Abstract
Introduction
Pauci-immune crescentic glomerulonephritis (GN) is nearly synonymous with antineutrophil cytoplasmic antibody (ANCA)–associated disease. Cases with immune complex deposition create a diagnostic conundrum leading to suspicion for concurrent infection or autoimmune disease. Small case series have demonstrated myeloperoxidase (MPO) in the immune deposits in patients with membranous nephropathy (MN) and ANCA-associated disease. However, the specificity of MPO staining to characterize immune deposits in crescentic GN has not been thoroughly evaluated.
Methods
We performed MPO immunostaining of 143 kidney biopsies, including pauci-immune crescentic GN (n = 15), ANCA with immune-complex crescentic GN (n = 20), MN without crescents (n = 24), endocarditis-associated crescentic GN (n = 25), hydralazine-associated crescentic GN (n = 11), and concurrent crescentic GN and MN without phospholipase A2 receptor (PLA2R) (n = 38) and with PLA2R (n = 10). MPO immunohistochemistry (IHC) was evaluated for positivity, character, and location of MPO immune deposits by 4 blinded pathologists.
Results
In patients with dual crescentic GN and MN without PLA2R, 84.2% were MPO-IHC positive. Crescentic GN with mesangial IgG was MPO-IHC positive in 40%. Crescentic GN related to hydralazine exposure was MPO-IHC positive in 72.7%. All cases with pauci-immune crescentic GN, endocarditis-associated cases, and MN cases with known antigens were negative for MPO.
Conclusion
Our study demonstrated that glomerular immune deposits in patients with crescentic GN with positive MPO serology demonstrated MPO positivity in the pattern of immune deposits in the majority of cases. Glomerular immune complexes in patients with MPO-positive crescentic GN therefore represent MPO-IgG immune complexes and should be thought of as one disease rather than a second disease process.
Keywords: crescentic glomerulonephritis, membranous nephropathy, myeloperoxidase
Graphical abstract
See Commentary on Page 1321
ANCA-associated GN is a leading cause of rapidly progressive renal failure worldwide. The majority of patients are serologically positive for MPO/p-ANCA and/or proteinase 3 (PR3)/c-ANCA, which are proteins expressed on the cell surface of cytokine-primed neutrophils.1 On kidney biopsy, the characteristic findings are a necrotizing and/or crescentic GN without significant immune complex deposition, that is, “pauci-immune.” However, many patients with ANCA-associated GN have some immune complex deposition (reported in up to 54% of cases).2 Immune deposition in ANCA-associated disease can be mesangial and/or subepithelial, and in some cases has a pattern of an MN.
Crescentic GN with positive staining for IgG creates a diagnostic conundrum with the suspicion of other disease etiologies2 such as endocarditis or other infections, drug nephrotoxicity, or autoimmune diseases. Because crescentic GN is a pattern of injury on kidney biopsy, determining a precise etiology is essential for clinical management. ANCA-associated disease can require aggressive immunosuppression, whereas infection-associated etiologies require treatment with antimicrobial therapies and could be exacerbated with immunosuppression. Drugs can also induce crescentic GN, including the presence of a positive ANCA serology; for example, hydralazine,3 TNF alpha inhibitors,4 and levamisole-adulterated cocaine,5, 6, 7 among others. In this setting, the avoidance of the inciting drug is essential.
There are small case series and reports demonstrating that MPO is present within immune complexes in patients with ANCA-associated GN and concurrent MN.8, 9, 10 Recently, MPO was identified as the predominant autoantigen in cases of crescentic GN and MN by mass spectrometry of isolated glomeruli.10 This suggests that MN in the setting of crescentic GN represents one disease process. MPO immunostaining of kidney biopsies could be useful in practice if specific to ANCA-associated disease. Here, we evaluate the specificity of MPO immunostaining and further characterize patients with dual ANCA-associated GN and MN.
Methods
Cohort
A total of 143 renal biopsies from the archives of Arkana Laboratories between January 2010 and August 2024, were retrospectively studied. These included cases of ANCA–associated pauci-immune crescentic GN with completely negative immunofluorescence studies (referred to as pauci-immune) (n = 15), ANCA with immune-complex crescentic GN (with 1+ or greater staining intensity by immunofluorescence, n = 20), MN without crescents (n = 24), endocarditis-associated crescentic GN (n = 25), hydralazine-associated crescentic GN (n = 11), and concurrent crescentic GN and MN without PLA2R (n = 38) and with PLA2R staining (n = 10).
The concurrent crescentic GN with MN without PLA2R cases included every case identified between 2010 and 2024 (n = 38, comprising 0.07% of total kidney biopsies received in that time period). Patients with anti-GBM disease were excluded. Use of medications associated with vasculitis was not an exclusion criterion. For the MN cases, 5 cases each of MN with known antigens (PLA2R, exostosin 2, neural epidermal growth factor-like 1, thrombospondin type 1 domain-containing 7A) were included, as well as 4 cases negative for these antigens in patients with a positive MPO serology (without crescentic GN). In addition, 10 cases with PLA2R+ MN with crescents were examined for staining specificity. For cases that had unknown serology results using enzyme-linked immunosorbent assay, we used c-ANCA and p-ANCA positivity as a surrogate for MPO/PR3.
Patients with endocarditis-associated GN had a known diagnosis of endocarditis with valvular vegetations identified on echocardiography and positive blood cultures. Patients with hydralazine-associated crescentic GN included those with a history of hydralazine use in temporal association with crescentic GN, often with a concurrent positive ANCA serology. Further details of each cohort are included in the Supplementary Data. The study was approved by Solutions Institutional Review Board and adhered to the principles of the Declaration of Helinski.
Renal Biopsy Processing
Processing of the kidney biopsies were prepared as per standard kidney pathology assessment and details are included in the Supplementary Data.11
Immunostaining for MPO and MN Antigens
For IHC staining for MPO, formalin fixed paraffin-embedded tissue sections were cut at 3 μm, deparaffinized, and antigen retrieval was performed by incubation at 99°C. Sections were reacted with rabbit polyclonal MPO antibody at 1:200 (Sigma-Aldrich, cat # 475915) for 40 minutes, washed in phosphate-buffered saline, and reacted with antirabbit IgG-HRP antibody at 1:100 (Jackson Immunoresearch) for 20 minutes. Slides were then washed, dehydrated, coverslipped, and evaluated by standard light microscopy.
Cases of MN were immunophenotyped by staining 3 μm formalin fixed paraffin-embedded tissue sections with antibodies forPLA2R, (rabbit polyclonal, Sigma-Aldrich, cat # HPA012657, 1:50), thrombospondin type 1 domain-containing 7A (rabbit polyclonal, Atlas antibodies, cat # AMAB91234, 1:50), neural epidermal growth factor-like 1 (rabbit polyclonal, Thermo Fisher Scientific, cat #PA5-27958, 1:100), and exostosin 1 (rabbit polyclonal, Invitrogen, cat # PA5-60699, 1:50), as above.
Assessment of MPO Staining
MPO immunostaining was assessed by 4 blinded kidney pathologists (RG, TNC, AB-R, and CPL). Glomeruli were evaluated for MPO positivity by the distribution of deposits within glomeruli (capillary wall and/or mesangium), extent of deposits within glomeruli including global (> 50%) versus segmental (< 50%) staining, and the proportion of glomeruli involved (diffuse vs. focal involvement). Cases of acute pyelonephritis were used as a control for MPO staining, with MPO positive in neutrophils without staining within glomeruli or within the interstitium. Only granular capillary loop or granular mesangial staining within glomeruli was considered a positive result (Supplementary Figure S1). Staining was considered positive or negative and was not quantitative.
Clinical and Laboratory Data
Medical records were reviewed for demographics, clinical presentation, and clinical features of systemic vasculitis (such as a purpuric rash or hemoptysis, presence/absence of peripheral edema, and laboratory values). Laboratory values included serum creatinine, quantitative proteinuria if available (dipstick, urine protein-to-creatinine ratio or 24-hour urine collection), urinalysis findings, and serologic studies.
Statistics
Continuous variables were assessed using mean ± SD for parametric continuous variables and sample medians for nonparametric variables. Kappa statistics were used to compare interrater concordance in the interpretation of immunostaining scores read by 4 blinded pathologists.
Results
Clinical and Laboratory Characteristics of Patients With Concurrent Crescentic GN and MN
Thirty-eight patients with concurrent crescentic GN and MN without PLA2R were identified, with a mean age of 65.7 (range: 25–85) years and no sex predominance (Table 1). No patients had prior known ANCA-associated disease or a prior kidney biopsy. The most common clinical comorbidities included hypertension (n = 24), diabetes (n = 6), and chronic kidney disease (n = 10). Eight patients had evidence of systemic vasculitis; 7 with hemoptysis and 1 with a purpuric rash consistent with leukocytoclastic vasculitis. No patients had active known infections and only 1 patient was taking medications (hydralazine) associated with inciting ANCA-associated GN or MN.
Table 1.
Clinical features of patients with crescentic glomerulonephritis with concurrent membranous nephropathy in comparison to patients with other forms of crescentic glomerulonephritis or membranous nephropathy without crescents
| Diagnosis | Crescentic GN + MN | MPO+ crescentic GN w/ mesangial deposits | PR3+ cres GN w/ deposits | Pauci-immune crescentic GN | Endocarditis associated-GN | MN without crescents | PLA2R+ MN with crescents | Hydralazine associated cres GN |
|---|---|---|---|---|---|---|---|---|
| n | 38 | 10 | 10 | 15 | 25 | 24 | 10 | 11 |
| Demographics | ||||||||
| Age | 65.7 (25–85) | 66.8 (26–81) | 60.3 (33–74) | 57.9 (26–86) | 49.6 (16–84) | 51.8 (20–78) | 55 (33–64) | 78.7 (63–88) |
| Sex | 19 (M), 19 (F) | 2 (M), 8 (F) | 5 (M), 5 (F) | 8 (M), 7 (F) | 16 (M), 9 (F) | 13 (M), 11 (F) | 6 (M), 4 (F) | 0 (M), 11 (F) |
| Clinical | ||||||||
| PMH | HTN (24/38), CKD (10/38), DM (6/38), | HTN (5/10), CKD (2/10), DM (2/10), | HTN (7/10), CKD (5/10), DM (4/10) | HTN (2/15), CKD (3/15), DM (1/15) | Valve replacement (10/25), i.v. drug use (9/25), Heart failure (2/25) | HTN (8/24), CKD (2/24), DM (5/24), | HTN (4/10), DM (2/10), cancer (1/10) | Hydralazine (11/11), HTN (11/11), cancer (2/11), ANA+ (7/11) |
| Presentation | AKI (38/38) | AKI (9/10), hematuria (1/10) | AKI (10/10) | AKI (15/15) | AKI (25/25) | CKD (1/24), Prot (23/24) | AKI (1/10), Prot (10/10) | AKI (10/11), hematuria (1/11) |
| Systemic vasculitis | Rash (1/38), pulmonary hemorrhage (7/38) | Rash (2/10), pulmonary hemorrhage (3/10) | 0/10 | Hemoptysis (1/15), sinusitis (1/15) | Rash (3/25) | Rash (1/24) | 0/10 | Rash (1/11) |
| Laboratory data | ||||||||
| Creatinine (mg/dl) | 4.3 (1.2–12.0) | 4.4 (0.9–14.3) | 4.3 (3.2–5.7) | 4.1 (1.3–8) | 4.4 (1.4–12.2) | 1.4 (0.5–3.4) | 4.7 (4.0–11.4) | 3.5 (0.9–5.4) |
| Proteinuria | 27/27 | 5/6 | 5/5 | 10/12 | 12/12 | 22/23 | 10/10 | 6/6 |
| Hematuria | 18/19 | 5/7 | 4/4 | 8/11 | 6/6 | 3/23 | 10/10 | 8/8 |
| ANCA | 34/38 | 10/10 | 10/10 | 15/15 | 10/14 | 4/24 | 1/10 | 10/11 |
| MPO-IHC | 32/38 | 4/10 | 2/10 | 0/15 | 0/25 | 1/24 | 0/10 | 8/11 |
AKI, acute kidney injury; ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; F, female; GN, glomerulonephritis; HTN, hypertension; IHC, immunohistochemistry; M, male; MN, membranous nephropathy; MPO, myeloperoxidase; MS, multiple sclerosis; PE, pulmonary embolus; PLA2R, phospholipase A2 receptor; PMH, past medical history; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; w/, with.
All patients presented with acute kidney injury, with an elevated creatinine (mean: 4.3 mg/dl, range: 1.2–12 mg/dl) and proteinuria (mean: 2.5 g/d, range: 0.5–7.4 g/d). ANCA testing showed MPO/p-ANCA positivity in 33 of 35 patients with known data (94.3%) and 1 patient was PR3 positive (Table 1 and Supplementary Table S1). When compared with ANCA-associated disease pauci-immune type (n = 15), patients with concurrent crescentic GN and MN without PLA2R had a similar serum creatinine (4.1 mg/dl vs. 4.3 mg/dl), but higher degree of proteinuria (1.7 vs. 2.5 g/d).
The majority of MN without PLA2R cases in patients with concurrent crescentic GN had segmental IgG deposits (22/38, 57.9%) and 42.1% (16/38) had global immune deposits. In comparison, in the MN group without crescentic GN, 19 of 24 showed global deposits and 5 of 24 showed segmental deposits (segmental deposits were within the neural epidermal growth factor-like 1 and antigen-negative groups). Half of patients with crescentic GN and MN without PLA2R (19/38) had fibrous crescents in addition to active cellular or fibrocellular crescents with a mean of 25.7% of glomeruli involved (range: 4%–70% of those with fibrous crescents, Table 2 and Supplementary Table S2). MPO-IHC showed positivity within glomeruli in 32 of 38 patients (84.2%, Table 1 and Figure 1). Only 3 patients with known MPO positive serology had negative MPO-IHC staining (Supplementary Table S2).
Table 2.
Histopathologic findings of patients with crescentic glomerulonephritis with concurrent membranous nephropathy in comparison to patients with other forms of crescentic glomerulonephritis or membranous nephropathy without crescents
| Diagnosis | Crescentic GN + MN | MPO+ crescentic GN w/ mesangial deposits | PR3+ crescentic GN w/ deposits | Pauci-immune crescentic GN | Endocarditis associated-GN | MN without crescents | PLA2R+ MN with crescents | Hydralazine-associated crescentic GN |
|---|---|---|---|---|---|---|---|---|
| n | 38 | 10 | 10 | 15 | 25 | 24 | 10 | 11 |
| Light microscopy | ||||||||
| % GGS | 27.3 (0–72) | 32.9 (0–63) | 20.2 (0–46) | 21.4 (0–54) | 25.2 (0–78) | 18.6 (0–75) | 14.1 (0–47) | 27.0 (11–53) |
| % Crescents | 40.6 (5–100) | 31.0 (6–67) | 42.5 (13–80) | 44.4 (5–89) | 20 (5–78) | N/A | 31 (13–76) | 22 (4–50) |
| Endocapillary hypercellularity | 4/38 | 0/10 | 0/10 | 0/15 | 12/25 | 2/24 | 0/10 | 1/11 |
| GBM spikes/holes | 16/38 | 0/10 | 1/10 | 0/15 | 0/25 | 16/24 | 10/10 | 0/11 |
| IF/TA - absent to mild | 15/38 | 4/10 | 2/10 | 7/15 | 18/25 | 13/24 | 5/10 | 5/11 |
| IF/TA - moderate to severe | 23/38 | 6/10 | 8/10 | 8/15 | 7/25 | 11/24 | 5/10 | 6/11 |
| Arteriosclerosis - absent to mild | 14/38 | 4/10 | 4/10 | 7/15 | 19/25 | 8/23 | 5/9 | 3/10 |
| Arteriosclerosis - moderate to severe | 24/38 | 6/10 | 6/10 | 8/15 | 6/25 | 15/23 | 4/9 | 7/10 |
| Immunofluorescence | ||||||||
| IgG | Trace (1/38),1+ (7/38), 2+ (17/38), 3+ (13/38) | 1+ (5/10), 2+ (5/10) | Trace (1/10), 1+ (2/10), 2+ (7/10) | 0/15 | Trace (1/25), 1+ (3/25), 2+ (2/25) | Trace (1/24), 1+ (1/24), 2+ (3/24), 3+ (19/24) | 2+ (1/10) 3+ (9/10) | Trace (2/11) 1+ (2/11) 2+ (4/11) |
| IgM | Trace (2/38), 1+ (6/38), 2+ (7/38), 3+ (1/38) | 1+ (4/10), 2+ (2/10) | Trace (2/10), 1+ (1/10), 2+ (2/10) | Trace (1/15) | 1+ (3/25), 2+ (6/25), 3+ (6/25) | Trace (1/24), 1+ (3/24), 2+ (5/24), 3+ (1/24) | 1+ (1/10) 2+ (1/10) | 1+ (6/11) 2+ (3/11) |
| IgA | Trace (3/38), 1+ (3/38), 2+ (3/38), 3+ (1/38) | Trace (2/10), 1+ (1/10) | 1+ (3/10) | 0/15 | Trace (1/25), 1+ (2/25) 2+ (1/25) | Trace (1/24), 1+ (1/24), 2+ (1/24), 3+ (2/24) | 1+ (2/10) 2+ (1/10) | 0/11 |
| C3 | Trace (3/38), 1+ (10/38), 2+ (13/38), 3+ (7/38) | 1+ (3/10), 2+ (5/10) | Trace (3/10) 1+ (1/10), 2+ (1/10), 3+ (1/10) | Trace (1/15) | 1+ (3/25), 2+ (11/25), 3+ (11/25) | Trace (5/24), 1+ (4/24), 2+ (5/24), 3+ (5/24) | 1+ (2/10) 2+ (1/10) 3+ (5/10) | Trace (2/11) 1+ (3/11) 2+ (4/11) 3+ (2/11) |
| C1q | Trace (1/38), 1+ (1/38), 2+ (1/38) | 2+ (1/10) | 1+ (1/10) | 0/15 | Trace (2/25), 1+ (7/25), 2+ (1/25) | 1+ (1/24), 3+ (1/24) | Trace (1/10) | Trace (1/11) |
| Electron microscopy | ||||||||
| Subepi deposits (any) | 31/33 | 1/9 | 3/9 | 0/11 | 3/24 | 23/23 | 10/10 | 2/11 |
| Subendo deposits | 3/33 | 0/9 | 0/9 | 0/11 | 1/24 | 3/23 | 0/10 | 1/11 |
| Mesangial deposits | 26/33 | 7/9 | 5/9 | 0/11 | 22/24 | 15/23 | 4/10 | 7/11 |
| Severe Foot process effacement | 16/33 | 1/9 | 5/9 | 2/11 | 9/24 | 19/23 | 9/10 | 3/11 |
GBM, glomerular basement membrane; GGS, global glomerulosclerosis; GN, glomerulonephritis; IF/TA, interstitial fibrosis and tubular atrophy; MN, membranous nephropathy; MPO, myeloperoxidase; N/A, not available; PLA2R, phospholipase A2 receptor; Subendo, subendothelial; Subepi, subepithelial.
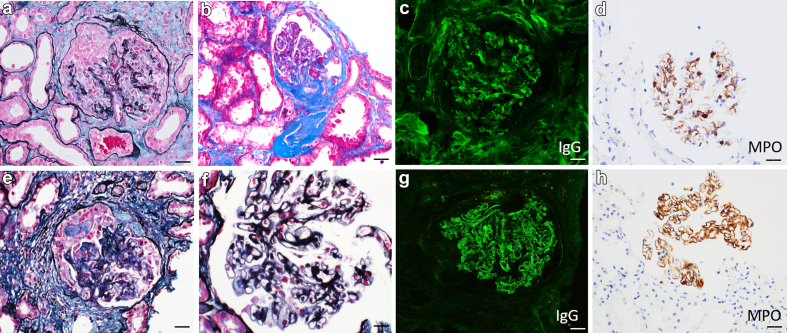
Figure 1.
Myeloperoxidase immunohistochemistry positive case examples. (a–d) Crescentic glomerulonephritis with mesangial MPO-positive immune complex deposits. (a) Glomerulus containing a cellular crescent, silver methanamine Masson-Trichrome (SMMT) stain. (b) Glomerulus containing a fibrous crescent, Masson-Trichrome stain. (c) Glomerulus with granular mesangial staining for IgG, direct immunofluorescence. (d) Positive staining for MPO within glomerular mesangium, MPO immunoperoxidase. (e–h) Concurrent crescentic glomerulonephritis and membranous nephropathy with MPO-positive immune deposits. (e) Glomerulus with a fibrocellular crescent, silver methanamine Masson-Trichrome (SMMT) stain. (f) Thickened glomerular basement membranes with punctate holes along the capillary loops, Jones methenamine silver stain. (g) Glomerulus with granular capillary loop staining for IgG, direct immunofluorescence. (h) Positive MPO staining along the glomerular capillary loops, MPO immunoperoxidase. Images are at 400× and scale bars are 20 μm. MPO, myeloperoxidase.
Specificity of MPO Staining to Comparison Cohorts
To examine the specificity of MPO immunostaining, multiple disease cohorts were evaluated in a blinded fashion. These included 14 patients with crescentic GN and MN without PLA2R (a subset from the above cohort), 15 patients with pauci-immune crescentic GN, 20 with ANCA with immune-complex crescentic GN, 24 patients with MN without crescents, and 14 cases with endocarditis-associated crescentic GN (a subset from the total cohort). Detailed descriptions of all comparison cohorts are included in Supplementary Tables S3 to S6.
MPO -IHC was evaluated by 4 independent pathologists with good interobserver concordance for positivity (kappa = 0.857). Discordance was noted in 2 PLA2R MN, 2 exostosin MN, 2 pauci-immune crescentic GN, and 2 in ANCA-associated immune complex GN. All of the discordant cases had weak and variable staining within glomeruli with examples shown in Supplementary Figure S2.
Of concurrent crescentic GN and MN without PLA2R cases, 12 of 14 (85.7%) showed positive MPO-IHC staining along within the glomerular capillary loops, of which 7 of 14 (50%) showed only capillary loop staining, and 5 of 14 (35.7%) showed both capillary loop and mesangial staining (Table 3).
Table 3.
Myeloperoxidase staining results in patients with concurrent crescentic glomerulonephritis with membranous nephropathy in comparison to patients with other forms of crescentic glomerulonephritis or membranous nephropathy without crescents
| Category | Immune deposits by IF | ANCA/MPO/PR3 Serology | Positive MPO staining cases | Capillary loop staining only | Mesangial staining only | Capillary loop and mesangial staining |
|---|---|---|---|---|---|---|
| Crescentic GN without known infection | None | + MPO (p-ANCA) | 0/10 | N/A | N/A | N/A |
| None | + PR3 (c-ANCA) | 0/5 | N/A | N/A | N/A | |
| IgG in membranous pattern | + MPO (p-ANCA) | 12/14 | 7/14 | 0 | 5/14 | |
| IgG in mesangial pattern | + MPO (p-ANCA) | 4/10 | 0 | 4/10 | 0 | |
| IgG in membranous and/or mesangial pattern | + PR3 (c-ANCA) | 2/10 | 1/10 | 0 | 1/10 | |
| Endocarditis-associated crescentic GN | None | 2/20 MPO+ 2/20 PR3+/c-ANCA, 3/20 ANCA positive |
0/20 | N/A | N/A | N/A |
| IgG | 1/5 MPO+, 1/5 MPO and PR3+, 1/5 PR3+ | 0/5 | N/A | N/A | N/A | |
| Antigen type | Serology | |||||
| Membranous pattern without crescents | PLA2R | MPO negative | 0/5 | N/A | N/A | N/A |
| EXT2 | MPO negative | 0/5 | N/A | N/A | N/A | |
| NELL1 | MPO negative | 0/5 | N/A | N/A | N/A | |
| THSD7A | MPO negative | 0/5 | N/A | N/A | N/A | |
| Unknown (PLA2R, EXT2, NELL1, and THSD7A negative) | MPO Positive | 1/4 | 1/4 | 0 | 0 |
ANCA, antineutrophil cytoplasmic antibodies; EXT, exostosin 1/2; GN, glomerulonephritis; MPO, myeloperoxidase; N/A, not available; NELL1, neural epidermal growth factor-like 1; PLA2R, phospholipase A2 receptor; PR3, proteinase 3; THSD7A, thrombospondin domain containing 7A.
In patients with positive serology for MPO+/pANCA and a crescentic GN with mesangial IgG deposits (n = 10), 4 were MPO-IHC positive. Of 10 patients with a PR3+/c-ANCA serology, 2 were MPO-IHC positive within immune deposits. MPO-IHC staining was negative in all patients with a clinical history of endocarditis (n = 25), 10 out of 14 with known serologies had a positive ANCA result. All patients with pauci-immune ANCA-associated GN were MPO-IHC negative (n = 15, including 10 with MPO/pANCA and 5 with PR3/c-ANCA serology). MN cases without crescentic GN (n = 24, including 5 PLA2R+, 5 exostosin1/2+, 5 neural epidermal growth factor-like 1+, 5 thrombospondin type 1 domain-containing 7A+, and 4 PLA2R negative cases with a MPO positive serology) were also evaluated. All cases of known antigen type were MPO-negative, and 1 case with MPO positive serology was MPO-IHC positive along the capillary loops (Table 3). In this 1 MN patient with a MPO-positive serology with MPO-IHC positivity, there was no history of systemic vasculitis, but only 10 glomeruli were present by light microscopy; thus, an unsampled crescentic GN cannot be entirely excluded.
In addition, 10 PLA2R positive MN cases with crescents were evaluated and found to have no positive MPO-IHC staining (0/10, Supplementary Table S7). Eleven cases with hydralazine-associated vasculitis were tested and there was MPO-IHC positivity in the majority (Table 1 and Supplementary Table S8). Of note, 2 of the 3 hydralazine-associated cases that were negative for MPO-IHC staining, showed no immunoglobulin immunofluorescence staining.
Discussion
Crescentic GN with immune complex deposition creates a clinical challenge because it creates a wide differential diagnosis. Small case series have demonstrated MPO within immune deposits in patients with concurrent crescentic GN and MN. Colocalization of MPO and IgG (but not IgM) has been shown in multiple studies.8,9,12 MPO has been identified within glomeruli by mass spectrometry, without other dominant protein antigens in patients with concurrent crescentic GN and MN, demonstrating that MPO is the target antigen in these patients.10 MPO in this study as well as in our cohort was in a granular pattern within glomeruli in the same distribution as IgG to be considered positive. Extraglomerular MPO staining can occur in neutrophils and macrophages,13 neutrophil extracellular traps,13 and reactive endothelium,14 and should not be considered a positive result.
In our study, we observed that the glomerular immune deposits in biopsies with concurrent crescentic GN and MN were MPO-IHC positive in the majority of cases. In all cases of MN (pure MN and crescentic MN with PLA2R), where the deposits were positive for a different antigen, the MPO-IHC was negative, showing good specificity for the stain. However, the stain did not show specificity regarding a primary ANCA vasculitis and a secondary trigger for ANCA vasculitis such as hydralazine GN. This demonstrates that immune complexes in patients with MPO-positive crescentic GN represent MPO-IgG immune deposits and should be thought of as one disease. All endocarditis crescentic GN cases were negative with MPO-IHC despite 10 cases showing positive ANCA serologies. This could perhaps shed light on the role of MPO-ANCA in different types of primary and secondary crescentic diseases with immune complexes. It may suggest that in hydralazine GN, the glomerular immune complexes are truly MPO positive indicating that the MPO-ANCA could be involved in the pathogenesis of the immune complexes in that setting. Whereas in endocarditis, the immune complexes may be composed of something else given that they are MPO negative. There have been various clinical presentations reported with dual ANCA-associated crescentic GN and MN. One study showed greater proteinuria, more fibrous crescents, and lower MPO-ANCA titer in patients with dual ANCA-associated crescentic GN and MN compared with patients with ANCA-associated GN only.10 Others found a greater incidence of acute kidney injury, higher proteinuria, and increased active crescents in patients with dual disease.2,15 In our series, patients with crescentic GN with MN had a similar serum creatinine and proportion of crescents, but higher proteinuria (Table 1, Table 2).
A total of 6 out of 38 concurrent crescentic GN and MN without PLA2R were negative for MPO-IHC staining, 3 of which had positive MPO serology. We cannot rule out a secondary disease process in these cases or the MN being due to a different target antigen than MPO. There was not a clear secondary disease process from the clinical histories identified for these cases. In addition, we cannot rule out MPO-IHC staining being below the limit of detection, but do not suspect this to be the explanation because there was not weak IgG staining in each of these cases (5/6 had 2+ or greater IgG staining by immunofluorescence).
The presence of MPO-IHC staining was noted in 2 cases (20%) with crescentic GN that were positive for c-ANCA. However, the MPO antibody titer was unknown in these cases and the possibility of dual MPO and PR3 antibodies cannot be ruled out. Other possibilities for this finding includes that perhaps MPO-positive immune complexes can occur in both PR3- or MPO-ANCA, or that the crescentic GN may trigger glomerular inflammation that induces nonspecific trapping of MPO within preexisting immune complexes.
Limitations of this study include a lack of follow-up data and the limited number of comparison cohorts. A further limitation is the unknown enzyme-linked immunosorbent assay serology as well as the ANCA serology status in a subset of patients, particularly in the endocarditis and PLA2R+ MN with crescents cohorts. Additional patients with endocarditis or known infections would be needed to exclude infection in patients with MPO-IHC staining, because the sample size may be too small for definitive conclusions. Further work is needed to establish specificity within other immune complex–mediated kidney diseases, such as lupus or other drug-induced etiologies. Further work is required to identify potential mechanisms of MPO deposition within glomeruli.
In conclusion, we report the largest clinical cohort of concurrent crescentic GN and MN cases to date. MPO immunostaining may be helpful for cases with immune complex deposition greater than typically expected in a pauci-immune crescentic GN or in a membranous pattern. Our study shows IHC for MPO to be specific, establishing the immune deposition to be related to ANCA-associated disease over infection. In addition, evaluation for MPO-IHC as the target antigen within glomeruli can simplify the workup of MN in patients with concurrent crescentic GN.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was previously presented in abstract form at the United States and Canadian Academy of Pathology Annual Scientific Meeting in 2024. Research reported in this publication was supported by the National Institute on Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R44DK130702-03 (to CL and TC).
Footnotes
Supplementary Methods
Supplementary Data
Figure S1. Myeloperoxidase (MPO) immunohistochemical staining examples.
Figure S2. Examples of myeloperoxidase staining in cases with interobserver concordance or variability.
Table S1. Clinical characteristics of patients with crescentic glomerulonephritis and membranous nephropathy.
Table S2. Histopathologic characteristics of patients with crescentic glomerulonephritis and membranous nephropathy.
Table S3. Clinical and histopathologic characteristics of the pauci-immune crescentic glomerulonephritis patient cohort.
Table S4. Clinical and histopathologic characteristics of the infective endocarditis patient cohort.
Table S5. Clinical and histopathologic characteristics of the membranous nephropathy patient cohorts.
Table S6. Clinical and histopathologic characteristics of patients with ANCA with immune complex crescentic glomerulonephritis (1+ or greater immune complex deposition on kidney biopsy).
Table S7. Clinical and histopathologic characteristics of the PLA2R+ MN patient cohort with concurrent crescentic glomerulonephritis.
Table S8. Clinical and histopathologic characteristics of the hydralazine-associated crescentic glomerulonephritis patient cohort.
Supplementary Material
Supplementary Methods. Supplementary Data. Figure S1. Myeloperoxidase (MPO) immunohistochemical staining examples. Figure S2. Examples of myeloperoxidase staining in cases with interobserver concordance or variability. Table S1. Clinical characteristics of patients with crescentic glomerulonephritis and membranous nephropathy. Table S2. Histopathologic characteristics of patients with crescentic glomerulonephritis and membranous nephropathy. Table S3. Clinical and histopathologic characteristics of the pauci-immune crescentic glomerulonephritis patient cohort. Table S4. Clinical and histopathologic characteristics of the infective endocarditis patient cohort. Table S5. Clinical and histopathologic characteristics of the membranous nephropathy patient cohorts. Table S6. Clinical and histopathologic characteristics of patients with ANCA with immune complex crescentic glomerulonephritis (1+ or greater immune complex deposition on kidney biopsy). Table S7. Clinical and histopathologic characteristics of the PLA2R+ MN patient cohort with concurrent crescentic glomerulonephritis. Table S8. Clinical and histopathologic characteristics of the hydralazine-associated crescentic glomerulonephritis patient cohort.
References
- 1.Falk R.J., Jennette J.C. ANCA disease: where is the field heading? J Am Soc Nephrol. 2010;21:745–752. doi: 10.1681/ASN.2009121238. [DOI] [PubMed] [Google Scholar]
- 2.Haas M., Eustace J.A. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int. 2004;65:2145–2152. doi: 10.1111/j.1523-1755.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- 3.Santoriello D., Bomback A.S., Kudose S., et al. Anti-neutrophil cytoplasmic antibody associated glomerulonephritis complicating treatment with hydralazine. Kidney Int. 2021;100:440–446. doi: 10.1016/j.kint.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Usui J., Salvatore S.P., Yamagata K., Seshan S.V. Clinicopathologic spectrum of renal lesions following anti-TNF- α inhibitor therapy: a single center experience. Kidney360. 2023;4:363–373. doi: 10.34067/KID.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moinuddin I., Madhrira M., Bracamonte E., Thajudeen B., Sussman A. Membranous nephropathy with crescents associated with levamisole-induced MPO-ANCA vasculitis. Pathol Res Pract. 2016;212:650–653. doi: 10.1016/j.prp.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Nolan A.L., Jen K.Y. Pathologic manifestations of levamisole-adulterated cocaine exposure. DiagnPathol. 2015;10:48. doi: 10.1186/s13000-015-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collister D., Sathianathan C., Ryz K., Karpinski M., Bernstein K., Gibson I.W. ANCA associated vasculitis secondary to levamisole-adulteredcocaine with associated membranous nephropathy: a case series. Am J Nephrol. 2017;45:209–216. doi: 10.1159/000456553. [DOI] [PubMed] [Google Scholar]
- 8.Hanamura K., Tojo A., Kinugasa S., et al. Detection of myeloperoxidase in membranous nephropathy-like deposits in patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Hum Pathol. 2011;42:649–658. doi: 10.1016/j.humpath.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto K., Honda H., Shibata T., et al. MPO-ANCA crescentic glomerulonephritis complicated by membranous nephropathy: MPO demonstrated in epimembranous deposits. NDT Plus. 2009;2:461–465. doi: 10.1093/ndtplus/sfp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tominaga K., Toda E., Takeuchi K., et al. Myeloperoxidase-associated membranous nephropathy in antineutrophil cytoplasmic antibody-associated glomerulonephritis. Kidney Int Rep. 2024;9:2240–2249. doi: 10.1016/j.ekir.2024.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker P.D., Cavallo T., Bonsib S.M., Ad Hoc Committee on Renal Biopsy Guidelines of the Renal Pathology Society Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555–1563. doi: 10.1038/modpathol.3800239. [DOI] [PubMed] [Google Scholar]
- 12.Hirose O., Itabashi M., Takei T., Honda K., Nitta K. Antineutrophil cytoplasmic antibody-associated glomerulonephritis with immunoglobulin deposition. Clin Exp Nephrol. 2017;21:643–650. doi: 10.1007/s10157-016-1341-1. [DOI] [PubMed] [Google Scholar]
- 13.O’Sullivan K.M., Lo C.Y., Summers S.A., et al. Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int. 2015;88:1030–1046. doi: 10.1038/ki.2015.202. [DOI] [PubMed] [Google Scholar]
- 14.Blatt N.B., Kumar T., Wickman L.T., Kanaan H.D., Chang A., Zhang P.L. Myeloperoxidase immunohistochemical staining can identify glomerular endothelial cell injury in dense deposit disease. Pediatr Nephrol. 2021;36:4003–4007. doi: 10.1007/s00467-021-05262-x. [DOI] [PubMed] [Google Scholar]
- 15.Nasr S.H., Said S.M., Valeri A.M., et al. Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol. 2009;4:299–308. doi: 10.2215/CJN.04060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Supplementary Data. Figure S1. Myeloperoxidase (MPO) immunohistochemical staining examples. Figure S2. Examples of myeloperoxidase staining in cases with interobserver concordance or variability. Table S1. Clinical characteristics of patients with crescentic glomerulonephritis and membranous nephropathy. Table S2. Histopathologic characteristics of patients with crescentic glomerulonephritis and membranous nephropathy. Table S3. Clinical and histopathologic characteristics of the pauci-immune crescentic glomerulonephritis patient cohort. Table S4. Clinical and histopathologic characteristics of the infective endocarditis patient cohort. Table S5. Clinical and histopathologic characteristics of the membranous nephropathy patient cohorts. Table S6. Clinical and histopathologic characteristics of patients with ANCA with immune complex crescentic glomerulonephritis (1+ or greater immune complex deposition on kidney biopsy). Table S7. Clinical and histopathologic characteristics of the PLA2R+ MN patient cohort with concurrent crescentic glomerulonephritis. Table S8. Clinical and histopathologic characteristics of the hydralazine-associated crescentic glomerulonephritis patient cohort.