Abstract
Introduction and aims
This study aimed to examine the causal link between oral microbiome and the risk of oral and oropharyngeal squamous cell carcinoma (OOPSCC) using Mendelian randomization (MR).
Methods
Utilizing single nucleotide polymorphisms as instrumental variables, we applied the MR inverse-variance weighted approach to assess the impact of salivary and tongue microbiome on OOPSCC. The data were obtained from the CNGBdb database and the UK Biobank, and analytical procedures were performed using the R package ‘TwoSampleMR’. To ensure the robustness of our findings, we conducted sensitivity studies, which included the MR-Egger intercept test, to establish strong correlations and eliminate the phenomenon of horizontal pleiotropy.
Result
Our large-scale MR study revealed a genetically predisposed causal relationship between 13 microbial taxa, each from saliva and tongue, with OOPSCC. Notably, microbial taxa from six genera, including Prevotella, Neisseria, Veillonella, Granulicatella, Treponema, and Streptococcus, in both salivary and tongue microbiomes, showed this relationship. Conversely, several taxa, including Hemophilus, Solobacterium, Campylobacter, and Porphyromonas, predominantly demonstrated an inverse relationship, suggesting a protective effect. The robustness of our findings was further confirmed through sensitivity analyses, providing additional confidence in our results.
Conclusion
Our MR study indicates that the oral microbiota has a significant causal impact on the risk of oral and oropharyngeal cancers. The microbial biomarkers we identified, which are linked to OOPSCC, have the potential to uncover the underlying mechanisms and pave the way for new therapeutic approaches for targeted treatment of these malignancies.
Key words: Oral microbiome, Mendelian randomisation, Genetics, Causal, Risk factor
Introduction
Oral and oropharyngeal squamous cell carcinoma (OOPSCC) is one of the most common kinds of cancer globally and is linked to high rates of morbidity.1,2 Approximately 60% of cases of oral malignancies are detected in a late stage, leading to a survival probability of fewer than 50% during a 5-year period.1,2 Established risk factors implicated in the development of OOPSCC include tobacco use, excessive alcohol consumption, betel nut chewing, and human papillomavirus, especially for SCC of the oro-pharynx.3 However, there has been a rise in the incidence of OOPSCC cases without these conventional risk factors in recent years.4 Hence, it is essential to promptly uncover the additional risk factors that may substantially influence the prognosis in patients with these malignancies and enable early detection and prevention.
The oral microbiome is a highly significant and intricate collection of microorganisms within the human body. It is considered one of the top five areas of focus in the human microbiome project, alongside the nasal cavity, vagina, intestine, and skin.5,6 Mounting data substantiates the correlation between the oral microbiota and human systemic disorders.7 The correlation between these two factors can be ascribed to the capacity of several oral microorganisms to impact the inflammatory microenvironment. Extensive data over the past two decades has conclusively demonstrated a strong correlation between bacteria and the development of tumours.8,9 Several pathogens have been implicated in the development of various types of cancer. For instance, Helicobacter pylori with gastric cancer, Chlamydia pneumoniae with lung cancer, Salmonella typhi with gallbladder cancer, Streptococcus bovis and Bacteroides fragilis with unspecified types of cancer, and Fusobacterium nucleatum (F. nucleatum) with colon cancer.10,11 Moreover, numerous studies have suggested that oral bacteria might play a role in carcinogenesis through both direct and indirect mechanisms.12,13
Microbial dysbiosis, defined as the imbalance in the microbial equilibrium, has been suggested to play a significant role in the development of OOPSCC; notable changes in the diversity of specific genera or species have been reported by several studies in OOPSCC.8,14 These investigations have indicated the potential involvement of several bacteria in the development of OOPSCC, and the subsequent research findings offer some evidence to substantiate this hypothesis. Nevertheless, the makeup of oral bacterial populations varies depending on the saliva and specific locations within the mouth cavity.15 In addition, the established etiological factors, including betel quid chewing, tobacco, and alcohol, can potentially affect the population of oral microorganisms, which makes the relationship more complicated.16 Thus, the observational research in this particular area is impeded by confounding factors and reverse causality. Considering the growing data suggesting that the human microbiome might cause cancer, exploring the role of oral microbial dysbiosis in OOPSCC may help explain why some individuals who are not exposed to established risk factors nevertheless develop cancer. Further, clarifying the potential cause-and-effect relationship between the oral microbiome and OOPSCC is crucial to improving the prevention and prognosis.
Mendelian randomization (MR) is a flexible tool that employs whole-genome sequencing data to investigate causal correlations in epidemiology.17,18 Genetic variants strongly linked to the exposure are used as instrumental variables (IVs) in MR to establish causality and reduce the impact of confounding biases.19, 20, 21 In previous studies, scientists have demonstrated the influence of human genetics on the composition of microorganisms in the mouth. A study on the oral microbiome in twins showed that the oral microbiome is inherited, with more than 50% of the microbiome traits showing heritability.22 Despite limited understanding, earlier genome-wide association study (GWAS) investigations have found specific genetic loci linked to the composition and stability of the oral microbiome, highlighting the influence of host genetics.23 However, our understanding of the causal influence of oral microbiota and cancers is still in its early developmental phases.24 The presence of such research gaps not only restricts our comprehension of the correlation between the oral microbiome and cancer but also obstructs the identification of possible preventative and therapeutic approaches. In this study, we performed a two-sample MR analysis to examine the causal link between salivary and tongue microbiome with OOPSCC using publicly available GWAS summary statistics.
Methods
Study design and data sources
This two-sample MR analysis utilized a previously published GWAS focused on the oral microbiota of East Asian individuals – CNGBdb database.25, 26, 27 This GWAS is notable for being the first large-scale study within this population, targeting 2017 tongue dorsum samples and 1915 salivary samples, utilizing high-depth whole-genome sequencing (PMID: 34873157). For summary statistics, we used the publicly available ieu-b-4962 dataset that includes OOPSCC genetic data from a large European cohort.
Oral microbiota data collection and processing
The study dataset included tongue dorsum microbiomes (N = 2017) and salivary microbiomes (N = 1915) (Table S1). Samples were subjected to rigorous inclusion criteria to ensure data quality, including a variant calling rate of at least 98%, a mean sequencing depth of over 20×, absence of population stratification in principle component analysis, and the exclusion of related individuals based on pairwise identity by descent estimates. Additional stringent criteria were applied, such as a minimum mean depth of 8×, Hardy–Weinberg equilibrium values greater than 10^ to 5, and a genotype calling rate above 98% for analysed variations. Following these stringent quality control protocols, a comprehensive cohort of 2984 participants was established, comprising 2017 individuals with tongue dorsum samples and 1915 individuals with salivary samples. This dataset included approximately 10 million variants, covering common and low-frequency variants with a minor allele frequency of at least 0.5%, maintained for further analysis. Raw sequencing data were processed to generate operational taxonomic units, which were then taxonomically classified using reference databases. Data standardization and normalization procedures were implemented to ensure comparability across different datasets and sequencing platforms.
Genetic data and IV selection
Genetic data for both datasets were obtained from GWAS. Single nucleotide polymorphisms (SNPs) significantly associated with oral microbiota taxa (P < 5 × 10^-7) were selected as IVs for the MR analyses. These SNPs were chosen based on their relevance to the microbial taxa of interest and their availability in both datasets. To ensure the validity of the IVs, we excluded SNPs with pleiotropic effects unrelated to the microbiota.
MR analyses
We employed the inverse-variance weighted (IVW) method as the primary analysis, complemented by MR-Egger regression, weighted median, simple median, and weighted mode methods to account for potential pleiotropy and provide robust estimates. The IVW method combines the effect estimates of the IVs, assuming no pleiotropy, while complementary methods offer sensitivity analyses to detect and adjust for pleiotropy.
Heterogeneity and pleiotropy assessments
To assess the robustness of our findings, we performed heterogeneity and pleiotropy analyses. Cochran's Q test about IVW and Eggar methods was used to evaluate the heterogeneity among the selected SNPs, with significant heterogeneity indicating variability in the IV effects. The MR-Egger intercept test was employed to detect directional pleiotropy, where a significant intercept suggests the presence of pleiotropy. Additionally, the MR-PRESSO (Pleiotropy RESidual Sum and Outlier) test was used to identify and correct for outliers, further ensuring the reliability of the MR findings.18,28,29 The Steiger analysis was used to detect the causal direction of the MR analysis between exposure OM and outcome OOPSCC.
Statistical analyses
All statistical analyses were performed using R software, with ‘TwoSampleMR’ and ‘MR-PRESSO’ packages specifically designed for MR analyses. The significance threshold for the MR analyses was set at P < .05. Results were visualized using forest and bubble plots to illustrate the effect sizes and confidence intervals (CI) of the associations between microbiota taxa and OOPSCC risk. These visualizations facilitate the interpretation of the findings and highlight the most significant associations.
Results
In the MR analysis considering saliva microbiota and tongue microbiota as an exposure, our study identified associations with one phylum, two classes, eight orders, 17 families, 15 genera, and 412 species. Regarding the saliva microbiota, we found associations with two phyla, 1 class, four orders, 13 families, 96 genera, and 424 species. With 9080 SNPs and 8661 SNPs with genome-wide significance were selected. The calculated F-statistics for the selected SNPs related to saliva microbiota and tongue microbiota exceeded the conventional threshold of 20 (Tables S2 and S3), indicating that these selected SNPs may be reliable representatives of saliva microbiota and tongue microbiota.
Associations between saliva microbiota and OOPSCC
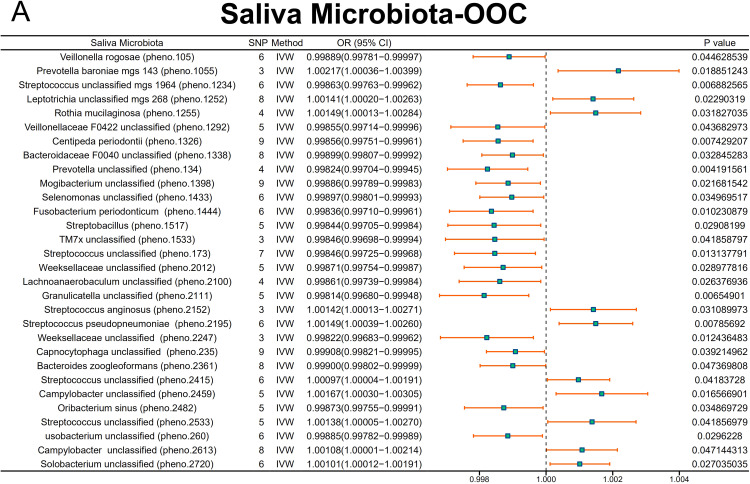
The MR analyses revealed several significant associations between saliva microbiota taxa and oral cancer risk. Notably, Veillonella unclassified (pheno.1057) (odds ratios [OR] = 1.00125, 95% CI = 1.00012-1.00238, P = 0.03045), Granulicatella unclassified (pheno.1308) (OR = 1.00187, 95% CI = 1.00095-1.00278, P = .00006), Streptococcus unclassified (pheno.134) (OR = 1.00121, 95% CI = 1.00013-1.00229, P = .02753), Streptococcus sp000411475 (pheno.1435) (OR = 1.00083, 95% CI = 1.00003-1.00162, P = .04085), Streptococcus unclassified (pheno.1713) (OR = 1.00150, 95% CI = 1.00024-1.00277, P = .02012), Streptococcus unclassified (pheno.1771) (OR = 1.00247, 95% CI = 1.00094-1.00399, P = .00153), Prevotella veroralis (pheno.1822) (OR = 1.00152, 95% CI = 1.00025-1.00279, P = .01864), Neisseria unclassified (pheno.2211) (OR = 1.00136, 95% CI = 1.00021-1.00252, P = .02015), Kingella unclassified (pheno.2262) (OR = 1.00103, 95% CI = 1.00004-1.00201, P = .04092), Haemophilus unclassified (pheno.2373) (OR = 1.00127, 95% CI = 1.00001-1.00253, P = .04779), Streptococcus unclassified (pheno.2374) (OR = 1.00173, 95% CI = 1.00058-1.00289, P = .00330), Streptococcus unclassified (pheno.250) (OR = 1.00238, 95% CI = 1.00050-1.00426, P = .01290), and Treponema vincentii (pheno.2566) (OR = 1.00143, 95% CI = 1.00018-1.00268, P = .02513 were significantly associated with an increased risk of OOPSCC (Figure 1).
Fig. 1.
Primary MR analysis (IVW) of salivary microbiome associations with OOPSCC risk. This figure presents the effect estimates (odds ratios) and 95% confidence intervals for the associations between oral microbiota taxa and OOPSCC risk using MR-IVW methods across the ieu-b-4962 datasets.
On the contrary, Campylobacter unclassified (pheno.1053) (OR = 0.99826, 95% CI = 0.99655-0.99997, P = .04578), Aggregatibacter segnis (pheno.1100) (OR = 0.99862, 95% CI = 0.99741-0.99984, P = .02600), Prevotella unclassified (pheno.1299) (OR = 0.99906, 95% CI = 0.99819-0.99994, P = .03679), Streptococcus unclassified (pheno.1388) (OR = 0.99850, 95% CI = 0.99710-0.99991, P = .03657), Mogibacterium unclassified (pheno.1415) (OR = 0.99892, 95% CI = 0.99787-0.99998, P = .04622), Saccharimonadaceae unclassified (pheno.1447) (OR = 0.99833, 95% CI = 0.99697-0.99969, P = .01613), Campylobacter unclassified (pheno.1972) (OR = 0.99842, 95% CI = 0.99695-0.99988, P = .03421), Pauljensenia cellulosilytica (pheno.2054) (OR = 0.99864, 95% CI = 0.99747-0.99982, P = .02357), Solobacterium unclassified (pheno.2178) (OR = 0.99880, 95% CI = 0.99781-0.99979, P = .01793), Streptococcus sinensis (pheno.2416) (OR = 0.99822, 95% CI = 0.99703-0.99941, P = .00343), and Streptococcus unclassified (pheno.2437) (OR = 0.99864, 95% CI = 0.99745-0.99983, P = .02506) was associated with an reduced risk (Figure 1). The detailed effect estimates (β), standard errors, OR, 95% CI, and P values for these associations of saliva microbiota are provided in Supplementary Table 2.
Associations between tongue microbiota and OOPSCC
In addition, the tongue microbiota also demonstrated significant associations with OOPSCC. MR analysis illustrates these significant associations, highlighting the effect sizes (OR) and statistical significance for these associations across OOPSCC datasets and tongue microbiota types. Prevotella veroralis (pheno.1822) (OR = 1.00152, 95% CI = 1.00025-1.00279, P = .01864) and Treponema vincentii (pheno.2566) (OR = 1.00143, 95% CI = 1.00018-1.00268, P = .02513) was positively associated with OOPSCC risk, while Veillonella unclassified (pheno.1057) (OR = 1.00125, 95% CI = 1.00012-1.00238, P = .03045), Granulicatella unclassified (pheno.1308) (OR = 1.00187, 95% CI = 1.00095-1.00278, P = .00006), Streptococcus unclassified (pheno.134) (OR = 1.00121, 95% CI = 1.00013-1.00229, P = .02753), Streptococcus sp000411475 (pheno.1435) (OR = 1.00083, 95% CI = 1.00003-1.00162, P = .04085), Streptococcus unclassified (pheno.1713) (OR = 1.00150, 95% CI = 1.00024-1.00277, P = .02012), Streptococcus unclassified (pheno.1771) (OR = 1.00247, 95% CI = 1.00094-1.00399, P = .00153), Neisseria unclassified (pheno.2211) (OR = 1.00136, 95% CI = 1.00021-1.00252, P = .02015), Kingella unclassified (pheno.2262) (OR = 1.00103, 95% CI = 1.00004-1.00201, P = .04092), Haemophilus unclassified (pheno.2373) (OR = 1.00127, 95% CI = 1.00001-1.00253, P = .04779), Streptococcus unclassified (pheno.2374) (OR = 1.00173, 95% CI = 1.00058-1.00289, P = .00330), Streptococcus unclassified (pheno.250) (OR = 1.00238, 95% CI = 1.00050-1.00426, P = .01290), showed similar patterns (Figure 2).
Fig. 2.
Primary MR analysis (IVW) of tongue microbiome associations with OOPSCC Risk. This figure presents the effect estimates (odds ratios) and 95% confidence intervals for the associations between oral microbiota taxa and OOPSCC risk using MR-IVW methods across the ieu-b-4962 datasets.
However, Porphyromonas asaccharolytica (OR = 0.712, 95% CI = 0.541-0.936, P = .015) from the ieu-b-4962 dataset was negatively associated with OOPSCC risk. Additionally, Centipeda noxia (OR = 0.406, 95% CI = 0.194-0.852, P = .017), Clostridia (OR = 0.389, 95% CI = 0.177-0.855, P = .019), Gemella unclassified (OR = 0.415, 95% CI = 0.190-0.905, P = .027), Haemophilus unclassified (OR = 0.494, 95% CI = 0.256-0.952, P = .035), Lachnoanaerobaculum saburreum (OR = 0.518, 95% CI = 0.303-0.885, P = .016), Lachnoanaerobaculum unclassified (OR = 0.455, 95% CI = 0.212-0.976, P = .043), and Veillonella rogosae (OR = 0.385, 95% CI = 0.175-0.843, P = .017) showed similar patterns (Figure 2). The circos plot, which displays the complex relationships between different microbiota taxa and OOPSCC risk, providing a comprehensive overview of the microbiota's influence on OOPSCC across various datasets, is provided in Figure 3. The detailed effect estimates (β), standard errors, OR, 95% CI, and P values for these associations of tongue microbiota are provided in Supplementary Table 3.
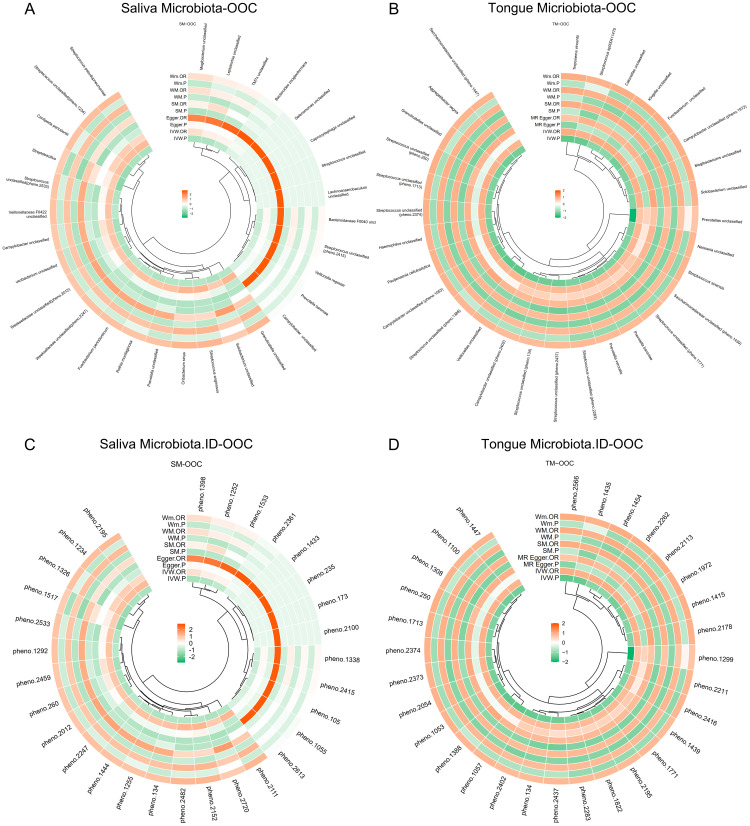
Fig. 3.
Mendelian randomization estimates (microbial taxa) visualised as Circos plot for Tongue microbiota (A) and Salivary microbiota (B). Mendelian randomization estimates (database ID of the microbes) visualised for Circos plot Tongue microbiota (C) and Salivary microbiota (D) *OOC: Oral and Oropharyngeal Cancer.
Heterogeneity and pleiotropy analyses
To ensure the robustness of our findings, we performed heterogeneity and pleiotropy analyses. The results of Cochran's Q test, which indicated no significant heterogeneity across most associations, supporting the consistency of the IVs used in the analyses, are presented in Supplementary Tables S4 and S5. Additionally, the MR-Egger intercept tests did not show significant evidence of directional pleiotropy for the significant associations. This suggests that the results are unlikely to be biased by horizontal pleiotropy (Supplementary Tables S6 and S7).
Discussion
This study utilized primary MR analyses (IVW) to investigate the causal relationships between microbiota from the oral cavity (saliva and tongue) and oral and oropharyngeal cancer (OOPSCC), employing comprehensive datasets. The analyses aimed to identify significant microbial taxa associated with OOPSCC risk across different microbiota and datasets, highlighting the integrated role. This large-scale MR study identified 13 microbial taxa each from saliva and tongue, showing a genetically predisposed causal relationship with OOPSCC.
Among the taxa associated with increased risk, several ones, including Prevotella, Neisseria, Veillonella, and Treponema, have already been implicated in cancer development. The correlation between Prevotella and oral carcinogenesis has been acknowledged for over two decades. In 2005, Mager et al30 observed an elevated presence of P. melaninogenica in the saliva of individuals affected by the condition. More recently, Zhang et al31 observed that oral squamous cell carcinoma (OSCC) tumour samples exhibit an increased presence of Prevotella intermedia. Torralba et al32 also observed an elevation in the concentrations of Prevotella in certain patients with OSCC. Ganly et al33 have also seen an increase in Prevotella levels in nonsmoking, human papillomavirus-negative OSCC patients, similar to Alloprevotella. Muto et al34 demonstrated that the genus Neisseria exhibited exceptionally high ADH activity and generated substantial quantities of acetaldehyde in a laboratory setting. Neisseria has been proposed to serve as a local reservoir of carcinogenic acetaldehyde and thereby have a crucial function in the development of alcohol-related cancer in humans. It is widely believed that chronic inflammation and immunomodulation produced by Treponema denticola (T. denticola) may represent the underlying mechanism by which these bacteria contribute to developing oro-digestive cancers.35,36 Invitro experiments have shown that T. denticola could promote the development of OSCC by activating the TGF-β pathway.37
A majority of taxa which was significantly associated with OOPSCC in our analysis belonged to the genus Streptococcus; they could not be identified at the species level because oral microbiome GWAS are still in their early stages since they have limited sample sizes, resulting in inadequate information at the species or strain level. Several species belonging to the genus Streptococcus have been previously implicated in having a role in cancers of the oral-gut axis. Streptococcus anginosus is more commonly discovered in esophageal cancer samples than oral cancer and is also higher in relative abundance.38 One of the earlier reports by Tateda et al39 documented the presence of S. anginosus in cancer samples collected from patients’ oral and pharyngeal cavities. After 5 years, Sasaki et al40 observed that S. anginosus is increased in patients with oral cancer but not in patients with other malignancies. In 2004, Narikiyo et al41 documented that patients with esophageal cancer exhibited a predilection and high occurrence of S. anginosus and S. mitis infections. The study conducted by Rai et al42 revealed an increased presence of S. anginosus in individuals with OSCC, underscoring the ongoing significance of this bacterium in the development of oral cancer. Another recent study suggested that S. anginosus could be used as a noninvasive biomarker for oropharyngeal cancer.43 It has been suggested that S. anginosus induces the recruitment of neutrophils and monocytes, potentially disrupting epithelial cells and subsequent development of cancerous growths.41 Furthermore, evidence supports the transformation of ethanol into acetaldehyde by S. anginosus.44 Acetaldehyde is recognized to contribute to cancer development by causing DNA damage and generating point mutations that occur during its repair.35,45 An increased presence of S mitis, another type of streptococci, has also been reported in patients with OSCC.30 In recent years, there has been compelling evidence linking oral cancer to the following streptococci: Streptococcus constellatus, Streptococcus salivarius, Streptococcus gordonii, and Streptococcus parasanguinis.46,47
The present study also suggests that specific taxa belonging to the genus Hemophilus, may possess a mitigating or protective influence on the incidence of OOPSCC. One possible explanation for this phenomenon is their influence on safeguarding impact on the overall balance of oral microbiota. Previous studies have shown that H. influenzae infection leads to increased expression of the NLRP3 inflammasome gene, thereby protecting the overall balance of gut microbiota and decreasing the likelihood of colorectal cancer.48 Exploring this connection is crucial in order to comprehend and evaluate the function of NLRP3 as a possible target for oncological protection against OOPSCC. Another species found to be protective is P. asaccharolytica, a Gram-negative, obligate anaerobic bacterium with black pigmentation, classified under the Bacteriodaceae family. Little is known about this species and its role in cancers. On the contrary, another species belonging to the same genus, Porphyromonas gingivalis, is a pivotal pathogen involved in the development of periodontitis, which is marked by persistent inflammation and imbalance of microbiota. Several studies have highlighted the role of P. gingivalis in orodigestive cancers.49
One notable observation in our analysis is the absence of F. nucleatum, which has been previously linked to oral carcinogenesis by several microbiome studies.50 The rationale for this could be explained by the fact that, specific taxa-associated carcinogenesis is uncommon in the general population and hence it may not be present in the GWAS of healthy individuals. On the contrast, increased microbial richness and diversity in microbiome studies do not always suggest taxonomic presence; it could also be because of the variation in methodology and the databases used for bioinformatic analysis in these studies.51
The human microbiome's composition is affected by several environmental influences.14 Alterations in host nutrition influence oral microbiome communities both taxonomically and functionally.52 Furthermore, the consumption of pharmaceuticals and antibiotics can influence the oral microbial flora.53 These impacts of environmental influences on individuals will be influenced by their genotype and the distinct effects of both genotype and environmental factors. Simultaneously, the influence of genetic elements is also contingent upon environmental variables.54 We employed the MR methodology to mitigate these certain confounders frequently encountered in epidemiological research.55 Furthermore, our SNPs exhibited a robust correlation with microbiota and were analysed with various cancer databases. Another strength of this work lies in the use of extensive GWAS data that spans the salivary microbiome, tongue microbiome, and OOPSCC. This comprehensive data collection guarantees strong statistical power and produces many outcomes. Furthermore, this article utilized a meticulously crafted analytical framework to examine the causal connections, and the work utilizes several methods of MR analysis to establish causal relationships and performed sensitivity studies to guarantee the strength of the results, reducing the impact of horizontal pleiotropy and other variables.
The limitation of this study, which is inherent to several MR studies, is the assumption of linearity in the causal link between oral microbiome and OOPSCC. Nevertheless, this connection may be more complex, encompassing environmental elements and other genetic determinants. Although we have identified putative mediators of the causative association between tongue and salivary microbiome, it is essential to note that our analysis may not include all hypothetical mediation pathways due to the intricate biological processes involved. Given the unique nature of GWAS investigations, there is a shortage of covariate adjustment for the cohort from which, the data was initially obtained. Further, the study sample primarily comprises persons of European descent, potentially constraining the applicability of results to the general population. Moreover, the database we employed categorized oral and oropharyngeal malignancies under a single broad classification despite recent evidence highlighting their distinct risk factors, behaviours, and treatment outcomes.3,56
Our findings have provided plausible biomarkers that can be further explored as noninvasive biomarkers for diagnosis or intervention targets for the prevention of oral and oropharyngeal cancers.57 Integrating our study results with what is currently known about the oral microbiome and cancer strengthens the microbiome's importance in cancer research and creates new interdisciplinary opportunities for comprehending and addressing disease. The comprehensive amalgamation of microbiological and conventional risk factors can enhance cancer prevention, diagnosis, and treatment methods, resulting in more individualized and efficient healthcare solutions.
Conclusion
While we have demonstrated the possible causative links between the oral microbiota and OOPSCC, we have also emphasized the necessity for further research to address the current knowledge gaps. Furthermore, future research should strive to investigate these correlations in varied populations and use longitudinal methodologies to comprehend the impact of temporal alterations in the microbiome on the risk and advancement of cancer.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.identj.2025.01.017.
Appendix. Supplementary materials
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coletta RD, Yeudall WA, Salo T. Grand challenges in oral cancers. Front Oral Health. 2020;1:3. doi: 10.3389/froh.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santacroce L, Passarelli PC, Azzolino D, et al. Oral microbiota in human health and disease: a perspective. Exp Biol Med (Maywood) 2023;248(15):1288–1301. doi: 10.1177/15353702231187645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunath BJ, De Rudder C, Laczny CC, Letellier E, Wilmes P. The oral-gut microbiome axis in health and disease. Nat Rev Microbiol. 2024;22(12):791–805. doi: 10.1038/s41579-024-01075-5. [DOI] [PubMed] [Google Scholar]
- 7.Pisano M. Oral dysbiosis and systemic diseases: a two-way relationship? Medicina (Kaunas) 2023;59(11):1933. doi: 10.3390/medicina59111933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Mun L, Wye Lum S, Kong Yuiin Sze G, et al. Association of microbiome with oral squamous cell carcinoma: a systematic review of the metagenomic studies. IJERPH. 2021;18(14):7224. doi: 10.3390/ijerph18147224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menati Rashno M, Mehraban H, Naji B, Radmehr M. Microbiome in human cancers. Access Microbiol. 2021;3(8) doi: 10.1099/acmi.0.000247. https://www.microbiologyresearch.org/content/journal/acmi/10.1099/acmi.0.000247 [cited 2025 Jan 4]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan Z, Liu WJ, Cui H, et al. The role of oral microbiota in cancer. Front Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1253025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irfan M, Delgado RZR, Frias-Lopez J. The oral microbiome and cancer. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.591088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10(3) doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang AH, Parsonnet J. Role of bacteria in oncogenesis. Clin Microbiol Rev. 2010;23(4):837–857. doi: 10.1128/CMR.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopinath D, Menon RK, Banerjee M, Su Yuxiong R, Botelho MG, Johnson NW. Culture-independent studies on bacterial dysbiosis in oral and oropharyngeal squamous cell carcinoma: a systematic review. Crit Rev Oncol Hematol. 2019;139:31–40. doi: 10.1016/j.critrevonc.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Gopinath D, Menon RK, Wie CC, et al. Differences in the bacteriome of swab, saliva, and tissue biopsies in oral cancer. Sci Rep. 2021;11(1):1181. doi: 10.1038/s41598-020-80859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Liu Y, Yang X, Li C, Song Z. The oral microbiota: community composition, influencing factors, pathogenesis, and interventions. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.895537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. 2019;10(4):486–496. doi: 10.1002/jrsm.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess S, Foley CN, Zuber V. Inferring causal relationships between risk factors and outcomes from genome-wide association study data. Annu Rev Genomics Hum Genet. 2018;19:303–327. doi: 10.1146/annurev-genom-083117-021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith GD. Mendelian randomization for strengthening causal inference in observational studies: application to gene × environment interactions. Perspect Psychol Sci. 2010;5(5):527–545. doi: 10.1177/1745691610383505. [DOI] [PubMed] [Google Scholar]
- 20.Weed DL, Hursting SD. Biologic plausibility in causal inference: current method and practice. Am J Epidemiol. 1998;147(5):415–425. doi: 10.1093/oxfordjournals.aje.a009466. [DOI] [PubMed] [Google Scholar]
- 21.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 22.Demmitt BA, Corley RP, Huibregtse BM, et al. Genetic influences on the human oral microbiome. BMC Genomics. 2017;18(1):659. doi: 10.1186/s12864-017-4008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Tong X, Zhu J, et al. Metagenome-genome-wide association studies reveal human genetic impact on the oral microbiome. Cell Discov. 2021;7(1):117. doi: 10.1038/s41421-021-00356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teles FRF, Alawi F, Castilho RM, Wang Y. Association or causation? Exploring the oral microbiome and cancer links. J Dent Res. 2020;99(13):1411–1424. doi: 10.1177/0022034520945242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu Y, Jiang L, Shui M, et al. Revealing the association between East Asian oral microbiome and colorectal cancer through Mendelian randomization and multi-omics analysis. Front Cell Infect Microbiol. 2024;14 doi: 10.3389/fcimb.2024.1452392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao C, Chen F, Li Q, Zhang W, Peng L, Yue C. Causal relationship between oral microbiota and epilepsy risk: evidence from Mendelian randomization analysis in East Asians. Epilepsia Open. 2024;9(6):2419–2428. doi: 10.1002/epi4.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boehm FJ, Zhou X. Statistical methods for Mendelian randomization in genome-wide association studies: a review. Comput Struct Biotechnol J. 2022;20:2338–2351. doi: 10.1016/j.csbj.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The Salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3(27) doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Liu Y, Zheng HJ, Zhang CP. The oral microbiota may have influence on oral cancer. Front Cell Infect Microbiol. 2020;9:476. doi: 10.3389/fcimb.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torralba MG, Aleti G, Li W, et al. Oral microbial species and virulence factors associated with oral squamous cell carcinoma. Microb Ecol. 2021;82(4):1030–1046. doi: 10.1007/s00248-020-01596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganly I, Yang L, Giese RA, et al. Periodontal pathogens are a risk factor of oral cavity squamous cell carcinoma, independent of tobacco and alcohol and human papillomavirus. Int J Cancer. 2019;145(3):775–784. doi: 10.1002/ijc.32152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muto M, Hitomi Y, Ohtsu A, et al. Acetaldehyde production by non-pathogenic Neisseria in human oral microflora: implications for carcinogenesis in upper aerodigestive tract. Int J Cancer. 2000;88(3):342–350. [PubMed] [Google Scholar]
- 35.Zhang WL, Wang SS, Wang HF, Tang YJ, Tang YL, Liang XH. Who is who in oral cancer? Exp Cell Res. 2019;384(2) doi: 10.1016/j.yexcr.2019.111634. [DOI] [PubMed] [Google Scholar]
- 36.Nieminen MT, Listyarifah D, Hagström J, et al. Treponema denticola chymotrypsin-like proteinase may contribute to orodigestive carcinogenesis through immunomodulation. Br J Cancer. 2018;118(3):428–434. doi: 10.1038/bjc.2017.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng RT, Sun Y, Zhou XD, et al. Treponema denticola promotes OSCC development via the TGF-β signaling pathway. J Dent Res. 2022;101(6):704–713. doi: 10.1177/00220345211067401. [DOI] [PubMed] [Google Scholar]
- 38.Morita E, Narikiyo M, Yano A, Nishimura E, Igaki H, Sasaki H. Different frequencies of Streptococcus anginosus infection in oral cancer and esophageal cancer. Cancer Sci. 2003;94(6):492–496. doi: 10.1111/j.1349-7006.2003.tb01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tateda M, Shiga K, Saijo S, et al. Streptococcus anginosus in head and neck squamous cell carcinoma: implication in carcinogenesis. Int J Mol Med. 2000;6(6):699–1402. doi: 10.3892/ijmm.6.6.699. http://www.spandidos-publications.com/10.3892/ijmm.6.6.699 [cited 2024 Aug 29]; Available from: [DOI] [PubMed] [Google Scholar]
- 40.Sasaki M, Yamaura C, Ohara-Nemoto Y, et al. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005;11(3):151–156. doi: 10.1111/j.1601-0825.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 41.Narikiyo M, Tanabe C, Yamada Y, Igaki H, Tachimori Y, Kato H. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 2004;95(7):569–574. doi: 10.1111/j.1349-7006.2004.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rai AK, Panda M, Das AK, et al. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch Microbiol. 2021;203(1):137–152. doi: 10.1007/s00203-020-02011-w. [DOI] [PubMed] [Google Scholar]
- 43.Panda M, Rai AK, Rahman T, et al. Alterations of salivary microbial community associated with oropharyngeal and hypopharyngeal squamous cell carcinoma patients. Arch Microbiol. 2020;202(4):785–805. doi: 10.1007/s00203-019-01790-1. [DOI] [PubMed] [Google Scholar]
- 44.Moritani K, Takeshita T, Shibata Y, Ninomiya T, Kiyohara Y, Yamashita Y. Acetaldehyde production by major oral microbes. Oral Dis. 2015;21(6):748–754. doi: 10.1111/odi.12341. [DOI] [PubMed] [Google Scholar]
- 45.Mizumoto A, Ohashi S, Hirohashi K, Amanuma Y, Matsuda T, Muto M. Molecular mechanisms of acetaldehyde-mediated carcinogenesis in squamous epithelium. IJMS. 2017;18(9):1943. doi: 10.3390/ijms18091943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pushalkar S, Ji X, Li Y, et al. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12(1):144. doi: 10.1186/1471-2180-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang CY, Yeh YM, Yu HY, Chin CY, Hsu CW, Liu H. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front Microbiol. 2018;9(862) doi: 10.3389/fmicb.2018.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fortoul MC, Kim E, Ardeljan AD, Frankel L, Takabe K, Rashid OM. The role of hemophilus influenzae infection and its relationship with colorectal cancer. World J Oncol. 2023;14(3):188–194. doi: 10.14740/wjon1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsen I, Yilmaz O. Possible role of Porphyromonas gingivalis in orodigestive cancers. J Oral Microbiol. 2019;11(1):1563410. doi: 10.1080/20002297.2018.1563410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McIlvanna E, Linden GJ, Craig SG, Lundy FT, James JA. Fusobacterium nucleatum and oral cancer: a critical review. BMC Cancer. 2021;21(1):1212. doi: 10.1186/s12885-021-08903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nearing JT, Douglas GM, Hayes MG, et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat Commun. 2022;13(1):342. doi: 10.1038/s41467-022-28034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santonocito S, Giudice A, Polizzi A, et al. A cross-talk between diet and the oral microbiome: balance of nutrition on inflammation and immune system's response during periodontitis. Nutrients. 2022;14(12):2426. doi: 10.3390/nu14122426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon RK, Gomez A, Brandt BW, et al. Long-term impact of oral surgery with or without amoxicillin on the oral microbiome-A prospective cohort study. Sci Rep. 2019;9(1):18761. doi: 10.1038/s41598-019-55056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mbemi A, Khanna S, Njiki S, Yedjou CG, Tchounwou PB. Impact of gene–environment interactions on cancer development. IJERPH. 2020;17(21):8089. doi: 10.3390/ijerph17218089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekula P, Del Greco M F, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27(11):3253–3265. doi: 10.1681/ASN.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gopinath D, Kunnath Menon R. Unravelling the molecular signatures in HNSCC: is the homogenous paradigm becoming obsolete? Oral Oncol. 2018;82:195. doi: 10.1016/j.oraloncology.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Zhou X, Hao Y, Peng X, et al. The clinical potential of oral microbiota as a screening tool for oral squamous cell carcinomas. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.728933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.