Abstract
Circadian (≅24-h) rhythms are governed by endogenous biochemical oscillators (clocks) that in a wide variety of organisms can be phase shifted (i.e., delayed or advanced) by brief exposure to light and changes in temperature. However, how changes in temperature reset circadian timekeeping mechanisms is not known. To begin to address this issue, we measured the effects of short-duration heat pulses on the protein and mRNA products from the Drosophila circadian clock genes period (per) and timeless (tim). Heat pulses at all times in a daily cycle elicited dramatic and rapid decreases in the levels of PER and TIM proteins. PER is sensitive to heat but not light, indicating that individual clock components can markedly differ in sensitivity to environmental stimuli. A similar resetting mechanism involving delays in the per-tim transcriptional-translational feedback loop likely underlies the observation that when heat and light signals are administered in the early night, they both evoke phase delays in behavioral rhythms. However, whereas previous studies showed that the light-induced degradation of TIM in the late night is accompanied by stable phase advances in the temporal regulation of the PER and TIM biochemical rhythms, the heat-induced degradation of PER and TIM at these times in a daily cycle results in little, if any, long-term perturbation in the cycles of these clock proteins. Rather, the initial heat-induced degradation of PER and TIM in the late night is followed by a transient and rapid increase in the speed of the PER-TIM temporal program. The net effect of these heat-induced changes results in an oscillatory mechanism with a steady-state phase similar to that of the unperturbed control situation. These findings can account for the lack of apparent steady-state shifts in Drosophila behavioral rhythms by heat pulses applied in the late night and strongly suggest that stimulus-induced changes in the speed of circadian clocks can contribute to phase-shifting responses.
Circadian (≅24-h) rhythms are governed by endogenous biochemical oscillators, or clocks (reviewed in references 18 and 46). Although these rhythms persist under constant environmental conditions, they can be entrained (synchronized) by external time cues (zeitgebers), most notably the daily light/dark and temperature cycles. The adaptive ability of circadian clocks to be reset by external cues enables organisms to maintain temporal alignment between their endogenously driven rhythms and biologically advantageous times in a daily cycle. A powerful strategy for probing this resetting feature is based on the ability of short pulses of environmental stimuli or other agents to elicit phase shifts in circadian pacemakers. These perturbation experiments revealed that the direction (i.e., delay or advance) and magnitude of a phase shift are functions of the time in a daily cycle that the zeitgeber is administered. Plotting the average phase shift as a function of time that the stimulus was applied yields a phase-response curve (PRC) that describes the resetting behavior of the clock for that particular agent. A major challenge in the study of circadian biology is to elucidate how timekeeping mechanisms are reset by changes in environmental conditions.
With the likely exception of light, temperature is the most predominant entraining cue in nature (18). Although early studies revealed that even in poikilotherms the free-running periods of circadian rhythms are essentially invariant over a wide range of constant temperatures, these rhythms can be phase shifted by changes in temperature (pulses and steps) and entrained by daily temperature cycles. This thermosensitivity has been demonstrated not only in poikilotherms (2, 12, 37, 55) but also in some homeotherms (47). In addition to the entraining capabilities of temperature, it appears that clocks can function only within a restricted range of temperatures and that outside these limits, timekeeping mechanisms stop or are held constant (recently reviewed in reference 28). Although the wide-ranging effects of temperature on circadian rhythms have been the subject of great interest, the molecular underpinnings governing how clocks are regulated by temperature are not well understood. A contributing factor to the paucity of information on this important topic is that in Drosophila melanogaster and Neurospora crassa, currently the best-characterized model systems for investigating how circadian clocks operate, very few studies have analyzed the effects of temperature on the underlying oscillatory mechanisms. Work in Drosophila has largely been limited to investigating the molecular basis for temperature compensation (14, 20). In N. crassa, very recent studies addressed how temperature limits that are permissive for rhythmicity are established (13, 28). However, there are no reports describing how circadian clocks are perturbed by changes in temperature. A related fundamental issue that has not been addressed is whether temperature and light signals regulate circadian oscillators in similar or different manners.
The isolation of clock mutants and genes in D. melanogaster makes this species an attractive system for investigating the response of a circadian timekeeping mechanism to fluctuations in temperature. Two genes, called period (per) and timeless (tim), have been shown to be required for circadian rhythms in locomotor activity and eclosion (emergence from pupal cases) (24, 43). In the adult fly head (the anatomical location of the fruit fly circadian pacemaker underlying rhythms in locomotor activity [10, 11, 23, 50]), the PER and TIM proteins undergo daily fluctuations in abundance (8, 21, 32, 45, 52–54), phosphorylation state (8, 53), subcellular distribution (5, 21, 25, 32), and native size (25, 53), consistent with an important role in pacemaker function. Furthermore, per and tim mRNAs oscillate (16, 17, 44) by means of a feedback loop, likely negative (16, 25, 31, 44, 52), that depends on the presence of both PER and TIM (17, 44), suggesting that a shared mechanism participates in the autoregulation of per and (presumably) tim transcription. The observation that PER and TIM physically interact to form a functional complex that enters the nucleus (5, 14, 25, 41, 53) likely explains this reciprocal autoregulation (44, 53). Although the biochemical functions of PER and TIM are not known, numerous lines of evidence indicate that the temporally ordered interdigitation of the various per and tim protein and RNA cycles yields a self-sustaining and entrainable transcription-translation negative feedback loop of ∼24 h that is the core of a circadian timekeeping mechanism in D. melanogaster (for a recent review, see reference 40). In an analogous manner, the RNA and protein products from the Neurospora clock gene frequency (frq) also comprise a transcription-translation-based autoinhibitory loop that is central to the oscillatory mechanism in this species (4, 13).
Importantly, the per, tim, and frq gene products satisfy most, if not all, of the criteria for bona fide state variables of a clock (4, 40). (A state variable is a clock component whose rhythmic changes in abundance or activity, not mere presence in the cell, is a necessary element of the timekeeping mechanism [4].) Understanding how state variables are modulated by external stimuli should provide significant insights into the mechanisms underlying the resetting and entrainment of circadian clocks. Indeed, recent studies have shown that light signals elicit rapid alterations in the levels of TIM (21, 32, 53) and frq RNA (4), strongly suggesting that these changes are the initial clock-specific events mediating photic entrainment in Drosophila and Neurospora, respectively. Together these findings appear to validate the most widely accepted model for nonparametric entrainment (entrainment by brief pulses of environmental stimuli); a major tenet of this model is that entraining agents reset the phase of the clock by inducing rapid and discrete changes in the level or activity of one or more state variables rather than by evoking longer lasting alterations in the speed of the clock (34, 55). Furthermore, stimulus-induced changes in state variables yield time-of-day-specific shifts that differ in magnitude and direction because these molecular signals are differentially interpreted by the dynamics of the oscillatory mechanism. For example, the light-induced degradation of TIM in the early or late night either delays or advances, respectively, the PER-TIM temporal program (21, 25, 32, 53). These findings can explain the main features of the light PRC for Drosophila behavioral rhythms, with its characteristic nonresponsive zone in the subjective day, phase delays in the early night, and phase advances in the late night (6, 26, 32, 34, 42).
Intriguingly, although brief heat pulses at elevated temperatures also elicit phase delays when applied in the early night, they do not evoke significant phase shifts in the late night (references 7 and 29 and this report). To address the molecular basis for this modality-specific response and to begin to understand how changes in temperature perturb circadian time-keeping mechanisms, we determined the effects of heat pulses on the protein and mRNA products of per and tim. The results indicate that heat signals elicit rapid decreases in the levels of PER and TIM at all times in a daily cycle. Surprisingly, heat pulses in the late night are also accompanied by transient and very rapid increases in the speed of the PER-TIM cycles in abundance and phosphorylation. The net effect of the heat-induced changes in the PER-TIM temporal program by temperature treatments in the late night yields an oscillatory mechanism with a steady-state phase similar to that of the unperturbed control situation. These findings can account for the observation that heat pulses in the late night elicit little, if any, steady-state phase shifts in behavioral rhythms. Thus, although the initial clock-specific response to light or heat signals is likely the rapid degradation of clock proteins, at certain times in a daily cycle these two modalities produce remarkably different long-term effects on the Drosophila circadian time-keeping mechanism.
MATERIALS AND METHODS
Fly maintenance and heat treatments.
The wild-type Canton-S (CS) flies and the mutant per01 flies used in this study were descendants of stocks originally maintained in the laboratory of M. Rosbash (Brandeis University, Waltham, Mass.) and were previously described (8). The tim0 flies were descendants of stocks originally maintained in the laboratory of A. Sehgal (University of Pennsylvania Medical School, Philadelphia) and were previously described (43). All flies were grown and maintained in vials containing standard agar-cornmeal-sugar-yeast-tegosept medium. For heat pulse experiments, vials containing ∼100 young (2- to 6-day-old) adult flies were placed in incubators (Precision Scientific) at 25 or 18°C (see Fig. 2), exposed to four cycles of 12 h of light/12 h of dark (LD; where lights on is zeitgeber time zero [ZT0] and lights off is ZT12), and subsequently maintained in the dark (DD). Between ZT0 and ZT1 of the third LD cycle, flies were transferred to fresh vials containing 2% agar–5% sugar medium. At selected times during constant dark conditions, vials were carefully placed in water baths (for all temperatures used in this study, the maximum variation between experiments was ±0.5°C). The water level was above the highest point that flies could reach inside the vials. Vials containing control untreated flies were handled similarly except that they were not placed in water baths. Following heat treatment for the indicated times, vials were removed from the water bath and either (i) flies were collected immediately by freezing or (ii) the vials were kept in the incubator and flies were subsequently collected at selected times by freezing.
FIG. 2.
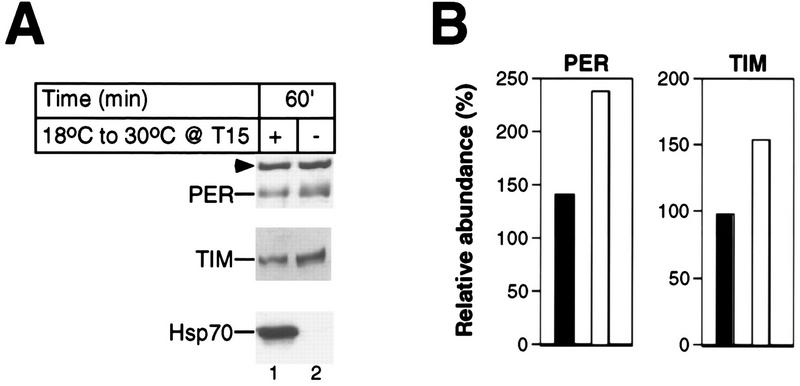
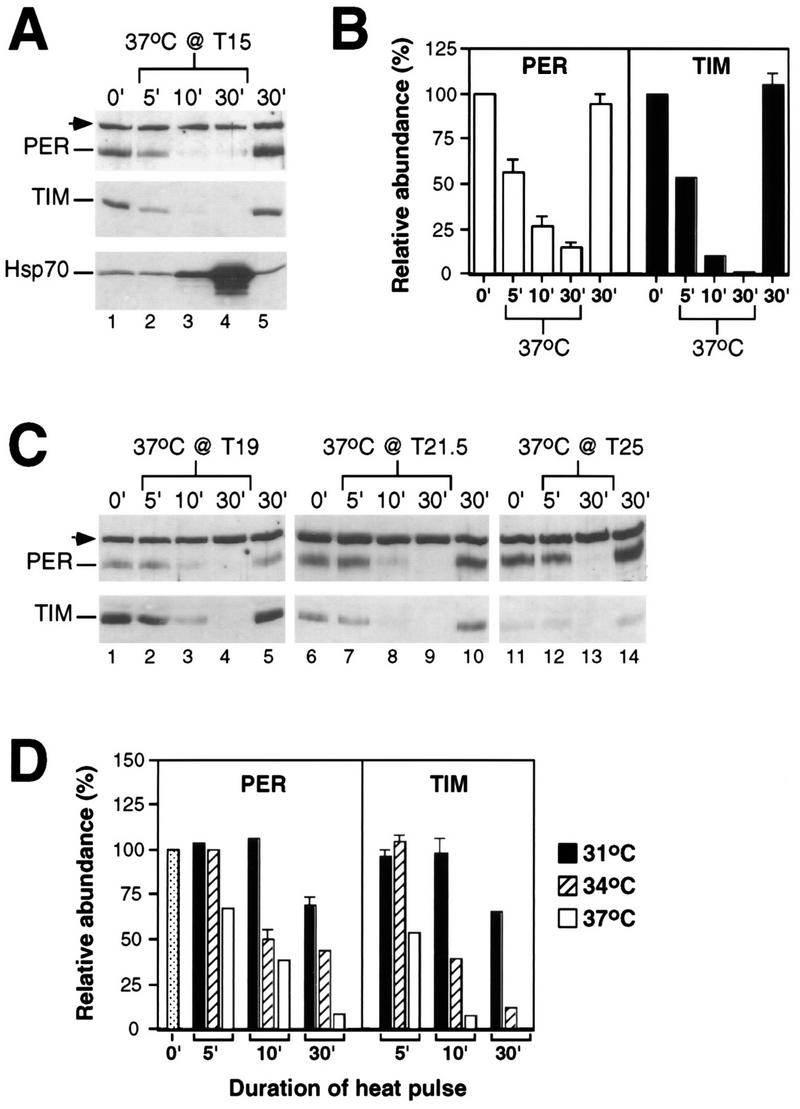
Large temperature changes are not sufficient to evoke rapid reductions in the levels of PER and TIM. Two groups of wild-type CS flies were kept at 18°C during LD and DD conditions. One group was exposed to a 1-h 30°C heat pulse beginning at T15; another group served as controls. (A) Total protein extracts prepared from isolated heads were analyzed by immunoblotting in the presence of antibodies to PER (top), TIM (middle), or Hsp70 (bottom). Above the panels are shown time of fly collection in minutes since the start of the heat pulse and whether flies were heat pulsed (+) or untreated (−). The positions of PER, TIM, and Hsp70 are shown at the left. Arrowhead, cross-reacting size standard. Similar results were obtained in four independent experiments (data not shown), and a representative example is shown. (B) Quantitation of data shown in panel A for heat-pulsed flies (black bars) and control flies (white bars).
Locomotor activity rhythms.
Locomotor activity was monitored by placing individual adult flies in glass tubes and using a Trikinetics (Waltham, Mass.) system interfaced with an Apple computer as previously described (15). The flies were maintained under identical LD and DD conditions in incubators at 25°C as described above. To measure the effects of heat pulses on the locomotor activity rhythm, tubes containing individual flies were carefully removed from the activity monitors (Trikinetics) at selected times in DD and heat pulsed at the indicated temperature in water baths as described above for flies kept in vials. Following heat treatment, the tubes were returned to the appropriate activity monitor and locomotor activity was recorded for another 7 to 10 days in DD. To eliminate complications arising from nonspecific startle effects of heat on the activity of flies during the day of temperature treatment (data not shown), we used data recorded between the third and last days of DD. Activity data were recorded in 30-min bins and stored until analyzed with the Phase program (15). Using this program, we calculated the phase of the locomotor activity rhythm by measuring the time in each consecutive 24-h cycle that the peak of activity occurred. For each individual fly, the peak time of activity for each day was averaged and pooled with the average values from other individual flies of the same genotype that were treated under identical conditions. Finally, the phase shift was calculated by subtracting the values obtained from untreated control flies with those from heat-pulsed flies (Table 1).
TABLE 1.
Average phase shift in locomotor activity induced by heat pulses at different temperatures and times in a daily cycle
| Heat pulse treatmenta | Peak activity (CTe ± SEM [n]) | Phase shift (h)b |
|---|---|---|
| Control | 12.7 ± 0.2 (58) | |
| T15.0, 37°C | 15.0 ± 0.2 (47)c | −2.3 |
| T15.0, 34°C | 13.5 ± 0.3 (29)c | −0.8 |
| T15.0, 31°C | 12.6 ± 0.2 (28)d | 0.1 |
| T20.5, 37°C | 12.5 ± 0.3 (16)d | 0.2 |
| T21.5, 37°C | 12.5 ± 0.2 (27)d | 0.2 |
| T23.0, 37°C | 12.9 ± 0.3 (14)d | −0.2 |
Flies were exposed to several LD cycles at 25°C and heat pulsed for 30 min at the indicated temperatures in the dark phase of the last LD cycle. Heat-pulsed flies were then returned to 25°C, and locomotor activity was continuously recorded during the following 7 to 10 days of DD.
Calculated by subtracting the time in a day when peak activity occurred in control untreated flies from the values obtained for heat-pulsed flies. A negative value indicates a phase delay.
Daily peak of activity significantly different from the control (P < 0.005 by two-tailed t test).
Daily peak of activity not significantly different from the control (P ≥ 0.5 by two-tailed t test).
CT, circadian time, which is equivalent to zeitgeber time (ZT).
Immunoblotting.
Total fly head extract was prepared essentially as described elsewhere (8, 25, 30). Briefly, for each time point ∼30-μl aliquots of heads isolated from frozen flies were placed in a microcentrifuge tube and homogenized at 4°C in 3 volumes (relative to the starting volume of heads) of extraction solution 1 (ES1; 100 mM KCl, 20 mM HEPES [pH 7.5], 5% glycerol, 5 mM EDTA, 1 mM dithiothreitol, 0.1% Triton X-100, 10 μg of aprotonin per ml, 5 μg of leupeptin per ml, 1 μg of pepstatin A per ml), using a battery-operated minihomogenizer (Kontes). Subsequently, homogenates were centrifuged (12 min at 12,000 × g) and clarified supernatants were removed to new tubes. Protein concentration was determined by using a Coomassie protein assay as instructed by the manufacturer (Pierce). An equal volume of 2× sodium dodecyl sulfate (SDS) sample buffer was added to the supernatant fraction, and the mixture was boiled. In some cases, pelleted material was resuspended by sonication in 3 volumes (relative to the starting volume of heads) of both ES1 and 2× SDS sample buffer followed by boiling. Equal amounts of protein (total of ∼40 μg) from clarified supernatant fractions were resolved by electrophoresis on SDS–5.7% polyacrylamide gels as described previously (8, 25). To determine the relative distribution of PER and TIM in the clarified supernatant and pellet material (see Fig. 3), equal volumes of resuspended pellet and corresponding supernatant fractions (resulting in equal number of cells) were used.
FIG. 3.
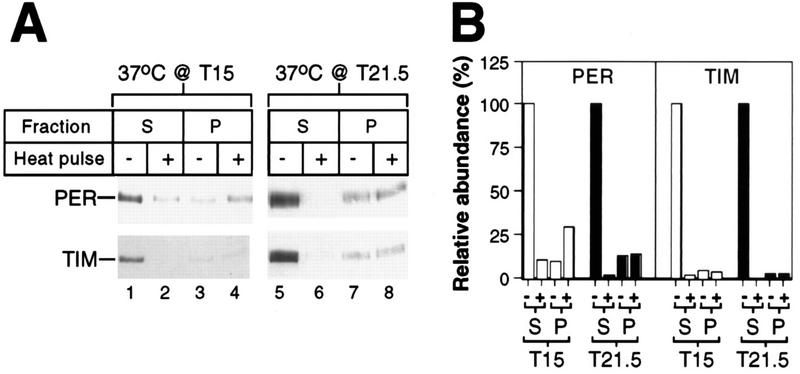
Relative levels of PER and TIM in supernatant and pellet fractions prepared from control and heat-pulsed flies. (A) During the last dark period of LD, two groups of wild-type CS flies were exposed to a 30-min 37°C heat pulse (+) beginning at either T15 or T21.5; another group served as controls (−). Supernatant (S) and pellet (P) fractions from an equal number of cells were analyzed by immunoblotting in the presence of antibodies to PER or TIM. The positions of PER and TIM are indicated at the left. Each experiment was done at least two independent times (data not shown), and representative examples are shown. (B) Quantitation of data shown in panel A for supernatant and pellet. The levels of PER and TIM at T15.5 and T22 in the supernatant fractions prepared from control untreated flies were set at 100.
Immunoblotting and visualization using chemiluminescence (ECL kit; Amersham) were done essentially as described elsewhere (8, 25, 30). One of the antibodies to PER used in this study (herein referred to as RP) was described previously (25) and cross-reacts with a high-molecular-weight nonspecific band (the preimmune serum also reacts with this band [data not shown]) that acts as a convenient internal size standard (see Fig. 1, 2, 4, 5, and 6) (25). In addition, we generated new antibodies to PER by using PCR to amplify per cDNA sequences that encode amino acids 1 to 240 and cloned the PCR fragment upstream of sequences that encode a polyhistidine stretch (His) in the expression vector pET23b (Novagen). Likewise, a similar strategy was used to subclone tim cDNA sequences that encode amino acids 222 to 577 (32) in the pET23b vector. The PER-His and TIM-His fusion proteins were produced in bacteria as recommended by the manufacturer (Novagen) and purified under denaturing conditions (8 M urea), using the TALON metal affinity resin from Clontech. Purified fusion proteins were used to produce antibodies in rats and guinea pigs (Cocalico Biologicals, Reamstown, Pa.). Numerous control experiments verified the specificities of the anti-PER and anti-TIM antibodies used in this study (e.g., Fig. 4 to 7). The PER immunoblots shown in this report were generated mainly by using a combination of two rat anti-PER antibodies (final concentrations of 1:20,000 [RP] and 1:2,000 [PR5-2]; the RP antibody was added because it detects a high-molecular-weight band that acts as a convenient internal size marker). To visualize TIM, immunoblots were incubated with a rat anti-TIM antibody (TR1-3E2) that was diluted to a final concentration of 1:2,000. In some cases (Fig. 6), immunoblots developed with antibodies to PER were stripped and reprobed with antibodies to TIM. The anti-Hsp70 (70-kDa heat shock protein) monoclonal antibody (7FB) used in this study was obtained from S. Lindquist (University of Chicago) and was previously described (29). Bands on autoradiographs were quantified by using a densitometer (Computing Densitometer Scan v 5.0) and ImageQuant software (Molecular Dynamics). Scanned images of autoradiographs were manipulated with Adobe Photoshop 3.0 and Canvas 5.1 software.
FIG. 1.
Heat pulses elicit rapid decreases in the levels of PER and TIM. During the last dark period of LD, a group of wild-type CS flies was exposed to a 30-min heat pulse at the indicated temperature and time; another group served as controls. Total protein extracts were prepared from isolated heads and analyzed by immunoblotting in the presence of antibodies to PER, TIM, or Hsp70. (A and C) Above each lane is shown the time of fly collection in minutes since the start of the heat pulse. The positions of PER, TIM, and Hsp70 are indicated at the left. The arrowhead marks a cross-reacting size standard; this band also reacts with preimmune antibodies to PER (25). Each experiment was done at least three independent times (data not shown), and representative examples are shown. (B and D) Relative levels of PER and TIM in flies heat pulsed at T15 for 30 min and in control untreated flies collected at the indicated times (in minutes since T15). For each of three independent experiments, the levels of PER and TIM at T15 were set to 100. Shown are the average results from at least three independent experiments. The standard error of the mean is shown above each bar. (D) Flies were heat pulsed at either 31, 34, or 37°C.
FIG. 4.
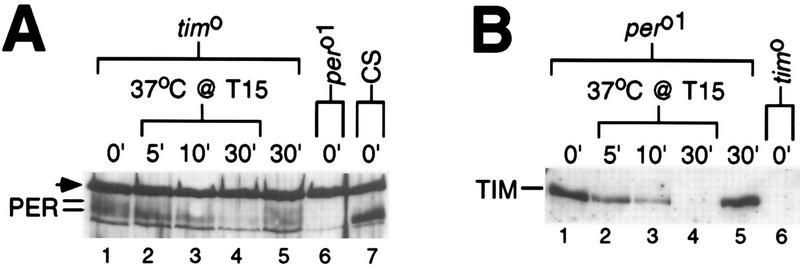
PER and TIM can be independently regulated by heat. Several different strains of flies (genotypes indicated at the top) were maintained under LD conditions. For each genotype, a group of flies was exposed to a 37°C heat pulse at T15; another group served as controls. Head extracts were prepared and immunoblots were incubated with antibodies to either PER (A) or TIM (B). Above each lane is shown the time of fly collection in minutes since the start of the heat pulse. (A) Arrowhead, cross-reacting size standard. Note that multiple PER species differing in electrophoretic mobility are detected in tim0 flies (compare lanes 5 and 7) (36). In the experiment shown, at least two major isoforms of PER (indicated at the left) are visible.
FIG. 5.
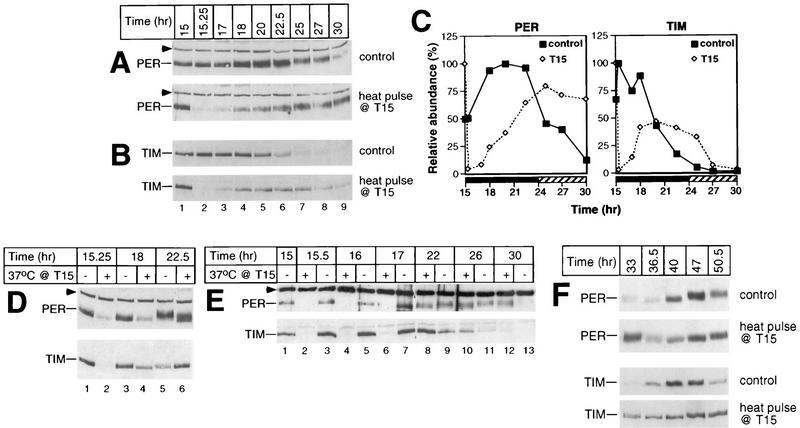
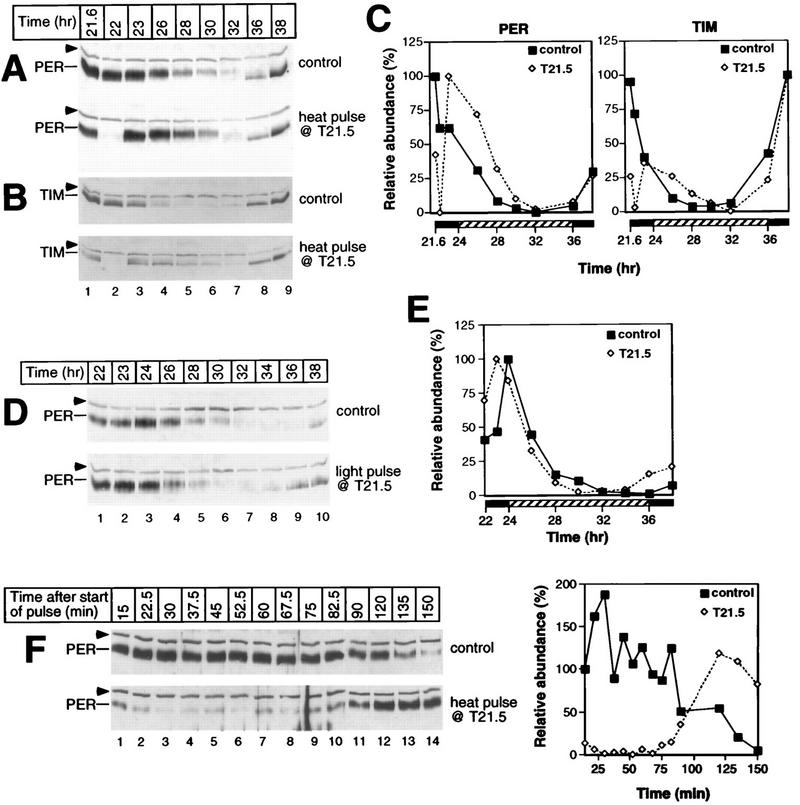
Heat pulses in the early night are accompanied by delays in the PER and TIM biochemical cycles. During the last dark period of LD, a group of CS flies was exposed to a 37°C heat pulse of 30 min beginning at T15; another group served as controls. Total protein extracts were prepared from isolated heads and analyzed by immunoblotting in the presence of antibodies to either PER (A and top panels in D, E, and F) or TIM (B and bottom panels in D, E, and F). The time of fly collection in hours since the last dark-light transition at ZT0 is shown above the panels. The positions of PER and TIM are indicated at the left. Arrowheads mark the cross-reacting size standard. (D and E) Above each lane is shown whether flies were heat pulsed (+) or untreated (−). Each experiment was done at least six times (data not shown), and representative examples are shown. (C) Quantitation of results shown in panels A and B. Depicted are the relative levels of PER and TIM in either heat-pulsed (T15) or control flies as a function of time (hours since the last dark-light transition at ZT0). The peak value for each group of flies was set at 100.
FIG. 6.
Pulses of heat and light at T21.5 produce remarkably different effects on the PER and TIM biochemical cycles. Following entrainment in LD, a group of CS flies was exposed at T21.5 to either a 30-min heat pulse at 37°C (A, B, and F) or a 30-min light pulse (D); for each treatment, another group served as controls. Total protein extracts were prepared from isolated heads and analyzed by immunoblotting in the presence of antibodies to either PER (A, D, and F) or TIM (B). The positions of PER and TIM are indicated at the left. Arrowheads mark the cross-reacting size standard. (A, B, and D) At the top is shown the time of fly collection (in hours since the last dark-light transition at ZT0). (F) At the top is shown the time of fly collection in minutes since the start of the heat pulse at T21.5. Each experiment was done at least two independent times (data not shown), and representative examples are shown. (C) Quantitation of results shown in panels A and B. (E) Quantitation of results shown in panel D. The peak value for each group of control flies was set at 100 except in panel F, where the abundance of PER in control flies collected 15 min after the start of the heat pulse (lane 1, top left panel) was set at 100.
FIG. 7.
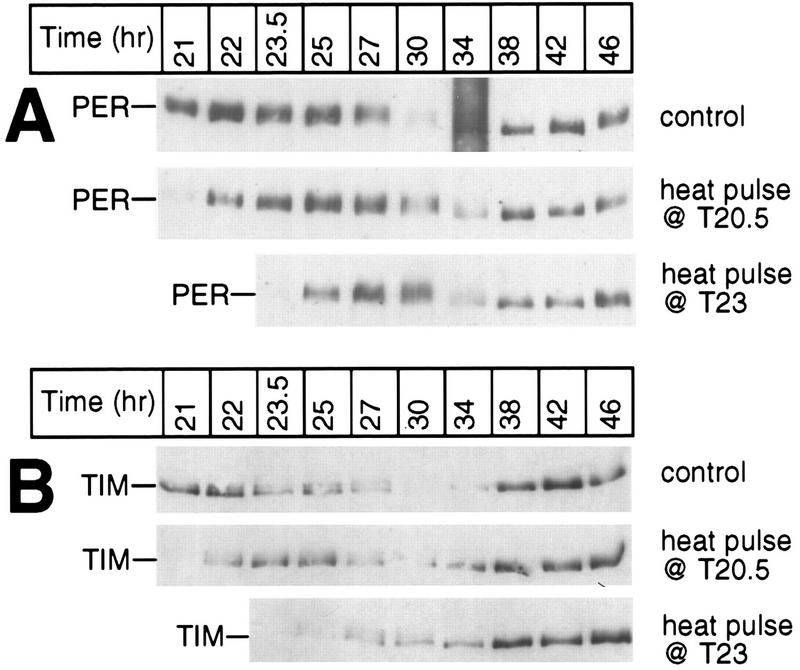
Heat-induced changes in the PER and TIM biochemical cycles similar to those observed at T21.5 also occur at other times in the late night. During the last dark period of LD, two groups of wild-type CS flies were exposed to a 30-min 37°C heat pulse beginning at either T20.5 or T23; another group served as controls. Total protein extracts were prepared from isolated heads and analyzed by immunoblotting in the presence of antibodies to either PER (A) or TIM (B). The positions of PER and TIM are indicated at the left; the time of fly collection (in hours since the last dark-light transition at ZT0) is shown at the top. Each experiment was done at least two independent times (data not shown), and representative examples are shown.
RNase protection assay.
For each time point, total RNA was extracted from ∼10 μl of fly heads by using Tri Reagent (Sigma) and the manufacturer’s recommended procedure (30). The amounts of per and tim transcripts were determined by RNase protection assays (17) performed with the modifications described by Zeng et al. (52). per and tim RNA levels were determined by using the per 2/3 probe (17) and an antisense tim probe that contains nucleotides 4413 to 4270 (numbering based on tim sequence submitted under accession no. U37018) (33), respectively. As a control for RNA loading in each lane, a ribosomal protein probe (RP49) was included in each protection assay (17). Protected bands were quantified with a PhosphorImager from Molecular Dynamics.
RESULTS
Rapid degradation of PER and TIM by heat pulses.
As an initial attempt to understand how changes in temperature perturb circadian oscillatory mechanisms, we sought to compare the effects of short-duration heat pulses on the D. melanogaster clock with those recently reported for light pulses in this species (21, 25, 32, 53). A significant objective was to understand the molecular basis for the different PRCs induced by pulses of light and heat (2, 7, 21, 25, 29, 53, 55). Heat pulses were based on a previous study showing that brief treatments at 37°C elicit phase shifts in the clock-controlled locomotor activity rhythms of D. melanogaster (7). Wild-type CS flies were entrained by LD followed by DD. At selected times in DD, flies were heat pulsed at 37°C and returned to 25°C. Untreated control and heat-pulsed flies were collected at various times, and head extracts were probed for PER and TIM by immunoblotting (Fig. 1).
Heat treatment at 15 h after the last dark-light transition at ZT0 (T15) elicited the rapid disappearance of both PER and TIM proteins (Fig. 1A and B). Clear reductions were first observed between 3 to 5 min following the start of the 37°C incubation, and essentially undetectable levels were reached after 10 to 15 min of heat treatment (Fig. 1A and B and data not shown). The rapid induction of Hsp70 (Fig. 1A) indicates that the flies quickly reached the pulse temperature (48). Similar heat-induced decreases in the levels of both proteins were observed at all times in a daily cycle (Fig. 1C). No changes in the levels of either per or tim transcripts were detected during the first 20 min of the heat pulses (see Fig. 8; also data not shown), strongly suggesting that a posttranscriptional mechanism is solely responsible for mediating the heat-induced decreases in the levels of PER and TIM. Figure 1D shows that reductions in the levels of PER and TIM are proportional to the temperature of the pulse. The magnitude of the phase shift in the locomotor activity rhythm is also proportional to the temperature of the pulse (Table 1), consistent with a causal relationship between the heat-induced degradation of PER, TIM, or both and phase resetting (see Discussion). The small decreases in the levels of PER and TIM observed in flies treated for 30 min at 31°C (Fig. 1D) are not solely due to a reduced temperature differential, as flies entrained at 18°C and pulsed at 30°C (a 12°C difference identical in magnitude to that of the 25-to-37°C pulse) showed less than twofold reductions in the levels of PER and TIM after 1 h of incubation (Fig. 2). Induction of Hsp70 by the 18-to-30°C treatment (Fig. 2A) confirmed that the flies responded to the temperature change. Thus, although longer incubations under more modest temperature increases are accompanied by reductions in the levels of PER and TIM (Fig. 1 and 2 and data not shown), it appears that only high temperatures are effective in eliciting rapid and dramatic decreases in the levels of these two clock proteins.
FIG. 8.
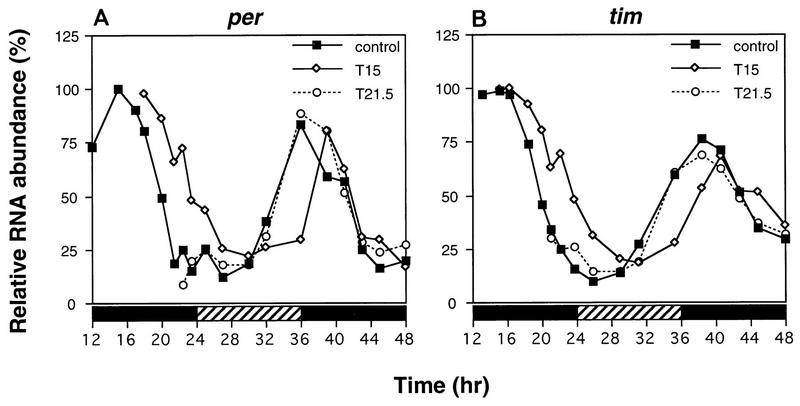
Heat pulses administered at times in a daily cycle that phase shift behavioral rhythms also elicit perturbations in the per and tim transcript cycles. Three groups of LD-entrained flies were maintained under constant dark conditions; one group was heat pulsed at 37°C for 30 min beginning at T15, whereas another group was treated with an identical heat pulse beginning at T21.5; the remaining group served as controls. RNase protection assays were performed on head RNA collected from flies frozen at the indicated times (hours in DD following the last dark-light transition at ZT0). Relative RNA abundance refers to either per/RP49 (A) or tim/RP49 (B) values. The control values for per and tim at T15 were set at 100. Closed bar, subjective night; striped bar, subjective day. This experiment was done three times with similar results, and a representative example is shown.
Because the preparation of total head extracts involves a centrifugation step to remove particulate material (see Materials and Methods), it is possible that the disappearance of PER and/or TIM in extracts prepared from heat-pulsed flies (Fig. 1) is due to heat-induced changes in the centrifugation properties of these clock proteins. To address this possibility, we analyzed the supernatant and pellet fractions processed from flies that were either untreated or heat pulsed at several different times in a daily cycle (Fig. 3 and data not shown). Although we occasionally detected more PER and TIM in pellets derived from heat-pulsed flies than in equivalent material from untreated flies (Fig. 3A, top panel [compare lanes 4 and 3] and data not shown), these differences cannot account for the dramatic decreases in the staining intensities of these two clock proteins in supernatant fractions prepared from heat-pulsed flies (Fig. 3B). These findings strongly suggest that, at the very least, the majority of the heat-induced disappearance of PER and TIM is due to degradation. The physiological significance for the observation that a fraction of PER and TIM appears to be in a high-molecular-weight complex that does not undergo heat-induced reductions in levels is not clear.
Together these observations (Fig. 1 to 3) suggest that degradation of PER and TIM is the initial clock-specific event induced by exposure of flies to elevated temperatures. Moreover, PER is sensitive to heat but not light (25, 53), indicating that individual clock components can markedly differ in sensitivity to the two most important entraining cues in nature.
PER and TIM can be independently degraded by heat pulses.
PER and TIM associate in a functional complex (14, 25, 41, 53), raising the possibility that this interaction is necessary for the heat-induced degradation of one or both proteins. To address this possibility, we determined the thermosensitivity of each protein in the absence of the other by using two different arrhythmic null mutants that do not produce either PER (per01) (24) or TIM (tim0) (43). The results indicate that PER (Fig. 4A) and TIM (Fig. 4B) can be independently regulated by heat and that this degradation does not require a functional clock. This result is reminiscent of the finding that TIM remains sensitive to light in per01 flies (32, 53).
Heat-induced phase delays in behavioral rhythms are accompanied by long-term delays in the PER and TIM biochemical oscillations.
What might account for the observation that although heat pulses in the late night appear to elicit little or no phase shifts in behavioral rhythms (reference 7 and Table 1), they nevertheless are accompanied by rapid decreases in the levels of key state variables (Fig. 1 and 3)? To investigate this apparent contradiction, we sought to determine the long-term effects of heat pulses on the PER and TIM biochemical cycles. We reasoned that stable phase shifts in downstream rhythms (i.e., locomotor activity) should be accompanied by long-term perturbations in the oscillations of key state variables. For example, light pulses perturb the per RNA and protein cycles in a manner consistent with the magnitude and direction of the phase shift in locomotor activity rhythms (25). To examine this issue, flies were heat pulsed at either T15 (Fig. 5) or T21.5 (Fig. 6) and returned to 25°C for several hours. At both times, PER and TIM are present (Fig. 1); however, 37°C heat pulses yield relatively large phase delays in locomotor activity rhythms at T15 but little if any shift at T21.5 (reference 7 and Table 1).
Incubating flies at T15 with a 37°C heat pulse lasting 30 min elicits long-term delays in the PER and TIM cycles in abundance and phosphorylation (Fig. 5); for example, peak levels of PER are attained at ca. T25 in heat-pulsed flies (Fig. 5A [bottom panel] and C [left panel]) compared to ca. T20 in untreated flies (Fig. 5A [top panel] and C [left panel]). Likewise, TIM in the pulsed flies reaches peak concentrations at ca. T20 (Fig. 5B [bottom panel] and C [right panel]) compared to the control case of T15 to T18 (Fig. 5B [top panel] and C [right panel]). Although the cycle in TIM abundance is delayed by heat treatment, it remains advanced relative to the timing of oscillations in PER abundance, similar to the phase angle observed in untreated flies (31). In the experiment shown, the amplitude of the cycle in TIM concentration is ∼2-fold lower in the heat-pulsed flies than in control flies (Fig. 5C, right panel). However, this was not always the case (e.g., Fig. 5E, bottom panel, compare lanes 8 and 7), and we never detected amplitude reductions of greater than 2.5-fold (the reason for the variability in results is not clear). Following the rapid heat-induced decreases in the levels of PER and TIM (Fig. 5A and B [compare lanes 2 in top and bottom panels], D [compare lanes 2 and 1], and E [compare lanes 2 and 3]), these two proteins (re)accumulate over several hours at rates that appear somewhat faster than those observed for the untreated control situation. For example, in a normal circadian cycle, TIM reaches trough levels around CT3 (equivalent to T27), requiring approximately 13 h to reach peak values at ∼CT16 (Fig. 5B and C). However, it takes only 5 to 7 h for TIM to reach peak concentrations following a heat pulse at T15 (Fig. 5B to E). Higher levels of per and tim transcripts at T15 compared to times in a daily cycle when these two proteins are normally at trough levels (see Fig. 8) could account for the faster-accumulation phases observed in the heat-pulsed flies (see Discussion).
In addition to changes in the timing of PER and TIM abundance, side-by-side analysis of head extracts prepared from T15 heat-pulsed and control flies collected at the same times in a daily cycle clearly demonstrates that the temperature treatment retards the progressive phosphorylation of PER (Fig. 5D [top panel, lanes 6 and 5] and E [top panel, lanes 8 to 11]; compare the distance between PER and an internal size standard in heat-pulsed and control flies). Although less obvious, the temporal regulation of TIM phosphorylation also appears to be delayed by heat treatment (Fig. 5D, bottom panel, compare the relative electrophoretic mobilities in lanes 4 and 3). The heat-induced delays in the temporal regulation of PER and TIM abundance and phosphorylation were stable for at least 2 days after the environmental perturbation (Fig. 5F). For example, in untreated control flies the timing of PER and TIM degradation begins between T47 and T50.5, whereas in T15 heat-pulsed flies no detectable decreases were observed at these times.
Together these results indicate that a 37°C heat pulse at T15 evokes stable phase delays of several hours in the biochemical oscillations of PER and TIM, consistent with the magnitude and direction of the phase shift in locomotor activity rhythms produced by identical temperature treatments (reference 7 and Table 1). This heat-induced delay in the temporal regulation of PER and TIM abundance and phosphorylation is very reminiscent of the effects of light pulses applied at T15 (21, 25, 32, 53), suggesting that in the early night, photic and heat signals delay the Drosophila clock by a common mechanism (see Discussion).
Heat pulses in the late night elicit transient and rapid increases in the speed of the PER-TIM cycles.
Heat pulses at T21.5 (Fig. 6) are accompanied by several changes in the PER and TIM biochemical cycles that are strikingly different from those observed in T15-treated flies (Fig. 5). Most notably, following the rapid heat-induced degradation of PER and TIM (Fig. 6A and B, compare lanes 2 in top and bottom panels), in T21.5-treated flies these two clock proteins (re)accumulate extremely quickly (Fig. 6A and B, bottom panels, compare lanes 3 and 2). This remarkably rapid accumulation phase is quickly followed by another round of degradation (between T23 to T32) that is somewhat delayed compared to the untreated control (Fig. 6A to C). Despite these dramatic changes, significant long-term perturbations in the steady-state cycles of PER and TIM were not detected. For example, following the heat-induced rapid oscillation in the levels of PER and TIM, the accumulation profiles of both clock proteins are similar, if not identical, to those observed in untreated flies (Fig. 6A and B [compare lanes 8 and 9 in top and bottom panels] and C). Moreover, the per and tim RNA oscillations are indistinguishable in T21.5 heat-pulsed flies and control flies (see Fig. 8). These findings are consistent with the apparently unperturbed activity rhythms in flies treated under identical conditions (reference 7 and Table 1).
In sharp contrast, whereas a light pulse at T21.5 results in both the premature disappearance of TIM (21, 32, 53) and an advanced timing in the degradation of PER (25, 53) (Fig. 6D [compare lanes 6 in top and bottom panels]; quantitation shown in Fig. 6E), these events are not accompanied by rapid increases in the levels of these clock proteins. Rather, PER and TIM remain at trough levels for several hours and during the next daily cycle accumulate earlier than in untreated controls (Fig. 6D, top and bottom panels, compare lanes 9 and 10). The net effect is that light pulses at T21.5 elicit ∼2-h advances in the PER and TIM biochemical oscillations, consistent with the direction and magnitude of the phase shift in activity rhythms observed in flies treated under identical conditions (6, 26, 32, 42).
Although heat pulses at T21.5 do not elicit significant long-term perturbations in the timing of the PER and TIM biochemical changes, in agreement with the lack of a shift in behavioral rhythms, we were very surprised by the extremely rapid accumulation of these clock proteins following the temperature treatment. To confirm and extend these findings, we performed a high-resolution time course (Fig. 6F). The results clearly indicate that PER begins to increase in abundance approximately 75 min after the start of the heat pulse (Fig. 6F, bottom panel, lane 9) and that it takes only 45 min to reach levels comparable to the peak amounts detected in control flies (compare lane 12 in bottom panel to lanes 1 to 10 in top panels). Similarly rapid increases in the abundance of TIM were also obtained (Fig. 6B, bottom panel, and data not shown). The rapidity of these increases in abundance is in sharp contrast to the normal situation whereby it takes 10 to 12 h for the levels of these two clock proteins to increase from trough to maximal values. Thus, PER and TIM accumulate ∼15-fold faster in the heat-pulsed flies than in control flies. This is likely an underestimate of the real differences in synthesis rates because at T21.5 the levels of per and tim transcripts are very low in contrast to the situation occurring during the normal accumulation phase of PER and TIM proteins (see Fig. 8). Furthermore, PER undergoes temporal changes in phosphorylation during the heat-induced rapid increases and decreases in its abundance (Fig. 6F, bottom panel, compare the distance between the highest isoform of PER and an internal size standard in lanes 7 and 12). Thus, it appears that the rapid biochemical oscillation in PER and TIM following a heat pulse in the late night might encompass most, if not all, of the characteristic features comprising a normal daily cycle in the PER-TIM temporal program.
Similar short- and long-term effects on the time course of PER and TIM abundance changes were also obtained with heat pulses applied at other times in the late night (Fig. 7) and with temperature treatments differing in duration (data not shown). Thus, although heat pulses in the late night elicit a series of rapid and dramatic changes in the metabolism of PER and TIM, the net result is that the steady-state phases of these clock protein rhythms are similar to those found in control flies, in agreement with the behavioral data (reference 7 and Table 1).
The timing of per and tim mRNA cycles is perturbed by heat pulses in a manner consistent with the direction and magnitude of the behavioral phase shift.
As another measure of the timekeeping mechanism, we determined the effects of heat pulses on the per and tim RNA fluctuations (Fig. 8). In flies exposed to a heat pulse at T15, the daily oscillations in the levels of per (Fig. 8A) and tim (Fig. 8B) transcripts were delayed by 2 to 3 h, consistent with both (i) the magnitude and direction of the phase shift in behavioral rhythms observed in flies treated under identical conditions (reference 7 and Table 1) and (ii) the long-term changes in the timing of the fluctuations in the abundance and phosphorylation of PER and TIM (Fig. 5). Furthermore, these results support the contention that per expression and tim expression are regulated by the same negative transcriptional feedback loop (44). Delays in the per transcript cycle were also detected in flies exposed to light pulses at T15 (25), supporting the contention that a similar mechanism underlies light- and heat-induced phase delays. By analogy with results obtained in assays using light pulses, the delayed decline in the levels of per and tim transcripts following a heat pulse at T15 is likely caused by the retarded nuclear entry of the PER-TIM complex (25) (see Discussion). No changes in the per and tim RNA oscillations were observed in flies heat pulsed at T21.5 (Fig. 8), in agreement with the relatively unperturbed locomotor activity rhythm (reference 7 and Table 1) and the lack of significant long-term effects on the PER and TIM biochemical rhythms (Fig. 6 and 7). These results also indicate that the rapid accumulation and subsequent degradation of PER and TIM following a heat pulse in the late night (Fig. 6 and 7) are mediated solely by a posttranscriptional mechanism(s).
DISCUSSION
In this study, we sought to understand the molecular basis for the different phase-response curves induced by short-duration pulses of light and heat on D. melanogaster behavioral rhythms. We show that elevated temperature treatments at all times in a daily cycle elicit rapid and dramatic reductions in the levels of PER and TIM (Fig. 1 to 3), strongly suggesting that the enhanced degradation of these two proteins is the initial clock-specific event induced by heat signals. The thermosensitivities of PER and TIM are independent of each other and do not require a functional clock (Fig. 4). In addition, we show that the long-term effects of heat on the per-tim transcription-translation feedback loop (Fig. 5 to 8) parallel the phase shifts in behavioral rhythms (Table 1). These data begin to explain the molecular underpinnings for the Drosophila heat PRC, with its characteristic phase delay zone in the early night and relatively insensitive zone in the late night. Our findings also reveal several unanticipated and novel features of clocks and their component factors. First, there are time-of-day-specific differences in how clocks interact with photic and heat signals. Second, rapid changes in the levels of key state variables in a clock are not necessarily accompanied by evident steady-state shifts in the phase of the oscillator. Third, it appears that external stimuli can elicit transient changes in the speed of timekeeping mechanisms. Finally, individual clock proteins can markedly differ in their sensitivities to different zeitgebers.
Although the mechanism(s) responsible for the heat-induced decreases in the levels of PER and TIM is not known, we did not observe early changes in the levels of per and tim transcripts following the application of heat pulses (Fig. 8). These results indicate that posttranscriptional events solely mediate the thermosensitivities of PER and TIM. Because of the rapidity and magnitude of the decreases in the levels of both clock proteins, it is highly likely that heat somehow results in the enhanced proteolysis of PER and TIM. In this context, it is noteworthy that brief pulses at 37°C elicit a full-blown heat shock response in D. melanogaster (27, 48), raising the possibility that this pathway participates in mediating the enhanced degradation of PER and/or TIM. For example, thermally denatured proteins are prime targets for proteolysis by the ubiquitin-proteasome system (reviewed in references 19 and 22). Indeed, a possible link between the heat or stress response and the regulation of circadian timing systems has been suggested previously (38, 39). This suggestion was based on several lines of evidence demonstrating that numerous agents that elicit the heat or stress response also perturb the phases of a variety of circadian rhythms. It will be of interest to determine whether components of the heat shock pathway are involved in transducing heat signals to the intracellular timekeeping mechanisms underlying circadian clocks.
Nevertheless, the heat shock response is accompanied by numerous changes in cell physiology and gene expression that could conceivably perturb the dynamics of an oscillatory mechanism in a nonspecific manner. These considerations raise the formal possibility that the heat-induced degradations of PER and TIM are epiphenomena unrelated to the resetting mechanism. Although we cannot rule out this possibility, several lines of evidence strongly support a causal link between the heat-induced degradation of PER, TIM, or both and phase resetting. First, the rapidity with which heat elicits decreases in the levels of PER and TIM (Fig. 1) suggests that these two proteins are the initial clock-specific components perturbed by heat. Second, there is a direct relationship between the magnitude of heat-induced phase delays in locomotor activity rhythms and the extent of PER and TIM disappearance (Table 1 and Fig. 1). Third, there is a very tight correlation between the heat-induced (i) time-of-day-specific changes in the biochemical cycles of both clock proteins and (ii) magnitude and direction of the phase shifts in activity rhythms (Table 1 and Fig. 5 to 7). Because PER and TIM satisfy most, if not all, of the criteria for bona fide state variables in a clock (4, 40), we believe that the most likely interpretation that can account for these biochemical and behavioral observations is that the heat-induced perturbations in the PER and/or TIM cycles directly contribute to, rather than merely correlate with, the effects of heat on behavioral rhythms.
Photic stimuli also perturb the per-tim feedback loop in a manner that parallels the magnitude and direction of the phase shift evoked in behavioral rhythms (25, 32). Recent work has suggested a model that can explain the salient features of the Drosophila light PRC (21, 25, 32, 53). In brief, the light-induced degradation of cytoplasmic TIM during the early night disrupts the PER-TIM complex prior to nuclear entry. Hence TIM must reaccumulate in order to reassemble a functional PER-TIM complex that eventually enters the nucleus, resulting in phase delays. Conversely, the premature disappearance of nuclear TIM by photic cues in the late night is also accompanied by the earlier hyperphosphorylation and degradation of PER. The advanced timing in the disappearance of TIM, PER, or both in the nucleus likely relieves the autoregulatory transcriptional inhibition resulting in phase advances. Finally, the dead zone occurs during times in a daily cycle with little or no TIM and PER, likely explaining the insensitivity of the clock to light during this phase.
Heat pulses in the early night delay the per and tim protein and RNA cycles (Fig. 5 and 8), similar to the effects of light pulses applied at the same times in a daily cycle (25, 32, 53). Light and heat apparently elicit delays in the per-tim transcription-translation feedback loop by a common mechanism involving the rapid degradation of one or more key clock proteins in the cytoplasm. Although our data do not directly address whether the heat-induced degradation of PER, TIM, or both is required to elicit a phase delay in the timekeeping mechanism, it is likely that decreases in either clock protein are sufficient. In support of this contention are findings demonstrating that the light-induced degradation of TIM appears sufficient to evoke phase delays (21, 32, 53) and that rapid increase in the abundance of PER in transgenic flies bearing an inducible version of per converts the phase delay region to a phase advance region (7). Furthermore, PER and TIM require the presence of each other to enter the nucleus (32, 41, 49), strongly suggesting that the temporary absence of either clock protein in the cytoplasm will delay the entire cycle. Presumably, removal of the heat stimulus enables de novo syntheses of PER and TIM. As previously proposed for light pulses, the reaccumulation of PER and TIM in the cytoplasm following a heat pulse might be facilitated by the presence of peak amounts of per and tim transcripts at these times in a daily cycle (32, 53).
Heat and light pulses in the late night have very different effects on behavioral rhythms, resulting in either minor or no shifts or relatively large phase advances, respectively. Yet both modalities elicit rapid decreases in the levels of key state variables (21, 32, 53) (Fig. 1, 6, and 7). This apparent contradiction is resolved by analyzing the long-term effects of light and heat on the oscillatory mechanism. Following the premature disappearance of PER and TIM by light pulses in the late night (Fig. 6D), both clock proteins remain at trough levels for ∼6 h and undergo the next round of accumulation ∼2 h earlier than in the control untreated flies. Heat-induced decreases in the levels of PER and TIM, however, are followed by a very rapid cycle of increases and decreases (Fig. 6 and 7). Subsequently, PER and TIM accumulate in a manner very similar to that observed in untreated flies. The net effect of the changes evoked by heat results in little or no apparent long-term perturbation in the PER-TIM temporal program. Thus, it appears as though the intercalation of a rapid oscillation counteracts the initial heat-induced decreases, resulting in a timekeeping mechanism with essentially unperturbed dynamics. That the timekeeping mechanism has not been stably shifted is further supported by the observation that the waveforms in the per and tim RNA rhythms are essentially indistinguishable in T21.5-treated flies and control untreated flies (Fig. 8).
A limitation of this report is that we do not have a satisfactory explanation for the remarkably rapid and unexpected increases in the levels of PER and TIM following the heat induced degradation of these two proteins in the late night. Similar increases were not observed in flies exposed to heat pulses at T15 (Fig. 5), indicating that this response is gated in a circadian manner. This time-of-day difference is even more perplexing in light of the observation that at T15 there is approximately three- to fivefold more per and tim transcripts than at T21.5 (17, 44) (Fig. 8). These considerations might suggest that in the late night, the combination of a heat pulse followed by the return to normal temperatures results in changes in the cellular milieu that greatly enhance the accumulation rates of PER and TIM.
Our findings strongly suggest that under certain conditions, one or more aspects of the normal PER-TIM temporal program can be greatly accelerated. This heat-induced change in the speed of the PER and TIM biochemical cycles has a theoretical precedent. Two alternative models that in principle can account for the time-of-day-specific differences in the magnitude and direction of phase shifts elicited by brief stimuli have been suggested (34, 55). In nonparametric entrainment, stimuli primarily cause rapid (relative to 24 h) changes in the concentration (or activity) of one or more key state variables, promptly resetting the oscillator to a new phase essentially dictated by the poststimulus concentration(s) of the affected state variable(s). Alternatively, in parametric entrainment, stimuli elicit phase shifts by causing longer-acting changes in the speed of the driving oscillator that eventually affects the metabolism of key clock components. Our data raise the intriguing possibility that, at least during certain times in a daily cycle, a parametric mechanism also participates in the response of the Drosophila oscillator to heat signals. We suggest that although the induction of rapid and discrete changes in one or more state variables by entraining agents is likely a conserved feature of all phase-shifting mechanisms, in certain cases, accompanying transitory changes in the speed of the clock can contribute to the phase-shifting response by enhancing, diminishing, or even negating the initial stimulus-induced changes in state variables.
An issue not directly addressed by our studies is the ability to entrain Drosophila behavioral rhythms with daily cycles of moderately high and low temperatures. For example, D. melanogaster locomotor activity rhythms are efficiently entrained by daily cycles of 12 h at 25°C followed by 12 h at 28°C (51). Yet our results indicate that brief exposure of flies to moderate increases in temperature evoke little changes in the levels of PER and TIM (Fig. 1 and 2). To shift the Drosophila clock by modest increases in temperature appears to require longer incubation times lasting several hours (2, 55). The ability to shift the Drosophila clock by the rapid heat-induced degradation of PER and TIM described in this study is specific for elevated temperatures. Interestingly, very recent studies of Neurospora have shown that the RNA and protein products from the clock gene frq are induced by nonrecurrent step increases in temperature (3, 28). Although how these heat-induced changes in the abundance of FRQ might perturb the frq-based transcriptional-translational feedback loop was not investigated, in both Drosophila and Neurospora, heat elicits rapid changes in the levels of key state variables.
An intriguing observation is that PER is sensitive to heat (Fig. 1 and 4) but not light (21, 25, 53), whereas TIM is sensitive to both stimuli (Fig. 1 and 4) (21, 32, 53). Thus, individual clock components can markedly differ in sensitivity to the two most important environmental entraining cues. Although not well studied, it is highly likely that under natural conditions a wide variety of organisms manifest circadian rhythms that are influenced by multiple temporal cues (1, 9, 35). In the case of Drosophila, it appears that the photic and heat signal transduction pathways converge at the level of regulating the stability of one or more key clock proteins. The observation that PER and TIM interact to form a functional complex (14, 25, 41, 53) that is involved in an autoregulatory circuit central to the timekeeping mechanism might ensure that the effects of light and temperature (and possibly other stimuli) on individual clock proteins are combined into a coherent temporal cue resulting in daily rhythms that are optimally adapted to the precise local conditions. It will be of interest to determine whether other circadian timekeeping devices are assembled with components that differ in sensitivity to different environmental entraining cues.
ACKNOWLEDGMENTS
We thank P. Lobel, C. Pikielny, A. Shatkin, and M. Toledano for critical reading of the manuscript. We thank members of S. Lindquist’s laboratory for antibodies to Hsp70, S. Hitchcock-DeGregori for the use of her densitometer, and A. Sehgal for providing tim0 flies.
This work was partially supported by National Institutes of Health grant NS34958 to I.E. J.M. was supported by a neuroscience training grant from the National Institute of Mental Health.
REFERENCES
- 1.Bateman M A. The effects of light and temperature on the rhythm of pupal ecdysis in the Queensland fruit-fly Dacus (strumet) tryoni (frogg.) Aust J Zool. 1955;3:22–33. [Google Scholar]
- 2.Chandrasherkan M K. Phase shifts in the Drosophila pseudoobscura circadian rhythm evoked by temperature pulses of varying durations. J Interdiscip Cycle Res. 1974;5:371–380. [Google Scholar]
- 3.Crosthwaite S K, Dunlap J C, Loros J J. Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- 4.Crosthwaite S K, Loros J J, Dunlap J C. Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell. 1995;81:1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 5.Curtin K D, Huang Z J, Rosbash M. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron. 1995;14:365–372. doi: 10.1016/0896-6273(95)90292-9. [DOI] [PubMed] [Google Scholar]
- 6.Dushay M S, Konopka R J, Orr D, Greenacre M L, Kyriacou C P, Rosbash M, Hall J C. Phenotypic and genetic analysis of Clock, a new circadian rhythm mutant in Drosophila melanogaster. Genetics. 1990;125:557–578. doi: 10.1093/genetics/125.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edery I, Rutila J E, Rosbash M. Phase shifting of the circadian clock by induction of the Drosophila period protein. Science. 1994;263:237–240. doi: 10.1126/science.8284676. [DOI] [PubMed] [Google Scholar]
- 8.Edery I, Zwiebel L J, Dembinska M E, Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci USA. 1994;91:2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans K J. Responses of the locomotor activity rhythms of lizards to simultaneous light and temperature cycles. Comp Biochem Physiol. 1966;19:91–103. doi: 10.1016/0010-406x(66)90549-4. [DOI] [PubMed] [Google Scholar]
- 10.Ewer J, Frisch B, Hamblen-Coyle M J, Rosbash M, Hall J C. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells’ influence on circadian behavioral rhythms. J Neurosci. 1992;12:3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisch B, Hardin P E, Hamblen-Coyle M J, Rosbash M, Hall J C. A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the Drosophila nervous system. Neuron. 1994;12:555–570. doi: 10.1016/0896-6273(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 12.Gander P H. The circadian locomotor activity rhythm of Hemideina thoracica (orthoptera): the effects of temperature pertubations. Int J Chronobiol. 1979;6:243–262. [Google Scholar]
- 13.Garceau N Y, Liu Y, Loros J J, Dunlap J C. Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- 14.Gekakis N, Saez L, Delahaye-Brown A M, Myers M P, Sehgal A, Young M W, Weitz C J. Isolation of timeless by PER protein interaction: defective interaction between timeless protein and long-period mutant PERL. Science. 1995;270:811–815. doi: 10.1126/science.270.5237.811. [DOI] [PubMed] [Google Scholar]
- 15.Hamblen-Coyle M J, Wheeler D A, Rutila J E, Rosbash M, Hall J C. Behavior of period-altered rhythm mutants of Drosophila in light:dark cycles. J Insect Behav. 1992;5:417–446. [Google Scholar]
- 16.Hardin P E, Hall J C, Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci USA. 1992;89:11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardin P E, Hall J C, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343:536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- 18.Hastings J W, Rusak B, Boulos Z. Circadian rhythms: the physiology of biological timing. In: Prosser C L, editor. Neural and integrative animal physiology. New York, N.Y: Wiley-Liss, Inc.; 1991. pp. 435–546. [Google Scholar]
- 19.Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z J, Curtin K D, Rosbash M. PER protein interactions and temperature compensation of a circadian clock in Drosophila. Science. 1995;267:1169–1172. doi: 10.1126/science.7855598. [DOI] [PubMed] [Google Scholar]
- 21.Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- 22.Jentsch S. The ubiquitin-conjugation system. Annu Rev Genet. 1992;26:179–207. doi: 10.1146/annurev.ge.26.120192.001143. [DOI] [PubMed] [Google Scholar]
- 23.Konopka R, Wells S, Lee T. Mosaic analysis of a Drosophila clock mutant. Mol Gen Genet. 1983;190:284–288. [Google Scholar]
- 24.Konopka R J, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C, Parikh V, Itsukaichi T, Bae K, Edery I. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science. 1996;271:1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- 26.Levine J D, Casey C I, Kalderon D D, Jackson F R. Altered circadian pacemaker functions and cyclic AMP rhythms in the Drosophila learning mutant dunce. Neuron. 1994;13:967–974. doi: 10.1016/0896-6273(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 27.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Garceau N Y, Loros J J, Dunlap J C. Thermally regulated translational control of FRQ mediates aspects of temperature responses in the Neurospora circadian clock. Cell. 1997;89:1–20. doi: 10.1016/s0092-8674(00)80228-7. [DOI] [PubMed] [Google Scholar]
- 29.Maier R W. Phase shifting of the circadian rhythm of eclosion in Drosophila pseudoobscura with temperature pulses. J Interdiscip Cycle Res. 1973;4:125–135. [Google Scholar]
- 30.Majercak J, Kalderon D, Edery I. Drosophila melanogaster deficient in protein kinase A manifests behavior-specific arrhythmia but normal clock function. Mol Cell Biol. 1997;17:5915–5922. doi: 10.1128/mcb.17.10.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrus S B, Hongkui Z, Rosbash M. Effect of constant light and circadian entrainment of perS flies: evidence for light-mediated delay of the negative feedback loop in Drosophila. EMBO J. 1996;15:6877–6886. [PMC free article] [PubMed] [Google Scholar]
- 32.Myers M P, Wager-Smith K, Rothenfluh-Hilfiker A, Young M W. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 33.Myers M P, Wager-Smith K, Wesley C S, Young M W, Sehgal A. Positional cloning and sequence analysis of the Drosophila clock gene, timeless. Science. 1995;270:805–808. doi: 10.1126/science.270.5237.805. [DOI] [PubMed] [Google Scholar]
- 34.Pittendrigh C S. Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Pittendrigh C S, Bruce V, Kaus P. On the significance of transients in daily rhythms. Proc Natl Acad Sci USA. 1958;44:965–973. doi: 10.1073/pnas.44.9.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price J L, Dembinska M E, Young M W, Rosbash M. Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. EMBO J. 1995;14:4044–4049. doi: 10.1002/j.1460-2075.1995.tb00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rence B, Loher W. Arrhythmically singing crickets: thermoperiodic reentrainment after bilobectomy. Science. 1975;190:385–387. doi: 10.1126/science.1179217. [DOI] [PubMed] [Google Scholar]
- 38.Rensing L, Bos A, Kroeger J, Cornelius G. Possible link between circadian rhythm and heat shock response in Neurospora crassa. Chronobiol Int. 1987;4:543–549. doi: 10.3109/07420528709078546. [DOI] [PubMed] [Google Scholar]
- 39.Rensing L, Hardeland R. The cellular mechanism of circadian rhythms—a view on evidence, hypotheses and problems. Chronobiol Int. 1990;7:353–370. doi: 10.3109/07420529009059146. [DOI] [PubMed] [Google Scholar]
- 40.Rosbash M, Allada R, Dembinska M, Guo W Q, Le M, Marrus S, Qian Z, Rutila J, Yaglom J, Zeng H. A Drosophila circadian clock. Cold Spring Harbor Symp Quant Biol. 1996;61:265–278. [PubMed] [Google Scholar]
- 41.Saez L, Young M W. Regulation of nuclear entry of the Drosophila clock proteins period and timeless. Neuron. 1996;17:911–920. doi: 10.1016/s0896-6273(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 42.Saunders D S, Gillanders S W, Lewis R D. Light pulse phase response curves for the locomotor activity rhythm in period mutants. J Insect Physiol. 1994;40:957–968. [Google Scholar]
- 43.Sehgal A, Price J L, Man B, Young M W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- 44.Sehgal A, Rothenfluh-Hilfiker A, Hunter-Ensor M, Chen Y, Myers M P, Young M W. Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science. 1995;270:808–810. doi: 10.1126/science.270.5237.808. [DOI] [PubMed] [Google Scholar]
- 45.Siwicki K K, Eastman C, Petersen G, Rosbash M, Hall J C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988;1:141–150. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi J S. Circadian rhythms: from gene expression to behavior. Curr Opin Neurobiol. 1991;1:556–561. doi: 10.1016/s0959-4388(05)80028-5. [DOI] [PubMed] [Google Scholar]
- 47.Tokura H, Aschoff J. Effects of temperature on the circadian rhythm of pig-tailed macaques Macaca nemestrina. Am J Physiol. 1983;245:R800–R804. doi: 10.1152/ajpregu.1983.245.6.R800. [DOI] [PubMed] [Google Scholar]
- 48.Velazquez J M, Sonoda S, Bugaisky G, Lindquist S. Is the major Drosophila heat shock protein present in cells that have not been heat shocked? J Cell Biol. 1983;96:286–290. doi: 10.1083/jcb.96.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vosshall L B, Price J L, Sehgal A, Saez L, Young M W. Block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994;263:1606–1609. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- 50.Vosshall L B, Young M W. Circadian rhythms in Drosophila can be driven by period expression in a restricted group of central brain cells. Neuron. 1995;15:345–360. doi: 10.1016/0896-6273(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 51.Wheeler D A, Hamblen-Coyle M J, Dushay M S, Hall J C. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J Biol Rhythms. 1993;8:67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- 52.Zeng H, Hardin P E, Rosbash M. Constitutive overexpression of the Drosophila period protein inhibits period mRNA cycling. EMBO J. 1994;13:3590–3598. doi: 10.1002/j.1460-2075.1994.tb06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng H, Qian Z, Myers M P, Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature. 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
- 54.Zerr D M, Hall J C, Rosbash M, Siwicki K K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990;10:2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerman W F, Pittendrigh C S, Pavlidis T. Temperature compensation of the circadian oscillation in Drosophila pseudoobscura and its entrainment by temperature cycles. J Insect Physiol. 1968;14:669–684. doi: 10.1016/0022-1910(68)90226-6. [DOI] [PubMed] [Google Scholar]