Abstract
Introduction and Aims
Caregiver's oral health literacy (OHL) has a multidimensional impact on child oral health outcomes. In infants and toddlers, oral health behaviours (OHB) play a crucial role in the development of early childhood caries and could be related to the caregiver's OHL. A simple OHL assessment may help identify children at risk of caries. This study aimed to investigate the association between maternal OHL and the child OHBs during the first 2 years of life.
Methods
A cross-sectional study was conducted with 398 mothers of children aged 6 to 24 months. Maternal OHL was assessed using the ThREALD-30. Maternal demographics, oral health knowledge, and child OHBs were collected through self-administered questionnaires. ThREALD-30 scores were compared using the Mann–Whitney U and Kruskal–Wallis tests. Spearman's rank correlation and gamma generalized linear models were used to identify factors associated with OHL. Binary logistic regression examined the association between maternal OHL and child OHBs. The Receiver Operating Characteristics curve determined a cut-off OHL score for severely deleterious child OHBs, and Chi-square analyses assessed the association with the cut-off.
Results
The mean maternal ThREALD-30 was 23.7 (SD = 5.0), showing a strong positive correlation with oral health knowledge (r = 0.81, P < .001) and associations with low education and family income (P < .001). Logistic regression showed that lower ThREALD-30 levels were associated with poor child OHBs, including nighttime feeding, sugary bottle feeding, and no oral cleaning (P < .05). ThREALD-30 score ≤21 related to severely deleterious child OHBs.
Conclusion
Lower maternal OHL was strongly associated with poor child OHBs, with a ThREALD-30 score ≤21 indicating a high risk of caries.
Clinical Relevance
ThREALD-30 may be a useful screening tool for assessing mothers with young children at risk of early childhood caries.
Key words: Oral health literacy, Early childhood caries, Oral health knowledge, Child oral health behaviour, REALD-30, ThREALD-30
Introduction
Early childhood caries has been a major oral health problem in Thailand1 despite the implementation of numerous policies, campaigns, and projects on oral health promotion and dental caries prevention.2 It is so extensive that cavitated lesions have been found as early as 10 months old3 and continue to increase with age. Oral health-related behaviours, including improper tooth brushing habit,4 consumption of sugar-added milk and beverages,5, 6, 7 and nighttime bottle feeding,5,8,9 are identified as risk factors in most caries risk assessment tools worldwide.10
Fortunately, if not underestimated, the prevalence of cavitated dental caries appears relatively low in infants and toddlers.1,3,11 However, it sharply rises to alarming levels during the preschool years. The challenge lies in detecting caries at its nascent stage, as the caries process unfolds gradually over time. Clinical examination for dental caries in this age group also faces constraints related to personnel, accessibility, and techniques. Identifying unhealthy child oral health behaviour at an early age could serve as a pivotal indicator of caries development.10 This aspect deserves special attention and intervention before caries manifest. This window of time before the surge in prevalence offers an opportunity for early intervention.
Oral health literacy (OHL), oral health knowledge, and oral health behaviour have emerged as influential factors in determining oral health outcomes among adults.12 Research indicates that enhancing OHL levels has the potential to lower the prevalence of dental caries and periodontal disease in adults.13, 14, 15, 16 This effect is mediated through improvements in oral health-related behaviours. Over the past decade, many studies worldwide have demonstrated the association between caregivers’ OHL and a child oral health status. Lower caregivers’ OHL is related to poorer knowledge17 and poorer child oral health status.18, 19, 20, 21, 22 Mothers play a role in shaping child oral health behaviour during early childhood.23 Maternal OHL and knowledge, concerning early childhood caries, can directly impact oral health-related behaviour, subsequently influencing a child oral health and vulnerability to dental caries. Since young children are dependent on their caregivers, the OHL of the caregiver becomes crucial.
The Rapid Estimate of Adult Literacy in Dentistry (REALD-30)24 is the predominant tool for investigating adult OHL.25 It consists of 30 dental terminologies selected from the American Dental Association glossary and has demonstrated robust validity through psychometric analysis. Recently, in 2019, the Thai Version of Rapid Estimate of Adult Literacy in Dentistry (ThREALD-30) underwent testing, affirming its reliability and validity. ThREALD-30 emerges as a valuable resource for gauging oral health outcomes in the adult population.26 Although the literacy rate among Thai adults, aged 15 and above, reached 94% in 2021 national report,27 this hasn't necessarily translated into OHL or positive oral health outcomes or a strong grasp of utilizing health information to enhance oral health. The persistent prevalence of unhealthy oral health practices among children, such as bottle feeding, consumption of sugar-added milk, and inadequate tooth brushing, is evident through survey data.1 Consequently, addressing health literacy has risen to the forefront of the national agenda in Thailand.28 To improve the chances of being cavity-free in children, healthy oral health behaviour need to be practised at early age. Mothers usually dictate child-rearing, including oral health behaviour. Maternal OHL deserves more attention and should be assessed, and its association with early childhood caries risk factors should be tested. There remains a gap in research regarding OHL in caregivers, especially among mothers, and oral health behaviour in infants and toddlers in Thailand, as well as their potential interrelations.
This study aimed to: (1) evaluate the OHL of Thai mothers and its related factors; (2) examine the association between maternal OHL and their child oral health behaviour; and (3) identify a cut-off OHL score to detect mothers at risk of having a child with deleterious oral health behaviour that are known to negatively impact the child oral health. This could potentially serve as a screening tool for identifying children at risk of caries.
Material and methods
Sample size and subject recruitment
The sample size was calculated using G*Power software (version 3.1.9.4, Franz Faul) based on a statistical test for logistic regression to determine the minimum sample required to detect an odds ratio of at least 2.32, with a statistical power of 95% (β = 0.05) and a significance level of 0.05. The calculated sample size was 378, and after accounting for an expected dropout rate of 10%, the recruitment of 416 mothers was required.
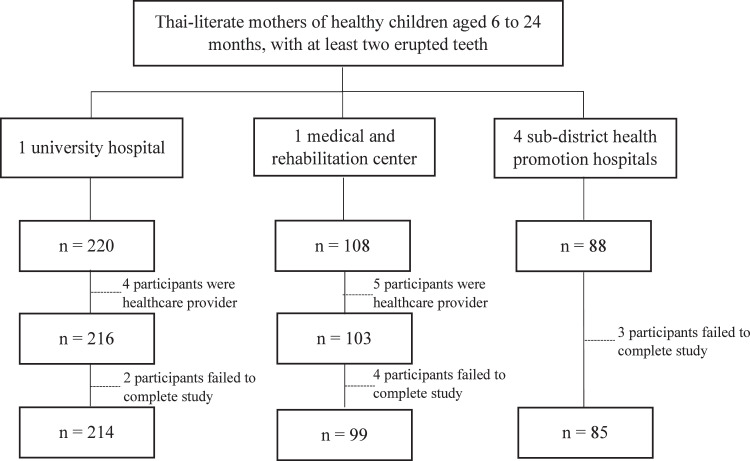
Study sites were selected through convenience sampling across six hospitals in Pathum Thani Province, Thailand, including one university hospital, one medical and rehabilitation center (primary care), and four subdistrict health promotion hospitals (primary care). Thai-literate mothers who brought their healthy children aged 6 to 24 months, with at least two erupted teeth, to the hospitals for well-baby clinics or minor medical purposes were approached using quota sampling. Mothers who were healthcare providers or unable to complete online data collection were excluded. Participants were fully informed about the study, including the risks, benefits, and the right to withdraw, prior to providing consent. Participant recruitment, inclusion, and exclusion are displayed in Figure 1.
Fig. 1.
The flow diagram of participants enrolled in the study (n = 398).
Assessment of maternal OHL
Maternal OHL was assessed using ThREALD-30.26 A previous study demonstrated that the Thai version showed good reliability (Cronbach's alpha coefficient = 0.95; intraclass correlation coefficient = 0.97, 95% CI: 0.94-1.00) and predictive validity with oral health status, including decayed, missing, and filled teeth (r = −0.28, P < .001). The ThREALD-30 test score ranges from 0 to 30, reflecting the ability to recognize and pronounce 30 certain selected dental-related words. One point was assigned for each word correctly pronounced, with higher scores indicating a higher level of OHL.
Assessment of maternal oral health knowledge and child oral health behaviour
An online self-administered questionnaire was created, comprising three sections: (1) sociodemographic characteristics of the mother and child, (2) the child oral health behaviours regarding dietary habits, oral hygiene care, and dental utilization, and (3) maternal oral health knowledge, assessed through 10 questions derived from the ‘Maternal and Child Health Handbook: MCHH version 2019 & 2020’ provided by the Ministry of Public Health, Thailand. The questions tested on caries aetiology, vertical transmission of cariogenic bacteria, initial caries appearance, understanding of caries as a disease, appropriate fluoride concentration in children's toothpaste, nutrition-rich of plain milk, crispy starchy snack and caries, caries related with sleeping with the bottle, unnecessary nighttime feeding, regular dental visits. The developed questionnaire was tested for content validity using the Index of Item-Objective Congruence by paediatric dentistry and dental public health/community dentistry specialists, with revisions made until all items achieved an Item-Objective Congruence score of 1. The reliability testing of oral health knowledge questionnaire, conducted with 20 subjects, demonstrated good internal consistency, with a Cronbach's alpha of 0.84.
Data collection
Prior to data collection, one examiner conducted intracalibration for the ThREALD-30 test with 20 subjects, achieving 100% agreement. To minimize personal contact during the COVID-19 pandemic in 2022, data collection was conducted online. An online ThREALD-30 test was created using the Line application, a primary mobile messenger app in Thailand. After obtaining written informed consent, participants were invited to scan a QR code to access the study, where they were scheduled for synchronized online testing.
To assess reading ability (by one examiner), 30 words were displayed, one at a time on a shared screen, during a video call on the Line application for the participant to read aloud. Afterwards, they were asked to complete the online self-administered structured questionnaire using the online survey software provided at www.surveymonkey.com (SurveyMonkey Inc.).
Study variables
To analyse the factors associated with maternal OHL (objective 1), we examined five categorical independent variables: type of hospital utilized by mothers (primary care, university hospital), maternal education (junior high school or lower, senior high school or vocational certificate, bachelor's degree or higher), living with a spouse (yes, no), family income (low, moderate, or high), and birth order of child (first, second, or third to fifth order). Additionally, two continuous independent variables were included: maternal age and oral health knowledge score. The dependent variable was the ThREALD-30 score.
To analyse the association between maternal OHL (ThREALD-30 scores) and child oral health behaviours (objective 2), we first compared the scores of mothers who practised each of the child oral health behaviour. Dichotomous dependent variables included exclusive breastfeeding, nighttime feeding after the age of 6 months, putting sugar-added liquid in the baby's bottle, appropriate age (≤18 months) of bottle weaning or plan, use of fluoridated toothpaste, use of the appropriate amount of toothpaste, continuing to brush even though the child is crying, and the child dental utilization. The other two were categorical variables, including the time oral cleaning started (before/at the first tooth eruption, after several teeth eruption or not yet started), and the frequency of brushing by caregiver (≥2 times/d, 1 time or no brushing). Afterwards, we selected significant behavioural dependent variables for further analysis, including prolonged nighttime feeding, sugar-added bottle feeding, between-meal sugar consumption, no oral cleaning, and irregular dental utilization. ThREALD-30 score and oral health knowledge score were used as independent variables.
Data analysis
SPSS version 27 for Windows (IBM; 2020) was used for all statistical analyses, with a significance level set at 0.05. Descriptive statistics were used to describe demographic data and OHL score. The Kolmogorov–Smirnov test revealed that ThREALD-30 scores were not normally distributed (P < .001).
The factors associated with maternal OHL were examined using the Mann–Whitney U test and Kruskal–Wallis test, followed by post-hoc Dunn's test, to compare differences in the ThREALD-30 score across categorized variables, including type of hospital, education level, living with a spouse, family income, and birth order of the child, while Spearman's rank correlation coefficient was applied to assess relationships between the ThREALD-30 and maternal age, as well as the ThREALD-30 and oral health knowledge score. Finally, gamma generalized linear models (GLMs) was used to determine the factors significantly associated with OHL.
To analyse the association between maternal OHL and child oral health behaviour, an initial nonparametric comparison analysis, including the Mann–Whitney U test and the Kruskal–Wallis test, followed by post-hoc Dunn's test, was conducted to identify the outcome variables (child oral health behaviours) that were strongly related to early childhood caries. Subsequently, binary logistic regression was used to investigate the relationship between maternal OHL and outcomes regarding child oral health-related behaviours.
To assess the diagnostic performance of the ThREALD-30 in identifying high-risk behaviours (objective 3), mothers exhibiting all three of the following were categorized as having ‘severely deleterious child oral health behaviour’: prolonged nighttime feeding after 6 months, sugar-added bottle feeding, and improper oral hygiene care (including neglecting to brush their child's teeth, brushing without toothpaste, or using non-fluoridated toothpaste). The receiving operating characteristics curve with area under the curve was utilized to assess the diagnostic performance, and a cut-off score was established based on Youden's index.29 Then, the Chi-square test was used to determine if the ‘cut-off’ score was associated with child oral health behaviour.
Results
Demography
A total of 398 Thai mothers, with a mean age of 29.7 years (SD = 5.8; range = 15-45 years), completed the process. Their children's ages ranged from 6 to 24 months, with a mean age of 15.1 months (SD = 5.8). The sociodemographic characteristics are presented in Table 1.
Table 1.
Maternal OHL scores distribution by socio-demographic variables.
| Variables | n | % | OHL score |
P value | |
|---|---|---|---|---|---|
| Mean ± SD (95% CI) | Median (range) | ||||
| Types of hospital | <.001* | ||||
| Primary care | 184 | 46.2 | 22.5 ± 5.0 (21.8, 23.2) | 22 (11-30) | |
| University hospital | 214 | 53.8 | 24.8 ± 4.8 (24.2, 25.5) | 25.5 (10-30) | |
| Education‡ | <.001† | ||||
| Junior high school or lower | 106 | 26.6 | 19.0 ± 3.9 (18.2, 19.8) | 19 (10-28) | |
| Senior high school/Voc. Cert. | 100 | 25.1 | 22.6 ± 4.2 (21.8, 23.4) | 22 (12-30) | |
| Bachelor's degree or higher | 192 | 48.2 | 26.9 ± 3.4 (26.5, 27.4) | 28 (12-30) | |
| Living with a spouse | <.001* | ||||
| Yes | 316 | 79.4 | 24.6 ± 4.8 (24.1, 25.1) | 25 (11-30) | |
| No | 82 | 20.6 | 20.2 ± 4.6 (19.2, 21.2) | 20 (10-30) | |
| Family income/mo‡ | <.001† | ||||
| Low (≤30,000 ฿, approx. USD 835) | 215 | 54.0 | 20.9 ± 4.3 (20.3, 21.4) | 20 (10-30) | |
| Moderate (30,001-50,000 ฿) | 130 | 32.7 | 26.7 ± 3.8 (26.0, 27.3) | 28 (12-30) | |
| High (>50,000 ฿, approx. USD 1400) | 53 | 13.3 | 28.2 ± 2.6 (27.5, 28.9) | 29 (21-30) | |
| Birth order of child | .511† | ||||
| 1st | 188 | 47.2 | 24.0 ± 5.0 (23.2, 24.7) | 24.5 (10-30) | |
| 2nd | 139 | 34.9 | 23.9 ± 4.7 (23.1, 24.7) | 24 (11-30) | |
| 3rd-5th | 71 | 17.8 | 22.9 ± 5.9 (21.5, 24.3) | 23 (12-30) | |
Significant level 0.05.
Mann–Whitney U test.
Kruskal–Wallis test.
Post-hoc Dunn's test indicates a significant difference among all the compared values.
Maternal OHL
The mean ThREALD-30 score was 23.7 (SD = 5.0), with a median of 24 (range = 10-30). Eighteen per cent of participants achieved a full ThREALD-30 score of 30. Two words that all participants read correctly were ‘sugar’ and ‘caries’ in Thai. The three Thai words least correctly read were ‘restoration’ (58.5%), ‘sealant’ (48.7%,), and ‘periodontal’ (47.5%).
Maternal oral health knowledge
Maternal oral health knowledge scores ranged from 0 to 10 (full score), with a mean of 6.7 (SD = 2.4). Approximately one-fifth of the mothers (19.6%) achieved a full knowledge score and had a very high mean ThREALD-30 score of 29.3 (SD = 1.2). Mothers exhibiting the greatest understanding of caries aetiology and its sugar-related aspects had the highest correct response rate at 95.7%. In contrast, recognition of chipped and rotten primary teeth as a pathology received the lowest correct response rate, at 37.9%.
Factors related to maternal OHL
Maternal oral health knowledge was strongly correlated with the ThREALD-30 score (r = 0.81, P < .001). Mothers who attended a university hospital, had higher education levels, had higher family income, and lived with their spouse exhibited significantly higher OHL scores (P < .001), as detailed in Table 1. However, maternal age showed a weak correlation with the ThREALD-30 score (r = 0.11, P = .034).
Type of hospital utilized by mother, education level, living with a spouse, family income, maternal age, and oral health knowledge, which were initially found to be significantly related to maternal OHL (P < .001), were tested in the GLMs. The final GLMs (Table 2) revealed that oral health knowledge (P < .001), education level (Reference: Junior high school or lower; Senior high school/Vocational Certificate, P < .001; Bachelor's degree or higher, P < .001), and family income (Reference: Low; Moderate, P = .069; High, P = .011) were significantly related to ThREALD-30 scores. Conversely, type of hospital, spousal cohabitation, and maternal age showed no significant association with ThREALD-30 scores in the regression model.
Table 2.
Gamma generalized linear models after excluding nonsignificant variables (n = 398).
| Variables | Coef. | S.E. | 95% CI | P value |
|---|---|---|---|---|
| Oral health knowledge | –0.002 | 0.0002 | (–0.002, –0.002) | <.0001 |
| Education level | ||||
| Junior high school or lower | Ref. | |||
| Senior high school/Voc. Cert. | –0.004 | 0.0009 | (–0.006, –0.002) | <.001 |
| Bachelor's degree or higher | –0.006 | 0.0010 | (–0.008, –0.004) | <.001 |
| Family income | ||||
| Low (≤30,000 ฿, approx. USD 835) | Ref. | |||
| Moderate (30,001-50,000 ฿) | –0.001 | 0.0008 | (–0.003, 0.000) | .069 |
| High (>50,000 ฿, approx. USD 1400) | –0.003 | 0.0010 | (–0.004, –0.001) | .011 |
| Constants | 0.063 | 0.0010 | (0.061, 0.065) | <.001 |
AIC = 3315.899, link function: power (–1), significant level 0.05.
Cut-off ThREALD-30 score consideration for detecting risk behaviours
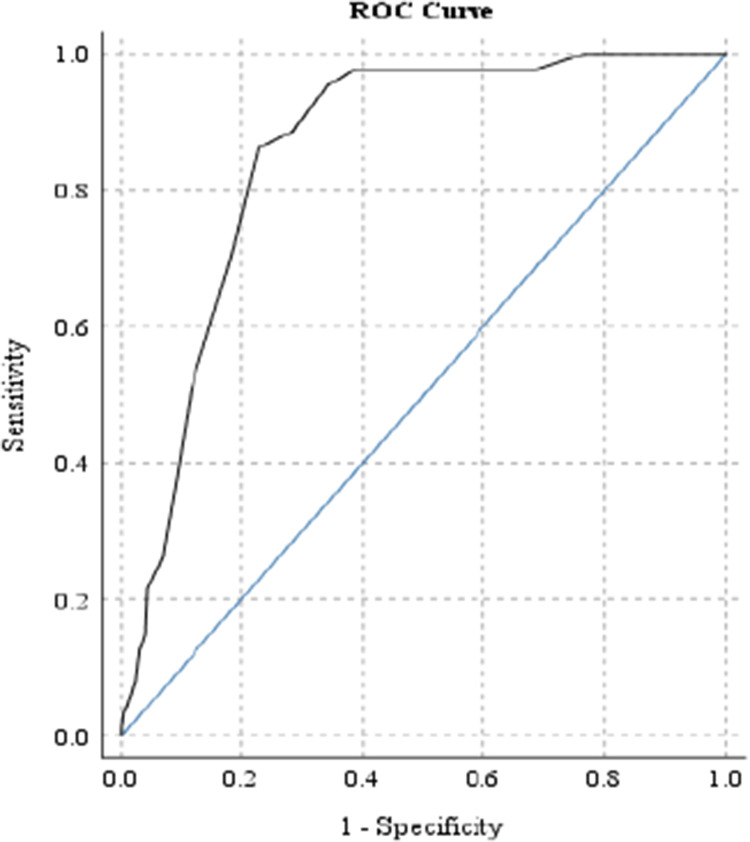
The receiving operating characteristics curve analysis, displayed in Figure 2, yielded an area under the curve of 0.86 (P < .001, 95% CI = 0.82-0.89), indicating that the ThREALD-30 test has good diagnostic performance. Specifically, a ThREALD-30 score of 21, which yielded the greatest sum of sensitivity (Se) and specificity (Sp), was found to be an effective cut-off score for detecting ‘severe deleterious child oral health behaviour’, with Se and Sp values of 0.86 and 0.77, respectively.
Fig. 2.
The ROC curve of ThREALD-30 scores to predict ‘severely deleterious child oral health behaviours’ for considering appropriate cut-off score. The model can accurately discriminate 86% of the cases (P < .001, 95% CI = 0.82-0.89).
Association between maternal OHL and child oral health behaviour
Mothers who practised healthy oral health behaviour for their child had significantly higher ThREALD-30 scores (P < .001), as shown in Table 3. Mothers with higher ThREALD-30 scores were significantly less likely to put sugar-added liquids in the bottle and provided cariogenic snacks or beverages less frequently to their children (P < .001). They also brushed their children's teeth more frequently, attempted to brush even with the child's resistance, and utilized dental care services regularly (P < .001). Interestingly, while most mothers used an appropriate amount of toothpaste for young children, regardless of OHL scores (P = .198), mothers with high OHL tended to use non-fluoridated toothpaste for their young children (P < .001). Exclusive breastfeeding was practised by only 8.8% of the mothers, and there was no significant difference in OHL scores between those who did and did not practice it (P = .932). The mothers were grouped by the ‘cut-off’ ThREALD-30 score (≤21 or >21), as shown in Table 3. The chi-square analysis indicated its influence on all child oral health behaviour, except for exclusive breastfeeding and the use of fluoridated toothpaste.
Table 3.
Maternal OHL scores distribution by child oral health behaviours.
| Variables | n | % | OHL score |
P value | OHL score |
P value‡ | ||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD (95% CI) | Median (range) | ≤21 n (%) (95% CI) |
>21 n (%) (95% CI) |
|||||
|
Dietary habit |
||||||||
| Exclusive breastfeeding | .932* | .979 | ||||||
| Yes | 35 | 8.8 | 23.9 ± 5.1 (22.1, 25.6) |
25 (14-30) |
13 (37) (20, 50) |
22 (63) (46, 80) |
||
| No | 363 | 91.2 | 23.7 ± 5.0 (23.2, 24.2) |
24 (10-30) |
134 (37) (32, 42) |
229 (63) (58, 68) |
||
| Nighttime feeding after the age of 6 mo | <.001* | <.001 | ||||||
| Yes | 232 | 58.3 | 21.1 ± 4.4 (20.5, 21.7) |
21 (10-30) |
138 (59) (53, 66) |
94 (41) (34, 47) |
||
| No | 166 | 41.7 | 27.4 ± 3.2 (26.9, 27.9) |
28 (13-30) |
9 (5) (2, 9) |
157 (95) (91, 98) |
||
| Putting sugar-added liquid in baby's bottle║ | <.001* | <.001 | ||||||
| Yes | 126 | 34.6 | 20.0 ± 4.7 (19.3, 20.7) |
20 (10-30) |
92 (73) (65, 81) |
34 (27) (19, 35) |
||
| No | 238 | 65.4 | 25.6 ± 4.1 (25.1, 26.2) |
27 (12-30) |
43 (18) (13, 23) |
195 (82) (77, 87) |
||
| Bottle weaning or plan to wean at the age ≤18 mo║ | <.001* | <.001 | ||||||
| Yes | 111 | 30.6 | 27.8 ± 3.3 (27.2, 28.4) |
29 (11-30) |
4 (4) (0, 7) |
107 (96) (93, 100) |
||
| No | 252 | 69.4 | 21.8 ± 4.6 (21.2, 22.4) |
21 (10-30) |
131 (52) (46, 58) |
121 (48) (42, 54) |
||
| Frequency per day of cariogenic snacks or beverages between meals | <.001† | <.001 | ||||||
| ≥3 times | 78 | 19.6 | 21.2 ± 5.8 (19.9, 22.5) |
20§ (10-30) |
47 (60) (49, 71) |
31 (40) (29, 51) |
||
| 1-2 times | 171 | 43.0 | 22.2 ± 4.6 (21.5, 22.9) |
21§ (12-30) |
86 (50) (43, 58) |
85 (50) (42, 57) |
||
| No | 149 | 37.4 | 26.8 ± 3.3 (26.3, 27.3) |
28 (16-30) |
14 (9) (5, 14) |
135 (91) (86, 95) |
||
| Oral hygiene practices | ||||||||
| Time to start oral cleaning | <.001† | <.001 | ||||||
| Before/at the first tooth eruption | 276 | 69.3 | 26.0 ± 3.8 (25.5, 26.4) |
27 (15-30) |
45 (16) (12, 21) |
231 (84) (79, 88) |
||
| After several teeth eruption | 101 | 25.4 | 18.8 ± 3.4 (18.2, 19.4) |
19¶ (11-26) |
85 (84) (77, 91) |
16 (16) (9, 23) |
||
| Not yet started | 21 | 5.3 | 18.0 ± 4.6 (15.9, 20.0) |
20¶ (10-25) |
17 (81) (63, 99) |
4 (19) (1, 37) |
||
| Frequency per day of brushing by caregiver | <.001* | <.001 | ||||||
| ≥2 times | 212 | 53.3 | 26.5 ± 4.0 (26.0, 27.0) |
28 (11-30) |
29 (14) (9, 18) |
183 (86) (82, 91) |
||
| 1 time or no brushing | 186 | 46.7 | 20.6 ± 4.3 (20.0, 21.2) |
20 (10-30) |
118 (63) (56, 70) |
68 (37) (30, 44) |
||
| Use of fluoridated toothpaste║ | <.001* | .001 | ||||||
| Yes | 250 | 90.9 | 24.5 ± 5.0 (23.9, 25.1) |
25.5 (11-30) |
76 (30) (25, 36) |
174 (70) (64, 75) |
||
| No | 25 | 9.1 | 28.4 ± 2.1 (27.5, 29.3) |
29 (23-30) |
0 (0) | 25 (100) | ||
| Use of the appropriate amount of toothpaste║ | .198* | .838 | ||||||
| Yes | 222 | 77.9 | 24.9 ± 5 (24.2, 25.5) |
26.5 (11-30) |
64 (29) (23, 35) |
158 (71) (65, 77) |
||
| No | 63 | 22.1 | 24.2 ± 4.8 (23.0, 25.4) |
25 (12-30) |
19 (30) (19, 42) |
44 (70) (58, 81) |
||
| Continue to brush even though the child is crying | <.001* | <.001 | ||||||
| Yes | 320 | 80.4 | 25.0 ± 4.5 (24.5, 25.5) |
26 (11-30) |
78 (24) (20, 29) |
242 (76) (71, 80) |
||
| No | 78 | 19.6 | 18.7 ± 3.7 (17.8, 19.5) |
19 (10-30) |
69 (88) (81, 96) |
9 (12) (4, 19) |
||
| Dental utilization | ||||||||
| Child dental utilization | <.001* | <.001 | ||||||
| Yes | 120 | 30.2 | 27.2 ± 3.7 (26.6, 27.9) |
29 (13-30) |
12 (10) (5, 15) |
108 (90) (85, 95) |
||
| No | 278 | 69.8 | 22.2 ± 4.8 (21.7, 22.8) |
21 (10-30) |
135 (49) (43, 54) |
143 (51) (46, 57) |
||
Significant level 0.05.
Mann–Whitney U test.
Kruskal–Wallis test.
Chi-square test.
Post-hoc Dunn's test indicates no significant difference between the values labeled with the same character.
Post-hoc Dunn's test indicates no significant difference between the values labeled with the same character.
Column totals may not add to total due to missing data.
The binary logistic regression analysis revealed a significant association between low maternal OHL and poor child oral health behaviour (Table 4), including prolonged nighttime feeding (>6 months old), sugar-added bottle feeding, and no oral cleaning (P < .05). These associations remained significant even after adjusting the odds ratio for education level and family income (P < .05). In contrast, no significant association was found in between-meal sugar consumption and irregular dental utilization. However, the result indicate that oral health knowledge had an impact on all risk oral health behaviour (P < .05).
Table 4.
Binary logistic regression to predict the child oral health behaviours.
| Variables | Oral health literacy |
Oral health knowledge |
||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | |
| Prolonged nighttime feeding |
0.68 (0.63, 0.73) |
0.70 (0.64, 0.80) |
0.46 (0.39, 0.53) |
0.51 (0.43, 0.60) |
| Sugar-added bottle feeding |
0.76 (0.71, 0.81) |
0.78 (0.72, 0.84) |
0.52 (0.45, 0.60) |
0.55 (0.50, 0.65) |
| Between-meal sugar consumption | 1.02 (0.94, 1.10) |
0.99 (0.91, 1.09) |
0.90 (0.82, 0.97) |
0.90 (0.84, 0.97) |
| No oral cleaning |
0.78 (0.71, 0.87) |
0.68 (0.59, 0.79) |
0.61 (0.48, 0.77) |
0.53 (0.40, 0.70) |
| Irregular dental utilization | 1.04 (0.95, 1.15) |
1.04 (0.93, 1.15) |
0.86 (0.78, 0.94) |
0.88 (0.79, 0.99) |
Significant level 0.05. The significant associations are in bold.
Adjusted for maternal education level and family income.
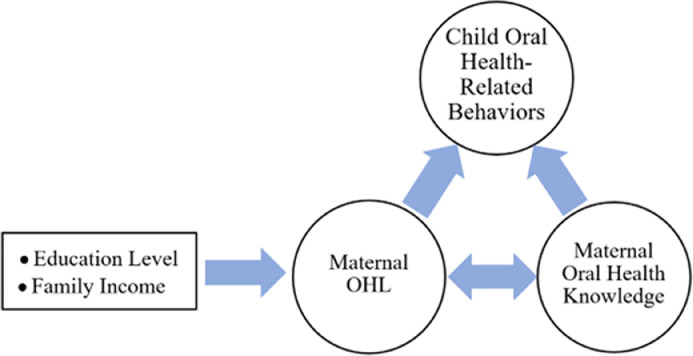
The findings on factors related to maternal OHL, the correlation between maternal OHL and oral health knowledge, and their association with child oral health-related behaviors demonstrate a chain of effects among these variables. A detailed summary of these findings is presented in Figure 3.
Fig. 3.
A flow diagram depicting the summary of the findings.
Discussion
To promote a child health requires attentive behaviour from the caregiver, especially during the crucial first 1000-day period of life, including oral health.30 This period is foundational not only for physical and cognitive development but also for the establishment of long-term oral health habits. A child oral health-related behaviours become critically important once the first tooth erupts, as it completes the host factor in dental caries aetiology. The prevalence of caries among preschoolers is high and continues to increase sharply.1 This rise may be attributed to prolonged exposure to primary causes of caries. Detecting caries at an early age is not easy, especially for non-dental providers. Our study focused on child oral health-related behaviours practised by mother as a surrogate outcome for child oral health status, providing substantial evidence of its significant impact on early childhood caries.31 Our results align with previous studies32; one study, conducted in a group of children of a similar age to ours and focusing on female caregivers (but not specifically mothers), found that female caregivers with lower levels of OHL were associated with deleterious oral health behaviours in children.33 Two other studies also identified an association between lower caregiver's OHL and prolonged bottle feeding,34,35 while another study found no significant relationship.19 Currently, no studies have specifically examined the practice of feeding children with sugar-added bottles. Notably, our study identified a significant association between this behaviour and lower levels of maternal OHL. In contrast, when analysing the frequency of cariogenic snacks or beverages between meals using regression analysis, no significant association was found, consistent with the previous study.33 Regarding tooth brushing behaviour, our study's results differed from previous studies.19,35 We observed an association that aligns with one study,33 while prior studies did not. This discrepancy may be attributed to the studies being conducted among different age groups of children, who are highly dependent on their caregivers for daily routines, including oral care. In research involving younger children, the influence of caregivers is more pronounced, and OHL tends to have a greater impact on the child oral health behaviour. Additionally, our studies found that most participants used fluoridated toothpaste, regardless of their OHL level. A small group, with higher OHL and education, chose non-fluoridated toothpaste. Misinformation regarding fluoride toxicity and benign ignorance of recent changes in recommendations and evidence may contribute to this discrepancy. This aspect of fluoride use needs to be explored, emphasized, and individualized for some specific groups.
To identify mothers with low OHL and children at risk of early childhood caries at a very young age is important, and it is essential to make attempts to modify risk factors. Our study not only aimed to investigate the relationships but also aimed to find score values to assess the high-risk group. Previous studies have established cut-off score for REALD-30 to differentiate between low OHL (less than 13) and high OHL (13 or more).33,34,36 However, these studies were conducted in different populations, it may be inappropriate to apply their findings directly to our target group (Thai mothers) due to potential variations in cultural and socioeconomic factors. Our study noted that a ThREALD-30 score of 21 or lower may serve as a screening tool for identifying mothers with severely poor child oral health behaviour and whose children are predisposed to a high risk of developing caries. In the future, the cut-off score for caregivers may need to be studied in other aspects, especially across different child age groups. Additionally, research should also explore how these scores relate to the child oral health status to better understand the direct impact on oral health outcomes. Comparative studies involving oral health status, when oral examination is permitted, would provide valuable insights.
OHL is a type of health literacy37 that was classified into three levels,38 based on individual cognitive and social skills that influence the motivation and ability to understand and utilize information for maintaining good oral health. These include basic (or functional), communicative (or interactive), and critical literacy. The importance of health literacy and its levels has been widely recognized in addressing health issues. Various tools have been developed to evaluate OHL at one or more levels. However, assessing levels beyond the basic level is complex. To date, there is no single instrument that can assess all three levels of health literacy.39 Selecting simple and effective tools can enable efficient and swift screening of high-risk groups. Identifying individuals with low levels of OHL and providing them with oral health education or intervention may assist in prevention efforts. REALD-30,24 a word recognition instrument, has been successfully used in adults across various countries40, 41, 42, 43 to assess basic literacy through reading ability. Its validity, simplicity, and minimal time requirement of 3 minutes or less per person have been thoroughly documented in many studies. The word recognition test has been established as an efficient proxy for health literacy. This study used the Thai version of REALD-30 (ThREALD-30), adapted for the local language, and data were collected online through video calls during the COVID-19 pandemic, differing from the original version that involved face-to-face testing.
The mean ThREALD-30 score in this study (23.7, SD = 5.0) as well as findings from other studies in Thailand using the ThREALD-30 (20.1, SD = 8.7,26 26.7, SD = 3.7644), was higher than the scores reported in studies utilizing the original REALD-30 by Lee et al24 (19.8 ± 6.4) and Vann et al33 (15.8, SD = 5.3) who also studied female caregivers in a similar age group to our study. This may be due to cross-cultural influences; while the translated words are valid in content and meaning, they may not be comparable in reading difficulty to the original English version. Most of the Thai translations consisted of compound words formed from basic vocabulary. For instance, the term ‘apicoectomy’ is translated into simpler, easily readable components as ‘cutting-tip-(of the)-root’. The original REALD-30 includes 30 words, ranging from common to technical terminology, organized in ascending order of language difficulty. Transitioning these words to Thai may cause the loss of that incremental language difficulty. Additionally, half of our participants achieved a bachelor's degree or higher, in contrast to only 5% of those in the study conducted by Vann et al.33 Higher education is consistently associated with higher level of OHL35,36,45,46 and our study similarly found that education significantly influences these levels. A previous study found higher scores among participants recruited in dental clinic settings compared to those from community-based general health settings.35 Therefore, our study was conducted in nondental clinics and excluded mothers employed as healthcare personnel to minimize biases and ensure a more representative sample of the general population. High oral health knowledge and family income positively influence OHL score, as observed in other studies involving mothers.17,33,47 Although our results find a weak association between the mother's age and OHL, it is noteworthy that all 14 teenage mothers (age ≤19 years) were from low-income families and had significantly lower mean scores of 19.9 (SD = 3.6) (P < .001).
Our findings confirm a positive relationship between OHL and oral health knowledge. The knowledge questions in our study were based exclusively on information sourced from the ‘Maternal and Child Health Handbook’, an interactive booklet provided by the Ministry of Public Health to all pregnant women during prenatal care. This handbook contains information on safe pregnancy, child development, and healthy childcare. It was assumed that the content of this handbook would be educational and have a protective effect against early childhood caries. However, our results indicate that more than half of mothers still lack knowledge regarding dental caries in primary teeth and vertical transmission of Streptococcus mutans. Additionally, while some mothers possessed knowledge, they did not consistently apply it. Interestingly, most mothers were aware that starch and sugar are contributing factors to dental caries; nevertheless, more than half of them continued to provide sugary snacks between meals to their children. These findings can help guide improvement of strategies and the identification of key content for oral health education aimed at caregivers. Access to dental care needs to be enhanced, and there should be vigorous advocacy for effective self-care practices to cultivate good oral health behaviour. Additionally, oral health knowledge related to early childhood caries should be delivered in a timely, clear, and practical manner to effectively reach caregivers with low levels of OHL.
The limitations of this study include a sample size confined to a specific population in Pathum Thani Province, Thailand, potentially restricting the generalizability of the findings to other regions or populations with different socioeconomic, cultural, or environmental conditions. Future studies should consider more diverse populations to enhance the applicability of the results. Additionally, this study utilized a cross-sectional design and relied on self-reported information from mothers, possibly leading to responses that reflect socially acceptable behaviours rather than their true practices. Oral examinations of the children were not conducted, as the prevalence of dental caries during this period was found to be low. Furthermore, data collection occurred during the COVID-19 pandemic, potentially limiting access to in-person examinations. Conducting oral assessments and implementing longitudinal studies could have uncovered additional relationships and led to new insights, ultimately contributing to improvements in public health systems and promoting better oral health among the population.
The study suggests that the ThREALD-30 demonstrates clinical feasibility and requires minimal time for testing, taking 3 minutes or less per person. A low ThREALD-30 score may serve as one of predictive indicators for poor oral health-related behaviours in children. This approach could help identify young children at risk of early childhood caries before the disease occurs or becomes detectable. Based on this assessment, a tailored package of oral health knowledge should be developed and delivered according to the individual's appropriate level of OHL.
Conclusion
Lower maternal OHL, as assessed by ThREALD-30, was associated with poor oral health behaviour of the child. Maternal education, family income, and oral health knowledge were associated with OHL. These findings imply that ThREALD-30 could be a feasible screening tool for mothers to early identify young children at risk of caries.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
Author contributions
KK contributed to the study design, data collection, conducted the statistical analyses, and wrote the manuscript. PL contributed to the formulation of the research question, study design, interpretation of the results, supervision, writing, and revision of the manuscript. JL contributed to the study design and manuscript revision. All authors have read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
This cross-sectional study was approved by the Institutional Review Board of the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University (No. MU-DT/PY-IRB 2021/062.0207), and the Human Research Ethics Committee of Thammasat University Hospital (No. HREC-TUH 015/2564).
Acknowledgements
We would like to express our gratitude to Assoc. Prof. Dr Varangkanar Jirarattanasopha from Mahidol University, Thailand, and Dr Dusit Chaiprasithikul from Silpakorn University, Thailand, for their valuable consultation and assistance with data analysis. We also extend our thanks to Thammasat University, Thammasat University Hospital, and the Pediatric Dental Residency Training Program at the Faculty of Dentistry, Mahidol University, Thailand, for their generous support.
References
- 1.Thai Bureau of Dental Health. The 9th National Oral Health Survey in Thailand [in Thai]. Department of Health, Ministry of Public Health, https://dental.anamai.moph.go.th/th/national-oral-health-survey-report/4952#wow-book/[accessed 14 June 2024].
- 2.Thai Bureau of Dental Health . Department of Health, Ministry of Public Health; 2024. Draf Thailand National Oral Health Plan 2023-2037 [in Thai]https://dental.anamai.moph.go.th/th/oral-health-plan/download?id=115982&mid=38393&mkey=m_document&lang=th&did=34545 [accessed 14 August 2024] [Google Scholar]
- 3.Vachirarojpisan T, Shinada K, Kawaguchi Y, Laungwechakan P, Somkote T, Detsomboonrat P. Early childhood caries in children aged 6–19 months. Community Dent Oral Epidemiol. 2004;32(2):133–142. doi: 10.1111/j.0301-5661.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Tadakamadla J, Johnson NW. Effect of toothbrushing frequency on incidence and increment of dental caries: a systematic review and meta-analysis. J Dent Res. 2016;95(11):1230–1236. doi: 10.1177/0022034516655315. [DOI] [PubMed] [Google Scholar]
- 5.Detsomboonrat P, Pisarnturakit PP. Dental caries and related oral health factors among 9 to 18 month old Thai children. Southeast Asian J Trop Med Public Health. 2015;46(4):786–797. PMID: 26867399. [PubMed] [Google Scholar]
- 6.Feldens CA, Rodrigues PH, de Anastácio G, Vítolo MR, Chaffee BW. Feeding frequency in infancy and dental caries in childhood: a prospective cohort study. Int Dent J. 2018;68(2):113–121. doi: 10.1111/idj.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JG, Messer LB. Intake of sweet drinks and sweet treats versus reported and observed caries experience. Eur Arch Paediatr Dent. 2010;11(1):5–17. doi: 10.1007/BF03262704. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo TD, Bezerra AC, de Toledo OA. Feeding habits and severe early childhood caries in Brazilian preschool children. Pediatr Dent. 2005;27(1):28–33. PMID: 15839392. [PubMed] [Google Scholar]
- 9.Suparattanapong P, Chankanka O, Matangkasombut O, Govitvattana N. Dental caries and associated risk factors in 13- to 18-month-old infants receiving breast or formula milk feeding: a cross-sectional study. Int J Paediatr Dent. 2022;32(4):527–537. doi: 10.1111/ipd.12930. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatric Dentistry . The reference manual of pediatric dentistry. American Academy of Pediatric Dentistry; Chicago, IL: 2023. Caries-risk assessment and management for infants, children, and adolescents; pp. 301–307. [Google Scholar]
- 11.Thitasomakul S, Thearmontree A, Piwat S, et al. A longitudinal study of early childhood caries in 9- to 18-month-old Thai infants. Community Dent Oral Epidemiol. 2006;34(6):429–436. doi: 10.1111/j.1600-0528.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 12.The invisible barrier: literacy and its relationship with oral health A report of a workgroup sponsored by the National Institute of Dental and Craniofacial Research, National Institute of Health, U.S. Public Health Service, Department of Health and Human Services. J Public Health Dent. 2005;65(3):174–182. doi: 10.1111/j.1752-7325.2005.tb02808.x. [DOI] [PubMed] [Google Scholar]
- 13.Dutra LDC, de Lima LCM, Neves É TB, et al. Adolescents with worse levels of oral health literacy have more cavitated carious lesions. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0225176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wehmeyer MM, Corwin CL, Guthmiller JM, Lee JY. The impact of oral health literacy on periodontal health status. J Public Health Dent. 2014;74(1):80–87. doi: 10.1111/j.1752-7325.2012.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baskaradoss JK. Relationship between oral health literacy and oral health status. BMC Oral Health. 2018;18(1):172. doi: 10.1186/s12903-018-0640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phetnin DN. Oral health literacy and oral health status among Thai elderly. Int Dent J. 2023;73:S41. doi: 10.1016/j.identj.2023.07.660. [DOI] [Google Scholar]
- 17.Hom JM, Lee JY, Divaris K, Baker AD, Vann WF., Jr Oral health literacy and knowledge among patients who are pregnant for the first time. J Am Dent Assoc (1939) 2012;143(9):972–980. doi: 10.14219/jada.archive.2012.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montes G, Bonotto DV, Ferreira FM, Menezes J, Calixto Fraiz F. Caregiver's oral health literacy is associated with prevalence of untreated dental caries in preschool children. Cien Saude Colet. 2019;24:2737–2744. doi: 10.1590/1413-81232018247.18752017. [DOI] [PubMed] [Google Scholar]
- 19.Miller E, Lee JY, DeWalt DA, Vann WF., Jr Impact of caregiver literacy on children's oral health outcomes. Pediatrics. 2010;126(1):107–114. doi: 10.1542/peds.2009-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrett GM, Citi AM, Gansky SA. Parental functional health literacy relates to skip pattern questionnaire error and to child oral health. J Calif Dent Assoc. 2012;40(5):423–430. doi: 10.1080/19424396.2012.12220912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridges SM, Parthasarathy DS, Wong HM, Yiu CK, Au TK, McGrath CP. The relationship between caregiver functional oral health literacy and child oral health status. Patient Educ Couns. 2014;94(3):411–416. doi: 10.1016/j.pec.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Sowmya KR, Puranik MP, Aparna KS. Association between mother's behaviour, oral health literacy and children's oral health outcomes: a cross-sectional study. Indian J Dent Res. 2021;32(2):147–152. doi: 10.4103/ijdr.IJDR_676_18. [DOI] [PubMed] [Google Scholar]
- 23.Alamoudi R, Showlag R, Almujil N, et al. The impact of parental oral health behaviors on the oral health of children. Int J Commun Med Public Health. 2023;10:4451–4456. doi: 10.18203/2394-6040.ijcmph20233149. [DOI] [Google Scholar]
- 24.Lee JY, Rozier RG, Lee SY, Bender D, Ruiz RE. Development of a word recognition instrument to test health literacy in dentistry: the REALD-30—a brief communication. J Public Health Dent. 2007;67(2):94–98. doi: 10.1111/j.1752-7325.2007.00021.x. [DOI] [PubMed] [Google Scholar]
- 25.Dickson-Swift V, Kenny A, Farmer J, Gussy M, Larkins S. Measuring oral health literacy: a scoping review of existing tools. BMC Oral Health. 2014;14(1):148. doi: 10.1186/1472-6831-14-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeraksa S, Chaichit R, Muktabhant B, Udompanich S. Reliability and validity of the Thai version of rapid estimate of adult literacy in dentistry. J Int Oral Health. 2019;11:132. doi: 10.4103/jioh.jioh_51_19. [DOI] [Google Scholar]
- 27.The World Bank. Literacy rate, adult total (% of people ages 15 and above) in Thailand, https://data.worldbank.org/indicator/SE.ADT.LITR.ZS/; 2021 [accessed 24 September 2021].
- 28.Strategy and Planning Division. Twenty-year National Strategic Plan for Public Health (2017-2036) in Thailand. Ministry of Public Health, https://spd.moph.go.th/wp-content/uploads/2022/09/Ebook-MOPH-20-yrs-plan-2017-Final-Eng-120961.pdf; 2018 [accessed 1 September 2024].
- 29.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the "first 1000 days". J Pediatr. 2016;175:16–21. doi: 10.1016/j.jpeds.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simancas-Pallares MA, Ginnis J, Vann WF, Jr., et al. Children's oral health-related behaviours and early childhood caries: a latent class analysis. Community Dent Oral Epidemiol. 2022;50(3):147–155. doi: 10.1111/cdoe.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Firmino RT, Ferreira FM, Martins CC, Granville-Garcia AF, Fraiz FC, Paiva SM. Is parental oral health literacy a predictor of children’s oral health outcomes? Systematic review of the literature. Int J Paediatr Dent. 2018 doi: 10.1111/ipd.12378. Published online July 8. [DOI] [PubMed] [Google Scholar]
- 33.Vann WF, Jr., Lee JY, Baker D, Divaris K. Oral health literacy among female caregivers: impact on oral health outcomes in early childhood. J Dent Res. 2010;89(12):1395–1400. doi: 10.1177/0022034510379601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divaris K, Lee JY, Baker AD, Vann WF., Jr. Caregivers’ oral health literacy and their young children's oral health-related quality-of-life. Acta Odontol Scand. 2012;70(5):390–397. doi: 10.3109/00016357.2011.629627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanzone L, Lee J, Divaris K, Dewalt D, Baker A, Vann W. A cross sectional study examining social desirability bias in caregiver reporting of children's oral health behaviors. BMC Oral Health. 2013;13:24. doi: 10.1186/1472-6831-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vann WF, Jr., Divaris K, Gizlice Z, Baker AD, Lee JY. Caregivers’ health literacy and their young children's oral-health-related expenditures. J Dent Res. 2013;92(7 Suppl):55s–62s. doi: 10.1177/00220345134843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isman B. National Institute of Dental and Craniofacial Research; Bethesda, MD: 2000. National Institute of Dental and Craniofacial Research (U.S.); Centers for Disease Control and Prevention (U.S.). Healthy People 2010. Oral Health Toolkit. [Google Scholar]
- 38.Nutbeam D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259–267. doi: 10.1093/heapro/15.3.259. [DOI] [Google Scholar]
- 39.Ghaffari M, Rakhshanderou S, Ramezankhani A, Mehrabi Y, Safari-Moradabadi A. Systematic review of the tools of oral and dental health literacy: assessment of conceptual dimensions and psychometric properties. BMC Oral Health. 2020;20(1):186. doi: 10.1186/s12903-020-01170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima LCM, Neves É TB, Dutra LDC, et al. Psychometric properties of BREALD-30 for assessing adolescents’ oral health literacy. Rev Saude Publica. 2019;53:53. doi: 10.11606/s1518-8787.2019053000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peker K, Köse TE, Güray B, Uysal Ö, Erdem TL. Reliability and validity of the Turkish version of the Rapid Estimate of Adult Literacy in Dentistry (TREALD-30) Acta Odontol Scand. 2017;75(3):198–207. doi: 10.1080/00016357.2016.1278079. [DOI] [PubMed] [Google Scholar]
- 42.Tadakamadla SK, Quadri MF, Pakpour AH, et al. Reliability and validity of Arabic Rapid Estimate of Adult Literacy in Dentistry (AREALD-30) in Saudi Arabia. BMC Oral Health. 2014;14:120. doi: 10.1186/1472-6831-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong HM, Bridges SM, Yiu CK, McGrath CP, Au TK, Parthasarathy DS. Development and validation of Hong Kong Rapid Estimate of Adult Literacy in Dentistry. J Investig Clin Dent. 2012;3(2):118–127. doi: 10.1111/j.2041-1626.2012.00113.x. [DOI] [PubMed] [Google Scholar]
- 44.Chaisombat M, Kootrakul B, Wanichsaithong P, Chatiketu P. Associations between oral health literacy and oral health status among patients attending comprehensive dental clinic. Chiang Mai Dent J. 2023;44:34–41. doi: 10.12982/CMDENTJ.2023.010. [DOI] [Google Scholar]
- 45.Mohammadi TM, Malekmohammadi M, Hajizamani HR, Mahani SA. Oral health literacy and its determinants among adults in Southeast Iran. Eur J Dent. 2018;12(3):439–442. doi: 10.4103/ejd.ejd_429_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiu Fong Lai S, Kok Wun Wong M, Ming Wong H, Kar Yung Yiu C. Parental oral health literacy of children with severe early childhood caries in Hong Kong. Eur J Paediatr Dent. 2017;18(4):326–331. doi: 10.23804/ejpd.2017.18.04.11. [DOI] [PubMed] [Google Scholar]
- 47.Vichayanrat T, Sittipasoppon T, Rujiraphan T, Meeprasert N, Kaveepansakol P, Atamasirikun Y. Oral health literacy among mothers of pre-school children oral health literacy among mothers of pre-school children. M Dent J. 2014;34:243–252. [Google Scholar]