Abstract
The modulation of the chaperone activity of the heat shock cognate Hsc70 protein in mammalian cells involves cooperation with chaperone cofactors, such as Hsp40; BAG-1; the Hsc70-interacting protein, Hip; and the Hsc70-Hsp90-organizing protein, Hop. By employing the yeast two-hybrid system and in vitro interaction assays, we have provided insight into the structural basis that underlies Hsc70’s cooperation with different cofactors. The carboxy-terminal domain of Hsc70, previously shown to form a lid over the peptide binding pocket of the chaperone protein, mediates the interaction of Hsc70 with Hsp40 and Hop. Remarkably, the two cofactors bind to the carboxy terminus of Hsc70 in a noncompetitive manner, revealing the existence of distinct binding sites for Hsp40 and Hop within this domain. In contrast, Hip interacts exclusively with the amino-terminal ATPase domain of Hsc70. Hence, Hsc70 possesses separate nonoverlapping binding sites for Hsp40, Hip, and Hop. This appears to enable the chaperone protein to cooperate simultaneously with multiple cofactors. On the other hand, BAG-1 and Hip have recently been shown to compete in binding to the ATPase domain. Our data thus establish the existence of a network of cooperating and competing cofactors regulating the chaperone activity of Hsc70 in the mammalian cell.
Molecular chaperones of the 70-kDa heat shock protein (Hsp70) family participate in various cellular processes under normal growth conditions as well as under cellular stress (7, 15). Hsp70 apparently stabilizes nonnative polypeptides through the binding of hydrophobic peptide segments, which are exposed during protein synthesis, protein translocation, and protein degradation (26). Recognition of a polypeptide substrate is mediated by Hsp70’s 18-kDa peptide binding domain and appears to involve a 10-kDa carboxy-terminal domain that forms a lid over the peptide binding pocket (26, 34). Substrate recognition is controlled by the binding of ATP to the amino-terminal 45-kDa ATPase domain of Hsp70, ATP hydrolysis, and nucleotide exchange (8, 21, 26). The ATP-bound form of Hsp70 binds and releases substrates rapidly, resulting in a low overall affinity, whereas the ADP form binds substrates slowly but more stably (24, 27). The cycling of Hsp70 between the different nucleotide states is regulated by chaperone cofactors, which modulate the ATPase activity of Hsp70 and thus influence the affinity of the chaperone for polypeptide substrates (12, 15, 21, 26).
We have recently characterized cofactors that participate in the regulation of the cytosolic and nuclear heat shock cognate Hsc70 in mammals (16–18, 22). Efficient association of Hsc70 with a polypeptide substrate is achieved through the cooperation of the chaperone protein with Hsp40 (also known as Hdj-1). Hsp40 stimulates the conversion of Hsc70-bound ATP to ADP, resulting in the stable association of Hsc70 with a polypeptide substrate (11, 22). Subsequent ADP release and ATP rebinding can occur spontaneously to a significant extent when Hsc70 cooperates with Hsp40 (16, 22). However, the ADP-bound state of Hsc70, generated during interaction with Hsp40, can be affected by the Hsc70-interacting protein, Hip (also known as p48), and the BAG-1 cofactor (also known as Rap46 and Hap46) in opposite ways (16, 17). Both proteins bind to the ATPase domain of Hsc70 (16, 31, 33). However, while Hip appears to stabilize the ADP-bound conformation of Hsc70, BAG-1 has been shown to stimulate ADP release from the chaperone protein (16). The physiological consequences of the regulation of Hsc70 by BAG-1 remain to be established. On the other hand, Hip-mediated stabilization of the ADP-bound form of Hsc70 may be of particular importance for the cooperation of Hsc70 with other chaperone systems in the mammalian cell (12, 35). For example, Hsc70 cooperates with the abundant cytosolic chaperone Hsp90 during the conformational regulation of signaling molecules, such as steroid hormone receptors and certain protein kinases (2, 12). Intriguingly, Hip was found to be associated with the progesterone receptor and the oncogenic tyrosine kinase pp60v-src, respectively, as part of an Hsc70-Hsp90 chaperone complex (17, 25, 30). It was therefore speculated that Hip stabilizes the interaction of Hsc70 with the signaling molecule until Hsp90 is recruited to the chaperone-substrate complex (12, 35). Notably, the cooperation of Hsc70 with Hsp90 also involves the Hsc70-Hsp90-organizing protein, Hop (also known as p60 in mammals and Sti1 in Saccharomyces cerevisiae) (23, 29). Hop has been identified as a component of chaperone complexes containing Hsp70 and Hsp90 in mammals and yeast and appears to act as a coupling factor mediating the interaction between the chaperone proteins (4–6, 29, 30).
Here we provide insight into structural and functional aspects of the interaction of Hsc70 with different cofactors. We show that the 10-kDa carboxy-terminal domain of Hsc70 provides nonoverlapping binding sites for Hop (p60/Sti1) and Hsp40 (Hdj-1). In contrast, the binding of Hip to Hsc70 is exclusively mediated by the amino-terminal ATPase domain of Hsc70. The existence of distinct binding sites for different Hsc70 cofactors appears to provide the structural basis for the functional cooperation of Hsc70 with multiple chaperone cofactors as it occurs during Hsc70-Hsp90-mediated processes.
MATERIALS AND METHODS
Cloning of the rat hop cDNA.
To investigate the interaction of Hop with rat Hsc70, we cloned the rat hop cDNA by PCR, with a rat liver cDNA library (Clontech Laboratories) as a template. Primers covering the 3′ and 5′ regions were designed based on the nucleotide sequences of mouse and human hop as follows: 5′-GTGCGGACCGGCCGTCGACCTATGGAGCAGGTGAATGAGC-3′ and 5′-GGTGTGCCTCTAGAATATTCACCGAATTGCGATGAGACC-3′. To exclude PCR-induced mutations, four independently isolated clones were completely sequenced and found to be identical. The deduced primary structure of the rat Hop protein displays 99.1% similarity to mouse Hop and 97.4% similarity to human Hop (mouse and human Hop proteins have 97.2% similarity). The open reading frame of rat hop was subcloned into plasmid pAD in frame with the GAL4 activation domain coding region (pAD-hop). pAD is based on plasmid pGAD-GH (Clontech Laboratories) but carries the 695-bp HindIII/HindIII cassette, including the multiple cloning site of plasmid pGAD424 (Clontech Laboratories). For expression of Hop in Sf9 insect cells after infection with recombinant baculovirus, the open reading frame of hop was subcloned into vector pVL1392 (PharMingen).
Construction of plasmids containing hsc70.
The complete open reading frame of rat hsc70, including a portion of the 3′ noncoding region, was obtained by restriction enzyme digestion of plasmid pET-hsc70 (28) and was subcloned into vector pGBT9 (Clontech Laboratories) (pGBT-hsc70). Fragments of hsc70 encoding the carboxy-terminal 139 and 110 amino acids and a mutant form of the 70C139 domain lacking the EEVD motif were amplified by PCR and subcloned into pGBT9 (pGBT-70C139, pGBT-70C110, and pGBT-70C139ΔEEVD). PCR-amplified fragments were sequenced to confirm the wild-type character. The construction of a plasmid encoding the GAL4 DNA-binding domain fused to the Hsc70 ATPase domain was previously described (17) (it is designated pGBT-70N383 in this paper). For expression as glutathione S-transferase (GST) fusion proteins, hsc70 fragments were subcloned into vector pGEX-4T-1 (Pharmacia).
Protein expression and purification.
Rat Hsc70, rat Hip, human Hsp40, and human BAG-1 were purified after recombinant expression in insect cells (Hsc70, Hip, and BAG-1) or bacteria (Hsp40) as previously described (16). Rat Hop was expressed in Sf9 insect cells after infection with recombinant baculovirus. After growing for ∼60 h, the infected cells were disrupted with a French pressure cell (Aminco) and spun at 100,000 × g for 30 min. Hop was purified by chromatography of the supernatant fraction on DEAE-Sepharose (Pharmacia), Bio-Gel HT hydroxyapatite (Bio-Rad), butyl-Sepharose (Pharmacia), and a MonoP column (Pharmacia). Prior to final concentration and dialysis, the Hop preparation was passed over an ATP-agarose column (Sigma) to remove minute amounts of associated Hsp70. Nontagged GST and GST fusion proteins were expressed by addition of 0.1 mM isopropyl-1-thio-β-d-galactopyranoside to Escherichia coli TG1 cells transformed with corresponding pGEX-4T-1 plasmids. After induction at 37°C for 3 h, the cells were disrupted with a French pressure cell and centrifuged at 100,000 × g. GST, GST-70C139, and GST-70C110 were purified on glutathione-Sepharose (Pharmacia) as described by the manufacturer.
Localization of cofactor binding sites on Hsc70 by the yeast two-hybrid system.
To analyze the interaction of Hip and Hop with Hsc70 and Hsc70 fragments in the two-hybrid assay, yeast strain HF7c (Clontech Laboratories) was transformed with corresponding two-hybrid constructs. Expression of GAL4 DNA-binding domain and GAL4 activation domain fusion proteins was investigated by immunoblotting of crude cell extracts after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with monoclonal anti-GAL4 activation domain and anti-GAL4 DNA-binding domain antibodies (Clontech Laboratories), revealing similar expression levels of the different fusion proteins. Interaction with Hsc70 or Hsc70 fragments was analyzed by growth of corresponding transformants on synthetic dextrose minimal medium lacking histidine, leucine, and tryptophan for 7 days at 30°C (1).
In vitro binding assays.
For in vitro binding assays, GST, GST-70C139, GST-70C110, and GST-70C139ΔEEVD were purified as described above on glutathione-Sepharose without final elution with glutathione. The proteins were stored at 4°C bound to the Sepharose beads in an equal amount of solution containing 20 mM HEPES-KOH (pH 7.2), 25 mM KCl, 1 mM EDTA, 1 mM NaN3, and 0.002% phenylmethylsulfonyl fluoride. Immobilized GST, GST-70C139, and GST-70C110 (160 μg each) were incubated with 45 μg of Hop, 35 μg of Hip, 35 μg of BAG-1, or 30 μg of Hsp40 in 500 μl of buffer A (20 mM HEPES-KOH [pH 7.2], 50 mM KCl) at 4°C for 2 h. Following collection of the flowthrough fraction, the Sepharose beads were washed five times with 500 μl of buffer A. Bound protein was finally eluted by addition of 500 μl of buffer A containing 100 mM glutathione. All fractions were trichloroacetic acid-precipitated and subsequently analyzed by SDS-PAGE. Hop, Hip, and BAG-1 were detected by Coomassie blue staining. Due to the similar molecular masses of Hsp40 and the GST fusion proteins, Hsp40 was detected in the eluted fractions by immunoblotting with a polyclonal rabbit anti-Hsp40 antibody. Competition between full-length Hsc70 and GST-70C139 in binding to Hsp40 was analyzed by incubation of 40 μg of immobilized GST-70C139 with 10 μg of Hsp40, as well as 73 μg of Hsc70 when indicated. Binding of Hop to immobilized GST-70C139 in the presence of Hsp40 was analyzed by incubation of 40 μg of immobilized GST-70C139 with 20 μg of Hop (corresponding to 0.67 μM) and the indicated amount of Hsp40. About 45% of the added Hop protein was bound to GST-70C139 after incubation for 2 h at 4°C.
Complexes of Hop and Hsc70 were formed by incubation of 60 μg of Hop and 73 μg of Hsc70 in 50 μl of buffer A containing 5% glycerol–1 mM EDTA for 1 h at 4°C. Samples were centrifuged at 100,000 × g for 20 min and subsequently size fractionated on a Superose 12 column (Pharmacia) equilibrated in the same buffer. Fractions were trichloroacetic acid-precipitated and analyzed by SDS-PAGE. The amounts of Hop and Hsc70 were quantified after Coomassie blue staining.
Miscellaneous.
Rates of ATP hydrolysis were determined as described previously (20). Protein concentrations were determined with the Bio-Rad Bradford assay reagent with gamma globulin as the standard. Recombinant DNA techniques were performed as described previously (1).
Nucleotide sequence accession number.
The nucleotide sequence of the rat hop open reading frame has been submitted to the EMBL Nucleotide Sequence Database (accession no. Y15068).
RESULTS
Hip and Hop recognize distinct regions of the Hsc70 chaperone.
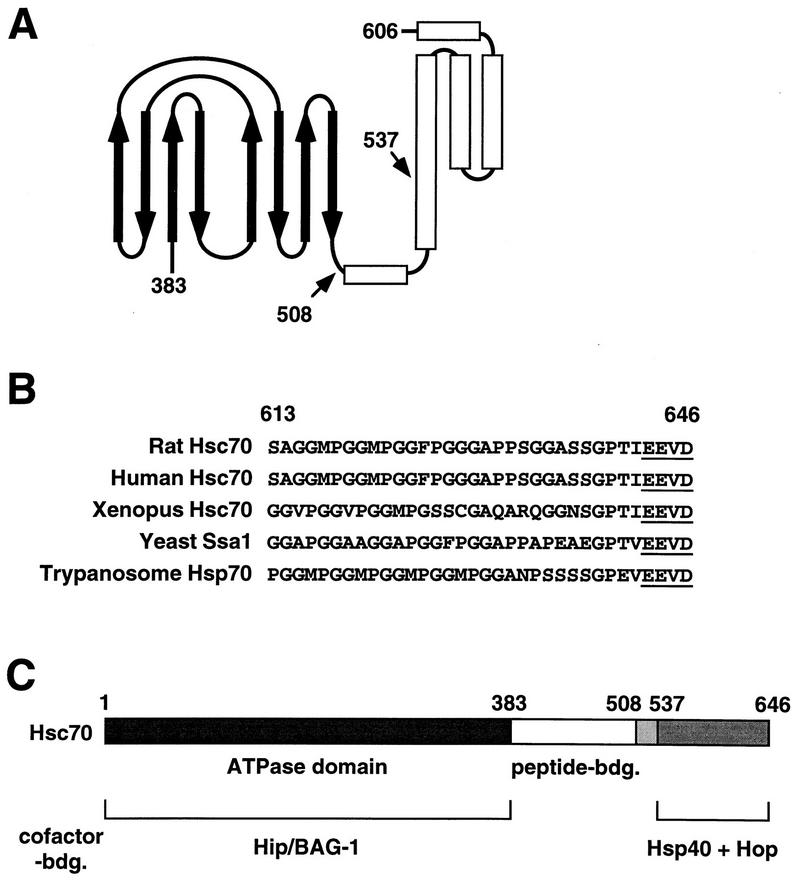
We investigated the capacity of the carboxy terminus of Hsc70 to mediate an interaction with chaperone cofactors. For this purpose, we expressed deletion fragments of rat Hsc70 which comprise the carboxy-terminal 110 (70C110) and 139 (70C139) amino acids (residues 537 to 646 and 508 to 646, respectively) (Fig. 1A). Residues 508 and 537 are located at the junction between the peptide binding pocket and the carboxy terminus of Hsc70. Each fragment includes a tightly folded α-helical subdomain that forms a lid over the peptide binding site (34), multiple degenerate repeats of the tetrapeptide GGMP, and the regulatory EEVD motif at the extreme carboxy terminus of Hsc70 (3, 9). Interaction with the Hsc70 cofactors Hip and Hop was analyzed by the yeast two-hybrid system (Fig. 1B). We have previously shown that rat Hip interacts with the ATPase domain of Hsc70 in the two-hybrid assay (17, 18). In contrast, an interaction of Hip with the two carboxy-terminal fragments of Hsc70 was not observed (Fig. 1B), although these fragments were expressed at levels similar to that of the ATPase domain construct (data not shown). To analyze the interaction of Hop with rat Hsc70, we cloned the hop cDNA from a rat liver cDNA library (see Materials and Methods). Similarly to Hip, the Hop protein interacted with full-length Hsc70 in the two-hybrid assay (Fig. 1B). However, Hop did not bind to Hsc70’s ATPase domain. Instead, it efficiently recognized the carboxy-terminal fragments of Hsc70, 70C110, and 70C139. Encouraged by these results, we attempted to further localize the region within the carboxy terminus of Hsc70 that mediates binding to Hop. For this purpose the following deletion fragments of Hsc70 were expressed in the two-hybrid system and examined for their interaction with Hop: (i) a carboxy-terminal fragment that lacks the EEVD motif (amino acids 508 to 642), (ii) a fragment that comprises primarily the α-helical subdomain (amino acids 508 to 612), and (iii) a fragment that comprises only the GGMP repeats and the EEVD motif (amino acids 613 to 646). All of the deletion fragments were expressed to levels similar to those of the 70C139 and 70C110 fragments in the two-hybrid system (data not shown), yet an interaction with Hop was not observed (data not shown). It appears that the α-helical subdomain, the GGMP repeats, and the carboxy-terminal EEVD motif of Hsc70 form a structural entity that mediates interaction with Hop. Thus, distinct binding sites have evolved in Hsc70 for the chaperone cofactors Hip and Hop.
FIG. 1.
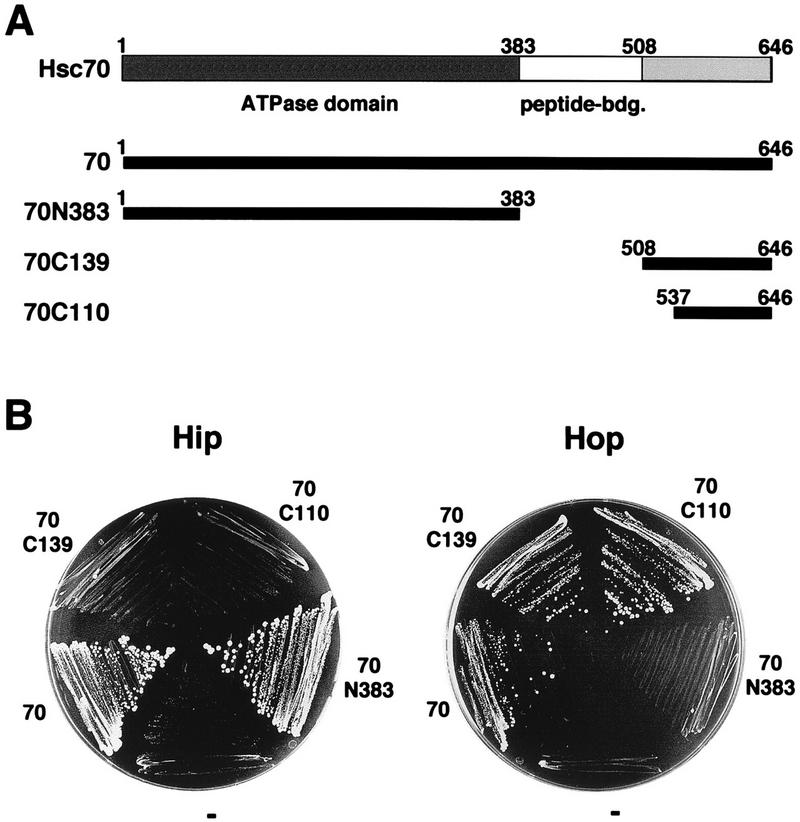
Interaction of Hsc70 and Hsc70 deletion fragments with Hip and Hop in the yeast two-hybrid assay. (A) Full-length rat Hsc70 (amino acids 1 to 646) (70) and deletion fragments of Hsc70 (70N383, 70C139, and 70C110) were fused to the Gal4 DNA-binding domain of plasmid pGBT9 and expressed in yeast strain HF7c. Peptide-bdg., peptide-binding domain. (B) Interaction with Hip and Hop was analyzed after growth of double transformants, carrying pAD-Hip (Hip) or pAD-Hop (Hop) and the indicated Hsc70 construct, on synthetic dextrose minimal medium lacking histidine for 7 days at 30°C. “−” indicates a control strain that carries the unmodified plasmid pGBT9 instead of an Hsc70 construct.
Hop directly interacts with Hsc70’s carboxy terminus.
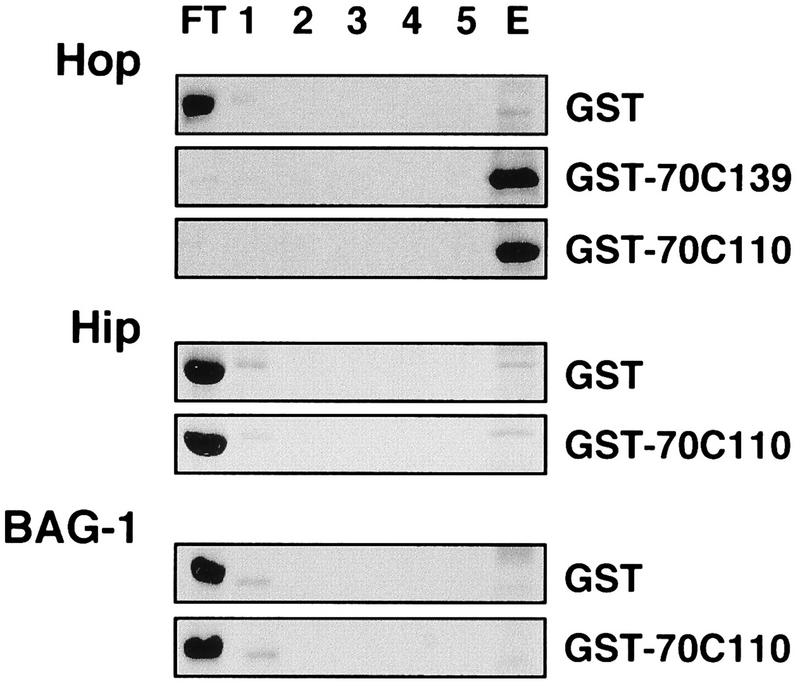
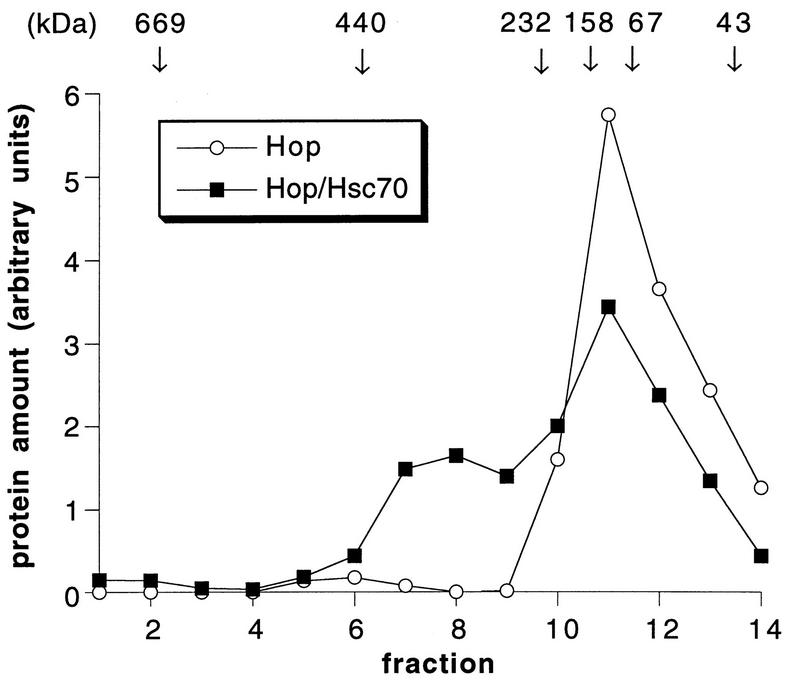
To exclude the possibility that the interaction between Hop and the carboxy terminus of Hsc70 observed in the two-hybrid assay involved additional proteins, a biochemical interaction assay was developed. Hop was purified to homogeneity after expression in insect cells infected with a recombinant baculovirus. The carboxy-terminal fragments of Hsc70 fused to GST were expressed in E. coli and affinity purified. While purified Hop did not significantly bind to unmodified GST immobilized on glutathione-Sepharose, the chaperone cofactor was quantitatively retained on immobilized GST-70C139 and GST-70C110 (Fig. 2). In contrast, purified Hip and BAG-1, previously shown to bind to Hsc70’s ATPase domain (16, 17, 31, 33), did not interact with the carboxy terminus of Hsc70 (Fig. 2). The in vitro assay thus confirms the specific recognition of Hsc70’s carboxy terminus by Hop and reveals that Hop interacts directly with the carboxy terminus without a requirement for additional protein components. Direct interaction between Hop and Hsc70 was also observed with purified full-length Hsc70. Incubation of Hop with Hsc70 gave rise to an ∼300-kDa Hop-Hsc70 complex that was detectable after gel filtration chromatography (Fig. 3). About 40% of the added Hsc70 coeluted with Hop in fractions 6 to 9. Notably, Hop alone eluted from the gel filtration column with an apparent native mass of ∼130 kDa, which would be consistent with a dimeric structure of the chaperone cofactor. Moreover, the apparent mass of the Hop-Hsc70 complex (∼300 kDa) may reflect the binding of two molecules of Hsc70 to the dimeric Hop protein. However, additional structural data are required to determine the exact stoichiometry of Hop-Hsc70 complexes.
FIG. 2.
In vitro binding assays with immobilized GST or GST fusion proteins comprising carboxy-terminal fragments of Hsc70 and purified Hop, Hip, and BAG-1. Immobilized GST, GST-70C139, and GST-70C110 were incubated with purified Hop, Hip, and BAG-1 as indicated for 2 h at 4°C. After collection of the flowthrough fraction (FT), the resin was washed five times (1 to 5) and bound protein was eluted with glutathione (E). Hop, Hip, and BAG-1 were detected by Coomassie blue staining after SDS-PAGE analysis of the collected fractions.
FIG. 3.
Gel filtration chromatography of Hop and Hop-Hsc70 complex. Hop (60 μg) was incubated alone (Hop) or in the presence of Hsc70 (73 μg) (Hop/Hsc70) for 1 h at 4°C, followed by gel filtration chromatography on a Superose 12 column. Amounts of Hop were quantified by densitometry of Coomassie blue-stained SDS-polyacrylamide gels.
The carboxy terminus of Hsc70 provides a binding site for Hsp40.
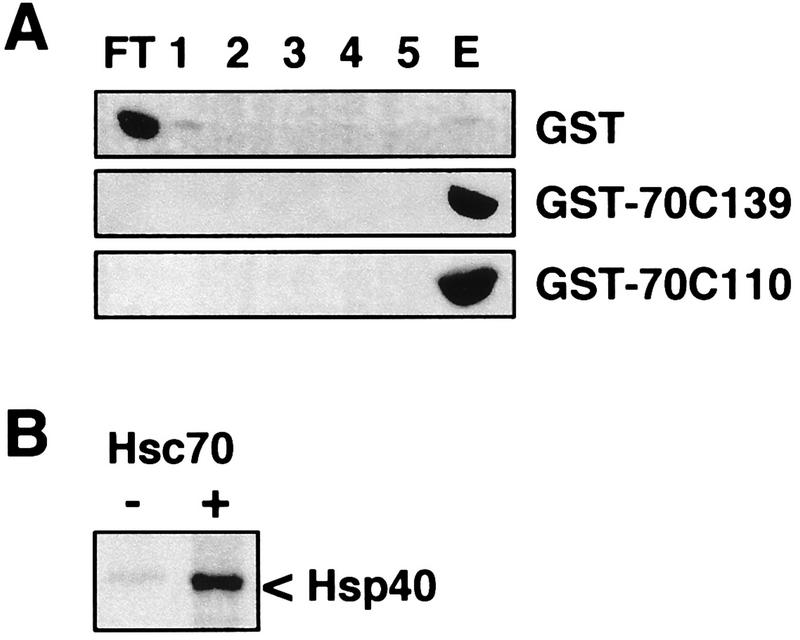
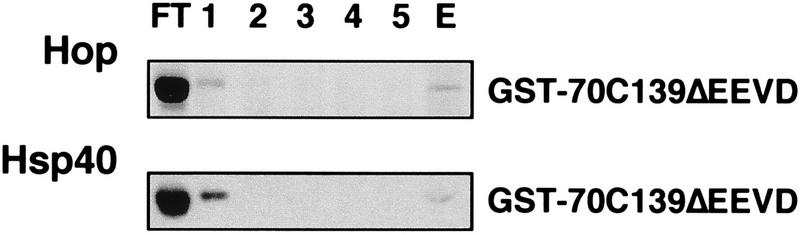
It has been shown that the carboxy-terminal EEVD motif of Hsc70 is essential for the regulation of the chaperone protein by Hsp40 (9). We therefore analyzed the binding of purified Hsp40 to the carboxy terminus of Hsc70, using immobilized GST-70C139 and GST-70C110. Hsp40 was quantitatively retained on both fusion proteins but did not bind to a significant extent to GST alone (Fig. 4A). The data reveal the existence of a binding site for Hsp40 within the carboxy-terminal domain of Hsc70. This was further supported when the binding of Hsp40 to GST-70C139 was performed in the presence of purified full-length Hsc70. Under these conditions a reduced amount of Hsp40 associated with the immobilized fusion protein, and Hsp40 was detectable in the flowthrough fraction (Fig. 4B). Apparently, full-length Hsc70 and the carboxy-terminal domain of the chaperone protein compete in binding to Hsp40. We also constructed a fusion protein which comprised GST and the carboxy-terminal domain of Hsc70 lacking the EEVD motif (GST-70C139ΔEEVD). An interaction of Hsp40 with the immobilized ΔEEVD carboxy-terminal domain of Hsc70 was not observed (Fig. 5). This is consistent with the loss of functional cooperation between Hsp40 and Hsc70 observed upon removal of the EEVD motif from full-length Hsc70 (9). Furthermore, Hop was unable to bind to the truncated carboxy-terminal domain of Hsc70 (Fig. 5).
FIG. 4.
In vitro binding assays with immobilized GST or GST fusion proteins comprising carboxy-terminal fragments of Hsc70 and purified Hsp40. (A) Immobilized GST, GST-70C139, and GST-70C110 were incubated with purified Hsp40. Binding and elution conditions were as described in the legend of Fig. 2. Hsp40 was detected with a polyclonal anti-Hsp40 antibody after immunoblotting. (B) Binding of Hsp40 to GST-70C139 was performed in the absence (−) of Hsc70 or in the presence (+) of equimolar amounts of Hsc70 and the GST fusion protein, and Hsp40 was detected in the flowthrough fractions by Coomassie blue staining.
FIG. 5.
In vitro binding assays with an immobilized GST fusion protein comprising the 70C139 carboxy-terminal domain of Hsc70 lacking the EEVD motif (GST-70C139ΔEEVD). The binding of Hop and Hsp40 and subsequent elution was performed as described in the legends of Fig. 2 and 4.
Hop and Hsp40 interact with Hsc70’s carboxy terminus in a noncompetitive manner.
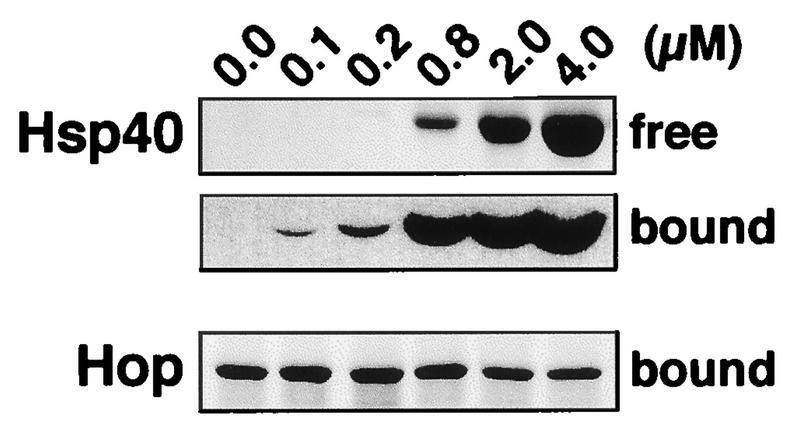
Since Hsp40 and Hop both interact with the carboxy terminus of Hsc70, we asked whether the two cofactors compete in binding to Hsc70. To answer this question, the binding of Hop to immobilized GST-70C139 was analyzed in the presence of increasing concentrations of Hsp40 (Fig. 6). GST-70C139 was incubated with saturating amounts of Hop and different amounts of Hsp40. Remarkably, even in the presence of a sixfold molar excess of Hsp40 over Hop, the amount of Hop associated with the carboxy terminus of Hsc70 was not significantly reduced. In this situation GST-70C139 was already saturated with Hsp40, as indicated by the detection of Hsp40 in the flowthrough fraction (Fig. 6). Moreover, under saturating conditions equimolar amounts of Hsp40 and Hop were found associated with the immobilized carboxy-terminal domain of Hsc70. It thus appears that Hop and Hsp40 interact with the carboxy terminus of the chaperone molecule in a noncompetitive manner.
FIG. 6.
Interaction of purified Hop with immobilized GST-70C139 in the presence of different concentrations of Hsp40. Immobilized GST-70C139 was incubated with 0.67 μM Hop and with different concentrations of Hsp40 as indicated. After incubation for 2 h at 4°C, the flowthrough fraction was collected (free), the resin was washed five times, and bound protein was eluted by addition of 100 mM glutathione (bound). Proteins were detected by Coomassie blue staining (free Hsp40 and bound Hop) and by immunoblotting (bound Hsp40) after SDS-PAGE analysis.
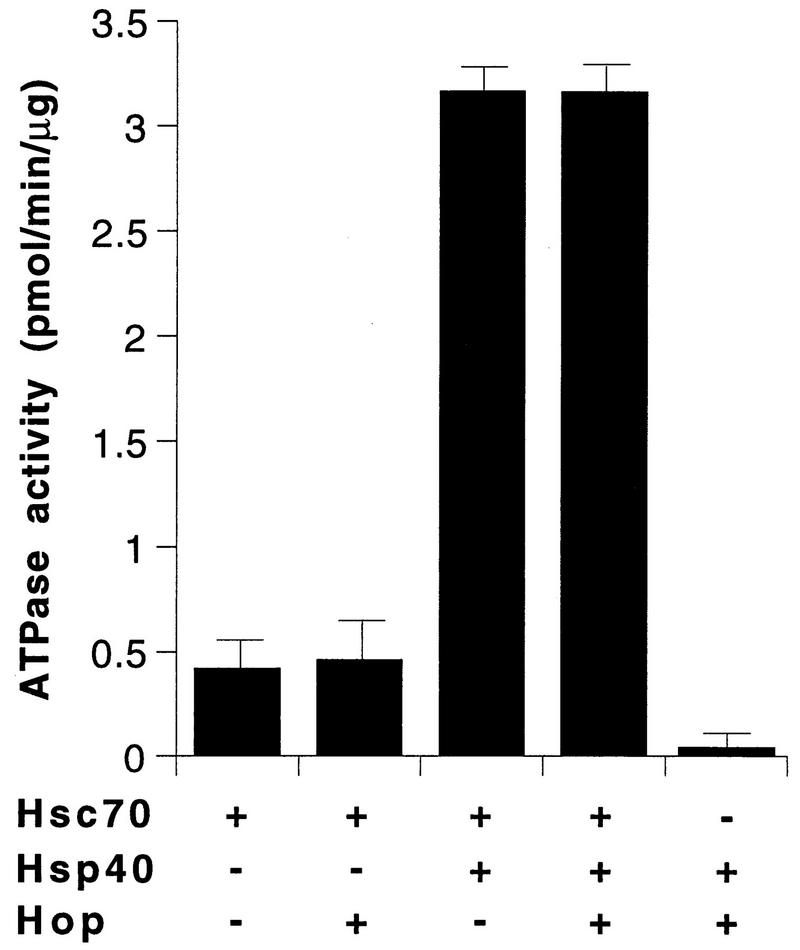
Hop does not affect the regulation of Hsc70 by Hsp40.
Chaperone cofactors are involved in the regulation of Hsc70’s ATPase activity and thus modulate the affinity of the chaperone for polypeptide substrates (12, 15, 26). However, it appears that Hop does not participate in the regulation of the Hsc70 ATPase cycle. When Hsc70 was coincubated with an equimolar amount of Hop, the cofactor did not alter the steady-state rate of ATP hydrolysis by Hsc70 (Fig. 7). In contrast, addition of Hsp40 led to an ∼7-fold stimulation of Hsc70’s ATPase activity. Again, the Hsp40-mediated stimulation was not affected by further addition of Hop (Fig. 7). Even at a 25-fold molar excess of Hop over Hsp40, Hop did not inhibit the regulation of Hsc70 by Hsp40 (data not shown). These data are consistent with the previous notion that Hop acts as a structural component mediating Hsc70-Hsp90 cooperation (4–6). Notably, the functional assays were performed under conditions that allowed Hop to interact with Hsc70 (Fig. 3). It thus appears that the binding of Hop to Hsc70 does not interfere with the functional cooperation of Hsc70 with Hsp40. The existence of nonoverlapping binding sites for Hop and Hsp40 apparently enables Hsc70 to interact simultaneously with both cofactors.
FIG. 7.
Steady-state ATP-hydrolytic activity of Hsc70 in the presence of Hsp40 and Hop. Hsc70 (3 μM) was incubated at 30°C with Hsp40 (1.5 μM) and Hop (3 μM) as indicated (+, present; −, absent), and the rates of ATP hydrolysis were determined. The bars represent averages of values (plus standard deviations) from four independent experiments, with six samples taken during the linear course of the reaction in each experiment.
DISCUSSION
In this study we show that the 10-kDa carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of cofactors, i.e., Hsp40 (Hdj-1) and Hop (p60/Sti1). The carboxy-terminal domain comprises a tightly folded α-helical subdomain (residues 537 to 606 of rat Hsc70), multiple degenerate repeats of the tetrapeptide GGMP of unknown function (residues 613 to 640), and the EEVD motif at the extreme carboxy terminus (Fig. 8). Determination of the crystal structure of the peptide binding domain of the bacterial Hsp70 homolog DnaK revealed that the α-helical subdomain is not directly involved in peptide recognition (34). A peptide substrate is bound by the chaperone protein via a channel formed by two sheets of four antiparallel β strands that are connected by extended loop structures (Fig. 8) (26, 34). The α-helical subdomain does not directly contact the peptide but apparently forms a lid over the peptide binding site. Through a latch-like mechanism, the subdomain may regulate the access of a substrate to the peptide binding pocket (26, 34). Secondary structure predictions and structural data obtained for rat Hsc70 indicate that the overall tertiary structures of the peptide binding site and the α-helical subdomain are largely conserved (3, 34). Still, the α-helical subdomain is less conserved on the amino acid level than the ATPase domain and the peptide binding pocket (3, 34). Furthermore, cytosolic eukaryotic Hsp70s possess GGMP repeats and the EEVD motif at the carboxy terminus whereas other Hsp70 family members lack such structural elements (3, 9). Thus, it appears that the carboxy terminus of the chaperone molecule is a rather variable domain which may determine the functional specificity of individual Hsp70s. This notion is supported by recent studies on cytosolic Hsp70s of the yeast S. cerevisiae (19). The exchange of the variable carboxy-terminal domains between distinct Hsp70s was found to alter the functional specificities of the resultant chimeras. In this regard, it is intriguing that the carboxy terminus of Hsc70 provides binding sites for the chaperone cofactors Hop and Hsp40 (Fig. 1, 2, and 4). Apparently, the recruitment of a distinct set of cofactors mediated by the carboxy-terminal domain participates in establishing the specific functions of individual Hsp70 family members.
FIG. 8.
(A) Schematic representation of the peptide binding region of bacterial Hsp70. The peptide binding site is formed by two sheets of four antiparallel β-strands (thick arrows) connected by extended loops. The β sandwich is followed by five α helices (open bars) that appear to form a lid over the peptide binding site. Together, the carboxy-terminal half of the second helix and helices 3 to 5 form a tightly folded subdomain. The carboxy-terminal deletion fragments of Hsc70 used in this study start at positions 508 and 537 at the junction between the peptide binding site and the carboxy terminus (arrows). The numbers indicate the corresponding residues of rat Hsc70. The schematic representation does not include the GGMP repeats and the extreme carboxy terminus of Hsc70. (B) Multiple degenerate repeats of the tetrapeptide GGMP and the EEVD motif (underlined) are found at the carboxy terminus of eukaryotic cytosolic members of the Hsp70 protein family. The numbers indicate residues of rat Hsc70. (C) Domains of Hsc70 mediating interactions with chaperone cofactors. While Hip and BAG-1 bind to the ATPase domain of Hsc70 (amino acids 1 to 383) in a mutually exclusive manner, Hsp40 and Hop recognize distinct binding sites within the carboxy terminus of the chaperone protein (amino acids 537 to 646). bdg., binding.
While our data reveal an Hsp40 binding site within the carboxy-terminal domain of Hsc70, the existence of an additional binding site for the cofactor cannot be excluded. A second binding site for Hsp40 may be jointly formed by the peptide binding domain and the ATPase domain of Hsc70, similar to the recently proposed binding site on Hsc70 for the Hsp40-related auxilin (32). In fact, we observed that the ATPase activity of the chymotryptic 60-kDa fragment of Hsc70, which lacks the carboxy terminus, can still be stimulated 2.5-fold by the addition of Hsp40 (data not shown). Remarkably, however, a deletion mutant of Hsc70 lacking the carboxy-terminal EEVD motif is no longer regulated by Hsp40 (9). Since deletion of the EEVD motif abolishes the binding of Hsp40 to the carboxy-terminal domain of Hsc70 (Fig. 5), cooperation of Hsc70 with Hsp40 may require an initial interaction of the cofactor with Hsc70’s carboxy terminus to gain access to a second binding site for Hsp40. In the 60-kDa fragment, such a binding site for Hsp40 may be permanently exposed. It remains to be seen whether the EEVD motif directly mediates the interaction between Hsp40 and Hsc70. Alternatively, the motif could be important for the correct folding of the carboxy-terminal domain of Hsc70. Notably, the interaction of Hop with the carboxy terminus of Hsc70 is also abolished by a deletion of the EEVD motif, but it is similarly affected when the α-helical subdomain is removed. This may indicate that the α-helical subdomain, the GGMP repeats, and the EEVD motif together form a structural entity mediating cofactor binding.
Remarkably, Hsp40 and Hop did not compete in binding to the carboxy terminus of Hsc70 (Fig. 6). Nonoverlapping binding sites have apparently evolved for both cofactors within the carboxy-terminal domain. This may allow Hsp40 to cooperate functionally with Hsc70 even when the chaperone interacts with Hop. In fact, the presence of Hop did not affect the stimulation of Hsc70’s ATPase activity by Hsp40 (Fig. 7) and Hop did not interfere with the cooperation of Hsc70 and Hsp40 during in vitro refolding of denatured β-galactosidase (10) and firefly luciferase (data not shown). Notably, Hop has previously been purified from rabbit reticulocyte lysate during an attempt to identify a factor that promotes the recycling of Hsc70 (13, 14). This factor stimulated the release of ADP from Hsc70 through an interaction with the ATPase domain of the chaperone protein. In light of the data presented here, it appears unlikely that Hop fulfills such a function in the Hsc70 reaction cycle. Hop did not alter the ATPase activity of Hsc70, either alone or when coincubated with Hsp40 (Fig. 7), and Hop did not interact with Hsc70’s ATPase domain in the two-hybrid assay (Fig. 1) and biochemical binding assays (data not shown). We would therefore conclude that Hop acts primarily as a structural component that promotes the cooperation of Hsc70 with Hsp90.
Analysis of the Hsc70-Hsp90-mediated activation of steroid hormone receptors emphasizes the importance of the interaction of Hsc70 with cofactors such as Hsp40, Hip, and Hop (2, 30). The progesterone receptor, for example, enters its activation pathway through an initial recognition by Hsc70, which apparently cooperates with Hsp40 at this stage (12, 25, 30). Cooperation of Hsc70 with Hsp40 also stimulates the association of the chaperone with Hip (17). Conceivably, a transient complex may form that comprises both cofactors bound to the Hsc70-receptor complex via their distinct binding sites on Hsc70. In fact, the retention of Hsp40 on immobilized Hip was recently observed when it was coincubated with Hsc70 (17). This demonstrates that Hsc70 can interact simultaneously with Hip and Hsp40. Further activation of the progesterone receptor requires the recruitment of Hsp90 to the chaperone-receptor complex. Recruitment of Hsp90 appears to be promoted by a Hip-mediated stabilization of the interaction of Hsc70 with the receptor molecule and by the action of the Hop protein. Hop is able to bind to Hsc70 and Hsp90, and may thus mediate the coupling of the chaperone proteins (4, 6). At this stage a simultaneous interaction of Hop and Hip with Hsc70 may be essential for the efficient cooperation of Hsc70 with Hsp90. An Hsc70-Hsp90 chaperone complex containing Hip and Hop has in fact been identified as an intermediate complex during receptor activation (30). Moreover, in immunoprecipitation experiments Hop was coprecipitated with Hsc70 when a Hip-specific antibody was used (25). The identification of distinct binding sites for Hip and Hop now provides us with the structural basis for the simultaneous interaction of Hsc70 with both cofactors.
The recent characterization of the BAG-1 protein as a binding partner of Hsc70 revealed that competition between distinct cofactors offers additional means for Hsc70 regulation (16, 31, 33). While BAG-1 cooperates with Hsp40 to efficiently stimulate the ATPase activity of Hsc70, it competes with Hip in binding to Hsc70’s ATPase domain (16). Hence, a network of competing and cooperating cofactors appears to modulate the chaperone activity of Hsc70 in the mammalian cell.
ACKNOWLEDGMENTS
We thank Jorgos Pyrowolakis for construction of plasmid pAD, Stefan Jentsch for helpful discussions, and Helle Ulrich and Joachim Rassow for critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (HO 1518/2-1 and HO 1518/2-2).
REFERENCES
- 1.Ausubel F J, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1989. [Google Scholar]
- 2.Bohen S P, Yamamoto K R. Modulation of steroid receptor signal transduction by heat shock proteins. In: Morimoto R I, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 313–334. [Google Scholar]
- 3.Boorstein W R, Ziegelhoffer T, Craig E A. Molecular evolution of the HSP70 multigene family. J Mol Evol. 1994;38:1–17. doi: 10.1007/BF00175490. [DOI] [PubMed] [Google Scholar]
- 4.Chang J H-C, Lindquist S. Conservation of Hsp90 macromolecular complexes in Saccharomyces cerevisiae. J Biol Chem. 1994;269:24983–24988. [PubMed] [Google Scholar]
- 5.Chang J H-C, Nathan D F, Lindquist S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60) Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Prapapanich V, Rimerman R A, Honore B, Smith D F. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins Hsp90 and Hsp70. Mol Endocrinol. 1996;10:682–693. doi: 10.1210/mend.10.6.8776728. [DOI] [PubMed] [Google Scholar]
- 7.Craig E A, Baxter B K, Becker J, Halladay J, Ziegelhoffer T. Cytosolic hsp70s of Saccharomyces cerevisiae: a role in protein synthesis, protein translocation, proteolysis, and regulation. In: Morimoto R I, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 31–52. [Google Scholar]
- 8.Flaherty K M, DeLuca-Flaherty C, McKay D B. Three-dimensional structure of the ATPase fragment of the 70 K heat-shock cognate protein. Nature. 1990;346:623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- 9.Freeman B C, Myers M P, Schumacher R, Morimoto R I. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman B C, Toft D O, Morimoto R I. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- 11.Frydman J, Nimmesgern E, Ohtsuka K, Hartl F-U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370:111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- 12.Frydman J, Höhfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- 13.Gross M, Hessefort S, Olin A, Reddy G. Extensive sequencing of tryptic peptides of a rabbit reticulocyte 66-kDa protein that promotes recycling of Hsp70. J Biol Chem. 1996;271:16842–16849. doi: 10.1074/jbc.271.28.16842. [DOI] [PubMed] [Google Scholar]
- 14.Gross M, Hessefort S. Purification and characterization of a 66-kDa protein from rabbit reticulocyte lysate which promotes the recycling of Hsp70. J Biol Chem. 1996;271:16833–16841. doi: 10.1074/jbc.271.28.16833. [DOI] [PubMed] [Google Scholar]
- 15.Hartl F-U. Molecular chaperones in protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 16.Höhfeld J, Jentsch S. GrpE-like regulation of the Hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höhfeld J, Minami Y, Hartl F-U. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 18.Irmer H, Höhfeld J. Characterization of functional domains of the eukaryotic co-chaperone Hip. J Biol Chem. 1997;272:2230–2235. doi: 10.1074/jbc.272.4.2230. [DOI] [PubMed] [Google Scholar]
- 19.James P, Pfund C, Craig E A. Functional specificity among Hsp70 molecular chaperones. Science. 1997;275:387–389. doi: 10.1126/science.275.5298.387. [DOI] [PubMed] [Google Scholar]
- 20.Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci USA. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarty J S, Buchberger A, Reinstein J, Bukau B. The role of ATP in the functional cycle of the DnaK chaperone system. J Mol Biol. 1995;249:126–137. doi: 10.1006/jmbi.1995.0284. [DOI] [PubMed] [Google Scholar]
- 22.Minami Y, Höhfeld J, Ohtsuka K, Hartl F-U. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- 23.Nicolet C M, Craig E A. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:3638–3646. doi: 10.1128/mcb.9.9.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palleros D R, Welch W J, Fink A L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci USA. 1991;88:5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prapapanich V, Chen S, Nair S C, Rimerman R A, Smith D F. Molecular cloning of human p48, a transient component of progesterone receptor complexes and an Hsp70-binding protein. Mol Endocrinol. 1996;10:420–431. doi: 10.1210/mend.10.4.8721986. [DOI] [PubMed] [Google Scholar]
- 26.Rüdiger S, Buchberger A, Bukau B. Interaction of Hsp70 chaperones with substrates. Nat Struct Biol. 1997;4:342–349. doi: 10.1038/nsb0597-342. [DOI] [PubMed] [Google Scholar]
- 27.Schmid D, Baici A, Gehring H, Christen P. Kinetics of molecular chaperone action. Science. 1994;263:971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, Thomas J O. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992;12:2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith D F, Sullivan W P, Marion T N, Zaitsu K, Madden B, McCormick D J, Toft D O. Identification of a 60-kilodalton stress-related protein, p60, which interacts with hsp90 and hsp70. Mol Cell Biol. 1993;13:869–876. doi: 10.1128/mcb.13.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith D F, Whitesell L, Nair S C, Chen S, Prapapanich V, Rimerman R A. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takayama S, Bimston D N, Matsuzawa S, Freeman B C, Aime-Sempe C, Xie Z, Morimoto R I, Reed J C. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ungewickell E, Ungewickell H, Holstein S E H. Functional interaction of the auxilin J domain with the nucleotide- and substrate-binding modules of Hsc70. J Biol Chem. 1997;272:19594–19600. doi: 10.1074/jbc.272.31.19594. [DOI] [PubMed] [Google Scholar]
- 33.Zeiner M, Gebauer M, Gehring U. Mammalian protein RAP46; an interaction partner and modulator of 70 kDa heat shock proteins. EMBO J. 1997;16:5483–5490. doi: 10.1093/emboj/16.18.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X, Zhao X, Burkholder W F, Gragerov A, Ogata C, Gottesman M E, Hendrickson W A. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegelhoffer T, Johnson J L, Craig E A. Protein folding: chaperones get Hip. Curr Biol. 1996;6:272–275. doi: 10.1016/s0960-9822(02)00476-1. [DOI] [PubMed] [Google Scholar]