Abstract
Background
Biofilm formation in dental unit waterlines (DUWLs) and the consequent microbial contamination of dental chair unit (DCU) water is a significant challenge. The South African government has no explicit requirements for water quality supplied to DCUs or for disinfection protocols for DUWLs.
Aim
To assess bacterial water quality and presence of biofilm-associated organisms in DUWLs of open and closed system DCUs.
Methods
Standard water sampling was followed in accordance with the South African National Standard for drinking water (SANS 241:1) and used as reference for microbial water quality to measure heterotrophic plate counts (HPC) and total coliforms for possible water contamination. Pseudomonas aeruginosa and Legionella spp. are common opportunistic pathogens found in DUWL and were also assessed using selective media.
Results
HPC exceeded the national standard of <10 × 103 CFU mL−1 in water from both open and closed systems (1.48-6.94 × 104 CFU mL−1 and 1.71 × 104 CFU mL−1). P. aeruginosa was detected in fast handpieces, reservoir bottles, and distiller bottles of closed system DCUs. Legionella spp. (22 CFU mL−1) were present in the output water from one fast handpiece of an open system DCU. Internal surfaces of taps, fast handpieces, distiller bottles and reservoir bottles also exhibited mean HPC counts which exceeded the national standard. Total coliforms were identified in the fast handpieces of open system DCUs (5.09 × 103 CFU 100 mL−1) and distiller bottles (6.23 × 103 CFU 100 mL−1) of closed systems. P. aeruginosa (3.64 × 104 CFU mL−1), was detected on the internal surfaces of the municipal tap supplying water to open system DCUs as well as, internal surfaces of reservoir bottles (5.9 × 101 CFU 100 mL−1) and fast handpieces (1.5×101 CFU 100 mL−1) of closed system DCUs.
Conclusion
Contamination levels of DUWL water and surfaces of open and closed system DCUs were high, highlighting the need for national regulations of DUWL quality and decontamination protocols in South Africa.
Keywords: Dental unit waterlines, Water quality, Biofilm, Contamination, Microorganisms
Introduction
Globally, infection control continues to be one of the most critical issues in the provision of healthcare services. Infection prevention and the control of potential cross-infection are important if a risk-free environment for patients and healthcare workers is to be established in healthcare facilities in general and in oral healthcare facilities in particular.1 Oral healthcare providers and their patients are said to be at risk of infections caused by a plethora of microorganisms. Cross-infection can easily occur while oral healthcare procedures are performed.2 The risk of infection may occur directly through contact with blood, splatter, oral fluids, and other secretions, or indirectly through contact with contaminated instruments and environmental surfaces.3
Direct transmission of infection to patients, oral healthcare providers (OHCPs), and oral healthcare personnel may occur by exposure to contaminated dental chair unit (DCU) water through aspiration or contact with microorganisms present in aerosols or splatters of oral and respiratory fluids that are channelled through dental unit waterlines (DUWLs).4,5 Water is an indispensable commodity for the execution of oral healthcare procedures because it is used for the irrigation and cooling of both DCU ancillary equipment and tooth surfaces, while it is also used for oral rinsing. Water is used to prevent the tooth from overheating during cavity preparations or finishing by irrigating the tooth surfaces and it is also used for the rinsing the oral cavity. Water is supplied via the narrow-bore plastic tubing of dental unit waterlines to handpieces, three-in-one syringes, and ultrasonic scalers and supplied to DCUs either directly from a municipal source or via tanks or bottles connected to the DCU. The water travels from the source to the various handpieces of the DCU via waterlines which form an interconnected network of narrow bore plastic tubing.6,7
Dental chair unit water systems are classified as either open or closed based on the water source supplied to the DCU. The open system is directly connected to the municipal water supply while the closed system uses refillable storage tanks or bottled water reservoirs that are fitted to the DCU or placed nearby.8 DUWLs are equipped with a dual water supply system that permits the supply of only municipal water or reservoir bottle water, or both. The use of different types of water sources are regulated by a bypass button on the control panel and are selected based on the water volume required or is used as a system of choice by the OHCP.6
Open DCU systems that receive their water directly from municipal water supplies often contain low, but a diverse number of bacterial species. Municipal water with low bacterial counts enters DUWLs, the bacteria attach to the lumens of waterlines, and this eventually gives rise to multispecies biofilm. Biofilms are highly dynamic microbial communities that can attach to both abiotic and biotic surfaces. It is evident that biofilm formation in man-made tubing attracts microorganisms from the water that flows through them, resulting in biofilm formation and water contamination.9 The architecture of DUWLs consists of plastic tubing with extremely narrow diameters (2-3 mm) and a system that allows very slow flow rates in handpieces (2-10 mm per minute). These measures favour the deposition of organic nutrients and the adhesion of microorganisms to the surface of the DCU tubing, promoting the formation and proliferation of biofilm on the lumens of DCU waterlines.6,10 The bacteria and other microorganisms that form biofilm inside the DUWL tubing act as a primary reservoir for continuous contamination of the water used in oral healthcare procedures.10
DUWL surfaces and narrow tube diameters with their internal aqueous environment provide optimal conditions for the development of microbial biofilms. The source of microorganisms for biofilm formation in DUWLs may be microbial load of source water piped into a DUWL and the retraction of patients’ saliva into the waterline because of retraction valve failure.11 In Latvia it was observed that, within 5 days of DCU installation, microbial counts reached levels of 2.0 × 105 colony forming units per millilitre (CFU mL−1) in the water exiting handpieces.12
The majority of DCUs currently on the market have independent reservoir bottles as a default option, and this system is classified as a closed-water system.13 The reservoir isolates the DCU from the municipal water supply and draws fluid from a reservoir bottle holding the supplier's choice of water; or the OHCP can control the quality of the water that is being introduced into the DUWL system as independent reservoirs can be filled with sterile or distilled water or with water directly from the municipal supply (tap) in the oral healthcare facility.14 The quality of this water is dependent on the sanitation processes and schedules of the oral healthcare facility and can lead to contamination or even infection.14
Opportunistic pathogens like Pseudomonas aeruginosa and Legionella spp. are widely reported to be present in hospital water systems and for the fact that they contaminate DUWLs. Their presence as biofilm colonisers in DUWLs is of significance in the nosocomial infection of patients who may be immunocompromised, while they may also affect OHCPs and their personnel. These individuals are at risk of being infected with these opportunistic pathogens by cross-infection or when aerosols are dispersed into the environment by handpieces.15
The acceptable microbial quality of DCU water is therefore of fundamental importance in the treatment of patients and for OHCPs who perform oral healthcare procedures.16 Drinking water quality in South Africa is regulated by the Department of Water Affairs (DWA) in respect of the Water Services Act No. 108 of 1997 and related regulations promulgated under Section 9 of the Act: Norms and Oral Standards for Quality Water Services. The South African National Standard (SANS) 241:1 of 201517 Drinking Water Specification provides the minimum requirements for potable water to be considered safe for human consumption in this country. The South African government currently has no explicit requirements for the quality of water, which is supplied to DCUs, and neither has it issued an infection control policy that regulates or guides OHCPs to protect their patients and oral healthcare personnel. The National Health Policy also does not contain any regulations related to oral health aspects to curb the transmission of infectious diseases or for infection control issues related to the practice of oral healthcare.18
Infection prevention and control guidelines have been developed by the United States Centre for Disease Control and Prevention (CDC) through a careful, rigorous, evidence-based process overseen by the Health Infection Control Practice Advisory Committee (HICPAC) Division of Healthcare Quality Promotion of the National Centre for Infectious Diseases.19 The CDC recommends that water used for non-surgical oral healthcare procedures should be less than or equal to 500 CFU mL−1 of aerobic heterotrophic bacteria.20, 21, 22 The CDC further states that no more than 5% of water samples that are tested when monitoring DCUs should be contaminated with total coliforms.23 The CDC Guidelines are considered the most definitive Standard of Care for Infection Control to prevent the transmission of diseases from the patient to the OHCP, from the OHCP to the patient, and from patient to patient during oral healthcare procedures.18 The standard required by the American Dental Association (ADA) for water delivered to patients for non-surgical oral healthcare procedures for heterotrophic bacteria should be equal to, or not exceed, 200 CFU mL−1.24 The aim of this study was to assess the bacterial water quality in DCU waterlines and to determine the presence of biofilm-associated organisms in the waterlines of both open and closed systems in the DCUs.
Inclusion and exclusion criteria
A convenience sampling strategy was followed. Thus, all participants willing to partake in the study who completed the questionnaire were included in the study. INCLUSION: We conducted the study based on a voluntary basis – we did not have specific criteria except that the oral healthcare facility had to be in the Mangaung region and perform routine oral healthcare procedures. EXCLUSION: None.
Materials and methods
Study area
The water quality and biofilm-associated organisms that occur in dental unit waterlines in oral healthcare facilities in Mangaung, Free State Province, South Africa was the focus of this study. The study involved six private and government oral healthcare facilities to examine and evaluate the water quality of the dental chair units (DCUs) from both private and government operated oral healthcare facilities.
A total of six oral healthcare facilities were recruited to participate in the study. Three used an open-water system, (O facilities), which received water directly from the municipal mains. Two DCUs were investigated at oral healthcare facility O1, four DCUs were investigated at O2, and four DCUs were investigated at O3. Water was supplied directly from the municipal mains to these oral healthcare facilities’ DCUs. The remaining three facilities all used a closed system, (C facilities), where the reservoir bottles were filled with distilled water and attached to the DCUs or fitted at a designated location within the oral healthcare facility to ensure water supply. At the C-facilities, the DCUs used a closed system. Three DCUs were sampled at C1, five DCUs were sampled at C2, and three DCUs were sampled at C3. A different number of dental chair units (DCUs) were included in sampling as indicated in Figure 1.
Fig. 1.
Schematic representation of open (purple) and closed (green) system facilities sampled. Chair numbers at each facility are designated Oxx and Cxx for open and closed systems, respectively. Sampling sites included the main tap, where water is supplied by the municipality, and handpieces at all facilities and additional sampling points (distiller- and reservoir bottles) at facilities with closed systems. The ages of the chairs varied from 5 to 30 years. No further information was provided regarding servicing or maintenance of the DUWLs relative to the age of the DCUs.
The study followed a quantitative research approach, and a purposeful method of sampling was executed (Figure 1). Oral healthcare providers of an already existing network in Mangaung were approached as part of the process of informed consent to carry out this study at their oral healthcare facilities. The dental chair and ancillary equipment at all oral healthcare facilities are used for routine general oral healthcare procedures. These procedures include oral examinations, dental extractions, endodontics, prosthodontic procedures, restorations and prophylaxis among others. The OHCPs received a letter informing them of the research project and describing the aims and objectives of the study. They were assured of their privacy and anonymity throughout the study and in subsequent reports and articles.
Sampling protocol
Oral healthcare facilities participating in the study signed a letter of consent and completed a set of questions. The consent letter explained that the study would test the water that was used within the oral healthcare facility and selected DCUs and, more specifically, that the bacterial water quality of the water in the waterlines would be tested for biofilm-associated organisms. Biofilm swabs would be taken of the municipal water supply, distiller tanks, reservoir bottles, and fast handpiece to test for the presence of biofilm-associated organisms. It was also explained that a set of questions will be asked about the age of the DCU being sampled as well as any cleaning regimes and chemicals that are used for disinfection.
Collection of water samples
The exterior surfaces of taps, fast handpieces and DCU tubing were disinfected with an alcohol swab to avoid any other contamination before sampling commenced.25 Water samples (500 mL) were collected aseptically in wide-mouthed sterile glass bottles. The municipal water source water (tap) to the DCU in the oral healthcare facility and water exiting the fast handpieces was allowed to run for ten seconds before a water sample was collected.26 It was noted that distilled water was used to fill reservoir bottles that were attached to the closed-water system DCUs to supply water to the handpieces. Water samples were additionally collected from distiller bottles as well as from reservoir bottles that were used for closed-water system DCUs. The disinfectant property of the residual chlorine in the water samples was neutralised with the addition of 0.5 mL of 1% sodium thiosulphate at a final concentration of 0,01%.27 Water samples were placed in a cooler box on ice and sustained at a temperature not exceeding 4°C28 and transported to the Centre for Applied Food Sustainability and Biotechnology (CAFSaB) laboratory at the Central University of Technology (CUT) and processed within 24 hours. Legionella falls within the Animal Biosafety Level-2 (ABSL-2) category and was sent to SANAS accredited Aspirita Laboratories for testing.
Collection of biofilm samples
The conventional swabbing method of field testing with a cotton swab on surfaces like stainless steel, wood, and plastic for detecting pathogenic bacteria was used.29,30
The exterior surfaces of taps, fast handpieces and DCU tubing were disinfected with an alcohol swab to avoid any other contamination before sampling commenced.25 Swab samples were collected from the internal surfaces of distal outlets of taps and the fast handpieces of open and closed system DCUs. To swab the fast handpieces, the coupling was removed to expose the surface of the waterline that supplied the fast handpiece with water. Distilled water was used to fill the reservoir bottles that were attached to the closed-water system DCUs to supply the handpieces with water for use during oral healthcare procedures. Swab samples were therefore additionally collected from internal surfaces of empty distiller bottles and reservoir bottles in the case of closed-water system DCUs. Water that is distributed by the municipality to the public is used to fill distiller bottles to distill water used in reserviour bottles. Reserviour bottles with distilled water is attached to closed system DCUs and it is naturally assumed that this water has been tested prior to public distribution and that it consequently meets the SANS 241:117 standard for microbial water quality. The microbial load of distilled water that is used to fill reservoir bottles for closed system DCUs should be the same as, if not lower than that of municipal water.
Swabs of internal surfaces of sampling sites were collected aseptically using a pre-moistened cotton swab (COPAN SRK) containing 1% sodium thiosulphate using a perpendicular back and forth motion. The tip of the swab was broken off and suspended in 1 mL of sterile buffered saline containing 1% sodium thiosulphate with a final concentration of 0,01% to neutralise residual chlorine.23 Swab samples were placed in a cooler box on ice with a temperature not exceeding 4°C. Samples were transported to the CAFSaB laboratory at the CUT and processed within 24 hours. The spread plate method was used for the propagation and enumeration of swab samples for heterotrophic bacteria, total coliforms, and Pseudomonas aeruginosa from both open and closed system DCUs.31
Microbial analysis
Heterotrophic plate counts
A 1 mL sample of water, or buffered saline containing the swab tip, in an Eppendorf tube was agitated by vortexing for 15 seconds at the highest speed.11 A volume of 0,1 mL of the water sample was platted onto R2A agar using the constant function of the easySpiral Pro automatic plater (interscience) and spreading it evenly over the agar.32 This procedure was performed in duplicate for all water samples. Plates were incubated at a temperature of 27°C for 5 days.33 Colonies were counted using the Scan 1200 Automatic HD colony counter (interscience) and reported as colony forming units per millilitre (CFU mL−1), as stipulated in SANS 241:1.17
Coliforms and Escherichia coli
The membrane filtration technique was performed for the propagation and enumeration of total coliform bacteria. A volume of 100 mL of each water sample was filtered through a Millipore Filtration assembly with a sterile membrane nitrocellulose grid filter with a 0.45 µm pore size. The water was filtered using a vacuum venturi system attached to a tap. Membrane filters were placed grid side facing up on RAPID’E. coli 2 agar (BIO-RAD Laboratories). RAPID’E. coli 2 agar for water testing is composed of the RAPID’E. coli 2 chromogenic medium base and a selective supplement. Agar plates were incubated at 37°C for 24 hours. Resulting colonies on the surface of the membrane filter were counted. Purple to violet colonies represented Escherichia coli colonies while and green to blue colonies represented coliform colonies. Colonies were counted collectively to obtain total coliform counts. Colonies were counted with the Scan 1200 Automatic HD colony counter (interscience) and reported as colony forming units per 100 millilitre (CFU mL−1) as stipulated in SANS 241:1.17 The spread plate method was used for the propagation and enumeration of swab samples as described for heterotrophic plate counts.
Propagation and enumeration of Pseudomonas aeruginosa
Propagation and enumeration of P. aeruginosa were completed using the membrane filtration technique similar as described above for detection of total coliforms. The membrane filter was placed grid side up onto Cephalothin-Sodium Fusidate-Cetrimide agar (CFC). The plates were incubated at 30°C for 48 hours. The plates were placed under an ultraviolet (UV) light after their incubation period for the detection of P. aeruginosa colonies which could be identified by green fluorescence. Colonies were counted with the Scan 1200 Automatic HD colony counter (interscience). P. aeruginosa was propagated and enumerated from swab samples as described for total coliforms and plated on the same CFC selective media use for membrane filtration.
Propagation and enumeration of Legionella spp.
The membrane filtration technique was used for the propagation and enumeration of Legionella spp. using SANS 7218, which presents the general requirements for and guides microbiological examinations, and ISO 11731 of 2017 (water quality enumeration of Legionella). Water samples were collected aseptically in wide-mouthed sterile glass bottles with a volume of 200 mL. Water samples were collected from open and closed system fast handpieces. Legionella spp. were isolated using the membrane filtration method. A volume of 100 mL of each water sample was filtered through a Millipore Filtration assembly with a sterile membrane nitrocellulose grid filter with a 0.45 µm pore size. The filter was subject to acid treatment, and then plated onto non-selective charcoal yeast extract (CYE) agar, followed by the addition of a selective GVPC (glycine, vancomysin, polymyxinB, cycloheximide) agar. Plates were incubated for up to 10 days at 36°C. Positive colonies fluoresced when placed under a UV lamp at 360 nm. Legionella was further confirmed using Matrix Assisted Laser Desorption Ionisation Time-of-Flight (MALDI-TOF) technology on the VITEK MS (BioMerieux).
Questionnaire administration
A list of questions was administered to the manager of each oral healthcare facility, or their representative, to determine the number of DCUs in each facility and to gather information about the cleaning procedures each facility employed to ensure the health safety of patients and personnel. The aim of the questionnaire was to establish baseline information for all the facilities who volunteered to participate in the study. The questionnaires had been prepared in advance, adhered to ethical procedures, and were completed voluntarily. The information gathered from the questionnaire responses was used to augment the findings regarding the cleaning and decontamination protocols, if any, that were followed at each facility.
Data analysis
Descriptive statistics were conducted. Levels of microbial contamination were summarised using descriptive statistics (mean, standard deviation, minimum, median, maximum) separately by type, location, and system. SAS procedure TABULATE (SAS, 2017) software as well as IBM SPSS Statistics Version 25 were used for statistical analyses.
Comparison of levels of contamination of heterotrophic plate count (HPC) samples
The levels of contamination with HPC were compared between open and closed systems using a mixed model. SAS procedure MIXED (SAS, 2017) software was used for mixed model analysis. From the mixed model, the mean contamination levels were estimated for the two systems, as well as the mean difference between the two systems and the associated 95% confidence interval (CI) and p-value. Where the p-value was less than .05 (<.05), the null hypothesis was rejected. and it could be concluded that there was a difference in the mean count between the 2 systems.
Comparison of levels of contamination of total coliforms and Pseudomonas aeruginosa
Fisher's Exact test was used to analyse the comparison of the proportions of compliant samples between the two systems. SAS procedure SUMMARY (SAS, 2017) software was used for mixed model analysis. The null hypothesis was that the relative proportions of one variable (closed system) were independent of the second variable (open system). The null hypothesis was that the probability of getting the same number of chairs meeting the SANS 241:117 was the same whether the open or closed system was used. Therefore, if the p-value was <.05, the null hypothesis was in favour of the alternative, which would suggest a difference between the two variables, that is, there was a difference between the two systems that met the SANS 241:1.17
Results
The microbial contamination of water samples and presence of biofilm associated organisms from swab samples were collected at six oral healthcare facilities in Mangaung, Free State, South Africa. Three oral healthcare facilities used an open-water system where samples were collected from ten DCUs at the outlets of municipal taps and dental handpieces. The other samples were collected from three facilities that used closed system DCUs and samples collected from outlets of municipal taps, handpieces, distiller bottles and reservoir bottles.
Water and swab samples were measured against the South African National Standard, SANS 241:1 (2015)17 for HPC microbial drinking water quality and total coliforms. Heterotrophic plate counts in both water and swab samples indicated mean counts significantly exceeding the SANS 241:1,17 which stipulates that water should not contain more than 1 × 103 CFU mL−1 of heterotrophic bacteria. The mean HPC count for water quality in closed system DCUs was the highest, 1.74 × 104 CFU mL−1 for municipal tap water and 6.93 × 104 CFU mL−1 in water exiting the handpieces. The mean HPC for water quality of open system municipal tap water was 1.48 × 104 CFU mL−1 and 3.52 × 104 CFU mL−1 for water exiting handpieces. There was a statistical significant difference (p = .040) between the municipal water of open and closed system DCUs. The mean HPC of closed system DCUs of distiller bottles was 4.76 × 104 CFU mL−1 and 5.92 × 104 CFU ml1 for reservoir bottles.
The high HPCs of the supply water suggest optimal conditions, the opportunity for biofilm growth, and the proliferation of opportunistic pathogens. These were indicated by the microbial load detected on the internal surfaces of municipal taps, distiller bottles, reservoir bottles, and handpieces. Inner municipal tap surfaces of open system DCUs (1.47 × 104 CFU mL−1) was higher than closed system DCUs (4 × 103 CFU mL−1). The two DCU systems were compared for biofilm associated with HPC, and the one order magnitude of difference between the two systems was statistically significant (p = .013). The inner surfaces of closed system DCU handpieces were however higher (3.96 × 104 CFU mL−1) than that of open system handpieces (1.39 × 104 CFU mL−1) and exceeded the SANS 241:117 recommendation for HPC of less than 1 × 103 CFU mL−1. When the HPCs of the inner surfaces of fast handpieces for the two DCU systems were compared, the mean HPC did not differ significantly (p = .270). The inner surfaces of reservoir bottles and distiller bottles were also not compliant with the SANS 241:1 recommendation for HPC of 1×103 CFU mL−1.17 The respective counts were 8.7 × 104 CFU mL−1 and 2.08 × 104 CFU mL−1. Results for the two DCU systems were compared, and it was found that HPCs for the inner surfaces of municipal taps of the closed system were lower than that of the inner surfaces of municipal taps of open system DCUs. The one order magnitude of difference between the two systems was statistically significant (p = .013).
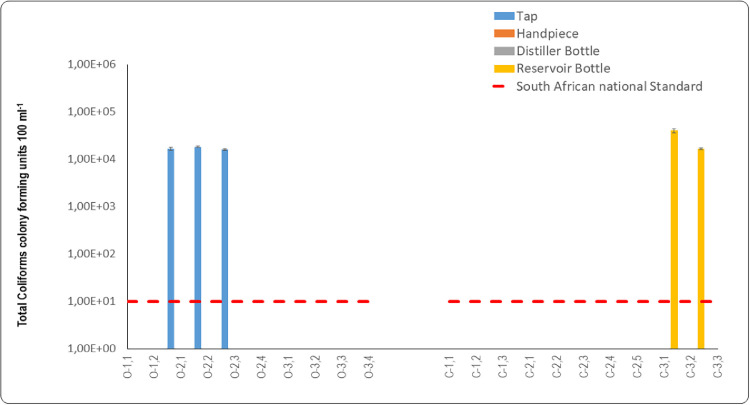
Total coliforms were determined as an indicator organism of contamination and possible detection of potentially pathogenic bacteria in the DCUs at the various sampling sites. The South African National Standard, SANS 241:1, for total coliforms stipulates that water should not contain more than 1 × 101 CFU 100 mL−1 considered safe for human consumption.17 Based on the measurement of the microbial load of total coliform bacteria, the findings indicated that municipal taps supplying closed system DCUs (9 × 101 CFU 100 mL−1) and water exiting handpieces (8.63 × 101 CFU 100 mL−1) did not comply with SANS 241:1.17 The municipal taps (9 × 100 CFU 100 mL−1) supplying closed system DCUs were compliant with SANS 241:1.17 However, the handpieces (6 × 101 CFU 100 mL−1) exceeded the recommendation for total coliforms of 1 × 101 CFU 100ml−1. Reservoir bottles and distiller bottles with total coliform counts of 6 × 101 CFU 100 mL−1 and 1.36 × 101 CFU 100 mL−1 exceeded the SANS 241:1 limitation of 1 × 101 CFU 100 mL−1.17 The mean total coliform counts of open and closed system DCUS were compared for the inner surfaces of taps that supplied source water to the DCUs. The inner surfaces of municipal tap swab samples differed significantly (p = .032) and the inner surfaces of the municipal taps of open system DCUs displayed total coliform counts of 5.09 × 103 CFU 100 mL−1, while the municipal taps of the closed system DCUs did not have any total coliforms present on their inner surfaces. The inner surfaces of the fast handpieces of both the open and closed system DCUs did not contain any total coliforms. Total coliforms were found present on the inner surfaces of reservoir bottles, 6.23 × 103 CFU 100 mL−1, and exceeded the SANS 241:1 recommendation of 1 × 1 × 101 CFU 100 mL−1.17 No total coliforms were detected on the inner surfaces of reservoir bottles.
Opportunistic pathogens such as Pseudomonas aeruginosa and Legionella spp. are prevalent in dental unit water and waterlines. However, there are no legislated parameters relating to the presence of these organisms and microbial water quality, which is an oversight as their presence can encourage the growth of other microorganisms whose presence contravenes the limitations for microbial water quality.34 P. aeruginosa was present in the source water and handpieces of open system DCUs, while no P. aeruginosa was detected in the municipal tap water of closed system DCUs. P. aeruginosa was however present in the water exiting handpieces (1.41 × 10° CFU 100 mL−1), reservoir bottles (3.80 × 10° CFU 100 mL−1) and distiller bottles (1.5 × 101 CFU 100 mL−1) of closed system DCUs.
The comparison of the inner surfaces of the municipal taps supplying source water to open and closed system DCUs indicated a statistical significance difference (p = .015), where the inner surfaces of municipal taps of open system contained P. aeruginosa (3.64 × 104 CFU 100 mL−1) and the inner surfaces of closed system DCUs contained no P. aeruginosa which likely indicated the presence of biofilm on the inner tap surfaces of open system DCUs. The inner surfaces of fast handpieces showed no statistical significant difference (p = .173), although the fast handpieces of closed system DCUs showed the presence of P. aeruginosa with a count of 1.5 × 10° CFU 100 mL−1. This is an indication that biofilm could have been present in the DUWLs of the closed system DCUs. Swab samples from the inner surfaces of reservoir bottles had a microbial count of 5.91 × 101 CFU 100 mL−1 and P. aeruginosa was not detected in the distiller bottles.
Only the water exiting handpieces were examined for Legionella spp., and it was detected in one open system DCU (2.2 × 100 CFU 100 mL−1).
Discussion
Bacterial water quality of dental unit waterlines
Heterotrophic plate counts
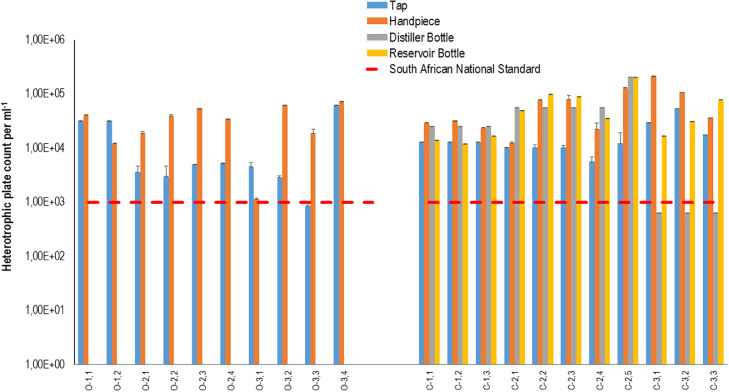
The study found that the water sourced by the municipality (municipal tap water) used to supply both the open and closed system DCUs exceeded the required SANS 241:117 heterotrophic bacteria limit for safe drinking water (Figure 2). Zhang and co-workers35 argued that elevated HPCs in municipal water supply may serve as a precursor for further bacterial growth within DUWLs where they propagate the formation of biofilm.
Fig. 2.
Graphic representation of data from the oral healthcare facilities for heterotrophic plate counts for water quality of open and closed system dental units. Water samples were taken from taps (blue) and fast handpieces (orange) in open system DCUs (O1.1-O3.4). Water samples were taken from taps (blue), distiller bottles (grey), reservoir bottles (yellow) and fast handpieces (orange) used in closed system DCUs (C1.1-C3.3). The dashed lines represent recommendations by the South African National Standard, SANS 241:1 (2015) for drinking water quality limitations for heterotrophic bacteria per millilitre. Water samples should not contain more than 1 × 103 CFU mL−1 of heterotrophic bacteria.
The municipal tap water that supplies dental chair units with water for functioning of fast handpieces during oral healthcare procedures contained high heterotrophic bacterial counts. It was therefore reasonable to assume that the HPCs from the fast handpieces fed by this water would also exceed the allowed HPC SANS 241:1 limit of 1 × 103 CFU mL−1.17 This was indeed the case, as the HPC values obtained from the fast handpieces of open system DCUs ranged from 1.15×103 CFU mL−1 to 7.20×104 CFU mL−1, and those from the closed system DCUs ranged from 1.24×104 CFU mL−1 to 2.11×105 CFU mL−1 (Figure 2). For DUWLs, earlier studies reported an average viable microbial count for water exiting dental handpieces of 1×105 CFU mL−1.36,37
There was no statistically significant difference between the water exiting distal outlets of fast handpieces in open and closed system DCUs (p = .099). The HPCs of fast handpieces in both open and closed system DCUs were high and exceeded the SANS 241:117 limitation, suggesting that further contamination occurred in the DCUs of both systems. Similar to the range of HPCs in this study, a study conducted on the water of distal outlets of the three-in-one syringes of DCUs at the oral health centre of Sefako Makgatho Health Sciences University (a dental school and a comprehensive care referral hospital on the outskirts of Pretoria, South Africa) found that the baseline mean bacterial count was 1.8 × 104 CFU mL−1 in the distal outlet water.38
The water used to supply closed system DCUs was subject to a distillation process. The microbial load of distilled water that is used to fill reservoir bottles for closed system DCUs should be the same as, if not lower than that of municipal water. However, the findings showed this was not the case, as there was statistical significance between the municipal tap water supplied to closed system DCUs and the municipal tap water supplied to distiller bottles. Facility C1 and C2 where the HPCs of water in the distilled bottles were higher than those of the source water, and this finding was thus statistically significant (p = 0,004). Additionally, the HPCs of the water distilled at facility C1 and C2 may also have been elevated because of the initial microbial load in the source water, thus rendering the distillation process ineffective in eliminating or reducing the microbial load in the water that would be used in the reservoir bottles. Similarly, the one order magnitude of difference regarding municipal tap water supplied to open system DCUs and closed system DCUs, and the water exiting the distal outlets of fast handpieces, was statistically significant for heterotrophic bacteria.
The microbial load in distiller and reservoir bottles should be different, as reservoir bottles should have low to no microbial load. The HPCs for the water in the distiller bottles at facility C3 all complied with SANS 241:117 for HPCs after the distillation process. However, the water in all the reservoir bottles of the DCUs at facility C3 displayed elevated HPC values compared to those of the water in the distiller bottles (Figure 2). Although there was an observed difference in HPCs between the water in distilled and reservoir bottles, this difference was not statistically significant (p = 0.052). Based on the information received from the oral healthcare practitioner at facility C3 (Table 1), the DUWLs were flushed daily, and shock treated weekly. However, regardless of measures to disinfect the waterlines, the reservoir bottles were contaminated and therefore most likely contained biofilm. It was observed that reservoir bottles were disconnected from DCUs and filled at facility C3 without using gloves, which could have contributed to the contamination of the reservoir bottles. The elevated HPCs may have occurred because of biofilm present in the distiller bottles due to ineffective disinfection or lack of aseptic handling.13
Table 1.
Results of questionnaire administered to OHCPs at the various dental healthcare facilities.
| Facility | Chair Number | Age of dental unit (in years) | Is water distilled | What water source is used to fill reservoir bottles | Do you use a cleaning regime to clean DUWLs | If yes, list the chemicals used to clean DUWLs | Indicate the frequency of cleaning regime for the DCU (hours/days/months) | Areas of concern or comments by OHCPs |
|---|---|---|---|---|---|---|---|---|
| Open System DCUs | ||||||||
| 1 | O-1.1 | 5+ | N/A | N/A | Yes | Sodium hypochlorite (bleach) | Daily at the beginning and end of each working day | There are times when the water coming from the taps in the clinical areas are not clean. Sometimes the water is brownish. |
| 1 | O-1.2 | 5+ | N/A | N/A | Yes | Sodium hypochlorite (bleach) | Daily at the beginning and end of each working day | There are times when the water coming from the taps in the clinical areas are not clean. Sometimes the water is brownish. |
| 2 | O-2.1 | 6+ | N/A | N/A | Yes | Qualiclean Sodium hypochlorite |
Daily at the beginning and end of each working day | Handpieces are autoclaved along with cold sterilisation |
| 2 | O-2.2 | 6+ | N/A | N/A | Yes | Qualiclean Sodium hypochlorite |
Daily at the beginning and end of each working day | Handpieces are autoclaved along with cold sterilisation |
| 2 | O-2.3 | 15 | N/A | N/A | Yes | Qualiclean Sodium hypochlorite |
Daily at the beginning and end of each working day |
Chair is not used as frequently as other chairs in the clinic. Chair is on the 'older' side of the clinic. Handpieces are autoclaved along with cold sterilisation |
| 2 | O-2.4 | 6+ | N/A | N/A | Yes | Qualiclean Sodium hypochlorite |
Daily at the beginning and end of each working day | Handpieces are autoclaved along with cold sterilisation |
| 3 | O-3.1 | 7 | N/A | N/A | Yes | Steri201 Quaternary Ammonium Compound/ Biguanide blend (99,3%) | Oral healthcare provider did not comment | None |
| 3 | O-3.2 | 7 | N/A | N/A | Yes | Steri201 Quaternary Ammonium Compound/ Biguanide blend (99,3%) | Oral healthcare provider did not comment | None |
| 3 | O-3.3 | 7 | N/A | N/A | Yes | Steri201 Quaternary Ammonium Compound/ Biguanide blend (99,3%) | Oral healthcare provider did not comment | None |
| 3 | O-3.4 | 7 | N/A | N/A | Yes | Steri201 Quaternary Ammonium Compound/ Biguanide blend (99,3%) | Oral healthcare provider did not comment | None |
| Closed System DCUs | ||||||||
| 4 | C-1.1 | 30 | Yes | Distilled | Yes | Diluted Milton (Active ingredient hypo chloride) | Every 3 months | None |
| 4 | C-1.2 | 30 | Yes | Distilled | Yes | Diluted Milton (Active ingredient hypochlorite) | Every 3 months | None |
| 4 | C-1.3 | 30 | Yes | Distilled | Yes | Diluted Milton (Active ingredient hypochlorite) | Every 3 months | None |
| 5 | C-2.1 | 5+ | Yes | Distilled | Yes | E20 waterline treatment. Hydrogen peroxide 1-2% | Daily at the beginning and end of each day. Monthly shock treatment | There is a distilled water tank in each clinical area from which the reservoir bottles are filled. |
| 5 | C-2.2 | 5+ | Yes | Distilled | Yes | E20 waterline treatment. Hydrogen peroxide 1-2% | Daily at the beginning and end of each day. Monthly shock treatment | There is a distilled water tank in each clinical area from which the reservoir bottles are filled. |
| 5 | C-2.3 | 5+ | Yes | Distilled | Yes | E20 waterline treatment. Hydrogen peroxide 1-2% | Daily at the beginning and end of each day. Monthly shock treatment | There is a distilled water tank in each clinical area from which the reservoir bottles are filled. |
| 5 | C-2.4 | 5+ | Yes | Distilled | Yes | E20 waterline treatment. Hydrogen peroxide 1-2% | Daily at the beginning and end of each day. Monthly shock treatment | There is a distilled water tank in each clinical area from which the reservoir bottles are filled. |
| 5 | C-2.5 | 5+ | Yes | Distilled | Yes | E20 waterline treatment. Hydrogen peroxide 1-2% | Daily at the beginning and end of each day. Monthly shock treatment | This chair uses a different distiller than the other chairs in the oral healthcare facility. Water is poured into the reservoir bottle. |
| 6 | C-3.1 | 6 | Yes | Distilled | Yes | San-O-Dent (Water, sodium chloride, HOCl - Hypochlorous Acid) | Flush beginning and end of day. Weekly clean/shock |
It was observed that the protocol for filling reservoir bottles from distiller bottles was that the distiller bottle cap was placed face down on the counter. The entire contents of the distiller bottle were not emptied into the reservoir bottle and the distiller bottle was closed for later use. The RB was removed from O3.1, and dental assistant did not wear gloves when removing or replacing the RB to the DCU |
| 6 | C-3.2 | 6 | Yes | Distilled | Yes | San-O-Dent (Water, sodium chloride, HOCl - Hypochlorous Acid) | Flush beginning and end of day. Weekly clean/shock |
|
| 6 | C-3.3 | 6 | Yes | Distilled | Yes | San-O-Dent (Water, sodium chloride, HOCl - Hypochlorous Acid) | Flush beginning and end of day. Weekly clean/shock |
|
HPCs for reservoir bottles ranged between a minimum count of 1.20×104 CFU mL−1 to a maximum count of 2.05×105 CFU mL−1. Counts for handpieces displayed a similar range with a minimum of 1.24×104 CFU mL−1 and a maximum of 2.11×105 CFU mL−1. These data support the suggestion that the distillers were not functioning optimally, perhaps because the initial microbial load was too high for the distillation process to have eliminated all microbes in the water supply. In addition, the reservoir bottles may have been contaminated due to improper handling, ineffective decontamination and possibly contained biofilm.
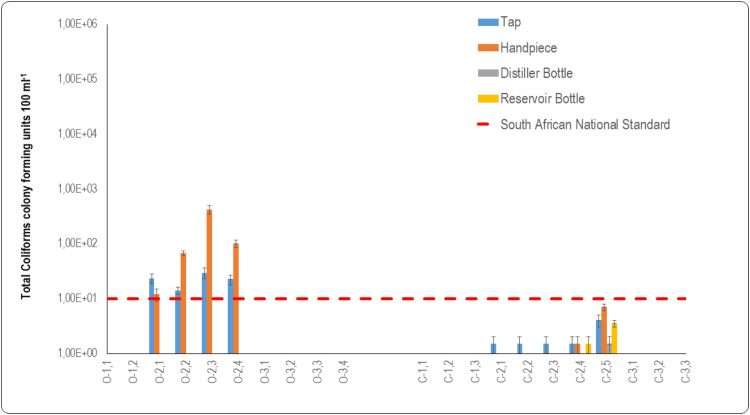
Total coliforms as indicators of water quality
Mean total coliform counts recorded for municipal tap water supplied to the open system DCUs were: O2.1 = 2.35 × 101 CFU 100 mL−1, O2.2 = 1.40 × 101 CFU 100 mL−1, O2.3 = 2.95 × 101 CFU 100 mL−1, and O2.4 = 2.25 × 101 CFU 100 mL−1. These levels did not comply with the SANS 241:117 limit for total coliform bacteria of 1 × 101 CFU 100 mL−1 in water (Figure 3). The presence of coliforms in the source water that is channelled to DCUs can serve as a precursor for the formation of biofilm in DCUs’ DUWLs, where these organisms attach to waterline lumens and form biofilm colonies that further multiply in DUWLs. Water samples of municipal taps of open system DCUs at O1.1, O1.2, O1.3 to O3.4 did not contain any total coliforms. The water samples of municipal taps of closed system DCUs at C1.1 to C1.3 and C3.1 to C3.3 similarly did not contain total coliforms and were compliant with the SANS 241:117 for total coliforms which should not exceed 1 × 101 CFU 100 mL−1. The one order magnitude of difference between tap water used in open system DCUs and in closed system DCUs was statistically significant (p = .043). When comparing the 2 DCU systems, the tap water supplying open system DCUs had higher total coliform counts than those of the closed system DCUs. However, there was no statistically significant difference between the tap water samples of the two DCU systems (p = .669).
Fig. 3.
Graphic representation of data from oral healthcare facilities for total coliform counts for water quality of open and closed system dental units. Water samples were taken from taps and fast handpieces in open system dental chair units (O1.1-O3.4). Water samples were also taken from taps, distiller bottles, reservoir bottles, and fast handpieces of closed system dental chair units (C1.1-C3.3). The dashed red line represents the SANS 241:1 [17] limit for drinking water quality for total coliforms per 100 mL.
The mean coliform counts for the fast handpieces of the DCUs were: O2.1 = 1.2 × 101 CFU 100 mL−1, O2.2 = 6.7× 101 CFU 100 mL−1, O2.3 = 4.19 × 102 CFU 100 mL−1, and O2.4 = 1.0×102 CFU 100 mL−1. These results indeed displayed total coliform counts exceeding the limitation as specified by SANS 241:117 for total coliforms that should not exceed 1 × 101 CFU 100 mL−1 of total coliform bacteria (Figure 3). The mean total coliform count for the fast handpiece of DCU O2.1 (1.20 × 101 CFU 100 mL−1) was lower than that of the source water supplied to this DCU (2.35 × 101 CFU 100 mL−1) (Figure 3). The lower total coliform count in this fast handpiece could be attributed to flushing of the DUWL system before sampling, or the decontamination of the waterline prior to sampling. In a study of 16 private and 10 government dental healthcare facilities in Bushehr, water samples taken from the municipal water distribution system and two sites of DUWLs, including the three-in-one syringes and high-speed handpieces results revealed that total coliform samples were negative in all municipal water samples, while 25% of high-speed handpieces and 25.8% of three-in-one syringe samples tested positive for total coliforms.35 In this instance, water contamination may have been caused by total coliforms that were present in the source water (Figure 3). Water samples measured from handpieces of open system DCUs at O1.1, O1.2, O1.3 to O3.4 did not contain any total coliforms. The water samples measured from handpieces of closed system DCUs at C1.1 to C1.3 and C3.1 to C3.3 similarly did not contain total coliforms.
Total coliform counts that were recorded for the closed system DCUs were all within the limits of 1 × 101 CFU 100 mL−1, as regulated by SANS 241:117 for total coliforms in drinking water (Figure 3). However, despite these counts falling below this limitation, total coliforms were detected in the municipal tap water of the closed system DCUs: C2.1, C2.2, C2.3, C2.4 = 1.5 × 101 CFU 100 mL−1, C2.2 = 1.50 × 101 CFU 100 mL−1 and C2.5 = 4.0 × 101 CFU 100 mL−1. Water in most of the distiller bottles was compliant with SANS 241:1.17 Total coliforms were present in the water of one DCU (C2.5 = 1.5 × 101 CFU 100 mL−1), but this level was below the SANS 241:117 limit.
There was a noticeable increase in the mean count of total coliforms in the reservoir bottle water of DCU C2.5 (Figure 3). It is reasonable to assume that the presence of the total coliforms in this reservoir bottle was because both the source water and the distiller bottle contained total coliforms. According to information presented in Table 1, an oral healthcare practitioner reported daily flushing and weekly shock treatment of the DUWLs. However, despite these protocols there was still contamination by total coliforms in the reservoir bottles originating from the source water.
The total coliform count in the reservoir bottle of DCU C2.5 (3.50×10° CFU 100 mL−1) was within the SANS 241:117 limit (Figure 3). The contamination in this instance may have resulted because of lax hand hygiene protocols and improper handling of the reservoir bottle when it was removed from the DCU to refill it with distilled water and when reattached to the DCU. The opportunity for such contamination can occur when the reservoir bottle on the dental unit is being filled or removed, or when the water source bottles are handled or being prepared.13,39,40
The water emitted from all the fast handpieces in the closed system DCUs complied with the SANS 241:117 limitation for total coliforms of 1 × 101 CFU 100 mL−1 (Figure 3). The presence of these coliforms may be attributed to the introduction of coliform bacteria by the contaminated reservoir bottles that were supplying these fast handpieces with water, and by inappropriate handling procedures. Dreyer and Hauman41 demonstrated that internal surfaces of dental handpieces became contaminated during normal oral procedures, with waterlines attached to the handpiece displaying the densest contamination. They concluded that autoclaving handpieces could possibly be the only effective way to sterilise both internal and external surfaces. A study that was conducted in Brazil on 30 DCUs collected samples from three-in-one syringes, high-speed handpieces coupled to tubing, the tubing of high-speed handpieces without them being coupled, water reservoir bottles, and the source water supplying these reservoir bottles. The findings revealed that total coliforms were detected in nine out of thirty (30%) water samples.26
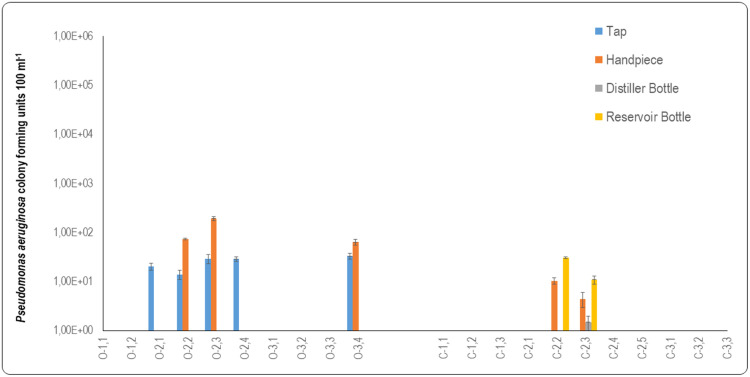
Pseudomonas aeruginosa detection of water quality
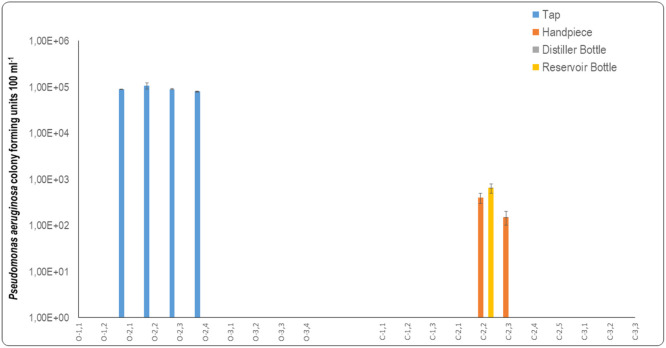
P. aeruginosa was present in the source water from municipal taps and handpieces of open system DCUs, while none was detected in the municipal tap water of closed system DCUs (Figure 4). The presence of P. aeruginosa in the municipal tap water supplying open system DCUs can act as an initiator for colonisation and subsequent biofilm formation in open system DCUs. Moreover, the safety of the water that exists the fast handpiece can be greatly compromised if the municipal tap water supplied to the DCUs is contaminated from the onset.
Fig. 4.
Graphic representation of data from oral healthcare facilities for Pseudomonas aeruginosa counts to determine the water quality of open and closed system DCUs. Water samples were taken from taps and fast handpieces for open system dental DCUs (O1.1-O3.4). Water samples were taken from taps, distiller bottles, reservoir bottles, and fast handpieces for closed system DCUs (C1.1-C3.3).
Water supplied to the open system DCUs for chairs O2.1, O2.2, O2.3 and O3.4 all contained P. aeruginosa (Figure 4). It can be assumed that, because the water supplying these fast handpieces was contaminated with P. aeruginosa, the water exiting the distal outlets of the fast handpieces would be similarly contaminated. The P. aeruginosa mean counts measured for the handpieces of the open system DCUs revealed that P. aeruginosa was present in DCUs O1.1 (3.5 × 100 CFU 100 mL−1), O.2 (7.45 × 101 CFU 100 mL−1), O2.3 (1.93 × 102 CFU 100 mL−1), and O3.4 (6.45 × 101 CFU 100 mL−1) (Figure 4). The P. aeruginosa results for these fast handpieces were similar to those reported in a study that assessed three-in-one syringes (5.29×101 CFU 100 mL−1) and high-speed handpieces (6.78 × 101 CFU 100 mL−1) that were supplied by water collected from 20 DCUs.42
In the current study, no P. aeruginosa was detected in the water sampled at the distal outlet of the fast handpieces of DCU O2.1 and O2.4, even though the municipal tap water supplying these DCUs contained P. aeruginosa (Figure 4). However, the municipal tap water supplying DCUs O2.2 and O2.3 contained P. aeruginosa and the fast handpieces of these DCUs exhibited elevated P. aeruginosa mean counts. It could be assumed that the increase in P. aeruginosa in the waterlines of DCUs O2.2 and O2.3 were colonised by P. aeruginosa to form biofilm. The biofilm was possibly sloughed off and was fed into the water supply of the fast handpieces where it contaminated the water at the distal outlets of these instruments.
The mean P. aeruginosa count in DCU O1.1 (3.5 × 10° CFU 100 mL−1) was attributed to a biofilm that may have been resident within the waterlines of this DCU. No P. aeruginosa was detected in the source water supplying this DCU. Moreover, no statistical difference (p = .21) was measured between the P. aeruginosa counts of the municipal tap water and the water exiting the distal outlet of the fast handpiece of this open system. The P. aeruginosa counts in closed system DCUs revealed that none was present in the municipal tap water that was used to fill the distiller bottles. Distilled water samples were also generally free from P. aeruginosa. The only distilled water that contained P. aeruginosa was found in DCU C2.3 (1.5 × 101 CFU 100 mL−1).
The reservoir bottles that were attached to the closed system DCUs that supplied the fast handpieces with water generally did not contain P. aeruginosa. The water contained in the two reservoir bottles attached to DCU C2.2 and C2.3 had mean counts of 3.1 × 101 CFU 100 mL−1 and 1.1 × 101 CFU 100 mL−1, respectively (Figure 4). Reservoir bottles could have become contaminated due to improper disinfection of reservoir bottles and water stagnation. There was no statistically significant difference (p = .151) between the P. aeruginosa counts of the water of distiller bottles and those of the reservoir bottles of the closed system DCUs.
The origin of bacteria that contaminate DUWLs can be attributed to 2 factors:
(i) contaminated municipal water that is used in DCUs, and (ii) the suck back of patients’ saliva into the DUWL because of ineffective or faulty anti-retraction valves.43,44 Anti-retraction valves are incorporated into high-speed handpieces and their couplings. They are meant to prevent the reverse flow of organic material caused by centrifugal suction when the handpiece is switched off. Anti-retraction valves, when functioning adequately, prevent the reverse flow (suck back) of organic material and subsequent bacterial growth. However, their efficiency relies on proper maintenance of both the anti-retraction valves and the handpiece.45 When comparing the two DCU systems, no statistical significance (p = .19) was observed between the municipal tap water supplied to the DCUs and the water exiting the fast handpieces (p = .12). The internal architecture of DUWLs comprises a network of small bores and narrow lumens that create an optimal environment for P. aeruginosa colonisation when even a small amount is present in a municipal water system. P. aeruginosa has been recovered from oral cavities of approximately 4% of healthy individuals.46 It is therefore possible that some of these bacteria are aspirated into DUWLs. However, if water is monitored and maintained according to procedural controls, the prevalence of P. aeruginosa in the municipal water supply should be extremely low.46
Detection of Legionella spp. in water samples
Water samples sourced only at the distal outlets of fast handpieces used in both open and closed system DCUs were analysed for the presence of Legionella spp., and it was detected in the water that exited the distal outlet of fast handpiece O2.1. No Legionella spp. was detected in the water samples from fast handpieces in closed water system DCUs. The presence of Legionella spp. in the water of fast handpiece O2.1 was considered a high risk, especially if this water would be aerosolised and aspirated by patients who were immunocompromised. Legionella spp. were confirmed using the VITEK MS (BioMerieux) and was reported to the environmental health officer at the oral healthcare facility where it was detected, and the Free State Department of Health. The current study was put on hold and the DCU was suspended for use of oral procedures for a period of 14 days until a suitable intervention could be agreed upon and executed. The waterlines of DCU O2.1 were flushed daily with 50 mL of 0.2% chlorhexidine mouthwash for two weeks.
Following the 14 days of suspension of use of the DCU, the water at the distal outlet of the fast handpiece of DCU O2.1 was sampled and analysed for the presence of Legionella spp. Upon examination, no Legionella spp. was detected in the water of this fast handpiece, and hence the daily flushing with 0.2% chlorhexidine mouthwash was assumed to have eliminated the Legionella spp. from the water. Agahi and co-workers conducted a study where similar results were demonstrated with 50 mL of 0.2% chlorhexidine to reduce microbial counts in DUWLs.47 In their study, the water exiting the high-speed handpiece and three-in-one syringe of 35 DCUs was analysed. The DUWL water from all DCUs was reported to be contaminated before chlorhexodine use, but no further contamination was detected after flushing with chlorhexodine. Twenty-eight of the 30 treated DUWLs had microbial values of less than 2 × 102 CFU mL−1 after flushing daily for 1 week.
Several other studies have also reported the presence of Legionella spp. in dental unit water. In one case study the authors reported a case of Legionnaires’ disease in a 35-year-old dental receptionist in Cape Town, South Africa.48 It was found that she had inhaled contaminated aerosols in the oral healthcare facility where she worked. Legionella spp. concentrations at 2 sites in the oral healthcare facility were reported at concentrations of 4.00×102 CFU 100 mL−1 for Legionella pneumophila serogroup 1 and 8.8 × 103 CFU 100 mL−1 for Legionella pneumophila serogroup 2-14.48
Biofilm
The water quality of samples taken from DCUs in this study revealed that water entering the DCUs and exiting from the distal outlets of handpieces largely measured high bacterial counts that could possibly indicate the presence of biofilm in the DUWLs and in DCU water. While it is important to monitor the water quality of DUWLs for the assessment of possible bacterial contamination, the detection and analysis of biofilms in DUWLs are equally important.49,50 To further analyse the microbial load and the presence of biofilm-associated organisms in DCUs, surface swabs were taken at the distal outlets of open and closed DCUs as well as from the inner surfaces of distiller and reservoir bottles. The HPC results were compared to the SANS 241:117 standard for the presence of heterotrophic bacteria, SANS 241:117 (1 × 103 CFU mL−1) to be considered safe for human consumption.
HPC for the detection of biofilm
Swab samples were taken of the inner distal surfaces of municipal taps supplying water to open and closed DCUs. It has been reported that drinking water distribution systems have a propensity to form biofilm on the lumens of pipelines that distribute DCU water. The biofilm on the pipeline lumens can enhance the proliferation of microorganisms on the inner surfaces of taps, which contributes to the microbial loading of organisms that are transferred from water supplied to open system DCUs to their DUWLs.51,52
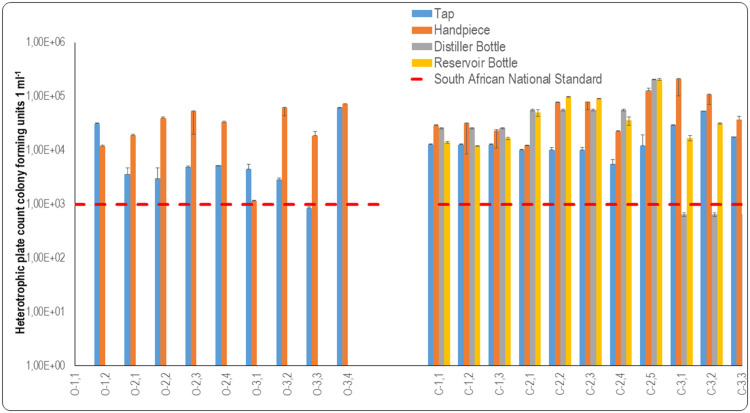
Biofilm-associated HPCs of open system DCUs
Microbial load on the inner surfaces of DUWLs could affect the water exiting from fast handpieces as this water passes over tap surfaces supplied by the municipality to the oral healthcare facilities.
Based on the information received from oral healthcare providers (Table 1), it was expected that there would be a difference in the microbial load on the surface swab samples taken at the various facilities. These results indicated that the inner surfaces of the taps at facility O1 had a high microbial load, but the microbial load on the inner surfaces of the fast handpieces was lower than that of the taps (Figure 5). As reported, this oral healthcare facility flushed the DUWL of DCUs daily; however, the HPCs of the inner surfaces of the fast handpieces were still above the SANS 241:117 recommendation of 1 × 103 CFU 1 mL−1. Flushing may have had an effect by reducing the microbial load, but it was evidently not effective enough to eliminate the biofilm that was likely to be present in the DUWL. In a study conducted to determine the efficacy of flushing waterlines on the quality of water, and obtained mean HPC counts of 6.33×104 CFU mL−1 before flushing and 8.36 × 103 CFU mL−1 post flushing.53 Reduced HPCs were observed after flushing the DUWLs, but HPC counts were not reduced to a level where the mean count adhered to the standard stipulated by the Environmental Protection Agency. The reason for this is the use of the DCUs over time and biofilm that is already resident within DUWLs.
Fig. 5.
Graphic representation of data from oral healthcare facilities for heterotrophic plate counts from swab samples to assess biofilm formation in open and closed system dental units. Samples were taken from taps (blue) and fast handpieces (orange) in open system DCUs (O1.1 to O3.4). Samples were taken from taps (blue), distiller bottles (grey), reservoir bottles (yellow) and fast handpieces (orange) of closed system DCUs (C1.1-C3.3). The dashed lines represent recommendations by the South African National Standard, SANS 241:1 (2015) for drinking water quality limitations for heterotrophic bacteria per millilitre. Water samples should not contain more than 1 × 103 CFU mL−1 of heterotrophic bacteria.
The internal surfaces of the taps at facility O2 had HPCs that were above the acceptable limit (Figure 5), which indicated the possible presence of biofilm within the pipelines of these taps. The oral healthcare practitioner reported (Table 1) that waterlines were flushed daily and shocked weekly with sodium hypochlorite. Dental chair units O2.2 and O2.3 were the only chairs at facility O2 where the internal surfaces of the fast handpieces recorded lower HPCs than the internal surfaces of the taps, as displayed in Figure 5. However, the HPCs of internal surfaces of the municipal tap in the oral healthcare facility and fast handpieces were not statistically different (p = .06).
The internal surfaces of the fast handpieces of DCUs O3.2, O3.3, and O3.4 all displayed a high microbial load which was higher than that of the internal surfaces of the taps that supplied water to these DCUs. Like a study conducted at a dental school in Tehran University of Medical Sciences, the results showed that the contamination of the three-in-one syringe was higher than that of the municipal tap water supplying the dental units54 There was no statistical significance difference between the internal municipal tap surfaces and the fast handpieces in facility O3 (p = .24).
The pipelines supplying water to the DCUs, as well as the internal surfaces of the fast handpieces, should be monitored regularly. Upon reflection of the findings, it may be argued that the presence of biofilms may be one of the predominant causes of high microbial loads, which was in all probability the case for the fast handpieces at this oral healthcare facility. The one order magnitude of difference between inner municipal tap surfaces and the surfaces of the distal outlets of the fast handpieces in open system DCUs was not statistically significant (p = .337).
Biofilm-associated HPCs of closed system DCUs
Swab samples of the inner distal municipal tap surfaces had a mean count of 4×103 CFU mL−1, which did not comply with the prescribed SANS 241:117 limit of 1 × 103 CFU mL−1. However, there were some inner surfaces of municipal taps supplying water to DCUs that did meet the SANS 241:117 limit: O.3.1 (5.50 × 102 CFU mL−1), O3.2 (4.50 × 102 CFU mL−1), and O3.3 (3.50 × 102 CFU mL−1) (Figure 5). This was the case even though the water flowing from the distal outlet of these taps was not compliant with SANS 241:117 (1 × 103 CFU mL−1). The internal surfaces of the taps at facility C1 indicated elevated microbial loads as can be seen in Figure 5. When considering the age of the DCUs and the frequency of disinfection reported by the oral healthcare practitioner (Table 1), it was unsurprising that biofilm would be present in the DUWLs, as can be observed in Figure 5. The HPCs of the internal surfaces of the fast handpieces are also presented.
When the HPCs are considered, it appears that the microbial load on the inner surfaces of the taps suggests that biofilm may have been present in the pipeline supplying water to the municipal taps, as observed in Figure 5. The disinfection protocol for C2.4 seemed to influence the microbial load in that the HPCs were marginally within the limit for the internal municipal tap surface and within the limit for the fast handpiece. The oral healthcare practitioner utilising C2.5 reported (Table 1) that the water used for this chair was not the same as that used for the others in the facility, and this may explain why the HPC for the internal surface of the fast handpiece of this unit was extremely high (Figure 5). There may have been biofilm present in the DUWLs of this unit as the internal surface of the fast handpiece displayed a much higher HPC than the count for the internal surface of the tap.
The HPCs of the inner surfaces of the waterline attached to fast handpieces of the closed system DCUs measured a mean count of 3.96 × 104 CFU mL−1, which exceeded the limit prescribed by SANS 241:117 of 1 × 103 CFU 1ml−1 for heterotrophic bacteria. However, the water of some waterline surfaces of the fast handpieces of the closed system DCUs did comply with the SANS limitation, namely C2.1 (6.50 × 102 CFU mL−1), C2.4 (3.00 × 102 CFU mL−1), C3.1 (5.50 × 102 CFU mL−1), and C3.3 (4.00 × 102 CFU mL−1) (Figure 5). These fast handpiece waterlines could have been decontaminated or flushed prior to sampling, thus resulting in low microbial load counts on the inner surfaces of the waterlines supplying these fast handpieces. In a study conducted on three-in-one syringes, high-speed air turbine hand pieces, an ultrasonic scalar, and oral rinse sources, higher HPCs were observed prior to flushing than after flushing. This suggested that flushing these instruments for a period of 3 minutes did not completely eliminate heterotrophic bacteria, but it caused a noticeable reduction in their number.55
Municipal tap water supplied to the oral healthcare facilities was used to fill the distiller bottles of the closed system DCUs, and therefore the organisms that may have been present on the inner surfaces of the taps as well as in the municipal tap water and may have influenced the HPCs of the distilled water and the internal surfaces of the distiller bottles. The internal surfaces of the distiller bottles at facility C1 showed no HPC, however, the reservoir bottles’ HPCs were high (Figure 5). This may indicate that the reservoir bottles were contaminated or could have contained biofilm. Conversely, the internal surfaces of distiller bottles at facility C2 were all compliant with the HPC limitation, except C2.5. The oral healthcare practitioner reported (Table 1) that a separate distillation room was used to distil water and fill the reservoir bottle of this DCU. The internal surface results varied for DCUs at facility C2 in terms of distiller bottles and reservoir bottles, and no statistical significance was evident (p = .33) when they were compared. It is important to observe that the internal surface of the distiller bottle of C2.5 had a high microbial load and was contaminated from the onset, which appeared to have influenced the HPC and microbial load of the reservoir bottle where biofilm may have formed. The condition and cleanliness of the distiller used to fill the reservoir bottle of this chair may not have been optimal for the distillation of water, and the distiller bottle may have contained biofilm that could have been transferred to the reservoir bottle. The microbiological quality of the water used in the reservoir bottles may thus have been compromised by the storage conditions and the condition of the plastic container that was used repeatedly.56
The oral healthcare providers at facility C3 reported that they flushed the waterlines daily and did a weekly shock treatment as part of their disinfection protocol (Table 1). The internal surfaces of the distiller bottles that were used at the facility were within the limit for HPCs, but the reservoir bottles showed elevated levels of HPCs (Figure 5). The internal surfaces of the distiller and reservoir bottles showed statistical significance (p = .26). The elevated HPCs for the internal surfaces of the reservoir bottles at facility C3 may have been an indication of contamination or the presence of biofilm in the reservoir bottles.
The internal surface swabs of reservoir bottles C1.1, C1.2, C1.3, C2.3, and C2.4 did not present any heterotrophic bacteria. The inner surfaces of the distiller bottles also did not indicate high HPCs even though the distilled water contained high HPCs. The mean count for the surface swabs indicated that the distilled water quality may have affected the HPCs of the surfaces of the reservoir bottles, or that the reservoir bottles could have become contaminated due to inadequate disinfection or improper handling when they were removed from the DCUs, filled with distilled water, and attached to the units again. Biofilm growth on the inner surfaces of reservoir bottles can cause rapid deterioration of the microbiological quality of the water in these bottles that supply water to closed system DCUs.56 The oral healthcare providers did not report any cleaning or disinfection protocols for the reservoir bottles. The one order magnitude of difference between internal reservoir bottle surfaces and the inner surfaces of fast handpiece waterlines in the closed system DCUs was not statistically significant (p = .356).
Total coliforms associated with biofilm
The biofilm results pertaining to open and closed system DCUs were characterised by analysing the concentration of total coliform bacteria from swab samples taken at various points of use and comparing the total coliform results to the SANS 241:1.17 Requirement for the presence of total coliform bacteria SANS 241:117 specifies that water should not contain more than 1 × 101 CFU mL−1 of total coliform bacteria to be considered safe for human consumption.
Swabs were taken of the inner distal surfaces of municipal taps in oral healthcare facilities supplying water to open and closed DCUs and total coliform counts were obtained. The total coliform counts of the inner distal municipal tap surfaces supplying water to open system DCUs presented a mean count of 5.09 × 103 CFU 100 mL−1, which was not in compliance with SANS 241:1.17 The open system DCUs that did not comply with SANS 241:117 for total coliforms had total coliform counts of O2.1 = 1.66 × 104 CFU 100 mL−1, O2.2 = 1.84 × 104 CFU 100 mL−1, and O2.3 = 1.61 × 104 CFU 100 mL−1 (Figure 6). All the municipal taps supplying water to DCUs at facility O2 contained total coliforms except O2.4, which evidently did not contain biofilm as did the other DCUs in this facility. No coliform bacteria were detected on any other municipal tap surfaces supplying water to open system DCUs.
Fig. 6.
Graphic representation of data from oral healthcare facilities for biofilm- associated total coliform counts for swab samples of open and closed system DCUs. Samples were taken from taps and fast handpieces in open system DCUs (O1.1-O3.4) and taps, distiller bottles, reservoir bottles, and fast handpieces of closed system DCUs (C1.1-C3.3). No counts were detected from distiller bottles and reservoir bottles. The dashed red line represents the SANS 241:1 [17] for drinking water quality for total coliforms per 100 millilitres.
No coliforms were present on the inner tap surfaces that supplied water to distiller bottles in open system DCUs. The inner surfaces of the handpieces of the closed system DCUs all complied with SANS for total coliforms as no coliforms were detected (Figure 6). Total coliforms measured in closed system DCUs showed a total absence of this microbial group on the inner surfaces of taps, the inner surfaces of distiller bottles, and the inner surfaces of reservoir bottles. Total coliforms were present on the inner surfaces of reservoir bottles of closed system DCUs at a mean count of 6.23× 03 CFU 100 mL−1. Total coliforms were also present on the inner surfaces of reservoir bottles in open system DCUs, and specifically at C3.1 (4.07 × 104 CFU 100 mL−1), C3.2 (1.66 × 104 CFU 100 mL−1), and C3.3 (1.13 × 104 CFU mL−1). No total coliforms were present on the inner surfaces of the fast handpieces of the closed system DCUs.
Pseudomonas aeruginosa associated with biofilm
P. aeruginosa was detected on the inner surfaces of municipal taps supplying DCUs O2.1, O2.2, O2.3, and O2.4. However, no P. aeruginosa was detected on any inner surfaces of the waterlines supplying the fast handpieces of open system DCUs with water.
The absence of P. aeruginosa on the inner surfaces of the fast handpieces could indicate that flushing of waterlines and/or the use of decontaminating agents may have resulted in the absence of P. aeruginosa in this instance. An earlier study investigated biofilm production on high-speed handpiece surfaces by cutting aluminium fragments from them and testing these fragments. The number of adhered cells for P. aeruginosa on the surfaces of the aluminium pieces was 9 × 106 CFU cm–2.42
No P. aeruginosa was present on the inner surfaces of the taps that were used to fill distiller bottles, and the microbe was also not detected on the inner surfaces of distiller bottles (Figure 7). However, P. aeruginosa was detected in reservoir bottles of closed system DCUs, at a mean count of 5.91×101 CFU 100 mL−1. P. aeruginosa was also detected on the inner surface of the reservoir bottle attached to DCU C2.2 (6.50 × 102 CFU 100 mL−1). This reservoir bottle may have become contaminated due to ineffective decontamination practices or by improper handling. The water in this reservoir bottle was also contaminated with P. aeruginosa and may have colonised the reservoir bottle surface.
Fig. 7.
Graphic representation of data from oral healthcare facilities for biofilm- associated Pseudomonas aeruginosa counts for swab samples of open and closed system dental units. Samples were taken from the inner surfaces of taps and fast handpieces in open system dental chair units (O1.1-O3.4) and from the inner surfaces of taps, distiller bottles, reservoir bottles, and fast handpieces of closed system dental chair units (C1.1-C3.3). No counts were detected from the inner surfaces of distiller bottles and reservoir bottles.
It was expected that the surface of the fast handpiece in DCU C2.2 would house P. aeruginosa because it was present on the inner surface of the reservoir bottle and in the water that filled the reservoir bottle of this DCU (Figure 7). P. aeruginosa was indeed present on the inner surface of the fast handpiece waterline of this unit at a count of 4.00 × 102 CFU mL−1. P. aeruginosa was also present on the inner surface of the fast handpiece of another closed system DCU, namely C2.3 at 1.50 × 102 CFU mL−1.
Conclusion
This study highlights the environmental risk of contaminated source water that is supplied to dental chair units, as well as the risk of contaminated water that exits distal outlets of fast handpieces attached to open and closed system DCUs. It also highlights the disparity that exists for heterotrophic bacteria and total coliforms as stipulated by the South African National Standard, SANS 241:1 (SANS, 2015) and the actual conditions at oral healthcare facilities, where these standards are not met in certain instances. The study also exposes a lack of legislation and guidelines to steer water quality assurance in oral healthcare facilities. The current study was only exploratory and did not physically access waterlines to determine the actual rates of contamination and biofilm on their internal surfaces. The microbial diversity in DCU waterlines may be much more complex and requires investigation on genomic level.
Conflict of interests
None disclosed.
Acknowledgments
Acknowledgements
The authors acknowledge the Central University of Technology for financial support to conduct this research.
Author contributions
CBK master's research candidate. CBK conducted sampling and laboratory analyses. ODS and JO conceptualised the experimental design, CBK and ODS conducted data analysis and drafted figures and tables. CBK drafted the manuscript. All authors critically reviewed and approved the submission of the manuscript.
References
- 1.Dagher J, Sfeir C, Abdallah A, Majzoub Z. Infection control measures in private dental clinics in Lebanon. Int J Dent. 2017;2017:1–11. doi: 10.1155/2017/5057248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim NK, Alwafi HA, Sangoof SO, Turkistani AK, Alattas BM. Cross-infection and infection control in dentistry: Knowledge, attitude and practice of patients attended dental clinics in King Abdulaziz University Hospital, Jeddah, Saudi Arabia. J Infect Public Health. 2017;10(4):438–445. doi: 10.1016/j.jiph.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahiya P, Kamal R, Sharma V, Kaur S. “Hepatitis” - Prevention and management in dental practice. J Educ Health Promot. 2015;4:33. doi: 10.4103/2277-9531.157188. http://www.ncbi.nlm.nih.gov/pubmed/26097847 Available from: Accessed 17 March 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristina ML, Spagnolo AM, Sartini M, et al. Investigation of organizational and hygiene features in dentistry: a pilot study. J Prev Med Hyg. 2009;50(3):175–180. [PubMed] [Google Scholar]
- 5.Dallolio L, Scuderi A, Rini MS, et al. Effect of different disinfection protocols on microbial and biofilm contamination of dental unit waterlines in community dental practices. Int J Environ Res Public Health. 2014;11(2):2064–2076. doi: 10.3390/ijerph110202064. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3945585&tool=pmcentrez&rendertype=abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lizzadro J, Mazzotta M, Girolamini L, Dormi A, Pellati T, Cristino S. Comparison between two types of dental unit waterlines: How evaluation of microbiological contamination can support risk containment. Int J Environ Res Public Health Article. 2019;16(328):2–13. doi: 10.3390/ijerph16030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paramashivaiah R, Prabhuji M. Ultrasonic scaling: Associated risks. J Dent Oral Biosci. 2011;2(2):40–44. [Google Scholar]
- 8.Weissfeld AS. Infection control in the dental office. Clin Microbiol News. 2014;36(11):79–84. http://linkinghub.elsevier.com/retrieve/pii/S0196439914000361 Available from: [Google Scholar]
- 9.Lal S, Singhrao SK, Achilles-Day UEM, Glyn Morton LH, Pearce M, Crean SJ. Risk assessment for the spread of serratia marcescens within dental-unit waterline systems using vermamoeba vermiformis. Curr Microbiol. 2015;71(4):434–442. doi: 10.1007/s00284-015-0872-0. [DOI] [PubMed] [Google Scholar]
- 10.Yadav SS, Priyanka Y, Ch A, Gayatri N, Gopi P. ABCDE OF DUWL (Alternate Biofilm Chair side Disinfection Efficacy of Dental Unit Waterline) EC Dent Sci. 2015;1(3):145–151. [Google Scholar]
- 11.Nikaeen M, Hatamzadeh H, Sabzevar Z, Zareh O. Microbial quality of water in dental unit waterlines. J Res Med Sci. 2009;14(5):297–300. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L355665278%0Ahttp://journals.mui.ac.ir/jrms/article/view/2801/1731 Available from: [PMC free article] [PubMed] [Google Scholar]
- 12.Valcina O, Pule D, Makarova S, et al. Occurrence of Legionella Pneumophila in water distribution systems in dental practices in Latvia. Med Basic Sci. 2014:33–39. [Google Scholar]
- 13.Mills S, Porteous N, Zawada J. Dental unit water quality: organization for safety, asepsis and prevention white paper and recommendations–2018. J Dental Infect Contr Saf. 2018;1(1) [Google Scholar]
- 14.Ghosh S, Mallick SK. Microbial biofilm: contamination in dental chair unit. Ind Med Gazette. 2012:383–387. [Google Scholar]
- 15.Spagnolo AM, Sartini M, Cristina ML. Pseudomonas aeruginosa in the healthcare facility setting. Rev Med Microbiol. 2021;32(3):169–175. [Google Scholar]
- 16.Zhang Y, Ping Y, Zhou R, Wang J, Zhang G. High throughput sequencing-based analysis of microbial diversity in dental unit waterlines supports the importance of providing safe water for clinical use. J Infect Public Health. 2018;11(3):357–363. doi: 10.1016/j.jiph.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 17.SANS . South African National Standard; South Africa: 2015. SANS 241-1: 2015 South African National Standard Drinking Water Part 1: Microbiological, physical , aesthetic and chemical determinants. [Google Scholar]
- 18.Hartshorne J. Contaminated dentistry and infection control standards of care. Int Dent S Afr. 2010;12(6):46–50. http://osap.site-ym.com/resource/resmgr/International/hartshorne_INTERNATIONAL_DEN.pdf Available from. [Google Scholar]
- 19.Naik S, Khanagar S, Kumar A, Vadavadagi S, Mayakonda H. Knowledge, attitude, and practice of hand hygiene among dentists practicing in Bangalore city – A cross-sectional survey. J Int Soc Prevent Commun Dent. 2014;4(3):159–163. doi: 10.4103/2231-0762.142013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michałkiewicz M, Ginter-kramarczyk D, Kruszelnicka I. Is water in dental units microbiologically safe? Med Pr. 2015;66(6):763–770. doi: 10.13075/mp.5893.00148. [DOI] [PubMed] [Google Scholar]
- 21.CDC Summary of infection prevention practices in dental settings basic expectations. Centers for Disease Control and Prevention (CDC) 2016;43 http://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care.pdf Available from: Accessed 12 July 2019. [Google Scholar]
- 22.Alkhulaifi MM, Alotaibi DH, Alajlan H, Binshoail T. Assessment of nosocomial bacterial contamination in dental unit waterlines: impact of flushing. Saudi Dent J. 2019:3–8. doi: 10.1016/j.sdentj.2019.07.003. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chate RAC. An audit improves the quality of water within the dental unit water lines of general dental practices across the East of England. Br Dent J. 2010;209(7):1–8. doi: 10.1038/sj.bdj.2010.885. [DOI] [PubMed] [Google Scholar]
- 24.Ma MS, Yunus Z, Yunus ARM, Ahmad Z, Fatah FA. A comparison of different disinfectants on the microbiological quality of water from the dental unit waterlines of a military hospital. Sains Malays. 2015;44(2):187–192. [Google Scholar]
- 25.Rodrigues S, Suvarna S, Suvarna J, Saralaya V, Saldanha S, Shenoy V. Microbial assessment of dental unit waterlines in an institutional setup in Karnataka, South India. Ind J Dent Res. 2017;28(5):555. doi: 10.4103/ijdr.IJDR_775_16. [DOI] [PubMed] [Google Scholar]
- 26.Lisboa GM, Lisboa YRM, Pinheiro TML, Stegun RC, Silva-filho EA. Microbial diversity in dental unit waterlines. Acta Odontológica Latinoamericana. 2014;27:110–114. doi: 10.1590/S1852-48342014000300002. [DOI] [PubMed] [Google Scholar]
- 27.Bedada TL, Mezemior WD, Dera FA, et al. Virological and bacteriological quality of drinking water in Ethiopia. Appl Water Sci. 2018;8(2):1–6. doi: 10.1007/s13201-018-0716-8. Available from: [DOI] [Google Scholar]
- 28.Chua CS, Fathilah AR, Himratul-Aznita WH. Health risk of dental unit waterline system to dental patients – an issue of concern. Ann Dent. 2018;20(1):20–26. [Google Scholar]
- 29.Ismaïl R, Aviat F, Michel V, et al. Methods for recovering microorganisms from solid surfaces used in the food industry: a review of the literature. Int J Environ Res Public Health. 2013;10(11):6169–6183. doi: 10.3390/ijerph10116169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansson L, Akel Y, Eriksson R, Lavander M, Hedman J. Impact of swab material on microbial surface sampling. J Microbiol Methods. 2020;176 doi: 10.1016/j.mimet.2020.106006. Available from: [DOI] [PubMed] [Google Scholar]
- 31.Porteous N, Sun Y, Dang S, Schoolfield J. A comparison of 2 laboratory methods to test dental unit waterline water quality. Diagn Microbiol Infect Dis. 2013;77(3):206–208. doi: 10.1016/j.diagmicrobio.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa D, Mercier A, Gravouil K, Lesobre J, Verdon J, Imbert C. Occurrence and diversity of both bacterial and fungal communities in dental unit waterlines subjected to disinfectants. Pathog Dis. 2016;74(7):1–11. doi: 10.1093/femspd/ftw094. [DOI] [PubMed] [Google Scholar]
- 33.Abdallah SA, Khalil AI. Impact of cleaning regimes on dental water unit contamination. J Water Health. 2011;9(4):647–652. doi: 10.2166/wh.2011.184. [DOI] [PubMed] [Google Scholar]
- 34.Coleman DC, O’Donnell MJ, Shore a C, Russell RJ. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol. 2009;106(5):1424–1437. doi: 10.1111/j.1365-2672.2008.04100.x. http://www.ncbi.nlm.nih.gov/pubmed/19187140 Available from: Accessed 25 July 2014. [DOI] [PubMed] [Google Scholar]
- 35.Dobaradaran S, Nabipour I, Ramavandi B, et al. Microbial contamination of dental unit waterlines in Bushehr. Iran. Fresenius Environ Bull. 2014;23(4):1000–1005. [Google Scholar]
- 36.Walker JT, Bradshaw DJ, Bennett AM, Fulford MR, Martin MV, Marsh PD. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl Environ Microbiol. 2000;66(8):3363–3367. doi: 10.1128/aem.66.8.3363-3367.2000. Accessed 16 June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker JT, Marsh PD. A review of biofilms and their role in microbial contamination of dental unit water systems (DUWS) Int Biodeterior Biodegradation. 2004;54(2–3):87–98. http://linkinghub.elsevier.com/retrieve/pii/S0964830504000587 Available from: Accessed 16 June 2014. [Google Scholar]
- 38.Kgabi SP, Mthethwa SR. The effect of A-dec ICX TM on microbiological water quality in self-contained dental units’ water systems. S Afr Dent J. 2020;75(7):367–372. [Google Scholar]
- 39.Mills SE. The dental unit waterline controversy. J Am Dent Assoc. 2001;131:1427–1441. doi: 10.14219/jada.archive.2000.0054. [DOI] [PubMed] [Google Scholar]
- 40.Szymańska J, Sitkowska J. Bacterial contamination of dental unit waterlines. Environ Monit Assess. 2013;185(5):3603–3611. doi: 10.1007/s10661-012-2812-9. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3613572&tool=pmcentrez&rendertype=abstract Available from: Accessed 23 June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dreyer AG, Hauman CH. Bacterial contamination of dental handpieces. J S Afr Dent Assoc. 2001;56(11):510–512. [PubMed] [Google Scholar]
- 42.Freitas VD, van der Sand ST, Simonetti AB. In vitro biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus on the surface of high-speed dental handpieces. Revista de Odontologia da UNESP. 2013;39(4):193–200. [Google Scholar]
- 43.Montebugnoli LL, Dolci GG. Contamination: an ’in vitro’ and clinical ’study’. 2002; 4:1–4. [DOI] [PMC free article] [PubMed]
- 44.Loganathan K, Shankar Narayan G, Kumar RA, et al. Dental handpiece-maintenance, contamination control and sterilization. Int J Curr Aspects. 2023 doi: 10.24941/ijcr.34208.02.2019. [DOI] [Google Scholar]
- 45.Samaranayake L, Fakhruddin K, Sobon N, Osathanon T. Dental unit waterlines: disinfection and management. Int Dent J. 2024;74:S437–S445. doi: 10.1016/j.identj.2024.07.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barben J, Schmid J. Dental units as infection sources of Pseudomonas aeruginosa. Eur Respirat J. 2008;32(4):1119–1122. doi: 10.1183/09031936.00072808. [DOI] [PubMed] [Google Scholar]
- 47.Agahi RH, Hashemipour MA, Kalantari M, Ayatollah-Mosavi A, Aghassi H, Nassab AHG. Effect of 0.2% chlorhexidine on microbial and fungal contamination of dental unit waterlines. Dent Res J (Isfahan) 2014;11(3):351–356. http://www.ncbi.nlm.nih.gov/pubmed/25097645%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4119368 Available from: Accessed 28 June 2021. [PMC free article] [PubMed] [Google Scholar]
- 48.Chikte U, Khondowe O, Gildenhuys I. A case study of a dental receptionist diagnosed with Legionnaires’ disease. S Afr Dent J. 2011;66(6):284–287. [PubMed] [Google Scholar]
- 49.Figueiredo-Filho AO de, Bem JSP, Weber Sobrinho CR, Souza FB de. Microbiological Water Evaluation from Biofilm Adhered to Dental Unit Waterlines. Int J Odontostomatol. 2019;13(3):357–362. [Google Scholar]
- 50.Göksay D, Cotuk A, Zeybek Z. Microbial contamination of dental unit waterlines in Istanbul. Turkey. Environ Monit Assess. 2008;147(1–3):265–269. doi: 10.1007/s10661-007-0118-0. http://www.ncbi.nlm.nih.gov/pubmed/18210208 Available from: Accessed 30 July 2014. [DOI] [PubMed] [Google Scholar]
- 51.Chaves Simões L, Simões M. Biofilms in drinking water: Problems and solutions. RSC Adv. 2013;3(8):2520–2533. [Google Scholar]
- 52.Pareek S, Nagaraj A, Sharma P, Atri M, Walia S, Naidu S, et al. Disinfection of dental unit water line using aloe vera: In vitro study. Int J Dent. 2013;2013 doi: 10.1155/2013/618962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shahabudin S, Hami R, Lee-Sim L, Salleh A. The Effect of Dental Unit Waterline Flushing on the Quality of Water in Dental Teaching Center in. J Biomed Clin Sci. 2018;3(June):72–74. [Google Scholar]
- 54.Mansourian A, J MB, Jabalameli F, Tohidast Z, S BS, Khorshidian A, et al. Detection and quantification of bacterial flora in dental unit water systems and the effect of flushing on its reduction. 2011; 11:40–6.
- 55.Zahir S, Zahir H, Khurshid Z, Akhther S. Is flushing of dental water lines effective in removing microbial is flushing of dental water lines effective in removing microbial contaminants? Pak Oral Dent J. 2016;36(June):305–307. [Google Scholar]
- 56.O’Donnell MJ, Boyle M a, Russell RJ, Coleman DC. Management of dental unit waterline biofilms in the 21st century. Future Microbiol. 2011;6(10):1209–1226. doi: 10.2217/fmb.11.104. http://www.ncbi.nlm.nih.gov/pubmed/22004039 Available from: Accessed 18 July 2014. [DOI] [PubMed] [Google Scholar]