Abstract
Background
The Dictyostelium greenbeard pathway is mediated by two polymorphic transmembrane proteins, the TgrC1 ligand and the TgrB1 receptor. These proteins mediate allorecognition, altruism, and the developmental transition to multicellularity. A genetic suppressor screen revealed activating mutations in tgrB1 and inactivating mutations in rapgapB, a regulator of the GTPase protein RapA. Inactivation of either tgrB1, tgrC1, or rapgapB leads to developmental defects, but the respective double-mutant strains rapgapB–tgrB1– and rapgapB–tgrC1– develop well and produce spores. This mutual suppression could result from inducing an alternative pathway or from restoring wild-type development, but morphological analyses alone could not resolve this question.
Results
Here, we show that the mutual suppression between rapgapB– and tgrB1– restores wild-type development. We also analyzed an activated tgrB1 allele in the wild-type background and found evidence for interactions between the wild-type and the activated alleles. Using RNA-sequencing analyses, we compared the transcriptomes of the wild type to those of several mutant strains and found that the single-gene mutations attenuated transcriptome progression over developmental time, whereas the double-gene mutation strain rapgapB–tgrB1– and the activated tgrB1 mutation exhibited near wild-type transcriptomes. Our findings suggest that tgrB1, tgrC1, and rapgapB are involved in a pathway in which rapgapB negatively regulates tgrB1 and tgrC1 expression, whereas tgrB1 and tgrC1 positively regulate rapgapB expression.
Conclusions
These findings suggest that the Dictyostelium greenbeard pathway interfaces with the central RapGAPB-RapA regulatory pathway, providing molecular insight into a mutual suppression mechanism in which two deleterious mutations restore wild-type behavior.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-025-11745-0.
Keywords: Mutual suppression, Greenbeard pathway, Dictyostelium allorecognition, Dictyostelium development, Transcriptome phenotyping
Background
Dictyostelium discoideum development begins with starvation and continues with the cessation of replicative cell division and aggregation of thousands of cells into distinct multicellular structures. The cells differentiate into spores and stalks in a 24-hour process that involves coordinated cell movement and vast changes in gene expression [1]. The aggregative nature of development can bring together individual cells with different genotypes, so that it can be viewed as a model for sociality [2]. Indeed, developing D. discoideum cells exhibit cooperation, altruism, cheating, allorecognition, and nepotism [2, 3, 4]. Allorecognition is mediated by the tgrB1 and tgrC1 genes. These genes reside on chromosome 3 in a head-to-head configuration, separated by a 524 bp segment that includes their coordinately regulated promoters [5]. They are necessary and sufficient for allorecognition and cooperative aggregation [6]. Inactivation of either tgrB1 or tgrC1 leads to developmental attenuation at the loose aggregate stage, followed by repeated aggregation and disaggregation without the formation of spores and stalks [5, 7]. This dual activity of tgrB1 and tgrC1 in development and allorecognition is related to their function as a receptor-ligand pair that mediates the transition between unicellularity and multicellularity [8, 9].
The TgrC1 ligand and the TgrB1 receptor do not have close homologs outside the Dictyostelium group of amoebae, so there is little information about their mechanism of action. A screen for genetic suppressors that modify tgrB1-tgrC1 disfunction revealed several mutations that included inactivation of rapgapB and activation of tgrB1 [10]. RapGAPB regulates RapA during development, including the late aggregation stages in which TgrB1 and TgrC1 exert their function [11]. RapA is a Ras-family GTPase protein and a key component in D. discoideum growth and development [12]. It participates in functions such as cell division, cell-substrate adhesion, and regulation of gene expression, and it is regulated by multiple effectors. It is, therefore, possible that RapGAPB is a signal-transduction element that functions downstream of the TgrB1-TgrC1 ligand-receptor system (Fig. S1).
The TgrB1-TgrC1 system has been described as a polychromatic greenbeard in D. discoideum [13]. Greenbeards are genetic elements that confer three key properties in social systems, namely the ability to display an unusual signal, the ability to recognize that signal in others, and a tendency to act altruistically toward individuals that display the same signal [14, 15]. The D. discoideum tgrB1 and tgrC1 genes are highly polymorphic in natural populations, and the degree of polymorphism is directly related to cooperation and altruism [5, 13]. The constitutively active alleles of tgrB1 include tgrB1L846F, which carries a mutation in the domain that encodes the cytoplasmic domain of the TgrB1 protein [10]. A strain that carries tgrB1L846F in addition to the wild-type tgrB1 allele behaves altruistically when mixed with wild-type cells by making more than its fair share of the prestalk and stalk cells and by increasing the sporulation efficiency of the mixing partner [16]. Conversely, the inactivation of tgrB1 causes cheating. Mixing with the wild type allows tgrB1– cells to increase their own sporulation efficiency without contributing to the prestalk and stalk cell populations. These findings demonstrated that tgrB1 is part of the signal reception element of the greenbeard system, as well as part of the altruistic pathway [16].
Exploration of social interactions revealed that inactivation of rapgapB causes cheating in an allotype-specific manner [17]. This finding, together with the suppressor screen findings [10], suggests that rapgapB is an altruism element in the D. discoideum greenbeard pathway. One of the surprising findings was that rapgapB– is a mutual suppressor of tgrB1– and tgrC1–. Strains that carry one mutation, either rapgapB–, tgrB1–, or tgrC1–, were unable to complete development, but the double mutant strains rapgapB–tgrB1– and rapgapB–tgrC1– developed well and produced spores and stalks in fruiting bodies that resembled the wild type. Mutual suppressors are rather uncommon, and they usually reveal genes that encode elements of protein complexes or regulatory pathways [17, 18, 19]. There is no evidence that RapGAPB might be physically associated with TgrB1 or TgrC1 proteins, and they do not seem to have opposite functions like several other mutual suppressors. Therefore, the finding that rapgapB– is a mutual suppressor of tgrB1– and tgrC1– was unexpected. We have considered two possibilities. First, the combination of a rapgapB– mutation and a tgrB1– or a tgrC1– mutation diverts the cells from the normal wild-type pathway of development and leads to fruiting body formation through an alternative pathway. Second, the combination of a rapgapB– mutation and a tgrB1– or a tgrC1– mutation restores normal or near-normal wild-type development. The morphological analysis alone could not resolve this issue [17].
Here, we tested the strains with RNA-seq, which provides a detailed molecular measurement that can be used as a general quantitative phenotype [20, 21]. It can also be divided into smaller elements that indicate progression through developmental milestones, as well as the differentiation of specific cell types [22]. In the case of the mutual suppressors, the detailed comparisons of the individual elements showed that the single-mutation strains were different from the wild type and similar to one another, whereas the double-mutant strain resembled the wild type. This finding supports the second hypothesis, that the combination of the two mutations bypasses the arrested stage to restore wild-type development rather than divert the cells to a specific alternative developmental pathway. In the case of the activated tgrB1 mutation, we found that the tgrB1L846F allele induced slightly precocious development. Analyses of the entire transcriptome and of specific genes within suggest that tgrB1, tgrC1 and rapgapB are involved in an intimate feedback loop that regulates their own mRNA abundance as well as the developmental and social behavior of the cells.
Results
Experimental setup
To explore the functional relationships between tgrB1, tgrC1, and rapgapB, we used several published strains. AX4 is the laboratory wild type [23]. tgrB1–, tgrC1– and rapgapB– are AX4 derivatives in which the respective genes were inactivated by CRISPR-Cas9 mutagenesis [17]. AX4 tgrB1L846F is an AX4 derivative that expresses a constitutively active form of tgrB1 [8, 16]. The double mutant strain rapgapB–tgrB1– was generated by CRISPR-Cas9 mutagenesis of rapgapB in the tgrB1– strain [17], so all the strains are genetically identical except for the indicated mutations. We chose the rapgapB–tgrB1– strain for this analysis because tgrB1 functions downstream of tgrC1 (Fig. S1) and because the phenotypes of rapgapB–tgrB1– and rapgapB–tgrC1– are quite similar to each other [17]. We developed the cells for 20 h and collected samples at 4-hour intervals. We did not include the terminal time point (24 h) in our analysis because extraction from spores and stalks is different from RNA extraction from the developing amoebae.
Transcriptional dependence between tgrB1, tgrC1, and RapgapB
The tgrB1 and tgrC1 mRNAs are coordinately regulated by a common intergenic region of 524 bp, but their abundance levels are independent of each other, such that if one gene is inactivated, the other is still expressed [5]. Previous studies have shown that tgrB1 and tgrC1 are mutual suppressors of rapgapB [17], suggesting that the three genes might be involved in mutual regulation. To test that possibility, we examined the mRNA levels of rapgapB, tgrB1, and tgrC1 in the six strains. Figure 1a shows the inferred transcriptional feedback loop and the rest of the panels in Fig. 1 show the data that support it. In AX4, rapgapB mRNA levels increased between 4 and 12 h and then decreased between 12 and 20 h of development. In the tgrB1– and tgrC1– strains, rapgapB mRNA levels increased between 4 and 8 h, like in AX4, but decreased by 12 h and remained low thereafter (Fig. 1b). These data suggest that tgrB1 and tgrC1 positively regulate rapgapB mRNA levels after eight hours of development (Fig. 1a). In the AX4 tgrB1L846F strain, where tgrB1 is constitutively active, rapgapB mRNA levels increased more rapidly and to higher levels than AX4, and then declined to near-wild-type levels (Fig. 1b). These data further suggest that tgrB1 positively regulates rapgapB mRNA levels (Fig. 1a).
Fig. 1.
A feedback loop controls the mRNA abundance of tgrB1, tgrC1 and rapgapB. We traced the mRNA abundance of three transcripts, tgrB1, tgrC1 and rapgapB, in the developmental time courses of six strains as indicated in the legend. AX4 is the wild type; tgrB1–, tgrC1–, and rapgapB– are knockout strains for the respective single genes; rapgapB–tgrB1– is a strain in which the respective two genes were knocked out; AX4 L846F is a wild-type strain that carries an additional allele of activated tgrB1L846F. (a) A schematic description of the transcriptional feedback loop where arrowheads indicate positive regulation and barred lines indicate negative regulation after eight hours of development. The dashed lines indicate that the regulatory relationships are indirect. (b) The mRNA abundance of rapgapB; (c) The mRNA abundance of tgrB1; (d) The mRNA abundance of tgrC1. In b, c, and d, the x-axis depicts developmental time (hours) and the y-axis depicts mRNA abundance in Reads Per Kilobase per Million (RPKM). Note that the y-axis in b is different from the y-axes in c and d. Each point represents the mean of three independent replications and the error bars represent the standard error of the mean
In AX4, tgrB1 and tgrC1 mRNAs accumulated to a peak between 4 and 8 h of development. They remained high until 12 h, declined between 12 and 16 h, and remained low between 16 and 20 h (Fig. 1c and d). In the rapgapB– strain, tgrB1 and tgrC1 mRNAs were not downregulated after the 8–12 h peak. Instead, the mRNA abundance remained high until 16 h and increased further between 16 and 20 h (Fig. 1c and d). These data suggest that rapgapB negatively regulates tgrB1 and tgrC1 mRNA levels after 8 h of development (Fig. 1b).
The levels of rapgapB mRNA were reduced in the rapgapB– and rapgapB–tgrB1– strains compared to the wild type (Fig. 1b). Likewise, tgrB1 mRNA levels were greatly reduced in tgrB1– and in rapgapB–tgrB1– (Fig. 1c) and tgrC1 mRNA levels were greatly reduced in tgrC1– (Fig. 1d). These results suggest that the mutations, which were designed to disrupt the open reading frames, also caused reduced mRNA accumulation. In the rapgapB–tgrB1– strain, tgrC1 levels resembled AX4 albeit somewhat lower at the 8–12 h peak as well (Fig. 1d). We also traced the mRNA abundance of rapgapB, tgrB1 and tgrC1 in a higher temporal resolution dataset of AX4 [17, 24]. The data show that tgrB1 and tgrC1 mRNAs begin to accumulate slightly before rapgapB in the wild type, and they reach higher abundance at their peaks, but the general trajectories are similar (Fig. S2). Together, these results support the model proposed in Fig. 1a, in which rapgapB activity reduces tgrB1 and tgrC1 mRNA abundance, and tgrB1 and tgrC1 activities increase rapgapB mRNA abundance (Fig. 1a). We note that the AX4 tgrB1L846F strain accumulated high levels of tgrB1 mRNA and exhibited precocious accumulation of tgrB1 mRNA (Fig. 1c).
Global transcriptome phenotypes of the tgrB1-tgrC1-rapgapB pathway
Transcriptomes provide detailed, quantitative phenotypes for comparison and analysis of different mutant strains [20, 21]. To compare the transcriptional phenotypes of the five mutant strains to the AX4 wild type and associate each mutant gene expression profile with a developmental time of the wild-type strain, we developed a method akin to multidimensional scaling (MDS). The method includes gene expression normalization followed by comparing each mutant gene expression profile to AX4 using a cost function that incorporates gene expression changes and wild-type temporal progression. The main difference between MDS and our method lies in the constraints expressed in the cost function, which are related to the considered time positions. These constraints help the approach avoid undesired placement effects of reference profiles that differ substantially from the mutant profile. By weighting expression similarity with chronological proximity, the algorithm anchors each mutant sample to the most likely developmental time, preventing implausible jumps that standard MDS can introduce when distant reference profiles are similar. A detailed description of the method is provided in Additional File 1 and results obtained with standard MDS analysis are provided in Fig. S3 for comparison.
We first compared the single gene mutation strains, tgrB1–, tgrC1–, and rapgapB– to AX4, considering all the transcripts in the transcriptome. Figure 2a shows that comparing AX4 to itself exhibits a near-straight line across the diagonal, as expected. This comparison suggests that the global transcriptome changes monotonically with a slight delay around 8–12 h of development. The profiles of tgrB1– and tgrC1– were very similar to one another but different from AX4. During the first eight hours, the profiles were nearly identical to AX4, but from that time on, the mutant profiles continued to resemble the 8-hour time point of AX4 (Fig. 2a). These results are consistent with previous findings and the developmental attenuation of the tgrB1– and tgrC1– at the loose aggregate stage [22]. They validate the experimental approach and the analysis method and provide a basis for comparison with the rest of the mutants.
Fig. 2.
Comparison between the strains over the entire transcriptome. We compared the transcriptomes of six strains using an MDS-like method. The black line in both panels represents the wild type (AX4) and the colored lines represent the mutant strains. tgrB1–, tgrC1–, and rapgapB– are knockout strains for the respective single genes. rapgapB–tgrB1– is a strain in which the respective two genes were knocked out. AX4 L846F is a wild-type strain that carries an additional allele of activated tgrB1L846F. In both panels, the x-axis represents the developmental time (hours) of the listed strain and the y-axis represents the developmental time (hours) of the wild-type reference strain (AX4). Colored lines above and to the left of the black line represent precocious developmental progression. Colored lines below and to the right represent attenuated developmental progression. (a) Comparison of the three single-mutant strains tgrB1–, tgrC1–, and rapgapB– to the wild type. (b) Comparison of the other strains to the wild type. Each time point on the graph represents the mean of three independent replications and the error bars represent the respective standard errors of the mean. Statistical significance between each strain and AX4 was assessed at each time point using a two-sample t-test. P-values were adjusted using the Benjamini-Hochberg correction to account for multiple comparisons. Asterisks at the bottom of the plot indicate significant differences: * for p < 0.05, ** for p < 0.01, and *** for p < 0.001. The calculated p-values for all the instances are provided in Additional File 6. The separation of (a) and (b) has no functional significance. It was done to simplify the visualization
The rapgapB– transcriptome was very similar to AX4 during the first 8 h of development as well. It was then delayed for 4 h between 12 and 16 h, albeit more advanced than tgrB1– and tgrC1–, and slow progression resumed between 16 and 20 h (Fig. 2a). These results are consistent with the delay seen in the morphogenesis of rapgapB– after the formation of tight aggregates at 12 h and the terminal morphology of the mutant, which is multi-tipped aggregates with a few small fruiting bodies [17].
The double mutant rapgapB–tgrB1– exhibited a pattern similar to AX4 and different from the respective single-mutant strains tgrB1– and rapgapB– (Fig. 2b). The similarity to AX4 is consistent with the morphogenesis and developmental patterns of the double mutant strain [17]. It also suggests that the mutual suppression observed between this gene pair is accompanied by bypassing the arrested stage and restoring a near wild-type phenotype rather than the induction of a hypothetical developmental pathway that is not normally seen in the wild type.
The transcriptional pattern of the activated tgrB1 strain AX4 tgrB1L846F was slightly accelerated compared to AX4 (Fig. 2b), which is not consistent with its morphogenesis either [16]. This mild acceleration might suggest a dosage effect due to the presence of the activated allele in addition to the resident wild-type allele. It is also consistent with the precocious accumulation of the tgrB1 mRNA in this strain (Fig. 1c).
Transcriptional milestones in the tgrB1-tgrC1-rapgapB pathway
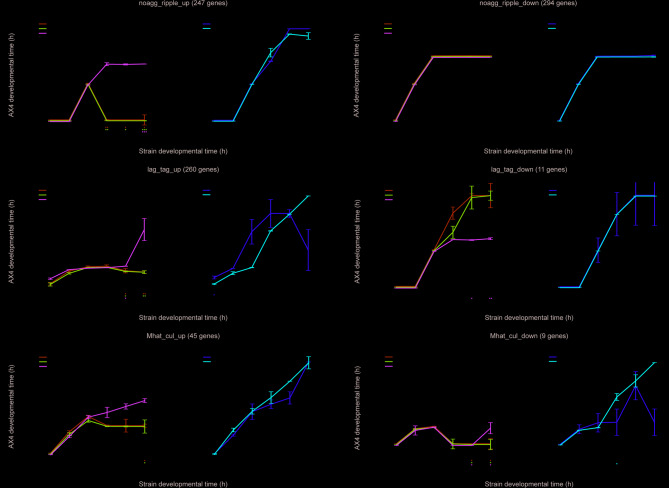
When D. discoideum cells develop on a solid substrate, they exhibit several morphological transitions. First, they appear as a flat, featureless field of unaggregated cells (noagg). Then, ripples appear as the cells begin to aggregate. The first multicellular structures appear as loose aggregates (lag) that transform into tight aggregates (tag) after about 12 h of development. Prespore and prestalk cells differentiate around that time, and the developing prestalk cells accumulate at the top of the aggregate as the structure becomes a tipped aggregate (tip). The structure then elongates and assumes a finger-like shape that can topple over and form a migrating slug. These structures eventually settle and transform their shape until they resemble a Mexican hat (Mhat). In the final stages, the structure culminates (cul) as the prestalk cells from the top of the Mexican hat structure begin to form a stalk that elongates through the prespore cell mass toward the solid substrate. The ultimate structure is a fruiting body (FB), in which a slender cellular stalk carries a ball of spores. These morphological transitions are accompanied by transcriptional changes and eight groups of transcripts serve as milestones that exhibit typical changes whenever these transitions occur [22]. To refine the analysis of the transcriptional differences between the mutants and the wild type in this study, we focused the comparison on the transcriptional milestones. Fig. S4 shows the results of the comparison between the wild type and all the mutants as well as the average transcriptional pattern of the respective genes in the wild type [22]. In each case, we considered a group of up-regulated genes that was designated ‘up’, and a group of down-regulated genes that was designated ‘down’ in the context of the developmental transition. For example, noagg_ripple_up refers to a group of genes that are up-regulated during the transition from no aggregation to ripples.
Figure 3 shows three of the most informative milestones. In the first transition, from noagg to ripples (Fig. 3a), the up group profiles of the tgrB1– and tgrC1– strains are similar to the wild type in the first 8 h. Then, they turn back to the 0-hour pattern between 12 and 20 h. These findings are consistent with the observation that the two mutant strains arrest and dedifferentiate around 8–12 h of development [22]. The rapgapB– strain is attenuated around 12 h of development, which is also consistent with its morphogenesis [17], and the double mutant rapgapB–tgrB1– and the activated tgrB1 strain AX4 tgrB1L846F exhibited near-normal patterns. The downregulated group is almost identical between the different mutants, suggesting that they all have a similar starvation response, which is not different from the wild type. The loose-aggregate to tight-aggregate (lag-tag) milestone group is shown in Fig. 3b. The patterns are consistent with the failure of the tgrB1– and tgrC1– mutants to transition from loose aggregates to tight aggregates in that they resemble AX4 until the 8-hour time point and then maintain that profile throughout the time course. The rapgapB– strain pattern is consistent with the attenuated morphogenesis of rapgapB– during the first 16 h, with a subsequent trajectory that parallels the AX4 trajectory, suggesting that some of the cells can enter a tight-aggregate phase, which is equivalent to the wild-type mid-development stage, albeit later than the wild type. The double mutant rapgapB–tgrB1– exhibited more advanced profiles than the respective single-gene mutants, consistent with the mutual suppression relationships [17] and the activated tgrB1 strain AX4 tgrB1L846F exhibited a near-normal pattern. The lag-tag downregulated group is rather small, but it suggests that the tgrB1– and tgrC1– mutants are capable of turning off the expression of the respective genes, whereas the rapgapB– strain is not. The Mexican-hat to culmination milestone (Mhat-cul) is shown in Fig. 3c. In the up group, the three single-gene mutation strains tgrB1–, tgrC1–, and rapgapB– resembled AX4 for the first 8 h of development and exhibited various levels of attenuation thereafter. The double mutant rapgapB–tgrB1– resembled the wild type with some attenuation. The downregulated group is too small to be meaningful, but it shows a similar set of trends. The overall pattern of the activated tgrB1 strain AX4 tgrB1L846F was similar to the wild type in most cases, which is consistent with its morphogenesis. More detailed explanations for the images in Fig. S4 are provided in Additional File 1.
Fig. 3.
Comparison between the strains over selected milestone genes. We compared the six strains using an MDS-like method with three milestone gene groups. The black line represents the wild type (AX4) and the colored lines represent the mutant strains. tgrB1–, tgrC1–, and rapgapB– are knockout strains for the respective single genes. rapgapB–tgrB1– is a strain in which the respective two genes were knocked out. AX4 L846F is a wild-type strain that carries an additional allele of activated tgrB1L846F. The x-axis represents the developmental time (hours) of the listed strain and the y-axis represents the developmental time (hours) of the wild-type reference strain (AX4). Colored lines above and to the left of the black line represent precocious developmental progression. Colored lines below and to the right represent attenuated developmental progression. The first two panels in each row represent genes that are up-regulated during the milestone transition and the next two panels represent genes that are down-regulated during the milestone transition. (a) The milestone group represents the no-aggregation to ripples transition (noagg_ripple). The upregulated group (_up) consists of 247 genes and the downregulated group (_down) consists of 294 genes. (b) The milestone group represents the loose-aggregate to tight-aggregate transition (lag_tag). The upregulated group (_up) consists of 260 genes and the downregulated group (_down) consists of 11 genes. (c) The milestone group represents the Mexican hat to culmination transition (Mhat_cul). The upregulated group (_up) consists of 45 genes and the downregulated group (_down) consists of 9 genes. All eight milestone-based comparisons, including the above groups as well as information about the average developmental trajectory of the respective genes, are described in Fig. S4. Each time point on the graph represents the mean of three independent replications and the error bars represent the respective standard errors of the mean. Statistical significance between each strain and AX4 was assessed at each time point using a two-sample t-test. P-values were adjusted using the Benjamini-Hochberg correction to account for multiple comparisons. Asterisks at the bottom of the plot indicate significant differences: * for p < 0.05, ** for p < 0.01, and *** for p < 0.001. The calculated p-values for all the instances are provided in Additional File 6. The separation between the left and right panels in each section has no functional significance. It was done to simplify the visualization
In some cases, the double mutant rapgapB–tgrB1– patterns deviated from AX4. In the lag_tag_up (Fig. 3b), tag_tip_up (Fig. S4d), tip_slug_up (Fig. S4e), and Mhat_cul_down (Fig. 3c), we observed a down-turn between 16 and 20 h of development compared to AX4. The relatively small number of genes in these groups might explain why they did not cause a global down-turn in the overall pattern (Fig. 2b). While there is no morphological delay or regression in the developmental patterns of these mutants during late development [17], the downturn in these specific milestone genes might suggest that the mutual suppression is incomplete at that stage.
Overall, the data shown in Fig. 3 and Fig. S4 support and refine the conclusions made based on the entire transcriptome in Fig. 2. Most importantly, they indicate that the single gene mutation strains undergo starvation and initiate development like the wild type, but then attenuate or arrest at the time that the respective genes would become functional. They also show that the double mutation in the rapgapB–tgrB1– strain largely restores wild-type behavior. The activated tgrB1 strain AX4 tgrB1L846F exhibits aspects of precocious development (Fig. 3).
Cell-type specific transcripts
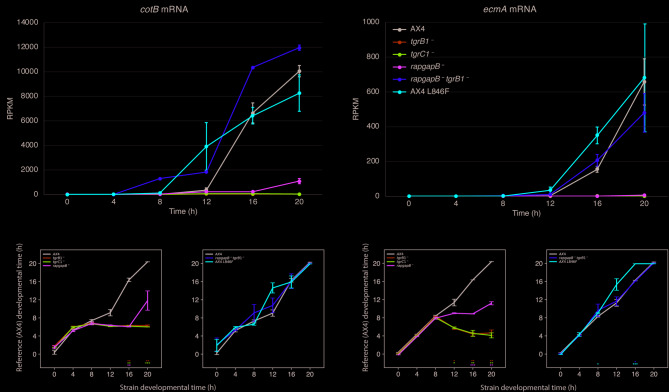
Allotype recognition, which is mediated by matching pairs of tgrB1 and tgrC1, is required for proper expression of the prespore gene cotB and the prestalk gene ecmA [8, 9]. Previous analysis of tgrB1– and tgrC1– cells during development revealed that these mutants do not express significant mRNA levels of these two cell-type-specific genes [22]. It was, therefore, interesting to examine the mRNA abundance of cotB and ecmA as well as other prespore- and prestalk-enriched genes in the relevant mutant strains. Figure 4a shows the mRNA abundance of cotB, which is initially induced between 8 and 12 h of development and continues to accumulate up to 20 h in AX4. The tgrB1– and tgrC1– cells did not accumulate significant mRNA levels of cotB, as expected, and the rapgapB– accumulated minute levels of cotB mRNA with a minor rise between 16 and 20 h. This finding is consistent with low expression of the [cotB]: mCherry marker in rapgapB– cells [17]. The double mutant strain rapgapB–tgrB1– accumulated much more cotB mRNA than the respective single mutants. In fact, rapgapB–tgrB1– cells exhibited elevated levels and precocious accumulation of cotB mRNA compared to the wild type. The AX4 tgrB1L846F cells exhibited precocious accumulation of cotB mRNA between 8 and 12 h and a slight decline between 16 and 20 h (Fig. 4a). Similar patterns were observed with ecmA mRNA, which begins to accumulate after the 12-hour time point and continues to increase thereafter in the wild type (Fig. 4b). The single gene mutation strains, tgrB1–, tgrC1–, and rapgapB–, did not accumulate significant levels of ecmA mRNA, as expected [8, 9, 17], and the double mutant strain rapgapB–tgrB1– accumulated ecmA mRNA at near wild-type levels. The AX4 tgrB1L846F cells exhibited precocious accumulation of ecmA mRNA (Fig. 4b). These results are consistent with the previous findings [22] and with the overall transcriptional progression of the mutants (Fig. 2). It is especially interesting to note that expression of cotB and ecmA was restored to near wild-type levels in the double mutant strain rapgapB–tgrB1–, whereas the respective single mutations, tgrB1– and rapgapB–, nearly abolished the expression of these cell-type specific genes.
Fig. 4.
Comparison between the strains over cell-type specific genes. We traced the mRNA abundance of two transcripts, the prespore gene cotB (a), and the prestalk gene ecmA (b), in the developmental time courses of six strains as indicated in the legend. The mRNA abundance (RPKM, y-axis) is plotted as a function of developmental time (hours, x-axis). AX4 is the wild type; tgrB1–, tgrC1–, and rapgapB– are knockout strains for the respective single genes; rapgapB–tgrB1– is a strain in which the respective two genes were knocked out; AX4 L846F is a wild-type strain that carries an additional allele of activated tgrB1L846F. Each point in all panels represents the mean of three independent replications and the error bars represent the standard errors of the mean. We also compared the six strains using an MDS-like method with two cell-type specific gene groups (c and d). The black line in all panels represents the wild type (AX4) and the colored lines represent the mutant strains. tgrB1–, tgrC1–, and rapgapB– are knockout strains for the respective single genes. rapgapB–tgrB1– is a strain in which the respective two genes were knocked out. AX4 L846F is a wild-type strain that carries an additional allele of activated tgrB1L846F. In the two panels, the x-axis represents the developmental time (hours) of the listed strain and the y-axis represents the developmental time (hours) of the wild-type reference strain (AX4). Colored lines above and to the left of the black line represent precocious developmental progression. Colored lines below and to the right represent attenuated developmental progression. c. The prespore-specific gene group consists of 48 genes. d. The prestalk-specific gene group consists of 87 genes. Statistical significance between each strain and AX4 was assessed at each time point using a two-sample t-test. P-values were adjusted using the Benjamini-Hochberg correction to account for multiple comparisons. Asterisks at the bottom of the plot indicate significant differences: * for p < 0.05, ** for p < 0.01, and *** for p < 0.001. The calculated p-values for all the instances are provided in Additional File 6. The separation of the mutants into two panels in c and d has no functional significance. It was done to simplify the visualization
We expanded the analysis by examining the transcriptional patterns of the strains while focusing on a group of 48 prespore genes (Fig. 4c) and 87 prestalk genes (Fig. 4d). These genes were previously identified by in-situ mRNA hybridization [25] and validated by RNA-seq of mechanically separated prespore and prestalk cells [25, 26]. They are listed in Additional File 2. In the three single-gene knockout strains, tgrB1–, tgrC1–, and rapgapB–, the prespore and prestalk gene patterns resembled AX4 during the first 8 h of development (Fig. 4c and d). This finding is expected because there is almost no prespore or prestalk gene expression during that time. In subsequent time points, the three mutants exhibited an arrest or a delay compared to AX4, which is consistent with their morphological progression patterns. The rapgapB– pattern was more advanced than the other two mutants, as expected from the morphological progression [17]. The double mutant strain rapgapB–tgrB1– was similar to AX4 throughout development (Fig. 4c and d), further supporting the conclusion that the mutual suppression restores normal development [17]. The AX4 tgrB1L846F cells exhibited a slightly precocious and more advanced pattern than AX4, which is consistent with its overall transcriptional progression (Fig. 2). Overall, the results presented in Fig. 4 are congruent with the previous analyses and indicate that prespore and prestalk differentiation patterns are consistent with the overall transcriptional and morphological progression of the mutant strains.
Differentially expressed genes
We searched for differentially expressed genes among the six strains. Figure 5a and b show that most of the genes (10,132) in the six strains were not differentially expressed. Nevertheless, the circles that represent the single-gene mutation strains tgrB1–, tgrC1–, and rapgapB– are skewed to the right of the Venn diagram (Fig. 5a). This finding is consistent with the morphological similarity between these strains and their differences from the strains that progress further in development, AX4, rapgapB–tgrB1– and AX4 tgrB1L846F (Fig. 2).
Fig. 5.
Differentially expressed genes and networks. We compared the transcriptomes of the six strains to one another and performed differential gene expression analysis using a significance threshold of p < 0.0001. In the Venn diagram (a), each circle represents a strain and the overlapping regions represent genes that are not differentially expressed. The strain names are indicated in colored text next to the respective circles and the number of genes in selected sections is indicated in black text. The bar graph (b) shows the number of genes in the respective intersections, the number above each bar indicates the number of commonly (non-differentially) expressed genes, and the dot-and-line chart below each bar indicates which overlapping strains refer to the respective bar (black dots and solid line connectors represent included strains; grey dots represent excluded strains). For example, the third bar represents 424 genes that were commonly expressed in the AX4, rapgapB–tgrB1–, and AX4 tgrB1L846F strains (black dots) and different from the tgrB1– and tgrC1– strains. We then used the differential expression data to compare the wild type AX4 to two single-mutation strains, tgrB1– (c) and rapgapB– (d), and to the respective double mutant rapgapB–tgrB1– (e). We used the differentially expressed genes to perform gene-set enrichment analysis using the dictyBase mutant phenotype category to identify common annotations among the differentially expressed genes. We performed network analysis to view the annotations of the top 100 gene groups and generated a narrative using an artificial intelligence tool. Each node in the network represents a group of genes with a common annotation and the lengths of the arcs are directly proportional to the distance between the nodes. The colored nodes highlight the most significant clusters and the colored text is the respective AI-derived narrative. There is no relationship between the colors in the different subpanels. The respective data are provided in Additional File 4. The data shown in panels c, d, and e are also shown in Fig. S5 in the context of other mutant strains
We then analyzed the differentially expressed gene groups to identify enriched gene sets with common annotations, and performed network analysis to detect community structures among the gene sets [27]. We used the large language model GPT4 to extract the most common annotations and describe them in a coherent manner [28]. For common annotations, we considered the Gene Ontology (GO) biological process [29, 30] as well as the phenotypes induced by mutating the annotated genes as listed in dictyBase [31, 32]. The differential gene expression (DGE) results are provided in Additional File 3, the gene set enrichment results are provided in Additional File 4 (dictyBase mutant phenotypes) and Additional File 5 (GO biological process), and the graphs are shown in Fig. S5 (dictyBase Mutant Phenotypes) and Fig. S6 (GO Biological Process). Text descriptions of the results are provided in Additional File 1. Figure 5 illustrates a characteristic result of the dictyBase Mutant Phenotype analysis using the case of tgrB1–, rapgapB–, and rapgapB–tgrB1–.
The differences between tgrB1– and AX4 yielded four major categories (Fig. 5c). The first one was “Variations in developmental stages, cell morphology, motility, and response to environmental signals”. The second was “Changes in cellular signaling pathways, chemotaxis and cell polarity”. The third was, “Differences in cell differentiation particularly in prestalk and prespore cells” and the fourth one was “Alterations in cell differentiation, vacuole size, and response to environmental conditions”. This outcome is consistent with the hypothesis that tgrB1– is required for morphogenesis and development past the loose aggregate stage [22]. More specifically, observing “chemotaxis and cell polarity” among the annotations is consistent with previous findings that the tgrB1-tgrC1 system is essential for chemotactic cell movement [33] and cell polarization in rotating aggregates [9].
The differences between rapgapB– and AX4 yielded three major categories (Fig. 5d). The first one was “Alterations in cellular response to external stress and signaling regulation”. The second one was “Changes in cellular morphology, signaling pathways, and actin dynamics”. The third one was “Variations in gene expression, cell differentiation, and developmental processes”. These categories, especially the relationship with cell morphology and actin dynamics, are consistent with the role of RapGAPB as a regulator of RapA, because RapA is a master regulator of various cytoskeletal and signaling functions during growth and development [11, 12].
The differences between rapgapB–tgrB1– and AX4 yielded three categories as well (Fig. 5e). The first one included “Variations in autophagy, mitochondrial function and cellular responses”. The second one consisted of “General cellular changes, including morphology, motility, and developmental stages”. The third one included “Metabolic changes, particularly in glycogen and amino acid levels”. The relationship to autophagy and mitochondrial function are particularly relevant because autophagy gene function is tightly related to sporulation [34] and mitochondrial activity is highly enriched in prespore cells [35, 36]. Nevertheless, there is no similarity with either one of the two single-gene mutant strains (Fig. 5 and Fig. S5), supporting the hypothesis that the double-gene knockout represents mutual suppression that restores wild-type-like development rather than diverting the cells to an alternative developmental pathway. The differentially expressed genes in Fig. 5e did not include canonical developmental pathways such as those that lead to prespore and prestalk differentiation, further supporting the conclusion that the mutual suppressors restored wild-type-like development. Similar findings were made with the GO Biological Process networks (Fig. S6), further supporting these conclusions and indicating that they are applicable to more than one annotation method.
Data on the expression of specific genes in the different mutant strains are available for exploration on dictyExpress (https://app.dictyexpress.org), where one can explore individual genes or small groups of genes and compare their expression patterns between various mutants [37].
Discussion
Our data support the conclusion that tgrB1, tgrC1, and rapgapB are components of a regulatory feedback loop in which rapgapB activity negatively regulates tgrB1 and tgrC1 expression whereas tgrB1 and tgrC1 activities positively regulate rapgapB expression. These transcriptional regulatory relationships are probably mediated through RapA activity, as indicated in Fig. S1, and various transcriptional regulators. They also support the hypothesis that the mutual suppression between tgrB1 and rapgapB restores wild-type like development rather than divert the cells to an alternative developmental pathway. The possibility of alternative pathways in development stems from the observation that D. discoideum can respond to starvation in several ways, including induction of a sexual cycle [38, 39] and the formation of aspidocytes [40], both of which are characterized by transcriptional signatures that are distinct from the multicellular development signature [40, 41]. The original mutual suppression findings relied on developmental morphology and social behavior [10, 17], but they could not distinguish between restoration of the wild-type pathway and induction of an alternative pathway. Our transcriptome data support the conclusion that the wild-type developmental pathway was restored at the macro level of overall gene expression, as well as at the granular level of restored developmental milestones and restored prespore and prestalk gene expression. Moreover, the differential gene expression analysis revealed similarities between the single-gene mutation strains and similarities between the double-gene mutation strain and the wild type.
Mutual suppressors are uncommon in molecular genetics. They usually indicate that the participating genes encode components of a large protein complex or elements of regulatory pathways [18, 19]. Our findings support the possibility that tgrB1, tgrC1, and rapgapB participate in a regulatory pathway, probably indirectly. While we have some knowledge of the mechanistic function of tgrB1 and tgrC1 and the proteins they encode [8, 9], rapgapB probably participates in various pathways, because it regulates the multifaceted effector RapA during development [11, 12]. It is hard to explain how the combination of two deleterious mutations in two different genes can restore a near wild-type phenotype, but the finding that the tgrB1, tgrC1, and rapgapB pathway involves both positive and negative regulatory relationships might hold the key to solving the mechanistic details of this observation.
The activated tgrB1 allele tgrB1L846F exhibited two interesting behaviors. First, the level of tgrB1 mRNA was greatly increased, suggesting that the mutation contributed to increased mRNA synthesis and/or stability. Second, expression of tgrB1L846F induced precocious development. When cells with compatible allotypes interact, cis-heterodimers of TgrC1 on the membrane of one cell induce homodimerization of TgrB1 on the membrane of the interacting cell [42]. It is possible that the protein encoded by tgrB1L846F in the wild-type background can interact with the resident TgrB1 protein in cis, inducing more rapid activation of the developmental pathways that are regulated by TgrB1 and TgrC1, including the transition from unicellularity to multicellularity [8, 9].
Conclusions
We conclude that tgrB1, tgrC1, and rapgapB participate in a transcriptional feedback pathway, where rapgapB negatively regulates tgrB1 and tgrC1 whereas tgrB1 and tgrC1 positively regulate rapgapB. We also conclude that the mutual suppression between tgrB1 and rapgapB restores wild-type development.
Methods
Cell culture, strains, strain maintenance, development and cell collection
All the D. discoideum strains were derivatives of AX4 [23]. The tgrB1– and tgrC1– strains were generated by CRISPR/Cas9 mutagenesis as described [16]. In tgrB1, the sequence between nucleotides 701–741 was replaced by a single ‘C’. In tgrC1, a single ‘A’ was inserted at nucleotide 403. The rapgapB– strain and the double mutant rapgapB–tgrB1– were generated as described [17]. The AX4 tgrB1L846F strain was generated as described [8]. We grew the cells, developed them and collected samples at 0, 4, 8, 12, 16 and 20 h as described [22]. Each strain was grown and developed three independent times.
RNA-sequencing
Total RNA was extracted at each indicated developmental time point by resuspending approximately 2.5 × 107 cells in 1 mL of TRIzol® (Invitrogen) and following the manufacturer’s recommended protocol. To isolate mRNA, we performed two rounds of poly(A) selection using the Dynabeads™ mRNA Purification Kit (Invitrogen). The resulting mRNA (100 ng) was fragmented to an optimal length of 200 nucleotides and reverse-transcribed with random priming to generate double-stranded cDNA. Indexed Illumina libraries were prepared from each sample and pooled in equal amounts. The multiplexed libraries were sequenced on a NovaSeq 6000 platform using a 150 bp paired-end sequencing approach. The sequencing data for two of the strains, rapgapB– and rapgapB–tgrB1–, exhibited lower coverage, so their libraries were sequenced twice and their FASTQ files were combined before further processing. The sequencing data are available from the NCBI Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE279943.
Data analysis
After demultiplexing, we used the Genialis platform (https://www.genialis.com) to perform data processing. All the reads were filtered with BBDuk (paired-end) to remove adapters and low-quality bases. Filtered reads were aligned uniquely to the D. discoideum reference genome using Bowtie (Dicty). Unmapped reads were processed further by repeated alignment after trimming two bases from the end of the reads. Mapped reads that uniquely aligned to gene exons were then summed to generate a raw count per gene. Because some genomic regions cannot be uniquely mapped (e.g., repeat elements), only the mappable regions of each exon were used to calculate the gene length. For normalization, mRNA abundance data were reported in Reads Per Kilobase per Million (RPKM).
Extraction of specific mRNA abundance profiles from the dataset and from published data sets were done with the Orange Data Mining software (v3.36) [43] and plotted with Microsoft Excel (v16.84). The MDS-like method for comparing gene expression patterns is described in Additional File 1. The milestone groups of genes were previously defined [22] and the prespore and prestalk genes were previously defined [25] and validated [26]. They are listed in Additional File 2.
Differential Gene Expression was performed using a natural cubic spline regression model (R package splineTimeR) with one treatment factor and time as a continuous variable fitted to the experimental time-course data [27, 44]. In each case, one mutant dataset was compared to the wild type AX4 dataset with a p-value cutoff of 0.0001. Parameters of the fitted spline regression models were used to define differentially expressed genes over time and the Top 500 genes were used for gene set enrichment analysis.
Gene Set Enrichment Analysis was carried out using the enrichPath function from the R package splineTimeR, the same package used for finding the differentially expressed genes. We considered the top 50 gene sets under the dictyBase Mutant Phenotype annotation [32], and the top 100 gene sets under the GO Biological Process annotation [30]. Gene set annotations were downloaded from dictyBase (http://dictybase.org/Downloads, “All curated mutants with phenotypes”) and from the Gene Ontology database (Gene Ontology DOI: 10.5281/zenodo.7942786, data from 2023-05-10). We used the Infomap algorithm [45] to identify communities among the enriched gene sets and to generate network images. We used the large language model GPT4 to summarize the most common annotations and describe them in a concise and coherent manner [28].
The custom code used to analyze the data and a ReadMe file are available at https://github.com/lenatr99/RNAseq_dicty.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- RNA-seq
Ribo Nucleic Acid sequencing
- MDS
Multidimensional scaling
- Noagg
No aggregation
- lag
Loose aggregate
- tag
Tight aggregate
- tip
Tipped aggregate
- Mhat
Mexican hat
- cul
Culminant
- FB
Fruiting body
- GO
Gene Ontology
- DGE
Differential Gene Expression
Author contributions
M.K.-K., G.S. and B.Z. designed the experiments; M.K.-K. and P.L. performed the experiments; M.K.-K. performed the RNA-seq analysis; M.K.-K. and L.T. performed the data analysis; L.T. and B.Z. developed and implemented the MDS-like method for comparing gene expression patterns; M.K.-K., L.T., and G.S. prepared the figures and tables; G.S. wrote the manuscript; M.K.-K., L.T., P.L., and B.Z. reviewed and edited the manuscript.
Funding
This work was supported by the National Institutes of Health (NIH) grant number R35 GM152113 and the Slovenian Research and Innovation Agency, grant numbers L2-60154 and P2-0209.
Data availability
All raw and processed sequencing data generated in this study have been submitted to the NCBI Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE279943. The data are also available for online exploration on dictyExpress at https://app.dictyexpress.org. Materials are available from the corresponding author upon reasonable request. The custom code used to analyze the data and a ReadMe file are available at https://github.com/lenatr99/RNAseq_dicty.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kessin RH. Dictyostelium - Evolution, cell biology, and the development of multicellularity. Cambridge, UK: Cambridge Univ. Press; 2001. [Google Scholar]
- 2.Strassmann JE, Gilbert OM, Queller DC. Kin discrimination and Cooperation in microbes. Annu Rev Microbiol. 2011;65:349–67. [DOI] [PubMed] [Google Scholar]
- 3.Shaulsky G, Kessin RH. The cold war of the social amoebae. Curr Biology: CB. 2007;17(16):R684–692. [DOI] [PubMed] [Google Scholar]
- 4.Kundert P, Shaulsky G. Cellular allorecognition and its roles in Dictyostelium development and social evolution. Int J Dev Biol. 2019;63(8–9–10):383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benabentos R, Hirose S, Sucgang R, Curk T, Katoh M, Ostrowski EA, Strassmann JE, Queller DC, Zupan B, Shaulsky G, et al. Polymorphic members of the lag gene family mediate kin discrimination in Dictyostelium. Curr Biology: CB. 2009;19(7):567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirose S, Benabentos R, Ho HI, Kuspa A, Shaulsky G. Self-recognition in social amoebae is mediated by allelic pairs of tiger genes. Science. 2011;333(6041):467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dynes JL, Clark AM, Shaulsky G, Kuspa A, Loomis WF, Firtel RA. LagC is required for cell-cell interactions that are essential for cell-type differentiation in Dictyostelium. Genes Dev. 1994;8(8):948–58. [DOI] [PubMed] [Google Scholar]
- 8.Hirose S, Chen G, Kuspa A, Shaulsky G. The polymorphic proteins TgrB1 and TgrC1 function as a ligand-receptor pair in Dictyostelium allorecognition. J Cell Sci. 2017;130(23):4002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirose S, Santhanam B, Katoh-Kurosawa M, Shaulsky G, Kuspa A. Allorecognition, via TgrB1 and TgrC1, mediates the transition from unicellularity to multicellularity in the social amoeba Dictyostelium discoideum. Development. 2015;142(20):3561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CL, Santhanam B, Webb AN, Zupan B, Shaulsky G. Gene discovery by chemical mutagenesis and whole-genome sequencing in Dictyostelium. Genome Res. 2016;26(9):1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkinson K, Bolourani P, Traynor D, Aldren NL, Kay RR, Weeks G, Thompson CR. Regulation of Rap1 activity is required for differential adhesion, cell-type patterning and morphogenesis in Dictyostelium. J Cell Sci. 2009;122(Pt 3):335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilbi H, Kortholt A. Role of the small GTPase Rap1 in signal transduction, cell dynamics and bacterial infection. Small GTPases. 2019;10(5):336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruenheit N, Parkinson K, Stewart B, Howie JA, Wolf JB, Thompson CR. A polychromatic ‘greenbeard’ locus determines patterns of Cooperation in a social amoeba. Nat Commun. 2017;8:14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madgwick PG, Belcher LJ, Wolf JB. Greenbeard genes: theory and reality. Trends Ecol Evol. 2019;34(12):1092–103. [DOI] [PubMed] [Google Scholar]
- 15.Gardner A. The Greenbeard effect. Curr Biology: CB. 2019;29(11):R430–1. [DOI] [PubMed] [Google Scholar]
- 16.Katoh-Kurasawa M, Lehmann P, Shaulsky G. The Greenbeard gene tgrB1 regulates altruism and cheating in Dictyostelium discoideum. Nat Commun. 2024;15(1):3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann P, Katoh-Kurasawa M, Kundert P, Shaulsky G. Going against the family: perturbation of a Greenbeard pathway leads to falsebeard cheating. iScience. 2024;27(11):111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Leeuwen J, Pons C, Boone C, Andrews BJ. Mechanisms of suppression: the wiring of genetic resilience. BioEssays 2017, 39(7):1700042. [DOI] [PMC free article] [PubMed]
- 19.Prelich G. Suppression mechanisms: themes from variations. Trends Genet. 1999;15(7):261–6. [DOI] [PubMed] [Google Scholar]
- 20.Hughes TR, Marton MJ, Jones AR, Roberts CJ, Stoughton R, Armour CD, Bennett HA, Coffey E, Dai H, He YD, et al. Functional discovery via a compendium of expression profiles. Cell. 2000;102(1):109–26. [DOI] [PubMed] [Google Scholar]
- 21.Van Driessche N, Demsar J, Booth EO, Hill P, Juvan P, Zupan B, Kuspa A, Shaulsky G. Epistasis analysis with global transcriptional phenotypes. Nat Genet. 2005;37(5):471–7. [DOI] [PubMed] [Google Scholar]
- 22.Katoh-Kurasawa M, Hrovatin K, Hirose S, Webb A, Ho HI, Zupan B, Shaulsky G. Transcriptional milestones in Dictyostelium development. Genome Res. 2021;31(8):1498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knecht DA, Cohen SM, Loomis WF, Lodish HF. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986;6(11):3973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosengarten RD, Santhanam B, Fuller D, Katoh-Kurasawa M, Loomis WF, Zupan B, Shaulsky G. Leaps and lulls in the developmental transcriptome of Dictyostelium discoideum. BMC Genomics. 2015;16(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda M, Sakamoto H, Iranfar N, Fuller D, Maruo T, Ogihara S, Morio T, Urushihara H, Tanaka Y, Loomis WF. Changing patterns of gene expression in Dictyostelium prestalk cell subtypes recognized by in situ hybridization with genes from microarray analyses. Eukaryot Cell. 2003;2(3):627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh A, Miranda ER, Katoh-Kurasawa M, Fuller D, Rot G, Zagar L, Curk T, Sucgang R, Chen R, Zupan B, et al. Conserved developmental transcriptomes in evolutionarily divergent species. Genome Biol. 2010;11(3):R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spies D, Renz PF, Beyer TA, Ciaudo C. Comparative analysis of differential gene expression tools for RNA sequencing time course data. Brief Bioinform. 2019;20(1):288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joachimiak MP, Caufield JH, Harris NL, Kim H, Mungall CJ. Gene Set Summarization Using Large Language Models. ArXiv 2024.
- 29.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25(1):25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gene Ontology C, Aleksander SA, Balhoff J, Carbon S, Cherry JM, Drabkin HJ, Ebert D, Feuermann M, Gaudet P, Harris NL et al. The gene ontology knowledgebase in 2023. Genetics 2023, 224(1):iyad031. [DOI] [PMC free article] [PubMed]
- 31.Kreppel L, Fey P, Gaudet P, Just E, Kibbe WA, Chisholm RL, Kimmel AR. DictyBase: a new Dictyostelium discoideum genome database. Nucleic Acids Res. 2004;32(Database issue):D332–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fey P, Dodson RJ, Basu S, Hartline EC, Chisholm RL. DictyBase and the dicty stock center (version 2.0) - a progress report. Int J Dev Biol. 2019;63(8–9–10):563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukumaran S, Brown JM, Firtel RA, McNally JG. lagC-null and gbf-null cells define key steps in the morphogenesis of Dictyostelium mounds. Dev Biol. 1998;200(1):16–26. [DOI] [PubMed] [Google Scholar]
- 34.Yamada Y, Schaap P. The proppin Bcas3 and its interactor KinkyA localize to the early phagophore and regulate autophagy. Autophagy. 2021;17(3):640–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaulsky G, Loomis WF. Mitochondrial DNA replication but no nuclear DNA replication during development of Dictyostelium. Proc Natl Acad Sci USA. 1995;92(12):5660–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi I. The correlation of cellular changes with succinic dehydrogenase and cytochrome oxidase activities in the development of the cellular slime molds. Dev Biol. 1960;2(4):343–66. [DOI] [PubMed] [Google Scholar]
- 37.Stajdohar M, Rosengarten RD, Kokosar J, Jeran L, Blenkus D, Shaulsky G, Zupan B. DictyExpress: a web-based platform for sequence data management and analytics in Dictyostelium and beyond. BMC Bioinformatics. 2017;18(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Day DH, Keszei A. Signalling and sex in the social amoebozoans. Biol Rev Camb Philos Soc. 2012;87(2):313–29. [DOI] [PubMed] [Google Scholar]
- 39.Sinha U, Ashworth JM. Evidence for the existence of elements of a para-sexual cycle in the cellular slime mould, Dictyostelium discoideum. Proc R Soc Lond B. 1969;173:531–40. [Google Scholar]
- 40.Serafimidis I, Bloomfield G, Skelton J, Ivens A, Kay RR. A new environmentally resistant cell type from Dictyostelium. Microbiol (Reading). 2007;153(Pt 2):619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muramoto T, Suzuki K, Shimizu H, Kohara Y, Kohriki E, Obara S, Tanaka Y, Urushihara H. Construction of a gamete-enriched gene pool and RNAi-mediated functional analysis in Dictyostelium discoideum. Mech Dev. 2003;120(8):965–75. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Xu X, Wu X, Thomson A, Siu CH. Assembly of the TgrB1-TgrC1 cell adhesion complex during Dictyostelium discoideum development. Biochem J. 2014;459(2):241–9. [DOI] [PubMed] [Google Scholar]
- 43.Demšar J, Curk T, Erjavec A, Gorup Č, Hočevar T, Milutinovič M, Možina M, Polajnar M, Toplak M, Starič A. Orange: data mining toolbox in Python. J Mach Learn Res. 2013;14(1):2349–53. [Google Scholar]
- 44.Michna A. splineTimeR: Time-course differential gene expression data analysis using spline regression models followed by gene association network reconstruction. In. Edited by Bioconductor, Bioconductor version: Release (3.18); R package version 1.30.0 edn; 2023.
- 45.Rita L, Francisco A, Carriço J, Borges V. Community finding with applications on phylogenetic networks. In: 2020; Cham. Springer International Publishing; 2020. pp. 1935–53.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw and processed sequencing data generated in this study have been submitted to the NCBI Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE279943. The data are also available for online exploration on dictyExpress at https://app.dictyexpress.org. Materials are available from the corresponding author upon reasonable request. The custom code used to analyze the data and a ReadMe file are available at https://github.com/lenatr99/RNAseq_dicty.