Abstract
Mammalian brains have extremely high levels of aerobic metabolism and typically suffer irreversible damage after brief periods of oxygen deprivation such as occur during stroke or cardiac arrest. Here we report that brain tissue from naked mole-rats, rodents that live in a chronically low-oxygen environment, is remarkably resistant to hypoxia: naked mole-rat neurons maintain synaptic transmission much longer than mouse neurons and can recover from periods of anoxia exceeding 30 min. We suggest that brain tolerance to hypoxia may result from slowed or arrested brain development in these extremely long-lived animals.
Keywords: adenosine, anoxia, hippocampus, hypoxia, paired-pulse facilitation, synaptic transmission
Introduction
African naked mole-rats (Heterocephalus glaber) display a number of anatomical, physiological, and behavioral adaptations to subterranean life in large underground colonies with up to 300 animals per colony [1]. Specializations include an insect-like social structure termed eusociality [2], greatly reduced thermoregulation that reduces metabolic demand [3], and altered renal function to retain water [4].
Naked mole-rats also have adaptations consistent with living under low-oxygen (O2) and high-carbon dioxide (CO2) conditions. Air in mammalian burrows is low in O2 and high in CO2 in general because of poor gas exchange through soil. However, it seems to be an extreme problem for naked mole-rats because of the large number of animals sharing a limited air supply [1].
Naked mole-rats have specialized circulatory and metabolic functions that help them cope with hypoxic/hypercapnic conditions. First, their weight-specific metabolic rate is only about half that of other rodents [5], reducing oxygen consumption. Second, their blood respiratory properties include high oxygen affinity hemoglobin to extract as much oxygen as possible from the atmosphere, and a pronounced acid buffering capacity to neutralize the formation of carbonic acid from carbon dioxide [6].
These considerations suggest that brain tissue from this species might also show adaptive characteristics for living under chronic hypoxia. Compared with other tissues, brain is extremely sensitive to hypoxia because it is highly metabolic yet maintains very little in the way of nonoxidative energy stores [7]. A well-established and sensitive technique for assessing the effects of hypoxia challenge on brain tissue involves recording evoked activity from hippocampal slices [8]. In this study, we recorded evoked responses before, during, and after exposure to a variety of O2 concentrations. A brief report on these experiments appeared previously in abstract form [9].
Methods
Animals
Experiments were performed with 2–4-month-old male CD-1 mice (Charles River Laboratories, Wilmington, Massachusetts, USA) and 1–10-year-old naked mole-rats. Maximum life span in naked mole-rats approaches 30 years; the animals used are considered to be adults but not senescent. A few experiments used blind mole-rats (Spalax spp.) caught in the wild as adults. Animal protocols were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Electrophysiology
Transverse hippocampal slices were prepared in the conventional manner. Briefly, mice and naked mole-rats were decapitated and the brains were rapidly removed into ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl, 124; KCl, 3; KH2PO4, 1.2; NaHCO3, 26; MgSO4, 2.5; CaCl2, 3.4; Na-ascorbate, 2; and D-glucose, 10, gassed with 95% O2 and 5% CO2. The tissue was then sliced at 400 μm on a tissue chopper. Slices were placed in an interface chamber and constantly perfused (1.0 ml/min) with ACSF at either 30 or 35°C. One mouse and one naked mole-rat were used on each day and slices from each were maintained in parallel in the same chamber for experiments.
Stimulation electrodes were placed in the stratum radiatum of subfield CA1c to activate Schaffer-commissural fibers. Population recordings of synaptic field potentials (excitatory postsynaptic potentials or EPSPs) were made with micropipettes positioned in the stratum radiatum of CA1b. Evoked responses were digitized by microcomputer and analyzed online using custom software. Field EPSPs (fEPSPs) were evoked alternately in the mouse and naked mole-rats slices at 10 s intervals throughout experiments. Baseline stimulus intensity was set to evoke a half-maximal fEPSP in each slice. Baseline recordings were taken for at least 10 min before manipulations. Initial slope and peak amplitude were calculated for each fEPSP and normalized to the baseline average in each slice.
Episodes of hypoxia were induced by replacing the O2 content of the chamber atmosphere and perfusion ACSF with various concentrations of nitrogen (N2). In some experiments, O2 was totally replaced with N2 (nominal anoxia). In these experiments, the time required to completely eliminate fiber volleys evoked by stimulation was determined as an estimate of anoxic depolarization (AD) in both mouse and naked mole-rat slices. In other experiments, mixtures of O2 and N2 in various proportions (graded hypoxia) were applied for a 30-min period. In these cases, the percentage O2 content refers to the proportion of the non-CO2 component of the gas supply (CO2 was always maintained at 5%). For example, ‘25% O2’ contains 5% CO2, 25% of 95% O2 or 23.75% O2 and 75% of 95% N2 or 71.25% N2. The percent change in fEPSP amplitude at the end of the 30-min period was taken as the acute response to hypoxia, and the degree of recovery after reinstatement of 95% O2 was determined after 30–60 min.
Results
Partial hypoxia
To test the effects of partial hypoxia on synaptic function in hippocampal slices, some of the O2 in the slice chamber atmosphere and perfusion medium was replaced with N2 for a 30-min period. For example, data from two partial hypoxia experiments are shown in Fig. 1a and b. The data in Fig. 1a are from an experiment where the O2 level was reduced to 15% (see Methods). The graphs plot the amplitude of fEPSPs evoked alternately in mouse and naked mole-rat slices; the bar labeled ‘15% O2’ corresponds to the 30-min period of hypoxia. The most notable feature of this graph is the difference between the naked mole-rat slice and the mouse slice during hypoxia. The amplitude of the fEPSP from mouse (open circles) declined to about half that of the baseline shortly after the application of hypoxia. In contrast, the amplitude of the evoked potential from naked mole-rat (filled circles) was completely unperturbed.
Fig. 1.
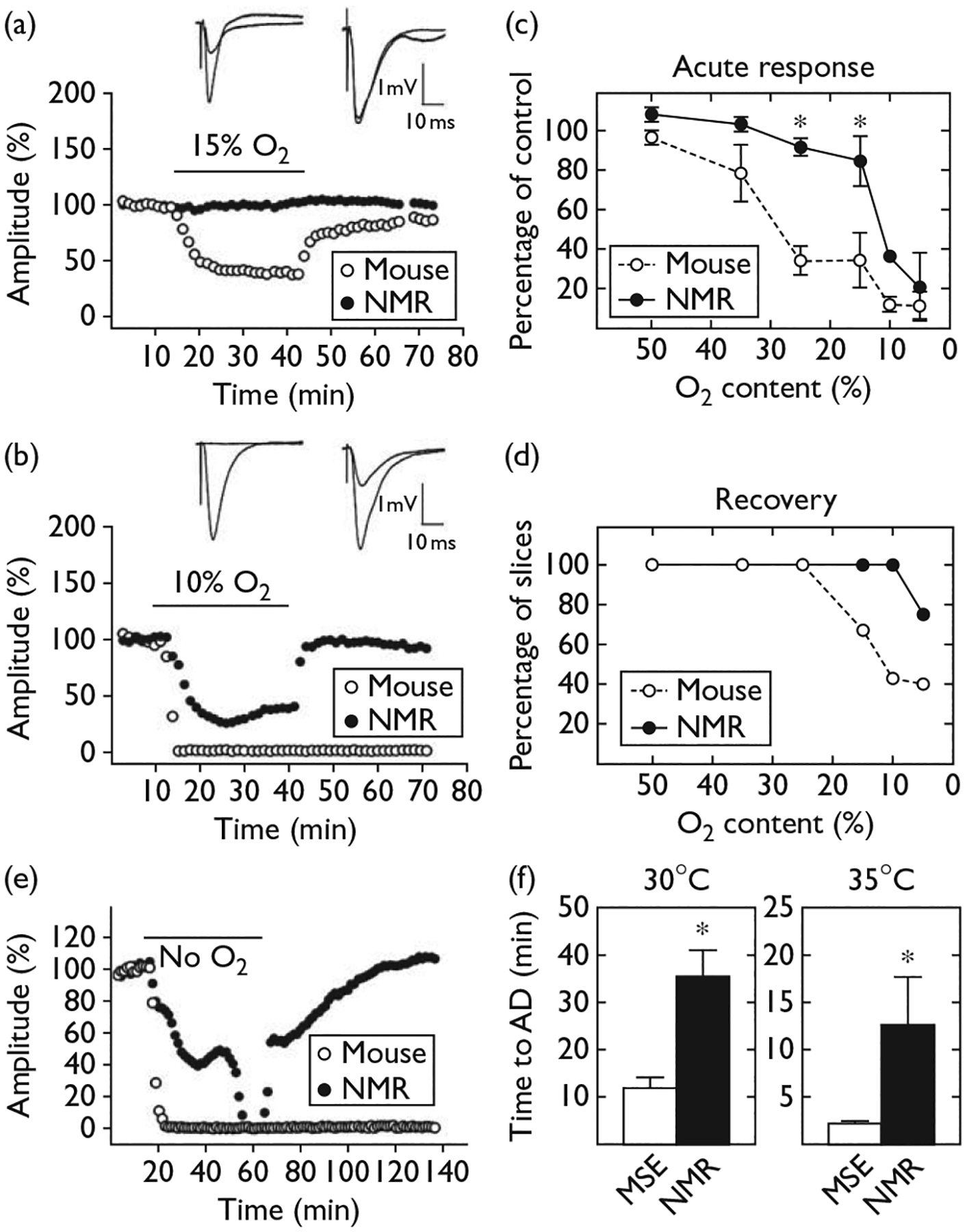
Resistance of naked mole-rat (NMR) hippocampus to hypoxia. Field excitatory postsynaptic potential (EPSP) amplitude in hippocampal slices before, during (horizontal bar), and after replacement of 95% oxygen (O2) in the slice chamber with 15% O2/80% N2 (a) or 10% O2/85% N2 (b) for 30 min. Inset traces show EPSPs recorded before and during hypoxia for mouse (left) and mole-rat (right). (c) Decrease in field EPSP amplitude in NMR (n = 25) and mouse (n = 48) slices at varying O2 content (mean ± SEM). (d) Percent of slices showing any functional recovery after reoxygenation. (e) Response to removal of all O2 from the chamber atmosphere. (f) Duration of exposure to O2-free atmosphere needed to produce anoxic depolarization (AD) in slices from mice (MSE, n = 19) and NMR (n = 13) incubated at 30 or 35°C. All NMRs were at least 1 year old and mice were at least 2 months old. *P < 0.01.
Evoked potentials from naked mole-rat slices showed an effect from hypoxia, but only under more severe hypoxic conditions. An example experiment using 10% O2 is shown in Fig. 1b. Evoked potentials from both mouse and naked mole-rat declined in amplitude during hypoxia. However, the decline was much more rapid and extensive for the mouse compared with the naked mole-rat. In addition, after the 30-min period of hypoxia, the amplitude of the fEPSP from the naked mole-rat recovered to baseline levels whereas the amplitude of the fEPSP from the mouse did not recover at all.
Summary data from multiple slices tested with six O2 levels from 50 to 5% are presented in Fig. 1c and d. The acute response curves (Fig. 1c) indicate the average fEPSP amplitudes corresponding to each O2 level. The curves show that progressively decreasing O2 content progressively decreased the amplitude of synaptic potentials for both naked mole-rats and mice. However, the amplitude for mice (open circles) declined much more rapidly than the amplitude for naked mole-rats (filled circles). The curves for mouse and naked mole-rat are particularly divergent at O2 contents of 25 and 15%. Under extreme hypoxia with only 5% O2, both naked mole-rat and mouse showed a major decline in fEPSP amplitude. However, the majority of slices from naked mole-rat tested with 5% O2 recovered after reoxygenation while the majority of mouse slices did not.
Data on recovery after hypoxia are displayed in Fig. 1d that plots the percent of slices whose fEPSP amplitude recovered to baseline levels after the 30 min exposure to hypoxia for each of the O2 levels tested. This graph shows that the majority of naked mole-rat slices that were exposed to 10 and 5% O2 recovered. In contrast, the majority of mouse slices exposed to these O2 levels did not recover after reoxygenation.
Anoxia (nominally zero oxygen)
In the second set of experiments, the O2 in the chamber atmosphere and perfusion ACSF was completely replaced with N2. We refer to this condition as nominal anoxia as the slice chamber is an open system and probably picks up some O2 from the outside air. In these experiments, nominal anoxia was maintained until both mouse and naked mole-rat slices displayed an AD, signaled by the loss of all synaptic response, a large DC shift in the field potential, and abolition of the presynaptic fiber volley [10]. Again, slices from both the species were tested in the same chamber under identical conditions. Example data from an anoxia experiment are shown in Fig. 1e. As one would predict from the previous experiments, the fEPSP amplitude for the mouse slice decreased much more rapidly than the fEPSP amplitude for the naked mole-rat slice and anoxia abolished all electrical activity in the mouse slice within a few minutes. In this example, the AD in the mouse slice occurred after 7 min and 2 s of nominal anoxia; the naked mole-rat slice did not show AD until 42 min and 50 s of anoxia had transpired. In contrast to the abrupt effect in the mouse slice, the naked mole-rat slice showed an initial slow decline in synaptic transmission to a plateau that was maintained for many minutes before a sharp AD occurred. Reoxygenation resulted in the recovery of synaptic transmission in the mole-rat slice but not in the mouse slice. The duration of anoxia required to elicit AD in mouse and naked mole-rat tissue is summarized in Fig. 1f (left hand panel); naked mole-rats persisted more than three times as long as mice.
The data presented thus far were collected from slices maintained at 30°C – the normal body temperature in H. glaber. However, temperature greatly affects responses to hypoxia [11]. Hence, additional slices were tested at 35°C – nearer physiological temperature for mice and mammals in general. Under these conditions, both mouse and naked mole-rat slices lost synaptic function and membrane potentials more rapidly than at 30°C, as one would expect, but the naked mole-rat slices still maintained function approximately four times longer than mouse slices (Fig. 1f, right hand panel).
To determine whether extreme hypoxia tolerance is a common trait among mole-rat species, we tested blind mole-rats (Spalax spp.), which are also fully subterranean, but unlike naked mole-rats, lead solitary lifestyles [1]. We found that slices from Spalax spp. responded very similarly to those from mice, showing AD after less than 2 min anoxia at 35°C (1.58 ± 0.17 min).
Paired-pulse facilitation and response to adenosine
The extreme tolerance of adult naked mole-rat brain to hypoxia is reminiscent of the tolerance of neonatal rat brain [12]. Another physiological characteristic of adult naked mole-rat hippocampus that resembles neonatal rat is the absence of paired-pulse synaptic facilitation [13,14] (Fig. 2a). This could reflect an elevated probability of transmitter release, possibly because of altered intracellular calcium regulation in presynaptic terminals. Consistent with this scenario, synaptic transmission in naked mole-rat slices was significantly less sensitive to extracellular adenosine application (Fig. 2b), as expected if release probability is elevated by higher intraterminal calcium [15].
Fig. 2.

Immature properties of adult naked mole-rat neurophysiology. (a) Lack of paired-pulse facilitation. Graphs show excitatory postsynaptic potential (EPSP) initial slope for second response as a percentage of the first response of the pair with interpulse intervals (IPIs) of 50–1600 ms. Slices from mouse (n = 42) show robust facilitation; slices from naked mole-rat (NMR, n = 13) show only a small facilitation at 50 ms and depression at longer intervals. Standard error bars are smaller than symbols. (b) Attenuated response to adenosine in NMR slices. Histograms show average (mean ± SEM) percent change in field EPSP amplitude after application of 300 μM adenosine. Slices from NMR showed significantly less inhibition than slices from mice. *P < 0.05.
Discussion
The main finding of this study is that isolated brain tissue from the naked mole-rat is remarkably tolerant to hypoxia and anoxia. This finding is consistent with the naked mole-rats’ chronically hypoxic natural environment and previously documented adaptations for living under hypoxic conditions (high O2 affinity hemoglobin and low metabolic rate). The present results complement those of a recent report on cell survival in naked mole-rat hippocampal explant cultures exposed to combined oxygen and nutrient deprivation [16]. That study showed that the percentage of cells that died from O2/nutrient deprivation was significantly greater in cultures from laboratory rats compared with cultures from naked mole-rats. Hence, brain tolerance in the naked mole-rat is evident both physiologically and anatomically.
Other mammalian preparations that show brain resistance to low O2 include the hibernating ground squirrel during periods of hibernation [17] and neonatal mammals in general [18]. The neonatal model may be the most relevant to the present results. Embryonic mammalian brain is tolerant to the significantly lower O2 levels in utero compared with the atmosphere, and this tolerance persists into neonatal life. Neonatal mammalian brain is also characterized by a lack of paired-pulse facilitation and insensitivity to adenosine – a constellation of features associated with higher levels of intracellular calcium in neonatal brain cells. It is intriguing that adult naked mole-rats share this constellation of features with neonatal mammals. The naked mole-rat may represent a case of slowed or arrested brain development, perhaps to maintain brain function in a chronically hypoxic environment. One other consequence of an extremely slow brain development may be the extremely long life span exhibited by these animals: naked mole-rats can live more than 28 years, compared with 3 years for a rat or mouse [19].
Hypoxia/anoxia tolerance in animals that have evolved to live in hypoxic environments is of interest to scientists studying how nervous systems have adapted to a specific environmental challenge. However, there are also clinical implications associated with identifying a new model system of brain tolerance. The goal now is to identify the mechanisms underlying tolerance in the naked mole-rat and to determine whether similar mechanisms can be invoked in humans challenged by pathological conditions.
Conclusion
Synaptic transmission in hippocampal slices from naked mole-rat brains was much less affected by hypoxia than transmission in mouse slices. Similarly, the electrical activity of naked mole-rat brain slices exposed to a nominally anoxic atmosphere persisted much longer than that of mouse slices, and mole-rat slices could recover from anoxic periods greater than 30 min. Resistance to hypoxia seems to be an adaptation of naked mole-rat brain to an environment chronically low in oxygen. Hypoxia tolerance may result from a slowing or arrest of the development of oxygen-sensitive, metabolically active brain processes in naked mole-rats. These adaptations may also be related to the extremely long maximum lifespan observed for this species.
Acknowledgements
This study was supported by grants from the National Institutes of Health (DC005793) and the National Science Foundation (0744979).
References
- 1.Bennett NC, Faulkes CG. African mole-rats: ecology and eusociality. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 2.Jarvis JU. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science 1981; 212:571–573. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis JUM, Bennett NC. Ecology and behavior of the family Bathyergidae. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The biology of the naked mole-rat. Princeton: Princeton University Press; 1991. pp. 66–96. [Google Scholar]
- 4.Urison NT, Buffenstein R. Kidney concentrating ability of a subterranean xeric rodent, the naked mole-rat (Heterocephalus glaber). J Comp Physiol B 1994; 163:676–681. [DOI] [PubMed] [Google Scholar]
- 5.McNab BK. The influence of body size on the energetics and distribution of fossorial and burrowing mammals. Ecology 1979; 60:1010–1021. [Google Scholar]
- 6.Johansen K, Lykkeboe G, Weber RE, Maloiy GM. Blood respiratory properties in the naked mole rat Heterocephalus glaber, a mammal of low body temperature. Respir Physiol 1976; 28:303–314. [DOI] [PubMed] [Google Scholar]
- 7.Siesjo BK. Historical overview. Calcium, ischemia, and death of brain cells. Ann NY Acad Sci 1988; 522:638–661. [DOI] [PubMed] [Google Scholar]
- 8.Kass IS, Lipton P. Mechanisms involved in irreversible anoxic damage to the in vitro rat hippocampal slice. J Physiol 1982; 332:459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park TJ, Jessen RE, Smalheiser NR, Larson J. Neuronal specializations in the hippocampus of the naked mole-rat. Abstracts, Society for Neuroscience 2005; 205:9. [Google Scholar]
- 10.Arai A, Larson J, Lynch G. Anoxia reveals a vulnerable period in the development of long-term potentiation. Brain Res 1990; 511:353–357. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Chambers G, Cottrell JE, Kass IS. Differential fall in ATP accounts for effects of temperature on hypoxic damage in rat hippocampal slices. J Neurophysiol 2000; 83:3462–3472. [DOI] [PubMed] [Google Scholar]
- 12.Cherubini E, Ben-Ari Y, Krnjevic K. Anoxia produces smaller changes in synaptic transmission, membrane potential, and input resistance in immature rat hippocampus. J Neurophysiol 1989; 62:882–895. [DOI] [PubMed] [Google Scholar]
- 13.Muller D, Oliver M, Lynch G. Developmental changes in synaptic properties in hippocampus of neonatal rats. Dev Brain Res 1989; 49:5–114. [DOI] [PubMed] [Google Scholar]
- 14.Wasling P, Hanse E, Gustafsson B. Developmental changes in release properties of the CA3-CA1 glutamate synapse in rat hippocampus. J Neurophysiol 2004; 92:2714–2724. [DOI] [PubMed] [Google Scholar]
- 15.Smith DA, Dunwiddie TV. Effects of bivalent cations on adenosine sensitivity in the rat hippocampal slice. Brain Res 1993; 617:61–68. [DOI] [PubMed] [Google Scholar]
- 16.Nathaniel TI, Saras A, Umesiri FE, Olajuyigbe F. Tolerance to oxygen nutrient deprivation in the hippocampal slices of the naked mole rats. J Integr Neurosci 2009; 8:123–136. [DOI] [PubMed] [Google Scholar]
- 17.Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab 1998; 18:168–175. [DOI] [PubMed] [Google Scholar]
- 18.Bickler PE, Fahlman CS, Taylor DM. Oxygen sensitivity of NMDA receptors: relationship to NR2 subunit composition and hypoxia tolerance of neonatal neurons. Neuroscience 2003; 118:25–35. [DOI] [PubMed] [Google Scholar]
- 19.Buffenstein R The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci 2005; 60:1369–1377. [DOI] [PubMed] [Google Scholar]


