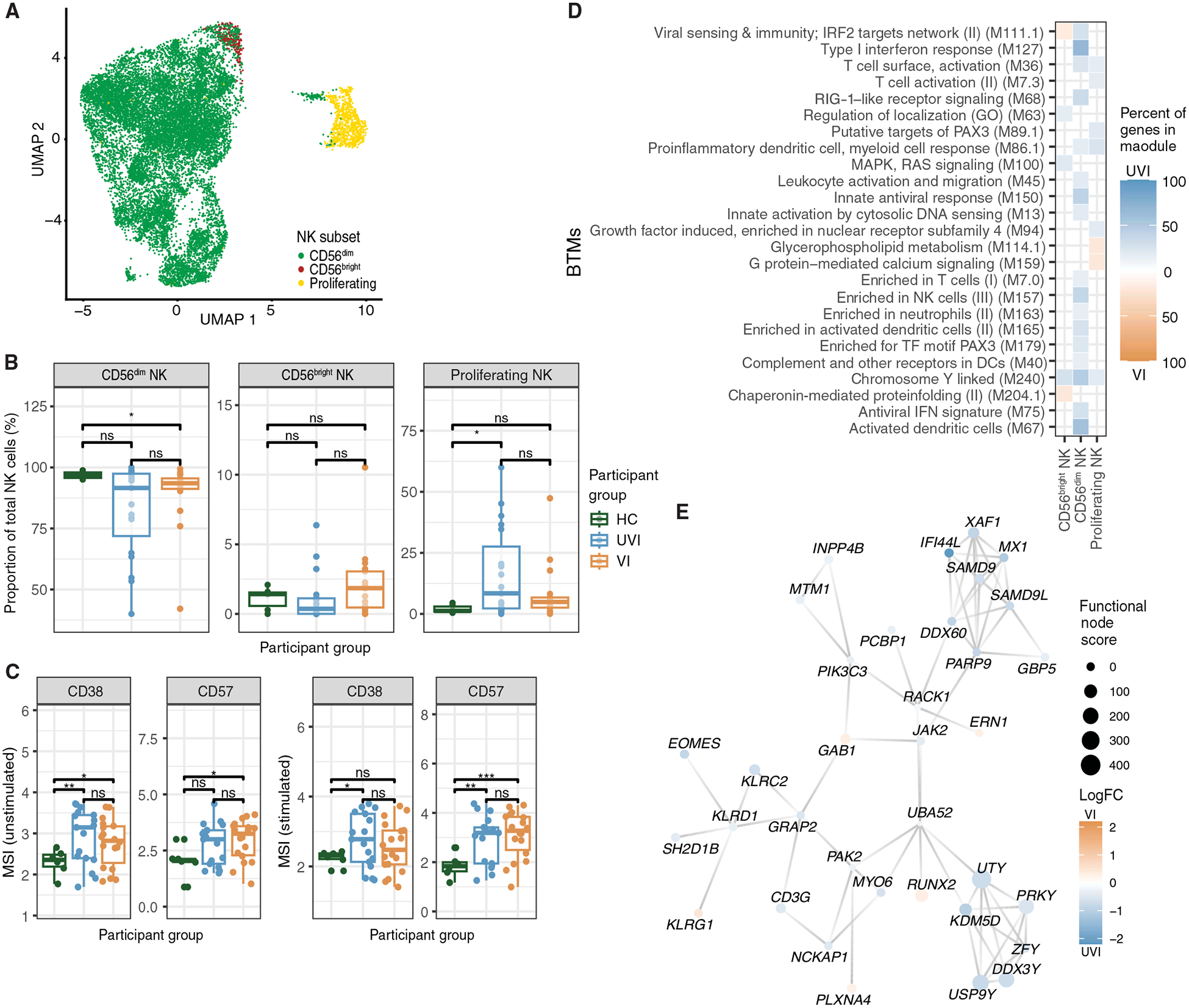
(A) UMAP projection of NK cells in the scRNA-seq dataset colored by NK cell subsets. (B) Proportion of NK cell subsets out of total NK cells in each sample in scRNA-seq dataset (n = 6 HCs, n = 19 UVI participants, n = 17 VI participants). (C) Box plots depicting the arcsinh-transformed MSI of protein expression in total NK cells (n = 6 HCs, n = 19 UVI participants, n = 17 VI participants). (D) Heatmap of the top 30 enriched BTMs in NK cell subsets identified as in Fig. 1F. (E) Network map of differentially expressed genes in CD56dim NK cells mapped to known protein-protein networks derived from the human STRING database as described in Fig. 2C (orange: up-regulated in samples from VI participants; blue: up-regulated in samples from UVI participants). *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 by the Wilcoxon rank sum test with Benjamini-Hochberg’s correction for multiple hypothesis testing. PAX3, paired box 3; RAS, rat sarcoma; GO, Gene Ontology.
Within the majority CD56dim NK subset from both UVI and VI participants, we observed up-regulated genes enriched in type I interferon response and activation-related BTMs relative to HCs (fig. S7, A and B). However, samples from UVI participants further up-regulated genes enriched in viral sensing, type I interferon response, and activation-related BTMs compared with those from VI participants (Fig. 5D). Consistent with this, samples from UVI participants up-regulated ISG [XAF1, IFI44L, DExD/H-box helicase 60 (DDX60), MX1, SAMD9, and SAMD9L] and NK receptor and signaling [killer cell lectin-like receptor D1 (KLRD1), killer cell lectin-like receptor C2 (KLRC2), CD3 gamma subunit of T cell receptor complex (CD3G), janus kinase 2 (JAK2), SH2 domain containing 1B (SH2D1B), and GRB2-related adaptor protein 2 (GRAP2)] gene hubs, indicating greater CD56dim NK cell activation during primary compared with breakthrough infection (Fig. 5E). These observations in CD56dim NK cells also extended to proliferating NK cells, where samples from UVI participants up-regulated proinflammatory and T cell activation–related BTMs compared with samples from VI participants (Fig. 5D). The T cell activation BTM includes the genes NKG7 (natural killer cell granule protein 7), IFNG (interferon gamma), GZMA (granzyme A), CD3E (CD3 epsilon subunit of T cell receptor complex), and CD8A (CD8 subunit A), which are expressed in both NK cells and T cells. Together, these findings support an active role of NK cells in SARS-CoV-2 infection with a greater inflammatory and activated response in unvaccinated individuals compared with vaccinated individuals.
Next, we investigated potential mechanisms contributing to these differences in primary versus breakthrough infections by evaluating the signals NK cells receive from other immune cells (Fig. 6, A and B). This analysis inferred that NK cells receive signals from other NK cells, CD4+ and CD8+ T cells, and monocytes through CD6, LGALS3 (galectin 3), and HBEGF (heparin binding EGF-like growth factor) in samples from the UVI group. In samples from the VI group, NK cells received signals primarily from B cells, CD8+ T cells, monocytes, and NK cells. In samples from the UVI group, a prominently up-regulated interaction was from CD6 on NK cells and CD4+ and CD8+ T cells to ALCAM (activated leukocyte cell adhesion molecule) on NK cells (Fig. 6, A and C). This inferred communication pathway was correlated with an up-regulation of downstream target genes enriched for cell cycle–related functions, including BRCA2 (BRCA2 DNA repair associated), CDC6 (cell division cycle 6), CDCA7 (cell division cycle associated 7), DTL (denticleless E3 ubiquitin protein ligase homolog), PCNA (proliferating cell nuclear antigen), and POLA1 (DNA polymerase alpha 1) (Fig. 6C and fig. S8A), suggesting that NK cells receive signals inducing a strong proliferative response during primary infection. In contrast, in samples from VI participants, cell cycle–related genes DTL and RANBP1 (RNA binding protein 1) were down-regulated (Fig. 6D and fig. S8B). As in monocytes, TXNIP was also down-regulated in NK cells from UVI participants and up-regulated in NK cells from VI participants (Fig. 6, C to E). Thus, this analysis predicts that NK cells from UVI participants receive ligand-receptor interactions promoting cell proliferation and chemoattractant secretion, whereas NK cells from VI participants receive ligand-receptor interactions that limit NK cell proliferation. This is consistent with the significantly elevated proportion of proliferating NK cells observed in UVI participants compared with that in HCs (adjusted P value ≤ 0.05; Fig. 5B). Collectively, these findings suggest that the NK response to SARS-CoV-2 infection differed between participants who had or had not been previously vaccinated.
Fig. 6. Differential cell-cell interactions are predicted to enhance NK cell proliferation in primary infections and down-regulate proliferation in breakthrough infections.
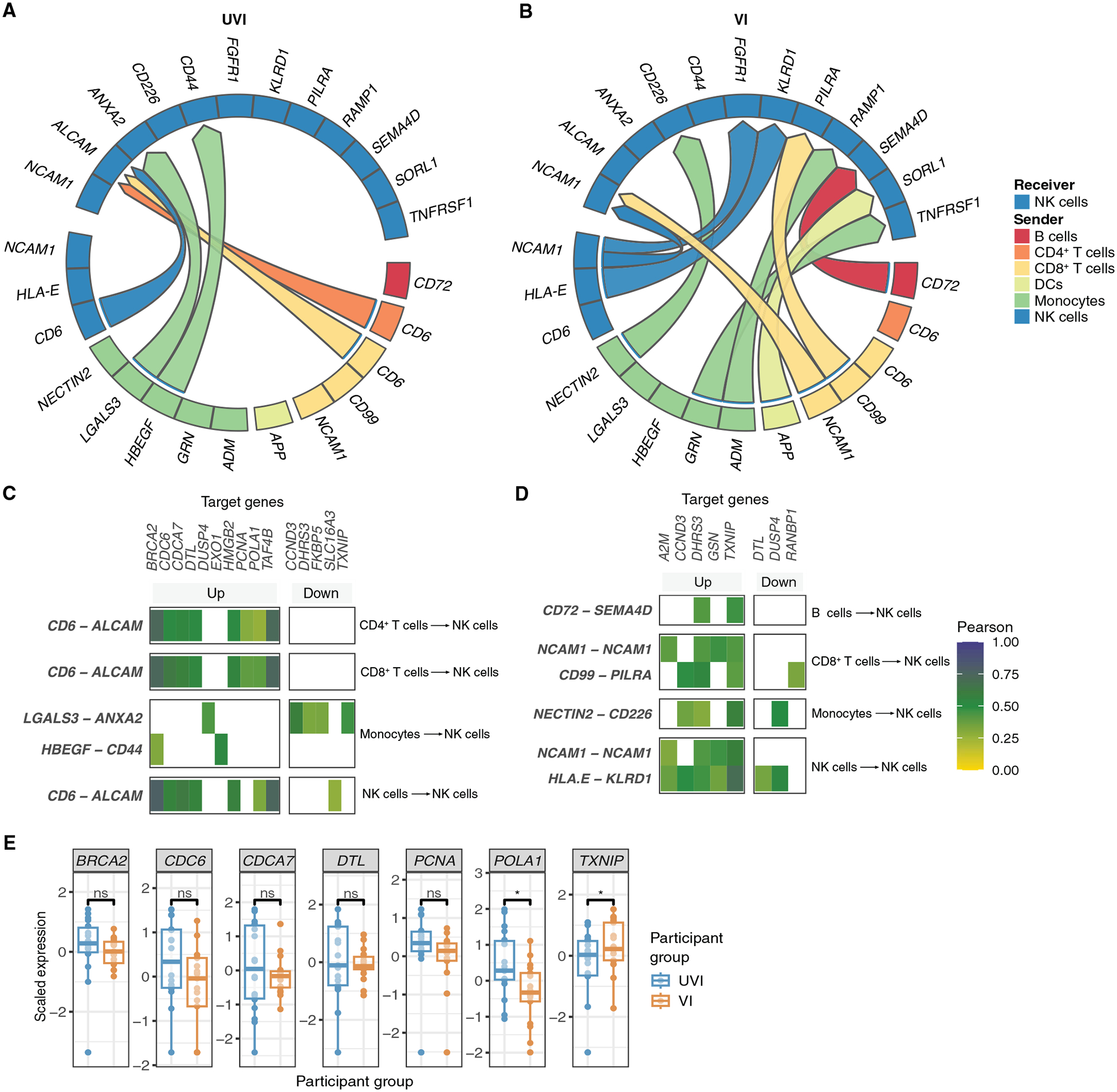
(A and B) Circos plots of the top 15 predicted differential cell-cell communications received by NK cells from all annotated PBMC coarse cell types in UVI (n = 19) (A) and VI (n = 17) (B) participants. (C and D) Heatmap depicting the top target genes correlated to the up-regulated cell-cell interactions received by NK cells in UVI (C) and VI (D) participants. Pearson correlation coefficients were calculated between the log-transformed normalized pseudobulk expression values of the genes encoding for each ligand-receptor pair of interest and the corresponding downstream target gene in the receiver cell type. (E) Box plots depicting scaled expression of cell-cell interactions’ target genes. *P ≤ 0.05 by t test with Benjamini-Hochberg’s correction for multiple hypothesis testing.
Innate immune responses are predicted to differentially regulate adaptive responses in COVID-19 breakthrough infections
Because innate immune cells can regulate adaptive immune responses (28–30) and were differentially activated during breakthrough infection, we investigated differences in adaptive immune responses in UVI and VI participants. There were no significant differences in the frequencies of naïve B cells, memory B cells, intermediate B cells, and plasmablasts (adjusted P value > 0.05; fig. S9, A and B) or in plasma neutralization activity against the SARS-CoV-2 Delta pseudovirus (fig. S9C) in samples from UVI and VI participants. On the transcriptomic level, the up-regulation of genes enriched in type I interferon response and activation-related BTMs in naïve and memory B cells in samples from UVI and VI participants, respectively, points to a more inflammatory role of these B cell subsets in primary infections (fig. S9D).
In the T cell compartment, there were no significant differences in the frequencies of CD4+ T or CD8+ T cell subsets (adjusted P value > 0.05; fig. S10, A to D). Prior reports suggested that SARS-CoV-2–specific CD4+ T cells in peripheral blood typically had a central memory phenotype, whereas CD8+ T cells had a more effector phenotype (52, 53). Thus, it is noteworthy that transcriptomic differences in the T cell compartment were predominantly found in the CD4+ TCM (central memory) subset and the CD8+ TEM (effector memory) subset (fig. S10E). CD4+ TCM cells from UVI participants up-regulated genes enriched in growth factor–induced and chemokine BTMs (fig. S10E). CD8+ TEM cells from UVI participants up-regulated genes enriched in multiple BTMs related to proinflammatory responses and activation (fig. S10E). These findings demonstrate that prior vaccination results in reduced transcriptomic activation in naïve B cell, intermediate B cell, memory B cell, CD4+ TCM, and CD8+ TEM subsets in breakthrough infections compared with primary infections.
To elucidate how changes in innate immune cell activity may contribute to these transcriptomic differences, we specified innate immune cells (monocytes, DCs, and NK cells) as the sender cells and adaptive immune cells (B, CD4+ T, and CD8+ T cells) as the receiver cells in the cell-cell communication analysis (Fig. 7, A and B). Although the role of monocytes in modulating adaptive immune responses has previously been less well-known compared with the roles of DCs and NK cells, we found that monocytes participated in 12 of the top 25 differential cell-cell interactions sent by innate immune cells (Fig. 7, A and B). B cells from VI participants received communication correlated with up-regulation of AREG (amphiregulin) and CCND3 (cyclin D3), genes related to cell proliferation, and down-regulation of SLAMF7 (SLAM family member 7), which encodes an activating receptor (Fig. 7, C and D). CD4+ T cells from UVI participants received communication correlated with up-regulation of TNF (tumor necrosis factor) (Fig. 7, E and F, and fig. S10F). In CD8+ T cells, cell-cell communication from NK cells in UVI participants was correlated with down-regulation of B3GAT1 (beta-1,3-glucuronyltransferase 1), which encodes for CD57, a marker for replicative senescence in T cells (Fig. 7G and fig. S10G) (54). In samples from VI participants, CD8+ T cells received communication correlated with down-regulation of EGR1 (early growth response 1) and PMEPA1 (prostate transmembrane protein, androgen induced 1), a negative regulator of transforming growth factor–β signaling (Fig. 7H). Collectively, these interactions demonstrate that innate immune cells induce greater activity in adaptive immune cells in UVI participants compared with VI participants.
Fig. 7. Innate immune cells differentially regulate adaptive immune responses in breakthrough infections.

(A and B) Circos plot of the top 25 predicted differential cell-cell communications sent by DCs, monocytes, and NK cells to B cells, CD4+ T cells, and CD8+ T cells in UVI (n = 19) (A) and VI (n = 17) (B) participants. (C to H) Heatmaps depicting the top target genes correlated to the up-regulated cell-cell interactions received by B cells [(C) and (D)], CD4+ T cells [(E) and (F)], and CD8+ T cells [(G) and (H)] in UVI [(C), (E), and (G)] and VI [(D), (F), and (H)] participants. Pearson correlation coefficients were calculated between the log-transformed normalized pseudobulk expression values of the genes encoding for each ligand-receptor pair of interest and the corresponding downstream target gene in the receiver cell type.
Females with breakthrough infections exhibited increased transcriptomic activation of innate immune cells
Given differences in COVID-19 outcomes between males and females (55–57), we next evaluated sex differences in the innate immune response to breakthrough infections. In our cohort, 40% of UVI participants were female, whereas 46% of VI participants were female (table S1). Across multiple immune cell subsets, a chromosome Y–linked BTM was up-regulated in samples from UVI participants relative to VI participants, suggesting sex differences in the immune response to primary and breakthrough infections (Fig. 1F). Sex-stratified analysis revealed a female-specific up-regulation of genes enriched in viral sensing, type I interferon, antiviral response, and activation BTMs in CD14+ monocytes, CD16+ monocytes, and type 2 conventional dendritic cells in samples from VI participants compared with UVI participants (Fig. 8, A and B). In male participants, these modules were only enriched in genes up-regulated in samples from UVI participants (Fig. 8B).
Fig. 8. Transcriptomic sex differences in innate immune cells are present between primary and breakthrough infections.

(A and B) Heatmaps of enriched BTMs in innate immune subsets as described in Fig. 1F in female (A) and male (B) infected UVI (n = 19) versus VI (n = 17) participants from the scRNA-seq dataset with the top 20 BTMs displayed. (C and D) Box plots depicting the arcsinh-transformed MSI of protein expression in NK cells from UVI (C) and VI (D) participants under unstimulated conditions from the mass cytometry dataset (n = 19 UVI participants and n = 17 VI participants). (E and F) Box plots depicting the normalized protein expression of plasma proteins in UVI (n = 30) (E) and VI (n = 24) (F) participants from the Olink dataset. *P ≤ 0.05 and **P ≤ 0.01 by t test with Benjamini-Hochberg’s correction for multiple hypothesis testing. ITK, IL2 inducible T cell kinase; RA, retinoic acid; PKC, protein kinase C; CSF, colony-stimulating factor.
Within the NK cell compartment, mass cytometry analysis revealed increased CD11c and HLA-DR protein expression in samples from female VI participants compared with male VI participants, whereas no differences were found in samples from male and female UVI participants (Fig. 8, C and D). CD11c and HLA-DR have previously been shown to be up-regulated on NK cells in proinflammatory environments (58). In addition, NK cells in female VI participants had increased protein expression of CD38 and CXCR5, markers of activation and migration (Fig. 8D), suggesting that NK cells in females are more activated in breakthrough infection than NK cells in males.
In participants’ plasma, no differences were noted in cytokine concentrations between male and female UVI participants (Fig. 8E). However, in samples from VI participants, females had elevated protein expression of the inflammatory markers artemin, CD244, CXCL6, fibroblast growth factor 21, IL-2RB, tumor necrosis factor–β (TNF-β), TNF-related apoptosis-inducing ligand (TRAIL), and TNF-related activated-induced cytokine (TRANCE) (Fig. 8F). TRANCE has been shown to promote the longevity and abundance of mature DCs at the site of T cell priming (59). This substantiates our scRNA-seq and mass cytometry findings that SARS-CoV-2 infection elicited a more robust innate immune response in vaccinated females.
We next compared differences in immunomodulatory interactions sent by innate immune cells to adaptive immune cells between male and female VI participants. By directly comparing samples from male versus female VI participants, we identified several interactions from monocytes and NK cells that were up-regulated in samples from male VI participants (fig. S11, A and B). These interactions were correlated with up-regulation of genes related to cytolytic functions, including FASLG (Fas ligand), PRF1 (perforin 1), and SYTL2 (synaptotagmin-like 2) in CD8+ T cells (fig. S11B). These predicted interactions suggest that CD8+ T cells in breakthrough infections are more activated in male participants than in female participants. Because monocytes and NK cells from female VI participants were more activated during breakthrough infections than those in males, this suggests that these innate immune cells may drive greater immunosuppression of adaptive immunity in female VI participants. Overall, these data suggest that sex differences result in greater innate immune activity in female participants than male participants with breakthrough infection.
Attenuated inflammatory responses in breakthrough infections are generalizable to other COVID-19 vaccination types
Participants in this study primarily received either the Pfizer BNT162b2 or the Moderna (Spikevax) COVID-19 mRNA vaccine. To validate our findings, we turned to a study of the ChAdOx1-nCOV-199 vaccine (AZD1222) conducted in UK healthy adult volunteers (registered at ClinicalTrials.gov, NCT04324606). In stage 1 of this study, bulk RNA-seq data were collected from study participants on day 0 after a positive COVID-19 nucleic acid amplification test by reverse transcription polymerase chain reaction (RT-PCR) (60). Of these 16 study participants, 7 had received the ChAdOx1-nCOV-199 vaccine, whereas 9 had received a placebo vaccine (60). Vaccination in this cohort resulted in reduced inflammatory responses during breakthrough COVID-19 between June and December 2020, when the main SARS-CoV-2 variants circulating were ancestral and Alpha (60). Here, we used CIBERSORTx to infer activation profiles within specific immune cell types from the bulk RNA-seq data provided from this study (61). Because of the limited number of VI participants (ChAdOx1-nCOV-199–vaccinated, SARS-CoV-2–infected) included in this study, we did not further stratify by sex to analyze sex-based differences.
In monocytes, we found reduced gene expression of several ISGs in samples from VI participants (fig. S12A). Consistent with our cell-cell communication analysis, samples from VI participants from the ChAdOx1-nCOV-199 cohort had reduced VEGFA gene expression in monocytes (fig. S12B). These VI participants also down-regulated the gene expression of C3AR1 (complement C3a receptor 1), a complement receptor stimulating chemotaxis, and CD68, involved in macrophage recruitment to tissues and organs, further corroborating our findings of reduced migratory potential in monocytes in VI participants (fig. S12B). Monocytes in UVI participants (placebo-vaccinated, SARS-CoV-2–infected) from the ChAdOx1-nCOV-199 cohort also up-regulated CD74, involved in antigen presentation (fig. S12B), which is consistent with the up-regulation of genes enriched in antigen presentation BTMs in CD16+ monocytes in our cohort (Fig. 2B).
In the NK cell compartment, samples from VI participants in the ChAdOx1-nCOV-199 cohort had reduced expression of the gene encoding the activation marker CD38, as well as reduced gene expression of IL12RB2 (interleukin-12 receptor subunit beta 2) and IL2RB (interleukin-2 receptor subunit beta) (fig. S12C). The genes encoding the cytolytic proteins granzyme B and perforin were also down-regulated in samples from VI participants, suggesting reduced NK cytolytic activity. TRAF4 (TNF receptor–associated factor 4), promoting cell proliferation, was also down-regulated in samples from VI participants (fig. S12C), consistent with our cell-cell communication analysis predicting that NK cells in VI participants receive signals limiting proliferation (Fig. 5D). Together, these findings demonstrate that multiple types of SARS-CoV-2 vaccination attenuate inflammatory responses in breakthrough infections in independent cohorts.
DISCUSSION
To understand the determinants of protective versus pathogenic immune responses to SARS-CoV-2, it is critical to understand how prior exposure, through vaccination or infection, shapes immunity. Prior studies have focused primarily on antigen-specific B and T responses (2–7), yet innate immune responses can be altered by prior exposure and have a marked impact on immune function (11–16). To identify the impact of prior exposure on both innate and adaptive immune responses to SARS-CoV-2, we identified individuals with primary and breakthrough SARS-CoV-2 infections during the Delta wave of the COVID-19 pandemic when vaccines were first available. All participants had mild-to-moderate disease. We show that prior vaccination can profoundly alter activation of innate immune responses. Although monocytes and NK cells from both infected groups were more activated compared with those of HCs, our findings revealed that monocytes and NK cells in breakthrough infection were less activated compared with their counterparts in primary infection. We further found that multiple communication pathways predicted to promote monocyte migration and NK cell proliferation were down-regulated during breakthrough infection compared with primary infection. The ability to clear infection without driving marked innate immune activation may be key to preventing severe disease, given that dysregulation of innate immune responses is strongly associated with COVID-19 disease severity (23–27). This understanding opens new avenues for future research, particularly in exploring the role of prior exposure in suppressing dysregulated innate immune responses.
Previous work comparing SARS-CoV-2–unvaccinated and SARS-CoV-2–vaccinated, infected patients revealed a higher percentage of HLA-DR+ monocytes and CD83+ monocytes in vaccinated patients (18). Here, we found that the total monocyte proportion is elevated in the setting of breakthrough infection, possibly because of enhanced myelopoiesis primed by prior vaccination, consistent with trained immunity (62). On the transcriptomic level, we found that CD14+ monocytes exhibited reduced activation and expression of ISGs in breakthrough infections. Consistent with these transcriptional findings, we found reduced ex vivo proinflammatory IP-10 responses in monocytes in breakthrough infections. In CD14+ monocytes, targeted metabolic BTM analysis revealed down-regulation of genes in lipid metabolism, purine nucleotide biosynthesis, and respiratory electron transport chain BTMs in VI participants compared with UVI participants, suggesting reduced metabolic activity in vaccinated individuals with breakthrough infections. Unlike CD14+ monocytes, CD16+ monocytes in both unvaccinated and vaccinated, infected participants up-regulated ISGs and genes enriched in activation BTMs. This corroborates findings in Omicron breakthrough infections, where CD16+ monocytes, but not CD14+ monocytes, exhibited a high expression of cytokine and interferon signatures (17). Given that nonclassical CD16+ monocytes are associated with wound healing and produce fewer inflammatory cytokines than both classical CD14+ monocytes and intermediate CD14+CD16+ monocytes (63, 64), this suggests an overall attenuated inflammatory response in breakthrough infections. In CD16+ monocytes, the up-regulation of genes enriched in the inositol phosphate metabolism BTM in the UVI group further pointed to differences in immunometabolism between the UVI and VI groups. Vaccination-induced changes in immunometabolism have previously been documented and contribute to trained immunity in monocytes (41–43). However, future metabolomic studies would be needed to further investigate how vaccination affects immunometabolism in breakthrough infections.
Prior work has demonstrated that higher NK cell frequency during the early course of SARS-CoV-2 infection is correlated with a lower abundance of SARS-CoV-2 viral RNA (52), pointing to the potential role of NK cells in resolving SARS-CoV-2 infection. Here, we have shown that, although increased abundance of proliferating NK cells is characteristic of both infection groups regardless of vaccination status, prior exposure alters the responsiveness of NK cells to SARS-CoV-2 infection. Our transcriptomic analyses highlighted reduced gene expression of several BTMs related to NK activation and type I interferon responses and enrichment of pathways limiting NK proliferation in breakthrough infections, indicating a role for prior vaccination in downmodulating NK cell responses. This is corroborated in network analysis, where in addition to ISGs, genes encoding for NKG2C (KLRC2; an activating NK receptor), growth factor receptor–binding protein (GRAP2), and SH2 domain–containing 1B (SH2D1B; a cytoplasmic adapter downstream of the NK activating receptor 2B4) were up-regulated in samples from primary infection compared with breakthrough infection.
In addition to their roles as early responders in viral infections, innate immune cells are also major regulators of adaptive immunity (28–30). We therefore applied cell-cell communication analyses to dissect differences in innate immune cell communication with adaptive immune cells in UVI (primary) and VI (breakthrough) participants. Our findings revealed that signals sent by monocytes, DCs, and NK cells are predicted to prevent overactive adaptive immune responses in VI participants, suggesting that vaccination-induced immunosuppressive effects may be at play. Although the down-regulation of activating receptors and up-regulation of CD57 were correlated with these immunosuppressive interactions, the mechanisms by which innate immune cells regulate adaptive immune responses after SARS-CoV-2 vaccination and infection will need to be elucidated in future studies. Nonetheless, these findings underscore the importance of evaluating immunoregulatory effects on adaptive immunity when designing vaccinations to harness innate immune cell functions.
Although it has long been documented that females generate a more robust immune response against viruses and vaccines (65, 66), the COVID-19 pandemic has further unveiled sex differences in COVID-19 outcomes (55–57). Prior work demonstrated that female patients mounted a stronger T cell response than male patients during SARS-CoV-2 infection (57). Here, we have shown that multiple innate immune cell subsets were transcriptionally more activated in females in breakthrough infections than in primary infections, whereas innate immune cells in male participants have a more activated transcriptomic profile exclusively in primary infections. These results were recapitulated in both mass cytometry profiling of PBMCs and plasma proteomic analysis, where females had elevated protein expression of activation and inflammatory markers compared with males in breakthrough infections. Thus, these results reveal important sex differences in how prior exposure primes the innate immune response to SARS-CoV-2 infection with implications for sex-based vaccination and treatment plans that seek to modulate the magnitude of innate immune responses.
There are several limitations to this study. First, we did not have longitudinal samples to compare pre- and posttime points for vaccination and infection. Our work controlled for the confounding factor of different variants of concern (VOCs) by restricting participants to those infected during the SARS-CoV-2 Delta variant wave, but the VOC was not confirmed by sequencing. Although participants included in this study did not have documented cases of prior SARS-CoV-2 infection, we cannot rule out the possibility of asymptomatic or mild COVID-19 cases. However, our ability to detect prevalent differences between UVI and VI participants across multiple cell types and profiling modalities demonstrates the overall impact of vaccination on the peripheral immune response in breakthrough infections. Cell-cell communication analysis in this study provided important insights to potential mechanisms underlying observed immune differences in unvaccinated and vaccinated, infected individuals, but future work would be needed to functionally validate these cell-cell interactions. Given that only peripheral blood was available, this work does not capture the immune response at the site of infection in the respiratory tract.
In summary, this analysis of SARS-CoV-2 breakthrough infections provides insights into both innate and adaptive immune responses. Our findings revealed subdued transcriptomic and proteomic activation of monocytes and NK cells in breakthrough infections that may in part be explained by immunoregulatory cellular communication coordinated to prevent an overactivated innate immune response. These findings contribute to our understanding of how prior exposure can induce innate immune cells to mediate protective, rather than pathologic, immune responses.
MATERIALS AND METHODS
Study design
The aim of this study was to identify the role of prior vaccination on peripheral innate and adaptive immune responses in COVID-19 breakthrough infections. Cryopreserved PBMCs were obtained from individuals with COVID-19 enrolled in the Stanford University COVID-19 Biobanking studies (Stanford Institutional Review Board approvals #55650 and #68301) from April to December 2021. Infected participants’ disease severity was scored using the WHO severity score (0 to 8), as previously described (27). All infected participants were assigned a WHO score of 3 or 4 and had a documented vaccine date and type, and none had a documented prior COVID-19 diagnosis. A score of 3 refers to individuals with mild-to-moderate disease who were hospitalized but did not require supplemental oxygen, and a score of 4 was assigned to hospitalized patients requiring noninvasive supplemental oxygen.
Mass cytometry, scRNA-seq, and plasma proteomic assays were performed on acquired PBMC and plasma samples. Participants with clinically documented immunosuppression were excluded from the study. The n value was not controlled and was dependent on the availability of samples during the primary Delta wave from April to December 2021. Samples were processed in a randomized manner in all arms of the study. Investigators were not blinded to samples’ vaccination status during data analysis.
Validation analysis was performed in an independent cohort that is part of an observer-blinded, randomized controlled trial (registered at ClinicalTrials.gov, NCT04324606) designed to determine the safety and efficacy of the Oxford-AstraZeneca COVID-19 vaccine ChAdOx1 nCoV-19 (60). Publicly available bulk RNA-seq data were obtained on day 0 of positive COVID-19 nucleic acid amplification test by RT-PCR from UK healthy adult volunteers receiving either a placebo vaccine (n = 35) or the ChAdOx1-nCOV-199 vaccine (n = 26) (60). Samples included in this dataset spanned from June to December 2020, when the primary circulating SARS-CoV-2 variants were ancestral and Alpha. Participants included in this cohort had no history of laboratory-confirmed SARS-CoV-2 infection or COVID-19–like symptoms before study enrollment. All cases of COVID-19 in this study were either mild or moderate.
Statistical analysis
Individual-level data for experiments where n < 20 are presented in data file S1. Differences between groups were assessed by the Wilcoxon rank sum test (two-sided) with Benjamini-Hochberg’s correction for multiple hypothesis testing. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001. All data analysis was performed using R versions 4.2.0 and 4.2.2.
Supplementary Material
The PDF file includes:
Other Supplementary Material for this manuscript includes the following:
Data files S1 to S4
Acknowledgments:
We are grateful to all participants in this cohort. We appreciate M. Davis and his laboratory for allowing us to use the Helios mass cytometer. Special thanks to A. Rustagi for assistance with the Parse Biosciences scRNA-seq library preparation and preprocessing pipeline. We thank N. Neff and A. Seng at the Chan Zuckerberg Biohub for assistance with sequencing. We thank T. T. Nguyen at Stanford University’s Human Immune Monitoring Core for performing the Olink plasma proteomic assay. We are grateful to I. de los Rios Kobara for the gift of SARS-CoV-2 Delta pseudovirus and assistance with the pseudovirus neutralization assay protocol. We thank P. Kim, J. Bloom, and D. Xu for the gift of HeLa-ACE2-TMPRSS2 cells. Figure illustrations were created using BioRender.com.
Funding:
This work was supported by NIH-funded institutional training grants 5T32AI007290-37 (to L.C.), F31AI179125 (to L.C.), T32AI007502 (to R.E.H.), and 5T32HL129970 (to S.M.P.); the Bill and Melinda Gates Foundation OPP113682 (to C.A.B.); NIH/NIAID K23 HL124663 (to A.J.R.); Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease 1016687 (to C.A.B.); a gift from the Quattrone family (to C.A.B.); NIH/NIAID U19AI057229-17W1 COVID SUPP #2 (to C.A.B.); the Chan Zuckerberg Biohub Investigator Program (to C.A.B.); and the Mercatus Center (to C.A.B.).
Footnotes
Competing interests: C.A.B. is a scientific advisory board member of ImmuneBridge, DeepCell Inc., and Qihan Bio on topics unrelated to this manuscript. B.P. served on the external immunology board of GSK and on the scientific advisory boards of Sanofi, Medicago, Boehringer Ingelheim, Pharmajet, Icosavax, and Ed-Jen. K.C.N. consults for Excellergy, Red Tree Ventures, Before Brands, Alladapt, Cour Pharma, Latitude, Regeneron, and IgGenix; is a cofounder of Before Brands, Alladpt, Latitude, and IgGenix; and is a national scientific committee member at Immune Tolerance Network (ITN) and NIH clinical research centers. All other authors declare that they have no competing interests.
Data and materials availability:
All data associated with this study are present in the paper or the Supplementary Materials. Mass cytometry FCS files with deidentified metadata supporting this publication are available at Flow Repository (http://flowrepository.org) under repository ID: FR-FCM-Z77Q. Data from scRNA-seq are deposited in the Gene Expression Omnibus (GEO) database under accession no. GSE261862. Olink data are available in data file S1. Data from the ChAdOx1-nCOV-199 vaccination validation cohort are deposited in GEO under accession no. GSE228842. A Zenodo repository for all original code used for analysis and visualization is available at DOI: 10.5281/zenodo.13972674.
REFERENCES AND NOTES
- 1.Al-Aly Z, Bowe B, Xie Y, Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med 28, 1461–1467 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan Q, Shi J, Yang Y, Tang G, Jiang M, Li J, Tang J, Li L, Wen X, Zhang L, Deng X, Wang Y, Lan Y, Li L, Peng P, Tong Y, Lu H, Yan L, Liu Y, Cai S, Li Y, Mo X, Li M, Deng X, Hu Z, Yu H, Hu F, Liu J, Tang X, Li F, Clinical characteristics and immune profile alterations in vaccinated individuals with breakthrough Delta SARS-CoV-2 infections. Nat. Commun 13, 3979 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collier A-RY, Brown CM, Mcmahan K, Yu J, Liu J, Jacob-Dolan C, Chandrashekar A, Tierney D, Ansel JL, Rowe M, Sellers D, Ahmad K, Aguayo R, Anioke T, Gardner S, Siamatu M, Bermudez Rivera L, Hacker MR, Madoff LC, Barouch DH, Immune responses in fully vaccinated individuals following breakthrough infection with the SARS-CoV-2 Delta variant in Provincetown, Massachusetts. medRxiv 21265113 [Preprint] (2021). 10.1101/2021.10.18.21265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koutsakos M, Lee WS, Reynaldi A, Tan H-X, Gare G, Kinsella P, Liew KC, Taiaroa G, Williamson DA, Kent HE, Stadler E, Cromer D, Khoury DS, Wheatley AK, Juno JA, Davenport MP, Kent SJ, The magnitude and timing of recalled immunity after breakthrough infection is shaped by SARS-CoV-2 variants. Immunity 55, 1316–1326.e4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pušnik J, Monzon-Posadas WO, Zorn J, Peters K, Baum M, Proksch H, Schlüter CB, Alter G, Menting T, Streeck H, SARS-CoV-2 humoral and cellular immunity following different combinations of vaccination and breakthrough infection. Nat. Commun 14, 572 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Painter MM, Johnston TS, Lundgreen KA, Santos JJS, Qin JS, Goel RR, Apostolidis SA, Mathew D, Fulmer B, Williams JC, McKeague ML, Pattekar A, Goode A, Nasta S, Baxter AE, Giles JR, Skelly AN, Felley LE, McLaughlin M, Weaver J, BioBank PM, Kuthuru O, Dougherty J, Adamski S, Long S, Kee M, Clendenin C, da Silva Antunes R, Grifoni A, Weiskopf D, Sette A, Huang AC, Rader DJ, Hensley SE, Bates P, Greenplate AR, Wherry EJ, Prior vaccination promotes early activation of memory T cells and enhances immune responses during SARS-CoV-2 breakthrough infection. Nat. Immunol 24, 1711–1724 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Koutsakos M, Reynaldi A, Lee WS, Nguyen J, Amarasena T, Taiaroa G, Kinsella P, Liew KC, Tran T, Kent HE, Tan H-X, Rowntree LC, Nguyen THO, Thomas PG, Kedzierska K, Petersen J, Rossjohn J, Williamson DA, Khoury D, Davenport MP, Kent SJ, Wheatley AK, Juno JA, SARS-CoV-2 breakthrough infection induces rapid memory and de novo T cell responses. Immunity 56, 879–892.e4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paniskaki K, Anft M, Meister TL, Marheinecke C, Pfaender S, Skrzypczyk S, Seibert FS, Thieme CJ, Konik MJ, Dolff S, Anastasiou O, Holzer B, Dittmer U, Queren C, Fricke L, Rohn H, Westhoff TH, Witzke O, Stervbo U, Roch T, Babel N, Immune response in moderate to critical breakthrough COVID-19 infection after mRNA vaccination. Front. Immunol 13, 816220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sejdic A, Hartling HJ, Holler JG, Klingen Gjærde L, Lindegaard B, Dungu AM, Gnesin F, Møller MEE, Teglgaard RS, Niemann CU, Brooks PT, Jørgensen CS, Franck KT, Fischer TK, Marquart HV, Harboe ZB, Ostrowski SR, Immune cell populations and induced immune responses at admission in patients hospitalized with vaccine breakthrough SARS-CoV-2 infections. Front. Immunol 15, 1360843 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay MZ, Rouers A, Fong S-W, Goh YS, Chan Y-H, Chang ZW, Xu W, Tan CW, Chia WN, Torres-Ruesta A, Amrun SN, Huang Y, Hor PX, Loh CY, Yeo NK-W, Wang B, Ngoh EZX, Salleh SNM, Chavatte J-M, Lim AJ, Maurer-Stroh S, Wang L-F, Lin RVTP, Wang C-I, Tan S-Y, Young BE, Leo Y-S, Lye DC, Renia L, Ng LF, Decreased memory B cell frequencies in COVID-19 delta variant vaccine breakthrough infection. EMBO Mol. Med 14, e15227 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikzad R, Angelo LS, Aviles-Padilla K, Le DT, Singh VK, Bimler L, Vukmanovic-Stejic M, Vendrame E, Ranganath T, Simpson L, Haigwood NL, Blish CA, Akbar AN, Paust S, Human natural killer cells mediate adaptive immunity to viral antigens. Sci. Immunol 4, eaat8116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier J-C, Mailhot-Léonard F, Ahmed E, Belle J, Besla R, Mazer B, King IL, Nijnik A, Robbins CS, Barreiro LB, Divangahi M, BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190.e19 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JWM, van Crevel R, Netea MG, Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U.S.A 109, 17537–17542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, Cheng S-C, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JWM, Joosten LAB, Wijmenga C, Martens JHA, Xavier RJ, Logie C, Netea MG, Stunnenberg HG, Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wimmers F, Donato M, Kuo A, Ashuach T, Gupta S, Li C, Dvorak M, Foecke MH, Chang SE, Hagan T, De Jong SE, Maecker HT, van der Most R, Cheung P, Cortese M, Bosinger SE, Davis M, Rouphael N, Subramaniam S, Yosef N, Utz PJ, Khatri P, Pulendran B, The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell 184, 3915–3935.e21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun JC, Beilke JN, Lanier LL, Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Liu C, Xie X, Niu M, Wang Y, Cheng X, Zhang B, Zhang D, Liu M, Sun R, Ma Y, Ma S, Wang H, Zhu G, Lu Y, Huang B, Su P, Chen X, Zhao J, Wang H, Shen L, Fu L, Huang Q, Yang Y, Wang H, Wu C, Ge W, Chen C, Huo Q, Wang Q, Wang Y, Geng L, Xie Y, Xie Y, Liu L, Qi J, Chen H, Wu J, Jiang E, Jiang W, Wang X, Shen Z, Guo T, Zhou J, Zhu P, Cheng T, Multi-omics blood atlas reveals unique features of immune and platelet responses to SARS-CoV-2 Omicron breakthrough infection. Immunity 56, 1410–1428.e8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huapaya JA, Higgins J, Kanth S, Demirkale CY, Gairhe S, Aboye EA, Regenold D, Sahagun SJ, Pastor G, Swaim D, Dewar R, Rehman T, Highbarger HC, Lallemand P, Laverdure S, Adelsberger J, Rupert A, Li W, Krack J, Teferi G, Kuruppu J, Strich JR, Davey R, Childs R, Chertow D, Kovacs JA, Barnett C, Torabi-Parizi P, Suffredini AF, COVID-ARC Study Group, Vaccination ameliorates cellular inflammatory responses in SARS-CoV-2 breakthrough infections. J. Infect. Dis 228, 46–58 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arunachalam PS, Scott MKD, Hagan T, Li C, Feng Y, Wimmers F, Grigoryan L, Trisal M, Edara VV, Lai L, Chang SE, Feng A, Dhingra S, Shah M, Lee AS, Chinthrajah S, Sindher SB, Mallajosyula V, Gao F, Sigal N, Kowli S, Gupta S, Pellegrini K, Tharp G, Maysel-Auslender S, Hamilton S, Aoued H, Hrusovsky K, Roskey M, Bosinger SE, Maecker HT, Boyd SD, Davis MM, Utz PJ, Suthar MS, Khatri P, Nadeau KC, Pulendran B, Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596, 410–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saresella M, Piancone F, Marventano I, Hernis A, Trabattoni D, Invernizzi M, La Rosa F, Clerici M, Innate immune responses to three doses of the BNT162b2 mRNA SARS-CoV-2 vaccine. Front. Immunol 13, 947320 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maucourant C, Filipovic I, Ponzetta A, Aleman S, Cornillet M, Hertwig L, Strunz B, Lentini A, Reinius B, Brownlie D, Cuapio A, Ask EH, Hull RM, Haroun-Izquierdo A, Schaffer M, Klingström J, Folkesson E, Buggert M, Sandberg JK, Eriksson LI, Rooyackers O, Ljunggren H-G, Malmberg K-J, Michaëlsson J, Marquardt N, Hammer Q, Strålin K, Björkström NK, Karolinska COVID-19 Study Group, Natural killer cell immunotypes related to COVID-19 disease severity. Sci. Immunol 5, eabd6832 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alrubayyi A, Touizer E, Hameiri-Bowen D, Charlton B, Gea-Mallorquí E, Hussain N, da Costa KAS, Ford R, Rees-Spear C, Fox TA, Williams I, Waters L, Barber TJ, Burns F, Kinloch S, Morris E, Rowland-Jones S, McCoy LE, Peppa D, Natural killer cell responses during SARS-CoV-2 infection and vaccination in people living with HIV-1. Sci. Rep 13, 18994 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venet M, Ribeiro MS, Décembre E, Bellomo A, Joshi G, Nuovo C, Villard M, Cluet D, Perret M, Pescamona R, Paidassi H, Walzer T, Allatif O, Belot A, Trouillet-Assant S, Ricci EP, Dreux M, Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2. Nat. Commun 14, 694 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, Krämer B, Krammer T, Brumhard S, Bonaguro L, De Domenico E, Wendisch D, Grasshoff M, Kapellos TS, Beckstette M, Pecht T, Saglam A, Dietrich O, Mei HE, Schulz AR, Conrad C, Kunkel D, Vafadarnejad E, Xu C-J, Horne A, Herbert M, Drews A, Thibeault C, Pfeiffer M, Hippenstiel S, Hocke A, Müller-Redetzky H, Heim K-M, Machleidt F, Uhrig A, Bosquillon de Jarcy L, Jürgens L, Stegemann M, Glösenkamp CR, Volk H-D, Goffinet C, Landthaler M, Wyler E, Georg P, Schneider M, Dang-Heine C, Neuwinger N, Kappert K, Tauber R, Corman V, Raabe J, Kaiser KM, Vinh MT, Rieke G, Meisel C, Ulas T, Becker M, Geffers R, Witzenrath M, Drosten C, Suttorp N, von Kalle C, Kurth F, Händler K, Schultze JL, Aschenbrenner AC, Li Y, Nattermann J, Sawitzki B, Saliba A-E, Sander LE, Angelov A, Bals R, Bartholomäus A, Becker A, Bezdan D, Bonifacio E, Bork P, Clavel T, Colome-Tatche M, Diefenbach A, Dilthey A, Fischer N, Förstner K, Frick J-S, Gagneur J, Goesmann A, Hain T, Hummel M, Janssen S, Kalinowski J, Kallies R, Kehr B, Keller A, Kim-Hellmuth S, Klein C, Kohlbacher O, Korbel JO, Kurth I, Landthaler M, Li Y, Ludwig K, Makarewicz O, Marz M, McHardy A, Mertes C, Nöthen M, Nürnberg P, Ohler U, Ossowski S, Overmann J, Peter S, Pfeffer K, Poetsch AR, Pühler A, Rajewsky N, Ralser M, Rieß O, Ripke S, Nunes da Rocha U, Rosenstiel P, Saliba A-E, Sander LE, Sawitzki B, Schiffer P, Schulte E-C, Schultze JL, Sczyrba A, Stegle O, Stoye J, Theis F, Vehreschild J, Vogel J, von Kleist M, Walker A, Walter J, Wieczorek D, Ziebuhr J, Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 182, 1419–1440.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann ER, Menon M, Knight SB, Konkel JE, Jagger C, Shaw TN, Krishnan S, Rattray M, Ustianowski A, Bakerly ND, Dark P, Lord G, Simpson A, Felton T, Ho L-P, NIHR Respiratory TRC, Feldmann M, CIRCO, Grainger JR, Hussell T, Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci. Immunol 5, eabd6197 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetité D, Tavares LA, Paiva IM, Rosales R, Colón D, Martins R, Castro IA, Almeida GM, Lopes MIF, Benatti MN, Bonjorno LP, Giannini MC, Luppino-Assad R, Almeida SL, Vilar F, Santana R, Bollela VR, Auxiliadora-Martins M, Borges M, Miranda CH, Pazin-Filho A, da Silva LLP, Cunha LD, Zamboni DS, Dal-Pizzol F, Leiria LO, Siyuan L, Batah S, Fabro A, Mauad T, Dolhnikoff M, Duarte-Neto A, Saldiva P, Cunha TM, Alves-Filho JC, Arruda E, Louzada-Junior P, Oliveira RD, Cunha FQ, SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med 217, e20201129 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilk AJ, Lee MJ, Wei B, Parks B, Pi R, Martínez-Colón GJ, Ranganath T, Zhao NQ, Taylor S, Becker W, Stanford COVID-19 Biobank, Jimenez-Morales D, Blomkalns AL, O’Hara R, Ashley EA, Nadeau KC, Yang S, Holmes S, Rabinovitch M, Rogers AJ, Greenleaf WJ, Blish CA, Multi-omic profiling reveals widespread dysregulation of innate immunity and hematopoiesis in COVID-19. J. Exp. Med 218, e20210582 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banchereau J, Steinman RM, Dendritic cells and the control of immunity. Nature 392, 245–252 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Schuster IS, Coudert JD, Andoniou CE, Degli-Esposti MA, “Natural regulators”: NK cells as modulators of T cell immunity. Front. Immunol 7, 235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gyurova IE, Ali A, Waggoner SN, Natural killer cell regulation of B cell responses in the context of viral infection. Viral Immunol. 33, 334–341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazdani M, Gholizadeh Z, Nikpoor AR, Hatamipour M, Alani B, Nikzad H, Mohamadian Roshan N, Verdi J, Jaafari MR, Noureddini M, Badiee A, Vaccination with dendritic cells pulsed ex vivo with gp100 peptide-decorated liposomes enhances the efficacy of anti PD-1 therapy in a mouse model of melanoma. Vaccine 38, 5665–5677 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Gu Y, Zhao X, Song X, Ex vivo pulsed dendritic cell vaccination against cancer. Acta Pharmacol. Sin 41, 959–969 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boonnak K, Vogel L, Orandle M, Zimmerman D, Talor E, Subbarao K, Antigen-activated dendritic cells ameliorate influenza A infections. J. Clin. Invest 123, 2850–2861 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García F, Climent N, Guardo AC, Gil C, León A, Autran B, Lifson JD, Martínez-Picado J, Dalmau J, Clotet B, Gatell JM, Plana M, Gallart T, DCV2/MANON07-ORVACS Study Group, A dendritic cell–based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci. Transl. Med 5, 166ra2 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R, Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arunachalam PS, Wimmers F, Mok CKP, Perera RAPM, Scott M, Hagan T, Sigal N, Feng Y, Bristow L, Tak-Yin Tsang O, Wagh D, Coller J, Pellegrini KL, Kazmin D, Alaaeddine G, Leung WS, Chan JMC, Chik TSH, Choi CYC, Huerta C, Paine McCullough M, Lv H, Anderson E, Edupuganti S, Upadhyay AA, Bosinger SE, Maecker HT, Khatri P, Rouphael N, Peiris M, Pulendran B, Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369, 1210–1220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, Blish CA, A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med 26, 1070–1076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, Kasturi S, Carlone GM, Quinn C, Chaussabel D, Palucka AK, Mulligan MJ, Ahmed R, Stephens DS, Nakaya HI, Pulendran B, Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol 15, 195–204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvin A, Chapuis N, Dunsmore G, Goubet A-G, Dubuisson A, Derosa L, Almire C, Hénon C, Kosmider O, Droin N, Rameau P, Catelain C, Alfaro A, Dussiau C, Friedrich C, Sourdeau E, Marin N, Szwebel T-A, Cantin D, Mouthon L, Borderie D, Deloger M, Bredel D, Mouraud S, Drubay D, Andrieu M, Lhonneur A-S, Saada V, Stoclin A, Willekens C, Pommeret F, Griscelli F, Ng LG, Zhang Z, Bost P, Amit I, Barlesi F, Marabelle A, Pène F, Gachot B, André F, Zitvogel L, Ginhoux F, Fontenay M, Solary E, Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell 182, 1401–1418.e18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin S, Jiang Y, Wei X, Liu X, Guan J, Chen Y, Lu H, Qian J, Wang Z, Lin X, Dynamic changes in monocytes subsets in COVID-19 patients. Hum. Immunol 82, 170–176 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Gonçalves LG, Belinha A, Cunha C, Oosting M, Joosten LAB, Matarese G, Van Crevel R, Netea MG, Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 17, 2562–2571 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S, Sullivan NL, Rouphael N, Yu T, Banton S, Maddur MS, McCausland M, Chiu C, Canniff J, Dubey S, Liu K, Tran V, Hagan T, Duraisingham S, Wieland A, Mehta AK, Whitaker JA, Subramaniam S, Jones DP, Sette A, Vora K, Weinberg A, Mulligan MJ, Nakaya HI, Levin M, Ahmed R, Pulendran B, Metabolic phenotypes of response to vaccination in humans. Cell 169, 862–877.e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan KR, Gan ES, Chan CYY, Liang C, Low JZH, Zhang SL-X, Ong EZ, Bhatta A, Wijaya L, Lee YH, Low JG-H, Ooi EE, Metabolic perturbations and cellular stress underpin susceptibility to symptomatic live-attenuated yellow fever infection. Nat. Med 25, 1218–1224 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Timblin Greg. A., Tharp Kevin. M., Hoeve J. Ten, Kantner DS, Baydemir I, Noel EA, Khantwal C, Singh PK, Farahzad JN, Domínguez-Andrés J, Vance RE, Snyder NW, Weaver VM, Coenzyme A governs proinflammatory macrophage metabolism. bioRxiv 505732 [Preprint] (2022). 10.1101/2022.08.30.505732. [DOI] [Google Scholar]
- 45.Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, Brenner T, Uhle F, Iwamoto Y, Robbins CS, Noiret L, Maier SL, Zönnchen T, Rahbari NN, Schölch S, Klotzsche-von Ameln A, Chavakis T, Weitz J, Hofer S, Weigand MA, Nahrendorf M, Weissleder R, Swirski FK, Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 347, 1260–1265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMahan RS, Birkland TP, Smigiel KS, Vandivort TC, Rohani MG, Manicone AM, McGuire JK, Gharib SA, Parks WC, Stromelysin-2 (MMP10) moderates inflammation by controlling macrophage activation. J. Immunol 197, 899–909 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Browaeys R, Gilis J, Sang-Aram C, De Bleser P, Hoste L, Tavernier S, Lambrechts D, Seurinck R, Saeys Y, MultiNicheNet: A flexible framework for differential cell-cell communication analysis from multi-sample multi-condition single-cell transcriptomics data. bioRxiv 544751 [Preprint] (2023); 10.1101/2023.06.13.544751. [DOI] [Google Scholar]
- 48.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marmé D, Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87, 3336–3343 (1996). [PubMed] [Google Scholar]
- 49.Eubank TD, Galloway M, Montague CM, Waldman WJ, Marsh CB, M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes. J. Immunol. Baltim. Md 171, 2637–2643 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J, Zhang J, Chao J, Porphyromonas gingivalis promotes monocyte migration by activating MMP-9. J. Periodontal Res 47, 236–242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertini R, Zack Howard OM, Dong H-F, Oppenheim JJ, Bizzarri C, Sergi R, Caselli G, Pagliei S, Romines B, Wilshire JA, Mengozzi M, Nakamura H, Yodoi J, Pekkari K, Gurunath R, Holmgren A, Herzenberg LA, Herzenberg LA, Ghezzi P, Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med 189, 1783–1789 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witkowski M, Tizian C, Ferreira-Gomes M, Niemeyer D, Jones TC, Heinrich F, Frischbutter S, Angermair S, Hohnstein T, Mattiola I, Nawrath P, McEwen S, Zocche S, Viviano E, Heinz GA, Maurer M, Kölsch U, Chua RL, Aschman T, Meisel C, Radke J, Sawitzki B, Roehmel J, Allers K, Moos V, Schneider T, Hanitsch L, Mall MA, Conrad C, Radbruch H, Duerr CU, Trapani JA, Marcenaro E, Kallinich T, Corman VM, Kurth F, Sander LE, Drosten C, Treskatsch S, Durek P, Kruglov A, Radbruch A, Mashreghi M-F, Diefenbach A, Untimely TGFβ responses in COVID-19 limit antiviral functions of NK cells. Nature 600, 295–301 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Ferreira-Gomes M, Kruglov A, Durek P, Heinrich F, Tizian C, Heinz GA, Pascual-Reguant A, Du W, Mothes R, Fan C, Frischbutter S, Habenicht K, Budzinski L, Ninnemann J, Jani PK, Guerra GM, Lehmann K, Matz M, Ostendorf L, Heiberger L, Chang H-D, Bauherr S, Maurer M, Schönrich G, Raftery M, Kallinich T, Mall MA, Angermair S, Treskatsch S, Dörner T, Corman VM, Diefenbach A, Volk H-D, Elezkurtaj S, Winkler TH, Dong J, Hauser AE, Radbruch H, Witkowski M, Melchers F, Radbruch A, Mashreghi M-F, SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat. Commun 12, 1961 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA, Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Meng Y, Wu P, Lu W, Liu K, Ma K, Huang L, Cai J, Zhang H, Qin Y, Sun H, Ding W, Gui L, Wu P, Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLOS Pathog. 16, e1008520 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT, Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun 11, 6317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh JE, Tokuyama M, Lu P, Venkataraman A, Park A, Liu F, Meir A, Sun J, Wang EY, Casanovas-Massana A, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Yale IMPACT Research Team, Shaw A, Fournier JB, Odio CD, Farhadian S, Cruz C. Dela, Grubaugh ND, Schulz WL, Ring AM, Ko AI, Omer SB, Iwasaki A, Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 588, 315–320 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aranami T, Miyake S, Yamamura T, Differential expression of CD11c by peripheral blood NK cells reflects temporal activity of multiple sclerosis. J. Immunol 177, 5659–5667 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Josien R, Li H-L, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y, Trance, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J. Exp. Med 191, 495–502 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drury RE, Camara S, Chelysheva I, Bibi S, Sanders K, Felle S, Emary K, Phillips D, Voysey M, Ferreira DM, Klenerman P, Gilbert SC, Lambe T, Pollard AJ, O’Connor D, Multi-omics analysis reveals COVID-19 vaccine induced attenuation of inflammatory responses during breakthrough disease. Nat. Commun 15, 3402 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, Diehn M, Alizadeh AA, Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol 37, 773–782 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitroulis I, Ruppova K, Wang B, Chen L-S, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, Chatzigeorgiou A, Schlitzer A, Beyer M, Joosten LAB, Isermann B, Lesche M, Petzold A, Simons K, Henry I, Dahl A, Schultze JL, Wielockx B, Zamboni N, Mirtschink P, Coskun Ü, Hajishengallis G, Netea MG, Chavakis T, Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161.e12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, Jordan BT, Peirce SM, Botchwey EA, Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci. Rep 7, 447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM, Phenotype, function, and differentiation potential of human monocyte subsets. PLOS ONE 12, e0176460 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghosh S, Klein RS, Sex drives dimorphic immune responses to viral infections. J. Immunol. Baltim. Md 198, 1782–1790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fink AL, Engle K, Ursin RL, Tang W-Y, Klein SL, Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl. Acad. Sci. U.S.A 115, 12477–12482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hafemeister C, Satija R, Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, Slichter CK, Miller HW, McElrath MJ, Prlic M, Linsley PS, Gottardo R, MAST: A flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beisser D, Klau GW, Dandekar T, Müller T, Dittrich MT, BioNet: An R-Package for the functional analysis of biological networks. Bioinformatics 26, 1129–1130 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Liu YE, Darrah PA, Zeppa JJ, Kamath M, Laboune F, Douek DC, Maiello P, Roederer M, Flynn JL, Seder RA, Khatri P, Blood transcriptional correlates of BCG-induced protection against tuberculosis in rhesus macaques. Cell Rep. Med 4, 101096 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cerami EG, Gross BE, Demir E, Rodchenkov I, Babur O, Anwar N, Schultz N, Bader GD, Sander C, Pathway Commons, a web resource for biological pathway data. Nucleic Acids Res. 39, D685–D690 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammal F, de Langen P, Bergon A, Lopez F, Ballester B, ReMap 2022: A database of Human, Mouse, Drosophila and Arabidopsis regulatory regions from an integrative analysis of DNA-binding sequencing experiments. Nucleic Acids Res. 50, D316–D325 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng C, Song C, Liu Y, Qian F, Gao Y, Ning Z, Wang Q, Jiang Y, Li Y, Li M, Chen J, Zhang J, Li C, KnockTF: A comprehensive human gene expression profile database with knockdown/knockout of transcription factors. Nucleic Acids Res. 48, D93–D100 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia-Alonso L, Holland CH, Ibrahim MM, Turei D, Saez-Rodriguez J, Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res. 29, 1363–1375 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hwang S, Kim CY, Yang S, Kim E, Hart T, Marcotte EM, Lee I, HumanNet v2: Human gene networks for disease research. Nucleic Acids Res. 47, D573–D580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Browaeys R, Saelens W, Saeys Y, NicheNet: Modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Jiang P, Zhang Y, Ru B, Yang Y, Vu T, Paul R, Mirza A, Altan-Bonnet G, Liu L, Ruppin E, Wakefield L, Wucherpfennig KW, Systematic investigation of cytokine signaling activity at the tissue and single-cell levels. Nat. Methods 18, 1181–1191 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, Pe’er D, Nolan GP, Bendall SC, Normalization of mass cytometry data with bead standards. Cytometry A 83, 483–494 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zunder ER, Finck R, Behbehani GK, Amir E-AD, Krishnaswamy S, Gonzalez VD, Lorang CG, Bjornson Z, Spitzer MH, Bodenmiller B, Fantl WJ, Pe’er D, Nolan GP, Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc 10, 316–333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Gassen S, Gaudilliere B, Angst MS, Saeys Y, Aghaeepour N, CytoNorm: A normalization algorithm for cytometry data. Cytometry A 97, 268–278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stassen SV, Siu DMD, Lee KCM, Ho JWK, So HKH, Tsia KK, PARC: Ultrafast and accurate clustering of phenotypic data of millions of single cells. Bioinformatics 36, 2778–2786 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Breiman L, Cutler A, Liaw A, Wiener M, randomForest.pdf (2002); https://CRAN.R-project.org/doc/Rnews/.
- 83.Van Gassen S, Callebaut B, Van Helden MJ, Lambrecht BN, Demeester P, Dhaene T, Saeys Y, FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A 87, 636–645 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Assarsson E, Lundberg M, Holmquist G, Björkesten J, Bucht Thorsen S, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson A-C, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S, Homogenous 96-Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLOS ONE 9, e95192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. Mass cytometry FCS files with deidentified metadata supporting this publication are available at Flow Repository (http://flowrepository.org) under repository ID: FR-FCM-Z77Q. Data from scRNA-seq are deposited in the Gene Expression Omnibus (GEO) database under accession no. GSE261862. Olink data are available in data file S1. Data from the ChAdOx1-nCOV-199 vaccination validation cohort are deposited in GEO under accession no. GSE228842. A Zenodo repository for all original code used for analysis and visualization is available at DOI: 10.5281/zenodo.13972674.


