Abstract
Velvet antler (VA) has been widely used in traditional Chinese medicine more than thousand years. Several pharmacological properties of VA have been demonstrated, such as anti-osteoporosis, anti-osteoarthritis, anti-aging, and anti-ischemia-hypoxia effects. Recently, many studies applied different omics in VA studies to illustrate biological pathways or processes, identify bioactive compounds, and discover pharmacological properties. This mini-review article summarizes the application of different omics for investigating therapeutic effects of VA. The limitation and future challenges facing the application of multi-omics in VA is also briefly discussed.
Keywords: Velvet antler, Therapeutic effects, Microbiomics, Proteomics, Transcriptomics
Graphical abstract

1. Introduction
Velvet antler (VA), a cartilaginous tissue without full calcification, has been used in traditional Chinese medicine more than thousand years. Several pharmacological properties of VA have been demonstrated, such as anti-osteoporosis, wound healing-promoting effects and anti-inflammation.1,2 Specifically, the regeneration property of VA constitutive tissues (bone, cartilage, skin, nerves and blood vessels) with high abundance of growth factors (vascular endothelial growth factor, epidermal growth factor, fibroblast growth factor and nerve growth factor) makes researchers draw more attention for evaluating bone/tissue regeneration-promoting properties.3, 4, 5 Additionally, VA possesses more than 50 % of the dry weight in protein, which is a good source of bioactive peptides.2,6 The peptides derived from VA have showed anti-inflammatory,7, 8, 9 hypoglycemic,10 barrier integrity,11 anti-allergy,12 anti-aging,13 anti-tumor,14 bone regeneration-promoting properties,15,16 and anti-neurological disease.15,16 Besides peptides, velvet antler contains common chemical substances such as mineral elements, amino acids, proteins, peptides, saccharides, lipids, polyamines, uracil, hypoxanthine, and undine.17 Additionally, a previous study identified a diverse array of compounds in velvet antler extracts from Cervus nippon Temminck var. mantchurieus Sainhoe, including one lignan, 30 terpenoids (of which 20 are triterpenes), 39 steroids, 8 alkaloids, 4 organic acids, 5 esters, and 16 phospholipids.18 Different deer species also demonstrated different levels of bioactive components. Comparative bioactive components of VA water extracts by proteomics showed that the level of Apolipoprotein A1 (APOA-I) in sambar deer was higher than that in red deer.9 APOA-I possessed anti-inflammatory ability on human monocytes, macrophages, adipocytes, and endothelial.
The application of multi-omics brings biological research into a new era. The different omic approaches, such as genomics (complete collection of DNA),19, 20, 21 transcriptomics (complete collection of RNA in a cell or collection of cell),19,22, 23, 24 proteomics (complete collection of protein in a cell/tissue/organism),21,25 metabolomics (complete collection of small molecules in a cell/tissue/organism),26, 27, 28 microbiomics (complete collection of genes of microbes in the organism),29,30 could assist at clarifying a particular biological mechanisms in biological issues.22,31,32 Table 1 highline the principles, instrument and limitation of omic approaches. Recently, many studies applied different omics in VA studies to illustrate biological pathways or processes, identify bioactive compounds, and discover pharmacological properties. This mini-review article summarizes the application of different omics for investigating therapeutic effects of VA. The limitation and future challenges facing the application of multi-omics in deer VA is also briefly discussed. Results were obtained and selected from PubMed and Science Citation Index Expanded (SCIE) databases in December 2023, by searching “deer antler proteomics/microbiomics/genomics/transcriptomics/metabolomics”.
Table 1.
Summarize the studies in therapeutic effect of deer velvet antler using multi-omics strategies.
| Omics | Principle | Instruments | Limitations | References |
|---|---|---|---|---|
| Genomics | Studies the entire set of genes in an organism, including their structure, function, evolution, and interactions | High-throughput sequencers, PCR machines | High cost, complex data analysis | 19, 20, 21 |
| Transcriptomics | Studies RNA molecules, especially messenger RNA (mRNA), to understand how genes are regulated under different conditions | RNA sequencers (e.g., Illumina HiSeq), Real-time quantitative PCR (qPCR) | High sample quality requirement, complex data processing | 19,22, 23, 24 |
| Proteomics | Studies the structure, function, and interactions of proteins | Mass spectrometers (e.g., LC-MS/MS), Gel-based approach | Difficulty in protein extraction and purification, complex data analysis | 21,25 |
| Metabolomics | Studies small molecule metabolites within an organism to understand metabolic pathways and biological processes | Nuclear Magnetic Resonance (NMR) spectroscopy, Mass spectrometers (e.g., GC-MS, LC-MS) | Sample stability issues, complex data interpretation | 26, 27, 28 |
| Microbiomics | Studies the composition and function of microbial communities in different environments | Next-generation sequencing, 16S rRNA gene sequencing, Metagenomic sequencing | Complex data analysis, need for comprehensive databases, sample contamination | 29,30 |
2. Application of omics in therapeutic effects of VA
Different omics, including microbiomics, proteomics, transcriptomics and metabolomics. have been applied to investigate various therapeutic effects of VA. Table 2 summarizes the studies of therapeutic effects of VA by omics.
Table 2.
Summarize the studies in therapeutic effect of deer velvet antler using multi-omics strategies.
| VA sample | Therapeutic effect | Experimental subject | Omics | Major finding | Reference |
|---|---|---|---|---|---|
| Anti-oxidative and immunoregulatory effects | |||||
| Red deer (Cervus elaphus) VA | Anti-oxidation | VA | Quantitative proteomic analysis | The antler samples recognized 259 differentially abundant proteins between tips and middle section major associated with antioxidant metabolic mechanisms. | 33 |
| VA | Immunoregulatory effect | VA | Analyzing the GeneCards, OMIM database, BATMAN-TCM and the STRING database via network pharmacology, molecular docking, and molecular dynamics simulation techniques | Four substances and 130 target genes might be related in immunomodulatory effect. From the KEGG analysis, the PI3K-Akt signaling pathway, regulating T cell function, was one of enriched pathways. MAPK3–17 beta estradiol complex was also identified, that might be important in immune regulation. | 34 |
| Effect on ischemia-hypoxia | |||||
| Sika deer VA protein: water extract | Hypoxic-ischemic cardiac disease | Hypoxic-ischemic cardiac microvascular endothelial cells (CMECs) | Proteomics | PI3K/Akt signaling pathway might involve in the protective effect of VA protein on CMEC | 41 |
| Pilose antler polypeptides (powder form) | Hypoxic-ischemic encephalopathy | Hypoxic-ischemic injured rats | Microbiomics | Alternation of gut microbiota contributes an inhibitory effect of VA on the inflammatory response mainly associated with reduction in serum LPS and inflammatory factors, and maintenance of the integrity of the intestinal barrier. Shifting gut microbiota by VA polypeptide also led to a functional change in the microbial communities. | 36 |
| Effect on bone and cartilage | |||||
| The antler tip of Chinese Sika deer | cCartilage formation, development, and growth. | The antler tip | Transcriptomics | Numerous functional genes, such as extracellular matrices, growth factors and transcription factors, are involved in cartilage formation, development, and growth. | 63 |
| Deer antler stem cells from sika deer | Bone regeneration | VA and deer blastocysts | Transcriptomics | Antler stem cells expressed classic marrow stromal cells markers, suggesting that they have some attributes of the embryonic stem cells. Through comprehensively characterizing the antler stem cells could benefit the future human regenerative medicine. | 55 |
| VA extract from Chinese sika deer containing water-soluble proteins | Treatment of cartilage -related disease | Primary chondrocyte from 4-day-old neonatal mice | Proteomics | Promote proliferation and preventing apoptosis/differentiation of chondrocyte by controlling chromatin structure and dynamics, transcription, translation, posttranslational modification, signal transduction and alterations of the cytoskeletal structure in chondrocytes. | 65 |
| VA extract from Chinese sika deer containing water-soluble proteins | Treatment of cartilage -related disease | Sprague-Dawley rats | Transcriptomics | Downregulate the expression of genes associated with the pathophysiology of osteoarthritis and upregulate the expression of functional genes involved in cartilage formation, growth, and repair. | 67 |
| Antler blastema progenitor cells from sika deer (Cervus nippon) | Bone repair. | Femoral condyle defect repair in a rabbit model | Transcriptomics | Antler blastema progenitor cells could produce osteochondral lineage cells with strong self-renewal ability in vitro and in vivo, suggesting a potential application in clinical bone repair. | 54 |
| Velvet collagen hydrolysate from sika deer | Anti- osteoporosis effect | Urine samples of rats in the osteoporosis model | Metabolomics | Identify five biomarkers, including taurine, saccharopine, Trp, Asn, and Arg. | 73 |
| VA extract from Chinese sika deer containing water-soluble proteins | Anti- osteoporosis effect | Serum samples of rats with VA extract | Proteomics | Identified that 23 upregulated protein and 10 downregulated protein were mapped in a complex network, which affect the dynamic balance between osteoblasts and osteoclasts. | 74 |
| VA extract from sika deer | Anti- osteoporosis effect | The ovariectomized mice | Microbiomics | The gut dysbiosis caused by ovariectomy was restored after VA protein treatment with decreasing the ratio of Firmicutes to Bacteroidetes in mice. | 76 |
| Antler stem cells (ASCs) from sika deer | Anti-osteoarthritis effect | The anterior cruciate ligament transection (ACLT)-induced mouse model | Proteomics and Transcriptomics | ASC-derived exosomes exert their geroprotective effects by promoting cell division and repressing the senescence-associated inflammation. | 57 |
| Other effects | |||||
| VA polypeptides (powder form) | Anti-aging effect | D-galactose induced anti-aging mice | Microbiomics | Regulation of gut microbial composition by VA could affect the fatty acid degradation pathway, such as downregulating fatty acid by enhancing fatty acid decomposition. Reduction of fatty acid could contribute to increase in ATP and decrease in the expression of APOE4 in the brain. | 81 |
| VA extract | Anti-fatigue | Mouse model with forced swimming test | Transcriptomics | Nine pathways were regulated. Seven pathways involved in systematic effects with regulating sugar and energy metabolism through modifying two other local tissue pathways. | 87 |
| Water extracts of Formosan sambar deer (SVAE) and red deer (RVAE) | Anti-colitis | SDS induced mouse colitis model | Microbiomics and metabolomics | Anti-colitis effects might involve in the shift in microbiota. The potentially bioactive components of SVAE and RVAE were identified as small molecules | 88 |
| A chitosan/sodium alginate/velvet antler blood peptides hydrogel (CAVBPH) | Wound healing | A skin wound mouse model | Proteomics | the mechanisms of CAVBPH might be associated with the activation of the PI3K/AKT/mTOR and SIRT1/NF-κB pathways. | 90 |
| A velvet antler protein (VA-pro) extracted from velvet antler (Cervus elaphus residue by simulating the gastrointestinal digestion | Anti-tumor | Murine sarcoma 180 (S180) cells | Proteomics | A velvet antler protein (VA-pro), extracted from velvet antler residue by simulating the gastrointestinal digestion the velvet with average molecular weight of 22.589 kDa, could inhibit the proliferation and promote apoptosis of solid S180 tumors by inducing S phase cell cycle arrest of tumor cells mediated through mitochondria. | 92 |
Results were obtained and selected from PubMed and SCIE databases in December 2023 (Table 2), by searching “deer antler proteomics/microbiomics/genomics/transcriptomics/metabolomics”.
2.1. Anti-oxidative and immunoregulatory effects
Numerous studies have confirmed that the therapeutic effects of VA were due to its antioxidative and immunoregulating properties. Quantitative proteomic analysis of the antler samples of red deer recognized 259 differentially abundant proteins between tips and middle section primarily associated with antioxidant metabolic mechanisms. This result indicated that anti-oxidant/redox regulating proteins at VA tip are important during deer antler regeneration due to high metabolic and growth rate with subsequent risk of oxidative stress.33 However, if higher anti-oxidative proteins at VA tips possess better anti-oxidative effect than other VA sections, it still needs more studies to verify it.
Additionally, immunomodulatory mechanism of deer antler active compounds has also been studied by analyzing the GeneCards, OMIM database, BATMAN-TCM and the STRING database via network pharmacology, molecular docking, and molecular dynamics simulation techniques. Four substances and 130 target genes might be related in immunomodulatory effect. From the KEGG analysis, the PI3K-Akt signaling pathway, regulating T cell function, was one of enriched pathways. MAPK3–17 beta estradiol complex was also identified, that might be important in immune regulation.34
2.2. Ischemia-hypoxia
VA has demonstrated beneficial effect in cardiovascular diseases including arrhythmia,35 ischemic disease,36 and heart failure37 in cell models and animal models. The various mechanisms involve reducing the release of endothelin,38 promoting superoxide dismutase activities, decreasing serum malondialdehyde levels,39 and increasing the levels of nitric oxide and calcitonin-gene-related peptide.40
Several studies showed that VA protein/polypeptides provided a therapeutic effect for hypoxic-ischemic diseases including myocardial infarction41 and hypoxic-ischemic encephalopathy.42 Recently, a novel velvet antler polypeptide, PNP1, has been identified, that demonstrated the anti-ischemic cerebral injury effect in the ischemia reperfusion mouse model.43 Although the pathophysiological mechanisms and pathway of hypoxic-ischemic diseases are still needed to be clarified, a couple of studies indicated that inflammation, excitotoxicity, and oxidative stress were involved.44,45 Administration of VA polypeptides could reduce hepatic lipid accumulation, systemic oxidative stress and inflammation, as well as impaired gut barrier function via modification of gut microbiota observing in a hypoxic-ischemic rat model.36 At the genus level, the abundance of Lachnospiraceae NK4A136, Pectinophilus, Ruminiclostridium and Butyricicoccus were downregulated, and the abundance of Ruminiclostridium_5, Lachnoclostridium, Veillonella and Bacteroides were upregulated after intervention of VA polypeptides. Several studies indicated that lipid polysaccharide (LPS)-mediated inflammation affects gut microbiota-mediated lipid metabolism.46 Alternation of gut microbiota contributes an inhibitory effect of VA on the inflammatory response mainly associated with reduction in serum LPS and inflammatory factors, and maintenance of the integrity of the intestinal barrier. Shifting gut microbiota by VA polypeptide also led to a functional change in the microbial communities, such as attenuated susceptibility to bacterial infections, decreased antibiotic synthesis and changed cellular processes and signaling, that generate the protective effects in hypoxic-ischemic disease.36
Endothelial dysfunction is also an important factor relating myocardial infarction, and myocardial ischemia reperfusion.47 A cell study using hypoxic-ischemic cardiac microvascular endothelial cells (CMECs) indicated that VA protein could reverse the change in expression of Akt, caspase-3, Bcl-2, and Bax induced by ischemia-hypoxia.41 Bcl-2 is an anti-apoptotic protein, whereas Bax is a pro-apoptotic protein. Caspase-3 can generate DNA degradation. The ratial between the apoptotic proteins is crucial for cell survival against injuries caused by ischemia-hypoxia.48 The change in LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor, was also observed, indicating that the PI3K/Akt signaling pathway might involve in the protective effect of VA protein on CMECs.41 Further proteomic analysis of VA protein, 21 proteins were directly related to the PI3K/Akt signaling pathway, which was paralleled with the Western blot findings.41
2.3. Effect on bone and cartilage
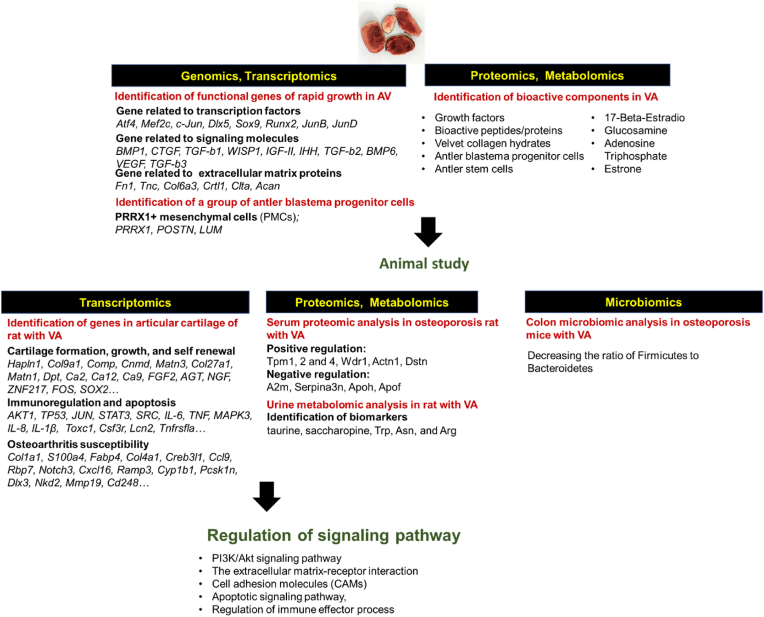
The investigation of deer antler osteogenesis could be application for the study of bone damage repair due to its regrowth feature.49 Numerous evidences also demonstrated that VA play key roles in promoting bone growth, repairing and development.50, 51, 52 The underlying molecular mechanisms that control the pharmacological activity of VA involving in anti-osteoporosis and osteoarthritis have been investigated using different omics. Fig. 1 characterizes therapeutic effect of deer velvet antler using multi-omics strategies in bone and cartilage.
Fig. 1.
Characterizing therapeutic effect of deer velvet antler using multi-omics strategies in bone and cartilage.
2.3.1. Regeneration of deer antler and antler stem cell
Antler stem cells (ASCs), continuing self-renewal and differentiation into multi-lineage cells to form an organ,53 could be a potential treatment for bone damage. A group of “antler blastema progenitor cells” (ABPCs), recognized by genomics, could produce osteochondral lineage cells with strong self-renewal ability in vitro and in vivo, suggesting a potential application in clinical bone repair.54 Other studies in deer antler proteomics and transcriptomics indicated that ASCs expressed classic marrow stromal cells markers, suggesting that they have some attributes of the embryonic stem cells.55 HIF-1and PI3K-AKT signaling pathways might involve in initiating/regulating the regeneration of antlers.55,56
A recent in-vitro study showed that exosomes derived from ASCs improved senescence in human mesenchymal stem cells (hMSCs).57 Further animal study demonstrated that intra-articular injection of exosomes derived from ASCs alleviated osteoarthritis in the anterior cruciate ligament transection (ACLT)-induced mouse model. After proteomic and transcriptomic analysis, ASC-derived exosomes exert their geroprotective effects by promoting cell division and repressing the senescence-associated inflammation.57
2.3.2. Anti-osteoarthritis
The inflammatory factors, including matrix degrading enzymes, growth factors and inflammatory cytokines are one of major pathogenesis of osteoarthritis. The chondrocyte clusters release these inflammatory factors, that affects the surrounding chondrocytes and joint tissue to increase progression of osteoarthritis. The inflammatory factors also promote cartilage degradation by activating cells in the superficial layer of the cartilage to synthesize matrix metalloproteinases (MMPs).58 Besides, oxidative stress also involves in the pathological process of osteoarthritis, that induces local intra-articular lesions and chondrocyte anabolism. The damage by free radicals to chondrocytes has also been reported.59, 60, 61 VA and its extract have been reported to alleviate the progressing of osteoarthritis. Cervus peptides, extracted from Sika deer, could improve the therapeutic efficacy of rheumatoid arthritis patients in combination with umbilical cord mesenchymal stem cell clinically. The possible mechanism could be associated with the downregulation of inflammatory cytokines, regulation of immunity, and enhancement of microcirculation.62
Several studies analyzing the functional genes of VA extracts using transcriptomics identified numerous functional genes, such as extracellular matrices, growth factors and transcription factors, that are involved in cartilage formation, development, and growth.63, 64, 65, 66 Further animal study verified that VA extracts containing water-soluble proteins could significantly downregulate the expression of genes associated with the pathophysiology of osteoarthritis and upregulate the expression of functional genes involved in cartilage formation, growth, and repair in rats.67 Additional examining the protein expression profiles by proteomics revealed that intervention of VA extracts could promote proliferation and preventing apoptosis/differentiation of chondrocyte by controlling chromatin structure and dynamics, transcription, translation, posttranslational modification, signal transduction and alterations of the cytoskeletal structure in chondrocytes to maintain genomic stability and regulate epigenetic alterations, protein synthesis, degradation and modification.67 The findings in transcriptomics and proteomics suggest that the VA extracts may play crucial roles in regulating chondrocyte homeostasis.
2.3.3. Anti-osteoporosis
Osteoporosis is characterized by low bone mass and microarchitectural deterioration of bone tissue, resulting in increased bone fragility and susceptibility to fracture.68,69 The pathogenetic mechanisms include failure to achieve a skeleton of optimal strength during growth and development; excessive bone resorption; and failure to replace lost bone due to defects in bone formation.70 After menopause, the reduction of estrogen also causes excessive bone resorption by osteoclasts, leading to postmenopausal osteoporosis.71
Intervention with VA base collagens for 90 days increased bone mineral density and the hydroxyproline content in the serum as comparing with osteoporosis control in rats.72 In clinical practice, Cervus polypeptide ameliorated the bone metabolic biochemical markers in patients with osteoporosis induced by glucocorticoids. To investigate the underlying mechanism of anti-osteoporosis effect of VA products, different omics strategies were conducted. A urine metabolomics analysis in an osteoporosis rat model identified five compounds, including taurine, saccharopine, tryptophan (Trp), asparagine (Asn), and arginine (Arg), that could be used as biomarkers.73 Another serum proteomic analysis of rats in the osteoporosis model indicated that VA polypeptides upregulated 47 and downregulate 32 proteins. Further protein-protein interaction analysis identified that 23 upregulated protein and 10 downregulated protein were mapped in a complex network, including positively regulating Tropomyosins (Tpm1, 2 and 4), WD repeat-containing protein 1 (Wdr1), Alpha-actinin-1 (Actn1) and Destrin (Dstn), and negatively regulating Alpha-2-macroglobulin (A2m), Serine protease inhibitor A3 N (Serpina3n) and Apolipoproteins (Apoh and Apof), which affect the dynamic balance between osteoblasts and osteoclasts.74 By transcriptional sequencing analysis, the bone strengthening effect of VA also involved in the upregulation of genes in regulating the skeletal system, including S100a8 and S100a9 (calcifying the cartilage matrix), lipocalin 2 (Lcn2, promoting bone regeneration), transferrin (Tf, transporting iron to the bone marrow), Serpinb1a (inhibiting osteoclast formation and osteoblast differentiation), and lymphocyte cytosolic protein 1 (Lcp1, regulating the intracellular calcium during osteoblast differentiation).75
Additionally, in an ovariectomized mice study, treatment of Sika deer velvet antler protein extract for 12 weeks suggested a therapeutic effect on osteoporosis. The further gut microbiota analysis revealed that the gut dysbiosis caused by ovariectomy was restored after VA protein treatment with decreasing the ratio of Firmicutes to Bacteroidetes in mice. The upregulation of beneficial bacteria genus including Akkermansia and Lactobacillus were also observed.76 Another study constructed a drug-component-target-disease network by analyzing the GeneCards (The Human Gene Database), OMIM database (human genes and genetic phenotypes) and BATMAN-TCM (a database designed to store known and predicted linkages between traditional Chinese medicine ingredients and target proteins), and found that the major components involved in the treatment of postmenopausal osteoporosis were 17β-E2, adenosine triphosphate, and oestrone. These components targeted on 21 key genes and regulated the PI3K/Akt-signaling and MAPK-signaling pathways.71
The omics findings suggested that the mechanism of VA products on anti-osteoporosis may involve in the restoration of dysbiosis of gut microbiota, the regulation of amino acid metabolism and phospholipid metabolism, and control signaling pathways responsible for bone formation and remodeling.
2.4. Other therapeutic effects
2.4.1. Anti-aging effect
With an extension of lifespan, aging is becoming one of the most important public health issues worldwide.77 Oxidative stress results in accumulation of mitochondrial reactive oxygen species (ROS) and free radicals, leading to dysfunctions of cognitive, memory, and learning abilities.78,79 Numerous studies have confirmed that VA processes antioxidant and anti-aging effects with preventing neurodegenerative diseases including inhibit neuronal apoptosis via the thalamus-pituitary-adrenal axis80 and downregulating the concentration of the glucocorticoid receptor, miner-alocorticoid receptor and corticotropin-releasing hormone.81 VA polypeptide could enrich antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in the serum and downregulate the level of lipid peroxide malondialdehyde (MDA), leading to effectively eliminate free radicals and peroxides accumulation, positively impacting the aging process.80,81
Further investigating the molecular mechanisms reveals that modification of gut microbiota plays an important role for antiaging effect of VA polypeptides. The VA polypeptide could shift the intestinal microbial composition of aging mice to the normal mice with significantly increasing the abundance of Lactobacillus in D-galactose inducing aging model.80 Lactobacillus has been demonstrated a protective effect on antiaging and cognitive functions.82,83 Regulation of gut microbial composition by VA could also affect the fatty acid degradation pathway, such as downregulating fatty acid by enhancing fatty acid decomposition. Reduction of fatty acid could contribute to increase in ATP and decrease in the expression of human apolipoprotein E (APOE) 4 in the brain,81 thereby improving the cognitive impairment caused by aging, alleviating learning ability, and protecting neurons. Additionally, apoE4 reduction could also attenuate neuroinflammation and improve amyloid and tau pathologies in the Alzheimer's disease brain, providing a possible therapeutic approach.84 Steroid hormone receptors such as glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) also play an important role in preventing neurodegenerative diseases including inhibit neuronal apoptosis via the thalamus-pituitary-adrenal axis.85,86
2.4.2. Anti-fatigue
Physical fatigue is contributed by energy source reduction and surplus metabolite accumulation. A VA tip extract from Formosan sambar deer demonstrated an anti-fatigue effect in a mouse model. The transcriptomic analysis in the biceps skeletal muscle of mice indicated that nine pathways were regulated. Seven pathways (prolactin signalling pathway, serum response factor mediated pathway, hypothalamic gonadotropin-releasing hormone signalling pathway, citrate cycle, insulin signalling pathway, insulin-like growth factor signalling pathway, and adipocytokine signalling pathway) involved in systematic effects with regulating sugar and energy metabolism through modifying two other local tissue pathways. (tight junction and adherents junction).87
2.4.3. Anti-colitis
In a dextran sulphate sodium (DSS)-induced colitis mouse model, both water extracts of Formosan sambar deer (SVAE) and red deer (RVAE) possessed anti-colitis effects. After microbiome and metabolome analysis, the shift in microbiota might be driven by genera Gemella, Ruminococcaceae_UCG_014, Clostridium_sensu_stricto_1, Curtobacterium, Rikenellaceae_RC9_gut_group, and Lachnoclostridium. The potentially bioactive components of SVAE and RVAE were identified as small molecules, including L-carnitine, hypoxanthine, adrenic acid, creatinine, gamma-aminobutyric-lysine, oleic acid, glycine, poly-γ-glutamic acid, and eicosapentaenoic acid.88
2.4.4. Wound healing
VA protein and stem cells have wound healing effect due to their organ regeneration and rapid tissue growth.89 A chitosan/sodium alginate/velvet antler blood peptides hydrogel (CAVBPH) significantly accelerated skin wound healing in mice. After proteomics analysis, the mechanisms of CAVBPH might be associated with the activation of the PI3K/AKT/mTOR and SIRT1/NF-κB pathways.90
2.4.5. Anti-tumor
Deer antler has been used in the treatment of cancer.91 A velvet antler protein (VA-pro), extracted from velvet antler residue by simulating the gastrointestinal digestion the velvet with average molecular weight of 22.589 kDa, could inhibit the proliferation and promote apoptosis of solid S180 tumors by inducing S phase cell cycle arrest of tumor cells mediated through mitochondria.92 Another study, engineered antler stem cells (ASCs)-derived exosomes, termed as M2Pep-Exo (pIC) were constructed for improved tumor immunotherapy through modifying with M2 macrophage targeting peptide (M2Pep) and toll-like receptor 3 (TLR3) agonist poly(I:C). Result demonstrated that the M2Pep-Exo(pIC) could enhance systemic anti-tumor immune responses, suggesting that the natural nanocarrier exosomes from ASCs might have potential to suppress tumor growth and improve immunotherapy effect for tumors.93 By analyzing the GeneCards, OMIM database, BATMAN-TCM and the STRING database, a total of 66 target gene were annotated into the cancer pathway,34 supporting the anti-cancer effects of deer antler.
3. The limitation and future challenges
The therapeutic effects of VA have been intensive study. However, characterization of underlying mechanisms using different omics is still few and limits in single omic approach. Although each type of omics data could provide useful information for biomarkers and biological pathways or processes for therapeutic effects of VA. Analysis by single omic is restricted to correlations. Integration of different omics data types could elucidate potential molecular mechanism of VA intervention.
VA, a substance revered both in traditional and modern medicine for its therapeutic properties. While VA's rich composition of amino acids, minerals, and other bioactive compounds underscores its therapeutic potential, we must also address its limitations. These include the need for more rigorous clinical trials to substantiate traditional claims, challenges in standardizing extracts, and sustainability concerns regarding sourcing. The future challenges in integrating VA into mainstream medical practices, such as regulatory hurdles and the need for more comprehensive pharmacological studies. By providing a balanced view of VA's medical applications, we aim to pave the way for future research and potential therapeutic innovations. Additionally, VA samples from different deer species, physiological conditions, VA sections, preparation methods, and storage conditions could significantly affect the therapeutic effects, which limit to generate a consistent omic data in different batch samples. In the future, the parameters affecting VA therapeutic effects with bioactive compounds should be identified. Further multi-omics study, including microbiomics, metabolomics, transcriptomics, and proteomics, is necessary for each therapeutic effect to clarify what bioactive components modulate which signaling pathways via alternation of specific microbial composition and metabolites. This information is also important for future development of VA as precision medicine.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Wu F., Li H., Jin L., et al. Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology. J Ethnopharmacol. 2013;145:13. doi: 10.1016/j.jep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Sui Z., Zhang L., Huo Y., Yukui Z. Bioactive components of velvet antlers and their pharmacological properties. J Pharm Biomed Anal. 2014;87:12. doi: 10.1016/j.jpba.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Clark D.E., Li C., Wang W., Martin S.K., Suttie J.M. Vascular localization and proliferation in the growing tip of the deer antler. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:973–981. doi: 10.1002/ar.a.20364. [DOI] [PubMed] [Google Scholar]
- 4.Clark D.E., Lord E.A., Suttie J.M. Expression of VEGF and pleiotrophin in deer antler. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1281–1293. doi: 10.1002/ar.a.20393. [DOI] [PubMed] [Google Scholar]
- 5.Pita-Thomas W., Fernandez-Martos C., Yunta M., et al. Gene expression of axon growth promoting factors in the deer antler. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y., Jin Y., Wang F.J., Yan J.Z., Qi Y.X., Ye M.L. Protein digestomic analysis reveals the bioactivity of deer antler velvet in simulated gastrointestinal digestion. Food Res Int. 2017;96:182–190. doi: 10.1016/j.foodres.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y., Liu L., Shan X., et al. Pilose antler peptide attenuates LPS-induced inflammatory reaction. Int J Biol Macromol. 2018;108:272–276. doi: 10.1016/j.ijbiomac.2017.11.176. [DOI] [PubMed] [Google Scholar]
- 8.Dai T.Y., Wang C.H., Chen K.N., et al. The antiinfective effects of velvet antler of Formosan sambar deer (Cervus unicolor swinhoei) on Staphylococcus aureus-infected mice. Evi Based Comple Alternat Med. 2011;2011 doi: 10.1155/2011/534069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo C., Cheng Y., Ho S., Yu C., Chen M. Comparison of anti-inflammatory effect and protein profile between the water extracts from Formosan sambar deer and red deer. J Food Drug Anal. 2018;26:8. doi: 10.1016/j.jfda.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Zhang J., Yang X., Huang F. Hypoglycemic activity of CPU2206: a novel peptide from sika (Cervus nippon Temminck) antler. J Food Biochem. 2019;43 doi: 10.1111/jfbc.13063. [DOI] [PubMed] [Google Scholar]
- 11.Hung Y.K., Ho S.T., Kuo C.Y., Chen M.J. In vitro effects of velvet antler water extracts from Formosan Sambar deer and red deer on barrier integrity in Caco-2 cell. Int J Med Sci. 2021;18:1778–1785. doi: 10.7150/ijms.53599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo C.Y., Wang T., Dai T.Y., et al. Effect of the velvet antler of Formosan sambar deer (Cervus unicolor swinhoei) on the prevention of an allergic airway response in mice. Evi Based Comple Alternat Med. 2012;2012 doi: 10.1155/2012/481318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zang Z.J., Tang H.F., Tuo Y., et al. Effects of velvet antler polypeptide on sexual behavior and testosterone synthesis in aging male mice. Asian J Androl. 2016;18:613–619. doi: 10.4103/1008-682X.166435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng K., Li Q., Lin D., et al. Peptidomic analysis of pilose antler and its inhibitory effect on triple-negative breast cancer at multiple sites. Food Funct. 2020;11:14. doi: 10.1039/d0fo01531h. [DOI] [PubMed] [Google Scholar]
- 15.Yang C., Cai W., Wen H., et al. Pilose antler peptide protects osteoblasts from inflammatory and oxidative injury through EGF/EGFR signaling. Int J Biol Macromol. 2017;99:6. doi: 10.1016/j.ijbiomac.2017.02.056. [DOI] [PubMed] [Google Scholar]
- 16.Xin J.L., Zhang Y., Li Y., Zhang L.Z., Lin Y., Zheng L.W. Protective effects of Cervus nippon Temminck velvet antler polypeptides against MPP+-induced cytotoxicity in SH-SY5Y neuroblastoma cells. Mol Med Rep. 2017;16:5143–5150. doi: 10.3892/mmr.2017.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui Z., Zhang L., Huo Y., Zhang Y. Bioactive components of velvet antlers and their pharmacological properties. J Pharm Biomed Anal. 2014;87:229–240. doi: 10.1016/j.jpba.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L.Q., Wang J., Li T., et al. Determination of the chemical components and phospholipids of velvet antler using UPLC/QTOF-MS coupled with UNIFI software. Exp Ther Med. 2019;17:3789–3799. doi: 10.3892/etm.2019.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai X., Shen L. Advances and trends in omics technology development. Front Med. 2022;9 doi: 10.3389/fmed.2022.911861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karahalil B. Overview of systems biology and omics technologies. Curr Med Chem. 2016;23:4221–4230. doi: 10.2174/0929867323666160926150617. [DOI] [PubMed] [Google Scholar]
- 21.Hasin Y., Seldin M., Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krassowski M., Das V., Sahu S.K., Misra B.B. State of the field in multi-omics research: from computational needs to data mining and sharing. Front Genet. 2020;11 doi: 10.3389/fgene.2020.610798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe R., Shirley N., Bleackley M., Dolan S., Shafee T. Transcriptomics technologies. PLoS Comput Biol. 2017;13 doi: 10.1371/journal.pcbi.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z., Gerstein M., Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Amrani S., Al-Jabri Z., Al-Zaabi A., Alshekaili J., Al-Khabori M. Proteomics: concepts and applications in human medicine. World J Biol Chem. 2021;12:57–69. doi: 10.4331/wjbc.v12.i5.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aderemi A.V., Ayeleso A.O., Oyedapo O.O., Mukwevho E. Metabolomics: a scoping review of its role as a tool for disease biomarker discovery in selected non-communicable diseases. Metabolites. 2021;11 doi: 10.3390/metabo11070418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couvillion S.P., Zhu Y., Nagy G., et al. New mass spectrometry technologies contributing towards comprehensive and high throughput omics analyses of single cells. Analyst. 2019;144:794–807. doi: 10.1039/c8an01574k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson C.H., Ivanisevic J., Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg G., Rybakova D., Fischer D., et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8:103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galloway-Peña J., Hanson B. Tools for analysis of the microbiome. Dig Dis Sci. 2020;65:674–685. doi: 10.1007/s10620-020-06091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandita D., Pandita A. Omics technology for the promotion of nutraceuticals and functional foods. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.817247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasin Y., Seldin M., Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Pedrouso M., Lorenzo J.M., Landete-Castillejos T., et al. SWATH-MS quantitative proteomic analysis of deer antler from two regenerating and mineralizing sections. Biology. 2021;10 doi: 10.3390/biology10070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L., Jiao Y., Yang M., Wu L., Long G., Hu W. Network pharmacology, molecular docking and molecular dynamics to explore the potential immunomodulatory mechanisms of deer antler. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241210370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Han W.X., Liu L. Clinical research on the Xin-Bao pills treatment for chronic arrhythmia. China J Traditi Chinese Med Pharm. 2014;20:2. [Google Scholar]
- 36.Ni Y., Wang Z., Ma L., Yang L., Wu T., Fu Z. Pilose antler polypeptides ameliorate inflammation and oxidative stress and improves gut microbiota in hypoxic-ischemic injured rats. Nutr Res. 2019;64:93–108. doi: 10.1016/j.nutres.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Shao M.J., Wang S.R., Zhao M.J., et al. The effects of velvet antler of deer on cardiac functions of rats with heart failure following myocardial infarction. Evi Based Comple Alternat Med. 2012;2012 doi: 10.1155/2012/825056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Huang X., Sun J. Protective effect of alcholic extractive of Cornu Cervi against myocardial damage of acute myocardial infarction model rats and influence on plasma ET. J Tradit Chin Med. 2007;14:2. [Google Scholar]
- 39.Ling Y., Feng B., Zhang Y-h. vol. 23. Journal of Changchun University of Traditional Chinese Medicine; 2007. p. 2. (The Effection of Cornu Cervi on the Content of SOD ,MDA during the Later Stage of Aacute Myocardial Ischemic in Rat). [Google Scholar]
- 40.Gu T., Ma Y., Zhang X ea. vol. 28. Journal of China Medical University; 1999. p. 4. (Protective Effects of Pantocrine on Rat Myocardium with Ischemia Reperfusion Injury by Modifying the Activity of Na+-K+-ATPase). [Google Scholar]
- 41.Xiao X., Xu S., Li L., et al. The effect of velvet antler proteins on cardiac microvascular endothelial cells challenged with ischemia-hypoxia. Front Pharmacol. 2017;8:601. doi: 10.3389/fphar.2017.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu T., Yang L., Chen Y., et al. Pilose antler polypeptides ameliorates hypoxic-ischemic encephalopathy by activated neurotrophic factors and SDF1/CXCR4 axis in rats. Acta Biochim Biophys Sin. 2018;50:254–262. doi: 10.1093/abbs/gmy005. [DOI] [PubMed] [Google Scholar]
- 43.Pei H., Du R., He Z., et al. Protection of a novel velvet antler polypeptide PNP1 against cerebral ischemia-reperfusion injury. Int J Biol Macromol. 2023;247 doi: 10.1016/j.ijbiomac.2023.125815. [DOI] [PubMed] [Google Scholar]
- 44.Bennet L., Tan S., Van den Heuij L., et al. Cell therapy for neonatal hypoxia-ischemia and cerebral palsy. Ann Neurol. 2012;71:589–600. doi: 10.1002/ana.22670. [DOI] [PubMed] [Google Scholar]
- 45.Dixon B.J., Reis C., Ho W.M., Tang J., Zhang J.H. Neuroprotective strategies after neonatal hypoxic ischemic encephalopathy. Int J Mol Sci. 2015;16:22368–22401. doi: 10.3390/ijms160922368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia P., Liu D., Jiao Y., et al. Health effects of peptides extracted from deer antler. Nutrients. 2022;14 doi: 10.3390/nu14194183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao X., Li L., Xu S., et al. Evaluation of velvet antler total protein effect on bone marrow-derived endothelial progenitor cells. Mol Med Rep. 2017;16:3161–3168. doi: 10.3892/mmr.2017.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustafsson A.B., Gottlieb R.A. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 49.Dong Z., Coates D. Bioactive molecular discovery using deer antlers as a model of mammalian regeneration. J Proteome Res. 2021;20:2167–2181. doi: 10.1021/acs.jproteome.1c00003. [DOI] [PubMed] [Google Scholar]
- 50.Shi B., Li G., Wang P., et al. Effect of antler extract on corticosteroid-induced avascular necrosis of the femoral head in rats. J Ethnopharmacol. 2010;127:124–129. doi: 10.1016/j.jep.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 51.Lee H.S., Kim M.K., Kim Y.K., et al. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by antler and fermented antler using Cordyceps militaris. J Ethnopharmacol. 2011;133:710–717. doi: 10.1016/j.jep.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 52.Chen J., Yang Y., Abbasi S., Hajinezhad D., Kontulainen S., Honaramooz A. The effects of elk velvet antler dietary supplementation on physical growth and bone development in growing rats. Evi Based Comple Alternat Med. 2015;2015 doi: 10.1155/2015/819520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sui Z., Sun H., Weng Y., et al. Quantitative proteomics analysis of deer antlerogenic periosteal cells reveals potential bioactive factors in velvet antlers. J Chromatogr A. 2020;1609 doi: 10.1016/j.chroma.2019.460496. [DOI] [PubMed] [Google Scholar]
- 54.Qin T., Zhang G., Zheng Y., et al. A population of stem cells with strong regenerative potential discovered in deer antlers. Science. 2023;379:840–847. doi: 10.1126/science.add0488. [DOI] [PubMed] [Google Scholar]
- 55.Wang D.T., Berg D., Ba H.X., Sun H.M., Wang Z., Li C.Y. Deer antler stem cells are a novel type of cells that sustain full regeneration of a mammalian organ-deer antler. Cell Death Dis. 2019;10 doi: 10.1038/s41419-019-1686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong Z., Ba H.X., Zhang W., Coates D., Li C.Y. iTRAQ-based quantitative proteomic analysis of the potentiated and dormant antler stem cells. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lei J., Jiang X., Li W., et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. 2022;13:220–226. doi: 10.1007/s13238-021-00860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drevet S., Gavazzi G., Grange L., Dupuy C., Lardy B. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Exp Gerontol. 2018;111:107–117. doi: 10.1016/j.exger.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Lepetsos P., Papavassiliou A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862:576–591. doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Ansari M.Y., Ahmad N., Haqqi T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ling X., Luo T., Wang L., et al. Potential of food protein-derived peptides for the improvement of osteoarthritis. Trends Food Sci Technol. 2022;129:14. [Google Scholar]
- 62.Qi T., Gao H., Dang Y., Huang S., Peng M. Cervus and cucumis peptides combined umbilical cord mesenchymal stem cells therapy for rheumatoid arthritis. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000021222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao B., Zhao Y., Wang Q., et al. De novo characterization of the antler tip of Chinese Sika deer transcriptome and analysis of gene expression related to rapid growth. Mol Cell Biochem. 2012;364:93–100. doi: 10.1007/s11010-011-1209-3. [DOI] [PubMed] [Google Scholar]
- 64.Yao B., Zhang M., Liu M., Wang Q., Liu M., Zhao Y. Sox9 functions as a master regulator of antler growth by controlling multiple cell lineages. DNA Cell Biol. 2018;37 doi: 10.1089/dna.2017.3885. [DOI] [PubMed] [Google Scholar]
- 65.Yao B., Zhang M., Leng X., Zhao D. Proteomic analysis of the effects of antler extract on chondrocyte proliferation, differentiation and apoptosis. Mol Biol Rep. 2019;46:1635–1648. doi: 10.1007/s11033-019-04612-1. [DOI] [PubMed] [Google Scholar]
- 66.Yao B., Zhao Y., Zhang H., et al. Sequencing and de novo analysis of the Chinese Sika deer antler-tip transcriptome during the ossification stage using Illumina RNA-Seq technology. Biotechnol Lett. 2012;34:813–822. doi: 10.1007/s10529-011-0841-z. [DOI] [PubMed] [Google Scholar]
- 67.Yao B., Zhou Z., Zhang M., Leng X., Zhao D. Investigating the molecular control of deer antler extract on articular cartilage. J Orthop Surg Res. 2021;16:8. doi: 10.1186/s13018-020-02148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimai H.P., Fahrleitner-Pammer A. Osteoporosis and Fragility Fractures: currently available pharmacological options and future directions. Best Pract Res Clin Rheumatol. 2022;36 doi: 10.1016/j.berh.2022.101780. [DOI] [PubMed] [Google Scholar]
- 69.Atmaca A., Kleerekoper M., Bayraktar M., Kucuk O. Soy isoflavones in the management of postmenopausal osteoporosis. Menopause. 2008;15:748–757. doi: 10.1097/gme.0b013e31815c1e7f. [DOI] [PubMed] [Google Scholar]
- 70.Raisz L.G. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo K., Wang T., Luo E., Leng X., Yao B. Use of network pharmacology and molecular docking technology to analyze the mechanism of action of velvet antler in the treatment of postmenopausal osteoporosis. Evi Based Comple Alternat Med. 2021;2021 doi: 10.1155/2021/7144529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niu F.-Q., Zhao Y., Xu Y., Zhang H. Therapeutic effects of collagen of antler plate on osteoporosis in ovariectomized rats. Chinese J Moder Applie Pharm. 2012;29:5. [Google Scholar]
- 73.Na Li LZ., Lin Zhe, Li Jing, et al. Metabonomics study of the anti-osteoporosis effect of velvet collagen hydrolysate using rapid resolution liquid chromatography combined with quadrupole time-of-flight tandem mass spectrometry. J Liq Chrom Relat Tech. 2015;38:6. [Google Scholar]
- 74.Yao B., Gao H., Liu J., Zhang M., Leng X., Zhao D. Identification of potential therapeutic targets of deer antler extract on bone regulation based on serum proteomic analysis. Mol Biol Rep. 2019;46:4861–4872. doi: 10.1007/s11033-019-04934-0. [DOI] [PubMed] [Google Scholar]
- 75.Dong Z., Coates D. Bioactive molecular discovery using deer antlers as a model of mammalian regeneration. J Proteome Res. 2021;20:15. doi: 10.1021/acs.jproteome.1c00003. [DOI] [PubMed] [Google Scholar]
- 76.Pan W., Du J., An L., et al. Sika deer velvet antler protein extract modulater bone metabolism and the structure of gut microbiota in ovariectomized mice. Food Sci Nutr. 2023;11:3309–3319. doi: 10.1002/fsn3.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brayne C., Miller B. Dementia and aging populations-A global priority for contextualized research and health policy. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cenini G., Lloret A., Cascella R. Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen P., Chen F., Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018;8:1465. doi: 10.1038/s41598-018-19732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Q., Lin J.-N., Sui X., et al. Antiapoptotic effects of velvet antler polypeptides on damaged neurons through the hypothalamic-pituitary-adrenal axis. J Integr Neurosci. 2020;19:9. doi: 10.31083/j.jin.2020.03.167. [DOI] [PubMed] [Google Scholar]
- 81.Liu X., Yang Q., Li H., et al. The anti-aging effect of velvet antler polypeptide is dependent on modulation of the gut microbiota and regulation of the PPARα/APOE4 pathway. J Integr Neurosci. 2021;20:11. doi: 10.31083/j.jin2003061. [DOI] [PubMed] [Google Scholar]
- 82.Hor Y.Y., Lew L.C., Jaafar M.H., et al. Lactobacillus sp. improved microbiota and metabolite profiles of aging rats. Pharmacol Res. 2019;146 doi: 10.1016/j.phrs.2019.104312. [DOI] [PubMed] [Google Scholar]
- 83.Ho S.T., Hsieh Y.T., Wang S.Y., Chen M.J. Improving effect of a probiotic mixture on memory and learning abilities in d-galactose-treated aging mice. J Dairy Sci. 2019;102:1901–1909. doi: 10.3168/jds.2018-15811. [DOI] [PubMed] [Google Scholar]
- 84.Li Y., Macyczko J.R., Liu C.C., Bu G. ApoE4 reduction: an emerging and promising therapeutic strategy for Alzheimer's disease. Neurobiol Aging. 2022;115:20–28. doi: 10.1016/j.neurobiolaging.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang T., Zhong S., Li T., Zhang J. Saponins as modulators of nuclear receptors. Crit Rev Food Sci Nutr. 2020;60:94–107. doi: 10.1080/10408398.2018.1514580. [DOI] [PubMed] [Google Scholar]
- 86.Zhang T., Liang Y., Zhang J. Natural and synthetic compounds as dissociated agonists of glucocorticoid receptor. Pharmacol Res. 2020;156 doi: 10.1016/j.phrs.2020.104802. [DOI] [PubMed] [Google Scholar]
- 87.Chen J., Hsiang C.H., Lin Y., Ho T. Deer antler extract improves fatigue effect through altering the expression of genes related to muscle strength in skeletal muscle of mice. Evi Based Comple Alternat Med. 2014;2014 doi: 10.1155/2014/540580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hung Y.K., Ho S.T., Kuo C.Y., Chen M.J. Multiomics strategy reveals the mechanism of action and ameliorating effect of deer velvet antler water extracts on DSS-induced colitis. Biomedicines. 2023;11 doi: 10.3390/biomedicines11071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang G., Wang D., Ren J., et al. Velvet antler peptides reduce scarring via inhibiting the TGF-beta signaling pathway during wound healing. Front Med. 2021;8 doi: 10.3389/fmed.2021.799789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hao M., Peng X., Sun S., Ding C., Liu W. Chitosan/sodium alginate/velvet antler blood peptides hydrogel promoted wound healing by regulating PI3K/AKT/mTOR and SIRT1/NF-kappaB pathways. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.913408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu G., Zhao H., Xu J., Zhang Y., Qi X., Shi A. Hard antler extract inhibits invasion and epithelial-mesenchymal transition of triple-negative and Her-2(+) breast cancer cells by attenuating nuclear factor-kappaB signaling. J Ethnopharmacol. 2021;269 doi: 10.1016/j.jep.2020.113705. [DOI] [PubMed] [Google Scholar]
- 92.Cao T.Q., An H.X., Ma R.J., et al. Structural characteristics of a low molecular weight velvet antler protein and the anti-tumor activity on S180 tumor-bearing mice. Bioorg Chem. 2023;131 doi: 10.1016/j.bioorg.2022.106304. [DOI] [PubMed] [Google Scholar]
- 93.Yang M., Pu L., Yang S., et al. Engineered antler stem cells derived exosomes potentiate anti-tumor efficacy of immune checkpoint inhibitor by reprogramming immunosuppressive tumor microenvironment. Chem Eng J. 2024;497:16. [Google Scholar]